Abstract
Introduction
Post-contrast acute kidney injury (PC-AKI) is a major complication of contrast media usage; risks for PC-AKI are generally evaluated before computed tomography (CT) with contrast at the emergency department (ED). Although persistent hypotension (systolic blood pressure [sBP] <80 mm Hg for 1 h) is associated with increased PC-AKI incidence, it remains unclear whether transient hypotension that is haemodynamically stabilized before CT is a risk of PC-AKI. We hypothesized that hypotension on ED arrival would be associated with higher PC-AKI incidence even if CT with contrast was performed after patients are appropriately resuscitated.
Methods
This multicentre retrospective observational study was conducted at three tertiary care centres during 2013–2014. We identified 280 patients who underwent CT with contrast at the ED. Patients were classified into two groups based on sBP on arrival (<80 vs. ≥80 mm Hg); hypotension was considered as transient because CT with contrast has always been performed after patients were stabilized at participating hospitals. PC-AKI incidence was compared between the groups; inverse probability weighting (IPW) was conducted to adjust background characteristics.
Results
Eighteen patients were excluded due to chronic haemodialysis, cardiac arrest on arrival, or death within 72 h; 262 were eligible for this study. PC-AKI incidence was higher in the transient hypotension group than the normotension group {7/27 (28.6%) vs. 24/235 (10.2%), odds ratio (OR) 3.08 (95% confidence interval [CI] 1.18–8.03), p = 0.026}, which was confirmed by IPW (OR 3.25 [95% CI 1.99–5.29], p < 0.001).
Conclusion
Transient hypotension at the ED was associated with PC-AKI development.
Keywords: Transient hypotension, Computed tomography with contrast, Emergency department, Post-contrast acute kidney injury
Introduction
Acute kidney injury (AKI) is a new-onset or worsening renal dysfunction that can be caused by variable factors damaging the kidney, such as cytotoxic agents, hypoxemic distress due to atherosclerosis, and hypoperfusion due to haemodynamic instability [1, 2, 3, 4]. Post-contrast AKI (PC-AKI) is a subset of AKI following the intravascular administration of contrast media, previously known as contrast-induced AKI. It has been extensively reported that PC-AKI is associated with increased short- and long-term mortality and prolonged hospital stay [5]. Although various study groups have investigated the prevalence of PC-AKI, approximately 5–7% of patients who underwent percutaneous coronary angiography using contrast media were found to develop PC-AKI [6, 7].
Computed tomography (CT) is an important imaging modality, and more than 75.6 million CT scans are performed in the USA annually [8]. It is frequently performed with contrast media, particularly in the emergency department (ED) with increasing popularity [9] and sometimes followed by emergency interventional radiology that uses additional contrast media if major bleeding is detected. Although such increased exposure to contrast media in the ED has introduced PC-AKI among a considerable number of patients, no effective treatment for PC-AKI has been validated. The only strategy available for physicians would be to avoid unnecessary contrast media exposure during the CT scan, particularly in patients at high risk for PC-AKI.
Although risk stratification of patients to predict the development of PC-AKI has been exclusively investigated, persistent hypotension, defined as systolic blood pressure (sBP) of <80 mm Hg for at least 1 h requiring inotropic agents, has been suggested as one of the risk factors for PC-AKI [10, 11]. Nevertheless, it remains unclear whether transient hypotension would affect the incidence of PC-AKI even after patients are haemodynamically stabilized. Considering that patients with haemodynamic instability in the ED would often undergo CT with contrast after stabilization, we investigated whether the CT scan using contrast media is associated with a high incidence of PC-AKI among patients who have a transient hypotensive episode. Our hypothesis was that transient hypotension, recognized on arrival at the ED and resuscitated adequately, is associated with PC-AKI following the contrast-enhanced CT scan.
Materials and Methods
Study Design and Settings
This was a multicentre retrospective observational study conducted at three tertiary care centres between April 2013 and March 2014. The study protocol was reviewed and approved by the Institutional Review Board (IRB) for the Conduct of Human Research at each participating hospital (application number of IRB at Keio University School of Medicine: 20140447) and complied with the Declaration of Helsinki. Requirement for individual patient informed consent was waived by the IRB because of the retrospective design of this study.
