Abstract
Many plant-derived flavonoids are known for their anti-neuroinflammatory and anti-neurodegenerative effects. The fruits and leaves of the black currant (BC, Ribes nigrum) contain these phytochemicals with therapeutic benefits. The current study presents a report on a standardized BC gemmotherapy extract (BC-GTE) that is prepared from fresh buds. It provides details about the phytoconstituent profile specific to the extract as well as the associated antioxidant and anti-neuroinflammatory properties. The reported BC-GTE was found to contain approximately 133 phytonutrients, making it unique in its composition. Furthermore, this is the first report to quantify the presence of significant flavonoids such as luteolin, quercetin, apigenin, and kaempferol. Drosophila melanogaster-based tests revealed no cytotoxic but nutritive effects. We also demonstrated that adult male Wistar rats, pretreated with the analyzed BC-GTE and assessed after lipopolysaccharide (LPS) injection, did not show any apparent increase in body size in the microglial cells located in the hippocampal CA1 region, while in control experiments, the activation of microglia was evident. Moreover, no elevated levels of serum-specific TNF-α were observed under the LPS-induced neuroinflammatory condition. The analyzed BC-GTE’s specific flavonoid content, along with the experimental data based on an LPS-induced inflammatory model, suggest that it possesses anti-neuroinflammatory/neuroprotective properties. This indicates that the studied BC-GTE has the potential to be used as a GTE-based complementary therapeutic approach.
Keywords: Ribes nigrum, gemmotherapy, neuroinflammation, TNF-α, microglia
1. Introduction
In general terms, research on flavonoids involves the isolation, identification, characterization, and understanding of their functions, along with investigating potential health benefits associated with their consumption [1]. Flavonoids are an important class of natural phytonutrients and are well known for their protective effects against neurodegeneration and neuroinflammation [2,3]. The cytokine TNF-α (tumor necrosis factor alpha) is a pro-inflammatory molecule of the Th1-class and is known to play a crucial role in brain development by affecting the development and function of the hippocampus [4]. However, elevated levels of TNF-α can indicate the presence of inflammation. The neuroprotective effect of flavonoid-rich foods or drinks has been linked to enhanced neuronal connection and communication, including an ability to suppress neuroinflammation. These effects have the potential to promote memory, learning, and cognitive function in the hippocampus [5,6,7]. Growing evidence suggests that sustained neuroinflammation, caused by microglia activation, is implicated in the development of several neurological disorders [8]. The black currant (BC, Ribes nigrum) emerges as a superfood due to its phytonutrient profile and the associated health effects [9]. Some BC extracts prepared from fruits or leaves could elicit anti-inflammatory and antioxidant properties, respectively. A study on BC fruit juice had shown to lower TNF-α gene expression in LPS (lipopolysaccharide) induced cultured macrophages [10]. Other studies carried out on diet-induced obese mice demonstrated that a BC fruit extract reduced obesity-induced inflammation in adipose tissue and splenocytes by lowering TNF-α transcription [11]. Furthermore, BC fruit extract repressed obesity-associated M1 polarization of both murine and human macrophages by reducing the expression of pro-inflammatory genes such as TNF-α [12]. The above-mentioned studies, which mainly focused on in vitro experiments, suggested that BC extracts made from fruits or leaves had anti-inflammatory properties. However, no clear correlations were established between the biological effect and the extract-specific phytoconstituent profile, which remained largely unknown.
Therefore, we have proposed to obtain a BC-GTE that is standardized with respect to its flavonoid content and antioxidant activity, as these features would greatly facilitate the reproducibility of experiments. Next, we used a Drosophila melanogaster-based in vivo nutritional test system to assess the BC-GTE effect on viability. Furthermore, to investigate the anti-neuroinflammatory properties of our BC-GTE, we conducted an in vivo test using rats (Rattus norvegicus), where lipopolysaccharide was applied to induce inflammation through microglia activation [13]. The fact that natural flavonoids have anti-neuroinflammatory properties [14], along with the flavonoid content of the BC-GTE, provided strong justification for conducting experiments. Therefore, we examine the effect of BC-GTE pretreatment on microglia activation and TNF-α production during LPS-induced inflammation.
2. Results
2.1. The BC-GTE Contains Important Flavonoids and Features Relevant In Vitro Antioxidant Properties
The BC-GTE was subjected to a HPLC–ESI-MS analysis, which detected the presence of 133 phytonutrients. These phytonutrients were primarily from the flavonoid class of polyphenols, although other chemical compounds such as carboxylic acids, amino acids, vitamins, alkaloids, esters, terpenes, and others were also identified (Table 1). Our qualitative analysis of the GTE confirmed the presence of several phytonutrients that are specific to BC, including acacetin, ampelopsin, apigenin, astragalin, eriodictyol, genkwanin, isorhamnetin, narcissin, naringenin, rhamnetin, sakuranetin, tetrahydroxychalcone, galloylglucose, phloretin, phlorizin, tetrahydroxy-methoxy chalcone, and abscisic acid. This is the first time that the presence of these compounds has been confirmed through our analysis. Surprisingly, no anthocyanins could be revealed in the BC-GTE, though others have already reported their presence in BC fruits. The trigonelline and 4-hydroxy isoleucine were detected in the BC-GTE, while their incidence in the fenugreek (Trigonella foenum-graecum) was invoked to explain the associated antidiabetic properties [15]. Based on this similarity, it seems logical to predict an antidiabetic property for the BC-GTE, whereas this has already been confirmed for the fruits of BC [9]. Furthermore, the fact that kynurenic acid and tryptophan are present in BC-GTE suggests a potential neuroprotective function, as these compounds have been extensively studied for their implications in brain function and protection [16]. The identified components that are BC-GTE-specific suggest that its administration could produce several biological effects. It is also possible that the generated effects would have either synergistic, cumulative or even antagonistic features that could affect the viability of animal/human individuals fed/treated with BC-GTE.
Table 1.
The phytonutrient profile of BC-GTE.
| Chemical Classification | Bioactive Compounds |
|---|---|
| Alkaloids | Kynurenic acid |
| Trigonelline | |
| Amino Acids | 4-Guanidinobutyric acid |
| 4-Hydroxyisoleucine | |
| Asparagine | |
| Glutamic acid | |
| Isoleucine or Leucine | |
| Phenylalanine | |
| Proline | |
| Threonine | |
| Tryptophan | |
| Others | Hydroxybenzaldehyde |
| Indole-4-carbaldehyde | |
| Esters | Ethyl gallate |
| Sinapoyl-methoxyphenol | |
| Flavonoids | Acacetin |
| Ampelopsin (Ampeloptin, Dihydromyricetin) | |
| Apigenin | |
| Astragalin (Kaempferol-3-O-glucoside) | |
| Catechin | |
| Chrysoeriol | |
| Dihydrokaempferol (Aromadendrin, Katuranin) | |
| Dihydroxy-dimethoxyflavone isomer 1 | |
| Flavonoids (Continue)Dihydroxy-dimethoxyflavone isomer 2 | |
| Flavonoids (Continue) | Dihydroxy-dimethoxyisoflavan |
| Dihydroxy-methoxyflavanone-O-hexoside isomer 1 | |
| Dihydroxy-methoxyflavanone-O-hexoside isomer 2 | |
| Dihydroxy-trimethoxyflavone isomer 1 | |
| Dihydroxy-trimethoxyflavone isomer 2 | |
| Epicatechin | |
| Epigallocatechin | |
| Eriodictyol | |
| Genkwanin (Apigenin-7-O-methyl ether) | |
| Hydroxy-dimethoxyflavone | |
