Abstract
Background:
Five-year survival after childhood cancer does not fully describe life years lost due to childhood cancer as there are a large number of deaths occurring beyond five-years (late mortality) related to cancer and cancer treatment. Specific causes of health-related (non-recurrence, non-external) late mortality and risk reduction through modifiable lifestyle and cardiovascular risk factors (CVRF) are not well-described.
Methods:
Late mortality (death ≥5 years from diagnosis) and specific causes of death were evaluated in 34,230 five-year survivors of childhood cancer diagnosed <21 years of age from 1970-1999; median follow-up from diagnosis 29 years (range 5-48) in the Childhood Cancer Survivor Study (CCSS; a North American, hospital-based cohort study). Demographic, self-reported modifiable lifestyle (smoking, alcohol, physical activity, BMI) and CVRFs (hypertension, diabetes, dyslipidemia) associated with health-related mortality (excludes death from primary cancer and external causes; includes death from late effects of cancer therapy) were evaluated.
Findings:
40-year cumulative all-cause mortality was 23·3% (95% CI 22·7–24·0) with 51% of 5,916 deaths from health-related causes. Survivors ≥40 years from diagnosis experienced 131 excess health-related deaths per 10,000 person-years (95% CI 111-163), including those due to the top three causes of health-related death in the general population: cancer (54/10,000, 95% CI 41 – 68), heart disease (27/10,000, 95% CI 18 – 38), and cerebrovascular disease (10/10,000, 95% CI 5 – 17). Healthy lifestyle and absence of hypertension and diabetes were each associated with a 20-30% reduction in health-related mortality independent of other factors (all p-values ≤0·002).
Interpretation:
Survivors of childhood cancer are at excess risk of late mortality even 40 years from diagnosis, due to many of the leading causes of death in the US population. Modifiable lifestyle and CVRFs associated with reduced risk for late mortality should be part of future interventions.
Funding:
US National Cancer Institute and the American Lebanese Syrian Associated Charities.
Keywords: Pediatric, Cancer, Mortality, Modifiable Risk, Survivor, Survivorship
Introduction
Survivors of childhood cancer have the potential for decades of life beyond cancer.1 Although five-year survival now exceeds 85% in the United States (US),2 long-term survivors experience excess morbidity and late mortality (death beyond five years from cancer diagnosis) compared to the general population attributable to late effects of treatment.3-11 To reduce premature mortality, a better understanding of the specific causes of and risk factors for excess late mortality compared to the general population is needed as the first generation of survivors now enter their fifth, sixth, and seventh decades of life.
A prior study utilizing the Childhood Cancer Survivor Study (CCSS) cohort identified improvements in late mortality attributable to reductions in treatment exposures and fewer deaths from recurrence among survivors diagnosed in more recent eras.6 While there were substantial reductions in late occurring deaths from the primary cancer, the incremental improvement in health-related late mortality (deaths not caused by the primary cancer or external/accidental causes but including chronic health conditions acquired due to cancer treatment) was only modest. A recent report using data from the Surveillance, Epidemiology, and End Results (SEER) Program highlighted the importance of evaluating survival among aging survivors using the metric of excess deaths. Despite a 20% absolute increase in five-year survival the total number of annual excess deaths due to childhood cancer between 1985 and 2016 remained largely unchanged attributable to a dramatic increase in excess deaths occurring more than ten years from diagnosis.12 Further, although modifiable lifestyle and cardiovascular risk factors (CVRF) impact risk of death in the general population, 13,14 their association with late mortality risk in survivors is largely unknown.
Our previous publication documented death in the CCSS cohort through 2007,6 with an additional ten years of observation, nearly 2,000 new deaths have occurred. Thus, utilizing a well-characterized cohort of five-year survivors of the most common childhood cancers diagnosed between 1970-1999, we evaluated specific health-related causes of late mortality and excess deaths compared to the general US population and identified targets to reduce future risk.
Methods
Study Population
The CCSS is a multi-institutional, hospital-based, retrospective cohort with longitudinal follow-up of survivors treated at 31 institutions in the US and Canada (https://ccss.stjude.org/). Eligibility included five-year or greater survival from cancer (including leukemia, central nervous system tumors, lymphoma, Wilms tumor, neuroblastoma, soft tissue or bone sarcoma) diagnosed before the age of 21 years and between January 1, 1970 and December 31, 1999. The 34,230 survivors included in this analysis represent approximately 20% of all survivors of childhood cancer in the US during the study period.6 CCSS was approved by institutional review boards. Participants or their proxies provided informed consent. A detailed description of cohort methodology and study design has been published.15,16
Cause of Death
Date and cause of death through December 2017 were ascertained through linkage with the National Death Index (NDI) and classified using the International Classification of Disease 9th and 10th revisions (ICD-9 and −10) into three mutually exclusive categories: (1) recurrence or progression of primary cancer; (2) health-related cause (subsequent neoplasms, cardiac, pulmonary and all other deaths due to a medical condition; excludes deaths from the primary cancer); and (3) external cause (accidents, suicides, and other external causes). Specific causes of death were then categorized using 113 established causes of death according to ICD-9 and −10,17 modified to emphasize risks specific to childhood cancer survivors (e.g. cardiomyopathy evaluated with heart failure instead of “other cardiac causes”, Table S1). For deaths that predated the NDI (N=129 deaths from 1975-1978), state death certificates were ascertained. Because no information on specific cause of death was available for Canadian survivors (N=1,293), they were excluded from this analysis resulting in 34,230 evaluable survivors (Figure S1).
Cancer Treatment Information
For the 21,418 survivors who provided consent for treatment abstraction, chemotherapy doses and body region-specific radiation dosimetry were abstracted from medical records by treating institutions utilizing standardized protocols.15,18 For survivors with missing treatment information, we applied multiple imputation,19 consistent with prior reports.6
Lifestyle and Modifiable Cardiovascular Risk Factors
Lifestyle factors, including smoking, alcohol use, physical activity, and unhealthy weight, were assigned a score of 0 (unhealthy) or 1 (healthy) and combined to create a lifestyle score ranging from 0-4 for each survey time-point. We defined unhealthy as: ever smoked more than 100 cigarettes, heavy or risky drinking (>7 drinks/week or >3 drinks/day for women, >14 drinks/week or >4 drinks/day for men), body mass index <18·5 (underweight) or ≥30 (obese) kg/m2, and sedentary (0-3 MET-h/week of activity). For low physical activity (3-6 MET-h/week) a score of 0·5 was assigned.20 The lifestyle score was categorized as unhealthy (0-2), moderately healthy (2·5-3), and healthy (3·5-4). Presence or absence of hypertension, diabetes, and dyslipidemia were defined through self-report of a diagnosis by a healthcare provider plus taking medication for the condition(s) at each survey time-point, consistent with prior reports.21
Statistical Analysis
The study sample size was based on the eligible population of the CCSS cohort from the 31 institutions described above which was maximized based on funding support from the National Cancer Institute for a wide range of late effect outcomes. As such, the sample size for this analysis was not determined by study-power considerations for specific hypothesis tests. Cumulative incidence (risk) of all-cause and cause-specific death was estimated overall, by primary cancer diagnosis, and by time from diagnosis in 5-year intervals. Cumulative cause-specific mortality curves were estimated by the cumulative incidence method, taking the other causes of death as competing risk events. The cumulative all-cause mortality curve was estimated as the complement of the Kaplan–Meier product-limit estimate of the survival curve. Standardized mortality ratios (SMR) and rate difference (commonly referred to as “absolute excess risk”, AER, and thus hereafter designated AER) per 10,000 person-years were estimated with 95% confidence intervals based on exact Poisson distributions for all-cause and cause-specific death, compared to the age-, sex-, and calendar year-specific rates in the US population derived from the CDC WONDER (Centers for Disease Control and Prevention Wide-Ranging Online Data for Epidemiologic Research) database.22 Follow-up time at risk for death started five years post-diagnosis and ended at the earlier of the date of death or December 31, 2017, the latter being the last coverage date for the NDI data. The proportions censored at end of follow-up time (administrative censoring) were 83% among all eligible and 90% among adult CCSS participants in lifestyle analyses.
