Abstract
Electrical stimulation (ES) has been frequently used in different biomedical applications both in vitro and in vivo. Numerous studies have demonstrated positive effects of ES on cellular functions, including metabolism, proliferation, and differentiation. The application of ES to cartilage tissue for increasing extracellular matrix formation is of interest, as cartilage is not able to restore its lesions owing to its avascular nature and lack of cells. Various ES approaches have been used to stimulate chondrogenic differentiation in chondrocytes and stem cells; however, there is a huge gap in systematizing ES protocols used for chondrogenic differentiation of cells. This review focuses on the application of ES for chondrocyte and mesenchymal stem cell chondrogenesis for cartilage tissue regeneration. The effects of different types of ES on cellular functions and chondrogenic differentiation are reviewed, systematically providing ES protocols and their advantageous effects. Moreover, cartilage 3D modeling using cells in scaffolds/hydrogels under ES are observed, and recommendations on reporting about the use of ES in different studies are provided to ensure adequate consolidation of knowledge in the area of ES. This review brings novel insights into the further application of ES in in vitro studies, which are promising for further cartilage repair techniques.
Keywords: cartilage, osteoarthritis, chondrogenesis, electrical stimulation, mesenchymal stem cells
1. Introduction
Electrical stimulation (ES) has attracted a lot of attention as a physical stimulus used for tissue engineering and treatment of various diseases, such as movement, psychiatric and seizure disorders, in order to reduce pain and to improve the quality of life [1,2,3]. ES is frequently used for the stimulation of cells in vitro and in vivo, inducing a number of intracellular pathways involved in the regulation of cell metabolism, proliferation, migration and differentiation [4,5]. A meta-analysis of clinical trials showed that neuromuscular electrical stimulation or interferential current can improve pain management and physical function in knee osteoarthritis patients [6,7].
ES has already been proven as a useful tool in cartilage tissue engineering. Cartilage is composed of only non-excitable cells—chondrocytes [8,9]. Due to a lack of voltage-gated Na+ and Ca2+ channels, these non-excitable cells cannot generate action potential as a response to membrane depolarization [10]. Since the number of chondrocytes in cartilage is very low and their ability to restore damage to the extracellular matrix (ECM) is very weak, cartilage is prone to the development of degenerative diseases such as osteoarthritis (OA) [11]. Various arthroscopic cartilage intervention procedures such as chondroplasty, microfracture or mosaicplasty [12,13,14], as well as more modern technologies such as autologous chondrocyte implantation [15] are currently under development; however, these methods have not yet been approved for clinical use because their efficacy is still to be confirmed. Stem cell-based tissue engineering technologies, specifically those utilizing adult tissue-derived mesenchymal stem cells (MSCs), which can differentiate into chondrocytes, seem to be a promising therapeutic approach for cartilage damage repair. ES has been shown to be an important part in stem cell-based cartilage engineering, as it stimulates chondrogenic differentiation of MSCs even in the absence of growth factors [16,17].
The lack of standardized protocols for ES in tissue engineering introduces challenges in characterizing the exact mechanisms of its effect, since the electrical parameters (applied voltage, pulse or stimulus duration, frequency and field strength) can vary by several orders of magnitude.
This review summarizes ES applied in chondrogenic differentiation experiments. ES regimens, such as continuous, static, cyclic or pulsed stimulation, are described, emphasizing their beneficial effects and limitations. ES parameters and effects in the context of chondrogenesis are presented in order to improve the consolidation of knowledge in this area and direct the research towards development of optimized and more standardized protocols.
2. Electrical Stimulation Overview
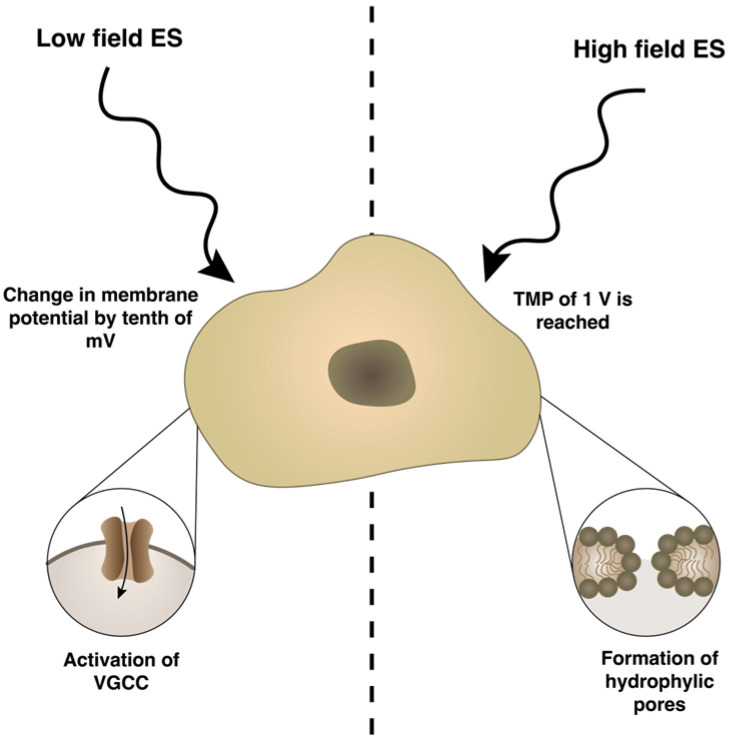
ES induces electrically mediated stress in cells and changes their membrane potential, which, depending on the protocol used, may lead to either activation of ion channels and other voltage sensitive proteins, or permeabilization of the plasma membrane. These processes result in a flux of different ions across the plasma membrane [18]. Altered ion concentration leads to the activation of different gene expression [19], production and secretion of growth and transcription factors [16,20], cell adhesion [21] and cell-cell interaction molecules [22]. Typically, low electric fields are used in applying ES, which leads to moderate change in the membrane potential by a tenth of mV and initiates the movement of voltage-sensing domains, resulting in conformational changes and the opening of voltage-gated ion channels (Figure 1). The most important voltage-gated channel for chondrogenic differentiation is L-type voltage-gated calcium channel (VGCC), which regulates expression of chondrogenesis markers (SOX9, COL2A1, Ihh) in vitro and limb development in vivo [23]. However, when the cell is exposed to a high-intensity pulsed electric field (PEF), the cell plasma membrane is polarized and a significant transmembrane potential (TMP) is induced [24,25]. When a critical TMP threshold is reached, which is frequently referred as 1 V [26], hydrophilic pores are formed in the membrane, resulting in increased membrane permeability to exogeneous molecules [27]. This phenomenon, called electroporation or electropermeabilization [28], is used for gene and drug delivery, tissue ablation, protein extraction and food processing. Depending on the PEF parameters, electroporation can be reversible or irreversible [29,30]. In mammalian cells, electroporation can be triggered at a 400–800 V/cm PEF [31], while lower field (<400 V/cm) can induce a phenomenon known as electroendocytosis, which means enhanced absorption of macromolecules after cell exposure to low electric fields [32,33]. Scientific papers focusing on the stimulating effects of high-intensity PEF also have started to appear in recent years.
Figure 1.
The mechanism of different types of electrical stimulation (ES) on cells membrane. TMP—transmembrane potential, VGCC—voltage-gated calcium channel.
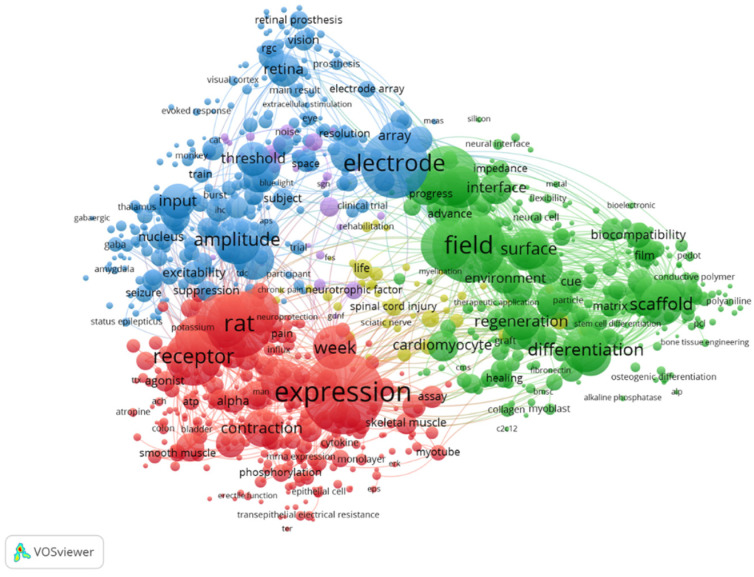
ES is widely applied in various fields of cellular research. After the analysis of Clarivate Analytics Web of Science using keywords “electrical stimulation” and “cells”, 11,180 papers published since 2008 were filtered. The most dominating keywords are related to electrical aspects (electrode, device, current and amplitude), while for biological aspects—expression, receptor, differentiation and inhibition are most widely used (Figure 2). The dominating keyword “rat” shows the application of ES for in vivo studies with rats as the model organism.
Figure 2.
Keyword map of electrical stimulation studies during last 15 years. Visualized using VOSviewer, version 1.6.18.
The cell membrane is the first to respond to ES, and is critical in maintaining membrane potential, cellular homeostasis and controlling the exchange of nutrients, waste products and chemical molecules important for signaling [34]. ES activates several independent signal transduction pathways; therefore, it is difficult to establish a direct link between ES and specific cellular responses. It is known that ES activates JNK/CREB-STAT3, ERK/JNK/STAT3 and wnt/β-catenin signaling pathways, leading to enhanced phosphorylation of JNK, CREB and STAT3 [35] and the expression of β-catenin protein [36].
There are hypotheses that electric fields and fluid shear stress activate similar signaling pathways that involve integrin receptors [37]. One of the main cell responses to ES is the opening of voltage-gated calcium channels (VGCC) and subsequent increase of calcium (Ca2+) inside the cell [38]. Both chondrocytes and mesenchymal stem cells have VGCC that can be regulated by external chemical or physical stimuli [39,40]. Such increase of intracellular Ca2+ was also observed in vitro after mechanical stimulation [41]. Furthermore, cytoskeletal structure reorganization, including denser f-actin texture and aligned actin filament orientation, has been observed in response to ES [42] as well as inverse when mechanical stimulation causes intracellular electrical signals through mechanotransduction [37]. The interconnectedness of the effects of ES and mechanical loading might be used in cartilage tissue engineering as scaffolds that cannot withstand mechanical pressure and could be instead stimulated with electrical fields, causing a similar effect as mechanical loads.
