Abstract
BACKGROUND:
Patients with food allergy may be advised to introduce specific foods into their diets, both to gradually increase tolerance and as next steps after completing oral immunotherapy or other therapeutic interventions. However, the safe use of retail foods is dependent on the ability to establish the specific allergen protein content of these foods.
OBJECTIVE:
To develop a systematic approach to estimate the protein content of peanut, milk, egg, wheat, cashew, hazelnut, and walnut in a variety of retail food equivalents for each allergen and associated patient education materials.
METHOD:
We created an algorithm that used a multi-step process with information from product food labels, nutrient databases, independent weighing and measuring of foods, and information provided by manufacturers, including Certificates of Analysis, and e-mail communication to estimate allergen protein content of multiple retail foods for each of seven allergens. Once a variety of retail food equivalents for each allergen and allergen serving size was determined, we developed participant education handouts, which were reviewed by study teams at 10 food allergy centers, NIAID, and the CoFAR coordinating center. After one year of use, multiple queries were addressed and the retail food equivalents and educational materials were reviewed and edited.
RESULTS:
We identified a variety of retail food equivalents for seven allergens at six serving sizes, and created 48 unique patient education materials.
CONCLUSION:
Our results provide extensive guidance on a variety of retail equivalents for seven foods, and a method to systematically estimate retail food protein equivalents with ongoing reassessment.
Keywords: Oral immunotherapy, OUtMATCH, CoFAR, omalizumab, retail food equivalents, allergens, diet, nutrition, food allergy
INTRODUCTION
Food allergy is a global health concern that is increasing in prevalence. Dietary avoidance remains the standard of care but carries social(1), nutritional(2), and financial(3) burdens. Strict avoidance of allergens is not a simple undertaking and allergic reactions due to accidental ingestion are common(4–6). Patients and their families living with food allergies must contend with the burden of the risk of an allergic reaction from accidental ingestion(7). As such, alternative approaches to allergen avoidance, including a variety of treatment methods, are being evaluated. Oral immunotherapy (OIT) is one such therapeutic approach in which increasing amounts of a food allergen are ingested with the goal of raising the individual’s reaction threshold. The United States Food and Drug Administration (FDA) and European Medicines Agency (EMA) recently approved the first commercially available peanut OIT product, Palforzia™. FDA approved OIT products for other common allergens are not currently available, although research is ongoing.
Clinical research protocols for OIT ideally use well-characterized, food proteins (typically flours and powders) manufactured with Good Manufacturing Practices with FDA oversight (8–10). Retail food equivalents (RFE) differ from food proteins used in research in that they are foods purchased on the retail market and used as equivalent allergen protein dosing. However, in clinical practice, RFE (e.g. peanut puffs, peanut or nut butters or flours, candies, nut milks, hen’s egg, and cow’s milk) are increasingly offered for OIT(11–14) and several professional societies have published clinical practice guidelines for OIT recommending the use of “normal” foods purchased on the retail market(15–19). The safe use of RFE, however, is dependent on the ability to establish the equivalent allergen protein content of these foods.
RFE are not regulated as drug products, thus there is natural variation in allergen protein content. For example, Leroux et al(20) evaluated the size and variability of peanuts in chocolate-coated peanut candies to assess their suitability for OIT and compared these peanuts to standard whole roasted cocktail peanuts. They found that peanuts used in candies were 41% smaller and more variable in size than standard cocktail peanuts. Mack et al(21) evaluated peanut protein content of peanut M&M’s® (Mars Chocolate, Hackettstown, NJ) by weighing 294 whole candies and the peanuts therein. Fourteen (4.8 %) candies contained either partial, multiple, or no peanut and one contained almond. They also observed significant variability in the weight of individual peanuts within the candies. In both studies, the coefficient of variation decreased with increasing doses, suggesting that RFE may be more suited to larger maintenance doses rather than smaller initial OIT up-doses.
The focus of this endeavor was to address a critical need to establish a standardized approach to determine allergenic protein content in RFE that could be used for maintenance dosing after OIT or other food allergy treatments. While there is a similar need for use in OIT, this was not an objective of this study. The lowest protein serving size we developed was 300 mg for each allergen. This should not be adapted for smaller doses due to the greater effect of variability at lower doses and the increased risk of reactions with small variations in doses. In this paper, we describe the process by which RFE dosing protocols for peanut, milk, egg, wheat, cashew, hazelnut, and walnut and participant-oriented educational materials were developed for the Consortium for Food Allergy Research (CoFAR)-11 study, Omalizumab as Monotherapy and as Adjunct Therapy to Multi-Allergen OIT in Food Allergic Children and Adults (OUtMATCH).
METHODS
OUtMATCH trial design
The OUtMATCH study is a phase III, multi-center, randomized, double-blind, placebo-controlled study to determine if omalizumab injections alone or in combination with multi-allergen OIT will enable participants with multiple food allergies to consume foods without dose-limiting symptoms during double-blind, placebo-controlled food challenges (DBPCFC) (Wood, 2022 JACI Global In Press). The OUtMATCH study includes a post-treatment evaluation of safety with daily dietary consumption of up to three of a participant’s allergens including peanut plus two of the following: milk, egg, wheat, cashew, walnut and hazelnut. The target serving size (300, 600, 1000, 2000, 4000, or 6000 mg allergen protein) for dietary consumption is determined by the end-of-treatment DBPCFC. Based upon end-of-treatment DBPCFC, the maximum serving size for daily dietary consumption with RFE is determined per protocol as no more than 75% of the cumulative tolerated amount (Table I). Before beginning home dietary consumption with RFE, an observed open feeding is performed to each food, with the participant consuming at least the minimum serving size of 300 mg, and no more than their maximum serving size using RFE. Based on how well the participant tolerates the open feeding, the participant and site PI then determine the maximum amount for daily dietary consumption at home. Food-specific detailed education and participant-oriented written educational materials for each eligible serving size are provided by study personnel after the open feeding.
Table I.
