Abstract
The respiratory tree maintains sterilizing immunity against human fungal pathogens. Humans inhale ubiquitous filamentous molds and geographically restricted dimorphic fungal pathogens that form small airborne conidia. In addition, pathogenic yeasts, exemplified by encapsulated Cryptococcus species, and Pneumocystis pose significant fungal threats to the lung. Classically, fungal pneumonia occurs in immune compromised individuals, specifically in patients with HIV/AIDS, in patients with hematologic malignancies, in organ transplant recipients, and in patients treated with corticosteroids and targeted biologics that impair fungal immune surveillance in the lung. The emergence of fungal co-infections during severe influenza and COVID-19 underscores the impairment of fungus-specific host defense pathways in the lung by respiratory viruses and by medical therapies to treat viral infections. Beyond life-threatening invasive syndromes, fungal antigen exposure can exacerbate allergenic disease in the lung. In this review, we discuss emerging principles of lung-specific antifungal immunity, integrate the contributions and cooperation of lung epithelial, innate immune, and adaptive immune cells to mucosal barrier immunity, and highlight the pathogenesis of fungal-associated allergenic disease. Improved understanding of fungus-specific immunity in the respiratory tree has paved the way to develop improved diagnostic, pre-emptive, therapeutic, and vaccine approaches for fungal diseases of the lung.
Keywords: Lung, Pneumonia, Infection, Fungus, Aspergillus, Cryptococcus, Histoplasma, Blastomyces, Talaromyces, Paracoccidioides, Mucorales, Mycosis, Mucomycosis, Immunity, Innate, Macrophage, Neutrophil, Monocyte, T cell, Lymphocyte
1. Common human fungal pathogens and fungus-associated diseases in the lung
Many fungi form small airborne propagules, termed conidia (i.e., vegetative spores) for dispersal in habitats on Earth. Humans and terrestrial animals inhale fungal cells daily, with silent and asymptomatic clearance being the norm. Only a small subset of the > 1 million fungal species can cause invasive disease in the lung [1,2]. Chief among these are the filamentous molds (Aspergillus species, Fusarium species, agents of mucormycosis) dimorphic fungi (Blastomyces dermatitidis, Histoplasma capsulatum, Coccidioides immitis and C. posadasii, Paracoccidioides brasiliensis, and Talaromyces marnefeii), the encapsulated yeasts Cryptococcus neoformans and C. gattii, and Pneumocystis jirovecii. Notably, Candida species are not associated with invasive disease in the lower respiratory tract, though can cause mucosal candidiasis in the oropharynx. Table 1 summarizes major human respiratory fungal pathogens, physical attributes of inhaled conidia and associated clinical syndromes. Thermotolerance to human body temperature and the small size of the infectious propagules (typically range from 1 to 10 μm) represent core properties of the respiratory fungal pathogens listed above [3].
Table 1.
Common fungal agents, characteristic growth patterns, associated pulmonary clinical syndromes, and risk factors for human pulmonary mycoses.
| Agent of Lung Disease and Geographic Range | Infectious Propagule | Morphotype | Pulmonary Clinical Syndromes | Common Patient Populations |
|---|---|---|---|---|
|
Aspergillosis
Aspergillus fumigatus, A. flavus, A. niger, A. terreus, A. versicolor, A. ustus, A. lentulus, A. nidulans (chronic granulomatous disease) Range: Ubiquitous |
Airborne conidia (2–3 μm diameter) | Mold forms tissue-invasive hyphae |
Pneumonia Tracheobronchitis (CNS dissemination) Chronic cavitaryaspergillosis (hyphae in pre-existing lung cavities) Allergenic disease Allergic bronchopulmonary aspergillosis (ABPA), extrinsic allergic alveolitis |
Hematologic malignancy or aplastic anemia, hematopoietic cell transplant; lung and heart transplant, patients with severe COVID-19 or severe influenza, chronic granulomatous disease. Patients with structural lung damage, COPD, prior tuberculosis, bronchiectasis, sarcoidosis ABPA occurs primarily in patients with atopic asthma and cystic fibrosis |
|
Mucormycosis
(Rhizopus, Mucor,Rhizomucor, Lichtheimia spp.) Range: Ubiquitous |
Conidia (variable size based on species) | Mold | Pneumonia | Acute hematologic malignancy or bone marrow failure, hematopoietic cell transplant recipients; lung and heart transplant recipients, patients with severe COVID-19, diabetic ketoacidosis. |
|
Cryptococcosis
(Cryptococcus neoformans,C. gattii) Range: C. neoformans: ubiquitous, C. gattii: Australia, Papua New Guinea,US Pacific Coast and British Columbia |
Desiccated yeast cells | Encapsulated yeast (variable capsule size, rare Titan cells up to 100 μm diameter) | Pneumonia (CNS dissemination) | Patients with HIV/AIDS, patients on lymphodepleting therapies for hematologic malignancies. |
|
Blastomycosis
(Blastomyces dermatitidis) Range: Eastern US and Canada; emergomycosis in Africa |
Conidia (2–10 μm) | Dimorphic, 8–12 μm yeast with broad-based buds in tissue | Pneumonia (self-limiting to life-threatening disease, mucosal disease, skin) | Patients with HIV/AIDS, 50% of cases in immune competent patients; patients of Laotian ancestry in endemic area |
|
Coccidioidomycosis
(Coccidioides immitis, C. posadasii) Range: CA, OR, WA, AZ, Mexico |
Arthroconidia (3 × 5 μm, boxcar-shaped) | Dimorphic, 20–80 μm thick-walled spherules contain 2–5 μm yeast cells in tissue | Pneumonia (self-limiting to life-threatening disease, mucosal disease, CNS, erythema nodosum) | Patients with HIV/AIDS, patients on lymphodepleting therapies. Pregnant women, African-American and Filipino ancestry in endemic area |
|
Histoplasmosis
(Histoplasma capsulatum) Range: OH and MS Valleys in US, Canada, Central and South America, sub-Saharan Africa, SE Asia, Australia |
Conidia (microconida & macroconidia) | Dimorphic, Intracellular yeasts tissue; Hc var. capsulatum 2–4 μm; Hc var. duboisii 8–15 μm) | Pneumonia (self-limiting to life-threatening disease) | Patients with HIV/AIDS, patients on lymphodepleting therapies. |
|
Paracoccidioidomycosis
(Paracoccidioides brasiliensis) Range: Central and South America (20th parallel north to 35th parallel south of equator) |
Conidia | Dimorphic, yeast cells with multiple buds in tissue | Pneumonia (mucosal disease, skin) | Patients with HIV/AIDS, patients on lymphodepleting therapies. |
|
Talaromycosis
(Talaromyces marneffei) Range: SE Asia |
Conidia | Dimorphic, yeast cells in tissue | Pneumonia (mucosal disease) | Patients with HIV/AIDS, patients on lymphodepleting therapies. |
|
Pneumocystosis
(Pneumocystis jirovecii) Range: Likely ubiquitous, non-culturable, no known reservoir outside of humans |
Unknown | Trophozoites, Cysts | Pneumonia (characterized by hypoxemia) | Patients with HIV/AIDS, patients on lymphodepleting therapies. |
The most common clinical syndrome associated with respiratory fungal pathogens is pneumonia, characterized by the triad of fever, cough, and radiographic findings of lower respiratory tract disease. Hypoxemic respiratory failure is a hallmark of Pneumocystis pneumonia and is uncommon in fungal pneumonia caused by filamentous molds. Fungal pneumonia occurs primarily in immune compromised individuals, in which injury to neutrophils and other myeloid cells is pivotal for the development of invasive mold infections (Aspergillus, agents of mucormycosis). In contrast, injury to T cell function and to the process of granulomatous inflammation is critical for susceptibility to the genera Cryptococcus, Pneumocystis, and dimorphic fungi, all of which represent causative agents of AIDS-defining pulmonary opportunistic infections. Rarely, immune competent individuals can become susceptible to primary lung infections by dimorphic fungal pathogens.
Classic risk factors for pulmonary mold infections include prolonged neutropenia and/or monocytopenia (e.g., GATA2 deficiency), functional myeloid cell defects (e.g., chronic granulomatous disease), receipt of systemic corticosteroids, and diabetic ketoacidosis (agents of mucormycosis). Novel risk groups have emerged due to advances in medical technologies, specifically small molecule targeted biologics that interfere with fungal surveillance pathways beyond their intended role to treat autoimmunity and cancer [4,5]. Patients with severe viral infections, in particular influenza [6] and COVID-19 [7], as well as ICU and COPD patients all exhibit heightened risk for lung fungal infections.
Recent years have seen a rapid advance in our understanding of molecular and cellular response pathways to pulmonary fungal pathogens, driven in part by the study of immune deficiency disorders that confer susceptibility to these infections, the development of appropriate mouse models of disease, and the creation of fungal antigen-specific T cells to examine cell-mediated immunity and experimental vaccine immunity to these pathogens. This review focuses on broad themes that have arisen in studies of pulmonary antifungal immunity. We emphasize the role of different anatomic sites and cellular crosstalk in maintaining sterilizing anti-fungal immunity in the lung and in the pathogenesis of fungus-associated allergenic disease. The reader is referred to outstanding reviews that summarize host genetic risk and pharmacologic susceptibility to pulmonary mycoses [8,9].
2. Architecture of the lung
Humans breathe 10–15 m3 of air daily. Inhaled air passes through the upper respiratory tract (URT) that includes the nasal cavity, pharynx, and larynx, past the vocal cords, and into the lower respiratory tract (LRT). The airways in the URT consist primarily of ciliated columnar epithelial cells, secretory Goblet cells, chemosensory Tuft cells (aka brush cells), pulmonary neuroendocrine cells, and undifferentiated basal cells (Fig. 1). The mucociliary elevator harnesses the rhythmic, beating action of ciliated epithelial cells and mechanical entrapment of inhaled particles in the mucus layer to clear and expel inhaled particles (e.g., fungal cells, dust, and allergens) via cough.
Fig. 1.
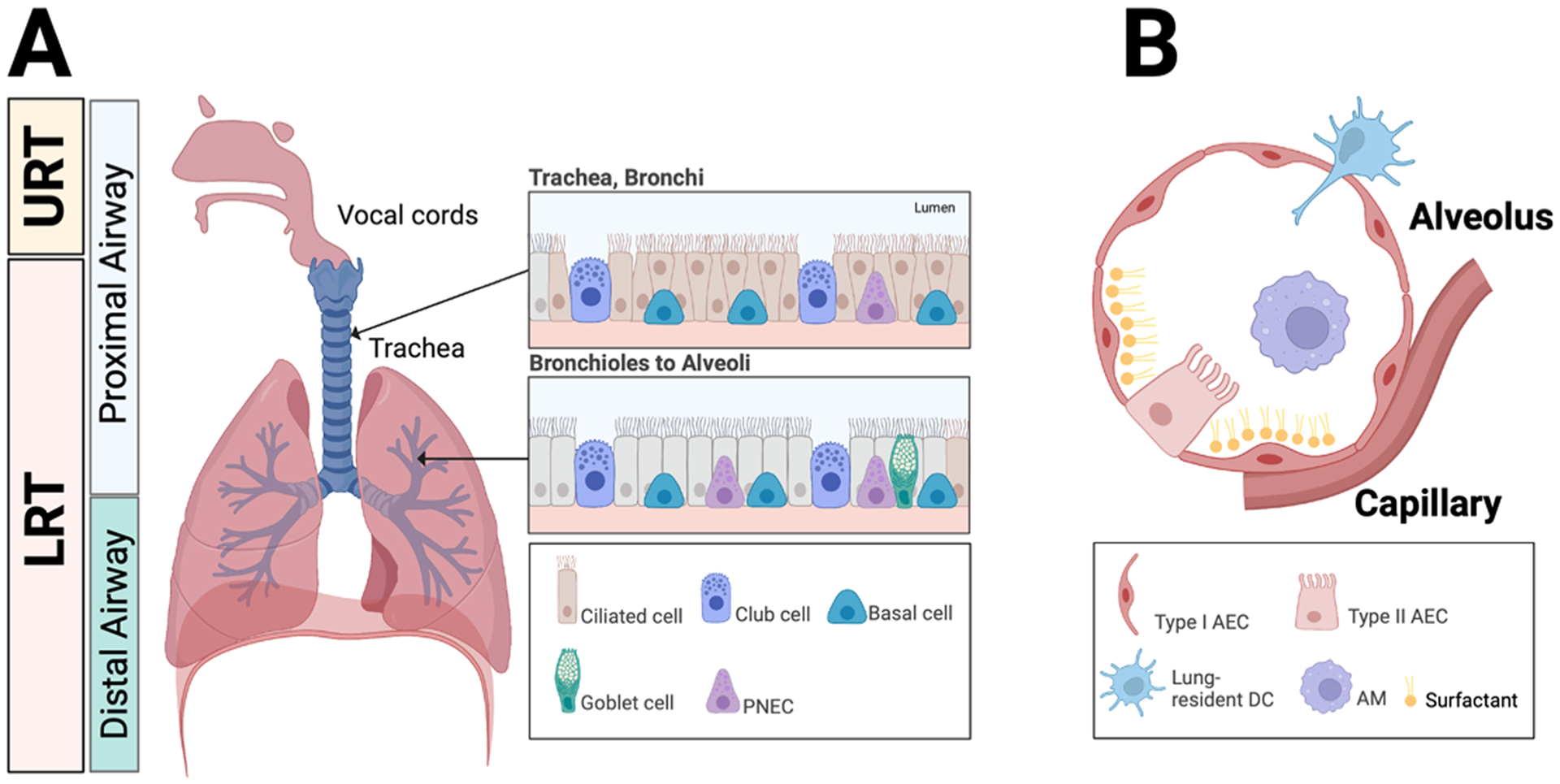
Anatomy of the respiratory tract and lung. A. The vocal cords in the human respiratory tract form the boundary between the upper and lower respiratory tracts. Proximal airways function largely to conduct air to the distal airways. Distal airways are comprised of the respiratory bronchioles, alveolar ducts, and alveoli where gas exchange occurs. The inset depicts the epithelial architecture of the trachea and bronchi (upper panel) as well as the bronchioles (lower panel). PNEC, pulmonary neuroendocrine cell. B. The schematic depicts the cellular architecture of alveoli that includes type I and type II alveolar epithelial cells (AEC), alveolar macrophages, surfactant, and lung-resident dendritic cells. Gas exchange occurs via adjacent capillaries.
The conducting airways of the LRT consist of the trachea, bronchi, and bronchioles. Non-ciliated club cells join epithelial and secretory cells to form the lining of bronchi and bronchioles. Basal cells in the tracheobronchial zone can regenerate columnar epithelial cells following tissue injury. In humans and other large mammals, proximal conducting airways transition into the distal respiratory zone, which consists of respiratory bronchioles and alveolar ducts. Finally, air reaches the terminal alveolar sacs. Gas exchange and blood oxygenation occurs in this distal airway and alveolar respiratory zone, with a 1 μm cellular barrier separating the alveolar lumen from lung capillaries. The human lungs contain approximately 300 million alveoli that measure 400–450 μm in diameter [10]. Type I alveolar epithelial cells that derive from surfactant-producing type II alveolar epithelial cells collectively form the cellular lining in alveoli that are surrounded by capillaries, thin interstitial tissue, and nerve cells.
Single-cell sequencing approaches have identified rare cell types in human and murine airways. In the trachea, Foxj1+ pulmonary ionocytes express the cystic fibrosis transmembrane regulator including multi-potent respiratory airway secretory cells that give rise to type II alveolar epithelial cells via Notch and Wnt signaling; these cells can be distinguished from other secretory cells by a distinct secretoglobulin (SCGB) expression profile [11]. Ascl1+ pulmonary neuroendocrine cells (<0.5 % of airway epithelium) receive innervation from efferent and afferent neurons, stimulate type 2 innate lymphoid cells via calcitonin gene-related peptide release, and amplify allergic asthma responses [12]. In general, the functional role of these rare lung cell types in antifungal immunity and fungus-associated allergenic disease remains to be elucidated.
The respiratory tree harbors collections of steady-state and inducible immune cell populations. Frontline, steady-state immunity includes the positioning of alveolar (AMs) and interstitial macrophages (IMs), conventional dendritic cell (cDC) 1 and 2 subsets either in or adjacent to the airways. Single-cell technologies and the use of fluorescent reporters have revealed heterogeneity among previously uniform cell populations. For example, during Aspergillus and Cryptococcus infection AMs segregate into CXCL2+ and into CXCL2− subsets [13]. CXCL2+ AMs appear to be pro-inflammatory in phenotype while their CXCL2− counterparts are characterized by production of C1q and the regulatory cytokine IL-10. CXCL2− AMs exhibit a transcriptional profile that is more consistent with a regulatory phenotype [13]. Thus, AMs assume context-specific roles as sentinels in the induction of pro-inflammatory responses as well as in the maintenance of tolerance and tissue homeostasis.
