Abstract
The current 2022 mpox (monkeypox) outbreak has been officially recognized as a public health emergency. The mpox clinical symptoms include high fever, fatigue, chills, headache, swollen lymph nodes, muscle aches, and a disseminated painful rash. However, recent cases of mpox have shown a shift in clinical symptoms, with anogenital skin lesions emerging as the predominant feature. Due to the predominant skin manifestations of mpox, dermatologists could be crucial in detecting new mpox cases and educating frontline healthcare professionals about mpox. The mpox virus is continuously evolving and has several variants. Genome sequencing has revealed that the Clade IIb variant is responsible for the 2022 mpox outbreak. Mpox spread may occur through animal-to-human and human-to-human transmission; however, unlike coronavirus disease 2019 (COVID-19), long-range airborne transmission has not been reported. Healthcare professionals are at higher risk of becoming infected since they are usually in close contact with both the patients and potentially contaminated fomites (e.g., examination table, gowns, gloves). Both public and healthcare professionals should take preventive and avoidance measures to limit the spread. Mpox is usually self-limiting and may require only symptomatic treatment; however, it may cause severe complications in special populations such as immunocompromised individuals. For severe infection, clinicians may consider antiviral drugs (off-label), tecovirimat and brincidofovir, originally approved for smallpox treatment. Two smallpox vaccines, ACAM2000® and JYNNEOSTM, can be used as pre-exposure prophylaxis against mpox. JYNNEOSTM, which carries approval for mpox use, has less adverse effect potential than ACAM2000®, and may also be used as post-exposure prophylaxis, preferably within 4 days of exposure.
Key Points
| Genital skin lesions are a dominant symptom in the recently reported mpox cases. |
| Dermatologists and healthcare professionals should be more cautious and take certain preventive measures since they are usually in close contact with patients infected with the mpox virus. |
| Clinicians usually recommend symptomatic treatment for mpox patients; however, tecovirimat and brincidofovir, two smallpox drugs, can be recommended off-label for severe mpox infections. |
| ACAM2000® and JYNNEOSTM, two smallpox vaccines, can be used as prophylaxis against mpox infection, however JYNNEOSTM possesses less adverse effect potential than ACAM2000®. |
Introduction
The 2022 monkeypox (mpox) outbreak has initially raised fears of another upcoming pandemic following the recent coronavirus disease 2019 (COVID-19) pandemic [1]. The World Health Organization (WHO) declared the current mpox outbreak as a Public Health Emergency of International Concern (PHEIC) on 23 July 2022 [2]. At that time, there were only about 16,000 cases worldwide, reported from 75 countries; many more cases have been reported to date. The mpox virus was first isolated in the State Serum Institute, Copenhagen, Denmark, in 1958 from a captive cynomolgus monkey and was named after the host animal [1]. Notably, the WHO has recently recommended using a new preferred term, ‘mpox’, for monkeypox [3]. In this very first reported instance, the virus did not infect humans working in the research laboratory. However, other hosts, including rope squirrels, tree squirrels, Gambian pouched rats, and dormice, appeared as natural reservoirs of the mpox virus [4]. In 1970, the first human case was identified in the Democratic Republic of the Congo [1, 5]. Thus far, 110 countries, including the US, Canada, and the UK, have reported mpox cases since January 2022, whereas previously, mpox infections were mainly observed in just a few countries located on the African continent [4, 6].
Patients with mpox infection routinely exhibit cutaneous symptoms [7]. Therefore, dermatologists are well-placed to establish this diagnosis. Their knowledge base and experience may allow them to educate and prepare frontline healthcare professionals to presumptively identify new cases [7]. This article reviews differential diagnosis, how symptoms of 2022 mpox infections differ from past cases, its mode of transmission, prevention, and current treatments.
Virology of Monkeypox (Mpox) Virus
Genome sequencing to date has identified two mpox variants or clades; clade 1 (formerly known as the Congo Basin or Central African clade) and clade II (formerly known as the West African Clade) [8]. Clade II also has two subclades, clade IIa and IIb [8]. Clade I is reported to be more transmissible and virulent than clade II [9]. Notably, Clade II, more specifically clade IIb, has caused the current 2022 mpox outbreak [10]. Likos and co-workers tried to differentiate between two clades and identified multiple candidate genes that may be responsible for the observed differences [9].
Additionally, mpox variants are continuously evolving. A recent study reported possible microevolution of the mpox virus, which may enhance the virus’s human adaptation [11]. Mutations in APOBEC3 (apolipoprotein B mRNA-editing catalytic polypeptide-like 3), a host genome editing enzyme, may be responsible for this microevolution [11]. Further studies are required to determine whether APOBEC3-mediated genome editing is causing excess mutations observed in the 2022 mpox outbreak [11].
Diagnosis, Disease Progression, and Prognosis of Mpox Infection
Clinical Symptoms and Differential Diagnosis
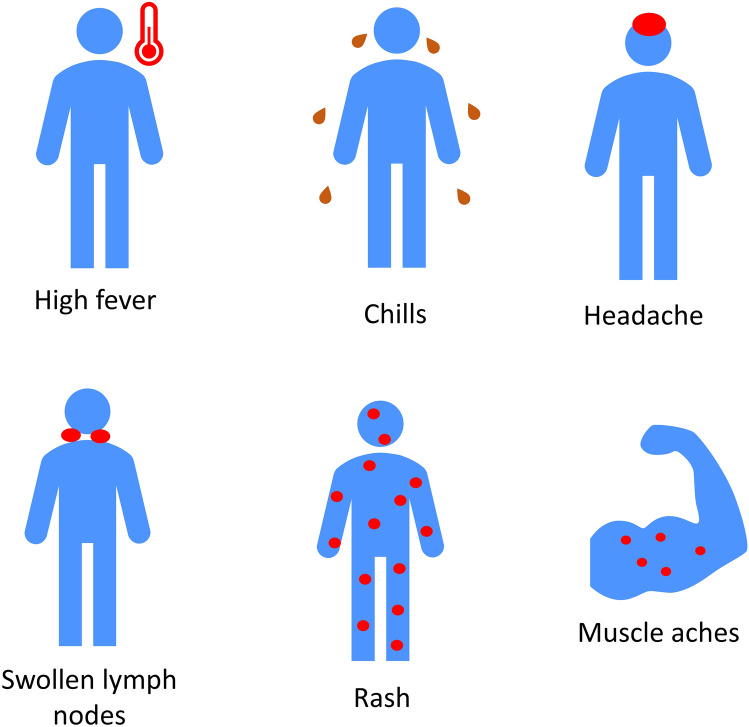
The clinical features of mpox, chickenpox, and smallpox are relatively similar [12]. Table 1 compares mpox, smallpox and chickenpox, and presents their differential diagnosis [12–15]. The common symptoms of mpox include high fever, chills, headache, swollen lymph nodes, muscle aches, and a painful cutaneous eruption that usually appears as raised bumps on the skin and tends to distribute on the face, genitals, and extremities (Fig. 1) [12, 15, 16]. The eruption typically progresses rapidly from macules to papules, papules to vesicles, vesicles to pustules, pustules to umbilicated pustules, and finally to a crusted residual [13, 17]. Mpox patients may not have all types of lesions during an eruption, and not all of their lesions may not be in the same stage [13]. However, a study that examined 185 mpox cases in Spain in 2022 found that the first lesions were uniform and papular (pseudo-pustules), not pustules [18].
