Abstract
It is more effective to maintain good health than to regain it after losing it. This work focuses on the biochemical defense mechanisms against free radicals and their role in building and maintaining antioxidant shields, aiming to show how to balance, as much as possible, the situations in which we are exposed to free radicals. To achieve this aim, foods, fruits, and marine algae with a high antioxidant content should constitute the basis of nutritional elements, since natural products are known to have significantly greater assimilation efficiency. This review also gives the perspective in which the use of antioxidants can extend the life of food products, by protecting them from damage caused by oxidation as well as their use as food additives.
Keywords: biochemistry, health, antioxidants, free radicals, fruits, vitamin C, food, marine algae, food additives
1. Introduction
Antioxidants are a class of a multitude of chemical substances clearly associated with large health benefits and lower risks of various age-related diseases.
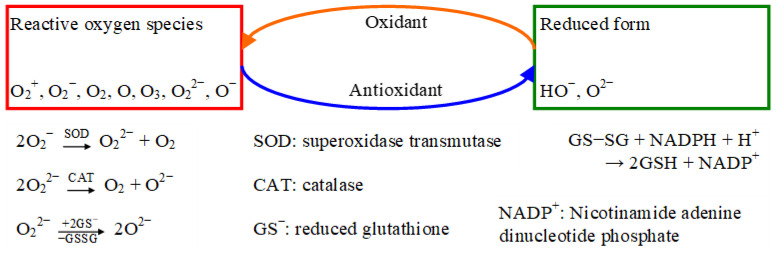
They also can stop the damaging actions of reactive oxygen species (ROS) [1,2], which include partially reduced or “energized” forms of oxygen, some of them as “free radicals”, with an unpaired electron included in an orbital, while others as “nonradical species”, such as hydrogen peroxide and singlet oxygen, whose reactivity is even greater than that of the ground state of molecular oxygen [1,3]. A schematic way of antioxidants action neutralizing free radicals by reacting together is shown in Figure 1, adapted from [2,4,5,6].
Figure 1.
Biochemistry of antioxidants (formation and equilibrium reactions in Ref. [4]; oxyl radical detection in [5]; antioxidants reduction in [6]).
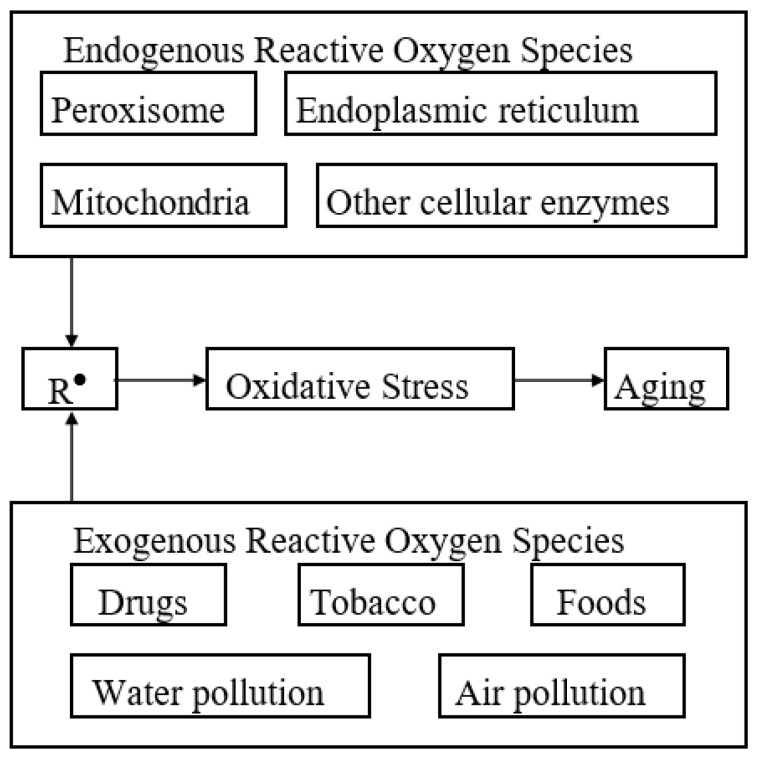
Endogenous and exogenous sources of free radicals are presented in Figure 2, while some of their damaging actions are schematically presented in Figure 3 (adapted from [7]).
Figure 2.
Endogenous and exogenous sources contributing to aging (free radicals are the leverage in oxidative stress and aging).
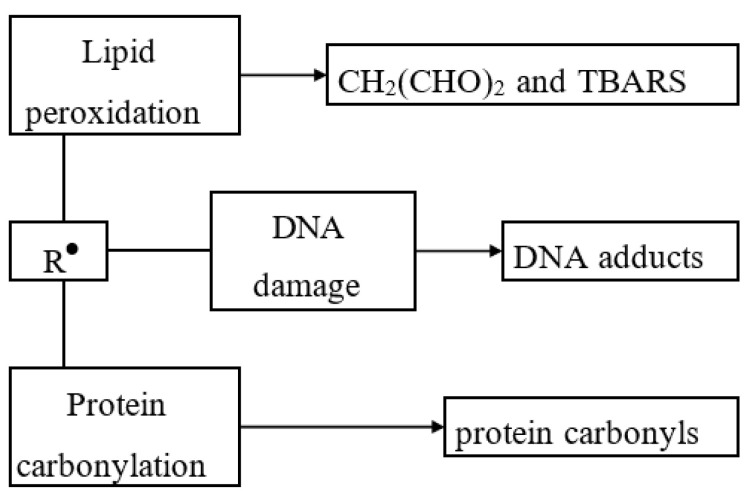
Figure 3.
Damaging actions of free radicals (most organic radicals have short lifetimes; many radicals spontaneously dimerize; however, during their short lifetime, due to the presence of unpaired electrons, the radicals are highly chemically reactive and may damage the biological tissue). TBARS, thiobarbituric acid reactive substance (formed as a result of lipid peroxidation).
ROS are produced by normal aerobic metabolism, via environmental factors, such as smoke and radiation, an excess of drugs, or an incorrect nutritional style [8,9,10]. Free radicals damage nuclear DNA, proteins, and the lipid matrix of cells [11,12,13]. Once they enter the body, they not only cause aberrant cell development but can also cause genetic changes that are the basis of relentless disease [14,15].
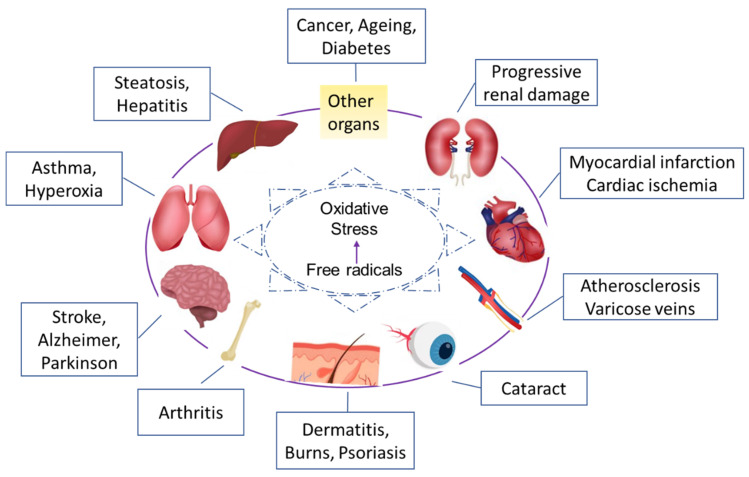
Studies have shown that reactive oxygen molecules are involved in more than 50 medical conditions, including various forms of cancer [16], heart disease [17,18], premature aging [19], cataracts [20], and even AIDS [21], or in pregnancy [22] (Figure 4).
Figure 4.
Most frequent pathologies related to oxidative stress.
It is estimated that a single cell is exposed to free radical damage 10,000 times a day [23]. Many of the resulting injuries are repaired by the body, but some accumulate. Some researchers claim that aging is due to the accumulation during the life of unrepaired damage to the deoxyribonucleic acid inside the mitochondria, damage caused mostly by the action of free radicals [24,25]. Free radicals are usually destroyed by our body’s natural antioxidant system [26]. There are both stable and unstable molecules of oxygen in the body: While stable oxygen is essential for sustaining life, unstable oxygen molecules (free radicals) can also be useful because they can be harnessed to fight inflammation, and bacteria, and control muscle tone, regulating the functioning of internal organs and blood vessels [26,27].
The problem with free radicals lies in their imbalance, their regulation often being compromised [28]. Many of the body’s natural biological processes, such as breathing, digesting food, neutralizing alcohol, and drugs, and converting fat into energy produce harmful free radicals [8,9,10]. These free radicals can trigger a negative chain reaction in the body, a reaction that destroys the cell membrane, blocks the action of the main enzymes, prevents cellular processes and normal cell division, destroys cellular DNA, and blocks energy generation [26,27,28].
Understanding these mechanisms and their biochemistry at the molecular level allows us to further explain and emphasize the importance of antioxidants intake for combating the negative effects of ROS, which belong to the category of oxidants. With this purpose, in our review, we elaborate on these causes and effects situations (ROS vs. antioxidants) and the health benefits of these. In this regard, we will further present in the next sections, numerous studies that have highlighted the importance of a diet rich in antioxidant compounds (i.e., polyphenols, thiols, vitamins C and E, as well as some minerals) for the prevention of various chronic-degenerative diseases related to an increase in oxidative stress, caused by free radicals, but also we will present the aspects of promising studies currently elaborated for delivering new resources of antioxidants, such as the marine extracts.
More than this, another important aspect that we considered in this review is to clarify the antioxidants terminology, since this refers to multiple sides and has different meanings in food and health science as follows [29].
2. Biochemistry of Antioxidants and Their Mode of Action
Endogenous antioxidants are body products. In contrast with the exogenous antioxidants, the body possesses enzyme systems with an antioxidant action (superoxide dismutase, glutathione peroxidase, and catalysis), co-participating in the deactivation of some free radicals that are formed in the body [30,31]. As a defense against oxidative stress imbalances, the body has produced so-called endogenous antioxidants, enzyme systems capable of annihilating free oxygen molecules, preventing the production of negative effects in the body [32,33]. Among the endogenous antioxidants, we mention superoxide-dismutase, catalase, glutathione peroxidase, and hydropersulfides [34,35]. Some subtypes of glutathione peroxidase are selenium (Se)-dependent, and recent studies [36,37,38,39,40] show that an increased intake of Se is associated with protection against the development of cancer and other chronic diseases.
Antioxidants as food additives are referring to some natural or synthetic (established) antioxidants which are also widely used in the food industry to prevent a reduction in the oxidation of fats or other components present in food, during the preservation period [41,42].
Exogenous antioxidants are introduced with food and are referred to the established or natural antioxidants. Because it is much more effective, and cheaper, to maintain good health rather than to regain it, the best protection against free radicals is to build and maintain “antioxidant shields”, through a regimen of adequate food with little fat, rich in digestive fibers, and in antioxidant substances, such as vitamin E, vitamin C, and beta-carotene, combined with regular exercise [43,44], and through a life program [45] aimed at avoiding, as much as possible, the situations in which we are exposed to the attack of free radicals. Foods with a high content of antioxidants constitute the basis of nutritional strategies that we can take from external sources [46,47]. Food of plant origin was associated with a high content of antioxidants [47,48]. Importantly, exogenous antioxidants that can be taken in the diet have the same role in reducing the excessive number of free radicals. The most important of these external (or exogenous) antioxidants are vitamin C, vitamin E, and beta-carotene [49].
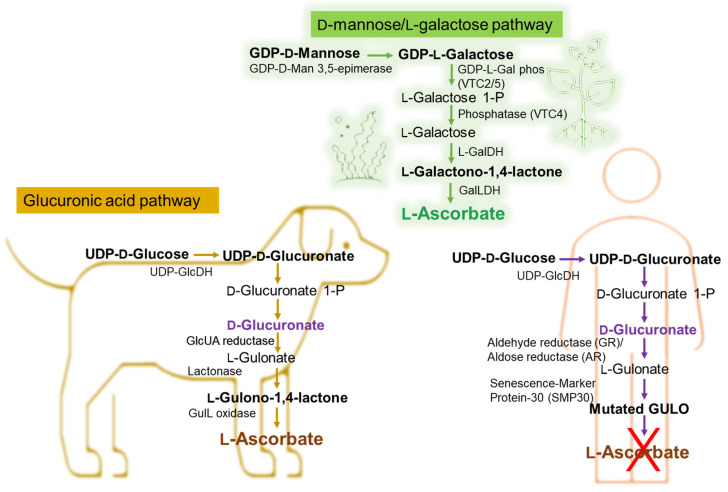
Taking the example of vitamin C’s different pathways to biosynthesis in marine algae or plants, animals, and the human bodies, we can emphasize the importance of the exogenous addition of antioxidants such as vitamin C in the human body. The Smirnoff–Wheeler pathway, in which vitamin C is synthesized from D-mannose and L-galactose (D-mannose/L-galactose pathway) [50], represents the major route of vitamin C biosynthesis in marine algae and plants, at the cellular level (Figure 5), the other three involved routes being the glucose, myoinositol, and the galacturonate pathways [51,52,53,54]. Most animals produce relatively high levels of ascorbic acid from glucose in the liver via the glucuronic acid pathway (Figure 5) [55,56,57,58]. Humans are unable to synthesize vitamin C and must ingest this vitamin [59,60].
Figure 5.
Biochemical pathway of vitamin C synthesis in animals vs. plants/green algae and humans. GDP-L-Gal phos, guanosine diphosphate-L-galactose phosphorylase; GalDH, galactose dehydrogenase; UDP-GlcDH, uridine diphosphate glucose dehydrogenase; GlcUA reductase, glucuronic acid reductase; GulL oxidase (GULO), L-gulonolactone oxidase. This figure is based on information from references [61,62,63,64,65].
As shown in Figure 5, in certain vertebrates (i.e., dogs), L-Ascorbate synthesis involves three enzymatic steps starting from the conversion of D-Glucuronate, with L-Gulonate and L-Gulono-γ-lactone (L-Gulono-1,4-lactone) as intermediate metabolites [61,62,63,64,65,66]. If in this case, the final enzymatic step is catalyzed by L-Gulono-γ-lactone oxidase (GULO), converting L-Gulonate to L-Ascorbate, in humans, the GULO enzyme is mutated (Figure 5) and not functional in primates also including guinea pigs and some spontaneous mutant mouse and rat models [65,67]. Instead, the conversion of L-Gulonate to L-Gulono-1,4-lactone occurs via Senescence-Marker Protein-30 (SMP30) (Figure 5) also known as regucalcin [62,65]. Likewise, in the humans’ case, the conversion of D-Glucuronate to L-Gulonate occurs mainly through aldehyde reductase (GR) and to a smaller extent with aldose reductase’ (AR) contribution (Figure 5) [51,65,66]. Considering that the common molecular mechanism of the body’s limited ability to synthesize vitamin C is the absence of GULO [68,69,70,71], genetically, it is considered that the loss of synthesizing the ascorbic acid is likely due to the complete loss of the L-gulono-γ-lactone oxidase (GULO) gene.
Combinations of all these facts bring us to the conclusion of an important balanced diet associated with antioxidant supplements, potentiating each other’s effects and influencing the prevention of diseases, such as heart disease, arthritis, visual impairment, stroke, and premature aging of the skin, enhancing well-being.
Membrane lipids represent a major target of the radical attack, due to the presence of double bonds in the structures of the polyunsaturated fatty acids which comprise them. Membrane phospholipids most frequently contain unsaturated fatty acids, i.e., linoleic acid, linolenic acid, and arachidonic acid [27,28]. Membrane lipid peroxidation affects the structure and functions of the plasma membrane and the membranes of intracytoplasmic organelles so that transmembrane potentials, ion fluxes, and transmembrane transport are disturbed, and membrane receptors are inactivated and signaling pathways are deregulated [71,72].
The process of lipid peroxidation changes not only the lipid components of membranes but also the proteins, following the reaction of some amino acids with the aldehyde products of peroxidation [71]. Oxidative changes in proteins under the action of reactive oxygen species can also cause the inactivation of enzymes and membrane proteins [73], thereby producing structural changes that lead to the destabilization of cell morphology. The products generated because of lipid peroxidation are involved in inflammatory diseases [74], aging [75], hepatotoxicity [76], hemolysis [77], and all phases of carcinogenesis during the appearance of malignant tumors and metastases [78].
The effect of reactive oxygen species on enzymes includes, for the most part, decreased catalytic capacity, often caused by the oxidation of sulfhydryl groups and the modification of amino groups [79,80]. Some free radicals result from normal cellular processes, for example, when cells use oxygen as fuel for energy production, free radicals appear as secondary products of this metabolic process necessary to sustain life [9]. On the other hand, both the environment, in which we live, and the living environment are other main factors causing reactive oxygen species [9,12]. Antioxidants can interrupt the sequence of oxidation reactions before it is initiated. In general, antioxidants have a high reduction potential, releasing hydrogen ions, with the inhibition process proceeding as shown in the following representation (Equation (1)) [81]:
| InH + RO2− → RO2H + In− | (1) |
where InH is an antioxidant, RO−2 is a free hydroperoxide radical ion, RO2H is hydroperoxide of, e.g., a fatty acid, and In− is an inactive or weakly active radical ion.
But in all cases, with the increase in the inactivation duration, there is a decrease in the number of antioxidants—the increase in the peroxide index is found only after there has been a significant decrease in the added antioxidant [2,82,83]. Taking here the α-tocopherol (Vitamin E) as an example of lipid-soluble antioxidant, which acts as a “chain breakerf” to intercept lipid peroxyl radicals (LOO˙) and to terminate the lipid peroxidation chain reactions (Equation (2)) [79], it can be seen that the mechanism of action is much more complex, as the antioxidants can act at successive steps of initiation, propagation, and chain termination of the oxidative radical process [80].
| OO˙ + α-tocopherol–OH → LOOH + α-tocopherol–O˙ | (2) |
It can be explained that there is a close correlation between the structure of antioxidants and their mode of action, determined by factors as follows [84,85]:
The presence of the aromatic nucleus of phenol or naphthol, of a secondary or tertiary hydroxyl group which increases the effectiveness (most antioxidants have a phenolic structure).
The presence of allylic groups in the ortho or para position compared to the hydroxyl groups which have a favorable effect.
The antioxidant effect increases proportionally with the length of the chain.
Alkylation in the meta position is less effective.
The esterification of the hydroxyl groups which causes a total disappearance of the antioxidant activity.
A classification of antioxidants according to their mode of action is presented in Table 1 [83,86].
Table 1.
