Abstract
Long-term sequelae in hospitalized Coronavirus Disease 2019 (COVID-19) patients may result in limited quality of life. The current study aimed to determine health-related quality of life (HRQoL) after COVID-19 hospitalization in non-intensive care unit (ICU) and ICU patients. This is a single-center study at the University Hospital of Wuerzburg, Germany. Patients eligible were hospitalized with COVID-19 between March 2020 and December 2020. Patients were interviewed 3 and 12 months after hospital discharge. Questionnaires included the European Quality of Life 5 Dimensions 5 Level (EQ-5D-5L), patient health questionnaire-9 (PHQ-9), the generalized anxiety disorder 7 scale (GAD-7), FACIT fatigue scale, perceived stress scale (PSS-10) and posttraumatic symptom scale 10 (PTSS-10). 85 patients were included in the study. The EQ5D-5L-Index significantly differed between non-ICU (0.78 ± 0.33 and 0.84 ± 0.23) and ICU (0.71 ± 0.27; 0.74 ± 0.2) patients after 3- and 12-months. Of non-ICU 87% and 80% of ICU survivors lived at home without support after 12 months. One-third of ICU and half of the non-ICU patients returned to work. A higher percentage of ICU patients was limited in their activities of daily living compared to non-ICU patients. Depression and fatigue were present in one fifth of the ICU patients. Stress levels remained high with only 24% of non-ICU and 3% of ICU patients (p = 0.0186) having low perceived stress. Posttraumatic symptoms were present in 5% of non-ICU and 10% of ICU patients. HRQoL is limited in COVID-19 ICU patients 3- and 12-months post COVID-19 hospitalization, with significantly less improvement at 12-months compared to non-ICU patients. Mental disorders were common highlighting the complexity of post-COVID-19 symptoms as well as the necessity to educate patients and primary care providers about monitoring mental well-being post COVID-19.
Subject terms: Health care, Public health, Quality of life
Introduction
Coronavirus disease 2019 (COVID-19) ranges from asymptomatic infection up to critical illness requiring hospitalization and intensive care unit (ICU) treatment. In a recent meta-analysis of 13,398 patients, the mortality rate of all COVID-19 patients admitted to the hospital was 11.5% and 40.5% among critically ill ICU patients1. However, COVID-19 does not end with survival to hospital discharge. Symptoms and organ dysfunction can persist as a post-COVID-19 syndrome. Problems include fatigue, muscle weakness, cognitive dysfunction, and posttraumatic stress disorder (PTSD)2. In a substantial part of the patients these long-term conditions may impact quality of life. Recent systematic reviews with a limited number of heterogenic studies and varying follow-ups up to 6 months concluded that post-COVID-19 health-related quality of life (HRQoL) remained poor3,4. In a multicenter study from six hospitals in China, HRQoL was still impaired in a 3-month follow-up. Most of these patients had mild to moderate COVID-19, whereas female gender and older age were risk factors for a reduced HRQoL5.
Quality of life itself is a multidimensional construct, which includes universal and emic properties. The World Health Organization definition of quality of life includes physical and mental health, social conditions, income, and environmental quality6. Nevertheless, in case of illness, almost all aspects can become health related. Moreover, physiologic measures often correlate poorly with functional capacity and well-being in daily activities7. As such, the evaluation of HRQoL as a self-perceived physical and mental status over time is increasingly recognized as a pivotal outcome complementing traditional parameters, such as mortality8.
The current study aimed to determine HRQoL after COVID-19 hospitalization utilizing a longitudinal assessment in non-ICU and ICU COVID-19 patients. Instruments providing an overall summary of HRQoL and specific instruments focusing on depression, anxiety, stress, and fatigue were utilized.
Methods
Study design, patient population and data collection
This is a single-center longitudinal study conducted at the University Hospital of Würzburg, Germany. Potential patients were identified from a prospective, daily screening log between March and December 2020. Adult patients ≥ 18 years of age were eligible for enrollment if they required inpatient treatment at the University Hospital of Würzburg with COVID-19. All COVID-19 patients admitted to the University Hospital of Würzburg during the respective time were evaluated for study inclusion. SARS-CoV-2 infection had to be confirmed with real-time reverse transcriptase polymerase chain reaction (RT-PCR) testing. None of the patients was vaccinated against SARS-CoV2 during acute illness, as vaccines were not available during the respective time. Baseline assessment was done on the day of hospital admission. Patients were categorized according to the need of ICU or non-ICU treatment, respectively.
Patient socio-demographics (including gender, age, body mass index -BMI, prior work status, and education), comorbidities, clinical characteristics (e.g. invasive mechanical ventilation, non-invasive mechanical ventilation, high-flow oxygen, tracheostomy, extracorporeal membrane oxygenation -ECMO), complications, and outcome were recorded at the time of inpatient COVID-19 treatment. All data were retrieved via patient data management systems (PDMS) (COPRA6 RM1.0, COPRA System GmbH, Berlin, Germany; Meona Produktion, Mesalvo Freiburg GmbH, Freiburg, Germany), as well as personal communication. Data were documented within a standardized electronic case report form (REDCap®, Vanderbilt University).
Follow-up protocol and instruments
We evaluated patients 3 and 12 months after discharge from the University Hospital of Würzburg. At each visit, the patient was interviewed by telephone. The telephone interviews were scripted and performed by trained personnel. Questionnaires included the European Quality of Life 5 Dimensions 5 Level (EQ-5D-5L) as a general instrument of patient reported HRQoL. The five dimensions of the EQ-5D-5L are: mobility, self-care, usual activities, pain/discomfort, anxiety/depression. The EQ-5D-5L index value was calculated using the German value set. The patient health questionnaire-9 (PHQ-9) was used to measure depression. The PHQ-9 scores each of the 9 Diagnostic and Statistical Manual of Mental Disorders Version IV (DSM-IV) criteria as “0” (not at all) to “3” (nearly every day) as the respective module of the patient health questionnaire. The PHQ-9 has been found to have acceptable diagnostic properties for detecting major depressive disorder for cut-off scores between 8 and 119. Based on this diagnostic window a cut-off value of ≥ 10 was chosen in the current study. The generalized anxiety disorder 7 (GAD-7) screened for the presence of a generalized anxiety disorder utilizing 7 items scored from “0” (not at all) to “3” (nearly every day). A score of 10 or greater on the GAD-7 represents a reasonable cut point for identifying cases of GAD10. Individual’s level of fatigue was assessed with the FACIT fatigue scale containing 13-items. The level of fatigue is measured on a scale from “4” (not at all fatigued) to “0” (very much fatigued). Scores < 30 indicate severe fatigue; higher scores indicate less fatigue. Stress assessment was done with the perceived stress scale (PSS-10). The PSS-10 contains 10 questions scored from “0” (never) to “4” (very often), whereas scores for questions 4, 5, 7, 8 are reversed. Scores ranging from 0 to 13 would be considered low stress, scores ranging from 14 to 26 would be considered moderate stress, scores ranging from 27 to 40 would be considered high perceived stress, respectively.
