Abstract
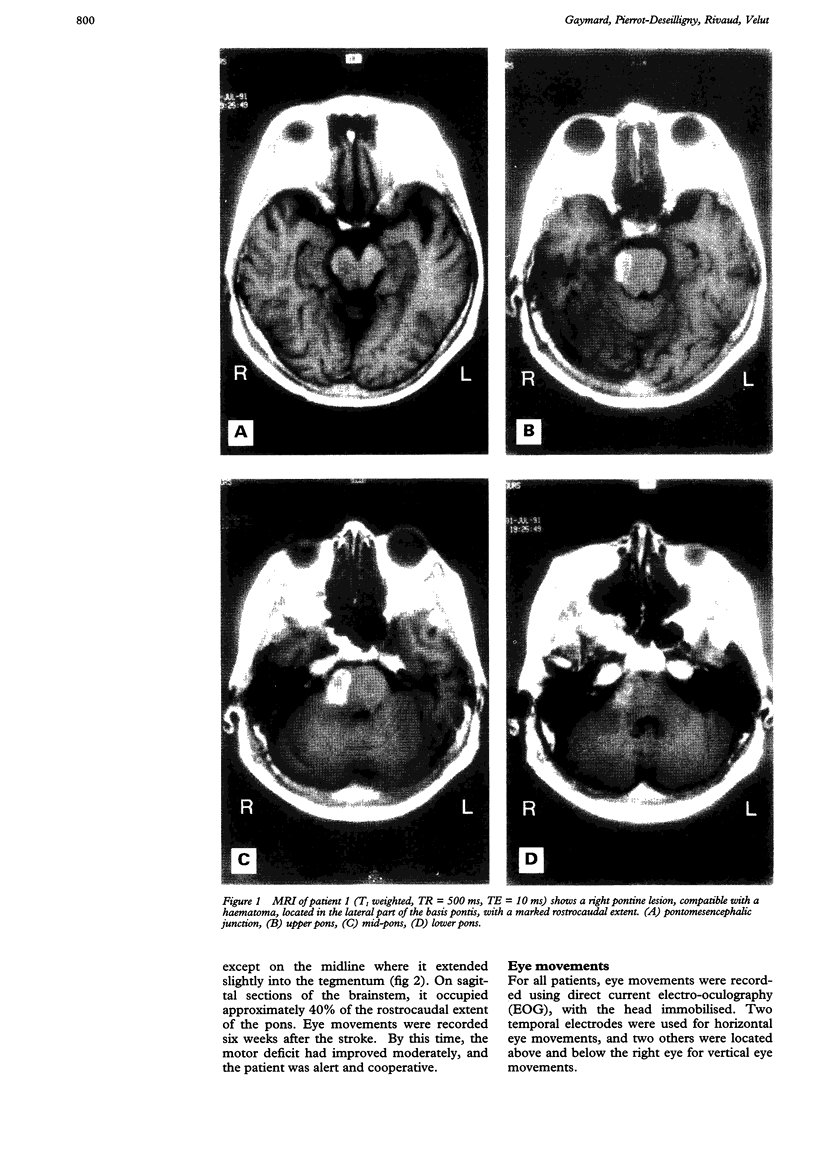
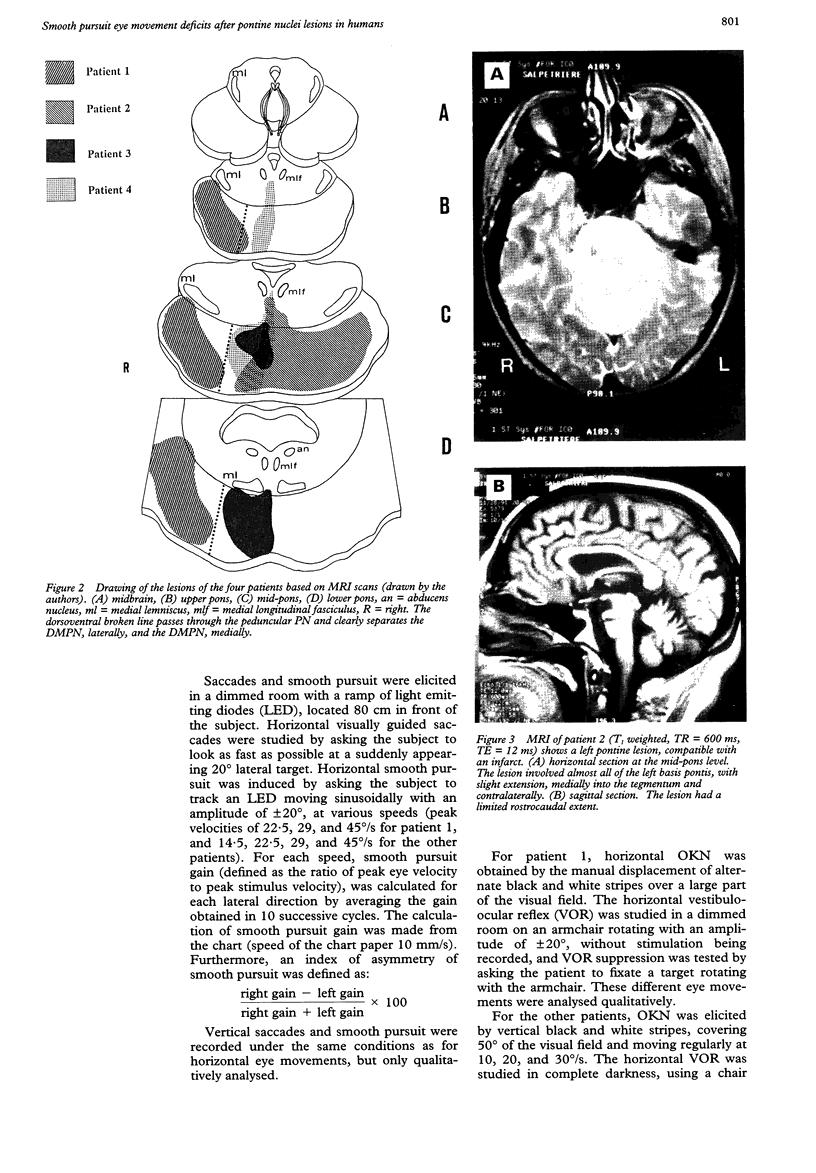
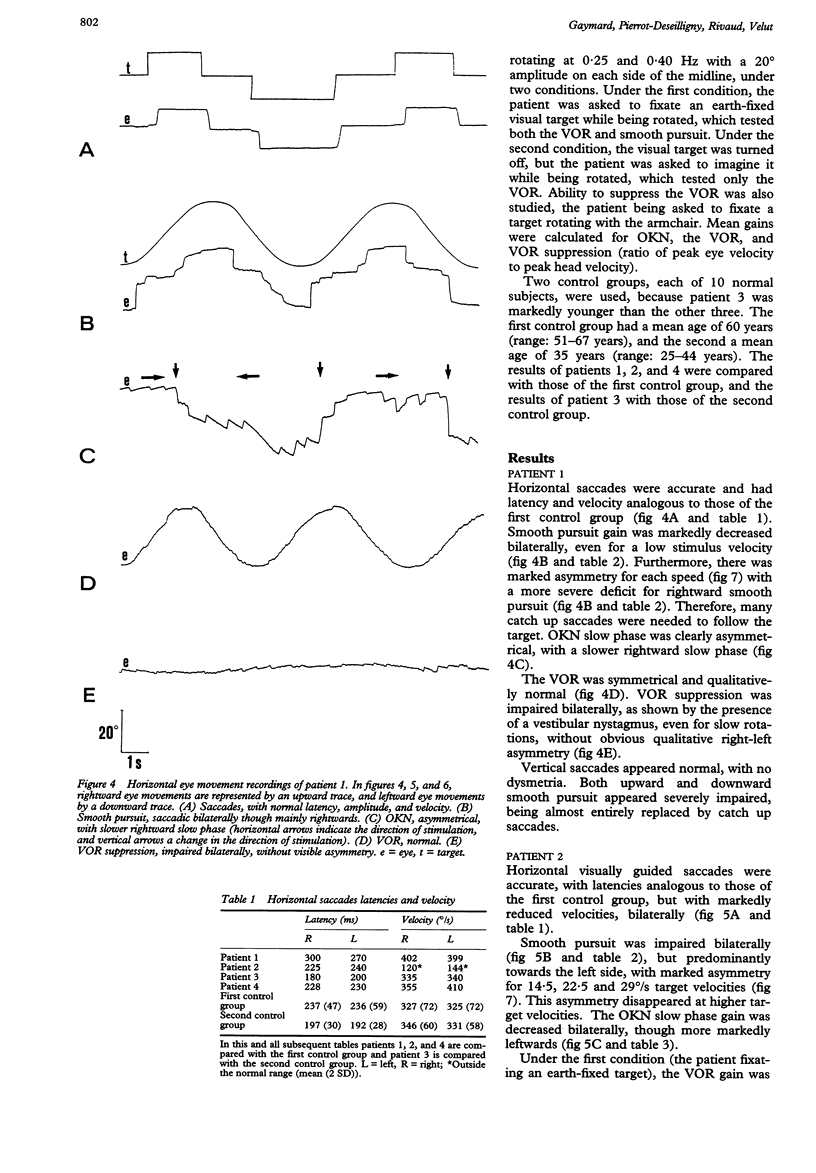
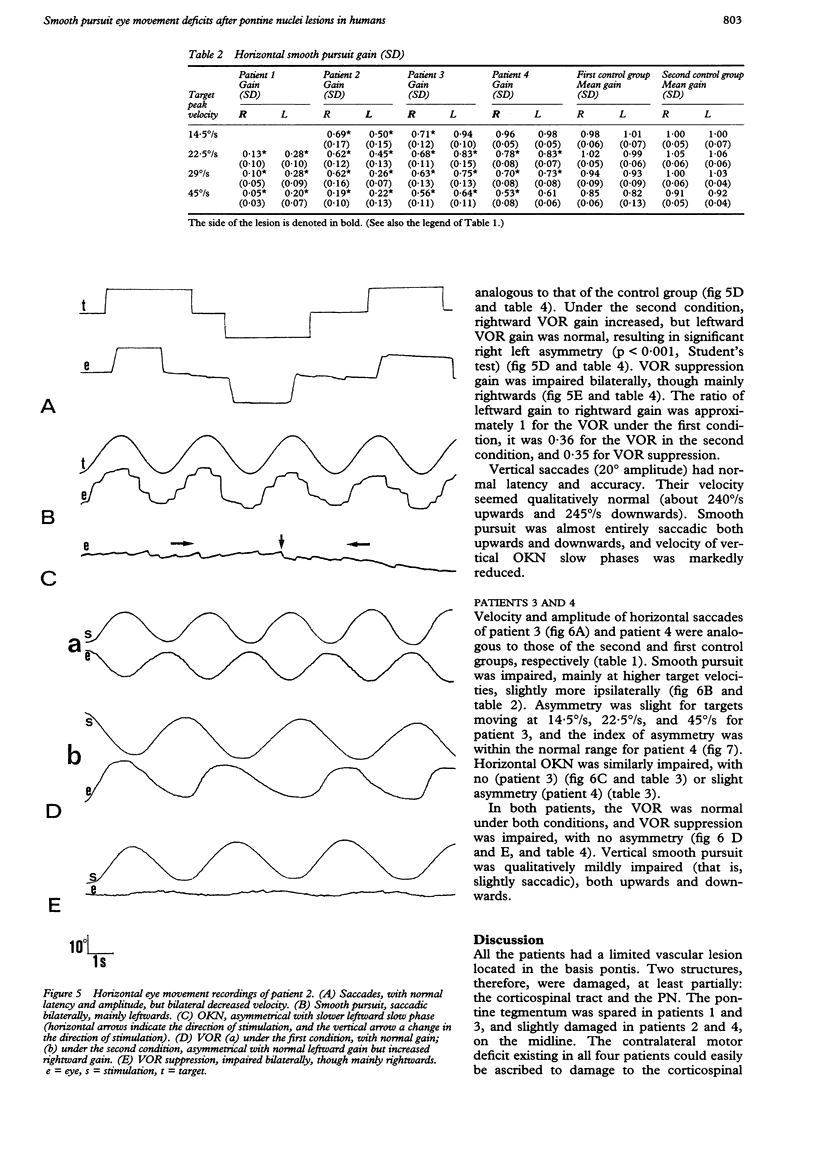