Inhibitors of lipid oxidation reactions.
| Type | Mode of Action | Examples |
|---|---|---|
| “Metal scavenger” | Chelates metal ions such as copper and iron, forming inactive complexes |
Chelating agents such as EDTA, citric acid, phospholipids, polyphosphates |
| “Oxygen scavenger” | Reacts with oxygen; reduces oxygen | Ascorbic acid, ascorbyl-palmitate |
| Antioxidant (AH) | Interrupts propagation stages in the case of oxidation reactions; donates a hydrogen atom | Phenolic compounds such as BHA, BHT, TBHQ, PG, tocopherols, hydroxytyrosol, caffeic acid, carnosol, etc. |
| Reducing agents (RSH) | Regenerates phenols (synergism) | Ascorbic acid |
| Enzymatic antioxidant | Removes dissolved oxygen or oxidative species | Superoxide dismutase, glutathione peroxidase, glucose-oxidase-catalase |
| Antioxidants with multiple functions | Regenerates primary antioxidants chelated with metals; reduces hydroperoxides | Phospholipids (phosphatidyl-ethanol amine- fish oil), products of the Maillard reaction |
| Methyl-silicone and ethylidene phytosterols | They prevent oxidative polymerization in heated oils |
Polydimethylsiloxane, citrostadienol |
Glutathione peroxidase (GSHPx), catalase (CAT), and superoxide dismutase (SOD) (mentioned in Figure 1) act as the first-line defense antioxidants, as their importance is especially related to superoxide anion radical (*O2) which is perpetually generated in normal body metabolism, particularly through the mitochondrial energy production pathway (MEPP) and their fundamental role in preventing oxidative stress and the cellular damage [31,79,82].
Glutathione is a nonenzymatic antioxidant that is found in most cells, and tissues of plants and animals, and in humans, the highest levels are in the liver, lens, pancreas, spleen, and kidney [87,88]. It is mainly synthesized by the body [88], and it can increase the level of cytotoxic T cells in lymphocytes and neutralize free radicals [89,90]. Given that glutathione has a tripeptide composition of cysteine, glutamate, and glycine, it has an active thiol (SH−) within the cysteine structure [32,34]. In the cell, >98% of glutathione is found in the reduced thiol form (GSH) [31,88], but due to the cysteine residues that can be easily oxidized nonenzymatically by various electrophilic substances (free radicals, reactive oxygen, and nitrogen species), it is also present in the oxidized form as glutathione disulfide (GSSG) or glutathione peroxidase [31,88]. After synthesis, it is distributed to intracellular compartments and the extracellular space for use by other cells and tissues [88,90]. The rate of GSH synthesis is largely controlled by the degree of expression and catalytic activity of the enzyme γ-glutamyl-cysteine synthetase (GCS) and the cellular availability of cysteine [31,82,88,89,90]. Oxidative stress, inflammatory cytokines, cancer, chemotherapy, ionizing radiation, heat shock, inhibition of GCS activity, GSH depletion, GSH conjugation, heavy metals, antioxidants, and insulin increase γ-glutamyl-cysteine synthase transcription or activity in a wide variety of cells [91,92]. In contrast, protein deficiency, dexamethasone, erythropoietin, TNF-β (tumor necrosis factor), hyperglycemia, and GCS phosphorylation decrease GCS transcription or activity [91,93,94]. The glutathione system also represents a “capture system” for peroxides from water metabolism and lipid peroxides permanently formed in the cell, metabolizing them with the formation of water and oxygen [31,88,89,90]. It provides important protection for the mitochondrial and cell membrane against the harmful effects of reactive oxygen species (oxidative stress) [31], protects the tertiary structure of proteins, and activates the transport of amino acids through the cell membrane [31,95]. The cellular level of glutathione is stimulated by alpha lipoic acid, glutamine, colostrum, selenium, and vitamins C, B6, and B2, and the effectiveness of vitamins C, E, and coenzyme Q10 depends on the level of glutathione in the body [96]. Food sources rich in GSH are generally green leafy vegetables, such as spinach, parsley, and broccoli. However, glutathione from food is only partially absorbed, being mostly hydrolyzed by peptidases [96,97,98]. However, the diet plays an important role in the exogenous intake of glutathione by providing important cofactors, such as Se, Mn, Zn, and S-containing amino acids. GSH has a dual role in our health and pathology as an antioxidant and in the detoxification of certain xenobiotics [96,97,98].
Catalases. While GSHPxs are cytosolic residents, catalases are mainly found in peroxisomes, in the liver, and erythrocytes, but some catalases are found in all tissues [82,99], being the first characterized antioxidant enzymes [88] and being one of the crucial antioxidant enzymes that mitigate oxidative stress to a considerable extent by destroying cellular hydrogen peroxide to produce water and oxygen by using either iron or manganese as a cofactor [31,100]. Basically, they are present in almost all living tissues that utilize oxygen [31]. Due to its chemical structure of four subunits, each containing a heme group and a molecule of NADPH, catalase basically catalyzes the conversion of hydrogen peroxide to water and oxygen [82], while superoxide dismutase (one of the most potent intracellular enzymatic antioxidants) catalyzes the conversion of superoxide anions to dioxygen and hydrogen peroxide [82]. Hence, all three, catalase, glutathione peroxidase, and superoxide dismutase, are functionally interconnected due to the hydrogen peroxide (H2O2), which is produced as a result of the reaction catalyzed by SOD, H2O2 being the substrate of both CAT and GSHPx [87]. Deficiency or malfunction of catalase causes aging disorders and pathogenesis of degenerative diseases, such as diabetes mellitus, hypertension, anemia, vitiligo, Alzheimer’s disease, Parkinson’s disease, bipolar disorder, cancer, schizophrenia, or even male infertility [100,101].
Superoxide dismutase. Depending on its expressed activity, superoxide dismutase may act either as an antioxidant or as a prooxidant [88] as exists in several isoforms, differing in the active metal center, amino acid composition, cofactors, and other properties [82], and neutralizes superoxide ions by going through successive oxidative and reductive cycles of transition metal ions at its active site [82]. In humans, three forms of SOD are present: cytosolic Cu, Zn-SOD (consisting of a dinuclear metal cluster with copper and zinc ions, which catalyzes the dismutation of the superoxide anion to oxygen and water), the mitochondrial Mn-SOD (a homotetramer that includes one manganese atom per subunit, which partitions the superoxide anion), and the extracellular superoxide dismutase containing copper and zinc (a tetrameric secretary glycoprotein having a high affinity for certain glycosaminoglycans) [82,102,103]. In contrast with the fact that superoxide dismutase is indispensable to cellular health, that is protecting body cells from oxidative stress, and that helps in the process of aging or cell death, superoxide dismutase enzyme deficiency is quite common, and more than this, levels of superoxide dismutase decline with age, whereas free radical formation increases [31]. As a result, plant sources of SOD and SOD supplementation became of interest for health enhancement [103,104]. It has been reported that a considerable and adequate daily SOD supplementation protects the immune system and significantly reduces the chances of degenerative diseases and aging pathogenesis, and there are several natural resources that can assure the daily intake of SOD, such as cabbage, Brussels sprouts, wheat grass, barley grass, or broccoli [31].
If the first-line antioxidants act to suppress or prevent the formation of free radicals or reactive species in cells being very fast in neutralizing molecules with the potential of developing into a free radical or neutralizing any free radical with the ability to induce the production of other radicals [31], the second-line defense antioxidants (scavenging antioxidants) are neutralizing or scavenging free radicals by donating an electron to them, becoming free radicals themselves but of lesser damaging effects [31,82]. These are mainly represented by hydrophilic antioxidants, such as ascorbic acid, uric acid, and glutathione, and by lipophilic antioxidants, such as alpha-tocopherol (vitamin E) and ubiquinol [31,82].
After free radical damage has occurred, a third category of antioxidants (de novo enzymes), such as polymerases, glycosylases, nucleases, proteinases, proteases, and peptidases, are acting towards repairing the damage caused to biomolecules and reconstitute the damaged cell membrane [31], while a fourth-line defense antioxidants can prevent the formation or reaction of free radicals [31,82].
3. Antioxidants as Food Additives
Food additives that are approved for use in Europe are annotated with an E, which is followed by at least three digits. There are several categories of additives, one being antioxidants. According to the directives of the European Parliament and the Board of Directors 95/2EEC and 98/85/EEC, the antioxidants authorized to be used in food products are those between E-300 and E-321 and include a series of natural compounds (E-300, L-ascorbic acid; E-306, the natural extract rich in tocopherols) but especially synthetic compounds, with toxic potential [103]. Table 2 lists those antioxidants commonly used as food additives using the nomenclature of [6].
Table 2.
Inhibitors of lipid oxidation reactions.
| Annotation | Antioxidant | Annotation | Antioxidant |
|---|---|---|---|
| E300 | Ascorbic acid | E310 | Propyl gallate |
| E301 | Sodium ascorbate | E315 | Erythorbic acid |
| E302 | Calcium ascorbate | E316 | Sodium erythorbate |
| E304 | Fatty acid esters of ascorbic acid | E319 | Tertiary-butyl hydroquinone |
| (TBHQ) | |||
| E306 | Tocopherols | E320 | Butylated hydroxyanisole |
| (BHA) | |||
| E307 | α-tocopherol | E321 | Butylated hydroxytoluene |
| (BHT) | |||
| E308 | γ-tocopherol | E392 | Extracts of rosemary |
| E309 | δ-tocopherol | E586 | 4-Hexylresorcinol |
A wide range of antioxidants can be used to stabilize food products, but their use is limited by health regulations. The conditions that an antioxidant must fulfil to benefit from the legal permission for use in food products are the following [42,105,106,107,108]:
-
➢
The addition of the antioxidant must be authorized by the legislation of the country where the food products will be consumed.
-
➢
The action of the antioxidant must not be limited only to finding it as such and must be limited to food products in which the respective fat was later incorporated as an ingredient.
-
➢
The addition of the antioxidant must be simple, without lengthy or complicated manipulations.
-
➢
The appearance or taste of the respective product must not be modified in any way by the presence of the antioxidant.
-
➢
No negative effect on the human body is allowed even after continuous and prolonged incorporation in the daily food ration.
-
➢
The antioxidant must be effective in very small quantities so that its addition exerts an insignificant influence on the cost price of the respective product.
-
➢
The presence of the antioxidant in fats or other food products must be able to be determined through simple analysis, preferably both quantitatively and qualitatively.
Currently, the focus in industry and research is not only to find new antioxidants to further fulfil these legal conditions but also to replace as many synthetic food additives [109] as possible with ones based on natural antioxidants [110] from vegetable sources, and special attention is given to the ones of marine origin [111] for lipid systems. From the biochemical point of view, it is known that antioxidants can be for lipid systems or for hydrophilic systems [82,87,110], the ones for lipid systems are widely used in the food industry [109], and some examples are given below [86,109].
Butylhydroxyanisole (BHA) is composed of a mixture of two isomers 2 and 3 tributyl-4-hydroxyanisole (C11H16O2). It is a white-yellow crystalline substance insoluble in water, but soluble in ethyl alcohol and other organic solvents [112,113]. It has good resistance to high temperatures, and as a result, it can be used for frying, boiling, and baking products. It is carefully used in lower concentrations of 0.01%–0.02% for its antioxidant effect [112,113]. Despite its favorable properties, rational use of BHA must be considered [114], as at higher concentrations, it can cause carcinogenicity, cytotoxicity, oxidative stress induction, endocrine disruption [114,115], and important side effects of tert-butylhydroxyanisole, such as reducing hepatic enzymes or toxic effects in lung tissue [116].
Butylhydroxytoluene (C15H24O), also known as BHT, is presented in the form of white crystals or sequins, with a weak phenolic smell. It is insoluble in water, but soluble in alcohol [114]. A dose-related increased incidence of the severity of toxic nephrosis, nephrotoxicity, and pneumotoxicity, and marked congestion of the liver and kidney [114], as well as diffuse enlargement of the liver with rounded borders and rupture with hemorrhaging, were cited as toxic effects of BHT in mice [117,118] or in rats [119,120]. Fourteen metabolites or degradation products of BHT were at an increased level of concern about their toxic effects (BHT-CH2OH, BHT-CHO, BHT-COOH, BHT-Q, BHT-QM, DBP, BHT-OH, BHT-OOH, TBP, BHQ, BHT-OH(t), BHT-OH(t)QM, 2-BHT, and 2-BHT-QM), with reviewed effect on in vitro DNA cleavage for BHT-Q at the lowest concentration [121]; BHT-CHO and BHT-OOH were also cited with such ability to cause DNA cleavage, but not for BHT, BHT-CH2OH, BHT-COOH, and BHT-QM [121]. Mice-fed dietary BHT for a year were known to develop marked hyperplasia of the hepatic bile ducts with associated subacute cholangitis [117,118]. Dose-related increases in hepatocellular adenomas and carcinomas were also cited in the case of rats [119,120,121].
Gallic acid esters are widely used as antioxidants [122,123,124,125], being derivatives of a naturally occurring low-molecular-weight triphenolic compound, the gallic acid (3,4,5-trihydroxy-benzoic acid) has a strong antioxidant and an efficient apoptosis-inducing agent effects [125]. Some reports on their contact-sensitizing ability (using topically applied products) correlated with the side chain length were documented, while a maximum of sensitization occurrence was tested for 12 carbon atoms length of the molecule (dodecyl gallate) [126,127]. Currently, the use of long-chain gallic acid esters, such as octal and dodecyl gallates, is preferred [122,123,124,125,126,127], which have a much better distribution coefficient and are more effective for the protection of fat/water systems.
Tocopherols are widely distributed in nature, having the role of natural antioxidants, and being represented by vitamin E. Among the isomers of tocopherol, δ-tocopherol has the greatest effectiveness as an antioxidant [128,129]. It is presented in the form of viscous, yellowish oil [128]. Δ-tocopherol was reported as more active than α- or γ-tocopherol in inhibiting tumor growth, possibly through trapping reactive oxygen and nitrogen species and inducing apoptosis [130,131].
Ascorbyl palmitate is the ester of palmitic acid with ascorbic acid and is obtained by the synthesis of two components that are naturally found in food [132]. Concentrations of 0.01% ascorbyl palmitate [133] were reported to provide a useful increase in the shelf-life of vegetable oils [132], with a better action in this regard when compared to butylated hydroxytoluene and butylated hydroxyanisole [134]. Moreover, combinations with other known antioxidants were shown to improve the shelf-life of all vegetable oils, as well as potato chips [135,136].
Among the antioxidants for hydrophilic systems, especially for use in wines, juices, and fruit derivatives, two have found a wide application so far: SO2 and ascorbic acid [137]. The sulfur dioxide exerts a double effect as it inhibits oxidizing enzymes and at the same time has a strong reducing action [138]. It has been established that the oxidizing enzymes, polyphenoloxidases, peroxidases, and ascorbinoxidases are inhibited in the case of using large doses of sulfur dioxide, acting on the prosthetic groups found in the enzymes [138]. Sulfur dioxide has a bleaching effect, a process that is, however, irreversible. In human consumers, allergies caused by sulfites (SO2-derived compounds) were documented, including symptoms of their expression, such as headaches, nausea, gastric irritation, and breathing difficulties in asthma patients [139].
Ascorbic acid, due to its reducing properties, achieves the inhibition of oxidation processes in concentrations of 100–200 mg/L. It is used at lower concentrations than sulfur dioxide to avoid toxicity, since at higher concentrations the ascorbic acid was cited to act as an antioxidant, while at lower concentrations, as a pro-oxidant [140,141]. Although the combined use of ascorbic acid and sulfur dioxide was initially assumed as with many advantages over the use of either compound alone, later reviewed studies suggested that ascorbic acid may not be the ideal complement to sulfur dioxide as first considered [142].
Along with antioxidants, in the food industry, synergistic substances are also used, which do not have antioxidant action, but promote it, including citric acid, phosphoric acid, and ethylenediaminetetraacetic acid (EDTA) [143,144,145].
There are also those known as antioxidant combinations meant to ensure an optimal effect, in which mixtures of two or more antioxidants are used, associated with synergists, ensuring optimal product stability [143,144,145]. Thus, for animal fats, a solution containing 20% BHA, 6% propellant gallate, and 4% citric acid is used. French fries and fries with a high fat or oil content can be very well protected by a mixture of BHA, BHT, propellant gallate, and citric acid. In the case of meat dishes, the mixture of BHA and citric acid works best. Antioxidants can also be used to extend the shelf life of frozen fish, preventing the appearance of yellow-brown color due to the oxidation and polymerization of fats [110].
As industry tends to shift from synthetically produced preservatives to natural preservatives, more and more studies are established in this direction, the interest being to lower the unnecessary chemical burden on health and to naturally prevent food degradation. There are reports showing that natural antioxidants from fruits (grapes, pomegranate, date, kinnow, plums, avocado, and tomato), herbs and spices (tea, rosemary, oregano, cinnamon, sage, thyme, mint, ginger, and clove), vegetables extract from broccoli, potatoes, drumstick, pumpkin, curry, and nettle are used as additional additives or antioxidants in food preservation [146,147].
Due to their high content of phenolic compounds, they provide alternatives to currently used conventional antioxidants, prolonging the shelf life of foods [146,147]. For example, in the meat industry, these natural antioxidant extracts are used for improving the quality of fresh and processed meat and meat products by decreasing lipid oxidation [146,148,149,150,151]. Other examples are the use of terpenoids and polyphenols in the prevention of lipid oxidation of meat, fish, or vegetable food with different amounts of saturated/polyunsaturated fat for ensuring high sensorial quality and food preservation [107,152]. Moreover, it was shown that powdered leaves of matcha green tea and moringa added to white chocolate during the tempering process improved its antioxidant capacity due to the high amounts of polyphenols in green tea [146,153]. Another recent study showed that lemongrass essential oil has a higher applicability as a food preservative due to its content in terpenes [154]. From natural marine algae, seaweeds are known to have several properties to act as natural preservatives extending the shelf life of perishable foods and at the same time not affecting their quality or causing side effects. An applicable example is the seaweed gel coating used to protect tomatoes from perishing [155].
On the other hand, active food packaging containing natural antioxidants, such as α-tocopherol, caffeic acid, catechin, quercetin, carvacrol, and plant extracts (e.g., rosemary extract), has been implemented in recent years as there is more advantage than the addition of antioxidants directly to the food [156]. One important interest is in active biodegradable/compostable packaging and edible films to reduce environmental impact, minimize food loss and contaminants from industrial production, and reutilize by-products [156], active packaging being biocompatible and eco-friendly [157], having also other properties, such as antimicrobial, antioxidant, UV blocking, oxygen scavenging, and water vapor permeability effects [158].
4. Natural Antioxidants and Their Benefits
In addition to established antioxidants, which fall into the category of additives, there are natural antioxidants that can be obtained through extraction from different plants [86]. Extracts from rosemary, aloe vera, fenugreek (Trigonella foenum), ginseng, mustard, sage, oregano, horseradish, hyssop, basil, marjoram, mint, thyme, ginger, cumin, cloves, nutmeg, curry, cinnamon, black pepper, green tea, coffee, grape skin and seeds, and pine bark have been used with various food groups [86], giving equivalent or better results than those obtained with synthetic antioxidants (BHA, BHT, and gallates). However, the use of these extracts is limited by the intensity of their flavor which influences the taste and smell of the products to which they are added. Essential antioxidants include vitamins A, C, E, and beta-carotene, which are helpful in slowing down the aging process and protecting the body from cancer, heart disease, and pollution. They also help strengthen the immune system and increase the body’s resistance to infections. Every year, more and more antioxidants are discovered [110,111,159]. Among them, there are substances from forest fruits, grapes, tomatoes, broccoli, and mustard, as well as those from medicinal plants, such as turmeric and Ginkgo Biloba but also algae extract. In addition to the already well-known antioxidant role of vitamins A, C, and E, there are other important antioxidants, including lipoic acid, carnitine, lutein, and lycopene [160,161,162], and currently, there is still a high interest in discovering novel antioxidants from fruits and vegetables as they are a main source of antioxidants, known by their antioxidant expression values.
