Abstract
The world population’s significant increase has promoted a higher consumption of poultry products, which must meet the specified demand while maintaining their quality and safety. It is well known that conventional antimicrobials (antibiotics) have been used in livestock production, including poultry, as a preventive measure against or for the treatment of infectious bacterial diseases. Unfortunately, the use and misuse of these compounds has led to the development and dissemination of antimicrobial drug resistance, which is currently a serious public health concern. Multidrug-resistant bacteria are on the rise, being responsible for serious infections in humans and animals; hence, the goal of this review is to discuss the consequences of antimicrobial drug resistance in poultry production, focusing on the current status of this agroeconomic sector. Novel bacterial control strategies under investigation for application in this industry are also described. These innovative approaches include antimicrobial peptides, bacteriophages, probiotics and nanoparticles. Challenges related to the application of these methods are also discussed.
Keywords: poultry production, microbiota, antibiotics, antibiotic alternatives, antimicrobial resistance, food safety
1. Introduction
The human population is continually increasing, rendering food security a major concern; thus, it is necessary to ensure that food production systems can support this population increase [1]. Animal food products, including meat, play an important role in the human diet. The demand for this foodstuff is on the rise, and meat consumption has increased more than 4-fold in the last 50 years [2].
Nowadays, poultry is one of the most consumed meats worldwide, being the second most produced and consumed meat in the European Union (EU) after pork [3]. In addition, global meat production has increased over the years [2]. From a global perspective, and according to the FAO, in 2020, the production of poultry meat represented almost 40% of global meat production [4]. Consequently, there has been a global shift towards intensive farming systems in which infections, including zoonosis, are transmitted more easily, affecting animal health and productivity [2,5].
Along with the apprehension related to food safety, this increase leads to concerns regarding production sustainability and safety. The production of animal-derived products have inherent impacts to One Health, such as an increase in greenhouse gases, the contamination of drinking water, environmental contamination, the dissemination of antimicrobial drug resistance, and the emergence and re-emergence of zoonotic diseases [6,7]. The production of sufficient amounts of food for the global population is one of the major current challenges [7].
Due to the increasing concentration of animals in intensive farms and the use of conventional antibiotics to safeguard the health of animals and animal products, antimicrobial resistance has developed and spread, which has led to a global public health concern. This review aims to focus on the role of poultry production in the development of AMR and the main bacterial pathogens that affect poultry, and to discuss the potential role of innovative antimicrobial compounds as an alternative or complementary strategy to the use of conventional antibiotics and, consequently, for the reduction and dissemination of AMR between animals, humans and the environment in a One Health Approach.
2. Antimicrobial Drug Resistance
2.1. Global Scenario
Antibiotics are natural, semisynthetic or synthetic substances, which interfere with the growth or survival of bacterial microorganisms, and are used to prevent or treat the associated infections [8,9]. Although traditional antimicrobial compounds have been recognized for thousands of years since their discovery by ancient civilizations, it was only in 1928 that the first antibiotic, penicillin, was developed by Alexander Flemming [8].
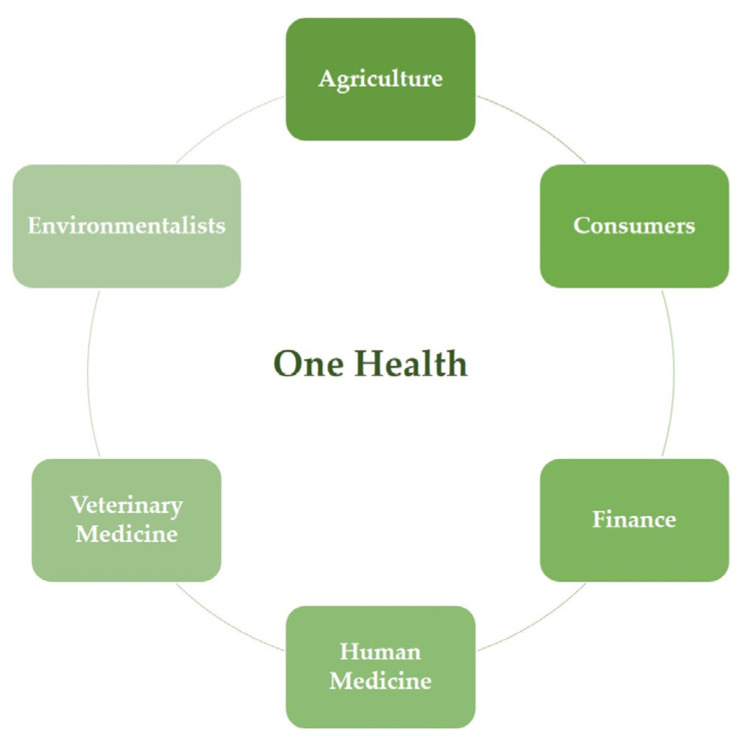
The advent of antibiotics revolutionized medicine due to their ability to combat bacterial infections, allowing an increase in the average life expectancy of humans and animals, the control of infectious diseases and the reduction in morbidity and mortality, while also contributing to food safety [8,9]. Unfortunately, due to the extensive use of these compounds, multidrug resistant (MDR) microorganisms have emerged and disseminated, which is currently a global concern [10]. If the rate of development of MDR bacteria continues to increase, it is estimated that in 2050 the mortality rate caused by resistant bacterial infections will exceed the mortality rate caused by cancer [11]. In 2000, the World Health Organization (WHO) classified antimicrobial drug resistance (AMR) as a global public health concern. As such, it is urgent to find strategies for the control and mitigation of these strains [11,12]. In 2015, the World Health Assembly (WHA), which is the decision-making body of the WHO, adopted a global action plan focused on AMR based on five objectives: improve awareness of antimicrobial drug resistance; strengthen knowledge about it through surveillance and research; reduce the incidence of infection by effective sanitation, hygiene and infection prevention measures; optimize the use of antimicrobials in human and veterinary medicine; and increase investment in the development of new medicines, diagnostic tools and vaccines, taking into consideration the necessities of all countries. This action plan highlights the need for an effective One Health approach to tackle this issue and requires coordination among several sectors and groups, including human and veterinary doctors, farmers, economists, environmentalists and informed consumers [13] (Figure 1).
Figure 1.
Schematic representation of the coordination between different groups required for a One Health approach.
To help control AMR dissemination, the European Medicine Agency (EMA) developed a categorization of the conventional antibiotics used in veterinary medicine in order to promote their responsible use, focusing on the protection of public and animal health. As such, antibiotics were classified as category A (“Avoid”), which includes antibiotics that are not authorized in veterinary medicine; category B (“Restrict”), which includes critically important compounds for human medicine for which use in animals should be restricted; category C (“Caution”), which includes antibiotics for which alternatives in human medicine generally exist and can be applied in the veterinary settings in the absence of alternatives belonging to category D; and category D (“Prudence”), which includes the antibiotics that should be used for first-line treatments in animals [14].
2.2. Antibiotics in Poultry Production
Antibiotics have been used in animal production for over fifty years as therapeutic and metaphylactic/prophylactic agents or as growth promoters [15]. The efficacy and cost-effectiveness of the majority of these compounds led to their indiscriminate usage [16]. Consequently, the misuse and overuse of these antimicrobials promoted the establishment of microbial reservoirs carrying AMR determinants in livestock, including poultry. As some of the antimicrobials applied to animals are the same as those administrated to humans, AMR dissemination poses a serious threat to the effective treatment of serious bacterial infections in humans, leading to higher medical costs, prolonged hospital stays and increased mortality [17,18,19].
Antimicrobial growth promoters (AGPs) started being applied in 1951, when the United States (US) Food and Drug Administration (FDA) approved the use of antibiotics as animal additives without prescription, followed by European Union (EU) countries, which approved their own regulations on the use of those substances in animal production [18]. AGPs are antibiotics administrated at subtherapeutic doses, aiming to modify the animal’s intestinal microbiota to attain a better performance. AGP dissemination contributes to selecting intestinal bacteria, reducing competition for nutrients and improving animal growth rates. Some authors defend these benefits, arguing that they are important in the early stages of production or that they are useful in the presence of sub-optimal hygiene conditions [20], while others report that they increase productivity, highlighting the importance of good husbandry in animal production [19].
AGP use has contributed to the evolution and spread of AMR in intestinal microbiota [5,21], prompting some countries to ban their application in animal production. Sweden was the first country to prohibit the inclusion of AGPs in animal feed in 1986. In 2006, the EU banned the use of 25 AGPs from animal production. Moreover, EU’s decision to ban AGPs has been adopted by several other countries, such as Mexico, New Zealand and South Korea. On the other hand, the USA, Australia, Japan and Canada implemented laws to partially ban or exclude some antibiotic-derived additives [22]. In fact, some important human medicine antimicrobials have been prevented from being used as AGPs in the US since 2016 [19]. Despite these actions, antibiotics are still relevant for the prevention and treatment of bacterial infections, contributing to animal welfare and to the reduction in zoonotic diseases [5,15,19].
2.3. Development of Antimicrobial Drug Resistance
Antimicrobial drug resistance relates to the capacity of a microorganism to survive the inhibitory or killing activity of an antimicrobial compound [10]. This phenomenon has been reported since the discovery of antibiotics [12]. When an antibiotic is administrated, susceptible bacteria are eliminated, favoring the selection of resistant strains. These strains become the predominant bacterial population, allowing the transmission of genetic resistance determinants to clonal descendants, to other isolates of the same species, or even to members of other bacterial species. This phenomenon occurs either in commensal or pathogenic bacteria from humans, animals and the environment [9].
There are two main pathways associated with the evolution and development of antimicrobial drug resistance. The first is related to resistance mediated by pre-existing phenotypes in natural bacterial populations. During the evolutionary process, bacteria accumulate genetic errors in existing genes (present in the bacterial chromosome or in plasmids) and transfer those genetic determinants responsible for innate/natural or intrinsic resistance to progeny cells via vertical gene transfer (VGT) [23]. The second scenario refers to acquired resistance, which may develop via a direct pathway, which involves gene mutations, or an indirect pathway, by the acquisition of DNA fragments coding for resistance (namely, transposons, integrons, phages, plasmids or insertion sequences) by horizontal gene transfer (HGT) mechanisms that may occur between the same or different bacterial species. HGT takes place via either conjugation, transformation or transduction [19,23,24]. VGT and HGT can occur in a variety of settings [19]. As such, farms in which animals and vegetables are produced can act as reservoirs of antibiotic resistant bacteria as the food chain comprises distinct ecological niches, including those in which antibiotics are used and bacteria coexist [25].
2.4. Transmission of Antimicrobial Drug Resistance
Drug resistance can disseminate along the food chain through direct or indirect contact between the different actors and settings, both of which are also considered routes of transmission for zoonotic diseases. Direct contact occurs when humans come into contact with resistant bacteria present in animals or in their biological products such as urine, feces, blood, saliva and semen. Occupational workers, such as veterinarians, farmers, abattoir workers and food handlers, and others who have contact with them, have a higher risk of being colonized or infected with resistant strains. At present, it is well established that occupational workers and their families are an entryway for resistant bacteria into the community [9,26]. Alternatively, indirect contact can also lead to infection, and includes the handling and consumption of contaminated food products, such as meat and eggs, in the case of the poultry industry [17,19].
Additionally, a large proportion of antibiotics are not totally degraded, nor are transformed into inactive compounds by animals and humans, and retain their activity after being excreted in urine and feces. The active antibiotic, related metabolites or degradation products, named antibiotic residues, can accumulate in soils, wastewater and manure, causing profound impacts [9,17]. Hence, the dissemination of antibiotic-resistant bacteria and antibiotic residues via food and animal waste turn the environment into an important reservoir of antimicrobial drug resistance [9,27]. In fact, it is known that the disposal of manure from animal pens has a significant role in the promotion of HGT of resistance genes among soil bacteria. This way, natural soil can also play a role as a reservoir of resistance determinants [24]. In addition to commensal and environmental bacteria, foodborne pathogens also carry AMR genes [28].
3. Important Pathogens in Poultry Production
The presence of a wide variety of microorganisms is surveilled in several food producing animals due to their importance to public health, namely in broilers and laying hens. The pathogens most relevant in this industry, and associated with antimicrobial drug resistance, include Salmonella enterica, Campylobacter spp. (specially C. jejuni), Escherichia coli, Enterococcus spp. and methicillin-resistant Staphylococcus aureus (MRSA) [29,30].
3.1. Salmonella Enterica
Salmonella enterica causes foodborne enteric disease worldwide, representing the second most commonly reported zoonotic pathogen in the EU [31,32]. It is responsible for disease outbreaks associated with significant morbidity and mortality [33], and up to 25% of human Salmonella outbreaks, illnesses and hospitalizations are related to poultry sources [32,34].
Salmonella are gram-negative, facultative anaerobic bacteria belonging to the Enterobacteriaceae family, and are considered commensals of the gut microbiota of mammals, birds, reptiles, amphibians, fish and shellfish [35] (Figure 2).
Figure 2.
S. enterica in Hektoen agar (Oxoid, Hampshire, UK).
S. enterica includes more than 2650 serovars [36], in which several have been previously described as contaminants of poultry meat and eggs, representing a serious concern for public health [37].
In poultry, diseases promoted by S. enterica are divided into three conditions: fowl typhoid (promoted by S. enterica subsp. enterica serovar Gallinarum biovar Gallinarum), pullorum disease (by S. enterica subsp. enterica serovar Gallinarum biovar Pullorum) and avian paratyphoid, which results from infection caused by other S. enterica serovars, including S. Enteriditis, S. Typhimurium and S. Infantis [30]. These bacteria can be transmitted via both vertical (from infected breeders) and horizontal (from other birds in a flock or from the environment) routes [33]. Young poultry are particularly susceptible to gastrointestinal tract (GIT) colonization by S. enterica. Its excretion in feces may result in the contamination of the environment and the infection of nearby birds [32,35]. Moreover, poultry meat contaminated with digesta during slaughter is a major risk to public health [32,38].
S. enterica can be transmitted from animals to humans through the consumption of contaminated animal derived products, such as meat, and of other foodstuffs contaminated with fecal matter, or through direct or indirect contact with colonized animals or contaminated water. This agent is considered moderately resistant to certain environmental conditions, such as freezing, acidic pH and dehydration, which contribute to its high transmissibility. When infecting humans, Salmonella attaches and colonizes the intestinal columnar epithelial cells, resulting in fever, nausea, diarrhea, vomiting and abdominal pain. The disease is usually self-limiting in healthy adults, but it can lead to septicemia and death in severe cases, especially in children, the elderly and immunocompromised patients [33,35]. In these cases, antimicrobial treatment is advised, and fluoroquinolones, macrolides and third-generation cephalosporins can be used to treat S. enterica infection [35].
Several studies comprising the evaluation of the antimicrobial drug resistance profiles of more than 4000 isolates of Salmonella spp. were revised by Saraiva et al., 2022 [30]. Higher frequencies of resistance were observed towards nalidixic acid, amoxicillin, ampicillin, erythromycin, penicillin G, sulfamethoxazole and tetracycline, while higher susceptibilities were associated with the aminoglycosides spectinomycin and gentamicin.
In the case of paratyphoid Salmonella, multidrug resistance is a concern because it can lead to treatment failure. The most common resistance patterns associated with this pathogen include important therapeutic antimicrobial classes used in human medicine, such as penicillins, tetracyclines, cephalosporins and fluoroquinolones, and this association represents a public health concern [30].
3.2. Campylobacter spp.
Campylobacter spp. are ubiquitous bacteria that can be found in various environments, including soil and water, and as commensals of the GIT of poultry. Despite this, they can cause disease in animals and humans and constitute an important cause of foodborne diseases worldwide [39,40,41]. This bacterial genus can be responsible for acute bacterial diarrhea, which is mainly caused by C. jejuni and C. coli. Although other sources can be responsible for human infection, poultry products are considered the predominant source of human campylobacteriosis [33] (Figure 3).
Figure 3.
C. jejuni in Columbia Agar + 5% sheep blood (bioMérieux, Marcy-l’Etoile, France).
Campylobacter spp. can be introduced in the production farms by wild animals, pests or humans. When infecting poultry, it colonizes the animal’s intestine, invades the intestinal epithelium and multiplies rapidly in the intestinal mucus, avoiding clearance and persisting in the animal’s GIT [41,42]. In this way, avian hosts constitute a natural reservoir for Campylobacter spp., namely C. jejuni and C. coli [41]. According to the European Food Safety Authority (EFSA) and European Centre for Disease Prevention and Control (ECDC), the highest prevalence of Campylobacter is observed in fresh meat from broilers (37.5%) [31]. Although carriers of Campylobacter spp., chickens generally do not exhibit clinical signs [41]. Antibiotics have a limited role in the elimination of Campylobacter spp. by these animals, due to its high occurrence and commensal character in avian species, and can promote the emergence of resistant strains; therefore, biosecurity practices are the most important method for reducing Campylobacter infection at the production level [43].
Human infections are usually associated with the handling, preparation and consumption of contaminated poultry products, and occupational transmission has also been observed [39]. In humans, these pathogens cause gastroenteritis associated with diarrhea, abdominal pain, fever, nausea and vomiting, which usually occur between two and five days after infection. Symptoms are often mild and self-limiting. Antibiotic treatment is not usually required, but severe cases may be treated with macrolides, such as clarithromycin, azithromycin and erythromycin. Ciprofloxacin is not currently used, as resistance to quinolones is now considered to be too high for these antibiotics to be used as an empirical treatment [33,39,41,44]. Studies on the antimicrobial drug resistance profile of Campylobacter spp. isolated from broilers, laying hens, chicken carcasses and chicken meat revealed high frequencies of resistance to nalidixic acid, ampicillin, cephalexin, ciprofloxacin, erythromycin, gentamicin and tetracycline [30].
3.3. Escherichia coli
E. coli is a gram-negative bacillus belonging to the Enterobacteriaceae family [45]. It is an important bacterial species in the human–animal–environment triad, since it is a commensal inhabitant of the digestive tract of animals, including birds, being widely disseminated via fecal material [46]. This species is often studied as a marker of antimicrobial drug resistance, mainly due to its widespread distribution and capacity to harbor several genes in mobile genetic elements, serving as a source of antimicrobial drug resistance determinants to other bacteria [47].
