Abstract
Background
Novel combination therapies to overcome anti-PD-1 resistance are required. Enadenotucirev, a tumor-selective blood stable adenoviral vector, has demonstrated a manageable safety profile and ability to increase tumor immune-cell infiltration in phase I studies in solid tumors.
Methods
We conducted a phase I multicenter study of intravenous enadenotucirev plus nivolumab in patients with advanced/metastatic epithelial cancer not responding to standard therapy. Co-primary objectives were safety/tolerability and maximum tolerated dose and/or maximum feasible dose (MTD/MFD) of enadenotucirev plus nivolumab. Additional endpoints included response rate, cytokine responses, and anti-tumor immune responses.
Results
Overall, 51 heavily pre-treated patients were treated, 45/51 (88%) of whom had colorectal cancer (35/35 patients with information available were microsatellite instability-low/microsatellite stable) and 6/51 (12%) had squamous cell carcinoma of the head and neck. The MTD/MFD of enadenotucirev plus nivolumab was not reached, with the highest dose level tested (1×1012 vp day 1; 6×1012 vp days 3 and 5) shown to be tolerable. Overall, 31/51 (61%) patients experienced a grade 3–4 treatment-emergent adverse event (TEAE), most frequently anemia (12%), infusion-related reaction (8%), hyponatremia (6%), and large intestinal obstruction (6%). Seven (14%) patients experienced serious TEAEs related to enadenotucirev; the only serious TEAE related to enadenotucirev occurring in >1 patient was infusion-related reaction (n=2). Among the 47 patients included in efficacy analyses, median progression-free survival was 1.6 months, objective response rate was 2% (one partial response for 10 months), and 45% of patients achieved stable disease. Median overall survival was 16.0 months; 69% of patients were alive at 12 months. Persistent increases in Th1 and related cytokines (IFNγ, IL-12p70, IL-17A) were seen from ~day 15 in two patients, one of whom had a partial response. Among the 14 patients with matching pre-tumor and post-tumor biopsies, 12 had an increase in intra-tumoral CD8+ T-cell infiltration and 7 had increased markers of CD8 T-cell cytolytic activity.
Conclusions
Intravenously dosed enadenotucirev plus nivolumab demonstrated manageable tolerability, an encouraging overall survival and induced immune cell infiltration and activation in patients with advanced/metastatic epithelial cancer. Studies of next-generation variants of enadenotucirev (T-SIGn vectors) designed to further re-program the tumor microenvironment by expressing immune-enhancer transgenes are ongoing.
Trial registration number
Keywords: immunotherapy; clinical trials as topic; oncolytic viruses; therapies, investigational
WHAT IS ALREADY KNOWN ON THIS TOPIC
The efficacy of single-agent immunotherapy has been suboptimal in numerous cancer types, particularly those typically characterized as having tumor microenvironments lacking T-cell infiltration. Therefore, a critical need remains to identify new therapeutic combinations that can modify the tumor microenvironment and promote favorable immune responses.
WHAT THIS STUDY ADDS
Encouragingly, data from this study have shown that enadenotucirev in combination with nivolumab can induce immune cell infiltration and activation, with increases in measures such as intratumoral CD8+ T cells and cytolytic activity seen in the majority of evaluable patients.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE, OR POLICY
The results of this study suggest that enadenotucirev is a promising tumor-selective viral platform that can be used to create vectors that encode immunostimulatory payloads to increase tumor microenvironment re-programming and clinical efficacy. Studies of next-generation variants of enadenotucirev (T-SIGn vectors) designed to further re-program the tumor microenvironment by expressing immune-enhancer transgenes are ongoing.
Background
PD-1 checkpoint inhibitors are considered a key facet of treatment algorithms in a range of cancer indications. However, despite driving meaningful improvements in outcomes since their introduction, more than half of patients with solid tumors do not experience an objective response following treatment with PD-1 inhibitors.1–4 Response rates are particularly low among patients with cancers typically characterized by immunosuppressive tumor microenvironments that lack T-cell infiltration, for example, microsatellite stable metastatic colorectal cancer (CRC), even when combined with other investigational therapies.5 Even for those patients who do respond, secondary resistance and disease progression typically occur eventually.
Enadenotucirev is a group B Ad11p/Ad3 chimeric adenoviral vector that was generated by directed evolution to have potent tumor-selective replication and cytotoxicity.6 Enadenotucirev has a high level of stability in human blood7 and selectively replicates in cells derived from epithelial tumors, leading to local amplification and specific killing of malignant cells by a rapid non-apoptotic immunogenic mechanism.6 8 While enadenotucirev has no inserted transgenes, clinical studies of modified variants known as “Tumor-Specific Immuno Gene Therapy” (T-SIGn) vectors are ongoing. Results from previous phase I studies of enadenotucirev in epithelial malignancies have demonstrated that intravenous dosing of enadenotucirev leads to selective and sustained delivery, with evidence of viral persistence ~7 weeks after dosing,9 and a manageable tolerability profile.9–11 In addition, enadenotucirev appears to stimulate tumor immune-cell infiltration.9 11
In murine models, tumor-selective viruses have been shown to reverse tumor insensitivity to checkpoint inhibitors (CTLA-4 blockade)12 and have been shown to reverse resistance to PD-1 inhibitors by eliciting a broader spectrum of cytotoxic T-cell responses to tumor-associated antigens.13 Combining a checkpoint inhibitor with a tumor-selective virus such as enadenotucirev may provide a unique ability to increase the efficacy of the checkpoint inhibitor. Therefore, we conducted a phase I dose-escalation study of enadenotucirev, in combination with nivolumab, in patients with advanced/metastatic epithelial cancer.
Methods
Study design
This multicenter, open-label, non-randomized study (NCT02636036) included phase Ia and 1b dose escalation and dose expansion stages. However, the study was terminated early prior to opening of the dose expansion stage. Dose escalation in phase Ia was conducted using a standard “3+3” design, with patients receiving increasing dose levels and/or cycles of enadenotucirev in combination with a PD-1 inhibitor. A total of seven cohorts of patients were enrolled. Patients in cohort 1 were to receive up to 6 cycles of enadenotucirev in combination with pembrolizumab (1 mg/kg every 3 weeks). A study amendment replaced pembrolizumab with nivolumab, for non-safety related reasons, from cohort 2 onward. Patients in cohorts 2–7A were to receive up to 8 cycles of treatment involving 1–2 cycles of enadenotucirev and up to 8 cycles of nivolumab (360 mg every 3 weeks). Patients could receive additional cycles of nivolumab beyond cycle 8, with the Sponsor’s agreement and provided that the Investigator believed that the potential benefits outweighed the potential risks. Dose levels are shown in online supplemental figure 1 and figure 1.
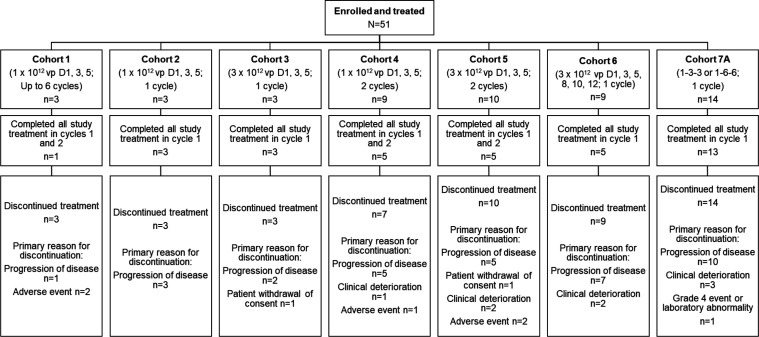
Figure 1.