Maximum Amount of Daily Allergenic Protein Allowed Per Protocol*
| Final Dose Consumed Without Dose-Limiting Symptoms During Most recent OFC (mg protein) | Cumulative Dose Consumed Without Dose-Limiting Symptoms During Most recent OFC (mg protein) | Minimum Amount to Ingest Daily (mg protein of food) | Maximum Amount to Ingest Daily (mg protein of food) |
|---|---|---|---|
| 600 | 1044 | 300 | 600 |
| 1000 | 2044 | 300 | 1000 |
| 1st dose of 2000 | 4044 | 300 | 2000 |
| 2nd dose of 2000 | 6044 | 300 | 4000 |
| 3rd dose of 2000 | 8044 | 300 | 6000 |
This approach is being evaluated in this study and should not be construed as an evidence-based approach to post-OIT management
The OUtMATCH Dietary Sub-Committee
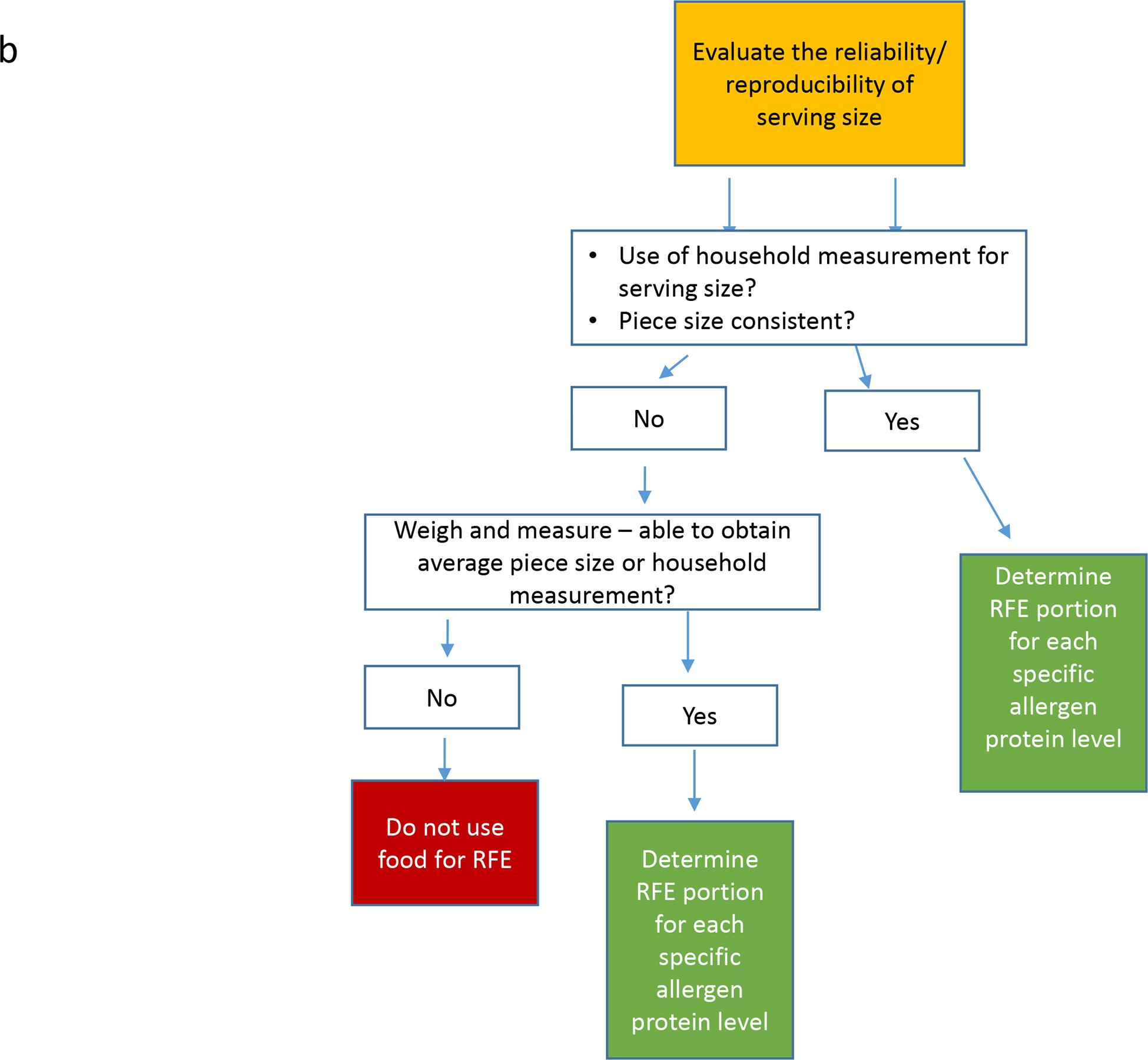
The OUtMATCH Dietary Sub-Committee (DSC) consisted of four registered dietitians [MG, MW, BG and AS] and one registered nurse [KM], all with extensive food allergy expertise. The DSC reviewed retail foods suggested from six food allergy research centers and using a systematic approach, created an algorithm (Figure 1a and 1b) to estimate protein content in RFE for the seven OUtMATCH allergens at the six serving sizes. The systematic approach included evaluation of product food labels, food and nutrient databases, independent weighing and measuring of foods, independent protein analysis testing, and/or information provided by the manufacturer, including Certificates of Analysis (COA) and e-mail communication with food manufacturers. When available, published studies were reviewed to assist with the RFE protein estimates(20–22). Once the allergen protein contents were estimated for the RFE for each of the seven OUtMATCH allergens, educational materials were developed to provide participant-oriented, detailed guidance on the introduction of RFE after the end-of-treatment DBPCFC.
Figure I.
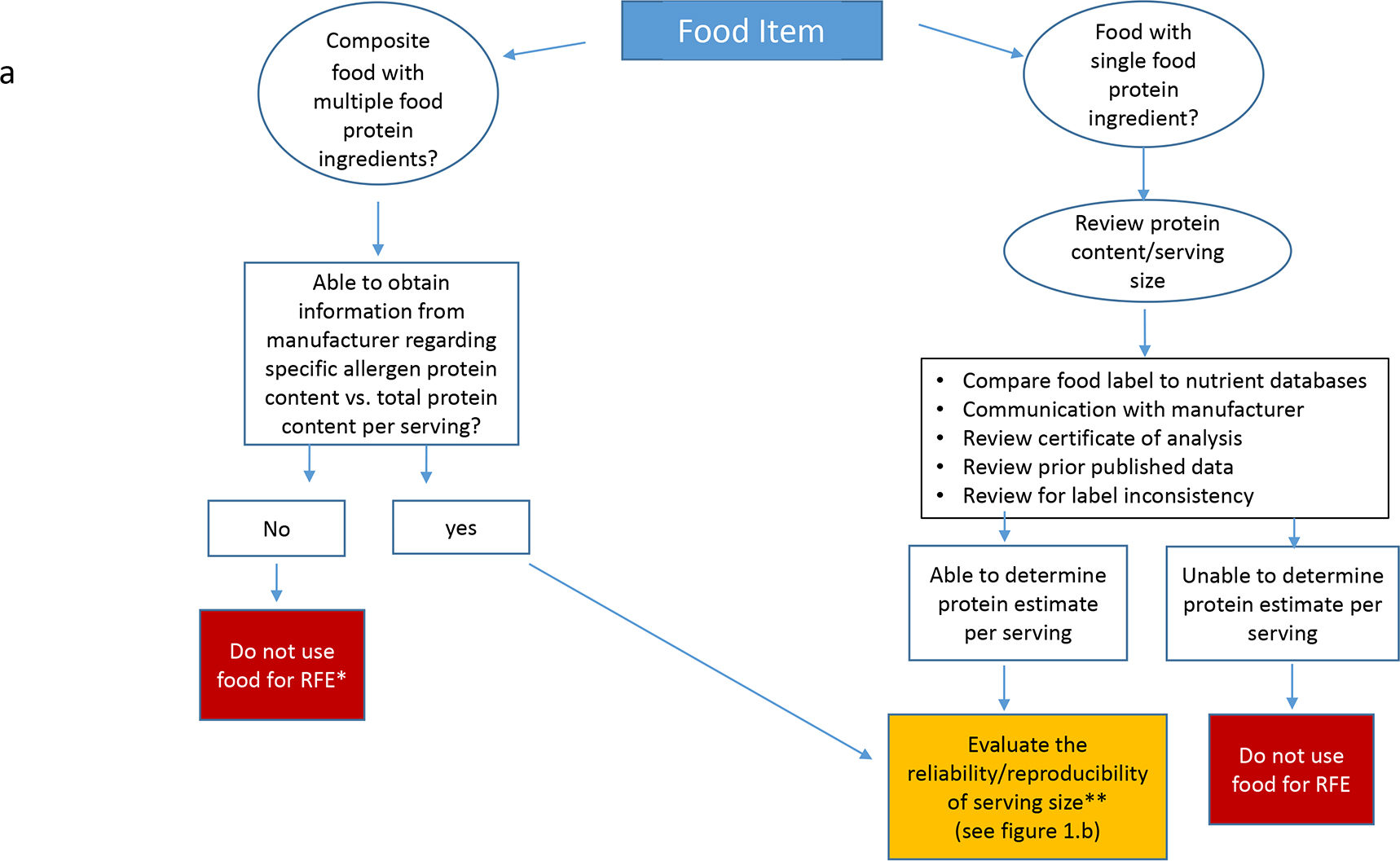
a. Systematic Approach to Estimate Allergen Protein Content
*RFE= Retail Food Equivalent
** See Figure I.b
b. Reliability of Serving Size
Determining allergen protein content
Protein content listed on a product’s food label includes proteins from all sources in the food. Even in non-composite foods, when the total protein value reflects the allergenic protein only (e.g., peanut butter or peanut flour), what is listed on the label is often rounded to the nearest whole gram. The serving size may also be rounded to the nearest half or whole serving depending on the number of servings per package.
