Abstract
Objective
To review the comparative effectiveness of osteoporosis treatments, including the bone anabolic agents, abaloparatide and romosozumab, on reducing the risk of fractures in postmenopausal women, and to characterise the effect of antiosteoporosis drug treatments on the risk of fractures according to baseline risk factors.
Design
Systematic review, network meta-analysis, and meta-regression analysis of randomised clinical trials.
Data sources
Medline, Embase, and Cochrane Library to identify randomised controlled trials published between 1 January 1996 and 24 November 2021 that examined the effect of bisphosphonates, denosumab, selective oestrogen receptor modulators, parathyroid hormone receptor agonists, and romosozumab compared with placebo or active comparator.
Eligibility criteria for selecting studies
Randomised controlled trials that included non-Asian postmenopausal women with no restriction on age, when interventions looked at bone quality in a broad perspective. The primary outcome was clinical fractures. Secondary outcomes were vertebral, non-vertebral, hip, and major osteoporotic fractures, all cause mortality, adverse events, and serious cardiovascular adverse events.
Results
The results were based on 69 trials (>80 000 patients). For clinical fractures, synthesis of the results showed a protective effect of bisphosphonates, parathyroid hormone receptor agonists, and romosozumab compared with placebo. Compared with parathyroid hormone receptor agonists, bisphosphonates were less effective in reducing clinical fractures (odds ratio 1.49, 95% confidence interval 1.12 to 2.00). Compared with parathyroid hormone receptor agonists and romosozumab, denosumab was less effective in reducing clinical fractures (odds ratio 1.85, 1.18 to 2.92 for denosumab v parathyroid hormone receptor agonists and 1.56, 1.02 to 2.39 for denosumab v romosozumab). An effect of all treatments on vertebral fractures compared with placebo was found. In the active treatment comparisons, denosumab, parathyroid hormone receptor agonists, and romosozumab were more effective than oral bisphosphonates in preventing vertebral fractures. The effect of all treatments was unaffected by baseline risk indicators, except for antiresorptive treatments that showed a greater reduction of clinical fractures compared with placebo with increasing mean age (number of studies=17; β=0.98, 95% confidence interval 0.96 to 0.99). No harm outcomes were seen. The certainty in the effect estimates was moderate to low for all individual outcomes, mainly because of limitations in reporting, nominally indicating a serious risk of bias and imprecision.
Conclusions
The evidence indicated a benefit of a range of treatments for osteoporosis in postmenopausal women for clinical and vertebral fractures. Bone anabolic treatments were more effective than bisphosphonates in the prevention of clinical and vertebral fractures, irrespective of baseline risk indicators. Hence this analysis provided no clinical evidence for restricting the use of anabolic treatment to patients with a very high risk of fractures.
Systematic review registration
PROSPERO CRD42019128391.
Introduction
Advances in research have led to a more accurate assessment of the risk of fractures, and the range of treatment options available to prevent fractures has expanded. Algorithms on the risk of fractures that combine clinical risk factors and bone mineral density are now widely used in clinical practice to target treatment to individuals at high risk of fractures.1 Although drug treatments targeted at osteoporosis consistently improve bone mineral density, preventing fractures is the most relevant patient outcome.2 Heterogeneity has been noted for the magnitude of the reduction in the risk of vertebral, non-vertebral, hip, and clinical fractures between treatments. Few active comparator trials have directly compared the effects on fracture endpoints.3 4 Greater understanding of the differences in the effects of treatments across clinical trials would influence estimates of the benefits of treatment and should therefore be considered among the evidence base that drives guideline recommendations.
Moreover, most randomised controlled trials included patients with an estimated high baseline risk of fractures, but this varied between treatments and over time. Existing post hoc analyses indicate that the antifracture efficacy of some treatments for osteoporosis differ according to estimates of the baseline risk of fractures of individuals in the study, typically, but not exclusively, calculated with the fracture risk assessment tool (FRAX).5 6 7 8 9 10 Therefore, factors such as history of fractures, age, bone mineral density, and body mass index, among others, might be potential effect modifiers.
In this analysis, we looked at several baseline risk indicators associated with the efficacy of drug treatments to assess the evidence of the effect and harms of available osteoporosis treatments on primary and secondary reduction of the risk of fractures among postmenopausal women. We also critically appraised the internal validity of the randomised controlled trials.11 We used meta-regression analyses to explore the evidence of the effect of antiosteoporosis drug treatments on the risk of fracture according to recognised baseline risk factors.
Methods
Our results are reported, and our analyses conducted, in accordance with the guidelines of the Cochrane Collaboration and the Preferred Reporting Items for Systematic review and Meta-analysis (PRISMA) for Network Meta-Analysis,12 13 and structured according to the population, intervention, comparison, and outcome framework.14 The protocol was registered and accepted in March 2019. Minor protocol changes were made: harm outcomes were evaluated post hoc and antiresorptive or bone anabolic drugs were grouped in the meta-regression analyses to increase statistical power.
Eligibility criteria
We considered randomised controlled trials that included postmenopausal women (with no restriction on the definition of sex or gender), with no restriction on age, and where interventions considered bone mineral density or fractures. Because the doses used in randomised controlled trials in Asian settings are different from doses used in the rest of the world, we excluded studies performed exclusively in Asian settings. Trials in mixed populations were included if the data were reported for the populations of interest separately.
The primary outcome was all clinical fractures (excluding fingers and toes), and secondary outcomes were vertebral fractures (clinical, morphometric, or both), non-vertebral fractures, hip fractures, and major osteoporotic fractures, as defined in the randomised controlled trials. Harm outcomes were all cause mortality, number of patients with any adverse events, and number of patients with serious cardiovascular adverse events. The time frame was the longest follow-up after the start of the preplanned intervention. Interventions considered for inclusion were bisphosphonates (alendronate, risedronate, ibandronate, and zoledronate), denosumab, selective oestrogen receptor modulators (raloxifene hydrochloride, bazedoxifene, and bazedoxifene with conjugated oestrogen), parathyroid hormone receptor agonists (teriparatide and abaloparatide), and sclerostin inhibitor (romosozumab). Studies were included if they examined the effects compared with placebo or with an active comparator. Calcium and vitamin D supplementation were allowed as co-interventions. Studies examining sequential treatment or combination treatment were also considered for inclusion. No restrictions were set on dose or length of treatment. The baseline risk indicators considered were previous history of fractures, mean age, mean spine T score, mean body mass index, and mean FRAX score for major osteoporotic fractures.
Information sources and search strategy
One of the authors (MNH) performed the literature search on 24 November 2021. Databases searched were Medline and Embase via Ovid, and the Cochrane Central Register of Controlled Trials (CENTRAL)15 (acceptable coverage for musculoskeletal disorders has been shown16). The search strategy (table S1) included medical subject headings and text words related to the population, intervention, comparison, and outcome framework, and was restricted to human and published studies written in English from 1 January 1996 onwards. Reference lists of previous published systematic reviews and meta-analysis, and of the included studies were screened. Content experts ensured that any relevant studies were not missed by the search.
Selection of studies
Duplicates were identified and excluded in EndNote. The remaining references were imported to Covidence (www.covidence.org/home); two reviewers independently screened titles and abstracts, followed by screening of the full text. Disagreements were resolved by discussion. Conference abstracts were considered if data were not published elsewhere.
Data extraction
Study data were extracted with a predefined extraction template in Covidence. Extraction of background data was performed by one reviewer and extraction of quantitative data was independently performed by two reviewers. Disagreements were resolved by discussion. Information from journal article(s), conference abstract(s), trial protocol, or trial registry record was used as sources in the data extraction and the risk of bias assessment. Authors were contacted by email to provide more information to resolve uncertainties or obtain missing data (table S2). No deadlines were given. One author provided data on hip fractures among postmenopausal women.17 When multiple reports of one study were identified, the publication with the longest follow-up and the most complete data was included, and if all studies had complete information, these studies were treated as one study with reference made to all of the publications. Intention-to-treat analyses were prioritised in the data extraction. In multi-arm trials, results from treatments that were the same but at various doses were combined into one group. In four studies,18 19 20 21 missing data on lumbar spine bone mineral density T score were replaced with estimates calculated by Bouxsein et al.22
Critical appraisal of reporting in individual studies
The Cochrane risk of bias tool 223 (parallel trials) was used for critical appraisal of the reporting of the included studies. Two reviewers independently conducted a risk of bias appraisal. Discrepancies were resolved by discussion.
Statistical analysis
For the meta-analysis, dichotomous outcomes were analysed by calculating the relative risk for the direct comparisons (with 95% confidence intervals). Relative risk was also converted into the corresponding anticipated absolute risk in the study population, for each 1000 individuals,24 calculated as the difference between the baseline risk of the outcome (median in the control group) and the risk of outcome after the intervention was applied. The I2 statistic was used to measure the proportion of total variability caused by heterogeneity between the trials.25 Heterogeneity between studies was quantified by the estimate τ2. An inverse variance random effects model was applied as the default to allow for heterogeneity in treatment effects across trials.
Subgroup analysis by risk of bias was planned but was not considered feasible because most of the included studies were rated as having some concerns or had a high risk of bias. The meta-analyses, funnel plots, and forest plots were produced in Review Manager Software (version 5.2, Nordic Cochrane Centre, Cochrane Collaboration, Copenhagen, Denmark). Statistical power is usually too low to distinguish chance from real asymmetry, so we did not perform any tests for funnel plot asymmetry because most of the meta-analysis subgroups were based on <10 trials (data points).
In a network meta-analysis, when two or more drugs are compared with a common standard, the difference in effect between these drugs with respect to the common standard forms the basis of indirect comparisons (ie, formation of a star network). In our analysis, most drug treatments were compared with placebo and the same baseline treatment. We used the star design26 for indirect treatment comparisons and included one active and one placebo group from each available trial, independent of concomitant drug treatment use.27 To analyse fracture outcomes, we calculated odds ratios by default after use of a random effects network meta-analysis model with binomial likelihood and logit link. For the primary (arm based) network meta-analyses, we used generalised linear mixed models combining a series of 2×2 tables, with the odds ratio modelled as a linear combination of study level covariates and random effects, representing variation between studies.28 Although the prior choice for heterogeneity between studies is critical in bayesian network meta-analysis with empirical Bayes methods,29 the prior probability distribution for heterogeneity between studies is estimated from the data.30 Fitting the network meta-analysis model estimates the summary treatment effects for each drug relative to others, allowing for clustering of patients and drugs within trials, and for heterogeneity between trials in treatment effects (as measured by τ2, assuming the same for every treatment effect). Furthermore, to assess the robustness of these results, we performed sensitivity analyses adjusting each group for the length of the study multiplied by the specific number of participants randomly allocated (ie, a proxy for patient years).
We ranked clinical efficacy with rankograms, surface under the cumulative ranking, and average ranks. The transitivity assumption was evaluated by comparing the distribution of clinical and methodological variables that could act as effect modifiers, whereas statistical consistency (ie, agreement between direct and indirect evidence) was evaluated with node splitting.
In the meta-regression analysis, for each combination of outcome and baseline risk indicator, we performed a meta-regression with restricted maximum likelihood estimation as part of mixed linear models. The resulting slope β indicates the increase (or decrease) in treatment effect in terms of log risk ratio. For ease of interpretation, we used back transformation, so the slope is interpreted as the proportional increase (or decrease) in the treatment effect (ie, risk ratio) per unit increase in the baseline risk indicator. A slope of exp(β)=1 indicates no association with the treatment effect. For brevity, exp(β) is β.
We performed separate analyses by type of treatment and comparator group in the following groups to increase statistical power: antiresorptive drugs (selective oestrogen receptor modulators, bisphosphonates, and denosumab) versus placebo; bisphosphonates versus placebo; anabolic treatments (romosozumab and parathyroid hormone receptor agonists) versus placebo; and anabolic treatments (romosozumab and parathyroid hormone receptor agonists) versus bisphosphonates. We quantified inconsistency across trials with the standard I2 statistic, describing the percentage of total variation caused by heterogeneity rather than chance.31 We estimated the variation explained by each baseline risk indicator by %τ2 explained=(τ2 0−τ2)/τ2 0×100%, where τ2 0 is the variation between trials for the meta-regression without the baseline risk indicator in the model. Statistical analyses were performed in R (version 3.6.1)32 33 and SAS (version 9.4). Grading of recommendations, assessment, development, and evaluations (GRADE) fitted to the network meta-analysis was used to rate the overall certainty of evidence for each outcome.34
Patient and public involvement
Owing to lack of funding, patients and members of the public were not involved in the design, conduct, or reporting of this study.
Results
Technical assessment
Literature search and study selection
We identified 6244 records after removing duplicates. Screening of titles and abstracts excluded 5447 records, and in the remaining 797, the full text was screened, resulting in exclusion of 639 references. When references were screened, we identified three more records eligible for inclusion. Table S3 lists the reasons for exclusion. In total, we identified 161 references3 4 5 7 8 9 10 17 18 19 20 21 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99 100 101 102 103 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 119 120 121 122 123 124 125 126 127 128 129 130 131 132 133 134 135 136 137 138 139 140 141 142 143 144 145 146 147 148 149 150 151 152 153 154 155 156 157 158 159 160 161 162 163 164 165 166 167 168 169 170 171 172 173 174 175 176 177 178 179 180 181 182 183 providing information about 69 distinct trials. Figure 1 is a flowchart of the included studies.
Fig 1.
