Abstract
The gut microbiota is critical for maintaining human health and the immunological system. Several neuroscientific studies have shown the significance of microbiota in developing brain systems. The gut microbiota and the brain are interconnected in a bidirectional relationship, as research on the microbiome–gut–brain axis shows. Significant evidence links anxiety and depression disorders to the community of microbes that live in the gastrointestinal system. Modified diet, fish and omega-3 fatty acid intake, macro- and micro-nutrient intake, prebiotics, probiotics, synbiotics, postbiotics, fecal microbiota transplantation, and 5-HTP regulation may all be utilized to alter the gut microbiota as a treatment approach. There are few preclinical and clinical research studies on the effectiveness and reliability of various therapeutic approaches for depression and anxiety. This article highlights relevant research on the association of gut microbiota with depression and anxiety and the different therapeutic possibilities of gut microbiota modification.
Keywords: gut–brain axis, microbiota, anxiety, depression, probiotics
1. Introduction
Depression and anxiety impact people’s everyday life, health, and economic position. According to research, depression may overtake heart failure as the most prevalent disease in the world by 2030 [1,2]. Anxiety and stress are often present in conjunction with depressive disorders; these coexist in approximately 75% of children and adolescents [2,3]. Anxiety disorders are thought to be 47–58% more likely to develop during a depressive episode, and 56% of people with anxiety disorders experience depression [4,5]. Patients’ unexpectedly unpleasant life situations occur around 50% of the time before depressive episodes [6,7,8,9,10]. Unfortunately, everyone experiences stress at some point in their life. With lifetime incidence rates of 14% and 12%, respectively, anxiety and depressive disorders are common mental health conditions globally [11]. It is undoubtedly challenging for the patient to operate in society when suffering from anxiety or despair. The common risk factors for depression are poverty, unemployment, traumatic life events, physical disease, and drug and alcohol addiction, although anybody may suffer from depression. The individual who suffers from anxiety or depression loses their sense of self-worth. In psychiatry, patient observation and medical interviews with the patient and their immediate family members are the primary methods for diagnosing stress, anxiety, and depression [12,13]. In addition to the adverse effects on the individuals affected, these disorders place a significant financial strain on society due to high healthcare costs [14,15]. These points make it evident that there is a need for effective treatments.
Pharmacotherapy is the keystone of current therapies for depression. Selective serotonin reuptake inhibitors (SSRIs) are the most popular first-line medication, although monoamine oxidase and serotonin-norepinephrine reuptake inhibitors are also used. However, the effectiveness of currently available antidepressant medications used in clinics for symptom relief and prevention seems inconsistent [16]. In addition, it has been shown that tolerance develops during follow-up care; using the same medication on the same patient repeatedly leads to decreased efficacy. Up to 35% of people are estimated to experience treatment-resistant depression [17]. Therefore, there is a need to explore novel therapies for preserving the quality of life for all people suffering from depression.
According to studies, there is a correlation between diet, nutrition and anxiety and depression. Preliminary studies suggested that dietary changes may be an alternate treatment or preventative measure for anxiety and depression [18,19]. The correlation between unhealthy diet and the propensity to develop mental illnesses has received more attention in recent years [20]. “Western” dietary patterns with low consumption of fruits and vegetables and high consumption of refined grains, fried and processed meals, red meat, and high-fat dairy products are linked to anxiety and depression [21,22]. At the same time, many correlative studies in healthy adults demonstrate a lower incidence of depression in those who adhere to “healthy” dietary patterns, such as the Norwegian diet [18], Japanese diet [23,24], and Mediterranean diet [25,26], which are focused on the abundant consumption of vegetables, fruits, cereals, nuts, seeds, pulses, dairy, eggs, fish, and unsaturated fats [27]. Stress and depression may also affect dietary preferences, how sweet and fatty foods are perceived [28,29], and taste thresholds [30]. A 10-year longitudinal research study conducted in France demonstrated a correlation between poor nutrition and depression incidence and healthy diet pattern associated with lower depressive symptoms [31]. Randomized controlled trials and prospective research have both shown inconsistent findings when attempting to establish the direction of the correlation. A high-quality diet, independent of its form, with greater fish and vegetable consumption, was related to a decreased incidence of depression, with a dose-type association with compliance with the healthy diet, according to a meta-analysis of prospective studies. In contrast, the meta-analysis revealed that a poor diet was not linked to an increased risk of depression, and the findings revealed significant variability across trials [32].
The scientific findings are not consistent regarding diet and mental health. Maybe there are bidirectional processes behind how the diet might impact anxiety and depression. The micronutrients, such as zinc, magnesium, selenium, iron, and the vitamins B-6, B-12, D, E, and folate, are deficient in those who have depression or are at a greater risk of developing depression and anxiety [33,34,35].
More than 3.8 × 1013 bacteria exist in the human gut microbiota [36]. Microflora dysbiosis is associated with increased intestinal permeability and systemic inflammation [37]. The human gut has the second-highest concentration of neurons after the brain [38].
Therefore, studies have been conducted to find the association between gut microbiota and depression. Naseribafrouci et al. confirmed the correlations between this mental disorder and intestinal flora. They showed that individuals with major depressive disorder have higher levels of the genera Oscillibacter and Alistipes [39]. Lower propionic acid levels and enormous amounts of isocaproic acid were found in individuals diagnosed with depression [40].
Although the etiology of depression is complicated, the gut microbiome’s potential to affect depression development has been examined in several research studies. Intestinal microbiota disturbances might result in the onset of anxiety and depression [41]. After injecting high sugar, high fat, and antibiotic doses into mice, David et al. discovered changes in the mice’s behavior and intestinal microbiota [42]. The quantity of Bacteroides spp. dramatically dropped, while Clostridium spp. numbers significantly increased [43]. The c-FOS proto-oncogene may be produced by Campylobacter jejuni, which can also cause anxiety and depression [44]. Lactobacillus and Bifidobacterium species were found to be capable of reducing depression to a large extent [45,46]. The points mentioned above suggest that gut microbiota has a role in developing anxiety and depression. Anxiety and depression are gut–brain axis disorders that can be treated with the help of the gut microbiota, which offers a unique approach to modifying neurotransmitter regulation in the brain [47,48].
Studies have shown links between anxiety and depression and the development or diagnosis of certain metabolic disorders [49], cardiovascular diseases [49], cancers [50], atopic illnesses [51], and chronic pain syndromes [52]. Numerous studies on humans and animals have shown the importance of physiological mechanisms underlying inflammatory and stress responses in the etiology of depression and anxiety [53,54]. Extensive research has shown the connection between inflammation and depression [55]. Consumption of a high-fat diet leads to chronic systemic inflammation [56]. Anti-inflammatory medications are beneficial in treating depression [57]. They may change neurotransmitters’ metabolism by lowering precursors’ availability and stimulating the hypothalamic–pituitary–adrenal (HPA) axis. Studies have shown that certain depressed individuals have higher levels of pro-inflammatory cytokines than healthy controls [58]. High levels of pro-inflammatory cytokines indicate a future risk of depression [59]. Studies of individuals with clinically diagnosed anxiety have shown higher levels of pro-inflammatory cytokines than healthy people [58]. Furthermore, it has been shown that triggering an immunological response makes humans [60] anxious and shows anxiety-like phenotypes in animals [61]. On a molecular level, it has been shown that stress-induced interleukin (IL)-6 activity changes the expression of certain genes in monocytes and results in anxiety-like behavior in mice [62]. Though further investigations are required, observational and experimental studies generally provide evidence for the theory that inflammation plays a role in anxiety and depression. This article emphasizes the relationship between gut microbiota and anxiety or depression. We provide a potential strategy for alleviating anxiety and depression that involves modified diets, fish, and omega-3 fatty acids (FA) intake, probiotics, prebiotics, synbiotics, postbiotics, fecal microbiota transplantation, and 5-hydroxytryptophan regulation. The main objective of this article is to summarize the recent evidence linking gut microbiota to anxiety and depression and possible ways to alleviate the symptoms of these disorders.
2. Material and Methods
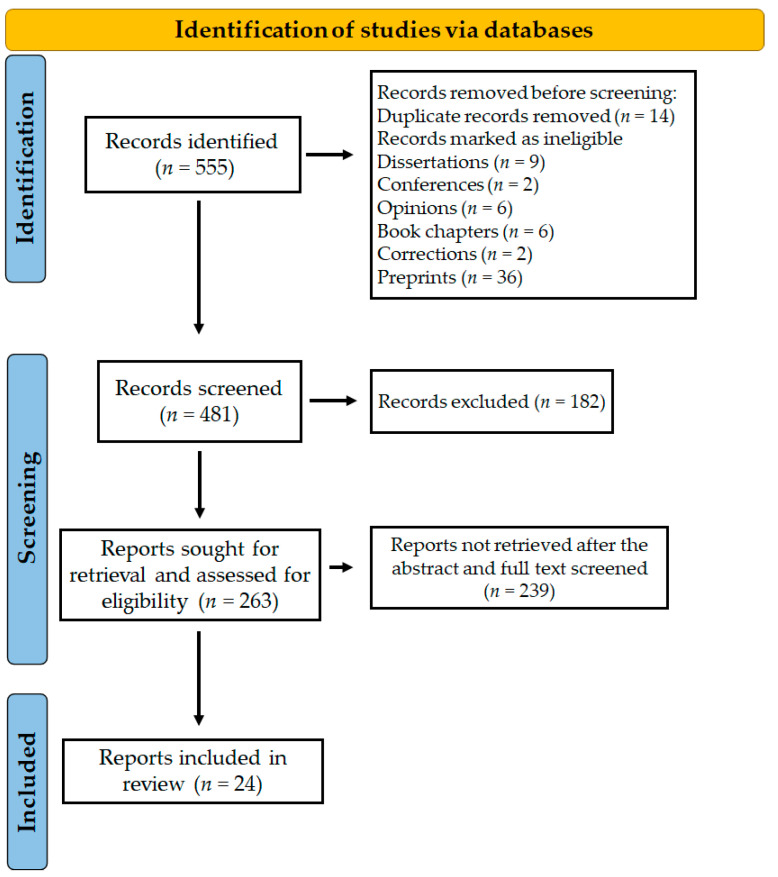
Whether there is a link between the gastrointestinal microbiota and anxiety or depression was the major concern of this review. Does the gut microbiota impact the onset, progression, and treatment of anxiety or depression? From 1 November to 31 December 2022, the databases Scopus, Google Scholar, and PubMed were examined using the phrases title:(microbiome OR microbiota) AND title:(depression OR depressive OR anxiety). We provide a thorough summary of the information published on this subject. Figure 1 represents preclinical and clinical data on the relationships of depression and anxiety with alterations in the gut microbiota. This review included 24 papers, most published between 2019 and 2022, indicating a dramatic rise in interest in this field.
Figure 1.
PRISMA diagram explaining the screening and selection of the literature for the study.
3. Epidemiology of Depression and Anxiety
3.1. The Gut–Brain Axis in Depression and Anxiety
Several studies showed that the gut–brain axis influences the development of anxiety and depression. The gut–brain axis is a network that transmits information in a bidirectional pattern between the gut and the brain and is controlled by neuroendocrine and neuroimmune mechanisms [63,64,65]. Gamma-aminobutyric acid (GABA) neurotransmitters [66], secondary bile acids [67], short-chain fatty acids [68], and tryptophan metabolites generated from the microbiota are only a few of the molecules that regulate these mechanisms [66,69,70]. The gut–brain axis is dysregulated and linked to neuroinflammation and altered blood–brain barrier (BBB) permeability during gut microbiota dysbiosis or disturbance in the gut ecosystem [71]. According to research using rodent models, the BBB becomes more permeable when the normal intestinal microbiota is lost or disturbed [72]. Increased BBB permeability and possible subsequent development of Alzheimer’s disease with amyloid-peptide accumulation may be associated with metabolic illnesses [73]. It has been discovered that microbial dysbiosis affects the protective properties of the BBB, including permeability modulation [72] via tight junction expression [74], and causes behavioral alterations [75].
Studies showed that changes in gut microbiota increased the level of harmful compounds such as p-cresol, which may compromise the integrity of the BBB [76,77]. Earlier research demonstrated that p-cresol was considerably higher in the prefrontal cortex of susceptible mice that previously exhibited anxiety-like phenotypes [78]. Moreover, the gut-derived metabolite 4-ethyl phenyl sulfate (4EPS) affects brain activity and causes anxiety-like behaviors [79]. The gut microbiota generates reduced levels of neurotoxic metabolites after administration of Bacteroides fragilis, including 4-EPS, serum glycolate, and imidazole propionate, improving gut permeability and reducing anxiety-like behavior [80]. Serotonin and dopamine release, brain-derived neurotrophic factor levels, the HPA axis, and the production of inflammatory cytokines may all be affected by disturbances in the gut microbiota during depression and anxiety [81]. For instance, C-reactive protein (CRP) and cytokines, including interleukin-1, interleukin-2, interleukin 6, interleukin-1β, and interferon-γ, were released in response to depression [82].
According to studies, patients with inflammatory disorders are more likely to experience depression. Episodes of depressive symptoms are expected with severe inflammatory bowel disease conditions, possibly related to disrupting the pathways involved in the gut–brain axis [83]. Persistent neuroinflammation alters brain functioning and affects a person’s mood and behavior [56]. The cure for inflammation-mediated depression and vice versa has not been found yet, and managing mental disorders and severe inflammation is challenging [84,85]. People with depression who resist medical treatment might have severe inflammation and gut dysbiosis [86,87].
The research by Guida et al. revealed that the consumption of the probiotic Lactobacillus casei was able to alleviate the depression and overall inflammatory state that was caused by antibiotic-induced dysbiosis in mice [88]. It has also been shown that feces from people with depression may cause a depressive-like phenotype in animals with altered gut microbiota [89]. Preliminary evidence from observational studies has demonstrated that patients with depression and anxiety disorders have significantly different gut microbiome profiles compared to healthy individuals [90].
3.2. The Relationships between Epigenetics, Gut Microbiota, Depression, and Anxiety
Microorganisms inhabit the human gut in a synbiotic manner. The prokaryotic organisms that comprise the “human holobiont” are essential for preserving homeostasis and proper functioning. Various neurological illnesses, including Alzheimer’s disease, Parkinson’s disease, depression, etc., have been related to disturbances in the gut microbiota composition. Numerous sophisticated molecular mechanisms, including immune system modification [91], metabolic signaling [92], neuroendocrine signaling [93], vagal nerve signaling [94], and epigenetics, are used by these microorganisms to maintain normal homeostasis [95,96]. Epigenetics plays a significant role in controlling host physiology by modifying the metabolic activity of the gut microbiome, which is influenced by environment and nutrition. For example, cofactors for the activity of enzyme acetylases and methylases, which control histone modification and DNA methylation, originate from the gut microbiome. The metabolites generated by the gut microbiota function as cofactors and substrates for numerous enzyme activities [95]. Epigenetic regulation is a dynamic process affected by changes in diet, activity, and microbiota composition [97]. Epigenetics means “in addition to genetics”. Instead of looking at the DNA sequence, it includes analyzing chromosome-level changes in gene expression. Both modifications are persistent and heritable. Epigenetics primarily control chromosomal superstructure changes and chemical modifications to nitrogenous bases without directly affecting the DNA sequence. Epigenetics may result from several molecular processes, but the primary ones include histone modification, DNA methylation and acetylation, and RNA-associated silencing [98].
4. Risk Factors Associated with Depression and Anxiety
There are various risk factors for the onset of depression and anxiety, such as biological [99], genetic [100], personality trait-related [101], social [102], economic [103], and lifestyle-related factors [99]. Factors that may contribute to depressive symptoms in the elderly include aging, living alone, being a woman, having less education, getting divorced, having comorbid physical illnesses, having functional disorders, using tobacco and alcohol, and having lower-level cognitive dysfunction [104]. Another meta-analysis found that having a chronic disease and feeling unwell increased the chance of depression [105]. There is a high prevalence of depression in people with physical and chronic illnesses [106]. Several research studies have investigated risk factors for depression and anxiety in students. These factors include age [107], grade [108], ethnicity [109], being an only child [110], attitude toward future career [111], academic pressure [112], smoking addiction [113], alcoholism [113], family financial status [111,112], and social support [114].
