Abstract
This article reviews recent developments in the application of cell-free DNA-based liquid biopsies to neurological diseases.
Over the past few decades, an explosion of interest in the use of accessible biofluids to identify and track molecular disease has revolutionized the fields of oncology, prenatal medicine and others. More recently, technological advances in signal detection have allowed for informative analysis of biofluids that are typically sparse in cells and other circulating components, such as CSF. In parallel, advancements in epigenetic profiling have allowed for novel applications of liquid biopsies to diseases without characteristic mutational profiles, including many degenerative, autoimmune, inflammatory, ischaemic and infectious disorders. These events have paved the way for a wide array of neurological conditions to benefit from enhanced diagnostic, prognostic, and treatment abilities through the use of liquid biomarkers: a ‘liquid biopsy’ approach.
This review includes an overview of types of liquid biopsy targets with a focus on circulating cell-free DNA, methods used to identify and probe potential liquid biomarkers, and recent applications of such biomarkers to a variety of complex neurological conditions including CNS tumours, stroke, traumatic brain injury, Alzheimer’s disease, epilepsy, multiple sclerosis and neuroinfectious disease. Finally, the challenges of translating liquid biopsies to use in clinical neurology settings—and the opportunities for improvement in disease management that such translation may provide—are discussed.
Keywords: liquid biopsy, neurology, cfDNA, epigenetics, methylation
Gaitsch et al. review recent developments in the identification and application of cell-free DNA-based liquid biopsies to neurological diseases, including CNS tumours, stroke, traumatic brain injury, Alzheimer’s disease, epilepsy, multiple sclerosis, and neuroinfectious disease.
Introduction
Basics of liquid biopsy
Liquid biopsy, an alternative to solid tissue biopsy, involves sampling body fluids, usually for molecular components released from cells.1 Biofluids used for liquid biopsy commonly include plasma, serum and CSF but also extend to urine, saliva, pleural effusions and others (Fig. 1).2,3 The liquid biopsy approach has many advantages, including being relatively less invasive and less expensive compared to tissue biopsy and offering a more panoramic view of disease origins via analysis of genetic and epigenetic markers.4–6 Liquid biopsies have long been appreciated as potential tools to resolve the genesis of cancers of unknown origin and to detect occult tissue damage stemming from complex pathologies.7 Additionally, serial biopsies can be taken to determine treatment effects,8 a feat difficult to achieve with traditional tissue biopsies and nearly impossible in some tissues, such as brain and spinal cord. Liquid biopsies can sample numerous molecular entities within the blood, including free nucleic acids, such as cell-free nuclear DNA (cfDNA), cell-free mitochondrial DNA (cf-mtDNA), circulating tumour DNA (ctDNA) and cell-free RNA (cfRNA); extracellular vesicles, often containing nucleic acid components; proteins; and metabolites. Circulating nucleic acids (CNAs) were first described in the 1940s,9 though their usefulness was not fully appreciated until several decades later.
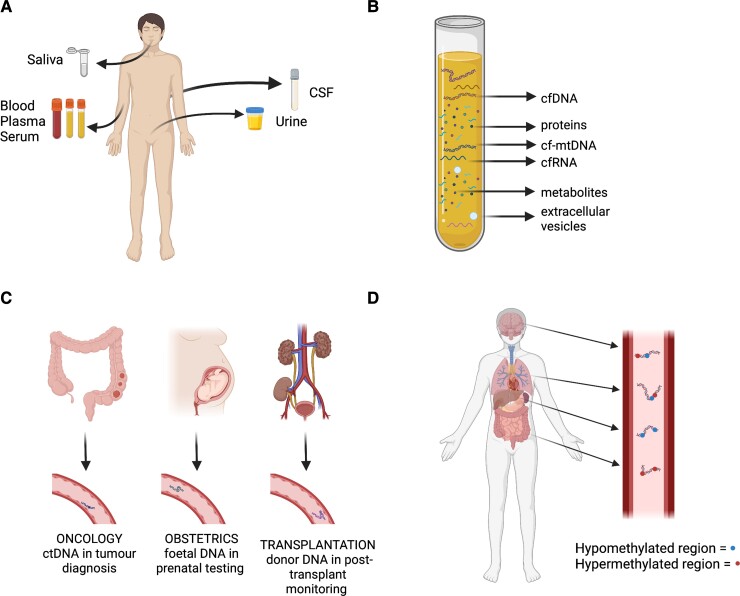
Figure 1.
Overview of liquid biopsy, existing applications and target molecules. (A) Body fluids used for liquid biopsy include whole blood, plasma, serum, urine, CSF, saliva and others. The choice of biofluid depends on the specific clinical application, extent of disease, biomarker target, signal-to-noise tolerability and patient characteristics. (B) Biofluid samples can be used to determine the presence and levels of different molecular contents, including specific cell-free circulating nucleic acid fragments, extracellular vesicles, proteins and metabolites. (C) The liquid biopsy approach is currently being applied in several fields of medicine, including oncology, obstetrics and transplant medicine. These applications often rely on identification of specific mutations or polymorphisms in circulating DNA fragments, such as tumorigenic mutations. (D) The non-genetic features of cfDNA, including CpG methylation patterns, can allow for identification of their tissue and cellular origin. Additionally, these features may permit the application of liquid biopsy in neurological pathologies that are primarily characterized by degeneration, inflammation and ischaemia.
Existing applications of liquid biopsy
Recent decades have seen an explosion in research investigating CNA-based liquid biopsies in a wide variety of human diseases.10 The field of obstetrics, for instance, often relies on non-invasive prenatal testing to determine foetal sex and the presence of chromosomal abnormalities.11–14 Similarly, the field of oncology has found numerous liquid biopsy applications,15–17 such as the United States Food and Drug Administration-approved Epi proColon test that analyses methylation patterns in cfDNA for population-wide colorectal cancer screening.18 The liquid biopsy approach has also been used for early detection of graft rejection in human organ transplants,19–21 and to evaluate tissue damage in patients with COVID-19.7 While neuro-oncology has made significant strides in developing liquid biopsies for CNS tumours, other neurological diseases are in need of non-invasive methods for diagnosis and prognosis. Many of the applications listed above rely on genetic anomalies in CNAs; however, many neurological conditions are not characterized by DNA sequence alterations.
Strategies for liquid biomarker identification
Sequence differences in ctDNA, including known tumorigenic mutations, have been of particular interest in oncology for their potential as prognostic indicators and early-stage screening biomarkers.22 However, the non-genetic characteristics of CNAs, and cfDNA in particular, can provide information applicable to diseases without genetic mutations (Fig. 1). Epigenetic signatures, such as DNA methylation patterns, can be used to identify the tissue and even cell type of origin for cfDNA samples.4,6,23 Given that the human genome contains ∼28 million unique DNA methylation sites,24 the use of methylation array technology and deconvolution algorithms make it possible to identify differentially methylated regions (DMRs) corresponding to various cellular sources and gene expression levels.25 These approaches open the possibility of detecting cfDNA released from cell types that comprise small percentages of the total circulating cfDNA, such as CNS tissue-derived cfDNA in plasma. Remarkably, even cfDNA derived from minority cell populations in tissues, such as oligodendroglial lineage cells in multiple sclerosis, have become targets for liquid biopsy development.
Basis of epigenetic liquid biomarkers
DNA methylation is an epigenetic process by which specific genes are silenced and thus prevented from being transcribed. DNA methylation is thought to physically interfere with transcription factor binding and to promote the formation of heterochromatin via recruitment of histone deacetylases and other proteins necessary for chromatin remodelling.26 The normal physiological functions of DNA methylation are wide-ranging, including embryonic development, suppression of transposable elements, genomic imprinting and X chromosome inactivation. Aberrant DNA methylation is associated with pathological states such as cancer and various age-related diseases.27
The primary targets of methylation are dinucleotide segments of DNA, termed ‘CpG’ units, in which cytosine residues are located adjacent to guanine residues and connected via a phosphate bond. Methylation of DNA in promoter regions tends to repress gene transcription, while methylation of DNA in the gene body is associated with transcript splicing alterations.28 Thus, epigenetic processes allow for selective gene expression, enabling cell types to have unique functions. As DMRs underlie the unique methylation signatures of specific cell types, CpG methylation patterns are a marker of cell identity.
DNA methylation and other epigenetic patterns are important because the genetic sequence alone cannot give information about non-cancer pathologies such as neurodegeneration, inflammation and ischaemia. Epigenetic analysis of cfDNA may also allow for the determination of collateral tissue injury (e.g. off-target drug effects) and monitoring of treatment response during clinical trials. DNA methylation signatures can be used to map the source of the cfDNA released from cells during times of tissue damage, as in the case of tumour metastasis,29 since these fragments retain the methylation signatures characteristic of their tissue of origin. Recent advancements in methylome analysis have led to high-resolution deconvolution algorithms, which can match cfDNA fragments to specific tissues and cells based on a given set of DMRs.7,23,30
Challenges to clinical application of liquid biopsies
Despite the advantages of liquid biopsy over tissue biopsy, several technical hurdles have slowed its translation. ctDNA is released from tumours in minimal amounts in early-stage disease, often rendering it below the limit of detection.31 Additional roadblocks include the need for standardization of sample collection protocols and target amplification procedures,32–34 and more cost-effective molecular profiling methods. Furthermore, epigenetic signatures may change throughout disease, as is seen in CNS tumours, where the signatures used to identify initial disease are not optimal for recurring or treatment-resistant disease.35 Finally, confounding factors, such as comorbidities or risk factors, may inhibit liquid biopsy development by rendering studies difficult to compare. Ideal biomarker targets for liquid biopsy—indeed, for any biopsy—are stable and consistently present in high enough amounts to achieve detection with current technologies. Identifying markers that fit this profile will ensure the reproducibility needed for clinical application.
Cell-free nuclear DNA-based targets in liquid biopsy
This review focuses on cfDNA-based liquid biopsies, one of the most heavily studied liquid biomarkers given the plethora of techniques available to analyse nucleic acids and to probe epigenetic features of DNA. However, rapid advances in genomic and transcriptomic techniques, combined with ongoing reductions in sequencing costs, have led to the identification of several additional types of CNA biomarkers. While not covered in this article, cfRNA—including cell-free mRNA, tRNA, long non-coding RNA and microRNA (miRNA)—and non-nucleic acid circulating components, such as proteins, metabolites, tumour-educated platelets, extracellular vesicles and whole tumour cells, provide promising targets for liquid biopsy in neurological and other disease contexts.36
Cell-free nuclear DNA
Cell-free nuclear DNA fragments are primarily derived from intra- and extracellular nuclease cleavage of DNA.37 They are present in small amounts in healthy individuals, with the majority derived from haematopoietic cells.23 Although cfDNA consists mainly of linear double-stranded DNA, it can also be present in the form of circular and single-stranded DNA.38 Double-stranded cfDNA molecules are the best-studied and most prevalent in plasma. Human plasma DNA consists of a mixture of DNA fragments of different sizes, with the modal size being approximately 166 base pairs (bp). The quantity of cfDNA changes during disease, when it is primarily released from dying cells or by active secretion.7 cfDNA itself can also precipitate tissue injury by serving as a damage-associated molecular pattern, as in renal tubular cells, via the production of mitochondrial reactive oxygen species. Fragments of cfDNA and chromatin can be taken up by mammalian cells and integrate into the genome, thus behaving like mobile genetic elements.39 This uptake suggests that cfDNA fragments might, in some contexts, serve as endogenous contributors to DNA damage associated with ageing.40
In non-disease states, healthy individuals typically have plasma cfDNA levels lower than 10 ng/ml, whereas individuals with acute or chronic disease can have levels that are orders of magnitude higher.1 Levels of up to 50× the normal range have been reported in patients with a history of myocardial infarction, stroke, diabetes mellitus and cancer. Given that the half-life of cfDNA has been estimated to be between 4 min and 12 h, it can offer a ‘real-time’ snapshot of a tumour profile.41 In addition to specific disease states, age appears to play a significant role in the total cfDNA present in the bloodstream,23 likely due to decreased clearance ability with age, rather than increased cfDNA release from dying cells. In either case, the composition of cfDNA appears to be the same in healthy young and old individuals.
