Abstract
Mechanical forces are central to how cancer treatments such as chemotherapeutics and immunotherapies interact with cells and tissues. At the simplest level, electrostatic forces underlie the binding events that are critical to therapeutic function. However, a growing body of literature points to mechanical factors that also affect whether a drug or an immune cell can reach a target, and to interactions between a cell and its environment affecting therapeutic efficacy. These factors affect cell processes ranging from cytoskeletal and extracellular matrix remodeling to transduction of signals by the nucleus to metastasis of cells. This review presents and critiques the state of the art of our understanding of how mechanobiology impacts drug and immunotherapy resistance and responsiveness, and of the in vitro systems that have been of value in the discovery of these effects.
Keywords: hydrogel, stiffness, substrate compliance, drug responsiveness, chemotherapy
Graphical Abstract
1. Introduction
Biological systems react to force through diverse responses including growth, death, differentiation, remodeling, and changes to regulation that can, recursively, change forces and promote further and enhanced force responses 1–3. Such changes range from adaptive to pathological and are a major focus of the emerging field of mechanobiology 4,5. Pathologies including certain cancers can accentuate or change the responses of cells to mechanics 6–8, and can thereby alter the stiffness and permeability of a tissue, as well as cell-cell and cell-extracellular matrix (ECM) connections. A side effect of these modulations is that they can change the ability of drug or immunotherapy agents to reach a target site, the ability of cells at the target site to take up these drugs, and the ability of a cell to respond to a drug. A growing body of evidence points to a set of pathologies in which this often self-reinforcing cascade of events can prevent drug treatment from being effective. This review aims to link together these pathologies into a single class of bioengineering challenges in which mechanobiology governs the success of drug treatment. The hope is that by studying key phenomena from this unified perspective, these pathologies of mechanobiology will begin to be recognized as the critical, integrated, cross-scale barriers to healthcare that we believe them to be, and that cross-cutting strategies to address them can begin to emerge.
The review begins with several examples of pathologies in which mechanobiological factors block the delivery or efficacy of drugs that might otherwise be effective. A key theme is that, while it has long been known that force can drive physiology and pathophysiology through mechanobiological factors, the discovery of mechanobiological pathways affecting drug delivery and efficacy has the potential to lead to new mechanobiological approaches to improved treatment. These mechanisms are often multifactorial, and to date linked only loosely in the medical literature. Because of the complexity of these mechanisms, many have been discovered only recently with the advent of simplified in vitro systems. The review concludes with a summary of these in vitro systems and of the continuing value of these systems for both identification and amelioration of mechanobiological effects on cancer cell responsiveness to drug and immunotherapy.
2. Mechanobiology in drug and immunotherapy resistance and responsiveness
2.1. Roles of cell-cell interactions
2.1.1. Chemotherapy resistance and responsiveness
We begin with observations across a broad range of cancers. Many cancerous cells interact with a diverse range of components of their surrounding microenvironment, including other cells 9. Those interactions can determine how cancer cells respond to treatment. For example, interactions between cancerous cells and nearby stromal cells have been shown to increase cancer cell survival 7. Epithelial ovarian cancer cells interacting with stromal cells display chemoresistance to platin and taxans, which indicates that the presence of stromal cells within a patient’s tumor might enhance chemoresistance 10.
One mechanism for drug resistance arising from cell-cell interactions is the activation of anti-apoptotic signaling. Binding of the integrin receptors of cancer cells to ligands in extracellular matrix proteins secreted by surrounding stromal cells can activate such ant-apoptotic signaling 11. A key example occurs in patients with acute myelogenous leukemia. Here, the survival of even a few cancer cells in the bone marrow can cause minimal residual disease, meaning a relapse of the cancer after chemotherapy. In this case, drug resistance is induced by the ligation of the protein very late antigen 4, a member of the integrin family (α4β1) on leukemic cells to fibronectin associated with bone-marrow stromal cells 12.
Direct cell-cell interactions can similarly lead to drug resistance through a phenomenon known as cell adhesion-mediated drug resistance (CAM-DR). CAM-DR was first described in human myeloma cell lines at the turn of the century 13, and has since been discovered in a variety of other tumor types. Acute lymphoblastic leukemia cells adhere directly to the integrin α4 of bone marrow stromal cells, leading to CAM-DR and implicating α4 as a therapeutic target for drug resistant leukemia 14. Ovarian cancer cells in physical contact with mesenchymal stromal cells exhibit a pro-metastatic and chemoresistant profile 15–18. Glioblastoma multiforme (GBM) cells, in the absence of ECM proteins, employ an alternative mode of CAM-DR by forming spheres that undergo CAM-DR via cell–cell interactions, implicating the role of gap junctions in chemoresistance 19. Note that cell-cell interaction-based drug resistance is found to be more significant in 3D culture models than in 2D models. For example, resistance to cisplatin and sorafenib by cancer spheroids and cancer-associated fibroblasts is higher in 3D than 2D culture models 20.
Chemokines can trigger chemoresistance in cancer cells even in the absence of contact-based chemoresistance. Mesenchymal stromal cells can induce chemoresistance in ovarian cancer cells without contact through interleukin-6 (IL-6) 21. Other chemokines secreted by cancer-associated fibroblasts that play an important role in cytokine-mediated chemoresistance of cancer cells include interleukin-17 (IL-17), which is overexpressed by colorectal cancer-associated fibroblasts in response to chemotherapy 22. Similarly, interleukin-11 (IL-11) can promote cancer cell chemoresistance by protecting cancer cells from cisplatin-induced apoptosis 23. Taken together, these results show that both contact-based and noncontact-based interactions of cancer cells with surrounding cells can confer drug resistance and promote cancer cell survival.
Tumor-stroma mechanical interactions in the form of compressive stresses can reduce drug efficacy 24. Cell-cell contacts can also affect responsiveness to chemical modulators in human hepatocytes 25. Specifically, decreased expression and localization of intercellular gap junctions and E-cadherin-mediated cell adhesions correlates with decreased constitutive and rifampicin-induced levels of cytochrome P450 3A4 activity. In general, cell-cell interactions are key to preserving the function of primary hepatocytes in culture, which can then be used to detect hepatotoxicity and drug–drug interactions 26.
2.1.2. Immunotherapy resistance and responsiveness
While there is a rich literature on cell-cell interactions in the context of immunotherapy and immunotherapy resistance 27,28, here we focus the discussion on interactions driven by mechanical stimuli. It is now understood that while cancer tissues are generally stiffer than normal tissues, cancer cells are typically softer compared to normal cells 29. Cancer cells have been shown to rearrange their cytoskeletal network and soften their membrane to allow them to move through confined spaces 30, which contributes to their malignancy and metastatic potential 31. The question is whether such changes in cancer cell mechanics can directly influence response to immunotherapy. For example, cytotoxic T lymphocytes need to directly interact with the surface of the target cells to kill them and it is known that T cells can respond to the stiffness of their microenvironment 32. Recent work has shown that T cells also respond to the stiffness of cancer cells, which the authors termed a “mechanical immune checkpoint” and suggested that it could become a therapeutic target 33. Specifically, Lei and co-workers showed that T-cell mediated cancer cell killing was less efficient for soft cancer cells, which had cholesterol enriched membranes, compared to stiff cancer cells, which had cholesterol depleted membranes 33. Interestingly, cancer cell stiffness had no effect on T cell signaling and cytolytic protein production, but it impaired T cell mechanical forces at the immunological synapse (Figure 1) 33. Immunological synapse is the physically active structure, capable of exerting a mechanical force, that forms between a cytotoxic T lymphocyte and a cancer cell 34. To kill cancer cells, T cells are known to exert a mechanical force at the immunological synapse, which is associated with enhanced perforin pore formation on the target cancer cell due to increase in target cell tension 34. Traction force microscopy studies have shown that the increase in mechanical forces correlates with local increases in actin density 35.
Figure 1:
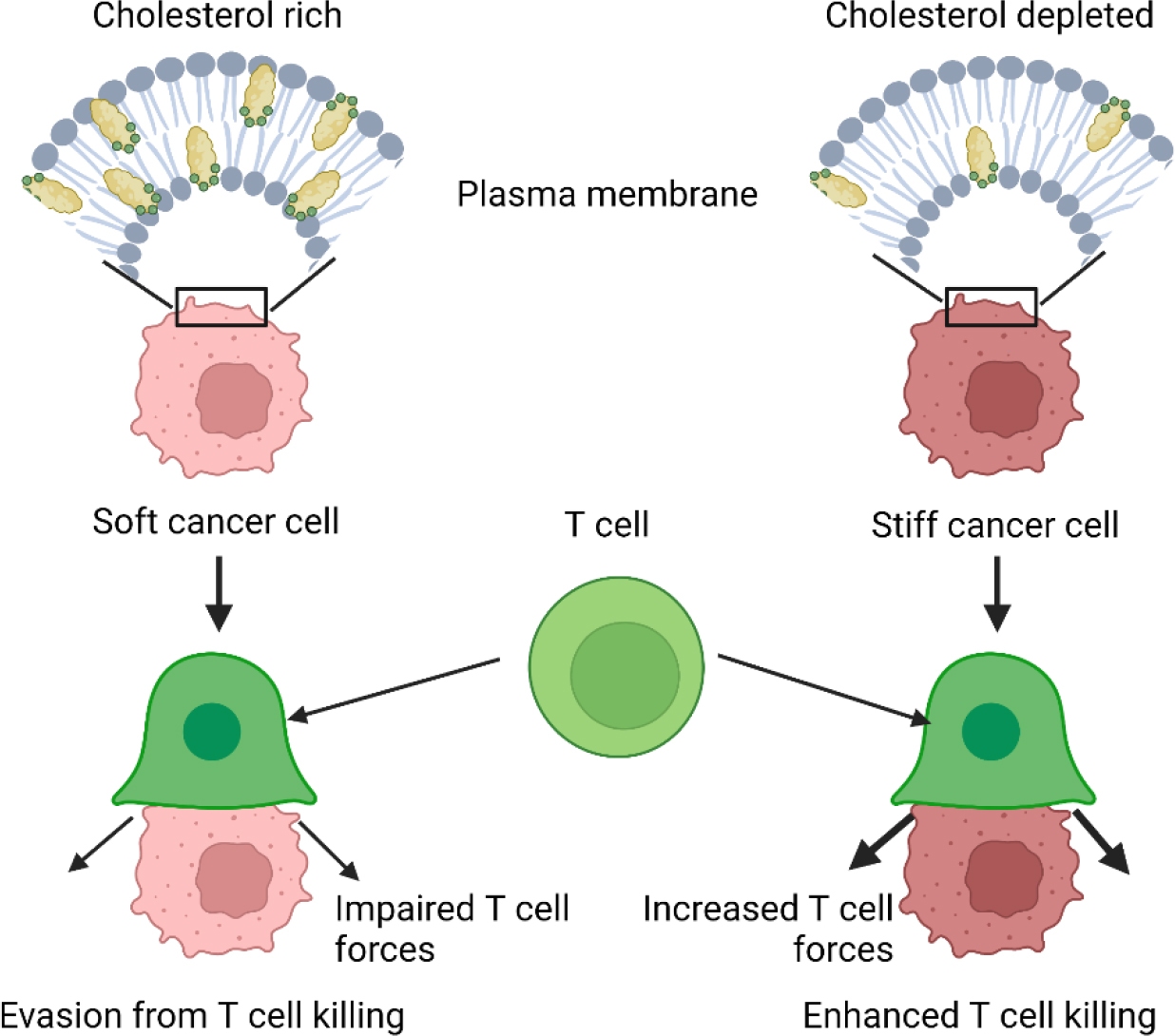
Stiffening of cancer cells due to membrane cholesterol depletion enhances cancer cell killing by T-cells. Adapted from Lei et al. 33.
Another recent study used melanoma and breast cancer cells to show that myocardin-related transcription factors (MRTFs) A and B, which are essential for cancer cell migration and metastasis, also improve cancer cells responsiveness to immune checkpoint blockade antibodies 36. The authors showed that cancer cells overexpressing MRTFs induce stronger cytotoxic T lymphocyte activation and cytotoxicity because they have more rigid filamentous actin cytoskeleton 36. The authors further suggested that this mechanical dimension of the immunosurveillance, which they termed mechanosurveillance, might be relevant for the targeting of metastatic disease. Immunosurveillance, the process by which immune cells detect and eliminate cancer cells, plays a critical role in immunotherapy treatments for cancer 36.
2.2. Roles of cell-extracellular matrix interactions
2.2.1. Chemotherapy resistance and responsiveness
Cells are known to sense the physical cues from their extracellular matrix (ECM), including mechanical forces, dimension, stiffness, viscosity, plasticity, shape, and confinement 1–3,37–41. More importantly, cells respond to these physical cues by regulating their cytoskeletal and nuclear components 4,42–48, which in turn affect almost every aspect of cellular behavior including migration, differentiation, proliferation, signaling, adhesion, and gene expression 8,49–51. As cells adapt themselves to the physical properties of their ECM, it is important to understand how these ECM-induced cellular changes impact the resistance and responsiveness of cancer cells to different drugs. In fact, interactions between cancer cell surface integrins and ECM components have been linked to drug resistance to various agents, from DNA damaging agents to kinase inhibitors, suggesting that integrin antagonists could sensitize tumor cells when used in combination with standard chemotherapy 52. The chemoresistance conferred by the cancer microenvironment has led to various therapies targeting cell-ECM interactions being investigated as an adjuvant, combination or stand-alone treatments 53.
To sense the physical cues from the ECM, cells first need to be connected to the extracellular environment through focal adhesions 54, which together with the cytoskeleton and the nucleus compose a three-way feedback loop through which physical signals are transmitted from the ECM to the nucleus 55. Thus, various experimental approaches have been used to study how cell-matrix adhesion impacts the responsiveness of cells to different drugs. Most of these studies showed that cell-matrix adhesion increases the resistance of both normal and cancer cells to drugs 13,56–58. For example, compared with cells in suspension, human myeloma cells attached to fibronectin exhibit higher resistance to the apoptotic effects of melphalan and doxorubicin 13. As integrins play an important role in cell-matrix adhesion 59, different studies have addressed the specific contribution of integrins to drug resistance 60. For example, dependence of cell survival on β1-integrin ligands fibronectin and laminin was tested in human lung cancer, MDA-MB-231 breast cancer cells, and normal fibroblasts upon the Ukrain drug treatment, and it was shown that fibronectin and laminin significantly increase resistance to the cytotoxic drug 61. Other studies have demonstrated that in MDA-MB-231 and MDA-MB-435 breast cancer cells, β1 integrin signaling inhibits paclitaxel- and vincristine-induced apoptosis by inhibiting the release of cytochrome c from the mitochondria, which in turn is dependent on the activation of the PI 3-kinase/Akt pathway 62. In a clinical study of 249 breast cancer patients with a median follow-up of 8.4 years, high levels of β1 integrin expression and co-expression of fibronectin were linked to more aggressive and invasive breast cancer and it was suggested that such patients could benefit from targeted therapy 63. For a detailed recent review on the role of integrins in breast cancer on drug resistance and how targeting specific integrins and integrin-binding proteins may lead to new therapies, the readers are referred to the following review 64. Adhesion through β1 integrins to fibronectin, laminin and collagen IV of small-cell lung cancer cells, has also been linked to chemotherapy resistance due to stimulating protein tyrosine kinase (PTK) signaling downstream of DNA damage 65,66. In ovarian cancer, drug resistance to chemotherapeutic drugs such as cisplatin as well as poor patient outcomes, have been linked to the upregulation of integrin α-6 (ITGA6) 67. A clinical study has correlated high expression of Lewis y antigen and integrin α5β1 in ovarian carcinoma tissues to chemotherapeutic drug resistance 68. For a review on tumor targeting via integrin ligands, specifically integrins recognizing the RGD cell adhesive sequence, the readers are referred to the following review 69.
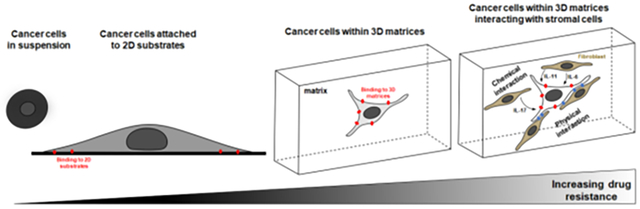
As cell-matrix adhesion within three-dimensional (3D) environments exhibit a few key differences from the one on two-dimensional (2D) substrates 70, it is expected that cells within 3D matrices show different levels of resistance to chemotherapy. Indeed, experimental studies show that cells cultured in 3D are usually more chemoresistant compared to cells cultured on flat 2D substrates (Figure 2) 71,72. Note that while not the focus here, matrix composition in addition to matrix stiffness can affect cancer cell responsiveness to drugs 73. For example, one study developed a high-throughput ECM microarray to investigate the effect of ECM composition on lung adenocarcinoma cell drug response and identified ECM proteins (e.g. fibronectin) that mediated resistance to cisplatin and sunitinib 74. In other examples, only in type 1 collagen matrices, cell-matrix interaction is regulated by many different parameters including collagen concentration, degree of nonlinear stiffening of the ECM, matrix pore size, cell density, ECM crosslinking, matrix constraint, ECM degradability, and growth factors 45,75–78. How each of these parameters changes the resistance of cells to chemotherapy remains a difficult question to answer as most of these parameters are intertwined and cannot be varied independently of others (e.g., collagen pore size decreases with increasing collagen concentration).
Figure 2:
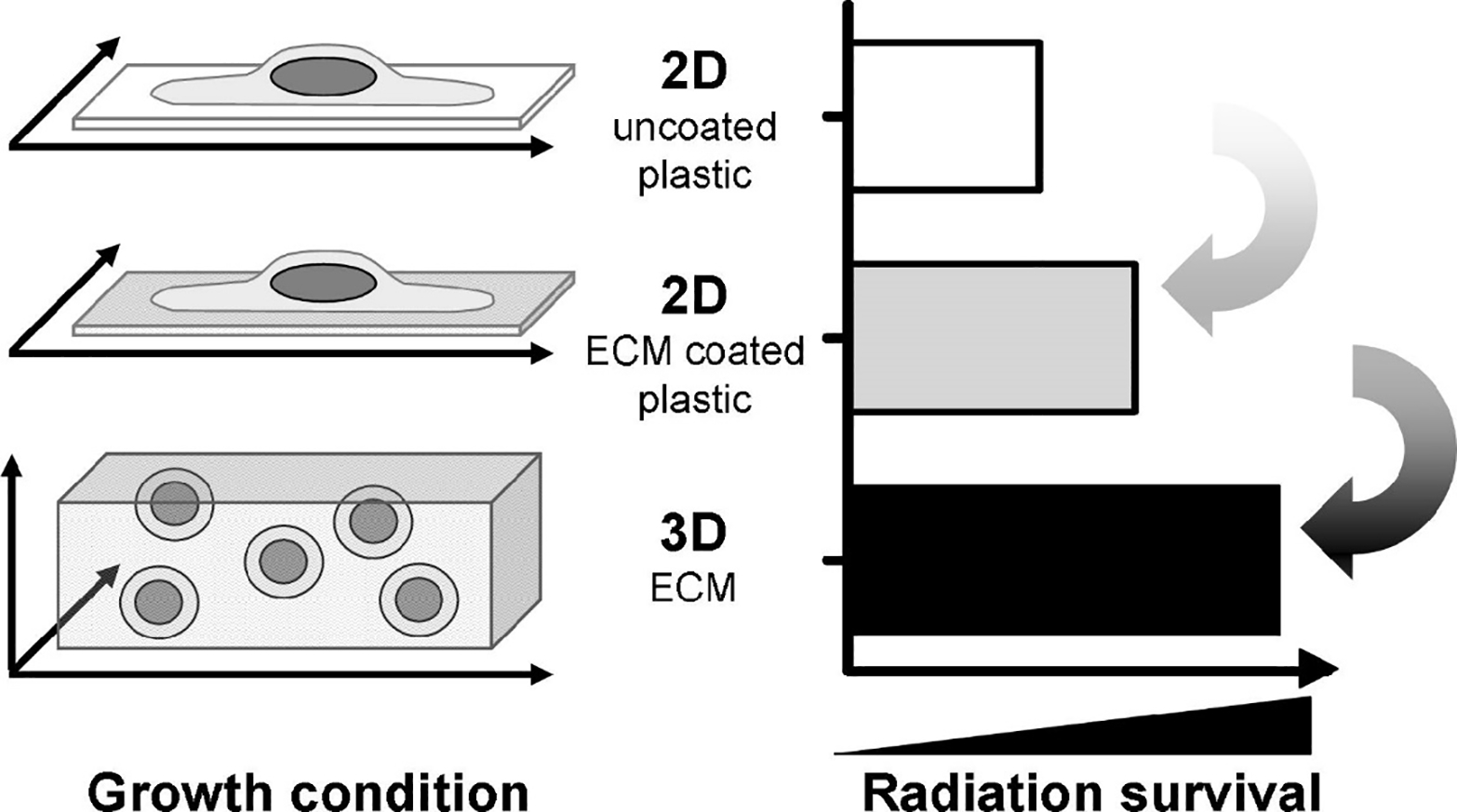
Growth conditions modulate cellular radiation survival. Comparison of the survival of irradiated cells grown on cell culture plastic, on ECM-coated plastic or in 3D ECM. ECM, extracellular matrix. Illustration adapted with permission from Eke et at. 71.
In both 2D and 3D, adherent cells can sense the stiffness of their microenvironment through exerting contractile forces and usually adjust the magnitude and the direction of these contractile forces in response to the stiffness of the microenvironment 1,79. Interestingly, these cellular forces can themselves alter the stiffness of the microenvironment leading to a positive feedback loop between cell contractile forces and ECM stiffness 80. For solid tumors in particular, a feedback loop between cell contractility and matrix alignment and stiffening has been noted, which enables cells to polarize and become more invasive due to their increased contractility 81. On the other hand, for many cancers such as glioblastoma, increased invasiveness has been associated with increased stemness and drug resistance 82. However, the specific role of the positive feedback loop between matrix stiffening and cancer cell contractility and potentially invasiveness, and its effect of drug responsiveness needs further investigation.
