Abstract
Background
Previous studies on the relationship between fructose intake and cardiometabolic biomarkers have yielded inconsistent results, and the metabolic effects of fructose are likely to vary across food sources such as fruit versus sugar-sweetened beverages (SSB).
Objectives
We aimed to examine associations of fructose from 3 major sources (SSB, fruit juice, and fruit) with 14 insulinemic/glycemic, inflammatory, and lipid markers.
Methods
We utilized cross-sectional data from 6858 men in the Health Professionals Follow-up Study, 15,400 women in NHS, and 19,456 women in NHSII who were free of type 2 diabetes, CVDs, and cancer at blood draw. Fructose intake was assessed via a validated FFQ. Multivariable linear regression was used to estimate the percentage differences of biomarker concentrations according to fructose intake.
Results
We found a 20 g/d increase in total fructose intake was associated with 1.5%– 1.9% higher concentrations of proinflammatory markers plus 3.5% lower adiponectin, as well as 5.9% higher TG/HDL cholesterol ratio. Unfavorable profiles of most biomarkers were only associated with fructose from SSB and juice. In contrast, fruit fructose was associated with lower concentrations of C-peptide, CRP, IL-6, leptin, and total cholesterol. Substituting 20 g/d fruit fructose for SSB fructose was associated with 10.1% lower C-peptide, 2.7%–14.5% lower proinflammatory markers and 1.8%–5.2% lower blood lipids.
Conclusions
Beverage fructose intake was associated with adverse profiles of multiple cardiometabolic biomarkers.
Keywords: fructose, fruit, fruit juice, sugar-sweetened beverages, glycemic control, insulin, inflammation, lipids
Introduction
Fructose is present as a monosaccharide rich in fruit and high fructose corn syrup (HFCS, a glucose-fructose mixture sweetener containing 42%–55% fructose) or as a part of a disaccharide, sucrose, with glucose in a 1:1 ratio. The national survey data from the United States in 1999–2004 reported an average fructose intake of 49 g/d that accounted for ∼10% of total energy intake for both sexes and all age groups [1].
A close parallel between the rise in HFCS consumption and the increases in obesity and type 2 diabetes in the United States over the past 3 to 4 decades have stimulated investigations on the effect of fructose. In animal studies, fructose appears to impair glycemic control, promote inflammation, and trigger lipogenesis in the liver [2]. In human, some randomized controlled trials (RCTs) assessed the effect of short-term fructose feeding on cardiometabolic biomarkers, yet the results were inconsistent [[3], [4], [5]]. The inconsistency might be related to different food sources of fructose. Sugar-sweetened beverages (SSBs) are the most common source in US diets, providing 46.0% of total fructose, whereas fruit only provides 13.4% [1]. Consumption of SSBs was consistently found to be associated with an unhealthful lipid profile and an increased concentration of insulin and inflammatory markers [6, 7]. Conversely, fruit intake is featured in several healthy dietary patterns such as the Mediterranean diet and was shown to be anti-inflammatory [8]. Only one study to date has investigated the metabolic effects of fructose from different food sources and found only fructose from SSBs and juice, but not fructose from fruit, was associated with higher intrahepatic lipid contents [9].
In the present study, we examined the cross-sectional associations of fructose intake with insulinemic/glycemic and inflammatory markers as well as blood lipids in 3 large prospective cohorts of US adults, the Health Professionals Follow-up Study (HPFS), NHS, and NHSII. We also separately examined the associations of SSB fructose, fruit juice fructose, and fruit fructose with these biomarkers.
Method
Study population
The HPFS was initiated in 1986 with 51,529 male health professionals, aged 40–75 y at enrollment [10]. The NHS was initiated in 1976 with 121,700 female registered nurses, aged 30–55 y at enrollment. The NHSII was initiated in 1989 with 116,429 nurses, aged 25–42 y at enrollment [11]. For each cohort, participants were followed up with mailed questionnaires about medical, lifestyle, and other health-related information biennially, with follow-up rates of >90% for each 2-y cycle. Blood samples were collected from 18,159 men in the HPFS between 1993 and 1995, from 32,826 women in the NHS between 1989 and 1990 and from 29,611 women in the NHSII between 1996 and 1999. As previously reported [12], participants who provided blood samples were generally similar with those who did not in terms of demographics and lifestyle characteristics. For the current study, we included participants who provided a blood sample and had their biomarkers measured when involved in prior nested case-control analyses of type 2 diabetes, CVD, and several cancers (for example, breast, colorectum, endometrium, ovary, and prostate) [[12], [13], [14]]. We excluded participants who had a history of diabetes (n = 1779), CVD (n = 1302) or cancer (n = 1475) at blood draw. Participants with missing data on fructose intake (n = 1077) were further excluded. A total of 41,714 individuals (6858 from HPFS, 15,400 from NHS, and 19,456 from NHSII) were included in the final analysis. Supplemental Figure 1 illustrates how study population was generated. The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health. The return of the questionnaires was considered to imply informed consent.
Assessment of fructose and major sources of fructose intake
Dietary intake was assessed via validated FFQs administered every 4 y. In each FFQ, participants were asked the average frequency of intake over the previous year for consuming a standard portion size of each food item, ranging from “never or less than once per month” to “6 or more times per day.” Nutrient intake was calculated by multiplying the frequency of consuming each food by its nutrient contents (from US Department of Agriculture food composition database) [15], and then summing contributions from all foods. The nutrient value was adjusted for total energy intake using residual method. Fructose intake was calculated as free fructose intake plus half sucrose intake because sucrose is digested into glucose and fructose rapidly in the small intestine.
SSBs in this study included carbonated (for example, Coke, Pepsi, 7-Up) and noncarbonated soft drinks (for example, Hawaiian Punch). Fruit juice fructose was calculated as the sum of fructose contributions from apple juice or cider, orange juice, grapefruit juice, and other fruit juices. Fruit fructose was calculated as the sum of fructose from 16 fruit items.
The validity of the FFQs has been evaluated in a subgroup of participants whose intake was also assessed by multiple diet records. The correlation coefficient between the FFQs and diet records was 0.54 for sucrose but was not evaluated for fructose. However, the correlations were generally high for SSBs, fruit juice, and fruit: 0.84 for sugar-sweetened cola, 0.84 for orange juice, 0.79 for banana, and 0.80 for apple [16, 17]. In the current study, to reduce within-person measurement error and better reflect usual diet, we used the average intake from the last 2 FFQs administered before blood collection (1990 and 1994 for HPFS, 1986 and 1990 for NHS, and 1995 and 1999 for NHSII). On average, the midpoint of these 2 FFQs preceded blood collection by 22, 18, and 6 mo in HPFS, NHS, and NHSII, respectively.
Biochemical analyses
Detailed procedures for blood collection, handling, and storage have been reported elsewhere [13]. In the current study, we focused on 14 insulinemic/glycemic and inflammatory biomarkers and blood lipids that have been shown to be related to the etiology of type 2 diabetes, CVD, or cancer in previous studies and were measured in our cohorts: C-peptide, hemoglobin A1c, CRP, IL-6, TNF-R1, TNF-R2, ICAM-1, adiponectin, leptin, total cholesterol (TC), HDL cholesterol, LDL cholesterol, TG, and TG/HDL cholesterol ratio. All biomarkers were measured using standard methods [14, 18, 19]. Quality-control samples were randomly interspersed among the case-control samples. The intra-assay coefficients of variation ranged from 1% to 20% for all biomarkers across batches. Laboratory personnel were masked to quality-control samples and case-control status.
