Abstract
Inulin-type fructans (ITFs) have been shown to possess various biological activities. However, studies on their safety and side effects are limited. Hence, we aimed to evaluate the possible effects of burdock ITFs on the physiological indices of healthy mice and their filial generation when fed for six months. Thirty-two C57BL/6J mice were randomly divided into two groups; a normal control (NC) and an ITFs group. The parental generations were kept in one cage with free access to a normal diet and double-distilled water (P-NC group) or burdock ITFs drinking water (P-ITFs group, 2% w/v). The filial generations (F-NC group and F-ITFs group) were kept separately and were fed as their parental generation. Behavior, organ/body weight, serum indices, histopathology, time of production, and number of pup births were observed. There were no significant adverse effects on these indices. Functional indices of the spleen, lung, heart, and pancreas of the ITFs groups were higher than those of the NC groups, respectively. Interestingly, the serum glucose (GLU), total cholesterol (TC), uric acid (UA) and creatine kinase (CK) levels of the ITFs groups were lower than those of the NC groups. Meanwhile, the pregnancy number and pup birth number of the P-ITFs group were more than those of P-NC group. Therefore, long-term consumption of burdock ITFs has no obvious adverse effects on the health of parental mice and their offspring, but may contribute to reproductive capacity, fatigue reduction, and risk reduction of renal disease.
Keywords: burdock, inulin-type fructans, physiology, safety
Worldwide, people live mainly on three starch-rich foods, rice, corn, and wheat, which represent over 60% of the world’s food intake [9, 30, 35]. The reliance on single crops as staple foods poses a threat to population health in many countries [1]. The World Health Organization has recognized the widespread prevalence of inadequate nutrient intakes or nutrient imbalances, also termed “hidden hunger” [14, 18]. The United Nations Food and Agriculture Organization has reported that approximately 2 billion people around the world suffered from hidden hunger in 2019, of which 300 million were from China [9]. Excessive intakes of calories, fat, protein, and salt are the main cause of chronic non-communicable diseases including diabetes mellitus, cardiovascular disease, cancer, and chronic respiratory diseases, which represent a serious threat to human health [33]. In China, 70% of chronic diseases are associated with hidden hunger, accounting for 88% of all deaths. Chronic non-infectious diseases account for over 70% of the total disease burden in China [37]. Many studies have revealed that a lack of dietary fiber is linked with the onset of chronic non-infectious diseases [2]. Dietary fiber was declared as a nutrient by the Nutrition Labeling and Education Act in 1990 [11].
With the recent increased demand for prebiotic fiber and lower-energy foods, the use of non-starchy polysaccharides from the Asteraceae family such as Jerusalem artichoke (Helianthus tuberosus L.) tubers and chicory (Cichorium intybus) roots, which are known to contain high amounts of inulin-type fructans (ITFs; consisting of fructose subunits via β-(2→1) linkages with an α-glucose terminal unit) have been on the rise [4, 23, 34]. Due to the β-(2→1) glycosidic bonds, ITFs cannot be broken down by human digestive enzymes in the mouth, stomach, or small intestine, but can be fermented by gut bacteria into the major short-chain fatty acids (SCFAs) acetate, propionate, and butyrate in the colon. Changes in the intestinal flora caused by ITFs fermentation influence the immune function of the intestinal lymphoid tissue. Meanwhile, the changes in the intestinal flora on account of ITFs fermentation help to prevent and treat chronic non-infectious diseases. Due to these benefits, ITFs are eaten as a dietary fiber source by humans in their daily diet [3, 16, 21, 32].
Burdock (Arctium lappa) belongs to the Asteraceae family, as does chicory and Jerusalem artichoke [27, 42]. Burdock roots are known sources of prebiotic ITFs and are consumed raw or cooked in Asia, Europe, and the United States [8, 26]. Dried burdock root flour has been used as a prebiotic ingredient with whole wheat flour in the manufacture of bakery products and is available as a herbal tincture and tea in many Asian countries [38]. Burdock roots contain approximately 15% ITFs (approximately 80% moisture), which are the main bioactive components [27]. These ITFs exhibit hypolipidemic, hypoglycemic [40], antithrombotic [29], antitussive [17], free radical-scavenging, antioxidant [22], anti-inflammatory [41], and prebiotic properties [12, 27]. Therefore, burdock root is considered as a functional food with homology to medicine, which has led to it being widely planted in many countries [13]. Despite these reported positive properties, one month supplementation with ITFs had a negative effect on the liver morphology of non-diabetic rats and plasma glucose levels in both diabetic and non-diabetic rats [25]. ITFs have also been shown to cause adverse hypersensitivity reactions associated with the quality and purification of the product extracted from plants [6]. These studies demonstrate that not all sources and forms of ITFs are healthy and safe, and that a diet enriched with fermentable fibers displays different effects in different organisms [25]. Moreover, over-intake of ITFs induces an increase in gastrointestinal and/or other serious side effects such as inflammation, compensatory cell proliferation, and liver cancer [23, 34]. Few studies have evaluated the safety of burdock ITFs, though some have looked at chicory root and Jerusalem artichoke [6, 7, 34]. Therefore, we aimed to evaluate the long-term effects of burdock ITFs on the physiological indexes of healthy mice. The results presented here help span the knowledge gap on the dietary and medicinal applications of burdock root as a fiber source.
MATERIALS AND METHODS
Animal ethics statement
The experimental animals, designs, and animal management in the present study were approved by the Laboratory Animals Ethical Committee of Wannan Medical College (Wuhu, China; approval number: LLSC-2022-041) and complied with the recommendations of the ARRIVE Guidelines for the Care and Use of Laboratory Animals.
Preparation and structural characterization of burdock ITFs
Fresh burdock root was purchased from a plantation based in Fengxian, Xuzhou city, Jiangsu province, China (Fig. 1). The obtained burdock root was classed as “Liuchuanlixiang,” a representative variety of burdock that was widely planted in Fengxian and Peixian in Xuzhou City of China. To obtain a high concentration of ITFs, fresh burdock roots were harvested before first flowering and stored in polyethylene packages at 2°C [42]. ITFs were extracted from fresh burdock roots. The extraction process was performed as previously reported [31, 40]. Briefly, fresh burdock roots were washed, peeled, sliced into small pieces, submerged in water (1 g per 10 mL), and heated for 120 min at 70°C with constant stirring. The aqueous extracts were filtered using a vacuum water pump (SHZ-D (III), Gongyi Yuhua Instrument Co., Ltd., Gongyi, China). This extraction was repeated three times. The filtrates were combined and concentrated using a rotary evaporator (N-1100D-WB, EYELA, Tokyo, Japan) at 60°C. The concentrated extract was precipitated with a four-fold volume of 95% alcohol for 12 hr at 4°C. The precipitate was then collected and dissolved in distilled water, followed by centrifugation at 5,000 rpm. The supernatant was deproteinated using the Sevag method (chloroform:n-butanol, 4:1 v/v). The deproteinated polysaccharide aqueous extract was then decolorized with a D101-macroporous resin (Henghuibio Co., Ltd., Beijing, China), followed by concentration, dialysis (Mw cut-off: 1000 Da), and lyophilization. The obtained crude ITFs (10 mg/mL) were purified using a DEAE-52 chromatographic column (2.5 × 40 cm), wherein the sample volume was 20 mL and double-distilled water as the eluent (1.0 mL/min), and a G-75 chromatographic column (1.5 × 60 cm) where the sample volume was 5 mL (5 mg/mL) and double-distilled water was the eluent (0.5 mL/min). Total carbohydrates in the eluate (6 mL per tube) were measured using the phenol-sulfuric acid method. Polysaccharides from the same single peak were collected, dialyzed, and lyophilized to obtain pure burdock ITFs. The pure burdock ITFs were characterized using nuclear magnetic resonance (NMR) with a JNM-ECZ600R/S1 spectrometer (JEOL, Tokyo, Japan) at 600 MHz. Burdock ITFs (20 mg) were dissolved in 0.5 mL D2O (99.9%), and One-dimensional 1H (under water suppression), 13C, and Distortionless Enhancement by Polarization Transfer 135 (DEPT-135) NMR spectra were recorded and analyzed using Mest ReNova software (version 6.1.0-6224; Mestrelab Research, Santiago de Compostela, Spain) [31].