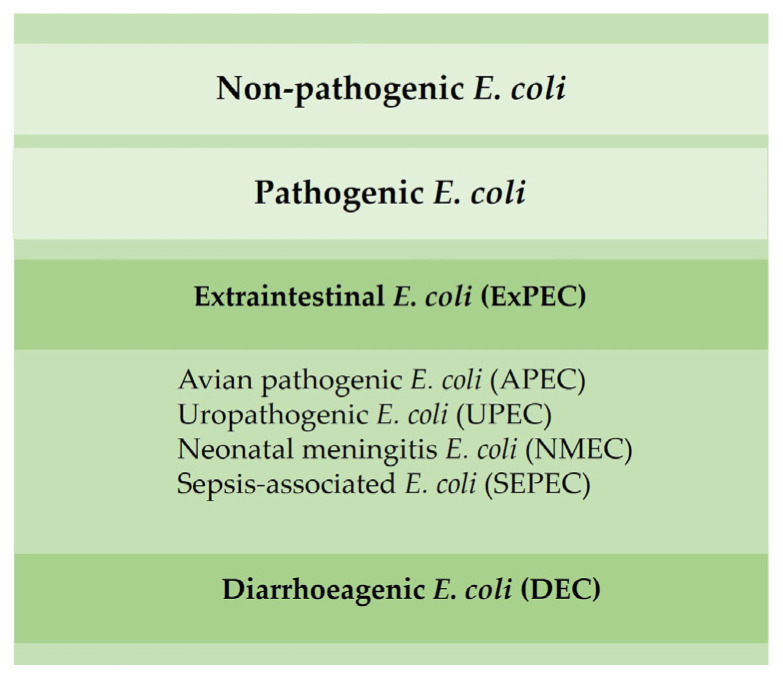
Most E. coli are nonpathogenic; however, certain pathogenic serotypes may induce disease. There are several E. coli pathotypes, which can be divided into extraintestinal E. coli (ExPEC) and diarrhoeagenic E. coli (DEC). Avian pathogenic E. coli (APEC), an ExPEC, may induce colibacillosis in domestic birds, a disease characterized as a local or systemic syndrome that can be transmitted by oral or vertical routes or through inhalation. E. coli-associated infections are widely distributed among poultry of all ages. Birds can be asymptomatic until sudden death or present various forms of disease, such as septicemia, coligranuloma (Hjarre’s disease), air sac disease (chronic respiratory disease), swollen-head syndrome, venereal colibacillosis, cellulitis, peritonitis, salpingitis, orchitis, osteomyelitis/synovitis, panophthalmitis, omphalitis/yolk sac infection and enteritis [48]. Colibacillosis constitutes the most frequent infectious bacterial disease found in poultry, being responsible for significant economic losses due to the loss of productivity, increased mortality and condemnations of carcasses [29,48].
Other ExPEC pathotypes, such as uropathogenic (UPEC), neonatal meningitis (NMEC) and sepsis-associated E. coli (SEPEC), have already been identified in poultry and can promote disease in humans (Figure 4) [49]. E. coli isolated from poultry may be resistant to aminoglycosides, β-lactam groups (penicillins and cephalosporins) and fluoroquinolones [30].
Figure 4.
Main E. coli pathotypes found in poultry.
3.4. Enterococcus spp.
Enterococcus species are ubiquitous and are commensals of the gastrointestinal microbiota of both humans and animals [50]. Some enterococcal strains have been used as probiotics [51], while others are known to be pathogenic, including in birds [52].
The transmission of enterococci can occur via vertical and horizontal routes. E. cecorum and E. faecalis are the most important species associated with avian disease. Pathogenic strains of E. cecorum have been associated with free thoracic vertebra (FTV) osteomyelitis in broilers [53], resulting in the paralysis of the posterior limbs, and with septicemia related to pericarditis or hepatitis, which can lead to death. In turn, E. faecalis can cause omphalitis and yolk sacculitis, which can lead to sepsis and the death of chicks in the first week of life. Surviving animals may develop chronic diseases, such as valve endocarditis, which can also lead to death [30].
Enterococcus spp. can easily acquire resistance determinants and, therefore, play a central role in AMR dissemination. Vancomycin-resistant Enterococcus (VRE) has been associated with economic losses in animal production and healthcare and associated with infections in humans [54]. Humans are exposed to enterococci from a variety of sources, including other humans, the environment and foods contaminated with animal’s intestinal microbiota. Certain species, such as E. faecalis and E. faecium, are a prominent cause of opportunistic infections in hospitalized humans, causing mild to fatal diseases, such as endocarditis, urinary tract infections or septicemia [50,52]. Studies previously performed have identified high levels of resistance against aminoglycosides (streptomycin), tetracyclines (doxycycline and tetracycline) and quinolones (ciprofloxacin and enrofloxacin) in enterococci isolated from poultry [30]. Vancomycin resistance, which is reported as infrequent, can be higher in isolates from chickens affected with FTV.
3.5. Methicillin-Resistant Staphylococcus aureus
S. aureus is considered the most common and pathogenic staphylococcal species isolated from poultry. Staphylococci are natural inhabitants of the skin and mucous membranes of healthy birds, being ubiquitous in the poultry environment [29,30]. The presence of S. aureus that is resistant to antimicrobials in production animals is a global health concern affecting both humans and animals [55]. Staphylococcal infections caused by S. aureus are a worldwide problem in poultry production, causing economic losses due to decreased production, increased mortality and the condemnation of carcasses. Infections caused by S. aureus include arthritis, synovitis, chondronecrosis, osteomyelitis, gangrenous dermatitis, subdermal abscesses (bumblefoot) and septicemia [29,30]. Moreover, some enterotoxin-producing strains can cause food poisoning in humans. Poultry-associated food poisoning can occur due to the contamination of carcasses with S. aureus at the processing phase, especially with enterotoxin-producing strains [56]. Regarding the presence of S. aureus in poultry, the major concern is the emergence of MRSA strains. Although they are infrequently isolated from poultry, MRSA can still be transmitted to humans through direct contact or through meat consumption [57]. Staphylococci from poultry can be resistant to amoxicillin, amoxicillin-clavulanic acid, ampicillin, cefoxitin, kanamycin, penicillin and tetracycline [30].
4. Strategies to Reduce Antimicrobial Drug Resistance in Poultry Production
Since the consumption of poultry meat is growing, the high density of animals in production flocks increases the risk of the transmission of infectious agents, including AMR bacteria. This prompts the need to find alternatives to replace or complement antibiotic usage in those settings and to evolve to a “post-antibiotic era” [58].
As previously described, there are several pathogens that are difficult to eliminate from poultry flocks, poultry meat and egg products, requiring improvements in all phases of the poultry production system. In the production phase, the optimization of cleaning procedures, improvement of biosecurity and implementation of adequate hazard analysis and critical control points are fundamental. At the retail level, it is crucial to take action on food handling and worker training, together with consumers’ education, to improve food safety awareness. Collectively, these actions offer opportunities to limit foodborne pathogen dissemination and reduce the risk of exposure to susceptible individuals; however, these measures may still be insufficient to protect humans from foodborne pathogens [33].
Interventions in poultry production can be grouped into two categories: pre-harvest and post-harvest interventions. At pre-harvest, measures to ensure animal health are applied primarily to prevent colonization and broiler infection by foodborne pathogens, via, for example, the administration of compounds in feed or drinking water. At post-harvest, measures applied aim to reduce or eliminate pathogens on carcasses or egg products. These measures focus on direct application on food, food packaging, surfaces and food processing equipment with the goal of minimizing the colonization or multiplication of pathogens and the spoilage of microorganisms during storage or retail [33,59].
Despite the availability and research on new substances, investigations usually focus on new methods to be applied at the flock production level, rather than on postharvest operations. This approach can be beneficial for two reasons. First, the ban of AGPs from poultry production led to the emergence of a market opportunity for alternative feed compounds showing health and performance benefits. Second, and from an overall food safety perspective, although reducing foodborne pathogens in processing plants is important, the focus should be on the live bird sector in order to reduce the pathogen loads before they enter the processing plants. However, the administration of alternative antimicrobial compounds to live birds through feed amendments has proven to be more challenging than anticipated [33]. In this sense, this review will focus on the pre-harvest application of nonconventional antimicrobial compounds, approaching, with greater depth, the reduction and eradication of pathogens at the flock level for the improvement of the flock’s health.
4.1. Husbandry
As previously described, one of the main objectives of the global action proposed by the WHO in 2015 is the reduction in infection incidence via effective sanitation and the application of hygiene and infection preventive measures [13]. The poultry industry needs to control infectious diseases, primarily through good husbandry and good farm management. If this point is addressed, all the other measures directed to pathogens and disease control can consequently be reduced [17]. This is valid for every step of the poultry production system from farm to fork. For example, when birds are transported to the processing facilities, if stressed, they excrete loose feces, which contaminate the bird itself and its surroundings, contributing to cross-contamination [33]. This highlights the importance of good transportation conditions. Another example is the importance of controlling flock density. Rawson, Dawkins and Bonsall, 2019 [60] concluded that high flock density is an essential factor responsible for Campylobacter spp. high microbial counts. Campylobacter becomes well established in large commercial flocks and becomes difficult to eradicate [33].
Hence, it is essential to develop innovative hygienic and management practices (focusing, for example, on housing and feeding systems) aiming to reduce, or even stop, the use of antimicrobials; develop and identify alternatives to antimicrobials, such as vaccines and supplements; and educate farmers and veterinarians to be in favor of a conscious attitude towards the importance of husbandry and the application of good practices [11].
4.2. Non-Conventional Antimicrobial Compounds
Due to the emergence of resistant microorganisms, research has focused on finding strategies alternative to conventional antibiotics, such as antimicrobial peptides, bacteriophages, probiotics and nanoparticles, as well as the use of alternative treatments. These solutions are considered fundamental to combating the dissemination of resistant microorganisms [17].
4.2.1. Antimicrobial Peptides
Antimicrobial peptides (AMPs) are small proteins that can be found in almost every living organism. They evolved as a host defense mechanism against microorganisms and are important to innate immunity [17,61]. Generally, AMPs are small molecules (<10 kDa) with 12–50 amino acids, presenting broad-spectrum antimicrobial activity against bacteria, fungi, protozoa and viruses [62,63]. AMPs have several advantages: they can present several modes of action, are easily degraded in nature, present reduced accumulation, contribute to the enhancement of host immunity, have the ability to neutralize the activity of many microorganisms and seem to present a low resistance frequency [17,64]. The majority of AMPs act by disrupting the bacterial membrane via several mechanisms, including electroporation, non-lytic membrane depolarization, membrane destabilization, pore formation, membrane thinning or thickening, and oxidized lipid targeting [65]. However, some AMPs can also interact with intracellular targets, by inhibiting the cell wall, protein and acid nucleic synthesis, and by interfering with the bacterial metabolic turnover [66].
The healthy functioning of poultry’s GITs depends on the homeostasis between physical, chemical, microbiological and immunological components [5]. The lymphoid tissue present in the GIT (gut associated lymphoid tissue (GALT)) is responsible for the interaction with antigens and the establishment of an immune response [45]. The interactions between intestinal components, such as mucosa and glycocalyx, where AMPs can be naturally found, lead to the maturation of the GIT immune system [5]. These AMPs that naturally occur in the GIT of poultry can serve as a template for new antimicrobials, and rapid advances in peptide synthesis technology make the future prospect of the industrial application of synthetic AMPs in poultry promising [5,45]. Due to their bacteriostatic or bactericidal effect, AMPs may be used in the prophylactic control, or for the treatment, of bacterial infections, while simultaneously promoting broiler’s growth. AMPs have been shown to significantly reduce pathogenic bacterial loads within the avian gut, while increasing the population of beneficial bacteria and modulating intestinal microbiota [17,45]. The use of synthetic and recombinant AMPs as feed additives may result in benefits for poultry production by promoting immunomodulation, controlling potential pathogen outbreaks, reducing the development of AMR and decreasing the risk of antibiotic-resistant foodborne pathogen consumption by humans [5]. In some situations, various functions can be attributed to the same AMP. Table 1 gathers information on previous studies regarding the use of AMPs in poultry production. In addition, AMPs can act synergistically with conventional antimicrobials, allowing the improvement of the antimicrobial activity of these compounds [14].
The GIT encompasses various organs responsible for digestion and immunity. The microbiota of the GIT is related to the growth performance of animals since the presence of specific microorganisms may promote the absorption of nutrients. Moreover, by reducing the presence of pathogenic species and altering the microbiota, AMPs may help decrease the frequency and lethality of some infections [67]. Additionally, AMPs can contribute to improving animals’ growth by reducing the competition for nutrients in the small intestine, the production of growth-depressing metabolites and the production of intestinal proinflammatory factors, which, in turn, can enhance poultry production parameters and feed intake [68].
Several diseases affect livestock production by causing intestinal mucosa inflammation and diarrhea associated with morphological changes in the intestinal epithelium [69]. AMP utilization has been demonstrated to contribute to health recovery by stabilizing the epithelial barrier integrity and boosting intestinal epithelium colonization. Furthermore, some AMPs can act by inhibiting pro-inflammatory cytokine production or modulating dendritic and T-cell response [17].
AMP Use as Growth Promoters
Several studies have already been carried out to determine the potential activity of AMPs as growth promoters [5]. Microcin J25 (MccJ25), a bacteriocin produced by a fecal E. coli with strong inhibition ability against other E. coli and Salmonella, has been shown to promote growth performance, influence intestinal microbiota and improve intestinal health [70,71]. Nisin, a bacteriocin produced by Lactococcus lactis, is currently being used in the food industry as an additive (GRAS, E234 in EU) due to its inhibitory capacity against putative bacterial pathogens. Although some studies have been performed aiming at its use in poultry [72,73,74], the EFSA has not approved its utilization in those settings. As such, nisin for use in livestock diets is still forbidden, including in poultry, and is not registered as a feed additive [72]. Various studies have demonstrated that the inclusion of nisin in feed changes the animal’s GIT microbiota, reducing potential pathogens in the ileum, such as the Bacteroides-Prevotella cluster, Enterobacteriaceae [73] and Clostridium perfringens [74]. This culminates in a reduction in pathogens in the GIT, lowering the competition for nutrients between the bacteria and the host, and, thus, improving energy utilization [72]. AS7, a bacteriocin produced by Carnobacterium divergens and investigated as a feed supplement, was demonstrated to have a positive role in growth promotion and antibacterial effects in broiler chickens [75]. The addition of Cecropin A-D-Asn, a recombinant AMP, to broilers’ feed, was shown to inhibit gut bacterial growth, improve nutrient utilization and intestine structure, and promote broiler growth [76]. Furthermore, the use of the synthetic AMPs A3 and P5 was able to increase the growth performance of broilers, with additional benefits such as increased nutrient uptake and reduced intestinal damage [77,78].
AMP Use as Immunomodulators
Other studies have reported a similar effect of several AMPs, derived from different sources, to reduce bacterial infections in broiler flocks through immune modulation [78,79]. AMPs can modulate the intestinal expression of proinflammatory cytokines, such as IL-2 and IL-6, and of anti-proinflammatory molecules, such as IL-10 [45,80]. Moreover, AMP supplementation improves intestinal morphometric parameters, including villus height and villus surface area, and productive parameters, such as feed conversion ratio [45]. Notably, supplementation of feed with exogenous AMPs mimics the physiological release of endogenous AMP, such as cathelicidin-B1 [80]. Therefore, AMPs can be considered major alternatives to maintaining intestinal balance in avian species [5]. Kogut, Genovese, He, Swaggerty and Jiang, 2013 [81] studied the application of a group of small cationic peptides (BT) with known immune modulatory properties produced by a Gram-positive bacterial species from soil, Brevibacillus texasporus, to broiler chickens. The authors demonstrated that these AMPs may be useful alternatives to antibiotics, acting as local immune modulators in neonatal poultry and providing prophylactic protection against Salmonella infections. A study by Aguirre et al., 2015 [82] demonstrated that bovine lactoferrin (bLf) had beneficial effects in broiler chickens, promoting the improvement of body weight and feed conversion and the enhancement of intestinal morphology. Liu et al., 2008 [83] determined the effect of the oral administration of rabbit Sacculus rotundus antimicrobial peptides (RSRP) on the intestinal mucosal immune responses in broilers. The results indicated that the presence of RSRP improved the structure of the intestine and stimulated intestinal mucosal immunity during growth. Ma et al., 2020 [84] studied the effects of diet supplementation with recombinant plectasin in broilers, showing its beneficial effects on growth performance, intestinal health and innate immunity.
AMPs Used for Infection Therapy in Poultry
Finally, AMPs also present an enormous potential in controlling poultry infectious diseases [85].
Salmonella
Cathelicidins were shown to present relevant antimicrobial activity against Salmonella. For example, synthetic human cathelicidin LL-37, released by neutrophils, has demonstrated strong antimicrobial activity against different Salmonella strains and immunomodulation effects [86]. Cathelicidin BF is one of the most potent cathelicidins known. Its administration in mice reduced infection by Salmonella resistant to streptomycin, accentuating the potential of AMPs in eliminating antibiotic resistant bacteria. Another cathelicidin, CATH-2, which is derived from chickens, showed in vitro antibacterial activity against S. Enteritidis and S. Typhimurium [87]. An in vivo study performed by Roque-Borda et al., 2021 [64] evaluated the effect of a peptide derived from the skin of the amphibian Hypsiboas albopunctatus, Ctx(Ile21)-Ha, known for its high antimicrobial activity against some important public health pathogens in laying hens. The authors concluded that after a challenge with S. Enteriditis and AMP treatment there was a reduction in younger chicks’ mortality in the first days of life. However, the investigation of the potential of avian AMP to reduce Salmonella’s infections in poultry is still ongoing.