In general, it has been shown that cellular drug resistance in both 2D and 3D increases with matrix stiffness 83–86, with a some exceptions such as for osteosarcoma cells for which stemness and drug resistance were enhanced on softer substrates due to miR-29 downregulation 87. For example, Liu et al., 88 studied the effect of matrix stiffness on cellular drug resistance within a 3D context. In this study, hepatocellular carcinoma cells were cultured in 3D alginate gels with different stiffness (21, 75, and 105 kPa) and treated with paclitaxel, 5-FU, and cisplatin. It was shown that cells within the stiffest matrix show higher resistance to the drugs indicating that an increase in matrix stiffness can decrease the effectiveness of cancer therapy. The fact that matrix stiffness negatively affects the effectiveness of cancer therapy becomes of significant importance in the context of chemotherapy knowing that tumor tissues are significantly stiffer than healthy tissues 6,89. In another study on the effect of matrix stiffness on cellular drug resistance, MDA-MB-231 breast cancer cells were cultured in 3D alginate gels with different stiffness. The chemoresistance of cells to doxorubicin in the stiff 2000 Pa gel was found to be three-fold higher compared with cells in the soft 200 Pa gel. Interestingly, MCF7 breast carcinoma cells cultured in the same gels did not show stiffness-dependent resistance to the chemotherapeutic doxorubicin 90. Using 3D alginate-based scaffolds with different stiffness and adhesive ligand, it was shown that both matrix stiffness and cell-matrix adhesions can strongly influence cell responses to toxins 91. Human breast cancer cell lines (MDA-MB-231, BT549, and SkBr3) showed the same response when they were cultured on substrates with different stiffness 92. Similar to cells within 3D matrices, cells on stiffer substrates showed more resistance against sorafenib (Raf kinase inhibitor) independent of ROCK activity 92. In agreement with these results, hepatocellular carcinoma cells on stiff substrates showed reduced apoptosis upon cisplatin treatment 93. However, surviving cells from soft substrates exhibited higher clonogenic capacity than surviving cells from stiff substrates 93, indicating a higher metastatic potential (Figure 3).
Figure 3:
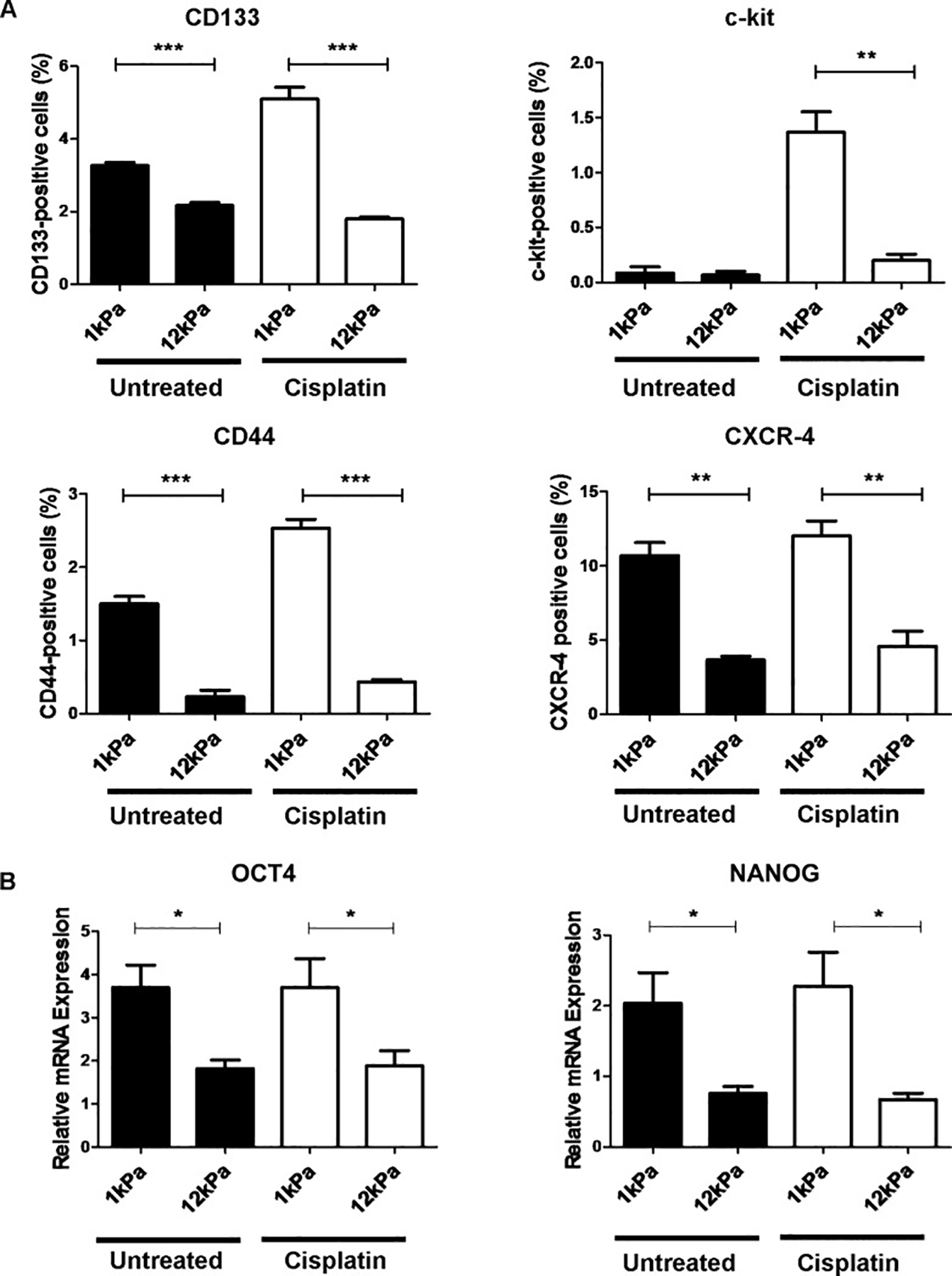
Matrix stiffness and chemotherapy regulate stem cell marker expression in HepG2 cells. (A) Quantification by flow cytometric analysis of putative cancer stem cell markers CD133, c-kit, CD44 and CXCR-4 in HepG2 cells cultured for 5 days on soft (1 kPa) or stiff (12 kPa) supports. Cells were either left untreated (black) or treated for 24 hours with cisplatin (white). Results are representative of three independent experiments. (B) Real-time quantitative PCR analysis of octamer-4 (OCT4) (left panel) and NANOG (right panel) expression in HepG2 cells cultured for 5-days on soft (1 kPa) or stiff (12 kPa) supports. Cells were either left untreated (black) or treated for 24 hours with cisplatin (white). Expression is relative to the 18S housekeeping gene. In each case, error bars represent SEM, *P < 0.05, **P < 0.01, and ***P < 0.001. Adapted with permission from Schrader et al. 93.
As noted above, various studies have shown that substrate stiffness significantly affects focal adhesion complexes, cell force generation, cytoskeletal organization, cell stiffness, nuclear morphology, cell spreading, and cell migration. As these substrate-induced changes often involve the Rho-Rho-associated protein kinases (ROCK) pathway, it is important to study whether the Rho-ROCK pathway is also involved in cellular drug resistance 94. For example, the Rho-ROCK pathway has been implicated in greater malignancy and chemoresistance of metastatic ovarian cancer cells on soft substrates 95, in regulating motility and metastasis in gastric cancer 96, and greater malignancy in breast cancer 97. Note that Rho-GTPases are known to promote the tumor metastasis by disrupting epithelial-sheet organization, increasing cell motility and promoting ECM degradation 98. For a review on the role of the Rho-ROCK pathway in cancer and tumor invasion and metastasis, the readers are referred to the following review 99. Cytoskeletal organization due to Rho-GTPases has been also linked to intrinsic and acquired drug resistance of cancer cells 100. For example, inhibition of the Rho/ROCK pathway has been shown to enhance responsiveness to cisplatin for ovarian cancer cells by blocking hypoxia-inducible factor-1α signal transduction 101. Inhibition of ROCK signaling has also been shown to enhance cisplatin resistance in neuroblastoma cells 102. For a review on the potential of the Rho-ROCK pathway as a target for cancer therapy including immunotherapy, the readers are referred to the following review 103,104.
2.2.2. Immunotherapy resistance and responsiveness
Similarly to chemotherapy and radiotherapy responsiveness, cell-ECM interactions can also affect the effectiveness of cancer immunotherapy 105. For example, even though therapies such as chimeric antigen receptor (CAR) T-cells and checkpoint inhibitors have been successful in treating cancer 106, many patients show therapy resistance stemming in part from excessive ECM deposition and cancer cell-ECM interactions 107,108. In some cases, the explanation could be that the dense ECM serves as a physical barrier between immune and tumor cells, preventing immune cells from getting deep into the tumor and in contact with the cancer cells even when they are attracted to the tumor site via chemokine gradients. For example, in vitro studies have shown that ECM presence significantly influenced migration and cytotoxicity of cytotoxic lymphocytes compared to 2D cultures and, hence, their ability to kill cancer cells 109. Other in vivo studies have shown that cytotoxic lymphocytes can get trapped and accumulate in the dense tumor ECM without being able to reach the tumor cells 110. In a study of urothelial patients, it was shown that a lack of response to programmed death-ligand 1 (PD-L1) checkpoint inhibition treatment correlated with accumulation (i.e. trapping) of cytotoxic lymphocytes into the tumor ECM 111. Further, many immunomodulatory drugs are antibodies of large hydrodynamic radius (e.g. ipilimumab and pembrolizumab), whose diffusion into the tumor would also be impeded by the dense ECM, hence reducing their efficacy. The dense ECM and obstructed transport also leads to hypoxia, which in turn is known to upregulate immunosuppressive factors like IL-10, CCL18, CCL22, TGF-β, and prostaglandin E2, as well as to inhibit T cell proliferation and macrophage phagocytosis 112.
Importantly, denser and highly crosslinked ECM also translates into a matrix of higher compliance or stiffness. A recent in vitro study on the immune escape of melanoma cells showed that a stiffer matrix enhanced immune escape of A375 cells due to overexpression of SNF5 (a core subunit of the SWI/SNF chromatin remodeling complexes), which activated the STAT-3 pathway and elevated the level of tumor-infiltrating CD8+T cells (Figure 4) 113. Another recent study of HCC827 lung adenocarcinoma cells on polyacrylamide hydrogels of 2 kPa (soft) and 25 kPa (stiff), showed a higher immune escape on stiffer substrates linked to elevated expression of PD-L1 via actin-dependent mechanisms (cell treatment with cytochalasin D, an actin polymerization inhibitor, reduced PD-L1) 114. Certain cancers, such as the pancreas, prostate, colon and others, are highly fibrotic with dense collagen matrix of high stiffness and high numbers of CAFs, limiting the efficacy of immunotherapy 115. As discussed above, the dense and stiff fibrotic ECM may act as a physical barrier to cytotoxic T cell infiltration into tumors and impede T cell velocity and migration. Further, in breast cancer, fibrosis has been shown to correlate with tumor-associated macrophages (TAMs) infiltration due to the overexpression of chemoattractants CCL2 and CSF-1 by tumor cells or CAFs in response to stiff collagen-rich ECM 116. Fibrosis-induced hypoxia can further suppress T cell infiltration and function and lead to constant activation of HIF-1α and increased NF-κB activation 117.
Figure 4:
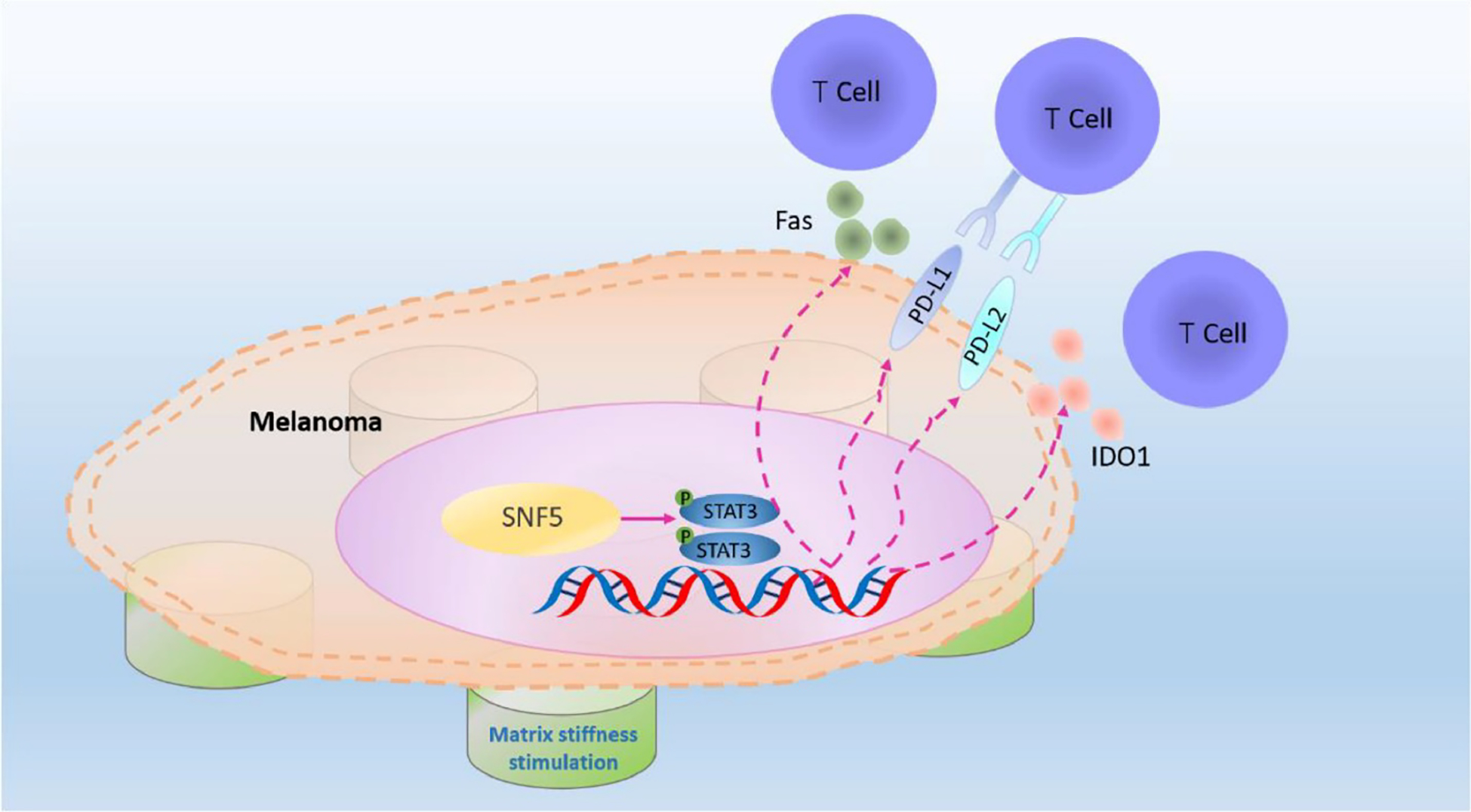
Proposed model of the role of SNF5 in regulating immune escape upon mechanical stimulation in melanoma. The upregulated expression of SNF5 on the stiffer matrix activates the expression of immune escape genes by activating the phosphorylation of STAT3, thereby inhibiting T cells recognition and infiltration. Illustration adapted with permission from Chen et al. 113.
Multiple studies have also shown that the tumor ECM type can affect immune cell motility, myeloid polarization, T-cell phenotype and immune cell metabolism and survival 118. TAMs, which are abundant in the tumor microenvironment and mediate adaptive immune response in cancer, can be immunosuppressive and pro-tumorigenic (M2 polarization) or anti-tumorigenic (M1 polarization) based on interactions with the ECM. For example, ECM molecules such as hyaluronic acid, collagen Type I and tenascin-C have been shown to drive M2 polarization in TAMs 119–121, while fibronectin has been shown to drive M1 polarization 122. Overall, tumors with high infiltration of TAMs are associated with poor patient prognosis and resistance to therapies, suggesting that TAMs depletion or re-polarization could be a successful therapeutic strategy 123. It is also known that collagens, which are functional ligands for the inhibitory immune receptor leukocyte-associated immunoglobulin-like receptor (LAIR)-1, promote immune invasion by interacting with LAIR-1 expressed on immune cells 124 and can also act as reservoirs for TGF-β and other immunosuppressive factors. A recent large-scale analyses found a distinct set of ECM genes upregulated in cancer, which correlated with the activation of TGF-β signaling in CAFs, were linked to immunosuppression in otherwise immunologically active tumors, suggesting that those genes could be targeted using TGF-β blockade to enhance responses to immune-checkpoint blockade 125. For more details on the immunosuppressive properties of TGF-β, the readers are referred to the following review 126. Consequently, strategies that target the ECM in order to improve the efficacy of immunotherapy are gaining momentum and represent an exciting direction for the field 127. For a recent review on mechanical immunoengineering and potential therapeutic applications in the context of T cells, the readers are referred to the following review 128.
2.3. Cell-tissue interactions: the role of vasculature in drug and immunotherapy responsiveness
The integration of cells and extracellular matrix with vasculature defines tissue-level structure. The health and spatial disposition of the vasculature are primary determinants of the success or failure of delivering both drug and immunotherapy to cancerous cells. Interruptions to these have been exploited to enhance drug efficacy and targeting, but also can lead to reduced efficacy. We describe two key examples: diabetes mellitus, and leaky vasculature associated with cancer.
2.3.1. Diabetes mellitus
An emerging example of a pathology that transforms the cell, ECM, transport, and tissue properties that are critical for delivery of therapeutic agents to tumors is diabetes mellitus. Here, cells, cell-cell interactions, ECM and vasculature all change in response to glycation from high blood sugar levels 129. For diabetes patients, this translates to substantially diminished prognosis.
At the core of these pathologies is the process of glycation, in which ECM proteins undergo glycation that leads to stiffening of tissues. Here, the high blood sugar levels in diabetes cause an oxidative and non-enzymatic reaction between glucose and collagen 130–132, which in turn can affect the physical properties of the extracellular matrix 133,134. For the critical case of type I collagen, the effects are easily measurable, with glycated type I collagen matrices exhibiting significantly higher stiffness under shear testing 45. Similarly, ECM stiffness increases with incubation in glucose and ribose 135. These effects can change myocardial and liver function, and can damage vasculature 130,136. This damage to the vasculature and these changes to the ECM have broad and well-characterized deleterious effects on the delivery of drugs to tissues, with the archetypal example being reduced access of antibiotics and the patient’s own immune system to tissues affected by foot ulcers and infection; the result is the spontaneous foot ulcers, chronic wounds, infections, and ischemic tissue necrosis that are a hallmark of the disease 137,138.
Although the effects of these glycation on treatments for cancer are less well understood, increases in ECM stiffness are, as discussed earlier, associated with cellular drug resistance, and are therefore expected to increase cancer chemoresistance. Hyperglycemia is associated with poor responses to chemotherapy, with high blood glucose a part of the metabolic syndrome that is associated with a poor response to chemotherapy in breast cancer 139. Similarly, high glucose enhances cell proliferation, migration, and invasion in gastric cancer,140 and increases gastric cancer chemoresistance both in vivo and in vitro 141. Hyperglycemia reduces the antiproliferative effect of 5-Fluorouracil (5-FU) on colon cancer cells 142 and inhibits the apoptosis of prostate cancer cells induced by docetaxel 143.
Although the causality is, again, unclear at present, much circumstantial evidence exists for a role of diabetes and its associated mechanical effects on tissues in poor cancer treatment outcomes. Chemotherapy in diabetes mellitus patients results in lower survival and lower reduction in tumor mass following pancreatic cancer 144. Diabetes and its mechanobiological effects increase complications of adjuvant chemotherapy in certain populations for breast cancer 145. Because complications such as neutropenia are also higher among cancer patients with diabetes, chemotherapy can be less efficacious due to the need to attenuate its severity 146. Overall, the mechanobiological effects of diabetes mellitus appear to affect the delivery, uptake, and dosage of drug and immunotherapies.
2.3.2. Leaky vasculature
In many cancers, tumor growth is accompanied by hastily formed irregular vasculature with endothelial cells that do not connect as well as those in healthy tissues 147–149. The gaps between endothelial cells give rise to a “leaky vasculature” that leads to the “enhanced permeability and retention” (EPR) effect. This leakiness and the associated retention of nanoparticles and small molecules has long been proposed and exploited as a mechanism for delivering drugs specifically to the tumor site 147,150. The leaky and aberrant blood vessels also lead to decreased blood supply to tumors causing hypoxia that is associated with drug resistance.
Irregular and leaky tumor vasculature is also responsible for increased interstitial pressure in the tumor microenvironment, which in turn affects the tumor growth and metastasis as well as drug delivery. For example, a recent modeling study of glioma showed that leaky vasculature and elevated interstitial fluid pressure (due also to lack of lymphatic drainage) produced tensile stress within the tumor in opposition to the compressive stress produced by tumor growth, leading to elevated stiffness in the tumor rim 151. Cancer cells respond to the elevated interstitial pressure in the tumor by altering their proliferation, apoptosis, migration, and metastasis. For a review on the role of the cancer cell cytoskeleton and the nucleus in mediating cancer cell response to elevated interstitial pressure, the readers are referred to the following article 152. For a review on the role of fluid mechanics in cancer and cancer therapy, the readers are referred to the following article 153.
Besides the leaky vasculature changing tumor tissue mechanics, it is now being understood that it could also be caused by changed mechanics. For example, a study of cancer-associated fibroblasts (CAFs), which have enhanced mechanical activity regulated by the Rho-ROCK pathway (compared to normal fibroblasts), lead to increased vascularization in a 3D gel-based model of vasculogenesis compared to normal fibroblasts, due in part to increased mechanical deformations of the 3D gel (Figure 5) 154. In another study by the same group, the authors developed a microfluidic device to mimic vascularized tumors and allow for decoupling of interstitial flow and mechanical strain and showed that higher mechanical strain induced by CAFs promoted tumor angiogenesis, even though it prevented diffusion of soluble factors to stimulate the growing vasculature 155. A recent screening study of a novel drug that induces apoptosis in CAFs, has shown that in addition to decreased cancer cell proliferation and apoptotic resistance, the drug reduced intratumoral collagen and eliminated leaky tumor angiogenic vessels, which consequently reduced tumor hypoxia and improved drug delivery 156. Such findings imply that the tumor vasculature could affect tumor drug and immunotherapy responsiveness not only by allowing nanoparticle accumulation (leaky vasculature) or hindering efficient chemotherapy delivery (aberrant vasculature), but through mechanical signaling. For a comprehensive review on the how mechanical cues from the tumor microenvironment promote aberrant tumor angiogenesis and its impact on tumor progression and therapeutic treatment, the readers are referred to the following article 157.
