Abstract
Antimicrobial resistance (AMR) is one of the Global Health challenges of the 21st century. The inclusion of AMR on the global map parallels the scientific, technological, and organizational progress of the healthcare system and the socioeconomic changes of the last 100 years. Available knowledge about AMR has mostly come from large healthcare institutions in high-income countries and is scattered in studies across various fields, focused on patient safety (infectious diseases), transmission pathways and pathogen reservoirs (molecular epidemiology), the extent of the problem at a population level (public health), their management and cost (health economics), cultural issues (community psychology), and events associated with historical periods (history of science). However, there is little dialogue between the aspects that facilitate the development, spread, and evolution of AMR and various stakeholders (patients, clinicians, public health professionals, scientists, economic sectors, and funding agencies). This study consists of four complementary sections. The first reviews the socioeconomic factors that have contributed to building the current Global Healthcare system, the scientific framework in which AMR has traditionally been approached in such a system, and the novel scientific and organizational challenges of approaching AMR in the fourth globalization scenario. The second discusses the need to reframe AMR in the current public health and global health contexts. Given that the implementation of policies and guidelines are greatly influenced by AMR information from surveillance systems, in the third section, we review the unit of analysis (“the what” and “the who”) and the indicators (the “operational units of surveillance”) used in AMR and discuss the factors that affect the validity, reliability, and comparability of the information to be applied in various healthcare (primary, secondary, and tertiary), demographic, and economic contexts (local, regional, global, and inter-sectorial levels). Finally, we discuss the disparities and similarities between distinct stakeholders’ objectives and the gaps and challenges of combatting AMR at various levels. In summary, this is a comprehensive but not exhaustive revision of the known unknowns about how to analyze the heterogeneities of hosts, microbes, and hospital patches, the role of surrounding ecosystems, and the challenges they represent for surveillance, antimicrobial stewardship, and infection control programs, which are the traditional cornerstones for controlling AMR in human health.
Keywords: antimicrobial resistance, disease ecology, syndemic, antimicrobial stewardship, lateral public health, social health, global health, one health, ecosystem, metasystem
1. Antibiotic Resistance in the Global Healthcare Network: A Multifaceted Problem Involving Many Stakeholders of Disparate Sectors
Antibiotic resistance (AMR, see Appendix A for definitions of words in blue-italics) is recognized as one of the major Global Health challenges of the 21st century [1]. The inclusion of AMR in the global health map parallels the scientific, technological, and organizational progresses of the healthcare system and reflects the colossal socioeconomic changes of the last 100 years. Studies originating in various fields have examined particular aspects of the AMR problem, focused on patient safety (infectious diseases and infection control) [2], transmission pathways, and pathogen reservoirs (molecular epidemiology) [2,3,4], the extent of the problem at a population level (public health) [5,6,7], their management and cost (health economics) [8,9,10,11], behavioral aspects related to infection prevention, management, antibiotic prescription and antibiotic use (social sciences) [12,13], antimicrobial pollution (ecotoxicology and biodiversity) [14], and episodes associated with historical events (history of science) [15,16,17,18]. However, we lack an essential dialogue between all these aspects that have an important role in the development, transmission, and evolution of AMR. This work discusses the challenges of AMR in the global healthcare system, considering the heterogeneities of its (ecosystem) structure, the instruments employed to detect and monitor AMR, and the disparities and similarities of the objectives of distinct stakeholders. It also describes the changes in the theoretical framework and the effects of knowledge fragmentation in interpreting the AMR information throughout history.
1.1. The Modern Healthcare System: A Story of “Social Construction” with an Impact on AMR
Between the end of the 19th century and the early 20th century, medical treatment moved from providing care in particular houses, philanthropic organizations, religious charities (including primitive hospitals and quarantine stations), and ad hoc foundations for particular diseases to care centered in advanced hospitals [19]. This change was driven by industrialization and rapid urbanization which dramatically increased the numbers of poor, wounded, and ill people who had moved from the countryside to the cities and transformed charitable actions into a public health issue. The need for therapeutic solutions to counteract major epidemics of infectious diseases led to the birth of the “chemotherapy era” with the introduction of arsphenamine (Salvarsan) in 1910, sulfonamides in the 1930s, and natural antibiotics in the 1940s [15,16,18]. Advances in the pharmaceutical sector during these decades (such as the adoption of production models from the food industry, company alliances, and strong licensing restrictions by large Western companies) would influence the further development, production, and consumption of antibiotics in Western societies [20,21,22,23]. The birth and colossal development of health industries (hospital infrastructure and health services), communication media, and transportation, enabled the rapid, generalized, and massive therapeutic and prophylactic use of antimicrobials in hospitals and community settings. World Wars (WW) occurring between the “first globalization” (second half of the 19th century to 1910) and “the second globalization” (1945 to 1990) also triggered the development of logistics to control infectious diseases epidemics and illness in international campaigns, which included mass drug administration of antibiotics and antiseptics to troops [16]. The serendipitous discovery of the effects of antibiotic byproducts (vitamin B12 coproduced with streptomycin, aureomycin derived from tetracycline) on animal growth by the early 1940s drastically changed the farming sector and food industry and widened the big pharma business sector [17,18,24,25]. By the end of WWII, the global human population had directly and/or indirectly been exposed to diverse antimicrobials and antiseptics for decades [15,16]. Soon after, the global spread of multidrug-resistant (MDR) organisms was a reality [26,27].
The second (1945–1989) and the third globalization (1989–2008) waves reflect two major global socioeconomic timeframes that greatly impacted the healthcare system and AMR in different ways. The bimodal economy models established after WWII and represented by the free markets from Europe and America, and the centralized planning markets from the Soviet Union and China resulted in major differences between the health sectors (and other industrial sectors) of the countries aligned with these two models. The 1978 Alma-Ata declaration’s vision for societal health pinpointed the need to reorientate health systems toward primary care to address the social and environmental determinants of health and inequality [28]. The Alma-Ata declaration used the definition of health given in the 1948 United Nations declaration [29], which added a political dimension and the need to involve various sectors to counteract health inequalities (a change from the traditional vertical public health perspective towards horizontal “health promotion”). However, this interpretation of health has not been fully understood by major health stakeholders [28]. The end of the Cold War and the birth of the World Trade Organization in the 1990s (with the late inclusion of China in 2002) meant that the breakdown of national barriers to trade and migration, the generalization of international traveling, and the implementation of the internet provided unique opportunities for global expansion of hosts, food, goods, and microbes [14,30,31,32,33]. In 2005, the Organization for Economic Co-operation and Development (OECD) alerted the public to the involvement of economic growth sectors in the problem of AMR and the need for intersectoral cooperation at a global level to fight the AMR problem [34]. A few years later, the “O’Neill reports” highlighted how AMR could influence emergent economies, grouped under the terms BRICS (Brazil, Russia, India, China, and South Africa) and MINT (Mexico, Indonesia, Nigeria, and Turkey) [10,35].
The fourth wave of globalization (the Fourth Industrial Revolution, 4IR) brought novel challenges, such as the rapid emergence of ecological threats, the rising of the absolute number of the human population (with a global increase in the elderly), the increased social atomization, the political polarization, and social inequality [36]. In 2018, the Astana declaration (Alma-Ata 2.0) emphasized once more the need to reorient health systems toward primary care to provide universal health coverage instead of hospital-focused systems or low investment in health [28]. The novelty of the Astana declaration was to link health with the achievement of sustainable development goals (SDGs) [28]. AMR is also linked to SDGs by the quadripartite World Health Organization (WHO)–Food and Agriculture Organization of the United Nations (FAO)–World Organization for Animal Health (OIE)–United Nations Environmental Programme (UNEP) [37,38].
Other than inequalities, the current social atomization also impacts the increasing knowledge fragmentation, the loss of a comprehensive vision of the sociocultural frame, and the rapid obsolescence of social habits that greatly influence the vision and the way to approach health and scientific questions [36,39,40].
1.2. The Understanding of AMR in the Healthcare Network: From Disease Ecology to Evolutionary Biology and Social Sciences
AMR has been analyzed through the lens of different disciplines and conceptual frames. The belief that infectious diseases could be eradicated by novel “miracle” drugs was soon replaced by increasing evidence that unnecessary antibiotic use contributes to the evolution of infectious pathogens. This warning, originally raised in the mid-1940s by René Dubos [41,42], contributed to the ecological understanding of infectious diseases’ causation, now adding chemotherapeutic agents and technological advances of modern medicine to other environmental factors that have forced bacterial adaptation. Dubos was the first to conceive of microbial evolution within a large-scale and long-term scenario and greatly influenced clinical microbiologists and infectious disease physicians after the publication of his seminal book, The Mirage of Health [43]. They started to apply disease ecology principles to short-term and local events derived from AMR in hospitals and farms. Early data collection noted a change in the causal agents of hospital infections, now commensal opportunistic pathogens, such as staphylococci, Gram-negative microorganisms, and fungi [44,45], which often become resistant to multiple antibiotics. Early studies have also shown the vulnerability of hospitalized infected patients [45,46,47] and technology’s role in creating novel ecological niches that favor the unforeseen and abrupt emergence of infections [47,48]. Adopting the disease ecology perspective for infectious diseases led to hospitals being considered as environments and patients as structured populations. Standardization of the hospital infrastructure and medical practices during these early years in the US constituted the first step of current “evidence-based medicine”. However, these standards would not be employed by all medical practices or implemented at a global level until the 1980s and 1990s [2].
The swift escalation of antibiotic-resistant clinical isolates, especially after the global epidemics of penicillin-resistant Staphylococcus aureus in the 1950s, followed by a continuing endemic situation, motivated the arrival of public health-based and population-based principles to improve hospital hygiene performance in the United Kingdom and the United States by the mid-1960s [49]. Hospital hygiene became a discipline, and large hospital-acquired infection (HAI) control programs based on a risk-factor analysis and antibiotic stewardship policies (restriction of antibiotic use and treatment based on laboratory susceptibility information) were implemented [50,51,52]. More importantly, it meant that hospitals were defined as communities in which public health principles could be used to prevent and control HAIs [52].
By the late 1970s, the medical community had realized how rapid bacterial adaptation could occur through plasmids as vectors of antibiotic resistance genes (ARGs), enabling AMR to cross species and borders [18,26,27]. Although AMR started to be formally discussed at WHO during the mid 1970s [53,54], what placed AMR in the global scene were the initiatives led by Stuart Levy a few years later. At the closing session of the “Molecular Biology, Pathogenicity, and Ecology of Bacterial Plasmids” conference he organized in Santo Domingo, Dominican Republic, in 1981, over 200 clinicians and scientists from 27 countries signed a joint “Statement Regarding Worldwide Antibiotic Misuse” where AMR was firstly categorized as a “worldwide public health problem” and awareness of the consequences of antibiotic misuse at all levels of usage were firstly highlighted [55]. This seminal statement led to the foundation of the Alliance for the Prudent Use of Antibiotics (APUA), a global nonprofit organization with the common concern and vision of educating the medical profession and public alike about appropriate antibiotic use, promoting the global uniformity of regulation regarding antibiotic sales and labeling, and turning attention to the need for more standardized and coordinated surveillance of AMR [56]. Then, AMR began to be defined as a “global” (pandemic) and “ecological” problem [33,57,58]. Moreover, at a conference in Paris in 1988, Joshua Lederberg first included the evolution of AMR in a new category he named “emergent diseases” [59], in part to recognize and recall the landmark contribution of René Dubos’s ideas to the fields of AMR and disease ecology. Although this categorization primarily highlighted the epidemiological connotations of AMR, it also referred to the rapidly evolving processes followed by long-term changes in bacterial ecology.
Until the 1990s, the control of infectious diseases had been prioritized over analyzing the biological evolution of microorganisms. During this decade, we witnessed the arrival of molecular epidemiology and “-omics” developments and the adoption of a population genetics lens to recognize the impact of various units of selection (multilevel populations) in the evolution of AMR [40,60]. These advances brought an explosion of descriptive genomic studies that exhaustively addressed the ubiquity and diversity of species and ARGs and also revealed the AMR transmission pathways between and within the One Health sectors with impact on the way to face the control and prevention of AMR [3,4,61,62]. Afterward, the analysis of AMR in microbiomes and the birth of the resistome concept [63,64] changed the way of defining and approaching AMR (Table S1). Variations in microbiome composition were recently associated with the host’s susceptibility to antibiotics and to pathogen translocation/invasion [65,66]. Moreover, the application of an ecological framework is promising for understanding host–microbiome interactions at different scales (living and abiotic environments) and, thus, selection and transmission events [67]. However, it implies the revision of some of the Henle-Koch’s postulates, such as the causality of infectious diseases by a single “etiological organism” [68,69]. In a formal epidemiological sense, alterations of the microbiome, but also hereditary traits and immunity, act as confounding variables on the expression of an infectious organism.
Most of the studies published during the 1990s and 2000s were disconnected from the control of the disease, which led to the categorization of this time as “the obscure era for infectious diseases” [2,70], despite the great advance in basic knowledge they provided on the ecology and evolutionary pathways of microbial entities. In the 2010s, many voices claimed the inclusion of evolutionary biology as a basic science for medicine [71]. Nonetheless, ecology remains to be essential to understand the fundamentals of disease in the era of the microbiome [67,72,73,74]. Multi and transdisciplinary approaches taking into account social sciences are increasingly demanded to understand and face Global Health challenges, including AMR [13,75,76,77].
1.3. AMR at the Healthcare Network in the 21st Century: Novel Challenges in a Global World
Socioeconomic changes in the 20th century have contributed to abruptly modifying the fluxes of humans, animals, goods, and microbes that affect the emergence, transmission, and burden of AMR at the local, national, regional, and global levels [14,30,31,32,78]. Human migrations and/or international travel play an important role in the global spread of MDR bacterial strains of certain species, such as Mycobacterium tuberculosis [79], Salmonella typhi [80], or Enterobacteriaceae resistant to third-generation cephalosporins, carbapenemases, colistin, or fluoroquinolones, which are easily recognized when introduced into low-incidence countries [81,82,83]. Changes in social, individual, and collective habits influence issues, such as antibiotic use [12] or the emergence and transmission of novel threats (e.g., Shigella in HIV-negative men who have sex with men) [84,85]. Novel food habits have also triggered the mass production of food animals and the use of antibiotics in veterinary and affect the rates of AMR at a local level [86,87]. Demographic changes, including the rise in elderly and life expectancy rates, are predominantly affected by infections caused by MDR strains of commensal opportunistic pathogens [88]. Moreover, pandemics of both non-communicable and communicable diseases [77], emerging infectious diseases, and other global health challenges are now part of a colossal global syndemic landscape [75,89,90,91,92] in which the altered conditions of a particular ecosystem are readily (and synergistically) transmitted to others. The results of such interactive globality modify antimicrobial usage, infection control programs, and transmission pathways that affect the emergence, transmission, and burden of AMR in healthcare centers and the community [93,94,95]. Today, AMR disproportionally impacts novel disparate vulnerable populations (e.g., children, the elderly, hematologic-oncologic, immunocompromised, or undernourished populations, migrants, and citizens with inadequate sanitation infrastructures, or those in war zones) who require medical care, drug therapy, and/or chemoprophylaxis to prevent or control different infectious diseases but with disparate access to antibiotics and health coverage [94,96,97]. Climate change is predicted to increase the rate of human infections, particularly those caused by MDR pathogens [98,99,100].
In summary, interactions between heterogeneous microbial communities vary in non-additive ways that may influence pathogen invasion and persistence at different spatial levels [74,78]. The estimation of the relative contributions of heterogeneities across scales (hosts, pathogens, environmental-healthcare patches, systemic and institutional drivers) determining pathogen transmission and persistence [101] is a pending issue of disease ecology and, thus, for the adoption of appropriate measures to combat/control infectious diseases and AMR.
2. Reframing AMR and Infectious Diseases in a Global Syndemic Scenario
AMR has been a public health priority for decades. In 2019, the WHO included AMR as one of the top ten threats to global health [1]. Today, all international organizations stated that AMR should be approached from the perspectives of One-Health and Global Health [35,38,61]. However, the implementation of efficient measures in different socioeconomic and geopolitical timeframes remains a major challenge.
2.1. Public Health to Control AMR in a Global Syndemic Scenario
Public health principles have been applied to prevent and control HAIs and AMR in the healthcare network [52]. Surveillance, antibiotic stewardship, and hygiene control programs have been the cornerstones to mitigate these challenges. Surveillance shows limitations related to the difficulties of the diagnostics to validate endpoints (e.g., definition and interpretation of HAIs cases, the effect of underlying conditions, etc.) (see Section 3 and Section 4.1). The COVID-19 pandemic has fueled the AMR global crisis due to “the overuse of antibiotics to treat COVID-19 patients, disruptions to infection prevention and control practices in overwhelmed health systems, and diversion of human and financial resources away from monitoring and responding to AMR threats” [102]. Concurrent infectious diseases or pandemics would have similar effects [43,54,55]. This “novel” reality represents a change from the traditional vertical public health perspective focused on reducing the spread of single pathogens towards horizontal “health promotion”, also accounting for socioeconomic and environmental factors [3]. More transversal programs (coined as “Lateral Public Health”) to enhance the alerts between healthcare centers and community hotspots by surveillance of vulnerable populations in community health centers (CHCs) would help to meet challenges in the 4IR [75,103]. The influence of the socioeconomic and cultural context in behaviors on antimicrobial stewardship and use of healthcare systems (e.g., healthcare-seeking behavior, financial reimbursement systems, institutional quality management, governmental incentive systems, regional hospital network structures, and hospital referral practices) impact the implementation of public health measures and, thus, the prevalence and diversity of HAIs and the transmission of AMR pathogens. A scientific approach to explore and understand the influences of these factors is starting to be adopted [13,104,105,106].