5. The Gut Microbiota, Depression, and Anxiety
5.1. Animal Studies
Numerous studies have looked at the relationship between alterations in the composition and diversity of the gut microbiome and anxiety [115,116,117] and depression [116,118,119]. Most of these studies used rodent models and antibiotics to diminish the gut microbiota [120,121], and the results demonstrated varied behavioral and physiological changes. It is well-acknowledged that stress contributes to the pathophysiology of depression and shows adaptive adjustments in many pathways, including brain-derived neurotrophic factor (BDNF), inflammatory cytokines, and the spleen, to promote resilience [122,123,124]. Learned helplessness susceptible rats had much lower levels of acetic and propionic acid in their feces than the control. The learned helplessness resilient rats had significantly larger relative abundances of the genera Lactobacillus, Clostridium cluster III, and Anaerofustis [125]. Interestingly, resistance to chronic social defeat stress in mice was correlated with antibiotic-induced gut dysbiosis [126]. These results confirm that the brain–gut–microbiota axis influences stress resiliency and vulnerability [123,127]. Preclinical research is accumulating evidence that the brain–gut–microbiota axis is critical in the development of depression [128,129]. Stress-induced depression in rodents has been shown to be accompanied by abnormal levels of gut microbiota-related short-chain fatty acids and other metabolites such as alanine, isoleucine, L-threonine, serine, and tyrosine, which may be connected to altered levels of 5-hydroxytryptamine (5-HT) in the brain and depressive-like phenotypes [130,131]. The brain–gut–microbiota axis is thought to have a bidirectional impact on depression.
The brain, the gut microbiota, and the immune system are connected through the vagus nerve [132]. Vagus nerve signaling, connected to inflammatory control and modification by neuroactive chemicals, is linked to depression [133]. According to Bravo et al., vagotomy prevented the effects of Lactobacillus rhamnosus on brain chemistry and depressive-like phenotypes and decreased stress-induced depression-like phenotypes in mice [92]. Subdiaphragmatic vagotomy (SDV) was shown to reduce depression-like phenotypes, levels of pro-inflammatory cytokines, expression of synaptic proteins, and aberrant gut microbiota composition in mice following lipopolysaccharide (LPS) treatment [134]. Researchers have used mouse models of depression to examine how the probiotic Lactobacillus rhamnosus affects neural function. One investigation discovered a dependent or independent relationship between variations in the c-Fos protein in certain brain areas and vagal signaling [135]. Considering these results, pinpointing the vagus nerve’s exact pathway to the brain–gut–microbiota axis in depression is of tremendous interest. A stimulating electrode emitting low-frequency, irregular electrical pulses [136] or gut bacteria [137] may stimulate the vagus nerve. Vagus nerve stimulation (VNS) has potent anti-inflammatory actions [138,139]. Patients’ moods improved because of vagus nerve stimulation, first used to treat refractory epilepsy. It is now recognized as a treatment option for those with refractory depression [140].
According to several research studies, it has been proved that the administration of microbiota or their metabolites may exacerbate or ameliorate depression. Fecal microbiota transplantation of the “depression-related microbiome” induced depression-like phenotypes in mice [141,142]. On the other hand, it has been shown that therapy with a combination of short-chain fatty acids, including acetate, butyrate, and propionate, reduces stress-induced depressive behaviors [143]. The altered protein profiles were anticipated to perform roles in the inflammatory immune response and metabolic regulation [144]. The case for the brain–gut axis’ involvement in depression may be strengthened by a better knowledge of the significant changes that occur from the gut to the brain or from the brain to the gut [145,146,147]. In recent research, rats who received feces from depressed human patients displayed depressed behavior and were depleted of Coproccocus, indicating that Coproccocus may have a causative relationship with depression [141]. To examine and evaluate the effects of microbiota depletion on anxiety-like behavior and depression in rodent models, as well as to identify the research that needs to be performed to assist future translatability, gathering and summarizing the available data is required. Table 1 represents the alteration in microbial composition during anxiety and depression.
Table 1.
The microbiota changes in depression and anxiety.
| Disease | Subject | Sample Size and Characteristics | Sequencing Platforms |
Observations and Changes in Microbiota | Ref. |
|---|---|---|---|---|---|
| MDD (with anxiety) | Human | Control: n = 10 (Mean age: 33 years, 60% female); Psychiatric subjects: n = 60 (major depressive disorder and anxiety: n = 38, anxiety: n = 8, depression: n = 14). | 16S rRNA sequencing using Roche 454 Titanium platform. | ↓ Clostridia in people with depression. ↓ Bacteroides are more closely linked to the prevalence of anxiety than depression. |
[90] |
| Depression | Flinders sensitive line rats | 24 FRL rats (weight 327.8 ± 40.7 g, and 10.6 ± 1.1 weeks old). | 16S rRNA sequencing using Illumina MiSeq. | ↓ Phyla Elusimicrobia and Saccharibacteria ↑ Proteobacteria ↑ Blautia and Subdoligranulum ↓ Candidatus Saccharimonas, Alistipes, and Roseburia |
[145] |
| Depression | Human | Belgian Flemish Gut Flora Project population (n = 1054 subjects). | Shotgun sequencing | In depression, ↓ Coprococcus and Dialister
after restricting the antidepressant medication effect. ↓ Fusicatenibacter and Butyricicoccus after controlling for antidepressant treatment. ↑ Phascolarctobacterium, Lactobacillus, Parabacteroides, Holdemania ↓ Dialister, Coprococcus, Turicibacter, and Faecalibacterium |
[148] |
| Bipolar depression | Human | Healthy controls: n = 45; Bipolar depression patients: n = 72. | 16S rRNA sequencing using Illumina MiSeq. | ↑ Parabacteroides, Bacteroides, Weissella, and Halomonas. | [149] |
| MDD | Human | Healthy controls: n = 71; Major depressive disorder patients n = 70. | 16S rRNA sequencing using Roche 454 Titanium platform. | ↓ Bacteroidetes, Actinobacteria, and Firmicutes | [150] |
| Anxiety and depression | Human | Control: n = 46; Anxiety and depression group: n = 23. | 16S rRNA sequencing using MiniSeq. | Reduction in Gemmiger, Ruminococcus, and Veillonella. | [151] |
| Generalized anxiety disorder | Human | Healthy controls: n = 36; Generalized anxiety disorder patients: n = 40. | 16S rRNA sequencing using Illumina MiSeq. | ↑ Fusobacterium, Escherichia-Shigella, and Ruminococcus gnavus | [152] |
| Chronic paradoxical sleep deprivation-induced depression | Wistar rats | - | 16S rRNA pyrosequencing. | ↓ Akkermansia, Phascolarctobacterium, and Ruminococcus
↑ Parabacteroides, Oscillospira, and Aggregatibacter |
[153] |
| MDD | Human | Healthy controls: n = 10 (age: 24–65 years, women: n = 5); Major depressive disorder patients: n = 10 (age: 18–56 years, women: n = 5). | - | ↑Actinobacteria, Firmicutes, and Lachnospiraceae ↓ Bacteroidetes and Proteobacteria ↓ Faecalibacterium |
[154] |
| Late-life depression | Human | Healthy controls: n = 17; Late-life depression patients: n = 36. | 16S rRNA sequencing using Illumina MiSeq. | ↑ Akkermansiaceae and Akkermansia. | [155] |
| MDD | Human | Healthy controls: n = 37; Major depressive disorder patients: n = 36. | 16S rRNA sequencing using Illumina MiSeq. | ↑ Actinobacteria and Firmicutes ↑ Bifidobacterium, and Blautia ↓ Prevotella |
[156] |
| MDD | Human | Healthy controls: n = 10; Major depressive disorder patients: n = 10. | 16S rRNA sequencing using Illumina MiSeq. | ↓ Bifidobacterium and Dialister
↑ Bacteroidetes and Bacteroides |
[157] |
| Depression | Human | Controls: n = 31; Ulcerative colitis without depression: n = 31; Ulcerative colitis with depression: n = 31. | 16S rRNA pyrosequencing. | ↑ Proteobacteria, gamma proteobacteria ↓ Firmicutes, Clostridia, and Clostridiales |
[158] |
| Postpartum depressive disorder | Human | Healthy controls: n = 16; Postpartum depressive disorder patients: n = 28. | 16S rRNA sequencing using Illumina MiSeq. | ↓ Faecalibacterium, Phascolarctobacterium, Butyricicoccus, and Lachnospiraceae ↑ Enterobacteriaceae |
[159] |
| Systemic lupus erythematosus with depression | Human | Healthy controls: n = 32; Systemic lupus erythematosus with depression patients: n = 21. | 16S rRNA using Illumina Novaseq 6000 sequencing. | ↓ Ratios of the genera Faecalibacterium to Roseburia and phyla Firmicutes to Bacteroidetes. | [160] |
| MDD | Human | Healthy controls: n = 43; First-episode, drug-naïve major depressive disorder patients: n = 66. | 16S rRNA sequencing using Illumina Novaseq PE250 platform. | ↑ Deinococcus and Odoribacter ↓ Bacteroides, Alistipes, Turicibacter, Clostridium, Roseburia, and Enterobacter |
[161] |
| MDD | Human | Healthy controls: n = 28; Major depressive disorder patients: n = 26. | 16S rRNA sequencing using Illumina HiSeq 2500 platform. | ↓ Firmicutes ↑ Proteobacteria and Actinobacteria. |
[162] |
| MDD | Human | Healthy controls: n = 45; Current active major depressive disorder patients: n = 46; Remission or with only mild symptoms of major depressive disorder: n = 22. | 16S rRNA sequencing using Illumina MiSeq. | ↑ Bilophila and Alistipes
↓ Anaerostipes and Dialister |
[163] |
| MDD | Human | Healthy controls: n = 27; Major depressive disorder patients: n = 27. | 16S rRNA sequencing using Illumina HiSeq2500. | ↓ Lachnospiraceae, Ruminococcaceae, Coprococcus, Blautia, Clostridiaceae, and Dorea
↑ Oxalobacter, Pseudomonas, Parvimonas, Bulleidia, Peptostreptococcus, and Gemella |
[164] |
| MDD | Human | Healthy female controls: n = 24; First-episode drug-naïve major depressive disorder female patients: n = 24; Healthy male controls: n = 20; First-episode drug-naive major depressive disorder male patients: n = 20. |
16S rRNA sequences using Roche 454. | ↓ Bacteroidetes and Proteobacteria
↑ Firmicutes and Actinobacteria |
[165] |
| MDD | Human | Healthy controls: n = 29; Major depressive disorder patients: n = 26. | 16S rRNA sequencing using Illumina HiSeq 2500 platform. | ↑ Bifidobacterium, Enterococcus, Megasphaera, Coriobacterium, Streptococcus, Slackia, Heliobacterium, Lactobacillus, Oscillibacter, Olsenella, Sphaerochaeta, Desulfitobacterium, Acidaminococcus, Eggerthella, Lachnoclostridium, Atopobium, Rothia. ↓ Sphingobacterium, Bacteroides. ↑ Clostridium saccharolyticum, Megasphaera elsdenii, Acidaminococcus fermentans, Streptococcus parasanguinis, Eggerthella lenta, Desulfovibrio vulgaris, Lactobacillus crispatus, Bifidobacterium adolescentis, Enterococcus faecium, B. longum, Atopobium parvulum, B. bifidum. ↓ Bacteroides helcogenes. |
[166] |
| MDD | Human | Healthy controls: n = 30; Major depressive disorder patients: n = 31; Bipolar disorder with current major depressive episode patients: n = 30. | 16S rRNA sequencing using Illumina HiSeq 2500 platform. | ↓ Firmicutes, Bacteroidota ↑Actinobacteria ↑ Bacteroides, Clostridium, Bifidobacterium, Oscillibacter, and Streptococcus |
[167] |
MDD: Major depressive disorder; ↑: Increased; ↓: Decreased.
5.2. Human Studies
The pathogenic effect of gut dysbiosis is linked, in many clinical investigations, to depressive and anxious behaviors [168,169]. Patients with depression typically have disturbed gastrointestinal (GI) symptoms such as constipation, abdominal discomfort, vomiting, nausea, and bloating [170]. Irritable bowel syndrome (IBS) symptoms or psychological distress are often observed in anxious patients [171,172,173]. Bacteroides, Prevotella/Prevotellaceae, and Proteobacteria were more prevalent in comorbid IBS and anxiety/depression patients than in healthy individuals [174]. According to earlier literature, the gut bacterial strains Coprococcus, Subdoligranulum, Eggerthella, and Ruminococcaceae are linked to depression [175]. It was consistently shown that Eggerthella levels were higher in those with depression and anxiety. It was shown that those with depression and generalized anxiety disorder had decreased levels of Subdoligranulum and Coprococcus [148]. Both unipolar and bipolar depression patients have been discovered to have low levels of the genus and family Ruminococcaceae [148,149,176]. Bosch et al. study also showed a similar trend, with numerous taxa from the family Ruminococcaceae being decreased among individuals reporting more depressive symptoms. Depression symptoms were strongly correlated with Sellimonas and Hungatella, and higher levels of Lachnoclostridium were linked to greater depression symptoms [175].
Some of the key points must be considered in microbiome studies. In detail, genetic variation affects the gut microbial composition and diversity and disease incidences in humans [177]. The study revealed that despite differences in microbial composition between humans and mice, the results of animal studies aid in improving the understanding of the interplay between host genetics and gut microbiota [178]. We must consider the study population’s genetic makeup, age, traditions, and geography when we study their microbial composition. Additionally, lifestyle and food habits affect microbial compositional variation [179].
6. Potential Therapy Involved in the Treatment of Depression and Anxiety
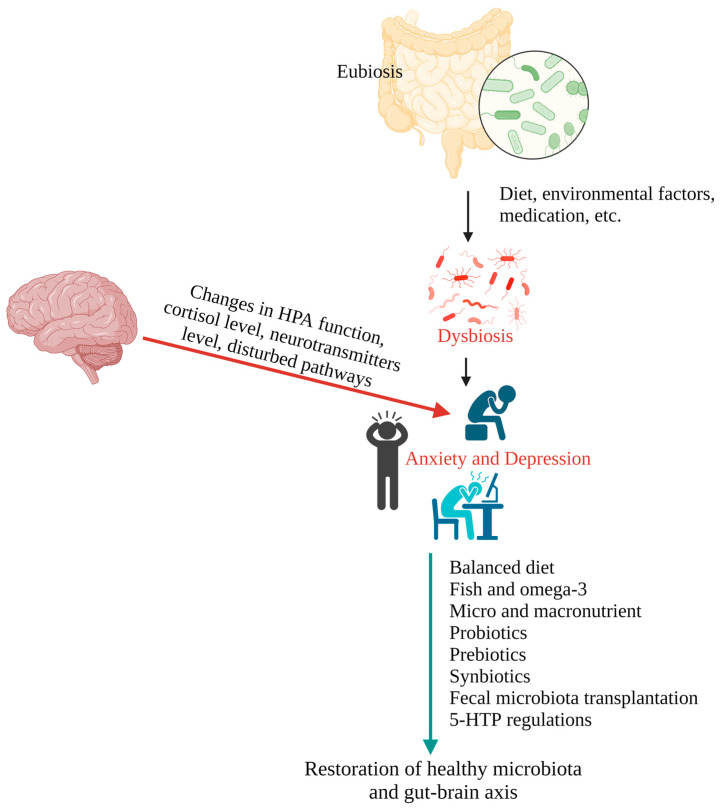
Human depression may be treated with various synthesized drugs, though their effectiveness varies depending on several factors [180]. The recent alternative potential strategies currently being widely considered include modified diets, fish and omega-3 fatty acids intake, probiotics, prebiotics, synbiotics, postbiotics, fecal microbiota transplantation, and 5-hydroxytryptophan regulation (Table 2). These approaches directly or indirectly restore healthy gut microbial composition and diversity. Figure 2 represents the different methods of restoration of gut microbiota to prevent and treat anxiety and depression.
Table 2.
Supplements that aid in the management of depression and anxiety.
| Intervention | Subjects | Main Results | Ref. |
|---|---|---|---|
| L. rhamnosus | Mice | Lowered stress-induced anxiety and depressive-like behavior | [92] |
| Lactobacillus acidophilus, B. longum, B. lactis, B. bifidum, galacto-oligosaccharides (GOS), inulin and fructo-oligosaccharides (FOS) | Human | The synbiotic intervention reduced the depression and anxiety symptoms effectively | [181] |
| Bacillus coagulans | Human | Reduction of depression and irritable bowel syndrome symptoms | [182] |
| Bifidobacterium longum | Human | Improved the quality of life and lessened depression, but not anxiety, in patients with irritable bowel syndrome. | [183] |
| Clostridium butyricum | Human | Enhanced the effectiveness of traditional treatment for depression | [184] |
| L. acidophilus, L. casei, and B. bifidum | Human | Reduction of Beck’s Depression Inventory score | [185] |
| L. helveticus R0052 and B. longum | Human | Effectively reduced symptoms of depression and decreased anxiety | [186] |
|
L. helveticus and B. longum |
Human | Improvement of Beck’s Depression Inventory score | [187,188] |
| L. plantarum | Human | Improvement in symptoms and psychological scores | [189] |
|
B. longum subsp. longum
BAMA-B05/Bau-B1024, B. lactis BAMA-B06/Bau-B0111, B. adolescentis, Streptococcus thermophiles, L. acidophilus, and L. delbrueckii subsp. bulgaricus |
Human | Decreased anxiety. | [190] |
| S. thermophiles, B. longum, B. breve, L. rhamnosus, L. bulgaricus, L. acidophilus, L. casei, and FOS | Human | Improved depressive symptoms in major depressive disorder | [191] |
| Short-chain FOS | Human | Improved depression and anxiety score in patients with irritable bowel syndrome | [192] |
| FOS and GOS | Mice | Improved depression and anxiety | [193] |
| Fecal microbiota transplantation | Mice | Reduced stress-associated depressive-like behavior | [194] |
| Oligosaccharides 3′sialyllactose (3′SL) or 6′sialyllactose (6′SL) | Mice | Lower nervous anxiety-related reactions and preventative impact on anxious behavior. | [195] |
| Polysaccharide of okra | Mice | Restored the gut microbiota | [196] |
Figure 2.
