Abstract
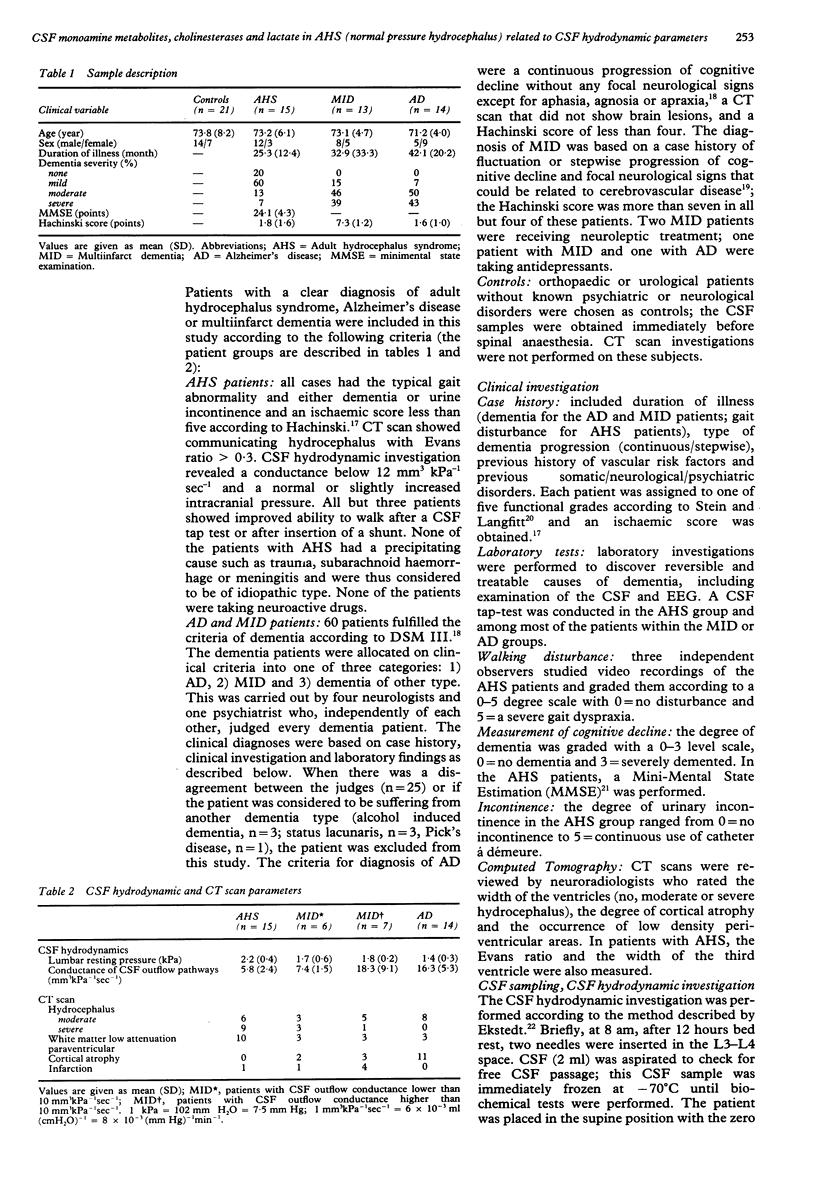
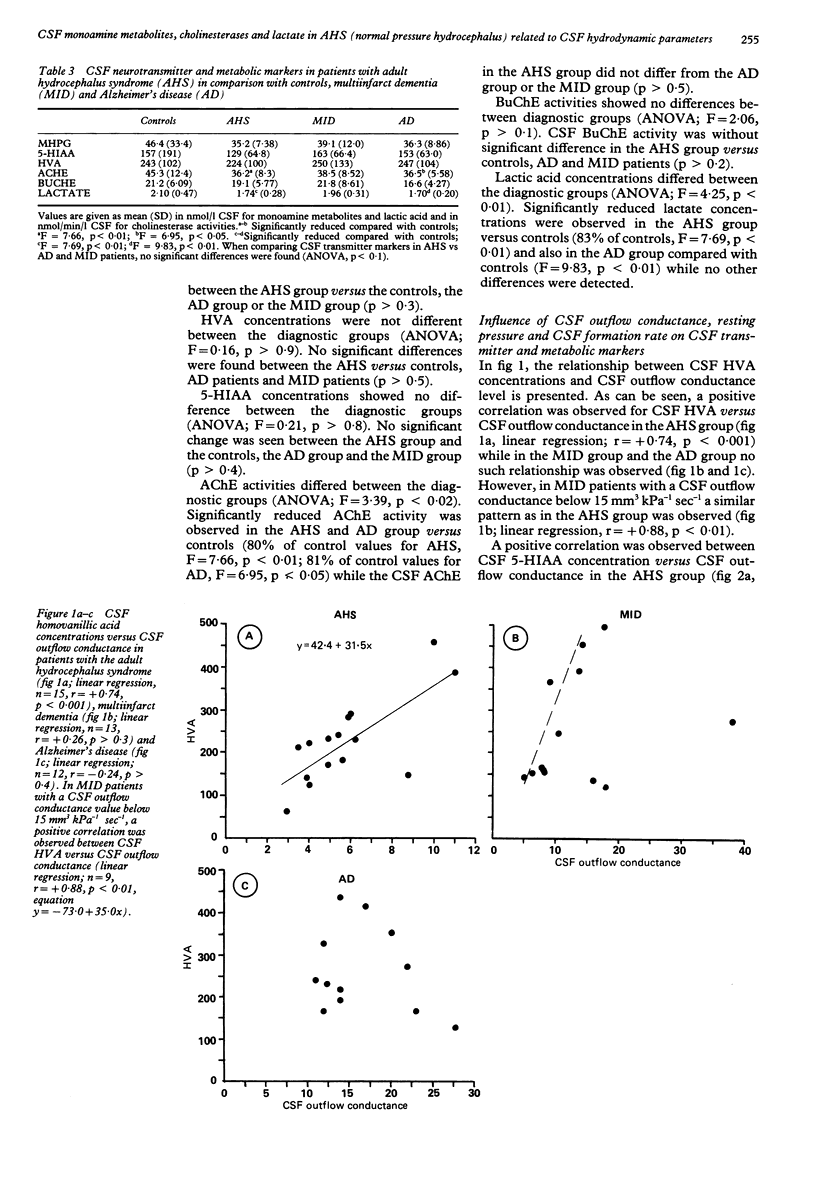
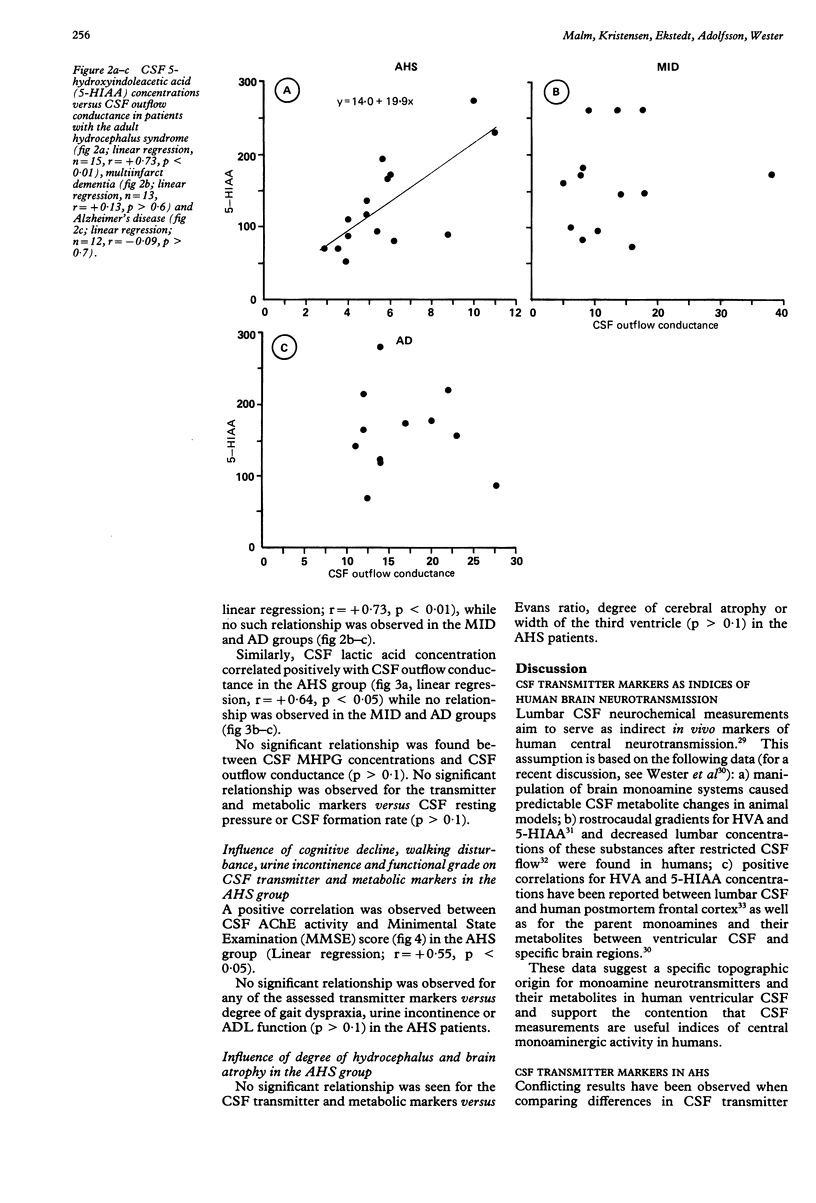
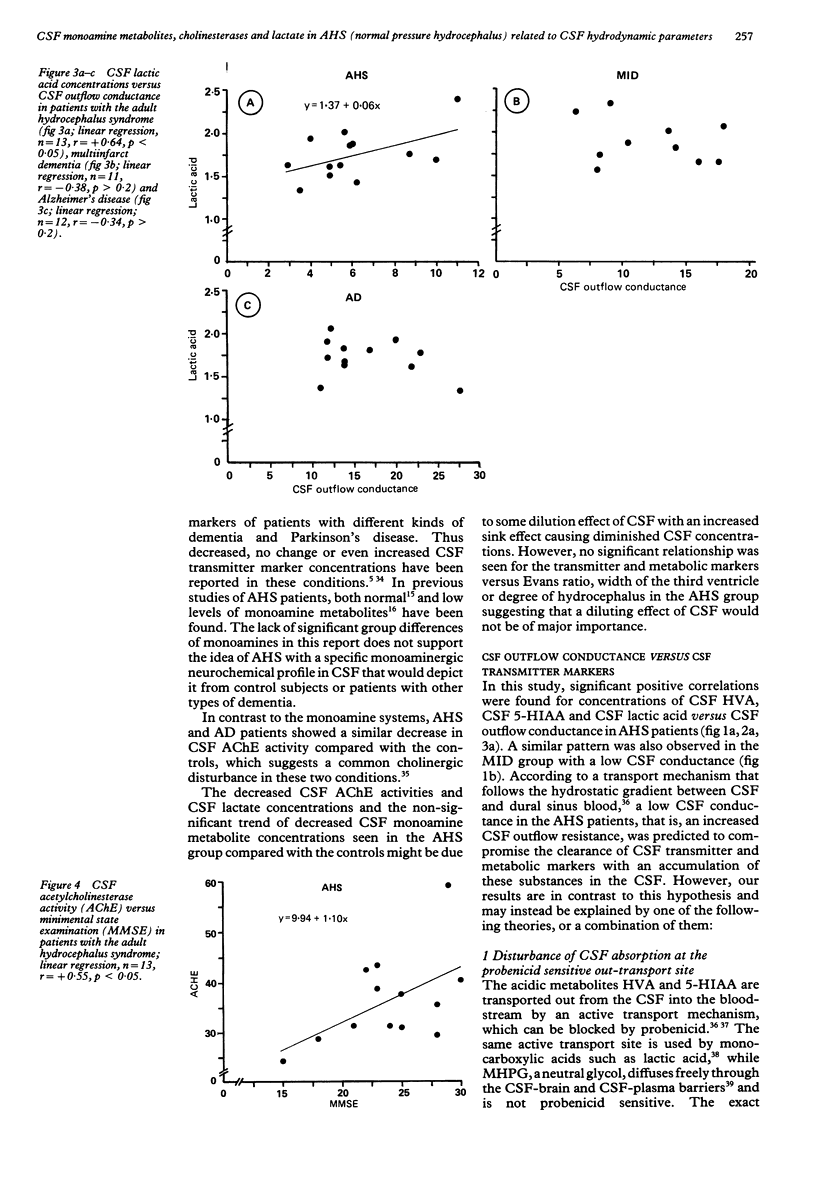
Monoamine metabolites, cholinesterases and lactic acid in lumbar cerebrospinal fluid (CSF) were investigated on patients with the adult hydrocephalus syndrome (idiopathic normal pressure syndrome; AHS, n = 15), Alzheimer's disease (AD, n = 14), multi-infarct dementia (MID, n = 13) and controls (n = 21). Patients had clinical and CSF hydrodynamic investigations. Monoamine concentrations were determined by reversed-phase liquid chromatography, cholinesterases and lactate were determined photometrically. In the AHS patients, CSF monoamine concentrations were not significantly different compared with controls, AD or MID patients. AHS and AD patients showed a similar reduction of CSF acetylcholinesterase activity compared with controls. Positive correlations were found in concentrations of CSF homovanillic acid, CSF 5-hydroxyindoleacetic acid and CSF lactic acid versus CSF outflow conductance (that is, resistance against CSF outflow) in the AHS patients. A similar pattern was observed in a subgroup of MID patients characterised by dilated ventricles and disturbed CSF hydrodynamics. These data suggest that a low CSF outflow conductance may facilitate the clearance of acidic substances from the arachnoid space at the probenecid sensitive active transport site. Alternative explanations would be that a pathologically low CSF outflow conductance is accompanied by an inverse caudorostral flow of CSF or a compromised trans-ependymal diffusion.