Abstract
BACKGROUND
Interventions to reduce sexually transmitted infections (STIs) among men who have sex with men (MSM) are needed.
METHODS
We conducted an open-label, randomized study involving MSM and transgender women who were taking preexposure prophylaxis (PrEP) against human immunodeficiency virus (HIV) infection (PrEP cohort) or living with HIV infection (persons living with HIV infection [PLWH] cohort) and who had had Neisseria gonorrhoeae (gonorrhea), Chlamydia trachomatis (chlamydia), or syphilis in the past year. Participants were randomly assigned in a 2:1 ratio to take 200 mg of doxycycline within 72 hours after condomless sex (doxycycline postexposure prophylaxis) or receive standard care without doxycycline. STI testing was performed quarterly. The primary end point was the incidence of at least one STI per follow-up quarter.
RESULTS
Of 501 participants (327 in the PrEP cohort and 174 in the PLWH cohort), 67% were White, 7% Black, 11% Asian or Pacific Islander, and 30% Hispanic or Latino. In the PrEP cohort, an STI was diagnosed in 61 of 570 quarterly visits (10.7%) in the doxycycline group and 82 of 257 quarterly visits (31.9%) in the standard-care group, for an absolute difference of −21.2 percentage points and a relative risk of 0.34 (95% confidence interval [CI], 0.24 to 0.46; P<0.001). In the PLWH cohort, an STI was diagnosed in 36 of 305 quarterly visits (11.8%) in the doxycycline group and 39 of 128 quarterly visits (30.5%) in the standard-care group, for an absolute difference of −18.7 percentage points and a relative risk of 0.38 (95% CI, 0.24 to 0.60; P<0.001). The incidences of the three evaluated STIs were lower with doxycycline than with standard care; in the PrEP cohort, the relative risks were 0.45 (95% CI, 0.32 to 0.65) for gonorrhea, 0.12 (95% CI, 0.05 to 0.25) for chlamydia, and 0.13 (95% CI, 0.03 to 0.59) for syphilis, and in the PLWH cohort, the relative risks were 0.43 (95% CI, 0.26 to 0.71), 0.26 (95% CI, 0.12 to 0.57), and 0.23 (95% CI, 0.04 to 1.29), respectively. Five grade 3 adverse events and no serious adverse events were attributed to doxycycline. Of the participants with gonorrhea culture available, tetracycline-resistant gonorrhea occurred in 5 of 13 in the doxycycline groups and 2 of 16 in the standard-care groups.
CONCLUSIONS
The combined incidence of gonorrhea, chlamydia, and syphilis was lower by two thirds with doxycycline postexposure prophylaxis than with standard care, a finding that supports its use among MSM with recent bacterial STIs. (Funded by the National Institutes of Health; DoxyPEP ClinicalTrials.gov number, NCT03980223.)
Rates of bacterial sexually transmitted infections (STIs) are increasing in the United States,1 with cisgender men who have sex with men (MSM) and transgender women being disproportionately affected. Increasing STI rates have been associated with a rise in serious illness, including blindness related to syphilitic ocular complications2 and congenital syphilis.1 Antimicrobial-resistant Neisseria gonorrhoeae (gonorrhea) continues to rise, leading to limited options for oral treatment,3,4 and is an emerging public health threat.5–7 Untreated STIs increase the risk of human immunodeficiency virus (HIV) acquisition among persons exposed to HIV and STI transmission from persons with HIV infection without virologic suppression.8–11
Effective and acceptable interventions are needed to halt the rise in syphilis, gonorrhea, and Chlamydia trachomatis (chlamydia).12 Doxycycline is effective against chlamydia and syphilis, is not used for gonorrhea treatment, has an excellent safety profile and low-cost generic formulations, and has limited drug–drug interactions, factors that make it a strong candidate for STI prophylaxis. Doxycycline postexposure prophylaxis (doxy-PEP) was associated with a 47% reduction in STI incidence in the IPERGAY (Intervention Preventive de l’Exposition aux Risques avec et pour les Gays) study among MSM taking event-driven preexposure prophylaxis (PrEP) against HIV infection.13 The relative reduction was almost 70% for syphilis and chlamydia, but there was no reduction in gonorrhea.14 A pilot study of doxy-PEP involving 30 MSM living with HIV infection showed a 73% reduction in a composite end point of bacterial STIs, warranting additional investigation.15 The DoxyPEP study was designed to assess the effectiveness, safety, acceptability, and effect on antimicrobial resistance of doxy-PEP in MSM and transgender women taking HIV PrEP or living with HIV infection for the prevention of bacterial STIs.
METHODS
STUDY DESIGN AND POPULATION
Participants were randomly assigned in a 2:1 ratio to receive doxy-PEP (doxycycline group) or standard care without doxy-PEP (standard-care group), to maximize data on adverse-event profile, adherence, and antibacterial resistance. The study was open-label to evaluate the net effectiveness of the intervention, including biologic efficacy and potential changes in sexual behavior. Randomization was performed according to study clinic with the use of variable block size and was stratified according to site. The study was powered to separately assess doxy-PEP effectiveness in persons receiving PrEP and persons living with HIV infection (PLWH) because of potential differences in adverse-event profile, adherence, STI incidence, sexual networks, sexual practices, and background antimicrobial resistance.
The study was conducted at two HIV clinics and two sexual health clinics in San Francisco and Seattle. Participants were eligible if they were at least 18 years of age, were assigned male sex at birth, had received a diagnosis of HIV or were taking or planning to start HIV PrEP, had a history of condomless anal or oral sex with a man in the previous 12 months, and had received a diagnosis of gonorrhea, chlamydia, or early syphilis in the previous 12 months. Participants were not eligible if they reported a tetracycline allergy, were taking medications with drug interactions with doxycycline, or were planning to take doxycycline for an extended period.
The authors vouch for the completeness and accuracy of the reported data and for the fidelity of the study to the protocol, which is available with the full text of this article at NEJM.org. All the participants provided written informed consent. The study protocol was approved by the University of California, San Francisco, institutional review board, which served as the primary institutional review board.
PROCEDURES
At enrollment, participants in the doxycycline groups received three bottles with 30 delayed-release tablets containing 200 mg of doxycycline hyclate. Participants subsequently received doxycycline tablets at quarterly intervals, as determined by the number of bottles used and participant request on the basis of frequency of sex. Participants were counseled to take 200 mg of doxycycline ideally within 24 hours but no later than 72 hours after any condomless anogenital, vaginal, or oral sex and to take no more than one dose every 24 hours.
Participants had quarterly visits with additional interim visits as needed for a total of 12 months. Sexual activity and symptoms during the previous 3 months were ascertained through computer-assisted surveys at scheduled quarterly visits. The doxycycline groups had quarterly assessments of adherence to the study regimen with different types of sex, side effects associated with doxycycline, and acceptability of doxycycline at the 6- and 12-month visits. Safety was assessed on the basis of Division of AIDS (DAIDS) adverse-event reporting16 for grade 2 and higher hematologic and hepatic laboratory abnormalities, for all DAIDS grade 3 and 4 adverse events that were deemed by the site investigators to be associated with doxycycline, and all serious adverse events.
