Abstract
Hematological toxicity represents the most common grade ≥3 toxicity after chimeric antigen receptor (CAR) T-cell therapy. However, its underlying pathophysiology is incompletely understood and its grading and management remains ill-defined. To inform the forthcoming European Hematology Association/European Society for Blood and Marrow Transplantation (EHA/EBMT) guidelines on the management of “immune effector cell-associated hematotoxicity” (ICAHT), we undertook a survey of experienced clinicians using an online survey focusing on (1) grading, (2) risk-stratification and diagnostic work-up, (3) short-term, and (4) long-term management of ICAHT. There were 81 survey respondents across 18 countries. A high degree of variability was noted for cytopenia grading in regards to depth, duration, and time from CAR-T infusion. The majority of experts favored pre-CAR-T bone marrow studies, especially in case of a high-risk profile. Most respondents felt that the work-up for patients with severe hematotoxicity should rule-out viral infections (96%), substrate deficiency (80%), or coincident sHLH/MAS (serum ferritin, 92%), and should include bone marrow aspiration (86%) and/or biopsy (61%). Clinicians were divided as to whether the occurrence of coincident immunotoxicity should influence the decision to apply G-CSF, and when to initiate G-CSF support. In case of prolonged thrombocytopenia, most survey participants favored thrombopoietin agonists (86%). Conversely, autologous hematopoietic cell boosts represented the preferred choice for neutropenia (63%), although they were frequently not available and no consensus was reached regarding the optimal trigger point. These findings underline the current heterogeneity of practice patterns regarding ICAHT and invite the development of consensus guidelines, which may harmonize grading, establish standard operating procedures for diagnosis, and set management guidelines.
INTRODUCTION
Following decades of preclinical evaluation and clinical development, the early 2020s have firmly established the role of chimeric antigen receptor (CAR) T-cell therapies for the treatment of a broad range of refractory B-cell malignancies.1–5 CARs are synthetic receptors that redirect immune effector cells against a target antigen on the tumor cell surface such as CD19.6,7 Although remarkable response rates have been reported, this form of adoptive cell therapy is also accompanied by a unique side effect profile, which typically includes cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS).8–10 Although initially under-reported in terms of clinical significance, real-world evidence has further highlighted the importance of hematological toxicity, referring to profound and often long-lasting cytopenias.11 These represent the most frequent Common Terminology Criteria for Adverse Events (CTCAE) grade ≥3 toxicity of CAR T-cell therapy, often follow a biphasic trajectory of count recovery, and can persist long after the resolution of clinical CRS.12–14 The underlying pathomechanism remains ill-defined, although recent evidence points towards a critical role of the host inflammatory state and hematopoietic reserve, as well as the inflammatory stress conferred by severe CRS and the associated cytokine patterns.14–17 Importantly, cytopenias compound the immunosuppression conferred by B-cell aplasia and hypogammaglobulinemia. Indeed, infections substantially contribute to morbidity, can prolong hospital stays, and represent the dominant cause of non-relapse mortality in the real-world setting.18–20
Currently, there remains a high degree of heterogeneity in terms of both the grading and the management of cytopenias. Notably, most grading systems, such as the CTCAE describe cytopenias mainly in quantitative terms, and thus fail to capture the unique quality of post-CAR-T cytopenias such as the biphasic and/or delayed course. Furthermore, a numeric value only incompletely reflects the true clinical severity of hematotoxicity in an equal manner to other side effects of CAR-T such as CRS or ICANS. To inform the forthcoming European Hematology Association (EHA)/European Society for Blood and Marrow Transplantation (EBMT) recommendations on the management of immune effector cell-associated hematotoxicity, termed ICAHT, in adult patients receiving autologous CAR T-cell therapy, we undertook a survey of experienced clinicians to assess their opinions on grading, risk stratification, and short-term and long-term management of cytopenias.
METHODOLOGY
The EBMT Practice Harmonization and Guidelines committee together with the guidelines committee of the EHA proposed that a joint EBMT/EHA-wide group be formed to produce a framework for grading and risk stratification, and to suggest practical clinical recommendations for ICAHT following CAR T-cell therapy. The initiative was adopted by the EHA and EBMT board after which experts in the field were invited to participate.
As we did for the previous European guidelines,21,22 an international survey was conceived and sent to physicians with experience in applying CAR T-cell therapies and managing the concomitant toxicities, to provide a reference point for deliberations and to solicit feedback on current strategies for the topics covered in the forthcoming guidelines. The survey followed the following structure:
General information about the participating center and attendant affiliations.
Grading of immune effector cell-associated cytopenia according to the depth and duration.
Risk stratification and diagnostic work-up: role of bone marrow biopsy prior and after CAR T-cell therapy, use of the CAR-HEMATOTOX score as a risk stratification tool, routine diagnostic studies in case of severe hematotoxicity, criteria to identify secondary Hemophagocytic Lymphohistiocytosis/Macrophage Activation Syndrome (sHLH/MAS) as a pertinent differential diagnosis, and work-up for clonal hematopoiesis of indeterminate potential (CHiP) or myelodysplastic syndrome (MDS) such as next-generation sequencing.
Short-term management: general utilization and trigger point for the use of growth factors such as granulocyte colony-stimulating factor (G-CSF).
Long-term management: alternative strategies for mitigation of ICAHT including thrombopoietin (TPO) agonists, immunosuppressive agents, autologous and allogeneic hematopoietic cell boost. Specific analysis of the patterns of hematopoietic cell boost utilization such as availability, optimal date of initiation, pre-emptive application, necessity of conditioning before reinfusion of the graft.
To ensure that a broad range of opinions were incorporated, we sent the survey to EBMT centers and >50 worldwide centers with an experience in the use of both commercially available and investigational products across 18 countries in 5 geographic regions (Asia, Middle East, Europe, Australia, and United States). Participants were provided a short deadline of 30 days to complete the survey and the pooled results were analyzed on December 22, 2022. We received 81 initial survey responses; however, 19 respondents either did not treat CAR-T patients regularly or did not complete the majority of survey questions, leaving 62 evaluable physicians that responded within the deadline. The survey was then closed and the responses were aggregated accordingly. To help with the interpretation of the tables, the most frequently chosen response is highlighted in bold. The responding participants and their affiliations are listed at the end of the article.
Countries and center characteristics
The countries of the survey respondents are provided in Table 1. As expected, the majority of centers were located in Europe (46%), followed by the United States (35%). Considering the number of active trials, China was under-represented in this survey (5%). An overview of center characteristics is shown in Suppl. Table S1. Only answers of the 72 centers with experience in administering CAR T-cell therapies, including commercially approved CAR-T products, were analyzed. Only 9% of centers reported lack of FACT-JACIE accreditation, and 40% reported being fully FACT-JACIE accredited for immune effector cell therapy (including CAR T-cell therapy). In terms of the CAR-T setting, the vast majority were a mixed transplant/CAR-T unit (73.8%), followed by general hematology/oncology ward (13.9%), and dedicated CAR-T unit (10.8%) (Suppl. Figure S1).
Table 1.