The lung environment rapidly and profoundly alters its composition after fungal pathogens trigger an inflammatory response. One example is the presence of inducible tissue-resident immune cells, e.g., mucosa-associated (MALT) and bronchus-associated lymphoid tissue (BALT), that collectively play an important role in immune surveillance and in the rapid orchestration of innate and adaptive immunity to fungal pathogens. In upper airways, microfold cells (M cells) associate with MALT and can facilitate the entry and dissemination of bacterial pathogens [14]. Fungal recognition and growth in the lung trigger the rapid deployment of circulating immune cells, primarily neutrophils and monocytes, to the site of infection, leading to the induction of fungal antigen-specific immune responses. In the ensuing sections, the review will focus on lung-specific mechanisms of host defense and allergy and on the role of crosstalk between lung parenchymal cells and immune cells in these processes.
3. Fungal recognition and innate immune activation in the lung
Although pulmonary fungal pathogens vary widely in cellular morphology, the cell wall consists primarily of conserved polysaccharides that include immune-reactive β-1,3- and β-1,6-linked glucans, chitin, and mannans. Typically, chitin is found in the innermost proximal layer of the cell wall that extends over the plasma membrane, with β-glucans found in intermediate layers, and mannans and mannoproteins in more external layers. The reader is referred to excellent reviews that summarize current knowledge of fungal cell wall biogenesis and assembly [15].
The cell wall of Aspergillus species illustrates dynamic changes that occur during fungal cell growth in the lung. Inhaled A. fumigatus conidia contain a proteinaceous rodlet layer and a dihydroxynaphthalene (DHN)-melanin pigment, both of which are shed to expose β-glucans during the process of germination. Following germination, A. fumigatus hyphae secrete an exopolysaccharide, galactosaminogalactan (GAG), which confers adhesive properties and promotes resistance to neutrophil extracellular traps, as discussed below [16]. GAG can activate inflammasomes in macrophages through its binding to ribosomal proteins, likely inducing pyropoptic host cell death [17], though its mechanism of entry into host cells remains undefined. Most lung fungal pathogens also synthesize polysaccharide moieties that do not trigger robust innate immune responses, exemplified by α-glucans. In the case of Histoplasma, an external α-glucan layer can shield β-glucans and undergo active remodeling by glucanases [18]. The encapsulated yeast Cryptococcus forms a large glucuronoxylomannan and galactoxylomannan capsule which conceals underlying immune-reactive glycoprotein components and blunts host innate immune activation [19].
Within the respiratory tree, inhaled fungal cells are opsonized with soluble pattern recognition receptors, surfactant, complement, and antibodies. These interactions facilitate the induction of antifungal effector systems and signaling responses by membrane-bound receptors. For example, the soluble receptor pentraxin-3 (PTX3) binds to galactomannan found on Aspergillus conidia and promotes FcγRIIA (Cd32)-dependent conidial uptake and subsequent inactivation by neutrophils [20]. Ptx3 polymorphisms are associated with susceptibility to invasive pulmonary aspergillosis in bone marrow transplant patients, underscoring the importance of this molecule in pulmonary antifungal immunity [21,22]. The soluble mannose receptor binds to mannose moieties on fungal cells, though genetic deficiency does not increase mortality in a murine model of Pneumocystis pneumonia [23], consistent with functional redundancy in vivo.
Surfactant consists primarily of phosphatidylcholine (approx. 80%), phosphatidylglycerol (5–10%), cholesterol (5–10%), and surfactant proteins A-D (2–5%). Genetic deletion of individual surfactant proteins causes mild or no defects in antifungal immunity in mouse models of aspergillosis [24]. In fact, surfactant protein D can be harnessed by C. neoformans for protection against oxidative damage [25]. However, pulmonary alveolar proteinosis (PAP) can lead to substantial susceptibility to lung fungal infections by dimorphic fungi and, more rarely, by filamentous molds [26]. PAP patients accumulate enormous quantities of surfactant due to defective granulocyte-macrophage colony-stimulating factor (GM-CSF)-dependent surfactant catabolism by alveolar macrophages. These studies link surfactant homeostasis to antifungal immunity in the lung.
Although terminal complement deficiency has not been linked to susceptibility to fungal disease, the complement inhibitor eculizumab, a treatment for hemolytic uremic syndrome and paroxysmal nocturnal hemoglobinemia, is associated with invasive pulmonary fungal infections [27–30]. Thus, pharmacologic C5a blockade likely impairs myeloid phagocyte recruitment and activation in response to fungal exposure. The inbred DBA/2 and A/Sn mouse strains lack C5 complement and represent susceptible strains compared to resistant C57BL/6 and Balb/c mice in a corticosteroid model of aspergillosis, with delayed lung neutrophil influx as a key difference in the host response [31]. Aspergillus secreted proteases have the capacity to cleave human complement proteins [32,33] which reduces conidial complement opsonization and may represent an immune evasion mechanism.
The membrane-spanning C-type lectin receptors (CLRs) Dectin-1 (Clec7a), Dectin-2 (Clec6a in humans, Clec4n in mice), and Dectin-3 (Clec4d) recognize fungal β-glucans (Dectin-1) and mannans/mannoproteins (Dectin-2/−3), respectively, that are widely exposed by fungal pathogens during growth and morphologic transitions that occur in the respiratory tree (see [34] for detailed review on CLR signaling). For example, Coccidioides arthroconidia expose β-glucans prior to their dimorphic transition into mature spherules that contain endospores. Recent work has expanded the repertoire of CLR ligands expressed by lung fungal pathogens. For example, an O-linked C-terminal mannan moiety on Blastomyces endoglucanase acts as a novel Dectin-2 ligand and potential vaccine adjuvant [35,36]. Dectin-1, −2, and −3 signaling converge on spleen tyrosine kinase, protein kinase C-δ, and CARD9, resulting in canonical NF-κB activation to promote the release of pro-inflammatory cytokines that include TNF, pro-IL-1β, IL-6, and neutrophil-and monocyte-recruiting chemokines such as CXCL2 and CCL2. CARD9 signaling further controls the induction of T helper 17 cells during respiratory and systemic fungal infections [37]. Beyond Dectin-1, CD23 (Fcer2) [38], a low affinity IgE receptor, EphA2 [39–41], and lactosyl ceramide [42] may bind to fungal β-glucans and initiate inflammatory responses, though the in vivo role of these receptors and contributions to collective β-glucan-sensing in pulmonary host defense remain largely undefined.
Brown and colleagues functionally characterized Mel-Lec (Clec1a), a CLR that binds fungal DHN-melanin [43]. In contrast to Dectin-1, -2, and -3 which are primarily expressed by murine lung-resident macrophages and dendritic cells as well as lung-infiltrating monocytes and neutrophils, Mel-Lec is expressed by murine lung endothelial cells and protects against disseminated aspergillosis, but not against pulmonary aspergillosis in otherwise immune competent mice. In humans, myeloid cells exhibit Mel-Lec expression, though the downstream signaling events remain unknown. The identification of a Clec1a polymorphism as a risk factor for aspergillosis in hematopoietic cell transplant patients argues for a functional role in host defense [43].
CARD9 deficiency is the only known primary immune deficiency disorder that exclusively manifests with life-threatening invasive fungal disease, particularly at extrapulmonary sites including the brain, dermis, and visceral organs [44–47]. These clinical observations imply that alternate innate defense pathways can compensate for CARD9 defects more effectively in the respiratory tree than at extrapulmonary sites. The rapid release of IL-1α following pulmonary Aspergillus infection represents an alternate mechanism of rapid innate immune activation, in parallel to the NLRP3 and AIM2 inflammasome-dependent activation of caspase-1 and the induction of caspase-8 activity which results in IL-1β release [41,48]. IL-1α and IL-1β activate IL-1 receptor/MyD88 signaling in radioresistant lung epithelial cells to regulate the quantity and anti-fungal activities of myeloid phagocytes, as discussed in the ensuing section.
4. The epithelium and orchestration of antifungal Immunity
The respiratory epithelium plays a key role in the initiation and amplification of the innate immune response to lung fungal pathogens. During Aspergillus infection, chemokine-dependent neutrophil recruitment occurs in two distinct phases. First, IL-1R/MyD88 signaling in club cells leads to the rapid production of CXCL1 and CXCL5 and the initial neutrophil influx into infected airways [49] (Fig. 2A). Second, Card9 signaling in leukocytes sustains chemokine-dependent neutrophil recruitment, highlighting compartment-specific and collaborative control of neutrophil influx in the fungus-infected lung [49]. Beyond IL-1R/MyD88- and CARD9-dependent neutrophil recruitment, 5-lipoxygenase (5-LO) activity in hematopoietic cells generates leukotriene B4 (LTB4) which can mediate neutrophil swarming via LTB4 receptor signaling and promote neutrophil influx in the Aspergillus-infected lung [50,51]. Lung stroma-derived soluble galectin-3 further promotes neutrophil homing into infected airways [52].
Fig. 2.
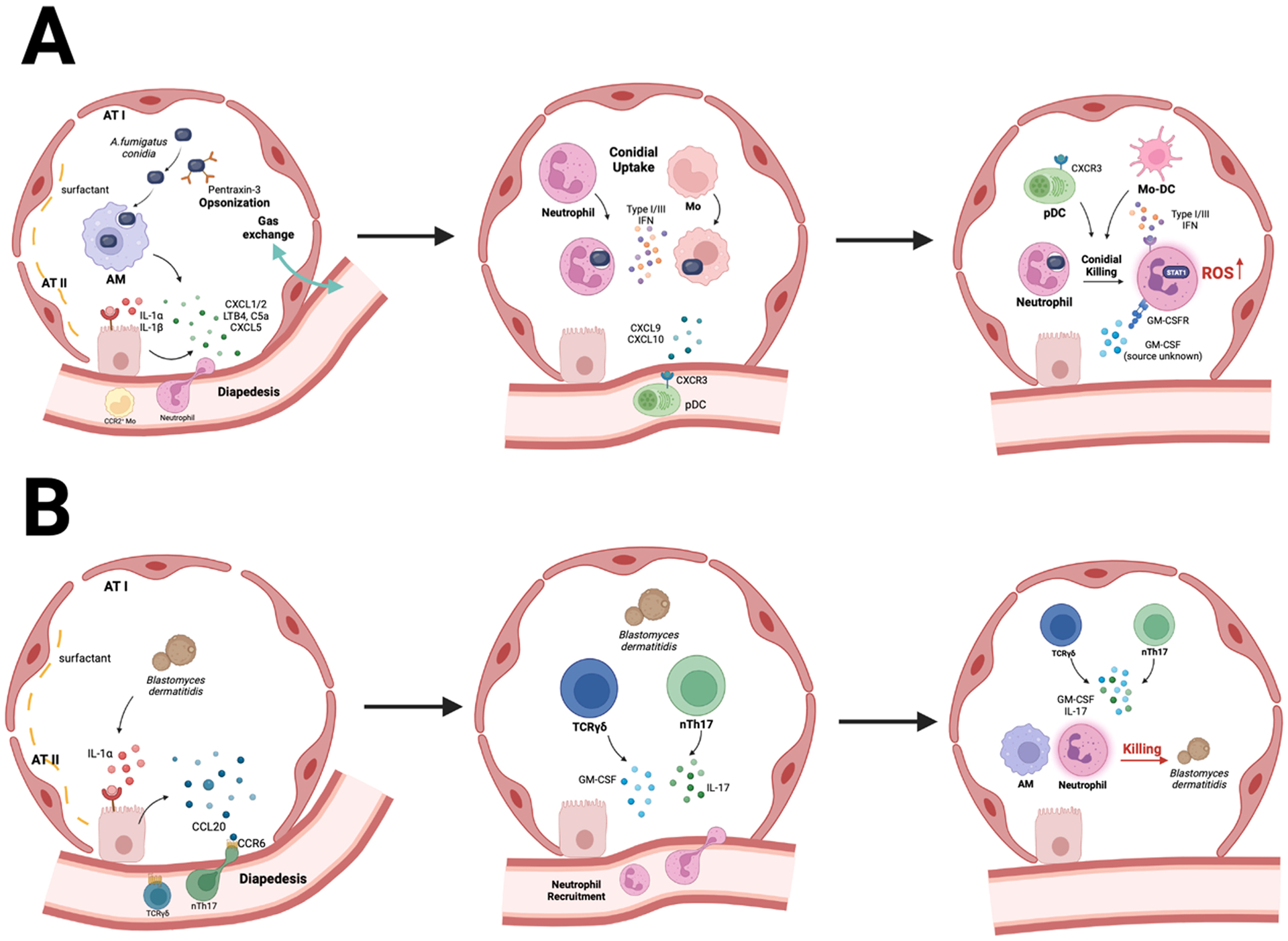
Immune crosstalk is a central feature of pulmonary antifungal immunity. A. Aspergillus conidia induce the rapid release of IL-1 α/β by lung-resident AMs and DCs. This process activates IL-1R/MyD88 signaling in pulmonary epithelial cells which causes the release of neutrophil chemoattractants, primarily CXCL1 and CXCL5. CXC- chemokines collaborate with LTB4, C5a, and galectin-3 to mediate neutrophil influx to infected airways. CCL2, –7, and –12 mediate the ensuing influx of CCR2+ monocytes into the lung parenchyma. Neutrophils, monocytes, and monocyte-derived dendritic cells (Mo-DCs) engulf conidia into phagolysosomes and inactivate fungal cells via products of NADPH oxidase. Monocyte-regulated type I and type III IFNs enhance fungal killing. Fungus-engaged neutrophils and Mo-DCs release CXCL9 and CXCL10 which recruits CXCR3+ plasmacytoid DCs from the circulation into the lung. Plasmacytoid DCs (pDC) do not bind conidia but enhance the oxidative burst to boost conidial killing in neutrophils. GM-CSF enhances this process as well. B. During Blastomyces infection, early IL-1α/β release triggers epithelial CCL20 production which acts on CCR6+ ILCs and mediates their trafficking to the infected lung. ILCs and natural T helper 17 cells produce IL-17 and GM-CSF which potentiate yeast cell killing by neutrophils and Mo-DCs.
In addition to its role in neutrophil trafficking, the pulmonary epithelium orchestrates myeloid phagocyte effector activity and adaptive immune responses. During Blastomyces infection, IL-1R and NF-κB signaling in lung epithelial cells releases CCL20 that regulates the influx of CCR6+ innate lymphoid cells (ILCs) (Fig. 2B). ILCs and natural T helper 17 cells produce IL-17A and GM-CSF that enhances fungal killing by phagocytic cells [53]. Binding of Pneumocystis to type II alveolar epithelial cells induces IL-1R and NF-κB signaling that regulates T helper 17 and B cell adaptive immune responses required for fungal clearance [42,54–56]. Pulmonary infection with Cryptococcus neoformans induces epithelial cell production of IL-25, and disruption of IL-25R signaling in Il17rb−/− mice improves survival by suppressing T helper 2 responses, promoting T helper 1 responses, and reducing dissemination of the fungus to the central nervous system [57]. Thus, cytokine-driven epithelial activation is a key feature of immune activation and fungal clearance in the respiratory tree.
The role of the respiratory epithelium and its cellular constituents in direct fungal sensing and recognition remains poorly defined, despite the discovery that human epithelial cells in bronchioles and alveoli can express Dectin-1 [58]. To study these interactions, researchers have turned to cell lines (e.g., BEAS-2B cells as a model for human bronchial epithelial cells or A549 adenocarcinoma cells as a model for type II alveolar epithelial cells) and to air-liquid interface (ALI) cultures of primary airway epithelial cells [59]. Aspergillus can induce Dectin-1 expression in a human bronchial epithelium cell line in a TLR2-dependent manner, resulting in the production of pro-inflammatory cytokines such as TNF, antimicrobial peptides, and reactive oxygen species (ROS) [60]. Dectin-1 facilitates the uptake of Aspergillus conidia into A549 cells through phospholipase D signaling [61,62]. While the majority of Aspergillus conidia ingested by A549 cells are killed, surviving conidia can germinate and form invasive hyphae [63]. Blocking Dectin-1 protects A549 cells from monolayer detachment upon challenge with Aspergillus, but infected Clec7a−/− mice demonstrate worse pulmonary epithelial damage than control mice [62]. Studies with epithelial cells maintained in ALI cultures indicate that an Aspergillus cell wall component initiates neutrophil recruitment and trans-migration to the apical airway surface [59], implying that direct epithelial fungal sensing may facilitate leukocyte chemotaxis across epithelial layers. While these results suggest that pulmonary epithelial cells may participate in beneficial or deleterious immune responses during fungal infections, it remains largely unproven whether fungal sensing or uptake by epithelial cells represents a core feature of the pulmonary immune response, since in vitro observations may not recapitulate in vivo properties of primary lung epithelial cells. Definitive experimental evidence for candidate fungal receptors and signal transducers rests on conditional gene deletion strategies in defined lung epithelial subsets and rigorous analysis of infectious outcomes in relevant animal models.