Fig. 1.
Image of the commercial fresh burdock root.
Experimental animals, diets, and design
Thirty-two weanling C57BL/6J mice (six-weeks-old, 16 males and 16 females, average weight 22 g) were obtained from Sibf Biotechnology Co., Ltd. (Anyang, China) and housed at the specific pathogen-free Animal Center of Wannan Medical College. Mice were randomly divided into two groups: the normal control group and the ITFs-group (P-NC and P-ITFs groups, respectively). The experiment began with eight male and eight female mice per group as the parental generation. The parental generation was maintained in one cage with free access to a normal chow diet (lab mice diet #SWS9102, Jiangsu Syony Pharmaceutical Biological Engineering Co., Ltd., Nanjing, China) and double distilled water (P-NC group) or burdock ITFs drinking water (2% w/v, P-ITFs group) throughout the study [7, 20, 28]. The only difference between the groups was the burdock ITF supplement intake. The filial generations of both groups (termed F-NC and F-ITFs from the P-NC and P-ITFs groups, respectively) were kept separately in one cage and fed as their parental generation for six months. Food intake and water consumption were recorded daily. The environmental conditions of the animal room were at 23–25°C, 12 hr light/dark cycle, and 50 ± 10% relative humidity.
Sample collection and analysis
After six months, the four groups (P-NC, P-ITFs, F-NC, and F-ITFs) were fasted overnight. All animals were weighed, anesthetized with pentobarbital sodium (40 mg/kg, 0.036 g/mL), and then sacrificed. The nose-to-tail tip lengths of all animals were recorded. Blood samples were collected from the retro-orbital sinus, and the serum was separated via centrifugation for 10 min at 1500 rpm and 4°C. Serum was then stored at −80°C for the future measurement of total protein (TP), albumin (ALB), globulin (GLOB), total bilirubin (TBIL), direct bilirubin (DBIL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), urea nitrogen (Bun), creatinine (Cr), uric acid (UA), glucose (GLU), total cholesterol (TC), triglycerides (TG), high density lipoprotein (HDL), low density lipoprotein (LDL), creatine kinase (CK), lactate dehydrogenase (LDH), amylase (AMY), calcium (Ca), phosphorus (P), and magnesium (Mg), which were measured using an automatic biochemical analyzer (7080, HITACHI, Tokyo, Japan).
The brain, lung, heart, liver, spleen, kidneys, pancreas, colon, and abdominal fat were collected. The selected organs from the four groups of animals were weighed immediately and the organ/body weight ratio was calculated. After being weighed, the specimens were frozen in liquid nitrogen or fixed in neutral-buffered formalin for morphological analysis. Paraffin sections (5 μm) of the kidney and liver samples were stained with hematoxylin and eosin for histological analysis. Photomicrographs (400× magnification) of the stained tissues were evaluated for steatosis and tissue damage using an Olympus BX53M optical microscope (Olympus Corp., Tokyo, Japan) and a 40× objective lens.
Statistical analysis
Data variables were expressed as the mean ± standard deviation and were analyzed using GraphPad Prism 7.0 software (GraphPad Software, Inc., San Diego, CA, USA). Comparisons between P-NC and P-ITFs groups, or F-NC and F-ITFs groups were performed using one-way analysis of variance and the Student’s t-test. A value of P<0.05 was considered statistically significant.
