Abstract
Introduction
Senile osteoporosis is one of the most common age-related diseases worldwide. Glucagon like peptide-2 (GLP-2), a naturally occurring gastrointestinal peptide, possesses therapeutic effects on bone loss in postmenopausal women and ovariectomized rats. However, the role of GLP-2 in senile osteoporosis and underlying mechanisms has not been explored.
Methods
GLP-2 was subcutaneously injected into the 6-month-old male senile osteoporosis model of senescence-accelerated mouse prone 6 (SAMP6) mice for 6 weeks. SAMP6 subjected to normal saline and senescence-accelerated mouse resistant 1 served as control groups. Micro-computed tomography was performed to evaluate the bone mass and microarchitecture of the mice. Osteoblastic and osteoclastic activities were determined by biochemical, quantitative real-time PCR, histological, and histomorphometric analyses combined with hematoxylin-eosin, toluidine blue, and tartrate-resistant acid phosphatase staining. We also examined the proteins and structure of intestinal tight junction using immunohistochemical assay as well as a transmission electron microscope. Serum inflammation marker levels were measured using ELISA. Additionally, anti-oxidative enzymes GPX-4 and SOD-2 and receptors of GLP-2 and vitamin D expression in the ileum and colon were detected under immunofluorescence staining.
Results
Six-week GLP-2 treatment attenuated bone loss in SAMP6 mice, as evidenced by increased bone mineral density, improved microarchitecture in femora, and enhanced osteogenic activities. In contrast, the activity of osteoclastic activity was not obviously inhibited. Moreover, GLP-2 ameliorated tight junction structure and protein expression in the intestinal barrier, which was accompanied by the reduction of TNF-α level. The expression of receptors of intestinal GLP-2 and vitamin D in the ileum was elevated. Furthermore, the oxidative stress in the intestines was improved by increasing the GPX-4 and SOD-2 signaling.
Conclusion
Our findings suggest that GLP-2 could ameliorate age-associated bone loss, tight junction structure, and improved antioxidant enzyme activity in the gut in SAMP6 mice. Amelioration of gut barrier dysfunction may potentially contribute to improving bone formation and provide evidence for targeting the entero-bone axis in the treatment of senile osteoporosis.
Keywords: Glucagon like peptide-2, Senile osteoporosis, Intestinal barrier function, Inflammation, Oxidative stress, Senescence-accelerated mouse prone 6 mice
Introduction
Osteoporosis is a common metabolic bone disease characterized by reduced bone mass, deterioration of bone structure, and consequently an increased susceptibility to fractures. It affects more than 200 million people worldwide due to the demographic shift as a result of aging populations [1]. As an age-related degenerative disease, senile osteoporosis has become an important public health concern because of the compromised quality of life and high mortality associated with osteoporotic fractures in the elderly [2, 3]. Senile osteoporosis typically occurs in women over the age of 65 years and men over 70 years. It represents a deficit in bone formation relative to bone resorption, which differs from postmenopausal osteoporosis with strong bone resorption relative to bone formation due to estrogen deficiency [4, 5, 6]. Most currently available therapeutics for treating osteoporosis are bone-resorptive inhibitors, and parathyroid hormone peptide is the only bone formation agent [7]. Therefore, unmet needs have led to efforts for the development of new anabolic agents for osteoporosis.
Glucagon like peptide-2 (GLP-2) is a naturally occurring peptide mainly co-secreted with GLP-1 by intestinal enteroendocrine L-cells and by neurons in the brainstem projected to the hypothalamus discretely [8]. It was found to function as reparative action for the gut, evidenced by cytoprotection and regeneration of the epithelial surface, upregulation of intestinal blood flow, and intestinal integrity and barrier function maintenance [9]. The analog teduglutide has been approved for the treatment of short bowel syndrome (SBS) since 2012. Subsequently, GLP-2 acts as an important modulator of bone turnover has been initially identified by the Holst group in Denmark [10]. They found that decreased bone resorption (measured as CTX) postprandially is inversely related to the plasma concentrations of GLP-2 [11]. In healthy subjects, prolonged exposure (achieved by s.c. injection) of GLP-2 was more effective in reducing circulating CTX level than acute high concentrations (obtained by i.v. injection) [12]. Short-term 5-week native GLP-2 administration significantly increased spinal bone mineral density (BMD), intestinal calcium absorption rates, and levels of serum ionized calcium in short bowel patients with no colon [10]. Moreover, exogenous subcutaneous injection of GLP-2 inhibited bone resorption (CTX) in a dose-dependent manner in postmenopausal women, while bone formation markers osteocalcin (OCN) or PINP were unaffected or modest but nonsignificant increases in OCN levels [11, 13, 14, 15]. We previously showed GLP-2 exerts a positive impact on postmenopausal osteoporosis in seven-month ovariectomized rats by promoting bone formation and inhibiting bone resorption [16].
Recently, the regulation properties of bone turnover of GLP-2 have been reported in various human studies [17, 18, 19, 20]. However, the effect of GLP-2 on senile osteoporosis, another type of primary osteoporosis with distinct etiology and pathophysiological characteristics, has not been investigated.
GLP-2 action is initiated by binding to GLP-2R, which is mainly confined to the gastrointestinal tract and has not been identified in mature human osteoclast and osteoblast [21, 22, 23, 24]. It implicates that bone effect might be mediated by GLP-2 indirectly, probably involving intestinal factor [25]. It has gradually been revealed that gut microbiota is involved in the process of osteoporosis [26, 27]. Treatment with GLP-2 resulted in a significant reduction in the prevalence of pathogenic bacterial genera and an increase in some potentially beneficial bacteria in aged rats [28]. Meanwhile, other endogenous intestinal hormones, glucose-dependent insulinotropic polypeptide (GIP) and GLP-1, participate in postprandial bone homeostasis as part of the so-called gut-bone axis [29, 30]. Intriguingly, no reduction in postprandial bone resorption was observed in the SBS with colectomy and patients with jejunostomy who were absent of endogenous GLP-2 secretion [31, 32]. Meanwhile, the decreased bone resorption in response to GLP-2 injections could not be elicited in colectomized patients with jejunostomy [32, 33]. Together, this all indicates that an intact bowel anatomy is essential for GLP-2-mediated reduction in bone resorption. Therefore, the mechanism underlying regulation of bone remodeling through the crosstalk between bone and gut should be further explored.
Senescence-accelerated mouse prone 6 (SAMP6), a naturally occurring mouse line that displays a phenotype of accelerated aging, exhibits decreased bone loss with defective osteoblastogenesis compared to the same strain of SAMP2 and control senescence-accelerated mouse resistant 1 (SAMR1) strain [34]. The line has been cultivated since 1970 by investigators at Kyoto University using selective inbreeding of AKR/J mice over more than 50 generations. Due to the fact that spontaneous bone traits are equivalent to the senile osteoporosis in humans identified by microdensitometric, chemical, and histological analysis, it serves as an ideal model for senile osteoporosis [35].
In this study, we first explore the effect of GLP-2 on osteoporosis in a SAMP6 model for senile osteoporosis. Intestinal factors were evaluated to preliminarily classify the underlying entero-bone endocrine axis in the pathogenesis of osteoporosis.
Materials and Methods
Animals and Treatments
All experiments and animal care procedures were approved by the Zhongshan Hospital Fudan University Animal Care and Use Committee (No. ZS20150212032. Shanghai, China). Sixteen 6-month-old male SAMP6 mice (SPF level) were used as a senile osteoporosis model in this study. The age-matched male SAMR1 (non-osteopenic, SPF level) was established as the control strain (samR group) [36]. All animals were obtained from First Teaching Hospital of Tianjin University of Traditional Chinese Medicine (Tianjin, China) and housed under controlled ambient conditions (12-h light/dark cycles, lights on at 6:00 a.m.) with free access to a standard rodent diet and distilled water.
After 1 week of acclimatization, the SAMP6 mice were randomly divided into two groups, which were injected once a day with GLP-2 (200 μg/kg, samP-G group) or an equal volume of normal saline (samP-C group) subcutaneously for 6 weeks. After 6 weeks of intervention, the mice fasted for 12 h, were euthanized, and then blood and organs were harvested for analysis as previously described [16].
Micro-Computed Tomography Analysis
The analysis of bone ultrastructure was conducted as outlined in our previous research [16]. Briefly, a high-resolution micro-computed tomography (micro-CT) scan (SkyScan 1176; Bruker, Knotich, Belgium) was performed to obtain the micro-CT images of the right femur at a resolution of 18 μm voxel size, with a voltage of 75 kV, a current of 333 μA, and 0.6-degree rotation steps (180-degree angular range). The visualized results of two-dimensional and three-dimensional (3D) reconstruction images (NRecon v1.6) were then analyzed (CTAn v1.13). The following morphometric parameters in femoral trabecula were measured: BMD, bone volume/tissue volume (BV/TV), bone surface/tissue volume (BS/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), trabecular pattern factor, and structure model indices; parameters in femoral cortex included BMD, BV/TV, and cortical thickness (Ct.Th).
Biochemical Analysis
Serum levels of bone turnover markers were measured using commercially available ELISA kits. For alkaline phosphatase (ALP), an osteogenic marker, a BALP ELISA Kit (BioAssay Systems; Hayward, CA, USA) was used. The C-terminal cross-linked telopeptides of type I collagen (CTX-1) were chosen as a classic osteoclast marker (CTX-1; ImmunoWay, Plano, TX, USA). Serum inflammatory markers (Multisciences, Hangzhou, Zhejiang, China) included tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), and interferon-γ (IFN-γ).
Histology and Histomorphometry Analysis
Pathological sections of the left femur were prepared according to the standard procedure for bone histology and histomorphometry analysis. Dissected left femur was isolated and fixed with 4% paraformaldehyde, decalcified in 10% EDTA, and then embedded in paraffin. Afterward, the processed samples were sectioned into 5-μm thick slices and stained with hematoxylin-eosin stain (H&E, solarbio, Beijing, China), toluidine blue (solarbio, Beijing, China), and tartrate-resistant acid phosphatase (TRAP, Sigma-Aldrich, USA). The analysis indexes including the number of osteoblast/trabecular perimeter, number of osteoclast/trabecular perimeter, and osteoclast surface/trabecular surface ratio were measured with the ImageJ software.
Immunohistochemistry and Immunofluorescence in the Intestine
Immunohistochemistry was performed to identify the protein expression of ZO-1 and occludin and immunofluorescence to detect oxidative stress (glutathione peroxidase 4, GPX-4 and superoxide dismutase 2, SOD-2) and receptors of GLP-2 (GLP-2R) and vitamin D (VDR) signaling in ileum and colon samples. Briefly, deparaffinization, rehydration, antigen retrieval, and endogenous peroxidase quenching (autofluorescence quenching for immunofluorescence) were sequentially applied to the 4.0-μm thick paraffin sections using a routine procedure. Then, the sections were incubated with primary antibodies: anti-ZO-1 antibody (Abcam, ab221547), anti-occludin antibody (Abcam, ab216327), anti-GPX-4 antibody (Abcam, ab125066), anti-SOD-2 antibody (Abcam, ab137037), anti-GLP-2R (Absin, abs127046), anti-VDR (Absin, abs131708), followed by incubation with the secondary antibodies (HRP conjugated for histomorphometry, FITC conjugated and Cy3 conjugated for immunofluorescence). For immunohistochemistry analysis, the peroxidase substrate DAB kit (DA1010, Solarbio, Beijing, China) was utilized for qualitative identification of antigens. Meanwhile, staining of nuclei was done with DAPI (C0065, Solarbio, Beijing, China) in immunofluorescence.