Patient disposition. Patients in cohort 1 received enadenotucirev in combination with pembrolizumab. Patients in cohort 2 to cohort 7A received enadenotucirev in combination with nivolumab. Per protocol, the end of study was defined as when all patients had completed all study visits, had otherwise discontinued from the study, or the last patients treated in the study all had at least 9 months of follow-up after their first dose of study treatment. Since there was no set definition of the timing of study completion rates across all patients, study completion rates would not be a meaningful measure, nor appropriate to compare across cohorts, and therefore have not been provided. Instead, number of patients completing treatment is summarized in the figure. 1-3-3, 1×1012 vp on day 1 followed by 3×1012 vp on days 3 and 5; 1-6-6, 1×1012 vp on day 1 followed by 6×1012 vp on days 3 and 5.
jitc-2022-006561supp001.pdf (4.3MB, pdf)
Participants
Eligible participants had one of the following metastatic or advanced cancers not responding to therapy or for which there were no standard of care options: CRC, urothelial cell cancer, squamous cell cancer of the head and neck (SCCHN), salivary gland cancer, or non-small cell lung cancer. Patients who had received prior treatment with a PD-1 or PD-L1 inhibitor were not eligible for inclusion in cohorts 1–6. Patients who had received prior PD-1 or PD-L1 therapy were eligible for inclusion in cohort 7A if it was their most recent/current line of therapy and treatment was for ≥6 weeks and ≤4 months, with best response of stable disease or progressive disease. Additional eligibility criteria included age ≥18 years; Eastern Cooperative Oncology Group performance status of 0–1; and adequate renal, hepatic, bone marrow, and coagulation function. Key exclusion criteria included history or evidence of significant immunodeficiency due to underlying disease or medication (eg, systemic corticosteroids in the 4 weeks prior to day 1), renal or autoimmune disease, or recent use of antiviral agents (ribavirin, adefovir, lamivudine, or cidofovir within 7 days prior to day 1; pegylated IFN within 4 weeks of day 1).
Procedures
Safety and tolerability
The reporting period for all adverse events (AEs) began on the date informed consent was provided and continued until at least 100 days after the last administration of any study treatment. The incidence, type, and severity of AEs were characterized using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03. A Safety Review Committee composed of an independent oncologist, all Principal Investigators or their delegates, and medically qualified representatives of the Sponsors reviewed all safety data (including any dose-limiting toxicities (DLTs)) and determined whether additional patients were enrolled at the current dose level or whether a higher dose level cohort was opened. During the 42-day DLT period, any of the following toxicities were defined as DLTs if they were considered at least possibly attributed to enadenotucirev, whether given as monotherapy or in combination with a PD-1 inhibitor: grade ≥3 non-hematological toxicity except for activated partial thromboplastin time (aPTT) prolongation, nausea, fatigue, headache, and chills (grade ≥3 nausea, headache, and chills lasting >3 days and grade ≥3 fatigue lasting >7 days were considered DLTs); grade ≥3 infusion reaction that did not resolve within 72 hours with appropriate treatment; grade 3 hematological toxicities lasting >3 days and grade 4 hematological adverse event or laboratory abnormality (with the exception of grade 4 lymphocytopenia, amylase or lipase abnormalities not associated with symptoms or clinical manifestations); clotting event (ie, deep vein thrombosis, pulmonary embolism) occurring concurrently with grade 2 or 3 aPTT prolongation; clinically significant bleeding event (requiring blood transfusion or hospitalization) occurring concurrently with grade 2 or 3 aPTT prolongation (unless there was a clear explanation for the event, such as tumor-related bleeding); any toxicity managed by discontinuation of nivolumab.
Efficacy assessments
Tumor imaging (CT) with oral and intravenous contrast or MRI was performed prior to treatment, as clinically indicated, and at 6-week intervals (7 weeks in cohort 4 only) during the study. A second scan after ≥4 weeks was required to confirm responses or disease progression. All response-based endpoints (and the derived progression-free survival (PFS) endpoint) were based on an independent reviewer’s assessments per Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 and immune-related (ir) RECIST v1.14 15
Pharmacokinetics
Whole blood samples for measurement of enadenotucirev pharmacokinetics were taken on each enadenotucirev dosing day (pre-dose and post-dose (up to 10 min after the end of the infusion)), each nivolumab dosing day (pre-dose) of each cycle, and at the end of study treatment visit. The concentration of enadenotucirev in the blood was measured using qPCR to detect viral genomic DNA as previously described.9 11
Pharmacodynamics
Anti-enadenotucirev antibodies
Serum samples for anti-enadenotucirev antibody status were taken pre-dose on the first enadenotucirev dosing day in combination treatment cycles, pre-dose on each nivolumab dosing day in all cycles, at the end of study treatment visit, and at the first follow-up visit. Serum anti-enadenotucirev antibody response was assessed using an electrochemiluminescence ligand binding assay on a Meso Scale Discovery platform by BioOutsource (Glasgow, UK).9 11
Detection of enadenotucirev in tumor samples by qPCR
Detection of enadenotucirev in frozen tumor tissue from pre-treatment and post-treatment biopsies was performed by qPCR of the E3 gene (enadenotucirev has a nearly complete E3 region deletion),6 as previously described.11
Cytokine responses
Blood samples for serum cytokine analysis were drawn on each enadenotucirev dosing day (pre-dose and ~8 hours post-dose) and each nivolumab dosing day (cycle 1 and cycle 2 only). Serum cytokine levels were determined using a 17-analyte multiplex Luminex assay for the following analytes: IL-2, IL-5, IL-6, IL-10, IL-17A, MCP-1, TNF-α, IFNγ, IL-13, IL-15, CXCL9 (MIG), CXCL10 (IP-10), CXCL11 (I-TAC), IFNα2, MIP1α, IL-8, and IL-12p70.
Histopathology and immunohistochemistry
Tumor biopsies were collected at screening and between days 8 and 15 of cycle 2. Sections were stained for viral hexon, pan-cytokeratin (PanCK)/CD8, CD8/Granzyme B, Foxp3, PD-L1, PD-1, and CD8/Ki67 (duplex immunofluorescence), as previously described.11 A combination of automated image analysis and qualitative evaluation by a pathologist was used to generate immunohistochemistry data outputs. Tumor immune phenotype (desert, excluded, excluded at invasive margin, or inflamed) was assessed by a pathologist-led scoring system (online supplemental appendix).
Immunophenotyping of immune cells subsets
Blood samples for peripheral blood mononuclear cell (PBMC) immunophenotyping were drawn pre-dose on cycle 1 day 1, cycle 3 day 15, and at end of treatment. PBMCs were analyzed using 17-color or 18-color multiparameter flow cytometry. The panels of antibodies included the following markers: CD3, CD4, CD8, CD25, CD20, CD56, CD11c, HLA-DR, CD38, PD-1, CD86, CD40, CD14, CD16, CD123, CD127, viability dye, and CD45 (18-color only).
Objectives and endpoints
The co-primary objectives were safety/tolerability and maximum tolerated dose (MTD) and/or maximum feasible dose (MFD) of enadenotucirev plus nivolumab. Secondary endpoints included PFS, overall survival (OS), objective response rate (ORR), best overall response (BoR), duration of response (DoR), and pharmacokinetics. Key exploratory endpoints included cytokine responses, immunogenicity, and anti-tumor immune responses.
Statistical analyses
No formal sample size calculations were performed for this study. Sample size was determined by clinical rather than statistical considerations with a minimum of three patients per cohort. No formal statistical hypothesis testing was performed.
The safety analysis set included all patients who received ≥1 dose of study treatment (enadenotucirev, nivolumab, or pembrolizumab). This population was used for all safety, OS, and pharmacodynamic and pharmacokinetic analyses. All efficacy analyses, except for OS, were assessed using the full analysis set, which included all patients with ≥1 dose of study treatment, as well as a baseline and at least one post-baseline efficacy assessment.
