Abstract
Thioredoxin and thioredoxin reductase are evolutionarily conserved antioxidant enzymes that protect organisms from oxidative stress. These proteins also play roles in redox signaling and can act as a redox-independent cellular chaperone. In most organisms, there is a cytoplasmic and mitochondrial thioredoxin system. A number of studies have examined the role of thioredoxin and thioredoxin reductase in determining longevity. Disruption of either thioredoxin or thioredoxin reductase is sufficient to shorten lifespan in model organisms including yeast, worms, flies and mice, thereby indicating conservation across species. Similarly, increasing the expression of thioredoxin or thioredoxin reductase can extend longevity in multiple model organisms. In humans, there is an association between a specific genetic variant of thioredoxin reductase and lifespan. Overall, the cytoplasmic and mitochondrial thioredoxin systems are both important for longevity.
Keywords: aging, lifespan, thioredoxin, reactive oxygen species, redox signaling, animal models, C. elegans, Drosophila, mouse models, genetics
1. Introduction
Aging is an intrinsic process that causes a progressive loss of function over time that increases the probability of death. While the aging process remains incompletely understood, research in multiple model organisms has begun to unravel the molecular mechanisms involved. In yeast, worms, flies and mice, modulating the expression of a single gene out of thousands of genes is sufficient to affect lifespan, thereby providing insight into the genetic pathways that determine longevity.
The Free Radical Theory of Aging proposes that aging results primarily from the accumulation of oxidative damage caused by reactive oxygen species (ROS) [1]. ROS are highly reactive oxygen-containing molecules that can damage cellular components, including DNA, proteins, and lipids. ROS are generated during normal cellular metabolism, but their levels can be increased through exposure to environmental stressors or internal stressors such as inflammation or metabolic dysfunction. In order to detoxify ROS and repair ROS-mediated damage, organisms have evolved to express antioxidant enzymes, including thioredoxin (TRX/TXN) and thioredoxin reductase (TRXR/TXNRD).
Thioredoxin and thioredoxin reductase combine to form thioredoxin systems in different compartments of the cell and play a crucial role in regulating cellular redox homeostasis [2]. The thioredoxin system acts to reduce proteins, both to repair oxidative stress and modulate their activity. Thioredoxin also plays an important role in intracellular signaling. In this review, we discuss the different roles of thioredoxin and thioredoxin reductase in the cell and how these proteins affect longevity in different model organisms.
2. Antioxidant Roles of Thioredoxin and Thioredoxin Reductase
The thioredoxin system is a crucial redox regulatory system consisting of thioredoxin and its reducing partner thioredoxin reductase, which uses nicotinamide adenine dinucleotide phosphate (NADPH) as an electron donor to reduce thioredoxin (Figure 1). Thioredoxin possesses a unique tertiary structure composed of five β-strands forming the internal core of the protein, four α-helices and a short stretch of helix surrounding the central β-sheets [3] (Figure 2). The active site disulfide is located after the β2-sheet and forms the N-terminal portion of α2. A cis-proline located in a loop preceding β-strand 4 is crucial for the stability and function of thioredoxin. Thioredoxins are the main protein disulfide reductases in the cell and act as electron donors for enzymes via the reversible oxidation of two cysteine thiol groups (-CGPC-, also called CXXC motif, thioredoxin motif and thioredoxin fold) to a disulfide, which is crucial in the thiol-dependent antioxidant system [4]. The thioredoxin fold structure of thioredoxin is shared among a group of proteins that serve as key players in redox signaling and control, all of which can act to reduce disulfides. These proteins include glutaredoxin (GRX), glutathione peroxidase (GPX), glutathione transferase (GST), thioredoxin peroxidase (also known as peroxiredoxin or PRX) and protein disulfide isomerase (PDI) [5,6].
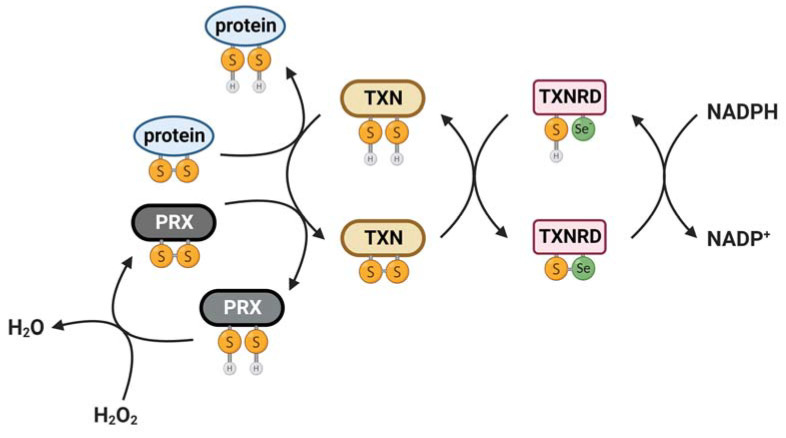
Figure 1.
Role of thioredoxin system in antioxidant defense. Thioredoxin (TXN) catalyzes the reduction of disulfides (S-S) within oxidized cellular proteins, which can act to restore protein function. An important target of thioredoxin is the antioxidant peroxiredoxin (PRX), which can detoxify hydrogen peroxide. In reducing target proteins, thioredoxin becomes oxidized. In order to reactivate thioredoxin, thioredoxin reductase (TXNRD) reduces TXN using reducing equivalents obtained from NADPH. S-S = oxidized form. SH = reduced form.
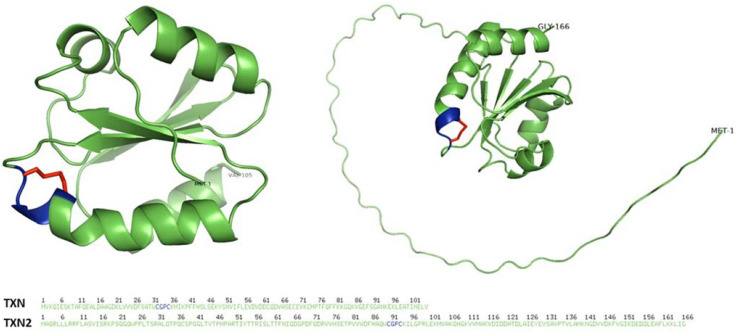
Figure 2.
Three-dimensional structure of human thioredoxins. The 3D structure of human cytoplasmic thioredoxin (TXN) (left) and mitochondrial thioredoxin 2 (TXN2) (right), in their oxidized forms, are shown (UniProt accession P10599 and Q99757, respectively). The 3D representation includes labeled N- and C-termini and highlights the active site thioredoxin fold in blue and the disulfide bond in red. The structures were generated using PyMOL (http://www.pymol.org/pymol, accessed on 31 March 2023) after retrieving TXN1 and TXN2 protein data bank (PDB) formats from AlphaFold [7,8].
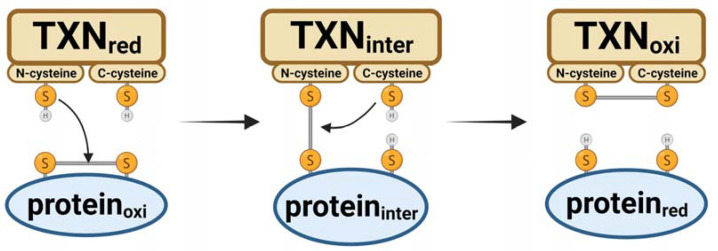
The main antioxidant function of thioredoxin involves the transfer of two electrons and two protons, resulting in the covalent interconversion of a disulfide and a dithiol. During this reaction, cysteines located at positions 32 and 35 of thioredoxin execute a bimolecular nucleophilic substitution mechanism to transfer electrons from thioredoxin to the substrate protein [5]. Firstly, the N-terminal cysteine of thioredoxin initiates a nucleophilic attack on the substrate protein’s disulfide bond, leading to the formation of a mixed disulfide bond between thioredoxin and the substrate protein (Figure 3). Subsequently, the C-terminal cysteine of thioredoxin initiates a nucleophilic attack on the intermediate intermolecular disulfide bond, forming a disulfide bond in the oxidized thioredoxin and breaking the disulfide bond in the reduced substrate protein.
Figure 3.
Mechanism of thioredoxin reaction to reduce oxidized proteins. The reduced (red) form of thioredoxin (TXN) can transfer two electrons and two protons to convert an oxidized protein’s disulfide to a dithiol. This reaction involves cysteines at positions 32 (N-terminus) and 35 (C-terminus) of thioredoxin that execute a bimolecular nucleophilic substitution to transfer electrons to the substrate protein. The process ends with an oxidized (oxi) thioredoxin. Inter = intermediate reactant. S-S = oxidized form. SH = reduced form.
Thioredoxin is maintained in its active and reduced form primarily by thioredoxin reductase but can also be reactivated by glutaredoxin in the glutathione (GSH) system [9,10]. Thioredoxin can act as an antioxidant either directly by quenching singlet oxygen and scavenging of hydroxyl radicals or indirectly by reducing proteins oxidized by ROS [11]. One of the most important targets of thioredoxin is peroxiredoxin, which acts to directly reduce peroxides such as H2O2 and various alkyl hydroperoxides [12,13]. Once peroxiredoxin reduces its target, thioredoxin restores peroxiredoxin activity by recycling the oxidized form of peroxiredoxin back to its reduced state.