We performed quarterly nucleic acid amplification testing of samples obtained from the pharynx, rectum, and urine to check for the presence of gonorrhea and chlamydia using Aptima (Hologic) or Xpert CT/NG (Cepheid). Blood was obtained for syphilis serologic studies, according to the Centers for Disease Control and Prevention (CDC) guidelines for serologic diagnosis.17 STI treatment was provided through the local standard of care, in accordance with current CDC guidelines for STIs.17 Before treatment, participants with a positive nucleic acid test for N. gonorrhoeae were asked to return for swabs for gonococcal culture with phenotypic resistance testing through CDC gonorrhea surveillance programs (Strengthening the United States Response to Resistant Gonorrhea and Antimicrobial Resistance Laboratory Network).18 The latter CDC program tests for resistance to tetracycline but not to doxycycline. However, in antimicrobial resistance testing, tetracycline is often used as a surrogate for doxycycline, a tetracycline-class antibiotic agent. Tetracycline resistance was defined as a minimum inhibitory concentration (MIC) of 2.0 μg per milliliter or greater by the agar dilution method.19 In the doxycycline groups, complete blood counts and liver-enzyme levels were measured at 3 and 9 months. In all groups, swabs from the anterior nares and oropharynx were obtained at baseline, 6 months, and 12 months for Staphylococcus aureus culture and doxycycline-resistance testing, with resistance defined as an MIC of 16 μg per milliliter or greater by ETEST (bioMérieux).20
END POINTS
The primary effectiveness end point was the incidence of at least one bacterial STI (gonorrhea, chlamydia, or syphilis) each quarter on the basis of testing during study visits or other clinical testing outside the study site. An independent committee whose members were unaware of the study-group assignments adjudicated all STI end-point events. The primary antimicrobial-resistance outcomes were tetracycline resistance in N. gonorrhoeae and S. aureus isolated at baseline as compared with organisms isolated during study follow-up. Secondary outcomes were the incidence of each individual STI (gonorrhea, chlamydia, and syphilis), safety, adverse-event profile, and acceptability.
STATISTICAL ANALYSIS
We estimated that a sample of 390 participants in each cohort (PrEP cohort and PLWH cohort) would provide the study with 80% power, at a two-sided type I error of 0.05, to detect a 50% lower combined incidence of gonorrhea, chlamydia, and early syphilis per quarter with doxy-PEP than with standard care, assuming a 10% annual loss to follow-up and an intraclass correlation of 0.2. These values correspond to an annual incidence of STIs of 34% with standard care and 19% with doxy-PEP. For each quarter, incident STIs were analyzed as binary outcomes; participants with multiple STIs in a quarter contributed once to each quarterly end point.
An independent data and safety monitoring board reviewed study progress and safety and effectiveness data every 6 months, with a single interim effectiveness analysis at approximately 50% of follow-up time. O’Brien–Fleming stopping boundaries for effectiveness were based on a one-sided alpha level of 0.025 for each cohort. The data and safety monitoring board recommended early discontinuation of the standard-care groups on May 13, 2022, because both the PrEP and PLWH cohorts had crossed the stopping boundary for effectiveness, and recommended that all the participants assigned to receive standard care be offered doxy-PEP. This report covers the period between enrollment and the end of the randomized part of the study.
The modified intention-to-treat population comprises all randomly assigned participants who were enrolled long enough to qualify for at least one quarter of follow-up at the interim efficacy review. Prespecified subgroups for effectiveness included age (≤30 and >30 years) and one STI as compared with more than one STI in the previous year. STI incidence according to study group was compared by estimating relative risks of any STI at quarterly visits; we used a modified Poisson model fitted according to generalized-estimating-equation methods to account for repeated observations within individual participants, assuming an independent covariance structure, with city and study group as covariates. P values and 95% confidence intervals were computed with the use of robust standard errors. The same analysis was conducted for individual STIs. Confidence intervals were not adjusted for multiple comparisons.
RESULTS
STUDY PARTICIPANTS
From August 19, 2020, through May 13, 2022, the study enrolled 637 participants, with 501 in the modified intention-to-treat population (Fig. 1). In the 3 months before enrollment, participants reported a median of 9 sexual partners (interquartile range, 4 to 17), a median of 5 sexual acts per month (interquartile range, 1.7 to 10.7), and 90.1% of sex as condomless. At enrollment, 30% of the participants had an STI diagnosed, and 43% had two or more STIs diagnosed in the previous year (Table 1).
Figure 1. Enrollment and Follow-up of the Study Participants.
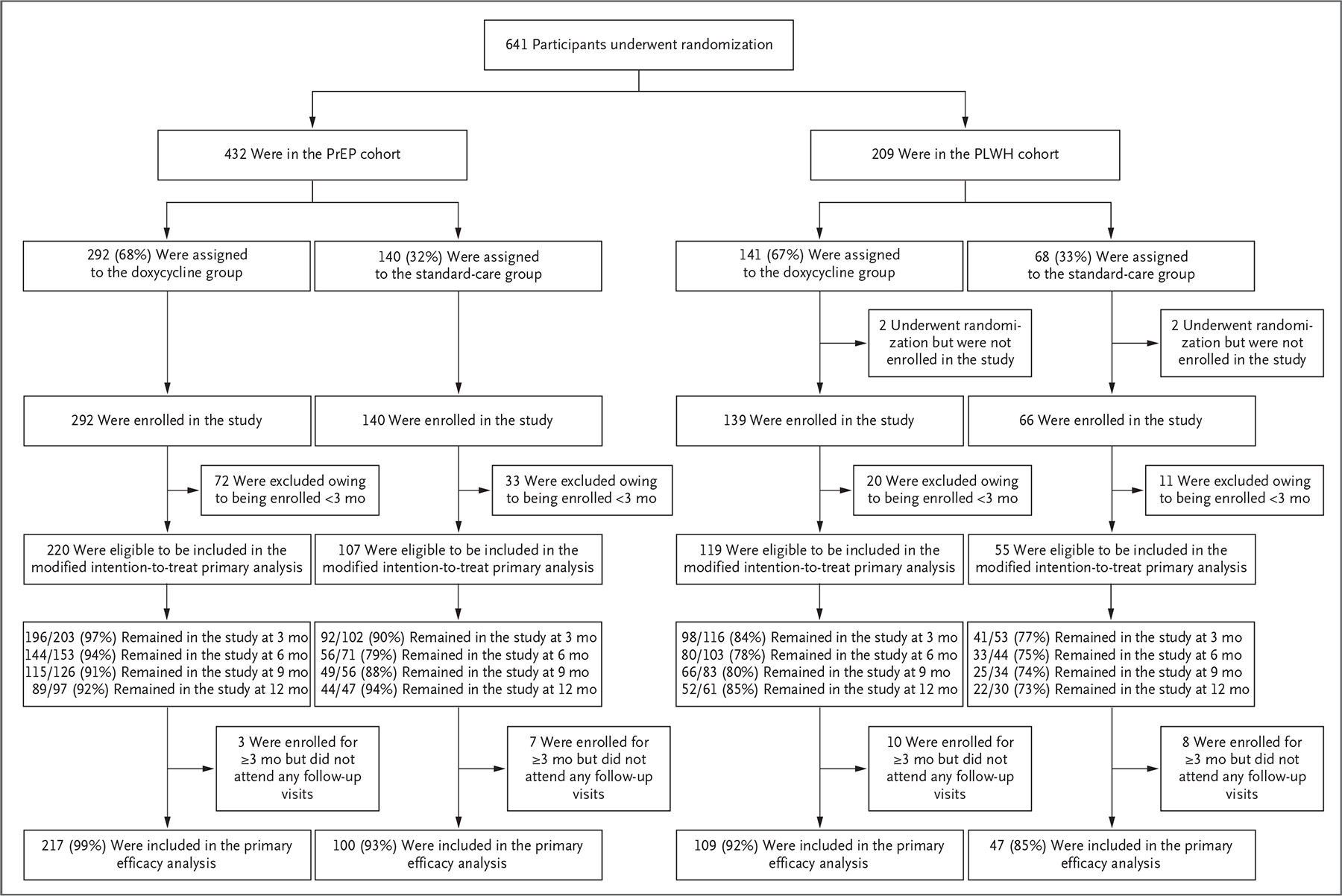
