Abstract
Background
Sufficient and timely spinal cord decompression is a critical surgical objective for neurological recovery in spinal cord injury (SCI). Residual cord compression may be associated with disturbed cerebrospinal fluid pressure (CSFP) dynamics.
Objectives
This study aims to assess whether intrathecal CSFP dynamics in SCI following surgical decompression are feasible and safe, and to explore the diagnostic utility.
Methods
Prospective cohort study. Bedside lumbar CSFP dynamics and cervical MRI were obtained following surgical decompression in N = 9 with mostly cervical acute-subacute SCI and N = 2 patients with non-traumatic SCI. CSFP measurements included mean CSFP, cardiac-driven CSFP peak-to-valley amplitudes (CSFPp), Valsalva maneuver, and Queckenstedt’s test (firm pressure on jugular veins, QT). From QT, proxies for cerebrospinal fluid pulsatility curve were calculated (ie, relative pulse pressure coefficient; RPPC-Q). CSFP metrics were compared to spine-healthy patients. computer tomography (CT)-myelography was done in 3/8 simultaneous to CSFP measurements.
Results
Mean age was 45 ± 9 years (range 17-67; 3F), SCI was complete (AIS A, N = 5) or incomplete (AIS B-D, N = 6). No adverse events related to CSFP assessments. CSFP rise during QT was induced in all patients [range 9.6-26.6 mmHg]. However, CSFPp was reduced in 3/11 (0.1-0.3 mmHg), and in 3/11 RPPC-Q was abnormal (0.01-0.05). Valsalva response was reduced in 8/11 (2.6-23.4 mmHg). CSFP dynamics corresponded to CT-myelography.
Conclusions
Comprehensive bedside lumbar CSFP dynamics in SCI following decompression are safe, feasible, and can reveal distinct patterns of residual spinal cord compression. Longitudinal studies are required to define critical thresholds of impaired CSFP dynamics that may impact neurological recovery and requiring surgical revisions.
Keywords: spinal cord injury, spinal cord compression, cerebrospinal fluid pressure, craniospinal compliance, compression biomarker, spine surgery
Abbreviations
| ASIA | American Spinal Injury Association |
| AIS | American Spinal Injury Association Impairment Scale |
| CSFP | Cerebrospinal fluid pressure |
| CSFPp | Cardiac-driven CSFP peak-to-valley amplitude |
| IQR | Interquartile range |
| ISNCSCI | International Standards for Neurological Classification of Spinal Cord Injury |
| ISP | Intraspinal pressure |
| LP | Lumbar puncture |
| MARS | metal artifact reduction sequence |
| MRI | Magnetic resonance imaging |
| NISCI | Nogo Inhibition in Spinal Cord Injury |
| RPPC-Q | Relative pulse pressure coefficient computed through Queckenstedt’s test |
| SCI | Spinal cord injury |
Introduction
Spinal cord injury (SCI) is associated with neurological impairment of varying severity, ranging from complete paralysis to incomplete sensorimotor deficits. 1 Spinal cord compression is considered a key mechanism for primary and secondary damage in SCI. 2 Therefore, timely and sufficient surgical decompression is required in most patients and it is associated with improved functional outcomes. 3 Following the acute surgical and medical management, patients are referred to specialized SCI rehabilitation centers to promote neuroplastic reorganization and compensation strategies. 4 To improve neurological outcomes in SCI, intrathecal or intraspinal administered drug, cell, or stem-cell therapies have been investigated, yet without reproducible evidence for patient benefit.5,6
However, insufficient decompression is a typical surgical adverse event that may prompt revision surgery, and which may confound the effects of neurorestorative therapies through restricted drug circulation and limited regenerative capacity due to persistent cord compression.7-9 Perioperative intraspinal pressure (ISP) and intrathecal cerebrospinal fluid pressure (CSFP) monitoring have been investigated as a tool to determine sufficient decompression10,11 and to determine the optimum spinal cord perfusion pressure. 12
Bedside assessments of CSFP dynamics had a long tradition with the goal to quantify spinal canal blockage from chronic degenerative or tumorous lesions, before being replaced by magnetic resonance imaging (MRI).13-15 Queckenstedt’s test was the first CSFP readout used in suspected spinal cord compression about 100 years ago and was routinely performed to test for static and dynamic spinal cord compression in patients with clinical signs of myelopathy before neuroimaging provided a measure of spinal canal diameter. 14 In a recent study we demonstrated that CSFP dynamics reveal signs of effective spinal canal narrowing in ambiguous spine conditions. 16 In this study, we aimed to explore the safety and feasibility, as well as the diagnostic utility of this method, in sub-acute and chronic SCI. We hypothesize that bedside CSFP assessments may be useful in SCI as part of clinical trial protocols involving intrathecal drug administration, to reveal a non-obstructed cerebrospinal fluid compartment in patients with suspicion of residual cord compression.
Methods
Study Overview and Clinical Examinations
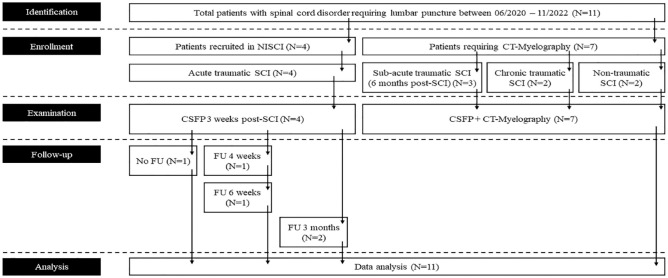
From the Spinal Cord Injury Center and Department of Neurology and Neurophysiology at Balgrist University Hospital, patients with SCI were consecutively enrolled between 2020 and 2022 (Figure 1). Measurements were part of a comprehensive study that investigates CSFP dynamics in patients with spinal cord compression (NCT02170155). 17 CSFP recordings were performed before intrathecal drug administration as part of the Nogo Inhibition in Spinal Cord Injury (NISCI) study (NCT03935321) (ID1-3,6), in patients with cord compression of unclear significance prior to computer tomography (CT-) myelography (ID4,7,8-11), or during baclofen testing (ID5). Follow-up measurements were available from NISCI patients during repetitive intrathecal drug administration. Patients were examined according to the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI), and injury was classified with the American Spinal Injury Association (ASIA) Impairment Scale (AIS). 18 Spinal cord MRI was available for most patients and included optimized metal artifact reduction sequence (MARS). 19 Three patients had MRI contraindications (ID9-11). The study protocol conformed to the latest revision of the Declaration of Helsinki and was approved by the local Ethics Committee of the University Hospital of Zurich (KEK-ZH number PB-2016-00623).
Figure 1.
STROBE flow chart of sample recruitment.
Abbreviations: FU, follow-up; NISCI, Nogo inhibition in spinal cord injury; SCI, spinal cord injury; STROBE, strengthening the reporting of observational studies in epidemiology.
