Abstract
Screening for perinatal-occurring obsessive-compulsive disorder (OCD) is rare. We sought to evaluate the Dimensional Obsessive-Compulsive Scale (DOCS) as a screening tool for perinatal OCD and compare the screening accuracy of the DOCS with the commonly recommended Edinburgh Postnatal Depression Scale (EPDS). English-speaking, pregnant individuals aged 19+ (N = 574) completed online questionnaires and diagnostic interviews to assess for OCD prenatally and twice postpartum. The DOCS total score demonstrated the highest level of accuracy. Neither the EPDS-Full nor the three-item Anxiety subscale of the EPDS (EPDS-3A) met the criteria of a sufficiently accurate screening tool for OCD at any of the assessment points. Findings provide support for the DOCS as a screening tool for perinatal OCD and indicate a need for disorder-specific screening for perinatal anxiety and their related disorders (AD). Generalizability of findings is limited to Canada only. Future research would benefit from comparisons with measures of perinatal OCD (e.g., the Perinatal Obsessive-Compulsive Scale).
Keywords: obsessive-compulsive disorder, perinatal mental health, screening
Obsessive-compulsive disorder (OCD) is an anxiety-related mental health disorder marked by obsessions and compulsions (Coluccia et al., 2016; Stasik et al., 2012). Obsessions are recurrent, unwanted, and distressing thoughts, images, or impulses. Compulsions are repetitive mental or behavioral acts that the person engages in, in an effort to cope with the obsessions. Obsessions typically involve content related to contamination, violence, sex, religion, and/or being responsible for harm to others (Handelzalts et al., 2012). Compulsive behaviors connected to obsessions often involve checking, reassurance seeking, washing and cleaning rituals, and repetitive mental acts (e.g., mentally undoing a negative thought or image). Avoidance of situations related to one’s obsessions is also common.
Frequently, those affected by OCD experience heightened levels of distress and impairment due to their obsessions and/or compulsions (Monk, 2001; Nerum et al., 2013; Newham et al., 2014; Nieminen et al., 2009, 2016). OCD is associated with impaired social functioning, marital difficulties, family relationship problems, increased health care utilization, compromised work functioning, financial problems (Nieminen et al., 2016), and poorer quality of life (Monk, 2001; Nerum et al., 2013; Newham et al., 2014; Nieminen et al., 2009, 2016). As obsessions are often perceived as socially unacceptable to OCD sufferers, they often conceal their obsessions from others, including health care providers (Challacombe & Wroe, 2013), thus impeding access to effective interventions.
Consistent data from individual investigations and a recent meta-analysis indicate an increased risk of OCD during the perinatal period (Fawcett et al., 2019; Ruscio et al., 2010; Russell et al., 2013). Indeed, one in seven perinatal people (15%) experience OCD at some point during pregnancy or the first 6 months postpartum (Fairbrother et al., 2021). This increased prevalence has been found to peak in the weeks following childbirth, after which it declines (Brok et al., 2017; Fairbrother et al., 2021). When OCD occurs postpartum, it is frequently characterized by obsessions of harm related to the newborn (Fairbrother & Abramowitz, 2016), specifically thoughts and images of harming (intentionally or accidentally) the infant. Overt compulsions are less common in postpartum OCD (ppOCD) compared with non-perinatal OCD. However, avoidance of the infant or avoidance of specific activities with one’s infant (e.g., bathing the infant or using knives near the infant) is common. When compulsions are present, they are often in the form of checking (e.g., checking on the infant’s breathing or checking the internet for reassurance) and mental compulsions (e.g., mentally undoing the obsession by imagining one’s infant to be well) (Fairbrother & Abramowitz, 2016; Russell et al., 2013).
For perinatal people, OCD has negative implications for fetal and newborn health, parenting, and infant development (Saisto et al., 2001; Salomonsson et al., 2013; Stoll et al., 2016; Straub et al., 2012; Sydsjö et al., 2014, 2015; Takegata et al., 2015). The consequences of ppOCD may involve impaired functioning, reduced ability to complete daily tasks, strain on relationships, and negative effects to fetal, newborn, and infant well-being (Brander et al., 2016; Challacombe et al., 2016; Coplan et al., 2005; House et al., 2016; Uguz et al., 2015). In addition, comorbid depressed mood is common among people affected by ppOCD (Miller et al., 2015).
Screening for Anxiety and Anxiety-Related Disorders Among Perinatal People
Over the past few years, there have been urgent calls from health care agencies around the world (e.g., the United States, Canada, and Australia) for accurate and reliable screening tools for perinatal anxiety and their related disorders (AD). Typically, this includes the core anxiety conditions listed in the Diagnostic and Statistical Manual of Mental Disorders (5th ed.; DSM-5; American Psychiatric Association, 2013), as well as OCD and posttraumatic stress disorder (PTSD). Despite an urgent need and calls for evidence-based screening measures, the overwhelming consensus among scientists working in this area is that the current evidence is too weak to support recommending any specific tool to screen for perinatal AD. A key difficulty in identifying an accurate and reliable screening tool for perinatal AD has been the methodological quality of the studies conducted. While a large number of studies have evaluated the screening accuracy of various tools, few to none have employed high-quality methodology.
Further, to merit broad implementation, screening tools should meet minimum criteria of accuracy. The empirical literature supports the following minimum criteria be met for a screening tool to be deemed “sufficiently accurate” as to merit implementation (Bossuyt et al., 2015; Cohen et al., 2016; Zimmerman et al., 2004). Specifically, to be recommended for widespread use, a screening tool should demonstrate an area under the curve (AUC) ≥0.8 (≥0.8 is generally considered excellent; Mandrekar, 2010), a Youden’s Index of ≥0.5 (i.e., when sensitivity = 0.75, specificity = ≥0.75), a negative predictive value (NPV) of ≥0.8, and a positive likelihood ratio (LR+) of ≥4.0. An LR+ of 4.0 means that with a positive test result, the probability the person has the disease increases 25% over pretest probability (Sackett et al., 2000).
Using the Edinburgh Postnatal Depression Scale (EPDS) to Screen for Perinatal OCD
The EPDS (Cox et al., 1987) is a 10-item measure of depressed mood among perinatal period. It is arguably the most commonly used measure of perinatal depression (Matthey et al., 2013). Three items of the EPDS have been identified as measuring anxiety and these three items have been labeled the three-item Anxiety subscale of the EPDS (EPDS-3A; Matthey, 2008; Matthey et al., 2013). Both the 10-item EPDS-Full and the EPDS-3A have been evaluated as screening tools for both perinatal depression and perinatal anxiety and anxiety-related disorders (Cox et al., 1987; Grigoriadis et al., 2011). To date, all studies of the EPDS/EPDS-3A as screening tools for the anxiety and their related conditions have evaluated these conditions as a group and not individually (Fairbrother et al., 2019; van Heyningen et al., 2018).
Only four studies have assessed the EPDS as a screening tool for perinatal AD employing some Gold Standard methodology. Of these, all four evaluated the EPDS-3A and one the EPDS-Full version. In the one study to assess the EPDS-Full version, it failed to meet the criteria of a sufficiently accurate screening tool. Among the four studies to assess the EPDS-3A, only one demonstrated an AUC of greater than 0.80. Although NPV values were sufficient across the three studies that reported them, in none of the four studies was the J index ≥0.50.