Circulating tumour DNA
When cfDNA is released from tumour cells, it is referred to as ctDNA.38 While cancer is a disease often characterized by high levels of blood cfDNA, not all cancers shed DNA into the blood at the same rate.1 Thus, it is likely that disease states differ significantly in their levels of cell death and, therefore, cfDNA release.
Cell-free mitochondrial DNA
Relative to cfDNA, levels of circulating cf-mtDNA do not appear to vary directly with cell death—in fact, several neurodegenerative diseases feature decreased circulating cf-mtDNA levels—and may play a more complex role in neuroinflammatory pathology.42
Methods used to identify, detect and analyse cfDNA-based liquid biopsy targets
Biofluid selection and collection
The low concentration of cfDNA in human biofluids, combined with the high potential for contamination by intracellular nucleic acids, necessitates careful handling during sample extraction. Consideration should be given to the biofluid being sampled, as levels of contamination are affected by the specimen type. For instance, while the levels of cfDNA isolated from plasma and serum are similar, serum samples have increased contamination from large DNA fragments and contain lower levels of ctDNA.43 Overall, plasma appears to be the preferable blood fraction for cfDNA isolation. Additional factors to consider include biofluid volume, accessibility and minimization of potentially painful procedures such as lumbar punctures (LPs).
For all specimen types, it is vital to minimize contamination by sterilizing the collection site and preventing excessive trauma. For blood sampling, anticoagulant-containing blood collection tubes (BCTs) should be employed. Commonly used BCTs include ethylenediamine tetraacetic acid BCTs and cfDNA BCTs (Streck), the latter containing a preservative to prevent cell lysis and allow more extended periods of sample preservation. Following whole blood collection, plasma or serum can be fractioned via centrifugation.
Cell-free nuclear DNA isolation and purification
A variety of commercial kits and ‘homemade’ bead-based protocols can be used to isolate cfDNA from biofluids.44 Isolation kits vary in their volume handling abilities and must be chosen to suit the desired application (e.g. array preparation versus PCR amplification). Automated platforms such as QIAsymphony (Qiagen) can be used for high-throughput cfDNA isolation with comparable performance to manual approaches.45 Concentrations of purified cfDNA can be quantified using fluorometric instruments or assays such as the Quant-iT assay (Thermo Fisher). Instruments such as the 2100 Bioanalyzer (Agilent) can be used to assess sample purity and fragment size distribution.
Target discovery
Cell-free nuclear DNA expression can be interrogated using microarrays designed to characterize mutational profiles or epigenetic alterations. Commonly used epigenome-wide arrays include the Infinium MethylationEPIC BeadChip (Illumina) and its predecessor, the Infinium Methylation450K BeadChip, covering over 850 000 and 450 000 CpG sites, respectively.46,47 These array platforms represent major technical advancements in the field of cfDNA methylation profiling.
While methylation array-based strategies can be appropriate for discovery studies, the low levels of cfDNA in many samples limit such an approach, which requires a relatively high sample input. Additionally, these platforms only cover a small percentage of the total CpG sites in the human genome, and methylation at each site is measured independently, failing to account for methylation haplotype blocks.48 Indeed, a recent largescale sequencing-based study reported that most cell type-specific, uniquely demethylated CpG regions identified were not represented in the single-CpG Infinium arrays.30 These characteristics make sequencing-based approaches indispensable, particularly in situations when the CpG target is already known or an exhaustive search for DMRs desired.
In addition to commonly-used next-generation sequencing (NGS) approaches,49 two novel sequencing techniques employed in epigenetic liquid biopsy studies include whole-genome bisulfite sequencing (WGBS) and cell-free methylated DNA immunoprecipitation and high-throughput sequencing (cfMeDIP-seq). Whole-genome bisulfite sequencing is used to uncover the methylation state of all cytosines.1 It can be performed on DNA samples as small as 30 ng and provides the methylation state for all cytosines, even in regions of low CpG density and non-CpG sites. The process of WGBS begins when bisulfite salts are used to deaminate unmethylated cytosine residues to uracil, leaving methylated cytosines unchanged; this allows for subsequent PCR amplification and sequencing to identify methylated regions of DNA. One caveat is that most DNA is degraded during this process, though there is no known degradation bias toward methylated versus unmethylated fragments. The newer methodology, cfMeDIP-seq, enriches for methylated cfDNA fragments and allows comprehensive profiling of methylated cfDNA to detect and classify a range of tumours.50,51 One advantage of cfMeDIP-seq over WGBS is that it allows for omission of the bisulfite conversion step, thereby preventing significant sample loss.
Target amplification
Quantitative PCR
Quantitative PCR (qPCR) can be adapted to study epigenetic patterns using methylation-specific primer designs targeting loci identified in array and sequencing studies. It can be used for methylation validation by employing techniques such as methylation-specific high-resolution DNA melting (MS-HSM), which quantifies the methylation status of a locus by exploiting the different melting temperatures of methylated and unmethylated DNA.52
Droplet digital PCR
Droplet-digital PCR (ddPCR) is an ultrasensitive qPCR method that can detect low copy numbers of DNA. This method is unique in that it is based on a system of partitioning DNA molecules into individual droplets before undergoing PCR amplification. Thus, ddPCR is ideal for the analysis of small nucleic acid inputs.
Cell-free nuclear DNA origin analysis
It is often useful to determine the anatomical origins of cfDNA fragments using epigenetic signatures; examples include investigations of cancers of unknown origin or localization of off-target treatment effects. Unique cellular methylation patterns have been employed to establish rates of death among specific cell types, including oligodendrocytes, from circulating cfDNA.53 The development of unbiased deconvolution algorithms for DNA54 and, more recently, cfDNA55 has been vital to harness the tissue- and cell-specific nature of epigenetic patterning.
In 2018, Moss et al.23 reported the creation of a methylation atlas for 25 human tissue and cell types of origin for circulating cfDNA. The cfDNA of healthy volunteers was characterized as originating mainly from white blood cells (55%), erythrocyte progenitors (30%), vascular endothelial cells (10%) and hepatocytes (1%). Clinical laboratory tests, namely complete blood count and liver function tests, correlate well with plasma blood cell- and hepatocyte-derived cfDNA proportions, respectively. Focusing on CNS-derived cfDNA, a separate proof-of-concept study showed that increased cfDNA from glia and neurons could be identified in plasma from military personnel trained to work with explosives following occupational training exposure.25
Advancing this work, Loyfer et al.30 recently reported the creation of a comprehensive human DNA methylation atlas, based on deep WGBS, containing a large collection of potential biomarkers that could serve as liquid biopsy targets. Importantly, the resolution of this atlas allows for identification of tissue and cell type transcriptional enhancers and other gene regulatory elements. Thirty-nine cell types were profiled, including neurons and oligodendrocytes. In silico experiments using mixtures of sequenced reads demonstrated that the use of 25 methylation markers per cell type was sufficient to accurately detect DNA from a given source when it constituted only 0.03–0.1% of the total DNA mixture, thus dramatically lowering the signal-to-noise ratio required for target cfDNA detection in biofluids. These results further open the possibilities of applying the liquid biopsy approach to neurological diseases, in which the target cell types of origin contribute to a small minority of overall cfDNA in the bloodstream (Fig. 2). Such deconvolution analyses can be reproduced for alternative experimental setups using publicly available GitHub resources.7,23,25
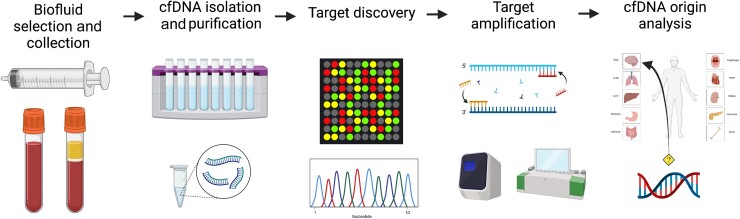
Figure 2.
General strategy for cfDNA-based liquid biomarker discovery. A generalized workflow to identify and validate cfDNA-based liquid biopsy targets consists of the following steps: biofluid selection and collection, cfDNA isolation and purification, target discovery, target amplification and cfDNA origin analysis. While this pipeline is generally followed, a wide variety of specific methods to identify and isolate cfDNA biomarkers are reported in the literature. This variation may account for some of the difficulty in translating cfDNA-based liquid biopsies for neurological diseases to the clinic, especially given the low concentration of CNS-derived cfDNA in blood, the most common substrate for liquid biopsy.
Recent developments and applications of cfDNA-based liquid biopsies in neurological disease contexts
CNS tumours
The potential to diagnose and characterize CNS tumours, replace or complement ambiguous imaging studies and monitor treatment response in a minimally invasive manner provides exciting opportunities for liquid biopsy development (Fig. 3). In the case of gliomas, the most common group of primary CNS tumours, progress in screening and treatment efficacy remains low.56 While advances in genetic profiling of tumours allow for intricate diagnosis,57,58 a key component of oncologic precision medicine, obtaining a histopathological diagnosis necessitates brain biopsy, a risky and sometimes impossible procedure depending upon the surgical accessibility of the tumour. Brain biopsies have even been implicated in glioma recurrence via disruption of the tumour microenvironment, leading to the initiation of signalling cascades that make the tumour more aggressive.59 Non-invasive diagnostic approaches, such as CT and MRI neuroimaging modalities, are sensitive to most spinal and intracranial tumours but can be difficult to interpret in certain situations. Pseudoprogression, for instance, occurs when imaging studies suggest progression in patients undergoing other processes such as necrosis or reactive tissue damage; the confusion caused by this phenomenon might be ameliorated by a confirmatory liquid biopsy test. While surgical intervention will remain essential to CNS tumour treatment, liquid biopsies in these contexts have the potential to play a vital role in understanding tumour genetics and spatial heterogeneity, guiding treatment decision-making, monitoring therapeutic response, and surveying for treatment resistance.60
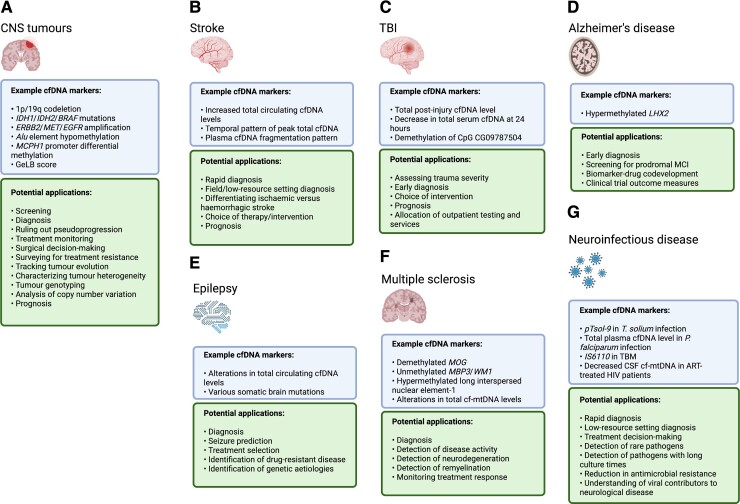
Figure 3.
Recent developments and applications of liquid biopsy in neurological disease contexts. There is an unmet need for diagnostic, prognostic, and treatment-monitoring liquid biomarkers in a wide variety of neurological diseases. Recent work highlighting potential cfDNA-based liquid biopsy targets in several common neurological conditions is summarized here. Conditions including (A) CNS tumours, (B) stroke, (C) traumatic brain injury, (D) Alzheimer’s disease, (E) epilepsy, (F) multiple sclerosis, and (G) neuroinfectious disease each have characteristics that could uniquely benefit from information provided by liquid biopsy.
Given the characteristic mutational burden of many CNS tumours, the literature surrounding liquid biopsies in neuro-oncology primarily focuses on targeted sequencing of known driver mutations in ctDNA. This broad interest in using ctDNA for early tumour detection has prompted investigations of basic ctDNA dynamics, including turnover rates and the relationship between biofluid concentration and tumour location. Stallard et al.61 demonstrated that CSF-derived ctDNA from patients with diffuse intrinsic pontine glioma (DIPG) could be used to quantify tumour growth by targeting the tumour-specific H3F3A K27M mutation. To simulate ctDNA release into the CSF, a co-culture model of DIPG007 cells and normal human astrocytes was used to show that increased levels of tumour cell proliferation corresponded to increased ctDNA in the culture media, even when the media was changed frequently to approximate the constant production and resorption of CSF. Further experiments found that irradiation of cells resulted in a dramatic increase in ctDNA for ∼72–120 h post-treatment before tapering off, likely due to the rapid death of cells and the concurrent release of ctDNA fragments.