CT Myelography
All punctures were performed by a board-certified, fellowship trained musculoskeletal radiologist with 10 years of experience in CT-guided spinal procedures. The patient was placed prone on the CT table. A low-dose CT scan of the lumbar spine was performed from L2 to S1 to identify a suitable segment for puncture of the lumbar subarachnoid space, below the anatomic conus level. After skin preparation in standard fashion, a 23 Gauge spinal needle was used for thecal sac puncturing using an interlaminar approach. The needle was advanced under intermittent CT guidance. Accurate needle tip location was confirmed with the injection of 0.5 mL of nonionic iodinated contrast medium (Iopamidol, with 200 mg/mL of iodine), demonstrating free intrathecal contrast distribution. Then, measurement of the intrathecal CSFP was performed. For the subsequent myelography, 15 to 20 mL of nonionic iodinated contrast medium (mixture of Iopamidol with 200 and 300 mg/mL iodine at a ratio of 1:1) were injected. After reinserting the stylet and removing the needle, the patient was moved to a tilting table in Trendelenburg position. Intermittent right-to-left patient rotation was performed to support homogenous contrast opacification of the cerebrospinal fluid. After 10 to 30 minutes, a regular dose CT scan was performed over the spinal region of interest, including axial, coronal, and sagittal reformation images in bone and soft tissue kernel with slice thicknesses from 0.6 to 2 mm. From these patients (ID4,7-11), MRI was available from 2 weeks after CT-myelography (ID4), or 4 weeks before (ID7) and 9 days before CT-myelography (ID8), while the remaining patients only had CT-scan (ID9-11), which was acquired on the same day.
CSFP Recordings
Patients underwent bedside lumbar puncture (LP), or in some cases LP on CT table, and assessment of CSFP dynamics, before any cerebrospinal fluid (CSF) was drained. LP was done in lateral position with 20 to 22 Gauge Sprotte® needles, or in prone position on CT table. The needle was connected to an analogue digital pressure converter (Neuromedex VentrEX), and the digitized signal linked to a Philips X2-Pat Interface+MX 700 Monitor, connected to a recording software ICM+ (University of Cambridge). In 1 patient (ID5), CSFP was measured from a lumbar catheter (Neuromedex Lumbalkatheter 4.5F) that was inserted to perform external baclofen pump testing. The assessment of CSFP dynamics included recording of 30 to 60 seconds time window during resting-state and manual jugular vein compression (Queckenstedt’s test). Lastly, patients were asked to blow against a blocked syringe (Valsalva maneuver). Valsalva maneuver was performed to test for correct needle placement and to investigate if sufficient abdominal force can be recruited, but it was not considered to inform about cord compression due to other physiological mechanisms involved.
CSFP Analysis
Data were analyzed with MATLAB software. The signal was decomposed into different frequency bins using discrete wavelet decomposition to extract mean CSFP and cardiac-driven CSFP peak-to-valley amplitude (CSFPp). The first 4 frequency bins (0-0.5 Hz) were used to reconstruct mean CSFP. For CSFPp, defined as the difference between the systolic peak and the associated diastolic valley of CSFP, the next 4 frequency bins (0.5-8 Hz) were used. For the extraction of the relative pulse pressure coefficient, computed from Queckenstedt’s test (RPPC-Q), a regression line was fitted to the CSFPp versus mean CSFP curve (pulsatility curve). This curve was formed by individual subjects’ data points during the resting state and Queckenstedt’s test. RPPC-Q was the slope of this regression line.
Ranges of CSFP Dynamics in Patients Without Spinal Cord Compression
We previously obtained CSFP dynamics in patients who underwent LP for reasons other than spinal cord compression (N = 14; mean age 59.7 ± 9.6 years, range 39-73 years; 6 females; mean body-mass index (BMI) was 25 ± 3, range 18-30). Patients were in stable medical condition and the examination was done in an outpatient setting. There was no evidence for stenosis of the cervical spinal canal. LP was done for suspicion of demyelinating disease in most patients (N = 7), and for peripheral neuropathy or infectious CNS disease in the remaining. Mean CSFP during resting state had a median of 12.3 [Interquartile range, IQR 3.2] mmHg, ranging from 8.6 to 18.9, and median CSFPp was 1.0 mmHg [0.5], ranging from 0.4 to 2.1 mmHg. Mean CSFP rise during Queckenstedt’s test had a median of 12.5 [7.3] mmHg, range from 5.3 to 34.1 mmHg. Mean CSFP rise during Valsalva maneuver had a median of 38.4 [15.5] mmHg, range from 27.4 to 60.8 mmHg. Median RPPC-Q was 0.18 [0.04], range from 0.1 to 0.4.
For comparison, normal values were determined with regards to the lowest values obtained in the spine-healthy cohort. This implies that findings were considered abnormal if baseline CSFP was <8.6 mmHg, CSFPp was <0.4 mmHg, Queckenstedt’s test rise was <5 mmHg, a Valsalva rise was <27 mmHg, or RPPC-Q was <0.1. Owing to the case-based approach, a precise statistical analysis was not required. Furthermore, a statistical group level analysis was not deemed appropriate in this cohort with variable clinical and imaging findings. However, in more homogenous cohorts, we also advocate group level statistics for CSFP dynamics.
Results
Patient Characteristics
Eleven patients with a mean age of 45 ± 9 years were prospectively enrolled (range 17-67; 3F) and underwent CSFP assessments without adverse events. Individuals with SCI were younger than spine-healthy patients (P = .009). Mean BMI was 24 ± 3, range 17 to 30, and not different from the spine-healthy cohort (P = .311). Patients had complete (AIS A, N = 5) or sensorimotor incomplete SCI (AIS B, C, or D, N = 6). The neurological level was between C3 and C7 for most patients (N = 7), between T2 and T7 in some (N = 3), and 1 patient had tandem injury (C5 and T8). Most patients had traumatic SCI (9 out of 11 patients) and time since injury was weeks to months in most, except for 3 patients, who were in late chronic stages (3.5, 9, and >10 years after injury). All patients with traumatic SCI underwent decompressive surgery and fusion within 72 hours of the injury. One patient had spondylodiscitis, with an onset of 2 weeks before the assessments and was examined 1 week after surgical decompression. In addition to SCI, 1 patient had syringomyelia (ID7) and 1 patient had suspicion of adjacent level stenosis (ID8). In 3 patients (ID9-11) there was clinical suspicion of residual cord compression following surgery. MRI was not feasible due to metal splinter from war injury in patients 9 and 11, and due to cardiac pacemaker in patient 10. Therefore, CT-myelography was performed. All patients were fully awake and breathing without assistance except for 1 patient (ID4), who was tracheotomized and mildly sedated during the examination. Clinical characteristics and detailed CSFP findings are summarized in Table 1. The causes of trauma and surgical techniques are shown in Table S1.
