Abstract
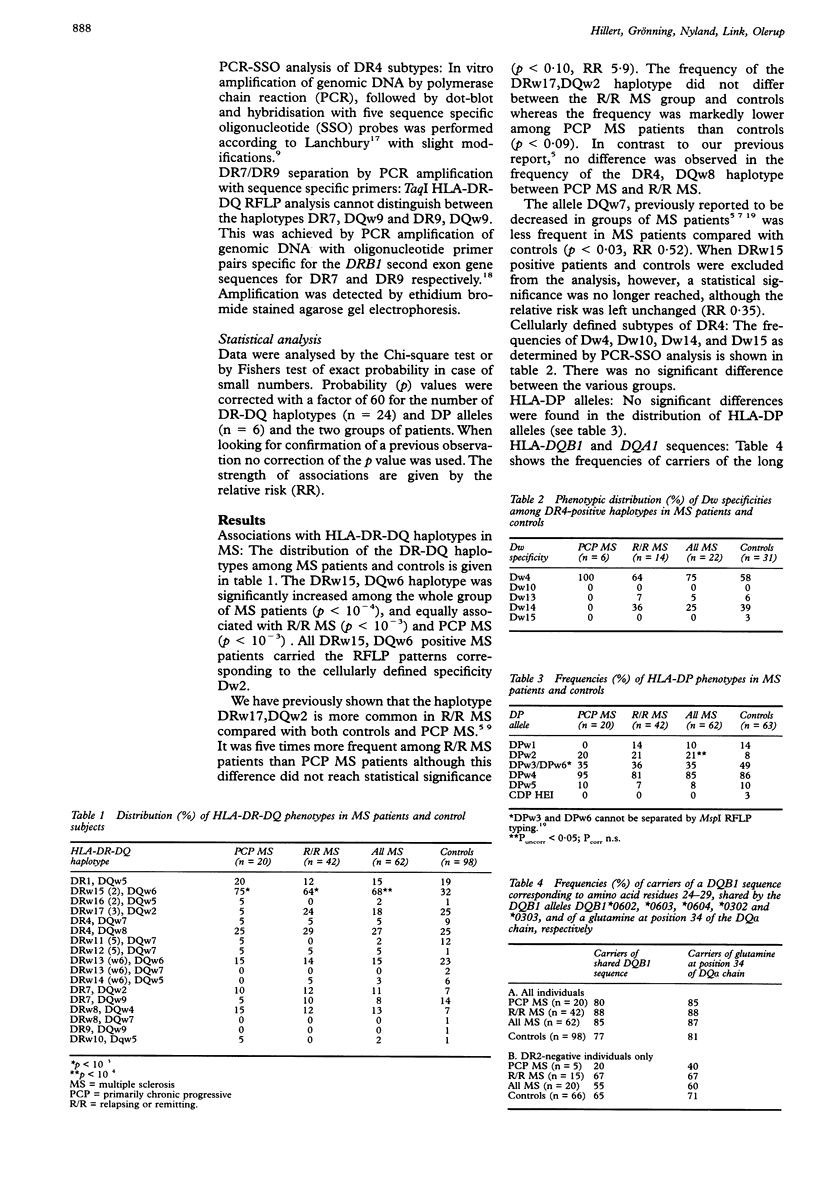
Two clinical forms of multiple sclerosis (MS), primarily chronic progressive MS (PCP MS) and relapsing/remitting MS (R/R MS) have been shown to differ in several respects. The results of genomic HLA class II typing with restriction fragment length polymorphism analysis of 62 MS patients from Western Norway, 42 with R/R MS and 20 PCP MS, are reported on here. As in previous studies of Swedish patients, the haplotype DRw17(3), DQw2 was found to be five times more common in R/R MS than in PCP MS. This finding supports the hypothesis that R/R and PCP MS are immunogenetically separate entities. In contrast with a previous investigation of Norwegian MS patients, no association of MS with glutamine at position 34 of the HLA-DQ alpha chain or with defined sequences of the HLA-DQB1 gene was found.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bidwell J. L., Bidwell E. A., Laundy G. J., Klouda P. T., Bradley B. A. Allogenotypes defined by short DQ alpha and DQ beta cDNA probes correlate with and define splits of HLA-DQ serological specificities. Mol Immunol. 1987 May;24(5):513–522. doi: 10.1016/0161-5890(87)90026-5. [DOI] [PubMed] [Google Scholar]
- Carlsson B., Wallin J., Böhme J., Möller E. HLA-DR-DQ haplotypes defined by restriction fragment analysis. Correlation to serology. Hum Immunol. 1987 Oct;20(2):95–113. doi: 10.1016/0198-8859(87)90025-5. [DOI] [PubMed] [Google Scholar]
- Confavreux C., Aimard G., Devic M. Course and prognosis of multiple sclerosis assessed by the computerized data processing of 349 patients. Brain. 1980 Jun;103(2):281–300. doi: 10.1093/brain/103.2.281. [DOI] [PubMed] [Google Scholar]
- Cullen C. G., Middleton D., Savage D. A., Hawkins S. HLA-DR and DQ DNA genotyping in multiple sclerosis patients in Northern Ireland. Hum Immunol. 1991 Jan;30(1):1–6. doi: 10.1016/0198-8859(91)90062-e. [DOI] [PubMed] [Google Scholar]
- Gregersen P. K., Silver J., Winchester R. J. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987 Nov;30(11):1205–1213. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- Hyldig-Nielsen J. J., Odum N., Morling N., Svejgaard A. Restriction fragment polymorphism (RFLP) of a "new" HLA-DP specificity, CDP-HEI. Tissue Antigens. 1988 Mar;31(3):161–163. doi: 10.1111/j.1399-0039.1988.tb02077.x. [DOI] [PubMed] [Google Scholar]