E. coli
An in vivo study performed by Daneshmand et al., 2019 [45] demonstrated that the use of cLF36, a lactoferrin-derived peptide, is capable of decreasing infection in broilers challenged with enterotoxigenic E. coli by modulating the expression of cytokines IL-2 and IL-6 and mucine. The addition of cLF36 to feed reduced the population of E. coli and Clostridium spp. by 25% and 20%, respectively. Additionally, the number of Lactobacillus spp. and Bifidobacterium spp., species beneficial to poultry’s GIT microbiota, increased up to 36% [45]. Another study has also evaluated the application of a recombinant peptide derived from camel milk, cLFchimera, which presented a strong antimicrobial effect against two different E. coli strains in birds [88].
Campylobacter spp.
An in vitro study performed by Line et al., 2022 [61] showed that six AMPs, namely, NRC-13 Pleurocidin, RL-37, Temporin L, Cecropin A-Magainin 2 hybrid, Dermaseptin and C12K-2β12, had the capacity to inhibit Campylobacter growth. One of these AMPs, C12K-2β12, is heat- and acid-stable, making it an attractive compound for in vivo delivery to poultry. Moreover, another in vitro study showed that three purified bacteriocins produced by Paenibacillus polymyxa and one from Bacillus circulans NRRL B-30644 presented antagonistic activity against Campylobacter from broiler chickens [89]. Nevertheless, it is important to note that further in vivo studies, aiming at evaluating the inhibition of Campylobacter spp. infections in poultry, must be conducted.
C. perfringens
Pediocin A, a bacteriocin produced by Pediococcus pentaceus FBB61 [90], has demonstrated antimicrobial activity against Gram-positive bacteria, such as C. perfringens type A. Pediocin A was administered via feed to broilers infected with C. perfringens type A, which resulted in the increased growth of the animals [85,91]. In another study, Sublacin, a peptide produced by Bacillus subtilis 168, was administered to poultry via drinking water. Authors demonstrated that this AMP could be used as a potential antimicrobial agent to control necrotic enteritis caused by C. perfringens without causing changes in the Lactobacillus community [92].
Table 1.
In vitro and in vivo studies evaluating AMPs activity against poultry-associated foodborne pathogens (nd—not determined; na—not applicable).
| AMP | Origin | Target (Gram) | Trial | Dosage | Effect | Study |
|---|---|---|---|---|---|---|
| 3 bacteriocins |
Paenibacillus polymyxa
B. circulans |
Campylobacter (−) | In vitro | na | Antimicrobial activity | [89] |
| Gallinacin-6 | Gallus gallus domesticus | C. jejuni (−), S. enterica (−), C. perfringens (+), E. coli (−) | In vitro | na | Antimicrobial activity | [93] |
| RSRP | Oryctolagus cuniculus—sacculus rotundus | E. coli (−) | In vivo | 0.1 mg of RSRP on d 7, 14, 21 and 28 | Immunomodulation; alteration of intestinal morphology |
[83] |
| AS7 | Carnobacterium divergens AS7 | C. perfringens (+) | In vivo | 200 AU/g of feed for 42 days | Improvement of growth performance; alteration of intestinal microbiota |
[94] |
| A-D-Asn | Pichia pastoris | - | In vivo | Basal diets with a A-D-Asn supplementation at 0, 2, 4, 6 and 8 mL/kg | Improvement of growth performance | [76] |
| BT | Brevibacillus texasporus | S. Enteritidis (−) | In vivo | 24 ppm BT peptide-supplemented diet | Immunomodulation in neonatal poultry; antimicrobial activity (prophylactic protection against Salmonella infections) |
[81] |
| Nisin | Lactococcus lactis subsp. lactis | - | In vivo | Diet supplemented with various concentrations of nisin (100, 300, 900 and 2700 IU/g) | Improvement of growth performance; modulation of the GIT microbial ecology |
[73] |
| CATH-2 | Chicken |
S. Enteritidis (−) S. Typhimurium (−) |
In vitro | na | Antimicrobial activity | [87] |
| A3 | Analog of Helicobacter pylori 2–20 | - | In vivo | Basal diet supplemented with 60 or 90 mg/kg AMP-A3 | Improvement of growth performance; alteration of intestinal microbiota |
[77] |
| P5 | Analog of hybrid AMP CA-MA | - | In vivo | Basal diet supplemented with 40 and 60 mg/kg AMP-P5 | Improvement of growth performance; alteration of intestinal microbiota |
[78] |
| Sublacin | B. subtilis 168 | C. perfringens (+) | In vivo | Chickens supplemented with sublancin at 2.88, 5.76 or 11.52 mg activity/L of water | Antimicrobial activity; alteration of intestinal morphology |
[92] |
| Lactoferrin (bLf) | Bos taurus |
E. coli (−) Salmonella (−) |
In vivo | Diets with 130, 260 and 520 mg bLf/kg feed during the starter stage | Alteration of intestinal morphology | [82] |
| Nisin | Lactococcus lactis subsp. lactis | - | In vivo | 35-day administration of nisin at 2700 IU kg−1 through diet | Improvement of growth performance | [74] |
| Microcin J25 (Mcc) | Fecal strain of E. coli |
E. coli AZ1 (−) Salmonella CVCC519(−) |
In vivo | Basal diet with 0, 5 and 1 mg/kg Mcc J25 | Decrease in intestinal inflammation; alteration of intestinal microbiota; improvement of growth performance |
[70] |
| Nisin | Lactococcus lactis subsp. lactis | - | In vivo | Administration of nisin in 2700 IU/kg via diet | Improvement of growth performance | [95] |
| cLFcuimera | Camel lactoferrin-derived peptide |
E. coli (−), S. Enteritidis (−), S. aureus (+) |
In vitro | na | Antimicrobial activity on both Gram-positive and Gram-negative avian pathogenic bacteria | [88] |
| cLF36 | Camel lactoferrin-derived peptide |
E. coli (−) Clostridium spp. (+) |
In vivo | 20 mg/kg AMP | Improvement of growth performance; immune modulator; alteration of intestinal microbiota |
[45] |
| Plectasin (Ple) | Pseudoplectania nigrella | - | In vivo | Basal diet supplemented with 100 and 200 mg Ple/kg | Immunomodulation | [84] |
| Ctx(Ile21)-Ha | Hypsiboas albopunctatus | S. Enteriditis (−) | In vivo | 20 and 40 mg of Ctx(Ile21)-Ha/kg were included in the diet for 28 days | Antimicrobial activity | [64] |
| 11 AMPs | Chemically synthesized | C. jejuni (−) | In vitro | na | Antimicrobial activity | [61] |
4.2.2. Bacteriophages
There has been an increased interest in research regarding the application of bacteriophages in the poultry industry [29]. Bacteriophages (or phages) are viruses that specifically target and infect bacteria. Phages were discovered in 1915 [96], but research focusing on their usage as antimicrobials decreased with the spread of antibiotic use [16].
Phages are globally ubiquitarian in the environment, being found in all habitats, including in water, plants and food, and are relatively easy to isolate. They are frequently consumed by humans (via food or water) and are considered non-pathogenic. Moreover, phages have been recognized as important components of the human microbiome, being prevalent in the human gut virome [97]. Bacteriophages may be present in high concentrations in the farm environment, but only a small percentage may have specific action against a target pathogen [98]. The best source of phages is the environment where the host bacterium is prevalent [99,100].
Phages are obligate bacterial parasites, using the prokaryotic cell to replicate. Depending on their interactions with bacteria and their life cycle, phages can be divided into two types: lytic (or virulent) and lysogenic. During the lytic cycle, a bacteriophage infects a target bacterium, replicates, kills the bacterium through lysis, and releases multiple progeny phages. These progeny phages can infect other bacterial cells, thereby repeating the cycle [101]. Lytic phages have several potential applications. In contrast, the lysogenic cycle does not result in the lysis of the host cell, nor in progeny production. Instead, it leads to the integration of phage genetic material into the bacterial genome, and its transmission into new cells upon cell division. Finally, certain bacteriophages have the ability to perform both lytic and lysogenic cycles, depending on environmental triggers [102].
Bacteriophages are classified into many orders and 15 families. The vast majority of phages (96%) belong to the order Caudovirales, which correspond to phages with tails. This order is divided into three families: Siphoviridae (including 61% of tailed phages), Myoviridae (25%), and Podoviridae (14%) [103]. Phages possess very high specificity for one (monovalent phage) or similar (polyvalent phage) bacterial species. On one hand, the selective ability of phages to attack certain bacteria allows for the elimination of specific microorganisms; on the other hand, it restricts their use for broad therapeutic purposes [29]. Phage specificity towards target bacteria is dependent on cell surface receptors, such as outer membrane and lipopolysaccharide proteins and flagella components [104].
In addition to therapies using only bacteriophages, it is described that phages can be more effective when applied in combination with conventional antibiotics. This phenomenon is known as phage–antibiotic synergy. These combinations allow the application of antibiotics in sub-inhibitory doses, leading to a reduction in the harmful effects of antibiotics in animals and humans. In addition, the combination of phages with antibiotics can potentiate antibiotic function, prolonging or restoring their activity against bacteria [105,106]. To the authors’ best knowledge, this synergism is not fully characterized regarding the application of phages in poultry production; however, it is a factor that should be considered in the development of protocols for the treatment of bacterial infections using bacteriophages.
Several studies have addressed the efficacy of phages in reducing bacterial counts or controlling bacterial infections in poultry. Phages can be applied in the poultry industry with three different aims: food biocontrol, disinfection (post-harvest) and phage therapy directed to infections (pre-harvest) [29].
Regarding food biocontrol, phages are used to reduce food contamination, promoting the removal and neutralization (inactivation) of microorganisms in food products and delaying putrefaction. Specifically, phages may be used as food biopreservatives, via the direct application on food or as food packaging, including on raw meat and ready-to eat (RTE) products. In fact, there are some phages being commercialized as biopreservation agent products acting against Listeria monocytogenes, Salmonella, Shigella and E. coli [29,59].
For disinfection, phages can be applied to the decontamination of food-contact surfaces, equipment and the skin of poultry carcasses, with the aim of reducing bacterial loads [59].
Phage-Mediated Control at Pre-Harvest
Bacteriophages function in more specific ways compared to antibiotics; however, only lytic bacteriophages are suitable for phage therapy. It should be noted that antibiotic treatment not only kills the pathogenic bacteria but also affects the normal intestinal microbiota, potentially leading to dysbiosis, immunosuppression and secondary infections [107]. In this way, bacteriophage treatment represents an excellent tool for the treatment of bacterial infections in poultry. Table 2 shows the results from studies conducted about the use of bacteriophages against pathogens in poultry production.
Salmonella
Since Salmonella is one of the most important foodborne pathogens, the search for bacteriophages directed towards this agent is of major importance. In 1991, an experiment was conducted on newly hatched chickens challenged with S. Typhimurium [108]. After bacterial challenging and phage inoculation, a reduction in the microbial load was observed in some portions of the GIT. Bardina, Spricigo, Cortés and Llagostera, 2012 [109] concluded that bacteriophage cocktails composed of various phages are more effective in promoting Salmonella inhibition than the administration of just one phage, promoting a significant reduction in Salmonella counts in chicken cecum after repeated treatment. Hong et al., 2013 [110] showed that bacteriophages may be effective alternatives to antibiotics for the control of fowl typhoid disease caused by S. Gallinarum in layer chickens. In this study, chickens were fed with bacteriophages for 7 days before a bacterial challenge and for 21 days after the challenge, and mortality rates significantly decreased in the challenged chickens treated with bacteriophages. Nabil, Tawakol and Hassan, 2018 [111] reported the presence of Salmonella-specific bacteriophages in sewage samples from a poultry farm. Those bacteriophages were administrated to chicks before oral Salmonella challenge, and were subsequently followed by four successive phage treatments. At the end of the trial (after the fifth and last dose), no bacteria were detected in the cecum, indicating that Salmonella was eliminated by phage treatment.
The world’s first commercially available bacteriophage product for application in poultry production is the Biotector S1® (CJ Cheil Jedang Research Institute of Biotechnology, Seoul, Republic of Korea), which can be used as a feed additive to control S. Pullorum and S. Gallinarum in poultry. In a study using this product, the mortality rate in chickens challenged with Salmonella and receiving Biotector S1® decreased and performance was improved in the phage treated group [59]. Another phage cocktail available, SalmoFREE®(Theseo, Laval, France), is considered safe for administration via drinking water, which does not affect animals’ production parameters. This product can reduce Salmonella levels in cloaca to 0% after a 33-day treatment [112]. Bafasal® (Proteon Pharmaceuticals, Poland), another phage-based product, can also be administrated via drinking water to birds and has the ability to reduce Salmonella levels, improve feed conversion rates and reduce mortality. In addition, it has a prophylactic and a post-infection effect and its administration does not require a waiting period for meat and eggs [113,114].
Campylobacter spp.
Most of the phages exhibiting specificity for Campylobacter belong to the family Myoviridae, and to a lesser extent, to Siphoviridae [115]. Campylobacter phages have been isolated from retail poultry, the feces and intestines of chickens and ducks, abattoir effluents, sewage, human feces and poultry manure [99]. There are already some in vivo studies on the use of phage treatment for the reduction in Campylobacter GIT colonization and infection in poultry. In 2005, Wagenaar, Bergen, Mueller, Wassenaar and Carlton [116] compared the efficacy of a single Campylobacter phage with a cocktail of two-phages, administered to live broilers, concluding that phage-treatment can decrease C. jejuni colonization in broiler caeca when phages are used in a preventive way, as well as therapeutically, and that a cocktail of two phages can reduce the rate of phage-resistant mutant development, when compared to single phage usage. Loc Carrillo et al., 2005 [117] studied the effectiveness of two phages towards Campylobacter strains, demonstrating that the best reduction in bacterial load was achieved after 24–48 h, and that this decrease depended on phage amount and time of administration; however, substantial differences were identified between in vitro and in vivo results, which highlights the importance of developing in vivo studies. In 2009, El-Shibiny et al. [118] evaluated the application of the CP220 phage to broilers colonized with C. coli or C. jejuni, concluding that only high doses of phages were able to reduce C. coli caecal counts within 48 h, while a more extensive reduction in C. jejuni HPC5 levels occurred at 24 h post-administration. In 2010, Carvalho et al. [119] tested the efficacy of a phage cocktail composed of three phages for the control of Campylobacter infections in poultry and also evaluated the effectiveness of two administration routes (oral gavage and feed supplementation). The authors observed that the cocktail targeted both C. jejuni and C. coli and that administration via feed led to an early and more sustainable reduction in Campylobacter compared to administration by oral gavage. Kittler, Fischer, Abdulmawjood, Glünder and Kleina, 2013 [120] tested a phage cocktail composed of four group III phages in three commercial broiler farms. In this study, one of the experimental groups showed a significant reduction in C. jejuni, with a maximum reduction being achieved 1–4 days prior to slaughter. The fact that sometimes genotypically identical C. jejuni strains within flocks exhibit different phage susceptibility profiles was another important conclusion from this study, linking the lack of efficacy with the genotypic variability of C. jejuni isolates. In another study, Fischer, Kittler, Klein and Glünder, 2013 [121] tested a phage cocktail, as well as a single phage, observing a significant reduction in Campylobacter after one to four weeks of treatment and also concluding that phage cocktail administration delayed the emergence of phage resistance.
Currently, to the authors’ knowledge, there are no commercially available phage-based products directed to Campylobacter. As previously mentioned, there are several studies being performed on Campylobacter phage treatment; however, since multiple genotypes of Campylobacter can be present in a flock, this genetic diversity can contribute to the decreased effectiveness of phage therapy and resistance development.
E. coli
In 1998, Barrow, Lovell and Berchieru [122] studied the efficacy of a bacteriophage isolated from human sewage, bacteriophage R, in preventing and treating septicemia, cerebritis and meningitis in chickens inoculated intramuscularly or intracranially with E. coli. The results showed that phages could reach the animals’ brain and that animals treated with phages before the E. coli challenge did not develop disease, which may indicate that phages can persist long enough in the tissues to allow for their application as a prophylactic measure for colibacillosis, as well as for infection treatment. Huff, Huff, Rath, Balog and Donoghue, 2003 [123] tested the application of a two-bacteriophage mixture as an aerosol spray and intramuscular injection treatment to birds immediately after challenging the animals with E. coli, observing that this method provided significant protection to the birds and decreased mortality from 50 to 20%. However, the bacteriophage aerosol spray was not effective when applied 24 or 48 h after the birds were challenged with E. coli. In fact, treating this severe respiratory E. coli infection with a single injection into the thigh muscle was found to be much more effective, significantly reducing mortality when administered immediately after the E. coli challenge, as well as at 24 and 48 h after the challenge. In 2009, the same authors [124] demonstrated that bacteriophage administration via an aerosol spray to seven-day-old chicks prior to a challenge with E. coli could prevent airsacculitis caused by this bacterial agent. Moreover, the effectiveness of treatment with bacteriophage seems to be dependent on the circulating bacteriophage titers. After the administration of phages via an aerosol spray, only a few animals had detectable phage levels in the blood, in contrast to the animals subjected to intramuscular injection.
Other authors compared the efficacy of an antibiotic (chloramphenicol) and oral phage therapy (phage Esc-A, isolated from sewage) against enteropathogenic E. coli in 20-day-old chickens [125]. In the second week of treatment, the birds receiving phages presented no diarrhea, while 12.4% of the birds receiving the antibiotic presented diarrhea, along with 25.2% of those in the control group (treated with water). The death rate was 14.8% in the control group, which was two and five times higher than in the antibiotic and phage groups, respectively. In addition, oral phage therapy caused no secondary effects in the chickens when compared with antibiotic treatments. This can be explained by the fact that the phage treatment has a higher specificity and does not affect beneficial bacteria present in the gut, which is very important for maintaining intestinal homeostasis.
To our knowledge, phage-based products for colibacillosis treatment in poultry are still not available on the market; however, there are two substances available that can be applied topically to live animals for the reduction in E. coli loads, Ecolicide PX™ (Intralytix, Columbia, MD, USA) and Finalyse® (Arm & Hammer Animal and Food Production, Princeton, NJ, USA) [59].