Descriptive statistics were used to summarize ORR and clinical benefit rate (CBR). BoR, CBR, and ORR were calculated from date of first dose to date of first objectively documented progression or date of subsequent therapy, whichever occurred first. CBR was calculated as the proportion of patients who achieved a BoR of complete response or partial response (PR) at any time, or stable disease (SD) ≥12 weeks. The Kaplan-Meier method was used to analyze DoR, PFS, and OS. PFS was defined as the time from the start date of treatment to the date of documented clinical/radiological progression or date of death. Patients were censored at the date of the last adequate tumor assessment (or at the time of first dose if no post-baseline assessments were performed) if no documented disease progression was reported or at the date of subsequent anti-cancer therapy (where initiated prior to any recorded disease progression).
Results
Patient characteristics, disposition, and study drug exposure
A total of 51 patients were enrolled and treated across six sites in the USA. All patients were included in the safety analysis set and 47 patients were included in the full analysis set.
No clinically important differences were identified between dose cohorts in terms of demographics or baseline disease characteristics (table 1). In total, 45/51 (88%) patients had CRC (including 3 patients with appendiceal tumors) and 6/51 (12%) patients had SCCHN. Of the 35/45 patients with CRC and information on MSI status available, all were microsatellite instability-low or microsatellite stable (MSI-L/MSS; MSI-L and MSS statuses were grouped as one in electronic case report forms). While data on presence of liver metastases were not specifically collected at baseline, 55% (26/47) of patients had either target or non-target liver lesions documented. All patients had received prior systemic therapy, 65% (33/51) of whom had ≥3 prior systemic regimens and 20% (10/51) had received >5 regimens. In addition, 92% (47/51) of patients had a prior cancer-related surgery and 47% (24/51) of patients had received radiotherapy. No patients received prior treatment with a PD-1/PD-L1 inhibitor.
Table 1.
Baseline demographics and disease characteristics
| Characteristic | Overall (N=51) |
Cohort 1 (n=3) |
Cohort 2 (n=3) |
Cohort 3 (n=3) |
Cohort 4 (n=9) |
Cohort 5 (n=10) |
Cohort 6 (n=9) |
Cohort 7A (n=14) |
| Median age, years (min, max) | 59 (22, 83) | 67 (56, 76) | 55 (43, 66) | 58 (56, 83) | 67 (22, 79) | 57 (38, 75) | 52 (42, 62) | 62 (41, 78) |
| Male, n (%) | 33 (64.7) | 2 (66.7) | 3 (100) | 2 (66.7) | 7 (77.8) | 6 (60.0) | 7 (77.8) | 6 (42.9) |
| Tumor type, n (%) | ||||||||
| CRC | 45 (88.2) | 3 (100) | 3 (100) | 3 (100) | 7 (77.8) | 8 (80.0) | 7 (77.8) | 14 (100) |
| SCCHN | 6 (11.8) | 0 | 0 | 0 | 2 (22.2) | 2 (20.0) | 2 (22.2) | 0 |
| Time since first cytological or histopathological diagnosis (years), median (min, max) | 2.6 (0.4, 9.8) | 2.4 (0.6, 5.2) | 2.4 (1.6, 3.9) | 3.7 (1.0, 6.0) | 2.4 (1.5, 9.8) | 3.9 (1.0, 8.4) | 2.4 (0.4, 9.8) | 2.5 (1.2, 8.0) |
| Time since first metastatic disease diagnosis (years), median (min, max) | n=49 2.4 (0.1, 9.8) |
n=3 2.4 (0.6, 3.2) |
n=3 1.6 (0.9, 3.9) |
n=3 3.7 (1.9, 6.0) |
n=8 2.3 (0.1, 9.8) |
n=10 3.2 (1.0, 5.0) |
n=9 2.4 (0.4, 4.3) |
n=13 2.3 (0.3, 8.0) |
| Tumor stage at baseline, n (%) | ||||||||
| IIA | 2 (3.9) | 0 | 0 | 1 (33.3) | 1 (11.1) | 0 | 0 | 0 |
| IIIB | 1 (2.0) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (7.1) |
| IIIC | 1 (2.0) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (7.1) |
| IVA | 19 (37.3) | 2 (66.7) | 2 (66.7) | 0 | 4 (44.4) | 5 (50.0) | 2 (22.2) | 4 (28.6) |
| IVB | 27 (52.9) | 0 | 1 (33.3) | 2 (66.7) | 4 (44.4) | 5 (50.0) | 7 (77.8) | 8 (57.1) |
| Unknown | 1 (2.0) | 1 (33.3) | 0 | 0 | 0 | 0 | 0 | 0 |
| No of prior systemic regimens, n (%) | ||||||||
| 1 | 6 (11.8) | 1 (33.3) | 0 | 1 (33.3) | 2 (22.2) | 1 (10.0) | 1 (11.1) | 0 |
| 2 | 12 (23.5) | 0 | 1 (33.3) | 0 | 2 (22.2) | 3 (30.0) | 3 (33.3) | 3 (21.4) |
| 3 | 7 (13.7) | 0 | 1 (33.3) | 0 | 3 (33.3) | 0 | 2 (22.2) | 1 (7.1) |
| 4 | 9 (17.6) | 0 | 1 (33.3) | 0 | 1 (11.1) | 1 (10.0) | 2 (22.2) | 4 (28.6) |
| 5 | 7 (13.7) | 1 (33.3) | 0 | 1 (33.3) | 0 | 3 (30.0) | 1 (11.1) | 1 (7.1) |
| >5 | 10 (19.6) | 1 (33.3) | 0 | 1 (33.3) | 1 (11.1) | 2 (20.0) | 0 | 5 (35.7) |
Patient numbers provided for characteristic for which not all patients had data available.
CRC, colorectal cancer; SCCHN, squamous cell cancer of the head and neck.
Patient disposition is summarized in figure 1. Overall, 34/51 (67%) of patients received all planned doses of enadenotucirev. Most patients received at least one cycle/dose of pembrolizumab or nivolumab (48/51 [94%]), with nine patients receiving ≥8 cycles. Median number of cycles of pembrolizumab or nivolumab was 3 (range: 0 to 23).
Dose escalation and DLTs
Three patients were enrolled to receive enadenotucirev 1×1012 vp (days 1, 3, and 5; up to 6 cycles) in combination with pembrolizumab (cohort 1). DLTs were reported in 2/3 patients treated in cohort 1. Following review of the cohort 1 data by the Safety Review Committee, the study design was modified to reduce the number of cycles of enadenotucirev from six to a maximum of two. In addition, pembrolizumab was switched for nivolumab from cohort 2 onwards (non-safety-related decision); patients in cohorts 2–7A all received enadenotucirev in combination with nivolumab. Cohort 2 (1×1012 vp on days 1, 3, and 5 (1 cycle); n=3) and cohort 3 (3×1012 vp on days 1, 3, and 5 (1 cycle); n=3) completed with no DLTs. A total 9 patients were enrolled in cohort 4 (1×1012 vp on days 1, 3, and 5 (2 cycles)); enrollment into cohort 3 and cohort 4 was in parallel. Following a DLT of hypoxia in cohort 4, a dosing schedule modification was introduced for cohort 6 to administer all six doses of virus in cycle 1. In addition, prophylactic treatment with steroids and diphenhydramine was implemented in the study on enadenotucirev dosing days to help minimize acute reactions to viral particle infusion. Cohorts 5 and 6 enrolled patients at the same 3×1012 vp dose but with differing schedules (cohort 5: dosing on days 1, 3, and 5 (2 cycles); cohort 6: dosing on days 1, 3, 5, 8, 10, and 12 (1 cycle)). DLTs occurred in 2/10 and 1/9 patients, respectively. A dose of 3×1012 vp was considered tolerable, but the implementation of additional renal monitoring was recommended, with guidance to hold administration of enadenotucirev should proteinuria be detected, and the use of a “low-high-high” dosing regimen (where a lower dose is given on day 1, followed by higher doses on days 3 and 5). Subsequently, a total of 14 patients were enrolled in cohort 7A (1×1012 vp (day 1) followed by 3×1012 or 6×1012 vp ((days 3 and 5); n=7 per dose level). One DLT was reported in cohort 7A. Both dose schedules were deemed tolerable; thus, a MTD of enadenotucirev in combination with nivolumab was not determined. A decision was made to halt further enrollment due to limited clinical activity noted on study and the low likelihood of observing additional clinical signals from further escalation.