2.2. AMR and Health, One-Health, and Global Health
The term “One Health” focuses on the risk assessment of AMR’s emergence, transmission, and maintenance at the interface between humans, animals, and their related environments. The One Heath sectors comprise heterogeneous populations with varying interaction intensities and levels of agency [61,78,107]. Various studies in Western countries have demonstrated distinguishable transmission cycles in each One-Health segment [4,108] with much less cross-sector impact [3,4,109,110]. More specifically, AMR transmission pathways predominantly occur within “hothouses” (e.g., hospitals or farms) or associated networks (e.g., primary-secondary-tertiary healthcare levels), which accumulate some heterogeneous and synergistic risk factors, such as crowding, selective pressure, vulnerable populations, environmental reservoirs of MDR pathogens, or transmission vectors, such as chronic patients or medical workers with high (inter) healthcare exposure. Wastewater treatment plants are increasingly recognized as AMR hotspots [38,111] (Figure 1).
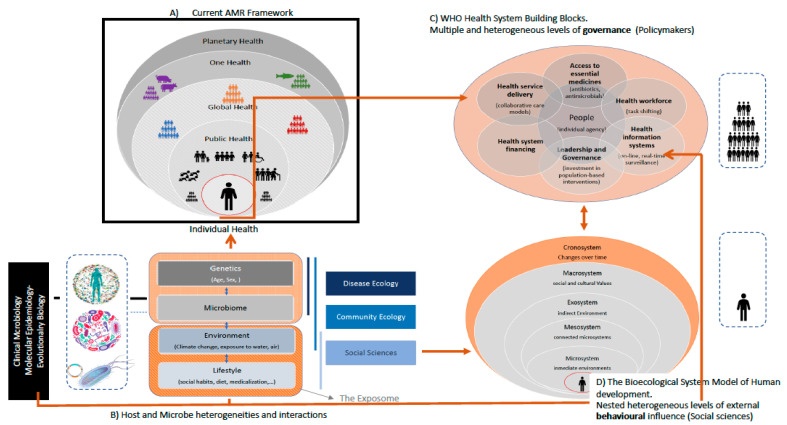
Figure 1.
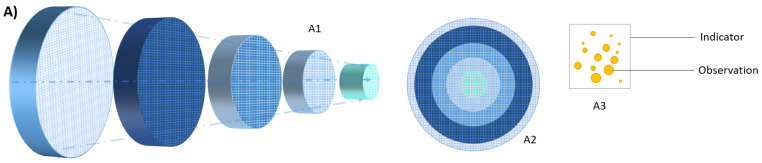
A framework for antimicrobial resistance in the healthcare network of the 21st century. A multidisciplinary approach for the analysis of AMR in a host metasystem landscape. (A) The current framework to approach health and global health challenges, which includes antimicrobial resistance (AMR) and pandemics [61]. (B) Multidisciplinary approaches to analyzing host and microbe heterogeneity and their interactions in the context of individual human health, which is influenced by intrinsic individual traits (e.g., genetics and physiology) and the exposome (exposure to environment/s, social habits, and contact with abiotic and non-abiotic entities). Individual microbial heterogeneity at sub-specific (gene, plasmid, clone) and supra-specific (microbiome) hierarchical levels is the focus of clinical microbiology and molecular epidemiology; host-microbe interactions and dynamics are analyzed by disease ecology and community ecology. (C) The WHO Health System Building Blocks framework, which was developed to promote a common understanding of the health system [112]. This is relevant for public health investments and results to feed global decision-making. (D) The Bioecological System Model of Human development. It establishes different levels (systems) of exposure to social groups [113,114]. These levels overlap those of microbial exposure. Orange-colored areas represent the influence of time in all systems (human, microbial, individual species, and institutions). Brown arrows represent connections between the various levels. Dotted boxes reflect the central targeted unit, namely humans, in (A,D); human groups in (C); and microbes and hosts in (B).
The social and economic inequalities triggered the adoption of universal plans and policies under the “Global Health” umbrella [28,37,62]. However, the 20th century’s general improvements in health, such as care systems, infrastructures, or governance resulted in byproducts of social prosperity and inclusion (e.g., increased life expectancy and changes in the health care system focused on the elderly or on pediatrics) has given way in the 21st century to narrow approaches based on targeted interventions and narrow international aid (e.g., “mass drug administration programs”, support to war conflicts, and humanitarian health assistance). Moreover, the current global economic system prioritizes “wealth” over “health”. In fact, the effectiveness of interventions and analysis of AMR outcomes are usually expressed in cost by different official bodies, such as the Centers for Disease Control and Prevention (CDC), European Centre for Disease Control and Prevention (ECDC), OECD, WHO, World Bank, and social independent corporations, such as the ReACT group RAND Corporation (Research ANd Development) [8,34,90,97,115,116,117]. Again, this is probably influenced by the misinterpretation of the health definition that tends to indistinctly consider “disease care” and “health care” [91].
2.3. Ecosystems and Their Feedback Loops: The Environmental Dimensions of AMR
AMR control is heterogeneously influenced by the One Health sectors, their associated economic growth groups, and their loops (Figure 1 and Figure S1). The time of variation within each compartment or ecosystem is linked to changes in the behavior and culture of these human communities [113,114]. According to “the biological ecological model of human development”, the temporal scale of changes decreases from microsystems (such as interaction within families or friends) to medium-sized ecosystems (mesosystems, such as hospitals or workplaces), and larger ecosystems (macrosystems, mostly involving activities, such as immigration, tourism, or changes in the food or pharma industry) [113,114]. The “sustainability and management ecosystems” are based on the control of others (metasystems, including social norms, such as family status, professional/educational status, culture, behavior, cultural norms, beliefs, and religious practices), policies and interventions, and economic structure [118]. These ecosystems are not only linked by a hierarchical structure, but they continuously change—each dependent on the others—linking in-loop dynamics of health, business, governance, and ecological activities. Indeed, the outcomes of such dynamics are directed toward improving human and environmental health and well-being, scientific development, economic prosperity, and social equity. In all these ecosystem dimensions, AMR plays a significant role, and, in turn, all of them influence AMR’s evolution.
Other than the need to include the socioeconomic and cultural context in planning public health interventions mentioned above, the time dimension, barely considered in previous operational conceptual frames, will be a key factor for planning and decisionmaking. Muti-level ecological systems considering social traits, including age and family structure, space and time location of individuals, and social dynamics, in general, have been included in the computational simulation of AMR evolution [119].
3. Challenges in Measuring AMR in the Heterogeneous Healthcare Network
AMR information is mostly provided by hospital records that also feed local and regional surveillance systems. Correlation of these data with demographic information from whom the pathogens are isolated (to date, mostly humans and mostly inpatients) offers insight into the underlying epidemiology and facilitates the formulation of rational interventions aimed at reducing the burden of AMR (diagnosis, treatment, prevention) and its monitorization [56]. The available information on AMR came from relatively few sources using similar indicators, methodologies, and models for disparate purposes (“a one-for-all surveillance system”). Some studies have categorized the various surveillance programs according to their scale, scope, and structure [120,121,122,123,124]. However, a reflection on the validity and reliability of the AMR information to be applied in various healthcare (primary, secondary, tertiary), demographic, and economic contexts (local, regional, global, and inter-sectorial levels) has been neglected to date.
“Validity” (or “construct validity”) defines how well a test, indicator, or experiment measures up to its claims. It refers to whether the operational definition of a variable actually reflects the true theoretical meaning of a concept. In other words, does the indicator or the instrument measure what it is supposed to measure? “Construct validity” comprises all other types of validity evidence, such as content validity, instrument validity, and criterion validity. “Reliability” (or reproducibility) in statistics refers to consistency in measurement: the capacity to produce the same result for two identical states; or, more operationally, the closeness of the initial estimated value(s) to the subsequent estimated value(s) (see Section 3.3).
This section defines the unit of analysis and indicators used in AMR and discusses the factors that affect their validity and reliability (Figure 2).
3.1. The Sample (The Unit of Analysis)
Microbial infectious diseases and AMR disproportionally impact populations and settings, and the “who” or “what” that might occur needs analysis. The units of analysis in AMR are human groups (such as single individuals, social, professional, or health-risk groups), healthcare settings (tertiary, secondary, primary, or single wards), and economic sectors (One Health, economic growth sectors) [125].
Age, sex, body health condition, genetics, and the exposome influence host susceptibility, host immunity, and social behavior [113]. The classical high-risk populations prone to acquire AMR are individuals with severe underlying diseases, immunocompromised conditions (children and elderly), living in poor socioeconomic structural environments, or significantly exposed to factors that increase the risk of selecting or spreading AMR (e.g., invasive procedures). In low- and middle-income countries (LMICs), risk groups for AMR also include those enrolled in mass drug administration programs to prevent infectious diseases, such as children younger than five years (to prevent trachoma or group B streptococci infections), HIV contacts, or those affected by respiratory and enteric infections [125,126,127]. Some individuals can be responsible for a large number of secondary contacts (“superspreaders”), although the factors beyond this phenomenon remain unclear [128,129].
The risk of acquiring and spreading AMR also varies at different healthcare levels. Traditionally, populations are analyzed at tertiary and secondary healthcare centers (hospitals and long-term care facilities), where the concentration of the high-risk groups of people and online diagnostic and life-sustaining equipment (such as ventilators), indwelling catheterization, and frequent use of drugs facilitate the emergence and local evolution of AMR. Within hospitals, some wards concentrate on groups and factors at higher risk than others [130,131]. Primary healthcare is greatly influenced by structural, governance, and management networks [105,106,132]. AMR in health system loops and One Health sectors has recently begun to be analyzed [133,134] (See Section 3.2 and Section 4).
3.2. The Indicators
In AMR surveillance, indicators (also called Operational Units of Surveillance, OUSs) [120] refer to the percentage of pathogens resistant to a particular antibiotic (pathogen–antibiotic pairs) at particular sites; the proportion of AMR-HAIs cases in population-based studies as markers for significant emergent threats; and the density of antimicrobial use (AMU) and/or consumption (AMC) as drivers of AMR. Hospital laboratories identify the microorganisms of any clinical sample they receive and have access to hospital AMC/AMU information. Surveillance programs at local, regional, global, or sectorial levels are fed with the OUS’ information (sentinel species) from non-categorized patients in large hospitals and with AMU/AMC from selected institutions/departments [121,122,135].
Sentinel microbial species. Only a few bacterial and fungal species acquire and successfully spread ARGs of medical relevance, following the Pareto principle (80% of consequences come from 20% of causes), as occurs for causal agents of many infectious diseases [125]. These species constituted the “watch lists” or “priority lists” of antibiotic threats of the WHO and the CDC, which only include a few traditional causes of major “classical” human infectious diseases (Neisseria gonorrhoeae, M. tuberculosis, Streptococcus group A, and Streptococcus pneumoniae), all affecting specific risk groups of community-based people and often associated with syndemic processes affected by social structures. The lists also included some commensal opportunistic pathogens, such as Enterobacterales, enterococci, and staphylococci, or environmental organisms (Pseudomonas aeruginosa and Acinetobacter baumannii). Recently, the Division of Healthcare Quality Promotion (DHQP) at the CDC reported that more than one fifth of HAIs are non-Legionella water-related opportunistic pathogens and warned of a possible major underestimation of these numbers [136]. The DHQP comprised multidrug-resistant Enterobacterales already included in the above-mentioned watch lists and other increasingly reported opportunistic pathogens (e.g., Elizabethkingia, Achromobacter, and Burkholderia) able to cause significant hospital outbreaks [137]. What all these prioritization guidelines reflect is a healthcare landscape comprising multiple patches of microbial species and host populations and the neglected analysis of the “hospital-built environmental patches” (sinks, wastewaters) in the emergence, transmission, and persistence of AMR [138].
None of the available “watch lists” consider the heterogeneity within a species. Again (Pareto’s principle), only a limited number of specialized lineages within each species significantly contribute to hosting and transmitting AMR. These have been identified as high-risk clonal complexes (HiRCCs) [139,140,141] or “pandemic clones” [142], which are operational designations helping target precision and preemptive interventions (e.g., control hospital outbreaks and prevent infections in the individual). However, the increased percentage of healthy human carriers of HiRCCs [143,144] has raised the possibility that these populations (even before acquiring AMR) are a natural part of the commensal flora, which can be spread to relatives when the population size increases, and the shedding is high [145]. The increase in number and exposure to various hosts and environments necessarily tends toward an increase in internal genetic diversity, which includes the emergence of hybrid lineages, such as ST258 Klebsiella pneumoniae, ST239 S. aureus [146], or EHEC Escherichia coli [147], or “jumps” of AMR bacterial lineages between humans and non-human hosts [109]. Both diversity within clonal complexes and clonal fluctuations with periodic emergences of new genotypes enable a permanent bacterial diversity that makes it difficult to rank and predict the risks of critical sub-populations. Genomic typing to track AMR and outbreak investigations is progressively implemented in national and international surveillance programs [148]. It demands a high level of agreement between genotypes and phenotypes, which seem to work for the most common pathogens and antibiotics included in the WHO’s and CDC’s “watch lists” [149].
Genes, plasmids, and mobile elements. ARGs can be rapidly spread across bacterial species, genera, or microbial kingdoms of various human, animal, and environmental backgrounds through transposable elements, often embedded in plasmid entities [78,150,151,152]. Once again, only a few members of plasmid, transposon, integron, or insertion sequence families are responsible for the spread of ARGs [151,153,154]. However, its diversification (e.g., F plasmids in Enterobacterales) [153,155,156,157] and promiscuity (e.g., carbapenemase encoding genes and the mobile genetic elements in which they are located) [158,159] often result in mosaic elements that difficult the choice of diagnostic tools, the identification of transmission pathways, the implementation and monitoring of interventions to target AMR plasmids. There are plenty of unresolved questions that require a deeper understanding of the dynamics of ARGs and plasmids in microbiomes beyond the analysis of every single entity [160].
Microbiomes and resistomes. Although not included in any surveillance program, metagenomics-enabled surveillance methods offer the opportunity to improve the detection of both known and yet-to-emerge pathogens [161]. The analysis of the intestinal and respiratory microbiomes and the resistomes may also allow clarifying fundamental questions, such as the host’s susceptibility to infection, therapeutic regiments, fecal transplantation, long-term AMR colonization, or health response to antimicrobial disturbances [65,162,163,164,165]. Other relevant and unexplored aspects are related to the microbiome of the hospital-built environment, an area of increasing interest in research agendas [166,167]. Unmet questions include the distribution of microorganisms between hospitalized patients and the “ward/room environment”, or the impact of the “hospital-built environmental microbiome” on patient health and indoor and outdoor environmental contamination [66,168,169]. Knowledge in this area is still scarce, and the tools and instruments are insufficient for acquiring valid and reliable information [170,171].
Antimicrobial consumption (AMC) and antimicrobial use (AMU) reflect how antimicrobials are dispensed and prescribed. Although they are often used interchangeably, they measure different things. AMC reflects the volumes of antimicrobials distributed (e.g., at a hospital or pharmacy), and AMU tells us how antimicrobials are used at the patient level (e.g., what diseases/pathogens are targeted, routes of administration). AMC/AMU data are primarily relevant in combination with local AMR trends and the preparation of antibiotic stewardship guidelines. Protocols and tools to properly collect AMC/AMU information are currently being debated [130,131,132]. Nonetheless, AMU data are still unavailable in most countries and hospitals, and antimicrobial exposure is generally reflected as AMC [94]. Surprisingly, only a few guidelines clearly state which antibiotics should be routinely monitored. A consensus list based on the antibiotics most used or included in the “Watch” and “Reserve” categories of the WHO’s Essential Medicines List AWARE index has been suggested to harmonize information [131,132].
AMU/AMC variations and shifts at local levels (i.e., outpatient stewardship) and regional levels reflect cultural and socioeconomic differences or secondary public health problems [13,94]. For example, cephalosporins and broad-spectrum agents, such as fluoroquinolones, macrolides, and second-line agents, such as oxazolidinones, have increased in LMICs and decreased in high income countries (HICs). The dramatic increase in the use of cephalosporins is due not only to economic growth, but also to changing prescribing practices for respiratory tract infections, skin and soft tissue infections, gonococcal infections, and enteric fever, in which cephalosporins have replaced penicillins and quinolones for infection management due to rising resistance [90]. Such differences in AMC affect the selection, emergence, and further transmission of AMR between distant geographical areas. Counterfeit and substandard antibiotics represent up to one third of the available pharmaceuticals in LMICs (42% of all reports received by the WHO Global Surveillance and Monitoring System on substandard and falsified medicines worldwide came from Africa, most corresponding to antibiotics and antimalarials) [32,172]. Of special concern is the scope of hospital and outpatient drug stewardship programs because many non-antibiotic drugs have an antibiotic effect and can contribute to the selection of MDR (bystander selection) [163,173,174] (Figure 2).