Figure 5:
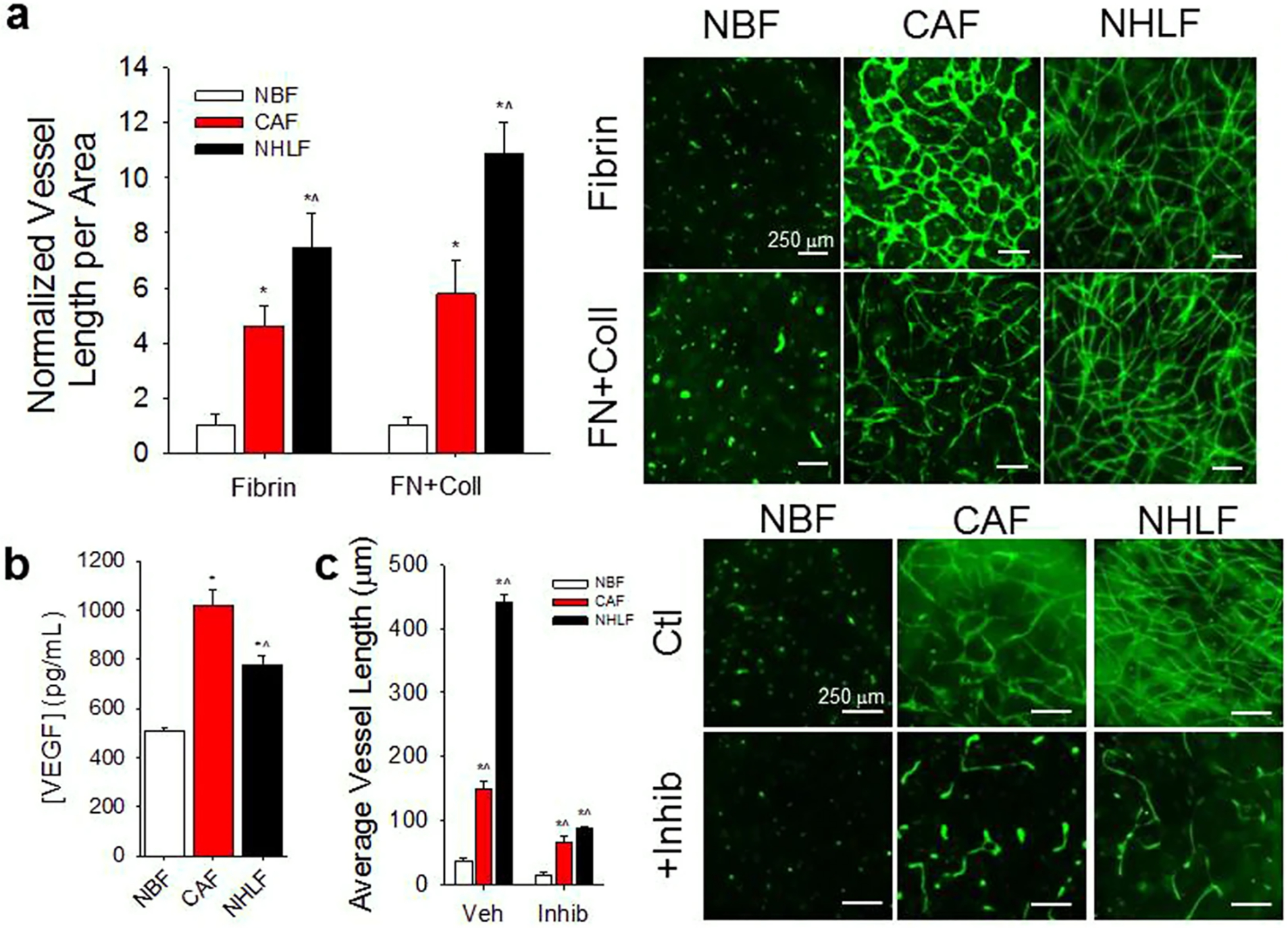
CAFs support vascularization in 3D microtissues. (a) When co-cultured with ECs in Fibrin or combination Fibrin-Collagen (FN + Coll) gels, CAFs support significantly more vascular growth compared to NBFs. Normal human lung fibroblasts (NHLF) also demonstrate significantly higher vascularization potential compared to normal breast fibroblasts (NBFs). Data are presented as total vessel length per unit area, normalized to NBF in Fibrin: 0.0014 ± 0.0002 μm−1; or NBF in FN + Coll: 0.0018 ± 0.0006 μm−1. *p < 0.01 vs. NBF; ^p < 0.01 vs. CAF for same gel type. (Right) Immunofluorescent images of CD31 staining of 3D vessel systems show interconnected vascular networks in CAF & NHLF samples, but not in NBF samples. (b) CAFs in co-culture with ECs (CAFs/ECs) demonstrate higher steady state levels of soluble VEGF than NBFs/ECs. NHLF/EC co-cultures exhibit significantly lower levels of VEGF compared to CAF/EC samples. *p < 0.01 vs. NBF; ^p < 0.01 vs. CAF. (c) Inhibition of VEGFRs suppresses CAF- and NHLF-supported vascular growth compared to vehicle treated controls but shows significantly larger average vessel growth compared to NBF vehicle controls. Data are presented as average vessel length in μm. *p < 0.01 vs. NBF vehicle; ^p < 0.01 vs. NBF + inhibitor (Right) Immunofluorescent images of CD31 staining show vascular fragments of >100 μm in length present in CAF samples with inhibited VEGFR. Scale bars = 250 μm. Figure adapted with permission from Sewell-Loftin et al. 154.
3. Biomaterial platforms to study the role of mechanobiology in cancer cell responsiveness to chemotherapy and immunotherapy
It has been broadly recognized that screening cell lines on unnaturally rigid plastic substrates does not properly recapitulate in situ cell responsiveness to therapies 158. Consequently, more complex platforms have been developed to study cell mechanobiology and associated chemotherapy and immunotherapy responsiveness (Figure 6). The simplest and most heavily used platforms include hydrogels and other biomaterials spanning a wide range of stiffnesses in either a discrete fashion or in the form of a gradient or a pattern. A more recent innovation is developing biomaterial platforms with dynamically switchable stiffness. In either 2D or 3D context, such dynamic materials can be stiffened or softened in the presence of cells. Other platforms that provide for mechanical manipulation of cells and manipulation of cell-ECM interactions include microfluidic devices and custom bioreactors. Added benefit of such biomaterial-based platforms is that they enable cell co-cultures with support or immune cells. Here we give a brief description of these major platforms and focus on the ways in which they have enabled the study of drug and immunotherapy resistance and responsiveness.
Figure 6:
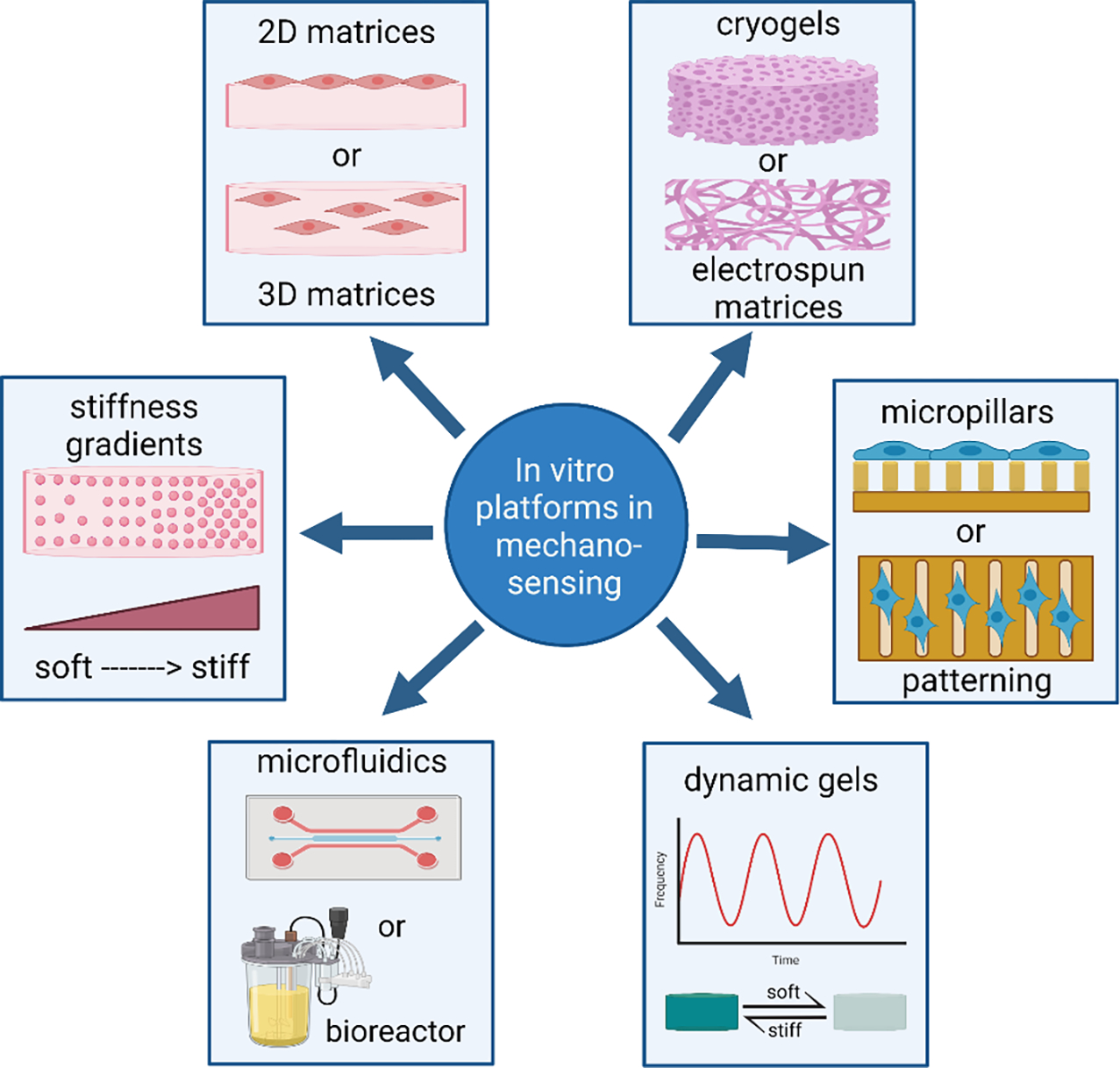
Schematic representation summarizing the diversity of biomaterial-based platforms that have proven beneficial in mechanosensing and mechanobiology studies.
3.1. Two-dimensional hydrogel platforms
Perhaps the most heavily utilized and arguably simplest platform to study cell mechanobiology has been the polyacrylamide gel, which has led to many seminal discoveries 3,159. Polyacrylamide gels are formed by free radical polymerization between an acrylate monomer (Ac) and a bisacrylate (Bis) crosslinker, where stiffness can be modulated by varying the concentration of each component as well the ratio between the two 85,160. Once polymerized, polyacrylamide gels are non-cytotoxic and span a Young’s modulus of ~0.1 – 300 kPa, which encompasses the stiffness of most biological tissues 161. Polyacrylamide gels can also be fabricated via photopolymerization, where stiffness gradients can be created by simply adjusting light exposure time for different parts of the same gel 162. Further, to facilitate the high-throughput requirement for drug screening and the study of stiffness-dependent cell biology, we and others have developed multi-well polyacrylamide gel platforms (Figure 7) 83,163. Despite its broad use, polyacrylamide gels have some limitations. For example, because of the toxicity of the individual monomers and the nanoporosity of the resulting gel, polyacrylamide gels cannot be used as 3D substrates. Further, polyacrylamide gels are synthetic materials and adhesive ligands need to be added to elicit cell attachment. While a limitation, the gel inertness allows for decoupling biomechanical and biochemical contributions to cell behaviors, which is of particular importance in the study of cell mechanobiology.
Figure 7:
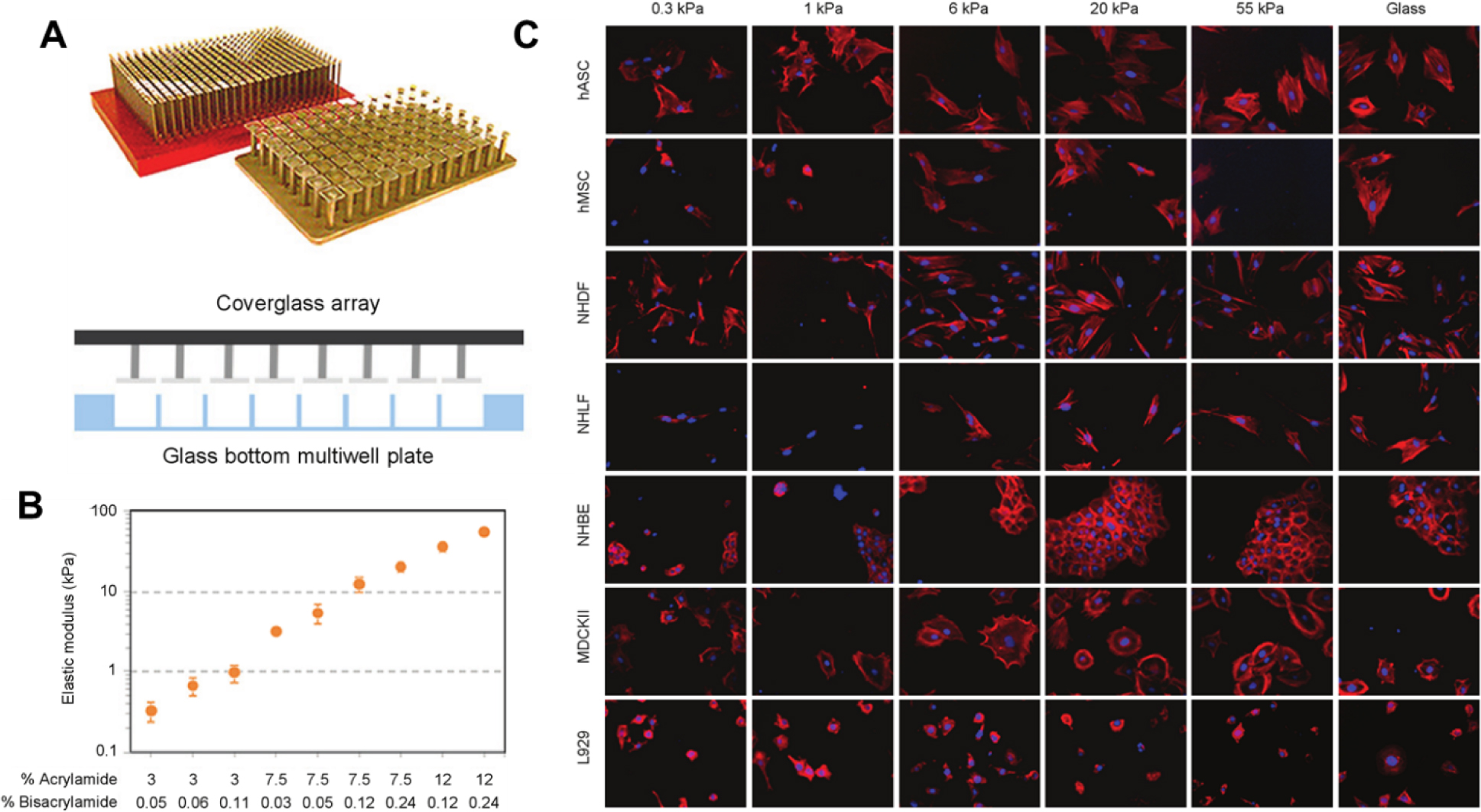
A) Schematic of polyacrylamide gel incorporation into a multiwell plate. PA gels are cast using an array of coverglass to sandwich polymerization solutions within a multiwell plate, followed by ligand conjugation and sterilization. B) Measurement of substrate elastic modulus. Acrylamide: bisacrylamide content was chosen to target a broad physiologically relevant stiffness range. Young’s modulus was determined by AFM microindentation of gels cast within three separate 96 well plates. Data are mean ± SD (n = 3). C) Automated imaging of cell morphology in a 384 well plate. Seven cell types were cultured across increasing substrate stiffness, stained for F-actin (red) and nuclei (blue). Images were obtained at 200X magnification. Figure adapted with permission from Mih et al. 163.
Not surprisingly, polyacrylamide gels have been used extensively to study cell responsiveness to drugs. For example, we have previously shown that drug responsiveness is cell-type dependent 83. In another study, fibronectin-coated polyacrylamide gels of increasing stiffness were used to study chemotherapeutic responses of primary and immortalized breast cancer cells 158. The authors demonstrated that primary cells underwent phenotypic changes when cultured on stiff rigid substrates, which further led to high susceptibility to the chemotherapeutic drugs paclitaxel and doxorubicin. On the other hand, when the same cells were cultured on soft substrates, they had similar gene expression profiles to in situ tumor cells and low susceptibility to paclitaxel and doxorubicin. In another study collagen I-coated polyacrylamide gels (0.4 – 40 kPa) were used to study HER2-amplified breast cancer cells response to the a HER2 receptor tyrosine kinase inhibitor lapatinib 164. Yes-associated protein (YAP) and WW-domain-containing transcription regulator 1 (WWTR1; also known as TAZ) activation correlated with resistance to lapatinib, and when YAP was knocked out in orthotopically implanted tumors grown in mice, tumor growth slowed, and they became more sensitive to lapatinib.
Another simple 2D hydrogel platform for studying the role of mechanosensing in cellular responses to drugs is the synthetic polydimethylsiloxane (PDMS) gel. PDMS gels offer orders-of-magnitude modulus tunability from kPa to MPa, controlled independently of other material properties, which is not achievable by most any other hydrogel system 165. PDMS gradient gels can be prepared with high fidelity by means of a temperature gradient during curing 166. Methods are also being developed to produce high quality 2D and 3D PDMS substrates by additive manufacturing techniques such as electrohydrodynamic inkjet printing 167. In one study PDMS substrates with different stiffness (mimicking articular cartilage, collagenous bone and mammary tumor, respectively) were used to study the responses of breast cancer MCF-7 cells to the antitumor drugs, cisplatin and paclitaxel 168. The authors showed that cell sensitivity to the drugs was highly enhanced on the stiff compared to the soft substrates, which was attributed to increased cell cycle progression on stiff substrates.
3.2. Three-dimensional hydrogel matrices
Multiple studies have shown that cells cultured in 3D environments, which are more physiologically relevant, are more resistant to drugs then their monolayer counterparts 71,72,169. On the other hand, studies have shown that a fully confluent cell monolayer can show increased drug resistance similar to a 3D culture due to both decreased drug penetration and higher intrinsic resistance of confluent cells 170,171. Note that cells are typically seeded at sparse density (typically 4×104 cells/mL on a 2D substrate) for drug screening. Despite that, 3D hydrogel cultures provide conditions not available in 2D monolayers, such as cell-ECM interactions, tethering of growth factors and other biomolecules directly to the gel to guide cell fates, and the ability to form co-cultures with precise spatial patterns. Multiple hydrogels with tunable stiffness, including gelatin methacrylamide, polyethylene glycol (PEG), alginate, silk, and hyaluronic acid (HA) have been developed 172 and could be adapted for use in drug screening platforms. However, hydrogels are intrinsically soft and excessive crosslinking, which is typically used to achieve higher stiffness, could also lead to diminished nutrient and oxygen diffusion.
While it has been established that 3D cell culture technologies can improve precision in drug discovery, there are multiple challenges in applying 3D cultures for high-throughput screening (HTS) of drugs and immunotherapies. Those include labor intensiveness and material cost, scalability to 384- and 1,536-well plates, reproducibility, incorporation into an automated screening setup (e.g. liquid handlers), compatibility with currently available assay and detection methods, and visualization of 3D structures with automated imaging systems 173. Development of new 3D platforms for drug discovery should take into account HTS compatibility, while also being versatile and tunable to emulate the in vivo microenvironment 174. Synthetic hydrogels are typically preferred for drug screening applications due to their reproducibility and their wide range of properties, which can be tuned with high precision. Biochemical cues can be selectively added to synthetic hydrogels to support cell adhesion and other desirable cell behaviors and to emulate the in vivo environment with more fidelity. While some natural hydrogels can be fabricated with reproducible properties (e.g. agarose, alginate), most cell-adhesive natural hydrogels (e.g. Matrigel, collagen) suffer from batch-to-batch variability and cannot be tuned to cover a wide range of mechanical properties. Hence, their use in high-throughput drug screening applications is limited. Lastly, the number of high-throughput methods applicable to stiffness appear to be limited because the range of stiffness is often inadequate due to the choice of polymer and crosslinking methodology. Here is a recent review on high-throughput fabrication of 3D cell laden biomaterials 175.
For many nanoporous hydrogels, cells need to be added to the hydrogel precursor solution and encapsulated during gelation. While this assures immediate and homogeneous cell distribution within the hydrogel, it does not allow the preparation of hydrogels in multiwell plates and other high-throughput formats in advance. To decouple hydrogel production from cell seeding, Zang et al. 176 developed a 96-well plate containing pre-cast, MMP-degradable PEG hydrogels with in-depth density gradient at the surface to promote the infiltration of cells deposited on top of it. The one drawback of the system was the time required to accomplish cell infiltration: it took 3 days for the cells to reach a depth of 200 μm and 10 days to reach a depth of 500 μm. Despite the above limitations, 3D platforms have been invaluable in understanding the role of mechanosensing on cell drug responsiveness 84. For example Shin et al. 177 used alginate hydrogels to demonstrate in vitro and in vivo that matrix softening accelerated cancer growth kinetics and caused resistance to standard chemotherapy in myeloid leukemia cells (Figure 8). In another study, a methoxypolyethylene glycol (mPEG)-modified chitosan hydrogel was used to show that increased hydrogel stiffness promoted increased resistance of breast cancer cells to doxorubicin 178.