During the study period, the indication for CT with contrast was decided by the treating physician and imaging was performed 30–60 min after ED arrival. Patients were transferred to CT room after sBP was increased to >90 mm Hg by fluid resuscitation and/or vasopressor usage and also the treating physicians confirmed that patients could safely leave the ED considering their conditions, such as lactate clearance. While the treating physician decided the resuscitation strategy before the CT scan, taking into consideration the possible diagnosis and haemodynamic status, patients with hypotension were administered 500–1,000 mL of fluid on arrival. Furthermore, patients with a known history of chronic kidney disease (CKD) were administered at least 500 mL of fluid before their CT scan to prevent PC-AKI.
Study Population
We retrospectively identified patients who arrived at the ED and were then admitted to each participating hospital during the study period. After the screening process, 299 patients who underwent the CT scan with contrast were identified during the study period. Inclusion criteria were as follows: (1) patients aged ≥18 years; (2) those who underwent a CT scan with contrast media in the ED; and (3) those whose serum creatinine (sCr) levels were measured before and 1–3 days after the CT scan. Patients on haemodialysis, those who arrived with cardiac arrest, and those who died within 72 h after admission were excluded.
Data Collection and Definitions
Data were collected from medical records by investigators, which included age, sex, comorbidities, vital signs on hospital arrival, sCr levels, estimated glomerular filtration rate (eGFR) before and after the CT scan, haematocrit, acute physiology and chronic health evaluation (APACHE) II score on admission, renal replacement therapy (RRT) performed after admission, hospital length of stay (LOS), and survival status at discharge. sCr and eGFR initially measured at the ED were defined as baseline sCr and eGFR.
Based on the frequently used threshold of hypotension as a risk for PC-AKI, transient hypotension was defined as an sBP of <80 mm Hg on hospital arrival or the initiation of vasopressors before the CT scan; hypotension was considered to be transient because all patients were resuscitated before the CT scan (sBP was increased to >90 mm Hg) as mentioned earlier. PC-AKI was defined based on the most frequently published criteria, i.e., an absolute increase in the sCr level by ≥0.5 mg/dL or ≥25% increase over baseline sCr level within 72 h after CT with contrast [12]. Anaemia was defined as a haematocrit value of <39% for men and <36% for women [10]. CKD was defined as an eGFR of <60 mL/min/1.73 m2.
Outcome Measures
The primary outcome was the development of PC-AKI. The secondary outcomes included in-hospital mortality, RRT within 30 days after admission, and LOS.
Statistical Analysis
Patient data were divided between transient hypotension (sBP <80 mm Hg on hospital arrival) and normotension groups (sBP ≥80 mm Hg on hospital arrival). To adjust covariates between the two groups, inverse probability weighting (IPW) analyses with propensity scores were performed to compare the primary outcome. The propensity score was developed using the logistic regression model to estimate the probability of being in the transient hypotension group compared with the normotension group. Relevant covariates were carefully selected from known or possible AKI predictors based on previous studies, which included age, sex, presence of anaemia, and comorbidities, including congestive heart failure, coronary artery disease, diabetes mellitus, stroke, and CKD [10, 11, 13]. All these variables were entered into the propensity model, where patients with missing covariates were excluded from the propensity score calculation. The precision of discrimination and propensity score calibration were analysed using the c-statistic and Hosmer-Lemeshow goodness-of-fit test [14]. IPW analyses were then performed to compare the primary and secondary outcomes between the two groups using χ2 test and ordinal logistic regression analysis.
Sensitivity analyses were conducted to validate the primary results. An IPW analysis with restriction was performed without using patient data with <0.1 or >0.9 of the propensity score to avoid extreme weights. Logistic regression analysis was also performed using the propensity score as covariate to confirm whether the results were independent of the IPW method. In addition, to assess the robustness of the study results, multivariate logistic regression was performed using the same covariates for the calculation of propensity scores.
Subgroup analyses were conducted to investigate the relationships between the effect of transient hypotension on the development of PC-AKI and a baseline kidney function, age, sex, anaemia, and APACHE II score. Propensity score development and IPW analyses on the primary outcome were repeated in each subgroup divided by the baseline eGFR, age, sex, anaemia, and APACHE II score.