All slices were scanned with a high-resolution slide scanning system (Pannoramic MID, 3DHISTECH Ltd., Hungary) and photographed with corresponding CaseViewer 2.21 software. We used the software Adobe Photoshop 20.0.4, Adobe Illustrator 23.0.3 (San Jose, CA, USA), and ImageJ 1.53a (Rawak Software Inc., Stuttgart, Germany) for image processing, editing, and analysis.
Transmission Electron Microscopy Examination
As previously specified [37], the intestinal specimens were fixed with 2.5% glutaraldehyde and processed for transmission electron microscopy (TEM) following standard procedures. The embedded sections were sectioned into 75-nm slices and stained with uranyl acetate and lead citrate double staining and then examined in a JEM-1200 transmission electron microscope (Jeol Ltd., Tokyo, Japan).
Quantitative Real-Time PCR Analysis in Bone
Bilateral tibias were homogenized in liquid nitrogen to collect the mRNA samples. Total RNA was extracted using Trizol (Invitrogen, Carlsbad, USA) following the manufacturer's protocol. Then, cDNA was synthesized using the Prime Script RT reagent kit with DNA Eraser (Takara Bio, Shiga, Japan). Equal volumes of cDNA were amplified in a real-time detection system (ABI7500, USA) with FastStart Universal SYBR Premix (Yeasen, Shanghai, China) and primers (Sangon Biotech, Shanghai, China). Relative mRNA expression was calculated using the relative standard curve method (2−ΔΔCT) with β-actin as the endogenous control. The primer sequence of RANK, ALP, collagen type I (Col-1), runt-related transcription factor 2 (Runx2), osteocalcin (OCN), osteoprotegerin (OPG), RANKL, NFATC1, and c-fos are listed in online supplementary Table 1 (for all online suppl. material, see www.karger.com/doi/10.1159/000527502).
Statistical Analysis
The data were presented as the mean ± standard deviation (SD) and analyzed using GraphPad Prism version 8.2.0 (GraphPad Software Inc., San Diego, CA, USA). For normal distribution data, statistical evaluation between groups was identified using one-way analysis of variance followed by Dunnett's posttest, and a Kruskal-Wallis test followed by Dunn's multiple comparison post hoc test was used for nonparametric data. A p value <0.05 was considered to indicate statistical significance.
Results
GLP-2 Improved Bone Trabecular Microarchitecture in SAMP6 Mice
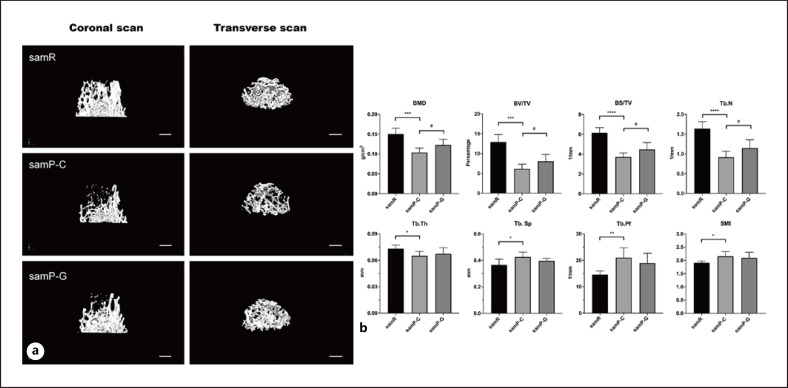
Micro-CT scanning showed that SAMP6 mice had reduced trabecular bone mass in the distal femur relative to SAMR1 mice, whereas subcutaneous administration of GLP-2 to SAMP6 mice for 6 weeks alleviated this bone loss (Fig. 1a). Quantification analysis revealed that the values of BMD, BV/TV, BS/TV, Tb.N, and Tb.Th in SAMP6 mice were significantly lower than those in the SAMR1 group, which together indicated a reduced and more separated trabecular bone network in this senile model. Meanwhile, all of these downregulated parameters significantly improved after GLP-2 treatment with the exception of the Tb.Th (Fig. 1b). Likewise, SMAP6 mice exhibited higher Tb.Sp, trabecular pattern factor, and structure model index compared with SAMR1 mice, and GLP-2 reversed these increased indexes to some degree, but the trend had no statistical significance (Fig. 1b).
Fig. 1.
a Representative images of trabecular microarchitecture of distal femurs in mice evaluated by micro-CT. Both coronal and transverse image reconstruction demonstrate the bone loss in SAMP6 mice relative to SAMR1 mice, while the bone loss was alleviated after administration of GLP-2 in SAMP6 mice. n = 11 for samR and n = 6 per group for samP-C and samP-G mice. Bar: 500 μm. b Quantitative histomorphometric analysis of bone mineral density (BMD), bone volume/tissue volume (BV/TV), bone surface/tissue volume (BS/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), trabecular pattern factor (Tb.Pf), and structure model index (SMI) in each group. n = 6 for samR and n = 8 per group for samP-C and samP-G mice. Data are presented as mean ± SD *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 versus samR group, #p < 0.05 versus samP-G group. samR, senescence-accelerated mouse resistant 1 (SAMR1); samP-C, senescence-accelerated mouse prone 6 treated with normal saline (SAMP6+NS); samP-G, senescence-accelerated mouse prone 6 treated with GLP-2 (SAMP6+GLP-2).
Representative 3D and two-dimensional images of the femoral cortex are shown in Figure 2. Compared to SAMR1 mice, SAMP6 mice showed remarkable cortical bone loss phenotypes, as indicated by significantly decreased BV/TV and cortical bone thickness (Fig. 2c). However, no significant improvement in these two cortical morphometric parameters was observed in the SAMP6 group following GLP-2 treatment.
Fig. 2.
Micro-CT and quantification of bone microarchitecture in the femoral cortex. a–b Representative 3D and 2D micro-CT reconstructed images of the femora from samR, samP-C, and samP-G mice. c Quantification of cortical bone microarchitecture. Left: bone volume/tissue volume (BV/TV), right: cortical thickness (Ct.Th). n = 6 for samR and n = 8 per group for samP-C and samP-G mice. Data are mean ± SD *p < 0.05; **p < 0.01; ***p < 0.001. Bar: 2a, 500 μm; 2b, 1 mm. Micro-CT, micro-computed tomography; samR; senescence-accelerated mouse resistant 1 (SAMR1); samP-C, senescence-accelerated mouse prone 6 treated with normal saline (SAMP6+NS); samP-G, senescence-accelerated mouse prone 6 treated with GLP-2 (SAMP6+GLP-2); 2D, two-dimensional.
GLP-2 Increased Serum Bone Formation Marker in SAMP6 Mice
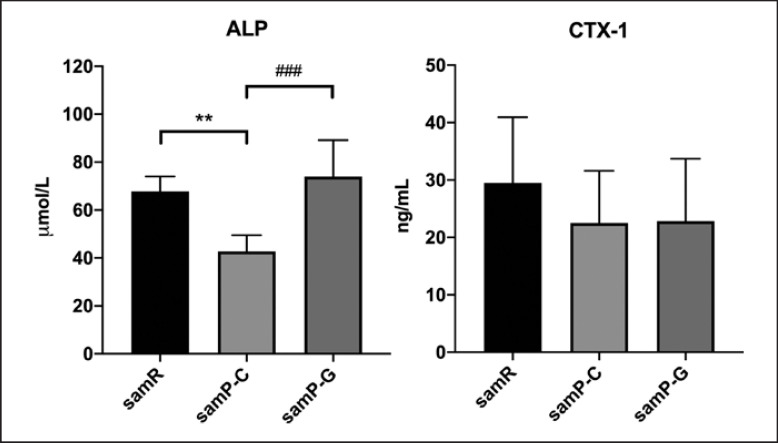
Serum representative biochemical markers for osteoporosis were examined. The level of bone formation marker ALP was remarkably lower in the SAMP6 group than in the SAMR1 group. However, GLP-2 significantly increased the ALP level by 42.2% (Fig. 3). Concomitant with ALP reduction, the level of bone resorption indicated by CTX-1 was decreased in SAMP6 mice. However, there was no statistically significant difference in the level of CTX-1 among each group, demonstrating that bone resorption was not altered by GLP-2 treatment. These data suggest that GLP-2 may prevent osteoporosis by increasing bone volume and maintaining bone formation rather than inhibiting bone resorption.
Fig. 3.
Serum levels of bone turnover biomarkers including ALP and CTX-1 in different groups were assessed using ELISA. Data are depicted as mean ± SD n = 5 for samR and n = 6 per group for samP-C and samP-G mice. Statistical analysis was performed by one-way ANOVA. *p < 0.05, **p < 0.01 versus samR group, ###p < 0.001 versus samP-G group. ALP, alkaline phosphatase; CTX-1, C-terminal cross-linked telopeptides of type I collagen; samR, senescence-accelerated mouse resistant 1 (SAMR1); samP-C, senescence-accelerated mouse prone 6 treated with normal saline (SAMP6+NS); samP-G, senescence-accelerated mouse prone 6 treated with GLP-2 (SAMP6+GLP-2); ANOVA, analysis of variance.