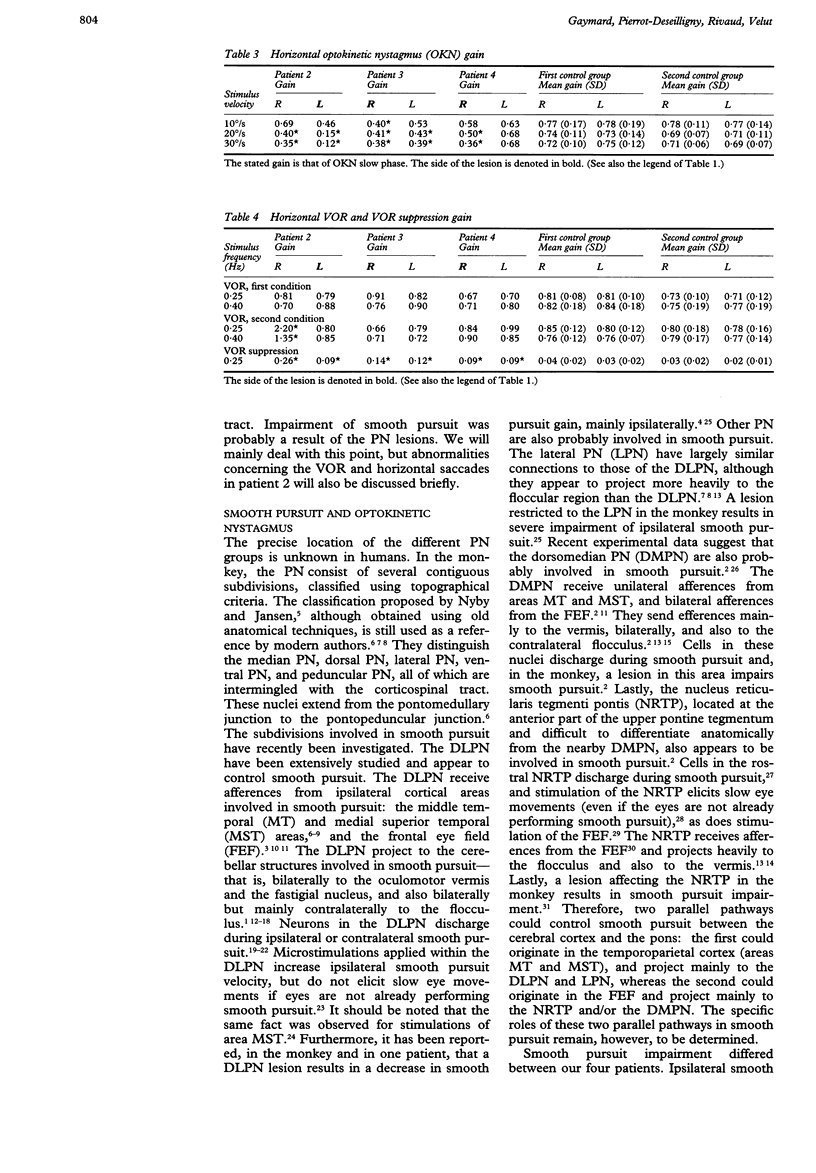
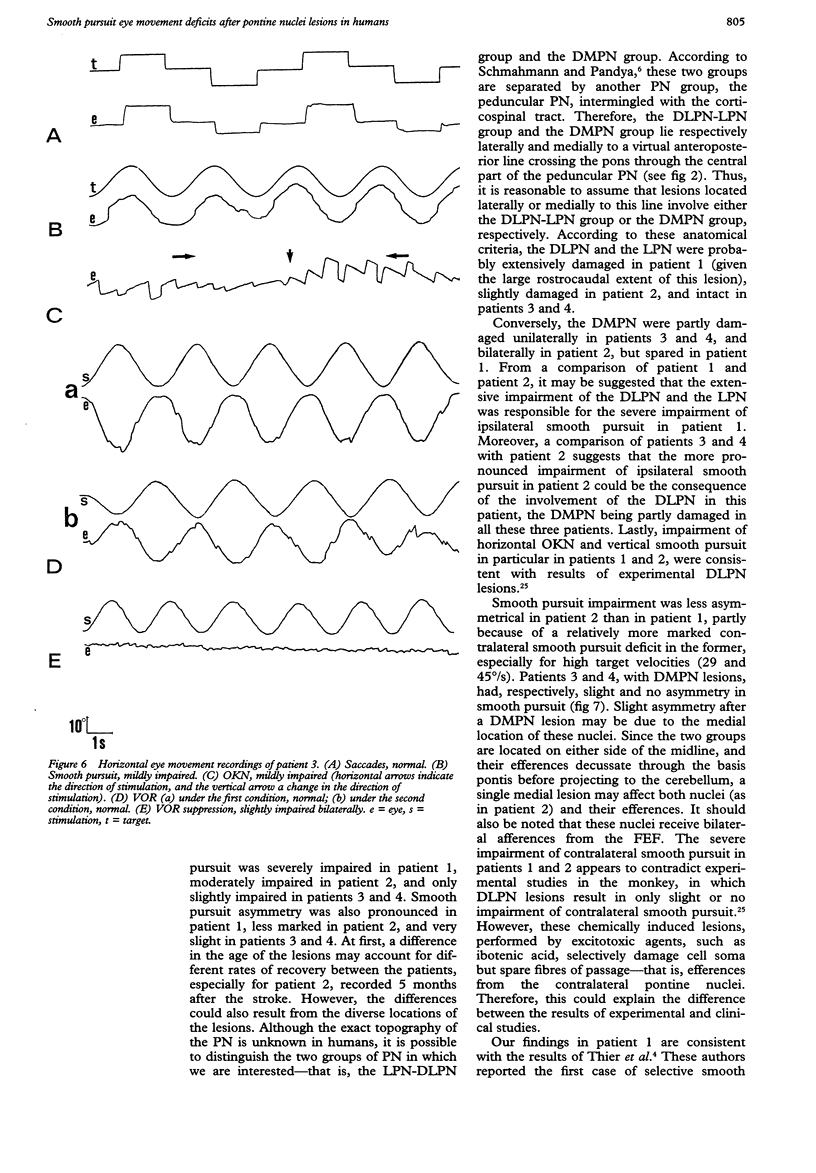
Eye movements were recorded electroculographically in four patients with basal pontine lesions, demonstrated by MRI. The most prominent eye movement abnormality observed was mild to severe impairment of smooth pursuit and optokinetic nystagmus, mainly ipsilateral to the lesion. This abnormality is thought to result from damage to the pontine nuclei, which form a crucial relay between the cerebral cortex and the cerebellum controlling smooth pursuit. Abnormalities of saccades and the vestibulo-ocular reflex in one patient are also discussed.
