Abstract
Carbapenem-resistant Acinetobacter baumannii-calcoaceticus complex (CRAB) is one of the top-priority pathogens for new antibiotic development. Unlike other antibiotic-resistant threats, none of the available therapies have been shown to consistently reduce mortality or improve patient outcomes in clinical trials. Antibiotic combination therapy is routinely used in clinical practice; however, the preferred combination has not been defined. This narrative review focuses on evidence-based solutions for the treatment of invasive CRAB infections. We dissect the promise and perils of traditional agents used in combination, such as colistin, sulbactam, and the tetracyclines, and offer clinical pearls based on our interpretation of the available data. Next, we investigate the merits of newly developed β-lactam agents like cefiderocol and sulbactam-durlobactam, which have demonstrated contrasting results in recent randomized clinical trials. The review concludes with the authors’ perspective on the evolving treatment landscape for CRAB infections, which is complicated by limited clinical data, imperfect treatment options, and a need for future clinical trials. We propose that effective treatment for CRAB infections requires a personalized approach that incorporates host factors, the site of infection, pharmacokinetic-pharmacodynamic principles, local molecular epidemiology of CRAB isolates, and careful interpretation of antibiotic susceptibility testing results. In most clinical scenarios, a dose-optimized, sulbactam-based regimen is recommended with the addition of at least one other in vitro active agent. Should sulbactam-durlobactam receive regulatory approval, recommendations will need to be re-evaluated with the most recent evidence.
Keywords: Acinetobacter, sulbactam, durlobactam, colistin, cefiderocol
Acinetobacter baumannii-calcoaceticus complex isolates commonly cause of nosocomial infections. This organism displays clonal dissemination and is able to accumulate resistance traits by horizontal gene transfer, natural transformation, acquisition of mutations, and mobilization of genetic elements that modulate gene expression.
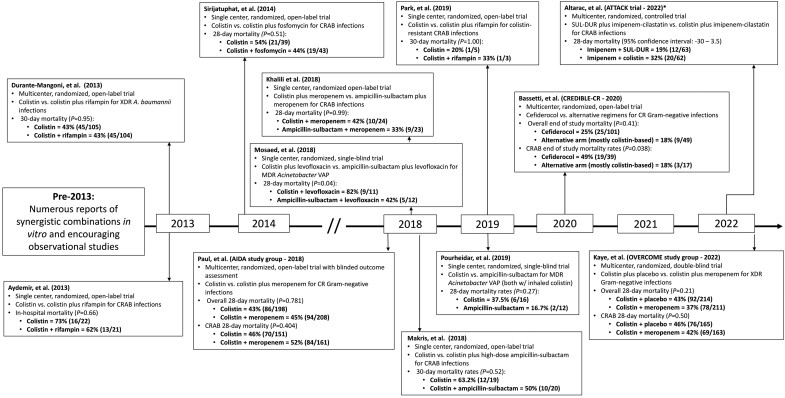
Carbapenem-resistant Acinetobacter baumannii-calcoaceticus complex (CRAB) remains one of the foremost public health challenges of the 21st century. Largely regarded as a top-priority pathogen globally for new antibiotic development [1, 2], CRAB are notorious for their ability to survive in hospital environments, evade host immunity, acquire new antibiotic-resistance mechanisms, and defy therapeutic countermeasures [3]. Unlike other antibiotic-resistant pathogens [4], no available treatments have been shown to substantially lower mortality or significantly improve the outcome of patients with invasive CRAB infections [5, 6]. As a result, 28-day mortality rates among patients enrolled in randomized clinical trials investigating CRAB therapeutics exceed 45% (Figure 1). These data underscore disproportionately increased rates of death associated with CRAB infections when compared with other carbapenem-resistant pathogens [5, 7, 8]. Indeed, CRAB infections are the fourth-leading cause of death attributable to antimicrobial resistance globally [9].
Figure 1.
A brief timeline of noteworthy CRAB clinical trials. *Full results have not yet been published. Abbreviations: CR, carbapenem-resistant; CRAB, carbapenem-resistant Acinetobacter baumannii-calcoaceticus complex; MDR, multidrug-resistant; SUL-DUR, sulbactam-durlobactam; VAP, ventilator-associated pneumonia; XDR, extensively drug-resistant.
Beyond its public health relevance, assessment and management of individual patients from whom CRAB is isolated remains a major challenge to clinicians for several reasons. First, differentiating critically ill patients with respiratory colonization from those with acute infections is rarely intuitive [10], and is particularly difficult among patients with severe immunocompromise and other high-risk conditions. Accordingly, CRAB-directed treatment is often initiated in the face of diagnostic uncertainty. Second, the majority of CRAB infections are pneumonia, and rapid molecular tests that identify CRAB from respiratory specimens are not routinely used in regions where CRAB is highly prevalent. This results in delayed treatment of CRAB pneumonia that contributes to poor outcomes [11]. Third, CRAB pneumonia requires optimization of antimicrobial dosing to achieve pharmacokinetic-pharmacodynamic (PK-PD) targets within the epithelial lining fluid (ELF) and lung parenchyma. Unfortunately, many of the available antimicrobial agents with in vitro activity against CRAB are limited by poor penetration into the lungs and dose-dependent toxicities (Table 1). Fourth, biofilm formation associated with CRAB infections is commonly associated with resistant phenotypes and enhanced virulence [12], highlighting the importance of removing indwelling devices contaminated with CRAB [13]. Finally, clinical breakpoints to determine antibiotic susceptibility against CRAB isolates have either not been established, require revision, or vary across professional organizations [14–16].
Table 1.
The promise, perils, and pearls of the preferred antibiotic options for the treatment of CRAB infections
| Agent | Percentages of In Vitro Activity Against CRAB Isolates [17, 19, 26, 40, 90, 141, 142] |
Promise | Perils | Pearls in the Management of Invasive CRAB Infections |
|---|---|---|---|---|
| Polymyxins | ||||
| Polymyxin B | 80.3–99.3% (Defined as colistin MICs ≤2 mg/L) |
|
|
|
| Tetracyclines | ||||
| Minocycline | 54–72.1% (Defined by the CLSI breakpoint of ≤4 mg/L) |
|
|
|
| Tigecycline | No breakpoints established; MIC50 = 1–4 mg/L; MIC90 = 2–8 mg/L |
|
|
|
| β-lactams | ||||
| Cefiderocol | 89.7–96.1% (Defined by the CLSI breakpoint of ≤4 mg/L) |
|
|
|
| β-lactams–β-lactamase inhibitors | ||||
| Ampicillin-sulbactam | 3.7–24.3% (Defined by CLSI ampicillin-sulbactam breakpoint of ≤8/4 mg/L) |
|
|
|
| Sulbactam-durlobactam | 96.7–96.8% (Based on FDA provisional breakpoint of ≤4 mg/L) |
|
|
|
Abbreviations: CLSI, Clinical and Laboratory Standards Institute; CRAB, carbapenem-resistant Acinetobacter baumannii-calcoaceticus complex; EUCAST, European Committee on Antimicrobial Susceptibility Testing; FDA, Food and Drug Administration; fT > MIC, time free antibiotic concentrations are above the minimum inhibitory concentration; MIC, minimum inhibitory concentration.
Global proportions of carbapenem resistance against A. baumannii vary between 30% and 80%, and are the highest in Asia, Eastern Europe, and Latin America [17–19]. Corresponding proportions of resistance in the United States range from 30% to 50% [18, 19]. Antibiotic resistance in CRAB is mediated through complex mechanisms that include intrinsic and acquired β-lactamases, upregulation of efflux pumps, decreased outer membrane permeability, and antibiotic target site modifications [2, 18, 20]. Carbapenem resistance is commonly associated with the horizontal transfer of genes encoding oxacillinase (OXA) carbapenemases including OXA-23 and OXA-24/40 enzymes [21, 22]. Importantly, rates and underlying mechanisms of carbapenem resistance are geographically specific [17], which confounds the interpretation of the data from single centers or certain regions. In a recent analysis of CRAB isolates collected from 4 US healthcare systems, several distinct CRAB lineages were identified, and each varied by hospital and resistance phenotype [22]. Whole-genome phylogeny studies uncovered a diverse population structure that likely manifested over time due to recombination events and plasmid transmission between endemic strains [22]. When compared with prior investigations [23], it is notable that the predominant CRAB clonal types have shifted over time [24]. Concerningly, rates of non-susceptibility to key antibiotics like ampicillin-sulbactam and colistin are increasing in the United States [22] and worldwide [25].
Agents demonstrating the highest rates of in vitro activity in rank order, include the polymyxins (colistin, polymyxin B), tetracyclines (eravacycline, minocycline, tigecycline), and β-lactams (ampicillin-sulbactam, carbapenems). Novel β-lactams like cefiderocol and sulbactam-durlobactam show potent in vitro activity across diverse isolates [19, 26]; however, susceptibility breakpoints vary or have not yet been established, respectively. Shortcomings for all of these agents have been reviewed elsewhere [18, 20, 27, 28], and are summarized in Table 1.
Combination therapy is generally preferred for invasive CRAB infections. The rationale for combination therapy is dependent upon the poor efficacy and considerable toxicities and/or PK limitations for each of the individual treatment options with anticipated in vitro activity. Supporting evidence stems from in vitro synergy studies of various combinations and its theoretical benefit in suppressing the emergence of further antibiotic resistance in CRAB, although supportive data are lacking to indicate that this benefit translates in vivo [29]. Moreover, there is a general recognition that patients with invasive CRAB infections are susceptible to poor clinical outcomes. Thus, the potential benefits of antibiotic combination therapy seemingly outweigh the risks of potentially suboptimal treatment with a single agent [20]. Indeed, both the Infectious Diseases Society of America (IDSA) antimicrobial-resistance (AMR) treatment guidance document and European Society of Clinical Microbiology and Infectious Diseases (ESCMID) antimicrobial-resistance treatment guidelines suggest combination therapy with at least 2 in vitro active agents (when available) for severe CRAB infections [30, 31].
This narrative review will focus on evidence-based solutions for key questions surrounding the management of invasive CRAB infections. The objective is to highlight antibiotic combinations with the most comprehensive or promising data, and not combinations unlikely to improve patient outcomes, such as colistin plus fosfomycin [32], colistin plus rifampin [33–35], and aminoglycoside-based combinations [36].
IS IT TIME TO RETIRE COLISTIN-MEROPENEM COMBINATIONS FOR CRAB?
The short answer is “yes” for colistin plus meropenem alone; however, unanswered questions remain for the use of these 2 agents plus other potentially active drugs in combination [29, 37, 38]. It is unclear if data for the combination of colistin plus meropenem for CRAB infections are representative of outcomes for polymyxin B plus meropenem [39]. The genesis of colistin as a backbone for combination therapy stems from high rates of in vitro activity against genetically diverse isolates collected worldwide [17, 19, 22, 26, 40]. While the limitations of colistin are well known [41], the rationale for partnering colistin with a carbapenem has been justified by high rates of in vitro synergy across numerous studies [38, 42–44]. A mechanistic explanation for observed synergy exists whereby colistin potentiates the activity of carbapenems through depolarization of the outer cell membrane, allowing for increased access of carbapenems to their target sites within the periplasmic space.