Participating Countries
| Country | N(%) |
|---|---|
| Austria | 3 (4.8) |
| Australia | 1 (1.6) |
| Belgium | 1 (1.6) |
| China | 3 (4.8) |
| Czech Republic | 1 (1.6) |
| France | 7 (11) |
| Germany | 10 (16) |
| Greece | 1 (1.6) |
| Israel | 1 (1.6) |
| Italy | 6 (9.5) |
| Poland | 2 (3.1) |
| Romania | 1 (1.6) |
| Saudi Arabia | 2 (3.1) |
| Singapore | 1 (1.6) |
| Spain | 10 (16) |
| Sweden | 3 (4.8) |
| The Netherlands | 2 (3.1) |
| USA | 8 (12.6) |
| Total (18) | 63 (100) |
RESULTS
Grading
We found that the large majority of survey participants currently use the CTCAE criteria (version 5.0) to grade post-CAR-T cytopenias (84%, Suppl. Table S2). This was followed by the CIBMTR reporting guidelines for hematopoietic recovery (10%) and the phenotypes of neutrophil recovery (6%).14,15 To initiate the development of a clinically relevant grading system, we queried respondents on their opinions concerning both depth and duration of cytopenia.
Immune effector cell-associated thrombocytopenia
In terms of depth, the most popular answers for mild and moderate thrombocytopenia were a platelet count of <100 × 109/L (56% of respondents) and <50 × 109/L (54% of respondents), respectively (Table 2). The most popular threshold for severe (grade 3) thrombocytopenia was a platelet count <20 × 109/L (34%), although no clear consensus was reached and 30% of respondents chose a platelet count <50 × 109/L. Life-threatening (grade 4) thrombocytopenia was characterized as either a bleeding event requiring urgent intervention (42%), followed by transfusion dependency with >1 transfusion weekly (38%), while 20% respondents voted for a threshold-based definition.
Table 2.
IEC-associated Thrombocytopenia by Depth (Top) and Duration (Bottom)
| Immune Effector Cell-associated Thrombocytopenia by Depth and Duration | ||||
|---|---|---|---|---|
| Grade 1: Mild, N (%) | Grade 2: Moderate, N (%) | Grade 3: Severe, N (%) | Grade 4: Life-Threatening, N (%) | Grade 5: Fatal, N (%) |
| Platelet count <150 109/L, 13 (26) | Platelet count <100 109/L, 10 (20) | Platelet count <50 109/L, 15 (30) | Transfusion dependency (>1 transfusion weekly), 19 (38) | Death directly attributable to thrombocytopenia-related event, 49 (98) |
| Platelet count <100 109/L, 28 (56) | Platelet count <50 109/L, 27 (54) | Platelet count <30 109/L, 6 (12) | Bleeding event requiring urgent intervention, 21 (42) | Other, 1 (2) |
| Platelet count <50 109/L, 2 (4) | Platelet count <30 109/L, 0 (0) | Platelet count <20 109/L, 17 (34) | Other, 10 (20) | |
| Platelet count <30 109/L, 1 (2) | Platelet count <20 109/L, 3(6) | Platelet count <10 109/L, 3 (6) | ||
| Platelet count <20 109/L, 0 (0) | Requiring no more than a single transfusion weekly, 0 (0) | Transfusion dependency (>1 weekly), 1 (2) | ||
| Other, 6 (12) | Other, 10 (20) | Bleeding event (not immediately life-threatening), = (0) | ||
| Other, 8 (16) | ||||
| Grade 1: Very Short, N (%) | Grade 2: Short, N (%) | Grade 3: Long, N (%) | Grade 4: Very Long, N (%) | |
| ≤4 d, 25 (50) | ≤7 d, 20 (40) | >7 d, 9 (18) | >10 d, 0 (0) | |
| ≤7 d, 16 (32) | ≤10 d, 9 (18) | >10 d, 15 (10) | >14 d, 6 (12) | |
| ≤10 d, 7 (14) | ≤14 d, 17 (34) | >14 d, 15 (30) | >21 d, 6 (12) | |
| Other, 2 (4) | Other, 4 (8) | >21 d, 6 (12) | >28 d, 19 (38) | |
| >28 d, 12 (24) | >60 d, 15 (30) | |||
| Other 3 (6) | Requiring stem cell boost, 2 (4) | |||
| Other, 2 (4) | ||||
The most popular survey choice is highlighted in bold.
In terms of duration, very short (grade 1) thrombocytopenia was most commonly classified as ≤4 days (50% of respondents), short (grade 2) thrombocytopenia as ≤7 days (40% of respondents), long (grade 3) thrombocytopenia as ≥14 days (30% of respondents), and very long (grade 4) thrombocytopenia as ≥28 days (38% of respondents).
Immune effector cell-associated anemia
An overview of the consensus definitions for the classification of anemia is provided in Table 3. Mild anemia (grade 1) was most commonly defined as a hemoglobin (Hb) below the lower limit of normal (52% of respondents), followed by a Hb <10 g/dL (44%). The most popular threshold for moderate anemia (grade 2) was a Hb <10 g/dL (56%), followed by a Hb <8 g/dL (34%). The preference choice for severe anemia (grade 3) was a Hb <8 g/dL, mirroring current CTCAE definitions. However, 22% of respondents also chose a transfusion-based definition: either ≥1 transfusion weekly or requiring <1 transfusion weekly, respectively. Life-threatening anemia (grade 4) was classified as a severe complication related to anemia (eg, cardiac arrhythmia, heart failure, and respiratory insufficiency) for 44% of survey participants, followed by severe transfusion dependency defined as approximately daily (28%) and transfusion dependency with a frequency of ≥1 packed red blood cell (pRBC) transfusion weekly (26%).
Table 3.
IEC-associated Anemia by Depth (Top) and Duration (Bottom)
| Immune Effector Cell-associated Anemia by Depth and Duration | ||||
|---|---|---|---|---|
| Grade 1: Mild, N (%) | Grade 2: Moderate, N (%) | Grade 3: Severe, N (%) | Grade 4: Life-threatening, N (%) | Grade 5: Fatal, N (%) |
| Hemoglobin < LLN, 26 (52) | Hemoglobin <10 g/dL, 28 (56) | Hemoglobin <8 g/dL, 24 (48) | Transfusion dependency (>1 transfusion weekly), 13 (26) | Death directly attributable to anemia-related event, 48 (96) |
| Hemoglobin <10 g/dL, 22 (44) | Hemoglobin <8 g/dL, 17 (34) | Requiring no more than a single transfusion weekly, 11 (22) | Severe transfusion dependency (approximately daily), 14 (28) | Other, 2 (4) |
| Hemoglobin <8 g/dL, 0 (0) | Requiring no more than a single transfusion weekly, 3 (6) | Transfusion dependency (>1 transfusion weekly), 11 (22) | Severe complication related to anemia requiring urgent intervention, 22 (44) | |
| Other, 2 (4) | Other, 2 (4) | Other, 4 (8) | Other, 1 (2) | |
| Grade 1: Very Short, N (%) | Grade 2: Short, N (%) | Grade 3: Long, N (%) | Grade 4: Very Long, N (%) | |
| ≤7 d, 37 (74) | ≤14 d, 32 (64) | >14 d, 9 (18) | >21 d, 3 (6) | |
| ≤14 d, 9 (18) | ≤21 d, 6 (12) | >21 d, 10 (20) | >28 d, 14 (28) | |
| ≤21 d, 2 (4) | ≤28 d, 9 (18) | >28 d, 24 (48) | >60 d, 17 (34) | |
| Other, 2 (4) | Other, 3 (6) | >60 d, 5 (10) | >90 d, 14 (28) | |
| Other, 2 (4) | Other, 2 (4) | |||
The most popular survey choice is highlighted in bold.
In terms of duration, very short (grade 1) and short (grade 2) anemia were most commonly defined as ≤7 days (74%) and ≤14 days (64%), respectively. The preference for long (grade 3) anemia was ≥28 days (48%), although a majority opinion was not reached and other survey participants voted for a threshold of ≥21 days (20%) or ≥14 days (18%). No clear consensus was established for very long (grade 4) anemia. The most popular choice was ≥60 days (34%), followed by ≥28 days and ≥90 days (both 28%, respectively).