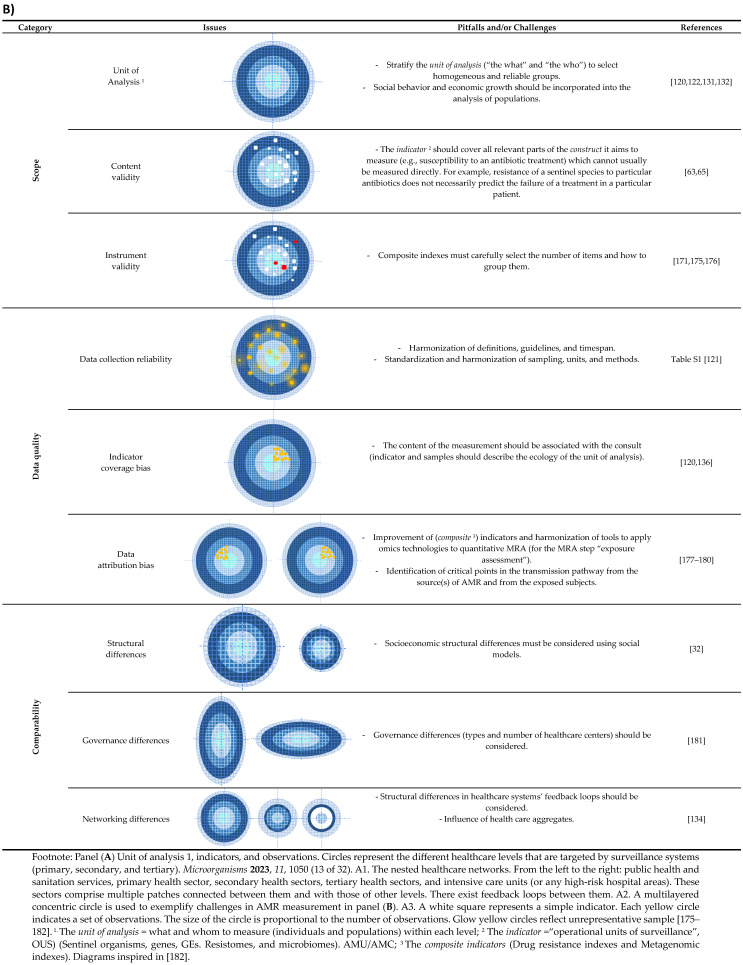
Figure 2.
Challenges of AMR measurements. The validity, reliability, and comparability of the information [175,176,177,178,179,180,181,182].
3.3. Validity, Reliability, and Comparability of the Information
All AMR stakeholders use different indicators for their analysis. The pitfalls of multi-indicator analysis can be grouped into the categories of scope, data quality, and comparability (Figure 2). An evaluation of the suitability of multi-indicators in decision making has been neglected for the AMR field, although it is widely used in social sciences.
Scope issues. The “validity” of the information. The scope issues tell us the extent to which we are able to collect the appropriate indicators (content validity) to analyze various individuals, populations, or setting levels (units of analysis) and in a balanced and discriminated way (instrument validity). Although AMR surveillance employs different indicators/ OUSs to describe trends for a specific unit of analysis (individual patients, populations, healthcare institutions, and settings), traditional OUSs information is often too narrow to consider the full richness of such a context. AMR composite indicators are needed to analyze the population (e.g., resistome and microbiome indexes) [171,175], settings (e.g., “drug resistance index”) [176], or different sectors [133]. A clear prior conceptual understanding of the indicators is necessary to avoid redundancy (hierarchy), disbalance, or coverage bias. The ratio of sample size/number of indicators is a sensitive issue. The development of such indicators is still in its infancy.
Data quality issues. The reliability of the information relies on the quality of the underlying data. Variations in the unit of analysis, indicators, measurement methods, definitions (e.g., AMR, see Table S1), guidelines (e.g., European Committee for Antimicrobial Susceptibility Testing vs. Clinical Laboratory Standards Institute antibiotic susceptibility breakpoints), and/or the application/use of an indicator greatly influence the quality of the information and preclude its comparability. For example, current estimations on AMR trends (in terms of the impact of AMC) or disease outcomes (HAI mortality predictions) came from mathematical models using epidemiological counts that do not consider disparities in the incidence or prevalence of infectious diseases or the antibiotics used [117]. Temporal series also influence data quality. Long-term series may be dotted with changes in definitions and guidelines that occur during the study (this is especially relevant when historical series should be analyzed or compared). This confusing trend might increase in the near future, given that new pharmacodynamic parameters will be considered to complement the minimal inhibitory concentration [183].
AMR information is frequently valid and reliable, but it is greatly biased by the structure of the dataset (indicator coverage bias). This bias often occurs for AMR in the health sector if the ecology or physiopathology of the sentinel organisms does not match that of the unit of analysis. Blood and cerebrospinal fluids are predominantly screened in AMR surveillance, not only because of their clinical relevance but because this choice prevents some of the inconsistencies that arise from differences in clinical cases, different sampling frames, or heterogeneous healthcare utilization. However, invasive isolates might not represent isolates of the same bacterial species producing other infections. In addition, variations in blood culture frequency (non-differential sampling vs. differential sampling, linked to diagnostic practices and the frequency with which blood cultures are taken) result in an increasing uncertainty when comparing resistance percentages between hospitals, CHCs, and other settings. On the other hand, severe infections are uncommonly reported in CHCs, such as LTCFs, where monitoring AMR usually uses urine or respiratory samples [184]. (See also sentinel organisms in Section 3.2).
Finally, the assignation of an observation to a particular class (data attribution) is one of the most important challenges in AMR and infectious diseases that has traditionally been approached by microbial risk assessment [178,180]. Such an association is not always obvious. Observations can be attributed to wrong classes because the most adequate class is still unknown or to multiple causes. Research on AMR makes inferences at the level of bigger aggregates, such as healthcare networks, countries, geographical areas, One Health sectors, or industry/economic sectors. Practical problems with MRA include identifying the number of observations needed (e.g., critical points in the transmission chain) and how to group them under these aggregates, which is problematic if they are cross-border [133,134].
Comparability issues. The “structural” information. The comparison of the unit of analysis should take into account (socioeconomic) structural, governance, and specialization differences, which are conceptually linked. Socioeconomic structural differences have been pointed out as affecting AMR [35,96,185,186]. The O’Neill report highlighted how AMR would affect LMICs, especially BRICS and MINT, a term grouping some countries according to their economies. However, they differ in their underlying socioeconomic structures that are not considered in any analysis [35,185].
Healthcare systems also have relevant semi-structural differences in terms of the structure and functioning of countries. Only the OECD statistics provide useful information for understanding the dynamics of the healthcare business and hospital systems in various countries; however, it is restricted to the last three decades [19]. The availability of beds per hospital normalized by 1000 persons, the most used indicator, revealed a broad variety of systems (e.g., a high density of small hospitals in Asian HICs vs. a small group of large hospitals in Western countries) with a major impact on the diffusion of medical technology and healthcare consumption. The impact of these structures has not been evaluated.
Specialization/networking differences. In addition to the structural and governance differences, the units of analyses can also differ due to e.g., patch dependency and feedback loops. Properties that define complex systems are emergence, feedback, and adaptation. Such properties have been analyzed in the context of “microbial complex systems” [7,151] but not in the context of “social or economic systems” that influence microbial fluxes. Emergence describes the properties of the system but cannot be inferred from the elements that comprise it (e.g., heterogeneity in AMR distribution can be conceptualized as an emergent property of the healthcare system, but also the pharma, food, transport, economic, and other systems). Feedback describes the effect of a change on further changes [134]. Finally, adaptation refers to the adjustments in behavior in response to intervention (e.g., restriction in antibiotic availability triggers the “black antibiotic market” and the trade of counterfeit, substandard, or old antibiotics in LMICs). Tools for approaching “complex public health systems” are still in their infancy [91,107,187] (Figure S1).
4. Heterogeneity of Stakeholders Involved in the Global Health Network: Activities, Objectives and Challenges
AMR research and policies have involved many stakeholders with disparate interests and objectives. Grundmann suggested a wise and interesting transversal frame to approach the problem and join the efforts of stakeholders with common objectives [120]. On this basis, he identified three groups of “targeted stakeholders”: (i) directly and immediately affected by adverse healthcare outcomes (patients and families, doctors, clinical microbiologists, drug prescribers, and drug dispensers); (ii) those affected by the social impact of healthcare outcomes (policymakers, politicians, public health workers, health insurance companies, and infection-control experts); and (iii) those with societal and corporate responsibility (scientists and researchers, pharma industry, funding agencies, global health, and research donors and sponsors). Table 1 shows the objectives, activities, and information sources of stakeholders at these levels. Gaps and challenges to achieve such objectives (in fact, to face the AMR problem) are highlighted using this framework. Some strategic aspects are discussed below.
4.1. Patient-Centered (Addressing Clinical Demands)
The hospital is the most important landscape to detect the emergence and facilitate the evolution of AMR. On average, HAIs occur in 7% and 10% of hospitalized patients in LMICs and HICs, respectively [188]. Different estimations consider the high rate of HAIs caused by AMR organisms [189] and predict rising mortality rates due to AMR [35]. Still, the risk of acquiring infections during hospitalization greatly varies between wards (5–10 times higher in ICUs than in general wards) [47], and between institutions [94,115,190]. The elucidation of the hospital indoor microbiome to the health and safety of the patients would help the detection of critical points and assessment of infection control and intervention strategies [191,192,193]. Quantification of individual risks according to the patient (e.g., microbiota composition and host factors) and the built environment (hospital abiotic reservoirs and ward metrics) are some of the current major challenges not fully addressed in surveillance programs [171,175,194].
One of the major challenges continues to be the definition and interpretation of HAIs cases because it undermines valid estimates of infection rates. Nosocomial acquisition is arbitrarily defined 48–72h after hospital admission, but it can also occur after the patient’s discharge, creating a flux of microbes between healthcare centers, and other community ecosystems [195,196]. The increase in health centers (including CHCs) hosting heterogeneous risk populations (e.g., elderly, migrants, the poor, and migrant agricultural workers) enlarged the health network and led to the development of the category “community-onset healthcare-associated infections” to accurately address the complexity of the acquisition/transmission pathways of AMR [196,197]. The increasing number of studies that demonstrate long-term colonization of discharged patients and households [165] confirms previous studies that suggested the transmission of AMR between healthcare and the community [195,198].
4.2. Population-Centered (Addressing Policy Demands)
Considerable investments in surveillance, design of guidelines, and policies to contain the AMR problem in the global arena have been conducted with limited success. In May 2015, the 68th World Health Assembly adopted the “global action plan on AMR” [62] and called on WHO member states to establish national action plans (NAPs) aimed at obtaining sustainable access to effective antibiotics. More than 85% of the world’s population lives in countries with developed or are in the process of developing NAPs-AMR although only very few NAPs in LMICs are based on a situational analysis. Recently, the environmental dimensions of AMR, neglected in the WHO-Global Action Plan, have been revised by the UNEP and supported by the WHO-FAO-OIE-UNEP quadripartite [38]. Other white papers by the World Bank [199], the OECD [97], and many other international organisms have identified gaps and challenges at various socioeconomic levels. A governance framework has been suggested, conceptualized as a cyclical process between the three governance areas: policy design, implementation tools, and monitoring and evaluation considering various domains and indicators to implement goals and challenges from various organizations [181].
One of the key objectives of the WHO global plan to fight AMR is to improve awareness and understanding of AMR through effective communication, education, and training [62]. Communication campaigns have played a critical role in the history of AMR, from enthusiastically encouraging the use of antibiotics among humans and animals in the 1930s, 1940s, and 1950s, to clearly recommending the prudent use of them in all NAPs nowadays. In early years, the press greatly contributed to the popularization of the “miracle” effect of antibiotics and stimulated its consumption and mass production. Two articles reflect such social effects. One, published in the New York Times in 1936, reported the successful recovery of President Roosevelt’s son after treatment for deadly sepsis with sulfa drugs [200] and stimulated the sales of the drug, which were sold at low cost and without prescription since the early 1930s [11]. Other lay press, published in The Time in 1941, echoed the “miracle” outcome of several infection cases treated by penicillin and the problem and cost of mass production of the drug. Such article triggered a response by the Royal Army Medical Corps and forced the British government to consider this issue a national emergency [201] Afterwards, the serendipitous discovery of the effects of antibiotic byproducts (vitamin B12 coproduced with streptomycin, aureomycin derived from tetracycline) on animal growth by the early 1940s drastically changed the farming sector and food industry and widened the big pharma business sector. In the 1950s, the Food and Drug Administration (FDA) greatly contributed to the encouragement of antibiotic use for growth promotion with massive communication and awareness campaigns. [17,18,24,25]. Such a position was defended by many scientists beyond the publication of the Swann report in 1969 [202]. Until the 1970s, no voices claimed the prudent use of antibiotics (see Section 1.2). Since 2015, the WHO has implemented an annual global awareness campaign called ‘Antibiotics: Handle with Care,’ which take place during the World Antimicrobial Awareness Week (WAAW). Results differ between countries, and sound recommendations for tailored campaigns adapted to various cultural models and sectors and involving experts in health communication and social marketing seems crucial [203].
4.3. Microbial-Centered (Addressing Novel and Old Infection-Control Needs and Community Demands)
Many fundamental scientific questions relevant to understanding the development, selection, transmission, and persistence of ARGs remain to be unraveled. The Joint Programming Initiative on Antimicrobial Resistance (JPIAMR), an international collaborative platform coordinating and funding research on AMR, provides a research and innovation framework for joint actions, outlining key areas that should be addressed, and providing guidance for countries to align their AMR research agendas at national and international levels [204]. The Scientific Research and Innovation Agenda (SRIA) of the JPIAMR adopted the One Health perspective and is included in the WHO Global Action Plan on AMR [62] as a recommendation for national research plans. More specifically, the SRIA defines priority topics through which coordinated research activities are translated into new and/or improved strategies to address AMR (development of new therapies, stewardship of new and existing antimicrobials, and strategies to monitor and prevent the selection and spread of AMR between humans, animals, and the environment). Gaps and challenges of specific AMR topics have been highlighted in various workshops supported by the JPIAMR in the last years [38,130,131,132,204,205], as well as in reports by other policymakers, such as UNEP [38,97,206].
Although a colossal advance in knowledge has been achieved in recent decades with the support of public and private funding, we face new challenges associated with the 4IR, such as extended life expectancy, economic growth of pharma and food industries (see Table 1) that led to consumptogenic systems, intensified livestock practices, exacerbation of diseases by climate change, and loss of biodiversity by environmental pollution. They will open novel research areas and will also require the commitment of economic and social sectors [38,206] (Table 1). Still, we have not established the fundamental knowledge to understand the selection, transmission and persistence of AMR pathogens, the dynamic of complex systems (e.g., microbial interkingdom interactions) [150] and the ecotoxicity of pharmaceuticals and drug mixtures on selection and biodiversity [38,207].
Table 1.
Frame to approach the AMR problem according to stakeholders’ groups with common objectives. Gaps and challenges.
| Level | Stakeholders | Objectives | Required Information |
Scope | Information Source | Gaps and Challenges |
|---|---|---|---|---|---|---|
| Patient-centered (addressing clinical demands) |
Patients (and their families), doctors, clinical microbiologists, prescribers, and drug dispensers (pharmacists), and infection control practitioners. | Improving patient treatment. Design and implement standard treatment guidelines and essential drug lists. Expected burden of disease (BoD) at any geo-administrative level (individual setting). |
Fine-scale information of individual risks for infection, colonization, and/or expected treatment outcome. Fine-scale information at setting level (identification of risks areas). |
OUSs 1 Composite indexes (DRI, microbiome indexes) AMU |
Real-time collection of local and stratified patient and AMC/AMU data. Lateral Public Health [208] |
Develop criteria to define HAIs cases, still based on 48–72 h admission time [195,196]. Microbiome precision medicine [161,194]. Develop composite indexes [171,175]. Combine AMR and AMC/AMU trends in categorized patient populations [122,130,131,132,209,210]. Understand microbial transmission (plasmid, clones, and microbial consortiums). Plasmid surveillance: criteria, tools, and databases [156,160,211,212]. Evaluate the impact of the indoor microbiome on the health and safety of patients [164]. Implement AMU interventions based on behavioral models [13,213]. |
| Population-centered (addressing policy demands) |
Health managers, infection control practitioners, public health experts, and health insurance companies. Politicians, policymakers, economists, and economic growth sectors. |
To estimate the impact of AMR at national, regional, or international levels (trends and benchmarking). Identifying the leading causes for AMR emergence and spread. Quantifying AMC (pharmaco-epidemiology, sales). |
Information aligned with validity, reliability, and comparability data from local, regional AMR surveillance networks. | Surveillance. OUS (list of priority pathogens). AMC (sales). Composite indexes. |
Real-time collection of local and stratified patient data linked to local, regional, and/or national databases. Active population-based surveillance at local and regional levels |
Identify population-level factors/groups linked to the emergence of AMR Granularity of the information to extrapolate estimates. Harmonize units of analysis and indicators (with appropriate corrector indexes. See Section 3 (data quality and comparability issues). Omic tools and infrastructures (capacity building, harmonized and standardized tools) [149,161]. Put AMR in the context of other Public Health threats (syndemics). Real-time surveillance only available in a few countries [121]. Community level surveillance adapted to healthcare loops (hospital wastewater, and insurance health networks,) [138,198,214]. |
| To design guidelines and policies. To monitor the effect of interventions. |
Information aligned with validity, reliability and comparability data from regional AMR surveillance networks. | Aligned with National Antibiotic Plans (NAPs). | Active OneHealth sector-based surveillance aligned with NAPs | Harmonize NAPs according to local data and governance [181]. | ||
| Estimating the cost–benefit impact on public health, environment, and economy (BoD, biodiversity). | Return estimation ISOR. Social impact. |
Public Health economics. | Public Health and Social data repositories. | Integrate the human, animal, and environmental policy outcomes with the economy markers. | ||
| Efficient awareness tools and campaigns between stakeholders and lay public to inform alerts or interventions (control, drug policies,…). | Awareness through feedback influencing consumers, policies, and investment. | Awareness Tailored according to social and cultural norms. |
Consumers-public polls and enquiries. Data from patient’s associations (ITUs). |
Develop efficient communication tools and channels (intra and inter-sectorial) [203]. Assure updating of messages [203]. Revise prescription models. |
||
| Revise market and marketing practices (pharma and food industry). | Evolution of sales and prescriptions. Control good practices. |
Market and marketing. | National Health and Consumer services. | Strengthening governance, management, innovation, and financing [181]. | ||
| Pathogen-centered (addressing infection-control demands) 2 |
Scientists and researchers, pharmaceutical industry, funding bodies, and global health donors. |
Identification of transmission routes and microorganisms. Identify diagnostic biomarkers. Identification of reservoirs of MDR bacteria, plasmids, and hotspots for horizontal gene tranfer Identify PD parameters as minimal selective concentrations. Identify PD parameters as ecotoxicological concentrations. |
Scientific publications, grey literature, workshop reports, international dossiers, white papers, and society reports. | Strains of microbial species (bacteria, fungi) of biomedical relevance from catalogued repositories and type strain collections. | Determine ethe effects of AMU (antibiotics and non-antibiotic antimicrobials) on dysbiosis patterns [164,215]. Understand microbial transmission (plasmid, clones, and microbial consortiums) Understand transmission pathways. (e.g., effects of particulate matters in the bacterial and fungal transmission, particularly in water–soil edges [216]. Plasmid surveillance: criteria, tools and databases [156,160,211,212]. Involve engineers in Public Health policies and guidelines. Environmental impact of sanitation measures [38]. Effects of interkingdom microbial interactions in the dynamics of MDR bacteria and ARGs [150]. Actions targeting the reduction of pharmaceuticals in the environment including the design, synthesis, and production of pharmaceuticals [38]. Determine the concentration of selective antibiotics for AMR in local environments [205,217,218]. Determine the impact of drugs in the environment to regulate microbial residue limits (MRLs) [218] 3. |
1 The number of Operational Units of Surveillance (OUSs) tested greatly varies for different units of analysis. Although routine clinical laboratories measure all pathogens against a battery of antibiotics in order to guide therapy of individual patients, most surveillance studies are based on the collection of these data. 2 The increasing annual global growth of the pharma sector, prescriptions, and sales of pharmaceuticals at a rate greater than the increase of the population and with up to more than 50% of people in Western countries taking pharmaceuticals prescribed from more than one specialist, will force us to revise pharma workflow [38] as the effects of novel AMU/AMC patterns. 3 For most licensed medicines (+88%), data on environmental toxicity is not available [38].