To determine the best estimate of the specific allergen protein content of non-composite foods, we referenced several nutrient databases: Nutrition Data System for Research (NDSR, version 2020, developed by the University of Minnesota, Minneapolis, MN) and three databases available on United States Department of Agriculture FoodData Central (FDC): The National Nutrient Database for Standard Reference, Legacy Release (SR), the Food and Nutrient Database for Dietary Studies (FNDDS, 2017–2018), and the Global Branded Food Products Database (GBFPD).
NDSR contains information from SR, scientific literature, and information from food manufacturers. SR has been the primary food composition data source in the US for many years and contains values derived from analysis, imputation and/or scientific literature(23). The last update was released in 2018, and no future updates are currently planned. FNDDS is updated and released every two years. The GBFPD contains nutrient data provided voluntarily by the food industry and is updated monthly(23).
We compared the protein content values from these databases to each other and to the information printed on food labels for a variety of products for each allergen. When there were discrepancies in the protein content of a food product across data sources, such that we could not arrive at a reasonable protein estimate per serving, the product was not used for RFE (e.g., American cheese product).
A COA was used to verify protein content of RFE when available from the manufacturer (e.g., hazelnut flour) and verifies the analytical testing conducted on the food product and describes the specifications of safety (e.g., microbial, mold content) and quality (e.g., protein content) of a food. Due to batch-to-batch variation, protein content may change slightly and this is reflected in a COA, which is updated regularly, when available. However, a COA is an internal document that, while highly recommended, it not required and therefore is not always available and manufacturers are not always willing to share this internal document.
In one case, additional testing (Kjeldhal titration assay) for determining protein content was conducted on a cashew flour that was new to the market and therefore not listed in the nutrient databases and no COA was available from the company. This product was preferable as it was manufactured in a dedicated cashew only facility.
We reviewed prior published allergen protein estimates, when available, but did not always use the previously published processes. Leroux et al(20) visually assessed cocktail peanut size and eliminated the largest and smallest cocktail peanuts prior to determining average size. One of the goals of our process was to minimize participant burden by simplifying the selection and measurement of RFE, so this process was not adopted.
If protein content listed on the product’s food label was provided per weight (grams/ounces) rather than household measurement (teaspoons/cups), the product was weighed and measured to ensure a household measurement could be used. Pieces (e.g., peanuts) were also weighed to determine the average piece size weight prior to estimating protein content per piece.
For composite foods, the manufacturer was contacted about the protein content of the relevant allergen since the label lists the total protein content. If the manufacturer provided the information, the food was further evaluated for serving size consistency and average piece size (e.g., candy containing peanut).
Developing participant education materials
The DSC initially focused on developing handouts for 300 mg of protein for each of the seven OUtMATCH allergens using a variety of RFE for each. The 300 mg handouts were reviewed by the protocol co-chairs, NIAID, and coordinating center and then by the medical, nursing, and dietary staff at the 10 OUtMATCH food allergy research sites. This review yielded 52 unique queries on dosing amounts, suggestions for additional foods, and a few requests for clarification on form instructions resulting in further communication with manufacturers, weighing and measuring food items, and revision of handouts. Lastly, handouts for the five remaining serving sizes (600–6000 mg) for the seven allergens were created. We initially created 42 participant education handouts (seven allergens, each with six serving sizes), and written instructional materials to provide further guidance on introducing RFE. After the education handouts had been used by the 10 OUtMATCH sites for one year, we collected additional queries and comments and further revised the handouts, which are presented here.
RESULTS
Retail food equivalents
The allergen protein content was estimated for each RFE (Tables II–IV showing peanut, milk and egg respectively; Tables E1–E4 showing wheat, walnut, cashew, and hazelnut respectively, available in this article’s online repository) using the developed systematic approach as described in Figure 1a and 1b. The seven OUtMATCH allergens had multiple RFE identified for each of the six serving sizes. For each RFE serving size, the estimated allergen protein goal was within a 10% estimated margin of error. We made efforts to simplify the serving size measurement when possible. For instance, if a more accurate measurement required 1 tablespoon, plus 2 teaspoons, plus 1/16th teaspoon of a RFE, we would instead round up to 1 tablespoon, plus 2 ½ teaspoons or round down to 1 tablespoon plus 2 teaspoons, whichever was closest to the 10% estimated margin of error. As participants were assigned a maximum daily serving size that was no more than 75% of the cumulative tolerated dose at the end-of-treatment DBPCFC, it was determined to be low risk to adjust serving sizes to simplify measuring and mimic normal feeding rather than “dosing” with RFE.
Table II.