5. Epithelial invasion and tissue damage during mucormycosis
Epithelial and endothelial cells can be hijacked by invading fungi to facilitate tissue invasion and necrosis, most prominently by angioinvasive Mucorales fungi. These agents of mucormycosis cause devastating, disfiguring rhino-orbital infections in patients with uncontrolled diabetic ketoacidosis (DKA) as well as pulmonary infections in patients with hematologic malignancies. Biochemical pull-down assays initially identified glucose-regulated protein 78 (GRP78) as an endothelial cell receptor for Rhizopus oryzae [64]. GRP78 binding to spore coat protein homologs 2 and 3 (CotH2, CotH3) facilitated fungal endocytosis by endothelial cells, leading to tissue damage [64,65]. Mucorales fungi also produce mucoricin, a 17 kDa ricin-like toxin that injures cultured lung epithelial cells by damaging membrane permeability and impairing protein synthetic function [66]. Treatment of infected DKA mice with antibodies against GRP78, CotH, or mucoricin significantly improved survival rates [64].
Further research has advanced the concept that differential expression of fungal receptors by epithelial cells along the respiratory tract may determine the site of fungal invasion. Under hyperglycemic conditions, cultured nasal epithelial cells highly upregulate GRP78 which binds to R. delemar via CotH3 [67]. In contrast, A549 cells express the integrin α3β1 that binds to R. delemar via CotH7, and the expression of α3β1 is not regulated by DKA conditions. Infected, neutropenic mice have improved survival when treated with antibodies against the integrin β1 subunit [67]. Similarly, the Aspergillus thaumatin-like protein CalA protein can bind to the α5β1 integrin that is expressed by epithelial and endothelial cells. The ΔCalA deletion mutant exhibits reduced virulence in a corticosteroid model of disease [68]. These findings may explain why patients with hematologic malignancies tend to develop pulmonary, rather than rhino-orbital mold infections, unlike patients with DKA. Thus, Mucorales and other molds can hijack cellular receptors within epithelial and endothelial cells in the respiratory tree to cause local tissue damage and necrosis.
6. Innate antifungal effector mechanisms
Sterilizing mucosal barrier immunity in the lungs is critical to preventing invasive fungal infections. For example, patients with chronic granulomatous disease (CGD), a primary immunodeficiency disorder in which NADPH oxidase activity and superoxide anion production is impaired in phagocytic cells, have an exquisite susceptibility to infections by filamentous fungi, particularly Aspergillus and Mucorales, and to systemic Candida infections [69]. During a robust host immune response, myeloid cell-derived ROS induce a form of regulated cell death in Aspergillus conidia [70]. Thus, host-derived ROS can exploit gene-dependent cell death pathways in fungi to mediate sterilizing immunity in the respiratory tree. However, the Aspergillus anti-cell death protein Bir1 can counteract this process. Bir1 overexpression promotes fungal survival in myeloid cells and facilitates hyphal tissue invasion [70]. The production of mitochondrial ROS in alveolar macrophages can enhance Aspergillus conidial killing, but catalase-dependent neutralization of mitochondrial-derived H2O2 is insufficient to alter murine susceptibility to aspergillosis, both in the presence and absence of NADPH oxidase activity [71,72].
When neutrophils encounter fungal cells that are too large to phagocytose, NADPH oxidase activity is critical for the formation of neutrophil extracellular traps (NETs) [73]. NETosis is a form of regulated cell death, in which neutrophils extrude chromatin threads decorated with cationic histones and DNA and release proteinaceous chelators of essential ions (e.g., calprotectin which traps Fe2+, Zn2+, and Mn2+) to immobilize fungal filaments and deplete essential nutrients in their immediate environment. [73] The release of extracellular ROS by neutrophils in response to large filaments amplifies IL-1β release which in turn boosts additional neutrophil recruitment to the site of infection [74]. In contrast, fungal cell phagocytosis and the generation of intracellular ROS limit IL-1β release and the ensuing formation of neutrophil clusters [74]. Neutrophils preferentially undergo NETosis in response to Aspergillus hyphal forms over conidia, causing a fungistatic effect that helps to prevent dissemination [75,76].
Nutritional immunity, the sequestration of essential ions from scavenging microbial pathogens, or alternatively, the use of concentrated metal ion levels to create a toxic environment for pathogens, operates within fungus-containing phagosomes as well. Rhizopus conidia establish intracellular persistence in alveolar macrophages but fail to germinate due to intracellular iron restriction [77]. Similarly, neutrophils from CGD patients can restrict Aspergillus growth by iron chelation using secreted lactoferrin [78]. In the case of Histoplasma, GM-CSF-responsive macrophages control the transcription and intracellular localization of metallothioneins and zinc transporters to withdraw zinc from the Histoplasma-containing phagosome, to regulate the activity of a phagosomal proton pump, and to enhance the oxidative burst [79,80].
Fungi have developed ways to subvert host effector mechanisms to their advantage. For example, mold conidia that contain DHN-melanin arrest the process of LC3-associated phagocytosis, delaying glycolytic flux, macrophage phagosomal maturation, and exposure to the respiratory burst [81,82]. During primary infection, B. dermatitidis is resistant to killing by alveolar macrophages because of its ability to inhibit NO production [83], and in a B. dermatitidis vaccination model, inducible NOS (iNOS) deficiency impairs host clearance of the fungus [84]. A Blastomyces di-peptidyl peptidase (i.e., Dpp IVA) can cleave host-derived chemokines that mediate the influx of CCR2+ monocytes. This immune evasion mechanism bypasses their effector activity [85]. Cryptococcus species are facultative intracellular organisms that can harness macrophages to replicate and disseminate in the host [86,87]. Recently, a secreted Cryptococcus protein, Cpl1, was discovered to polarize interstitial macrophages to a non-protective type 2 phenotype in a process that requires TLR4 signaling and facilitates tissue invasion [88]. Talaromyces yeast cells can parasitize macrophages to avoid exposure to neutrophil oxidative products that are toxic to this fungus [89]. In addition, Talaromyces can induce β-glucan-dependent shuttling between neutrophils and macrophages, though the contribution of yeast cell transfer between leukocytes to immune evasion and infectious outcomes remains uncertain [90].
7. Innate immune crosstalk regulates antifungal effector activity in the lung
Emerging studies have advanced the concept that lung-infiltrating myeloid phagocytes engage in multi-directional crosstalk and condition the inflammatory environment in the lung to boost the recruitment and antifungal properties of other innate immune cells. Inflammatory monocytes are important early responders and central players in the control of pulmonary fungal pathogens including Aspergillus, Blastomyces, Cryptococcus neoformans, Coccidioides, and Histoplasma [91]. The role of monocytes in antifungal immunity is protective, with the notable exception of cryptococcosis, in which monocytes may have harmful or protective roles depending on host and fungal factors [92–95]. In the lung, monocytes differentiate into monocyte-derived DCs (Mo-DCs) and macrophages (Mo-Macs) to directly kill fungal cells [91]. Monocytes also direct the recruitment of other innate cells to the site of infection and establish an inflammatory environment in the lung conducive to fungal cell elimination. Important monocyte-derived cytokines and chemokines include TNF, IL-1α/β, CXCL1, CXCL2, CXCL9 and CXCL10, among others [41,96,97]. These inflammatory mediators are important for the establishment of a protective milieu in the lung and enhance the recruitment of other innate cells, including neutrophils. Fungus-engaged Mo-DCs cooperate with fungus-engaged neutrophils to produce CXCL9 and CXCL10 for the CXCR3-dependent recruitment of plasmacytoid DCs in the Aspergillus-infected lung [98] (Fig. 2A).
Beyond their role in innate immune cell recruitment, monocytes and Mo-DCs control a type I and type III interferon cascade in the Aspergillus-infected lung that is essential for the optimal antifungal response of neutrophils [99]. The activation of STAT1-dependent signals on neutrophils downstream of the type III IFN receptor is required for optimal ROS activation and Aspergillus killing [99] (Fig. 2A). Whether this pathway is also involved in the regulation of antifungal neutrophils in response to other fungal pathogens remains to be established, but it is clearly involved in other inflammatory processes, most notably in response to viral infection [100–103]. The monocyte-dependent recruitment of plasmacytoid DCs, prototypic anti-viral and type I IFN-producing cells, into the fungus-infected lung complements and augments monocyte-dependent regulation of neutrophil NADPH oxidase activity. This provides a feedforward amplification mechanism to boost the antifungal properties of neighboring myeloid phagocytes by infected innate immune cells (Fig. 2A). Innate immune crosstalk is multidirectional, with neutrophils also acting as important regulators of other innate cells [104], specifically for the optimal antifungal activation of monocytes which includes monocyte maturation and Mo-DC ROS formation [105]. The common involvement of IFN activation during fungal and viral infections may be an intriguing basis for secondary susceptibility to fungal infection post influenza and SARS-Cov2 infection [106]. Future studies will be needed to unravel the complex mechanisms involved in the maintenance of antifungal immunity in the context of infection with other pathogens.
8. Cell-mediated immunity to lung fungal pathogens and implications for vaccine strategies
Pneumocystis, Cryptococcus, and dimorphic fungal pathogens observed in patients living with AIDS underscores the importance of T cells to anti-fungal immunity. CD4+ T helper cells represent the critical T cell lineage implicated in antifungal immunity or allergy-associated tissue injury (discussed in ensuing section) in three general ways [107]. First, the activation and differentiation of T helper cells into Tbet+ T helper 1 cells depends on fungal antigen transport to draining lymph nodes and interaction with IL-12 producing dendritic cells. In turn, T helper 1 cells migrate to the lung and provide cytokines, primarily IFN-γ, that activate the antifungal activities of macrophages. This circuit of cross-regulation is central to the potential utility of antifungal vaccines [108].
Second, GATA-3+ T helper 2 cells that produce IL-4, IL-5, and IL-13 drive characteristics of allergenic responses that include eosinophilia, Goblet cell hyperplasia, and IgE class switching. Augmentation of the endogenous T helper 2 response either through administration of IL-2/IL-2 antibody complexes or disruption of regulatory T cell-dependent suppression of T helper 2 cells accelerated lethal disease in a model of invasive cryptococcosis [109,110]. Thus, T helper 2 cell responses do not simply fail to eliminate fungi from the respiratory tree but directly contribute to immunopathology associated with uncontrolled pulmonary fungal infection. This concept can be exploited for therapeutic purposes, as illustrated in a recent case of a 4-year-old child with disseminated coccidioidomycosis [111]. This patient suffered from a primary immune deficiency disorder caused by abnormal expression of a short, nonfunctional isoform of the IL-12 receptor, resulting in insufficient type 1 immunity and augmented type 2 immunity. The boy responded clinically to dupilumab, a mAb directed against IL-4 that suppresses type 2 immunity, and recombinant IFN-γ which enhances type 1 immunity.
Third, RORγt+ T helper 17 cells combat extracellular fungi by secreting IL-17 that can stimulate epithelial cells in the oral and respiratory tract to release neutrophil chemokines and to produce antifungal peptides [112]. Tissue-resident γδ T, natural T helper 17, and type 3 ILCs represent additional lymphocyte subsets that contribute to rapid IL-17-dependent host defense in murine models of oropharyngeal candidiasis and pulmonary blastomycosis [53,113–115]. Recent human data indicates that fungus-specific T helper 17 cells may drive pathologic allergenic inflammation as well, as discussed in the ensuing section [116].
T helper cell responses to respiratory fungal infection are typically mixed in character. Innate and adaptive lymphocytes respond to fungal infection and coordinate recruitment of neutrophilic and eosinophilic granulocytes. During cryptococcosis, the granulocyte response can be experimentally manipulated and polarized by interfering with lymphocyte differentiation [117]. Mice with highly polarized eosinophil or neutrophil responses due to lymphocyte subset impairments succumbed more rapidly to infection than wild type mice with a mixture of lymphocytes and a more balanced granulocyte population. With the exception of Histoplasma [118], most fungi can survive and replicate outside of myeloid phagocytes. Thus, balanced T cell responses that target the free-ranging lifestyle of most lung fungal pathogens are likely to be most advantageous to the host.
The development of T helper cell receptor transgenic (TCR-tg) mice has advanced our understanding of T helper cell priming, activation, and memory formation by allowing for the precise tracking of fungal antigen-specific CD4 T cells at various stages of activation, differentiation, and trafficking [119,120]. Following fungal trafficking to lung-draining mediastinal lymph nodes by monocytes and Mo-DCs [121], Aspergillus-specific Af3.16 TCR-tg T helper cells proliferated rapidly, migrated to the infected lung and differentiated primarily into IFN-γ-producing T helper 1 cells independently of TLR/MyD88-mediated signals and into a smaller subset of IL-17-producing T helper 17 cells [119]. Dectin-1-mediated signals modulated T-bet expression in Af3.16 TCR-tg cells and controlled the balance of T helper 1 and T helper 17 differentiation [122]. Intriguingly, Mo-DCs are required for the sustained expression of T-bet and maintenance of T helper 1 differentiation during Aspergillus and Blastomyces lung infection [122,123].
The use of Blastomyces-specific 1807 TCR-tg cells allowed for the identification of broadly protective T helper cells that, following adoptive transfer, controlled infections by multiple dimorphic fungi, including Histoplasma and Coccidioides [120,124]. 1807 TCR-tg cells recognize fungal calnexin, a broadly conserved antigen expressed by fungi that belong to the Ascomycetes family [125]. This finding provides proof-of-principle evidence that a cross-reactive vaccine based on this antigen may protect against multiple fungi via the stimulation of broadly protective T helper cells [126]. The use of TCR-tg cells in these models and in other fungal infections [127] will undoubtedly continue to help advance our mechanistic understanding of critical factors that shape pulmonary antifungal immunity against fungal pathogens.
Despite the potency of cell-mediated immunity and the induction of T cell memory, a select number of fungi cause disease in immunocompetent individuals. Consequently, there is tremendous interest in developing vaccines to primary fungal pathogens, such as Blastomyces, Coccidioides, Histoplasma, and Cryptococcus gattii. While there are currently no approved fungal vaccines, experimental candidates against pulmonary fungal pathogens are summarized in the following review [108]. For pulmonary fungal pathogens, an effective vaccine would prime a robust T cell response, which was largely unachievable prior to the development of encapsulated mRNA-based vaccines for SARS-CoV2 [128]. Due to safety concerns with attenuated live fungal vaccine candidates, significant research effort is directed at the development of protein- or peptide-based subunit vaccines. This approach must overcome the challenge of delivering T cell epitopes that accommodate the enormous human MHC allelic diversity and must be formulated with an adjuvant that provokes strong T helper cell memory responses.
Researchers have made advances to overcome these challenges. In a murine model of cryptococcosis, researchers recently identified fungal peptides from in silico MHC II binding algorithms on the basis of their ability to prime protective T cell responses to lethal pulmonary infection with Cryptococcus [129]. Several peptides successfully protected Balb/c mice in vaccine formulations, but only one provided comparable protection in C57BL/6 mice. Although these studies represent a step forward in identifying protective peptide T cell epitopes, they highlight the challenges and limitations of peptide-based subunit vaccines. Glucan particles have been used as an experimental preclinical vaccine adjuvant that was effective when co-formulated with candidate fungal vaccine strains that protect against lethal Blastomyces, Coccidioides, and Histoplasma challenge [130–132]. As discussed earlier, the Blastomyces mannoprotein endoglucanase-1 acts a Dectin-2 agonist by virtue of C-terminal O-linked mannans and exhibits adjuvant properties to promote experimental vaccine resistance to Blastomyces and Cryptococcus [36]. Thus, the co-development of novel adjuvant and peptide- or protein-based candidates appears to represent the most promising avenue to generate robust vaccine immunity against lung fungal pathogens.
An alternative approach, tested in animal models, is the use of heat-killed, whole cell preparations that contain a complex mixture of immunogenic antigens as well as fungal PAMPs for the activation of innate receptors and the effective programming of protective, antifungal T helper 1 and T helper 17 cells. Several laboratories have shown proof-of-principle data that demonstrate the effective use of heat-killed, mutant Cryptococcus strains as vaccine formulations for the activation of potent protection [133–136] that can operate even in the context of CD4 deficiency [133,135]. Importantly, some Cryptococcus whole cell formulations confer heterologous vaccine-induced protection [134,135, 137]. In the case of the HK-fbp1 vaccine, heterologous protection was observed not only against C. gattii but also against Aspergillus in a drug-induced model of susceptibility [135]. These findings provide support for the development of vaccine formulations capable of protecting against multiple fungal pathogens, even in the context of defined immune deficiency. Unfortunately, a lack of investment in this field by vaccine manufacturers remains a major impediment to the development of a fungal vaccine. The most promising candidate to date, a formulation of the Candida albicans invasin Als3 conjugated with alum demonstrated therapeutic and preventative effects in a phase II trial in vaginal candidiasis [138].