Unfortunately, 2 large randomized clinical trials have demonstrated that this approach is no better than treating patients with colistin alone for invasive CRAB infections [6, 45]. In the first of these 2 trials, patients were randomized to receive colistin alone or colistin in combination with dose-optimized meropenem (2 g every 8 hours given as a 3-hour infusion) for treatment of severe infections caused by carbapenem-resistant, gram-negative pathogens. Clinical outcomes were assessed by 2 investigators blinded to the treatment arm. The primary outcome was clinical success at 14 days defined as survival with improvement or stability in signs and symptoms of infection. In total, 198 patients were assigned to receive colistin monotherapy and 208 patients to receive colistin plus meropenem. The main pathogen in the study was CRAB (77%), and common infections included pneumonia (45%) and bacteremia (43%). Overall, no significant differences were observed for clinical success or survival among patients who received colistin monotherapy or combination therapy. For patients infected with CRAB specifically, proportions of clinical failure were 83% (125/151) and 81% (130/161) among patients who received colistin alone or in combination with meropenem, respectively. The corresponding 28-day mortality rates following CRAB infections were 46% (70/151) and 52% (84/161), respectively.
These trial findings were largely consistent with results of a second double-blind, placebo-controlled trial to evaluate patients who received colistin alone versus colistin in combination with meropenem (1 g every 8 hours as a 30-minute infusion [4 patients received imipenem-cilastatin]) for the treatment of bacteremia or pneumonia due to extensively drug-resistant, gram-negative pathogens [45]. In total, 423 patients were included in the modified intent-to-treat analysis. The most frequently isolated pathogens were A. baumannii (78%), carbapenem-resistant Enterobacterales (16%), and Pseudomonas aeruginosa (10%); over 90% of all isolates were carbapenem resistant. The primary outcome of the study was 28-day all-cause mortality, which did not differ between patients who received colistin monotherapy or combination therapy across all pathogens (43% vs 37%), including those specifically infected with A. baumannii (46% vs 42%). A composite definition of clinical failure did not unveil any potential benefit to treatment with colistin combination therapy, resulting in clinical failure rates of 70% and 64% for patients infected with A. baumannii treated with colistin or colistin plus a carbapenem, respectively. These data underscore the likely futility of combining colistin with a carbapenem. In fact, post hoc analyses of both trials were unable to associate in vitro synergy with improved clinical outcomes [42, 43].
Overall these data may not be surprising given that both studies were conducted among critically ill patients who predominantly had lower respiratory tract infections, a site at which colistin is unlikely to achieve therapeutic levels [46, 47], and corresponding isolates from patients uniformly showed high-level carbapenem resistance [42, 43]. On the other hand, it is surprising that nearly all of the available clinical data to date have been generated with colistin, and not polymyxin B—a derivative with advantages that include less interpatient variability in drug exposures and improved safety for patients [39, 41]. It is unclear if polymyxin B more readily concentrates in the ELF and lung parenchyma than colistin, given the dearth of human PK studies [48]. Nonetheless, polymyxin B clearly achieves steady-state concentrations faster and more reliably than the pro-drug colistin (administered as colistimethate), and demonstrates consistent exposures across patients with or without renal impairment [39]. For these reasons, polymyxin B is preferred over colistin against CRAB infections when a polymyxin agent is used [30, 31]; however, given the lack of clinical data, the combination of polymyxin B with a carbapenem for treatment of CRAB infections is not advised.
How to best use the polymyxins then is still a matter of debate. One approach is the use of a 3-drug combination that includes ampicillin-sulbactam, carbapenems, and polymyxins. Mechanistically, polymyxins likely facilitate increased access for both ampicillin-sulbactam and carbapenems, enabling each to saturate complementary penicillin-binding proteins (PBP1/3 and PBP2, respectively). Time-kill studies have demonstrated increased killing with this 3-drug combination when compared with 2-drug combinations [29], particularly against isolates collected from patients previously treated with colistin-carbapenem regimens [38]. Clinical data are sparse, but use of the combination has been motivated by a small single-center observation of lower 30-day mortality rates for patients who received a 3-drug regimen of ampicillin-sulbactam, colistin, and a carbapenem (0%; 0/7) compared with other regimens (60%; 6/10) (P = .03) for treatment of colistin-resistant CRAB infections [37]. A study conducted during a single-center outbreak of CRAB infections among patients with coronavirus disease 2019 (COVID-19) used dose-optimized treatment with ampicillin-sulbactam, polymyxin B, and meropenem, which resulted in overall low 30-day mortality rates (23% [3/13]) relative to previous clinical trials; importantly, the combination appeared to suppress the emergence of further antibiotic resistance [49]. These data are supported by more rapid bactericidal killing of 3-drug combinations in a dynamic hollow-fiber infection model when compared with monotherapy or 2-drug combinations against CRAB [29]. It is unclear if these preliminary data may ultimately identify an effective 3-drug combination for patients with invasive CRAB infections, or if they serve as further evidence to support the utility of sulbactam in constructing effective combinations. Despite the lack of robust clinical data, the authors consider combination therapy with high-dose ampicillin-sulbactam, polymyxin B with high-dose, extended infusion meropenem as a reasonable treatment option for invasive CRAB infections that have either recurred following primary treatment or in the setting of documented resistance to other available treatment options. Close monitoring for toxicity is warranted given the use of dual β-lactams and polymyxin B. The 2023 IDSA AMR Guidance document does not advocate for the use of carbapenem therapy as a component of combination therapy for the treatment of CRAB infections [30].
SULBACTAM OR BUST FOR TREATMENT OF INVASIVE CRAB INFECTIONS?
A compelling case for the use of ampicillin-sulbactam can be made from the available evidence. This case hinges upon the safety profile of high-dose β-lactams and the unique activity of sulbactam against CRAB. Sulbactam targets and saturates PBP1a, PBP1b, and PBP3 in A. baumannii-calcoaceticus complex. Its utility, however, is dependent upon achieving PD targets for sulbactam with the commercially available 2:1 formulation of ampicillin-sulbactam (2 g of ampicillin, 1 g of sulbactam). Like other β-lactams, the time free sulbactam concentrations are above the minimum inhibitory concentration (fT > MIC) is the driver of efficacy in murine infection models [50, 51]; however, drug exposures vary widely across critically ill patients [52]. Using a target of 60% fT > MIC for patients with a creatinine clearance ranging between 90 and 120 mL/minute, sulbactam doses of 1 g every 6 hours or 2 g every 8 hours as a 4-hour prolonged infusion are needed to achieve a more than 90% probability of target attainment when MICs are less than 4 mg/L [52]. In the more likely event that sulbactam MICs are 16 mg/L or greater [19], dosing regimens equivalent to 9 g/day of sulbactam are needed and have been shown to be safe in patients [53]. This directly translates to ampicillin-sulbactam dosing regimens of 9 g every 8 hours as a prolonged 4-hour infusion or 27 g as a continuous infusion [30]. Recently, an alternative target of 25% fT > MIC has been associated with 1-log killing in a murine neutropenic lung infection model [52]. Using this target, sulbactam doses of 1 g every 4 hours are needed to achieve more than 90% target attainment for MICs up to 8 mg/L. These data provide support for an ampicillin-sulbactam regimen of 3 g every 4 hours when isolates test susceptible or intermediate to ampicillin-sulbactam. For isolates testing resistant (MIC ≥16 mg/L), however, ampicillin-sulbactam optimized regimens of 9 g every 8 hours administered as a 4-hour infusion are needed to achieve PK-PD targets [51]. The importance of sulbactam dose optimization cannot be understated given that most clinical isolates of CRAB test non-susceptible to ampicillin-sulbactam when applying Clinical and Laboratory Standards Institute (CLSI) interpretive criteria [19]. When optimized doses are used, ampicillin-sulbactam eradicates CRAB in hollow-fiber infection models [29], and the frequency of spontaneous sulbactam resistance selection appears to be low [54].
Clinical evidence in support of dose-optimized ampicillin-sulbactam for the treatment of CRAB infections has been mounting over the past 2 decades [53, 55, 56]. The vast majority of clinical data, however, have come from observational studies rather than rigorously controlled randomized trials. As a result, the data are highly heterogeneous and ampicillin-sulbactam dosing regimens vary significantly across studies. In an effort to elucidate important differences across studies, several meta-analyses have been undertaken comparing sulbactam-based combinations with other combinations [57–60]. The most recent network meta-analysis and systematic review included 7 randomized clinical trials and 11 observational studies to evaluate endpoints of clinical improvement, clinical cure, microbiologic eradication, and all-cause mortality [59]. The investigators found that sulbactam (≥6 g/day) plus another active antibiotic (either levofloxacin or tigecycline) resulted in higher rates of clinical improvement when compared with colistin alone, colistin plus a carbapenem, or colistin with another active agent (relative risk [RR] = 2.99 [95% confidence interval (CI): 1.08–8.24], 3.12 [1.14–8.60], and 3.06 [1.13–8.29], respectively); however, no regimen was associated with significant improvements in survival. An earlier Bayesian network meta-analysis of 23 studies across 2118 patients compared 15 treatment regimens for the primary outcome of all-cause mortality [58]. The analysis showed that sulbactam (3–8 g/day) and high-dose sulbactam (≥9 g/day) as monotherapy resulted in the highest probability of reducing mortality when compared with other treatments; however, the combination of sulbactam plus colistin ranked as the lowest. In the same study, sulbactam was superior to colistin monotherapy for reducing all-cause mortality by Bayesian posterior probability estimates (odds ratio [OR] = .27; 95% CI: .06–.91), but high-dose sulbactam (OR = .56; 95% CI: .09–3.17) and sulbactam plus colistin (OR = 2.58; 95% CI: .71–9.88) were not. A third network meta-analysis found that colistin-based combinations were associated with lower all-cause mortality than sulbactam-based combinations [57]. These conflicting results add to the mystery of defining the best treatment options against CRAB, particularly when comparing observational studies that do not have standardized methods or standardized dosing of colistin or sulbactam. Thus, some reliance is needed upon individual studies where standardized approaches are used. In an interim analysis of 23 patients randomized to receive colistin plus levofloxacin (n = 11) or continuous infusion ampicillin-sulbactam (24 g daily; equivalent to 8 g of sulbactam) plus levofloxacin (n = 12) for CRAB pneumonia, significantly higher rates of clinical cure (83% vs 27%; P = .007) and lower rates of 28-day mortality (42% vs 82%; P = .04) were identified among patients randomized to receive ampicillin-sulbactam [55]. Numerically lower rates of death (17% vs 38%) were also reported among 28 patients randomized to continuous infusion ampicillin-sulbactam or colistin (both administered with inhaled colistin) for multidrug-resistant (MDR) Acinetobacter pneumonia [61].
