Abstract
T cells are critical in destroying cancer cells by recognizing antigens presented by MHC molecules on cancer cells or antigen-presenting cells. Identifying and targeting cancer-specific or overexpressed self-antigens is essential for redirecting T cells against tumors, leading to tumor regression. This is achieved through the identification of mutated or overexpressed self-proteins in cancer cells, which guide the recognition of cancer cells by T-cell receptors. There are two main approaches to T cell-based immunotherapy: HLA-restricted and HLA-non-restricted Immunotherapy. Significant progress has been made in T cell-based immunotherapy over the past decade, using naturally occurring or genetically engineered T cells to target cancer antigens in hematological malignancies and solid tumors. However, limited specificity, longevity, and toxicity have limited success rates. This review provides an overview of T cells as a therapeutic tool for cancer, highlighting the advantages and future strategies for developing effective T cell cancer immunotherapy. The challenges associated with identifying T cells and their corresponding antigens, such as their low frequency, are also discussed. The review further examines the current state of T cell-based immunotherapy and potential future strategies, such as the use of combination therapy and the optimization of T cell properties, to overcome current limitations and improve clinical outcomes.
Keywords: T cells, immunotherapy, neoantigens, cancer antigens, TCR engineering, HLA restriction, chimeric antigen receptor, CAR NK cells, CAR MIAT
1. Introduction
Cancer is a major public health challenge, affecting millions of people worldwide. Despite significant advances in cancer therapy, cancer remains a leading cause of death, accounting for approximately 10 million deaths annually. The immune system plays a vital role in protecting the host from malignant cells, and harnessing its potential has been a focus of cancer therapy research for decades. T cells are a critical component of the adaptive immune system, and their ability to recognize and eliminate cancer cells has led to significant advances in cancer immunotherapy. In the mid-twentieth century, the concept of lymphocytes as mediators of anti-tumor surveillance was first proposed by Thomas and Burnett, but it was not until later that T cells were identified as the major mediators of adaptive immunity [1,2,3,4,5,6,7]. Today, the role of T cells in cancer immunotherapy is a rapidly expanding area of research.
The development of T cells begins in the bone marrow, where they originate from progenitors that migrate to the thymus for maturation. Upon maturation in the thymus, T cells differentiate into two main lineages—conventional αβ T cells and unconventional γδ T cells—that can be distinguished by the expression of αβ or γδ T cell receptors, respectively. Conventional αβ T cells comprise various subsets, including cytotoxic T cells, helper T cells, regulatory T cells, natural killer T cells, and memory T cells, each with distinct functions in immune response. While the thymus output of naive T cells decreases with age, human naive T cells have a long intrinsic lifespan and turnover rate, making them a promising candidate for immunotherapy [8,9,10,11,12].
T cells have revolutionized cancer treatment, and their use as adoptive T cell therapy has shown great success for some cancers. However, the success of adoptive T cell therapy depends on various factors, such as the choice of T cell subset, target antigen, and immune evasion mechanisms employed by cancer cells. Additionally, the tumor microenvironment can influence T cell function and promote immune escape, highlighting the need for combination therapies targeting both the cancer cells and the immune system. Nevertheless, the potential of T cells to recognize and eliminate cancer cells has opened new avenues for cancer immunotherapy, providing hope for a future where cancer can be effectively treated and cured. This review provides an overview of T cells as a therapeutic tool for cancer, highlighting the advantages and future strategies for developing effective T cell cancer immunotherapy. We discuss the role of T cells in mediating immunity against cancer and recent advancements in T cell-based immunotherapy. We also highlight the challenges that remain, such as limited specificity, longevity, and toxicity, and outline future strategies for developing effective T cell cancer immunotherapy.
2. Antigenic Targets for T Cell Immunotherapy
Antigenic targets for T cell immunotherapy are crucial for the development of effective cancer treatments. The first human tumor antigen was discovered by Van der Bruggen et al. in 1991; it was named MZ2-E and was expressed in melanoma cells [7]. Consequently, cytotoxic T lymphocytes from melanoma patients recognized the melanoma cell line of HLA-A1 patients expressing the MZ2-E. Therefore, their group proposed that precise immunotherapy could be provided to HLA-A1+ melanoma patients expressing the MZ2-E antigen. Since then, various biochemical and genetic approaches have been developed to identify tumor antigens recognized by T cells. The biochemical approach involves eluting the HLA-peptide complex from tumor cells, followed by mass spectrometric sequencing to identify the antigenic peptides presented by the HLA molecules. In contrast, the genetic approach comprises two strategies: forward immunology and reverse immunology. The forward immunology approach involves cloning tumor-reactive T cells from patients and screening a cDNA library from patient-derived tumor cells to identify the antigens recognized by these T cells. On the other hand, the reverse immunology approach uses genome sequencing to identify mutations and predict potential HLA-binding antigens in the patient’s tumor. The peptide candidates are then screened for their ability to activate T cells in vitro [13]. T cells can target both HLA-restricted and HLA-non-restricted antigens found on the target cells. HLA-independent T cell immunotherapy targets antigens expressed on the surface of tumor cells. In contrast, HLA-dependent T cell immunotherapy targets antigens bound to HLA molecules on the surface of cells, known as the immunopeptidome.
2.1. Identification of Tumor Antigenic Epitopes for T Cell Immunotherapy
T cell immunotherapy is a promising approach for cancer treatment, but identifying tumor-reactive T cells and their target antigens is crucial for its success. There are two approaches to identifying these antigens: the indirect method, which involves screening candidate antigen targets of T cells from tumors in the laboratory, and the direct method, which involves isolating and identifying tumor-reactive T cells from tumor-infiltrating lymphocytes (TILs) or peripheral blood lymphocytes (PBLs) in patients. It is crucial to validate the reactivity of T cells towards tumors by studying the interactions between cancer cells and T cells in more depth. However, identifying T cells and their corresponding receptors with the ideal affinity for cancer antigens is challenging, as these antigens are often unmutated self-proteins expressed at higher levels in cancer cells than in normal tissues.
To generate antitumor T cells with optimal affinity of T cell receptor (TCR) to cancer-associated antigens, Obenaus et al. (2015) reported the potential of using antigen-negative humanized mice to generate T cells with a diverse human TCR repertoire and isolated the TCR specific to cancer antigens MAGE-A1 and NY-ESO-1, which have better affinity compared to human-derived TCRs [14]. Several other studies have attempted to enrich naturally occurring T cells from cancer patients by co-culturing TILs or PBLs with autologous tumors and expanding tumor-specific T cells before infusing them back into the patient [15,16,17,18]. However, the low frequency of T cells specific for these antigens makes it difficult to identify TCR T cells specific for cancer antigens. More recently, Arnaud et al. (2022) described a prediction method called NeoScreen that potentially identifies rare tumor antigens and their cognate TCR from TILs, enabling the selective expansion of antigen-specific T cells [19]. They reported that T cells transduced with target specific TCR identification via NeoScreen mediate tumor regression in preclinical models of cancer. Several T cell-based strategies have been developed to target tumor antigens that are well tolerated and show clinical promise in cancer patients. Overall, identifying tumor antigenic epitopes for T cell immunotherapy is crucial for the success of this promising approach to cancer treatment.
2.2. HLA Restricted T Cell-Based Immunotherapy
HLA dependent T cell-based immunotherapy employs the use of lymphocytes with minimally modified TCR or naturally occurring TCR directed against the tumor-specific antigen processed and presented by antigen-presenting cells via HLA molecules. This approach utilizes the ability of T cells to recognize the tumor antigens including cancer-associated or tumor-specific antigens presented by the antigen-presenting molecules on the cell surface. Many approaches have been employed for cancer immunotherapy in patients, such as endogenous T cells targeting undefined or defined antigens including cancer-associated or cancer-specific antigens.
2.2.1. Endogenous T Cells Targeting Undefined Antigens
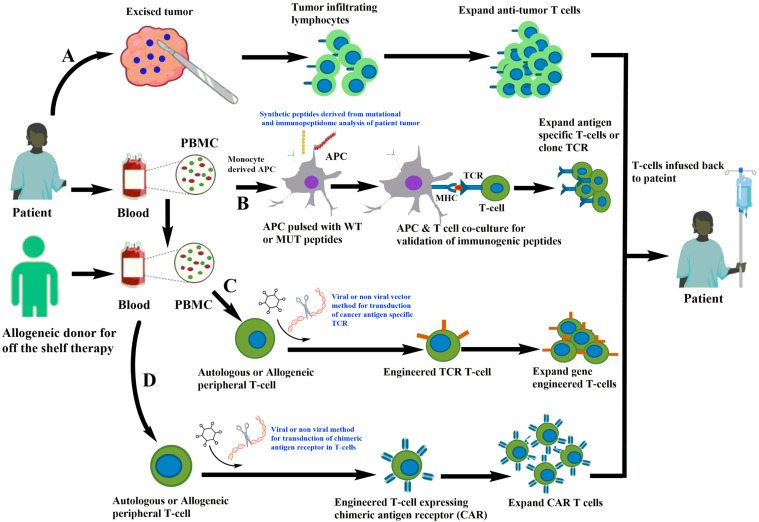
Endogenous T cell immunotherapy is a patient-specific type of therapy that involves using autologous tumor-infiltrating lymphocytes to treat tumors. In this type of T cell therapy, the tumor is surgically removed from the patient and TILs are dissected from the tumor. These TILs are then further expanded in the laboratory in the presence of cytokines, such as IL-2, which promotes the growth of T cells to reach a sufficient number for infusion back into the patient (Figure 1A). These naturally occurring T cells have the advantage of targeting multiple undefined antigens and can generate a nearly clonal or oligoclonal T cell response. In clinical trials, TILs have demonstrated success in a limited number of immunogenic cancers, such as melanoma and human papilloma-associated cancers. Moreover, TILs have a great amount of variability in their specificity and avidity for undefined antigens. In melanoma, TILS and PBL from patients that were stimulated in vitro using the patient’s tumor were found to kill the autologous tumor [20,21,22]. However, TIL infusion was associated with toxicity, and the most common side effects observed with TIL infusion included fever, hypotension, and flu-like symptoms, which were likely due to the high-dose interleukin-2 (IL-2) therapy that was used to support TIL expansion and activation [23]. Additionally, some patients experienced serious autoimmune toxicities, such as thyroiditis, colitis, and hepatitis, which were likely due to the reactivity of TILs against normal tissues in the body [24]. Overall, despite the variability in TIL specificity and avidity, the early TIL studies showed promise for treating certain types of advanced cancers but were limited by the significant toxicities associated with the therapy. Therefore, further research is needed to optimize TIL expansion, increase specificity and efficacy, decrease toxicity, and improve patient selection for this therapy.