Table 1.
| Criteria | Mpox | Smallpox (eradicated) | Chickenpox | ||
|---|---|---|---|---|---|
| Pathogen | Mpox virus, orthopoxvirus family | Variola virus, orthopoxvirus family | Varicella zoster virus | ||
| Incubation period (days) | 7–17 | 7–17 | 12–14 | ||
| Prodrome period (days) | 1–4 | 2–4 | 0–2 | ||
| Symptom | Fever severity | Moderate | Severe | Mild or none | |
| Malaise severity | Moderate | Moderate | Mild | ||
| Headache severity | Moderate | Severe | Mild | ||
| Lymphadenopathy severity | Moderate | None | None | ||
| Lesions | Depth (diameter in mm) | Superficial to deep (4–6) | Deep (4–6) |
Superficial (2–4) |
|
| Distribution |
Prior outbreaks: centrifugal 2022 outbreak: Mainly penile, anorectal, and oral-pharyngeal lesions |
Centrifugal | Centripetal | ||
| Evaluation |
2022 outbreak: Usually polymorphic rash |
Monomorphic rash | Polymorphic rash | ||
| Desquamation (days) | 14–21 | 14–21 | 6–14 | ||
| Frequency of lesions on palms or soles of the feet |
Prior outbreaks: Common 2022 outbreak: Less common |
Common | Rare | ||
| Death rate |
1–10% of cases (depends on strain) |
~ 30% of cases (depends on the type) |
Rare | ||
Mpox monkeypox
Fig. 1.
Common symptoms of monkeypox infection
A recent cohort study conducted on 70 male mpox patients who had sex with men found that over one-third of the patients had proctitis [19]. In two-thirds of those cases, no sign of typical rash was evident, and in one-fifth, there was no visible sign of rash, making it challenging to diagnose. However, a polymerase chain reaction (PCR) of a rectal swab could help diagnose the mpox infection.
Huhn and colleagues studied 34 patients with confirmed mpox infection during the US outbreak in 2003 [20]. The majority of the patients reported rash (97%), followed by fever (85%), chills (71%), adenopathy (71%), headache (65%), and myalgias (56%) [20]. Approximately 68% of patients (n = 31) had a monomorphic rash, whereas 29% exhibited a pleomorphic rash. This is a key differentiating feature from smallpox, where monomorphic pustules or crusting lesions (but not both at the same time) are virtually always seen (Fig. 2). Additionally, generalized centrifugal rash distribution was more common (48.4%) than localized distribution (25.8%) [20].
Fig. 2.
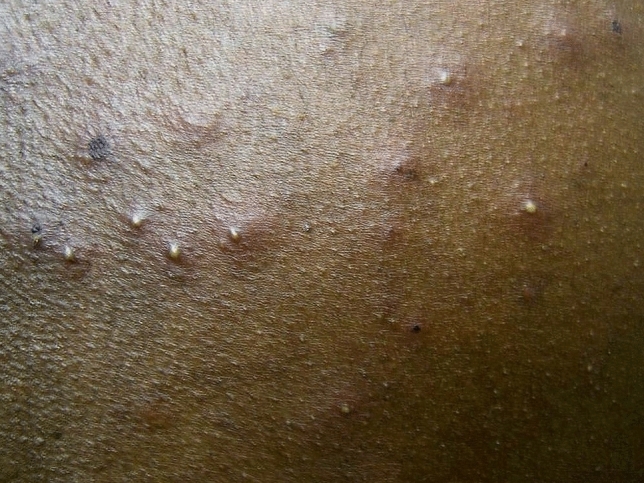
Polymerase chain reaction-verified pleomorphic monkeypox demonstrates both pustules and crusts.
2022 Mpox Outbreak
As of 25 March 2023, 110 countries have reported 86,646 confirmed and several thousand probable cases of mpox infection, including 112 deaths [6]. Based on the available data, approximately 96.4% of patients were male, with a median age of 34 years. This indicates that the young male population (18–44 years) had a higher incidence of infection in the recent outbreak [6, 21]. In 84.2% of cases, patients identified their sexual orientation as men who have sex with men (MSM) [6, 21]. Approximately 48.4% were HIV-positive among the patients who disclosed their HIV status [6, 21]. Additional epidemiological risk factors identified in retrospective case analysis included recent exposure to multiple, often anonymous, sexual partners and attending pride events [21].
Currently, the number of newly reported mpox cases is trending negative, both globally and in the US [6]. The global number peaked between 8 and 14 August 2022; 7477 new mpox cases were reported.
Anogenital Skin Lesions are a Dominant Symptom in the 2022 Outbreak
An observational study published in The Lancet Infectious Diseases found that mpox infection symptoms in recent cases were different than in past outbreaks [22]. The presence of one or more anogenital skin lesions was a dominant symptom [22]. All 54 patients included in the study had skin lesions, of which 94% (51/54 patients) were anogenital, 89% of patients reported lesions presence in more than one anatomical site, and 7% had oropharyngeal lesions [22]. On the other hand, 67% experienced fatigue, 57% reported fever, and 18% did not present with any prodromal symptoms [22]. In some instances, the genital ulcers were painless with bilateral inguinal lymphadenopathy, mimicking primary syphilis [23].
An international collaborative group of physicians who monitored 528 mpox infections in 2022 also found anogenital lesions as one of the predominant symptoms [21]. While 73% of patients reported anogenital lesions, mucosal lesions appeared in 41% of the patients [21]. Rash appeared in 95% of mpox patients, and other common symptoms were fever (62%), lymphadenopathy (56%), lethargy (41%), myalgia (31%), and headache (27%) [21].
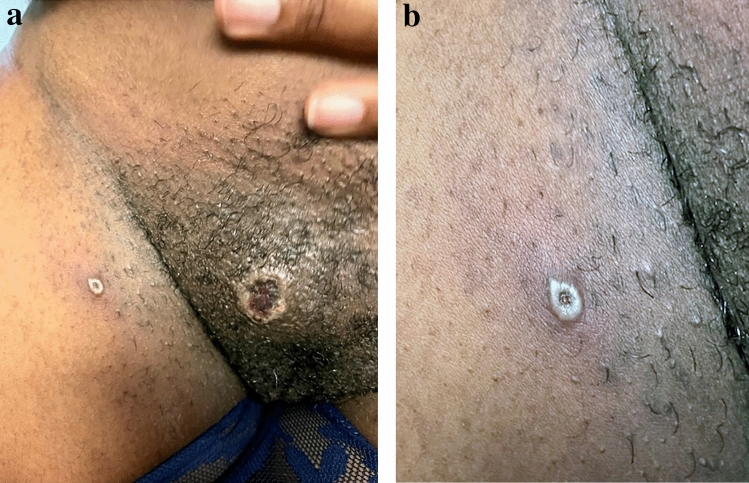
Since clinical symptoms are variable and different in the 2022 outbreak, Tarín-Vicente and colleagues opined that dermatologists and healthcare professionals should maintain a low threshold when examining patients with possible mpox infection [24]. This same research group pointed out that the location of lesions in the present outbreak most often correlated with the type of sexual activity performed [24]. Perianal lesions, for example, would occur in an individual who had receptive anal intercourse (Fig. 3a, b). Oral lesions result from oral sex performed on an already infected individual, while facial lesions may result from performing rimming on an infected partner or simply from kissing (Fig. 4).
Fig. 3.
a Perigenital PCR-verified mpox lesions in a woman following vaginal intercourse with a bisexual man who was subsequently diagnosed with penile and suprapubic mpox. b PCR-verified umbilicated pustule typical of mpox. PCR polymerase chain reaction, mpox monkeypox
Fig. 4.