5. Conclusions
AMR is one of the Global Health challenges of the 21st century that also threatens the achievement of several SDGs. The “Great acceleration” period has entirely transformed the health sector and health problems. While it led to the advance of human health, it also made humans increasingly vulnerable to contemporary challenges (e.g., AMR, emerging and re-emerging infectious diseases and non-communicable diseases), which are further impacted by climate change, poverty, conflict, migration, the increased atomization of the society, and knowledge fragmentation. Such a syndemic scenario showcases microbial communities in convergent vulnerable populations and disparate settings interacting with varying intensities that increase the risks to the health and safety of humans. The increasing lack of therapeutic options to treat MDR pathogens, the changes in the global drug market, the consequent decreasing interest of pharmaceutical companies in the development of new antimicrobials, and the spread of AMR across humans and animals worsen this scenario [18,48,219]. To date, the recommendations and guidelines related to the control of AMR in the healthcare sector rely on information provided by clinical laboratories and surveillance systems using similar indicators but poorly harmonized tools. The limitations about the validity, reliability and comparability of the information have been largely neglected and need to be revised in light of new challenges and taking into account methodological problems common to statistics in science under a multidisciplinary scientific framework. Public Health and Global Health policies and guidelines should be reframed to consider the novel problems in the 4IR and involve the global health network, including major economic sectors.
This review has only tried to bring together many aspects that are often considered separately. AMR is a public health problem resulting from social construction in the last centuries. Thus, the understanding of the history, the conceptual scientific frames, and the behavior of humans to face health problems are necessary to identify the current gaps and challenges and to resolve many already unresolved problems (the “known unknowns”).
Acknowledgments
The authors acknowledge Cruz Soriano (Servicio de Medicina Intensiva, Hospital Universitario and Ramón y Cajal, Madrid, Spain), and Ana Alastruey (Instituto de Salud Carlos III, Madrid, Spain) for the critical reading of the manuscript. The authors wish to acknowledge the referees for careful revision, relevant comments, and suggestions to improve the paper. During the performance of this study, A-E.P.-C was supported by the Program Attraction of Talent of the Regional Government of Madrid (Grant No. 2019-T2/BMD-12874); and M.D.F.D.B. by the Instituto de Salud Carlos III (pFIS F19/00366).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms11041050/s1, Table S1. AMR definitions, applications, and gaps. Figure S1. Antimicrobial resistance in the healthcare sector from a systems perspective [220,221,222,223].
Appendix A
Table A1.
Antimicrobial resistance, infection, and medical microbiology. Interconnected fields influenced by other disciplines. Glossary.
| Terms | Definition/Comments |
|---|---|
| Abiotic reservoirs | The part of an ecosystem where a microorganism or a chemical accumulates or is stockpiled outside of living organisms. Abiotic surfaces are composed of a diversity of materials. The impact of environmental factors on the selection of bacteria on abiotic reservoirs is largely unexplored [38,217]. |
| AMR surveillance system | A structured and systematic procedure to measure the prevalence or incidence of AMR through continuous or periodical surveillance performed with a defined methodology and with specified indicators. Heterogeneities in the sampling, methods, or targets, for example, made current AMR surveillance systems inefficient for decision-making [85]. |
| Antibiotic resistance | See Table S1. |
| Bystander selection | The inadvertent pressure imposed by therapeutic treatments on microbes other than the targeted pathogen [215]. The effects of antibiotics on human microbiomes (at individual levels or at micro- and mesosystems) and on environmental microbiomes remain unexplored. |
| Disease ecology | The ecological study of host–pathogen interactions within the context of their environment and evolution as relating to the impact of diseases on populations. This field grows out of the awareness of the pervasive role of pathogens in ecosystems. While medical researchers, such as Theobald Smith, Frank Macfarlane Burnet, and Frank Fenner, approached ecological interactions at the level of human populations, René Dubos focused on the interface of human hosts and microbes in the physiological environments of individuals [59]. This view influenced the way to approach medical microbiology. |
| Community ecology | A subfield of ecology that investigates the factors that influence biodiversity, community structure, and the distribution and abundance of species (e.g., in the microbiosphere, interactions with the abiotic world, interactions between species, and between individuals of the same species (microbiome heterogeneity). Community ecology is the framework to analyze and interpret the human microbiome and resistome, including microbiomes of hospitalized patients, and hospital-built environment [73,169]. |
| Consumptogenic systems | The systems that promote the consumption of goods and services to the detriment of either population or environmental health. Excess consumption is arguably a product of societal pressures from cultural and economic actors encouraging market transactions to individual consumers that forge daily routines around consumption and trading money for goods and symbols to reflect their social status. An emphasis on the consumptogenic system attempts to shift the focus away from just individual choices to consume to the structural conditions that enable and promote excess consumption. |
| Environment, ecosystem, and assignation of these terms to the hospital space | The concept of “hospitals as environments” implies “hospitals as ecosystems”, comprising populations of individuals. Environment refers to “the sum total of all the conditions which surround man at a given space and time” [224], and, accordingly with the classic definition of ecosystem “the system resulting from the integration of all the living and non-living factors of the environment” [225,226]. |
| Exposome | The measure of all the exposures from our environment (diet, lifestyle, and professional) of an individual in a lifetime and how those exposures interact with our own unique characteristics (e.g., genetics, physiology, and epigenetics) relate to health [227]. |
| Evolutionary biology | A branch of biology focused on understanding the drivers’ (what/how) causal and stochastic processes and determined the evolutionary mechanisms (natural selection, common descent, and speciation-clonalization) that led to the current biodiversity of organisms on our planet [71]. |
| Globalization | The growing interdependence of the world’s economies, cultures, and populations brought about by cross-border trade in goods and services, technology, and flows of investment, people, and information [different articles/analysis by the World Bank, https://www.worldbank.org/, accessed on 28 March 2023 or World Economic Forum, https://www.weforum.org/, accessed on 28 March 2023]. |
| The Great Acceleration and the Anthropocene | The 1950s are the starting point of “The Great Acceleration” period which defines the sharp increase of the human activities (population, economy, resource use, and technologies) that drove rapid and unprecedented changes to the structure and functioning of the Earth System, such as the climate change. The “Great Acceleration” is the basis for a proposed new geologic epoch in Earth history, “the Anthropocene”. Both the Great Acceleration and the Anthropocene are often linked to the problem of AMR [14,228]. |
| Feedback loops | The part of a system in which some part (or all) of the system’s output is used as input for future actions. |
| Hybrid lineages | Biological lineages as bacterial species and clones, or mobile genetic elements that resulted from hybridization between two or more distinct lineages, frequently favored by DNA exchange [146]. |
| Health | The WHO defines it as “a state of complete physical, mental and social wellbeing and not merely the absence of disease or infirmity” in its Constitution of 1948 [29]. The definition implies to see health as a fundamental human right. However, the definition remains inadequately implemented at the level of national law and standardized codes of practice despite WHO requests, and with significant consequences (identify “health” with “wellbeing” and “health care” with “disease care [229]. Thus, it inadvertently contributes to the ‘over-medicalization’ of the population. It also allows a platform for industry, medical technologies, and professionals to redefine our health status. This results in unequal access to health care and ultimately social inequality by excluding most potential users from the development of standards and policy. |
| Health system | The WHO defines them as the totality of “organizations, people and actions whose primary intent is to promote, restore or maintain health”. The WHO health systems framework identifies the building blocks of a health system as related to governance, financial arrangements, medicines and technologies, health information systems, human resources, and health service delivery. This health system view is essential to approach the AMR problem [229]. |
| Health center or community-health centers (CHCs) |
Community-based and patient-directed organizations that deliver comprehensive, culturally competent, high-quality primary healthcare services to the nation’s most vulnerable individuals and families, including people experiencing homelessness, agricultural workers, residents of public housing, and veterans. Health centers integrate access to pharmacy, mental health, substance use disorder, and oral health services in areas where economic, geographic, or cultural barriers limit access to affordable health care. |
| Hothouses | Closed and regulated environments with a high density and diversity of biological entities, favoring interactions between them. |
| Individual agency, agency relationship |
Individual agency is the level of freedom and self-decision to act whereby people act as individual members of society (within cultures, countries) or given collective community (practitioners). Factors that determine or limit individual agency include social class, religion, gender, ethnicity, ability, customs, and professional sector. Individualism represents a cultural shift from and juxtaposition to collectivism. Agency relationship is a fiduciary relationship where one person (called the “principal”) allows an agent to act on his or her behalf (e.g., practitioner and patients). These terms are derived from sociology and are increasingly relevant in AMU/AMC policies [145,146]. |
| Koch’s postulates (or the Henle-Koch postulates) |
Koch’s postulates (or Henle–Koch postulates) are four criteria designed to establish a causal relationship between a causative microbe and a disease. They were originally formulated by Friedrich Gustav Jacob Henle in the mid 1880s and refined and published by Robert Koch in 1890. Although they are still valid for a relatively small number of defined circumstances in which bacteria can be precisely tied to the cause of a particular clinical syndrome, they are revised under the light of available current knowledge [68,230]. |
| Miracle drugs | Sulfa drugs were coined as “magic bullets” and a “growing miracle” because they were credited with declines in mortality from childbirth, pneumonia, and other diseases by the late 1930s [11,201]. Shortly after, penicillin was hailed as a “miracle drug” when it proved its efficiency in curing untreatable infection episodes of septicemia [201]. These adjectives have been applied to drugs with antibiotic effect. |
| Risk assessment | A systematic and science-based approach to estimate the risks to the health and safety of persons arising out or in contact with AMR genes/pathogens, and also to support authorities in policymaking about the detection of critical points, and assessment of control and intervention strategies. Used in food safety [178] and infection control [180]. |
| Pandemics | A pandemic is “an epidemic occurring worldwide, or over a very wide area, crossing international boundaries, and usually affecting a large number of people” [231]. Although the definition has traditionally been associated with infectious diseases, it is increasingly applied to communicable and non-communicable diseases [77]. |
| Patches, landscape, and patches landscape | A patch is an area in a landscape that is different from surrounding areas. The patches generate what is called landscape heterogeneity, the diversity within a landscape. The diversity and uneven distribution that defines a landscape’s heterogeneity is based on many different features of an ecosystem (e.g., species and resources distribution and land-use patterns). The changes that occur in the separate spatial components of an ecosystem are described as patch dynamics. Patch dynamics is also a theoretical approach according to which the dynamics of an ecosystem can be understood through the analysis of its smaller spatial components (“patches”) that interact mutually. |
| Plasmid | The term “plasmid” was coined by Joshua Lederberg in 1952 to define any extrachromosomal determinant of heredity [232]. Outbreaks of MDR organisms in hospitals during 1950s or in farms in 1960s, demonstrated the transferability of single or combined (multiresistance) AMR phenotypes and represented the landmark discovery of non-Mendelian heredity. They are unique MGEs because of their ability to cross species barriers, to generate novel entities resulting from recombination events, and to increase the copy number (and thus the mutation rate) after gene acquisition [26,151,233]. |
| Population | The term “population” applies to the sets of individuals or evolutionary units with replication capacity that, at the subcellular (genes, plasmids, transposons, clones) and supracellular (bacterial communities, microbiomes, holobiome) level, are genetically and demographically connected. Thus, the ecological conditions (selection) in the system affect the adaptability of the units at each level (selective units) individually or cooperatively according to their degree of connectivity within a dynamic system (“evolution by association) [139,234]. |
| Resistome | Initially defined by D’Costa in 2006 as the set of genes in the pan genome which encode antibiotic resistance [235]. Other genes coding for resistance to antimicrobials that co-select AMR (heavy metals, antiseptics,…) could join this category [236]. |
| Social atomism | Atomism (or social atomism) is a sociological theory that refers to “the tendency for society to be made up of a collection of self-interested and largely self-sufficient individuals, operating as separate atoms”. Therefore, all social values, institutions, developments, and procedures evolve entirely out of the interests and actions of the individuals who inhabit any particular society. |
| Social behaviour components and layers | See Figure S1. |
| Syndemic or synergistic epidemic | The aggregation of two or more concurrent or sequential epidemics or diseases in a population with biological, psychological, or societal interactions and sharing common underlying societal drivers that exacerbate the prognosis and burden of disease. The syndemic approach contrasts with the biomedical approach to diseases that diagnostically isolate, study, and treat diseases as distinct entities separate from other diseases and independent of social contexts [75]. AMR is increasingly being approached as a syndemic threat (COVID, climate change, and emerging co-occurring infection). |
| SDGs | The Sustainable Development Goals (SDGs), also known as the Global Goals, were adopted by the United Nations in 2015 as a universal call to action to end poverty, protect the planet, and ensure that by 2030 all people enjoy peace and prosperity. The 17 SDGs are integrated—they recognize that action in one area will affect outcomes in others, and that development must balance social, economic, and environmental sustainability. |
| Therapeutic solutions to early major Public Health problems | Salvarsan represented the first effective treatment against syphilis; sulfonamides were the first effective options against infectious diseases of high mortality and morbidity, such as puerperal fever, pneumonia, scarlet fever, meningitis, gonorrhea, and erysipelas; and natural antibiotics (such as streptomycin and other aminoglycosides, penicillin, and tetracycline) were the first effective options for tuberculosis and safe alternatives to combat many infectious diseases. All were major public health problems in the early 20th century [15,16,18]. |
| “Watch lists” or “priority lists” of pathogens for which new antibiotics are urgently needed | They are catalogues of bacteria/fungi families that pose the greatest threat to human health. Their goal is to guide and promote research and development (R and D) of new antibiotics, to align R and D priorities with public health needs, and to coordinate the fight against AMR bacteria based on epidemiologic control of the infectious agent, its accurate surveillance and detection, and implementation of effective treatments [237]. In 2013, the Centers for Disease Control and Prevention (CDC) published the first “watch list of antibiotic threats” (AMR bacteria) which ranked them as urgent, serious, or concerning threats to humans [189,238]. In 2017, the WHO categorized the same pathogens into critical, high, and medium-priority groups in what they called priority pathogen lists [237,239]. The first watch list of fungal antimicrobial resistance threats has been available since late 2022 [239]. |
| World Antimicrobial Awareness Week (WAAW) | The WAAW is a global campaign that is celebrated annually to improve awareness and understanding of AMR and encourage best practices among the public, One Health stakeholders, and policymakers, who all play a critical role in reducing the further emergence and spread of AMR. |
Author Contributions
T.M.C., conceptualization, writing original drafts, and final editions; F.B., editing and review; R.C., A.E.P.-C. and M.D.F.-d.-B. review, and contributions to figures and tables. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
Work in the ecoevobiome lab (www.ecoevobiome.org) is supported by the European Commission (MISTAR AC21_2/00041) and the Instituto de Salud Carlos III (ISCIII) of Spain, cofinanced by the European Development Regional Fund (A Way to Achieve Europe program; Spanish Network for Research in Infectious Diseases grants PI18/1942; PI21/02027), CIBERINFEC (CB21/13/00084), the Fundación Ramón Areces (BioMetasep project), the Fundación Francisco Soria Melguizo (microCarbaFlux project), and the Ramón Cajal Institute for Health-IRYCIS (grant IMP21-C22).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.EClinicalMedicine Antimicrobial Resistance: A Top Ten Global Public Health Threat. EClinicalMedicine. 2021;41:101221. doi: 10.1016/j.eclinm.2021.101221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gradmann C. From Lighthouse to Hothouse: Hospital Hygiene, Antibiotics and the Evolution of Infectious Disease, 1950–1990. Hist. Philos. Life Sci. 2017;40:8. doi: 10.1007/s40656-017-0176-8. [DOI] [PubMed] [Google Scholar]
- 3.Mughini-Gras L., Dorado-García A., van Duijkeren E., van den Bunt G., Dierikx C.M., Bonten M.J.M., Bootsma M.C.J., Schmitt H., Hald T., Evers E.G., et al. Attributable Sources of Community-Acquired Carriage of Escherichia Coli Containing β-Lactam Antibiotic Resistance Genes: A Population-Based Modelling Study. Lancet Planet Health. 2019;3:e357–e369. doi: 10.1016/S2542-5196(19)30130-5. [DOI] [PubMed] [Google Scholar]
- 4.Dorado-García A., Smid J.H., van Pelt W., Bonten M.J.M., Fluit A.C., van den Bunt G., Wagenaar J.A., Hordijk J., Dierikx C.M., Veldman K.T., et al. Molecular Relatedness of ESBL/AmpC-Producing Escherichia coli from Humans, Animals, Food and the Environment: A Pooled Analysis. J. Antimicrob. Chemother. 2018;73:339–347. doi: 10.1093/jac/dkx397. [DOI] [PubMed] [Google Scholar]
- 5.Baquero F., Cantón R., Cornaglia G. Public Health Microbiology, a Challenge for Europe. Clin. Microbiol. Infect. 2010;16:123–125. doi: 10.1111/j.1469-0691.2009.03126.x. [DOI] [PubMed] [Google Scholar]
- 6.Antimicrobial Resistance-The Role of Public Health Organizations in Addressing Public Health Problems in Europe-NCBI Bookshelf. [(accessed on 28 March 2023)]; Available online: https://www.ncbi.nlm.nih.gov/books/NBK536193/
- 7.Baquero F., Lanza V.F., Cantón R., Coque T.M. Public Health Evolutionary Biology of Antimicrobial Resistance: Priorities for Intervention. Evol. Appl. 2015;8:223–239. doi: 10.1111/eva.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Bank Drug-resistant infections: A threat to our economic future (Vol. 2): Final report (English). HNP/Agriculture Global Antimicrobial Resistance Initiative Washington, D.C.: World Bank Group. [(accessed on 28 March 2023)]. Available online: http://documents.worldbank.org/curated/en/323311493396993758/final-report.