Illustration representing the factors associated with the development of anxiety and depression and the methods of gut microbiota restoration for the prevention and treatment of anxiety and depression.
6.1. Modified Diet
Nutritional study has shifted away from concentrating on individual nutrients since they are hardly taken in the isolated form [197]. According to research, nutrient-dense food supports physical and mental health [138]. Proper brain function is supported by dietary nutrients such as vitamins, minerals, polyunsaturated fats, and amino acids in a balanced diet [140,141]. Numerous nutrients act as enzyme cofactors, producing neurotransmitters, cell signaling, and metabolic pathways [198]. Several different diets might aid in reducing anxiety and sadness. A decreased risk of anxiety or depression was linked to consuming a Mediterranean diet [199,200,201]. In individuals with low levels of anxiety and depression, this diet exhibits a preventive effect against unfavorable cardiovascular disease events [201]. The Mediterranean diet assures enough essential nutrients to prevent depression, such as fruits, nuts, vegetables, grains, legumes, and seafood. Intake of folate was negatively correlated with the frequency of depression in males, particularly smokers. Intake of the B12 vitamin was negatively correlated with depression in women, particularly in smokers and physically active women [202]. In addition, the Mediterranean diet alters the gut flora [203], which may be a plausible mechanism for reducing anxiety and depression. With an exposure–response connection, the “healthy Japanese” pattern may be inversely related to depressive symptoms. In addition to vegetables, fish/shellfish, and fruit, the “healthy Japanese” diet also featured potatoes, seaweed, mushrooms, and soy products. These seem to provide a dietary pattern less likely to cause inflammation. This aspect may be linked to improved psychological health via the production of monoamines and gut flora [204]. Even a fiber-rich diet reduces intestinal pH, preventing harmful bacteria from overgrowing [205]. Extensive research has shown the potential advantages of prebiotics, probiotics, and special dietary therapies in treating depression by modulating gut microbiota and depression through the gut–brain axis [206,207].
6.2. Fish and Omega-3 FA Intake
Healthy dietary habits that include fish have been linked to a decreased incidence of depression [22,208,209,210]. Both clinical and preclinical investigations have shown that fish oil is rich in omega-3 and exhibits antidepressant properties [211,212]. A meta-analysis of 13 randomized clinical trials found that fish oil demonstrated potential for treating serious depression [213]. A supplemental diet rich in omega-3 and omega-6 polyunsaturated fatty acids boosts Bifidobacterium and Lactobacillus and controls microbial metabolism, particularly during early life stress [212,214]. Omega-3 fatty acids, such as docosahexaenoic acid and eicosapentaenoic acid, have also been shown to improve cognition in adulthood and reduce stress and depression [212,215].
6.3. Micronutrient Intake
Many micronutrients are provided through the host’s diet and are also needed for the microbes. Therefore, the host’s micronutrient consumption may impact the composition and functioning of the intestinal flora. Mice receiving a diet low in magnesium had altered gut flora, which was linked to increased depression-like phenotypes [216]. Many bacteria need iron; therefore, iron consumption via diet impacts the diversity of the intestinal flora [217]. In addition, iron deficiency also has an impact on some neurotransmitter levels in the hippocampus and the corpus striatum [218]. People with depression are more iron-deficient than healthy individuals [219]. This result may explain the need for iron to produce neurotransmitters implicated in the pathophysiology of depression. Vitamin B alleviates anxiety, depression, and stress [173,174,220], and vitamin D3 alleviates depression [221,222,223,224].
6.4. Macronutrient Intake
A greater incidence of depression is substantially linked to a lower protein consumption than recommended. A 10% increase in protein consumption was shown to reduce the incidence of depression considerably in South Korea and in the United States [225]. Several biological explanations have linked the intake of protein and depression. These theories are supported by the fact that tryptophan, an amino acid, is a precursor of serotonin. Although consuming a large amount of protein may raise the plasma content of tryptophan [226], other neural amino acids can compete with tryptophan for brain cellular absorption [227]. As a result, increased protein consumption does not always result in higher levels of tryptophan in the brain. The finding that increased protein consumption protects against depression through boosting serotonin in the brain is challenging to understand because of this contradictory impact of protein intake on the tryptophan content. Additionally, other macronutrients have the power to control tryptophan levels and synthesis; for example, consuming carbs or receiving an insulin injection has been shown to raise plasma tryptophan levels [228]. Focusing on the impacts of macronutrients on the intestinal flora has led to an increasing convergence in nutrition [229]. Plant protein, unsaturated fats, and fiber encourage a healthy gut flora compared to excessive animal protein intake, saturated fats, and simple or artificial carbohydrates.
The quality of macronutrients, particularly dietary carbohydrates, is another factor to consider. The impact of high- and low-glycemic-load meals on depression symptoms was investigated in nondepressed persons. According to this research, eating a diet high in glycemic load may result in overall mood changes, more tiredness, and depression symptoms than eating a diet low in glycemic load [230]. The physiological effects of fatty acids vary depending on their type. However, no clinical trial data are available on how fatty acids affect depression or depressed symptoms depending on their saturation level. Since inflammation and endothelial dysfunction are significant risk factors for depression and cardiovascular disease, dietary advice for preventing cardiovascular disease may be beneficial for managing and preventing depression. The prevention and treatment of depression may be aided by replacing saturated fats with unsaturated fatty acids [231], although more research is required to confirm this statement.
6.5. Probiotics
Probiotics, living microorganisms, encourage the development of beneficial bacteria [54,232,233]. When the probiotic L. rhamnosus was administered to stressed mice, it decreased corticosterone levels and the stress-induced gamma-aminobutyric acid 2 mRNA expression. It did not affect gamma-aminobutyric acid 2 expression in the hippocampus [92,234]. Treatment with the probiotic L. farciminis reduced gut barrier leakiness [91]. In animal models, administering a probiotic such as Bifidobacterium longum restored hippocampus BDNF levels and decreased inflammation-induced anxiety-like behavior [183,234]. Probiotics have been observed to lessen depressive-like behavior in IBS patients, but not anxiety [235]. The Oscillibacter strain aids in treating insomnia and anxiety [236,237]. B. longum is beneficial in reducing stress-induced cortisol levels and daily self-reported stress levels [238]. Probiotics including B. bifidum, B. lactis, Lactococcus lactis, L. casei, L. salivarius, L. brevis, and L. acidophilus showed promising results in reducing negative thoughts and behavior in another study [239]. These results from studies demonstrate that probiotics may be used to treat depression and anxiety.
6.6. Prebiotics
Prebiotics are specific substrates that support the development and activity of certain advantageous gut microorganisms [240,241]. In healthy young volunteers, dietary prebiotics, including fructo-oligosaccharides (FOS) and galacto-oligosaccharides (GOS), encouraged the growth of advantageous bacteria such as B. longum. They decreased the hypothalamic–pituitary–adrenal axis activation caused by stress [206,242,243]. Rats’ anxiety and depressive-like behavior caused by lipopolysaccharides were decreased after receiving GOS [207]. Crocin-I enhanced gut microbiota composition and short-chain fatty acid levels and improved the brain-derived neurotrophic factor level in mice with depression-like phenotypes [244].
6.7. Synbiotics
Synbiotics may improve gut microbial activity. Malondialdehyde and hydrogen peroxide concentrations in human plasma were significantly reduced after taking syn-biotics [245]. Women consuming synbiotics had considerably greater plasma levels of glutathione and free sulfhydryl groups than males [246,247]. According to a randomized trial, synbiotic FOS, GOS, and inulin combined with a probiotic mixture containing B. lactis, B. bifidum, L. acidophilus, and B. longum reduced depression and increased serum levels of a brain-derived neurotrophic factor in depressed patients compared to controls. According to this study, synbiotics reduced depressive symptoms more than probiotics alone [181].
6.8. Postbiotics
Postbiotics have therapeutic effects similar to those of probiotics in that they support the integrity of the epithelial barrier function, restore the microbiota’s diversity and composition, control immunological responses, and regulate signaling along the gut–brain axis [248,249]. The administration of a heat-killed L. helveticus strain reduced anxiety- or depression-like phenotypes in adolescent male mice. It improved the genes involved in neuron differentiation and development and signal transduction in the nucleus accumbens [250]. Adult male mice were given heat-killed Enterococcus fecalis along with diet, which decreased depressive and anxious-like behaviors, increased the expression of the genes for the neurotransmitter receptors, and increased the density of Butyricicoccus and Enterococcus content in the gut [251]. Young individuals subjected to chronic stress were given two tablets of heat-inactivated L. gasseri daily for 24 weeks to lower their anxiety, improve their sleep, produce more n-valeric acid, and restore the balance of their microbiome [252].
6.9. Fecal Microbiota Transplantation (FMT)
FMT repairs gut diversity by transferring healthy microflora to the patient’s gut. FMT was developed to achieve healthy gut microbial composition and function, much like probiotics. When healthy donors’ fecal microbiota was transferred to anxious mice, it resulted in a reduction in the symptoms of anxiety and depression. FMT is now one of the approaches most often used to treat gastrointestinal and neuropsychiatric diseases [253]. IBS and other GI tract-related issues have been linked to depression in clinical trials. FMT from a healthy donor reduced IBS patients’ depressive and anxious-like behavior, and Clostridium difficile infection was also reduced in older patients [254,255]. However, if fecal microbiota is transferred from an unhealthy individual, it may cause adverse side effects such as depression. For example, according to a report, fecal microbiota transferred from rheumatoid arthritis patients with depression caused depression-like behaviors in mice via systemic inflammation [256]. Therefore, extra precautions are required for FMT procedures.
6.10. Bifidobacterium and 5-HTP Regulation
In a study, oral 5-HTP treatment markedly improved gut microbiota dysbiosis in mice exhibiting depression-like phenotypes. When 5-HTP was used to treat depression in rats, it helped maintain levels of short-chain fatty acids and brain-derived neurotrophic factors [257]. In a different study, mice underwent a 5-week trial of chronic moderate stress and received LAB (B. longum subsp. infantis E41 and B. breve M2CF22M7). E41 and M2CF22M7 dramatically decreased depression-like phenotypes in the mice by increasing Tph1 expression and 5-HTP secretion in RIN14B cells. 5-HTP and brain-derived neurotrophic factor concentrations in the brain were elevated after E41 and M2CF22M7 administration [258].
7. Advantages and Limitations of Drug Therapy and Gut Microbiota-Based Approaches
Tricyclic antidepressants (TCAs), antihistamines, SSRIs, serotonin-norepinephrine reuptake inhibitors (SNRIs), benzodiazepines (BZDs), and monoamine oxidase inhibitors (MAOI) are FDA-approved anti-depressants [259]. However, there are several drawbacks to popular anti-depressants, including drug tolerance, delayed action, inadequate effectiveness, and side effects [260]. Therefore, it is essential to find novel antidepressant approaches to guarantee the quality of life for all patients with depression and anxiety.
Microbial dysbiosis has a role in the pathophysiology of several chronic illnesses, including depression and anxiety. The above-stated therapies are based on microbiome restoration for treating and preventing depression and anxiety. However, procedures for the restoration of gut microbiota have several pros and cons, which are given below:
7.1. Advantages
These therapies may correct certain forms of dysbiosis and promote health-promoting microbial loads.
The effectiveness of the above-stated therapies has been proved by experiments using animal models and human subjects.
The therapies are relatively effective and produce a long-term cure for the illness.
7.2. Limitations
It is challenging to justify timing and dose regimens because conceptual, mechanistic, and ecological knowledge of the above-stated therapies is currently poor.
Exposing patients’ immune systems to allogenic strains may be harmful if they suffer from disorders such as allergy disease, IBD, or autoimmune diseases characterized by pathologic immune responses.
They are time-consuming procedures compared to drug therapies.
8. Conclusions and Future Prospects
Many studies have examined the gut microbiota in anxiety and depression disorders to elucidate underlying microbial relationships and guide potential diagnostic and therapeutic approaches for these issues. Worldwide, depression and anxiety are the most prevalent diseases and are associated with a reduction in patients’ quality of life. Epidemiology research has demonstrated the protective benefits of modified diets, fish and omega-3 fatty acid intake, probiotics, prebiotics, synbiotics, postbiotics, fecal microbiota transplantation, and 5-hydroxytryptophan regulation against anxiety and depression. In preclinical and clinical studies, the anti-depressive effects of these therapies occurred through various mechanisms, including the upregulation of neuroactive substance expression, the control of monoamine neurotransmitter levels, the reduction of oxidative stress and inflammation, and the modulation of the hypothalamic–pituitary–adrenal axis and gut–brain axis. Apart from the above-mentioned modalities, vagus nerve stimulation is one of the effective methods to attenuate anxiety and depression. Still, detailed studies are required to demonstrate its efficiency in improving mental health.
The available studies have some limitations. For example, the fundamental concepts underlying the processes by which the gut microbiome contributes to depression and anxiety and the efficacy of microbial restoration therapies need further understanding. Additionally, knowledge is poor regarding the effectiveness and safety of probiotics, prebiotics, synbiotics, postbiotics, and fecal microbiota transplantation. The most efficient dosages of fish and omega-3 fatty acids, probiotics, prebiotics, synbiotics, postbiotics, and fecal microbiota transplantation, as well as the possible function of adjuncts such as antidepressant drugs, have not been determined.
Further studies are needed to assess the effectiveness of microbial restoration therapy in anxiety and depression among relevant patient groups. Furthermore, adequately powered clinical and follow-up studies are required to determine the response stability and short- and long-term safety. In addition, basic research is required to understand the mechanisms behind microbiota-based treatments for mental illness, especially for anxiety and depression.
Acknowledgments
B.S.S. and C.C. wish to thank Chiang Mai University, Chiang Mai, Thailand, for its support. B.G.P. wishes to thank Ganpat University, Gujarat, India, for its support.