Moreover, posttraumatic stress (PTS) was investigated with the posttraumatic symptom scale 10 (PTSS-10). Response alternatives were ranked from “0” (never) to “3” (often). The PTSS-10 total score (score range = 0–30) constitutes the summation of the ratings and was interpreted according to the following levels of PTS-symptoms: 0 to 11 (mild to moderate) and 12 to 30 (moderate to severe range). A score of 18 or more represented a “case” and a score of 12 or above regarded as the need for psychological help. FACIT, PSS-10 and PTSS-10 were only collected at 12 months. All questionnaires were available as validated German language versions11–15.
Functional status
Items on functional status included the ability of returning to work, reasons not to return to work, part in life as compared to prior to COVID-19 hospitalization (and housing status) at 3- and 12-months after hospital discharge. Clinical frailty was assessed as a baseline characteristic, as well as during the 3- and 12-months follow-ups.
Statistical analysis
Continuous variables are presented as median and interquartile range (IQR), categorical variables as percentages. Two-tailed matched pairs Wilcoxon signed rank test was used for the analysis of ordinal data, two-tailed Mann–Whitney U test for unpaired ordinal data, Pearson’s Chi-squared test for categorical variables or McNemar testing to compare paired proportions, respectively. Wilcoxon signed-rank as a more powerful test was preferred over the sign test. Spearman correlation was utilized for correlation analysis. A p-value of ≤ 0.05 was considered statistically significant. Statistical calculations were performed using GraphPad Prism 9 (San Diego, California USA, 2020).
Ethics
The study was performed in accordance with the Declaration of Helsinki. The Ethics Committee of the Medical Faculty of the Julius-Maximilians-University of Würzburg approved the study protocol (89/20-sc). Written informed consent was obtained from each patient or its legal representative prior to study inclusion.
Results
Consent was not obtainable in 104 out of 189 eligible patients. A total of 85 patients were included in the study. 45 patients had critical COVID-19 with the need for ICU treatment. 3-month follow-up was available in 35 and 12-month follow-up in 38 non-ICU patients. A total of 27 ICU patients were evaluated during the 3-month follow-up, 30 ICU patients were evaluated during the 12-month follow-up (Fig. 1). COVID-19 was the primary diagnosis for hospital admission in 72 (85%) of the patients.
Figure 1.
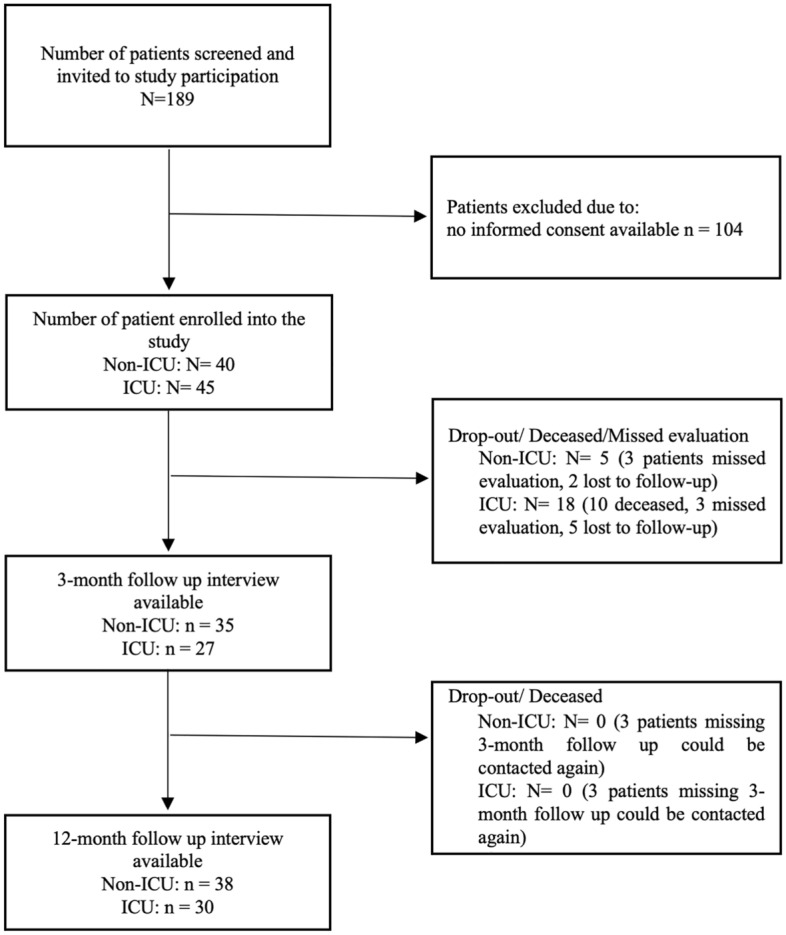
Screening and follow-up. Patients with COVID-19 treated at the University hospital of Würzburg were prospectively screened for study inclusion upon admission. After written consent, baseline characteristics were obtained and follow-up telephone interviews were conducted after 3- and 12-months, respectively.
Patient demographics
Patient demographics and comorbidities are depicted in Table 1. Median age was 63 (54–73) in non-ICU patients and 61 (52–68) in ICU patients, respectively. BMI was significantly higher in ICU (29 kg/m2) compared to non-ICU patients (26 kg/m2) (p = 0.0007). Age and BMI only had weak correlations to the questionnaire scores (Fig. S3). Arterial hypertension was the most common comorbidity. Most patients had 5 or 6-year secondary school education. Prior to hospitalization 74% non-ICU and 62% ICU patients categorized in a clinical frailty scale of 1–3 (very fit – managing well), with no significant differences between the two groups (p = 0.0998).
Table 1.
Patient demographics and comorbidities at baseline.
| Characteristic | Non-ICU (N = 40) | ICU (N = 45) |
|---|---|---|
| Age (years) | 63 (54–73) | 61 (52–68) |
| Male (%) | 70 | 64 |
| BMI (kg/m2) | 26 (24–29) (N = 39) | 29 (26–35) (N = 42) |
| Education n (%) | (N = 34) | (N = 30) |
| None | 2 (6) | 2 (7) |
| Five-year secondary school | 10 (29) | 10 (33) |
| Six-year secondary school | 7 (21) | 10 (33) |
| Grammar school | 6 (18) | 3 (10) |
| University/college degree | 9 (26) | 5 (17) |
| Cardiovascular risk factors n (%) | ||
| Arterial hypertension | 19 (48) | 26 (58) |
| Coronary artery disease | 4 (10) | 5 (11) |
| Heart failure | 4 (10) | 0 |
| Pulmonary risk factors n (%) | ||
| Chronic obstructive pulmonary disease | 5 (13) | 2 (4) |
| Bronchial asthma | 7 (18) | 5 (11) |
| Obstructive sleep apnea | 0 | 2 (4) |
| Neurologic disorder n (%) | ||
| Apoplex cerebri | 0 | 0 |
| Parkinson’s disease | 2 (5) | 2 (4) |
| Multiple sclerosis | 0 | 0 |
| Psychatric disorder n (%) | ||
| Dementia | 2 (5) | 0 |
| Depression | 3 (8) | 2 (4) |
| Psychosis | 1 (3) | 0 |
| Anxiety disorder | 0 | 1 (2) |
| Clinical frailty scale n (%) | (N = 35) | (N = 34) |
| Very fit (1) | 4 (11) | 2 (6) |
| Well (2) | 15 (43) | 10 (29) |
| Managing well (3) | 7 (20) | 9 (26) |
| Vulnerable (4) | 4 (11) | 6 (18) |
| Mildly frail (5) | 4 (11) | 1 (3) |
| Moderately frail (6) | 0 | 2 (6) |
| Severely frail (7) | 1 (3) | 3 (9) |
| Very severely frail (8) | 0 | 1 (3) |
| Terminally ill (9) | 0 | 0 |
Values are depicted as median plus interquartile range, or percentage of patients as indicated.