Immune effector cell-associated neutropenia
A majority of respondents coalesced around a definition for mild (ANC <1.5 × 109/L), moderate (ANC <1.0 × 109/L), and severe (ANC <0.5 × 109/L) neutropenia (Table 4). Life-threatening neutropenia (grade 4) was most commonly classified as resulting in a life-threatening infection with or without respiratory insufficiency or hemodynamic instability. However, a significant proportion of experts also picked “neutropenia refractory to any therapeutic measures” (30%), or provided a threshold-based definition (16%).
Table 4.
IEC-associated Neutropenia by Depth (Top) and Duration (Bottom)
| Immune Effector Cell-associated Neutropenia by Depth and Duration | ||||
|---|---|---|---|---|
| Grade 1: Mild, N (%) | Grade 2: Moderate, N (%) | Grade 3: Severe, N (%) | Grade 4: Life-threatening, N (%) | Grade 5: Fatal, N (%) |
| ANC <1.5 109/L, 29 (58) | ANC <1.0 109/L, 28 (56) | ANC <0.8 109/L, 3 (6) | Neutropenia refractory to therapeutic measures (eg, growth factor, TPO-agonist, immunosuppressive therapy, 15 (30) | Death directly attributable to neutropenia-related event, 49 (98) |
| ANC <1.0 109/L, 11 (22) | ANC <0.8 109/L, 8 (16) | ANC <0.5 109/L, 32 (64) | Life-threatening Infections with or without respiratory insufficiency (requiring high-flow O2 support) or hemodynamic instability (requiring vasopressor support), 24 (48) | Other, 1 (2) |
| ANC <0.8 109/L, 2 (4) | ANC <0.5 109/L, 7 (14) | ANC <0.1 109/L, 7 (14) | Life-threatening Infections with respiratory insufficiency (requiring high-flow O2 support) and/or hemodynamic instability (requiring vasopressor support), 3 (6) | |
| ANC <0.5 109/L, 0 (0) | ANC <0.1 109/L, 0 (0) | Other, 8 (16) | Other, 8 (16) | |
| Other, 8 (16) | Other, 7 (14) | |||
| Grade 1: Very Short, N (%) | Grade 2: Short, N (%) | Grade 3: Long, N (%) | Grade 4: Very Long, N (%) | |
| ≤4 d, 24 (48) | ≤7 d, 18 (36) | >7 d, 7 (14) | >14 d, 5 (10) | |
| ≤7 d, 22 (44) | ≤10 d, 13 (26) | >10 d, 4 (8) | >21 d, 6 (12) | |
| ≤10 d, 2 (4) | ≤14 d, 17 (34) | >14 d, 19 (38) | >28 d, 23 (46) | |
| Other, 2 (4) | Other, 2 (4) | >21 d, 5 (10) | >42 d, 11 (22) | |
| >28 d, 12 (24) | Requiring stem cell boost, 1 (2) | |||
| Other, 3 (6) | Other, 4 (8) | |||
| Definition of persistent/prolonged immune effector cell-associated neutropenia | ||||
| ANC <0.5 109/L | ANC <1.0 109/L | Other | ||
| ANC <0.5 109/L after day +14 post-CAR-T, 2 (4) | ANC <1.0 109/L after day +14 post-CAR-T, 0 (0) | Other, 3 (6) | ||
| ANC <0.5 109/L after day +28 post-CAR-T, 19 (38) | ANC <1.0 109/L after day +28 post-CAR-T, 10 (20) | |||
| ANC <0.5 109/L after day +60 post-CAR-T, 6 (12) | ANC <1.0 109/L after day +60 post-CAR-T, 3 (6) | |||
| ANC <0.5 109/L after day +90 post-CAR-T, 4 (8) | ANC <1.0 109/L after day +90 post-CAR-T, 3 (6) | |||
The most popular survey choice is highlighted in bold.
A similar percentage of respondents defined very short (grade 1) neutropenia as ≤4 days (48%) and ≤7 days (44%). Similarly, no clear consensus was reached for short (grade 2) neutropenia: ≤7 days (36%), ≤10 days (26%), and ≤14 days (34%). The most popular selection for long neutropenia (grade 3) was ≥14 days (38%), followed by ≥28 days (24%) as the second most popular choice. Very long neutropenia (grade 4) was described as ≥28 days by 48% of respondents. To account for the often delayed and biphasic nature of neutropenia, we also surveyed how international experts would define persistent/prolonged neutropenia following CAR T-cell therapy. While no clear consensus emerged, the two most popular choices were an ANC <0.5 109/L at day +28 (38%) and an ANC <1.0 109/L at day +28 (20%), indicating that day +28 after CAR-T infusion was the preferred time point to define this toxicity category.
Risk-stratification and diagnostic work-up
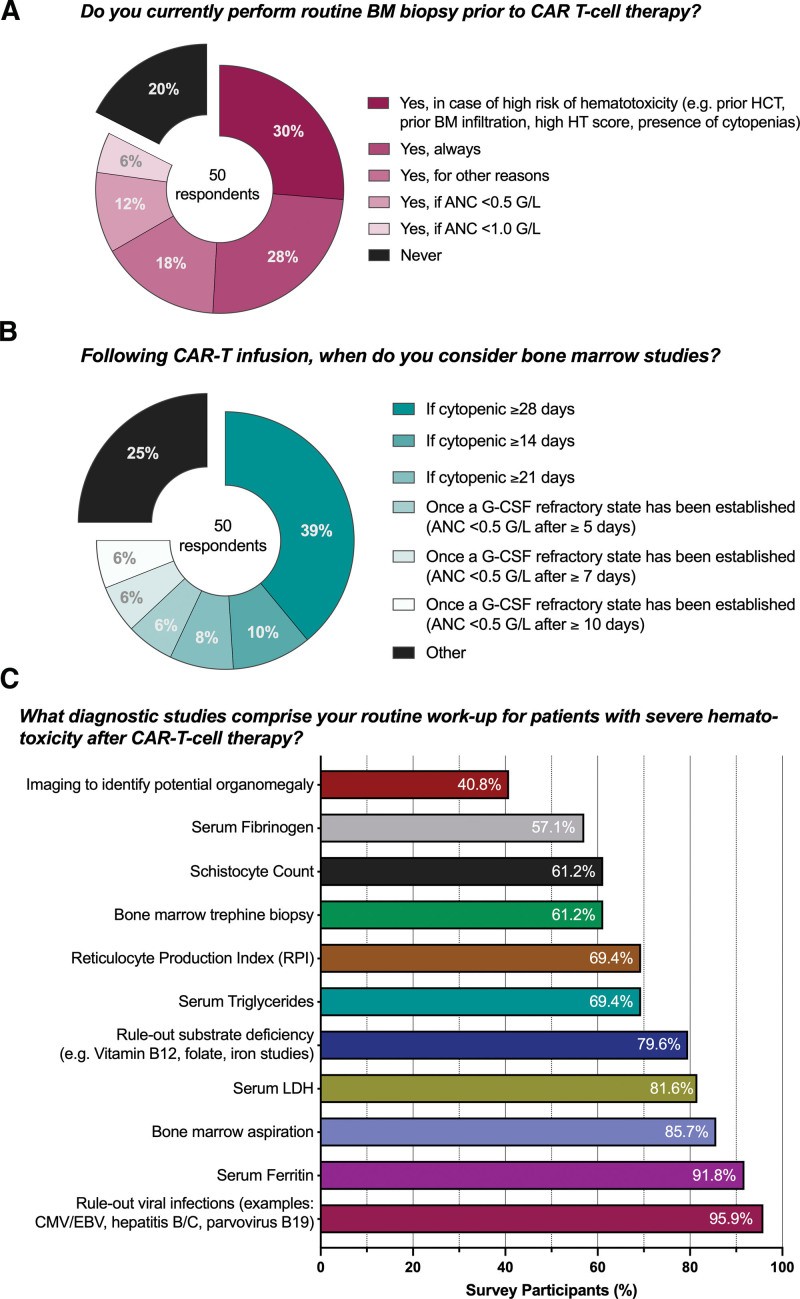
Before lymphodepleting chemotherapy, 36% of respondents described using a risk-stratification system to determine the patient-individual risk of hematological toxicity, which was almost exclusively the CAR-HEMATOTOX score (Suppl. Table S3, online calculator: https://www.german-lymphoma-alliance.de/Scores.html).19 This score incorporates factors related to hematopoietic reserve (ANC, Hb, and platelet count) and baseline inflammatory state (C-reactive protein [CRP] and ferritin). Routine bone marrow biopsy before CAR T-cell therapy was most commonly performed in case of a high-risk profile for hematotoxicity (30% of respondents, eg, prior hematopoietic cell transplantation, high CAR-HEMATOTOX score, and presence of cytopenia) (Fig. 1A). Still, 28% of survey participants reported always attaining pre-CAR-T bone marrow studies, while a further 18% reported using an ANC-based threshold (12%: <0.5 109/L; 6%: <1.0 109/L). Only 1 out of 5 reported never performing bone marrow analysis. The threshold to consider bone marrow analysis following CAR-T infusion varied by center (Fig. 1B): if cytopenic ≥28 days (39%), if cytopenic ≥14 days (10%), if cytopenic ≥21 day (8%), followed by the establishment of a G-CSF refractory state (≥5/≥7/≥10 days of G-CSF support, all 6%).