In vitro studies show that ES can increase Ca2+-driven ATP oscillations, leading to condensation of cells—the initial step of chondrogenic differentiation [22,43]. Furthermore, ES elevates the secretion of growth factors (transforming growth factor beta 1 (TGF-β1), platelet-derived growth factor (PDGF)-AA, and insulin-like growth factor-binding protein 2 and 3 (IGFBP-2 and 3) [22], which further drive the production of ECM, creating a microenvironment for chondrocyte attachment and interactions [44,45].
ES is a powerful physical stimulus that triggers different cell behaviors; however, due to a lack of a systemic approach to characterize the ES parametric protocols, the effects on different cell cultures and scaffolds are hardly predictable.
3. Electromechanics of Articular Cartilage
Cartilage is a biphasic tissue, composed of a solid phase, which consists of a charged porous collagen-proteoglycan matrix, and an interstitial fluid phase [46]. Changes in cartilage composition and arrangement of collagen fibers result in different biomechanical properties. It was shown that resistivity gradually increases from the superficial to the deep zone of cartilage, which means that the superficial zone of cartilage contains more mobile charged particles, than the deeper zones, and conducts electrical charge more efficiently. The elastic modulus also increases going from the superficial to the deep zone while the permeability of cartilage decreases [47]. There is not much data about the conductivity of articular cartilage (Table 1). More studies in this field are needed because part of the data were obtained using animal models, in which possibilities to reflect human data are limited.
Table 1.
The conductivity of cartilage in different models.
| Type of Cartilage | Conductivity | Reference |
|---|---|---|
| Baseline—post-exercise | [48] | |
| All cartilages, | 1.12–2.98 S/m; | |
| Patellar cartilage, | 1.11–2.80 S/m; | |
| Trochlear cartilage | 1.51–2.98 S/m | |
| Humeral head bovine articular cartilage * | 1.14 ± 0.11 S/m | [49] |
| Articular cartilage ** | 0.88 ± 0.08 S/m | [49] |
S/m—Siemens per meter. * Results from a 1- to 2-year-old steer. ** Results from a 4-year-old cow.
Articular cartilage undergoes a number of biomechanical and physiochemical changes related to age, obesity, injury or development of OA. One of the most important pathological changes of cartilage in OA is hypertrophic differentiation and fibrillation, when the tissue loses ECM (proteoglycans and collagen) fibers. Cell senescence induces inflammation, which triggers chondrocytes to produce cytokines and catabolic agents (matrix metalloproteinases (MMPs) and aggrecanases (ADAMTS-4 and ADAMTS-5), leading to the destruction of pericellular and intercellular matrix [50]. Cartilage breakdown products activate synovium, resulting in subsequent production of cytokines and infiltration of immune cells, such as macrophages [51]. Similar secretion of pro-inflammatory cytokines and proteinases can be found in cartilage after joint trauma, which can also be a cause of OA [52].
Based on that, ES has been proposed as a tool in cartilage tissue engineering to improve regenerative, mechanical and other properties of engineered tissue. Basic mathematical models of electrical behavior in cartilage have been described previously [46]. Cartilage itself is an electrically charged tissue that exhibits electromechanical, depth-dependent properties [53]. Exercise and other weight-bearing movements cause mechanical deformation of the tissue, producing electrical signals through the flow of positively charged particles across negatively charged ECM of the cartilage [54]. Age, injury-, or pathology-caused reduction of cartilage mobility can reduce endogenous electrical signals. Therefore, the application of external ES can mimic the endogenous electrical signals of the tissue, produce compression and deformation, which results in tissue recovery upon ES [53,55,56].
It was shown that elevated extracellular calcium (eCa2+) concentration inhibits chondrogenesis [57] while eCa2+ oscillations trigger Ca2+-depending transcription factors and signaling pathways and are associated with modifications in cartilage ECM synthesis [58]. This suggests that different functional responses are determined via specific calcium signaling patterns.
In conclusion, this is why externally applied ES, through the regulation of calcium signaling pathway, is a promising, non-invasive stimuli treating OA. Even though ES studies on cartilage have been carried out in vitro, further investigation is still required.
Therefore, electrical signals should be considered as an important component of cartilage function, and the application of external ES may restore Ca2+ homeostasis and cartilage tissue integrity after damage or disease.
4. Types of ES Application and Their Effects Activating Cellular Mechanisms and Functions
There are several ways how ES can be delivered to cells. The electric field can be applied to cells using electrodes directly inserted into the culture media or using capacitive or inductive coupling (reviewed in Chen, 2019) [1]. In this review, we focused mainly on the research where direct and capacitive coupling was used. Direct coupling is easy to operate but electrode contact with the medium can cause changes in medium temperature, pH, and the generation of reactive oxygen species. Capacitive coupling is non-invasive; electrodes are outside the plate at opposing ends, providing a relatively uniform electrical field to a cell monolayer on a scaffold [1].
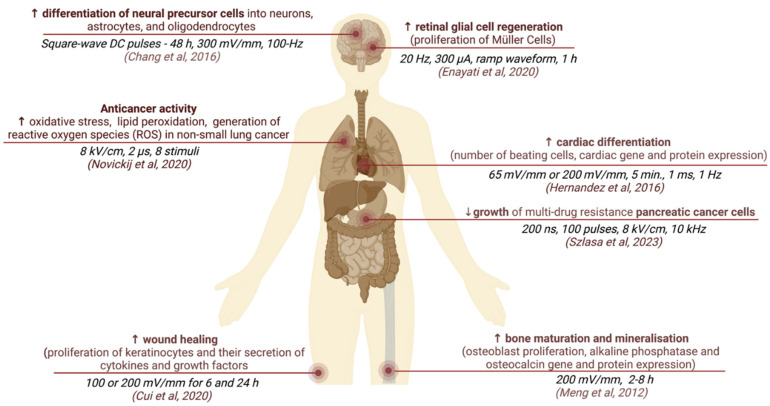
The effects of ES on cellular behavior depend on the electrical field parameters and stimulus protocols used. Such stimulus can be directly applied as straightforward continuous static voltage on tissue culture as well as more complicated stimulation with pulses of various waveforms. The main parameters describing ES pulses are: the strength of the electric field (mV/mm), pulse shape (monophasic or biphasic), and duration frequency (Hz). These pulses can be applied in various regimens, and in case of weak electric fields, the total stimulation duration can last hours or even days. Some stimulation protocols may have a complex on–off cycle structure (for example ES 3 h a day for 21 days). Examples of different parameters and the corresponding effects of ES are described in Figure 3 [59,60,61,62,63,64,65].
Figure 3.
Examples of different ES parameter settings used on different cell types and associated cellular behavior [59,60,61,62,63,64,65].
ES parameters are determined not just by cell types, but also by different study end-goals and ES delivery methods. It is established that low electric fields (either continuous or periodic) alter cell transmembrane potential, which leads to the opening of voltage-gated ion channels or changes in the enzymatic activity of phosphatases, containing a voltage-sensor domain [66]. High electric fields might have thermal effects and cause tissue damage; however, when high-intensity stimulation is applied for short intervals, it can trigger positive effects, similar to those induced by longer low electric field strength stimulation [67]. Depending on the pulsed electric field strength, the duration and the number of pulses can be reduced, providing flexibility in the development of parametric protocols (Table 2) [68]. For example, nanosecond PEFs (nsPEFs) feature an extremely high electric field strength (i.e., up 100 kV/cm), which can be induced in 1 to a few hundred nanoseconds. Microsecond pulses can cause reversible electroporation with a field strength between 400 and 600 V/cm, while irreversible electroporation of neurons and cardiomyocytes is caused by electric field of 1–2 kV/cm with at least 30–50 pulses [69,70]. Exceeding the electroporation threshold causes the development of hydrophilic pores in the cell membrane, which leads to increased concentration of intracellular Ca2+, altered proliferation, differentiation or even cell apoptosis [71,72]. Therefore, the duration of cell membrane polarization plays an important role in the dynamics of electroporation. For example, in the supra-electroporation range (where pulse duration is shorter than the duration of membrane polarization), PEF intensities of 2–10 kV/cm can still induce reversible damage to the cell. Nevertheless, an increase in the number of pulses (e.g., 10 kV/cm × 900 ns × 600 pulses delivered at 2 Hz) can trigger apoptosis [73]. The application of the same protocol with shorter pulses improves cell viability, which is in accordance with established knowledge. Both microsecond and nsPEF can be used for plasma membrane permeabilization [68], which potentially enables mimicking a spontaneous cellular oscillation with a Ca2+ spike [74]. The application of extremely short pulses (typically sub-100 ns) also enable selective permeabilization of internal cell membranes, such as the endoplasmic reticulum, mitochondria or nucleus and induces Ca2+ release from the endoplasmic reticulum through IP3-dependent Ca2+ channels or permeabilize the membrane of the ER [35,72,75,76].
Table 2.
The effect of different stimulus parameters on cell membranes.
| Cell Type | ES Conditions | Result | Reference | |
|---|---|---|---|---|
| Nanosecond pulse | Cancer cell lines CT-26 and EL-4 | 300 and 100 pulses (200 ns, 7 kV/cm, 10 Hz) | Induced ER stress | [75] |
| Porcine bone marrow-derived stromal cells (pBM-MSCs) | 10 ns at 20 kV/cm, 100 ns at 10 kV/cm | Affects intracellular signaling pathways (JNK, P38, ERK, and Wnt signaling pathways) | [35] | |
| TPC-1 (papillary thyroid carcinoma cell line) | 900 ns | Reduced viability and proliferation, induced apoptosis | [73] | |
| Microsecond pulse | Tumor cell lines (DC3F, IGROV 1, SA-1, MCF7, B16F0, TBL.Cl2, TBL.Cl2 PT, HeLa, IGROV 1/DDP, B16F1, MM46T, EAT) | 400–600 V/cm, 1 HZ, 100 μs | Reversible plasmic membrane electroporation | [77] |
| HL1 cardiomyocytes, PC12, F11, and SH-S5Y5 neural cells | 1000–1250 V/cm, 100 μs 30–50 pulses | Irreversible plasmic membrane electroporation | [70] | |
| Human adipose mesenchymal stem cells (haMSC) | One single micropulse of 100 μs | Induced spontaneous Ca2+ oscillations | [74] | |
| haMSC | One single micropulse of 100 μs | Permeabilization of ER membrane | [76] |
At the same time, stimuli that are below the electroporation threshold can mimic cell-signaling mechanisms, such as Ca2+ signaling pathways as well as mitochondria- and caspase-dependent mechanisms [72].
