Abstract
The clinical manifestations of mitochondrial encephalomyopathy are described in four generations of a single kindred. The age of onset of major neurological disturbance varied from 3-70 years. In some patients, deafness was the only manifestation; in others, recurrent bouts of status epilepticus associated with focal neurological deficits and headache, caused severe disability or death. Examples of all three adult forms of mitochondrial encephalomyopathy: MELAS, MERFF and Kearns Sayre syndrome, were represented within the kindred. Associated features included deafness, short stature, non-insulin-dependent diabetes mellitus, migraine, peptic ulceration and severe constipation. The nt 3243 A-G MELAS mutation was detected in two members of the kindred. This study highlights the diversity of clinical expression of a mitochondrial mutation within a single kindred.
Full text
PDF


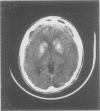
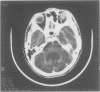
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cervera R., Bruix J., Bayes A., Blesa R., Illa I., Coll J., Garcia-Puges A. M. Chronic intestinal pseudoobstruction and ophthalmoplegia in a patient with mitochondrial myopathy. Gut. 1988 Apr;29(4):544–547. doi: 10.1136/gut.29.4.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darley-Usmar V. M. The molecular aetiology of human mitochondrial myopathies. Biochem Soc Trans. 1987 Feb;15(1):102–103. doi: 10.1042/bst0150102. [DOI] [PubMed] [Google Scholar]
- DiMauro S., Bonilla E., Zeviani M., Nakagawa M., DeVivo D. C. Mitochondrial myopathies. Ann Neurol. 1985 Jun;17(6):521–538. doi: 10.1002/ana.410170602. [DOI] [PubMed] [Google Scholar]
- DiMauro S., Zeviani M., Servidei S., Bonilla E., Miranda A. F., Prelle A., Schon E. A. Cytochrome oxidase deficiency: clinical and biochemical heterogeneity. Ann N Y Acad Sci. 1986;488:19–32. doi: 10.1111/j.1749-6632.1986.tb46545.x. [DOI] [PubMed] [Google Scholar]
- Driscoll P. F., Larsen P. D., Gruber A. B. MELAS syndrome involving a mother and two children. Arch Neurol. 1987 Sep;44(9):971–973. doi: 10.1001/archneur.1987.00520210065021. [DOI] [PubMed] [Google Scholar]
- Edwards R. H., Round J. M., Jones D. A. Needle biopsy of skeletal muscle: a review of 10 years experience. Muscle Nerve. 1983 Nov-Dec;6(9):676–683. doi: 10.1002/mus.880060910. [DOI] [PubMed] [Google Scholar]
- Fukuhara N. Strokelike episodes in MERRF. Ann Neurol. 1985 Sep;18(3):368–368. doi: 10.1002/ana.410180323. [DOI] [PubMed] [Google Scholar]
- Fukuhara N., Tokiguchi S., Shirakawa K., Tsubaki T. Myoclonus epilepsy associated with ragged-red fibres (mitochondrial abnormalities ): disease entity or a syndrome? Light-and electron-microscopic studies of two cases and review of literature. J Neurol Sci. 1980 Jul;47(1):117–133. doi: 10.1016/0022-510x(80)90031-3. [DOI] [PubMed] [Google Scholar]
- Gambetti P., Mellman W. J., Gonatas N. K. Familial spongy degeneration of the central nervous system (Van Bogaert-Bertrand disease). An ultrastructural study. Acta Neuropathol. 1969 Jan 31;12(2):103–115. doi: 10.1007/BF00692500. [DOI] [PubMed] [Google Scholar]
- Goto Y., Nonaka I., Horai S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1990 Dec 13;348(6302):651–653. doi: 10.1038/348651a0. [DOI] [PubMed] [Google Scholar]
- Grivell L. A. Mitochondrial DNA. Small, beautiful and essential. Nature. 1989 Oct 19;341(6243):569–571. doi: 10.1038/341569a0. [DOI] [PubMed] [Google Scholar]