Covariate assessment
Overall diet quality was assessed by the alternative healthy eating index (AHEI)-2010, with a higher score indicating a better diet quality [20]. Information on nondietary covariates was updated in biennial follow-up questionnaires. Physical activity (PA) was calculated by multiplying the hours spent on various forms of exercise per week and the metabolic equivalent of task (MET) score of each activity and then summing up the MET-hours for all activities to obtain a value of total weekly MET-hours. The reproducibility and validity of PA have been described previously [21, 22]. The cumulative average PA and BMI (from 1990 to 1994 questionnaires for HPFS, 1986 and 1988 questionnaires for NHS, and 1995–1999 questionnaires for NHSII) were used in the analyses. For other nondietary covariates, we used questionnaires closest to the blood draw (1994 for HPFS, 1990 for NHS, and 1999 for NHSII).
Statistical analyses
To account for variation in sample handling and laboratory drift among batches, all biomarker concentrations were recalibrated across batches within each cohort to the value of an “average batch” using the method of Rosner et al. [23]. We constructed multivariable generalized linear models to evaluate the associations between fructose intake and biomarker concentrations. All biomarkers were natural log-transformed to improve normality. Model 1 was adjusted for cohort (HPFS/NHS/NHSII), age at blood draw (continuous), sex (male/female), case-control status (case/control), fasting status (fasting/nonfasting), and total energy intake (quartiles). Model 2 was additionally adjusted for PA (quartiles); smoking status and intensity (never smoker; former smoker, pack-years <30; former smoker, pack-years ≥30; current smoker, pack-years <30; current smoker, pack-years ≥30); alcohol intake (0, 0.1–4.9, 5.0–9.9, 10.0–14.9, ≥15.0 g/d); AHEI-2010 score excluding SSBs, fruit juice, and fruit (quartiles); hypertension (yes/no); hypercholesterolemia (yes/no); use of cholesterol-lowering drugs (yes/no); regular use of aspirin or nonsteroidal anti-inflammatory drugs (yes/no); BMI (<23.0, 23.0–24.9, 25.0–27.4, 27.5–29.9, ≥30.0 kg/m2); and additionally, for women, menopausal status (premenopausal/postmenopausal/unknown) and menopausal hormone therapy (never/past/current use). The results were presented as percentage differences calculated using the equation: [exp (β-coefficient) − 1] × 100%. We performed restricted cubic spline analyses and used a likelihood ratio test to compare the model with only the linear term of fructose with the model with both the linear and spline terms. We did not find strong statistical evidence for nonlinearity and thus presented the results for a 20g/d increase in fructose assuming linear relationships between fructose and log-transformed biomarker concentrations.
We further examined associations between fructose intake and biomarker concentrations stratified by sex (male/female), age at blood draw (<60/≥60 y), smoking status (never/ever), alcohol intake (below/above median), PA (quartiles), and BMI (<25.0/25.0–29.9/≥30.0 kg/m2). Potential effect modification was assessed by likelihood ratio tests comparing the model with and without the product term between 20 g/d fructose and each of the stratified variables above.
In sensitivity analyses, we restricted the analysis to participants who provided fasting blood, those who were selected as controls in previous case-control studies, those who reported no history of hypertension or hypercholesterolemia at blood draw, and those whose FFQ preceded blood draw by ≥1 y. Considering potential confounding by dietary fiber intake and mediation by BMI, we conducted an analysis additionally adjusting for fiber intake and an analysis not adjusting for BMI to test the robustness of results.
Finally, we examined the associations of 20 g/d SSB fructose, fruit juice fructose, and fruit fructose with these biomarkers. The multivariable models were adjusted for the same set of covariates as in Model 2, and each fructose source was mutually adjusted for other sources. We also estimated the percentage differences in biomarkers when substituting 20 g/d fruit fructose for 20 g/d SSB fructose and juice fructose, respectively, on the basis of the partition model approach [24].
We considered 2-sided P < 0.005 to be statistically significant and 0.005 ≤ P < 0.05 to be suggestively significant [25]. SAS version 9.4 (SAS institute) was used for all statistical analyses.
Results
Total fructose intake was higher in the male cohort and later cohort than the female and earlier cohort, with mean intakes of 48.6 g/d in HPFS and 43.4 g/d in NHSII compared with 39.2 g/d in NHS. Fruit was the major contributor to fructose in all 3 cohorts. The contribution from SSBs was larger in the male and later cohort than the female and earlier cohort. For example, 12% and 17% of fructose intakes were from SSBs in HPFS and NHSII, respectively, compared with 9% in NHS, whereas 28% and 24% of fructose intakes were from fruit in HPFS and NHSII, respectively, compared with 35% in NHS. The top 10 food contributors in each cohort are presented in Supplemental Table 1. Table 1 demonstrates the characteristics of study participants at blood draw according to quartiles of total fructose intake. Across all cohorts, participants who had a higher total fructose intake were less likely to be current smokers, drank substantially less alcohol, did more PA, and had lower BMI. This pattern was also observed in participants who had a higher fruit fructose and juice fructose intake but not in participants who had a higher SSB fructose intake (Supplemental Table 2). Biomarker concentrations according to total fructose quartiles are presented in Supplemental Table 3.
TABLE 1.