Multivariable piecewise-exponential models estimated relative rates (RR) and 95% confidence intervals (CI) of death by specific therapy exposures with a second analysis limited to those who had survived at least 30 years from diagnosis to evaluate for persistence of associations in late survivorship. Piecewise-exponential models also estimated relative rates (RR) of death by modifiable lifestyle and CVRFs, limited to CCSS participants who had completed at least one survey at age 18 or greater, starting the at-risk status of the analyses at the first survey. All regression analyses were adjusted for sex, age at diagnosis, attained age during follow-up and race/ethnicity, while modifiable risk analyses further included treatment exposures, educational attainment, income, and insurance status. As modifiable risk factors and socioeconomic circumstances are dynamic and assessed at multiple survey time-points, they were included as time-varying covariates so that changes over time were accounted for in analyses. Further, because lifestyle factors impact health over prolonged time periods and to minimize the possibility of bias due to reverse causality from lifestyle change or weight loss prompted by chronic illness (such as cachexia associated with a second malignancy or low physical activity due to progressive heart failure), the lifestyle score was shifted back five years. A directed acyclic graph (Figure S2) shows the relationships between covariates considered in our multivariable analyses. Sampling weights were applied using inverse probability weighting to account for the under-sampling of acute lymphoblastic leukemia survivors diagnosed between 1987 and 1999 in the CCSS expansion cohort. Additional details regarding the cohort, lifestyle score and statistical analysis included in Supplementary Methods.
The funding agencies had no role in the study design, data collection, data analysis, data interpretation, or writing of the report
Results
Among 34,230 CCSS-eligible five-year survivors (median age at diagnosis 6·0 years; median follow-up from diagnosis, 29·1 years, interquartile range [IQR] 22·0 to 36·2 years, range 5·0 to 48·0 years; Table 1) there were 5,916 deaths with 2,009 (34·0%) attributable to recurrence or progression of the primary cancer and 3,061 (51·2%) attributable to non-recurrence, health-related causes, including subsequent neoplasms (n=1,458), cardiac (n=504), pulmonary (n=238), and other health-related causes (n=861; Table S1). At 40-years from diagnosis, cumulative all-cause mortality was 23·3% (95% CI 22·7-24·0) for survivors compared to <5% expected in the general US population of comparable age-, sex-, and calendar-year (Table S2, Figure S3a). Assessment by decade of diagnosis, and specific cancer type are provided (Figure S3b-S8, Table S2).
Table 1.
Demographic, cancer diagnosis and treatment characteristics of five-year survivors of childhood cancer by vital status
| All eligible survivors (N=34,230) | |||
|---|---|---|---|
| Total n (%) |
Alive n (%) |
Dead n (%) |
|
| 34230 | 28314 | 5916 | |
| Sex | |||
| Male | 19093 (56) | 15618 (55) | 3475 (59) |
| Race/Ethnicity | |||
| Non-Hispanic white | 21567 (64) | 18189 (65) | 3378 (58) |
| Non-Hispanic black | 2116 (6) | 1773 (6) | 343 (6) |
| Hispanic | 2591 (9) | 2282 (9) | 309 (6) |
| Other | 2213 (7) | 1857 (7) | 356 (6) |
| Unknown | 5743 (15) | 4213 (13) | 1530 (25) |
| Age at diagnosis (years) | |||
| Median (IQR) | 6.0 (3.0-12.2) | 5.7 (2.9-11.5) | 9.3 (4.0-14.8) |
| 0-4 | 13803 (43) | 12097 (46) | 1706 (30) |
| 5-9 | 7738 (24) | 6440 (24) | 1298 (22) |
| 10-14 | 7110 (19) | 5685 (18) | 1425 (23) |
| 15-21 | 5579 (14) | 4092 (12) | 1487 (24) |
| Age at last follow-up (years) | |||
| 5-14 | 896 (3) | 0 (0) | 896 (17) |
| 15-24 | 3956 (13) | 2314 (10) | 1642 (28) |
| 25-34 | 9605 (33) | 8188 (35) | 1417 (24) |
| 35-44 | 11613 (31) | 10511 (33) | 1102 (18) |
| 45-54 | 6532 (17) | 5842 (18) | 690 (11) |
| ≥55 | 1628 (4) | 1459 (4) | 169 (3) |
| Survival after diagnosis (years) | |||
| 5-9 | 1776 (5) | 0 (0) | 1776 (31) |
| 10-19 | 4085 (12) | 2515 (10) | 1570 (27) |
| 20-29 | 12231 (41) | 10951 (45) | 1280 (22) |
| 30-39 | 11588 (30) | 10571 (33) | 1017 (16) |
| ≥40 | 4550 (11) | 4277 (13) | 273 (4) |
| Decade of diagnosis | |||
| 1970-79 | 8770 (22) | 6095 (18) | 2675 (43) |
| 1980-89 | 12768 (35) | 10645 (35) | 2123 (36) |
| 1990-99 | 12692 (43) | 11574 (47) | 1118 (21) |
| Diagnosis | |||
| Leukemia | 10587 (40) | 9042 (42) | 1545 (30) |
| Acute lymphoblastic leukemia | 8756 (36) | 7534 (38) | 1222 (25) |
| Acute myeloid leukemia | 1264 (3) | 1078 (3) | 186 (3) |
| Other leukemia | 567 (1) | 430 (1) | 137 (2) |