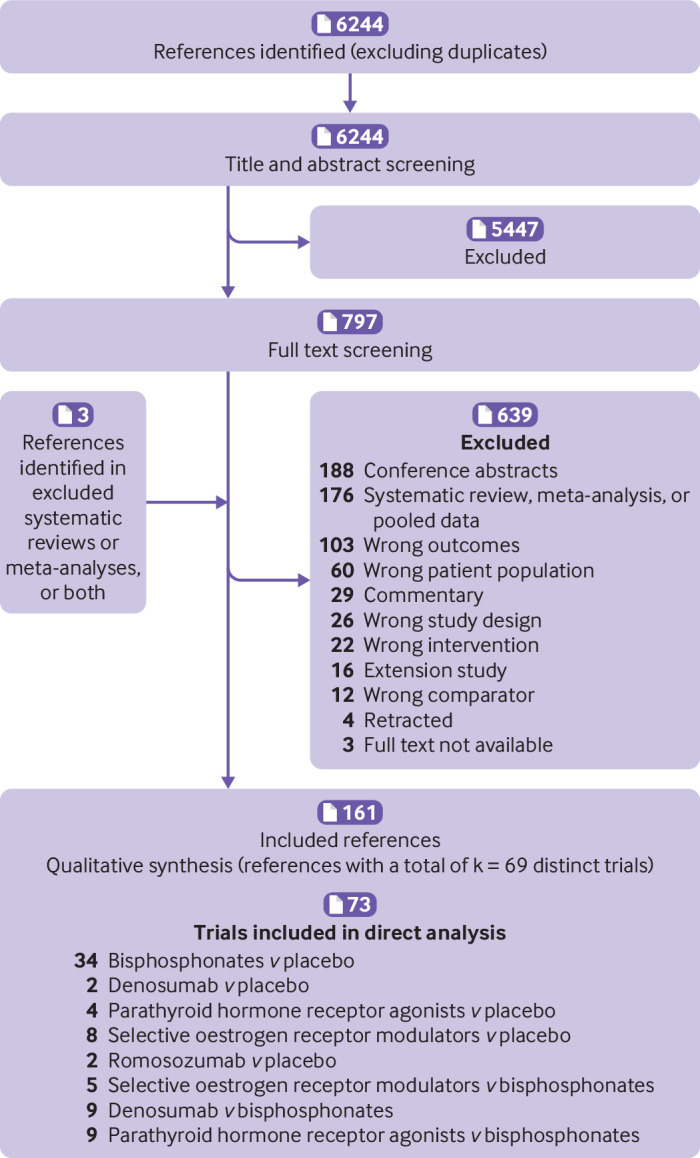
Flowchart of included studies. Numbers=number of records; k=number of distinct trials
Study characteristics
The included studies were published between 1 January 1996 and 24 November 2021. Table S4 provides a further description of the included studies. Table S5 details the role of funding sources and potential conflicts of interest.
Data completeness
Thirty four studies reported on clinical fractures, 40 reported on vertebral fractures, 52 on non-vertebral fractures, 30 on hip fractures, and nine studies reported on major osteoporotic fractures. For data completeness for the baseline risk indicators, 52 studies reported on a history of fractures (75%), all studies reported mean age (100%), 49 studies reported spine T scores (71%), 51 studies reported body mass index (74%), and six studies reported FRAX scores (9%). The prevalence of a history of fractures ranged from 0 to 100% in the study populations, mean age from 51.5 to 85.5 years, mean spine T score from −0.4 to −3.9, mean body mass index from 23.7 to 29.1, and mean FRAX score for the probability of a major osteoporotic fracture within the next 10 years from 13.2% to 30%.
Certainty of evidence
The overall GRADE evaluation of the certainty in the effect estimates was moderate to low for all individual outcomes because of the serious risk of bias and imprecision (table 1 and secondary outcomes in table S6).
Table 1.
Estimates of effects and quality ratings for comparison of drug treatments for osteoporosis to prevent clinical fractures
| Comparison | Direct evidence | Network meta-analysis | |||
|---|---|---|---|---|---|
| Relative risk (95% CI) |
Absolute risk difference* (95% CI) |
Odds ratio (95% CI) |
Certainty of evidence | ||
| Parathyroid hormone receptor agonists v placebo | 0.58 (0.35 to 0.95) | 35 fewer per 1000 (39 fewer to 3 fewer)† | — | Moderate¶ | |
| Selective oestrogen receptor modulators v placebo | 0.41 (0.10 to 1.69) | — | — | Low¶ ** | |
| Romosozumab v placebo | 0.64 (0.47 to 0.89) | 9 fewer per 1000 (13 fewer to 3 fewer)‡ | — | Low¶ ** | |
| Parathyroid hormone receptor agonists v bisphosphonates | 0.61 (0.39 to 0.94) | — | — | Moderate¶ | |
| Denosumab v bisphosphonates | 1.28 (0.91 to 1.81) | — | — | Low¶ ** | |
| Romosozumab v bisphosphonates | 0.82 (0.68 to 0.99) | — | — | Low¶ ** | |
| Romosozumab v parathyroid hormone receptor agonists | 0.88 (0.32 to 2.37) | — | — | Low¶ ** | |
| Bisphosphonates v denosumab | — | — | 0.81 (0.57 to 1.15) | Low¶ ** | |
| Bisphosphonates v placebo | 0.81 (0.72 to 0.91) | 14 fewer per 1000 (21 fewer to 7 fewer)§ | 0.79 (0.70 to 0.89) | Moderate¶ | |
| Bisphosphonates v parathyroid hormone receptor agonists | — | — | 1.49 (1.12 to 2.00) | Moderate¶ | |
| Bisphosphonates v romosozumab | — | — | 1.26 (0.99 to 1.60) | Low¶ ** | |
| Bisphosphonates v selective oestrogen receptor modulators | 0.96 (0.48 to 1.94) | — | 1.40 (0.72 to 2.71) | Low¶ ** | |
| Denosumab v placebo | 3.08 (0.42 to 22.33) | — | 0.98 (0.68 to 1.41) | Low¶ ** | |
| Denosumab v parathyroid hormone receptor agonists | — | — | 1.85 (1.18 to 2.92) | Moderate¶ | |
| Denosumab v romosozumab | — | — | 1.56 (1.02 to 2.39) | Moderate¶ | |
| Denosumab v selective oestrogen receptor modulators | — | — | 1.74 (0.82 to 3.66) | Low¶ ** | |
| Placebo v parathyroid hormone receptor agonists | — | — | 1.90 (1.41 to 2.55) | Moderate¶ | |
| Placebo v romosozumab | — | — | 1.60 (1.24 to 2.05) | Low¶ ** | |
| Placebo v selective oestrogen receptor modulators | — | — | 1.78 (0.91 to 3.47) | Low¶ ** | |
| Parathyroid hormone receptor agonists v romosozumab | — | — | 0.84 (0.59 to 1.21) | Low¶ ** | |
| Parathyroid hormone receptor agonists v selective oestrogen receptor modulators | — | — | 0.94 (0.46 to 1.93) | Low¶ ** | |
| Romosozumab v selective oestrogen receptor modulators | — | — | 1.11 (0.55 to 2.25) | Low¶ ** | |
CI=confidence interval.
Absolute measure of intervention effects is difference between baseline risk of outcome (median in control group) and risk of outcome after intervention is applied.
The serious risk of bias was mainly because of unclear reporting of how random sequence and allocation concealment were performed (table S7). Some studies also had incomplete outcome data74 83 105 129 145 151 and selective outcome reporting74 83 105 129 145 151 (table S7). Potential involvement of the funding parties was judged to increase the risk of bias related to conflict of interest. A serious risk of imprecision was assigned for outcomes where data were available from one study only. From visual inspection of the funnel plots, we did not detect evidence of small study effects (fig S1).
Clinical efficacy
Synthesis of results
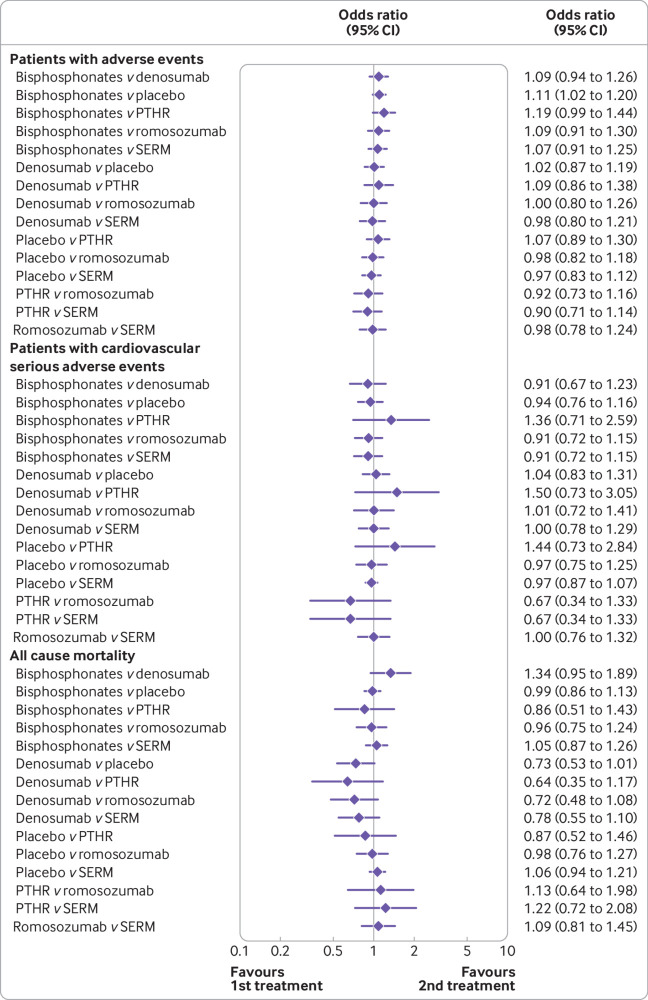
All osteoporosis treatments had at least one placebo controlled trial, and all treatments were directly compared with at least one active drug in any of the networks (fig 2), except in the analyses of major osteoporotic fractures. Figure 3 and figure 4 show the results from the network meta-analysis for all outcomes. Table S8 shows the sensitivity analyses. Figure S4, table S9, and table S10 present the rankogram, mean ranks, and surface under the cumulative ranking values that summarise the evidence and comparisons. Parathyroid hormone receptor agonists had the highest rankogram and surface under the cumulative ranking value, and the lowest mean rank, indicating better ranking of the treatment (fig S4).
Fig 2.
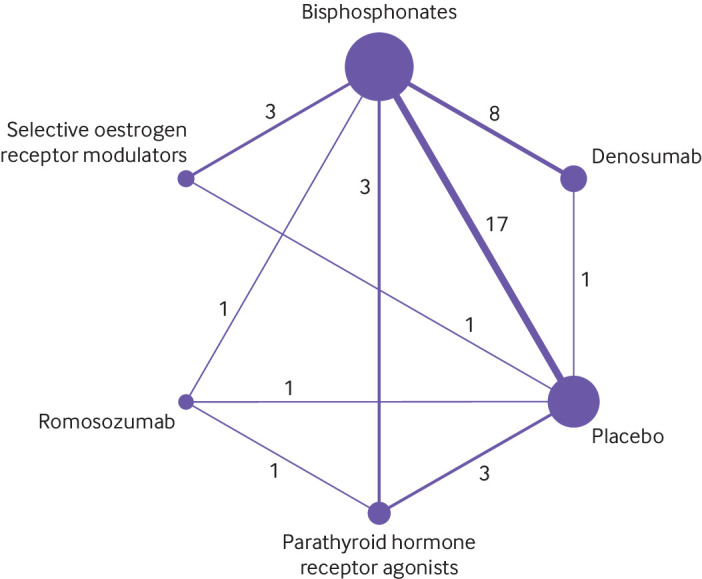
Network plot of studies included in network meta-analysis on clinical fractures. Each circle represents an intervention and is referred to as a node. Nodes are sized proportionally to the number of trials that included each intervention. Lines between nodes represent direct comparisons, and their thickness is proportional to the number of trials contributing to each comparison. Number of trials for each direct comparison is shown. No connecting line between two treatments indicates no direct comparison
Fig 3.
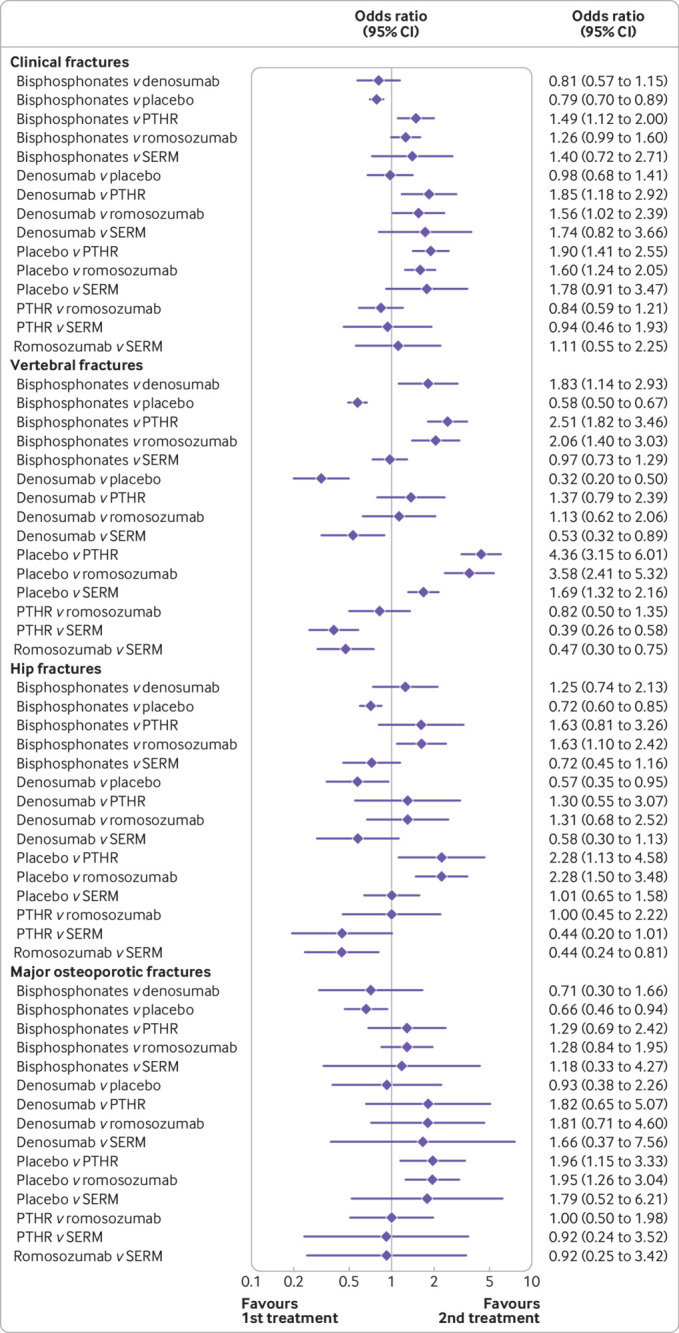
Network meta-analysis for clinical, vertebral, hip, and major osteoporotic fractures. PTHR=parathyroid hormone receptor agonists; SERM=selective oestrogen receptor modulators; CI=confidence interval
Fig 4.