- 9.Roope L.S.J., Smith R.D., Pouwels K.B., Buchanan J., Abel L., Eibich P., Butler C.C., Tan P.S., Sarah Walker A., Robotham J.V., et al. The Challenge of Antimicrobial Resistance: What Economics Can Contribute. Science. 2019;364:eaau4679. doi: 10.1126/science.aau4679. [DOI] [PubMed] [Google Scholar]
- 10.O’Neill J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. Review on Antimicrobial Resistance. Wellcome Trust; London, UK: 2014. [(accessed on 28 March 2023)]. Available online: https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf. [Google Scholar]
- 11.Jayachandran S., Lleras-Muney A., Smith K.V. Modern Medicine and the Twentieth Century Decline in Mortality: Evidence on the Impact of Sulfa Drugs. Am. Econ. J. Appl. Econ. 2010;2:118–146. doi: 10.1257/app.2.2.118. [DOI] [Google Scholar]
- 12.Dionisio F., Baquero F., Fuertes M. Psychological and Cultural Factors Influencing Antibiotic Prescription. [(accessed on 28 March 2023)];Trends Microbiol. 2023 29 doi: 10.1016/j.tim.2022.12.010. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0966842X22003468. [DOI] [PubMed] [Google Scholar]
- 13.Borek A.J., Santillo M., Wanat M., Butler C.C., Tonkin-Crine S. How Can Behavioural Science Contribute to Qualitative Research on Antimicrobial Stewardship in Primary Care? JAC Antimicrob. Resist. 2022;4:dlac007. doi: 10.1093/jacamr/dlac007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jørgensen P.S., Aktipis A., Brown Z., Carrière Y., Downes S., Dunn R.R., Epstein G., Frisvold G.B., Hawthorne D., Gröhn Y.T., et al. Antibiotic and Pesticide Susceptibility and the Anthropocene Operating Space. Nat. Sustain. 2018;1:632–641. [Google Scholar]
- 15.Landecker H. Antibiotic Resistance and the Biology of History. Body Soc. 2016;22:19–52. doi: 10.1177/1357034X14561341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Landecker H. Antimicrobials before Antibiotics: War, Peace, and Disinfectants. Palgrave Commun. 2019;5:45. doi: 10.1057/s41599-019-0251-8. [DOI] [Google Scholar]
- 17.Podolsky S.H. Historical Perspective on the Rise and Fall and Rise of Antibiotics and Human Weight Gain. Ann. Intern Med. 2017;166:133–138. doi: 10.7326/M16-1855. [DOI] [PubMed] [Google Scholar]
- 18.Podolsky S.H. The Evolving Response to Antibiotic Resistance (1945–2018) Palgrave Commun. 2018;4:124. doi: 10.1057/s41599-018-0181-x. [DOI] [Google Scholar]
- 19.Donzé P.Y., Fernández Pérez P. Health Industries in the Twentieth Century. Bus. Hist. 2019;61:385–403. doi: 10.1080/00076791.2019.1572116. [DOI] [Google Scholar]
- 20.American Chemical Society Pfizer’s Work on Penicillin for World War II Becomes a National Historic Chemical Landmark. [(accessed on 28 March 2023)]. Available online: https://www.acs.org/pressroom/newsreleases/2008/june/pfizers-work-on-penicillin-for-world-war-ii-becomes-a-national-historic-chemical-landmark.html.
- 21.Williams K.J. The Introduction of ‘Chemotherapy’ Using Arsphenamine—the First Magic Bullet. J. R. Soc. Med. 2009;102:343. doi: 10.1258/jrsm.2009.09k036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohr K.I. History of Antibiotics Research. Curr. Top Microbiol. Immunol. 2016;398:237–272. doi: 10.1007/82_2016_499. [DOI] [PubMed] [Google Scholar]
- 23.Tansey E.M. Medicines and Men: Burroughs, Wellcome & Co, and the British Drug Industry before the Second World War. J. R. Soc. Med. 2002;95:411. doi: 10.1258/jrsm.95.8.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.UNDARK “Big Chicken”: A 1948 Antibiotic Experiment That Shook the World. [(accessed on 10 June 2017)]. Available online: https://undark.org/2017/10/06/chicken-experiment-shook-world/
- 25.Thoms U. Between Promise and Threat: Antibiotics in Foods in West Germany 1950-1980. NTM Int. J. Hist. Ethics Nat. Sci. Technol. Med. 2012;20:181–214. doi: 10.1007/s00048-012-0073-x. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe T. Infective Heredity of Multiple Drug Resistance in Bacteria. Bacteriol. Rev. 1963;27:87–115. doi: 10.1128/br.27.1.87-115.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farrar W.E. Antibiotic Resistance in Developing Countries. J. Infect. Dis. 1985;152:1103–1106. doi: 10.1093/infdis/152.6.1103. [DOI] [PubMed] [Google Scholar]
- 28.Hone T., Macinko J., Millett C. Revisiting Alma-Ata: What Is the Role of Primary Health Care in Achieving the Sustainable Development Goals? Lancet. 2018;392:1461–1472. doi: 10.1016/S0140-6736(18)31829-4. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization (WHO) Constitution of the World Health Organization. [(accessed on 28 March 2023)]. Available online: https://www.who.int/about/governance/constitution.
- 30.Jones K.E., Patel N.G., Levy M.A., Storeygard A., Balk D., Gittleman J.L., Daszak P. Global Trends in Emerging Infectious Diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu Y.G., Gillings M., Simonet P., Stekel D., Banwart S., Penuelas J. Microbial Mass Movements. Science. 2017;357:1099–1100. doi: 10.1126/science.aao3007. [DOI] [PubMed] [Google Scholar]
- 32.Hernando-Amado S., Coque T.M., Baquero F., Martínez J.L. Antibiotic Resistance: Moving From Individual Health Norms to Social Norms in One Health and Global Health. Front. Microbiol. 2020;11:1914. doi: 10.3389/fmicb.2020.01914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Institute of Medicine (US) Committee on Emerging Microbial Threats to Health in the 21st Century . In: Microbial Threats to Health: Emergence, Detection, and Response. Smolinski M.S., Hamburg M.A., Lederberg J., editors. National Academies Press (US); Washington, DC, USA: 2003. [PubMed] [Google Scholar]
- 34.Cecchini M., Langer J., Slawomirski L. In: Antimicrobial Resistance in G7 Countries and beyond: Economic Issues, Policies and Options for Action. Cecchini M., Langer J., Slawomirski L., editors. Organization for Economic Co-operation and Development (OECD); 2015. [(accessed on 28 March 2023)]. pp. 1–75. Available online: https://www.oecd.org/els/health-systems/Antimicrobial-Resistance-in-G7-Countries-and-Beyond.pdf. [Google Scholar]
- 35.O’Neill J. Tackling a Crisis for the Health and Wealth of nations_1.pdf. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Wellcome Trust; London, UK: 2016. [Google Scholar]
- 36.Minh Pham T., Kondor I., Hanel R., Thurner S. The Effect of Social Balance on Social Fragmentation. J. R. Soc. Interface. 2020;17:20200752. doi: 10.1098/rsif.2020.0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Antimicrobial Resistance and the United Nations Sustainable Development Cooperation Framework: Guidance for United Nations Country Teams. World Health Organization (WHO), Food and Agriculture Organization (FAO), World Organisation for Animal Health (OIE), UN Environment Programme (UNEP) eds. 2021. [(accessed on 28 March 2023)]. Available online: https://sdg.iisd.org/news/antimicrobial-resistance-threatens-development-sdgs-tripartite-report.
- 38.United Nations Environment Programme Bracing for Superbugs: Strengthening Environmental Action in the One Health Response to Antimicrobial Resistance. 2023. [(accessed on 28 March 2023)]. Available online: https://www.unep.org/resources/superbugs/environmental-action.
- 39.Culturico The Spreading Process of Social Atomization. [(accessed on 28 March 2023)]. Available online: https://culturico.com/2019/03/01/the-spreading-process-of-social-atomization/
- 40.Lewontin R.C. Biology as Ideology: The Doctrine of DNA. Volume 148. Harper Perennial Press; New York, NY, USA: 1991. pp. 148–162. [Google Scholar]
- 41.Dubos R.J. Microbiology. Annu. Rev. Biochem. 1942;11:659–678. doi: 10.1146/annurev.bi.11.070142.003303. [DOI] [Google Scholar]
- 42.Moberg C.L. René Dubos, a Harbinger of Microbial Resistance to Antibiotics. Perspect Biol. Med. 1999;42:559–580. doi: 10.1353/pbm.1999.0011. [DOI] [PubMed] [Google Scholar]
- 43.Dubos R. Mirage of Health: Utopias, Progress, and Biological Change. Anchor Books; Garden City, NY, USA: 1959. [Google Scholar]
- 44.Rogers D.E. The Changing Pattern of Life-Threatening Microbial Disease. N. Engl. J. Med. 1959;261:677–683. doi: 10.1056/NEJM195910012611401. [DOI] [PubMed] [Google Scholar]
- 45.Williams R.E.O., Shooter R.A. In: Infection in Hospitals. Epidemiology and Control. A Symposium. Williams R.E.O., Shooter R.A., editors. Blackwell Scientific Publications Ltd.; Oxford, UK: 1963. [Google Scholar]
- 46.McDermott W. The Problem of Staphylococcal Infection. Br. Med. J. 1956;2:837. doi: 10.1136/bmj.2.4997.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wenzel R. Stalking Microbes: A Relentless Pursuit of Infection Control. AuthorHouse Ltd.; Bloomington, IN, USA: 2005. [Google Scholar]
- 48.Schaffner W. Priorities in Infection Control: The Impact of New Technology. J. Hosp. Infect. 1984;5:1–5. doi: 10.1016/0195-6701(84)90023-9. [DOI] [PubMed] [Google Scholar]
- 49.Williams R.E.O., Blowers R., Garrod L.P., Shooter R.A. Hospital Infection, Causes and Prevention. Lloyd-Luke (Medical Books) Ltd.; London, UK: 1960. [Google Scholar]
- 50.Freeman J., McGowan J.E., Freeman J., McGowan J.E. Risk Factors for Nosocomial Infection. J. Infect. Dis. 1978;138:811–819. doi: 10.1093/infdis/138.6.811. [DOI] [PubMed] [Google Scholar]
- 51.Barber M., Dutton A.A.C., Beard M.A., Elmes P.C., Williams R. Reversal of Antibiotic Resistance in Hospital Staphylococcal Infection. Br. Med. J. 1960;1:11. doi: 10.1136/bmj.1.5165.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dixon R.E. Control of Health-Care--Associated Infections, 1961–2011. Morbility Mortal. Wkly. Rep. MMWR Suppl. 2011;60:58–63. [PubMed] [Google Scholar]
- 53.World Health Organization (WHO) Surveillance for the Prevention and Control of Health Hazards due to Antibiotic-Resistant Enterobacteria: Report of a WHO Meeting. 1978. [(accessed on 28 March 2023)]. Available online: https://apps.who.int/iris/handle/10665/41319. [PubMed]
- 54.World Health Organization (WHO) Public health aspects of antibiotic-resistant bacteria in the environment: Report on a consultation meeting, Brussels, Belgium, 9–12 December 1975. [(accessed on 28 March 2023)]. Available online: https://agris.fao.org/agris-search/search.do?recordID=XF2015030555.
- 55.Levy S.B., Clowes R.C., Koenig E.L. Molecular Biology, Pathogenicity, and Ecology of Bacterial Plasmids. Plenum; New York, NY, USA: 1981. Statement Regarding Worldwide Antibiotic Misuse; pp. 679–681. [Google Scholar]
- 56.Alliance for the Prudent Use of Antibiotics (APUA). [(accessed on 28 March 2023)]. Available online: https://apua.org/ourhistory.
- 57.Anderson R.M. The Pandemic of Antibiotic Resistance. Nature Med. 1999;5:147–149. doi: 10.1038/5507. [DOI] [PubMed] [Google Scholar]
- 58.Levy S.B. The Antibiotic Paradox. Springer; New York, NY, USA: 1992. [Google Scholar]
- 59.Honigsbaum M. Antibiotic Antagonist: The Curious Career of René Dubos. Lancet. 2016;387:118. doi: 10.1016/S0140-6736(15)00840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lipsitch M., Levin B.R. Population Dynamics of Tuberculosis Treatment: Mathematical Models of the Roles of Non-Compliance and Bacterial Heterogeneity in the Evolution of Drug Resistance. Int. Union Against Tuberc. Lung Dis. 1998;2:187–199. [PubMed] [Google Scholar]
- 61.Hernando-Amado S., Coque T.M., Baquero F., Martínez J.L. Defining and Combating Antibiotic Resistance from One Health and Global Health Perspectives. Nat. Microbiol. 2019;4:1432–1442. doi: 10.1038/s41564-019-0503-9. [DOI] [PubMed] [Google Scholar]
- 62.World Health Organization (WHO) Global Action Plan on Antimicrobial Resistance. [(accessed on 1 January 2016)]. Available online: https://www.who.int/publications/i/item/9789241509763.