Full text
PDF
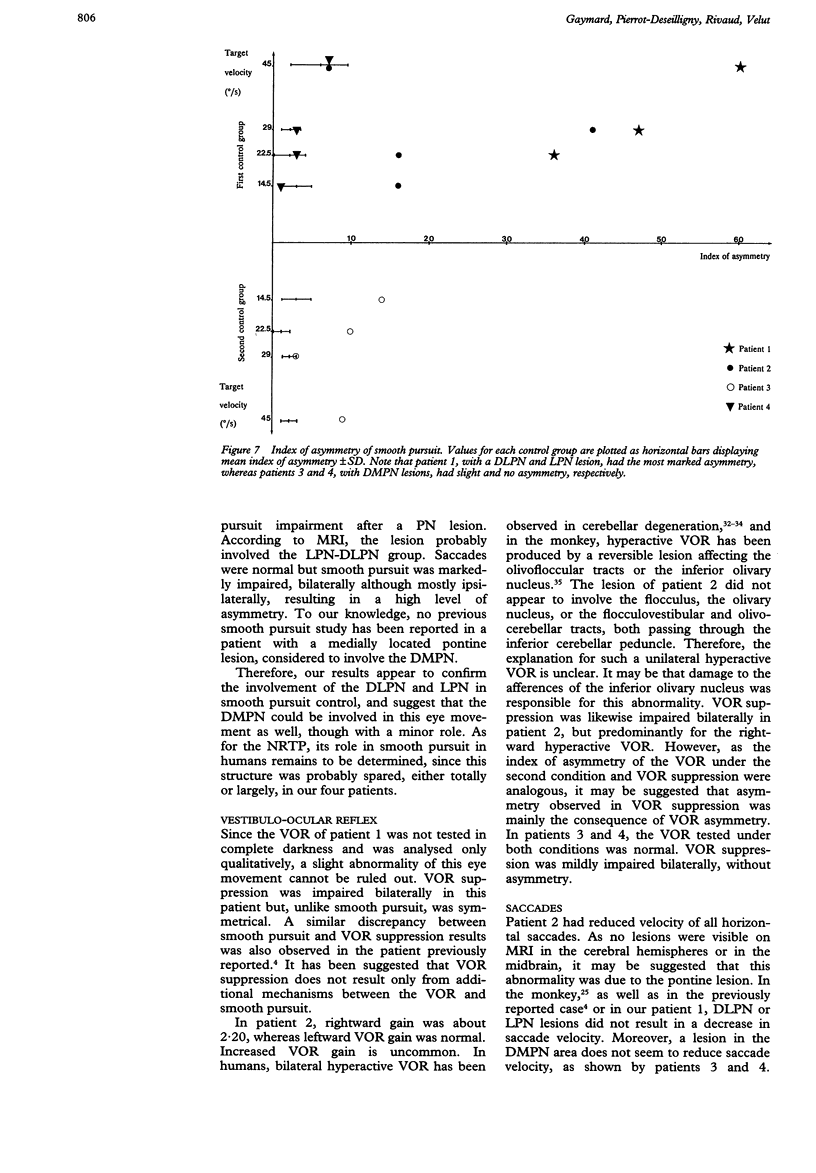

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baloh R. W., Konrad H. R., Honrubia V. Vestibulo-ocular function in patients with cerebellar atrophy. Neurology. 1975 Feb;25(2):160–168. doi: 10.1212/wnl.25.2.160. [DOI] [PubMed] [Google Scholar]
- Brodal P. The pontocerebellar projection in the rhesus monkey: an experimental study with retrograde axonal transport of horseradish peroxidase. Neuroscience. 1979;4(2):193–208. doi: 10.1016/0306-4522(79)90082-4. [DOI] [PubMed] [Google Scholar]
- Bruce C. J., Goldberg M. E., Bushnell M. C., Stanton G. B. Primate frontal eye fields. II. Physiological and anatomical correlates of electrically evoked eye movements. J Neurophysiol. 1985 Sep;54(3):714–734. doi: 10.1152/jn.1985.54.3.714. [DOI] [PubMed] [Google Scholar]
- Büttner U., Fuchs A. F., Markert-Schwab G., Buckmaster P. Fastigial nucleus activity in the alert monkey during slow eye and head movements. J Neurophysiol. 1991 Jun;65(6):1360–1371. doi: 10.1152/jn.1991.65.6.1360. [DOI] [PubMed] [Google Scholar]
- Demer J. L., Robinson D. A. Effects of reversible lesions and stimulation of olivocerebellar system on vestibuloocular reflex plasticity. J Neurophysiol. 1982 Jun;47(6):1084–1107. doi: 10.1152/jn.1982.47.6.1084. [DOI] [PubMed] [Google Scholar]
- Glickstein M., May J. G., 3rd, Mercier B. E. Corticopontine projection in the macaque: the distribution of labelled cortical cells after large injections of horseradish peroxidase in the pontine nuclei. J Comp Neurol. 1985 May 15;235(3):343–359. doi: 10.1002/cne.902350306. [DOI] [PubMed] [Google Scholar]
- Hoddevik G. H., Brodal A., Kawamura K., Hashikawa T. The pontine projection to the cerebellar vermal visual area studied by means of the retrograde axonal transport of horseradish peroxidase. Brain Res. 1977 Mar 11;123(2):209–227. doi: 10.1016/0006-8993(77)90475-9. [DOI] [PubMed] [Google Scholar]
- Keller E. L., Crandall W. F. Neuronal responses to optokinetic stimuli in pontine nuclei of behaving monkey. J Neurophysiol. 1983 Jan;49(1):169–187. doi: 10.1152/jn.1983.49.1.169. [DOI] [PubMed] [Google Scholar]
- Keller E. L., Heinen S. J. Generation of smooth-pursuit eye movements: neuronal mechanisms and pathways. Neurosci Res. 1991 Jul;11(2):79–107. doi: 10.1016/0168-0102(91)90048-4. [DOI] [PubMed] [Google Scholar]