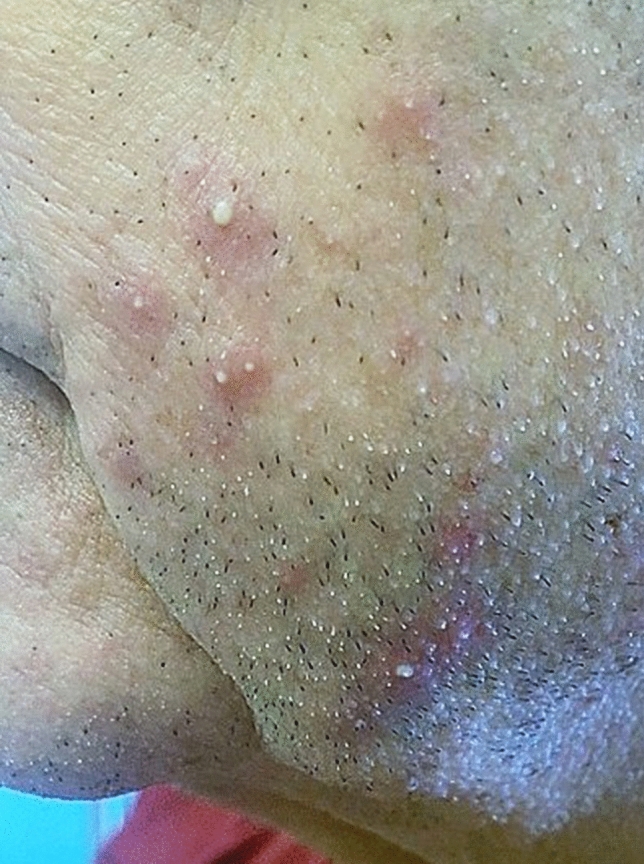
Facial monkeypox following ‘rimming’ an infected partner
Laboratory Testing
Timely and accurate testing of mpox infection is vital for breaking the transmission chains and thus stopping the outbreak [25]. Clinicians should recommend appropriate laboratory testing for any patients with suspected mpox infection. The test uses real-time or conventional PCR, alone or in combination with sequencing, to detect unique viral DNA sequence [25]. Laboratory specimens can be lesion surface swabs and/or exudate, roofs collected from more than one lesion, or lesion crusts [25]. It is important that the swab used to collect exudate or lesion base specimens be made of dacron, nylon or polyester, and not of cotton, to ensure proper transfer to testing media. The person who collects the specimen, and the laboratory staff, should handle it carefully and follow a risk-based approach. Depending on the laboratory, one may receive a positive report that reads ‘non-variola orthopox virus detected’. Since no other orthopoxviruses are currently of epidemiologic significance, this is equivalent to a presumptive diagnosis of mpox. WHO recommends notifying all test results, positive or negative, to the national authorities [25].
Disease Progression and Prognosis
Although mpox infection is often self-limited, generally lasting 2–4 weeks, it may lead to severe complications in some cases [4]. Special populations, such as patients younger than 8 years of age, pregnant women, and immunocompromised individuals (particularly those with untreated or poorly controlled concurrent HIV infection), are more prone to develop mpox-related complications [4]. Such complications may include confluent and necrotic skin lesions, fatigue, sore throat, headache [26], secondary bacterial sepsis, ocular infection leading to keratitis, encephalitis, and myocarditis. The mortality rate of mpox infection has been estimated to be between 0 and 11% in the general population of African countries [27], with the highest mortality rate being observed in young children [27]. As of 25 March 2023, the fatality rate of the current 2022 outbreak was approximately 0.13% (112/86,646) [6]. Moreover, individuals under 40–50 years of age may be more susceptible to mpox infection due to the discontinuation of routine smallpox immunization in the early 1980s following smallpox worldwide eradication [27].
Transmission of the Mpox Virus
Mpox does not spread through long-range airborne transmission like COVID-19 [28]. In airborne transmission, small virus particles stay suspended in the air for an extended period and become a potential source of infection [28]. However, mpox may theoretically spread through droplet transmissions (e.g., an infected individual’s respiratory secretions or saliva) [28]. Of note, Peiró-Mestres and co-workers identified mpox in saliva in 12/12 specimens tested; the clinical significance of this finding as it relates to transmission is still uncertain [29].
Mpox spread occurs through both animal-to-human (zoonotic) and human-to-human transmission [30]. Some examples include (1) contact with infected animals (monkeys, wild rats, or rope squirrels); (2) close contact with an mpox patient; (3) transmission through clothes, bedding, or towels used by an infected individual; (4) prolonged exposure to respiratory secretions, droplet or saliva of an mpox patient; (5) direct contact with skin lesions, lesion exudate or crust; and (6) butchering, handling or consuming meats of infected animals [28, 30, 31]. Mpox infection may also transmit from mother to fetus through the placenta [30]. While the zoonotic transmission route remains important in cases seen in Africa, the current global outbreak seems to be primarily spread by intimate human-to-human contact (i.e., sexual contact).
However, walking by an infected individual in a shopping mall or having a casual conversation poses a very low risk of transmission [28]. Although it is still unclear, mpox may spread through sexual routes such as contact with vaginal secretions or semen. Researchers are also actively investigating via infection modeling whether presymptomatic mpox-infected patients can spread the disease, which appears to be both likely and common [28, 32].
Prevention of Mpox
Clinicians and Healthcare Professionals
The prevention of mpox infection in clinicians or healthcare professionals is more challenging since they are usually in close contact with infected patients [4]. To minimize the infection risk, a healthcare professional should follow the recommendations such as (1) avoiding direct contact with skin lesions or materials used by mpox patients; (2) wearing personal protective equipment, including a gown, gloves, eye protection, and a fitted N95 mask when treating a patient with skin rash; (3) masking the patient with suspected or confirmed mpox infection immediately, covering the skin lesions with a gown or cloth, and placing the patient in isolation, preferably in a single-person room; (4) placing the patient in a negative-pressure room in the hospital (if hospital care is required); and (5) wearing gloves while handling the laundry of mpox patients (Fig. 5a) [4].
Fig. 5.
a Recommendations to minimize the mpox infection in clinicians and healthcare professionals. b Recommendations for individuals to limit the spreading of mpox infection. mpox monkeypox
Individuals
An individual may limit the spread of mpox by paying attention to the following: (1) a patient with suspected or confirmed infection should stay home and limit contact with others; an immunocompetent individual, with mild mpox symptoms, should stay away from others for 3–6 weeks [33]; (2) an individual should avoid intimate contact, including sexual contact, with someone infected or exposed to the mpox virus; (3) individuals should maintain good hand hygiene and respiratory etiquette, such as wearing a fitted mask and covering coughs and sneezes with the bend of arm or a piece of tissue or cloth; and (4) after having visitors at home, proper cleaning and disinfection of high-touch areas are recommended [34]. Additionally, using condoms, practicing safe sex, and having fewer sexual partners may help reduce mpox spread (Fig. 5b) [34]. In the US, a recent Centers for Disease Control and Prevention (CDC) survey study showed that about half of those at the highest risk have in fact already modified their sexual practices and number of sexual partners [35].
Pre-exposure Prophylaxis
The available data indicate that smallpox vaccination may have 85% cross-protection against mpox [36]. Some experts opined that ending routine smallpox vaccination after the disease was declared eradicated in 1980 could be partly responsible for the recent mpox resurgence [37]. ACAM2000® (smallpox [vaccinia] vaccine, live) [38] and JYNNEOSTM (vaccinia virus modified strain ankara-bavarian nordic non-replicating antigen) [39] are the two licensed vaccines currently available in the US for smallpox prevention (Table 2) [36, 38, 40].
