Abstract
This study uses data from a Drug Enforcement Administration list of Drug Addiction Treatment Act (DATA)–waivered clinicians to examine trends in DATA-waivered clinicians’ active participation in prescribing buprenorphine overall and by patient limits between January 2017 and May 2021.
Increasing the workforce available to treat opioid use disorder (OUD) remains a priority in light of high opioid mortality and percentages of people with OUD who do not receive treatment.1 The Drug Addiction Treatment Act (DATA) of 2000 created a pathway for clinicians to obtain waivers (“DATA waivers”) to simultaneously treat up to 30, 100, or 275 patients with OUD outside of an opioid treatment program, provided the clinicians met certification and training requirements. Policies in 2021 reduced2 waiver requirements and in 2023 removed waiver requirements,3 leading to expectations of increased OUD treatment. It is important to understand historical clinician prescribing patterns prior to these policy changes to track the specific effects going forward. Prior research mainly relies on DATA-waiver status4 or prescription data5 alone to estimate the workforce, leading to likely overestimates. To address these limitations, this study examined trends in DATA-waivered clinicians’ active participation in prescribing buprenorphine, overall and by patient limits, between January 2017 and May 2021.
Methods
We used a Drug Enforcement Administration list of DATA-waivered clinicians between January 2017 and May 2021 with clinicians’ patient limit (30, 100, or 275) and status change dates and matched the records with a National Provider Identifier (NPI) using name, address, and clinician specialty. Using NPIs, we merged the DATA-waivered roster with clinician-level monthly IQVIA Xponent prescription data collected from 93% of US retail pharmacies to identify prescriptions by DATA-waivered clinicians between January 2017 and May 2021 for buprenorphine medications approved for OUD. We then compared monthly counts of DATA-waivered clinicians with “active prescribers” with 1 or more buprenorphine prescriptions in a given month. Then we used χ2 tests to compare by patient limit in May 2021 the proportion of DATA-waivered prescribers actively prescribing, percentage in the active workforce, and percentage of monthly prescription volume. We used STATA version 17 (StataCorp), with statistical significance set at P < .05 (2-sided). The George Washington University institutional review board ruled the study exempt and waived informed consent.
Results
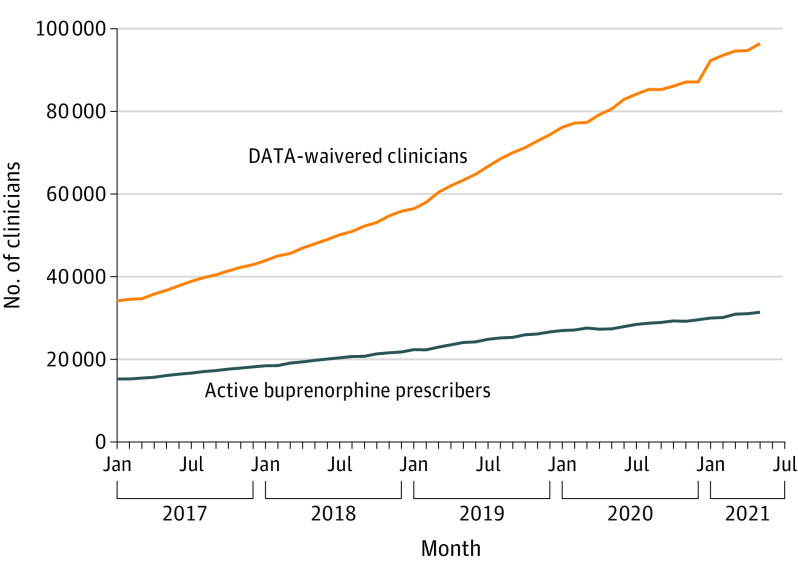
A total of 101 501 (99.7%) of the DATA-waivered clinicians were matched with an NPI. Between January 2017 and May 2021, waivered clinicians increased from 34 149 to 96 415, and clinicians actively prescribing buprenorphine increased from 15 232 to 31 391 but represented a declining proportion of DATA-waivered clinicians (44.6% to 32.6%; P < .001) (Figure 1).
Figure 1. Change in Number of DATA-Waivered Clinicians and Active Buprenorphine Prescribers, January 2017-May 2021.
Active prescribers included any Drug Enforcement Administration Drug Addiction Treatment Act (DATA)–waivered clinician with 1 or more prescriptions for buprenorphine with an indication for opioid use disorder in a given month. Figure generated from authors’ analysis of IQVIA Xponent data January 2017 to May 2021 and the DATA-waivered practitioner roster January 2017 to May 2021.