There was no separate screening visit; thus, reasons for screening failure were not collected. The study had two cohorts: those who were taking preexposure prophylaxis (PrEP) against human immunodeficiency virus (HIV) infection (PrEP cohort) and persons living with HIV infection (PLWH cohort). Within each cohort, participants in the doxycycline group were assigned to take doxycycline within 72 hours after condomless sex (doxycycline postexposure prophylaxis [doxy-PEP]), and participants in the standard-care group were assigned to receive standard care without doxycycline. Of the 4 participants in the PLWH cohort who underwent randomization but were not enrolled in the study, 3 immediately withdrew consent after receiving their randomization assignment and 1 was withdrawn at the investigator’s discretion. A total of 136 participants were enrolled but had not yet reached the month 3 visit at the time of this analysis. Visit attendance indicates the percentage who had completed visits for which they were eligible at the time that the data and safety monitoring board met. A total of 28 participants remained in follow-up but had not completed a follow-up visit. A total of 473 of 501 participants (94%) in the modified intention-to-treat population contributed follow-up visit data for the primary efficacy analysis. A total of 18 participants discontinued the study early: 5 in the doxycycline groups (1 moved, 1 had a new job, 1 was in a monogamous relationship, and 2 gave no reason) and 13 in the standard-care groups (2 moved, 1 had a new job, 6 wanted doxy-PEP, 1 had a concern about coronavirus disease 2019, and 3 gave no reason).
Table 1.
Characteristics of the Participants at Baseline (Modified Intention-to-Treat Population).*
| Characteristic | PrEP Cohort | PLWH Cohort | Total (N =501) | ||
|---|---|---|---|---|---|
| Doxycycline Group (N = 220) |
Standard-Care Group (N = 107) |
Doxycycline Group (N = 119) |
Standard-Care Group (N =55) |
||
| Median age (IQR) —yr | 36 (31–42) | 36 (31–42) | 43 (36–54) | 42 (37–50) | 38 (32–47) |
| Race — no./total no. (%)† | |||||
| White | 144/209 (69) | 66/104 (63) | 74/116 (64) | 37/53 (70) | 321/482 (67) |
| Black | 9/209 (4) | 5/104 (5) | 15/116 (13) | 7/53 (13) | 36/482 (7) |
| Asian or Pacific Islander | 33/209 (16) | 12/104 (12) | 7/116 (6) | 1/53 (2) | 53/482 (11) |
| Multiple races or other | 23/209 (11) | 21/104 (20) | 20/116 (17) | 8/53 (15) | 72/482 (15) |
| Hispanic or Latino ethnic group — no. (%)† | 55 (25) | 41 (38) | 41 (34) | 14 (25) | 151 (30) |
| Gender identity — no. (%) | |||||
| Man | 212 (96) | 107 (100) | 109 (92) | 54 (98) | 482 (96) |
| Transgender woman or gender-diverse | 8 (4) | 0 | 10 (8) | 1 (2) | 19 (4) |
| Gender of sexual partners — no./total no. (%) | |||||
| Men only | 191/220 (87) | 90/107 (84) | 105/118 (89) | 48/55 (87) | 434/500 (87) |
| Multiple genders | 29/220 (13) | 17/107 (16) | 13/118 (11) | 7/55 (13) | 66/500 (13) |
| Annual income— no./total no. (%) | |||||
| <$20,000 | 31/219 (14) | 13/106 (12) | 42/119 (35) | 17/55 (31) | 103/499 (21) |
| $20,001-$50,000 | 64/219 (29) | 39/106 (37) | 40/119 (34) | 22/55 (40) | 165/499 (33) |
| $50,001-$75,000 | 45/219 (21) | 14/106 (13) | 22/119 (18) | 5/55 (9) | 86/499 (17) |
| >$75,000 | 79/219 (36) | 40/106 (38) | 15/119 (13) | 11/55 (20) | 145/499 (29) |
| STI in the past 12 mo — no. (%) | |||||
| Gonorrhea | 155 (70) | 78 (73) | 71 (60) | 39 (71) | 343 (68) |
| Chlamydia | 144 (65) | 63 (59) | 58 (49) | 27 (49) | 292 (58) |
| Syphilis‡ | 32 (15) | 16 (1) | 35 (29) | 17 (31) | 100 (20) |
| Two or more STIs in the past 12 mo — no. (%) | 106 (48) | 44 (41) | 39 (33) | 26 (47) | 215 (43) |
| Any STI at baseline — no./total no. (%) | 65/219 (30) | 27/106 (25) | 34/114 (30) | 20/55 (36) | 146/494 (30) |
| Gonorrhea | 40/218 (18) | 20/107 (19) | 25/117 (21) | 14/54 (26) | 99/496 (20) |
| Chlamydia | 31/219 (14) | 11/107 (10) | 11/117 (9) | 8/54 (15) | 61/497 (12) |
| Syphilis | 5/219 (2) | 1/107 (1) | 11/117 (9) | 4/55 (7) | 21/498 (4) |
| Median no. of sexual partners in the past 3 mo (IQR) | 8 (4–17) | 10 (5–16.5) | 7 (3–18.5) | 10.5 (3–20) | 9 (4–17) |
| Transactional sex during lifetime — no./total no. (%)§ | 47/219 (21) | 28/107 (26) | 47/116 (41) | 21/49 (43) | 143/491 (29) |
| Substance use in the past 3 mo — no./total no. (%) | 112/216 (52) | 66/107 (62) | 77/117 (66) | 38/53 (72) | 293/493 (59) |
| Stimulants: methamphetamine, cocaine, or crack | 51/216 (24) | 22/107 (21) | 50/117 (43) | 23/53 (43) | 146/493 (30) |
| Heroin or other opioids | 2/216 (1) | 1/107 (1) | 9/117 (8) | 2/53 (4) | 14/493 (3) |
| Ecstasy, GHB, or ketamine | 63/216 (29) | 34/107 (32) | 39/117 (33) | 21/53 (40) | 157/493 (32) |
| Amyl nitrates, also known as poppers | 93/216 (43) | 47/107 (44) | 56/117 (48) | 28/53 (53) | 224/493 (45) |
| Marijuana | 96/216 (44) | 56/107 (52) | 60/117 (51) | 27/53 (51) | 239/493 (48) |
The study had two cohorts: those who were taking preexposure prophylaxis (PrEP) against human immunodeficiency virus (HIV) infection (PrEP cohort) and persons living with HIV infection (PLWH cohort). Within each cohort, participants in the doxycycline group were assigned to take doxycycline within 72 hours after condomless sex (doxycycline postexposure prophylaxis, or doxy-PEP]), and participants in the standard-care group were assigned to receive standard care without doxycycline. The modified intention-to-treat population includes all the participants with at least one follow-up visit expected by May 13, 2022 (date of early discontinuation of the standard-care groups by the data and safety monitoring board). GHB denotes γ-hydroxybutyrate, IQR interquartile range, and STI sexually transmitted infection.