9. Role of fungi in allergenic disease in the lung
Over half of all individuals with allergic asthma are sensitized to fungi or fungal components [139–142]. T helper cells represent central mediators of allergic inflammation; however, unlike anti-fungal immunity that requires balanced T helper 1 and T helper 17 cells, pathologic T helper 2 or T helper 17 cells dominate the response in allergic asthma. In the ensuing section, we discuss recent advances in our understanding of how fungi and the proteolytic activity of common fungal aeroallergens contribute to allergic airway inflammation. A comprehensive review highlighted the multifaceted role of pulmonary macrophages and dendritic cells in allergenic airway inflammation [143].
Common household molds such as Alternaria, Aspergillus, Cladosporium and Penicillium account for a large proportion of the fungal allergens that sensitize asthma [144]. Determining how these allergens interact with the host to instigate allergic inflammation has been the focus of intense investigation, starting with the key insight that many fungal allergens are proteases [145]. Aspergillus and Penicillium secrete the clinically important allergen, alkaline protease 1 (also known as Asp f 13, Pen c 13). The respiratory epithelium is an early target of these inhaled aeroallergens and generates inflammatory signals that recruit and activate allergy- and wound healing-promoting immune cells to diseased airways [146] (Fig. 3). The proteolytic activity of Asp f 13 disrupts bronchiolar epithelial cell junctions, and club cells that line the bronchioles respond to this damage by releasing calcium fluxes [147]. The phosphatase calcineurin responds to the rise in cytosolic calcium and indirectly activates proinflammatory cytokine secretion that supports T helper 2 cell priming and the ensuing eosinophilia. The mechano-sensitive calcium channel, TRPV4, functions in epistasis with calcineurin, suggesting that club cells detect junctional injury as mechanical force. The homologous protease, Pen n 13, also disrupts epithelial cell junctions, triggering a potent allergic response, likely by analogous mechanisms [148]. Finally, Asp f 13 exerts inflammation-independent airway hyperreactivity and bronchoconstriction by disrupting the interaction of extracellular matrix and smooth muscle cells subjacent to bronchioles. Protease-damaged smooth muscle cells signal via RhoA and ROCK, suggesting transduction of mechanical forces in this cascade as well [149]. A central theme of these studies that fungal protease-dependent tissue injury triggers and aggravates allergic responses.
Fig. 3.
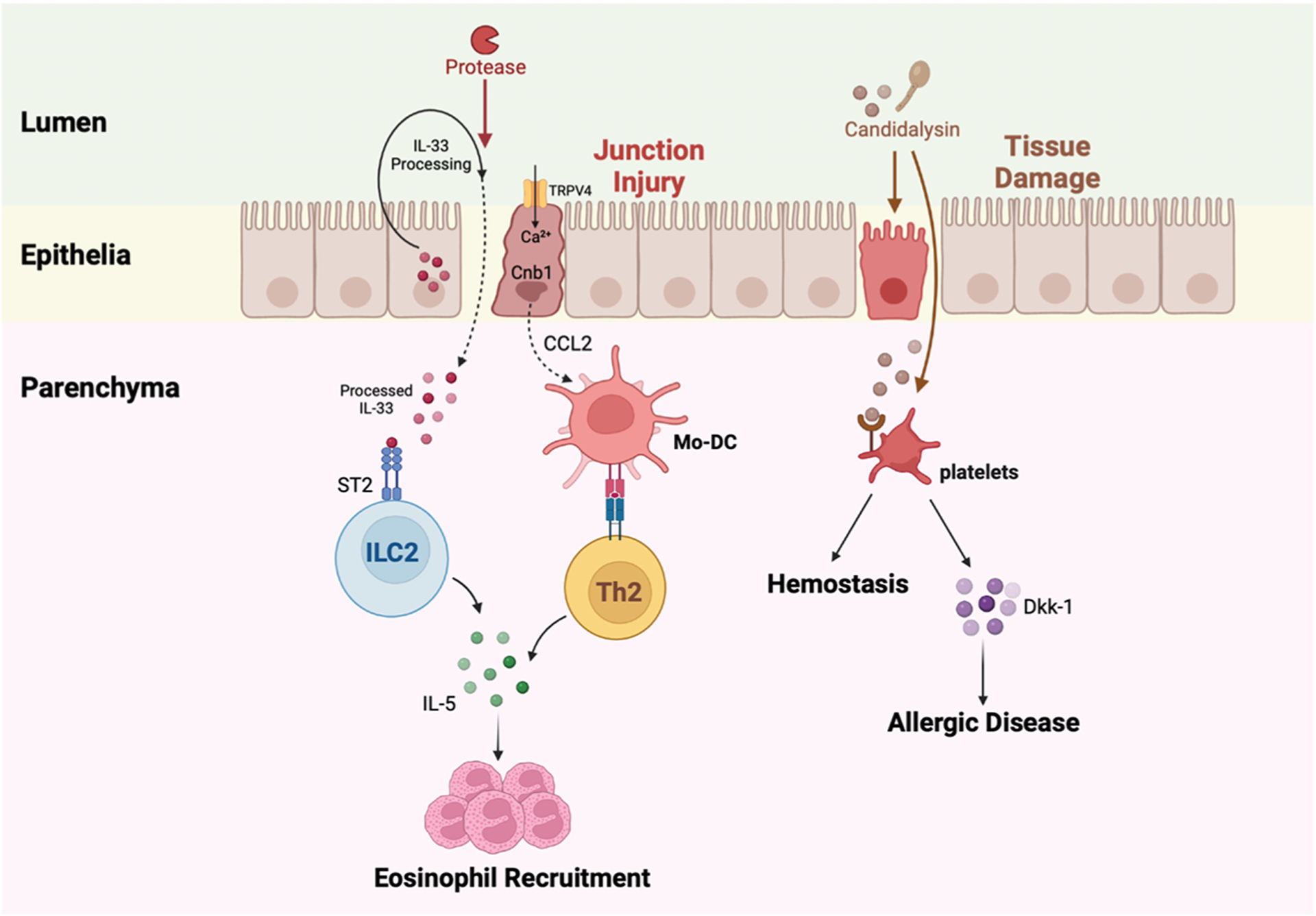
Fungal proteases and candidalysin can initiate allergenic inflammation. A fungal protease cleaves IL-33 and disrupts epithelial junctions. Processed IL-33 penetrates the barrier into the lung parenchyma, binds to ST2 receptors on type 2 innate lymphoid cells (ILC2), and drives the production of IL-5 by ILC2. This process drives eosinophil recruitment. Protease injury to the epithelial cell junctions also activates TRPV4 and causes calcium to flux into epithelial cells. Calcium signals through calcineurin and promotes CCL2 release from epithelial cells, resulting in the recruitment of Mo-DCs. Mo-DCs present antigen to T helper 2 cells in the lungs that secrete IL-5 and coordinate eosinophil influx and allergenic disease. Candidalysin, a peptide toxin released by Candida albicans, can damage lung epithelial cells in the upper airway. Platelets that arrive to limit hemorrhage interact with candidalysin, release dickkopf-1 (Dkk-1), and Dkk-1 promotes T helper 2-and T helper 17-dependent allergic responses.
Interleukin-33, a prototypical alarmin and ST2 ligand, was recently proposed to act as a soluble protease sensor. In this model, protease sensing enables host cells to react to the threat of tissue injury. Uncharacterized Aspergillus and Alternaria proteases cleave IL-33 into a highly active form that activates group 2 ILCs via the ST2 receptor to produce type 2 cytokines and drive IL-5-dependent eosinophil recruitment to the lungs [150] (Fig. 3). However, IL-33 signaling does not affect the generation of T helper 2 responses to the main protease constituent of Aspergillus extract, Asp f 13 [146]. It is possible to reconcile these seemingly contradictory observations since type 2 cytokine responses may be beneficial in the context of wound repair [151]. Thus, protease sensors may serve a beneficial function by signaling innate immune cells to coordinate transient and moderated wound repair while a more substantial barrier injury may prime a permanent and anamnestic T cell response which contributes to lifelong allergenic disease.
Beyond direct injury to epithelial cells, fungal proteases have also been implicated in host fibrinogen cleavage to generate fibrin split products. Fibrin split products activate macrophage-dependent fungistatic activity via an integrin receptor Mac-1 (CD11b/CD18) and TLR4 signaling-dependent pathway to boost epithelial cell responsiveness to T helper 2-derived cytokines, such as IL-13, and to promote mucin production. This pathway does not appear to influence the formation of T helper 2 cells [152]. Although Candida species are not a cause of invasive disease in the lung, patients with cystic fibrosis can harbor Candida within diseased airways; this finding has been associated with long-term declines in pulmonary function [153,154]. C. albicans and C. dubliniensis pseudohyphae secrete a 31 amino acid cytolytic peptide toxin, candidalysin, that mediates mucosal infection [155]. Candidalysin, a product of the Ece1 gene, damages epithelial cells and triggers protective immune responses by inducing mitogen-activated protein kinase and NF-κB signaling [156]. In a murine model of allergic airway inflammation, candidalysin-secreting C. albicans strains induced platelet activation in the lung via a direct candidalysin-von Willebrand factor glycoprotein 1ba interaction that stimulates the release of the Wnt agonist Dickkopf-1 (Dkk-1) which in turn promoted T helper 2 and T helper 17 responses [157] (Fig. 3). Surprisingly, these signaling events reduced the lung fungal burden and promoted platelet-dependent hemostasis and tissue protection, a critical property to prevent lethal hemorrhage during invasive fungal disease in the lung [157,158].
Beyond local effects on allergenic airway disease, fungi can exert long range effects by promoting pathologic T helper cell subsets at distant sites. A seminal study revealed that the intestinal- and mucosal tissue-resident fungus C. albicans can prime and induce the majority of human T helper 17 cells [116], while other fungal constituents of the microbiota and human fungal pathogens only induce small and variable fractions of IL-17-producing T helper cells, the majority of which are cross-reactive with Candida antigens and can respond to homologous peptides expressed by other ascomycetes. In patients with inflammatory bowel disease, the frequency of Candida-reactive T helper cells was increased compared to healthy controls; this finding correlates with a parallel increase in Aspergillus-reactive T helper 17 cells. In patients with allergic bronchopulmonary aspergillosis, these Candida-induced, and Aspergillus cross-reactive T helper 17 cells were expanded and mediated pathologic inflammation prior to receipt of antifungal drugs and corticosteroid therapy. This study supports the concept that fungal antigen exposure at extrapulmonary sites can generate cross-reactive, pathologic Th17 cells that respond to heterologous fungi in the lung.
Further evidence for the concept of a fungal gut-lung axis that regulates extraintestinal inflammation comes from studies in murine models of house dust mite-induced allergy. Prolonged oral administration of the antifungal drug fluconazole (which targets most yeasts, but not molds) led to an outgrowth of three drug-resistant filamentous fungal taxa, Aspergillus amstelodami, Epicoccum nigrum and Wallemia sebi in the murine intestinal tract, leading to fungal dysbiosis. Drug therapy was associated with exacerbated colitis and heightened pulmonary house dust mite-triggered allergenic responses. This finding was recapitulated in germ-free mice reconstituted with the three identified taxa [159,160]. In this model, the induction of heightened allergenic responses in the lung depended on Syk and CARD9 signaling in intestinal CX3CR1+ mononuclear phagocytes; these mononuclear phagocytes represent the major subset of fungus-responsive sentinels in the intestinal tract [161].
10. Fungal co-infections in the context of severe viral disease
Viral infections can increase the susceptibility of patients to fungal co-infections, even in the absence of pre-existing immune deficits. For example, influenza-associated pulmonary aspergillosis (IAPA) and COVID-19-associated pulmonary aspergillosis (CAPA) have emerged as distinct entities of post-viral respiratory fungal infections that can manifest in previously healthy patients [162–167]. In transplant patients, cytomegalovirus (CMV) disease or a discordant serostatus (CMV-seropositive donor and CMV seronegative recipient) increases the risk of fatal invasive fungal infections, including respiratory infections by Aspergillus, Pneumocystis, and Cryptococcus [168,169].
There are several proposed mechanisms by which these viruses may predispose patients to fungal infections in the lungs. This topic has recently been comprehensively reviewed [170], so we highlight some key concepts and findings here. As respiratory pathogens themselves, influenza virus and SARS-CoV2, the causative agent of COVID-19, can directly damage the respiratory epithelium and endothelium, which may lead to disruption of these physical barriers along with their roles in coordinating pulmonary antifungal responses [171–173]. These viruses may also alter the presence and effector functions of immune cells in the lungs that are important for fungal recognition and clearance. In a murine model of IAPA, influenza suppressed neutrophil recruitment to the lungs through the induction of IFN-STAT1 signaling, leading to increased susceptibility to invasive pulmonary aspergillosis [174]. CMV-infected human monocytes have reduced expression of genes encoding fungal pattern recognition receptors CD36, mannose receptor, complement receptor 3, and TLR6, as well as impaired phagocytosis of fungal organisms, including Cryptococcus [175]. SARS-CoV2 causes a dysregulation in monocyte and neutrophil responses, including an overwhelming influx of these cells into the lungs during severe disease [176,177]. This excessive inflammatory response contributes to significant immunopathology in the lungs during severe COVID-19 disease, which in turn may facilitate the development of CAPA [176,177]. However, this mechanism and other correlative observations have not yet been directly studied.
Therapies used to treat viral infections have also been investigated for their potential role in increasing the risk for fungal co-infections. Oseltamivir, a neuraminidase inhibitor that is used to treat influenza, has been found to impair the anti-fungal activity of mouse splenocytes and human peripheral blood mononuclear cells (PBMC) against Aspergillus through the inhibition of host neuraminidase activity and prevention of sialic acid recognition by immune receptors [178]. Frontline treatments that emerged during the COVID-19 pandemic included corticosteroids and the IL-6R inhibitor tocilizumab, both of which can increase susceptibility to fungal infections when given at high doses and/or for prolonged periods of time [179–183]. Notably, short courses of these drugs are recommended for COVID-19 treatment [184]. Tocilizumab has not clearly been shown to increase the risk for CAPA [185]. Studies on the contribution of corticosteroids to CAPA have had conflicting results [186–191]. However, a recent study suggests that dexamethasone increases the risk for CAPA three-fold [192].
Other fungal co-infections have been described, including COVID-19-associated mucormycosis (CAM), which came to prominence in India [193,194]. The incidence of cryptococcosis after COVID-19 has been studied, but the data suggest these patients already have typical risk factors for cryptococcosis [195]. Further studies are needed to determine if additional pathogens can increase the risk for respiratory fungal infections and to investigate the potential immune mechanisms that mediate this increased susceptibility.
11. Conclusions and future perspectives
The past decade has witnessed substantial advances in deciphering the molecular and cellular components of antifungal immunity in the lung, notably with respect to integrating parenchymal, innate, and adaptive immune cells into cooperative models of organ-specific immunity and fungus-associated allergenic disease. Preclinical vaccine studies have identified complex mixtures of fungal-derived antigens and novel adjuvant combinations that confer vaccine-mediated protection, and in individual cases, sterilizing immunity. The stubborn remaining hurdles in fungal vaccine studies relate to advancing these studies into the clinical realm, harnessing mRNA-based vaccine platforms, and harnessing broader interest and support from the biotechnology sector. Important and substantial knowledge gaps remain. For example, the sensory nervous system is a key contributor to cutaneous antifungal immunity and pulmonary immunity to bacterial pathogens [196–198]. However, the role of neuro-immune interactions in pulmonary anti-fungal immunity remains largely unknown. In the case of concurrent polymicrobial infections with fungi and other non-fungal pathogens and in the case of secondary fungal infections following severe bacterial and viral infections, it is largely unclear how concurrent and antecedent infections with other pathogenic microbes disrupt core features of antifungal immunity. Recent studies have highlighted contributions of intestinal fungi to protective antifungal immunity [199–201] and to invasive disease [202]. Candida albicans, a member of the intestinal-resident fungal community, promotes the formation of fungus-specific IgG antibodies [199] and the expansion of fungus-specific T helper 17 cells [200], both which exert protective functions during murine systemic candidiasis. The relationship of endogenous fungal communities in the intestine and at extraintestinal sites with susceptibility to pulmonary fungal infections remains another important question for future investigation. Ongoing progress in these many areas is critical to formulate improved strategies to benefit patients with life-threatening pulmonary fungal infections.