- Komatsu H., Wurtz R. H. Modulation of pursuit eye movements by stimulation of cortical areas MT and MST. J Neurophysiol. 1989 Jul;62(1):31–47. doi: 10.1152/jn.1989.62.1.31. [DOI] [PubMed] [Google Scholar]
- Langer T., Fuchs A. F., Scudder C. A., Chubb M. C. Afferents to the flocculus of the cerebellum in the rhesus macaque as revealed by retrograde transport of horseradish peroxidase. J Comp Neurol. 1985 May 1;235(1):1–25. doi: 10.1002/cne.902350102. [DOI] [PubMed] [Google Scholar]
- Leichnetz G. R. Inferior frontal eye field projections to the pursuit-related dorsolateral pontine nucleus and middle temporal area (MT) in the monkey. Vis Neurosci. 1989 Aug;3(2):171–180. doi: 10.1017/s0952523800004478. [DOI] [PubMed] [Google Scholar]
- May J. G., Andersen R. A. Different patterns of corticopontine projections from separate cortical fields within the inferior parietal lobule and dorsal prelunate gyrus of the macaque. Exp Brain Res. 1986;63(2):265–278. doi: 10.1007/BF00236844. [DOI] [PubMed] [Google Scholar]
- May J. G., Keller E. L., Suzuki D. A. Smooth-pursuit eye movement deficits with chemical lesions in the dorsolateral pontine nucleus of the monkey. J Neurophysiol. 1988 Mar;59(3):952–977. doi: 10.1152/jn.1988.59.3.952. [DOI] [PubMed] [Google Scholar]
- Mustari M. J., Fuchs A. F., Wallman J. Response properties of dorsolateral pontine units during smooth pursuit in the rhesus macaque. J Neurophysiol. 1988 Aug;60(2):664–686. doi: 10.1152/jn.1988.60.2.664. [DOI] [PubMed] [Google Scholar]
- Noda H., Sugita S., Ikeda Y. Afferent and efferent connections of the oculomotor region of the fastigial nucleus in the macaque monkey. J Comp Neurol. 1990 Dec 8;302(2):330–348. doi: 10.1002/cne.903020211. [DOI] [PubMed] [Google Scholar]
- Schmahmann J. D., Pandya D. N. Projections to the basis pontis from the superior temporal sulcus and superior temporal region in the rhesus monkey. J Comp Neurol. 1991 Jun 8;308(2):224–248. doi: 10.1002/cne.903080209. [DOI] [PubMed] [Google Scholar]
- Stanton G. B., Goldberg M. E., Bruce C. J. Frontal eye field efferents in the macaque monkey: II. Topography of terminal fields in midbrain and pons. J Comp Neurol. 1988 May 22;271(4):493–506. doi: 10.1002/cne.902710403. [DOI] [PubMed] [Google Scholar]