Race and ethnic group were reported by the participant.
Syphilis includes only primary, secondary, or early latent syphilis.
Transactional sex includes either paying for sex or being paid for sex.
FOLLOW-UP AND RETENTION
The median time in the study as of the interim efficacy review was 270 days (interquartile range, 104 to 371). Of the 501 participants eligible for a follow-up visit by the time of the interim analysis, 473 (94%) had at least one follow-up visit (Fig. 1). A total of 18 participants discontinued the study early: 5 in the doxycycline groups and 13 in the standard-care groups, including 6 who discontinued in order to receive doxy-PEP outside the study.
EFFECTIVENESS AGAINST INCIDENT STIS
In the PrEP cohort, at least one STI was diagnosed in 61 of 570 quarterly visits (10.7%) in the doxycycline group and 82 of 257 quarterly visits (31.9%) in the standard-care group, for an absolute difference of −21.2 percentage points and a relative risk of 0.34 (95% confidence interval [CI], 0.24 to 0.46; P<0.001) (Fig. 2A). In the PLWH cohort, at least one STI was diagnosed in 36 of 305 quarterly visits (11.8%) in the doxycycline group and 39 of 128 quarterly visits (30.5%) in the standard-care group, for an absolute difference of −18.7 percentage points and a relative risk of 0.38 (95% CI, 0.24 to 0.60; P<0.001) (Fig. 2B). The number needed to treat to prevent a quarter with an incident STI was 4.7 in the PrEP cohort and 5.3 in the PLWH cohort.
Figure 2. Primary, Secondary, and Subgroup Analyses of Effectiveness against Incident Sexually Transmitted Infections (STIs).
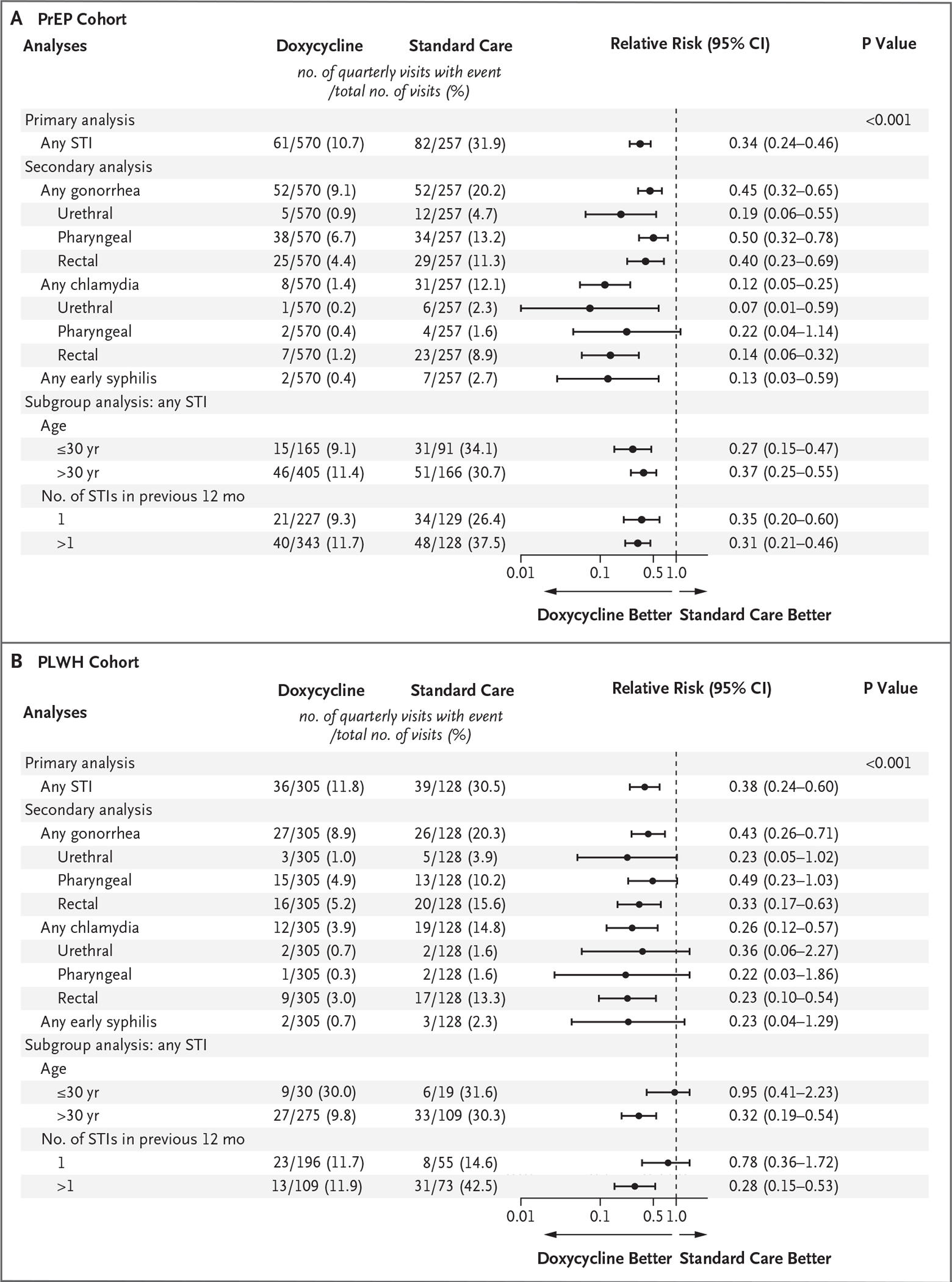
Confidence intervals have not been adjusted for multiple testing.