Network meta-analysis for safety outcomes. PTHR=parathyroid hormone receptor agonists; SERM=selective oestrogen receptor modulators; CI=confidence interval
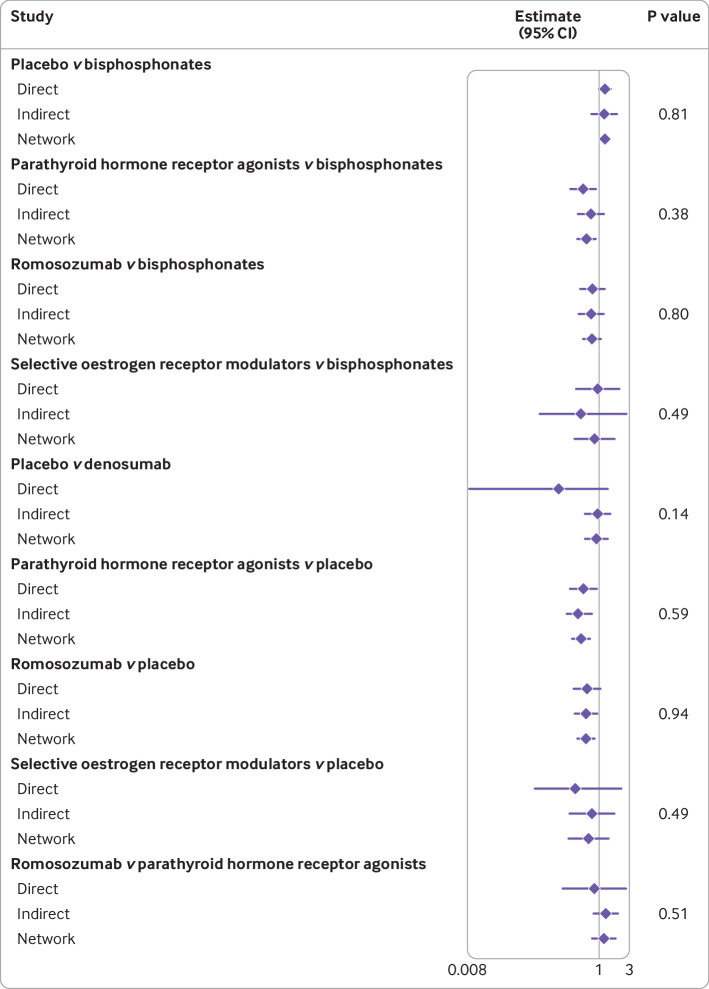
Figure 5 is a forest plot illustrating the results of node splitting, comparing the direct, indirect, and network estimates. For our network meta-analysis, we found no indication of inconsistency between direct and indirect evidence (fig 5), and we considered that the relevant effect modifiers were balanced across the different comparisons. Figure S3 reports the potential baseline risk indicators facilitating the judgments about the assumption of transitivity.
Fig 5.
Forest plot illustrating the result of node splitting, comparing direct, indirect, and network estimates. CI=confidence interval
Clinical fractures (prespecified primary outcome)
For clinical fractures, the network meta-analysis showed a protective effect of bisphosphonates, parathyroid hormone receptor agonists, and romosozumab compared with placebo, but not of denosumab and selective oestrogen receptor modulators (fig 3). Analysis of the data for denosumab did not include the FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months) pivotal trial, however, because of lack of aggregated data for clinical fractures in the publication. Compared with parathyroid hormone receptor agonists, bisphosphonates were less effective in reducing clinical fractures (odds ratio for bisphosphonates v parathyroid hormone receptor agonist 1.49, 95% confidence interval 1.12 to 2.00). Compared with parathyroid hormone receptor agonists and romosozumab, denosumab was less effective in reducing clinical fractures (odds ratios for denosumab v parathyroid hormone receptor agonist1.85, 95% confidence interval 1.18 to 2.92 and v romosozumab 1.56, 1.02 to 2.39). The results were robust after adjustment for patient years (table S8).
Vertebral fractures (secondary outcome)
We found an effect of all treatments on vertebral fractures compared with placebo. In the active treatment comparisons, denosumab, parathyroid hormone receptor agonists, and romosozumab were more effective in preventing vertebral fractures than bisphosphonates (fig 3). The results were robust to adjustment for patient years (table S8).
Non-vertebral fractures (secondary meta-analysis outcome)
Network meta-analyses could not be performed for non-vertebral fractures.
Hip fractures (secondary outcome)
The network meta-analysis showed a protective effect of bisphosphonates, denosumab, parathyroid hormone receptor agonists, and romosozumab for hip fractures compared with placebo, but not of selective oestrogen receptor modulators (fig 3). In the active treatment comparisons, romosozumab was more effective in preventing hip fractures than oral bisphosphonates or selective oestrogen receptor modulators (fig 3). The results were robust to adjustment for patient years (table S8).
Major osteoporotic fractures (secondary outcome)
This outcome was reported in only a small number of trials, which limited the power of the analysis. For major osteoporotic fractures, the network meta-analysis showed a protective effect of bisphosphonates, parathyroid hormone receptor agonists, and romosozumab compared with placebo, but not of denosumab or selective oestrogen receptor modulators (fig 3). We found no differences in the active treatment comparisons.
Safety outcomes
Compared with placebo or other comparators, the active treatments did not increase the risk of all cause mortality, number of patients with any adverse events, or number of patients with serious cardiovascular adverse events (fig 4 and table S8).
Meta-regression analyses
The effect of all treatments was unaffected by the baseline risk indicators (table S11), except for antiresorptive treatments that showed a greater reduction of clinical fractures compared with placebo with increasing mean age (β=0.98, 95% confidence interval 0.96 to 0.99, τ2 explained=97%, P=0.031, based on 17 studies) (fig 6 and table S11).
Fig 6.
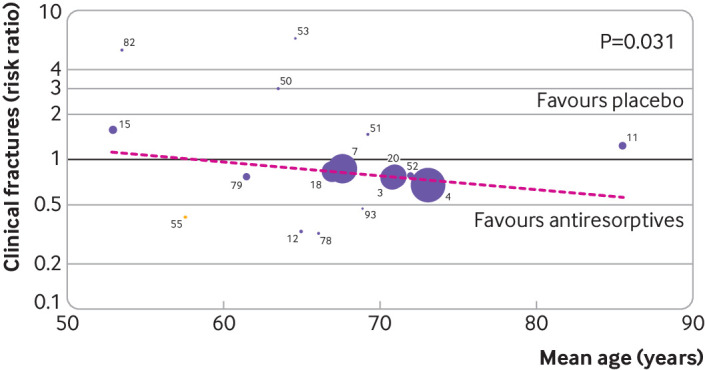
Meta-regression on risk of clinical fractures with baseline mean age as risk indicator, for antiresorptive agents (selective oestrogen receptor modulators, bisphosphonates, and denosumab) versus placebo. Based on restricted maximum likelihood based meta-regression for association between (log risk ratio) clinical fractures and mean age. Bisphosphonates versus placebo are indicated by purple dots and selective oestrogen receptor modulators versus placebo by yellow dot; colours do not reflect the applied model and are only for illustrative purposes. Meta-regression was done on log risk ratio scale, but for ease of interpretation, the back transformed risk ratio is shown. Identification number in figure, trial name, and reference: 3=FIT1 (Fracture Intervention Trial 1)18; 4=HORIZON-PFT (Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly Pivotal Fracture Trial)19; 7=FIT2 (Fracture Intervention Trial 2)20; 11=ZEST (Zoledronic acid in frail Elders to STrengthen bone)85; 12=ACTRN1260700057642687; 15=Hosking 199821; 18=IBAN IV183; 20=ACTRN1260900593235139; 50=ACTRN1260500027863988; 51=Hosking 200393; 52=Greenspan 200386; 53=Downs 200073; 55=Miller 2008121; 78=Bell 200244; 79=Bone 200049; 82=McClung 2009182; 93=NCT00271713165
Discussion
Treatment options for postmenopausal osteoporosis have increased considerably in the past 20 years. No Cochrane-type reviews or meta-analyses on this topic, however, have been done recently.184 185 Although effective, relatively safe, and affordable treatments are available,186 187 188 189 190 191 192 193 194 195 196 197 198 199 the treatment of patients at high risk of fractures needs to be looked at and the best interventions identified. Our network meta-analyses support a beneficial effect of most, but not all, treatments on all fracture outcomes compared with placebo. These treatments have already been approved by the appropriate authorities in Europe, the US, and elsewhere for the treatment of postmenopausal osteoporosis.
Although most randomised controlled trials have preferentially included patients with a high baseline risk of fractures, the prevalence of these patients has varied between different treatments and studies. Higher baseline risk is a major factor for the absolute risk of fractures, but in most studies the relative reduction in the risk of fractures was found to be mostly independent of baseline risk factors. Confirmation that the approved treatments reduce the risk of fractures compared with placebo is not surprising, but whether all treatments are equally effective is an interesting question. The network meta-analyses showed that bone anabolic treatments (teriparatide62 63 83 89 129 200 and romosozumab4 113) reduced the risk of clinical and vertebral fractures compared with bisphosphonates. The certainty of the pooled results on bone anabolic treatments, especially romosozumab, however, was found to be low because of the small number of studies identified.
Head-to-head randomised controlled trials are more challenging than studies comparing active treatment with placebo. In head-to-head trials, the number of individuals with fractures is much lower, and therefore these studies might have low statistical power and are also unlikely to be replicated because of the financial and operational challenges. Hence most head-to-head randomised controlled trials were done comparing anabolic and antiresorptive treatments, where a clinically relevant difference in antifracture efficacy was anticipated.
Because of the lack of consistent reporting on non-vertebral fractures, hip fractures, and major osteoporotic fractures across studies, we could not draw more definite conclusions. The VERO (VERtebral fracture treatment comparisons in Osteoporotic women) trial is an illustrative example of the effect of different definitions of groups of fractures. When non-vertebral major osteoporotic fractures were defined according to the European Medicine Agency (hip, radius, humerus, ribs, pelvis, femur, and tibia), the reduction seen with teriparatide compared with risedronate was not significant, but when major osteoporotic fractures were defined according to FRAX (clinical, vertebral, hip, humerus, and forearm fractures), the reduction was significant (hazard ratio 0.40, P<0.001).168 Estimates for non-vertebral, hip, and major osteoporotic fractures were somewhat uncertain because of low statistical power and varying definitions, but the results were largely in agreement with estimates for clinical and vertebral fractures.
Meta-regression analysis
The benefits of antiresorptive agents in general, and bisphosphonates in particular, as well as bone anabolic treatments, seemed to be independent of baseline risk indicators at the study level. Nevertheless, the meta-regression analyses showed that antiresorptive treatments (bisphosphonates, selective oestrogen receptor modulators, and denosumab) seemed more effective in reducing the risk of clinical fractures with increasing mean age (mean age reported in studies ranged from 50 to 85 years), indicated by the estimated slope <1 and that including mean age in the model reduced the variance between studies. This observation is important because a common belief is that the oldest patients might not benefit from osteoporosis treatment, whereas the evidence provided here suggests that antiresorptive treatments might be even more effective in reducing clinical fractures in this high risk population. The results of the meta-regression are vulnerable to aggregation bias and study level confounding, however, and need to be confirmed in studies of individual patient data.
Bone anabolic treatments reduced the risk of fractures more than antiresorptive agents in postmenopausal women, and their comparative efficacy was largely independent of baseline risk indicators. The results for bone anabolic treatments were based on seven included studies, however, and only a modest spread of risk factors between studies was found, making it more difficult to detect these associations. Individual comparison trials have shown the greater benefits of teriparatide or romosozumab compared with oral bisphosphonates alone in high risk groups, characterised by 100% presence of vertebral fractures at baseline.3 4 83 Our data suggest that the advantage of bone anabolic treatment versus antiresorptive treatment might not be restricted to the highest risk groups. The reason for recommending bone anabolic treatments specifically in patients at high risk of fractures is therefore based more on cost considerations (ie, lower cost per fracture avoided if the fracture rate is high) than on robust evidence favouring its use in this group over others. With the introduction of biosimilars and generics of teriparatide at a lower cost, our results could prompt a review of current guidelines for an earlier use of these agents in the treatment of osteoporosis.