Table 1.
Clinical Characteristics of Patients With Spinal Cord Injury (SCI) (ID1-11).
| ID | Type of disease | AIS | Time since injury | NLI | CT-myelography | RS CSFP†/†† | RS CSFPp†/†† | VM rise † | QT rise † | RPPC-Q †† |
|---|---|---|---|---|---|---|---|---|---|---|
| Range from spine-healthy | NA | NA | NA | NA | NA | 8.6-18.9 | 0.4-2.2 | 27.4-60.8 | 5.3-34.1 | 0.10-0.44 |
| 1 | tSCI | A | 3 weeks | C5 | NP | 23.4 (0.3) | 1.1 (0.2) | 13.0 | 23.1 | 0.12 |
| 1 FU | tSCI | A | 3 months | C5 | NP | 13.2 (0.2) | 2.3 (0.4) | NP | 23.1 | 0.16 |
| 2 | tSCI | D | 3 weeks | C5 | NP | 14.0 (1.0) | 0.6 (0.3) | 15.5 | 22.9 | 0.08 |
| 2 FU | tSCI | D | 3 months | C5 | NP | 10.6 (0.5) | 0.3 (0.1) | 16.2 | 26.6 | 0.10 |
| 3 | tSCI | D | 3 weeks | C4 (central cord syndrome) | NP | 3.2 (1.8) | 0.2 (0.05) | 34.3 | 9.6 | 0.12 |
| 4 | Spondylodiscitis | A | 2 weeks | C3 | Stenosis | 15.8 (1.5) | 0.9 (0.4) | 1.6 § | 13.3 | 0.01 |
| 5 | tSCI | C | 6 months | C5 (tandem injury, more severe at level T8/9) | NP | 8.9 (0.6) | 1.9 (0.1) | 29.2 | 12.2 | 0.05 |
| 6 | tSCI | B | 3 weeks | C4 | NP | 5.8 (0.2) | 0.1 (0.0) | 2.6 | 15.1 | 0.03 |
| 6 FU | tSCI | B | 4 weeks | C4 | NP | 13.4 (0.8) | 0.5 (0.1) | 4.3 | 13.0 | 0.10 |
| 6 FU | tSCI | B | 6 weeks | C4 | NP | 9.9 (0.4) | 0.2 (0.1) | 4.0 | 15.2 | 0.04 |
| 7 | tSCI, syringomyelia | A | 3.5 years | T2 | Normal | 5.5 (0.2) | 0.8 (0.1) | 11.0 | 12.5 | 0.13 |
| 8 | tSCI | D | >10 years | C7 | Normal | 14.6 (0.4) | 1.2 (0.5) | 6.3 | 14.4 | 0.26 |
| 9 | tSCI | D | 6 months | C7 | Normal | 13.8 (0.6) | 0.7 (0.1) | 17.9 | 22.2 | 0.10 |
| 10 | Ischemia | D | 9 years | T6 | Normal | 4.8 (0.6) | 1.3 (0.5) | 23.4 | 13.9 | 0.15 |
| 11 | tSCI | A | 6 months | T7 | Normal | 6.2 (0.4) | 0.5 (0.1) | 10.0 | 17.9 | 0.10 |
Abbreviations: AIS, ASIA impairment scale; CSFP, cerebrospinal fluid pressure; CSFPp, cardiac-driven CSFP peak-to-valley amplitude; NLI, neurological level of injury; NP, not performed; SCI, spinal cord injury; tSCI, traumatic SCI.
Follow-up measurements were available in ID1,2,6. All CSF pressure (CSFP) assessments were performed in lateral decubital position through lumbar spinal needle, and in 1 case through lumbar catheter (ID5).
mmHg.
Median (interquartile range).
Inspiration hold maneuver.
Safety and Feasibility
None of the patients had post-puncture syndrome or other adverse events associated with the LP. Hence, the additional CSFP dynamic maneuvers did not lead to a higher rate of adverse events during LP. Protocol adherence was assured in all patients. The CSFP assessment were done in short time and total examination time was not significantly delayed. Mild discomfort was felt during Queckenstedt’s test, because it involves firm pressure on the neck.
CSFP Dynamics
Mean CSFP had a range of 3.2 to 23.4 mmHg, with CSFPp ranging from 0.1 to 2.3 mmHg. Compared to spine-healthy subjects, we found borderline or reduced CSFPp in N = 3 (ID2,3,6; 0.1-0.3 mmHg). Two of those patients also had comparably low mean CSFP (3.2 and 5.8 mmHg). Mean CSFP rise during Queckenstedt’s test ranged between 9.6 and 23.1 mmHg, with an RPPC-Q of 0.01 to 0.26. None of the patients had spinal block, as defined by absence of CSFP rise during Queckenstedt’s test. It was notable that despite adequate CSFP response, CSFPp was weakly modulated on top of Queckenstedt’s response in N = 3 (ID4-6; RPPC-Q between 0.01 and 0.05), significantly less in comparison to the spine-healthy subjects. Reduced CSFPp was not paralleled with reduced RPPC-Q; only 1 patient showed reduction in both parameters (ID6). This patient also showed a delayed return to CSFP baseline following release of Queckenstedt’s test, so called “valvular effect” according to previous reports. 20
Neuroimaging and CSFP Findings
An overview of cervical and thoracic spine MRI at the time of measurements is shown in Figure 2. An overview of pre- and 72 hours post-operative cervical and thoracic cervical and thoracic spine MRI or CT is shown in Figure S1. Patients had emergency CT- and/or MR-imaging according to the individual patient need. Patients ID8-11 are not included here as there was no imaging available from ID8 due to the long time passed since the accident, from ID 9 and 11 due to injuries occurring during warfare, and from ID10 who did not have traumatic injury. Acute stage MRI showed typical tissue changes (eg, cord swelling and cord edema) and findings related to surgery (eg, seroma and spinal instrumentation). In chronic stage MRI, demarcated T2-hyperintense spinal cord tissue signals were seen. In 2 patients (ID1,5), there was no evidence of residual cervical cord compression, corresponding to normal CSFP dynamics, except for valvular effect in ID1 (Figure 3). In 1 case, there was possible posterior compression from seroma (ID2), with CSFP dynamics being normal. There was diagnostic uncertainty if sufficient decompression could be achieved in 5 patients (ID3,4,6,9,11) (ID4: Figure 4, ID6: Figure 5) and suspected lumbar stenosis in 1 patient (ID10). In some of these patients, CSFP dynamics were clearly altered (ID3,4,6). In the remaining patients with suspected cord compression (ID9-11), in the patient with syringomyelia of unclear clinical significance (ID7) and the other patient with suspicion of adjacent level stenosis (ID8), CSFP dynamics were normal. CT-myelography results were in line with CSFP findings, suggesting effective spinal canal compression in 1 patient (ID4) (Figure 4F) and normal passage of contrast agent in the others (ID7-11).
