Abstract
A failure of central immune tolerance driven by autoantigen specific T regulatory (Treg) cells is a major cause of many autoimmune diseases. Restoration of proper autoantigen Treg specific response holds promise as a highly effective, long-term therapy for a wide variety of autoimmune diseases. Generating autoantigen specific Tregs remains a challenge due to the non-specific nature of most tolerizing agents and the complexities of generating Tregs in vivo. Here we show a new push/pull method for inducing antigen-specific Treg tolerance via induction of tolerogenic dendritic cells (tolDCs). We identified a combination of three tolerogenic drugs, dexamethasone, simvastatin and SC-514, which when used in combination with toll-like-receptor (TLR) agonists induces an active tolDC phenotype. When the tolerogenic combination was packaged into a liposome with a model antigen such as ovalbumin (OVA), these tolDCs induce differentiation of OVA specific Tregs both ex vivo and in vivo. We examined the tolerizing potential of the combination in an experimental autoimmune encephalomyelitis (EAE) disease model. Given the antigen specificity of this technique, this paper presents an attractive preclinical autoimmune therapy.
Keywords: Dendritic cells, Autoimmunity, Liposome, T regulatory cells
1. Introduction
Generating autoantigen specific suppression remains a major challenge in developing effective autoimmune disease treatments. Despite increased knowledge of how autoimmune diseases arise the most effective clinically used therapies are still broadly immunosuppressive [1]. These drugs have significant side effects and still do not treat the root cause of the disease: generation of autoantigen specific T and B cells [2]. Many new autoimmune therapies have been developed that generate autoantigen specific immune suppression, yet many challenges remain to generate active and antigen specific T regulatory cells (Tregs) [3]. T regulatory cells maintain long lasting tolerance for autoimmune patients, as they suppress autoreactive T and B cells and promote their anergy and deletion, while sparing other essential immune cells [4]. This Treg is a proposed key goal of the field of autoimmune research, as it typically yields long lasting tolerance without the need for general immune suppression [5].
Current research in generating autoantigen specific Tregs is focused on DNA delivery, using modified autoantigens and tolerance inducing nano/micro particles [3]. DNA for disease relevant proteins in multiple sclerosis (MS) and type I diabetes (T1D) have been developed but have had limited success in clinical applications [6]. Likewise, modified whole autoantigens, such as CTLA-4 antibodies conjugated to autoantigens, have had limited success in clinical trials [7]. The most promising of these translational strategies is packaging autoantigens and immunomodulators into nano/micro carriers [8–10]. The goal of these carriers is to facilitate a tolerogenic state of antigen presenting cells (APC), typically dendritic cells (DCs), which in turn facilitates the development of T regs [11]. Tolerogenic DCs (TolDCs) are a specialized DCs subset which overexpresses tolerance markers such as PD-L1/2 and lacks T cell co-stimulatory markers such as CD80, CD86 or CD40 and release tolerogenic cytokines like IL-10 [12]. Recent research has shown that microparticles containing tolerogenic cytokines, IL-10 and TGF-β, along with an epitope for an autoantigen can be used as a therapy in a mouse model of MS and has the potential for treating T1D by inducing tolDCs [13,14]. However, current methods can only differentiate immature DC cells ex vivo into tolDC-like phenotypes (induced or itolDCs) and show autoimmune suppression. Some notable examples include using NF-kB inhibitors, steroid immune suppressants (dexamethasone or rapamycin) or exogenous IL-10 to generate tolDCs [8, 15–18]). Some of these procedures are limited because they require harvesting immature DCs from a patient (since allotransplants are impossible due to cross-reactivity issues) and reintroducing them after ex vivo treatment, which is difficult. In vivo therapeutics, meanwhile, have difficultly in only activating Treg tolerance due to the presence of non-specific immunosuppressive therapies. Furthermore, itolDCs are typically short lived, potentially limiting long-term clinical viability of ex vivo tolDCs [19]. Furthermore cytokines such as IL-10 and TGF-β may have many immunomodulatory effects of their own and remain difficult to manufacture and store, making this formulation challenging to move to the clinic.
This study presents a new method of inducing tolDCs, which can induce long-term and effective autoantigen Treg tolerance. While most nanoparticulate formulations used as tolerance therapies include just an antigen and an immunomodulator to suppress DC activation, here we show, somewhat counter-intuitively, that this combination can be improved by including an immune activator, such as a toll-like-receptor (TLR) agonists. This push/pull approach considers generating tolDCs as they arise physiologically. Instead of one suppressive signal, DCs differentiate into tolDCs by integrating both activating (push) and inhibitory signals (pull) and interpreting an insufficient activation response [20]. We first tested this “push/pull” hypothesis using a set of immunomodulators and TLR agonists, a primary mechanism for APC activation [21]. Furthermore, using TLR agonists to trigger tolerance is well documented in the literature. Small doses of lipopolysaccharide (LPS) desensitizes cells to other TLR agonists and lead to tolerance and increase tolerogenic cytokines such as IL-10, but this is dangerous as LPS can also trigger strong inflammatory responses [22]. Therefore, we sought to 1) identify a combination of TLR agonist and immunomodulator that can generate tolDCs in vitro and 2) design an appropriate nanocarrier system to implement this combination with an autoantigen to generate Treg responses in vivo.
2. Results
2.1. Identifying a combination of TLR agonists/immunomodulators that generate tolDCs in vitro
By incorporating a TLR agonist, immunomodulator and antigen into a single nanoparticle, we hypothesized that we could generate more effective tolDC responses, which in turn would generate both more effective Treg tolerance and better autoimmune suppression (Fig. 1A). While TLR agonists are widely used in vaccine research, using TLR agonists or inhibitors to trigger tolerance is also well documented in the literature. Small doses of lipopolysaccharide (LPS) can desensitize cells to other TLR agonists and lead to tolerance and increase tolerogenic cytokines such as IL-10, but this is dangerous as LPS can also trigger strong inflammatory responses [22]. Another potential benefit of TLR activation for tolDCs is that optimal Treg responses is achieved from suboptimal, not non-existent, T cell co-stimulation [23]. This means that tolDCs need to generate a small amount of CD28 or CD40 expression in combination with PD-L1. Such positive co-stimulation is best achieved via TLR activation [24]. Furthermore, exposure to TLR agonists leads to DC maturation, leading to longer lived phenotypes and upregulation of survival signals [25]. Our hypothesis was that exposure to the appropriate combination of TLR agonist and immunomodulators would generate tolDCs that are long-lived and which actively and strongly stimulate Treg differentiation. The challenge in this study was to find a combination of TLR agonist and immunomodulators that generated this tolDC phenotype.
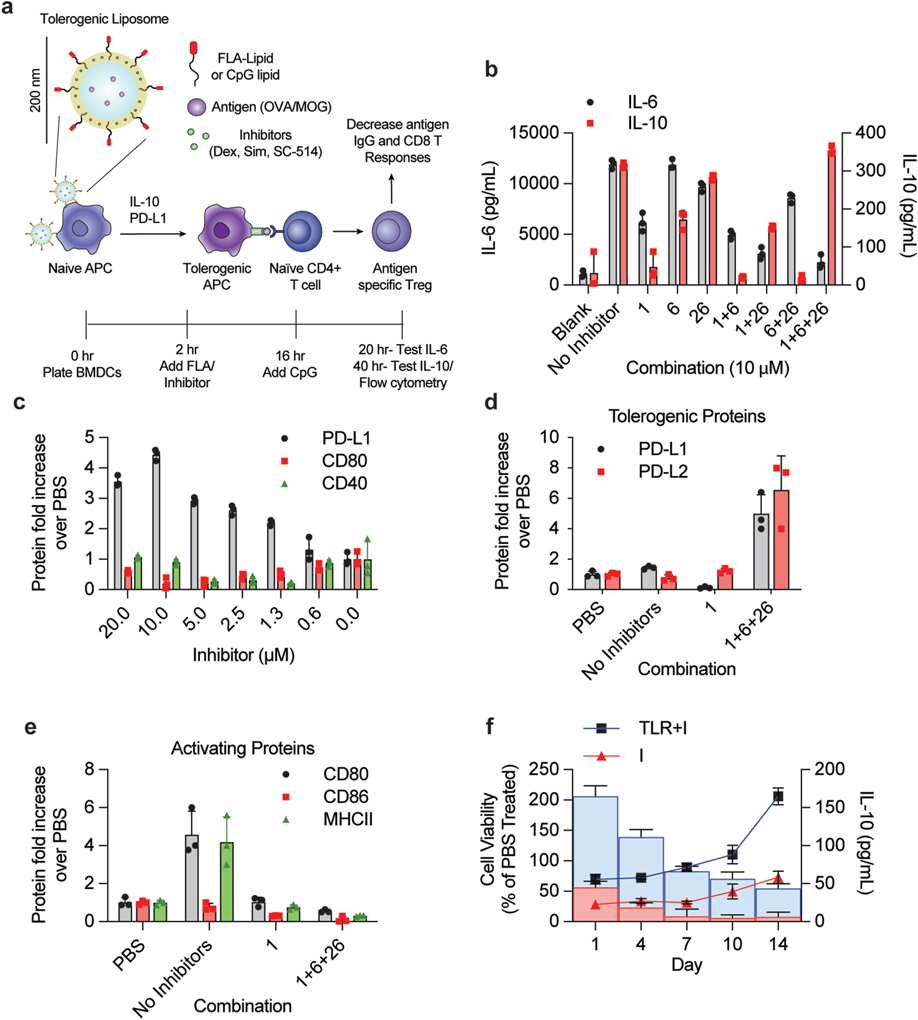
Fig. 1. Combining TLR agonist and immunomodulatory drugs generates tolerogenic APCs in vitro.