Strengths and limitations of included studies
We used standardised methods allowing us to evaluate the certainty of the results. The potential risks of bias identified across several of the included studies, predominantly in the form of selective reporting, lowered the rate of certainty in the effect estimates of the outcomes. Other reporting items might have favoured newer studies because older studies would not always have anticipated a future standard. Reporting items that might have favoured newer studies were domains related to the description of randomisation sequence generation and allocation concealment (selection bias), but not the domains related to incomplete outcome data, selective outcome reporting (post hoc analyses), and potential active involvement of funding parties. We adhered to best practice and reviewed only the quality of published scientific papers. The supposedly poor quality of reporting in randomised controlled trials is not unique to drug trials of osteoporosis and is a common problem across medical disciplines.201 202 Efforts towards more transparent and stringent reporting are urgently needed.
Strengths and limitations of systematic review
Along with the 2020 update to the Endocrine Society’s guidelines for the treatment of postmenopausal osteoporosis,203 204 our analysis included the recently launched drugs, abaloparatide and romosozumab, and presented an up-to-date and comprehensive systematic review of all available head-to-head trials in this field. A major strength of our systematic review was that the methods were rigorous and transparent, with a priori defined criteria in accordance with standardised guidelines. Other strengths were the large number of randomised controlled trials included and restriction of our patient population to postmenopausal women, which ensured robust results and reduced the heterogeneity caused by sex and comorbidity.
The network meta-analysis and meta-regression analysis were limited by a substantial amount of missing data on outcomes and baseline risk indicators of interest, which required combining treatment groups on an ad hoc basis to make the best use of the number of data points. Also, we did not differentiate between outcomes reported as adverse events, or primary or secondary outcomes, resulting in various and non-standardised definitions of fractures across studies. In our network meta-analysis, denosumab did not significantly reduce the risk of clinical fractures compared with placebo. Critically, the FREEDOM study from 2009, which was pivotal for almost all approvals made for denosumab, did not provide the outcomes included in the analysis. We cannot exclude the possibility that data on adverse events could have been inadequately monitored and infrequently reported, further introducing bias. Because we relied on published mean baseline characteristics, instead of individual patient data, a risk of aggregation bias exists that could increase or decrease the associations found. Furthermore, meta-regression analyses, despite including only randomised controlled trials, are observational and the results might be confounded by other characteristics.205 Interpretation might also be complicated by overlapping outcomes (eg, in some studies non-vertebral fractures would also count as clinical fractures80 87 104) and overlapping treatment groups.
Directions for future research
Future research could include individual patient data from the trials to advance our understanding of the influence of baseline risk indicators on the efficacy of treatments. Progress has been made for antiresorptive treatments by the Foundation for the National Institutes of Health Bone Quality project, where individual patient data were collected for 28 000 participants in 11 trials of bisphosphonates and selective oestrogen receptor modulators.206 We encourage randomised controlled trial data to be made available to provide the evidence needed for a personalised approach to the management of osteoporosis.
Conclusion
The current available evidence indicates that, despite the varying quality of the reported studies, most approved treatments for postmenopausal osteoporosis are beneficial for all types of fractures, with head-to-head trials favouring bone anabolic treatments over bisphosphonates in the prevention of clinical and vertebral fractures, and romosozumab followed by alendronate over alendronate in the prevention of hip fractures in patients at high risk of fractures. Overall, the nominal certainty in the evidence was rated down based on the GRADE criteria because of the serious risk of bias across all treatment combinations and outcomes. For the bone anabolic treatments, a risk of imprecision also existed because only a few studies were available.
The results of the meta-regression analysis showed that treatments were beneficial in reducing the risk of fractures in postmenopausal women, and the effect was mostly independent of baseline risk indicators. Treatment with bone anabolic agents effectively reduced clinical and vertebral fractures, irrespective of mean age and baseline risk, whereas antiresorptive treatments seemed more effective in older patients. Because bone anabolic treatments were more effective than bisphosphonates, irrespective of the baseline risk, no evidence from clinical trials exists supporting the view that bone anabolic treatment should be limited to patients at very high risk of fractures because of efficacy.
What is already known on this topic
Treatment options for postmenopausal osteoporosis have increased considerably in the past 20 years
No Cochrane-type reviews or meta-analyses on this topic have been done recently
Treatment of patients at high risk of fractures needs to be looked at and the best interventions identified
What this study adds
Most approved treatments of postmenopausal osteoporosis for all types of fractures were beneficial, with head-to-head trials favouring bone anabolic treatments over bisphosphonates in the prevention of clinical and vertebral fractures
Overall, the nominal certainty of the evidence was rated down because of the serious risk of bias and risk of imprecision
Results from the meta-regression analysis showed that treatments were beneficial in reducing the risk of fractures in postmenopausal women, and the effect was mostly independent of baseline risk indicators
Web extra.
Extra material supplied by authors
Web appendix: Supplementary material
Contributors: SLF, BL, and BA share last authorship. All authors made substantial contributions to the conception and design. MNH developed the search strategy, and the search strategy was approved by all authors. All authors were involved in conducting the research, including identification of relevant studies. MNH, IC, CvB, JFR, and AU extracted the data. MNH, SMN, and RC performed the statistical analyses. The original draft was prepared by MNH. All authors contributed to data analysis and interpretation, critically revised the manuscript, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work. MNH is the guarantor (the contributor who accepts full responsibility for the finished article, had access to any data, and controlled the decision to submit for publish). MNH, IC, CvB, JFT, AU, SMN, and RC had full access to the data, and the data were available to all authors on request. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: Resources to conduct and publish the systematic review were provided by UCB (Allée de la Recherche 60, 1070 Brussels, Belgium) and Amgen (Thousand Oaks, CA 91320-1799). The Parker Institute, Bispebjerg and Frederiksberg Hospital, is supported by a core grant from the Oak Foundation (OCAY-18-774-OFIL). None of the funding sources had any influence on study design, or preparation of this systematic review, and had no influence on the collection, analysis, interpretation of the data, writing of the systematic review, or decisions on publishing the results.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare support from UCB and Amgen for the submitted work: MNH, IC, CvB, JFR, AU, SMN, and RC report grants from UCB and Amgen paid to the Parker Institute to conduct the study; J-JB reports personal fees from UCB during the conduct of the study and personal fees from UCB and Sandoz outside the submitted work; MLB reports fees as honorarium, speaker, grants, and consultant from Amgen, Bruno Farmaceutici, Calcilytix, Kyowa Kirin, UCB, Abiogen, Alexion, Echolight, Eli Lilly, Kyowa Kirin, SPA, Theramex, and Amolyt outside the submitted work; AD-P has received speaker fees from Amgen, Lilly, and Theramex and is a shareholder of Active Life; PH reports personal fees from UCB during the conduct of the study and personal fees from UCB, Amgen, Gedeon Richter, Stada, and Theramex outside the submitted work; MKJ reports personal fees and non-financial support from UCB during the conduct of the study, and grants, personal fees, and non-financial support from Amgen and UCB outside the submitted work; MKJ was also supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre and the views expressed are those of the author and not necessarily those of the NHS, the NIHR, or the Department of Health; WFL reports speakers fee/advisory board from UCB, Amgen, Pfizer, and Lilly; XN has received fees for consulting from UCB and Amgen and for lectures from UCB, Amgen, and Lilly; CR reports personal fees from UCB during the conduct of the study, and grants and personal fees from Alexion, Regeneron, Sanofi, and Amgen outside the submitted work; SM reports fees as speaker and advisory board from Abiogen, Amgen, Bruno Farmaceutici, Diasorin, Eli Lilly, Shire, Sandoz, Takeda, Abiogen, Kyowa Kirin, Pfizer and UCB outside the submitted work; TT reports personal fees from UCB during the conduct of the study, personal fees from Amgen, Arrow, and Biogen, personal fees from Grunenthal, Jansen, LCA, Lilly, MSD, Nordic, Novartis, Pfizer, Sanofi, Thuasne, and Theramex, grants and personal fees from Chugai and UCB, and grants from Bone Therapeutics outside the submitted work; SLF reports grants from Amgen, consulting and lecture honorarium from UCB, consulting honorarium from Radius, and grants and consulting honorarium from Agnovos outside the submitted work; DP-A's department received consultancy fees related to this work, DP-A reports grants and fees for speaker services and advisory board membership from Amgen, grants, non-financial support, and fees for consultancy services from UCB Biopharma, grants from Les Laboratoires Servier and UCB outside the submitted work, DP-A is an HTA Funding Committee membership, and Janssen, on behalf of Innovative Medicines Initiative (IMI) funded European Health Data and Evidence Network (EHDEN) and European Medical Information Framework (EMIF) consortiums, and Synapse Management Partners, have supported training programmes organised by DP-A's department that are open to external participants; BL reports personal fees from UCB during the conduct of the study and BL has received funding to her institution from Amgen and Novo Nordisk and personal fees from Amgen, UCB, Eli Lilly, Gedeon-Richter, and Gilead outside the submitted work; BA reports personal fees from UCB during the conduct of the study, grants and personal fees from UCB and Kyowa-Kirin UK, personal fees from Amgen, grants from Novartis, and grants and personal fees from Pharmacosmos outside the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
The lead author (the manuscript’s guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned and registered have been explained.
Dissemination to participants and related patient and public communities: This research will be reported in press releases and presented at scientific meetings, including the Cochrane Colloquium. The research will be shared with the Committee of National Societies of the International Osteoporosis Foundation (IOF) for communication to patient societies.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
Not required.
Data availability statement
The statistical code and dataset are available from the corresponding author.
References
- 1. Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet 2019;393:364-76. 10.1016/S0140-6736(18)32112-3 [DOI] [PubMed] [Google Scholar]
- 2. Wells GA, Cranney A, Peterson J, et al. Alendronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev 2008;(1):CD001155. 10.1002/14651858.CD001155.pub2 [DOI] [PubMed] [Google Scholar]
- 3. Kendler DL, Marin F, Zerbini CAF, et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet 2018;391:230-40. 10.1016/S0140-6736(17)32137-2 [DOI] [PubMed] [Google Scholar]
- 4. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or Alendronate for Fracture Prevention in Women with Osteoporosis. N Engl J Med 2017;377:1417-27. 10.1056/NEJMoa1708322 [DOI] [PubMed] [Google Scholar]
- 5. Harvey NC, Kanis JA, Odén A, et al. Efficacy of weekly teriparatide does not vary by baseline fracture probability calculated using FRAX. Osteoporos Int 2015;26:2347-53. 10.1007/s00198-015-3129-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kanis JA, Johansson H, Oden A, McCloskey EV. A meta-analysis of the effect of strontium ranelate on the risk of vertebral and non-vertebral fracture in postmenopausal osteoporosis and the interaction with FRAX. Osteoporos Int 2011;22:2347-55. 10.1007/s00198-010-1474-0 [DOI] [PubMed] [Google Scholar]
- 7. Kaufman JM, Palacios S, Silverman S, Sutradhar S, Chines A. An evaluation of the Fracture Risk Assessment Tool (FRAX) as an indicator of treatment efficacy: the effects of bazedoxifene and raloxifene on vertebral, nonvertebral, and all clinical fractures as a function of baseline fracture risk assessed by FRAX. Osteoporos Int 2013;24:2561-9. 10.1007/s00198-013-2341-6 [DOI] [PubMed] [Google Scholar]
- 8. McCloskey EV, Johansson H, Oden A, et al. Denosumab reduces the risk of osteoporotic fractures in postmenopausal women, particularly in those with moderate to high fracture risk as assessed with FRAX. J Bone Miner Res 2012;27:1480-6. 10.1002/jbmr.1606 [DOI] [PubMed] [Google Scholar]
- 9. McCloskey EV, Johansson H, Oden A, et al. The Effect of Abaloparatide-SC on Fracture Risk Is Independent of Baseline FRAX Fracture Probability: A Post Hoc Analysis of the ACTIVE Study. J Bone Miner Res 2017;32:1625-31. 10.1002/jbmr.3163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cosman F, Crittenden DB, Ferrari S, et al. Romosozumab FRAME Study: A Post Hoc Analysis of the Role of Regional Background Fracture Risk on Nonvertebral Fracture Outcome. J Bone Miner Res 2018;33:1407-16. 10.1002/jbmr.3439 [DOI] [PubMed] [Google Scholar]
- 11. Schünemann HJ, Tugwell P, Reeves BC, et al. Non-randomized studies as a source of complementary, sequential or replacement evidence for randomized controlled trials in systematic reviews on the effects of interventions. Res Synth Methods 2013;4:49-62. 10.1002/jrsm.1078 [DOI] [PubMed] [Google Scholar]
- 12. Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 2021;372:n160. 10.1136/bmj.n160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777-84. 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 14. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol 2011;64:395-400. 10.1016/j.jclinepi.2010.09.012 [DOI] [PubMed] [Google Scholar]
- 15.Lefebvre C, Glanville J, Briscoe S, et al. Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, et al (eds). Cochrane Handbook for Systematic Reviews of Interventions version 6.2. Cochrane, 2021. https://www.training.cochrane.org/handbook.
