Abstract
Microneurography was performed in median nerve sensory fascicles with concentric needle electrodes and with conventional tungsten microneedles. The latter electrodes preferentially recorded activity from the myelinated fibres in the whole fascicle. By contrast, due to its special design, a concentric needle can record activity selectively from even a small part of a fascicle. High amplitude signals in C fibres can be discriminated close to Schwann cells that envelope unmyelinated axons. Apart from being biased for activity in thin fibres, the concentric needles can also record signals from nearby myelinated fibres. The palmar receptive fields of such fibre groups were not congruent with the areas traditionally attributed to multiunit skin afferents in humans, namely the innervation zone(s) of one or two adjacent digital nerve(s). Instead, the multiunit fields often comprised small parts of a digital nerve innervation area, frequently only the pulp of a finger. Single units were always localised within previously screened multiunit areas. Contrary to some previously accepted tenets it is probable that single unit activity in myelinated fibres in these studies is recorded extra-axonally near to a node of Ranvier. The findings also suggest the presence of a somatotopy in human limb nerve fascicles, comparable to that previously established in the spinal cord and the somatosensory cortex.
Full text
PDF

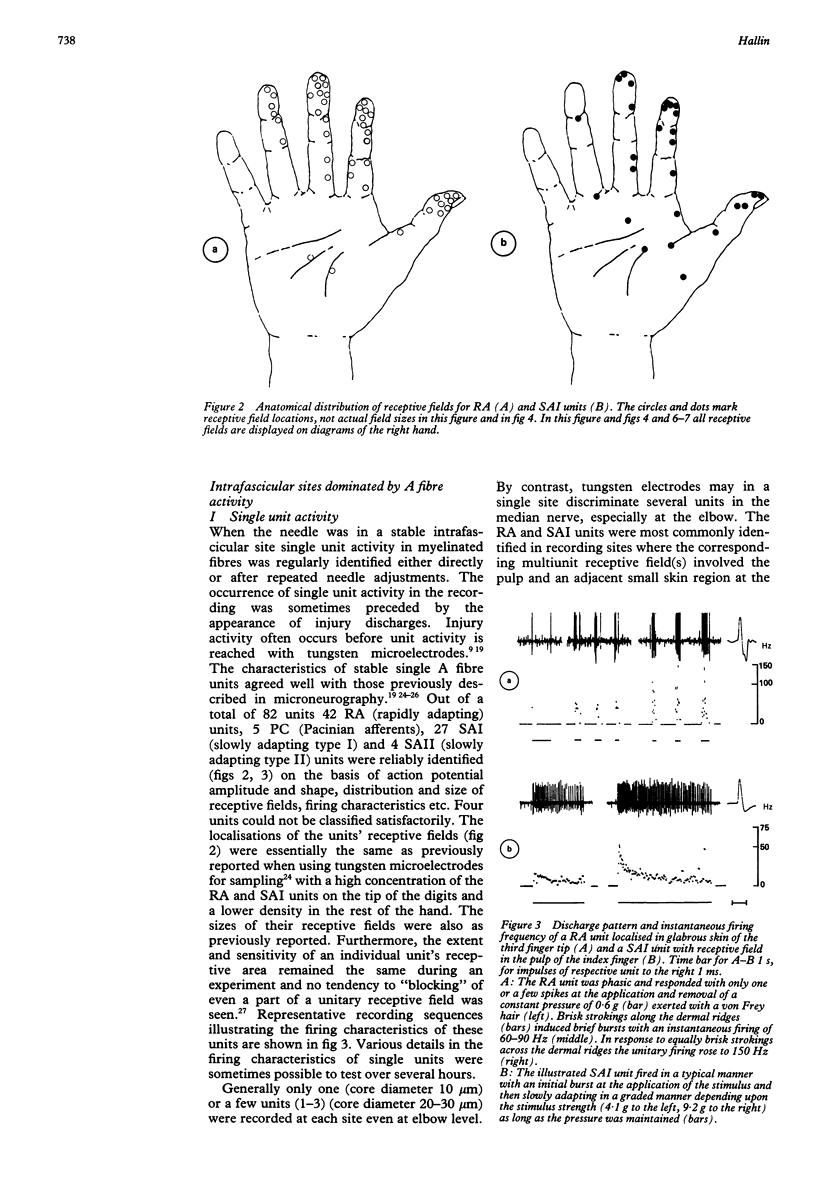
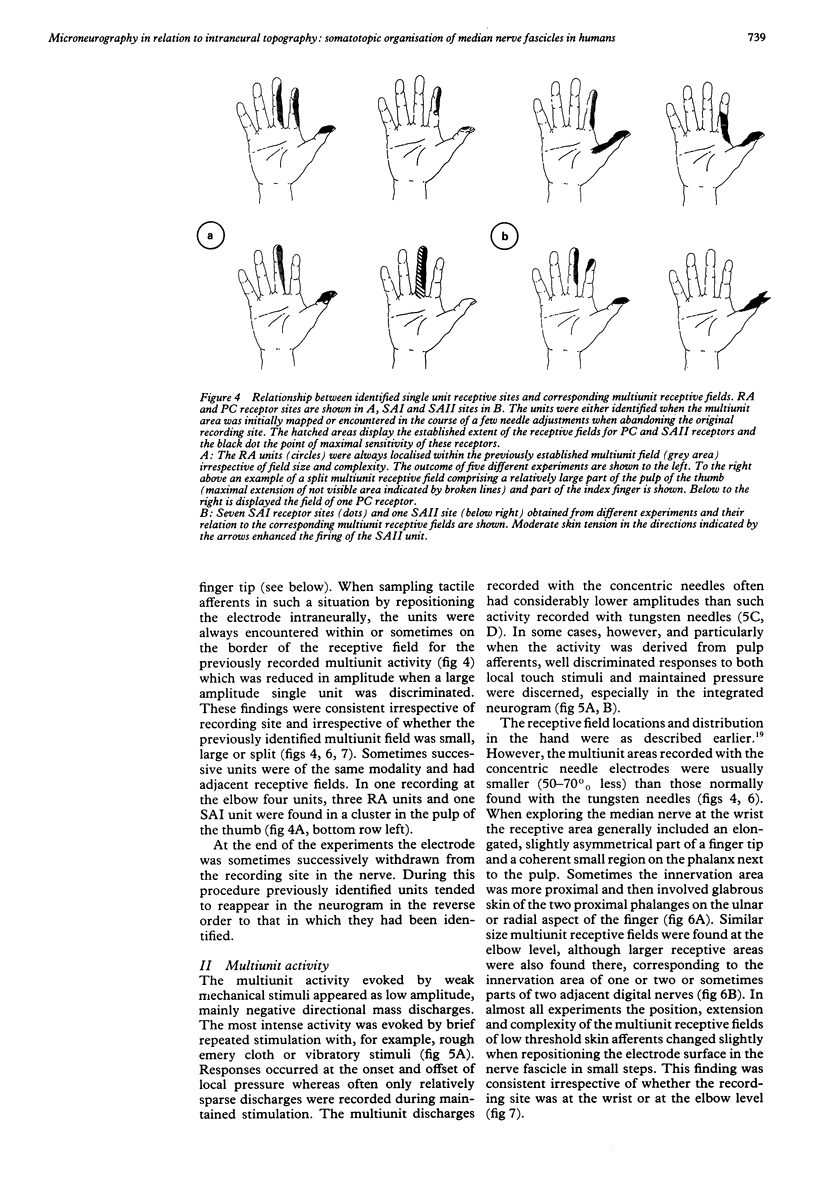
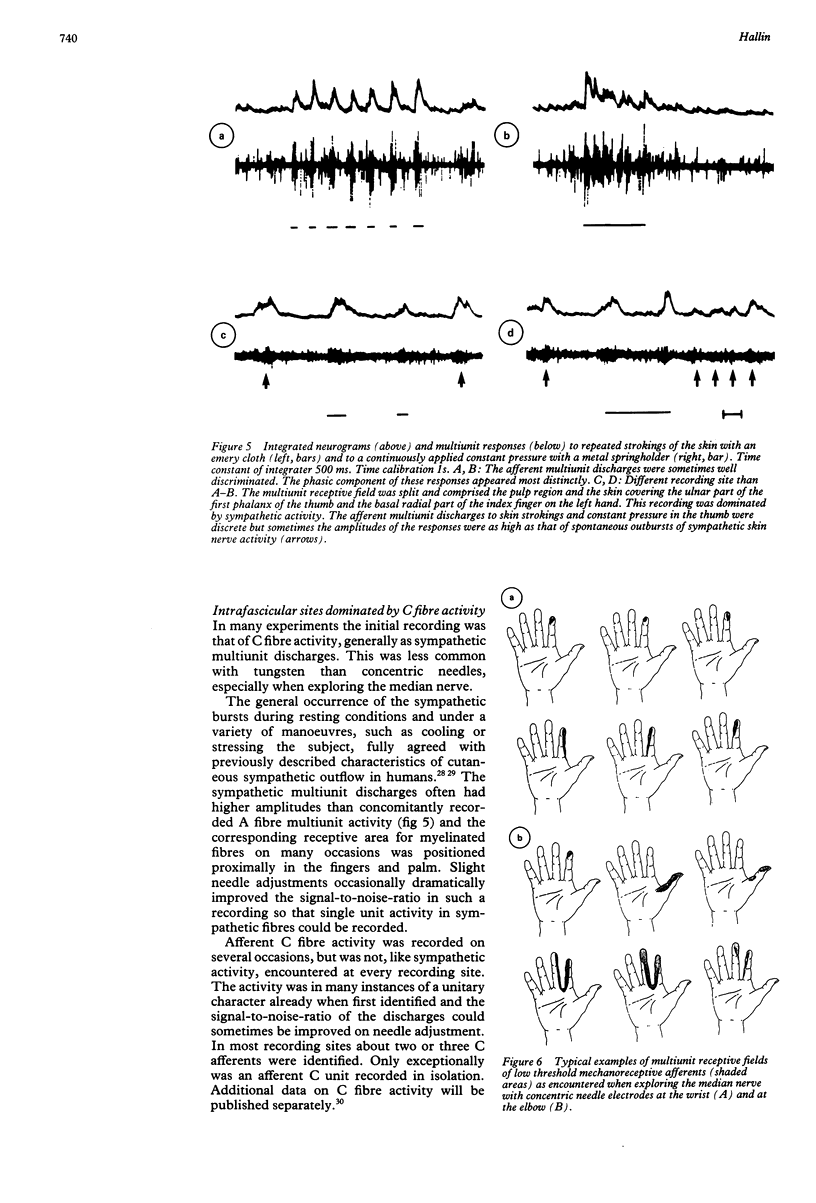



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAWSON G. D., SCOTT J. W. The recording of nerve action potentials through skin in man. J Neurol Neurosurg Psychiatry. 1949 Nov;12(4):259–267. doi: 10.1136/jnnp.12.4.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON G. D. The relative excitability and conduction velocity of sensory and motor nerve fibres in man. J Physiol. 