One way of expressing the antioxidant values of food products is in ORAC units (the oxygen radical absorption capacity expressed in units), a unit of measure approved by the National Institute for the Elderly within the National Institute of Health (INS) [163]. An ORAC unit is expressed in micromoles of Trolox equivalents (TE) per 100 g of sample. The ORAC scale presents in ascending order the antioxidant values that foods have up to the present time. Recently, an evaluation of antioxidant capacity (ABTS and CUPRAC) and total phenolic content (Folin–Ciocalteu) assays of selected fruits, vegetables, and spices has also been established [164,165]. While the ORAC value is a method of measuring the antioxidant potential of different food products and supplements [163], these recent scales highlight the common foods that have increased antioxidant effects.
One ORAC unit destroys free radicals in addition to the fact that our body naturally destroys free radicals only through its production of antioxidant enzymes. FDA’s recommendation of ORAC units per day administration is the amount between 3000 and 5000 µmol TE per day (ORAC) from plant foods, especially in the presence of constant pro-oxidant factors [166] but has been shown that ≥10,000 µmol TE per day (ORAC) has positive health effects [163]. This amount is necessary for the prevention of various diseases and to maintain health. Moreover, in the situations of people with diseases, such as degenerative diseases, acute diseases, cancer, lymphomas, leukemias, and AIDS, a higher intake of ORAC units/day is recommended [163,166].
In order to inform about the total antioxidant content of foods, there is an available database that comprises antioxidant food content [167], based on several assays, such as 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox-equivalent antioxidant capacity (TEAC)) assay, the ferric-reducing ability of plasma (FRAP), and the oxygen radical absorbance capacity assay (ORAC) [168].
4.1. Food and Fruits Antioxidants
Various studies on nutritional issues have revealed that a diet rich in fruits and vegetables is important due to their sources of nutrients and nonnutritive food constituents, showing that a high daily intake of fruits and vegetables promotes health [169]. In the meantime, it is known that low fruit and vegetable consumption is linked with an increased risk of death from vascular disease and cancer; these benefits are attributed in part to antioxidants, vitamins, and phytochemicals [170]. Although phytotherapeutic substances and antioxidants exist in their purest form in fruits, vegetables, and cereals, a balance of the daily calories’ intake must be considered as well, as to metabolize calories, the body burns oxygen, generating free radicals [12,171,172]; hence, the more calories are consumed, more free radicals are generated. A balance in the antioxidant uptake, as well as certain pathological conditions such as cancer, must be considered since there are studies showing risks of an imbalanced antioxidant uptake for cancer patients, since both excess and lack of antioxidants can affect and negatively influence the normal cellular processes [173,174,175].
There are over 2000 phytotherapeutic substances contained in food, and these are defined as natural compounds that act as a plant defense system [176]. Various types of food are rich in antioxidants and phytotherapeutic substances such as:
Tomatoes—One medium tomato contains 26 calories and 0 g of fat. Biochemically speaking, tomatoes are rich in lycopene and are a great source of vitamin C [176,177].
Spinach—100 g of spinach contains 41 calories and 0 g of fat and is rich in iron, folic acid, and B vitamins [178]. In addition, spinach contains two phytotherapeutic substances, lutein and zeaxanthin, which are very important for eye health [176,178]. It is recommended to be eaten, as much as possible, raw or scalded [178].
Nuts—30 g of nuts contain 12 g of fat and 150 calories. Although they are high in fat, walnuts contain good monosaturated and polyunsaturated fats [179]. When eaten in place of red meat or high-fat foods, walnuts help lower bad cholesterol (LDL) and increase good cholesterol (HDL) [176,179]. Nuts also contain a well-known antioxidant, vitamin E. However, they contain higher calories, and therefore, their consumption is indicated in limited quantities [176,179].
Broccoli—100 g of cooked broccoli contains 44 calories and 0 g of fat. Broccoli is an important source of vitamin C, fiber, and calcium [180,181]. This vegetable also provides phytotherapeutic substances used as natural antibiotics, antiviral drugs, and antimycotics, contributing to protection from DNA damage and preventing the formation of cancer cells [176,180,181]. The deep thermal processing of this vegetable destroys the enzymes and the corresponding nutrients and therefore it is recommended to consume it fresh or just scalded [176].
Blueberries—100 g of blueberries contain 81 calories and 0 g of fat and represents a significant source of antioxidants [176,182]. Anthocyanin is the phytotherapeutic substance that gives the dark blue color, specific to blueberries, which prevents the formation of cancer cells and has a curative effect on urinary infections [182]. A regular, moderate intake of blueberries and/or anthocyanins was associated with a reduced risk of cardiovascular disease, and type 2 diabetes, and with improved weight maintenance and neuroprotection [182,183].
Green tea—In Asia, green tea is consumed in the same amount as coffee in the Western world [176]. Studies correlate the consumption of green tea with the low incidence of stomach, esophagus, and liver cancers [176,184,185,186,187]. Polyphenol, the phytotherapeutic substance in green tea, has been identified as an anticancer agent [184,185,186,187].
Adding to the above examples, Table 3 also displays other foods containing important antioxidants [168,188,189,190].
Table 3.
Antioxidants in foods.
| Anthocyanins | β-Carotene | Catechins | Cryptoxanthines | Copper | Flavonoids | Indoles | Isoflavonoides | Lignans | Lutein | Lycopene | Manganese | Polyphenols | Selenium | Sulphur | Vit. A | Vit. C | Vit. E | Zinc | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acai berries | ✔ | ||||||||||||||||||
| Almonds | ✔ | ||||||||||||||||||
| Apricots | ✔ | ||||||||||||||||||
| Artichokes | ✔ | ||||||||||||||||||
| Avocados | ✔ | ✔ | |||||||||||||||||
| Barley | ✔ | ||||||||||||||||||
| Black currants | ✔ | ||||||||||||||||||
| Black rice | ✔ | ||||||||||||||||||
| Blackberries | ✔ | ||||||||||||||||||
| Blueberries | ✔ | ||||||||||||||||||
| Brazil nuts | ✔ | ||||||||||||||||||
| Broccoli | ✔ | ✔ | |||||||||||||||||
| Brown rice | ✔ | ||||||||||||||||||
| Brussels sprouts | ✔ | ||||||||||||||||||
| Butter | ✔ | ||||||||||||||||||
| Cabbage | ✔ | ||||||||||||||||||
| Carrots | ✔ | ✔ | |||||||||||||||||
| Cauliflower | ✔ | ✔ | |||||||||||||||||
| Cherries | ✔ | ||||||||||||||||||
| Chicken | ✔ | ||||||||||||||||||
| Chickpeas | ✔ | ||||||||||||||||||
| Chives | ✔ | ||||||||||||||||||
| Citrus | ✔ | ✔ | ✔ | ||||||||||||||||
| Cocoa | ✔ | ||||||||||||||||||
| Coffee | ✔ | ||||||||||||||||||
| Cottage cheese | ✔ | ||||||||||||||||||
| Dark chocolate | ✔ | ||||||||||||||||||
| Egg yolk | ✔ | ✔ | |||||||||||||||||
| Eggplant | ✔ | ||||||||||||||||||
| Eggs | ✔ | ||||||||||||||||||
| Extra virgin olive oil | ✔ | ||||||||||||||||||
| Flaxseed | ✔ | ||||||||||||||||||
| Garlic | ✔ | ||||||||||||||||||
| Grapefruit | ✔ | ||||||||||||||||||
| Grapes | ✔ | ||||||||||||||||||
| Green tea | ✔ | ||||||||||||||||||
| Kale | ✔ | ✔ | ✔ | ||||||||||||||||
| Kiwi | ✔ | ||||||||||||||||||
| Leeks | ✔ | ||||||||||||||||||
| Lentils | ✔ | ||||||||||||||||||
| Liver | ✔ | ✔ | |||||||||||||||||
| Lobster | ✔ | ||||||||||||||||||
| Mangoes | ✔ | ✔ | |||||||||||||||||
| Mushrooms | ✔ | ||||||||||||||||||
| Mustard seed | ✔ | ||||||||||||||||||
| Oatmeal | ✔ | ||||||||||||||||||
| Onions | ✔ | ✔ | |||||||||||||||||
| Oregano | ✔ | ||||||||||||||||||
| Oysters | ✔ | ||||||||||||||||||
| Papaya | ✔ | ✔ | |||||||||||||||||
| Parsley | ✔ | ✔ | ✔ | ||||||||||||||||
| Peanuts | ✔ | ||||||||||||||||||
| Peas | ✔ | ||||||||||||||||||
| Pecans | ✔ | ||||||||||||||||||
| Pineapples | ✔ | ||||||||||||||||||
| Pinto beans | ✔ | ||||||||||||||||||
| Pistachios | ✔ | ||||||||||||||||||
| Pork | ✔ | ✔ | |||||||||||||||||
| Pumpkins | ✔ | ||||||||||||||||||
| Raspberries | ✔ | ||||||||||||||||||
| Red bell peppers | ✔ | ||||||||||||||||||
| Red grapes | ✔ | ||||||||||||||||||
| Rye | ✔ | ||||||||||||||||||
| Sardines | ✔ | ||||||||||||||||||
| Sesame seeds | ✔ | ||||||||||||||||||
| Shiitake mushrooms | ✔ | ✔ | |||||||||||||||||
| Shrimp | ✔ | ||||||||||||||||||
| Soybeans * | ✔ | ||||||||||||||||||
| Spinach | ✔ | ✔ | |||||||||||||||||
| Spirulina | ✔ | ||||||||||||||||||
| Squash | ✔ | ||||||||||||||||||
| Strawberries | ✔ | ||||||||||||||||||
| Sweet potatoes | ✔ | ✔ | |||||||||||||||||
| Tea | ✔ | ||||||||||||||||||
| Thyme | ✔ | ||||||||||||||||||
| Tomatoes | ✔ | ||||||||||||||||||
| Turnips | ✔ | ||||||||||||||||||
| Watermelon | ✔ | ||||||||||||||||||
| Whole wheat bread | ✔ | ||||||||||||||||||
| Wine | ✔ |
* Soybeans include Tofu, Edamame, and Tempeh. ✔ indicates the presence of the compound.
Anthocyanins are part of the phenolic group with the pigments (red, purple, and blue) in glycosylated forms and are found in black rice, blackberries, blueberries, eggplant, grapes, and raspberry [191,192] (Table 3) with the most abundant pigment in plants, the cyanidin-3-glucoside. Apart from being used as a natural food colorant, anthocyanins are considered pharmaceutical ingredients that give various beneficial health effects, being reported as having antidiabetic, anticancer, anti-inflammatory, antimicrobial, and antiobesity effects [192,193,194].
Beta-carotene is part of the carotenoid family. It reaches our body partially transformed into vitamin A, found mainly in colorful vegetables and fruits [195,196] (Table 3). Β-carotene administered orally was reported to be metabolized in the animal or human body to form vitamin A, which is subsequently stored in the liver [195,196].
Catechins are flavanols found in wine, green tea, and cocoa [197,198], which have potent antioxidant properties, in the meantime being known as reactive oxygen species (ROS) scavengers and metal ion chelators. They have indirect antioxidant activities comprising induction of antioxidant enzymes, inhibition of pro-oxidant enzymes, production of detoxification enzymes and antioxidant enzymes. For these reasons, they are being considered beneficial in preventing and protecting against diseases caused by oxidative stress [197,198].
Cryptoxanthines are found in butter, citrus, egg yolk, papaya, red bell peppers, and pumpkins [199,200], having relatively high bioavailability from these natural resources [199]. Among other carotenoids, β-cryptoxanthin has high antioxidant activity and promotes free radical scavenging, protecting against chronic diseases [200].
Copper is found in dark chocolate, liver, lobster, oysters, spirulina, and shiitake mushrooms [201,202], and it was shown that dietary copper could improve antioxidant capacity and immune state [202].
Flavonoids are abundant in broccoli, citrus, kale, parsley, onions, strawberries, and tea [203,204,205,206], and their antioxidant capacity has been intensely proven in the last years [203,204,205,206]. They are a large group of diverse polyphenolic compounds of plant origin classified into major classes, including flavonols, flavones, flavanones, flavanols, anthocyanidins, isoflavones, and chalcones [206], and nowadays are considered an indispensable component in a variety of medicinal, pharmaceutical, nutraceutical, and cosmetic preparations [203,204,205,206].
Indoles are found in broccoli, cabbage, cauliflower, mustard seeds, and turnips [207,208]. They are known for their chemopreventive effects on hormone-dependent cancers and inhibit proliferation, migration, and invasion of cancer cells in vitro studies [207,208].
Isoflavonoids can be found in chickpeas, peanuts, pistachio, and soybeans [209,210], and they are very well known for their therapeutic properties, having anti-inflammatory, estrogenic, antiestrogenic, anticancer, antibiotic, and radical scavenging activities [209] and being involved as well in the prevention of cancer [210].
Lignans, in barley, flaxseed, rye, and sesame seeds [211,212], are used as pharmacological agents in disorders related to oxidative stress and inflammation [211], as they possess a strong anti-inflammatory and antioxidant capacity [211,212].
Lutein is one of the few xanthophyll carotenoids that is found in high concentration in the macula of the human retina, and as it cannot be de novo synthesis within the human body, lutein must be obtained from natural food, such as kale, and parsley, peas, spinach, and tomatoes [213,214].
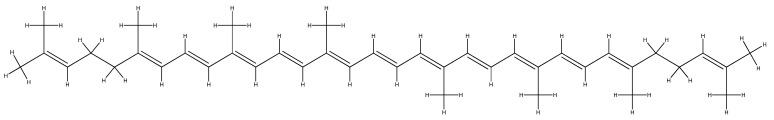
Lycopene is a carotenoid contained especially in tomatoes, with antioxidant and detoxifying properties. It helps the proper functioning of the cell growth process and has a beneficial influence on the skin and mucous membranes [215,216]. Studies show that lycopene inhibits the development of esophageal, stomach, colon, breast, and prostate cancer cells [215,216]. The antioxidant action of carotenoids includes their ability to capture singlet oxygen, an action possible due to their chemical structure (Figure 6); lycopene, for example, is the most effective of carotenoids in capturing the reactive oxygen species [215,216].
Figure 6.
The molecular structure of lycopene.
Manganese is found in acai berries, almonds, brown rice, pecans, pineapples, pinto beans, and whole wheat bread [217,218]. Due to its component integration for Mn superoxide dismutase (MnSOD), it is mainly responsible for scavenging reactive oxygen species (ROS) in mitochondrial oxidative stress [217]. Avoiding its deficiency and intoxication is essential as otherwise, the imbalance can lead to associated adverse metabolic and neuropsychiatric effects [217,218].
Polyphenols stimulate the body’s natural defense capacity and protect human cells from oxidative stress [219,220]. They prevent skin aging and degenerative diseases, such as cardiovascular diseases, and osteoporosis [219] and have anticarcinogenic properties by suppressing tumor formation and progression [221,222,223]. Polyphenols, such as quercetin, resveratrol, and curcumin, are more potent antioxidants than vitamins C and E, having a faster antioxidant action [224,225], and enhancing the therapeutic profiles [226]. Polyphenols are represented by several thousand plant-based molecules with antioxidant properties [227], classified into flavonoids (i.e., anthocyanins, flavanols, flavanones, flavonols, flavonones, flavones, and isoflavones) and non-flavonoids (i.e., phenolic acids, xanthones, stilbens, lignans, and tannins), and known for their high potential application in food preservation and for therapeutic beneficial use [227].
Selenium is the main mineral antioxidant and deactivator of free radicals, and it is effective in preventing degenerative diseases, including cancer and cardiovascular diseases [228,229]. As an antioxidant, selenium acts by intervening in the activation of glutathione peroxidase [230], an enzyme with a role in the detoxification of lipid and organic peroxides in the cell, thus preventing alterations caused by peroxidation of cellular macromolecules [230]. Another action of selenium is influencing the activity of DNA polymerase and nucleotide kinases or inducing the synthesis of selenoproteins [231,232]. Depending on the dosage and chemical form of selenium and the nature of the carcinogenic stress, selenium is proposed as an anticarcinogenic agent, owing, among other factors, to reversible or irreversible inhibition of protein and DNA synthesis [230]. Selenium is an essential mineral for both humans and animals, found in all types of soil around the world [228,229]. Plants and small animal organisms convert selenium found in the soil into organic components, such as seleno-methionine or chelated selenium, the form in which selenium is found in food [233,234]. It binds to proteins and is absorbed into the body in its original form without undergoing metabolic changes [233,234]. The human body needs this mineral in a small amount, as a too high level can have toxic effects on the body, so the recommended daily allowance of selenium is 55 mcg/day for women and 70 mcg/day for men, and the tolerable upper limit is 400 mcg/day, while the deficiency is defined as less than 30 mcg/day [235]. Selenium deficiency has been reported quite rarely, and the need for selenium supplements is not necessary and is available only by prescription in some cases [233,234].
Sulphur is a structural component in chives, garlic, leeks, and onions [236,237]. It is an important element in biological systems as being integrated into proteins as the redox-active cysteine residue or in vital antioxidant molecules, such as glutathione, thioredoxin, and glutaredoxin [236,237].
Vitamin A or all-trans-retinal, which accumulates in the retina because of the absorption of light by visual pigments, is found in carrots, egg yolk, liver, and sweet potatoes [238,239]. Being a liposoluble vitamin, a balance in its intake is to be considered [239].
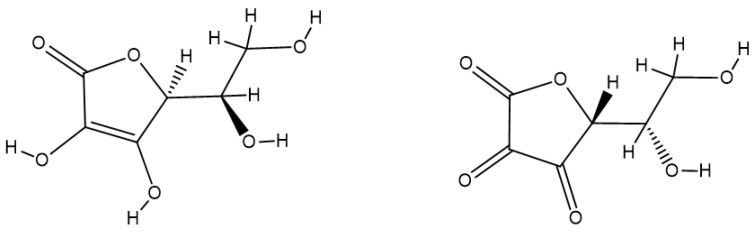
Vitamin C or L-ascorbic acid is essential in maintaining health as it prevents and fights infections, promotes wound healing, and prevents stress and fatigue [224,225]. Vitamin C is also involved in skin health and protection, preventing the appearance of wrinkles as well [240]. Vitamin C is a water-soluble vitamin and is also considered one of the most important antioxidants, acting at the level of extracellular fluids [224,225]. Due to its properties, its lack in the body leads to a drastic decrease in the immune system in the fight against infections [241]. In contrast to most animals, the human body cannot synthesize vitamin C, because of a mutation in the last enzyme required for ascorbate biosynthesis [58,242]. Thus, vitamin C must be obtained from our daily diet [241]. The oxidized form of ascorbic acid is represented by dehydroascorbic acid (Figure 7), and the regeneration of ascorbate from this oxidized form is necessary to maintain sufficient tissue levels of the reduced form of vitamin C [243].