| Hydroxy-tetramethoxyflavone (Retusin) | |
| Hydroxy-trimethoxyflavone (Salvigenin) | |
| Hyperoside (Quercetin-3-O-galactoside, Hyperin) | |
| Isoquercitrin (Hirsutrin, Quercetin-3-O-glucoside) | |
| Isorhamnetin | |
| Isorhamnetin-3-O-glucoside | |
| Isorhamnetin-3-O-rutinoside (Narcissin) | |
| Isorhamnetin-O-hexoside isomer 1 | |
| Isorhamnetin-O-hexoside isomer 2 | |
| Isorhamnetin-O-hexoside-O-pentoside | |
| Kaempferol | |
| Kaempferol-3-O-rutinoside (Nicotiflorin) | |
| Kaempferol-O-hexoside | |
| Kaempferol-O-hexoside-di-O-deoxyhexoside | |
| Kaempferol-O-hexoside-O-pentoside-O-deoxyhexoside | |
| Kaempferol-O-pentoside | |
| Luteolin | |
| MyricetiFlavonoids (Continue)n | |
| Flavonoids (Continue) | Myricetin-3′-O-glucoside (Cannabiscitrin) |
| Myricetin-3-O-rutinoside | |
| Myricetin-O-(malonyl)glucoside | |
| Myricetin-O-arabinoside | |
| Myricetin-O-xyloside | |
| Naringenin | |
| Naringenin-6,8-di-C-glucoside | |
| Pentahydroxyflavanone | |
| Pentahydroxyflavanone isomer 1 | |
| Pentahydroxyflavanone isomer 2 | |
| Pentahydroxy-methoxyflavon-O-hexoside isomer 1 | |
| Pentahydroxy-methoxyflavon-O-hexoside isomer 2 | |
| Pentahydroxy-methoxyflavon-O-rutinoside | |
| Prodelphinidin B | |
| Prodelphinidin C isomer 1 | |
| Prodelphinidin C isomer 2 | |
| Prodelphinidin C isomer 3 | |
| Prunin (Naringenin 7-O-glucoside) | |
| Quercetin | |
| Quercetin-3-O-rutinoside-7-O-glucoside | |
| Quercetin-di-O-hexoside | |
| Quercetin-O-(acetyl)hexoside | |
| Quercetin-O-(coumaroyl)hexoside | |
| Quercetin-O-hexoside-di-O-deoxyhexoside | |
| Quercetin-O-hexoside-O-pentoside-O-deoxyhexoside | |
| Quercetin-O-pentoside isomer 1 | |
| Quercetin-O-pentoside isomer 2 | |
| Rutin (Quercetin-3-O-rutinoside) | |
| Sakuranetin (4′,5-Dihydroxy-7-methoxyflavanone) | |
| Taxifolin (Dihydroquercetin) | |
| Tetrahydroxychalcone (Butein) | |
| Tetrahydroxy-dimethoxyflavone isomer 1 | |
| Tetrahydroxy-dimethoxyflavone isomer 2 | |
| Tetrahydroxy-dimethoxyflavone-O-hexoside isomer 1 | |
| Tetrahydroxy-dimethoxyflavone-O-hexoside isomer 2 | |
| Tetrahydroxy-dimethoxyflavone-O-hexoside-O-deoxyhexoside | |
| Tetrahydroxyflavanone-O-hexoside | |
| Tetrahydroxyflavone-O-(pentosyl)hexoside | |
| Trihydroxy-dimethoxyflavone | |
| Trihydroxy-methoxyflavone | |
| Trihydroxy-trimethoxyflavone-O-hexoside | |
| Trihydroxy-trimethoxyflavone-O-hexoside isomer 1 | |
| Trihydroxy-trimethoxyflavone-O-hexoside isomer 2 | |
| Carboxylic Acids | Caffeic acid |
| cis-Aconitic acid | |
| Dihydroxy-methoxybenzoic acid isomer 1 | |
| Dihydroxy-methoxybenzoic acid isomer 2 | |
| Ferulic acid | |
| Hydroxyhexadecanoic acid (hydroxypalmitic acid) | |
| Jasmonic acid | |
| trans-Aconitic acid | |
| Tuberonic acid | |
| α-Linolenic acid | |
| 5-O-p-Coumaroylnigrumin | |
| Carboxylic Acids (Continue)Caffeoylglucose isomer 1 | |
| Carboxylic Acids (Continue) | Caffeoylglucose isomer 2 |
| Caffeoylglucose isomer 3 | |
| Chlorogenic acid (3-O-Caffeoylquinic acid) | |
| Chryptochlorogenic acid (4-O-Caffeoylquinic acid) | |
| Coumaroyl-glucose isomer 1 | |
| Coumaroyl-glucose isomer 2 | |
| Coumaroyl-glucose isomer 3 | |
| Coumaroylquinic acid isomer 1 | |
| Coumaroylquinic acid isomer 2 | |
| Coumaroylquinic acid isomer 3 | |
| Feruloylquinic acid isomer 1 | |
| Feruloylquinic acid isomer 2 | |
| Feruloylquinic acid isomer 3 | |
| Galloylglucose isomer 1 | |
| Galloylglucose isomer 2 | |
| Neochlorogenic acid (5-O-Caffeoylquinic acid) | |
| Phloretin | |
| Phlorizin (Phloridzin) | |
| Tetrahydroxy-methoxy chalcone | |
| Tuberonic acid glucoside | |
| Terpenes | Abscisic acid (ABA) |
| Geranylgeraniol | |
| Vitamins | Adenine (B4) |
| Nicotinic acid (Niacin, B3) | |
| Pyridoxine (B6) | |
| Riboflavin (B2) |
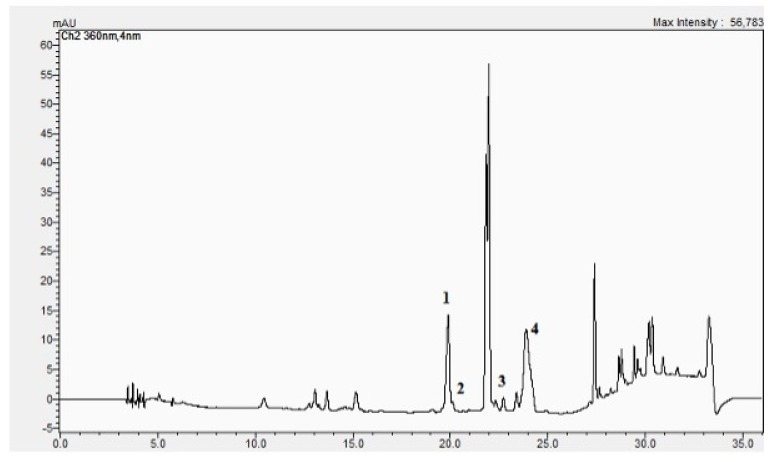
Noticeably, our study indicates that the flavonoids are the most numerous phytonutrients in the BC-GTE since we could identify about 80 of them, comprising both non-glycosidic and glycoside types. The most important non-glycosidic flavonoids are luteolin, quercetin, and apigenin, which together quantitatively represent 91.7% of all flavonoids, whereas the presence of kaempferol looks diminished (see Table 2 and Figure 1). Noteworthy, the BC-GTE showed relevant in vitro antioxidant properties as demonstrated by all four methods used (see Materials and Methods Section).
Table 2.
BC-GTE flavonoid content and antioxidant activity.
| BC-GTE Flavonoids Content | BC-GTE Antioxidant Capacity | |||||||
|---|---|---|---|---|---|---|---|---|
| Total Flavonoids [mg/mL] | Apigenin [mg/mL] | Kaempferol [µg/mL] | Luteolin [mg/mL] | Quercetin [mg/mL] | FRAP 1 | CUPRAC 1 | Superoxid Radical Inhibition 2 | Xanthin-Oxidase Inhibition 3 |
| 0.82 ± 0.013 | 0.152 ± 0.002 | 3.9 ± 0.04 | 0.34 ± 0.003 | 0.26 ± 0.003 | 672 ± 7.1 | 4565 ± 48.8 | 4512 ± 49.6 | 41 ± 0.4 |
| 18.5% | 41.5% | 31.7% | ||||||
1 mM TE/100 mL extract. 2 µM TE/100 mL extract. 3 mg AE/mL extract.
Figure 1.
The HPLC chromatogram of BC-GTE. 1 = quercetine, 2 = luteolin, 3 = kaempferol, 4 = apigenin.
2.2. The BC-GTE Does Not Feature Cytotoxicity in the Case of Drosophila melanogaster
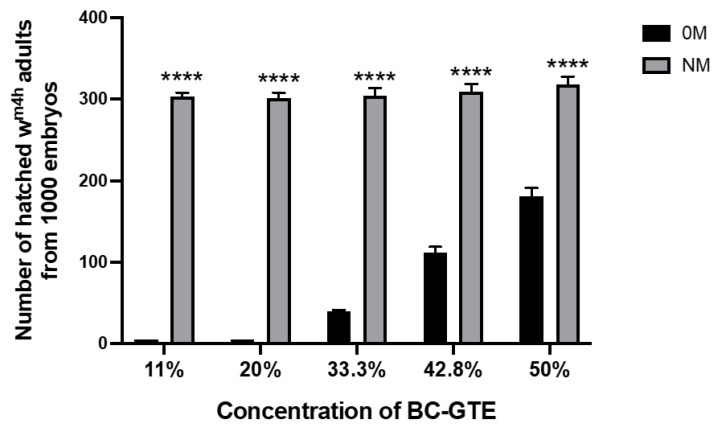
To assess the putative viability or cytotoxic properties of the BC-GTE, we have used a Drosophila melanogaster-based nutritive test system where embryos of the same age and genotype were put on 0M and NM culture conditions supplemented with the BC-GTE at different concentrations (see Materials and Methods). Therefore, by mixing 0.5 mL, 1 mL, 2 mL, 3 mL, and 4 mL of BC-GTE stock solution with 4 mL of 0M or NM culture media, we were able to assess the BC-GTE-generated effect at concentrations such as 11, 20, 33.3, 42.8, and 50%. The 0M represented a dietary condition that did not provide nutrients to support the larval development of fruit flies, and consequently, the hatched first-instar larvae would die instantly due to the lack of nutrients. Interestingly, when the 0M contained 11% and 20% of BC-GTE, no 2nd instar larvae were observed in the culture vessels, suggesting that the extract was not able to cover the nutritional requirements of the early phase of larval development (Figure 2). However, when 33.3%, 42.8%, and 50% of BC-GTE concentrations were applied in the case of the 0M condition, we could observe the adult-specific survival rates increasing from 4% to 11% and 18%, respectively. The surviving fruit fly adults showed normal body sizes, and no variegating white phenotype was visible for the wm4h strain. This suggests that the BC-GTE did not affect the expression of the white gene. Noteworthy, increasing the BC-GTE concentrations over 50% did not yield higher adult viability in combination with the 0M. Furthermore, we also assessed the effects of the above-mentioned BC-GTE concentrations among the NM type of dietary conditions. The normal medium is considered to provide the optimal (standard) nutrient composition for the larval development of fruit flies. When the NM was supplemented with the BC-GTE, it did not significantly affect the about 30% viability level of the wm4h individuals. These observations indicate that under normal dietary conditions, the BC-GTE does not have any effect on enhancing viability, but in the case of 0M dietary restrictions, it may provide some support to the development of fruit fly larvae. Taken together, we must conclude that the BC-GTE does not feature cytotoxicity and should contain relevant nutrients for larval development. It should be noted that in our evaluation of several GTEs for their nutritional properties on a 0M-based diet, we observed that all extracts containing tryptophan, an essential amino acid, were able to support the development of fruit fly larvae to adulthood to some extent, as shown in unpublished results by Máthé E. Besides the rich phytonutrient profile, the nutrition-based viability tests all indicate that the BC-GTE is a fairly complex matrix that requires thoughtful examination if the associated biological effects are to be assessed.