- 63.Perry J.A., Westman E.L., Wright G.D. The Antibiotic Resistome: What’s New? Curr. Opin. Microbiol. 2014;21:45–50. doi: 10.1016/j.mib.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 64.Perry J.A., Wright G.D. The Antibiotic Resistance “Mobilome”: Searching for the Link between Environment and Clinic. Front. Microbiol. 2013;4:138. doi: 10.3389/fmicb.2013.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ruppé E., Ghozlane A., Tap J., Pons N., Alvarez A.S., Maziers N., Cuesta T., Hernando-Amado S., Clares I., Martínez J.L., et al. Prediction of the Intestinal Resistome by a Three-Dimensional Structure-Based Method. Nat. Microbiol. 2019;4:112–123. doi: 10.1038/s41564-018-0292-6. [DOI] [PubMed] [Google Scholar]
- 66.Lax S., Gilbert J.A. Hospital-Associated Microbiota and Implications for Nosocomial Infections. Trends Mol. Med. 2015;21:427–432. doi: 10.1016/j.molmed.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 67.Miller E.T., Svanbäck R., Bohannan B.J.M. Microbiomes as Metacommunities: Understanding Host-Associated Microbes through Metacommunity Ecology. Trends Ecol. Evol. 2018;33:926–935. doi: 10.1016/j.tree.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 68.Byrd A.L., Segre J.A. Adapting Koch’s Postulates. Science. 2016;351:224–226. doi: 10.1126/science.aad6753. [DOI] [PubMed] [Google Scholar]
- 69.Koskella B., Hall L.J., Metcalf C.J.E. The Microbiome beyond the Horizon of Ecological and Evolutionary Theory. Nat. Ecol. Evol. 2017;1:1606–1615. doi: 10.1038/s41559-017-0340-2. [DOI] [PubMed] [Google Scholar]
- 70.Tibayrenc M. The Golden Age of Genetics and the Dark Age of Infectious Diseases. Infect. Genet. Evol. 2001;1:1–2. doi: 10.1016/S1567-1348(01)00012-0. [DOI] [PubMed] [Google Scholar]
- 71.Nesse R.M., Bergstrom C.T., Ellison P.T., Flier J.S., Gluckman P., Govindaraju D.R., Niethammer D., Omenn G.S., Perlman R.L., Schwartz M.D., et al. Making Evolutionary Biology a Basic Science for Medicine. Proc. Natl. Acad. Sci. USA. 2010;107:1800–1807. doi: 10.1073/pnas.0906224106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schlechte J., Zucoloto A.Z., Yu I.L., Doig C.J., Dunbar M.J., McCoy K.D., McDonald B. Dysbiosis of a Microbiota–Immune Metasystem in Critical Illness Is Associated with Nosocomial Infections. Nat. Med. 2023:1–11. doi: 10.1038/s41591-023-02243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gilbert J.A., Lynch S.V. Community Ecology as a Framework for Human Microbiome Research. Nat. Med. 2019;25:884–889. doi: 10.1038/s41591-019-0464-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johnson P.T.J., De Roode J.C., Fenton A. Why Infectious Disease Research Needs Community Ecology. Science. 2015;349:1259504. doi: 10.1126/science.1259504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Singer M., Clair S. Syndemics and Public Health: Reconceptualizing Disease in Bio-Social Context. Med. Anthropol. Q. 2003;17:423–441. doi: 10.1525/maq.2003.17.4.423. [DOI] [PubMed] [Google Scholar]
- 76.Lewis C.M., Akinyi M.Y., DeWitte S.N., Stone A.C. Ancient Pathogens Provide a Window into Health and Well-Being. Proc. Natl. Acad. Sci. USA. 2023;120:e2209476119. doi: 10.1073/pnas.2209476119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stephen C. Rethinking Pandemic Preparedness in the Anthropocene. Healthc. Manag. Forum. 2020;33:153–157. doi: 10.1177/0840470419867347. [DOI] [PubMed] [Google Scholar]
- 78.Baquero F., Coque T.M., Martínez J.L., Aracil-Gisbert S., Lanza V.F. Gene Transmission in the One Health Microbiosphere and the Channels of Antimicrobial Resistance. Front. Microbiol. 2019;10:2892. doi: 10.3389/fmicb.2019.02892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eimer J., Patimeteeporn C., Jensenius M., Gkrania-Klotsas E., Duvignaud A., Barnett E.D., Hochberg N.S., Chen L.H., Trigo-Esteban E., Gertler M., et al. Multidrug-Resistant Tuberculosis Imported into Low-Incidence Countries—a GeoSentinel Analysis, 2008–2020. J. Travel. Med. 2021;28:taab069. doi: 10.1093/jtm/taab069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chatham-Stephens K., Medalla F., Hughes M., Appiah G.D., Aubert R.D., Caidi H., Angelo K.M., Walker A.T., Hatley N., Masani S., et al. Emergence of Extensively Drug-Resistant Salmonella Typhi Infections Among Travelers to or from Pakistan — United States, 2016–2018. MMWR Morb. Mortal. Wkly. Rep. 2019;68:11–13. doi: 10.15585/mmwr.mm6801a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Voor In ’T Holt A.F., Mourik K., Beishuizen B., van der Schoor A.S., Verbon A., Vos M.C., Severin J.A. Acquisition of Multidrug-Resistant Enterobacterales during International Travel: A Systematic Review of Clinical and Microbiological Characteristics and Meta-Analyses of Risk Factors. Antimicrob. Resist. Infect. Control. 2020;9:71. doi: 10.1186/s13756-020-00733-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nielsen R.T., Köse G., Sloth L., Andersen C.Ø., Petersen J.H., Norredam M. Pathogen Distribution and Antimicrobial Resistance in Infections in Migrants and Non-migrants in Denmark, a Cross-Sectional Study. Trop. Med. Int. Health. 2022;27:999–1008. doi: 10.1111/tmi.13820. [DOI] [PubMed] [Google Scholar]
- 83.Rodríguez-Molina D., Berglund F., Blaak H., Flach C.F., Kemper M., Marutescu L., Gradisteanu G.P., Popa M., Spießberger B., Wengenroth L., et al. International Travel as a Risk Factor for Carriage of Extended-Spectrum β-Lactamase-Producing Escherichia coli in a Large Sample of European Individuals—The AWARE Study. Int. J. Environ. Res. Public Health. 2022;19:4758. doi: 10.3390/ijerph19084758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mcneil C.J., Kirkcaldy R.D., Workowski K. Enteric Infections in Men Who Have Sex With Men. Clin. Infect. Dis. 2022;74:S169–S178. doi: 10.1093/cid/ciac061. [DOI] [PubMed] [Google Scholar]
- 85.Charles H., Prochazka M., Thorley K., Crewdson A., Greig D.R., Jenkins C., Painset A., Fifer H., Browning L., Cabrey P., et al. Outbreak of Sexually Transmitted, Extensively Drug-Resistant Shigella sonnei in the UK, 2021–2022: A Descriptive Epidemiological Study. Lancet Infect. Dis. 2022;22:1503–1510. doi: 10.1016/S1473-3099(22)00370-X. [DOI] [PubMed] [Google Scholar]
- 86.Ngaruka G.B., Neema B.B., Mitima T.K., Kishabongo A.S., Kashongwe O.B. Animal Source Food Eating Habits of Outpatients with Antimicrobial Resistance in Bukavu, D.R. Congo. Antimicrob. Resist. Infect. Control. 2021;10:124. doi: 10.1186/s13756-021-00991-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yamaichi Y., Chao M.C., Sasabe J., Clark L., Davis B.M., Yamamoto N., Mori H., Kurokawa K., Waldor M.K. High-Resolution Genetic Analysis of the Requirements for Horizontal Transmission of the ESBL Plasmid from Escherichia coli O104:H4. Nucleic Acids Res. 2014;43:348–360. doi: 10.1093/nar/gku1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yoshikawa T.T. Antimicrobial Resistance and Aging: Beginning of the End of the Antibiotic Era? J. Am. Geriatr. Soc. 2002;50:226–229. doi: 10.1046/j.1532-5415.50.7s.2.x. [DOI] [PubMed] [Google Scholar]
- 89.Mendenhall E., Kohrt B.A., Logie C.H., Tsai A.C. Syndemics and Clinical Science. Nat. Med. 2022;28:1359–1362. doi: 10.1038/s41591-022-01888-y. [DOI] [PubMed] [Google Scholar]
- 90.REAct Tracking Antimicrobial Resistance in the Sustainable Development Goals. 2019. [(accessed on 28 March 2023)]. Available online: https://www.reactgroup.org/news-and-views/news-and-opinions/year-2019/tracking-antimicrobial-resistance-in-the-sustainable-development-goals/
- 91.Swinburn B.A., Kraak V.I., Allender S., Atkins V.J., Baker P.I., Bogard J.R., Brinsden H., Calvillo A., de Schutter O., Devarajan R., et al. The Global Syndemic of Obesity, Undernutrition, and Climate Change: The Lancet Commission Report. Lancet. 2019;393:791–846. doi: 10.1016/S0140-6736(18)32822-8. [DOI] [PubMed] [Google Scholar]
- 92.Allen L. Are We Facing a Noncommunicable Disease Pandemic? J. Epidemiol. Glob. Health. 2017;7:5–9. doi: 10.1016/j.jegh.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Knight G.M., Glover R.E., McQuaid C.F., Olaru I.D., Gallandat K., Leclerc Q.J., Fuller N.M., Willcocks S.J., Hasan R., van Kleef E., et al. Antimicrobial Resistance and Covid-19: Intersections and Implications. Elife. 2021;10:1–27. doi: 10.7554/eLife.64139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Klein E.Y., van Boeckel T.P., Martinez E.M., Pant S., Gandra S., Levin S.A., Goossens H., Laxminarayan R. Global Increase and Geographic Convergence in Antibiotic Consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA. 2018;115:E3463–E3470. doi: 10.1073/pnas.1717295115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kenyon C., Kenyon C., Manoharan-Basil S.S., van Dijck C. Gonococcal Resistance Can Be Viewed Productively as Part of a Syndemic of Antimicrobial Resistance: An Ecological Analysis of 30 European Countries. Antimicrob. Resist. Infect. Control. 2020;9:1–9. doi: 10.1186/s13756-020-00764-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Goldberg J., Uyen-Cateriano A., Van Gassen G. Making Matters Worse: Antimicrobial Resistance in Conflict Settings. [(accessed on 23 June 2022)]. Available online: https://revive.gardp.org/making-matters-worse-antimicrobial-resistance-in-conflict-settings/
- 97.Organization for Economic Co-Operation and Development (OECD) Stemming the Superbug Tide: Just A Few Dollars More. [(accessed on 7 November 2018)]. Available online: https://www.oecd-ilibrary.org/social-issues-migration-health/stemming-the-superbug-tide_9789264307599-en?itemId=/content/publication/9789264307599-en&_csp_=33f7c05cefd0845031c4db9085aa0f90&itemIGO=oecd&itemContentType=book.
- 98.Burnham J.P. Climate Change and Antibiotic Resistance: A Deadly Combination. Ther. Adv. Infect. Dis. 2021;8:2049936121991374. doi: 10.1177/2049936121991374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.MacFadden D.R., McGough S.F., Fisman D., Santillana M., Brownstein J.S. Antibiotic Resistance Increases with Local Temperature. Nature Climate Chang. 2018;8:510–514. doi: 10.1038/s41558-018-0161-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cavicchioli R., Ripple W.J., Timmis K.N., Azam F., Bakken L.R., Baylis M., Behrenfeld M.J., Boetius A., Boyd P.W., Classen A.T., et al. Scientists’ Warning to Humanity: Microorganisms and Climate Change. Nat. Rev. Microbiol. 2019;17:569–586. doi: 10.1038/s41579-019-0222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Paull S.H., Song S., McClure K.M., Sackett L.C., Kilpatrick A.M., Johnson P.T.J. From Superspreaders to Disease Hotspots: Linking Transmission across Hosts and Space. Front. Ecol. Environ. 2012;10:75. doi: 10.1890/110111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pan American Health Organization (PAHO) Antimicrobial Resistance, Fueled by the COVID-19 Pandemic. Policy Brief November 2021. 2022. [(accessed on 28 March 2023)]. Available online: https://iris.paho.org/handle/10665.2/55864.
- 103.Liebana E., Carattoli A., Coque T.M., Hasman H., Magiorakos A.-P., Mevius D., Peixe L., Poirel L., Schuepbach-Regula G., Torneke K., et al. Public Health Risks of Enterobacterial Isolates Producing Extended-Spectrum β-Lactamases or AmpC β-Lactamases in Food and Food-Producing Animals: An EU Perspective of Epidemiology, Analytical Methods, Risk Factors, and Control Options. Clin. Infect. Dis. 2013;56:1030–1037. doi: 10.1093/cid/cis1043. [DOI] [PubMed] [Google Scholar]
- 104.Gaygısız Ü., Lajunen T., Gaygısız E. Socio-Economic Factors, Cultural Values, National Personality and Antibiotics Use: A Cross-Cultural Study among European Countries. J. Infect. Public Health. 2017;10:755–760. doi: 10.1016/j.jiph.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 105.Park S., Kang J.E., Choi H.J., Kim C.J., Chung E.K., Kim S.A., Rhie S.J. Antimicrobial Stewardship Programs in Community Health Systems Perceived by Physicians and Pharmacists: A Qualitative Study with Gap Analysis. Antibiotics. 2019;8:252. doi: 10.3390/antibiotics8040252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mitchell J., Cooke P., Ahorlu C., Arjyal A., Baral S., Carter L., Dasgupta R., Fieroze F., Fonseca-Braga M., Huque R., et al. Community Engagement: The Key to Tackling Antimicrobial Resistance (AMR) across a One Health Context? Glob. Public Health. 2022;17:2647–2664. doi: 10.1080/17441692.2021.2003839. [DOI] [PubMed] [Google Scholar]
- 107.Rutter H., Savona N., Glonti K., Bibby J., Cummins S., Finegood D.T., Greaves F., Harper L., Hawe P., Moore L., et al. The Need for a Complex Systems Model of Evidence for Public Health. Lancet. 2017;390:2602–2604. doi: 10.1016/S0140-6736(17)31267-9. [DOI] [PubMed] [Google Scholar]
- 108.Van den Bunt G., Fluit A.C., Spaninks M.P., Timmerman A.J., Geurts Y., Kant A., Scharringa J., Mevius D., Wagenaar J.A., Bonten M.J.M., et al. Faecal Carriage, Risk Factors, Acquisition and Persistence of ESBL-Producing Enterobacteriaceae in Dogs and Cats and Co-Carriage with Humans Belonging to the Same Household. J. Antimicrob. Chemother. 2020;75:342–350. doi: 10.1093/jac/dkz462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sheppard S.K., Guttman D.S., Fitzgerald J.R. Population Genomics of Bacterial Host Adaptation. Nat. Rev. Gen. 2018;19:549–565. doi: 10.1038/s41576-018-0032-z. [DOI] [PubMed] [Google Scholar]
- 110.Bonten M.J.M., Mevius D. Less Evidence for an Important Role of Food-Producing Animals as Source of Antibiotic Resistance in Humans. Clin. Infect. Dis. 2015;60:1867. doi: 10.1093/cid/civ275. [DOI] [PubMed] [Google Scholar]
- 111.Pruden A., Larsson D.G.J., Amézquita A., Collignon P., Brandt K.K., Graham D.W., Lazorchak J.M., Suzuki S., Silley P., Snape J.R., et al. Management Options for Reducing the Release of Antibiotics and Antibiotic Resistance Genes to the Environment. Environ. Health Perspect. 2013;121:878–885. doi: 10.1289/ehp.1206446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mounier-Jack S., Griffiths U.K., Closser S., Burchett H., Marchal B. Measuring the Health Systems Impact of Disease Control Programmes: A Critical Reflection on the WHO Building Blocks Framework. BMC Public Health. 2014;14:1–8. doi: 10.1186/1471-2458-14-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sunsern R., Lawang W. Bronfenbrenner’s Ecological Model: Theoretical Lens for a Community-Based Research. J. Health Sci. Altern. Med. 2019;1:4–7. [Google Scholar]
- 114.Bronfenbrenner U., Morris P.A. Handbook of Child Psychology. Theoretical Models of Human Development. Volume 1. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2007. The Bioecological Model of Human Development; p. 793. [Google Scholar]
- 115.de Kraker M.E.A., Stewardson A.J., Harbarth S. Will 10 Million People Die a Year Due to Antimicrobial Resistance by 2050? PLoS Med. 2016;13:e1002184. doi: 10.1371/journal.pmed.1002184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jernigan J.A., Hatfield K.M., Wolford H., Nelson R.E., Olubajo B., Reddy S.C., McCarthy N., Paul P., McDonald L.C., Kallen A., et al. Multidrug-Resistant Bacterial Infections in U.S. Hospitalized Patients, 2012–2017. N. Engl. J. Med. 2022;382:1309–1319. doi: 10.1056/NEJMoa1914433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Infectious Diseases Society of America (IDSA) Drug-Resistant Infections Led to $1.9 Billion in Health Care Costs, More Than 10,000 Deaths Among Older Adults in One Year. [(accessed on 21 January 2023)]. Available online: https://www.idsociety.org/news--publications-new/articles/2021/drug-resistant-infections-led-to-$1.9-billion-in-health-care-costs-more-than-10000-deaths-among-older-adults-in-one-year/
- 118.Grimshaw A., Ferrari A., Lindahl G., Holcomb K. Metasystems. Commun. ACM. 1998;41:46–47. doi: 10.1145/287831.287839. [DOI] [Google Scholar]
- 119.Campos M., Capilla R., Naya F., Futami R., Coque T., Moya A., Fernandez-Lanza V., Cantón R., Sempere J.M., Llorens C., et al. Simulating Multilevel Dynamics of Antimicrobial Resistance in a Membrane Computing Model. mBio. 2019;10:e02460-18. doi: 10.1128/mBio.02460-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Grundmann H. Towards a Global Antibiotic Resistance Surveillance System: A Primer for a Roadmap. Ups J. Med. Sci. 2014;119:87–95. doi: 10.3109/03009734.2014.904458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Diallo O.O., Baron S.A., Abat C., Colson P., Chaudet H., Rolain J.M. Antibiotic Resistance Surveillance Systems: A Review. J. Glob. Antimicrob. Resist. 2020;23:430–438. doi: 10.1016/j.jgar.2020.10.009. [DOI] [PubMed] [Google Scholar]
- 122.Tacconelli E., Sifakis F., Harbarth S., Schrijver R., van Mourik M., Voss A., Sharland M., Rajendran N.B., Rodríguez-Baño J., Bielicki J., et al. Surveillance for Control of Antimicrobial Resistance. Lancet Infect Dis. 2018;18:e99–e106. doi: 10.1016/S1473-3099(17)30485-1. [DOI] [PubMed] [Google Scholar]
- 123.Johnson A.P. Surveillance of Antibiotic Resistance. Philos. Trans. R. Soc. B Biol. Sci. 2015;370:20140080. doi: 10.1098/rstb.2014.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Frost I., Kapoor G., Craig J., Liu D., Laxminarayan R. Status, Challenges and Gaps in Antimicrobial Resistance Surveillance around the World. J. Glob. Antimicrob. Resist. 2021;25:222–226. doi: 10.1016/j.jgar.2021.03.016. [DOI] [PubMed] [Google Scholar]