Thioredoxin reductase is an oxidoreductase that uses NADPH to reduce the active-site disulfide of thioredoxin, thereby restoring thioredoxin’s activity [14]. Thioredoxin reductase contains flavin adenine dinucleotide (FAD) and pyridine nucleotide disulfide. Thioredoxin reductase exists as an antiparallel homodimer with both subunits playing a crucial role in the normal redox reaction during the catalytic cycle. Unlike bacteria and archaea, the active site of thioredoxin reductase in mammalian and multicellular eukaryotes comprises a conserved selenocysteine (Sec) that replaces the Cys2 residue located at the penultimate C-terminal position in its X-Cys1-Cys2-X motif (X is usually Gly or Ser), which is essential for its catalytic function [15]. This substitution confers several advantages including the superior nucleophilicity of Sec, which arises from its ionization under physiological conditions compared to the protonated Cys [16]. Additionally, the position of Sec in the C-terminus provides conformational flexibility that enables it to function as a cellular redox sensor [17].
The first step of the reductive half-reaction of the enzyme involves reduction of the enzyme-bound flavine adenine dinucleotide by NADPH in one subunit [14,18]. From there, the reducing equivalents are transferred to the Cys-Val-Asn-Val-Gly-Cys active site motif of the same subunit, forming a dithiol motif. This dithiol motif reduces the C-terminal selenenyl sulfide motif of the other subunit of the dimer, forming a dithiol or selenolthiol motif [14]. This reduced motif can then reduce the substrates of thioredoxin reductase, including the active site disulfide between positions 32 and 35 of thioredoxin, glutaredoxin 2 (GRX2), PDI, thioredoxin-like-1, granulysin [19,20] and some small molecule substrates such as selenite [21], dehydroascorbate [22], lipoic acid [23], ubiquinone [24], cytochrome C [25] or the cancer drugs motexafin gadolinium [26] and alloxan [27]. Thioredoxin reductase can function as an antioxidant given that it provides electrons to small molecules that can react directly with H2O2 [20,28]. Thus, the thioredoxin system is an essential redox regulatory system that interacts and collaborates with the glutathione system to maintain the redox balance and protect against oxidative stress in the organism.
3. Additional Roles of Thioredoxin and Thioredoxin Reductase: Redox Signaling
In addition to its roles in antioxidant defense, thioredoxin also affects metabolism and intracellular signaling by regulating protein activity (Figure 4). In transferring reducing equivalents from NADPH to target proteins, thioredoxin can modulate their function, structure or stability [29]. The modulation of enzyme activity can result in the binding of substrates or allosteric effectors, leading to metabolic changes. In addition, thioredoxin can activate several transcription factors through redox regulation by modulating their DNA-binding activities.
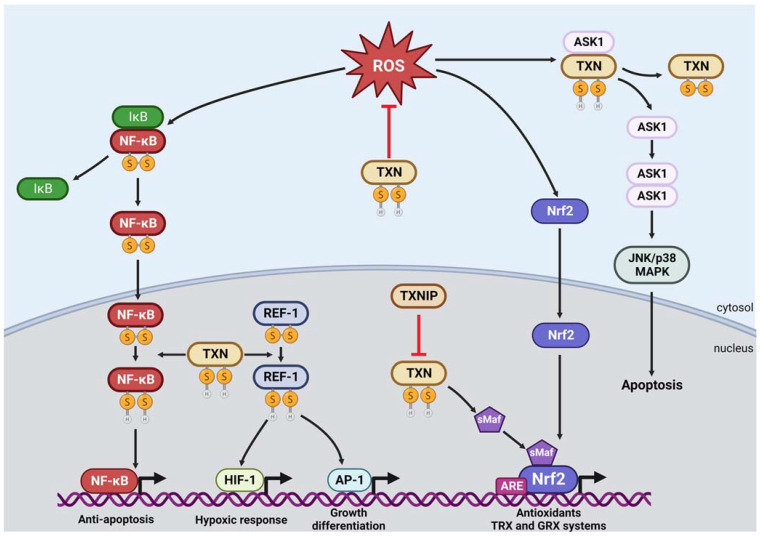
Figure 4.
Role of thioredoxin in redox signaling. Thioredoxin (TXN) negatively regulates apoptosis via redox regulation of ASK-1 and inhibition of Iκβ degradation by scavenging ROS in the cytoplasm. In the nucleus, TXN increases the DNA-binding activity of NF-κβ and can enhance the binding of Nrf2 to the antioxidant response element (ARE) through small Maf proteins (sMaf) via reduction of their cysteine residues. TXN also increases the DNA-binding activity of other transcription factors, such as AP-1 and HIF-1, indirectly via the reduction of intermediate Ref-1 cysteine residues. Thioredoxin-interacting protein (TXNIP) can inhibit TXN function by forming a mixed disulfide bond with its reduced form. Note that although thioredoxin reductase is not depicted in this figure, it is important for the reduction of thioredoxin into its reduced, active form. It is the reduced, active form of thioredoxin that contributes to redox signaling. Red lines indicate an inhibitory effect. S-S = oxidized form. SH = reduced form.
Under basal conditions, the redox-regulated apoptosis-signal kinase (ASK1), a member of the MAPKKK family, is directly bound to TXN and TXN2 to maintain low levels of ROS while the thioredoxin-interacting protein TXNIP resides in the nucleus. However, in response to oxidative stress, TXNIP translocates to the cytoplasm and mitochondria and disrupts the binding of TXN-ASK1 and TXN2-ASK1, respectively [30,31]. The disruption can also occur through increased ROS and lead to an overall ROS buildup, mitochondrial distress signaling and eventually an apoptotic signaling cascade. The cascade begins with the phosphorylation of unbound ASK1, leading to the release of cytochrome C and cleavage of caspase-3, initiating downstream apoptotic signaling.
Additionally, TXNIP inhibits TXN2 protection of mitochondria against ROS [32], leading to mtDNA oxidation and binding of the NOD-like receptor protein 3 (NLRP3) inflammasome [33], ultimately activating the inflammasome [34]. In addition, TXNIP expression is induced by the endoplasmic reticulum unfolded protein response (ERUPR) under the IRE1α and PERK-eIF2α pathways [35]. This activation results in the cleavage of pro-interleukin-1β to its active, mature form by caspase-1 and its subsequent production and secretion. Overall, the interaction between TXNIP and thioredoxin plays a crucial role in regulating cellular responses to oxidative stress, including apoptosis and inflammation [36].
Thioredoxin can directly reduce some transcription factors to negatively regulate apoptosis. For example, thioredoxin can activate the nuclear factor (NF)-κB, which regulates the expression of genes that antagonize cell death [37]. Under normal conditions, thioredoxin scavenges ROS in the cytoplasm and inhibits the degradation of IκB. However, increased ROS mediates the degradation of IκB and the nuclear translocation of NF-κB [38]. Nuclear thioredoxin then directly reduces a cysteine of NF-κB and allows NF-κB-dependent gene expression [39]. The role of thioredoxin in inhibiting the ASK-1 and NF-κB pathways suggests that ROS-induced apoptosis may serve as a protective mechanism against chronic oxidative stress.
Thioredoxin can indirectly activate transcription factors responsible for promoting cell viability in response to adverse conditions such as oxidative stress and hypoxia [6]. The DNA-binding activity of these transcription factors is regulated by specific Cys residues, which are reduced by reducing redox factor-1 (Ref-1) as an intermediate in the nucleus. In order for Ref-1 to catalyze this reduction, it needs to be in its reduced form, which is catalyzed by thioredoxin [40]. In addition, Ref-1 is a DNA-repair endonuclease that is involved in the base excision repair (BER) pathway, which is responsible for repair of apurinic/apyrimidinic sites in DNA caused by ROS [41]. Notably, expression of Ref-1 is upregulated in response to oxidative stress [42]. One example of a transcription complex dependent on thioredoxin/Ref-1 interaction is Activator protein-1 (AP-1), the basic region-leucine zipper (bZIP) family of Jun and Fos [43]. Thioredoxin reduces AP-1 cysteines indirectly via Ref-1 and thereby increases the DNA-binding activity of AP-1 to regulate cell growth, differentiation and apoptosis. Another example of thioredoxin’s role in regulating cellular responses is the reduction of a single cysteine residue of the hypoxia-inducible factor 1α (HIF-1α) subunit by thioredoxin/Ref-1 during hypoxia [44,45]. This redox modification is essential for HIF-1 binding with CBP/p300 co-activator to initiate the hypoxic response element (HRE) target genes expression [45]. Like in the previous example, thioredoxin and thioredoxin reductase inhibitors have been shown to downregulate expression of HIF-1α and its subsequent activity [46]. Moreover, studies have demonstrated that thioredoxin and related redox proteins are upregulated in response to hypoxia [47,48], which further emphasizes thioredoxin’s role in HIF-1α regulation. Therefore, thioredoxin possesses multiple important functions in protecting cells from both oxidative stress and the hypoxic-stress response via Ref-1.
Overall, thioredoxin plays an important role in redox signaling in diverse cellular processes, including cell proliferation, differentiation, apoptosis and responses to oxidative stress.
4. Regulation of the Thioredoxin System
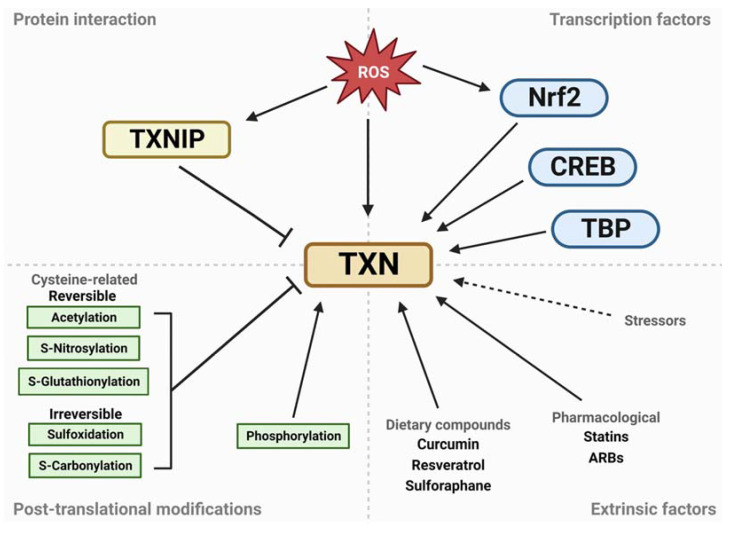
The activity of the thioredoxin system is regulated at multiple levels, including gene expression, post-translational modifications, and protein–protein interactions (Figure 5). These regulatory mechanisms allow the system to respond to changes in the cellular redox environment and adapt to different physiological or pathological conditions.