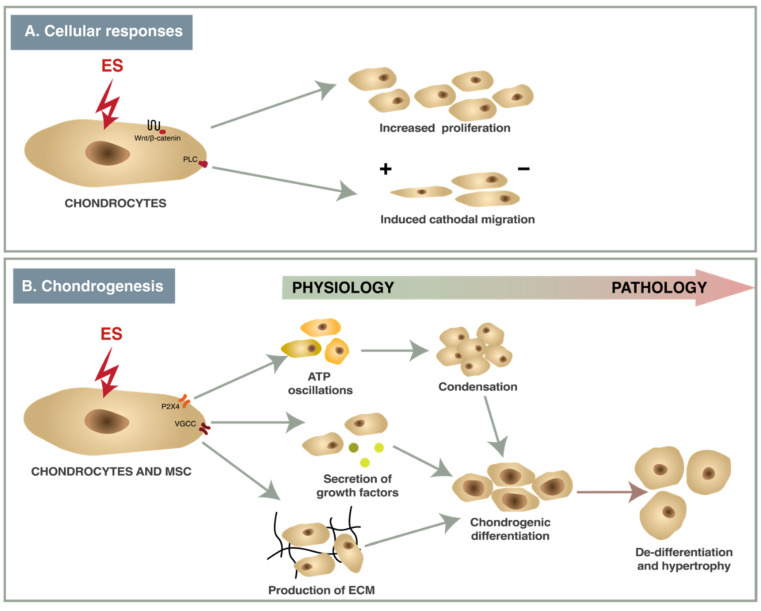
A wide variety of ES protocols with different parameters have been used in studies with chondrocytes or MSCs in vitro causing contrasting effects. Electric fields of different strengths and frequencies have multiple effects in chondrocytes and MSCs (Figure 2). ES of 2–500 mV/mm acting via ion channels (VGCC, P2X4) and enzymes (phospholipase-C (PLC)) causes changes in gene transcription (Figure 4), resulting in increased cell proliferation, directional migration and differentiation, while nsPEF modulates signaling pathways (Wnt/β-catenin, JNK/CREB-STAT3, ERK/JNK/STAT3) and also affects mRNA expression [16,44,78]. The effects that ES have on chondrocytes and chondrogenesis are summarized below.
Figure 4.
The effects of ES to responses (A) and chondrogenesis (B).
Proliferation and metabolic activity. Proliferation of chondrocytes is necessary for cartilage repair [79]. A total of 100 mVRMS (corresponds to 5.2 × 10−5 mV/cm) electric fields increased the production of ECM components (collagen type II, glycosaminoglycans (GAGs)) and proliferation in chondrocytes isolated from post-traumatic, non-OA human cartilage [45]. The opposite results were obtained using nsPEF—the proliferation of porcine chondrocytes was increased, but dedifferentiation of chondrocytes was also enhanced without showing any cytotoxicity or signs of changing cell morphology. This effect was reduced by inhibiting the wnt/β-catenin pathway [36]. Krueger et al. also evaluated that together with increased proliferation low-intensity (100 mVRMS (corresponds to 5.2 × 10−5 mV/cm), ES decreased the metabolic activity of chondrocytes, isolated from osteoarthritic and non-degenerative chondrocytes [45], while alternating electric field (1 kHz, 0.7 VRMS) did not change the metabolic activity of human chondrocytes or bone marrow MSCs (BMMSCs) but increased expression of chondrogenic genes (collagen type II, aggrecan) [80].
Migration. There is much data about the stimulatory effect of ES to cell directional migration due to the negative cell surface potential [1], which was also shown in chondrocytes. Direct current of an at least 80 mV/mm electric field for 2 h induced the inositol phospholipid pathway-dependent cathodal migration of primary bovine chondrocytes, cultivated in a monolayer [78]. Physiologically relevant ES stimulates phospholipase C (PLC)-coupled surface receptors; PLC hydrolyses phosphatidylinositol 4,5-bisphosphate into inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 induces iCa2+ release from the endoplasmic reticulum and activates gene expression [81,82]. ES also changed the morphology and alignment of chondrocytes—it caused cell elongation and perpendicular alignment to minimize the electric field gradient across the cell [78].
ATP Oscillations. Ca2+-driven ATP oscillations via P2X4 receptors and cAMP/PKA signaling are necessary for prechondrogenic condensation [43]. Electrical stimulation of 500 mV/mm, 10 Hz, 8 ms was observed to cause ATP oscillations via the P2X4 receptor, leading to mouse MSC condensation and increased expression of chondrogenic markers during chondrogenic differentiation [16].
Condensation. Condensation is the initial step of chondrogenic differentiation when a microenvironment for cell differentiation is created [83]. ES of 500 mV/mm, 10 Hz, 8 ms for 3 days induced prechondrogenic condensation in micromass cultures of mouse MSCs and human dermal fibroblasts [16,22]. ES induces prechondrogenic condensation via TGF-β signaling and Ca2+/ATP oscillations, which can be inhibited by the paracrine factor secretion inhibitor BFA and gap junction inhibitor carbenoxolone, while bone morphogenetic protein-2 (BMP-2) signaling inhibitor noggin did not exhibit any effect [16].
Secretion of growth factors. Many growth factors play a significant role in chondrogenic differentiation [84]. Some studies showed increased gene expression and secretion of TGF-β1, a key factor for chondrogenic differentiation in vitro, in canine adipose-derived MSCs (ADSCs) mouse MSCs and human fibroblasts after 3 days of ES [16,20,22]. ES of 500 mV/mm, 10 Hz, 8 ms also elevated secretion of PDGF-AA, IGFBP-2 and 3 in human dermal fibroblasts during chondrogenic differentiation [22], and gene expression of BMP2 in mouse MSCs [16] (Table 3).
Table 3.
The effects of different growth factors on chondrogenesis.
| Growth Factor | Function in Chondrogenesis | Reference |
|---|---|---|
| Transforming growth factor (TGF)-β1 | Induces condensation of MSCs; Induces the production of fibronectin and N-cadherin |
[16] |
| Platelet-derived growth factor (PDGF)-AA | Promotes MSC osteogenic differentiation and migration; Promotes chondrogenesis in the early stages of limb development |
[85,86] |
| Insulin-like growth factor-binding protein (IGFPB)-2 | Reduces proliferation of chondrocytes; Stimulates expression of prehypertrophy marker Indian hedgehog; Inhibits chondrogenic differentiation and ECM synthesis of micromass cultures |
[87,88] |
| IGFPB-3 | Reduces proliferation of chondrocytes; Might diminish the synthesis of matrix collagen and aggrecan |
[89,90] |
| BMP-2 | Increases chondrogenic differentiation by increasing Sox9a and Runx2 proteins expression in vitro, Increases hypertrophy and expression of osteogenic markers: type I collagen, type X collagen |
[91,92] |
Production of ECM and MMPs. ECM creates a microenvironment that facilitates chondrocyte attachment and induces chondrogenic differentiation. Components of ECM (collagen II, aggrecan, GAGs) are widely analyzed as biomarkers of chondrogenic differentiation [93]. ES can affect the production of ECM in two ways—by changing the expression of ECM components or by affecting the degradation of ECM by MMPs. Low-intensity electric field stimulation (100 mVRMS (corresponds to 5.2 × 10−5 mV/cm), 1 kHz) increased gene expression of aggrecan (ACAN) and GAGs in human chondrocytes, seeded on collagen elastin scaffolds, in comparison to unstimulated control [45]. An electrical field of 500 mV/mm, 10 Hz, 8 ms increased gene expression of chondrogenic markers (collagen II, aggrecan, SOX9), increased protein expression of collagen II, increased GAGs content and decreased gene and protein expression of hypertrophic marker collagen I in murine MSCs and human dermal fibroblasts [22]. Optimized conditions of coupled electrical field (60 kHz, 2 mV/mm cyclic stimulation) when applied for 1 h increased aggrecan mRNA expression, and for 6 h—collagen type II; 30 min continuous stimulation reduced expression of MMP-1, MMP-3, MMP-13 and ADAMTS-4, and ADAMTS-5. These effects can be completely blocked by inhibitors of VGCCs, calmodulin activation, calcineurin activity, phospholipase C activity and prostaglandin PGE2 synthesis. These results show that the effects of cyclic stimulation act mostly via Ca2+ signaling [44]. ES also has different effects in hypoxic and normoxic conditions. While ES increased collagen type II and aggrecan mRNA expression in BMMSCs in both conditions, ES increased collagen type II synthesis in chondrocytes only in hypoxic conditions [80]. The optimized conditions of nsPEF increased gene expression levels of collagen type II gene, SOX9, and ACAN via the JNK/CREB-STAT3 signaling pathway [35].
De-differentiation and hypertrophy. In some studies, the positive effect of ES is observed along with increased expression of hypertrophic genes. Low-intensity ES increased the expression of COL1 and alkaline phosphatase (ALP) and protein expression of collagen type I [45]; an alternating electric field (700 mVRMS, 1 kHz) also increased the expression of COL1 [80]. Nanosecond-pulsed ES had the strongest hypertrophic effect; 5 pulses of 100 ns at 10 kV/cm or 20 kV/cm downregulated collagen II and SOX9 gene expression and increased expression of collagen I and collagen X mRNA in chondrocytes, but this effect can be partially decreased by blocking the wnt/β-catenin pathway [36]. Another study showed that 60 ns pulse of 5–20 kV/cm increased collagen type X and collagen type I expressions [35].
Thus, the application of ES might result in a range of cellular responses, leading to diverse functional outcomes depending on the protocol and ES type used. ES parameters should be equilibrated based on the utilized ES methods and specific investigated cell types in order to optimize the production of engineered tissue.
5. The Effects of ES on Chondrogenesis In Vitro
Current in vitro studies using different ES protocols have generated controversial results on the effect of ES to chondrogenesis (Table 4).