Figure 2.
Effects of exposure to different concentrations of BC-GTE on the survival of Drosophila melanogaster. Abbreviations: 0M-zero media, NM-normal media; (****) p < 0.0001 zero media vs. normal media group. The values are mean ± SEM, n = 1000.
2.3. The BC-GTE Contains Many Phytonutrients with Reported Anti-Inflammatory and Neuroprotective Properties
Several previously published studies indicated that flavonoids possess important anti-inflammatory properties [14]. In the following, we will consider some of the most relevant flavonoids identified in BC-GTE with regard to their proven physiological effects. The luteolin could activate M2 and suppress M1 macrophages [17]. It suppressed the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) [18], p53 [19], and phosphoinositide 3-kinases/protein kinase B (PI3K/Akt) [20] signaling pathways in inflammation-associated pathological conditions. The positive implication of quercetin in regulating macrophage activation and M1/M2 polarization was revealed [21,22], while the LPS counteracting anti-inflammatory effect was also identified [23,24]. Apigenin was found to have anti-inflammatory properties when microglia were stimulated by LPS, as it induced the glycogen synthase kinase-3 beta/nuclear factor erythroid 2–related factor 2 (GSK3β/Nrf2) signaling pathway [25] and modulated the expression of enzymes involved in the tryptophan/kyneurin pathway [26]. Kaempferol was shown to exert neuroprotective [27] and anti-neuroinflammatory [28] properties, and it would reduce the interleukin-1β (IL-1β)-induced inflammation by suppressing the NF-κB pathway [29]. Rutin and naringenin are two flavonoids identified at low concentrations in BC-GTE. Rutin, which is a glycoside of the flavonoid quercetin, was shown to have a strong protective effect in the case of neurodegenerative diseases [30], and it is also appreciated for its anticancer, anti-diabetic, and antimicrobial properties [31]. The compound naringenin had protective effects in the case of liver diseases [32]. Additionally, it has demonstrated neuroprotective [33] and anti-diabetic [34] properties.
Further to flavonoids, another numerous category of phytonutrients seen in the BC-GTE is the one with different carboxylic acids (CA). Interestingly, the most extensively studied compounds in this group have been found to possess anti-inflammatory properties, such as caffeic acid, which has been shown to be relevant for intestinal inflammation [35] and metabolic syndrome [36]. Other CAs found in BC-GTE were the ferulic acid, with anti-inflammatory and neuroprotective roles [37], while the jasmonic acid, showing structural similarity to prostaglandins, was able to inhibit inflammation through several mechanisms [38]. Chlorogenic acid was also identified in the BC-GTE, and its neuroprotective role was associated with anti-oxidant and anti-inflammatory mechanisms [39]. It has been demonstrated that, in combination with other plant-derived bioactive compounds such as epigallocatechin-3-O-gallate, resveratrol, and curcumin, it could alleviate neurodegenerative diseases [40]. Among the BC-GTE identified Cas, we should also mention that α-linolenic acid, which is an omega-3 type of fatty acid, has many experimentally proven health benefits, including neuroprotection [41].
It is essential to notice that the multiple anti-inflammatory effects reported in the case of individual flavonoids and carboxylic acids have not been thoroughly reviewed. Nevertheless, the presence and combination of these phytonutrients strongly indicate that the BC-GTE might possess neuroprotective and/or anti-inflammatory properties.
2.4. The BC-GTE Pretreatment Overcomes the Swelling of Rat Microglial Cell Body, following LPS Activation
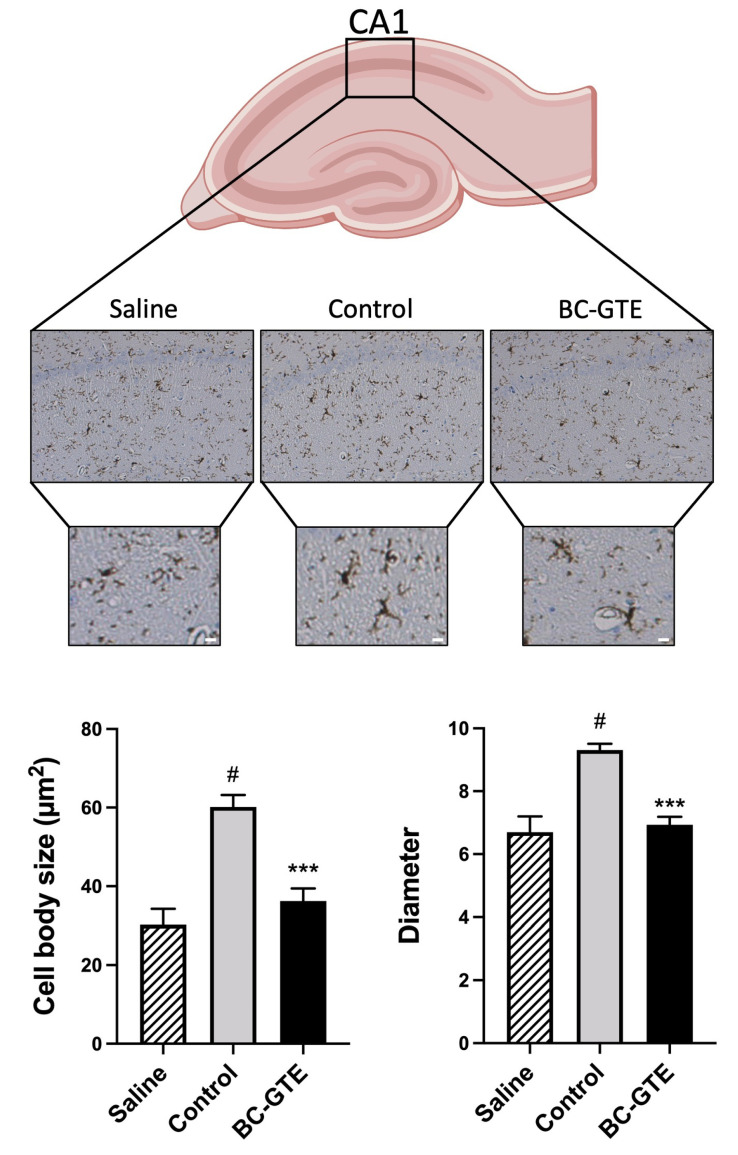
After observing the absence of cytotoxicity in vivo through Drosophila melanogaster-based tests, as well as the significant flavonoid content and relevant in vitro antioxidant activity, we decided to investigate the anti-inflammatory potential of the BC-GTE. For this purpose, we utilized the rat (Rattus norvegicus)-specific LPS-induced neuroinflammatory model, which is widely accepted in the field of inflammation research (for review, see [42]). Firstly, in this pilot study, we aimed to measure the acute response of the peripheral immune system to LPS administration in rats. It was previously described that the concentrations of cytokines and chemokines markedly increased after 3 h via 1 mg/kg body weight LPS administration [43]. Our second aim was to study microglia activation in the rat brain, and we decided on the 72 h time point after LPS injection. It has already been demonstrated that mice infected with live E. coli showed microglial activation 72 h post-inoculation, with increased cell number in the cortex, hippocampus, and thalamus as compared to controls. At 72 h, flow cytometry of microglia from E. coli-infected mice showed increased cell size [13,44]. Interestingly, in many rodent-based experiments, peripheral inflammatory stimuli such as the LPS could activate microglial cells and increase the TNF-α level in the brain. These are features that are specific for neuroinflammation [13,45]. It is well documented that activated microglial cells play a determinative role in brain inflammation, leading to the swelling of the microglial cell body, thickening of proximal processes, and reduction in distal ramifications [46]. These phenomena can be seen in the case of aging and neurodegenerating brains too [47,48].