Descriptive statistics were expressed as mean, median (interquartile range), or number (percentage) and were compared using unpaired t tests, Mann-Whitney U tests, χ2 tests, or Fisher's exact tests, as appropriate. For testing all hypotheses, a two-sided α threshold of 0.05 was considered statistically significant. All statistical analyses were conducted using SPSS, version 26.0 (IBM, Armonk, NY, USA), and Microsoft Excel (Microsoft, Redmond, WA, USA).
Results
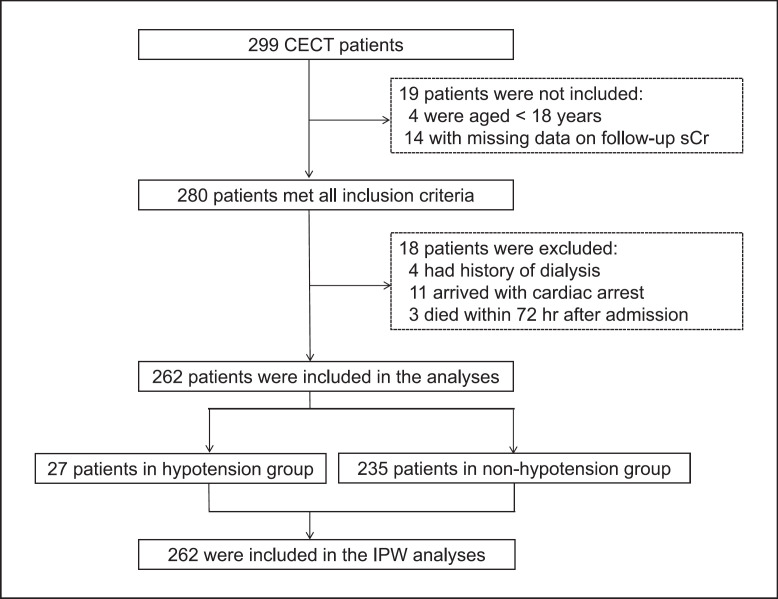
Although 280 patients satisfied all the inclusion criteria, 4 patients on haemodialysis and 11 with out-of-hospital cardiac arrest were excluded. Figure 1 summarizes the patient flow diagram.
Fig. 1.
Patient flow diagram. Of the 299 patients who underwent CT scan with contrast, 262 were eligible for this study. Among them, 27 (10%) had transient hypotension. CECT, contrast-enhanced computed tomography; IPW, inverse probability weighting; sCr, serum creatinine.
A total of 262 patients were eligible for this study, of whom 27 (10.2%) had transient hypotension and 235 did not have transient hypotension. The characteristics of the patients are summarized in Table 1. Regarding the diagnosis on hospital admission, 90 (34.4%) patients had trauma, 82 (31.3%) had gastrointestinal diseases, 29 (11.1%) had gastrointestinal bleeding, 18 (6.9%) had sepsis, 11 (4.2%) had stroke, and 6 (2.3%) had status epilepticus. Compared to the patients in the normotension group, more patients in the transient hypotension group had high APACHE II scores on admission (8 [29.6%] vs. 34 [14.5%]). Moreover, compared to the normotension group, fewer patients in the transient hypotension group had congestive heart failure or coronary artery disease (2 [7.4%] vs. 30 [12.8%]).
Table 1.