Figure 5.
Regulation of the thioredoxin system. The regulation of the thioredoxin system occurs at multiple levels, including gene expression, post-translational modifications, protein–protein interactions and extrinsic factors. The thioredoxin system is regulated at the gene expression level by transcription factors, including Nrf2, TBP and CREB, which bind to specific cis-regulatory elements located in the promoter regions of the genes that encode thioredoxin and thioredoxin reductase. Post-translational modifications, such as phosphorylation, acetylation or S-nitrosylation, can also modulate the activity of the thioredoxin system. Protein–protein interactions are also important for the regulation of the thioredoxin system, with TXNIP being an important endogenous molecule that negatively regulates the function of thioredoxin. In addition to these intrinsic regulatory mechanisms, the thioredoxin system can also be modulated by extrinsic factors such as dietary interventions, drugs or environmental stressors. The figure shows the different regulatory mechanisms that control the activity of the thioredoxin system and their effects on its function.
The thioredoxin system is regulated at the gene expression level by transcription factors, including the nuclear factor erythroid 2–related factor 2 (Nrf2), TATA-binding protein (TBP) and cAMP response element-binding protein (CREB) [49,50]. These transcription factors bind to specific cis-regulatory elements located in the promoter regions of the genes that encode thioredoxin and thioredoxin reductase and are activated in response to various stressors, including oxidative stress and inflammation. This activation induces the expression of the thioredoxin system components, which form part of the cellular stress response. For instance, oxidative stress triggers Nrf2 binding to the antioxidant response element (ARE) present in the thioredoxin promoter [51]. Similarly, the thioredoxin reductase and peroxiredoxin promoters also contain ARE elements that mediate upregulation of their expression in response to oxidative stress [50]. Notably, the reduced form of thioredoxin enhances the binding of Nrf2 to ARE by reducing conserved cysteine residues in the DNA-binding domains of small Maf proteins (sMaf), thereby activating Nrf2-transcription [51,52]. As a result, oxidative stress leads to an increase in thioredoxin levels, which in turn activates the transcription factors responsible for inducing even higher levels of thioredoxin and other antioxidants.
Post-translational modifications, such as phosphorylation, acetylation or S-nitrosylation, can also modulate the activity of the thioredoxin system. For example, phosphorylation of thioredoxin at specific residues has been shown to increase its activity or alter its substrate specificity [53], while S-nitrosylation [54] and glutathionylation [55] can impair its function by interfering with the formation of disulfide bonds.
Protein–protein interactions are also important for the regulation of the thioredoxin system. For instance, TXNIP, also known as thioredoxin binding protein-2 (TBP-2), is an important endogenous molecule that negatively regulates the function of thioredoxin [56,57]. There are two mechanisms through which TXNIP inhibits thioredoxin function and activity. Firstly, TXNIP competes with thioredoxin for binding sites, which removes thioredoxin from proteins that are inhibited by the steric effect of TXN1 binding, such as redox-regulated apoptosis-signal kinase 1 (ASK1) [30]. TXNIP inhibits thioredoxin activity in a redox-dependent manner by forming a mixed disulfide bond with reduced thioredoxin active site thiols through thioredoxin active site Cys32 and TXNIP Cys247 [57]. Secondly, the increased and overexpressed TXNIP, as seen in response to factors such as disturbed flow and high glucose [58,59], results in a reduction in the thioredoxin system activity. Thus, the increased formation of TXNIP-TXN complexes leads to a higher concentration of oxidized proteins when exposed to oxidative stress.
In addition to these intrinsic regulatory mechanisms, the thioredoxin system can also be modulated by extrinsic factors such as dietary interventions, drugs or environmental stressors. For example, several dietary compounds, such as resveratrol [60], curcumin [61,62] or sulforaphane [63], have been shown to modulate the expression and the activity of thioredoxin and other antioxidant enzymes. Similarly, some drugs, such as statins [64,65] or angiotensin receptor blockers [66,67], have been reported to enhance the activity of the thioredoxin system and reduce oxidative stress.
5. Cytoplasmic Thioredoxin System Contributes to Lifespan in Yeast
The thioredoxin system is evolutionarily conserved with multiple forms distributed in different compartments of the cell [68]. Yeast possesses a cytoplasmic thioredoxin system consisting of the thioredoxin TRX1 and thioredoxin reductase TRR1 and a mitochondrial thioredoxin system that includes the thioredoxin TRX3 and thioredoxin reductase TRR2 (Table S1). In yeast, replicative lifespan is measured as the number of cell divisions that a mother cell can undertake to produce daughter cells. Chronologic lifespan is the length of time that a yeast cell maintains the ability to generate new colonies in a non-dividing state, for example, when a specific cell density is reached in liquid culture. Disruption of the cytoplasmic thioredoxin gene TRX1 results in decreased chronologic lifespan [69,70], while disruption of the mitochondrial thioredoxin gene TRX3 has no effect on chronologic or replicative lifespan [71,72] (Table 1). Similarly, disruption of the cytoplasmic thioredoxin reductase gene TRR1 reduces chronologic lifespan [73], while loss of the mitochondrial thioredoxin reductase gene TRR2 does not affect chronologic or replicative lifespan [71,72]. Together, this indicates that the cytoplasmic thioredoxin system is required for normal longevity in yeast, while the mitochondrial thioredoxin system is dispensable.
6. Cytoplasmic Thioredoxin Is Important for Longevity in Caenorhabditis elegans
In C. elegans, there are at least five different thioredoxins (TRX-1, TRX-2, TRX-3, TRX-4 and TRX-5) and two thioredoxin reductases (TRXR-1 and TRXR-2). TRX-1 and TRXR-1 make up the cytoplasmic thioredoxin system, while TRX-2 and TRXR-2 form the mitochondrial thioredoxin system (Table S1). In C. elegans, disruption of the cytoplasmic thioredoxin gene trx-1 results in a clear decrease in lifespan in wild-type worms and multiple long-lived mutant strains [74,75,76,77] (Table 1). Deletion of trx-1 also results in decreased resistance to exogenous stressors and elevated levels of reactive oxygen species [74,75]. The large effect of trx-1 disruption on lifespan is perhaps surprising given that its expression is primarily limited to a small number of neurons (ASI and ASJ) and part of the intestine [75,76] and accounts for less than 1% of the total thioredoxin mRNA [74]. A role for trx-1 in determining lifespan is supported by the observation that overexpression of trx-1 is sufficient to extend longevity [77]. In contrast to trx-1, disruption of the cytoplasmic thioredoxin reductase gene trxr-1 does not affect lifespan [74,78,79], and trxr-1 mutants do not exhibit decreased survival after exposure to oxidative stress or other exogenous stressors [74,78,79]. Together, this suggests that trx-1 performs functions in the cell that are independent of trxr-1 and are important for lifespan and cellular resilience. It is possible that trx-1 can be re-activated by another enzyme or that redox-independent functions of trx-1 are important for stress resistance and longevity.
As in yeast, deletion of the mitochondrial thioredoxin gene trx-2 does not affect lifespan in wild-type worms [74,80], though it is required for lifespan extension in the long-lived mitochondrial mutants nuo-6 and isp-1 [74]. Disruption of trx-2 also has little or no detrimental effect on resistance to oxidative or other stresses in wild-type animals [74,80]. The lifespan of trxr-2 mitochondrial thioredoxin reductase mutants is equivalent to or slightly decreased compared to wild-type worms, depending on the precise experimental conditions [74,79,80]. Similar to trx-2, trxr-2 is specifically required for the extended longevity of nuo-6 and isp-1 mutants [74] but not long-lived daf-2 insulin/IGF-1 receptor mutants [80]. The loss of trxr-2 does not decrease resistance to oxidative or other stresses [74,80], despite resulting in increased levels of ROS [74,79].
Deletion of trx-3 does not affect lifespan or resistance to stress, while trx-3 overexpression provides modest protection against exposure to bacterial pathogens [81]. Overall, cytoplasmic thioredoxin is required for both lifespan and resistance to stress in C. elegans, while cytoplasmic thioredoxin reductase and both components of the mitochondrial thioredoxin system are dispensable. Although trx-1 is required to achieve a normal lifespan in C. elegans, this gene is not essential, as trx-1 mutants are viable, fertile and develop to adulthood.
7. Contribution of Thioredoxin Systems to Lifespan in Drosophila
Drosophila melanogaster possess a male-specific thioredoxin, thioredoxinT (TrxT), and a female specific thioredoxin, Deadhead (Dhd) and both of these are present in the nucleus in the germline. Drosophila also possess thioredoxin 2 (Trx-2), which is present in the nucleus. There are two thioredoxin reductases in Drosophila: Trxr-1 possess isoforms present in both the cytoplasm and mitochondria, while Trxr-2 is expressed in the mitochondria (Table S1). Disruption of either the male-specific thioredoxinT (TrxT) or female-specific deadhead (dhd) thioredoxin genes does not affect longevity in Drosophila [82] (Table 1). Overexpression of TrxT in all neurons results in increased lifespan and enhanced resistance to oxidative stress [83]. Disruption of the thioredoxin gene Trx-2 decreases lifespan [82,84], while overexpression of this gene extends longevity [82]. Trx-2 mutants have been shown to possess increased resistance to hydrogen-peroxide-mediated oxidative stress [82] but decreased resistance to paraquat-mediated oxidative stress [84]. Trx-2 overexpression flies show increased resistance to both types of oxidative stress [82,84], while flies lacking all three thioredoxin genes (TrxT, dhd and Trx-2) show decreased resistance to hydrogen-peroxide-mediated oxidative stress compared to wild-type flies [82].