1956 Feb 28;131(2):436–451. doi: 10.1113/jphysiol.1956.sp005473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delius W., Hagbarth K. E., Hongell A., Wallin B. G. Manoeuvres affecting sympathetic outflow in human skin nerves. Acta Physiol Scand. 1972 Feb;84(2):177–186. doi: 10.1111/j.1748-1716.1972.tb05168.x. [DOI] [PubMed] [Google Scholar]
- Dykes R. W., Rasmusson D. D., Hoeltzell P. B. Organization of primary somatosensory cortex in the cat. J Neurophysiol. 1980 Jun;43(6):1527–1546. doi: 10.1152/jn.1980.43.6.1527. [DOI] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. Saltatory conduction in myelinated nerve fibres. J Physiol. 1952 Sep;118(1):107–112. doi: 10.1113/jphysiol.1952.sp004776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friede R. L., Meier T., Diem M. How is the exact length of an internode determined. J Neurol Sci. 1981 May;50(2):217–228. doi: 10.1016/0022-510x(81)90168-4. [DOI] [PubMed] [Google Scholar]
- GILLIATT R. W., MELVILLE I. D., VELATE A. S., WILLISON R. G. A STUDY OF NORMAL NERVE ACTION POTENTIALS USING AN AVERAGING TECHNIQUE (BARRIER GRID STORAGE TUBE). J Neurol Neurosurg Psychiatry. 1965 Jun;28:191–200. doi: 10.1136/jnnp.28.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabb W. C., Bement S. L., Koepke G. H., Green R. A. Comparison of methods of peripheral nerve suturing in monkeys. Plast Reconstr Surg. 1970 Jul;46(1):31–38. [PubMed] [Google Scholar]
- HENSEL H., BOMAN K. K. Afferent impulses in cutaneous sensory nerves in human subjects. J Neurophysiol. 1960 Sep;23:564–578. doi: 10.1152/jn.1960.23.5.564. [DOI] [PubMed] [Google Scholar]
- Hagbarth K. E., Hallin R. G., Hongell A., Torebjörk H. E., Wallin B. G. General characteristics of sympathetic activity in human skin nerves. Acta Physiol Scand. 1972 Feb;84(2):164–176. doi: 10.1111/j.1748-1716.1972.tb05167.x. [DOI] [PubMed] [Google Scholar]