Perhaps the most compelling data in support of ampicillin-sulbactam come from an open-label, prospective randomized study in 2 Greek intensive care units (ICUs) [62]. In this study, 39 patients with CRAB pneumonia were randomized to receive colistin alone or colistin plus high-dose ampicillin-sulbactam (6 g every 6 hours; equivalent to 8 g of sulbactam daily). To be included in the study, patients were required to be infected with colistin and ampicillin-sulbactam susceptible CRAB; ampicillin-sulbactam susceptibility was defined as an MIC of 8 mg/L or less. Clinical response was defined as an improvement in symptoms for at least 48 hours and was assessed by the unblinded treating physician. Initial clinical response was demonstrated in 16% (3/19) and 70% (14/20) of patients receiving colistin alone and colistin plus ampicillin-sulbactam, respectively (OR = 12.4; 95% CI: 2.6–59.3; P = .001). The treating clinician was allowed to change therapy if it was determined to be unsuccessful after the fourth day, resulting in changes for 16 patients in the colistin-alone arm and 3 changes in the combination arm of the study. Among those who initially received colistin alone, a favorable clinical response was observed in 38% (6/16) when ampicillin-sulbactam was added. Altogether, 28-day mortality rates did not differ between patients who received colistin alone (63%) or colistin plus ampicillin-sulbactam (50%) (P = .52). Given the open-label design and physician-assigned outcomes in the study, caution should be exercised in extrapolating the findings. Moreover, these data highlight a broader challenge of identifying effective treatment for CRAB in the setting of critical illness and high baseline mortality rates.
Conceptually speaking, the notion that an in vitro active β-lactam antibiotic like ampicillin-sulbactam would be more effective than colistin for the treatment of CRAB infections, particularly pneumonia, is somewhat intuitive given the known PK limitations and toxicity associated with colistin [41]. Unfortunately, such hypotheses are not supported by the available clinical data. This is due, in part, to the very low rates of in vitro activity for sulbactam against CRAB in international surveillance studies [19]. It is important to recognize, and not extrapolate, the differential activity of sulbactam against all A. baumannii-calcoaceticus complex isolates when compared with carbapenem-resistant isolates where median MICs are 8 and 64 mg/L, respectively [19]. Moreover, the sulbactam MIC against A. baumannii clinical isolates with varying β-lactamases ranges anywhere from 0.5 to 64 mg/L [54]. When applying the current CLSI interpretative breakpoint of ≤8/4 mg/L (corresonding to a sulbactam MIC ≤4 mg/L) for ampicillin-sulbactam against Acinetobacter spp., less than 5% of CRAB isolates test susceptible [19]. When more liberal sulbactam breakpoints of ≤8 or ≤16 mg/L are considered, still less than half of CRAB isolates are categorized as susceptible [52]. These data serve as a valuable reminder that sulbactam does not inhibit, but rather is a substrate for, hydrolysis by TEM-1, ADC-30, and numerous OXA enzymes that are produced by CRAB isolates [63–65]. Thus, the utility of ampicillin-sulbactam can be best summarized as an arms race between the ability of sulbactam to reach PBP targets before degradation by β-lactamases within the periplasmic space. Increasing doses of sulbactam may improve the likelihood that sufficient saturation occurs prior to degradation, but this is not a guarantee across diverse clinical isolates.
A similar paradigm was previously established for the treatment of Klebsiella pneumoniae carbapenemase (KPC)–producing Enterobacterales, where the utility of dual carbapenem therapy [66], or even extremely high doses of meropenem [67], to overcome KPC-mediated hydrolysis was investigated. While some clinical reports were encouraging [68], efficacy ultimately depended upon the use of carbapenems in combination with other in vitro active agents when carbapenem MICs were 8 mg/L or less [69]. Even with ideal circumstances, patient outcomes remained poor [70]. These strategies have largely been abandoned since the introduction of novel β-lactamase inhibitors like avibactam, relebactam, and vaborbactam, that inhibit KPC-mediated hydrolysis and protect partner β-lactam agents. Use of these novel agents has led to dramatically improved clinical outcomes and lower mortality for patients with KPC-producing Enterobacterales infections when compared with traditional combination approaches [71–73]. A compelling hypothesis based on these data can be proposed for sulbactam, such that optimal use of sulbactam against CRAB is to similarly protect this agent from hydrolysis with a β-lactamase inhibitor. Until such options are clinically available and the evidence is fully evaluated, high-dose ampicillin-sulbactam (defined as regimens of at least 9 g/day of sulbactam) should be used in combination with at least 1 other in vitro active agent, and potentially 2 in vitro active agents when sulbactam MICs are either unknown or ≥16 mg/L. Which agents to use in combination is best informed by the infection site and patient-specific risk factors as summarized below [74].
IS THERE A ROLE FOR TETRACYCLINES IN TREATMENT ALGORITHMS FOR INVASIVE CRAB INFECTIONS?
Unlike polymyxin- or sulbactam-based combinations, no randomized clinical trials have compared tetracycline-based regimens with other treatments for CRAB infections. Observational studies have yielded mixed results due to small sample sizes, non-standardized dosing regimens, variable infection types, and use of tetracyclines both as monotherapy and in combination. Most clinical data have been reported for tigecycline against CRAB infections (Table 1), and several notable findings have been described. First, tigecycline monotherapy is associated with higher all-cause mortality when compared with other treatment options, particularly in the setting of bacteremia or pneumonia [58, 75–77]. Second, when used in combination with colistin, the benefit of tigecycline is most consistently demonstrated when MICs are less than 2 mg/L [78, 79]. Notwithstanding, the preferred tigecycline-based combination has not been defined; a 3-drug combination that includes colistin, sulbactam, and tigecycline was associated with the highest rate of clinical cure when compared with other regimens in a meta-analysis of 29 studies and 2529 patients [57]. Finally, higher tigecycline doses (200 mg loading dose followed by 100 mg every 12 hours) have been associated with improved outcomes compared with standard doses [80, 81]. Importantly, however, high-dose regimens have been used almost exclusively in combination with other in vitro active antibiotics, so its role as monotherapy is less clear. These findings should be considered in the context of tigecycline drug exposures that are suboptimal in respiratory tract, serum, and urine, and impacted by nonlinear plasma protein binding [82, 83]. Moreover, neither CLSI nor the European Committee on Antimicrobial Susceptibility Testing (EUCAST) have established a clinical breakpoint for tigecycline against Acinetobacter spp. As a result, the Food and Drug Administration (FDA) susceptibility breakpoint against Enterobacterales (defined as MIC ≤2 mg/L) is often adopted erroneously in clinical practice.
When standard doses are used, tigecycline PK-PD targets (fAUC:MIC) are only achieved with a more than 90% probability when MICs are 1 mg/L or less in critically ill patients, and higher doses of at least 100 mg twice daily are needed to meet the same target when tigecycline MICs are ≥2 mg/L [83]. Conflicting data have been reported in a PK study of serum and ELF concentrations among 32 critically ill patients receiving high-dose tigecycline where the PK-PD target attainment for pneumonia was only achieved reliably when tigecycline MICs were ≤0.5 mg/L [82]. Only 31% and 69% of international CRAB isolates demonstrated tigecycline MICs ≤0.5 and 1 mg/L, respectively [17]. These data support the recommendation to only use high-dose tigecycline, as opposed to standard-dose tigecycline, for the treatment of CRAB infections, and as a component of combination therapy [30, 31]. Efficacy may be limited when MICs are >1 mg/L; however, further studies are needed to define a reliable clinical breakpoint.
Minocycline has also been studied in case series and observational studies against invasive CRAB infections, and used almost exclusively in combination with other agents [84, 85]. In the largest study to date [86], 55 patients received minocycline alone (n = 3) or in various combinations (n = 52) at a single center. Proportions of clinical success and infection-related mortality were 73% and 25%, respectively. Although encouraging, results of this study are very difficult to interpret given the wide variety of combinations used and the use of conservative minocycline dosing with 100 mg twice daily. In fact, minocycline was initially approved in the 1960s, and surprisingly little has been known about the PK of the agent in critically ill patients, or more importantly, if PK-PD targets in the setting of pneumonia are achieved [87]. To this end, a PK study of critically ill patients who received a single 200-mg intravenous dose was conducted and a population PK model was developed [88]. The analysis showed that a dosing regimen of minocycline 200 mg intravenously every 12 hours would only exceed a 90% probability of PK-PD target attainment if MICs are ≤1 mg/L when applying a bacteriostasis target. When a 1-log kill PK-PD target was assessed, more than 90% target attainment was only achieved for isolates with minocycline MICs ≤0.5 mg/L [88]. These findings have a profound impact on the potential utility of minocycline for the treatment of CRAB infections, given that median MICs are 8-fold higher against MDR A. baumannii-calcoaceticus complex isolates when compared with all A. baumannii isolates (MIC50 = 2 and 0.25 mg/L, respectively) [40]. Indeed, if a more conservative susceptibility breakpoint of ≤1 mg/L is applied, rates of susceptibility would fall below 40% [19, 40]. As with tigecycline, the role of minocycline appears to be best placed as a combination regimen when MICs are low and infection-site–specific PK-PD targets can be met. An in vitro study demonstrated a benefit with a 3-drug regimen that included dose-optimized minocycline with polymyxin B and sulbactam [89]; however, limited data are available to support dose-optimized minocycline in combination [85].
Eravacycline is a novel, synthetic fluorocycline that demonstrates lower MICs than minocycline or tigecycline in surveillance studies [90, 91]. Like tigecycline, however, no clinical breakpoints have been defined by CLSI or EUCAST, leaving an important knowledge gap between susceptibility testing and clinical adoption of this agent for invasive CRAB infections. Based on PK-PD studies in a murine thigh-infection model [92], mean fAUC:MIC targets for net bacteriostasis and 1-log kill endpoints against Escherichia coli were 28 and 33, respectively. These targets are notably higher than fAUC:MIC targets for minocycline and tigecycline by comparison [87, 93, 94]; further PK-PD investigations for eravacycline against A. baumannii specifically are needed. Clinical data supporting eravacycline use for CRAB are scant. A single case series reported 32 patients with various CRAB infections [95]. The majority of patients in this study received eravacycline in combination with other antibiotics and were infected by other pathogens in addition to CRAB. Altogether, the 30-day mortality was 22%; however, few clinical correlations can be gleaned from these observational data. In the only comparison study of eravacycline [96], the outcomes of 27 patients who received eravacycline-based treatment for CRAB pneumonia were compared with those of 66 patients who received a variety of alternative therapies across 6 hospitals. Overall 30-day, in-hospital mortality rates were 33% (9/27) and 15% (10/66) for patients who received eravacycline or alternative regimens, respectively (P = .048). Clinical cure was numerically higher among patients who received alternative therapy, including fewer days on mechanical ventilation (P = .016). Notably, patients in the eravacycline group were more likely to have COVID-19 (22% vs 2%) and CRAB bacteremia (15% vs 3%) than the alternative-therapy arm. Future studies are needed before clinical use of eravacycline alone or in combination can be recommended.