While there is an urgent need to identify accurate and valid screening tools for perinatal AD as a whole (Massachusetts General Hospital Centre for Women’s Mental Health, 2019), it is equally important to know how well they perform in detecting individual disorders, in particular those with low prevalence. It is possible for a screening tool to perform well for perinatal AD overall and yet neglect one or more low prevalence conditions, consequently neglecting to detect those with these difficulties. Should the EPDS-3A prove to be an accurate screening tool for perinatal anxiety, this would have significant practical implications: as the EPDS is currently in widespread use as a screening tool for perinatal depression, implementation of the EPDS requires additional scoring only but not additional administration. Consequently, motivation to evaluate the EPDS-3A as a potential screening tool for perinatal AD is high. In addition to appearing to perform poorly, the EPDS-3A has yet to be evaluated as a screening tool specifically for perinatal OCD.
Screening for OCD
The majority of studies evaluating the screening accuracy of self-report measures of OCD in adults have assessed the Obsessive-Compulsive Inventory–Revised (OCI-R; Abramowitz & Deacon, 2006; Abramowitz et al., 2010; Foa et al., 2002; Gönner et al., 2007; Tang et al., 2015; Williams et al., 2013; Wootton et al., 2015), and the Dimensional Obsessive-Compulsive Scale (DOCS; Abramowitz et al., 2010; López-Solà et al., 2014; Thibodeau et al., 2015) or the DOCS–Short Form (DOCS-SF; Eilertsen et al., 2017). While both the OCI-R and the DOCS have been documented as possessing strong screening metrics, available data indicate that the DOCS (including the DOCS-SF) is the more accurate of the two (Abramowitz et al., 2010; Eilertsen et al., 2017; López-Solà et al., 2014; Rapp et al., 2016). To date, only the Perinatal Obsessive-Compulsive Scale (POCS) and the Yale Brown Obsessive-Compulsive Scale–Self-Report (Y-BOCS self-report) have been evaluated as potential screening tools for perinatal OCD (Lord et al., 2011). The POCS resulted in stronger screening metrics (i.e., AUC = 0.81, J = 0.78) compared with the Y-BOCS (i.e., AUC = 0.75, J = 0.73). The screening accuracy of the DOCS has yet to be evaluated.
The purpose of this study was to evaluate the DOCS as screening tool for perinatal OCD and to compare the accuracy of the DOCS with (a) the criteria for a “sufficiently accurate” measure and (b) the accuracy of the EPDS and the EPDS-3A in screening for OCD among perinatal people. We hypothesized that disorder-specific screening for OCD (i.e., the DOCS) would result in superior accuracy to generic screening for OCD (i.e., the EPDS-Full and EPDS-3A), both in pregnancy and the postpartum, and that disorder-specific screening may be needed to achieve the standard of a sufficiently accurate measure.
Method
This research was conducted as part of a larger study, for which a detailed study protocol has been published (Collardeau et al., 2019).
Ethics
This province-wide study was approved by the University of British Columbia Behavioral Research Ethics Board, the Island Health, Health Research Ethics Board, the Fraser Health Research Ethics Board, and Vancouver Coastal Health. Due to the sensitive nature of some aspects of the broader study, written consent was obtained at both the initial prenatal assessment and the first postpartum assessment. Oral consent was also provided at the beginning of each study interview. A study debriefing letter was provided to all participants upon completion of participation.
Participants
All English-speaking, pregnant individuals over the age of 19 and living in the Canadian Province of British Columbia (BC) were eligible to take part in this study. In total, 763 individuals participated. A total of 574 of these contributed data to the current study. Data collection occurred from February 9, 2014 until February 14, 2017.
Recruitment
To promote sample representativeness, we employed a variety of recruitment strategies, including hospital-based recruitment (85.3%), community-based recruitment (13.3%), and approaches focused specifically on rural areas of BC (1.4%). For hospital-based recruitment, we recruited proportionally across hospitals in BC in which 1,500 or more live births occur annually and used primarily direct approach methods (i.e., approaching pregnant individuals waiting for routine antenatal appointments). Additional participants were recruited using direct and/or indirect recruitment methods at private clinics, trade shows, community events, and prenatal centers throughout BC. Individuals who expressed an interest via telephone, email, or in-person, and met the study eligibility requirements, were invited to participate.
Procedures
Participants were followed from the third trimester of pregnancy (at the earliest 32 weeks’ gestation) to a maximum of 9 months postpartum. They were asked to complete online questionnaires followed by a telephone interview at three separate timepoints—once in late pregnancy (M = 36.89 weeks, SD = 1.96) and twice postpartum (M = 9.09 weeks, SD = 1.94; and M = 21.27 weeks, SD = 3.83). Questionnaires were primarily completed online; however, if necessary, participants could choose to complete questionnaires via paper hardcopy sent to their home to be returned by mail. Participants who did not complete a questionnaire and/or interview at earlier timepoints were nevertheless eligible to complete the assessments at subsequent stages.
Not all 574 participants provided data for all three assessment points (e.g., some participants may have completed all three interviews but missed one of the questionnaire assessments). Of the 574 participants who contributed data to the present inquiry, 573 provided data for the prenatal assessment, 542 for the early postpartum assessment, and 394 for the late postpartum assessment.
Measures
Demographic (age, marital status, occupation, education, income, race/ethnicity, and language), pregnancy (medical and pregnancy complications, and reproductive history), and birth (baby’s date of birth, mode and location of delivery, birth weight, pregnancy and birth complications, neonatal health, and infant feeding) information was collected via self-report.
The DOCS (Abramowitz et al., 2010) is a 20-item self-report measure used to assess the four most consistently replicated OCD symptoms, using a four-factor model corresponding to the measure’s subscales: (a) Germs and Contamination; (b) Responsibility for Harm, Injury, or Mistakes; (c) Unacceptable Obsessional Thoughts; and (d) Symmetry, Completeness, and Ordering. Five items (rated 0–4) assess the parameters of severity of time occupied by obsessions and rituals, avoidance, distress, functional interference, and difficulty disregarding the obsessive thoughts and refraining from the compulsions within each symptom dimension (Abramowitz et al., 2010; Enander et al., 2012; Thibodeau et al., 2015). DOCS subscales have excellent test-retest reliability in clinical samples (α = .87–.96) and in student samples (α = .82–.93) as well as strong convergent, discriminant, and construct validity (Abramowitz et al., 2010; Enander et al., 2012; Thibodeau et al., 2015). The measure is sensitive to changes over time and incremental increases on the DOCS represent actual increases in OCD symptoms (Thibodeau et al., 2015). Evidence suggests the DOCS is accurate in detecting OCD from healthy controls, with AUCs consistently exceeding 0.8 (Abramowitz et al., 2010; Eilertsen et al., 2017; López-Solà et al., 2014; Thibodeau et al., 2015).