- Hagbarth K. E., Hongell A., Hallin R. G., Torebjörk H. E. Afferent impulses in median nerve fascicles evoked by tactile stimuli of the human hand. Brain Res. 1970 Dec 18;24(3):423–442. doi: 10.1016/0006-8993(70)90183-6. [DOI] [PubMed] [Google Scholar]
- Hagbarth K. E., Vallbo A. B. Pulse and respiratory grouping of sympathetic impulses in human muscle-nerves. Acta Physiol Scand. 1968 Sep-Oct;74(1):96–108. doi: 10.1111/j.1748-1716.1968.tb04218.x. [DOI] [PubMed] [Google Scholar]
- Hallin R. G., Torebjörk H. E. Afferent and efferent C units recorded from human skin nerves in situ. A preliminary report. Acta Soc Med Ups. 1970;75(5-6):277–281. [PubMed] [Google Scholar]
- Hallin R. G., Torebjörk H. E. C-fibre components in electrically evoked compound potentials recorded from human median nerve fascicles in situ. A preliminary report. Acta Soc Med Ups. 1970;75(1-2):77–80. [PubMed] [Google Scholar]
- Hallin R. G., Torebjörk H. E. Electrically induced A and C fibre responses in intact human skin nerves. Exp Brain Res. 1973 Jan 29;16(3):309–320. doi: 10.1007/BF00233333. [DOI] [PubMed] [Google Scholar]
- Hallin R. G., Wiesenfeld-Hallin Z., Duranti R. Percutaneous microneurography in man does not cause pressure block of almost all axons in the impaled nerve fascicle. Neurosci Lett. 1986 Aug 4;68(3):356–361. doi: 10.1016/0304-3940(86)90516-1. [DOI] [PubMed] [Google Scholar]
- Hallin R. G., Wiesenfeld Z. A standardized electrode for percutaneous recording of A and C fibre units in conscious man. Acta Physiol Scand. 1981 Dec;113(4):561–563. doi: 10.1111/j.1748-1716.1981.tb06940.x. [DOI] [PubMed] [Google Scholar]
- Huxley A. F., Stämpfli R. Evidence for saltatory conduction in peripheral myelinated nerve fibres. J Physiol. 1949 May 15;108(3):315–339. [PMC free article] [PubMed] [Google Scholar]
- Jahnke M. T. Properties of a microcoaxial electrode designed for unit recording from human peripheral nerves. J Neurosci Methods. 1982 Nov;6(4):335–346. doi: 10.1016/0165-0270(82)90034-6. [DOI] [PubMed] [Google Scholar]