Figure 1.
Diagnostic studies for ICAHT prior and after CAR T-cell infusion. CAR = chimeric antigen receptor; HT = CAR-HEMATOTOX score; ICAHT = immune effector cell-associated hematotoxicity.
In terms of diagnostic studies for severe hematotoxicity following CAR-T infusion (Fig. 1C), the large majority of respondents described ruling-out viral infections such as CMV, EBV, hepatitis B/C, and parvovirus B19 (95.9%) as well as substrate deficiency (79.6%).
Bone marrow aspiration was more commonly performed (85.7%) than bone marrow trephine biopsy (61.2%). A majority of survey participants also incorporated a work-up for sHLH/MAS including serum fibrinogen (57.1%), serum triglycerides (69.4%), and serum ferritin levels (91.8%). To discriminate sHLH/MAS in patients with severe hematotoxicity, the experts most frequently employed the EBMT/EHA criteria23 (61%), followed by the MD Anderson criteria24 (27%) and the HScore25 (22%) (Suppl. Table S4). Other examinations included analyses for underlying hemolytic conditions including reticulocyte production index (69.4%) and serum LDH (81.6%) and microangiopathy markers such as schistocyte count (61.2%). Conversely, screening for potential organomegaly (eg, abdominal ultrasound) was only performed in 40.8% of respondents. Of interest, we found that a plurality of centers currently does not perform an extensive work-up for Clonal Hematopoiesis of indeterminate Potential (CHiP)26 or MDS in patients with persistent hematotoxicity (Suppl. Table S4). However, ~30% of respondents described initiating diagnostic evaluation using next-generation sequencing with a targeted gene panel in case of persistent hematotoxicity after ~3 months following CAR-T infusion.
Management of ICAHT
Short-term (day 0 to day +14)
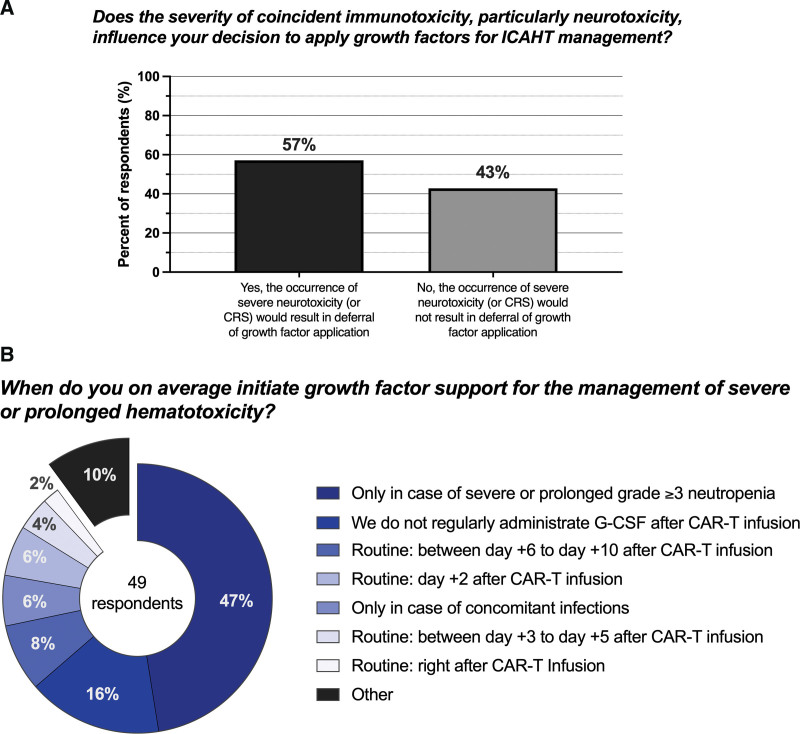
We noted marked heterogeneity in the practice patterns concerning G-CSF use for ICAHT. Notably, 57% of survey participants were hesitant to apply growth factors in the setting of coincident immunotoxicity, particularly ICANS (Fig. 2A). Concomitantly, the most popular survey response for application of growth factors during the first 14 days post-infusion was deferment due to concerns of exacerbating neurotoxicity (24.5%) (Table 5).27 However, other respondents stated that they regularly applied G-CSF in >50% of cases (20.4%), or administered growth factors in case of prolonged neutropenia. Consistent with this response, most survey respondents did not state a fixed day after CAR-T infusion on which growth factors were initiated, but rather administered G-CSF for severe or prolonged grade ≥3 neutropenia, although routine use on a specific day after CAR-T infusion was described: day +6–10 (8%), day +3–5 (4%), and right after CAR-T infusion (8%) (Fig. 2B).
Figure 2.
Practice patterns concerning G-CSF use for prolonged cytopenias. G-CSF = granulocyte colony-stimulating factor.
Table 5.
Growth Factors
| Do you regularly apply growth factors for the management of neutropenia occurring within 14 d after CAR T-cell infusion? N (%) | |
|---|---|
| No, because of concerns of exacerbating neurotoxicity | 12 (24.5) |
| Yes, but I try to avoid growth factors when possible due to concerns of exacerbating neurotoxicity | 4 (8) |
| Yes, in ~30% of patients | 4 (8) |
| Yes, in ~50% of patients | 1 (2) |
| Yes, in >50% of patients | 10 (20.5) |
| Only, in case of prolonged neutropenia with or without concomitant infection | 9 (18) |
| Only, in case of prolonged neutropenia with concomitant infection | 6 (12) |
| Other | 3 (6) |
| Do you regularly apply growth factors for the management of neutropenia occurring between day +14 and day +28 after CAR T-cell infusion? N (%) | |
| No, because of concerns of exacerbating neurotoxicity | 4 (8) |
| Yes, but I try to avoid growth factors when possible due to concerns of exacerbating neurotoxicity | 5 (10) |
| Yes, in ~30% of patients | 6 (12) |
| Yes, in ~50% of patients | 4 (8) |
| Yes, in >50% of patients | 10 (20.5) |
| Only, in case of prolonged neutropenia with or without concomitant infection | 12 (24.5) |
| Only, in case of prolonged neutropenia with concomitant infection | 5 (10) |
| Other | 3 (6) |
The most popular survey choice is highlighted in bold.