The EPDS (Cox et al., 1987) is a self-report measure widely used to screen for postnatal depression (Jomeen & Martin, 2005). The EPDS contains 10 items with four response options each rated 0 to 3. As it has been developed for use in postpartum samples, it de-emphasizes the somatic symptoms that may overlap with depressive symptoms but would be considered normative during this period (Gaynes et al., 2005). It has demonstrated good to excellent psychometric properties and acceptable ranges of sensitivity and specificity (70%–100% for sensitivity and 74%–97% for specificity in the antenatal period; 65%–100% for sensitivity and 49%–100% for specificity in the postnatal period; Kozinszky & Dudas, 2015). Factor analytic studies of the EPDS support a distinct three-item Anxiety subscale (EPDS-3A; Matthey, 2008). Both the EPDS and the EPDS-3A have acceptable internal consistency reliability among perinatal people (Swalm et al., 2010) and the EPDS-3A is effective in screening for perinatal anxiety (Fairbrother et al., 2019). Literature assessing EPDS-3A with sufficient methodological criteria indicates moderate accuracy; Fairbrother et al. (2019) report an AUC of 0.76, and Van Heyningen and others (2018) report an AUC of 0.69.
Diagnostic Interviews
The Structured Clinical Interview for DSM-5 (SCID-5; First et al., 2016) is a well-validated, semi-structured interview for DSM-5 diagnoses. Interviewers were trained to strict criteria by the principal investigator. Symptom severity was rated from 0 (none) to 8 (very severe/disabling), with “0” representing a diagnostic status of absent or in full remission, “0.5–2.5” representing partial remission, “3.0–3.5” representing subclinical diagnoses, and “4.0–8.0” representing the range of diagnostic severity for those whose symptoms met full diagnostic criteria.
At each interview, participants were asked about obsessive-compulsive (OC) symptoms experienced in the 2 weeks prior. In addition, in all but the first postpartum interview, participants were asked to identify the 2-week period (prenatal or postpartum) during which their OC symptoms were most intense. This permitted an assessment of prenatal and postpartum prevalence (reported in a separate publication; Fairbrother et al., 2021). Diagnostic status and diagnostic severity ratings were specified for both current and most intense time periods. In addition to standard diagnostic questions, during the postpartum interviews, participants were also asked about any thoughts of infant-related harm (both accidental and intentional harm) and associated behaviors. The overall evaluation of OCD diagnostic status and severity included evaluations of obsessions and compulsions of infant-related harm.
Upon completion of data collection, reliability checks were undertaken by a senior interviewer and two external OCD specialists. A quarter of all audio-recorded interviews with individuals diagnosed with significant OCD symptomatology (subclinical, clinical, partial remission) were reviewed for reliability, along with 5% of interviews in which no diagnosis of OCD was reported. Interviews from each of the 10 interviewers were proportionally and randomly sampled at each timepoint. Interrater reliability among the three raters was rated as good to excellent using an intraclass correlation coefficient with a two-way random effect model for consistency, with scores ranging from .75 to .98 across the three timepoints.
Statistical Analyses
Analyses were carried out using SPSS 2017 (IBM Corp., 2017) and R software (R Core Team, 2020). Descriptive statistics are presented as means, standard deviations, and correlations. Receiver operating characteristic (ROC) curves were constructed for all DOCS subscales, the total DOCS scores, the EPDS, and the EPDS-3A. ROC curves were constructed using the “cutpointr” package (Thiele, 2020) in R (R Core Team, 2020). The optimal cutpoint was estimated by maximizing Youden’s index over 5,000 bootstrap replicates at each timepoint for each subscale. OCD was defined as meeting full diagnostic criteria. The number of completed measures differs across assessment points.
Results
Participants
Participant demographic and reproductive data are presented in Table 1. Participants in this study differed somewhat from the larger study population in terms of parity, χ2(1, N = 133,636) = 26.9, p < .001; mode of delivery, χ2(1, N = 133,600) = 6.2, p = .01; and age, χ2(6, N = 133,637) = 28.5, p < .001. Specifically, when compared with provincial data, our sample contained a greater proportion of people who had never given birth (55% vs. 46%), were slightly older (63% 30- to 40-year-olds vs. 58%), and gave birth via Cesarian section (35% vs. 33%). Eleven (3.6%) met full criteria for OCD in pregnancy, 43 (10.6%) at 2 months postpartum, and 22 (5.8%) at 5 months postpartum.
Table 1.
Demographic Information, Reproductive History, and Medical and Pregnancy Complications (N = 574).
| Demographic characteristics | % of total sample |