Figure 7.
The molecular structure of ascorbic acid (left) and dehydroascorbic acid (right).
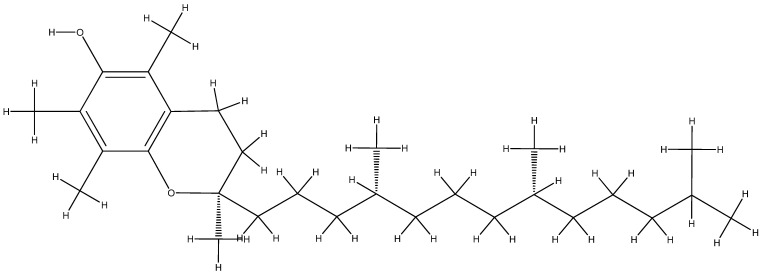
Vitamin E is one of the best natural antiaging remedies, comprising eight forms of tocopherols and tocotrienols [224,225]. Its action is particularly related to the genital sphere, having an important role in the fecundity mechanism [244], stimulating cell regeneration, protecting cells and tissues from the action of free radicals, and having a therapeutic role in cancer [245]. Depending on the number and position of the methyl groups attached to the chromanol ring, they are named by joining the Latin letters α, β, γ, and δ; α-tocopherol (Figure 8) and γ-tocopherol being the most important forms of vitamin E [246] are found in vegetable oils, sunflower seeds, vegetables, and fruits [240]. Soybean oil contains a mixture of γ, δ, and α-tocopherol, with a cited tocopherol content in corn oil and soybean oil of 77% and 70% γ-tocopherol, 2% and 23% δ-tocopherol, and 14% and 7% of α-tocopherol, respectively [247].
Figure 8.
The molecular structure of vitamin E.
Zinc is an important mineral as the human body cannot store zinc reserves, so this needs to be added to the diet, especially because zinc is influencing the immune system, transcription factors, cell differentiation and proliferation, DNA and RNA synthesis and repair, enzyme activation or inhibition, the regulation of cellular signaling, and the stabilization of the cell structure and membranes [248,249]. Avocados, chicken, oatmeal, pork, and Shiitake mushrooms present higher amounts of zinc [248,249].
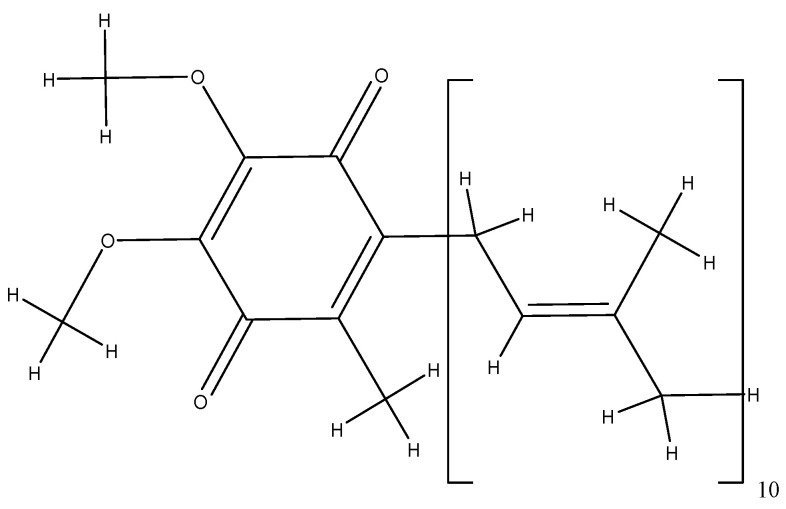
Coenzyme Q10 is a fat-soluble antioxidant that ensures the transport of oxygen inside the cells and the production of energy becoming amphiphilic following electron and proton interactions [250]. Q10 represents its molecular structure, made up of 10 isoprene units around the quinone ring, and the number of isoprene units in the side chain varies with the species (Figure 9) [250,251]. Coenzyme Q10 is a natural compound, which is produced in the body following the consumption of fish, sardines, nuts, green vegetables, soybeans, and oilseeds [252]. It is a good ally against skin aging [253], and it is effective in preventing and treating cardiovascular diseases, especially heart rhythm disorders [254,255], and considerably reduces the risk of breast, uterine, lung, and colon cancers [256,257,258,259,260]. In addition to the antioxidant function, coenzyme Q10 has the capacity to donate electrons, contributing to the production of bioenergy by oxidizing glucose in the mitochondria, where the free radicals are produced during the process of cellular respiration [89,90,91,92,261].
Figure 9.
The molecular structure of coenzyme Q10.
The plant-based diet has become the equivalent of many curative and prophylactic treatments [48,109,262,263]. The importance of the consumption of vegetables and fruits is explained by the beneficial intake of antioxidants as they are being active in numerous biological processes of the body at the cellular level [109,264]. Moreover, most studies [265,266,267] indicate that animal foods should be substantially reduced and replaced with fresh, minimally processed plant foods to reduce the prevalence of cancer.
4.2. Marine Algae Antioxidants
Considerable attention must be paid to the algae antioxidants as many recent studies showed their high beneficial potential and importance (Figure 10 and Figure 11) [268,269].
A classification of marine macroalgae is known as brown algae (Phaeophyceae), red algae (Phylum Rhodophyta), and green algae (Phylum Chlorophyta), according to their pigmentation [270,271,272]. Representative species of brown, red, and green algae with higher antioxidant activity are presented in Figure 10. Marine antioxidants from seaweeds have a very high antioxidant potency, currently being known as the mechanisms of antioxidative action for at least 301 macroalgal metabolites [270,273].
As shown in Figure 10, the trend of antioxidant potential is going from the highest antioxidant activity of brown algae extract followed by red and green algae extract as shown in several studies [270,274,275]. Their high antioxidant activity is probably due to the synergetic coexistence of polyphenols and alkaloids [276,277].
Figure 10.
Antioxidant activity of marine algae and representative species, with higher antioxidant activity range from brown algae to green algae. This figure is based on information from references [270,274,275,278].
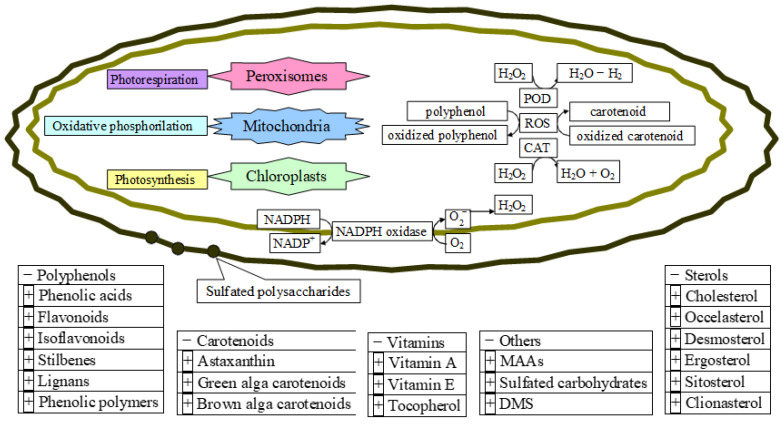
Figure 11.
The microalgae antioxidants and their mechanism of action at the cellular level. POD, peroxidase; CAT, catalase.
The future of marine algae extracts providing resources as food antioxidants additives is very promising since they are containing compounds from polyphenols to carotenoids, sterols, vitamins, and several others from at least 50,000 species of known marine microalgal classes [273,279,280,281] (Figure 11). Moreover, the favorable biological activities of microalgae are due to their intrinsic antioxidant, anti-inflammatory, and antitumoral features [279,282].
Their intrinsic mechanism of taking up H2O and CO2 combined with the sunlight, and converting them to complex organic compounds, makes them subsequently kept inside or released from the cell. Figure 11 represents the biological background of antioxidant formation in microalgae as a response to oxidative stress, at the cellular level, listing the main antioxidant compounds found in microalgae [270,273,279,280,281,282,283,284,285,286,287].
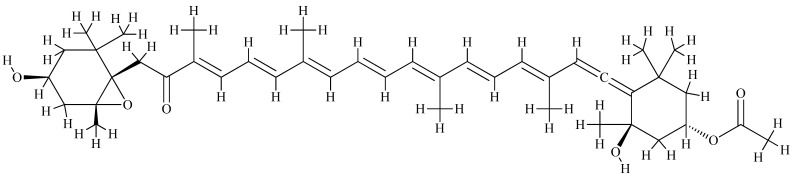
Fucoxanthin, for example, has a particularly interesting and unique molecular structure, exhibiting antioxidant properties due to a long-conjugated backbone characteristic of all carotenoids (Figure 12) [288], but possessing an unusual terminal allenic bond and conjugated carbonyl groups [289]. Fucoxanthin is a member of the xanthophyll class of carotenoids and is present at high concentrations in the brown algae Saccharina sp. [288,289,290,291], where it plays a key role in light harvesting and radiation protection [291].
Figure 12.
The molecular structure of fucoxanthin (cis).
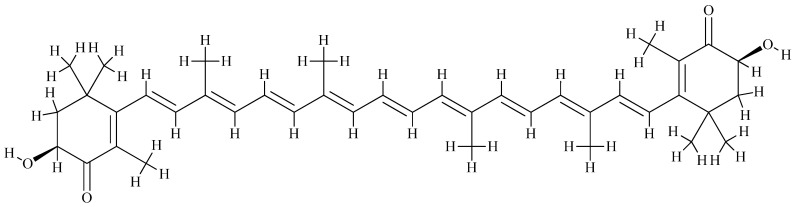
Astaxanthin (Figure 13) is another fat-soluble xanthophyll carotenoid with a red pigmentation, which is found in various microorganisms and marine animals and algae [292,293] such as Haematococcus pluvialis, a green microalga, which accumulates high astaxanthin amounts under stress conditions of high salinity, nitrogen deficiency, high temperature, and light [294,295,296]. In a few years, it has been approved to be used as a food colorant in animal and fish feed such as salmon, trout, and shrimp [292,297] and used as a nutritional supplement and has been rapidly growing in foods, feeds, nutraceuticals, and pharmaceuticals [298,299].
Figure 13.
The molecular structure of astaxanthin (trans).
Other potential resources of marine macroalgae components known to exhibit significant antioxidant activities are marine secondary metabolites such as bromophenols (containing one or several phenols with one or more bromine atoms), present in all three algae types, red, brown, and green algae, phlorotannins [273,300], terpenoids, and meroterpenoids [301], which are to be further investigated.
5. Conclusions
From endogenous to exogenous antioxidants mentioned in this review, we have outlined the special importance of the presence of antioxidants in food, fruits, and their consumption, as well as their use as food additives.
Given that over years, some food additives proved to have carcinogenic effects or to cause other health issues, exploring the use of alternative antioxidants in the food industry became a high priority.
Understanding the biochemical defense mechanisms against free radicals and the mechanisms of antioxidant activity, altogether with the intracellular antioxidant balance, is complex as has been illustrated in this review.
Marine resources of possible new food antioxidants are of much current interest due to their high biodiversity as well as their ability to adapt and colonize very different types of aquatic ecosystems. Further exploration of their properties via testing the antioxidant activity in vitro, and in vivo, including economic and environmental concerns and possible negative side effects such as toxicological issues is hence warranted.
Author Contributions
Conceptualization, L.L.P.; methodology, L.L.P.; validation, D.S.B.; formal analysis, L.J.; investigation, L.L.P.; resources, L.J.; data curation, D.S.B.; writing—original draft preparation, L.L.P. and A.C.G.; writing—review and editing, L.J.; visualization, L.J.; supervision, D.S.B. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The data is contained within the article.
Conflicts of Interest
L.L.P., L.J. and A.C.G. declare no conflict of interest. D.S.B. is the CEO and share owner at IOTA Pharmaceuticals. The funders had no role in the design of the study.
Funding Statement
L.L.P was partially funded by PN-III-P2-2.1-PTE-2021—Development of toxicogenomics applications for screening and risk assessment when exposed to contaminants (arsenic, lead, and cadmium) in the environmental media—PROMETHEuS (107PTE/2022).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Benzie I.F.F., Choi S.W. Antioxidants in food: Content measurement, significance, action, cautions, caveats, and research needs (Chapter 1) Adv. Food Nutr. 2014;71:1–53. doi: 10.1016/B978-0-12-800270-4.00001-8. [DOI] [PubMed] [Google Scholar]
- 2.Lü J.M., Lin P.H., Yao Q., Chen C. Chemical and Molecular Mechanisms of Antioxidants: Experimental Approaches and Model Systems. J. Cell. Mol. Med. 2010;14:840–860. doi: 10.1111/j.1582-4934.2009.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halliwell B. Reactive oxygen species (ROS), oxygen radicals and antioxidants: Where are we now, where is the field going and where should we go? Biochem. Biophys. Res. Commun. 2022;633:17–19. doi: 10.1016/j.bbrc.2022.08.098. [DOI] [PubMed] [Google Scholar]
- 4.Larson R.A., McCord J.M. Environmental chemistry of reactive oxygen species. CRC Crit. Rev. Env. Control. 1977;8:197–246. doi: 10.1080/10643387709381663. [DOI] [Google Scholar]
- 5.Herlihy D., Waegele M., Chen X., Pemmaraju C.D., Prendergast D., Cuk T. Detecting the oxyl radical of photocatalytic water oxidation at an n-SrTiO3/aqueous interface through its subsurface vibration. Nat. Chem. 2016;8:549–555. doi: 10.1038/nchem.2497. [DOI] [PubMed] [Google Scholar]
- 6.Stone W.L., Pham T., Mohiuddin S.S. StatPearls [Internet] StatPearls Publishing; Treasure Island, FL, USA: 2022. [(accessed on 29 January 2023)]. Biochemistry, Antioxidants. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541064/ [Google Scholar]
- 7.Weidinger A., Kozlov A.V. Biological Activities of Reactive Oxygen and Nitrogen Species: Oxidative Stress versus Signal Transduction. Biomolecules. 2015;5:472–484. doi: 10.3390/biom5020472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang M.Y., Jiang Y.X., Yang Y.C., Liu J.Y., Huo C., Ji X.L., Qu Y.Q. Cigarette smoke extract induces pyroptosis in human bronchial epithelial cells through the ROS/NLRP3/caspase-1 pathway. Life Sci. 2021;269:119090. doi: 10.1016/j.lfs.2021.119090. [DOI] [PubMed] [Google Scholar]
- 9.Jakubczyk K., Dec K., Kałduńska J., Kawczuga D., Kochman J., Janda K. Reactive oxygen species—Sources, functions, oxidative damage. Pol. Merkur. Lekarski. 2020;48:124–127. [PubMed] [Google Scholar]
- 10.de Jager T.L., Cockrell A.E., Du Plessis S.S. Ultraviolet Light Induced Generation of Reactive Oxygen Species. Adv. Exp. Med. Biol. 2017;996:15–23. doi: 10.1007/978-3-319-56017-5_2. [DOI] [PubMed] [Google Scholar]
- 11.Wang H., Mu X., He H., Zhang X.D. Cancer Radiosensitizers. Trends Pharmacol. Sci. 2018;39:24–48. doi: 10.1016/j.tips.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Phaniendra A., Jestadi D.B., Periyasamy L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015;30:11–26. doi: 10.1007/s12291-014-0446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Upadhyayula S.S., Bryce W.Q.T., Balamurugan A.V., Anand D.J. ROS and the DNA damage response in cancer. Redox Biol. 2019;25:101084. doi: 10.1016/j.redox.2018.101084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee S.M., Kim-Ha J., Choi W.Y., Lee J., Kim D., Lee J., Choi E., Kim Y.-J. Interplay of genetic and epigenetic alterations in hepatocellular carcinoma. Epigenomics. 2016;8:993–1005. doi: 10.2217/epi-2016-0027. [DOI] [PubMed] [Google Scholar]
- 15.Moloney J.N., Cotter T.G. ROS signaling in the biology of cancer. Semin. Cell Dev. Biol. 2018;80:50–64. doi: 10.1016/j.semcdb.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 16.Cheung E.C., Vousden K.H. The role of ROS in tumour development and progression. Nat. Rev. Cancer. 2022;22:280–297. doi: 10.1038/s41568-021-00435-0. [DOI] [PubMed] [Google Scholar]
- 17.Wang C., Chen J., Wang M., Naruse K., Takahashi K. Role of the TRPM4 channel in mitochondrial function, calcium release, and ROS generation in oxidative stress. Biochem. Biophys. Res. Commun. 2021;566:190–196. doi: 10.1016/j.bbrc.2021.03.077. [DOI] [PubMed] [Google Scholar]
- 18.Karekar P., Jensen H.N., Russart K.L.G., Ponnalagu D., Seeley S., Sanghvi S., Smith S.A., Pyter L.M., Singh H., Gururaja R.S. Tumor-Induced Cardiac Dysfunction: A Potential Role of ROS. Antioxidants. 2021;10:1299. doi: 10.3390/antiox10081299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaiprasongsuk A., Panich U. Role of Phytochemicals in Skin Photoprotection via Regulation of Nrf2. Front. Pharmacol. 2022;13:823–881. doi: 10.3389/fphar.2022.823881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen L., Chen Y., Ding W., Zhan T., Zhu J., Zhang L., Wang H., Shen B., Wang Y. Oxidative Stress-Induced TRPV2 Expression Increase Is Involved in Diabetic Cataracts and Apoptosis of Lens Epithelial Cells in a High-Glucose Environment. Cells. 2022;11:1196. doi: 10.3390/cells11071196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maandi S.C., Maandi M.T., Patel A., Manville R.W., Mabley J.G. Divergent effects of HIV reverse transcriptase inhibitors on pancreatic beta-cell function and survival: Potential role of oxidative stress and mitochondrial dysfunction. Life Sci. 2022;294:120–329. doi: 10.1016/j.lfs.2022.120329. [DOI] [PubMed] [Google Scholar]
- 22.Hussain T., Murtaza G., Metwally E., Kalhoro D.H., Kalhoro M.S., Rahu B.A., Sahito R.G.A., Yin Y., Yang H., Chughtai M.I., et al. The Role of Oxidative Stress and Antioxidant Balance in Pregnancy. Mediat. Inflamm. 2021;2021:9962860. doi: 10.1155/2021/9962860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson S.P., Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cadet J., Davies K.J.A. Oxidative DNA damage & repair: An introduction. Free Radic. Biol. Med. 2017;107:2–12. doi: 10.1016/j.freeradbiomed.2017.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Reest J., Nardini Cecchino G., Haigis M.C., Kordowitzki P. Mitochondria: Their relevance during oocyte ageing. Ageing Res. Rev. 2021;70:101378. doi: 10.1016/j.arr.2021.101378. [DOI] [PubMed] [Google Scholar]
- 26.Martemucci G., Costagliola C., Mariano M., D’andrea L., Napolitano P., D’Alessandro A.G. Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen. 2022;2:48–78. doi: 10.3390/oxygen2020006. [DOI] [Google Scholar]
- 27.Juan C.A., Pérez de la Lastra J.M., Plou F.J., Pérez-Lebeña E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021;22:4642. doi: 10.3390/ijms22094642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adwas A.A., Elsayed A.S.I., Azab A.E., Quwaydir F.A. Oxidative stress and antioxidant mechanisms in human body. J. Appl. Biotechnol. Bioeng. 2019;6:43–47. [Google Scholar]
- 29.Yang C.S., Ho C.T., Zhang J., Wan X., Zhang K., Lim J. Antioxidants: Differing Meanings in Food Science and Health Science. J. Agric. Food Chem. 2018;66:3063–3068. doi: 10.1021/acs.jafc.7b05830. [DOI] [PubMed] [Google Scholar]
- 30.Yu S., Huang X., Miao L., Zhu J., Yin Y., Luo Q., Xu J., Shen J., Liu J. A supramolecular bifunctional artificial enzyme with superoxid dismutase and glutathione peroxidase activities. Bioorg. Chem. 2010;38:159–164. doi: 10.1016/j.bioorg.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Ighodaro O.M., Akinloye O.A. First time defense antioxidants—Superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx): Their functional role in the entire antioxidant defense grid. Alex. J. Med. 2018;54:287–293. [Google Scholar]
- 32.Mirończuk-Chodakowska I., Witkowska A.M., Zujko M.E. Endogenous non-enzymatic antioxidants in the human body. Adv. Med. Sci. 2018;63:68–78. doi: 10.1016/j.advms.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Lange M., Olzmann J.A. Hydropersulfides are endogenous antioxidants that inhibit ferroptosis. Cell Chem. Biol. 2022;29:1661–1663. doi: 10.1016/j.chembiol.2022.11.011. [DOI] [PubMed] [Google Scholar]
- 34.Wu Z., Khodade V.S., Chauvin J.R., Rodriguez D., Toscano J.P., Pratt D.A. Hydropersulfides Inhibit Lipid Peroxidation and Protect Cells from Ferroptosis. J. Am. Chem. Soc. 2022;144:15825–15837. doi: 10.1021/jacs.2c06804. [DOI] [PubMed] [Google Scholar]