Figure 8:
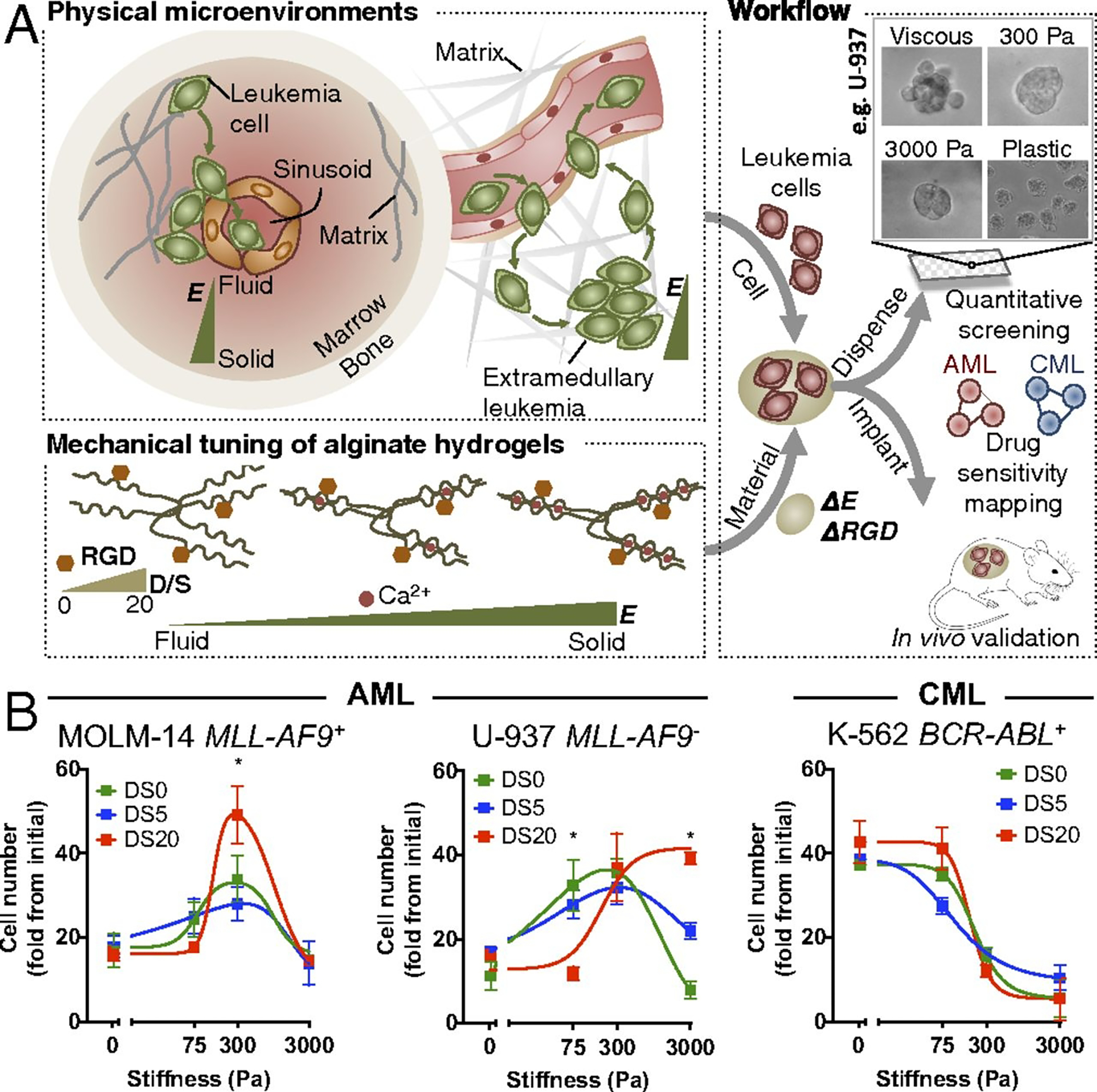
Development of an integrative approach to systematically investigate the role of matrix mechanics in myeloid leukemias. (A) Schematic showing recapitulation of mechanical properties relevant to the hematopoietic system by ionic cross-linking of alginate hydrogels, followed by adaptation of the 3D hydrogels into quantitative screening and animal validation. (B) Different myeloid leukemia subtypes show distinct proliferative responses against matrix mechanics and ligand density. Ligand density is controlled by “degree of substitution” (DS), which indicates the number of RGD peptides conjugated per alginate molecule (0~20). The whole cell population was used for viability analysis. The data were fit to biphasic dose–response curves for AML cells and standard dose–response inhibition curves for CML cells. *P < 0.05 from one-way ANOVA with Tukey’s honestly significant difference (HSD) test. Figure adapted with permission from Shin et al. 177.
3.3. Electrospun scaffolds and cryogels
Electrospun matrices and cryogels are characterized by microporosity to macroporosity and high permeability, which makes them excellent cell scaffolds. Due to their large pores, cells can move, proliferate, and infiltrate the scaffold and experience minimal gradients of nutrients and oxygen. It could be argued that macroporous scaffolds represent a bridge between 2D and 3D materials as a typical cell would only “see” the surface of the pore and might not experience a true third dimension. To control stiffness of such materials, in addition to manipulation of polymer concentration or crosslinking, one can control the fiber diameter or wall thickness with thicker walls and fibers leading to higher stiffness. Another general rule is that higher porosity or larger pores (void spaces) typically correlate with lower modulus materials. For electrospun scaffolds, polymeric composition, followed by fiber orientation and fiber diameter (in that order) are the main factors that determine their elastic modulus 179. Additionally, processing parameters such as polymer weight ratio (for multipolymeric scaffolds), mandrel speed and orientation can have an indirect impact on the modulus by affecting fiber diameter or orientation. Other techniques to control the mechanical properties of electrospun scaffolds are sintering (increases modulus), salt leaching (decreases modulus) 180, or ice crystal formation (decreases modulus) 181. For cryogels, mechanical properties are modulated through controlling the rate of freezing, ice crystal formation, and polymer concentration, where smaller pores and highly concentrated polymer phase, correspond to higher modulus 182,183.
One study developed elecrospun scaffolds to mimic the native environment of prostate cancer bone metastatic cells 184. The authors showed that cells on electrospun substrates were more resistant to docetaxel and camptothecin compared to cells grown on collagen-coated tissue culture polystyrene. Another study used coaxial electrospinning of gelatin and polycaprolactone (the most widely used material combination for electrospun scaffolds) with tunable mechanical properties (modulus ranged between ~2 and 60 MPa) as 3D osteosarcoma models 185. Osteosarcoma cells responded to decrease in substrate stiffness by increasing nuclear localization of YAP and TAZ (Hippo pathway effectors), while downregulating total YAP and increasing resistance to combination chemotherapy compared to monolayer controls. In another study, a cryogel made of PEG-diacrylate and gelatin methacrylamide was shown to support the formation of breast cancer spheroids in the absence of additional growth factors, but only when a low concentration of gelatin methacrylamide (1% w/v) was used 186. The cryogel-grown spheroids exhibited more resistance to paclitaxel compared to 2D grown cells, which the authors attributed partially to the epithelial to mesenchymal transition observed in spheroids. Another group used cryogel scaffolds to develop high-throughput platforms for drug screening applications and again showed significantly higher drug resistance in the cryogels compared to cells seeded on 2D plastic dishes 187. In another interesting approach, cryogels of micro sizes (microcryogels) were fabricated, loaded with cells to create microtissues and assembled on a chip into 3D microtissue arrays for high-throughput drug screening 188.
3.4. Hydrogels with stiffness gradients
A variety of gradient stiffness hydrogels have been developed and have brought excellent insights into cell mechanosensing 189. For recent detailed reviews on gradient stiffness hydrogels, the readers are referred to the following reviews 190,191. Stiffness gradients in hydrogels can be made by controlling the degree of crosslinking 162 or polymer concentration, by controlling gel thickness 192, by layering hydrogels of different stiffness 193, by blending different polymers 194, or by using a syringe pump in combination with photocrosslinking to flow more polymer solution during active polymerization 195. Stiffness gradient hydrogels can be coupled with microfluidic devices 196 and be presented as both 2D substrates 162 and 3D matrices 197. Stiffness gradients could also be coupled with biochemical and other physical gradients such as porosity 198 or even with gradients of soluble biomolecular cues 199 or oxygen 200 using microfluidic approaches. They can also be made compatible with high-throughput screening technologies 197.
While stiffness gradient gels have been used extensively in the study of cell mechanosensing, they have rarely been used in drug screening, where single stiffness gels are preferred. This could be due to the fact that most cell viability assays (e.g. colorimetric assays) used to determine drug efficacy (e.g. 50% effective concentration or IC50), give results on all cells in a particular area, making it hard to pinpoint the role of stiffness in a gradient gel. On the other hand, viability assays that allow for probing specific cells in defined gel areas (e.g. live/dead staining) are usually time consuming and more costly to perform. Also, since cells are known to migrate along the gradient, viability data in relation to gel stiffness might be hard to interpret. Still, several groups have performed drug screening on cells seeded on gradient gels. In one example, Lam et al. 201 seeded MDA-MB-231 breast cancer spheroids at the interface of dual stiffness collagen gels, namely 0.3–1.2 kPa and 0.3–6.0 kPa. The spheroids infiltrated the softer matrix more significantly than the stiffer matrix. They also suffered from apoptosis earlier when treated with paclitaxel compared to cells in stiffer matrices, suggesting that reduced invasion in the stiffer matrix could be linked to reduced drug sensitivity. Our lab has shown that when U87 GBM spheroids were seeded at the stiffness interface in dual-stiffness PEG hydrogels, spheroid cell invasion was observed away from the interface with only individual cells migrating along the stiffness interface 202. Spheroids also showed similar responses to TMZ treatment in the soft and stiff gels, but cell viability was higher in the spheroid periphery than the core for stiff gels and in the core for soft gels 202. In another study, glioblastoma xenograft cells were seeded in gradient PEG hydrogels with 5 stiffness zones spanning a range from ~150 to 1300 Pa (Figure 9) 195. Cells were cultured for 21 days and either formed spheroids in the stiffer regions or interconnected networks in the softer regions and showed higher susceptibility to temozolomide in the softer compared to the stiffer gel regions. The study did not address cells at the stiffness interfaces. Lastly, Wang et al. 200 developed a microfluidic device to generate a stiffness gradient over an oxygen gradient created by using oxygen scavengers. The authors treated lung cancer A549 cells with the hypoxia sensitive anti-cancer drug triapazamine and demonstrated matrix stiffness-dependent cell drug resistance and hypoxia-induced cytotoxicity of triapazamine.
Figure 9:
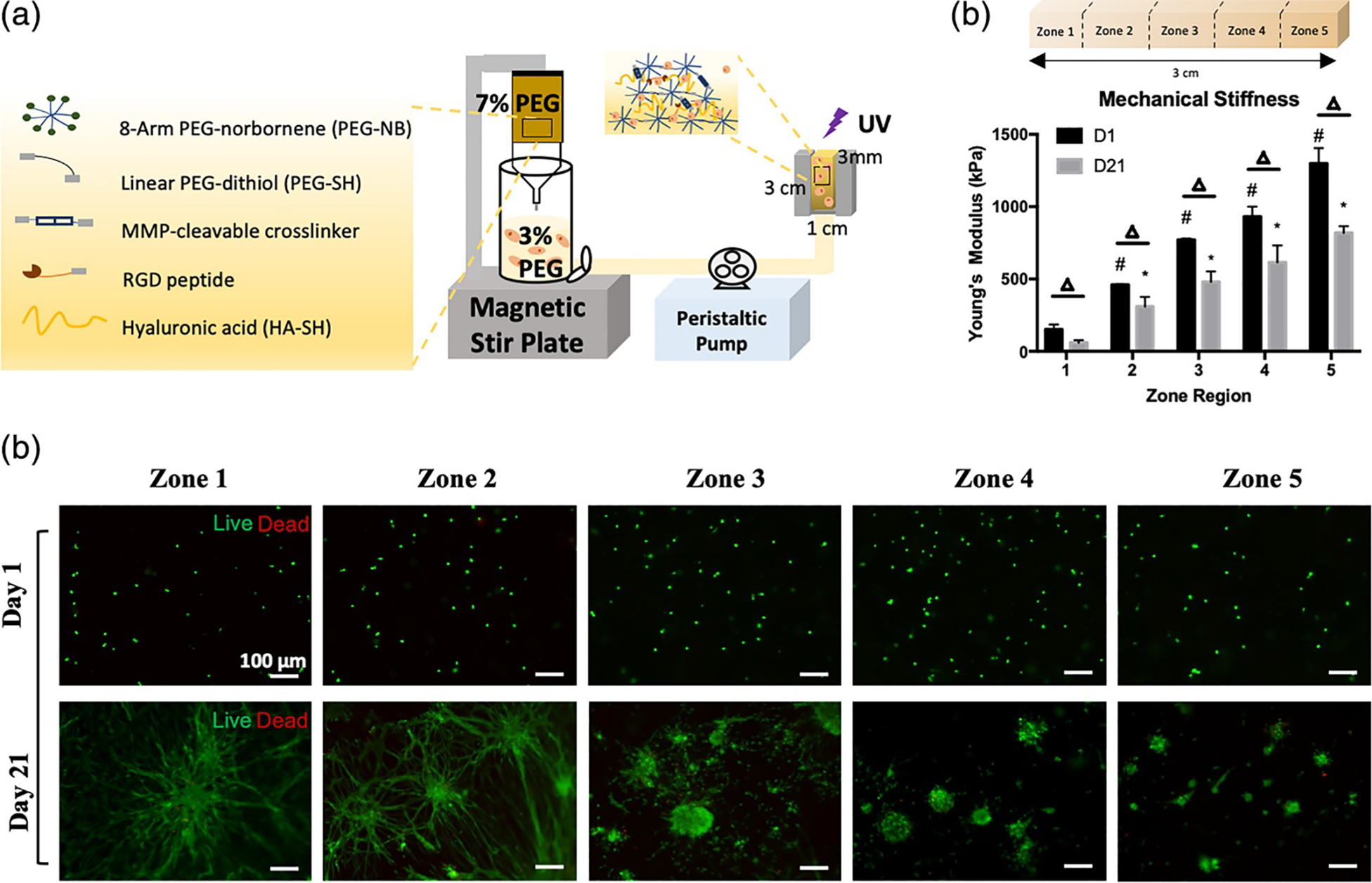
(a) Schematic representation of the hydrogel composition and syringe-pump/gradient maker system used to create the 3D gradient hydrogel. The vertical cylindrical chamber holds 3% (w/v) hydrogel precursor solution. The syringe pump holds the 7% (w/v) hydrogel precursor solution. The peristaltic pump pushes the mixed solutions through to form a gradient in the customizable mold. (b) Unconfined compression test of PDTX GBM-laden cellular gradient hydrogels stiffness (Pa) on day 1 and day 21 (n = 3). # one-way ANOVA followed by Tukey’s post hoc t-tests, p < .05 against D1 zone 1 stiffness; * one-way ANOVA followed by Tukey’s post hoc t-tests, p < .05 against D21 zone 1 stiffness; Δ multiple students’ t-tests between D1 and D21 in each zone, p < .05. (c) Live/dead assay of PDTX GBM cells 1 day and 21 days after encapsulation in gradient hydrogels. Live: green; dead: red. Scale bar = 100 μm. Figure adapted with permission from Zhu et al. 195.
3.5. Patterning and topography
Substrates with topographical features, such as microposts, micropillars or microridges have also been used extensively to study cell mechanotransduction; various fabrication techniques and uses in cell mechanics and mechanobiological studies are reviewed in the following articles 203,204. It should be noted that micropillar arrays affect both cell and nuclear shape and both need to be considered in studies of cells on topological surfaces 205. Micropillar arrays are usually made of PDMS or silicone 206 and offer reproducible and well-defined cell microenvironments. Substrate stiffness can be modulated by changing parameters such as micropillar height, width and spacing 206 as well as polymer concentration or crosslink density 207. The micropillar technique can also be adapted to develop stiffness gradient gels by using gradients in micropillar height 208 or spacing 209. A gradient gel has also been achieved by embedding magnetic beads in a micropillar array and then applying a magnetic field gradient in its vicinity 210. Also, multiple approaches have been developed to mimic the hierarchical structure of the natural extracellular matrix by combining both nanoscale and microscale topographies in the same substrate to influence cell behaviors 211. Lastly, micro- and nanopillar arrays can be fabricated in multi-well plates amenable to semiautomated acquisition, detection, and quantification, and hence, adapted for high-throughput screening of therapeutics 212.
It should be noted that surface topography alone can modulate cell stiffness and mechanical forces. For example, studies have shown that microtopography-induced cell shape changes lead to differential single cell stiffness 213. It has also been shown that nanotopography alters integrin clustering and focal adhesion assembly, which in turn leads to changes in cytoskeletal organization and single cell stiffness 214. Other exciting studies have used micropatterning to examine the interplay between substrate stiffness and geometric confinement and have shown that interfacial cues guide CAFs migration and direct cancer cell assembly 215. In another study, substrate curvature and confinement have been shown to lead to a higher probability of cancer cells expressing stemness markers 216. While micro- and nanopillar arrays are mostly used to study cells on 2D environments, patterning could be used to provide spatiotemporal control of stiffness and viscoelasticity in 3D matrices 217. A common approach to pattern 3D materials and enable stiffening of defined regions is by photoillumination 218 or click chemistry 219.
Micropillar arrays assembled in multi-well plates offer a great potential to be used in high-throughput anti-cancer drug screening. For example, a recent study has shown that micropillar arrays, through modulation of micropillar rigidity and topography, could induce epithelial-to-mesenchymal transition (EMT) without the use of exogenous cytokines, highlighting the utility of such arrays as drug screening platform (Figure 10) 220. In yet another study it was shown that pillar-based mechanical stimuli can be used to induce enhanced ameboid-like migration in A549 cells and more aggressive tumorigenic cancer cell models in general 221. In another study A549 cells incubated on micropillars showed EMT-like behavior and FAK activation - a hallmark of cancer cell adhesion and migration, typically induced by TGF-β 222. The authors then used this platform to screen a drug candidate with activity against TGF-β-induced cancer cell metastasis with favorable results. In another interesting application, PDMS micropillars of high aspect ratio were used as peripheral flexible force sensors by trapping tumor spheroids within a micropillar circle, showing potential for use in drug screening 223.
Figure 10:
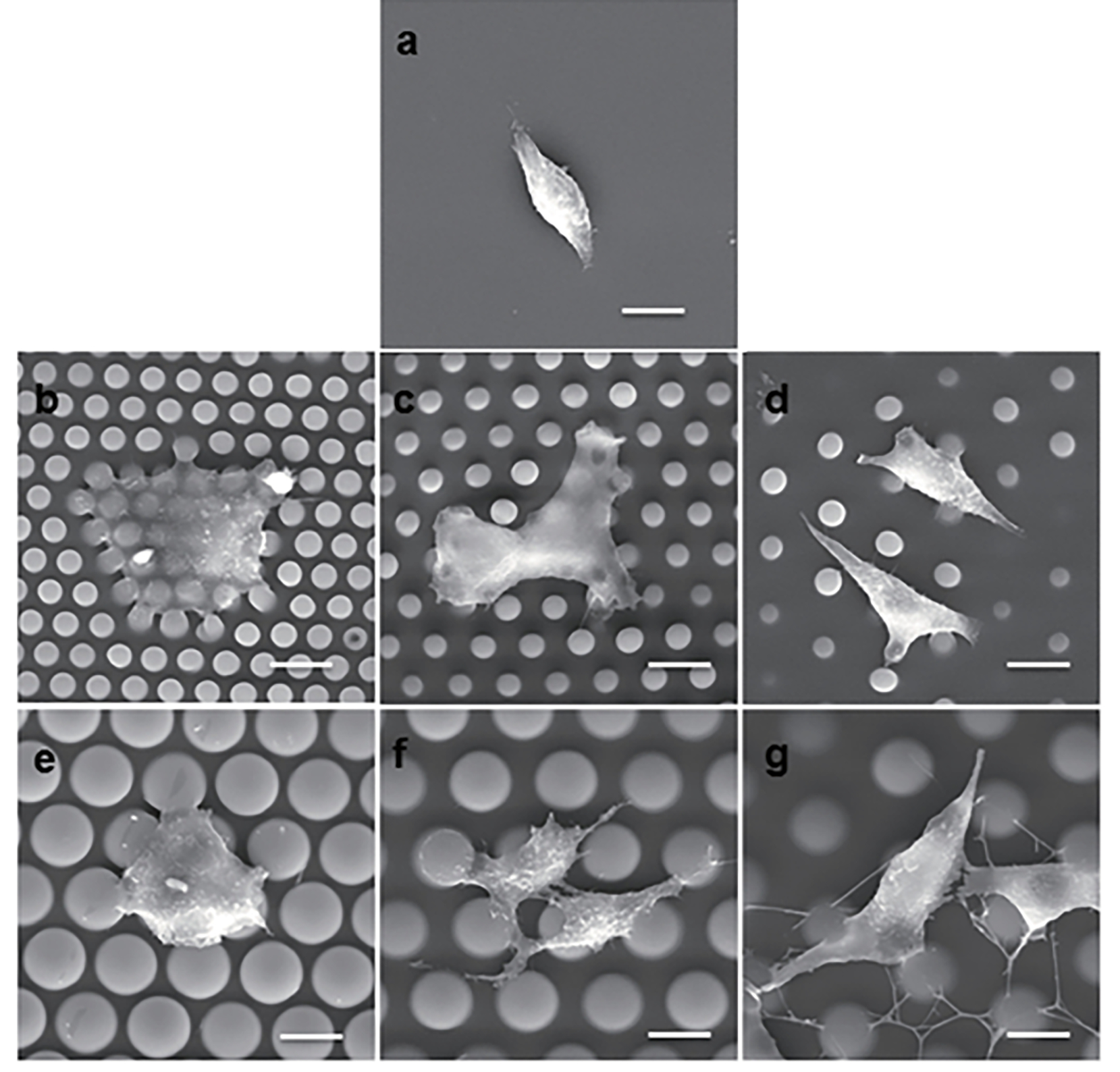
Cellular morphology on micropillar arrays and planar. A: Micropillar-induced cellular morphogenesis. (a) An SEM micrograph shows A549 cells on the flat PDMS substrate. (b–g) SEM micrographs show A549 cells on the 4–2 μm (b), 4–4 μm (c), 4–7 μm (d), 10–2 μm (e), 10–4 μm (f), and 10–7 μm (g) micropillar arrayed substrates. Scale bar =10 μm. Figure reproduced with permission from Xu et al. 220.