- Stanton G. B., Goldberg M. E., Bruce C. J. Frontal eye field efferents in the macaque monkey: II. Topography of terminal fields in midbrain and pons. J Comp Neurol. 1988 May 22;271(4):493–506. doi: 10.1002/cne.902710403. [DOI] [PubMed] [Google Scholar]
- Suzuki D. A., Keller E. L. The role of the posterior vermis of monkey cerebellum in smooth-pursuit eye movement control. I. Eye and head movement-related activity. J Neurophysiol. 1988 Jan;59(1):1–18. doi: 10.1152/jn.1988.59.1.1. [DOI] [PubMed] [Google Scholar]
- Suzuki D. A., Keller E. L. Visual signals in the dorsolateral pontine nucleus of the alert monkey: their relationship to smooth-pursuit eye movements. Exp Brain Res. 1984;53(2):473–478. doi: 10.1007/BF00238178. [DOI] [PubMed] [Google Scholar]
- Suzuki D. A., May J. G., Keller E. L., Yee R. D. Visual motion response properties of neurons in dorsolateral pontine nucleus of alert monkey. J Neurophysiol. 1990 Jan;63(1):37–59. doi: 10.1152/jn.1990.63.1.37. [DOI] [PubMed] [Google Scholar]
- Thier P., Bachor A., Faiss J., Dichgans J., Koenig E. Selective impairment of smooth-pursuit eye movements due to an ischemic lesion of the basal pons. Ann Neurol. 1991 Apr;29(4):443–448. doi: 10.1002/ana.410290419. [DOI] [PubMed] [Google Scholar]
- Thier P., Koehler W., Buettner U. W. Neuronal activity in the dorsolateral pontine nucleus of the alert monkey modulated by visual stimuli and eye movements. Exp Brain Res. 1988;70(3):496–512. doi: 10.1007/BF00247598. [DOI] [PubMed] [Google Scholar]
- Ungerleider L. G., Desimone R., Galkin T. W., Mishkin M. Subcortical projections of area MT in the macaque. J Comp Neurol. 1984 Mar 1;223(3):368–386. doi: 10.1002/cne.902230304. [DOI] [PubMed] [Google Scholar]
- Yamada J., Noda H. Afferent and efferent connections of the oculomotor cerebellar vermis in the macaque monkey. J Comp Neurol. 1987 Nov 8;265(2):224–241. doi: 10.1002/cne.902650207. [DOI] [PubMed] [Google Scholar]
- Zee D. S., Yamazaki A., Butler P. H., Gücer G. Effects of ablation of flocculus and paraflocculus of eye movements in primate. J Neurophysiol. 1981 Oct;46(4):878–899. doi: 10.1152/jn.1981.46.4.878. [DOI] [PubMed] [Google Scholar]
- Zee D. S., Yee R. D., Cogan D. G., Robinson D. A., Engel W. K. Ocular motor abnormalities in hereditary cerebellar ataxia. Brain. 1976 Jun;99(2):207–234. doi: 10.1093/brain/99.2.207. [DOI] [PubMed] [Google Scholar]