Abstract
Psychopathology emerges from the dynamic interplay of physiological and mental processes and ecological context. It can be seen as a failure of recursive, homeostatic processes to achieve adaptive re-equilibrium. This general statement can be actualized with consideration of polygenic liability, early exposures, and multi-unit (multi-“level”) analysis of the psychological action and the associated physiological and neural operations, all in the context of the developmental exposome. This essay begins by identifying key principles and clarifying key terms necessary to mental disorder theory. It then ventures a sketch of a model that highlights epigenetic dynamics and proposes a common pathways hypothesis toward psychopathology. An epigenetic perspective elevates the importance of developmental context and adaptive systems, particularly in early life, while opening the door to new mechanistic discovery. The key proposal is that a finite number of homeostatic biological and psychological mechanisms are shared across most risky environments (and possibly many genetic liabilities) for psychopathology. Perturbation of these mediating mechanisms leads to development of psychopathology. A focus on dynamic changes in these homeostatic mechanisms across multiple units of analysis and time points can render the problem of explaining psychopathology tractable. Key questions include the mapping of recursive processes over time, at adequate density, as mental disorders unfold across development.
Keywords: epigenetics, theory, common pathways, developmental psychopathology
General Scientific Summary.
Mental disorders develop in the midst of complex processes. A focus on causal mechanism can benefit from a focus on regulatory processes and on common pathways of effect across multiple risks and conditions.
Static models of psychopathology are as a set of fixed entities are outdated. Instead, psychopathology must be understood dynamically. In that context, an epigenetic lens can open powerful avenues toward mechanistic discovery. That lens also harmonizes with the developmental reality. The onset of psychopathology disproportionately targets developing children, adolescents, and young adults (Homberg et al., 2016; Kessler et al., 2005). In turn, the dynamic, asynchronous process of human development is exquisitely sensitive to ecological context. Thus, like development itself, although some elements are static/stable, the entire syndrome is not; psychopathology emerges from dynamic cybernetic and transactional processes (Bronfenbrenner & Ceci, 1994; Cicchetti, 1984, 2016; Cicchetti & Natsuaki, 2014; Cicchetti & Toth, 2009; Hyman, 2018; Kendler, 2008; Miller, 1996; Thomas & Sharp, 2019).
In Part I, I set the stage for theory. The first subsection, “The Phenomena” provides a crucial context not only for this but for any theory attempt in psychopathology, addressing the realities that characterize the phenomena of interest: its complexity, development-in-context, genotype-environment interplay, the importance of the exposome, and the non-ignorable element of individual construal of experience. My intent is to orient the reader to the conceptual layers necessary to any theory of psychopathology. In the second subsection, “Conceptual Clarifications,” I define key multivalent concepts for present purposes. The third and final subsection of Part I, “Etiology and Epigenetics” provides context for the epigenetics element.
In Part II, I provide a theoretical sketch or schema of an epigenetic-centered model, then introduce and explain a multi-level, common pathways hypothesis that can render the complexity of the problem tractable.1 I include example hypotheses and highlight epigenetic process, self-regulation, and the importance of integrating different units of analysis.2
Part I: Setting the Conceptual Context
The Phenomena
The problem is compelling: mental health disorders, addiction, and neurodevelopmental conditions collectively constitute the largest single source of health burden in the world in terms of years of life lost to disability (Whiteford et al., 2013). Any theory of mental disorder must account for the multiple known features of the phenomenon: complexity, development, environmental/social context, and self-regulating mental evaluations of experience.
Complexity
First, the conditions described in the DSM are not discrete diseases with discrete causes but are partially related, both phenotypically and biologically (Anttila et al., 2018; Borsboom, Cramer, Schmittmann, Epskamp, & Waldorp, 2011; Cross-Disorder Group of the Psychiatric Genomics Consortium, 2019; de Jonge et al., 2018; Kotov et al., 2017; Plana-Ripoll et al., 2019; Radonjić et al., 2021). At least for descriptive purposes, they may be organized hierarchically (Achenbach, 2020; Kotov et al., 2017; Kotov et al., 2021), or alternatively, in a small-world network perspective (Borsboom et al., 2011). However, beyond stipulating the phenotypic and biological relatedness of the many conditions, my argument does not depend on a particular nosological architecture.
Yet, second, heterogeneity is also the rule. Even within putative disorders, etiological and phenotypic variability require characterization, to maximize mechanism discovery and clinical value. Indeed, as required by polygenic theory (Wray, Wijmenga, Sullivan, Yang, & Visscher, 2018), even specific genetic etiology is heterogeneous, despite the partial genetic overlaps among conditions. Likewise, the clinical phenomena are protean: for any syndrome or dimension, features and expression can vary noticeably by age or social or incentive context.
Third, much psychopathology is on a continuum with normal function (Burns, Becker, Geiser, Leopold, & Willcutt, 2019; Haslam, Holland, & Kuppens, 2012; Hyman, 2010; Shankman et al., 2009; Waszczuk et al., 2020). However, properly describing its dimensional (or perhaps nonlinear) structure remains a methodological challenge (Borsboom et al., 2016). Further, the dimension model is incomplete, because some psychopathology likely reflects non-continuous causality, such as early neural injury or rare gene mutation of strong effect.
Fourth, aside from such rare structural DNA variants or major teratogenic exposures, biological signals are weak, with very small associations of genes or neural signatures with clinical conditions. Although mapping at the group level of biological and neural correlates remains important, and may be aided decisively by phenotype refinement (Nigg, Karalunas, Feczko, & Fair, 2020) so as to support clinical care, the long search for definitive biomarkers of individual cases (or disorders) appears ill conceived for the vast majority (Harrington, 2019; Kapur, Phillips, & Insel, 2012; Miller, 1996). No isomorphism exists between the main population of psychopathology phenotypes and biology. This is not to devalue the role of the biological lens (see below), but to highlight the necessity of a multi-unit perspective (Cicchetti, 2016).3
Development in Relation to Expectable and Expected Environment
Liability may be present before or at birth (Gustafsson et al., 2020; Neumann et al., 2020), but psychopathology itself is not—it emerges in stages, with recognizable homo- and heterotypic continuities. Some conditions emerge early in development (ADHD, ASD, ODD, learning disorders) and are seen as “neurodevelopmental”. Others have peak onset from the pubertal transition through the adolescent-young adult transition (mood disorders, psychoses, addictions, eating disorders) (Martel, 2013)--although they may also have early neurodevelopmental roots (Corsi-Zuelli & Deakin, 2021; Eyles, 2021; Kloiber et al., 2020; Mansur, Lee, McIntyre, & Brietzke, 2020; Schmidt & Mirnics, 2015). Intervention and prevention differ across developmental periods (preschool, adolescent transition, early adult transition (e.g. Nigg, Sibley, Thapar, & Karalunas, 2020)).
Development proceeds in the context of a species-wide expectable environment, or range of environments, within which normative programmed processes unfold. For example, humans “expect” speech from which they can learn language; they “expect” certain nutrients and are ready to utilize them. They do not expect extreme emotional neglect, or measurable levels of lead and organophosphate pesticides. The contemporary environment includes rare as well as common non-expectable exposures (see the Exposome, later).
Related to this, developmental origins theory posits that the fetus responds dynamically to physiological signals from the mother, which themselves are indices of her environment. The fetal physiological and epigenetic response constitutes re-programming of development in anticipation of a refined expected post-natal environment. In this view, maladjustment occurs not only when the environment is outside of the species-general expectable environment, but also when the specific attempted predication and adaptation from the actual maternal cues is mismatched with the actual postnatal environment. For example, a baby “expecting” a violent environment (e.g., due to material emotional trauma) develops with enhanced impulsivity and aggressiveness but is born into a relatively stable and safe setting (e.g., the mother has relocated to a better setting or the child is adopted) for which those traits are poorly suited.4 Research on these early programming origins of psychopathology remains nascent, but promising data are emerging (Lester et al., 2018; Monk, Lugo-Candelas, & Trumpff, 2019; Morgan et al., 2020; Ostlund et al., 2019). Developmental origins theory is amenable to a dynamic systems perspective (Kaliush et al., 2020) and to epigenetics (Conradt et al., 2018).
Critical developmental unknowns include (a) timing of exposure, (b) when and whether reprogramming changes risk versus responsivity to environment (Belsky & Pluess, 2009; Belsky et al., 2019), and (c) when and how the post-natal environment, in particular the infant-caregiver relationship in early life, moderates prenatal programming.
Genotype-Environment Interplay
That genotype is important in psychopathology is beyond dispute--but it generally confers liability, not psychopathology per se. While recognizing the role of rare gene variants of large effect in some cases, the field’s focus is now on polygenic liability involving many genes of small effect, and on the form of genotype-environment interplay.
Genotype-environment interaction
Genotype-environment interaction (GxE) can mean a specific physiological interplay of genotype and environment (e.g., genotypic regulation of how rapidly nutrients or toxicants are metabolized). In the polygenic context it means statistical dependency rather than biological process. In the decomposition of twin data, heritability can be inflated by GxE involving shared environments (Purcell, 2002). These family-wide shared effects may well be widespread exposures. Thus, to the extent GxE is involved, the typically robust heritability of major psychiatric conditions elevates the importance of common exposures, especially those that are outside the evolutionary expectable range. Important examples in contemporary societies known to be relevant to psychopathology include ubiquitous chemical toxicants and over- and under-nutrition, as documented in Table S-1. Necessary are contextually-informed rather than context-free biomarker, mechanism, and genetic studies. When GxE is mechanistic, one mechanism is epigenetic, that is regulation of gene expression (see “Epigenetics”, below).
Genotype-environment correlation
Genotype-environment correlation (rGE) has two meanings. One is that environment can mediate genetic effects (called genetic nurturance) (Armstrong-Carter et al., 2020; Kong et al., 2018). For example, postnatal caregiving can transmit genetic effects even when alleles are not passed on (Armstrong-Carter et al., 2020; Kong et al., 2018), potentially reversing or amplifying genetic liability (Garg et al., 2018; Ribas-Fitó et al., 2003). In the offspring this is a purely environmental effect. The second meaning occurs when the environment is correlated with the transmitted alleles, i.e., confounded with genotype. This correlation can be passive (parents pass on genes for high IQ and also read more to their child), evocative (child evokes certain response related to child personality), or active (child chooses certain activities, influenced by personality, itself genetically influenced). These effects may be more notable for proximal (e.g., caregiving) than distal (e.g., air pollution) exposures. Crucially, the unknown frequency, timing, and magnitude of rGE effects in psychopathology leaves unresolved the mechanism behind many genetic and exposure findings as well as some GxE findings.
Exposome
The preceding highlights the importance of the exposome—the diversity of exposures to “synthetic chemicals, dietary constituents, psychosocial stressors, and physical factors” (Vermeulen, Schymanski, Barabási, & Miller, 2020; Wild, 2005) that influence human development. Examples of developmental variation in response to both social context (e.g., rearing environment) and genotype/biology are legion (Fenesy, Teh, & Lee, 2019; Gottlieb, 2007; Henry et al., 2018; Hinshaw & Arnold, 2015; Meaney, 2010; O’Donnell & Meaney, 2020; Trentacosta et al., 2019; Tung & Lee, 2017). The importance of the exposome is underscored by the burgeoning literatures on social determinants of health (Alegría, NeMoyer, Falgàs Bagué, Wang, & Alvarez, 2018) and on cultural variation in development (Causadias & Cicchetti, 2018). To realize the potential in the industrious mapping of the genome and the neural connectome, then, the field needs a proper account of the exposome (Rager et al., 2020; Vermeulen et al., 2020). Again, see Table S-1 for selected examples.
Efforts to establish a multi-unit developmental account of the exposome for psychological development are foundational (Bronfenbrenner, 1977, 1979; Bronfenbrenner & Ceci, 1994; Fearon, 2018; Wachs, 1999). The work of Bronfenbrenner (1979) and its subsequent elaborations laid a crucial framework. Marking progress in the field, Wachs, Georgieff, Cusick, and McEwen (2014) provided a detailed overview of the importance of early environment, allostatic load, and different sensitive periods for development (e.g., early life may be salient for neural development, longer periods salient for psychosocial and cognitive development). They also gave examples of early biological (e.g., nutrients) and psychosocial influences. In the past decade, fruits of this line of work have enabled developmental scientists to document many early exposures and interventions that impair or facilitate development, and to guide prevention interventions (Britto et al., 2017; Britto & Pérez-Escamilla, 2013; Wachs et al., 2014). That progress dovetails with more recent work across a range of health outcomes applying newer computational tools (e.g., network analysis) to organize understanding of the exposome and associated, overlapping physiological responses (Vermeulen et al., 2020).
Yet the challenges are daunting. Whereas some exposures are causal, for most substantial work remains to evaluate causal effects versus those that may be explained by genotype-environment correlation or other confound (Nigg, Elmore, Natarajan, Friderici, & Nikolas, 2016; Riglin et al., 2020; Thapar & Rice, 2020). Further, environmental continually moderates development (e.g., school environment, neighborhoods, peer relations, and parenting style) (Wachs et al., 2014). Here too, the degree of causal influence remains poorly described. Finally, effect sizes and success rates for prevention interventions remain unsatisfying. Additional theoretical elaboration may open the door to superior mechanistic intervention and prevention, motivating both this essay and others in this issue.
The field thus faces the extraordinary complexity of environmental inputs and their dynamic interplay with genotype and one another over time. High-level taxonomies of exposure (Wachs, 1999) are necessary, yet bely the deep granularity that a full accounting would require. For example, potentially hundreds of neuro-active chemicals are in commercial use and found in children’s environments (Grandjean & Landrigan, 2014); only a handful have undergone serious scientific study for effects on child brain development. Studying all of them and interactions among them and with genetic risk, diet, stress, and other factors is prohibitive. That foreshadows my subsequent emphasis on hypothesized common mechanistic pathways.