RESULTS
Structural characteristics of burdock ITFs
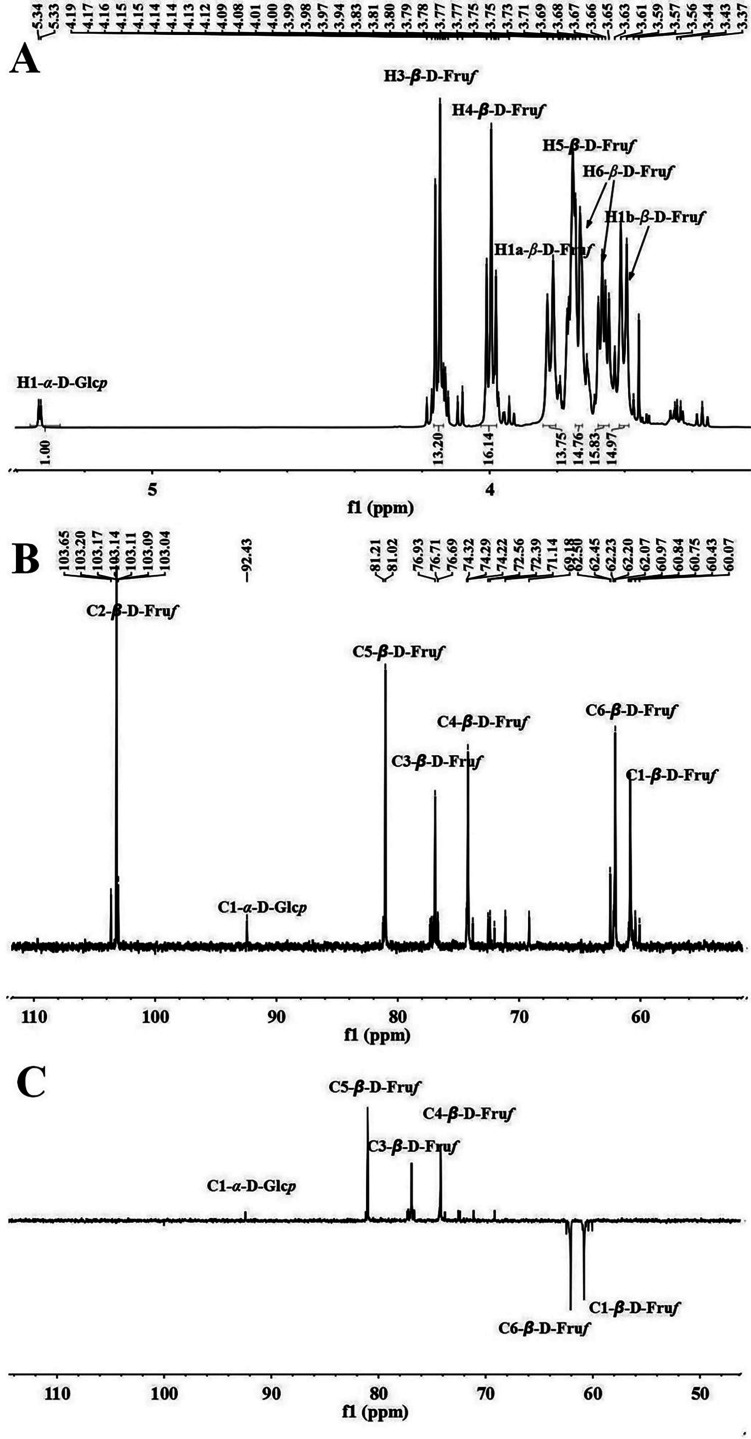
1H, 13C, and DEPT-135 NMR spectra were recorded to confirm the structure of ITFs. In the 1H NMR spectrum (Fig. 2A), the peaks in the region of δ 4.7–5.5 ppm are attributed to the α-glycosidical configuration anomeric carbon hydrogen signals, and the β-glycosidical configuration anomeric carbon hydrogen signals usually appear in the δ 4.4–4.8 ppm region. In the anomeric region, the weak peaks at δ 5.33 ppm (d, J=3.6 Hz) were assigned to the H1 signal of α-D-Glcp (1→. The strong signals in the region of δ 3.20–4.30 ppm were sugar-ring-proton peaks. The peaks at δ 3.82 ppm (d, J=10.8 Hz), δ 3.60 ppm (d, J=10.8 Hz), δ 4.16 ppm (d, J=9.0 Hz), δ 3.99 ppm (t, J=8.4 Hz), and δ 3.65 ppm (q, J=6 Hz) were characteristic H1a, H1b, H3, H4, and H6 signals of D-Fruf, respectively. The H5 and H6a signals of the fructosyl hydrogen were assigned between δ 3.73–3.76 region. The weak signals in a similar region are attributed to the α-D-Glcp (1→. Based on these reports, the chain length of burdock ITFs was estimated at 14 according to the integration ratio of the H-3 signal of Fruf and the H-1 signal of Glcp from the 1H NMR spectrum, in accordance with literatures [28, 31]. In the 13C NMR spectrum (Fig. 2B), the α-glycosidical configuration anomeric carbon chemical shift ranged from δ 95–101 ppm, while the β-glycosidical bond anomeric carbon chemical shift was in the region of δ 101–112 ppm. Six predominant carbon signals were assigned to the carbon chemical shifts of β-D-Fruf. The strong peak at δ 103.14 ppm corresponds to the C2 signal of (2,1)-β-D-Fruf. The methylene group (CH2) peaked at δ 60.79 and 62.07 ppm for C1 and C6 of (2,1)-β-D-Fruf, respectively. The C-H group peaks at δ 74.12, 76.93, and 81.02 ppm were assigned to C4, C3, and C5 of (2,1)-β-D-Fruf, respectively. The weak peak at δ 92.40 ppm in the anomeric carbon region is the C1 signal of α-D-Glcp (1→ residue. These were confirmed by the DEPT 135 experiment (Fig. 2C). The quaternary carbon had no peak, the tertiary carbon (CH) had a positive peak, and the secondary carbon (CH2) had an inverted peak.
Fig. 2.
Nuclear magnetic resonance (NMR) spectra of burdock inulin-type fructans (ITFs). (A) 1H, (B) 13C, and (C) Distortionless Enhancement by Polarization Transfer 135 (DEPT-135).
Effects of burdock ITFs supplementation on behavior and weight
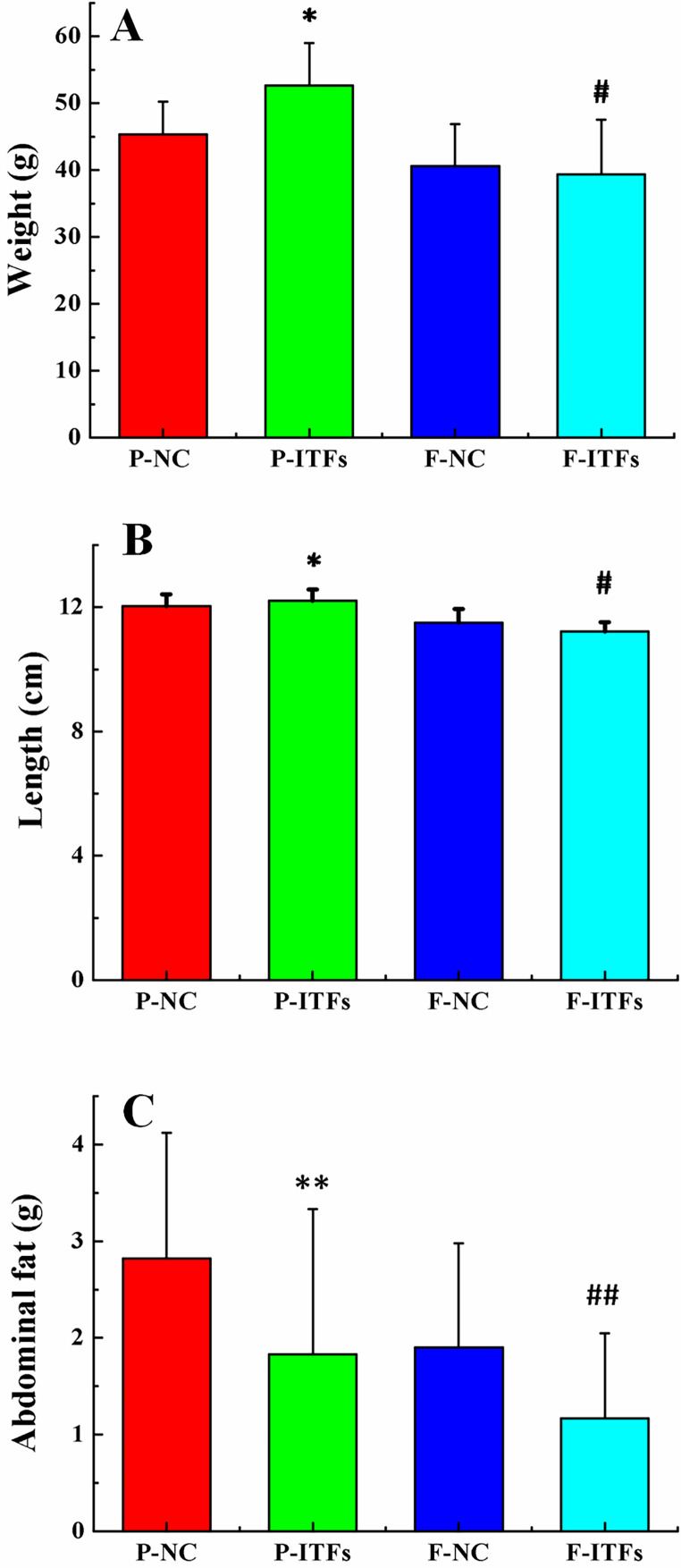
The P-ITFs and F-ITFs groups received free access to a normal chow diet and burdock ITFs drinking water (2% w/v) throughout the study. The P-NC and F-NC groups were fed a normal chow diet and double distilled water. No deaths were observed during the study. The animals in the ITFs groups were more active. When handled by their tails, the male mice were more likely to bite the handler. The increased aggression was observed during the experiment. No abnormalities were observed in the general state of the mice. It was concluded that ITFs did not affect the general health of the mice. A slight increase in the average feed intake of the ITFs groups was observed. After six months, all mice gained weight and length, and the weights and lengths were similar among the groups as shown in Fig. 3A and 3B. A slight decrease in abdominal fat was observed in the P-ITFs and F-ITFs groups compared to the P-NC and F-NC groups, respectively (Fig. 3C).
