Abstract
Vitamin D or calciferol is a fat-soluble vitamin that has a unique feature of synthesizing in the body mainly by exposure to UV from the sunlight and then transformed to 25 (OH) D by the liver and finally to a vital form 1,25-dihydroxyvitamin D by the kidneys. Vitamin D receptor gene polymorphism (FokI-rs2228570) has been proposed as the major cause of anemia. The present study aimed to discover the association between vitamin D deficiency and vitamin D receptor gene polymorphism (FokI-rs2228570) in patients with anemia. A total of 120 men with anemia and no kidney disorders have been compared with 60 healthy controls in the present case-control study. A single nucleotide polymorphism (SNP) FokI-rs2228570 was detected by PCR and PCR-RFLP techniques. Levels of serum vitamin D, erythropoietin, and some biochemical parameters were detected by the ELISA assay technique. The mutant homozygous genotype ff was more frequent in patients with anemia (45%) than in the controls (15%). Also, the frequency of the f allele was associated with a significant decrease in the levels of vitamin D and hemoglobin in patients (0.62%); therefore, the mutant allele is a risk factor for developing anemia compared with genetic patterns FF and Ff. Vitamin D deficiency is common in those with anemia which is often associated with low hemoglobin and high levels of erythropoietin. Additionally, the genetic frequencies also affect the level of vitamin D which is indicated by low levels of mutant patterns (Ff, ff) in which patients suffer from severe anemia.
Keywords: Anemia, Vitamin D, Vitamin D deficiency, Vitamin D Receptor Gene Polymorphism
1. Introduction
Vitamin D or calciferol is a fat-soluble vitamin that has a unique feature of synthesizing in the body mainly by exposure to UV from the sunlight and then transformed to 25(OH)D by the liver and finally to a vital form 1,25-dihydroxy D by the kidney ( 1 ), which plays a key role in keeping steady the levels of calcium and phosphorus required for bone growth ( 2 ). Furthermore, several studies have proven the biological activities of vitamin D in inducing and inhibiting cell growth and proliferation, it also reinforces the defense system as an anti-inflammatory factor ( 3 ). The fundamental role of vitamin D has been proven in metabolic processing and the function of endothelial tissues ( 4 ). Similarly, other studies have focused on the important role of calciferol in erythropoiesis and its association with anemia ( 5 ).
Vitamin D deficiency causes several health problems such as anemia and low hemoglobin levels. An association was observed between vitamin D deficiency and anemia in which the active pattern of calciferol binds to the vitamin D receptor (VDR) meaning that VDR is also involved in decreasing hemoglobin levels in patients with anemia ( 6 , 7 ).
Anemia is a global public health problem affecting many countries. World Health Organization (WHO) defined anemia as a condition in which the number of red blood cells or the hemoglobin concentration is lower than normal to meet the physiological requirements of the body ( 8 ). Several reasons stimulate the low ability of red blood cells to carry the oxygen and cause anemia, such as iron deficiency, insufficient globin synthesis, kidney failure, inflammation, menorrhagia, and malabsorption ( 9 ). In addition, various studies have been conducted on the relationship between vitamin D receptor gene polymorphisms and hemoglobin concentration in individuals with anemia and no chronic kidney disease ( 10 ).
The vast majority of biological actions of vitamin D were performed by binding to high-affinity receptors. VDR gene is located on chromosome 12 (q12-14) at 75 kb with "11 exons", where 2 and 3 exons are expressed for amino acids involved in DNA bounding, while 7, 8, and 9 exons are involved in vitamin D bounding. Single nucleotide polymorphisms (SNPs) of FokI-rs2228570, TaqI-rs731236, ApaI-rs7975232, and BsmI-rs1544410 are the well-known SNPs of VDR gene polymorphism ( 11 ). The FokI SNP represents a T-to-C transition (ATG to ACG) in exon 2 of the VDR gene, and this ATG represents the translation-initiation codon resulting in the generation of a protein shortened by three amino acids with higher transcriptional activity than the original ( 12 ). The present study aimed to investigate the relationship between vitamin D deficiency and vitamin D receptor gene polymorphism (FokI-rs2228570) in patients without any kidney diseases.