ICU intensive care unit, BMI body mass index.
Clinical characteristics and outcome
Nearly half of the patients required high-flow oxygen treatment during non-ICU care, while 89% of the ICU patients were mechanically ventilated. Moreover, ECMO support was necessary in 44% of the ICU patients and renal replacement therapy in 36%. Pulmonary co-infections were found in 5% of non-ICU (two patients) and 38% of the ICU patients. Bloodstream co-infections were present in 8% of non-ICU (three patients) and 38% of the ICU patients. All non-ICU patients survived to hospital discharge or transfer to another hospital/skilled care facility, respectively. Mortality of ICU treatment was 22% (10 patients). There were no additional deaths in either group during the 12-month follow-up period. (Table S1).
European quality of life 5 dimensions 5 level (EQ5D-5L)
The mean EQ5D-5L-index as an overall measure of the HRQoL differed between non-ICU (0.78 ± 0.33 and 0.84 ± 0.23) and ICU (0.71 ± 0.27; 0.74 ± 0.2) patients after 3- and 12-months with a significant difference after 12-months (p = 0.0511 and p = 0.0013, respectively) (Fig. S1 and Table 2). Mobility significantly differed between ICU and non-ICU patients after 12-months (p = 0.0155). Mobility was reported as no problem by one-third of ICU patients, whereas slight or moderate problems were present in a total of 40%, severe problems in 23%, respectively. However, the percentage of ICU patients with severe problems or unable to walk about decreased between the follow-ups. Usual activities were also significantly impaired in ICU compared to non-ICU patients at 3- (p = 0.0334) and 12-months (p = 0.0021), respectively. In non-ICU patients 58% were able to resume their usual activities after 12-months, one-third still suffered from slight or moderate problems. More than half of ICU patients had slight or moderate problems after 12-months, 20% had severe problems or were unable to perform their usual activities.
Table 2.
Functional status as well as questionnaire results for general HRQoL.
| Parameter | Non-ICU | ICU | p-value | |||||
|---|---|---|---|---|---|---|---|---|
| 3-month follow-up (N = 35) | 12-month follow-up (N = 38) | 3-month follow-up (N = 27) | 12-month follow-up (N = 30) | Non-ICU 3-month vs. 12-month follow-up | ICU 3-month vs. 12-month follow-up | ICU vs. Non-ICU 3-month follow-up | ICU vs. Non-ICU 12-month follow-up | |
| Living situation n (%) | > 0.999 | 0.2188 | 0.3328 | 05004 | ||||
| At home independent | 29 (83) | 33 (87) | 19 (70) | 24 (80) | ||||
| At home dependent | 4 (11) | 3 (8) | 6 (22) | 5 (17) | ||||
| Nursing home | 2 (6) | 2 (5) | 2 (7) | 1 (0.3) | ||||
| Change of living situation n (%) | 6 (16) | 6 (20) | 0.7501 | |||||
| Daily activities n (%) | (N = 34) | 0.7402 | 0.0151 | 0.0033 | 0.0942 | |||
| Unchanged to pre-COVID-19 | 18 (53) | 20 (53) | 4 (15) | 10 (33) | ||||
| Limited | 10 (29) | 14 (37) | 13 (48) | 15 (50) | ||||
| Severely limited | 6 (18) | 4 (12) | 10 (37) | 5 (17) | ||||
| Returned to work n (%) | 16 (46) | 18 (47) | 10 (37) | 11 (37) | > 0.999 | 0.3447 | 0.60599 | 0.3265 |
| Reason if not | (N = 18) | (N = 19) | (N = 17) | (N = 19) | ||||
| Retired | 16 (88) | 17 (89) | 10 (59) | 11 (58) | ||||
| Unemployed | 1 (6) | 0 | 0 | 1 (5) | ||||
| Disability | 1 (6) | 2 (11) | 7 (41) | 7 (37) | ||||
| Clinical frailty scale n (%) | (N = 29) | 0.2125 | 0.0339 | 0.0014 | 0.0553 | |||
| Very fit (1) | 6 (17) | 5 (13) | 0 | 2 (7) | ||||
| Well (2) | 9 (26) | 10 (26) | 2 (7) | 4 (14) | ||||
| Managing well (3) | 8 (23) | 10 (26) | 9 (33) | 6 (21) | ||||
| Vulnerable (4) | 8 (23) | 7 (18) | 5 (19) | 11 (38) | ||||
| Mildly frail (5) | 2 (6) | 1 (3) | 5 (19) | 2 (7) | ||||
| Moderately frail (6) | 1 (3) | 3 (8) | 4 (15) | 2 (7) | ||||
| Severely frail (7) | 0 | 1 (3) | 1 (4) | 1 (3) | ||||
| Very severely frail (8) | 1 (3) | 1 (3) | 1 (4) | 1 (3) | ||||
| Terminally ill (9) | 0 | 0 | 0 | 0 | ||||
| EQ-5D-5L (score) | ||||||||
| Mobility | 1 (1–3) | 1 (1–2) | 2 (1–3) | 2 (1–3) | 0.1712 | > 0.999 | 0.3021 | 0.0155 |
| Self-care | 1 (1–1) | 1 (1–1) | 1 (1–2) | 1 (1–2) | 0.1250 | 0.8281 | 0.222 | 0.1136 |
| Usual activities | 1 (1–2) | 1 (1–2) | 2 (1–3) | 2 (2–3) | 0.7812 | > 0.999 | 0.0334 | 0.0021 |
| Pain/discomfort | 2 (1–3) | 1 (1–2) | 2 (2–3) | 2 (1–3) | 0.0774 | 0.7964 | 0.4445 | 0.061 |
| Anxiety/depression | 1 (1–3) | 1 (1–2) | 2 (1–3) | 2 (1–2) | 0.4447 | > 0.999 | 0.4671 | 0.2664 |
| EQ VAS score | 70 (60–84) | 70 (52–84) | 68 (50–80) | 65 (50–76) | 0.3357 | 0.9937 | 0.4132 | 0.1164 |
| EQ-5D-5L-index [mean ± SD] | 0.78 ± 0.33 | 0.84 ± 0.23 | 0.71 ± 0.27 | 0.74 ± 0.2 | 0.2965 | 0.7906 | 0.0511 | 0.0013 |
| PHQ-9 (score) | (N = 34) | |||||||
| Little interest or pleasure in doing things? | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) | ||||
| Feeling down, depressed, or hopeless? | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) | ||||
| Trouble falling or staying asleep, or sleeping too much? | 1 (0–2) | 1 (0–2) | 1 (0–3) | 1 (0–2) | ||||
| Tiredness, feeling of missing energy? | 1 (1–2.5) | 1 (0–2) | 1 (0–3) | 1 (0–2) | ||||
| Poor appetite or overeating? | 0 (0–0.5) | 0 (0–0) | 0 (0–1) | 0 (0–0) | ||||
| Feeling bad about yourself — or that you are a failure or have let yourself or your family down? | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | ||||
| Trouble concentrating on things? | 0 (0–1) | 0 (0–1) | 0 (0–0.5) | 1 (0–1) | ||||
| Moving or speaking so slowly that other people could have noticed? Or so fidgety or restless that you have been moving a lot more than usual? | 0 (0–0) | 0 (0–0) | 0 (0–1) | 1 (0–0.8) | ||||