Long-term (after day +14)
When asked if growth factors are regularly applied for the management of neutropenia during days +14 to +28, the most popular choice was “only in the case of prolonged neutropenia with or without concomitant infection” (24.5%), with an additional 10.2% stating “only in the case of prolonged neutropenia with concomitant infections (Table 5). Still, G-CSF was commonly applied for neutropenia in ≥50% of cases (20.4%), ~50% of cases (8.2%), and between 30% and 50% of cases (12.2%), highlighting that G-CSF currently represents a mainstay for the management of hematological toxicity following CAR T-cell therapy.
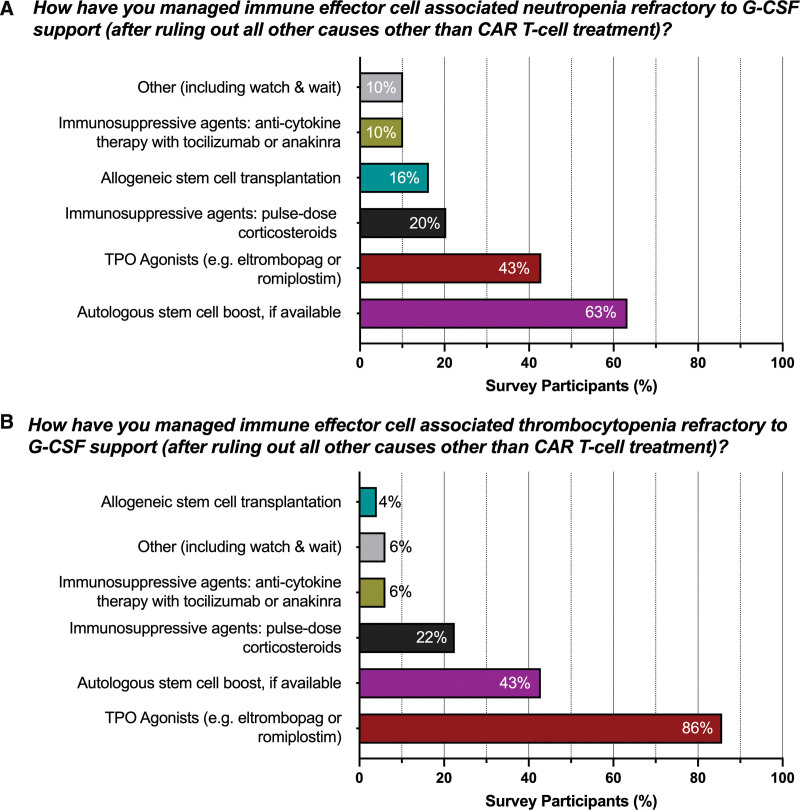
Next, we asked how centers approach immune effector cell-associated cytopenia that is refractory to growth factor support (after ruling out all other potential causes, Fig. 2). For neutropenia, the most frequently encountered strategy was an autologous hematopoietic cell boost, when available (63%, Fig. 3A), followed by TPO agonists (43%) and immunosuppression using pulse-dose glucocorticoids (20%). Allogeneic hematopoietic cell transplantation was employed in 16% of cases, while every 10th survey participant favored either anticytokine therapy or other options such as a watch and wait approach. In contrast, the top 2 choices were inverted for the management of thrombocytopenia (Fig. 3B): TPO agonists were the most popular choice (86%) followed by autologous hematopoietic cell boost, if available (43%). Pulse-dose glucocorticoids were applied in 22% of survey participants, while all other options were used in <10% of cases.
Figure 3.
Management strategies for G-CSF refractory immune effector cell-associated thrombocytopenia and neutropenia. G-CSF = granulocyte colony-stimulating factor.
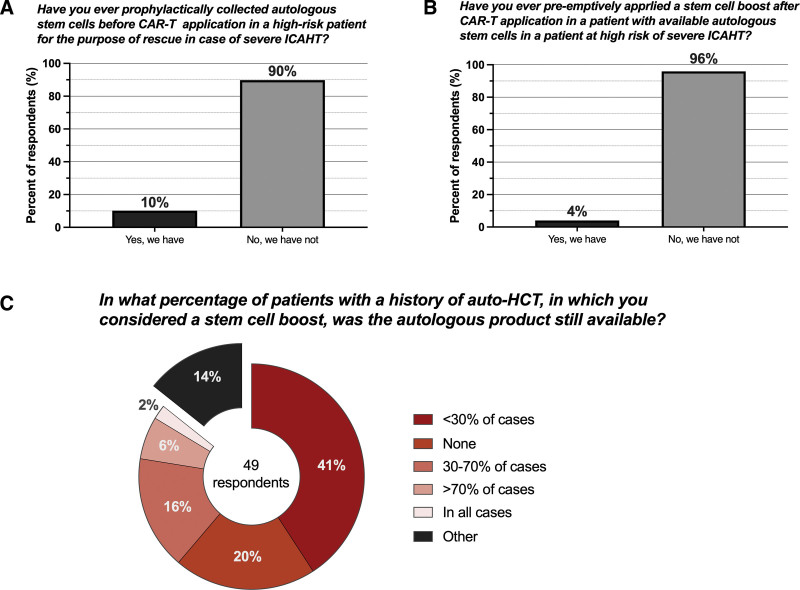
When focusing specifically on practice patterns for autologous hematopoietic cell boost, we found that prophylactic collection of CD34+ hematopoietic cells before CAR-T infusion for high-risk patients for the purpose of rescue in case of severe ICAHT was a rare proposition (only 10%, Fig. 4A). Pre-emptive, early application of an available hematopoietic cell boost in high-risk patients was even more rare (4%, Fig. 4B). Of interest, even if a hematopoietic cell boost represented a viable treatment option, in the majority of patients with a prior history of autologous stem cell transplantation (ASCT), hematopoietic cell boosts were either available in <30% of cases (41%) or not available at all (20%) (Fig. 4C). The stated preference to trigger hematopoietic cell boost for neutropenia and thrombocytopenia varied across all sites (Table 6). For neutropenia, the most common answer was ≥90 days (39%), followed by ≥28 days (20%), and ≥60 days (14%) after CAR-T infusion. Similarly, the preferred trigger was ≥90 days for refractory thrombocytopenia (35%), followed by ≥28 days (16%), and then either day +60 or day +180 post-CAR-T (both 12%, respectively). Other answers included refractoriness to platelet transfusions, failure to respond to TPO agonists, or deciding based on clinical need or in case of severe complications. Finally, most respondents stated that they preferred to simply re-infuse the graft (76%), while a minority indicated that they applied conditioning chemotherapy before reinfusion (4%). Furthermore, 14% stated that they “do not know” how they would apply an autologous hematopoietic cell boost.
Figure 4.
Practice patterns concerning the use of autologous stem cell boost for the management of ICAHT. ICAHT = immune effector cell-associated hematotoxicity.
Table 6.
