Abstract
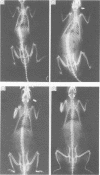
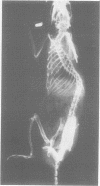
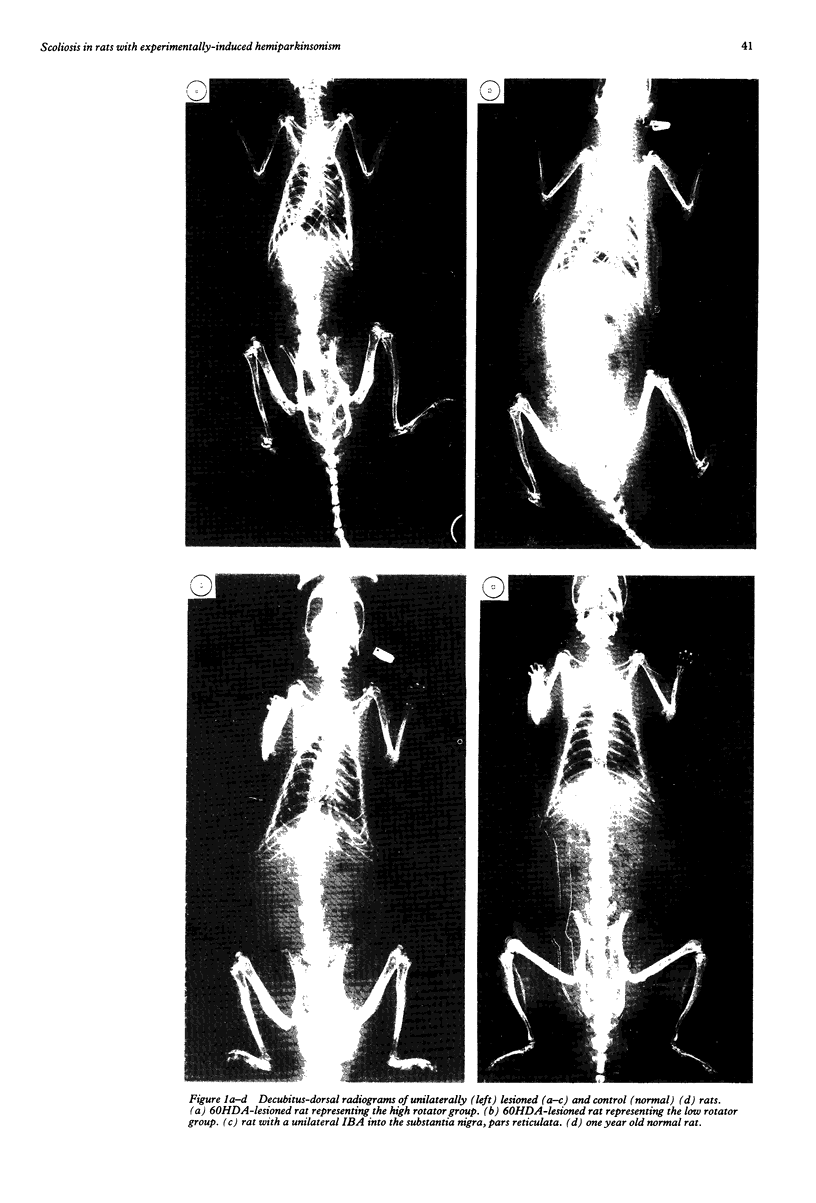
Rats suffering from experimental hemiparkinsonism induced by a unilateral injection of 6-hydroxydopamine into the left area ventralis tegmenti showed a strong ipsilateral deviation and scoliosis-like skeletal deformity. The rats often showed single rotatory curves affecting the thoracic and lumbar regions, although cases with multiple curves were also found. The severity of the scoliosis was closely related to a decrease in extracellular striatal dopamine measured with microdialysis and to the development of postsynaptic dopamine receptor supersensitivity, functionally evaluated with rotational behaviour elicited with apomorphine. Indeed, rats with the strongest dopamine depletion (greater than 95%) and the strongest rotational responses showed the sharpest spinal deviation and skeletal deformity. These findings agree with the clinical observations that scoliosis occurs in patients with Parkinson's disease and its direction is correlated with the side of the major signs and symptoms of parkinsonism.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews C. J., Burke D., Lance J. W. The response to muscle stretch and shortening in Parkinsonian rigidity. Brain. 1972;95(4):795–812. doi: 10.1093/brain/95.4.795. [DOI] [PubMed] [Google Scholar]
- Andén N. E., Johnels B. Effect of local application of apomorphine to the corpus striatum and to the nucleus accumbens on the reserpine-induced rigidity in rats. Brain Res. 1977 Sep 16;133(2):386–389. doi: 10.1016/0006-8993(77)90777-6. [DOI] [PubMed] [Google Scholar]
- Christensson-Nylander I., Herrera-Marschitz M., Staines W., Hökfelt T., Terenius L., Ungerstedt U., Cuello C., Oertel W. H., Goldstein M. Striato-nigral dynorphin and substance P pathways in the rat. I. Biochemical and immunohistochemical studies. Exp Brain Res. 1986;64(1):169–192. doi: 10.1007/BF00238213. [DOI] [PubMed] [Google Scholar]
- Duvoisin R. C., Marsden C. D. Note on the scoliosis of Parkinsonism. J Neurol Neurosurg Psychiatry. 1975 Aug;38(8):787–793. doi: 10.1136/jnnp.38.8.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes J. D., Hassan M. N., Trent G., Halle D., Armstrong G. W. Clinical and radiographic features of scoliosis in Parkinson's disease. Adv Neurol. 1987;45:353–355. [PubMed] [Google Scholar]