Overall, treatment-emergent adverse events (TEAEs) classified as DLTs were reported in 7/51 (14%) patients. A total of four DLTs (acute cardiac failure, infusion-related reaction, acute kidney injury, and hypoxia) were also considered serious adverse events (SAEs). Further DLTs that were not considered SAEs included grade 3 fatigue (n=1; event occurred in the same patient with acute cardiac failure), grade 3 hypoxia (n=1), and grade 1 proteinuria (n=2).
Safety and tolerability
A summary of safety is presented in table 2. All patients experienced at least one TEAE, and 31/51 (61%) patients experienced at least one grade 3–4 TEAE. The grade ≥3 TEAEs reported in the highest proportion of patients were anemia (6/51; 12%), infusion-related reaction (4/51; 8%), hyponatremia (3/51; 6%) and large intestinal obstruction (3/51; 6%).
Table 2.
Overall safety summary and TEAEs occurring in >15% of patients (safety analysis set)
| Patients with at least one: | Overall (N=51) |
Cohort 1 (n=3) |
Cohort 2 (n=3) |
Cohort 3 (n=3) |
Cohort 4 (n=9) |
Cohort 5 (n=10) |
Cohort 6 (n=9) |
Cohort 7A (n=14) |
| Serious TEAE | 13 (25.5) | 2 (66.7) | 1 (33.3) | 0 | 1 (11.1) | 2 (20.0) | 1 (11.1) | 6 (42.9) |
| Serious TEAE related to enadenotucirev | 7 (13.7) | 2 (66.7) | 1 (33.3) | 0 | 0 | 1 (10.0) | 1 (11.1) | 2 (14.3) |
| TEAE with toxicity grade 3–4 | 31 (61.0) | 2 (66.7) | 2 (66.7) | 0 | 4 (44.4) | 6 (60.0) | 7 (77.8) | 10 (71.4) |
| TEAE classified as a dose limiting toxicity | 7 (13.7) | 2 (66.7) | 0 | 0 | 1 (11.1) | 2 (20.0) | 1 (11.1) | 1 (11.1) |
| TEAE leading to discontinuation of enadenotucirev | 12 (23.5) | 2 (66.7) | 0 | 0 | 3 (33.3) | 4 (40.0) | 1 (11.1) | 2 (14.3) |
| General disorders and administration site conditions | 46 (90.2) | 3 (100) | 3 (100) | 2 (66.7) | 8 (88.9) | 10 (100) | 8 (88.9) | 12 (85.7) |
| Fever | 25 (49.0) | 1 (33.3) | 1 (33.3) | 0 | 4 (44.4) | 6 (60.0) | 5 (55.6) | 8 (57.1) |
| Chills | 22 (43.1) | 0 | 0 | 0 | 5 (55.6) | 5 (50.0) | 4 (44.4) | 8 (57.1) |
| Fatigue | 21 (41.2) | 2 (66.7) | 3 (100) | 1 (33.3) | 1 (11.1) | 6 (60.0) | 5 (55.6) | 3 (21.4) |
| Influenza like illness | 11 (21.6) | 3 (100) | 2 (66.7) | 1 (33.3) | 2 (22.2) | 1 (10.0) | 0 | 2 (14.3) |
| Edema peripheral | 10 (19.6) | 0 | 0 | 1 (33.3) | 2 (22.2) | 2 (20.0) | 1 (11.1) | 4 (28.6) |
| Gastrointestinal disorders | 40 (78.4) | 3 (100) | 3 (100) | 1 (33.3) | 8 (88.9) | 10 (100) | 5 (55.6) | 10 (71.4) |
| Nausea | 18 (35.3) | 1 (33.3) | 3 (100) | 0 | 4 (44.4) | 4 (40.0) | 1 (11.1) | 5 (35.7) |
| Diarrhea | 16 (31.4) | 2 (66.7) | 1 (33.3) | 0 | 5 (55.6) | 3 (30.0) | 1 (11.1) | 4 (28.6) |
| Vomiting | 13 (25.5) | 1 (33.3) | 0 | 0 | 2 (22.2) | 3 (30.0) | 1 (11.1) | 6 (42.9) |
| Abdominal pain | 9 (17.6) | 1 (33.3) | 1 (33.3) | 0 | 3 (33.3) | 1 (10.0) | 1 (11.1) | 2 (14.3) |
| Constipation | 9 (17.6) | 0 | 1 (33.3) | 1 (33.3) | 1 (11.1) | 3 (30.0) | 2 (22.2) | 1 (7.1) |
| Abdominal pain upper | 8 (15.7) | 1 (33.3) | 0 | 0 | 1 (11.1) | 2 (20.0) | 1 (11.1) | 3 (21.4) |
| Respiratory, thoracic, and mediastinal disorders | 31 (60.8) | 2 (66.7) | 0 | 2 (66.7) | 5 (55.6) | 7 (70.0) | 6 (66.7) | 9 (64.3) |
| Dyspnea | 13 (25.5) | 1 (33.3) | 0 | 1 (33.3) | 2 (22.2) | 2 (20.0) | 4 (44.4) | 3 (21.4) |
| Cough | 12 (23.5) | 1 (33.3) | 0 | 1 (33.3) | 0 | 2 (20.0) | 3 (33.3) | 5 (35.7) |
| Metabolism and nutrition disorders | 30 (58.8) | 2 (66.7) | 2 (66.7) | 1 (33.3) | 4 (44.4) | 7 (70.0) | 4 (44.4) | 10 (71.4) |
| Decreased appetite | 17 (33.3) | 0 | 2 (66.7) | 0 | 2 (22.2) | 4 (40.0) | 3 (33.3) | 6 (42.9) |
| Hypokalemia | 8 (15.7) | 0 | 0 | 1 (33.3) | 1 (11.1) | 2 (20.0) | 1 (11.1) | 3 (21.4) |
| Musculoskeletal and connective tissue disorders | 24 (47.1) | 2 (66.7) | 2 (66.7) | 1 (33.3) | 6 (66.7) | 6 (60.0) | 3 (33.3) | 4 (28.6) |
| Myalgia | 13 (25.5) | 1 (33.3) | 1 (33.3) | 0 | 3 (33.3) | 4 (40.0) | 1 (11.1) | 3 (21.4) |
| Back pain | 8 (15.7) | 1 (33.3) | 2 (66.7) | 0 | 2 (22.2) | 1 (10.0) | 0 | 2 (14.3) |
| Nervous system disorders | 21 (41.2) | 3 (100) | 1 (33.3) | 1 (33.3) | 5 (55.6) | 5 (50.0) | 3 (33.3) | 3 (21.4) |
| Headache | 13 (25.5) | 1 (33.3) | 1 (33.3) | 0 | 3 (33.3) | 4 (40.0) | 2 (22.2) | 2 (14.3) |
| Blood and lymphatic system disorders | 15 (29.4) | 2 (66.7) | 0 | 1 (33.3) | 3 (33.3) | 3 (30.0) | 2 (22.2) | 4 (28.6) |
| Anemia | 10 (19.6) | 2 (66.7) | 0 | 1 (33.3) | 1 (11.1) | 1 (10.0) | 2 (22.2) | 3 (21.4) |
TEAE, treatment-emergent adverse event.
Thirteen patients (25%) experienced a total of 23 serious TEAEs. The serious TEAEs reported in the highest proportion of patients were sepsis, infusion-related reaction, acute kidney injury, and small intestinal obstruction (2 patients (4%) each); all other serious TEAEs were reported for a single patient only. The only serious TEAE related to enadenotucirev reported in more than one patient was infusion-related reaction (n=2).
Overall, 12/51 patients (24%) had TEAEs leading to discontinuation of enadenotucirev. The only TEAEs leading to discontinuation of enadenotucirev that were reported in more than one patient were hypoxia, proteinuria, and infusion-related reaction (all n=2).