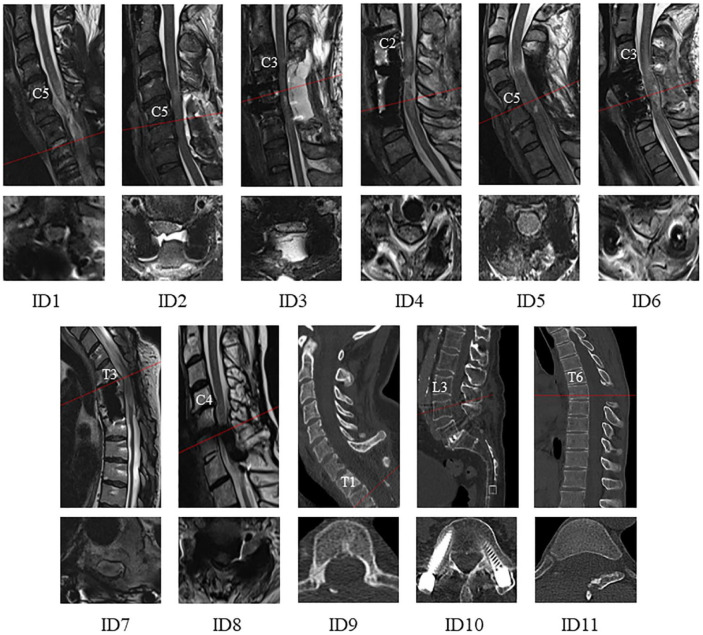
Figure 2.
Sagittal (upper row) and axial (lower row) cervical T2-weighted MRI from the time of CSF pressure (CSFP) assessment shown for all subjects (ID1-11). Patients were examined at 2 to 3 weeks (ID1-4 and 6), several weeks up to 6 months (ID5,9,11), and in late chronic stages several years following SCI (ID7,8,10).
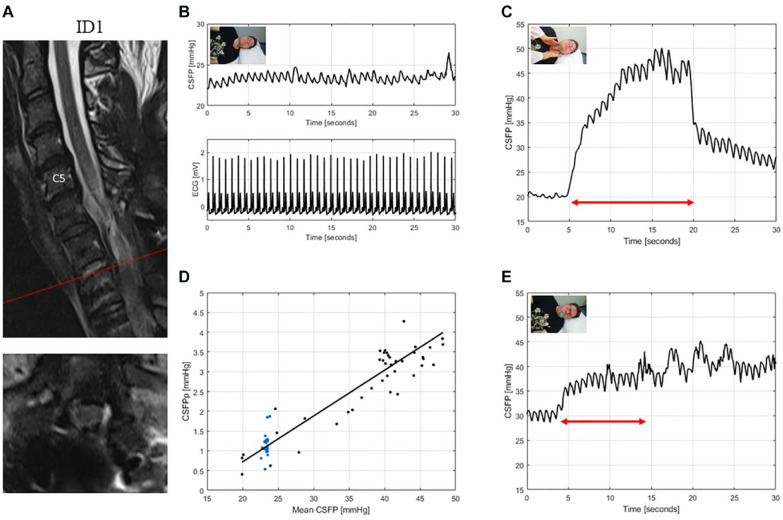
Figure 3.
T2-weighted MRI of the cervical spine for ID1 (patient with traumatic spinal cord injury, AIS: A) (A). The interpretation of the cervical MRI was challenging, but upon radiological judgement there was no clear sign of residual cord compression. CSFP and electrocardiogram (ECG) are shown during resting state (B). CSFP were normal at baseline and rise of 9.6 mmHg during Queckenstedt’s test showed a clear valvular effect (C). Cardiac-driven CSFP peak-to-valley amplitude (CSFPp) at resting state (blue dots) and during Queckenstedt’s test (black dots) is plotted against mean CSFP (D). The regression line (in black) showed a normal relative pulse pressure coefficient (RPPC-Q) equal to 0.12. Response to Valsalva maneuver is reduced with a CSFP rise of 13.0 mmHg (E).
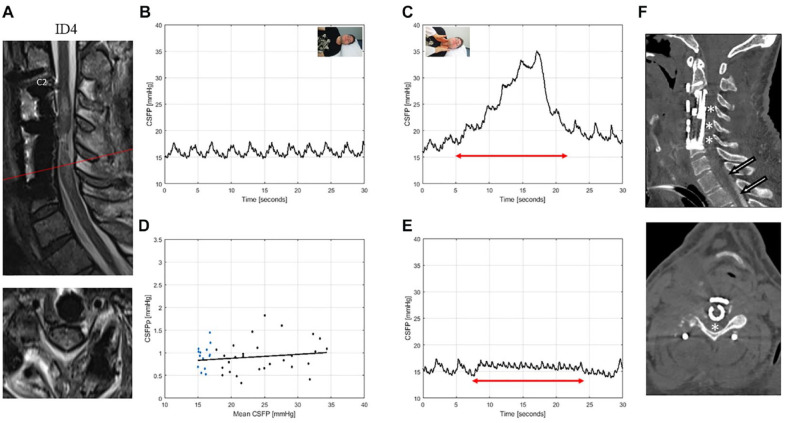
Figure 4.
T2-weighted MRI of the cervical spine for ID4 (patient with spondylodiscitis, AIS: A) (A). This patient was tracheotomized and mildly sedated during the examination. Sagittal and axial images showed edema and were suggestive of residual cord compression related to cord swelling. CSFP was normal during resting state (B) and Queckenstedt’s test (rise 13.3 mmHg) (C). Cardiac-driven CSFP peak-to-valley amplitude (CSFPp) at resting state (blue dots) and during Queckenstedt’s test (black dots) is plotted against mean CSFP (D). The regression line (in black) was pathologically reduced with a relative pulse pressure coefficient (RPPC-Q) equal to 0.01. Response to inspiration hold maneuver is low with a CSFP rise of 1.6 mmHg (E). Sagittal and axial CT myelography reformation images of a cervical spine (F) after prolonged Trendelenburg position of 60 minutes show partial cerebrospinal fluid opacification extending to T1 (arrows), but not beyond. The constellation of findings suggests focal central C4 to C6 stenosis (asterisks), causing cranial cerebrospinal fluid flow restriction.
Figure 5.