(A) Conceptual diagram representing tolerogenic liposome for generation of T regulatory response – a combination of inhibitors and TLR agonists generate more active Tregs through a push/pull mechanism. (B) Analysis of IL-6 and IL-10 production from combinations of immunomodulatory drugs and TLR agonists. 100 k BMDCs were plated and treated with a combination of 0.1 μg/mL FLA (TLR5 agonist) and immunoinhibitory drugs. After a 14 h incubation, cells were washed and treated with 0.5 μM CpG. Supernatants were drawn 4 h later (for IL-6) and 20 h later (for IL-10). Cytokine secretion was measured by ELISA. (C) Analysis of cell surface marker response to combination in BMDCs. Cells were treated with varying concentrations of a 1:1:1 combination of dex, sim, and sc-514 (concentration indicated on x axis is concentration of all inhibitors). Cells were analyzed via flow 24 h after CpG addition for PD-L1, CD80 and CD40. (D–E) Analysis of tolerogenic response on naïve spleenocytes. Methods from C was repeated on naïve spleenocytes (1 million per sample) in triplicate from three separate mice and then analyzed via flow. CD45+, MHCII+, CD11c+, CD19− cells (DCs) were gated and then stained for (D) PD-L1 and PD-L2 or (E) CD80, CD86 and MHCII. (F) Analysis of TLR agonist/Inhibitor combination lifetime in tolDC phenotypes. BMDCs were plated into 5 different 96 well plates (200 k cells per well) and incubated with 1 μM inhibitor combination (I, 1:1:1 combination of dex:sim:sc-514) in combination with 0.1 μg/mL FLA for 16 h. Cells were then washed and treated with 0.5 μM CpG+1 μM I. 20 h later, on day 1, one plate of cells was tested for viability using MTT assay and for IL-10 secretion via CBA. The remaining plates were washed and incubated with fresh media. On day 3, one plate of cells was challenged with 0.5 μM CpG for 20 h then analyzed for cell viability (line plots) and IL-10 secretion (bar graphs). This procedure was repeated on day 6, 9 and 13 with remaining plates. The TLR agonist and inhibitor treated samples (TLR + I, shown in blue) were compared to cells treated with just inhibitor combination (I, shown in red). Error bars indicate ± of SD of biological triplicate experiments. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
First, we obtained a library of TLR agonists and immunomodulators to test in vitro with APC cell lines and BMDCs to optimize this TLR agonist/immunomodulator combination. After validating these combinations of TLR agonists and modulators do indeed increase tolDC phenotypes, we purchased a library of TLR agonists and immunomodulators based on previous literature (Table S-1). We dosed mouse bone marrow derived dendritic cells (BMDCs) or RAW Blue cells with varying concentrations and combinations of TLR agonists/modulators (Table S-1). To test the potential for a tolerogenic response, cells were challenged after 16 h with the TLR9 agonist CpG (ODN 1826). The levels of PD-L1, CD80, CD40, IL-10 and IL-6 were measured for BMDCs and the levels of NF-κB activity was measured using SEAP reporter assay on the RAW Blue cells. A tolerance score was calculated by assessing the relative contributions of tolerizing activity (PD-L1, IL-10) to inflammatory activity (CD80, CD40, IL-6 and NF-κB) (Table S-2, see methods). Three compounds were identified from this preliminary screen for further testing as they showed some tolerizing effects: dexamethasone (compound 1), simvastatin (compound 6), and SC-514 (compound 26). While dexamethasone and simvastatin have previous clinical data in autoimmune treatment, SC-514 (a NF-κB inhibitor) has almost no data and has yet to be analyzed clinically [26–28]. We identified that tolDCs are optimally generated with treated with this cocktail of inhibitors and the TLR agonist Flagellin (FLA) and then subsequently treated a combination of TLR agonist CpG (ODN 1826) and the same cocktail of inhibitors on the following day (Fig. 1B and C).
We optimized the dosing strategy and determined that adding the inhibitors with FLA on day 1, washing and then adding CpG with inhibitors on day 2 resulted in the highest tolerance score (Fig. S-1). This formulation and dosing regimen also led to the greatest increase in tolDC phenotype in BMDCs. We observed similar tolDC phenotype markers in DCs extracted from mouse spleens and innate immune cell lines such as DC2.4 and RAW264.2 (Fig. 1D, E, Fig. S-2). During the investigation of this study, we tested many ratios and concentrations of all three tolerogenic drugs and TLR agonists in BMDCs, but found that the most effective formulation ranged between 10 and 1 μM total inhibitor concentration (of all three drugs at a 1:1:1 ratio) combined with either 0.1 μg/mL of FLA or 0.5 μM of CpG (Fig. S-3). For clarity, we denote formulations with TLR agonists containing 1:1:1 Dex:Sim:SC-514 as (TLR + I), formulations containing just TLR agonists as (TLR) and formulations containing just inhibitor as (I). Note that the treatment regimen of dosing with FLA containing formulations on day 1 followed by CpG containing formulations on day 2 will be used in all the experiments of this study unless noted otherwise.
We hypothesized that the inclusion of TLR agonists with immunomodulators would increase tolDC viability and lifetime in addition to generating stronger tolDC phenotypes. To test this hypothesis, we treated BMDCs in a similar fashion as our previous experiment with a dose regiment of both concentration of TLR + I and time (Fig. 1D/E). After the addition of the final TLR + I, cells were incubated for 24 h and tested for IL-10 and cytotoxicity using an MTT assay on day 1. Similarly treated cells were washed on day 3, challenged for 24 h with 0.5 μM of CpG and tested for IL-10 secretion and cytotoxicity on day 4. This procedure was subsequently repeated on day 6, 9 and 13. The results show that BMDC viability is low for inhibitor treated cells on day 1, but rises over time when compared to PBS control cells (Fig. 1F). BMDCs treated with both inhibitors and TLR agonists (TLR + I) had greater viability and longevity when compared to BMDCs treated with just inhibitors and no agonists (I). TLR + I treated cells also had an increase in IL-10 secretion, indicating that these cells were more actively tolerizing for longer than inhibitor only group.
“Push/Pull” tolerizing liposomes generate tolDC phenotypes in vitro.
After optimizing our TLR agonist-inhibitor formulation, we sought to incorporate this formulation in an appropriate nanocarrier. A nanocarrier was required for any in vivo study for two reasons 1): in order to ensure that all components of the inhibitor formulation were co-delivered and 2)provide antigen specificity [10]. We chose liposomes as our nanocarriers as liposomes provide good passive targeting of innate immune cells and have been widely used in the clinic for other diseases and have shown success in generating robust tolerance([8,29]). Also, liposomes can readily be formulated with all three tolerance drugs (Dex, Sim and SC-514, Fig. S-4). However, the TLR agonists FLA and CpG are too hydrophilic to reliably be incorporated into liposomes. Furthermore, we want the TLR agonists displayed on the surface of a liposome to ensure the agonists stimulate TLRs during phagocytosis. To achieve this, we conjugated FLA and CpG with lipid (C16) tails using established chemistries, purified them using HPLC and confirmed that FLA-lipid and CpG-lipid conjugates are incorporated into 200 nm diameter liposomes at >95% efficiency (Fig. S-4) [30]. Cells and mice were both applied using the same protocols as previous free drug formulations, receiving FLA/inhibitor liposomes first, then CpG/inhibitor liposomes the following day. For groups with no TLR agonist, cells/mice were treated on consecutive days with the same liposome (with or without inhibitors). We denoted groups treated with liposomes bearing TLR agonists and inhibitors as LipoTLR + I, groups treated with inhibitor loaded liposomes with no TLR agonist as LipoI and groups treated with TLR agonist bearing liposomes only as LipoTLR.
After validating that our tolerogenic formulations contained the expected levels of compounds, we sought to show that these formulations induce antigen specific tolDCs both in vitro and in vivo. We first validated that LipoTLR + I and LipoTLR formulations generate similar tolerogenic DC phenotypes as free formulations of TLR/inhibitors in vitro using BMDCs (Fig. S-5). Next, using the fluorescently labeled model antigen Ovalbumin (OVA), we observed OVA uptake in BMDCs with or without our inhibitor formulations both in free and liposomal formulations (Fig. 2A, S-6). There was no decrease in OVA uptake for LipoTLR + I compared to blank liposomes (LipoBlank). We observed an increase in the OVA major MHC I epitope presentation for LipoTLR + I groups compared to LipoBlank (Fig. S-6). Interestingly, there was also a decrease in OVA uptake and MHCI epitope presentation for BMDCs treated with free tolerogenic inhibitors, indicating that a liposomal formulation may enhance effective antigen presentation in vivo. Finally, we tested if FLA or CpG bearing liposomes were selectively uptaken by any DC subset or if they selectively targeted mature or immature DCs. We incubated FLA liposomes or CpG liposomes or Blank liposomes for 30 min with splenocytes isolated from mice and observed no significant uptake between the three groups for any DC subset (Figure S-6). Interestingly we observed that each liposome formulation was more readily uptaken by cells bearing markers of DC maturity (CD80,CD86,CCR7, CD40 or OX40L) but not CD8+ cDC1 or CD11b + cDC2 subsets, but this selective uptake of cationic liposomes by mature rather than naïve DCs is already established [31–33].