Across tetracycline agents, some unique benefits are worth noting. First, minocycline is the only agent with reliable in vitro activity against CRAB that is available as an oral formulation, an advantage for de-escalation in the setting of noninvasive infections. Omadacycline is a new aminomethylcycline agent that is also available as an oral formulation; however, clinical data for treatment of CRAB infections have not been reported. Further, clinical breakpoints have not been established against A. baumannii-calcoaceticus complex, and it is unlikely that fAUC:MIC efficacy targets can be reached with licensed dosing regimens [97, 98]. Second, the tetracycline class generally penetrates soft tissues, bone, and biofilms well, which offers a suitable therapeutic option in the setting of osteoarticular or retained hardware infections [74, 99, 100]. The PK benefits of tetracyclines should be weighed against the potential limitations of low concentrations in the urine, serum, and ELF. Third, tetracyclines are associated with a decreased risk of Clostridioides difficile infections when compared with other antimicrobial classes [101]. Reasonably, use of tetracycline-based combinations could be preferred among patients at high risk of C. difficile infection. Finally, the class is associated with less nephrotoxicity than polymyxin-based combinations. It may be reasonable to prioritize polymyxin-sparing combinations with use of the tetracycline-based regimens for vulnerable patients at risk of nephrotoxicity [74].
HAVE HIGH HOPES FOR CEFIDEROCOL TO TREAT CRAB INFECTIONS BEEN DASHED?
Cefiderocol was developed to overcome various mechanisms of carbapenem resistance, and envisioned as a preferred agent against CRAB infections [102]. Surveillance studies have demonstrated universally high rates of susceptibility against CRAB when defined by the CLSI breakpoint of 4 mg/L or less [26, 103, 104], including against isolates with varying molecular mechanisms of resistance [105]. Unfortunately, the in vitro activity of cefiderocol has not translated into superior clinical efficacy against CRAB infections [102, 106]. In an open-label phase 3 trial, patients were randomly assigned (2:1) to receive cefiderocol or an alternative therapy for the treatment of infections due to carbapenem-resistant gram-negative pathogens. Among 118 patients in the microbiologic intent-to-treat population, 80 were treated with cefiderocol (83% as monotherapy) and 38 with alternative regimens that included colistin alone or in combination with other agents in 16% and 50%, respectively. Overall, 56 patients were infected with CRAB, and unexpectedly, mortality among patients treated with cefiderocol (n = 39) was numerically higher than in those who received alternative regimens (n = 17) at the end-of-study visit (49% vs 18%; P = .04). Patients assigned to the cefiderocol arm were more likely to be in the ICU at the time of randomization and have ongoing septic shock than those in the alternative-therapy arm, which may explain, in part, the imbalanced mortality proportions. In a subsequent randomized phase 3 trial of patients with nosocomial pneumonia, cefiderocol was shown to be noninferior to dose-optimized meropenem among 292 patients [107]. Thirty-six patients were infected with CRAB (n = 18 in each arm), for whom 28-day mortality rates did not differ among those treated with cefiderocol or meropenem (33% and 39%, respectively).
Since publication of these trials, several observational studies have been published [102, 108–112]. The largest comparative study was reported from a single center in Italy [109]. In this study, 124 consecutive patients with CRAB infections were treated with cefiderocol-based (n = 47) or colistin-based (n = 77) regimens and compared through an inverse probability of treatment weighting (IPTW) analysis. The primary outcome was 30-day all-cause mortality. Nearly all patients were in the ICU at the time of CRAB infection with median APACHE-II scores between 16 to 18 across patients. Among those who received cefiderocol, 68% received cefiderocol in combination with another agent, which was most commonly high-dose tigecycline (66%; 21/32 of combinations). By comparison, 84% (65/77) of patients in the colistin arm received colistin-containing combinations that included tigecycline in 91% (59/65) of instances. In an IPTW-adjusted analysis, treatment with cefiderocol was associated with a lower risk of 30-day mortality (hazard ratio [HR] = .44; 95% CI: .22–.66) [109]. Findings were consistent for subgroups that included only patients with monomicrobial infections (P = .04) or bacteremia (P = .007), but not those with pneumonia (P = .918). Notably, most patients with pneumonia had COVID-19, which may have confounded the interpretation of clinical response and contributed to high mortality rates. Likewise, 107 patients admitted to the ICU with severe COVID-19 and nosocomial CRAB infections were evaluated through an earlier multicenter, retrospective study in Italy [112]. Here, the outcomes of consecutive patients treated with cefiderocol monotherapy (n = 42) or colistin-based combination therapy (n = 65) were compared. No differences were identified in all-cause 28-day mortality when stratified by treatment regimen (57% vs 55%), including among the subset of patients with bacteremia (n = 62). Collectively, the real-world evidence offers mixed results that underscores the complexity of defining treatment outcomes.
The real-world evidence to support cefiderocol combination therapy provides a glimmer of optimism despite notable study design limitations and residual selection bias [109, 113]. The collective data also provide even more reasons for caution with cefiderocol. In particular, the single-center experience from Italy reported that microbiologic failures were higher for patients who received cefiderocol compared with colistin (17% vs 7%, respectively), and half of patients who experienced microbiologic failures (8.5%; 4/47 overall) were infected with isolates demonstrating cefiderocol resistance [109]. Microbiologic failures occurred more commonly with cefiderocol monotherapy (43% [6/14]) than combination therapy (6% [2/32]) (P = .006). Indeed, treatment-emergent resistance to cefiderocol in the setting of CRAB infections has been reported elsewhere [5, 108], and appears to be associated with numerous molecular mechanisms [108, 114, 115]. The use of combination therapy may mitigate the emergence of cefiderocol resistance [109], although this hypothesis has not been confirmed by clinical data. In vitro, various agents demonstrate synergy in combination with cefiderocol [104, 116, 117]. In a murine thigh-infection model, the combination of cefiderocol with ampicillin-sulbactam, ceftazidime-avibactam, or meropenem showed enhanced killing of 15 CRAB isolates compared with cefiderocol alone, and combinations of cefiderocol plus ampicillin-sulbactam or ceftazidime-avibactam prevented the emergence of cefiderocol resistance [118].
Cefiderocol PK-PD studies demonstrate comparable ELF exposures to other cephalosporins in ventilated patients [119]. Population PK modeling suggests a high probability of achieving PK-PD targets (fT > MIC) in ELF when cefiderocol MICs are ≤2 mg/L [120]; however, it is notable that targets are considerably higher for CRAB (88% fT > MIC) than for other carbapenem-resistant pathogens (≤70% fT > MIC) [121]. Moreover, determining accurate cefiderocol susceptibility results in clinical practice has proven to be a major challenge given concerns with the reproducibility of results, which is further complicated by varying breakpoints set forth by CLSI, EUCAST, and the FDA [102]. Putting these data together, it is clear that we have yet to define the optimal role of cefiderocol for treatment of invasive CRAB infections. Given the available evidence, and notwithstanding the disappointing results of a large clinical trial [5], it is reasonable to consider cefiderocol as part of combination regimens due to its likely in vitro activity, safety profile, and potentially favorable real-world evidence when used in combination. Monotherapy should be discouraged [5, 109], and among all patients treated with cefiderocol, close observation for the emergence of resistance is highly recommended [108].
ARE WE CLOSE TO ENTERING A NEW ERA IN THE TREATMENT OF CRAB INFECTIONS?
None of the novel β-lactamase inhibitors currently approved by the FDA (avibactam, relebactam, or vaborbactam) reliably inhibit OXA carbapenemases. Durlobactam (formerly known as ETX2514), is a next-generation diazabicyclooctanone (DBO) β-lactamase inhibitor that was chemically optimized to inhibit class D OXA carbapenemases [122]. Like other DBOs (avibactam, relebactam), durlobactam is also a potent inhibitor of class A and C serine β-lactamases. When combined with sulbactam, durlobactam potentiates sulbactam’s activity against CRAB clinical isolates [19]. Durlobactam is tested using a fixed concentration of 4 mg/L and lowers median sulbactam MICs by 32-fold against CRAB. The resulting MIC50 and MIC90 of sulbactam-durlobactam against 2570 global CRAB isolates was 1 and 4 mg/L, respectively [19]. These in vitro data are supported by preclinical PK-PD and safety studies [123] that have led to clinical development of sulbactam-durlobactam dosed at 1 g of sulbactam and 1 g of durlobactam administered every 6 hours as a 3-hour infusion. The dosing regimen that includes 4 g/day of sulbactam was validated through robust population PK studies of both infected patients (n = 162) and healthy subjects (n = 211) using free-drug plasma targets associated with 1-log killing in murine infection models of 50% fT > MIC for sulbactam and an AUC:MIC target of 10 for durlobactam [124]. Target attainment rates across all targets investigated were greater than 90% for isolates with sulbactam-durlobactam MICs of ≤4 mg/L. Penetration into ELF relative to total drug concentrations for sulbactam and durlobactam was 53% and 37%, respectively.
Preliminary results from a phase 3 clinical trial evaluating the safety and efficacy of sulbactam-durlobactam for the treatment of CRAB infections have been reported [125]. Patients with CRAB infections were randomized to receive sulbactam-durlobactam plus imipenem-cilastatin or the combination of colistin plus imipenem-cilastatin. Imipenem-cilastatin was added to sulbactam-durlobactam to expand coverage beyond CRAB for concomitant gram-negative pathogens. Overall, 181 patients were randomized from 95 trial sites across 17 countries; 128 patients were included in the microbiologic intent-to-treat analysis. Among this population, all but 3 patients had pneumonia, 69% (88/128) were in the ICU at the time of randomization, and mean APACHE-II scores ranged from 16 to 17 across study arms. Sulbactam-durlobactam met the primary noninferiority endpoint of 28-day all-cause mortality compared with colistin. Most notably, however, the 28-day mortality rates showed a trend towards lower mortality among patients who received sulbactam-durlobactam (19% [12/63]) compared with colistin (32% [20/62]) (95% CI: –30.0% to 3.5%). At the test-of-cure visit, clinical cure was 62% and 40% for patients who received sulbactam-durlobactam and colistin, respectively (95% CI: 2.9–40.3%). In a parallel, open-label arm for patients who had either failed colistin or were infected by colistin-resistant CRAB, another 28 patients received sulbactam-durlobactam. In this arm, 61% (17/28) had bacteremia and the overall mortality was 18% (5/28)—results in line with those from the main cohort. The study also met its primary safety objective showing a significant reduction in nephrotoxicity among patients who received at least 1 dose of sulbactam-durlobactam or colistin, reporting rates of 13% (12/91) and 38% (32/85), respectively (P = .0002). This study provides an exciting glimpse into the future of improving the outcomes of patients with CRAB infections should the agent ultimately be approved by the FDA. It should be noted, however, that full study results have not yet been published and the findings have not undergone peer review. Future studies are needed to determine the relative contribution, if any, of imipenem-cilastatin to the observed efficacy of sulbactam-durlobactam for the treatment of CRAB infections. To this end, alternative agents have been used successfully in combination with sulbactam-durlobactam to treat invasive CRAB infections through an expanded access program [126, 127].