Figure 1.
Different strategies of T cell immunotherapies. (A) Isolation of tumor-infiltrating lymphocytes from patient tumor followed by expansion of anti-tumor T cells in vitro and infusion back to the patients. (B) Generation of antigen-presenting cells from monocytes derived from patient PBMC and loaded with potential antigens derived from the mutational and immunopeptidome analysis of patient tumor. The antigen-loaded APC are co-cultured with autologous T cells that are screened for anti-tumor T cells and further expanded. (C) TCR is cloned and used to gene-engineer TCR in fresh autologous or allogeneic T cells for expansion in laboratory conditions and infusion back to the patient. (D) Isolation of T cells from patient or allogeneic donor and gene-editing approaches for engineering of chimeric antigen receptors targeting cancer cells.
Additionally, newer technologies such as genetic engineering of T cell receptors are being developed to enhance the efficacy of T cell therapy. T cells are being engineered for cancer immunotherapy through the introduction of T cell receptor (TCR) alpha and beta chains (Figure 1C) or chimeric antigen receptors (CARs) to target cancer cells (Figure 1D). The use of engineered TCRs is not without challenges, however, as the introduced TCRs can mispair with endogenous TCRs, potentially resulting in unpredictable specificity [25]. To minimize this risk, approaches that reduce mispairing and increase specificity are being developed. PBMCs obtained from either autologous or allogeneic donors through leukapheresis are used to introduce the transgene into the T cells through viruses using retro and lentiviral constructs or non-viral methods of delivery such as CRISPR-Cas9-based insertion of TCR [26]. The transduced T cells are then expanded in vitro under good manufacturing practices (GMP) before being infused back into the patient (Figure 1). The method of delivery for TCR transfer is critical, with viral methods being more effective than non-viral methods. However, challenges still exist in maximizing TCR expression, reducing mispairing between introduced and endogenous TCRs, and enhancing functional avidity of transduced T cells. Therefore, preclinical evaluation and screening of engineered T cells is crucial before proceeding to the clinical setting. With continued development and optimization, gene-engineered T cell therapy may hold promise as a highly targeted and effective approach to cancer treatment.
2.2.2. T Cells Targeting Defined Cancer-Associated Antigens
Cancer-associated antigens (CAAs) are proteins that are overexpressed in tumor cells but rarely in normal cells [27,28]. Many CAAs have been identified through cDNA-based libraries, and targeting CAAs with T cells for adoptive cell therapy in patients expressing these antigens is a promising approach for treating advanced cancers. CDC27 is the first tumor-associated antigen identified that is well recognized by CD4+ TILS. Nagarsheth et al. (2021) reported the first in human, phase 1 clinical trial of T cells targeting CAA, HPV-16 E7 in metastatic human papilloma virus-associated epithelial cancers. The study displayed in vivo persistence and mediated robust tumor regression with an objective clinical response in 50% of patients [29]. T cells targeting tumor antigens in the tumor microenvironment have been reported in many cancers, and depending on whether the tumors are hot or cold, the frequency of these CAAs varies. However, chronic stimulation of tumor-associated antigen-specific T cells that bear high affinity TCR results in the elimination during negative selection in the thymus and hence loss of their anti-tumor ability. Nevertheless, not all high-affinity TCRs are eliminated by the negative selection and can be found in the body.
Other CAAs, such as cancer testis antigens (CTAs), are expressed in germline and placental trophoblast cells but are epigenetically silenced in normal cells. However, these CTAs have been found to be expressed in several cancers and elicit T cell-mediated responses. For example, T cells targeting NY-ESO1 is one of the successful immunotherapies used in a wide range of malignancies and has shown transient regression in more than 50% of patients with no off-target toxicity [30,31,32,33,34]. Similarly, CD4+ T cells engineered to target MHC class II displayed MAGE-A3 yielded positive responses in MAGE-A3-positive tumors with no toxicity but produced severe off-target toxicity when high-affinity TCR or MHC-I-restricted TCR was employed for target killing of cancer cells [35,36]. KK-LC-1 is also a CTA that is reported to be highly expressed in gastric cancer, breast cancer, lung cancer, and is currently being explored for safety and tolerability in clinical trials against KK-LC-1 highly-expressing tumors in clinical trials for many cancers (Table 1). The number of CTAs is more than 400 genes, and the list is ever-increasing in the CTA database [30]. Other CTAs such as GAGE, XAGE, BAGE and PAGE families, SSX1, SSX2 largely remain unexplored in many cancers, while targeting tumor-associated antigens such as MART-1, gp-100 with TCR T cell-directed therapy, also called tissue differentiation antigens, mediated tumor regression in 30% and 19% of patients, while at the same time targeting the normal tissues expressing cognate antigens in patients leads to off-target toxicity [37]. HER2, hTERT, and CEA are overexpressed in many epithelial cancers, but targeting them in T cell therapies carries a risk of toxicity. For instance, in a clinical trial for metastatic colorectal cancer patients, T cells that targeted CEA induced tumor regression but also caused severe transient inflammatory colitis [38]. Similarly, Oncofetal antigens which are CAAs include PSA, AFP, and WT1, and it is reported that T cells targeting WTI prevented AML relapse post-transplant with 100% relapse-free survival at a median of 44 months following infusion, showing promise for preventing AML recurrence [39].
Table 1.
List of HLA-restricted and non-HLA restricted T cell cancer immunotherapies in clinical trials targeting many different antigens.