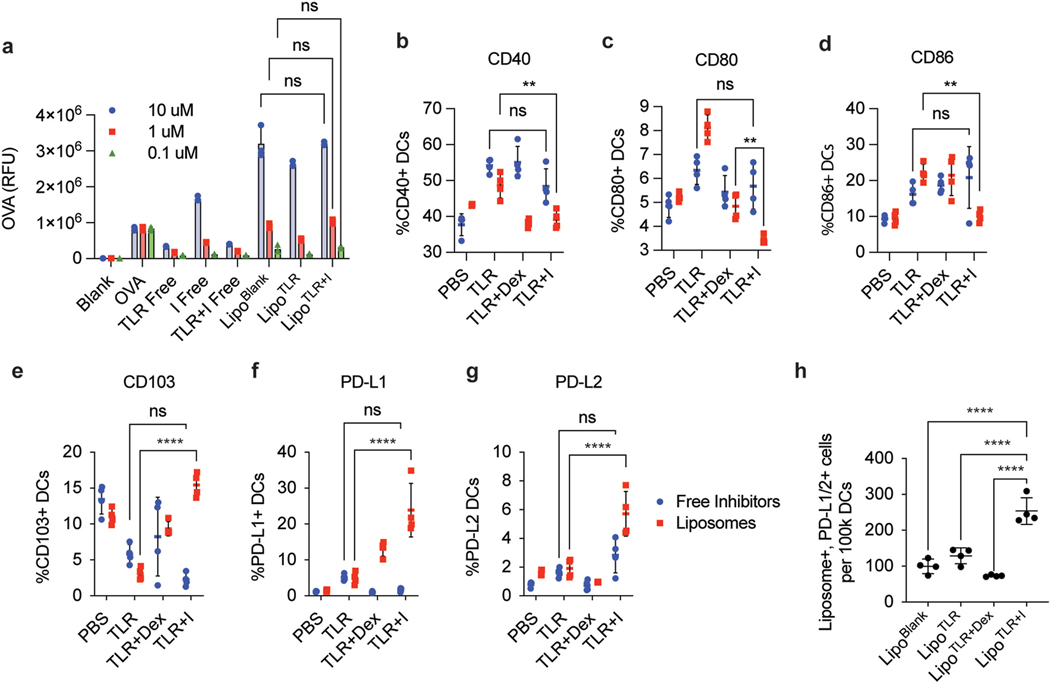
Fig. 2.
LipoTLR + I generate tolDCs that actively uptake antigen in vitro and in vivo. (A) Analysis of LipoTLR + I In Vitro. 100 k BMDCs were treated with free or liposomal formulations of TLR + I, TLR or I and analyzed via flow cytometry for liposomal uptake. Liposomes were synthesized with AF647-OVA for a total of 1 μg of OVA per .1 μM of inhibitor (using loading procedure from Figure S-4). Free inhibitor formulations were treated with equivalent OVA dose. 1 h after second treatment, cells were washed and analyzed via flow for OVA internalization (B) In Vivo Analysis of LipoTLR + I uptake. C57BL/6 mice (4 per group) were injected with either free or Lipo formulations of OVA and combinations of inhibitors to for the following categories (100 μg OVA/mouse, 10 umols inhibitor/mouse, 1 μg FLA/mouse, 10 μg CpG/mouse) [1]: OVA alone (PBS) [2], OVA + TLR agonists (TLR), OVA + TLR agonists + dexsamethasone only (TLR + Dex) or OVA + Inhibitor combination (TLR + I). For Lipo formulations, DiD was added at 0.01% total lipid loading to allow fluorescent analysis. Mice were injected with either Lipo or free combinations i.p. with FLA then CpG formulations on consecutive days. 24 h after final CpG injection, mice were sacrificed, popliteal and inguinal lymph nodes harvested, disassociated and stained for various immune cell markers. Lymph cells were analyzed via spectral flow and DC populations (CD45+, MHCII+, CD11c+, CD19−) analyzed for CD40, (C) CD80 (D) CD86 (E) CD103 (F) PD-L1 (G) PD-L2. (H) Mice treated with liposomes were gated on Liposome+ and PD-L1/2 + cell populations. Error bars indicate ± of SD of each mouse group (N = 4). Significance was determined by a two-way ANOVA with Tukey post hoc test for multiple comparisons. *p < 0.5, **p < 0.01, ***p < 1 × 10−4, ****p < 1 × 10−5..
“Push/Pull” liposomes generate tolDCs and antigen specific Treg populations in vivo.
Next, we sought to demonstrate that the tolerogenic liposomes can generate tolDC populations in vivo. Mice were injected with either free or liposomal formulations of all three inhibitors (TLR + I), dexamethasone with TLR agonists (TLR + Dex), with only TLR agonists (TLR) or blank PBS controls (free PBS or blank liposome). We chose dex only containing formulations as a control because dex has been used in several previous studies for tolerance, and we wanted to observe the effects of the drug combination compared to dex [34]. All mouse groups were injected with FLA formulations first, then 24 h later injected with CpG formulations (except PBS, which was injected with just PBS on both days) then sacrificed and lymph nodes analyzed 24 h after CpG injection. This dosing regimen mirrored the dosing strategy optimized in vitro (Table S-2). All formulations contained 100 μg of OVA. DCs from mouse lymph nodes were analyzed via spectral flow cytometry for DC inflammatory markers CD40, CD80 and CD86 and DC tolerogenic markers such as CD103, PD-L1 and PD-L2 (Fig. 2B–G, Fig. S-7 for gating strategy). We observed a significant increase in all tolerogenic markers and a decrease in all inflammatory markers in mice treated with the combination of tolerogenic liposomes when compared to non-liposomal formulations. To monitor which DCs phagocytose the liposomes, all liposomes were labeled with a DiD dye. Tracking this dye, we determined that LipoTLR + I significantly increased the number of liposome+, PD-L1/2+ tolDCs (Fig. 2H).
After demonstrating that LipoTLR + I generated tolDCs, our next goal was to show that these tolDCs reduce antigen specific immunity and generate antigen specific Tregs. Similar to our previous experiment, mice were injected with OVA + LipoTLR + I, OVA + LipoTLR or the same mixture of molecules not in liposomes. Lymph cells were also stained with a tetramer for the major MHCI epitope of OVA and analyzed via flow. While we did not observe a change in the overall CD8 or CD4 ratios, there was a decrease in CD8+, MHCI-epitope tetramer positive T effector cells in tolerogenic liposome treated mice (Fig. 3A, Fig. S-8). Furthermore, there was an increase in MHCII-tetramer positive Treg cells for LipoTLR + I group (Fig. 3B). 10 days after the second injection, mice were sacrificed and serum analyzed for OVA specific IgGs, showing a significant decrease in IgGs for LipoTLR + I (Fig. 3C). This result indicates that the liposomal treatment did not alter global T cell populations, but rather antigen specific T cells were selectively removed through OVA antigen specific Tregs. Finally, we wanted to evaluate the functionality of the Tregs – specifically how well they suppress antigen specific T effector cell function. To do this, we extracted spleens, incubated splenocytes with BMDCs presenting OVA and observed T cell proliferation and cytokine release after 48 h. We observed a significant decrease in T cell proliferation in the spleens of mice administered LipoTLR + I (Fig. 3D). This suppression was recovered when generated Tregs were removed via a magnetic cell sorter, further indicating that Tregs were responsible for T cell suppression. A similar trend was also seen in the IL-2 and IFN-γ release (Fig. S-9).
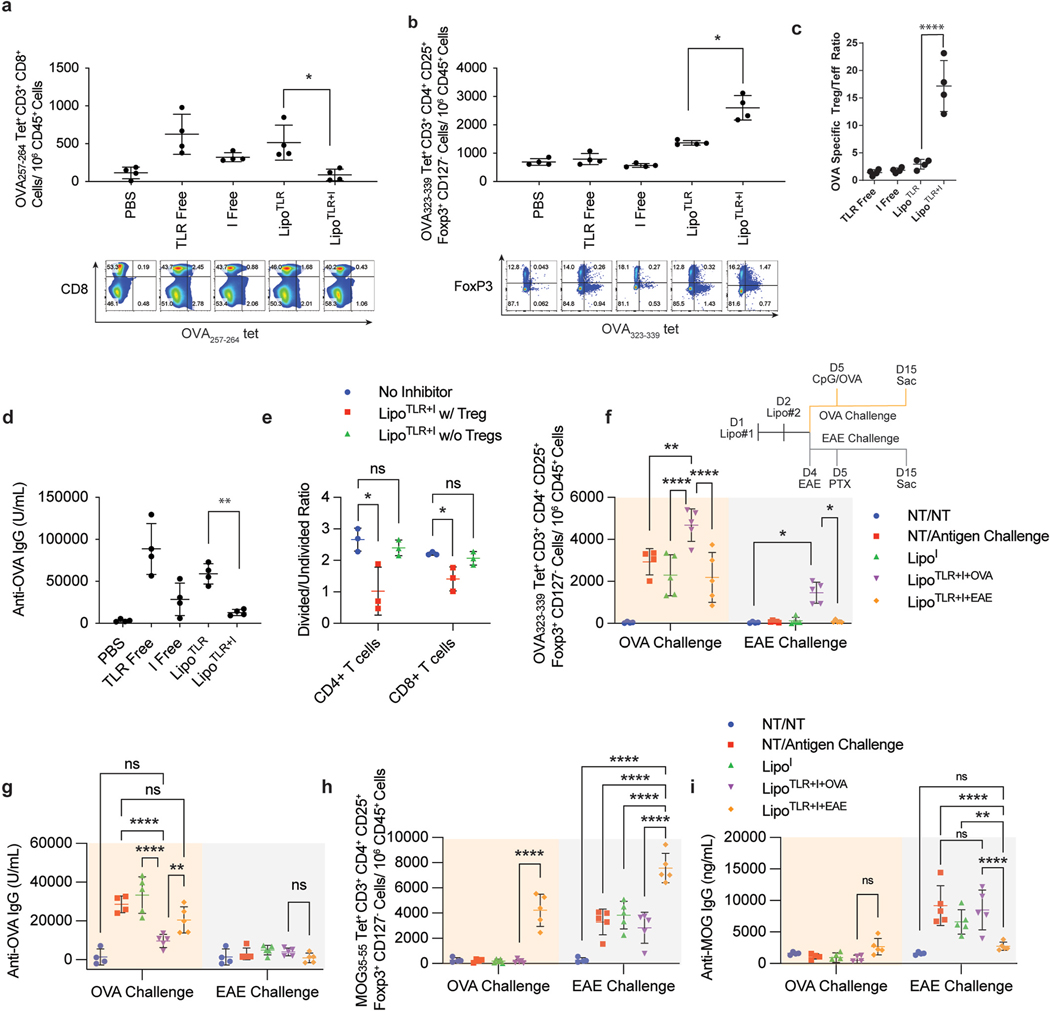
Fig. 3.