| Hodgkin lymphoma | 4380 (11) | 3153 (9) | 1227 (20) |
| Non-Hodgkin lymphoma | 2853 (7) | 2495 (7) | 358 (6) |
| CNS tumor | 6144 (16) | 4789 (14) | 1355 (22) |
| Astrocytoma | 3822 (10) | 3039 (9) | 783 (13) |
| Medulloblastoma | 1367 (3) | 996 (3) | 371 (6) |
| Other CNS | 955 (2) | 754 (2) | 201 (3) |
| Kidney tumors | 3072 (8) | 2805 (8) | 267 (4) |
| Neuroblastoma | 2630 (7) | 2373 (7) | 257 (4) |
| Soft tissue sarcoma | 1695 (4) | 1422 (4) | 273 (4) |
| Bone tumors | 2869 (7) | 2235 (7) | 634 (10) |
| Ewing sarcoma | 999 (3) | 744 (2) | 255 (4) |
| Osteosarcoma | 1789 (5) | 1419 (4) | 370 (6) |
| Other bone tumors | 81 (0) | 72 (0) | 9 (0) |
| Treatment exposures | |||
| Radiation exposure | |||
| Any radiation | 18858 (55) | 14321 (46) | 4525 (76) |
| Cranial radiation (Gy)a | |||
| Any exposure | 10183 (30) | 7750 (27) | 2452 (42) |
| Median dose (IQR) | 24·0 (18·0 - 50·0) | 24·0 (18·0 - 50·0) | 39·4 (23·4 - 53·5) |
| Chest radiation (Gy)a | |||
| Any exposure | 8658 (25) | 6124 (19) | 2535 (42) |
| Median dose (IQR) | 25·0 (15·0 - 36·0) | 23·4 (15·0 - 36·0) | 35·0 (20·0 - 40·0) |
| TBI | 921 (3) | 705 (3) | 224 (4) |
| Chemotherapy | |||
| Anthracycline (mg/m2)b | |||
| Any exposure | 16365 (48) | 13875 (54) | 2470 (44) |
| Median dose (IQR) | 159 (83 - 286) | 153 (77 - 267) | 227 (126 - 358) |
| Alkylating agents (mg/m2)c | |||
| Any exposure | 18735 (55) | 15156 (55) | 3459 (59) |
| Median dose (IQR) | 7215 (3095 - 12105) | 6836 (2993 - 11270) | 9534 (5149 - 15098) |
| Bleomycin (mg/m2) | |||
| Any exposure | 2373 (7) | 1892 (6) | 448 (7) |
| Median dose (IQR) | 62 (41 - 103) | 62 (42 - 102) | 72 (40 - 108) |
| Epipodophyllotoxin (mg/m2)d | |||
| Any exposure | 6169 (18) | 5211 (21) | 956 (18) |
| Median dose (IQR) | 2003 (1004 - 4458) | 1980 (1008 - 4280) | 2208 (986 - 4997) |
| Platinume | |||
| Any exposure | 3916 (11) | 3130 (9) | 787 (13) |
| Median dose (IQR) | 469 (315 - 676) | 469 (313 - 673) | 471 (319 - 686) |
| Methotrexate (mg/m2)f | |||
| Any exposure | 8061 (24) | 6875 (29) | 1184 (22) |
| Median dose (IQR) | 5921 (1506 - 21020) | 6400 (1606 - 21023) | 3719 (663 - 21015) |
CNS = Central nervous system; TBI = Total body irradiation
Analyses, including reported percentages and means/medians, were weighted to account for under-sampling of acute lymphoblastic leukemia (ALL) survivors (1987-1999).
Cranial radiation and chest radiation are all excluding scatter to body site. Cranial radiation is maxTD (maximum target dose), taken as the sum of the prescribed dose for all overlapping brain fields.
Cumulative dose median and IQR are among participants who received the agent of interest.
Anthracycline dose reported as doxorubicin equivalent dose where conversions are idarubicin x 3, daunorubicin x 0·5, mitoxantrone x 10 and epirubicin x 0·67.23
Alkylator dose reported as cyclophosphamide equivalent dose conversions are ifosfamide x 0·244, procarbazine x 0·857, BCNU x 15, CCNU x 16, melphalan x 40, Thio-TEPA x 50, nitrogen mustard x 100 and Busulfan and 8·823.24
Epipodophyllotoxin dose is the sum of teniposide and etoposide cumulative doses.
Platinum dose is the sum of the cumulative carboplatin dose divided by 4 and the cisplatin dose.
Methotrexate include all systemic methotrexate (IV, IM).
Mortality and excess deaths compared to US population
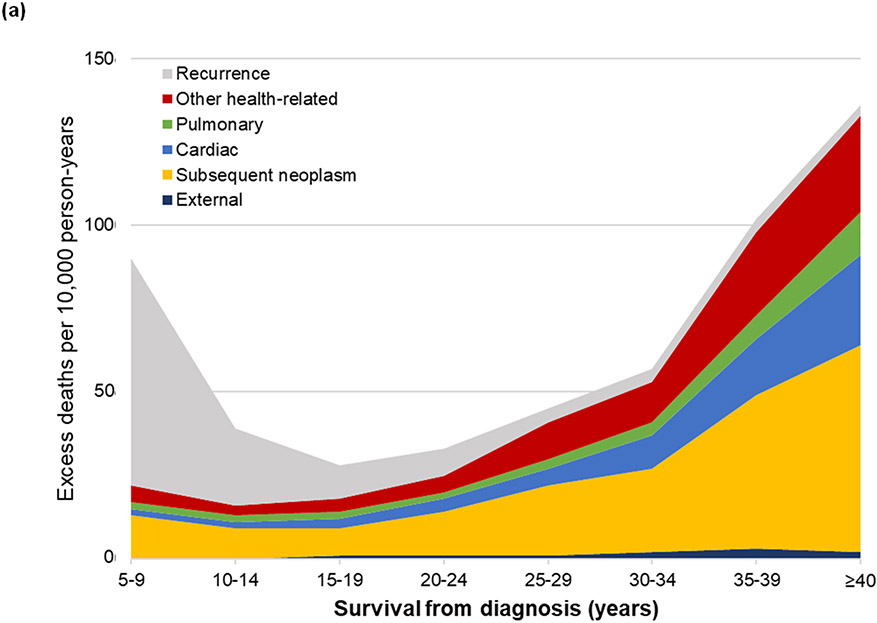
Compared to the general population, survivors were at elevated risk of death (SMR=5·6, 95% CI 5·4-5·7), which decreased from 18·1-fold (95% CI 17·3-18·9) at 5-9 years from diagnosis plateauing at a 4-fold increased risk (20 years from diagnosis: SMR=3·9, 95% CI 3·6-4·2, ≥40 years from diagnosis: SMR=4·0, 95% CI 3·5-4·5) attributable to a persistent 4·4-fold increased risk for health-related mortality ≥40 years (95% CI 3·9-5·0; Figure S9, Table S3). The absolute excess risk of all-cause mortality among survivors was 56 deaths per 10,000 person-years (95% CI 54-57). While survivors 5-9 years from diagnosis had 95 excess deaths per 10,000 person-years (95% CI 95-100) with only 21 (95% CI 19-23) attributable to health-related causes, beyond 40 years from diagnosis there were 138 excess deaths per 10,000 person-years (95% CI 117-161), with 131 (95% CI 111-153) attributable to health-related causes (Table S4; Figure 1a). Absolute excess risk of death differed by diagnosis (Figure S10). Survivors of acute lymphoblastic leukemia experienced <100 excess deaths per 10,000 person-years at all follow-up time points, while survivors of Hodgkin lymphoma experienced 100 excess deaths per 10,000 person-years 25 years from diagnosis and nearly 400 per 10,000 person-years ≥40 years from diagnosis.
Figure 1.