HOW SHOULD ADJUNCTIVE THERAPIES FOR CRAB BE IMPLEMENTED IN CLINICAL PRACTICE?
Given the complexities surrounding the treatment of invasive CRAB infections, several adjunctive therapies have been explored, including the use of inhaled or aerosolized antibiotics, bacteriophages, and monoclonal antibodies. There are currently limited data to support any of these approaches outside of extenuating circumstances [30, 31].
For patients with CRAB pneumonia, inhaled aminoglycosides and colistin decrease bacterial burdens, but have not been shown to significantly improve clinical outcomes [128, 129]. Use is best reserved for clinical scenarios where reducing the bacterial burden of CRAB may offer benefit for chronically infected patients, including those with structural lung disease. Lung transplant patients, for instance, with upper airway colonization may serve as a reasonable cohort of patients to administer inhaled antibiotics targeted against CRAB, but it should be noted that this practice is not well supported by clinical data [130]. There is likely a role for future clinical trials evaluating inhaled colistin using vibrating mesh nebulizers in combination with intravenous antibiotics to determine whether this adjunctive therapy provides clinical benefit in patients with pneumonia [131].
Bacteriophages have garnered much attention for CRAB on the basis on a single, remarkable case report [132]; however, the broader efficacy of bacteriophages in combination with antibiotics has not yet been demonstrated. Novel regulatory pathways will also need to be developed prior to clinical adoption. That said, several investigations are underway [133, 134] and expanded-access programs are available.
Enhancing innate immunity against CRAB is a novel approach to overcome infection due to virulent strains. To this end, several monoclonal antibodies (MAbs) have been recently investigated [135–137], and 2 particularly promising agents, MAb 8 and MAb 65, have demonstrated the ability to improve mortality in murine models [136, 137]. While encouraging, both MAbs combined only bind to less than 50% of CRAB isolates screened, suggesting that future immunotherapeutics will need to combine multiple MAbs into a single cocktail to be effective against diverse strains.
PREFERRED APPROACHES FOR TREATMENT OF INVASIVE CRAB INFECTIONS
For nearly 2 decades clinicians have relied heavily on anecdotes and observations to guide treatment selection for CRAB infections. The field is now benefiting from results of several recent randomized clinical trials (Figure 1), contemporary PK-PD studies, and the development of novel antibiotics. The general paradigm of combination therapy for all patients remains [30, 31, 74]; however, we are nearing a point where effective, colistin-sparing regimens can be feasibly constructed [74]. Equally as important, optimized dosing regimens for ampicillin-sulbactam, polymyxin B, and tigecycline have been defined [30], as have proposed updates to modernize susceptibility breakpoints against Acinetobacter spp. [41, 82, 88]. Cefiderocol may not be the paradigm-shifting agent against CRAB that many had once hoped for, but in combination, it offers a well-tolerated, in vitro active β-lactam agent that has been associated with encouraging real-world clinical use in a limited number of patients [109]. The most promising data for patients with CRAB infections have been reported with sulbactam-durlobactam in a randomized clinical trial [125]. Other agents demonstrating in vitro activity against CRAB are currently in preclinical development and have been reviewed previously [138–140]. Taken together, the tide is slowly, but surely shifting towards improved management of CRAB infections.
In most scenarios moving forward, treatment of CRAB infections should be tailored around a sulbactam backbone [30], either with the potent β-lactamase inhibitor durlobactam (if it becomes FDA-approved) or in combination with 1 or more in vitro active antibiotics. Defining in vitro activity should not solely rely upon susceptibility breakpoints, but rather an advanced understanding of antimicrobial PK-PD targets and drug exposures at the site of infection. For example, minocycline may be categorized as susceptible based on the current CLSI breakpoint (MIC ≤4 mg/L), but is unlikely to achieve exposure targets when MICs are >1 mg/L [88]. Moreover, proposed clinical breakpoints for cefiderocol vary by organization and have not been established for sulbactam or tigecycline. In fact, most CRAB isolates will be categorized as non-susceptible to ampicillin-sulbactam, which should not deter use, but rather promote dose optimization with at least 9 g/day of sulbactam. Taking these factors into consideration, individualized treatment regimens will need to be constructed based on susceptibility testing results, the site of infection, and knowledge of the local epidemiology for CRAB [22].
Recommendations for front-line treatment of invasive CRAB infections vary across organizations [30, 31, 74]. Our preferred regimen consists of high-dose ampicillin-sulbactam, in combination with either cefiderocol, polymyxin B, or tigecycline stratified by PK-PD optimized dosing, susceptibility testing results, and the site of infection. For example, during treatment of pneumonia, ampicillin-sulbactam with tigecycline or cefiderocol is preferred; for bloodstream infections, ampicillin-sulbactam with cefiderocol or polymyxin B is preferred; and for osteoarticular infections, ampicillin-sulbactam with tigecycline is preferred. Similar combinations can be used for less-common infection types, including intra-abdominal infections where ampicillin-sulbactam with tigecycline or cefiderocol is recommended, and for urinary tract infections with the use of ampicillin-sulbactam and colistin. Until further data are available, it is suggested to reserve the addition of a third agent for patients with delayed clinical responses or recurrent infections. As with any treatment regimen selected, timely source control and close monitoring for clinical response and toxicity are required. If sulbactam-durlobactam is approved for clinical use by the FDA, the authors are optimistic that it can be positioned as a front-line agent for the treatment of invasive CRAB infections. Sulbactam-durlobactam may be best used in combination with another in vitro active agent until additional studies have been performed to evaluate efficacy as monotherapy or in combination with specific agents.
Supplementary Material
Contributor Information
Ryan K Shields, Department of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
David L Paterson, ADVANCE-ID, Saw Swee Hock School of Public Health, National University of Singapore, Singapore.
Pranita D Tamma, Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Notes
Financial support . This work was sponsored by Entasis Therapeutics, a wholly owned subsidiary of Innoviva, Inc. The authors did not receive any fees for authorship.
Supplement sponsorship . This article appears as part of the supplement “Sulbactam-durlobactam, a Targeted β-lactam/β-lactamase Inhibitor, for MDR Acinetobacter,” sponsored by Entasis Therapeutics Inc., a wholly owned subsidiary of Innoviva, Inc.
References
- 1. Tacconelli E, Carrara E, Savoldi A, et al. . Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 2018; 18(3):318–27. [DOI] [PubMed] [Google Scholar]
- 2. Perez F, Hujer AM, Hujer KM, Decker BK, Rather PN, Bonomo RA. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 2007; 51(10):3471–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wong D, Nielsen TB, Bonomo RA, Pantapalangkoor P, Luna B, Spellberg B. Clinical and pathophysiological overview of Acinetobacter infections: a century of challenges. Clin Microbiol Rev 2017; 30(1):409–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States . Available at: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf. Accessed 11 January 2022.
- 5. Bassetti M, Echols R, Matsunaga Y, et al. . Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant gram-negative bacteria (CREDIBLE-CR): a randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect Dis 2021; 21(2):226–40. [DOI] [PubMed] [Google Scholar]
- 6. Paul M, Daikos GL, Durante-Mangoni E, et al. . Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis 2018; 18(4):391–400. [DOI] [PubMed] [Google Scholar]
- 7. Babiker A, Clarke LG, Saul M, et al. . Changing epidemiology and decreased mortality associated with carbapenem-resistant gram-negative bacteria from 2000-2017. Clin Infect Dis 2021; 73(11):e4521–e4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kadri SS, Adjemian J, Lai YL, et al. . Difficult-to-treat resistance in gram-negative bacteremia at 173 US hospitals: retrospective cohort analysis of prevalence, predictors, and outcome of resistance to all first-line agents. Clin Infect Dis 2018; 67(12):1803–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Antimicrobial Resistance Collaborators . Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 2022; 399(10325):629–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bartal C, Rolston KVI, Nesher L. Carbapenem-resistant Acinetobacter baumannii: colonization, infection and current treatment options. Infect Dis Ther 2022; 11(2):683–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang ST, Chiang MC, Kuo SC, et al. . Risk factors and clinical outcomes of patients with carbapenem-resistant Acinetobacter baumannii bacteremia. J Microbiol Immunol Infect 2012; 45(5):356–62. [DOI] [PubMed] [Google Scholar]
- 12. Bardbari AM, Arabestani MR, Karami M, Keramat F, Alikhani MY, Bagheri KP. Correlation between ability of biofilm formation with their responsible genes and MDR patterns in clinical and environmental Acinetobacter baumannii isolates. Microb Pathog 2017; 108:122–8. [DOI] [PubMed] [Google Scholar]