| Thoughts that you would be better off dead, or thoughts of hurting yourself in some way? | 0 (0–0) | 1 (1–2) | 0 (0–0) | 2 (1–2) | ||||
| Total | 4 (2–7.5) | 3.5 (1–6) | 5 (1.5–9.5) | 5 (1.3–7.8) | 0.1772 | 0.7002 | 0.7156 | 0.0003 |
| Score ≥ 10 n (%) | 6 (17) | 7 (18) | 7(26) | 6 (20) | 0.4329 | 0.5944 | ||
| GAD-7 | ||||||||
| Feeling nervous, anxious, or on edge | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0.5 (0–1) | ||||
| Not being able to stop or control worrying | 0 (0–1) | 0 (0–0) | 0 (0–0) | 0 (0–1) | ||||
| Worrying too much about different things | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) | ||||
| Trouble relaxing | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) | ||||
| Being so restless that it's hard to sit still | 0 (0–1) | 0 (0–0) | 0 (0–0.5) | 0 (0–0) | ||||
| Becoming easily annoyed or irritable | 0 (0–1) | 0 (0–1) | 0 (0–0) | 0 (0–1) | ||||
| Feeling afraid as if something awful might happen | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–1) | ||||
| Total | 3 (1–6.5) | 2 (0–4) | 2 (0–4.5) | 2.5 (0–5) | 0.3544 | 0.6436 | 0.7236 | 0.6930 |
| Score ≥ 10 n (%) | 1 (3) | 0 | 4 (15) | 4 (13) | 0.0864 | 0.0203 | ||
EQ-5D-5L (European Quality of Life 5 Dimensions 5 Level Version), PHQ-9 (Patient Health Questionnaire-9) and GAD-7 (Generalized Anxiety Disorder 7) Each of the five dimensions comprising the EQ-5D is divided into five levels of perceived problems (1: indicating no problem, 2: indicating slight problems, 3: indicating moderate problems, 4: indicating severe problems, 5: indicating unable to/extreme problems). EQ-5D-5L VAS is scaled from 0 (the worst health you can imagine) to 100 (the best health you can imagine). PHQ-9 and GAD-7 are a self-report measures, whereas respondents are asked to rate each of the items on a scale of 0 to 3 based on how much a symptom has bothered them over the last 2 weeks (0: not at all, 1: several days, 2: more than half the days, 3: nearly every day). Scores for each item are added up to get a total. A PHQ-9 score ≥ 10 was utilized for detecting major depressive disorder. A GAD-7 score ≥ 10 represents a reasonable cut point for identifying cases of GAD. Results are median plus interquartile range or percentage of patients as indicated. EQ-5D-5L-index values calculated using the German value set are mean ± SD.
Significant values are in bold.
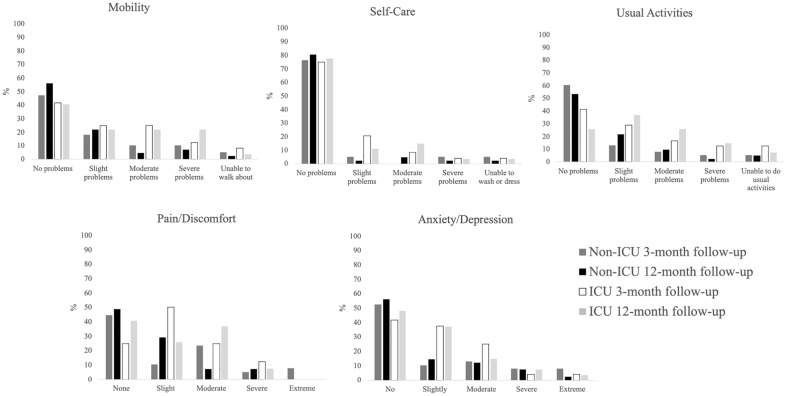
Self-care, pain/discomfort, anxiety/depression did not significantly differ between non-ICU and ICU patients, as well as between the 3-months and 12-months follow-up. No problems in self-care were present in most patients in both groups, whereas a higher percentage of ICU patients suffered from slight or moderate pain/discomfort or anxiety/depression. Severe or extreme problems were only present in a small percentage in both groups (Fig. 2).
Figure 2.
EQ5D-5L dimensions. Each of the five dimensions of the EQ5D-5L displayed as the percentage of patients within the respective category at the 3- and 12-month follow-ups. More than half of ICU patients were limited in their mobility with severe problems in 23% vs. 7% in non-ICU patients. In non-ICU patients 56% had no mobility problems vs. 41% in ICU patients. Moderate or slight problems in self-care were more common in ICU patients. However, in both ICU and non-ICU patients around 80% reported no problems after 12 months. Nevertheless, 15% of ICU patients had severe problems to perform their usual activities compared to 2% in non-ICU patients. Only 25% of the ICU patients reported no problems, compared to 53% in non-ICU patients after 12-months. Extreme pain was not observed in any group, while severe pain was present in 7% of both ICU and non-ICU patients. However, moderate pain remained in 37% of ICU patients compared to only 7% in non-ICU patients. Anxiety/depression was reported in similar percentages in both groups along all categories, with 10 to 11% having severe or extreme problems in total.
Functional status
Only two non-ICU and one ICU patient resided in a nursing home after 12 months. A significantly higher percentage of ICU patients remained limited in their daily activities after 3-months (p = 0.0033). However, recuperation happened between 3 and 12-months follow-up with five (17%) ICU patients not being severely limited anymore, and 6 (18%) more returning to their usual daily activities. Although clinical frailty was not significantly different at baseline (p = 0.0998), in non-ICU patients 10.5% of the patients fell into the categories moderately limited or worse after 12 months as a significant difference compared to baseline (p = 0.047). This was not the case in ICU patients (p = 0.0749), whereas clinical frailty significantly improved between the 3- and 12-month follow-up (p = 0.0339). Nevertheless, a high percentage of ICU patients remained vulnerable after 12-months with 38% still vulnerable. Only 6 percent were severely or very severely frail. Overall frailty in non-ICU patients did not change comparing 3- and 12-months follow-ups, with a total of 36% being very fit or well and another 26% managing well after 12-months. Only 37% of ICU patients and half of the non-ICU patients returned to work. Reason for not returning to work was retirement in 89% non-ICU and 58% ICU patients, whereas 11% of non-ICU and 37% of ICU patients were unable to work due to disability. Of non-ICU 87% and 80% of ICU patients were independently living at home after 12 months (Table 2).