- 125.Woolhouse M.E.J., Dye C., Etard J.F., Smith T., Charlwood J.D., Garnett G.P., Hagan P., Hii J.L.K., Ndhlovu P.D., Quinnell R.J., et al. Heterogeneities in the Transmission of Infectious Agents: Implications for the Design of Control Programs. Proc. Natl. Acad. Sci. USA. 1997;94:338. doi: 10.1073/pnas.94.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gough E.K. The Impact of Mass Drug Administration of Antibiotics on the Gut Microbiota of Target Populations. Infect Dis. Poverty. 2022;11:76. doi: 10.1186/s40249-022-00999-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nellums L.B., Thompson H., Holmes A., Castro-Sánchez E., Otter J.A., Norredam M., Friedland J.S., Hargreaves S. Antimicrobial Resistance among Migrants in Europe: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2018;18:796–811. doi: 10.1016/S1473-3099(18)30219-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lloyd-Smith J.O., Schreiber S.J., Kopp P.E., Getz W.M. Superspreading and the Effect of Individual Variation on Disease Emergence. Nature. 2005;438:355. doi: 10.1038/nature04153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Baquero F., Saralegui C., Marcos-Mencía D., Ballestero L., Vañó-Galván S., Moreno-Arrones Ó.M., del Campo R. Epidermis as a Platform for Bacterial Transmission. Front. Immunol. 2021;12:4935. doi: 10.3389/fimmu.2021.774018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sibani M., Mazzaferri F., Carrara E., Pezzani M.D., Arieti F., Göpel S., Paul M., Tacconelli E., Mutters N.T., Voss A. White Paper: Bridging the Gap between Surveillance Data and Antimicrobial Stewardship in Long-Term Care Facilities—Practical Guidance from the JPIAMR ARCH and COMBACTE-MAGNET EPI-Net Networks. J. Antimicrob. Chemother. 2020;75:ii33. doi: 10.1093/jac/dkaa427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pezzani M.D., Carrara E., Sibani M., Presterl E., Gastmeier P., Renk H., Kanj S.S., Velavan T.P., Song L.H., Leibovici L., et al. White Paper: Bridging the Gap between Human and Animal Surveillance Data, Antibiotic Policy and Stewardship in the Hospital Sector-Practical Guidance from the JPIAMR ARCH and COMBACTE-MAGNET EPI-Net Networks. J. Antimicrob. Chemother. 2020;75:II20–II32. doi: 10.1093/jac/dkaa426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Arieti F., Göpel S., Sibani M., Carrara E., Pezzani M.D., Murri R., Mutters N.T., Lòpez-Cerero L., Voss A., Cauda R., et al. White Paper: Bridging the Gap between Surveillance Data and Antimicrobial Stewardship in the Outpatient Sector-Practical Guidance from the JPIAMR ARCH and COMBACTE-MAGNET EPI-Net Networks. J. Antimicrob. Chemother. 2020;75:II42–II51. doi: 10.1093/jac/dkaa428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Feng J., Guo Z., Ai L., Liu J., Zhang X., Cao C., Xu J., Xia S., Zhou X.N., Chen J., et al. Establishment of an Indicator Framework for Global One Health Intrinsic Drivers Index Based on the Grounded Theory and Fuzzy Analytical Hierarchy-Entropy Weight Method. Infect. Dis. Poverty. 2022;11:121. doi: 10.1186/s40249-022-01042-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Naylor N.R., Lines J., Waage J., Wieland B., Knight G.M. Quantitatively Evaluating the Cross-Sectoral and One Health Impact of Interventions: A Scoping Review and Case Study of Antimicrobial Resistance. One Health. 2020;11:100194. doi: 10.1016/j.onehlt.2020.100194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ashley E.A., Recht J., Chua A., Dance D., Dhorda M., Thomas N.V., Ranganathan N., Turner P., Guerin P.J., White N.J., et al. An Inventory of Supranational Antimicrobial Resistance Surveillance Networks Involving Low- and Middle-Income Countries since 2000. J. Antimicrob. Chemother. 2018;73:1737–1749. doi: 10.1093/jac/dky026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Perkins K.M., Reddy S.C., Fagan R., Arduino M.J., Perz J.F. Investigation of Healthcare Infection Risks from Water-Related Organisms: Summary of CDC Consultations, 2014–2017. Infect. Control Hosp. Epidemiol. 2019;40:621–626. doi: 10.1017/ice.2019.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McTaggart L.R., Stapleton P.J., Eshaghi A., Soares D., Brisse S., Patel S.N., Kus J.V. Application of Whole Genome Sequencing to Query a Potential Outbreak of Elizabethkingia Anophelis in Ontario, Canada. Access Microbiol. 2019;1:e000017. doi: 10.1099/acmi.0.000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liguori K., Keenum I., Davis B.C., Calarco J., Milligan E., Harwood V.J., Pruden A. Antimicrobial Resistance Monitoring of Water Environments: A Framework for Standardized Methods and Quality Control. Environ. Sci. Technol. 2022;56:9149–9160. doi: 10.1021/acs.est.1c08918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fernando B., Coque T.M. Multilevel Population Genetics in Antibiotic Resistance. FEMS Microbiol. Rev. 2011;35:705–706. doi: 10.1111/j.1574-6976.2011.00293.x. [DOI] [PubMed] [Google Scholar]
- 140.Willems R.J.L., Hanage W.P., Bessen D.E., Feil E.J. Population Biology of Gram-Positive Pathogens: High-Risk Clones for Dissemination of Antibiotic Resistance. FEMS Microbiol. Rev. 2011;35:872–900. doi: 10.1111/j.1574-6976.2011.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Woodford N., Turton J.F., Livermore D.M. Multiresistant Gram-Negative Bacteria: The Role of High-Risk Clones in the Dissemination of Antibiotic Resistance. FEMS Microbiol. Rev. 2011;35:736–755. doi: 10.1111/j.1574-6976.2011.00268.x. [DOI] [PubMed] [Google Scholar]
- 142.Riley L.W. Pandemic Lineages of Extraintestinal Pathogenic Escherichia coli. Clin. Microbiol. Infect. 2014;20:380–390. doi: 10.1111/1469-0691.12646. [DOI] [PubMed] [Google Scholar]
- 143.Tedim A.P., Ruiz-Garbajosa P., Corander J., Rodríguez C.M., Cantón R., Willems R., Baquero F., Coque T.M. Population Biology of Enterococcus from Intestinal Colonization in Hospitalized and Non-Hospitalized Individuals in Different Age Groups. Appl. Environ. Microbiol. 2014;81:AEM-03661. doi: 10.1128/AEM.03661-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Valverde A., Grill F., Coque T.M., Pintado V., Baquero F., Canton R., Cobo J. High Rate of Intestinal Colonization with Extended-Spectrum- -Lactamase-Producing Organisms in Household Contacts of Infected Community Patients. J. Clin. Microbiol. 2008;46:2796–2799. doi: 10.1128/JCM.01008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Faith J.J., Colombel J.F., Gordon J.I. Identifying Strains That Contribute to Complex Diseases through the Study of Microbial Inheritance. Proc. Natl. Acad. Sci. USA. 2015;112:633–640. doi: 10.1073/pnas.1418781112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Croucher N.J., Klugman K.P. The Emergence of Bacterial “Hopeful Monsters”. MBio. 2014;5:e01550-14. doi: 10.1128/mBio.01550-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cointe A., Birgy A., Mariani-Kurkdjian P., Liguori S., Courroux C., Blanco J., Delannoy S., Fach P., Loukiadis E., Bidet P., et al. Emerging Multidrug-Resistant Hybrid Pathotype Shiga Toxin–Producing Escherichia coli O80 and Related Strains of Clonal Complex 165, Europe. Emerg. Infect. Dis. 2018;24:2262. doi: 10.3201/eid2412.180272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.European Centre for Disease Prevention and Control (ECDC) ECDC strategic framework for the integration of molecular and genomic typing into European surveillance and multi-country outbreak investigations 2019–2021. Stockholm: ECDC; 2019. [(accessed on 28 March 2023)]. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/framework-for-genomic-surveillance.pdf.
- 149.Hendriksen R.S., Bortolaia V., Tate H., Tyson G.H., Aarestrup F.M., McDermott P.F. Using Genomics to Track Global Antimicrobial Resistance. Front. Public Health. 2019;7:242. doi: 10.3389/fpubh.2019.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Amaro F., Martín-González A. Microbial Warfare in the Wild-the Impact of Protists on the Evolution and Virulence of Bacterial Pathogens. Int. Microbiol. 2021;24:559–571. doi: 10.1007/s10123-021-00192-y. [DOI] [PubMed] [Google Scholar]
- 151.Baquero F., Martínez J.L., Lanza V.F., Rodríguez-Beltrán J., Galán J.C., San Millán A., Cantón R., Coque T.M. Evolutionary Pathways and Trajectories in Antibiotic Resistance. Clin. Microbiol. Rev. 2021;34:e00050-19. doi: 10.1128/CMR.00050-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Constantinides B., Chau K.K., Phuong Quan T., Rodger G., Andersson M.I., Jeffery K., Lipworth S., Gweon H.S., Peniket A., Pike G., et al. Genomic Surveillance of Escherichia coli and Klebsiella spp. in Hospital Sink Drains and Patients. Microb. Genom. 2020;6:4–16. doi: 10.1099/mgen.0.000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lipworth S., Matlock W., Shaw L., Rodger G., Chau K., Barker L., George S., Kavanagh J., Davies T., Vaughan A., et al. The Mobilome Associated with Gram-Negative Bloodstream Infections: A Large-Scale Observational Hybrid Sequencing Based Study. medRxiv. :2022. doi: 10.1101/2022.04.03.22273290. [DOI] [Google Scholar]
- 154.Rodrigues C., Lanza V.F., Peixe L., Coque T.M., Novais Â., the Epidemiological Markers Study Group (ESGEM) Phylogenomics of Globally Spread Clonal Groups 14 and 15 of Klebsiella pneumoniae. bioRxiv. :2022. doi: 10.1101/2022.08.30.505806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Johnson T.J., Danzeisen J.L., Youmans B., Case K., Llop K., Munoz-Aguayo J., Flores-Figueroa C., Aziz M., Stoesser N., Sokurenko E., et al. Separate F-Type Plasmids Have Shaped the Evolution of the H30 Subclone of Escherichia coli Sequence Type 131. MSphere. 2016;1:e00121-16. doi: 10.1128/mSphere.00121-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lanza V.F., Tedim A.P., Martínez J.L., Baquero F., Coque T.M. The Plasmidome of Firmicutes: Impact on the Emergence and the Spread of Resistance to Antimicrobials. Microbiol. Spectr. 2015;3:379–419. doi: 10.1128/microbiolspec.PLAS-0039-2014. [DOI] [PubMed] [Google Scholar]
- 157.León-Sampedro R., DelaFuente J., Díaz-Agero C., Crellen T., Musicha P., Rodríguez-Beltrán J., de la Vega C., Hernández-García M., López-Fresneña N., Ruiz-Garbajosa P., et al. Pervasive Transmission of a Carbapenem Resistance Plasmid in the Gut Microbiota of Hospitalized Patients. Nat. Microbiol. 2021;6:606–616. doi: 10.1038/s41564-021-00879-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tato M., Coque T.M., Ruíz-Garbajosa P., Pintado V., Cobo J., Sader H.S., Jones R.N., Baquero F., Cantón R. Complex Clonal and Plasmid Epidemiology in the First Outbreak of Enterobacteriaceae Infection Involving VIM-1 Metallo-Beta-Lactamase in Spain: Toward Endemicity? Clin. Infect. Dis. 2007;45:1171–1178. doi: 10.1086/522288. [DOI] [PubMed] [Google Scholar]
- 159.David S., Cohen V., Reuter S., Sheppard A.E., Giani T., Parkhill J., Rossolini G.M., Feil E.J., Grundmann H., Aanensen D.M. Integrated Chromosomal and Plasmid Sequence Analyses Reveal Diverse Modes of Carbapenemase Gene Spread among Klebsiella pneumoniae. Proc. Natl. Acad. Sci. USA. 2020;117:25043–25054. doi: 10.1073/pnas.2003407117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Joint Programming Initiative on Antimicrobial Resistance (JPIAMR) TEAPOTS (Tools for the Epidemiology of AMR Plasmids, One-Health Transmission and Surveillance) [(accessed on 28 March 2023)]. Available online: https://www.jpiamr.eu/api-projects/teapots/
- 161.Ko K.K.K., Chng K.R., Nagarajan N. Metagenomics-Enabled Microbial Surveillance. Nat. Microbiol. 2022;7:486–496. doi: 10.1038/s41564-022-01089-w. [DOI] [PubMed] [Google Scholar]
- 162.Pérez-Cobas A.E., Baquero F., de Pablo R., Soriano M.C., Coque T.M. Altered Ecology of the Respiratory Tract Microbiome and Nosocomial Pneumonia. Front. Microbiol. 2022;12:4295. doi: 10.3389/fmicb.2021.709421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lee K., Raguideau S., Sirén K., Asnicar F., Cumbo F., Hildebrand F., Segata N., Cha C.J., Quince C. Population-Level Impacts of Antibiotic Usage on the Human Gut Microbiome. Nature Commun. 2023;14:1–19. doi: 10.1038/s41467-023-36633-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fierer N., Ferrenberg S., Flores G.E., González A., Kueneman J., Legg T., Lynch R.C., McDonald D., Mihaljevic J.R., O’Neill S.P., et al. From Animalcules to an Ecosystem: Application of Ecological Concepts to the Human Microbiome. Ann. Rev. Ecol. Evol Syst. 2012;43:137–155. doi: 10.1146/annurev-ecolsys-110411-160307. [DOI] [Google Scholar]
- 165.Kang J.T.L., Teo J.J.Y., Bertrand D., Ng A., Ravikrishnan A., Yong M., Ng O.T., Marimuthu K., Chen S.L., Chng K.R., et al. Long-Term Ecological and Evolutionary Dynamics in the Gut Microbiomes of Carbapenemase-Producing Enterobacteriaceae Colonized Subjects. Nature Microbiol. 2022;7:1516–1524. doi: 10.1038/s41564-022-01221-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.National Academies of Sciences, Engineering, and Medicine . Microbiomes of the Built Environment: A Research Agenda for Indoor Microbiology, Human Health, and Buildings. The National Academies Press; Washington, DC, USA: 2017. [PubMed] [Google Scholar]
- 167.Mahnert A., Moissl-Eichinger C., Zojer M., Bogumil D., Mizrahi I., Rattei T., Martinez J.L., Berg G. Man-Made Microbial Resistances in Built Environments. Nat. Commun. 2019;10:968. doi: 10.1038/s41467-019-08864-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gibbons S.M. The Built Environment Is a Microbial Wasteland. mSystems. 2016;1:e00033-16. doi: 10.1128/mSystems.00033-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gilbert J.A. Ecological Medicine. Environ. Microbiol. 2018;20:1917–1919. doi: 10.1111/1462-2920.14115. [DOI] [PubMed] [Google Scholar]
- 170.Jeffery I.B., Claesson M.J., O’Toole P.W., Shanahan F. Categorization of the Gut Microbiota: Enterotypes or Gradients? Nat. Rev. Microbiol. 2012;10:591–592. doi: 10.1038/nrmicro2859. [DOI] [PubMed] [Google Scholar]
- 171.Santiago M., Eysenbach L., Allegretti J., Aroniadis O., Brandt L.J., Fischer M., Grinspan A., Kelly C., Morrow C., Rodriguez M., et al. Microbiome Predictors of Dysbiosis and VRE Decolonization in Patients with Recurrent Clostridium difficile Infections in a Multi-Center Retrospective Study. AIMS Microbiol. 2019;5:1–18. doi: 10.3934/microbiol.2019.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Popoola O.O., Madhur G., Mehrim M.M., Omondi M.O., Owusu-Mensah P., Mamtani S.A., Etukakpan A.U. A Literature Review on the Global Burden and Impact of Substandard and Falsified Medicine. Ann. Public Health Issues. 2022;2:16–31. doi: 10.2478/aphi-2022-0003. [DOI] [Google Scholar]
- 173.Tedijanto C., Grad Y.H., Lipsitch M. Potential Impact of Outpatient Stewardship Interventions on Antibiotic Exposures of Common Bacterial Pathogens. Elife. 2020;9:e52307. doi: 10.7554/eLife.52307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tedijanto C., Olesen S.W., Grad Y.H., Lipsitch M. Estimating the Proportion of Bystander Selection for Antibiotic Resistance among Potentially Pathogenic Bacterial Flora. Proc. Natl. Acad. Sci. USA. 2018;115:E11988–E11995. doi: 10.1073/pnas.1810840115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wei S., Bahl M.I., Baunwall S.M.D., Hvas C.L., Licht T.R. Determining Gut Microbial Dysbiosis: A Review of Applied Indexes for Assessment of Intestinal Microbiota Imbalances. Appl. Environ. Microbiol. 2021;87:1–13. doi: 10.1128/AEM.00395-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Laxminarayan R., Klugman K.P. Communicating Trends in Resistance Using a Drug Resistance Index. BMJ Open. 2011;1:e000135. doi: 10.1136/bmjopen-2011-000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ashbolt N.J., Amézquita A., Backhaus T., Borriello P., Brandt K.K., Collignon P., Coors A., Finley R., Gaze W.H., Heberer T., et al. Human Health Risk Assessment (HHRA) for Environmental Development and Transfer of Antibiotic Resistance. Environ. Health Perspect. 2013;121:993–1001. doi: 10.1289/ehp.1206316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pires S.M., Duarte A.S., Hald T. Source Attribution and Risk Assessment of Antimicrobial Resistance. Microbiol. Spectr. 2018;6:3. doi: 10.1128/microbiolspec.ARBA-0027-2017. [DOI] [PubMed] [Google Scholar]
- 179.Brul S., Bassett J., Cook P., Kathariou S., McClure P., Jasti P.R., Betts R. ‘Omics’ Technologies in Quantitative Microbial Risk Assessment. Trends Food Sci. Technol. 2012;27:12–24. doi: 10.1016/j.tifs.2012.04.004. [DOI] [Google Scholar]
- 180.Quality Compliance Systems. Infection Control Policy and the Importance of Risk Assessment. [(accessed on 28 March 2023)]. Available online: https://www.qcs.co.uk/infection-control-policy-importance-risk-assessment/
- 181.Anderson M., Schulze K., Cassini A., Plachouras D., Mossialos E. A Governance Framework for Development and Assessment of National Action Plans on Antimicrobial Resistance. Lancet Infect. Dis. 2019;19:e371–e384. doi: 10.1016/S1473-3099(19)30415-3. [DOI] [PubMed] [Google Scholar]
- 182.Der Hertog P., Jager C.-J., Vankan A., te Velde R., Veldkamp J., Aksnes D.W., Sivertsen G., van Leuween T., van Wijk E. Science, Technology and Innovation Indicators. Thematic paper 1. Challenges in Measuring the Efficiency of National Science, Technology & Innovation Systems. 2014. [(accessed on 28 March 2023)]. Available online: www.sti2.nl.