|---|---|
| Relationship status | |
| Married or living with a romantic partner | 95.3 |
| Single | 3.6 |
| Divorced/separated | 1.1 |
| Education | |
| Did not complete high school | 2.0 |
| Completed high school | 7.1 |
| Some undergraduate education | 51.1 |
| Some graduate education | 39.8 |
| Cultural heritage | |
| European | 56.5 |
| East Asian | 11.5 |
| South Asian | 6.2 |
| Southeast Asian | 5.6 |
| Indigenous | 2.7 |
| Other | 17.4 |
| Age in years | M = 32.5 (SD = 4.9), range = 18–47 |
| Reproductive history | % of total sample |
| First pregnancy | 39.9 |
| Prior history of miscarriage | 25.5 |
| Prior history of late loss | 1.3 |
| Primiparous | 69.9 |
| Current pregnancy and birth | % of total sample |
| Mode of delivery | |
| Vaginal | 62.1 |
| Cesarean (before the onset of labor) | 37.9 |
| Complications during labor | 30.5 |
| Episiotomy performed | 9.1 |
| Readmission to the hospital (parent who carried pregnancy) | 8.1 |
| Baby admitted to intensive or special care unit | 12.3 |
ROC Curves and Diagnostic Accuracy
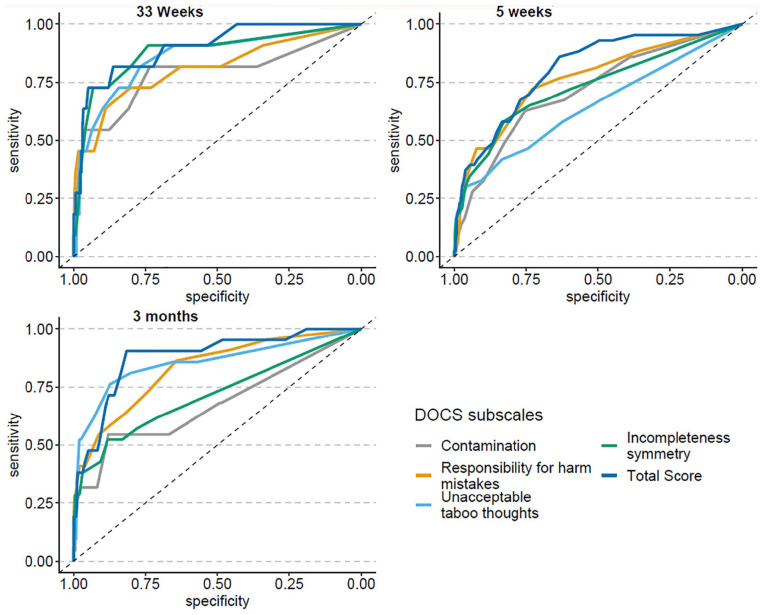
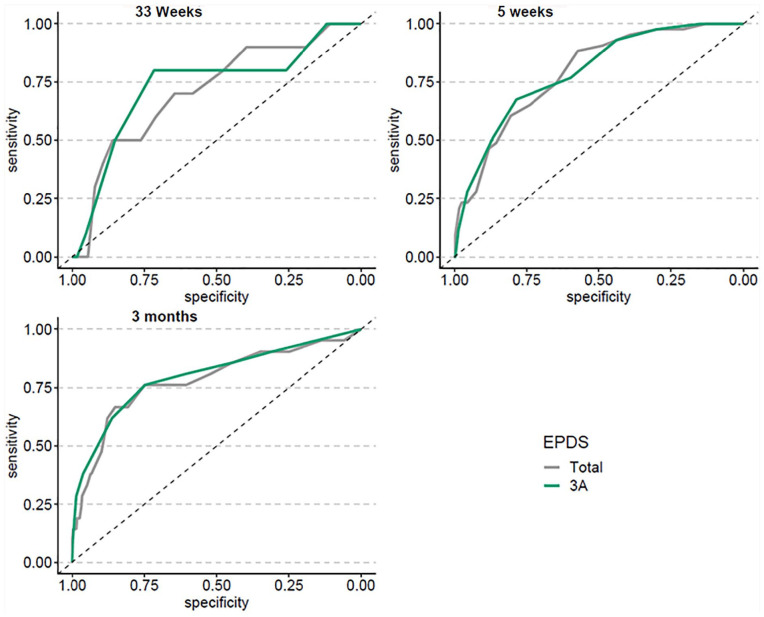
ROC curves for the DOCS (the four subscales and the total score) and the EPDS-Full and EPDS-3A are shown in Figures 1 and 2, respectively. Means and standard deviations for scales and subscales are listed in Table 2, and indices of diagnostic accuracy are shown in Table 3 (for the DOCS) and Table 4 (for the EPDS-Full and the EPDS-3A). ROC curves show the AUC in pregnancy and at approximately 2 and 5 months postpartum, for each of the DOCS subscales and the DOCS total score (Figure 1) as well as the EPDS-Full and the EPDS-3A (Figure 2). AUC values ranged from 0.65 to 0.88 for the individual DOCS subscales, and from 0.81 to 0.90 for the full scale. For the EPDS, AUC values ranged from 0.71 to 0.79 for the EPDS-Full, and from 0.73 to 0.80 for the EPDS-3A.
Figure 1.
ROC Curves for DOCS Total and Subscale Scores.
Note. ROC = Receiver Operating Characteristic; DOCS = Dimensional Obsessive-Compulsive Scale.
Figure 2.
ROC Curves for EPDS-Full and EPDS-3A Scores.
Note. ROC = Receiver Operating Characteristic; EPDS = Edinburgh Postnatal Depression Scale; EPDS-3A = three-item Anxiety subscale of the EPDS.
Table 2.
Means and Standard Deviations for Scales and Subscales.
| DOCS and EPDS Totals and Subscales | Prenatal (n = 573) M (SD) |
Early Postpartum (n = 542) M (SD) |
Late Postpartum (n = 394) M (SD) |
|---|---|---|---|
| DOCS Contamination | 2.85 (2.90) | 2.72 (2.76) | 2.08 (2.56) |
| DOCS Responsibility/Harm | 2.74 (2.91) | 2.72 (2.98) | 2.46 (2.50) |
| DOCS Unacceptable Thoughts | 2.13 (3.00) | 1.96 (2.75) | 1.56 (2.29) |
| DOCS Incompleteness/Symmetry | 2.02 (2.87) | 1.95 (2.89) | 1.22 (2.27) |
| DOCS Total | 9.81 (9.46) | 9.33 (9.28) | 7.31 (7.74) |
| EPDS-Full | 7.15 (4.81) | 7.00 (4.67) | 6.36 (4.81) |
| EPDS-3A | 3.38 (2.14) | 3.23 (2.15) | 3.09 (2.18) |
Note. DOCS = Dimensional Obsessive-Compulsive Scale; EPDS = Edinburgh Postnatal Depression Scale; EPDS-3A = three-item Anxiety subscale of the EPDS.
Table 3.
DOCS ROC Analysis Data.
| DOCS Total and Subscales by Assessment Point | AUC [95% CI] | J | Cutpoint | Sensitivity | Specificity | PPV | NPV | LR+ |
|---|---|---|---|---|---|---|---|---|
| DOCS Contamination | ||||||||
| Prenatal | 0.79 [0.60, 0.97] | 0.42 | 5.41 | 0.55 | 0.88 | 0.14 | 0.98 | 4.58 |
| Early postpartum | 0.72 [0.63, 0.80] | 0.38 | 3.56 | 0.63 | 0.75 | 0.23 | 0.94 | 2.52 |
| Late postpartum | 0.68 [0.54, 0.82] | 0.42 | 4.70 | 0.55 | 0.88 | 0.22 | 0.97 | 4.58 |
| DOCS Responsibility/Harm | ||||||||
| Prenatal | 0.81 [0.64, 0.98] | 0.53 | 5.34 | 0.64 | 0.89 | 0.18 | 0.99 | 5.82 |
| Early postpartum | 0.77 [0.68, 0.85] | 0.45 | 3.96 | 0.72 | 0.73 | 0.24 | 0.96 | 2.67 |
| Late postpartum | 0.83 [0.73, 0.93] | 0.47 | 3.71 | 0.73 | 0.74 | 0.15 | 0.98 | 2.81 |
| DOCS Unacceptable Thoughts | ||||||||
| Prenatal | 0.86 [0.72, 0.99] | 0.54 | 3.74 | 0.73 | 0.81 | 0.12 | 0.99 | 3.84 |
| Early postpartum | 0.65 [0.55, 0.74] | 0.23 | 4.43 | 0.33 | 0.91 | 0.30 | 0.92 | 3.67 |
| Late postpartum | 0.85 [0.75, 0.96] | 0.63 | 3.46 | 0.76 | 0.87 | 0.26 | 0.98 | 5.85 |
| DOCS Incompleteness/Symmetry | ||||||||
| Late prenatal | 0.88 [0.75, 1.0] | 0.61 | 4.25 | 0.73 | 0.88 | 0.18 | 0.99 | 6.08 |
| Early postpartum | 0.73 [0.64, 0.82] | 0.41 | 3.20 | 0.58 | 0.83 | 0.29 | 0.94 | 3.41 |
| Late postpartum | 0.72 [0.56, 0.84] | 0.40 | 3.66 | 0.52 | 0.88 | 0.20 | 0.97 | 4.33 |
| DOCS Total | ||||||||
| Late prenatal | 0.90 [0.80, 1.0] | 0.64 | 18.60 | 0.73 | 0.91 | 0.23 | 0.99 | 8.11 |
| Early postpartum | 0.81 [0.74, 0.88] | 0.46 | 8.84 | 0.79 | 0.67 | 0.22 | 0.96 | 2.39 |
| Late postpartum | 0.88 [0.80, 0.96] | 0.72 | 10.82 | 0.90 | 0.82 | 0.23 | 0.99 | 5.00 |
Note. DOCS = Dimensional Obsessive-Compulsive Scale; ROC = receiver operating characteristic; AUC = area under the curve; CI = confidence interval; LR+ = positive likelihood ratio; PPV = positive predictive value; NPV = negative predictive value.