Mental Appraisal and Evaluation of Experience
It is not just the “objective” exposome that matters. How people interpret and evaluate their situation is also important. News headlines alone testify to the central role in psychopathology of existential issues (meaning, value) and mental evaluations. For example, in some demographic groups in the United States, life expectancy has declined due to opioid addiction, suicide, and poor health. These early deaths have been referred to as “diseases and deaths of despair” (Case & Deaton, 2020). Despair is existential. In another example, excessive expectations on children to achieve have been suggested as one factor in rising rates of ADHD identification in western societies (Scheffler & Hinshaw, 2014).
Mental processes like appraisal or evaluation, both automatized and deliberate, play an important role in depression (Hammen, 2018), anxiety (LeDoux & Pine, 2016; Newman, Llera, Erickson, Przeworski, & Castonguay, 2013), aggression (Allen, Anderson, & Bushman, 2018; Benjamin, Kepes, & Bushman, 2018), and drug use and addiction (Neighbors, Tomkins, Lembo Riggs, Angosta, & Weinstein, 2019; Verdejo-Garcia, Garcia-Fernandez, & Dom, 2019). Likewise, mental construal is associated with successful treatment or recovery from depression or anxiety, as well as associated remodeling of brain function or even structure (Lissemore et al., 2018; Matsuda, Makinodan, Morimoto, & Kishimoto, 2019; Porto et al., 2009; Yoshino et al., 2018). The power of placebo or social bonds to enhance health is well-known (Ysseldyk, McQuaid, McInnis, Anisman, & Matheson, 2018).
In short, people ascribe meaning to their experience using narrative, symbol, schema, appraisal or evaluation, and goal-referents. Doing so is part of self-regulation (below). Such appraisals support adaptive stability, adjusting cognition, emotion and behavior in ways that (when successful) improve resilience (overcoming challenge) and overall adaptation (Yao & Hsieh, 2019); (also see Table S-2). Any account of mental disorder must address psychological self-regulation including mental evaluations (García-Mieres, Niño-Robles, Ochoa, & Feixas, 2019; Izard, 1993; Rachman, 2016; Salsman et al., 2020; Trope & Liberman, 2003).
Admittedly and crucially, the mental level is also idiographic and subjective. It stretches from cognitive evaluations (emphasized herein) to the human capacity for self-transcendence (art, music, religious ritual, symbol, experience) and the unassailable authority of subjective knowledge (knowing how I feel, who I love). The subjective experience is not directly accessible to scientific observation. Yet it cannot be simply dispensed with or theorized away. Rather, the interior aspect of mental disorder requires consideration in a scientific model to the extent possible. Its inclusion need not prevent a tractable theory (for discussion, see Schaffner, 2020).
Summary
A theory of mental illness has to address four domains: (a) systems biology (pathophysiology via neuroscience, genetics, and physiology), (b) the exposome, including the proximal social-relational environment, nutrition, and wider social and toxicant exposures, (c) developmental timing and process and behavioral analysis (early origins and programming, behavioral learning history, developmental trajectory), and (d) mental/psychological processes (attributions, evaluations, beliefs, meanings, and subjective phenomenology; herein I emphasize evaluations). I next clarify other key concepts before offering a theory sketch. To help navigate the next section, Table S-2 provides a glossary of relevant, multivalent terms as I use them.
Conceptual Clarifications and Elements
Dynamic system
Full articulation of a specific dynamic systems model is beyond the scope of this paper (see Kalisch et al., 2019; Nelson, McGorry, Wichers, Wigman, & Hartmann, 2017; Tay & Lim, 2020). Figure 1 points to a simplified model to describe, at different units of analysis. an epigenetic, common pathways framework of psychopathology. Dynamic system models are familiar in systems biology, neuroscience, clinical psychology, and developmental psychopathology (Cicchetti, 1984; Granic, Hollenstein, & Lichtwarck-Aschoff, 2016; Mascolo, van Geert, Steenbeek, & Fischer, 2016). Key axioms, drawing from developmental psychopathology and Craver’s (2005, 2007) neuroscience-based view, include:
Lower (smaller) units constrain but do not explain higher (larger or more complex) levels or units of analysis.
Emergent properties are unique to the system as a whole and not reducible to its elements (see Table S-2, for notation on issues with the idea of emergence). Thus, mental activity and behavior and their dysfunctions are not static elements of a person, but emerge from internal processes interacting with one another and with the functional context (Miller & Bartholomew, 2020). This view rejects eliminative reductionism (Table S-2), which posits that smaller units of analysis will explain, and thus render superfluous, larger units. Craver (2005, 2007) points out that whereas eliminative reductionism (not his term) was highly productive in physics, chemistry, and molecular biology, it fails with dynamic systems (such as brain functioning or complex human behavior). At the same time, as noted in point “a”, smaller units do still constrain the possibilities for larger units.
Systems phenomena cannot be fully explained without including their own level of analysis (for psychopathology, that level is mental or psychological activity).
The function of multi-level system components (whether ideations, emotions, behaviors, neurons or genes) can be different in, and thus cannot be understood apart from, a particular functional context (Bertolaso & Ratti, 2018). The system is non-determinative, as well, due to participation of stochastic processes (e.g., in neurodevelopment) (White, 2019). In biology, “Cell to cell variability is an inherent and emergent property of populations of cells…no two genetically identical cells behave and look identical” (Xavier da Silveira dos Santos & Liberali, 2019). In relation to psychopathology, see (Miller, 1996; Miller & Bartholomew, 2020).
Mutual regulation occurs among system components (component co-action). For example, top-down and bottom up processes mutually regulate one another.
Thus, mechanism here means causal explanation (Nicholson, 2012; Thomas & Sharp, 2019), not a machine-like linear process (See Table S-2).
Nonlinear change that includes phase transitions (organizational state changes) is incorporated across development.
The system is self-organizing, and develops from lesser to greater complexity through cybernetic processes (feedback loops). The cybernetic principle is central to the present proposal. I highlight the importance of recursive adaptive processes as the system seeks to maintain homeostasis (equilibrium), thus providing both a ground of stability and canalization as well as dynamic development and change.
Figure 1: Schematic for a mechanistic-cybernetic model of psychopathology.
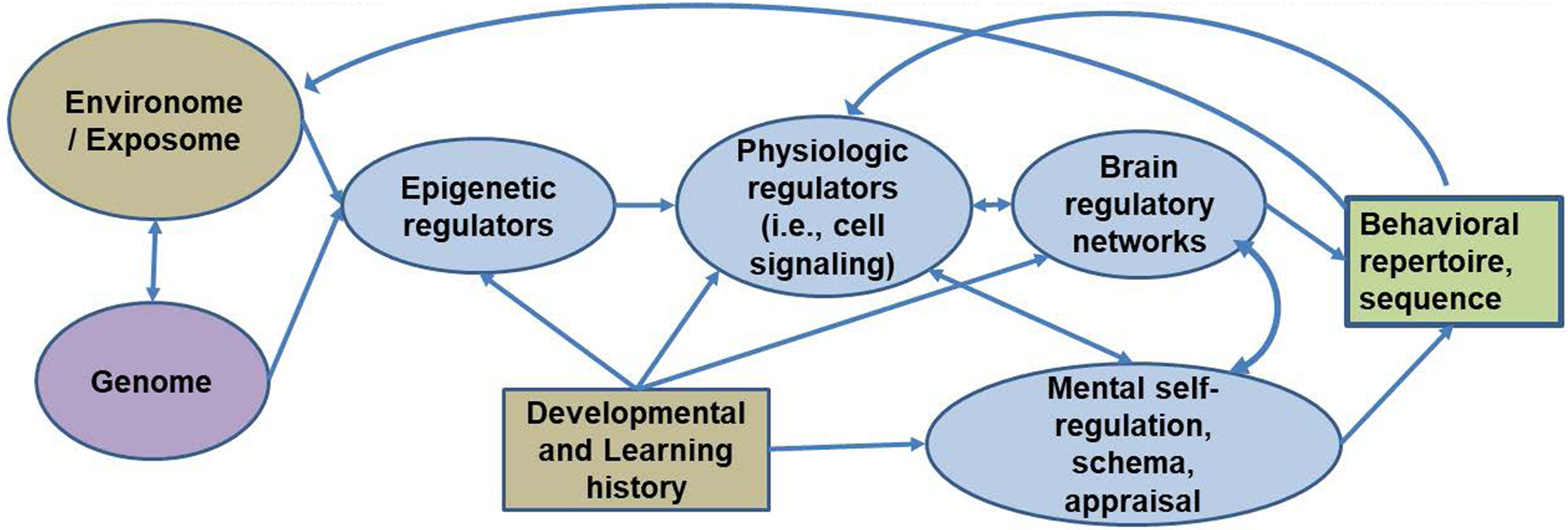
Each domain shown can be decomposed into component parts and relations (e.g., interacting elements of exposome, physiology, neural organization, or psychological self-regulation). The homeostatic system fails to adequately adapt to inputs from one or more of the perturbations (tan). Individual adaptation is supported or degraded by alterations in extrinsic (brown) risks and supports or intrinsic (blue) processes. Genetics can be a support or a risk so is in violet and is mediated and moderated as shown.
two-headed straight arrow=bidirectional influence; bidirectional curved arrow=correlated but not causal association (implementation); one-directional arrows = directional influences.
The idea of psychopathology emerging in the context of dynamic system properties is not new (Granic, 2005; Lichtwarck-Aschoff, Kunnen, & van Geert, 2009). However, empirical evidence that psychopathology actually behaves like a dynamic system has accrued only recently (Kendler, Zachar, & Craver, 2011; Kuranova et al., 2020; Lichtwarck-Aschoff et al., 2009; Nelson et al., 2017; Schiepek, Heinzel, Karch, Plöderl, & Strunk, 2016; Schiepek, Tominschek, & Heinzel, 2014; Wichers, Wigman, & Myin-Germeys, 2015). With those data in support, I stipulate that psychopathology is not a static entity responding to linear, unidirectional inputs. Rather it emerges from dynamic system interplay (Miller & Bartholomew, 2020). It is best understood at multiple units of analysis, with recursive dynamics over time (including processes of change and stabilizing/canalizing processes), and in relation to the ecological context.
Stressor and Perturbation
The exposome provides support and scaffolding for development, but also introduces stress, challenge, or perturbation. In theories of early development and in the concept of allostasis and allostatic load, “stressor” is broadly defined as any pressure strong enough to activate an adaptive compensation (Guidi, Lucente, Sonino, & Fava, 2021; Monk et al., 2019; Palego, Giannaccini, & Betti, 2020; Wachs et al., 2014). To avoid confusion with psychological stress alone, I use perturbation to reference the panoply of chemical and social challenges to development from the exposome that are strong enough to require an adaptive psychobiological response (see Table S2). Perturbation and adaptation are universal and continuous, rather than isolated to a subset of individuals in emotional distress. When the process diverts into maladaptation, it is psychopathology.
Self-Regulation
From a systems perspective, self-regulation means the adjustment of affect, thought, or action to maintain adaptation (Nigg, 2017)(Table S-2). It draws upon both extrinsic factors, such as social supports, and intrinsic factors, such as physiological responses and cognitive evaluations, operating in a mutually-regulating fashion. It depends on both (a) automatized bottom-up processes (typically anchored in physiological or subcortical-to-cortical response to perturbation) and (b) deliberate, top-down, goal-directed processes (explicit mental responses such as attribution, evaluation, planning, and problem solving, related to frontal cortical to subcortical neural projections). Top-down aspects of self-regulation (also referred to as self-control, Table S-2) involve attentional control and executive function (Nigg, 2017).
I propose self-regulation as a common or shared psychological mechanism across most of psychopathology. For example, via either automatic or cognitive routes, anxiety and mood disorders are related to failure to regulate affect, ADHD to dysregulation of impulse, and schizophrenia to dysregulation of motivation. As a result, it is not surprising that partially overlapping neural circuitry also is involved in these disparate conditions (Table S-3).
Regulatory mental and physiological processes (homeostatic/allostatic) are ubiquitous yet finite. Importantly, they can be mapped conceptually (McRae & Gross, 2020; Nigg, 2017; Sheppes, Suri, & Gross, 2015), and computationally (Petzschner, Garfinkel, Paulus, Koch, & Khalsa, 2021). Thus, a focus on self-regulation as a shared psychological or psychophysiological mechanism in psychopathology opens the door to modeling change as well as stability, creates a finite focus for mechanism study across a range of environmental perturbations and behavioral outcomes, and is amendable to computational study.
Etiology and Behavioral Epigenetics
Epigenetics: Overview
A common biological pathway linking genetic and environmental influences is gene regulation. Sustained regulation of gene expression can result from epigenetic changes—heritable (across cell division, not human generations per se) changes to the genome that do not change the DNA sequence. Epigenetic changes can be caused by genes, by stochastic process, and crucially, by exposures. In the last case, they are a mechanism for implementation of GxE and a liability-exposure model. Indeed, at highly variable genetic sites, GxE or G+E appears to best explain many DNA methylation changes (Czamara et al., 2019; Czamara et al., 2021). I use epigenetics to mean biological mechanisms that modulate or at least tag gene expression changes (transcription modification) (Table S-2 provides further context).
Behavioral epigenetics
Behavioral epigenetics (Moore, 2015) has emerged in the past decade following more established lines of work in cellular and tissue epigenetics. Interest in epigenetic influence on complex behavior was activated by fundamental discoveries in the 1970s (in animals) that epigenetic changes could be related to exposures and preserved over time, yet also reversed. It is now recognized that epigenetic effects, including in response to environments, play a major role in brain development (M. Li et al., 2018) and that genetic and exposome influences on behavior and psychopathology are in part mediated by altered gene expression (Barker, Walton, & Cecil, 2018; Smigielski, Jagannath, Rössler, Walitza, & Grünblatt, 2020; Wheater et al., 2020). The elucidation of a biological mechanism by which experience modifies gene action thus remains integrative and evocative for the field.