Depression, anxiety, fatigue and stress
None of the non-ICU patients had a GAD-7 score ≥ 10 at 12-month follow-up. Four ICU patients suffered from GAD at 3- and 12-months follow-up, which was significantly different compared to the non-ICU group (p = 0.0203) after 12-months. Results of the GAD-7 for anxiety did not significantly differ between non-ICU and ICU patients, as well as between the 3- and 12-months follow-up. PHQ-9 for depression significantly differed between ICU and non-ICU patients. However, considering a cutoff value of ≥ 10 for the PHQ-9, 26% of ICU patients had a depression after 3-months, which decreased to 20% after 12-months. This was not significantly different to non-ICU patients with percentages of 17% and 18%, respectively (Table 2).
FACIT fatigue scale was only assessed at the 12-months follow-up and did not result in significant differences between non-ICU and ICU patients, as 30% of the ICU patients and 21% of non-ICU patients fulfilled the diagnostic criterion for fatigue (Table 3).
Table 3.
Questionnaire results testing for perceived stress, fatigue, and posttraumatic stress symptoms at the 12-month follow-up.
| Parameter | Non-ICU | ICU | p-value |
|---|---|---|---|
| 12-month follow-up | |||
| PSS-10 (score) | (N = 38) | (N = 30) | |
| In the last month, how often have you been upset because of something that happened unexpectedly? | 2 (1–2) | 2 (1–3) | |
| In the last month, how often have you felt that you were unable to control the important things in your life? | 1 (1–2) | 1 (1–3) | |
| In the last month, how often have you felt nervous and stressed? | 2 (2–3) | 3 (2–3) | |
| In the last month, how often have you felt confident about your ability to handle your personal problems? | 5 (4–5) | 4.5 (3–5) | |
| In the last month, how often have you felt that things were going your way? | 4 (4–5) | 4 (3–4) | |
| In the last month, how often have you found that you could not cope with all the things that you had to do? | 2 (1–3) | 3 (1–3) | |
| In the last month, how often have you been able to control irritations in your life? | 4 (3–5) | 3 (3–4) | |
| In the last month, how often have you felt that you were on top of things? | 5 (4–5) | 3 (3–5) | |
| In the last month, how often have you been angered because of things that happened that were outside of your control? | 3 (2–3) | 3 (-3) | |
| In the last month, how often have you felt difficulties were piling up so high that you could not overcome them? | 1 (1–2) | 2 (1–2) | |
| Total | 19 (14–24) | 20 (17–29.75) | 0.0547 |
| Score ≥ 27 n (%) | 7 (18) | 9 (30) | 0.2637 |
| Score ≤ 13 n (%) | 9 (24) | 1 (3) | 0.0186 |
| FACIT-F (score) | (N = 38) | (N = 30) | |
| I feel fatigued | 1 (0–2) | 1 (0–3) | |
| I feel weak all over | 1 (0–2) | 1 (0–3) | |
| I feel listless (“washed out”) | 1 (0–2) | 1 (0–2) | |
| I feel tired | 0 (0–2) | 2 (1–3) | |
| I have trouble starting things because I am tired | 1 (0–2) | 0.5 (0–2) | |
| I have trouble finishing things because I am tired | 0 (0–1) | 0 (0–2) | |
| I have energy | 2 (2–3) | 2 (1–3) | |
| I am able to do my usual activities | 4 (2–4) | 3 (1–4) | |
| I need to sleep during the day | 1 (0–3) | 2 (0–3) | |
| I am too tired to eat | 0 (0–0) | 0 (0–0) | |
| I need help doing my usual activities | 0 (0–0) | 0 (0–2) | |
| I am frustrated by being too tired to do the things I want to do | 0 (0–1) | 0 (0–1) | |
| I have to limit my social activity because I am tired | 0 (0–1) | 0 (0–1) | |
| Total | 42 (33–50) | 36 (23.5–47) | 0.3268 |
| Score ≤ 30 n (%) | 8 (21) | 9 (30) | 0.3975 |
| PTSS-10 (score) | (N = 38) | (N = 30) | |
| Sleep problems | 1 (0–2) | 2 (0–3) | |
| Nightmares about the events | 0 (0–0) | 0 (0–1) | |
| I feel dejected/downtrodden | 0 (0–1) | 1 (0–2) | |
| Jumpiness, I am easily frightened by sudden sounds I hear or sudden movements I see | 0 (0–1) | 0 (0–1) | |
| The need to withdraw from others | 0 (0–1) | 0 (0–1) | |
| Irritability | 0 (0–1) | 0.5 (0–2) | |
| Frequent mood swings | 0 (0–2) | 1 (0–2) | |
| Bad conscience, blame me, have guilt feelings | 0 (0–0) | 0 (0–1) | |
| Fear of places and situations, which remind me of the events | 0 (0–0) | 0 (0–0) | |
| Muscular tension | 0 (0–1) | 1 (0–2) | |
| Total | 5 (0–10) | 6.5 (4–11) | 0.1471 |
| Score ≥ 12 n (%) | 6 (16) | 7 (23) | 0.4322 |
| Score ≥ 18 n (%) | 2 (5) | 3 (10) | 0.4574 |
PSS-10 (Perceived Stress Scale-10) respondents are asked to rate each of the items on a scale of 0 to 4 based on how much a symptom has bothered them during the last month (0: never, 1: almost never, 2: sometimes 3: fairly often 4: very often). Scores for questions 4, 5, 7, and 8 are inverted. On these 4 questions, the scores are changed like this: 0 = 4, 1 = 3, 2 = 2, 3 = 1, 4 = 0. Scores are subsequently added up to get a total. Scores ranging from 0 to 13 would be low stress, scores ranging from 14 to 26 would be considered moderate stress and scores ranging from 27 to 40 would be considered high-perceived stress, respectively. FACIT-F (Functional Assessment of Chronic Illness Therapy – Fatigue) respondents are asked to rate each of the items on a scale of 0 to 4 based on the past 7 days. Scores on items 7 and 8 are reversed like this: 0 = 4, 1 = 3, 2 = 2, 3 = 1, 4 = 0. Scores are subsequently added up to a total. Scores < 30 indicate severe fatigue. PTSS-10 (Posttraumatic Symptom Scale-10) respondents are asked to rate each of the items on a scale of 0 to 3 (0: never, 1: rarely, 2: sometimes 3: often). PTSS-10 scores are subsequently added up to get a total.scores interpreted according to the following levels: 0 to 11 (mild to moderate) and 12 to 30 (moderate to severe). A score ≥ 18 represented a “case” and a score of ≥ 12 indicates the need for psychological help. Results are median plus interquartile range or percentage of patients as indicated.
Significant values are in bold.
Stress levels remained high in both non-ICU and ICU patients. Only 24% of non-ICU and 3% of ICU patients (p = 0.0186) had low perceived stress on the PSS. Relative risk of ICU patients not having a PSS-score ≤ 13 was 7.1 (CI 95% 1.3–42.2). High perceived stress was present in 30% of the ICU patients and 18% of non-ICU patients at 12-months.
Posttraumatic symptoms were not present in as many patients, with only 5% of non-ICU and 10% of ICU patients having a PTSS-10 ≥ 18, indicating a posttraumatic stress disorder. There was no significant difference between the patient groups (Table 3). The 12-months follow-up results for depression, anxiety, fatigue, and stress are visualized in Fig. S2.