LipoTLR + I generate antigen specific Treg in vivo and Reduce Effector T Cells and IgGs. (A–C) C57BL/6 mice (4 per group) were injected with the following formulations: PBS control (PBS), OVA + free TLR agonists (TLR Free), OVA + Inhibitor free (I), liposomal OVA + TLR (LipoTLR) or liposomal OVA + Inhibitor combination (LipoTLR + I). Each formulation included combinations of 100 μg OVA/mouse, 10 μmol inhibitors/mouse, 1 μg FLA/mouse, 10 μg CpG/mouse. (A) On day 10, mice were sacrificed, their lymph nodes disassociated, the removed cells were stained and analyzed via flow. Lymph cells were analyzed for the number of CD45+, CD3+, CD8+, MHCI OVA epitope tetramer positive effector T cells were calculated. Representative flow plots of CD45+, CD3+, CD8+ cells showing distribution of CD8 (y axis) and major OVA257–264 MHCI tetramer signal (x axis). (B) Lymph cells from part A were also analyzed for the number of CD3+, CD4+, CD127−, FoxP3+, and OVA323–339 MHCII tetramer positive cells (OVA specific T regs) were calculated. Below are representative flow plots of CD45+, CD3+, CD4+ cells showing distribution of major OVA323–339 MHCII tetramer signal (x axis) and FoxP3 (y axis). (C) OVA specific Treg (from part A)/OVA specific T effector (from part B) ratio. (D) 10 days after last injection, serum was sampled and analyzed via ELISA for anti-OVA IgG. (E) Splenocytes from mice in part A were isolated, stained with CFSE and allowed to incubate with BMDCs for 16 h (3:1 spleenocytes to BMDCs). The cell mixture was then incubated with either the major MHCI epitope (for CD8 cells) or MHCII epitope (for CD4 cells) from OVA for 48 h. T-cell proliferation of spleenocytes was assessed via CFSE assay for both CD4 and CD8 cells. (F–I) LipoTLR + I treatment is selective for treatment antigen. Liposome formulations similar to part A were loaded with either OVA (100 μg/mouse) or MOG35–55 peptide (10 μg/mouse) and injected into C57Bl/6 mice on day 1 (FLA formulation) and day 2 (CpG formulation) (N = 5). Mice were then either challenged with CpG/OVA (10 μg/mouse/100 μg/mouse) on day 5 or injected to induce MOG specific EAE disease on day 4 and 5 (see methods). See experiment schematic (lower right of part E). On day 15, all mice were sac’d, popliteal lymph nodes analyzed via flow cytometery for antigen specific T cell populations and blood analyzed for anti-MOG or OVA IgG titers. (E) OVA323–339 MHCII tetramer + CD4+ T cell populations, (F) Anti-OVA IgG concentrations on day 15. (G) MOG35–55 peptide MHCII tetramer + T reg cells. (H) MOG35–55 peptide specific IgGs. Error bars indicate ± of SD of each mouse group (N = 4–5). Significance was determined by a two-way ANOVA with Tukey post hoc test for multiple comparisons. *p < 0.5, **p < 0.01, ***p < 1 × 10−4, ****p < 1 × 10−5..
While this data strongly supports that the tolerogenic liposome formulation suppresses immunity and generates Tregs and tolDCs, we sought to confirm that this suppression is selectively antigen specific. To show this selectivity, we prepared two different antigen-bearing formulations of LipoTLR + I, one with OVA and one with the major peptide epitope of myelin oligodendrocyte glycoprotein (MOG35–55). Mice were then challenged with either CpG/OVA or with the mouse model experimental autoimmune encephalomyelitis (EAE) that generate specific immune responses against MOG (Fig. 3E schematic). We hypothesized that mice tolerized against OVA would not prevent usual immune responses against EAE and vice versa, but would generate tolerance against the antigen included in the treatment. After mice were sacrificed on day 15, blood was analyzed for MOG and OVA specific IgGs and inguinal lymph nodes analyzed for antigen specific Tregs using tetramers to the major MHCII epitopes of MOG and OVA (Fig. 3E–H) As expected, OVA-LipoTLR + I significantly increased OVA-specific Treg populations compared to CpG/OVA challenge alone, while MOG-LipoTLR + I did not (Fig. 3E). A similar trend was seen with anti-OVA IgG, with OVA specific treatments reducing OVA-IgGs but MOG specific treatments not (Fig. 3F). The antigen specificity was further confirmed when MOG-LipoTLR + I only increased MOG specific Tregs and decreased MOG IgGs (Fig. 3G and H, see Fig. S-10 for further analysis). Antigen specificity was also seen in intracellular staining (ICS) of splenocytes from these mice, although it should be noted that while LipoTLR + I significantly reduced INFγ+ and IL17A + CD4 T cells and INFγ+CD8 T cells in an antigen specific fashion, liposomal treatments had less of an effect on IL4+ CD4+ T cells (Fig. S-10). Overall, these data provides strong evidence that LipoTLR + I generates antigen specific tolerance.
2.2. Tolerogenic liposomes generate lasting Treg mediated suppression in EAE animal models
The last goal of our study was to validate our tolerance system in a mouse model of an autoimmune disease. We chose the EAE – a model of multiple sclerosis (MS) [35]. In this model, a small peptide specific to MOG in mice is injected with a combination of strong immune stimulants to generate antigen specific T and IgG responses that destroy mouse oligodendrocytes – ultimately resulting in severe paralysis. EAE was chosen because 1) of its wide use as a tolerance model of a major and life-threatening autoimmune disease (MS), 2) it has commercially available tools to assess antigen specific responses and a well-established protocol for assessing disease progression, and 3) recent work in tolerizing mRNA systems have employed it as a model [13,36,37]. First, we performed a small pilot study where groups of 10 mice were given a prophylactic treatment of liposomes with tolerance compounds then induced to generate EAE autoimmune responses and compared to no pretreatment. This study determined the optimal method for inducing EAE and that our inhibitor combination can reduce EAE responses in a pretreatment (Fig. S-11).
A more clinically valuable experiment is induced EAE responses that are then treated as most autoimmune diseases are not diagnosed until after symptoms arise. We took mice (n = 14–15 per group) and induced EAE, then injected with either the MOG35–55+LipoTLR + I, MOG35–55+LipoI, free I, or PBS mock injections. Mice were injected with the two-dosing regimen similar to Fig. 3 on day 4/5 and repeated the treatment on day 6/7, then EAE symptoms were monitored for 30 days after final injection (Fig. 4A). LipoTLR + I and LipoI formulations prevented strong EAE symptoms from appearing almost entirely, but after three weeks the LipoI formulation began to show slight symptoms while the LipoTLR + I formulation showed no significant symptoms. This is important to note, as the push/pull system of TLR + I provided longer term symptom protection than just I alone which seemed to deteriorate after 3 weeks. Groups of 3–5 mice were similarly treated but sacrificed on day 14 after EAE induction to observe anti-MOG IgG titers, CD4+ EAE tetramer+ T cell and MOG35–55 Tetramer+ Treg populations. The LipoTLR + I group showed the lowest anti-MOG IgG, lowest CD4+ MOG35–55 tetramer+ T cells and highest MOG35–55 tetramer+ Treg populations, demonstrating that our T formulation was protecting mice from symptoms through a Treg mechanism (Fig. 4B–D). Finally, similar to Fig. 3, splenocytes from these groups had the smallest levels of T cell proliferation in response to antigen challenge and lowest levels of inflammatory/T cell cytokines (Fig. S-12).
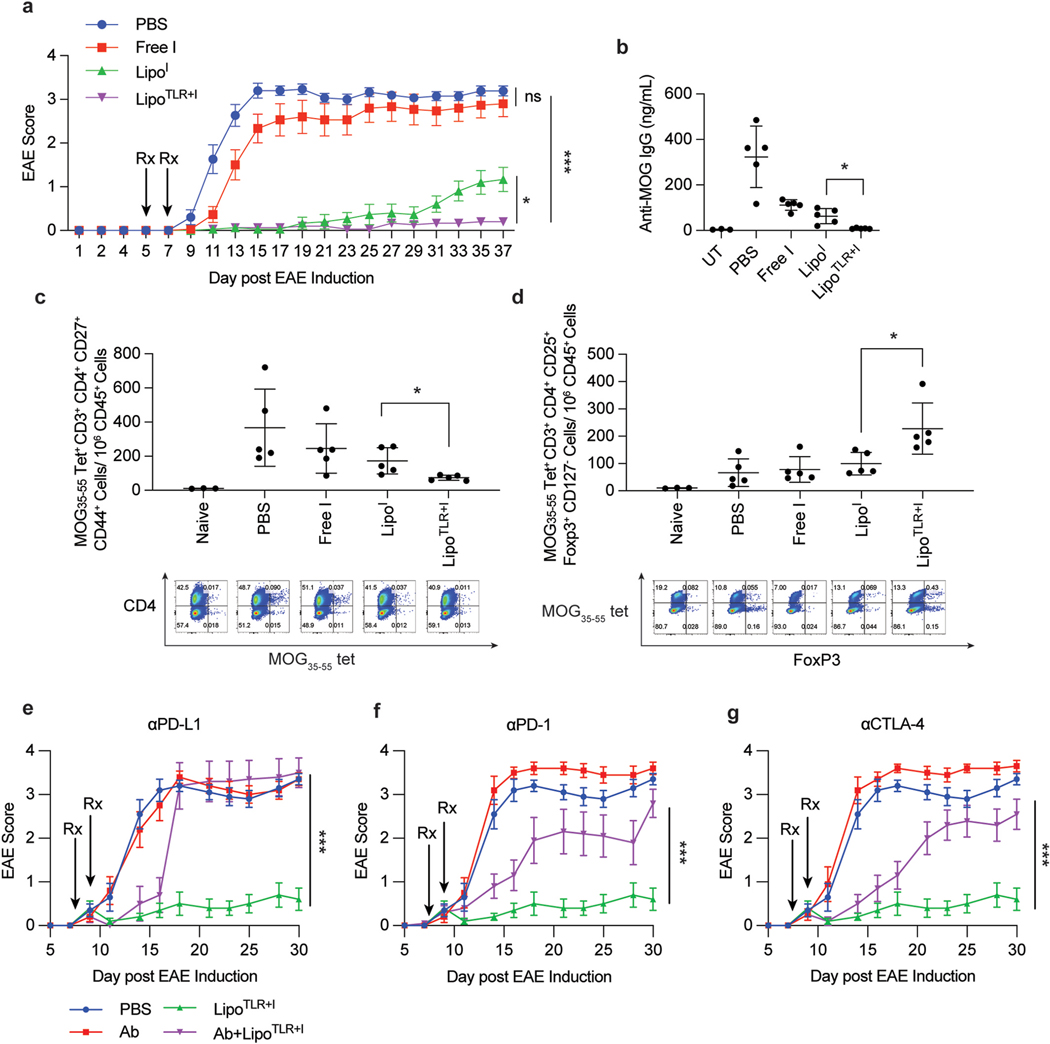
Fig. 4.