Epigenetics: Challenges and Cautions
Epigenetics are too often assumed, even by psychologists, to reflect mainly environmental input. While this assumption does have some support for psychopathology as noted above, genetic and stochastic processes also contribute (White, 2019). For example, newer studies that include genotype (MWAS+GWAS, Table S-2) have indicated that peripheral DNA methylation in developmental psychopathologies like ADHD are also mediating genetic effects (Meijer et al., 2020; Mooney et al., 2020).
A key challenge for an epigenetic hypothesis is small size of biological effects. Probes in human peripheral tissue reveal only very small associations with psychopathology. However, aggregated scores (e.g., MWAS principal components, region scores, or polyepigenetic scores targeting an exposure) may be more informative (O’Donnell & Meaney, 2020; Pries, Gülöksüz, & Kenis, 2017; Stevenson et al., 2020; Suarez et al., 2020). (Note that like polygenic risk scores, they are not particularly informative about one particular mechanisms, but instead summarize various regulatory influences).
A further limitation is that the epigenetic demonstrations relevant to psychopathology and the exposome emphasized maternal emotional stress or childhood maltreatment (O’Donnell & Meaney, 2020). The logic in that fascinating work needs to be extended to inform a wider range of exposures and outcomes (Table S-1 and S-3). Further, most basic knowledge about environmentally triggered epigenetic remodeling influences on behavior is from animal and insect studies. These are powerful due to their experimental control, but generalizing to humans is problematic. The human environment does not resemble the environment of a laboratory animal, and human capacity to adapt to a wide range of environments substantially exceeds that of other animals.
Finally, studies in human behavior remain difficult for methodological reasons. DNA methylation varies across development (Mulder et al., 2021), with potential timing-specific effects (Cecil et al., 2016). Moreover, epigenetic signals vary by tissue and cell type. It is difficult to generalize from peripheral tissue to the brain (Bakulski, Halladay, Hu, Mill, & Fallin, 2016; Conradt et al., 2018; Lappalainen & Greally, 2017; Mooney et al., 2020; Oldenburg, O’Shea, & Fry, 2020). Yet the living human brain is relatively inaccessible, so scientists seeking systemic effects rely mainly on indirect methods, each with important limitations (post-mortem brains, animal models, peripheral tissue assays).
Epigenetics: Prospects and Importance
The challenges are real. Yet prospects for epigenetic insight into psychopathology are sufficiently compelling as to commend integration in a dynamic model. The critical element is that epigenetic remodeling is dynamically responsive to the environment; it can exemplify experiential programming of gene expression (Meaney, 2010; O’Donnell & Meaney, 2020). As Table S-3 documents, epigenetic involvement has begun to be identified in a range of psychopathologies. Likewise, several psychopathology-relevant exposures have epigenetic sequelae (albeit, often studied only in non-human animals).
Epigenetics elevates the importance of the exposome in development and anchors it back to biology. Epigenetic markers can index relevant exposures (in the context of genotype) as well as link to psychopathology (Richetto & Meyer, 2020) (Again, see Table S-3 for examples). In theory, common exposures in early development can provoke systemic epigenetic response (e.g., DNA methylation of an immunological gene network). Such a response might then be common to many psychopathologies, and detected in peripheral tissue later, even though the mechanistic effect was in the central nervous system. It would thus serve as a biomarker of exposure and potentially of a mechanism. Such biomarker identification is promising for prior exposures (Noor et al., 2019; Richetto & Meyer, 2020), even in peripheral tissue (Aghagoli et al., 2019). It could even be an exposome biomarker for a causal (mechanistic) effect, if system wide effects occurred and were known. More difficult but promising is that algorithmic epigenetic scores can be phenotype biomarkers (Stevenson et al., 2020; Suarez et al., 2020). In sharp contrast to these near term prospects, finding mechanisms of epigenetic influence on complex phenotypes is a more distal possibility.5
Part II. Sketch of the Theory and Hypotheses
Theory Elements and Sketch: Overview and Recap of Perspective
The preceding discussion of exposome and mechanisms raises the level of complexity of to a potentially intractable degree. This state of affairs bedevils any non-simplistic theory of psychopathology. However, if we stipulate that psychopathology emerges from dynamic adaptation attempts or their failure within a dynamic system, it becomes possible to propose common mechanisms cutting across psychopathology. Doing so makes the problem tractable. Here then is a sketch of a theory following on that premise.
Process
Human development is dynamic and non-linear, unfolding in an emergent manner via greater and greater complexity, differentiation, and organization from conception forward, during which new properties emerge as complexity increases. Dynamically, a partially definable genetic architecture (Cross-Disorder Group of the Psychiatric Genomics Consortium, 2019) sets a liability for what we call psychopathology later. That genetic structure immediately encounters epigenetic changes in the germline from exposures to the parents, pre-conception, followed by the prenatal exposome. Then, throughout development, exposures continually serve to either stabilize or destabilize the dynamic processes of adaptation. Continually, the child and then the adult (the dynamic person-biology system), adapts more or less successfully in biological, physiological, and as the child matures, psychological (mental) spheres. This adaptive response includes alterations in neural development, mediated by chemical signaling (from mother-via-placenta-to fetus), and fetal and subsequent epigenetic change. Some of the epigenetic programming may be systemic and thus detectable as a biomarker in peripheral tissue later.
The adaptive process is recursive and continuous (i.e., cybernetic). Most perturbation is subtle enough that adaptation is easily attained or even strengthened. However, when the perturbation exceeds the capabilities of the system to adapt, then epigenetic, neural, and mental processes modify recursively in an attempt at adaptation that proves costly or becomes rigid or self-reinforcing, such that the individual is unable to adapt further. This sets the stage for or emerges as psychopathology, either relatively early (ADHD) or somewhat later and gradually (schizophrenia), perhaps only after further perturbation. Mental disorder thus emerges from a perturbation of a dynamic system that is unable to regain an adaptive equilibrium (or homeostasis) and instead canalizes into a maladaptive or unstable developmental course, culminating in frank psychopathology.
If genetic liability is high, then the perturbation may seem relatively minor (e.g., background toxicant exposure or sub-optimal diet, maternal distress, losses that happen to everyone in life, temporary experimentation with drugs) such that perhaps most individuals would adapt without psychopathology. But an ill-timed or strong perturbation (frank physical abuse, moderately elevated lead exposure, perinatal complications, extreme maternal trauma, an unusual series of losses, or a rare genetic mutation of large effect) may disrupt adaptation and lead to psychopathology even when liability is only moderate. Figure 1 schematizes the model to this point.
Common Pathway Hypothesis
A major obstacle to explaining psychopathology is the vastness of etiological inputs to the extraordinarily complex recursive processes that govern human behavior and cognition and maintain functional adaptation. To address this, a common pathways hypothesis proposes that a circumscribed set of characteristic mechanisms can be identified within the relevant units of analysis, all in the regulatory domain. I hypothesize that the universe of mechanistic human self-regulatory processes is smaller than the universe of inputs or the complex combinations of behavioral and mental outputs. Figure 2 illustrates this logic. If correct, a finite and tractable set of homeostatic processes would emerge as shared across a wide range of exposures and psychopathologies, across multiple units of analysis: genetically (e.g., neurodevelopmental pathways), biologically (e.g., chemical signaling), neurally (e.g., control systems), and psychologically (e.g., self-regulation processes and mental evaluations) (Table S-3).
Figure 2: Funnel Visualization.
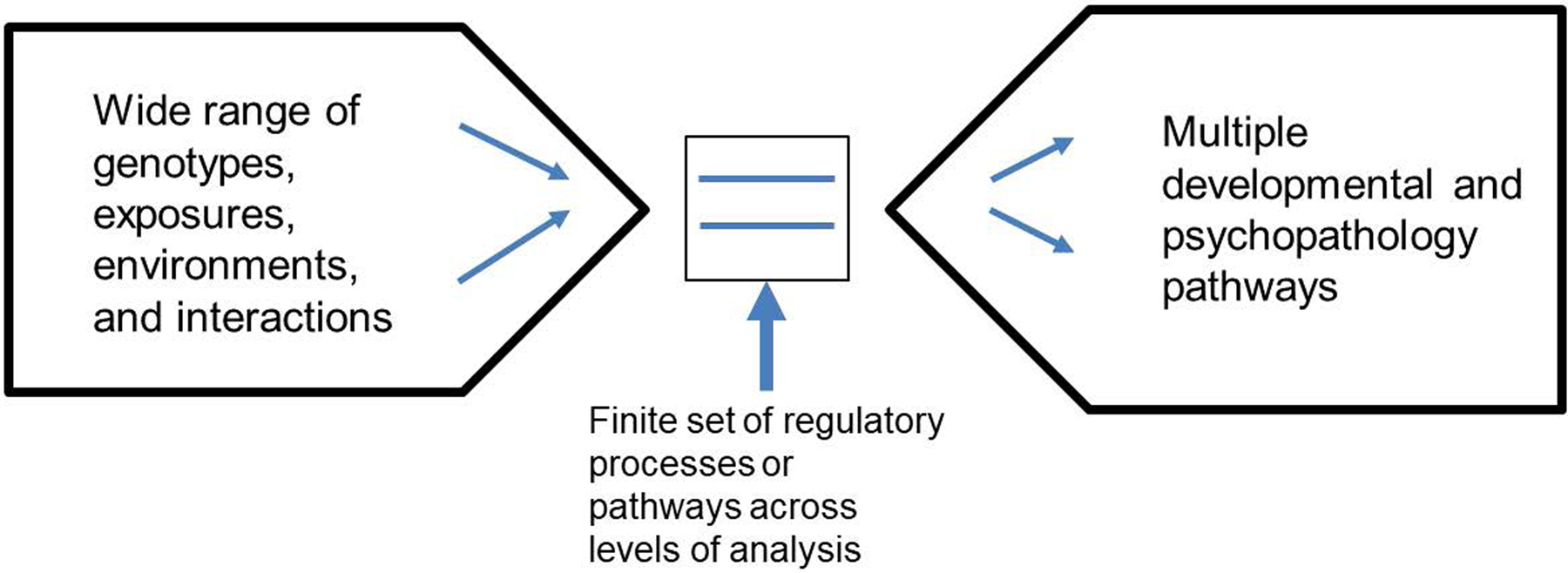
The available exposures, genotypes, and biochemical pathways potentially involved include the entire human biome and beyond (social context). However, it is hypothesized here that a small set of common regulatory or homeostatic mechanisms at the psychological and biological levels of analysis will participate in a disproportionate share of perturbations related to psychopathology.
At the same time, the “funnel” content in Figure 2 is not singular (I do not propose to boil mental disorder down to a brain system, or a physiological system, or a mental process). It requires mechanistic description across multiple units, each of which can then become avenues for potential intervention. The most appropriate unit or explanatory perspective can vary across conditions or individuals, yet can be constrained. Key nodes of causal action may occur biologically (placental modification in response to infection, leading to alteration, or perhaps cost, in neural development); or cognitively (rumination in depression, sustained attribution-driven arousal in aggression or anxiety, body-perception in anorexia) and contribute to unfolding and sustained behavioral and biological change.
As an analogy, consider the myriad bacteria and viruses that can cause illness—yet a single (multi-unit) immune system responds to all of them. The response product (e.g., antibodies) is specific, but the response process is shared. Similarly, the cortical networks involved in higher order analytic thought and planning are activated for an infinite range of complex problems or goal conflicts. The content solution a person thinks of is particular, but the supporting process is shared across difficult problems. From an evolutionary perspective, this efficiency of process is the only way for an organism to meet a potentially unlimited array of challenges—to have an all-purpose regulatory response that can adapt itself to an unknown array of different challenges.
To flesh out this idea, Figure 3 illustrates four critical levels or units of analysis and offers exemplar hypotheses for common mechanisms at each level. These can be considered as example working hypotheses. Space limitations preclude their critical evaluation here but Table S-3 provides selective empirical support for their reasonableness. In network terminology, the task is to differentiate (a) the most important spatial nodes (unit of analysis), and (b) the most sensitive temporal nodes (third trimester, pubertal transition, other) (Jones et al., 2019).
Figure 3: Proposed contents of common pathway or funnel.