2. Materials and Methods
2.1. Study Subjects
Venous blood samples were taken from 120 men with anemia and no kidney diseases and 60 healthy men as control using 5 ml disposable syringes. Then, 2 ml of blood samples were deposited in EDTA tubes and gently mixed for 3 minutes. The samples were divided into two parts: the first part was used for serum tests, and the second was stored at –20 Cº for later use in genetic analysis. Serum levels of albumin, iron, ferritin, transferrin, and Hb were estimated for each sample. Blood levels of vitamin D and erythropoietin were determined using the ELISA kit.
DNAs were extracted from frozen blood using various components of the extraction kit according to the protocol of the manufacturer (Geneaid/Korea). The purity of isolated DNA was assessed by a Nano-drop. PCR-RFLP was used to detect polymorphism in the vitamin D receptor gene (FokI-rs2228570) by specific primers:
[Forward: 5'-GATGCCAGCTGGCCCTGGCACTG-3']
[Reverse: 5' -ATGGAAACACCTTGCTTCTTCTCCCTC-3'] ( 13 ).
In a 25 µl total reaction volume, the PCR reaction mixture consisted of 250 ng/l template DNA, 400 M of each dNTP, 12.5 µl buffer with 1 U Go Taq DNA polymerase (Promega), 10 M of each primer, and 3 mM MgCl2. The GTS series thermal cycler (Cleaver Scientific/UK) was used to perform the amplification processes. Initial denaturation was performed at 96 ºC for 5 min, followed by 40 PCR cycles (denaturation at 94 ºC for 45 s, primer annealing at 59.5 ºC for 30 s, and template elongation at 72 ºC for 10 min). After PCR, the product was digested by the FokI restriction enzyme at 37 ºC, overnight. Ethidium bromide staining was used in 3% agarose gel electrophoresis visualized using a UV-trans illuminator to detect the presence or absence of VDR FokI-rs2228570 gene polymorphism. The size of the FokI gene was detected with a 100-1000 bp DNA marker.
2.2. Statistical Analysis
Data analysis was performed using SPSS software (version 23). Mean±SD was used to depict continuous variables. Also, a student’s t-test was used to compare the means of the two groups.
3. Results and Discussion
3.1. DNA Extraction
According to the findings of the present genetic study, genomic (DNA) isolated from blood samples has a molecular weight of 50-200 ng/l and purity of 1.7-2.2 (Figure 1).
Figure 1.
Electrophoresis of DNA extracted from the blood of the patients and control groups. (Lane: 1-6 DNA from patients, lane: 7-10 from controls; 1% agarose, 75 V, 20 Am for 1 h)
3.2. FokI-rs2228570 Genotyping
The results of VDR FokI-rs2228570 gene genotyping revealed that the outcome of PCR consisted of a band (approximately 273 bp) for patients and controls (Figure 2).
Figure 2.
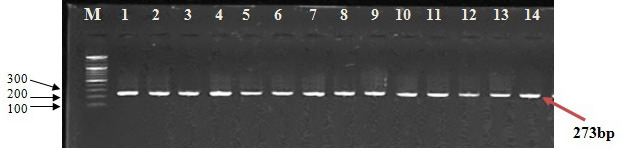
Electrophoresis of PCR product of VDR FokI-rs2228570 gene amplification.
(M: DNA marker [100 bp], lane 1-7 from patients with anemia, lane 8-14 from controls; 1% agarose, 75 V, 20 mA for 1 h)
3.3. PCR-RFLP of FokI-rs2228570 Gene
The results of PCR-RFLP of the FokI-rs2228570 gene in men with anemia and controls using the FokI-restriction enzyme revealed that the homozygous FF pattern has one band around (273bp), the homozygous FF pattern has two bands around (75 bp and 198 bp), and the heterozygous Ff pattern has three bands around (75, 198, and 273 bp) (Figure 3).
Figure 3.
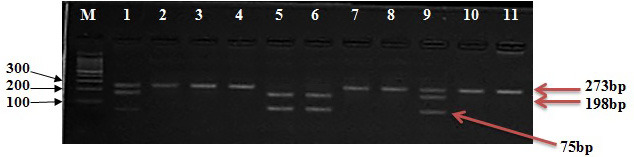
Electrophoresis of PCR-RFLP for PCR product (273bp) with a FokI restriction enzyme, 3% agarose, 75 V, 20 mA for 2 h.