Tolerogenic Liposomes Prevent EAE Disease Progression via Antigen Specific Tolerance. (A) C57BL/6 mice (14–15 mice per group) were injected on consecutive days to induce EAE following procedure in methods section. Following final injection, the disease was allowed to progress for 5 days, at which point mice were injected with treatment candidates on day 4 and 5, allowed to rest for 48 h then treated again on day 7 and 8. Treatment groups were: PBS (on both days), Free I, LipoI, or LipoTLR + I. For all TLR containing formulations, the FLA formulation was given first (e.g day 4), then 24 h later the CpG formulation was administered (e.g. day 5). All formulations (except PBS) contained 10 μg of MOG peptide in each 100 μL injection. and MOG35–55 peptide was fully encapsulated in liposomal formulations. After final treatment, mice were monitored for 37 days and disease progression tracked. Error bars represent ±SEM of disease score. (B) Groups of 5 mice were treated similarly to part A, but sacrificed on day 14 following EAE induction. Serum was taken and analyzed for and MOG35–55 peptide specific IgG levels via ELISA. UT denotes an “untreated” mouse, a naïve C57BL/6 mouse without and MOG35–55 exposure (C) Lymph nodes from mice in part B were dissociated and lymph cells were stained for analysis of T-cells. The number of EAE peptide tetramer positive CD4+ activated T cells is shown. A representative flow plots of CD45+, CD3+, CD4+ cells show a distribution of CD4 (y axis) and MOG35–55 peptide tetramer (x axis). (D) and MOG35–55 peptide tetramer positive T reg cells from lymph nodes in part C. Below is representative flow plots of CD45+, CD3+, CD4+ cells showing distribution of MHCII-MOG peptide tetramer (y axis) and FoxP3 (x axis). (E–G) C57Bl/6 mice (N = 10) were similarly treated as in part A with LipoTLR + I, but with treatment starting on day 8 after EAE induction. Mice were then injected i.p with PBS or antibodies against mouse (E) PD-L1, (F)PD-1 or (G) CTLA-4. Mice were injected with 500 μg antibody on day 7 and day 10 and 250 μg antibody on day 14 and day 17. Statistical significance of AUC differences of EAE disease development curves was assessed by using one-way ANOVA and Tukey’s multiple comparison test in (A) and (E–G). Significance for B-D was determined by a two-way ANOVA with Tukey post hoc test for multiple comparisons. *p < 0.5, **p < 0.01, ***p < 1 × 10−4, ****p < 1 × 10−5..
This data provides strong evidence that the LipoTLR + I formulation can abrogate autoimmune responses in a relevant disease model and given prior data strongly suggest this is Treg driven. To provide additional evidence that the EAE protection is due to Treg mediated tolerance, we induced EAE in C57Bl/6 mice, waited 7 days, then treated with MOG-LipoTLR + I or MOG-LipoTLR + I + blocking antibodies against PD-L1, PD-1 or CTLA-4. First, this experiment would demonstrate that LipoTLR + I treatment could protect against EAE even after symptoms have begun and allow the mice to recover. Second, if this protection is Treg driven, blocking PD-1 or CTLA-4 would diminish the protection, and if tolDCs are important for developing EAE protection, blocking PD-L1 would reduce EAE protection [11,23]. The results show that all three markers are critical for generation and maintenance of LipoTLR + I EAE protection, as mice co-treated with liposomes and antibodies had reduced or non-existent protection (Fig. 4E–G). αPD-L1 treated mice had rapid deterioration of protection, indicating that initial delay in symptoms could be due to non-specific immune suppression of the inhibitors in the liposome, but lacked any Treg generation. αPD-1 and αCTLA-4 treated mice had some protection, suggesting that Tregs developed but were rendered ineffective due to inhibitory receptor blocking. Finally, this data critically shows that LipoTLR + I treatment can recover mice who have already begun to show initial symptoms of EAE.
3. Conclusions
Here we present evidence of a new method for inducing antigen specific Tregs as a treatment for autoimmune diseases via tolerogenic liposomes containing immunomodulators and TLR agonists. This method of combining TLR agonists and inhibitory compounds is a versatile therapy and could potentially be adapted to many autoimmune conditions as long as the antigen of interest is known. This offers the potential for tailoring this approach to many promising clinical applications. Also of importance, this paper presents new immunological findings. First, we showed that a cocktail of three immunomodulators, dexamethasone, simvastatin and SC-514, work synergistically to generate tolDCs. Dexamethasone and simvastatin have been used in combination for treatment of rheumatoid arthritis. Simvastatin in combination with other steroids can generate Treg cells [38,39]. Simva statin can increase the IL-10 production in the clinic by increasing transcription of indoleamine 2,3-dioxygenase and it is likely that this increase in combination with the anti-inflammatory properties of dexamethasone contribute to tolDC differentiation [40].
However, in these new finding we present that SC-514, an NF-kB inhibitor, and a TLR agonist work synergistically, in a proposed push/pull system, with these drugs to increase tolDCs. One possible explanation for these results is that the increase in tolDCs is due to the effect of silencing NF-kB driven STAT4/TLR signaling – though this is currently just a conjectured mechanism. The resulting phenotype of the cells is a triggered immune “activation” and antigen presentation, pushing the system, without suppressed inflammatory signals resulting in incomplete naïve CD4 T cell activation – pulling it toward tolerance. There is some prior evidence that combining a TLR agonist with an immune modulator can activate already existing tolDCs to more actively suppress immunity and generate Tregs [41]. Therefore, we hypothesize that by selectively attenuating the TLR response with SC-514, we further drive DCs into a more tolerogenic phenotype while still promoting antigen presentation [42]. The result is generation of strong tolDC phenotypes that also present the autoantigen of interest and can more readily generate antigen specific Tregs. A recent study by Krienke et al. achieved tolDC phenotypes through a similar insufficient activation mechanism by modifying mRNA for EAE antigens to reduce their immunogenicity, demonstrating another mechanism for tolDC induction with similar promising results in the EAE model [37]. Both finding support the idea that an insufficient activation mechanism may provide a new avenue for inducing immune tolerance. Our system also has a distinct advantage in that it does not require cold storage for administration.
Despite the successes of this study, there are questions that remain to be answers and potential improvements needed. While we have shown this tolerizing cocktail of TLR agonist, SC-514, simvastatin and dexamethasone to be highly effective in inducing tolDCs, we do not know the mechanism through which this phenotype is attained. Furthermore, we have limited data on how these cocktails effect other immune cells; while there is some effect of DC “targeting” via TLR agonists, TLRs exist on many immune cells and these all could be involved in secondary effects, although our EAE antibody blocking studies demonstrate how critical Treg involvement is for disease protection. Our initial studies have shown the promise of this approach and further study of the mechanism will help expand the potential of this approach.
In terms of clinical potential, the tolerogenic liposome formulation is highly promising; boasting reliable generation of antigen specific Tregs with minimal non-specific immune suppression. Still, there are several areas where this approach could be improved. The majority of these issues, such as lack of data on human cells or different autoimmune models, can potentially be remedied with further research. Furthermore, there is potential room for optimization of 1) the ratio of drugs and 2) type and size of nanocarrier formulation. One immutable drawback of this approach is that any effective tolerizing formulation will require a previous identification of a limited number autoimmune related self-protein or epitope to achieve antigen specificity, which is not the case for all autoimmune conditions. Given that some autoimmune self-antigens for major autoimmune diseases like RA, T1D and MS, have been identified and are fairly well conserved in human populations, we believe this strategy will still be useful in the clinic and will prove to be more useful as more antigens are discovered [43].
Notwithstanding the lack of detailed mechanistic insight on how our tolerogenic liposomal cocktail functions, it is clear from our data that it does induce antigen specific immune suppression via Tregs and has potential for clinical application. In conclusion, we showed that a combination of immune modulations and TLR agonists induce tolDC populations from naïve APCs and that these tolDCs induce Tregs in vivo. Finally, in a relevant disease model, we presented data that therapeutic treatment with tolerogenic liposomes is high effective in preventing autoimmune disease symptoms and removing auto-reactive IgG and T cells. With further research, LipoTLR + I could be a novel class of autoimmune therapies which can generate auto-antigen specific immune suppression.