Abbreviations
| IBD | Inflammatory bowel disease |
| CRP | C-reactive protein |
| 4EPS | 4-ethyl phenyl sulfate |
| BBB | Blood–brain barrier |
| GABA | Gamma-aminobutyric acid |
| IL | Interleukin |
| HPA | Hypothalamic–pituitary–adrenal |
| DNA | Deoxyribonucleic acid |
| RNA | Ribonucleic acid |
| BDNF | Brain-derived neurotrophic factor |
| 5-HTP | 5-hydroxytryptamine |
| SDV | Subdiaphragmatic vagotomy |
| LPS | Lipopolysaccharides |
| MDD | Major depressive disorder |
| SSRIs | Selective serotonin reuptake inhibitors |
| FRL | Flinders resistant line |
| GI | Gastrointestinal |
| IBS | Irritable bowel syndrome |
| FA | Fatty acids |
| TCAs | Tricyclic antidepressants |
| SNRIs | Serotonin-norepinephrine reuptake inhibitors |
| BZDs | Benzodiazepines |
| MAOI | Monoamine oxidase inhibitors |
| FDA | Food and Drug Administration |
| FMT | Fecal microbiota transplantation |
| FOS | Fructo-oligosaccharides |
| GOS | Galacto-oligosaccharides |
| mRNA | Messenger ribonucleic acid |
Author Contributions
Conceptualization, B.G.P., B.S.S. and C.C.; methodology, A.K.; software, J.P.; validation, A.K., J.P., N.G. and D.C.; formal analysis, investigation, B.G.P., B.S.S. and C.C.; data curation, A.K., J.P., N.G. and D.C.; writing—original draft preparation, B.G.P., B.S.S. and C.C.; writing—review and editing, B.G.P., B.S.S. and C.C.; supervision, B.G.P.; project administration, B.G.P.; funding acquisition, C.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This project was supported by the fundamental research fund, Chiang Mai University, Chiang Mai, Thailand.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Wang P.S., Aguilar-Gaxiola S., Alonso J., Angermeyer M.C., Borges G., Bromet E.J., Bruffaerts R., de Girolamo G., de Graaf R., Gureje O., et al. Use of Mental Health Services for Anxiety, Mood, and Substance Disorders in 17 Countries in the WHO World Mental Health Surveys. Lancet. 2007;370:841–850. doi: 10.1016/S0140-6736(07)61414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nobis A., Zalewski D., Waszkiewicz N. Peripheral Markers of Depression. J. Clin. Med. 2020;9:3793. doi: 10.3390/jcm9123793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angola A., Costello E.J. Depressive Comorbidity in Children and Adolescents: Empirical, Theoretical, and Methodological Issues. Am. J. Psychiatry. 1993;150:1779–1791. doi: 10.1176/ajp.150.12.1779. [DOI] [PubMed] [Google Scholar]
- 4.de Graaf R., Bijl R.V., Spijker J., Beekman A.T.F., Vollebergh W.A.M. Temporal Sequencing of Lifetime Mood Disorders in Relation to Comorbid Anxiety and Substance Use Disorders–Findings from the Netherlands Mental Health Survey and Incidence Study. Soc. Psychiatry Psychiatr. Epidemiol. 2003;38:1–11. doi: 10.1007/s00127-003-0597-4. [DOI] [PubMed] [Google Scholar]
- 5.Wittchen H.U., Kessler R.C., Pfister H., Höfler M., Lieb R. Why Do People with Anxiety Disorders Become Depressed? A Prospective-Longitudinal Community Study. Acta Psychiatr. Scand. 2000;406:14–23. doi: 10.1111/j.0065-1591.2000.acp29-03.x. [DOI] [PubMed] [Google Scholar]
- 6.Beaglehole B., Mulder R.T., Frampton C.M., Boden J.M., Newton-Howes G., Bell C.J. Psychological Distress and Psychiatric Disorder after Natural Disasters: Systematic Review and Meta-Analysis. Br. J. Psychiatry. 2018;213:716–722. doi: 10.1192/bjp.2018.210. [DOI] [PubMed] [Google Scholar]
- 7.Chaves C., Castellanos T., Abrams M., Vazquez C. The Impact of Economic Recessions on Depression and Individual and Social Well-Being: The Case of Spain (2006–2013) Soc. Psychiatry Psychiatr Epidemiol. 2018;53:977–986. doi: 10.1007/s00127-018-1558-2. [DOI] [PubMed] [Google Scholar]
- 8.Middeldorp C.M., Cath D.C., van Dyck R., Boomsma D.I. The Co-Morbidity of Anxiety and Depression in the Perspective of Genetic Epidemiology. A Review of Twin and Family Studies. Psychol. Med. 2005;35:611–624. doi: 10.1017/S003329170400412X. [DOI] [PubMed] [Google Scholar]
- 9.Boyer P. Do Anxiety and Depression Have a Common Pathophysiological Mechanism? Acta Psychiatr. Scand. Suppl. 2000;406:24–29. doi: 10.1111/j.0065-1591.2000.acp29-04.x. [DOI] [PubMed] [Google Scholar]
- 10.Wen B.M. Stress, Adaptation, and Disease: Allostasis and Allostatic Load. Ann. N. Y. Acad. Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 11.Johannsen M., Nissen E.R., Lundorff M., O’Toole M.S. Mediators of Acceptance and Mindfulness-Based Therapies for Anxiety and Depression: A Systematic Review and Meta-Analysis. Clin. Psychol. Rev. 2022;94:102156. doi: 10.1016/j.cpr.2022.102156. [DOI] [PubMed] [Google Scholar]
- 12.Montgomery S.A., Asberg M. A New Depression Scale Designed to Be Sensitive to Change. Br. J. Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 13.Beck A.T., Ward C.H., Mendelson M., Mock J., Erbaugh J. An Inventory for Measuring Depression. Arch. Gen. Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 14.Vasiliadis H.M., Dionne P.A., Préville M., Gentil L., Berbiche D., Latimer E. The Excess Healthcare Costs Associated with Depression and Anxiety in Elderly Living in the Community. Am. J. Geriatr. Psychiatry. 2013;21:536–548. doi: 10.1016/j.jagp.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Simon G., Ormel J., VonKorff M., Barlow W. Health Care Costs Associated with Depressive and Anxiety Disorders in Primary Care. Am. J. Psychiatry. 1995;152:352–357. doi: 10.1176/ajp.152.3.352. [DOI] [PubMed] [Google Scholar]
- 16.Leucht S., Hierl S., Kissling W., Dold M., Davis J.M. Putting the Efficacy of Psychiatric and General Medicine Medication into Perspective: Review of Meta-Analyses. Br. J. Psychiatry. 2012;200:97–106. doi: 10.1192/bjp.bp.111.096594. [DOI] [PubMed] [Google Scholar]
- 17.Rush A.J., Kraemer H.C., Sackeim H.A., Fava M., Trivedi M.H., Frank E., Ninan P.T., Thase M.E., Gelenberg A.J., Kupfer D.J., et al. Report by the ACNP Task Force on Response and Remission in Major Depressive Disorder. Neuropsychopharmacology. 2006;31:1841–1853. doi: 10.1038/sj.npp.1301131. [DOI] [PubMed] [Google Scholar]
- 18.Bear T.L.K., Dalziel J.E., Coad J., Roy N.C., Butts C.A., Gopal P.K. The Role of the Gut Microbiota in Dietary Interventions for Depression and Anxiety. Adv. Nutr. 2020;11:890–907. doi: 10.1093/advances/nmaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shabbir M.A., Mehak F., Khan Z.M., Ahmed W., Haq S.M., Khan M.R., Bhat Z.F., Aadil R.M. Delving the role of nutritional psychiatry to mitigate the COVID-19 Pandemic Induced Stress, Anxiety and Depression. Trends Food Sci. Technol. 2022;120:25–35. doi: 10.1016/j.tifs.2021.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Álvarez S.A., Rocha-Guzmán N.E., González-Laredo R.F., Gallegos-Infante J.A., Moreno-Jiménez M.R., Bravo-Muñoz M. Ancestral Food Sources Rich in Polyphenols, Their Metabolism, and the Potential Influence of Gut Microbiota in the Management of Depression and Anxiety. J. Agric. Food Chem. 2022;70:944–956. doi: 10.1021/acs.jafc.1c06151. [DOI] [PubMed] [Google Scholar]
- 21.Akbaraly T.N., Brunner E.J., Ferrie J.E., Marmot M.G., Kivimaki M., Singh-Manoux A. Dietary Pattern and Depressive Symptoms in Middle Age. Br. J. Psychiatry. 2009;195:408–413. doi: 10.1192/bjp.bp.108.058925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruusunen A., Lehto S.M., Mursu J., Tolmunen T., Tuomainen T.P., Kauhanen J., Voutilainen S. Dietary Patterns Are Associated with the Prevalence of Elevated Depressive Symptoms and the Risk of Getting a Hospital Discharge Diagnosis of Depression in Middle-Aged or Older Finnish Men. J. Affect. Disord. 2014;159:1–6. doi: 10.1016/j.jad.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 23.Nanri A., Kimura Y., Matsushita Y., Ohta M., Sato M., Mishima N., Sasaki S., Mizoue T. Dietary Patterns and Depressive Symptoms among Japanese Men and Women. Eur. J. Clin. Nutr. 2010;64:832–839. doi: 10.1038/ejcn.2010.86. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki T., Miyaki K., Tsutsumi A., Hashimoto H., Kawakami N., Takahashi M., Shimazu A., Inoue A., Kurioka S., Kakehashi M., et al. Japanese Dietary Pattern Consistently Relates to Low Depressive Symptoms and It Is Modified by Job Strain and Worksite Supports. J. Affect. Disord. 2013;150:490–498. doi: 10.1016/j.jad.2013.04.044. [DOI] [PubMed] [Google Scholar]
- 25.Ford P.A., Jaceldo-Siegl K., Lee J.W., Youngberg W., Tonstad S. Intake of Mediterranean Foods Associated with Positive Affect and Low Negative Affect. J. Psychosom. Res. 2013;74:142–148. doi: 10.1016/j.jpsychores.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crichton G.E., Bryan J., Hodgson J.M., Murphy K.J. Mediterranean Diet Adherence and Self-Reported Psychological Functioning in an Australian Sample. Appetite. 2013;70:53–59. doi: 10.1016/j.appet.2013.06.088. [DOI] [PubMed] [Google Scholar]
- 27.Lai J.S., Hiles S., Bisquera A., Hure A.J., McEvoy M., Attia J. A Systematic Review and Meta-Analysis of Dietary Patterns and Depression in Community-Dwelling Adults. Am. J. Clin. Nutr. 2014;99:181–197. doi: 10.3945/ajcn.113.069880. [DOI] [PubMed] [Google Scholar]
- 28.Platte P., Herbert C., Pauli P., Breslin P.A.S. Oral Perceptions of Fat and Taste Stimuli Are Modulated by Affect and Mood Induction. PLoS ONE. 2013;8:e65006. doi: 10.1371/journal.pone.0065006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noel C., Dando R. The Effect of Emotional State on Taste Perception. Appetite. 2015;95:89–95. doi: 10.1016/j.appet.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Heath T.P., Melichar J.K., Nutt D.J., Donaldson L.F. Human Taste Thresholds Are Modulated by Serotonin and Noradrenaline. J. Neurosci. 2006;26:12664–12671. doi: 10.1523/JNEUROSCI.3459-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.le Port A., Gueguen A., Kesse-Guyot E., Melchior M., Lemogne C., Nabi H., Goldberg M., Zins M., Czernichow S. Association between Dietary Patterns and Depressive Symptoms Over Time: A 10-Year Follow-Up Study of the GAZEL Cohort. PLoS ONE. 2012;7:e51593. doi: 10.1371/journal.pone.0051593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molendijk M., Molero P., Ortuño Sánchez-Pedreño F., van der Does W., Angel Martínez-González M. Diet Quality and Depression Risk: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. J. Affect. Disord. 2018;226:346–354. doi: 10.1016/j.jad.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 33.Wang J., Um P., Dickerman B.A., Liu J. Zinc, Magnesium, Selenium and Depression: A Review of the Evidence, Potential Mechanisms and Implications. Nutrients. 2018;10:584. doi: 10.3390/nu10050584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gougeon L., Payette H., Morais J.A., Gaudreau P., Shatenstein B., Gray-Donald K. Intakes of Folate, Vitamin B6 and B12 and Risk of Depression in Community-Dwelling Older Adults: The Quebec Longitudinal Study on Nutrition and Aging. Eur. J. Clin. Nutr. 2016;70:380–385. doi: 10.1038/ejcn.2015.202. [DOI] [PubMed] [Google Scholar]
- 35.Vulser H., Wiernik E., Hoertel N., Thomas F., Pannier B., Czernichow S., Hanon O., Simon T., Simon J.M., Danchin N., et al. Association between Depression and Anemia in Otherwise Healthy Adults. Acta Psychiatr. Scand. 2016;134:150–160. doi: 10.1111/acps.12595. [DOI] [PubMed] [Google Scholar]
- 36.Sender R., Fuchs S., Milo R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016;14:e1002533. doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang B., Chen T., Cao M., Yuan C., Reiter R.J., Zhao Z., Zhao Y., Chen L., Fan W., Wang X., et al. Gut Microbiota Dysbiosis Induced by Decreasing Endogenous Melatonin Mediates the Pathogenesis of Alzheimer’s Disease and Obesity. Front. Immunol. 2022;13:2161. doi: 10.3389/fimmu.2022.900132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rothschild D., Weissbrod O., Barkan E., Kurilshikov A., Korem T., Zeevi D., Costea P.I., Godneva A., Kalka I.N., Bar N., et al. Environment Dominates over Host Genetics in Shaping Human Gut Microbiota. Nature. 2018;555:210–215. doi: 10.1038/nature25973. [DOI] [PubMed] [Google Scholar]
- 39.Naseribafrouei A., Hestad K., Avershina E., Sekelja M., Linløkken A., Wilson R., Rudi K. Correlation between the Human Fecal Microbiota and Depression. Neurogastroenterol. Motil. 2014;26:1155–1162. doi: 10.1111/nmo.12378. [DOI] [PubMed] [Google Scholar]
- 40.Skonieczna-żydecka K., Grochans E., Maciejewska D., Szkup M., Schneider-Matyka D., Jurczak A., Łoniewski I., Kaczmarczyk M., Marlicz W., Czerwińska-Rogowska M., et al. Faecal Short Chain Fatty Acids Profile Is Changed in Polish Depressive Women. Nutrients. 2018;10:1939. doi: 10.3390/nu10121939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carabotti M., Scirocco A., Maselli M.A., Severi C. The Gut-Brain Axis: Interactions between Enteric Microbiota, Central and Enteric Nervous Systems. Ann. Gastroenterol. 2015;28:203–209. [PMC free article] [PubMed] [Google Scholar]
- 42.David L.A., Maurice C.F., Carmody R.N., Gootenberg D.B., Button J.E., Wolfe B.E., Ling A.V., Devlin A.S., Varma Y., Fischbach M.A., et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature. 2013;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mayer E.A., Knight R., Mazmanian S.K., Cryan J.F., Tillisch K. Gut Microbes and the Brain: Paradigm Shift in Neuroscience. J. Neurosci. 2014;34:15490–15496. doi: 10.1523/JNEUROSCI.3299-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goehler L.E., Gaykema R.P.A., Opitz N., Reddaway R., Badr N., Lyte M. Activation in Vagal Afferents and Central Autonomic Pathways: Early Responses to Intestinal Infection with Campylobacter Jejuni. Brain Behav. Immun. 2005;19:334–344. doi: 10.1016/j.bbi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Abildgaard A., Elfving B., Hokland M., Wegener G., Lund S. Probiotic Treatment Reduces Depressive-like Behaviour in Rats Independently of Diet. Psychoneuroendocrinology. 2017;79:40–48. doi: 10.1016/j.psyneuen.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 46.Thangaleela S., Sivamaruthi B.S., Kesika P., Chaiyasut C. Role of Probiotics and Diet in the Management of Neurological Diseases and Mood States: A Review. Microorganisms. 2022;10:2268. doi: 10.3390/microorganisms10112268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mangiola F., Ianiro G., Franceschi F., Fagiuoli S., Gasbarrini G., Gasbarrini A. Gut Microbiota in Autism and Mood Disorders. World J. Gastroenterol. 2016;22:361–368. doi: 10.3748/wjg.v22.i1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang F., Wu X. Brain Neurotransmitter Modulation by Gut Microbiota in Anxiety and Depression. Front. Cell Dev. Biol. 2021;9:472. doi: 10.3389/fcell.2021.649103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Birk J.L., Kronish I.M., Moise N., Falzon L., Yoon S., Davidson K.W. Depression and Multimorbidity: Considering Temporal Characteristics of the Associations between Depression and Multiple Chronic Diseases. Health Psychol. 2019;38:802–811. doi: 10.1037/hea0000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chida Y., Hamer M., Wardle J., Steptoe A. Do Stress-Related Psychosocial Factors Contribute to Cancer Incidence and Survival? Nat. Clin. Pract. Oncol. 2008;5:466–475. doi: 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- 51.Chida Y., Hamer M., Steptoe A. A Bidirectional Relationship between Psychosocial Factors and Atopic Disorders: A Systematic Review and Meta-Analysis. Psychosom. Med. 2008;70:102–116. doi: 10.1097/PSY.0b013e31815c1b71. [DOI] [PubMed] [Google Scholar]