Table 1.
Effect of thioredoxin systems on lifespan in model organisms.
| Organism | Gene | Location | Modulation | Effect on Lifespan | References |
|---|---|---|---|---|---|
| Yeast | TRX1 | Cytoplasm | Disruption | ↓ | [69,70] |
| TRR1 | Cytoplasm | Disruption | ↓ | [73] | |
| TRX3 | Mitochondria | Disruption | = | [71,72] | |
| TRR2 | Mitochondria | Disruption | = | [71,72] | |
| C. elegans | trx-1 | Cytoplasm | Disruption | ↓ | [74,75,76,77] |
| trx-1::GFP | Cytoplasm | Overexpression | ↑ | [77] | |
| trxr-1 | Cytoplasm | Disruption | = | [74,78,79] | |
| trx-2 | Mitochondria | Disruption | = | [74,80] | |
| trxr-2 | Mitochondria | Disruption | =/↓ | [74,79,80] | |
| trx-3 | Cytoplasm/Nucleus Intestine |
Disruption | = | [81] | |
| Drosophila | TrxT | Nucleus, male specific | Disruption | = | [82] |
| TrxT | Nucleus, male specific | Overexpression in all neurons | ↑ | [83] | |
| dhd | Nucleus, female specific | Disruption | = | [82] | |
| Trx-2 | Nucleus | Disruption | ↓ | [82,84] | |
| Trx-2 | Nucleus | Overexpression | ↑ | [82] | |
| TrxT-dhd-Trx-2 | Nucleus | Disruption | =/↓ | [82] | |
| Trxr-1 | Cytoplasm and Mitochondria | Disruption | Larval lethality | [85,86] | |
| Trxr-1 | Cytoplasm and Mitochondria | Overexpression | = | [87] | |
| Trxr-2 | Mitochondria | Overexpression | ↑ | [87] | |
| Vdup1 | Cytoplasm/Nucleus | Downregulation | ↑ | [88] | |
| Vdup1 | Cytoplasm/Nucleus | Overexpression | ↓ | [88] | |
| Mice | Txn1/Trx1 | Cytoplasmic | Disruption | Embryonic lethal | [89] |
| Txn1+/− | Cytoplasmic | Heterozygous disruption | = | [90,91] | |
| Human TXN | Cytoplasmic | Overexpression | ↑ | [92] | |
| Human TXN | Cytoplasmic | Overexpression | ↑ early lifespanin males | [93] | |
| Human TXN | Cytoplasmic | Overexpression | = | [94] | |
| Txnrd1 | Cytoplasmic | Disruption | Embryonic lethal | [95] | |
| Txn2/Trx2 | Mitochondria | Disruption | Embryonic lethal | [96] | |
| Txn2+/− | Mitochondria | Heterozygous disruption | = | [97] | |
| Human TXN2 | Mitochondria | Overexpression | ↑ early lifespan | [98] | |
| Human TXN1 + human TXN2 | Cytoplasmic and Mitochondria | Overexpression | ↓ | [99] | |
| Txn+/−; Txn2+/− | Cytoplasmic and Mitochondria | Heterozygous disruption | ↑ | [91] | |
| Txnrd2 | Mitochondria | Disruption | Embryonic lethal | [100] | |
| Rats | Human TXN | Cytoplasmic | Overexpression | = | [91] |
“↓” lifespan decreased compared to WT. “↑” lifespan increased compared to WT. “=” lifespan equivalent to WT.
A null mutation in the cytoplasmic thioredoxin reductase gene Trxr-1 results in larval lethality, while mutations that decrease Trxr-1 activity markedly reduce adult lifespan [85]. The lifespan deficit in Trxr-1 mutants is partially rescued by overexpression of catalase, suggesting that diminished oxidative stress defense contributes to the decrease in longevity [85]. Interestingly, Trxr-1 encodes a cytoplasmic and mitochondrial isoform, both of which affect longevity independently of the other isoform [86]. While overexpression of Trxr-1 is not sufficient to extend longevity [87], overexpression of the mitochondrial thioredoxin reductase gene Trxr-2 increases lifespan [87].
The thioredoxin-interacting protein TXNIP is a negative regulator of thioredoxin. Knockdown of Vdup1, the Drosophila homolog of TXNIP, with RNAi increases thioredoxin activity and results in an increase in mean lifespan and a slight increase in resistance to oxidative stress [88]. Overexpression of Vdup1 results in the opposite effect, decreasing thioredoxin activity, decreasing lifespan and decreasing oxidative stress resistance [88].
Overall, Drosophila lifespan is highly dependent on TRXR-1 activity, while TRX-2 modestly contributes to longevity.
8. Cytoplasmic and Mitochondrial Thioredoxin Systems Are Required for Survival in Mice
In mice, there is a cytoplasmic thioredoxin system consisting of the thioredoxin TXN1 and the thioredoxin reductase TXNRD1 and mitochondrial thioredoxin system consisting of the thioredoxin TXN2 and the thioredoxin reductase TXNRD2. Mice also have a third thioredoxin reductase TXNRD3, which is present in the cytoplasm and nucleus (Table S1). Mice that are homozygous for the targeted inactivation of the cytoplasmic thioredoxin gene Txn1/Trx1 are embryonic lethal [89], while heterozygous Txn1+/− mice have a wild-type lifespan [90,91] (Table 1). The targeted inactivation of the cytoplasmic thioredoxin reductase gene Txnrd1/TrxR1 also results in embryonic lethality [95]. Although one study reported that overexpression of human TXN under the human β-actin promoter significantly extends longevity in mice [92], a subsequent study using the same mice found that only early life survival in male mice was significantly increased via TXN overexpression [93]. This difference may have arisen due to a relatively short wild-type mouse lifespan in the earlier study. Nonetheless, both studies reported beneficial effects of TXN overexpression, including protection against focal ischemia [101], increased survival of isolated cells after UV stress [92], decreased oxidative damage [93] and increased resistance to oxidative stress [93]. A third study examined the effect of overexpressing human TXN under the endogenous TXN promoter, as expression from the β-actin promoter decreases with age, which may have accounted for the lack of effect on maximum lifespan in the second study. While there appeared to be a mild increase in survival at early time points, there was overall no statistically significant difference between TXN overexpressing mice and wild-type animals [94].As with the cytoplasmic thioredoxin system, the mitochondrial thioredoxin system is also essential for embryonic development in mice. Targeted inactivation of the mitochondrial thioredoxin gene Txn2/Trx2 [96] or the mitochondrial thioredoxin reductase gene Txnrd2/TrxR2 [100] results in embryonic lethality. Mice heterozygous for the Txn2 gene (Txn2+/− mice) possess a wild-type lifespan [102], despite exhibiting elevated levels of ROS and increased oxidative damage to DNA, protein and lipids [97]. Similar to overexpression of TXN, transgenic mice expressing increased levels of human TXN2 under the human endogenous promoter show a small increase in lifespan early in life but no change in maximum lifespan [98].
Interestingly, simultaneous overexpression of human TXN and TXN2 decreases lifespan in mice, despite the overexpression of each gene individually mildly increasing early lifespan [99]. The detrimental effect on longevity was attributed to an increased incidence of cancer. Consistent with this result, mice that are heterozygous for the inactivation of both Txn1 and Txn2 exhibit a significant increase in lifespan, which may be due to a decrease in cancer incidence [91]. Txnrd2-transgenic mice have recently been generated but their lifespan has yet to be determined [103]. Cells derived from the Txnrd2-transgenic mice exhibit increased resistance to oxidative stress. It will be interesting to determine the lifespan of these animals and the extent to which overexpression of Txnrd1 will affect longevity.
In summary, all components of the cytoplasmic and mitochondrial thioredoxin systems are essential for embryonic development in mouse models. It is unclear whether the lack of survival during embryonic development results from a severe shortening of lifespan or whether functional thioredoxin systems are required for important processes in embryonic development. To examine the effect of thioredoxin-system disruption on lifespan independent of embryonic development, one could generate adult-only knockout animals using a Cre/lox system or use an inducible expression system to express thioredoxin during development in a thioredoxin-knockout background. Results from the overexpression studies indicate that modulation of thioredoxin genes affects longevity in mice.
9. Thioredoxin Reductase Variant Is Associated with Longevity in Humans
Similar to mice, humans possess TXN and TXNRD1 in the cytoplasm and TXN2 and TXNRD2 in the mitochondria with a third thioredoxin reductase TXNRD3 present in the cytoplasm and nucleus (Table S1). While it is not possible to genetically manipulate the expression levels of thioredoxin system genes in humans, multiple studies have identified genetic variants that are associated with extended longevity. In a study comparing oldest–old individuals (age 92–93) with middle-aged Danes, an allele of the cytoplasmic thioredoxin reductase gene TXNRD1 was found to be associated with longevity [104]. A subsequent study found that genetic variation in TXNRD1 is associated with physical and cognitive performance in very old individuals [105]. The association of TXNRD1 with physical performance in old age was confirmed in a cohort from Southern Italy [106], while the association of TXNRD1 with longevity was supported by results from a Dutch cohort, which showed the same relationship but failed to reach significance [104]. Taken together, these results suggest that the thioredoxin system may also contribute to longevity in humans. As the number of studies examining the role of the thioredoxin system in longevity in humans is currently limited, additional evidence would strengthen this conclusion.