(LaneM: DNA marker [100 bp], Lane: 2, 3, 4, 7, 8, 10, 11 homozygote type FF genotype, Lane: 1,9 heterozygote mutant type Ff genotype, Lane: 5,6 homozygote mutant type ff genotype)
The results of the present study revealed that the frequency of mutant homozygote pattern ff was higher in patients with anemia (45%) than in healthy subjects (15%). Also, the frequency of the f mutant allele was higher in the patients (0.62%) compared to the controls (0.25%) which suggest that the pattern of mutant homozygote was mainly associated with anemia (Table 1).
Table 1.
Frequencies of genotypes and allele of the VDR FokI-rs2228570 in patients with anemia and control group
| Genotype Pattern | Genotype Frequency (%) | ||||
|---|---|---|---|---|---|
| Patients with Anemia No:120 | Control No: 60 | P-Value | Odds Ratio | 95% CI | |
| FF | 27 (23%) | 40 (67%) | Reference | ||
| Ff | 39 (32%) | 11 (18%) | 0.03* | 2.448 | 1.0057-4.574 |
| ff | 54 (45%) | 9 (15%) | 0.0001** | 4.6364 | 2.0944-10.2637 |
| Allele Frequency% | F (0.38) | F (0.75) | |||
| f (0.62) | f (0.25) | ||||
*P-value ≤ 0.05
**P-value ≤ 0.001
3.4. Physiological Parameters
The levels of vitamin D and hemoglobin were significantly lower (P≤0.01) in patients with anemia than in control individuals, while the levels of erythropoietin were significantly higher (P≤ QUOTE ≤ 0.01) in patients than in controls. The results revealed no significant differences in albumin, iron, ferritin, and transferrin levels in both groups (Table 2).
Table 2.
Some physiological parameters in patients with anemia and control groups
| Indices | Control (Mean ± SD) | Patients with Anemia (Mean ± SD) | P-Value |
|---|---|---|---|
| Age (Year) | 47.61 ± 7.91 | 48.26 ± 8.47 | 0.73 |
| Albumin (g/dl) | 5.66 ± 1.93 | 5.18 ± 1.88 | 0.27 |
| Serum Iron (mg/dl) | 83.46 ± 5.63 | 81.44 ± 4.76 | 0.09 |
| Ferritin (ng/ml) | 30.23 ± 5.12 | 29.86 ± 5.49 | 0.76 |
| Transferrin (ng/ml) | 30.00 ± 6.22 | 30.60 ± 5.34 | 0.64 |
| Vitamin D (ng/ml) | 21.38 ± 2.24 | 13.39 ± 4.20 | 0.0001** |
| Erythropoietin (mu/ml) | 5.97 ± 2.65 | 22.57 ± 5.11 | 0.0001** |
| Hb (mg/dl) | 16.53 ± 2.44 | 9.28 ± 2.62 | 0.0001** |
SD: standard deviation; * Signification at P-value ≤ 0.01
3.5. The effect of the FokI-rs2228570 gene polymorphisms
A significant difference was observed between non-mutant wild-type-FF and mutant-type-Ff and ff in patients with anemia. The levels of vitamin D and hemoglobin decreased (P≤0.01) in the mutant type, while the levels of erythropoietin significantly increased (P≤0.01) (Table 3).
Table 3.
The effect of VDR FokI-rs2228570 gene polymorphisms on the levels of vitamin D, erythropoietin, and Hb in patients with anemia
| Indices | VDR FokI-rs2228570 gene | P-Value | |
|---|---|---|---|
| Wild type-FF (Mean ± S.D.) | Mutant type-Ff, ff (Mean ± S.D.) | ||
| Vitamin D (ng/ml) | 16.75 ± 2.05 | 9.25 ± 2.30 | 0.001* |
| Erythropoietin (mu/ml) | 16.50 ± 2.74 | 27.33 ± 2.64 | 0.001* |
| Hb (mg/dl) | 12.00 ± 1.85 | 7.75 ± 1.91 | 0.001* |
S.D.: standard deviation; * Signification at P-value ≤ 0.01
The present study focuses on the role of vitamin D receptor gene polymorphism (FokI-rs2228570) in men with anemia in Babylon, Iraq to elucidate the association between vitamin D deficiency and genetic variation of vitamin D receptor in developing anemia. Vitamin D receptor gene polymorphisms can be associated with developing anemia as calciferol, the vital component of vitamin D plays a mediating role in erythropoiesis ( 14 ). The present study is based on the idea that the gene polymorphism of the FokI receptor may indicate the levels of erythropoietin and hemoglobin which are needed in patients with anemia ( 15 ).