Having in mind that we were able to reproduce the above-described neuro-inflammatory scenario, we designed another experimental setup where, before the LPS injection, the rats received ad libitum water with BC-GTE (see Materials and Methods). Analyzing the hippocampal CA1 area of the rats 72 h after LPS injection, we observed a significant reduction in the body size of microglia in the case of BC-GTE pretreatments compared to controls (p < 0.001; Figure 3). The LPS-injected control animals had significantly greater body sizes compared to the non-LPS-induced saline group (p < 0.01), an effect that indicates the inflammatory potential of the LPS. Moreover, no significant differences are apparent among all samples regarding the microglia number and the thickening of the proximal processes of microglial cells in all analyzed samples (Figure 3). Therefore, our observations are suggesting that the pre-treatment with BC-GTE could efficiently prevent/suppress the microglial activation-induced swelling of the cell body in the hippocampus upon the LPS-induced neuroinflammation.
Figure 3.
Experimental data presenting the physiological effects of BC-GTE on LPS-induced adult rats. The microglial cell body swelling (B, C). Abbreviations: BC-GTE—extract treated animal group; (***) p < 0.001 control vs. BC-GTE group; (#) p < 0.01 control vs. saline group. The values are mean ± SEM, n = 30–60. Scale bar: 10 µm.
2.5. The BC-GTE Pretreatment Leads to Diminished Serum TNF-α Level in the Serum
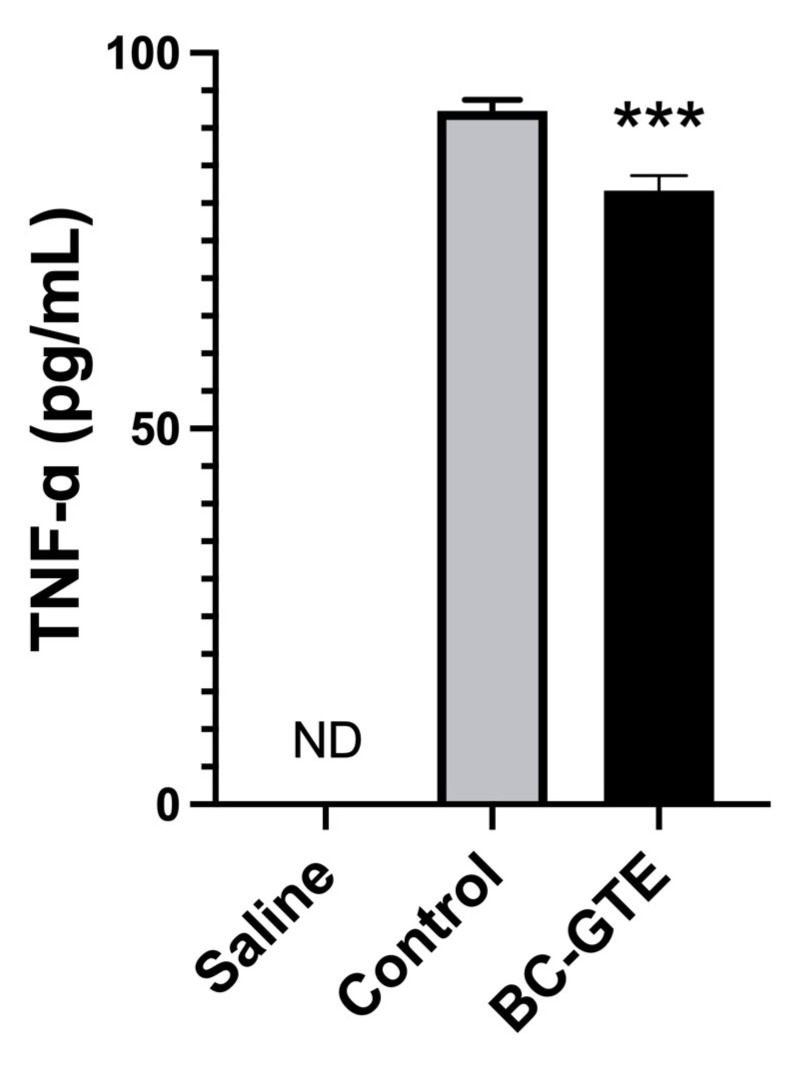
As mentioned earlier, luteolin, quercetin, apigenin, and kaempferol, the most important flavonoids identified in BC-GTE when assessed independently, were reported to have immunomodulatory and anti-inflammatory properties. Other studies indicated that the stimulation of the innate immune system through LPS injection could activate microglial cells. Their morphological changes would be accompanied by secreting pro-inflammatory cytokines such as TNF-α [13], eventually leading to neuroinflammation [45]. To further substantiate the anti-inflammatory properties of the BC-GTE, we analyzed the level of TNF-α in rat serum-specific after LPS injection. We observed that in the BC-GTE pretreated animals, the serum-specific concentration of TNF-α appeared significantly diminished compared to the control animals or the BC-GTE non-pretreated control individuals (Figure 4). Moreover, we could not detect TNF-α in three types of samples: (i) the “saline” group, where animals were pretreated with the solvent mix of the BC-GTE and the LPS was replaced with the saline injection; (ii) the control animals before LPS injection; and (iii) the animals pretreated with BC-GTE before LPS injection. Our results indicated that the pretreatment with BC-GTE prevented the rise of the circulating TNF-α levels following LPS injection, further suggesting that the BC-GTE would possess a relevant anti-neuroinflammatory effect.
Figure 4.
Experimental data presenting the serum TNF-α levels of BC-GTE on LPS-induced adult rats. Abbreviations: BC-GTE—extract treated animal group; ND—not detected. (***) p < 0.001 control vs. BC-GTE group. The values are mean ± SEM, n = 6.
3. Materials and Methods
3.1. Preparation of BC-GTE
Fresh buds of BC were harvested annually around the February-March period in Cluj, Romania, from an organic crop culture (ECOINSPECT certificate Ro-008) for three consecutive years. Each time the extractions were performed at cold temperatures by periodic mixing of the fresh vegetal material with the solvent (96% vol. ethanol—100% glycerol = 1:1) for 20 days. The extraction ratio was 1:20, while the extracted solutions were separated from the vegetal rests by decantation and pressing at 400 atm. The obtained BC-GTEs could be stored at room temperature for at least 2 years without affecting their total flavonoid content and antioxidant activity.
3.2. UHPLC–ESI-MS Analysis of BC-GTE
A Dionex Ultimate 3000RS UHPLC (ultra high-performance liquid chromatography) system equipped with a Thermo Accucore C18 column, 100/2.1 with a particle size of 2.6 μm, was coupled to a Thermo Q Exactive Orbitrap mass spectrometer equipped with an electrospray ionization source (ESI), and the measurement accuracy was within 5 ppm.
3.3. Phytochemical Analysis of BC-GTE
The total flavonoid content (TFC) was evaluated by the spectrophotometric method as described in the Romanian Pharmacopoeia (1993) at 430 nm with AlCl3 as a coloring agent. The flavonoids were determined quantitatively using a Shimadzu Nexera-i HPLC equipped with Fortis C18 columns (150 × 2.1 mm × 3 µm) and a UV–Vis DAD detector at 360 nm. The elution was performed with a solvent gradient (see Supplementary Table S1). The standards for the identification of apigenin, kaempferol, luteolin, and quercetin were obtained from Phytolab (Germany), while calibration curves were required for their quantitation (see Table 3). The antioxidant capacity of the BC-GTE was evaluated using methods such as FRAP (Ferric reducing antioxidant power), CUPRAC (cupric reducing antioxidant capacity), superoxide radical, and xanthinoxidase inhibition [49,50,51]. FRAP and CUPRAC are spectral methods to evaluate the antioxidant capacity. FRAP is the ferric-reducing ability of plasma, while CUPRAC is the cupric ion-reducing antioxidant capacity. All assessments were performed in technical triplicates.
Table 3.
Calibration curves data.
| Standard | Concentration 1 | Calibration Curve Equation 2 | Correlation Factor | Detection Limit 1 | Quantification Limit 1 |
|---|---|---|---|---|---|
| Apigenin | 50–360 | 42,007 × c[µg/mL] – 218,952 | 0.9990 | 15.6 | 26.1 |
| Kaempferol | 40–300 | 38,007 × c[µg/mL] + 772,543 | 0.9799 | 40.6 | 81.3 |
| Luteolin | 40–300 | 32,470 × c[µg/mL] + 441,347 | 0.9945 | 27.2 | 54.4 |
| Quercetin | 50–400 | 10,272 × c[µg/mL] + 17,725 | 0.9926 | 3.5 | 6.9 |
1 µg/mL. 2 area.
3.4. Cytotoxicity Studies on Drosophila Melanogaster
To assess the putative cytotoxicity of BC-GTE, Drosophila melanogaster-based nutrition tests were carried out using the wm4h (white mottled 4) mutant strain. These experiments were performed in parallel at 25 °C and by applying two different dietary conditions: [1] zero medium (0M) and [2] normal medium (NM). For this reason, synchronized 0–2 h old embryos were collected and placed on the two types of dietary setups, while the survival of embryos to adulthood was monitored. The number of adults was scored daily until no hatched adults were found. Meanwhile, the BC-GTE was assessed at 5 different concentrations: 11%, 20%, 33.3%, 42.8%, and 50% (by mixing 0.5 mL, 1 mL, 2 mL, 3 mL, and 4 mL of the GTE stock solution with 4 mL of both 0M and NM culture media). It is important to pinpoint that the wm4h individuals tested had the same genotype and age, and the experiments were conducted in triplicates. Thus, approximately 1000 embryos were examined for each evaluated BC-GTE concentration, ensuring that the results obtained were fully comparable.