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS R. D., FISHER C. M., HAKIM S., OJEMANN R. G., SWEET W. H. SYMPTOMATIC OCCULT HYDROCEPHALUS WITH "NORMAL" CEREBROSPINAL-FLUID PRESSURE.A TREATABLE SYNDROME. N Engl J Med. 1965 Jul 15;273:117–126. doi: 10.1056/NEJM196507152730301. [DOI] [PubMed] [Google Scholar]
- Bannister R., Gilford E., Kocen R. Isotope encephalography in the diagnosis of dementia due to communicating hydrocephalus. Lancet. 1967 Nov 11;2(7524):1014–1017. doi: 10.1016/s0140-6736(67)90288-7. [DOI] [PubMed] [Google Scholar]
- Bowen D. M., Smith C. B., White P., Davison A. N. Neurotransmitter-related enzymes and indices of hypoxia in senile dementia and other abiotrophies. Brain. 1976 Sep;99(3):459–496. doi: 10.1093/brain/99.3.459. [DOI] [PubMed] [Google Scholar]
- Brun A., Englund E. Regional pattern of degeneration in Alzheimer's disease: neuronal loss and histopathological grading. Histopathology. 1981 Sep;5(5):549–564. doi: 10.1111/j.1365-2559.1981.tb01818.x. [DOI] [PubMed] [Google Scholar]
- Børgesen S. E. Conductance to outflow of CSF in normal pressure hydrocephalus. Acta Neurochir (Wien) 1984;71(1-2):1–45. doi: 10.1007/BF01401149. [DOI] [PubMed] [Google Scholar]
- Clough C. G. A case of normal pressure hydrocephalus presenting as levodopa responsive parkinsonism. J Neurol Neurosurg Psychiatry. 1987 Feb;50(2):234–234. doi: 10.1136/jnnp.50.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserr H. F., Ostrach L. H. Bulk flow of interstitial fluid after intracranial injection of blue dextran 2000. Exp Neurol. 1974 Oct;45(1):50–60. doi: 10.1016/0014-4886(74)90099-5. [DOI] [PubMed] [Google Scholar]