Peanut Food Protein Equivalents
| Food | 300mg | 600mg | 1000mg | 2000mg | 4000mg | 6000mg |
|---|---|---|---|---|---|---|
|
Cocktail peanuts Planters |
1 and ½ peanuts | 2 and ½ peanuts | 4 and ½ peanuts | 9 peanuts | 18 peanuts | 27 peanuts |
|
Peanuts in the shell Hampton Farms |
1 and ½ peanuts | 2 and ½ peanuts | 4 and ½ peanuts | 9 peanuts | 17 peanuts | 25 peanuts |
|
Regular peanut butter Jif or Skippy, smooth |
¼ teaspoon | ½ teaspoon | 1 teaspoon | 1 and ¾ teaspoons | 1 Tablespoon plus ½ teaspoon | 1 Tablespoon plus 2 teaspoons |
|
Natural peanut butter Teddie, smooth |
¼ teaspoon | ½ teaspoon | ¾ teaspoon | 1 and ½ teaspoons | 1 Tablespoon | 1 Tablespoon plus 1 and 2/3 teaspoons |
|
12% Light Roast peanut flour Byrd Mill |
1/3 teaspoon | 2/3 teaspoon | 1 teaspoon | 2 teaspoons | 1 Tablespoon plus 1 teaspoon | 2 Tablespoons |
|
PB2 powdered peanut butter Bell Plantation |
1/3 teaspoon | 2/3 teaspoon | 1 teaspoon | 2 teaspoons | 1 Tablespoon plus 1 teaspoon | 2 Tablespoons |
|
Bamba Osem |
3 pieces | 6 pieces | 10 pieces | 21 pieces | 42 pieces | 63 pieces |
| Reese’s Minis unwrapped | 1 and ½ minis | 3 and ½ minis | 5 and ½ minis | 11 minis | 22 minis | 33 minis |
| Reese’s Miniatures wrapped | ½ miniature | 1 miniature | 1 and ½ miniatures | 3 and ½ miniatures | 6 and ½ miniatures | 10 miniatures |
|
Reese’s Peanut Butter (PB)
Cup standard size |
n/a | 1/3 a PB cup | ½ a PB cup | 1 PB cup | 2 PB cups | 3 and 1/3 PB cups |
| Reese’s Pieces | 4 pieces | 7 pieces | 12 pieces | 24 pieces | 47 pieces | 71 pieces |
| Peanut M&M | 2 pieces | 4 pieces | 7 pieces | 14 pieces | 27 pieces | 41 pieces |
| Pasokin peanut butter bites | n/a | n/a | ½ piece | 1 piece | 2 pieces | 3 pieces |
Table IV.
Egg Food Protein Equivalents
| Food | 300mg | 600mg | 1000mg | 2000mg | 4000mg | 6000mg |
|---|---|---|---|---|---|---|
|
Hen’s Egg Generic, Large |
1/12th egg* | 1/6th egg* | ¼ egg* | ½ egg* | 1 egg* | 1 and 2/3 egg* |
|
Hen’s Egg Generic, Extra Large |
n/a | n/a | n/a | n/a | n/a | 1 and ½ egg |
|
Meringue Cookie Trader Joe’s |
1 cookie | 2 cookies | 4 cookies | 8 cookies | 16 cookies | 24 cookies |
|
Liquid Egg Whites Egg Beaters |
1/2 teaspoon | 1 teaspoon | 2 teaspoons | 1 Tablespoon plus ¾ teaspoon | 2 Tablespoons plus 1 teaspoon | 3 Tablespoons plus 1 and ¾ teaspoons |
|
Egg White Protein OvaEasy |
1/8th teaspoon | ¼ teaspoon | ½ teaspoon | 1 teaspoon | 1 and ¾ teaspoon | 2 and 2/3 teaspoons |
Serving size is based on egg white protein, not whole egg protein
Participant education handouts
A sample education handout is provided in Figure II. (For the full set of education handouts see Figures E1–8 in this article’s online repository.) Participants were given multiple RFE options for each serving size and encouraged to make nutritionally sound choices. The amount of RFE participants were instructed to eat was expressed in written language (e.g., ½ teaspoon, 2 nuts, 1 cookie) and occasionally illustrated to underscore differences between whole and partial nuts or demonstrate correct division of whole foods (e.g., bread, egg, cheese stick). Guidance was provided on how to read food label information to help participants compare and choose alternative brands for most foods. Participants were advised however to use only the specific brands listed for peanut RFE because of the variability in peanut protein content in similar peanut foods from different manufacturers. For example, Hindley et al(24) found Bamba brand peanut puffs were well-formulated with consistent total peanut protein content and distribution of the major allergenic component proteins between lots. Another brand tested had 3–4 fold less peanut allergen content than Bamba. We chose a light roast, 12% fat peanut flour to eliminate risk of variability in peanut protein (and peanut component protein) between various brands, roasts and fat content. Additionally, many of the peanut candies are composite protein foods and therefore the manufacturers input on formulation was vital to the peanut protein estimate and applied only to that specific peanut candy brand.
Figure II.
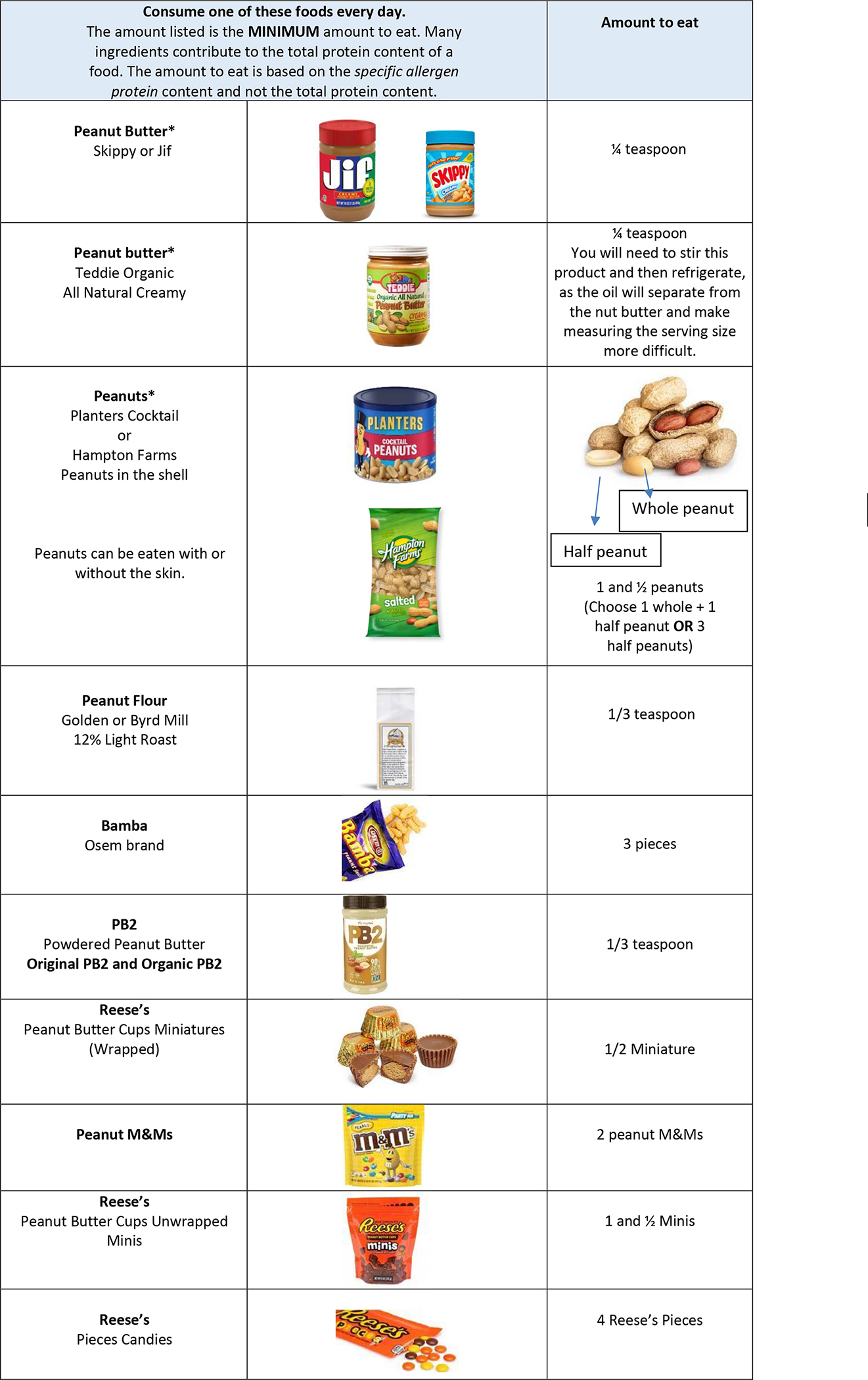

Sample Participant Education Handout
The participant education handouts included reminders to read food labels with every purchase and continue to avoid any additional allergens. Participants eligible for and consuming a study allergen had tolerated a cumulative dose of at least 1044 mg of the allergenic protein during the end-of-treatment DBPCFC and therefore they were not required to avoid foods with precautionary allergen labeling (PAL) to that allergen. Handouts included guidance on avoiding PAL for other allergens for which strict avoidance was advised for management. To help participants incorporate the RFE into their diets, serving suggestions were provided on each handout with some containing recipes.