GLP-2 Ameliorated Bone Loss by Enhancing Bone Formation in SAMP6 Mice
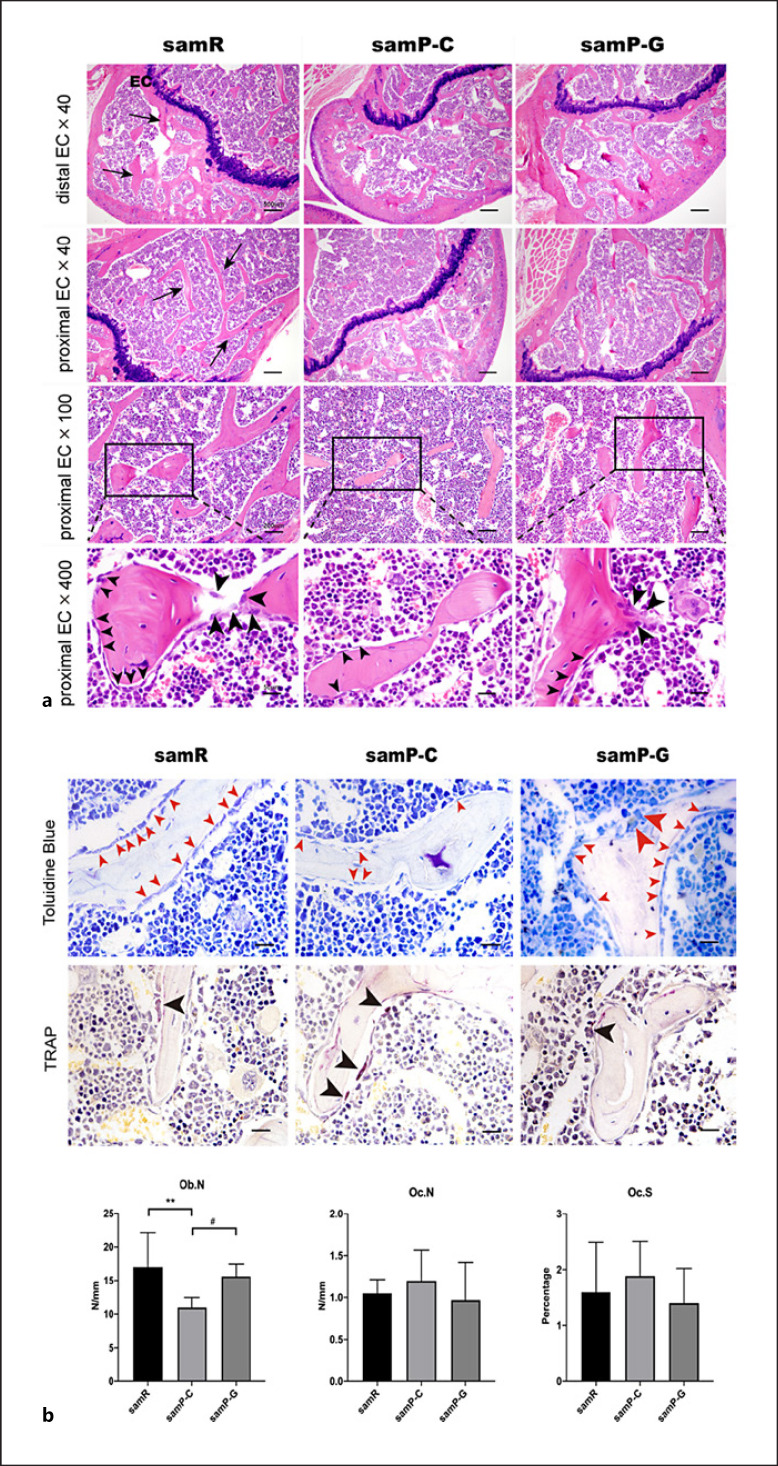
Histological analysis of femoral metaphysis was evaluated by hematoxylin and eosin (H&E), toluidine blue, and TRAP staining, as shown in Figure 4. Consistent with the results of the micro-CT, the cancellous bone volume of the distal femora of SAMP6 mice was significantly decreased, relative to that of the SAMR1 group. Decreased cancellous bone volume was due to a reduction in Tb.N and a lower Tb.Th. Meanwhile, administration of GLP-2 attenuated the bone loss of the distal femora (Fig. 4a).
Fig. 4.
a Histological evaluation of GLP-2 treatment on the proximal femur in different groups using H&E staining. The above two rows presented the changes of bone trabecula (black arrows) in distal and proximal epiphysis, respectively. The last row was the enlarged view of the rectangles in the third row showed that the osteoblasts rimmed around the surface of trabeculae (arrowheads). The bigger arrowheads indicate the active osteoblasts, small arrowheads indicate quiescent osteoblasts. n = 6 for samR and n = 8 per group for samP-C and samP-G mice. EC, epiphyseal cartilage; samR, SAMR1 mice; samP-C, SAMP6 treated with normal saline; samP-G, SAMP6 treated with GLP-2. b Representative toluidine blue and tartrate-resistant acid phosphatase (TRAP) staining images of femora in different groups. Toluidine blue and TRAP-positive staining, respectively, indicate osteoblast (red arrowheads) and osteoclast (black arrowheads). The bigger red arrowheads indicate the active osteoblasts. Quantitative analysis of osteoblast number per trabecular perimeter (Ob. N), osteoclast number per trabecular perimeter (Oc. N), and the osteoclast surface relative to the bone surface (Oc.S) in the femoral bones of each group mice under 400× visual field. Data are expressed as mean ± SD (n = 6 for samR and n = 8 per group for samP-C and samP-G mice). Statistically significant difference: *p < 0.05, **p < 0.01 versus the samR group, #p < 0.05 versus the samP-G group. Scale bar: 50 μm. samR, SAMR1 mice; samP-C, SAMP6 treated with normal saline; samP-G, SAMP6 treated with GLP-2.
H&E staining of the SAMP6 group showed a reduction in the number of osteoblasts as compared with that of the SAMR1 control group, and GLP-2 treatment increased the osteoblast number in SAMP6 mice. In particular, active columnar osteoblasts rimmed around the surface of trabeculae (lager black arrows in Fig. 4a), from which cellular processes extend through the developing bone. Histomorphometry based on toluidine blue (osteoblast-specific staining) consistently demonstrated that samP-G group had a 29.8% increase in the number of osteoblast/trabecular perimeter as compared with SAMR1 mice (Fig. 4b). TRAP staining (osteoclast-specific staining) of distal femora showed that, in all sections of trabecular bone area, TRAP-positive osteoclast number, and osteoclast surface per bone surface displayed no significant change among the three groups (Fig. 4b).
These data suggest that senile SAMP6 mice exhibited low bone turnover with decreased bone formation at a more remarkable level than increased bone resorption. GLP-2 exerts a protective effect against SAMP6-induced senile osteoporosis.
GLP-2 Affected the Expression of Genes Related to Bone Formation and Bone Resorption
Figure 5 shows the mRNA levels related to osteoblast markers, including ALP, Col-1, Runx2, and OCN by qRT-RCR. The expression of ALP and Col-1 was clearly downregulated in SAMP6 mice compared to SAMR1 mice, while the other two markers showed no statistical significance. The downregulated levels of early markers of bone formation encompassing ALP, Col-1, and Runx2 were markedly increased after 6 weeks of treatment with GLP-2. The effect of GLP-2 on increasing the late marker bone formation OCN noted no statistically significant difference.
Fig. 5.
a ALP, Col-1, Runx2, and OCN mRNA expressions in femora of samR, samP-C, and samP-G mice. Total RNA was isolated and qRT-PCR was performed to determine mRNA expressions, which were normalized to that of β-actin. Values are expressed as mean ± SD (n = 6 for samR; n = 8 for samP-C, samP-G mice per group); *p < 0.05, #p < 0.05 compared with samP-C mice. b OPG, RANKL, NFATC1, and c-fos mRNA expressions and ratio of OPG to RANKL in the femora of samR, samP-C, and samP-G mice. Total RNA was isolated and qRT-PCR was performed to determine mRNA expressions, which were normalized to that of β-actin. Values are expressed as mean ± SD (n = 6 for samR; n = 8 for samP-C, samP-G mice per group); *p < 0.05, **p < 0.01 and #p < 0.05, ##p < 0.01 compared with samP-C mice. ALP, alkaline phosphatase; Col-1, type 1 collagen; Runx2, runt-related transcription factor 2; OCN, osteocalcin; OPG, osteoprotegerin; RANKL, receptor activator for nuclear factor-κB ligand; NFATC1, nuclear factor of activated T cells 1; samR, SAMR1 mice; samP-C, SAMP6 treated with normal saline; samP-G, SAMP6 treated with GLP-2.
In SAMP6 mice, mRNA expression of RANKL, the essential cue for osteoclast differentiation, was significantly increased compared with the SAMR1 group. The expression of OPG and OPG/RANKL showed no significant difference between the two groups. However, GLP-2 significantly decreased RANKL mRNA expression and increased OPG mRNA expression, thereby resulting in a notable upregulation of the OPG/RANKL ratio in SAMP6 mice. However, GLP-2 had no effect on the expression of osteoclast-specific differentiation genes Nfatc1 and c-fos (Fig. 5).
Effect of GLP-2 on Serum Inflammation Cytokines
To explore the mechanism underlying GLP-2 in osteoporosis prevention, we then examined the serum inflammation markers in each group. As shown in Figure 6, no statistical difference was detected in the serum level of IFN-γ between the SAMP6 and SAMR1 groups, while the levels of inflammation cytokines including TNF-α, IL-6, and IL-1β were markedly increased in SAMP6 mice relative to SAMR1 mice. Six weeks of treatment of GLP-2 notably decreased the TNF-α level, while no improvement was observed in the IFN-γ, IL-6, and IL-1β.
Fig. 6.
Effects of 6 weeks of treatment with GLP-2 on inflammation markers. TNF-α, tumor necrosis factor α; IL-6, interleukin-6; IL-1β, interleukin-1β; IFN-γ, interferon-γ. Data are depicted in terms of mean ± SD n = 5 for samR; n = 6 for samP-C, samP-G mice per group. Statistically significant difference: *p < 0.05, **p < 0.01 versus the samR group, #p < 0.05 versus the samP-G group. samR, SAMR1 mice; samP-C, SAMP6 treated with normal saline; samP-G, SAMP6 treated with GLP-2.
GLP-2 Upregulated Expression of Intestinal Epithelial Tight Junction Proteins in SAMP6 Mice
The tight junction (TJ) proteins zonula occludens 1 (ZO-1) and occludin were analyzed in intestinal sections in order to detect the integrity of the epithelium (Fig. 7). In the SAMR1 group, the expression of ZO-1 was observed to be located in the apical junction complex, which was both in the villi border and at the colonal glands. Occludin was primarily expressed in intercellular junctional molecules on opposing epithelial cells in the ileum and colon. However, such stains were substantially weak or absent in SAMP6 mice, suggesting a low percentage of TJ protein expression. GLP-2 supplementation effectively sustained the basolateral and partial apical staining of ZO-1 and occludin in the SAMP6 ileum, except for the occludin signal in the colon sections. The data suggest the application of GLP-2 led to improvement in the percentage of TJ protein expression and helped maintain the integrity of the intestinal barrier.
Fig. 7.
Immunohistochemical detection of zonula occludens 1 (ZO-1) and occludin and its mean density in sections of ileum and colon in each group. Data are expressed as mean ± SD (n = 6 for samR; n = 8 for samP-C, samP-G mice per group). Statistically significant difference: *p < 0.05, **p < 0.01, ***p < 0.001 versus the samR group, #p < 0.05, ##p < 0.01 versus the samP-G group. Scale bar: 20 μm for each group. samR, SAMR1 mice; samP-C, SAMP6 treated with normal saline; samP-G, SAMP6 treated with GLP-2.
GLP-2 Improved TJ Structure of Jejunum and Ileum in SAMP6 Mice by TEM
Furthermore, TEM was performed to identify the ultrastructural changes in intercellular TJs (Fig. 8). In the SAMR1 group, closely arranged rows of epithelial cells, regularly aligned microvilli in the intestinal epithelium, integrated mitochondria, and rough endoplasmic reticulum were observed. A series of junctional complexes, including TJ, adherens junction, and desmosome between epithelial cells with narrow paracellular space, were intact and clear. In SAMP6 mice, intestinal epithelial cells were shrunken. The widening of the paracellular spaces was attributed to obscured TJ strand, widened AJ, and decreased amount of desmosomes. In addition, the amount of microvilli was decreased with irregular length and arrangement. The mitochondria were swollen and cracked with vacuolated cristae. GLP-2 treatment improved disrupted junctional complexes concerning structural integration and closeness of intercellular connection.