- 52.Bobo W.V., Grossardt B.R., Virani S., St Sauver J.L., Boyd C.M., Rocca W.A. Association of Depression and Anxiety With the Accumulation of Chronic Conditions. JAMA Netw. Open. 2022;5:e229817. doi: 10.1001/jamanetworkopen.2022.9817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nemeth C.L., Reddy R., Bekhbat M., Bailey J., Neigh G.N. Microglial Activation Occurs in the Absence of Anxiety-like Behavior Following Microembolic Stroke in Female, but Not Male, Rats. J. Neuroinflamm. 2014;11:174. doi: 10.1186/s12974-014-0174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chaiyasut C., Sivamaruthi B.S. Influence of Probiotic Supplementation on Brain Function: Involvement of Gut Microbiome, Inflammation, and Stress Pathway. In: Evrensel A., Unsalver B.O., editors. Gut Microbiota–Brain Axis. 1st ed. Volume 1. Intechopen Limited; London, UK: 2018. pp. 19–33. [Google Scholar]
- 55.Kim Y.K., Na K.S., Myint A.M., Leonard B.E. The Role of Pro-Inflammatory Cytokines in Neuroinflammation, Neurogenesis and the Neuroendocrine System in Major Depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2016;64:277–284. doi: 10.1016/j.pnpbp.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 56.Schachter J., Martel J., Lin C.S., Chang C.J., Wu T.R., Lu C.C., Ko Y.F., Lai H.C., Ojcius D.M., Young J.D. Effects of Obesity on Depression: A Role for Inflammation and the Gut Microbiota. Brain Behav. Immun. 2018;69:1–8. doi: 10.1016/j.bbi.2017.08.026. [DOI] [PubMed] [Google Scholar]
- 57.Bear T., Dalziel J., Coad J., Roy N., Butts C., Gopal P. The Microbiome-Gut-Brain Axis and Resilience to Developing Anxiety or Depression under Stress. Microorganisms. 2021;9:723. doi: 10.3390/microorganisms9040723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duivis H.E., Vogelzangs N., Kupper N., de Jonge P., Penninx B.W.J.H. Differential Association of Somatic and Cognitive Symptoms of Depression and Anxiety with Inflammation: Findings from the Netherlands Study of Depression and Anxiety (NESDA) Psychoneuroendocrinology. 2013;38:1573–1585. doi: 10.1016/j.psyneuen.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 59.Peirce J.M., Alviña K. The Role of Inflammation and the Gut Microbiome in Depression and Anxiety. J. Neurosci. Res. 2019;97:1223–1241. doi: 10.1002/jnr.24476. [DOI] [PubMed] [Google Scholar]
- 60.Reichenberg A., Yirmiya R., Schuld A., Kraus T., Haack M., Morag A., Pollmächer T. Cytokine-Associated Emotional and Cognitive Disturbances in Humans. Arch. Gen. Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- 61.Sominsky L., Fuller E.A., Bondarenko E., Ong L.K., Averell L., Nalivaiko E., Dunkley P.R., Dickson P.W., Hodgson D.M. Functional Programming of the Autonomic Nervous System by Early Life Immune Exposure: Implications for Anxiety. PLoS ONE. 2013;8:e57700. doi: 10.1371/journal.pone.0057700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Niraula A., Witcher K.G., Sheridan J.F., Godbout J.P. Interleukin-6 Induced by Social Stress Promotes a Unique Transcriptional Signature in the Monocytes That Facilitate Anxiety. Biol. Psychiatry. 2019;85:679–689. doi: 10.1016/j.biopsych.2018.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alli S.R., Gorbovskaya I., Liu J.C.W., Kolla N.J., Brown L., Müller D.J. The Gut Microbiome in Depression and Potential Benefit of Prebiotics, Probiotics and Synbiotics: A Systematic Review of Clinical Trials and Observational Studies. Int. J. Mol. Sci. 2022;23:4494. doi: 10.3390/ijms23094494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith P.M., Howitt M.R., Panikov N., Michaud M., Gallini C.A., Bohlooly-Y M., Glickman J.N., Garrett W.S. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic T Reg Cell Homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cash H.L., Whitham C.V., Behrendt C.L., Hooper L.V. Symbiotic Bacteria Direct Expression of an Intestinal Bactericidal Lectin. Science. 2006;313:1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shishov V.A., Kirovskaya T.A., Kudrin V.S., Oleskin A.V. Amine Neuromediators, Their Precursors, and Oxidation Products in the Culture of Escherichia Coli k-12. Appl. Biochem. Microbiol. 2009;45:494–497. doi: 10.1134/S0003683809050068. [DOI] [PubMed] [Google Scholar]
- 67.Barrett E., Ross R.P., O’Toole P.W., Fitzgerald G.F., Stanton C. γ-Aminobutyric Acid Production by Culturable Bacteria from the Human Intestine. J. Appl. Microbiol. 2012;113:411–417. doi: 10.1111/j.1365-2672.2012.05344.x. [DOI] [PubMed] [Google Scholar]
- 68.Silva Y.P., Bernardi A., Frozza R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020;11:25. doi: 10.3389/fendo.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wikoff W.R., Anfora A.T., Liu J., Schultz P.G., Lesley S.A., Peters E.C., Siuzdak G. Metabolomics Analysis Reveals Large Effects of Gut Microflora on Mammalian Blood Metabolites. Proc. Natl. Acad. Sci. USA. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yano J.M., Yu K., Donaldson G.P., Shastri G.G., Ann P., Ma L., Nagler C.R., Ismagilov R.F., Mazmanian S.K., Hsiao E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rutsch A., Kantsjö J.B., Ronchi F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front. Immunol. 2020;11:3237. doi: 10.3389/fimmu.2020.604179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Braniste V., Al-Asmakh M., Kowal C., Anuar F., Abbaspour A., Tóth M., Korecka A., Bakocevic N., Guan N.L., Kundu P., et al. The Gut Microbiota Influences Blood-Brain Barrier Permeability in Mice. Sci. Transl. Med. 2014;6:263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Acharya N.K., Levin E.C., Clifford P.M., Han M., Tourtellotte R., Chamberlain D., Pollaro M., Coretti N.J., Kosciuk M.C., Nagele E.P., et al. Diabetes and Hypercholesterolemia Increase Blood-Brain Barrier Permeability and Brain Amyloid Deposition: Beneficial Effects of the LpPLA2 Inhibitor Darapladib. J. Alzheimer’s Dis. 2013;35:179–198. doi: 10.3233/JAD-122254. [DOI] [PubMed] [Google Scholar]
- 74.Lee S.W., Kim W.J., Choi Y.K., Song H.S., Son M.J., Gelman I.H., Kim Y.J., Kim K.W. SSeCKS Regulates Angiogenesis and Tight Junction Formation in Blood-Brain Barrier. Nat. Med. 2003;9:900–906. doi: 10.1038/nm889. [DOI] [PubMed] [Google Scholar]
- 75.Spadoni I., Fornasa G., Rescigno M. Organ-Specific Protection Mediated by Cooperation between Vascular and Epithelial Barriers. Nat. Rev. Immunol. 2017;17:761–773. doi: 10.1038/nri.2017.100. [DOI] [PubMed] [Google Scholar]
- 76.Shah S.N., Knausenberger T.B.-A., Connell E., Le Gall G., Hardy T.A.J., Randall D.W., McCafferty K., Yaqoob M.M., Solito E., Müller M., et al. Cerebrovascular Damage Caused by the Gut Microbe-Derived Uraemic Toxinp-Cresol Sulfate Is Prevented by Blockade of the Epidermal Growth Factor Receptor. bioRxiv. 2022 doi: 10.1101/2022.11.12.516113. [DOI] [Google Scholar]
- 77.Stachulski A.V., Knausenberger T.B.A., Shah S.N., Hoyles L., McArthur S. A Host-Gut Microbial Amino Acid Co-Metabolite, p-Cresol Glucuronide, Promotes Blood-Brain Barrier Integrity in Vivo. Tissue Barriers. 2023;11:2073175. doi: 10.1080/21688370.2022.2073175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Torrisi S.A., Lavanco G., Maurel O.M., Gulisano W., Laudani S., Geraci F., Grasso M., Barbagallo C., Caraci F., Bucolo C., et al. A Novel Arousal-Based Individual Screening Reveals Susceptibility and Resilience to PTSD-like Phenotypes in Mice. Neurobiol. Stress. 2020;14:100286. doi: 10.1016/j.ynstr.2020.100286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Needham B.D., Funabashi M., Adame M.D., Wang Z., Boktor J.C., Haney J., Wu W.L., Rabut C., Ladinsky M.S., Hwang S.J., et al. A Gut-Derived Metabolite Alters Brain Activity and Anxiety Behaviour in Mice. Nature. 2022;602:647–653. doi: 10.1038/s41586-022-04396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hsiao E.Y., McBride S.W., Hsien S., Sharon G., Hyde E.R., McCue T., Codelli J.A., Chow J., Reisman S.E., Petrosino J.F., et al. Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopmental Disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Du Y., Gao X.R., Peng L., Ge J.F. Crosstalk between the Microbiota-Gut-Brain Axis and Depression. Heliyon. 2020;6:e04097. doi: 10.1016/j.heliyon.2020.e04097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Farooq R.K., Asghar K., Kanwal S., Zulqernain A. Role of Inflammatory Cytokines in Depression: Focus on Interleukin-1β (Review) Biomed. Rep. 2017;6:15–20. doi: 10.3892/br.2016.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moulton C.D., Pavlidis P., Norton C., Norton S., Pariante C., Hayee B., Powell N. Depressive Symptoms in Inflammatory Bowel Disease: An Extraintestinal Manifestation of Inflammation? Clin. Exp. Immunol. 2019;197:308–318. doi: 10.1111/cei.13276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kiecolt-Glaser J.K., Derry H.M., Fagundes C.P. Inflammation: Depression Fans the Flames and Feasts on the Heat. Am. J. Psychiatry. 2015;172:1075–1091. doi: 10.1176/appi.ajp.2015.15020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Martin-Subero M., Anderson G., Kanchanatawan B., Berk M., Maes M. Comorbidity between Depression and Inflammatory Bowel Disease Explained by Immune-Inflammatory, Oxidative, and Nitrosative Stress; Tryptophan Catabolite; and Gut–Brain Pathways. CNS Spectr. 2016;21:184–198. doi: 10.1017/S1092852915000449. [DOI] [PubMed] [Google Scholar]
- 86.Yang C., Wardenaar K.J., Bosker F.J., Li J., Schoevers R.A. Inflammatory Markers and Treatment Outcome in Treatment Resistant Depression: A Systematic Review. J. Affect. Disord. 2019;257:640–649. doi: 10.1016/j.jad.2019.07.045. [DOI] [PubMed] [Google Scholar]
- 87.Strawbridge R., Hodsoll J., Powell T.R., Hotopf M., Hatch S.L., Breen G., Cleare A.J. Inflammatory Profiles of Severe Treatment-Resistant Depression. J. Affect. Disord. 2019;246:42–51. doi: 10.1016/j.jad.2018.12.037. [DOI] [PubMed] [Google Scholar]
- 88.Guida F., Turco F., Iannotta M., de Gregorio D., Palumbo I., Sarnelli G., Furiano A., Napolitano F., Boccella S., Luongo L., et al. Antibiotic-Induced Microbiota Perturbation Causes Gut Endocannabinoidome Changes, Hippocampal Neuroglial Reorganization and Depression in Mice. Brain Behav. Immun. 2018;67:230–245. doi: 10.1016/j.bbi.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 89.Kelly J.R., Borre Y., O’ Brien C., Patterson E., el Aidy S., Deane J., Kennedy P.J., Beers S., Scott K., Moloney G., et al. Transferring the Blues: Depression-Associated Gut Microbiota Induces Neurobehavioural Changes in the Rat. J. Psychiatr. Res. 2016;82:109–118. doi: 10.1016/j.jpsychires.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 90.Mason B.L., Li Q., Minhajuddin A., Czysz A.H., Coughlin L.A., Hussain S.K., Koh A.Y., Trivedi M.H. Reduced Anti-Inflammatory Gut Microbiota Are Associated with Depression and Anhedonia. J. Affect. Disord. 2020;266:394–401. doi: 10.1016/j.jad.2020.01.137. [DOI] [PubMed] [Google Scholar]
- 91.Cryan J.F., Dinan T.G. Mind-Altering Microorganisms: The Impact of the Gut Microbiota on Brain and Behaviour. Nat. Rev. Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 92.Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M., Dinan T.G., Bienenstock J., Cryan J.F. Ingestion of Lactobacillus Strain Regulates Emotional Behavior and Central GABA Receptor Expression in a Mouse via the Vagus Nerve. Proc. Natl. Acad. Sci. USA. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jokela M., Virtanen M., David Batty G., Kivimaki M. Inflammation and Specific Symptoms of Depression. JAMA Psychiatry. 2016;73:87–88. doi: 10.1001/jamapsychiatry.2015.1977. [DOI] [PubMed] [Google Scholar]
- 94.Berk M., Williams L.J., Jacka F.N., O’Neil A., Pasco J.A., Moylan S., Allen N.B., Stuart A.L., Hayley A.C., Byrne M.L., et al. So Depression Is an Inflammatory Disease, but Where Does the Inflammation Come From? BMC Med. 2013;11:200. doi: 10.1186/1741-7015-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Abe-Higuchi N., Uchida S., Yamagata H., Higuchi F., Hobara T., Hara K., Kobayashi A., Watanabe Y. Hippocampal Sirtuin 1 Signaling Mediates Depression-like Behavior. Biol. Psychiatry. 2016;80:815–826. doi: 10.1016/j.biopsych.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 96.Higuchi F., Uchida S., Yamagata H., Abe-Higuchi N., Hobara T., Hara K., Kobayashi A., Shintaku T., Itoh Y., Suzuki T., et al. Hippocampal MicroRNA-124 Enhances Chronic Stress Resilience in Mice. J. Neurosci. 2016;36:7253–7267. doi: 10.1523/JNEUROSCI.0319-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Libert S., Pointer K., Bell E.L., Das A., Cohen D.E., Asara J.M., Kapur K., Bergmann S., Preisig M., Otowa T., et al. SIRT1 Activates MAO-A in the Brain to Mediate Anxiety and Exploratory Drive. Cell. 2011;147:1459–1472. doi: 10.1016/j.cell.2011.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Begum N., Mandhare A., Tryphena K.P., Srivastava S., Shaikh M.F., Singh S.B., Khatri D.K. Epigenetics in Depression and Gut-Brain Axis: A Molecular Crosstalk. Front. Aging Neurosci. 2022;14:1433. doi: 10.3389/fnagi.2022.1048333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ghaedrahmati M., Kazemi A., Kheirabadi G., Ebrahimi A., Bahrami M. Postpartum Depression Risk Factors: A Narrative Review. J. Educ. Health Promot. 2017;6:60. doi: 10.4103/jehp.jehp_9_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alshaya D.S. Genetic and Epigenetic Factors Associated with Depression: An Updated Overview. Saudi J. Biol. Sci. 2022;29:103311. doi: 10.1016/j.sjbs.2022.103311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Puyané M., Subirà S., Torres A., Roca A., Garcia-Esteve L., Gelabert E. Personality Traits as a Risk Factor for Postpartum Depression: A Systematic Review and Meta-Analysis. J. Affect. Disord. 2022;298:577–589. doi: 10.1016/j.jad.2021.11.010. [DOI] [PubMed] [Google Scholar]
- 102.Feng Z., Jones K., Wang W.W. An Exploratory Discrete-Time Multilevel Analysis of the Effect of Social Support on the Survival of Elderly People in China. Soc. Sci. Med. 2015;130:181–189. doi: 10.1016/j.socscimed.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mirza I., Jenkins R. Risk Factors, Prevalence, and Treatment of Anxiety and Depressive Disorders in Pakistan: Systematic Review. BMJ. 2004;328:794. doi: 10.1136/bmj.328.7443.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Weyerer S., Eifflaender-Gorfer S., Köhler L., Jessen F., Maier W., Fuchs A., Pentzek M., Kaduszkiewicz H., Bachmann C., Angermeyer M.C., et al. Prevalence and Risk Factors for Depression in Non-Demented Primary Care Attenders Aged 75 Years and Older. J. Affect. Disord. 2008;111:153–163. doi: 10.1016/j.jad.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 105.Chang-Quan H., Zheng-Rong W., Yong-Hong L., Yi-Zhou X., Qing-Xiu L. Education and Risk for Late Life Depression: A Meta-Analysis of Published Literature. Int. J. Psychiatry Med. 2010;40:109–124. doi: 10.2190/PM.40.1.i. [DOI] [PubMed] [Google Scholar]
- 106.Alsaadi T., Kassie S., el Hammasi K., Shahrour T.M., Shakra M., Turkawi L., Nasreddine W., Raoof M. Potential Factors Impacting Health-Related Quality of Life among Patients with Epilepsy: Results from the United Arab Emirates. Seizure. 2017;53:13–17. doi: 10.1016/j.seizure.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 107.Rotenstein L.S., Ramos M.A., Torre M., Bradley Segal J., Peluso M.J., Guille C., Sen S., Mata D.A. Prevalence of Depression, Depressive Symptoms, and Suicidal Ideation Among Medical Students: A Systematic Review and Meta-Analysis. JAMA. 2016;316:2214–2236. doi: 10.1001/jama.2016.17324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Elkins C., Kyle P., Germain L., Elkins C. Burnout and Depression in MS1 and MS3 Years. Fam. Med. 2017;49:456–459. [PubMed] [Google Scholar]