Fig. 3.
Terminal body weight (A), Length (B), and abdominal fat (C) of the normal control and experimental groups and their filial generations. *P<0.05, **P<0.01, compared to the P-NC group; #P<0.05, ##P<0.01, compared to the F-NC group. P-NC: parental normal control mice; P-ITFs: parental burdock inulin-type fructans supplemented mice; F-NC: filial normal control mice; F-ITFs: filial burdock inulin-type fructans supplemented mice.
Effects of burdock ITFs supplement on organ weight
No significant differences were observed between the normal control and experimental groups with respect to their filial generations, as illustrated by the organ/body weight ratios (Table 1). However, the P-ITFs and F-ITFs groups showed a trend of slight increase in the spleen, lung, heart, and pancreas when compared to the P-NC and F-NC groups, respectively (Fig. 4).
Table 1. Organ/body weight ratios of the normal control and experimental groups with their filial generations.
| Parameter | P-NC group | P-ITFs group | F-NC group | F-ITFs group |
|---|---|---|---|---|
| Liver (mg/g) | 53.77 ± 7.95 | 56.69 ± 13.81* | 57.66 ± 9.90 | 51.97 ± 8.04# |
| Spleen (mg/g) | 2.97 ± 1.96 | 3.18 ± 1.58* | 2.77 ± 1.26 | 3.45 ± 1.69# |
| Left kidney (mg/g) | 9.36 ± 1.21 | 10.44 ± 1.64 | 10.12 ± 2.29 | 8.90 ± 2.22 |
| Right kidney (mg/g) | 9.49 ± 1.64 | 10.61 ± 1.52 | 11.19 ± 2.69 | 9.09 ± 2.84 |
| Lung (mg/g) | 7.28 ± 2.74 | 8.32 ± 2.34* | 7.12 ± 1.86 | 7.65 ± 1.80# |
| Heart (mg/g) | 4.83 ± 0.39 | 5.36 ± 0.42** | 5.11 ± 0.50 | 5.43 ± 0.80## |
| Brain (mg/g) | 13.90 ± 1.63 | 11.96 ± 1.35* | 14.66 ± 1.79 | 15.53 ± 2.62# |
| Pancreas (mg/g) | 13.87 ± 6.40 | 16.52 ± 2.56** | 9.87 ± 3.46 | 13.98 ± 3.95## |
| Mesenterium (mg/g) | 28.29 ± 9.95 | 19.56 ± 6.79 | 13.16 ± 7.42 | 17.57 ± 15.90 |
| Colon (cm) | 9.69 ± 1.68 | 11.33 ± 1.78 | 10.93 ± 0.68 | 7.81 ± 1.12 |
Mean ± SD, N=6. *P<0.05, **P<0.01, compared with P-NC group. #P<0.05, ##P<0.01, compared with the F-NC group. P-NC: parental normal control mice; P-ITFs: parental burdock inulin-type fructans supplemented mice; F-NC: filial normal control mice; F-ITFs: filial burdock inulin-type fructans supplemented mice.
Fig. 4.
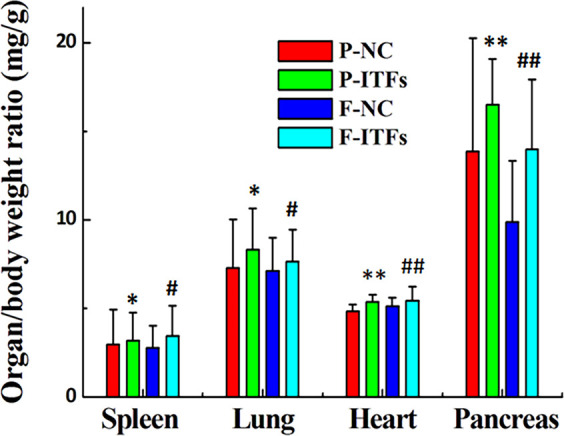
Organ/body weight ratios of normal control and experimental groups and their filial generations. *P<0.05, **P<0.01, compared to the P-NC group; #P<0.05, ##P<0.01, compared to the F-NC group. P-NC: parental normal control mice; P-ITFs: parental burdock inulin-type fructans supplemented mice; F-NC: filial normal control mice; F-ITFs: filial burdock inulin-type fructans supplemented mice.
Effects of burdock ITFs supplementation on serum indexes
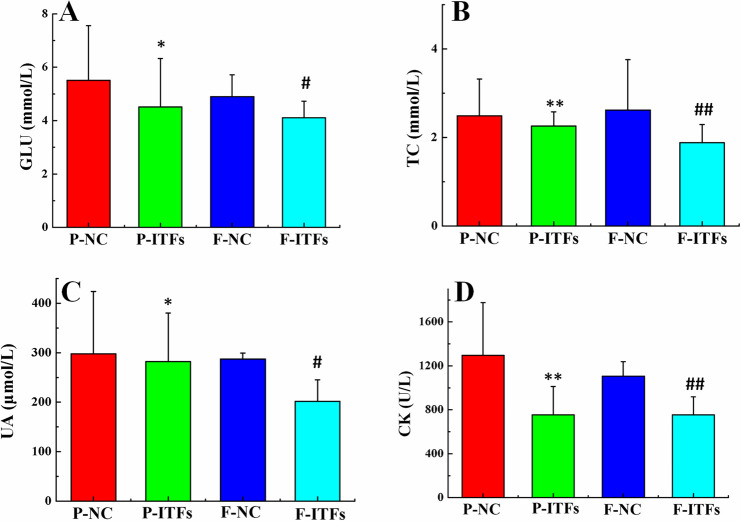
The serum biochemical indices are shown in Table 2. No significant differences were observed in serum parameters. Most serum indices for the P-ITFs and F-ITFs groups were similar with those of the P-NC and F-NC groups. As shown in Fig. 5, we observed a trend of decrease in serum GLU, TC, UA, and CK levels in the P-ITFs and F-ITFs groups compared to those in the control groups.
Table 2. Serum biochemical values of the normal control and experimental groups with their filial generations.