Of the individual STI end points, gonorrhea was the most frequent. In the PrEP cohort, the quarterly incidence of gonorrhea was 9.1% (52 of 570 quarters) in the doxycycline group and 20.2% (52 of 257 quarters) in the standard-care group (relative risk, 0.45; 95% CI, 0.32 to 0.65). In the PLWH cohort, the quarterly incidence of gonorrhea was 8.9% (27 of 305 quarters) and 20.3% (26 of 128 quarters), respectively (relative risk, 0.43; 95% CI, 0.26 to 0.71), with a substantially lower risk of gonorrhea at all anatomical sites with doxy-PEP than with standard care in the PrEP cohort and a substantially lower risk of rectal gonorrhea, the most common site of gonorrhea, with doxy-PEP than with standard care in the PLWH cohort.
Chlamydia was diagnosed in 70 quarters. In the PrEP cohort, the quarterly incidence of chlamydia was 1.4% (8 of 570 quarters) in the doxycycline group and 12.1% (31 of 257 quarters) in the standard-care group (relative risk, 0.12; 95% CI, 0.05 to 0.25). In the PLWH cohort, the quarterly incidence of chlamydia was 3.9% (12 of 305 quarters) in the doxycycline group and 14.8% (19 of 128 quarters) in the standard-care group (relative risk, 0.26; 95% CI, 0.12 to 0.57).
Early syphilis was diagnosed in 14 quarters. In the PrEP cohort, the quarterly incidence of syphilis was 0.4% (2 of 570 quarters) in the doxycycline group and 2.7% (7 of 257 quarters) in the standard-care group (relative risk, 0.13; 95% CI, 0.03 to 0.59). In the PLWH cohort, the quarterly incidence of syphilis was 0.7% (2 of 305 quarters) and 2.3% (3 of 128 quarters), respectively (relative risk, 0.23; 95% CI, 0.04 to 1.29).
In a secondary analysis of time to first STI, the incidence was lower by 66% with doxy-PEP than with standard care in the PrEP cohort (hazard ratio, 0.34, 95% CI, 0.23 to 0.51) and by 52% in the PLWH cohort (hazard ratio, 0.48; 95% CI, 0.28 to 0.83) (Fig. 3). The per-protocol analyses involving participants with no doxycycline discontinuation and participant report of always using doxy-PEP indicate similar effectiveness of doxy-PEP in reducing the incidence of STIs (see the Supplementary Appendix, available at NEJM.org).
Figure 3. Kaplan–Meier Estimate of Time to First STI Diagnosis.
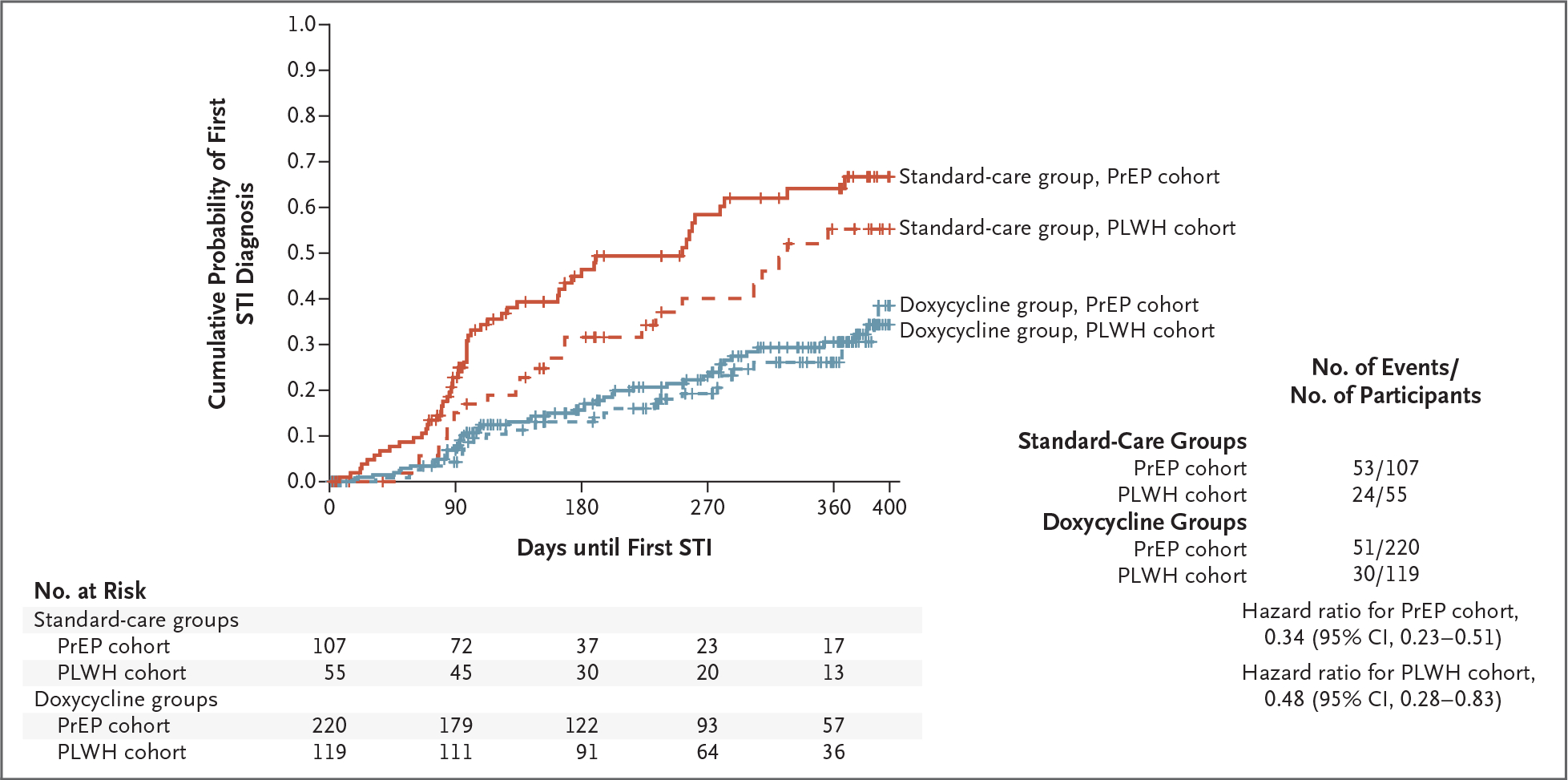
The cumulative probability of any incident bacterial STI (chlamydia, gonorrhea, or syphilis) is shown according to study group (doxycycline and standard care) and participant cohort (PrEP and PLWH).