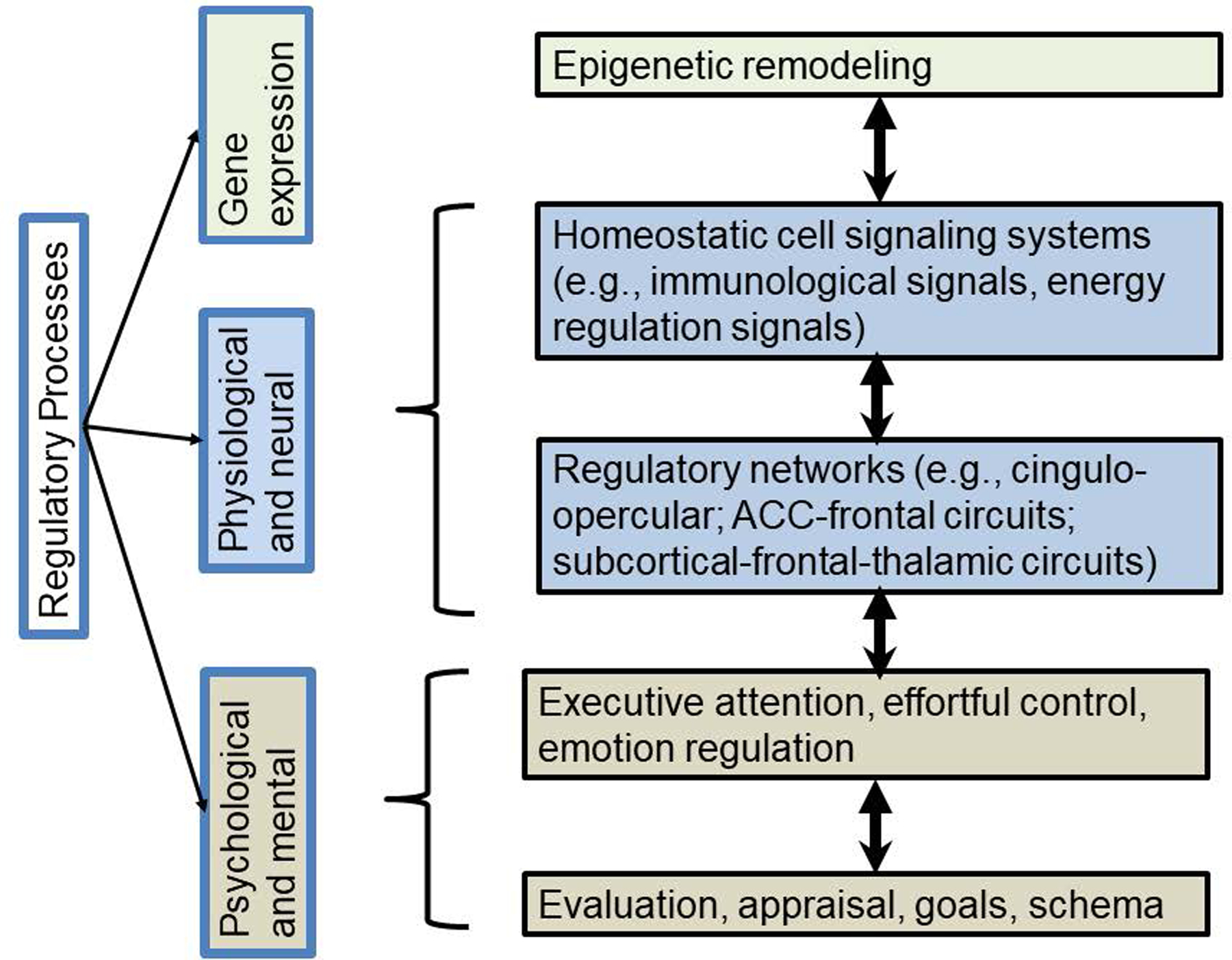
elements, represented at different levels of size, abstraction, or analysis. Mechanisms to be described at each level are hypothesized to be finite and fewer than the extrinsic inputs or behavioral and psychological outputs involved in psychopathology. Within level increasing granularity is used to explain mechanism.
Challenges and Considerations
I have noted that the most relevant level of analysis for a given subset of psychopathology may be at the mental/psychological level in some instances, but the biological or genetic level in others. This raises two important considerations.
First, I reiterate that although it may seem that the emphasis on environmental perturbation restricts this proposal to trauma or stress-related disorders like depression, anxiety, or PTSD, I propose that perturbations outside the evolutionary expected environment are common (see Exposome section).6 These may represent non-shared environment in twin studies and contribute to heritability estimates if they are involved in GxE; I hypothesize that they in fact provoke epigenetic change and can lead to re-organization or, in dynamic systems language, a phase change toward atypical development. Because development is sequential and cumulative, with later skills relying on earlier ones to be in place, early negative7 perturbations can lead to psychopathology at a later point (schizophrenia, bipolar disorder).
A second potential misunderstanding of my argument is that difficulties in adaptation may seem reminiscent of difficulties in coping, and most readily applicable to conditions like ADHD, oppositional defiant disorder, anxiety, or depression. They may seem too weak an explanation for the dramatic breakdowns in perception, motivation, and circadian processes in schizophrenia, bipolar disorder, or severe depression. I re-emphasize that adaptation includes physiological and epigenetic change, not only psychological coping. For example, schizophrenia likely has roots in early neurodevelopment, perhaps involving neuro-inflammatory inputs (Corsi-Zuelli & Deakin, 2021; Eyles, 2021; Schmidt & Mirnics, 2015). A similar story may emerge for bipolar disorder, perhaps involving metabolic pathways rather than inflammatory signaling systems (Kloiber et al., 2020; Mansur et al., 2020). These possibilities fit the current theory well.
Summary
A central hypothesis is that multiple conditions will share common biological, neural, and psychological mechanisms (Figure 2, Figure 3). The corollary hypothesis is that these will be involved in maintaining homeostasis (regulatory). Thus, recursive processes are involved in change and can be mapped dynamically across time. If this hypothesis is correct, substantial explanatory power will accrue through a finite set of routes.
Hypotheses
Research Design Implications
Below, I present hypotheses in schematic form. However, these come in the context of research design considerations. Many design recommendations from dynamic systems theorists are infeasible (e.g., requiring very dense time series data, which would be cost prohibitive for many multi-unit studies). However, I note the following principles of design that follow from the present perspective:
(a) Emphasis on change over time (whether on very short time scales, as in dynamic regulation of heart rate or synchronization of neural networks), medium scales (e.g., changes across menstrual cycles), or long scales (development over several years). (b) Emphasis on cybernetic processes as can be represented in multiple ways in graphical and other tools, such as network modeling (Jones, Ma, & McNally, 2019; McNally, 2011). (c) Designs that place biomarkers in ecological and interpersonal/psychosocial context, and environmental studies in genetic and physiological context and consider their interplay. (d) Dynamic systems models offer a suite of graphical analysis tools (e.g., state-space grids) that may be helpful when sufficient data are available, as noted by Kaliush et al. (2020). For further design suggestions related to dynamic systems in developmental psychopathology, see (Granic et al., 2016).
What follows extends from Figure 3 and is given partial support in Table S-3. The hypotheses discussed in this section are also summarized in Table 1 for reference. Tests of each hypothesis would involve confirming their shared involvement in extended types of psychopathology and across extended ranges of exposome pressures and determining whether effect sizes are sufficient to be helpful in explanation. Their tests involve confirming or rejecting the relevance of the proposed recursive effects in onset and course of disorder.
Table 1:
Example Hypotheses at Four Scales, Levels, or Units of Analysis
| Genetic and Epigenetic |
| a) Epigenetic measures (e.g., DNA methylation profiles, algorithms, or principal components) will be detectable in relation to exposures that are associated with psychopathology (Smigielski et al., 2020). They can introduce both stable markings of exposure (e.g., DNA methylation scores of lifetime smoking or in utero smoking exposure) and at the same time change dynamically in response to exposure over time, in which case change will in turn be associated with behavior or exposure change in recursive pattern until stability is reached. |
| b) Epigenetic signals associated with a common adaptive system such as immunological function will be identified across multiple psychopathologies, as recently seen for schizophrenia (Smigielski et al., 2020) and ADHD (Neumann et al., 2020). However, these signals at the specific level will include both general (most psychopathologies) and specific (to particular kinds of psychopathology) signals. |
| c) Epigenetic algorithms that index exposure will map to exposures related to psychopathology (Richetto & Meyer, 2020) using peripheral tissue measures and ultimately be verified in relation to neurodevelopment, indexing a biomarker of system-wide compensatory response. Similar approaches can be used to index treatment response or responsivity (Gardea-Resendez et al., 2020; Goud Alladi, Etain, Bellivier, & Marie-Claire, 2018). For example, it will be possible to create epigenetic profiles related to stress, inflammatory activity, smoking, alcohol exposure, or other exposures (Stevenson et al., 2020) that will be combinable to map a risk or exposome profile for a given child and then related to brain or cognitive development. |
| (d) Change over time in epigenetic scores (using summary scores for systems) will recursively interact with physiological, cognitive, or behavioral change, thus predicting individual differences related to psychopathology. |
| Physiological/cellular |
| a) Higher systemic inflammation /immune response (or alternative signaling response system) will be seen in women whose offspring go on to develop psychopathology. |
| b) The same common signals seen in women whose offspring develop psychopathology will also be seen elevated in individuals with psychopathology (Goldsmith & Rapaport, 2020). |
| c) Anti-inflammatory intervention in early life will mitigate impacts of multiple known risk factors (e.g., dietary, metabolic, toxicant) for multiple conditions (Thompson et al., 2018). |
| d) Such intervention may also reduce psychopathology symptoms across multiple disorders (Fond, Lançon, Korchia, Auquier, & Boyer, 2020). |
| e) Microbiome signals (perhaps related to dietary response) (Bordeleau, Fernández de Cossío, Chakravarty, & Tremblay, 2020) will emerge as an influence in multiple psychopathologies (Tilg et al., 2020). |
| d) Dynamic change in cell signaling summary scores or targeted mechanistic measures will interact with epigenetic or neural change in dynamic, recursive fashion. Individual variation in change of the dynamic direction or set point will relate to psychopathology risk or severity. |
| Neural (multiple levels exist within neural of course; here only two are noted) |
| a) Changes in brain regulatory structures or networks, either at the macro level (e.g., control networks) or micro level (e.g. microglia function) will change recursively with change in epigenetic or physiological signals and mediate behavioral change in dynamic, bidirectional fashion over time. |
| Psychological (also called mental, cognitive) |
| a) Self-regulation of cognition, affect, or behavior can be identified as a functional mechanism in multiple psychopathologies. The weak version of this hypothesis is that either bottom up or top down aspects of self-regulation are commonly identified. The strong or risky version of this hypothesis is that top-down self-regulation mechanisms are shared. Hence, the corollary risky hypothesis is: |
| b) Cognitive evaluation (appraisal) of experience meanings can be identified as a functional mechanism within self-regulation across multiple psychopathologies. |
| c) Cognitive evaluation mechanisms will respond dynamically to exposome challenge and parallel physiological change bi-directionally, until a stable pattern is reached over time. Individual differences in particular patterns of change will index response to clinical intervention or change in clinical course. |
Epigenetic-Genetic Unit of Analysis
The smallest level is gene regulation. The theme is that DNA structure may set liability but the operative mechanism is regulation of gene expression (for an interesting perspective here see Turkheimer, Pettersson, & Horn, 2014). Gene expression is genetically and epigenetically regulated. Epigenetic regulation serves a homeostatic function and should show dynamic associations with onset and course over time. Table 1 provides the very simple epigenetic hypotheses that follow here if the model is correct. The ideas in Table 1 have initial support, albeit mostly in studies of non-human animals (see Table S-3). For research and clinical applicability, a dynamic model perspective would ask about change over time and recursive effects relative to risk and adaptation. For example, it would be useful to discover when and to what extent epigenetic remodeling follows or precedes behavioral change in relation to exposures. The hypothesis is that epigenetic alteration (or else gene expression itself) will show dynamic directional effects on behavior or on physiological or neural correlates of behavior, in a recursive manner. (This would be refuted if directional change in epigenetic score or finding was not mirrored by change in the target behavioral outcome; though again, small effect sizes are still expected). If supported, then composite scores reflecting system-wide activity could function in clinical algorithms or in research models (Stevenson et al., 2020).
The Physiological Unit of Analysis
Systems biology identifies tens of thousands of potentially relevant molecules, proteins, and enzymes. However, it can be hypothesized that chemical signals critical to maintaining homeostasis are crucial (E. Sullivan, personal communication; Dunn, Loftis, & Sullivan, 2020). A salient example is immunological signalers such as cytokines and chemokines (Dunn et al., 2020; Izvolskaia, Sharova, & Zakharova, 2020; Oppenheim, 2018). Table S-3 illustrates the potential widespread involvement of immunological signaling molecules as common pathways in psychopathology. Of course, these interact in complex ways with related systems such as the microbiome, the aforementioned epigenetic signals, mitochondrial alterations, and brain glial cells, among other linkages. I hypothesize that, at the physiological level, this family or another like it will account for meaningful variance in early development and subsequently in relation to multiple psychopathologies (Na, Jung, & Kim, 2014). Other key ligands include hormones regulating response to stress and energy balance. Clearly, the interplay of these examples with oxidative stress, HPA axis, and other systems invites a more extensive, yet still finite, mapping.
Specific hypotheses are again listed in Table 1. In those hypotheses, I use immunological/inflammatory signaling as the primary common pathway; this is a stand in for the clumsier statement that one or more common inter-cellular signaling systems should emerge as commonly affected across multiple kinds of exposures and psychopathologies. It may be that the particular signaling system affected varies across psychopathologies (e.g., immunological, HPA, energetic). In this example, immunological functioning interacts closely with the microbiome (Tilg, Zmora, Adolph, & Elinav, 2020), so an associated hypothesis is included in Table 1. Dynamically, these hypotheses would expand to enable assessment of component change in relation to other units (e.g., neural re-organization, epigenetic remodeling, immunological signaling units).
The Neural Unit of Analysis
I do not attempt to propose a new neural framework for regulatory functioning or homeostatic maintenance of mental life. However, recall from early in the paper that while it is possible to conceive at least heuristically of disorder-specific neural networks (for one attempt, see Kandel, 2018), recent neuroimaging data strongly qualify such distinctions (T. Li et al., 2020). Instead, overlap and non-specificity seem to be the rule. The relevant higher-order regulatory neural systems can be conceived in terms of functional networks (e.g., the cingulo-opercular network, the default mode network, the executive control network) (Petersen & Posner, 2012; Posner, Rothbart, & Voelker, 2016) (Table S-3 citations); as frontal-subcortical-thalamic loops (Bonelli & Cummings, 2007); or as key nodes within regulatory networks (e.g., the anterior cingulate cortex) (Posner et al., 2016). The primary hypothesis is that they will reorganize in response to perturbation, with individual differences related to emergence or course of psychopathology over time.
Mediating neural mechanisms can also be identified at a more granular level. For example, staying with the immunological example, an important candidate is neuroinflammation (Dunn et al., 2020), with associated involvement of glial cells and astrocytes responsive to brain chemokine and cytokine signaling. Microglia play a homeostatic role in the brain (Cowan & Petri, 2018; Nayak, Roth, & McGavern, 2014; Wright-Jin & Gutmann, 2019), making them a promising common mechanism in a dynamic neural model of mental disorder. The dynamic question concerns change how over time and recursive modification occurs until health – or decompensation—emerge in a new stabilization. I hypothesize that the process would correlate with behavioral and mental self-regulation (or breakdowns of regulation) over time.