4. Materials and methods
4.1. Study design
Sample size:
Experiments for all in vitro analysis were performed in biological triplicate on different days using different splits of the cell lines or from BMDCs derived from different animals. For in vivo assays, studies were performed in groups of at least 4 for all experimental groups and at least 3 for untreated control mice. Sample sizes were selected based on available materials and to observe preliminary results. No power analysis was performed to select the sample size. No outliers were removed or excluded from analysis.
Rules for stopping data collection:
For EAE experiments, mice were sacrificed when either they achieved an EAE score of 4 or when 20% of initial body weight was lost in accordance with our IACUC protocol.
Replicates:
In vitro data was performed in biological triplicate but with no replicates. ELISA data, CBA analysis and ICS data were performed in technical triplicates.
Research objectives:
Our primary hypothesis was that a combination of TLR stimulation and immune inhibition via small molecules generated more robust, long lived tolDCs that can generate more robust antigen specific T cell responses.
Research subjects or units of investigation.
Describe the type of research subjects (e.g., cancer patients, healthy volunteers), animals, or experimental units (e.g., cell cultures) studied.
Experimental design:
Initial identification of potent tolDC drug combinations were performed analyzing drug treated BMDCs via flow cytometry, cytokine release via ELISA, RAW blue assay, and a cell viability assay. Further analysis of tolerance liposomes were performed in vivo by injecting mice and waiting 1, 7 or 14 days post treatment, then analyzing antibody titers with ELISA, T cell responses via ICS and tetramer staining. Finally an EAE model was performed to assess effect of liposomes on clinical autoimmune disease models.
Randomization/Blinding-
Most in vitro work was not blinded or randomized, but in vivo work was performed in manually randomized groups. EAE experiment were performed in a double blinded fashion via an independent scorer from UChicago’s Animal Resource Center.
Materials
All chemicals and reagents unless noted otherwise were purchased from Sigma. ELISA kits and all fluorescently tagged antibodies were purchased from BioLegend. Live/Dead Aqua was purchased from Thermo Fisher and used according to manufacturer’s instructions. MHC I tetramer (OVA257–264-BV480 conjugate) was purchased from Tetramer Shop (Denmark). MHCII Tetramers (OVA323-339 –PE conjugate and MOG35–55-PE conjugate) were purchased from MBL International (Woburn, MA). All in vivo blocking antibodies were purchased from Bio X Cell. EAE kits and MOG peptide was purchased from Hookes Laboratory. Flagellin and ODN 1826 (CpG) and all other TLR agonists were purchased from Invivogen. RAW Blue cells and reagents were purchased from Invivogen. Liposome extruder equipment, membranes, filter supports, 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy (polyethylene glycol)-2000] (ammonium salt) (PEG2000 lipid), MBP-PE and succinyl-PE were purchased from Avanti Polar Lipids.
Cell Culture
All cells were kept at 5% CO2 and 37 °C during all incubations and maintained in either RPMI or DMEM supplanted with penicillin/streptomycin and 10% FBS (or heat inactivated FBS during assays). RAW 264.2 and RAW Blue cells were cultured at 5 × 106 cells per mL in DMEM and split 1:5 twice per week. DC2.4 cells were cultured at 2 × 106 cells per mL in RPMI and split three times per week.
BMDC Cell Culture
BMDCs were cultured according to a previously published protocol. Cells were used between 5 and 7 days after isolation [44].
Initial In Vitro Validation of Inhibitors
Combinations of inhibitors and TLR agonists were tested on 2 × 106 BMDCs and incubated for 20 h. BMDCs were then washed with PBS and challenged with a second TLR agonist (see Table S-1 for concentrations of inhibitors and agonists used). BMDCs were then tested for IL-10, IL-6 via ELISA, and CD103, CD80, and PD-L1 via flow cytometry.
RAW Blue NF-κB Assay:
RAW-Blue NF-κB cells (Invivogen) were challenged similarly to BMDCs as above. After 18 h of second TLR agonist challenge, 20 μL of the cell supernatant was placed in 180 μL freshly prepared QuantiBlue (Invivogen) solution and incubated at 37 °C/5% CO2 for up to 2 h. The plate was analyzed every hour using a Multiskan FC plate reader (Thermo Scientific) and absorbance was measured at 620 nm.
Flow Cytometry
Flow cytometry was performed on a ACEA NovaCyte Flow cytometer unless otherwise noted (6 channels, 2 laser). 1 million cells per sample were treated with liposomes/MPs/antibodies, washed and placed in HBSS+ 2% HIFBS +0.1 mM EDTA. Samples were gated on FCS and SCC for live and single cells. Samples were compensated based on sample with single stains and calculated using FlowJo.
ELISA-
TNFα, IL-6, TGF-β, and IL-10 levels were measured by commercially kits from BioLegend. TNFα and IL-6 supernatants were analyzed 4 h after CpG challenge and TGF-β and IL-10 supernatants were analyzed 16 h after CpG challenge.
Tolerance Score Calculation
The tolerance score from Table S-2 was calculated by combining data from IL-10, PD-L1, CD103, CD80, IL-6, CD40 and NFkB Raw blue data. Each type data was normalized between values of 1–100 for all combinations of TLR agonists and inhibitors. The tolerance score was calculated by adding “positive” tolerance values of IL-10, CD103 and PD-L1 and subtracting the negative tolerance values of CD80, IL-6, and NFkB Raw blue data then dividing by 6.
Animals
6 week C57BL/6 female mice were purchased from Jackson Laboratory and housed and treated according to our approved IACUC protocol.
Splenocyte/Lymph Node extraction
Splenocyte/Lymph Node were dissected into 1 mm sized portions and placed in disassociation media (0.5 mg/mL collagenase D, 0.1 mg/mL DNAase I in RPMI) for 30 min at room temp, then incubated at 37 °C for 30 min, then passed through a 70 μm filter. Spleenocytes were treated with RBC lysis buffer (Invitrogen) prior to final wash.
Cytokine Bead Array
a Mouse Inflammation CBA kit was purchased from BD Biosciences and used according to the manufacturer’s instructions. Mouse blood was spun down at 10,000 g for 10 min to remove cells and the supernatant tested undiluted. Supernatant from cell culture experiments was also used with no dilution.
Aurora Spectral Flow Analysis
A 5 laser Aurora spectral flow cytometer was used for phenotyping of all in vivo and ex vivo experiments. Primary splenocytes or lymph node samples were washed using cell staining buffer (StemCell Technologies), then resuspended in 100 μL (10 million cells for splenocytes and 1 million of lymph nodes), stained for live dead staining, washed, stained with Tetramers (if used) and FcX blocking antibody on ice for 15 min, washed 2x then stained for surface marker on ice for 1 h and immediately run on flow. Single stained compensation controls were run first and then unmixed using the Aurora spectral flow software. Data was further analyzed in FlowJo.
Flagellin-lipid (FLA-lipid) Conjugation
0.5 mg of Succinyl-PE (C16, Avanti) was incubated with 1 mg of 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and 1 mg of N-hydroxysulfosuccinimide in PBS (pH 7.4) for 10 min, then incubated with 0.5 mg of FLA for 1 h. FLA-lipid was dialyzed with a 3.5 kDa membrane for 24 h against PBS. FLA-lipid was analyzed via LC-MS to confirm approximately 1 conjugation per protein (+796 Da from un-conjugated protein). SDS-PAGE analysis was performed on a 4–15% Mini-PROTEAN TGX precast gel (Bio-Rad) and stained with Coomassie blue. >95% of FLA was increased in molecular weight as seen in Fig. S-4.
GpG-Lipid Conjugation
The CpG-lipid procedure was adopted from a previous published source.(30) 0.5 mg of MPB-PE (C16, Avanti) was dissolved in 15 mL diethyl ether and Thiol conjugated CpG made with phosphodiester bonds (purchased from Integrated DNA Technologies) (CpG-SH) was dissolved in 5 mL water. The two solutions were combined and vortexed for 16 h, the aqueous layer was retained, washed three times with chloroform, lyophilized, rehydrated in PBS and purified with a 3.5 kDa dialysis membrane against PBS. GpG lipid was analyzed by SDS-PAGE and run on a 2.5% agarose-TBE gel and stained with Sybr Safe DNA stain (thermo) and determined to have approximately 40% purity. CpG lipid was also analyzed via LC-MS to confirm one lipid addition per CpG (7793.8 Da measured (M – 1H), 7794.8 Da expected).
Liposome Synthesis
Liposomes were synthesized via membrane extrusion method using a setup from Avanti polar lipids and 200 nm extrusion filters. Blank liposomes were synthesized by combining 2 μmols of total lipid (DSPC, PEG2000 PE, Cholesterol at a 90/5/5 ratio), drying via rotoevaporation followed by lyophilization, and rehydrated in PBS to make a 10 mM total lipid, 200 μL solution. TLR agonist-lipids were added during initial mixing to final concentration of 10 μg/mL FLA-lipid or 100 μg/mL CpG-lipid. Inhibitors (Dex, sim and SC-514) were dissolved in EtOH and added to final concentrations of 10 mM (total inhibitor) prior to drying. 1 mg/mL OVA in PBS was added during rehydration. Solutions were gently rotated at 67 °C and then passed through a 70 °C 200 nm filter 5 times. All liposomes were dialyzed against PBS (1:10,000) with a 100,000 Da filter at 4 °C for 24 h prior to use.
LC-MS Analysis of Liposome Loading
Liposomes loaded with FLA or CpG lipids, OVA/EAE peptide and inhibitors or any combinations of these were tested for loading via LC-MS analysis. Initial loading (Figure S-4E and S-4F) was calculated by analyzing liposome solutions for presence of TLR-lipids, inhibitor or antigens before and after 24 h dialysis at 4 °C against a 100 kDa membrane. For liposome release study (Figure S–4G), liposomes already dialyzed for 24 h at 4 °C were further dialyzed against a 100 kDa membrane for 1, 2, or 4 h at 37 °C, then liposome solutions analyzed via HPLC. Stock solutions of liposomes were diluted down to 1 mM total lipid and then 5 μL of this solution was injected on a C8 analytical HPLC column with a gradient of 10–90% ACN in 20 min and the effluent observed by an Agilent 6135BAR LCMS XT mass spectrometer and a diode array detector at 220 nm. Signal from various compounds in liposome formulations were compared to standard curves at 220 nm (for antigens or FLA-lipid) or 254 nm (for inhibitors or CpG lipid).