- 109.Wang Y.H., Shi Z.T., Luo Q.Y. Association of Depressive Symptoms and Suicidal Ideation among University Students in China: A Systematic Review and Meta-Analysis. Medicine. 2017;96:e6476. doi: 10.1097/MD.0000000000006476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sun X.J., Niu G.F., You Z.Q., Zhou Z.K., Tang Y. Gender, Negative Life Events and Coping on Different Stages of Depression Severity: A Cross-Sectional Study among Chinese University Students. J. Affect. Disord. 2017;209:177–181. doi: 10.1016/j.jad.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 111.Waqas A., Rehman A., Malik A., Muhammad U., Khan S., Mahmood N., Waqas A., Rehman A., Malik A., Muhammad U., et al. Association of Ego Defense Mechanisms with Academic Performance, Anxiety and Depression in Medical Students: A Mixed Methods Study. Cureus. 2015;7:e337. doi: 10.7759/cureus.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Romo-Nava F., Tafoya S.A., Gutiérrez-Soriano J., Osorio Y., Carriedo P., Ocampo B., Bobadilla R.I., Heinze G. The Association between Chronotype and Perceived Academic Stress to Depression in Medical Students. Chronobiol. Int. 2016;33:1359–1368. doi: 10.1080/07420528.2016.1217230. [DOI] [PubMed] [Google Scholar]
- 113.Piumatti G. Motivation, Health-Related Lifestyles and Depression among University Students: A Longitudinal Analysis. Psychiatry Res. 2018;260:412–417. doi: 10.1016/j.psychres.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 114.Iqbal S., Gupta S., Venkatarao E. Stress, Anxiety & Depression among Medical Undergraduate Students & Their Socio-Demographic Correlates. Indian J. Med. Res. 2015;141:354. doi: 10.4103/0971-5916.156571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hebert J.C., Radford-Smith D.E., Probert F., Ilott N., Chan K.W., Anthony D.C., Burnet P.W.J. Mom’s Diet Matters: Maternal Prebiotic Intake in Mice Reduces Anxiety and Alters Brain Gene Expression and the Fecal Microbiome in Offspring. Brain Behav. Immun. 2021;91:230–244. doi: 10.1016/j.bbi.2020.09.034. [DOI] [PubMed] [Google Scholar]
- 116.Sovijit W.N., Sovijit W.E., Pu S., Usuda K., Inoue R., Watanabe G., Yamaguchi H., Nagaoka K. Ovarian Progesterone Suppresses Depression and Anxiety-like Behaviors by Increasing the Lactobacillus Population of Gut Microbiota in Ovariectomized Mice. Neurosci. Res. 2021;168:76–82. doi: 10.1016/j.neures.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 117.Yang Q., Tian L., Li S., Huo J., Jiang Y., Chen L., Wang W. Semen Sojae Praeparatum Improves Anxiety in Mice by Inhibiting HPA Axis Hyperactivity and Modulating Gut Microbiota. J. Funct. Foods. 2022;98:105282. doi: 10.1016/j.jff.2022.105282. [DOI] [Google Scholar]
- 118.Wang S., Ishima T., Qu Y., Shan J., Chang L., Wei Y., Zhang J., Pu Y., Fujita Y., Tan Y., et al. Ingestion of Faecalibaculum Rodentium Causes Depression-like Phenotypes in Resilient Ephx2 Knock-out Mice: A Role of Brain–Gut–Microbiota Axis via the Subdiaphragmatic Vagus Nerve. J. Affect. Disord. 2021;292:565–573. doi: 10.1016/j.jad.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li H., Xiang Y., Zhu Z., Wang W., Jiang Z., Zhao M., Cheng S., Pan F., Liu D., Ho R.C.M., et al. Rifaximin-Mediated Gut Microbiota Regulation Modulates the Function of Microglia and Protects against CUMS-Induced Depression-like Behaviors in Adolescent Rat. J. Neuroinflamm. 2021;18:254. doi: 10.1186/s12974-021-02303-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Glover M.E., Cohen J.L., Singer J.R., Sabbagh M.N., Rainville J.R., Hyland M.T., Morrow C.D., Weaver C.T., Hodes G.E., Kerman I.A., et al. Examining the Role of Microbiota in Emotional Behavior: Antibiotic Treatment Exacerbates Anxiety in High Anxiety-Prone Male Rats. Neuroscience. 2021;459:179–197. doi: 10.1016/j.neuroscience.2021.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hao W., Wu J., Yuan N., Gong L., Huang J., Ma Q., Zhu H., Gan H., Da X., Deng L., et al. Xiaoyaosan Improves Antibiotic-Induced Depressive-Like and Anxiety-Like Behavior in Mice Through Modulating the Gut Microbiota and Regulating the NLRP3 Inflammasome in the Colon. Front. Pharmacol. 2021;12:773. doi: 10.3389/fphar.2021.619103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hashimoto K. Rapid-Acting Antidepressant Ketamine, Its Metabolites and Other Candidates: A Historical Overview and Future Perspective. Psychiatry Clin. Neurosci. 2019;73:613–627. doi: 10.1111/pcn.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hashimoto K. Molecular Mechanisms of the Rapid-Acting and Long-Lasting Antidepressant Actions of (R)-Ketamine. Biochem. Pharmacol. 2020;177:113935. doi: 10.1016/j.bcp.2020.113935. [DOI] [PubMed] [Google Scholar]
- 124.Zhang K., Sakamoto A., Chang L., Qu Y., Wang S., Pu Y., Tan Y., Wang X., Fujita Y., Ishima T., et al. Splenic NKG2D Confers Resilience versus Susceptibility in Mice after Chronic Social Defeat Stress: Beneficial Effects of (R)-Ketamine. Eur. Arch. Psychiatry Clin. Neurosci. 2021;271:447–456. doi: 10.1007/s00406-019-01092-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang K., Fujita Y., Chang L., Qu Y., Pu Y., Wang S., Shirayama Y., Hashimoto K. Abnormal Composition of Gut Microbiota Is Associated with Resilience versus Susceptibility to Inescapable Electric Stress. Transl. Psychiatry. 2019;9:231. doi: 10.1038/s41398-019-0571-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang S., Qu Y., Chang L., Pu Y., Zhang K., Hashimoto K. Antibiotic-Induced Microbiome Depletion Is Associated with Resilience in Mice after Chronic Social Defeat Stress. J. Affect. Disord. 2020;260:448–457. doi: 10.1016/j.jad.2019.09.064. [DOI] [PubMed] [Google Scholar]
- 127.Wei Y., Chang L., Hashimoto K. Molecular Mechanisms Underlying the Antidepressant Actions of Arketamine: Beyond the NMDA Receptor. Mol. Psychiatry. 2021;27:559–573. doi: 10.1038/s41380-021-01121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chevalier G., Siopi E., Guenin-Macé L., Pascal M., Laval T., Rifflet A., Boneca I.G., Demangel C., Colsch B., Pruvost A., et al. Effect of Gut Microbiota on Depressive-like Behaviors in Mice Is Mediated by the Endocannabinoid System. Nat. Commun. 2020;11:6363. doi: 10.1038/s41467-020-19931-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Siopi E., Chevalier G., Katsimpardi L., Saha S., Bigot M., Moigneu C., Eberl G., Lledo P.M. Changes in Gut Microbiota by Chronic Stress Impair the Efficacy of Fluoxetine. Cell Rep. 2020;30:3682–3690.e6. doi: 10.1016/j.celrep.2020.02.099. [DOI] [PubMed] [Google Scholar]
- 130.Jianguo L., Xueyang J., Cui W., Changxin W., Xuemei Q. Altered Gut Metabolome Contributes to Depression-like Behaviors in Rats Exposed to Chronic Unpredictable Mild Stress. Transl. Psychiatry. 2019;9:40. doi: 10.1038/s41398-019-0391-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wu M., Tian T., Mao Q., Zou T., Zhou C.J., Xie J., Chen J.J. Associations between Disordered Gut Microbiota and Changes of Neurotransmitters and Short-Chain Fatty Acids in Depressed Mice. Transl. Psychiatry. 2020;10:350. doi: 10.1038/s41398-020-01038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu Y., Forsythe P. Vagotomy and Insights into the Microbiota-Gut-Brain Axis. Neurosci. Res. 2021;168:20–27. doi: 10.1016/j.neures.2021.04.001. [DOI] [PubMed] [Google Scholar]
- 133.Breit S., Kupferberg A., Rogler G., Hasler G. Vagus Nerve as Modulator of the Brain-Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry. 2018;9:44. doi: 10.3389/fpsyt.2018.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang J., Ma L., Chang L., Pu Y., Qu Y., Hashimoto K. A Key Role of the Subdiaphragmatic Vagus Nerve in the Depression-like Phenotype and Abnormal Composition of Gut Microbiota in Mice after Lipopolysaccharide Administration. Transl. Psychiatry. 2020;10:186. doi: 10.1038/s41398-020-00878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bharwani A., West C., Champagne-Jorgensen K., McVey Neufeld K.A., Ruberto J., Kunze W.A., Bienenstock J., Forsythe P. The Vagus Nerve Is Necessary for the Rapid and Widespread Neuronal Activation in the Brain Following Oral Administration of Psychoactive Bacteria. Neuropharmacology. 2020;170:108067. doi: 10.1016/j.neuropharm.2020.108067. [DOI] [PubMed] [Google Scholar]
- 136.Guo B., Zhang M., Hao W., Wang Y., Zhang T., Liu C. Neuroinflammation Mechanisms of Neuromodulation Therapies for Anxiety and Depression. Transl. Psychiatry. 2023;13:5. doi: 10.1038/s41398-022-02297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Han Y., Wang B., Gao H., He C., Hua R., Liang C., Zhang S., Wang Y., Xin S., Xu J. Vagus Nerve and Underlying Impact on the Gut Microbiota-Brain Axis in Behavior and Neurodegenerative Diseases. J. Inflamm. Res. 2022;15:6213–6230. doi: 10.2147/JIR.S384949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Borovikova L.V., Ivanova S., Zhang M., Yang H., Botchkina G.I., Watkins L.R., Wang H., Abumrad N., Eaton J.W., Tracey K.J. Vagus Nerve Stimulation Attenuates the Systemic Inflammatory Response to Endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 139.Bonaz B., Sinniger V., Pellissier S. Anti-inflammatory properties of the vagus nerve: Potential therapeutic implications of vagus nerve stimulation. J. Physiol. 2016;594:5781–5790. doi: 10.1113/JP271539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fülling C., Dinan T.G., Cryan J.F. Gut Microbe to Brain Signaling: What Happens in Vagus…. Neuron. 2019;101:998–1002. doi: 10.1016/j.neuron.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 141.Knudsen J.K., Michaelsen T.Y., Bundgaard-Nielsen C., Nielsen R.E., Hjerrild S., Leutscher P., Wegener G., Sørensen S. Faecal Microbiota Transplantation from Patients with Depression or Healthy Individuals into Rats Modulates Mood-Related Behaviour. Sci. Rep. 2021;11:21869. doi: 10.1038/s41598-021-01248-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang S., Ishima T., Zhang J., Qu Y., Chang L., Pu Y., Fujita Y., Tan Y., Wang X., Hashimoto K. Ingestion of Lactobacillus intestinalis and Lactobacillus reuteri Causes Depression- and Anhedonia-like Phenotypes in Antibiotic-Treated Mice via the Vagus Nerve. J. Neuroinflamm. 2020;17:241. doi: 10.1186/s12974-020-01916-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.van de Wouw M., Boehme M., Lyte J.M., Wiley N., Strain C., O’Sullivan O., Clarke G., Stanton C., Dinan T.G., Cryan J.F. Short-Chain Fatty Acids: Microbial Metabolites That Alleviate Stress-Induced Brain–Gut Axis Alterations. J. Physiol. 2018;596:4923–4944. doi: 10.1113/JP276431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu Y., Wang H., Gui S., Zeng B., Pu J., Zheng P., Zeng L., Luo Y., Wu Y., Zhou C., et al. Proteomics Analysis of the Gut–Brain Axis in a Gut Microbiota-Dysbiosis Model of Depression. Transl. Psychiatry. 2021;11:568. doi: 10.1038/s41398-021-01689-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tillmann S., Abildgaard A., Winther G., Wegener G. Altered Fecal Microbiota Composition in the Flinders Sensitive Line Rat Model of Depression. Psychopharmacology. 2019;236:1445–1457. doi: 10.1007/s00213-018-5094-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yang J., Zheng P., Li Y., Wu J., Tan X., Zhou J., Sun Z., Chen X., Zhang G., Zhang H., et al. Landscapes of Bacterial and Metabolic Signatures and Their Interaction in Major Depressive Disorders. Sci. Adv. 2020;6:eaba8555. doi: 10.1126/sciadv.aba8555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zheng P., Yang J., Li Y., Wu J., Liang W., Yin B., Tan X., Huang Y., Chai T., Zhang H., et al. Gut Microbial Signatures Can Discriminate Unipolar from Bipolar Depression. Adv. Sci. 2020;7:1902862. doi: 10.1002/advs.201902862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Valles-Colomer M., Falony G., Darzi Y., Tigchelaar E.F., Wang J., Tito R.Y., Schiweck C., Kurilshikov A., Joossens M., Wijmenga C., et al. The Neuroactive Potential of the Human Gut Microbiota in Quality of Life and Depression. Nat. Microbiol. 2019;4:623–632. doi: 10.1038/s41564-018-0337-x. [DOI] [PubMed] [Google Scholar]
- 149.Hu S., Li A., Huang T., Lai J., Li J., Sublette M.E., Lu H., Lu Q., Du Y., Hu Z., et al. Gut Microbiota Changes in Patients with Bipolar Depression. Adv. Sci. 2019;6:1900752. doi: 10.1002/advs.201900752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chen J.J., He S., Fang L., Wang B., Bai S.J., Xie J., Zhou C.J., Wang W., Xie P. Age-Specific Differential Changes on Gut Microbiota Composition in Patients with Major Depressive Disorder. Aging. 2020;12:2764–2776. doi: 10.18632/aging.102775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhu J., Li M., Shao D., Ma S., Wei W. Altered Fecal Microbiota Signatures in Patients With Anxiety and Depression in the Gastrointestinal Cancer Screening: A Case-Control Study. Front. Psychiatry. 2021;12:1832. doi: 10.3389/fpsyt.2021.757139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jiang H.Y., Zhang X., Yu Z.H., Zhang Z., Deng M., Zhao J.H., Ruan B. Altered Gut Microbiota Profile in Patients with Generalized Anxiety Disorder. J. Psychiatr. Res. 2018;104:130–136. doi: 10.1016/j.jpsychires.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 153.Ma W., Song J., Wang H., Shi F., Zhou N., Jiang J., Xu Y., Zhang L., Yang L., Zhou M. Chronic Paradoxical Sleep Deprivation-Induced Depressionlike Behavior, Energy Metabolism and Microbial Changes in Rats. Life Sci. 2019;225:88–97. doi: 10.1016/j.lfs.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 154.Chen Z., Li J., Gui S., Zhou C., Chen J., Yang C., Hu Z., Wang H., Zhong X., Zeng L., et al. Comparative Metaproteomics Analysis Shows Altered Fecal Microbiota Signatures in Patients with Major Depressive Disorder. Neuroreport. 2018;29:417–425. doi: 10.1097/WNR.0000000000000985. [DOI] [PubMed] [Google Scholar]
- 155.Tsai C.F., Chuang C.H., Wang Y.P., Lin Y.B., Tu P.C., Liu P.Y., Wu P.S., Lin C.Y., Lu C.L. Differences in Gut Microbiota Correlate with Symptoms and Regional Brain Volumes in Patients with Late-Life Depression. Front. Aging Neurosci. 2022;14:838. doi: 10.3389/fnagi.2022.885393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chung Y.C.E., Chen H.C., Chou H.C.L., Chen I.M., Lee M.S., Chuang L.C., Liu Y.W., Lu M.L., Chen C.H., Wu C.H., et al. Exploration of Microbiota Targets for Major Depressive Disorder and Mood Related Traits. J. Psychiatr. Res. 2019;111:74–82. doi: 10.1016/j.jpsychires.2019.01.016. [DOI] [PubMed] [Google Scholar]