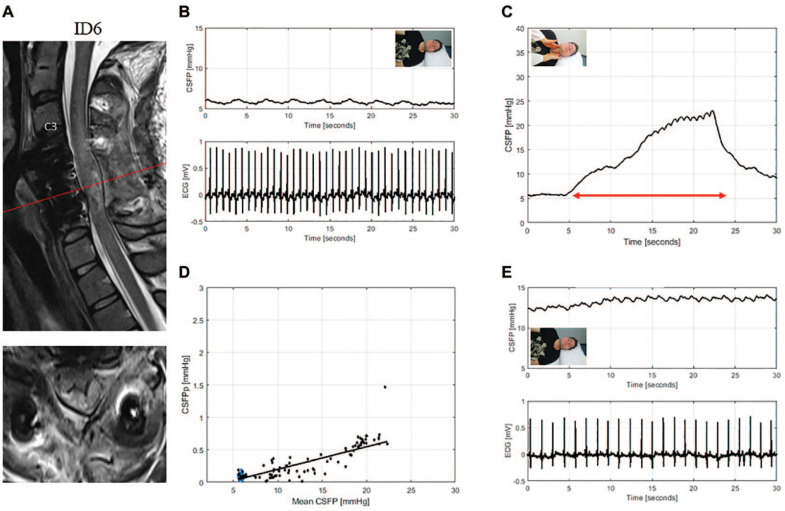
T2-weighted MRI of the cervical spine for ID6 (patient with traumatic spinal cord injury, AIS: B) (A). Sagittal and axial images showed beginning cyst formation and edema, and were suggestive of spinal cord swelling against the dura despite sufficient bony decompression. CSFP is severely affected during resting state (B) but with a responsive Queckenstedt’s test (rise 15.1 mmHg) (C). Cardiac-driven CSFP peak-to-valley amplitude (CSFPp) at resting state (blue dots) and during Queckenstedt’s test (black dots) is plotted against mean CSFP (D). The regression line (in black) is reduced with a relative pulse pressure coefficient (RPPC-Q) equal to 0.03. Resting state CSFPp was recovered at the first follow-up session (E).
Response to Valsalva Maneuver
Valsalva maneuver was not considered to provide information on spinal cord compression but on thoracoabdominal muscle recruitment. In 5 patients, we found lower mean CSFP rise during Valsalva maneuver compared to the spine-healthy controls (2.6-23.4 mmHg). Three of them were graded AIS A or B, 2 were graded AIS D. In addition, these patients had higher mean CSFP rise during Queckenstedt’s test than Valsalva maneuver (2 of them reproducibly in follow-up measurements), which was found in none of our spine-healthy cases, therefore indicating abnormal CSFP response during Valsalva maneuver.
Follow-Up Measurements
In 2 patients (ID1,2) data were recorded 3 weeks and 3 months after the initial assessment. In 1 patient (ID6) data were recorded 3-, 4-, and 6-weeks following SCI. CSFP rise during Queckenstedt’s test was well reproducible in all patients. CSFPp was variable and appeared to decrease in 1 patient (ID2), whereas an increase was seen in another (ID6). Follow-up imaging did not show relevant changes compared to the baseline assessment.
Discussion
Summary of Main Findings
In this study we investigated CSFP dynamics in patients with subacute and chronic SCI who previously underwent decompressive surgery. The assessment of CSFP dynamics was safe and feasible in all individuals with SCI. CSFPp was reduced in 3 patients compared to a spine-healthy cohort, indicative of restricted CSF circulation. Restricted CSF circulation aligned with abnormal CT-myelography findings, while patients with normal CSFP assessment had normal CT-myelography. The range of CSFP rise during Queckenstedt’s test was not different from spine-healthy controls. However, in 3 patients with otherwise normal CSFP dynamics, proxies for craniospinal elastance, as calculated from Queckenstedt’s test (RPPC-Q), were abnormal, pointing toward changes in the functional properties of the CSF compartment following SCI. Patients with cervical SCI commonly had reduced response to Valsalva maneuver, which was related to reduced abdominal and thoracic muscle force. These findings suggest that CSFP dynamics post-injury may be used to characterize CSF circulation and to assess the degree of residual cord compression. The advantage of CSFP assessment is its applicability as a bedside assessment tool.
Pathophysiology of Spinal Cord Compression and Altered CSFP Dynamics
CSF surrounds the brain and the spinal cord in vertebrates and has numerous functions in the central nervous system, for example, buoyancy and homeostasis. 21 The CSF space is a dynamic pressure system maintained by a finely regulated CSF secretion and absorption. 22 CSFP dynamics communicate between the cranial and the spinal CSF compartments under physiological conditions. 23 Regarding the main driver of CSFPp, there are 2 opposing theories mainly derived from animal models. One is that intracranial arterial pulsations are transmitted to the CSF compartment,24,25 and the other, that CSFPp partly or even mainly arise from spinal arterial pulsations.26-28 For instance, in dogs, it has been demonstrated that the removal of the choroid plexus is associated with a severe drop in CSFPp, 25 indicating that the choroid plexus was the chief site of arterial pulsation transfer to the CSF. In the presence of spinal canal narrowing the cranial and spinal compartments become separated, and CSFP dynamics measured at lumbar level dissociate from supra-stenotic pressure conditions. 15 Simultaneous cisternal and LP provided strong evidence in favor of the disconnected CSF compartments theory, since patients with spinal canal narrowing had an increase of cisternal CSFP, but a diminished response in lumbar CSFP.15,29 Notably, also pachy-arachnoiditis with adhesions and infectious cord swelling may result in disturbed CSFP dynamics. Except for patient ID3 and 4, none of the patients had clinical signs of inflammation of the arachnoid mater and subarachnoid space. Therefore, in patient ID3 and 4, infectious, in addition to mechanical compression may have contributed to disturbed CSFP dynamics. In addition to other abnormalities of the Queckenstedt’s test, there was a persistent CSFP rise in 2 patients after the release of the neck compression (Figures 3 and 5), that is not found in spine-healthy patients, previously called the valvular effect. 20 This might be another signature of the dissociation between the cerebral and lumbar compartment related to the stenosis. Alternatively, the altered vessel tone, for example, of the epidural veins, might contribute to this finding.
CSFP Dynamics in Acute and Chronic Spinal Canal Narrowing
CSFP dynamics were more significantly altered in acute SCI compared to findings from a study we conducted previously in patients with chronic-degenerative spinal canal narrowing. 16 Resting-state CSFP was not reduced in any patient with chronic cord compression, whereas in SCI it was reduced in 3 patients. The CSFPp and RPPC-Q were more severely reduced in SCI patients compared to chronic cord compression. Altogether, these findings are suggestive of pronounced CSFP abnormalities in SCI compared to more subtle findings in chronic cord compression. This is certainly related to the initial severity of the primary injury in SCI, which also leads to swelling of the spinal cord against the dura, which may in turn interrupt the CSF flow. 30 Furthermore, cord atrophy is present in chronic spinal canal narrowing, 31 which could be associated with partially restored CSF flow over time. Lastly, the dura remains intact in patients with degenerative spinal canal narrowing, which could explain that proxies for craniospinal elastance were not altered as severe as in acute SCI, where dura tearing and leakage may occur.