Fig. 8.
Representative photographs of transmission electron microscope of intestinal mucosa from each group. samR, SAMR1 mice; samP-C, SAMP6 treated with normal saline; samP-G, SAMP6 treated with GLP-2. n = 4 per group. Thick arrows: tight junctions. Arrow heads: adherence junctions. De, desmosome; Asterisks, microvilli; Mt, mitochondria; ER, endoplasmic reticulum. Scale bar: 500 nm.
GLP-2 Increased the Expression of GLP-2R in the Ileum and Colon, Enhanced VDR Expression in the Ileum but Not in the Colon
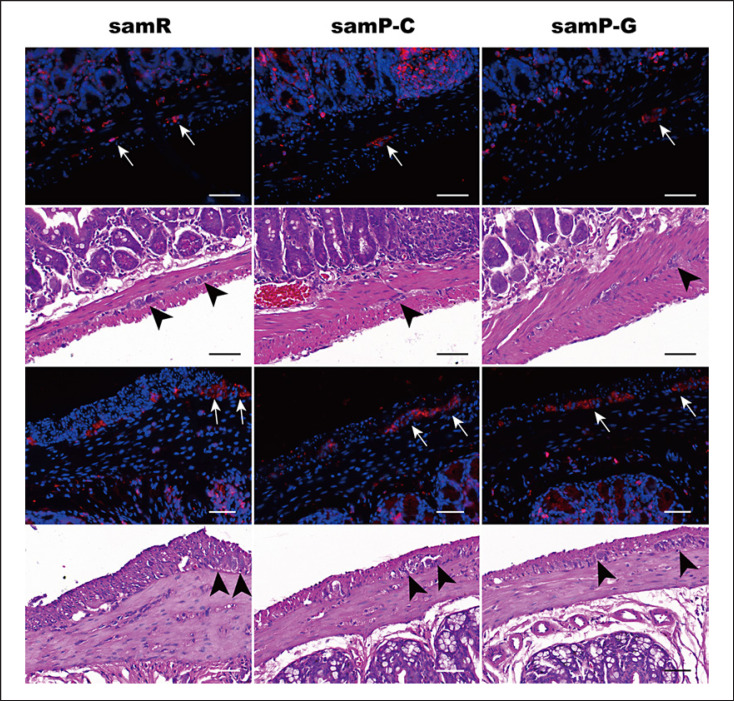
Both GLP-2R and VDR are involved in the intestinal epithelial barrier function and regulation of bone metabolism. Therefore, the expression in intestines was investigated under immunofluorescence. Figures 9 and 10 show that in the SAMR1 group, the GLP-2R was localized on the surface of apical villi of ileal mucosal epithelium and in the myenteric plexus. In the colon, it was expressed in the myenteric plexus, whereas the signal intensity in enterocytes was significantly decreased. Meanwhile, GLP-2R expression is more pronounced in the ileum than in the colon. Negative controls were shown in the muscularis and submucosal vascular smooth muscle (online suppl. Fig. 1). VDR resided in the epithelial nuclei of the mucosa and glandular ducts. However, signals of both GLP-2R and VDR in the ileum were significantly reduced in SAMP6 mice, an effect which was improved following GLP-2 administration. However, no significant difference in VDR quantitation was found in the colon (online suppl. Fig. 2).
Fig. 9.
Immunofluorescence analysis of the effect of GLP-2 administration on expression of receptors of GLP-2 (GLP-2R) and vitamin D (VDR) in the ileum in SAMP6 mice. samR as the control group. GLP-2R (red), VDR stain (green), DAPI (blue), and merged images are presented. Arrows indicate mucosal epithelium, arrowheads indicate glandular epithelium. In samP-C group, the red fluorescence line fractured and disappeared, the green fluorescence was weakened. Data are expressed as mean ± SD (n = 3 for samR, n = 5 for samP-C and n = 3 for samP-G). Statistically significant difference: *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 versus the samR group, #p < 0.05, ##p < 0.01 versus the samP-G group. Scale bar: 20 μm for each group. samR, SAMR1 mice; samP-C, SAMP6 treated with normal saline; samP-G, SAMP6 treated with GLP-2.
Fig. 10.
Immunofluorescence and corresponding hematoxylin and eosin staining map the GLP-2 receptor (GLP-2R) locations in the three groups of myenteric plexus of the ileum (top two rows) and the colon (bottom two rows). Arrowheads show cells that stain positive for GLP-2R. Black arrows indicate myenteric plexus. GLP-2R (red) and DAPI (blue) of merged immunofluorescence images are presented. Scale bar: 50 μm for each group. samR, SAMR1 mice; samP-C, SAMP6 treated with normal saline; samP-G, SAMP6 treated with GLP-2.
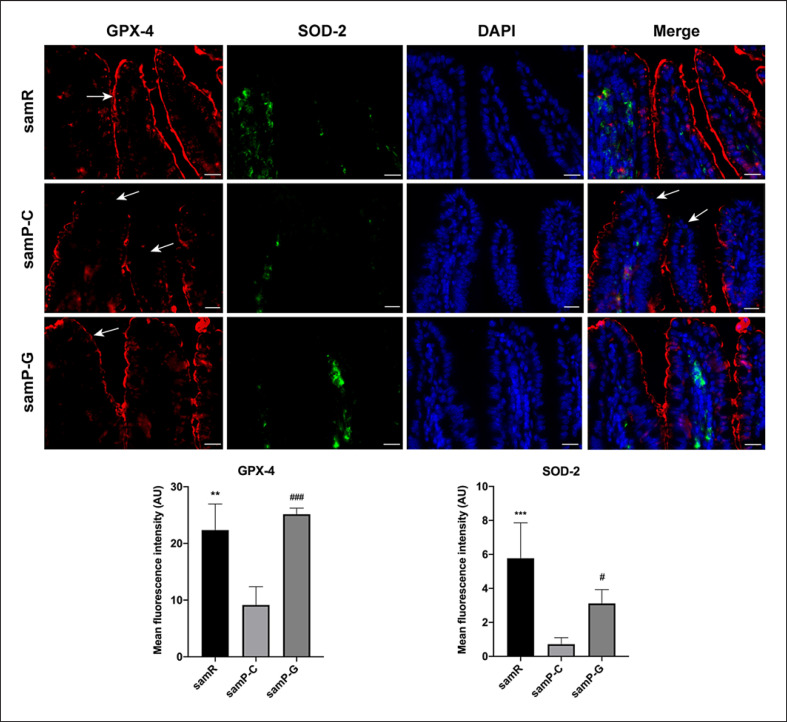
GLP-2 Enhanced GPX-4 and SOD-2 Signaling in the Ileum and Colon
Since oxidative stress has been implicated in senile osteoporosis and the close relationship between gut and bone, changes in markers of oxidative stress in intestinal tissue were detected using immunofluorescence. GPX-4 and SOD-2 are two main antioxidant enzymes that scavenge oxidant compounds and thereby protect cells against lipid peroxidation damage. As shown in Figure 11, GPX-4 appeared as continuous bands along the apical villi and was distributed partially in the epithelial nuclei in the SAMR1 ileum. It tended to reside more pronouncedly in the nuclei of the colon. SOD was mainly located in the lamina propria in both the ileum and colon. However, in SAMP6 mice, the distribution of GPX-4 in the ileum was utterly disrupted. The signals of SOD-2 were remarkedly reduced in the intestine tissue of the SAMP6 group. Treatment of GLP-2 could primarily ameliorate the destruction and enhance the intensity of staining of intestinal GPX-4 and SOD-2 in the ileum (Fig. 11) and colon (online suppl. Fig. 3).
Fig. 11.
Representative images of GPX-4 and SOD-2 expression in the ileum among different groups by immunofluorescence analysis. Arrows indicate sites of staining. Data are expressed as mean ± SD (n = 3 for samR, n = 5 for samP-C and n = 3 for samP-G). Statistically significant difference: *p < 0.05, **p < 0.01, ***p < 0.001 versus the samR group, #p < 0.05, ##p < 0.01, ###p < 0.001 versus the samP-G group. Scale bar: 50 μm for each group. GPX-4, glutathione peroxidase 4; SOD-2, superoxide dismutase 2; samR, SAMR1 mice; samP-C, SAMP6 treated with normal saline; samP-G, SAMP6 treated with GLP-2.
Discussion
Senile osteoporosis, an age-related degenerative disease, is characterized by a low bone turnover state, principally with decreased bone formation. The SAMP6 strain mice with senile osteoporosis, in our present study, exhibited an impaired bone loss as compared to the control age-matched SAMR1 mice. We found for the first time that exogenous administration of GLP-2 had a beneficial effect on the senile osteoporosis model, as evidenced by increased BMD, improved microarchitecture in the femora, and enhanced osteogenic activities. Such effects function by accelerating the functional properties of osteoblasts rather than by the inhibition of osteoclastic activity. Improved epithelial barrier dysfunction in line with remission of inflammation state, elevated receptors of GLP-2 and vitamin D in the ileum and colon, as well as amelioration of oxidative stress by enhancing GPX-4 and SOD-2 activities in the gut from GLP-2 may play a potential role contributing to improving bone formation.
The gut-derived peptide hormones could affect bone remodeling either directly or indirectly and vice versa. This integrative system between the crosstalk in the gastrointestinal tract and those involved in bone was termed the entero-osseous axis. The entero-bone endocrine axis has gradually been recognized as an important regulator in the pathogenesis of osteoporosis [30, 38]. Therefore, the potential mechanism by which GLP-2 exerts bone-sparing effect via the entero-bone axis was investigated.
GLP-2 is a naturally occurring peptide mainly co-secreted with GLP-1 by intestinal enteroendocrine L-cells and neurons in the brainstem projected to the hypothalamus discretely [8]. Its analog teduglutide has been approved for the treatment of SBS since 2012 due to the reparative action for the gut, evidenced by cytoprotection and regeneration of the epithelial surface, upregulation of intestinal blood flow, and maintenance of intestinal integrity and barrier function. The hormone acts directly via the GLP-2 receptors, which are predominantly present in the gastrointestinal tract. The distribution of GLP-2R in intestinal tracts remains controversial in different reports. The challenges are derived from the accurate detection methods, specificity of reagents (predominantly antisera), and species-specific differences in GLP-2R expression [9, 22, 39, 40]. The expression discrepancy occurred when an antibody was directed against the N- or C-terminal terminus of the GLP-2R [39, 41, 42], even though research has used C-terminal-specific antibodies [41, 42]. SAM strains are interrelated recombinant inbred mice and carry their own gene mutations, causing age-related pathological phenotypes unique to each strain. Since there are no reports on detecting GLP-2R expression in SAM strains, a fair comparison with other studies is needed. A suitable validated antibody is required in further work. GLP-2R expression is more pronounced in the ileum than in the colon. This expression pattern is consistent with previous reports of greatest intense immunostaining in the proximal bowel [42, 43].