| T Cell Therapies | Antigen/Type | Disease | HLA Subtype | Status | Reference/Clinical Trial Identifier |
|---|---|---|---|---|---|
| TCR-T | KK-LC-1/cancer testis antigen Kita-kyushu Lung Cancer Antigen 1 |
Gastric, breast, cervical, lung, other KK-LC-1 and other positive epithelial cancers | HLA-A01:01 | Phase I |
NCT05483491 NCT05035407 NCT03778814 |
| E7 TCR cells | HPV-16 E7 | Human papillomavirus (HPV)-16+ cancers (cervical, vulvar, vaginal, penile, anal, and oropharyngeal cancers | HLA-A02:01 | phase I/II | NCT02858310 |
| TCR-transduced CD4+ and CD8+ T-cells FH-MCVA2TCR | MCPyV T | Merkel cell cancer | HLA-A02 | phase I/II trial | NCT03747484 |
| LMP2 Antigen-specific TCR T cells | LMP2 | Recurrent and metastatic nasopharyngeal carcinoma | HLA-A2, HLA-A11, HLA-A24 | Phase 1 | NCT03925896 |
| NY-ESO-1 TCR-T | NY-ESO-1 | Advanced soft tissue sarcoma | HLA-A 02/01 | Recruiting | NCT05620693 |
| TCR-T therapy | KRAS G12V or G12D | Pancreatic cancer | HLA-A*11:01 | Phase 1 | NCT05438667 |
| EBV-Specific Anti-PD1 TCR-T Cells | EBV antigen | Head and neck squamous cell carcinoma | Unknown | Phase I/II trial | NCT04139057 |
| Tumor-specific TCR-T cells | Patient specific | Solid tumor | Unknown | Phase 1 | NCT03891706 |
| HPV TCR-T | HPV E6 | HPV-positive head and neck carcinoma or cervical cancer | Unknown | Phase 1 | NCT03578406 |
| TCR-transduced T cells | TSA-reactive TCR-engineered T cells | Malignant epithelial neoplasms | Unknown | Phase 1 | NCT04520711 |
| CRTE7A2-01 TCR-T Cell | HPV16 | Cervical, anal, head and neck cancers |
HLA-A*02:01 | Phase 1 | NCT05122221 |
| MC2 TCR T cells | MAGE-C2 | Melanoma melanoma, uveal head and neck cancer |
HLA-A2 | Phase 1/2 | NCT04729543 |
| Neoantigen specific TCR-T cells | Patient specific | Gynecologic cancer colorectal, pancreatic cancer, non-small cell lung cancer, cholangiocarcinoma, ovarian cancer, ovary neoplasm, squamous cell lung cancer, adenocarcinoma of lung, adenosquamous cell lung cancer |
Unknown | Phase I/II | NCT05292859 |
| KSH01-TCR-T cells | Unknown | Refractory/recurrent solid tumors | HLA-A*02 | Early Phase 1 | NCT05539833 |
| Neoantigen specific TCR-T cell | KRAS G12D, KRAS G12V, TP53 R175H, TP53 R248W, TP53 Y220C, EGFR E746-A750del |
Gynecologic, colorectal, pancreatic, non-small cell lung cancer, cholangiocarcinoma ovarian, endometrial cancer, ovary neoplasm, squamous cell lung cancer, adenocarcinoma of lung, adenosquamous cell lung cancer |
HLA-A*11:01 HLA-C*08:02 HLA-A*11:01 HLA-C*01:02 HLA-A*02:01, HLA-DRB1*13:01, HLA-A*68:01, HLA-DRB3*02:02, HLA-DPA1*02:01, DPB1*01:01 |
Phase I/II | NCT05194735 |
| KK-LC-1 TCR T | KK-LC-1 | Gastric, breast, cervical, lung cancer | HLA-A01:01 | Phase 1 | NCT05035407 |
| FH-TCR-Tᴍsʟɴ cells | Mesothelin | Metastatic pancreatic ductal adenocarcinoma | HLA-A*02:01 | Phase 1 | NCT04809766 |
| NY-ESO-1 T cell receptor (TCR) engineered T cells | NY-ESO-1 | Ovarian, Fallopian tube, or primary peritoneal cancer | HLA-A*0201, HLA-DP*04 | Phase 1 | NCT03691376 |
| fhB7H3.CAR-Ts | B7H3 | Ovarian cancer | Non-HLA | Phase I/II | NCT05211557 |
| Anti-MUC1 CAR-T cells | MUC1 | Advanced esophageal cancer | Non-HLA | Phase I/II | NCT03706326 |
| Anti-CEA-CAR T | CEA | Colorectal cancer | Non-HLA | Phase I | NCT04513431 |
| anti-MESO CAR-T cells | Mesothelin | Ovarian cancer | Non-HLA | Phase 1/2 | NCT03916679 |
| EpCAM CAR-T cells | EpCAM | Advanced solid tumors | Non-HLA | Phase 1 | NCT02915445 |
| CAR-T cells | PSCA, MUC1, TGFβ, HER2, Mesothelin, Lewis-Y, GPC3, AXL, EGFR, Claudin18.2, or B7-H3 | Lung cancer | Non-HLA | Phase 1 | NCT03198052 |
| CD70-targeted CAR-T Therapy | CD70 | Advanced renal cancer | Non-HLA | Phase 1 | NCT05420519 |
| Dual Targeted CAR T-cells | CD20/CD22 | Relapsed or refractory lymphoid malignancies | Non-HLA | Phase 1 | NCT04283006 |
| MOv19-BBz CAR T Cells | Alpha folate receptor | Recurrent high-grade serous ovarian, Fallopian tube, or primary peritoneal cancer | Non-HLA | Phase 1 | NCT03585764 |
| U87 CAR-T | U87 | Pancreatic cancer | Non-HLA | Phase 1 | NCT05605197 |
| LMP1 CAR T-cells | LMP1 | Hematological malignancies | Non-HLA | Phase 1 | NCT04657965 |
| IM83 CAR-T Cells | IM83 | Liver cancer | Non-HLA | Phase 1 | NCT05123209 |
| CAR T cells | ICAM-1 | Relapsed/refractory thyroid cancer | Non-HLA | Phase 1 | NCT04420754 |
| anti- MESO CAR-T cells | Mesothelin | Ovarian cancer | Non-HLA | Phase 1 | NCT03814447 |
| huCART-meso cells | Mesothelin | Pancreatic cancer | Non-HLA | Phase 1 | NCT03323944 |
| Anti-CD33-CAR-transduced T cells | CD33 | Myeloid malignancies | Non-HLA | Phase 1/2 | NCT02958397 |
| Anti-CD7 CAR-T | CD7 | Hematological malignancies | Non-HLA | Phase 2 | NCT05454241 |
| B4T2-001 Autologous CAR T cells | BT-001 | Advanced solid tumor | Non-HLA | Phase 1 | NCT05621486 |
| CEA CAR-T cells | CEA | Solid tumor, lung cancer, colorectal cancer, liver cancer, pancreatic cancer, gastric cancer, breast cancer, |
Non-HLA | Phase 1/2 | NCT04348643 |
| PSCA- CAR T cells | PSCA | Metastatic castration-resistant prostate cancer | Non-HLA | Phase 1 | NCT03873805 |
| PRGN-3005 Ultra CAR-T cells | BRCA | Advanced, recurrent platinum-resistant ovarian, Fallopian tube or primary peritoneal cancer | Non-HLA | Phase 1 | NCT03907527 |
| P-PSMA-101 CAR-T cells | prostate-specific membrane antigen (PSMA) | Metastatic castration-resistant prostate cancer and advanced salivary gland cancers | Non-HLA | Phase 1 | NCT04249947 |
| BPX-601 CAR-T cells | PSCA | Metastatic castration-resistant prostate cancer | Non-HLA | Phase 1/2 | NCT02744287 |
| Allogeneic γδ T cells in combination with standard drugs | Unknown | Neuroblastoma | Non-HLA | Phase 1 | NCT05400603 |
| CAR γδ T-cells | NKG2DL | Malignant cancer | Non-HLA | Phase 1 | NCT05302037 |
| CAR γδ T-cells | CD7 | Malignant cancer | Non-HLA | Phase 1 | NCT04702841 |
| Autologous and allogeneic γδ T-cells | Unknown | Glioblastoma | Non-HLA | Phase 1/2 | NCT05664243 |
| Allogeneic γδ T-cells | Unknown | Non-Hodgkin’s lymphoma, peripheral T cell lymphoma | Non-HLA | Phase 1 | NCT04696705 |
2.2.3. T Cells Targeting Defined Cancer-Specific Antigens
T cells targeting defined cancer-specific antigens (CSA) are highly tumor-specific and arise due to tumor-specific genomic alterations or from post-translational modifications. Neoantigens, also known as CSAs, are a well-characterized type of antigen that is expressed exclusively by cancer cells. They are generated by tumor-specific DNA alterations in the coding part of the genome, which creates a pool of new epitopes that are not present in somatic tissues. Neoantigens can be generated not only by DNA-specific alterations but also by other mechanisms such as viral infections, post-translational modifications, or gene rearrangements [40,41,42]. The advent of next-generation sequencing technology has enabled researchers to identify tumor-specific mutations and, in combination with binding prediction algorithms, has narrowed down the mutated peptides to potential immunogenic epitopes. Tumor mutational burden is a crude indicator of the number of cancer-specific antigens present in a tumor, and it is often the quality, rather than the quantity, of neoantigens that is critical for inducing an effective anti-tumor T cell response via the TCR.
The discovery of the first neoantigen dates back to 1988 when De Plaen and colleagues used a cDNA library screening to identify P91A, which differs from the normal gene by only one nucleotide and is recognized by cytolytic T lymphocytes [43]. In 1996, neoantigens derived from somatic mutations were reported in melanoma and renal cell carcinoma. With the rapid development of next-generation sequencing, the identification of mutated DNA sequences from normal and tumor cells became less labor-intensive. The steps in the identification of neoantigens involve the identification of non-synonymous mutations using whole-exome sequencing and RNA sequencing, selection of candidate neoantigens for immunogenicity prediction, and evaluation of immunogenicity of these neoantigens in a wet laboratory using different immunological assays. The mutations from the patient’s tumor can be identified, and binding to MHCs or immunogenicity of neoantigens can be predicted using high-throughput algorithmic platforms and validated in in vitro experimental setups (Figure 1B). Neoantigens that are immunogenic can induce an immune response via CD4+ and CD8+ T cell responses and generate neoantigen-specific clonotypes.
Comprehensive mapping of the mutational landscape of common forms of cancer is occurring at a rapid pace, leading to the identification of potential neoantigens, but strategies need to be developed to combine sequencing with mass spectrometry to yield true neoantigens that are relevant to T cell-based immunotherapy for the clinical benefit of cancer patients. The neoantigens are an important target for T cell therapy, but tumors can evade by depleting the neoantigens either at the DNA level by loss of copy number or at the RNA level by suppressing the RNA transcripts of neoantigens or at the epigenetic level via hypermethylation of genes coding neoantigens through post-translational mechanisms [44]. In summary, targeting defined cancer-specific antigens, particularly neoantigens, using T cell-based immunotherapy, has shown great promise in the treatment of cancer. However, further research is needed to improve the identification of neoantigens, as well as to overcome tumor escape mechanisms, to improve the efficacy of this approach.
Personalized and Shared Neoantigens as Targets of T Cell Therapy
Neoantigens can be classified as either personalized or shared. Personalized neoantigens are unique to each patient and have been found to drive anti-tumor CD8+ T cell responses in immune checkpoint blockade and TIL therapy, resulting in durable clinical benefits and improved progression-free survival in cancer patients [45,46,47,48]. In a study by Tran et al., a cancer-specific antigen called ERBB2IP was identified in a patient with metastatic cholangiocarcinoma. CD4+ T helper (Th1) cells from the patient were expanded and activated in vitro and then reinfused into the patient, resulting in significant tumor reduction and even tumor disappearance in some cases [18].