Handouts for milk and egg also incorporated instructions on consuming heated and baked forms. Per protocol, all participants consuming milk RFE were instructed to consume at least 300 mg of unheated milk protein, while the remainder of their serving size if >300 mg could be consumed as unheated, heated, or baked forms of milk. Definitions and examples of each form were included on the handouts. Consuming raw egg is a safety hazard and not recommended. There was a perceived difficulty of participant’s willingness to use cooked egg to meet their daily dietary consumption goal. Therefore, per protocol, the target amount of egg was allowed to be consumed using solely home baked goods with egg, if desired, and all egg handouts included this explanation. In addition to their minimum/maximum daily serving size, participants were given the option to consume commercially baked products with milk and/or egg as a minor ingredient. Because of the difficulty determining the protein content of milk and egg in commercially baked items, these foods were allowed, but did not count towards a participant’s daily serving size. Commercially baked items with milk or egg as a minor ingredient were defined by the following criteria: 1) The product must contain milk or egg as the third ingredient or further down on the ingredient list, and 2) The product must contain a grain ingredient (wheat flour or gluten-free flour) and 3) be fully baked in the oven to form a dry crumb, baked-good texture. Examples of “commercially baked items” include muffins, cookies, crackers, breads, or rolls.
Annual Review
After the education handouts had been in use for approximately one year, revisions were made based on annual review of products and feedback from the 10 OUtMATCH sites. RFE that were no longer readily available or had experienced labeling or formulation changes and were no longer appropriate per the algorithm were removed. See Table E5 for a list of RFE that were changed. Additionally, a list of “tolerated mix-ins” (e.g., dry cereal, granola, chocolate chips and coconut flakes) was added to peanut and tree nut handouts to help mask textures and flavors. A second set of peanut handouts were developed for participants on a milk-free diet and those able to eat limited-milk, as part of the study, increasing the number of handouts from 42 to 48. A dried egg white crystal RFE replaced a difficult to measure dried egg powder and a notation was added for hen’s egg to clarify that only egg white protein is included in egg white protein calculations. We attempted to communicate with the manufacturer of a hazelnut spread to ascertain the milk content so that participants could use the spread for both their milk and hazelnut daily servings, but we were unable to secure this information. Once edited, all handouts underwent final review by the DSC.
DISCUSSION
Our results provide guidance to participants for home consumption of daily allergenic food quantities using RFE. Importantly, we provide an algorithm to evaluate RFE for other foods if desired. We found that a single approach to estimate allergen protein content of RFE was imprecise due to rounding on product labels, disagreement between product labels and nutrient databases, lack of COA, inadequate product information from manufacturers, and difficulty in accurately dividing foods for appropriate RFE serving sizes. Additionally, allergen protein estimates can be a moving target as protein content on product labels and even the formulations can change. The time and resources required for the development of the RFE allergen protein estimates, education handouts, and the annual review process was substantial.
RFE have reportedly been used successfully in OIT in clinical practice (14, 25), however we are concerned that variability may pose a far greater risk when precision is needed, as with smaller OIT updoses rather than larger maintenance doses. OUtMATCH participants were advised to consume a daily amount, with a minimum serving size of 300 mg and a maximum that was at most 75% of the cumulative tolerated dose during a post treatment DBPCFC (Table I), therefore an exact pharmaceutically accurate dose was deemed unnecessary.
Caution is warranted as foods are not regulated as drug products and as such, the allergen protein content of a food is an estimate and cannot be considered an exact amount. Variation in protein content has been reported in a number of studies of peanut products (11, 20, 21, 24). Even if foods have equivalent allergenic protein content, processing may have an impact on allergenicity. There is extensive research on the impact on allergenicity of heating food proteins, for instance, such as milk and egg (26–28). Unbaked milk products may also contain varying quantities of the milk proteins with cheese and yogurt typically containing more casein than whey compared to fluid milk. Detailed information on processing related to the allergenicity of many foods is lacking.
An area we did not address in our work was the immunodominant allergen content of the RFE. Ara h 1, Ara h 2, and Ara h 6 are immunodominant allergens among those with peanut allergy. One study found that the composition and concentration of peanut component protein varied among peanut powders, flours, and butters (29). Additionally, there was variable protein concentration among different flours. The major difference in flours resulted primarily from the degree of roasting (light vs. dark), which is why we specified a light roast peanut flour and did not allow other peanut flours. The lack of assessment of immunodominant equivalency between our RFE products was a limitation to our approach. However, this was not only beyond the scope of this project but also inconsistent with our effort to provide a real-world approach to our study participants, allowing for consumption of a variety of normal foods.
Another limitation is that allergen protein estimates can be a moving target as product labels and even the ingredients and formulations can change. We have planned annual reviews of our RFE, however the time and resources required for the development and review of allergen protein estimates and education handouts is substantial and requires a multidisciplinary approach. Of note, of the 61 food products used as RFE, only two products (an ice cream and a bread) required removal after the annual review due to formulation or product label changes. Commercially baked products with milk or egg as a minor ingredient were not RFE but were listed on our education handouts and allowed to be eaten freely. Of all the foods listed on the patient education handouts, baked goods were most likely to have formulation changes with five products requiring removal after the annual review. Additionally, it must be noted that our RFE results are product-specific and may not apply to similar products from other companies. The same product, if available in other countries, may not have the same formulation. We noted in our assessment that even the exact product (a vanilla ice cream brand) from one manufacturer had a different formulation dependent on the region for sale in the US. Therefore, our results should not be broadly applied without local assessment.