Table 4.
The effects of different types of ES on chondrogenesis.
| Cell Type | ES Conditions | Beneficial Effects | Adverse Effects | References |
|---|---|---|---|---|
| Continuous Stimulation | ||||
| Human dermal fibroblasts (HDFs) | 100–500 mV/mm electric field, bipolar square-wave pulse, 6–10 ms at 5 Hz for 3 days | 8 ms pulse: ↑ condensation; ↑ expression of chondrogenic genes (COL2A1, ACAN, SOX9;) ↓ expression of COL1A1, COL1A2 genes; ↑level of COL2 protein, GAGs; ↑ secretion of growth factors (TGF-β1, PDGF-AA, IGFBP-2 and 3) |
Pulse duration 6 ms: No condensation Pulse duration 10 ms: Damaged cells, No compact condensation |
[22] |
| Murine BMMSCs | 100–2500 mV/mm electric field, bipolar square-wave pulse, 8 ms at 5 Hz for 3 days | Electrical field of 500 mV/mm: ↑ condensation; ↑ expression of chondrogenic genes (COL2A1, ACAN, SOX9); ↓ expression of COL1 gene; ↑ level of COL2 protein, GAGs; ↑ expression of TGF-β1 and BMP2. |
Electrical field of 100 and 2500 mV/mm: ↓ Ca2+/ATP oscillations; ↓ condensation |
[16] |
| Human ADSCs are extracted from subcutaneous abdominal adipose tissue | 1 kHz, 20 mv/cm for 20 min. | ↑ aggrecan secretion ↑ expression of COLII and SOX9 genes ↓ expression of COLX gene |
[94] | |
| Bovine chondrocytes | 0.02–4 mV/mm, sine-wave with a frequency of 60 kHz; stimulation time 0.5 h | 0.5-h of 2 mV/mm (1 min on (1′ ON), 7 off (7′ OFF)—30 cycles and 1′ ON/1′ OFF 30 cycles), harvest after 3.5 h of ES: ↑ expression of ACAN gene. 2 mV/mm, regimen—1′ ON/7′ OFF—30 cycles and continuous stimulation, harvest after 5.5 h: ↑ expression of COL2 gene. |
2–6 h of stimulation, 0.1, 0.5, 4 mV/mm amplitude: No change in expression of ACAN gene 0.02, 0.1, 0.5, 1, 4 mV/mm amplitude: No change in expression of COL2 gene |
[95] |
| Explants from human osteoarthritic cartilage | 2 mV/mm, static for 30 min followed by 8.33 μs square wave pulse at 60 kHz for 1 h × 4 times a day (gap 5 h) for 7 or 14 days |
↑ proteoglycan and collagen content; ↑ expression of ACAN and COL2 genes; ↓ expression of IL-1β induced MMP-1, MMP-3, MMP-13, ADAM-TS4 genes. |
- | [19] |
| Human ADSCs were extracted from subcutaneous abdominal adipose tissue | Capacitively electric fields (20 mV/cm, 60 KHz) pulsed wave applied for 20 min daily for 7 days. | ↑ expression of COLII, SOX9 genes; ↓ expression of COL X and COLI genes ↑ secretion of aggrecan No difference in cell viability |
- | [96] |
| Articular chondrocytes were isolated from adult bovine patellae | 2 mV/mm, 8.33 μs square wave pulse at 60 kHz for 1–6 h 1′ ON/7′ OFF) for 1 h for aggrecan and 1′ ON/1′ OFF for 6 h for collagen II; for MMPs—30 min static stimulation at 2 mV/mm |
↑ expression of ACAN, COLII genes ↓ expression of IL-1β induced MMP-1, MMP-3, MMP-13, ADAMTS-4, ADAMTS-5 genes |
- | [44] |
| Nanosecond pulsed electrical field | ||||
| Normal human chondrocytes (#CC2550, Lonza) | Asymmetrical biphasic rectangular pulses, 210/30 ms in each polarity, respectively, repeating at 4150 Hz, delivered in 10-ms bursts 15 times per second for 30 min. PEF generated peak changes in current of 2.7 mAmps and an electric field in culture media of 0.2 mV/cm |
↑ proliferation ↑ NO and cGMP |
[97] | |
| Chondrocytes from porcine articular cartilage tissue | 1–2 × 106 mV/mm, square wave with transients; 5 × 100 ns, 1 Hz | ↑ proliferation of chondrocytes. | ↓ GAG production; ↓ expression of COLII, SOX9; ↑ expression COLI gene and COLX. |
[36] |
| Porcine BMMSCs | 0.5–3 × 106 mV/mm, square wave with transients; 5 × 10−300 ns, 1 Hz | 10 ns at 2 × 106 mV/mm, 60 ns at 5 and 2 × 106 mV/mm, 100 ns at 1 × 106 mV/mm: ↑ expression of COLII, SOX9, and ACAN genes; 10 ns at × 106 mV/mm and 100 ns at 10 × 106 mV/mm: ↑ production of GAGs |
2 and 3 × 106 mV/mm with longer pulse duration (100, 300 ns): ↓ viability; 60 ns at 0.5–2 × 106 mV/mm: ↑ expression of COLI and COLX genes |
[35] |
| Rat BMMSCs | 100 ns duration, 10 kV cm−1, 1 Hz) | ↑ OCT4 and NANOG expression Together with grelin ↑ expression of SOX9, COLII, ACAN genes ↑ de novo cartilage regeneration (smoother cartilage surface in defect area, ↑ ICRS histology score) |
- | [98] |
| Porcine BMMSCs and human BMMSCs | 5 pulses of nsPEFs (10 ns at 20 kV/cm, 60 ns at 5 kV/cm, 60 ns at 10 kV/cm, 60 ns at 20 kV/cm, and 100 ns at 10 kV/cm, 1 Hz) with 1 s time interval between two pulses | 10 ns at 20 kV/cm, and 100 ns at 10 kV/cm in both types of cells: ↑ enhance trilineage differentiation potential; -No influence on proliferation; ↑ expression of OCT4 and NANOG genes; |
- | [99] |
| Porcine BMMSCs | Stimulation was carried out with 5 pulses of nsPEFs (10~25 kV/cm, 10~100 ns), and the time interval between each pulse was 1 s. |
Four 100 ns at 10 kV/cm pulse on cells cultured on PLLA/CNT films: ↑ tri-lineage differentiation 4 times of PES ↑ expression of pluripotency genes (OCT4, NANOG, SOX2) |
Single 100 ns at 10 kV/cm pulse on cells cultured on PLLA/CNT films: ↑ expression of pluripotency genes (OCT4, NANOG, SOX2) 3 days after pulse |
[100] |
Continuous and cyclic low-voltage ES with optimized conditions can be a valuable tool for the induction of ATP oscillations, resulting in the condensation of cells and increased expression of chondrogenic genes (COL1, ACAN, SOX9) in HDFs, murine BMMSCs and chondrocytes [16,22]. Wang et al. [95] showed that the highest expression of different chondrogenic genes can be achieved in different time points, so each case needs optimization research. High-intensity and low-voltage electrical field stimulation also had a chondroprotective effect because of the reduced expression of interleukin 1β (IL-1β), which induced MMPs.
NsPEF causes mixed effects on chondrogenesis—a shorter pulse (10 ns at 2 × 106 mV/mm, 60 ns at 5 and 2 × 106 mV/mm, 100 ns at 1 × 106 mV/mm) increased the expression of chondrogenic genes in porcine chondrocytes, while a longer pulse (60 ns) with EF of 0.5–2 × 106 mV/mm) mostly increased de-differentiation and hypertrophy of porcine MSC and chondrocytes [35,36]. EF of 2 and 3 × 106 mV/mm with longer pulse duration (100, 300 ns) also decreased the viability of porcine MSCs while similar conditions increased the proliferation of porcine chondrocytes [35]. Mixed results can be explained by the different size of MSC and chondrocytes, which reacts differently to electrical stimuli; also, different signaling pathways are activated by diverse electrical fields.
The mechanism of ES on chondrogenesis is not well understood yet. It is known that ES directly affects membrane channels (e.g., VGCC) and receptors (e.g., P2X4), affecting cAMP/PKA signaling. The studies also show that the inhibition of some signaling pathways (JNK/CREB-STAT3, wnt/β-catenin) decreases or diminishes the effect of ES [43].
Until now, only a few studies have focused on the effects of ES on chondrogenesis in 3D structures (Table 5). Biocompatible and electroconductive scaffolds must be used for such studies. ES was mostly applied on hydrogels or scaffolds containing natural cartilage ECM components (collagen, elastin, or hyaluronic acid) [45,101,102]; however, some studies have been performed with synthetic scaffolds [103]. Both biological and synthetic scaffolds have their own advantages, as natural polymer structures are more biocompatible while synthetic counterparts are mechanically stronger and easier to produce and manipulate [38], which makes it possible to improve conductive properties of the scaffold by, for example, incorporating conductive materials. Moreover, different scaffold manufacturing methods such as 3D printing and electrospinning result in scaffolds exhibiting varying properties (porosity, cell adhesion, etc.) that might affect conductivity [104]. Another possible future prospect of cartilage tissue engineering research is the development of electroactive hydrogels that can be shaped by applying noncytotoxic voltages [105].
Table 5.