- Kausz M., Réthelyi M. Lamellar arrangement of neuronal somata in the dorsal root ganglion of the cat. Somatosens Res. 1985;2(3):193–204. doi: 10.3109/07367228509144563. [DOI] [PubMed] [Google Scholar]
- Knibestöl M. Stimulus-response functions of rapidly adapting mechanoreceptors in human glabrous skin area. J Physiol. 1973 Aug;232(3):427–452. doi: 10.1113/jphysiol.1973.sp010279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knibestöl M. Stimulus-response functions of slowly adapting mechanoreceptors in the human glabrous skin area. J Physiol. 1975 Feb;245(1):63–80. doi: 10.1113/jphysiol.1975.sp010835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod J. G. Digital nerve conduction in the carpal tunnel syndrome after mechanical stimulation of the finger. J Neurol Neurosurg Psychiatry. 1966 Feb;29(1):12–22. doi: 10.1136/jnnp.29.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa J., Torebjörk E. Sensations evoked by intraneural microstimulation of single mechanoreceptor units innervating the human hand. J Physiol. 1983 Sep;342:633–654. doi: 10.1113/jphysiol.1983.sp014873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röper J., Schwarz J. R. Heterogeneous distribution of fast and slow potassium channels in myelinated rat nerve fibres. J Physiol. 1989 Sep;416:93–110. doi: 10.1113/jphysiol.1989.sp017751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schady W. J., Torebjörk H. E., Ochoa J. L. Peripheral projections of nerve fibres in the human median nerve. Brain Res. 1983 Oct 31;277(2):249–261. doi: 10.1016/0006-8993(83)90932-0. [DOI] [PubMed] [Google Scholar]
- TASAKI I. Properties of myelinated fibers in frog sciatic nerve and in spinal cord as examined with micro-electrodes. Jpn J Physiol. 1952 Nov;3(1):73–94. doi: 10.2170/jjphysiol.3.73. [DOI] [PubMed] [Google Scholar]