Characteristics of participants at blood draw according to total fructose quartiles in HPFS, NHS and NHSII1
| HPFS (n = 6858) |
NHS (n = 15,400) |
NHSII (n = 19,456) |
||||
|---|---|---|---|---|---|---|
| Quartile 1 |
Quartile 4 |
Quartile 1 |
Quartile 4 |
Quartile 1 |
Quartile 4 |
|
| n = 1714 | n = 1714 | n = 3849 | n = 3849 | n = 4864 | n = 4863 | |
| Age at blood draw, y | 61.1 ± 8.1 | 62.6 ± 8.8 | 56.2 ± 7.0 | 58.4 ± 6.8 | 44.0 ± 4.5 | 44.0 ± 4.7 |
| Fructose intake, g/d | 31.5 ± 5.7 | 68.2 ± 10.6 | 26.4 ± 4.6 | 53.4 ± 7.6 | 27.4 ± 4.7 | 63.3 ± 12.5 |
| SSB fructose intake, g/d | 2.1 ± 3.9 | 14.4 ± 19.1 | 1.4 ± 3.1 | 9.5 ± 14.8 | 2.0 ± 2.9 | 22.8 ± 21.7 |
| Juice fructose intake, g/d | 3.7 ± 3.7 | 9.9 ± 8.3 | 3.3 ± 3.7 | 9.2 ± 8.5 | 2.7 ± 3.0 | 9.3 ± 9.6 |
| Fruit fructose intake, g/d | 8.3 ± 5.0 | 20.2 ± 12.5 | 8.6 ± 4.6 | 18.4 ± 10.3 | 7.0 ± 4.4 | 11.6 ± 9.1 |
| Percentage fructose from SSB, % | 6.6 | 20.3 | 5.1 | 16.8 | 6.9 | 33.5 |
| Percentage fructose from juice, % | 11.4 | 14.5 | 12.4 | 17.2 | 9.5 | 14.9 |
| Percentage fructose from fruit, % | 25.9 | 29.8 | 32.1 | 34.7 | 25.0 | 19.3 |
| Percentage energy from fructose, % | 6.3 | 13.6 | 6.7 | 13.5 | 6.1 | 14.0 |
| Percentage energy from total carbohydrates, % | 42.5 | 57.6 | 42.5 | 55.8 | 45.3 | 58.1 |
| Percentage energy from fat, % | 33.2 | 27.2 | 34.9 | 28.6 | 33.7 | 27.0 |
| Total energy intake, kcal/d | 1999 ± 566 | 1954 ± 547 | 1769 ± 473 | 1736 ± 483 | 1832 ± 520 | 1804 ± 527 |
| AHEI score2 | 45.1 ± 9.1 | 48.6 ± 8.8 | 44.2 ± 8.7 | 46.0 ± 8.6 | 44.4 ± 8.7 | 44.1 ± 8.4 |
| Physical activity, MET-h/wk | 28.8 ± 22.2 | 32.6 ± 24.4 | 13.0 ± 14.0 | 15.7 ± 16.0 | 16.4 ± 18.2 | 16.9 ± 19.2 |
| BMI, kg/m2 | 26.0 ± 3.3 | 25.1 ± 2.9 | 25.7 ± 4.8 | 24.9 ± 4.4 | 26.8 ± 6.1 | 25.5 ± 5.6 |
| Current smokers, % | 8.8 | 5.1 | 20.4 | 11.6 | 10.6 | 8.1 |
| Pack-years of smoking among ever-smokers | 16.3 ± 19.9 | 8.6 ± 15.1 | 18.0 ± 22.2 | 9.7 ± 16.3 | 5.7 ± 9.8 | 4.2 ± 9.0 |
| Alcohol intake, g/d | 21.1 ± 18.9 | 5.5 ± 8.3 | 11.1 ± 14.2 | 2.9 ± 5.2 | 6.4 ± 9.6 | 2.1 ± 4.0 |
| Hypertension, % | 25.2 | 23.0 | 24.8 | 23.2 | 13.2 | 12.9 |
| Hypercholesterolemia, % | 39.0 | 36.4 | 35.6 | 40.8 | 22.0 | 23.2 |
| Cholesterol-lowering drug use, % | 7.6 | 6.9 | 2.0 | 2.8 | 3.4 | 4.4 |
| Regular aspirin or NSAID use3, % | 60.8 | 54.2 | 61.3 | 57.8 | 45.0 | 41.3 |
| Postmenopausal, % | — | — | 74.8 | 75.0 | 18.0 | 19.9 |
| Current menopausal hormone use4, % | — | — | 31.1 | 30.2 | 13.4 | 14.8 |
AHEI, Alternative Healthy Eating Index; HPFS, Health Professionals Follow-up Study; MET, metabolic equivalent of task; NSAID, nonsteroidal anti-inflammatory drug; SSB, sugar-sweetened beverages.
Values are means ± SDs for continuous variables and percentages for categorical variables unless otherwise specified. All variables were standardized by age at blood draw.
Excluded SSB, fruit juice, and fruit in the score calculation.
Regular users were defined as ≥2 tablets of aspirin (325 mg/tablet) or NSAIDs per week.
Defined in menopausal women only.
Table 2 shows the associations between total fructose intake and biomarker concentrations. In the combined cohort, a 20 g/d increase in fructose intake was associated with 1.5% to 5.9% higher concentrations of IL-6, TNF-R1, TNF-R2, ICAM-1, TG, TG/HDL cholesterol ratio, and 1.9% to 3.5% lower concentrations of adiponectin and HDL cholesterol. Slightly stronger associations were observed for men than women (P-interaction < 0.05 for TNF-R1 and TNF-R2).
TABLE 2.
Percentage of differences (95% CIs) in biomarker concentrations associated with 20 g/d of total fructose among participants from HPFS, NHS, and NHSII1
| 3 cohorts combined (n = 41,714) | HPFS (n = 6858) | NHS (n = 15,400) | NHSII (n = 19,456) | |
|---|---|---|---|---|
| Insulinemic/glycemic markers | ||||
| C-peptide | ||||
| n | 12,433 | 3946 | 6550 | 1937 |
| Model 1 | −2.1 (−3.5, −0.7)∗∗ | −2.3 (−4.5, −0.1)∗ | −3.1 (−5.4, −0.9)∗ | −0.2 (−3.1, 2.7) |
| Model 2 | 0.9 (−0.5, 2.4) | 1.8 (−0.6, 4.3) | 0.0 (−2.2, 2.4) | 0.6 (−2.2, 3.4) |
| HbA1c | ||||
| n | 9912 | 1919 | 5711 | 2282 |
| Model 1 | −0.1 (−0.3, 0.1) | −0.2 (−0.7, 0.3) | −0.2 (−0.5, 0.1) | 0.1 (−0.2, 0.5) |
| Model 2 | −0.2 (−0.4, 0.0) | −0.1 (−0.7, 0.4) | −0.3 (−0.6, 0.0)∗ | 0.1 (−0.2, 0.4) |
| Inflammatory markers | ||||
| CRP | ||||
| n | 16,810 | 4109 | 9054 | 3647 |
| Model 1 | −5.9 (−8.3, −3.4)∗∗∗ | −6.6 (−10.3, −2.8)∗∗∗ | −8.0 (−11.8, −4.0)∗∗∗ | −1.0 (−6.0, 4.4) |
| Model 2 | 0.3 (−2.2, 2.8) | −0.6 (−4.7, 3.7) | −0.5 (−4.5, 3.6) | 3.0 (−1.7, 7.9) |
| IL-6 | ||||
| n | 11,011 | 2037 | 5926 | 3048 |
| Model 1 | −0.7 (−2.5, 1.0) | −3.7 (−6.7, −0.6)∗ | −1.6 (−4.5, 1.4) | 2.2 (−0.6, 5.1) |