Acknowledgements
This work was supported by the National Institutes of Health (NIH) grant nos. R37 AI093808 (T.M.H.), (T.M.H.), R01 AI142639 (T.M.H.), R21 AI156157 (T.M.H.), R01 AI169769 (A.R.), R01 AI141368 (A.R.), R01 AI162765 (L.J.H.), K08 AI130366 (L.J.H.), and K22 AI153678 (D.L. W.), by an Investigator in the Pathogenesis of Infectious Diseases Awards from the Burroughs Wellcome Fund (A.R.), and by NIH P30 CA008748 to Memorial Sloan Kettering Cancer Center.
References
- [1].Kohler JR, Hube B, Puccia R, Casadevall A, Perfect JR, Fungi that Infect Humans, Microbiol. Spectr. 5 (3) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lionakis MS, Iliev ID, Hohl TM, Immunity against fungi, JCI Insight 2 (11) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Casadevall A, Immunity to invasive fungal diseases, Annu. Rev. Immunol 40 (2022) 121–141. [DOI] [PubMed] [Google Scholar]
- [4].Chamilos G, Lionakis MS, Kontoyiannis DP, Call for action: invasive fungal infections associated with ibrutinib and other small molecule kinase inhibitors targeting immune signaling pathways, Clin. Infect. Dis 66 (1) (2018) 140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zarakas MA, Desai JV, Chamilos G, Lionakis MS, Fungal infections with ibrutinib and other small-molecule kinase inhibitors, Curr. Fungal Infect. Rep 13 (3) (2019) 86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].van de Veerdonk FL, Kolwijck E, Lestrade PP, Hodiamont CJ, Rijnders BJ, van Paassen J, Haas PJ, Oliveira Dos Santos C, Kampinga GA, Bergmans DC, van Dijk K, de Haan AF, van Dissel J, van der Hoeven HG, Verweij PE, Dutch G, Mycoses study, influenza-associated aspergillosis in critically Ill patients, Am. J. Respir. Crit. Care Med 196 (4) (2017) 524–527. [DOI] [PubMed] [Google Scholar]
- [7].Hoenigl M, Seidel D, Sprute R, Cunha C, Oliverio M, Goldman GH, Ibrahim AS, Carvalho A, COVID-19-associated fungal infections, Nat. Microbiol. 7 (8) (2022) 1127–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lionakis MS, Genetic variation and fungal infection risk: state of the art, Curr. Fungal Infect. Rep 13 (4) (2019) 250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Maus MV, Lionakis MS, Infections associated with the new ‘nibs and mabs’ and cellular therapies, Curr. Opin. Infect. Dis 33 (4) (2020) 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Weibel ER, Gomez DM, Architecture of the human lung. Use of quantitative methods establishes fundamental relations between size and number of lung structures, Science 137 (3530) (1962) 577–585. [DOI] [PubMed] [Google Scholar]
- [11].Basil MC, Cardenas-Diaz FL, Kathiriya JJ, Morley MP, Carl J, Brumwell AN, Katzen J, Slovik KJ, Babu A, Zhou S, Kremp MM, McCauley KB, Li S, Planer JD, Hussain SS, Liu X, Windmueller R, Ying Y, Stewart KM, Oyster M, Christie JD, Diamond JM, Engelhardt JF, Cantu E, Rowe SM, Kotton DN, Chapman HA, Morrisey EE, Human distal airways contain a multi-potent secretory cell that can regenerate alveoli, Nature 604 (7904) (2022) 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sui P, Wiesner DL, Xu J, Zhang Y, Lee J, Van Dyken S, Lashua A, Yu C, Klein BS, Locksley RM, Deutsch G, Sun X, Pulmonary neuroendocrine cells amplify allergic asthma responses, Science 360 (6393) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Xu-Vanpala S, Deerhake ME, Wheaton JD, Parker ME, Juvvadi PR, MacIver N, Ciofani M, Shinohara ML, Functional heterogeneity of alveolar macrophage population based on expression of CXCL2, Sci. Immunol. 5 (50) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nair VR, Franco LH, Zacharia VM, Khan HS, Stamm CE, You W, Marciano DK, Yagita H, Levine B, Shiloh MU, Microfold cells actively translocate mycobacterium tuberculosis to initiate infection, Cell Rep. 16 (5) (2016) 1253–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gow NAR, Latge JP, Munro CA, The fungal cell wall: structure, biosynthesis, and function, Microbiol. Spectr. 5 (3) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lee MJ, Liu H, Barker BM, Snarr BD, Gravelat FN, Al Abdallah Q, Gavino C, Baistrocchi SR, Ostapska H, Xiao T, Ralph B, Solis NV, Lehoux M, Baptista SD, Thammahong A, Cerone RP, Kaminskyj SG, Guiot MC, Latge JP, Fontaine T, Vinh DC, Filler SG, Sheppard DC, The fungal exopolysaccharide galactosaminogalactan mediates virulence by enhancing resistance to neutrophil extracellular traps, PLoS Pathog. 11 (10) (2015), e1005187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Briard B, Fontaine T, Samir P, Place DE, Muszkieta L, Malireddi RKS, Karki R, Christgen S, Bomme P, Vogel P, Beau R, Mellado E, Ibrahim-Granet O, Henrissat B, Kalathur RC, Robinson C, Latge JP, Kanneganti TD, Galactosaminogalactan activates the inflammasome to provide host protection, Nature 588 (7839) (2020) 688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Garfoot AL, Shen Q, Wuthrich M, Klein BS, Rappleye CA, The Eng1 beta-glucanase enhances histoplasma virulence by reducing beta-glucan exposure, MBio 7 (2) (2016) e01388–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Casadevall A, Coelho C, Cordero RJ, Dragotakes Q, Jung E, Vij R, Wear MP, The capsule of Cryptococcus neoformans, Virulence 10 (1) (2019) 822–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Moalli F, Doni A, Deban L, Zelante T, Zagarella S, Bottazzi B, Romani L, Mantovani A, Garlanda C, Role of complement and Fc{gamma} receptors in the protective activity of the long pentraxin PTX3 against Aspergillus fumigatus, Blood 116 (24) (2010) 5170–5180. [DOI] [PubMed] [Google Scholar]
- [21].Cunha C, Aversa F, Lacerda JF, Busca A, Kurzai O, Grube M, Loffler J, Maertens JA, Bell AS, Inforzato A, Barbati E, Almeida B, Santos e Sousa P, Barbui A, Potenza L, Caira M, Rodrigues F, Salvatori G, Pagano L, Luppi M, Mantovani A, Velardi A, Romani L, Carvalho A, Genetic PTX3 deficiency and aspergillosis in stem-cell transplantation, N. Engl. J. Med 370 (5) (2014) 421–432. [DOI] [PubMed] [Google Scholar]
- [22].Fisher CE, Hohl TM, Fan W, Storer BE, Levine DM, Zhao LP, Martin PJ, Warren EH, Boeckh M, Hansen JA, Validation of single nucleotide polymorphisms in invasive aspergillosis following hematopoietic cell transplantation, Blood 129 (19) (2017) 2693–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Swain SD, Lee SJ, Nussenzweig MC, Harmsen AG, Absence of the macrophage mannose receptor in mice does not increase susceptibility to Pneumocystis carinii infection in vivo, Infect. Immun 71 (11) (2003) 6213–6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Madan T, Reid KB, Clark H, Singh M, Nayak A, Sarma PU, Hawgood S, Kishore U, Susceptibility of mice genetically deficient in SP-A or SP-D gene to invasive pulmonary aspergillosis, Mol. Immunol 47 (10) (2010) 1923–1930. [DOI] [PubMed] [Google Scholar]
- [25].Geunes-Boyer S, Beers MF, Perfect JR, Heitman J, Wright JR, Surfactant protein D facilitates Cryptococcus neoformans infection, Infect. Immun 80 (7) (2012) 2444–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Punatar AD, Kusne S, Blair JE, Seville MT, Vikram HR, Opportunistic infections in patients with pulmonary alveolar proteinosis, J. Infect 65 (2) (2012) 173–179. [DOI] [PubMed] [Google Scholar]
- [27].de Andrade LGM, Contti MM, Nga HS, Bravin AM, Takase HM, Viero RM, da Silva TN, Chagas KN, Palma LMP, Long-term outcomes of the atypical hemolytic uremic syndrome after kidney transplantation treated with eculizumab as first choice, PLoS One 12 (11) (2017), e0188155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Clancy M, McGhan R, Gitomer J, Inocencio AM, Aldrich C, Iaderosa R, Stevens R, Disseminated cryptococcosis associated with administration of eculizumab, Am. J. Health Syst. Pharm. 75 (14) (2018) 1018–1022. [DOI] [PubMed] [Google Scholar]
- [29].Vellanki VS, Bargman JM, Aspergillus Niger peritonitis in a peritoneal dialysis patient treated with eculizumab, Ren. Fail 36 (4) (2014) 631–633. [DOI] [PubMed] [Google Scholar]
- [30].Hirama T, Santiago RM, John R, Chaparro C, Pulmonary blastomycosis following eculizumab therapy in a lung transplant recipient, Exp. Clin. Transpl 18 (3) (2020) 410–413. [DOI] [PubMed] [Google Scholar]
- [31].Svirshchevskaya EV, Shevchenko MA, Huet D, Femenia F, Latge JP, Boireau P, Berkova NP, Susceptibility of mice to invasive aspergillosis correlates with delayed cell influx into the lungs, Int. J. Immunogenet 36 (5) (2009) 289–299. [DOI] [PubMed] [Google Scholar]
- [32].Behnsen J, Lessing F, Schindler S, Wartenberg D, Jacobsen ID, Thoen M, Zipfel PF, Brakhage AA, Secreted Aspergillus fumigatus protease Alp1 degrades human complement proteins C3, C4, and C5, Infect. Immun 78 (8) (2010) 3585–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Shende R, Wong SSW, Rapole S, Beau R, Ibrahim-Granet O, Monod M, Guhrs KH, Pal JK, Latge JP, Madan T, Aimanianda V, Sahu A, Aspergillus fumigatus conidial metalloprotease Mep1p cleaves host complement proteins, J. Biol. Chem 293 (40) (2018) 15538–15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nikolakopoulou C, Willment JA, Brown GD, C-type lectin receptors in antifungal immunity, Adv. Exp. Med. Biol 1204 (2020) 1–30. [DOI] [PubMed] [Google Scholar]
- [35].Wang H, Lee TJ, Fites SJ, Merkhofer R, Zarnowski R, Brandhorst T, Galles K, Klein B, Wuthrich M, Ligation of Dectin-2 with a novel microbial ligand promotes adjuvant activity for vaccination, PLoS Pathog. 13 (8) (2017), e1006568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Dos Santos Dias L, Dobson HE, Bakke BK, Kujoth GC, Huang J, Kohn EM, Taira CL, Wang H, Supekar NT, Fites JS, Gates D, Gomez CL, Specht CA, Levitz SM, Azadi P, Li L, Suresh M, Klein BS, Wuthrich M, Structural basis of Blastomyces Endoglucanase-2 adjuvancy in anti-fungal and -viral immunity, PLoS Pathog. 17 (3) (2021), e1009324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, Schweighoffer E, Tybulewicz V, Brown GD, Ruland J, Reis e Sousa C, Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17, Nat. Immunol 8 (6) (2007) 630–638. [DOI] [PubMed] [Google Scholar]
- [38].Guo Y, Chang Q, Cheng L, Xiong S, Jia X, Lin X, Zhao X, C-type lectin receptor CD23 is required for host defense against Candida albicans and Aspergillus fumigatus infection, J. Immunol 201 (8) (2018) 2427–2440. [DOI] [PubMed] [Google Scholar]
- [39].Swidergall M, Solis NV, Lionakis MS, Filler SG, EphA2 is an epithelial cell pattern recognition receptor for fungal beta-glucans, Nat. Microbiol 3 (1) (2018) 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Swidergall M, Solis NV, Wang Z, Phan QT, Marshall ME, Lionakis MS, Pearlman E, Filler SG, EphA2 is a neutrophil receptor for candida albicans that stimulates antifungal activity during oropharyngeal infection, Cell Rep. 28 (2) (2019) 423–433, e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Caffrey AK, Lehmann MM, Zickovich JM, Espinosa V, Shepardson KM, Watschke CP, Hilmer KM, Thammahong A, Barker BM, Rivera A, Cramer RA, Obar JJ, IL-1alpha signaling is critical for leukocyte recruitment after pulmonary Aspergillus fumigatus challenge, PLoS Pathog. 11 (1) (2015), e1004625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Evans SE, Hahn PY, McCann F, Kottom TJ, Pavlovic ZV, Limper AH, Pneumocystis cell wall beta-glucans stimulate alveolar epithelial cell chemokine generation through nuclear factor-kappaB-dependent mechanisms, Am. J. Respir. Cell. Mol. Biol 32 (6) (2005) 490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Stappers MHT, Clark AE, Aimanianda V, Bidula S, Reid DM, Asamaphan P, Hardison SE, Dambuza IM, Valsecchi I, Kerscher B, Plato A, Wallace CA, Yuecel R, Hebecker B, da M Gloria Teixeira Sousa, Cunha C, Liu Y, Feizi T, Brakhage AA, Kwon-Chung KJ, Gow NAR, Zanda M, Piras M, Zanato C, Jaeger M, Netea MG, van de Veerdonk FL, Lacerda JF, Campos A, Carvalho A, Willment JA, Latge JP, Brown GD, Recognition of DHN-melanin by a C-type lectin receptor is required for immunity to Aspergillus, Nature 555 (7696) (2018) 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gavino C, Cotter A, Lichtenstein D, Lejtenyi D, Fortin C, Legault C, Alirezaie N, Majewski J, Sheppard DC, Behr MA, Foulkes WD, Vinh DC, CARD9 deficiency and spontaneous central nervous system candidiasis: complete clinical remission with GM-CSF therapy, Clin. Infect. Dis 59 (1) (2014) 81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Drummond RA, Collar AL, Swamydas M, Rodriguez CA, Lim JK, Mendez LM, Fink DL, Hsu AP, Zhai B, Karauzum H, Mikelis CM, Rose SR, Ferre EM, Yockey L, Lemberg K, Kuehn HS, Rosenzweig SD, Lin X, Chittiboina P, Datta SK, Belhorn TH, Weimer ET, Hernandez ML, Hohl TM, Kuhns DB, Lionakis MS, CARD9-dependent neutrophil recruitment protects against fungal invasion of the central nervous system, PLoS Pathog 11 (12) (2015), e1005293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Rieber N, Gazendam RP, Freeman AF, Hsu AP, Collar AL, Sugui JA, Drummond RA, Rongkavilit C, Hoffman K, Henderson C, Clark L, Mezger M, Swamydas M, Engeholm M, Schule R, Neumayer B, Ebel F, Mikelis CM, Pittaluga S, Prasad VK, Singh A, Milner JD, Williams KW, Lim JK, Kwon-Chung KJ, Holland SM, Hartl D, Kuijpers TW, Lionakis MS, Extrapulmonary Aspergillus infection in patients with CARD9 deficiency, JCI Insight 1 (17) (2016), e89890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Drummond RA, Swamydas M, Oikonomou V, Zhai B, Dambuza IM, Schaefer BC, Bohrer AC, Mayer-Barber KD, Lira SA, Iwakura Y, Filler SG, Brown GD, Hube B, Naglik JR, Hohl TM, Lionakis MS, CARD9(+) microglia promote antifungal immunity via IL-1beta- and CXCL1-mediated neutrophil recruitment, Nat. Immunol 20 (5) (2019) 559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Karki R, Man SM, Malireddi RK, Gurung P, Vogel P, Lamkanfi M, Kanneganti TD, Concerted activation of the AIM2 and NLRP3 inflammasomes orchestrates host protection against Aspergillus infection, Cell Host Microbe 17 (3) (2015) 357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Jhingran A, Kasahara S, Shepardson KM, Junecko BA, Heung LJ, Kumasaka DK, Knoblaugh SE, Lin X, Kazmierczak BI, Reinhart TA, Cramer RA, Hohl TM, Compartment-specific and sequential role of MyD88 and CARD9 in chemokine induction and innate defense during respiratory fungal infection, PLoS Pathog. 11 (1) (2015), e1004589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Lammermann T, Afonso PV, Angermann BR, Wang JM, Kastenmuller W, Parent CA, Germain RN, Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo, Nature 498 (7454) (2013) 371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Caffrey-Carr AK, Hilmer KM, Kowalski CH, Shepardson KM, Temple RM, Cramer RA, Obar JJ, Host-derived leukotriene B(4) is critical for resistance against invasive pulmonary Aspergillosis, Front. Immunol 8 (2017) 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Snarr BD, St-Pierre G, Ralph B, Lehoux M, Sato Y, Rancourt A, Takazono T, Baistrocchi SR, Corsini R, Cheng MP, Sugrue M, Baden LR, Izumikawa K, Mukae H, Wingard JR, King IL, Divangahi M, Satoh MS, Yipp BG, Sato S, Sheppard DC, Galectin-3 enhances neutrophil motility and extravasation into the airways during Aspergillus fumigatus infection, PLoS Pathog. 16 (8) (2020), e1008741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hernandez-Santos N, Wiesner DL, Fites JS, McDermott AJ, Warner T, Wuthrich M, Klein BS, Lung epithelial cells coordinate innate lymphocytes and immunity against pulmonary fungal infection, Cell Host Microbe 23 (4) (2018) 511–522, e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Perez-Nazario N, Rangel-Moreno J, O’Reilly MA, Pasparakis M, Gigliotti F, Wright TW, Selective ablation of lung epithelial IKK2 impairs pulmonary Th17 responses and delays the clearance of Pneumocystis, J. Immunol 191 (9) (2013) 4720–4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Bello-Irizarry SN, Wang J, Olsen K, Gigliotti F, Wright TW, The alveolar epithelial cell chemokine response to pneumocystis requires adaptor molecule MyD88 and interleukin-1 receptor but not toll-like receptor 2 or 4, Infect. Immun 80 (11) (2012) 3912–3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Wang J, Gigliotti F, Maggirwar S, Johnston C, Finkelstein JN, Wright TW, Pneumocystis carinii activates the NF-kappaB signaling pathway in alveolar epithelial cells, Infect. Immun 73 (5) (2005) 2766–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hansakon A, Jeerawattanawart S, Pattanapanyasat K, Angkasekwinai P, IL-25 receptor signaling modulates host defense against cryptococcus neoformans infection, J. Immunol 205 (3) (2020) 674–685. [DOI] [PubMed] [Google Scholar]