Staphylococcus aureus
Phages infecting Staphylococcus aureus belong to the Podoviridae, Siphoviridae and Myoviridae families. From the therapeutic point of view, Myoviruses are considered the most promissing phages against S. aureus. Although Podoviruses are strictly lytic, they rarely occur in nature. Despite showing strong specificity to S. aureus, these bacteriophages may carry enterotoxigenic genes, which make them pointless for phage therapy [126]. To our knowledge, there are no phage preparations commercially available for the prophylaxis, or the treatment, of S. aureus infections in poultry.
Table 2.
In vivo studies evaluating bacteriophages activity against poultry-associated pathogens (nd—not determined).
| Phage | Origin | Target (Gram) | Administration Route | Dosage (PFU—Plaque-Forming Unit) |
Effect | Study |
|---|---|---|---|---|---|---|
| Phage type 14, type 40 and type 141 | Raw human sewage | S. Typhimurium (−) | Feed | 105 and 1010 PFU/mL | Reduction in the viable numbers of S. Typhimurium in the crop, small intestine and caeca for up to 12 h after inoculation with smaller reductions in the liver at 24 and 48 h after infection | [108] |
| Phage R | Human sewage | E. coli (−) | Intramuscular Intracranial |
102–106 PFU 106–108 PFU |
Long persistence of phages in tissues, which may be useful in prophylaxis and treatment of colibacillosis | [122] |
| DAF6 and SPR02 | Municipal and poultry processing waste |
E. coli (−) | Aerosol Intramuscular |
Aerosol spray: 7.65 × 108 (DAF6) and 2.83 × 109 (SPR02) PFU/mL; injection: 1.88 × 109 (DAF6) and 6.35 × 108 (SPR02) | Successful treatment of E. coli infection by intramuscular injection, which also could be used to prevent colibacillosis in poultry | [123] |
| Phage 69 (NCTC 12669) and Phage 71 (NCTC 12671) | National Collection of Type Cultures in the UK | C. jejuni (−) | Feed | 4 × 109 to 2 × 1010 PFU |
Significant decrease in Campylobacter colonization | [116] |
| CP8 and CP34 | Ceca and upper and lower intestines of chicken | Campylobacter (−) | Feed | log10 5, 7 and 9 PFU | Decrease in the bacterial load depending on the amount of phage and time of administration | [117] |
| Esc-A | Sewage | E. coli (−) | Oral | 105 PFU | More efficient decrease in the death rate compared to chloramphenicol treatment | [125] |
| CP220 | nd |
C. jejuni (−) C. coli (−) |
Oral gavage | log10 5, 7 and 9 PFU | Decrease in Campylobacter colonization | [118] |
| phiCcoIBB35, phiCcoIBB37 and phiCcoIBB12 | Poultry intestinal contents |
C. jejuni (−) C. coli (−) |
Oral gavage/feed | Phage cocktail with 1 × 106–1.5 × 107 PFU | Reduction in the number of C. jejuni (Experiment 1) and C. coli (Experiment 2) colonization in chickens | [119] |
| UAB_Phi20, UAB_Phi78 and UAB_Phi87 | Chicken cloacae and pig rectal swabs | Salmonella spp. (−) | Oral | Phage cocktail with 1010 PFU/animal | The frequent treatment of the chickens with bacteriophages, especially prior to colonization of the intestinal tract by Salmonella, is required to achieve effective bacterial reduction over time | [109] |
| ST4, L13 and SG3 | Sewage water treatment | S. Gallinarum (−) | Feed | Phage cocktail with 108 PFU/kg |
Significant decrease in bacterial isolation from the organs and mortality in chickens treated with the bacteriophages | [110] |
| Phages NCTC12672, 12673, 12674 and 12678 | British phage typing scheme | C. jejuni (−) | Drinking water | Phage cocktail with log10 5.8 to 7.5 PFU/bird | Decrease in Campylobacter load | [120] |
| Phages 1 (NCTC 12673), 2 (NCTC 12674), 5 (NCTC 12678) and 13 (NCTC 12672) | National Collection of Type Cultures | C. jejuni (−) | Oral | Single phage or a four-phage cocktail (107 PFU/bird) |
Permanent reduction in Campylobacter load by the phage cocktail, as well as by the single phage. However, the cocktail delayed the emergence of phage resistance | [121] |
| nd | Sewage water taken at broiler farm |
S. Typhimurium (−) S. Enteritidis (−) |
Oral | 1.18 × 1011 PFU/chick to1.03 × 1012 PFU/chick | No detection of Salmonella in the cecum after the last (5th) dose | [111] |
4.2.3. Probiotics
The prohibition of antibiotics supplementation of poultry feed in the EU and other countries has led to a shift towards the use of new substances for the prophylactic control of some pathogens in broiler chickens at the farm level [127].
According to the WHO, probiotics are “live microorganisms which when administered in adequate amounts confer a health benefit on the host” [22,128]. A probiotic preparation must respect some requirements to be considered functional: probiotic bacteria should be resistant to the acidic pH of the environment; easily adhere to the intestinal epithelium; and maintain the intestinal microbiota at the appropriate physiological level [22,51]. Being live microorganisms, probiotics can stimulate gut microbiota, improving the host’s health [127]. These beneficial microbes can act via several mechanisms, including the maintenance of the normal intestinal microbiota, the competitive use of nutrients or sites of bacterial adhesion; metabolism change, by increasing digestive enzyme activity and decreasing bacterial enzyme activity and ammonia production; improvement of feed intake and digestion; and stimulation of the immune system [51,127]. Most of the effective probiotics are lactic acid bacteria (LAB), including those from the genera Lactobacillus and Pediococcus, which are normally found in the GIT of vertebrates and invertebrates [129]. Another type of probiotics, allochthonous probiotics, include microorganisms that are not usually found in the GIT, such as Saccharomyces and spore-forming Bacillus [129].
In broilers, probiotic species belonging to Lactobacillus, Streptococcus, Bacillus, Bifidobacterium, Enterococcus, Aspergillus, Candida and Saccharomyces have been shown to present a beneficial effect on performance, the modulation of intestinal microbiota and pathogen inhibition, intestinal histological changes, immunomodulation and on the microbiological meat quality, and have been used to integrate probiotic formulations. Contrarily to antibiotics, probiotics can be employed as growth promoters [51]. To our knowledge, thirty probiotic preparations are currently registered in the EU [22,51].
Lactic acid bacteria are known to contribute to a healthy intestinal environment, delivering enzymes and other beneficial substances to the GIT [51]. Several studies have already been performed aiming to evaluate LAB contribution to the normal microbiota of chickens [126,127,128,129,130]. Table 3 briefly presents several studies on the use of probiotics in poultry production.
The probiotic FloraMax-B11® (Pacific Vet Group, Fayetteville, AR, USA), composed of 11 Lactobacillus strains (three of L. bulgaricus, three of Limosilactobacillus fermentum (ex Lactobacillus fermentum [131]), two of Lacticaseibacillus casei (ex Lactobacillus casei [131]), two of Limosilactobacillus fermentum (ex Lactobacillus cellobiosus [131]), and one of L. helveticus), was shown to successfully be able to reduce Salmonella spp. when applied via drinking water [129]. A study by Vicente et al., 2007 [132] also investigated the effect of the previously referred probiotic after administration via drinking water, observing that it significantly reduced mortality in poultry farms and increased animals’ performance. Another study evaluating FloraMax-B11® in Salmonella-challenged broiler chickens also observed a reduction in bacterial loads in the GIT [133]. A study by Shivaramaiah et al., 2011 [134] also demonstrated that heat-resistant spore-forming Bacillus can markedly reduce Salmonella and Clostridium when administered in high loads. In fact, several studies have been performed to evaluate the effects of probiotics on Salmonella infections [133,134,135,136,137,138,139,140,141], and probiotics are already being used in the poultry industry for preventing or reducing Salmonella colonization orinfections, and enhancing growth performance in broiler chickens [127,134]. Moreover, several studies have already been performed that aimed at determining the potential role of probiotics in Campylobacter inhibition [142,143,144,145,146], and demonstrated that lactobacilli and bifidobacteria have the potential to inhibit Campylobacter spp. growth [147].
Table 3.
In vivo studies evaluating effects of probiotics on poultry’s health (nd—not determined; na—not applicable).
| Probiotic | Microorganisms | Target (Gram) | Dosage (CFU—Colony-Forming Unit) | Effect | Study |
|---|---|---|---|---|---|
| FM-B11® | Lactobacillus spp. | - | 106 CFU/mL via drinking water | Significant reduction in mortality and increase in broiler chick performance | [132] |
| FM-B11® | Eleven lactic acid bacterial isolates | S. Enteritidis (−) | Oral doses of 104,106 and 108 CFU/bird | Possible reduction in Salmonella Enteritidis in neonatal chicks. | [133] |
| FM-B11® | Lactobacillus spp. | S. Enteritidis (−) | 4 × 106 CFU/mL via oral gavage | Significant reductions in the concentrations of S. Enteritidis within the ceca, and the timing of FM-B11® treatment affects S. Enteritidis-associated reductions | [135] |
| - | Bacillus spp. | S. Typhimurium (−) | Directly fed microbials at 106 spores/g of feed |
Significantly lower cecal S. Typhimurium load and increased performance |
[134] |
| Gallipro® | B. subtilis | S. Enteritidis (−) | 0.02% probiotic of diet supplementation | No significant effect at non-contaminated environment, showing a greater efficacy at a pathogen contaminated environment and improving immune response of infected chickens | [136] |
| - |
E. faecium PXN33 Ligilactobacillus salivarius (ex Lactobacillus salivarius [131]) 59 |
S. Enteritidis (−) | Oral gavage with 1 × 109 CFU of probiotics | Prevention of S. Enteritidis colonization of poultry | [137] |
| - | Bacillus spp. | S. Typhimurium (−) | na | Reinstatement of the microbial genera displaced by S. Typhimurium challenge | [138] |
| - | Bacillus spp. | S. Enteritidis (−) | Feed supplemented in concentration of 454 g/ton | Reduction in the load of Salmonella in the ceca. | [139] |
| - | B. subtilis CSL2 | Salmonella (−) | Feed supplemented in concentration of 1.0 × 107 CFU/g of feed | Modulation in the microbiota, potentially protecting against S. Gallinarum infection | [140] |
| - | L. salivarius | S. Pullorum (−) | Feed supplemented in concentrations of 107, 108 and 109 CFU/kg of feed | Enhancement in S. Pullorum infection resistance in broilers challenged with Aflatoxin B1 | [141] |
| PrimaLac (Star Labs, St. Joseph, MO, USA) | Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium thermophilus and Enterococcus faecium | C. jejuni (−) | Minimum of 1.04 × 108 CFU/g |
Reduction in the presence of C. jejuni, but no significant effect on the growth performance of broilers | [142] |
| - | Lactobacillus plantarum PCS 20 and Bifidobacterium longum PCB 133 | C. jejuni (−) | Oral gavages in concentration of 108 CFU for 15 days | B. longum PCB 133 led to significant reduction in C. jejuni concentration in poultry feces. | [143] |
| - | Lactobacillus gasseri SBT2055 | C. jejuni (−) | Oral gavages in concentration of 1 × 108 CFU CFU for 14 days | Significant reduction in cecum colonization by C. jejuni at 14 days after infection. | [144] |
| - | Lactobacillus gasseri SBT2055 | C. jejuni (−) | na | Significant reduction in cecum colonization by C. jejuni. | [145] |
| - |
Bacillus spp. L. salivarus subsp. salivarius L. salivarus subsp. salicinus |
Campylobacter(−) | na | These strains had significantly reduced C. jejuni counts at 14 days after infection. | [146] |
4.2.4. Nanoparticles
Nanotechnology is an innovative technology with various biomedical applications, and its implementation in the poultry industry has been studied [148]. Nanoparticles (NPs) have unique physical and chemical properties, which make them a target of attention due to their potential use in a range of diverse areas [149,150]. Based on their composition, NPs can be classified as inorganic/organic, carbon-based and hybrid [58]. The inorganic group comprises metal/metal oxide NPs and quantum dots. Organic nanomaterials include polymeric NPs, liposomes and lipid-based NPs that can be used for drug and bioactive delivery [151,152], antimicrobial use, bioimaging and tissue regeneration. Carbon-based nanomaterials comprise carbon black, nanotubes, graphene, nanofibers, nanodots, fullerenes, nano-diamond, carbon onions and carbon rings [58].
Nanoparticle synthesis can be performed by different methods, including physical, chemical and green methods. Physical and chemical approaches usually involve the use of toxic chemicals, which are potentially hazardous to humans and the environment. Compared to conventional methods, biological methods are considered safer and more sustainable for nanomaterial fabrication; therefore, an eco- and environmentally friendly approach for the synthesis of nanoparticles using microorganisms and different plants, commonly referred to as a Green Approach, should be considered [153].
NPs may be used in vaccine production and immunostimulation [154], diagnostic techniques for various diseases, and as disinfectants, growth promoters, antimicrobials (antibacterial, antiviral, antiparasitic and antifungal) and antimycotoxin agents, with one of their most interesting properties being their bacteriostatic activity [69,148].
The use of nanoscale materials as nanobiotics and nano-drug delivery systems can be applied to the production of new antibiotics. Several studies also demonstrated that some NPs, such as AgNPs, can enhance the effect of antibiotics against susceptible and resistant bacteria, and decrease bacterial adhesion and biofilm formation [155,156], revealing a synergy between these compounds. In fact, the use of nanometric size materials can result in greater contact between the compound and the bacteria with increased bioavailability and absorption [157].
To the authors’ knowledge, there are still no licensed nanoparticles for application in the poultry industry. However, studies have been performed with this purpose. The NPs most frequently studied for application in the poultry industry are inorganic NPs, such as copper (CuNPs), zinc (ZnNPs), zinc oxide (ZnONPs), gold (AuNPs), silver (AgNPs) and selenium (SeNPs) NPs. These NPs have been widely explored as antibacterial agents due to their distinctive physicochemical and biological properties [148,149]. Some NPs, especially metallic ones, have antibacterial activity against various bacterial pathogens. Several in vitro and in vivo studies have shown their inhibitory potential against Gram-positive and Gram-negative bacteria, such as E. coli, S. aureus, S. Enteriditis, Aeromonas, Bacillus, Flavobacterium, Klebsiella and Pseudomonas aeruginosa [148], but only a few studies have been performed directly with poultry. Table 4 briefly describes the studies carried out on the in vivo and in vitro application of these NPs against important pathogens in broiler production.
ZnONPs exhibit important antibacterial properties against a wide range of microorganisms, including Gram-positive and Gram-negative bacteria. The properties of ZnONPs (big surface area, biocompatibility, biodegradability, semiconductor behavior and a UV light barrier) contribute to their vast application. These inorganic metal oxide NPs have been used as antimicrobial agents applied via topical creams and animal feed due to their strong bactericidal effect together with small particle size and higher surface energies [150,158]. An in vitro study evaluated the antibacterial activity of biologically synthesized ZnONPs against poultry-associated foodborne pathogens such as Salmonella spp., E. coli and S. aureus [149]. The study revealed that ZnONPs exhibit effective antibacterial activity against poultry-associated foodborne pathogens with S. aureus being the most susceptible. Moreover, ZnONPs can be used as a feed supplement to reduce the effects of MRSA-induced footpad dermatitis in broiler chickens [148]. Therefore, biosynthesized ZnONPs have great potential to be used as alternative antibacterial agents (nanobiotics) in poultry production to control the gut burden of poultry-associated foodborne pathogens, although further studies are required to evaluate their in vivo antibacterial efficacy [149].
AgNPs are one of the most promising nanotechnology products. AgNPs have been reported to have a wide range of antibacterial activities against both Gram-positive and Gram-negative bacteria, including major foodborne pathogens. For example, in 2014, Devi and Bhimba [159] investigated the antimicrobial effect of AgNPs synthesized using the marine algae Hypnea muciformis, with the goal of increasing silver nitrate stability. These NPs were tested against several microorganisms, some of them potentially pathogenic to poultry and humans, like E. coli, B. subtilis, Klebsiella pneumoniae, S. aureus and P. aeruginosa. Currently, there are several commercial products containing AgNPs with broad-spectrum antimicrobial properties. In fact, in human medicine, silver has become a popular additive for many medical applications, such as surgical devices, implants, shunts, catheters and wound dressings [160], and in human dentistry, for preventing the recolonization of bacteria and diminishing biofilm formation [161]. Regarding the poultry industry, AgNPs were shown to promote a reduction in pathogenic bacteria in broiler litter; however, these particles showed no inhibitory activities towards Campylobacter in broilers [148]. On the other hand, an in vivo study by Salem, Ismael and Shaalan, 2021 [162] evaluated the influence of AgNPs in necrotic enteritis caused by C. perfringens in boiler chickens. The authors concluded that these NPs had a positive impact on animals’ gut health integrity and no impact on immune organs. Although AgNPs can accumulate in muscles, further studies are needed to establish the particle concentration and size, the route of administration and the withdrawal time to ensure the safety of chicken meat for human consumption. In fact, Song, Lv, Sheikhahmadi, Uerlings and Everaert, 2017 [163] reported that AgNPs, used as disinfectants in the poultry industry, can have negative effects on chicken health, such as growth and performance impairment. As for other NPs, a trial has been performed to evaluate the effect of the oral administration of Fe3O4 to chickens challenged with S. Enteritidis, and observed a significant decrease in pathogen invasion, as well as reduced inflammatory response associated with infection [69]. Another example is the in vitro study by Ali and Bakheet, 2020 [164], which demonstrated that a chitosan NP, a biodegradable polymer, exhibited higher antimicrobial activity against biofilm-forming E. coli isolated from cases of omphalitis, in comparison to some antibiotics. A previous report described that zinc, copper and selenium NPs administered in ovo at the 18th day of incubation via an amniotic route did not harm the developing embryo, nor affect hatchability [165].