ADHERENCE TO DOXY-PEP
In the doxycycline groups, 86% of participants reported taking doxy-PEP consistently (always or often) within 72 hours after condomless anal or vaginal sex and 71% reported never missing doxycycline after condomless sex. On the basis of quarterly computer-assisted questionnaires, the median number of doxycycline doses taken after condomless anal or vaginal sex per month was estimated to be 4.0 doses (interquartile range, 1.0 to 10.0).
SAFETY, ADVERSE EVENT, AND ACCEPTABILITY
One grade 2 laboratory abnormality (transaminitis) and five grade 3 adverse events (three diarrheal events and two headaches or migraines) occurred that were possibly or probably related to doxycycline. No serious adverse events were attributed by the site investigators to doxycycline (Table S4 in the Supplementary Appendix). Of participants assigned to the doxycycline groups, 2% discontinued because of unacceptable adverse events or patient preference. The observed difference in annualized mean absolute weight change, adjusted for baseline weight, was not substantial: −0.78 kg (95% CI, −2.12 to 0.54) in the doxycycline groups and 0.20 kg (95% CI, −1.32 to 1.72) in the standard-care groups. Among participants in the doxycycline groups, 89% reported that taking doxy-PEP was acceptable or very acceptable.
ANTIMICROBIAL RESISTANCE
Among the gonorrhea diagnoses at study baseline and during follow-up, 44 of 256 (17.2%) had results for phenotypic resistance to tetracycline. Cultures were limited owing to an inability to collect culture before gonorrhea treatment in half the participants as well as a lack of culture growth, which is more common with extragenital gonorrhea. At baseline, tetracycline resistance was observed in 4 of 15 N. gonorrhoeae isolates (27%); after enrollment, it was observed in 5 of 13 isolates (38%) in the doxycycline groups and 2 of 16 (12%) in the standard-care groups (Fig. 4A).
Figure 4. Antimicrobial Resistance and Culture Positivity in Neisseria gonorrhoeae and Staphylococcus aureus.
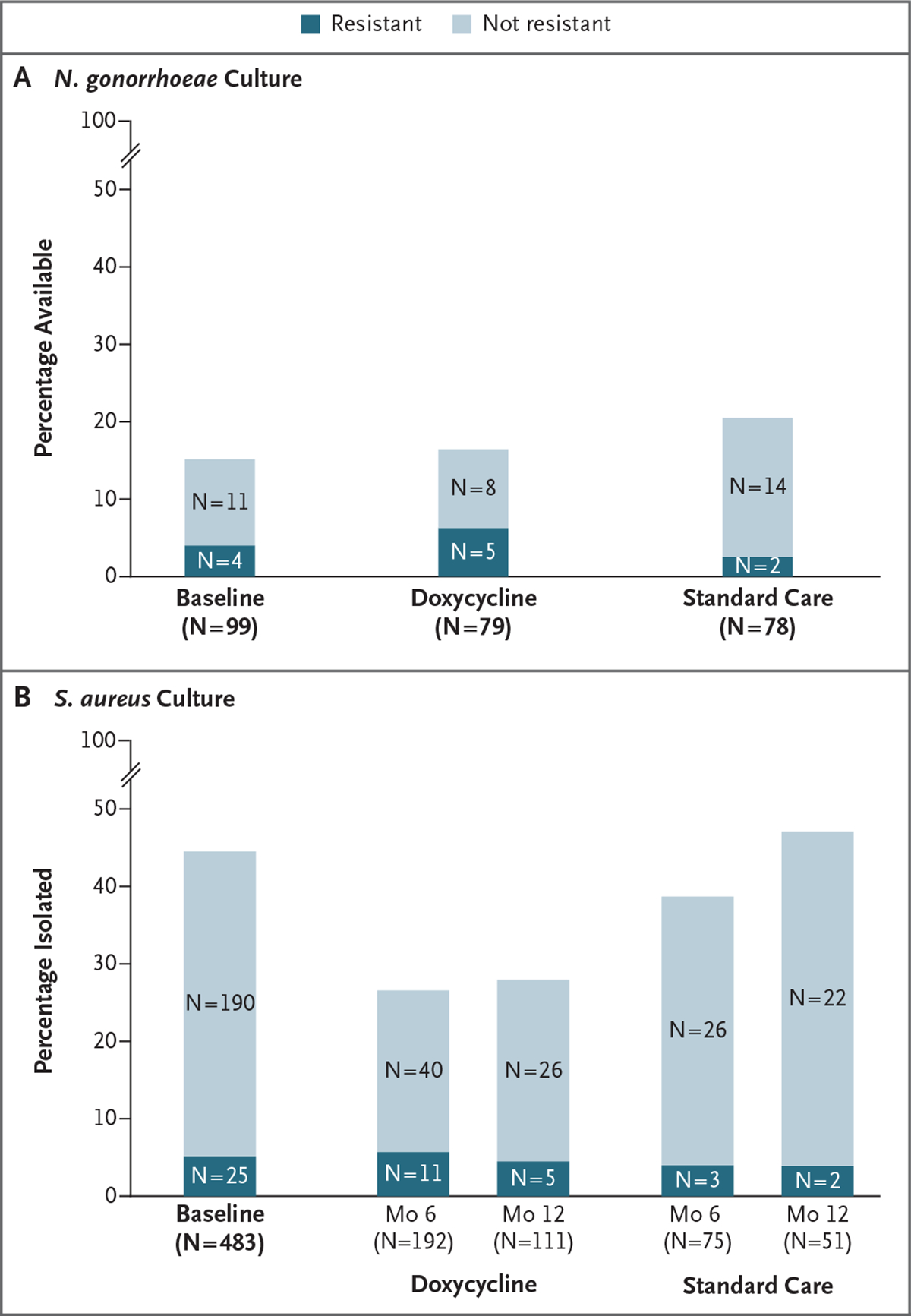
In Panel A, the bar height represents N. gonorrhoeae cultures obtained from participants with lab-confirmed gonorrhea at baseline and for adjudicated gonorrhea end points according to study group during follow-up. Of the gonorrhea diagnoses, 44 of 256 N. gonorrhoeae infections (17.2%) had data available for resistance testing. The dark shading represents high-level tetracycline resistance (minimum inhibitory concentration [MIC], ≥2 μg per milliliter). The light shading represents N. gonorrhoeae without high-level tetracycline resistance. Gonorrhea culture was performed through the Centers for Disease Control and Prevention (CDC) Strengthening the United States Response to Resistant Gonorrhea program, and tetracycline-resistance testing was performed by agar dilution through the CDC Antimicrobial Resistance Laboratory Network. With respect to Panel B, all the participants had oronasopharyngeal swabs obtained at enrollment and at months 6 and 12, which were cultured for S. aureus. The bar height represents the percentage culture-positive for S. aureus, and the dark shading represents specimens with doxycycline resistance by ETEST (MIC, ≥16 μg per milliliter).