ISP and CSFP Monitoring in SCI
A recent intraoperative CSFP monitoring study found that CSFP and CSFPp increased following surgical decompression. 10 Our study expanded CSFP examination to the postoperative setting in SCI. Despite surgical decompression, CSFP dynamics were altered in several patients, thus suggesting that sufficient decompression was not achieved. This can be related to residual extradural bony compression or swelling of the cord against the dura. 30 Our findings of severely altered CSFP dynamics in some patients, despite surgical decompression, indicate that intraoperative methods may be helpful to determine effective decompression. To this end, intraoperative monitoring of CSFP and ISP, 2 distinct procedures, each measuring a different compartment and providing specific information, have been pioneered in spine surgery. ISP monitoring was specifically used to determine intradural pressure, spinal cord perfusion, and to monitor the effects of expansion duroplasty.11,32 Currently, the randomized controlled trial DISCUS (Duroplasty for Injured Cervical Spinal Cord with Uncontrolled Swelling, NCT04936620) investigates whether performing duroplasty improves outcomes after acute SCI. For the surgical management of patients with acute spinal cord compression, intrathecal pressure monitoring at the lumbar level could serve as an easily applicable screening and monitoring method that does not require an intervention at the injury site. However, a well-informed data interpretation based on sound understanding of dynamics within the CSF compartment is required to interpret CSFPp in patients and their potential in guiding the surgical procedures. 33 Further investigation of intrathecal postoperative CSFP dynamics in SCI is of interest to inform about the spatial conditions in the spinal canal at bedside. This may be particularly helpful in the post-surgery setting to inform if revision surgery is needed, as MRI is more difficult to perform (eg, mechanically ventilated patients) and to interpret (eg, following instrumentation). In addition to the clinical applications, studies that investigate the efficacy of intrathecal regenerative therapies may easily obtain CSFP dynamics during administration, which provide an additional surrogate marker for factors that may confound the treatment response. For example, patients with residual cord compression might be less responsive to treatment due to locally impaired perfusion. In addition to baseline evaluation, our findings indicate a potential use for follow-up examinations. Given that CSFP assessments enabled identification of patients with cord compression in this series, we consider the clinical application useful in selected cases, where other methods do not allow for an informed decision.
Advanced Queckenstedt’s Test Analysis
It was notable that the rise of CSFP during Queckenstedt’s test alone did not sufficiently capture pathological dynamics, as evident from patients with normal mean CSFP rise from baseline but abnormal CSFPp increase at the peak of CSFP response to the Queckenstedt’s test (Figures 4 and 5). Furthermore, CSFP abnormalities were not consistently present across all parameters (ie, normal findings during Queckenstedt’s test but not steady state or vice versa). We advocate a comprehensive CSFP assessment, including an advanced Queckenstedt’s test analysis with RPPC-Q and detection of valvular effect. Further research on RPPC-Q is needed in spinal cord disorders. In this cohort, the ranges were generally low compared to the values we previously obtained from patients without spinal cord compression using the same technical setup. RPPC evaluation is traditionally done with infusion testing to evaluate craniospinal compliance, 34 a metric for the ability of the craniospinal compartment to accommodate volume changes without substantial increase in CSFP. 35 Possibly, craniospinal compliance remains partially disturbed following spine injury and surgery, presumably due to dysfunctional biomechanics of the CSF compartment.
CSFP Response During Valsalva Maneuver in SCI
Due to multiple physiological mechanisms involved, the Valsalva maneuver does not inform about the degree of spinal cord compression. The Valsalva maneuver requires expiratory muscle contraction and diaphragm elevation, 36 which results in intrathoracic pressure increase. This rise, in turn, is transmitted to the venous system (ultimately translating into impeded venous return into the thorax) and subsequently to the CSF spaces, since CSFP depends, among other factors, on the venous pressure in the dural sinuses. 37 An abnormal CSFP response during Valsalva maneuver was present in several SCI patients. Firstly, this is related to the reduced ability to contract abdominal muscles in traumatic tetraplegics, 38 which are required to successfully execute the Valsalva maneuver. 39 Moreover, diaphragm and respiratory muscles contribute to the Valsalva maneuver. Previous studies have indeed shown that mainly patients with high-cervical motor-complete SCI are also less capable to sufficiently perform the Valsalva maneuver. 40 Therefore, our findings support the concept that CSFP response during Queckenstedt’s test is due to a relative intracranial volume change, whereas the Valsalva maneuver is mainly related to intrathoracic and intraabdominal pressure changes, associated with an increased intracranial venous filling, but also with a reverse flow to the spinal venous plexus.
Strengths and Limitations
This is the first study investigating bedside CSFP dynamics in SCI following surgery. All patients received a standardized clinical examination from spinal cord specialists and a complete neuroimaging workup. Data from spine-healthy patients was available for comparison, and the measurement system was tested previously to allow for inter-trial comparability. Despite these strengths, some limitations need to be mentioned. First, this was a small sample group to explore the potential applicability of CSFP assessments. However, it appeared that CSFP patterns were informative on a single subject level. Second, the execution of the Valsalva maneuver was pragmatic, and respiratory monitoring (eg, airway pressure) was not done. With the same pragmatic approach, we previously obtained a much larger CSFP response to Valsalva in all spine-healthy patients. Importantly, future investigations should further evaluate metrics for reproducibility and diagnostic accuracy. For this purpose, larger cohorts that compare CSFP dynamics to CT-myelography might be highly valuable. Lastly, potential confounders to CSFP dynamics such as arterial blood pressure, BMI, or age were not systematically analyzed, owed to the case-based setup. This approach was preferred over a group-based approach to account for individual patient characteristics. We do not consider the age difference between the individuals with SCI and spine-healthy cohort to account for the findings here, because abnormal CSFP dynamics were observed across the whole age span.
Conclusions
This was the first study to investigate the post-surgical signatures of CSFP dynamics in SCI. Bedside measurements of intrathecal CSFP dynamics allowed to identify cases with disturbed dynamics and altered craniospinal elastance. This study provides the proof-of-concept that CSFP dynamics can determine suspected residual cord compression associated with restricted CSF circulation. In patients with diagnostic uncertainty, further investigation of CSFP dynamics as a complementary examination tool may help personalized clinical decision-making. Clinical trials that involve intrathecal drug administration in patients with acute or chronic cord compression should consider inclusion of CSFP assessments to identify patients with residual compression, and thereby specify individual profiles of treatment responders.