3.6. Hydrogels for in situ dynamic stiffness modulation
Many groups have developed both 2D and 3D hydrogel systems that enable dynamic modulation of substrate modulus in the presence of cells. We give a brief summary of several such systems and refer the readers to some excellent detailed reviews on dynamic hydrogels to emulate ECM complexity 224–227. It is important to note that such dynamic systems are yet to be used in drug screening applications and could represent an exciting new frontier.
As mentioned earlier, single stiffness PDMS gels have already been used in drug screening applications and some new developments have led to dynamically switchable PDMS gels. Yeh et al. 228 developed a 2D PDMS substrate with tunable mechanical stiffness spanning from 3 to 200 kPa. This was achieved by a two-step reaction, where PDMS was first gelled by platinum-catalyzed crosslinking and then a thiol-ene click photopolymerization reaction was used to increase the crosslinking and stiffen the substrate. The photopolymerization reaction was carried out in the presence of cells and increased the modulus up to 10-fold within minutes. Some limitations of this system include the irreversibility of the stiffening reaction and the fact that this process could only be done once. A PDMS with reversible dynamic stiffening and softening was also developed by incorporating magnetic particles in the substrate 229. The soft PDMS was stiffened and then softened nearly instantaneously in the presence of cells by applying a magnetic gradient, where the magnetic field was manipulated by the distance of the magnet from the substrate. This allowed an incremental increase in modulus from 10 to 60 kPa, which was completely reversible.
A 3D thermoresponsive hydrogel where reversible stiffness has been incorporated consisted of gelatin methacrylamide hydrogel network interpenetrated by a poly(N-isopropylacrylamide-co-2-hydroxyethyl methacrylate) (NIPAM-HEMA) nanogel 230. The stiffness of this material was then regulated in situ in the presence of cells by reversibly stiffening soft niches via multicyclic temperature changes from 25 to 37 °C. Further, based on the initial concentration of gelatin methacrylamide, the stiffness ranges achieved by this system ranged from 80–120 Pa in G’ (for 1.5 w/v% gelatin methacrylamide) to 800–4000 Pa in G’ (for 3.5 w/v% gelatin methacrylamide). A similar thermoresponsive gelatin-based hydrogel with dynamic modulus regulation has been developed by another group 231. Here the authors used norbornene substituted gelatin (GelNB) photocrosslinked with the thermoresponsive poly(N-isopropylacrylamide-s-2-hydroxypropyl methacrylate-s-mercaptoethyl acrylate) using thiol–norbornene reactions. A storage modulus of ~5–19 kPa was achieved by varying the thiol to ene stoichiometric ratios and the hydrogels could be softened by 2.7–3.5 kPa using thiol–disulfide exchange reactions in the presence of cells.
Polyethylene glycol (PEG)-based hydrogels have also been developed as both 2D and 3D materials with dynamic compliance modulation. Kloxin et al. developed a 2D photodegradable PEG hydrogel which could be degraded in the presence of cells via UV irradiation generating a range of 32 to 7 kPa in Young’s modulus 232,233. Another type of a 3D in situ softening PEG hydrogels were developed by crosslinking PEG with heparin-based polymers via Michael-type addition and then degrading those by externally applied light 234. Dynamically stiffening 3D PEG hydrogels (from 0.2 to 13 kPa) have also been developed by using MMP-degradable 8-arm PEG-norbornene hydrogels and then stiffening them in situ via a second, photoinitiated thiol-ene polymerization with 8-arm PEG-thiol 235. More details on hydrogels with photoswitchable stiffness in particular can be found in the following book chapter 236. Stowers et al. 237 developed a different hydrogel system where light was used to temporally soften or stiffen the material in situ. The authors used calcium-crosslinked alginate where stiffness was modulated by calcium concentration. The dynamic nature of the modulation was achieved by embedding temperature-sensitive liposomes loaded with gold nanorods and either CaCl2 (calcium crosslinker; stiffening) or DTPA (calcium chelator; softening) and irradiating the gel with near-infrared light to trigger the release of CaCl2 or DTPA. That led to a reversible 3D gel stiffening in the presence of cells achieving storage moduli from 91 Pa to 1,179 Pa after 180 s of irradiation.
Another interesting chemistry for developing 3D hydrogel matrices with on-demand tunable stiffness is by using host-guest interactions. Shih et al. developed hydrogels formed by thiol–allylether photo-click reaction between thiolated poly(vinyl alcohol) (TPVA), 4-arm poly(ethylene glycol)-allylether (PEG4AE), and mono-functional β-cyclodextrin-allylether (βCDAE) 238. Hydrogels were stiffened by soaking in adamantane-functionalized 4-arm PEG (PEG4AD) and softened by soaking in unmodified βCD. The process was fully reversible and cytocompatible and resulted in a moduli range of 0.03 to 6 kPa. Another team developed coumarin-functionalized hydrogels formed via host–guest mediated self-assembly with cucurbituril that could photo-switch to covalent gels and reversibly toggle between the two states spanning a storage modulus range of 0.074 kPa to 4.1 kPa 239.
Other approaches for dynamically tuning hydrogel stiffness include DNA crosslinking of the polymer chains. In one system two DNA strands were covalently attached to polyacrylamide polymer chains and crosslinker DNA strand base-paired with two other strands, forming a crosslink (similar to a zip) 240. The gelation was reversed by introducing a complement to one of the DNA strands attached to the polymer. Lastly, while the above described systems span a moduli range from Pa to kPa, dynamic modulation in the MPa range has been described in polycaprolactone (PCL) polymers 241. The authors achieved a modulus of ~1.4 to 61.1 MPa at 37 °C by heating and then cooling PCL in a narrow temperature range of 30–43 °C allowing a phase change between crystalline and amorphous domains.
3.7. Platforms to study immunotherapies
Immunotherapies, such as heat shock protein-based therapies, immune checkpoint inhibitors, poly [ADP-ribose] polymerase inhibitors, dendritic cell vaccines, (CAR) T cell therapy, targeted delivery of stimulatory cytokines, adoptive transfer methods and combination therapies 242–245, are gaining momentum for the treatment of cancer. Unlike chemotherapy treatments which target tumor cells, immunotherapies generally work by restoring the immune system’s ability to eliminate the tumors. At the same time, it has been established that mechanical forces play an important role in regulating the interaction and function of immunoreceptor-ligand pairs for a variety of immune cells 246. Further, activated B cells, which secrete antigen-specific antibodies and cytokines that exert regulatory stimuli on other immune cells, can play an active role in the treatment of cancers and are known to respond to substrate topology and stiffness 247. Overall, immunotherapy has been less successful against solid tumors, partly because of its focus on the biological and chemical mechanisms and less on the physical and mechanical mechanisms involved in combating cancer. Mechanoimmunology studies have recently emerged to fill this gap and deepen our understanding of the role of mechanosignaling, mechanosensing, and mechanotransduction in immunotherapy efficacy 248.
The correlation between cell mechanosensing and cancer response to immunotherapy could be further investigated trough the development of biomaterial-based platforms that include components of the immune system, such as cells and/or biomolecules. Such platforms could resemble the ones already discussed in this review with the added complexity of immune system component incorporation. For example, microfluidic platforms have shown useful for evaluating cancer-immune cell interactions with the goal of assessing the efficacy of emerging immunotherapies 249. Microfluidic platforms allow versatile set-ups, where immune cells could be perfused above adherent or matrix embedded cancer cells, immune and cancer cells could be localized in adjacent chambers, immune cells could be “recruited” through chemotactic gradients, etc., to answer different mechanistic questions 249. In another study, an injection molded plastic array culture device was integrated within a 96-well plate to allow for high-throughput screening (Figure 11) 109. Here cancer cells were embedded in collagen and exposed to cytotoxic lymphocytes in the culture media, to mimic the physical barrier presented by the extracellular matrix which hinders the migration, access to cancer cells and therapeutic efficacy of cytotoxic lymphocytes in vivo 109. Further, ex vivo models of fresh tumor biopsies and surgical excisions that preserve the cellular and microenvironment heterogeneity including the immune compartment, have been used to predict the clinical efficacy of anti-cancer and immuno-oncology drugs, such as immune checkpoint inhibitors 250,251. Other ex-vivo models include tumor organoids from patient derived cells that could facilitate screening of immunotherapies including cytokines, checkpoint inhibitors, or CAR T therapies on an individual basis 252. Note that for ex vivo models, timing is important because initially preserved immune cells and microenvironmental characteristics can be lost and diluted over as cells adapt to in vitro culture conditions. For a more detailed review on the engineering approaches and 3D models for screening immunomodulatory drugs, the readers are referred to the following reviews 253,254.
Figure 11:
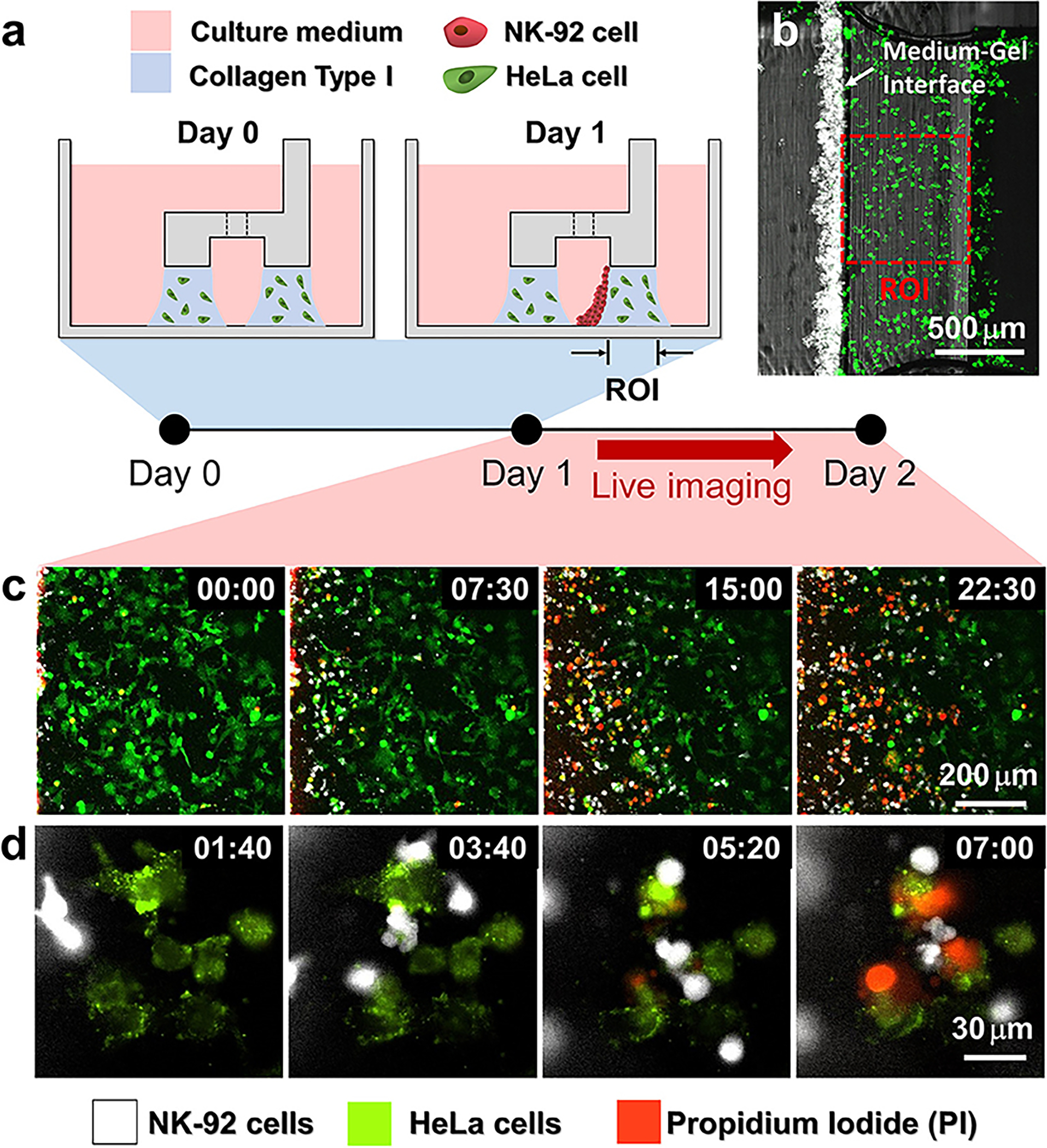
Procedure of 3D cytotoxicity assay and its outputs. (a) Schematic process of the assay. HeLa cells embedded in collagen were patterned under low rails (Day 0). After 24 h of cultivation, NK-92 cells were loaded into a microchannel formed by the hydrogel. By tilting the device at an angle of 90°, NK-92 cells were deposited on a collagen block (Day 1) and cultured for additional 24 h to observe migration and cytotoxic activity of NK cells. (b) Initial state of the assay (Day 1). (c,d) Live monitoring of migration and cytotoxic activity of NK-92 cells. Time is indicated in HH:MM in the top right corner of each image. Figure reproduced with permission from Park et al. 109.
4. Animal models to study the role of mechanobiology in cancer chemotherapy and immunotherapy
Although the in vitro models are far more effective and less expensive for screening chemo- and immunotherapeutic agents, in vivo models are essential prior to translation of candidate drugs to patients. At present, the use of animal models for evaluating the effects of mechanobiology on cancer cell responsiveness to chemotherapy and immunotherapy is very limited. However, these efforts are developing and we provide a review here of challenges and opportunities associated with the using for this purpose two of the most common small animal models in cancer research: mice and zebra fish.
Small animal models are generally cheaper, less time consuming, easier to maintain than large animal models. They reproduce more often, allowing for higher throughout studies. Because of this, they are desirable in chemotherapy and immunotherapy screening applications. Tumor models using these small animals can be xenografted (including patient-derived xenografts), syngeneic, transgenic, carcinogen-induced or spontaneous. Each of these offers different levels of complexity, physiological relevance, and predictability of the human response. For recent comprehensive reviews on animal models in cancer research, readers are referred to the following 255,256. Our focus here is the state of efforts to identify roles of mechanobiology in treatments using these models.
4.1. Mouse models
Mouse models are the most common models used in cancer research and have proven useful in understanding the molecular and cellular mechanisms of tumor initiation and growth as well as serving as pre-clinical models for therapy testing 257. Some limitations of mouse models include inability to fully reflect the complex human tumor, low-throughput, and limited modalities for in vivo imaging and data analysis. Different mouse models include syngeneic or xenograft cancer cells implanted subcutaneously or orthotopically in mice, which are widely used due to their low cost and availability. Transgenic models can be developed by constitutively or conditionally expressing oncogenes, silencing tumor-suppressor genes, or through CRISPR/Cas9 genome editing 258 and offer a designer approach to mimic various aspects of human cancers but are more costly. Various mouse models have also been developed or could be adapted for cancer immunotherapy research 259. Specifically, refined and humanized genetically engineered mouse models could be invaluable in anticancer drug development, including target validation, assessment of tumor response or resistance to therapy and investigation of drug pharmacokinetics and pharmacodynamics 260.
Mouse models have been used to highlight the effect of mechanobiology on cancer treatment, where tissue mechanics could be modulated through methods such as external mechanical loading or targeted softening of the tumor microenvironment. For example, it has been shown that increased mechanical stimuli from exercise and controlled mechanical loading can have antitumorigenic effect and can mitigate metastatic tumor-induced bone disease 261. In a different example, a breast cancer xenograft mouse model was treated with free paclitaxel or reactive oxygen species-activatable nanoenzyme (SP-NE) developed by the group to show that matrix softening sensitized the tumor to chemotherapy 262. These dual-action nanoenzymes were disassociated in the presence of reactive oxygen species, leading to collagenase release and generation of paclitaxel prodrug. The authors demonstrated an enhanced chemotherapeutic efficacy of SP-NE (compared to paclitaxel alone) due to downregulation of integrin-FAK-RhoA and integrin-FAK-pERK 1/2 signaling 262. Another study used a similar approach of matrix softening to sensitize liver-metastasized colorectal cancer cells to the drug bevacizumab, based on the observation that in liver metastasized tumor, matrix stiffness is higher compared to the primary colorectal tumor 263.
Matrix stiffness can be used to improve drug delivery to cancer tissues. For example, a patient-derived orthotopic xenograft mouse model of glioblastoma has been used to implant mechanically matched (between the implant and the brain) hyaluronic acid hydrogels for chemotherapeutic delivery 264. Doxorubicin and gemcitabine-releasing hydrogels with mechanical properties tailored to lie within the range associated with brain parenchyma yield improved drug bioavailability and increased survival rate of up to 45% 264.
A very promising advance in the application of mechanobiology to cancer treatment has been enabled by the study of mouse models. Mouse models reveal a correlation between the ECM stiffening associated with recovery from cancer resection surgery and the subsequent increase in cancer metastasis to the lungs 265. Here, both mice that underwent surgery and control mice that were pre-conditioned with plasma from mice that underwent surgery had lower survival rates following injection with EMT/6-GFP+ breast cancer cells that metastasize to the lungs. This was attributed to increased lysyl oxidase activity and expression (due to hypoxia at the surgical site), which in turn leads to collagen crosslinking, focal adhesion signaling, and finally matrix stiffening and increased cancer cell metastasis to the lungs. The effect could be reversed when the matrix was softened via treatment with a collagen crosslinking inhibitor, showing a potential mechanobiological pathway for chemotherapeutic treatment.
An additional example is that lysyl oxidase and matrix stiffening promote metastasis of mouse mammary carcinomas deficient in transforming growth factor-β.266 This implies that the rigidity of the matrix of a potential metastatic site can influence cancer cell homing and secondary tumor formation, and could be a treatment target.
4.2. Zebra fish models
Zebra fish models are useful alternative models as they can support multiplexed or high throughput studies and are associated with lower costs and time investment compared to other animal models. They are amenable to pharmacological testing, and they have transparent bodies which allow for real-time live imaging of cancer progression. In addition, the majority of human genes have at least one zebrafish orthologue 267. Not surprisingly, multiple zebra fish cancer models have been developed, including tumors in various organ sites that resemble human tumors histologically and genetically.268,269 They have been used as drug discovery platforms 270, and transgenic and xenograft models exist for the potential development of personalized chemotherapy treatment regimens 271,272.
In the context of mechanobiology, mechanotransduction pathways can be replicated and studied in zebra fish. For example, using a transgenic model, Chew et al. have shown that signaling crosstalk between Kras and RhoA regulate liver overgrowth and tumorigenesis and that Rho activation could suppress Kras-induced liver malignancies 273. Zebra fish can also be used to study the effect of the tumor microenvironment on cancer cells. For example, cancer cells have been implanted in fish tissues to study a range of mechanical and biochemical characteristics, and transgenic models exist to silenced or overexpress specific genes or to tune the microenvironment 274. The optical transparency of zebrafish has enabled the study of the tumor cell-vascular interface 275, the extravasation of tumor cells across the vasculature 276, and the contribution of biomechanics to the extravasation of circulating tumor cells showing a direct link between hemodynamic forces and metastasis 277.
In another example, Paul et al. showed that several human brain- and bone-homing breast cancer subclones colonize analogous tissues in zebra fish larvae 278. They then showed that bone marrow homing was related to high integrin expression and focal adhesions associated with mechanosensing, while brain homing was guided by vessel topography during extravasation 278. In addition, transgenic zebra fish models can be created where host immune cells endogenously express fluorescent proteins to enable the studies of host cell-tumor interactions 279. For example, Roh-Johnson et al. showed that macrophages transfer cytoplasm to tumor cells, which correlated with melanoma cell motility and dissemination 280.
Strengths of zebra fish models thus include their modest expense, short intergenerational time, genetic control, and optical transparency. These have been used to discover and modulate aspects of mechanobiology in the zebra fish, and to control the spread and growth of cancer. These models seem to hold potential that has not been tapped for study of aspects of mechanobiology on resistance to chemo- and immunotherapy.
5. Modulation of mechanical factors to improve chemotherapy and immunotherapy
Mechanosensing happens between cells and between cells and their microenvironment. Understanding the mechanisms underlying the differential responsiveness could lead to the development of targeted, stand-alone or adjuvant therapies for the treatment of cancer. Many of the attempts fall under the heading of “mechano-medicine,” a phrase coined by Ning Wang to encompass the application of mechanobiology to intelligently manipulate mechanical factors for positive therapeutic outcomes 55.
A key example that has been attempted in the literature for decades is the usage of ultrasound to target cells or manipulate the cell microenvironment to selectively kill cancerous cells (e.g. 281). However, these direct mechanical modulation efforts have yet to provide their first clinical treatment of a cancer, with the key challenge being delivering energy to cancer cells without injuring so much of the surrounding tissue as to cause substantial side effects. At the core of these difficulties is the challenge that the key factor in energy absorption – the mismatch of acoustic impedance between the components of a cancerous cell and its surrounding environment – is not sufficiently strong to enable targeted ablation 282.
Further along the pathway to potential application are efforts to manipulate the mechanical properties of tissues for therapeutic effect. Although here, too, no cures for cancer have reached the clinic, several promising results can be found. Many of these are in the category of the aforementioned demonstration improved cancer prognosis in a mouse model following drugs administered to modulate ECM properties as part of a treatment regime,265 in which simply changing mechanobiological factors can alter the progression of pathology. However, our focus here is examples in which mechanical modulations enable
A key example is the heat shock protein (Hsp) 47, a collagen-binding glycoprotein essential in the maturation of collagens, but that leads to fibrosis in overabundance and to pathologies including osteogenesis imperfecta when mutated 283. Hsp47 has been explored as a target for tumors with dense ECM, such as pancreatic ductal adenocarcinoma, where activated pancreatic stellate cells (PSCs) are responsible for excessive ECM production 284. In this study, a dual-action therapy of PEGylated polyethylenimine-coated gold nanoparticles that delivered both all-trans retinoic acid (to induce PSC quiescence) and siRNA to target Hsp47 led to decreased ECM density, which in turn supported drug delivery and enhanced chemotherapy efficacy.