Receptors can be constantly metabolized in a state of dynamic equilibrium, and their quantity, affinity, and potency are modulated by a multitude of pharmacological and pathophysiological factors. In the present study, we found increased GLP-2R expression in the epithelium of the ileum and colon in the group injected with exogenous GLP-2. Consistent with our result, infusion of GLP-2 leads to a 3-fold increase in GLP-2R expression in the ileum of resected rats compared to the controls given saline. A study suggests that GLP-2R expression only undergoes a significant upregulation at the higher plasma GLP-2 levels [44]. Similarly, a single injection of the [Gly2]pGLP-2 microspheres significantly increased colonic GLP-2R mRNA expression in DSS-induced colitis mice [45]. Another study observed increased GLP-2R gene expression in the intestines of mice with acute graft-versus-host disease treated with teduglutide compared with the vehicle group [46]. The mechanism of receptor upregulation has not been determined. The expression of the receptor subunit-encoding genes would be expected to cause the upregulation of GLP-2R. It is well known that the action of GLP-2 is initiated by binding to GLP-2R. In our present study, SAMP6 mice showed decreased GLP-2R signaling, which limited the therapeutic effects of endogenous GLP-2. GLP-2 substitution engages in the upregulation to increase the number of receptors of target cells. The process allows cells to be more sensitive to the hormone, which enhances the therapeutic effects of GLP-2 [45]. In addition, except for acting as a trophic factor regulating intestinal epithelial proliferation, GLP-2 increases the number and proportion of neurons expressing VIP within the colonic submucosal plexus [47, 48]. GLP-2 stimulates the proliferation of primary colonic cancer-derived myofibroblasts [49]. They promoted regeneration of enteric neurons, and subepithelial myofibroblast on which GLP-2R is located specifically may suggest new receptor synthesis. It is contrasted with our previous finding that there is no increase in GLP-2R expression neither in young rats nor in old rats by GLP-2 treatment [37]. Another study reported that GLP-2 injection reduced the expression of GLP-2R mRNA and GLP-2R-positive cells in the ileum, while did not affect the GLP-2R in the duodenum in LPS-induced piglets [50]. The short-term duration of treatment and diverse animal species may contribute to the deviation. Likewise, the controversial location reported in different animals may provide another interpretation that makes the difference.
Consistent with previous research works, GLP-2 produces a substantial reduction in bone resorption, while the effect was inhibited in colectomized patients with jejunostomy, indicating intact bowel anatomy is essential for GLP-2-mediated reduction in bone resorption [32]. It is therefore concluded that GLP-2 originating from the gastrointestinal tract exerts protection of osteoporosis through intestinal feedback via GLP-2R signaling pathway activation. This integrative system denotes the direct effect of entero-osseous axis (Fig. 12).
Fig. 12.
Model representing the GLP-2 role on ameliorating senile osteoporosis may be through regulation of entero-bone axis. VDR, receptors of vitamin D.
The apical cell membrane of the enterocytes which accompanies intestinal TJ complexes that exist between epithelium serves as an essential structure for the integrity of the intestinal barrier [51]. ZO-1 and occludin are major components of TJ, which are implicated in maintaining the barrier function and modulating TJ permeability [52, 53].
Compromises in intestinal epithelial gate and fence functions are common in senile osteoporosis [54, 55]. Consistently, we observed a lower density of ZO-1 protein expression distributed in the ileum and colonic mucosa in SAMP6 mice by immunohistochemical analysis. The lack of change in occludin level in the colon among each group may be due to low TJ structure in the colon physiologically. Disrupted series junctional complex structures alone with other degenerative manifestations such as swollen mitochondria with cracked and vacuolated cristae were also visualized under TEM examination in SAMP6. However, GLP-2 treatment partially improved disrupted junctional complexes concerning structural integration and closeness of intercellular connection.
Intestinal barrier and inflammatory state are two conditions which could affect each other. Intestinal barrier failure may lead to systemic inflammation in patients with severe injury [56] and Crohn's disease [57]. Referring to the pivotal role of inflammatory cytokines on the regulation of gastrointestinal barrier function and permeability [58], it is reported that TNF-α had a dose-dependent increasing effect on the permeability of the epithelium [59], downregulating the expression of TJ proteins, and disrupting TJ structure in vivo and in vitro [60, 61].
Senile osteoporosis is a model of the central role of pro-inflammation in determining bone resorption [62]. A longitudinal study checked inflammatory markers at baseline and their changes at 2.9-year follow-up. An association was found between them and bone loss/resorption in older adults. Results showed that total body BMD change was associated with baseline CRP, IL-6, and TNF-α, as well as change in CRP and IL-6. Meanwhile, IL-6 predicted the change of hip and spinal BMD sites, TNF-α predicted spinal BMD changes [63]. In healthy elderly individuals, higher CRP serum levels are associated with a higher bone turnover rate, which results in lower bone mass [64]. During senescence, the elevated inflammatory cytokines, including IL-6, TNF-α, and IL-1, induce osteoclast activation and increased catabolic signals driven by inflamm-aging, which could cause age-related osteoporosis [65, 66]. Inflamm-aging itself has been implicated in bone remodeling through pro-inflammatory cytokines [67], which disturb the balance of the OPG/RANK/RANKL system. Modulation of inflammatory response may become a new target for targeting the design of a therapeutic approach to prevent bone loss during senescence [62]. Currently, anti-TNF-α treatment shows a distinct effect concerning hip and lumbar spine BMD and vertebral fractures in different patients [68, 69, 70, 71, 72, 73, 74]. Among older patients using medium to high doses of the anti-inflammatory agent, alendronate treatment for a median duration of 2.9 years was associated with a significantly lower risk of hip fracture [75]. In the present study, the excess secretion of inflammatory cytokines, such as TNF-α, was corrected by GLP-2.
Therefore, the improvement in bone loss by GLP-2 may be attributed to the partial amelioration of intestinal barrier function in concert with inflammatory responses. It suggested a mechanism mediating GLP-2 anabolic effects on bone mass through an enteroendocrine-osseous axis may be via the intestinal barrier (Fig. 12).
In addition, oxidative stress, defined as the excess of reactive oxygen species (ROS) alongside low antioxidant status, plays a pivotal role in the etiology of many chronic diseases including senile osteoporosis [76, 77]. Antioxidant therapy has been investigated as a potential therapeutic in the prevention of osteoporosis [78, 79]. Antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), are principal biomarkers of antioxidant systems in the body [80]. In our present study, GPX-4 and SOD antioxidant activity was significantly lower in the gut in SAMP6 mice, which was consistent with previous research works [76]. The mechanism of intestinal antioxidant affecting bone formation is poorly understood. Because ROS are important signaling molecules at physiological levels, we speculate excessive intestinal ROS accumulation might have systemic effects [81]. Meanwhile, inflammation could trigger ROS production by immune cells [82], leading to oxidation of other organs, such as the bone. Improvement of antioxidant activity in the intestine may therefore show a positive effect on bone directly or indirectly by altering the gut microbial profile or through modulation of immunity [81, 83]. Otherwise, the accumulation of oxidative stress decreases epithelial barrier function by compromising the molecular fence function of the TJs. GLP-2 improves antioxidant activity by increasing SOD-2 and GPX-4 levels in intestinal tissue, implicating its role in repressing oxidative stress indirectly. Therefore, the protection of osteoporosis mediated by GLP-2 may function by regulating antioxidant activity and improving intestinal impairment induced by concurrent oxidative stress. The relationship between oxidative stress, intestinal integrity, and inflammation in osteoporosis suggests that targeting intestinal barrier function may constitute an important route for therapy. Taken together, it may provide another way of understanding mechanisms of action of GLP-2 in relation to bone homeostasis.
Malabsorption of calcium is quite common in elderly patients, accompanied by a reduction in the level of VDR [84]. The genomic actions involving classical VDR participant in mediating the intestinal Ca2+ transport are regulated by 1α,25(OH)2D3. 1α,25(OH)2D3-VDR binding is reduced in old avian and mammalian intestinal cells [84]. This is corroborated by our results that the signal of VDR in the ileum was significantly attenuated in aged SAMP6 mice. The VDR expression was increased in the ileum, a major calcium absorption site, following GLP-2 administration.
Previous studies showed that a decrease in the amount of intestinal VDR with age leads to a decreased responsiveness of intestinal cells to 1α,25(OH)2D [85]. VDR polymorphism may independently determine the risk of diminished BMD [86]. Meanwhile, transgenic expression of the human VDR in the duodenum of VDR-null mice attenuates the age-dependent decline in calcium absorption [87]. The results were in concert with our data that the increased intestinal VDR could be beneficial to bone metabolism to alleviate osteoporosis.
In addition, other than the direct role in Ca2+ endocrinology or bone formation, 1α,25(OH)2D3-VDR-RXR complex has essential consequences for intestinal cell proliferation [84]. Therefore, the correction of intestinal VDR may promote bone health either through direct or indirect mechanisms in which entero-bone endocrine axis plays a salient role.
Micro-CT provides accurate measures of bone microarchitecture and phenotypic data related to cortical and trabecular morphology; thus, the pathology of osteoporosis and the effects of treatments can be well evaluated. In line with other research, nearly 8-month-old SAMP6 mice in our study presented deterioration of trabecular bone structure and bone volume in the femur, including decreased BMD, BV/TV, BS/TV, Th.N, Tb.Th and increased Tb.Sp. BV/TV and BS/TV are direct and indirect parameters representing bone volume. The indexes of Th.N, Tb.Th, and Tb.Sp reflect the spatial morphology and architecture of bone trabeculae, which is correspondent with pathological findings in bone histology. All the reduction indicated an imbalance of bone resorption and formation, resulting in bone loss and osteoporosis. Our data demonstrated that GLP-2 clearly increased BMD and prevented loss of trabecular volume. The results were consistently confirmed under the pathological examination.
However, with regards to the cortical bone, although BV/TV and Ct.Th were decreased in SAMP6, cortical BMD did not differ significantly compared to SAMR1 mice of the same age, which is consistent with previous research [88]. It is known that cortex changes come later than that of trabeculae. It was found that peak total bone mass of SAMP6 mice was observed at about 4–5 months of age and then gradually decreased with age [34]. The difference in BMD change in the femoral cortex in these 8-month SAMP6 mice has not yet been presented as compared to SAMR1 mice.
Similar to direct histomorphometric observations in mice, the improvement of bone mass mediated by GLP-2 included biochemical markers, histology, and expressions of genes related to bone metabolism. All these actions were independent of the promotion of osteogenesis.
Our study detected no evident elevated levels of serum CTX-1, which seems contradictory to the deterioration of trabecular bone structure measurements causing bone breakdown. Three possibilities may explain this finding. SAMP6 is a model of senile osteoporosis which exhibits a low bone turnover state with remarkably decreased bone formation. The substantial reduction of ALP is concomitantly paralleled with decreased CTX-1 level as bone formation and bone resorption are coupled. In addition, as confirmed by corresponding BMD examination, cortical bones initially increase in width, thinning and delamination, then loosen, and eventually become threadlike during the process of osteoporosis. As viewed over time, the changes in the bone cortex are not in the initial stage due to the reduction value of Ct.Th in our study. It may be in a stable period of bone loss. Therefore, no remarkable difference in CTX-1 was found. It may be part of the explanation that we did not find an ameliorating effect of GLP-2 on CTX-1 in SAMP6 mice. However, the results are inconsistent with previous studies that GLP-2 can reduce CTX-1 but has no significant increases in levels of OCN or PINP in human postmenopausal women [11, 13, 14]. Several interpretations allow for this difference. Since the formation process of new bone matrix usually lasts for at least 4–6 weeks, it seems unlikely that the increased rate of bone formation has not yet occurred in human studies [10]. Role of GLP-2 in other rodent models for osteoporosis, so far, has only been reported in a single study, where it exerts a positive impact on seven-month ovariectomized rats by promoting bone formation and inhibiting bone resorption [16]. Estrogen withdrawal induced by menopause or oophorectomy results in high bone turnover with increased bone formation and resorption. The distinct etiology and pathophysiological characteristics between postmenopausal osteoporosis and senile osteoporosis may contribute to inconsistent action derived from GLP-2 [4]. Last but not least, the effect of GLP-2 may differ between humans and mice and between mice and rats [89].