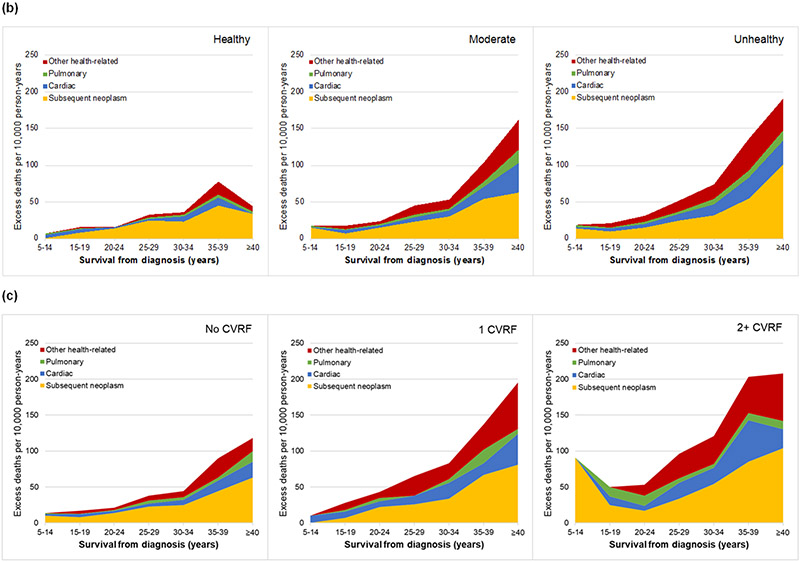
Absolute excess risk of death per 10,000 person years by survival time as cause-specific mortality (a) among all eligible survivors including recurrence, external/accidental, and health-related causes of death and (b, c) among 20,051 adult survivors included in the modifiable risk analysis as health-related causes of death (b) by lifestyle score (healthy, moderately healthy, unhealthy), and (c) number of cardiovascular risk factors (0, 1, 2+).
Associations with treatment
Specific treatment exposures were independently associated with a dose-dependent, increase in risk for all-cause and health-related mortality (Table S5). When limited to 16,139 survivors who had survived at least 30-years from diagnosis, independent associations between increased risk of health-related death and treatment persisted for chest irradiation per 10 Gy (subsequent neoplasm: RR=1·34, 95% CI 1·26-1·42; cardiac: RR=1·60, 95% CI 1·48-1·72; pulmonary: RR=1·41, 95% CI 1·22-1·62; other health-related cause: RR=1·21, 95% CI 1·12-1·31), cranial irradiation per 10 Gy (subsequent neoplasm: RR=1·15, 95% CI 1·09-1·22; other-health related cause: RR=1·24, 95% CI 1·17-1·32), anthracycline chemotherapy (cardiac: RR=1·10, 95% CI 1·01-1·20 per 100 mg/m2 in doxorubicin equivalent dose)23, and alkylator chemotherapy (subsequent neoplasm: RR=1·07, 95% CI 1·03-1·12 per 5,000 mg/m2 in cyclophosphamide equivalent dose;24 Table S6).
Specific Causes of Death
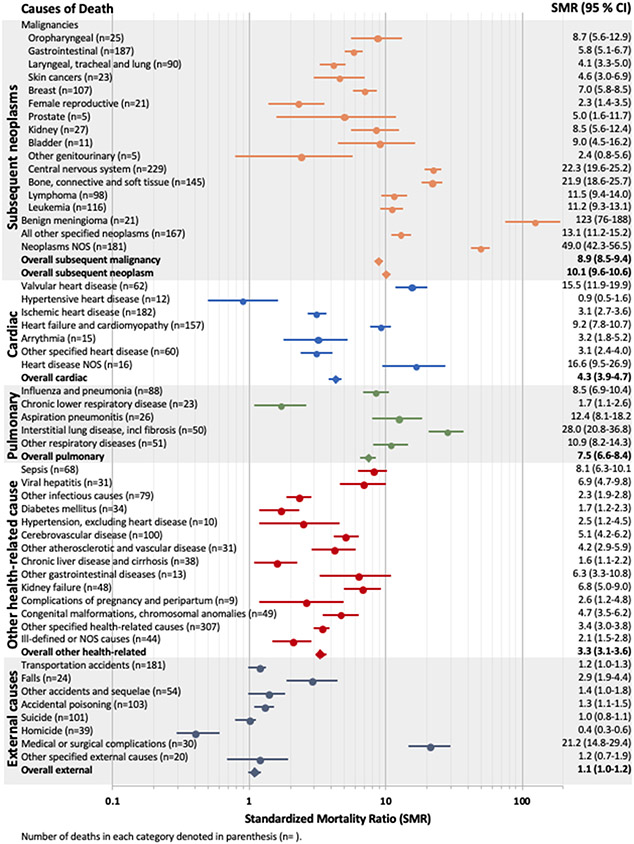
Compared to the general population, survivors of childhood cancer were at significantly increased risk for nearly all specific health-related causes of death (Table S7; Figure 2). Notably, survivors had a significantly increased risk of death for nine of the ten leading causes of health-related death in the general US adult population <60 years:23 subsequent cancer (SMR=8·9, 95% CI 8·5-9·4), heart disease (SMR=4·3, 95% CI 3·9-4·7), cerebrovascular disease (SMR=5·1, 95% CI 4·2-6·2), sepsis (SMR=8·1, 95% CI 6·3-10·1), influenza and pneumonia (SMR=8·5, 95% CI 6·9-10·4), kidney failure (SMR=6·8, 95% CI 5·0-9·0), diabetes mellitus (SMR=1·7, 95% CI 1·2-2·3), liver disease (SMR=1·6, 95% CI 1·1-2·2), and chronic lower respiratory disease (SMR=1·7, 95% CI 1·1-2·6). Although survivors were not at an overall increased risk for external or accidental causes of death, they were at increased risk of death from a fall (SMR=2·9, 95% CI 1·9-4·4) or a complication of medical or surgical care (SMR=21·2, 95% CI 14·8-29·4).
Figure 2.
Standardized mortality ratios of specific causes of death among survivors compared to US population.
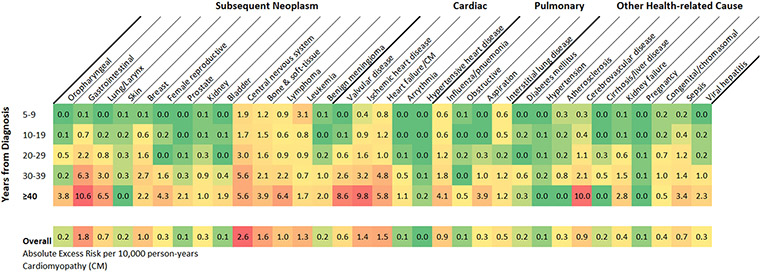
At ≥40 years from diagnosis, two-thirds of 131 excess deaths from health-related causes were due to the top three causes of health-related death in the general population: cancer (AER/10,000 person-years=54, 95% CI 41-68), heart disease (AER=27, 95% CI 18-38), and cerebrovascular disease (AER=10, 95% CI 5-17). The largest contributions from individual causes were due to gastrointestinal cancers (AER=11, 95% CI 5-18), cerebrovascular disease (AER=10, 95% CI 5-17), ischemic heart disease (AER=10, 95% CI 4-18), and valvular heart disease (AER=9, 95% CI 5-15; Figure 3).
Figure 3.