- Herrera-Marschitz M., Forster C., Ungerstedt U. Rotational behaviour elicited by intracerebral injections of apomorphine and pergolide in 6-hydroxy-dopamine-lesioned rats. I: Comparison between systemic and intrastriatal injections. Acta Physiol Scand. 1985 Nov;125(3):519–527. doi: 10.1111/j.1748-1716.1985.tb07750.x. [DOI] [PubMed] [Google Scholar]
- Herrera-Marschitz M., Goiny M., Utsumi H., Ungerstedt U. Mesencephalic dopamine innervation of the frontoparietal (sensorimotor) cortex of the rat: a microdialysis study. Neurosci Lett. 1989 Feb 27;97(3):266–270. doi: 10.1016/0304-3940(89)90608-3. [DOI] [PubMed] [Google Scholar]
- Herrera-Marschitz M., Ungerstedt U. Evidence that apomorphine and pergolide induce rotation in rats by different actions on D1 and D2 receptor sites. Eur J Pharmacol. 1984 Feb 17;98(2):165–176. doi: 10.1016/0014-2999(84)90587-9. [DOI] [PubMed] [Google Scholar]
- Herrera-Marschitz M., Ungerstedt U. Evidence that striatal efferents relate to different dopamine receptors. Brain Res. 1984 Dec 10;323(2):269–278. doi: 10.1016/0006-8993(84)90297-x. [DOI] [PubMed] [Google Scholar]
- Johnels B. Reserpine-induced rigidity in rats: drug effects on muscle tone from corpus striatum and nucleus accumbens. Pharmacol Biochem Behav. 1983 Sep;19(3):463–470. doi: 10.1016/0091-3057(83)90121-1. [DOI] [PubMed] [Google Scholar]
- Robin G. C., Span Y., Steinberg R., Makin M., Menczel J. Scoliosis in the elderly: a follow-up study. Spine (Phila Pa 1976) 1982 Jul-Aug;7(4):355–359. doi: 10.1097/00007632-198207000-00005. [DOI] [PubMed] [Google Scholar]
- Schwarcz R., Hökfelt T., Fuxe K., Jonsson G., Goldstein M., Terenius L. Ibotenic acid-induced neuronal degeneration: a morphological and neurochemical study. Exp Brain Res. 1979 Oct;37(2):199–216. doi: 10.1007/BF00237708. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U., Arbuthnott G. W. Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Res. 1970 Dec 18;24(3):485–493. doi: 10.1016/0006-8993(70)90187-3. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Postsynaptic supersensitivity after 6-hydroxy-dopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand Suppl. 1971;367:69–93. doi: 10.1111/j.1365-201x.1971.tb11000.x. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiol Scand Suppl. 1971;367:1–48. doi: 10.1111/j.1365-201x.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Striatal dopamine release after amphetamine or nerve degeneration revealed by rotational behaviour. Acta Physiol Scand Suppl. 1971;367:49–68. doi: 10.1111/j.1365-201x.1971.tb10999.x. [DOI] [PubMed] [Google Scholar]