Abstract
PREVENT-19, the pivotal phase 3 trial of the Novavax adjuvanted, recombinant spike protein COVID-19 vaccine (NVX-CoV2373), demonstrated that the vaccine was well tolerated and efficacious (vaccine efficacy, VE = 90%) for the prevention of symptomatic COVID-19. In the trial, participants were randomly assigned in a 2:1 ratio to receive 2 doses of NVX-CoV2373 or placebo 21 days apart. Throughout the study, the predominant SARS-CoV-2 variant was alpha, but additional variants were in circulation (i.e., beta, gamma, epsilon, and iota). VE among the per-protocol efficacy analysis population was calculated according to pre-specified disease severity (mild, moderate, or severe) criteria, but the impact on the risk of COVID-19–associated hospitalization was not specifically investigated. During this analysis period (January 25, 2021, to April 30, 2021 [95 days]), 4 hospitalizations occurred among the 77 events analyzed for the primary endpoint using the per-protocol population, 0 among vaccine recipients and 4 among placebo recipients, yielding a post hoc VE against hospitalization of 100% (95% CI: 28.8, 100). Among an expanded efficacy population, also identified post hoc, which included COVID-19–associated hospitalizations without a requirement for diagnostic polymerase chain reaction testing performed at the study central laboratory, 12 total hospitalizations were identified, 0 among vaccine recipients and 12 among placebo recipients, yielding a post hoc VE against hospitalization of 100% (95% CI: 83.1, 100). These additional data from the PREVENT-19 trial provide relevant public health information concerning the attributes of NVX-CoV2373.
Keywords: COVID-19–associated hospitalization, Vaccine efficacy, NVX-CoV2373, Novavax
1. Introduction
Since early 2020, COVID-19–associated hospitalization has placed a significant burden on the US healthcare system, requiring considerable resources, and resulting in overcrowding and other strains on hospital staff [1]. Further, it has been linked to health risks such as hospital-acquired infections [2], and substantial financial costs for individuals, families, and healthcare systems [1], [3]. The risk of hospitalization with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is understood to be heavily impacted by certain health conditions, older age, obesity, race/ethnicity, history of smoking, and occupation [4], [5], as well as social and cultural influences such as social distancing, living conditions, travel, and public mobility [6]. For COVID-19 vaccines, which have been deployed with remarkable success, the ability to prevent hospitalization is of critical importance. Despite this, vaccine efficacy estimates against hospitalization were absent from the publications associated with the pivotal trials of most widely utilized COVID-19 vaccines [7], [8].
The US-based phase 3 COVID-19 vaccine clinical trials were primarily conducted during the early pandemic stages of 2020, prior to widespread prevalence of variant strains. These trials generally reported high vaccine efficacy against symptomatic and severe disease [7], [8]. In the phase 3 trial of BNT162b2 (Pfizer/BioNTech, July 2020–November 2020), vaccine efficacy against severe COVID-19 occurrence after dose 1 was reported as 88.9% (95% CI: 20.1, 99.7), and as 75% (95% CI: −152.6, 99.5) ≥ 7 days after dose 2 [8]. The COVE study, a phase 3 trial of mRNA-1273 (Moderna, July 2020–October 2020), evaluated the prevention of severe COVID-19 ≥ 14 days after dose 2 and found a vaccine efficacy of 100% (95% CI: could not be estimated, 1.0) in the adjudicated per-protocol group, and 95.1% (95% CI: 91.1, 97.3) in a secondary case definition group [7]. Neither of these pivotal trials reported efficacy against hospitalization in the associated publications [9]. Due to widespread uptake of both mRNA vaccines, recent real-world evidence data have provided new insight into their effectiveness against hospitalization. Notably, BNT162b2 effectiveness against hospitalization from December 20, 2020, to February 1, 2021, was reported to be 87% (95% CI: 55, 100) ≥ 7 days after dose 2 [10]. Other studies investigating mRNA COVID-19 vaccines effectiveness against hospitalization have found a range of estimates and are complicated by highly variable vaccination history, timing of infections relative to the receipt of each vaccine dose, and incidental COVID-19 hospitalizations [11]. Though reductions in vaccine effectiveness against new SARS-CoV-2 variant strains (currently various omicron subvariants) have been observed, the real-world benefit of COVID-19 vaccination to reduce hospitalization rates has well been demonstrated [11], [12], [13], [14], [15], [16], [17], [18], [19].