- Curzon G., Gumpert E. J., Sharpe D. M. Amine metabolites in the lumbar cerebrospinal fluid of humans with restricted flow of cerebrospinal fluid. Nat New Biol. 1971 Jun 9;231(23):189–191. doi: 10.1038/newbio231189a0. [DOI] [PubMed] [Google Scholar]
- Davies P., Maloney A. J. Selective loss of central cholinergic neurons in Alzheimer's disease. Lancet. 1976 Dec 25;2(8000):1403–1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- Di Rocco C., Di Trapani G., Maira G., Bentivoglio M., Macchi G., Rossi G. F. Anatomo-clinical correlations in normotensive hydrocephalus. Reports on three cases. J Neurol Sci. 1977 Sep;33(3):437–452. doi: 10.1016/0022-510x(77)90139-3. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Ekstedt J. CSF hydrodynamic studies in man. 1. Method of constant pressure CSF infusion. J Neurol Neurosurg Psychiatry. 1977 Feb;40(2):105–119. doi: 10.1136/jnnp.40.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein M. F., Folstein S. E., McHugh P. R. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975 Nov;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Hakim S., Adams R. D. The special clinical problem of symptomatic hydrocephalus with normal cerebrospinal fluid pressure. Observations on cerebrospinal fluid hydrodynamics. J Neurol Sci. 1965 Jul-Aug;2(4):307–327. doi: 10.1016/0022-510x(65)90016-x. [DOI] [PubMed] [Google Scholar]
- Hollander E., Mohs R. C., Davis K. L. Antemortem markers of Alzheimer's disease. Neurobiol Aging. 1986 Sep-Oct;7(5):367–407. doi: 10.1016/0197-4580(86)90164-8. [DOI] [PubMed] [Google Scholar]