Different effects of ES on cells grown in scaffolds or hydrogels.
| Scaffold Characteristics | Cell Type | Electrical Stimulation Parameters | Beneficial Effects | Adverse Effects | Reference |
|---|---|---|---|---|---|
| 3D collagen I -elastin scaffolds | Human chondrocytes (OA and control) |
5.2 × 10−6 and 5.2 × 10−5 mV/mm, sine wave 1 kHz. 3 times in a day for 45 min for 7 days |
5.2 × 10−6 mV/mm ↑ GAG, COLII protein synthesis |
↑ COLI protein synthesis | [45] |
| CNT (carbon nanotubes)/PCU polycarbonate urethane | Human chondrocytes (Cell Applications) | Alternating current (AC) stimulation of voltages was generated at 10 lA with 10 Hz throughout the entire cell experiment but with different stimulation times (specifically, either 3 or 6 h) |
Both 3 and 6 h stimulation ↑ proliferation of chondrocytes | - | [38] |
| Collagen I-elastin scaffold | Chondrocytes (non-degraded) | 0.005–2.5 mV/mm bipolar wave delivered at 1 or 60 kHz for 45 min per day for seven days. |
↑ chondrogenic re-differentiation at the gene and protein level of human de-differentiated chondrocytes | - | [106] |
| Graphene-Containing PCL/Bioactive Glass Bilayer | Pre-osteoblastic MC3T3-E1 and chondrogenic ATDC5 | 2 mV/mm, sine wave at 60 kHz 30 min/day for 3 days | ↑ viability of ATDC5 cells | ↓ viability of MC3T3-E1 cells | [103] |
| Injectable hyaluronic acid—gelatin hydrogel | Porcine MSCs | 0.9–1.2 mV/mm sine wave at 60 kHz, 30 min 4 times a day, 21 days | ↑ SOX9 and aggrecan and COLII protein |
↓ production of GAG | [101] |
In addition to classic applications of ES, pulsed supra-physiological electrical field ES can also lead to increased chondrogenic (collagen, aggrecan) gene and protein expression when used on different origin chondrocytes and MSCs, making it a valuable tool for cartilage tissue engineering.
6. Recommendations on Reporting ES in the Context of Chondrogenesis
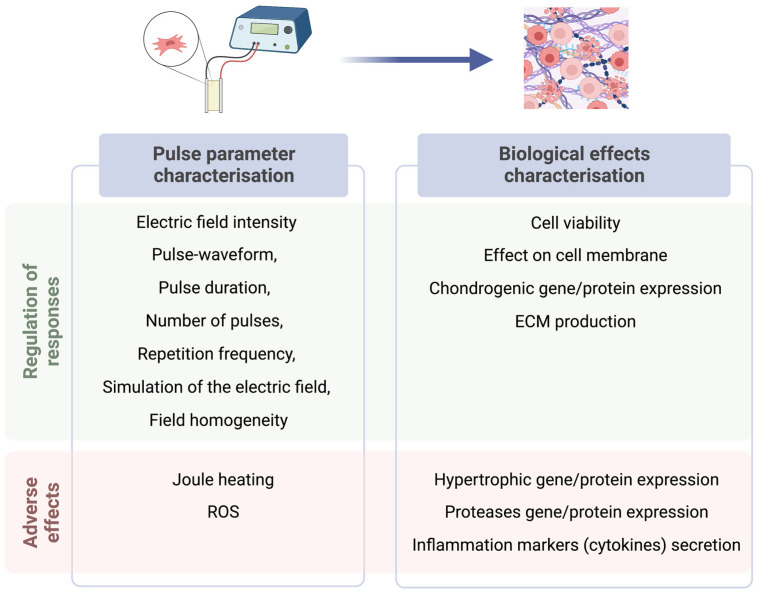
In order to improve the reproducibility of the results and ensure adequate consolidation of knowledge in the area of ES, pulse parameters should be properly characterized (Figure 5). One of the main parameters is the electric field intensity, which is characterized by electric field strength, pulse-waveform, pulse duration, number of pulses and the repetition frequency. However, other influencing factors are often overlooked. Since ES is cell membrane polarization-based phenomenon, the dynamics of the cell membrane polarization are influenced by the dielectric parameters of cell and the surrounding medium and the frequency of the externally applied electric field [66]. Therefore, comparison of the induced biological effects is non-straightforward and often impossible if the pulsing conditions are not properly reported. One of the solutions is to use the guidelines for reporting data on pulsed electric field-based treatments [77,107,108]. Additionally, simulation of the electric field and the field homogeneity should be reported, since they strongly depend on the structure of the applicators (i.e., electrodes) [109]. Finally, the effects of Joule heating [110,111] should be estimated in case of long-term treatments; thus, the input energy of the whole protocol should be presented.
Figure 5.
Recommendations on electrical parameters and biological effects that must be characterized in order to get reliable results on chondrogenesis experiments.
To exclude possible cytotoxic effects, it is necessary to prove cell viability and proliferative activity after ES, which can be performed via fluorescent imaging (Live/Dead, Calcein AM, EdU) or metabolic assays (CCK-8, AlamarBlue etc.) [112]. The direct effect of electrical stimulation on the cell membrane should be monitored using VGCC inhibitors, while the creation of micropores can be proved via the Yo-Pro-1 uptake [113]. During chondrogenesis assays, it is necessary to not only show chondrogenic gene (SOX9, Col II, Aggrecan), but also hypertrophic gene (Col X, Col I, MMP-13) expression [36]. To prove successful chondrogenic differentiation, tissue GAG expression can be evaluated using histology (AlcianBlue or Safranin-O) or its release quantified in the cell supernatant [45,114]. In addition to the markers of chondrogenic differentiation, signs of undesired effects must also be analyzed. Cytokines such as IL-6 can be monitored as signs of inflammation [115], while tissue degradation can be detected via the expression of proteases such as MMP-13, ADAMTS or the downregulation of previously mentioned chondrogenic genes and proteins [116].
Another important aspect of studies using ES on engineered cartilage tissue is the selection of scaffolds as they can both affect the strength of ES exerted on the cells as well as influence cell viability and chondrogenesis. Properties such as mechanical strength, conductivity and biocompatibility of the scaffolding material should be evaluated in the context of the experiment [38]. Furthermore, other scaffold parameters, such as porosity, and the introduction of parallel mechanical stimulation could play a role in the outcomes of ES studies.
7. Conclusions
Electrostimulation is still a novel technique that has a high potential in tissue engineering. Electrical impulses have various effects on cellular processes, including the opening of voltage-gated membrane channels and signaling pathway activation. Recent studies showed that ES can be used to increase chondrogenic gene and protein expression in 2D and 3D systems, while some conditions can induce cell hypertrophy or cell damage. Thus, ES is a promising tool for chondrogenesis studies and has the potential for cartilage repair.
Author Contributions
Conceptualization, all authors; original draft writing, all authors; editing, all authors; figure creation, R.V.; critical revision, A.A., V.N. and E.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was funded by the European Structural and Social Funds (ES Struktūrinės Paramos) through the Research Council of Lithuania (Lietuvos Mokslo Taryba) according to the activity Attracting Foreign Researchers for Research Implementation (2018–2022), Grant No 01.2.2-LMT-K-718-02-0022 and HORIZON Europe project: HORIZON-WIDERA-2021-ACCESS-03-01, nr. 101079489-TwInflAg.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Chen C., Bai X., Ding Y., Lee I.-S. Electrical stimulation as a novel tool for regulating cell behavior in tissue engineering. Biomater. Res. 2019;23:25. doi: 10.1186/s40824-019-0176-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu L., Klein J.D., Hassounah F., Cai H., Zhang C., Xu P., Wang X.H. Low-frequency electrical stimulation attenuates muscle atrophy in CKD—A potential treatment strategy. J. Am. Soc. Nephrol. 2015;26:626–635. doi: 10.1681/ASN.2014020144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faingold C.L. Electrical stimulation therapies for CNS disorders and pain are mediated by competition between different neuronal networks in the brain. Med. Hypotheses. 2008;71:668–681. doi: 10.1016/j.mehy.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang X., Das R., Patel A., Nguyen T.D. Physical Stimulations for Bone and Cartilage Regeneration. Regen. Eng. Transl. Med. 2018;4:216–237. doi: 10.1007/s40883-018-0064-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuan X., Arkonac D.E., Chao P.H., Vunjak-Novakovic G. Electrical stimulation enhances cell migration and integrative repair in the meniscus. Sci. Rep. 2014;4:3674. doi: 10.1038/srep03674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giggins O., Fullen B., Coughlan G. Neuromuscular electrical stimulation in the treatment of knee osteoarthritis: A systematic review and meta-analysis. Clin. Rehabil. 2012;26:867–881. doi: 10.1177/0269215511431902. [DOI] [PubMed] [Google Scholar]
- 7.Zeng C., Li H., Yang T., Deng Z.H., Yang Y., Zhang Y., Lei G.H. Electrical stimulation for pain relief in knee osteoarthritis: Systematic review and network meta-analysis. Osteoarthr. Cartil. 2015;23:189–202. doi: 10.1016/j.joca.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Mobasheri A., Matta C., Uzieliene I., Budd E., Martin-Vasallo P., Bernotiene E. The chondrocyte channelome: A narrative review. Jt. Bone Spine. 2019;86:29–35. doi: 10.1016/j.jbspin.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Uzieliene I., Bernotas P., Mobasheri A., Bernotiene E. The Role of Physical Stimuli on Calcium Channels in Chondrogenic Differentiation of Mesenchymal Stem Cells. Int. J. Mol. Sci. 2018;19:2998. doi: 10.3390/ijms19102998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahaut-Smith M.P., Hussain J.F., Mason M.J. Depolarization-evoked Ca2+ release in a non-excitable cell, the rat megakaryocyte. J. Physiol. 1999;515:385–390. doi: 10.1111/j.1469-7793.1999.385ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sophia Fox A.J., Bedi A., Rodeo S.A. The basic science of articular cartilage: Structure, composition, and function. Sport Health. 2009;1:461–468. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomoll A.H. Microfracture and augments. J. Knee Surg. 2012;25:9–15. doi: 10.1055/s-0031-1299654. [DOI] [PubMed] [Google Scholar]
- 13.Carey J.L. Fibrocartilage following microfracture is not as robust as native articular cartilage: Commentary on an article by Aaron, J.; Krych, M.D.; et al. Activity levels are higher after osteochondral autograft transfer mosaicplasty than after microfracture for articular cartilage defects of the knee. A retrospective comparative study. J. Bone Joint. Surg. Am. 2012;94:e80. doi: 10.2106/jbjs.L.00319. [DOI] [PubMed] [Google Scholar]