The PREVENT-19 trial was the pivotal phase 3 trial of the Matrix-M−adjuvanted, recombinant spike protein COVID-19 vaccine (NVX-CoV2373), which demonstrated an efficacy of 90% for the prevention of mild, moderate, or severe COVID-19 due to circulating SARS-CoV-2 strains at the time of the trial. Although the vaccine efficacy against moderate-to-severe disease assessed by pre-defined criteria was 100% (95% CI: 87.0, 100), the efficacy in prevention of COVID-19–associated hospitalization was not evaluated [20]. Due to the relatively short time span since the authorization of NVX-CoV2373, real-world effectiveness data that may inform its impact on hospitalization are not yet available.
This post hoc analysis aimed to identify all hospitalizations among the PREVENT-19 efficacy analysis population and to include additional COVID-19–associated hospitalizations that had been excluded from the per-protocol population due to absence of infection confirmation by polymerase chain reaction (PCR) testing at the central study laboratory. Further, we sought to detail clinical narratives of all hospitalized patients to better understand the underlying risk factors for COVID-19–associated hospitalization in trial participants.
2. Methods
2.1. Trial design
The methodology, protocol, demographic information, and clinical characteristics for this trial have been previously published (Dunkle et al. NEJM 2022; clinicaltrials.gov NCT04611802) [20]. Briefly, we assessed the safety and efficacy of two doses of NVX-CoV2373 (5 μg of SARS-CoV-2 recombinant spike protein with 50 μg of Matrix-M™ adjuvant, from Novavax, Inc. [Gaithersburg, MD]) or placebo, administered intramuscularly (deltoid muscle, using a 1″ 25-gauge needle) 21 days apart (vaccine lots Par 28003 and Par 28004). This phase 3 randomized, observer-blinded, placebo-controlled trial was conducted at sites across the United States and Mexico. Participants were randomly assigned in a 2:1 ratio via block randomization to receive two doses of NVX-CoV2373 or placebo (normal saline) using a centralized Interactive Response Technology system. As part of the trial, a blinded cross-over was introduced, which ended the placebo-controlled period of the study. In the pre-crossover efficacy period, the per-protocol endpoints for this analysis accrued from January 25, 2021, to April 30, 2021. During this period 10 moderate and 4 severe COVID-19 cases were identified, all of which were among placebo recipients, yielding vaccine efficacy against moderate-to-severe disease of 100% (95% CI: 87.0, 100). Disease severity was defined according to FDA-specified criteria in Dunkle et al. NEJM 2022. Briefly, moderate COVID-19 was defined by fever, evidence of lower respiratory tract infection (i.e., shortness of breath, significant hypoxemia, or abnormal chest X-ray), and/or increased respiratory rate. Severe COVID-19 case definitions included markedly abnormal vital signs, and/or requirement for high–flow oxygen therapy or mechanical ventilation. Severe case criteria also included organ system dysfunction or failure, thrombotic events, admission to the ICU, or death. Any potentially severe per-protocol endpoint cases were reviewed by an external, independent Endpoint Review Committee (iERC) to confirm principal investigator–assigned severity. Neither set of severity criteria specified the need for hospitalization, and thus the specific impact of NVX-CoV2373 on hospitalizations was not reported in prior publications.
In PREVENT-19, the pre-specified analysis of efficacy within the per-protocol population required endpoint events to have a PCR-positive SARS-CoV-2 infection confirmation from a nasal swab tested at the study central laboratory (University of Washington, Seattle, Washington). This protocol requirement led to the exclusion of a number of hospitalizations for COVID-19 in the primary publication [20]. To include these hospitalizations in the current analysis, vaccine efficacy was estimated with this methodology or with a relaxed methodology which allowed for COVID-19 to be diagnosed by any PCR test, rapid antigen test, or confirmed by an unspecified diagnostic assay test. All participants who received two doses of NVX-CoV2373 or placebo and were hospitalized with COVID-19 confirmed by any diagnostic assay test were included in the population hereafter referred to as the “expanded efficacy population”. Cases uniquely identified from this expanded efficacy population (those that were not part of the per-protocol efficacy analysis population) were not reviewed by the external iERC. Clinical narratives of the identified participants were obtained from the clinical study report, reviewed, and summarized.