10. Discussion
10.1. Relative Importance of Cytoplasmic and Mitochondrial Thioredoxin Systems Differs between Species
The role of the cytoplasmic and mitochondrial thioredoxin systems in determining lifespan has been examined in multiple genetic model organisms through increasing or decreasing the expression of thioredoxin or thioredoxin reductase. While there is evidence for a contribution of the thioredoxin systems to longevity in yeast, worms, flies, mice and humans, the relative importance of each component of these systems varies between species. In yeast and C. elegans, disruption of the cytoplasmic thioredoxin system results in the largest detrimental effect on longevity, while disruption of the mitochondrial thioredoxin system has minimal impact on lifespan. In Drosophila, both the cytoplasmic and mitochondrial thioredoxin systems affect lifespan, with the largest effect observed with the cytoplasmic thioredoxin reductase. In mice, both the cytoplasmic and mitochondrial thioredoxin systems are essential for life as disruption of any of the components results in embryonic lethality. Thus, it appears that in more-complex organisms, there is a greater reliance on thioredoxin systems for survival and an increased importance of the mitochondrial thioredoxin system compared to less-complex organisms.
10.2. Cytoplasmic and Mitochondrial Thioredoxin Systems Act Independently to Affect Longevity
In reviewing the literature, we found that disrupting the cytoplasmic or mitochondrial thioredoxin systems often resulted in different effects on longevity, especially in yeast and C. elegans. This clearly indicates that the cytoplasmic and mitochondrial thioredoxin systems do not perform redundant functions. It is important to possess a functioning thioredoxin system in both of these compartments of the cell. As one of thioredoxin’s main functions is to reduce disulfide bonds in oxidized proteins, thioredoxin is required to reduce proteins in all parts of the cell. Precise subcellular localization is likely also important for thioredoxin’s roles in intracellular signaling and as a molecular chaperone.
10.3. Thioredoxin and Thioredoxin Reductase can Affect Lifespan Independently
Although in some cases modulating the expression of the thioredoxin gene resulted in the same effect as modulating the expression of the corresponding thioredoxin reductase gene on lifespan, there were also examples in which different effects were observed. For example, in C. elegans, deletion of the cytoplasmic thioredoxin gene trx-1 decreases lifespan and stress resistance, while loss of the cytoplasmic thioredoxin reductase gene trxr-1 does not reduce longevity or resistance to stress. In Drosophila, disruption of TrxT or dhd does not affect lifespan, while loss of Trxr-1 results in larval lethality. In cases where thioredoxin disruption produces a phenotype while disruption of the corresponding thioredoxin reductase does not, this suggests that the thioredoxin possesses a thioredoxin-reductase-independent function or that multiple enzymes can restore thioredoxin activity. As thioredoxins have been shown to possess redox-independent functions [107,108,109,110,111], disruption of thioredoxin and thioredoxin reductase would produce different results if it is a redox-independent function of thioredoxin that is contributing to its effect on lifespan. In cases where the phenotype resulting from disruption of thioredoxin reductase is more severe than disruption of the corresponding thioredoxin, it is possible that the thioredoxin reductase is also acting on other targets, leading to a broader effect than just disrupting the thioredoxin.
10.4. The Effect of Thioredoxin and Thioredoxin Reductase on Lifespan Is Correlated with Effect on Resistance to Oxidative Stress
As thioredoxin performs multiple functions within the cell as an antioxidant, signaling molecule and molecular chaperone, it is important to determine the relative contributions of each of these functions to longevity. In general, it has been observed that resistance to oxidative stress is modulated in the same direction as lifespan. Disruption of TRR1 in yeast, deletion trx-1 in C. elegans, disruption of Trx-2 in Drosophila and overexpression of Vdup1 in Drosophila all result in decreased lifespan and decreased resistance to oxidative stress. Deletion of trxr-1, trx-2, trxr-2 or trx-3 in C. elegans does not decrease lifespan or oxidative stress resistance. Overexpression of TrxT or Trx-2 or downregulation of Vdup1 in Drosophila increases lifespan and resistance to oxidative stress. Taken together, these results indicate a correlation between resistance to oxidative stress and lifespan and are consistent with the conclusion that one of the mechanisms by which the thioredoxin systems affect longevity is through modulation of resistance to oxidative stress.
11. Conclusions
Overall, this review highlights the importance of both the cytoplasmic and mitochondrial thioredoxin systems in determining lifespan, which is conserved across species. Disruption of thioredoxin or thioredoxin reductase can have detrimental effects on lifespan in yeast, worms, flies and mice. In addition, overexpression of individual thioredoxin or thioredoxin reductase genes is sufficient to extend longevity in worms, flies and mice. In future studies, it will be important to define the precise molecular mechanisms by which each thioredoxin system affects lifespan.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox12040944/s1, Table S1: Overview of thioredoxin systems in different organisms.
Author Contributions
Conceptualization: A.A. and J.M.V.R.; Visualization: A.A. and J.M.V.R.; Writing—original draft: A.A. and J.M.V.R.; Writing—review and editing: A.A. and J.M.V.R.; Supervision: J.M.V.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the content of the manuscript, the writing of the manuscript or in the decision to publish.
Funding Statement
This work was supported by the Canadian Institutes of Health Research (CIHR; J.M.V.R.) and the Natural Sciences and Engineering Research Council of Canada (NSERC; J.M.V.R.). J.M.V.R. is the recipient of a Senior Research Scholar career award from the Fonds de Recherche du Québec Santé (FRQS) and Parkinson Quebec. A.A. received a doctoral scholarship from Healthy Brains Healthy Lives (HBHL).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Harman D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 2.Holmgren A. Thioredoxin. 6. The amino acid sequence of the protein from Escherichia coli B. Eur. J. Biochem. 1968;6:475–484. doi: 10.1111/j.1432-1033.1968.tb00470.x. [DOI] [PubMed] [Google Scholar]
- 3.Martin J.L. Thioredoxin—A fold for all reasons. Structure. 1995;3:245–250. doi: 10.1016/S0969-2126(01)00154-X. [DOI] [PubMed] [Google Scholar]
- 4.Horibe T., Gomi M., Iguchi D., Ito H., Kitamura Y., Masuoka T., Tsujimoto I., Kimura T., Kikuchi M. Different contributions of the three CXXC motifs of human protein-disulfide isomerase-related protein to isomerase activity and oxidative refolding. J. Biol. Chem. 2004;279:4604–4611. doi: 10.1074/jbc.M310922200. [DOI] [PubMed] [Google Scholar]
- 5.Collet J.F., Messens J. Structure, function, and mechanism of thioredoxin proteins. Antioxid. Redox Signal. 2010;13:1205–1216. doi: 10.1089/ars.2010.3114. [DOI] [PubMed] [Google Scholar]
- 6.Lillig C.H., Holmgren A. Thioredoxin and related molecules—From biology to health and disease. Antioxid. Redox Signal. 2007;9:25–47. doi: 10.1089/ars.2007.9.25. [DOI] [PubMed] [Google Scholar]
- 7.Jumper J., Evans R., Pritzel A., Green T., Figurnov M., Ronneberger O., Tunyasuvunakool K., Bates R., Zidek A., Potapenko A., et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varadi M., Anyango S., Deshpande M., Nair S., Natassia C., Yordanova G., Yuan D., Stroe O., Wood G., Laydon A., et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022;50:D439–D444. doi: 10.1093/nar/gkab1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du Y., Zhang H., Lu J., Holmgren A. Glutathione and glutaredoxin act as a backup of human thioredoxin reductase 1 to reduce thioredoxin 1 preventing cell death by aurothioglucose. J. Biol. Chem. 2012;287:38210–38219. doi: 10.1074/jbc.M112.392225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H., Du Y., Zhang X., Lu J., Holmgren A. Glutaredoxin 2 reduces both thioredoxin 2 and thioredoxin 1 and protects cells from apoptosis induced by auranofin and 4-hydroxynonenal. Antioxid. Redox Signal. 2014;21:669–681. doi: 10.1089/ars.2013.5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das K.C., Das C.K. Thioredoxin, a singlet oxygen quencher and hydroxyl radical scavenger: Redox independent functions. Biochem. Biophys. Res. Commun. 2000;277:443–447. doi: 10.1006/bbrc.2000.3689. [DOI] [PubMed] [Google Scholar]
- 12.Rhee S.G., Woo H.A., Kil I.S., Bae S.H. Peroxiredoxin functions as a peroxidase and a regulator and sensor of local peroxides. J. Biol. Chem. 2012;287:4403–4410. doi: 10.1074/jbc.R111.283432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujii J., Ikeda Y. Advances in our understanding of peroxiredoxin, a multifunctional, mammalian redox protein. Redox Rep. 2002;7:123–130. doi: 10.1179/135100002125000352. [DOI] [PubMed] [Google Scholar]