- 14.Devitt B.M., Bell S.W., Webster K.E., Feller J.A., Whitehead T.S. Surgical treatments of cartilage defects of the knee: Systematic review of randomised controlled trials. Knee. 2017;24:508–517. doi: 10.1016/j.knee.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Urlić I., Ivković A. Cell Sources for Cartilage Repair-Biological and Clinical Perspective. Cells. 2021;10:2496. doi: 10.3390/cells10092496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwon H.J., Lee G.S., Chun H. Electrical stimulation drives chondrogenesis of mesenchymal stem cells in the absence of exogenous growth factors. Sci. Rep. 2016;6:39302. doi: 10.1038/srep39302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Csaki C., Schneider P.R., Shakibaei M. Mesenchymal stem cells as a potential pool for cartilage tissue engineering. Ann. Anat. 2008;190:395–412. doi: 10.1016/j.aanat.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Ciobanu F., Golzio M., Kovacs E., Teissié J. Control by Low Levels of Calcium of Mammalian Cell Membrane Electropermeabilization. J. Membr. Biol. 2018;251:221–228. doi: 10.1007/s00232-017-9981-y. [DOI] [PubMed] [Google Scholar]
- 19.Brighton C.T., Wang W., Clark C.C. The effect of electrical fields on gene and protein expression in human osteoarthritic cartilage explants. J. Bone Jt. Surg. Am. 2008;90:833–848. doi: 10.2106/JBJS.F.01437. [DOI] [PubMed] [Google Scholar]
- 20.Chang C., Park J., Noh J. Electrical stimulation confers pre-chondrogenic differentiation by modulating TGF- β1 levels in canine adipose-derived mesenchymal stem cells. Cytotherapy. 2020;22:S72. doi: 10.1016/j.jcyt.2020.03.113. [DOI] [Google Scholar]
- 21.Park H.J., Rouabhia M., Lavertu D., Zhang Z. Electrical Stimulation Modulates the Expression of Multiple Wound Healing Genes in Primary Human Dermal Fibroblasts. Tissue Eng. Part A. 2015;21:1982–1990. doi: 10.1089/ten.tea.2014.0687. [DOI] [PubMed] [Google Scholar]
- 22.Lee G.S., Kim M.G., Kwon H.J. Electrical stimulation induces direct reprogramming of human dermal fibroblasts into hyaline chondrogenic cells. Biochem. Biophys. Res. Commun. 2019;513:990–996. doi: 10.1016/j.bbrc.2019.04.027. [DOI] [PubMed] [Google Scholar]
- 23.Atsuta Y., Tomizawa R.R., Levin M., Tabin C.J. L-type voltage-gated Ca2+ channel CaV1.2 regulates chondrogenesis during limb development. Proc. Natl. Acad. Sci. USA. 2019;116:21592–21601. doi: 10.1073/pnas.1908981116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marszalek P., Liu D.S., Tsong T.Y. Schwan equation and transmembrane potential induced by alternating electric field. Biophys. J. 1990;58:1053–1058. doi: 10.1016/S0006-3495(90)82447-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arena C.B., Sano M.B., Rossmeisl J.H., Jr., Caldwell J.L., Garcia P.A., Rylander M.N., Davalos R.V. High-frequency irreversible electroporation (H-FIRE) for non-thermal ablation without muscle contraction. Biomed. Eng. Online. 2011;10:102. doi: 10.1186/1475-925X-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aycock K.N., Zhao Y., Lorenzo M.F., Davalos R.V. A Theoretical Argument for Extended Interpulse Delays in Therapeutic High-Frequency Irreversible Electroporation Treatments. IEEE Trans. Biomed. Eng. 2021;68:1999–2010. doi: 10.1109/TBME.2021.3049221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kotnik T., Rems L., Tarek M., Miklavčič D. Membrane Electroporation and Electropermeabilization: Mechanisms and Models. Annu. Rev. Biophys. 2019;48:63–91. doi: 10.1146/annurev-biophys-052118-115451. [DOI] [PubMed] [Google Scholar]
- 28.Tekle E., Astumian R.D., Chock P.B. Electro-permeabilization of cell membranes: Effect of the resting membrane potential. Biochem. Biophys. Res. Commun. 1990;172:282–287. doi: 10.1016/S0006-291X(05)80206-2. [DOI] [PubMed] [Google Scholar]
- 29.Kotnik T., Kramar P., Pucihar G., Miklavcic D., Tarek M. Cell membrane electroporation- Part 1: The phenomenon. IEEE Electr. Insul. Mag. 2012;28:14–23. doi: 10.1109/MEI.2012.6268438. [DOI] [Google Scholar]
- 30.Geboers B., Scheffer H.J., Graybill P.M., Ruarus A.H., Nieuwenhuizen S., Puijk R.S., van den Tol P.M., Davalos R.V., Rubinsky B., de Gruijl T.D., et al. High-Voltage Electrical Pulses in Oncology: Irreversible Electroporation, Electrochemotherapy, Gene Electrotransfer, Electrofusion, and Electroimmunotherapy. Radiology. 2020;295:254–272. doi: 10.1148/radiol.2020192190. [DOI] [PubMed] [Google Scholar]
- 31.Wells J.M., Li L.H., Sen A., Jahreis G.P., Hui S.W. Electroporation-enhanced gene delivery in mammary tumors. Gene Ther. 2000;7:541–547. doi: 10.1038/sj.gt.3301141. [DOI] [PubMed] [Google Scholar]
- 32.Antov Y., Barbul A., Mantsur H., Korenstein R. Electroendocytosis: Exposure of cells to pulsed low electric fields enhances adsorption and uptake of macromolecules. Biophys. J. 2005;88:2206–2223. doi: 10.1529/biophysj.104.051268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin R., Chang D.C., Lee Y.K. Single-cell electroendocytosis on a micro chip using in situ fluorescence microscopy. Biomed. Microdevices. 2011;13:1063–1073. doi: 10.1007/s10544-011-9576-9. [DOI] [PubMed] [Google Scholar]
- 34.Cho I., Jackson M.R., Swift J. Roles of Cross-Membrane Transport and Signaling in the Maintenance of Cellular Homeostasis. Cell. Mol. Bioeng. 2016;9:234–246. doi: 10.1007/s12195-016-0439-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ning T., Guo J., Zhang K., Li K., Zhang J., Yang Z., Ge Z. Nanosecond pulsed electric fields enhanced chondrogenic potential of mesenchymal stem cells via JNK/CREB-STAT3 signaling pathway. Stem Cell Res. Ther. 2019;10:45. doi: 10.1186/s13287-019-1133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang K., Guo J., Ge Z., Zhang J. Nanosecond pulsed electric fields (nsPEFs) regulate phenotypes of chondrocytes through Wnt/β-catenin signaling pathway. Sci. Rep. 2014;4:5836. doi: 10.1038/srep05836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thrivikraman G., Boda S.K., Basu B. Unraveling the mechanistic effects of electric field stimulation towards directing stem cell fate and function: A tissue engineering perspective. Biomaterials. 2018;150:60–86. doi: 10.1016/j.biomaterials.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Miguel F., Barbosa F., Ferreira F.C., Silva J.C. Electrically Conductive Hydrogels for Articular Cartilage Tissue Engineering. Gels. 2022;8:710. doi: 10.3390/gels8110710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matta C., Zákány R., Mobasheri A. Voltage-dependent calcium channels in chondrocytes: Roles in health and disease. Curr. Rheumatol. Rep. 2015;17:43. doi: 10.1007/s11926-015-0521-4. [DOI] [PubMed] [Google Scholar]
- 40.Wen L., Wang Y., Wang H., Kong L., Zhang L., Chen X., Ding Y. L-type calcium channels play a crucial role in the proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2012;424:439–445. doi: 10.1016/j.bbrc.2012.06.128. [DOI] [PubMed] [Google Scholar]
- 41.Uzieliene I., Bironaite D., Bernotas P., Sobolev A., Bernotiene E. Mechanotransducive Biomimetic Systems for Chondrogenic Differentiation In Vitro. Int. J. Mol. Sci. 2021;22:9690. doi: 10.3390/ijms22189690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mobini S., Talts U.-L., Xue R., Cassidy N., Cartmell S. Electrical Stimulation Changes Human Mesenchymal Stem Cells Orientation and Cytoskeleton Organization. J. Biomater. Tissue Eng. 2017;7:829–833. doi: 10.1166/jbt.2017.1631. [DOI] [Google Scholar]
- 43.Kwon H.J. Extracellular ATP signaling via P2X(4) receptor and cAMP/PKA signaling mediate ATP oscillations essential for prechondrogenic condensation. J. Endocrinol. 2012;214:337–348. doi: 10.1530/JOE-12-0131. [DOI] [PubMed] [Google Scholar]
- 44.Xu J., Wang W., Clark C.C., Brighton C.T. Signal transduction in electrically stimulated articular chondrocytes involves translocation of extracellular calcium through voltage-gated channels. Osteoarthr. Cartil. 2009;17:397–405. doi: 10.1016/j.joca.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Krueger S., Achilles S., Zimmermann J., Tischer T., Bader R., Jonitz-Heincke A. Re-Differentiation Capacity of Human Chondrocytes in Vitro Following Electrical Stimulation with Capacitively Coupled Fields. J. Clin. Med. 2019;8:1771. doi: 10.3390/jcm8111771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farooqi A.R., Bader R., van Rienen U. Numerical Study on Electromechanics in Cartilage Tissue with Respect to Its Electrical Properties. Tissue Eng. Part B Rev. 2019;25:152–166. doi: 10.1089/ten.teb.2018.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun Y., Zhang K., Dong H., Wang Y., Yan Y., Yu J., Wu X., Zhang M., Wang Y., Chen W. Layered mechanical and electrical properties of porcine articular cartilage. Med. Biol. Eng. Comput. 2022;60:3019–3028. doi: 10.1007/s11517-022-02653-6. [DOI] [PubMed] [Google Scholar]
- 48.Lee J.H., Yoon Y.C., Kim H.S., Lee J., Kim E., Findeklee C., Katscher U. In vivo electrical conductivity measurement of muscle, cartilage, and peripheral nerve around knee joint using MR-electrical properties tomography. Sci. Rep. 2022;12:73. doi: 10.1038/s41598-021-03928-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Binette J.S., Garon M., Savard P., McKee M.D., Buschmann M.D. Tetrapolar Measurement of Electrical Conductivity and Thickness of Articular Cartilage. J. Biomech. Eng. 2004;126:475–484. doi: 10.1115/1.1785805. [DOI] [PubMed] [Google Scholar]