2.2. Statistical analysis
To ensure datasets accurately mirrored those used in PREVENT-19 following processing and interpretation, the per-protocol efficacy analysis from the trial was confirmed ( Fig. 1 , Supplemental S1.1-S1.3 ). Subsequently, two analyses with different data inclusion criteria were performed. For both, all participants in the per-protocol population (n = 25,482: NVX-CoV2373, n = 17,312; placebo, n = 8,140) within the date range (01/25/21 to 04/30/21 [95 days]) and with hospitalization events ≥ 7 days after dose 2 were included. In the per-protocol efficacy analysis population, participants were screened for PCR-positive SARS-CoV-2 infection confirmed by the central laboratory. Among these participants all hospitalizations were identified ( Fig. 1 , Supplemental 1.4 ). In the expanded efficacy population analysis, the per-protocol population (n = 25,482) was used to identify participants who were hospitalized with diagnosed COVID-19 by any PCR test, rapid antigen test, or confirmed by an unspecified diagnostic assay test, irrespective of testing at the central laboratory ( Fig. 1 , Supplemental 1.5 ). Vaccine efficacy was defined as one minus a measure of relative risk. For both the per-protocol efficacy and expanded efficacy populations the vaccine efficacy and 95% confidence intervals were calculated using an exact conditional binomial method using the Clopper-Pearson method ( Supplemental Fig. 1 ) [21]. This method was chosen as both populations had zero events in the vaccine arm. The method involves conditioning on the total number of events across both treatment groups, where the number of events in the active group is generated from a single binomial distribution. The point estimate and corresponding confidence intervals, generated from this single binomial distribution, were then transformed back to relative risks. Data processing and interpretation were carried out using Python (Fredericksburg, VA, version 3.9.12), Pandas (Fredericksburg, VA, version 1.4.2), and Jupyter Notebook (version 6.4.8), and were recorded and described in detail. Key term abbreviations were standardized and used throughout the analysis ( Supplemental Table 1 ).
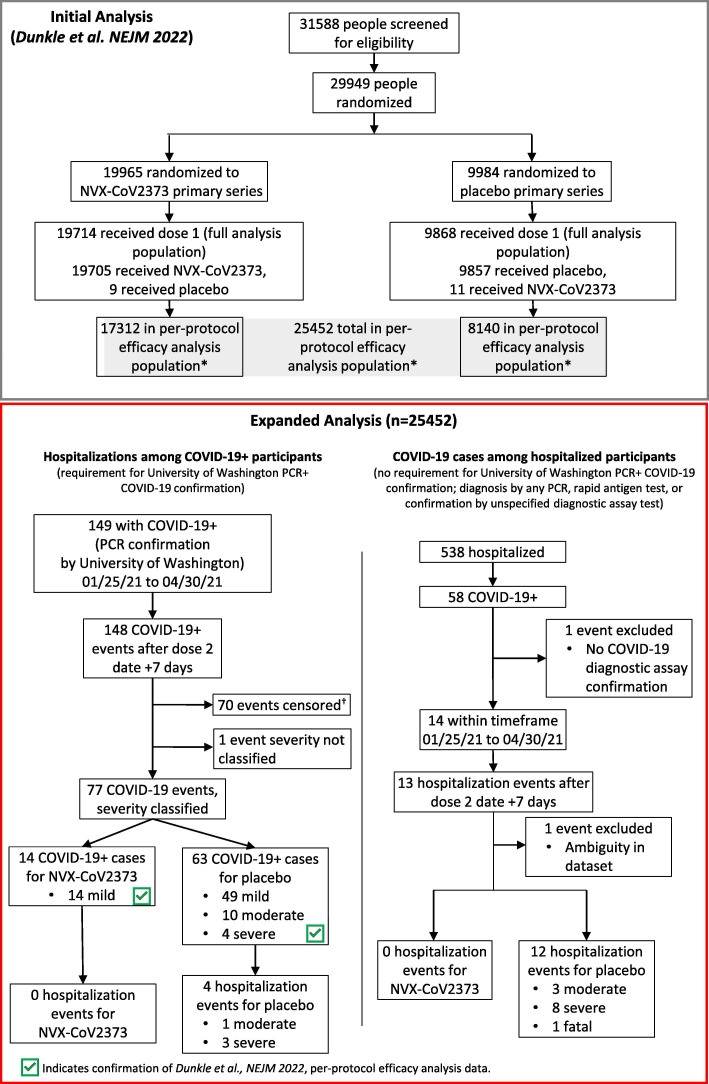
Fig. 1.
CONSORT Diagram for Original Trial and Expanded Analysis. CONSORT diagram for original trial participants and event identification in the expanded analysis. The 4 identified hospitalized participants are among the 77 events analyzed for the PREVENT-19 per-protocol primary efficacy analysis endpoint. COVID-19+ indicated diagnosis of COVID-19. *Participants received both doses as assigned, were seronegative for anti–severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nucleocapsid protein and had a SARS-CoV-2 RNA reverse transcriptase–polymerase chain reaction (RT-PCR)–negative nasal swab at baseline and did not have a censoring event at any time before 7 days after the second injection. †Censoring events for exclusion of participants from the per-protocol analysis population as described by Dunkle et al. [20] NEJM 2022 (Supplemental S1.3).