- MacMillan V. Effect of probenecid on cerebral and cisternal cerebrospinal fluid lactate content. J Cereb Blood Flow Metab. 1987 Feb;7(1):118–123. doi: 10.1038/jcbfm.1987.17. [DOI] [PubMed] [Google Scholar]
- Maira G., Bareggi S. R., Di Rocco C., Calderini G., Morselli P. L. Monoamine acid metabolites and cerebrospinal fluid dynamics in normal pressure hydrocephalus: preliminary results. J Neurol Neurosurg Psychiatry. 1975 Feb;38(2):123–128. doi: 10.1136/jnnp.38.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E. M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984 Jul;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Sjöström R., Ekstedt J., Anggård E. Concentration gradients of monoamine metabolites in human cerebrospinal fluid. J Neurol Neurosurg Psychiatry. 1975 Jul;38(7):666–668. doi: 10.1136/jnnp.38.7.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley M., Traskman-Bendz L., Dorovini-Zis K. Correlations between aminergic metabolites simultaneously obtained from human CSF and brain. Life Sci. 1985 Oct 7;37(14):1279–1286. doi: 10.1016/0024-3205(85)90242-5. [DOI] [PubMed] [Google Scholar]
- Stein S. C., Langfitt T. W. Normal-pressure hydrocephalus. Predicting the results of cerebrospinal fluid shunting. J Neurosurg. 1974 Oct;41(4):463–470. doi: 10.3171/jns.1974.41.4.0463. [DOI] [PubMed] [Google Scholar]
- Sudarsky L., Simon S. Gait disorder in late-life hydrocephalus. Arch Neurol. 1987 Mar;44(3):263–267. doi: 10.1001/archneur.1987.00520150019012. [DOI] [PubMed] [Google Scholar]