3.5. Rattus Norvegicus Based Experimental Design
Eight months old, male Wistar rats were housed in groups (2 animals/cage) so that a standard laboratory diet and water were provided ad libitum at a 12 h light/dark cycle. The animals were randomly divided into three groups, of which two were the saline group (n = 6), the control group (C, n = 6), and the BC-GTE group (n = 6). The saline group and the control group received a diluted stock solution that contained 40% ethanol, 40% vegetable glycerin, and 20% water. The third group (BC-GTE) received a specific drink: a diluted stock solution of BC-GTE having 40% ethanol, 40% vegetable glycerin, and 20% BC bud material extract for 4 weeks. The contents of the drinking vessels were replaced daily with 250 mL of freshly prepared drinks, while the stock solutions were diluted at 1:7500 based on the recommendations of the gemmotherapy literature [52].
All experimental procedures on animals were approved by the Animal Examination Ethics Council of the Animal Protection Advisory Board at Semmelweis University (Budapest, Hungary), which fully complied with the principles of EU Directive 2010/63/EU for animal experiments.
3.6. The LPS-Induced Inflammatory Reaction in Rats
After a four-week long administration period, there were collected blood samples from the right foot saphenous vein. During the following day, the two groups of animals (Control and BC-GTE) were administered an intraperitoneal injection of LPS (lipopolysaccharide from Escherichia coli, Sigma-Aldrich Co., St. Louis, MO, USA) at a dose of 1 mg/kg body weight. This concentration has been used in most inflammation-related animal studies [43,53,54]. The third group was injected with saline. Three hours after injection, blood samples were collected from the left foot saphenous vein and centrifuged at 1500× g for 15 min at 4 °C. Following the supernatant removal, the samples were kept on ice until they were assayed.
3.7. Quantification of the Serum Specific TNF-α Levels
The concentrations of TNF-α were measured by a commercial TNF-α ELISA kit, and the protocol provided by the vendor was followed (#KRC3011, Invitrogen Co., Carlsbad, CA, USA). We measured the optical density (OD) in each sample with a microplate reader, and we calculated the mean OD from the triplicates. The OD of the sample was compared to a standard curve according to the manufacturer’s instructions. The inter- and intra-assay precisions were <10% (% coefficient of variation).
3.8. Immunohistochemistry Analysis
We sacrificed the animals by transcardial perfusion with saline containing heparin under CO2 anesthesia after 72 h of the LPS injection. The obtained brain samples were processed for immunohistochemical evaluation and stained with the IBA-1 antibody as described in [45]. Briefly, immersion-fixed 30 μm sections were pretreated with 0.3% H2O2 and incubated with 1:2500 rabbit-anti IBA-1 (#019-19741, Wako Chemicals GmbH, Neuss, Germany) in 2% BSA and 0.1% TX overnight at 4 °C. On the next day, we continued the protocol with 3 × 10 min PBS washing. We used 1:500 goat anti-rabbit secondary antibody (#8114S, Cell Signaling, Boston, MA, USA) for two hours at room temperature and avidin–biotin peroxidase complex (#PK-4004, Vectastain ABC Kit, Vector, Burlingame, CA, USA) for one hour. Sections were DAB-labeled (0.075 mg/mL DAB).
3.9. Analysis of Microglial Activation
Two immunohistochemically labeled pictures per rat were obtained from the hippocampal region-specific CA1 area (20× and 200×, Leica Application Suite V4.12). In each picture, a square was drawn, covering the equivalent of 50.000 µm2 in the case of the original microscopic section, and from 5 to 10 microglial cells were analyzed in each square. The ramified microglia can transform into an “activated state”, characterized by swollen ramified cells with a larger cell body and shorter, thicker processes, or alternatively, the microglia can adopt a “reactive state”, typically characterized by small, spherical cells. These morphological features of the immunostained microglia were assessed by image analysis software (Fiji, National Institute of Mental Health, Bethesda, MD, USA).
3.10. Statistical Analysis
The Statistica 13.5.0.17 soft was applied to assess the laboratory animal experiments’ specific data. The immunohistochemistry and ELISA (enzyme-linked immunosorbent assay) results were evaluated using a Student’s t-test. All numerical data were represented as mean ± SEM (standard error of the mean). Differences were considered significant for p < 0.05.
4. Discussion
In this paper, we present the first analysis of a BC-based GTE obtained from fresh buds. We report the extract’s chemical composition and demonstrate its anti-neuroinflammatory/neuroprotective effect. In addition to its significant antioxidant activity, the studied BC-GTE contains about 133 phytoconstituents, of which the flavonoids are the most numerous category, with more than 80 representatives, outnumbering all the other assessed bioactive compound categories. We also show that quantitatively, the BC-GTE flavonoid profile excels in luteolin, quercetin, apigenin, and kaempferol, but the presence of rutin and naringenin is also noticeable. The interference of flavonoids with inflammation and the immune system has gained much attention in the past decade [55]. For the relevant flavonoids seen in BC-GTE and when assessed individually, different types of anti-inflammatory properties could be observed [56,57,58].
Our study aims to shed light on the possible implication of the BC-GTE in overcoming neuroinflammation because the flavonoids seen in our extract were revealed to generate anti-neuroinflammatory properties [14]. Neuroinflammation should be envisioned as a central nervous system-related immune response that is triggered by injuries, PAMPs (pathogen-associated molecular patterns), or DAMPs (damage-associated molecular patterns), and conditions such as hypoxia [59,60]. After initiation, neuroinflammation can progress to the chronic phase through various factors and mechanisms. Microglial activation and the subsequent production of chemokines and cytokines are involved in the recruitment of further innate and adaptive immune cells to the inflammatory site(s) [61]. If the initial phase of a brain injury is not resolved, the above-mentioned scenario continues since no suppression/inhibition occurs. Following the establishment of chronic neuroinflammation, the aggravation of brain-related pathological conditions facilitates the irreversible progression of neurodegenerative diseases and psycho-affective disorders. According to our current knowledge, the microglia are the principal innate immune cells distributed all over the brain and regulate brain homeostasis, including all aspects of neuroinflammation, starting from the initial injury responses and ultimately protecting against PAMPs and DAMPs [62]. During the initiation of neuroinflammation, microglia are activated, reaching a pro-inflammatory status through different cellular mechanisms. This is accompanied by relevant morphological and secretory changes, which are meant to restore brain homeostasis but could also be considered markers indicating the progression of neuroinflammation. It is well known that flavonoids such as luteolin [63], quercetin [21,22], apigenin [25,26], kaempferol [28], rutin [63], and naringenin [64] have been reported to inhibit the microglia activation, which plays a crucial role in neuroinflammation. The presence of these flavonoids in the BC-GTE, we decided to assess the anti-neuroinflammatory effect of the BC-GTE led to an assessment of their anti-neuroinflammatory effect upon LPS induction. Several already reported mice- and rat-based experiments clearly demonstrated that the peripheral inflammatory LPS stimuli would cause brain-specific microglial activation associated with an increased serum-specific TNF-α protein level [13]. It has been demonstrated that under in vitro conditions, microglia but not astrocytes produce TNF-α due to LPS induction. This means that the TNF-α level could be considered indicative of microglial activation and neuroinflammation [65].
The above-mentioned experimental approach successfully recapitulates neuroinflammation in laboratory conditions. Therefore, it could serve as a valuable tool for defining novel therapeutic strategies to treat/prevent neurodegenerative diseases and/or psycho-affective disorders with inflammatory components [42]. Our reported experiments indicated that the BC-GTE administered before LPS injection would prevent to some extent the transformation of hippocampal microglia, which is further corroborated by the reduced serum specific TNF-α level. Therefore, our results suggested that the BC-GTE, through its phytoconstituent profile, could overcome the early phase of neuroinflammation, probably by not prolonging the pro-inflammatory status of microglia. It is also possible that other compounds of the BC-GTE, such as tryptophan and kynurenic acid, the flavonoid naringenin [66], or carboxylic acids such as caffeic, ferrulic, jasmonic, chlorogenic, and α-linoleic acids, might induce further neuroprotective mechanisms that would mitigate the progression of neuroinflammation.
5. Conclusions
Our research was based on a standardized BC-GTE whose phytonutrient profile was thoroughly analyzed both qualitatively and quantitatively. This approach could provide much-needed consistency and continuity for future studies. In addition to flavonoids, the BC-GTE contained other polyphenols, carboxylic acids, and essential amino acids and featured nutritive properties as revealed by Drosophila melanogaster tests. The administration of BC-GTE prior to the LPS-induced neuroinflammation appeared to decrease microglia activation in the adult rat brains and the serum-specific TNF-α levels. These latter results are promising but, based more on a pilot study, would require in-depth analyses regarding the elicited anti-neuroinflammatory effect. Though further studies are needed to define the cellular mechanisms behind the generated biological effects, the BC-GTE-based approach holds the promise of complex therapy in the case of damaged brain functions and related diseases.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28083571/s1. Table S1: Solvent gradient.