Heat map of excess risk of specific causes of health-related mortality among all eligible five-year survivors overall and by years from diagnosis as absolute excess risk per 10,000 person-years· A green to red color gradient was applied with lowest value indicated by the deepest green, the midpoint (by percentile) in yellow, and the highest value the darkest red.
Modifiable Risk
Among 20,051 adult survivors (median age 39·0 years, IQR 32·6-46·1, range 18·7-67·7) completing an average of 2·1 surveys including modifiable risk factors (range 1-4), 19% reported at least one CVRF (13% (n=2842) hypertension, 9% (n=1974) dyslipidemia, 5% (n=955) diabetes) and only 27% (n=5250) reported a healthy lifestyle (Table S8, Figure S11). With 1,476 health-related deaths occurring after completion of their first CCSS survey, health-related mortality risk was lower among those with a healthy vs. unhealthy lifestyle (SMR=3·5, 95% CI 3·1-3·9 vs. SMR=6·2, 95% CI 5·7-6·7) and high among survivors with both hypertension and diabetes (SMR=13·0, 95% CI 9·2-18·0; Table S9). Stratified by lifestyle score and then CVRFs, the absolute excess risk of health-related death was lowest in survivors with a healthy lifestyle (Figure 1b, 1c; Table S10). In multivariable models, adjusted for therapy exposures and sociodemographic factors, a healthy lifestyle (vs. unhealthy) was associated with a 20% decreased risk of health-related mortality independent of traditional CVRFs (p-value 0·002) and a moderately healthy lifestyle a 10% decreased risk (p-value 0·031, Table 2). Absence of reported hypertension or diabetes were each associated with a 30% decreased risk of health-related mortality overall (p-values ≤0·001) including a 30-50% decreased risk of cardiac mortality independent of lifestyle and other CVRFs (p-values ≤0·053). Absence of dyslipidemia did not demonstrate a protective effect and associations, including interaction with other CVRFs, were further explored in a supplemental model (Table S11).
Table 2.
Relative rate of health-related late mortality and association with modifiable lifestyle and cardiovascular risk factors among CCSS participants who completed at least one survey at age ≥18 years
| Health-related cause | Subsequent neoplasm | Cardiac | Pulmonary | Other | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RR | 95% CI | p-value | RR | 95% CI | p-value | RR | 95% CI | p-value | RR | 95% CI | p-value | RR | 95% CI | p-value | |
| Healthy lifestyle score | |||||||||||||||
| Unhealthy (0-2) | Ref | Ref | Ref | Ref | Ref | ||||||||||
| Moderate (2·5-3) | 0·9 | 0·8 - 1·0 | 0·031 | 0·9 | 0·7 - 1·1 | 0·25 | 0·9 | 0·7 - 1·2 | 0·45 | 0·8 | 0·5 - 1·4 | 0·47 | 0·8 | 0·6 - 1·0 | 0·11 |
| Healthy (3·5-4) | 0·8 | 0·7 - 0·9 | 0·002 | 0·9 | 0·8 - 1·2 | 0·57 | 0·8 | 0·5 - 1·2 | 0·26 | 0·6 | 0·3 - 1·1 | 0·093 | 0·5 | 0·3 - 0·7 | <·001 |
| Cardiovascular risk factors | |||||||||||||||
| Hypertension No vs. Yes | 0·7 | 0·6 - 0·8 | <·001 | 0·9 | 0·7 - 1·1 | 0·33 | 0·7 | 0·5 - 1·0 | 0·053 | 0·7 | 0·4 - 1·1 | 0·13 | 0·5 | 0·3 - 0·6 | <·001 |
| Diabetes No vs. Yes | 0·7 | 0·6 - 0·9 | <·001 | 0·9 | 0·7 - 1·3 | 0·65 | 0·5 | 0·3 - 0·8 | 0·004 | 0·7 | 0·3 - 1·4 | 0·27 | 0·6 | 0·4 - 0·9 | 0·007 |
| Dyslipidemia No vs. Yes | 1·2 | 1·0 - 1·4 | 0·059 | 0·9 | 0·8 - 1·2 | 0·64 | 1·2 | 0·8 - 1·8 | 0·29 | 1·9 | 0·9 - 3·8 | 0·070 | 1·5 | 1·0 - 2·1 | 0·036 |
Relative rates are adjusted for treatment exposures: cranial irradiation dose, chest irradiation dose, anthracycline dose, and alkylating agent dose, and sociodemographic factors including: age at diagnosis, race/ethnicity, sex, and attained age, education, income, and insurance status.
Lifestyle factors, including smoking status, alcohol use, physical activity, and unhealthy weight, were assigned a score of 0 (unhealthy) to 1 (healthy) and combined to create a lifestyle score ranging from 0-4 which was further categorized as unhealthy (0-2), moderately healthy (2·5-3), and healthy (3·5-4).
Covariates presented in the table (lifestyle category and chronic conditions) and socioeconomic factors (education, income, insurance) factors were included in the model as time-dependent variables.
Discussion
Despite the 20% increase in five-year survival in childhood cancer from 61·5% in 1975-1977 to 80·3% in 1999-2001,2 it is clear that five-year survival alone fails to describe the full influence of cancer and its therapy. Using the CCSS cohort, we present, to our knowledge, the most comprehensive assessment of specific causes of excess late mortality and novel associations with modifiable lifestyle and cardiovascular risk factors and excess risk of death among survivors of childhood cancer. We now demonstrate that excess deaths observed among survivors, previously noted to increase >10 years from diagnosis,12 accelerate in late survivorship and are highest among those at least 40 years from diagnosis (median age 50 years). In contrast to the period 5-9 years from diagnosis when most deaths are due to the primary cancer, the excess deaths beyond ten years from diagnosis are primarily attributable to early onset of specific, health-related causes of death commonly observed in the middle-aged US population including cancer, heart disease, cerebrovascular disease, influenza and pneumonia, sepsis, and kidney failure.25,26 The result is 138 excess deaths per 10,000 survivor-years of follow-up beyond 40 years from diagnosis, of which, 131 are attributable to health-related causes including deaths from late effects of cancer therapy. While prior studies reported on health-related mortality among survivors of childhood cancer limited to six5-10 and fifteen11 cause categories, the large size of the CCSS cohort and continued follow-up into middle- and late-adulthood has allowed the current study to specifically identify these causes of death within larger categories (e.g. type of cancer or heart disease related death) and include information about the relative and absolute excess risk of death by cause compared to the general population. Further, even decades from diagnosis, cancer-related therapeutic exposures remain significantly associated with this increased risk of death. Importantly, however, we also identified that healthy lifestyle and absence of cardiovascular risk factors are independently associated with a reduced risk of death among survivors and should inform the design of future prevention and intervention strategies aimed to reduce mortality.