One concern of the DSC was the nutritional impact of daily consumption of some of the RFE. At the 300 mg level, we agreed there would likely be minor impact, but as serving sizes increased the calorie, saturated fat, and sugar content could become excessive, in particular for spreads, candies and other sweet treats. Therefore, participants were encouraged to use candies, cookies, spreads and sweets as occasional treats. Although we included full serving sizes for RFE on all handouts, our aim was for participants to use the amount listed on the handout as a guide to calculate and consume only a portion of their daily serving from “treats” while making up the difference using healthier options. This was explained on the general instruction education handout and on participant education handouts with the following statement, “Use a variety of foods to meet your daily serving goals. Choosing only candy, sweet spreads, cookies and other ‘treats’ is not appropriate to meet serving goals at higher serving sizes. When you eat these foods, eat them as a portion of your daily serving. Choose healthier options as much as possible.”
In summary, protein estimates of RFE are required for safe use in clinical and research settings. A single method to estimate protein content of RFE is imprecise. Our education handouts were created using a systematic approach, reviewed by physicians, nurses, and dietitians in 10 food allergy research centers, and edited again after 12 months post implementation in a participant population (see Table V). Our goal was to develop a systematic process to estimate allergen protein of RFE and to provide detailed educational materials with feedback from multiple stakeholders to support participant comprehension and safe RFE consumption as part of the daily diet.
Table V.
Considerations for Estimating Retail Food Protein Equivalents
| • Use multiple sources to estimate protein content. |
| • Consider total protein does not always equal specific allergenic protein content. |
| • Include registered dietitians on your protocol development team. |
| • Consider regional availability of food and differences in product labels. |
| • Create patient facing education handouts. |
| • Create a system for feedback from users. |
| • Review and update at least annually. |
Supplementary Material
Table III.
Milk Food Protein Equivalents
| Food | 300mg | 600mg | 1000mg | 2000mg | 4000mg | 6000mg |
|---|---|---|---|---|---|---|
|
Fluid Cow’s Milk Dairy Pure 1% Fat Milk |
2 teaspoons | 1 Tablespoon plus ½ teaspoon | 2 Tablespoons | ¼ cup | ½ cup | ¾ cup |
|
Mozzarella Cheese Sargento String Cheese |
1/24 of a cheese stick | 1/12 of a cheese stick | 1/6 of a cheese stick | 7/24 of a cheese stick | 7/12 of a cheese stick | 5/6 of a cheese stick |
|
Shredded Mozzarella Cheese Kraft Natural Mozzarella Shredded Low Moisture Part Skim |
½ teaspoon | 1 teaspoon | 1 and ¾ teaspoon | 1 Tablespoon plus ½ teaspoon | 2 Tablespoons plus 1 teaspoon | 3 and ½ Tablespoons |
|
Parmesan Cheese grated Kraft |
1/3 teaspoon | 2/3 teaspoon | 1 teaspoon | 2 teaspoons | 1 Tablespoon plus 1 teaspoon | 2 Tablespoons |
|
Cream Cheese Philadelphia Regular Cream Cheese |
1 teaspoon | 1 and ¾ teaspoon | 1 Tablespoon | 2 Tablespoons | 4 Tablespoons (1/4 cup) | 6 Tablespoons |
|
Whipped Cream Cheese Philadelphia Whipped Cream Cheese |
2 teaspoons | 1 Tablespoon plus ½ teaspoon | 2 Tablespoons | 4 Tablespoons | 8 Tablespoons (½ cup) | 12 Tablespoons |
|
Non-Fat Plain Greek Yogurt Chobani (no fruit flavors) |
3/4 teaspoon | 1 and 1/3 teaspoon | 2 and ¼ teaspoons | 1 and ½ Tablespoons | 3 Tablespoons | 4 and ½ Tablespoons |
|
Regular Yogurt Yoplait Original |
2 teaspoons | 1 Tablespoon plus ¾ teaspoon | 2 Tablespoons | 4 Tablespoons | 4 ounces or ½ cup | 1 single serve (6 ounces) (3/4 cup) |
|
Instant Nonfat Dry milks Carnation |
1/2 teaspoon | ¼ teaspoon plus 2/3 teaspoon | 1 and ½ teaspoons (or ½ Tablespoon) | 1 Tablespoon | 2 Tablespoons | 3 Tablespoons |
|
Ice Cream Classic Vanilla Bean Edy’s or Dryer’s |
1 Tablespoon plus ½ teaspoon | 2 Tablespoons plus ½ teaspoon | ¼ cup (or 4 Tablespoons) | ¼ cup plus 3 Tablespoons | ¾ cup plus 2 Tablespoons | 1 and 1/3 cups |
|
Milk Chocolate Chips Hershey’s |
16 chips | 32 chips | 53 chips | 109 chips (3 and ½ Tablespoons) | 217 chips (¼ cup plus 3 Tablespoons) | 325 chips (½ cup plus 2½ Tablespoons) |
1. What is already known about this topic?
Although foods are increasingly being used for therapeutic purposes in food allergy, only limited guidance on retail food protein equivalent doses have been published.
2. What does this article add to our knowledge?
Here we describe a process, by which multiple retail food equivalents and participant-oriented educational materials were developed for a post-treatment clinical research trial for the following allergens: peanut, milk, egg, wheat, cashew, hazelnut, and walnut.
3. How does this study impact current management guidelines?
Our results provide extensive guidance and retail food equivalent variety to research participants and a method to systematically estimate retail food equivalents for maintenance of home feeding of daily allergen protein quantities.
Acknowledgements:
Donald Leung, MD, PhD
Andrew Long, PharmD
Diane Dunham
Tricia Lee, MD
Rashelle Berry, MPH, MS, RDN, LD
Ashley Dulson CCRP
Mary Vess, RN CCRP
Jim Rhodes, PharmD
Caitlin Waddle, MS, RDN, LD
Julie Wang, MD
Jaime Ross, RN
Makeda Pinnock, RN
Ashley Gutierrez
Noreen Dugan
Amy M. Scurlock, MD
Amika Sood, MD
Aprile Wicker, RN
Marissa Clancy, RD
Molly Boone, RD
A Wesley Burks, MD
Corinne Keet, MD PhD
Yamini Virkud, MD MPH
Andrew Winslow, MD
Holly Barber, RN MSN CPNP
Sarah Bennick, RN MSN CPNP
Emily English, RN MSN CPNP
Deanna Hamilton, RN
Lauren Herlihy, RN MSN CPNP
Amy L. Arneson
Kaylee Lirely
Melissa Zamudio
Christopher Parrish
Maria Crain, RN MSN CPNP
Sayantani Sindher, MD
Katharine Fast, MD
William Collins, MD
Kathleen Jia, MD
Kari C. Nadeau, MD, PhD
Laurie Kost, MS
Frida Calderon
We acknowledge with gratitude the OUtMATCH study participants and their families for their dedication to the advancement of food allergy science.