On the other hand, shared neoantigens are present in multiple types of cancer, such as KRAS, which is found in 60–70% of pancreatic adenocarcinomas and 20–30% of colorectal cancers [49,50]. A recent study reported that a shared neoantigen, tumor protein 53, in which arginine is replaced with histidine at position 175 (p53R175H), can be targeted by a highly specific TCR-mimic antibody that is HLA-A*0201-restricted p53R175H and can lyse tumor cells expressing the neoantigen [51]. Similarly, Kim et al. (2022) identified a pan-cancer epitope, collagen type VI α-3 (COL6A3), that was present in 30 tumor tissues derived from eight different cancer types. They also identified several TCRs that could recognize the shared neoantigen and efficiently eliminate tumors in preclinical models of cancer [52]. In a recent clinical trial, a 71-year-old woman with progressive pancreatic adenocarcinoma received a single infusion of engineered T cells expressing two allogeneic HLA-C*08:02-restricted TCRs targeting the KRASG12D neoantigen. The treatment resulted in an overall 72% objective partial tumor regression at 6 months, with the engineered T cells representing 2% of the total circulating T cells [53]. Targeting neoantigens has shown promise in leading to long-lasting clinical responses in many solid tumors, including epithelial cancers that account for more than 90% of cancer mortality rates in the United States [54].
Targeting shared neoantigens among solid tumors broadens the applicability of adoptive T cell therapy utilizing TCR T cells specific for common neoantigens. For example, out of 163 metastatic solid tumor patients screened for mutations by whole-exome sequencing, TP53 mutations were found in 78 patients [55]. Kim et al. (2022) reported a library of 39 TCRs that recognize tumor cells expressing TP53 mutations shared among more than 7% of patients with solid tumors, using in vitro and in vivo models. Moreover, the allogeneic use of TCR T cells targeting TP53 mutations in a chemo-refractory breast cancer patient showed that autologous PBLs engineered with R175H-TCR at 6 weeks post-treatment infiltrated the patient’s tumor and had acquired a central memory phenotype with stem-like features, suggesting the longevity of these cells. However, initial treatment with autologous PBLs engineered with R175H-TCR showed 55% objective regression that lasted only 6 months, with new cutaneous metastasis on the bilateral breast followed by death at 8 months due to other complications. Biopsies, WES, and RNAscope of progressing lesions confirmed the presence of the neoantigen TP53 on the tumor but loss of HLA-A*02:01 expression. Therefore, testing TCR T cell therapy directed against more than one shared neoantigen should be exploited, and off-target effects need to be evaluated in patient clinical trials to make the off-shelf T cell therapy available to advanced cancers with the mutated antigens.
Additionally, cancer cells can escape recognition by T cell-based immunotherapies by expressing the checkpoint molecules such as PD-L1, TIM3, LAG3 or downregulating the HLA expression which can further dampen T cell activity [56]. Thus, the expression of these molecules on cancer cells is an important factor to consider when designing T cell-based immunotherapies. Strategies to overcome PD-L1-mediated T cell suppression, such as the use of combination therapies that target multiple immune checkpoints, are being explored to enhance the efficacy of T cell-based immunotherapies in cancer treatment. Additionally, ongoing research is focused on identifying new targets and pathways to improve the effectiveness of T cell-based immunotherapies for cancer.
Moreover, using autologous T cells for T cell engineering has its own unique advantages and disadvantages over allogeneic T cell immunotherapies such as low risk of graft-versus-host disease (GVHD), since the T cells are derived from the patient’s own immune system. However, this therapy can be limited by the patient’s own T cell quality, quantity, and function. Additionally, it can be time-consuming and expensive to manufacture personalized T cell therapies for each patient. Allogeneic T cell immunotherapies, on the other hand, use T cells from a healthy donor, which can be modified to recognize and kill cancer cells (Figure 1C,D). The main advantage of allogeneic T cell immunotherapy is the potential for off-the-shelf availability and scalability, as a single donor can provide T cells for multiple patients. However, the major disadvantage of allogeneic T cell immunotherapy is the risk of GVHD, a serious complication where the donor T cells attack the recipient’s healthy cells. To address this risk, researchers are developing methods to mitigate the risk of GVHD, such as using gene-editing approaches to delete or downregulate genes that are involved in T cell activation and proliferation, such as CD52 and CD70, or engineering the T cells to express a suicide gene that can be triggered in case of GVHD [57,58,59]. Another approach is to use partially matched donors, which may reduce the risk of GVHD while still providing effective therapy.
2.3. Non-HLA Restricted T Cell-Based Immunotherapy
HLA-independent TCR-based T cell immunotherapy employs use of lymphocytes modified with TCR that are directed against the tumor antigens directly presented on the tumor cells. This approach utilizes a chimeric receptor introduced into the immune effector cells, such as T cells, natural killer cells or gamma delta (γδ) T cells to recognize tumor cell surface proteins and are commonly called as chimeric antigen receptor T or natural killer cells.
2.3.1. Chimeric Antigen Receptor (CAR) T Cells Targeting Tumor-Defined Antigens
CAR T cell therapy is a type of immunotherapy that involves genetically engineering T cells to produce synthetic chimeric receptors that have an antigen-binding domain and a T cell-activating domain. This approach was first tested for HIV infection, but the CAR T cells showed no clinical improvement in HIV-infected individuals [60]. First-generation CARs were also tested in solid tumors targeting the MUC1 antigen but did not show longevity or persistence in clinical trials. The addition of a second co-stimulatory signal in CAR T cells, called second-generation CARs, was found to enhance the anti-tumor effect and persistence of CAR T cells in preclinical models of leukemia [61,62,63].
The team led by Carl June at the University of Pennsylvania involved in the early clinical trials of CAR T cell therapy played a critical role in the development of the first FDA-approved CAR T cell therapy, tisagenlecleucel. They designed CAR T cells targeting the B cell antigen CD19, coupled with CD137 and CD3-zeta, showing a low dose of these CAR T cells in a chronic lymphoid leukemia patient who showed a complete response after 3 weeks of treatment [64]. Similarly, they observed that CAR T cells with specificity to CD19 and a T cell-signaling molecule resulted in durable remission of acute lymphoblastic leukemia (ALL) in two pediatric patients with refractory and relapsed pre-B cell ALL [65]. The development of CAR T cells targeting the CD19 antigen resulted in a remission rate of close to 90% in r/r B-ALL, and 30–50% in chronic lymphocytic leukemia (CLL) and non-Hodgkin’s lymphoma in a phase I clinical trial. Consequently, researchers were able to circumvent the limits of conventional approaches by treating patients with CAR T-cells targeting the CD19 antigen, which stimulated recovery despite recurring malignancies [66,67]. CD19 CAR T cell treatment also has a significant anti-tumor effect in follicular lymphoma (FL), diffuse large B-cell lymphoma (DLBCL), mantle cell lymphoma (MCL), chronic lymphocytic leukemia (CLL), and multiple myeloma (MM) [68,69]. Tumor antigens evaluated in recent years for CAR T therapy include CD138, BCMA, CD123, LeY [70], and several others are in clinical trials (Table 1). Mesothelin CAR T cells have shown a positive response in a clinical study using patients with advanced mesothelioma and pancreatic cancer with no off-target effects [71]. Typically, the third-generation CARs are an expansion of the second generation and are composed of an antigen-binding unit, a spacer (hinge), a transmembrane domain and endodomain. Liu et al. (2017) evaluated the impact of the third generation of CAR T-cells that precisely target CD20 antigens, a glycosylated phosphoprotein expressed on the surface of activated B cells [72]. Patients with a wide range of B-lymphocyte-related lymphomas who were included in specialized clinical studies established positive clinical outcomes that were attributed to a well-executed therapy strategy. The concentration of antigen used in this therapy must meet a certain threshold for it to work properly; beyond that concentration, the treatment may have no impact on the progression of cancer in patients [73,74]. The fourth-generation CARs, also called ‘T cell redirected for universal cytokine-mediated killing’ (TRUCKs), are also based on the second-generation CARs with the addition of cytokine, IL-12, which is constitutively expressed, or have inducible expression promoting the tumor-killing via synergistic mechanisms. Similarly, fifth-generation CARs currently being explored have a truncated IL-2 receptor beta chain which upon activation triggers TCR and cytokine signaling via JAK-STAT/3/5, which drives the activation and proliferation of T cells.
Despite demonstrating promising results in preclinical and clinical trials, the efficacy of CAR T cell therapy may be limited by several unique characteristics of solid tumors. These characteristics include a deficiency in target antigens, heterogeneity of tumor antigens, inadequate trafficking and infiltration, and challenges imposed by the tumor microenvironment (TME), such as physical and metabolic barriers, the existence of soluble factors, and immunosuppressive cells. Several novel ways have been taken so far to overcome these obstacles, but the current methods are insufficient, and many more efforts are underway, such as combining CAR T cell technology with genome editing and adjuvant medicines, to discover a solution for more efficient therapeutics [75]. To date, a total of six CAR-T cell therapies directed against either the CD19 or B cell maturation antigens on B cells have been approved by FDA for the treatment of liquid tumors including lymphoma, leukemia, and multiple myeloma. These CAR T therapies are customized for each patient by collecting the blood and isolating T cells from patients, inserting the gene-encoding chimeric antigen receptor using vectors followed by selection, expansion in laboratory and infusion of these cells back to the patient (Figure 1D).