Considering these findings, efforts to improve the health and lifespan of childhood cancer survivors should focus on two key areas. First, changes in primary cancer therapy that reduce exposure to treatments known to cause late effects decades after treatment should remain a priority. Even after surviving 30 years from diagnosis, radiation exposure to the brain or chest, and alkylator chemotherapy remained significantly associated with increased all-cause and health-related mortality and anthracycline chemotherapy with cardiac-specific mortality. Some high-risk treatments like cranial irradiation are used more sparingly in contemporary therapy (e.g. the near elimination of cranial irradiation in pediatric acute lymphoblastic leukemia).27 However, it remains the backbone of treatment for medulloblastoma and other central nervous system tumors, where improved survival achieved with intensification of chemotherapy and continued cranial irradiation has unfortunately increased treatment-related late effects in recent eras.28 Despite excellent five-year survival, survivors of Hodgkin lymphoma treated as recently as the 1990s remain at increased risk for subsequent cancer, cardiac, pulmonary, and other health-related causes of death as most received >15 Gy chest irradiation and >100 mg/m2 of anthracycline.29 Fortunately, recent trials may reduce risk while maintaining excellent survival, with use of novel chemotherapy agents, PET-adapted response criteria, proton beam irradiation, and reduced radiation field exposure, with up to 40% of intermediate and high-risk patients spared any radiation.30-32 Similarly, therapeutic trials for other common pediatric cancers, including acute lymphoblastic leukemia, utilizing targeted therapy and immunotherapy may allow for reductions in traditional chemotherapeutic agents among specific populations in the future.33,34 While doing so, monitoring for unanticipated late-effects of these newer agents will be essential to ensure we are not simply exchanging one set of toxicities for another.
Second, it is imperative to promote risk reduction for childhood cancer survivors across their lifespan. This includes early detection of anticipated late effects of therapy, investigation into rare causes of excess external/accidental deaths (falls and complications of medical/surgical care; not included as health-related deaths based on ICD coding but may relate to treatment-associated complications), and, importantly, prevention and control of modifiable CVRF and promotion of a healthy lifestyle, as our study demonstrates these measures may independently reduce late mortality risk. Based on the increased risk for heart failure, valvular heart disease, hypertension, dyslipidemia, diabetes, obesity, and sedentary lifestyle, consensus-based screening recommendations exist.35-39 However, optimal screening intervals or prevention and treatment approaches for many of these conditions are not known. A limited number of randomized trials are ongoing, including evaluation of carvedilol for cardiomyopathy prevention in survivors exposed to anthracycline,40 but more are needed. For example, survivors who received chest irradiation are at high risk for atherosclerotic cardiovascular disease (ASCVD) later in life, and survivor-specific risk-prediction tools exist.41,42 Although it is unknown whether approaches to management of traditional CVRFs used in the general adult population (e.g. high-dose statins) demonstrate equivalent safety and efficacy, our results suggesting a protective effect of treated dyslipidemia among survivors with both hypertension and diabetes (high-risk for cardiovascular mortality) call for investigation into the impact of statin therapy (often first-line for dyslipidemia management). Survivors whose CVRFs are largely related to exposure to cancer therapy may require treatment initiation at a younger age, but the general-population guidelines for statin therapy for ASCVD risk do not emphasize use until 40 years of age,43 30 years after treatment for most survivors. Similarly, there are few survivor-specific studies of behavioral modification for lifestyle change including smoking cessation,44 physical activity and nutrition,45,46 and those that exist are limited by the short duration of interventions, small study size, and difficulty demonstrating a durable response. Our findings that survivors with a healthy lifestyle and no CVRFs are at reduced risk for late mortality provides initial evidence that strategies that promote CVRF control, smoking cessation, weight loss, and physical activity should be tested.
We must consider limitations. First, because some rare diagnostic groups are not included in the cohort and participating centers, although geographically diverse, are largely academic which may impact treatment exposures and five-year survival, findings may not be generalizable to all survivors of childhood cancer. However, the inclusion of a significant proportion of all five-year survivors diagnosed in the country from 1970-1999, previously shown to be similar in demographic characteristics and cancer type to population-level data from the SEER database,47 leads us to believe these findings are robust and reasonably representative of the broader US population. Second, analyses of modifiable lifestyle and CVRFs were subject to a potential bias as they were limited to those who completed at least one survey at age 18 or greater. Further, the observational and self-reported nature of these outcomes was subject to possible unmeasured confounding and measurement error (in variables including smoking or BMI), and we were unable to fully address the possibility of feedback between closely related lifestyle variables, including physical activity and weight, and complex time-varying confounding caused by it. Third, although NDI data is widely used and allows mortality comparisons to the general population, it is at risk for mis-classification of cause of death from death certificate data. Finally, it is important to acknowledge that patterns of death will continue to evolve as more recently treated survivors age into middle- and late-adulthood.
Despite improvements in five-year survival, long-term survivors of childhood cancer remain at four times the risk of death compared to the general, aging population. Cancer treatment exposures remain associated with death decades after treatment. Further, the excess risk of death beyond five-year survival was greatest, and increasing, at 40 years from diagnosis, due to excess deaths from many of the leading causes of death in the general population. Fortunately, the potential exists to mitigate this risk by healthy lifestyle and absence of traditional CVRFs. Continued reductions in intensity of primary cancer therapy and future research targeting interventions for modifiable lifestyle and CVRFs in survivors may offer an opportunity to reduce morbidity and extend the lifespan for survivors.
Supplementary Material
Research in context.
Evidence before this study
Five-year survival after childhood cancer has improved dramatically over the past 50 years and survivors have the potential for decades of life after cancer. However, it is well known that cancer and cancer treatment contribute to an increased risk of late morbidity and mortality among long-term survivors. Although prior reports have demonstrated improvements in the relative risk of late mortality among five-year survivors of childhood cancer treated in more recent eras, there has been little change in the absolute number of excess deaths attributable to childhood cancer (from diagnosis through survivorship) over the past 30 years in the United States. This is in part due to a growing and aging population of long-term survivors leading to an increase in excess deaths occurring more than ten years from diagnosis. However, specific causes of late mortality across survival times and the contribution of modifiable risk factors to these late deaths have not been well described. We searched PubMed from database inception until January 2022 using the search terms “childhood cancer OR pediatric cancer”, “survivor OR five-year survivor”, and “mortality OR death” to identify studies that investigated late mortality among five-year survivors of childhood cancer. While a large number of studies across international cohorts have described late mortality (death beyond five-years from diagnosis), most have used six causes of death (primary cancer, subsequent cancer, cardiac, respiratory, external/accidental and other) or organ system specific causes (e.g. malignancy, cardiac, endocrine, gastrointestinal) and have not included therapy exposures or modifiable risk factors.
Added value of this study
To our knowledge, this study is the first to report on specific causes of late mortality among five-year survivors of childhood cancer beyond broad, systems-based categories such as cardiac, respiratory, or infectious causes. Since the most recent report from the Childhood Cancer Survivor Study (CCSS) in 2016, there has been an additional ten years of mortality data with nearly 2,000 additional deaths reported, most due to health-related causes (includes deaths related to late-effects of cancer therapy but excludes deaths due to the primary cancer or external/accidental causes). With this added follow-up, we were able to report on over 70 discrete causes of death using a modification of 113 established causes of death according to ICD-9 and −10 codes from the Centers for Disease Control and National Vital Statistics Report and compare them to the general United States population including measures of relative and absolute excess risk. This adds to the existing literature by further detailing specific causes of death (e.g. valvular heart disease, ischemic heart disease, heart failure, and hypertensive heart disease all are cardiac deaths but have differing magnitudes of excess risk and patterns of change over survival time). This detailed classification also allowed us to demonstrate, for the first time, that many specific causes of death experienced in late survivorship are the same as the major causes of death in the general population; however, survivors are dying at a younger age and higher rate. Further, the CCSS includes detailed treatment data and self-report of potentially modifiable lifestyle and traditional cardiovascular risk factors, information that is not routinely available in other large survivor cohorts. This allowed us to identify associations between healthy lifestyle, absence of cardiovascular risk factors, and reduced risk for health-related late mortality among survivors, independent of prior cancer treatment. Collectively, these findings better characterize excess late mortality and the impact of modifiable lifestyle and cardiovascular risk factors and identify opportunities for future research into interventions to mitigate risk for these common causes of death among survivors who have uniquely high risks associated with their prior cancer treatment.