Funding:
This work was supported by the National Institutes of Health (NIH) [grant numbers UM2AI130836, UM1AI130838, UL1TR003098, UM1AI130570, UM1 AI130936-06, UL1TR002489, UL1 TR002535, 5UM1AI130780-04, UM1AI130781, UL1TR003107, UM1AI130934, 1UL1TR001102, 8UL1TR000170, 1UL1RR025758]
Abbreviations:
- ED
Emergency department
- OIT
Oral immunotherapy
- FDA
US Food and Drug Administration
- RFE
Retail food equivalents
- OUtMATCH
Omalizumab as Monotherapy and as Adjunct Therapy to Multi-Allergen OIT in Food Allergic Children and Adults
- DBPCFC
Double-blind, placebo-controlled food challenge
- COA
Certificate of Analysis
- CoFAR
Consortium for Food Allergy Research
- DSC
Dietary Subcommittee
- DC
CoFAR Dietary Committee
- NDSR
Nutrition Data System for Research
- FDC
FoodData Central
- SR
The National Nutrient Database for Standard Reference Studies, Legacy Release
- FNDDS
The Food and Nutrient Database for Dietary Studies
- PAL
Precautionary Allergen Labeling
- GBFPD
Global Branded Food Products Database
Footnotes
Conflict of interest:
M.E. Groetch receives royalties from UpToDate, FARE, and Academy of Nutrition and Dietetics; serves on the Medical Advisory Board of IFPIES, as a Senior Advisor to FARE, as a Health Sciences Advisor for APFED and on the editorial board of Journal of Food Allergy; she has no commercial interests to disclose.
K. Mudd has no conflict of interest to report
M. Woch has no conflict of interest to report.
A. Schaible has no conflict of interest to report.
B.E. Gray has no conflict of interest to report and acknowledges her work is supported by Grant Number 1UL1TR002541-01.
J.A. Bird reports grants from NIH-NIAID, Genentech, Aimmune, Astellas, DBV Technologies, Food Allergy Research and Education (FARE), Novartis and Regeneron and personal fees from AllerGenis, Allergy Therapeutics, Before Brands, DBV Technologies, FARE, HAL Allergy, Novartis, and Nutricia.
S.M. Jones reports grants from NIH-NIAID, Food Allergy Research & Education (FARE), Aimmune Therapeutic, DBV Technologies, Astellas, Inc., Sanofi, Inc., Regeneron, Inc., and Genentech, Inc. and personal fees from Food Allergy Research and Education, Aimmune Therapeutics
E.H. Kim reports ad board: ALK, DBV Technologies, Kenota Health, Ukko Inc and consultant to AllerGenis, Allergy Therapeutics Ltd, Belhaven Pharma, Duke Clinical Research Institute, Genentech, Nutricia
B.J. Lanser reports grants and personal fees from Aimmune Therapeutics, DBV Technologies, and Genentech; grants from Regeneron; personal fees from Allergenis, and GlaxoSmithKline; and grant support to his institution from the NIH/NIAID.
J. Poyser co-authorship of this publication does not necessarily constitute endorsement by the NIAID, the NIH or any other agency of the US government.
A.K. Rudman Spergel co-authorship of this publication does not necessarily constitute endorsement by the NIAID, the NIH or any other agency of the US government.
N. Rogers has no conflict of interest to report.
D.C. Babineau has no conflict of interest to report.
W.G.Shreffler reports grants from Aimmune, Angany, DBV, FARE, FASI, Novartis, NIAID, Regeneron, personal fees from Aimmune, DBV, Merk, Novartis, Regeneron and UpToDate
S.H. Sicherer reports royalty payments from UpToDate and from Johns Hopkins University Press; grants to his institution from the National Institute of Allergy and Infectious Diseases, and from Food Allergy Research and Education; and personal fees from the American Academy of Allergy, Asthma and Immunology as Deputy Editor of the Journal of Allergy and Clinical Immunology: In Practice, outside of the submitted work.
J.M.Spergel reports grant support from Novartis, Regeneron-Sanofi, National Institute of Allergy and Infectious Diseases and Food Allergy Research Education; royalties from Uptodate; and consultant agreements from Regeneron, Sanofi, and Novartis.
B.P.Vickery reports grants from Abbott, grants and personal fees from Aimmune, grants from Alladapt, personal fees from AllerGenis, personal fees from Aravax, grants and personal fees from DBV, grants and personal fees from FARE, grants from Genentech, personal fees from Moonlight Therapeutics, grants from NIH-NIAID, personal fees from Reacta Biosciences, grants and personal fees from Regeneron, grants from Siolta.
R.S. Chinthrajah reports grants from NIAID, CoFAR, Aimmune Therapeutics, DBV Technologies, Astellas, Regeneron, FARE, Stanford Maternal and Child Health Research Institute (MCHRI); is an advisory board member for Alladapt Therapeutics, Novartis, Genentech, Sanofi, Allergenis, Intrommune Therapeutics, and Nutricia, outside the submitted work.