Discussion
Severe COVID-19 is associated with high in-hospital mortality and long-lasting limitations. In the current study HRQoL was reduced in COVID-19 ICU patients 3- and 12-months post COVID-19 hospitalization, with significantly less improvement at 12-months compared to non-ICU patients. Mobility and usual activities were most severely impeded. Approximately one-fifth of both non-ICU and ICU patients suffered from depression. Severe fatigue and stress were also present in a high number of patients, whereas GAD and PTS were only observed in a small fraction.
The utilized EQ5D-5L is widely applicable as an instrument of general HRQoL and can be used in quality-adjusted life year (QALY) calculations. Mean EQ5D-5L indexes in non-ICU patients were similar to what has been described in the German population aged 55- 64 years (0.8716) or > 65 years (0.84–0.8816,17), respectively. Although our cohort might not be directly comparable to the general population of the same age, the percentage of non-ICU patients reporting no problems in the five different EQ-5D-5L dimensions was lower. In the general population up to two-thirds report no problems across all dimensions18. This indicates that in non-ICU cases, although recovery to a decent HRQoL was observed, long-term sequelae are common. In ICU patients all dimensions were strikingly limited. Only one-third reported no problems with mobility, pain/discomfort, and anxiety/depression. Less than one fourth were able to do all their usual activities. In conjunction with low median VAS scores and low EQ-5D-5L index scores, our data indicate that COVID-19 ICU patients still suffer from a significantly lower HRQoL after 12-months. EQ-5D-5L index scores were like what has been reported in mild heart failure (NYHA class I/II)19 or survivors of other causes of acute respiratory distress syndrome (ARDS). Results in non-COVID-19 ARDS survivors were like our data with EQ-5D-VAS scores of 69 and index scores of 0.7520. This is further corroborated by a systematic review of 4408 patients identifying ICU treatment as a risk factor of low HRQoL21. A meta-analysis showed that ARDS, prolonged mechanical ventilation, and sepsis lead to significant impairments in long-term QoL, whereas survivors of cardiac arrest, severe pancreatitis, esophagectomy, and acute kidney injury had a comparable or even better QoL than age- and gender-matched populations. It is important to notice, that reduced HRQoL is not equal to an unacceptable outcome. In a study of 1453 post-ICU patients, 95% self-reported their outcome acceptable irrespective of a reduced HRQoL with an EQ-5D-index of 0.81. Those reporting an unacceptable outcome had an EQ-5D-index of 0.57 while EQ-5D-index cut-off values could not be defined. Besides a low EQ-5D-index, symptoms of anxiety and depression had the strongest association with self-reported unacceptable outcome one year after ICU discharge22. Although these data originate from non-COVID-19 patients, it may be reasonable to assume a similar perception in our study cohort. Nevertheless, while physical improvement may take years, mental and emotional aspects are often stagnant or decline even further23.
Long-term mental health problems are recognized as COVID-19 consequences impeding full-recovery24. Of our non-ICU patients 18% and 20% of our ICU patients tested at risk for a major depressive disorder after 12-months, whereas it needs to be considered that the PHQ-9 cutoff-value of 10 may result in many false negatives in hospital settings and more false positives in primary care9. Nevertheless, a large community-based study in Great Britain found a similar overall prevalence of anxiety/depression (26.4%) and only a small positive association to SARS-CoV-2 infection25. Depression is highly interconnected to fatigue26, which was found in a similar percentage of patients. Fatigue may be debilitating. and although it improves over time, a meta-analysis showed that population references scores are seldomly reached27. In our cohort, a lower fraction of ICU patients had fatigue compared to what had been previously reported in prolonged critical illness with critical illness polyneuropathy/myopathy28. Moreover, the percentage of ICU patients with a suspected anxiety disorder was lower than what has been reported at one month follow-up24 or in the COVID symptom study25. Anxiety is often associated with younger adults and females, whereas the median age in our study was older and two-thirds were male. Furthermore, as depression or anxiety symptoms increased in the general population during the pandemic due to disrupted work or social life, the overall assessment of depression, fatigue and anxiety as specific post-COVID-19 symptoms is complicated29 and cannot be reasoned from our data.
Another important aftermath of severe disease and hospitalization is perceived stress or posttraumatic stress disorder (PTSD). PTSD has been described in up to 20% of general ICU survivors, whereas it is less common in non-ICU patients30,31. Trauma of hospitalization is furthermore associated with greater risk of 30-day readmission32. In our cohort, 10% of COVID-19 ICU patients had suspected PTSD, while a total of 23% were at risk. This in line with results from the BRAIN-ICU trial, finding PTSD in 7% of patients after 3 and 12 months33. It is of note, that we found a comparably high rate of suspected PTSD in non-ICU patients. While symptoms of PTSD, i.e. evidenced by the presence of flashbacks or nightmares, jumpiness, or fear of reminiscent situations were altogether rarely observed, symptoms of perceived stress were three times as common in ICU patients. Perceived stress is characterized by an individual’s appraise of situations in their lives as excessively uncontrollable and the extent to which they feel overloaded. Not only a higher percentage of ICU patients had high levels of stress, although non-significant, but also a significantly lower percentage had low levels of stress compared to non-ICU treatment. Low levels of stress were rarely observed in ICU patients, indicating that nearly all ICU survivors experience moderate to high levels of stress one-year after discharge.
Limitations of our study include the single-center design, with a high percentage of ECMO patients as the University hospital of Würzburg is a regional ECMO/ARDS referral center. Nearly half of our ICU cohort required ECMO support, which had been associated with long-term limitations in daily life34. However, others have also shown an overall positive long‐term HRQoL outcome for ECMO survivors with only few pulmonary and mental limitations35,36. We also found a high level of bacterial co-infections in this severely ill cohort. Both factors could potentially limit external validity. Furthermore, we found a significantly higher baseline BMI in ICU compared to non-ICU patients. In prior studies increasing BMI was associated with a higher probability of low self-perceived HRQoL and self-reported problems in all five EQ5D-5L dimensions. Effects of BMI on HRQoL were strongest in patients with severe to morbid obesity (BMI ≥ 35) and primarily affected mobility and pain/discomfort37. Hence, we cannot exclude that the higher BMI in ICU patients to some extent explains the lower HRQoL. Nevertheless, we only found a negligible negative correlation between EQ5D-5L-Index and BMI. Nutrition problems in ICU patients are complex and it needs to be considered that critically ill patients mostly lose lean body mass during ICU treatment. Although weight is subsequently gained back, nearly all of this is fat mass and not functional lean body mass. A catabolic/hypermetabolic state can maintain for up to 2 years after ICU discharge38,39, emphasizing the importance of individual nutrition management post ICU.