The Psychological Unit of Analysis
In the funnel model, I do not suggest that mental contents are finite, but that the processes relevant to psychopathology are. Within a finite set of key processes, specific contents are unique to the individual (particular ways of making meaning, or interpreting their experience). An analogy might be the finding that the equation for reward discounting appears almost species universal, whereas parameter weights vary across species and incentive context. Two related processes, each able to be mechanistically decomposed (at least in principle), are salient.
The first, overarching process is intrinsic self-regulatory processes. I previously noted that self-regulation involves an interplay of automatic and intentional (goal directed) processes or mental activities (Nigg, 2017). Top down elements appear to be shared across a range of psychopathologies. For example, attentional control (Burgoyne & Engle, 2020) supports executive functioning, emotional regulation, and fluid intelligence; its breakdowns are observed in in ADHD, anxiety, depression, schizophrenia, bipolar disorder, and PTSD (Table S-3).
The second, a component of the first, is appraisal schema, or evaluations. Evaluations are both implicit (non-conscious) and explicit. They serve to help regulate emotion, confer meaning, and thus guide behavior (Tamir, 2021). Implicit evaluative representations can occur in a wide range of psychopathologies (Teachman, Clerkin, Cunningham, Dreyer-Oren, & Werntz, 2019) both a mechanism and an outcome. Recent theories propose a continuous iterative process by which mental evaluations are continuously updated, with implicit processes followed up by explicit (conscious) revision (Cunningham & Zelazo, 2007; Ehret, Monroe, & Read, 2015; Tamir, 2021). Implicit evaluations are difficult but not impossible to measure (Teachman et al., 2019). Mental evaluations can be thought of as a seamless element of the continuous dynamic of homeostatic stabilization that constitutes psychological regulation. They are a common pathway for much psychopathology (see Figure 3, Table S-3, and Table 1).
Clinical and Prevention Relevance
Can a multi-level dynamic proposal inform applied problems? I noted early on the relevance of multi-level models to risk prevention efforts. All of the mechanisms noted in this essay are already recognized as potential mechanisms for intervention; proposed here is the potential to integrate them around epigenetic and dynamic processes. Mascolo et al. (2016) provide extensive elaboration on how their approach to dynamic systems should inform clinical practice; many of their suggestions hold here. A central one is a shift in conceptualization away from static conditions with linear inputs, and toward an emergent formulation (this is consistent with a developmental psychopathology perspective as well). It also highlights the value of sequential behavioral analysis in clinical evaluation. Kaliush et al. (2020) suggest that clearer identification of phase-state transition periods, particularly in early life, could enrich a prevention focus. As mathematical description of these processes rapidly improves, targeted intervention should improve as well.8 Further, clinical algorithms could utilize composite scores reflecting system-wide activity in brain, such as a polyvertex score (Zhao et al., 2019) or related score for functional networks (Mooney et al., 2021 under review), or epigenetic or physiological summary scores (Stevenson et al., 2020). These would target regulatory process and could become indices of clinical change (the complexity of interpreting poly-scores notwithstanding).
Conclusion
The goal of mental health intervention at a human level is to support, heal, and help a struggling person. Re-stating the goal in the dry language of a schematic theory, it is to re-establish homeostasis, perhaps at a new set point, for a faltering dynamic system. Doing so can include introducing new information (biologically or cognitively), adding supports (e.g., social), or providing opportunity for re-organization (e.g., new environmental niches).
Key gaps in knowledge include: (a) insufficient mapping of the exposome, (b) under-emphasis on causally informative designs, (c) insufficient cross-fertilization of genetic, physiological, psychological, and exposure studies (i.e., the problem of decontextualized biomarker studies), (d) insufficient joining of prenatal and postnatal influences in the same cohorts (which is beginning to change), and (e) slow application to developmental studies of psychopathology of network and other system-modeling tools or to multi-unit dynamics.
Developing the present sketch or theory schema would entail mapping the dynamics of a complex, multilevel system that includes genetic liability, exposures of varying significance, epigenetic response and regulation of brain development, and psychological processes involved in self-regulation including mental evaluations. A key challenge concerns how to map patterns of recursive feedback (cybernetic processes) over time within and across units of analysis. Initial developmental trajectories may be reversible in many instances once these mechanisms are better understood. However, at present sensitive periods of development (where in dynamic systems terms, phase shifts are likely to occur), and mechanisms of stabilizing someone in a maladaptive state, are still only partially elucidated.
In terms of priorities for the field, the complexity of the bio-psychological system of the person can be focused for scientific purposes on (a) mapping of the exposome (as in the ECHO consortium effort), (b) testing of mechanistic hypotheses involved in the epigenome and in key chemical communication systems (inflammatory factors and stress hormones), (c) more use of longitudinal network and other models that can track recursive processes over time in relation to phenotypic expression. I have sketched the beginnings of testable hypotheses.
Overall, the core hypothesis is that neurobiological and psychological pathways of influence are finite and represent shared mechanisms across many psychopathologies. I hypothesized that this is because their function is regulatory, and that the human bio-psychological system has a finite number of multi-purpose processes that adapt to varying pressures (immune response is an exemplar). Epigenetic regulation of gene expression in the face of environmental pressure is an essential mechanism to include, and not merely a metaphor for development, even though its central role remains to be fully determined. The principle of common regulatory processes is reflected in different units of analysis such as the physiological (cell signaling), the psychological (evaluations and other intrinsic self-regulatory mental processes), and their corresponding implementing neural networks.
A finite (albeit complex) set of these processes respond to the array of inputs relevant to psychopathology and produce its specific forms as their content, as schematized in Figure 2 and Figure 3. In this dynamic context, while the vast complexity of neural and psychological processes is involved, and the potential inputs and outputs are vast, the critical mechanisms (defined in dynamic systems theory as controlling processes) shaping mental disorder are finite and isolated to regulatory systems.
Testing this logic requires a shift away from studies of single inputs (just the genome, or just a given environmental risk) or single domains of psychopathology, toward integrated study of multiple psychopathology and risk factors. It can be targeted on a-priori hypotheses of which the possibilities can be limited to adaptive processes. While multiple psychopathologies should be studied, units can be studied in subsets so long as they are subsequently connected to other units in an integrated explanation. Thus, the tools are already in place to undertake novel tests and to develop the approach outlined in this essay.
Supplementary Material
Acknowledgments
The author was supported in this work by NIH grant R01-MHR3759105. He declares no conflicts of interest with the material herein. I thank Charlotte Cecil, Sarah Karalunas, Michael Mooney, Philip Shaw, and Elinor Sullivan for critical comments on aspects of this work. The opinions presented are the author’s and do not necessarily reflect the official views of the National Institutes of Health, or the views of commentators on this work. Material in this paper is original and has not been presented in whole or in part elsewhere. Any citations of the author’s research involved studies that had full ethics and human subject approval.
Footnotes
Thomas and Sharp (2019) suggest that theory develops from sketch, to schema, to testable model. I propose a sketch that may be close to a schema. I bypass definitions of mental disorder, defining normal versus abnormal, and essentialism. See (McNally, 2011) and (Wakefield, 1992, 1999). The “illness” metaphor is fraught (Hudson, 1993) but may pertain for psychopathology as complex illness.
With regard to distal causes, my argument is compatible with an evolutionary-adaptationist perspective (Ellis & Del Giudice, 2019) although not reducible to it. The focus herein is more proximal.
The classic view in developmental psychopathology (paralleled in neuroscience) refers to levels of analysis, such as social, psychological, neural, and genetic. Recent thinking has moved away from this language (Thomas & Sharp, 2019). I use the term “units” to refer to these heretofore putative scales or levels of examination (see section on Mechanism and reduction later).
In human research, pre- and post-natal risk environments often display some continuity, e.g., psychosocial adversity. Even so, fetal adaptation may be insufficient to what the child later encounters.
Studies using bead-based arrays have provided tantalizing clues on both fronts (Smigielski et al., 2020). However, whole methylome and other sequencing may be necessary to describe mechanisms.
Beyond the scope of this essay is whether they were just as common in ancient times, when many psychopathologies were already known and have only become a little more common.
Of course, positive exposures also occur that enhance resilience, such as extra breastfeeding or exceptional parenting, which are side-stepped here in the interest of length limits.
Mathematical description of neural, cognitive, and behavioral dynamic processes is advancing rapidly. It will be increasingly tractable to quantify perturbations and the recursive process around them while considering individual variations in parameter weightings for finite (if extensive) process equations.
References
- Achenbach TM (2020). Bottom-Up and Top-Down Paradigms for Psychopathology: A Half-Century Odyssey. Annu Rev Clin Psychol, 16, 1–24. doi: 10.1146/annurev-clinpsy-071119-115831 [DOI] [PubMed] [Google Scholar]
- Aghagoli G, Conradt E, Padbury JF, Sheinkopf SJ, Tokadjian H, Dansereau LM, … Lester BM (2019). Social Stress-Related Epigenetic Changes Associated With Increased Heart Rate Variability in Infants. Frontiers in Behavioral Neuroscience, 13, 294. doi: 10.3389/fnbeh.2019.00294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alegría M, NeMoyer A, Falgàs Bagué I, Wang Y, & Alvarez K (2018). Social Determinants of Mental Health: Where We Are and Where We Need to Go. Curr Psychiatry Rep, 20(11), 95. doi: 10.1007/s11920-018-0969-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JJ, Anderson CA, & Bushman BJ (2018). The General Aggression Model. Curr Opin Psychol, 19, 75–80. doi: 10.1016/j.copsyc.2017.03.034 [DOI] [PubMed] [Google Scholar]
- Anttila V, Bulik-Sullivan B, Finucane HK, Walters RK, Bras J, Duncan L, … Murray R (2018). Analysis of shared heritability in common disorders of the brain. Science, 360(6395). doi: 10.1126/science.aap8757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong-Carter E, Trejo S, Hill LJB, Crossley KL, Mason D, & Domingue BW (2020). The Earliest Origins of Genetic Nurture: The Prenatal Environment Mediates the Association Between Maternal Genetics and Child Development. Psychological Science, 31(7), 781–791. doi: 10.1177/0956797620917209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakulski KM, Halladay A, Hu VW, Mill J, & Fallin MD (2016). Epigenetic Research in Neuropsychiatric Disorders: the “Tissue Issue”. Curr Behav Neurosci Rep, 3(3), 264–274. doi: 10.1007/s40473-016-0083-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker ED, Walton E, & Cecil CAM (2018). Annual Research Review: DNA methylation as a mediator in the association between risk exposure and child and adolescent psychopathology. J Child Psychol Psychiatry, 59(4), 303–322. doi: 10.1111/jcpp.12782 [DOI] [PubMed] [Google Scholar]
- Belsky J, & Pluess M (2009). Beyond diathesis stress: differential susceptibility to environmental influences. Psychological Bulletin, 135(6), 885–908. doi: 10.1037/a0017376 [DOI] [PubMed] [Google Scholar]
- Belsky J, Pokhvisneva I, Rema ASS, Broekman BFP, Pluess M, O’Donnell KJ, … Silveira PP (2019). Polygenic differential susceptibility to prenatal adversity. Development and Psychopathology, 31(2), 439–441. doi: 10.1017/s0954579418000378 [DOI] [PubMed] [Google Scholar]
- Benjamin AJ Jr., Kepes S, & Bushman BJ (2018). Effects of Weapons on Aggressive Thoughts, Angry Feelings, Hostile Appraisals, and Aggressive Behavior: A Meta-Analytic Review of the Weapons Effect Literature. Personality and Social Psychology Review, 22(4), 347–377. doi: 10.1177/1088868317725419 [DOI] [PubMed] [Google Scholar]
- Bertolaso M, & Ratti E (2018). Conceptual Challenges in the Theoretical Foundations of Systems Biology. Methods in Molecular Biology, 1702, 1–13. doi: 10.1007/978-1-4939-7456-6_1 [DOI] [PubMed] [Google Scholar]