Characteristics of patients who underwent CECT scan at the ED
| Before IPW |
After IPW |
|||||
|---|---|---|---|---|---|---|
| transient hypotension | normotension | standardized difference | transient hypotension | normotension | standardized difference | |
| Case | 27 | 235 | ||||
| Age, median (IQR) | 64 (51–76) | 64 (48–77) | 70 (54–78) | 64 (49–77) | ||
| 65-70, n (%) | 2 (7.4) | 18 (7.7) | 0.010 | 18 (8.3) | 23 (8.7) | 0.024 |
| 70-75, n (%) | 3 (11.1) | 19 (8.1) | 0.103 | 15 (6.7) | 21 (8.0) | 0.048 |
| 75-85, n (%) | 4 (14.8) | 43 (18.3) | 0.094 | 48 (21.5) | 48 (18.3) | 0.082 |
| 85-, n (%) | 3 (11.1) | 27 (11.5) | 0.111 | 29 (13.0) | 30 (11.4) | 0.053 |
| Male, n (%) | 19 (70.4) | 171 (72.8) | 0.053 | 170 (76.2) | 191 (72.6) | 0.083 |
| sCr, median (IQR), mg/dL | 0.87 (0.60–1.31) | 0.79 (0.66–1.01) | 0.360 | 0.81 (0.64–1.05) | 0.80 (0.66–1.03) | 0.096 |
| eGFR, median (IQR), mL/min/1.73 m2 | 66 (45–95) | 74 (55–91) | 0.151 | 72 (48–95) | 73 (54–91) | 0.021 |
| eGFR <60 mL/min/1.73 m2, n (%) | 12 (44.4) | 67 (28.5) | 0.336 | 75 (29.1) | 79 (30.3) | 0.026 |
| eGFR <30 mL/min/1.73 m2, n (%) | 4 (14.8) | 9 (3.8) | 0.385 | 11 (4.9) | 14 (5.3) | 0.018 |
| Anaemia, n (%) | 12 (44.4) | 111 (47.2) | 0.056 | 100 (44.8) | 124 (47.1) | 0.046 |
| Comorbidities, n (%) | ||||||
| CHF/CAD | 2 (7.4) | 30 (12.8) | 0.179 | 25 (11.2) | 22 (8.4) | 0.096 |
| Diabetes | 3 (11.1) | 31 (13.2) | 0.064 | 30 (13.5) | 34 (12.9) | 0.016 |
| Stroke | 3 (11.1) | 24 (10.2) | 0.029 | 22 (9.9) | 27 (10.3) | 0.013 |
| APACHE II score ≥ 18, n (%) | 8 (29.6) | 34 (14.5) | 0.372 | 41 (18.4) | 46 (17.5) | 0.023 |
APACHE II score, acute physiology and chronic health evaluation II score; CAD, coronary artery disease; CHF, congestive heart failure; eGFR, estimated glomerular filtration rate; IPW, inverse probability weighting; IQR, interquartile range; sCr, serum creatinine.
The propensity model predicting allocation to the transient hypotension group was validated to have sufficient discrimination and calibration (c-statistic = 0.783 and Hosmer-Lemeshow goodness-of-fit p = 0.690), and no patients were excluded from IPW analyses due to missing covariates. The characteristics of the patients after IPW are summarized in Table 1, in which all covariates were successfully adjusted.
The incidence of PC-AKI was significantly higher among patients with transient hypotension than among patients with normotension on ED arrival in the unadjusted analysis (7 [28.6%] vs. 24 [10.2%]; odds ratio [OR], 3.08; 95% confidence interval [CI], 1.18–8.03; p = 0.026; Table 2); these results were validated by the IPW analysis (27.8% vs. 10.6%; OR, 3.25; 95% CI, 1.99–5.29; p < 0.001; Table 2). Adjusted analyses for secondary outcomes showed that in-hospital mortality and RRT within 30 days after admission were significantly higher among patients with transient hypotension than among patients with normotension (11.7% vs. 3.8%; OR, 3.34; 95% CI, 1.57–7.09; p = 0.001 and 8.5% vs. 1.5%; OR, 6.03; 95% CI, 2.02–18.00; p < 0.001, respectively; Table 2). The transient hypotension on arrival was also associated with longer hospital stay (mean LOS = 24 vs. 14 days; coefficient = 11 days; 95% CI, 1–20 days; Table 2).
Table 2.