| Model 2 | 1.9 (0.1, 3.7)∗ | −0.7 (−4.1, 2.8) | 2.2 (−0.8, 5.4) | 3.3 (0.7, 6.1)∗ |
| TNF-R1 | ||||
| n | 3314 | 250 | 1729 | 1335 |
| Model 1 | 1.9 (0.6, 3.2)∗ | 5.7 (1.3, 10.3)∗ | 2.6 (0.3, 5.0)∗ | 0.5 (−1.1, 2.1) |
| Model 2 | 1.7 (0.3, 3.0) ∗ | 6.7 (1.7, 12.0)∗ | 2.1 (−0.2, 4.5) | 0.3 (−1.3, 1.8) |
| TNF-R2 | ||||
| n | 10,708 | 2126 | 5847 | 2735 |
| Model 1 | 2.4 (1.6, 3.1)∗∗∗ | 3.6 (2.2, 5.0)∗∗∗ | 2.2 (0.9, 3.6)∗∗∗ | 1.5 (0.4, 2.6)∗ |
| Model 2 | 1.9 (1.1, 2.7)∗∗∗ | 3.2 (1.7, 4.8)∗∗∗ | 1.7 (0.4, 3.1)∗ | 1.1 (0.1, 2.2)∗ |
| ICAM-1 | ||||
| n | 6248 | 2148 | 3130 | 970 |
| Model 1 | 0.5 (−0.4, 1.4) | 1.0 (−0.3, 2.3) | −0.9 (−2.4, 0.6) | 2.2 (0.4, 4.1)∗ |
| Model 2 | 1.5 (0.6, 2.4)∗∗∗ | 1.2 (−0.2, 2.7) | 1.5 (0.0, 3.0) | 1.6 (−0.2, 3.4) |
| Adiponectin | ||||
| n | 13,893 | 2455 | 8100 | 3338 |
| Model 1 | −3.1 (−4.3, −1.9)∗∗∗ | −4.1 (−6.5, −1.6)∗∗ | −2.5 (−4.2, −0.8)∗∗ | −3.5 (−5.7, −1.3)∗∗ |
| Model 2 | −3.5 (−4.7, −2.3)∗∗∗ | −4.2 (−6.8, −1.4)∗∗ | −3.2 (−4.9, −1.5)∗∗∗ | −3.5 (−5.7, −1.3)∗∗ |
| Leptin | ||||
| n | 9831 | 2762 | 3372 | 3697 |
| Model 1 | −6.4 (−8.4, −4.4)∗∗∗ | −9.6 (−12.9, −6.1)∗∗∗ | −8.0 (−12.0, −3.8)∗∗∗ | −2.8 (−5.9, 0.4) |
| Model 2 | −0.9 (−2.7, 0.9) | −1.0 (−4.3, 2.4) | −2.0 (−5.4, 1.5) | 0.4 (−2.2, 3.1) |
| Lipid markers | ||||
| TC | ||||
| n | 14,979 | 2612 | 6741 | 5626 |
| Model 1 | −0.4 (−0.9, 0.0)∗ | −0.7 (−1.6, 0.3) | −0.3 (−1.0, 0.5) | −0.4 (−1.0, 0.2) |
| Model 2 | −0.1 (−0.5, 0.3) | 0.5 (−0.4, 1.5) | −0.2 (−1.0, 0.5) | −0.2 (−0.8, 0.4) |
| HDL-C | ||||
| n | 8623 | 1738 | 5565 | 1320 |
| Model 1 | −3.6 (−4.5, −2.7)∗∗∗ | −3.5 (−5.1, −1.9)∗∗∗ | −3.1 (−4.4, −1.9)∗∗∗ | −4.7 (−6.5, −2.8)∗∗∗ |
| Model 2 | −1.9 (−2.8, −1.0)∗∗∗ | −1.9 (−3.6, −0.1)∗ | −1.6 (−2.9, −0.3)∗ | −2.7 (−4.4, −1.0)∗∗ |
| LDL-C | ||||
| n | 7169 | 2094 | 3757 | 1318 |
| Model 1 | 0.5 (−0.5, 1.5) | 0.3 (−1.3, 1.9) | 0.2 (−1.4, 1.7) | 1.5 (−0.5, 3.6) |
| Model 2 | 0.6 (−0.4, 1.6) | 1.5 (−0.1, 3.2) | −0.6 (−2.1, 0.9) | 1.4 (−0.5, 3.4) |
| TG | ||||
| n | 10,234 | 1598 | 4428 | 4208 |
| Model 1 | 1.6 (0.2, 3.1)∗ | 2.0 (−1.1, 5.3) | 1.7 (−0.8, 4.4) | 1.4 (−0.7, 3.5) |
| Model 2 | 2.8 (1.4, 4.2)∗∗∗ | 4.6 (1.3, 8.0)∗ | 3.8 (1.3, 6.5)∗∗ | 1.5 (−0.4, 3.4) |
| TG/HDL-C ratio | ||||
| n | 6092 | 1516 | 3293 | 1283 |
| Model 1 | 5.4 (2.7, 8.1)∗∗∗ | 5.7 (1.1, 10.5)∗ | 3.2 (−0.7, 7.3) | 8.3 (3.1, 13.8)∗∗ |
| Model 2 | 5.9 (3.3, 8.5)∗∗∗ | 7.0 (2.4, 11.9)∗∗ | 4.8 (0.9, 8.9)∗ | 5.4 (0.7, 10.2)∗ |
HDL-C, HDL cholesterol; HPFS, Health Professionals Follow-up Study; LDL-C, LDL cholesterol; ref, reference; TC, total cholesterol.
∗0.005 ≤ P < 0.05, ∗∗0.001 ≤ P < 0.005, ∗∗∗P < 0.001.
Model 1 was adjusted for cohort (HPFS/NHS/NHSII), age at blood draw (continuous), sex (male/female), case-control status (case/control), fasting status (fasting/not fasting), and total energy intake (quartiles). Model 2 was additionally adjusted for physical activity (quartiles); smoking status and intensity (never smoker; former smoker, pack-years <30; former smoker, pack-years ≥30; current smoker, pack-years <30; current smoker, pack-years ≥30); alcohol intake (0, 0.1–4.9, 5.0–9.9, 10.0–14.9, ≥15.0 g/d); AHEI score excluding fruit, fruit juice, and SSB (quartiles); hypertension (yes/no); hypercholesterolemia (yes/no); use of cholesterol-lowering drugs (yes/no); regular use of aspirin or nonsteroidal anti-inflammatory drug use (yes/no); BMI (<23.0, 23.0–24.9, 25.0–27.4, 27.5–29.9, ≥30.0 kg/m2); and for women, menopausal status (premenopausal/postmenopausal/unknown) and menopausal hormone therapy (never/past/current use).
Low PA and high BMI similarly interacted with total fructose on inflammatory markers, especially CRP and adiponectin (Supplemental Figure 2). The proinflammatory differences associated with fructose appeared to be more profound in participants with low PA and high BMI (P-interaction = .04 for adiponectin stratified by BMI). We did not observe clear patterns when stratified by age, smoking, or alcohol intake (Supplemental Figure 3).
The results did not change substantially when restricting analyses to participants who provided fasting blood, those who were selected as controls in previous case-control studies, those who reported no history of hypertension or hypercholesterolemia, and those whose FFQ preceded blood draw by ≥1 y (Supplemental Table 4). Adjusting for fiber intake slightly attenuated the associations for fruit fructose yet did not alter other results (Supplemental Table 5). Not adjusting for BMI attenuated the associations for SSBs and juice fructose, whereas it enhanced the associations for fruit fructose and with leptin (Supplemental Table 6).