The unique role of the blood–brain barrier (BBB) in CNS tumours and its dynamic permeability in several neurological conditions necessitate a more nuanced understanding of the relationship between tumour location and biofluid cfDNA concentration. Remodelling of the BBB, a known feature of glioma evolution62 and an outcome of radiation therapy,63 potentially facilitates the passage of cfDNA into the peripheral circulation. Multifocal CSF sampling from different anatomical reservoirs indicates that closer tumour proximity to the ventricles or subarachnoid cisterns is correlated with higher levels of mutation-containing ctDNA.61,64 Correspondingly, direct sampling of CSF from DIPG patients at autopsy found that samples taken directly from the ventricles contained higher levels of K27M-containing ctDNA than samples collected via LP. In comparing the glioma-derived ctDNA content of CSF versus plasma, several studies indicate that CSF is a more sensitive liquid biopsy method for detecting tumour mutations, particularly given the lack of background signal from blood cell-derived cfDNA.60,65,66 However, even if plasma contains a relatively lower concentration of mutation-containing ctDNA, it may be helpful as an alternative sampling route for clinical situations in which an LP is contraindicated (e.g. brain herniation). Urine is another biofluid of interest, with preliminary results suggesting that urine ctDNA fragmentation patterns can be used to detect glioma.67
In addition to tumour detection, genotyping of gliomas65,66,68–70 and identification of mutation-specific therapy options and relevant clinical trials71 can be accomplished by sequencing CSF-derived ctDNA. Whole exome sequencing of ctDNA collected from glioblastoma patient CSF can be used to detect mutations preoperatively.72 This is beneficial given that different glioblastoma mutational profiles warrant different treatment strategies; for instance, IDH1 mutant glioblastoma has a survival benefit associated with maximal surgical resection.73 Mutational characterization of CNS germ cell tumours,74 paediatric diffuse midline glioma,75–77 and paediatric medulloblastoma78,79 is also feasible using CSF-derived ctDNA, as is detection of tumour-associated copy number variations.80 The range of mutations detectable by these methods—including alterations occurring early in tumorigenesis, such as codeletion of chromosome arms 1p/19q, mutations in IDH1 and IDH2, and amplifications in ERBB2, MET and EGFR—largely overlaps with those found in traditional tissue biopsy. However, intriguingly, many mutations that are detectable in CSF-derived ctDNA are not regularly detected through tissue biopsy, highlighting the ‘panoramic’ view provided by liquid biopsies,64 particularly for highly heterogeneous tumours like glioblastoma. The presence of ctDNA in glioma patient CSF is associated with an increased risk of death, independent of IDH status, tumour burden at the time of LP, or extent of resection; however, no significant associations have been observed between ctDNA-positive CSF and tumour grade, disease duration, or prior therapy.
Liquid biopsy of CSF-derived ctDNA has a sensitivity advantage over CSF cytology, given that many glioma patients with detectable CSF ctDNA do not have detectable CSF tumour cells. Additionally, prospective clinical studies have found that CSF-derived ctDNA contains more variants and higher variant frequencies than genomic DNA extracted from circulating tumour cells, making it superior for molecular profiling.81 Plasma- and serum-based ctDNA sequencing can also provide information about glioma mutational profile, including driver mutations such as BRAF.82,83
Advances in characterizing ctDNA methylation patterns have expanded the possibilities of using liquid biopsies for the clinical management of CNS tumours, especially for tumours with a low frequency of oncogenic mutations. Global methylation markers represent one way of monitoring epigenetic cfDNA alterations in disease. Given its high abundance in DNA, hypomethylation of the Alu element—a short, interspersed genomic element—has been used as a global marker of methylation status.84 Significantly lower methylation levels in the Alu element can be detected in the serum-derived ctDNA of glioma patients, effectively separating them from healthy controls and patients with benign intracranial tumours using a liquid chip assay.85 In addition to distinguishing cases of glioma, methylation of the Alu element in ctDNA is negatively correlated with glioma severity and positively correlated with survival. However, a rigorous survey of Alu methylation levels in other diseases has not been completed and therefore it may not be a specific glioma marker.
Disease-specific methylation changes can also be gleaned from ctDNA analysis. For instance, the MCPH1 promotor in serum-derived ctDNA correlates with both glioma size and grade.86 Epigenetic signatures, based on patterns of DMRs present in CSF-derived ctDNA from medulloblastoma, can be used for tumour detection and monitoring of treatment effects.87 In addition to ctDNA methylation patterns, hydroxymethylation patterns identified via highly sensitive chemical labelling of plasma ctDNA have shown promise in distinguishing glioblastoma from lower-grade astrocytomas.88
Epigenetic characteristics of ctDNA can also be used to discriminate between intracranial CNS tumour types, an impressive feat given that these tumours often have similar cell types of origin. Nassiri et al.89 used cfMeDIP-seq to characterize the epigenetic cfDNA profiles of plasma samples from patients with diffuse gliomas, patients with extracranial tumours and healthy controls. DMRs identified in this analysis were used to build a classifier capable of assigning a methylation score to each sample. This score could differentiate gliomas from other cancer types and healthy controls and, when calculated for over 100 additional CNS tumour samples, accurately discriminated between gliomas, meningiomas, hemangiopericytomas and brain metastases. A similar study, this time using a methylation array, found that the epigenetic signatures present in glioma-derived ctDNA isolated from patient CSF and plasma can be used to establish an initial diagnosis prior to surgical intervention.90 To this end, a machine-learning-based model was used to develop a glioma-epigenetic-liquid biopsy (GeLB) score to indicate the likelihood that a given serum sample is from a glioma patient. While this score was shown to have high sensitivity and specificity in recognizing patients during initial disease, most patients had decreased GeLB scores at recurrence, possibly due to a treatment-induced DNA methylation shift in glioma cells. Recent work has also shown that genetic alterations attributable to clonal haematopoiesis can be detected in the plasma cfDNA of patients with a history of temozolomide chemotherapy.91 These findings highlight another challenge in tumour biomarker development: the continual evolution of tumours themselves.
Liquid biopsy developments in neuro-oncology have been reviewed extensively elsewhere.92–100
Stroke
The importance of rapid stroke diagnosis cannot be overstated, given that each minute without intervention corresponds to increased brain tissue damage.101 The two main types of stroke, ischaemic and haemorrhagic, have fundamentally different treatment approaches, highlighting the importance of accurate aetiological determination. Current diagnosis of acute stroke relies heavily on neuroimaging techniques, including CT and MRI. While acute imaging and angiography will undoubtedly remain vital for in-hospital treatment decision-making—for example, to rule out haemorrhagic stroke and to detect large-vessel intracranial occlusions amenable to mechanical endovascular thrombectomy—these strategies may not be sensitive enough to catch early-stage ischaemic strokes when they are most receptive to intervention via intravenous thrombolysis and/or thrombectomy, and to regaining function.102 Indeed, approximately half of acute stroke or transient ischaemic attack (TIA) patients lack detectable abnormalities in initial imaging studies.103 While MRI provides a greater sensitivity for detecting ischaemic stroke when compared to CT, it is not used in many emergency room settings due to real or perceived104 time constraints as well as hardware availability in less resource-rich settings. Additionally, imaging can be costly and may not be suitable for restless or acutely distressed patients. Thus, there is a distinct need for clinically validated, rapidly assayed blood biomarkers to assess ischaemia severity in stroke, similar to those available for assessment of myocardial infarction.105
While several serum protein biomarkers have been associated with stroke, including S100, NSE and CRP, among others, these markers only become elevated several hours following symptom onset and are therefore not ideal for rapid biomarkers.102 Some protein markers, including GFAP in the case of haemorrhagic stroke, are released into the bloodstream much faster; however, variability in diagnostic performance has limited their progression to clinical use.106 Simple cfDNA-based tests that could be used by emergency personnel in the field or in the ambulance might allow for immediate initiation of treatment, such as administration of blood pressure lowering medications, and more informed decision-making regarding transport to qualified stroke centres. Even when compared to protein biomarkers at the same timepoints post-stroke, cfDNA has been found to provide sensitive, independent prognostic information, especially in the case of severe stroke outcomes.107
In acute ischaemic stroke (AIS), the most common stroke subtype, lack of perfusion quickly leads to death of brain cells and disruption of the BBB. Therefore, it has been hypothesized that this post-injury environment is characterized by cfDNA released into the peripheral circulation by cells undergoing necrosis and apoptosis.108 This hypothesis is supported by the finding that the total amount of circulating cfDNA measured at the time of AIS patient admission correlates with both stroke severity (assessed using the National Institutes of Health Stroke Scale) and medium-term prognosis (assessed using the modified Rankin Scale at three months post-infarction).109 Furthermore, circulating cfDNA threshold values can be used to differentiate actual AIS patients from stroke mimics110 and to classify the patients most likely to experience neurological improvement following mechanical thrombectomy or intravenous thrombolysis.111 Notably, these increased cfDNA levels in stroke patients may signify intraparenchymal brain damage, increased damage to the BBB, or both. Interestingly, the temporal dynamics of circulating cfDNA differ between ischaemic and haemorrhagic stroke.112 Mirroring the two different pathologies, haemorrhagic stroke results in a rapid and temporary increase in plasma cfDNA, whereas cfDNA levels in ischaemic stroke increase in parallel with hypoxia- and reperfusion-induced cellular damage over the course of days. These differences may be useful in developing rapid cfDNA-based tests for grouping patients with stroke symptoms based on aetiology and prognosis.
Given the frequency of pre-existing cardiovascular conditions in stroke patients, tissue-of-origin analysis of cfDNA samples is particularly relevant. For instance, it is possible that alterations in stroke patient cfDNA levels are driven by nucleic acid release from endothelial cells or activated platelets, as opposed to dying brain cells. The finding of elevated levels of such cfDNA fragments might serve as a ‘proxy’ for the identification of concurrent or subsequent pathological processes in a patient. In this way, stroke presents a particularly challenging scenario for standardized biomarker development, given its many different causes and the frequency of patient comorbidities. Despite these challenges, there remains a clinical need for a rapid (results within minutes) blood test to diagnose stroke and guide treatment decision-making, either in combination with current imaging studies or as a stand-alone test in low-resource settings that lack access to imaging equipment or trained imaging technicians. Additionally, such blood tests may be used to monitor treatment effects as a measure of ongoing CNS cellular damage, to predict long-term outcomes and even to guide the pursuit of rehabilitation regimens.
Additional liquid biopsy developments relating to stroke are reviewed elsewhere.102,106,113
Traumatic brain injury
Severe traumatic brain injury (TBI) is a critical neurological condition that contributes to death and disability in all age groups, and patient clinical courses can be highly unpredictable. Secondary brain injuries due to TBI such as elevated intracranial pressure can cause additional long-lasting neurological damage beyond the initial insult.114 Even patients with mild TBI can develop post-concussion syndrome (PCS)115 characterized by symptoms that often last for months after the initial insult. These include personality changes and cognitive difficulties that can affect employment and personal relationships. Despite the evaluation of several protein biomarkers for TBI,116,117 no broadly applicable TBI damage marker has been identified. Therefore, TBI patient outcomes would benefit from rapid and accurate classification of the extent of tissue damage, allowing for proper resource allocation and appropriate clinical interventions.
Overall levels of post-injury serum cfDNA positively correlate with the severity of TBI.118 The decrease in overall cfDNA serum levels at 24 h post-admission has been shown to be important for predicting TBI patient outcomes, with the diminution ratio of serum cfDNA being predictive of fatal outcomes. Additionally, cfDNA levels have been shown to predict disability post-injury in mild TBI. A prospective pilot study using a fluorescence assay to measure cfDNA levels (correlating highly with a traditional qPCR assay of β-globin) in the serum of mild TBI patients found that overall cfDNA levels at hospital admission positively correlated with levels of cognitive impairment after adjusting for age and education level.119 This method has been proposed as a potential rapid screening system in emergency care settings to determine patients at risk of PCS. Another strategy using an alternating current electrokinetic microchip to test a variety of plasma biomarkers, including cfDNA levels, in mild TBI patients has also shown promise in predicting the risk of PCS-associated symptoms.120 Such real-time screening might enable rapid allocation of medical and social support, including regular cognitive testing and social services, to those patients who need them most. However, the use of such diagnostic tests should also be examined in patients with multitrauma, given that a significant percentage of TBI cases occur alongside other bodily injuries (e.g. car accidents, falls, assaults), leading to widespread tissue damage.