Table 2.
Vaccines for monkeypox preventiona
| Agents | Dose | Contraindications | Warning and precautions | Adverse reactions | Use in specific populations |
|---|---|---|---|---|---|
| ACAM2000 (live vaccine) |
A droplet (0.0025 mL) of reconstituted vaccine percutaneously administered using 15 jabs of a bifurcated needle A booster dose (every 3 years) is recommended for high-risk individuals |
Immunodeficient individuals, such as patients receiving bone marrow transplantation or individuals with primary or acquired immunodeficiency | Myocarditis and pericarditis, encephalitis, encephalomyelitis, encephalopathy, progressive vaccinia, generalized vaccinia, severe vaccinial skin infections, erythema multiforme major (including Stevens–Johnson syndrome), eczema vaccinatum resulting in permanent sequelae or death, ocular complications, blindness, and fetal death |
Injection site reaction, lymphadenitis, constitutional symptoms (e.g., malaise, fatigue, fever, myalgia, and headache) Adverse events are more frequent in persons receiving first-time vaccination |
Not studied in infants (< 12 months of age) or children. Higher risk of severe complications Not studied in pregnant women. May cause fetal infection |
| JYNNEOS (live, attenuated vaccine) | 2 doses (0.5 mL each) subcutaneously administered 4 weeks apart |
Patients allergic to JYNNEOS Immunocompromised individuals |
Severe allergic reaction, immunocompromised individuals | Injection site reactions, muscle pain, headache, fatigue, nausea, and chills |
Use in pregnancy: Human data are insufficient, but animal studies revealed no embryo-fetal toxicity Nursing mothers: No data are available. Clinicians should consider whether the benefits outweigh the risk Pediatric use: Safety is not well-established in patients aged < 18 years Geriatric use: Data are insufficient to determine whether the response differs in the geriatric population compared with younger subjects |
aDisclaimer: Table 2 is for general information only and does not constitute professional advice. Please consult the relevant up-to-date guidelines, package inserts, and authoritative texts before making a clinical decision
A recent study analyzed 2054 men who were eligible to receive a vaccine [41]. During the study period, 50% of the men were vaccinated with JYNNEOSTM and followed up for at least 90 days. During that time, 5 vaccinated and 16 unvaccinated individuals were confirmed to have been infected with the mpox virus. The study estimated the adjusted vaccine effectiveness of JYNNEOSTM to be 86%, with a 95% confidence interval of 59–95% [41]. This indicates that the risk of getting infected with the mpox virus was significantly lower for those who received a single dose of the subcutaneous JYNNEOSTM, which was administered to the high-risk cohort in the study.
Notably, the US FDA has approved only JYNNEOSTM for mpox prevention, which has less adverse effect potential than ACAM2000® [4, 36]. Both ACAM2000® (administered by pricking the skin with a two-pronged or bifurcated needle) and JYNNEOSTM (administered intradermally) vaccines exhibit cutaneous adverse effects. According to the Vaccine Adverse Event Reporting System (VAERS) database, ACAM2000® is mainly associated with skin rash (11.73%), erythema (7.66%), skin lesion (7.07%), pruritus (7.02%), and injection site erythema (6.91%). In comparison, JYNNEOSTM predominantly exhibits injection site erythema (10.16%), injection site swelling (8.64%), injection site pain (6.52%), urticaria (6.27%), erythema (5.84%), injection site pruritus (5.59%) and pruritus (4.06%) [42].
Currently, the CDC does not recommend pre-exposure prophylaxis against mpox for the general population [43]. According to the Advisory Committee and Immunization Practices (ACIP), eligible groups for pre-exposure mpox immunization include (1) individuals who are at risk of occupational exposure (i.e., research laboratory personnel, technicians performing orthopoxvirus diagnostic testing, members of a designated response team, and healthcare professionals administering ACAM2000® or caring for patients with orthopoxvirus infection); (2) gay, bisexual, other MSM, transgender or nonbinary individuals diagnosed with nationally reportable sexually transmitted diseases (i.e., gonorrhea, syphilis, acute HIV, chancroid, or chlamydia) in the past 6 months and have had multiple sex partners; (3) individuals who had sex at a commercial sex place or at large social gatherings in a geographic area where mpox infection is prevalent; and (4) individuals who have sexual partners falling in the category 2 or 3; and individuals who may experience the risks listed in category 2 or 3 [43].
Post-exposure Prophylaxis
Mpox is transmissible when an individual is exposed to an mpox patient for a prolonged period. Brief exposure, especially if the exposed individual uses appropriate personal protective equipment, may not require post-exposure prophylaxis [44]. An individual may be considered for JYNNEOSTM vaccination depending on the extent of exposure [4]. Table 3 details the CDC’s recommendations for high-, intermediate-, and low-degree exposure. The first dose of the vaccine should be administered within 4 days of exposure [45]. If administered late, within 4–14 days, the vaccination may not prevent disease but will reduce the symptom severity [44].
Table 3.
Risk assessment and recommendations for post-exposure prophylaxis in patients exposed to mpox infections
| Degree of exposure | Characteristics | Recommendations |
|---|---|---|
| Higher |
Intimate or sexual contact with an mpox patient Contact between an individual’s mucous membrane or broken or damaged skin with the mpox patient’s skin lesions or body fluid Contact between an individual’s mucous membrane or damaged skin with the materials (clothing, linens, sex toys, etc.) contaminated by an mpox patient’s skin lesions or body fluids |
Monitoring: yes PEP: recommended |
| Intermediate |
Not maintaining social distancing and being within 6 feet for ≥ 3 h of an unmasked mpox patient without having a surgical mask or respirator Contact between the intact skin of an individual and an mpox patient’s skin lesions or body fluid Contact between the intact skin of an individual with the materials (e.g., sex toys, cloths, linens) contaminated by an mpox patient’s skin lesions or body fluids Contact between the clothing of an individual with the materials (e.g., sex toys, cloths, linens) contaminated by an mpox patient’s skin lesions or body fluids |
Monitoring: yes PEP: based on the individual case. Considered if the benefits of PEP outweigh the risk |
| Lower | Entering into the living space of an mpox patient and in the absence of any exposures listed above |
Monitoring: yes PEP: no |
mpox monkeypox, PEP post-exposure prophylaxis
Treatment of Mpox
Currently, mpox infection has no approved treatments, therefore clinicians mostly recommend symptomatic treatment [4, 44, 46]. However, special cases such as patients with severe mpox infection, immunocompromised individuals, children younger than 8 years of age, and pregnant women may require antiviral treatment [4, 46]. The CDC recommends two antiviral drugs, tecovirimat and brincidofovir, for treating mpox infection (Table 4) [4, 44, 46–55]. Additionally, Table 4 lists the possible treatments for mpox and chickenpox infections [44, 47–55].
Table 4.