- 157.Chen H.M., Chung Y.C.E., Chen H.C., Liu Y.W., Chen I.M., Lu M.L., Hsiao F.S.H., Chen C.H., Huang M.C., Shih W.L., et al. Exploration of the Relationship between Gut Microbiota and Fecal MicroRNAs in Patients with Major Depressive Disorder. Sci. Rep. 2022;12:20977. doi: 10.1038/s41598-022-24773-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen D.L., Dai Y.C., Zheng L., Chen Y.L., Zhang Y.L., Tang Z.P. Features of the Gut Microbiota in Ulcerative Colitis Patients with Depression: A Pilot Study. Medicine. 2021;100:e24845. doi: 10.1097/MD.0000000000024845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhou Y., Chen C., Yu H., Yang Z. Fecal Microbiota Changes in Patients With Postpartum Depressive Disorder. Front. Cell. Infect. Microbiol. 2020;10:511. doi: 10.3389/fcimb.2020.567268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yao H., Yang H., Wang Y., Xing Q., Yan L., Chai Y. Gut Microbiome and Fecal Metabolic Alteration in Systemic Lupus Erythematosus Patients with Depression. Front. Cell. Infect. Microbiol. 2022;12:1766. doi: 10.3389/fcimb.2022.1040211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Liu P., Gao M., Liu Z., Zhang Y., Tu H., Lei L., Wu P., Zhang A., Yang C., Li G., et al. Gut Microbiome Composition Linked to Inflammatory Factors and Cognitive Functions in First-Episode, Drug-Naive Major Depressive Disorder Patients. Front. Neurosci. 2022;15:1918. doi: 10.3389/fnins.2021.800764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ye X., Wang D., Zhu H., Wang D., Li J., Tang Y., Wu J. Gut Microbiota Changes in Patients With Major Depressive Disorder Treated With Vortioxetine. Front. Psychiatry. 2021;12:664. doi: 10.3389/fpsyt.2021.641491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Caso J.R., MacDowell K.S., González-Pinto A., García S., de Diego-Adeliño J., Carceller-Sindreu M., Sarramea F., Caballero-Villarraso J., Gracia-García P., de la Cámara C., et al. Gut Microbiota, Innate Immune Pathways, and Inflammatory Control Mechanisms in Patients with Major Depressive Disorder. Transl. Psychiatry. 2021;11:645. doi: 10.1038/s41398-021-01755-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Huang Y., Shi X., Li Z., Shen Y., Shi X., Wang L., Li G., Yuan Y., Wang J., Zhang Y., et al. Possible Association of Firmicutes in the Gut Microbiota of Patients with Major Depressive Disorder. Neuropsychiatr. Dis. Treat. 2018;14:3329–3337. doi: 10.2147/NDT.S188340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chen J.J., Zheng P., Liu Y.Y., Zhong X.G., Wang H.Y., Guo Y.J., Xie P. Sex Differences in Gut Microbiota in Patients with Major Depressive Disorder. Neuropsychiatr. Dis. Treat. 2018;14:647–655. doi: 10.2147/NDT.S159322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lai W.T., Deng W.F., Xu S.X., Zhao J., Xu D., Liu Y.H., Guo Y.Y., Wang M.B., He F.S., Ye S.W., et al. Shotgun Metagenomics Reveals Both Taxonomic and Tryptophan Pathway Differences of Gut Microbiota in Major Depressive Disorder Patients. Psychol. Med. 2021;51:90–101. doi: 10.1017/S0033291719003027. [DOI] [PubMed] [Google Scholar]
- 167.Rong H., Xie X.H., Zhao J., Lai W.T., Wang M.B., Xu D., Liu Y.H., Guo Y.Y., Xu S.X., Deng W.F., et al. Similarly in Depression, Nuances of Gut Microbiota: Evidences from a Shotgun Metagenomics Sequencing Study on Major Depressive Disorder versus Bipolar Disorder with Current Major Depressive Episode Patients. J. Psychiatr. Res. 2019;113:90–99. doi: 10.1016/j.jpsychires.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 168.Kiecolt-Glaser J.K., Wilson S.J., Shrout M.R., Madison A.A., Andridge R., Peng J., Malarkey W.B., Bailey M.T. The Gut Reaction to Couples’ Relationship Troubles: A Route to Gut Dysbiosis through Changes in Depressive Symptoms. Psychoneuroendocrinology. 2021;125:105132. doi: 10.1016/j.psyneuen.2021.105132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Liu Y., Yu X., Yu L., Tian F., Zhao J., Zhang H., Qian L., Wang Q., Xue Z., Zhai Q., et al. Lactobacillus Plantarum CCFM8610 Alleviates Irritable Bowel Syndrome and Prevents Gut Microbiota Dysbiosis: A Randomized, Double-Blind, Placebo-Controlled, Pilot Clinical Trial. Engineering. 2021;7:376–385. doi: 10.1016/j.eng.2020.06.026. [DOI] [Google Scholar]
- 170.Capuco A., Urits I., Hasoon J., Chun R., Gerald B., Wang J.K., Kassem H., Ngo A.L., Abd-Elsayed A., Simopoulos T., et al. Current Perspectives on Gut Microbiome Dysbiosis and Depression. Adv. Ther. 2020;37:1328–1346. doi: 10.1007/s12325-020-01272-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lin H., Guo Q., Wen Z., Tan S., Chen J., Lin L., Chen P., He J., Wen J., Chen Y. The Multiple Effects of Fecal Microbiota Transplantation on Diarrhea-Predominant Irritable Bowel Syndrome (IBS-D) Patients with Anxiety and Depression Behaviors. Microb. Cell Fact. 2021;20:233. doi: 10.1186/s12934-021-01720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Qin X., Pan C., Cai Q., Zhao Y., He D., Wei W., Zhang N., Shi S., Chu X., Zhang F. Assessing the Effect of Interaction between Gut Microbiome and Inflammatory Bowel Disease on the Risks of Depression. Brain Behav. Immun. Health. 2022;26:100557. doi: 10.1016/j.bbih.2022.100557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Miranda P.M., Bertolini F., Kadarmideen H.N. Investigation of Gut Microbiome Association with Inflammatory Bowel Disease and Depression: A Machine Learning Approach. F1000Research. 2019;7:702. doi: 10.12688/f1000research.15091.2. [DOI] [Google Scholar]
- 174.Simpson C.A., Mu A., Haslam N., Schwartz O.S., Simmons J.G. Feeling down? A Systematic Review of the Gut Microbiota in Anxiety/Depression and Irritable Bowel Syndrome. J. Affect. Disord. 2020;266:429–446. doi: 10.1016/j.jad.2020.01.124. [DOI] [PubMed] [Google Scholar]
- 175.Radjabzadeh D., Bosch J.A., Uitterlinden A.G., Zwinderman A.H., Ikram M.A., van Meurs J.B.J., Luik A.I., Nieuwdorp M., Lok A., van Duijn C.M., et al. Gut Microbiome-Wide Association Study of Depressive Symptoms. Nat. Commun. 2022;13:7128. doi: 10.1038/s41467-022-34502-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liu R.T., Rowan-Nash A.D., Sheehan A.E., Walsh R.F.L., Sanzari C.M., Korry B.J., Belenky P. Reductions in Anti-Inflammatory Gut Bacteria Are Associated with Depression in a Sample of Young Adults. Brain Behav. Immun. 2020;88:308–324. doi: 10.1016/j.bbi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Qin Y., Havulinna A.S., Liu Y., Jousilahti P., Ritchie S.C., Tokolyi A., Sanders J.G., Valsta L., Brożyńska M., Zhu Q., et al. Combined Effects of Host Genetics and Diet on Human Gut Microbiota and Incident Disease in a Single Population Cohort. Nat. Genet. 2022;54:134–142. doi: 10.1038/s41588-021-00991-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cahana I., Iraqi F.A. Impact of Host Genetics on Gut Microbiome: Take-home Lessons from Human and Mouse Studies. Anim. Model Exp. Med. 2020;3:229. doi: 10.1002/ame2.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yatsunenko T., Rey F.E., Manary M.J., Trehan I., Dominguez-Bello M.G., Contreras M., Magris M., Hidalgo G., Baldassano R.N., Anokhin A.P., et al. Human Gut Microbiome Viewed across Age and Geography. Nature. 2012;486:222. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fleshner M., Frank M., Maier S.F. Danger Signals and Inflammasomes: Stress-Evoked Sterile Inflammation in Mood Disorders. Neuropsychopharmacology. 2016;42:36–4525. doi: 10.1038/npp.2016.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Haghighat N., Rajabi S., Mohammadshahi M. Effect of Synbiotic and Probiotic Supplementation on Serum Brain-Derived Neurotrophic Factor Level, Depression and Anxiety Symptoms in Hemodialysis Patients: A Randomized, Double-Blinded, Clinical Trial. Nutr. Neurosci. 2019;24:490–499. doi: 10.1080/1028415X.2019.1646975. [DOI] [PubMed] [Google Scholar]
- 182.Majeed M., Nagabhushanam K., Arumugam S., Majeed S., Ali F. Bacillus coagulans MTCC 5856 for the Management of Major Depression with Irritable Bowel Syndrome: A Randomised, Double blind, Placebo Controlled, Multi-Centre, Pilot Clinical Study. Food Nutr. Res. 2018;62:1218. doi: 10.29219/fnr.v62.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pinto-Sanchez M.I., Hall G.B., Ghajar K., Nardelli A., Bolino C., Lau J.T., Martin F.P., Cominetti O., Welsh C., Rieder A., et al. Probiotic Bifidobacterium Longum NCC3001 Reduces Depression Scores and Alters Brain Activity: A Pilot Study in Patients With Irritable Bowel Syndrome. Gastroenterology. 2017;153:448–459.e8. doi: 10.1053/j.gastro.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 184.Miyaoka T., Kanayama M., Wake R., Hashioka S., Hayashida M., Nagahama M., Okazaki S., Yamashita S., Miura S., Miki H., et al. Clostridium Butyricum MIYAIRI 588 as Adjunctive Therapy for Treatment-Resistant Major Depressive Disorder: A Prospective Open-Label Trial. Clin. Neuropharmacol. 2018;41:151–155. doi: 10.1097/WNF.0000000000000299. [DOI] [PubMed] [Google Scholar]
- 185.Akkasheh G., Kashani-Poor Z., Tajabadi-Ebrahimi M., Jafari P., Akbari H., Taghizadeh M., Memarzadeh M.R., Asemi Z., Esmaillzadeh A. Clinical and Metabolic Response to Probiotic Administration in Patients with Major Depressive Disorder: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrition. 2016;32:315–320. doi: 10.1016/j.nut.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 186.Messaoudi M., Violle N., Bisson J.F., Desor D., Javelot H., Rougeot C. Beneficial Psychological Effects of a Probiotic Formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in Healthy Human Volunteers. Gut Microbes. 2011;2:256–261. doi: 10.4161/gmic.2.4.16108. [DOI] [PubMed] [Google Scholar]
- 187.Romijn A.R., Rucklidge J.J., Kuijer R.G., Frampton C. A Double-Blind, Randomized, Placebo-Controlled Trial of Lactobacillus helveticus and Bifidobacterium longum for the Symptoms of Depression. Aust. N. Z. J. Psychiatry. 2017;51:810–821. doi: 10.1177/0004867416686694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kazemi A., Noorbala A.A., Azam K., Eskandari M.H., Djafarian K. Effect of Probiotic and Prebiotic vs Placebo on Psychological Outcomes in Patients with Major Depressive Disorder: A Randomized Clinical Trial. Clin. Nutr. 2019;38:522–528. doi: 10.1016/j.clnu.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 189.Chong H.X., Yusoff N.A.A., Hor Y.Y., Lew L.C., Jaafar M.H., Choi S.B., Yusoff M.S.B., Wahid N., Abdullah M.F.I.L., Zakaria N., et al. Lactobacillus plantarum DR7 Alleviates Stress and Anxiety in Adults: A Randomised, Double-Blind, Placebo-Controlled Study. Benef. Microbes. 2019;10:355–373. doi: 10.3920/BM2018.0135. [DOI] [PubMed] [Google Scholar]
- 190.Qin Q., Liu H., Yang Y., Wang Y., Xia C., Tian P., Wei J., Li S., Chen T. Probiotic Supplement Preparation Relieves Test Anxiety by Regulating Intestinal Microbiota in College Students. Dis. Markers. 2021;2021:e5597401. doi: 10.1155/2021/5597401. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 191.Ghorbani Z., Nazari S., Etesam F., Nourimajd S., Ahmadpanah M., Jahromi S.R. The Effect of Synbiotic as an Adjuvant Therapy to Fluoxetine in Moderate Depression: A Randomized Multicenter Trial. Arch. Neurosci. 2018;5:60507. doi: 10.5812/archneurosci.60507. [DOI] [Google Scholar]
- 192.Azpiroz F., Dubray C., Bernalier-Donadille A., Cardot J.M., Accarino A., Serra J., Wagner A., Respondek F., Dapoigny M. Effects of ScFOS on the Composition of Fecal Microbiota and Anxiety in Patients with Irritable Bowel Syndrome: A Randomized, Double Blind, Placebo Controlled Study. Neurogastroenterol. Motil. 2017;29:e12911. doi: 10.1111/nmo.12911. [DOI] [PubMed] [Google Scholar]
- 193.Vulevic J., Juric A., Walton G.E., Claus S.P., Tzortzis G., Toward R.E., Gibson G.R. Influence of Galacto-Oligosaccharide Mixture (B-GOS) on Gut Microbiota, Immune Parameters and Metabonomics in Elderly Persons. Br. J. Nutr. 2015;114:586–595. doi: 10.1017/S0007114515001889. [DOI] [PubMed] [Google Scholar]
- 194.Rao J., Qiao Y., Xie R., Lin L., Jiang J., Wang C., Li G. Fecal Microbiota Transplantation Ameliorates Stress-Induced Depression-like Behaviors Associated with the Inhibition of Glial and NLRP3 Inflammasome in Rat Brain. J. Psychiatr. Res. 2021;137:147–157. doi: 10.1016/j.jpsychires.2021.02.057. [DOI] [PubMed] [Google Scholar]
- 195.Tarr A.J., Galley J.D., Fisher S.E., Chichlowski M., Berg B.M., Bailey M.T. The Prebiotics 3′Sialyllactose and 6′Sialyllactose Diminish Stressor-Induced Anxiety-like Behavior and Colonic Microbiota Alterations: Evidence for Effects on the Gut–Brain Axis. Brain Behav. Immun. 2015;50:166–177. doi: 10.1016/j.bbi.2015.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yan T., Nian T., Liao Z., Xiao F., Wu B., Bi K., He B., Jia Y. Antidepressant Effects of a Polysaccharide from Okra (Abelmoschus esculentus (L) Moench) by Anti-Inflammation and Rebalancing the Gut Microbiota. Int. J. Biol. Macromol. 2020;144:427–440. doi: 10.1016/j.ijbiomac.2019.12.138. [DOI] [PubMed] [Google Scholar]
- 197.Jacka F.N. Nutritional Psychiatry: Where to Next? EBioMedicine. 2017;17:24–29. doi: 10.1016/j.ebiom.2017.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Tardy A.L., Pouteau E., Marquez D., Yilmaz C., Scholey A. Vitamins and Minerals for Energy, Fatigue and Cognition: A Narrative Review of the Biochemical and Clinical Evidence. Nutrients. 2020;12:228. doi: 10.3390/nu12010228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sánchez-Villegas A., Delgado-Rodríguez M., Alonso A., Schlatter J., Lahortiga F., Serra-Majem L., Martínez-González M.A. Association of the Mediterranean Dietary Pattern With the Incidence of Depression: The Seguimiento Universidad de Navarra/University of Navarra Follow-up (SUN) Cohort. Arch. Gen. Psychiatry. 2009;66:1090–1098. doi: 10.1001/archgenpsychiatry.2009.129. [DOI] [PubMed] [Google Scholar]
- 200.Féart C., Samieri C., Rondeau V., Amieva H., Portet F., Dartigues J.F., Scarmeas N., Barberger-Gateau P. Adherence to a Mediterranean Diet, Cognitive Decline, and Risk of Dementia. JAMA. 2009;302:638–648. doi: 10.1001/jama.2009.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ν Georgousopoulou E., Kastorini C.-M., Milionis H.J., Ntziou E., Kostapanos M., Nikolaou V., Vemmos K., Goudevenos J., Panagiotakos D.B. Association Between Mediterranean Diet and Non-Fatal Cardiovascular Events, in the Context of Anxiety and Depression Disorders: A Case/Case-Control Study. Hell. J. Cardiol. 2014;55:24–31. [PubMed] [Google Scholar]
- 202.Sánchez-Villegas A., Henríquez P., Bes-Rastrollo M., Doreste J. Mediterranean Diet and Depression. Public Health Nutr. 2006;9:1104–1109. doi: 10.1017/S1368980007668578. [DOI] [PubMed] [Google Scholar]
- 203.Merra G., Noce A., Marrone G., Cintoni M., Tarsitano M.G., Capacci A., de Lorenzo A. Influence of Mediterranean Diet on Human Gut Microbiota. Nutrients. 2020;13:7. doi: 10.3390/nu13010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Konishi K. Associations between Healthy Japanese Dietary Patterns and Depression in Japanese Women. Public Health Nutr. 2021;24:1753–1765. doi: 10.1017/S1368980020001548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lin H.V., Frassetto A., Kowalik E.J., Nawrocki A.R., Lu M.M., Kosinski J.R., Hubert J.A., Szeto D., Yao X., Forrest G., et al. Butyrate and Propionate Protect against Diet-Induced Obesity and Regulate Gut Hormones via Free Fatty Acid Receptor 3-Independent Mechanisms. PLoS ONE. 2012;7:e35240. doi: 10.1371/journal.pone.0035240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Burokas A., Arboleya S., Moloney R.D., Peterson V.L., Murphy K., Clarke G., Stanton C., Dinan T.G., Cryan J.F. Targeting the Microbiota-Gut-Brain Axis: Prebiotics Have Anxiolytic and Antidepressant-like Effects and Reverse the Impact of Chronic Stress in Mice. Biol. Psychiatry. 2017;82:472–487. doi: 10.1016/j.biopsych.2016.12.031. [DOI] [PubMed] [Google Scholar]