Author Contributions
E.M. (Emőke Mihok), Z.C. and N.-K.O. carried out the chemical studies; T.T. and C.N. designed and performed the rat-based experiments; E.M. (Emőke Mihok) and E.M. (Endre Máthé) designed and performed the fruit fly experiments; T.T., E.M. (Emőke Mihok), N.-K.O. and E.M. (Endre Máthé) analyzed the data; T.T., E.M. (Emőke Mihok), N.-K.O., C.N. and E.M. (Endre Máthé) wrote and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The animal study protocol was approved by the Animal Examination Ethics Council of the Animal Protection Advisory Board at Semmelweis University (Budapest, Hungary), which fully complied with the principles of EU Directive 2010/63/EU for animal experiments.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are available from the authors upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the ÚNKP-18-3 New National Excellence Program of the Ministry of Human Capacities.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Panche A.N., Diwan A.D., Chandra S.R. Flavonoids: An overview. [(accessed on 18 November 2022)];J. Nutr. Sci. 2016 5:e47. doi: 10.1017/jns.2016.41. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5465813/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calis Z., Mogulkoc R., Baltaci A.K. The Roles of Flavonols/Flavonoids in Neurodegeneration and Neuroinflammation. [(accessed on 18 November 2022)];Mini Rev. Med. Chem. 2020 20:1475–1488. doi: 10.2174/1389557519666190617150051. Available online: https://pubmed.ncbi.nlm.nih.gov/31288717/ [DOI] [PubMed] [Google Scholar]
- 3.Arulselvan P., Fard M.T., Tan W.S., Gothai S., Fakurazi S., Norhaizan M.E., Kumar S.S. Role of Antioxidants and Natural Products in Inflammation. [(accessed on 18 November 2022)];Oxid. Med. Cell. Longev. 2016 2016:5276130. doi: 10.1155/2016/5276130. Available online: /pmc/articles/PMC5075620/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golan H., Levav T., Mendelsohn A., Huleihel M. Involvement of Tumor Necrosis Factor Alpha in Hippocampal Development and Function. [(accessed on 18 November 2022)];Cereb. Cortex. 2004 14:97–105. doi: 10.1093/cercor/bhg108. Available online: https://academic.oup.com/cercor/article/14/1/97/433504. [DOI] [PubMed] [Google Scholar]
- 5.Spencer J.P.E. Flavonoids and brain health: Multiple effects underpinned by common mechanisms. [(accessed on 18 November 2022)];Genes Nutr. 2009 4:243. doi: 10.1007/s12263-009-0136-3. Available online: /pmc/articles/PMC2775888/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vauzour D., Vafeiadou K., Rodriguez-Mateos A., Rendeiro C., Spencer J.P.E. The neuroprotective potential of flavonoids: A multiplicity of effects. [(accessed on 18 November 2022)];Genes Nutr. 2008 3:115–126. doi: 10.1007/s12263-008-0091-4. Available online: https://genesandnutrition.biomedcentral.com/articles/10.1007/s12263-008-0091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rendeiro C., Spencer J.P.E., Vauzour D., Butler L.T., Ellis J.A., Williams C.M. The impact of flavonoids on spatial memory in rodents: From behaviour to underlying hippocampal mechanisms. [(accessed on 18 November 2022)];Genes Nutr. 2009 4:251. doi: 10.1007/s12263-009-0137-2. Available online: /pmc/articles/PMC2775889/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brás J.P., Bravo J., Freitas J., Barbosa M.A., Santos S.G., Summavielle T., Almeida M.I. TNF-alpha-induced microglia activation requires miR-342: Impact on NF-kB signaling and neurotoxicity. [(accessed on 17 November 2022)];Cell Death Dis. 2020 11:415. doi: 10.1038/s41419-020-2626-6. Available online: https://www.nature.com/articles/s41419-020-2626-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortez R.E., Mejia E.G. de Blackcurrants (Ribes nigrum): A Review on Chemistry, Processing, and Health Benefits. [(accessed on 11 October 2022)];J. Food Sci. 2019 84:2387–2401. doi: 10.1111/1750-3841.14781. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/1750-3841.14781. [DOI] [PubMed] [Google Scholar]
- 10.Huebbe P., Giller K., de Pascual-Teresa S., Arkenau A., Adolphi B., Portius S., Arkenau C.N., Rimbach G. Effects of blackcurrant-based juice on atherosclerosis-related biomarkers in cultured macrophages and in human subjects after consumption of a high-energy meal. [(accessed on 22 May 2019)];Br. J. Nutr. 2012 108:234–244. doi: 10.1017/S0007114511005642. Available online: http://www.ncbi.nlm.nih.gov/pubmed/22011640. [DOI] [PubMed] [Google Scholar]
- 11.Benn T., Kim B., Park Y.K., Wegner C.J., Harness E., Nam T.G., Kim D.O., Lee J.S., Lee J.Y. Polyphenol-rich blackcurrant extract prevents inflammation in diet-induced obese mice. J. Nutr. Biochem. 2014;25:1019–1025. doi: 10.1016/j.jnutbio.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Lee Y., Lee J.-Y. Blackcurrant (Ribes nigrum) Extract Exerts an Anti-Inflammatory Action by Modulating Macrophage Phenotypes. [(accessed on 4 June 2019)];Nutrients. 2019 11:975. doi: 10.3390/nu11050975. Available online: http://www.ncbi.nlm.nih.gov/pubmed/31035378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoogland I.C.M., Houbolt C., van Westerloo D.J., van Gool W.A., van de Beek D. Systemic inflammation and microglial activation: Systematic review of animal experiments. [(accessed on 22 November 2022)];J. Neuroinflamm. 2015 12:114. doi: 10.1186/s12974-015-0332-6. Available online: https://pubmed.ncbi.nlm.nih.gov/26048578/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y., Peng F., Xing Z., Chen J., Peng C., Li D. Beneficial effects of natural flavonoids on neuroinflammation. [(accessed on 13 March 2023)];Front. Immunol. 2022 13:6267. doi: 10.3389/fimmu.2022.1006434. Available online: https://pubmed.ncbi.nlm.nih.gov/36353622/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wadhwa G., Krishna K.V., Taliyan R., Tandon N., Yadav S.S., Banerjee D., Narwaria A., Katiyar C., Dubey S.K. A novel UPLC-MS/MS method for simultaneous quantification of trigonelline, 4-hydroxyisoleucine, and diosgenin from Trigonella foenum-graecum extract: Application to pharmacokinetic study in healthy and type 2 diabetic rats. [(accessed on 13 March 2023)];Biomed. Chromatogr. 2022 36:e5275. doi: 10.1002/bmc.5275. Available online: https://pubmed.ncbi.nlm.nih.gov/34738247/ [DOI] [PubMed] [Google Scholar]
- 16.Ostapiuk A., Urbanska E.M. Kynurenic acid in neurodegenerative disorders-unique neuroprotection or double-edged sword? [(accessed on 13 March 2023)];CNS Neurosci. Ther. 2022 28:19–35. doi: 10.1111/cns.13768. Available online: https://pubmed.ncbi.nlm.nih.gov/34862742/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gong B., Zheng Y., Li J., Lei H., Liu K., Tang J., Peng Y. Luteolin activates M2 macrophages and suppresses M1 macrophages by upregulation of hsa_circ_0001326 in THP-1 derived macrophages. [(accessed on 13 March 2023)];Bioengineered. 2022 13:5079–5090. doi: 10.1080/21655979.2022.2036897. Available online: https://pubmed.ncbi.nlm.nih.gov/35152837/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J.X., Xing J.G., Wang L.L., Jiang H.L., Guo S.L., Liu R. Luteolin Inhibits Fibrillary β-Amyloid1-40-Induced Inflammation in a Human Blood-Brain Barrier Model by Suppressing the p38 MAPK-Mediated NF-κB Signaling Pathways. [(accessed on 13 March 2023)];Molecules. 2017 22:334. doi: 10.3390/molecules22030334. Available online: https://pubmed.ncbi.nlm.nih.gov/28245546/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang K.K., Wang H., Qu D., Chen L.J., Wang L.B., Li J.H., Liu J.L., Xu L.L., Yoshida J.S., Xu J.T., et al. Luteolin Alleviates Methamphetamine-Induced Hepatotoxicity by Suppressing the p53 Pathway-Mediated Apoptosis, Autophagy, and Inflammation in Rats. [(accessed on 13 March 2023)];Front. Pharmacol. 2021 12:641917. doi: 10.3389/fphar.2021.641917. Available online: https://pubmed.ncbi.nlm.nih.gov/33679421/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan X.H., Zhang K.K., Xu J.T., Qu D., Chen L.J., Li J.H., Wang Q., Wang H.J., Xie X.L. Luteolin alleviates methamphetamine-induced neurotoxicity by suppressing PI3K/Akt pathway-modulated apoptosis and autophagy in rats. [(accessed on 13 March 2023)];Food Chem. Toxicol. 2020 137:111179. doi: 10.1016/j.fct.2020.111179. Available online: https://pubmed.ncbi.nlm.nih.gov/32035215/ [DOI] [PubMed] [Google Scholar]