Implications of all the available evidence
Despite improvements in five-year survival from childhood cancer and reduced late mortality among those treated in more recent eras, the excess risk of death among survivors continues to increase in late survivorship. While deaths within the first ten years from diagnosis are primarily due to recurrence or progression of the primary cancer, those occurring beyond ten years from diagnosis are most often related to early onset of many of the leading causes of death commonly observed in the middle-aged and older adult population including age-related cancers, heart disease, cerebrovascular disease, and specific infectious causes of death. As a result, by 40 years from diagnosis survivors of childhood cancer in the CCSS experienced 138 excess deaths per 10,000 person-years of follow-up compared to the general population of the United States. Further, even decades after treatment, many therapy exposures remain associated with a dose dependent increase in risk of death among survivors and further motivate efforts to reduce exposure to these agents while maintaining five-year survival. Finally, the absence of potentially modifiable lifestyle and cardiovascular risk factors were found to be associated with a reduced risk of late mortality and may be a target for future interventions.
Acknowledgements
We are grateful to the funding agencies who supported this work including support for the CCSS by the National Cancer Institute (CA55727) and additional support to St. Jude Children’s Research Hospital provided by the Cancer Center Support (CORE) grant (CA21765) and the American Lebanese-Syrian Associated Charities (ALSAC).
Footnotes
Declaration of interests
The authors have no conflicts to declare.
Partial results from this research were previously presented as an oral presentation at The American Society of Clinical Oncology (ASCO) Annual Meeting 2021, virtual format with additional results presented in a poster at The American Society of Clinical Oncology (ASCO) Annual Meeting 2022.
Contributor Information
Stephanie B. Dixon, Department of Oncology, St. Jude Children’s Research Hospital, Memphis, TN; Department of Epidemiology and Cancer Control, St. Jude Children’s Research Hospital, Memphis, TN
Qi Liu, School of Public Health, University of Alberta, Edmonton, Alberta, Canada
Eric J. Chow, Cancer Prevention, Fred Hutchinson Cancer Research Center, Seattle, WA
Kevin C. Oeffinger, Department of Medicine, Duke University, Durham, NC.
Paul C. Nathan, Division of Hematology/Oncology, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada.
Rebecca M. Howell, Radiation Physics Department, The University of Texas at MD Anderson Cancer Center, Houston, TX.
Wendy M. Leisenring, Cancer Prevention, Fred Hutchinson Cancer Research Center, Seattle, WA; Clinical Statistics Programs, Fred Hutchinson Cancer Research Center, Seattle, WA.
Matthew J. Ehrhardt, Department of Oncology, St. Jude Children’s Research Hospital, Memphis, TN; Department of Epidemiology and Cancer Control, St. Jude Children’s Research Hospital, Memphis, TN
Kirsten K. Ness, Department of Epidemiology and Cancer Control, St. Jude Children’s Research Hospital, Memphis, TN.
Kevin R. Krull, Department of Epidemiology and Cancer Control, St. Jude Children’s Research Hospital, Memphis, TN; Department of Psychology, St. Jude Children’s Research Hospital, Memphis, TN.
Ann C. Mertens, Department of Pediatrics, Emory University School of Medicine, Atlanta, GA.
Melissa M. Hudson, Department of Oncology, St. Jude Children’s Research Hospital, Memphis, TN; Department of Epidemiology and Cancer Control, St. Jude Children’s Research Hospital, Memphis, TN.
Leslie L Robison, Department of Epidemiology and Cancer Control, St. Jude Children’s Research Hospital, Memphis, TN.
Yutaka Yasui, Department of Epidemiology and Cancer Control, St. Jude Children’s Research Hospital, Memphis, TN.
Gregory T. Armstrong, Department of Oncology, St. Jude Children’s Research Hospital, Memphis, TN; Department of Epidemiology and Cancer Control, St. Jude Children’s Research Hospital, Memphis, TN.
Data sharing statement
The Childhood Cancer Survivor Study is an NCI-funded resource (U24 CA55727) to promote and facilitate research among long-term survivors of cancer diagnosed during childhood and adolescence. Access to the CCSS data is considered on a case-by-case basis. For all types of access, a research application of intent followed by an analysis concept proposal must be submitted for evaluation by the CCSS Publications Committee. Users interested in potential uses of this resource are encouraged to visit http://ccss.stjude.org. In addition, CCSS data is publicly available on dbGaP, accession number: phs001327.v2.p1. Part of the data are shared on St. Jude Cloud at https://www.stjude.cloud/research-domains/cancer-survivorship.
References
- 1.Yeh JM, Ward ZJ, Chaudhry A, et al. Life expectancy of adult survivors of childhood cancer over 3 decades. JAMA Oncol 2020;6:350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howlader N NA, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2018. Bethesda, MD: National Cancer Institute; 2021. [Google Scholar]
- 3.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 2006;355:1572–82. [DOI] [PubMed] [Google Scholar]
- 4.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA 2013;309:2371–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst 2008;100:1368–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong GT, Chen Y, Yasui Y, et al. Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med 2016;374:833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schindler M, Spycher BD, Ammann RA, et al. Cause-specific long-term mortality in survivors of childhood cancer in Switzerland: A population-based study. Int J Cancer 2016;139:322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reulen RC, Winter DL, Frobisher C, et al. Long-term cause-specific mortality among survivors of childhood cancer. JAMA 2010;304:172–9. [DOI] [PubMed] [Google Scholar]
- 9.Merzenich H, Baaken D, Schneider A, et al. Mortality risk among 5-Year survivors of childhood cancer in Germany - Results from the CVSS study. Int J Cancer 2022;150:67–72. [DOI] [PubMed] [Google Scholar]
- 10.Byrne J, Schmidtmann I, Rashid H, et al. Impact of era of diagnosis on cause-specific late mortality among 77 423 five-year European survivors of childhood and adolescent cancer: The PanCareSurFup consortium. Int J Cancer 2022;150:406–19. [DOI] [PubMed] [Google Scholar]
- 11.Möller TR, Garwicz S, Barlow L, et al. Decreasing late mortality among five-year survivors of cancer in childhood and adolescence: A population-based study in the Nordic countries. J Clin Oncol 2001;19:3173–81. [DOI] [PubMed] [Google Scholar]
- 12.Williams AM, Liu Q, Bhakta N, et al. Rethinking success in pediatric oncology: Beyond 5-year survival. J Clin Oncol 2021;39:2227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yusuf S, Joseph P, Rangarajan S, et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet 2020;395:795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Pan A, Wang DD, et al. Impact of healthy lifestyle factors on life expectancies in the US population. Circulation 2018;138:345–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: experience of the Childhood Cancer Survivor Study. J Clin Oncol 2009;27:2319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol 2009;27:2308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson RN, Minino AM, Hoyert DL, Rosenberg HM. Comparability of cause of death between ICD-9 and ICD-10: preliminary estimates. Natl Vital Stat Rep 2001;49:1–32. [PubMed] [Google Scholar]
- 18.Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res 2006;166:141–57. [DOI] [PubMed] [Google Scholar]
- 19.Little RJA, Rubin DB. Statistical Analysis with Missing Data, Second Edition. 2nd ed: John Wiley & Sons, Inc.; 2002. [Google Scholar]
- 20.Scott JM, Li N, Liu Q, et al. Association of exercise with mortality in adult survivors of childhood cancer. JAMA Oncol 2018;4:1352–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armstrong GT, Oeffinger KC, Chen Y, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol 2013;31:3673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.United States Department of Health and Human Services Centers for Disease Control and Prevention NCfHS. Compressed mortality file on CDC Wonder Online Database; CMF 1999-2016, Series 20, No 2v, 2017; CMF 1968-1988, Series 20, No 2A, 2000; CMF 1989-1998, Series 20, No 2E, 2003.