- 35.Barayeu U., Schilling D., Eid M., da Silva T.N.X., Schlicker L., Mitreska N., Zapp C., Gräter F., Miller A.K., Kappl R., et al. Hydropersulfides inhibit lipid peroxidation and ferroptosis by scavenging radicals. Nat. Chem. Biol. 2023;19:28–37. doi: 10.1038/s41589-022-01145-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Razaghi A., Poorebrahim M., Sarhan D., Björnstedt M. Selenium stimulates the antitumour immunity: Insights to future research. Eur. J. Cancer. 2021;155:256–267. doi: 10.1016/j.ejca.2021.07.013. [DOI] [PubMed] [Google Scholar]
- 37.Hu W., Zhao C., Hu H., Yin S. Food Sources of Selenium and Its Relationship with Chronic Diseases. Nutrients. 2021;13:1739. doi: 10.3390/nu13051739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang N., Tan H.Y., Li S., Xu Y., Guo W., Feng Y. Supplementation of Micronutrient Selenium in Metabolic Diseases: Its Role as an Antioxidant. Oxidative Med. Cell. Longev. 2017;2017:7478523. doi: 10.1155/2017/7478523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cardoso B.R., Cominetti C., Seale L.A. Editorial: Selenium, Human Health and Chronic Disease. Front. Nutr. 2022;8:827759. doi: 10.3389/fnut.2021.827759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuršvietienė L., Mongirdienė A., Bernatonienė J., Šulinskienė J., Stanevičienė I. Selenium Anticancer Properties and Impact on Cellular Redox Status. Antioxidants. 2020;9:80. doi: 10.3390/antiox9010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silva M.M., Lidon F.C. An overview on applications and side effects of antioxidant food additives. Emir. J. Food Agric. 2016;28:823–832. doi: 10.9755/ejfa.2016-04-351. [DOI] [Google Scholar]
- 42.Franco R., Navarro G., Martínez-Pinilla E. Antioxidants versus Food Antioxidant Additives and Food Preservatives. Antioxidants. 2019;8:542. doi: 10.3390/antiox8110542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adams A.K., Best T.M. The role of antioxidants in exercise and disease prevention. Physician Sportsmed. 2015;30:37–44. doi: 10.3810/psm.2002.05.281. [DOI] [PubMed] [Google Scholar]
- 44.Baranowski B.J., Marko D.M., Fenech R.K., Yang A.J.T., MacPherson R.E.K. Healthy brain, healthy life: A review of diet and exercise interventions to promote brain health and reduce Alzheimer’s disease risk. Appl. Physiol. Nutr. Metab. 2020;45:1055–1065. doi: 10.1139/apnm-2019-0910. [DOI] [PubMed] [Google Scholar]
- 45.Sharifi-Rad M., Anil Kumar N.V., Zucca P., Varoni E.M., Dini L., Panzarini E., Rajkovic J., Tsouh Fokou P.V., Azzini E., Peluso I., et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020;11:694. doi: 10.3389/fphys.2020.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wahlqvist M.L. Antioxidant relevance to human health. Asia Pac. J. Clin. Nutr. 2013;22:171–176. doi: 10.6133/apjcn.2013.22.2.21. [DOI] [PubMed] [Google Scholar]
- 47.Zeb A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020;44:e13394. doi: 10.1111/jfbc.13394. [DOI] [PubMed] [Google Scholar]
- 48.Shaw K.A., Zello G.A., Rodgers C.D., Warkentin T.D., Baerwald A.R., Chilibeck P.D. Benefits of a plant-based diet and considerations for the athlete. Eur. J. Appl. Physiol. 2022;122:1163–1178. doi: 10.1007/s00421-022-04902-w. [DOI] [PubMed] [Google Scholar]
- 49.Serafini M., Peluso I. Functional Foods for Health: The Interrelated Antioxidant and Anti-Inflammatory Role of Fruits, Vegetables, Herbs, Spices and Cocoa in Humans. Curr. Pharm. Des. 2016;22:6701–6715. doi: 10.2174/1381612823666161123094235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paciolla C., Fortunato S., Dipierro N., Paradiso A., De Leonardis S., Mastropasqua L., de Pinto M.C. Vitamin C in Plants: From Functions to Biofortification. Antioxidants. 2019;8:519. doi: 10.3390/antiox8110519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smirnoff N. Ascorbic acid metabolism and functions: A comparison of plants and mammals. Free Radic. Biol. Med. 2018;122:116–129. doi: 10.1016/j.freeradbiomed.2018.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duan W., Ren J., Li Y., Liu T., Song X., Chen Z., Huang Z., Hou X., Li Y. Conservation and Expression Patterns Divergence of Ascorbic Acid d-mannose/l-galactose Pathway Genes in Brassica rapa. Front. Plant Sci. 2016;7:778. doi: 10.3389/fpls.2016.00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zheng X., Gong M., Zhang Q., Tan H., Li L., Tang Y., Li Z., Peng M., Deng W. Metabolism and Regulation of Ascorbic Acid in Fruits. Plants. 2022;11:1602. doi: 10.3390/plants11121602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Broad R.C., Bonneau J.P., Hellens R.P., Johnson A.A.T. Manipulation of Ascorbate Biosynthetic, Recycling, and Regulatory Pathways for Improved Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2020;21:1790. doi: 10.3390/ijms21051790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fujii J. Ascorbate is a multifunctional micronutrient whose synthesis is lacking in primates. J. Clin. Biochem. Nutr. 2021;69:1–15. doi: 10.3164/jcbn.20-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hishiyama N., Kayanuma H., Matsui T., Yano H., Suganuma T., Funaba M., Fujise H. Plasma concentration of vitamin C in dogs with a portosystemic shunt. Can. J. Vet. Res. 2006;70:305–307. [PMC free article] [PubMed] [Google Scholar]
- 57.Gordon D.S., Rudinsky A.J., Guillaumin J., Parker V.J., Creighton K.J. Vitamin C in Health and Disease: A Companion Animal Focus. Top. Companion Anim. Med. 2020;39:100432. doi: 10.1016/j.tcam.2020.100432. [DOI] [PubMed] [Google Scholar]
- 58.Drouin G., Godin J.R., Pagé B. The genetics of vitamin C loss in vertebrates. Curr. Genom. 2011;12:371–378. doi: 10.2174/138920211796429736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharma Y., Popescu A., Horwood C., Hakendorf P., Thompson C. Relationship between vitamin C deficiency and cognitive impairment in older hospitalised patients: A cross—Sectional study. Antioxidants. 2022;11:463. doi: 10.3390/antiox11030463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DePhillipo N.N., Aman Z.S., Kennedy M.I., Begley J.P., Moatshe G., LaPrade R.F. Efficacy of vitamin c supplementation on collagen synthesis and oxidative stress after musculoskeletal injuries: A systematic review. Orthop. J. Sports Med. 2018;6:2325967118804544. doi: 10.1177/2325967118804544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Linster C.L., Van Schaftingen E. Vitamin C biosynthesis, recycling and degradation in mammals. FEBS J. 2007;274:1–22. doi: 10.1111/j.1742-4658.2006.05607.x. [DOI] [PubMed] [Google Scholar]
- 62.Baek S.-M., Lee S.-W., Kim T.-U., Choi S.-K., Yun S., Lee W.-J., Han S.-H., Hong I.-H., Park S.-J., Kim T.-H., et al. Senescence Marker Protein 30 (SMP30): A Novel Pan-Species Diagnostic Marker for the Histopathological Diagnosis of Breast Cancer in Humans and Animals. Int. J. Mol. Sci. 2021;22:2340. doi: 10.3390/ijms22052340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tao J., Hao Z., Huang C. Molecular evolution of GDP-L-galactose phosphorylase, a key regulatory gene in plant ascorbate biosynthesis. AoB Plants. 2020;12:plaa055. doi: 10.1093/aobpla/plaa055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gabbay K.H., Bohren K.M., Morello R., Bertin T., Liu J., Vogel P. Ascorbate synthesis pathway: Dual role of ascorbate in bone homeostasis. J. Biol. Chem. 2010;285:19510–19520. doi: 10.1074/jbc.M110.110247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang H. Conserved or lost: Molecular evolution of the key gene GULO in vertebrate vitamin C biosynthesis. Biochem. Genet. 2013;51:413–425. doi: 10.1007/s10528-013-9574-0. [DOI] [PubMed] [Google Scholar]
- 66.Meščić Macan A., Gazivoda Kraljević T., Raić-Malić S. Therapeutic Perspective of Vitamin C and Its Derivatives. Antioxidants. 2019;8:247. doi: 10.3390/antiox8080247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nandi A., Mukhopadhyay C.K., Ghosh M.K., Chattopadhyay D.J., Chatterjee I.B. Evolutionary significance of vitamin C biosynthesis in terrestrial vertebrates. Free Radic. Biol. Med. 1997;22:1047–1054. doi: 10.1016/S0891-5849(96)00491-1. [DOI] [PubMed] [Google Scholar]
- 68.Schröder G.C., Meilleur F. Metalloprotein catalysis: Structural and mechanistic insights into oxidoreductases from neutron protein crystallography. Acta Crystallogr. Sect. D Struct. Biol. 2021;77:1251–1269. doi: 10.1107/S2059798321009025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fain O. Musculoskeletal manifestations of scurvy. Jt. Bone Spine. 2005;72:124–128. doi: 10.1016/j.jbspin.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 70.El Basuini M.F., Shahin S.A., Teiba I.I., Zaki M.A.A., El Hais A.M., Sewilam H., Almeer R., Abdelkhalek N., Dawood M.A.O. The influence of dietary coenzyme Q10 and vitamin C on the growth rate, immunity, oxidative-related genes, and the resistance against Streptococcus agalactiae of Nile tilapia (Oreochromis niloticus) Aquaculture. 2021;531:735862. doi: 10.1016/j.aquaculture.2020.735862. [DOI] [Google Scholar]
- 71.Li Y., Schellhorn H.E. New developments and novel therapeutic perspectives for vitamin C. J. Nutr. 2007;137:2171–2184. doi: 10.1093/jn/137.10.2171. [DOI] [PubMed] [Google Scholar]
- 72.Chen X., Li J., Kang R., Klionsky D.J., Tang D. Ferroptosis: Machinery and regulation. Autophagy. 2021;17:2054–2081. doi: 10.1080/15548627.2020.1810918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bhattacharjee S. Membrane lipid peroxidation and its conflict of interest: The two faces of oxidative stress. Curr. Sci. 2014;107:1811–1823. [Google Scholar]
- 74.Hawkins C.L., Davies M.J. Detection, identification, and quantification of oxidative protein modifications. J. Biol. Chem. 2019;20:19683–19708. doi: 10.1074/jbc.REV119.006217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu C., Liu Z., Xiao J. Ferroptosis: A Double-Edged Sword in Gastrointestinal Disease. Int. J. Mol. Sci. 2021;22:12403. doi: 10.3390/ijms222212403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nguyen-Powanda P., Robaire B. Aging and oxidative stress alter DNA repair mechanisms in male germ cells of superoxide dismutase-1 null mice. Biol. Reprod. 2021;105:944–957. doi: 10.1093/biolre/ioab114. [DOI] [PubMed] [Google Scholar]
- 77.Bárcena B., Salamanca A., Pintado C., Mazuecos L., Villar M., Moltó E., Bonzón-Kulichenko E., Vázquez J., Andrés A., Gallardo N. Aging Induces Hepatic Oxidative Stress and Nuclear Proteomic Remodeling in Liver from Wistar Rats. Antioxidants. 2021;10:1535. doi: 10.3390/antiox10101535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Menon A.V., Liu J., Tsai H.P., Zeng L., Yang S., Asnani A., Kim J. Excess heme upregulates heme oxygenase 1 and promotes cardiac ferroptosis in mice with sickle cell disease. Blood. 2022;139:936–941. doi: 10.1182/blood.2020008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jaganjac M., Cindric M., Jakovcevic A., Žarkovic K., Žarkovic N. Lipid peroxidation in brain tumors. Neurochem. Int. 2021;149:105–118. doi: 10.1016/j.neuint.2021.105118. [DOI] [PubMed] [Google Scholar]
- 80.Satish B.N., Dilipkumar P. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015;5:27986–28006. [Google Scholar]
- 81.Pisoschi A.M., Pop A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015;97:55–74. doi: 10.1016/j.ejmech.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 82.Prenzler P.D., Ryan D., Robards K. Handbook of Antioxidant Methodology: Approaches to Activity Determination. Royal Society of Chemistry; London, UK: 2021. Introduction to Basic Principles of Antioxidant Activity; pp. 1–62. [Google Scholar]
- 83.Kurutas E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016;15:71. doi: 10.1186/s12937-016-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Musakhanian J., Rodier J.D., Dave M. Oxidative Stability in Lipid Formulations: A Review of the Mechanisms, Drivers, and Inhibitors of Oxidation. AAPS PharmSciTech. 2022;23:151. doi: 10.1208/s12249-022-02282-0. [DOI] [PubMed] [Google Scholar]
- 85.Parcheta M., Świsłocka R., Orzechowska S., Akimowicz M., Choińska R., Lewandowski W. Recent Developments in Effective Antioxidants: The Structure and Antioxidant Properties. Materials. 2021;14:1984. doi: 10.3390/ma14081984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Losada-Barreiro S., Sezgin-Bayindir Z., Paiva-Martins F., Bravo-Díaz C. Biochemistry of Antioxidants: Mechanisms and Pharmaceutical Applications. Biomedicines. 2022;10:3051. doi: 10.3390/biomedicines10123051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Flieger J., Flieger W., Baj J., Maciejewski R. Antioxidants: Classification, Natural Sources, Activity/Capacity Measurements, and Usefulness for the Synthesis of Nanoparticles. Materials. 2021;14:4135. doi: 10.3390/ma14154135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Irato P., Santovito G. Enzymatic and Non-Enzymatic Molecules with Antioxidant Function. Antioxidants. 2021;10:579. doi: 10.3390/antiox10040579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lushchak V.I. Glutathione homeostasis and functions: Potential targets for medical interventions. J. Amino Acids. 2012;2012:736837. doi: 10.1155/2012/736837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kesarwani P., Murali A.K., Al-Khami A.A., Mehrotra S. Redox regulation of T-cell function: From molecular mechanisms to significance in human health and disease. Antioxid. Redox Signal. 2013;18:1497–1534. doi: 10.1089/ars.2011.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Galano A., Alvarez-Idaboy J.R. Glutathione: Mechanism and kinetics of its non-enzymatic defense action against free radicals. RSC Adv. 2011;1:1763–1771. doi: 10.1039/c1ra00474c. [DOI] [Google Scholar]
- 92.Du X., Yu J., Sun X., Qu S., Zhang H., Hu M., Yang S., Zhou P. Impact of epigallocatechin-3-gallate on expression of nuclear factor erythroid 2-related factor 2 and γ-glutamyl cysteine synthetase genes in oxidative stress-induced mouse renal tubular epithelial cells. Mol. Med. Rep. 2018;17:7952–7958. doi: 10.3892/mmr.2018.8798. [DOI] [PubMed] [Google Scholar]
- 93.Espinosa-Diez C., Fierro-Fernández M., Sánchez-Gómez F., Rodríguez-Pascual F., Alique M., Ruiz-Ortega M., Beraza N., Martínez-Chantar M.L., Fernández-Hernando C., Lamas S. Targeting of Gamma-Glutamyl-Cysteine Ligase by miR-433 Reduces Glutathione Biosynthesis and Promotes TGF-β-Dependent Fibrogenesis. Antioxid. Redox Signal. 2015;23:1092–1105. doi: 10.1089/ars.2014.6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lu S.C. Regulation of glutathione synthesis. Curr. Top. Cell. Regul. 2001;36:95–116. doi: 10.1016/s0070-2137(01)80004-2. [DOI] [PubMed] [Google Scholar]
- 95.Forman H.J., Zhang H., Rinna A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009;30:1–12. doi: 10.1016/j.mam.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ballatori N., Krance S.M., Notenboom S., Shi S., Tieu K., Hammond C.L. Glutathione dysregulation and the etiology and progression of human diseases. Biol. Chem. 2009;390:191–214. doi: 10.1515/BC.2009.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sitohang I.B.S., Anwar A.I., Jusuf N.K., Arimuko A., Norawati L., Veronica S. Evaluating Oral Glutathione Plus Ascorbic Acid, Alpha-lipoic Acid, and Zinc Aspartate as a Skin-lightening Agent: An Indonesian Multicenter, Randomized, Controlled Trial. J. Clin. Aesthetic Dermatol. 2021;14:53–58. [PMC free article] [PubMed] [Google Scholar]
- 98.Minich D.M., Brown B.I. A Review of Dietary (Phyto)Nutrients for Glutathione Support. Nutrients. 2019;11:2073. doi: 10.3390/nu11092073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hossain A., Khatun M.A., Islam M., Huque R. Enhancement of Antioxidant Quality of Green Leafy Vegetables upon Different Cooking Method. Prev. Nutr. Food Sci. 2017;22:216–222. doi: 10.3746/pnf.2017.22.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sies H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nandi A., Yan L.J., Jana C.K., Das N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxidative Med. Cell. Longev. 2019;2019:9613090. doi: 10.1155/2019/9613090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rubio-Riquelme N., Huerta-Retamal N., Gómez-Torres M.J., Martínez-Espinosa R.M. Catalase as a Molecular Target for Male Infertility Diagnosis and Monitoring: An Overview. Antioxidants. 2020;9:78. doi: 10.3390/antiox9010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sheng Y., Abreu I.A., Cabelli D.E., Maroney M.J., Miller A., Teixeira M., Valentine J.S. Superoxide Dismutases and Superoxide Reductases. Chem. Rev. 2014;114:3854–3918. doi: 10.1021/cr4005296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mondola P., Damiano S., Sasso A., Santillo M. The Cu, Zn Superoxide Dismutase: Not Only a Dismutase Enzyme. Front. Physiol. 2016;7:594. doi: 10.3389/fphys.2016.00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stephenie S., Chang Y.P., Gnanasekaran A., Esa N.M., Gnanaraj C. An insight on superoxide dismutase (SOD) from plants for mammalian health enhancement. J. Funct. Foods. 2020;68:103917. doi: 10.1016/j.jff.2020.103917. [DOI] [Google Scholar]