Treatments for mpox and chickenpox infectionsa
| Infection | Pathogen | Treatments | Dose and duration |
|---|---|---|---|
| Mpox |
Mpox virus • DNA virus • Taxonomy Family Poxviridae, subfamily Chordopoxvirinae, genus Orthopoxvirus Genome size: ~ 190 kb |
Usually self-limiting Oral/intravenous rehydration is recommended for patients with GIT symptoms (e.g., vomiting, diarrhea) Antiviral drugs Tecovirimat Brincidofovir Intravenous vaccinia immune globulin |
Antiviral drugs Tecovirimat Oral dosing: Patients weighing from 13 kg to < 25 kg: 200 mg (1 capsule) every 12 h for 14 days Weighing from 25 kg to < 40 kg: 400 mg (2 capsules) every 12 h for 14 days Weighing from 40 kg to < 120 kg: 600 mg (3 capsules) every 12 h for 14 days Weighing ≥ 120 kg: 600 mg (3 capsules) every 8 h for 14 days Intravenous dosing: Patients weighing from 3 kg to < 35 kg: 6 mg/kg every 12 h by intravenous infusion over 6 h for 14 days Weighing 35 kg to < 120 kg: 200 mg every 12 h by intravenous infusion over 6 h for 14 days Brincidofovir Pediatric patients weighing < 10 kg: 6 mg/kg oral suspension once weekly for two doses (on Days 1 and 8) Patients weighing from 10 kg to < 48 kg: 4 mg/kg oral suspension once weekly for two doses (on Days 1 and 8) Weighing 48 kg or above: 200 mg (2 tablets)/20 mL (100 mg/mL) oral suspension once weekly for two doses (on Days 1 and 8) Vaccinia immune globulin intravenous (VIGIV) Benefit is not proven May be considered in severe cases May be considered for prophylaxis use in exposed individuals with severe immunodeficiency in T-cell function for which vaccination is contraindicated Initial dose: 6000 U/kg intravenously; if the patient is not responsive to the initial dose, a higher dose (9000 U/kg or 24,000 U/kg) may be considered |
| Chickenpox |
VZV (also known as human herpesvirus 3) • DNA virus • Taxonomy: Family Herpesviridae, genus Varicellovirus Genome size: ~ 125 kb |
Usually self-limiting Acetaminophen to relieve pain; NSAIDs such as aspirin and ibuprofen are not recommended; aspirin (Reye’s syndrome in children); ibuprofen (may be associated with life-threatening skin infection) Drinking plenty of water to avoid dehydration Avoiding scratching to stop the spread or future scarring Antiviral (acyclovir) treatment is recommended for patients who are more likely to develop severe complications Varicella or chickenpox (VZV) vaccine (live attenuated): Varilrix≥12 months; Varivax III≥12 months VZIG may be considered for high-risk persons. |
Acyclovir Children: 80 mg/kg/day (dose divided into qid) Adult: 400 mg PO qid or 5 times/day Duration: 5 days (up to 10 days if immunocompromised) Intravenous acyclovir for severe, children and immunocompromised |
mpox monkey pox, GIT gastrointestinal tract, qid four times daily, PO by mouth, VZV varicella-zoster virus, NSAIDs non-steroidal anti-inflammatory drugs, VZIG varicella zoster immune globulin
aDisclaimer: Table 4 is for general information only and does not constitute professional advice. Please consult the relevant up-to-date guidelines, package inserts, and authoritative texts before making a clinical decision
Symptomatic Treatment
Pain Management
Managing pain is important when treating mpox patients because pain is a frequent and often severe symptom (e.g., rectal pain, headache, muscle aches, pain from lesions, and lymphadenopathy pain) [56]. Pain management should be tailored to the individual patient, with a combination of non-pharmacologic and pharmacologic therapies as needed [57]. Clinicians should assess the pain at the beginning and then regularly, and pain management should be adjusted as required [56].
Mild pain can be treated with over-the-counter medications such as acetaminophen or ibuprofen, while short-term management of severe pain (e.g., severe rectal pain due to proctitis) may require opioids such as tramadol or morphine, after assessing the benefits and risks of opioid use [56, 57]. Rinsing with salt water at least four times a day may help patients who have rash inside the mouth [50]. Additionally, local anesthetics (e.g., viscous lidocaine) or prescription mouthwashes can be recommended to relieve the pain [56, 57]. Starting treatment with tecovirimat early on may help alleviate pain in patients with severe proctitis, but more evidence is needed [56]. Long-term follow-up is also important to quickly diagnose prolonged nociceptive syndromes, and consultation with a pain specialist may be required for refractory cases [56].
Rash Relief
Patients must try hard not to touch or scratch the rash [57]. Topical benzocaine or lidocaine gels can provide temporary relief. Oral histamines (e.g., Benadryl), topical preparations (e.g., calamine lotion), or petroleum jelly can help relieve itching. Additionally, a warm bath can help patients with itching sensations. Patients having rash in the anus or genital area may also try sitz baths [57].
Tecovirimat
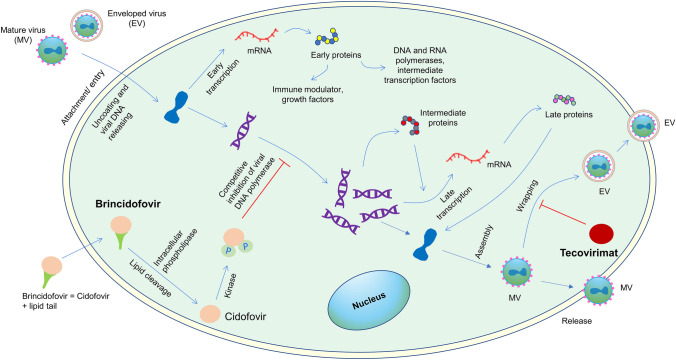
The FDA originally approved tecovirimat for treating smallpox infection in 2018 [4]. Table 5 lists pharmacokinetics, contraindications, warnings and precautions, adverse reactions of tecovirimat, and its use in specific populations [48, 51, 58]. The drug hinders viral envelope formation by inhibiting p37 protein, a highly conserved sequence among all orthopoxviruses (Fig. 6) [59–61]. Since mpox is an orthopoxvirus, tecovirimat may show antiviral activity against mpox. In the US, patients with mpox infection can access tecovirimat from city, county, or state health authorities via a CDC and FDA-approved expanded access–investigational new drug (EA-IND) protocol [4].
Table 5.