- 14.Mustacich D., Powis G. Thioredoxin reductase. Pt 1Biochem. J. 2000;346:1–8. doi: 10.1042/bj3460001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhong L., Arner E.S., Holmgren A. Structure and mechanism of mammalian thioredoxin reductase: The active site is a redox-active selenolthiol/selenenylsulfide formed from the conserved cysteine-selenocysteine sequence. Proc. Natl. Acad. Sci. USA. 2000;97:5854–5859. doi: 10.1073/pnas.100114897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reich H.J., Hondal R.J. Why nature chose selenium. ACS Chem. Biol. 2016;11:821–841. doi: 10.1021/acschembio.6b00031. [DOI] [PubMed] [Google Scholar]
- 17.Sun Q.A., Wu Y., Zappacosta F., Jeang K.T., Lee B.J., Hatfield D.L., Gladyshev V.N. Redox regulation of cell signaling by selenocysteine in mammalian thioredoxin reductases. J. Biol. Chem. 1999;274:24522–24530. doi: 10.1074/jbc.274.35.24522. [DOI] [PubMed] [Google Scholar]
- 18.Gladyshev V.N., Jeang K.T., Stadtman T.C. Selenocysteine, identified as the penultimate C-terminal residue in human T-cell thioredoxin reductase, corresponds to TGA in the human placental gene. Proc. Natl. Acad. Sci. USA. 1996;93:6146–6151. doi: 10.1073/pnas.93.12.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arner E.S. Focus on mammalian thioredoxin reductases—Important selenoproteins with versatile functions. Biochim. Biophys. Acta. 2009;1790:495–526. doi: 10.1016/j.bbagen.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Zhao R., Masayasu H., Holmgren A. Ebselen: A substrate for human thioredoxin reductase strongly stimulating its hydroperoxide reductase activity and a superfast thioredoxin oxidant. Proc. Natl. Acad. Sci. USA. 2002;99:8579–8584. doi: 10.1073/pnas.122061399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamura T., Sato K., Komori K., Imai T., Kuwahara M., Okugochi T., Mihara H., Esaki N., Inagaki K. Selenite reduction by the thioredoxin system: Kinetics and identification of protein-bound selenide. Biosci. Biotechnol. Biochem. 2011;75:1184–1187. doi: 10.1271/bbb.100847. [DOI] [PubMed] [Google Scholar]
- 22.May J.M., Mendiratta S., Hill K.E., Burk R.F. Reduction of dehydroascorbate to ascorbate by the selenoenzyme thioredoxin reductase. J. Biol. Chem. 1997;272:22607–22610. doi: 10.1074/jbc.272.36.22607. [DOI] [PubMed] [Google Scholar]
- 23.Sies H. Strategies of antioxidant defense. Eur. J. Biochem. 1993;215:213–219. doi: 10.1111/j.1432-1033.1993.tb18025.x. [DOI] [PubMed] [Google Scholar]
- 24.Xia L., Nordman T., Olsson J.M., Damdimopoulos A., Bjorkhem-Bergman L., Nalvarte I., Eriksson L.C., Arner E.S., Spyrou G., Bjornstedt M. The mammalian cytosolic selenoenzyme thioredoxin reductase reduces ubiquinone. A novel mechanism for defense against oxidative stress. J. Biol. Chem. 2003;278:2141–2146. doi: 10.1074/jbc.M210456200. [DOI] [PubMed] [Google Scholar]
- 25.Nalvarte I., Damdimopoulos A.E., Spyrou G. Human mitochondrial thioredoxin reductase reduces cytochrome c and confers resistance to complex III inhibition. Free Radic. Biol. Med. 2004;36:1270–1278. doi: 10.1016/j.freeradbiomed.2004.02.072. [DOI] [PubMed] [Google Scholar]
- 26.Hashemy S.I., Ungerstedt J.S., Zahedi Avval F., Holmgren A. Motexafin gadolinium, a tumor-selective drug targeting thioredoxin reductase and ribonucleotide reductase. J. Biol. Chem. 2006;281:10691–10697. doi: 10.1074/jbc.M511373200. [DOI] [PubMed] [Google Scholar]
- 27.Holmgren A., Lyckeborg C. Enzymatic reduction of alloxan by thioredoxin and NADPH-thioredoxin reductase. Proc. Natl. Acad. Sci. USA. 1980;77:5149–5152. doi: 10.1073/pnas.77.9.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holmgren A. Antioxidant function of thioredoxin and glutaredoxin systems. Antioxid. Redox Signal. 2000;2:811–820. doi: 10.1089/ars.2000.2.4-811. [DOI] [PubMed] [Google Scholar]
- 29.Lee S., Kim S.M., Lee R.T. Thioredoxin and thioredoxin target proteins: From molecular mechanisms to functional significance. Antioxid. Redox Signal. 2013;18:1165–1207. doi: 10.1089/ars.2011.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saitoh M., Nishitoh H., Fujii M., Takeda K., Tobiume K., Sawada Y., Kawabata M., Miyazono K., Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei B., Li F. Mechanisms of Trx2/ASK1-Mediated Mitochondrial Injury in Pemphigus Vulgaris. Biomed. Res. Int. 2021;2021:2471518. doi: 10.1155/2021/2471518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saxena G., Chen J., Shalev A. Intracellular shuttling and mitochondrial function of thioredoxin-interacting protein. J. Biol. Chem. 2010;285:3997–4005. doi: 10.1074/jbc.M109.034421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimada K., Crother T.R., Karlin J., Dagvadorj J., Chiba N., Chen S., Ramanujan V.K., Wolf A.J., Vergnes L., Ojcius D.M., et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Q., Zhang D., Hu D., Zhou X., Zhou Y. The role of mitochondria in NLRP3 inflammasome activation. Mol. Immunol. 2018;103:115–124. doi: 10.1016/j.molimm.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 35.Oslowski C.M., Hara T., O’Sullivan-Murphy B., Kanekura K., Lu S., Hara M., Ishigaki S., Zhu L.J., Hayashi E., Hui S.T., et al. Thioredoxin-interacting protein mediates ER stress-induced beta cell death through initiation of the inflammasome. Cell Metab. 2012;16:265–273. doi: 10.1016/j.cmet.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qayyum N., Haseeb M., Kim M.S., Choi S. Role of thioredoxin-interacting protein in diseases and its therapeutic outlook. Int. J. Mol. Sci. 2021;22:2754. doi: 10.3390/ijms22052754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang C.Y., Mayo M.W., Korneluk R.G., Goeddel D.V., Baldwin A.S., Jr. NF-kappaB antiapoptosis: Induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 38.Morgan M.J., Liu Z.G. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011;21:103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matthews J.R., Wakasugi N., Virelizier J.L., Yodoi J., Hay R.T. Thioredoxin regulates the DNA binding activity of NF-kappa B by reduction of a disulphide bond involving cysteine 62. Nucleic Acids Res. 1992;20:3821–3830. doi: 10.1093/nar/20.15.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ando K., Hirao S., Kabe Y., Ogura Y., Sato I., Yamaguchi Y., Wada T., Handa H. A new APE1/Ref-1-dependent pathway leading to reduction of NF-kappaB and AP-1, and activation of their DNA-binding activity. Nucleic Acids Res. 2008;36:4327–4336. doi: 10.1093/nar/gkn416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramana C.V., Boldogh I., Izumi T., Mitra S. Activation of apurinic/apyrimidinic endonuclease in human cells by reactive oxygen species and its correlation with their adaptive response to genotoxicity of free radicals. Proc. Natl. Acad. Sci. USA. 1998;95:5061–5066. doi: 10.1073/pnas.95.9.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morel Y., Barouki R. Repression of gene expression by oxidative stress. Pt 3Biochem. J. 1999;342:481–496. doi: 10.1042/bj3420481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirota K., Matsui M., Iwata S., Nishiyama A., Mori K., Yodoi J. AP-1 transcriptional activity is regulated by a direct association between thioredoxin and Ref-1. Proc. Natl. Acad. Sci. USA. 1997;94:3633–3638. doi: 10.1073/pnas.94.8.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang L.E., Arany Z., Livingston D.M., Bunn H.F. Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its alpha subunit. J. Biol. Chem. 1996;271:32253–32259. doi: 10.1074/jbc.271.50.32253. [DOI] [PubMed] [Google Scholar]
- 45.Ema M., Hirota K., Mimura J., Abe H., Yodoi J., Sogawa K., Poellinger L., Fujii-Kuriyama Y. Molecular mechanisms of transcription activation by HLF and HIF1alpha in response to hypoxia: Their stabilization and redox signal-induced interaction with CBP/p300. EMBO J. 1999;18:1905–1914. doi: 10.1093/emboj/18.7.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jastrzab A., Skrzydlewska E. Thioredoxin-dependent system. Application of inhibitors. J. Enzym. Inhib. Med. Chem. 2021;36:362–371. doi: 10.1080/14756366.2020.1867121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu J., Fu M., Gao J., Dai G., Guan Q., Du C. Upregulation of Thioredoxin Reductase 1 Expression by Flavan-3-Ols Protects Human Kidney Proximal Tubular Cells from Hypoxia-Induced Cell Death. Antioxidants. 2022;11:1399. doi: 10.3390/antiox11071399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karlenius T.C., Shah F., Di Trapani G., Clarke F.M., Tonissen K.F. Cycling hypoxia up-regulates thioredoxin levels in human MDA-MB-231 breast cancer cells. Biochem. Biophys. Res. Commun. 2012;419:350–355. doi: 10.1016/j.bbrc.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 49.Tonelli C., Chio I.I.C., Tuveson D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018;29:1727–1745. doi: 10.1089/ars.2017.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hawkes H.J., Karlenius T.C., Tonissen K.F. Regulation of the human thioredoxin gene promoter and its key substrates: A study of functional and putative regulatory elements. Biochim. Biophys. Acta. 2014;1840:303–314. doi: 10.1016/j.bbagen.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 51.Kim Y.C., Yamaguchi Y., Kondo N., Masutani H., Yodoi J. Thioredoxin-dependent redox regulation of the antioxidant responsive element (ARE) in electrophile response. Oncogene. 2003;22:1860–1865. doi: 10.1038/sj.onc.1206369. [DOI] [PubMed] [Google Scholar]