| Parameter | P-NC group | P-ITFs group | F-NC group | F-ITFs group |
|---|---|---|---|---|
| TP (g/L) | 58.47 ± 5.47 | 59.22 ± 5.70* | 58.83 ± 2.05 | 52.74 ± 5.60# |
| ALB (g/L) | 19.17 ± 3.67 | 19.7 ± 4.54* | 20.27 ± 1.27 | 20.09 ± 2.01# |
| GLOB (g/L) | 39.3 ± 4.35 | 39.52 ± 4.67* | 38.57 ± 1.31 | 32.66 ± 4.29 |
| ALB/GLOB | 0.49 ± 0.10 | 0.51 ± 0.14 | 0.53 ± 0.03 | 0.62 ± 0.07 |
| TBIL (μmol/L) | 1.01 ± 0.55 | 1.11 ± 0.92* | 1.50 ± 0.76 | 0.72 ± 0.21## |
| DBIL (μmol/L) | 0.56 ± 0.27 | 0.53 ± 0.45 | 0.63 ± 0.25 | 0.51 ± 0.21 |
| ALT (U/L) | 66.33 ± 68.13 | 34.5 ± 14.73 | 38 ± 4 | 33.71 ± 10.56 |
| AST (U/L) | 190.5 ± 137.31 | 114.67 ± 31.69 | 127 ± 21.70 | 122.71 ± 69.46 |
| AST/ALT | 3.57 ± 1.52 | 3.65 ± 1.37 | 3.36 ± 0.69 | 3.97 ± 2.53 |
| GGT (U/L) | 2.33 ± 0.82 | 2 ± 1.26 | 3.33 ± 1.15 | 2.14 ± 0.69 |
| ALP (U/L) | 60.5 ± 21.37 | 51.33 ± 19.39** | 84 ± 31.32 | 82 ± 21.77# |
| Bun (mmol/L) | 6.54 ± 2.14 | 8.03 ± 1.73 | 7.54 ± 2.81 | 7.41 ± 1.59 |
| Cr (μmol/L) | 5.88 ± 0.73 | 5.57 ± 0.63 | 6.5 ± 0.2 | 6.56 ± 1.46 |
| UA (μmol/L) | 297.65 ± 126.17 | 281.87 ± 98.19* | 287.27 ± 11.85 | 202 ± 43.09# |
| GLU (mmol/L) | 5.51 ± 2.05 | 4.51 ± 1.82* | 4.90 ± 0.82 | 4.11 ± 0.62# |
| TC (mmol/L) | 2.49 ± 0.83 | 2.26 ± 0.32** | 2.62 ± 1.14 | 1.88 ± 0.41## |
| TG (mmol/L) | 0.56 ± 0.20 | 0.62 ± 0.32 | 1.05 ± 0.61 | 0.71 ± 0.57 |
| HDL (mmol/L) | 1.76 ± 0.46 | 1.75 ± 0.19 | 2.00 ± 0.98 | 1.40 ± 0.24 |
| LDL (mmol/L) | 0.41 ± 0.27 | 0.23 ± 0.10 | 0.28 ± 0.09 | 0.29 ± 0.17 |
| CK (U/L) | 1,295.83 ± 479.24 | 754 ± 257.99* | 1,106.67 ± 132.08 | 754 ± 163.83## |
| LDH (U/L) | 1,378.33 ± 550.51 | 1,051 ± 502.08** | 1,202.67 ± 482.15 | 1,062.71 ± 155.78# |
| AMY (U/L) | 3,135.5 ± 1,580.72 | 3,195 ± 739.72* | 2,544.33 ± 243.10 | 3,357 ± 1,411.34## |
| Ca (mmol/L) | 2.19 ± 0.24 | 2.18 ± 0.19 | 2.24 ± 0.04 | 2.14 ± 0.08 |
| P (mmol/L) | 2.12 ± 0.38 | 2.16 ± 0.46 | 2.54 ± 0.42 | 2.16 ± 0.23## |
| Mg (mmol/L) | 1.24 ± 0.13 | 1.18 ± 0.10 | 1.29 ± 0.15 | 1.13 ± 0.10 |
Mean ± SD, N=6. *P<0.05, **P<0.01, compared with P-NC group. #P<0.05, ##P<0.01, compared with the F-NC group. P-NC: parental normal control mice; P-ITFs: parental burdock inulin-type fructans supplemented mice; F-NC: filial normal control mice; F-ITFs: filial burdock inulin-type fructans supplemented mice; total protein: TP; albumin: ALB; globulin: GLOB; total bilirubin: TBIL; direct bilirubi: DBIL; alanine aminotransferase: ALT; aspartate aminotransferase: AST; gamma glutamyl transpeptidase: GGT; alkaline phosphatase: ALP; urea nitrogen: Bun; creatinin: Cr; uric acid: UA; glucose: GLU; total cholesterol: TC; triglycerides: TG; high density lipoprotein: HDL; low density lipoprotein: LDL; creatine kinase: CK; lactate dehydrogenase: LDH; amylase: AMY; calcium: Ca; phosphorus: P; magnesium: Mg.
Fig. 5.
Levels of serum glucose (GLU) (A), total cholesterol (TC) (B), uric acid (UA) (C), and creatine kinase (CK) (D) of normal control and experimental groups and their filial generations. *P<0.05, **P<0.01, compared to the P-NC group; #P<0.05, ##P<0.01, compared to the F-NC group. P-NC: parental normal control mice; P-ITFs: parental burdock inulin-type fructans supplemented mice; F-NC: filial normal control mice; F-ITFs: filial burdock inulin-type fructans supplemented mice.
Effects of burdock ITFs supplementation on morphology of liver and kidney
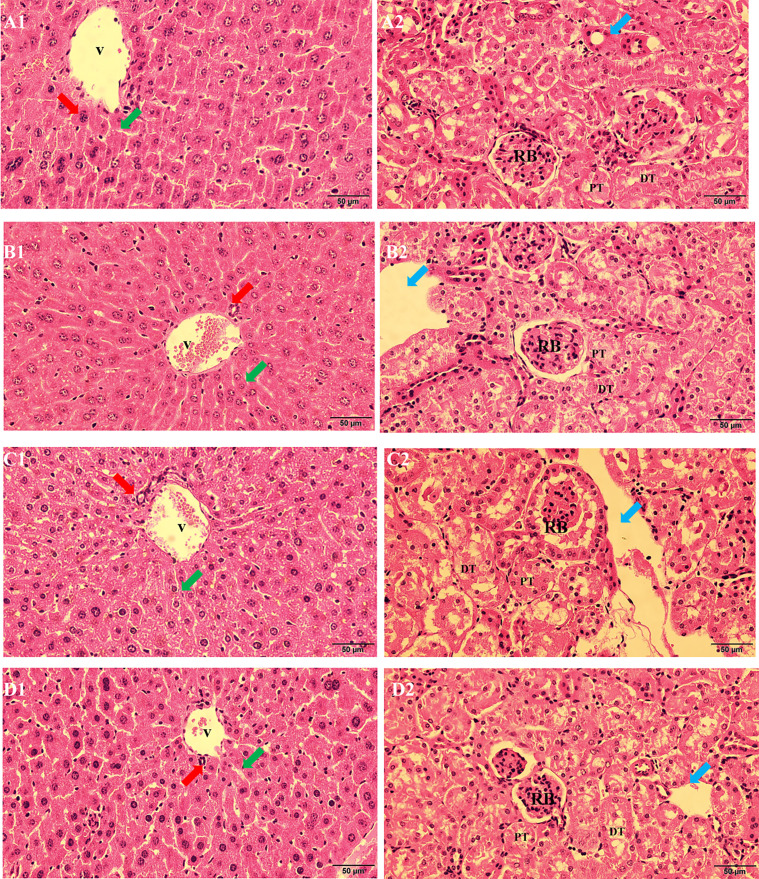
After a six-month exposure to burdock ITFs, macroscopic analysis of the liver and kidney were carried out (Fig. 6). The liver cells of all groups presented a normal appearance, size, color, and consistency without vacuoles. The portal triad showed a venule (represented by the acronym, V), bile duct (indicated by a red arrow), and sinusoid capillaries (indicated by a green arrow). Round hepatocyte nuclei (stained blue) were distinguished in the liver tissue. There was no hepatocyte cytoplasmic vacuolization, condensed nuclear chromatin, or sinusoidal widening damage (Fig. 6B1 and 6D1). Normal convoluted proximal and distal tubules (represented by the acronyms, PT and DT, respectively) with cellular debris in the lumen, blood vessels (indicated by a blue arrow), and renal bodies (represented by the acronym, RB) were observed in the kidney tissues of all groups. Kidney cells were arranged closely and were uniform in size and shape without loosening, edema, vacuoles, and necrosis (Fig. 6B2 and 6D2). Morphologically, the analyzed liver and kidney did not show any alterations in the P-ITFs and F- ITFs groups as compared to both P-NC and F-NC groups, respectively. These confirmed that the consumption of burdock ITFs caused no toxicity to the mouse liver and kidney.