Antivirals for mpox treatmenta
| Antivirals | Pharmacokinetics | Contraindications | Warning and precautions | Adverse reactions | Use in specific populations |
|---|---|---|---|---|---|
| Tecovirimat (TPOXX®) |
Absorption: median Tmax ≈ 6 h; food increased the absorption of orally administered tecovirimat by 39% Distribution: human plasma protein binding ≈ 77–82%; VD ≈ 383 L (200 mg intravenously), 1030 L (600 mg orally) Metabolism: hydrolysis; UGT1A1, UGT1A4 Excretion Cl ≈ 13 L/h (200 mg intravenously); 31 L/h (600 mg oral); t½ ≈ 21 h (200 mg intravenously), 19 h (600 mg oral); urine excretion (oral) ≈ 73% (mostly as metabolites); fecal excretion ≈ 23% (mostly as tecovirimat) |
Tecovirimat injection (not capsule) is contraindicated in patients with creatinine clearance below 30 mL/min |
Coadministration with repaglinide may lead to hypoglycemia Tecovirimat may reduce serum concentrations of tacrolimus and sirolimus, and a dose increase of these drugs may be required |
Oral (capsule): headache, nausea, abdominal pain, and vomiting (incidence ≥ 2%) Injection: injection site reactions, headache (incidence ≥ 4%) |
Use in pregnancy: human data are not available. Animal reproduction studies did not report any embryofetal developmental toxicity Pediatric use: no clinical studies are available due to ethical reasons. The dose is calculated based on weight Geriatric use: dose adjustment is not needed in patients ≥ 65 years of age Renal impairment: Tecovirimat capsules do not require dose adjustment in renally impaired patients. Tecovirimat injections also do not require dose adjustment in patients with mild (creatinine clearance 60–89 mL/min) or moderate (creatinine clearance 30–59 mL/min) renal impairment; however, it is contraindicated in patients with severe renal impairment Hepatic impairment: Dose adjustment is not required in patients with mild, moderate, or severe hepatic impairment |
| Brincidofovir (TEMBEXA®) |
Absorption: bioavailability ≈ 16.8% (oral suspension); 13.4% (tablet) Tmaxb ≈ 6 h; AUC∞ and Cmax decreased by 31% and 49%, respectively, when Brincidofovir tablet is taken with food Distribution: human plasma protein binding > 99.9%; VD ≈1230 L Metabolism Hydrolysis; CYP4F2 Metabolites Cidofovir and cidofovir diphosphate (active) Excretion Cl ≈ 44.1 L/h; t½ ≈ 19.3 h; Urine excretion ≈ 51% (mostly as metabolites); fecal excretion ≈ 40% (mostly as metabolites) |
– |
Serum transaminases (ALT or AST) and bilirubin may increase. Monitor hepatic parameters before and during brincidofovir treatment. Monitor brincidofovir-associated GI symptoms; if severe, do not provide the second and final dose Intravenous cidofovir should not be coadministered with brincidofovir May cause embryo-fetal toxicity Crushing or diving the tablets is not recommended due to its potential carcinogenicity May irreversibly impair male fertility Brincidofovir has drug interactions with several antiretroviral agents (protease inhibitors, cobicistat, and fostemsavir). ART regimen or dosing may need modification |
Diarrhea, nausea, vomiting, and abdominal pain (incidence ≥ 2%) |
Use in pregnancy: embryotoxicity has been reported in animal studies. Not recommended during pregnancy Pediatric use: the safety profiles are similar in adult and pediatric populations Geriatric use: dose adjustment is not required. The safety profiles are similar in patients older and younger than 65 years Renal impairment: dose adjustment is not required in renally impaired patients or patients with end-stage renal diseases receiving dialysis Hepatic impairment: liver function test is recommended before starting therapy and during the therapy. Dose adjustment is not required in mild, moderate, or severe hepatic impairment Lactation: brincidofovir is excreted in the milk (animal studies) |
| Cidofovir | AUCc ≈ 40.8 μg.h/mL; Cmaxc ≈ 19.6 μg/mL; VDc ≈ 410 mL/kg; clearancec ≈ 148 mL/min/1.73 m2; renal clearance ≈ 98.6 mL/min/1.73 m2 (usually administered with probenecid, which may increase plasma levels of other drugs) |
Contraindicated in patients with creatinine clearance ≤ 55 mL/min, serum creatinine > 1.5 mg/dL, or a urine protein ≥ 100 mg/dL Contraindicated in patients taking nephrotoxic drugs; must be stopped 7 days prior to starting the therapy Patients with cidofovir hypersensitivity |
Dose-dependent nephrotoxicity. A renal function test must be performed prior to each dose administration Neutrophil count should be monitored due to the risk of developing neutropenia Coadministration of cidofovir and tenofovir disoproxil fumarate is not recommended |
Neutropenia, headache, iritis, impaired hearing, dyspnea, nausea, vomiting, diarrhea, pancreatitis, alopecia, rash, proteinuria, asthenia, fever, chills | Data regarding cidofovir use in different age groups, sex, and race are not available |
mpox monkeypox, Tmax time to reach Cmax, VD volume of distribution, UGT1A1 uridine diphosphate (UDP)-glucuronosyl transferase family 1 member A1, UGT1A4 uridine diphosphate (UDP)-glucuronosyl transferase family 1 member A4, Cl clearance, ALT alanine transaminase, AST aspartate transaminase, AUC∞ area under the unbound plasma concentration-time curve extrapolated to infinity, Cmax maximum concentration, CYP4F2 cytochrome P450 family 4 subfamily F member 2, t½ terminal half-life, ART antiretroviral therapy, GI gastrointestinal
aDisclaimer: Table 5 is for general information only and does not constitute professional advice. Please consult the relevant up-to-date guidelines, package inserts, and authoritative texts before making a clinical decision
bAdministered under fasted conditions
c5 mg/kg cidofovir administered with probenecid
Fig. 6.
Mechanism of action of tecovirimat, brincidofovir and cidofovir. The diagram depicts a probable lifecycle of the mpox virus. Tecovirimat hinders viral envelope formation of orthopoxviruses (e.g., mpox and smallpox) by inhibiting p37 protein, a highly conserved sequence among all orthopoxviruses. Brincidofovir, a prodrug and lipid conjugate of cidofovir, can easily pass through the phospholipid bilayer compared with cidofovir. Cidofovir diphosphate competitively inhibits the viral DNA polymerase and thus hinders viral DNA replication. mpox monkey pox
Cidofovir and Brincidofovir
Brincidofovir is the prodrug of cidofovir (Table 5) [48, 51, 58]. The FDA approved cidofovir in 1996 for cytomegalovirus (CMV) retinitis in patients with acquired immunodeficiency syndrome (AIDS), and brincidofovir in 2021 for smallpox infection. Similar to tecovirimat, patients with mpox infection may get access to brincidofovir from health authorities through a CDC- and FDA-sanctioned EA-IND protocol [4]. Figure 6 illustrates the mechanism of action of brincidofovir and cidofovir [59–61]. Brincidofovir may have a better safety profile compared with cidofovir, especially with regard to the potential for renal toxicity [4]. Both of these agents have important drug–drug interactions with various antiretroviral medications, and caution is advised when administering either to an HIV-positive patient who is also taking an antiretroviral therapy (ART) regimen (see Table 5 for details).
Topical Cidofovir
In a prospective study that included 24 mpox patients, half of the patients received only supportive care and the other half received supportive care plus topical cidofovir 1% cream [62]. The patients who used the cream healed faster than the patients who did not. The cidofovir 1% users got rid of the lesions within 12 days (median), while it took the other group 18 days (median). After 2 weeks, only 10% of patients who used topical cidofovir 1% had PCR-positive skin lesions, while 62.5% of patients who did not use cidofovir 1% had PCR-positive skin lesions [62].
Vaccinia Immune Globulin Intravenous
Although the benefit is uncertain, clinicians may consider intravenous vaccinia immune globulin intravenous (VIGIV) for patients with severe mpox infection, or prophylaxis for patients with T-cell immunodeficiency for whom post-exposure vaccination is prohibited [46, 50]. VIGIV is not pre-positioned in state health departments and must be requested directly from the CDC on a case-by-case basis.
Conclusion
Although mpox is remarkably less transmissible than the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, we must act now to stop community transmission and avoid the development of additional animal reservoirs. The public, healthcare professionals, and government should be vigilant and promote preventive measures and appropriate diagnostic testing. By including mpox in the differential diagnosis of (1) papulovesicular or vesiculopustular lesions and (2) genital lesions/ulcers, dermatologists may play an essential role in the timely diagnosis and treatment of new cases, thereby preventing the spread of the disease [7, 23].
Declarations
Funding
No sources of funding were used to assist in the preparation of this article.
Conflict of interest
Aditya K. Gupta, Mesbah Talukder, Ted Rosen, and Vincent Piguet have no conflicts of interest to declare.
Ethics approval
The authors declare that human ethics approval was not required for this article.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors’ contributions
Manuscript conception was undertaken by AKG, and the work was drafted by MT, TR, and VP and substantively revised by AKG, MT, TR and VP.
Consent to participate
Not applicable.