- 106.Zehiroglu C., Ozturk Sarikaya S.B. The importance of antioxidants and place in today’s scientific and technological studies. J. Food Sci. Technol. 2019;56:4757–4774. doi: 10.1007/s13197-019-03952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.López-Pedrouso M., Lorenzo J.M., Franco D. Advances in Natural Antioxidants for Food Improvement. Antioxidants. 2022;11:1825. doi: 10.3390/antiox11091825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bellucci E.R.B., Bis-Souza C.V., Domínguez R., Bermúdez R., Barretto A.C.D.S. Addition of Natural Extracts with Antioxidant Function to Preserve the Quality of Meat Products. Biomolecules. 2022;12:1506. doi: 10.3390/biom12101506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kurek M., Benaida-Debbache N., Elez Garofulić I., Galić K., Avallone S., Voilley A., Waché Y. Antioxidants and Bioactive Compounds in Food: Critical Review of Issues and Prospects. Antioxidants. 2022;11:742. doi: 10.3390/antiox11040742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lourenço S.C., Moldão-Martins M., Alves V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules. 2019;24:4132. doi: 10.3390/molecules24224132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li J., Lin Y., He L., Ou R., Chen T., Zhang X., Li Q., Zeng Z., Long Q. Two New Isoprenoid Flavonoids from Sophora flavescens with Antioxidant and Cytotoxic Activities. Molecules. 2021;26:7228. doi: 10.3390/molecules26237228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Naheed N., Maher S., Saleem F., Khan A., Wadood A., Rasheed S., Choudhary M.I., Froeyen M., Abdullah I., Mirza M.U., et al. New isolate from Salvinia molesta with antioxidant and urease inhibitory activity. Drug Dev. Res. 2021;82:1169–1181. doi: 10.1002/ddr.21831. [DOI] [PubMed] [Google Scholar]
- 113.Arab Z., Jafarian S., Karimi-Maleh H., Roozbeh Nasiraie L., Ahmadi M. Monitoring of Butylated Hydroxyanisole in Food and Wastewater Samples Using Electroanalytical Two-Fold Amplified Sensor. Sustainability. 2022;14:2169. doi: 10.3390/su14042169. [DOI] [Google Scholar]
- 114.Delanghe T., Huyghe J., Lee S., Priem D., Van Coillie S., Gilbert B., Choi S.M., Vandenabeele P., Degterev A., Cuny G.D., et al. Antioxidant and food additive BHA prevents TNF cytotoxicity by acting as a direct RIPK1 inhibitor. Cell Death Dis. 2021;12:699. doi: 10.1038/s41419-021-03994-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xu X., Liu A., Hu S., Ares I., Martínez-Larrañaga M.-R., Wang X., Martínez M., Anadón A., Martínez M.-A. Synthetic phenolic antioxidants: Metabolism, hazards and mechanism of action. Food Chem. 2021;353:129488. doi: 10.1016/j.foodchem.2021.129488. [DOI] [PubMed] [Google Scholar]
- 116.Felter S.P., Zhang X., Thompson C. Butylated hydroxyanisole: Carcinogenic food additive to be avoided or harmless antioxidant important to protect food supply? Regul. Toxicol. Pharmacol. 2021;121:104887. doi: 10.1016/j.yrtph.2021.104887. [DOI] [PubMed] [Google Scholar]
- 117.Shamsadin-Azad Z., Taher M.A., Cheraghi S., Karimi-Maleh H. A nanostructure voltammetric platform amplified with ionic liquid for determination of tert-butylhydroxyanisole in the presence kojic acid. J. Food Meas. Charact. 2019;13:1781–1787. doi: 10.1007/s11694-019-00096-6. [DOI] [Google Scholar]
- 118.Zhang R., Li J., Cui X. Tissue distribution, excretion, and metabolism of 2,6-di-tert-butyl-hydroxytoluene in mice. Sci. Total Environ. 2020;739:139862. doi: 10.1016/j.scitotenv.2020.139862. [DOI] [PubMed] [Google Scholar]
- 119.Sun Z., Gao R., Chen X., Liu X., Ding Y., Geng Y., Mu X., Liu T., Li F., Wang Y., et al. Exposure to butylated hydroxytoluene compromises endometrial decidualization during early pregnancy. Environ. Sci. Pollut. Res. Int. 2021;28:42024–42036. doi: 10.1007/s11356-021-13720-0. [DOI] [PubMed] [Google Scholar]
- 120.Mizobuchi M., Ishidoh K., Kamemura N. A comparison of cell death mechanisms of antioxidants, butylated hydroxyanisole and butylated hydroxytoluene. Drug Chem. Toxicol. 2022;45:1899–1906. doi: 10.1080/01480545.2021.1894701. [DOI] [PubMed] [Google Scholar]
- 121.Abou-Hadeed A.H., Mohamed A.T., Hegab D.Y., Ghoneim M.H. Ethoxyquin and Butylated Hydroxy Toluene Distrub the Hematological Parameters and Induce Structural and Functional Alterations in Liver of Rats. Arch. Razi Inst. 2021;76:1765–1776. doi: 10.22092/ari.2021.356439.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nieva-Echevarría B., Manzanos M.J., Goicoechea E., Guillén M.D. 2,6-Di-Tert-Butyl-Hydroxytoluene and Its Metabolites in Foods. Compr. Rev. Food Sci. Food Saf. 2015;14:67–80. doi: 10.1111/1541-4337.12121. [DOI] [PubMed] [Google Scholar]
- 123.Zhao M.T., Liu Z.Y., Li A., Zhao G.H., Xie H.K., Zhou D.Y., Wang T. Gallic acid and its alkyl esters emerge as effective antioxidants against lipid oxidation during hot air drying process of Ostrea talienwhanensis. LWT. 2021;139:110551. doi: 10.1016/j.lwt.2020.110551. [DOI] [Google Scholar]
- 124.Choubey S., Varughese L.R., Kumar V., Beniwal V. Medicinal importance of gallic acid and its ester derivatives: A patent review. Pharm. Pat. Anal. 2015;4:305–315. doi: 10.4155/ppa.15.14. [DOI] [PubMed] [Google Scholar]
- 125.Kahkeshani N., Farzaei F., Fotouhi M., Alavi S.S., Bahramsoltani R., Naseri R., Momtaz S., Abbasabadi Z., Rahimi R., Farzaei M.H., et al. Pharmacological effects of gallic acid in health and diseases: A mechanistic review. Iran J. Basic Med. Sci. 2019;22:225–237. doi: 10.22038/ijbms.2019.32806.7897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Badhani B., Sharma N., Kakkar R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015;5:27540–27557. doi: 10.1039/C5RA01911G. [DOI] [Google Scholar]
- 127.Keramat M., Niakousari M., Golmakani M.T. Comparing the antioxidant activity of gallic acid and its alkyl esters in emulsion gel and non-gelled emulsion. Food Chem. 2023;407:135078. doi: 10.1016/j.foodchem.2022.135078. [DOI] [PubMed] [Google Scholar]
- 128.Perazzoli M.R., Perondi C.K., Baratto C.M., Winter E., Creczynski-Pasa T.B., Locatelli C. Gallic Acid and Dodecyl Gallate Prevents Carbon Tetrachloride-Induced Acute and Chronic Hepatotoxicity by Enhancing Hepatic Antioxidant Status and Increasing p53 Expression. Biol. Pharm. Bull. 2017;40:425–434. doi: 10.1248/bpb.b16-00782. [DOI] [PubMed] [Google Scholar]
- 129.Delgado A., Al-Hamimi S., Ramadan M.F., De Wit M., Durazzo A., Nyam K.L., Issaoui M. Contribution of Tocols to Food Sensorial Properties, Stability, and Overall Quality. J. Food Qual. 2020;2020:8885865. doi: 10.1155/2020/8885865. [DOI] [Google Scholar]
- 130.Shahidi F., de Camargo A.C. Tocopherols and Tocotrienols in Common and Emerging Dietary Sources: Occurrence, Applications, and Health Benefits. Int. J. Mol. Sci. 2016;17:1745. doi: 10.3390/ijms17101745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li G.X., Lee M.J., Liu A.B., Yang Z., Lin Y., Shih W.J., Yang C.S. δ-Tocopherol is more active than α- or γ-tocopherol in inhibiting lung tumorigenesis in vivo. Cancer Prev. Res. 2011;4:404–413. doi: 10.1158/1940-6207.CAPR-10-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jiang Q. Natural Forms of Vitamin E as Effective Agents for Cancer Prevention and Therapy. Adv. Nutr. 2017;8:850–867. doi: 10.3945/an.117.016329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tufiño C., Bernal C., Ottone C., Romero O., Illanes A., Wilson L. Synthesis with Immobilized Lipases and Downstream Processing of Ascorbyl Palmitate. Molecules. 2019;24:3227. doi: 10.3390/molecules24183227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ledinski M., Marić I., Peharec Štefanić P., Ladan I., Caput Mihalić K., Jurkin T., Gotić M., Urlić I. Synthesis and In Vitro Characterization of Ascorbyl Palmitate-Loaded Solid Lipid Nanoparticles. Polymers. 2022;14:1751. doi: 10.3390/polym14091751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zahid M.A., Seo J.K., Parvin R., Ko J., Yang H.S. Comparison of Butylated Hydroxytoluene, Ascorbic Acid, and Clove Extract as Antioxidants in Fresh Beef Patties at Refrigerated Storage. Food Sci. Anim. Resour. 2019;39:768–779. doi: 10.5851/kosfa.2019.e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Boroujeni L.S., Hojjatoleslamy M. Using Thymus carmanicus and Myrtus communis essential oils to enhance the physicochemical properties of potato chips. Food Sci. Nutr. 2018;6:1006–1014. doi: 10.1002/fsn3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pei X.C., Yin F.W., Zhong X., Liu H.L., Song L., Zhao G.H., Wang Y.F., Zhou D.Y. Effects of different antioxidants and their combinations on the oxidative stability of DHA algae oil and walnut oil. Food Sci. Nutr. 2022;10:2804–2812. doi: 10.1002/fsn3.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Badea G.A., Antoce A.O. Glutathione as a possible replacement of sulfur dioxide in winemaking technologies: A review. Sci. Pap. Ser. B Hortic. 2015;LIX:123–140. [Google Scholar]
- 139.Zhang D., Wang X., Tian X., Zhang L., Yang G., Tao Y., Liang C., Li K., Yu X., Tang X., et al. The Increased Endogenous Sulfur Dioxide Acts as a Compensatory Mechanism for the Downregulated Endogenous Hydrogen Sulfide Pathway in the Endothelial Cell Inflammation. Front. Immunol. 2018;9:882. doi: 10.3389/fimmu.2018.00882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Santos M.C., Nunes C., Saraiva J.A., Coimbra M.A. Chemical and physical methodologies for the replacement/reduction of sulfur dioxide use during winemaking: Review of their potentialities and limitations. Eur. Food Res. Technol. 2012;234:1–12. doi: 10.1007/s00217-011-1614-6. [DOI] [Google Scholar]
- 141.Padayatty S.J., Levine M. Vitamin C: The known and the unknown and Goldilocks. Oral Dis. 2016;22:463–493. doi: 10.1111/odi.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yin X., Chen K., Cheng H., Feng S., Song Y., Liang L. Chemical Stability of Ascorbic Acid Integrated into Commercial Products: A Review on Bioactivity and Delivery Technology. Antioxidants. 2022;11:153. doi: 10.3390/antiox11010153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bradshaw M.P., Barril C., Clark A.C., Prenzler P.D., Scollary G.R. Ascorbic acid: A review of its chemistry and reactivity in relation to a wine environment. Crit. Rev. Food Sci. Nutr. 2012;51:479–498. doi: 10.1080/10408391003690559. [DOI] [PubMed] [Google Scholar]
- 144.Mousavi A., Pourakbar L., Moghaddam S.S. Effects of malic acid and EDTA on oxidative stress and antioxidant enzymes of okra (Abelmoschus esculentus L.) exposed to cadmium stress. Ecotoxicol. Environ. Saf. 2022;248:114320. doi: 10.1016/j.ecoenv.2022.114320. [DOI] [PubMed] [Google Scholar]
- 145.Li J., Li F., Xu Y., Yang W., Qu L., Xiang Q., Liu C., Li D. Chemical composition and synergistic antioxidant activities of essential oils from Atractylodes macrocephala and Astragalus membranaceus. Nat. Prod. Commun. 2013;8:1321–1324. doi: 10.1177/1934578X1300800934. [DOI] [PubMed] [Google Scholar]
- 146.Karre L., Lopez K., Getty K.J.K. Natural antioxidants in meat and poultry products. Meat Sci. 2013;94:220–227. doi: 10.1016/j.meatsci.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 147.Poljsak B., Kovač V., Milisav I. Antioxidants, Food Processing and Health. Antioxidants. 2021;10:433. doi: 10.3390/antiox10030433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rather S.A., Masoodi F.A., Akhter R., Rather J.A., Shiekh K.A. Advances in use of Natural Antioxidants as Food Additives for improving the Oxidative Stability of Meat Products. Madr. J. Food Technol. 2016;1:10–17. [Google Scholar]
- 149.Tajik H., Farhangfar A., Moradi M., Rohani S.M.R. Effectiveness of clove essential oil and grape seed extract combination on microbial and lipid oxidation characteristics of raw buffalo patty during storage at abuse refrigeration temperature. J. Food Process. Preserv. 2014;38:31–38. doi: 10.1111/j.1745-4549.2012.00736.x. [DOI] [Google Scholar]
- 150.Das A.K., Rajkumar V., Verma A.K., Swarup D. Moringa oleifera leaves extract: A natural antioxidant for retarding lipid peroxidation in cooked goat meat patties. Int. J. Food Sci. Technol. 2012;47:585–591. doi: 10.1111/j.1365-2621.2011.02881.x. [DOI] [Google Scholar]
- 151.Jiang J., Xiong Y.L. Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: A review. Meat Sci. 2016;120:107–117. doi: 10.1016/j.meatsci.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 152.Gutiérrez-Del-río I., López-Ibáñez S., Magadán-Corpas P., Fernández-Calleja L., Pérez-Valero Á., Tuñón-Granda M., Miguélez E.M., Villar C.J., Lombó F. Terpenoids and polyphenols as natural antioxidant agents in food preservation. Antioxidants. 2021;10:1264. doi: 10.3390/antiox10081264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Poliński S., Topka P., Tańska M., Kowalska S., Czaplicki S., Szydłowska-Czerniak A. Impact of Bioactive Compounds of Plant Leaf Powders in White Chocolate Production: Changes in Antioxidant Properties during the Technological Processes. Antioxidants. 2022;11:752. doi: 10.3390/antiox11040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Faheem F., Liu Z.W., Rabail R., Haq I.U., Gul M., Bryła M., Roszko M., Kieliszek M., Din A., Aadil R.M. Uncovering the Industrial Potentials of Lemongrass Essential Oil as a Food Preservative: A Review. Antioxidants. 2022;11:720. doi: 10.3390/antiox11040720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Thahira Banu A., Sri Ramani P., Aswini M. Effect of seaweed coating on quality characteristics and shelf life of tomato (Lycopersicon esculentum mill) Food Sci. Hum. Wellness. 2020;9:176–183. doi: 10.1016/j.fshw.2020.03.002. [DOI] [Google Scholar]
- 156.Sanches-Silva A., Costa D., Albuquerque T.G., Buonocore G.G., Ramos F., Castilho M.C., Machado A.V., Costa H.S. Trends in the use of natural antioxidants in active food packaging: A review. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2014;31:374–395. doi: 10.1080/19440049.2013.879215. [DOI] [PubMed] [Google Scholar]
- 157.Rangaraj V., Rambabu K., Banat F., Mittal V. Natural antioxidants-based edible active food packaging: An overview of current advancements. Food Biosci. 2021;43:101251. doi: 10.1016/j.fbio.2021.101251. [DOI] [Google Scholar]
- 158.Vieira I.R.S., de Carvalho A.P.A., Conte-Junior C.A. Recent advances in biobased and biodegradable polymer nanocomposites, nanoparticles, and natural antioxidants for antibacterial and antioxidant food packaging applications. CRFSFS. 2022;21:3673–3716. doi: 10.1111/1541-4337.12990. [DOI] [PubMed] [Google Scholar]
- 159.Crespo Y.A., Bravo Sánchez L.R., Quintana Y.G., Cabrera A.S.T., Bermúdez Del Sol A., Mayancha D.M.G. Evaluation of the synergistic effects of antioxidant activity on mixtures of the essential oil from Apium graveolens L., Thymus vulgaris L. and Coriandrum sativum L. using simplex-lattice design. Heliyon. 2019;5:e01942. doi: 10.1016/j.heliyon.2019.e01942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pruteanu L.L., Kopanitsa L., Módos D., Kletnieks E., Samarova E., Bender A., Gomez L.D., Bailey D.S. Transcriptomics predicts compound synergy in drug and natural product treated glioblastoma cells. PLoS ONE. 2020;15:e0239551. doi: 10.1371/journal.pone.0239551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lopez-Maldonado A., Pastoriza S., Rufián-Henares J.Á. Assessing the antioxidant and metabolic effect of an alpha-lipoic acid and acetyl-L-carnitine nutraceutical. Curr. Res. Food Sci. 2021;4:336–344. doi: 10.1016/j.crfs.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ahn Y.J., Kim H. Lutein as a Modulator of Oxidative Stress-Mediated Inflammatory Diseases. Antioxidants. 2021;10:1448. doi: 10.3390/antiox10091448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bin-Jumah M.N., Nadeem M.S., Gilani S.J., Mubeen B., Ullah I., Alzarea S.I., Ghoneim M.M., Alshehri S., Al-Abbasi F.A., Kazmi I. Lycopene: A Natural Arsenal in the War against Oxidative Stress and Cardiovascular Diseases. Antioxidants. 2022;11:232. doi: 10.3390/antiox11020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Prior R.L. Oxygen radical absorbance capacity (ORAC): New horizons in relating dietary antioxidants/bioactives and health benefits. J. Funct. Foods. 2015;18:797–810. doi: 10.1016/j.jff.2014.12.018. [DOI] [Google Scholar]