- 16. Aagaard T, Lund H, Juhl C. Optimizing literature search in systematic reviews - are MEDLINE, EMBASE and CENTRAL enough for identifying effect studies within the area of musculoskeletal disorders? BMC Med Res Methodol 2016;16:161. 10.1186/s12874-016-0264-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Malouf-Sierra J, Tarantino U, García-Hernández PA, et al. Effect of Teriparatide or Risedronate in Elderly Patients With a Recent Pertrochanteric Hip Fracture: Final Results of a 78-Week Randomized Clinical Trial. J Bone Miner Res 2017;32:1040-51. 10.1002/jbmr.3067 [DOI] [PubMed] [Google Scholar]
- 18. Black DM, Cummings SR, Karpf DB, et al. Fracture Intervention Trial Research Group . Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 1996;348:1535-41. 10.1016/S0140-6736(96)07088-2 [DOI] [PubMed] [Google Scholar]
- 19. Black DM, Delmas PD, Eastell R, et al. HORIZON Pivotal Fracture Trial . Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007;356:1809-22. 10.1056/NEJMoa067312 [DOI] [PubMed] [Google Scholar]
- 20. Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 1998;280:2077-82. 10.1001/jama.280.24.2077 [DOI] [PubMed] [Google Scholar]
- 21. Hosking D, Chilvers CE, Christiansen C, et al. Early Postmenopausal Intervention Cohort Study Group . Prevention of bone loss with alendronate in postmenopausal women under 60 years of age. N Engl J Med 1998;338:485-92. 10.1056/NEJM199802193380801 [DOI] [PubMed] [Google Scholar]
- 22. Bouxsein ML, Eastell R, Lui LY, et al. FNIH Bone Quality Project . Change in Bone Density and Reduction in Fracture Risk: A Meta-Regression of Published Trials. J Bone Miner Res 2019;34:632-42. 10.1002/jbmr.3641 [DOI] [PubMed] [Google Scholar]
- 23. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 24. Guyatt GH, Oxman AD, Santesso N, et al. GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes. J Clin Epidemiol 2013;66:158-72. 10.1016/j.jclinepi.2012.01.012 [DOI] [PubMed] [Google Scholar]
- 25. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 26.Wells GA, Sultan SA, Chen L, Khan A, Coyle D. Indirect evidence: indirect treatment comparisons in meta-analysis. 1-94. 2009. Ottawa, Canada, Canadian Agency for Drugs and Technologies in Health. 7-2-2022. [Google Scholar]
- 27. Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 1997;50:683-91. 10.1016/S0895-4356(97)00049-8 [DOI] [PubMed] [Google Scholar]
- 28. Platt RW, Leroux BG, Breslow N. Generalized linear mixed models for meta-analysis. Stat Med 1999;18:643-54. [DOI] [PubMed] [Google Scholar]
- 29. Rosenberger KJ, Xing A, Murad MH, Chu H, Lin L. Prior Choices of Between-Study Heterogeneity in Contemporary Bayesian Network Meta-analyses: an Empirical Study. J Gen Intern Med 2021;36:1049-57. 10.1007/s11606-020-06357-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Casella G. An Introduction to Empirical Bayes Data Analysis. Am Stat 1985;39:83-87. [Google Scholar]
- 31. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. 2019.
- 33.R package 'metafor' https://www.jstatsoft.org/article/view/v036i03. 2020.
- 34. Puhan MA, Schünemann HJ, Murad MH, et al. GRADE Working Group . A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014;349:g5630. 10.1136/bmj.g5630 [DOI] [PubMed] [Google Scholar]
- 35. Adachi JD, Faraawi RY, O’Mahony MF, et al. Upper gastrointestinal tolerability of alendronate sodium monohydrate 10 mg once daily in postmenopausal women: a 12-week, randomized, double-blind, placebo-controlled, exploratory study. Clin Ther 2009;31:1747-53. 10.1016/j.clinthera.2009.08.016 [DOI] [PubMed] [Google Scholar]
- 36. Adami S, San Martin J, Muñoz-Torres M, et al. Effect of raloxifene after recombinant teriparatide [hPTH(1-34)] treatment in postmenopausal women with osteoporosis. Osteoporos Int 2008;19:87-94. 10.1007/s00198-007-0485-y [DOI] [PubMed] [Google Scholar]
- 37. Adami S, Libanati C, Boonen S, et al. FREEDOM Fracture-Healing Writing Group . Denosumab treatment in postmenopausal women with osteoporosis does not interfere with fracture-healing: results from the FREEDOM trial. J Bone Joint Surg Am 2012;94:2113-9. 10.2106/JBJS.K.00774 [DOI] [PubMed] [Google Scholar]
- 38. Agnusdei D, Iori N. Raloxifene: results from the MORE study. J Musculoskelet Neuronal Interact 2000;1:127-32. [PubMed] [Google Scholar]
- 39. Anastasilakis AD, Polyzos SA, Gkiomisi A, et al. Denosumab versus zoledronic acid in patients previously treated with zoledronic acid. Osteoporos Int 2015;26:2521-7. 10.1007/s00198-015-3174-2 [DOI] [PubMed] [Google Scholar]
- 40. Ascott-Evans BH, Guanabens N, Kivinen S, et al. Alendronate prevents loss of bone density associated with discontinuation of hormone replacement therapy: a randomized controlled trial. Arch Intern Med 2003;163:789-94. 10.1001/archinte.163.7.789 [DOI] [PubMed] [Google Scholar]
- 41. Austin M, Yang YC, Vittinghoff E, et al. FREEDOM Trial . Relationship between bone mineral density changes with denosumab treatment and risk reduction for vertebral and nonvertebral fractures. J Bone Miner Res 2012;27:687-93. 10.1002/jbmr.1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Barrett-Connor E, Cauley JA, Kulkarni PM, Sashegyi A, Cox DA, Geiger MJ. Risk-benefit profile for raloxifene: 4-year data From the Multiple Outcomes of Raloxifene Evaluation (MORE) randomized trial. J Bone Miner Res 2004;19:1270-5. 10.1359/JBMR.040406 [DOI] [PubMed] [Google Scholar]
- 43. Bauer DC, Garnero P, Hochberg MC, et al. Fracture Intervention Research Group . Pretreatment levels of bone turnover and the antifracture efficacy of alendronate: the fracture intervention trial. J Bone Miner Res 2006;21:292-9. 10.1359/JBMR.051018 [DOI] [PubMed] [Google Scholar]
- 44. Bell NH, Bilezikian JP, Bone HG, 3rd, Kaur A, Maragoto A, Santora AC, MK-063 Study Group . Alendronate increases bone mass and reduces bone markers in postmenopausal African-American women. J Clin Endocrinol Metab 2002;87:2792-7. 10.1210/jcem.87.6.8575 [DOI] [PubMed] [Google Scholar]
- 45. Bilezikian JP, Hattersley G, Mitlak BH, et al. Abaloparatide in patients with mild or moderate renal impairment: results from the ACTIVE phase 3 trial. Curr Med Res Opin 2019;35:2097-102. 10.1080/03007995.2019.1656955 [DOI] [PubMed] [Google Scholar]
- 46. Bjarnason NH, Sarkar S, Duong T, Mitlak B, Delmas PD, Christiansen C. Six and twelve month changes in bone turnover are related to reduction in vertebral fracture risk during 3 years of raloxifene treatment in postmenopausal osteoporosis. Osteoporos Int 2001;12:922-30. 10.1007/s001980170020 [DOI] [PubMed] [Google Scholar]
- 47. Body JJ, Gaich GA, Scheele WH, et al. A randomized double-blind trial to compare the efficacy of teriparatide [recombinant human parathyroid hormone (1-34)] with alendronate in postmenopausal women with osteoporosis. J Clin Endocrinol Metab 2002;87:4528-35. 10.1210/jc.2002-020334 [DOI] [PubMed] [Google Scholar]
- 48. Bone HG, Downs RW, Jr, Tucci JR, et al. Alendronate Elderly Osteoporosis Study Centers . Dose-response relationships for alendronate treatment in osteoporotic elderly women. J Clin Endocrinol Metab 1997;82:265-74. 10.1210/jc.82.1.265 [DOI] [PubMed] [Google Scholar]
- 49. Bone HG, Greenspan SL, McKeever C, et al. Alendronate/Estrogen Study Group . Alendronate and estrogen effects in postmenopausal women with low bone mineral density. J Clin Endocrinol Metab 2000;85:720-6. 10.1210/jc.85.2.720 [DOI] [PubMed] [Google Scholar]
- 50. Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women. J Clin Endocrinol Metab 2008;93:2149-57. 10.1210/jc.2007-2814 [DOI] [PubMed] [Google Scholar]
- 51. Boonen S, Marin F, Mellstrom D, et al. Safety and efficacy of teriparatide in elderly women with established osteoporosis: bone anabolic therapy from a geriatric perspective. J Am Geriatr Soc 2006;54:782-9. 10.1111/j.1532-5415.2006.00695.x [DOI] [PubMed] [Google Scholar]
- 52. Boonen S, Adachi JD, Man Z, et al. Treatment with denosumab reduces the incidence of new vertebral and hip fractures in postmenopausal women at high risk. J Clin Endocrinol Metab 2011;96:1727-36. 10.1210/jc.2010-2784 [DOI] [PubMed] [Google Scholar]
- 53. Brown JP, Prince RL, Deal C, et al. Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res 2009;24:153-61. 10.1359/jbmr.0809010 [DOI] [PubMed] [Google Scholar]
- 54. Bruyère O, Detilleux J, Chines A, Reginster JY. Relationships between changes in bone mineral density or bone turnover markers and vertebral fracture incidence in patients treated with bazedoxifene. Calcif Tissue Int 2012;91:244-9. 10.1007/s00223-012-9629-y [DOI] [PubMed] [Google Scholar]
- 55. Cauley JA, Black D, Boonen S, et al. HORIZON Pivotal Fracture Group . Once-yearly zoledronic acid and days of disability, bed rest, and back pain: randomized, controlled HORIZON Pivotal Fracture Trial. J Bone Miner Res 2011;26:984-92. 10.1002/jbmr.292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chapurlat RD, Palermo L, Ramsay P, Cummings SR. Risk of fracture among women who lose bone density during treatment with alendronate. The Fracture Intervention Trial. Osteoporos Int 2005;16:842-8. 10.1007/s00198-004-1770-7 [DOI] [PubMed] [Google Scholar]
- 57. Chen P, Miller PD, Delmas PD, Misurski DA, Krege JH. Change in lumbar spine BMD and vertebral fracture risk reduction in teriparatide-treated postmenopausal women with osteoporosis. J Bone Miner Res 2006;21:1785-90. 10.1359/jbmr.060802 [DOI] [PubMed] [Google Scholar]
- 58. Chesnut CH, Ettinger MP, Miller PD, et al. Ibandronate produces significant, similar antifracture efficacy in North American and European women: new clinical findings from BONE. Curr Med Res Opin 2005;21:391-401. 10.1185/030079905X30752 [DOI] [PubMed] [Google Scholar]
- 59. Chesnut CH, 3rd, Skag A, Christiansen C, et al. Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE) . Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res 2004;19:1241-9. 10.1359/JBMR.040325 [DOI] [PubMed] [Google Scholar]
- 60. Clemmesen B, Ravn P, Zegels B, Taquet AN, Christiansen C, Reginster JY. A 2-year phase II study with 1-year of follow-up of risedronate (NE-58095) in postmenopausal osteoporosis. Osteoporos Int 1997;7:488-95. 10.1007/PL00004152 [DOI] [PubMed] [Google Scholar]
- 61. Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N Engl J Med 2016;375:1532-43. 10.1056/NEJMoa1607948 [DOI] [PubMed] [Google Scholar]
- 62. Cosman F, Eriksen EF, Recknor C, et al. Effects of intravenous zoledronic acid plus subcutaneous teriparatide [rhPTH(1-34)] in postmenopausal osteoporosis. J Bone Miner Res 2011;26:503-11. 10.1002/jbmr.238 [DOI] [PubMed] [Google Scholar]
- 63. Cosman F, Nieves J, Zion M, Woelfert L, Luckey M, Lindsay R. Daily and cyclic parathyroid hormone in women receiving alendronate. N Engl J Med 2005;353:566-75. 10.1056/NEJMoa050157 [DOI] [PubMed] [Google Scholar]
- 64. Cosman F, Wermers RA, Recknor C, et al. Effects of teriparatide in postmenopausal women with osteoporosis on prior alendronate or raloxifene: differences between stopping and continuing the antiresorptive agent. J Clin Endocrinol Metab 2009;94:3772-80. 10.1210/jc.2008-2719 [DOI] [PubMed] [Google Scholar]
- 65. Cosman F, Nieves J, Woelfert L, et al. Parathyroid hormone added to established hormone therapy: effects on vertebral fracture and maintenance of bone mass after parathyroid hormone withdrawal. J Bone Miner Res 2001;16:925-31. 10.1359/jbmr.2001.16.5.925 [DOI] [PubMed] [Google Scholar]
- 66. Cosman F, Hattersley G, Hu MY, Williams GC, Fitzpatrick LA, Black DM. Effects of Abaloparatide-SC on Fractures and Bone Mineral Density in Subgroups of Postmenopausal Women With Osteoporosis and Varying Baseline Risk Factors. J Bone Miner Res 2017;32:17-23. 10.1002/jbmr.2991 [DOI] [PubMed] [Google Scholar]
- 67. Cosman F, Crittenden DB, Ferrari S, et al. FRAME Study: The Foundation Effect of Building Bone With 1 Year of Romosozumab Leads to Continued Lower Fracture Risk After Transition to Denosumab. J Bone Miner Res 2018;33:1219-26. 10.1002/jbmr.3427 [DOI] [PubMed] [Google Scholar]
- 68. Cummings SR, San Martin J, McClung MR, et al. FREEDOM Trial . Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009;361:756-65. 10.1056/NEJMoa0809493 [DOI] [PubMed] [Google Scholar]