One relatively common complication of TBI is intracranial haemorrhage (ICH).121 Current methods of monitoring ICH, including clinical exams, neuroimaging and intracranial pressure monitoring, are limited in their ability to accurately assess damage and predict prognosis. Plasma-derived cfDNA levels, measured using β-globin expression, have been found to positively correlate with patient Acute Physiology and Chronic Health Evaluation score and inversely correlate with patient Glasgow Coma Scale, indicating a relationship between overall cfDNA levels and ICH patient clinical status.122 Additionally, cfDNA samples taken on Day 3 of intensive care unit admission positively correlated with the duration of stay in intensive care, though this marker was not as predictive of patient outcome as the protein biomarker S100β. This increased cfDNA is thought to arise from neurons and glia damaged during CNS injury; indeed, investigation of epigenetic signatures indicates that demethylation at the brain-specific CpG CG09787504 can be readily detected in circulating cfDNA of patients after TBI, thus reporting on the rate of brain cell death.53
Additional liquid biomarkers of TBI are reviewed elsewhere.123
Alzheimer’s disease
The leading cause of dementia in the elderly is Alzheimer’s disease,124 a neurodegenerative disease characterized by loss of function primarily in the cognitive, language and memory domains.125 Alzheimer’s disease is one of several neurodegenerative conditions characterized by abnormal misfolded protein aggregation. Centering on the proteins highly associated with disease pathogenesis, an array of Alzheimer’s disease imaging and liquid protein biomarkers have been identified and validated.126 However, nucleic acid-based biomarkers for Alzheimer’s disease are less developed. With escalating pressure to identify a variety of circulating biomarkers of Alzheimer’s disease for use in both drug development and clinical practice, important questions have arisen involving specific biomarker contexts of use and relevance to disease pathology.127
Alzheimer’s disease is diagnosed primarily using clinical criteria and often begins with a prodrome involving mild cognitive impairment (MCI).128 Imaging modalities, including MRI and PET, can be used to confirm Alzheimer’s disease pathogenesis and even to identify cases during the prodromal period.129 Recent advancements in techniques that can identify presymptomatic disease and predict cognitive decline, including amyloid- and tau-based PET radiotracers,130–132 are particularly important given that disease progression may begin decades prior to clinically noticeable symptoms.133 Protein-based liquid biopsies of the CSF and blood have also demonstrated good diagnostic and prognostic performance by targeting molecules, such as amyloid-beta, tau, NfL and GFAP.134,135 Plasma tau is already being investigated in clinical trials and implemented in clinical practice as a predictor for future development of Alzheimer’s dementia in patients with MCI.136,137 Given the overall failure of disease-modifying therapies at treating symptomatic Alzheimer’s disease patients, it is likely that any progress to be made in slowing or preventing Alzheimer’s disease pathogenesis will occur by treating patients at the earliest stages of the disease, emphasizing the importance of biomarkers for early detection.
In addition to blood- and CSF- derived protein biomarkers of Alzheimer’s disease, such as the amyloid-β (Aβ)1–42/Aβ1–40 ratio, BACE1 enzyme activity, total-tau, phosphorylated-tau and NfL concentrations, putative cfDNA biomarkers of Alzheimer’s disease have begun to be identified.138 Given that LPs can be particularly challenging and distressing to perform in the elderly and those with severe cognitive deficits, many studies use peripheral blood as the biofluid substrate. Cell-free nuclear DNA with hypermethylated LHX2, an epigenetic pattern seen specifically in neural tissue, have been put forth as a marker of Alzheimer’s disease.139 Patterns of 5-hydroxymethylation are less commonly studied than 5-methylcytosine modifications in the context of liquid biopsy; however, their presence in serum-derived cfDNA could distinguish patients with late-onset Alzheimer’s disease from cognitively normal individuals in a recent case-control study using genome-wide profiling.140
Contrasting the progress in developing protein-based MCI biomarkers, the search for cfDNA-based MCI biomarkers has proven more difficult. A study of Finnish twin pairs found no significant epigenetic cfDNA markers associated with episodic memory impairment, possibly due to the low genomic coverage provided by plasma cfDNA fragments and the variation in cfDNA concentrations between individuals.141 The search for markers of MCI is particularly challenging due to the mildness of disease pathology compared to more severe neurodegenerative conditions, which might lead to less cfDNA being released from damaged neurons and, therefore, a blood signal that is harder to detect.
Both positive and negative predictive biomarkers may be helpful during Alzheimer’s disease drug development as part of biomarker-drug codevelopment programs.142 Such programs are particularly relevant given the high failure rate of putative Alzheimer’s disease drugs during clinical trials. Compared to CSF or neuroimaging markers, the use of easily accessible blood biomarkers might increase the number of sites able to participate in clinical trials and the number of patients able to be enrolled, thus expanding the statistical power of trials. This arrangement would constitute liquid biopsy-guided drug development. Biomarker panels may be used to stratify patients and help account for the heterogeneity of disease present in conditions like late-onset Alzheimer’s disease. Liquid biopsy findings may be further used to ‘stage’ complex neurodegenerative conditions like Alzheimer’s disease through the use of longitudinal studies enrolling cognitively healthy individuals at risk of developing Alzheimer’s disease and following them for several years.
At present, in the case of Alzheimer’s disease, the specificity and clinical utility of protein-based liquid biopsies is greater than those targeting circulating DNA. However, the development of cfDNA-based liquid biopsies in Alzheimer’s disease may still be advantageous in certain circumstances, at minimum due to their potential to serve as auxiliary biomarkers. For instance, disturbances in the homeostatic proportions of brain-derived cfDNA, identified via differential methylation profiling, may precede frank protein accumulation in the CSF or blood and therefore serve as an earlier disease marker. Furthermore, detection of the loss of specific types of neurons using characteristic DNA methylation patterns present in cfDNA may allow for a more nuanced understanding of neuronal vulnerability and disease pathogenesis compared to broad markers of neurodegeneration, such as NfL. This feature could assist in defining subtypes of disease affecting different neuronal populations, for example. Additionally, liquid biopsy assays targeting proteins in the blood can suffer from significant variation due to both degradation of brain-derived proteins by circulating proteases and non-specific assay detection of endogenous antibodies.126 This variation might be mitigated by the addition of non-protein biomarkers into diagnostic and prognostic frameworks. Finally, cfDNA-based liquid biopsies relevant to other related neurodegenerative diseases, such as frontotemporal dementia, would benefit from further research into identifying cfDNA targets in dementia.
Additional recent liquid biopsy developments relating to Alzheimer’s disease,126,143,144 and MCI145 are reviewed elsewhere.
Epilepsy
Epilepsy diagnosis is complex and presents unique challenges due to the temporary nature of seizure activity, which is often unwitnessed. While EEG and MRI assessments can aid diagnosis, molecular biomarkers could provide an effective way to confirm the diagnosis based on the clinical history, determine treatment regimens, and identify patients with potentially drug-resistant epilepsy.146 Neuronal death has been observed in patients with epilepsy following seizure activity and in animal models of induced status epilepticus,147–149 signifying the potential for increased release of cfDNA into circulating biofluids.
Despite its potential as a diagnostic and prognostic biomarker, studies of cfDNA in the context of epilepsy are limited. In one prospective study of focal epilepsy patients, cfDNA levels were reported to be higher in symptomatic refractory focal epilepsy compared to healthy controls.150 However, in a study of refractory epilepsy patients undergoing video-EEG monitoring, it was found that overall serum-derived cfDNA levels were lower in extratemporal lobe epilepsy compared to controls.151 Additionally, patients with a longer duration of epilepsy (18+ years) had lower cfDNA levels (measured as the difference between baseline and peak levels) compared to patients with a shorter history of epilepsy. These results indicate that cfDNA levels do not directly correlate with seizure burden and emphasize the need for further studies to clarify cfDNA differences between epilepsy subtypes and over the course of chronic disease.
Liquid biopsy may be useful in the molecular diagnosis of genetic causes of epilepsy, particularly in cases of intractable epilepsy due to brain malformations. It is possible that such an approach would decrease the need for brain tissue biopsy, allow for a more personalized approach to treatment based on patient mutational status, and even provide predictions of seizure relapse. A recent proof-of-principle study shows that somatic brain mutations can be identified by sequencing brain-derived cfDNA isolated from CSF samples.152 In another study, cfDNA was isolated from CSF collected during epilepsy surgery and used to detect somatic mutations consistent with those found by analysing resected tissue.153 Droplet digital PCR was used to detect several variants encompassing a wide array of pathological diagnoses; however, only a minority of patients had cfDNA mutations detectable in their collected CSF, indicating the need for substantial technical optimization and testing prior to clinical implementation of such a strategy.
Additional recent liquid biopsy developments in epilepsy are reviewed elsewhere.154
Multiple sclerosis
Multiple sclerosis is a neuroinflammatory and neurodegenerative condition that is a major cause of neurological disability, particularly in young adults.155 Its diverse symptomatology is caused by the development of demyelinating lesions in various regions of the brain and spinal cord that occur dynamically over time and space.156 The regenerative process of remyelination is often able to repair these lesions partially or completely in the early stages of the disease; however, this mechanism tends to fail at later stages of the disease, resulting in persistent lesions with increased damage to the surrounding tissue, axonal degeneration and progressive clinical course.157 Much remains to be understood regarding the molecular and cellular factors contributing to successful remyelination, how the potential for remyelination varies between individual patients and in different neuroanatomical locations, and how age might disrupt this process.158,159 Additionally, biomarkers for risk of neurodegeneration in multiple sclerosis patients are lacking.160
A major roadblock to evaluating potential regenerative multiple sclerosis therapies is the lack of ability to accurately track remyelination. To overcome this challenge, liquid biopsy tracking of oligodendrocyte lineage cell dynamics may provide a means of monitoring disease burden and even estimating remyelination activity. The development of liquid biopsies targeting molecular signatures of demyelination might also be useful for other demyelinating conditions, such as progressive multifocal leukoencephalopathy and acute disseminated encephalomyelitis. In multiple sclerosis, concurrent surveillance of patients with liquid biopsy may help clarify MRI findings, or vice versa, and reduce the number of imaging sessions needed for the patient, saving expense and time. Future longitudinal liquid biopsy studies of patients with relapsing-remitting multiple sclerosis may be useful for identifying prognostic signatures present in patients who eventually develop progressive disease.
MOG, a gene expressed specifically in mature oligodendrocytes, is demethylated in mouse and human oligodendrocytes when compared with other cell types.161 Measuring differential methylation of CNS-specific MOG cfDNA in plasma represents a viable way of quantifying oligodendrocyte death in rodent models and relapsing-remitting multiple sclerosis patients, without the need for sampling CSF. Similarly, cfDNA containing unmethylated MBP3 and WM1, also specific for oligodendrocytes, can be detected in the plasma of about 75% of patients with active (relapsing) multiple sclerosis, but not in patients with stable (remitting) disease.53 This may be due to the relapsing/remitting nature of multiple sclerosis, in which temporary inflammatory disruptions of the BBB might affect detection of cfDNA released from brain cells into the bloodstream. Differential methylation patterns of genetic elements beyond gene bodies and promotor regions can also be used to differentiate multiple sclerosis patients from healthy controls. For example, long interspersed nuclear element-1 is an autonomous retrotransposon containing CpG sites that are significantly hypermethylated in the serum-derived cfDNA of relapsing-remitting multiple sclerosis patients.162
Investigations of cf-mtDNA in the CSF of multiple sclerosis patients have found increased levels in both relapsing-remitting multiple sclerosis163 and progressive multiple sclerosis, the latter being associated with brain atrophy.164 In contrast, a separate study of post-mortem progressive multiple sclerosis patient ventricular CSF targeting two regions of cf-mtDNA (MTND1 and MTND4) found that decreased cf-mtDNA copies were associated with progressive multiple sclerosis.165 This study did not observe a correlation between cf-mtDNA and known neurodegenerative protein markers GFAP and S100β. This discrepancy may be due to living versus post-mortem sampling of CSF or the use of patients at a later stage of disease. However, it highlights the need for longitudinal studies over the course of complex and dynamic conditions like multiple sclerosis, as cf-mtDNA levels may fluctuate significantly during different disease stages.