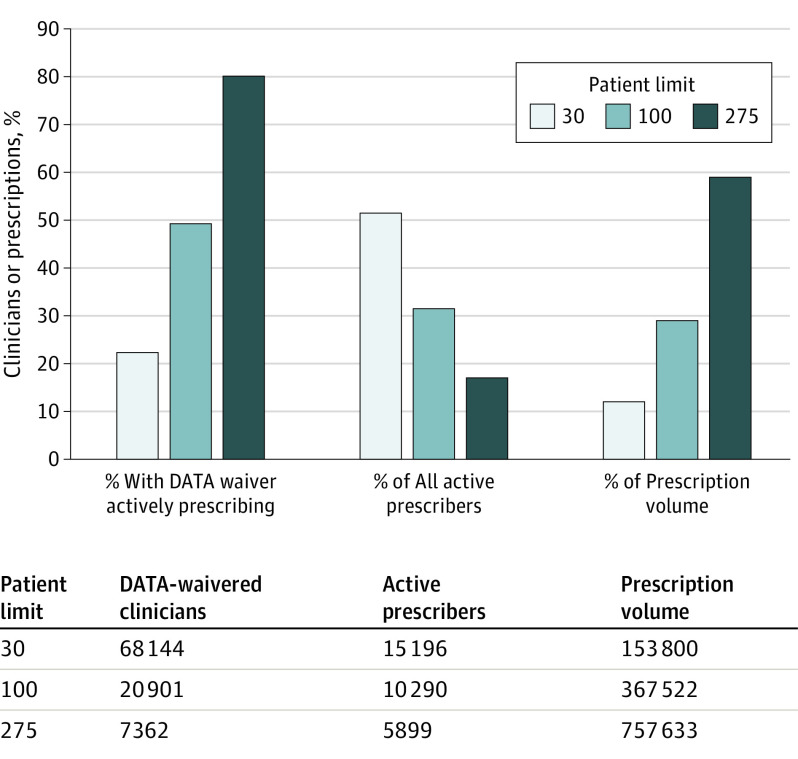
In May 2021, there were 68 144 clinicians with a 30-patient limit (monthly prescription volume, 153 800); 20 901 with a 100-patient limit (prescription volume, 367 522); and 7362 with 275-patient limit (prescription volume, 757 633). In May 2021, clinicians with a 30-patient limit were less likely to actively prescribe compared with clinicians with 100- and 275-patient limits (22.30% vs 49.23% and 80.13%, P < .001), represented the largest share of active prescribers (51.46% vs 31.50% and 17.04%), and prescribed the lowest percentage of monthly total prescription volume (12.02% vs 29.02% and 58.96%) (Figure 2).
Figure 2. Active Buprenorphine Prescribers and Prescription Volume by DATA Waiver Patient Limit, May 2021.
Active prescribers included any Drug Enforcement Administration Drug Addiction Treatment Act (DATA)–waivered clinician with 1 or more prescriptions of buprenorphine with an indication for opioid use disorder in May 2021. Figure generated from authors’ analysis of IQVIA Xponent data January 2017 to May 2021 and the DATA-waivered practitioner roster January 2017 to May 2021.
Discussion
This study found an increase in the number of DATA-waivered clinicians between 2017 and 2021 but limited participation in prescribing buprenorphine. Recent policies have reduced or removed DATA waiver requirements to prescribe buprenorphine, including the June 2021 elimination of training requirements to obtain a waiver to treat 30 patients simultaneously. This resulted in a 16% increase in waivered clinicians but not an acceleration in buprenorphine uptake.2 With the removal of the waiver entirely in the 2023 Consolidated Appropriations Act,3 this study brings into question the assumption that eliminating the DATA waiver will lead to substantial increases in buprenorphine access. While removal of this barrier may make it easier for clinicians to begin prescribing, additional investments may be needed to build and support clinicians treating OUD.
In addition, clinicians eligible to treat 275 patients accounted for nearly 60% of all buprenorphine prescriptions, while making up 17% of active prescribers. Therefore, it will also be important to fund practice models that encourage and support clinicians, including primary care physicians, nurse practitioners, and physician assistants,5,6 with an interest in specializing in OUD. Study limitations include active clinicians in states with a large market share of health maintenance organization pharmacies were not captured by IQVIA Xponent and that reasons for not prescribing were not available.
Section Editors: Jody W. Zylke, MD, Deputy Editor; Kristin Walter, MD, Senior Editor.
Data Sharing Statement
References
- 1.Samuels EA, Martin AF. Beyond the waiver: multilevel interventions needed to expand buprenorphine treatment. JAMA Netw Open. 2022;5(5):e2212425. doi: 10.1001/jamanetworkopen.2022.12425 [DOI] [PubMed] [Google Scholar]
- 2.Ali MM, Creedon T, Jacobus-Kantor L, et al. ; Office of the Assistant Secretary for Planning and Evaluation. Early changes in waivered clinicians and utilization of buprenorphine for opioid use disorder after implementation of the 2021 HHS buprenorphine practice guidelines. US Department of Health and Human Services. Published December 2, 2022. Accessed January 25, 2023. https://aspe.hhs.gov/reports/early-changes-after-2021-hhs-buprenorphine-practice-guidelines
- 3.Removal of DATA waiver (X-waiver) requirement. Substance Abuse and Mental Health Services Administration. Updated January 25, 2023. Accessed January 30, 2023. https://www.samhsa.gov/medications-substance-use-disorders/removal-data-waiver-requirement
- 4.Spetz J, Hailer L, Gay C, et al. Changes in US clinician waivers to prescribe buprenorphine management for opioid use disorder during the COVID-19 pandemic and after relaxation of training requirements. JAMA Netw Open. 2022;5(5):e225996-e225996. doi: 10.1001/jamanetworkopen.2022.5996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olfson M, Zhang V, Schoenbaum M, King M. Buprenorphine treatment by primary care providers, psychiatrists, addiction specialists, and others. Health Aff (Millwood). 2020;39(6):984-992. doi: 10.1377/hlthaff.2019.01622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spetz J, Hailer L, Gay C, et al. Buprenorphine treatment: advanced practice nurses add capacity. Health Aff (Millwood). 2022;41(9):1231-1237. doi: 10.1377/hlthaff.2022.00310 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Sharing Statement