- 183.Berryhill B.A., Gil-Gil T., Manuel J.A., Smith A.P., Margollis E., Baquero F., Levin B.R. What’s the Matter with MICs: The Contribution of Nutrients and Limiting Resources to the Pharmacodynamics of Antibiotics and Bacteria. bioRxiv. :2022. doi: 10.1101/2022.09.30.510422. [DOI] [Google Scholar]
- 184.Aschbacher R., Pagani L., Migliavacca R., Pagani L., Confalonieri M., Farina C., Fazii P., Luzzaro F., Rigoli R., Spalla M. Recommendations for the Surveillance of Multidrug-Resistant Bacteria in Italian Long-Term Care Facilities by the GLISTer Working Group of the Italian Association of Clinical Microbiologists (AMCLI) Antimicrob. Resist. Infect. Control. 2020;9:106. doi: 10.1186/s13756-020-00771-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.The BMJ Opinion Antimicrobial Resistance: More Quick Action Is Needed in BRICS and MINT Economic Transition Countries. [(accessed on 2 October 2019)]. Available online: https://blogs.bmj.com/bmj/2019/10/02/antimicrobial-resistance-more-quick-action-is-needed-in-brics-and-mint-economic-transition-countries/
- 186.Scherer L., de Koning A., Tukker A. BRIC and MINT Countries’ Environmental Impacts Rising despite Alleviative Consumption Patterns. Sci. Total Environ. 2019;665:52–60. doi: 10.1016/j.scitotenv.2019.02.103. [DOI] [PubMed] [Google Scholar]
- 187.Malik B., Bhattacharyya S. Antibiotic Drug-Resistance as a Complex System Driven by Socio-Economic Growth and Antibiotic Misuse. Sci. Rep. 2019;9:9788. doi: 10.1038/s41598-019-46078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Avershina E., Shapovalova V., Shipulin G. Fighting Antibiotic Resistance in Hospital-Acquired Infections: Current State and Emerging Technologies in Disease Prevention, Diagnostics and Therapy. Front. Microbiol. 2021;12:2044. doi: 10.3389/fmicb.2021.707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Centers for Disease Control and Prevention (CDC) Antibiotic Resistance Threats Report|CDC. [(accessed on 28 March 2023)];2019 Available online: https://www.cdc.gov/drugresistance/biggest-threats.html.
- 190.Abat C., Fournier P.E., Jimeno M.T., Rolain J.M., Raoult D. Extremely and Pandrug-Resistant Bacteria Extra-Deaths: Myth or Reality? Eur. J. Clin. Microbiol. Infect. Dis. 2018;37:1687–1697. doi: 10.1007/s10096-018-3300-0. [DOI] [PubMed] [Google Scholar]
- 191.U.S. Environmental Protection Agency The Indoor Microbiome. [(accessed on 28 March 2023)]; Available online: https://www.epa.gov/indoor-air-quality-iaq/indoor-microbiome#microbiome.
- 192.Dalton K.R., Rock C., Carroll K.C., Davis M.F. One Health in Hospitals: How Understanding the Dynamics of People, Animals, and the Hospital Built-Environment Can Be Used to Better Inform Interventions for Antimicrobial-Resistant Gram-Positive Infections. Antimicrob. Resist. Infect. Control. 2020;9:78. doi: 10.1186/s13756-020-00737-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Christoff A.P., Sereia A.F.R., Hernandes C., de Oliveira L.F.V. Uncovering the Hidden Microbiota in Hospital and Built Environments: New Approaches and Solutions. Exp. Biol. Med. 2019;244:534. doi: 10.1177/1535370218821857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kuntz T.M., Gilbert J.A. Introducing the Microbiome into Precision Medicine. Trends Pharmacol. Sci. 2017;38:81–91. doi: 10.1016/j.tips.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 195.Rodríguez I., Figueiredo A.S., Sousa M., Lanza V., Rodríguez C., Mingo P., Zamora J., Loza E., Brooks C., Cantón R., et al. A 21-Year Survey of Escherichia coli from Bloodstream Infections (BSIs) in a Tertiary Hospital Reveals How Community-Hospital Dynamics, Influence Local BSI Rates, the Trends of the B2 Phylogroup and the STc131 Pandemic Clone. mSphere. 2021;6:e0086821. doi: 10.1128/msphere.00868-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Horcajada J.P., Shaw E., Padilla B., Pintado V., Calbo E., Benito N., Gamallo R., Gozalo M., Rodríguez-Baño J. Healthcare-Associated, Community-Acquired and Hospital-Acquired Bacteraemic Urinary Tract Infections in Hospitalized Patients: A Prospective Multicentre Cohort Study in the Era of Antimicrobial Resistance. Clin. Microbiol. Infect. 2013;19:962–968. doi: 10.1111/1469-0691.12089. [DOI] [PubMed] [Google Scholar]
- 197.Laupland K.B., Church D.L. Population-Based Epidemiology and Microbiology of Community-Onset Bloodstream Infections. Clin. Microbiol. Rev. 2014;27:647–664. doi: 10.1128/CMR.00002-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Chau K.K., Barker L., Budgell E.P., Vihta K.D., Sims N., Kasprzyk-Hordern B., Harriss E., Crook D.W., Read D.S., Walker A.S., et al. Systematic Review of Wastewater Surveillance of Antimicrobial Resistance in Human Populations. Environ. Int. 2022;162:107171. doi: 10.1016/j.envint.2022.107171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Millennium Ecosystem Assessment, 2005. Island Press; Washington, DC, USA: 2005. Ecosystems and Human Well-being: Synthesis. [Google Scholar]
- 200.The New York Times. Young Roosevelt Saved By New Drug. [(accessed on 28 March 2023)]. Available online: https://www.nytimes.com/1936/12/17/archives/young-roosevelt-saved-by-new-drug-doctor-used-prontylin-in-fight-on.html.
- 201.TIME Mold for Infections. [(accessed on 28 March 2023)]. Available online: https://time.com/vault/issue/1941-09-15/page/57/
- 202.Report Joint Committee on the Use of Antibiotics in Animal Husbandry and Veterinary Medicine-Google Libros. [(accessed on 28 March 2023)]. Available online: https://books.google.es/books/about/Report_Joint_Committee_on_the_Use_of_Ant.html?id=JOtFtAEACAAJ&redir_esc=y.
- 203.Huttner B., Saam M., Moja L., Mah K., Sprenger M., Harbarth S., Magrini N. How to Improve Antibiotic Awareness Campaigns: Findings of a WHO Global Survey. BMJ Glob. Health. 2019;4:1239. doi: 10.1136/bmjgh-2018-001239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Joint Programming Initiative on Antimicrobial Resistance (JPIAMR) [(accessed on 28 March 2023)]. Available online: https://www.jpiamr.eu/
- 205.Larsson D.G.J., Andremont A., Bengtsson-Palme J., Brandt K.K., de Roda Husman A.M., Fagerstedt P., Fick J., Flach C.F., Gaze W.H., Kuroda M., et al. Critical Knowledge Gaps and Research Needs Related to the Environmental Dimensions of Antibiotic Resistance. Environ. Int. 2018;117:132–138. doi: 10.1016/j.envint.2018.04.041. [DOI] [PubMed] [Google Scholar]
- 206.United Nations Environmental Programme (UNEP) Summary for Policymakers-Environmental Dimensions of Antimicrobial Resistance. [(accessed on 28 February 2022)]. Available online: https://www.unep.org/resources/report/summary-policymakers-environmental-dimensions-antimicrobial-resistance.
- 207.Murray A.K., Stanton I., Gaze W.H., Snape J. Dawning of a New ERA: Environmental Risk Assessment of Antibiotics and Their Potential to Select for Antimicrobial Resistance. Water Res. 2021;200:117233. doi: 10.1016/j.watres.2021.117233. [DOI] [PubMed] [Google Scholar]
- 208.Semenza J.C. Lateral Public Health: Advancing Systemic Resilience to Climate Change. Lancet Reg. Health Eur. 2021;9:100231. doi: 10.1016/j.lanepe.2021.100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Pollack L.A., Srinivasan A. Core Elements of Hospital Antibiotic Stewardship Programs from the Centers for Disease Control and Prevention. Clin. Infect. Dis. 2014;59((Suppl. S3)):S97–S100. doi: 10.1093/cid/ciu542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Schuts E.C., Hulscher M.E.J.L., Mouton J.W., Verduin C.M., Stuart J.W.T.C., Overdiek H.W.P.M., van der Linden P.D., Natsch S., Hertogh C.M.P.M., Wolfs T.F.W., et al. Current Evidence on Hospital Antimicrobial Stewardship Objectives: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2016;16:847–856. doi: 10.1016/S1473-3099(16)00065-7. [DOI] [PubMed] [Google Scholar]
- 211.Villa L., Carattoli A. Methods in Molecular Biology. Volume 2075. Humana Press Inc.; New York, NY, USA: 2020. Plasmid Typing and Classification; pp. 309–321. [DOI] [PubMed] [Google Scholar]
- 212.Redondo-Salvo S., Fernández-López R., Ruiz R., Vielva L., de Toro M., Rocha E.P.C., Garcillán-Barcia M.P., de la Cruz F. Pathways for Horizontal Gene Transfer in Bacteria Revealed by a Global Map of Their Plasmids. Nat. Commun. 2020;11:3602. doi: 10.1038/s41467-020-17278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Workman C.L. Syndemics and Global Health. Nature Hum. Behav. 2021;6:25–26. doi: 10.1038/s41562-021-01249-8. [DOI] [PubMed] [Google Scholar]
- 214.Virginia M., Riquelme P., Garner E., Gupta S., Metch J., Zhu N., Blair M.F., Arango-Argoty G., Maile-Moskowitz A., Li A.-D., et al. Demonstrating a Comprehensive Wastewater-Based Surveillance Approach That Differentiates Globally Sourced Resistomes. Environ. Sci. Technol. 2022;56:14982–14993. doi: 10.1021/acs.est.1c08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Morley V.J., Woods R.J., Read A.F. Bystander Selection for Antimicrobial Resistance: Implications for Patient Health. Trends Microbiol. 2019;27:864–877. doi: 10.1016/j.tim.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Baquero F., Coque T.M., Guerra-Pinto N., Galán J.C., Jiménez-Lalana D., Tamames J., Pedrós-Alió C. The Influence of Coalescent Microbiotic Particles From Water and Soil on the Evolution and Spread of Antimicrobial Resistance. Front. Environ. Sci. 2022;10:385. doi: 10.3389/fenvs.2022.824963. [DOI] [Google Scholar]
- 217.Baquero F., Coque T.M., Martínez J.L. Natural Detoxification of Antibiotics in the Environment: A One Health Perspective. Front. Microbiol. 2022;13:1062399. doi: 10.3389/fmicb.2022.1062399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Murray A.K., Zhang L., Yin X., Zhang T., Buckling A., Snape J., Gaze W.H. Novel Insights into Selection for Antibiotic Resistance in Complex Microbial Communities. mBio. 2018;9:e00969-18. doi: 10.1128/mBio.00969-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Levy S.B., Fitzgerald G.B., Macone A.B. Spread of Antibiotic-Resistant Plasmids from Chicken to Chicken and from Chicken to Man. Nature. 1976;260:40–42. doi: 10.1038/260040a0. [DOI] [PubMed] [Google Scholar]
- 220.Martínez J.L., Coque T.M., Baquero F. What Is a Resistance Gene? Ranking Risk in Resistomes. Nat. Rev. Microbiol. 2015;13:116–123. doi: 10.1038/nrmicro3399. [DOI] [PubMed] [Google Scholar]
- 221.EUCAST: New S, I and R Definitions. [(accessed on 4 September 2022)]. Available online: https://www.eucast.org/newsiandr/
- 222.Davison H.C., Woolhouse M.E.J., Low J.C. What Is Antibiotic Resistance and How Can We Measure It? Trends Microbiol. 2000;8:554–559. doi: 10.1016/S0966-842X(00)01873-4. [DOI] [PubMed] [Google Scholar]
- 223.Cantón R. Antibiotic Resistance Genes from the Environment: A Perspective through Newly Identified Antibiotic Resistance Mechanisms in the Clinical Setting. Clin. Microbiol. Infect. 2009;15((Suppl. S1)):20–25. doi: 10.1111/j.1469-0691.2008.02679.x. [DOI] [PubMed] [Google Scholar]
- 224.Park C. In: The Environment: Principles and Applications. Gregory J., Walling E.K., editors. Routledge; London, UK: 2001. [Google Scholar]
- 225.Tansley A.G. “The Use and Abuse of Vegetational Concepts and Terms” (1935) In: Robin L., Sörlin S., Warde P., editors. The Future of Nature: Documents of Global Change. Yale University Press; New Haven, CT, USA: 2013. pp. 220–232. [DOI] [Google Scholar]
- 226.Willis A.J. The Ecosystem: An Evolving Concept Viewed Historically. Funct. Ecol. 1997;11:268–271. doi: 10.1111/j.1365-2435.1997.00081.x. [DOI] [Google Scholar]
- 227.Exposome and Exposomics|NIOSH|CDC. [(accessed on 11 September 2022)]; Available online: https://www.cdc.gov/niosh/topics/exposome/default.html.
- 228.Gillings M.R. Lateral Gene Transfer, Bacterial Genome Evolution, and the Anthropocene. Ann. N. Y. Acad. Sci. 2017;1389:20–36. doi: 10.1111/nyas.13213. [DOI] [PubMed] [Google Scholar]
- 229.Tomson G., Vlad I. The Need to Look at Antibiotic Resistance from a Health Systems Perspective. Ups J. Med. Sci. 2014;119:117. doi: 10.3109/03009734.2014.902879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Berman J.J. Changing How We Think about Infectious Diseases. Taxon. Guide Infect. Dis. 2019:321–365. doi: 10.1016/B978-0-12-817576-7.00008-0. [DOI] [Google Scholar]
- 231.Last J.M. Dictionary of Epidemiology. CMAJ Can. Med. Assoc. J. 1993;149:400. doi: 10.1136/jech.47.5.430. [DOI] [Google Scholar]
- 232.Lederberg J. Plasmid (1952–1997) Plasmid. 1998;39:1–9. doi: 10.1006/plas.1997.1320. [DOI] [PubMed] [Google Scholar]
- 233.Lederberg J., Lederberg E. Infection and Heredity. In: Rudnick D., editor. Cellular Mechanisms in Differentiation and Growth. Princeton University Press; Princeton, NJ, USA: 1956. pp. 101–124. [Google Scholar]
- 234.Baquero F. From Pieces to Patterns: Evolutionary Engineering in Bacterial Pathogens. Nat. Rev. Microbiol. 2004;2:510–518. doi: 10.1038/nrmicro909. [DOI] [PubMed] [Google Scholar]
- 235.D’Costa V.M., McGrann K.M., Hughes D.W., Wright G.D. Sampling the Antibiotic Resistome. Science (1979) 2006;311:374–377. doi: 10.1126/science.1120800. [DOI] [PubMed] [Google Scholar]
- 236.Lanza V.F., Baquero F., Martínez J.L., Ramos-Ruíz R., González-Zorn B., Andremont A., Sánchez-Valenzuela A., Ehrlich S.D., Kennedy S., Ruppé E., et al. In-Depth Resistome Analysis by Targeted Metagenomics. Microbiome. 2018;6:11. doi: 10.1186/s40168-017-0387-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Tacconelli E., Carrara E., Savoldi A., Harbarth S., Mendelson M., Monnet D.L., Pulcini C., Kahlmeter G., Kluytmans J., Carmeli Y., et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018;18:318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 238.Frieden T. Antibiotic Resistance Threats. CDC. 2013:22–50. [Google Scholar]
- 239.World Health Organization (WHO) WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action. [(accessed on 25 October 2022)]. Available online: https://www.who.int/publications/i/item/9789240060241.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.