Similarly, another study used proteases against collagen and other ECM proteins found in brain tumor tissue but not in normal brain parenchyma, and showed that direct protease pre-treatment enhanced the efficacy of adenovirus-mediated glioblastoma gene therapy by facilitating virus transfection 285. Although more work is needed to understand the mechanisms underlying these desirable effects, possible explanations include mechanobiological factors. Mechanical factors in the ECM that resist diffusion could possibly be affected by protease pre-treatment, and favorable cell mechanobiological responses might be triggered by proteolytic changes in ECM structure and mechanics.
Another therapeutic target for matrix softening has been lysyl oxidase (LOX), which catalyzes the conversion of lysine moieties into aldehydes that then crosslink collagen and elastin, resulting in ECM stiffening. Since LOX is upregulated by tumor cells and promotes metastasis and malignancy, LOX inhibitors (e.g. β-aminopropionitrile, aminomethylenethiophene, Simtuzumab) have been tested alone or in combination with chemotherapy and have shown antitumor effects against various cancers such as breast, colorectal and pancreatic cancer 286. In cases where ECM degradation does indeed prove efficacious in enhancing the function of chemo- and immunotherapies, LOX inhibition may hold potential as an adjuvant.
In addition to ECM softening, mechanotransducers such as integrins, present alternative therapeutic targets. One of the most sought after targets is integrin αv, because it is generally not expressed in epithelial cells, but is involved in tumor angiogenesis and metastasis. For example, the monoclonal antibody Intetumumab (CNTO 95) has been shown to interrupt αv-activated pathways associated with focal adhesions and cell motility, reducing breast cancer growth and metastasis in mouse models 287 and has been tested alone or in combination with chemotherapy for melanoma treatment in humans 288. Abituzumab, another monoclonal antibody that targets all αv integrins, has shown overall limited clinical efficacy in patients with metastatic colorectal cancer when administered in combination with EGFR inhibitor cetuximab and chemotherapeutic irinotecan 289. However, patients with high αvβ6 expression did benefit from the antibody, suggesting that a priori stratification for αvβ6 levels might be needed. Volociximab, an α5β1-inhibiting antibody, has also been shown to block angiogenesis and tumor growth in xenograft models, but has shown less efficacy in clinical trials for ovarian and peritoneal cancers 290. Small molecule integrin inhibitors, such as the RGD mimetic cilengitide, have also been tested alone or in combination with chemotherapy for prostate cancer, non-small cell lung carcinoma, and glioblastoma, but no clear positive outcomes have been noted 291. An exciting new frontier is the multiple natural agents currently being tested alone or in combination with other therapies as cancer treatments. For example, curcumin has been reported to regulate various integrins such as β1 and α6β4 in different cancers and has entered clinical trials 292. The chemo- and immunotherapeutic targeting of integrins, key mediators cell mechanobiological responses, suggests promise for targeting other mechanobiological pathways in cancer treatment. For a detailed review on integrins as therapeutic targets in cancer treatments, the readers are referred to the following review, reference 293.
A final mechanobiological target we will mention is the YAP and TAZ co-effectors of the Hippo pathway. These localize to the nucleus in response to mechanical stimuli to trigger downstream signaling 294–296 associated with matrix stiffness and tumorigenesis, and are thus an important therapeutic target. For example, the YAP inhibitor and photosensitizer verteporfin has been tested in patients with recurrent glioblastoma or advanced pancreatic carcinoma 297. YAP-TAZ signaling could also be achieved by reducing YAP/TAZ protein levels via proteolysis targeting chimeras (PROTACs) where some small molecule PROTACs have been evaluated in phase I clinical trials 298. Activation of YAP/TAZ and actin remodeling has been associated with chemotherapy resistance 299,300, and downregulation of YAP/TAZ with therapeutic molecules such as curcumin 301 could improve susceptibility of resistant cells to treatments.
6. Conclusions and future perspectives
In summary, mechanical forces play an important role in the cancer cell responsiveness to therapies, such as chemotherapy or immunotherapy, and mechanobiological factors serve as important therapeutic targets. Overall, matrix stiffening, activation of mechano-induced transcriptional regulators, and leaky vasculature have been linked to therapy resistance, suggesting that mechanosensing molecules and pathways could be viable therapeutic targets 302. Particularly compelling examples include softening of the tumor matrix via pharmaceuticals to reduce metastasis or sensitize cancer cells to chemotherapy.262,263 Repair of the tumor “leaky” vasculature via oxygen micorbubbles released via ultrasound 303 or aspirin 304 could reduce interstitial pressure and enhance perfusion to improve drug delivery efficiency. This may also suppress tumor cell extravasation and metastasis.
An important question to resolve is how ECM modulation affects the delivery of chemotherapeutic and immunotherapeutic agents. Certainly, clues are evident from the ways that ECM stiffening caused by other diseases impacts cancer treatments. In diabetes mellitus, high blood sugar levels alter the physical properties of the ECM and cause cellular drug resistance. However, further studies are still needed to determine whether the observed drug resistance is at least partially due to the alteration in the physical properties of the ECM. Alternative explanations are that this drug resistance arises from biochemical sources such as fatty acid synthase that has been reported to be the main cause of the hyperglycemic drug resistance in breast cancer cells 305. Overall, the mechanobiological effects of diabetes mellitus can affect the delivery, uptake, and dosage of drug and immunotherapies. Understanding and ameliorating these effects represent important frontiers.
Much progress in the field of mechanobiology owes its genesis to the development of biomaterial platforms that enable cells and tissue constructs to be studied under conditions of prescribed stiffness, surface chemistry, and mechanical loadings. Such platforms have been of broad utility in drug discovery 306 and are promising for discovery of drugs and immunotherapies that target cancer cells in different physical environments. However, increased complexity typically comes with higher screening costs, lower throughput and generally less assays and technology available for analysis. For example, most high-throughput systems and liquid handlers have been adapted to work with multiwell plates and most assays to study cell responses depend on dissociated cells or colorimetry. Image-analysis algorithms and in general microscopy technologies are limited for 3D systems. Simpler 2D assays that mimic the tumor ECM mechanically and compositionally, could be more easily adapted to work with the technologies currently developed to conduct large scale drug screening and are generally inexpensive and easy to use.
Further, many of the studies described here were performed with immortalized cell lines, which are well-characterized, cost-effective and allow for comparison of results between labs. However, cell lines are typically expanded on rigid polystyrene dishes and, thus, adapted to such mechanical environments. For example, recent work on breast cancer cells has shown that this adaptation leads to profound changes in cell growth, metastatic potential, and chemotherapeutic response, generally making them more susceptible to some chemotherapeutics 158. The use of primary cells or re-adaptation of immortalized cell lines to soft substrates 307 is recommended when testing the interplay between cell mechanosensing and drug responsiveness. Further, routine sequencing could be performed periodically to confirm that the cells are still matching the genetic profile of the original tumor.
Many of the challenges associated with tests on idealized cell lines are overcome by use of animal models. However, the physiological realism of animal models comes at the cost of reduced ability to control mechanobiological factors. In animal models, mechanobiology studies could be further facilitated by development of techniques capable of non-invasively measuring tissue stiffness and strain in vivo, and in real time. Some examples include techniques that actuate magnetics droplets to infer the viscoelastic properties of tissues 308,309, magnetic resonance elastography for estimating breast cancer or brain tissue stiffness 310,311, time resolved 3D ultrasound 312, and in situ calibration of optical tweezers for measuring hemodynamic forces in vivo 313. As the field of in vivo imaging for characterizing biomechanics and diffusion progresses 314, animal models may become increasing relevant to overcoming mechanobiological hurdles to chemotherapy and immunotherapy. In the meantime, innovation in vitro systems with prescribed mechanical properties appears poised to continue to lead the way.
Acknowledgements
This work was supported by grants NIH R01AR077793 and NSF CMMI 1548571 awarded to GG and T32-EB021955 awarded to DS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Discher DE, Janmey P & Wang Y.-l. Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Legant WR et al. Measurement of mechanical tractions exerted by cells in three-dimensional matrices. Nature methods 7, 969 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engler AJ, Sen S, Sweeney HL & Discher DE Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Eyckmans J, Boudou T, Yu X & Chen CS A hitchhiker’s guide to mechanobiology. Developmental cell 21, 35–47 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang JH-C & Thampatty BP An introductory review of cell mechanobiology. Biomechanics and modeling in mechanobiology 5, 1–16 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Butcher DT, Alliston T & Weaver VM A tense situation: forcing tumour progression. Nature Reviews Cancer 9, 108–122 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castells M, Thibault B, Delord J-P & Couderc B Implication of tumor microenvironment in chemoresistance: tumor-associated stromal cells protect tumor cells from cell death. International journal of molecular sciences 13, 9545–9571 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedl P & Alexander S Cancer invasion and the microenvironment: plasticity and reciprocity. Cell 147, 992–1009 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Liu AY et al. Cell–cell interaction in prostate gene regulation and cytodifferentiation. Proceedings of the National Academy of Sciences 94, 10705–10710 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rafii A et al. Oncologic trogocytosis of an original stromal cells induces chemoresistance of ovarian tumours. PloS one 3, e3894 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jo Y et al. Chemoresistance of cancer cells: requirements of tumor microenvironment-mimicking in vitro models in anti-cancer drug development. Theranostics 8, 5259 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsunaga T et al. Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nature medicine 9, 1158–1165 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Damiano JS, Cress AE, Hazlehurst LA, Shtil AA & Dalton WS Cell adhesion mediated drug resistance (CAM-DR): role of integrins and resistance to apoptosis in human myeloma cell lines. Blood, The Journal of the American Society of Hematology 93, 1658–1667 (1999). [PMC free article] [PubMed] [Google Scholar]
- 14.Shishido S, Bönig H & Kim Y-M Role of integrin alpha4 in drug resistance of leukemia. Frontiers in oncology 4, 99 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lis R et al. Mesenchymal cell interaction with ovarian cancer cells induces a background dependent pro-metastatic transcriptomic profile. Journal of translational medicine 12, 1–12 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lis R et al. Mesenchymal cell interaction with ovarian cancer cells triggers pro-metastatic properties. PloS one 7, e38340 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Touboul C et al. Mesenchymal stem cells enhance ovarian cancer cell infiltration through IL6 secretion in an amniochorionic membrane based 3D model. Journal of Translational Medicine 11, 1–12 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Touboul C, Vidal F, Pasquier J, Lis R & Rafii A Role of mesenchymal cells in the natural history of ovarian cancer: a review. Journal of translational medicine 12, 1–17 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westhoff M, Zhou S, Bachem M, Debatin K-M & Fulda S Identification of a novel switch in the dominant forms of cell adhesion-mediated drug resistance in glioblastoma cells. Oncogene 27, 5169–5181 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Lee J, Shin D & Roh J-L Development of an in vitro cell-sheet cancer model for chemotherapeutic screening. Theranostics 8, 3964 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasquier J et al. CCL2/CCL5 secreted by the stroma induce IL-6/PYK2 dependent chemoresistance in ovarian cancer. Molecular cancer 17, 1–14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lotti F et al. Chemotherapy activates cancer-associated fibroblasts to maintain colorectal cancer-initiating cells by IL-17A. Journal of Experimental Medicine 210, 2851–2872 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tao L et al. Cancer-associated fibroblasts treated with cisplatin facilitates chemoresistance of lung adenocarcinoma through IL-11/IL-11R/STAT3 signaling pathway. Scientific reports 6, 1–13 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rizzuti IF et al. Mechanical control of cell proliferation increases resistance to chemotherapeutic agents. Physical Review Letters 125, 128103 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Hamilton GA et al. Regulation of cell morphology and cytochrome P450 expression in human hepatocytes by extracellular matrix and cell-cell interactions. Cell and tissue research 306, 85–99 (2001). [DOI] [PubMed] [Google Scholar]
- 26.Li CY et al. Micropatterned cell–cell interactions enable functional encapsulation of primary hepatocytes in hydrogel microtissues. Tissue Engineering Part A 20, 2200–2212 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hofland T et al. Natural killer cell hypo-responsiveness in chronic lymphocytic leukemia can be circumvented in vitro by adequate activating signaling. Hemasphere 3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goc J et al. Dysregulation of ILC3s unleashes progression and immunotherapy resistance in colon cancer. Cell 184, 5015–5030. e5016 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cross SE, Jin Y-S, Rao J & Gimzewski JK Nanomechanical analysis of cells from cancer patients. Nature nanotechnology 2, 780–783 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Holle AW et al. Cancer cells invade confined microchannels via a self-directed mesenchymal-to-amoeboid transition. Nano letters 19, 2280–2290 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fritsch A et al. Are biomechanical changes necessary for tumour progression? Nature Physics 6, 730–732 (2010). [Google Scholar]
- 32.Majedi FS et al. T-cell activation is modulated by the 3D mechanical microenvironment. Biomaterials 252, 120058 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lei K et al. Cancer-cell stiffening via cholesterol depletion enhances adoptive T-cell immunotherapy. Nature Biomedical Engineering 5, 1411–1425 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basu R et al. Cytotoxic T cells use mechanical force to potentiate target cell killing. Cell 165, 100–110 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vorselen D et al. Microparticle traction force microscopy reveals subcellular force exertion patterns in immune cell–target interactions. Nature communications 11, 1–14 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tello-Lafoz M et al. Cytotoxic lymphocytes target characteristic biophysical vulnerabilities in cancer. Immunity 54, 1037–1054. e1037 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oakes PW, Banerjee S, Marchetti MC & Gardel ML Geometry regulates traction stresses in adherent cells. Biophysical journal 107, 825–833 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaudhuri O et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nature materials 15, 326–334 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wirtz D, Konstantopoulos K & Searson PC The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nature Reviews Cancer 11, 512–522 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Damodaran K et al. Compressive force induces reversible chromatin condensation and cell geometry–dependent transcriptional response. Molecular biology of the cell 29, 3039–3051 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamada KM & Sixt M Mechanisms of 3D cell migration. Nature Reviews Molecular Cell Biology 20, 738–752 (2019). [DOI] [PubMed] [Google Scholar]
- 42.Swift J et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 341 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jain N, Iyer KV, Kumar A & Shivashankar G Cell geometric constraints induce modular gene-expression patterns via redistribution of HDAC3 regulated by actomyosin contractility. Proceedings of the National Academy of Sciences 110, 11349–11354 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Versaevel M, Grevesse T & Gabriele S Spatial coordination between cell and nuclear shape within micropatterned endothelial cells. Nature communications 3, 1–11 (2012). [DOI] [PubMed] [Google Scholar]
- 45.Hall MS et al. Fibrous nonlinear elasticity enables positive mechanical feedback between cells and ECMs. Proceedings of the National Academy of Sciences 113, 14043–14048 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khatau SB et al. A perinuclear actin cap regulates nuclear shape. Proceedings of the National Academy of Sciences 106, 19017–19022 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J et al. Nuclear mechanics within intact cells is regulated by cytoskeletal network and internal nanostructures. Small 16, 1907688 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bao M, Xie J, Piruska A & Huck WT 3D microniches reveal the importance of cell size and shape. Nature communications 8, 1–12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Helvert S, Storm C & Friedl P Mechanoreciprocity in cell migration. Nature cell biology 20, 8–20 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petrie RJ, Koo H & Yamada KM Generation of compartmentalized pressure by a nuclear piston governs cell motility in a 3D matrix. Science 345, 1062–1065 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Charras G & Sahai E Physical influences of the extracellular environment on cell migration. Nature reviews Molecular cell biology 15, 813–824 (2014). [DOI] [PubMed] [Google Scholar]
- 52.Damiano JS Integrins as novel drug targets for overcoming innate drug resistance. Current cancer drug targets 2, 37–43 (2002). [DOI] [PubMed] [Google Scholar]
- 53.Holle AW, Young JL & Spatz JP In vitro cancer cell–ECM interactions inform in vivo cancer treatment. Advanced drug delivery reviews 97, 270–279 (2016). [DOI] [PubMed] [Google Scholar]
- 54.Prager-Khoutorsky M et al. Fibroblast polarization is a matrix-rigidity-dependent process controlled by focal adhesion mechanosensing. Nature cell biology 13, 1457–1465 (2011). [DOI] [PubMed] [Google Scholar]
- 55.Wang N, Tytell JD & Ingber DE Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nature reviews Molecular cell biology 10, 75–82 (2009). [DOI] [PubMed] [Google Scholar]
- 56.Deville SS & Cordes N The Extracellular, Cellular, and Nuclear Stiffness, a Trinity in the Cancer Resistome—A Review. Frontiers in oncology 9, 1376 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Damiano J, Hazlehurst L & Dalton W Cell adhesion-mediated drug resistance (CAM-DR) protects the K562 chronic myelogenous leukemia cell line from apoptosis induced by BCR/ABL inhibition, cytotoxic drugs, and γ-irradiation. Leukemia 15, 1232–1239 (2001). [DOI] [PubMed] [Google Scholar]
- 58.Shain KH & Dalton WS Cell adhesion is a key determinant in de novo multidrug resistance (MDR): new targets for the prevention of acquired MDR. Molecular cancer therapeutics 1, 69–78 (2001). [PubMed] [Google Scholar]
- 59.Berrier AL & Yamada KM Cell–matrix adhesion. Journal of cellular physiology 213, 565–573 (2007). [DOI] [PubMed] [Google Scholar]
- 60.Seguin L, Desgrosellier JS, Weis SM & Cheresh DA Integrins and cancer: regulators of cancer stemness, metastasis, and drug resistance. Trends in cell biology 25, 234–240 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cordes N, Blaese M, Plasswilm L, Rodemann H & Van Beuningen D Fibronectin and laminin increase resistance to ionizing radiation and the cytotoxic drug Ukrain® in human tumour and normal cells in vitro. International journal of radiation biology 79, 709–720 (2003). [DOI] [PubMed] [Google Scholar]
- 62.Aoudjit F & Vuori K Integrin signaling inhibits paclitaxel-induced apoptosis in breast cancer cells. Oncogene 20, 4995–5004 (2001). [DOI] [PubMed] [Google Scholar]
- 63.Yao ES et al. Increased β1 integrin is associated with decreased survival in invasive breast cancer. Cancer Research 67, 659–664 (2007). [DOI] [PubMed] [Google Scholar]
- 64.Yousefi H et al. Understanding the role of integrins in breast cancer invasion, metastasis, angiogenesis, and drug resistance. Oncogene 40, 1043–1063 (2021). [DOI] [PubMed] [Google Scholar]
- 65.Rintoul RC & Sethi T Extracellular matrix regulation of drug resistance in small-cell lung cancer. Clinical science 102, 417–424 (2002). [PubMed] [Google Scholar]
- 66.Sethi T et al. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo. Nature medicine 5, 662–668 (1999). [DOI] [PubMed] [Google Scholar]
- 67.Wei L, Yin F, Chen C & Li L Expression of integrin α-6 is associated with multi drug resistance and prognosis in ovarian cancer. Oncology letters 17, 3974–3980 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu Z et al. Elevated levels of Lewis y and integrin α5β1 correlate with chemotherapeutic drug resistance in epithelial ovarian carcinoma. International journal of molecular sciences 13, 15588–15600 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marelli UK, Rechenmacher F, Sobahi TRA, Mas-Moruno C & Kessler H Tumor targeting via integrin ligands. Frontiers in oncology 3, 222 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cukierman E, Pankov R, Stevens DR & Yamada KM Taking cell-matrix adhesions to the third dimension. Science 294, 1708–1712 (2001). [DOI] [PubMed] [Google Scholar]
- 71.Eke I & Cordes N Radiobiology goes 3D: how ECM and cell morphology impact on cell survival after irradiation. Radiotherapy and Oncology 99, 271–278 (2011). [DOI] [PubMed] [Google Scholar]
- 72.Chen Y et al. Microfluidic co-culture of liver tumor spheroids with stellate cells for the investigation of drug resistance and intercellular interactions. Analyst 144, 4233–4240 (2019). [DOI] [PubMed] [Google Scholar]
- 73.Hill L, Bruns J & Zustiak SP Hydrogel matrix presence and composition influence drug responses of encapsulated glioblastoma spheroids. Acta Biomaterialia (2021). [DOI] [PubMed] [Google Scholar]
- 74.Kaylan KB et al. Mapping lung tumor cell drug responses as a function of matrix context and genotype using cell microarrays. Integrative Biology 8, 1221–1231 (2016). [DOI] [PubMed] [Google Scholar]