Another NP application, which could prevent the development of antimicrobial drug resistance, is the production of antimicrobial NP carriers, which are usually based on liposomal, solid/lipid, terpenoid, polymeric, dendrimeric and inorganic materials. These carriers can improve the pharmacokinetics of the drugs, extending antimicrobials’ half-life and increasing the distribution volume at the site of infection, resulting in improving antimicrobial potency and eliminating bacterial pathogens at a lower dose. To the authors’ knowledge, there are still no applications of antimicrobial NP carriers available for the poultry production industry [58]. However, some studies have shown some beneficial effects of Aloe vera extracts carried by polymeric NPs in improving animals’ growth performance, constituting a possible alternative to reduce antibiotic usage as growth promoters [152]. Another study investigated the administration of nanoencapsulated Phaleria macrocarpa fruits extract (NEPM) in chitosan via drinking water. This plant is known for its antimicrobial properties, but has a low efficacy as a diet supplement, due to low solubility, fast degradation and low bioavailability, being thermolabile at body temperature. This study revealed that NEPM contributed to the increase in lactic-acid bacteria in the GIT, modulating the intestinal microbiota and limiting the growth of pathogenic bacteria in broiler chickens [166].
In conclusion, the antibacterial mechanisms of some NPs are still uncertain and their future application depends on complementary studies. Different bacterial strains, action periods, administration routes and NP characteristics have been examined in several studies, which makes the comparison of results difficult, constituting one limitation of the existing research on the antibacterial potential of NPs [167]. Therefore, the potential of NPs’ use as antimicrobial agents in poultry production must be further investigated.
Table 4.
In vitro and in vivo studies evaluating antimicrobial effects of NPs in poultry’s health (nd—not determined; na—not applicable).
| NP | Target (Gram) | Origin | Trial | Dosage | Effect | Study |
|---|---|---|---|---|---|---|
| ZnO | Salmonella spp. (−), E. coli (−), S. aureus (+) | Lactiplantibacillus plantarum (ex Lactobacillus plantarum [131] TA4 | In vitro | na | Effective antibacterial activity, particularly against S. aureus | [149] |
| Ag | C. jejuni (−) | High purity metals and high purity demineralized water |
In vivo | 50 ppm of AgNPs in the drinking water for 30 days | Reduced growth, impaired immune functions and no antibacterial effect on different intestinal bacterial groups | [168] |
| C. perfringens (+) | Chemical reduction of silver nitrate | In vivo | 150 µg of AgNPs via crop gavage for 5 days post infection |
Reduced colonization of C. perfringens in the intestine, positive impact on gut health integrity, and no impact on immune system | [162] | |
| Fe3O4 | S. Enteritidis (−) | FeCl3 and NaAc.3H2O | In vivo | Oral 50 mg/kg Fe3O4-NPs | Pretreatment could significantly decrease the invasion of S. Enteritidis in chickens and attenuate morphological changes caused by the infection | [69] |
| Chitosan | E. coli (−) | nd | In vitro | na | Antibacterial activity against biofilm-forming strains | [164] |
| Chitosan encapsulation | - | Crab shell and sodium triphosphate pentabasic | In vivo | Non-encapsulated (2) and nanoencapsulated extracts of Aloe vera, dill and nettle roots (3) in 0.02, 0.025 and 0.05% to starter, grower and finisher diets for 42 days | Nanoencapsulation of dill extract could improve growth performance and can be used as a substitute for AGPs in the diet | [152] |
| - | nd | In vivo | Diet with 2.5% of Phaleria macrocarpa fruit extract (2) with nanoencapsulated Phaleria, macrocarpa fruits extract (NEPM) 2.5% NEPM (3) and with 5.0% NEPM (4) |
Diet supplementation with 5.0% of NEMP had positive impact on modulation in the intestinal microbiota and limitation in the growth of pathogenic bacteria | [166] |
5. Challenges of the Application of These Innovative Compounds
5.1. Antimicrobial Peptides
In general, AMPs demonstrate effective activity against microbial pathogens due to their rapid, non-specific action against microorganisms, resulting in a low resistance rate [169]. It is known that bacteria have greater difficulty developing a resistance to AMPs than to antibiotics because most AMPs have the microbial membrane as the main target [170]. However, the overexposure of pathogens to AMPs can generate the development of AMP-resistant strains [169]. Compared with the challenging development of antibiotic resistance, AMP resistance is less worrying due to the lack of horizontal transmission of resistance genes. However, a possible increase in resistance after exposure to AMP-based substances should be expected [171].
There are several mechanisms by which bacteria can develop a resistance to AMPs. First, it is possible that alterations in bacterial membranes, cell walls and cellular metabolism may occur. In the case of membrane modification, the AMP target can be altered, decreasing the interaction of AMPs with membrane components and affecting membrane permeability. Another resistance mechanism results from the modification of bacterial ionic cell potential in specific interaction sites, affecting AMPs’ binding. Furthermore, it is also possible that AMPs activity against bacteria could generate metabolic stress, resulting in the modification of surface structures, such as the bacterial cell envelope, or biofilm production [17,171].
In addition to the possible development of resistances, another challenge inherent to AMPs is their high production costs, in comparison to antibiotics [172]. Lack of stability, susceptibility to enzymatic and pH degradation and low activity under physiological conditions are also recognized problems [169,173].
Due to the aforementioned issues, the application of AMPs to production animals is still limited. The disadvantages described above are responsible for the low number of peptides approved in clinical trials, since the in vitro efficacy of these molecules does not always correlate with the in vivo one. Nevertheless, AMPs remain a great option to control microbial infections [17].
5.2. Bacteriophages
One of the major challenges of bacteriophage therapy is the economic cost related to large-scale phage production that is necessary to cover the needs for the poultry production industry. Torres-Acosta et al., 2019 [174] have developed a bioprocess model for the economic analysis of different manufacture methodologies for bacteriophage products intended for use in poultry production, presenting an innovative approach for the development of phage therapy. This study also concluded that the production titer has a crucial impact on production costs. This parameter still requires optimization and improvement to decrease associated costs [174,175].
Furthermore, bacteriophage therapy can lead to the rapid development of resistance to these antimicrobials [16], which constitutes one of the biggest challenges of its use. Thus, bacterial susceptibility to phage, phage stability and phage efficiency should be monitored during treatment. Moreover, efforts should be undertaken to develop methods for preventing the spread of phage-resistant bacteria [29,176]. Resistance can occur through different mechanisms, such as spontaneous mutations, restriction modification systems and adaptative immunity through the CRISPR-Cas system. Spontaneous mutation is the most common mechanism of phage resistance, conferring resistance by modifying the structure of bacterial surface components, which act as phage receptors and also determine phage specificity. These include lipopolysaccharides (LPS), outer membrane proteins, cell wall teichoic acids, capsules and other bacterial appendices, such as flagella, many of which are involved in bacterial virulence. In fact, phage-resistant bacteria may become less virulent in the case of mutations in genes coding for surface virulence factors [176]. CRISPR-Cas is an adaptive immune system present in most bacteria, which confers protection against infection by phages and other foreign genetic elements [177].
Various strategies may be used to minimize the development of resistance, such as the administration of phage cocktails, instead of isolated phages, aiming to kill the same bacterial strain [178]. As mentioned before, one beneficial characteristic of bacteriophages is their specificity; however, this characteristic can also be detrimental, such as, for example, in the case of infections by Salmonella, which has more than 2650 serovars and multiple strains within each serovar, requiring the simultaneous use of multiple phages [16,33].
Another important consideration is that only strong lytic phages with known genomes should be used. Since lysogenic phages incorporate their genetic material into the bacterial genome, they can act as vehicles for HGT between the bacteria of animals or humans via the food chain. Many phage-based products use a combination of different phages, thus enlarging their lytic spectrum [29,179]. Various phage-based preparations have been commercialized and licensed for use in the US. In the EU, however, their implementation is highly regulated. Before therapeutic use, phages should be sequenced to demonstrate the impossibility of a lysogenic cycle and/or the presence of antimicrobial drug resistance genes. The EFSA also encourages research on bacteriophage persistence in foods and on the ability to prevent recontamination with the bacterial pathogen. Currently, there are no regulations or control methods aimed at monitoring bacteriophages and their consumption by humans [180].
5.3. Nanoparticles
NPs interact with pathogens via diverse mechanisms, which makes it harder for bacteria to generate resistance to these compounds, in comparison to antibiotics. Thus, numerous mutations are required for bacteria to develop nanoresistance. However, bacteria’s resistance to these substances is still an issue that requires attention [58,161,181]. The mechanisms of bacterial resistance towards NP can be intrinsic or extrinsic. The common resistance mechanisms involve ion efflux pumps, electrostatic repulsion, biofilm formation, enzyme detoxification followed by volatilization and mutations [161]. Nanoresistance changes the shape of bacteria and modulates the expression of membrane proteins, which reverses after the removal of NPs [182]. Although rare, resistance has been reported against inorganic NPs, such as AgNPs, CuNPs, AuNPs and ZnONPs, which can be attributed to increased expression of efflux pumps or alterations in membrane permeability [58,160,161,181,182].
As an example, AgNPs can interact with various targets in the bacterial cell, including the cell membrane, enzymes, proteins, lipids, DNA and plasmids, making the development of resistance to these nanoparticles complex. Despite this, resistance to AgNPs has already been reported [161,181]. Moreover, one of the first reports of bacterial resistance to silver relates to S. Typhimurium [183], an important pathogen in poultry production.
Another challenge that NPs face is related to safety. It is crucial that these substances are safe for animal and human application. In poultry production, it is important to first ensure that these substances do not harm the well-being and health of poultry, which are also reflected in the quality of the final product. Secondly, it is important to ensure that their residues are not harmful to humans after consumption of poultry products (eggs and meat). Investigations are now directed to evaluate the toxicity of NPs. Although some studies have shown a low ecotoxicity, others demonstrated some degree of adverse effects of these particles on the tested animals [148]. The toxicity of NPs depend on their concentration, size and charge. Exposure to NPs for long periods can lead to adverse effects on the animals’ immune systems, as well as to their accumulation in organs, such as the liver and spleen. NP aggregates are water soluble and can be harmful to useful bacterial due to low specificity. Metallic NPs can have toxic effects on animals’ tissue cells, as a result of using high concentrations or long-time application, even at a low dose. Thus, the toxicity and safety of these NPs should be evaluated before their addition to animals feed [148,184].
In poultry production, several studies concluded that AgNP overdoses could induce oxidative stress and damage to the liver and intestinal cells of broilers [148,163,185]. The accumulation of AgNPs in chicken liver, yolks, muscle, heart and the bursa of Fabricius is dose dependent and can possibly induce negative consequences in these organs and in the animals’ immune system [148,168]. An in vivo study performed by Vadalasetty et al., 2018 [168] investigated the application of AgNPs via drinking water in order to reduce the colonization of C. jejuni in challenged broilers. They concluded that, in a concentration of 50 ppm, AgNPs reduced broilers’ growth, impaired immune functions and had no antibacterial effect on several intestinal bacteria, such as C. jejuni, lactic acid bacteria, enterococci, C. perfringens, E. coli and lactose negative enterobacteria. These data may limit the applicability of the use of AgNPs against C. jejuni and other pathogens in broiler chickens. The toxicity of other inorganic NPs in poultry has not been well studied, but the inoculation of nanoiron has been found to decrease weight and promote neurologic degeneration, and even mortality, in embryos. Moreover, the in ovo injection of CuNPs can have deleterious effects on chicken embryos and hematological/biochemistry alterations in broilers [148].
Another challenge regarding the application of nanoparticles is the use of hazardous chemicals and the high cost of manufacturing, which support the need for the development of alternative methods for nanoparticle production [153]. Nevertheless, some studies have focused on the use of safer and more sustainable approaches for NP production, such as the one by Devi and Bhimba, 2014 [159], which showed that the biosynthesis of these NPs from seaweed can be cost effective and avoid the use of toxic chemicals. As such, further research needs to assess the advantages of the synthesis of these types of materials from sustainable and cost-effective sources.
Overall, information on nanoparticles’ properties, behavior and effects is still scarce, which limits their application in the food industry; however, metal NPs were already incorporated in some human foods and medications, allowing NP-based antibiotic delivery systems and topical application (wounds, dentistry) [184]. Therefore, the potential usage of nanotechnology in poultry production is limited by its possible toxicity and must be investigated in depth.
6. Conclusions
Bacterial infections are still a major cause of human and animal morbidity and mortality worldwide. Traditionally, antibiotics were exclusively used for the treatment of bacterial infections; however, their extensive use in human and veterinary medicine and in agriculture resulted in an increase in the selection pressure on bacterial populations in all types of environments [186], leading to the emergence of AMR, which in turn forced a radical change in attitudes in order to preserve the effectiveness of the available compounds and to guarantee global health. It is important that all sectors of the food chain use conventional antimicrobials in a responsible way via a reduced and improved application of these compounds. The effective regulation of antibiotic usage was proven to be highly successful in reducing AMR levels in several European countries [11]. Moreover, the global increase in resistance rates led to the need to investigate the application of alternative antimicrobial products, including in the poultry production setting. These alternatives should be more effective and present new action mechanisms, and include antimicrobial peptides, bacteriophages, probiotics and nanoparticles. Antimicrobial peptides are a large group of substances that can have several benefits in comparison to conventional antibiotics due to their unique action mechanisms and immunomodulatory effects. Bacteriophages also demonstrated a capacity to control some of the most important bacterial agents affecting poultry and, given their specificity, have the ability to not compromise the balance of the animals’ microbiota. Probiotics are already currently used in production animals because of their immunomodulatory activity and intestinal microbiota-modulation ability, both of which are associated with a reduced propensity to infectious disease development and growth-promoting action. Finally, nanoparticles are used not only due to their antimicrobial potential but also because they enhance the action of conventional antibiotics. Despite all their benefits, each of these innovative approaches also have limitations regarding their antimicrobial potential, resistance development, large-scale production costs and safety.
In conclusion, the application of these non-conventional antimicrobials can contribute to a decrease in antimicrobial use and AMR dissemination, with several of them being already in an advanced phase of research for application in human medicine. However, in vivo investigations regarding the poultry industry are still scarce and should be supported to slow the development of multidrug-resistant bacteria in these settings.
Author Contributions
Conceptualization, R.A. and T.S.-L.; methodology, T.S.-L. and M.O.; validation, T.S.-L., E.C., L.T. and M.O.; formal analysis, E.C. and M.O.; investigation, R.A. and T.S.-L.; resources, T.S.-L., L.T. and M.O.; writing—original draft preparation, R.A. and M.O.; writing—review and editing, E.C., L.T., T.S.-L. and M.O.; visualization, R.A. and T.S.-L.; supervision, T.S.-L. and M.O.; project administration, L.T. and M.O.; funding acquisition, T.S.-L., L.T. and M.O. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.United Nations Department of Economic and Social Affairs World Population Prospects 2022—Summary of Results. 2022. [(accessed on 15 November 2022)]. Available online: https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/wpp2022_summary_of_results.pdf.
- 2.Richie H., Rosado P., Roser M. Meat and Dairy Production. [(accessed on 16 November 2022)]. Available online: https://ourworldindata.org/meat-production.
- 3.EuroStat Agricultural Production—Livestock and Meat. [(accessed on 15 December 2022)]. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Agricultural_production_-_livestock_and_meat#Poultry.
- 4.FAO Gateway to Poultry Production and Products. [(accessed on 15 March 2023)]. Available online: https://www.fao.org/poultry-production-products/production/en/
- 5.Nazeer N., Uribe-Diaz S., Rodriguez-Lecompte J.C., Ahmed M. Antimicrobial Peptides as an Alternative to Relieve Antimicrobial Growth Promoters in Poultry. Br. Poult. Sci. 2021;62:672–685. doi: 10.1080/00071668.2021.1919993. [DOI] [PubMed] [Google Scholar]
- 6.Espinosa-Marrón A., Adams K., Sinno L., Cantu-Aldana A., Tamez M., Marrero A., Bhupathiraju S.N., Mattei J. Environmental Impact of Animal-Based Food Production and the Feasibility of a Shift toward Sustainable Plant-Based Diets in the United States. Front. Sustain. 2022;3:19. doi: 10.3389/frsus.2022.841106. [DOI] [Google Scholar]
- 7.Centers for Disease Control and Prevention One Health Basics. [(accessed on 30 November 2022)]; Available online: https://www.cdc.gov/onehealth/basics/index.html.
- 8.Mohr K.I. History of Antibiotics. Curr. Top. Microbiol. Immunol. 2016;398:237–272. doi: 10.1007/82_2016_499. [DOI] [PubMed] [Google Scholar]
- 9.Founou L.L., Founou R.C., Essack S.Y. Antibiotic Resistance in the Food Chain: A Developing Country-Perspective. Front. Microbiol. 2016;7:1881. doi: 10.3389/fmicb.2016.01881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verraes C., Van Boxstael S., Van Meervenne E., Van Coillie E., Butaye P., Catry B., de Schaetzen M.A., Van Huffel X., Imberechts H., Dierick K., et al. Antimicrobial Resistance in the Food Chain: A Review. Int. J. Environ. Res. Public Health. 2013;10:2643–2669. doi: 10.3390/ijerph10072643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.EIP AGRI Focus Group Reducing Antimicrobial Use in Poultry Farming. 2021. [(accessed on 2 November 2022)]. Available online: https://ec.europa.eu/eip/agriculture/sites/default/files/eip-agri_fg_reducing_antimicrobial_use_in_poultry_farming_final_report_2021_en.pdf.
- 12.Davies J., Davies D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. 2010;74:417–433. doi: 10.1128/MMBR.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization . Global Action Plan on Antimicrobial Resistance. World Health Organization; Geneva, Switzerland: 2015. pp. 1–28. [DOI] [PubMed] [Google Scholar]
- 14.EMA Categorisation of Antibiotics Used in Animals Promotes Responsible Use to Protect Public and Animal Health. [(accessed on 15 January 2023)]. Available online: https://www.ema.europa.eu/en/news/categorisation-antibiotics-used-animals-promotes-responsible-use-protect-public-animal-health.