Autologous Stem Cell Boost
| Appropriate Timing of Autologous Stem Cell Boost | Best Option for Autologous Stem Cell Boost in Refractory IEC-associated Cytopenia Patients (If a Cryopreserved Autologous Graft is Available) N (%) | |
|---|---|---|
| Refractory IEC-associated Neutropenia N (%) | Refractory IEC-Associated Thrombocytopenia N (%) | |
| ≥14 d, 2 (4) | ≥14 d, 0 (0) | Simple reinfusion of the graft, 37 (75.5) |
| ≥28 d, 10 (20) | ≥28 d, 8 (16) | Conditioning followed by re-infusion of the graft, 2 (4) |
| ≥60 d, 7 (14) | ≥60 d, 6 (12) | I don’t know, 7 (14) |
| ≥90 d, 19 (39) | ≥90 d, 17 (34) | Other, 3 (6) |
| ≥180 d, 2 (4) | ≥180 d, 6 (12) | |
| Other, 9 (18) | Other, 12 (24,5) | |
The most popular survey choice is highlighted in bold.
DISCUSSION
With CAR T-cell therapies revolutionizing the treatment landscape of multiple refractory hematological malignancies, a main focus in the field now relates to the optimization of the management of the associated side effects. Although hematological toxicity represents one of the very first dose-limiting side effects ever described for cytotoxic chemotherapy (described as early as 1946 by Goodman et al28), its renaissance in the field of novel immunotherapies makes it an old “new kid on the block.” Importantly, the nature of cytopenia after adoptive cell therapy is qualitatively unique and the underlying pathophysiology remains incompletely understood. Thus, classification systems and management guidelines that were developed in the setting of classic cytotoxic chemotherapies may not extend to patients receiving CAR T-cell therapies. In this multicenter international survey incorporating 72 centers across 18 countries, we surveyed current practice patterns regarding grading, risk stratification, and management of ICAHT.
As we had postulated, we observed considerable heterogeneity in the responses regarding how cytopenias ought to be graded across the participating sites, particularly for the severe and life-threatening cytopenia categories. This may relate to the fact that any cytopenia threshold remains arbitrary, as the severity grading requires further information on (1) total duration and (2) respective allotted time from CAR-T infusion. For example, the risk of infectious complications due to neutropenia is inherently time-dependent. Although a one-time measurement of an ANC <0.1 109/L may be worrisome, it is the sustained measurement of such a value for an extended period of time that particularly raises concern for severe infections.19 For this reason, the IDSA/ASCO consensus guidelines for the application of anti-infective prophylaxis (eg, antibacterial and mold-active azoles) in adult patients with cancer-related immunosuppression incorporate not only depth but also the duration of neutropenia.29 Furthermore, it is important to recognize that the underlying pathomechanism of cytopenia may be substantially different in patients receiving adoptive cell therapies than with classic chemotherapeutic agents. This is reflected by the typically biphasic time course of neutrophil recovery and the persistent nature of cytopenia.12–14 Any novel nomenclature or classification system of CAR T-cell-related hematotoxicity must, therefore, fully account for these peculiarities.
The survey results also underline that externally validated risk-stratification systems such as the CAR-HEMATOTOX score remain under-utilized.14,19,30–32 Interestingly, bone marrow studies were broadly performed before CAR-T infusion especially in high-risk candidates. A multitude of factors confer an increased risk profile for ICAHT, which can include prior hematopoietic cell transplantation, presence of bone marrow infiltration of the underlying malignancy, baseline cytopenias, and inflammatory state.12,14,23,33 Surprisingly, we found that clinicians were hesitant to apply early G-CSF (eg, day +5 or earlier), despite recent evidence pointing toward an acceptable safety profile with no deleterious impact on CAR-T expansion kinetics and anti-lymphoma activity.34–36 This likely stems from initial preclinical murine data that raised concern for exacerbating neurotoxicity with GM-CSF.27 Concomitantly, the survey indicated a high degree of variability in regards to the optimal trigger point for G-CSF application after CAR-T infusion, highlighting the need for harmonized consensus guidelines and ideally multicentric prospective data that establishes a new standard-of-care. Of interest, we did not observe significant differences between the participating European and non-Europeans centers in regards to their responses concerning practice patterns and the management of CAR-T-related cytopenias.
An interesting aspect of the survey relates to the utilization of hematopoietic cell boosts for the management of prolonged cytopenia following CAR T-cell therapy. Recently, three European groups delineated the safety and clinical feasibility of this approach for CAR-T patients with severe hematotoxicity.37–39 This survey represents the most detailed report outlining how autologous hematopoietic cell boosts are currently being employed. However, several open questions remain: should pre-emptive collection of CD34+ hematopoietic cells be considered in high-risk candidates before CAR T-cell infusion? If yes, according to what criteria? What represents the optimal trigger point for hematopoietic cell boost? Are there any unintended consequences on CAR T-cell expansion, function, or efficacy? It is notable that only 10% of survey respondents reported prophylactically collecting autologous stem cells in high-risk patients, although approximately two thirds would consider a boost for prolonged neutropenia following CAR-T therapy, if available. This disconnect suggests that physicians remain recalcitrant to prophylactically collect, potentially due to cost or logistic reasons. In the absence of clear empiric and especially prospective evidence, consensus expert recommendations are clearly needed. This is underlined by the increasing use of CAR T-cell products for disease entities such as multiple myeloma or mantle cell lymphoma, in which autologous hematopoietic cell transplantation remains established in the frontline treatment setting.40,41
One of the strengths of this survey relates to the large number of participants despite the still yet limited number of overall sites offering adoptive cell therapies. Although some questions were not answered by all centers, this may reflect the underlying ambiguity in regards to the management of ICAHT. Crucially, this survey represents a starting point for broader efforts to harmonize grading and develop consensus guidelines for ICAHT. For this purpose, an international panel of experts from different countries and belonging to EHA and EBMT convened in Lille, France in March 2023 to issue respective European recommendations. The large heterogeneity in practice patterns underlines that hematological toxicity remains an ill-defined toxicity category in need of harmonization and standardization. Such efforts would not only enable evidence-based management strategies tailored to the individual patient, but also would provide a blueprint for reporting for other novel immunotherapeutic agents such as bispecific antibodies and allogeneic CAR products. Still, this study has several relevant limitations. The respondents mainly were chosen based on their involvement in international societies such as EBMT, raising concern for inadvertent selection bias. Furthermore, not all queried centers responded, as is common for these types of surveys. The provided answers may only reflect the latest practice patterns at each individual center, as opposed to the average treatment choices over the last few years.
In conclusion, our multicenter survey introduces ICAHT as a novel toxicity category following CAR T-cell therapy and provides a snapshot of current practice patterns. The heterogenous survey results underline the current lack of uniform consensus for grading and management of profound and/or long-lasting cytopenias. As a result, the survey has informed the forthcoming joint EHA/EBMT/JACIE recommendations for the management of ICAHT following CAR T-cell therapy.