Supplemental Material
Supplemental material, sj-png-1-nnr-10.1177_15459683231159662 for Cerebrospinal Fluid Pressure Dynamics as a Bedside Test in Traumatic Spinal Cord Injury to Assess Surgical Spinal Cord Decompression: Safety, Feasibility, and Proof-of-Concept by Najmeh Kheram, Andrea Boraschi, Nikolai Pfender, Susanne Friedl, Maria Rasenack, Benjamin Fritz, Vartan Kurtcuoglu, Martin Schubert, Armin Curt and Carl M. Zipser in Neurorehabilitation and Neural Repair
Supplemental material, sj-png-2-nnr-10.1177_15459683231159662 for Cerebrospinal Fluid Pressure Dynamics as a Bedside Test in Traumatic Spinal Cord Injury to Assess Surgical Spinal Cord Decompression: Safety, Feasibility, and Proof-of-Concept by Najmeh Kheram, Andrea Boraschi, Nikolai Pfender, Susanne Friedl, Maria Rasenack, Benjamin Fritz, Vartan Kurtcuoglu, Martin Schubert, Armin Curt and Carl M. Zipser in Neurorehabilitation and Neural Repair
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: CZ, AC, and MS report a grant from the Swiss Paraplegia Foundation (FoKo_2019_01). CZ reports a grant from IRP - International Foundation for Research in Paraplegia (P190). VK reports a grant from the Swiss National Science Foundation (Project No. 182683).
ORCID iD: Carl M. Zipser  https://orcid.org/0000-0002-4396-4796
https://orcid.org/0000-0002-4396-4796
Supplementary material for this article is available on the Neurorehabilitation & Neural Repair website along with the online version of this article.
References
- 1.Ahuja CS, Wilson JR, Nori S, et al. Traumatic spinal cord injury. Nat Rev Dis Primers. 2017;3:1-21. doi: 10.1038/nrdp.2017.18 [DOI] [PubMed] [Google Scholar]
- 2.Alizadeh A, Dyck SM, Karimi-Abdolrezaee S. Traumatic spinal cord injury: an overview of pathophysiology, models and acute injury mechanisms. Front Neurol. 2019;10:1-25. doi: 10.3389/fneur.2019.00282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badhiwala JH, Wilson JR, Witiw CD, et al. The influence of timing of surgical decompression for acute spinal cord injury: a pooled analysis of individual patient data. Lancet Neurol. 2021;20(2):117-126. doi: 10.1016/S1474-4422(20)30406-3 [DOI] [PubMed] [Google Scholar]
- 4.Zipser CM, Curt A. Rehabilitation of acute spinal cord injury. In: Richard Winn H, ed. Youmans and Winn Neurological Surgery. 8th ed.Elsevier Inc.; 2022. doi: 10.1016/B978-0-323-66192-8.00345-1 [DOI] [Google Scholar]
- 5.Zipser CM, Cragg JJ, Guest JD, et al. Cell-based and stem-cell-based treatments for spinal cord injury: evidence from clinical trials. Lancet Neurol. 2022;21(7):659-670. doi: 10.1016/s1474-4422(21)00464-6 [DOI] [PubMed] [Google Scholar]
- 6.Willison AG, Smith S, Davies BM, Kotter MRN, Barnett SC. A scoping review of trials for cell-based therapies in human spinal cord injury. Spinal Cord. 2020;58(8):844-856. doi: 10.1038/s41393-020-0455-1 [DOI] [PubMed] [Google Scholar]
- 7.Knop C, Bastian L, Lange U, Oeser M, Zdichavsky M, Blauth M. Complications in surgical treatment of thoracolumbar injuries. Eur Spine J. 2002;11(3):214-226. doi: 10.1007/s00586-001-0382-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liebscher T, Ludwig J, Lübstorf T, et al. Cervical spine injuries with acute traumatic spinal cord injury: spinal surgery adverse events and their association with neurological and functional outcome. Spine. 2022;47(1):E16-E26. doi: 10.1097/BRS.0000000000004124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aarabi B, Olexa J, Chryssikos T, et al. Extent of spinal cord decompression in motor complete (American Spinal Injury Association Impairment Scale grades A and B) traumatic spinal cord injury patients: post-operative magnetic resonance imaging analysis of standard operative approaches. J Neurotrauma. 2019;36(6):862-876. doi: 10.1089/neu.2018.5834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwon BK, Curt AN, Belanger LM, et al. Intrathecal pressure monitoring and cerebrospinal fluid drainage in acute spinal cord injury: a prospective randomized trial. J Neurosurg Spine. 2009;10(3):181–193. doi: 10.3171/2008.10.SPINE08217 [DOI] [PubMed] [Google Scholar]
- 11.Werndle MC, Saadoun S, Phang I, et al. Monitoring of spinal cord perfusion pressure in acute spinal cord injury: initial findings of the injured spinal cord pressure Evaluation Study*. Crit Care Med. 2014;42(3):646-655. doi: 10.1097/CCM.0000000000000028 [DOI] [PubMed] [Google Scholar]
- 12.Chen S, Smielewski P, Czosnyka M, Papadopoulos MC, Saadoun S. Continuous monitoring and visualization of optimum spinal cord perfusion pressure in patients with acute cord injury. J Neurotrauma. 2017;34(21):2941-2949. doi: 10.1089/neu.2017.4982 [DOI] [PubMed] [Google Scholar]
- 13.Magnaes B, Tormod H. Surgery for myelopathy in cervical spondylosis: safety measures and preoperative factors related to outcome. Spine. 1980;5(3):211-214. [DOI] [PubMed] [Google Scholar]
- 14.Pearce JMS. Queckenstedt’s manoeuvre. J Neurol Neurosurg Psychiatry. 2006;77(6):728. doi: 10.1136/jnnp.2005.083618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ayer JB. Spinal subarachnoid block as determined by combined cistern and lumbar puncture: with special reference to the early diagnosis of cord tumor. Arch NeurPsych. 1922;7(1):38–52. [Google Scholar]
- 16.Kheram N, Pfender N, Boraschi A, et al. Cerebrospinal fluid pressure dynamics reveal signs of effective spinal canal narrowing in ambiguous spine conditions. Front Neurol. 2022;13: 951018. doi: 10.3389/fneur.2022.951018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zipser CM, Pfender N, Spirig JM, et al. Study protocol for an observational study of cerebrospinal fluid pressure in patients with degenerative cervical myelopathy undergoing surgical deCOMPression of the spinal CORD: the COMP-CORD study. BMJ Open. 2020;10(9):e037332. doi: 10.1136/bmjopen-2020-037332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirshblum S, Snider B, Rupp R, Read MS. Updates of the international standards for neurologic classification of spinal cord injury: 2015 and 2019. Phys Med Rehabil Clin N Am. 2020;31(3):319-330. doi: 10.1016/j.pmr.2020.03.005 [DOI] [PubMed] [Google Scholar]