Histology analysis visually revealed that GLP-2 increased amounts of trabecula and osteoblast, as detected by H&E and toluidine blue staining. The osteoblastic responses were corroborated in parallel by increased serum ALP activity and enhanced expression levels of osteogenesis-related genes including ALP, RUNX2, Col-1, and OCN. In contrast, the increased gene expression of OCN in the GLP-2 group did not reach statistical significance. ALP serves as a biochemical marker of osteoblast differentiation and facilitates osteoid mineralization [90]. Together with RUNX2 and Col-1, these represent the early bone turnover biomarkers for assessing bone formation rate. OCN, in contrast, is produced by mature osteoblasts during bone formation [91]. Principally, OCN forms in the bone matrix and only a small amount is secreted into circulation, which may be attributed to no difference in serum among groups.
Osteoclastogenesis is initiated by recognition of RANK in osteoclast precursor cells via binding to RANKL expressed on the osteoblast surface. Osteoblasts also produce OPG, a RANKL decoy receptor that inhibits osteoclast differentiation and functions by interrupting RANKL-RANK interaction. The modulation of osteoclastogenesis is determined by the ratio of OPG/RANKL expression [92]. In SAMP6 mice, although the mRNA level of RANKL increased, the ratio of OPG/RANKL showed no significant difference compared to SAMR1 mice. Therefore, no obvious changes were found in master transcription factor for RANKL-induced osteoclastogenesis of NFATC1 and c-fos mRNA expression level and the number of osteoclasts. Meanwhile, GLP-2 may suppress osteoclastic activity as exhibited by the ratio of OPG/RANKL mRNA expression while not affecting the genes of osteoclast-specific differentiation of NFATC1 and c-fos, which may be explained by the phenomenon that the amount of mRNA of OPG/RANKL may not be directly correlated with corresponding effective protein production [93]. Moreover, the process of osteoclast differentiation depends on RANKL-RANK signaling, which is temporally regulated by various adapter proteins and kinases. Any factor affecting signaling complexes that facilitates the regulation of signal transduction will result in the change of NFATC1 and c-fos mRNA expression [94]. It is corresponding with the results of no significant improvement in phenotypic traits of number of osteoclasts [95]. Based on the above findings, GLP-2 may play a role in promoting osteoblast formation instead of osteoclastogenic activity inhibition.
We acknowledge the following limitations in our study. First, oxidative stress occurs from the accumulation of oxidant radicals (reactive oxygen and nitrogen species, ROS and RNS) coupled with weakened antioxidant compounds. Since the half-life of unstable ROS is very short and difficult to detect, its end products or by-products of oxidizing substrates (nucleic acids, lipids, and proteins) are used in research to reflect the production of ROS indirectly and used as markers of oxidative stress. Although it is well described that senile osteoporosis shows a marked increase in ROS formation, we detect antioxidant compounds instead of directing ROS change in the gut in this research. We think biomarkers of ROS-induced peroxidation products will provide more robust evidence of alleviation of oxidative stress in the gut from GLP-2. Second, although our previous study evaluated serum GLP-2 level was lower in the old rats than in the young group (0.995 ± 0.341 vs. 0.544 ± 0.146 ng/mL, p < 0.01), presenting a physiological level change of GLP-2 in SAMP6 and SAMR1 mice would be valuable in our future work. Although the BMD measured reflects 70% of bone strength and the determination of mechanical properties of bone can be improved by combining the results of the 3D bone microarchitecture and its histomorphology as well as histological examinations in the present study, improvement of mechanical testing applied in future study will better test the effect of GLP-2 on bone strength.
Conclusion
For the first time, our results demonstrated that GLP-2 could ameliorate bone loss in senile osteoporosis of the SAMP6 model by accelerating osteoanabolic action rather than by inhibiting osteoclast activity. Amelioration of TJ structure and improved antioxidant enzyme activity in the gut from GLP-2 may potentially improve bone formation, which may provide evidence for targeting the entero-bone axis in the treatment of senile osteoporosis.
Statement of Ethics
All experiments and animal care procedures were approved by the Zhongshan Hospital Fudan University Animal Care and Use Committee (Shanghai, China). The approval number of the Ethics Committee is ZS20150212032.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study was supported by a grant from the National Natural Science Foundation of China (Grant No. 81570795). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
Yan-Mei Huang: conceptualization, data curation, formal analysis, and writing − original draft. Bing'er Xu: methodology, data curation, formal analysis, and validation. Zheng Kuai: data curation and writing − review and editing. Yu-Ting He: methodology and data curation. Yi Lu: formal analysis and data curation. Ji-Ping Shen, Ke-Fen Wu, and Jia-Yu Wu: data curation. Wei-Ying Ren: conceptualization, data curation, and validation. Yu Hu: conceptualization, funding acquisition, and writing − review and editing.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Funding Statement
This study was supported by a grant from the National Natural Science Foundation of China (Grant No. 81570795). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Fragility fracture epidemiology Available from: https://www.osteoporosis.foundation/health-professionals/fragility-fractures/epidemiology (accessed March 20, 2021)
- 2.Heidari B, Muhammadi A, Javadian Y, Bijani A, Hosseini R, Babaei M. Associated factors of bone mineral density and osteoporosis in elderly males. Int J Endocrinol Metab. 2017;15((1)):e39662. doi: 10.5812/ijem.39662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bliuc D, Nguyen ND, Alarkawi D, Nguyen TV, Eisman JA, Center JR. Accelerated bone loss and increased post-fracture mortality in elderly women and men. Osteoporos Int. 2015;26((4)):1331–1339. doi: 10.1007/s00198-014-3014-9. [DOI] [PubMed] [Google Scholar]
- 4.Ma X, Meng J, Jia M, Bi L, Zhou Y, Wang Y, et al. Exendin-4 a glucagon-like peptide-1 receptor agonist prevents osteopenia by promoting bone formation and suppressing bone resorption in aged ovariectomized rats. J Bone Miner Res. 2013;28((7)):1641–1652. doi: 10.1002/jbmr.1898. [DOI] [PubMed] [Google Scholar]
- 5.Rauner M, Sipos W, Pietschmann P. Age-dependent Wnt gene expression in bone and during the course of osteoblast differentiation. Age. 2008;30((4)):273–282. doi: 10.1007/s11357-008-9069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chao D, Qiang S. Research progress on relevant problems related to senile osteoporosis. Chin J Osteoporos. 2016;22((3)):372–375. [Google Scholar]
- 7.Appelman-Dijkstra NM, Papapoulos SE. Modulating bone resorption and bone formation in opposite directions in the treatment of postmenopausal osteoporosis. Drugs. 2015;75((10)):1049–1058. doi: 10.1007/s40265-015-0417-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amato A, Baldassano S, Mule F. GLP2 an underestimated signal for improving glycaemic control and insulin sensitivity. J Endocrinol. 2016;229((2)):R57–R66. doi: 10.1530/JOE-16-0035. [DOI] [PubMed] [Google Scholar]
- 9.Drucker DJ, Yusta B. Physiology and pharmacology of the enteroendocrine hormone glucagon-like peptide-2. Annu Rev Physiol. 2014;76((1)):561–583. doi: 10.1146/annurev-physiol-021113-170317. [DOI] [PubMed] [Google Scholar]
- 10.Haderslev KV, Jeppesen PB, Hartmann B, Thulesen J, Sorensen HA, Graff J, et al. Short-term administration of glucagon-like peptide-2. Effects on bone mineral density and markers of bone turnover in short-bowel patients with no colon. Scand J Gastroenterol. 2002;37((4)):392–398. doi: 10.1080/003655202317316006. [DOI] [PubMed] [Google Scholar]
- 11.Henriksen DB, Alexandersen P, Bjarnason NH, Vilsboll T, Hartmann B, Henriksen EE, et al. Role of gastrointestinal hormones in postprandial reduction of bone resorption. J Bone Miner Res. 2003;18((12)):2180–2189. doi: 10.1359/jbmr.2003.18.12.2180. [DOI] [PubMed] [Google Scholar]
- 12.Askov-Hansen C, Jeppesen PB, Lund P, Hartmann B, Holst JJ, Henriksen DB. Effect of glucagon-like peptide-2 exposure on bone resorption effectiveness of high concentration versus prolonged exposure. Regul Pept. 2013;181:4–8. doi: 10.1016/j.regpep.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Henriksen DB, Alexandersen P, Byrjalsen I, Hartmann B, Bone HG, Christiansen C, et al. Reduction of nocturnal rise in bone resorption by subcutaneous GLP-2. Bone. 2004;34((1)):140–147. doi: 10.1016/j.bone.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Henriksen DB, Alexandersen P, Hartmann B, Adrian CL, Byrjalsen I, Bone HG, et al. Disassociation of bone resorption and formation by GLP-2 a 14-day study in healthy postmenopausal women. Bone. 2007;40((3)):723–729. doi: 10.1016/j.bone.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 15.Henriksen DB, Alexandersen P, Hartmann B, Adrian CL, Byrjalsen I, Bone HG, et al. Four-month treatment with GLP-2 significantly increases hip BMD a randomized, placebo-controlled, dose-ranging study in postmenopausal women with low BMD. Bone. 2009;45((5)):833–842. doi: 10.1016/j.bone.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Xu B, He Y, Lu Y, Ren W, Shen J, Wu K, et al. Glucagon like peptide 2 has a positive impact on osteoporosis in ovariectomized rats. Life Sci. 2019;226:47–56. doi: 10.1016/j.lfs.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Schiellerup SP, Skov-Jeppesen K, Windelov JA, Svane MS, Holst JJ, Hartmann B, et al. Gut hormones and their effect on bone metabolism. Potential drug therapies in future osteoporosis treatment. Front Endocrinol. 2019;10:75. doi: 10.3389/fendo.2019.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skov-Jeppesen K, Svane MS, Martinussen C, Gabe MBN, Gasbjerg LS, Veedfald S, et al. GLP-2 and GIP exert separate effects on bone turnover a randomized, placebo-controlled, crossover study in healthy young men. Bone. 2019;125:178–185. doi: 10.1016/j.bone.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 19.Skov-Jeppesen K, Veedfald S, Madsbad S, Holst JJ, Rosenkilde MM, Hartmann B. Subcutaneous GIP and GLP-2 inhibit nightly bone resorption in postmenopausal women a preliminary study. Bone. 2021;152:116065. doi: 10.1016/j.bone.2021.116065. [DOI] [PubMed] [Google Scholar]