- 52.Rundlof A.K., Janard M., Miranda-Vizuete A., Arner E.S. Evidence for intriguingly complex transcription of human thioredoxin reductase 1. Free Radic. Biol. Med. 2004;36:641–656. doi: 10.1016/j.freeradbiomed.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 53.Chen X., Tang W., Liu S., Yu L., Chen Z. Thioredoxin-1 phosphorylated at T100 is needed for its anti-apoptotic activity in HepG2 cancer cells. Life Sci. 2010;87:254–260. doi: 10.1016/j.lfs.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 54.Sengupta R., Holmgren A. Thioredoxin and thioredoxin reductase in relation to reversible S-nitrosylation. Antioxid. Redox Signal. 2013;18:259–269. doi: 10.1089/ars.2012.4716. [DOI] [PubMed] [Google Scholar]
- 55.Casagrande S., Bonetto V., Fratelli M., Gianazza E., Eberini I., Massignan T., Salmona M., Chang G., Holmgren A., Ghezzi P. Glutathionylation of human thioredoxin: A possible crosstalk between the glutathione and thioredoxin systems. Proc. Natl. Acad. Sci. USA. 2002;99:9745–9749. doi: 10.1073/pnas.152168599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spindel O.N., World C., Berk B.C. Thioredoxin interacting protein: Redox dependent and independent regulatory mechanisms. Antioxid. Redox Signal. 2012;16:587–596. doi: 10.1089/ars.2011.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patwari P., Higgins L.J., Chutkow W.A., Yoshioka J., Lee R.T. The interaction of thioredoxin with Txnip. Evidence for formation of a mixed disulfide by disulfide exchange. J. Biol. Chem. 2006;281:21884–21891. doi: 10.1074/jbc.M600427200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zitman-Gal T., Green J., Pasmanik-Chor M., Oron-Karni V., Bernheim J. Endothelial pro-atherosclerotic response to extracellular diabetic-like environment: Possible role of thioredoxin-interacting protein. Nephrol. Dial. Transplant. 2010;25:2141–2149. doi: 10.1093/ndt/gfp768. [DOI] [PubMed] [Google Scholar]
- 59.Parikh H., Carlsson E., Chutkow W.A., Johansson L.E., Storgaard H., Poulsen P., Saxena R., Ladd C., Schulze P.C., Mazzini M.J., et al. TXNIP regulates peripheral glucose metabolism in humans. PLoS Med. 2007;4:e158. doi: 10.1371/journal.pmed.0040158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bedarida T., Baron S., Vibert F., Ayer A., Henrion D., Thioulouse E., Marchiol C., Beaudeux J.L., Cottart C.H., Nivet-Antoine V. resveratrol decreases TXNIP mRNA and protein nuclear expressions with an arterial function improvement in old mice. J. Gerontol. A Biol. Sci. Med. Sci. 2016;71:720–729. doi: 10.1093/gerona/glv071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cai W., Zhang B., Duan D., Wu J., Fang J. Curcumin targeting the thioredoxin system elevates oxidative stress in HeLa cells. Toxicol. Appl. Pharmacol. 2012;262:341–348. doi: 10.1016/j.taap.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 62.Fang J., Lu J., Holmgren A. Thioredoxin reductase is irreversibly modified by curcumin: A novel molecular mechanism for its anticancer activity. J. Biol. Chem. 2005;280:25284–25290. doi: 10.1074/jbc.M414645200. [DOI] [PubMed] [Google Scholar]
- 63.Wang W., Wang S., Howie A.F., Beckett G.J., Mithen R., Bao Y. Sulforaphane, erucin, and iberin up-regulate thioredoxin reductase 1 expression in human MCF-7 cells. J. Agric. Food Chem. 2005;53:1417–1421. doi: 10.1021/jf048153j. [DOI] [PubMed] [Google Scholar]
- 64.Skogastierna C., Johansson M., Parini P., Eriksson M., Eriksson L.C., Ekstrom L., Bjorkhem-Bergman L. Statins inhibit expression of thioredoxin reductase 1 in rat and human liver and reduce tumour development. Biochem. Biophys. Res. Commun. 2012;417:1046–1051. doi: 10.1016/j.bbrc.2011.12.091. [DOI] [PubMed] [Google Scholar]
- 65.Mansouri A., Reiner Z., Ruscica M., Tedeschi-Reiner E., Radbakhsh S., Bagheri Ekta M., Sahebkar A. Antioxidant effects of statins by modulating Nrf2 and Nrf2/HO-1 signaling in different diseases. J. Clin. Med. 2022;11:1313. doi: 10.3390/jcm11051313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ebrahimian T., Sairam M.R., Schiffrin E.L., Touyz R.M. Cardiac hypertrophy is associated with altered thioredoxin and ASK-1 signaling in a mouse model of menopause. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H1481–H1488. doi: 10.1152/ajpheart.00163.2008. [DOI] [PubMed] [Google Scholar]
- 67.Kim H., Baek C.H., Lee R.B., Chang J.W., Yang W.S., Lee S.K. Anti-Fibrotic Effect of Losartan, an Angiotensin II Receptor Blocker, Is Mediated through Inhibition of ER Stress via Up-Regulation of SIRT1, Followed by Induction of HO-1 and Thioredoxin. Int. J. Mol. Sci. 2017;18:305. doi: 10.3390/ijms18020305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gellert M., Hossain M.F., Berens F.J.F., Bruhn L.W., Urbainsky C., Liebscher V., Lillig C.H. Substrate specificity of thioredoxins and glutaredoxins—Towards a functional classification. Heliyon. 2019;5:e02943. doi: 10.1016/j.heliyon.2019.e02943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matecic M., Smith D.L., Pan X., Maqani N., Bekiranov S., Boeke J.D., Smith J.S. A microarray-based genetic screen for yeast chronological aging factors. PLoS Genet. 2010;6:e1000921. doi: 10.1371/journal.pgen.1000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Laschober G.T., Ruli D., Hofer E., Muck C., Carmona-Gutierrez D., Ring J., Hutter E., Ruckenstuhl C., Micutkova L., Brunauer R., et al. Identification of evolutionarily conserved genetic regulators of cellular aging. Aging Cell. 2010;9:1084–1097. doi: 10.1111/j.1474-9726.2010.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Unlu E.S., Koc A. Effects of deleting mitochondrial antioxidant genes on life span. Ann. N. Y. Acad. Sci. 2007;1100:505–509. doi: 10.1196/annals.1395.055. [DOI] [PubMed] [Google Scholar]
- 72.Demir A.B., Koc A. Assessment of chronological lifespan dependent molecular damages in yeast lacking mitochondrial antioxidant genes. Biochem. Biophys. Res. Commun. 2010;400:106–110. doi: 10.1016/j.bbrc.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 73.Picazo C., Matallana E., Aranda A. Yeast thioredoxin reductase Trr1p controls TORC1-regulated processes. Sci. Rep. 2018;8:16500. doi: 10.1038/s41598-018-34908-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harris-Gauthier N., Traa A., AlOkda A., Moldakozhayev A., Anglas U., Soo S.K., Van Raamsdonk J.M. Mitochondrial thioredoxin system is required for enhanced stress resistance and extended longevity in long-lived mitochondrial mutants. Redox Biol. 2022;53:102335. doi: 10.1016/j.redox.2022.102335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jee C., Vanoaica L., Lee J., Park B.J., Ahnn J. Thioredoxin is related to life span regulation and oxidative stress response in Caenorhabditis elegans. Genes Cells. 2005;10:1203–1210. doi: 10.1111/j.1365-2443.2005.00913.x. [DOI] [PubMed] [Google Scholar]
- 76.Miranda-Vizuete A., Fierro Gonzalez J.C., Gahmon G., Burghoorn J., Navas P., Swoboda P. Lifespan decrease in a Caenorhabditis elegans mutant lacking TRX-1, a thioredoxin expressed in ASJ sensory neurons. FEBS Lett. 2006;580:484–490. doi: 10.1016/j.febslet.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 77.Fierro-Gonzalez J.C., Gonzalez-Barrios M., Miranda-Vizuete A., Swoboda P. The thioredoxin TRX-1 regulates adult lifespan extension induced by dietary restriction in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 2011;406:478–482. doi: 10.1016/j.bbrc.2011.02.079. [DOI] [PubMed] [Google Scholar]
- 78.Stenvall J., Fierro-Gonzalez J.C., Swoboda P., Saamarthy K., Cheng Q., Cacho-Valadez B., Arner E.S., Persson O.P., Miranda-Vizuete A., Tuck S. Selenoprotein TRXR-1 and GSR-1 are essential for removal of old cuticle during molting in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2011;108:1064–1069. doi: 10.1073/pnas.1006328108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yu D., Pendergraff H., Liu J., Kordasiewicz H.B., Cleveland D.W., Swayze E.E., Lima W.F., Crooke S.T., Prakash T.P., Corey D.R. Single-Stranded RNAs Use RNAi to Potently and Allele-Selectively Inhibit Mutant Huntingtin Expression. Cell. 2012;150:895–908. doi: 10.1016/j.cell.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cacho-Valadez B., Munoz-Lobato F., Pedrajas J.R., Cabello J., Fierro-Gonzalez J.C., Navas P., Swoboda P., Link C.D., Miranda-Vizuete A. The characterization of the Caenorhabditis elegans mitochondrial thioredoxin system uncovers an unexpected protective role of thioredoxin reductase 2 in beta-amyloid peptide toxicity. Antioxid. Redox Signal. 2012;16:1384–1400. doi: 10.1089/ars.2011.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jimenez-Hidalgo M., Kurz C.L., Pedrajas J.R., Naranjo-Galindo F.J., Gonzalez-Barrios M., Cabello J., Saez A.G., Lozano E., Button E.L., Veal E.A., et al. Functional characterization of thioredoxin 3 (TRX-3), a Caenorhabditis elegans intestine-specific thioredoxin. Free Radic. Biol. Med. 2014;68:205–219. doi: 10.1016/j.freeradbiomed.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Svensson M.J., Larsson J. Thioredoxin-2 affects lifespan and oxidative stress in Drosophila. Hereditas. 2007;144:25–32. doi: 10.1111/j.2007.0018-0661.01990.x. [DOI] [PubMed] [Google Scholar]