References
- 1.Billioux BJ, Mbaya OT, Sejvar J, Nath A. Neurologic complications of smallpox and monkeypox: a review. JAMA Neurol. 2022;79(11):1180–1186. doi: 10.1001/jamaneurol.2022.3491. [DOI] [PubMed] [Google Scholar]
- 2.Nuzzo JB, Borio LL, Gostin LO. The WHO declaration of monkeypox as a global public health emergency. JAMA. 2022;328(7):615–617. doi: 10.1001/jama.2022.12513. [DOI] [PubMed] [Google Scholar]
- 3.WHO recommends new name for monkeypox disease. 2022. https://www.who.int/news/item/28-11-2022-who-recommends-new-name-for-monkeypox-disease#:~:text=Following%20a%20series%20of%20consultations,as%20a%20synonym%20for%20monkeypox. Accessed 14 Dec 2022.
- 4.Guarner J, Del Rio C, Malani PN. Monkeypox in 2022-what clinicians need to know. JAMA. 2022;328(2):139–140. doi: 10.1001/jama.2022.10802. [DOI] [PubMed] [Google Scholar]
- 5.Ladnyj ID, Ziegler P, Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ. 1972;46(5):593–597. [PMC free article] [PubMed] [Google Scholar]
- 6.2022 mpox (monkeypox) outbreak: global trends (World Health Organization). 2022. https://worldhealthorg.shinyapps.io/mpx_global/. Accessed 25 Mar 2023.
- 7.Bryer JS, Freeman EE, Rosenbach M. Monkeypox emerges on a global scale: a historical review and dermatological primer. J Am Acad Dermatol. 2022;87(5):1069–1074. doi: 10.1016/j.jaad.2022.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Mpox (monkeypox): experts give virus variants new names. 2022. https://www.who.int/news/item/12-08-2022-monkeypox--experts-give-virus-variants-new-names. Accessed 15 Sept 2022.
- 9.Likos AM, Sammons SA, Olson VA, Frace AM, Li Y, Olsen-Rasmussen M, et al. A tale of two clades: monkeypox viruses. J Gen Virol. 2005;86(10):2661–2672. doi: 10.1099/vir.0.81215-0. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Are there different types of mpox (monkeypox)? 2022. https://www.cdc.gov/poxvirus/monkeypox/about/faq.html. Accessed 15 Sept 2022.
- 11.Isidro J, Borges V, Pinto M, Sobral D, Santos JD, Nunes A, et al. Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus. Nat Med. 2022;28(8):1569–1572. doi: 10.1038/s41591-022-01907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinstein RA, Nalca A, Rimoin AW, Bavari S, Whitehouse CA. Reemergence of monkeypox: prevalence, diagnostics, and countermeasures. Clin Infect Dis. 2005;41(12):1765–1771. doi: 10.1086/498155. [DOI] [PubMed] [Google Scholar]
- 13.Barnes AH, Smith C, Dash A, Shishido AA. Mpox: special considerations in the immunocompromised host. Curr Treat Opt Infect Dis. 2022;14:1–24. [Google Scholar]
- 14.Long B, Liang SY, Carius BM, Chavez S, Gottlieb M, Koyfman A, et al. Mimics of Mpox: considerations for the emergency medicine clinician. Am J Emerg Med. 2023;65:172–178. doi: 10.1016/j.ajem.2023.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuster-Bruce C, Gal S. How monkeypox symptoms compare to smallpox and chickenpox. 2022. https://www.insider.com/monkeypox-smallpox-chickenpox-rash-symptoms-chart-virus-outbreak-2022-5. Accessed 15 Aug 2022.
- 16.Hagen A. Monkeypox: when to get tested and what to do if exposed. 2022. https://asm.org/Articles/2022/August/Monkeypox-When-to-Get-Tested-and-What-to-Do-if-Exp. Accessed 18 Aug 2022.
- 17.Centers for Disease Control and Prevention. Clinical recognition: key characteristics for identifying mpox. 2023. https://www.cdc.gov/poxvirus/mpox/clinicians/clinical-recognition.html. Accessed 7 Mar 2023.
- 18.Català A, Clavo-Escribano P, Riera-Monroig J, Martín-Ezquerra G, Fernandez-Gonzalez P, Revelles-Peñas L, et al. Monkeypox outbreak in Spain: clinical and epidemiological findings in a prospective cross-sectional study of 185 cases. Br J Dermatol. 2022;187(5):765–772. doi: 10.1111/bjd.21790. [DOI] [PubMed] [Google Scholar]
- 19.Yakubovsky M, Shasha D, Reich S, Tau L, Friedel N, Halutz O, et al. Mpox presenting as proctitis in men who have sex with men. Clin Infect Dis. 2023;76(3):528–530. doi: 10.1093/cid/ciac737. [DOI] [PubMed] [Google Scholar]
- 20.Huhn GD, Bauer AM, Yorita K, Graham MB, Sejvar J, Likos A, et al. Clinical characteristics of human monkeypox, and risk factors for severe disease. Clin Infect Dis. 2005;41(12):1742–1751. doi: 10.1086/498115. [DOI] [PubMed] [Google Scholar]
- 21.Thornhill JP, Barkati S, Walmsley S, Rockstroh J, Antinori A, Harrison LB, et al. Monkeypox virus infection in humans across 16 countries—April–June 2022. N Engl J Med. 2022;387(8):679–691. doi: 10.1056/NEJMoa2207323. [DOI] [PubMed] [Google Scholar]
- 22.Girometti N, Byrne R, Bracchi M, Heskin J, McOwan A, Tittle V, et al. Demographic and clinical characteristics of confirmed human monkeypox virus cases in individuals attending a sexual health centre in London, UK: an observational analysis. Lancet Infect Dis. 2022;22(9):1321–1328. doi: 10.1016/S1473-3099(22)00411-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffiths-Acha J, Vela-Ganuza M, Sarró-Fuente C, López-Estebaranz JL. Monkeypox: a new differential diagnosis when addressing genital ulcer disease. Br J Dermatol. 2022;187(6):1050–1052. doi: 10.1111/bjd.21834. [DOI] [PubMed] [Google Scholar]
- 24.Tarín-Vicente EJ, Alemany A, Agud-Dios M, Ubals M, Suñer C, Antón A, et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study. Lancet. 2022;400(10353):661–669. doi: 10.1016/S0140-6736(22)01436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. Laboratory testing for the monkeypox virus: interim guidance. 2022. https://www.who.int/publications/i/item/WHO-MPX-laboratory-2022.1. Accessed 27 Sept 2022.
- 26.de Sousa D, Frade J, Patrocínio J, Borges-Costa J, Filipe P. Monkeypox infection and bacterial cellulitis: a complication to look for. Int J Infect Dis. 2022;123:180–182. doi: 10.1016/j.ijid.2022.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martín-Delgado MC, Martin Sanchez F, Martinez-Selles M. Monkeypox in humans: a new outbreak. Rev Esp Quimioter. 2022;35(6):509–518. doi: 10.37201/req/059.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. How it spreads. 2022. https://www.cdc.gov/poxvirus/monkeypox/if-sick/transmission.html. Accessed 28 Sept 2022.
- 29.Peiró-Mestres A, Fuertes I, Camprubí-Ferrer D, Marcos M, Vilella A, Navarro M, et al. Frequent detection of monkeypox virus DNA in saliva, semen, and other clinical samples from 12 patients, Barcelona, Spain, May to June 2022. Euro Surveill. 2022;27(28):2200503. doi: 10.2807/1560-7917.ES.2022.27.28.2200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization. Mpox (monkeypox) 2022. https://www.who.int/news-room/fact-sheets/detail/monkeypox. Accessed 28 Sept 2022.