- 13. Burnham JP, Rojek RP, Kollef MH. Catheter removal and outcomes of multidrug-resistant central-line-associated bloodstream infection. Medicine (Baltimore) 2018; 97(42):e12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pogue JM, Jones RN, Bradley JS, et al. . Polymyxin susceptibility testing and interpretive breakpoints: recommendations from the United States Committee on Antimicrobial Susceptibility Testing (USCAST). Antimicrob Agents Chemother 2020; 64(2):e01495-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clinical and Laboratory Standards Institute . M100: Performance Standards for Antimicrobial Susceptibility Testing. 32nd ed.Wayne, PA: Clinical and Laboratory Standards Institute, 2022. [Google Scholar]
- 16. European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 13.0, 2023. Available a t:http://www.eucast.org. Accessed 11 May 2022. [Google Scholar]
- 17. Seifert H, Blondeau J, Lucassen K, Utt EA. Global update on the in vitro activity of tigecycline and comparators against isolates of Acinetobacter baumannii and rates of resistant phenotypes (2016-2018). J Glob Antimicrob Resist 2022; 31:82–9. [DOI] [PubMed] [Google Scholar]
- 18. O'Donnell JN, Putra V, Lodise TP. Treatment of patients with serious infections due to carbapenem-resistant Acinetobacter baumannii: how viable are the current options? Pharmacotherapy 2021; 41(9):762–80. [DOI] [PubMed] [Google Scholar]
- 19. Karlowsky JA, Hackel MA, McLeod SM, Miller AA. In vitro activity of sulbactam-durlobactam against global isolates of Acinetobacter baumannii-calcoaceticus complex collected from 2016 to 2021. Antimicrob Agents Chemother 2022; 66(9):e0078122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abdul-Mutakabbir JC, Griffith NC, Shields RK, Tverdek FP, Escobar ZK. Contemporary perspective on the treatment of Acinetobacter baumannii infections: insights from the Society of Infectious Diseases pharmacists. Infect Dis Ther 2021; 10(4):2177–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hujer AM, Hujer KM, Leonard DA, et al. . A comprehensive and contemporary “snapshot” of beta-lactamases in carbapenem resistant Acinetobacter baumannii. Diagn Microbiol Infect Dis 2021; 99(2):115242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Iovleva A, Mustapha MM, Griffith MP, et al. . Carbapenem-resistant Acinetobacter baumannii in U.S. Hospitals: diversification of circulating lineages and antimicrobial resistance. mBio 2022; 13(2):e0275921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Adams-Haduch JM, Onuoha EO, Bogdanovich T, et al. . Molecular epidemiology of carbapenem-nonsusceptible Acinetobacter baumannii in the United States. J Clin Microbiol 2011; 49(11):3849–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adams MD, Wright MS, Karichu JK, et al. . Rapid replacement of Acinetobacter baumannii strains accompanied by changes in lipooligosaccharide loci and resistance gene repertoire. mBio 2019; 10(2):e003356-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cai Y, Chai D, Wang R, Liang B, Bai N. Colistin resistance of Acinetobacter baumannii: clinical reports, mechanisms and antimicrobial strategies. J Antimicrob Chemother 2012; 67(7):1607–15. [DOI] [PubMed] [Google Scholar]
- 26. Hackel MA, Tsuji M, Yamano Y, Echols R, Karlowsky JA, Sahm DF. In vitro activity of the siderophore cephalosporin, cefiderocol, against carbapenem-nonsusceptible and multidrug-resistant isolates of gram-negative bacilli collected worldwide in 2014 to 2016. Antimicrob Agents Chemother 2018; 62(2):e01968-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Butler DA, Biagi M, Tan X, Qasmieh S, Bulman ZP, Wenzler E. Multidrug resistant Acinetobacter baumannii: resistance by any other name would still be hard to treat. Curr Infect Dis Rep 2019; 21(12):46. [DOI] [PubMed] [Google Scholar]
- 28. Viehman JA, Nguyen MH, Doi Y. Treatment options for carbapenem-resistant and extensively drug-resistant Acinetobacter baumannii infections. Drugs 2014; 74(12):1315–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lenhard JR, Smith NM, Bulman ZP, et al. . High-dose ampicillin-sulbactam combinations combat polymyxin-resistant Acinetobacter baumannii in a hollow-fiber infection model. Antimicrob Agents Chemother 2017; 61(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious Diseases Society of America guidance on the treatment of AmpC beta-lactamase-producing Enterobacterales, carbapenem-resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia infections. Clin Infect Dis 2022; 74(12):2089–114. [DOI] [PubMed] [Google Scholar]
- 31. Paul M, Carrara E, Retamar P, et al. . European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant gram-negative bacilli (endorsed by European Society of Intensive Care Medicine). Clin Microbiol Infect 2022; 28(4):521–47. [DOI] [PubMed] [Google Scholar]
- 32. Sirijatuphat R, Thamlikitkul V. Preliminary study of colistin versus colistin plus fosfomycin for treatment of carbapenem-resistant Acinetobacter baumannii infections. Antimicrob Agents Chemother 2014; 58(9):5598–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Park HJ, Cho JH, Kim HJ, Han SH, Jeong SH, Byun MK. Colistin monotherapy versus colistin/rifampicin combination therapy in pneumonia caused by colistin-resistant Acinetobacter baumannii: a randomised controlled trial. J Glob Antimicrob Resist 2019; 17:66–71. [DOI] [PubMed] [Google Scholar]
- 34. Durante-Mangoni E, Signoriello G, Andini R, et al. . Colistin and rifampicin compared with colistin alone for the treatment of serious infections due to extensively drug-resistant Acinetobacter baumannii: a multicenter, randomized clinical trial. Clin Infect Dis 2013; 57(3):349–58. [DOI] [PubMed] [Google Scholar]
- 35. Aydemir H, Akduman D, Piskin N, et al. . Colistin vs. the combination of colistin and rifampicin for the treatment of carbapenem-resistant Acinetobacter baumannii ventilator-associated pneumonia. Epidemiol Infect 2013; 141(6):1214–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bliziotis IA, Samonis G, Vardakas KZ, Chrysanthopoulou S, Falagas ME. Effect of aminoglycoside and beta-lactam combination therapy versus beta-lactam monotherapy on the emergence of antimicrobial resistance: a meta-analysis of randomized, controlled trials. Clin Infect Dis 2005; 41(2):149–58. [DOI] [PubMed] [Google Scholar]
- 37. Qureshi ZA, Hittle LE, O'Hara JA, et al. . Colistin-resistant Acinetobacter baumannii: beyond carbapenem resistance. Clin Infect Dis 2015; 60(9):1295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oleksiuk LM, Nguyen MH, Press EG, et al. . In vitro responses of Acinetobacter baumannii to two- and three-drug combinations following exposure to colistin and doripenem. Antimicrob Agents Chemother 2014; 58(2):1195–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nation RL, Velkov T, Li J. Colistin and polymyxin B: peas in a pod, or chalk and cheese? Clin Infect Dis 2014; 59(1):88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Flamm RK, Shortridge D, Castanheira M, Sader HS, Pfaller MA. In vitro activity of minocycline against U.S. isolates of Acinetobacter baumannii-Acinetobacter calcoaceticus species complex, Stenotrophomonas maltophilia, and Burkholderia cepacia complex: results from the SENTRY Antimicrobial Surveillance Program, 2014 to 2018. Antimicrob Agents Chemother 2019; 63(11):e01154-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tsuji BT, Pogue JM, Zavascki AP, et al. . International Consensus Guidelines for the Optimal Use of the Polymyxins: Endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy 2019; 39(1):10–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nutman A, Lellouche J, Temkin E, et al. . Colistin plus meropenem for carbapenem-resistant gram-negative infections: in vitro synergism is not associated with better clinical outcomes. Clin Microbiol Infect 2020; 26(9):1185–91. [DOI] [PubMed] [Google Scholar]
- 43. Pogue JM, Rybak MJ, Stamper K, et al. . The impact of in vitro synergy between colistin and meropenem on clinical outcomes in invasive carbapenem-resistant gram-negative infections: a report from the OVERCOME trial. Abstract #638. Presented at: IDWeek 2021. (Virtual Event, Sept. 29–Oct. 3).
- 44. Zusman O, Altunin S, Koppel F, Dishon Benattar Y, Gedik H, Paul M. Polymyxin monotherapy or in combination against carbapenem-resistant bacteria: systematic review and meta-analysis. J Antimicrob Chemother 2017; 72(1):29–39. [DOI] [PubMed] [Google Scholar]
- 45. Kaye KS, Marchaim D, Thamlikitkul V, et al. . Results from the OVERCOME trial: colistin monotherapy versus combination therapy for the treatment of pneumonoia or bloodstream infection due to extensively drug resistant gram-negative bacilli. Abstract #04773. Presented at: 31st ECC MID (virtual). 2021.
- 46. Boisson M, Jacobs M, Gregoire N, et al. . Comparison of intrapulmonary and systemic pharmacokinetics of colistin methanesulfonate (CMS) and colistin after aerosol delivery and intravenous administration of CMS in critically ill patients. Antimicrob Agents Chemother 2014; 58(12):7331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Imberti R, Cusato M, Villani P, et al. . Steady-state pharmacokinetics and BAL concentration of colistin in critically Ill patients after IV colistin methanesulfonate administration. Chest 2010; 138(6):1333–9. [DOI] [PubMed] [Google Scholar]
- 48. Manchandani P, Zhou J, Ledesma KR, et al. . Characterization of polymyxin B biodistribution and disposition in an animal model. Antimicrob Agents Chemother 2016; 60(2):1029–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Heil EL, Claeys KC, Kline EG, et al. . Utility of three-drug combinations for the treatment of carbapenem-resistant Acinetobacter baumannii infections among COVID-19 patients. Presented at: European Congress of Clinical Microbiology and Infectious Diseases (ECCMID; Lisbon, Portugal; 2022.
- 50. Yokoyama Y, Matsumoto K, Ikawa K, et al. . Pharmacokinetic/pharmacodynamic evaluation of sulbactam against Acinetobacter baumannii in in vitro and murine thigh and lung infection models. Int J Antimicrob Agents 2014; 43(6): 547–52. [DOI] [PubMed] [Google Scholar]
- 51. Abouelhassan Y, Kuti JL, Nicolau DP, Abdelraouf K. Sulbactam against Acinetobacter baumannii pneumonia: pharmacokinetic/pharmacodynamic appraisal of current dosing regimens. Presented at: IDWeek2022. (Washington, DC, Oct. 11–15).