- [58].Heyl KA, Klassert TE, Heinrich A, Muller MM, Klaile E, Dienemann H, Grunewald C, Bals R, Singer BB, Slevogt H, Dectin-1 is expressed in human lung and mediates the proinflammatory immune response to nontypeable Haemophilus influenzae, mBio 5 (5) (2014) e01492–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Feldman MB, Dutko RA, Wood MA, Ward RA, Leung HM, Snow RF, De La Flor DJ, Yonker LM, Reedy JL, Tearney GJ, Mou H, Hurley BP, Vyas JM, Aspergillus fumigatus cell wall promotes apical airway epithelial recruitment of human neutrophils, Infect. Immun 88 (2) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sun WK, Lu X, Li X, Sun QY, Su X, Song Y, Sun HM, Shi Y, Dectin-1 is inducible and plays a crucial role in Aspergillus-induced innate immune responses in human bronchial epithelial cells, Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol (2012). [DOI] [PubMed] [Google Scholar]
- [61].Han X, Yu R, Zhen D, Tao S, Schmidt M, Han L, Beta-1,3-glucan-induced host phospholipase D activation is involved in Aspergillus fumigatus internalization into type II human pneumocyte A549 cells, PLoS One 6 (7) (2011), e21468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Bertuzzi M, Schrettl M, Alcazar-Fuoli L, Cairns TC, Munoz A, Walker LA, Herbst S, Safari M, Cheverton AM, Chen D, Liu H, Saijo S, Fedorova ND, Armstrong-James D, Munro CA, Read ND, Filler SG, Espeso EA, Nierman WC, Haas H, Bignell EM, The pH-responsive PacC transcription factor of Aspergillus fumigatus governs epithelial entry and tissue invasion during pulmonary aspergillosis, PLoS Pathog. 10 (10) (2014), e1004413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Wasylnka JA, Moore MM, Aspergillus fumigatus conidia survive and germinate in acidic organelles of A549 epithelial cells, J. Cell Sci 116 (Pt 8) (2003) 1579–1587. [DOI] [PubMed] [Google Scholar]
- [64].Liu M, Spellberg B, Phan QT, Fu Y, Lee AS, Edwards JE Jr, Filler SG, Ibrahim AS, The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice, J. Clin. Investig 120 (6) (2010) 1914–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Gebremariam T, Liu M, Luo G, Bruno V, Phan QT, Waring AJ, Edwards JE Jr, Filler SG, Yeaman MR, Ibrahim AS, CotH3 mediates fungal invasion of host cells during mucormycosis, J. Clin. Investig 124 (1) (2014) 237–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Soliman SSM, Baldin C, Gu Y, Singh S, Gebremariam T, Swidergall M, Alqarihi A, Youssef EG, Alkhazraji S, Pikoulas A, Perske C, Venkataramani V, Rich A, Bruno VM, Hotopp JD, Mantis NJ, Edwards JE Jr, Filler SG, Chamilos G, Vitetta ES, Ibrahim AS, Mucoricin is a ricin-like toxin that is critical for the pathogenesis of mucormycosis, Nat. Microbiol 6 (3) (2021) 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Alqarihi A, Gebremariam T, Gu Y, Swidergall M, Alkhazraji S, Soliman SSM, Bruno VM, Edwards JE Jr., Filler SG, Uppuluri P, Ibrahim AS, GRP78 and integrins play different roles in host cell invasion during mucormycosis, mBio 11 (3) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Liu H, Lee MJ, Solis NV, Phan QT, Swidergall M, Ralph B, Ibrahim AS, Sheppard DC, Filler SG, Aspergillus fumigatus CalA binds to integrin alpha5beta1 and mediates host cell invasion, Nat. Microbiol 2 (2016) 16211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Holland SM, Chronic granulomatous disease, Hematol. Oncol. Clin. North Am 27 (1) (2013) 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Shlezinger N, Irmer H, Dhingra S, Beattie SR, Cramer RA, Braus GH, Sharon A, Hohl TM, Sterilizing immunity in the lung relies on targeting fungal apoptosis-like programmed cell death, Science 357 (6355) (2017) 1037–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Hatinguais R, Pradhan A, Brown GD, Brown AJP, Warris A, Shekhova E, Mitochondrial reactive oxygen species regulate immune responses of macrophages to Aspergillus fumigatus, Front. Immunol 12 (2021), 641495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Shlezinger N, Hohl TM, Mitochondrial reactive oxygen species enhance alveolar macrophage activity against Aspergillus fumigatus but are dispensable for host protection, mSphere 6 (3) (2021), e0026021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Branzk N, Lubojemska A, Hardison SE, Wang Q, Gutierrez MG, Brown GD, Papayannopoulos V, Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens, Nat. Immunol 15 (11) (2014) 1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Warnatsch A, Tsourouktsoglou TD, Branzk N, Wang Q, Reincke S, Herbst S, Gutierrez M, Papayannopoulos V, Reactive oxygen species localization programs inflammation to clear microbes of different size, Immunity 46 (3) (2017) 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].McCormick A, Heesemann L, Wagener J, Marcos V, Hartl D, Loeffler J, Heesemann J, Ebel F, NETs formed by human neutrophils inhibit growth of the pathogenic mold Aspergillus fumigatus, Microbes Infect. 12 (12–13) (2010) 928–936. [DOI] [PubMed] [Google Scholar]
- [76].Bruns S, Kniemeyer O, Hasenberg M, Aimanianda V, Nietzsche S, Thywissen A, Jeron A, Latge JP, Brakhage AA, Gunzer M, Production of extracellular traps against Aspergillus fumigatus in vitro and in infected lung tissue is dependent on invading neutrophils and influenced by hydrophobin RodA, PLoS Pathog. 6 (4) (2010), e1000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Andrianaki AM, Kyrmizi I, Thanopoulou K, Baldin C, Drakos E, Soliman SSM, Shetty AC, McCracken C, Akoumianaki T, Stylianou K, Ioannou P, Pontikoglou C, Papadaki HA, Tzardi M, Belle V, Etienne E, Beauvais A, Samonis G, Kontoyiannis DP, Andreakos E, Bruno VM, Ibrahim AS, Chamilos G, Iron restriction inside macrophages regulates pulmonary host defense against Rhizopus species, Nat. Commun 9 (1) (2018) 3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Zarember KA, Sugui JA, Chang YC, Kwon-Chung KJ, Gallin JI, Human polymorphonuclear leukocytes inhibit Aspergillus fumigatus conidial growth by lactoferrin-mediated iron depletion, J. Immunol 178 (10) (2007) 6367–6373. [DOI] [PubMed] [Google Scholar]
- [79].Subramanian Vignesh K, Landero Figueroa JA, Porollo A, Caruso JA, Deepe GS Jr., Granulocyte macrophage-colony stimulating factor induced Zn sequestration enhances macrophage superoxide and limits intracellular pathogen survival, Immunity 39 (4) (2013) 697–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Winters MS, Chan Q, Caruso JA, Deepe GS Jr., Metallomic analysis of macrophages infected with Histoplasma capsulatum reveals a fundamental role for zinc in host defenses, J. Infect. Dis 202 (7) (2010) 1136–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Akoumianaki T, Kyrmizi I, Valsecchi I, Gresnigt MS, Samonis G, Drakos E, Boumpas D, Muszkieta L, Prevost MC, Kontoyiannis DP, Chavakis T, Netea MG, van de Veerdonk FL, Brakhage AA, El-Benna J, Beauvais A, Latge JP, Chamilos G, Aspergillus cell wall melanin blocks LC3-associated phagocytosis to promote pathogenicity, Cell Host Microbe 19 (1) (2016) 79–90. [DOI] [PubMed] [Google Scholar]
- [82].Goncalves SM, Duarte-Oliveira C, Campos CF, Aimanianda V, Ter Horst R, Leite L, Mercier T, Pereira P, Fernandez-Garcia M, Antunes D, Rodrigues CS, Barbosa-Matos C, Gaifem J, Mesquita I, Marques A, Osorio NS, Torrado E, Rodrigues F, Costa S, Joosten LA, Lagrou K, Maertens J, Lacerda JF, Campos A Jr, Brown GD, Brakhage AA, Barbas C, Silvestre R, van de Veerdonk FL, Chamilos G, Netea MG, Latge JP, Cunha C, Carvalho A, Phagosomal removal of fungal melanin reprograms macrophage metabolism to promote antifungal immunity, Nat. Commun 11 (1) (2020) 2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Rocco NM, Carmen JC, Klein BS, Blastomyces dermatitidis yeast cells inhibit nitric oxide production by alveolar macrophage inducible nitric oxide synthase, Infect. Immun 79 (6) (2011) 2385–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Wuthrich M, Filutowicz HI, Warner T, Klein BS, Requisite elements in vaccine immunity to Blastomyces dermatitidis: plasticity uncovers vaccine potential in immune-deficient hosts, J. Immunol 169 (12) (2002) 6969–6976. [DOI] [PubMed] [Google Scholar]
- [85].Sterkel AK, Lorenzini JL, Fites JS, Subramanian Vignesh K, Sullivan TD, Wuthrich M, Brandhorst T, Hernandez-Santos N, Deepe GS Jr., Klein BS, Fungal mimicry of a mammalian aminopeptidase disables innate immunity and promotes pathogenicity, Cell Host Microbe 19 (3) (2016) 361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Charlier C, Nielsen K, Daou S, Brigitte M, Chretien F, Dromer F, Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans, Infect. Immun 77 (1) (2009) 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Nicola AM, Robertson EJ, Albuquerque P, Derengowski Lda S, Casadevall A, Nonlytic exocytosis of Cryptococcus neoformans from macrophages occurs in vivo and is influenced by phagosomal pH, mBio 2 (4) (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Dang EV, Lei S, Radkov A, Volk RF, Zaro BW, Madhani HD, Secreted fungal virulence effector triggers allergic inflammation via TLR4, Nature 608 (7921) (2022) 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Ellett F, Pazhakh V, Pase L, Benard EL, Weerasinghe H, Azabdaftari D, Alasmari S, Andrianopoulos A, Lieschke GJ, Macrophages protect Talaromyces marneffei conidia from myeloperoxidase-dependent neutrophil fungicidal activity during infection establishment in vivo, PLoS Pathog. 14 (6) (2018), e1007063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Pazhakh V, Ellett F, Croker BA, O’Donnell JA, Pase L, Schulze KE, Greulich RS, Gupta A, Reyes-Aldasoro CC, Andrianopoulos A, Lieschke GJ, Beta-glucan-dependent shuttling of conidia from neutrophils to macrophages occurs during fungal infection establishment, PLoS Biol. 17 (9) (2019), e3000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Heung LJ, Monocytes and the host response to fungal pathogens, Front. Cell Infect. Microbiol 10 (2020) 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Osterholzer JJ, Chen GH, Olszewski MA, Curtis JL, Huffnagle GB, Toews GB, Accumulation of CD11b+ lung dendritic cells in response to fungal infection results from the CCR2-mediated recruitment and differentiation of Ly-6Chigh monocytes, J. Immunol 183 (12) (2009) 8044–8053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Osterholzer JJ, Chen GH, Olszewski MA, Zhang YM, Curtis JL, Huffnagle GB, Toews GB, Chemokine receptor 2-mediated accumulation of fungicidal exudate macrophages in mice that clear cryptococcal lung infection, Am. J. Pathol 178 (1) (2011) 198–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Heung LJ, Hohl TM, Inflammatory monocytes are detrimental to the host immune response during acute infection with Cryptococcus neoformans, PLoS Pathog. 15 (3) (2019), e1007627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Traynor TR, Kuziel WA, Toews GB, Huffnagle GB, CCR2 expression determines T1 versus T2 polarization during pulmonary Cryptococcus neoformans infection, J. Immunol 164 (4) (2000) 2021–2027. [DOI] [PubMed] [Google Scholar]
- [96].Espinosa V, Jhingran A, Dutta O, Kasahara S, Donnelly R, Du P, Rosenfeld J, Leiner I, Chen CC, Ron Y, Hohl TM, Rivera A, Inflammatory monocytes orchestrate innate antifungal immunity in the lung, PLoS Pathog. 10 (2) (2014), e1003940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Arango Duque G, Descoteaux A, Macrophage cytokines: involvement in immunity and infectious diseases, Front. Immunol 5 (2014) 491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Guo Y, Kasahara S, Jhingran A, Tosini NL, Zhai B, Aufiero MA, Mills KAM, Gjonbalaj M, Espinosa V, Rivera A, Luster AD, Hohl TM, During Aspergillus infection, monocyte-derived DCs, neutrophils, and plasmacytoid DCs enhance innate immune defense through CXCR3-dependent crosstalk, Cell Host Microbe 28 (1) (2020) 104–116, e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Espinosa V, Dutta O, McElrath C, Du P, Chang YJ, Cicciarelli B, Pitler A, Whitehead I, Obar JJ, Durbin JE, Kotenko SV, Rivera A, Type III interferon is a critical regulator of innate antifungal immunity, Sci. Immunol 2 (16) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Ye L, Schnepf D, Staeheli P, Interferon-lambda orchestrates innate and adaptive mucosal immune responses, Nat. Rev. Immunol 19 (10) (2019) 614–625. [DOI] [PubMed] [Google Scholar]
- [101].Rivera A, Interferon Lambda’s new role as regulator of neutrophil function, J. Interferon Cytokine Res 39 (10) (2019) 609–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Broggi A, Tan Y, Granucci F, Zanoni I, IFN-lambda suppresses intestinal inflammation by non-translational regulation of neutrophil function, Nat. Immunol 18 (10) (2017) 1084–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Galani IE, Triantafyllia V, Eleminiadou EE, Koltsida O, Stavropoulos A, Manioudaki M, Thanos D, Doyle SE, Kotenko SV, Thanopoulou K, Andreakos E, Interferon-lambda mediates non-redundant front-line antiviral protection against influenza virus infection without compromising host fitness, Immunity 46 (5) (2017) 875–890, e6. [DOI] [PubMed] [Google Scholar]
- [104].Mocsai A, Diverse novel functions of neutrophils in immunity, inflammation, and beyond, J. Exp. Med 210 (7) (2013) 1283–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Espinosa V, Dutta O, Heung LJ, Wang K, Chang YJ, Soteropoulos P, Hohl TM, Siracusa MC, Rivera A, Cutting edge: neutrophils license the maturation of monocytes into effective antifungal effectors, J Immunol. 209 (10) (2022) 1827–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Dewi IM, Janssen NA, Rosati D, Bruno M, Netea MG, Bruggemann RJ, Verweij PE, van de Veerdonk FL, Invasive pulmonary aspergillosis associated with viral pneumonitis, Curr. Opin. Microbiol 62 (2021) 21–27. [DOI] [PubMed] [Google Scholar]
- [107].Romani L, Immunity to fungal infections, Nat. Rev. Immunol 11 (4) (2011) 275–288. [DOI] [PubMed] [Google Scholar]