- 20.Gabe MBN, Skov-Jeppesen K, Gasbjerg LS, Schiellerup SP, Martinussen C, Gadgaard S, et al. GIP and GLP-2 together improve bone turnover in humans supporting GIPR-GLP-2R co-agonists as future osteoporosis treatment. Pharmacol Res. 2022;176:106058. doi: 10.1016/j.phrs.2022.106058. [DOI] [PubMed] [Google Scholar]
- 21.Skov-Jeppesen K, Hepp N, Oeke J, Hansen MS, Jafari A, Svane MS, et al. The antiresorptive effect of GIP but not GLP-2 is preserved in patients with hypoparathyroidism-a randomized crossover study. J Bone Miner Res. 2021;36((8)):1448–1458. doi: 10.1002/jbmr.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yusta B, Matthews D, Koehler JA, Pujadas G, Kaur KD, Drucker DJ. Localization of glucagon-like peptide-2 receptor expression in the mouse. Endocrinology. 2019;160((8)):1950–1963. doi: 10.1210/en.2019-00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrow NM, Hanson AA, Mulvihill EE. Distinct identity of GLP-1R, GLP-2R, and GIPR expressing cells and signaling circuits within the gastrointestinal tract. Front Cell Dev Biol. 2021;9:703966. doi: 10.3389/fcell.2021.703966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Georgios K, Dimitriadis ADM. Glucagon Like Peptide 2 (GLP-2) 2018 [Google Scholar]
- 25.Ramsey W, Isales CM. Intestinal incretins and the regulation of bone physiology. Adv Exp Med Biol. 2017;1033:13–33. doi: 10.1007/978-3-319-66653-2_2. [DOI] [PubMed] [Google Scholar]
- 26.Sjogren K, Engdahl C, Henning P, Lerner UH, Tremaroli V, Lagerquist MK, et al. The gut microbiota regulates bone mass in mice. J Bone Miner Res. 2012;27((6)):1357–1367. doi: 10.1002/jbmr.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li B, Liu M, Wang Y, Gong S, Yao W, Li W, et al. Puerarin improves the bone micro-environment to inhibit OVX-induced osteoporosis via modulating SCFAs released by the gut microbiota and repairing intestinal mucosal integrity. Biomed Pharmacother. 2020;132:110923. doi: 10.1016/j.biopha.2020.110923. [DOI] [PubMed] [Google Scholar]
- 28.Wu J, Ren W, Li L, Luo M, Xu K, Shen J, et al. Effect of aging and glucagon-like peptide 2 on intestinal microbiota in SD rats. Aging Dis. 2018;9((4)):566–577. doi: 10.14336/AD.2017.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montes Castillo MC, Martinez Ramirez MJ, Soriano Arroyo R, Prieto Gomez I, Segarra Robles AB, Garrido-Martinez M, et al. Glucagon-like peptide 1 and Glucagon-like peptide 2 in relation to osteoporosis in non-diabetic postmenopausal women. Sci Rep. 2019;9((1)):13651. doi: 10.1038/s41598-019-50117-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Helsted MM, Gasbjerg LS, Lanng AR, Bergmann NC, Stensen S, Hartmann B, et al. The role of endogenous GIP and GLP-1 in postprandial bone homeostasis. Bone. 2020;140:115553. doi: 10.1016/j.bone.2020.115553. [DOI] [PubMed] [Google Scholar]
- 31.Holst JJ, Hartmann B, Gottschalck IB, Jeppesen PB, Miholic J, Henriksen DB. Bone resorption is decreased postprandially by intestinal factors and glucagon-like peptide-2 is a possible candidate. Scand J Gastroenterol. 2007;42((7)):814–820. doi: 10.1080/00365520601137272. [DOI] [PubMed] [Google Scholar]
- 32.Gottschalck IB, Jeppesen PB, Holst JJ, Henriksen DB. Reduction in bone resorption by exogenous glucagon-like peptide-2 administration requires an intact gastrointestinal tract. Scand J Gastroenterol. 2008;43((8)):929–937. doi: 10.1080/00365520801965381. [DOI] [PubMed] [Google Scholar]
- 33.Gottschalck IB, Jeppesen PB, Hartmann B, Holst JJ, Henriksen DB. Effects of treatment with glucagon-like peptide-2 on bone resorption in colectomized patients with distal ileostomy or jejunostomy and short-bowel syndrome. Scand J Gastroenterol. 2008;43((11)):1304–1310. doi: 10.1080/00365520802200028. [DOI] [PubMed] [Google Scholar]
- 34.Kasai S, Shimizu M, Matsumura T, Okudaira S, Matsushita M, Tsuboyama T, et al. Consistency of low bone density across bone sites in SAMP6 laboratory mice. J Bone Miner Metab. 2004;22((3)):207–214. doi: 10.1007/s00774-003-0471-1. [DOI] [PubMed] [Google Scholar]
- 35.Matsushita M, Tsuboyama T, Kasai R, Okumura H, Yamamuro T, Higuchi K, et al. Age-related changes in bone mass in the senescence-accelerated mouse (SAM). SAM-R/3 and SAM-P/6 as new murine models for senile osteoporosis. Am J Pathol. 1986;125((2)):276–283. [PMC free article] [PubMed] [Google Scholar]
- 36.Mirsaidi A, Kleinhans KN, Rimann M, Tiaden AN, Stauber M, Rudolph KL, et al. Telomere length telomerase activity and osteogenic differentiation are maintained in adipose-derived stromal cells from senile osteoporotic SAMP6 mice. J Tissue Eng Regen Med. 2012;6((5)):378–390. doi: 10.1002/term.440. [DOI] [PubMed] [Google Scholar]
- 37.Ren W, Wu J, Li L, Lu Y, Shao Y, Qi Y, et al. Glucagon-like peptide-2 improve intestinal mucosal barrier function in aged rats. J Nutr Health Aging. 2018;22((6)):731–738. doi: 10.1007/s12603-018-1022-8. [DOI] [PubMed] [Google Scholar]
- 38.Isales CM, Hamrick M. Nutritional hormones and the entero-osseous axis. J Musculoskelet Neuronal Interact. 2008;8((4)):348–350. [PubMed] [Google Scholar]
- 39.Guan X, Karpen HE, Stephens J, Bukowski JT, Niu S, Zhang G, et al. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology. 2006;130((1)):150–164. doi: 10.1053/j.gastro.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 40.El-Jamal N, Erdual E, Neunlist M, Koriche D, Dubuquoy C, Maggiotto F, et al. Glugacon-like peptide-2 broad receptor expression, limited therapeutic effect on intestinal inflammation and novel role in liver regeneration. Am J Physiol Gastrointest Liver Physiol. 2014;307((3)):G274–G285. doi: 10.1152/ajpgi.00389.2012. [DOI] [PubMed] [Google Scholar]
- 41.Yusta B, Huang L, Munroe D, Wolff G, Fantaske R, Sharma S, et al. Enteroendocrine localization of GLP-2 receptor expression in humans and rodents. Gastroenterology. 2000;119((3)):744–755. doi: 10.1053/gast.2000.16489. [DOI] [PubMed] [Google Scholar]
- 42.Orskov C, Hartmann B, Poulsen SS, Thulesen J, Hare KJ, Holst JJ. GLP-2 stimulates colonic growth via KGF released by subepithelial myofibroblasts with GLP-2 receptors. Regul Pept. 2005;124((1–3)):105–112. doi: 10.1016/j.regpep.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 43.Thulesen J, Hartmann B, Orskov C, Jeppesen PB, Holst JJ, Poulsen SS. Potential targets for glucagon-like peptide 2 (GLP-2) in the rat distribution and binding of i.v. injected (125)I-GLP-2. Peptides. 2000;21((10)):1511–1517. doi: 10.1016/s0196-9781(00)00305-3. [DOI] [PubMed] [Google Scholar]
- 44.Koopmann MC, Nelson DW, Murali SG, Liu X, Brownfield MS, Holst JJ, et al. Exogenous glucagon-like peptide-2 (GLP-2) augments GLP-2 receptor mRNA and maintains proglucagon mRNA levels in resected rats. JPEN J Parenter Enteral Nutr. 2008;32((3)):254–265. doi: 10.1177/0148607108316198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu J, Qi K, Xu Z, Wan J. Glucagon-like peptide-2-loaded microspheres as treatment for ulcerative colitis in the murine model. J Microencapsul. 2015;32((6)):598–607. doi: 10.3109/02652048.2015.1065923. [DOI] [PubMed] [Google Scholar]
- 46.Norona J, Apostolova P, Schmidt D, Ihlemann R, Reischmann N, Taylor G, et al. Glucagon-like peptide 2 for intestinal stem cell and Paneth cell repair during graft-versus-host disease in mice and humans. Blood. 2020;136((12)):1442–1455. doi: 10.1182/blood.2020005957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sigalet DL, Wallace L, De Heuval E, Sharkey KA. The effects of glucagon-like peptide 2 on enteric neurons in intestinal inflammation. Neurogastroenterol Motil. 2010;22((12)):1318–e350. doi: 10.1111/j.1365-2982.2010.01585.x. [DOI] [PubMed] [Google Scholar]
- 48.de Heuvel E, Wallace L, Sharkey KA, Sigalet DL. Glucagon-like peptide 2 induces vasoactive intestinal polypeptide expression in enteric neurons via phophatidylinositol 3-kinase-gamma signaling. Am J Physiol Endocrinol Metab. 2012;303((8)):E994–E1005. doi: 10.1152/ajpendo.00291.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shawe-Taylor M, Kumar JD, Holden W, Dodd S, Varga A, Giger O, et al. Glucagon-like petide-2 acts on colon cancer myofibroblasts to stimulate proliferation, migration and invasion of both myofibroblasts and cancer cells via the IGF pathway. Peptides. 2017;91:49–57. doi: 10.1016/j.peptides.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 50.Wu J, Qi KK, Xu ZW. Porcine glucagon-like peptide-2 microspheres ameliorate inflammation in lipopolysaccharide-challenged weaning piglets. J Anim Sci. 2016;94((12)):5286–5294. doi: 10.2527/jas.2016-1007. [DOI] [PubMed] [Google Scholar]
- 51.Quiros M, Nusrat A. RhoGTPases, actomyosin signaling and regulation of the epithelial Apical Junctional Complex. Semin Cell Dev Biol. 2014;36:194–203. doi: 10.1016/j.semcdb.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Itallie CM, Tietgens AJ, Anderson JM. Visualizing the dynamic coupling of claudin strands to the actin cytoskeleton through ZO-1. Mol Biol Cell. 2017;28((4)):524–534. doi: 10.1091/mbc.E16-10-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yano T, Kanoh H, Tamura A, Tsukita S. Apical cytoskeletons and junctional complexes as a combined system in epithelial cell sheets. Ann N Y Acad Sci. 2017;1405((1)):32–43. doi: 10.1111/nyas.13432. [DOI] [PubMed] [Google Scholar]
- 54.Ren WY, Wu KF, Li X, Luo M, Liu HC, Zhang SC, et al. Age-related changes in small intestinal mucosa epithelium architecture and epithelial tight junction in rat models. Aging Clin Exp Res. 2014;26((2)):183–191. doi: 10.1007/s40520-013-0148-0. [DOI] [PubMed] [Google Scholar]