- 69. Delmas PD, Licata AA, Reginster JY, et al. Fracture risk reduction during treatment with teriparatide is independent of pretreatment bone turnover. Bone 2006;39:237-43. 10.1016/j.bone.2006.02.003 [DOI] [PubMed] [Google Scholar]
- 70. Delmas PD, Recker RR, Chesnut CH, 3rd, et al. Daily and intermittent oral ibandronate normalize bone turnover and provide significant reduction in vertebral fracture risk: results from the BONE study. Osteoporos Int 2004;15:792-8. 10.1007/s00198-004-1602-9 [DOI] [PubMed] [Google Scholar]
- 71. Delmas PD, Genant HK, Crans GG, et al. Severity of prevalent vertebral fractures and the risk of subsequent vertebral and nonvertebral fractures: results from the MORE trial. Bone 2003;33:522-32. 10.1016/S8756-3282(03)00241-2 [DOI] [PubMed] [Google Scholar]
- 72. Donaldson MG, Palermo L, Ensrud KE, Hochberg MC, Schousboe JT, Cummings SR. Effect of alendronate for reducing fracture by FRAX score and femoral neck bone mineral density: the Fracture Intervention Trial. J Bone Miner Res 2012;27:1804-10. 10.1002/jbmr.1625 [DOI] [PubMed] [Google Scholar]
- 73. Downs RW, Jr, Bell NH, Ettinger MP, et al. Comparison of alendronate and intranasal calcitonin for treatment of osteoporosis in postmenopausal women. J Clin Endocrinol Metab 2000;85:1783-8. [DOI] [PubMed] [Google Scholar]
- 74. Dursun N, Dursun E, Yalçin S. Comparison of alendronate, calcitonin and calcium treatments in postmenopausal osteoporosis. Int J Clin Pract 2001;55:505-9. [PubMed] [Google Scholar]
- 75. Eastell R, Black DM, Boonen S, et al. HORIZON Pivotal Fracture Trial . Effect of once-yearly zoledronic acid five milligrams on fracture risk and change in femoral neck bone mineral density. J Clin Endocrinol Metab 2009;94:3215-25. 10.1210/jc.2008-2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ensrud KE, Stock JL, Barrett-Connor E, et al. Effects of raloxifene on fracture risk in postmenopausal women: the Raloxifene Use for the Heart Trial. J Bone Miner Res 2008;23:112-20. 10.1359/jbmr.070904 [DOI] [PubMed] [Google Scholar]
- 77. Ettinger B, Black DM, Mitlak BH, et al. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators . Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA 1999;282:637-45. 10.1001/jama.282.7.637 [DOI] [PubMed] [Google Scholar]
- 78. Felsenberg D, Miller P, Armbrecht G, Wilson K, Schimmer RC, Papapoulos SE. Oral ibandronate significantly reduces the risk of vertebral fractures of greater severity after 1, 2, and 3 years in postmenopausal women with osteoporosis. Bone 2005;37:651-4. 10.1016/j.bone.2005.05.004 [DOI] [PubMed] [Google Scholar]
- 79. Fogelman I, Ribot C, Smith R, Ethgen D, Sod E, Reginster JY, BMD-MN Study Group . Risedronate reverses bone loss in postmenopausal women with low bone mass: results from a multinational, double-blind, placebo-controlled trial. J Clin Endocrinol Metab 2000;85:1895-900. [DOI] [PubMed] [Google Scholar]
- 80. Freemantle N, Satram-Hoang S, Tang ET, et al. DAPS Investigators . Final results of the DAPS (Denosumab Adherence Preference Satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos Int 2012;23:317-26. 10.1007/s00198-011-1780-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Galesanu C. Biological therapy or bisphosphonates in postmenopausal women with osteoporosis? Calcif Tissue Int 2018;102(Suppl. 1):S137. [Google Scholar]
- 82. Gallagher JC, Genant HK, Crans GG, Vargas SJ, Krege JH. Teriparatide reduces the fracture risk associated with increasing number and severity of osteoporotic fractures. J Clin Endocrinol Metab 2005;90:1583-7. 10.1210/jc.2004-0826 [DOI] [PubMed] [Google Scholar]
- 83. Geusens P, Marin F, Kendler DL, et al. Effects of Teriparatide Compared with Risedronate on the Risk of Fractures in Subgroups of Postmenopausal Women with Severe Osteoporosis: The VERO Trial. J Bone Miner Res 2018;33:783-94. 10.1002/jbmr.3384 [DOI] [PubMed] [Google Scholar]
- 84. Greenspan SL, Parker RA, Ferguson L, Rosen HN, Maitland-Ramsey L, Karpf DB. Early changes in biochemical markers of bone turnover predict the long-term response to alendronate therapy in representative elderly women: a randomized clinical trial. J Bone Miner Res 1998;13:1431-8. 10.1359/jbmr.1998.13.9.1431 [DOI] [PubMed] [Google Scholar]
- 85. Greenspan SL, Perera S, Ferchak MA, Nace DA, Resnick NM. Efficacy and safety of single-dose zoledronic acid for osteoporosis in frail elderly women: a randomized clinical trial. JAMA Intern Med 2015;175:913-21. 10.1001/jamainternmed.2015.0747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Greenspan SL, Resnick NM, Parker RA. Combination therapy with hormone replacement and alendronate for prevention of bone loss in elderly women: a randomized controlled trial. JAMA 2003;289:2525-33. 10.1001/jama.289.19.2525 [DOI] [PubMed] [Google Scholar]
- 87. Grey A, Bolland M, Wong S, Horne A, Gamble G, Reid IR. Low-dose zoledronate in osteopenic postmenopausal women: a randomized controlled trial. J Clin Endocrinol Metab 2012;97:286-92. 10.1210/jc.2011-2081 [DOI] [PubMed] [Google Scholar]
- 88. Grey A, Bolland MJ, Wattie D, Horne A, Gamble G, Reid IR. The antiresorptive effects of a single dose of zoledronate persist for two years: a randomized, placebo-controlled trial in osteopenic postmenopausal women. J Clin Endocrinol Metab 2009;94:538-44. 10.1210/jc.2008-2241 [DOI] [PubMed] [Google Scholar]
- 89. Hadji P, Zanchetta JR, Russo L, et al. The effect of teriparatide compared with risedronate on reduction of back pain in postmenopausal women with osteoporotic vertebral fractures. Osteoporos Int 2012;23:2141-50. 10.1007/s00198-011-1856-y [DOI] [PubMed] [Google Scholar]
- 90. Harris ST, Watts NB, Genant HK, et al. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group . Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA 1999;282:1344-52. 10.1001/jama.282.14.1344 [DOI] [PubMed] [Google Scholar]
- 91. Hochberg MC, Thompson DE, Black DM, et al. FIT Research Group . Effect of alendronate on the age-specific incidence of symptomatic osteoporotic fractures. J Bone Miner Res 2005;20:971-6. 10.1359/JBMR.050104 [DOI] [PubMed] [Google Scholar]
- 92. Hooper MJ, Ebeling PR, Roberts AP, et al. Risedronate prevents bone loss in early postmenopausal women: a prospective randomized, placebo-controlled trial. Climacteric 2005;8:251-62. 10.1080/13697130500118126 [DOI] [PubMed] [Google Scholar]
- 93. Hosking D, Adami S, Felsenberg D, et al. Comparison of change in bone resorption and bone mineral density with once-weekly alendronate and daily risedronate: a randomised, placebo-controlled study. Curr Med Res Opin 2003;19:383-94. 10.1185/030079903125002009 [DOI] [PubMed] [Google Scholar]
- 94. Jacques RM, Boonen S, Cosman F, et al. Relationship of changes in total hip bone mineral density to vertebral and nonvertebral fracture risk in women with postmenopausal osteoporosis treated with once-yearly zoledronic acid 5 mg: the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res 2012;27:1627-34. 10.1002/jbmr.1644 [DOI] [PubMed] [Google Scholar]
- 95. Jamal SA, Ljunggren O, Stehman-Breen C, et al. Effects of denosumab on fracture and bone mineral density by level of kidney function. J Bone Miner Res 2011;26:1829-35. 10.1002/jbmr.403 [DOI] [PubMed] [Google Scholar]
- 96. Johnell O, Kanis JA, Black DM, et al. Associations between baseline risk factors and vertebral fracture risk in the Multiple Outcomes of Raloxifene Evaluation (MORE) Study. J Bone Miner Res 2004;19:764-72. 10.1359/jbmr.040211 [DOI] [PubMed] [Google Scholar]
- 97. Kanis JA, Johansson H, Oden A, McCloskey EV. A meta-analysis of the efficacy of raloxifene on all clinical and vertebral fractures and its dependency on FRAX. Bone 2010;47:729-35. 10.1016/j.bone.2010.06.009 [DOI] [PubMed] [Google Scholar]
- 98. Kanis JA, Johansson H, Oden A, McCloskey EV. Bazedoxifene reduces vertebral and clinical fractures in postmenopausal women at high risk assessed with FRAX. Bone 2009;44:1049-54. 10.1016/j.bone.2009.02.014 [DOI] [PubMed] [Google Scholar]
- 99. Kanis JA, Johnell O, Black DM, et al. Effect of raloxifene on the risk of new vertebral fracture in postmenopausal women with osteopenia or osteoporosis: a reanalysis of the Multiple Outcomes of Raloxifene Evaluation trial. Bone 2003;33:293-300. 10.1016/S8756-3282(03)00200-X [DOI] [PubMed] [Google Scholar]
- 100. Kanis JA, Barton IP, Johnell O. Risedronate decreases fracture risk in patients selected solely on the basis of prior vertebral fracture. Osteoporos Int 2005;16:475-82. 10.1007/s00198-004-1698-y [DOI] [PubMed] [Google Scholar]
- 101. Kendler DL, Roux C, Benhamou CL, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res 2010;25:72-81. 10.1359/jbmr.090716 [DOI] [PubMed] [Google Scholar]
- 102. Kendler DL, Chines A, Brandi ML, et al. The risk of subsequent osteoporotic fractures is decreased in subjects experiencing fracture while on denosumab: results from the FREEDOM and FREEDOM Extension studies. Osteoporos Int 2019;30:71-8. 10.1007/s00198-018-4687-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Krege JH, Wan X. Teriparatide and the risk of nonvertebral fractures in women with postmenopausal osteoporosis. Bone 2012;50:161-4. 10.1016/j.bone.2011.10.018 [DOI] [PubMed] [Google Scholar]
- 104. Langdahl BL, Libanati C, Crittenden DB, et al. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: a randomised, open-label, phase 3 trial. Lancet 2017;390:1585-94. 10.1016/S0140-6736(17)31613-6 [DOI] [PubMed] [Google Scholar]
- 105. Lewiecki EM, Miller PD, McClung MR, et al. AMG 162 Bone Loss Study Group . Two-year treatment with denosumab (AMG 162) in a randomized phase 2 study of postmenopausal women with low BMD. J Bone Miner Res 2007;22:1832-41. 10.1359/jbmr.070809 [DOI] [PubMed] [Google Scholar]
- 106. Lindsay R, Nieves J, Formica C, et al. Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet 1997;350:550-5. 10.1016/S0140-6736(97)02342-8 [DOI] [PubMed] [Google Scholar]
- 107. Lindsay R, Miller P, Pohl G, Glass EV, Chen P, Krege JH. Relationship between duration of teriparatide therapy and clinical outcomes in postmenopausal women with osteoporosis. Osteoporos Int 2009;20:943-8. 10.1007/s00198-008-0766-0 [DOI] [PubMed] [Google Scholar]
- 108. Lufkin EG, Whitaker MD, Nickelsen T, et al. Treatment of established postmenopausal osteoporosis with raloxifene: a randomized trial. J Bone Miner Res 1998;13:1747-54. 10.1359/jbmr.1998.13.11.1747 [DOI] [PubMed] [Google Scholar]
- 109. Marcus R, Wang O, Satterwhite J, Mitlak B. The skeletal response to teriparatide is largely independent of age, initial bone mineral density, and prevalent vertebral fractures in postmenopausal women with osteoporosis. J Bone Miner Res 2003;18:18-23. 10.1359/jbmr.2003.18.1.18 [DOI] [PubMed] [Google Scholar]
- 110. Maricic M, Adachi JD, Sarkar S, Wu W, Wong M, Harper KD. Early effects of raloxifene on clinical vertebral fractures at 12 months in postmenopausal women with osteoporosis. Arch Intern Med 2002;162:1140-3. 10.1001/archinte.162.10.1140 [DOI] [PubMed] [Google Scholar]
- 111. Masud T, McClung M, Geusens P. Reducing hip fracture risk with risedronate in elderly women with established osteoporosis. Clin Interv Aging 2009;4:445-9. 10.2147/CIA.S8200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. McClung MR, San Martin J, Miller PD, et al. Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med 2005;165:1762-8. 10.1001/archinte.165.15.1762 [DOI] [PubMed] [Google Scholar]
- 113. McClung MR, Grauer A, Boonen S, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med 2014;370:412-20. 10.1056/NEJMoa1305224 [DOI] [PubMed] [Google Scholar]
- 114. McClung MR, Harvey NC, Fitzpatrick LA, et al. Effects of abaloparatide on bone mineral density and risk of fracture in postmenopausal women aged 80 years or older with osteoporosis. Menopause 2018;25:767-71. 10.1097/GME.0000000000001080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. McClung MR, Williams GC, Hattersley G, Fitzpatrick LA, Wang Y, Miller PD. Geography of Fracture Incidence in Postmenopausal Women with Osteoporosis Treated with Abaloparatide. Calcif Tissue Int 2018;102:627-33. 10.1007/s00223-017-0375-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. McClung MR, Geusens P, Miller PD, et al. Hip Intervention Program Study Group . Effect of risedronate on the risk of hip fracture in elderly women. N Engl J Med 2001;344:333-40. 10.1056/NEJM200102013440503 [DOI] [PubMed] [Google Scholar]