ACKNOWLEDGMENTS
We are grateful for the support of all patients and the personnel who supported this work across all the participating centers. We want to particularly thank all the participants for their timely responses to this survey: MI KWON (Hematology Department, Hospital General Universitario Gregorio Marañon), David Beauvais (CHU Lille), Christian Koenecke (Hannover Medical School), Stig Lenhoff (Department of Hematology, SUS Lund), JUAN ALBERTO MARTIN GONZALEZ (TRANSPLANT AND CELL TERAPY CORDINATOR), Filippo Antonio Canale (Bone marrow transplantation unit of Grande Ospedale Metropolitano Bianchi-Melacrino-Morelli), Lioure B (Hematology, ICANS, Strasbourg, France), Massimo Martino (Stem Cell Transplant and Cellular Therapies Unit, GOM BMM), Werner Rabitsch (Vienna, Austria), Jordan Gauthier (Fred Hutchinson Cancer Center), Richard Mitchell (Transplant and Cellular Therapies Program, Kids Cancer Center), Magdalena Dutka (Medical University of Gdańsk, Hematolog and Transplatology Department), Valentín Ortiz-Maldonado (Hospital Clínic de Barcelona), Xavier Poiré (Institut Roi Albert II, Cliniques Universitaires St-Luc), Gwendolyn van Gorkom (Department of Internal Medicine, Division of Hematology, GROW School for Oncology and Developmental Biology, Maastricht University Medical Center), Ben-Niklas Bärmann (University Hospital Düsseldorf), Alberto Mussetti (Institut Català d’Oncologia-Hospital Duran i Reynals), Panagiotis Tsirigotis (ATTIKO University Hospital), Hildegard Greinix (Medical University Graz), Aloysius Y L Ho (Singapore General Hospital), Ignacio Español (Murcia, España), Jurgen Kuball (Department of Haematology, UMC Utrecht), MOHSEN ALZAHRANI (KING ABDULAZIZ MEDICAL CITY, MINISTRY OF NATIONAL GUARD-HEALTH AFFAIRS), Piera Angelillo (San Raffaele Scientifc Institute), Luca Castagna (Ospedale Villa Sofia Cervello), Jiri Sramek (Hemato-Oncology Department, University Hospital Pilsen), Simona Sica (Fondazione Policlinico Universitario Agostino Gemelli), Anca Colita (Fundeni Clinical Institute), Kristina Carlson (Department of Hematology, Uppsala University Hospital, Uppsala), Krzysztof Kalwak (Department of Pediatric Hematology, Oncology and BMT, Wroclaw Medical University), Lucía López-Corral (Hematology Department, University Hospital of Salamanca (HUS/IBSAL), CIBERONC and Cancer Research Institute of Salamanca-IBMCC (USAL-CSIC), 37007 Salamanca, Spain), RAFAEL HERNANI (Haematology Department, Hospital Clínico Universitario, INCLIVA Research Institute, Valencia, Spain), Carlos Panizo (Hospital Universitario Donostia. IIS Biodonostia), Jose Maria Sanchez-Pina (Madrid, Spain), Depei Wu (Soochow University), ~Xiao-Jun Huang (Peking University People’s Hospital), Jakob Rudzki (Medical University Hospital/Tirol Kliniken), He HUANG (Zhejiang University), Miguel-Angel Perales (Memorial Sloan Kettering Cancer Center), Cameron Turtle (Fred Hutchinson Cancer Center), BARUCHEL (Robert Debré University Hospital (APHP and Université Paris Cité), Fabian Müller (University Hospital of Erlangen), Bärmann (Universitätsklinikum Düsseldorf), Gloria Iacoboni (Vall d’Hebron Hospital), Prof Dr Bastian von Tresckow (Department of Hematology and Stem Cell Transplantation, University Hospital Essen), Roland Schroers (Ruhr Universität), Vladan Vucinic (University Leipzig, Medical Clinic I Hematology and Celltherapy), Prof Dr Stephan Mielke (Karolinska Institutet and University Hospital, CAST, LabMed, Karolinska Comprehensive Cancer Center), Wolff, Daniel (University Hospital Regensburg, Department of Medicine III), Navneet Majhail (Sarah Cannon Transplant and Cellular Therapy Program at TriStar Centennial), Michael Jain (Moffitt Cancer Center), Arnon Nagler (Chainm Sheba Medical Center), Mahmoud Aljurf (King Faisal Specialist Hospital and Research Center), Stephan Grupp (Children’s Hospital of Philadelphia), Catherine Thieblemont (Université de Paris; APHP, Hôpital Saint-Louis, Hémato-oncologie, Paris France), Mohamed Kharfan-Dabaja, MD, MBA (Mayo Clinic), Roch Houot (Department of Hematology, CHU de Rennes, Université de Rennes, Rennes, France), Nicolas Boissel (Hematology AYA Unit, Hôpital Saint-Louis, APHP), Jordan Gauthier (Fred Hutchinson Cancer Center), Kai Rejeski (Department of Medicine III - Hematology/Oncology), Raffaella Greco (IRCCS San Raffaele Institute), Ibrahim Yakoub-Agha (Lille University Hospital).
AUTHOR CONTRIBUTIONS
KR, IY-A, and MS did conceptualization. KR, RG, FO, ISO, CB, AS, JGG, IY-A, and MS did investigation. KR, RG, FO, ISO, AS, IY-A, and MS did formal analysis and visualization. KR, RG, FO, ISO, AS, IY-A, and MS contributed to methodology. KR did wrote the original draft. KR, JGG, IY-A, and MS did writing review and editing. All authors read and approved the final article.
DATA AVAILABILITY
For original data and material, please contact the corresponding authors.
DISCLOSURES
KR: Kite/Gilead: Research Funding and travel support; Novartis: Honoraria; BMS/Celgene: Consultancy, Honoraria; School of Oncology of the German Cancer Consortium (DKTK): fellowship; Else Kröner Forschungskolleg (EKFK) within the Munich Clinician Scientist Program (MCSP): funding. FO: Travel, Accommodations, Expenses: Takeda, Kyowa Kirin International, Medac. CB: inventor of different patents on cancer immunotherapy and genetic engineering; member of Advisory Boards and Consultant for, Intellia, Kite/Gilead, Miltenyi, Kiadis, QuellTx, Janssen, Chroma, Genyo, Pancancer-T, Alia, and received research support from Intellia Therapeutics. AS: Honoraria: Takeda, Bristol Myers Squibb, Merck Sharp and Dohme, Celgene, Janssen, Sanofi, Roche, Novartis, Gilead Sciences, Janssen-Cilag; Consulting or Advisory Role: Takeda, Bristol Myers Squibb, Gilead Sciences, Celgene, Janssen, Novartis; Speakers’ Bureau: Takeda; Travel, Accommodations, Expenses: Kite/Gilead. JGG: Received honoraria from Amgen, BMS Gilead/Kite, Janssen, Novartis, and received research funding from AZ, BMS and Janssen. IY-A: Received honoraria from Novartis, Gilead/Kite, BMS and Janssen. MS: Morphosys: Research Funding; Novartis: Consultancy, Research Funding; Janssen: Consultancy; Seattle Genetics: Research Funding; AMGEN: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria; Kite/Gilead: Consultancy, Honoraria, Research Funding; Roche AG: Consultancy, Research Funding. None of the mentioned conflicts of interest were related to financing of the content of this article. All the other authors have no conflicts of interest to disclose.
Supplementary Material
Footnotes
IY-A and MS have contributed equally to this work.
Supplemental digital content is available for this article.