- 75.Rhee S, Jiang H, Ho C-H & Grinnell F Microtubule function in fibroblast spreading is modulated according to the tension state of cell–matrix interactions. Proceedings of the National Academy of Sciences 104, 5425–5430 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Karamichos D, Lakshman N & Petroll WM Regulation of corneal fibroblast morphology and collagen reorganization by extracellular matrix mechanical properties. Investigative ophthalmology & visual science 48, 5030–5037 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shakiba D et al. The balance between actomyosin contractility and microtubule polymerization regulates hierarchical protrusions that govern efficient fibroblast–collagen interactions. ACS nano 14, 7868–7879 (2020). [DOI] [PubMed] [Google Scholar]
- 78.Flynn BP et al. Mechanical strain stabilizes reconstituted collagen fibrils against enzymatic degradation by mammalian collagenase matrix metalloproteinase 8 (MMP-8). PloS one 5, e12337 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Szulczewski JM et al. Directional cues in the tumor microenvironment due to cell contraction against aligned collagen fibers. Acta Biomaterialia (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang S & Ingber DE Cell tension, matrix mechanics, and cancer development. Cancer cell 8, 175–176 (2005). [DOI] [PubMed] [Google Scholar]
- 81.Ahmadzadeh H et al. Modeling the two-way feedback between contractility and matrix realignment reveals a nonlinear mode of cancer cell invasion. Proceedings of the National Academy of Sciences 114, E1617–E1626 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Molina JR, Hayashi Y, Stephens C & Georgescu M-M Invasive glioblastoma cells acquire stemness and increased Akt activation. Neoplasia 12, 453–IN455 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zustiak S, Nossal R & Sackett DL Multiwell stiffness assay for the study of cell responsiveness to cytotoxic drugs. Biotechnology and bioengineering 111, 396–403 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zustiak SP The role of matrix compliance on cell responses to drugs and toxins: towards predictive drug screening platforms. Macromolecular bioscience 15, 589–599 (2015). [DOI] [PubMed] [Google Scholar]
- 85.Syed S, Karadaghy A & Zustiak S Simple polyacrylamide-based multiwell stiffness assay for the study of stiffness-dependent cell responses. Journal of visualized experiments: JoVE (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Imaninezhad M, Hill L, Kolar G, Vogt K & Zustiak SP Templated macroporous polyethylene glycol hydrogels for spheroid and aggregate cell culture. Bioconjugate chemistry 30, 34–46 (2018). [DOI] [PubMed] [Google Scholar]
- 87.Li S et al. Soft Substrate Promotes Osteosarcoma Cell Self-Renewal, Differentiation, and Drug Resistance Through miR-29b and Its Target Protein Spin 1. ACS Biomaterials Science & Engineering 6, 5588–5598 (2020). [DOI] [PubMed] [Google Scholar]
- 88.Liu C et al. Role of three-dimensional matrix stiffness in regulating the chemoresistance of hepatocellular carcinoma cells. Biotechnology and applied biochemistry 62, 556–562 (2015). [DOI] [PubMed] [Google Scholar]
- 89.Plodinec M et al. The nanomechanical signature of breast cancer. Nature nanotechnology 7, 757–765 (2012). [DOI] [PubMed] [Google Scholar]
- 90.Joyce MH et al. Phenotypic basis for matrix stiffness-dependent chemoresistance of breast cancer cells to doxorubicin. Frontiers in oncology 8, 337 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zustiak SP et al. Three-dimensional matrix stiffness and adhesive ligands affect cancer cell response to toxins. Biotechnology and bioengineering 113, 443–452 (2016). [DOI] [PubMed] [Google Scholar]
- 92.Nguyen TV, Sleiman M, Moriarty T, Herrick WG & Peyton SR Sorafenib resistance and JNK signaling in carcinoma during extracellular matrix stiffening. Biomaterials 35, 5749–5759 (2014). [DOI] [PubMed] [Google Scholar]
- 93.Schrader J et al. Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology 53, 1192–1205 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wei L, Surma M, Shi S, Lambert-Cheatham N & Shi J Novel insights into the roles of Rho kinase in cancer. Archivum immunologiae et therapiae experimentalis 64, 259–278 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McGrail DJ, Kieu QMN & Dawson MR The malignancy of metastatic ovarian cancer cells is increased on soft matrices through a mechanosensitive Rho–ROCK pathway. Journal of cell science 127, 2621–2626 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Matsuoka T & Yashiro M Rho/ROCK signaling in motility and metastasis of gastric cancer. World journal of gastroenterology: WJG 20, 13756 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Matsubara M & Bissell MJ Inhibitors of Rho kinase (ROCK) signaling revert the malignant phenotype of breast cancer cells in 3D context. Oncotarget 7, 31602 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sahai E & Marshall CJ RHO–GTPases and cancer. Nature Reviews Cancer 2, 133–142 (2002). [DOI] [PubMed] [Google Scholar]
- 99.Rodriguez-Hernandez I, Cantelli G, Bruce F & Sanz-Moreno V Rho, ROCK and actomyosin contractility in metastasis as drug targets. F1000Research 5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mokady D & Meiri D RhoGTPases–a novel link between cytoskeleton organization and cisplatin resistance. Drug Resistance Updates 19, 22–32 (2015). [DOI] [PubMed] [Google Scholar]
- 101.Ohta T et al. Inhibition of the Rho/ROCK pathway enhances the efficacy of cisplatin through the blockage of hypoxia-inducible factor-1α in human ovarian cancer cells. Cancer biology & therapy 13, 25–33 (2012). [DOI] [PubMed] [Google Scholar]
- 102.Street CA et al. Pharmacological inhibition of Rho-kinase (ROCK) signaling enhances cisplatin resistance in neuroblastoma cells. International journal of oncology 37, 1297–1305 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim S, Kim SA, Han J & Kim I-S Rho-Kinase as a Target for Cancer Therapy and Its Immunotherapeutic Potential. International journal of molecular sciences 22, 12916 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cardama GA, González N, Maggio J, Menna PL & Gomez DE Rho GTPases as therapeutic targets in cancer. International journal of oncology 51, 1025–1034 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Henke E, Nandigama R & Ergün S Extracellular matrix in the tumor microenvironment and its impact on cancer therapy. Frontiers in molecular biosciences 6, 160 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hosseinkhani N et al. Immune checkpoints and CAR-T cells: the pioneers in future cancer therapies? International Journal of Molecular Sciences 21, 8305 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.O’Donnell JS, Teng MW & Smyth MJ Cancer immunoediting and resistance to T cell-based immunotherapy. Nature reviews Clinical oncology 16, 151–167 (2019). [DOI] [PubMed] [Google Scholar]
- 108.Fares CM, Van Allen EM, Drake CG, Allison JP & Hu-Lieskovan S Mechanisms of resistance to immune checkpoint blockade: why does checkpoint inhibitor immunotherapy not work for all patients? American Society of Clinical Oncology Educational Book 39, 147–164 (2019). [DOI] [PubMed] [Google Scholar]
- 109.Park D et al. High-throughput microfluidic 3D cytotoxicity assay for cancer immunotherapy (CACI-IMPACT platform). Frontiers in immunology 10, 1133 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Salmon H & Donnadieu E Within tumors, interactions between T cells and tumor cells are impeded by the extracellular matrix. Oncoimmunology 1, 992–994 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mariathasan S et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554, 544–548 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wei J et al. Hypoxia potentiates glioma-mediated immunosuppression. PloS one 6, e16195 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen Y et al. SNF5, a core subunit of SWI/SNF complex, regulates melanoma cancer cell growth, metastasis, and immune escape in response to matrix stiffness. Translational Oncology 17, 101335 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Miyazawa A et al. Regulation of PD-L1 expression by matrix stiffness in lung cancer cells. Biochemical and biophysical research communications 495, 2344–2349 (2018). [DOI] [PubMed] [Google Scholar]
- 115.Jiang H, Hegde S & DeNardo DG Tumor-associated fibrosis as a regulator of tumor immunity and response to immunotherapy. Cancer Immunology, Immunotherapy 66, 1037–1048 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Acerbi I et al. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integrative Biology 7, 1120–1134 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jiang C et al. Hypoxia-inducible factor 1α regulates a SOCS3-STAT3-adiponectin signal transduction pathway in adipocytes. Journal of Biological Chemistry 288, 3844–3857 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gordon-Weeks A & Yuzhalin AE Cancer extracellular matrix proteins regulate tumour immunity. Cancers 12, 3331 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim H, Cha J, Jang M & Kim P Hyaluronic acid-based extracellular matrix triggers spontaneous M2-like polarity of monocyte/macrophage. Biomaterials science 7, 2264–2271 (2019). [DOI] [PubMed] [Google Scholar]
- 120.Kaplan G In vitro differentiation of human monocytes. Monocytes cultured on glass are cytotoxic to tumor cells but monocytes cultured on collagen are not. The Journal of experimental medicine 157, 2061–2072 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sha H, Zhang D, Zhang Y, Wen Y & Wang Y ATF3 promotes migration and M1/M2 polarization of macrophages by activating tenascin-C via Wnt/β-catenin pathway. Molecular medicine reports 16, 3641–3647 (2017). [DOI] [PubMed] [Google Scholar]
- 122.Perri RT et al. Fibronectin enhances in vitro monocyte-macrophage-mediated tumoricidal activity. (1982). [PubMed] [Google Scholar]
- 123.Allavena P & Mantovani A Immunology in the clinic review series; focus on cancer: tumour-associated macrophages: undisputed stars of the inflammatory tumour microenvironment. Clinical & Experimental Immunology 167, 195–205 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ramos MIP et al. Cancer immunotherapy by NC410, a LAIR-2 Fc protein blocking human LAIR-collagen interaction. Elife 10, e62927 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chakravarthy A, Khan L, Bensler NP, Bose P & De Carvalho DD TGF-β-associated extracellular matrix genes link cancer-associated fibroblasts to immune evasion and immunotherapy failure. Nature communications 9, 1–10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Derynck R, Turley SJ & Akhurst RJ TGFβ biology in cancer progression and immunotherapy. Nature Reviews Clinical Oncology 18, 9–34 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang W et al. Chimeric antigen receptor macrophage therapy for breast tumours mediated by targeting the tumour extracellular matrix. British journal of cancer 121, 837–845 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lei K, Kurum A & Tang L Mechanical Immunoengineering of T cells for Therapeutic Applications. Accounts of Chemical Research 53, 2777–2790 (2020). [DOI] [PubMed] [Google Scholar]
- 129.Yudkin JS, Richter B & Gale EA Intensive treatment of hyperglycaemia: what are the objectives? The Lancet 376, 1462–1463 (2010). [DOI] [PubMed] [Google Scholar]
- 130.Snedeker JG & Gautieri A The role of collagen crosslinks in ageing and diabetes-the good, the bad, and the ugly. Muscles, ligaments and tendons journal 4, 303 (2014). [PMC free article] [PubMed] [Google Scholar]
- 131.Avery N & Bailey A The effects of the Maillard reaction on the physical properties and cell interactions of collagen. Pathologie Biologie 54, 387–395 (2006). [DOI] [PubMed] [Google Scholar]
- 132.Avery N & Bailey A Enzymic and non-enzymic cross-linking mechanisms in relation to turnover of collagen: relevance to aging and exercise. Scandinavian journal of medicine & science in sports 15, 231–240 (2005). [DOI] [PubMed] [Google Scholar]
- 133.Saito M & Marumo K Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporosis international 21, 195–214 (2010). [DOI] [PubMed] [Google Scholar]
- 134.Bank RA, Bayliss MT, Lafeber FP, Maroudas A & Tekoppele JM Ageing and zonal variation in post-translational modification of collagen in normal human articular cartilage: the age-related increase in non-enzymatic glycation affects biomechanical properties of cartilage. Biochemical Journal 330, 345–351 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sant S, Wang D, Agarwal R, Dillender S & Ferrell N Glycation alters the mechanical behavior of kidney extracellular matrix. Matrix biology plus 8, 100035 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Negre-Salvayre A, Salvayre R, Auge N, Pamplona R & Portero-Otin M Hyperglycemia and glycation in diabetic complications. Antioxidants & redox signaling 11, 3071–3109 (2009). [DOI] [PubMed] [Google Scholar]
- 137.Lévigne D, Tobalem M, Modarressi A & Pittet-Cuénod B Hyperglycemia increases susceptibility to ischemic necrosis. BioMed research international 2013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Joshi N, Caputo GM, Weitekamp MR & Karchmer A Infections in patients with diabetes mellitus. New England Journal of Medicine 341, 1906–1912 (1999). [DOI] [PubMed] [Google Scholar]
- 139.Stebbing J et al. A metabolic phenotyping approach to understanding relationships between metabolic syndrome and breast tumour responses to chemotherapy. Annals of Oncology 23, 860–866 (2012). [DOI] [PubMed] [Google Scholar]
- 140.Xu X et al. Hyperglycemia promotes Snail-induced epithelial–mesenchymal transition of gastric cancer via activating ENO1 expression. Cancer Cell International 19, 1–12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhao W et al. High glucose promotes gastric cancer chemoresistance in vivo and in vitro. Molecular medicine reports 12, 843–850 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ma Y-S et al. High glucose modulates antiproliferative effect and cytotoxicity of 5-fluorouracil in human colon cancer cells. DNA and cell biology 33, 64–72 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Biernacka K et al. Hyperglycaemia-induced chemoresistance of prostate cancer cells due to IGFBP2. Endocr Relat Cancer 20, 741–751 (2013). [DOI] [PubMed] [Google Scholar]
- 144.Ma J, Wang J, Ge L, Long B & Zhang J The impact of diabetes mellitus on clinical outcomes following chemotherapy for the patients with pancreatic cancer: a meta-analysis. Acta Diabetologica 56, 1103–1111 (2019). [DOI] [PubMed] [Google Scholar]
- 145.Srokowski TP, Fang S, Hortobagyi GN & Giordano SH Impact of diabetes mellitus on complications and outcomes of adjuvant chemotherapy in older patients with breast cancer. Journal of Clinical Oncology 27, 2170 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Alenzi EO & Kelley GA The association of hyperglycemia and diabetes mellitus and the risk of chemotherapy-induced neutropenia among cancer patients: a systematic review with meta-analysis. Journal of Diabetes and its Complications 31, 267–272 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Matsumura Y & Maeda H A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer research 46, 6387–6392 (1986). [PubMed] [Google Scholar]
- 148.Iyer AK, Khaled G, Fang J & Maeda H Exploiting the enhanced permeability and retention effect for tumor targeting. Drug discovery today 11, 812–818 (2006). [DOI] [PubMed] [Google Scholar]
- 149.Maeda H SMANCS and polymer-conjugated macromolecular drugs: advantages in cancer chemotherapy. Advanced drug delivery reviews 46, 169–185 (2001). [DOI] [PubMed] [Google Scholar]
- 150.Tee JK et al. Nanoparticles’ interactions with vasculature in diseases. Chemical Society Reviews 48, 5381–5407 (2019). [DOI] [PubMed] [Google Scholar]
- 151.Rey JA, Ewing JR & Sarntinoranont M A computational model of glioma reveals opposing, stiffness-sensitive effects of leaky vasculature and tumor growth on tissue mechanical stress and porosity. Biomechanics and modeling in mechanobiology 20, 1981–2000 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Purkayastha P, Jaiswal MK & Lele TP Molecular cancer cell responses to solid compressive stress and interstitial fluid pressure. Cytoskeleton 78, 312–322 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Koumoutsakos P, Pivkin I & Milde F The fluid mechanics of cancer and its therapy. Annual review of fluid mechanics 45, 325–355 (2013). [Google Scholar]
- 154.Sewell-Loftin MK et al. Cancer-associated fibroblasts support vascular growth through mechanical force. Scientific reports 7, 1–12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sewell-Loftin MK, Katz JB, George SC & Longmore GD Micro-strains in the extracellular matrix induce angiogenesis. Lab on a Chip 20, 2776–2787 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sharma M et al. Simultaneously targeting cancer-associated fibroblasts and angiogenic vessel as a treatment for TNBC. Journal of Experimental Medicine 218 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zanotelli MR & Reinhart-King CA Mechanical forces in tumor angiogenesis. Biomechanics in Oncology, 91–112 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Medina SH et al. Identification of a mechanogenetic link between substrate stiffness and chemotherapeutic response in breast cancer. Biomaterials 202, 1–11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pelham RJ & Wang Y.-l. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proceedings of the National Academy of Sciences 94, 13661–13665 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tse JR & Engler AJ Preparation of hydrogel substrates with tunable mechanical properties. Current protocols in cell biology 47, 10.16. 11–10.16. 16 (2010). [DOI] [PubMed] [Google Scholar]
- 161.Levental I, Georges PC & Janmey PA Soft biological materials and their impact on cell function. Soft Matter 3, 299–306 (2007). [DOI] [PubMed] [Google Scholar]
- 162.Sunyer R, Jin AJ, Nossal R & Sackett DL Fabrication of hydrogels with steep stiffness gradients for studying cell mechanical response. PloS one 7, e46107 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mih JD et al. A multiwell platform for studying stiffness-dependent cell biology. PloS one 6, e19929 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lin C-H et al. Microenvironment rigidity modulates responses to the HER2 receptor tyrosine kinase inhibitor lapatinib via YAP and TAZ transcription factors. Molecular biology of the cell 26, 3946–3953 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Palchesko RN, Zhang L, Sun Y & Feinberg AW Development of polydimethylsiloxane substrates with tunable elastic modulus to study cell mechanobiology in muscle and nerve. PloS one 7, e51499 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang P-Y, Tsai W-B & Voelcker NH Screening of rat mesenchymal stem cell behaviour on polydimethylsiloxane stiffness gradients. Acta biomaterialia 8, 519–530 (2012). [DOI] [PubMed] [Google Scholar]
- 167.Jiang L, Huang Y, Zhang X & Qin H Electrohydrodynamic inkjet printing of Polydimethylsiloxane (PDMS). Procedia Manufacturing 48, 90–94 (2020). [Google Scholar]
- 168.Feng J et al. Substrate stiffness influences the outcome of antitumor drug screening in vitro. Clinical hemorheology and microcirculation 55, 121–131 (2013). [DOI] [PubMed] [Google Scholar]
- 169.Yip D & Cho CH A multicellular 3D heterospheroid model of liver tumor and stromal cells in collagen gel for anti-cancer drug testing. Biochemical and biophysical research communications 433, 327–332 (2013). [DOI] [PubMed] [Google Scholar]
- 170.Garrido C et al. HSP27 as a mediator of confluence-dependent resistance to cell death induced by anticancer drugs. Cancer Research 57, 2661–2667 (1997). [PubMed] [Google Scholar]
- 171.Dimanche-Boitrel MT et al. Confluence-dependent resistance in human colon cancer cells: role of reduced drug accumulation and low intrinsic chemosensitivity of resting cells. International journal of cancer 50, 677–682 (1992). [DOI] [PubMed] [Google Scholar]
- 172.Baruffaldi D, Palmara G, Pirri C & Frascella F 3D Cell Culture: Recent Development in Materials with Tunable Stiffness. ACS Applied Bio Materials 4, 2233–2250 (2021). [DOI] [PubMed] [Google Scholar]
- 173.Langhans SA Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Frontiers in pharmacology 9, 6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Eglen RM & Randle DH Drug discovery goes three-dimensional: goodbye to flat high-throughput screening? Assay and drug development technologies 13, 262–265 (2015). [DOI] [PubMed] [Google Scholar]
- 175.Oliveira MB & Mano JF High-throughput screening for integrative biomaterials design: exploring advances and new trends. Trends in biotechnology 32, 627–636 (2014). [DOI] [PubMed] [Google Scholar]
- 176.Zhang N et al. Soft hydrogels featuring in-depth surface density gradients for the simple establishment of 3D tissue models for screening applications. SLAS DISCOVERY: Advancing Life Sciences R&D 22, 635–644 (2017). [DOI] [PubMed] [Google Scholar]
- 177.Shin J-W & Mooney DJ Extracellular matrix stiffness causes systematic variations in proliferation and chemosensitivity in myeloid leukemias. Proceedings of the National Academy of Sciences 113, 12126–12131 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chang F-C et al. PEG-chitosan hydrogel with tunable stiffness for study of drug response of breast cancer cells. Polymers 8, 112 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Vatankhah E et al. Artificial neural network for modeling the elastic modulus of electrospun polycaprolactone/gelatin scaffolds. Acta biomaterialia 10, 709–721 (2014). [DOI] [PubMed] [Google Scholar]
- 180.Wright L, Young R, Andric T & Freeman J Fabrication and mechanical characterization of 3D electrospun scaffolds for tissue engineering. Biomedical Materials 5, 055006 (2010). [DOI] [PubMed] [Google Scholar]
- 181.Simonet M et al. Tailoring the void space and mechanical properties in electrospun scaffolds towards physiological ranges. Journal of Materials Chemistry B 2, 305–313 (2014). [DOI] [PubMed] [Google Scholar]
- 182.Bruns J, McBride-Gagyi S & Zustiak SP Injectable and Cell-Adhesive Polyethylene Glycol Cryogel Scaffolds: Independent Control of Cryogel Microstructure and Composition. Macromolecular Materials and Engineering 303, 1800298 (2018). [Google Scholar]
- 183.Buchtová N, Pradille C, Bouvard J-L & Budtova T Mechanical properties of cellulose aerogels and cryogels. Soft matter 15, 7901–7908 (2019). [DOI] [PubMed] [Google Scholar]
- 184.Hartman O et al. Microfabricated electrospun collagen membranes for 3-D cancer models and drug screening applications. Biomacromolecules 10, 2019–2032 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Molina ER et al. Mechanically tunable coaxial electrospun models of YAP/TAZ mechanoresponse and IGF-1R activation in osteosarcoma. Acta biomaterialia 100, 38–51 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Singh A & Tayalia P Three-dimensional cryogel matrix for spheroid formation and anti-cancer drug screening. Journal of Biomedical Materials Research Part A 108, 365–376 (2020). [DOI] [PubMed] [Google Scholar]
- 187.Sarkar J & Kumar A Thermo-responsive polymer aided spheroid culture in cryogel based platform for high throughput drug screening. Analyst 141, 2553–2567 (2016). [DOI] [PubMed] [Google Scholar]
- 188.Li Y et al. 3D microtissues for injectable regenerative therapy and high-throughput drug screening. Journal of visualized experiments: JoVE (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hadden WJ et al. Stem cell migration and mechanotransduction on linear stiffness gradient hydrogels. Proceedings of the National Academy of Sciences 114, 5647–5652 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Xia T, Liu W & Yang L A review of gradient stiffness hydrogels used in tissue engineering and regenerative medicine. Journal of Biomedical Materials Research Part A 105, 1799–1812 (2017). [DOI] [PubMed] [Google Scholar]
- 191.Seidi A, Ramalingam M, Elloumi-Hannachi I, Ostrovidov S & Khademhosseini A Gradient biomaterials for soft-to-hard interface tissue engineering. Acta biomaterialia 7, 1441–1451 (2011). [DOI] [PubMed] [Google Scholar]