Table 4.
EPDS and EPDS-3A ROC Analysis Data.
| EPDS-Full and 3A by Assessment Point | AUC [95% CI] | J | Cutpoint | Sensitivity | Specificity | PPV | NPV | LR+ |
|---|---|---|---|---|---|---|---|---|
| EPDS-Full | ||||||||
| Prenatal | 0.71 [0.54, 0.88] | 0.26 | 9.05 | 0.50 | 0.76 | 0.07 | 0.98 | 2.08 |
| Early postpartum | 0.79 [0.72, 0.85] | 0.39 | 7.43 | 0.74 | 0.65 | 0.20 | 0.95 | 2.11 |
| Late postpartum | 0.78 [0.66, 0.90] | 0.52 | 10.01 | 0.67 | 0.85 | 0.22 | 0.98 | 4.47 |
| EPDS-3A | ||||||||
| Prenatal | 0.73 [0.55, 0.91] | 0.52 | 4.63 | 0.80 | 0.72 | 0.09 | 0.99 | 2.86 |
| Early postpartum | 0.79 [0.72, 0.86] | 0.46 | 4.35 | 0.67 | 0.79 | 0.28 | 0.95 | 3.19 |
| Late postpartum | 0.80 [0.68, 0.92] | 0.51 | 4.90 | 0.76 | 0.75 | 0.16 | 0.98 | 3.04 |
Note. EPDS = Edinburgh Postnatal Depression Scale; EPDS-3A = three-item Anxiety subscale of the EPDS; ROC = receiver operating characteristic; AUC = area under the curve; CI = confidence interval; LR+ = positive likelihood ratio; PPV = positive predictive value; NPV = negative predictive value.
Discussion
In this study of a representative sample of perinatal Canadians, we sought to assess and compare the accuracy of the DOCS and the EPDS (Full and 3A) as screening tools for perinatal OCD. The screening accuracy metrics of each of the evaluated measures were compared with the criteria for a “sufficiently accurate” measure.
The DOCS demonstrated a very high level of screening accuracy, significantly exceeding the criteria for a “sufficiently accurate” measure, at one or more assessment points, for three of the four subscales, and the DOCS total scores. Overall, the DOCS total score demonstrated the highest level of performance, with screening metrics mirroring those found in other, non-perinatal assessments of the DOCS as a screening tool for OCD (Abramowitz et al., 2010; Eilertsen et al., 2017; López-Solà et al., 2014; Thibodeau et al., 2015).
Of the four DOCS subscales, the Unacceptable Thoughts subscale demonstrated the highest level of accuracy. Specifically, at the late postpartum assessment, the Unacceptable Thoughts subscale exceeded the criteria for a “sufficiently accurate” measure across all three metrics (AUC = 0.85, J = 0.63, LR+ = 5.85), and at the prenatal assessment, it exceeded the criteria on two out of the three metrics (AUC = 0.86, J = 0.54, LR+ = 3.84). At the early postpartum assessment, however, it met only one criterion for a “sufficiently accurate” measure (i.e., AUC = 0.81). These findings (i.e., lower accuracy at the early postpartum assessment, and superior performance compared with other DOCS subscales) are consistent with our earlier findings from this research. Specifically, based on diagnostic interviews, we found a very high level of OC symptoms at the early postpartum assessment (Fairbrother et al., 2021), suggesting that some symptoms of OCD in the early postpartum may be a normative postpartum experience. It is probable that the high prevalence of OC symptoms at this assessment point may have diluted the screening accuracy of this subscale. In addition, the majority of participants in our research who met criteria for OCD (in particular at the postpartum assessment) reported obsessions involving unwanted, intrusive thoughts of infant-related harm. This is consistent with the finding that the DOCS Responsibility/Harm and Unacceptable Thoughts subscales demonstrated higher accuracy compared with the Contamination subscale. Finally, the Incompleteness/Symmetry subscale demonstrated a very high level of accuracy but only at the prenatal assessment. It is possible that this reflects the tendency for pregnant people to engage in high levels of “nesting” behavior (e.g., organizing, arranging, and preparing for the infant to arrive; Anderson & Rutherford, 2013).
As predicted, only the DOCS met the criteria of a “sufficiently accurate” measure. At none of the three assessment points did the EPDS-Full nor the EPDS-3A meet the criteria of a “sufficiently accurate” screening tool. The EPDS-3A demonstrated the highest level of accuracy at the late postpartum assessment where it met two of the three criteria for a “sufficiently accurate” measure. As expected, the DOCS (total and some subscales) outperformed both the EPDS-Full and the EPDS-3A as a screening tool for perinatal OCD.
Study findings provide strong evidence that the DOCS provides high accuracy in screening for perinatal OCD. What is also clear from the present findings is that, not surprisingly, disorder-specific measures (e.g., the DOCS) provide more accurate screening outcomes compared with more generic measures (e.g., the EPDS). What was perhaps more surprising was the fact that the EPDS-3A approached the standard of a “sufficiently accurate” screening tool, at least for the late postpartum assessment. The obtained metrics for the EPDS-3A suggest that while not fully sufficient, it is also not altogether inadequate as a screening tool for perinatal OCD. Given the ubiquity of the EPDS as a screening tool for perinatal depression, and more recently the EPDS-3A as a screening tool for perinatal anxiety (Cox et al., 1987; Grigoriadis et al., 2011), this is a welcome discovery.
While preliminary data indicate that the EPDS-3A may perform reasonably well as a screening instrument for perinatal anxiety and related disorders as a whole, it may perform less well for specific disorders and is also unable to provide any information regarding which disorder the person is most likely to be experiencing. A perhaps more promising and, to date, more accurate measure is the Anxiety Disorder-13 scale (AD-13; Fairbrother et al., 2019). If the AD-13 proves to be accurate in screening for individual disorders, and not only the anxiety and related disorders as a group, it has the ability to identify which specific disorders may require a follow-up screening instrument.
A curious aspect of our findings is the fact that all of the scales/subscales performed least well at the time of the early postpartum assessment. We suspect that this is a product of the fact that OC symptoms were highest at this time (Fairbrother et al., 2021) and may not always represent difficulties likely to persist. It is now well documented that unwanted, intrusive, infant-related thoughts, images, and impulses are ubiquitous in the early postpartum but tend to decrease later in the postpartum. It is likely that some OC symptoms in the early postpartum represent a normal postpartum phenomenon rather than any underlying psychopathology (Fairbrother & Woody, 2008). This phenomenon may interfere with OCD screening accuracy.