- 165.George J., Edwards D., Pun S., Williams D. Evaluation of Antioxidant Capacity (ABTS and CUPRAC) and Total Phenolic Content (Folin-Ciocalteu) Assays of Selected Fruit, Vegetables, and Spices. Int. J. Food Sci. 2022;2022:2581470. doi: 10.1155/2022/2581470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Apak R., Güçlü K., Demirata B., Ozyürek M., Celik S.E., Bektaşoğlu B., Berker K.I., Ozyurt D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules. 2007;12:1496–1547. doi: 10.3390/12071496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Caturla N., Funes L., Pérez-Fons L., Micol V. A randomized, double-blinded, placebo-controlled study of the effect of a combination of lemon verbena extract and fish oil omega-3 fatty acid on joint management. J. Altern. Complement Med. 2011;17:1051–1063. doi: 10.1089/acm.2010.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Carlsen M.H., Halvorsen B.L., Holte K., Bøhn S.K., Dragland S., Sampson L., Willey C., Senoo H., Umezono Y., Sanada C., et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr. J. 2010;9:3. doi: 10.1186/1475-2891-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Boeing H., Bechthold A., Bub A., Ellinger S., Haller D., Kroke A., Leschik-Bonnet E., Müller M.J., Oberritter H., Schulze M., et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012;51:637–663. doi: 10.1007/s00394-012-0380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Duthie S.J., Duthie G.G., Russell W.R.M., Kyle J.A., Macdiarmid J.I., Rungapamestry V., Stephen S., Megias-Baeza C., Kaniewska J.J., Shaw L., et al. Effect of increasing fruit and vegetable intake by dietary intervention on nutritional biomarkers and attitudes to dietary change: A randomised trial. Eur. J. Nutr. 2018;57:1855–1872. doi: 10.1007/s00394-017-1469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Key T.J., Bradbury K.E., Perez-Cornago A., Sinha R., Tsilidis K.K., Tsugane S. Diet, nutrition, and cancer risk: What do we know and what is the way forward? BMJ. 2020;368:511. doi: 10.1136/bmj.m511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Srivastava K.K., Kumar R. Stress, oxidative injury and disease. Indian J. Clin. Biochem. 2015;30:3–10. doi: 10.1007/s12291-014-0441-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Simioni C., Zauli G., Martelli A.M., Vitale M., Sacchetti G., Gonelli A., Neri L.M. Oxidative stress: Role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget. 2018;9:17181–17198. doi: 10.18632/oncotarget.24729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sayin V.I., Ibrahim M.X., Larsson E., Nilsson J.A., Lindahl P., Bergo M.O. Antioxidants accelerate lung cancer progression in mice. Sci. Transl. Med. 2014;6:221ra15. doi: 10.1126/scitranslmed.3007653. [DOI] [PubMed] [Google Scholar]
- 175.Zhang Y., Xu Y., Lu W., Li J., Yu S., Brown E.J., Stanger B.Z., Rabinowitz J.D., Yang X. G6PD-mediated increase in de novo NADP+ biosynthesis promotes antioxidant defense and tumor metastasis. Sci. Adv. 2022;8:29. doi: 10.1126/sciadv.abo0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Piskounova E., Agathocleous M., Murphy M.M., Hu Z., Huddlestun S.E., Zhao Z., Leitch A.M., Johnson T.M., DeBerardinis R.J., Morrison S.J. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature. 2015;527:186–191. doi: 10.1038/nature15726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Babich O., Larina V., Ivanova S., Tarasov A., Povydysh M., Orlova A., Strugar J., Sukhikh S. Phytotherapeutic Approaches to the Prevention of Age-Related Changes and the Extension of Active Longevity. Molecules. 2022;27:2276. doi: 10.3390/molecules27072276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jacob K., Periago M.J., Böhm V., Berruezo G.R. Influence of lycopene and vitamin C from tomato juice on biomarkers of oxidative stress and inflammation. Br. J. Nutr. 2008;99:137–146. doi: 10.1017/S0007114507791894. [DOI] [PubMed] [Google Scholar]
- 179.Metha D., Belemkar S. Pharmacological activity of Spinacia oleracea Linn.—A complete overview. Asian J. Pharm. 2014;2:83–93. [Google Scholar]
- 180.Banel D.K., Hu F.B. Effects of walnut consumption on blood lipids and other cardiovascular risk factors: A meta-analysis and systematic review. Am. J. Clin. Nutr. 2009;90:56–63. doi: 10.3945/ajcn.2009.27457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ayadi J., Debouba M., Rahmani R., Bouajila J. Brassica Genus Seeds: A Review on Phytochemical Screening and Pharmacological Properties. Molecules. 2022;27:6008. doi: 10.3390/molecules27186008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ke Y.Y., Shyu Y.T., Wu S.J. Evaluating the Anti-Inflammatory and Antioxidant Effects of Broccoli Treated with High Hydrostatic Pressure in Cell Models. Foods. 2021;10:167. doi: 10.3390/foods10010167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kalt W., Cassidy A., Howard L.R., Krikorian R., Stull A.J., Tremblay F., Zamora-Ros R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. 2020;11:224–236. doi: 10.1093/advances/nmz065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ribera-Fonseca A., Jiménez D., Leal P., Riquelme I., Roa J.C., Alberdi M., Peek R.M., Reyes-Díaz M. The Anti-Proliferative and Anti-Invasive Effect of Leaf Extracts of Blueberry Plants Treated with Methyl Jasmonate on Human Gastric Cancer In Vitro Is Related to Their Antioxidant Properties. Antioxidants. 2020;9:45. doi: 10.3390/antiox9010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yuan J.M. Green tea and prevention of esophageal and lung cancers. Mol. Nutr. Food Res. 2011;55:886–904. doi: 10.1002/mnfr.201000637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Huang Y., Chen H., Zhou L., Li G., Yi D., Zhang Y., Wu Y., Liu X., Wu X., Song Q., et al. Association between green tea intake and risk of gastric cancer: A systematic review and dose–response meta-analysis of observational studies. Public Health Nutr. 2017;20:3183–3192. doi: 10.1017/S1368980017002208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Fujiki H., Watanabe T., Sueoka E., Rawangkan A., Suganuma M. Cancer Prevention with Green Tea and Its Principal Constituent, EGCG: From Early Investigations to Current Focus on Human Cancer Stem Cells. Mol. Cells. 2018;41:73–82. doi: 10.14348/molcells.2018.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cheng Z., Zhang Z., Han Y., Wang J., Wang Y., Chen X., Shao Y., Cheng Y., Zhou W., Lu X., et al. A review on anti-cancer effect of green tea catechins. J. Funct. Foods. 2020;74:104172. doi: 10.1016/j.jff.2020.104172. [DOI] [Google Scholar]
- 189.Halvorsen B.L., Holte K., Myhrstad M.C.W., Barikmo I., Hvattum E., Remberg S.F., Wold A.B., Haffner K., Baugerod H., Andersen L.F., et al. A systematic screening of total antioxidants in dietary plants. J. Nutr. 2002;132:461–471. doi: 10.1093/jn/132.3.461. [DOI] [PubMed] [Google Scholar]
- 190.Dragland S., Senoo H., Wake K., Holte K., Blomhoff R. Several culinary and medicinal herbs are important sources of dietary antioxidants. J. Nutr. 2003;133:1286–1290. doi: 10.1093/jn/133.5.1286. [DOI] [PubMed] [Google Scholar]
- 191.Gonçalves A.C., Nunes A.R., Falcão A., Alves G., Silva L.R. Dietary Effects of Anthocyanins in Human Health: A Comprehensive Review. Pharmaceuticals. 2021;14:690. doi: 10.3390/ph14070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Khoo H.E., Azlan A., Tang S.T., Lim S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017;61:1361779. doi: 10.1080/16546628.2017.1361779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Salehi B., Cappellini F., Reiner Ž., Zorzan D., Imran M., Sener B., Kilic M., Fahmy N.M., Martorell M., Tonelli C., et al. The Therapeutic Potential of Anthocyanins: Current Approaches Based on Their Molecular Mechanism of Action. Front. Pharmacol. 2020;11:1300. doi: 10.3389/fphar.2020.01300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Mohammed H.A., Khan R.A. Anthocyanins: Traditional Uses, Structural and Functional Variations, Approaches to Increase Yields and Products’ Quality, Hepatoprotection, Liver Longevity, and Commercial Products. Int. J. Mol. Sci. 2022;23:2149. doi: 10.3390/ijms23042149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Pennington J.A.T., Fisher R.A. Classification of fruits and vegetables. J. Food Compos. Anal. 2009;22:S23–S31. doi: 10.1016/j.jfca.2008.11.012. [DOI] [Google Scholar]
- 196.Khoo H.E., Prasad K.N., Kong K.W., Jiang Y., Ismail A. Carotenoids and their isomers: Color pigments in fruits and vegetables. Molecules. 2011;16:1710–1738. doi: 10.3390/molecules16021710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mukai R., Fukuda T., Ohnishi A., Nikawa T., Furusawa M., Terao J. Chocolate as a food matrix reduces the bioavailability of galloylated catechins from green tea in healthy women. Food Funct. 2021;12:408–416. doi: 10.1039/D0FO02485F. [DOI] [PubMed] [Google Scholar]
- 198.Bernatoniene J., Kopustinskiene D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules. 2018;23:965. doi: 10.3390/molecules23040965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Burri B.J., La Frano M.R., Zhu C. Absorption, metabolism, and functions of β-cryptoxanthin. Nutr. Rev. 2016;74:69–82. doi: 10.1093/nutrit/nuv064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.González-Peña M.A., Ortega-Regules A.E., Anaya de Parrodi C., Lozada-Ramírez J.D. Chemistry, Occurrence, Properties, Applications, and Encapsulation of Carotenoids—A Review. Plants. 2023;12:313. doi: 10.3390/plants12020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mutalipassi M., Esposito R., Ruocco N., Viel T., Costantini M., Zupo V. Bioactive Compounds of Nutraceutical Value from Fishery and Aquaculture Discards. Foods. 2021;10:1495. doi: 10.3390/foods10071495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Liang H., Ji K., Ge X., Mi H., Xi B., Ren M. Effects of dietary copper on growth, antioxidant capacity and immune responses of juvenile blunt snout bream (Megalobrama amblycephala) as evidenced by pathological examination. Aquac. Rep. 2020;17:100296. doi: 10.1016/j.aqrep.2020.100296. [DOI] [Google Scholar]
- 203.Ramesh P., Jagadeesan R., Sekaran S., Dhanasekaran A., Vimalraj S. Flavonoids: Classification, Function, and Molecular Mechanisms Involved in Bone Remodelling. Front. Endocrinol. 2021;12:779638. doi: 10.3389/fendo.2021.779638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lu X., Zhao C., Shi H., Liao Y., Xu F., Du H., Xiao H., Zheng J. Nutrients and bioactives in citrus fruits: Different citrus varieties, fruit parts, and growth stages. Crit. Rev. Food Sci. Nutr. 2021:1–24. doi: 10.1080/10408398.2021.1969891. [DOI] [PubMed] [Google Scholar]
- 205.Wang Y., Liu X.J., Chen J.B., Cao J.P., Li X., Sun C.D. Citrus flavonoids and their antioxidant evaluation. Crit. Rev. Food Sci. Nutr. 2022;62:3833–3854. doi: 10.1080/10408398.2020.1870035. [DOI] [PubMed] [Google Scholar]
- 206.Khan J., Deb P.K., Priya S., Medina K.D., Devi R., Walode S.G., Rudrapal M. Dietary Flavonoids: Cardioprotective Potential with Antioxidant Effects and Their Pharmacokinetic, Toxicological and Therapeutic Concerns. Molecules. 2021;26:4021. doi: 10.3390/molecules26134021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Singh N.P., Singh U.P., Rouse M., Zhang J., Chatterjee S., Nagarkatti P.S., Nagarkatti M. Dietary Indoles Suppress Delayed-Type Hypersensitivity by Inducing a Switch from Proinflammatory Th17 Cells to Anti-Inflammatory Regulatory T Cells through Regulation of MicroRNA. J. Immunol. 2016;196:1108–1122. doi: 10.4049/jimmunol.1501727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wu Y., Li R.W., Huang H., Fletcher A., Yu L., Pham Q., Yu L., He Q., Wang T.T.Y. Inhibition of Tumor Growth by Dietary Indole-3-Carbinol in a Prostate Cancer Xenograft Model May Be Associated with Disrupted Gut Microbial Interactions. Nutrients. 2019;11:467. doi: 10.3390/nu11020467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Abreu A.C., Coqueiro A., Sultan A.R., Lemmens N., Kim H.K., Verpoorte R., van Wamel W.J.B., Simões M., Choi Y.H. Looking to nature for a new concept in antimicrobial treatments: Isoflavonoids from Cytisus striatus as antibiotic adjuvants against MRSA. Sci. Rep. 2017;7:3777. doi: 10.1038/s41598-017-03716-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Stanisławska I.J., Figat R., Kiss A.K., Bobrowska-Korczak B. Essential Elements and Isoflavonoids in the Prevention of Prostate Cancer. Nutrients. 2022;14:1225. doi: 10.3390/nu14061225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Osmakov D.I., Kalinovskii A.P., Belozerova O.A., Andreev Y.A., Kozlov S.A. Lignans as Pharmacological Agents in Disorders Related to Oxidative Stress and Inflammation: Chemical Synthesis Approaches and Biological Activities. Int. J. Mol. Sci. 2022;23:6031. doi: 10.3390/ijms23116031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Rodríguez-García C., Sánchez-Quesada C., Toledo E., Delgado-Rodríguez M., Gaforio J.J. Naturally Lignan-Rich Foods: A Dietary Tool for Health Promotion? Molecules. 2019;24:917. doi: 10.3390/molecules24050917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Li L.H., Lee J.C., Leung H.H., Lam W.C., Fu Z., Lo A.C.Y. Lutein Supplementation for Eye Diseases. Nutrients. 2020;12:1721. doi: 10.3390/nu12061721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Manzoor S., Rashid R., Prasad Panda B., Sharma V., Azhar M. Green extraction of lutein from marigold flower petals, process optimization and its potential to improve the oxidative stability of sunflower oil. Ultrason. Sonochem. 2022;85:105994. doi: 10.1016/j.ultsonch.2022.105994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Khan U.M., Sevindik M., Zarrabi A., Nami M., Ozdemir B., Kaplan D.N., Selamoglu Z., Hasan M., Kumar M., Alshehri M.M., et al. Lycopene: Food Sources, Biological Activities, and Human Health Benefits. Oxidative Med. Cell. Longev. 2021;2021:2713511. doi: 10.1155/2021/2713511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Mozos I., Stoian D., Caraba A., Malainer C., Horbańczuk J.O., Atanasov A.G. Lycopene and Vascular Health. Front. Pharmacol. 2018;9:521. doi: 10.3389/fphar.2018.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Li L., Yang X. The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions. Oxidative Med. Cell. Longev. 2018;2018:7580707. doi: 10.1155/2018/7580707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Goñi I., Hernández-Galiot A. Intake of Nutrient and Non-Nutrient Dietary Antioxidants. Contribution of Macromolecular Antioxidant Polyphenols in an Elderly Mediterranean Population. Nutrients. 2019;11:2165. doi: 10.3390/nu11092165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Nani A., Murtaza B., Sayed Khan A., Khan N.A., Hichami A. Antioxidant and Anti-Inflammatory Potential of Polyphenols Contained in Mediterranean Diet in Obesity: Molecular Mechanisms. Molecules. 2021;26:985. doi: 10.3390/molecules26040985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zhang Y., Liu K., Yan C., Yin Y., He S., Qiu L., Li G. Natural Polyphenols for Treatment of Colorectal Cancer. Molecules. 2022;27:8810. doi: 10.3390/molecules27248810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ahmad N., Qamar M., Yuan Y., Nazir Y., Wilairatana P., Mubarak M.S. Dietary Polyphenols: Extraction, Identification, Bioavailability, and Role for Prevention and Treatment of Colorectal and Prostate Cancers. Molecules. 2022;27:2831. doi: 10.3390/molecules27092831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Patra S., Pradhan B., Nayak R., Behera C., Das S., Patra S.K., Efferth T., Jena M., Bhutia S.K. Dietary polyphenols in chemoprevention and synergistic effect in cancer: Clinical evidences and molecular mechanisms of action. Phytomedicine. 2021;90:153554. doi: 10.1016/j.phymed.2021.153554. [DOI] [PubMed] [Google Scholar]
- 223.Intra J., Kuo S.M. Physiological levels of tea catechins increase cellular lipid antioxidant activity of vitamin C and vitamin E in human intestinal caco-2 cells. Chem. Biol. Interact. 2007;169:91–99. doi: 10.1016/j.cbi.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Gebicki J.M., Nauser T. Fast Antioxidant Reaction of Polyphenols and Their Metabolites. Antioxidants. 2021;10:1297. doi: 10.3390/antiox10081297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Khatoon S., Kalam N., Shaikh M.F., Hasnain M.S., Hafiz A.K., Ansari M.T. Nanoencapsulation of Polyphenols as Drugs and Supplements for Enhancing Therapeutic Profile—A Review. Curr. Mol. Pharmacol. 2022;15:77–107. doi: 10.2174/1874467214666210922120924. [DOI] [PubMed] [Google Scholar]
- 226.Hossen M.S., Ali M.Y., Jahurul M.H.A., Abdel-Daim M.M., Gan S.H., Khalil M.I. Beneficial roles of honey polyphenols against some human degenerative diseases: A review. Pharmacol. Rep. 2017;69:1194–1205. doi: 10.1016/j.pharep.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 227.Durazzo A., Lucarini M., Souto E.B., Cicala C., Caiazzo E., Izzo A.A., Novellino E., Santini A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019;33:2221–2243. doi: 10.1002/ptr.6419. [DOI] [PubMed] [Google Scholar]
- 228.Jenkins D.J.A., Kitts D., Giovannucci E.L., Sahye-Pudaruth S., Paquette M., Blanco Mejia S., Patel D., Kavanagh M., Tsirakis T., Kendall C.W.C., et al. Selenium, antioxidants, cardiovascular disease, and all-cause mortality: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2020;112:1642–1652. doi: 10.1093/ajcn/nqaa245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zheng Y., Xie T., Li S., Wang W., Wang Y., Cao Z., Yang H. Effects of Selenium as a Dietary Source on Performance, Inflammation, Cell Damage, and Reproduction of Livestock Induced by Heat Stress: A Review. Front. Immunol. 2022;12:820853. doi: 10.3389/fimmu.2021.820853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Flohé L. The glutathione peroxidase reaction: Molecular basis of the antioxidant function of selenium in mammals. Curr. Top. Cell. Regul. 1985;27:473–478. doi: 10.1016/b978-0-12-152827-0.50047-5. [DOI] [PubMed] [Google Scholar]