In the present study, genotypes Ff and ff of the FokI-rs2228570 were significantly associated with lower levels of vitamin D and hemoglobin than the wild-type FF. Improvement of FokI gene polymorphism is associated with vitamin D deficiency and altered red blood cell formation ( 6 ). However, the study of Singh, Kumar ( 12 ) showed no significant association between vitamin D deficiency and Hb levels with the FokI gene polymorphism in patients with anemia.
The present study found that the level of vitamin D in patients with anemia is lower than that of control as disorders are caused by deficiency of 25(OH) D in the liver leading to increased iron concentration and consequently liver problems ( 16 , 17 ).
Translation starts from the first [ATG] site in the mutant type ff which results in a long vitamin D receptor protein of 427 amino acids, while in the wild type FF, translation starts at the second [ATG] site which shortened protein chain to 424 amino acids ( 18 , 19 ). Changes in amino acid length of vitamin D receptor is significantly affected by the occurrence of polymorphism ( 20 , 21 ). Consequently, the f allele results in the creation of VDR with three more amino acids and decreased biological activity compared to the VDR of the F allele ( 22 ).
The present study indicated an association between vitamin D deficiency and vitamin D receptor gene polymorphism (FokI-rs2228570) in men with anemia.
Authors' Contribution
Study concept and design: A. H. M.
Acquisition of data: A. H. M.
Analysis and interpretation of data: R. S. A.
Drafting of the manuscript: A. H. A.
Critical revision of the manuscript for important intellectual content: R. S. A.
Statistical analysis: A. H. A.
Administrative, technical, and material support: A. H. M.
Ethics
All the procedures and the study protocol were approved by the ethics committee of Al-Mustaqbal University College, Hillah, Iraq.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Bischoff-Ferrari HA, Dietrich T, Orav EJ, Dawson-Hughes B. Positive association between 25-hydroxy vitamin D levels and bone mineral density: a population-based study of younger and older adults. Am J Med. 2004;116(9):634–9. doi: 10.1016/j.amjmed.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 2.Meier C, Woitge HW, Witte K, Lemmer B, Seibel MJ. Supplementation with oral vitamin D3 and calcium during winter prevents seasonal bone loss: a randomized controlled open‐label prospective trial. J Bone Miner Res. 2004;19(8):1221–30. doi: 10.1359/JBMR.040511. [DOI] [PubMed] [Google Scholar]
- 3.Tian J, Liu Y, Williams LA, de Zeeuw D. Potential role of active vitamin D in retarding the progression of chronic kidney disease. Nephrol Dial Transplant. 2007;22(2):321–8. doi: 10.1093/ndt/gfl595. [DOI] [PubMed] [Google Scholar]
- 4.Lee P. Vitamin D metabolism and deficiency in critical illness. Best Pract Res Clin Endocrinol Metab. 2011;25(5):769–81. doi: 10.1016/j.beem.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Kersey M, Chi M, Cutts DB. Anaemia, lead poisoning and vitamin D deficiency in low-income children: do current screening recommendations match the burden of illness? . Public Health Nutr. 2011;14(8):1424–8. doi: 10.1017/S1368980010003617. [DOI] [PubMed] [Google Scholar]
- 6.Dimitriadou M, Christoforidis A, Fidani L, Economou M, Perifanis V, Tsatra I, et al. Fok‐I gene polymorphism of vitamin D receptor in patients with beta‐thalassemia major and its effect on vitamin D status. Hematology. 2011;16(1):54–8. doi: 10.1179/102453311X12902908411878. [DOI] [PubMed] [Google Scholar]
- 7.Swamy N, Xu W, Paz N, Hsieh J-C, Haussler MR, Maalouf GJ, et al. Molecular modeling, affinity labeling, and site-directed mutagenesis define the key points of interaction between the ligand-binding domain of the vitamin D nuclear receptor and 1α, 25-dihydroxyvitamin D3. Biochemistry. 2000;39(40):12162–71. doi: 10.1021/bi0002131. [DOI] [PubMed] [Google Scholar]