Rates of PC-AKI and secondary outcomes
| Transient hypotension | Normotension | p value | OR/coefficients | 95% CI | |
|---|---|---|---|---|---|
| Rates of PC-AKI | |||||
| Unadjusted analysis, n/total (%) | 7/27 (28.6) | 24/235 (10.2) | 0.026 | 3.08 | 1.18–8.03 |
| After IPW, % (95% CI) | 27.8 (22.3–34.0) | 10.6 (7.39–14.9) | <0.001 | 3.25 | 1.99–5.29 |
| Secondary outcomes | |||||
| Emergent RRT, % (95% CI) | 8.5 (5.5–13.0) | 1.5 (0.50–4.0) | <0.001 | 6.03 | 2.02–18.00 |
| In-hospital mortality, % (95% CI) | 11.7 (8.0–16.6) | 3.8 (2.0–6.9) | 0.001 | 3.34 | 1.57–7.09 |
| LOS, mean, median (IQR), days | 24.2, 14.0 (8.0–37.0) | 13.8, 8.0 (5.0–16.0) | 10.5 | 0.76–20.1 | |
CI, confidence interval; IPW, inverse probability weighting; IQR, interquartile range; LOS, length of hospital stay; OR, odds ratio; PC-AKI, post-contrast acute kidney injury; RRT, renal replacement therapy.
Sensitivity analyses were performed to assure that the primary results were not dependent on the propensity score. The IPW analysis, excluding patient data with <0.1 or >0.9 of the propensity score, similarly revealed that transient hypotension was significantly associated with a higher incidence of PC-AKI (38.0% vs. 14.8%; OR, 3.54; 95% CI, 1.73–7.22; Table 3). Logistic regression with propensity score as a covariate also validated the association between transient hypotension and PC-AKI incidence (OR, 3.26; 95% CI, 1.99–5.33; Table 3). Furthermore, multivariate logistic regression using the same covariates for the propensity score calculation revealed similar results (OR, 3.02; 95% CI, 1.02–8.96; Table 3). Subgroup analyses demonstrated that the relationship between transient hypotension on admission and PC-AKI incidence existed among patients with eGFR <60 mL/min/1.73 m2, age ≥ 65, male, presence of anaemia, and APACHE II score <18 (Table 4).
Table 3.
Rate of PC-AKI in sensitivity analyses
| PC-AKI, % (95% CI) |
OR | 95% CI | ||
|---|---|---|---|---|
| transient hypotension | normotension | |||
| IPW with restriction (0.1-0.9 of PS) | 38.0 (29.1–47.8) | 14.8 (8.7–23.8) | 3.54 | 1.73–7.22 |
| Propensity score as covariate | 3.26 | 1.99–5.33 | ||
| Multiple regression analysis | 3.02 | 1.02–8.96 |
CI, confidence interval; IPW, inverse probability weighting; OR, odds ratio; PC-AKI, post-contrast acute kidney injury.
Table 4.
Rate of PC-AKI estimated using subgroup analysis
| PC-AKI, % (95% CI) |
OR | 95% CI | ||
|---|---|---|---|---|
| transient hypotension | normotension | |||
| eGFR <60 mL/min/1.73 m2 | 23.0 (14.1–35.0) | 1.2 (–0.4 to 7.1) | 24.43 | 3.11–191.68 |
| eGFR ≥60 mL/min/1.73 m2 | 17.9 (12.5–24.8) | 14.6 (10.1–20.6) | 1.27 | 0.71–2.29 |
| Age ≥65 | 27.0 (19.6–35.7) | 11.2 (6.7–18.0) | 2.93 | 1.47–5.84 |
| Age <65 | 17.6 (11.0–26.8) | 11.2 (6.8–17.8) | 1.70 | 0.79–3.62 |
| Male | 30.9 (24.3–38.3) | 11.1 (7.3–16.4) | 3.60 | 2.06–6.31 |
| Female | 22.6 (13.3–35.7) | 9.7 (4.5–19.0) | 2.72 | 0.99–7.47 |
| Anaemia (+) | 39.4 (30.3–49.6) | 8.7 (4.8–15.1) | 6.80 | 3.25–14.22 |
| Anaemia (–) | 19.8 (13.6–27.9) | 11.6 (7.2–18.1) | 1.89 | 0.95–3.75 |
| APACHE II score ≥18 | 21.9 (10.7–39.0) | 19.5 (10.0–34.3) | 1.16 | 0.37–3.61 |
| APACHE II score <18 | 26.8 (20.6–34.0) | 9.1 (5.9–13.7) | 3.64 | 2.05–6.50 |
CI, confidence interval; OR, odds ratio; PC-AKI, post-contrast acute kidney injury.