- 207.Savignac H.M., Couch Y., Stratford M., Bannerman D.M., Tzortzis G., Anthony D.C., Burnet P.W.J. Prebiotic Administration Normalizes Lipopolysaccharide (LPS)-Induced Anxiety and Cortical 5-HT2A Receptor and IL1-β Levels in Male Mice. Brain Behav. Immun. 2016;52:120–131. doi: 10.1016/j.bbi.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Rienks J., Dobson A.J., Mishra G.D. Mediterranean Dietary Pattern and Prevalence and Incidence of Depressive Symptoms in Mid-Aged Women: Results from a Large Community-Based Prospective Study. Eur. J. Clin. Nutr. 2013;67:75–82. doi: 10.1038/ejcn.2012.193. [DOI] [PubMed] [Google Scholar]
- 209.Mamplekou E., Bountziouka V., Psaltopoulou T., Zeimbekis A., Tsakoundakis N., Papaerakleous N., Gotsis E., Metallinos G., Pounis G., Polychronopoulos E., et al. Urban Environment, Physical Inactivity and Unhealthy Dietary Habits Correlate to Depression among Elderly Living in Eastern Mediterranean Islands: The MEDIS (MEDiterranean ISlands Elderly) Study. J. Nutr. Health Aging. 2010;14:449–455. doi: 10.1007/s12603-010-0091-0. [DOI] [PubMed] [Google Scholar]
- 210.Chatzi L., Melaki V., Sarri K., Apostolaki I., Roumeliotaki T., Georgiou V., Vassilaki M., Koutis A., Bitsios P., Kogevinas M. Dietary Patterns during Pregnancy and the Risk of Postpartum Depression: The Mother-Child “Rhea” Cohort in Crete, Greece. Public Health Nutr. 2011;14:1663–1670. doi: 10.1017/S1368980010003629. [DOI] [PubMed] [Google Scholar]
- 211.Réus G.Z., Maciel A.L., Abelaira H.M., de Moura A.B., de Souza T.G., dos Santos T.R., Darabas A.C., Parzianello M., Matos D., Abatti M., et al. ω-3 and Folic Acid Act against Depressive-like Behavior and Oxidative Damage in the Brain of Rats Subjected to Early- or Late-Life Stress. Nutrition. 2018;53:120–133. doi: 10.1016/j.nut.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 212.Robertson R.C., Seira Oriach C., Murphy K., Moloney G.M., Cryan J.F., Dinan T.G., Paul Ross R., Stanton C. Omega-3 Polyunsaturated Fatty Acids Critically Regulate Behaviour and Gut Microbiota Development in Adolescence and Adulthood. Brain Behav. Immun. 2017;59:21–37. doi: 10.1016/j.bbi.2016.07.145. [DOI] [PubMed] [Google Scholar]
- 213.Bastiaansen J.A., Munafò M.R., Appleton K.M., Oldehinkel A.J. The Efficacy of Fish Oil Supplements in the Treatment of Depression: Food for Thought. Transl. Psychiatry. 2016;6:e975. doi: 10.1038/tp.2016.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Costantini L., Molinari R., Farinon B., Merendino N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int. J. Mol. Sci. 2017;18:2645. doi: 10.3390/ijms18122645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Pusceddu M.M., Kelly P., Ariffin N., Cryan J.F., Clarke G., Dinan T.G. N-3 PUFAs Have Beneficial Effects on Anxiety and Cognition in Female Rats: Effects of Early Life Stress. Psychoneuroendocrinology. 2015;58:79–90. doi: 10.1016/j.psyneuen.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 216.Winther G., Pyndt Jørgensen B.M., Elfving B., Nielsen D.S., Kihl P., Lund S., Sorensen D.B., Wegener G. Dietary Magnesium Deficiency Alters Gut Microbiota and Leads to Depressive-like Behaviour. Acta Neuropsychiatr. 2015;27:168–176. doi: 10.1017/neu.2015.7. [DOI] [PubMed] [Google Scholar]
- 217.Kortman G.A.M., Raffatellu M., Swinkels D.W., Tjalsma H. Nutritional Iron Turned inside out: Intestinal Stress from a Gut Microbial Perspective. FEMS Microbiol. Rev. 2014;38:1202–1234. doi: 10.1111/1574-6976.12086. [DOI] [PubMed] [Google Scholar]
- 218.Shah H.E., Bhawnani N., Ethirajulu A., Alkasabera A., Onyali C.B., Anim-Koranteng C., Mostafa J.A., Shah H.E., Bhawnani N., Ethirajulu A., et al. Iron Deficiency-Induced Changes in the Hippocampus, Corpus Striatum, and Monoamines Levels That Lead to Anxiety, Depression, Sleep Disorders, and Psychotic Disorders. Cureus. 2021;13:e18138. doi: 10.7759/cureus.18138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Rucklidge J.J., Kaplan B.J. Broad-Spectrum Micronutrient Formulas for the Treatment of Psychiatric Symptoms: A Systematic Review. Expert Rev. Neurother. 2014;13:49–73. doi: 10.1586/ern.12.143. [DOI] [PubMed] [Google Scholar]
- 220.Coppen A., Bolander-Gouaille C. Treatment of Depression: Time to Consider Folic Acid and Vitamin B12. J. Psychopharmacol. 2016;19:59–65. doi: 10.1177/0269881105048899. [DOI] [PubMed] [Google Scholar]
- 221.Shaffer J.A., Edmondson D., Wasson L.T., Falzon L., Homma K., Ezeokoli N., Li P., Davidson K.W. Vitamin d Supplementation for Depressive Symptoms: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Psychosom. Med. 2014;76:190–196. doi: 10.1097/PSY.0000000000000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ekwaru J.P., Zwicker J.D., Holick M.F., Giovannucci E., Veugelers P.J. The Importance of Body Weight for the Dose Response Relationship of Oral Vitamin D Supplementation and Serum 25-Hydroxyvitamin D in Healthy Volunteers. PLoS ONE. 2014;9:e111265. doi: 10.1371/journal.pone.0111265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Tosunbayraktar G., Bas M., Kut A., Buyukkaragoz A.H. Low Serum 25(OH)D Levels Are Assocıated to Hıgher BMI and Metabolic Syndrome Parameters in Adult Subjects in Turkey. Afr. Health Sci. 2016;15:1161–1169. doi: 10.4314/ahs.v15i4.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kimball S.M., Mirhosseini N., Rucklidge J. Database Analysis of Depression and Anxiety in a Community Sample-Response to a Micronutrient Intervention. Nutrients. 2018;10:152. doi: 10.3390/nu10020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Oh J., Yun K., Chae J.H., Kim T.S. Association Between Macronutrients Intake and Depression in the United States and South Korea. Front. Psychiatry. 2020;11:207. doi: 10.3389/fpsyt.2020.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Fernstrom J.D., Wurtman R.J., Hammarstrom-Wiklund B., Rand W.M., Munro H.N., Davidson C.S. Diurnal Variations in Plasma Neutral Amino Acid Concentrations among Patients with Cirrhosis: Effect of Dietary Protein. Am. J. Clin. Nutr. 1979;32:1923–1933. doi: 10.1093/ajcn/32.9.1923. [DOI] [PubMed] [Google Scholar]
- 227.Wurtman J.J. Depression and Weight Gain: The Serotonin Connection. J. Affect. Disord. 1993;29:183–192. doi: 10.1016/0165-0327(93)90032-F. [DOI] [PubMed] [Google Scholar]
- 228.Fernstrom J.D., Wurtman R.J. Brain Serotonin Content: Increase Following Ingestion of Carbohydrate Diet. Science. 1971;174:1023–1025. doi: 10.1126/science.174.4013.1023. [DOI] [PubMed] [Google Scholar]
- 229.Maciej S., Becker F.G., Cleary M., Team R.M., Holtermann H., The D., Agenda N., Science P., Sk S.K., Hinnebusch R., et al. Systematic Review on Effects of Diet on Gut Microbiota in Relation to Metabolic Syndromes. J. Clin. Nutr. Metab. 2017;1:343–354. [Google Scholar]
- 230.Breymeyer K.L., Lampe J.W., McGregor B.A., Neuhouser M.L. Subjective Mood and Energy Levels of Healthy Weight and Overweight/Obese Healthy Adults on High-and Low-Glycemic Load Experimental Diets. Appetite. 2016;107:253–259. doi: 10.1016/j.appet.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kris-Etherton P.M., Petersen K.S., Hibbeln J.R., Hurley D., Kolick V., Peoples S., Rodriguez N., Woodward-Lopez G. Nutrition and Behavioral Health Disorders: Depression and Anxiety. Nutr. Rev. 2021;79:247. doi: 10.1093/nutrit/nuaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Alkasir R., Li J., Li X., Jin M., Zhu B. Human Gut Microbiota: The Links with Dementia Development. Protein Cell. 2016;8:90–102. doi: 10.1007/s13238-016-0338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sivamaruthi B.S., Prasanth M.I., Kesika P., Chaiyasut C. Probiotics in Human Mental Health and Diseases—A Mini Review. Trop. J. Pharm. Res. 2021;18:889–895. doi: 10.4314/tjpr.v18i4.29. [DOI] [Google Scholar]
- 234.Cheung S.G., Goldenthal A.R., Uhlemann A.C., Mann J.J., Miller J.M., Sublette M.E. Systematic Review of Gut Microbiota and Major Depression. Front. Psychiatry. 2019;10:34. doi: 10.3389/fpsyt.2019.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Sherwin E., Dinan T.G., Cryan J.F. Recent Developments in Understanding the Role of the Gut Microbiota in Brain Health and Disease. Ann. N. Y. Acad. Sci. 2018;1420:5–25. doi: 10.1111/nyas.13416. [DOI] [PubMed] [Google Scholar]
- 236.Saravana Babu C., Kesavanarayanan K.S., Kalaivani P., Ranju V., Ramanathan M. A Simple Densitometric Method for the Quantification of Inhibitory Neurotransmitter Gamma-Aminobutyric Acid (GABA) in Rat Brain Tissue. Chromatogr. Res. Int. 2011;2011:732409. doi: 10.4061/2011/732409. [DOI] [Google Scholar]
- 237.Katano Y., Fujinami S., Kawakoshi A., Nakazawa H., Oji S., Iino T., Oguchi A., Ankai A., Fukui S., Terui Y., et al. Complete Genome Sequence of Oscillibacter valericigenes Sjm18-20T (=NBRC 101213T) Stand. Genom. Sci. 2012;6:406. doi: 10.4056/sigs.2826118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Allen A.P., Hutch W., Borre Y.E., Kennedy P.J., Temko A., Boylan G., Murphy E., Cryan J.F., Dinan T.G., Clarke G. Bifidobacterium longum 1714 as a Translational Psychobiotic: Modulation of Stress, Electrophysiology and Neurocognition in Healthy Volunteers. Transl. Psychiatry. 2016;6:e939. doi: 10.1038/tp.2016.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Karen C., Shyu D.J.H., Rajan K.E. Lactobacillus paracasei Supplementation Prevents Early Life Stress-Induced Anxiety and Depressive-Like Behavior in Maternal Separation Model-Possible Involvement of Microbiota-Gut-Brain Axis in Differential Regulation of MicroRNA124a/132 and Glutamate Receptors. Front. Neurosci. 2021;15:1115. doi: 10.3389/fnins.2021.719933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Sonali S., Ray B., Tousif H.A., Rathipriya A.G., Sunanda T., Mahalakshmi A.M., Rungratanawanich W., Essa M.M., Qoronfleh M.W., Chidambaram S.B., et al. Mechanistic Insights into the Link between Gut Dysbiosis and Major Depression: An Extensive Review. Cells. 2022;11:1362. doi: 10.3390/cells11081362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kumar A., Prajapati B., Pramanik J. Prebiotics as Adjunctive Therapy in Diabetes: A Review of Prebiotics in Diabetes. Curr. Nutrac. 2022;3:e180822207618. [Google Scholar]
- 242.Schmidt K., Cowen P.J., Harmer C.J., Tzortzis G., Errington S., Burnet P.W.J. Prebiotic Intake Reduces the Waking Cortisol Response and Alters Emotional Bias in Healthy Volunteers. Psychopharmacology. 2015;232:1793–1801. doi: 10.1007/s00213-014-3810-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Dinan T.G., Cryan J.F. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol. Clin. N. Am. 2017;46:77–89. doi: 10.1016/j.gtc.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 244.Xiao Q., Shu R., Wu C., Tong Y., Xiong Z., Zhou J., Yu C., Xie X., Fu Z. Crocin-I Alleviates the Depression-like Behaviors Probably via Modulating “Microbiota-Gut-Brain” Axis in Mice Exposed to Chronic Restraint Stress. J. Affect. Disord. 2020;276:476–486. doi: 10.1016/j.jad.2020.07.041. [DOI] [PubMed] [Google Scholar]
- 245.Markowiak P., Ślizewska K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients. 2017;9:1021. doi: 10.3390/nu9091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kleniewska P., Pawliczak R. Influence of Synbiotics on Selected Oxidative Stress Parameters. Oxid. Med. Cell. Longev. 2017;2017:e9315375. doi: 10.1155/2017/9315375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Schröder J., Dören M., Schneider B., Oettel M. Are the Antioxidative Effects of 17β-Estradiol Modified by Concomitant Administration of a Progestin? Maturitas. 1996;25:133–139. doi: 10.1016/0378-5122(96)01049-3. [DOI] [PubMed] [Google Scholar]
- 248.Salminen S., Collado M.C., Endo A., Hill C., Lebeer S., Quigley E.M.M., Sanders M.E., Shamir R., Swann J.R., Szajewska H., et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021;18:649–667. doi: 10.1038/s41575-021-00440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Taverniti V., Guglielmetti S. The Immunomodulatory Properties of Probiotic Microorganisms beyond Their Viability (Ghost Probiotics: Proposal of Paraprobiotic Concept) Genes Nutr. 2011;6:261–274. doi: 10.1007/s12263-011-0218-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Maehata H., Kobayashi Y., Mitsuyama E., Kawase T., Kuhara T., Xiao J.Z., Tsukahara T., Toyoda A. Heat-Killed Lactobacillus Helveticus Strain MCC1848 Confers Resilience to Anxiety or Depression-like Symptoms Caused by Subchronic Social Defeat Stress in Mice. Biosci. Biotechnol. Biochem. 2019;83:1239–1247. doi: 10.1080/09168451.2019.1591263. [DOI] [PubMed] [Google Scholar]
- 251.Kambe J., Watcharin S., Makioka-Itaya Y., Inoue R., Watanabe G., Yamaguchi H., Nagaoka K. Heat-Killed Enterococcus Fecalis (EC-12) Supplement Alters the Expression of Neurotransmitter Receptor Genes in the Prefrontal Cortex and Alleviates Anxiety-like Behavior in Mice. Neurosci. Lett. 2020;720:134753. doi: 10.1016/j.neulet.2020.134753. [DOI] [PubMed] [Google Scholar]
- 252.Nishida K., Sawada D., Kuwano Y., Tanaka H., Rokutan K. Health Benefits of Lactobacillus Gasseri CP2305 Tablets in Young Adults Exposed to Chronic Stress: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients. 2019;11:1859. doi: 10.3390/nu11081859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Meyyappan A.C., Forth E., Wallace C.J.K., Milev R. Effect of Fecal Microbiota Transplant on Symptoms of Psychiatric Disorders: A Systematic Review. BMC Psychiatry. 2020;20:299. doi: 10.1186/s12888-020-02654-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Cai T., Shi X., Yuan L.Z., Tang D., Wang F. Fecal Microbiota Transplantation in an Elderly Patient with Mental Depression. Int. Psychogeriatr. 2019;31:1525–1526. doi: 10.1017/S1041610219000115. [DOI] [PubMed] [Google Scholar]
- 255.Nirmal J., Babu C.S., Harisudhan T., Ramanathan M. Evaluation of Behavioural and Antioxidant Activity of Cytisus Scoparius Link in Rats Exposed to Chronic Unpredictable Mild Stress. BMC Complement. Altern. Med. 2008;8:15. doi: 10.1186/1472-6882-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Pu Y., Zhang Q., Tang Z., Lu C., Wu L., Zhong Y., Chen Y., Hashimoto K., Luo Y., Liu Y. Fecal Microbiota Transplantation from Patients with Rheumatoid Arthritis Causes Depression-like Behaviors in Mice through Abnormal T Cells Activation. Transl. Psychiatry. 2022;12:223. doi: 10.1038/s41398-022-01993-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wu L., Ran L., Wu Y., Liang M., Zeng J., Ke F., Wang F., Yang J., Lao X., Liu L., et al. Oral Administration of 5-Hydroxytryptophan Restores Gut Microbiota Dysbiosis in a Mouse Model of Depression. Front. Microbiol. 2022;13:1419. doi: 10.3389/fmicb.2022.864571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Tian P., Wang G., Zhao J., Zhang H., Chen W. Bifidobacterium with the Role of 5-Hydroxytryptophan Synthesis Regulation Alleviates the Symptom of Depression and Related Microbiota Dysbiosis. J. Nutr. Biochem. 2019;66:43–51. doi: 10.1016/j.jnutbio.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 259.Murrough J.W., Yaqubi S., Sayed S., Charney D.S. Emerging Drugs for the Treatment of Anxiety. Expert Opin. Emerg. Drugs. 2015;20:393. doi: 10.1517/14728214.2015.1049996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Bennabi D., Aouizerate B., El-Hage W., Doumy O., Moliere F., Courtet P., Nieto I., Bellivier F., Bubrovsky M., Vaiva G., et al. Risk Factors for Treatment Resistance in Unipolar Depression: A Systematic Review. J. Affect. Disord. 2015;171:137–141. doi: 10.1016/j.jad.2014.09.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.