- TOMASCH J., BRITTON W. A. On the individual variability of fibre composition in human peripheral nerves. J Anat. 1956 Jul;90(3):337–349. [PMC free article] [PubMed] [Google Scholar]
- Talbot W. H., Darian-Smith I., Kornhuber H. H., Mountcastle V. B. The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. J Neurophysiol. 1968 Mar;31(2):301–334. doi: 10.1152/jn.1968.31.2.301. [DOI] [PubMed] [Google Scholar]
- Torebjörk H. E., Hallin R. G. C-fibre units recorded from human sensory nerve fascicles in situ. A preliminary report. Acta Soc Med Ups. 1970;75(1-2):81–84. [PubMed] [Google Scholar]
- Torebjörk H. E., Hallin R. G., Hongell A., Hagbarth K. E. Single unit potentials with complex waveform seen in microelectrode recordings from the human median nerve. Brain Res. 1970 Dec 18;24(3):443–450. doi: 10.1016/0006-8993(70)90184-8. [DOI] [PubMed] [Google Scholar]
- Torebjörk H. E., Hallin R. G. Identification of afferent C units in intact human skin nerves. Brain Res. 1974 Mar 8;67(3):387–403. doi: 10.1016/0006-8993(74)90489-2. [DOI] [PubMed] [Google Scholar]
- Tuczinski H. J., Friede R. L. Internodal length in ventral roots of bovine spinal nerves varies independently of fibre calibre. J Anat. 1984 May;138(Pt 3):423–433. [PMC free article] [PubMed] [Google Scholar]
- Vallbo A. B., Hagbarth K. E. Activity from skin mechanoreceptors recorded percutaneously in awake human subjects. Exp Neurol. 1968 Jul;21(3):270–289. doi: 10.1016/0014-4886(68)90041-1. [DOI] [PubMed] [Google Scholar]
- Wall P. D., McMahon S. B. Microneuronography and its relation to perceived sensation. A critical review. Pain. 1985 Mar;21(3):209–229. doi: 10.1016/0304-3959(85)90086-7. [DOI] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z., Hallin R. G. The influence of the sympathetic system on mechanoreception and nociception. A review. Hum Neurobiol. 1984;3(1):41–46. [PubMed] [Google Scholar]