Discussion/Conclusion
This retrospective study demonstrated that transient hypotension identified on hospital arrival was associated with a higher incidence of PC-AKI. This relationship was validated using IPW analysis, in which several AKI predictors were adjusted. Remarkably, the observed association was consistent across several sensitivity analyses, suggesting that the results were not dependent on the method of propensity score or the weighting.
Although the reason behind the relationship between transient hypotension and higher PC-AKI incidence remains inconclusive, several pathophysiological mechanisms could be considered based on previous studies. First, ischaemic damages to the nephron caused by decreased renal blood flow during transient hypotension would be persistent for considerable duration at least until contrast media was administered [15]. Considering that CT with contrast has been reported to introduce PC-AKI only among patients with a history of higher stage CKD [16, 17], clinically obvious AKI would have developed by contrast media in patients with preconditioning insults by transient hypotension in this study. Second, a reduction in the outer medullary blood flow has been reported to be critical even for a shorter period of time because renal tissue injury would be extended after reperfusion. Although blood flow in most kidney tissues, cortex, and inner medulla rapidly recovers after ischaemia, it takes more than 3 h in the outer medulla to be normalized after reperfusion [15]. Given that contrast media is a potent vasoconstrictive agent [18], the contrast media used for CT would further reduce the outer medullary blood flow.
Although the results of this study suggested transient hypotension as a risk factor for PC-AKI, the duration of hypotension that affects the kidney function before contrast media usage remains inconclusive. A retrospective study on 7,230 patients undergoing PCI showed that transient hypotension for <1 h occurred more often in patients with PC-AKI than in those without PC-AKI [19]. Another experimental study demonstrated a persistent reduction of blood flow in the outer medulla of rats after 30-min renal ischaemia [15]. The degree of impact depending on the duration of transient hypotension on the kidney function before CT with contrast needs to be further explored in a future study.
Although transient hypotension would predispose patients to develop AKI after CT with contrast, the present study does not deny intravenous hydration. Several studies have reported that the incidence of PC-AKI was significantly lower in patients who received appropriate hydration than in those without hydration [20, 21, 22, 23, 24], and intravenous fluid should be administered before and after the usage of contrast media, as a preventive strategy for PC-AKI.
In this study, the incidence of PC-AKI was 11.8%, which was higher than previous studies (5–6%). This is probably due to the fact that the current study only included patients who needed hospital admission, suggesting that the severity of diseases in this study was higher than in previous studies. AKI could have been caused by higher degree of dehydration or insufficient fluid resuscitation before the CT scan.
This study has some limitations. The results of this study must be interpreted within the context of the study design. Unfortunately, we did not record the amount of pre- or post-procedural fluid administration that would prevent AKI. However, patients with hypotension on ED arrival generally receive a considerable amount of fluid, which is probably more than that received by patients with normotension. In addition, most of the patients in this study were critical and body weight were not recorded in any patients. Another limitation is that we did not analyse variables relating to the indication for CT with contrast or diagnosis after the CT scan in this study. If the indication for CT or diagnosis-related hypotension was a strong predictor for AKI, the results would be different. In addition, we did not record the exact time when the CT scan was performed. Time between ED arrival and CT scan would affect the association of transient hypotension at the ED with subsequent risk of PC-AKI. However, at the study institutions, most patients underwent the CT scan 30–60 min after ED arrival. Moreover, we could not confirm the causal relationship between the contrast media and PC-AKI because of the lack of a control group, i.e., patients without contrast media usage; regardless of the contrast media administration, hypotension on arrival could have been the cause of AKI. However, a recent study has suggested that intravenous contrast was associated with higher risk of AKI compared with non-contrast group in ED patients undergoing CT [25]. In addition, if the short duration of hypotension would be a risk of AKI is still unclear [26]. Therefore, our results would at least indicate that transient hypotension renders patients susceptible to kidney injury after contrast media usage. Furthermore, the sample size was limited in this study. To validate the results in this study, further study with a large sample size should be conducted. Finally, due to the retrospective study design, our results are not conclusive. Residual confounding and unmeasured AKI predictors, such as degree of dehydration, the amount of fluid administration, and APACHE2 score, preclude the confirmation of our results.
In conclusion, our results suggest that transient hypotension in the ED was associated with the development of PC-AKI after the usage of contrast media. Therefore, a careful risk-benefit discussion must be performed before conducting CT with contrast for patients with hypotension on hospital arrival.