3. Results
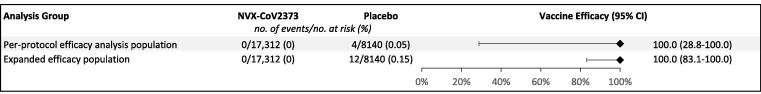
Of the 25,482 participants included in the per-protocol efficacy analysis population (NVX-CoV2373, n = 17,312; placebo, n = 8,140) 4 hospitalizations were identified in the 95-day follow-up period where there was a PCR-positive result from the central laboratory, 0 among vaccine recipients and 4 among placebo recipients ( Fig. 1 ), yielding vaccine efficacy against hospitalization of 100% (95% CI: 28.8, 100) ( Fig. 2 ). The 4 hospitalizations included 3 severe and 1 moderate case of COVID-19, as assessed by the iERC ( Fig. 1 ).
Fig. 2.
Vaccine Efficacy of NVX-CoV2373 Against COVID-19–Associated Hospitalization. Vaccine efficacy of NVX-CoV2373 against hospitalization in the per-protocol efficacy analysis and expanded efficacy populations.
With the removal of the central laboratory testing requirements pre-specified in the per-protocol efficacy analysis, 12 total (8 additional) COVID-19–associated hospitalizations were identified among the expanded efficacy population, 0 among vaccine recipients and 12 among placebo recipients ( Fig. 1 ), yielding vaccine efficacy against hospitalization of 100% (95% CI: 83.1, 100) ( Fig. 2 ). The 12 hospitalizations included 8 severe and 3 moderate cases of COVID-19 ( Fig. 1 ).
Among the 12 identified placebo-recipients with COVID-19–associated hospitalization, 11 resolved (3 moderate cases and 8 severe cases), and 1 resulted in death. Median (range) duration of hospitalization was 11 days (4 to 52 days). Median (range) time from dose 1 until the first day of hospitalization was 77 days (31 to 88 days). All participants were from the United States and were predominantly White. COVID was most often confirmed by PCR or an unspecified test. Participant ages ranged from 48 to 74 years old, with a mean (standard deviation) of 58.7 (8.2) years old ( Table 1 ). All participants had at least one pre-existing medical condition such as hypertension, hyperlipidemia, obesity, chronic obstructive pulmonary disease, gastroesophageal reflux disease and diabetes, and many were taking concurrent medications. Most participants (11, 92%) had a medical history of obesity or reported a body mass index (BMI) > 30 kg/m2. Of the 9 reported BMIs, the mean (standard deviation) was 37.5 (7.6) kg/m2 with 6 (66%) BMIs > 35 kg/m2 (extremely obese) ( Table 1 ). Only one participant had a BMI that did not indicate obesity, BMI = 22.3 kg/m2. While hospitalized, most of the participants were treated with various medications, including antivirals, antibiotics, analgesics, antipyretics, and glucocorticoids, primarily dexamethasone (10, 83%), remdesivir (10, 83%), and/or enoxaparin (6, 50%) ( Table 1 ). Two of the hospitalized participants experienced sequelae suggestive of long COVID—brain fog, fatigue, shortness of breath, and loss of taste and smell—after being discharged from the hospital, and several required home oxygen therapy or cardiopulmonary rehabilitation to regain lung function. A fatal COVID-19 case was described as severe COVID-19 pneumonia in a participant within the 55–64-year-old age range. The participant experienced shortness of breath, cough, loss of taste, chills, and fatigue for 4 days prior to being hospitalized. Despite aggressive therapeutic intervention, including bilevel positive airway pressure, broad-spectrum antibiotics, albuterol, glucocorticoids, remdesivir, immune supplements, and convalescent plasma, the participant’s respiratory status continued to decline and resulted in cardiopulmonary arrest, leading to death. The participant had pre-existing medical conditions, including obesity and primary hypertension.
Table 1.