- 117. McClung MR, Lewiecki EM, Cohen SB, et al. AMG 162 Bone Loss Study Group . Denosumab in postmenopausal women with low bone mineral density. N Engl J Med 2006;354:821-31. 10.1056/NEJMoa044459 [DOI] [PubMed] [Google Scholar]
- 118. McClung MR, Boonen S, Törring O, et al. Effect of denosumab treatment on the risk of fractures in subgroups of women with postmenopausal osteoporosis. J Bone Miner Res 2012;27:211-8. 10.1002/jbmr.536 [DOI] [PubMed] [Google Scholar]
- 119. Meunier PJ. Oral alendronate increases bone mineral density and reduces vertebral fracture incidence in postmenopausal osteoporosis. Br J Rheumatol 1997;36(Suppl. 1):15-9. [Google Scholar]
- 120. Miller PD, Hattersley G, Riis BJ, et al. ACTIVE Study Investigators . Effect of Abaloparatide vs Placebo on New Vertebral Fractures in Postmenopausal Women With Osteoporosis: A Randomized Clinical Trial. JAMA 2016;316:722-33. 10.1001/jama.2016.11136 [DOI] [PubMed] [Google Scholar]
- 121. Miller PD, Chines AA, Christiansen C, et al. Effects of bazedoxifene on BMD and bone turnover in postmenopausal women: 2-yr results of a randomized, double-blind, placebo-, and active-controlled study. J Bone Miner Res 2008;23:525-35. 10.1359/jbmr.071206 [DOI] [PubMed] [Google Scholar]
- 122. Miller PD, Pannacciulli N, Brown JP, et al. Denosumab or Zoledronic Acid in Postmenopausal Women With Osteoporosis Previously Treated With Oral Bisphosphonates. J Clin Endocrinol Metab 2016;101:3163-70. 10.1210/jc.2016-1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Minisola S, Marin F, Kendler DL, et al. Serum 25-hydroxy-vitamin D and the risk of fractures in the teriparatide versus risedronate VERO clinical trial. Arch Osteoporos 2019;14:10. 10.1007/s11657-019-0561-x [DOI] [PubMed] [Google Scholar]
- 124. Mortensen L, Charles P, Bekker PJ, Digennaro J, Johnston CC, Jr. Risedronate increases bone mass in an early postmenopausal population: two years of treatment plus one year of follow-up. J Clin Endocrinol Metab 1998;83:396-402. [DOI] [PubMed] [Google Scholar]
- 125. Muscoso E, Puglisi N, Mamazza C, et al. Antiresorption therapy and reduction in fracture susceptibility in the osteoporotic elderly patient: open study. Eur Rev Med Pharmacol Sci 2004;8:97-102. [PubMed] [Google Scholar]
- 126. Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001;344:1434-41. 10.1056/NEJM200105103441904 [DOI] [PubMed] [Google Scholar]
- 127. Nevitt MC, Ross PD, Palermo L, Musliner T, Genant HK, Thompson DE, The Fracture Intervention Trial Research Group . Association of prevalent vertebral fractures, bone density, and alendronate treatment with incident vertebral fractures: effect of number and spinal location of fractures. Bone 1999;25:613-9. 10.1016/S8756-3282(99)00202-1 [DOI] [PubMed] [Google Scholar]
- 128. Palacios S, Kalouche-Khalil L, Rizzoli R, et al. Treatment with denosumab reduces secondary fracture risk in women with postmenopausal osteoporosis. Climacteric 2015;18:805-12. 10.3109/13697137.2015.1045484 [DOI] [PubMed] [Google Scholar]
- 129. Panico A, Lupoli GA, Marciello F, et al. Teriparatide vs. alendronate as a treatment for osteoporosis: changes in biochemical markers of bone turnover, BMD and quality of life. Med Sci Monit 2011;17:CR442-8. 10.12659/MSM.881905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Pols HA, Felsenberg D, Hanley DA, et al. Fosamax International Trial Study Group . Multinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: results of the FOSIT study. Osteoporos Int 1999;9:461-8. 10.1007/PL00004171 [DOI] [PubMed] [Google Scholar]
- 131. Popp AW, Buffat H, Cavelti A, et al. Cortical bone loss at the tibia in postmenopausal women with osteoporosis is associated with incident non-vertebral fractures: results of a randomized controlled ancillary study of HORIZON. Maturitas 2014;77:287-93. 10.1016/j.maturitas.2013.12.013 [DOI] [PubMed] [Google Scholar]
- 132. Prevrhal S, Krege JH, Chen P, Genant H, Black DM. Teriparatide vertebral fracture risk reduction determined by quantitative and qualitative radiographic assessment. Curr Med Res Opin 2009;25:921-8. 10.1185/03007990902790993 [DOI] [PubMed] [Google Scholar]
- 133. Qu Y, Wong M, Thiebaud D, Stock JL. The effect of raloxifene therapy on the risk of new clinical vertebral fractures at three and six months: a secondary analysis of the MORE trial. Curr Med Res Opin 2005;21:1955-9. 10.1185/030079905X75032 [DOI] [PubMed] [Google Scholar]
- 134. Quandt SA, Thompson DE, Schneider DL, Nevitt MC, Black DM, Fracture Intervention Trial Research Group . Effect of alendronate on vertebral fracture risk in women with bone mineral density T scores of -1.6 to -2.5 at the femoral neck: the Fracture Intervention Trial. Mayo Clin Proc 2005;80:343-9. 10.4065/80.3.343 [DOI] [PubMed] [Google Scholar]
- 135. Recker RR, Kendler D, Recknor CP, et al. Comparative effects of raloxifene and alendronate on fracture outcomes in postmenopausal women with low bone mass. Bone 2007;40:843-51. 10.1016/j.bone.2006.11.001 [DOI] [PubMed] [Google Scholar]
- 136. Recknor C, Czerwinski E, Bone HG, et al. Denosumab compared with ibandronate in postmenopausal women previously treated with bisphosphonate therapy: a randomized open-label trial. Obstet Gynecol 2013;121:1291-9. 10.1097/AOG.0b013e318291718c [DOI] [PubMed] [Google Scholar]
- 137. Reginster J, Minne HW, Sorensen OH, et al. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group . Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Osteoporos Int 2000;11:83-91. 10.1007/s001980050010 [DOI] [PubMed] [Google Scholar]
- 138. Reginster JY, Felsenberg D, Pavo I, et al. Effect of raloxifene combined with monofluorophosphate as compared with monofluorophosphate alone in postmenopausal women with low bone mass: a randomized, controlled trial. Osteoporos Int 2003;14:741-9. 10.1007/s00198-003-1432-1 [DOI] [PubMed] [Google Scholar]
- 139. Reid IR, Horne AM, Mihov B, et al. Fracture Prevention with Zoledronate in Older Women with Osteopenia. N Engl J Med 2018;379:2407-16. 10.1056/NEJMoa1808082 [DOI] [PubMed] [Google Scholar]
- 140. Reid IR, Miller PD, Brown JP, et al. Denosumab Phase 3 Bone Histology Study Group . Effects of denosumab on bone histomorphometry: the FREEDOM and STAND studies. J Bone Miner Res 2010;25:2256-65. 10.1002/jbmr.149 [DOI] [PubMed] [Google Scholar]
- 141. Reid IR, Eastell R, Fogelman I, et al. A comparison of the effects of raloxifene and conjugated equine estrogen on bone and lipids in healthy postmenopausal women. Arch Intern Med 2004;164:871-9. 10.1001/archinte.164.8.871 [DOI] [PubMed] [Google Scholar]
- 142. Reid IR, Horne AM, Mihov B, et al. Anti-fracture efficacy of zoledronate in subgroups of osteopenic postmenopausal women: secondary analysis of a randomized controlled trial. J Intern Med 2019;286:221-9. 10.1111/joim.12901 [DOI] [PubMed] [Google Scholar]
- 143. Reid IR, Brown JP, Burckhardt P, et al. Intravenous zoledronic acid in postmenopausal women with low bone mineral density. N Engl J Med 2002;346:653-61. 10.1056/NEJMoa011807 [DOI] [PubMed] [Google Scholar]
- 144. Roux C, Goldstein JL, Zhou X, Klemes A, Lindsay R. Vertebral fracture efficacy during risedronate therapy in patients using proton pump inhibitors. Osteoporos Int 2012;23:277-84. 10.1007/s00198-011-1574-5 [DOI] [PubMed] [Google Scholar]
- 145. Roux C, Hofbauer LC, Ho PR, et al. Denosumab compared with risedronate in postmenopausal women suboptimally adherent to alendronate therapy: efficacy and safety results from a randomized open-label study. Bone 2014;58:48-54. 10.1016/j.bone.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 146. Ryder KM, Cummings SR, Palermo L, et al. Fracture Intervention Trial Research Group . Does a history of non-vertebral fracture identify women without osteoporosis for treatment? J Gen Intern Med 2008;23:1177-81. 10.1007/s11606-008-0622-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Sambrook PN, Geusens P, Ribot C, et al. Alendronate produces greater effects than raloxifene on bone density and bone turnover in postmenopausal women with low bone density: results of EFFECT (Efficacy of FOSAMAX versus EVISTA Comparison Trial) International. J Intern Med 2004;255:503-11. 10.1111/j.1365-2796.2004.01317.x [DOI] [PubMed] [Google Scholar]
- 148. Sarkar S, Reginster JY, Crans GG, Diez-Perez A, Pinette KV, Delmas PD. Relationship between changes in biochemical markers of bone turnover and BMD to predict vertebral fracture risk. J Bone Miner Res 2004;19:394-401. 10.1359/JBMR.0301243 [DOI] [PubMed] [Google Scholar]
- 149. Sarkar S, Mitlak BH, Wong M, Stock JL, Black DM, Harper KD. Relationships between bone mineral density and incident vertebral fracture risk with raloxifene therapy. J Bone Miner Res 2002;17:1-10. 10.1359/jbmr.2002.17.1.1 [DOI] [PubMed] [Google Scholar]
- 150. Seibel MJ, Naganathan V, Barton I, Grauer A. Relationship between pretreatment bone resorption and vertebral fracture incidence in postmenopausal osteoporotic women treated with risedronate. J Bone Miner Res 2004;19:323-9. 10.1359/JBMR.0301231 [DOI] [PubMed] [Google Scholar]
- 151. Silverman SL, Christiansen C, Genant HK, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res 2008;23:1923-34. 10.1359/jbmr.080710 [DOI] [PubMed] [Google Scholar]
- 152. Simon JA, Recknor C, Moffett AH, Jr, et al. Impact of denosumab on the peripheral skeleton of postmenopausal women with osteoporosis: bone density, mass, and strength of the radius, and wrist fracture. Menopause 2013;20:130-7. 10.1097/GME.0b013e318267f909 [DOI] [PubMed] [Google Scholar]
- 153. Siris E, Adachi JD, Lu Y, et al. Effects of raloxifene on fracture severity in postmenopausal women with osteoporosis: results from the MORE study. Multiple Outcomes of Raloxifene Evaluation. Osteoporos Int 2002;13:907-13. 10.1007/s001980200125 [DOI] [PubMed] [Google Scholar]
- 154. Siris ES, Simon JA, Barton IP, McClung MR, Grauer A. Effects of risedronate on fracture risk in postmenopausal women with osteopenia. Osteoporos Int 2008;19:681-6. 10.1007/s00198-007-0493-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Sontag A, Wan X, Krege JH. Benefits and risks of raloxifene by vertebral fracture status. Curr Med Res Opin 2010;26:71-6. 10.1185/03007990903427082 [DOI] [PubMed] [Google Scholar]
- 156. Tucci JR, Tonino RP, Emkey RD, Peverly CA, Kher U, Santora AC, 2nd. Effect of three years of oral alendronate treatment in postmenopausal women with osteoporosis. Am J Med 1996;101:488-501. 10.1016/S0002-9343(96)00282-3 [DOI] [PubMed] [Google Scholar]
- 157. Välimäki MJ, Farrerons-Minguella J, Halse J, et al. Effects of risedronate 5 mg/d on bone mineral density and bone turnover markers in late-postmenopausal women with osteopenia: a multinational, 24-month, randomized, double-blind, placebo-controlled, parallel-group, phase III trial. Clin Ther 2007;29:1937-49. 10.1016/j.clinthera.2007.09.017 [DOI] [PubMed] [Google Scholar]
- 158. van de Glind EM, Willems HC, Eslami S, et al. Estimating the Time to Benefit for Preventive Drugs with the Statistical Process Control Method: An Example with Alendronate. Drugs Aging 2016;33:347-53. 10.1007/s40266-016-0344-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Watts NB, Miller PD, Kohlmeier LA, et al. Vertebral fracture risk is reduced in women who lose femoral neck BMD with teriparatide treatment. J Bone Miner Res 2009;24:1125-31. 10.1359/jbmr.081256 [DOI] [PubMed] [Google Scholar]
- 160. Watts NB, Cooper C, Lindsay R, et al. Relationship between changes in bone mineral density and vertebral fracture risk associated with risedronate: greater increases in bone mineral density do not relate to greater decreases in fracture risk. J Clin Densitom 2004;7:255-61. 10.1385/JCD:7:3:255 [DOI] [PubMed] [Google Scholar]
- 161. Watts NB, Josse RG, Hamdy RC, et al. Risedronate prevents new vertebral fractures in postmenopausal women at high risk. J Clin Endocrinol Metab 2003;88:542-9. 10.1210/jc.2002-020400 [DOI] [PubMed] [Google Scholar]