R.A. Wood receives research support from NIAID, Aimmune, Astellas, DBV, FARE, Genentech, Novartis, Regeneron, and Siolta, and royalties from Up To Date
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Warren C, Dyer A, Lombard L, Dunn-Galvin A, Gupta R. The Psychosocial Burden of Food Allergy Among Adults: A US Population-Based Study. The journal of allergy and clinical immunology in practice. 2021;9(6):2452–60 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyer R, Wright K, Vieira MC, Chong KW, Chatchatee P, Vlieg-Boerstra BJ, et al. International survey on growth indices and impacting factors in children with food allergies. Journal of human nutrition and dietetics : the official journal of the British Dietetic Association. 2019;32(2):175–84. [DOI] [PubMed] [Google Scholar]
- 3.Dyer AA, Negris OR, Gupta RS, Bilaver LA. Food allergy: how expensive are they? Current opinion in allergy and clinical immunology. 2020;20(2):188–93. [DOI] [PubMed] [Google Scholar]
- 4.Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the Diagnosis and Management of Food Allergy in the United States: Summary of the NIAID-Sponsored Expert Panel Report. The Journal of allergy and clinical immunology. 2010;126(6):1105–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark S, Espinola J, Rudders SA, Banerji A, Camargo CA Jr Frequency of US emergency department visits for food-related acute allergic reactions. The Journal of allergy and clinical immunology. 2011;127(3):682–3. [DOI] [PubMed] [Google Scholar]
- 6.Dyer AA, Lau CH, Smith TL, Smith BM, Gupta RS. Pediatric emergency department visits and hospitalizations due to food-induced anaphylaxis in Illinois. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2015;115(1):56–62. [DOI] [PubMed] [Google Scholar]
- 7.Dunn Galvin A, Hourihane JO. Psychosocial Mediators of Change and Patient Selection Factors in Oral Immunotherapy Trials. Clinical reviews in allergy & immunology. 2018;55(2):217–36. [DOI] [PubMed] [Google Scholar]
- 8.Investigators PGoC Vickery BP, Vereda A Casale TB, Beyer K du Toit G, et al. AR101 Oral Immunotherapy for Peanut Allergy. The New England journal of medicine. 2018;379(21):1991–2001. [DOI] [PubMed] [Google Scholar]
- 9.Keet CA, Frischmeyer-Guerrerio PA, Thyagarajan A, Schroeder JT, Hamilton RG, Boden S, et al. The safety and efficacy of sublingual and oral immunotherapy for milk allergy. The Journal of allergy and clinical immunology. 2012;129(2):448–55, 55 e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burks AW, Jones SM, Wood RA, Fleischer DM, Sicherer SH, Lindblad RW, et al. Oral immunotherapy for treatment of egg allergy in children. The New England journal of medicine. 2012;367(3):233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soller L, Abrams EM, Carr S, Kapur S, Rex GA, Leo S, et al. First Real-World Safety Analysis of Preschool Peanut Oral Immunotherapy. The journal of allergy and clinical immunology in practice. 2019;7(8):2759–67 e5. [DOI] [PubMed] [Google Scholar]
- 12.Wasserman RL, Factor J, Windom HH, Abrams EM, Begin P, Chan ES, et al. An Approach to the Office-Based Practice of Food Oral Immunotherapy. The journal of allergy and clinical immunology in practice. 2021;9(5):1826–38 e8. [DOI] [PubMed] [Google Scholar]
- 13.Wasserman RL, Hague AR, Pence DM, Sugerman RW, Silvers SK, Rolen JG, et al. Real-World Experience with Peanut Oral Immunotherapy: Lessons Learned From 270 Patients. The journal of allergy and clinical immunology in practice. 2019;7(2):418–26 e4. [DOI] [PubMed] [Google Scholar]
- 14.Wasserman RL, Factor JM, Baker JW, Mansfield LE, Katz Y, Hague AR, et al. Oral immunotherapy for peanut allergy: multipractice experience with epinephrine-treated reactions. The journal of allergy and clinical immunology in practice. 2014;2(1):91–6. [DOI] [PubMed] [Google Scholar]
- 15.Pajno GB, Fernandez-Rivas M, Arasi S, Roberts G, Akdis CA, Alvaro-Lozano M, et al. EAACI Guidelines on allergen immunotherapy: IgE-mediated food allergy. Allergy. 2018;73(4):799–815. [DOI] [PubMed] [Google Scholar]
- 16.Begin P, Chan ES, Kim H, Wagner M, Cellier MS, Favron-Godbout C, et al. CSACI guidelines for the ethical, evidence-based and patient-oriented clinical practice of oral immunotherapy in IgE-mediated food allergy. Allergy, asthma, and clinical immunology : official journal of the Canadian Society of Allergy and Clinical Immunology. 2020;16:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pajno GB, Bernardini R, Peroni D, Arasi S, Martelli A, Landi M, et al. Clinical practice recommendations for allergen-specific immunotherapy in children: the Italian consensus report. Italian journal of pediatrics. 2017;43(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martorell A, Alonso E, Echeverria L, Escudero C, Garcia-Rodriguez R, Blasco C, et al. Oral Immunotherapy for Food Allergy: A Spanish Guideline. Egg and Milk Immunotherapy Spanish Guide (ITEMS GUIDE). Part II: Maintenance Phase of Cow Milk (CM) and Egg Oral Immunotherapy (OIT), Special Treatment Dosing Schedules. Models of Dosing Schedules of OIT With CM and Egg. Journal of investigational allergology & clinical immunology. 2017;27(5):279–90. [DOI] [PubMed] [Google Scholar]
- 19.Ebisawa M, Ito K, Fujisawa T, Committee for Japanese Pediatric Guideline for Food Allergy TJSoPA, Clinical I, Japanese Society of A. Japanese guidelines for food allergy 2020. Allergology international : official journal of the Japanese Society of Allergology. 2020;69(3):370–86. [DOI] [PubMed] [Google Scholar]
- 20.Leroux H, Langlois A, Paradis L, Des Roches A, Begin P. Visual assessment does not reliably predict peanut content in chocolate-covered peanut candies used for oral immunotherapy. The journal of allergy and clinical immunology in practice. 2020;8(1):368–70. [DOI] [PubMed] [Google Scholar]
- 21.Mack DP, Foster GA, Mack JD, Mack LC, Hanna MA. Weighing peanut candies used for oral immunotherapy mitigates variable peanut protein dose. The journal of allergy and clinical immunology in practice. 2021;9(1):521–3 e1. [DOI] [PubMed] [Google Scholar]
- 22.Feeney M, Du Toit G, Roberts G, Sayre PH, Lawson K, Bahnson HT, et al. Impact of peanut consumption in the LEAP Study: Feasibility, growth, and nutrition. The Journal of allergy and clinical immunology. 2016;138(4):1108–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukagawa NK, McKillop K, Pehrsson PR, Moshfegh A, Harnly J, Finley J. USDA’s FoodData Central: what is it and why is it needed today? The American journal of clinical nutrition. 2022;115(3):619–24. [DOI] [PubMed] [Google Scholar]
- 24.Hindley JP, Filep S, Block DS, King EM, Chapman MD. Dose of allergens in a peanut snack (Bamba) associated with prevention of peanut allergy. The Journal of allergy and clinical immunology. 2018;141(2):780–2. [DOI] [PubMed] [Google Scholar]
- 25.Abrams EM, Erdle SC, Cameron SB, Soller L, Chan ES. How to Incorporate Oral Immunotherapy into Your Clinical Practice. Current allergy and asthma reports. 2021;21(4):30. [DOI] [PubMed] [Google Scholar]
- 26.Kim EH, Perry TT, Wood RA, Leung DYM, Berin MC, Burks AW, et al. Induction of sustained unresponsiveness after egg oral immunotherapy compared to baked egg therapy in children with egg allergy. The Journal of allergy and clinical immunology. 2020;146(4):851–62 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leonard SA, Sampson HA, Sicherer SH, Noone S, Moshier EL, Godbold J, et al. Dietary baked egg accelerates resolution of egg allergy in children. The Journal of allergy and clinical immunology. 2012;130(2):473–80 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nowak-Wegrzyn A, Bloom KA, Sicherer SH, Shreffler WG, Noone S, Wanich N, et al. Tolerance to extensively heated milk in children with cow’s milk allergy. The Journal of allergy and clinical immunology. 2008;122(2):342–7, 7 e1–2. [DOI] [PubMed] [Google Scholar]
- 29.Filep S, Block DS, Smith BRE, King EM, Commins S, Kulis M, et al. Specific allergen profiles of peanut foods and diagnostic or therapeutic allergenic products. The Journal of allergy and clinical immunology. 2018;141(2):626–31 e7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