Consent was not obtainable in 55% of all eligible patients. Reasons include refusal to participate, as well as language barrier or unavailability of a legal representative amongst others. This low response rate could have potentially resulted in a selection bias. Due to the prospective design with the need for informed consent we cannot exclude a selection consent bias40. All patients included in the study were admitted as emergency hospitalizations. As such patients were not known prior to admission and prior recording of EQ-5D-5L was not possible. Overall clinical frailty was not significantly different at baseline. However, a higher proportion of ICU patients was moderately, severely, or very severely frail, which could have affected HRQoL at baseline, 3 and 12 months. The different EQ5D-5L health states were converted into a single index using country specific value sets, in our case representing the societal preferences of the German population41. We did not collect data on pulmonary function or six-minute walking distance. Hence, we cannot not correlate our data on HRQoL with follow-ups of physical status. Functional recovery after COVID-19 ARDS defined either by alterations on pulmonary function tests, a standardized 6 min walk test or fibrosis-like pulmonary findings will be further investigated in the “Functional Recovery From Acute Respiratory Distress Syndrome (ARDS) Due to COVID-19: Influence of Socio-Economic Status” (RECOVIDS) study42. The questionnaires used are generic scales, not specifically designed for HRQoL post COVID-19. They do not comprehensively consider all factors affecting HRQoL during the pandemic. Socioeconomics, lockdown measures, but also how COVID-19 affected family relationships (e.g. living alone or not, having relatives/friends who died) might have confounded the results. Furthermore, the presence of fatigue may be underrated as the FACIT-F was mainly developed for the geriatric population13. All results are based on univariate analysis and adjustment for possible confounders was not performed. Hence, we cannot exclude the possibility of interactions of the variables or one of the variables confounding other variables being studied.
In summary, our data highlight the complexity of post-COVID-19 symptoms as well as the necessity to educate patients and primary care providers about monitoring mental well-being. Particularly ICU survivors had a reduced HRQoL, like what has been described in ARDS from other causes. Mental disorders were common with high levels of perceived stress, depression, and fatigue. PTSD or anxiety on the contrary were less observed. However, our data also suggest that most patients were able to live independently at home after 12-months.
Supplementary Information
Acknowledgements
We would like to thank the teams of all participating departments of the WUE-COVID study (Maria-Elisabeth Göbeler, Bernhard Haring, Hartwig Klinker, Peter Kranke, Markus Kredel, Volker Kunzmann, Stefan Störk, Dirk Weismann) for their precious support. We explicitly thank our data management team (Anna Grau, Timo Ludwig, Udo Selig) for the prompt implementation of the database. We also thank the Interdisciplinary Center for Clinical Research (IZKF) Würzburg for supporting the study with a grant within their research funding program (Z-4/144).
Author contributions
J.H., K.M., K.H., P.H., P.M. and C.L. conceptualized the study. K.M., Q.N., M.E.H., A.H., J.S. performed telephone surveys. J.H., K.H., A.H., J.S. and C.L. analysed data and prepared figures. J.H., K.H., P.H., P.M. and C.L. wrote the manuscript. All authors reviewed the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-33783-y.
References
- 1.Macedo A, Goncalves N, Febra C. COVID-19 fatality rates in hospitalized patients: Systematic review and meta-analysis. Ann. Epidemiol. 2021;57:14–21. doi: 10.1016/j.annepidem.2021.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, Cook JR, Nordvig AS, Shalev D, Sehrawat TS, et al. Post-acute COVID-19 syndrome. Nat. Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Figueiredo EAB, Silva WT, Tsopanoglou SP, Vitorino DFM, Oliveira LFL, Silva KLS, Luz HDH, Avila MR, Oliveira LFF, Lacerda ACR, et al. The health-related quality of life in patients with post-COVID-19 after hospitalization: A systematic review. Rev. Soc. Bras. Med. Trop. 2022;55:e0741. doi: 10.1590/0037-8682-0741-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malik P, Patel K, Pinto C, Jaiswal R, Tirupathi R, Pillai S, Patel U. Post-acute COVID-19 syndrome (PCS) and health-related quality of life (HRQoL)-A systematic review and meta-analysis. J. Med. Virol. 2022;94(1):253–262. doi: 10.1002/jmv.27309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qu G, Zhen Q, Wang W, Fan S, Wu Q, Zhang C, Li B, Liu G, Yu Y, Li Y, et al. Health-related quality of life of COVID-19 patients after discharge: A multicenter follow-up study. J. Clin. Nurs. 2021;30(11–12):1742–1750. doi: 10.1111/jocn.15733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The World Health Organization Quality of Life assessment (WHOQOL): position paper from the World Health Organization. Soc. Sci. Med., 41(10):1403–1409 (1995). [DOI] [PubMed]
- 7.Tsukino M, Nishimura K, Ikeda A, Koyama H, Mishima M, Izumi T. Physiologic factors that determine the health-related quality of life in patients with COPD. Chest. 1996;110(4):896–903. doi: 10.1378/chest.110.4.896. [DOI] [PubMed] [Google Scholar]
- 8.Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann. Intern. Med. 1993;118(8):622–629. doi: 10.7326/0003-4819-118-8-199304150-00009. [DOI] [PubMed] [Google Scholar]
- 9.Manea L, Gilbody S, McMillan D. Optimal cut-off score for diagnosing depression with the Patient Health Questionnaire (PHQ-9): A meta-analysis. CMAJ. 2012;184(3):E191–196. doi: 10.1503/cmaj.110829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 11.Gräfe K, Zipfel S, Herzog W, Löwe B. Screening psychischer Störungen mit dem“Gesundheitsfragebogen für Patienten (PHQ-D)“. Diagnostica. 2004;50:171–181. doi: 10.1026/0012-1924.50.4.171. [DOI] [Google Scholar]
- 12.Lowe B, Decker O, Muller S, Brahler E, Schellberg D, Herzog W, Herzberg PY. Validation and standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the general population. Med. Care. 2008;46(3):266–274. doi: 10.1097/MLR.0b013e318160d093. [DOI] [PubMed] [Google Scholar]
- 13.Montan I, Lowe B, Cella D, Mehnert A, Hinz A. General population norms for the functional assessment of chronic illness therapy (FACIT)-fatigue scale. Value Health. 2018;21(11):1313–1321. doi: 10.1016/j.jval.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 14.Reis D, Lehr D, Heber E, Ebert DD. The German version of the perceived stress scale (PSS-10): Evaluation of dimensionality, validity, and measurement invariance with exploratory and confirmatory bifactor modeling. Assessment. 2019;26(7):1246–1259. doi: 10.1177/1073191117715731. [DOI] [PubMed] [Google Scholar]
- 15.Stoll C, Kapfhammer HP, Rothenhausler HB, Haller M, Briegel J, Schmidt M, Krauseneck T, Durst K, Schelling G. Sensitivity and specificity of a screening test to document traumatic experiences and to diagnose post-traumatic stress disorder in ARDS patients after intensive care treatment. Intensive Care Med. 1999;25(7):697–704. doi: 10.1007/s001340050932. [DOI] [PubMed] [Google Scholar]