COVID-19–Associated Hospitalized Participants Among Expanded Efficacy Analysis Population.
| Characteristics, n (%) | |
|---|---|
| Total Hospitalized | 12 |
| Trial Placebo Arm | 12 |
| COVID-19 Diagnostic Confirmation | |
| PCR Only | 3 (25%) |
| Rapid Antigen Test Only | 1 (8%) |
| Multiple Test Confirmations | 3 (25%) |
| Diagnostic Assay Confirmed, Unspecified Test | 5 (42%) |
| Severity | |
| Moderate | 3 (25%) |
| Severe | 9 (75%) |
| ICU Admission | |
| Yes | 2 (17%) |
| No/Not reported | 10 (83%) |
| Variant | |
| Alpha | 2 (16%) |
| Iota | 1 (8%) |
| Unknown | 9 (67%) |
| COVID-19 or Pneumonia–Related Hospital Meds | |
| Dexamethasone | 10 (83%) |
| Remdesivir | 10 (83%) |
| Enoxaparin | 6 (50%) |
| Clinical Outcome | |
| Resolved | 11 (91%) |
| Fatal | 1 (8%) |
| Age Range | 48–74 |
| Age Mean ± SD (years) | 58.7 ± 8.2 |
| Age 48–54 | 4 (33%) |
| Age 55–64 | 5 (42%) |
| Age ≥ 65 | 3 (25%) |
| Sex | |
| Female | 5 (42%) |
| Male | 7 (58%) |
| Race/Ethnicity | |
| Black or African American | 2 (17%) |
| White (non-Hispanic or Latino) | 8 (67%) |
| White Hispanic or Latino | 2 (17%) |
| Medical History | |
| Obesity* | 11 (91%) |
| Hypertension | 10 (83%) |
| Hyperlipidemia | 4 (33%) |
| GERD | 2 (17%) |
| Asthma | 1 (8%) |
| Pre-diabetes | 2 (17%) |
| Type I Diabetes | 1 (8%) |
| Type II Diabetes | 2 (17%) |
| COPD | 2 (17%) |
| Depression | 3 (25%) |
| Anxiety | 3 (25%) |
| Total BMI Reported | 9 |
| Mean BMI ± SD kg/m2 | 37.5 (7.6) |
| BMI > 30 kg/m2 (obese) | 2 (22%) |
| BMI > 35 kg/m2 (extremely obese) | 6 (67%) |
BMI, body mass index; COPD, chronic obstructive pulmonary disease; GERD, gastroesophageal reflux disease; ICU, intensive care unit; PCR, polymerase chain reaction; SD, standard deviation.
*Obesity was determined by BMI ≥ 30 kg/m2 and/or a reported clinical history of obesity.
One participant was excluded from the expanded efficacy population analysis due to the lack of any COVID-19 confirmation by diagnostic assay (PCR or rapid antigen) ( Fig. 1 ). Briefly, a female participant in the 48–54-year age range presented to the hospital for a pre-planned revision of her gastric sleeve and repair of a hiatal hernia with a gastroplasty approximately 25 days after receiving the second vaccination dose. During the hospitalization, she experienced sudden onset of shortness of breath which was characterized as viral pneumonia. A computed tomography scan of her chest demonstrated fibrosis due to possible pneumonitis. The participant was diagnosed with COVID-19 pneumonia based on clinical suspicion without a confirmatory COVID-19 test, and despite this, underwent gastric bypass surgery later that day. Post-operative respiratory treatments included ipratropium bromide/albuterol, which were administered for 4 days. The respiratory event was considered resolved and the participant was discharged from the hospital with no additional treatment for COVID-19 pneumonia. The principal investigator (PI) assessed the event of pneumonia as severe in intensity and not related to the study vaccine. The PI did not provide an alternative etiology for the event of pneumonia. This case was not reviewed by the iERC as it was not part of the per-protocol efficacy analysis population due to a lack of PCR performed at the central study laboratory. It is important to note that the participant had a significant medical history for hypertension and her calculated BMI was 34.1 kg/m2.
4. Discussion
The pivotal clinical trials of the Pfizer/BioNTech, Moderna, and Novavax COVID-19 vaccines did not report vaccine efficacy against hospitalization, but rather focused on the evaluation of efficacy against symptomatic mild, moderate, or severe disease [7], [8], [20]. Compared with efficacy against severe disease, vaccine efficacy estimates against hospitalization can provide a clearer understanding of the vaccine’s impact on healthcare systems, thus more accurately informing public health planning and resource allocation [22]. The review of clinical narratives from active vaccine and placebo recipients captured during clinical trials can provide insightful details and a more comprehensive understanding of the impact of a vaccine on disease outcomes and the natural history of the disease.