Dynamic Light Scattering Analysis of Liposomes
Hydrodynamic diameter of liposomes was confirmed using a Wyatt Möbiuζ DLS/ELS at 100 μM total liposome concentration.
Transmission Electron Microscopy
TEM was performed on a FEI Tecnai G2 F30 300 kV Super Twin Electron Microscope with assistance from UChicago’s Advanced Electron Microscopy Core. Briefly, blank liposomes, CpG-liposomes or FLA-liposomes were dried on plasma clean carbon grid films by adding 0.05 mg/mL liposome solution, diluted in 10 mM HEPES buffer (pH 7.4) briefly then drying. Films were then negatively stained with 1% uranyl acetate 3 times, dried and imaged at 10,000× and 25,000× magnifications. Images were processed in ImageJ, mean diameters calculated using the particle sizing wizard.
Liposome Ex vivo uptake experiment
Blank, CpG and FLA liposomes were tested for their initial uptake on splenocytes. Mouse spleens were extracted and disassociated using 0.5 mg/mL collagenase D and 0.1 mg/mL DNAse I in RPMI media for 30 min, mashed with the back side of a syringe and filtered with a 70 μm filter. RBCs were then lysed using ACK lysis buffer (1x) for 10 min. Remaining cells were washed 2x in HBSS, and diluted and allowed to rest in 1 mL of RPMI+ 10% HIFBS + 1X HEPES/BME for 2 h at 10 million cells/well. Splenocytes were then incubated with 10 μM total lipid of 200 nM Blank, FLA or CpG liposomes containing 0.1% DiD dye for 30 min at 37 °C. Splenocytes were then washed using flow cytometry buffer (Stem Cell Technologies), stained for live/dead (Live Dead Aqua) for 30 min, washed and stained for various cell surface markers for 1 h. Cells were washed and analyzed using an Aurora Spectral Flow Cytometer.
In vivo vaccination studies
200 nm liposomes were generated loaded with 1 mg/mL OVA, 10 mM of a 1:1:1 ratio of SC-514:Dex:Sim and either 10 μg/mL FLA-lipid or 100 μg/mL CpG-lipid. Liposomes were extruded and then dialyzed for 24 h against PBS. Loading verified by HPLC (Fig. S-4)indicated that inhibitors loaded at 15% for Dex or Sim and 2% for SC-514. Loading for OVA was 40% and >90% for CpG and FLA-lipids. Mice were injected with 100 μL of inhibitor liposome with FLA-lipid or liposomes with no inhibitor and FLA-lipid on day 1 and CpG-lipid formulations on day 2. For experiments in Fig. 2A–H, mice were sacrificed on day 3 and lymph and spleen cell analyzed for tolDC increase. For experiments in Fig. 2I–N, mice were sacrificed on day 10 and spleen and lymph node cells analyzed and serum tested for anti-OVA IgGs using an ELISA kit from Alpha Diagnostics.
T cell Proliferation Assay
T cells were extracted from mouse spleens taken on day 10 after in vivo vaccination study as described in the above protocol and magnetically sorted using a CD4+ T cell extraction kit from Stem Cell Technologies. Half of the T cell sample was further subjected to isolation of Treg cells using a Treg isolation kit from Stem Cell Technologies. 300 K of each of the three T cells populations (All T cell, Tregs only and Treg Depleted) were incubated in T cell culture media (RPMI+ 10% HIFBS + Pen/Strep + 1 mM HEPES + 50 μM beta mercaptoethanol) then stained with CFSE (10 μM) for 30 min then washed an incubated with 100 k BMDCs for 24 h. The following day, the cell mixture was incubated with either OVA 257–264 peptide (major MHC I epitope) or OVA 323–339 peptide (major MHCII epitope) for 48 h. Supernatant was analyzed via a Mouse Inflammation Cytokine Bead Array (CBA) from BD Biosciences. Cells were then stained for CD3, CD4, CD8 and CD11c and analyzed for proliferation of CD3+, CD4+, CD11c- and CD3+ CD8+, CD11c-populations based on CFSE decrease.
EAE
The Monophasic Model Experimental autoimmune encephalomyelitis (EAE) was established according to established protocols (35). C57BL/6 mice (20 per group) were induced with EAE using a kit from Hookes Laboratory. MOG peptide injection with complete Freud’s Adjuvant (CFA) was administered on day 1 by injection into all 4 hocks followed by pertussis toxin (200 ng per mouse) 1 h later and 24 h later. On day 4, mice were treated with FLA containing liposomes and on day 5 treated with CpG containing liposomes similar to experiments in Fig. 2. Groups with no TLR agonists were given the same injections on consecutive days. On day 14, 5 mice per group were sacrificed, anti-MOG IgG titers analyzed via ELISA, and spleen and lymph cells analyzed in similar fashion as experiments from Fig. 2. Mice from Fig. 4E–G were injected with EAE on day 8 and 9 and injected with antibodies on day 7 and day 10 (500 μg per mouse) and on day 14 and 17 (250 μg per mouse).
Statistical Analysis:
All of the data represent at least two independent experiments. All statistical analyses were performed using Prism 7. The statistical methods used are indicated in the corresponding Fig. captions. Statistically significant differences are indicated by asterisks.
Supplementary Material
Acknowledgments
We would like to thank the University of Chicago’s Cytometry and Antibody Technology Core and the Light Microscopy Core for use of their instruments and consultation. We would also like to thank The University of Chicago Advanced Electron Microscopy Core Facility (RRID:SCR_019198) for help with the TEM experiments. We would also like to thank the Animal Resource Center for their aid with animal experiments, especially Ani Solanki for weighing and grading EAE experiments.
Funding
National Institute of Health grant 7U01AI124286-03, 75N93019C00041 (AEK), National Institute of Health grant 5 F32 AI147517 (PED).
Abbreviations:
- DC
Dendritic Cell
- TolDC
Tolerogenic Dendritic Cell
- Treg
T regulatory Cell
- TLR
Toll-like-receptor
- OVA
Ovalbumin
- EAE
Experimental autoimmune encephalomyelitis
- MOG
Myelin oligodendrocyte glycoprotein
- MS
Multiple sclerosis
- T1D
Type 1 diabetes
- CBA
Cytokine bead array
- BMDC
Bone marrow derived dendritic cell
- FLA
flagellin
- CpG
ODN 1826
- dex
dexamethasone
- RA
rheumatoid arthritis
- ICS
intracellular staining
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.biomaterials.2022.121571.
Data and materials availability
All data are available in the main text or the supplementary materials.