Statement of Ethics
The study protocol was reviewed and approved by the Institutional Review Board (IRB) for the Conduct of Human Research at each participating hospital (application number of IRB at Keio University School of Medicine: 20140447) and complied with the Declaration of Helsinki. Requirement for individual patient informed consent was waived by the IRB because of the retrospective design of this study.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
There are no sources of financial support for the work.
Author Contributions
Jo Yoshizawa and Ryo Yamamoto designed the study. Jo Yoshizawa, Ryo Yamamoto, Koichiro Homma, Hanae Kamikura, Kazuhiko Sekine, Yosuke Kobayashi, and Tomohiro Funabiki performed data collection. Junichi Sasaki managed quality control. Jo Yoshiawa, Ryo Yamamoto, Koichiro Homma, Hanae Kamikura, Kazuhiko Sekine, Yosuke Kobayashi, Tomohiro Funabiki, and Junichi Sasaki performed data analysis, data interpretation, writing, and critical revision.
Data Availability Statement
The datasets generated and analysed during the current study are not publicly available due to privacy or ethical restrictions, but they are available from the corresponding author on reasonable request.
Supplementary Material
Supplementary data
Funding Statement
There are no sources of financial support for the work.
References
- 1.Vanmassenhove J, Kielstein J, Jörres A, Biesen WV. Management of patients at risk of acute kidney injury. Lancet. 2017;389((10084)):2139–2151. doi: 10.1016/S0140-6736(17)31329-6. [DOI] [PubMed] [Google Scholar]
- 2.Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol. 2012;2:1303–1353. doi: 10.1002/cphy.c110041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Izawa J, Kitamura T, Iwami T, Uchino S, Takinami M, Kellum JA, et al. Early-phase cumulative hypotension duration and severe-stage progression in oliguric acute kidney injury with and without sepsis: an observational study. Crit Care. 2016;20((1)):405. doi: 10.1186/s13054-016-1564-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lehman LW, Saeed M, Moody G, Mark R. Hypotension as a risk factor for acute kidney injury in ICU patients. Comput Cardiol. 2010;37:1095–1098. [PMC free article] [PubMed] [Google Scholar]
- 5.James MT, Samuel SM, Manning MA, Tonelli M, Ghali WA, Faris P, et al. Contrast-induced acute kidney injury and risk of adverse clinical outcomes after coronary angiography: a systematic review and meta-analysis. Circ Cardiovasc Interv. 2013;6((1)):37–43. doi: 10.1161/CIRCINTERVENTIONS.112.974493. [DOI] [PubMed] [Google Scholar]
- 6.Abe M, Morimoto T, Akao M, Furukawa Y, Nakagawa Y, Shizuta S, et al. Relation of contrast-induced nephropathy to long-term mortality after percutaneous coronary intervention. Am J Cardiol. 2014;114((3)):362–368. doi: 10.1016/j.amjcard.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Tsai TT, Patel UD, Chang TI, Kennedy KF, Masoudi FA, Matheny ME, et al. Contemporary incidence, predictors, and outcomes of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the NCDR Cath-PCI registry. JACC Cardiovasc Interv. 2014;7:1–9. doi: 10.1016/j.jcin.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.OECD Health at a glance 2015. OECD health statistics 2015. Available from:
- 9.Kocher KE, Meurer WJ, Fazel R, Scott PA, Krumholz HM, Nallamothu BK. National trends in use of computed tomography in the emergency department. Ann Emerg Med. 2011;58((5)):452–62.e3. doi: 10.1016/j.annemergmed.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 10.Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44((7)):1393–1399. doi: 10.1016/j.jacc.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 11.Nikolsky E, Mehran R, Lasic Z, Mintz GS, Lansky AJ, Na Y, et al. Low hematocrit predicts contrast-induced nephropathy after percutaneous coronary interventions. Kidney Int. 2005;67((2)):706–713. doi: 10.1111/j.1523-1755.2005.67131.x. [DOI] [PubMed] [Google Scholar]