At baseline, S. aureus was isolated from the oronasopharynx in 45% of the participants, and 12% had doxycycline-resistant S. aureus. At month 12, S. aureus was isolated in 28% in the doxycycline groups and 47% in the standard-care groups (P = 0.03), with doxycycline-resistant isolates in 16% and 8%, respectively (overall percentage with resistance, 5% in the doxycycline groups and 4% in the standard-care groups) (Fig. 4B).
DISCUSSION
Doxy-PEP taken within 72 hours after condomless sex decreased incident gonorrhea, chlamydia, and early syphilis by two thirds among MSM and transgender women who had had a bacterial STI in the previous year. Doxy-PEP substantially reduced the incidence of each bacterial STI, including gonorrhea, with consistent reductions in the PrEP and PLHV cohorts. No concerns with respect to adverse-event profile, safety, or acceptability were identified.
These results showed effectiveness of doxy-PEP among MSM regardless of HIV status in a socioeconomically and racially diverse population. Although the IPERGAY study of doxy-PEP showed no reduction in incident gonorrhea, the DoxyPEP study showed an approximately 55% reduction in incident gonorrhea, including pharyngeal gonorrhea. Potential hypotheses for the difference in effectiveness against gonorrhea in these studies include the amount of doxycycline taken, adherence, and the prevalence of tetracycline resistance in N. gonorrhoeae isolates. The IPERGAY study limited participants to a weekly maximum of 3 doses of 200-mg doxycycline; an average of 3.4 doses were taken per month.13 The DoxyPEP study permitted up to daily administration of doxycycline, with a median of 4 doses reported per month; however, 25% of the participants took 10 doses or more on the basis of the interquartile range, with 86% participant-reported adherence with condomless sex. Higher exposure to doxycycline may have improved effectiveness against gonorrhea, particularly in the pharynx, where higher concentrations of antibiotics are needed to eradicate N. gonorrhoeae.21 In addition, tetracycline resistance in gonorrhea was more prevalent in France when the IPERGAY study was conducted (56%,14 vs. 20% in the United States1). The higher proportion of incident gonorrhea with tetracycline resistance in the doxycycline groups than in the control groups suggests potential decreased protection against circulating tetracycline-resistant N. gonorrhoeae isolates; however, the number of available gonorrhea cultures was low. Given the potential for tetracycline resistance to reduce the effectiveness of doxy-PEP against gonorrhea, population-based surveillance of tetracycline-class susceptibility in N. gonorrhoeae isolates is important.
The effectiveness of doxy-PEP against chlamydia was 88% in the PrEP cohort and 74% in the PLWH cohort. Although the number of incident syphilis diagnoses was low, there was a reduction in the doxycycline group within the PrEP cohort and a trend toward a reduction in the PLWH cohort, findings consistent with relative reductions in syphilis shown in the IPERGAY study and in the doxycycline PrEP study involving PLWH.13,15
The effect of prophylactic antibiotic strategies on antimicrobial resistance is an important consideration. S. aureus carriage was 40% lower in the doxycycline groups than in the standard-care groups at month 12. Among the participants with colonization, a modestly higher proportion had doxycycline resistance in the doxycycline groups than in the standard-care groups, which could have clinical implications, because doxycycline can be used to treat methicillin-resistant S. aureus skin and soft-tissue infections. However, there was not a substantial difference in overall doxycycline-resistant S. aureus isolated between the doxycycline groups and the standard-care groups (5% and 4%, respectively). Tetracycline-class resistance has not been convincingly shown in C. trachomatis, although tetracycline resistance has been described in related Chlamydia suis.22 Chlamydial culture will be useful to monitor phenotypic resistance to doxycycline in break-through infections23 and is ongoing in this study. Additional research and longer follow-up are needed to determine whether doxy-PEP is associated with substantial selection of resistance in commensal oropharyngeal neisseria species, the gut microbiome, and other STI pathogens such as Mycoplasma genitalium.
Our study has several limitations. Measuring adherence to doxy-PEP was limited by the challenges of accurately ascertaining condomless sex and event-driven PEP use. Quarterly computer-assisted surveys recorded sexual activity and doxycycline use; however, these are limited by recall. Tetracycline susceptibility results were available in only 17% of gonorrhea end points, because only half the participants with incident gonorrhea infections during follow-up had N. gonorrhoeae culture obtained before treatment and because of lower cultivability from extragenital infections.24 The availability of N. gonorrhoeae culture was similar in the IPERGAY study.13 The 27% baseline tetracycline resistance in the DoxyPEP study is consistent with 19.7% resistance from the 2020 U.S. Gonococcal Isolate Surveillance Project data.1 Enrollment of transgender women was less than 5%, which limits generalizability in this population. This study was conducted in two West Coast cities; acceptability, adherence, and STI rates may vary in other settings. For generalizability considerations to broader MSM populations, see Table S7.
The effectiveness data provide evidence for doxy-PEP as an effective STI prevention intervention for MSM with recent STIs and ongoing condomless sex, regardless of HIV serostatus. The number needed to treat is approximately 5 to prevent a quarter with an STI end-point event, when doxy-PEP is offered to MSM and transgender women with a very elevated STI risk (30% quarterly incidence in the standard-care group). The role of doxy-PEP in other populations who are disproportionately affected by STIs, including cisgender women taking HIV PrEP, and in settings with a high prevalence of tetracycline resistance in N. gonorrhoeae isolates warrants further study.
Supplementary Material
Acknowledgments
Supported by a grant (R01 AI143439) from the Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health. Hologic and Cepheid donated N. gonorrhoeae and C. trachomatis testing kits, cartridges, and reagents, and Mayne Pharma donated doxycycline, but these entities did not provide financial support.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
We thank the participants for their altruism in participating in this study and the members of the data and safety monitoring board (Drs. Sheena McCormack [chair], Jim Hughes, James Kiarie, Kenneth Mayer, Frances Ndowa, and Kim Workowski) and the nonauthor members of the end-point adjudication committee (Oliver Bacon, Lindley Barbee, and Meena Ramchandani) for their oversight.
Footnotes
The members of the DoxyPEP Study Team are listed in the Supplementary Appendix, available at NEJM.org.
Contributor Information
Anne F. Luetkemeyer, Zuckerberg San Francisco General, Hospital and Trauma Center, San Francisco Department of Medicine, San Francisco.
Deborah Donnell, Fred Hutchinson Cancer Center, Seattle
Julia C. Dombrowski, Department of Medicine, Seattle University of Washington, and Public Health-Seattle and King County, Seattle.
Stephanie Cohen, Department of Medicine, San Francisco University of California, San Francisco, and San Francisco Department of Public Health, Population Health Division, San Francisco.