- 52. Jaruratanasirikul S, Nitchot W, Wongpoowarak W, Samaeng M, Nawakitrangsan M. Population pharmacokinetics and Monte Carlo simulations of sulbactam to optimize dosage regimens in patients with ventilator-associated pneumonia caused by Acinetobacter baumannii. Eur J Pharm Sci 2019; 136:104940. [DOI] [PubMed] [Google Scholar]
- 53. Betrosian AP, Frantzeskaki F, Xanthaki A, Georgiadis G. High-dose ampicillin-sulbactam as an alternative treatment of late-onset VAP from multidrug-resistant Acinetobacter baumannii. Scand J Infect Dis 2007; 39(1):38–43. [DOI] [PubMed] [Google Scholar]
- 54. Penwell WF, Shapiro AB, Giacobbe RA, et al. . Molecular mechanisms of sulbactam antibacterial activity and resistance determinants in Acinetobacter baumannii. Antimicrob Agents Chemother 2015; 59(3):1680–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mosaed R, Haghighi M, Kouchak M, et al. . Interim study: comparison of safety and efficacy of levofloxacin plus colistin regimen with levofloxacin plus high dose ampicillin/sulbactam infusion in treatment of ventilator-associated pneumonia due to multi drug resistant Acinetobacter. Iran J Pharm Res 2018; 17(Suppl 2):206–13. [PMC free article] [PubMed] [Google Scholar]
- 56. Betrosian AP, Frantzeskaki F, Xanthaki A, Douzinas EE. Efficacy and safety of high-dose ampicillin/sulbactam vs. colistin as monotherapy for the treatment of multidrug resistant Acinetobacter baumannii ventilator-associated pneumonia. J Infect 2008; 56(6):432–6. [DOI] [PubMed] [Google Scholar]
- 57. Kengkla K, Kongpakwattana K, Saokaew S, Apisarnthanarak A, Chaiyakunapruk N. Comparative efficacy and safety of treatment options for MDR and XDR Acinetobacter baumannii infections: a systematic review and network meta-analysis. J Antimicrob Chemother 2018; 73(1):22–32. [DOI] [PubMed] [Google Scholar]
- 58. Jung SY, Lee SH, Lee SY, et al. . Antimicrobials for the treatment of drug-resistant Acinetobacter baumannii pneumonia in critically ill patients: a systemic review and Bayesian network meta-analysis. Crit Care 2017; 21(1):319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Liu J, Shu Y, Zhu F, et al. . Comparative efficacy and safety of combination therapy with high-dose sulbactam or colistin with additional antibacterial agents for multiple drug-resistant and extensively drug-resistant Acinetobacter baumannii infections: a systematic review and network meta-analysis. J Glob Antimicrob Resist 2021; 24:136–47. [DOI] [PubMed] [Google Scholar]
- 60. Chu H, Zhao L, Wang M, Liu Y, Gui T, Zhang J. Sulbactam-based therapy for Acinetobacter baumannii infection: a systematic review and meta-analysis. Braz J Infect Dis 2013; 17(4):389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pourheidar E, Haghighi M, Kouchek M, et al. . Comparison of intravenous ampicillin-sulbactam plus nebulized colistin with intravenous colistin plus nebulized colistin in treatment of ventilator associated pneumonia caused by multi drug resistant Acinetobacter baumannii: randomized open label trial. Iran J Pharm Res 2019; 18(Suppl 1):269–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Makris D, Petinaki E, Tsolaki V, et al. . Colistin versus colistin combined with ampicillin-sulbactam for multiresistant Acinetobacter baumannii ventilator-associated pneumonia treatment: an open-label prospective study. Indian J Crit Care Med 2018; 22(2):67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Krizova L, Poirel L, Nordmann P, Nemec A. TEM-1 beta-lactamase as a source of resistance to sulbactam in clinical strains of Acinetobacter baumannii. J Antimicrob Chemother 2013; 68(12):2786–91. [DOI] [PubMed] [Google Scholar]
- 64. Kuo SC, Lee YT, Yang Lauderdale TL, et al. . Contribution of Acinetobacter-derived cephalosporinase-30 to sulbactam resistance in Acinetobacter baumannii. Front Microbiol 2015; 6:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Shapiro AB. Kinetics of sulbactam hydrolysis by beta-lactamases, and kinetics of beta-lactamase inhibition by sulbactam. Antimicrob Agents Chemother 2017; 61(12):e01612-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bulik CC, Nicolau DP. Double-carbapenem therapy for carbapenemase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 2011; 55(6): 3002–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cojutti P, Sartor A, Righi E, Scarparo C, Bassetti M, Pea F. Population pharmacokinetics of high-dose continuous-infusion meropenem and considerations for use in the treatment of infections due to KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 2017; 61(10):e00794-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. De Pascale G, Martucci G, Montini L, et al. . Double carbapenem as a rescue strategy for the treatment of severe carbapenemase-producing Klebsiella pneumoniae infections: a two-center, matched case-control study. Crit Care 2017; 21(1):173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tumbarello M, Viale P, Viscoli C, et al. . Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 2012; 55(7):943–50. [DOI] [PubMed] [Google Scholar]
- 70. Tzouvelekis LS, Markogiannakis A, Piperaki E, Souli M, Daikos GL. Treating infections caused by carbapenemase-producing Enterobacteriaceae. Clin Microbiol Infect 2014; 20(9):862–72. [DOI] [PubMed] [Google Scholar]
- 71. Shields RK, Nguyen MH, Chen L, et al. . Ceftazidime-avibactam is superior to other treatment regimens against carbapenem-resistant Klebsiella pneumoniae bacteremia. Antimicrob Agents Chemother 2017; 61(8):e00883-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. van Duin D, Lok JJ, Earley M, et al. . Colistin versus ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant Enterobacteriaceae. Clin Infect Dis 2018; 66(2):163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wunderink RG, Giamarellos-Bourboulis EJ, Rahav G, et al. . Effect and safety of meropenem-vaborbactam versus best-available therapy in patients with carbapenem-resistant enterobacteriaceae infections: the TANGO II randomized clinical trial. Infect Dis Ther 2018; 7(4):439–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Abdul-Mutakabbir JC, Griffith NC, Shields RK, Tverdek FP, Escobar ZK. Contemporary perspective on the treatment of Acinetobacter baumannii infections: insights from the Society of Infectious Diseases pharmacists. Infect Dis Ther 2021; 10(4):2177–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Liou BH, Lee YT, Kuo SC, Liu PY, Fung CP. Efficacy of tigecycline for secondary Acinetobacter bacteremia and factors associated with treatment failure. Antimicrob Agents Chemother 2015; 59(6):3637–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Niu T, Luo Q, Li Y, Zhou Y, Yu W, Xiao Y. Comparison of tigecycline or cefoperazone/sulbactam therapy for bloodstream infection due to carbapenem-resistant Acinetobacter baumannii. Antimicrob Resist Infect Control 2019; 8:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Liang CA, Lin YC, Lu PL, Chen HC, Chang HL, Sheu CC. Antibiotic strategies and clinical outcomes in critically ill patients with pneumonia caused by carbapenem-resistant Acinetobacter baumannii. Clin Microbiol Infect 2018; 24(8):908,.e1-e7. [DOI] [PubMed] [Google Scholar]
- 78. Cheng A, Chuang YC, Sun HY, et al. . Excess mortality associated with colistin-tigecycline compared with colistin-carbapenem combination therapy for extensively drug-resistant Acinetobacter baumannii bacteremia: a multicenter prospective observational study. Crit Care Med 2015; 43(6):1194–204. [DOI] [PubMed] [Google Scholar]
- 79. Cai X, Yang Z, Dai J, et al. . Pharmacodynamics of tigecycline alone and in combination with colistin against clinical isolates of multidrug-resistant Acinetobacter baumannii in an in vitro pharmacodynamic model. Int J Antimicrob Agents 2017; 49(5):609–16. [DOI] [PubMed] [Google Scholar]
- 80. Zha L, Pan L, Guo J, French N, Villanueva EV, Tefsen B. Effectiveness and safety of high dose tigecycline for the treatment of severe infections: a systematic review and meta-analysis. Adv Ther 2020; 37(3):1049–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. De Pascale G, Montini L, Pennisi M, et al. . High dose tigecycline in critically ill patients with severe infections due to multidrug-resistant bacteria. Crit Care 2014; 18(3):R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. De Pascale G, Lisi L, Ciotti GMP, et al. . Pharmacokinetics of high-dose tigecycline in critically ill patients with severe infections. Ann Intensive Care 2020; 10(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Xie J, Roberts JA, Alobaid AS, et al. . Population pharmacokinetics of tigecycline in critically ill patients with severe infections. Antimicrob Agents Chemother 2017; 61(8):e00345-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Fragkou PC, Poulakou G, Blizou A, et al. . The role of minocycline in the treatment of nosocomial infections caused by multidrug, extensively drug and pandrug resistant Acinetobacter baumannii: a systematic review of clinical evidence. Microorganisms 2019; 7(6):159–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lashinsky JN, Henig O, Pogue JM, Kaye KS. Minocycline for the treatment of multidrug and extensively drug-resistant A. baumannii: a review. Infect Dis Ther 2017; 6(2):199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Goff DA, Bauer KA, Mangino JE. Bad bugs need old drugs: a stewardship program's evaluation of minocycline for multidrug-resistant Acinetobacter baumannii infections. Clin Infect Dis 2014; 59(Suppl 6):S381–7. [DOI] [PubMed] [Google Scholar]
- 87. Tarazi Z, Sabet M, Dudley MN, Griffith DC. Pharmacodynamics of minocycline against Acinetobacter baumannii in a rat pneumonia model. Antimicrob Agents Chemother 2019; 63(2):e01671-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lodise TP, Van Wart S, Sund ZM, et al. . Pharmacokinetic and pharmacodynamic profiling of minocycline for injection following a single infusion in critically ill adults in a phase iv open-label multicenter study (ACUMIN). Antimicrob Agents Chemother 2021;65(3):e01809-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Beganovic M, Daffinee KE, Luther MK, LaPlante KL. Minocycline alone and in combination with polymyxin B, meropenem, and sulbactam against carbapenem-susceptible and -resistant Acinetobacter baumannii in an in vitro pharmacodynamic model. Antimicrob Agents Chemother 2021; 65(3):e01680-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Morrissey I, Olesky M, Hawser S, et al. . In vitro activity of eravacycline against gram-negative bacilli isolated in clinical laboratories worldwide from 2013 to 2017. Antimicrob Agents Chemother 2020; 64(3):e01699-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Livermore DM, Mushtaq S, Warner M, Woodford N. In vitro activity of eravacycline against carbapenem-resistant Enterobacteriaceae and Acinetobacter baumannii. Antimicrob Agents Chemother 2016; 60(6):3840–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zhao M, Lepak AJ, Marchillo K, VanHecker J, Andes DR. In vivo pharmacodynamic target assessment of eravacycline against Escherichia coli in a murine thigh infection model. Antimicrob Agents Chemother 2017; 61(7):e00250-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Bhavnani SM, Rubino CM, Hammel JP, et al. . Pharmacological and patient-specific response determinants in patients with hospital-acquired pneumonia treated with tigecycline. Antimicrob Agents Chemother 2012; 56(2):1065–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Koomanachai P, Kim A, Nicolau DP. Pharmacodynamic evaluation of tigecycline against Acinetobacter baumannii in a murine pneumonia model. J Antimicrob Chemother 2009; 63(5):982–7. [DOI] [PubMed] [Google Scholar]
- 95. Alosaimy S, Morrisette T, Lagnf AM, et al. . Clinical outcomes of eravacycline in patients treated predominately for carbapenem-resistant Acinetobacter baumannii. Microbiol Spectr 2022; 10(5):e0047922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Scott CJ, Zhu E, Jayakumar RA, Shan G, Viswesh V. Efficacy of eravacycline versus best previously available therapy for adults with pneumonia due to difficult-to-treat resistant (DTR) Acinetobacter baumannii. Ann Pharmacother 2022; 56(12):1299–307. [DOI] [PubMed] [Google Scholar]