- 83.Umeda-Kameyama Y., Tsuda M., Ohkura C., Matsuo T., Namba Y., Ohuchi Y., Aigaki T. Thioredoxin suppresses Parkin-associated endothelin receptor-like receptor-induced neurotoxicity and extends longevity in Drosophila. J. Biol. Chem. 2007;282:11180–11187. doi: 10.1074/jbc.M700937200. [DOI] [PubMed] [Google Scholar]
- 84.Tsuda M., Ootaka R., Ohkura C., Kishita Y., Seong K.H., Matsuo T., Aigaki T. Loss of Trx-2 enhances oxidative stress-dependent phenotypes in Drosophila. FEBS Lett. 2010;584:3398–3401. doi: 10.1016/j.febslet.2010.06.034. [DOI] [PubMed] [Google Scholar]
- 85.Missirlis F., Phillips J.P., Jackle H. Cooperative action of antioxidant defense systems in Drosophila. Curr. Biol. 2001;11:1272–1277. doi: 10.1016/S0960-9822(01)00393-1. [DOI] [PubMed] [Google Scholar]
- 86.Missirlis F., Ulschmid J.K., Hirosawa-Takamori M., Gronke S., Schafer U., Becker K., Phillips J.P., Jackle H. Mitochondrial and cytoplasmic thioredoxin reductase variants encoded by a single Drosophila gene are both essential for viability. J. Biol. Chem. 2002;277:11521–11526. doi: 10.1074/jbc.M111692200. [DOI] [PubMed] [Google Scholar]
- 87.Pickering A.M., Lehr M., Gendron C.M., Pletcher S.D., Miller R.A. Mitochondrial thioredoxin reductase 2 is elevated in long-lived primate as well as rodent species and extends fly mean lifespan. Aging Cell. 2017;16:683–692. doi: 10.1111/acel.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oberacker T., Bajorat J., Ziola S., Schroeder A., Roth D., Kastl L., Edgar B.A., Wagner W., Gulow K., Krammer P.H. Enhanced expression of thioredoxin-interacting-protein regulates oxidative DNA damage and aging. FEBS Lett. 2018;592:2297–2307. doi: 10.1002/1873-3468.13156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Matsui M., Oshima M., Oshima H., Takaku K., Maruyama T., Yodoi J., Taketo M.M. Early embryonic lethality caused by targeted disruption of the mouse thioredoxin gene. Dev. Biol. 1996;178:179–185. doi: 10.1006/dbio.1996.0208. [DOI] [PubMed] [Google Scholar]
- 90.Cunningham G.M., Roman M.G., Flores L.C., Hubbard G.B., Salmon A.B., Zhang Y., Gelfond J., Ikeno Y. The paradoxical role of thioredoxin on oxidative stress and aging. Arch. Biochem. Biophys. 2015;576:32–38. doi: 10.1016/j.abb.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 91.Roman M.G., Flores L.C., Cunningham G.M., Cheng C., Allen C., Hubbard G.B., Bai Y., Saunders T.L., Ikeno Y. Thioredoxin and aging: What have we learned from the survival studies? Aging Pathobiol. Ther. 2020;2:126–133. doi: 10.31491/APT.2020.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mitsui A., Hamuro J., Nakamura H., Kondo N., Hirabayashi Y., Ishizaki-Koizumi S., Hirakawa T., Inoue T., Yodoi J. Overexpression of human thioredoxin in transgenic mice controls oxidative stress and life span. Antioxid. Redox Signal. 2002;4:693–696. doi: 10.1089/15230860260220201. [DOI] [PubMed] [Google Scholar]
- 93.Perez V.I., Cortez L.A., Lew C.M., Rodriguez M., Webb C.R., Van Remmen H., Chaudhuri A., Qi W., Lee S., Bokov A., et al. Thioredoxin 1 overexpression extends mainly the earlier part of life span in mice. J. Gerontol. A Biol. Sci. Med. Sci. 2011;66:1286–1299. doi: 10.1093/gerona/glr125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Flores L.C., Roman M.G., Cunningham G.M., Cheng C., Dube S., Allen C., Van Remmen H., Hubbard G.B., Saunders T.L., Ikeno Y. Continuous overexpression of thioredoxin 1 enhances cancer development and does not extend maximum lifespan in male C57BL/6 mice. Pathobiol. Aging Age Relat. Dis. 2018;8:1533754. doi: 10.1080/20010001.2018.1533754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jakupoglu C., Przemeck G.K., Schneider M., Moreno S.G., Mayr N., Hatzopoulos A.K., de Angelis M.H., Wurst W., Bornkamm G.W., Brielmeier M., et al. Cytoplasmic thioredoxin reductase is essential for embryogenesis but dispensable for cardiac development. Mol. Cell. Biol. 2005;25:1980–1988. doi: 10.1128/MCB.25.5.1980-1988.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nonn L., Williams R.R., Erickson R.P., Powis G. The absence of mitochondrial thioredoxin 2 causes massive apoptosis, exencephaly, and early embryonic lethality in homozygous mice. Mol. Cell. Biol. 2003;23:916–922. doi: 10.1128/MCB.23.3.916-922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Perez V.I., Lew C.M., Cortez L.A., Webb C.R., Rodriguez M., Liu Y., Qi W., Li Y., Chaudhuri A., Van Remmen H., et al. Thioredoxin 2 haploinsufficiency in mice results in impaired mitochondrial function and increased oxidative stress. Free Radic. Biol. Med. 2008;44:882–892. doi: 10.1016/j.freeradbiomed.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 98.Roman M.G., Flores L.C., Cunningham G.M., Cheng C., Dube S., Allen C., Van Remmen H., Bai Y., Hubbard G.B., Saunders T.L., et al. Thioredoxin overexpression in mitochondria showed minimum effects on aging and age-related diseases in male C57BL/6 mice. Aging Pathobiol. Ther. 2020;2:20–31. doi: 10.31491/APT.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cunningham G.M., Flores L.C., Roman M.G., Cheng C., Dube S., Allen C., Valentine J.M., Hubbard G.B., Bai Y., Saunders T.L., et al. Thioredoxin overexpression in both the cytosol and mitochondria accelerates age-related disease and shortens lifespan in male C57BL/6 mice. Geroscience. 2018;40:453–468. doi: 10.1007/s11357-018-0039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Conrad M., Jakupoglu C., Moreno S.G., Lippl S., Banjac A., Schneider M., Beck H., Hatzopoulos A.K., Just U., Sinowatz F., et al. Essential role for mitochondrial thioredoxin reductase in hematopoiesis, heart development, and heart function. Mol. Cell. Biol. 2004;24:9414–9423. doi: 10.1128/MCB.24.21.9414-9423.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Takagi Y., Mitsui A., Nishiyama A., Nozaki K., Sono H., Gon Y., Hashimoto N., Yodoi J. Overexpression of thioredoxin in transgenic mice attenuates focal ischemic brain damage. Proc. Natl. Acad. Sci. USA. 1999;96:4131–4136. doi: 10.1073/pnas.96.7.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Perez V.I., Bokov A., Van Remmen H., Mele J., Ran Q., Ikeno Y., Richardson A. Is the oxidative stress theory of aging dead? Biochim. Et Biophys. Acta. 2009;1790:1005–1014. doi: 10.1016/j.bbagen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chocron E.S., Mdaki K., Jiang N., Cropper J., Pickering A.M. Mitochondrial TrxR2 regulates metabolism and protects from metabolic disease through enhanced TCA and ETC function. Commun. Biol. 2022;5:467. doi: 10.1038/s42003-022-03405-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Soerensen M., Dato S., Tan Q., Thinggaard M., Kleindorp R., Beekman M., Jacobsen R., Suchiman H.E., de Craen A.J., Westendorp R.G., et al. Human longevity and variation in GH/IGF-1/insulin signaling, DNA damage signaling and repair and pro/antioxidant pathway genes: Cross sectional and longitudinal studies. Exp. Gerontol. 2012;47:379–387. doi: 10.1016/j.exger.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dato S., Soerensen M., Lagani V., Montesanto A., Passarino G., Christensen K., Tan Q., Christiansen L. Contribution of genetic polymorphisms on functional status at very old age: A gene-based analysis of 38 genes (311 SNPs) in the oxidative stress pathway. Exp. Gerontol. 2014;52:23–29. doi: 10.1016/j.exger.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dato S., De Rango F., Crocco P., Passarino G., Rose G. Antioxidants and quality of aging: Further evidences for a major role of TXNRD1 gene variability on physical performance at old age. Oxid. Med. Cell. Longev. 2015;2015:926067. doi: 10.1155/2015/926067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jurado P., de Lorenzo V., Fernandez L.A. Thioredoxin fusions increase folding of single chain Fv antibodies in the cytoplasm of Escherichia coli: Evidence that chaperone activity is the prime effect of thioredoxin. J. Mol. Biol. 2006;357:49–61. doi: 10.1016/j.jmb.2005.12.058. [DOI] [PubMed] [Google Scholar]
- 108.Du H., Kim S., Hur Y.S., Lee M.S., Lee S.H., Cheon C.I. A cytosolic thioredoxin acts as a molecular chaperone for peroxisome matrix proteins as well as antioxidant in peroxisome. Mol. Cells. 2015;38:187–194. doi: 10.14348/molcells.2015.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu Y., Min W. Thioredoxin promotes ASK1 ubiquitination and degradation to inhibit ASK1-mediated apoptosis in a redox activity-independent manner. Circ. Res. 2002;90:1259–1266. doi: 10.1161/01.RES.0000022160.64355.62. [DOI] [PubMed] [Google Scholar]
- 110.Sanzo-Machuca Á., Monje Moreno J.M., Casado-Navarro R., Karakuzu O., Guerrero-Gómez D., Fierro-González J.C., Swoboda P., Muñoz M.J., Garsin D.A., Pedrajas J.R., et al. Redox-dependent and redox-independent functions of Caenorhabditis elegans thioredoxin 1. Redox Biol. 2019;24:101178. doi: 10.1016/j.redox.2019.101178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hao Y., Yang W., Ren J., Hall Q., Zhang Y., Kaplan J.M. Thioredoxin shapes the C. elegans sensory response to Pseudomonas produced nitric oxide. eLife. 2018;7:e36833. doi: 10.7554/eLife.36833. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.