- Lanchbury J. S., Hall M. A., Welsh K. I., Panayi G. S. Sequence analysis of HLA-DR4B1 subtypes: additional first domain variability is detected by oligonucleotide hybridization and nucleotide sequencing. Hum Immunol. 1990 Feb;27(2):136–144. doi: 10.1016/0198-8859(90)90110-b. [DOI] [PubMed] [Google Scholar]
- Larsen J. P., Kvaale G., Riise T., Nyland H., Aarli J. A. Multiple sclerosis--more than one disease? Acta Neurol Scand. 1985 Aug;72(2):145–150. doi: 10.1111/j.1600-0404.1985.tb00856.x. [DOI] [PubMed] [Google Scholar]
- Leibowitz U., Alter M. Clinical factors associated with increased disability in multiple sclerosis. Acta Neurol Scand. 1970;46(1):53–70. doi: 10.1111/j.1600-0404.1970.tb05604.x. [DOI] [PubMed] [Google Scholar]
- Madigand M., Oger J. J., Fauchet R., Sabouraud O., Genetet B. HLA profiles in multiple sclerosis suggest two forms of disease and the existence of protective haplotypes. J Neurol Sci. 1982 Mar;53(3):519–529. doi: 10.1016/0022-510x(82)90248-9. [DOI] [PubMed] [Google Scholar]
- Marcadet A., Massart C., Semana G., Fauchet R., Sabouraud O., Merienne M., Dausset J., Cohen D. Association of class II HLA-DQ beta chain DNA restriction fragments with multiple sclerosis. Immunogenetics. 1985;22(1):93–96. doi: 10.1007/BF00430598. [DOI] [PubMed] [Google Scholar]
- Marsh S. G., Bodmer J. G. HLA class II nucleotide sequences, 1991. Immunogenetics. 1991;33(5-6):321–334. doi: 10.1007/BF00216691. [DOI] [PubMed] [Google Scholar]
- Minderhoud J. M., van der Hoeven J. H., Prange A. J. Course and prognosis of chronic progressive multiple sclerosis. Results of an epidemiological study. Acta Neurol Scand. 1988 Jul;78(1):10–15. doi: 10.1111/j.1600-0404.1988.tb03611.x. [DOI] [PubMed] [Google Scholar]
- Noseworthy J., Paty D., Wonnacott T., Feasby T., Ebers G. Multiple sclerosis after age 50. Neurology. 1983 Dec;33(12):1537–1544. doi: 10.1212/wnl.33.12.1537. [DOI] [PubMed] [Google Scholar]
- Odum N., Hyldig-Nielsen J. J., Morling N., Sandberg-Wollheim M., Platz P., Svejgaard A. HLA-DP antigens are involved in the susceptibility to multiple sclerosis. Tissue Antigens. 1988 May;31(5):235–237. doi: 10.1111/j.1399-0039.1988.tb02088.x. [DOI] [PubMed] [Google Scholar]