References
- [1].Carballido JM, Regairaz C, Rauld C, Raad L, Picard D, Kammüller M, The emerging jamboree of transformative therapies for autoimmune diseases, Front. Immunol. 11 (2020), 10.3389/fimmu.2020.00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hayter SM, Cook MC, Updated assessment of the prevalence, spectrum and case definition of autoimmune disease, Autoimmun. Rev. 11 (2012) 754–765. [DOI] [PubMed] [Google Scholar]
- [3].Serra P, Santamaria P, Antigen-specific therapeutic approaches for autoimmunity, Nat. Biotechnol. 37 (2019) 238–251. [DOI] [PubMed] [Google Scholar]
- [4].Romano M, Fanelli G, Albany CJ, Giganti G, Lombardi G, Past, present, and future of regulatory T cell therapy in transplantation and autoimmunity, Front. Immunol. 10 (2019), 10.3389/fimmu.2019.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bystander Immunotherapy as a Strategy to Control Allergen-Driven Airway Inflammation | Mucosal Immunology, (available at: https://www.nature.com/articles/mi2014115). [DOI] [PMC free article] [PubMed]
- [6].Roep BO, Solvason N, Gottlieb PA, Abreu JRF, Harrison LC, Eisenbarth GS, Yu L, Leviten M, Hagopian WA, Buse JB, von Herrath M, Quan J, King RS, Robinson WH, Utz PJ, Garren H, BHT-3021 Investigators, L. Steinman, Plasmid-encoded proinsulin preserves C-peptide while specifically reducing proinsulin-specific CD8+ T cells in type 1 diabetes, Sci. Transl. Med. 5 (2013) 191ra82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chen J, Wang Q, Yin D, Vu V, Sciammas R, Chong AS, Cutting edge: CTLA-4Ig inhibits memory B cell responses and promotes allograft survival in sensitized recipients, J. Immunol. 195 (2015) 4069–4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kenison JE, Jhaveri A, Li Z, Khadse N, Tjon E, Tezza S, Nowakowska D, Plasencia A, Stanton VP, Sherr DH, Quintana FJ, Tolerogenic nanoparticles suppress central nervous system inflammation, Proc. Natl. Acad. Sci. Unit. States Am. 117 (2020) 32017–32028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Biomaterial Strategies for Immunomodulation | Annual Review of Biomedical Engineering, (available at: https://www.annualreviews.org/doi/abs/10.1146/annurev-bioeng-071813-104814). [DOI] [PMC free article] [PubMed]
- [10].Kishimoto TK, Maldonado RA, Nanoparticles for the induction of antigen-specific immunological tolerance, Front. Immunol. 9 (2018), 10.3389/fimmu.2018.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Domogalla MP, Rostan PV, Raker VK, Steinbrink K, Tolerance through education: how tolerogenic dendritic cells shape immunity, Front. Immunol. 8 (2017), 10.3389/fimmu.2017.01764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gregori S, Tomasoni D, Pacciani V, Scirpoli M, Battaglia M, Magnani CF, Hauben E, Roncarolo M-G, Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway, Blood 116 (2010) 935–944. [DOI] [PubMed] [Google Scholar]
- [13].Cho JJ, Stewart JM, Drashansky TT, Brusko MA, Zuniga AN, Lorentsen KJ, Keselowsky BG, Avram D, An antigen-specific semi-therapeutic treatment with local delivery of tolerogenic factors through a dual-sized microparticle system blocks experimental autoimmune encephalomyelitis, Biomaterials 143 (2017) 79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lewis JS, Stewart JM, Marshall GP, Carstens MR, Zhang Y, Dolgova NV, Xia C, Brusko TM, Wasserfall CH, Clare-Salzler MJ, Atkinson MA, Keselowsky BG, Dual-sized microparticle system for generating suppressive dendritic cells prevents and reverses type 1 diabetes in the nonobese diabetic mouse model, ACS Biomater. Sci. Eng. 5 (2019) 2631–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Odobasic D, Oudin V, Ito K, Gan P-Y, Kitching AR, Holdsworth SR, Tolerogenic dendritic cells attenuate experimental autoimmune antimyeloperoxidase glomerulonephritis, J. Am. Soc. Nephrol. 30 (2019) 2140–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Boks MA, Kager-Groenland JR, Haasjes MSP, Zwaginga JJ, van Ham SM, ten Brinke A, IL-10-generated tolerogenic dendritic cells are optimal for functional regulatory T cell induction — a comparative study of human clinical-applicable DC, Clin. Immunol. 142 (2012) 332–342. [DOI] [PubMed] [Google Scholar]
- [17].Pulendran B, Tang H, Manicassamy S, Programming dendritic cells to induce T (H)2 and tolerogenic responses, Nat. Immunol. 11 (2010) 647–655. [DOI] [PubMed] [Google Scholar]
- [18].Amiel E, Everts B, Fritz D, Beauchamp S, Ge B, Pearce EL, Pearce EJ, Mechanistic target of rapamycin inhibition extends cellular lifespan in dendritic cells by preserving mitochondrial function, J. Immunol. 193 (2014) 2821–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Obregon C, Kumar R, Pascual MA, Vassalli G, Golshayan D, Update on dendritic cell-induced immunological and clinical tolerance, Front. Immunol. 8 (2017), 10.3389/fimmu.2017.01514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Xing Y, Hogquist KA, T-cell tolerance: central and peripheral, Cold Spring Harbor Perspect. Biol. 4 (2012) a006957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Akira S, Hemmi H, Recognition of pathogen-associated molecular patterns by TLR family, Immunol. Lett. 85 (2003) 85–95. [DOI] [PubMed] [Google Scholar]
- [22].de Vos AF, Pater JM, van den Pangaart PS, de Kruif MD, van ‘t Veer C, van der Poll T, In vivo lipopolysaccharide exposure of human blood leukocytes induces cross-tolerance to multiple TLR ligands, J. Immunol. 183 (2009) 533–542. [DOI] [PubMed] [Google Scholar]
- [23].Frontiers | Generation and Function of Induced Regulatory T Cells | Immunology, (available at: https://www.frontiersin.org/articles/10.3389/fimmu.2013.00152/full). [DOI] [PMC free article] [PubMed]
- [24].Akira S, Takeda K, Toll-like receptor signalling, Nat. Rev. Immunol. 4 (2004) 499–511. [DOI] [PubMed] [Google Scholar]
- [25].Dudek AM, Martin S, Garg AD, Agostinis P, Immature, semi-mature, and fully mature dendritic cells: toward a DC-cancer cells interface that augments anticancer immunity, Front. Immunol. 4 (2013), 10.3389/fimmu.2013.00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].You Y, Qin Y, Lin X, Yang F, Li J, Sooranna SR, Pinhu L, Methylprednisolone attenuates lipopolysaccharide-induced Fractalkine expression in kidney of Lupus-prone MRL/lpr mice through the NF-kappaB pathway, BMC Nephrol. 16 (2015), 10.1186/s12882-015-0145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Plesner T, Arkenau H-T, Gimsing P, Krejcik J, Lemech C, Minnema MC, Lassen U, Laubach JP, Palumbo A, Lisby S, Basse L, Wang J, Sasser AK, Guckert ME, de Boer C, Khokhar NZ, Yeh H, Clemens PL, Ahmadi T, Lokhorst HM, Richardson PG, Phase 1/2 study of daratumumab, lenalidomide, and dexamethasone for relapsed multiple myeloma, Blood 128 (2016) 1821–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Aprahamian T, Bonegio R, Rizzo J, Perlman H, Lefer DJ, Rifkin IR, Walsh K, Simvastatin treatment ameliorates autoimmune disease associated with accelerated atherosclerosis in a murine lupus model, J. Immunol. 177 (2006) 3028–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Galea R, Nel HJ, Talekar M, Liu X, Ooi JD, Huynh M, Hadjigol S, Robson KJ, Ting YT, Cole S, Cochlin K, Hitchcock S, Zeng B, Yekollu S, Boks M, Goh N, Roberts H, Rossjohn J, Reid HH, Boyd BJ, Malaviya R, Shealy DJ, Baker DG, Madakamutil L, Kitching AR, O’Sullivan BJ, Thomas R, PD-L1– and calcitriol-dependent liposomal antigen-specific regulation of systemic inflammatory autoimmune disease, JCI Insight 4 (2019), 10.1172/jci.insight.126025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Andrews CD, Provoda CJ, Ott G, Lee K-D, Conjugation of lipid and CpG-containing oligonucleotide yields an efficient method for liposome incorporation, Bioconjugate Chem. 22 (2011) 1279–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Liu J, Zhang X, Cheng Y, Cao X, Dendritic cell migration in inflammation and immunity, Cell. Mol. Immunol. 18 (2021) 2461–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Macri C, Pang ES, Patton T, O’Keeffe M, Dendritic cell subsets, Semin. Cell Dev. Biol. 84 (2018) 11–21. [DOI] [PubMed] [Google Scholar]
- [33].Maji M, Mazumder S, Bhattacharya S, Choudhury ST, Sabur A, Shadab M, Bhattacharya P, Ali N, A lipid based antigen delivery system efficiently facilitates MHC class-I antigen presentation in dendritic cells to stimulate CD8+ T cells, Sci. Rep. 6 (2016) 27206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bartneck M, Peters FM, Warzecha KT, Bienert M, van Bloois L, Trautwein C, Lammers T, Tacke F, Liposomal encapsulation of dexamethasone modulates cytotoxicity, inflammatory cytokine response, and migratory properties of primary human macrophages, Nanomed. Nanotechnol. Biol. Med. 10 (2014) 1209–1220. [DOI] [PubMed] [Google Scholar]
- [35].Constantinescu CS, Farooqi N, O’Brien K, Gran B, Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS), Br. J. Pharmacol. 164 (2011) 1079–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nelson LM, Wallin MT, Marrie RA, Culpepper WJ, Langer-Gould A, Campbell J, Buka S, Tremlett H, Cutter G, Kaye W, Wagner L, Larocca NG, for the USMSP Workgroup, A new way to estimate neurologic disease prevalence in the United States: illustrated with MS, Neurology 92 (2019) 469–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Krienke C, Kolb L, Diken E, Streuber M, Kirchhoff S, Bukur T, Akilli-Öztürk Ö, Kranz LM, Berger H, Petschenka J, Diken M, Kreiter S, Yogev N, Waisman A, Karikó K, Türeci Ö, Sahin U, A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis, Science 371 (2021) 145–153. [DOI] [PubMed] [Google Scholar]
- [38].Ahmed YM, Messiha BAS, Abo-Saif AA, Protective effects of simvastatin and hesperidin against complete freund’s adjuvant-induced rheumatoid arthritis in rats, Pharmacology 96 (2015) 217–225. [DOI] [PubMed] [Google Scholar]
- [39].Maneechotesuwan K, Kasetsinsombat K, Wamanuttajinda V, Wongkajornsilp A, Barnes PJ, Statins enhance the effects of corticosteroids on the balance between regulatory T cells and Th17 cells, Clin. Exp. Allergy 43 (2013) 212–222. [DOI] [PubMed] [Google Scholar]
- [40].Maneechotesuwan K, Ekjiratrakul W, Kasetsinsombat K, Wongkajornsilp A, Barnes PJ, Statins enhance the anti-inflammatory effects of inhaled corticosteroids in asthmatic patients through increased induction of indoleamine 2, 3-dioxygenase, J. Allergy Clin. Immunol. 126 (2010) 754–762, e1. [DOI] [PubMed] [Google Scholar]
- [41].Chamorro S, García-Vallejo JJ, Unger WWJ, Fernandes RJ, Bruijns SCM, Laban S, Roep BO, ‘t Hart BA, van Kooyk Y , TLR triggering on tolerogenic dendritic cells results in TLR2 up-regulation and a reduced proinflammatory immune program, J. Immunol. 183 (2009) 2984–2994. [DOI] [PubMed] [Google Scholar]
- [42].Xue Y-L, Zhang S-X, Zheng C-F, Li Y-F, Zhang L-H, Hao Y-F, Wang S, Li X-W, Silencing of STAT4 protects against autoimmune myocarditis by regulating Th1/Th2 immune response via inactivation of the NF-κB pathway in rats, Inflammation 42 (2019) 1179–1189. [DOI] [PubMed] [Google Scholar]
- [43].Martini S, Nielsen M, Peters B, Sette A, The immune epitope database and analysis Resource program 2003–2018: reflections and outlook, Immunogenetics 72 (2020) 57–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Deak P, Kimani F, Cassaidy B, Esser-Kahn A, Determining whether agonist density or agonist number is more important for immune activation via micoparticle based assay, Front. Immunol. 11 (2020), 10.3389/fimmu.2020.00642. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the main text or the supplementary materials.