Despite the promising clinical results, current CAR T-cell therapies have also been associated with toxicities, but the nature and severity of these toxicities differ from those seen in early TIL studies. The most common toxicity associated with CAR T-cell therapy is cytokine release syndrome (CRS), which is caused by the release of large amounts of inflammatory cytokines as the CAR T-cells attack cancer cells [76,77]. CRS can cause fever, hypotension, and other flu-like symptoms, and can be severe or even life-threatening in some cases. Another common toxicity associated with CAR T-cell therapy is neurotoxicity, which can cause confusion, seizures, and other neurological symptoms [78]. However, these toxicities can often be managed with supportive care and, in some cases, with the use of immunosuppressive agents.
A potential alternative for CAR T cell therapy is CAR NK cell therapy by engineering natural killer cells, which are a type of innate immune cell that can recognize and eliminate abnormal cells including tumor cells. The advantages of using NK cells for immunotherapy include their ability to rapidly recognize and eliminate cancer cells, as well as their lack of requirement for prior sensitization, which can make them a more practical and accessible option than other types of immune cells. Additionally, NK cells have been shown to have a lower risk of causing graft-versus-host disease (GVHD) than T cells, which can be an important consideration in allogeneic transplant settings [79]. However, there are also some limitations and challenges associated with the use of NK cells in immunotherapy. For example, the efficacy of NK cell therapy can be affected by the immunosuppressive tumor microenvironment, which can impair NK cell function and limit their ability to eliminate cancer cells. Additionally, there is a need to identify reliable and effective methods for expanding and activating NK cells ex vivo for use in immunotherapy. Despite these challenges, ongoing research is focused on developing new strategies to overcome these limitations and improve the efficacy of NK cell-based immunotherapies [80]. These strategies include the use of combination therapies that target multiple immune pathways, the development of novel methods for NK cell expansion and activation, and the exploration of targeted delivery approaches to enhance NK cell infiltration and activity in the tumor microenvironment.
Similarly, in recent years mucosal-associated invariant T cells (MAIT cells) have been used as a potential source for CAR T cell therapy [81]. MAITs are a subset of T cells that recognize antigens presented by a non-classical major histocompatibility complex molecule, MR1. These cells are not HLA-restricted and are not expected to induce GVHD, which gives them huge potential as a source for off-the-shelf immunotherapy. Preclinical studies have shown that MAIT cells can be engineered to express CARs that target cancer cells and that these CAR MAIT cells are able to recognize and kill cancer cells in vitro and in vivo [82]. Additionally, MAIT cells have shown promise in treating solid tumors, which are often resistant to traditional CAR T cell therapy. However, there are still challenges to be addressed in the development of CAR MAIT cells. One of the main challenges is the limited understanding of the biology and function of MAIT cells, which makes it difficult to optimize the engineering and expansion of CAR MAIT cells. Additionally, the development of CAR MAIT cells requires the identification of suitable tumor-associated antigens that can be targeted by the CAR. Nonetheless, the potential of CAR MAIT cells as a novel immunotherapy approach for cancer treatment is an active area of research, and ongoing studies are focused on addressing these challenges and optimizing the use of these cells in the clinic.
2.3.2. Gamma Delta (γδ) T Cells as Weapons of Non-HLA-Restricted Immunotherapy
Gamma delta T cells (γδ) are a unique subgroup of T cells that make up 0.5–5% of all T cells and express T cell receptors composed of γ and δ chains. These cells have the ability to recognize non-HLA-restricted tumor antigens and can release antitumor cytokines, making them a promising candidate for T cell immunotherapy. In humans, there are four subgroups of γδ T cells based on the type of δ TCR chains (δ1, δ2, δ3, δ5) paired with seven different Vγ TCR chains (Vγ2, Vγ3, Vγ4, Vγ5, Vγ8, Vγ9). Recent research has shown that, depending on the subtype, γδ T cells can act as effector T cells playing a role in anti-tumor activity or promote the growth of the tumor by acting as regulatory cells, indicating a dual effect in cancer immunotherapy. Effector γδ T cells release granzyme B, perforin, IFN- γ and TNF-a, displaying a strong tumor-cell-killing activity. High infiltration of effector γδ T cells has been reported to improve the clinical outcome of patients with different malignancies [83,84,85,86,87]. Previous early clinical trials on the therapeutic potential of γδ T cells showed limited clinical benefit in renal, multiple myeloma, and non-small lung cancer progression, although they were well tolerated and safe [88,89,90]. More recent studies have revealed that γδ T cells, which express PD-1, are the primary effectors of immunotherapy in DNA mismatch repair-deficiency tumors that possess HLA class I defects [91]. This finding highlights the potential of these cells in HLA-non-restricted T cell immunotherapy, either alone or in conjunction with immune checkpoint blockade. Currently, various methods are being assessed to harness the potential of γδ T cells, including the development of γδ CAR-T cells (Table 1) to enhance therapeutic efficacy and minimize the risk of toxicity compared to αβ CAR-T cells [92,93,94,95,96,97]. Additionally, antibody-based approaches utilizing γδ TCR-specific engagers directed against antigens such as Her2 and CD123 have shown promise in killing tumor cells, as they have bispecific nanobody approaches targeting CD40, epidermal growth factor receptor in combination with γδ TCR T cells [98,99]. In summary, γδ T cells hold promise as a foundation for modifying the targeting of different cancer antigens and translating them into clinical benefits for patients. Further research and clinical trials are needed to fully realize their potential as a non-HLA-restricted immunotherapy.
3. Conclusions and Future Perspectives
In conclusion, cancer immunotherapies have shown great potential in harnessing the patient’s own immune system to fight cancer. The discovery of antitumor T cells infiltrating tumors has opened doors for T cell immunotherapy targeting tumor antigens. Over the years, T cell-based immunotherapy targeting antigens has been the focus of much research and development, with different approaches being employed for the treatment of cancer. This includes the use of autologous T cells isolated from the patient tumor or engineering the autologous or allogeneic donor T or NK cells to express tumor-specific T cell receptors (TCRs) or chimeric antigen receptors (CARs) that specifically target cancer cells. While HLA-restricted T cell therapy and non-restricted CAR T cell therapies have shown great potential in clinical trials, challenges such as the development of resistance to therapy and the identification of new targets for T cell-based immunotherapy remain. Additionally, off-target toxicity and tumor heterogeneity are concerns that need to be addressed. Identifying multiple targeted antigens and developing T cell-based therapies that express multiple antigen-specific CARs or TCRs specific for or shared among patients could overcome the tumor heterogeneity and increase effectiveness. Furthermore, part of a tumor may escape the immune response by upregulating check-point molecules that suppresses the effector T cell function or by losing HLA expression, causing a deficiency in the antigen processing and presentation machinery. Combination therapies targeting tumor antigens along with checkpoint inhibitors or use of alternative cell sources such as NK cells, MIAT cells, and γδ T cells may offer solutions. It is important to vigilantly monitor the safety associated with the T cell-based immunotherapies in preclinical settings before using them for the clinical benefit. Additionally, more research should be done employing the NK cells, MIAT cells and γδ T cells as a potential source for off-the-shelf antigen-directed therapies for cancer. Continued research, development, and refinement of these approaches could make it a standard treatment option for a wide range of cancers, improving patient outcomes and survival rates. While there is still much work to be done, the potential benefits of T cell-based immunotherapy are clear, and the field is poised to make significant strides towards more effective and safe cancer treatments.