Fig. 6.
Hematoxylin and eosin staining (×400) of the liver and kidney tissues of C57BL/6J mice (bar=50 µm). Liver (1) and kidney (2) tissues within the P-NC (A), P-ITFs (B), F-NC (C), and F-ITFs (D) groups. Bile ducts are indicated by red arrows, sinusoid capillaries are indicated by green arrows, and blood vessels are indicated by a blue arrow. V: venule; PT and DT: proximal and distal tubules; RB: renal bodies; P-NC: parental normal control mice; P-ITFs: parental burdock inulin-type fructans supplemented mice; F-NC: filial normal control mice; F-ITFs: filial burdock inulin-type fructans supplemented mice.
Effects of burdock ITFs supplementation on development and reproduction
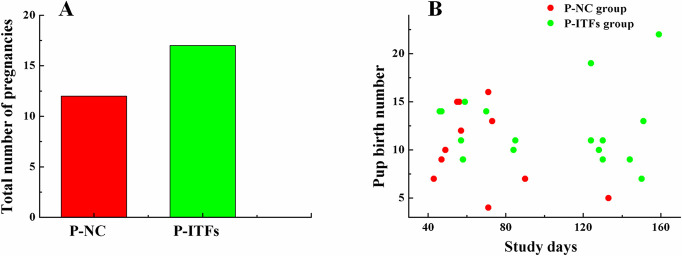
The total number of pregnancies and pup birth number of the P-ITFs group were higher than those of the P-NC group (Fig. 7).
Fig. 7.
Total number of pregnancies (A) and pup birth number (B) of the parental normal control and experimental groups in a six-month exposure study. P-NC: parental normal control mice; P-ITFs: parental burdock inulin-type fructans supplemented mice.
DISCUSSION
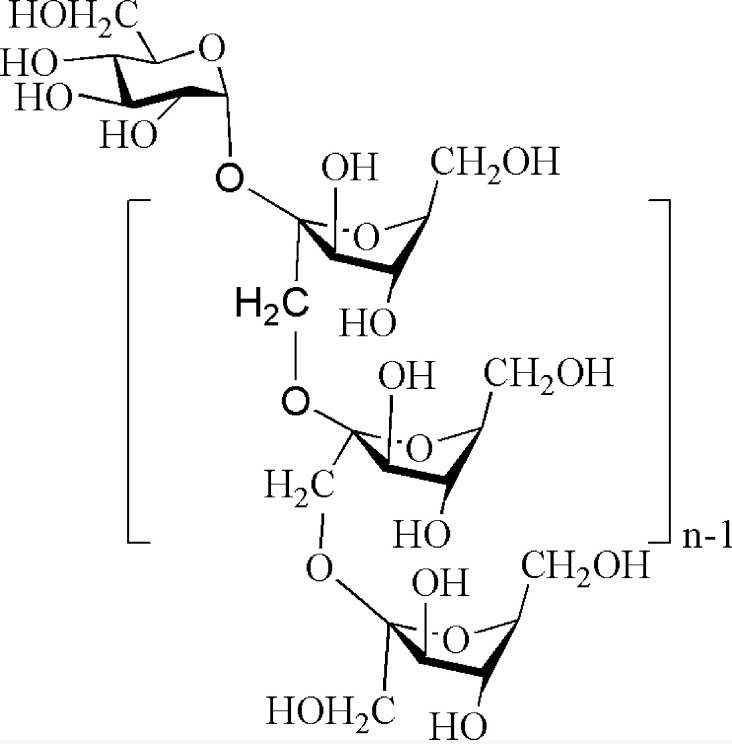
Burdock (Arctium lappa) belongs to the Asteraceae family, known as the sources of prebiotic ITFs. The most prominent nutrients in burdock root are ITFs [27]. The burdock ITFs are linear chains of fructose linked together by β-D-(2→1) bonds with a glucose bound to the free end of the chain by an α-D-(1→2) bond. Its molecular size is represented by the degree of polymerization, DPn, where n represents the number of fructose molecules. The average n of burdock ITFs is 12–14 [27]. These chemical shifts we observed were characteristic of a typical fruit residue according to previous reports [39]. All chemical shifts observed were uniform to the structural characteristics of ITFs, α-D-Glcp (1→2)-β-D-Frufn-1- (1→2)- β-D- Fruf [28], as shown in Fig. 8.
Fig. 8.
Chemical structure of burdock inulin-type fructans (ITFs).
According to the literature, prebiotic fiber ITFs can improve diet-induced obesity by regulating antimicrobial peptides [3, 27]. We observed a decrease in abdominal fat in ITFs-supplemented mice. This suggests that the administration of burdock ITFs could prevent the occurrence of obesity due to its low caloric value (approximately 1.5 kcal/g) and a decrease in the intake of carbohydrates and lipids due to dietary ITFs content [19]. In addition, some studies have shown that the inhibition of lipogenesis and stimulation of catabolism are the crucial cause for obesity reduction after administration of burdock extracts [8].
While we observed no physical differences in organ sizes when comparing parental and filial mice receiving the same treatment, ITFs-supplemented mice had increased spleen, lung, heart, and pancreas sizes when compared with controls of the same generation. In agreement with literature, these results reveal the contribution of burdock ITFs to body health [12, 26, 27].
Generally, the long-term consumption of burdock ITFs has a positive health effect on the body. Burdock ITFs have been shown reduce postprandial blood GLU and TC levels, endowing hypoglycemic and hypolipidemic activities as the result of the SCFAs [5, 27, 36, 40]. The results presented here suggest that burdock ITFs could reduce serum UA levels in mice originating from UA degradation by the intestinal microbiota [15]. Burdock ITFs may have a protective effect on renal function and lower the risk of renal disease, although further studies are needed to confirm this. Further, in mice, burdock ITFs decreased levels of CK, a muscle injury biomarker, showing a potential effect of burdock ITFs on preventing fatigue and elevating exercise performance [10].
Burdock ITFs exhibited positive effect on developmental and reproductive capacity of parental mice. According to previous reports, burdock ITFs enhance the body’s immune system, and improve the intestinal environment and body health [26]. Based on previously published literature, maternal intestinal microbiota can influence the bacterial colonization and intestinal properties of newborns and infants directly. Therefore, the maternal intake of prebiotics and dietary fiber during pregnancy and lactation can improve the health of both the mother and the offspring [24].
Here, we evaluated the effects of burdock ITFs on the physiological function indices of healthy mice and their filial generation. As expected, the results of serum biochemical parameters and liver and kidney pathological morphology revealed that no adverse effects on the development of the parental mice or their offspring in a six-month in vivo study. Additionally, our results suggest potential roles for ITFs in improving reproductive parameters, preventing renal disease, and aiding in combating fatigue. These positive results provide useful data for deeper research and development of burdock ITFs as functional foods and medicines. Further biochemical and pharmacological studies are needed to support this hypothesis.