- 21.Tsai C.F., Chen G.W., Chen Y.C., Shen C.K., Lu D.Y., Yang L.Y., Chen J.H., Yeh W.L. Regulatory Effects of Quercetin on M1/M2 Macrophage Polarization and Oxidative/Antioxidative Balance. [(accessed on 13 March 2023)];Nutrients. 2021 14:67. doi: 10.3390/nu14010067. Available online: https://pubmed.ncbi.nlm.nih.gov/35010945/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan H., Tang H.B., Shan L.Q., Liu S.C., Huang D.G., Chen X., Chen Z., Yang M., Yin X.H., Yang H., et al. Quercetin prevents necroptosis of oligodendrocytes by inhibiting macrophages/microglia polarization to M1 phenotype after spinal cord injury in rats. [(accessed on 13 March 2023)];J. Neuroinflamm. 2019 16:206. doi: 10.1186/s12974-019-1613-2. Available online: https://pubmed.ncbi.nlm.nih.gov/31699098/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiong G., Ji W., Wang F., Zhang F., Xue P., Cheng M., Sun Y., Wang X., Zhang T. Quercetin Inhibits Inflammatory Response Induced by LPS from Porphyromonas gingivalis in Human Gingival Fibroblasts via Suppressing NF-κB Signaling Pathway. [(accessed on 13 March 2023)];Biomed Res. Int. 2019 2019:6282635. doi: 10.1155/2019/6282635. Available online: /pmc/articles/PMC6720363/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai S.Q., Zhang Q., Zhao X.H., Shi J. The In Vitro Anti-Inflammatory Activities of Galangin and Quercetin towards the LPS-Injured Rat Intestinal Epithelial (IEC-6) Cells as Affected by Heat Treatment. [(accessed on 13 March 2023)];Molecules. 2021 26:7495. doi: 10.3390/molecules26247495. Available online: https://pubmed.ncbi.nlm.nih.gov/34946578/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen P., Huo X., Liu W., Li K., Sun Z., Tian J. Apigenin exhibits anti-inflammatory effects in LPS-stimulated BV2 microglia through activating GSK3β/Nrf2 signaling pathway. [(accessed on 13 March 2023)];Immunopharmacol. Immunotoxicol. 2020 42:9–16. doi: 10.1080/08923973.2019.1688345. Available online: https://pubmed.ncbi.nlm.nih.gov/31760890/ [DOI] [PubMed] [Google Scholar]
- 26.Kurniati D., Hirai S., Egashira Y. Effect of apigenin on tryptophan metabolic key enzymes expression in lipopolysaccharide-induced microglial cells and its mechanism. [(accessed on 13 March 2023)];Heliyon. 2022 9:e12743. doi: 10.1016/j.heliyon.2022.e12743. Available online: https://pubmed.ncbi.nlm.nih.gov/36685364/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang S., Li X., Zheng Y., Shi H., Zhang D., Jing B., Chen Z., Qian G., Zhao G. Kaempferol exerts a neuroprotective effect to reduce neuropathic pain through TLR4/NF-ĸB signaling pathway. [(accessed on 13 March 2023)];Phytother. Res. 2022 36:1678–1691. doi: 10.1002/ptr.7396. Available online: https://pubmed.ncbi.nlm.nih.gov/35234314/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park S.E., Sapkota K., Kim S., Kim H., Kim S.J. Kaempferol acts through mitogen-activated protein kinases and protein kinase B/AKT to elicit protection in a model of neuroinflammation in BV2 microglial cells. [(accessed on 13 March 2023)];Br. J. Pharmacol. 2011 164:1008–1025. doi: 10.1111/j.1476-5381.2011.01389.x. Available online: https://pubmed.ncbi.nlm.nih.gov/21449918/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhuang Z., Ye G., Huang B. Kaempferol Alleviates the Interleukin-1β-Induced Inflammation in Rat Osteoarthritis Chondrocytes via Suppression of NF-κB. [(accessed on 13 March 2023)];Med. Sci. Monit. 2017 23:3925. doi: 10.12659/MSM.902491. Available online: /pmc/articles/PMC5566200/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Enogieru A.B., Haylett W., Hiss D.C., Bardien S., Ekpo O.E. Rutin as a Potent Antioxidant: Implications for Neurodegenerative Disorders. [(accessed on 13 March 2023)];Oxid. Med. Cell. Longev. 2018 2018:6241017. doi: 10.1155/2018/6241017. Available online: https://pubmed.ncbi.nlm.nih.gov/30050657/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ganeshpurkar A., Saluja A.K. The Pharmacological Potential of Rutin. [(accessed on 13 March 2023)];Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2017 25:149–164. doi: 10.1016/j.jsps.2016.04.025. Available online: https://pubmed.ncbi.nlm.nih.gov/28344465/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hernández-Aquino E., Muriel P. Beneficial effects of naringenin in liver diseases: Molecular mechanisms. [(accessed on 13 March 2023)];World J. Gastroenterol. 2018 24:1679–1707. doi: 10.3748/wjg.v24.i16.1679. Available online: https://pubmed.ncbi.nlm.nih.gov/29713125/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nouri Z., Fakhri S., El-Senduny F.F., Sanadgol N., Abd-Elghani G.E., Farzaei M.H., Chen J.T. On the Neuroprotective Effects of Naringenin: Pharmacological Targets, Signaling Pathways, Molecular Mechanisms, and Clinical Perspective. [(accessed on 13 March 2023)];Biomolecules. 2019 9:690. doi: 10.3390/biom9110690. Available online: https://pubmed.ncbi.nlm.nih.gov/31684142/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartogh D.J.D., Tsiani E. Antidiabetic Properties of Naringenin: A Citrus Fruit Polyphenol. [(accessed on 13 March 2023)];Biomolecules. 2019 9:99. doi: 10.3390/biom9030099. Available online: /pmc/articles/PMC6468535/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zielińska D., Zieliński H., Laparra-Llopis J.M., Szawara-Nowak D., Honke J., Giménez-Bastida J.A. Caffeic Acid Modulates Processes Associated with Intestinal Inflammation. [(accessed on 13 March 2023)];Nutrients. 2021 13:554. doi: 10.3390/nu13020554. Available online: https://pubmed.ncbi.nlm.nih.gov/33567596/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muhammad Abdul Kadar N.N., Ahmad F., Teoh S.L., Yahaya M.F. Caffeic Acid on Metabolic Syndrome: A Review. [(accessed on 13 March 2023)];Molecules. 2021 26:5490. doi: 10.3390/molecules26185490. Available online: https://pubmed.ncbi.nlm.nih.gov/34576959/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stompor-Gorący M., Machaczka M. Recent Advances in Biological Activity, New Formulations and Prodrugs of Ferulic Acid. [(accessed on 13 March 2023)];Int. J. Mol. Sci. 2021 22:12889. doi: 10.3390/ijms222312889. Available online: https://pubmed.ncbi.nlm.nih.gov/34884693/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jarocka-karpowicz I., Markowska A. Therapeutic Potential of Jasmonic Acid and Its Derivatives. [(accessed on 13 March 2023)];Int. J. Mol. Sci. 2021 22:8437. doi: 10.3390/ijms22168437. Available online: https://pubmed.ncbi.nlm.nih.gov/34445138/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee T.K., Kang I.J., Kim B., Sim H.J., Kim D.W., Ahn J.H., Lee J.C., Ryoo S., Shin M.C., Cho J.H., et al. Experimental Pretreatment with Chlorogenic Acid Prevents Transient Ischemia-Induced Cognitive Decline and Neuronal Damage in the Hippocampus through Anti-Oxidative and Anti-Inflammatory Effects. [(accessed on 13 March 2023)];Molecules. 2020 25:3578. doi: 10.3390/molecules25163578. Available online: https://pubmed.ncbi.nlm.nih.gov/32781658/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fukutomi R., Ohishi T., Koyama Y., Pervin M., Nakamura Y., Isemura M. Beneficial Effects of Epigallocatechin-3- O-Gallate, Chlorogenic Acid, Resveratrol, and Curcumin on Neurodegenerative Diseases. [(accessed on 13 March 2023)];Molecules. 2021 26:415. doi: 10.3390/molecules26020415. Available online: https://pubmed.ncbi.nlm.nih.gov/33466849/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piermartiri T., Pan H., Figueiredo T.H., Marini A.M. α-Linolenic Acid, A Nutraceutical with Pleiotropic Properties That Targets Endogenous Neuroprotective Pathways to Protect against Organophosphate Nerve Agent-Induced Neuropathology. [(accessed on 13 March 2023)];Molecules. 2015 20:20355. doi: 10.3390/molecules201119698. Available online: /pmc/articles/PMC6332275/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skrzypczak-Wiercioch A., Sałat K. Lipopolysaccharide-Induced Model of Neuroinflammation: Mechanisms of Action, Research Application and Future Directions for Its Use. [(accessed on 13 March 2023)];Molecules. 2022 27:5481. doi: 10.3390/molecules27175481. Available online: https://pubmed.ncbi.nlm.nih.gov/36080253/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu S.Y., Wang T.F., Yu L., Jen C.J., Chuang J.I., Wu F.S., Wu C.W., Kuo Y.M. Running exercise protects the substantia nigra dopaminergic neurons against inflammation-induced degeneration via the activation of BDNF signaling pathway. [(accessed on 23 November 2022)];Brain. Behav. Immun. 2011 25:135–146. doi: 10.1016/j.bbi.2010.09.006. doi: 10.1016/j.bbi.2010.09.006. Available online: [DOI] [PubMed] [Google Scholar]