- [108].Rivera A, Lodge J, Xue C, Harnessing the immune response to fungal pathogens for vaccine development, Annu Rev. Microbiol (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Wiesner DL, Specht CA, Lee CK, Smith KD, Mukaremera L, Lee ST, Lee CG, Elias JA, Nielsen JN, Boulware DR, Bohjanen PR, Jenkins MK, Levitz SM, Nielsen K, Chitin recognition via chitotriosidase promotes pathologic type-2 helper T cell responses to cryptococcal infection, PLoS Pathog. 11 (3) (2015), e1004701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Wiesner DL, Smith KD, Kotov DI, Nielsen JN, Bohjanen PR, Nielsen K, Regulatory T cell induction and retention in the lungs drives suppression of detrimental type 2 Th cells during pulmonary cryptococcal infection, J. Immunol 196 (1) (2016) 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Tsai M, Thauland TJ, Huang AY, Bun C, Fitzwater S, Krogstad P, Douine ED, Nelson SF, Lee H, Garcia-Lloret MI, Butte MJ, Disseminated coccidioidomycosis treated with interferon-gamma and dupilumab, N. Engl. J. Med 382 (24) (2020) 2337–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Conti HR, Bruno VM, Childs EE, Daugherty S, Hunter JP, Mengesha BG, Saevig DL, Hendricks MR, Coleman BM, Brane L, Solis N, Cruz JA, Verma AH, Garg AV, Hise AG, Richardson JP, Naglik JR, Filler SG, Kolls JK, Sinha S, Gaffen SL, IL-17 receptor signaling in oral epithelial cells is critical for protection against oropharyngeal candidiasis, Cell Host Microbe 20 (5) (2016) 606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Gladiator A, Wangler N, Trautwein-Weidner K, LeibundGut-Landmann S, Cutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infection, J. Immunol 190 (2) (2013) 521–525. [DOI] [PubMed] [Google Scholar]
- [114].Conti HR, Peterson AC, Brane L, Huppler AR, Hernandez-Santos N, Whibley N, Garg AV, Simpson-Abelson MR, Gibson GA, Mamo AJ, Osborne LC, Bishu S, Ghilardi N, Siebenlist U, Watkins SC, Artis D, McGeachy MJ, Gaffen SL, Oral-resident natural Th17 cells and gammadelta T cells control opportunistic Candida albicans infections, J. Exp. Med 211 (10) (2014) 2075–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Gaffen SL, Moutsopoulos NM, Regulation of host-microbe interactions at oral mucosal barriers by type 17 immunity, Sci. Immunol 5 (43) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Bacher P, Hohnstein T, Beerbaum E, Rocker M, Blango MG, Kaufmann S, Rohmel J, Eschenhagen P, Grehn C, Seidel K, Rickerts V, Lozza L, Stervbo U, Nienen M, Babel N, Milleck J, Assenmacher M, Cornely OA, Ziegler M, Wisplinghoff H, Heine G, Worm M, Siegmund B, Maul J, Creutz P, Tabeling C, Ruwwe-Glosenkamp C, Sander LE, Knosalla C, Brunke S, Hube B, Kniemeyer O, Brakhage AA, Schwarz C, Scheffold A, Human anti-fungal Th17 immunity and pathology rely on cross-reactivity against Candida albicans, Cell 176 (6) (2019) 1340–1355, e15. [DOI] [PubMed] [Google Scholar]
- [117].Wiesner DL, Smith KD, Kashem SW, Bohjanen PR, Nielsen K, Different lymphocyte populations direct dichotomous eosinophil or neutrophil responses to pulmonary cryptococcus infection, J. Immunol 198 (4) (2017) 1627–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Deepe GS Jr., Gibbons RS, Smulian AG, Histoplasma capsulatum manifests preferential invasion of phagocytic subpopulations in murine lungs, J. Leukoc. Biol 84 (3) (2008) 669–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Rivera A, Ro G, Van Epps HL, Simpson T, Leiner I, Sant’Angelo DB, Pamer EG, Innate immune activation and CD4+ T cell priming during respiratory fungal infection, Immunity 25 (4) (2006) 665–675. [DOI] [PubMed] [Google Scholar]
- [120].Wuthrich M, Hung CY, Gern BH, Pick-Jacobs JC, Galles KJ, Filutowicz HI, Cole GT, Klein BS, A. TCR, transgenic mouse reactive with multiple systemic dimorphic fungi, J. Immunol 187 (3) (2011) 1421–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Hohl TM, Rivera A, Lipuma L, Gallegos A, Shi C, Mack M, Pamer EG, Inflammatory monocytes facilitate adaptive CD4 T cell responses during respiratory fungal infection, Cell Host Microbe 6 (5) (2009) 470–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Rivera A, Hohl TM, Collins N, Leiner I, Gallegos A, Saijo S, Coward JW, Iwakura Y, Pamer EG, Dectin-1 diversifies Aspergillus fumigatus-specific T cell responses by inhibiting T helper type 1 CD4 T cell differentiation, J. Exp. Med 208 (2) (2011) 369–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Wuthrich M, Ersland K, Sullivan T, Galles K, Klein BS, Fungi subvert vaccine T cell priming at the respiratory mucosa by preventing chemokine-induced influx of inflammatory monocytes, Immunity 36 (4) (2012) 680–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Wuthrich M, Gern B, Hung CY, Ersland K, Rocco N, Pick-Jacobs J, Galles K, Filutowicz H, Warner T, Evans M, Cole G, Klein B, Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice, J. Clin. Investig 121 (2) (2011) 554–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Wuthrich M, Brandhorst TT, Sullivan TD, Filutowicz H, Sterkel A, Stewart D, Li M, Lerksuthirat T, LeBert V, Shen ZT, Ostroff G, Deepe GS Jr., Hung CY, Cole G, Walter JA, Jenkins MK, Klein B, Calnexin induces expansion of antigen-specific CD4(+) T cells that confer immunity to fungal ascomycetes via conserved epitopes, Cell Host Microbe 17 (4) (2015) 452–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Rivera A, Hohl TM, Calnexin bridges the gap toward a pan-fungal vaccine, Cell Host Microbe 17 (4) (2015) 421–423. [DOI] [PubMed] [Google Scholar]
- [127].Trautwein-Weidner K, Gladiator A, Kirchner FR, Becattini S, Rulicke T, Sallusto F, LeibundGut-Landmann S, Antigen-specific Th17 cells are primed by distinct and complementary dendritic cell subsets in oropharyngeal candidiasis, PLoS Pathog. 11 (10) (2015), e1005164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Moss P, The T cell immune response against SARS-CoV-2, Nat. Immunol 23 (2) (2022) 186–193. [DOI] [PubMed] [Google Scholar]
- [129].Specht CA, Homan EJ, Lee CK, Mou Z, Gomez CL, Hester MM, Abraham A, Rus F, Ostroff GR, Levitz SM, Protection of mice against experimental cryptococcosis by synthesized peptides delivered in glucan particles, mBio (2022), e0336721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Campuzano A, Zhang H, Ostroff GR, Dos Santos Dias L, Wuthrich M, Klein BS, Yu JJ, Lara HH, Lopez-Ribot JL, Hung CY, CARD9-associated dectin-1 and dectin-2 are required for protective immunity of a multivalent vaccine against Coccidioides posadasii Infection, J. Immunol 204 (12) (2020) 3296–3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Deepe GS Jr, Buesing WR, Ostroff GR, Abraham A, Specht CA, Huang H, Levitz SM, Vaccination with an alkaline extract of Histoplasma capsulatum packaged in glucan particles confers protective immunity in mice, Vaccine 36 (23) (2018) 3359–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Dobson HE, Dias LDS, Kohn EM, Fites S, Wiesner DL, Dileepan T, Kujoth GC, Abraham A, Ostroff GR, Klein BS, Wuthrich M, Antigen discovery unveils resident memory and migratory cell roles in antifungal resistance, Mucosal Immunol. 13 (3) (2020) 518–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Normile TG, Del Poeta M, Three models of vaccination strategies against cryptococcosis in immunocompromised hosts using heat-killed Cryptococcus neoformans Deltasgl1, Front. Immunol 13 (2022), 868523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Upadhya R, Lam WC, Maybruck B, Specht CA, Levitz SM, Lodge JK, Induction of protective immunity to cryptococcal infection in mice by a heat-killed, chitosan-deficient strain of Cryptococcus neoformans, MBio 7 (3) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Wang Y, Wang K, Masso-Silva JA, Rivera A, Xue C, Heat-Killed A, Cryptococcus mutant strain induces host protection against multiple invasive mycoses in a murine vaccine model, mBio 10 (6) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Zhai B, Wozniak KL, Masso-Silva J, Upadhyay S, Hole C, Rivera A, Wormley FL Jr., Lin X, Development of protective inflammation and cell-mediated immunity against Cryptococcus neoformans after exposure to hyphal mutants, mBio 6 (5) (2015) e01433–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Van Dyke MCC, Chaturvedi AK, Hardison SE, Leopold Wager CM, Castro-Lopez N, Hole CR, Wozniak KL, Wormley FL Jr., Induction of Broad-spectrum protective immunity against disparate cryptococcus serotypes, Front. Immunol 8 (2017) 1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Edwards JE Jr, Schwartz MM, Schmidt CS, Sobel JD, Nyirjesy P, Schodel F, Marchus E, Lizakowski M, DeMontigny EA, Hoeg J, Holmberg T, Cooke MT, Hoover K, Edwards L, Jacobs M, Sussman S, Augenbraun M, Drusano M, Yeaman MR, Ibrahim AS, Filler SG, Hennessey JP Jr, A fungal immunotherapeutic vaccine (NDV-3A) for treatment of recurrent vulvovaginal candidiasis-a phase 2 randomized, double-blind, placebo-controlled trial, Clin. Infect. Dis 66 (12) (2018) 1928–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Denning DW, O’Driscoll BR, Hogaboam CM, Bowyer P, Niven RM, The link between fungi and severe asthma: a summary of the evidence, Eur. Respir. J 27 (3) (2006) 615–626. [DOI] [PubMed] [Google Scholar]
- [140].Denning DW, Pleuvry A, Cole DC, Global burden of allergic bronchopulmonary aspergillosis with asthma and its complication chronic pulmonary aspergillosis in adults, Med Mycol. 51 (4) (2013) 361–370. [DOI] [PubMed] [Google Scholar]
- [141].Hogan C, Denning DW, Allergic bronchopulmonary aspergillosis and related allergic syndromes, Semin Respir. Crit. Care Med 32 (6) (2011) 682–692. [DOI] [PubMed] [Google Scholar]
- [142].Knutsen AP, Bush RK, Demain JG, Denning DW, Dixit A, Fairs A, Greenberger PA, Kariuki B, Kita H, Kurup VP, Moss RB, Niven RM, Pashley CH, Slavin RG, Vijay HM, Wardlaw AJ, Fungi and allergic lower respiratory tract diseases, J. Allergy Clin. Immunol 129 (2) (2012) 280–291, quiz 292–3. [DOI] [PubMed] [Google Scholar]
- [143].Furlong-Silva J, Cook PC, Fungal-mediated lung allergic airway disease: the critical role of macrophages and dendritic cells, PLoS Pathog. 18 (7) (2022), e1010608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, Woodland DL, Lund FE, Randall TD, Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity, Nat. Med 10 (9) (2004) 927–934. [DOI] [PubMed] [Google Scholar]
- [145].Holgate ST, Epithelium dysfunction in asthma, J. Allergy Clin. Immunol 120 (6) (2007) 1233–1244, quiz 1245–6. [DOI] [PubMed] [Google Scholar]
- [146].Wiesner DL, Klein BS, Lung epithelium: barrier immunity to inhaled fungi and driver of fungal-associated allergic asthma, Curr. Opin. Microbiol 40 (2017) 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Wiesner DL, Merkhofer RM, Ober C, Kujoth GC, Niu M, Keller NP, Gern JE, Brockman-Schneider RA, Evans MD, Jackson DJ, Warner T, Jarjour NN, Esnault SJ, Feldman MB, Freeman M, Mou H, Vyas JM, Klein BS, Club cell TRPV4 serves as a damage sensor driving lung allergic inflammation, Cell Host Microbe 27 (4) (2020) 614–628, e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Chen JC, Chuang JG, Su YY, Chiang BL, Lin YS, Chow LP, The protease allergen Pen c 13 induces allergic airway inflammation and changes in epithelial barrier integrity and function in a murine model, J. Biol. Chem 286 (30) (2011) 26667–26679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Balenga NA, Klichinsky M, Xie Z, Chan EC, Zhao M, Jude J, Laviolette M, Panettieri RA Jr., Druey KM, A fungal protease allergen provokes airway hyper-responsiveness in asthma, Nat. Commun 6 (2015) 6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Cayrol C, Duval A, Schmitt P, Roga S, Camus M, Stella A, Burlet-Schiltz O, Gonzalez-de-Peredo A, Girard JP, Environmental allergens induce allergic inflammation through proteolytic maturation of IL-33, Nat. Immunol 19 (4) (2018) 375–385. [DOI] [PubMed] [Google Scholar]
- [151].Lambrecht BN, Hammad H, The immunology of asthma, Nat. Immunol 16 (1) (2015) 45–56. [DOI] [PubMed] [Google Scholar]
- [152].Millien VO, Lu W, Shaw J, Yuan X, Mak G, Roberts L, Song LZ, Knight JM, Creighton CJ, Luong A, Kheradmand F, Corry DB, Cleavage of fibrinogen by proteinases elicits allergic responses through Toll-like receptor 4, Science 341 (6147) (2013) 792–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Gileles-Hillel A, Shoseyov D, Polacheck I, Korem M, Kerem E, Cohen Cymberknoh M, Association of chronic Candida albicans respiratory infection with a more severe lung disease in patients with cystic fibrosis, Pediatr. Pulmonol 50 (11) (2015) 1082–1089. [DOI] [PubMed] [Google Scholar]
- [154].Al Shakirchi M, Klingspor L, Bergman P, Hjelte L, de Monestrol I, A 16-year retrospective study on fungal prevalence and diversity in patients with cystic fibrosis: Candida dubliniensis was associated with a decline in lung function, Int. J. Infect. Dis 96 (2020) 663–670. [DOI] [PubMed] [Google Scholar]