- Wester P., Bergström U., Eriksson A., Gezelius C., Hardy J., Winblad B. Ventricular cerebrospinal fluid monoamine transmitter and metabolite concentrations reflect human brain neurochemistry in autopsy cases. J Neurochem. 1990 Apr;54(4):1148–1156. doi: 10.1111/j.1471-4159.1990.tb01942.x. [DOI] [PubMed] [Google Scholar]
- Wester P., Gottfries J., Johansson K., Klintebäck F., Winblad B. Simultaneous liquid chromatographic determination of seventeen of the major monoamine neurotransmitters, precursors and metabolites. I. Optimization of the mobile phase using factorial designs and a computer program to predict chromatograms. J Chromatogr. 1987 Apr 10;415(2):261–274. doi: 10.1016/s0378-4347(00)83218-1. [DOI] [PubMed] [Google Scholar]
- Wester P., Gottfries J., Winblad B. Simultaneous liquid chromatographic determination of seventeen of the major monoamine neurotransmitters, precursors and metabolites. II. Assessment of human brain and cerebrospinal fluid concentrations. J Chromatogr. 1987 Apr 10;415(2):275–288. doi: 10.1016/s0378-4347(00)83219-3. [DOI] [PubMed] [Google Scholar]
- Wikkelsö C., Andersson H., Blomstrand C., Lindqvist G., Svendsen P. Normal pressure hydrocephalus. Predictive value of the cerebrospinal fluid tap-test. Acta Neurol Scand. 1986 Jun;73(6):566–573. doi: 10.1111/j.1600-0404.1986.tb04601.x. [DOI] [PubMed] [Google Scholar]
- Wolfson L. I., Escriva A. Clearance of 3-methoxy-4-hydroxyphenylglycol from the cerebrospinal fluid. Neurology. 1976 Aug;26(8):781–784. doi: 10.1212/wnl.26.8.781. [DOI] [PubMed] [Google Scholar]
- Wood J. H. Neurochemical analysis of cerebrospinal fluid. Neurology. 1980 Jun;30(6):645–651. doi: 10.1212/wnl.30.6.645. [DOI] [PubMed] [Google Scholar]