In response to a DLT of acute kidney injury early in the enrollment to cohort 5, acute kidney injury was considered to be a key safety signal and additional renal monitoring was implemented to help manage and mitigate against the risk. Throughout the course of the study, 34/51 (67%) patients had a urine protein dipstick result of 1+ or greater (CTCAE grade 1); of these 34 patients, 4 had a maximum result of 3+ and 1 had a maximum result of 4+. By end of study, proteinuria had resolved to <1+ in 18/34 (53%) patients. Serious TEAEs related to the risk of acute kidney injury were reported in 3/51 (6%) patients; one case was deemed to be secondary to obstruction from a tumor and therefore unrelated to study treatment.
Overall, 23/51 patients experienced ≥grade 2 aPTT prolongation at any time point during the study. By day 57, aPTT was normal or grade 1 in 33/38 patients with data available. Of the patients with prolongation, a positive result on antiphospholipid antibody (APLA) tests was seen in all three patients tested. Two of the three patients tested for APLAs were also tested for anti-cardiolipin and anti-beta 2 glycoprotein; both were negative on these tests. No apparent relationship between prolonged aPTT and clinical sequelae was seen.
Efficacy
Progression-free survival
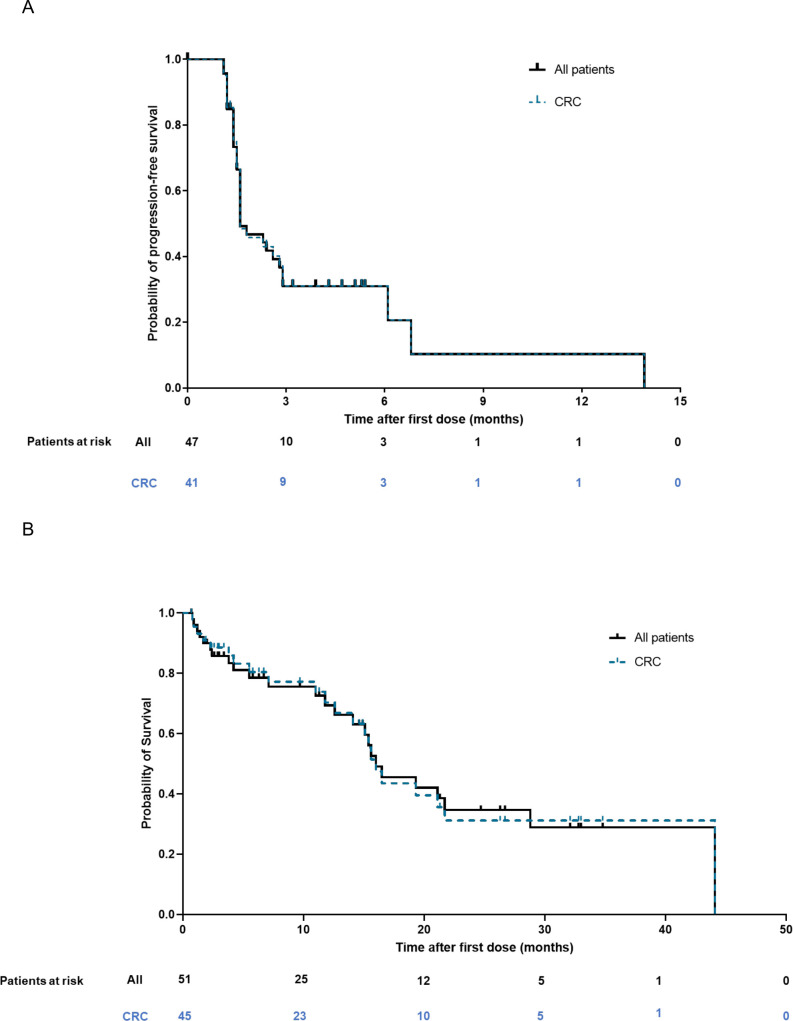
Among the 47 patients included in the full analysis set, 31% were progression-free at 6 months and 10% at 12 months, per RECIST v1.1 (figure 2A). Overall, median (95% CI) PFS was 1.6 (1.6, 2.9) months. Per irRECIST, median (95% CI) PFS was 1.6 (1.6, 6.1) months and the percentage of patients who were progression free at 6 and 12 months was 36% and 12%, respectively.
Figure 2.
(A) Progression-free survival (full analysis set) and (B) overall survival (safety analysis set). CRC, colorectal cancer.
Overall survival
Median (95% CI) OS was 16.0 (12.6, 28.8) months (figure 2B). OS was 79% and 69% at 6 and 12 months, respectively.
Response rate
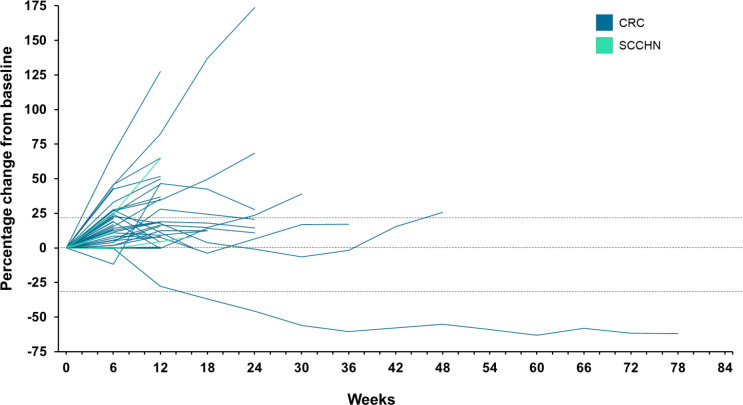
Overall, the ORR was 2% (table 3), with a single patient achieving a partial response (PR; duration of response: 9.8 months). In total, 45% (21/47) of patients had a BoR of SD, including three patients with SD for ≥24 weeks (figure 3). CBR (complete response or PR at any time, or SD ≥12 weeks) was assessed as 6% (3/47), as one of the patients with prolonged SD had an assessment of progressive disease (PD) before going on to achieve SD. As such, they were classed as having a BoR of PD. Response data per irRECIST were largely consistent with the responses per RECIST. Best change in target lesion burden is shown in online supplemental figure 2.
Table 3.
Response rate per RECIST v1.1 (full analysis set)
| Total (n=47) | Tumor type | ||
| CRC (n=41) | SCCHN (n=6) | ||
| Best overall response | |||
| CR | 0 | 0 | 0 |
| PR | 1 (2.1) | 1 (2.4) | 0 |
| SD | 21 (44.7) | 18 (43.9) | 3 (50.0) |
| PD | 23 (48.9) | 21 (51.2) | 2 (33.3) |
| No data | 2 (4.3) | 1 (2.4) | 1 (16.7) |
| Objective response rate | 1 (2.1) | 1 (2.4) | 0 |
| 95% CI | 0.05, 11.29 | 0.06, 12.86 | NE, NE |
| Clinical benefit rate | 3 (6.4) | 3 (7.3) | 0 |
| 95% CI | 1.34, 17.54 | 1.54, 19.92 | NE, NE |
Data are n (%) unless specified otherwise.
The BoR on-study was defined as the best response designation recorded between the date of first dose and the date of first objectively documented progression based on RECIST v1.1 criteria, or the date of subsequent therapy, whichever occurred first. CR or PR determinations must have been confirmed by a second scan no less than 4 weeks after the criteria for response are first met.
CBR was calculated as the proportion of patients who achieved a BoR of CR or PR at any time, or SD lasting ≥12 weeks.
BoR, best overall response; CBR, clinical benefit rate; CR, complete response; CRC, colorectal cancer; NE, Not evaluable; PD, progressive disease; PR, partial response; SCCHN, squamous cell cancer of the head and neck; SD, stable disease.
Figure 3.
Change in target lesion burden over time per RECIST v1.1 (full analysis set). CRC, colorectal cancer; SCCHN, squamous cell cancer of the head and neck.