- 97. Noel AR, Attwood M, Bowker KE, MacGowan AP. In vitro pharmacodynamics of omadacycline against Escherichia coli and Acinetobacter baumannii. J Antimicrob Chemother 2021; 76(3):667–70. [DOI] [PubMed] [Google Scholar]
- 98. Pfaller MA, Huband MD, Shortridge D, Flamm RK. Surveillance of omadacycline activity tested against clinical isolates from the United States and Europe: report from the SENTRY Antimicrobial Surveillance Program, 2016 to 2018. Antimicrob Agents Chemother 2020; 64(5):e02488-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Beganovic M, Luther MK, Daffinee KE, LaPlante KL. Biofilm prevention concentrations (BPC) of minocycline compared to polymyxin B, meropenem, and amikacin against Acinetobacter baumannii. Diagn Microbiol Infect Dis 2019; 94(3):223–6. [DOI] [PubMed] [Google Scholar]
- 100. Agwuh KN, MacGowan A. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J Antimicrob Chemother 2006; 58(2):256–65. [DOI] [PubMed] [Google Scholar]
- 101. Tariq R, Cho J, Kapoor S, et al. . Low risk of primary Clostridium difficile infection with tetracyclines: a systematic review and metaanalysis. Clin Infect Dis 2018; 66(4):514–22. [DOI] [PubMed] [Google Scholar]
- 102. McCreary EK, Heil EL, Tamma PD. New perspectives on antimicrobial agents: cefiderocol. Antimicrob Agents Chemother 2021; 65(8):e0217120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Yamano Y. In Vitro activity of cefiderocol against a broad range of clinically important gram-negative bacteria. Clin Infect Dis 2019; 69(Suppl 7):S544–S51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Abdul-Mutakabbir JC, Nguyen L, Maassen PT, et al. . In vitro antibacterial activity of cefiderocol against multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 2021; 65(9):e0264620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Longshaw C, Manissero D, Tsuji M, Echols R, Yamano Y. In vitro activity of the siderophore cephalosporin, cefiderocol, against molecularly characterized, carbapenem-non-susceptible gram-negative bacteria from Europe. JAC Antimicrob Resist 2020; 2(3):dlaa060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Shields RK. Case commentary: the need for cefiderocol is clear, but are the supporting clinical data? Antimicrob Agents Chemother 2020; 64(4):e00059-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wunderink RG, Matsunaga Y, Ariyasu M, et al. . Cefiderocol versus high-dose, extended-infusion meropenem for the treatment of gram-negative nosocomial pneumonia (APEKS-NP): a randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis 2021; 21(2):213–25. [DOI] [PubMed] [Google Scholar]
- 108. Smoke SM, Brophy A, Reveron S, et al. . Evolution and transmission of cefiderocol-resistant Acinetobacter baumannii during an outbreak in the burn intensive care unit. Clin Infect Dis 2023; 76(3):e1261-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Falcone M, Tiseo G, Leonildi A, et al. . Cefiderocol- compared to colistin-based regimens for the treatment of severe infections caused by carbapenem-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 2022; 66(5):e0214221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Falcone M, Tiseo G, Nicastro M, et al. . Cefiderocol as rescue therapy for Acinetobacter baumannii and other carbapenem-resistant gram-negative infections in intensive care unit patients. Clin Infect Dis 2021; 72(11):2021–4. [DOI] [PubMed] [Google Scholar]
- 111. Sansone P, Giaccari LG, Coppolino F, et al. . Cefiderocol for carbapenem-resistant bacteria: handle with care! A review of the real-world evidence. Antibiotics (Basel) 2022; 11(7):904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Rando E, Segala FV, Vargas J, et al. . Cefiderocol for severe carbapenem-resistant A. baumannii pneumonia: towards the comprehension of its place in therapy. Antibiotics (Basel) 2021; 11(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Karaba SM, Hirsch EB, Heil EL. In a pinch: cefiderocol for CRAB infections. Antimicrob Agents Chemother 2022; 66(5):e0006522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Malik S, Kaminski M, Landman D, Quale J. Cefiderocol resistance in Acinetobacter baumannii: Roles of beta-lactamases, siderophore receptors, and penicillin binding protein 3. Antimicrob Agents Chemother 2020; 64(11):e01221-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Poirel L, Sadek M, Nordmann P. Contribution of PER-type and NDM-type beta-lactamases to cefiderocol resistance in Acinetobacter baumannii. Antimicrob Agents Chemother 2021; 65(10):e0087721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Biagi M, Vialichka A, Jurkovic M, et al. . Activity of cefiderocol alone and in combination with levofloxacin, minocycline, polymyxin B, or trimethoprim-sulfamethoxazole against multidrug-resistant Stenotrophomonas maltophilia. Antimicrob Agents Chemother 2020; 64(9):e00559-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kobic E, Abouelhassan Y, Singaravelu K, Nicolau DP. Pharmacokinetic analysis and in vitro synergy evaluation of cefiderocol, sulbactam, and tigecycline in an extensively drug-resistant Acinetobacter baumannii pneumonia patient receiving continuous venovenous hemodiafiltration. Open Forum Infect Dis 2022; 9(10):ofac484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gill CM, Santini D, Takemura M, et al. In vivo efficacy and resistance prevention of cefiderocol in combination with ceftazidime/avibactam, meropenem, or ampicillin/sulbactam human simulating regimens using a 72-hour murine thigh infection model. Abstract #609. Presented at: IDWeek; Washington, DC; 2022 (Oct. 11–15).
- 119. Katsube T, Nicolau DP, Rodvold KA, et al. . Intrapulmonary pharmacokinetic profile of cefiderocol in mechanically ventilated patients with pneumonia. J Antimicrob Chemother 2021; 76(11):2902–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kawaguchi N, Katsube T, Echols R, Wajima T, Nicolau DP. Intrapulmonary pharmacokinetic modeling and simulation of cefiderocol, a parenteral siderophore cephalosporin, in patients with pneumonia and healthy subjects. J Clin Pharmacol 2022; 62(5):670–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Nakamura R, Ito-Horiyama T, Takemura M, et al. . In vivo pharmacodynamic study of cefiderocol, a novel parenteral siderophore cephalosporin, in murine thigh and lung infection models. Antimicrob Agents Chemother 2019; 63(9):e02031-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Durand-Reville TF, Guler S, Comita-Prevoir J, et al. ETX2514is a broad-spectrum beta-lactamase inhibitor for the treatment of drug-resistant gram-negative bacteria including Acinetobacter baumannii. Nat Microbiol 2017; 2:17104. [DOI] [PubMed] [Google Scholar]
- 123. Shapiro AB, Moussa SH, McLeod SM, Durand-Reville T, Miller AA. Durlobactam, a new diazabicyclooctane beta-lactamase inhibitor for the treatment of acinetobacter infections in combination with sulbactam. Front Microbiol 2021; 12:709974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Bhavnani SM, Rubino CM, Hammel JP, et al. . Population pharmacokinetics, pharmacodynamic/pharmacokinetic attainment and clinical pharmacokinetic/pharmacodynamic analyses for sulbactam-durlobactam to support dose selection for the treatment of Acinetobacter baumannii-calcoaceticus infections. Abstract LB2306. Presented at: IDWeek 2022. (Washington, DC; Oct 11–15).
- 125. Altarac D, Isaacs R, Srinivasan S, et al. . Efficacy and safety of sulbactam-durlobactam (SUL-DUR) versus colistin therpay in patients with Acinetobacter baumannii-calcoaceticus complex (ABC) infections: a global, randomised, active-controlled phase 3 trial (ATTACK). Abstract O2060. Presented at: 32nd ECCMID; Lisbon, Portugal; 2022, April 23–26.
- 126. Holger DJ, Kunz Coyne AJ, Zhao JJ, Sandhu A, Salimnia H, Rybak MJ. Novel combination therapy for extensively drug-resistant Acinetobacter baumannii necrotizing pneumonia complicated by empyema: a case report. Open Forum Infect Dis 2022; 9(4):ofac092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Zaidan N, Hornak JP, Reynoso D. Extensively drug-resistant Acinetobacter baumannii nosocomial pneumonia successfully treated with a novel antibiotic combination. Antimicrob Agents Chemother 2021; 65(11):e0092421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Vardakas KZ, Voulgaris GL, Samonis G, Falagas ME. Inhaled colistin monotherapy for respiratory tract infections in adults without cystic fibrosis: a systematic review and meta-analysis. Int J Antimicrob Agents 2018; 51(1):1–9. [DOI] [PubMed] [Google Scholar]
- 129. Niederman MS, Alder J, Bassetti M, et al. . Inhaled amikacin adjunctive to intravenous standard-of-care antibiotics in mechanically ventilated patients with gram-negative pneumonia (INHALE): a double-blind, randomised, placebo-controlled, phase 3, superiority trial. Lancet Infect Dis 2020; 20(3):330–40. [DOI] [PubMed] [Google Scholar]
- 130. Kalil AC, Metersky ML, Klompas M, et al. . Executive summary: management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016; 63(5):575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Rouby JJ, Zhu Y, Torres A, Rello J, Monsel A. Aerosolized polymyxins for ventilator-associated pneumonia caused by extensive drug resistant gram-negative bacteria: class, dose and manner should remain the trifecta. Ann Intensive Care 2022; 12(1): 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Schooley RT, Biswas B, Gill JJ, et al. . Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob Agents Chemother 2017; 61(10):e00954-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Jault P, Leclerc T, Jennes S, et al. . Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): a randomised, controlled, double-blind phase 1/2 trial. Lancet Infect Dis 2019; 19(1):35–45. [DOI] [PubMed] [Google Scholar]
- 134. Leitner L, Ujmajuridze A, Chanishvili N, et al. . Intravesical bacteriophages for treating urinary tract infections in patients undergoing transurethral resection of the prostate: a randomised, placebo-controlled, double-blind clinical trial. Lancet Infect Dis 2021; 21(3):427–36. [DOI] [PubMed] [Google Scholar]
- 135. Yeganeh O, Shabani M, Pakzad P, Mosaffa N, Hashemi A. Evaluation the reactivity of a peptide-based monoclonal antibody derived from OmpA with drug resistant pulsotypes of Acinetobacter baumannii as a potential therapeutic approach. Ann Clin Microbiol Antimicrob 2022; 21(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Nielsen TB, Yan J, Slarve M, et al. . Monoclonal antibody therapy against Acinetobacter baumannii. Infect Immun 2021; 89(10):e0016221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Nielsen TB, Pantapalangkoor P, Luna BM, et al. . Monoclonal antibody protects against Acinetobacter baumannii infection by enhancing bacterial clearance and evading sepsis. J Infect Dis 2017; 216(4):489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Arya R, Goldner BS, Shorr AF. Novel agents in development for multidrug-resistant gram-negative infections: potential new options facing multiple challenges. Curr Opin Infect Dis 2022; 35(6):589–94. [DOI] [PubMed] [Google Scholar]
- 139. Isler B, Doi Y, Bonomo RA, Paterson DL. New treatment options against carbapenem-resistant Acinetobacter baumannii infections. Antimicrob Agents Chemother 2019; 63(1):e01110-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Paterson DL, Isler B, Stewart A. New treatment options for multiresistant gram negatives. Curr Opin Infect Dis 2020; 33(2):214–23. [DOI] [PubMed] [Google Scholar]
- 141. Karlowsky JA, Hackel MA, Tsuji M, Yamano Y, Echols R, Sahm DF. In vitro activity of cefiderocol, a siderophore cephalosporin, against gram-negative bacilli isolated by clinical laboratories in North America and Europe in 2015-2016: SIDERO-WT-2015. Int J Antimicrob Agents 2019; 53(4):456–66. [DOI] [PubMed] [Google Scholar]
- 142. Seifert H, Muller C, Stefanik D, Higgins PG, Miller A, Kresken M. In vitro activity of sulbactam/durlobactam against global isolates of carbapenem-resistant Acinetobacter baumannii. J Antimicrob Chemother 2020; 75(9):2616–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.