- 50.Rim Y.A., Nam Y., Ju J.H. The Role of Chondrocyte Hypertrophy and Senescence in Osteoarthritis Initiation and Progression. Int. J. Mol. Sci. 2020;21:2358. doi: 10.3390/ijms21072358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van der Kraan P.M., van den Berg W.B. Chondrocyte hypertrophy and osteoarthritis: Role in initiation and progression of cartilage degeneration? Osteoarthr. Cartil. 2012;20:223–232. doi: 10.1016/j.joca.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 52.Heinegård D., Saxne T. The role of the cartilage matrix in osteoarthritis. Nat. Rev. Rheumatol. 2011;7:50–56. doi: 10.1038/nrrheum.2010.198. [DOI] [PubMed] [Google Scholar]
- 53.Mow V.C., Guo X.E. Mechano-electrochemical properties of articular cartilage: Their inhomogeneities and anisotropies. Annu. Rev. Biomed. Eng. 2002;4:175–209. doi: 10.1146/annurev.bioeng.4.110701.120309. [DOI] [PubMed] [Google Scholar]
- 54.Brady M.A., Waldman S.D., Ethier C.R. The application of multiple biophysical cues to engineer functional neocartilage for treatment of osteoarthritis. Part I: Cellular response. Tissue Eng. Part B Rev. 2015;21:1–19. doi: 10.1089/ten.teb.2013.0757. [DOI] [PubMed] [Google Scholar]
- 55.Mow V.C., Gibbs M.C., Lai W.M., Zhu W.B., Athanasiou K.A. Biphasic indentation of articular cartilage—II. A numerical algorithm and an experimental study. J. Biomech. 1989;22:853–861. doi: 10.1016/0021-9290(89)90069-9. [DOI] [PubMed] [Google Scholar]
- 56.Lai W.M., Mow V.C. Drag-induced compression of articular cartilage during a permeation experiment. Biorheology. 1980;17:111–123. doi: 10.3233/BIR-1980-171-213. [DOI] [PubMed] [Google Scholar]
- 57.Mellor L.F., Mohiti-Asli M., Williams J., Kannan A., Dent M.R., Guilak F., Loboa E.G. Extracellular Calcium Modulates Chondrogenic and Osteogenic Differentiation of Human Adipose-Derived Stem Cells: A Novel Approach for Osteochondral Tissue Engineering Using a Single Stem Cell Source. Tissue Eng. Part A. 2015;21:2323–2333. doi: 10.1089/ten.tea.2014.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matta C., Zakany R. Calcium signalling in chondrogenesis: Implications for cartilage repair. Front. Biosci. 2013;5:305–324. doi: 10.2741/S374. [DOI] [PubMed] [Google Scholar]
- 59.Chang H.F., Lee Y.S., Tang T.K., Cheng J.Y. Pulsed DC Electric Field-Induced Differentiation of Cortical Neural Precursor Cells. PLoS ONE. 2016;11:e0158133. doi: 10.1371/journal.pone.0158133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Enayati S., Chang K., Achour H., Cho K.-S., Xu F., Guo S., Enayati K.Z., Xie J., Zhao E., Turunen T., et al. Electrical Stimulation Induces Retinal Müller Cell Proliferation and Their Progenitor Cell Potential. Cells. 2020;9:781. doi: 10.3390/cells9030781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hernández D., Millard R., Sivakumaran P., Wong R.C.B., Crombie D.E., Hewitt A.W., Liang H., Hung S.S.C., Pébay A., Shepherd R.K., et al. Electrical Stimulation Promotes Cardiac Differentiation of Human Induced Pluripotent Stem Cells. Stem Cells Int. 2016;2016:1718041. doi: 10.1155/2016/1718041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Szlasa W., Michel O., Sauer N., Novickij V., Lewandowski D., Kasperkiewicz P., Tarek M., Saczko J., Kulbacka J. Nanosecond pulsed electric field suppresses growth and reduces multi-drug resistance effect in pancreatic cancer. Sci. Rep. 2023;13:351. doi: 10.1038/s41598-023-27605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meng S., Rouabhia M., Zhang Z. Electrical stimulation modulates osteoblast proliferation and bone protein production through heparin-bioactivated conductive scaffolds. Bioelectromagnetics. 2013;34:189–199. doi: 10.1002/bem.21766. [DOI] [PubMed] [Google Scholar]
- 64.Cui S., Rouabhia M., Semlali A., Zhang Z. Effects of electrical stimulation on human skin keratinocyte growth and the secretion of cytokines and growth factors. Biomed. Mater. 2021;16:065021. doi: 10.1088/1748-605X/ac2bba. [DOI] [PubMed] [Google Scholar]
- 65.Novickij V., Rembiałkowska N., Kasperkiewicz-Wasilewska P., Baczyńska D., Rzechonek A., Błasiak P., Kulbacka J. Pulsed electric fields with calcium ions stimulate oxidative alternations and lipid peroxidation in human non-small cell lung cancer. Biochim. Biophys. Acta Biomembr. 2022;1864:184055. doi: 10.1016/j.bbamem.2022.184055. [DOI] [PubMed] [Google Scholar]
- 66.Taghian T., Narmoneva D.A., Kogan A.B. Modulation of cell function by electric field: A high-resolution analysis. J. R. Soc. Interface. 2015;12:20150153. doi: 10.1098/rsif.2015.0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xiang X.-W., Liu H.-T., Liu W., Yan Z.-Y., Zeng Y.-L., Wang Y.-J., Liu J., Chen Y.-C., Yu S.-X., Zhu C.-H., et al. Microsecond pulse electrical stimulation modulates cell migration. bioRxiv. 2022 doi: 10.1101/2022.10.23.513372. [DOI] [Google Scholar]
- 68.Schoenbach K.H., Baum C.E., Joshi R.P., Beebe S.J. A Scaling Law for Bioelectric Effects of Nanosecond Pulses. IEEE Trans. Diel. Electr. Insul. 2009;16:1224–1235. doi: 10.1109/TDEI.2009.5293932. [DOI] [Google Scholar]
- 69.Kranjc M., Miklavčič D. Electric Field Distribution and Electroporation Threshold. In: Miklavčič D., editor. Handbook of Electroporation. Springer International Publishing; Cham, Switzerland: 2017. pp. 1043–1058. [Google Scholar]
- 70.Avazzadeh S., O’Brien B., Coffey K., O’Halloran M., Keane D., Quinlan L.R. Establishing Irreversible Electroporation Electric Field Potential Threshold in A Suspension In Vitro Model for Cardiac and Neuronal Cells. J. Clin. Med. 2021;10:5443. doi: 10.3390/jcm10225443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weaver J.C., Smith K.C., Esser A.T., Son R.S., Gowrishankar T.R. A brief overview of electroporation pulse strength-duration space: A region where additional intracellular effects are expected. Bioelectrochemistry. 2012;87:236–243. doi: 10.1016/j.bioelechem.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beebe S.J., Blackmore P.F., White J., Joshi R.P., Schoenbach K.H. Nanosecond pulsed electric fields modulate cell function through intracellular signal transduction mechanisms. Physiol. Meas. 2004;25:1077–1093. doi: 10.1088/0967-3334/25/4/023. [DOI] [PubMed] [Google Scholar]
- 73.Liu Z., Zou Y., Sun Y., Chen X., Chen X., Ren Z. Effects of Nanosecond Pulsed Electric Fields in Cell Vitality, Apoptosis, and Proliferation of TPC-1 Cells. Anal. Cell. Pathol. 2021;2021:9913716. doi: 10.1155/2021/9913716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hanna H., Andre F.M., Mir L.M. Electrical control of calcium oscillations in mesenchymal stem cells using microsecond pulsed electric fields. Stem Cell Res. Ther. 2017;8:91. doi: 10.1186/s13287-017-0536-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rossi A., Pakhomova O.N., Mollica P.A., Casciola M., Mangalanathan U., Pakhomov A.G., Muratori C. Nanosecond Pulsed Electric Fields Induce Endoplasmic Reticulum Stress Accompanied by Immunogenic Cell Death in Murine Models of Lymphoma and Colorectal Cancer. Cancers. 2019;11:2034. doi: 10.3390/cancers11122034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hanna H., Denzi A., Liberti M., André F.M., Mir L.M. Electropermeabilization of Inner and Outer Cell Membranes with Microsecond Pulsed Electric Fields: Quantitative Study with Calcium Ions. Sci. Rep. 2017;7:13079. doi: 10.1038/s41598-017-12960-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cemazar M., Sersa G., Frey W., Miklavcic D., Teissié J. Recommendations and requirements for reporting on applications of electric pulse delivery for electroporation of biological samples. Bioelectrochemistry. 2018;122:69–76. doi: 10.1016/j.bioelechem.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 78.Chao P.H., Roy R., Mauck R.L., Liu W., Valhmu W.B., Hung C.T. Chondrocyte translocation response to direct current electric fields. J. Biomech. Eng. 2000;122:261–267. doi: 10.1115/1.429661. [DOI] [PubMed] [Google Scholar]
- 79.Akkiraju H., Nohe A. Role of Chondrocytes in Cartilage Formation, Progression of Osteoarthritis and Cartilage Regeneration. J. Dev. Biol. 2015;3:177–192. doi: 10.3390/jdb3040177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hiemer B., Krogull M., Bender T., Ziebart J., Krueger S., Bader R., Jonitz-Heincke A. Effect of electric stimulation on human chondrocytes and mesenchymal stem cells under normoxia and hypoxia. Mol. Med. Rep. 2018;18:2133–2141. doi: 10.3892/mmr.2018.9174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khatib L., Golan D.E., Cho M. Physiologic electrical stimulation provokes intracellular calcium increase mediated by phospholipase C activation in human osteoblasts. FASEB J. 2004;18:1903–1905. doi: 10.1096/fj.04-1814fje. [DOI] [PubMed] [Google Scholar]
- 82.Berridge M.J. The Inositol Trisphosphate/Calcium Signaling Pathway in Health and Disease. Physiol. Rev. 2016;96:1261–1296. doi: 10.1152/physrev.00006.2016. [DOI] [PubMed] [Google Scholar]