Author Contributions
Conceptualization, M.Y.W.; methodology, M.Y.W. and Z.B.; software, M.Y.W.; investigation, M.Y.W.; writing—original draft preparation, M.Y.W.; writing—review and editing, Z.B., R.A.N.; supervision, M.Y.W. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Dunn G.P., Old L.J., Schreiber R.D. The three Es of cancer immunoediting. Annu. Rev. Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 2.Burnet M. Cancer: A biological approach. III. Viruses associated with neoplastic conditions. IV. Practical applications. Br. Med. J. 1957;1:841–847. doi: 10.1136/bmj.1.5023.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim R., Emi M., Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121:1–14. doi: 10.1111/j.1365-2567.2007.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller J.F., Mitchell G.F., Weiss N.S. Cellular basis of the immunological defects in thymectomized mice. Nature. 1967;214:992–997. doi: 10.1038/214992a0. [DOI] [PubMed] [Google Scholar]
- 5.Shankaran V., Ikeda H., Bruce A.T., White J.M., Swanson P.E., Old L.J., Schreiber R.D. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 6.Dunn G.P., Bruce A.T., Ikeda H., Old L.J., Schreiber R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 7.van der Bruggen P., Traversari C., Chomez P., Lurquin C., De Plaen E., van den Eynde B., Knuth A., Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 8.Douek D.C., McFarland R.D., Keiser P.H., Gage E.A., Massey J.M., Haynes B.F., Polis M.A., Haase A.T., Feinberg M.B., Sullivan J.L., et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 9.Junge S., Kloeckener-Gruissem B., Zufferey R., Keisker A., Salgo B., Fauchere J.C., Scherer F., Shalaby T., Grotzer M., Siler U., et al. Correlation between recent thymic emigrants and CD31+ (PECAM-1) CD4+ T cells in normal individuals during aging and in lymphopenic children. Eur. J. Immunol. 2007;37:3270–3280. doi: 10.1002/eji.200636976. [DOI] [PubMed] [Google Scholar]
- 10.Hazenberg M.D., Verschuren M.C., Hamann D., Miedema F., van Dongen J.J. T cell receptor excision circles as markers for recent thymic emigrants: Basic aspects, technical approach, and guidelines for interpretation. J. Mol. Med. 2001;79:631–640. doi: 10.1007/s001090100271. [DOI] [PubMed] [Google Scholar]
- 11.Vrisekoop N., den Braber I., de Boer A.B., Ruiter A.F., Ackermans M.T., van der Crabben S.N., Schrijver E.H., Spierenburg G., Sauerwein H.P., Hazenberg M.D., et al. Sparse production but preferential incorporation of recently produced naive T cells in the human peripheral pool. Proc. Natl. Acad. Sci. USA. 2008;105:6115–6120. doi: 10.1073/pnas.0709713105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nikolich-Zugich J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat. Rev. Immunol. 2008;8:512–522. doi: 10.1038/nri2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Want M.Y., Konstorum A., Huang R.Y., Jain V., Matsueda S., Tsuji T., Lugade A., Odunsi K., Koya R., Battaglia S. Neoantigens retention in patient derived xenograft models mediates autologous T cells activation in ovarian cancer. Oncoimmunology. 2019;8:e1586042. doi: 10.1080/2162402X.2019.1586042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obenaus M., Leitao C., Leisegang M., Chen X., Gavvovidis I., van der Bruggen P., Uckert W., Schendel D.J., Blankenstein T. Identification of human T-cell receptors with optimal affinity to cancer antigens using antigen-negative humanized mice. Nat. Biotechnol. 2015;33:402–407. doi: 10.1038/nbt.3147. [DOI] [PubMed] [Google Scholar]
- 15.Dijkstra K.K., Cattaneo C.M., Weeber F., Chalabi M., van de Haar J., Fanchi L.F., Slagter M., van der Velden D.L., Kaing S., Kelderman S., et al. Generation of Tumor-Reactive T Cells by Co-culture of Peripheral Blood Lymphocytes and Tumor Organoids. Cell. 2018;174:1586–1598. doi: 10.1016/j.cell.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen F., Zou Z., Du J., Su S., Shao J., Meng F., Yang J., Xu Q., Ding N., Yang Y., et al. Neoantigen identification strategies enable personalized immunotherapy in refractory solid tumors. J. Clin. Invest. 2019;129:2056–2070. doi: 10.1172/JCI99538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prickett T.D., Crystal J.S., Cohen C.J., Pasetto A., Parkhurst M.R., Gartner J.J., Yao X., Wang R., Gros A., Li Y.F., et al. Durable Complete Response from Metastatic Melanoma after Transfer of Autologous T Cells Recognizing 10 Mutated Tumor Antigens. Cancer Immunol. Res. 2016;4:669–678. doi: 10.1158/2326-6066.CIR-15-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tran E., Turcotte S., Gros A., Robbins P.F., Lu Y.C., Dudley M.E., Wunderlich J.R., Somerville R.P., Hogan K., Hinrichs C.S., et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–645. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnaud M., Chiffelle J., Genolet R., Navarro Rodrigo B., Perez M.A.S., Huber F., Magnin M., Nguyen-Ngoc T., Guillaume P., Baumgaertner P., et al. Sensitive identification of neoantigens and cognate TCRs in human solid tumors. Nat. Biotechnol. 2022;40:656–660. doi: 10.1038/s41587-021-01072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herin M., Lemoine C., Weynants P., Vessiere F., van Pel A., Knuth A., Devos R., Boon T. Production of stable cytolytic T-cell clones directed against autologous human melanoma. Int. J. Cancer. 1987;39:390–396. doi: 10.1002/ijc.2910390320. [DOI] [PubMed] [Google Scholar]
- 21.Knuth A., Danowski B., Oettgen H.F., Old L.J. T-cell-mediated cytotoxicity against autologous malignant melanoma: Analysis with interleukin 2-dependent T-cell cultures. Proc. Natl. Acad. Sci. USA. 1984;81:3511–3515. doi: 10.1073/pnas.81.11.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenberg S.A., Kawakami Y., Robbins P.F., Wang R. Identification of the genes encoding cancer antigens: Implications for cancer immunotherapy. Adv. Cancer Res. 1996;70:145–177. doi: 10.1016/s0065-230x(08)60874-x. [DOI] [PubMed] [Google Scholar]
- 23.Marabondo S., Kaufman H.L. High-dose interleukin-2 (IL-2) for the treatment of melanoma: Safety considerations and future directions. Expert. Opin. Drug. Saf. 2017;16:1347–1357. doi: 10.1080/14740338.2017.1382472. [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg S.A., Packard B.S., Aebersold P.M., Solomon D., Topalian S.L., Toy S.T., Simon P., Lotze M.T., Yang J.C., Seipp C.A., et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N. Engl. J. Med. 1988;319:1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 25.Cohen C.J., Zhao Y., Zheng Z., Rosenberg S.A., Morgan R.A. Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability. Cancer Res. 2006;66:8878–8886. doi: 10.1158/0008-5472.CAN-06-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roth T.L., Puig-Saus C., Yu R., Shifrut E., Carnevale J., Li P.J., Hiatt J., Saco J., Krystofinski P., Li H., et al. Reprogramming human T cell function and specificity with non-viral genome targeting. Nature. 2018;559:405–409. doi: 10.1038/s41586-018-0326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arber C., Feng X., Abhyankar H., Romero E., Wu M.F., Heslop H.E., Barth P., Dotti G., Savoldo B. Survivin-specific T cell receptor targets tumor but not T cells. J. Clin. Invest. 2015;125:157–168. doi: 10.1172/JCI75876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janelle V., Rulleau C., Del Testa S., Carli C., Delisle J.S. T-Cell Immunotherapies Targeting Histocompatibility and Tumor Antigens in Hematological Malignancies. Front. Immunol. 2020;11:276. doi: 10.3389/fimmu.2020.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagarsheth N.B., Norberg S.M., Sinkoe A.L., Adhikary S., Meyer T.J., Lack J.B., Warner A.C., Schweitzer C., Doran S.L., Korrapati S., et al. TCR-engineered T cells targeting E7 for patients with metastatic HPV-associated epithelial cancers. Nat. Med. 2021;27:419–425. doi: 10.1038/s41591-020-01225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Almeida L.G., Sakabe N.J., deOliveira A.R., Silva M.C., Mundstein A.S., Cohen T., Chen Y.T., Chua R., Gurung S., Gnjatic S., et al. CTdatabase: A knowledge-base of high-throughput and curated data on cancer-testis antigens. Nucleic. Acids. Res. 2009;37:D816–D819. doi: 10.1093/nar/gkn673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y.T., Boyer A.D., Viars C.S., Tsang S., Old L.J., Arden K.C. Genomic cloning and localization of CTAG, a gene encoding an autoimmunogenic cancer-testis antigen NY-ESO-1, to human chromosome Xq28. Cytogenet. Cell. Genet. 1997;79:237–240. doi: 10.1159/000134734. [DOI] [PubMed] [Google Scholar]