Limitations
Strengths of the current study include multiple assessments from pregnancy through to 5 months postpartum and the use of high-quality methodology to evaluate screening accuracy. A key limitation is the fact that we did not include a comparison of the DOCS with the POCS. This would have significantly strengthened this work and provided important additional information. This comparison should be undertaken in future research. Moreover, despite a recruitment method carefully designed to promote sample representativeness, data were collected in only one Canadian province, limiting the generalizability of findings to other cultures.
Conclusion and Clinical Implications
Our data suggest that screening for perinatal OCD may be most beneficial in pregnancy and at 4 to 6 months postpartum. As it can be challenging in health care settings to administer separate screening tools for each disorder of interest, it is very encouraging that both the Unacceptable Thoughts subscale of the DOCS and the EPDS-3A performed reasonably well as screening tools for perinatal OCD, with the DOCS Unacceptable Thoughts subscale outperforming the EPDS-3A. Decisions regarding whether to employ the DOCS full scale, the DOCS unacceptable thoughts, or the EPDS-3A should be made on the basis of available resources, burden on patients, and the objectives in the particular clinical context in which the measure is to be administered.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a Project Grant from the Canadian Institutes of Health Research (award number 123442).
ORCID iDs: Nichole Fairbrother  https://orcid.org/0000-0002-1939-5984
https://orcid.org/0000-0002-1939-5984
Rose B. Cameron  https://orcid.org/0000-0001-8237-2323
https://orcid.org/0000-0001-8237-2323
References
- Abramowitz J. S., Deacon B. J. (2006). Psychometric properties and construct validity of the Obsessive-Compulsive Inventory–Revised: Replication and extension with a clinical sample. Journal of Anxiety Disorders, 20(8), 1016–1035. 10.1016/j.janxdis.2006.03.001 [DOI] [PubMed] [Google Scholar]
- Abramowitz J. S., Deacon B. J., Olatunji B. O., Wheaton M. G., Berman N. C., Losardo D., Timpano K. R., McGrath P. B., Riemann B. C., Adams T., Björgvinsson T., Storch E. D., Hale L. R. (2010). Assessment of obsessive-compulsive symptom dimensions: Development and evaluation of the Dimensional Obsessive-Compulsive Scale. Psychological Assessment, 22(1), 180–198. 10.1037/a0018260 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). 10.1176/appi.books.9780890425596 [DOI]
- Anderson M. V., Rutherford M. D. (2013). Evidence of a nesting psychology during human pregnancy. Evolution and Human Behavior, 34(6), 390–397. [Google Scholar]
- Bossuyt P. M., Reitsma J. B., Bruns D. E., Gatsonis C. A., Glasziou P. P., Irwig L., Lijmer J. G., Moher D., Rennie D., de Vet H. C. W., Kressel H. Y., Rifai N., Golub R. M., Altman D. G., Hooft L., Korevaar D. A., Cohen J. F. (2015). STARD 2015: An updated list of essential items for reporting diagnostic accuracy studies. British Medical Journal, 351, Article h5527. 10.1136/bmj.h5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brander G., Rydell M., Kuja-Halkola R., Fernández de la Cruz L., Lichtenstein P., Serlachius E., Rück C., Almgvist C., D’Onofrio B. M., Larsson H., Mataix-Cols D. (2016). Association of perinatal risk factors with obsessive-compulsive disorder: A population-based birth cohort, sibling control study. JAMA Psychiatry, 71(11), 1135–1144. 10.1001/jamapsychiatry.2016.2095 [DOI] [PubMed] [Google Scholar]
- Brok E. C., Lok P., Oosterbaan D. B., Shene A. H., Tendolkar I., van Eijndhoven P. F. (2017). Infant-related intrusive thoughts of harm in the postpartum period: A critical review. The Journal of Clinical Psychiatry, 78(8), e913–e923. 10.4088/JCP.16r11083 [DOI] [PubMed] [Google Scholar]
- Challacombe F. L., Salkovskis P. M., Woolgar M., Wilkinson E. L., Read J., Acheson R. (2016). Parenting and mother-infant interactions in the context of maternal postpartum obsessive-compulsive disorder: Effects of obsessional symptoms and mood. Infant Behavior and Development, 44, 11–20. 10.1016/j.infbeh.2016.04.003 [DOI] [PubMed] [Google Scholar]
- Challacombe F. L., Wroe A. L. (2013). A hidden problem: Consequences of the misdiagnosis of perinatal obsessive-compulsive disorder. The British Journal of General Practice, 63(610), 275–276. 10.3399/bjgp13X667376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. F., Korevaar D. A., Altman D. G., Bruns D. E., Gatsonis C. A., Hooft L., Irwig L., Levine D., Reitsma J. B., de Vet H. C. W., Bossuyt P. M. (2016). STARD 2015 guidelines for reporting diagnostic accuracy studies: Explanation and elaboration. British Medical Journal Open, 6(11), Article e012799. 10.1136/bmjopen-2016-012799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collardeau F., Corbyn B., Abramowitz J. S., Janssen P. A., Woody S., Fairbrother N. (2019). Maternal unwanted and intrusive thoughts of infant-related harm, obsessive-compulsive disorder and depression in the perinatal period: Study protocol. BMC Psychiatry, 19(1), 94–94. 10.1186/s12888-019-2067-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coluccia A., Fagiolini A., Ferretti F., Pozza A., Costoloni G., Bolognesi S., Goracci A. (2016). Adult obsessive–compulsive disorder and quality of life outcomes: A systematic review and meta-analysis. Asian Journal of Psychiatry, 22, 41–52. 10.1016/j.ajp.2016.02.001 [DOI] [PubMed] [Google Scholar]
- Coplan R. J., O’Neil K., Arbeau K. A. (2005). Maternal anxiety during and after pregnancy and infant temperament at three months of age. Journal of Prenatal & Perinatal Psychology & Health, 19(3), 199–215. [Google Scholar]
- Cox J. L., Holden J. M., Sagovsky R. (1987). Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression Scale. The British Journal of Psychiatry, 150, 782–786. 10.1192/bjp.150.6.782 [DOI] [PubMed] [Google Scholar]
- Eilertsen T., Hansen B., Kvale G., Abramowitz J. S., Holm S. E. H., Solem S. (2017). The Dimensional Obsessive-Compulsive Scale: Development and validation of a short form (DOCS-SF). Frontiers in Psychology, 8, 1503–1503. 10.3389/fpsyg.2017.01503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enander J., Andersson E., Kaldo V., Lindefors N., Andersson G., Rück C. (2012). Internet administration of the Dimensional Obsessive-Compulsive Scale: A psychometric evaluation. Journal of Obsessive-Compulsive and Related Disorders, 1(4), 325–330. 10.1016/j.jocrd.2012.07.008 [DOI] [Google Scholar]