- 16.Grochtdreis T, Dams J, Konig HH, Konnopka A. Health-related quality of life measured with the EQ-5D-5L: Estimation of normative index values based on a representative German population sample and value set. Eur. J. Health Econ. 2019;20(6):933–944. doi: 10.1007/s10198-019-01054-1. [DOI] [PubMed] [Google Scholar]
- 17.Marten O, Greiner W. EQ-5D-5L reference values for the German general elderly population. Health Qual. Life Outcomes. 2021;19(1):76. doi: 10.1186/s12955-021-01719-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huber MB, Felix J, Vogelmann M, Leidl R. Health-related quality of life of the general german population in 2015: Results from the EQ-5D-5L. Int. J. Environ. Res. Public Health. 2017;14(4):426. doi: 10.3390/ijerph14040426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dyer MT, Goldsmith KA, Sharples LS, Buxton MJ. A review of health utilities using the EQ-5D in studies of cardiovascular disease. Health Qual. Life Outcomes. 2010;8:13. doi: 10.1186/1477-7525-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Granja C, Morujao E, Costa-Pereira A. Quality of life in acute respiratory distress syndrome survivors may be no worst than in other ICU survivors. Intensive Care Med. 2003;29(10):1744–1750. doi: 10.1007/s00134-003-1808-x. [DOI] [PubMed] [Google Scholar]
- 21.Nandasena H, Pathirathna ML, Atapattu A, Prasanga PTS. Quality of life of COVID 19 patients after discharge: Systematic review. PLoS One. 2022;17(2):e0263941. doi: 10.1371/journal.pone.0263941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerckhoffs MC, Kosasi FFL, Soliman IW, van Delden JJM, Cremer OL, de Lange DW, Slooter AJC, Kesecioglu J, van Dijk D. Determinants of self-reported unacceptable outcome of intensive care treatment 1 year after discharge. Intensive Care Med. 2019;45(6):806–814. doi: 10.1007/s00134-019-05583-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oeyen SG, Vandijck DM, Benoit DD, Annemans L, Decruyenaere JM. Quality of life after intensive care: A systematic review of the literature. Crit. Care Med. 2010;38(12):2386–2400. doi: 10.1097/CCM.0b013e3181f3dec5. [DOI] [PubMed] [Google Scholar]
- 24.Mazza MG, De Lorenzo R, Conte C, Poletti S, Vai B, Bollettini I, Melloni EMT, Furlan R, Ciceri F, Rovere-Querini P, et al. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav. Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klaser K, Thompson EJ, Nguyen LH, Sudre CH, Antonelli M, Murray B, Canas LS, Molteni E, Graham MS, Kerfoot E, et al. Anxiety and depression symptoms after COVID-19 infection: Results from the COVID symptom study app. J. Neurol. Neurosurg. Psychiatry. 2021;92(12):1254–1258. doi: 10.1136/jnnp-2021-327565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corfield EC, Martin NG, Nyholt DR. Co-occurrence and symptomatology of fatigue and depression. Compr. Psychiatry. 2016;71:1–10. doi: 10.1016/j.comppsych.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Bench S, Stayt L, Shah A, Dhiman P, Czuber-Dochan W. Prevalence and experience of fatigue in survivors of critical illness: A mixed-methods systematic review. Anaesthesia. 2021;76(9):1233–1244. doi: 10.1111/anae.15441. [DOI] [PubMed] [Google Scholar]
- 28.Wintermann GB, Rosendahl J, Weidner K, Strauss B, Hinz A, Petrowski K. Self-reported fatigue following intensive care of chronically critically ill patients: A prospective cohort study. J. Intensive Care. 2018;6:27. doi: 10.1186/s40560-018-0295-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hansel TC, Saltzman LY, Melton PA, Clark TL, Bordnick PS. COVID-19 behavioral health and quality of life. Sci. Rep. 2022;12(1):961. doi: 10.1038/s41598-022-05042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davydow DS, Gifford JM, Desai SV, Needham DM, Bienvenu OJ. Posttraumatic stress disorder in general intensive care unit survivors: A systematic review. Gen. Hosp. Psychiatry. 2008;30(5):421–434. doi: 10.1016/j.genhosppsych.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Righy C, Rosa RG, da Silva RTA, Kochhann R, Migliavaca CB, Robinson CC, Teche SP, Teixeira C, Bozza FA, Falavigna M. Prevalence of post-traumatic stress disorder symptoms in adult critical care survivors: A systematic review and meta-analysis. Crit. Care. 2019;23(1):213. doi: 10.1186/s13054-019-2489-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rawal S, Kwan JL, Razak F, Detsky AS, Guo Y, Lapointe-Shaw L, Tang T, Weinerman A, Laupacis A, Subramanian SV, et al. Association of the trauma of hospitalization with 30-day readmission or emergency department visit. JAMA Intern. Med. 2019;179(1):38–45. doi: 10.1001/jamainternmed.2018.5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson JC, Pandharipande PP, Girard TD, Brummel NE, Thompson JL, Hughes CG, Pun BT, Vasilevskis EE, Morandi A, Shintani AK, et al. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: A longitudinal cohort study. Lancet Respir. Med. 2014;2(5):369–379. doi: 10.1016/S2213-2600(14)70051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rossong H, Debreuil S, Yan W, Hiebert BM, Singal RK, Arora RC, Yamashita MH. Long-term survival and quality of life after extracorporeal membrane oxygenation. J. Thorac. Cardiovasc. Surg. 2022 doi: 10.1016/j.jtcvs.2021.10.077. [DOI] [PubMed] [Google Scholar]
- 35.Orbo MC, Karlsen SF, Pedersen EP, Hermansen SE, Ronning PB, Nergaard KA, Naesheim T, Myrmel T. Health-related quality of life after extracorporeal membrane oxygenation: A single centre’s experience. ESC Heart Fail. 2019;6(4):701–710. doi: 10.1002/ehf2.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rilinger J, Krotzsch K, Bemtgen X, Jackel M, Zotzmann V, Lang CN, Kaier K, Duerschmied D, Supady A, Bode C, et al. Long-term survival and health-related quality of life in patients with severe acute respiratory distress syndrome and veno-venous extracorporeal membrane oxygenation support. Crit. Care. 2021;25(1):410. doi: 10.1186/s13054-021-03821-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Busutil R, Espallardo O, Torres A, Martinez-Galdeano L, Zozaya N, Hidalgo-Vega A. The impact of obesity on health-related quality of life in Spain. Health Qual. Life Outcomes. 2017;15(1):197. doi: 10.1186/s12955-017-0773-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N. Engl. J. Med. 2003;348(8):683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 39.Wischmeyer PE, San-Millan I. Winning the war against ICU-acquired weakness: New innovations in nutrition and exercise physiology. Crit. Care. 2015;19(Suppl 3):S6. doi: 10.1186/cc14724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kho ME, Duffett M, Willison DJ, Cook DJ, Brouwers MC. Written informed consent and selection bias in observational studies using medical records: Systematic review. BMJ. 2009;338:b866. doi: 10.1136/bmj.b866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ludwig K, Graf von der Schulenburg JM, Greiner W. German value set for the EQ-5D-5L. Pharmacoeconomics. 2018;36(6):663–674. doi: 10.1007/s40273-018-0615-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Declercq PL, Fournel I, Demeyere M, Ksiazek E, Meunier-Beillard N, Riviere A, Clarot C, Maizel J, Schnell D, Plantefeve G, et al. Influence of socioeconomic status on functional recovery after ARDS caused by SARS-CoV-2: A multicentre, observational study. BMJ Open. 2022;12(4):e057368. doi: 10.1136/bmjopen-2021-057368. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.