- 32.D’Angelo S.P., Melchiori L., Merchant M.S., Bernstein D., Glod J., Kaplan R., Grupp S., Tap W.D., Chagin K., Binder G.K., et al. Antitumor Activity Associated with Prolonged Persistence of Adoptively Transferred NY-ESO-1 (c259)T Cells in Synovial Sarcoma. Cancer Discov. 2018;8:944–957. doi: 10.1158/2159-8290.CD-17-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nowicki T.S., Berent-Maoz B., Cheung-Lau G., Huang R.R., Wang X., Tsoi J., Kaplan-Lefko P., Cabrera P., Tran J., Pang J., et al. A Pilot Trial of the Combination of Transgenic NY-ESO-1-reactive Adoptive Cellular Therapy with Dendritic Cell Vaccination with or without Ipilimumab. Clin. Cancer Res. 2019;25:2096–2108. doi: 10.1158/1078-0432.CCR-18-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robbins P.F., Morgan R.A., Feldman S.A., Yang J.C., Sherry R.M., Dudley M.E., Wunderlich J.R., Nahvi A.V., Helman L.J., Mackall C.L., et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J. Clin. Oncol. 2011;29:917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu Y.C., Parker L.L., Lu T., Zheng Z., Toomey M.A., White D.E., Yao X., Li Y.F., Robbins P.F., Feldman S.A., et al. Treatment of Patients With Metastatic Cancer Using a Major Histocompatibility Complex Class II-Restricted T-Cell Receptor Targeting the Cancer Germline Antigen MAGE-A3. J. Clin. Oncol. 2017;35:3322–3329. doi: 10.1200/JCO.2017.74.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morgan R.A., Chinnasamy N., Abate-Daga D., Gros A., Robbins P.F., Zheng Z., Dudley M.E., Feldman S.A., Yang J.C., Sherry R.M., et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J. Immunother. 2013;36:133–151. doi: 10.1097/CJI.0b013e3182829903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson L.A., Morgan R.A., Dudley M.E., Cassard L., Yang J.C., Hughes M.S., Kammula U.S., Royal R.E., Sherry R.M., Wunderlich J.R., et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parkhurst M.R., Yang J.C., Langan R.C., Dudley M.E., Nathan D.A., Feldman S.A., Davis J.L., Morgan R.A., Merino M.J., Sherry R.M., et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol. Ther. 2011;19:620–626. doi: 10.1038/mt.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chapuis A.G., Egan D.N., Bar M., Schmitt T.M., McAfee M.S., Paulson K.G., Voillet V., Gottardo R., Ragnarsson G.B., Bleakley M., et al. T cell receptor gene therapy targeting WT1 prevents acute myeloid leukemia relapse post-transplant. Nat. Med. 2019;25:1064–1072. doi: 10.1038/s41591-019-0472-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y., Qian J., Gu C., Yang Y. Alternative splicing and cancer: A systematic review. Signal Transduct Target. 2021;6:78. doi: 10.1038/s41392-021-00486-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rathe S.K., Popescu F.E., Johnson J.E., Watson A.L., Marko T.A., Moriarity B.S., Ohlfest J.R., Largaespada D.A. Identification of candidate neoantigens produced by fusion transcripts in human osteosarcomas. Sci. Rep. 2019;9:358. doi: 10.1038/s41598-018-36840-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang G., Kang X., Chen K.S., Jehng T., Jones L., Chen J., Huang X.F., Chen S.Y. An engineered oncolytic virus expressing PD-L1 inhibitors activates tumor neoantigen-specific T cell responses. Nat. Commun. 2020;11:1395. doi: 10.1038/s41467-020-15229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Plaen E., Lurquin C., van Pel A., Mariame B., Szikora J.P., Wolfel T., Sibille C., Chomez P., Boon T. Immunogenic (tum-) variants of mouse tumor P815: Cloning of the gene of tum- antigen P91A and identification of the tum- mutation. Proc. Natl. Acad. Sci. USA. 1988;85:2274–2278. doi: 10.1073/pnas.85.7.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenthal R., Cadieux E.L., Salgado R., Bakir M.A., Moore D.A., Hiley C.T., Lund T., Tanic M., Reading J.L., Joshi K., et al. Neoantigen-directed immune escape in lung cancer evolution. Nature. 2019;567:479–485. doi: 10.1038/s41586-019-1032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu Y.C., Yao X., Crystal J.S., Li Y.F., El-Gamil M., Gross C., Davis L., Dudley M.E., Yang J.C., Samuels Y., et al. Efficient identification of mutated cancer antigens recognized by T cells associated with durable tumor regressions. Clin. Cancer Res. 2014;20:3401–3410. doi: 10.1158/1078-0432.CCR-14-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rizvi N.A., Hellmann M.D., Snyder A., Kvistborg P., Makarov V., Havel J.J., Lee W., Yuan J., Wong P., Ho T.S., et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Snyder A., Makarov V., Merghoub T., Yuan J., Zaretsky J.M., Desrichard A., Walsh L.A., Postow M.A., Wong P., Ho T.S., et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zacharakis N., Chinnasamy H., Black M., Xu H., Lu Y.C., Zheng Z., Pasetto A., Langhan M., Shelton T., Prickett T., et al. Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nat. Med. 2018;24:724–730. doi: 10.1038/s41591-018-0040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laghi L., Orbetegli O., Bianchi P., Zerbi A., Di Carlo V., Boland C.R., Malesci A. Common occurrence of multiple K-RAS mutations in pancreatic cancers with associated precursor lesions and in biliary cancers. Oncogene. 2002;21:4301–4306. doi: 10.1038/sj.onc.1205533. [DOI] [PubMed] [Google Scholar]
- 50.Coulie P.G., Lehmann F., Lethe B., Herman J., Lurquin C., Andrawiss M., Boon T. A mutated intron sequence codes for an antigenic peptide recognized by cytolytic T lymphocytes on a human melanoma. Proc. Natl. Acad. Sci. USA. 1995;92:7976–7980. doi: 10.1073/pnas.92.17.7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsiue E.H., Wright K.M., Douglass J., Hwang M.S., Mog B.J., Pearlman A.H., Paul S., DiNapoli S.R., Konig M.F., Wang Q., et al. Targeting a neoantigen derived from a common TP53 mutation. Science. 2021;371:6533. doi: 10.1126/science.abc8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim G.B., Fritsche J., Bunk S., Mahr A., Unverdorben F., Tosh K., Kong H., Maldini C.R., Lau C., Srivatsa S., et al. Quantitative immunopeptidomics reveals a tumor stroma-specific target for T cell therapy. Sci. Transl. Med. 2022;14:eabo6135. doi: 10.1126/scitranslmed.abo6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leidner R., Sanjuan Silva N., Huang H., Sprott D., Zheng C., Shih Y.P., Leung A., Payne R., Sutcliffe K., Cramer J., et al. Neoantigen T-Cell Receptor Gene Therapy in Pancreatic Cancer. N. Engl. J. Med. 2022;386:2112–2119. doi: 10.1056/NEJMoa2119662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA Cancer J. Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 55.Kim S.P., Vale N.R., Zacharakis N., Krishna S., Yu Z., Gasmi B., Gartner J.J., Sindiri S., Malekzadeh P., Deniger D.C., et al. Adoptive Cellular Therapy with Autologous Tumor-Infiltrating Lymphocytes and T-cell Receptor-Engineered T Cells Targeting Common p53 Neoantigens in Human Solid Tumors. Cancer Immunol. Res. 2022;10:932–946. doi: 10.1158/2326-6066.CIR-22-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Waldman A.D., Fritz J.M., Lenardo M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020;20:651–668. doi: 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Poirot L., Philip B., Schiffer-Mannioui C., Le Clerre D., Chion-Sotinel I., Derniame S., Potrel P., Bas C., Lemaire L., Galetto R., et al. Multiplex Genome-Edited T-cell Manufacturing Platform for "Off-the-Shelf" Adoptive T-cell Immunotherapies. Cancer Res. 2015;75:3853–3864. doi: 10.1158/0008-5472.CAN-14-3321. [DOI] [PubMed] [Google Scholar]
- 58.Greco R., Oliveira G., Stanghellini M.T., Vago L., Bondanza A., Peccatori J., Cieri N., Marktel S., Mastaglio S., Bordignon C., et al. Improving the safety of cell therapy with the TK-suicide gene. Front. Pharm. 2015;6:95. doi: 10.3389/fphar.2015.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gomes-Silva D., Srinivasan M., Sharma S., Lee C.M., Wagner D.L., Davis T.H., Rouce R.H., Bao G., Brenner M.K., Mamonkin M. CD7-edited T cells expressing a CD7-specific CAR for the therapy of T-cell malignancies. Blood. 2017;130:285–296. doi: 10.1182/blood-2017-01-761320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roberts M.R., Qin L., Zhang D., Smith D.H., Tran A.C., Dull T.J., Groopman J.E., Capon D.J., Byrn R.A., Finer M.H. Targeting of human immunodeficiency virus-infected cells by CD8+ T lymphocytes armed with universal T-cell receptors. Blood. 1994;84:2878–2889. doi: 10.1182/blood.V84.9.2878.2878. [DOI] [PubMed] [Google Scholar]
- 61.Kochenderfer J.N., Rosenberg S.A. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat. Rev. Clin. Oncol. 2013;10:267–276. doi: 10.1038/nrclinonc.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brentjens R.J., Santos E., Nikhamin Y., Yeh R., Matsushita M., La Perle K., Quintas-Cardama A., Larson S.M., Sadelain M. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin. Cancer. Res. 2007;13:5426–5435. doi: 10.1158/1078-0432.CCR-07-0674. [DOI] [PubMed] [Google Scholar]