- Fairbrother N., Abramowitz J. S. (2016). Obsessions and compulsions during pregnancy and the postpartum period. In Wenzel A. (Ed.), The Oxford handbook of perinatal psychology (pp. 167–181). Oxford University Press. [Google Scholar]
- Fairbrother N., Collardeau F., Albert A. Y., Challacombe F. L., Thordarson D. S., Woody S., Janssen P. (2021). High prevalence and incidence of OCD among women across pregnancy and postpartum. The Journal of Clinical Psychiatry, 82(2), Article 20m13398. [DOI] [PubMed] [Google Scholar]
- Fairbrother N., Corbyn B., Thordarson D. S., Ma A., Surm D. (2019). Screening for perinatal anxiety disorders: Room to grow. Journal of Affective Disorders, 250, 363–370. 10.1016/j.jad.2019.03.052 [DOI] [PubMed] [Google Scholar]
- Fairbrother N., Woody S. R. (2008). New mothers’ thoughts of harm related to the newborn. Archives of Women’s Mental Health, 11(3), 221–229. 10.1007/s00737-008-0016-7 [DOI] [PubMed] [Google Scholar]
- Fawcett E. J., Fairbrother N., Cox M. L., White I. R., Fawcett J. M. (2019). The prevalence of anxiety disorders during pregnancy and the postpartum period: A multivariate Bayesian meta-analysis. The Journal of Clinical Psychiatry, 80(4), 18r12527. 10.4088/JCP.18r12527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M. B., Williams J. B. W., Karg R. S., Spitzer R. L. (2016). User’s guide for the SCID-5-CV Structured Clinical Interview for DSM-5® disorders: Clinical version. American Psychiatric Publishing. [Google Scholar]
- Foa E. B., Huppert J. D., Leiberg S., Langner R., Kichic R., Hajcak G., Salkovskis P. M. (2002). The Obsessive-Compulsive Inventory: Development and validation of a short version. Psychological Assessment, 14(4), 485–496. 10.1037/1040-3590.14.4.485 [DOI] [PubMed] [Google Scholar]
- Gaynes B. N., Gavin N., Meltzer-Brody S., Lohr K. N., Swinson T., Gartlehner G., Brody S., Miller W. C. (2005). Perinatal depression: Prevalence, screening accuracy, and screening outcomes. Evidence Report/Technology Assessment (Summary), 119, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gönner S., Leonhart R., Ecker W. (2007). The Obsessive–Compulsive Inventory–Revised (OCI-R): Validation of the German version in a sample of patients with OCD, anxiety disorders, and depressive disorders. Journal of Anxiety Disorders, 22(4), 734–749. 10.1016/j.janxdis.2007.07.007 [DOI] [PubMed] [Google Scholar]
- Grigoriadis S., de Camps Meschino D., Barrons E., Bradley L., Eady A., Fishell A., Mamisachvili L., Cook G. S., O’Keefe M., Romans S., Ross L. E. (2011). Mood and anxiety disorders in a sample of Canadian perinatal women referred for psychiatric care. Archives of Women’s Mental Health, 14(4), 325–333. 10.1007/s00737-011-0223-5 [DOI] [PubMed] [Google Scholar]
- Handelzalts J., Fisher S., Lurie S., Shalev A., Golan A., Sadan O. (2012). Personality, fear of childbirth and cesarean delivery on demand. Acta Obstetricia et Gynecologica Scandinavica, 91(1), 16–21. 10.1111/j.1600-0412.2011.01287.x [DOI] [PubMed] [Google Scholar]
- House S. J., Tripathi S. P., Knight B. T., Morris N., Newport D. J., Stowe Z. N. (2016). Obsessive-compulsive disorder in pregnancy and the postpartum period: Course of illness and obstetrical outcome. Archives of Women’s Mental Health, 19(1), 3–10. 10.1007/s00737-015-0542-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp. (2017). IBM SPSS statistics for windows [computer software]. [Google Scholar]
- Jomeen J., Martin C. R. (2005). Confirmation of an occluded anxiety component within the Edinburgh Postnatal Depression Scale (EPDS) during early pregnancy. Journal of Reproductive and Infant Psychology, 23(2), 143–154. 10.1080/02646830500129297 [DOI] [Google Scholar]
- Kozinszky Z., Dudas R. B. (2015). Validation studies of the Edinburgh Postnatal Depression Scale for the antenatal period. Journal of Affective Disorders, 176, 95–105. 10.1016/j.jad.2015.01.044 [DOI] [PubMed] [Google Scholar]
- López-Solà C., Gutiérrez F., Alonso P., Rosado S., Taberner J., Segalàs C., Real E., Menchόn J. M., Fullana M. A. (2014). Spanish version of the Dimensional Obsessive–Compulsive Scale (DOCS): Psychometric properties and relation to obsessive beliefs. Comprehensive Psychiatry, 55(1), 206–214. 10.1016/j.comppsych.2013.08.015 [DOI] [PubMed] [Google Scholar]
- Lord C., Rieder A., Hall G. B. C., Soares C. N., Steiner M. (2011). Piloting the Perinatal Obsessive-Compulsive Scale (POCS): Development and validation. Journal of Anxiety Disorders, 25(8), 1079–1084. 10.1016/j.janxdis.2011.07.005 [DOI] [PubMed] [Google Scholar]
- Mandrekar J. N. (2010). Receiver Operating Characteristic curve in diagnostic test assessment. Journal of Thoracic Oncology, 5(9), 1315–1316. 10.1097/JTO.0b013e3181ec173d [DOI] [PubMed] [Google Scholar]
- Matthey S. (2008). Using the Edinburgh Postnatal Depression Scale to screen for anxiety disorders. Depression and Anxiety, 25(11), 926–931. 10.1002/da.20415 [DOI] [PubMed] [Google Scholar]
- Matthey S., Fisher J., Rowe H. (2013). Using the Edinburgh Postnatal Depression Scale to screen for anxiety disorders: Conceptual and methodological considerations. Journal of Affective Disorders, 146(2), 224–230. [DOI] [PubMed] [Google Scholar]
- Massachusetts General Hospital Centre for Women’s Mental Health. (2019). Women’s Preventive Services Initiative (WPSI) released draft recommendation on screening for anxiety. https://womensmentalhealth.org/posts/initiative-to-screen-all-women-for-anxiety-including-pregnant-and-postpartum-women/
- Miller E. S., Hoxha D., Wisner K. L., Gossett D. R. (2015). The impact of perinatal depression on the evolution of anxiety and obsessive-compulsive symptoms. Archives of Women’s Mental Health, 18(3), 457–461. 10.1007/s00737-014-0476-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C. (2001). Stress and mood disorders during pregnancy: Implications for child development. Psychiatric Quarterly, 72(4), 347–357. 10.1023/A:1010393316106 [DOI] [PubMed] [Google Scholar]
- Nerum H., Halvorsen L., Straume B., Sørlie T., Øian P. (2013). Different labour outcomes in primiparous women that have been subjected to childhood sexual abuse or rape in adulthood: A case–control study in a clinical cohort. British Journal of Obstetrics and Gynaecology, 120(4), 487–495. 10.1111/1471-0528.12053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newham J. J., Wittkowski A., Hurley J., Aplin J. D., Westwood M. (2014). Effects of antenatal yoga on maternal anxiety and depression: A randomized controlled trial. Depression and Anxiety, 31(8), 631–640. 10.1002/da.22268 [DOI] [PubMed] [Google Scholar]