REFERENCES
- 1.Locke FL, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386:640–654. [DOI] [PubMed] [Google Scholar]
- 2.Abramson JS, Solomon SR, Arnason J, et al. Lisocabtagene maraleucel as second-line therapy for large B-cell lymphoma: primary analysis of phase 3 TRANSFORM study. Blood. 2023;141:1675–1684. [DOI] [PubMed] [Google Scholar]
- 3.Shah BD, Ghobadi A, Oluwole OO, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021;398:491–502. [DOI] [PubMed] [Google Scholar]
- 4.Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382:1331–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raje N, Berdeja J, Lin Y, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. 2019;380:1726–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.June CH, Sadelain M. Chimeric antigen receptor therapy. N Engl J Med. 2018;379:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gauthier J, Yakoub-Agha I. Chimeric antigen-receptor T-cell therapy for hematological malignancies and solid tumors: clinical data to date, current limitations and perspectives. Curr Res Transl Med. 2017;65:93–102. [DOI] [PubMed] [Google Scholar]
- 8.Shimabukuro-Vornhagen A, Godel P, Subklewe M, et al. Cytokine release syndrome. J ImmunoTher Cancer. 2018;6:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karschnia P, Jordan JT, Forst DA, et al. Clinical presentation, management, and biomarkers of neurotoxicity after adoptive immunotherapy with CAR T cells. Blood. 2019;133:2212–2221. [DOI] [PubMed] [Google Scholar]
- 10.Gauthier J, Turtle CJ. Insights into cytokine release syndrome and neurotoxicity after CD19-specific CAR-T cell therapy. Curr Res Transl Med. 2018;66:50–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yassine F, Iqbal M, Murthy H, et al. Real world experience of approved chimeric antigen receptor T-cell therapies outside of clinical trials. Curr Res Transl Med. 2020;68:159–170. [DOI] [PubMed] [Google Scholar]
- 12.Fried S, Avigdor A, Bielorai B, et al. Early and late hematologic toxicity following CD19 CAR-T cells. Bone Marrow Transplant. 2019;54:1643–1650. [DOI] [PubMed] [Google Scholar]
- 13.Cordeiro A, Bezerra ED, Hirayama AV, et al. Late events after treatment with CD19-targeted chimeric antigen receptor modified T cells. Biol Blood Marrow Transplant. 2020;26:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rejeski K, Perez Perez A, Sesques P, et al. CAR-HEMATOTOX: a model for CAR T-cell related hematological toxicity in relapsed/refractory large B-cell lymphoma. Blood. 2021;138:2499–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juluri KR, Wu V, Voutsinas JM, et al. Severe cytokine release syndrome is associated with hematologic toxicity following CD19 CAR T-cell therapy. Blood Adv. 2022;6:2055–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain T, Knezevic A, Pennisi M, et al. Hematopoietic recovery in patients receiving chimeric antigen receptor T-cell therapy for hematologic malignancies. Blood Adv. 2020;4:3776–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rejeski K, Wu Z, Blumenberg V, et al. Oligoclonal T-cell expansion in a patient with bone marrow failure after CD19 CAR-T for richter transformed DLBCL. Blood. 2022;140:2175–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nastoupil LJ, Jain MD, Feng L, et al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US lymphoma CAR T consortium. J Clin Oncol. 2020;38:3119–3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rejeski K, Perez A, Iacoboni G, et al. The CAR-HEMATOTOX risk-stratifies patients for severe infections and disease progression after CD19 CAR-T in R/R LBCL. J ImmunoTher Cancer. 2022;10:e004475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bethge WA, Martus P, Schmitt M, et al. GLA/DRST real-world outcome analysis of CAR-T cell therapies for large B-cell lymphoma in Germany. Blood. 2022;140:349–358. [DOI] [PubMed] [Google Scholar]
- 21.Hayden PJ, Roddie C, Bader P, et al. Management of adults and children receiving CAR T-cell therapy: 2021 best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE) and the European Haematology Association (EHA). Ann Oncol. 2021;33:259–275. [DOI] [PubMed] [Google Scholar]
- 22.Hayden PJ, Sirait T, Koster L, et al. An international survey on the management of patients receiving CAR T-cell therapy for haematological malignancies on behalf of the Chronic Malignancies Working Party of EBMT. Curr Res Transl Med. 2019;67:79–88. [DOI] [PubMed] [Google Scholar]
- 23.Hill JA, Seo SK. How I prevent infections in patients receiving CD19-targeted chimeric antigen receptor T cells for B-cell malignancies. Blood. 2020;136:925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy – assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15:47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fardet L, Galicier L, Lambotte O, et al. Development and validation of the Hscore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66:2613–2620. [DOI] [PubMed] [Google Scholar]
- 26.Steensma DP, Bejar R, Jaiswal S, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sterner RM, Sakemura R, Cox MJ, et al. GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts. Blood. 2019;133:697–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodman LS, Wintrobe MM, et al. Nitrogen mustard therapy; use of methyl-bis (beta-chloroethyl) amine hydrochloride and tris (beta-chloroethyl) amine hydrochloride for Hodgkin’s disease, lymphosarcoma, leukemia and certain allied and miscellaneous disorders. J Am Med Assoc. 1946;132:126–132. [DOI] [PubMed] [Google Scholar]
- 29.Taplitz RA, Kennedy EB, Bow EJ, et al. Antimicrobial prophylaxis for adult patients with cancer-related immunosuppression: ASCO and IDSA clinical practice guideline update. J Clin Oncol. 2018;36:3043–3054. [DOI] [PubMed] [Google Scholar]
- 30.Rejeski K, Blumenberg V, Iacoboni G, et al. Identifying early infections in the setting of CRS with routine and exploratory serum proteomics and the HT10 score following CD19 CAR-T for relapsed/refractory B-NHL. HemaSphere. 2023;7:e858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rejeski K, Wang Y, Albanyan O, et al. The CAR-hematotox score identifies patients at high risk for hematological toxicity, infections and poor clinical outcomes following brexucabtagene autoleucel in relapsed/refractory mantle cell lymphoma. Blood. 2022;140(Supplement 1):651–653. [Google Scholar]
- 32.Rejeski K, Hansen DK, Bansal R, et al. The CAR-hematotox score as a prognostic model of toxicity and response in patients receiving BCMA-directed CAR-T for relapsed/refractory multiple myeloma. Blood. 2022;140(Supplement 1):7506–7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rejeski K, Perez Perez A, Iacoboni G, et al. Biphasic neutrophil recovery after CD19 CART in R/R LBCL is associated with superior PFS/OS, robust CAR T-cell expansion in relation to baseline tumor volume, and a decrease of systemic inflammation over time. Blood. 2022;140(Supplement 1):4549–4551. [Google Scholar]
- 34.Barreto JN, Bansal R, Hathcock MA, et al. The impact of granulocyte colony stimulating factor on patients receiving chimeric antigen receptor T-cell therapy. Am J Hematol. 2021;96:E399–E402. [DOI] [PubMed] [Google Scholar]
- 35.Lievin R, Di Blasi R, Morin F, et al. Effect of early granulocyte-colony-stimulating factor administration in the prevention of febrile neutropenia and impact on toxicity and efficacy of anti-CD19 CAR-T in patients with relapsed/refractory B-cell lymphoma. Bone Marrow Transplant. 2022;57:431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller KC, Johnson PC, Abramson JS, et al. Effect of granulocyte colony-stimulating factor on toxicities after CAR T cell therapy for lymphoma and myeloma. Blood Cancer J. 2022;12:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rejeski K, Burchert A, Iacoboni G, et al. Safety and feasibility of stem cell boost as a salvage therapy for severe hematotoxicity after CD19 CAR T-cell therapy. Blood Adv. 2022;6:4719–4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mullanfiroze K, Lazareva A, Chu J, et al. CD34+-selected stem cell boost can safely improve cytopenias following CAR T-cell therapy. Blood Adv. 2022;6:4715–4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gagelmann N, Wulf GG, Duell J, et al. Hematopoietic stem cell boost for persistent neutropenia after CAR-T cell therapy: a GLA/DRST study. Blood Adv. 2023;7:555–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hermine O, Hoster E, Walewski J, et al. Addition of high-dose cytarabine to immunochemotherapy before autologous stem-cell transplantation in patients aged 65 years or younger with mantle cell lymphoma (MCL Younger): a randomised, open-label, phase 3 trial of the European Mantle Cell Lymphoma Network. Lancet. 2016;388:565–575. [DOI] [PubMed] [Google Scholar]
- 41.Richardson PG, Jacobus SJ, Weller EA, et al. Triplet therapy, transplantation, and maintenance until progression in myeloma. N Engl J Med. 2022;387:132–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
For original data and material, please contact the corresponding authors.