CONFLICT OF INTEREST
The authors have no conflicts of interest.
Acknowledgments
This study was funded by the Major Projects of the Natural Science Foundation of the Department of Education of Anhui Province (KJ2021ZD0101), the Project of Anhui Provincial Engineering Research Center for Polysaccharide Drugs (WKGC202001), the Project of Anhui Laboratory of Molecule-Based Materials of the College of Chemistry and Materials Science of Anhui Normal University (fzj22016), and the Anhui Provincial Training Programs of Innovation and Entrepreneurship for Undergraduates (S202110368110).
REFERENCES
- 1.Bamji MS, Murty PVVS, Sudhir PD. 2021. Nutritionally sensitive agriculture-an approach to reducing hidden hunger. Eur J Clin Nutr 75: 1001–1009. doi: 10.1038/s41430-020-00760-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barber TM, Kabisch S, Pfeiffer AFH, Weickert MO. 2020. The health benefits of dietary fibre. Nutrients 12: 3209. doi: 10.3390/nu12103209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beisner J, Filipe Rosa L, Kaden-Volynets V, Stolzer I, Günther C, Bischoff SC. 2021. Prebiotic inulin and sodium butyrate attenuate obesity-induced intestinal barrier dysfunction by induction of antimicrobial peptides. Front Immunol 12: 678360. doi: 10.3389/fimmu.2021.678360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boillot J, Alamowitch C, Berger AM, Luo J, Bruzzo F, Bornet FR, Slama G. 1995. Effects of dietary propionate on hepatic glucose production, whole-body glucose utilization, carbohydrate and lipid metabolism in normal rats. Br J Nutr 73: 241–251. doi: 10.1079/BJN19950026 [DOI] [PubMed] [Google Scholar]
- 5.Boyle FG, Wrenn JM, Marsh BB, Anderson WI, Angelosanto FA, McCartney AL, Lien EL. 2008. Safety evaluation of oligofructose: 13 week rat study and in vitro mutagenicity. Food Chem Toxicol 46: 3132–3139. doi: 10.1016/j.fct.2008.06.079 [DOI] [PubMed] [Google Scholar]
- 6.Bui TV, Prot-Bertoye C, Ayari H, Baron S, Bertocchio JP, Bureau C, Davis P, Blanchard A, Houillier P, Prie D, Lillo-Le Louet A, Courbebaisse M. 2021. Safety of inulin and sinistrin: combining several sources for pharmacovigilance purposes. Front Pharmacol 12: 725417. doi: 10.3389/fphar.2021.725417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carabin IG, Flamm WG. 1999. Evaluation of safety of inulin and oligofructose as dietary fiber. Regul Toxicol Pharmacol 30: 268–282. doi: 10.1006/rtph.1999.1349 [DOI] [PubMed] [Google Scholar]
- 8.Chan YS, Cheng LN, Wu JH, Chan E, Kwan YW, Lee SM, Leung GP, Yu PH, Chan SW. 2011. A review of the pharmacological effects of Arctium lappa (burdock). Inflammopharmacology 19: 245–254. doi: 10.1007/s10787-010-0062-4 [DOI] [PubMed] [Google Scholar]
- 9.Chen D, Han Z, Si J. 2021. Huangjing (Polygonati rhizoma) is an emerging crop with great potential to fight chronic and hidden hunger. Sci China Life Sci 64: 1564–1566. doi: 10.1007/s11427-021-1958-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen WC, Hsu YJ, Lee MC, Li HS, Ho CS, Huang CC, Chen FA. 2017. Effect of burdock extract on physical performance and physiological fatigue in mice. J Vet Med Sci 79: 1698–1706. doi: 10.1292/jvms.17-0345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dumoitier A, Abbo V, Neuhofer ZT, McFadden BR. 2019. A review of nutrition labeling and food choice in the United States. Obes Sci Pract 5: 581–591. doi: 10.1002/osp4.374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evdokimova SA, Nokhaeva VS, Karetkin BA, Guseva EV, Khabibulina NV, Kornienko MA, Grosheva VD, Menshutina NV, Shakir IV, Panfilov VI. 2021. A study on the synbiotic composition of Bifidobacterium bifidum and Fructans from Arctium lappa roots and Helianthus tuberosus tubers against Staphylococcus aureus. Microorganisms 9: 930. doi: 10.3390/microorganisms9050930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan W, Wang S, Wang H, Wang A, Jiang F, Liu H, Zhao H, Xu D, Zhang Y. 2022. The genomes of chicory, endive, great burdock and yacon provide insights into Asteraceae palaeo-polyploidization history and plant inulin production. Mol Ecol Resour 22: 3124–3140. doi: 10.1111/1755-0998.13675 [DOI] [PubMed] [Google Scholar]
- 14.Harding KL, Aguayo VM, Webb P. 2018. Hidden hunger in South Asia: a review of recent trends and persistent challenges. Public Health Nutr 21: 785–795. doi: 10.1017/S1368980017003202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He S, Xiong Q, Tian C, Li L, Zhao J, Lin X, Guo X, He Y, Liang W, Zuo X, Ying C. 2022. Inulin-type prebiotics reduce serum uric acid levels via gut microbiota modulation: a randomized, controlled crossover trial in peritoneal dialysis patients. Eur J Nutr 61: 665–677. doi: 10.1007/s00394-021-02669-y [DOI] [PubMed] [Google Scholar]
- 16.Hiel S, Bindels LB, Pachikian BD, Kalala G, Broers V, Zamariola G, Chang BPI, Kambashi B, Rodriguez J, Cani PD, Neyrinck AM, Thissen JP, Luminet O, Bindelle J, Delzenne NM. 2019. Effects of a diet based on inulin-rich vegetables on gut health and nutritional behavior in healthy humans. Am J Clin Nutr 109: 1683–1695. doi: 10.1093/ajcn/nqz001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kardosová A, Ebringerová A, Alföldi J, Nosál’ová G, Franová S, Hríbalová V. 2003. A biologically active fructan from the roots of Arctium lappa L., var. Herkules. Int J Biol Macromol 33: 135–140. doi: 10.1016/S0141-8130(03)00079-5 [DOI] [PubMed] [Google Scholar]
- 18.Korompokis K, Verbeke K, Delcour JA. 2021. Structural factors governing starch digestion and glycemic responses and how they can be modified by enzymatic approaches: a review and a guide. Compr Rev Food Sci Food Saf 20: 5965–5991. doi: 10.1111/1541-4337.12847 [DOI] [PubMed] [Google Scholar]
- 19.Kumar SA, Ward LC, Brown L. 2016. Inulin oligofructose attenuates metabolic syndrome in high-carbohydrate, high-fat diet-fed rats. Br J Nutr 116: 1502–1511. doi: 10.1017/S0007114516003627 [DOI] [PubMed] [Google Scholar]
- 20.Lepczyński A, Herosimczyk A, Barszcz M, Ożgo M, Taciak M, Skomiał J. 2016. Inulin-type fructans trigger changes in iron concentration and activity of bone metabolism biomarkers in blood plasma of growing pigs. J Anim Feed Sci 25: 343–347. doi: 10.22358/jafs/67471/2016 [DOI] [Google Scholar]