- Olerup O., Hillert J., Fredrikson S., Olsson T., Kam-Hansen S., Möller E., Carlsson B., Wallin J. Primarily chronic progressive and relapsing/remitting multiple sclerosis: two immunogenetically distinct disease entities. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7113–7117. doi: 10.1073/pnas.86.18.7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olerup O., Hillert J. HLA class II-associated genetic susceptibility in multiple sclerosis: a critical evaluation. Tissue Antigens. 1991 Jul;38(1):1–15. doi: 10.1111/j.1399-0039.1991.tb02029.x. [DOI] [PubMed] [Google Scholar]
- Olerup O., Smith C. I., Hammarström L. Different amino acids at position 57 of the HLA-DQ beta chain associated with susceptibility and resistance to IgA deficiency. Nature. 1990 Sep 20;347(6290):289–290. doi: 10.1038/347289a0. [DOI] [PubMed] [Google Scholar]
- Schädlich H. J., Karbe H., Felgenhauer K. The prevalence of locally-synthesized virus antibodies in various forms of multiple sclerosis. J Neurol Sci. 1987 Sep;80(2-3):343–349. doi: 10.1016/0022-510x(87)90168-7. [DOI] [PubMed] [Google Scholar]
- Spurkland A., Rønningen K. S., Vandvik B., Thorsby E., Vartdal F. HLA-DQA1 and HLA-DQB1 genes may jointly determine susceptibility to develop multiple sclerosis. Hum Immunol. 1991 Jan;30(1):69–75. doi: 10.1016/0198-8859(91)90073-i. [DOI] [PubMed] [Google Scholar]
- Thompson A. J., Kermode A. G., MacManus D. G., Kingsley D. P., Kendall B. E., Moseley I. F., McDonald W. I. Pathogenesis of progressive multiple sclerosis. Lancet. 1989 Jun 10;1(8650):1322–1323. doi: 10.1016/s0140-6736(89)92710-4. [DOI] [PubMed] [Google Scholar]
- Thompson A. J., Kermode A. G., Wicks D., MacManus D. G., Kendall B. E., Kingsley D. P., McDonald W. I. Major differences in the dynamics of primary and secondary progressive multiple sclerosis. Ann Neurol. 1991 Jan;29(1):53–62. doi: 10.1002/ana.410290111. [DOI] [PubMed] [Google Scholar]
- Todd J. A., Bell J. I., McDevitt H. O. HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature. 1987 Oct 15;329(6140):599–604. doi: 10.1038/329599a0. [DOI] [PubMed] [Google Scholar]
- Van Lambalgen R., Sanders E. A., D'Amaro J. Sex distribution, age of onset and HLA profiles in two types of multiple sclerosis. A role for sex hormones and microbial infections in the development of autoimmunity? J Neurol Sci. 1986 Nov;76(1):13–21. doi: 10.1016/0022-510x(86)90138-3. [DOI] [PubMed] [Google Scholar]
- Vartdal F., Sollid L. M., Vandvik B., Markussen G., Thorsby E. Patients with multiple sclerosis carry DQB1 genes which encode shared polymorphic amino acid sequences. Hum Immunol. 1989 Jun;25(2):103–110. doi: 10.1016/0198-8859(89)90074-8. [DOI] [PubMed] [Google Scholar]