- 12.Isaka Y, Hayashi H, Aonuma K, Horio M, Terada Y, Doi K, et al. Guideline on the use of iodinated contrast media in patients with kidney disease 2018. Clin Exp Nephrol. 2020;24:1–44. doi: 10.1007/s10157-019-01750-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chong E, Poh KK, Liang S, Tan HC. Risk factors and clinical outcomes for contrast-induced nephropathy after percutaneous coronary intervention in patients with normal serum creatinine. Ann Acad Med Singap. 2010;39((5)):374–380. [PubMed] [Google Scholar]
- 14.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46((3)):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Regner KR, Roman RJ. Role of medullary blood flow in the pathogenesis of renal ischemia-reperfusion injury. Curr Opin Nephrol Hypertens. 2012;21((1)):33–38. doi: 10.1097/MNH.0b013e32834d085a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davenport MS, Khalatbari S, Dillman JR, Cohan RH, Caoili EM, Ellis JH. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material. Radiology. 2013;267((1)):94–105. doi: 10.1148/radiol.12121394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorelik Y, Bloch-Isenberg N, Yaseen H, Heyman SN, Khamaisi M. Acute kidney injury after radiocontrast-enhanced computerized tomography in hospitalized patients with advanced renal failure: a propensity-score-matching analysis. Invest Radiol. 2020;55((10)):677–687. doi: 10.1097/RLI.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 18.Bansal S, Patel RN. Pathophysiology of contrast-induced acute kidney injury. Interv Cardiol Clin. 2020;9((3)):293–298. doi: 10.1016/j.iccl.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Dangas G, Iakovou I, Nikolsky E, Aymong ED, Mintz GS, Kipshidze NN, et al. Contrast-induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. Am J Cardiol. 2005;95((1)):13–19. doi: 10.1016/j.amjcard.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 20.Jurado-Roman A, Hernandez-Hernandez F, Garcia-Tejada J, Granda-Nistal C, Molina J, Velázquez M, et al. Role of hydration in contrast-induced nephropathy in patients who underwent primary percutaneous coronary intervention. Am J Cardiol. 2015;115((9)):1174–1178. doi: 10.1016/j.amjcard.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Luo Y, Wang X, Ye Z, Lai Y, Yao Y, Li J, et al. Remedial hydration reduces the incidence of contrast-induced nephropathy and short-term adverse events in patients with ST-segment elevation myocardial infarction: a single-center, randomized trial. Intern Med. 2014;53((20)):2265–2272. doi: 10.2169/internalmedicine.53.1853. [DOI] [PubMed] [Google Scholar]
- 22.Maioli M, Toso A, Leoncini M, Gallopin M, Tedeschi D, Micheletti C, et al. Sodium bicarbonate versus saline for the prevention of contrast-induced nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. J Am Coll Cardiol. 2008;52((8)):599–604. doi: 10.1016/j.jacc.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 23.Maioli M, Toso A, Leoncini M, Micheletti C, Bellandi F. Effects of hydration in contrast-induced acute kidney injury after primary angioplasty: a randomized, controlled trial. Circ Cardiovasc Interv. 2011;4((5)):456–462. doi: 10.1161/CIRCINTERVENTIONS.111.961391. [DOI] [PubMed] [Google Scholar]
- 24.Trivedi HS, Moore H, Nasr S, Aggarwal K, Agrawal A, Goel P, et al. A randomized prospective trial to assess the role of saline hydration on the development of contrast nephrotoxicity. Nephron Clin Pract. 2003;93((1)):C29–C34. doi: 10.1159/000066641. [DOI] [PubMed] [Google Scholar]
- 25.Kene M, Arasu VA, Mahapatra AK, Huang J, Reed ME. Acute Kidney injury after CT in emergency patients with chronic kidney disease: a propensity score-matched analysis. West J Emerg Med. 2021;22((3)):614–622. doi: 10.5811/westjem.2021.1.50246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kluger MT, Collier JMK, Borotkanics R, van Schalkwyk JM, Rice DA. The effect of intra-operative hypotension on acute kidney injury, postoperative mortality and length of stay following emergency hip fracture surgery. Anaesthesia. 2022;77((2)):164–174. doi: 10.1111/anae.15555. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
The datasets generated and analysed during the current study are not publicly available due to privacy or ethical restrictions, but they are available from the corresponding author on reasonable request.