- 15.Al Sattar A., Chisty N.N., Irin N., Uddin M.H., Hasib F.M.Y., Hoque M.A. Knowledge and Practice of Antimicrobial Usage and Resistance among Poultry Farmers: A Systematic Review, Meta-Analysis, and Meta-Regression. Vet. Res. Commun. 2023 doi: 10.1007/s11259-023-10082-5. [DOI] [PubMed] [Google Scholar]
- 16.Joerger R.D. Alternatives to Antibiotics: Bacteriocins, Antimicrobial Peptides and Bacteriophages. Poult. Sci. 2003;82:640–647. doi: 10.1093/ps/82.4.640. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigues G., Santos L.S., Franco O.L. Antimicrobial Peptides Controlling Resistant Bacteria in Animal Production. Front. Microbiol. 2022;13:874153. doi: 10.3389/fmicb.2022.874153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castanon J.I.R. History of the Use of Antibiotic as Growth Promoters in European Poultry Feeds. Poult. Sci. 2007;86:2466–2471. doi: 10.3382/ps.2007-00249. [DOI] [PubMed] [Google Scholar]
- 19.Hudson J.A., Frewer L.J., Jones G., Brereton P.A., Whittingham M.J., Stewart G. The Agri-Food Chain and Antimicrobial Resistance: A Review. Trends Food Sci. Technol. 2017;69:131–147. doi: 10.1016/j.tifs.2017.09.007. [DOI] [Google Scholar]
- 20.Rushton J. Anti-Microbial Use in Animals: How to Assess the Trade-Offs. Zoonoses Public Health. 2015;62:10–21. doi: 10.1111/zph.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luiken R.E.C., Van Gompel L., Munk P., Sarrazin S., Joosten P., Dorado-García A., Borup Hansen R., Knudsen B.E., Bossers A., Wagenaar J.A., et al. Associations between Antimicrobial Use and the Faecal Resistome on Broiler Farms from Nine European Countries. J. Antimicrob. Chemother. 2019;74:2596–2604. doi: 10.1093/jac/dkz235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krysiak K., Konkol D., Korczyński M. Overview of the Use of Probiotics in Poultry Production. Animals. 2021;11:1620. doi: 10.3390/ani11061620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holmes A.H., Moore L.S.P., Sundsfjord A., Steinbakk M., Regmi S., Karkey A., Guerin P.J., Piddock L.J.V. Understanding the Mechanisms and Drivers of Antimicrobial Resistance. Lancet. 2016;387:176–187. doi: 10.1016/S0140-6736(15)00473-0. [DOI] [PubMed] [Google Scholar]
- 24.Bester L.A., Essack S.Y. Antibiotic Resistance via the Food Chain: Fact or Fiction? S. Afr. J. Sci. 2010;106:1–5. doi: 10.4102/sajs.v106i9/10.281. [DOI] [Google Scholar]
- 25.Acar J.F., Moulin G. Antimicrobial Resistance at Farm Level. Rev. Sci. Tech. 2006;25:775–792. doi: 10.20506/rst.25.2.1695. [DOI] [PubMed] [Google Scholar]
- 26.Marshall B.M., Levy S.B. Food Animals and Antimicrobials: Impacts on Human Health. Clin. Microbiol. Rev. 2011;24:718–733. doi: 10.1128/CMR.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Da Costa P.M., Loureiro L., Matos A.J.F. Transfer of Multidrug-Resistant Bacteria between Intermingled Ecological Niches: The Interface between Humans, Animals and the Environment. Int. J. Environ. Res. Public Health. 2013;10:278–294. doi: 10.3390/ijerph10010278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Djordjevic S.P., Stokes H.W., Chowdhury P.R. Mobile Elements, Zoonotic Pathogens and Commensal Bacteria: Conduits for the Delivery of Resistance Genes into Humans, Production Animals and Soil Microbiota. Front. Microbiol. 2013;4:86. doi: 10.3389/fmicb.2013.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Żbikowska K., Michalczuk M., Dolka B. The Use of Bacteriophages in the Poultry Industry. Animals. 2020;10:872. doi: 10.3390/ani10050872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Saraiva M.M.S., Lim K., do Monte D.F.M., Givisiez P.E.N., Alves L.B.R., de Neto O.C.F., Kariuki S., Júnior A.B., de Oliveira C.J.B., Gebreyes W.A. Antimicrobial Resistance in the Globalized Food Chain: A One Health Perspective Applied to the Poultry Industry. Braz. J. Microbiol. 2022;53:465–486. doi: 10.1007/s42770-021-00635-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.European T., One U., Report Z. The European Union One Health 2020 Zoonoses Report. EFSA J. 2021;19:e06971. doi: 10.2903/j.efsa.2021.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lorenzo-Rebenaque L., Malik D.J., Catalá-Gregori P., Torres J., Marin C., Sevilla-Navarro S. Microencapsulated Bacteriophages Incorporated in Feed for Salmonella Control in Broilers. Vet. Microbiol. 2022;274:109579. doi: 10.1016/j.vetmic.2022.109579. [DOI] [PubMed] [Google Scholar]
- 33.Ricke S.C. Strategies to Improve Poultry Food Safety, a Landscape Review. Annu. Rev. Anim. Biosci. 2021;9:379–400. doi: 10.1146/annurev-animal-061220-023200. [DOI] [PubMed] [Google Scholar]
- 34.Chai S.J., Cole D., Nisler A., Mahon B.E. Poultry: The Most Common Food in Outbreaks with Known Pathogens, United States, 1998–2012. Epidemiol. Infect. 2017;145:316–325. doi: 10.1017/S0950268816002375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cosby D.E., Cox N.A., Harrison M.A., Wilson J.L., Jeff Buhr R., Fedorka-Cray P.J. Salmonella and Antimicrobial Resistance in Broilers: A Review. J. Appl. Poult. Res. 2015;24:408–426. doi: 10.3382/japr/pfv038. [DOI] [Google Scholar]
- 36.Issenhuth-Jeanjean S., Roggentin P., Mikoleit M., Guibourdenche M., De Pinna E., Nair S., Fields P.I., Weill F.X. Supplement 2008–2010 (No. 48) to the White-Kauffmann-Le Minor Scheme. Res. Microbiol. 2014;165:526–530. doi: 10.1016/j.resmic.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Antunes P., Mourão J., Campos J., Peixe L. Salmonellosis: The Role of Poultry Meat. Clin. Microbiol. Infect. 2016;22:110–121. doi: 10.1016/j.cmi.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 38.Alali W.Q., Hofacre C.L. Preharvest Food Safety in Broiler Chicken Production. In: Thakur S., Kniel K.E., editors. Preharvest Food Safety. ASM Press; Washington, DC, USA: 2018. pp. 69–86. [DOI] [Google Scholar]
- 39.Veterinary Medicines Directorate . UK One Health Report—Joint Report on Antibiotic Use and Antibiotic Resistance, 2013–2017. Veterinary Medicines Directorate; Addlestone, UK: 2019. [(accessed on 20 December 2022)]. Available online: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/447319/One_Health_Report_July2015.pdf. [Google Scholar]
- 40.Silva J., Leite D., Fernandes M., Mena C., Gibbs P.A., Teixeira P. Campylobacter spp. As a Foodborne Pathogen: A Review. Front. Microbiol. 2011;2:200. doi: 10.3389/fmicb.2011.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sahin O., Kassem I.I., Shen Z., Lin J., Rajashekara G., Sahin O., Kassem A.I.I., Shen B.Z., Lin A.J., Rajashekara C.G., et al. Campylobacter in Poultry: Ecology and Potential Interventions. Avian Dis. 2015;59:185–200. doi: 10.1637/11072-032315-Review. [DOI] [PubMed] [Google Scholar]
- 42.Van Deun K., Pasmans F., Ducatelle R., Flahou B., Vissenberg K., Martel A., Van den Broeck W., Van Immerseel F., Haesebrouck F. Colonization Strategy of Campylobacter jejuni Results in Persistent Infection of the Chicken Gut. Vet. Microbiol. 2008;130:285–297. doi: 10.1016/j.vetmic.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 43.Hermans D., Pasmans F., Messens W., Martel A., Van Immerseel F., Rasschaert G., Heyndrickx M., Van Deun K., Haesebrouck F. Poultry as a Host for the Zoonotic Pathogen Campylobacter jejuni. Vector-Borne Zoonotic Dis. 2012;12:89–98. doi: 10.1089/vbz.2011.0676. [DOI] [PubMed] [Google Scholar]
- 44.Public Health England Summary of Antimicrobial Prescribing Guidance: Managing Common Infections. [(accessed on 20 January 2023)];2018 Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/994444/Common_Infect_PHE_context_references_and_rationale_May_2021_Bites_and_Eczema__1_.pdf.
- 45.Daneshmand A., Kermanshahi H., Sekhavati M.H., Javadmanesh A., Ahmadian M. Antimicrobial Peptide, CLF36, Affects Performance and Intestinal Morphology, Microflora, Junctional Proteins, and Immune Cells in Broilers Challenged with E. coli. Sci. Rep. 2019;9:14176. doi: 10.1038/s41598-019-50511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Islam M.S., Nayeem M.M.H., Sobur M.A., Ievy S., Islam M.A., Rahman S., Kafi M.A., Ashour H.M., Rahman M.T. Virulence Determinants and Multidrug Resistance of Escherichia coli Isolated from Migratory Birds. Antibiotics. 2021;10:190. doi: 10.3390/antibiotics10020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bass L., Liebert C.A., Lee M.D., Summers A.O., White D.G., Thayer S.G., Maurer J.J. Incidence and Characterization of Integrons, Genetic Elements Mediating Multiple-Drug Resistance, in Avian Escherichia coli. Antimicrob. Agents Chemother. 1999;43:2925–2929. doi: 10.1128/AAC.43.12.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nolan L.K., Barnes H.J., Vaillancourt J., Abdul-aziz T., Logue C.M. Diseases of Poultry. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2013. Colibacillosis; pp. 751–805. [Google Scholar]
- 49.Mitchell N.M., Johnson J.R., Johnston B., Curtiss R., Mellata M. Zoonotic Potential of Escherichia coli Isolates from Retail Chicken Meat Products and Eggs. Appl. Environ. Microbiol. 2015;81:1177–1187. doi: 10.1128/AEM.03524-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dea M.O., Sahibzada S., Jordan D., Laird T., Lee T., Hewson K., Pang S., Abraham R., Coombs G.W., Harris T., et al. Genomic, Antimicrobial Resistance, and Public Health Insights into Enterococcus spp. from Australian Chickens. J. Clin. Microbiol. 2019;57:e00319. doi: 10.1128/JCM.00319-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kabir S.M.L. The Role of Probiotics in the Poultry Industry. Int. J. Mol. Sci. 2009;10:3531–3546. doi: 10.3390/ijms10083531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Souillard R., Laurentie J., Kempf I., Le Caër V., Le Bouquin S., Serror P., Allain V. Increasing Incidence of Enterococcus-Associated Diseases in Poultry in France over the Past 15 Years. Vet. Microbiol. 2022;269:109426. doi: 10.1016/j.vetmic.2022.109426. [DOI] [PubMed] [Google Scholar]
- 53.Devriese L.A., Cawerts K., Hermans K., Wood A.M. Enterococcus cecorum Septicaemia as a Cause of Bone and Joint Lesions Resulting in Lameness in Broiler Chickens. Vlaams Diergeneeskd. Tijdschr. 2002;71:219–221. [Google Scholar]
- 54.Khan H.A., Ahmad A., Mehboob R. Nosocomial Infections and Their Control Strategies. Asian Pac. J. Trop. Biomed. 2015;5:509–514. doi: 10.1016/j.apjtb.2015.05.001. [DOI] [Google Scholar]
- 55.Lee G.Y., Lee S.I., Do Kim S., Park J.H., Kim G.-B., Yang S.-J. Clonal Distribution and Antimicrobial Resistance of Methicillin-Susceptible and -Resistant Staphylococcus aureus Strains Isolated from Broiler Farms, Slaughterhouses, and Retail Chicken Meat. Poult. Sci. 2020;101:102070. doi: 10.1016/j.psj.2022.102070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feßler A.T., Kadlec K., Hassel M., Hauschild T., Eidam C., Ehricht R., Monecke S., Schwarz S. Characterization of Methicillin-Resistant Staphylococcus aureus Isolates from Food and Food Products of Poultry Origin in Germany. Appl. Environ. Microbiol. 2011;77:7151–7157. doi: 10.1128/AEM.00561-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Verkade E., Kluytmans J. Livestock-Associated Staphylococcus aureus CC398: Animal Reservoirs and Human Infections. Infect. Genet. Evol. 2014;21:523–530. doi: 10.1016/j.meegid.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 58.Chakraborty N., Jha D., Roy I., Kumar P., Gaurav S.S., Marimuthu K., Ng O.T., Lakshminarayanan R., Verma N.K., Gautam H.K. Nanobiotics against Antimicrobial Resistance: Harnessing the Power of Nanoscale Materials and Technologies. J. Nanobiotechnol. 2022;20:375. doi: 10.1186/s12951-022-01573-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sommer J., Trautner C., Witte A.K., Fister S., Schoder D., Rossmanith P., Mester P.-J. Don’t Shut the Stable Door after the Phage Has Bolted—The Importance of Bacteriophage Inactivation in Food Environments. Viruses. 2019;11:468. doi: 10.3390/v11050468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rawson T., Dawkins M.S., Bonsall M.B. A Mathematical Model of Campylobacter Dynamics within a Broiler Flock. Front. Microbiol. 2019;10:1940. doi: 10.3389/fmicb.2019.01940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Line J.E., Seal B.S., Garrish J.K. Selected Antimicrobial Peptides Inhibit in Vitro Growth of Campylobacter spp. Appl. Microbiol. 2022;2:688–700. doi: 10.3390/applmicrobiol2040053. [DOI] [Google Scholar]
- 62.Cruz G.S., dos Santos A.T., de Brito E.H.S., Rádis-Baptista G. Cell-Penetrating Antimicrobial Peptides with Anti-Infective Activity against Intracellular Pathogens. Antibiotics. 2022;11:1772. doi: 10.3390/antibiotics11121772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lai Y., Gallo R.L. AMPed Up Immunity: How Antimicrobial Peptides Have Multiple Roles in Immune Defense. Trends Imunnology. 2009;30:131–141. doi: 10.1016/j.it.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roque-Borda C.A., Pereira L.P., Guastalli E.A.L., Soares N.M., Mac-Lean P.A.B., Salgado D.D., Meneguin A.B., Chorilli M., Vicente E.F. HPMCP-Coated Microcapsules Containing the Ctx(Ile21)-Ha Antimicrobial Peptide Reduce the Mortality Rate Caused by Resistant Salmonella enteritidis in Laying Hens. Antibiotics. 2021;10:616. doi: 10.3390/antibiotics10060616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Magana M., Pushpanathan M., Santos A.L., Leanse L., Fernandez M., Ioannidis A., Giulianotti M.A., Apidianakis Y., Bradfute S., Ferguson A.L., et al. The Value of Antimicrobial Peptides in the Age of Resistance. Lancet Infect. Dis. 2020;20:e216–e230. doi: 10.1016/S1473-3099(20)30327-3. [DOI] [PubMed] [Google Scholar]
- 66.Cunha E., Tavares L., Veiga A.S., Oliveira M. Advances in Medicine and Biology. Nova Science Publishers; Hauppauge, NY, USA: 2019. Fighting Antimicrobial Resistance: The Biomedical Use of the Antimicrobial Peptide Nisin; pp. 79–112. [Google Scholar]
- 67.Yadav S., Jha R. Strategies to Modulate the Intestinal Microbiota and Their Effects on Nutrient Utilization, Performance, and Health of Poultry. J. Anim. Sci. Biotechnol. 2019;10:2. doi: 10.1186/s40104-018-0310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Apajalahti J., Vienola K. Interaction between Chicken Intestinal Microbiota and Protein Digestion. Anim. Feed Sci. Technol. 2016;221:323–330. doi: 10.1016/j.anifeedsci.2016.05.004. [DOI] [Google Scholar]
- 69.Shen Y., Xiao Y., Zhang S., Wu S., Gao L., Shi S. Fe3O4 Nanoparticles Attenuated Salmonella Infection in Chicken Liver Through Reactive Oxygen and Autophagy via PI3K/Akt/MTOR Signaling. Front. Physiol. 2020;10:1580. doi: 10.3389/fphys.2019.01580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang G. Effect of Antimicrobial Peptide Microcin J25 on Growth Performance, Immune Regulation, and Intestinal Microbiota in Broiler Chickens Challenged with Escherichia coli and Salmonella. Animals. 2020;10:345. doi: 10.3390/ani10020345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Iseppi R., Lauková A., Sabia C. Bacteriocin-Producing Probiotic Bacteria: A Natural Solution for Increasing Efficiency and Safety of Livestock Food Production. Front. Microbiol. 2021;12:675483. doi: 10.3389/fmicb.2021.675483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kierończyk B., Rawski M., Mikołajczak Z., Świątkiewicz S., Józefiak D. Nisin as a Novel Feed Additive: The Efffects on Gut Microbial Modulation and Activity, Histological Parameters, and Growth Performance of Broiler Chickens. Animals. 2020;10:101. doi: 10.3390/ani10010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Józefiak D., Kierończyk B., Juśkiewicz J., Zduńczyk Z., Rawski M., Długosz J., Sip A., Højberg O. Dietary Nisin Modulates the Gastrointestinal Microbial Ecology and Enhances Growth Performance of the Broiler Chickens. PLoS ONE. 2013;8:e85347. doi: 10.1371/journal.pone.0085347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kierończyk B., Pruszyńska-Oszmałek E., Światkiewicz S., Rawski M., Długosz J., Engberg R.M., Józefiak D. The Nisin Improves Broiler Chicken Growth Performance and Interacts with Salinomycin in Terms of Gastrointestinal Tract Microbiota Composition. J. Anim. Feed Sci. 2016;25:309–316. doi: 10.22358/jafs/67802/2016. [DOI] [Google Scholar]