- 8.McLean E, Cogswell M, Egli I, Wojdyla D, De Benoist B. Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993–2005. Public Health Nutr. 2009;12(4):444–54. doi: 10.1017/S1368980008002401. [DOI] [PubMed] [Google Scholar]
- 9.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352(10):1011–23. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y-Z, Zhu Z, Zhang H-Y, Zhu M-Z, Xu X, Chen C-H, et al. Detection of hepatitis B virus A1762T/G1764A mutant by amplification refractory mutation system. Braz J Infect Dis. 2014;18:261–5. doi: 10.1016/j.bjid.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alimirah F, Peng X, Murillo G, Mehta RG. Functional significance of vitamin D receptor FokI polymorphism in human breast cancer cells. PloS One. 2011;6(1):16024. doi: 10.1371/journal.pone.0016024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh K, Kumar R, Shukla A, Phadke SR, Agarwal S. Status of 25-hydroxyvitamin D deficiency and effect of vitamin D receptor gene polymorphisms on bone mineral density in thalassemia patients of North India. Hematology. 2012;17(5):291–6. doi: 10.1179/1607845412Y.0000000017. [DOI] [PubMed] [Google Scholar]
- 13.Mishra DK, Wu Y, Sarkissyan M, Sarkissyan S, Chen Z, Shang X, et al. Vitamin D receptor gene polymorphisms and prognosis of breast cancer among African-American and Hispanic women. PLoS One. 2013;8(3):e57967. doi: 10.1371/journal.pone.0057967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sim JJ, Lac PT, Liu ILA, Meguerditchian SO, Kumar VA, Kujubu DA, et al. Vitamin D deficiency and anemia: a cross-sectional study. Ann Hematol. 2010;89(5):447–52. doi: 10.1007/s00277-009-0850-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohsen IH, Zaidan HK, Nassir IM, Al-Saadi AH, Al-Terehi MN, Al-Jboori MJ. Association of miR-499 Gene Polymorphism with Some Hormones in type 2 Diabetes Mellitus Patients. Indian J Public Health Res Dev. 2018;9(11) [Google Scholar]
- 16.El-Edel RH, Mostafa MS, Montaser BA, Ali YAE-H. Study of Apa-I vitamin D receptor gene polymorphism in patients with hepatocellular carcinoma. Menoufia Med J. 2017;30(2):619. [Google Scholar]
- 17.Zaidan HK, Asma'a HM. Glucagon like peptide-1 and C peptide level and their relationship with some physiological and biochemical variables of non-insulin dependent diabetes mellitus type 2 patient. Aust J Basic Appl Sci. 2014;8(17):595–600. [Google Scholar]
- 18.Ibrahim IH, Zaidan HK, Al Ameri QMA, Ewadh MJ, Al Saadi AH. The Prevalence of Microalbuminuria in Type 2 Diabetes Mellitus Patients in Al-Husain Hospital in Karbala Province-Iraq. Res Biotechnol. 2012;3(2) [Google Scholar]
- 19.Palomer X, González‐Clemente J, Blanco‐Vaca F, Mauricio D. Role of vitamin D in the pathogenesis of type 2 diabetes mellitus. Diabetes Obes Metab. 2008;10(3):185–97. doi: 10.1111/j.1463-1326.2007.00710.x. [DOI] [PubMed] [Google Scholar]
- 20.Deng HW, Shen H, Xu FH, Deng HY, Conway T, Zhang HT, et al. Tests of linkage and/or association of genes for vitamin D receptor, osteocalcin, and parathyroid hormone with bone mineral density. J Bone Miner Res. 2002;17(4):678–86. doi: 10.1359/jbmr.2002.17.4.678. [DOI] [PubMed] [Google Scholar]
- 21.Hadi M, Zaidan HK, Al-Saadi AH. Histopathological changes of pancreatic tissues in hyperglycemic male rats treated with mixture of plants extracts. Int J Chemtech Res. 2016;9(6):501–13. [Google Scholar]
- 22.Zaidan H, Wtwt M, Amira K, Al-Saadi A, Tahrear M. The Metabolic Syndrome and Disturbances in Sex Hormones Levels in both gender. Aust J Basic Appl Sci. 2014;8(13):27–33. [Google Scholar]