Neuroinfectious disease
Because CNS infections are often found in low-resource settings, testing for microbial nucleic acids present in readily accessible biofluids represents a promising strategy for rapid and inexpensive diagnosis. Additionally, this approach may allow for quick tailoring of treatment, lessening the side effects of prolonged empiric treatment. The clinical workflow for managing infectious diseases already employs biofluid collection: for example, the use of blood cultures and CSF analysis. However, cultures can take days to weeks to yield identification of the causative organism, and CSF analysis can fail to detect rare pathogens.166,167 Thus, the existing framework may be used to add additional targeted amplification of nucleic acid sequences released into body fluids by neuropathogens.
Parasites are a common cause of endemic CNS infectious disease around the globe. For example, neurocysticercosis is caused by ingestion of the Taenia solium (pork tapeworm) parasite, which forms cysts in the brain that can lead to the onset of seizures and other severe neurologic signs.168 Current diagnostic approaches for this urgent condition include imaging studies focused on identifying cysts, and detection of circulating parasite antigens or antibodies in the plasma or CSF. T. solium has been detected in patient CSF169 and urine170 samples using PCR primers targeting the pTsol-9 gene with subsequent amplicon sequencing to confirm positivity.
Another neurological consequence of parasitic infection is cerebral malaria, a life-threatening condition that can be unpredictable in onset and often affects young children infected with Plasmodium falciparum.171 A large-scale retrospective study examining plasma from Malawian children identified a straightforward potential predictive biomarker for the development of cerebral malaria: total plasma cfDNA (the sum of host and parasite cfDNA, of which the host makes a much larger contribution).172 This finding demonstrates that cfDNA released during host response to parasitic infection, as opposed to cfDNA released by the organism itself, can serve as a robust biomarker for disease severity and may allow for triage of at-risk patients.
Even with the current standard of CSF collection to diagnose cases of infectious meningitis and encephalitis, pathogen levels in the CSF can be below the limit of detection. This is often the case in tuberculosis meningitis (TBM),173 a life-threatening illness and one of the most serious manifestations of tuberculosis. In these circumstances, amplification of nucleic acid fragments like cfDNA can be used to detect microbes. Detection of Mycobacterium tuberculosis cfDNA (specifically, the IS6110 sequence) in the CSF has been reported to be more sensitive than traditional diagnostic methods, including microscopy, mycobacterial culture and the GeneXpert MTB/RIF Ultra assay, with similar specificity.174 A prospective multicentre study comparing GeneXpert to cfDNA assay for TBM found both to have optimal sensitivities and specificities (>90%),175 indicating that both approaches are useful for TBM diagnosis.
Circulating microbial cfDNA can be used to diagnose CNS infection when cultures and PCR testing for common organisms fail, as is demonstrated by a recent case study featuring a patient with a rare form of bacterial meningitis.176 Importantly, the diagnostic method was not impacted by antibiotic exposure, an essential characteristic of diagnostic tests for severe infections requiring immediate treatment. While the methodology used in this instance—applying NGS to cfDNA isolates from blood samples—is too expensive and time-consuming to be translatable to most clinical settings, it demonstrates the potential utility of identifying microbe-specific cfDNA signatures. A cfDNA-based liquid biopsy approach might be useful in cases where culture time is lengthy and causes a delay in the transition from empiric to narrow-spectrum treatment. This point also pertains to the rapidly growing problem of antibiotic resistance worldwide.
Viral diseases affecting the CNS have also been investigated using cfDNA-based liquid biopsy. In a large-scale study of the CNS HIV Antiretroviral Therapy (ART) Effects Research cohort, patients currently on ART or with a history of ART were found to have decreased levels of cf-mtDNA in their CSF samples.177 While levels of cf-mtDNA in the CSF were not associated with neurocognitive performance in HIV patients, cf-mtDNA levels were inversely correlated with proteins necessary for iron metabolism and angiogenesis, hinting at novel mechanisms of CNS damage due to HIV.
Beyond diagnosis of primary neuroinfectious disease, profiling of molecular by-products from microbes could enhance understanding of the underlying aetiologies of neuroinflammatory and neurodegenerative diseases. For instance, the growing consensus that Epstein–Barr virus (EBV) is part of the causal chain in the development of multiple sclerosis178 could be further investigated by targeting EBV-derived cfDNA released into patient CSF or plasma and comparing changes in these levels to neuroimaging biomarkers and patient clinical status.
Alzheimer’s disease has also been associated with multiple human herpesviruses,179 though the burden of proof is exceptionally high for claims that infection underlies the convoluted pathogenesis of Alzheimer’s disease. Indeed, a recent multicohort study found no difference in human herpesvirus 6 expression between healthy and non-Alzheimer’s disease control brains,180 suggesting that continuous viral pathogenesis is unlikely to be a primary contributor to this disease, but not ruling out other mechanisms by which viruses may influence or underlie Alzheimer’s disease pathology. A deeper understanding of viral cfDNA dynamics in relation to lesion and plaque development, and neuroaxonal degeneration, may prompt new insights into the complex aetiologies of these conditions.
The future of cell-free nuclear DNA-based liquid biopsy in neurological disease
In the future, combining multiple biomarkers may allow for more specific application of liquid biopsies, for instance, by targeting combinations of hypo- and hyper-methylated cfDNA regions (Fig. 4).181 Adding more loci per target region can enhance the power of the DNA methylation signature to serve as an accurate and stable biomarker of disease; doing so has already been observed to increase discriminative power by reducing interindividual phenotype-independent variation of DNA methylation levels and by increasing the accuracy of the tissue-of-origin assignment.53
Figure 4.
Future directions for liquid biomarker application in neurology. (A) Design considerations, such as combining multiple biomarkers or epigenetic loci, will allow for more specific application of liquid biopsies. (B) Logistical considerations, including reducing biofluid volumes necessary for biopsy, cost and turnaround time will allow for broader application of liquid biomarkers. (C) Translational considerations, including the execution of well-powered randomized controlled trials with subjects fitting target patient demographics and comorbidity profiles, will be necessary for the safe and efficient application of neurology-focused liquid biopsies. (D) Clinical considerations, such as screening and diagnosis, must be defined for each validated liquid biomarker based on proven sensitivity and specificity characteristics.
Future tests must be sensitive enough to use small volumes of biofluids with low levels of cfDNA, and, as with any translational approach, cost and timing must be considered. Tests must have a quick enough turnaround time to make them clinically useful during real-time medical decision-making. For markers that have reached a consensus in the pre-clinical realm, well-powered randomized controlled trials must be conducted for biomarker validation. Real-world patients are often different from those included in initial studies: exclusion criteria must be carefully selected while attempting to replicate real-world scenarios. For instance, many stroke patients also have hypertension or coronary artery disease. Thus, excluding patients who have these comorbidities might lead to wasted time and resources spent studying a stroke biomarker that may not be useful in the majority of stroke patients.
Additionally, it is vital to consider the potential clinical utility of such liquid biopsies: at what point in the process of screening, diagnosis, prognosis and treatment would they be useful? Are some of these markers more suited for screening versus diagnosis? How would the use of these markers be beneficial in place of (or in conjunction with) the current standard of care? Are certain tests better for discriminating between diseased versus healthy controls, whereas others may be more useful for classifying disease subtypes following initial diagnosis by conventional means?
One particularly exciting aspect of liquid biopsies is their ability to probe the anatomical, tissue and cellular origins of disorders with complex aetiologies. As seen in this review, cfDNA-based liquid biopsies for neurological disease need not only be based on material released from neuronal or glial cells themselves; in some cases, specific diseases are characterized by up- or downregulated levels of cfDNA released by other cell types, such as blood cells or endothelial cells. A deeper understanding of the role of cfDNA release during disease states may provide clues to underlying aetiologies and the dynamics of disease progression. Targeted sequencing of known mutations present in head and neck cancer-associated ctDNA has already been leveraged to create a multi-cancer diagnostic panel with the potential for liquid biopsy application,182 and high-sensitivity, patient-specific, ctDNA fingerprint panels for several different cancers have been developed to monitor treatment response, recurrence and drug-related mutations.183 Creating multi-disease liquid biomarker panels for other neurological conditions could aid in rapid discrimination between similar disease presentations, allowing for earlier initiation of treatment and preventing unnecessary, expensive and invasive diagnostic procedures. Analysis of serial liquid biopsies has the potential to provide real-time monitoring of disease pathogenesis, which is critical for optimal management of complex illnesses over time. Finally, liquid biomarkers may be leveraged to gain insight into the effects of traditional and experimental treatments on individual patients, providing a valuable method for personalizing medical care.
Conclusion
In clinical specialties focusing on neurological diseases, there is a significant unmet need for non-invasive, sensitive and specific ways to evaluate tissue damage and, by proxy, the presence of disease, whether occult (as in the case of early diagnosis and screening) or ambiguously symptomatic (as in the case of conditions like headache or dementia). If liquid biopsies, particularly those focusing on epigenetic signatures as targets, live up to their predicted potential, it is likely that they will be able to be applied to numerous neurological diseases at a variety of stages, from early diagnosis to monitoring effects of treatment, to predicting relapse, to serving as outcome measures during clinical trials for novel therapies. Furthermore, liquid biopsies may prove useful in further subtyping complex neurological conditions, such as stroke or Alzheimer’s disease, to better understand their aetiologies and develop more targeted treatment regimens. Lab-on-a-chip technologies could make liquid biopsies even more useful in clinics or low-resource settings, particularly in areas of the world where expensive imaging and tissue biopsy procedures are difficult to obtain. Multiplex assays may result in tests with higher sensitivity, such as in the case of cfDNA targets for which multiple informative fragments can be detected.
As with all efforts to validate diagnostic tests, preliminary studies in patients with neurological disorders must be followed by observational studies and clinical trials to determine whether the test can detect the disease in its prodrome or early stages. Likewise, prognostic tests and those designed to monitor treatment effects must be interrogated in appropriate longitudinal studies. In this way, putative biomarkers discovered in basic science settings can be applied to real-life clinical scenarios. Ultimately, the future of cfDNA-based liquid biopsies in neurological diseases will rely on further developments in the identification, detection and analysis of specific disease-associated genetic and epigenetic patterns along with universal standardization of these workflows. Before these technologies and workflows can be implemented in clinical settings, they must be engineered to account for the complexity of actual patients with comorbid conditions and heterogeneous disease pathologies. If the pace of recent advances in this field continues, liquid biopsies targeting informative cfDNA profiles in various readily accessible biofluids will represent a valuable tool for clinicians in diagnosing and caring for patients with a wide range of neurological conditions.
Acknowledgements
All figures were created with BioRender.com.
Contributor Information
Hallie Gaitsch, NIH-Oxford-Cambridge Scholars Program, Wellcome-MRC Cambridge Stem Cell Institute and Department of Clinical Neurosciences, University of Cambridge, Cambridge CB2 1TN, UK.
Robin J M Franklin, Altos Labs, Cambridge Institute of Science, Cambridge CB21 6GP, UK.
Daniel S Reich, Translational Neuroradiology Section, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD 20892, USA.
Funding
H.G. is supported by an NIH Intramural Research Training Award and a Gates-Cambridge Scholarship. D.S.R. is supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke (NIH) and the Adelson Medical Research Foundation.
Competing interests
The authors report no competing interests relevant to the specific content of this article.