- 75.Jozefiak D., Sip A., Rawski M., Rutkowski A., Kaczmarek S., Hojberg O., Jensen B.B., Engberg R.M. Dietary Divercin Modifies Gastrointestinal Microbiota and Improves Growth Performance in Broiler Chickens. Br. Poult. Sci. 2011;52:492–499. doi: 10.1080/00071668.2011.602963. [DOI] [PubMed] [Google Scholar]
- 76.Wen L.F., He J.G. Dose-Response Effects of an Antimicrobial Peptide, a Cecropin Hybrid, on Growth Performance, Nutrient Utilisation, Bacterial Counts in the Digesta and Intestinal Morphology in Broilers. Br. J. Nutr. 2012;108:1756–1763. doi: 10.1017/S0007114511007240. [DOI] [PubMed] [Google Scholar]
- 77.Choi S.C., Ingale S.L., Kim J.S., Park Y.K., Kwon I.K., Chae B.J. An Antimicrobial Peptide-A3: Effects on Growth Performance, Nutrient Retention, Intestinal and Faecal Microflora and Intestinal Morphology of Broilers. Br. Poult. Sci. 2013;54:738–746. doi: 10.1080/00071668.2013.838746. [DOI] [PubMed] [Google Scholar]
- 78.Choi S.C., Ingale S.L., Kim J.S., Park Y.K., Kwon I.K., Chae B.J. Effects of Dietary Supplementation with an Antimicrobial Peptide-P5 on Growth Performance, Nutrient Retention, Excreta and Intestinal Microflora and Intestinal Morphology of Broilers. Anim. Feed Sci. Technol. 2013;185:78–84. doi: 10.1016/j.anifeedsci.2013.07.005. [DOI] [Google Scholar]
- 79.Sochacki K.A., Barns K.J., Bucki R., Weisshaar J.C. Real-Time Attack on Single Escherichia coli Cells by the Human Antimicrobial Peptide LL-37. Proc. Natl. Acad. Sci. USA. 2011;108:E77–E81. doi: 10.1073/pnas.1101130108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Peng L., Scheenstra M.R., van Harten R.M., Haagsman H.P., Veldhuizen E.J.A. The Immunomodulatory Effect of Cathelicidin-B1 on Chicken Macrophages. Vet. Res. 2020;51:122. doi: 10.1186/s13567-020-00849-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kogut M.H., Genovese K.J., He H., Swaggerty C.L., Jiang Y. Modulation of Chicken Intestinal Immune Gene Expression by Small Cationic Peptides as Feed Additives during the First Week Posthatch. Clin. Vaccine Immunol. 2013;20:1440–1448. doi: 10.1128/CVI.00322-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aguirre A.A.T., Acda S.P., Angeles A.A., Cynthia Oliveros M.R., Merca F.E., Cruz F.A. Effect of Bovine Lactoferrin on Growth Performance and Intestinal Histologic Features of Broilers. Philipp. J. Vet. Anim. Sci. 2015;41:11–20. [Google Scholar]
- 83.Liu T., She R., Wang K., Bao H., Zhang Y., Luo D., Hu Y., Ding Y., Wang D., Peng K. Effects of Rabbit Sacculus Rotundus Antimicrobial Peptides on the Intestinal Mucosal Immunity in Chickens. Poult. Sci. 2008;87:250–254. doi: 10.3382/ps.2007-00353. [DOI] [PubMed] [Google Scholar]
- 84.Ma J.L., Zhao L.H., Sun D.D., Zhang J., Guo Y.P., Zhang Z.Q., Ma Q.G., Ji C., Zhao L.H. Effects of Dietary Supplementation of Recombinant Plectasin on Growth Performance, Intestinal Health and Innate Immunity Response in Broilers. Probiotics Antimicrob. Proteins. 2020;12:214–223. doi: 10.1007/s12602-019-9515-2. [DOI] [PubMed] [Google Scholar]
- 85.Hernández-González J.C., Martínez-Tapia A., Lazcano-Hernández G., García-Pérez B.E., Castrejón-Jiménez N.S. Bacteriocins from Lactic Acid Bacteria. A Powerful Alternative as Antimicrobials, Probiotics, and Immunomodulators in Veterinary Medicine. Animals. 2021;11:979. doi: 10.3390/ani11040979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Turner J., Cho Y., Dinh N.N., Waring A.J., Lehrer R.I. Activities of LL-37, a Cathelin-Associated Antimicrobial Peptide of Human Neutrophils. Antimicrob. Agents Chemother. 1998;42:2206–2214. doi: 10.1128/AAC.42.9.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Veldhuizen E.J.A., Brouwer E.C., Schneider V.A.F., Fluit A.C. Chicken Cathelicidins Display Antimicrobial Activity against Multiresistant Bacteria without Inducing Strong Resistance. PLoS ONE. 2013;8:e61964. doi: 10.1371/journal.pone.0061964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tanhaiean A., Azghandi M., Razmyar J., Mohammadi E., Sekhavati M.H. Recombinant Production of a Chimeric Antimicrobial Peptide in E. coli and Assessment of Its Activity against Some Avian Clinically Isolated Pathogens. Microb. Pathog. 2018;122:73–78. doi: 10.1016/j.micpath.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 89.Svetoch E.A., Stern N.J., Eruslanov B.V., Kovalev Y.N., Volodina L.I., Perelygin V.V., Mitsevich E.V., Mitsevich I.P., Pokhilenko V.D., Borzenkov V.N., et al. Isolation of Bacillus circulans and Paenibacillus polymyxa Strains Inhibitory to Campylobacter jejuni and Characterization of Associated Bacteriocins. J. Food Prot. 2005;68:11–17. doi: 10.4315/0362-028X-68.1.11. [DOI] [PubMed] [Google Scholar]
- 90.Daeschel M.A., Klaenhammer T.R. Association of a 13.6-Megadalton Plasmid in Pedicococcus pentosaceus with Bacteriocin Activity. Appl. Environ. Microbiol. 1985;50:1538–1541. doi: 10.1128/aem.50.6.1538-1541.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grilli E., Messina M., Catelli E., Morlacchini M., Piva A. Pediocin a Improves Growth Performance of Broilers Challenged with Clostridium perfringens. Poult. Sci. 2009;88:2152–2158. doi: 10.3382/ps.2009-00160. [DOI] [PubMed] [Google Scholar]
- 92.Wang S., Zeng X.F., Wang Q.W., Zhu J.L., Peng Q., Hou C., Thacker P., Qiao S.Y. The Antimicrobial Peptide Sublancin Ameliorates Necrotic Enteritis Induced by Clostridium perfringens in Broilers. Am. Soc. Anim. Sci. 2015;93:4750–4760. doi: 10.2527/jas2015-9284. [DOI] [PubMed] [Google Scholar]
- 93.Van Dijk A., Veldhuizen E.J.A., Kalkhove S.I.C., Tjeerdsma-Van Bokhoven J.L.M., Romijn R.A., Haagsman H.P. The β-Defensin Gallinacin-6 Is Expressed in the Chicken Digestive Tract and Has Antimicrobial Activity against Food-Borne Pathogens. Antimicrob. Agents Chemother. 2007;51:912–922. doi: 10.1128/AAC.00568-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Józefiak D., Sip A., Rutkowski A., Rawski M., Kaczmarek S., Wołuń-Cholewa M., Engberg R.M., Højberg O. Lyophilized Carnobacterium divergens AS7 Bacteriocin Preparation Improves Performance of Broiler Chickens Challenged with Clostridium perfringens. Poult. Sci. 2012;91:1899–1907. doi: 10.3382/ps.2012-02151. [DOI] [PubMed] [Google Scholar]
- 95.Kieronczyk B., Sassek M., Pruszynska-Oszmalek E., Kolodziejski P., Rawski M., Swiatkiewicz S., Józefiak D. The Physiological Response of Broiler Chickens to the Dietary Supplementation of the Bacteriocin Nisin and Ionophore Coccidiostats. Poult. Sci. 2017;96:4026–4037. doi: 10.3382/ps/pex234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Duckworth D.H. Who Discovered Bacteriophage? Bacteriol. Rev. 1976;40:793–802. doi: 10.1128/br.40.4.793-802.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Manrique P., Bolduc B., Walk S.T., Van Oost J.D., De Vos W.M., Young M.J. Healthy Human Gut Phageome. Proc. Natl. Acad. Sci. USA. 2016;113:10400–10405. doi: 10.1073/pnas.1601060113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Grant A., Hashem F., Parveen S. Salmonella and Campylobacter: Antimicrobial Resistance and Bacteriophage Control in Poultry. Food Microbiol. 2016;53:104–109. doi: 10.1016/j.fm.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 99.Janež N., Loc-Carrillo C. Use of Phages to Control Campylobacter spp. J. Microbiol. Methods. 2013;95:68–75. doi: 10.1016/j.mimet.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 100.Ushanov L., Lasareishvili B., Janashia I., Zautner A.E. Application of Campylobacter jejuni Phages: Challenges and Perspectives. Animals. 2020;10:279. doi: 10.3390/ani10020279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fortier L.-C., Sekulovic O. Importance of Prophages to Evolution and Virulence of Bacterial Pathogens. Virulence. 2013;4:354–365. doi: 10.4161/viru.24498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.O’Flaherty S., Ross R.P., Coffey A. Bacteriophage and Their Lysins for Elimination of Infectious Bacteria: Review Article. FEMS Microbiol. Rev. 2009;33:801–819. doi: 10.1111/j.1574-6976.2009.00176.x. [DOI] [PubMed] [Google Scholar]
- 103.Ackermann H.W. Bacteriophage Observations and Evolution. Res. Microbiol. 2003;154:245–251. doi: 10.1016/S0923-2508(03)00067-6. [DOI] [PubMed] [Google Scholar]
- 104.Stone E., Campbell K., Grant I., McAuliffe O. Understanding and Exploiting Phage-Host Interactions. Viruses. 2019;11:567. doi: 10.3390/v11060567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nikolic I., Vukovic D., Gavric D., Cvetanovic J., Aleksic Sabo V., Gostimirovic S., Narancic J., Knezevic P. An Optimized Checkerboard Method for Phage-Antibiotic Synergy Detection. Viruses. 2022;14:1542. doi: 10.3390/v14071542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Alaoui Mdarhri H., Benmessaoud R., Yacoubi H., Seffar L., Guennouni Assimi H., Hamam M., Boussettine R., Filali-Ansari N., Lahlou F.A., Diawara I., et al. Alternatives Therapeutic Approaches to Conventional Antibiotics: Advantages, Limitations and Potential Application in Medicine. Antibiotics. 2022;11:1826. doi: 10.3390/antibiotics11121826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lin D.M., Koskella B., Lin H.C. Phage Therapy: An Alternative to Antibiotics in the Age of Multi-Drug Resistance. World J. Gastrointest. Pharmacol. Ther. 2017;8:162. doi: 10.4292/wjgpt.v8.i3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Berchieri A., Lovell M.A., Barrow P.A. The Activity in the Chicken Alimentary Tract of Bacteriophages Lytic for Salmonella typhimurium. Res. Microbiol. 1991;142:541–549. doi: 10.1016/0923-2508(91)90187-F. [DOI] [PubMed] [Google Scholar]
- 109.Bardina C., Spricigo D.A., Cortés P., Llagostera M. Significance of the Bacteriophage Treatment Schedule in Reducing Salmonella Colonization of Poultry. Appl. Environ. Microbiol. 2012;78:6600–6607. doi: 10.1128/AEM.01257-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hong S.S., Jeong J., Lee J., Kim S., Min W., Myung H. Therapeutic Effects of Bacteriophages against Salmonella gallinarum Infection in Chickens. J. Microbiol. Biotechnol. 2013;23:1478–1483. doi: 10.4014/jmb.1304.04067. [DOI] [PubMed] [Google Scholar]
- 111.Nabil N.M., Tawakol M.M., Hassan H.M. Assessing the Impact of Bacteriophages in the Treatment of Salmonella in Broiler Chickens. Infect. Ecol. Epidemiol. 2018;8:1539056. doi: 10.1080/20008686.2018.1539056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Clavijo V., Baquero D., Hernandez S., Farfan J.C., Arias J., Arévalo A., Donado-Godoy P., Vives-Flores M. Phage Cocktail SalmoFREE® Reduces Salmonella on a Commercial Broiler Farm. Poult. Sci. 2019;98:5054–5063. doi: 10.3382/ps/pez251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wójcik E.A., Wojtasik A., Górecka E., Stańczyk M. Application of Bacteriophage Preparation Bafasal® Іn Broiler Chickens Experimentally Exposed to Salmonella spp. Наукoвo-технічний бюлетень Державнoгo наукoвo-дoсліднoгo кoнтрoльнoгo інституту ветеринарних препаратів та кoрмoвих дoбавoк і Інституту біoлoгії тварин. 2015;16:241–250. [Google Scholar]
- 114.Proteon-Pharmaceuticals Bafasal®. [(accessed on 28 November 2022)]. Available online: https://www.proteonpharma.com/bafasal-product/
- 115.Nowaczek A., Urban-Chmiel R., Dec M., Puchalski A., Stępień-Pyśniak D., Marek A., Pyzik E. Campylobacter spp. and Bacteriophages from Broiler Chickens: Characterization of Antibiotic Susceptibility Profiles and Lytic Bacteriophages. Microbiologyopen. 2019;8:e00784. doi: 10.1002/mbo3.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wagenaar J.A., Bergen M.A.P.V., Mueller M.A., Wassenaar T.M., Carlton R.M. Phage Therapy Reduces Campylobacter jejuni Colonization in Broilers. Vet. Microbiol. 2005;109:275–283. doi: 10.1016/j.vetmic.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 117.Loc Carrillo C., Atterbury R.J., El-Shibiny A., Connerton P.L., Dillon E., Scott A., Connerton I.F. Bacteriophage Therapy to Reduce Campylobacter jejuni Colonization of Broiler Chickens. Appl. Environ. Microbiol. 2005;71:6554–6563. doi: 10.1128/AEM.71.11.6554-6563.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.El-Shibiny A., Scott A., Timms A., Metawea Y., Connerton P., Connerton I. Application of a Group II Campylobacter Bacteriophage to Reduce Strains of Campylobacter jejuni and Campylobacter coli Colonizing Broiler Chickens. J. Food Prot. 2009;72:733–740. doi: 10.4315/0362-028X-72.4.733. [DOI] [PubMed] [Google Scholar]
- 119.Carvalho C.M., Gannon B.W., Halfhide D.E., Santos S.B., Hayes C.M., Roe J.M., Azeredo J. The in Vivo Efficacy of Two Administration Routes of a Phage Cocktail to Reduce Numbers of Campylobacter coli and Campylobacter jejuni in Chickens. BMC Microbiol. 2010;10:232. doi: 10.1186/1471-2180-10-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kittler S., Fischer S., Abdulmawjood A., Glünder G., Kleina G. Effect of Bacteriophage Application on Campylobacter jejuni Loads in Commercial Broiler Flocks. Appl. Environ. Microbiol. 2013;79:7525–7533. doi: 10.1128/AEM.02703-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fischer S., Kittler S., Klein G., Glünder G. Impact of a Single Phage and a Phage Cocktail Application in Broilers on Reduction of Campylobacter jejuni and Development of Resistance. PLoS ONE. 2013;8:e78543. doi: 10.1371/journal.pone.0078543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Barrow P., Lovell M., Berchieru A.J. Use of Lytic Bacteriophage for Control of Experimental Escherichia coli Septicemia and Meningitis in Chickens and Calves. Clin. Diagn. Lab. Immunol. 1998;5:89–114. doi: 10.1128/CDLI.5.3.294-298.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Huff W.E., Huff G.R., Rath N.C., Balog J.M., Donoghue A.M. Evaluation of Aerosol Spray and Intramuscular Injection of Bacteriophage to Treat an Escherichia coli Respiratory Infection. Poult. Sci. 2003;82:1108–1112. doi: 10.1093/ps/82.7.1108. [DOI] [PubMed] [Google Scholar]
- 124.Huff G., Huff W., Rath N., Donoghue A. Critical Evaluation of Bacteriophage to Prevent and Treat Colibacillosis in Poultry. J. Ark. Acad. Sci. 2009;63:93–98. [Google Scholar]
- 125.Xie H., Zhuang X., Kong J., Ma G., Zhang H. Bacteriophage Esc-A Is an Efficient Therapy for Escherichia coli 3-1 Caused Diarrhea in Chickens. J. Gen. Appl. Microbiol. 2005;51:159–163. doi: 10.2323/jgam.51.159. [DOI] [PubMed] [Google Scholar]
- 126.Marek A., Pyzik E., Stępień-Pyśniak D., Urban-Chmiel R., Nowaczek A. Characterization of Bacteriophages and Their Carriage in Staphylococcus aureus Isolated from Broilers in Poland. Br. Poult. Sci. 2019;60:373–380. doi: 10.1080/00071668.2018.1426831. [DOI] [PubMed] [Google Scholar]
- 127.Saint-Cyr M.J., Guyard-Nicodème M., Messaoudi S., Chemaly M., Cappelier J.M., Dousset X., Haddad N. Recent Advances in Screening of Anti-Campylobacter Activity in Probiotics for Use in Poultry. Front. Microbiol. 2016;7:553. doi: 10.3389/fmicb.2016.00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H.J., Salminen S., et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 129.Tellez G., Pixley C., Wolfenden R.E., Layton S.L., Hargis B.M. Probiotics/Direct Fed Microbials for Salmonella Control in Poultry. Food Res. Int. 2012;45:628–633. doi: 10.1016/j.foodres.2011.03.047. [DOI] [Google Scholar]
- 130.Farnell M.B., Donoghue A.M., Solis De Los Santos F., Blore P.J., Hargis B.M., Tellez G., Donoghue D.J. Upregulation of Oxidative Burst and Degranulation in Chicken Heterophils Stimulated with Probiotic Bacteria. Poult. Sci. 2006;85:1900–1906. doi: 10.1093/ps/85.11.1900. [DOI] [PubMed] [Google Scholar]