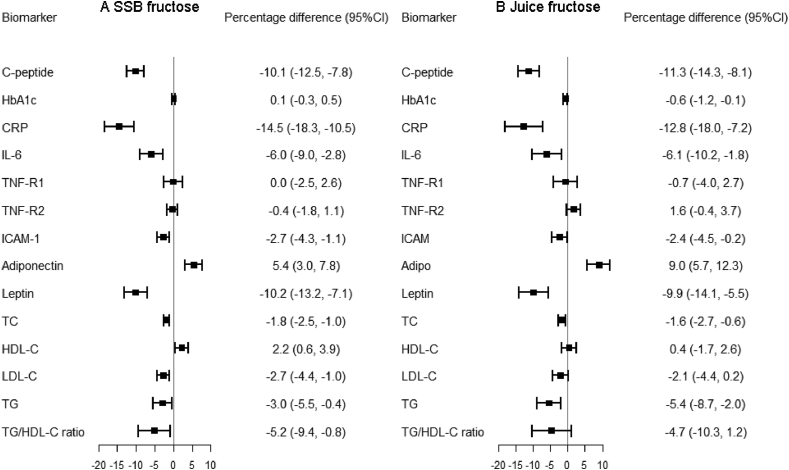
Table 3 shows the associations of 20 g/d SSB fructose, juice fructose, and fruit fructose with biomarker concentrations in the combined cohort. SSB fructose was associated with unfavorable profiles of most biomarkers. Juice fructose was associated with higher concentration of C-peptide, HbA1c, and TG, and lower concentration of adiponectin. In contrast, fruit fructose was associated with lower concentration of C-peptide, CRP, IL-6, leptin, and TC and higher concentration of TNF-R2. Similar patterns were observed in each cohort (Supplemental Tables 7–9). Figure 1 shows that substituting 20g/d fruit fructose for 20g/d SSB and juice fructose resulted in favorable metabolic profiles. For example, the substitution for SSB fructose was associated with 10.1% lower C-peptide, 2.7% to 14.5% lower proinflammatory markers, and 1.8% to 5.2% lower blood lipids.
TABLE 3.
Percentage of differences (95% CIs) in biomarker concentrations associated with 20 g/d of fructose from SSB, juice, and fruit in the combined cohort1
| SSB fructose | Juice fructose | Fruit fructose | |
|---|---|---|---|
| Insulinemic/glycemic markers | |||
| C-peptide (n = 12,433) | |||
| Model 1 | 6.1 (4.2, 8.1)∗∗∗ | 0.0 (−3.0, 3.0) | −11.2 (−13.1, −9.3)∗∗∗ |
| Model 2 | 3.4 (1.7, 5.2)∗∗∗ | 4.1 (1.2, 7.0)∗∗ | −6.9 (−8.9, −4.9)∗∗∗ |
| HbA1c (n = 9912) | |||
| Model 1 | 0.1 (−0.2, 0.3) | 0.3 (−0.1, 0.7) | −0.4 (−0.7, −0.1)∗ |
| Model 2 | −0.3 (−0.5, 0.0)∗ | 0.5 (0.1, 0.9)∗ | −0.2 (−0.5, 0.1) |
| Inflammatory markers | |||
| CRP (n = 16 810) | |||
| Model 1 | 12.7 (9.3, 16.2)∗∗∗ | −8.2 (−13.1, −3.1)∗∗ | −18.9 (−22.1, −15.6)∗∗∗ |
| Model 2 | 6.9 (3.9, 9.9)∗∗∗ | 3.3 (−1.6, 8.4) | −8.6 (−12.1, −4.9)∗∗∗ |
| IL-6 (n = 11 011) | |||
| Model 1 | 5.6 (3.5, 7.8)∗∗∗ | −2.9 (−6.4, 0.7) | −9.9 (−12.4, −7.4)∗∗∗ |
| Model 2 | 2.2 (0.2, 4.2)∗ | 1.9 (−1.6, 5.4) | −3.9 (−6.6, −1.1)∗ |
| TNF-R1 (n = 3314) | |||
| Model 1 | 2.1 (0.5, 3.7)∗ | 0.1 (−2.6, 2.8) | −0.8 (−2.9, 1.3) |
| Model 2 | 0.5 (−1.0, 2.1) | 1.2 (−1.4, 3.8) | 0.6 (−1.6, 2.8) |
| TNF-R2 (n = 10 708) | |||
| Model 1 | 3.1 (2.2, 4.0)∗∗∗ | −1.4 (−2.9, 0.2) | 0.0 (−1.2, 1.2) |
| Model 2 | 1.8 (0.9, 2.6)∗∗∗ | −0.6 (−2.1, 0.9) | 1.4 (0.1, 2.7)∗ |
| ICAM-1 (n = 6248) | |||
| Model 1 | 3.0 (1.9, 4.2)∗∗∗ | −1.2 (−3.0, 0.6) | −4.8 (−6.1, −3.5)∗∗∗ |
| Model 2 | 1.5 (0.5, 2.6)∗ | 0.8 (−0.9, 2.6) | −1.2 (−2.6, 0.2) |
| Adiponectin (n = 13 893) | |||
| Model 1 | −6.2 (−7.6, −4.8)∗∗∗ | −3.9 (−6.2, −1.5)∗∗ | 3.0 (1.1, 4.9)∗∗ |
| Model 2 | −4.2 (−5.6, −2.8)∗∗∗ | −6.7 (−8.9, −4.4)∗∗∗ | 0.7 (−1.2, 2.7) |
| Leptin (n = 9831) | |||
| Model 1 | 5.3 (2.7, 8.0)∗∗∗ | −9.7 (−13.8, −5.3)∗∗∗ | −17.3 (−20.2, −14.4)∗∗∗ |
| Model 2 | 1.8 (−0.2, 3.9) | 1.0 (−2.7, 4.8) | −8.6 (−11.3, −5.8)∗∗∗ |
| Lipid markers | |||
| TC (n = 14 979) | |||
| Model 1 | 0.8 (0.4, 1.3)∗∗∗ | −0.1 (−1.0, 0.8) | −2.2 (−2.9, −1.5)∗∗∗ |
| Model 2 | 0.4 (0.0, 0.9) | 0.2 (−0.6, 1.0) | −1.3 (−2.0, −0.6)∗∗∗ |
| HDL-C (n = 8623) | |||
| Model 1 | −4.3 (−5.4, −3.2)∗∗∗ | 0.5 (−1.4, 2.4) | 0.3 (−1.0, 1.7) |
| Model 2 | −2.0 (−3.1, −1.0)∗∗∗ | 0.2 (−1.5, 1.9) | 0.2 (−1.2, 1.6) |
| LDL-C (n = 7169) | |||
| Model 1 | 2.4 (1.1, 3.6)∗∗∗ | 0.6 (−1.4, 2.7) | −2.1 (−3.5, −0.6)∗∗ |
| Model 2 | 1.6 (0.4, 2.7)∗ | 0.7 (−1.2, 2.6) | −1.1 (−2.6, 0.3) |
| TG (n = 10 234) | |||
| Model 1 | 6.1 (4.4, 7.8)∗∗∗ | 1.0 (−1.9, 4.1) | −6.0 (−8.2, −3.7)∗∗∗ |
| Model 2 | 2.9 (1.4, 4.5)∗∗∗ | 4.9 (2.1, 7.7)∗∗∗ | 0.0 (−2.4, 2.4) |
| TG/HDL-C ratio (n = 6092) | |||
| Model 1 | 10.6 (7.3, 14.1)∗∗∗ | 1.7 (−3.4, 7.2) | −4.3 (−8.0, −0.5)∗ |
| Model 2 | 6.4 (3.4, 9.5)∗∗∗ | 4.5 (−0.3, 9.5) | 1.0 (−2.8, 5.0) |
HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; ref, reference; SSB, sugar-sweetened beverage; TC, total cholesterol.
∗0.005 ≤ P < 0.05, ∗∗0.001 ≤ P < 0.005, ∗∗∗P < 0.001.