References
- 1. Luo H, Wei W, Ye Z, Zheng J, Xu RH. Liquid biopsy of methylation biomarkers in cell-free DNA. Trends Mol Med. 2021;27:482–500. [DOI] [PubMed] [Google Scholar]
- 2. Ponti G, Manfredini M, Tomasi A. Non-blood sources of cell-free DNA for cancer molecular profiling in clinical pathology and oncology. Crit Rev Oncol Hematol. 2019;141:36–42. [DOI] [PubMed] [Google Scholar]
- 3. Fernández-Lázaro D, García Hernández JL, García AC, Córdova Martínez A, Mielgo-Ayuso J, Cruz-Hernández JJ. Liquid biopsy as novel tool in precision medicine: Origins, properties, identification and clinical perspective of cancer's biomarkers. Diagnostics (Basel). 2020;10:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sun K, Jiang P, Chan KC, et al. Plasma DNA tissue mapping by genome-wide methylation sequencing for noninvasive prenatal, cancer, and transplantation assessments. Proc Natl Acad Sci U S A. 2015;112:E5503-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tost J. Follow the trace of death: Methylation analysis of cell-free DNA for clinical applications in non-cancerous diseases. Epigenomics. 2016:8:1169–1172. [DOI] [PubMed] [Google Scholar]
- 6. Kang S, Li Q, Chen Q, et al. Cancerlocator: Non-invasive cancer diagnosis and tissue-of-origin prediction using methylation profiles of cell-free DNA. Genome Biol. 2017;18:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Andargie TE, Tsuji N, Seifuddin F, et al. Cell-free DNA maps COVID-19 tissue injury and risk of death and can cause tissue injury. JCI Insight. 2021;6:e147610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dietz S, Christopoulos P, Gu L, et al. Serial liquid biopsies for detection of treatment failure and profiling of resistance mechanisms in KLC1-ALK-rearranged lung cancer. Cold Spring Harb Mol Case Stud. 2019;5:a004630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mandel P, Metais P. Les acides nucléiques du plasma sanguin chez l'homme [nuclear acids in human blood plasma]. C R Seances Soc Biol Fil. 1948;142:241–243. [PubMed] [Google Scholar]
- 10. Swarup V, Rajeswari MR. Circulating (cell-free) nucleic acids–a promising, non-invasive tool for early detection of several human diseases. FEBS Lett. 2007;581:795–799. [DOI] [PubMed] [Google Scholar]
- 11. Lo YMDC, Sun KCA, Chen H, et al. Maternal plasma DNA sequencing reveals the genome-wide genetic and mutational profile of the Fetus. Sci Transl Med. 2010;2:61ra91. [DOI] [PubMed] [Google Scholar]
- 12. Fan HC, Gu W, Wang J, Blumenfeld YJ, El-Sayed YY, Quake SR. Non-invasive prenatal measurement of the fetal genome. Nature. 2012;487:320–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bianchi DW, Parker RL, Wentworth J, et al. DNA Sequencing versus standard prenatal aneuploidy screening. N Engl J Med. 2014;370:799–808. [DOI] [PubMed] [Google Scholar]
- 14. Wong FC, Lo YM. Prenatal diagnosis innovation: Genome sequencing of maternal plasma. Annu Rev Med. 2016;67:419–432. [DOI] [PubMed] [Google Scholar]
- 15. Schwarzenbach H, Hoon DSB, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11:426–437. [DOI] [PubMed] [Google Scholar]
- 16. Abbosh C, Birkbak NJ, Wilson GA, et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature. 2017;545:446–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heitzer E, Haque IS, Roberts CES, Speicher MR. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat Rev Genet. 2019;20:71–88. [DOI] [PubMed] [Google Scholar]
- 18. Lamb YN, Dhillon S. Epi proColon((R)) 2.0 CE: A blood-based screening test for colorectal cancer. Mol Diagn Ther. 2017;21:225–232. [DOI] [PubMed] [Google Scholar]
- 19. De Vlaminck I, Martin L, Kertesz M, et al. Noninvasive monitoring of infection and rejection after lung transplantation. Proc Natl Acad Sci U S A. 2015;112:13336–13341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. De Vlaminck I, Valantine HA, Snyder TM, et al. Circulating cell-free DNA enables noninvasive diagnosis of heart transplant rejection. Sci Transl Med. 2014;6:241ra77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gielis EM, Ledeganck KJ, De Winter BY, et al. Cell-Free DNA: An upcoming biomarker in transplantation. Am J Transplant. 2015;15:2541–2551. [DOI] [PubMed] [Google Scholar]
- 22. Bettegowda C, Sausen M, Leary RJ, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6:224ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moss J, Magenheim J, Neiman D, et al. Comprehensive human cell-type methylation atlas reveals origins of circulating cell-free DNA in health and disease. Nat Commun. 2018;9:5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stirzaker C, Taberlay PC, Statham AL, Clark SJ. Mining cancer methylomes: Prospects and challenges. Trends Genet. 2014;30:75–84. [DOI] [PubMed] [Google Scholar]
- 25. Chatterton Z, Mendelev N, Chen S, et al. Bisulfite amplicon sequencing can detect Glia and neuron cell-free DNA in blood plasma. Front Mol Neurosci. 2021;14:672614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dor Y, Cedar H. Principles of DNA methylation and their implications for biology and medicine. Lancet. 2018;392:777–786. [DOI] [PubMed] [Google Scholar]
- 27. Klutstein M, Nejman D, Greenfield R, Cedar H. DNA Methylation in cancer and aging. Cancer Res. 2016;76:3446–3450. [DOI] [PubMed] [Google Scholar]
- 28. Wang M, Ngo V, Wang W. Deciphering the genetic code of DNA methylation. Brief Bioinformatics. 2021;22:bbaa424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lubotzky A, Zemmour H, Neiman D, et al. Liquid biopsy reveals collateral tissue damage in cancer. JCI Insight. 2022;7:e153559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Loyfer N, Magenheim J, Peretz A, et al. A human DNA methylation atlas reveals principles of cell type-specific methylation and identifies thousands of cell type-specific regulatory elements. bioRxiv. Published online 25 January 2022. [Google Scholar]
- 31. Seton-Rogers S. Closing in on cfDNA-based detection and diagnosis. Nat Rev Cancer. 2020;20:481. [DOI] [PubMed] [Google Scholar]
- 32. O'Connell GC, Chantler PD, Barr TL. High interspecimen variability in nucleic acid extraction efficiency necessitates the use of spike-in control for accurate qPCR-based measurement of plasma cell-free DNA levels. Lab Med. 2017;48:332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ye Z, Scheffer IE, Berkovic SF, Hildebrand MS. Improving specificity of cerebrospinal fluid liquid biopsy for genetic testing. Ann Neurol. 2021;90:693–694. [DOI] [PubMed] [Google Scholar]
- 34. Kim SB, Baulac S, Lee JH. Reply to “improving specificity of cerebrospinal fluid liquid biopsy for genetic testing”. Ann Neurol. 2021;90:694–695. [DOI] [PubMed] [Google Scholar]
- 35. Johnson KC, Verhaak RGW. Serum cell-free DNA epigenetic biomarkers aid glioma diagnostics and monitoring. Neuro Oncol. 2021;23:1423–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Alix-Panabières C, Pantel K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 2016;6:479–491. [DOI] [PubMed] [Google Scholar]
- 37. Wan JCM, Massie C, Garcia-Corbacho J, et al. Liquid biopsies come of age: Towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17:223–238. [DOI] [PubMed] [Google Scholar]
- 38. Lo YMD, Han DSC, Jiang P, Chiu RWK. Epigenetics, fragmentomics, and topology of cell-free DNA in liquid biopsies. Science. 2021;372:eaaw3616. [DOI] [PubMed] [Google Scholar]
- 39. Mittra I, Khare NK, Raghuram GV. Circulating nucleic acids damage DNA of healthy cells by integrating into their genomes. J Biosci. 2015;40:91–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gravina S, Sedivy JM, Vijg J. The dark side of circulating nucleic acids. Aging Cell. 2016;15:398–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Khier S, Lohan L. Kinetics of circulating cell-free DNA for biomedical applications: Critical appraisal of the literature. Future Sci OA. 2018;4:FSO295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gambardella S, Limanaqi F, Ferese R, et al. ccf-mtDNA as a potential link between the brain and immune system in neuro-immunological disorders. Front Immunol. 2019;10:1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pittella-Silva F, Chin YM, Chan HT, et al. Plasma or Serum: Which is preferable for mutation detection in liquid biopsy? Clin Chem. 2020;66:946–957. [DOI] [PubMed] [Google Scholar]
- 44. Oberacker P, Stepper P, Bond DM, et al. Bio-On-Magnetic-Beads (BOMB): Open platform for high-throughput nucleic acid extraction and manipulation. PLoS Biol. 2019;17:e3000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van Dessel LF, Vitale SR, Helmijr JCA, et al. High-throughput isolation of circulating tumor DNA: A comparison of automated platforms. Mol Oncol. 2019;13:392–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wu YL, Jiang T, Huang W, Wu XY, Zhang PJ, Tian YP. Genome-wide methylation profiling of early colorectal cancer using an illumina infinium methylation EPIC BeadChip. World J Gastrointest Oncol. 2022;14:935–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dedeurwaerder S, Defrance M, Calonne E, Denis H, Sotiriou C, Fuks F. Evaluation of the infinium methylation 450K technology. Epigenomics. 2011;3:771–784. [DOI] [PubMed] [Google Scholar]
- 48. Guo S, Diep D, Plongthongkum N, Fung HL, Zhang K, Zhang K. Identification of methylation haplotype blocks aids in deconvolution of heterogeneous tissue samples and tumor tissue-of-origin mapping from plasma DNA. Nat Genet. 2017;49:635–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yohe S, Thyagarajan B. Review of clinical next-generation sequencing. Arch Pathol Lab Med. 2017;141:1544–1557. [DOI] [PubMed] [Google Scholar]
- 50. Shen SY, Burgener JM, Bratman SV, De Carvalho DD. Preparation of cfMeDIP-seq libraries for methylome profiling of plasma cell-free DNA. Nat Protoc. 2019;14:2749–2780. [DOI] [PubMed] [Google Scholar]
- 51. Shen SY, Singhania R, Fehringer G, et al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature. 2018;563:579–583. [DOI] [PubMed] [Google Scholar]
- 52. Šestáková Š, Šálek C, Remešová H. DNA Methylation validation methods: A coherent review with practical comparison. Biol Proced Online. 2019;21:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lehmann-Werman R, Neiman D, Zemmour H, et al. Identification of tissue-specific cell death using methylation patterns of circulating DNA. Proc Natl Acad Sci U S A. 2016;113:E1826–E1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Titus AJ, Gallimore RM, Salas LA, Christensen BC. Cell-type deconvolution from DNA methylation: A review of recent applications. Hum Mol Genet. 2017;26:R216–R224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Caggiano C, Celona B, Garton F, et al. Comprehensive cell type decomposition of circulating cell-free DNA with CelFiE. Nat Commun. 2021;12:2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang H, Xu T, Jiang Y, et al. The challenges and the promise of molecular targeted therapy in malignant gliomas. Neoplasia. 2015;17:239–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Network TCGRA . Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Louis DN, Perry A, Reifenberger G, et al. The 2016 world health organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016;131:803–820. [DOI] [PubMed] [Google Scholar]
- 59. Hamard L, Ratel D, Selek L, Berger F, van der Sanden B, Wion D. The brain tissue response to surgical injury and its possible contribution to glioma recurrence. J Neurooncol. 2016;128:1–8. [DOI] [PubMed] [Google Scholar]
- 60. Escudero L, Martinez-Ricarte F, Seoane J. Cerebrospinal fluid circulating tumour DNA as a liquid biopsy for central nervous system malignancies. Curr Opin Neurol. 2020;33:736–741. [DOI] [PubMed] [Google Scholar]
- 61. Stallard S, Savelieff MG, Wierzbicki K, et al. CSF H3f3a K27M circulating tumor DNA copy number quantifies tumor growth and in vitro treatment response. Acta Neuropathol Commun. 2018;6:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang Y, Zhang F, Xiong N, et al. Remodelling and treatment of the blood-brain barrier in glioma. Cancer Manag Res. 2021;13:4217–4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cao Y, Tsien CI, Shen Z, et al. Use of magnetic resonance imaging to assess blood-brain/blood-glioma barrier opening during conformal radiotherapy. J Clin Oncol. 2005;23:4127–4136. [DOI] [PubMed] [Google Scholar]