- Nieminen K., Berg I., Frankenstein K., Viita L., Larsson K., Persson U., Spånberger L., Wretman A., Silfvernagel K., Andersson G., Wijma K. (2016). Internet-provided cognitive behaviour therapy of posttraumatic stress symptoms following childbirth—A randomized controlled trial. Cognitive Behaviour Therapy, 45(4), 287–306. 10.1080/16506073.2016.1169626 [DOI] [PubMed] [Google Scholar]
- Nieminen K., Stephansson O., Ryding E. L. (2009). Women’s fear of childbirth and preference for cesarean section—A cross-sectional study at various stages of pregnancy in Sweden. Acta Obstetricia et Gynecologica Scandinavica, 88(7), 807–813. 10.1080/00016340902998436 [DOI] [PubMed] [Google Scholar]
- Rapp A. M., Bergman R. L., Piacentini J., McGuire J. F. (2016). Evidence-based assessment of obsessive-compulsive disorder. Journal of Central Nervous System Disease, 8(8), 13–29. 10.4137/JCNSD.S38359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2020). R: A language and environment for statistical computing [computer software]. R Foundation for Statistical Computing. [Google Scholar]
- Ruscio A. M., Stein D. J., Chiu W. T., Kessler R. C. (2010). The epidemiology of obsessive-compulsive disorder in the national comorbidity survey replication. Molecular Psychiatry, 15(1), 53–63. 10.1038/mp.2008.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell E. J., Fawcett J. M., Mazmanian D. (2013). Risk of obsessive-compulsive disorder in pregnant and postpartum women: A meta-analysis. The Journal of Clinical Psychiatry, 74(4), 377–385. 10.4088/JCP.12r07917 [DOI] [PubMed] [Google Scholar]
- Sackett D. L., Straus S., Richardson W. S., Rosenberg W., Haynes R. B. (2000). Evidence-based medicine: How to practise and teach EBM (2nd ed.). Churchill Livingstone. [Google Scholar]
- Saisto T., Salmela-Aro K., Nurmi J., Halmesmäki E. (2001). Psychosocial characteristics of women and their partners fearing vaginal childbirth. British Journal of Obstetrics and Gynaecology, 108(5), 492–498. 10.1111/j.1471-0528.2001.00122.x [DOI] [PubMed] [Google Scholar]
- Salomonsson B., Gullberg M. T., Alehagen S., Wijma K. (2013). Self-efficacy beliefs and fear of childbirth in nulliparous women. Journal of Psychosomatic Obstetrics & Gynecology, 34(3), 116–121. 10.3109/0167482X.2013.824418 [DOI] [PubMed] [Google Scholar]
- Stasik S. M., Naragon-Gainey K., Chmielewski M., Watson D. (2012). Core OCD symptoms: Exploration of specificity and relations with psychopathology. Journal of Anxiety Disorders, 26(8), 859–870. 10.1016/j.janxdis.2012.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll K., Hauck Y., Downe S., Edmonds J., Gross M. M., Malott A., McNiven P., Swift E., Thomson G., Hall W. A. (2016). Cross-cultural development and psychometric evaluation of a measure to assess fear of childbirth prior to pregnancy. Sexual & Reproductive Healthcare, 8, 49–54. 10.1016/j.srhc.2016.02.004 [DOI] [PubMed] [Google Scholar]
- Straub H., Adams M., Kim J. J., Silver R. K. (2012). Antenatal depressive symptoms increase the likelihood of preterm birth. American Journal of Obstetrics and Gynecology, 207(4), 329.e1–329.e4. 10.1016/j.ajog.2012.06.033 [DOI] [PubMed] [Google Scholar]
- Swalm D., Brooks J., Doherty D., Nathan E., Jacques A. (2010). Using the Edinburgh Postnatal Depression Scale to screen for perinatal anxiety. Archives of Women’s Mental Health, 13(6), 515–522. 10.1007/s00737-010-0170-6 [DOI] [PubMed] [Google Scholar]
- Sydsjö G., Bladh M., Lilliecreutz C., Persson A., Vyöni H., Josefsson A. (2014). Obstetric outcomes for nulliparous women who received routine individualized treatment for severe fear of childbirth—A retrospective case control study. BMC Pregnancy and Childbirth, 14(1), Article 126. 10.1186/1471-2393-14-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydsjö G., Blomberg M., Palmquist S., Angerbjörn L., Bladh M., Josefsson A. (2015). Effects of continuous midwifery labour support for women with severe fear of childbirth. BMC Pregnancy and Childbirth, 15(1), Article 115. 10.1186/s12884-015-0548-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takegata M., Haruna M., Matsuzaki M., Shiraishi M., Okano T., Severinsson E. (2015). Does antenatal fear of childbirth predict postnatal fear of childbirth? A study of Japanese women. Open Journal of Nursing, 5(2), 144–152. 10.4236/ojn.2015.52017 [DOI] [Google Scholar]
- Tang S., Yu W., He L., Wang J., Chasson G. S. (2015). Diagnostic utility of the Obsessive-Compulsive Inventory–Revised in China. Journal of Obsessive-Compulsive and Related Disorders, 5, 93–97. 10.1016/j.jocrd.2015.04.001 [DOI] [Google Scholar]
- Thibodeau M. A., Leonard R. C., Abramowitz J. S., Riemann B. C. (2015). Secondary psychometric examination of the Dimensional Obsessive-Compulsive Scale: Classical testing, item response theory, and differential item functioning. Assessment, 22(6), 681–689. 10.1177/1073191114559123 [DOI] [PubMed] [Google Scholar]
- Thiele C. (2020). Cutpointr: Determine and evaluate optimal cutpoints in binary classification tasks (R package version 1.0.32) [computer software]. [Google Scholar]
- Uguz F., Yuksel G., Karsidag C., Guncu H., Konak M. (2015). Birth weight and gestational age in newborns exposed to maternal obsessive-compulsive disorder. Psychiatry Research, 226(1), 396–398. 10.1016/j.psychres.2014.12.063 [DOI] [PubMed] [Google Scholar]
- van Heyningen T., Honikman S., Tomlinson M., Field S., Myer L. (2018). Comparison of mental health screening tools for detecting antenatal depression and anxiety disorders in South African women. PLOS ONE, 13(4), Article e0193697. 10.1371/journal.pone.0193697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M., Davis D. M., Thibodeau M. A., Bach N. (2013). Psychometric properties of the Obsessive-Compulsive Inventory Revised in African Americans with and without obsessive-compulsive disorder. Journal of Obsessive-Compulsive and Related Disorders, 2(4), 399–405. 10.1016/j.jocrd.2013.07.003 [DOI] [Google Scholar]
- Wootton B. M., Diefenbach G. J., Bragdon L. B., Steketee G., Frost R. O., Tolin D. F. (2015). A contemporary psychometric evaluation of the Obsessive Compulsive Inventory–Revised (OCI-R). Psychological Assessment, 27(3), 874–882. 10.1037/pas0000075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M., Posternak M. A., Chelminski I., Solomon D. A. (2004). Using questionnaires to screen for psychiatric disorders: A comment on a study of screening for bipolar disorder in the community. The Journal of Clinical Psychiatry, 65(5), 605–610. 10.4088/JCP.v65n0503 [DOI] [PubMed] [Google Scholar]