- 23.Feijen EAM, Leisenring WM, Stratton KL, et al. Derivation of anthracycline and anthraquinone equivalence ratios to doxorubicin for late-onset cardiotoxicity. JAMA Oncol 2019;5:864–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green DM, Nolan VG, Goodman PJ, et al. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: A report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer 2014;61:53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention, National Centers for Injury Prevention and Control. Web-based Injury Statistics Query and Reporting System (WISQARS) Leading Causes of Death Reports, 1981-2020 [online]. (2013-2017) {2022 June 1}. Available from: www.cdc.gov/injury/wisqars.
- 26.Heron M Deaths: Leading Causes for 2017. Natl Vital Stat Rep 2019;68:1–77. [PubMed] [Google Scholar]
- 27.Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med 2015;373:1541–52. [DOI] [PubMed] [Google Scholar]
- 28.Salloum R, Chen Y, Yasui Y, et al. Late morbidity and mortality among medulloblastoma survivors diagnosed across three decades: a report from the Childhood Cancer Survivor Study. J Clin Oncol 2019;37:731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oeffinger KC, Stratton KL, Hudson MM, et al. Impact of risk-adapted therapy for pediatric Hodgkin lymphoma on risk of long-term morbidity: A report from the Childhood Cancer Survivor Study. J Clin Oncol 2021;39:2266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mauz-Korholz C, Landman-Parker J, Balwierz W, et al. Response-adapted omission of radiotherapy and comparison of consolidation chemotherapy in children and adolescents with intermediate-stage and advanced-stage classical Hodgkin lymphoma (EuroNet-PHL-C1): a titration study with an open-label, embedded, multinational, non-inferiority, randomised controlled trial. Lancet Oncol 2022;23:125–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metzger ML, Link MP, Billett AL, et al. Excellent outcome for pediatric patients with high-risk Hodgkin lymphoma treated with brentuximab vedotin and risk-adapted residual node radiation. J Clin Oncol 2021;39:2276–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herrera AF, Li H, Castellino SM, et al. SWOG S1826: A phase III, randomized study of nivolumab plus AVD or brentuximab vedotin plus AVD in patients with newly diagnosed advanced stage classical Hodgkin lymphoma. Blood 2020;136:23–4. [Google Scholar]
- 33.Gökbuget N, Dombret H, Bonifacio M, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood 2018;131:1522–1531. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teachey DT, Hunger SP. Acute lymphoblastic leukaemia in 2017: Immunotherapy for ALL takes the world by storm. Nat Rev Clin Oncol 2018;15:69–70. [DOI] [PubMed] [Google Scholar]
- 35.Children's Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent and Young Adult Cancers, Version 5.0. Monrovia, CA: Children's Oncology Group; 2018. [Google Scholar]
- 36.Kremer LC, Mulder RL, Oeffinger KC, et al. A worldwide collaboration to harmonize guidelines for the long-term follow-up of childhood and young adult cancer survivors: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Pediatr Blood Cancer 2013;60:543–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Armenian SH, Hudson MM, Mulder RL, et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol 2015;16:e123–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bowers DC, Verbruggen LC, Kremer LCM, et al. Surveillance for subsequent neoplasms of the CNS for childhood, adolescent, and young adult cancer survivors: a systematic review and recommendations from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol 2021;22:e196–e206. [DOI] [PubMed] [Google Scholar]
- 39.van Dalen EC, Mulder RL, Suh E, et al. Coronary artery disease surveillance among childhood, adolescent and young adult cancer survivors: A systematic review and recommendations from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Eur J Cancer 2021;156:127–37. [DOI] [PubMed] [Google Scholar]
- 40.Armenian SH, Hudson MM, Chen MH, et al. Rationale and design of the Children's Oncology Group (COG) study ALTE1621: a randomized, placebo-controlled trial to determine if low-dose carvedilol can prevent anthracycline-related left ventricular remodeling in childhood cancer survivors at high risk for developing heart failure. BMC Cardiovasc Disord 2016;16:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chow EJ, Chen Y, Hudson MM, et al. Prediction of ischemic heart disease and stroke in survivors of childhood cancer. J Clin Oncol 2018;36:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Y, Chow EJ, Oeffinger KC, et al. Traditional cardiovascular risk factors and individual prediction of cardiovascular events in childhood cancer survivors. J Natl Cancer Inst 2020;112:256–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol. J Am Coll Cardiol 2019;73:e285–e350. [DOI] [PubMed] [Google Scholar]
- 44.Klesges RC, Krukowski RA, Klosky JL, et al. Efficacy of a tobacco quitline among adult survivors of childhood cancer. Nicotine Tob Res 2015;17:710–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang FF, Kelly MJ, Must A. Early Nutrition and Physical Activity Interventions in Childhood Cancer Survivors. Curr Obes Rep. 2017. Jun;6(2):168–177. doi: 10.1007/s13679-017-0260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheung AT, Li WHC, Ho LLK, Ho KY, Chan GCF, Chung JOK. Physical activity for pediatric cancer survivors: a systematic review of randomized controlled trials. J Cancer Surviv. 2021. Dec;15(6):876–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phillips SM, Padgett LS, Leisenring WM, et al. Survivors of Childhood Cancer in the United States: Prevalence and Burden of Morbidity. Cancer Epidemiol Biomarkers Prev. 2015; 24(4): 653–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Childhood Cancer Survivor Study is an NCI-funded resource (U24 CA55727) to promote and facilitate research among long-term survivors of cancer diagnosed during childhood and adolescence. Access to the CCSS data is considered on a case-by-case basis. For all types of access, a research application of intent followed by an analysis concept proposal must be submitted for evaluation by the CCSS Publications Committee. Users interested in potential uses of this resource are encouraged to visit http://ccss.stjude.org. In addition, CCSS data is publicly available on dbGaP, accession number: phs001327.v2.p1. Part of the data are shared on St. Jude Cloud at https://www.stjude.cloud/research-domains/cancer-survivorship.