Models were adjusted for the same set of covariates as in Table 2, and each fructose source was mutually adjusted for other sources.
FIGURE 1.
Percentage differences (95% CIs) in biomarker concentrations for substituting 20 g/d of fruit fructose for 20 g/d of (A) SSB fructose and (B) fruit juice fructose in the combined cohort (n = 41,714). Models were adjusted for the same set of covariates as in Table 2. Adipo, adiponectin; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; SSB, sugar-sweetened beverage; TC, total cholesterol.
Discussion
In these large cohorts of US men and women, fructose intake was associated with a worse metabolic profile including higher concentrations of IL-6, TNF-R1, TNF-R2, ICAM-1, TG, TG/HDL cholesterol ratio, and lower concentrations of adiponectin and HDL cholesterol. The proinflammatory differences were more profound in participants with low PA and high BMI. SSB fructose was associated with unfavorable profiles of most biomarkers, juice fructose was mainly associated with a few insulinemic markers and lipids, whereas fruit fructose and substituting fruit fructose for SSB and juice fructose were associated with favorable metabolic profiles.
Our findings of a worse cardiometabolic profile associated with fructose intake were generally in line with previous observational studies [[26], [27], [28]]. For example, a prospective cohort of 2369 participants showed baseline fructose intake was associated with higher insulin and lower HDL cholesterol concentration after 6.7 y of follow-up [26]. A prior study conducted in NHS and NHSII cohorts found women in the highest compared with lowest quintile of fructose intake had a 16.1% higher concentration of C-peptide [28]. Although most previous RCTs did not find significant effects induced by fructose feeding, the discrepancies in our findings are unlikely to be attributed to confounding. For example, we observed higher insulinemic markers and blood lipids associated with juice fructose intake. However, participants with a higher juice fructose intake generally lived a healthier lifestyle than those with a lower juice fructose intake, and thus the potential confounding could have biased the results toward the null. Rather, the null results in RCTs could be related to the small sample size (mostly <30) and relatively short feeding duration (mostly 1–2 mo) [[3], [4], [5]]. The median dose of fructose was either around 80 g/d in previous RCTs examining fructose and inflammation [5], or accounted for 25% of total energy in those examining fructose and lipids [3]. However, the average fructose intake in our participants was similar to the national survey data (∼49 g/d), and even the intake in the highest quartile (13.5% to 14.0% of total energy) was lower than the dose in previous RCTs. Therefore, our findings highlight that multifaceted metabolic harms might result from long-term consumption of fructose at an intake level common in the US population.
Intracellular fructose uptake is mediated by facilitated membrane transporters, glucose transporter (GLUT)5 and GLUT2, which are present in a wide set of tissues and not regulated by insulin, unlike glucose. This insulin-independent metabolism has been suspected to be responsible for some of the deleterious effects of fructose [29]. Stimulation of hepatic de novo lipogenesis might be partially responsible as well, although at the moderate intake level that was observed in our population, the proportion of fructose metabolized in liver would be small [29]. Another potential mechanism is that excess energy intake due to fructose intake leads to weight gain, increased visceral fat and downstream cardiometabolic disturbances [30]. RCTs found fructose could induce more severe metabolic disorder compared with isocaloric glucose [31, 32].
Our findings underscore that the metabolic effects of fructose could vary across food sources. A cross-sectional analysis of 3981 individuals specifically found fructose intake from SSBs and juice but not from fruit was associated with a higher intrahepatic lipid content [9]. Also, a prospective follow-up of 254 individuals found adolescent sugar intake from SSBs but not sugar from juice was associated with a higher inflammatory score in young adulthood [33]. The liquid versus solid form of the fructose sources might explain the different effects. Fructose from SSBs and juices is absorbed more quickly than fructose in fruit, which features higher fiber content and slower digestion. The rapid absorption of liquid fructose increases the rate of fructose uptake, hepatic fructose, and de novo lipogenesis [30]. Our findings of fiber mediating the fruit fructose associations are in line with this hypothesis. The various substances rich in fruit, such as vitamins and phytochemicals, can help decrease systemic inflammation [8]. These substances remaining in fruit juice may explain our findings of less unfavorable effects of juice fructose than SSB fructose, especially in inflammation.
Our findings of attenuated proinflammatory differences in higher PA groups might be due to increased release of glucose and lactate from liver to muscle [29]. Many previous studies reporting the adverse effects of a high fructose diet were conducted in low PA settings [[34], [35], [36]] whereas fructose-induced hepatic lipogenesis was prevented in more active settings [37, 38]. The interaction of BMI and fructose on inflammation has been understudied. A study found consuming SSBs for >351 mL/d was associated with higher CRP only in individuals with obesity but not in those of normal weight or overweight [39]. We hypothesized the low-grade systematic inflammation may predispose individuals with obesity to the effect of fructose. However, future studies are warranted to confirm these hypotheses.
Although 1.9% lower HDL cholesterol (∼1.09 mg/dL) and 2.8% higher TG (∼2.83 mg/dL) associated with every 20 g/d increase in fructose intake do not exceed the minimal clinically important difference [3.86 and 7.97 mg/dL for HDL cholesterol and TG respectively [40]], we need to consider the average intake in our population was 40–50 g/d and 25% of participants’ intakes were around 50–70 g/d. In addition, our estimate represents the average change in the population, and a proportion of individuals may have greater changes (for example, individuals with PA lower than median could have a 5.9% higher TG ∼5.96 mg/dL), and some sources of fructose may have greater effects (for example, juice was associated with 4.9% higher TG ∼4.95 mg/dL). The clinical relevance would be even larger as multiple inflammatory markers and blood lipids change simultaneously.
The strengths of the current study include providing a novel insight into effects of fructose from different food sources, the large sample size comprising both men and women, repeated assessment of dietary intake, comprehensive measurement of biomarkers, and detailed information on potential confounders and modifiers. Several limitations should also be considered. First, the cross-sectional design limited our ability to infer causality. We cannot exclude the possibility of residual confounding, but fructose intake was associated with overall healthy behaviors in our cohorts, which should have biased the results toward the null. Second, fructose intake was self-reported, raising the possibilities of misclassification and reporting bias. However, the FFQ has been validated; although individuals with hypertension, hypercholesteremia, and obesity may possibly underreport SSB intake, similar associations were found in those without hypertension and hypercholesteremia and even stronger associations in those with obesity. Third, our study participants were predominantly White health professionals living a healthier lifestyle than the general US population, limiting the generalizability of the findings. Fourth, multiple testing of the associations between different sources of fructose intake and multiple biomarkers might be a concern, but they involve correlated outcomes, and most of our findings are expected. If considered as 3 outcome groups, most our significant P values were <0.005 and would not be affected by a Bonferroni correction (adjusted α level as 0.05/3 = 0.017). Fifth, BMI could be a mediator in the fructose-biomarker relationship, especially for leptin and for fruit fructose, and thus we presented results not adjusted for BMI in the Supplementary Table 6 to prevent overadjustment and provide more information.
In conclusion, fructose intake was associated with adverse profiles of insulinemic/glycemic and inflammatory markers and blood lipids. The relationship with inflammatory markers was more profound in participants with low PA and high BMI. While fruit seems to be a preferred fructose source, juice fructose was associated with unfavorable metabolic profiles, and SSB fructose was even worse. Further studies in diverse racial/ethnic populations are warranted to confirm our findings and support policies restricting beverage fructose intake.