- Harrington M. G., Macpherson P., McIntosh W. B., Allam B. F., Bone I. The significance of the incidental finding of basal ganglia calcification on computed tomography. J Neurol Neurosurg Psychiatry. 1981 Dec;44(12):1168–1170. doi: 10.1136/jnnp.44.12.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEARNS T. P., SAYRE G. P. Retinitis pigmentosa, external ophthalmophegia, and complete heart block: unusual syndrome with histologic study in one of two cases. AMA Arch Ophthalmol. 1958 Aug;60(2):280–289. [PubMed] [Google Scholar]
- LEIGH D. Subacute necrotizing encephalomyelopathy in an infant. J Neurol Neurosurg Psychiatry. 1951 Aug;14(3):216–221. doi: 10.1136/jnnp.14.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legido A., Zimmerman R. A., Packer R. J., Bilaniuk L. T., Siegel K. R., D'Angio G. Significance of basal ganglia calcification on computed tomography in children. Pediatr Neurosci. 1988;14(2):64–70. doi: 10.1159/000120365. [DOI] [PubMed] [Google Scholar]
- Lertrit P., Noer A. S., Jean-Francois M. J., Kapsa R., Dennett X., Thyagarajan D., Lethlean K., Byrne E., Marzuki S. A new disease-related mutation for mitochondrial encephalopathy lactic acidosis and strokelike episodes (MELAS) syndrome affects the ND4 subunit of the respiratory complex I. Am J Hum Genet. 1992 Sep;51(3):457–468. [PMC free article] [PubMed] [Google Scholar]
- Montagna P., Gallassi R., Medori R., Govoni E., Zeviani M., Di Mauro S., Lugaresi E., Andermann F. MELAS syndrome: characteristic migrainous and epileptic features and maternal transmission. Neurology. 1988 May;38(5):751–754. doi: 10.1212/wnl.38.5.751. [DOI] [PubMed] [Google Scholar]
- Murphy M. J. Clinical correlations of CT scan-detected calcifications of the basal ganglia. Ann Neurol. 1979 Dec;6(6):507–511. doi: 10.1002/ana.410060608. [DOI] [PubMed] [Google Scholar]
- Noer A. S., Sudoyo H., Lertrit P., Thyagarajan D., Utthanaphol P., Kapsa R., Byrne E., Marzuki S. A tRNA(Lys) mutation in the mtDNA is the causal genetic lesion underlying myoclonic epilepsy and ragged-red fiber (MERRF) syndrome. Am J Hum Genet. 1991 Oct;49(4):715–722. [PMC free article] [PubMed] [Google Scholar]
- Pavlakis S. G., Phillips P. C., DiMauro S., De Vivo D. C., Rowland L. P. Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes: a distinctive clinical syndrome. Ann Neurol. 1984 Oct;16(4):481–488. doi: 10.1002/ana.410160409. [DOI] [PubMed] [Google Scholar]
- Peterson P. L., Martens M. E., Lee C. P. Mitochondrial encephalomyopathies. Neurol Clin. 1988 Aug;6(3):529–544. [PubMed] [Google Scholar]
- Petty R. K., Harding A. E., Morgan-Hughes J. A. The clinical features of mitochondrial myopathy. Brain. 1986 Oct;109(Pt 5):915–938. doi: 10.1093/brain/109.5.915. [DOI] [PubMed] [Google Scholar]
- Riggs J. E., Schochet S. S., Jr, Fakadej A. V., Papadimitriou A., DiMauro S., Crosby T. W., Gutmann L., Moxley R. T. Mitochondrial encephalomyopathy with decreased succinate-cytochrome c reductase activity. Neurology. 1984 Jan;34(1):48–53. doi: 10.1212/wnl.34.1.48. [DOI] [PubMed] [Google Scholar]
- Schon E. A., Rizzuto R., Moraes C. T., Nakase H., Zeviani M., DiMauro S. A direct repeat is a hotspot for large-scale deletion of human mitochondrial DNA. Science. 1989 Apr 21;244(4902):346–349. doi: 10.1126/science.2711184. [DOI] [PubMed] [Google Scholar]