Pharmacokinetics
Blood viral kinetics for patients receiving a single cycle (3 doses) of enadenotucirev were consistent with a previous study of enadenotucirev as a monotherapy.10 Multicycle dosing and 6-dose regimens did not appear to alter the overall pattern of viral kinetic responses compared with single-cycle 3-dose regimens, with viral concentration decreasing toward or below the lower limit of quantification within 48 hours after dosing across all regimens. Viral kinetics of patients in cohorts 3 (3×1012 vp on days 1, 3, and 5; 1 cycle), 5 (3×1012 vp on days 1, 3, and 5; 2 cycles), 6 (3×1012 vp on days 1, 3, 5, 8, 10, and 12; 1 cycle), and 7A (1×1012 vp on day 1 and 3×1012 vp or 6×1012 vp on days 3 and 5; 1 cycle) are shown in online supplemental figure 3. There were no apparent effects of nivolumab on enadenotucirev pharmacokinetics or vice versa (data not shown).
Pharmacodynamics
Anti-enadenotucirev antibodies
Response in peripheral blood was similar to that previously described for enadenotucirev at the intravenous dose of 1×1012 vp.10 11 Notably, the assay used only measures binding and not neutralization. All patients had no or low levels of antibodies to enadenotucirev at baseline. Following treatment, enadenotucirev antibody titers increased relative to baseline in all evaluable patients. There was little apparent relationship between antibody titer, dose, and number of cycles, and a wide inter-patient range was seen. Overall, there was a trend for the median titer to increase over time, before plateauing and decreasing. Across all cohorts with data available, median titer had decreased from peak value by end of treatment (data not shown).
Detection of enadenotucirev DNA in tumors
Enadenotucirev genomic DNA was detected in 5/26 (19%) patients with post-dose biopsies available (cycle 2 days 8–15 (29–36 days after first dose); data not shown). Of the biopsies with genomic DNA detected, three were from patients in cohort 7A, one was from a patient in cohort 6, and 1 was from a patient in cohort 2. Given the small number of samples available, no clear relationship between dose and positivity was seen.
Serum cytokines
Detection of serum cytokines provides information on acute post-dose response to virus infusion to support safety monitoring and dose selection strategies. In addition, in an exploratory setting, measurement of changes in immune analytes may serve to provide insights into treatment mechanisms and prognostic markers.
Overall, the pattern of acute (days 1–5) cytokine responses to flat doses (ie, the same dose at all time points) of enadenotucirev in peripheral blood were as previously described (online supplemental figure 4).10 Low-high-high dosing in cohort 7A appeared to allow higher doses of enadenotucirev (3×1012 and 6×1012 vp) to be administered on days 3 and 5 without the associated acute cytokine reactions to viral infusions seen with flat dosing at these levels. Cytokine responses with 1-3-3 dosing (1×1012 vp on day 1; 3×1012 vp on days 3 and 5), including after the first 3×1012 vp dose on day 3, were negligible. Cytokine responses after the first dose of 6×1012 vp in the 1-6-6 regimen were similar to those seen with the first dose of the flat 3×1012 vp regimen.
Persistent increases in Th1 and related cytokines (IFNγ, IL-12p70, IL-17A) were seen from ~day 15 in two patients from cohort 7A (one at 1-3-3 and one at 1-6-6), one of whom was classed as having a BoR of PR (online supplemental figure 5). Increases were sustained out to day 91 and day 544 (patient with PR), respectively. The small sample numbers limit further interpretation of these data but provides evidence of potential sustained enadenotucirev-mediated immune responses.
Anti-tumor immunity
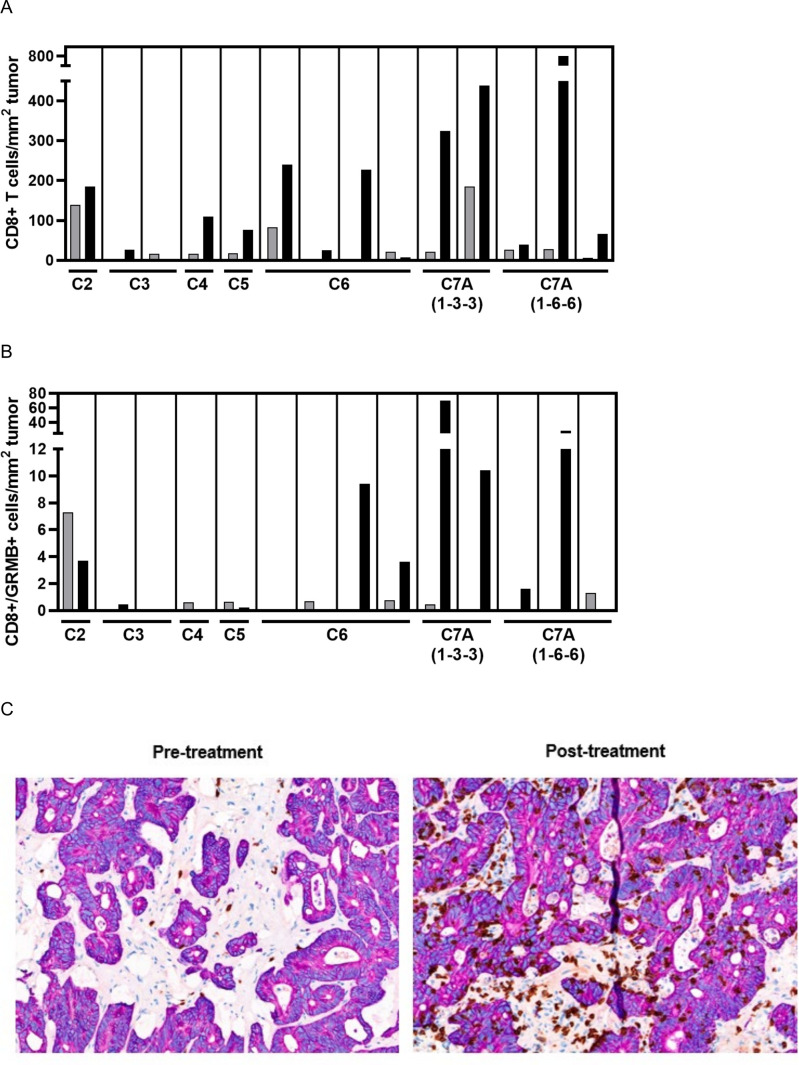
Overall, 14 patients (13 CRC (n=12 MSI-L/MSS; n=1 unknown); 1 SCCHN) had matching pre-treatment and post-treatment tumor biopsy samples with sufficient tumor tissue for immunohistochemistry staining. Twelve of 14 patients had increased post-dose intra-tumoral CD8+ T-cell infiltration (median (Q1–Q3) fold change 5.4× (1.7–22.8); figure 4A) Similarly, 12/14 patients had increased stromal CD8+ T-cell infiltration in post-treatment biopsies. Furthermore, the CD8+/FoxP3+ cell ratio increased in 12/14 patients and markers of CD8 T-cell cytolytic activity (Granzyme B) increased in 7/14 patients (figure 4B), with a trend of greater increases in the higher dose cohorts. Increases in tumor CD4 levels (11/14 patients), PD-L1+ immune cells (8/14 patients), and CD8+Ki67+ cells (8/10 patients) were observed. In addition, 4/14 patients showed conversion from a “desert” to “inflamed” phenotype post-dose (pathologist scored assessment of intra-epithelial and intra-tumoral stroma CD8-positive cell density; figure 4C); all four patients had MSI-L/MSS CRC. Among the four patients with conversion, two had a BoR of SD (DoR <12 weeks for both patients) and two had a BoR of PD. With limited patient numbers, no clear relationship between immune cell infiltration or activity and OS or response was seen.
Figure 4.