Author contribution
The contributions of the authors were as follows—XL and ELG conceived and designed the study. XL and H-KJ performed the statistical analysis. XL interpreted data and drafted the manuscript. All authors contributed substantially to the interpretation of the data and revision of the manuscript. ELG provided supervision. XL, H-KJ, and ELG had full access to all the data in the study and take primary responsibility for final content. All authors read and approved the final manuscript.
Conflict of interest
The study was started while KW was still employed at the Harvard T. H. Chan School of Public Health. KW is currently an employee of and holds stocks in Vertex Pharmaceuticals. This study was not funded by this commercial entity. All the other authors declare no conflict of interest.
Data Availability
Data described in the article, code book, and analytic code will be made available upon request pending approval by the Channing Division of Network Medicine at Brigham and Women’s Hospital and Harvard Medical School. Further information including the procedures to obtain and access data from the NHS and the Health Professionals Follow-Up Study is described at https://www.nurseshealthstudy.org/researchers (contact e-mail: nhsaccess@channing.harvard.edu) and https://sites.sph.harvard.edu/hpfs/for-collaborators/.
Acknowledgements
We would like to thank the participants and staff of the NHS, NHSII, and HPFS for their valuable contributions.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajcnut.2023.01.006.
Contributor Information
Xinyi Li, Email: xinyili@hsph.harvard.edu.
Hee-Kyung Joh, Email: hkjoh@snu.ac.kr.
Funding
The Nurses’ Health Study is supported by NIH grants UM1 CA186107 and R01 CA49449. The Nurses’ Health Study II is supported by NIH grants U01 CA176726 and R01 CA67262. The Health Professionals Follow-Up Study is supported by NIH grant U01 CA167552. This work was in addition supported by NIH grant R00 CA215314 (to Mingyang Song), American Cancer Society Mentored Research Scholar grant MRSG-17-220-01—NEC (to Mingyang Song), NIH grant K07 CA188126 (to Xuehong Zhang), American Cancer Society Research Scholar grant RSG NEC-130476 (to Xuehong Zhang) and NIH grant R37 CA246175 (to Yin Cao), NIH grants R03 CA197879, R21 CA222940 and R21 CA230873 (to Kana Wu), and an American Institute for Cancer Research Investigator-Initiated Research Grant (to Kana Wu). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors assume full responsibility for the analyses and interpretation of these data. The funding sources played no role in the study design, data collection, data analysis, and interpretation of results, or the decisions made in preparation and submission of the article.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Marriott B.P., Cole N., Lee E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J Nutr. 2009;139(6):1228s. doi: 10.3945/jn.108.098277. 35s. [DOI] [PubMed] [Google Scholar]
- 2.Tappy L., Lê K.A. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010;90(1):23–46. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- 3.Chiavaroli L., de Souza R.J., Ha V., Cozma A.I., Mirrahimi A., Wang D.D., Yu M., et al. Effect of fructose on established lipid targets: a systematic review and meta-analysis of controlled feeding trials. J Am Heart Assoc. 2015;4(9) doi: 10.1161/JAHA.114.001700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choo V.L., Viguiliouk E., Blanco Mejia S., Cozma A.I., Khan T.A., Ha V., et al. Food sources of fructose-containing sugars and glycaemic control: systematic review and meta-analysis of controlled intervention studies. BMJ (Clin Res Ed). 2018;363:k4644. doi: 10.1136/bmj.k4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Della Corte K.W., Perrar I., Penczynski K.J., Schwingshackl L., Herder C., Buyken A.E. Effect of dietary sugar intake on biomarkers of subclinical inflammation: a systematic review and meta-analysis of intervention studies. Nutrients. 2018;10(5):506. doi: 10.3390/nu10050606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Koning L., Malik V.S., Kellogg M.D., Rimm E.B., Willett W.C., Hu F.B. Sweetened beverage consumption, incident coronary heart disease, and biomarkers of risk in men. Circulation. 2012;125(14):1735–17341.s1. doi: 10.1161/CIRCULATIONAHA.111.067017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu Z., Ley S.H., Sun Q., Hu F.B., Malik V.S. Cross-sectional association between sugar-sweetened beverage intake and cardiometabolic biomarkers in US women. Br J Nutr. 2018;119(5):570–580. doi: 10.1017/S0007114517003841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hosseini B., Berthon B.S., Saedisomeolia A., Starkey M.R., Collison A., Wark P.A.B., et al. Effects of fruit and vegetable consumption on inflammatory biomarkers and immune cell populations: a systematic literature review and meta-analysis. Am J Clin Nutr. 2018;108(1):136–155. doi: 10.1093/ajcn/nqy082. [DOI] [PubMed] [Google Scholar]
- 9.Buziau A.M., Eussen S.J.P.M., Kooi M.E., van der Kallen C.J.H., van Dongen M.C.J.M., Schaper N.C., et al. Fructose intake from fruit juice and sugar-sweetened beverages is associated with higher intrahepatic lipid content: the Maastricht Study. Diabetes Care. 2022;45(5):1116–1123. doi: 10.2337/dc21-2123. [DOI] [PubMed] [Google Scholar]
- 10.Rimm E.B., Giovannucci E.L., Willett W.C., Colditz G.A., Ascherio A., Rosner B., et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338(8765):464–468. doi: 10.1016/0140-6736(91)90542-w. [DOI] [PubMed] [Google Scholar]
- 11.Bao Y., Bertoia M.L., Lenart E.B., Stampfer M.J., Willett W.C., Speizer F.E., et al. Origin, methods, and evolution of the three nurses’ health studies. Am J Public Health. 2016;106(9):1573–1581. doi: 10.2105/AJPH.2016.303338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunter D.J., Hankinson S.E., Hough H., Gertig D.M., Garcia-Closas M., Spiegelman D., et al. A prospective study of NAT2 acetylation genotype, cigarette smoking, and risk of breast cancer. Carcinogenesis. 1997;18(11):2127–2132. doi: 10.1093/carcin/18.11.2127. [DOI] [PubMed] [Google Scholar]
- 13.Chan A.T., Ogino S., Giovannucci E.L., Fuchs C.S. Inflammatory markers are associated with risk of colorectal cancer and chemopreventive response to anti-inflammatory drugs. Gastroenterology. 2011;140(3):799–808. doi: 10.1053/j.gastro.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pai J.K., Pischon T., Ma J., Manson J.E., Hankinson S.E., Joshipura K., et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351(25):2599–2610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- 15.USDA National Nutrient Database for Standard Reference, Release. Vol. 10. United States Department of Agriculture, Agricultural Research Service; 1993. [Internet] [date updated, date March 15th 2020] [Google Scholar]