- 192.Shu Y, Chan HN, Guan D, Wu H & Ma L A simple fabricated thickness-based stiffness gradient for cell studies. Science Bulletin 62, 222–228 (2017). [DOI] [PubMed] [Google Scholar]
- 193.Ko H et al. A simple layer-stacking technique to generate biomolecular and mechanical gradients in photocrosslinkable hydrogels. Biofabrication 11, 025014 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Guimarães CF et al. High-throughput fabrication of cell-laden 3D biomaterial gradients. Materials Horizons 7, 2414–2421 (2020). [Google Scholar]
- 195.Zhu D, Trinh P, Li J, Grant GA & Yang F Gradient hydrogels for screening stiffness effects on patient-derived glioblastoma xenograft cellfates in 3D. Journal of Biomedical Materials Research Part A 109, 1027–1035 (2021). [DOI] [PubMed] [Google Scholar]
- 196.Garcia S et al. Generation of stable orthogonal gradients of chemical concentration and substrate stiffness in a microfluidic device. Lab on a Chip 15, 2606–2614 (2015). [DOI] [PubMed] [Google Scholar]
- 197.Chatterjee K, F Young M & G Simon C Fabricating gradient hydrogel scaffolds for 3D cell culture. Combinatorial chemistry & high throughput screening 14, 227–236 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wang L et al. Hydrogel-based methods for engineering cellular microenvironment with spatiotemporal gradients. Critical reviews in biotechnology 36, 553–565 (2016). [DOI] [PubMed] [Google Scholar]
- 199.Dou J, Mao S, Li H & Lin J-M Combination stiffness gradient with chemical stimulation directs glioma cell migration on a microfluidic chip. Analytical chemistry 92, 892–898 (2019). [DOI] [PubMed] [Google Scholar]
- 200.Wang W, Li L, Ding M, Luo G & Liang Q A microfluidic hydrogel chip with orthogonal dual gradients of matrix stiffness and oxygen for cytotoxicity test. Biochip Journal 12, 93–101 (2018). [Google Scholar]
- 201.Lam CRI et al. A 3D biomimetic model of tissue stiffness interface for cancer drug testing. Molecular pharmaceutics 11, 2016–2021 (2014). [DOI] [PubMed] [Google Scholar]
- 202.Bruns J, Egan T, Mercier P & Zustiak SP Glioblastoma spheroid growth and chemotherapeutic responses in single and dual-stiffness hydrogels✰. Acta Biomaterialia (2022). [DOI] [PubMed] [Google Scholar]
- 203.Le Digabel J, Ghibaudo M, Trichet L, Richert A & Ladoux B Microfabricated substrates as a tool to study cell mechanotransduction. Medical & biological engineering & computing 48, 965–976 (2010). [DOI] [PubMed] [Google Scholar]
- 204.Xu Y & Zhao R in Micro and Nano Systems for Biophysical Studies of Cells and Small Organisms 23–42 (Elsevier, 2021). [Google Scholar]
- 205.Liu R, Yao X, Liu X & Ding J Proliferation of cells with severe nuclear deformation on a micropillar array. Langmuir 35, 284–299 (2018). [DOI] [PubMed] [Google Scholar]
- 206.Dickinson LE, Rand DR, Tsao J, Eberle W & Gerecht S Endothelial cell responses to micropillar substrates of varying dimensions and stiffness. Journal of Biomedical Materials Research Part A 100, 1457–1466 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.van Assenbergh P, Zhang K, Buijnsters JG & Dodou D Effect of lateral reinforcements on the adhesion and friction of micropillar adhesives. Applied Physics A 126, 1–9 (2020). [Google Scholar]
- 208.Wei J et al. Fabrication of adjacent micropillar arrays with different heights for cell studies. Microelectronic Engineering 158, 22–25 (2016). [Google Scholar]
- 209.Krishnamoorthy S, Zhang Z & Xu C Guided cell migration on a graded micropillar substrate. Bio-Design and Manufacturing 3, 60–70 (2020). [Google Scholar]
- 210.Le Digabel J et al. Magnetic micropillars as a tool to govern substrate deformations. Lab on a Chip 11, 2630–2636 (2011). [DOI] [PubMed] [Google Scholar]
- 211.Nguyen AT, Sathe SR & Yim EK From nano to micro: topographical scale and its impact on cell adhesion, morphology and contact guidance. Journal of Physics: Condensed Matter 28, 183001 (2016). [DOI] [PubMed] [Google Scholar]
- 212.Mei F et al. Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis. Nature medicine 20, 954–960 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yang L et al. Topography induced stiffness alteration of stem cells influences osteogenic differentiation. Biomaterials science 8, 2638–2652 (2020). [DOI] [PubMed] [Google Scholar]
- 214.Yim EK, Darling EM, Kulangara K, Guilak F & Leong KW Nanotopography-induced changes in focal adhesions, cytoskeletal organization, and mechanical properties of human mesenchymal stem cells. Biomaterials 31, 1299–1306 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Nemec S et al. Interfacial Curvature in Confined Coculture Directs Stromal Cell Activity with Spatial Corralling of Pancreatic Cancer Cells. Advanced Biology, 2000525 (2021). [DOI] [PubMed] [Google Scholar]
- 216.Weigelin B & Friedl P Stemness shaped by curvature. Nature materials 15, 827–828 (2016). [DOI] [PubMed] [Google Scholar]
- 217.Hui E, Gimeno KI, Guan G & Caliari SR Spatiotemporal control of viscoelasticity in phototunable hyaluronic acid hydrogels. Biomacromolecules 20, 4126–4134 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Keating M, Lim M, Hu Q & Botvinick E Selective stiffening of fibrin hydrogels with micron resolution via photocrosslinking. Acta biomaterialia 87, 88–96 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.DeForest CA, Sims EA & Anseth KS Peptide-functionalized click hydrogels with independently tunable mechanics and chemical functionality for 3D cell culture. Chemistry of materials 22, 4783–4790 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Xu X, Ma L, Wu Y & Tang L Micropillar-based culture platform induces epithelial–mesenchymal transition in the alveolar epithelial cell line. Journal of Biomedical Materials Research Part A 106, 3165–3174 (2018). [DOI] [PubMed] [Google Scholar]
- 221.Lee G et al. Pillar-Based Mechanical Induction of an Aggressive Tumorigenic Lung Cancer Cell Model. ACS Applied Materials & Interfaces (2021). [DOI] [PubMed] [Google Scholar]
- 222.Kim J et al. Micropillar arrays as potential drug screens: Inhibition of micropillar-mediated activation of the FAK–Src–paxillin signaling pathway by the CK2 inhibitor CX-4945. Acta biomaterialia 27, 13–20 (2015). [DOI] [PubMed] [Google Scholar]
- 223.Aoun L et al. Microdevice arrays of high aspect ratio poly (dimethylsiloxane) pillars for the investigation of multicellular tumour spheroid mechanical properties. Lab on a chip 14, 2344–2353 (2014). [DOI] [PubMed] [Google Scholar]
- 224.Rosales AM & Anseth KS The design of reversible hydrogels to capture extracellular matrix dynamics. Nature Reviews Materials 1, 1–15 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.LeValley PJ & Kloxin AM (ACS Publications, 2018). [Google Scholar]
- 226.Burdick JA & Murphy WL Moving from static to dynamic complexity in hydrogel design. Nature communications 3, 1–8 (2012). [DOI] [PubMed] [Google Scholar]
- 227.Uto K, Tsui JH, DeForest CA & Kim D-H Dynamically tunable cell culture platforms for tissue engineering and mechanobiology. Progress in polymer science 65, 53–82 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Yeh Y-C et al. Mechanically dynamic PDMS substrates to investigate changing cell environments. Biomaterials 145, 23–32 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Corbin EA et al. Tunable and reversible substrate stiffness reveals a dynamic mechanosensitivity of cardiomyocytes. ACS applied materials & interfaces 11, 20603–20614 (2019). [DOI] [PubMed] [Google Scholar]
- 230.Zhang J et al. Dynamic Mechanics-Modulated Hydrogels to Regulate the Differentiation of Stem-Cell Spheroids in Soft Microniches and Modeling of the Nonlinear Behavior. Small 15, 1901920 (2019). [DOI] [PubMed] [Google Scholar]
- 231.Perera MM, Fischesser DM, Molkentin JD & Ayres N Stiffness of thermoresponsive gelatin-based dynamic hydrogels affects fibroblast activation. Polymer Chemistry 10, 6360–6367 (2019). [Google Scholar]
- 232.Kloxin AM, Benton JA & Anseth KS In situ elasticity modulation with dynamic substrates to direct cell phenotype. Biomaterials 31, 1–8 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kloxin AM, Kasko AM, Salinas CN & Anseth KS Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science 324, 59–63 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kharkar PM et al. Controlling the release of small, bioactive proteins via dual mechanisms with therapeutic potential. Advanced healthcare materials 6, 1700713 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Mabry KM, Lawrence RL & Anseth KS Dynamic stiffening of poly (ethylene glycol)-based hydrogels to direct valvular interstitial cell phenotype in a three-dimensional environment. Biomaterials 49, 47–56 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Richards D, Swift J, Wong LS & Richardson SM Photoresponsive Hydrogels with Photoswitchable Stiffness: Emerging Platforms to Study Temporal Aspects of Mesenchymal Stem Cell Responses to Extracellular Stiffness Regulation. Cell Biology and Translational Medicine, Volume 5, 53–69 (2018). [DOI] [PubMed] [Google Scholar]
- 237.Stowers RS, Allen SC & Suggs LJ Dynamic phototuning of 3D hydrogel stiffness. Proceedings of the National Academy of Sciences 112, 1953–1958 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Shih H & Lin C-C Tuning stiffness of cell-laden hydrogel via host–guest interactions. Journal of Materials Chemistry B 4, 4969–4974 (2016). [DOI] [PubMed] [Google Scholar]
- 239.Tabet A, Forster RA, Parkins CC, Wu G & Scherman OA Modulating stiffness with photo-switchable supramolecular hydrogels. Polymer Chemistry 10, 467–472 (2019). [Google Scholar]
- 240.Jiang FX, Yurke B, Schloss RS, Firestein BL & Langrana NA Effect of dynamic stiffness of the substrates on neurite outgrowth by using a DNA-crosslinked hydrogel. Tissue Engineering Part A 16, 1873–1889 (2010). [DOI] [PubMed] [Google Scholar]
- 241.Uto K, Aoyagi T, DeForest C & Ebara M Dynamic alterations of hepatocellular function by on-demand elasticity and roughness modulation. Biomaterials science 6, 1002–1006 (2018). [DOI] [PubMed] [Google Scholar]
- 242.Shevtsov M & Multhoff G Heat shock protein–peptide and HSP-based immunotherapies for the treatment of cancer. Frontiers in immunology 7, 171 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Redman JM, Gulley JL & Madan RA in Urologic Oncology: Seminars and Original Investigations. 694–700 (Elsevier; ). [Google Scholar]
- 244.Hughes PE, Caenepeel S & Wu LC Targeted therapy and checkpoint immunotherapy combinations for the treatment of cancer. Trends in immunology 37, 462–476 (2016). [DOI] [PubMed] [Google Scholar]
- 245.Zhu L, Wu Y, Yoon CW & Wang Y Mechanogenetics for cellular engineering and cancer immunotherapy. Current Opinion in Biotechnology 66, 88–94 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Zhu C, Chen W, Lou J, Rittase W & Li K Mechanosensing through immunoreceptors. Nature immunology 20, 1269–1278 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Stoycheva D, Simsek H, Weber W, Hauser AE & Klotzsch E External cues to drive B cell function towards immunotherapy. Acta Biomaterialia (2021). [DOI] [PubMed] [Google Scholar]
- 248.Kristi N et al. Atomic force microscopy in mechanoimmunology analysis: a new perspective for cancer immunotherapy. Biotechnology journal 15, 1900559 (2020). [DOI] [PubMed] [Google Scholar]
- 249.Parlato S et al. Tumor-on-a-chip platforms to study cancer–immune system crosstalk in the era of immunotherapy. Lab on a Chip 21, 234–253 (2021). [DOI] [PubMed] [Google Scholar]
- 250.Majumder B et al. Predicting clinical response to anticancer drugs using an ex vivo platform that captures tumour heterogeneity. Nature communications 6, 1–14 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Radhakrishnan P et al. (AACR, 2017). [Google Scholar]
- 252.Jacob F et al. A patient-derived glioblastoma organoid model and biobank recapitulates inter-and intra-tumoral heterogeneity. Cell 180, 188–204. e122 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Zhou X et al. Screening Cancer Immunotherapy: When Engineering Approaches Meet Artificial Intelligence. Advanced Science 7, 2001447 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Di Modugno F et al. 3D models in the new era of immune oncology: focus on T cells, CAF and ECM. Journal of Experimental & Clinical Cancer Research 38, 1–14 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Li Z et al. Application of animal models in cancer research: Recent progress and future prospects. Cancer Management and Research 13, 2455 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Ruggeri BA, Camp F & Miknyoczki S Animal models of disease: pre-clinical animal models of cancer and their applications and utility in drug discovery. Biochemical pharmacology 87, 150–161 (2014). [DOI] [PubMed] [Google Scholar]
- 257.Cheon D-J & Orsulic S Mouse models of cancer. Annual Review of Pathology: Mechanisms of Disease 6, 95–119 (2011). [DOI] [PubMed] [Google Scholar]
- 258.Lampreht Tratar U, Horvat S & Cemazar M Transgenic mouse models in cancer research. Frontiers in oncology 8, 268 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Olson B, Li Y, Lin Y, Liu ET & Patnaik A Mouse Models for Cancer Immunotherapy ResearchCoclinical Mouse Models for Cancer Immunotherapy. Cancer discovery 8, 1358–1365 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Sharpless NE & DePinho RA The mighty mouse: genetically engineered mouse models in cancer drug development. Nature reviews Drug discovery 5, 741–754 (2006). [DOI] [PubMed] [Google Scholar]
- 261.Sarazin BA, Ihle CL, Owens P & Lynch ME Mechanobiology of Bone Metastatic Cancer. Current Osteoporosis Reports, 1–12 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Zhong Y et al. Tumor Microenvironment-Activatable Nanoenzymes for Mechanical Remodeling of Extracellular Matrix and Enhanced Tumor Chemotherapy. Advanced Functional Materials 31, 2007544 (2021). [Google Scholar]
- 263.Shen Y et al. Reduction of liver metastasis stiffness improves response to bevacizumab in metastatic colorectal cancer. Cancer cell 37, 800–817. e807 (2020). [DOI] [PubMed] [Google Scholar]
- 264.Parkins CC et al. Mechanically matching the rheological properties of brain tissue for drug-delivery in human glioblastoma models. Biomaterials 276, 120919 (2021). [DOI] [PubMed] [Google Scholar]
- 265.Rachman-Tzemah C et al. Blocking surgically induced lysyl oxidase activity reduces the risk of lung metastases. Cell reports 19, 774–784 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Pickup MW et al. Stromally Derived Lysyl Oxidase Promotes Metastasis of Transforming Growth Factor-β–Deficient Mouse Mammary CarcinomasStromal LOX Promotes TGFβR2-Null Breast Cancer Metastasis. Cancer research 73, 5336–5346 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.White R, Rose K & Zon L Zebrafish cancer: the state of the art and the path forward. Nature Reviews Cancer 13, 624–636 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Liu S & Leach SD Zebrafish models for cancer. Annual Review of Pathology: Mechanisms of Disease 6, 71–93 (2011). [DOI] [PubMed] [Google Scholar]
- 269.Stoletov K & Klemke R Catch of the day: zebrafish as a human cancer model. Oncogene 27, 4509–4520 (2008). [DOI] [PubMed] [Google Scholar]
- 270.Brown HK, Schiavone K, Tazzyman S, Heymann D & Chico TJ Zebrafish xenograft models of cancer and metastasis for drug discovery. Expert opinion on drug discovery 12, 379–389 (2017). [DOI] [PubMed] [Google Scholar]
- 271.Xiao J, Glasgow E & Agarwal S Zebrafish xenografts for drug discovery and personalized medicine. Trends in cancer 6, 569–579 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Di Franco G et al. Use of zebrafish embryos as avatar of patients with pancreatic cancer: A new xenotransplantation model towards personalized medicine. World journal of gastroenterology 26, 2792 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Chew TW et al. Crosstalk of Ras and Rho: activation of RhoA abates Kras-induced liver tumorigenesis in transgenic zebrafish models. Oncogene 33, 2717–2727 (2014). [DOI] [PubMed] [Google Scholar]
- 274.Chen X, Li Y, Yao T & Jia R Benefits of zebrafish xenograft models in cancer research. Frontiers in Cell and Developmental Biology 9, 616551 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Stoletov K, Montel V, Lester RD, Gonias SL & Klemke R High-resolution imaging of the dynamic tumor cell–vascular interface in transparent zebrafish. Proceedings of the National Academy of Sciences 104, 17406–17411 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Stoletov K et al. Visualizing extravasation dynamics of metastatic tumor cells. Journal of cell science 123, 2332–2341 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Follain G et al. Hemodynamic forces tune the arrest, adhesion, and extravasation of circulating tumor cells. Developmental cell 45, 33–52. e12 (2018). [DOI] [PubMed] [Google Scholar]
- 278.Paul CD et al. Tissue architectural cues drive organ targeting of tumor cells in zebrafish. Cell systems 9, 187–206. e116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Ellett F, Pase L, Hayman JW, Andrianopoulos A & Lieschke GJ mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood, The Journal of the American Society of Hematology 117, e49–e56 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Roh-Johnson M et al. Macrophage-dependent cytoplasmic transfer during melanoma invasion in vivo. Developmental cell 43, 549–562. e546 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Mittelstein DR et al. Selective ablation of cancer cells with low intensity pulsed ultrasound. Applied Physics Letters 116, 013701 (2020). [Google Scholar]
- 282.Peng X, He W, Xin F, Genin GM & Lu TJ The acoustic radiation force of a focused ultrasound beam on a suspended eukaryotic cell. Ultrasonics 108, 106205 (2020). [DOI] [PubMed] [Google Scholar]
- 283.Ishida Y & Nagata K in Methods in enzymology Vol. 499 167–182 (Elsevier, 2011). [DOI] [PubMed] [Google Scholar]
- 284.Han X et al. Reversal of pancreatic desmoplasia by re-educating stellate cells with a tumour microenvironment-activated nanosystem. Nature communications 9, 1–18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Kuriyama N, Kuriyama H, Julin C, Lamborn K & Israel M Pretreatment with protease is a useful experimental strategy for enhancing adenovirus-mediated cancer gene therapy. Human gene therapy 11, 2219–2230 (2000). [DOI] [PubMed] [Google Scholar]
- 286.Ferreira S, Saraiva N, Rijo P & Fernandes AS LOXL2 inhibitors and breast cancer progression. Antioxidants 10, 312 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Chen Q et al. CNTO 95, a fully human anti αv integrin antibody, inhibits cell signaling, migration, invasion, and spontaneous metastasis of human breast cancer cells. Clinical & experimental metastasis 25, 139–148 (2008). [DOI] [PubMed] [Google Scholar]
- 288.O’day S et al. A randomised, phase II study of intetumumab, an anti-αv-integrin mAb, alone and with dacarbazine in stage IV melanoma. British journal of cancer 105, 346–352 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Elez E et al. Abituzumab combined with cetuximab plus irinotecan versus cetuximab plus irinotecan alone for patients with KRAS wild-type metastatic colorectal cancer: the randomised phase I/II POSEIDON trial. Annals of Oncology 26, 132–140 (2015). [DOI] [PubMed] [Google Scholar]
- 290.Bell-McGuinn KM et al. A phase II, single-arm study of the anti-α5β1 integrin antibody volociximab as monotherapy in patients with platinum-resistant advanced epithelial ovarian or primary peritoneal cancer. Gynecologic oncology 121, 273–279 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Reardon DA et al. Randomized phase II study of cilengitide, an integrin-targeting arginine-glycine-aspartic acid peptide, in recurrent glioblastoma multiforme. Journal of clinical oncology 26, 5610–5617 (2008). [DOI] [PubMed] [Google Scholar]
- 292.Gupta SC, Patchva S & Aggarwal BB Therapeutic roles of curcumin: lessons learned from clinical trials. The AAPS journal 15, 195–218 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Xiong J et al. Integrins regulate stemness in solid tumor: an emerging therapeutic target. Journal of Hematology & Oncology 14, 1–18 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Cheng B et al. Predicting YAP/TAZ Nuclear Translocation in Response to ECM Mechanosensing. Biophysical Journal (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Driscoll TP, Cosgrove BD, Heo S-J, Shurden ZE & Mauck RL Cytoskeletal to nuclear strain transfer regulates YAP signaling in mesenchymal stem cells. Biophysical journal 108, 2783–2793 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Irianto J, Pfeifer CR, Ivanovska IL, Swift J & Discher DE Nuclear lamins in cancer. Cellular and molecular bioengineering 9, 258–267 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Huggett MT et al. Phase I/II study of verteporfin photodynamic therapy in locally advanced pancreatic cancer. British journal of cancer 110, 1698–1704 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Li X & Song Y Proteolysis-targeting chimera (PROTAC) for targeted protein degradation and cancer therapy. Journal of hematology & oncology 13, 1–14 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Zanconato F, Battilana G, Cordenonsi M & Piccolo S YAP/TAZ as therapeutic targets in cancer. Current opinion in pharmacology 29, 26–33 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Lin L et al. The Hippo effector YAP promotes resistance to RAF-and MEK-targeted cancer therapies. Nature genetics 47, 250–256 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Zhou X et al. Antitumor activity of curcumin is involved in down-regulation of YAP/TAZ expression in pancreatic cancer cells. Oncotarget 7, 79076 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Gargalionis AN, Basdra EK & Papavassiliou AG Tumor mechanosensing and its therapeutic potential. Journal of Cellular Biochemistry 119, 4304–4308 (2018). [DOI] [PubMed] [Google Scholar]
- 303.Ho Y-J et al. Normalization of tumor vasculature by oxygen microbubbles with ultrasound. Theranostics 9, 7370 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Maity G et al. Aspirin suppresses tumor cell-induced angiogenesis and their incongruity. Journal of cell communication and signaling 13, 491–502 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Zeng L et al. Hyperglycaemia confers resistance to chemotherapy on breast cancer cells: the role of fatty acid synthase. Endocrine-related cancer 17, 539–551 (2010). [DOI] [PubMed] [Google Scholar]
- 306.Elson EL & Genin GM Tissue constructs: platforms for basic research and drug discovery. Interface focus 6, 20150095 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Syed S, Schober J, Blanco A & Zustiak SP Morphological adaptations in breast cancer cells as a function of prolonged passaging on compliant substrates. Plos one 12, e0187853 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Serwane F et al. in APS March Meeting Abstracts. V37. 002. [Google Scholar]
- 309.Serwane F et al. In vivo quantification of spatially varying mechanical properties in developing tissues. Nature methods 14, 181–186 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Guertler CA et al. Mechanical properties of porcine brain tissue in vivo and ex vivo estimated by MR elastography. Journal of Biomechanics 69, 10–18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Sinkus R et al. Viscoelastic shear properties of in vivo breast lesions measured by MR elastography. Magnetic resonance imaging 23, 159–165 (2005). [DOI] [PubMed] [Google Scholar]
- 312.Boyle JJ et al. Regularization-free strain mapping in three dimensions, with application to cardiac ultrasound. Journal of biomechanical engineering 141, 011010 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Harlepp S, Thalmann F, Follain G & Goetz JG Hemodynamic forces can be accurately measured in vivo with optical tweezers. Molecular biology of the cell 28, 3252–3260 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Neu CP & Genin GM Handbook of imaging in biological mechanics. (CRC press, 2014). [Google Scholar]