- [155].Moyes DL, Wilson D, Richardson JP, Mogavero S, Tang SX, Wernecke J, Hofs S, Gratacap RL, Robbins J, Runglall M, Murciano C, Blagojevic M, Thavaraj S, Forster TM, Hebecker B, Kasper L, Vizcay G, Iancu SI, Kichik N, Hader A, Kurzai O, Luo T, Kruger T, Kniemeyer O, Cota E, Bader O, Wheeler RT, Gutsmann T, Hube B, Naglik JR, Candidalysin is a fungal peptide toxin critical for mucosal infection, Nature 532 (7597) (2016) 64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Naglik JR, Gaffen SL, Hube B, Candidalysin: discovery and function in Candida albicans infections, Curr. Opin. Microbiol 52 (2019) 100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Wu Y, Zeng Z, Guo Y, Song L, Weatherhead JE, Huang X, Zeng Y, Bimler L, Chang CY, Knight JM, Valladolid C, Sun H, Cruz MA, Hube B, Naglik JR, Luong AU, Kheradmand F, Corry DB, Candida albicans elicits protective allergic responses via platelet mediated T helper 2 and T helper 17 cell polarization, Immunity 54 (11) (2021) 2595–2610, e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Tischler BY, Tosini NL, Cramer RA, Hohl TM, Platelets are critical for survival and tissue integrity during murine pulmonary Aspergillus fumigatus infection, PLoS Pathog. 16 (5) (2020), e1008544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Wheeler ML, Limon JJ, Bar AS, Leal CA, Gargus M, Tang J, Brown J, Funari VA, Wang HL, Crother TR, Arditi M, Underhill DM, Iliev ID, Immunological consequences of intestinal fungal dysbiosis, Cell Host Microbe 19 (6) (2016) 865–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Li X, Leonardi I, Semon A, Doron I, Gao IH, Putzel GG, Kim Y, Kabata H, Artis D, Fiers WD, Ramer-Tait AE, Iliev ID, Response to fungal dysbiosis by gut-resident CX3CR1(+) mononuclear phagocytes aggravates allergic airway disease, Cell Host Microbe 24 (6) (2018) 847–856, e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Leonardi I, Li X, Semon A, Li D, Doron I, Putzel G, Bar A, Prieto D, Rescigno M, McGovern DPB, Pla J, Iliev ID, CX3CR1(+) mononuclear phagocytes control immunity to intestinal fungi, Science 359 (6372) (2018) 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Uyeki TM, Bernstein HH, Bradley JS, Englund JA, File TM, Fry AM, Gravenstein S, Hayden FG, Harper SA, Hirshon JM, Ison MG, Johnston BL, Knight SL, McGeer A, Riley LE, Wolfe CR, Alexander PE, Pavia AT, Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenzaa, Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am 68 (6) (2019) 895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, Clancy CJ, Wingard JR, Lockhart SR, Groll AH, Sorrell TC, Bassetti M, Akan H, Alexander BD, Andes D, Azoulay E, Bialek R, Bradsher RW, Bretagne S, Calandra T, Caliendo AM, Castagnola E, Cruciani M, Cuenca-Estrella M, Decker CF, Desai SR, Fisher B, Harrison T, Heussel CP, Jensen HE, Kibbler CC, Kontoyiannis DP, Kullberg BJ, Lagrou K, Lamoth F, Lehrnbecher T, Loeffler J, Lortholary O, Maertens J, Marchetti O, Marr KA, Masur H, Meis JF, Morrisey CO, Nucci M, Ostrosky-Zeichner L, Pagano L, Patterson TF, Perfect JR, Racil Z, Roilides E, Ruhnke M, Prokop CS, Shoham S, Slavin MA, Stevens DA, Thompson GR, Vazquez JA, Viscoli C, Walsh TJ, Warris A, Wheat LJ, White PL, Zaoutis TE, Pappas PG, Revision and update of the consensus definitions of invasive fungal disease from the european organization for research and treatment of cancer and the mycoses study group education and research consortium, Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am 71 (6) (2020) 1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Lahmer T, Kriescher S, Herner A, Rothe K, Spinner CD, Schneider J, Mayer U, Neuenhahn M, Hoffmann D, Geisler F, Heim M, Schneider G, Schmid RM, Huber W, Rasch S, Invasive pulmonary aspergillosis in critically ill patients with severe COVID-19 pneumonia: results from the prospective AspCOVID-19 study, PLoS One 16 (3) (2021), e0238825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Koehler P, Bassetti M, Chakrabarti A, Chen SCA, Colombo AL, Hoenigl M, Klimko N, Lass-Florl C, Oladele RO, Vinh DC, Zhu LP, Boll B, Bruggemann R, Gangneux JP, Perfect JR, Patterson TF, Persigehl T, Meis JF, Ostrosky-Zeichner L, White PL, Verweij PE, Cornely OA, M. European Confederation of Medical, M. International Society for Human Animal, G. Asia Fungal Working, I.L.I.W. Group, I.P.A.M.W. Group, M. European Society for Clinical, G. Infectious Diseases Fungal Infection Study, E.S.G.f.I.i.C.I. Patients, M. Interregional Association of Clinical, C. Antimicrobial, N. Medical Mycology Society of, A. Medical Mycology Society of China Medicine Education, H. Infectious Diseases Working Party of the German Society for, O. Medical, M. Association of Medical, C. Infectious Disease, Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance, Lancet Infect. Dis 21 (6) (2021) e149–e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Lamoth F, Lewis RE, Walsh TJ, Kontoyiannis DP, Navigating the uncertainties of COVID-19 associated aspergillosis (CAPA): a comparison with influenza associated aspergillosis (IAPA), J. Infect. Dis (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Crum-Cianflone NF, Invasive Aspergillosis associated with severe influenza infections, Open Forum Infect. Dis 3 (3) (2016) ofw171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].George MJ, Snydman DR, Werner BG, Griffith J, Falagas ME, Dougherty NN, Rubin RH, The independent role of cytomegalovirus as a risk factor for invasive fungal disease in orthotopic liver transplant recipients. Boston Center for Liver Transplantation CMVIG-Study Group. Cytogam, MedImmune, Inc. Gaithersburg, Maryland, Am. J. Med 103 (2) (1997) 106–113. [DOI] [PubMed] [Google Scholar]
- [169].Nichols WG, Corey L, Gooley T, Davis C, Boeckh M, High risk of death due to bacterial and fungal infection among cytomegalovirus (CMV)-seronegative recipients of stem cell transplants from seropositive donors: evidence for indirect effects of primary CMV infection, J. Infect. Dis 185 (3) (2002) 273–282. [DOI] [PubMed] [Google Scholar]
- [170].Salazar F, Bignell E, Brown GD, Cook PC, Warris A, Pathogenesis of respiratory viral and fungal coinfections, Clin. Microbiol. Rev 35 (1) (2022), e0009421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Herold S, Becker C, Ridge KM, Budinger GR, Influenza virus-induced lung injury: pathogenesis and implications for treatment, Eur. Respir. J 45 (5) (2015) 1463–1478. [DOI] [PubMed] [Google Scholar]
- [172].Delorey TM, Ziegler CGK, Heimberg G, Normand R, Yang Y, Segerstolpe A, Abbondanza D, Fleming SJ, Subramanian A, Montoro DT, Jagadeesh KA, Dey KK, Sen P, Slyper M, Pita-Juarez YH, Phillips D, Biermann J, Bloom-Ackermann Z, Barkas N, Ganna A, Gomez J, Melms JC, Katsyv I, Normandin E, Naderi P, Popov YV, Raju SS, Niezen S, Tsai LT, Siddle KJ, Sud M, Tran VM, Vellarikkal SK, Wang Y, Amir-Zilberstein L, Atri DS, Beechem J, Brook OR, Chen J, Divakar P, Dorceus P, Engreitz JM, Essene A, Fitzgerald DM, Fropf R, Gazal S, Gould J, Grzyb J, Harvey T, Hecht J, Hether T, Jane-Valbuena J, Leney-Greene M, Ma H, McCabe C, McLoughlin DE, Miller EM, Muus C, Niemi M, Padera R, Pan L, Pant D, Pe’er C, Pfiffner-Borges J, Pinto CJ, Plaisted J, Reeves J, Ross M, Rudy M, Rueckert EH, Siciliano M, Sturm A, Todres E, Waghray A, Warren S, Zhang S, Zollinger DR, Cosimi L, Gupta RM, Hacohen N, Hibshoosh H, Hide W, Price AL, Rajagopal J, Tata PR, Riedel S, Szabo G, Tickle TL, Ellinor PT, Hung D, Sabeti PC, Novak R, Rogers R, Ingber DE, Jiang ZG, Juric D, Babadi M, Farhi SL, Izar B, Stone JR, Vlachos IS, Solomon IH, Ashenberg O, Porter CBM, Li B, Shalek AK, Villani AC, Rozenblatt-Rosen O, Regev A, COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets, Nature 595 (7865) (2021) 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Chua RL, Lukassen S, Trump S, Hennig BP, Wendisch D, Pott F, Debnath O, Thurmann L, Kurth F, Volker MT, Kazmierski J, Timmermann B, Twardziok S, Schneider S, Machleidt F, Muller-Redetzky H, Maier M, Krannich A, Schmidt S, Balzer F, Liebig J, Loske J, Suttorp N, Eils J, Ishaque N, Liebert UG, von Kalle C, Hocke A, Witzenrath M, Goffinet C, Drosten C, Laudi S, Lehmann I, Conrad C, Sander LE, Eils R, COVID-19 severity correlates with airway epithelium-immune cell interactions identified by single-cell analysis, Nat. Biotechnol 38 (8) (2020) 970–979. [DOI] [PubMed] [Google Scholar]
- [174].Tobin JM, Nickolich KL, Ramanan K, Pilewski MJ, Lamens KD, Alcorn JF, Robinson KM, Influenza suppresses neutrophil recruitment to the lung and exacerbates secondary invasive pulmonary Aspergillosis, J. Immunol 205 (2) (2020) 480–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Sen P, Wilkie AR, Ji F, Yang Y, Taylor IJ, Velazquez-Palafox M, Vanni EAH, Pesola JM, Fernandez R, Chen H, Morsett LM, Abels ER, Piper M, Lane RJ, Hickman SE, Means TK, Rosenberg ES, Sadreyev RI, Li B, Coen DM, Fishman JA, El Khoury J, Linking indirect effects of cytomegalovirus in transplantation to modulation of monocyte innate immune function, Sci. Adv 6 (17) (2020) eaax9856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Merad M, Martin JC, Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages, Nat. Rev. Immunol 20 (6) (2020) 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Laforge M, Elbim C, Frere C, Hemadi M, Massaad C, Nuss P, Benoliel JJ, Becker C, Tissue damage from neutrophil-induced oxidative stress in COVID-19, Nat. Rev. Immunol 20 (9) (2020) 515–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Dewi IMW, Cunha C, Jaeger M, Gresnigt MS, Gkountzinopoulou ME, Garishah FM, Duarte-Oliveira C, Campos CF, Vanderbeke L, Sharpe AR, Bruggemann RJ, Verweij PE, Lagrou K, Vande Velde G, de Mast Q, Joosten LAB, Netea MG, van der Ven A, Wauters J, Carvalho A, van de Veerdonk FL, Neuraminidase and SIGLEC15 modulate the host defense against pulmonary aspergillosis, Cell Rep. Med 2 (5) (2021), 100289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Group RC, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ, Dexamethasone in hospitalized patients with Covid-19, N. Engl. J. Med 384 (8) (2021) 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Group RC, Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial, Lancet 397 (10285) (2021) 1637–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Lewis RE, Kontoyiannis DP, Invasive aspergillosis in glucocorticoid-treated patients, Med. Mycol. Off. Publ. Int. Soc. Hum. Anim. Mycol 47 (Suppl 1) (2009) S271–S281. [DOI] [PubMed] [Google Scholar]
- [182].Lionakis MS, Kontoyiannis DP, Glucocorticoids and invasive fungal infections, Lancet 362 (9398) (2003) 1828–1838. [DOI] [PubMed] [Google Scholar]
- [183].Schiff MH, Kremer JM, Jahreis A, Vernon E, Isaacs JD, van Vollenhoven RF, Integrated safety in tocilizumab clinical trials, Arthritis Res. Ther 13 (5) (2011) R141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Bhimraj A, Morgan RL, Shumaker AH, Lavergne V, Baden L, Cheng VC, Edwards KM, Gandhi R, Gallagher J, Muller WJ, O′Horo JC, Shoham S, Murad MH, Mustafa RA, Sultan S, Falck-Ytter Y, Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Infectious Diseases Society of America 2022; Version 9.0.1. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/. Available at https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/. (Accessed 24 August 2022). [DOI] [PMC free article] [PubMed]
- [185].Malgie J, Schoones JW, Pijls BG, Decreased mortality in coronavirus disease 2019 patients treated with tocilizumab: a rapid systematic review and meta-analysis of observational studies, Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am 72 (11) (2021) e742–e749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Permpalung N, Chiang TP, Massie AB, Zhang SX, Avery RK, Nematollahi S, Ostrander D, Segev DL, Marr KA, Coronavirus disease 2019-associated pulmonary Aspergillosis in mechanically ventilated patients, Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am 74 (1) (2022) 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Janssen NAF, Nyga R, Vanderbeke L, Jacobs C, Ergun M, Buil JB, van Dijk K, Altenburg J, Bouman CSC, van der Spoel HI, Rijnders BJA, Dunbar A, Schouten JA, Lagrou K, Bourgeois M, Reynders M, van Regenmortel N, Rutsaert L, Lormans P, Feys S, Debavaye Y, Tamion F, Costa D, Maizel J, Dupont H, Chouaki T, Nseir S, Sendid B, Bruggemann RJM, van de Veerdonk FL, Wauters J, Verweij PE, Multinational observational cohort study of COVID-19-associated pulmonary Aspergillosis(1), Emerg. Infect. Dis 27 (11) (2021) 2892–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Montrucchio G, Lupia T, Lombardo D, Stroffolini G, Corcione S, De Rosa FG, Brazzi L, Risk factors for invasive aspergillosis in ICU patients with COVID-19: current insights and new key elements, Ann. Intensive Care 11 (1) (2021) 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Bartoletti M, Pascale R, Cricca M, Rinaldi M, Maccaro A, Bussini L, Fornaro G, Tonetti T, Pizzilli G, Francalanci E, Giuntoli L, Rubin A, Moroni A, Ambretti S, Trapani F, Vatamanu O, Ranieri VM, Castelli A, Baiocchi M, Lewis R, Giannella M, Viale P, Group PS, Epidemiology of invasive pulmonary aspergillosis among intubated patients with COVID-19: a prospective study, Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am 73 (11) (2021) e3606–e3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Fekkar A, Lampros A, Mayaux J, Poignon C, Demeret S, Constantin JM, Marcelin AG, Monsel A, Luyt CE, Blaize M, Occurrence of invasive pulmonary fungal infections in patients with severe COVID-19 admitted to the ICU, Am. J. Respir. Crit. Care Med 203 (3) (2021) 307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Delliere S, Dudoignon E, Fodil S, Voicu S, Collet M, Oillic PA, Salmona M, Depret F, Ghelfenstein-Ferreira T, Plaud B, Chousterman B, Bretagne S, Azoulay E, Mebazaa A, Megarbane B, Alanio A, Risk factors associated with COVID-19-associated pulmonary aspergillosis in ICU patients: a French multicentric retrospective cohort, Clin. Microbiol Infect (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Leistner R, Schroeter L, Adam T, Poddubnyy D, Stegemann M, Siegmund B, Maechler F, Geffers C, Schwab F, Gastmeier P, Treskatsch S, Angermair S, Schneider T, Corticosteroids as risk factor for COVID-19-associated pulmonary aspergillosis in intensive care patients, Crit. Care 26 (1) (2022) 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Rudramurthy SM, Hoenigl M, Meis JF, Cornely OA, Muthu V, Gangneux JP, Perfect J, Chakrabarti A, Ecmm, Isham, ECMM/ISHAM recommendations for clinical management of COVID-19 associated mucormycosis in low- and middle-income countries, Mycoses 64 (9) (2021) 1028–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Garg D, Muthu V, Sehgal IS, Ramachandran R, Kaur H, Bhalla A, Puri GD, Chakrabarti A, Agarwal R, Coronavirus disease (Covid-19) associated mucormycosis (CAM): case report and systematic review of literature, Mycopathologia 186 (2) (2021) 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Chastain DB, Kung VM, Golpayegany S, Jackson BT, Franco-Paredes C, Vargas Barahona L, Thompson GR 3rd, Henao-Martinez AF, Cryptococcosis among hospitalised patients with COVID-19: a multicentre research network study, Mycoses 65 (8) (2022) 815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Kashem SW, Riedl MS, Yao C, Honda CN, Vulchanova L, Kaplan DH, Nociceptive sensory fibers drive interleukin-23 production from CD301b+ dermal dendritic cells and drive protective cutaneous immunity, Immunity 43 (3) (2015) 515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Cohen JA, Edwards TN, Liu AW, Hirai T, Jones MR, Wu J, Li Y, Zhang S, Ho J, Davis BM, Albers KM, Kaplan DH, Cutaneous TRPV1(+) neurons trigger protective innate type 17 anticipatory immunity, Cell 178 (4) (2019) 919–932, e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Baral P, Umans BD, Li L, Wallrapp A, Bist M, Kirschbaum T, Wei Y, Zhou Y, Kuchroo VK, Burkett PR, Yipp BG, Liberles SD, Chiu IM, Nociceptor sensory neurons suppress neutrophil and gammadelta T cell responses in bacterial lung infections and lethal pneumonia, Nat. Med 24 (4) (2018) 417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Doron I, Leonardi I, Li XV, Fiers WD, Semon A, Bialt-DeCelie M, Migaud M, Gao IH, Lin WY, Kusakabe T, Puel A, Iliev ID, Human gut mycobiota tune immunity via CARD9-dependent induction of anti-fungal IgG antibodies, Cell 184 (4) (2021) 1017–1031, e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Shao TY, Ang WXG, Jiang TT, Huang FS, Andersen H, Kinder JM, Pham G, Burg AR, Ruff B, Gonzalez T, Khurana Hershey GK, Haslam DB, Way SS, Commensal Candida albicans positively calibrates systemic Th17 immunological responses, Cell Host Microbe 25 (3) (2019) 404–417, e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Tso GHW, Reales-Calderon JA, Tan ASM, Sem X, Le GTT, Tan TG, Lai GC, Srinivasan KG, Yurieva M, Liao W, Poidinger M, Zolezzi F, Rancati G, Pavelka N, Experimental evolution of a fungal pathogen into a gut symbiont, Science 362 (6414) (2018) 589–595. [DOI] [PubMed] [Google Scholar]
- [202].Zhai B, Ola M, Rolling T, Tosini NL, Joshowitz S, Littmann ER, Amoretti LA, Fontana E, Wright RJ, Miranda E, Veelken CA, Morjaria SM, Peled JU, van den Brink MRM, Babady NE, Butler G, Taur Y, Hohl TM, High-resolution mycobiota analysis reveals dynamic intestinal translocation preceding invasive candidiasis, Nat. Med 26 (1) (2020) 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]