- Bonelli RM, & Cummings JL (2007). Frontal-subcortical circuitry and behavior. Dialogues in Clinical Neuroscience, 9(2), 141–151. doi: 10.31887/DCNS.2007.9.2/rbonelli [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordeleau M, Fernández de Cossío L, Chakravarty MM, & Tremblay M (2020). From Maternal Diet to Neurodevelopmental Disorders: A Story of Neuroinflammation. Frontiers in Cellular Neuroscience, 14, 612705. doi: 10.3389/fncel.2020.612705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsboom D, Cramer AO, Schmittmann VD, Epskamp S, & Waldorp LJ (2011). The small world of psychopathology. PloS One, 6(11), e27407. doi: 10.1371/journal.pone.0027407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsboom D, Rhemtulla M, Cramer AO, van der Maas HL, Scheffer M, & Dolan CV (2016). Kinds versus continua: a review of psychometric approaches to uncover the structure of psychiatric constructs. Psychological Medicine, 46(8), 1567–1579. doi: 10.1017/s0033291715001944 [DOI] [PubMed] [Google Scholar]
- Britto PR, Lye SJ, Proulx K, Yousafzai AK, Matthews SG, Vaivada T, … Bhutta ZA (2017). Nurturing care: promoting early childhood development. Lancet, 389(10064), 91–102. doi: 10.1016/s0140-6736(16)31390-3 [DOI] [PubMed] [Google Scholar]
- Britto PR, & Pérez-Escamilla R (2013). No second chances? Early critical periods in human development. Social Science and Medicine, 97, 238–240. doi: 10.1016/j.socscimed.2013.09.001 [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner U (1977). Toward an experimental ecology of human development. American Psychologist, 32(7), 513–531. [Google Scholar]
- Bronfenbrenner U (1979). The ecology of human development: Experiments by nature and design. Cambridge, MA: Harvard University Press. [Google Scholar]
- Bronfenbrenner U, & Ceci SJ (1994). Nature-nurture reconceptualized in developmental perspective: a bioecological model. Psychological Review, 101(4), 568–586. doi: 10.1037/0033-295x.101.4.568 [DOI] [PubMed] [Google Scholar]
- Burgoyne AP, & Engle RW (2020). Attention Control: A Cornerstone of Higher-Order Cognition. Current Directions in Psychological Science, 29(6), 624–630. doi: 10.1177/0963721420969371 [DOI] [Google Scholar]
- Burns GL, Becker SP, Geiser C, Leopold DR, & Willcutt EG (2019). Are Sluggish Cognitive Tempo, ADHD, and Oppositional Defiant Disorder Trait- or State-Like Constructs from Prekindergarten to Fourth Grade? Journal of Clinical Child and Adolescent Psychology, 1–9. doi: 10.1080/15374416.2019.1567348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case A, & Deaton A (2020). Deaths of Despair and the Future of Capitalism: Princeton University Press. [Google Scholar]
- Causadias JM, & Cicchetti D (2018). Cultural development and psychopathology. Development and Psychopathology, 30(5), 1549–1555. doi: 10.1017/s0954579418001220 [DOI] [PubMed] [Google Scholar]
- Cecil CAM, Walton E, Smith RG, Viding E, McCrory EJ, Relton CL, … Barker ED (2016). DNA methylation and substance-use risk: a prospective, genome-wide study spanning gestation to adolescence. Translational psychiatry, 6(12), e976–e976. doi: 10.1038/tp.2016.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D (1984). The emergence of developmental psychopathology. Child Development, 55(1), 1–7. [PubMed] [Google Scholar]
- Cicchetti D (2016). Socioemotional, Personality, and Biological Development: Illustrations from a Multilevel Developmental Psychopathology Perspective on Child Maltreatment. Annual Review of Psychology, 67, 187–211. doi: 10.1146/annurev-psych-122414-033259 [DOI] [PubMed] [Google Scholar]
- Cicchetti D, & Natsuaki MN (2014). Multilevel developmental perspectives toward understanding internalizing psychopathology: current research and future directions. Development and Psychopathology, 26(4 Pt 2), 1189–1190. doi: 10.1017/s0954579414000959 [DOI] [PubMed] [Google Scholar]
- Cicchetti D, & Toth SL (2009). The past achievements and future promises of developmental psychopathology: the coming of age of a discipline. Journal of Child Psychology and Psychiatry and Allied Disciplines, 50(1–2), 16–25. doi: 10.1111/j.1469-7610.2008.01979.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradt E, Adkins DE, Crowell SE, Raby KL, Diamond LM, & Ellis B (2018). Incorporating epigenetic mechanisms to advance fetal programming theories. Development and Psychopathology, 30(3), 807–824. doi: 10.1017/s0954579418000469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi-Zuelli F, & Deakin B (2021). Impaired regulatory T cell control of astroglial overdrive and microglial pruning in schizophrenia. Neuroscience and Biobehavioral Reviews. doi: 10.1016/j.neubiorev.2021.03.004 [DOI] [PubMed] [Google Scholar]
- Cowan M, & Petri WA Jr. (2018). Microglia: Immune Regulators of Neurodevelopment. Frontiers in Immunology, 9, 2576. doi: 10.3389/fimmu.2018.02576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craver CF (2005). Beyond reduction: mechanisms, multifield integration and the unity of neuroscience. Studies in History and Philosophy of Biological and Biomedical Sciences, 36(2), 373–395. doi: 10.1016/j.shpsc.2005.03.008 [DOI] [PubMed] [Google Scholar]
- Craver CF (2007). Explaining the brain: Mechanisms and the Mosaic Unity of Neuroscience. Oxford: Clarendon Press. [Google Scholar]
- Cross-Disorder Group of the Psychiatric Genomics Consortium. (2019). Genomic Relationships, Novel Loci, and Pleiotropic Mechanisms across Eight Psychiatric Disorders. Cell, 179(7), 1469–1482.e1411. doi: 10.1016/j.cell.2019.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham WA, & Zelazo PD (2007). Attitudes and evaluations: a social cognitive neuroscience perspective. Trends in Cognitive Sciences, 11(3), 97–104. doi: 10.1016/j.tics.2006.12.005 [DOI] [PubMed] [Google Scholar]
- Czamara D, Eraslan G, Page CM, Lahti J, Lahti-Pulkkinen M, Hämäläinen E, … Binder EB (2019). Integrated analysis of environmental and genetic influences on cord blood DNA methylation in new-borns. Nat Commun, 10(1), 2548. doi: 10.1038/s41467-019-10461-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czamara D, Tissink E, Tuhkanen J, Martins J, Awaloff Y, Drake AJ, … Binder EB (2021). Combined effects of genotype and childhood adversity shape variability of DNA methylation across age. Transl Psychiatry, 11(1), 88. doi: 10.1038/s41398-020-01147-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge P, Wardenaar KJ, Lim CCW, Aguilar-Gaxiola S, Alonso J, Andrade LH, … Scott K (2018). The cross-national structure of mental disorders: results from the World Mental Health Surveys. Psychological Medicine, 48(12), 2073–2084. doi: 10.1017/s0033291717003610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn GA, Loftis JM, & Sullivan EL (2020). Neuroinflammation in psychiatric disorders: An introductory primer. Pharmacology, Biochemistry and Behavior, 196, 172981. doi: 10.1016/j.pbb.2020.172981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret PJ, Monroe BM, & Read SJ (2015). Modeling the dynamics of evaluation: a multilevel neural network implementation of the iterative reprocessing model. Personality and Social Psychology Review, 19(2), 148–176. doi: 10.1177/1088868314544221 [DOI] [PubMed] [Google Scholar]
- Ellis BJ, & Del Giudice M (2019). Developmental Adaptation to Stress: An Evolutionary Perspective. Annual Review of Psychology, 70, 111–139. doi: 10.1146/annurev-psych-122216-011732 [DOI] [PubMed] [Google Scholar]
- Eyles DW (2021). How do established developmental risk-factors for schizophrenia change the way the brain develops? Transl Psychiatry, 11(1), 158. doi: 10.1038/s41398-021-01273-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon P (2018). Reimagining the environment in developmental psychopathology: from molecules to effective interventions. Journal of Child Psychology and Psychiatry and Allied Disciplines, 59(4), 299–302. doi: 10.1111/jcpp.12904 [DOI] [PubMed] [Google Scholar]
- Fenesy MC, Teh SE, & Lee SS (2019). Negative Parenting Moderates the Prospective Association of ADHD Symptoms and Youth Social Problems. Journal of Abnormal Child Psychology, 47(10), 1583–1597. doi: 10.1007/s10802-019-00542-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fond G, Lançon C, Korchia T, Auquier P, & Boyer L (2020). The Role of Inflammation in the Treatment of Schizophrenia. Front Psychiatry, 11, 160. doi: 10.3389/fpsyt.2020.00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Mieres H, Niño-Robles N, Ochoa S, & Feixas G (2019). Exploring identity and personal meanings in psychosis using the repertory grid technique: A systematic review. Clinical Psychology & Psychotherapy, 26(6), 717–733. doi: 10.1002/cpp.2394 [DOI] [PubMed] [Google Scholar]
- Gardea-Resendez M, Kucuker MU, Blacker CJ, Ho AM, Croarkin PE, Frye MA, & Veldic M (2020). Dissecting the Epigenetic Changes Induced by Non-Antipsychotic Mood Stabilizers on Schizophrenia and Affective Disorders: A Systematic Review. Frontiers in Pharmacology, 11, 467. doi: 10.3389/fphar.2020.00467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg E, Chen L, Nguyen TTT, Pokhvisneva I, Chen LM, Unternaehrer E, … O’Donnell KJ (2018). The early care environment and DNA methylome variation in childhood. Development and Psychopathology, 30(3), 891–903. doi: 10.1017/s0954579418000627 [DOI] [PubMed] [Google Scholar]
- Goldsmith DR, & Rapaport MH (2020). Inflammation and Negative Symptoms of Schizophrenia: Implications for Reward Processing and Motivational Deficits. Front Psychiatry, 11, 46. doi: 10.3389/fpsyt.2020.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb G (1998). Normally occurring environmental and behavioral influences on gene activity: from central dogma to probabilistic epigenesis. Psychological Review, 105(4), 792–802. doi: 10.1037/0033-295x.105.4.792-802 [DOI] [PubMed] [Google Scholar]
- Gottlieb G (2007). Probabilistic epigenesis. Dev Sci, 10(1), 1–11. doi: 10.1111/j.1467-7687.2007.00556.x [DOI] [PubMed] [Google Scholar]
- Goud Alladi C, Etain B, Bellivier F, & Marie-Claire C (2018). DNA Methylation as a Biomarker of Treatment Response Variability in Serious Mental Illnesses: A Systematic Review Focused on Bipolar Disorder, Schizophrenia, and Major Depressive Disorder. International Journal of Molecular Sciences, 19(10). doi: 10.3390/ijms19103026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, & Landrigan PJ (2014). Neurobehavioural effects of developmental toxicity. Lancet Neurology, 13(3), 330–338. doi: 10.1016/s1474-4422(13)70278-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granic I (2005). Timing is everything: Developmental psychopathology from a dynamic systems perspective. Developmental Review, 25(3), 386–407. doi: 10.1016/j.dr.2005.10.005 [DOI] [Google Scholar]
- Granic I, Hollenstein T, & Lichtwarck-Aschoff A (2016). A Survey of Dynamic Systems Methods for Developmental Psychopathology. In Developmental Psychopathology (pp. 1–43). [Google Scholar]
- Guidi J, Lucente M, Sonino N, & Fava GA (2021). Allostatic Load and Its Impact on Health: A Systematic Review. Psychotherapy and Psychosomatics, 90(1), 11–27. doi: 10.1159/000510696 [DOI] [PubMed] [Google Scholar]
- Gustafsson HC, Sullivan EL, Battison EAJ, Holton KF, Graham AM, Karalunas SL, … Nigg JT (2020). Evaluation of maternal inflammation as a marker of future offspring ADHD symptoms: A prospective investigation. Brain Behav Immun, 89, 350–356. doi: 10.1016/j.bbi.2020.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C (2018). Risk Factors for Depression: An Autobiographical Review. Annual Review of Clinical Psychology, 14, 1–28. doi: 10.1146/annurev-clinpsy-050817-084811 [DOI] [PubMed] [Google Scholar]
- Harrington A (2019). Mind fixers: psychiatry’s troubled search for the biology of mental illness. . New York: WW Norton & Co. [Google Scholar]
- Haslam N, Holland E, & Kuppens P (2012). Categories versus dimensions in personality and psychopathology: a quantitative review of taxometric research. Psychological Medicine, 42(5), 903–920. doi: 10.1017/s0033291711001966 [DOI] [PubMed] [Google Scholar]
- Henry J, Dionne G, Viding E, Vitaro F, Brendgen M, Tremblay RE, & Boivin M (2018). Early warm-rewarding parenting moderates the genetic contributions to callous-unemotional traits in childhood. Journal of Child Psychology and Psychiatry and Allied Disciplines, 59(12), 1282–1288. doi: 10.1111/jcpp.12918 [DOI] [PubMed] [Google Scholar]
- Hinshaw SP, & Arnold LE (2015). Attention-deficit hyperactivity disorder, multimodal treatment, and longitudinal outcome: evidence, paradox, and challenge. Wiley Interdisciplinary Reviews: Cognitive Science, 6(1), 39–52. doi: 10.1002/wcs.1324 [DOI] [PubMed] [Google Scholar]
- Homberg JR, Kyzar EJ, Scattoni ML, Norton WH, Pittman J, Gaikwad S, … Kalueff AV (2016). Genetic and environmental modulation of neurodevelopmental disorders: Translational insights from labs to beds. Brain Research Bulletin, 125, 79–91. doi: 10.1016/j.brainresbull.2016.04.015 [DOI] [PubMed] [Google Scholar]
- Hudson RP (1993). Concepts of Disease in the West. In Kiple KF (Ed.), The Cambridge World History of Human Disease (pp. 43–52). Cambridge: Cambridge University Press. [Google Scholar]
- Hyman SE (2010). The diagnosis of mental disorders: the problem of reification. Annual Review of Clinical Psychology, 6, 155–179. doi: 10.1146/annurev.clinpsy.3.022806.091532 [DOI] [PubMed] [Google Scholar]
- Hyman SE (2018). The daunting polygenicity of mental illness: making a new map. Philosophical Transactions of the Royal Society B: Biological Sciences, 373(1742), 20170031. doi:doi: 10.1098/rstb.2017.0031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izard CE (1993). Four systems for emotion activation: cognitive and noncognitive processes. Psychological Review, 100(1), 68–90. doi: 10.1037/0033-295x.100.1.68 [DOI] [PubMed] [Google Scholar]