- 64. Zhao Z, Zhang C, Li M, et al. Applications of cerebrospinal fluid circulating tumor DNA in the diagnosis of gliomas. Jpn J Clin Oncol. 2020;50:325–332. [DOI] [PubMed] [Google Scholar]
- 65. Pan W, Gu W, Nagpal S, Gephart MH, Quake SR. Brain tumor mutations detected in cerebral spinal fluid. Clin Chem. 2015;61:514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Miller AM, Shah RH, Pentsova EI, et al. Tracking tumour evolution in glioma through liquid biopsies of cerebrospinal fluid. Nature. 2019;565:654–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mouliere F, Smith CG, Heider K, et al. Fragmentation patterns and personalized sequencing of cell-free DNA in urine and plasma of glioma patients. EMBO Mol Med. 2021;13:e12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Martínez-Ricarte F, Mayor R, Martínez-Sáez E, et al. Molecular diagnosis of diffuse gliomas through sequencing of cell-free circulating tumor DNA from cerebrospinal fluid. Clin Cancer Res. 2018;24:2812–2819. [DOI] [PubMed] [Google Scholar]
- 69. Pan C, Diplas BH, Chen X, et al. Molecular profiling of tumors of the brainstem by sequencing of CSF-derived circulating tumor DNA. Acta Neuropathol. 2019;137:297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fujioka Y, Hata N, Akagi Y, et al. Molecular diagnosis of diffuse glioma using a chip-based digital PCR system to analyze IDH, TERT, and H3 mutations in the cerebrospinal fluid. J Neurooncol. 2021;152:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Piccioni DE, Achrol AS, Kiedrowski LA, et al. Analysis of cell-free circulating tumor DNA in 419 patients with glioblastoma and other primary brain tumors. CNS Oncol. 2019;8:CNS34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Duan H, Hu JL, Chen ZH, et al. Assessment of circulating tumor DNA in cerebrospinal fluid by whole exome sequencing to detect genomic alterations of glioblastoma. Chin Med J (Engl). 2020;133:1415–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Beiko J, Suki D, Hess KR, et al. IDH1 Mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro Oncol. 2014;16:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Takayasu T, Shah M, Dono A, et al. Cerebrospinal fluid ctDNA and metabolites are informative biomarkers for the evaluation of CNS germ cell tumors. Sci Rep. 2020;10:14326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Huang TY, Piunti A, Lulla RR, et al. Detection of histone H3 mutations in cerebrospinal fluid-derived tumor DNA from children with diffuse midline glioma. Acta Neuropathol Commun. 2017;5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Panditharatna E, Kilburn LB, Aboian MS, et al. Clinically relevant and minimally invasive tumor surveillance of pediatric diffuse midline gliomas using patient-derived liquid biopsy. Clin Cancer Res. 2018;24:5850–5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Izquierdo E, Proszek P, Pericoli G, et al. Droplet digital PCR-based detection of circulating tumor DNA from pediatric high grade and diffuse midline glioma patients. Neurooncol Adv. 2021;3:vdab013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Escudero L, Llort A, Arias A, et al. Circulating tumour DNA from the cerebrospinal fluid allows the characterisation and monitoring of medulloblastoma. Nat Commun. 2020;11:5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sun Y, Li M, Ren S, et al. Exploring genetic alterations in circulating tumor DNA from cerebrospinal fluid of pediatric medulloblastoma. Sci Rep. 2021;11:5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Liu APY, Smith KS, Kumar R, et al. Serial assessment of measurable residual disease in medulloblastoma liquid biopsies. Cancer Cell. 2021;39:1519–1530.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bale TA, Yang SR, Solomon JP, et al. Clinical experience of cerebrospinal fluid-based liquid biopsy demonstrates superiority of cell-free DNA over cell pellet genomic DNA for molecular profiling. J Mol Diagn. 2021;23:742–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. García-Romero N, Carrión-Navarro J, Areal-Hidalgo P, et al. BRAF V600e detection in liquid biopsies from pediatric central nervous system tumors. Cancers (Basel). 2019;12:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kang KM, Muralidharan K, Yekula A, et al. Blood-Based detection of BRAF V600E in gliomas and brain tumor metastasis. Cancers (Basel). 2021;13:1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Shi W, Lv C, Qi J, et al. Prognostic value of free DNA quantification in serum and cerebrospinal fluid in glioma patients. J Mol Neurosci. 2012;46:470–475. [DOI] [PubMed] [Google Scholar]
- 85. Chen J, Huan W, Zuo H, et al. Alu methylation serves as a biomarker for non-invasive diagnosis of glioma. Oncotarget. 2016;7:26099–26106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ghodsi M, Shahmohammadi M, Modarressi MH, Karami F. Investigation of promoter methylation of MCPH1 gene in circulating cell-free DNA of brain tumor patients. Exp Brain Res. 2020;238:1903–1909. [DOI] [PubMed] [Google Scholar]
- 87. Li J, Zhao S, Lee M, et al. Reliable tumor detection by whole-genome methylation sequencing of cell-free DNA in cerebrospinal fluid of pediatric medulloblastoma. Sci Adv. 2020;6:eabb5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Cai J, Zeng C, Hua W, et al. An integrative analysis of genome-wide 5-hydroxymethylcytosines in circulating cell-free DNA detects noninvasive diagnostic markers for gliomas. Neurooncol Adv. 2021;3(1):vdab049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Nassiri F, Chakravarthy A, Feng S, et al. Detection and discrimination of intracranial tumors using plasma cell-free DNA methylomes. Nat Med. 2020;26:1044–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sabedot TS, Malta TM, Snyder J, et al. A serum-based DNA methylation assay provides accurate detection of glioma. Neuro-Oncology. 2021;23:1494–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Okamura R, Piccioni DE, Boichard A, et al. High prevalence of clonal hematopoiesis-type genomic abnormalities in cell-free DNA in invasive gliomas after treatment. Int J Cancer. 2021;148:2839–2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Le Rhun E, Seoane J, Salzet M, Soffietti R, Weller M. Liquid biopsies for diagnosing and monitoring primary tumors of the central nervous system. Cancer Lett. 2020;480:24–28. [DOI] [PubMed] [Google Scholar]
- 93. Ali H, Harting R, de Vries R, Ali M, Wurdinger T, Best MG. Blood-Based biomarkers for glioma in the context of gliomagenesis: A systematic review. Front Oncol. 2021;11:665235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. An Y, Fan F, Jiang X, Sun K. Recent advances in liquid biopsy of brain cancers. Front Genet. 2021;12:720270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bunda S, Zuccato JA, Voisin MR, et al. Liquid biomarkers for improved diagnosis and classification of CNS tumors. Int J Mol Sci. 2021;22:4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Eibl RH, Schneemann M. Liquid biopsy and primary brain tumors. Cancers (Basel). 2021;13:5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Escudero L, Martinez-Ricarte F, Seoane J. ctDNA-Based liquid biopsy of cerebrospinal fluid in brain cancer. Cancers (Basel). 2021;13:1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gatto L, Franceschi E, Di Nunno V, Tosoni A, Lodi R, Brandes AA. Liquid biopsy in glioblastoma management: From current research to future perspectives. Oncologist. 2021;26:865–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Jelski W, Mroczko B. Molecular and circulating biomarkers of brain tumors. Int J Mol Sci. 2021;22:7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Mair R, Mouliere F. Cell-free DNA technologies for the analysis of brain cancer. Br J Cancer. 2022;126:371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Marler JR, Tilley BC, Lu M, et al. Early stroke treatment associated with better outcome: The NINDS rt-PA stroke study. Neurology. 2000;55:1649–1655. [DOI] [PubMed] [Google Scholar]
- 102. Wijerathne H, Witek MA, Baird AE, Soper SA. Liquid biopsy markers for stroke diagnosis. Expert Rev Mol Diagn. 2020;20:771–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Karakas M, Zeller T. A biomarker ocular: Circulating MicroRNAs toward diagnostics for acute ischemic stroke. Circ Res. 2017;121:905–907. [DOI] [PubMed] [Google Scholar]
- 104. Provost C, Soudant M, Legrand L, et al. Magnetic resonance imaging or computed tomography before treatment in acute ischemic stroke. Stroke. 2019;50:659–664. [DOI] [PubMed] [Google Scholar]
- 105. Chan D, Ng LL. Biomarkers in acute myocardial infarction. BMC Med. 2010;8:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kamtchum-Tatuene J, Jickling GC. Blood biomarkers for stroke diagnosis and management. Neuromolecular Med. 2019;21:344–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Geiger S, Holdenrieder S, Stieber P, et al. Nucleosomes as a new prognostic marker in early cerebral stroke. J Neurol. 2007;254:617–623. [DOI] [PubMed] [Google Scholar]
- 108. Tsai NW, Lin TK, Chen SD, et al. The value of serial plasma nuclear and mitochondrial DNA levels in patients with acute ischemic stroke. Clin Chim Acta. 2011;412:476–479. [DOI] [PubMed] [Google Scholar]
- 109. Vajpeyee A, Wijatmiko T, Vajpeyee M, Taywade O, Pandey S, Chauhan PS. Clinical usefulness of cell-free DNA as a prognostic marker in acute ischemic stroke. Neurologist. 2020;25:11–13. [DOI] [PubMed] [Google Scholar]
- 110. O'Connell GC, Petrone AB, Tennant CS, et al. Circulating extracellular DNA levels are acutely elevated in ischaemic stroke and associated with innate immune system activation. Brain Inj. 2017;31:1369–1375. [DOI] [PubMed] [Google Scholar]
- 111. Bustamante A, Mancha F, Macher HC, et al. Circulating cell-free DNA is a predictor of short-term neurological outcome in stroke patients treated with intravenous thrombolysis. J Circ Biomark. 2016;5:1849454416668791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Vasilyeva I, Bespalov V, Baranova A, Voznyuk I, Baranenko D. Differential dynamics of the levels of low molecular weight DNA fragments in the plasma of patients with ischemic and hemorrhagic strokes. Basic Clin Neurosci. 2020;11:805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Glebova KV, Veiko NN, Nikonov AA, Porokhovnik LN, Kostuyk SV. Cell-free DNA as a biomarker in stroke: Current status, problems and perspectives. Crit Rev Clin Lab Sci. 2018;55:55–70. [DOI] [PubMed] [Google Scholar]
- 114. Ghajar J. Traumatic brain injury. Lancet. 2000;356:923–929. [DOI] [PubMed] [Google Scholar]
- 115. Levin HS, Diaz-Arrastia RR. Diagnosis, prognosis, and clinical management of mild traumatic brain injury. Lancet Neurol. 2015;14:506–517. [DOI] [PubMed] [Google Scholar]
- 116. Zemlan FP, Jauch EC, Mulchahey JJ, et al. C-tau biomarker of neuronal damage in severe brain injured patients: Association with elevated intracranial pressure and clinical outcome. Brain Res. 2002;947:131–139. [DOI] [PubMed] [Google Scholar]
- 117. Kleindienst A, Hesse F, Bullock MR, Buchfelder M. The neurotrophic protein S100B: Value as a marker of brain damage and possible therapeutic implications. Prog Brain Res. 2007;161:317–325. [DOI] [PubMed] [Google Scholar]
- 118. Macher H, Egea-Guerrero JJ, Revuelto-Rey J, et al. Role of early cell-free DNA levels decrease as a predictive marker of fatal outcome after severe traumatic brain injury. Clin Chim Acta. 2012;414:12–17. [DOI] [PubMed] [Google Scholar]
- 119. Ben Zvi I, Harel OS, Douvdevani A, et al. Quick cell-free DNA testing for the prediction of postconcussion syndrome: A single-center prospective pilot trial. J Neurosurg. Published online 8 October 2021. [DOI] [PubMed] [Google Scholar]
- 120. Lewis JM, Dhawan S, Obirieze AC, et al. Plasma biomarker for post-concussive syndrome: A pilot study using an alternating current electro-kinetic platform. Front Neurol. 2020;11:685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Perel P, Roberts I, Bouamra O, Woodford M, Mooney J, Lecky F. Intracranial bleeding in patients with traumatic brain injury: A prognostic study. BMC Emerg Med. 2009;9:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Dilek A, Alacam H, Ulger F, et al. Comparison of predictive powers of S100B and cell-free plasma DNA values in intensive care unit patients with intracranial hemorrhage. J Crit Care. 2013;28:883.e1–883.e7. [DOI] [PubMed] [Google Scholar]