- 131.Zheng J., Wittouck S., Salvetti E., Franz C.M.A.P., Harris H.M.B., Mattarelli P., O’Toole P.W., Pot B., Vandamme P., Walte J., et al. A Taxonomic Note on the Genus Lactobacillus: Description of 23 Novel Genera, Emended Description of the Genus Lactobacillus Beijerinck 1901, and Union of Lactobacillaceae and Leuconostocaceae. Microbiol. Soc. 2020;70:2782–2858. doi: 10.1099/ijsem.0.004107. [DOI] [PubMed] [Google Scholar]
- 132.Vicente J.L., Aviña L., Torres-Rodriguez A., Hargis B., Tellez G. Effect of a Lactobacillus Spp-Based Probiotic Culture Product on Broiler Chicks Performance under Commercial Conditions. Int. J. Poult. Sci. 2007;6:154–156. doi: 10.3923/ijps.2007.154.156. [DOI] [Google Scholar]
- 133.Higgins S.E., Higgins J.P., Wolfenden A.D., Henderson S.N., Torres-Rodriguez A., Tellez G., Hargis B. Evaluation of a Lactobacillus-Based Probiotic Culture for the Reduction of Salmonella Enteritidis in Neonatal Broiler Chicks. Poult. Sci. 2008;87:27–31. doi: 10.3382/ps.2007-00210. [DOI] [PubMed] [Google Scholar]
- 134.Shivaramaiah S., Pumford N.R., Morgan M.J., Wolfenden R.E., Wolfenden A.D., Torres-Rodriguez A., Hargis B.M., Tellez G. Evaluation of Bacillus Species as Potential Candidates for Direct-Fed Microbials in Commercial Poultry. Poult. Sci. 2011;90:1574–1580. doi: 10.3382/ps.2010-00745. [DOI] [PubMed] [Google Scholar]
- 135.Higgins J.P., Higgins S.E., Wolfenden A.D., Henderson S.N., Torres-Rodriguez A., Vicente J.L., Hargis B.M., Tellez G. Effect of Lactic Acid Bacteria Probiotic Culture Treatment Timing on Salmonella Enteritidis in Neonatal Broilers. Poult. Sci. 2010;89:243–247. doi: 10.3382/ps.2009-00436. [DOI] [PubMed] [Google Scholar]
- 136.Sadeghi A.A., Shawrang P., Shakorzadeh S. Immune Response of Salmonella Challenged Broiler Chickens Fed Diets Containing Gallipro®, a Bacillus Subtilis Probiotic. Probiotics Antimicrob. Proteins. 2015;7:24–30. doi: 10.1007/s12602-014-9175-1. [DOI] [PubMed] [Google Scholar]
- 137.Carter A., Adams M., La Ragione R.M., Woodward M.J. Colonisation of Poultry by Salmonella Enteritidis S1400 Is Reduced by Combined Administration of Lactobacillus salivarius 59 and Enterococcus faecium PXN-33. Vet. Microbiol. 2017;199:100–107. doi: 10.1016/j.vetmic.2016.12.029. [DOI] [PubMed] [Google Scholar]
- 138.Khan S., Chousalkar K.K. Salmonella Typhimurium Infection Disrupts but Continuous Feeding of Bacillus Based Probiotic Restores Gut Microbiota in Infected Hens. J. Anim. Sci. Biotechnol. 2020;11:29. doi: 10.1186/s40104-020-0433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Price P.T., Gaydos T.A., Berghaus R.D., Baxter V., Hofacre C.L., Sims M.D. Salmonella Enteritidis Reduction in Layer Ceca with a Bacillus Probiotic. Vet. World. 2020;13:184–187. doi: 10.14202/vetworld.2020.184-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Oh J.K., Pajarillo E.A.B., Chae J.P., Kim I.H., Kang D.K. Protective Effects of Bacillus subtilis against Salmonella Infection in the Microbiome of Hy-Line Brown Layers. Asian-Australas. J. Anim. Sci. 2017;30:1332–1339. doi: 10.5713/ajas.17.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen X., Ishfaq M., Wang J. Effects of Lactobacillus salivarius Supplementation on the Growth Performance, Liver Function, Meat Quality, Immune Responses and Salmonella pullorum Infection Resistance of Broilers Challenged with Aflatoxin B1. Poult. Sci. 2022;101:101651. doi: 10.1016/j.psj.2021.101651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Willis W.L., Reid L. Investigating the Effects of Dietary Probiotic Feeding Regimens on Broiler Chicken Production and Campylobacter jejuni Presence. Poult. Sci. 2008;87:606–611. doi: 10.3382/ps.2006-00458. [DOI] [PubMed] [Google Scholar]
- 143.Santini C., Baffoni L., Gaggia F., Granata M., Gasbarri R., Di Gioia D., Biavati B. Characterization of Probiotic Strains: An Application as Feed Additives in Poultry against Campylobacter jejuni. Int. J. Food Microbiol. 2010;141:S98–S108. doi: 10.1016/j.ijfoodmicro.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 144.Nishiyama K., Seto Y., Yoshioka K., Kakuda T., Takai S., Yamamoto Y., Mukai T. Lactobacillus Gasseri SBT2055 Reduces Infection by and Colonization of Campylobacter jejuni. PLoS ONE. 2014;9:e108827. doi: 10.1371/journal.pone.0108827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nishiyama K., Nakazato A., Ueno S., Seto Y., Kakuda T., Takai S., Yamamoto Y., Mukai T. Cell Surface-associated Aggregation-promoting Factor from Lactobacillus gasseri SBT 2055 Facilitates Host Colonization and Competitive Exclusion of Campylobacter jejuni. Mol. Microbiol. 2015;98:712–726. doi: 10.1111/mmi.13153. [DOI] [PubMed] [Google Scholar]
- 146.Arsi K., Donoghue A.M., Woo-Ming A., Blore P.J., Donoghue D.J. The Efficacy of Selected Probiotic and Prebiotic Combinations in Reducing Campylobacter Colonization in Broiler Chickens. J. Appl. Poult. Res. 2015;24:327–334. doi: 10.3382/japr/pfv032. [DOI] [Google Scholar]
- 147.Mohan V. The Role of Probiotics in the Inhibition of Campylobacter jejuni Colonization and Virulence Attenuation. Eur. J. Clin. Microbiol. Infect. Dis. 2015;34:1503–1513. doi: 10.1007/s10096-015-2392-z. [DOI] [PubMed] [Google Scholar]
- 148.Abd El-Ghany W.A., Shaalan M., Salem H.M. Nanoparticles Applications in Poultry Production: An Updated Review. Worlds. Poult. Sci. J. 2021;77:1001–1025. doi: 10.1080/00439339.2021.1960235. [DOI] [Google Scholar]
- 149.Yusof H.M., Rahman N.A., Mohamad R., Zaidan U.H., Samsudin A.A. Antibacterial Potential of Biosynthesized Zinc Oxide An In Vitro Study. Animals. 2021;11:2093. doi: 10.3390/ani11072093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ye Q., Chen W., Huang H., Tang Y., Wang W., Meng F., Wang H., Zheng Y. Iron and Zinc Ions, Potent Weapons against Multidrug-Resistant Bacteria. Appl. Microbiol. Biotechnol. 2020;104:5213–5227. doi: 10.1007/s00253-020-10600-4. [DOI] [PubMed] [Google Scholar]
- 151.Siddiqui S.A., Bahmid N.A., Taha A., Abdel-Moneim A.M.E., Shehata A.M., Tan C., Kharazmi M.S., Li Y., Assadpour E., Castro-Muñoz R., et al. Bioactive-Loaded Nanodelivery Systems for the Feed and Drugs of Livestock; Purposes, Techniques and Applications. Adv. Colloid Interface Sci. 2022;308:102772. doi: 10.1016/j.cis.2022.102772. [DOI] [PubMed] [Google Scholar]
- 152.Meimandipour A., Emamzadeh A.N., Soleimani A. Effects of Nanoencapsulated Aloe Vera, Dill and Nettle Root Extract as Feed Antibiotic Substitutes in Broiler Chickens. Arch. Anim. Breed. 2017;60:1–7. doi: 10.5194/aab-60-1-2017. [DOI] [Google Scholar]
- 153.Soni M., Mehta P., Soni A., Goswami G.K. Green Nanoparticles: Synthesis and Applications. IOSR J. Biotechnol. Biochem. 2018;4:78–83. doi: 10.9790/264X-0403017883. [DOI] [Google Scholar]
- 154.Acevedo-Villanueva K.Y., Akerele G.O., Hakeem W.G.A., Renu S., Shanmugasundaram R., Selvaraj R.K. A Novel Approach against Salmonella: A Review of Polymeric Nanoparticle Vaccines for Broilers and Layers. Vaccines. 2021;9:1041. doi: 10.3390/vaccines9091041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tigabu B., Getachew A. Treatment of Antibiotic-Resistant Bacteria by Nanoparticles: Current Approaches and Prospects. Ann. Adv. Chem. 2022;6:001–009. doi: 10.29328/journal.aac.1001025. [DOI] [Google Scholar]
- 156.Kazemnia A., Ahmadi M., Mardani K., Moradi M., Darvishzadeh R. Combined Efficacy of Silver Nanoparticles and Commercial Antibiotics on Different Phylogenetic Groups of Escherichia coli. J. Hell. Vet. Med. Soc. 2019;70:1647–1654. doi: 10.12681/jhvms.21788. [DOI] [Google Scholar]
- 157.Lopez-Carrizales M., Velasco K.I., Castillo C., Flores A., Magaña M., Martinez-Castanon G.A., Martinez-Gutierrez F. In Vitro Synergism of Silver Nanoparticles with Antibiotics as an Alternative Treatment in Multiresistant Uropathogens. Antibiotics. 2018;7:50. doi: 10.3390/antibiotics7020050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ahmadi A., Ahmadi P., Ehsani A. Development of an Active Packaging System Containing Zinc Oxide Nanoparticles for the Extension of Chicken Fillet Shelf Life. Food Sci. Nutr. 2020;8:5461–5473. doi: 10.1002/fsn3.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Devi J.S., Bhimba B.V. Antibacterial and Antifungal Activity of Silver Nanoparticles Synthesized Using Hypnea Muciformis. Biosci. Biotechnol. Res. Asia. 2014;11:235–238. doi: 10.13005/bbra/1260. [DOI] [Google Scholar]
- 160.Finley P.J., Norton R., Austin C., Mitchell A., Zank S., Durham P. Unprecedented Silver Resistance in Clinically Isolated Enterobacteriaceae: Major Implications for Burn and Wound Management. Antimicrob. Agents Chemother. 2015;59:4734–4741. doi: 10.1128/AAC.00026-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Salas-Orozco M., Niño-Martínez N., Martínez-Castañón G.A., Méndez F.T., Jasso M.E.C., Ruiz F. Mechanisms of Resistance to Silver Nanoparticles in Endodontic Bacteria: A Literature Review. J. Nanomater. 2019;2019:7630316. doi: 10.1155/2019/7630316. [DOI] [Google Scholar]
- 162.Salem H.M., Ismael E., Shaalan M. Evaluation of the Effects of Silver Nanoparticles against Experimentally Induced Necrotic Enteritis in Broiler Chickens. Int. J. Nanomed. 2021;16:6783–6796. doi: 10.2147/IJN.S319708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Song Z., Lv J., Sheikhahmadi A., Uerlings J., Everaert N. Attenuating Effect of Zinc and Vitamin E on the Intestinal Oxidative Stress Induced by Silver Nanoparticles in Broiler Chickens. Biol. Trace Elem. Res. 2017;180:306–313. doi: 10.1007/s12011-017-1016-0. [DOI] [PubMed] [Google Scholar]
- 164.Ali N.M., Bakheet A.A. Evaluation of the Inhibitory Effect of Chitosan Nanoparticles on Biofilm Forming Escherichia coli Isolated from Omphalitis Cases. J. Adv. Vet. Res. 2020;10:213–218. [Google Scholar]
- 165.Patric Joshua P., Valli C., Balakrishnan V. Effect of in Ovo Supplementation of Nano Forms of Zinc, Copper, and Selenium on Post-Hatch Performance of Broiler Chicken. Vet. World. 2016;9:287–294. doi: 10.14202/vetworld.2016.287-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ningsih N., Ariyadi B., Dono N.D., Supadmo, Zuprizal The Effect of Nanoencapsulated Phaleria Macrocarpa Fruits Extract in Drinking Water on Jejunal Histomorphology of Broiler Chickens. Trop. Anim. Sci. J. 2019;42:106–112. doi: 10.5398/tasj.2019.42.2.106. [DOI] [Google Scholar]
- 167.Wang L., Hu C., Shao L. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int. J. Nanomed. 2017;12:1227–1249. doi: 10.2147/IJN.S121956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Vadalasetty K.P., Lauridsen C., Engberg R.M., Vadalasetty R., Kutwin M., Chwalibog A., Sawosz E. Influence of Silver Nanoparticles on Growth and Health of Broiler Chickens after Infection with Campylobacter jejuni. BMC Vet. Res. 2018;14:1. doi: 10.1186/s12917-017-1323-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Li Z., Hu Y., Yang Y., Lu Z., Wang Y. Antimicrobial Resistance in Livestock: Antimicrobial Peptides Provide a New Solution for a Growing Challenge. Anim. Front. 2018;8:21–29. doi: 10.1093/af/vfy005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Moretta A., Scieuzo C., Petrone A.M., Salvia R., Manniello M.D., Franco A., Lucchetti D., Vassallo A., Vogel H., Sgambato A., et al. Antimicrobial Peptides: A New Hope in Biomedical and Pharmaceutical Fields. Front. Cell. Infect. Microbiol. 2021;11:668632. doi: 10.3389/fcimb.2021.668632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Abdi M., Mirkalantari S., Amirmozafari N. Bacterial Resistance to Antimicrobial Peptides. J. Pept. Sci. 2019;25:e3210. doi: 10.1002/psc.3210. [DOI] [PubMed] [Google Scholar]
- 172.Kumar R., Ali S.A., Singh S.K., Bhushan V., Mathur M., Jamwal S., Mohanty A.K., Kaushik J.K., Kumar S. Antimicrobial Peptides in Farm Animals: An Updated Review on Its Diversity, Function, Modes of Action and Therapeutic Prospects. Vet. Sci. 2020;7:206. doi: 10.3390/vetsci7040206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Dijksteel G.S., Ulrich M.M.W., Middelkoop E., Boekema B.K.H.L. Review: Lessons Learned from Clinical Trials Using Antimicrobial Peptides (AMPs) Front. Microbiol. 2021;12:616979. doi: 10.3389/fmicb.2021.616979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Torres-Acosta M.A., Clavijo V., Vaglio C., González-Barrios A.F., Vives-Flórez M.J., Rito-Palomares M. Economic Evaluation of the Development of a Phage Therapy Product for the Control of Salmonella in Poultry. Biotechnol. Prog. 2019;35:e2852. doi: 10.1002/btpr.2852. [DOI] [PubMed] [Google Scholar]
- 175.Greer G.G. Bacteriophage Control of Foodborne Bacteria. J. Food Prot. 2005;68:1102–1111. doi: 10.4315/0362-028X-68.5.1102. [DOI] [PubMed] [Google Scholar]
- 176.Oechslin F. Resistance Development to Bacteriophages Occurring during Bacteriophage Therapy. Viruses. 2018;10:351. doi: 10.3390/v10070351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Xu Y., Li Z. CRISPR-Cas Systems: Overview, Innovations and Applications in Human Disease Research and Gene Therapy. Comput. Struct. Biotechnol. J. 2020;18:2401–2415. doi: 10.1016/j.csbj.2020.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sulakvelidze A. Using Lytic Bacteriophages to Eliminate or Significantly Reduce Contamination of Food by Foodborne Bacterial Pathogens. J. Sci. Food Agric. 2013;93:3137–3146. doi: 10.1002/jsfa.6222. [DOI] [PubMed] [Google Scholar]
- 179.Wernicki A., Nowaczek A., Urban-Chmiel R. Bacteriophage Therapy to Combat Bacterial Infections in Poultry. Virol. J. 2017;14:179. doi: 10.1186/s12985-017-0849-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gómez-Gómez C., Blanco-Picazo P., Brown-Jaque M., Quirós P., Rodríguez-Rubio L., Cerdà-Cuellar M., Muniesa M. Infectious Phage Particles Packaging Antibiotic Resistance Genes Found in Meat Products and Chicken Feces. Sci. Rep. 2019;9:13281. doi: 10.1038/s41598-019-49898-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Panáček A., Kvítek L., Smékalová M., Večeřová R., Kolář M., Röderová M., Dyčka F., Šebela M., Prucek R., Tomanec O., et al. Bacterial Resistance to Silver Nanoparticles and How to Overcome It. Nat. Nanotechnol. 2018;13:65–71. doi: 10.1038/s41565-017-0013-y. [DOI] [PubMed] [Google Scholar]
- 182.Zhang R., Carlsson F., Edman M., Hummelgård M., Jonsson B.G., Bylund D., Olin H. Escherichia coli Bacteria Develop Adaptive Resistance to Antibacterial ZnO Nanoparticles. Adv. Biosyst. 2018;2:1800019. doi: 10.1002/adbi.201800019. [DOI] [PubMed] [Google Scholar]
- 183.Larkin Mchugh G., Moellering R.C., Hopkins C.C., Swartz M.N. Salmonella Typhimurium Resistant to Silver Nitrate, Chloramphenicol, and Ampicillin: A New Threat in Burn Units? Lancet. 1975;305:235–240. doi: 10.1016/S0140-6736(75)91138-1. [DOI] [PubMed] [Google Scholar]
- 184.Fisinin V.I., Miroshnikov S.A., Sizova E.A., Ushakov A.S., Miroshnikova E.P. Metal Particles as Trace-Element Sources: Current State and Future Prospects. Worlds Poult. Sci. J. 2018;74:523–540. doi: 10.1017/S0043933918000491. [DOI] [Google Scholar]
- 185.Ognik K., Cholewińska E., Czech A., Kozłowski K., Wlazło Ł., Nowakowicz-Dębek B., Szlązak R., Tutaj K. Effect of Silver Nanoparticles on the Immune, Redox, and Lipid Status of Chicken Blood. Czech J. Anim. Sci. 2016;61:450–461. doi: 10.17221/80/2015-CJAS. [DOI] [Google Scholar]
- 186.Von Wintersdorff C.J.H., Penders J., Van Niekerk J.M., Mills N.D., Majumder S., Van Alphen L.B., Savelkoul P.H.M., Wolffs P.F.G. Dissemination of Antimicrobial Resistance in Microbial Ecosystems through Horizontal Gene Transfer. Front. Microbiol. 2016;7:173. doi: 10.3389/fmicb.2016.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.