- 55.Rios-Arce ND, Collins FL, Schepper JD, Steury MD, Raehtz S, Mallin H, et al. Epithelial barrier function in gut-bone signaling. Adv Exp Med Biol. 2017;1033:151–183. doi: 10.1007/978-3-319-66653-2_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheadle GA, Costantini TW, Lopez N, Bansal V, Eliceiri BP, Coimbra R. Enteric glia cells attenuate cytomix-induced intestinal epithelial barrier breakdown. PLoS One. 2013;8((7)):e69042. doi: 10.1371/journal.pone.0069042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, et al. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physioly. 2004;286((3)):G367–G376. doi: 10.1152/ajpgi.00173.2003. [DOI] [PubMed] [Google Scholar]
- 58.Luissint AC, Parkos CA, Nusrat A. Inflammation and the intestinal barrier leukocyte-epithelial cell interactions, cell junction remodeling, and mucosal repair. Gastroenterology. 2016;151((4)):616–632. doi: 10.1053/j.gastro.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cui W, Li LX, Sun CM, Wen Y, Zhou Y, Dong YL, et al. Tumor necrosis factor alpha increases epithelial barrier permeability by disrupting tight junctions in Caco-2 cells. Braz J Med Biol Res. 2010;43((4)):330–337. doi: 10.1590/S0100-879X2010007500020. [DOI] [PubMed] [Google Scholar]
- 60.Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005;166((2)):409–419. doi: 10.1016/s0002-9440(10)62264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clayburgh DR, Musch MW, Leitges M, Fu YX, Turner JR. Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo. J Clin Invest. 2006;116((10)):2682–2694. doi: 10.1172/JCI29218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Martinis M, Di Benedetto MC, Mengoli LP, Ginaldi L. Senile osteoporosis is it an immune-mediated disease? Inflamm Res. 2006;55((10)):399–404. doi: 10.1007/s00011-006-6034-x. [DOI] [PubMed] [Google Scholar]
- 63.Ding C, Parameswaran V, Udayan R, Burgess J, Jones G. Circulating levels of inflammatory markers predict change in bone mineral density and resorption in older adults a longitudinal study. J Clin Endocrinol Metab. 2008;93((5)):1952–1958. doi: 10.1210/jc.2007-2325. [DOI] [PubMed] [Google Scholar]
- 64.Ganesan K, Teklehaimanot S, Tran TH, Asuncion M, Norris K. Relationship of C-reactive protein and bone mineral density in community-dwelling elderly females. J Natl Med Assoc. 2005;97((3)):329–333. [PMC free article] [PubMed] [Google Scholar]
- 65.Roubenoff R. Catabolism of aging is it an inflammatory process? Curr Opin Clin Nutr Metab Care. 2003;6((3)):295–299. doi: 10.1097/01.mco.0000068965.34812.62. [DOI] [PubMed] [Google Scholar]
- 66.Pfeilschifter J. Role of cytokines in postmenopausal bone loss. Curr Osteoporos Rep. 2003;1((2)):53–58. doi: 10.1007/s11914-003-0009-4. [DOI] [PubMed] [Google Scholar]
- 67.Lia G, Paola ML, Maddalena SM, Massimo DM, Osteoporosis and aging . In: Handbook of immunosenescence: basic understanding and clinical implications. Fulop T, Franceschi C, Hirokawa K, Pawelec G, editors. Cham: Springer International Publishing; 2017. pp. p. 1–31. [Google Scholar]
- 68.van der Weijden MA, van Denderen JC, Lems WF, Nurmohamed MT, Dijkmans BA, van der Horst-Bruinsma IE. Etanercept increases bone mineral density in ankylosing spondylitis but does not prevent vertebral fractures results of a prospective observational cohort study. J Rheumatol. 2016;43((4)):758–764. doi: 10.3899/jrheum.150857. [DOI] [PubMed] [Google Scholar]
- 69.Dischereit G, Tarner IH, Muller-Ladner U, Lange U. Infliximab improves bone metabolism and bone mineral density in rheumatoid arthritis and ankylosing spondylitis a prospective 2-year study. Clin Rheumatol. 2013;32((3)):377–381. doi: 10.1007/s10067-012-2128-8. [DOI] [PubMed] [Google Scholar]
- 70.Beek KJ, Rusman T, van der Weijden MAC, Lems WF, van Denderen JC, Konsta M, et al. Long-term treatment with TNF-alpha inhibitors improves bone mineral density but not vertebral fracture progression in ankylosing spondylitis. J Bone Miner Res. 2019;34((6)):1041–1048. doi: 10.1002/jbmr.3684. [DOI] [PubMed] [Google Scholar]
- 71.Kawai VK, Stein CM, Perrien DS, Griffin MR. Effects of anti-tumor necrosis factor alpha agents on bone. Curr Opin Rheumatol. 2012;24((5)):576–585. doi: 10.1097/BOR.0b013e328356d212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abreu MT, Geller JL, Vasiliauskas EA, Kam LY, Vora P, Martyak LA, et al. Treatment with infliximab is associated with increased markers of bone formation in patients with Crohn's disease. J Clin Gastroenterol. 2006;40((1)):55–63. doi: 10.1097/01.mcg.0000190762.80615.d4. [DOI] [PubMed] [Google Scholar]
- 73.Lee JS, Lim DH, Oh JS, Kim YG, Lee CK, Yoo B, et al. Effect of TNF inhibitors on bone mineral density in rheumatoid arthritis patients receiving bisphosphonate a retrospective cohort study. Rheumatol Int. 2020;40((3)):481–487. doi: 10.1007/s00296-019-04418-1. [DOI] [PubMed] [Google Scholar]
- 74.Haroon NN, Sriganthan J, Al Ghanim N, Inman RD, Cheung AM. Effect of TNF-alpha inhibitor treatment on bone mineral density in patients with ankylosing spondylitis a systematic review and meta-analysis. Semin Arthritis Rheum. 2014;44((2)):155–161. doi: 10.1016/j.semarthrit.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 75.Axelsson KF, Nilsson AG, Wedel H, Lundh D, Lorentzon M. Association between alendronate use and hip fracture risk in older patients using oral prednisolone. JAMA. 2017;318((2)):146–155. doi: 10.1001/jama.2017.8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Manolagas SC. From estrogen-centric to aging and oxidative stress a revised perspective of the pathogenesis of osteoporosis. Endocr Rev. 2010;31((3)):266–300. doi: 10.1210/er.2009-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Callaway DA, Jiang JX. Reactive oxygen species and oxidative stress in osteoclastogenesis skeletal aging and bone diseases. J Bone Miner Metab. 2015;33((4)):359–370. doi: 10.1007/s00774-015-0656-4. [DOI] [PubMed] [Google Scholar]
- 78.Melough MM, Sun X, Chun OK. The role of AOPP in age-related bone loss and the potential benefits of berry anthocyanins. Nutrients. 2017;9((7)):789. doi: 10.3390/nu9070789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wilson C. Bone oxidative stress and osteoporosis. Nat Rev Endocrinol. 2014;10((1)):3. doi: 10.1038/nrendo.2013.225. [DOI] [PubMed] [Google Scholar]
- 80.Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, et al. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018;13:757–772. doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vaccaro A, Kaplan Dor Y, Nambara K, Pollina EA, Lin C, Greenberg ME, et al. Sleep loss can cause death through accumulation of reactive oxygen species in the gut. Cell. 2020;181((6)):1307.e15–1328.e15. doi: 10.1016/j.cell.2020.04.049. [DOI] [PubMed] [Google Scholar]
- 82.Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20((7)):1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Campbell EL, Colgan SP. Control and dysregulation of redox signalling in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol. 2019;16((2)):106–120. doi: 10.1038/s41575-018-0079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gonzalez Pardo V, Russo de Boland A. Age-related changes in the response of intestinal cells to 1alpha 25(OH)2-vitamin D3. Ageing Res Rev. 2013;12((1)):76–89. doi: 10.1016/j.arr.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 85.Ebeling PR, Sandgren ME, DiMagno EP, Lane AW, DeLuca HF, Riggs BL. Evidence of an age-related decrease in intestinal responsiveness to vitamin D relationship between serum 1, 25-dihydroxyvitamin D3 and intestinal vitamin D receptor concentrations in normal women. J Clin Endocrinol Metab. 1992;75((1)):176–182. doi: 10.1210/jcem.75.1.1320048. [DOI] [PubMed] [Google Scholar]
- 86.Thakkinstian A, D'Este C, Eisman J, Nguyen T, Attia J. Meta-analysis of molecular association studies vitamin D receptor gene polymorphisms and BMD as a case study. J Bone Miner Res. 2004;19((3)):419–428. doi: 10.1359/JBMR.0301265. [DOI] [PubMed] [Google Scholar]
- 87.Marks HD, Fleet JC, Peleg S. Transgenic expression of the human Vitamin D receptor (hVDR) in the duodenum of VDR-null mice attenuates the age-dependent decline in calcium absorption. J Steroid Biochem Mol Biol. 2007;103((3–5)):513–516. doi: 10.1016/j.jsbmb.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 88.Azuma K, Zhou Q, Kubo KY. Morphological and molecular characterization of the senile osteoporosis in senescence-accelerated mouse prone 6 (SAMP6) Med Mol Morphol. 2018;51((3)):139–146. doi: 10.1007/s00795-018-0188-9. [DOI] [PubMed] [Google Scholar]
- 89.Drucker DJ. The discovery of GLP-2 and development of teduglutide for short bowel syndrome. ACS Pharmacol Transl Sci. 2019;2((2)):134–142. doi: 10.1021/acsptsci.9b00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kuo TR, Chen CH. Bone biomarker for the clinical assessment of osteoporosis recent developments and future perspectives. Biomark Res. 2017;5((1)):18. doi: 10.1186/s40364-017-0097-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Greenblatt MB, Tsai JN, Wein MN. Bone turnover markers in the diagnosis and monitoring of metabolic bone disease. Clin Chem. 2017;63((2)):464–474. doi: 10.1373/clinchem.2016.259085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Udagawa N, Koide M, Nakamura M, Nakamichi Y, Yamashita T, Uehara S, et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J Bone Miner Metab. 2021;39((1)):19–26. doi: 10.1007/s00774-020-01162-6. [DOI] [PubMed] [Google Scholar]
- 93.Greenbaum D, Colangelo C, Williams K, Gerstein M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol. 2003;4((9)):117. doi: 10.1186/gb-2003-4-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Park JH, Lee NK, Lee SY. Current understanding of RANK signaling in osteoclast differentiation and maturation. Mol Cells. 2017;40((10)):706–713. doi: 10.14348/molcells.2017.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen H, Shoumura S, Emura S. Ultrastructural changes in bones of the senescence-accelerated mouse (SAMP6) a murine model for senile osteoporosis. Histol Histopathol. 2004;19((3)):677–685. doi: 10.14670/HH-19.677. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author.