- 31.Brown K, Leggat PA. Human monkeypox: current state of knowledge and implications for the future. Trop Med Infect Dis. 2016;1(1):8. doi: 10.3390/tropicalmed1010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ward T, Christie R, Paton RS, Cumming F, Overton CE. Transmission dynamics of monkeypox in the United Kingdom: contact tracing study. BMJ. 2022;2(379):e073153. doi: 10.1136/bmj-2022-073153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suñer C, Ubals M, Tarín-Vicente EJ, Mendoza A, Alemany A, Hernández-Rodríguez Á, et al. Viral dynamics in patients with monkeypox infection: a prospective cohort study in Spain. Lancet Infect Dis. 2023;23(4):445–453. doi: 10.1016/S1473-3099(22)00794-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Government of Canada. Mpox (monkeypox): how it spreads, prevention and risks. 2022. https://www.canada.ca/en/public-health/services/diseases/monkeypox/risks.html. Accessed 25 Aug 2022.
- 35.Centers for Disease Control and Prevention. Impact of monkeypox outbreak on select behaviors. 2022. https://www.cdc.gov/poxvirus/monkeypox/response/2022/amis-select-behaviors.html#:~:text=In%20an%20online%20survey%20of,encounters%2C%20and%2050%25%20reported%20reducing. Accessed 19 Oct 2022.
- 36.Centers for Disease Control and Prevention. Mpox (monkeypox) and smallpox vaccine guidance. 2022. https://www.cdc.gov/poxvirus/monkeypox/clinicians/smallpox-vaccine.html. Accessed 26 Sept 2022.
- 37.Rimoin AW, Mulembakani PM, Johnston SC, Lloyd Smith JO, Kisalu NK, Kinkela TL, et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc Natl Acad Sci USA. 2010;107(37):16262–16267. doi: 10.1073/pnas.1005769107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.US FDA. ACAM2000: highlights of prescribing information. 2018. https://www.fda.gov/vaccines-blood-biologics/vaccines/acam2000. Accessed 30 Aug 2022.
- 39.Drugs.com. Jynneos prescribing information. 2023. https://www.drugs.com/pro/jynneos.html. Accessed 6 Mar 2023.
- 40.US FDA. JYNNEOS :highlights of prescribing information. 2022. https://www.fda.gov/vaccines-blood-biologics/jynneos. Accessed 30 Aug 2022.
- 41.Sagy YW, Zucker R, Hammerman A, Markovits H, Arieh NG, Ahmad WA, et al. Real-world effectiveness of a single dose of mpox vaccine in males. Nat Med. 2023;29(3):748–752. doi: 10.1038/s41591-023-02229-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.US FDA. Vaccine adverse event reporting system (VAERS). 2022. http://wonder.cdc.gov/vaers.html. Accessed 30 Oct 2022.
- 43.Centers for Disease Control and Prevention. Vaccination. 2022. https://www.cdc.gov/poxvirus/monkeypox/interim-considerations/overview.html#:~:text=Currently%2C%20CDC%20is%20not%20recommending,monkeypox%20for%20the%20general%20public. Accessed 19 Oct 2022.
- 44.Rizk JG, Lippi G, Henry BM, Forthal DN, Rizk Y. Prevention and treatment of monkeypox. Drugs. 2022;82(9):957–963. doi: 10.1007/s40265-022-01742-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Centers for Disease Control and Prevention. Monitoring and risk assessment for persons exposed in the community. 2022. https://www.cdc.gov/poxvirus/monkeypox/clinicians/monitoring.html. Accessed 21 Sept 2022.
- 46.Centers for Disease Control and Prevention. Interim clinical guidance for the treatment of mpox (monkeypox). 2022. https://www.cdc.gov/poxvirus/monkeypox/clinicians/treatment.html. Accessed 22 Sept 2022.
- 47.European Centers for Disease Prevention and Control. Factsheet for health professionals on mpox (monkeypox). 2022. https://www.ecdc.europa.eu/en/all-topics-z/monkeypox/factsheet-health-professionals#:~:text=The%20pathogen,%2Drelated%20poxviruses%20%5B2%5D. Accessed 28 Aug 2022.
- 48.European Medicines Agency. Vistide, INN (cidofovir). 2007. https://www.ema.europa.eu/en/documents/product-information/vistide-epar-product-information_en.pdf. Accessed 30 Aug 2022.
- 49.Centre for Infectious Disease Research and Policy. Smallpox: agent and pathogenesis. 2014. https://www.cidrap.umn.edu/infectious-disease-topics/smallpox#overview. Accessed 28 Aug 2022.
- 50.US FDA. CNJ-016, Vaccinia Immune Globulin Intravenous (Human), sterile solution; highlights of prescribing information. 2010. https://www.fda.gov/media/78174/download. Accessed 22 Sept 2022.
- 51.US FDA. Highlights of prescribing information, TPOXX® (tecovirimat) capsules, for oral use and TPOXX® (tecovirimat) injection, for IV use. 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214518s000lbl.pdf. Accessed 30 Aug 2022.
- 52.Garcés-Ayala F, Rodríguez-Castillo A, Ortiz-Alcántara JM, Gonzalez-Durán E, Segura-Candelas JM, Pérez-Agüeros SI, et al. Full-genome sequence of a novel varicella-zoster virus clade isolated in Mexico. Genome Announc. 2015;3(4):e00752–e815. doi: 10.1128/genomeA.00752-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamilton K, Regier L, Jensen B. Anti-infectives for common infections: overview & management (RxFiles). 2021. http://www.RxFiles.ca. Accessed 18 Dec 2021.
- 54.Centers for Disease Control and Prevention. Prevention and treatment of chickenpox. 2021. https://www.cdc.gov/chickenpox/about/prevention-treatment.html. Accessed 25 Aug 2022.
- 55.Chickenpox (NHS inform). 2021. https://www.nhsinform.scot/illnesses-and-conditions/infections-and-poisoning/chickenpox#treating-chickenpox. Accessed 25 Aug 2022.
- 56.Mpox management approach: BMJ best practice. 2022. https://bestpractice.bmj.com/topics/en-us/1611/management-approach. Accessed 8 Mar 2023.
- 57.Mpox selfcare (CDC). 2022. https://www.cdc.gov/poxvirus/monkeypox/pdf/SelfCare-InfoSheet.pdf. Accessed 12 Dec 2022.
- 58.US FDA. Highlights of prescribing information, TEMBEXA (brincidofovir) tablets, for oral use and TEMBEXA (brincidofovir) oral suspension. 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214460s000,214461s000lbl.pdf. Accessed 30 Aug 2022.
- 59.Russo AT, Grosenbach DW, Chinsangaram J, Honeychurch KM, Long PG, Lovejoy C, et al. An overview of tecovirimat for smallpox treatment and expanded anti-orthopoxvirus applications. Expert Rev Anti Infect Ther. 2021;19(3):331–344. doi: 10.1080/14787210.2020.1819791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trevor AJ, Katzung BG, Masters SB, Kruidering-Hall M. Pharmacology examination & board review. New York: McGraw-Hill Medical New; 2010. [Google Scholar]
- 61.Huang Y, Mu L, Wang W. Monkeypox: epidemiology, pathogenesis, treatment and prevention. Signal Transduct Target Ther. 2022;7(1):1–22. doi: 10.1038/s41392-022-01215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sobral-Costas TG, Escudero-Tornero R, Servera-Negre G, Bernardino JI, Arroyo AG, Díaz-Menéndez M, et al. Human monkeypox outbreak: epidemiological data and therapeutic potential of topical cidofovir in a prospective cohort study. J Am Acad Dermatol. doi. 2022 doi: 10.1016/j.jaad.2022.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]