- 19.Hartley KG, Damon BM, Patterson GT, Long JH, Holt GE. MRI techniques: a review and update for the orthopaedic surgeon. J Am Acad Orthop Surg. 2012;20(12):775-787. doi: 10.5435/JAAOS-20-12-775 [DOI] [PubMed] [Google Scholar]
- 20.Antoni N. Pressure curves from the cerebrospinal fluid. Acta Med Scand. 1946;123(170 S):439-462. doi: 10.1111/j.0954-6820.1946.tb19257.x [DOI] [Google Scholar]
- 21.Spector R, Robert Snodgrass S, Johanson CE. A balanced view of the cerebrospinal fluid composition and functions: Focus on adult humans. Exp Neurol. 2015;273:57-68. doi: 10.1016/j.expneurol.2015.07.027 [DOI] [PubMed] [Google Scholar]
- 22.Sakka L, Coll G, Chazal J. Anatomy and physiology of cerebrospinal fluid. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128(6):309-316. doi: 10.1016/j.anorl.2011.03.002 [DOI] [PubMed] [Google Scholar]
- 23.Williams B.Simultaneous cerebral and spinal fluid pressure recordings - I. Technique, physiology, and normal results. Acta Neurochir. 1981;58(3-4):167-185. doi: 10.1007/BF01407124 [DOI] [PubMed] [Google Scholar]
- 24.Chopp M, Portnoy HD. Systems analysis of intracranial pressure. Comparison with volume-pressure test and CSF-pulse amplitude analysis. J Neurosurg. 1980;53(4):516-527. doi: 10.3171/jns.1980.53.4.0516 [DOI] [PubMed] [Google Scholar]
- 25.Bering EA. Choroid plexus and arterial pulsation of CSF. AMA Arch Neurol Psychiatry. 1955;73(2):165-172. [DOI] [PubMed] [Google Scholar]
- 26.Urayama K. Origin of lumbar cerebrospinal fluid pulse wave. Spine. 1994;19(4):441-445. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura K, Urayama K, Hoshino Y. Lumbar cerebrospinal fluid pulse wave rising from pulsations of both the spinal cord and the brain in humans. Spinal Cord. 1997;35(11):735-739. doi: 10.1038/sj.sc.3100548 [DOI] [PubMed] [Google Scholar]
- 28.Dunbar HS, Guthrie TC, Karpell B. A study of the cerebrospinal fluid pulse wave. Arch Neurol. 1966;14(6):624-630. [DOI] [PubMed] [Google Scholar]
- 29.Ayer JB. Puncture of the cisterna magna. Arch Neurol Psychiatry. 1920;4(5):529-541. [Google Scholar]
- 30.Saadoun S, Werndle MC, Lopez de, Heredia L, Papadopoulos MC. The dura causes spinal cord compression after spinal cord injury. Br J Neurosurg. 2016;30(5):582-584. doi: 10.3109/02688697.2016.1173191 [DOI] [PubMed] [Google Scholar]
- 31.Seif M, David G, Huber E, Vallotton K, Curt A, Freund P. Cervical cord neurodegeneration in traumatic and non-traumatic spinal cord injury. J Neurotrauma. 2020;37(6):860-867. doi: 10.1089/neu.2019.6694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phang I, Werndle MC, Saadoun S, et al. Expansion duroplasty improves intraspinal pressure, spinal cord perfusion pressure, and vascular pressure reactivity index in patients with traumatic spinal cord injury: Injured spinal cord pressure evaluation study. J Neurotrauma. 2015;32(12):865-874. doi: 10.1089/neu.2014.3668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hogg FRA, Gallagher MJ, Kearney S, Zoumprouli A, Papadopoulos MC, Saadoun S. Acute spinal cord injury: monitoring lumbar cerebrospinal fluid provides limited information about the injury site. J Neurotrauma. 2020;37(9):1156-1164. doi: 10.1089/neu.2019.6789 [DOI] [PubMed] [Google Scholar]
- 34.Malm J, Sundström N, Cesarini KG, et al. Implementation of a new CSF dynamic device: A multicenter feasibility study in 562 patients. Acta Neurol Scand. 2012;125(3):199-205. doi: 10.1111/j.1600-0404.2011.01533.x [DOI] [PubMed] [Google Scholar]
- 35.Marmarou A, Shulman K, LaMorgese J. Compartmental analysis of compliance and outflow resistance of the cerebrospinal fluid system. J Neurosurg. 1975;43(5):523-534. doi: 10.3171/jns.1975.43.5.0523 [DOI] [PubMed] [Google Scholar]
- 36.Talasz H, Kremser C, Kofler M, Kalchschmid E, Lechleitner M, Rudisch A. Proof of concept: Differential effects of Valsalva and straining maneuvers on the pelvic floor. Eur J Obstet Gynecol Reprod Biol. 2012;164(2):227-233. doi: 10.1016/j.ejogrb.2012.06.019 [DOI] [PubMed] [Google Scholar]
- 37.Davson H, Domer FR, Holungsworth JR. The mechanism of drainage of the cerebrospinal fluid. Brain. 1973;96(2):329-336. [DOI] [PubMed] [Google Scholar]
- 38.Estenne M, Pinet C, De Troyer A. Abdominal muscle strength in patients with tetraplegia. Am J Respir Crit Care Med. 2000;161(3 I):707-712. doi: 10.1164/ajrccm.161.3.9906020 [DOI] [PubMed] [Google Scholar]
- 39.Hackett DA, Chow CM. The Valsalva maneuver: its effect on intra-abdominal pressure and safety issues during resistance exercise. J Strength Cond Res. 2013;27(8):2338-2345. [DOI] [PubMed] [Google Scholar]
- 40.Legg Ditterline BE, Aslan SC, Randall DC, Harkema SJ, Ovechkin AV. Baroreceptor reflex during forced expiratory maneuvers in individuals with chronic spinal cord injury. Physiol Behav. 2016;229:65-70. doi: 10.1016/j.resp.2016.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-png-1-nnr-10.1177_15459683231159662 for Cerebrospinal Fluid Pressure Dynamics as a Bedside Test in Traumatic Spinal Cord Injury to Assess Surgical Spinal Cord Decompression: Safety, Feasibility, and Proof-of-Concept by Najmeh Kheram, Andrea Boraschi, Nikolai Pfender, Susanne Friedl, Maria Rasenack, Benjamin Fritz, Vartan Kurtcuoglu, Martin Schubert, Armin Curt and Carl M. Zipser in Neurorehabilitation and Neural Repair
Supplemental material, sj-png-2-nnr-10.1177_15459683231159662 for Cerebrospinal Fluid Pressure Dynamics as a Bedside Test in Traumatic Spinal Cord Injury to Assess Surgical Spinal Cord Decompression: Safety, Feasibility, and Proof-of-Concept by Najmeh Kheram, Andrea Boraschi, Nikolai Pfender, Susanne Friedl, Maria Rasenack, Benjamin Fritz, Vartan Kurtcuoglu, Martin Schubert, Armin Curt and Carl M. Zipser in Neurorehabilitation and Neural Repair