- Izvolskaia M, Sharova V, & Zakharova L (2020). Perinatal Inflammation Reprograms Neuroendocrine, Immune, and Reproductive Functions: Profile of Cytokine Biomarkers. Inflammation. doi: 10.1007/s10753-020-01220-1 [DOI] [PubMed] [Google Scholar]
- Jones PJ, Ma R, & McNally RJ (2019). Bridge Centrality: A Network Approach to Understanding Comorbidity. Multivariate Behav Res, 1–15. doi: 10.1080/00273171.2019.1614898 [DOI] [PubMed] [Google Scholar]
- Kalisch R, Cramer AOJ, Binder H, Fritz J, Leertouwer I, Lunansky G, … van Harmelen AL (2019). Deconstructing and Reconstructing Resilience: A Dynamic Network Approach. Perspectives on Psychological Science, 14(5), 765–777. doi: 10.1177/1745691619855637 [DOI] [PubMed] [Google Scholar]
- Kaliush PR, Gao MM, Vlisides-Henry RD, Thomas LR, Butner JE, Conradt E, & Crowell SE (2020). Perinatal foundations of personality pathology from a dynamical systems perspective. Curr Opin Psychol, 37, 121–128. doi: 10.1016/j.copsyc.2020.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER (2018). The Disordered Mind: What unusual brains tell us about ourselves. . New York: Farrar, Straus, & Giroux. [Google Scholar]
- Kapur S, Phillips AG, & Insel TR (2012). Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Molecular Psychiatry, 17(12), 1174–1179. doi: 10.1038/mp.2012.105 [DOI] [PubMed] [Google Scholar]
- Kendler KS (2008). Explanatory models for psychiatric illness. American Journal of Psychiatry, 165(6), 695–702. doi: 10.1176/appi.ajp.2008.07071061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Zachar P, & Craver C (2011). What kinds of things are psychiatric disorders? Psychological Medicine, 41(6), 1143–1150. doi: 10.1017/s0033291710001844 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, & Walters EE (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62(6), 593–602. doi: 10.1001/archpsyc.62.6.593 [DOI] [PubMed] [Google Scholar]
- Kloiber S, Rosenblat JD, Husain MI, Ortiz A, Berk M, Quevedo J, … Carvalho AF (2020). Neurodevelopmental pathways in bipolar disorder. Neuroscience and Biobehavioral Reviews, 112, 213–226. doi: 10.1016/j.neubiorev.2020.02.005 [DOI] [PubMed] [Google Scholar]
- Kong A, Thorleifsson G, Frigge ML, Vilhjalmsson BJ, Young AI, Thorgeirsson TE, … Stefansson K (2018). The nature of nurture: Effects of parental genotypes. Science, 359(6374), 424–428. doi: 10.1126/science.aan6877 [DOI] [PubMed] [Google Scholar]
- Kotov R, Krueger RF, Watson D, Achenbach TM, Althoff RR, Bagby RM, … Zimmerman M (2017). The Hierarchical Taxonomy of Psychopathology (HiTOP): A dimensional alternative to traditional nosologies. Journal of Abnormal Psychology, 126(4), 454–477. doi: 10.1037/abn0000258 [DOI] [PubMed] [Google Scholar]
- Kotov R, Krueger RF, Watson D, Cicero DC, Conway CC, DeYoung CG, … Wright AGC (2021). The Hierarchical Taxonomy of Psychopathology (HiTOP): A Quantitative Nosology Based on Consensus of Evidence. Annual Review of Clinical Psychology. doi: 10.1146/annurev-clinpsy-081219-093304 [DOI] [PubMed] [Google Scholar]
- Kuranova A, Booij SH, Menne-Lothmann C, Decoster J, van Winkel R, Delespaul P, … Wichers M (2020). Measuring resilience prospectively as the speed of affect recovery in daily life: a complex systems perspective on mental health. BMC Medicine, 18(1), 36. doi: 10.1186/s12916-020-1500-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappalainen T, & Greally JM (2017). Associating cellular epigenetic models with human phenotypes. Nat Rev Genet, 18(7), 441–451. doi: 10.1038/nrg.2017.32 [DOI] [PubMed] [Google Scholar]
- LeDoux JE, & Pine DS (2016). Using Neuroscience to Help Understand Fear and Anxiety: A Two-System Framework. American Journal of Psychiatry, 173(11), 1083–1093. doi: 10.1176/appi.ajp.2016.16030353 [DOI] [PubMed] [Google Scholar]
- Lester BM, Conradt E, LaGasse LL, Tronick EZ, Padbury JF, & Marsit CJ (2018). Epigenetic Programming by Maternal Behavior in the Human Infant. Pediatrics, 142(4). doi: 10.1542/peds.2017-1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Santpere G, Imamura Kawasawa Y, Evgrafov OV, Gulden FO, Pochareddy S, … Sestan N (2018). Integrative functional genomic analysis of human brain development and neuropsychiatric risks. Science, 362(6420). doi: 10.1126/science.aat7615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Wang L, Camilleri JA, Chen X, Li S, Stewart JL, … Feng C (2020). Mapping common grey matter volume deviation across child and adolescent psychiatric disorders. Neuroscience and Biobehavioral Reviews, 115, 273–284. doi: 10.1016/j.neubiorev.2020.05.015 [DOI] [PubMed] [Google Scholar]
- Lichtwarck-Aschoff A, Kunnen SE, & van Geert PL (2009). Here we go again: a dynamic systems perspective on emotional rigidity across parent-adolescent conflicts. Developmental Psychology, 45(5), 1364–1375. doi: 10.1037/a0016713 [DOI] [PubMed] [Google Scholar]
- Lissemore JI, Sookman D, Gravel P, Berney A, Barsoum A, Diksic M, … Benkelfat C (2018). Brain serotonin synthesis capacity in obsessive-compulsive disorder: effects of cognitive behavioral therapy and sertraline. Transl Psychiatry, 8(1), 82. doi: 10.1038/s41398-018-0128-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansur RB, Lee Y, McIntyre RS, & Brietzke E (2020). What is bipolar disorder? A disease model of dysregulated energy expenditure. Neuroscience and Biobehavioral Reviews, 113, 529–545. doi: 10.1016/j.neubiorev.2020.04.006 [DOI] [PubMed] [Google Scholar]
- Martel MM (2013). Sexual selection and sex differences in the prevalence of childhood externalizing and adolescent internalizing disorders. Psychological Bulletin, 139(6), 1221–1259. doi: 10.1037/a0032247 [DOI] [PubMed] [Google Scholar]
- Mascolo MF, van Geert P, Steenbeek H, & Fischer KW (2016). What Can Dynamic Systems Models of Development Offer to the Study of Developmental Psychopathology? In Developmental Psychopathology (pp. 1–52). [Google Scholar]
- Matsuda Y, Makinodan M, Morimoto T, & Kishimoto T (2019). Neural changes following cognitive remediation therapy for schizophrenia. Psychiatry and Clinical Neurosciences, 73(11), 676–684. doi: 10.1111/pcn.12912 [DOI] [PubMed] [Google Scholar]
- McNally RJ (2011). What Is Mental Illness? : Harvard University Press. [Google Scholar]
- McRae K, & Gross JJ (2020). Emotion regulation. Emotion, 20(1), 1–9. doi: 10.1037/emo0000703 [DOI] [PubMed] [Google Scholar]
- Meaney MJ (2010). Epigenetics and the biological definition of gene x environment interactions. Child Development, 81(1), 41–79. doi: 10.1111/j.1467-8624.2009.01381.x [DOI] [PubMed] [Google Scholar]
- Meijer M, Klein M, Hannon E, van der Meer D, Hartman C, Oosterlaan J, … Franke B (2020). Genome-Wide DNA Methylation Patterns in Persistent Attention-Deficit/Hyperactivity Disorder and in Association With Impulsive and Callous Traits. Front Genet, 11, 16. doi: 10.3389/fgene.2020.00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GA (1996). How we think about cognition, emotion, and biology in psychopathology. Psychophysiology, 33(6), 615–628. doi: 10.1111/j.1469-8986.1996.tb02356.x [DOI] [PubMed] [Google Scholar]
- Miller GA, & Bartholomew ME (2020). Challenges in the Relationships between Psychological and Biological Phenomena in Psychopathology. In Parnas J, Kendler KS, & Zachar P (Eds.), Levels of Analysis in Psychopathology: Cross-Disciplinary Perspectives (pp. 238–266). Cambridge: Cambridge University Press. [Google Scholar]
- Monk C, Lugo-Candelas C, & Trumpff C (2019). Prenatal Developmental Origins of Future Psychopathology: Mechanisms and Pathways. Annual Review of Clinical Psychology, 15, 317–344. doi: 10.1146/annurev-clinpsy-050718-095539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney MA, Bhatt P, Hermosillo RJM, Ryabinin P, Nikolas M, Faraone SV, … Nigg JT (2020). Smaller total brain volume but not subcortical structure volume related to common genetic risk for ADHD. Psychological Medicine, 1–10. doi: 10.1017/s0033291719004148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney MA, Hermosillo RJM, Feczko E, Miranda-Dominguez O, Moore L, Perrone A, … Fair DA (2021. under review). Cumulative effects of resting-state connectivity across all brain networks significantly correlate with ADHD symptoms. [DOI] [PMC free article] [PubMed]
- Moore DS (2015). The developing genome: An introduction to behavioral epigenetics. New York: Oxford University Press. [Google Scholar]
- Morgan JE, Lee SS, Mahrer NE, Guardino CM, Davis EP, Shalowitz MU, … Dunkel Schetter C (2020). Prenatal maternal C-reactive protein prospectively predicts child executive functioning at ages 4–6 years. Developmental Psychobiology. doi: 10.1002/dev.21982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder RH, Neumann A, Cecil CAM, Walton E, Houtepen LC, Simpkin AJ, … Suderman M (2021). Epigenome-wide change and variation in DNA methylation in childhood: trajectories from birth to late adolescence. Hum Mol Genet, 30(1), 119–134. doi: 10.1093/hmg/ddaa280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na KS, Jung HY, & Kim YK (2014). The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 48, 277–286. doi: 10.1016/j.pnpbp.2012.10.022 [DOI] [PubMed] [Google Scholar]
- Nayak D, Roth TL, & McGavern DB (2014). Microglia development and function. Annual Review of Immunology, 32, 367–402. doi: 10.1146/annurev-immunol-032713-120240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neighbors C, Tomkins MM, Lembo Riggs J, Angosta J, & Weinstein AP (2019). Cognitive factors and addiction. Curr Opin Psychol, 30, 128–133. doi: 10.1016/j.copsyc.2019.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson B, McGorry PD, Wichers M, Wigman JTW, & Hartmann JA (2017). Moving From Static to Dynamic Models of the Onset of Mental Disorder: A Review. JAMA Psychiatry, 74(5), 528–534. doi: 10.1001/jamapsychiatry.2017.0001 [DOI] [PubMed] [Google Scholar]
- Neumann A, Walton E, Alemany S, Cecil C, González JR, Jima DD, … Tiemeier H (2020). Association between DNA methylation and ADHD symptoms from birth to school age: a prospective meta-analysis. Transl Psychiatry, 10(1), 398. doi: 10.1038/s41398-020-01058-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman MG, Llera SJ, Erickson TM, Przeworski A, & Castonguay LG (2013). Worry and generalized anxiety disorder: a review and theoretical synthesis of evidence on nature, etiology, mechanisms, and treatment. Annual Review of Clinical Psychology, 9, 275–297. doi: 10.1146/annurev-clinpsy-050212-185544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson DJ (2012). The concept of mechanism in biology. Studies in History and Philosophy of Biological and Biomedical Sciences, 43(1), 152–163. doi: 10.1016/j.shpsc.2011.05.014 [DOI] [PubMed] [Google Scholar]
- Nigg JT (2017). Annual Research Review: On the relations among self-regulation, self-control, executive functioning, effortful control, cognitive control, impulsivity, risk-taking, and inhibition for developmental psychopathology. J Child Psychol Psychiatry, 58(4), 361–383. doi: 10.1111/jcpp.12675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Elmore AL, Natarajan N, Friderici KH, & Nikolas MA (2016). Variation in an Iron Metabolism Gene Moderates the Association Between Blood Lead Levels and Attention-Deficit/Hyperactivity Disorder in Children. Psychological Science, 27(2), 257–269. doi: 10.1177/0956797615618365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Karalunas SL, Feczko E, & Fair DA (2020). Toward a Revised Nosology for Attention-Deficit/Hyperactivity Disorder Heterogeneity. Biol Psychiatry Cogn Neurosci Neuroimaging. doi: 10.1016/j.bpsc.2020.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Sibley MH, Thapar A, & Karalunas SL (2020). Development of ADHD: Etiology, Heterogeneity, and Early Life Course. Annual Review of Developmental Psychology, 2(1), 559–583. doi: 10.1146/annurev-devpsych-060320-093413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor N, Cardenas A, Rifas-Shiman SL, Pan H, Dreyfuss JM, Oken E, … Isganaitis E (2019). Association of Periconception Paternal Body Mass Index With Persistent Changes in DNA Methylation of Offspring in Childhood. JAMA Netw Open, 2(12), e1916777. doi: 10.1001/jamanetworkopen.2019.16777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell KJ, & Meaney MJ (2020). Epigenetics, Development, and Psychopathology. Annual Review of Clinical Psychology, 16, 327–350. doi: 10.1146/annurev-clinpsy-050718-095530 [DOI] [PubMed] [Google Scholar]
- Oldenburg KS, O’Shea TM, & Fry RC (2020). Genetic and epigenetic factors and early life inflammation as predictors of neurodevelopmental outcomes. Seminars in Fetal & Neonatal Medicine, 101115. doi: 10.1016/j.siny.2020.101115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim JJ (2018). The Future of the Cytokine Discipline. Cold Spring Harbor Perspectives in Biology, 10(9). doi: 10.1101/cshperspect.a028498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund BD, Vlisides-Henry RD, Crowell SE, Raby KL, Terrell S, Brown MA, … Conradt E (2019). Intergenerational transmission of emotion dysregulation: Part II. Developmental origins of newborn neurobehavior. Development and Psychopathology, 31(3), 833–846. doi: 10.1017/s0954579419000440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palego L, Giannaccini G, & Betti L (2020). Neuroendocrine Response to Psychosocial Stressors, Inflammation Mediators and Brain-Periphery Pathways of Adaptation. Central Nervous System Agents in Medicinal Chemistry. doi: 10.2174/1871524920999201214231243 [DOI] [PubMed] [Google Scholar]