- 83.Ghosh S., Laha M., Mondal S., Sengupta S., Kaplan D.L. In vitro model of mesenchymal condensation during chondrogenic development. Biomaterials. 2009;30:6530–6540. doi: 10.1016/j.biomaterials.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yoon I.S., Chung C.W., Sung J.H., Cho H.J., Kim J.S., Shim W.S., Shim C.K., Chung S.J., Kim D.D. Proliferation and chondrogenic differentiation of human adipose-derived mesenchymal stem cells in porous hyaluronic acid scaffold. J. Biosci. Bioeng. 2011;112:402–408. doi: 10.1016/j.jbiosc.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 85.Li A., Xia X., Yeh J., Kua H., Liu H., Mishina Y., Hao A., Li B. PDGF-AA Promotes Osteogenic Differentiation and Migration of Mesenchymal Stem Cell by Down-Regulating PDGFRα and Derepressing BMP-Smad1/5/8 Signaling. PLoS ONE. 2014;9:e113785. doi: 10.1371/journal.pone.0113785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ataliotis P. Platelet-derived growth factor A modulates limb chondrogenesis both in vivo and in vitro. Mech. Dev. 2000;94:13–24. doi: 10.1016/S0925-4773(00)00321-X. [DOI] [PubMed] [Google Scholar]
- 87.Fisher M.C., Meyer C., Garber G., Dealy C.N. Role of IGFBP2, IGF-I and IGF-II in regulating long bone growth. Bone. 2005;37:741–750. doi: 10.1016/j.bone.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 88.McQueeney K., Dealy C.N. Roles of insulin-like growth factor-I (IGF-I) and IGF-I binding protein-2 (IGFBP2) and -5 (IGFBP5) in developing chick limbs. Growth Horm. IGF Res. 2001;11:346–363. doi: 10.1054/ghir.2001.0250. [DOI] [PubMed] [Google Scholar]
- 89.Gasparini G., De Gori M., Paonessa F., Chiefari E., Brunetti A., Galasso O. Functional relationship between high mobility group A1 (HMGA1) protein and insulin-like growth factor-binding protein 3 (IGFBP-3) in human chondrocytes. Arthritis Res. Ther. 2012;14:R207. doi: 10.1186/ar4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Eviatar T., Kauffman H., Maroudas A. Synthesis of insulin-like growth factor binding protein 3 in vitro in human articular cartilage cultures. Arthritis Rheum. 2003;48:410–417. doi: 10.1002/art.10761. [DOI] [PubMed] [Google Scholar]
- 91.Zhou N., Li Q., Lin X., Hu N., Liao J.-Y., Lin L.-B., Zhao C., Hu Z.-M., Liang X., Xu W., et al. BMP2 induces chondrogenic differentiation, osteogenic differentiation and endochondral ossification in stem cells. Cell Tissue Res. 2016;366:101–111. doi: 10.1007/s00441-016-2403-0. [DOI] [PubMed] [Google Scholar]
- 92.Majumdar M.K., Wang E., Morris E.A. BMP-2 and BMP-9 promotes chondrogenic differentiation of human multipotential mesenchymal cells and overcomes the inhibitory effect of IL-1. J. Cell. Physiol. 2001;189:275–284. doi: 10.1002/jcp.10025. [DOI] [PubMed] [Google Scholar]
- 93.Choi K.H., Choi B.H., Park S.R., Kim B.J., Min B.H. The chondrogenic differentiation of mesenchymal stem cells on an extracellular matrix scaffold derived from porcine chondrocytes. Biomaterials. 2010;31:5355–5365. doi: 10.1016/j.biomaterials.2010.03.053. [DOI] [PubMed] [Google Scholar]
- 94.Mardani M., Roshankhah S., Hashemibeni B., Salahshoor M., Naghsh E., Esfandiari E. Induction of chondrogenic differentiation of human adipose-derived stem cells by low frequency electric field. Adv. Biomed. Res. 2016;5:97. doi: 10.4103/2277-9175.183146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang W., Wang Z., Zhang G., Clark C.C., Brighton C.T. Up-regulation of chondrocyte matrix genes and products by electric fields. Clin. Orthop. Relat. Res. 2004;427:S163–S173. doi: 10.1097/01.blo.0000143837.53434.5c. [DOI] [PubMed] [Google Scholar]
- 96.Esfandiari E., Roshankhah S., Mardani M., Hashemibeni B., Naghsh E., Kazemi M., Salahshoor M. The effect of high frequency electric field on enhancement of chondrogenesis in human adipose-derived stem cells. Iran. J. Basic Med. Sci. 2014;17:571–576. [PMC free article] [PubMed] [Google Scholar]
- 97.Fitzsimmons R.J., Gordon S.L., Kronberg J., Ganey T., Pilla A.A. A pulsing electric field (PEF) increases human chondrocyte proliferation through a transduction pathway involving nitric oxide signaling. J. Orthop. Res. 2008;26:854–859. doi: 10.1002/jor.20590. [DOI] [PubMed] [Google Scholar]
- 98.Li K., Fan L., Lin J., Heng B.C., Deng Z., Zheng Q., Zhang J., Jiang Y., Ge Z. Nanosecond pulsed electric fields prime mesenchymal stem cells to peptide ghrelin and enhance chondrogenesis and osteochondral defect repair in vivo. Sci. China Life Sci. 2022;65:927–939. doi: 10.1007/s11427-021-1983-y. [DOI] [PubMed] [Google Scholar]
- 99.Li K., Ning T., Wang H., Jiang Y., Zhang J., Ge Z. Nanosecond pulsed electric fields enhance mesenchymal stem cells differentiation via DNMT1-regulated OCT4/NANOG gene expression. Stem Cell Res. Ther. 2020;11:308. doi: 10.1186/s13287-020-01821-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen J., Huang Y., Yang J., Li K., Jiang Y., Heng B.C., Cai Q., Zhang J., Ge Z. Multiple nanosecond pulsed electric fields stimulation with conductive poly(l-lactic acid)/carbon nanotubes films maintains the multipotency of mesenchymal stem cells during prolonged in vitro culture. J. Tissue Eng. Regen. Med. 2020;14:1136–1148. doi: 10.1002/term.3088. [DOI] [PubMed] [Google Scholar]
- 101.Vaca-González J.J., Clara-Trujillo S., Guillot-Ferriols M., Ródenas-Rochina J., Sanchis M.J., Ribelles J.L.G., Garzón-Alvarado D.A., Ferrer G.G. Effect of electrical stimulation on chondrogenic differentiation of mesenchymal stem cells cultured in hyaluronic acid—Gelatin injectable hydrogels. Bioelectrochemistry. 2020;134:107536. doi: 10.1016/j.bioelechem.2020.107536. [DOI] [PubMed] [Google Scholar]
- 102.Gavénis K., Andereya S., Schmidt-Rohlfing B., Mueller-Rath R., Silny J., Schneider U. Millicurrent stimulation of human articular chondrocytes cultivated in a collagen type-I gel and of human osteochondral explants. BMC Complement. Altern. Med. 2010;10:43. doi: 10.1186/1472-6882-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Deliormanlı A.M., Atmaca H. Biological Response of Osteoblastic and Chondrogenic Cells to Graphene-Containing PCL/Bioactive Glass Bilayered Scaffolds for Osteochondral Tissue Engineering Applications. Appl. Biochem. Biotechnol. 2018;186:972–989. doi: 10.1007/s12010-018-2758-7. [DOI] [PubMed] [Google Scholar]
- 104.Prasopthum A., Deng Z., Khan I.M., Yin Z., Guo B., Yang J. Three dimensional printed degradable and conductive polymer scaffolds promote chondrogenic differentiation of chondroprogenitor cells. Biomater. Sci. 2020;8:4287–4298. doi: 10.1039/D0BM00621A. [DOI] [PubMed] [Google Scholar]
- 105.Gupta K., Patel R., Dias M., Ishaque H., White K., Olabisi R. Development of an Electroactive Hydrogel as a Scaffold for Excitable Tissues. Int. J. Biomater. 2021;2021:6669504. doi: 10.1155/2021/6669504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Krueger S., Riess A., Jonitz-Heincke A., Weizel A., Seyfarth A., Seitz H., Bader R. Establishment of a New Device for Electrical Stimulation of Non-Degenerative Cartilage Cells In Vitro. Int. J. Mol. Sci. 2021;22:394. doi: 10.3390/ijms22010394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Raso J., Frey W., Ferrari G., Pataro G., Knorr D., Teissie J., Miklavčič D. Recommendations guidelines on the key information to be reported in studies of application of PEF technology in food and biotechnological processes. Innov. Food Sci. Emerg. Technol. 2016;37:312–321. doi: 10.1016/j.ifset.2016.08.003. [DOI] [Google Scholar]
- 108.Cvetkoska A., Pirc E., Reberšek M., Magjarevic R., Miklavčič D. Towards standardization of electroporation devices and protocols. IEEE Instrum. Meas. Mag. 2020;23:74–81. doi: 10.1109/MIM.2020.9062692. [DOI] [Google Scholar]
- 109.Aragón Á., Cebro-Márquez M., Perez E., Pazos A., Lage R., González-Juanatey J.R., Moscoso I., Bao-Varela C., Nieto D. Bioelectronics-on-a-chip for cardio myoblast proliferation enhancement using electric field stimulation. Biomater. Res. 2020;24:15. doi: 10.1186/s40824-020-00195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cao J., Cheng P., Hong F. Applications of electrohydrodynamics and Joule heating effects in microfluidic chips: A review. Sci. China Ser. E Technol. Sci. 2009;52:3477–3490. doi: 10.1007/s11431-009-0313-z. [DOI] [Google Scholar]
- 111.Hur J., Chung A.J. Microfluidic and Nanofluidic Intracellular Delivery. Adv. Sci. 2021;8:2004595. doi: 10.1002/advs.202004595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hu M., Hong L., He S., Huang G., Cheng Y., Chen Q. Effects of electrical stimulation on cell activity, cell cycle, cell apoptosis and β-catenin pathway in the injured dorsal root ganglion cell. Mol. Med. Rep. 2020;21:2385–2394. doi: 10.3892/mmr.2020.11058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Batista Napotnik T., Miklavčič D. In vitro electroporation detection methods—An overview. Bioelectrochemistry. 2018;120:166–182. doi: 10.1016/j.bioelechem.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 114.Tas J. The Alcian blue and combined Alcian blue—Safranin O staining of glycosaminoglycans studied in a model system and in mast cells. Histochem. J. 1977;9:205–230. doi: 10.1007/BF01003632. [DOI] [PubMed] [Google Scholar]
- 115.Chow Y.Y., Chin K.Y. The Role of Inflammation in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2020;2020:8293921. doi: 10.1155/2020/8293921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Murphy G., Lee M.H. What are the roles of metalloproteinases in cartilage and bone damage? Ann. Rheum. Dis. 2005;64:iv44. doi: 10.1136/ard.2005.042465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.