- 123. Wang KK, Yang Z, Zhu T, et al. An update on diagnostic and prognostic biomarkers for traumatic brain injury. Expert Rev Mol Diagn. 2018;18:165–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Blennow K, de Leon MJ, Zetterberg H. Alzheimer's disease. Lancet. 2006;368:387–403. [DOI] [PubMed] [Google Scholar]
- 125. Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer's disease. Lancet. 2011;377:1019–1031. [DOI] [PubMed] [Google Scholar]
- 126. Gong X, Zhang H, Liu X, et al. Is liquid biopsy mature enough for the diagnosis of Alzheimer's disease? Front Aging Neurosci. 2022;14:977999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Molinuevo JL, Ayton S, Batrla R, et al. Current state of Alzheimer's fluid biomarkers. Acta Neuropathol. 2018;136:821–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Beach TG, Monsell SE, Phillips LE, Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at national institute on aging Alzheimer disease centers, 2005-2010. J Neuropathol Exp Neurol. 2012;71:266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Huang J. Novel brain PET imaging agents: Strategies for imaging neuroinflammation in Alzheimer’s disease and mild cognitive impairment. Front Immunol. 2022;13:1010946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Chandra A, Valkimadi PE, Pagano G, et al. Applications of amyloid, tau, and neuroinflammation PET imaging to Alzheimer's disease and mild cognitive impairment. Hum Brain Mapp. 2019;40:5424–5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Cassinelli Petersen G, Roytman M, Chiang GC, Li Y, Gordon ML, Franceschi AM. Overview of tau PET molecular imaging. Curr Opin Neurol. 2022;35:230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Zhang K, Mizuma H, Zhang X, et al. PET Imaging of neural activity, beta-amyloid, and tau in Normal brain aging. Eur J Nucl Med Mol Imaging. 2021;48:3859–3871. [DOI] [PubMed] [Google Scholar]
- 133. Villemagne VL, Burnham S, Bourgeat P, et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: A prospective cohort study. Lancet Neurol. 2013;12:357–367. [DOI] [PubMed] [Google Scholar]
- 134. Park SA, Han SM, Kim CE. New fluid biomarkers tracking non-amyloid-beta and non-tau pathology in Alzheimer's disease. Exp Mol Med. 2020;52:556–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Moscoso A, Grothe MJ, Ashton NJ, et al. Longitudinal associations of blood phosphorylated Tau181 and neurofilament light chain with neurodegeneration in Alzheimer disease. JAMA Neurol. 2021;78:396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Palmqvist S, Janelidze S, Quiroz YT, et al. Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA. 2020;324:772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Ashton NJ, Pascoal TA, Karikari TK, et al. Plasma p-tau231: A new biomarker for incipient Alzheimer's disease pathology. Acta Neuropathol. 2021;141:709–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Baldacci F, Lista S, Vergallo A, Palermo G, Giorgi FS, Hampel H. A frontline defense against neurodegenerative diseases:The development of early disease detection methods. Expert Rev Mol Diagn. 2019;19:559–563. [DOI] [PubMed] [Google Scholar]
- 139. Pai MC, Kuo YM, Wang IF, Chiang PM, Tsai KJ. The role of methylated circulating nucleic acids as a potential biomarker in Alzheimer's disease. Mol Neurobiol. 2019;56:2440–2449. [DOI] [PubMed] [Google Scholar]
- 140. Chen L, Shen Q, Xu S, et al. 5-Hydroxymethylcytosine Signatures in circulating cell-free DNA as diagnostic biomarkers for late-onset Alzheimer's disease. J Alzheimers Dis. 2022;85:573–585. [DOI] [PubMed] [Google Scholar]
- 141. Konki M, Lindgren N, Kyläniemi M, et al. Plasma cell-free DNA methylation marks for episodic memory impairment: A pilot twin study. Sci Rep. 2020;10:14192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Hampel H, Goetzl EJ, Kapogiannis D, Lista S, Vergallo A. Biomarker-Drug and liquid biopsy co-development for disease staging and targeted therapy: Cornerstones for Alzheimer's precision medicine and pharmacology. Front Pharmacol. 2019;10:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Hampel H, Vergallo A, Caraci F, et al. Future avenues for Alzheimer's disease detection and therapy: Liquid biopsy, intracellular signaling modulation, systems pharmacology drug discovery. Neuropharmacology. 2021;185:108081. [DOI] [PubMed] [Google Scholar]
- 144. Soelter TM, Whitlock JH, Williams AS, Hardigan AA, Lasseigne BN. Nucleic acid liquid biopsies in Alzheimer's disease: Current state, challenges, and opportunities. Heliyon. 2022;8:e09239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Giau VV, Bagyinszky E, An SSA. Potential fluid biomarkers for the diagnosis of mild cognitive impairment. Int J Mol Sci. 2019;20:4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Kuhlmann L, Lehnertz K, Richardson MP, Schelter B, Zaveri HP. Seizure prediction—Ready for a new era. Nat Rev Neurol. 2018;14:618–630. [DOI] [PubMed] [Google Scholar]
- 147. Ekstrand JJ, Pouliot W, Scheerlinck P, Dudek FE. Lithium pilocarpine-induced status epilepticus in postnatal day 20 rats results in greater neuronal injury in ventral versus dorsal hippocampus. Neuroscience. 2011;192:699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Scholl EA, Dudek FE, Ekstrand JJ. Neuronal degeneration is observed in multiple regions outside the hippocampus after lithium pilocarpine-induced status epilepticus in the immature rat. Neuroscience. 2013;252:45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Mao XY, Zhou HH, Jin WL. Redox-Related neuronal death and crosstalk as drug targets: Focus on epilepsy. Front Neurosci. 2019;13:512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Liimatainen SP, Jylhävä J, Raitanen J, Peltola JT, Hurme MA. The concentration of cell-free DNA in focal epilepsy. Epilepsy Res. 2013;105:292–298. [DOI] [PubMed] [Google Scholar]
- 151. Alapirtti T, Jylhävä J, Raitanen J, et al. The concentration of cell-free DNA in video-EEG patients is dependent on the epilepsy syndrome and duration of epilepsy. Neurol Res. 2016;38:45–50. [DOI] [PubMed] [Google Scholar]
- 152. Ye Z, Chatterton Z, Pflueger J, et al. Cerebrospinal fluid liquid biopsy for detecting somatic mosaicism in brain. Brain Commun. 2021;3(1):fcaa235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Kim S, Baldassari S, Sim NS, et al. Detection of brain somatic mutations in cerebrospinal fluid from refractory epilepsy patients. Ann Neurol. 2021;89:1248–1252. [DOI] [PubMed] [Google Scholar]
- 154. Whitlock JH, Soelter TM, Williams AS, Hardigan AA, Lasseigne BN. Liquid biopsies in epilepsy: Biomarkers for etiology, diagnosis, prognosis, and therapeutics. Hum Cell. 2022;35:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Feigin VL, Nichols E, Alam T, et al. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18:459–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018;378:169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Franklin RJ. Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci. 2002;3:705–714. [DOI] [PubMed] [Google Scholar]
- 158. Williamson JM, Lyons DA. Myelin dynamics throughout life: An ever-changing landscape? Front Cell Neurosci. 2018;12:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Franklin RJM, Frisén J, Lyons DA. Revisiting remyelination: Towards a consensus on the regeneration of CNS myelin. Semin Cell Dev Biol. 2021;116:3–9. [DOI] [PubMed] [Google Scholar]
- 160. Harris VK, Tuddenham JF, Sadiq SA. Biomarkers of multiple sclerosis: Current findings. Degener Neurol Neuromuscul Dis. 2017;7:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Olsen JA, Kenna LA, Tipon RC, Spelios MG, Stecker MM, Akirav EM. A minimally-invasive blood-derived biomarker of oligodendrocyte cell-loss in multiple sclerosis. EBioMedicine. 2016;10:227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Dunaeva M, Derksen M, Pruijn GJM. LINE-1 Hypermethylation in Serum cell-free DNA of relapsing remitting multiple sclerosis patients. Mol Neurobiol. 2018;55:4681–4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Varhaug KN, Vedeler CA, Myhr KM, Aarseth JH, Tzoulis C, Bindoff LA. Increased levels of cell-free mitochondrial DNA in the cerebrospinal fluid of patients with multiple sclerosis. Mitochondrion. 2017;34:32–35. [DOI] [PubMed] [Google Scholar]
- 164. Leurs CE, Podlesniy P, Trullas R, et al. Cerebrospinal fluid mtDNA concentration is elevated in multiple sclerosis disease and responds to treatment. Mult Scler. 2018;24:472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Lowes H, Pyle A, Duddy M, Hudson G. Cell-free mitochondrial DNA in progressive multiple sclerosis. Mitochondrion. 2019;46:307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Benninger F, Steiner I. CSF In acute and chronic infectious diseases. Handb Clin Neurol. 2017;146:187–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Ramachandran PS, Wilson MR. Diagnostic testing of neurologic infections. Neurol Clin. 2018;36:687–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Garcia HH, Nash TE, Del Brutto OH. Clinical symptoms, diagnosis, and treatment of neurocysticercosis. Lancet Neurol. 2014;13:1202–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Almeida CR, Ojopi EP, Nunes CM, et al. Taenia solium DNA is present in the cerebrospinal fluid of neurocysticercosis patients and can be used for diagnosis. Eur Arch Psychiatry Clin Neurosci. 2006;256:307–310. [DOI] [PubMed] [Google Scholar]
- 170. Toribio L, Romano M, Scott AL, et al. Detection of taenia solium DNA in the urine of neurocysticercosis patients. Am J Trop Med Hyg. 2019;100:327–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Ashley EA, Pyae Phyo A, Woodrow CJ. Malaria. Lancet. 2018;391:1608–1621. [DOI] [PubMed] [Google Scholar]
- 172. Vera IM, Kessler A, Ting LM, et al. Plasma cell-free DNA predicts pediatric cerebral malaria severity. JCI Insight. 2020;5:e136279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Foppiano Palacios C, Saleeb PG. Challenges in the diagnosis of tuberculous meningitis. J Clin Tuberc Other Mycobact Dis. 2020;20:100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Cave MD, Eisenach KD, McDermott PF, Bates JH, Crawford JT. IS6110: Conservation of sequence in the Mycobacterium tuberculosis complex and its utilization in DNA fingerprinting. Mol Cell Probes. 6110;1991:73–80. [DOI] [PubMed] [Google Scholar]
- 175. Shao L, Qiu C, Zheng L, et al. Comparison of diagnostic accuracy of the GeneXpert ultra and cell-free nucleic acid assay for tuberculous meningitis: A multicentre prospective study. Int J Infect Dis. 2020;98:441–446. [DOI] [PubMed] [Google Scholar]
- 176. Asif AA, Roy M, Tellier BR, Ahmad S. Capnocytophaga canimorsus meningitis diagnosed using next- generation sequencing of microbial cell-free DNA. IDCases. 2021;24:e01126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Mehta SR, Pérez-Santiago J, Hulgan T, et al. Cerebrospinal fluid cell-free mitochondrial DNA is associated with HIV replication, iron transport, and mild HIV-associated neurocognitive impairment. J Neuroinflammation. 2017;14:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Bjornevik K. Longitudinal analysis reveals high prevalence of epstein-barr virus associated with multiple sclerosis. Science. 2022;375:296–301. [DOI] [PubMed] [Google Scholar]
- 179. Readhead B, Haure-Mirande JV, Funk CC, et al. Multiscale analysis of independent Alzheimer's cohorts finds disruption of molecular, genetic, and clinical networks by human herpesvirus. Neuron. 2018;99:64–82.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Allnutt MA, Johnson K, Bennett DA, et al. Human herpesvirus 6 detection in Alzheimer's disease cases and controls across multiple cohorts. Neuron. 2020;105:1027–1035.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Neiman D, Gillis D, Piyanzin S, et al. Multiplexing DNA methylation markers to detect circulating cell-free DNA derived from human pancreatic β cells. JCI Insight. 2020;5:e136579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. McCabe MJ, Gauthier MA, Chan CL, et al. Development and validation of a targeted gene sequencing panel for application to disparate cancers. Sci Rep. 2019;9:17052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Li J, Jiang W, Wei J, et al. Patient specific circulating tumor DNA fingerprints to monitor treatment response across multiple tumors. J Transl Med. 2020;18:293. [DOI] [PMC free article] [PubMed] [Google Scholar]