Cole Grabow, Department of Global Health, Seattle
Clare E. Brown, Department of Global Health, Seattle
Cheryl Malinski, Public Health–Seattle King County, Seattle University of Washington, and Public Health-Seattle and King County, Seattle.
Rodney Perkins, Department of Global Health, Seattle School of Nursing, Seattle.
Melody Nasser, University of California, San Francisco, and San Francisco Department of Public Health, Population Health Division, San Francisco
Carolina Lopez, Zuckerberg San Francisco General, Hospital and Trauma Center, San Francisco Department of Medicine, San Francisco.
Eric Vittinghoff, Department of Medicine, San Francisco
Susan P. Buchbinder, University of California, San Francisco, and San Francisco Department of Public Health, Population Health Division, San Francisco
Hyman Scott, University of California, San Francisco, and San Francisco Department of Public Health, Population Health Division, San Francisco
Edwin D. Charlebois, Department of Epidemiology and Biostatistics, San Francisco
Diane V. Havlir, Zuckerberg San Francisco General, Hospital and Trauma Center, San Francisco Department of Medicine, San Francisco.
Olusegun O. Soge, Department of Medicine, Seattle Department of Global Health, Seattle.
Connie Celum, Department of Medicine, Seattle Department of Global Health, Seattle; Department of Epidemiology, Seattle.
REFERENCES
- 1.Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2020. August 22, 2022. (https://www.cdc.gov/std/statistics/2020/default.htm).
- 2.Oliver SE, Aubin M, Atwell L, et al. Ocular syphilis — eight jurisdictions, United States, 2014–2015. MMWR Morb Mortal Wkly Rep 2016;65:1185–8. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2019: Gonococcal Isolate Surveillance Project (GISP) supplement and profiles. July 29, 2021. (https://www.cdc.gov/std/statistics/2019/gisp/docs/GISP_2019_Supplement_eClearance.pdf).
- 4.Wi T, Lahra MM, Ndowa F, et al. Antimicrobial resistance in Neisseria gonorrhoeae: global surveillance and a call for international collaborative action. PLoS Med 2017;14(7):e1002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbee LA, St Cyr SB. Management of Neisseria gonorrhoeae in the United States: summary of evidence from the development of the 2020 gonorrhea treatment recommendations and the 2021 Centers for Disease Control and prevention sexually transmitted infection treatment guidelines. Clin Infect Dis 2022; 74:Suppl 2:S95–S111. [DOI] [PubMed] [Google Scholar]
- 6.Schlanger K, Kirkcaldy RD. Rising to meet the programmatic public health challenges of emerging neisseria gonorrhoeae antimicrobial resistance: strengthening the United States response to resistant gonorrhea. Sex Transm Dis 2021;48:Suppl 2: S91–S92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quilter LAS, St Cyr SB, Hong J, et al. Antimicrobial susceptibility of urogenital and extragenital neisseria gonorrhoeae isolates among men who have sex with men: strengthening the US response to resistant gonorrhea and enhanced gonococcal isolate surveillance project, 2018 to 2019. Sex Transm Dis 2021;48:Suppl 2:S111–S117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malekinejad M, Barker EK, Merai R, et al. Risk of HIV acquisition among men who have sex with men infected with bacterial sexually transmitted infections: a systematic review and meta-analysis. Sex Transm Dis 2021;48(10):e138–e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelley CF, Vaughan AS, Luisi N, et al. The effect of high rates of bacterial sexually transmitted infections on HIV incidence in a cohort of black and white men who have sex with men in Atlanta, Georgia. AIDS Res Hum Retroviruses 2015;31:587–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lucar J, Hart R, Rayeed N, et al. Sexually transmitted infections among HIV-infected individuals in the District of Columbia and estimated HIV transmission risk: data from the DC cohort. Open Forum Infect Dis 2018;5(2):ofy017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pathela P, Jamison K, Braunstein SL, Schillinger JA, Varma JK, Blank S. Incidence and predictors of HIV infection among men who have sex with men attending public sexually transmitted disease clinics, New York City, 2007–2012. AIDS Behav 2017;21:1444–51. [DOI] [PubMed] [Google Scholar]
- 12.Eisinger RW, Erbelding E, Fauci AS. Re-focusing research on sexually transmitted infections. J Infect Dis 2020;222:1432–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molina JM, Charreau I, Chidiac C, et al. Post-exposure prophylaxis with doxycycline to prevent sexually transmitted infections in men who have sex with men: an open-label randomised substudy of the ANRS IPERGAY trial. Lancet Infect Dis 2018;18:308–17. [DOI] [PubMed] [Google Scholar]
- 14.La Ruche G, Goubard A, Bercot B, Cambau E, Semaille C, Sednaoui P. Gonococcal infections and emergence of gonococcal decreased susceptibility to cephalosporins in France, 2001 to 2012. Euro Surveill 2014;19:20885. [DOI] [PubMed] [Google Scholar]
- 15.Bolan RK, Beymer MR, Weiss RE, Flynn RP, Leibowitz AA, Klausner JD. Doxycycline prophylaxis to reduce incident syphilis among HIV-infected men who have sex with men who continue to engage in high-risk sex: a randomized, controlled pilot study. Sex Transm Dis 2015;42:98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Institutes of Health. DAIDS adverse event grading tables. February 1, 2018. (https://rsc.niaid.nih.gov/clinical-research-sites/daids-adverse-event-grading-tables). [Google Scholar]
- 17.Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Re-comm Rep 2021;70:1–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Combating the threat of antibiotic-resistant gonorrhea. December 23, 2022. (https://www.cdc.gov/std/gonorrhea/arg/carb.htm).
- 19.Centers for Disease Control and Prevention. Agar dilution antimicrobial susceptibility testing. December 10, 2013. (https://www.cdc.gov/std/gonorrhea/lab/agar.htm).
- 20.Performance standards for antimicrobial susceptibility testing, M100, 31st ed. Wayne, PA: Clinical and Laboratory Standards Institute, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bamberger DM, Graham G, Dennis L, Gerkovich MM. Extragenital gonorrhea and chlamydia among men and women according to type of sexual exposure. Sex Transm Dis 2019;46:329–34. [DOI] [PubMed] [Google Scholar]
- 22.Unterweger C, Schwarz L, Jelocnik M, et al. Isolation of tetracycline-resistant chlamydia suis from a pig herd affected by reproductive disorders and conjunctivitis. Antibiotics (Basel) 2020;9:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suchland RJ, Geisler WM, Stamm WE. Methodologies and cell lines used for antimicrobial susceptibility testing of chlamydia spp. Antimicrob Agents Chemother 2003;47:636–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nash EE, Pham CD, Raphael B, et al. Impact of anatomic site, specimen collection timing, and patient symptom status on neisseria gonorrhoeae culture recovery. Sex Transm Dis 2021;48:Suppl 2: S151–S156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