The Novavax vaccine arm showed no instances of hospitalization among participants in both the per-protocol efficacy analysis and expanded efficacy analysis populations. As a result, the vaccine demonstrated a 100% efficacy rate against hospitalization in both groups. The demographic information of nearly all identified hospitalized patients agreed with known risk factors of COVID-19 hospitalization, particularly obesity and presence of other underlying comorbid conditions including hypertension, hyperlipidemia, diabetes, chronic obstructive pulmonary disease, and psychiatric disorders [23], [24]. Compared to the per-protocol efficacy analysis population of PREVENT-19, the percentage of total participants with any coexisting conditions (47.3%) or with a medical history of obesity (37.2%) was higher among hospitalized participants from the expanded efficacy analysis population (100%, and 91%, respectively), though the latter only represents a small fraction of the former’s sample size [20]. Of hospitalized patients in the expanded efficacy population, 3 of 12 (25%) suffered from depression (compared to a US background rate of 9.5%) [25], underscoring the potential role of mental health in utilization of healthcare resources. Most hospitalized participants received COVID-19 pneumonia–related hospital medications (dexamethasone, 83%; remdesivir, 83%; and/or enoxaparin 50%). While these treatments offer great benefit to hospitalized patients, they are not without their own risks, further highlighting the importance of disease and hospitalization prevention [26], [27], [28], [29]. Notably, one vaccinated participant presented to the hospital for an unrelated indication, though it is evident that COVID-19 infection did not lead to hospitalization due to admission for pre-planned surgery. It is plausible that this was a case of incidental COVID-19 during unrelated hospitalization, as recent research by a US-based consortium shows that incidental hospitalization, where patients are admitted for unrelated reasons and later diagnosed with COVID-19, occurs in 26% of cases [30]. In the absence of any diagnostic test to confirm a diagnosis of COVID-19, this participant was excluded from the expanded efficacy analysis population.
There are several limitations to this post hoc analysis. Precision of vaccine efficacy estimates from the clinical trial data is affected by the low number of events, which despite the study having approximately 30,000 participants, only included 4 COVID-19–associated hospitalizations in the per-protocol efficacy population and 12 in the expanded efficacy population. At the time of the study, pandemic era restrictions, which prevented many social events and interactions, successfully reduced infections and hospitalization rates. The trial enrollment criteria excluded certain key demographics of interest, such as those with certain underlying health conditions (i.e., some immunocompromised populations), and may not have fully reflected the general population. Here, we were able to access a more inclusive population by expanding our search beyond the per-protocol efficacy analysis population to include all participants in the expanded efficacy population within PREVENT-19 participants. The limited duration of the study window did not permit assessment of long-term vaccine efficacy, and while alpha and other early variants were prevalent in the US and Mexico during the study, more information is needed to understand the impact of newer variants such as omicron. Though much information was attainable through clinical narrative reviews, these analyses were limited by what healthcare providers chose to include and may have omitted certain details such as complete medical histories. Interestingly, review of patient clinical narratives found that many hospitalized participants had common traits that may have contributed to their status. The future findings of currently ongoing real-world data and observational studies will help to provide a more complete picture of the vaccine’s effectiveness in preventing hospitalizations.
COVID-19 vaccines have played a crucial role in reducing hospitalizations by preventing the progression from mild to moderate and severe disease. Despite the impact of hospitalization on public health and economic outcomes, vaccine efficacy against hospitalization was not specifically reported in the pivotal trials of widely utilized COVID-19 vaccines. The results from this post hoc analysis of the PREVENT-19 study found that NVX-CoV2373 was effective in preventing hospitalization due to COVID-19. Nearly all hospitalized participants had pre-existing medical conditions such as hypertension, hyperlipidemia, diabetes, and obesity (BMI over 30 kg/m2). Importantly, the randomization scheme of PREVENT-19 resulted in well-balanced demographics between the vaccine and placebo treatment groups. Taken together, these results suggest that the vaccine is effective in reducing the risk of hospitalization due to COVID-19, especially in populations with pre-existing comorbid conditions and higher BMIs. Moving forward, real-world evidence studies on the effectiveness of NVX-CoV2373 in reducing hospitalization, particularly in high-risk populations, are needed to inform global public health policies and ensure the continued success of vaccination campaigns.
Notes
Funding: This work was supported by Novavax, Inc. The PREVENT-19 study was supported by Novavax, Inc.; the Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority (BARDA) [contract number: OWS: Novavax’s Project Agreement No. 1 under its Medical CBRN Defense Consortium (MCDC) Base Agreement No. 2020-530; Department of Defense (DoD) No. W911QY20C0077]; and the National Institute of Allergy and Infectious Diseases (NIAID), HIV Vaccine Trials Network (HVTN) Leadership and Operations Center [UM1 AI68614], the HVTN Statistics and Data Management Center [UM1 AI68635], the HVTN Laboratory Center [UM1 AI68618], the HIV Prevention Trials Network Leadership and Operations Center [UM1 AI68619], the AIDS Clinical Trials Group Leadership and Operations Center [UM1 AI68636], and the Infectious Diseases Clinical Research Consortium leadership group [UM1 AI148684].