Post-treatment increases in tumor immune cell infiltration. (A) Intra-tumoral increases in CD8+ T cells in patients with matched pre-treatment (gray bar) and post-treatment (black bar) biopsies from individual patients; (B) increases in CD8+/Granzyme B+ cell density in patients with matched pre-treatment (gray bar) and post-treatment (black bar) biopsies from individual patients; (C) representative immunohistochemistry staining of one lesion (liver) with conversion from “desert” to “inflamed” phenotype (T cells shown in brown).
Immune cell subsets in peripheral blood
PBMC samples from 10 patients (9 CRC, 1 SCCHN) were analyzed. T-Cell activation, as measured by increases in CD38 and HLA-DR expression on CD8+ T cells, was observed in 9/10 patients, including one patient who had only received enadenotucirev prior to sampling.
Discussion
This phase I study was conducted to assess the safety, tolerability, and MTD of enadenotucirev in combination with nivolumab in patients with advanced/metastatic epithelial cancer. In total, 88% of patients enrolled in this study had a primary diagnosis of CRC. Notably, the CRC tumor microenvironment is typically characterized as having a wound healing immune phenotype, defined as infiltrated with anti-inflammatory macrophages and Th2 cells.16 CRC is thus regarded as resistant to immunotherapy, as evidenced by lack of responsiveness to checkpoint inhibitors.17 18
This study demonstrated that intravenous enadenotucirev has a manageable tolerability profile when administered in combination with nivolumab. The frequency and type of AEs seen were largely consistent with previous enadenotucirev monotherapy studies suggesting no additive toxicity with nivolumab.9–11 In addition, the safety profile with one versus two cycles of enadenotucirev was similar. The maximum dose of enadenotucirev administered in this study (6×1012 vp as part of a 1-6-6 regimen) in combination with nivolumab was deemed tolerable. Thus, a MTD of enadenotucirev in combination with nivolumab was not determined. Administration of enadenotucirev using a “low-high-high” dosing schedule allowed higher doses of enadenotucirev (3×1012 and 6×1012 vp) to be administered on days 3 and 5 without the cytokine response associated with flat dosing at these levels. A dosing regimen of 1×1012 vp on day 1 followed by 6×1012 vp on days 3 and 5 is greater than the previously determined MTD of enadenotucirev—3×1012 vp when administered in a “flat” dosing regimen.10
In response to a DLT of acute kidney injury early in the enrollment to cohort 5, additional renal monitoring was implemented to help manage and mitigate against the risk of acute kidney injury. Notably, while proteinuria was recorded in a large proportion of patients in this study (67%), this was generally grade 1 or 2, transient, and not associated with clinical sequelae. A platform analysis of cases of acute kidney injury with enadenotucirev and related vectors has since revealed a pattern consistent with a diagnosis of post-infectious glomerulonephritis. Monitoring and risk mitigations have been successfully implemented in ongoing studies of T-SIGn vectors. Mitigation measures include frequent monitoring for proteinuria, blood pressure, and complement C3/C4, with prompt use of systemic corticosteroid therapy and nephrology referral if post-infectious glomerulonephritis is suspected. In addition, patients with prior grade 3–4 acute kidney injury or other clinically significant renal impairment are not considered eligible for T-SIGn studies.
During this study, a signal for frequently prolonged aPTT without discernible clinical sequelae was identified. Further analysis of this signal identified a pattern consistent with the presence of transient APLAs,19 which is a phenomenon known to occur following wild-type virus infection20 21 or treatment/vaccination with viral vectors.22–24 Infection-induced APLAs are generally transient and not associated with thrombotic risk21 and therefore do not meet the criteria established for diagnosing antiphospholipid syndrome.25 Based on the literature, the general level of risk of bleeding and clotting from infection-related APLAs is low, and therefore the risk to patients in clinical studies of enadenotucirev or other related vectors is believed to be small.19
Median PFS and ORR among patients with CRC in this study were similar to those seen in previous studies with regorafenib and immune checkpoint inhibitors in similar patient populations.17 18 26 A single patient achieved PR (duration of response: 9.8 months); this patient also demonstrated persistent increases in inflammatory cytokines (IFNγ, IL-12p70, IL-17A) from ~day 15, sustained out to day 544 (last measurement). Core needle biopsies were not available for the patient who achieved PR.
Median OS was longer in the SPICE study (16 months) than in the CORRECT study (median OS 6.4 months with regorafenib; 12-month OS rate 24%),26 and studies of immune checkpoint inhibitors in patients with metastatic CRC (range across studies: median OS 6.2–8.9 months).17 18 However, these data should be interpreted with caution for a number of reasons including differing patient populations and the potential impact of post-progression therapy, which was not available in detail following study treatment progression.
Enadenotucirev genomic DNA was detected in 19% patients with post-dose biopsies available (cycle 2 days 8–15); these data, together with those from previous studies,9 demonstrate that enadenotucirev can be effectively delivered intravenously with localization to tumors. The small size of core needle biopsies in this study and heterogenous nature of viral infection likely explain why the presence of virus or evidence of viral replication were not detected in small core needle biopsies from all patients.
Encouragingly, data from this study have shown that enadenotucirev in combination with nivolumab can induce immune cell infiltration and activation in some patients, with a high proportion of evaluable patients showing increased CD8+ T-cell infiltration (12/14 patients) and markers of T-cell cytolytic activity (7/14 patients) in post-treatment biopsies. Pro-inflammatory tumor phenotypes have been shown to have a prognostic impact in cancer.16 In addition, infiltration of immune cells into the tumor, immune cell activation, and immune cell proliferation have been identified as predictors of response to immunotherapy.27 Despite this, no relationship between CD8 T-cell infiltration and response or OS was seen in this small immature data set. These data highlight that in advanced cancer population, immune activation alone may not be sufficient to induce a response and that further targeting of resistance pathways and tumor intrinsic factors may be required.
Conclusions
This study demonstrated that intravenous enadenotucirev has a manageable tolerability profile when administered in combination with nivolumab. Enadenotucirev in combination with nivolumab demonstrated encouraging OS and induced immune cell infiltration and activation in this patient population, the majority of whom had MSS/MSI-L CRC. Together, these results suggest that enadenotucirev is a promising tumor-selective platform that can be further adapted to create viral vectors which encode immunostimulatory payloads to increase tumor microenvironment re-programming and clinical efficacy. Studies of next-generation variants of enadenotucirev (T-SIGn vectors) designed to further re-program the tumor microenvironment by expressing immune-enhancer transgenes are ongoing.
Acknowledgments
HB acknowledges the K-12 grant Program, K12CA090628, MCCC.
Footnotes
Contributors: All authors were involved in drafting of manuscript and revising it critically for important intellectual content and approved the final version to be published. Conception and design of the study: MF, WH, TL, DK, LR. Study supervision and collection of data: MF, WH, DM, HB, JB, JC, LR. Analysis and interpretation of data: MF, WH, DM, HB, JB, TL, DK, CC, MPa, LP, MPo, LR. TL is guarantor for this manuscript.
Funding: This study was funded by Akamis Bio Ltd, in collaboration with Bristol Myers Squibb.