- 21.Li J, Zhang X, Cao L, Ji J, Gao J. 2018. Three Inulin-Type Fructans from Codonopsis pilosula (Franch.) Nannf. Roots and Their Prebiotic Activity on Bifidobacterium longum. Molecules 23: 3123. doi: 10.3390/molecules23123123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li L, Qiu Z, Dong H, Ma C, Qiao Y, Zheng Z. 2021. Structural characterization and antioxidant activities of one neutral polysaccharide and three acid polysaccharides from the roots of Arctium lappa L.: a comparison. Int J Biol Macromol 182: 187–196. doi: 10.1016/j.ijbiomac.2021.03.177 [DOI] [PubMed] [Google Scholar]
- 23.Man S, Liu T, Yao Y, Lu Y, Ma L, Lu F. 2021. Friend or foe? The roles of inulin-type fructans. Carbohydr Polym 252: 117155. doi: 10.1016/j.carbpol.2020.117155 [DOI] [PubMed] [Google Scholar]
- 24.Mennitti LV, Oyama LM, de Oliveira JL, Hachul AC, Santamarina AB, de Santana AA, Okuda MH, Ribeiro EB, Oller do Nascimento CM, Pisani LP. 2015. Maternal supplementation with oligofructose (10%) during pregnancy and lactation leads to increased pro-inflammatory status of the 21-D-old offspring. PLoS One 10: e0132038. doi: 10.1371/journal.pone.0132038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Misiakiewicz-Has K, Maciejewska-Markiewicz D, Rzeszotek S, Pilutin A, Kolasa A, Szumilas P, Stachowska E, Wiszniewska B. 2021. The obscure effect of Tribulus terrestris saponins plus inulin on liver morphology, liver fatty acids, plasma glucose, and lipid profile in SD rats with and without induced type 2 diabetes mellitus. Int J Mol Sci 22: 8680. doi: 10.3390/ijms22168680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moro TMA, Celegatti CM, Pereira APA, Lopes AS, Barbin DF, Pastore GM, Clerici MTPS. 2018. Use of burdock root flour as a prebiotic ingredient in cookies. LWT 90: 540–546. [Google Scholar]
- 27.Moro TMA, T P S Clerici M. 2021. Burdock (Arctium lappa L) roots as a source of inulin-type fructans and other bioactive compounds: current knowledge and future perspectives for food and non-food applications. Food Res Int 141: 109889. doi: 10.1016/j.foodres.2020.109889 [DOI] [PubMed] [Google Scholar]
- 28.Roberfroid MB. 2005. Introducing inulin-type fructans. Br J Nutr 93Suppl 1: S13–S25. doi: 10.1079/BJN20041350 [DOI] [PubMed] [Google Scholar]
- 29.Ruan Y, Ding Y, Li X, Zhang C, Wang M, Liu M, Wang L, Xing J, Hu L, Zhao X, Ding Z, Dong J, Liu Y. 2022. Saccharides from Arctium lappa L. root reduce platelet activation and thrombus formation in a laser injury thrombosis mouse model. Exp Ther Med 23: 344. doi: 10.3892/etm.2022.11274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sabanis D, Tzia C. 2007. Effect of rice, corn and soy flour addition on characteristics of bread produced from different wheat cultivars. Food Bioprocess Technol 2: 68–79. doi: 10.1007/s11947-007-0037-7 [DOI] [Google Scholar]
- 31.Shao T, Yuan P, Zhang W, Dou D, Wang F, Hao C, Liu C, Han J, Chen K, Wang G. 2021. Preparation and characterization of sulfated inulin-type fructans from Jerusalem artichoke tubers and their antitumor activity. Carbohydr Res 509: 108422. doi: 10.1016/j.carres.2021.108422 [DOI] [PubMed] [Google Scholar]
- 32.Song J, Li Q, Everaert N, Liu R, Zheng M, Zhao G, Wen J. 2020. Dietary inulin supplementation modulates short-chain fatty acid levels and cecum microbiota composition and function in chickens infected with Salmonella. Front Microbiol 11: 584380. doi: 10.3389/fmicb.2020.584380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan BL, Norhaizan ME, Liew WP. 2018. Nutrients and oxidative stress: friend or foe? Oxid Med Cell Longev 2018: 9719584. doi: 10.1155/2018/9719584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tawfick MM, Xie H, Zhao C, Shao P, Farag MA. 2022. Inulin fructans in diet: Role in gut homeostasis, immunity, health outcomes and potential therapeutics. Int J Biol Macromol 208: 948–961. doi: 10.1016/j.ijbiomac.2022.03.218 [DOI] [PubMed] [Google Scholar]
- 35.Verma V, Vishal B, Kohli A, Kumar PP. 2021. Systems-based rice improvement approaches for sustainable food and nutritional security. Plant Cell Rep 40: 2021–2036. doi: 10.1007/s00299-021-02790-6 [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Nan X, Zhao Y, Jiang L, Wang H, Hua D, Zhang F, Wang Y, Liu J, Yao J, Xiong B. 2021. Dietary supplementation with inulin improves lactation performance and serum lipids by regulating the rumen microbiome and metabolome in dairy cows. Anim Nutr 7: 1189–1204. doi: 10.1016/j.aninu.2021.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams J, Allen L, Wickramasinghe K, Mikkelsen B, Roberts N, Townsend N. 2018. A systematic review of associations between non-communicable diseases and socioeconomic status within low- and lower-middle-income countries. J Glob Health 8: 020409. doi: 10.7189/jogh.08.020409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia J, Guo Z, Fang S, Gu J, Liang X. 2021. Effect of drying methods on volatile compounds of burdock (Arctium lappa L.) root tea as revealed by gas chromatography mass spectrometry-based metabolomics. Foods 10: 868. doi: 10.3390/foods10040868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu J, Chen D, Liu C, Wu XZ, Dong CX, Zhou J. 2016. Structural characterization and anti-tumor effects of an inulin-type fructan from Atractylodes chinensis. Int J Biol Macromol 82: 765–771. doi: 10.1016/j.ijbiomac.2015.10.082 [DOI] [PubMed] [Google Scholar]
- 40.Yuan Pc, Shao Tl, Han J, Liu Cy, Wang Gd, He Sg, Xu Sx, Nian Sh, Chen Ks. 2021. Burdock fructooligosaccharide as an α-glucosidase inhibitor and its antidiabetic effect on high-fat diet and streptozotocin-induced diabetic mice. J Funct Foods 86: 104703. doi: 10.1016/j.jff.2021.104703 [DOI] [Google Scholar]
- 41.Zhang N, Wang Y, Kan J, Wu X, Zhang X, Tang S, Sun R, Liu J, Qian C, Jin C. 2019. In vivo and in vitro anti-inflammatory effects of water-soluble polysaccharide from Arctium lappa. Int J Biol Macromol 135: 717–724. doi: 10.1016/j.ijbiomac.2019.05.171 [DOI] [PubMed] [Google Scholar]
- 42.Zhang X, Herrera-Balandrano DD, Huang W, Chai Z, Beta T, Wang J, Feng J, Li Y. 2021. Comparison of nutritional and nutraceutical properties of burdock roots cultivated in Fengxian and Peixian of China. Foods 10: 2095. doi: 10.3390/foods10092095 [DOI] [PMC free article] [PubMed] [Google Scholar]