- Petersen SE, & Posner MI (2012). The attention system of the human brain: 20 years after. Annual Review of Neuroscience, 35, 73–89. doi: 10.1146/annurev-neuro-062111-150525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzschner FH, Garfinkel SN, Paulus MP, Koch C, & Khalsa SS (2021). Computational Models of Interoception and Body Regulation. Trends in Neurosciences, 44(1), 63–76. doi: 10.1016/j.tins.2020.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plana-Ripoll O, Pedersen CB, Holtz Y, Benros ME, Dalsgaard S, de Jonge P, … McGrath JJ (2019). Exploring Comorbidity Within Mental Disorders Among a Danish National Population. JAMA Psychiatry, 76(3), 259–270. doi: 10.1001/jamapsychiatry.2018.3658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porto PR, Oliveira L, Mari J, Volchan E, Figueira I, & Ventura P (2009). Does cognitive behavioral therapy change the brain? A systematic review of neuroimaging in anxiety disorders. Journal of Neuropsychiatry and Clinical Neurosciences, 21(2), 114–125. doi: 10.1176/jnp.2009.21.2.114 [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK, & Voelker P (2016). Developing brain networks of attention. Curr Opin Pediatr, 28(6), 720–724. doi: 10.1097/mop.0000000000000413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pries LK, Gülöksüz S, & Kenis G (2017). DNA Methylation in Schizophrenia. Advances in Experimental Medicine and Biology, 978, 211–236. doi: 10.1007/978-3-319-53889-1_12 [DOI] [PubMed] [Google Scholar]
- Purcell S (2002). Variance components models for gene-environment interaction in twin analysis. Twin Research, 5(6), 554–571. doi: 10.1375/136905202762342026 [DOI] [PubMed] [Google Scholar]
- Rachman SJ (2016). Invited essay: Cognitive influences on the psychological immune system. Journal of Behavior Therapy and Experimental Psychiatry, 53, 2–8. doi: 10.1016/j.jbtep.2016.03.015 [DOI] [PubMed] [Google Scholar]
- Radonjić NV, Hess JL, Rovira P, Andreassen O, Buitelaar JK, Ching CRK, … Faraone SV (2021). Structural brain imaging studies offer clues about the effects of the shared genetic etiology among neuropsychiatric disorders. Mol Psychiatry, 26(6), 2101–2110. doi: 10.1038/s41380-020-01002-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rager JE, Bangma J, Carberry C, Chao A, Grossman J, Lu K, … Fry RC (2020). Review of the environmental prenatal exposome and its relationship to maternal and fetal health. Reproductive Toxicology. doi: 10.1016/j.reprotox.2020.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas-Fitó N, Cardo E, Sala M, Eulàlia de Muga M, Mazón C, Verdú A, … Sunyer J (2003). Breastfeeding, exposure to organochlorine compounds, and neurodevelopment in infants. Pediatrics, 111(5 Pt 1), e580–585. doi: 10.1542/peds.111.5.e580 [DOI] [PubMed] [Google Scholar]
- Richetto J, & Meyer U (2020). Epigenetic Modifications in Schizophrenia and Related Disorders: Molecular Scars of Environmental Exposures and Source of Phenotypic Variability. Biological Psychiatry. doi: 10.1016/j.biopsych.2020.03.008 [DOI] [PubMed] [Google Scholar]
- Riglin L, Leppert B, Dardani C, Thapar AK, Rice F, O’Donovan MC, … Thapar A (2020). ADHD and depression: investigating a causal explanation. Psychol Med, 1–8. doi: 10.1017/s0033291720000665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salsman JM, Schalet BD, Park CL, George L, Steger MF, Hahn EA, … Cella D (2020). Assessing meaning & purpose in life: development and validation of an item bank and short forms for the NIH PROMIS(®). Quality of Life Research. doi: 10.1007/s11136-020-02489-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner KF (2020). Approaches to Multilevel Models of Fear: The What, Where, Why, How, and How Much? In Parnas J, Kendler KS, & Zachar P (Eds.), Levels of Analysis in Psychopathology: Cross-Disciplinary Perspectives (pp. 384–409). Cambridge: Cambridge University Press. [Google Scholar]
- Scheffler R, & Hinshaw SP (2014). The ADHD Explosion and today’s push for performance: Myths, medication, and money. New York: Oxford University Press. [Google Scholar]
- Schiepek GK, Heinzel S, Karch S, Plöderl M, & Strunk G (2016, 2016//). Synergetics in Psychology: Patterns and Pattern Transitions in Human Change Processes. Paper presented at the Selforganization in Complex Systems: The Past, Present, and Future of Synergetics, Cham. [Google Scholar]
- Schiepek GK, Tominschek I, & Heinzel S (2014). Self-organization in psychotherapy: testing the synergetic model of change processes. Frontiers in Psychology, 5, 1089. doi: 10.3389/fpsyg.2014.01089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MJ, & Mirnics K (2015). Neurodevelopment, GABA system dysfunction, and schizophrenia. Neuropsychopharmacology, 40(1), 190–206. doi: 10.1038/npp.2014.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Lewinsohn PM, Klein DN, Small JW, Seeley JR, & Altman SE (2009). Subthreshold conditions as precursors for full syndrome disorders: a 15-year longitudinal study of multiple diagnostic classes. Journal of child psychology and psychiatry, and allied disciplines, 50(12), 1485–1494. doi: 10.1111/j.1469-7610.2009.02117.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppes G, Suri G, & Gross JJ (2015). Emotion regulation and psychopathology. Annual Review of Clinical Psychology, 11, 379–405. doi: 10.1146/annurev-clinpsy-032814-112739 [DOI] [PubMed] [Google Scholar]
- Smigielski L, Jagannath V, Rössler W, Walitza S, & Grünblatt E (2020). Epigenetic mechanisms in schizophrenia and other psychotic disorders: a systematic review of empirical human findings. Molecular Psychiatry. doi: 10.1038/s41380-019-0601-3 [DOI] [PubMed] [Google Scholar]
- Stevenson AJ, McCartney DL, Hillary RF, Campbell A, Morris SW, Bermingham ML, … Marioni RE (2020). Characterisation of an inflammation-related epigenetic score and its association with cognitive ability. Clinical Epigenetics, 12(1), 113. doi: 10.1186/s13148-020-00903-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez A, Lahti J, Lahti-Pulkkinen M, Girchenko P, Czamara D, Arloth J, … Räikkönen K (2020). A polyepigenetic glucocorticoid exposure score at birth and childhood mental and behavioral disorders. Neurobiol Stress, 13, 100275. doi: 10.1016/j.ynstr.2020.100275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamir M (2021). Effortful Emotion Regulation as a Unique Form of Cybernetic Control. Perspectives on Psychological Science, 16(1), 94–117. doi: 10.1177/1745691620922199 [DOI] [PubMed] [Google Scholar]
- Tay PKC, & Lim KK (2020). Psychological Resilience as an Emergent Characteristic for Well-Being: A Pragmatic View. Gerontology, 66(5), 476–483. doi: 10.1159/000509210 [DOI] [PubMed] [Google Scholar]
- Teachman BA, Clerkin EM, Cunningham WA, Dreyer-Oren S, & Werntz A (2019). Implicit Cognition and Psychopathology: Looking Back and Looking Forward. Annual Review of Clinical Psychology, 15, 123–148. doi: 10.1146/annurev-clinpsy-050718-095718 [DOI] [PubMed] [Google Scholar]
- Thapar A, & Rice F (2020). Family-Based Designs that Disentangle Inherited Factors from Pre- and Postnatal Environmental Exposures: In Vitro Fertilization, Discordant Sibling Pairs, Maternal versus Paternal Comparisons, and Adoption Designs. Cold Spring Harbor Perspectives in Medicine. doi: 10.1101/cshperspect.a038877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JG, & Sharp PB (2019). Mechanistic Science: A New Approach to Comprehensive Psychopathology Research That Relates Psychological and Biological Phenomena. Clinical Psychological Science, 7(2), 196–215. doi: 10.1177/2167702618810223 [DOI] [Google Scholar]
- Thompson JR, Gustafsson HC, DeCapo M, Takahashi DL, Bagley JL, Dean TA, … Sullivan EL (2018). Maternal Diet, Metabolic State, and Inflammatory Response Exert Unique and Long-Lasting Influences on Offspring Behavior in Non-Human Primates. Frontiers in Endocrinology, 9, 161. doi: 10.3389/fendo.2018.00161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H, Zmora N, Adolph TE, & Elinav E (2020). The intestinal microbiota fuelling metabolic inflammation. Nature Reviews: Immunology, 20(1), 40–54. doi: 10.1038/s41577-019-0198-4 [DOI] [PubMed] [Google Scholar]
- Trentacosta CJ, Waller R, Neiderhiser JM, Shaw DS, Natsuaki MN, Ganiban JM, … Hyde LW (2019). Callous-Unemotional Behaviors and Harsh Parenting: Reciprocal Associations across Early Childhood and Moderation by Inherited Risk. Journal of Abnormal Child Psychology, 47(5), 811–823. doi: 10.1007/s10802-018-0482-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trope Y, & Liberman N (2003). Temporal construal. Psychological Review, 110(3), 403–421. doi: 10.1037/0033-295x.110.3.403 [DOI] [PubMed] [Google Scholar]
- Tung I, & Lee SS (2017). Latent trajectories of adolescent antisocial behavior: Serotonin transporter linked polymorphic region (5-HTTLPR) genotype influences sensitivity to perceived parental support. Development and Psychopathology, 29(1), 185–201. doi: 10.1017/s0954579416000031 [DOI] [PubMed] [Google Scholar]
- Turkheimer E, Pettersson E, & Horn EE (2014). A phenotypic null hypothesis for the genetics of personality. Annual Review of Psychology, 65, 515–540. doi: 10.1146/annurev-psych-113011-143752 [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Garcia-Fernandez G, & Dom G (2019). Cognition and addiction Dialogues in Clinical Neuroscience, 21(3), 281–290. doi: 10.31887/DCNS.2019.21.3/gdom [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen R, Schymanski EL, Barabási AL, & Miller GW (2020). The exposome and health: Where chemistry meets biology. Science, 367(6476), 392–396. doi: 10.1126/science.aay3164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachs TD (1999). Celebrating complexity: Conceptualization and assessment of the environment. In Friedman SL & Wachs TD (Eds.), Measuring environment across the life span: Emerging methods and concepts (pp. 357–392). Washington DC: American Psychological Association. [Google Scholar]
- Wachs TD, Georgieff M, Cusick S, & McEwen BS (2014). Issues in the timing of integrated early interventions: contributions from nutrition, neuroscience, and psychological research. Annals of the New York Academy of Sciences, 1308, 89–106. doi: 10.1111/nyas.12314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield JC (1992). The concept of mental disorder. On the boundary between biological facts and social values. American Psychologist, 47(3), 373–388. doi: 10.1037//0003-066x.47.3.373 [DOI] [PubMed] [Google Scholar]
- Wakefield JC (1999). Evolutionary versus prototype analyses of the concept of disorder. Journal of Abnormal Psychology, 108(3), 374–399. doi: 10.1037//0021-843x.108.3.374 [DOI] [PubMed] [Google Scholar]
- Waszczuk MA, Eaton NR, Krueger RF, Shackman AJ, Waldman ID, Zald DH, … Kotov R (2020). Redefining phenotypes to advance psychiatric genetics: Implications from hierarchical taxonomy of psychopathology. Journal of Abnormal Psychology, 129(2), 143–161. doi: 10.1037/abn0000486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheater ENW, Stoye DQ, Cox SR, Wardlaw JM, Drake AJ, Bastin ME, & Boardman JP (2020). DNA methylation and brain structure and function across the life course: A systematic review. Neuroscience and Biobehavioral Reviews, 113, 133–156. doi: 10.1016/j.neubiorev.2020.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJH (2019). Brain Development and Stochastic Processes During Prenatal and Early Life: You Can’t Lose It if You’ve Never Had It; But It’s Better to Have It and Lose It, Than Never to Have Had It at All. J Am Acad Child Adolesc Psychiatry, 58(11), 1042–1050. doi: 10.1016/j.jaac.2019.02.010 [DOI] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, … Vos T (2013). Global burden of disease attributable to mental and substance use disorders: Findings from the Global Burden of Disease Study 2010. Lancet, 382(9904), 1575–1586. doi: 10.1016/s0140-6736(13)61611-6 [DOI] [PubMed] [Google Scholar]
- Wichers M, Wigman JTW, & Myin-Germeys I (2015). Micro-Level Affect Dynamics in Psychopathology Viewed From Complex Dynamical System Theory. Emotion Review, 7(4), 362–367. doi: 10.1177/1754073915590623 [DOI] [Google Scholar]
- Wild CP (2005). Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev, 14(8), 1847–1850. doi: 10.1158/1055-9965.Epi-05-0456 [DOI] [PubMed] [Google Scholar]
- Wray NR, Wijmenga C, Sullivan PF, Yang J, & Visscher PM (2018). Common Disease Is More Complex Than Implied by the Core Gene Omnigenic Model. Cell, 173(7), 1573–1580. doi: 10.1016/j.cell.2018.05.051 [DOI] [PubMed] [Google Scholar]
- Wright-Jin EC, & Gutmann DH (2019). Microglia as Dynamic Cellular Mediators of Brain Function. Trends in Molecular Medicine, 25(11), 967–979. doi: 10.1016/j.molmed.2019.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier da Silveira dos Santos A, & Liberali P (2019). From single cells to tissue self-organization. The FEBS Journal, 286(8), 1495–1513. doi: 10.1111/febs.14694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao ZF, & Hsieh S (2019). Neurocognitive Mechanism of Human Resilience: A Conceptual Framework and Empirical Review. International Journal of Environmental Research and Public Health, 16(24). doi: 10.3390/ijerph16245123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino A, Okamoto Y, Okada G, Takamura M, Ichikawa N, Shibasaki C, … Yamawaki S (2018). Changes in resting-state brain networks after cognitive-behavioral therapy for chronic pain. Psychological Medicine, 48(7), 1148–1156. doi: 10.1017/s0033291717002598 [DOI] [PubMed] [Google Scholar]
- Ysseldyk R, McQuaid RJ, McInnis OA, Anisman H, & Matheson K (2018). The ties that bind: Ingroup ties are linked with diminished inflammatory immune responses and fewer mental health symptoms through less rumination. PloS One, 13(4), e0195237. doi: 10.1371/journal.pone.0195237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Palmer CE, Thompson WK, Jernigan TL, Dale AM, & Fan CC (2019). The Bayesian polyvertex score (PVS-B): a whole-brain phenotypic prediction framework for neuroimaging studies. bioRxiv, 813915. doi: 10.1101/813915 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