- 162. Xie Z, Chen Y, Gurbuz S, et al. Effects of teriparatide in Chinese and Caucasian women with osteoporosis: bridging study on efficacy. Clin Interv Aging 2019;14:959-68. 10.2147/CIA.S181929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Luckey M, Kagan R, Greenspan S, et al. Once-weekly alendronate 70 mg and raloxifene 60 mg daily in the treatment of postmenopausal osteoporosis. Menopause 2004;11:405-15. 10.1097/01.GME.0000119981.77837.1F [DOI] [PubMed] [Google Scholar]
- 164. Barrett-Connor E, Mosca L, Collins P, et al. Raloxifene Use for The Heart (RUTH) Trial Investigators . Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med 2006;355:125-37. 10.1056/NEJMoa062462 [DOI] [PubMed] [Google Scholar]
- 165. Bock O, Börst H, Beller G, et al. Impact of oral ibandronate 150 mg once monthly on bone structure and density in post-menopausal osteoporosis or osteopenia derived from in vivo μCT. Bone 2012;50:317-24. 10.1016/j.bone.2011.10.027 [DOI] [PubMed] [Google Scholar]
- 166. Bueno JAH, Arias L, Yu CR, Williams R, Komm BS. Efficacy and safety of bazedoxifene in postmenopausal Latino women with osteoporosis. Menopause 2017;24:1033-9. 10.1097/GME.0000000000000889 [DOI] [PubMed] [Google Scholar]
- 167. Black DM, Reid IR, Napoli N, et al. The Interaction of Acute-Phase Reaction and Efficacy for Osteoporosis After Zoledronic Acid: HORIZON Pivotal Fracture Trial. J Bone Miner Res 2022;37:21-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Body JJ, Marin F, Kendler DL, et al. Efficacy of teriparatide compared with risedronate on FRAX-defined major osteoporotic fractures: results of the VERO clinical trial. Osteoporos Int 2020;31:1935-42. 10.1007/s00198-020-05463-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Brown JP, Engelke K, Keaveny TM, et al. Romosozumab improves lumbar spine bone mass and bone strength parameters relative to alendronate in postmenopausal women: results from the Active-Controlled Fracture Study in Postmenopausal Women With Osteoporosis at High Risk (ARCH) trial. J Bone Miner Res 2021;36:2139-52. 10.1002/jbmr.4409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Cosman F, Lewiecki EM, Ebeling PR, et al. T-Score as an Indicator of Fracture Risk During Treatment With Romosozumab or Alendronate in the ARCH Trial. J Bone Miner Res 2020;35:1333-42. 10.1002/jbmr.3996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Cosman F, Peterson LR, Towler DA, Mitlak B, Wang Y, Cummings SR. Cardiovascular Safety of Abaloparatide in Postmenopausal Women With Osteoporosis: Analysis From the ACTIVE Phase 3 Trial. J Clin Endocrinol Metab 2020;105:3384-95. 10.1210/clinem/dgaa450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Geusens P, Kendler DL, Fahrleitner-Pammer A, López-Romero P, Marin F. Distribution of Prevalent and Incident Vertebral Fractures and Their Association with Bone Mineral Density in Postmenopausal Women in the Teriparatide Versus Risedronate VERO Clinical Trial. Calcif Tissue Int 2020;106:646-54. 10.1007/s00223-020-00683-6 [DOI] [PubMed] [Google Scholar]
- 173. Reid IR, Horne AM, Mihov B, et al. Effects of Zoledronate on Cancer, Cardiac Events, and Mortality in Osteopenic Older Women. J Bone Miner Res 2020;35:20-7. 10.1002/jbmr.3860 [DOI] [PubMed] [Google Scholar]
- 174. Kendler D, Chines A, Clark P, et al. Bone Mineral Density After Transitioning From Denosumab to Alendronate. J Clin Endocrinol Metab 2020;105:e255-64. 10.1210/clinem/dgz095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Kendler DL, Marin F, Geusens P, et al. Psychotropic medications and proton pump inhibitors and the risk of fractures in the teriparatide versus risedronate VERO clinical trial. Bone 2020;130:115113. 10.1016/j.bone.2019.115113 [DOI] [PubMed] [Google Scholar]
- 176. Kanis JA, Harvey NC, Lorentzon M, et al. Combining fracture outcomes in phase 3 trials of osteoporosis: an analysis of the effects of denosumab in postmenopausal women. Osteoporos Int 2021;32:165-71. 10.1007/s00198-020-05699-0 [DOI] [PubMed] [Google Scholar]
- 177. McCloskey EV, Johansson H, Harvey NC, Lorentzon M, Shi Y, Kanis JA. Romosozumab efficacy on fracture outcomes is greater in patients at high baseline fracture risk: a post hoc analysis of the first year of the frame study. Osteoporos Int 2021;32:1601-8. 10.1007/s00198-020-05815-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. McClung MR, Bolognese MA, Brown JP, et al. A single dose of zoledronate preserves bone mineral density for up to 2 years after a second course of romosozumab. Osteoporos Int 2020;31:2231-41. 10.1007/s00198-020-05502-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. McClung MR, Bolognese MA, Brown JP, et al. Skeletal responses to romosozumab after 12 months of denosumab. JBMR Plus 2021;5:e10512. 10.1002/jbm4.10512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Saag KG, Williams SA, Wang Y, Weiss RJ, Cauley JA. Effect of Abaloparatide on Bone Mineral Density and Fracture Incidence in a Subset of Younger Postmenopausal Women with Osteoporosis at High Risk for Fracture. Clin Ther 2020;42 10.1016/j.clinthera.2020.04.012. [DOI] [PubMed] [Google Scholar]
- 181. Watts NB, Hattersley G, Fitzpatrick LA, et al. Abaloparatide effect on forearm bone mineral density and wrist fracture risk in postmenopausal women with osteoporosis. Osteoporos Int 2019;30:1187-94. 10.1007/s00198-019-04890-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. McClung MR, Bolognese MA, Sedarati F, Recker RR, Miller PD. Efficacy and safety of monthly oral ibandronate in the prevention of postmenopausal bone loss. Bone 2009;44:418-22. 10.1016/j.bone.2008.09.011 [DOI] [PubMed] [Google Scholar]
- 183. Recker R, Stakkestad JA, Chesnut CH, 3rd, et al. Insufficiently dosed intravenous ibandronate injections are associated with suboptimal antifracture efficacy in postmenopausal osteoporosis. Bone 2004;34:890-9. 10.1016/j.bone.2004.01.008 [DOI] [PubMed] [Google Scholar]
- 184. Wells GA, Cranney A, Peterson J, et al. Alendronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev 2008;(1):CD001155. 10.1002/14651858.CD001155.pub2 [DOI] [PubMed] [Google Scholar]
- 185. Wells G, Cranney A, Peterson J, et al. Risedronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev 2008;(1):CD004523. 10.1002/14651858.CD004523.pub3 [DOI] [PubMed] [Google Scholar]
- 186. Reginster J, Bianic F, Campbell R, Martin M, Williams SA, Fitzpatrick LA. Abaloparatide for risk reduction of nonvertebral and vertebral fractures in postmenopausal women with osteoporosis: a network meta-analysis. Osteoporos Int 2019;30:1465-73. 10.1007/s00198-019-04947-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Barrionuevo P, Kapoor E, Asi N, et al. Efficacy of Pharmacological Therapies for the Prevention of Fractures in Postmenopausal Women: A Network Meta-Analysis. J Clin Endocrinol Metab 2019;104:1623-30. 10.1210/jc.2019-00192 [DOI] [PubMed] [Google Scholar]
- 188. Freemantle N, Cooper C, Diez-Perez A, et al. Results of indirect and mixed treatment comparison of fracture efficacy for osteoporosis treatments: a meta-analysis. Osteoporos Int 2013;24:209-17. 10.1007/s00198-012-2068-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Liu GF, Wang ZQ, Liu L, Zhang BT, Miao YY, Yu SN. A network meta-analysis on the short-term efficacy and adverse events of different anti-osteoporosis drugs for the treatment of postmenopausal osteoporosis. J Cell Biochem 2018;119:4469-81. 10.1002/jcb.26550 [DOI] [PubMed] [Google Scholar]
- 190. Messori A, Fadda V, Maratea D, Trippoli S, Marinai C. Anti-reabsorptive agents in women with osteoporosis: determining statistical equivalence according to evidence-based methods. J Endocrinol Invest 2014;37:769-73. 10.1007/s40618-014-0124-3 [DOI] [PubMed] [Google Scholar]
- 191. Zhou J, Wang T, Zhao X, Miller DR, Zhai S. Comparative Efficacy of Bisphosphonates to Prevent Fracture in Men with Osteoporosis: A Systematic Review with Network Meta-Analyses. Rheumatol Ther 2016;3:117-28. 10.1007/s40744-016-0030-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Ellis AG, Reginster JY, Luo X, et al. Bazedoxifene versus oral bisphosphonates for the prevention of nonvertebral fractures in postmenopausal women with osteoporosis at higher risk of fracture: a network meta-analysis. Value Health 2014;17:424-32. 10.1016/j.jval.2014.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Sanderson J, Martyn-St James M, Stevens J, et al. Clinical effectiveness of bisphosphonates for the prevention of fragility fractures: A systematic review and network meta-analysis. Bone 2016;89:52-8. 10.1016/j.bone.2016.05.013 [DOI] [PubMed] [Google Scholar]
- 194. Yang XC, Deng ZH, Wen T, et al. Network Meta-Analysis of Pharmacological Agents for Osteoporosis Treatment and Fracture Prevention. Cell Physiol Biochem 2016;40:781-95. 10.1159/000453138 [DOI] [PubMed] [Google Scholar]
- 195. Murad MH, Drake MT, Mullan RJ, et al. Clinical review. Comparative effectiveness of drug treatments to prevent fragility fractures: a systematic review and network meta-analysis. J Clin Endocrinol Metab 2012;97:1871-80. 10.1210/jc.2011-3060 [DOI] [PubMed] [Google Scholar]
- 196. Migliore A, Broccoli S, Massafra U, Cassol M, Frediani B. Ranking antireabsorptive agents to prevent vertebral fractures in postmenopausal osteoporosis by mixed treatment comparison meta-analysis. Eur Rev Med Pharmacol Sci 2013;17:658-67. [PubMed] [Google Scholar]
- 197. Zhang L, Pang Y, Shi Y, et al. Indirect comparison of teriparatide, denosumab, and oral bisphosphonates for the prevention of vertebral and nonvertebral fractures in postmenopausal women with osteoporosis. Menopause 2015;22:1021-5. 10.1097/GME.0000000000000466 [DOI] [PubMed] [Google Scholar]
- 198. Jansen JP, Bergman GJ, Huels J, Olson M. The efficacy of bisphosphonates in the prevention of vertebral, hip, and nonvertebral-nonhip fractures in osteoporosis: a network meta-analysis. Semin Arthritis Rheum 2011;40:275-84.e1, 2. 10.1016/j.semarthrit.2010.06.001 [DOI] [PubMed] [Google Scholar]
- 199. Hernandez AV, Pérez-López FR, Piscoya A, et al. Comparative efficacy of bone anabolic therapies in women with postmenopausal osteoporosis: A systematic review and network meta-analysis of randomized controlled trials. Maturitas 2019;129:12-22. 10.1016/j.maturitas.2019.08.003 [DOI] [PubMed] [Google Scholar]
- 200. Muschitz C, Kocijan R, Fahrleitner-Pammer A, Lung S, Resch H. Antiresorptives overlapping ongoing teriparatide treatment result in additional increases in bone mineral density. J Bone Miner Res 2013;28:196-205. 10.1002/jbmr.1716 [DOI] [PubMed] [Google Scholar]
- 201. Vinkers CH, Lamberink HJ, Tijdink JK, et al. The methodological quality of 176,620 randomized controlled trials published between 1966 and 2018 reveals a positive trend but also an urgent need for improvement. PLoS Biol 2021;19:e3001162. 10.1371/journal.pbio.3001162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Dechartres A, Trinquart L, Atal I, et al. Evolution of poor reporting and inadequate methods over time in 20 920 randomised controlled trials included in Cochrane reviews: research on research study. BMJ 2017;357:j2490. 10.1136/bmj.j2490 [DOI] [PubMed] [Google Scholar]
- 203. Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2019;104:1595-622. 10.1210/jc.2019-00221 [DOI] [PubMed] [Google Scholar]
- 204. Shoback D, Rosen CJ, Black DM, Cheung AM, Murad MH, Eastell R. Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society Guideline Update. J Clin Endocrinol Metab 2020;105:dgaa048. 10.1210/clinem/dgaa048 [DOI] [PubMed] [Google Scholar]
- 205. Thompson SG, Higgins JP. Treating individuals 4: can meta-analysis help target interventions at individuals most likely to benefit? Lancet 2005;365:341-6. 10.1016/S0140-6736(05)17790-3 [DOI] [PubMed] [Google Scholar]
- 206. Bauer DC, Black DM, Bouxsein ML, et al. Foundation for the National Institutes of Health (FNIH) Bone Quality Project . Treatment-Related Changes in Bone Turnover and Fracture Risk Reduction in Clinical Trials of Anti-Resorptive Drugs: A Meta-Regression. J Bone Miner Res 2018;33:634-42. 10.1002/jbmr.3355 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary material
Data Availability Statement
The statistical code and dataset are available from the corresponding author.