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: AMM, EU, XZ, JK, WW, AM, IC, HB, GA, ST, and LMD are or were employees at the time of study conduct and might be shareholders of Novavax Inc.
Acknowledgments
Acknowledgement
Research support was provided by Sharon Glass of Novavax, Inc. Editorial support was provided by Kelly Cameron, PhD and Rebecca Harris, PhD of Ashfield MedComms (New York, USA), an Inizio company, supported by Novavax, Inc.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2023.04.054.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Data availability
Study information is available at https://clinicaltrials.gov/ct2/show/NCT04611802 and requests will be considered.
References
- 1.Grimm CA. Hospitals Reported That the COVID-19 Pandemic Has Significantly Strained Health Care Delivery. In: U.S. Department of Health and Human Services Office of Inspector General. Published March 03, 2021. Accessed March 08, 2023. https://oig.hhs.gov/oei/reports/OEI-09-21-00140.pdf
- 2.Lastinger L.M., Alvarez C.R., Kofman A., Konnor R.Y., Kuhar D.T., Nkwata A., et al. Continued increases in the incidence of healthcare-associated infection (HAI) during the second year of the coronavirus disease 2019 (COVID-19) pandemic. Infect Control Hosp Epidemiol. 2022:1–5. doi: 10.1017/ice.2022.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graves J.A., Baig K., Buntin M. The Financial Effects and Consequences of COVID-19: A Gathering Storm. JAMA. 2021;326:1909–1910. doi: 10.1001/jama.2021.18863. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Hospitalization and Death by Age; 2023. Accessed March 08, 2023. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-age.html
- 5.Centers for Disease Control and Prevention. Factors That Affect Your Risk of Getting Very Sick from COVID-19; 2023. Accessed March 08, 2023. https://www.cdc.gov/coronavirus/2019-ncov/your-health/risks-getting-very-sick.html
- 6.Mahmoudi J., Xiong C. How social distancing, mobility, and preventive policies affect COVID-19 outcomes: Big data-driven evidence from the District of Columbia-Maryland-Virginia (DMV) megaregion. PLoS One. 2022;17:e0263820. doi: 10.1371/journal.pone.0263820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. NEJM. 2020;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. NEJM. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohammed I., Nauman A., Paul P., Ganesan S., Chen K.-H., Jalil S.M.S., et al. The efficacy and effectiveness of the COVID-19 vaccines in reducing infection, severity, hospitalization, and mortality: a systematic review. Hum Vaccin Immunother. 2022;18:2027160. doi: 10.1080/21645515.2022.2027160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., et al. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. NEJM. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chatzilena A., Hyams C., Challen R., Marlow R., King J., Adegbite D., et al. Effectiveness of BNT162b2 COVID-19 vaccination in prevention of hospitalisations and severe disease in adults with SARS-CoV-2 Delta (B.1.617.2) and Omicron (B.1.1.529) variant between June 2021 and July 2022: a prospective test negative case–control study. Lancet Reg Health Eur. 2023;25 doi: 10.1016/j.lanepe.2022.100552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams K., Rhoads J.P., Surie D., Gaglani M., Ginde A.A., McNeal T., et al. Vaccine effectiveness of primary series and booster doses against covid-19 associated hospital admissions in the United States: living test negative design study. BMJ. 2022;379:e072065. doi: 10.1136/bmj-2022-072065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maltezou H.C., Basoulis D., Bonelis K., Gamaletsou M.N., Giannouchos T.V., Karantoni E., et al. Effectiveness of full (booster) COVID-19 vaccination against severe outcomes and work absenteeism in hospitalized patients with COVID-19 during the Delta and Omicron waves in Greece. Vaccine. 2023;41:2343–2348. doi: 10.1016/j.vaccine.2023.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gram M.A., Emborg H.