- 63.Milone M.C., Fish J.D., Carpenito C., Carroll R.G., Binder G.K., Teachey D., Samanta M., Lakhal M., Gloss B., Danet-Desnoyers G., et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol.Ther. 2009;17:1453–1464. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Porter D.L., Levine B.L., Kalos M., Bagg A., June C.H. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grupp S.A., Kalos M., Barrett D., Aplenc R., Porter D.L., Rheingold S.R., Teachey D.T., Chew A., Hauck B., Wright J.F., et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sheykhhasan M., Manoochehri H., Dama P. Use of CAR T-cell for acute lymphoblastic leukemia (ALL) treatment: A review study. Cancer Gene. 2022;29:1080–1096. doi: 10.1038/s41417-021-00418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kalos M., Levine B.L., Porter D.L., Katz S., Grupp S.A., Bagg A., June C.H. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chavez J.C., Yassine F., Sandoval-Sus J., Kharfan-Dabaja M.A. Anti-CD19 chimeric antigen receptor T-cell therapy in B-cell lymphomas: Current status and future directions. Int. J. Hematol. Oncol. 2021;10:IJH33. doi: 10.2217/ijh-2020-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chavez J.C., Bachmeier C., Kharfan-Dabaja M.A. CAR T-cell therapy for B-cell lymphomas: Clinical trial results of available products. Adv. Hematol. 2019;10:2040620719841581. doi: 10.1177/2040620719841581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin W.Y., Wang H.H., Chen Y.W., Lin C.F., Fan H.C., Lee Y.Y. Gene Modified CAR-T Cellular Therapy for Hematologic Malignancies. Int. J. Mol. Sci. 2020;21:8655. doi: 10.3390/ijms21228655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tomar S., Zhang J., Khanal M., Hong J., Venugopalan A., Jiang Q., Sengupta M., Miettinen M., Li N., Pastan I., et al. Development of Highly Effective Anti-Mesothelin hYP218 Chimeric Antigen Receptor T Cells With Increased Tumor Infiltration and Persistence for Treating Solid Tumors. Mol. Cancer. 2022;21:1195–1206. doi: 10.1158/1535-7163.MCT-22-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pavlasova G., Mraz M. The regulation and function of CD20: An "enigma" of B-cell biology and targeted therapy. Haematologica. 2020;105:1494–1506. doi: 10.3324/haematol.2019.243543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vitale C., Strati P. CAR T-Cell Therapy for B-Cell non-Hodgkin Lymphoma and Chronic Lymphocytic Leukemia: Clinical Trials and Real-World Experiences. Front. Oncol. 2020;10:849. doi: 10.3389/fonc.2020.00849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Till B.G., Jensen M.C., Wang J., Qian X., Gopal A.K., Maloney D.G., Lindgren C.G., Lin Y., Pagel J.M., Budde L.E., et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: Pilot clinical trial results. Blood. 2012;119:3940–3950. doi: 10.1182/blood-2011-10-387969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dimitri A., Herbst F., Fraietta J.A. Engineering the next-generation of CAR T-cells with CRISPR-Cas9 gene editing. Mol. Cancer. 2022;21:78. doi: 10.1186/s12943-022-01559-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brentjens R.J., Davila M.L., Riviere I., Park J., Wang X., Cowell L.G., Bartido S., Stefanski J., Taylor C., Olszewska M., et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 2013;5:177ra138. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maude S.L., Frey N., Shaw P.A., Aplenc R., Barrett D.M., Bunin N.J., Chew A., Gonzalez V.E., Zheng Z., Lacey S.F., et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shalabi H., Martin S., Yates B., Wolters P.L., Kaplan C., Smith H., Sesi C.R., Jess J., Toledo-Tamula M.A., Struemph K., et al. Neurotoxicity following CD19/CD28zeta CAR T-cells in children and young adults with B-cell malignancies. Neuro. Oncol. 2022;24:1584–1597. doi: 10.1093/neuonc/noac034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Olson J.A., Leveson-Gower D.B., Gill S., Baker J., Beilhack A., Negrin R.S. NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood. 2010;115:4293–4301. doi: 10.1182/blood-2009-05-222190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tarannum M., Romee R., Shapiro R.M. Innovative Strategies to Improve the Clinical Application of NK Cell-Based Immunotherapy. Front. Immunol. 2022;13:859177. doi: 10.3389/fimmu.2022.859177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bohineust A., Tourret M., Derivry L., Caillat-Zucman S. Mucosal-associated invariant T (MAIT) cells, a new source of universal immune cells for chimeric antigen receptor (CAR)-cell therapy. Bull. Cancer. 2021;108:S92–S95. doi: 10.1016/j.bulcan.2021.07.003. [DOI] [PubMed] [Google Scholar]
- 82.Dogan M., Karhan E., Kozhaya L., Placek L., Chen X., Yigit M., Unutmaz D. Engineering Human MAIT Cells with Chimeric Antigen Receptors for Cancer Immunotherapy. J. Immunol. 2022;209:1523–1531. doi: 10.4049/jimmunol.2100856. [DOI] [PubMed] [Google Scholar]
- 83.Lu H., Dai W., Guo J., Wang D., Wen S., Yang L., Lin D., Xie W., Wen L., Fang J., et al. High Abundance of Intratumoral gammadelta T Cells Favors a Better Prognosis in Head and Neck Squamous Cell Carcinoma: A Bioinformatic Analysis. Front. Immunol. 2020;11:573920. doi: 10.3389/fimmu.2020.573920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tosolini M., Pont F., Poupot M., Vergez F., Nicolau-Travers M.L., Vermijlen D., Sarry J.E., Dieli F., Fournie J.J. Assessment of tumor-infiltrating TCRVgamma9Vdelta2 gammadelta lymphocyte abundance by deconvolution of human cancers microarrays. Oncoimmunology. 2017;6:e1284723. doi: 10.1080/2162402X.2017.1284723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao N., Dang H., Ma L., Martin S.P., Forgues M., Ylaya K., Hewitt S.M., Wang X.W. Intratumoral gammadelta T-Cell Infiltrates, Chemokine (C-C Motif) Ligand 4/Chemokine (C-C Motif) Ligand 5 Protein Expression and Survival in Patients With Hepatocellular Carcinoma. Hepatology. 2021;73:1045–1060. doi: 10.1002/hep.31412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Meraviglia S., Lo Presti E., Tosolini M., La Mendola C., Orlando V., Todaro M., Catalano V., Stassi G., Cicero G., Vieni S., et al. Distinctive features of tumor-infiltrating gammadelta T lymphocytes in human colorectal cancer. Oncoimmunology. 2017;6:e1347742. doi: 10.1080/2162402X.2017.1347742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Devaud C., Rousseau B., Netzer S., Pitard V., Paroissin C., Khairallah C., Costet P., Moreau J.F., Couillaud F., Dechanet-Merville J., et al. Anti-metastatic potential of human Vdelta1(+) gammadelta T cells in an orthotopic mouse xenograft model of colon carcinoma. Cancer Immunol. Immunother. 2013;62:1199–1210. doi: 10.1007/s00262-013-1402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kobayashi H., Tanaka Y., Yagi J., Minato N., Tanabe K. Phase I/II study of adoptive transfer of gammadelta T cells in combination with zoledronic acid and IL-2 to patients with advanced renal cell carcinoma. Cancer Immunol. Immunother. 2011;60:1075–1084. doi: 10.1007/s00262-011-1021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nakajima J., Murakawa T., Fukami T., Goto S., Kaneko T., Yoshida Y., Takamoto S., Kakimi K. A phase I study of adoptive immunotherapy for recurrent non-small-cell lung cancer patients with autologous gammadelta T cells. Eur. J. Cardiothorac. Surg. 2010;37:1191–1197. doi: 10.1016/j.ejcts.2009.11.051. [DOI] [PubMed] [Google Scholar]
- 90.Abe Y., Muto M., Nieda M., Nakagawa Y., Nicol A., Kaneko T., Goto S., Yokokawa K., Suzuki K. Clinical and immunological evaluation of zoledronate-activated Vgamma9gammadelta T-cell-based immunotherapy for patients with multiple myeloma. Exp. Hematol. 2009;37:956–968. doi: 10.1016/j.exphem.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 91.de Vries N.L., van de Haar J., Veninga V., Chalabi M., Ijsselsteijn M.E., van der Ploeg M., van den Bulk J., Ruano D., van den Berg J.G., Haanen J.B., et al. gammadelta T cells are effectors of immunotherapy in cancers with HLA class I defects. Nature. 2023;613:743–750. doi: 10.1038/s41586-022-05593-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rischer M., Pscherer S., Duwe S., Vormoor J., Jurgens H., Rossig C. Human gammadelta T cells as mediators of chimaeric-receptor redirected anti-tumour immunity. Br. J. Haematol. 2004;126:583–592. doi: 10.1111/j.1365-2141.2004.05077.x. [DOI] [PubMed] [Google Scholar]
- 93.Rozenbaum M., Meir A., Aharony Y., Itzhaki O., Schachter J., Bank I., Jacoby E., Besser M.J. Gamma-Delta CAR-T Cells Show CAR-Directed and Independent Activity Against Leukemia. Front. Immunol. 2020;11:1347. doi: 10.3389/fimmu.2020.01347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee D., Rosenthal C.J., Penn N.E., Dunn Z.S., Zhou Y., Yang L. Human gammadelta T Cell Subsets and Their Clinical Applications for Cancer Immunotherapy. Cancers. 2022;14:3005. doi: 10.3390/cancers14123005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ganapathy T., Radhakrishnan R., Sakshi S., Martin S. CAR gammadelta T cells for cancer immunotherapy. Is the field more yellow than green? Cancer Immunol. Immunother. 2023;72:277–286. doi: 10.1007/s00262-022-03260-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Makkouk A., Yang X.C., Barca T., Lucas A., Turkoz M., Wong J.T.S., Nishimoto K.P., Brodey M.M., Tabrizizad M., Gundurao S.R.Y., et al. Off-the-shelf Vdelta1 gamma delta T cells engineered with glypican-3 (GPC-3)-specific chimeric antigen receptor (CAR) and soluble IL-15 display robust antitumor efficacy against hepatocellular carcinoma. J. Immunother. Cancer. 2021;9:e003441. doi: 10.1136/jitc-2021-003441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Du Z., Zhu S., Zhang X., Gong Z., Wang S. Non-Conventional Allogeneic Anti-BCMA Chimeric Antigen Receptor-Based Immune Cell Therapies for Multiple Myeloma Treatment. Cancers. 2023;15:567. doi: 10.3390/cancers15030567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.de Bruin R.C.G., Veluchamy J.P., Lougheed S.M., Schneiders F.L., Lopez-Lastra S., Lameris R., Stam A.G., Sebestyen Z., Kuball J., Molthoff C.F.M., et al. A bispecific nanobody approach to leverage the potent and widely applicable tumor cytolytic capacity of Vgamma9Vdelta2-T cells. Oncoimmunology. 2017;7:e1375641. doi: 10.1080/2162402X.2017.1375641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.de Weerdt I., Lameris R., Scheffer G.L., Vree J., de Boer R., Stam A.G., van de Ven R., Levin M.D., Pals S.T., Roovers R.C., et al. A Bispecific Antibody Antagonizes Prosurvival CD40 Signaling and Promotes Vgamma9Vdelta2 T cell-Mediated Antitumor Responses in Human B-cell Malignancies. Cancer Immunol. Res. 2021;9:50–61. doi: 10.1158/2326-6066.CIR-20-0138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.