Competing interests: MF: Honoraria (advisory, speakers bureau): Amgen, Inc. Consulting: AstraZeneca, Bayer Corporation, Bristol Myers Squibb, Incyte Corporation, Mirati Therapeutics, Inc., Akamis Bio Ltd, Taiho Oncology. Advisory: Bayer Corporation, Roche/Genentech, Xenthera. Advisory board: Mirati Therapeutics Inc., Nouscom. Editorial board: Mirati Therapeutics Inc. Grants to institutions: Bristol Myers Squibb (study-related drugs provided to Institution), Genentech, Verastem. WH: None. DM: Speakers Bureau: Caris Life Sciences and Guardant Health. Steering Committee: Janssen. HB: Consultancy: Myovant, Corea Therapeutics, Novocure, Coherus BioSciences. Speakers Bureau: Guardant360. Research funds: Blue Earth, Novocure, Spirita Oncology. Tumor board: CARIS. Steering Committee/Medical Advisory Board: Novocure, Virogin Biotech, Idera, and JS InnoPharm. JB: Advisory board member: Insmed, Bayer, Mirati, Ipsen, QED, Oxford Biotherapeutics. Data and Safety Monitoring Board: Novocure, Pancreatic Cancer Action Network, Karyopharm. Research funding to institution: I-Mab, Dragonfly, Astellas, Atreca, AbbVie, Pfizer, Karyopharm, Boston Biomedical, Akamis Bio Ltd, EMD Serono, BMS; Grants (to Institution): Novartis, Abbvie, Bayer, Lilly, Incyte, EMD Serono, Dragonfly, I-Mab, Incyte, Pfizer, BMS, Transcenta, Totus, Tyra, 23 and me, Sumitomo Dainippon Pharma; Advisory board: Bayer, Mirati, Insmed, Oxford Biotherapeutics, Biosapien, EMD Serono, Ipsen, Merck, Merus; Data safety monitoring board: AstraZeneca, Novocure; Scientific and medical advisory board: Pancreatic Cancer Action Network, Debbie’s Dream Foundation; Nominating committee: ASCO. LR: Akamis Bio Ltd: Research Funding to Institution. TL, DK, CC, JC, MPa, LP, MPo: employees and stock options, Akamis Bio Ltd.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. The data sets generated and/or analyzed during the current study are not publicly available; however, any reasonable requests for access to available data underlying the results reported in this article will be considered. Such proposals should be submitted to the corresponding author.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by the WCG Institutional Review Board (IRB00000533), the UCLA Institutional Review Board (IRB00000173), the Vanderbilt Institutional Review Board (IRB00002125), and the Henry Ford Institutional Review Board (IRB00000253). Participants gave informed consent to participate in the study before taking part.
References
- 1.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627–39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leighl NB, Hellmann MD, Hui R, et al. Pembrolizumab in patients with advanced non-small-cell lung cancer (KEYNOTE-001): 3-year results from an open-label, phase 1 study. Lancet Respir Med 2019;7:347–57. 10.1016/S2213-2600(18)30500-9 [DOI] [PubMed] [Google Scholar]
- 3.Cohen EEW, Soulières D, Le Tourneau C, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet 2019;393:156–67. 10.1016/S0140-6736(18)31999-8 [DOI] [PubMed] [Google Scholar]
- 4.Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017;376:1015–26. 10.1056/NEJMoa1613683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawazoe A, Kuboki Y, Shinozaki E, et al. Multicenter phase I/II trial of napabucasin and pembrolizumab in patients with metastatic colorectal cancer (EPOC1503/SCOOP trial). Clin Cancer Res 2020;26:5887–94. 10.1158/1078-0432.CCR-20-1803 [DOI] [PubMed] [Google Scholar]
- 6.Kuhn I, Harden P, Bauzon M, et al. Directed evolution generates a novel oncolytic virus for the treatment of colon cancer. PLoS One 2008;3:e2409. 10.1371/journal.pone.0002409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Y, Seymour L, Fisher K. Activity of a group B oncolytic adenovirus (ColoAd1) in whole human blood. Gene Ther 2014;21:440–3. 10.1038/gt.2014.2 [DOI] [PubMed] [Google Scholar]
- 8.Dyer A, Di Y, Calderon H, et al. Oncolytic group B adenovirus enadenotucirev mediates non-apoptotic cell death with membrane disruption and release of inflammatory mediators. Mol Ther Oncolytics 2017;4:18–30. 10.1016/j.omto.2016.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Carbonero R, Salazar R, Duran I, et al. Phase 1 study of intravenous administration of the chimeric adenovirus enadenotucirev in patients undergoing primary tumor resection. J Immunother Cancer 2017;5:71. 10.1186/s40425-017-0277-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Machiels J-P, Salazar R, Rottey S, et al. A phase 1 dose escalation study of the oncolytic adenovirus enadenotucirev, administered intravenously to patients with epithelial solid tumors (EVOLVE). J Immunother Cancer 2019;7:20. 10.1186/s40425-019-0510-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moreno V, Barretina-Ginesta M-P, García-Donas J, et al. Safety and efficacy of the tumor-selective adenovirus enadenotucirev with or without paclitaxel in platinum-resistant ovarian cancer: a phase 1 clinical trial. J Immunother Cancer 2021;9:e003645. 10.1136/jitc-2021-003645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zamarin D, Holmgaard RB, Subudhi SK, et al. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci Transl Med 2014;6:226ra32. 10.1126/scitranslmed.3008095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woller N, Gürlevik E, Fleischmann-Mundt B, et al. Viral infection of tumors overcomes resistance to PD-1-immunotherapy by broadening neoantigenome-directed T-cell responses. Mol Ther 2015;23:1630–40. 10.1038/mt.2015.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European Journal of Cancer 2009;45:228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 15.Bohnsack O, Hoos A, Ludajic K. Adaptation of the immune related response criteria: irrecist. Ann Oncol 2014;25:iv369. 10.1093/annonc/mdu342.23 [DOI] [Google Scholar]
- 16.Thorsson V, Gibbs DL, Brown SD, et al. The immune landscape of cancer. Immunity 2018;48:812–30. 10.1016/j.immuni.2018.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eng C, Kim TW, Bendell J, et al. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol 2019;20:849–61. 10.1016/S1470-2045(19)30027-0 [DOI] [PubMed] [Google Scholar]
- 18.Lee JJ, Sun W, Bahary N, et al. Phase 2 study of pembrolizumab in combination with azacitidine in subjects with metastatic colorectal cancer. JCO 2017;35:3054. 10.1200/JCO.2017.35.15_suppl.3054 [DOI] [Google Scholar]
- 19.Khalil DN, Prieto González-Albo I, Rosen L, et al. A tumor-selective adenoviral vector platform induces transient antiphospholipid antibodies, without increased risk of thrombosis, in phase 1 clinical studies. Invest New Drugs 2023:1–7. 10.1007/s10637-023-01345-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaeger U, Kapiotis S, Pabinger I, et al. Transient lupus anticoagulant associated with hypoprothrombinemia and factor XII deficiency following adenovirus infection. Ann Hematol 1993;67:95–9. 10.1007/BF01788133 [DOI] [PubMed] [Google Scholar]
- 21.Asherson RA, Cervera R. Antiphospholipid antibodies and infections. Ann Rheum Dis 2003;62:388–93. 10.1136/ard.62.5.388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malaeb BS, Gardner TA, Margulis V, et al. Elevated activated partial thromboplastin time during administration of first-generation adenoviral vectors for gene therapy for prostate cancer: identification of lupus anticoagulants. Urology 2005;66:830–4. 10.1016/j.urology.2005.04.041 [DOI] [PubMed] [Google Scholar]
- 23.Ledgerwood JE, Costner P, Desai N, et al. A replication defective recombinant ad5 vaccine expressing ebola virus GP is safe and immunogenic in healthy adults. Vaccine 2010;29:304–13. 10.1016/j.vaccine.2010.10.037 [DOI] [PubMed] [Google Scholar]
- 24.Crank MC, Wilson EMP, Novik L, et al. Safety and immunogenicity of a rad35-enva prototype HIV-1 vaccine in combination with rad5-enva in healthy adults (VRC 012). PLoS One 2016;11:e0166393. 10.1371/journal.pone.0166393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006;4:295–306. 10.1111/j.1538-7836.2006.01753.x [DOI] [PubMed] [Google Scholar]
- 26.Grothey A, Cutsem EV, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. The Lancet 2013;381:303–12. 10.1016/S0140-6736(12)61900-X [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Endres S, Kobold S. Enhancing tumor T cell infiltration to enable cancer immunotherapy. Immunotherapy 2019;11:201–13. 10.2217/imt-2018-0111 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2022-006561supp001.pdf (4.3MB, pdf)
Data Availability Statement
Data are available upon reasonable request. The data sets generated and/or analyzed during the current study are not publicly available; however, any reasonable requests for access to available data underlying the results reported in this article will be considered. Such proposals should be submitted to the corresponding author.