-D., Schelde A.B., Friis N.U., Nielsen K.F., Moustsen-Helms I.R., et al. Vaccine effectiveness against SARS-CoV-2 infection or COVID-19 hospitalization with the Alpha, Delta, or Omicron SARS-CoV-2 variant: A nationwide Danish cohort study. PLoS Med. 2022;19:e1003992. doi: 10.1371/journal.pmed.1003992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Havers F.P., Pham H., Taylor C.A., Whitaker M., Patel K., Anglin O., et al. COVID-19-Associated Hospitalizations Among Vaccinated and Unvaccinated Adults 18 Years or Older in 13 US States, January 2021 to April 2022. JAMA Int Med. 2022;182:1071–1081. doi: 10.1001/jamainternmed.2022.4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plumb I.D., Feldstein L.R., Barkley E., Posner A.B., Bregman H.S., Hagen M.B., et al. Effectiveness of COVID-19 mRNA Vaccination in Preventing COVID-19–Associated Hospitalization Among Adults with Previous SARS-CoV-2 Infection — United States, June 2021–February 2022. MMWR. 2022;71:549–555. doi: 10.15585/mmwr.mm7115e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Surie D, Bonnell L, Adams K, Gaglani M, Ginde AA, Douin DJ, et al. Effectiveness of Monovalent mRNA Vaccines Against COVID-19–Associated Hospitalization Among Immunocompetent Adults During BA.1/BA.2 and BA.4/BA.5 Predominant Periods of SARS-CoV-2 Omicron Variant in the United States — IVY Network, 18 States, December 26, 2021–August 31, 2022. MMWR. 2022; 71: 1327-34. [DOI] [PMC free article] [PubMed]
- 18.Tenforde M.W., Self W.H., Adams K., Gaglani M., Ginde A.A., McNeal T., et al. Association Between mRNA Vaccination and COVID-19 Hospitalization and Disease Severity. JAMA. 2021;326:2043–2054. doi: 10.1001/jama.2021.19499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright B.J., Tideman S., Diaz G.A., French T., Parsons G.T., Robicsek A. Comparative vaccine effectiveness against severe COVID-19 over time in US hospital administrative data: a case-control study. Lancet Resp Med. 2022;10:557–565. doi: 10.1016/S2213-2600(22)00042-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunkle L.M., Kotloff K.L., Gay C.L., Áñez G., Adelglass J.M., Barrat Hernández A.Q., et al. Efficacy and Safety of NVX-CoV2373 in Adults in the United States and Mexico. NEJM. 2022;386:531–543. doi: 10.1056/NEJMoa2116185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clopper C.J., Pearson E.S. THE USE OF CONFIDENCE OR FIDUCIAL LIMITS ILLUSTRATED IN THE CASE OF THE BINOMIAL. Biometrika. 1934;26:404–413. [Google Scholar]
- 22.Farrenkopf P.M. The Cost of Ignoring Vaccines. Yale J Biol Med. 2022;95:265–269. [PMC free article] [PubMed] [Google Scholar]
- 23.Ceban F., Nogo D., Carvalho I.P., Lee Y., Nasri F., Xiong J., et al. Association Between Mood Disorders and Risk of COVID-19 Infection, Hospitalization, and Death: A Systematic Review and Meta-analysis. JAMA Psychiat. 2021;78:1079–1091. doi: 10.1001/jamapsychiatry.2021.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bailly L., Fabre R., Courjon J., Carles M., Dellamonica J., Pradier C. Obesity, diabetes, hypertension and severe outcomes among inpatients with coronavirus disease 2019: a nationwide study. Clin Microbiol Infect. 2022;28:114–123. doi: 10.1016/j.cmi.2021.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johns Hopkins Medicine. Mental Health Disorder Statistics; 2023. Accessed March 08, 2023. https://www.hopkinsmedicine.org/health/wellness-and-prevention/mental-health-disorder-statistics
- 26.Day L.B., Abdel-Qadir H., Fralick M. Bradycardia associated with remdesivir therapy for COVID-19 in a 59-year-old man. CMAJ. 2021;193:E612. doi: 10.1503/cmaj.210300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan Q., Zhang B., Ma J., Zhang S. Safety profile of the antiviral drug remdesivir: An update. Biomed & Pharmacother. 2020;130 doi: 10.1016/j.biopha.2020.110532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noreen S., Maqbool I., Madni A. Dexamethasone: Therapeutic potential, risks, and future projection during COVID-19 pandemic. Eur J Pharmacol. 2021;894 doi: 10.1016/j.ejphar.2021.173854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang C.-J., Wei Y.-J., Chang H.-L., Chang P.-Y., Tsai C.-C., Chen Y.-H., et al. Remdesivir use in the coronavirus disease 2019 pandemic: A mini-review. J Microbiol, Immunol Infect. 2021;54:27–36. doi: 10.1016/j.jmii.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klann J.G., Strasser Z.H., Hutch M.R., Kennedy C.J., Marwaha J.S., Morris M., et al. Distinguishing Admissions Specifically for COVID-19 From Incidental SARS-CoV-2 Admissions: National Retrospective Electronic Health Record Study. J Med Internet Res. 2022;24:e37931. doi: 10.2196/37931. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Study information is available at https://clinicaltrials.gov/ct2/show/NCT04611802 and requests will be considered.