- 44.Hoogland I.C.M., Westhoff D., Engelen-Lee J.Y., Melief J., Valls Serón M., Houben-Weerts J.H.M.P., Huitinga I., van Westerloo D.J., van der Poll T., van Gool W.A., et al. Microglial Activation After Systemic Stimulation With Lipopolysaccharide and Escherichia coli. [(accessed on 22 November 2022)];Front. Cell. Neurosci. 2018 12:110. doi: 10.3389/fncel.2018.00110. Available online: https://pubmed.ncbi.nlm.nih.gov/29755322/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hovens I.B., van Leeuwen B.L., Nyakas C., Heineman E., van der Zee E.A., Schoemaker R.G. Postoperative cognitive dysfunction and microglial activation in associated brain regions in old rats. Neurobiol. Learn. Mem. 2015;118:74–79. doi: 10.1016/j.nlm.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 46.Fernández-Arjona M. del M.; Grondona, J.M.; Granados-Durán, P.; Fernández-Llebrez, P.; López-Ávalos, M.D. Microglia Morphological Categorization in a Rat Model of Neuroinflammation by Hierarchical Cluster and Principal Components Analysis. Front. Cell. Neurosci. 2017;11:235. doi: 10.3389/fncel.2017.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Godbout J.P., Chen J., Abraham J., Richwine A.F., Berg B.M., Kelley K.W., Johnson R.W. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. [(accessed on 11 October 2021)];FASEB J. 2005 19:1329–1331. doi: 10.1096/fj.05-3776fje. Available online: https://pubmed.ncbi.nlm.nih.gov/15919760/ [DOI] [PubMed] [Google Scholar]
- 48.Heppner F.L., Ransohoff R.M., Becher B. Immune attack: The role of inflammation in Alzheimer disease. [(accessed on 11 October 2021)];Nat. Rev. Neurosci. 2015 16:358–372. doi: 10.1038/nrn3880. Available online: https://www.nature.com/articles/nrn3880. [DOI] [PubMed] [Google Scholar]
- 49.Alam M.N., Bristi N.J., Rafiquzzaman M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013;21:143–152. doi: 10.1016/j.jsps.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benzie I.F.F., Strain J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. [(accessed on 11 October 2021)];Methods Enzymol. 1999 299:15–27. doi: 10.1016/s0076-6879(99)99005-5. Available online: https://pubmed.ncbi.nlm.nih.gov/9916193/ [DOI] [PubMed] [Google Scholar]
- 51.Apak R., Güçlü K., Özyürek M., Çelik S.E. Mechanism of antioxidant capacity assays and the CUPRAC (cupric ion reducing antioxidant capacity) assay. [(accessed on 11 October 2021)];Microchim. Acta. 2007 160:413–419. doi: 10.1007/s00604-007-0777-0. Available online: https://link.springer.com/article/10.1007/s00604-007-0777-0. [DOI] [Google Scholar]
- 52.Tetau M. Gemmotherapy: A Clinical guide; Editions Similia: 2010; ISBN 9782842510503. [(accessed on 1 March 2023)]. Available online: https://www.abebooks.com/Gemmotherapy-Clinical-guide-2010-Edition-Max/31290232315/bd.
- 53.Liu L., Zhang Q., Cai Y., Sun D., He X., Wang L., Yu D., Li X., Xiong X., Xu H., et al. Resveratrol counteracts lipopolysaccharide-induced depressivelike behaviors via enhanced hippocampal neurogenesis. [(accessed on 14 January 2020)];Oncotarget. 2016 7:56045–56059. doi: 10.18632/oncotarget.11178. Available online: http://www.ncbi.nlm.nih.gov/pubmed/27517628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin H.Y., Huang C.C., Chang K.F. Lipopolysaccharide Preconditioning Reduces Neuroinflammation Against Hypoxic Ischemia and Provides Long-Term Outcome of Neuroprotection in Neonatal Rat. [(accessed on 17 November 2022)];Pediatr. Res. 2009 66:254–259. doi: 10.1203/PDR.0b013e3181b0d336. Available online: https://www.nature.com/articles/pr2009197. [DOI] [PubMed] [Google Scholar]
- 55.Pérez-Cano F.J., Castell M. Flavonoids, Inflammation and Immune System. [(accessed on 13 March 2023)];Nutrients. 2016 8:659. doi: 10.3390/nu8100659. Available online: /pmc/articles/PMC5084045/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Di Matteo V., Esposito E. Biochemical and therapeutic effects of antioxidants in the treatment of Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis. [(accessed on 18 November 2022)];Curr. Drug Targets. CNS Neurol. Disord. 2003 2:95–107. doi: 10.2174/1568007033482959. Available online: https://pubmed.ncbi.nlm.nih.gov/12769802/ [DOI] [PubMed] [Google Scholar]
- 57.Ullah A., Munir S., Badshah S.L., Khan N., Ghani L., Poulson B.G., Emwas A.H., Jaremko M. Important Flavonoids and Their Role as a Therapeutic Agent. [(accessed on 18 November 2022)];Molecules. 2020 25:5243. doi: 10.3390/molecules25225243. Available online: /pmc/articles/PMC7697716/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krishnaiah D., Sarbatly R., Nithyanandam R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011;89:217–233. doi: 10.1016/j.fbp.2010.04.008. [DOI] [Google Scholar]
- 59.Hambali A., Kumar J., Hashim N.F.M., Maniam S., Mehat M.Z., Cheema M.S., Mustapha M., Adenan M.I., Stanslas J., Hamid H.A. Hypoxia-Induced Neuroinflammation in Alzheimer’s Disease: Potential Neuroprotective Effects of Centella asiatica. Front. Physiol. 2021;12:1698. doi: 10.3389/fphys.2021.712317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pranzatelli M.R. Advances in biomarker-guided therapy for pediatric- and adult-onset neuroinflammatory disorders: Targeting chemokines/cytokines. Front. Immunol. 2018;9:557. doi: 10.3389/fimmu.2018.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kölliker-Frers R., Udovin L., Otero-Losada M., Kobiec T., Herrera M.I., Palacios J., Razzitte G., Capani F. Neuroinflammation: An Integrating Overview of Reactive-Neuroimmune Cell Interactions in Health and Disease. [(accessed on 13 March 2023)];Mediators Inflamm. 2021 2021:9999146. doi: 10.1155/2021/9999146. Available online: https://pubmed.ncbi.nlm.nih.gov/34158806/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deus C.M., Tavares H., Beatriz M., Mota S., Lopes C. Mitochondrial Damage-Associated Molecular Patterns Content in Extracellular Vesicles Promotes Early Inflammation in Neurodegenerative Disorders. [(accessed on 13 March 2023)];Cells. 2022 11:2364. doi: 10.3390/cells11152364. Available online: https://pubmed.ncbi.nlm.nih.gov/35954208/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang S., Cao M., Xu S., Shi J., Mao X., Yao X., Liu C. Luteolin Alters Macrophage Polarization to Inhibit Inflammation. [(accessed on 13 March 2023)];Inflammation. 2020 43:95–108. doi: 10.1007/s10753-019-01099-7. Available online: https://pubmed.ncbi.nlm.nih.gov/31673976/ [DOI] [PubMed] [Google Scholar]
- 64.Zhang B., Wei Y.Z., Wang G.Q., Li D.D., Shi J.S., Zhang F. Targeting MAPK pathways by naringenin modulates microglia M1/M2 polarization in lipopolysaccharide-stimulated cultures. Front. Cell. Neurosci. 2019;12:531. doi: 10.3389/fncel.2018.00531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Welser-Alves J.V., Milner R. Microglia are the major source of TNF-α and TGF-β in postnatal glial cultures; regulation by cytokines, lipopolysaccharide, and vitronectin. [(accessed on 18 November 2022)];Neurochem. Int. 2013 63:47–53. doi: 10.1016/j.neuint.2013.04.007. Available online: /pmc/articles/PMC3819935/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen C., Wei Y.Z., He X.M., Li D.D., Wang G.Q., Li J.J., Zhang F. Naringenin Produces Neuroprotection Against LPS-Induced Dopamine Neurotoxicity via the Inhibition of Microglial NLRP3 Inflammasome Activation. [(accessed on 13 March 2023)];Front. Immunol. 2019 10:936. doi: 10.3389/fimmu.2019.00936. Available online: https://pubmed.ncbi.nlm.nih.gov/31118933/ [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are available from the authors upon reasonable request.