- 16.Feskanich D., Rimm E.B., Giovannucci E.L., Colditz G.A., Stampfer M.J., Litin L.B., et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93(7):790–796. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 17.Salvini S., Hunter D.J., Sampson L., Stampfer M.J., Colditz G.A., Rosner B., et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18(4):858–867. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 18.Nimptsch K., Brand-Miller J.C., Franz M., Sampson L., Willett W.C., Giovannucci E. Dietary insulin index and insulin load in relation to biomarkers of glycemic control, plasma lipids, and inflammation markers. Am J Clin Nutr. 2011;94(1):182–190. doi: 10.3945/ajcn.110.009555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song M., Zhang X., Wu K., Ogino S., Fuchs C.S., Giovannucci E.L., et al. Plasma adiponectin and soluble leptin receptor and risk of colorectal cancer: a prospective study. Cancer Prev Res (Phila). 2013;6(9):875–885. doi: 10.1158/1940-6207.CAPR-13-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiuve S.E., Fung T.T., Rimm E.B., Hu F.B., McCullough M.L., Wang M., et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–1018. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chasan-Taber S., Rimm E.B., Stampfer M.J., Spiegelman D., Colditz G.A., Giovannucci E., et al. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996;7(1):81–86. doi: 10.1097/00001648-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Wolf A.M., Hunter D.J., Colditz G.A., Manson J.E., Stampfer M.J., Corsano K.A., et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23(5):991–999. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 23.Rosner B., Cook N., Portman R., Daniels S., Falkner B. Determination of blood pressure percentiles in normal-weight children: some methodological issues. Am J Epidemiol. 2008;167(6):653–666. doi: 10.1093/aje/kwm348. [DOI] [PubMed] [Google Scholar]
- 24.Song M., Giovannucci E. Substitution analysis in nutritional epidemiology: proceed with caution. Eur J Epidemiol. 2018;33(2):137–140. doi: 10.1007/s10654-018-0371-2. [DOI] [PubMed] [Google Scholar]
- 25.Benjamin D.J., Berger J.O., Johannesson M., Nosek B.A., Wagenmakers E.J., Berk R., et al. Redefine statistical significance. Nat Hum Behav. 2018;2(1):6–10. doi: 10.1038/s41562-017-0189-z. [DOI] [PubMed] [Google Scholar]
- 26.Bahadoran Z., Mirmiran P., Tohidi M., Azizi F. Longitudinal associations of high-fructose diet with cardiovascular events and potential risk factors: Tehran Lipid and Glucose Study. Nutrients. 2017;9(8):872. doi: 10.3390/nu9080872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Domínguez Coello S., Cabrera de León A., Rodríguez Pérez M.C., Borges Álamo C., Carrillo Fernández L., Almeida González D., et al. Association between glycemic index, glycemic load, and fructose with insulin resistance: the CDC of the Canary Islands study. Eur J Nutr. 2010;49(8):505–512. doi: 10.1007/s00394-010-0110-2. [DOI] [PubMed] [Google Scholar]
- 28.Wu T., Giovannucci E., Pischon T., Hankinson S.E., Ma J., Rifai N., et al. Fructose, glycemic load, and quantity and quality of carbohydrate in relation to plasma C-peptide concentrations in US women. Am J Clin Nutr. 2004;80(4):1043–1049. doi: 10.1093/ajcn/80.4.1043. [DOI] [PubMed] [Google Scholar]
- 29.Tappy L., Rosset R. Health outcomes of a high fructose intake: the importance of physical activity. J Physiol. 2019;597(14):3561–3571. doi: 10.1113/JP278246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malik V.S., Hu F.B. Fructose and cardiometabolic health: what the evidence from sugar-sweetened beverages tells us. J Am Coll Cardiol. 2015;66(14):1615–1624. doi: 10.1016/j.jacc.2015.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geidl-Flueck B., Hochuli M., Németh Á., Eberl A., Derron N., Köfeler H.C., et al. Fructose- and sucrose- but not glucose-sweetened beverages promote hepatic de novo lipogenesis: a randomized controlled trial. J Hepatol. 2021;75(1):46–54. doi: 10.1016/j.jhep.2021.02.027. [DOI] [PubMed] [Google Scholar]
- 32.Simons N., Veeraiah P., Simons P.I.H.G., Schaper N.C., Kooi M.E., Schrauwen-Hinderling V.B., et al. Effects of fructose restriction on liver steatosis (FRUITLESS); a double-blind randomized controlled trial. Am J Clin Nutr. 2021;113(2):391–400. doi: 10.1093/ajcn/nqaa332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Della Corte K.A., Penczynski K., Kuhnle G., Perrar I., Herder C., Roden M., et al. The prospective association of dietary sugar intake in adolescence with risk markers of type 2 diabetes in young adulthood. Front Nutr. 2020;7 doi: 10.3389/fnut.2020.615684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bantle J.P., Laine D.C., Thomas J.W. Metabolic effects of dietary fructose and sucrose in types I and II diabetic subjects. JAMA. 1986;256(23):3241–3246. [PubMed] [Google Scholar]
- 35.Cox C.L., Stanhope K.L., Schwarz J.M., Graham J.L., Hatcher B., Griffen S.C., et al. Circulating concentrations of monocyte chemoattractant protein-1, plasminogen activator inhibitor-1, and soluble leukocyte adhesion molecule-1 in overweight/obese men and women consuming fructose- or glucose-sweetened beverages for 10 weeks. J Clin Endocrinol Metab. 2011;96(12):E2034–E2038. doi: 10.1210/jc.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnston R.D., Stephenson M.C., Crossland H., Cordon S.M., Palcidi E., Cox E.F., et al. No difference between high-fructose and high-glucose diets on liver triacylglycerol or biochemistry in healthy overweight men. Gastroenterology. 2013;145(5):1016. doi: 10.1053/j.gastro.2013.07.012. 25.e2. [DOI] [PubMed] [Google Scholar]
- 37.Egli L., Lecoultre V., Theytaz F., Campos V., Hodson L., Schneiter P., et al. Exercise prevents fructose-induced hypertriglyceridemia in healthy young subjects. Diabetes. 2013;62(7):2259–2265. doi: 10.2337/db12-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koutsari C., Karpe F., Humphreys S.M., Frayn K.N., Hardman A.E. Exercise prevents the accumulation of triglyceride-rich lipoproteins and their remnants seen when changing to a high-carbohydrate diet. Arterioscler Thromb Vasc Biol. 2001;21(9):1520–1525. doi: 10.1161/hq0901.095553. [DOI] [PubMed] [Google Scholar]
- 39.Lin W.T., Kao Y.H., Sothern M.S., Seal D.W., Lee C.H., Lin H.Y., et al. The association between sugar-sweetened beverages intake, body mass index, and inflammation in US adults. Int J Public Health. 2020;65(1):45–53. doi: 10.1007/s00038-020-01330-5. [DOI] [PubMed] [Google Scholar]
- 40.Goldenberg J.Z., Day A., Brinkworth G.D., Sato J., Yamada S., Jönsson T., et al. Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: systematic review and meta-analysis of published and unpublished randomized trial data. BMJ (Clin Res Ed). 2021;372:m4743. doi: 10.1136/bmj.m4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the article, code book, and analytic code will be made available upon request pending approval by the Channing Division of Network Medicine at Brigham and Women’s Hospital and Harvard Medical School. Further information including the procedures to obtain and access data from the NHS and the Health Professionals Follow-Up Study is described at https://www.nurseshealthstudy.org/researchers (contact e-mail: nhsaccess@channing.harvard.edu) and https://sites.sph.harvard.edu/hpfs/for-collaborators/.