- 231.Tsuji P.A., Santesmasses D., Lee B.J., Gladyshev V.N., Hatfield D.L. Historical Roles of Selenium and Selenoproteins in Health and Development: The Good, the Bad and the Ugly. Int. J. Mol. Sci. 2021;23:5. doi: 10.3390/ijms23010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Labunskyy V.M., Hatfield D.L., Gladyshev V.N. Selenoproteins: Molecular pathways and physiological roles. Physiol. Rev. 2014;94:739–777. doi: 10.1152/physrev.00039.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kieliszek M. Selenium–Fascinating Microelement, Properties and Sources in Food. Molecules. 2019;24:1298. doi: 10.3390/molecules24071298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zhou X., Yang J., Kronzucker H.J., Shi W. Selenium Biofortification and Interaction With Other Elements in Plants: A Review. Front. Plant Sci. 2020;11:586421. doi: 10.3389/fpls.2020.586421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Connelly-Frost A., Poole C., Satia J.A., Kupper L.L., Millikan R., Sandler R.S. Selenium, folate, and colon cancer. Nutr. Cancer. 2009;61:165–178. doi: 10.1080/01635580802404188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Alfieri M.L., Panzella L., Amorati R., Cariola A., Valgimigli L., Napolitano A. Role of Sulphur and Heavier Chalcogens on the Antioxidant Power and Bioactivity of Natural Phenolic Compounds. Biomolecules. 2022;12:90. doi: 10.3390/biom12010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Mukwevho E., Ferreira Z., Ayeleso A. Potential role of sulfur-containing antioxidant systems in highly oxidative environments. Molecules. 2014;19:19376–19389. doi: 10.3390/molecules191219376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Beydoun M.A., Canas J.A., Fanelli-Kuczmarski M.T., Maldonado A.I., Shaked D., Kivimaki M., Evans M.K., Zonderman A.B. Association of Antioxidant Vitamins A, C, E and Carotenoids with Cognitive Performance over Time: A Cohort Study of Middle-Aged Adults. Nutrients. 2020;12:3558. doi: 10.3390/nu12113558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Różanowska M.B., Czuba-Pełech B., Różanowski B. Is There an Optimal Combination of AREDS2 Antioxidants Zeaxanthin, Vitamin E and Vitamin C on Light-Induced Toxicity of Vitamin A Aldehyde to the Retina? Antioxidants. 2022;11:1132. doi: 10.3390/antiox11061132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Pullar J.M., Carr A.C., Vissers M.C.M. The Roles of Vitamin C in Skin Health. Nutrients. 2017;9:866. doi: 10.3390/nu9080866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Reider C.A., Chung R.Y., Devarshi P.P., Grant R.W., Hazels Mitmesser S. Inadequacy of Immune Health Nutrients: Intakes in US Adults, the 2005–2016 NHANES. Nutrients. 2020;12:1735. doi: 10.3390/nu12061735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Hornung T.C., Biesalski H. Glut-1 explains the evolutionary advantage of the loss of endogenous vitamin C-synthesis. The electron transfer hypothesis. Evol. Med. Public Health. 2019;1:221–231. doi: 10.1093/emph/eoz024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Cisternas P., Silva-Alvarez C., Martínez F., Fernández E., Ferrada L., Oyarce K., Salazar K., Bolanos J.P., Nualart F. The oxidized form of vitamin C, dehydroascorbic acid, regulates neuronal energy metabolism. J. Neurochem. 2014;129:663–671. doi: 10.1111/jnc.12663. [DOI] [PubMed] [Google Scholar]
- 244.Doostabadi M.R., Hassanzadeh-Taheri M., Asgharzadeh M., Mohammadzadeh M. Protective effect of vitamin E on sperm parameters, chromatin quality, and DNA fragmentation in mice treated with different doses of ethanol: An experimental study. Int. J. Reprod. Biomed. 2021;19:525–536. doi: 10.18502/ijrm.v19i6.9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Donnelly J., Appathurai A., Yeoh H.-L., Driscoll K., Faisal W. Vitamin E in Cancer Treatment: A Review of Clinical Applications in Randomized Control Trials. Nutrients. 2022;14:4329. doi: 10.3390/nu14204329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Jiang Q., Im S., Wagner J.G., Hernandez M.L., Peden D.B. Gamma-tocopherol, a major form of vitamin E in diets: Insights into antioxidant and anti-inflammatory effects, mechanisms, and roles in disease management. Free Radic. Biol. Med. 2022;178:347–359. doi: 10.1016/j.freeradbiomed.2021.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Wen Y., Xu L., Xue C., Jiang X., Wei Z. Assessing the Impact of Oil Types and Grades on Tocopherol and Tocotrienol Contents in Vegetable Oils with Chemometric Methods. Molecules. 2020;25:5076. doi: 10.3390/molecules25215076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Skrajnowska D., Bobrowska-Korczak B. Role of Zinc in Immune System and Anti-Cancer Defense Mechanisms. Nutrients. 2019;11:2273. doi: 10.3390/nu11102273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Tupe R.S., Tupe S.G., Tarwadi K.V., Agte V.V. Effect of different dietary zinc levels on hepatic antioxidant and micronutrients indices under oxidative stress conditions. Metabolism. 2010;59:1603–1611. doi: 10.1016/j.metabol.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 250.Pelton R. Coenzyme Q10: A Miracle Nutrient Advances in Understanding. Integr. Med. 2020;19:16–20. [PMC free article] [PubMed] [Google Scholar]
- 251.Saini R. Coenzyme Q10: The essential nutrient. J. Pharm. Bioallied Sci. 2011;3:466–467. doi: 10.4103/0975-7406.84471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Sifuentes-Franco S., Sánchez-Macías D.C., Carrillo-Ibarra S., Rivera-Valdés J.J., Zuñiga L.Y., Sánchez-López V.A. Antioxidant and Anti-Inflammatory Effects of Coenzyme Q10 Supplementation on Infectious Diseases. Healthcare. 2022;10:487. doi: 10.3390/healthcare10030487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Ayunin Q., Miatmoko A., Soeratri W., Erawati T., Susanto J., Legowo D. Improving the anti-ageing activity of coenzyme Q10 through protransfersome-loaded emulgel. Sci. Rep. 2022;12:906. doi: 10.1038/s41598-021-04708-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Zozina V.I., Covantev S., Goroshko O.A., Krasnykh L.M., Kukes V.G. Coenzyme Q10 in Cardiovascular and Metabolic Diseases: Current State of the Problem. Curr. Cardiol. Rev. 2018;14:164–174. doi: 10.2174/1573403X14666180416115428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Gutierrez-Mariscal F.M., de la Cruz-Ares S., Torres-Peña J.D., Alcalá-Diaz J.F., Yubero-Serrano E.M., López-Miranda J. Coenzyme Q10 and Cardiovascular Diseases. Antioxidants. 2021;10:906. doi: 10.3390/antiox10060906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Li J., Zheng S., Cheng T., Li Y., Mai X., Jiang G., Yang Y., Zhang Q., Li J., Zheng L., et al. Decylubiquinone Inhibits Colorectal Cancer Growth Through Upregulating Sirtuin2. Front. Pharmacol. 2022;12:804265. doi: 10.3389/fphar.2021.804265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Thapa M., Dallmann G. Role of coenzymes in cancer metabolism. Semin. Cell Dev. Biol. 2020;98:44–53. doi: 10.1016/j.semcdb.2019.05.027. [DOI] [PubMed] [Google Scholar]
- 258.Shidal C., Yoon H.S., Zheng W., Wu J., Franke A.A., Blot W.J., Shu X., Cai Q. Prospective study of plasma levels of coenzyme Q10 and lung cancer risk in a low-income population in the Southeastern United States. Cancer Med. 2021;10:1439–1447. doi: 10.1002/cam4.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Kiremitli T., Kiremitli S., Akselim B., Yilmaz B., Mammadov R., Tor I.H., Yazici G.N., Gulaboglu M. Protective effect of Coenzyme Q10 on oxidative ovarian and uterine damage induced by methotrexate in rats. Hum. Exp. Toxicol. 2021;40:1537–1544. doi: 10.1177/09603271211002891. [DOI] [PubMed] [Google Scholar]
- 260.Alimohammadi M., Rahimi A., Faramarzi F., Golpour M., Jafari-Shakib R., Alizadeh-Navaei R., Rafiei A. Effects of coenzyme Q10 supplementation on inflammation, angiogenesis, and oxidative stress in breast cancer patients: A systematic review and meta-analysis of randomized controlled-trials. Inflammopharmacology. 2021;29:579–593. doi: 10.1007/s10787-021-00817-8. [DOI] [PubMed] [Google Scholar]
- 261.Alcázar-Fabra M., Navas P., Brea-Calvo G. Coenzyme Q biosynthesis and its role in the respiratory chain structure. Biochim. Biophys. Acta. 2016;1857:1073–1078. doi: 10.1016/j.bbabio.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 262.Fehér A., Gazdecki M., Véha M., Szakály M., Szakály Z. A Comprehensive Review of the Benefits of and the Barriers to the Switch to a Plant-Based Diet. Sustainability. 2020;12:4136. doi: 10.3390/su12104136. [DOI] [Google Scholar]
- 263.Dressler J., Storz M.A., Müller C., Kandil F.I., Kessler C.S., Michalsen A., Jeitler M. Does a Plant-Based Diet Stand Out for Its Favorable Composition for Heart Health? Dietary Intake Data from a Randomized Controlled Trial. Nutrients. 2022;14:4597. doi: 10.3390/nu14214597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Giromini C., Givens D.I. Benefits and Risks Associated with Meat Consumption during Key Life Processes and in Relation to the Risk of Chronic Diseases. Foods. 2022;11:2063. doi: 10.3390/foods11142063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Barthelmie R.J. Impact of Dietary Meat and Animal Products on GHG Footprints: The UK and the US. Climate. 2022;10:43. doi: 10.3390/cli10030043. [DOI] [Google Scholar]
- 266.Mathur M.B., Peacock J., Reichling D.B., Nadler J., Bain P.A., Gardner C.D., Robinson T.N. Interventions to reduce meat consumption by appealing to animal welfare: Meta-analysis and evidence-based recommendations. Appetite. 2021;164:105277. doi: 10.1016/j.appet.2021.105277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Bryant C.J. Plant-based animal product alternatives are healthier and more environmentally sustainable than animal products. Future Foods. 2022;6:100174. doi: 10.1016/j.fufo.2022.100174. [DOI] [Google Scholar]
- 268.Sansone C., Brunet C. Marine Algal Antioxidants. Antioxidants. 2020;2:206. doi: 10.3390/antiox9030206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Šimat V., Elabed N., Kulawik P., Ceylan Z., Jamroz E., Yazgan H., Čagalj M., Regenstein J.M., Özogul F. Recent Advances in Marine-Based Nutraceuticals and Their Health Benefits. Mar. Drugs. 2020;18:627. doi: 10.3390/md18120627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Vladkova T., Georgieva N., Staneva A., Gospodinova D. Recent Progress in Antioxidant Active Substances from Marine Biota. Antioxidants. 2022;11:439. doi: 10.3390/antiox11030439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Rezayian M., Niknam V., Ebrahimzadeh H. Oxidative Damage and Antioxidative System in Algae. Toxicol. Rep. 2019;6:1309–1313. doi: 10.1016/j.toxrep.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Nedumaran T., Arulbalachandran D. Environmental Sustainability: Role of Green Technologies. Springer; New Delhi, India: 2015. Seaweeds: A promising source for sustainable development; pp. 65–88. [Google Scholar]
- 273.Tziveleka L.A., Tammam M.A., Tzakou O., Roussis V., Ioannou E. Metabolites with Antioxidant Activity from Marine Macroalgae. Antioxidants. 2021;10:1431. doi: 10.3390/antiox10091431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Harb T.B., Pereira M.S., Cavalcanti M.I.L.G., Fujii M.T., Chow F. Antioxidant Activity and Related Chemical Composition of Extracts from Brazilian Beach-Cast Marine Algae: Opportunities of Turning a Waste into a Resource. J. Appl. Phycol. 2021;33:3383–3395. doi: 10.1007/s10811-021-02446-8. [DOI] [Google Scholar]
- 275.Begum R., Howlader S., Mamun-Or-Rashid A.N.M., Rafiquzzaman S.M., Ashraf G.M., Albadrani G.M., Sayed A.A., Peluso I., Abdel-Daim M.M., Uddin M.S. Antioxidant and Signal-Modulating Effects of Brown Seaweed-Derived Compounds against Oxidative Stress-Associated Pathology. Oxidative Med. Cell. Longev. 2021;2021:9974890. doi: 10.1155/2021/9974890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Kosanić M., Ranković B., Stanojković T. Biological Activities of Two Macroalgae from Adriatic Coast of Montenegro. Saudi J. Biol. Sci. 2015;22:390–397. doi: 10.1016/j.sjbs.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Hamdy O., Karim A.-, Farouk Gheda S., Ismail G.A., Abo-Shady A.M., Assistant Of Phycology T. Phytochemical Screening and Antioxidant Activity of Chlorella Vulgaris. Delta J. Sci. 2020;41:81–91. [Google Scholar]
- 278.Haq S.H., Al-Ruwaished G., Al-Mutlaq M.A., Naji S.A., Al-Mogren M., Al-Rashed S., Ain Q.T., Al-Amro A.A., Al-Mussallam A. Antioxidant, Anticancer Activity and Phytochemical Analysis of Green Algae, Chaetomorpha Collected from the Arabian Gulf. Sci. Rep. 2019;9:18906. doi: 10.1038/s41598-019-55309-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Sonani R.R., Rastogi R.P., Madamwar D. Chapter 5—Natural Antioxidants From Algae: A Therapeutic Perspective. In: Rastogi R.P., Madamwar D., Pandey A., editors. Algal Green Chemistry. Elsevier; Amsterdam, The Netherlands: 2017. pp. 91–120. [Google Scholar]
- 280.Galasso C., Corinaldesi C., Sansone C. Carotenoids from Marine Organisms: Biological Functions and Industrial Applications. Antioxidants. 2017;6:96. doi: 10.3390/antiox6040096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Coulombier N., Jauffrais T., Lebouvier N. Antioxidant Compounds from Microalgae: A Review. Mar. Drugs. 2021;19:549. doi: 10.3390/md19100549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Ferdous U.T., Yusof Z.N.B. Medicinal Prospects of Antioxidants From Algal Sources in Cancer Therapy. Front. Pharmacol. 2021;12:593116. doi: 10.3389/fphar.2021.593116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Wang Q., Decker E.A., Rao J., Chen B. A combination of monoacylglycerol crystalline network and hydrophilic antioxidants synergistically enhances the oxidative stability of gelled algae oil. Food Funct. 2019;1:315–324. doi: 10.1039/C8FO00997J. [DOI] [PubMed] [Google Scholar]
- 284.Lordan S., Ross R.P., Stanton C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases. Mar. Drugs. 2011;9:1056–1100. doi: 10.3390/md9061056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Pulz O., Gross W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004;65:635–648. doi: 10.1007/s00253-004-1647-x. [DOI] [PubMed] [Google Scholar]
- 286.Guedes A.C., Amaro H.M., Malcata F.X. Microalgae as Sources of Carotenoids. Mar. Drugs. 2011;9:625. doi: 10.3390/md9040625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Pradhan B., Nayak R., Patra S., Jit B.P., Ragusa A., Jena M. Bioactive Metabolites from Marine Algae as Potent Pharmacophores against Oxidative Stress-Associated Human Diseases: A Comprehensive Review. Molecules. 2020;26:37. doi: 10.3390/molecules26010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Sangeetha R.K., Bhaskar N., Baskaran V. Comparative effects of beta carotene and fucoxanthin on retinol deficiency induced oxidative stress in rats. Mol. Cell. Biochem. 2009;331:59–67. doi: 10.1007/s11010-009-0145-y. [DOI] [PubMed] [Google Scholar]
- 289.Dembitsky V.M., Maoka T. Allenic and cumulenic lipids. Prog. Lipid Res. 2007;46:328–375. doi: 10.1016/j.plipres.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 290.Din N.A.S., Mohd Alayudin S., Sofian-Seng N.S., Rahman H.A., Mohd Razali N.S., Lim S.J., Wan Mustapha W.A. Brown Algae as Functional Food Source of Fucoxanthin: A Review. Foods. 2022;11:2235. doi: 10.3390/foods11152235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Getachew A.T., Saravana P.S., Cho Y.J., Woo H.C., Chun B.S. Concurrent extraction of oil from roasted coffee (Coffea arabica) and fucoxanthin from brown seaweed (Saccharina japonica) using supercritical carbon dioxide. J. CO2 Util. 2018;25:137–146. doi: 10.1016/j.jcou.2018.03.018. [DOI] [Google Scholar]
- 292.Ambati R.R., Phang S.-M., Ravi S., Aswathanarayana R.G. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs. 2014;12:128–152. doi: 10.3390/md12010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Higuera-Ciapara I., Felix-Valenzuela L., Goycoolea F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006;46:185–196. doi: 10.1080/10408690590957188. [DOI] [PubMed] [Google Scholar]
- 294.Pashkow F.J., Watumull D.G., Campbell C.L. Astaxanthin: A novel potential treatment for oxidative stress and inflammation in cardiovascular disease. Am. J. Cardiol. 2008;101:58D–68D. doi: 10.1016/j.amjcard.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 295.Sarada R., Tripathi U., Ravishankar G.A. Influence of stress on astaxanthin production in Haematococcus pluvialis grown under different culture conditions. Process Biochem. 2002;37:623–627. doi: 10.1016/S0032-9592(01)00246-1. [DOI] [Google Scholar]
- 296.Ranga Rao A. Ph.D. Thesis. University of Mysore; Mysore, India: May 15, 2011. Production of Astaxanthin from Cultured Green Alga Haematococcus pluvialis and Its Biological Activities. [Google Scholar]
- 297.Sarada R., Ranga Rao A., Sandesh B.K., Dayananda C., Anila N., Chauhan V.S., Ravishankar G.A. Influence of different culture conditions on yield of biomass and value added products in microalgae. Dyn. Biochem. Proc. Biotechnol. Mol. Biol. 2012;6:77–85. [Google Scholar]
- 298.Stachowiak B., Szulc P. Astaxanthin for the Food Industry. Molecules. 2021;26:2666. doi: 10.3390/molecules26092666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Bjørklund G., Gasmi A., Lenchyk L., Shanaida M., Zafar S., Mujawdiya P.K., Lysiuk R., Antonyak H., Noor S., Akram M., et al. The Role of Astaxanthin as a Nutraceutical in Health and Age-Related Conditions. Molecules. 2022;27:7167. doi: 10.3390/molecules27217167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Liu M., Hansen P.E., Lin X. Bromophenols in marine algae and their bioactivities. Mar. Drugs. 2011;9:1273–1292. doi: 10.3390/md9071273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Gross H., König G.M. Terpenoids from marine organisms: Unique structures and their pharmacological potential. Phytochem. Rev. 2006;5:115–141. doi: 10.1007/s11101-005-5464-3. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data is contained within the article.