Abstract
The cell type-specific expression of key transcription factors is central to development. Brachyury/T/TBXT is a major transcription factor for gastrulation, tailbud patterning, and notochord formation; however, how its expression is controlled in the mammalian notochord has remained elusive. Here, we identify the complement of notochord-specific enhancers in the mammalian Brachyury/T/TBXT gene. Using transgenic assays in zebrafish, axolotl, and mouse, we discover three Brachyury-controlling notochord enhancers T3, C, and I in human, mouse, and marsupial genomes. Acting as Brachyury-responsive, auto-regulatory shadow enhancers, deletion of all three enhancers in mouse abolishes Brachyury/T expression selectively in the notochord, causing specific trunk and neural tube defects without gastrulation or tailbud defects. Sequence and functional conservation of Brachyury-driving notochord enhancers with the brachyury/tbxtb loci from diverse lineages of fishes dates their origin to the last common ancestor of jawed vertebrates. Our data define the enhancers for Brachyury/T/TBXTB notochord expression as ancient mechanism in axis development.
INTRODUCTION
The defining feature of the chordate body plan is the notochord, a principal structure formed by the axial or chorda mesoderm that provides stability and rigidity along the body axis (Corallo et al., 2015; Stemple, 2005). As mammals form an ossified spine, their notochord progressively regresses and its remnants form the nucleus pulposus within the intervertebral discs (Bagnat and Gray, 2020; Peck et al., 2017; Risbud et al., 2010; Stosiek et al., 1988; Wang et al., 2018). Notochord precursors emerge from the initial organizer and form in a stereotypical rostral-to-caudal trajectory as gastrulation proceeds, manifesting among the earliest visible structures in chordate embryos (Satoh et al., 2012; Stemple, 2005). The deeply conserved T-box transcription factor gene Brachyury (also called T or TBXT) is a key regulator of notochord formation. Originally identified as dominant mutation T that caused short tails in mice, Brachyury expression and function has been linked to notochord emergence across chordates (Corbo et al., 1997; Dobrovolskaia-Zavadskaia, 1927; Halpern et al., 1993; Herrmann, 1995; Holland et al., 1995; Schulte-Merker et al., 1994; Smith et al., 1991). In addition to its central role in notochord fate specification, the function of vertebrate Brachyury is required for proper primitive streak formation, tailbud specification, and subsequent neuromesodermal progenitor control (Henrique et al., 2015; Martin and Kimelman, 2008; Rivera-Pérez and Magnuson, 2005). However, how the expression of this central developmental transcription factor is selectively regulated to achieve its notochord activity in mammals remains unresolved.
The central contribution of the notochord and the tailbud to different morphological adaptions and locomotion strategies shows in the diversification of axial structures across vertebrates (Schwaner et al., 2021). Gain and loss of gene copies and of their associated gene-regulatory elements are major drivers of evolutionary innovation, and the Brachyury gene family itself is a prime example of this process. Brachyury predates the origin of, and was present as, a single copy gene in the chordate ancestor (Inoue et al., 2017; Sebé-Pedrós et al., 2013). Following two whole genome duplications in early vertebrates and the subsequent loss of one of four Brachyury paralogs, three gene paralogs were present in the jawed vertebrate ancestor: Tbxta, Tbxtb, and Tbx19 (Inoue et al., 2017). Tbxta became subsequently lost within the tetrapod lineage, resulting in mammals and birds ultimately only retaining Tbxtb (commonly called Brachyury/T/TBXT in tetrapods including humans) (Amemiya et al., 2013). In contrast, ray-finned fishes retained both tbxta/ntla and tbxtb/ntlb, the latter being the ortholog of the remaining human Brachyury/T/TBXT (de facto TBXTB) gene (Martin and Kimelman, 2008).
Curiously, tbxta/ntla has become the predominant functional Brachyury/T/TBXT gene in zebrafish, as documented in classic mutants for ntla (no tail a) that fail to form a tail and notochord (Halpern et al., 1993; Schulte-Merker et al., 1994). While no mutant for zebrafish tbxtb/ntlb has been reported to date, morpholino-based knockdown studies indicate that tbxtb function adds minimally to the dominant role of zebrafish tbxta (Martin and Kimelman, 2008). This variable copy number of Brachyury genes across vertebrates came along with selection and divergence of regulatory elements controlling Brachyury gene expression during distinct developmental timepoints and cell types. Promoter-proximal regions of Brachyury in the Ciona Brachyury gene and in the zebrafish tbxta gene drive early organizer and notochord activity (Corbo et al., 1997; Harvey et al., 2010). In contrast, the promoter-proximal region called Tstreak of T/Tbxtb in mouse, human, and Xenopus has previously been found to drive primitive streak expression in response to canonical Wnt/beta-catenin signaling, yet lacks any notochord-driving activity (Arnold et al., 2000; Clements et al., 1996; Latinkić et al., 1997).
Notably, recent work documented that deleting a large 37 kb-spanning region upstream of mouse Brachyury/T/Tbxtb leads to mutant phenotypes consistent with a selective loss of Brachyury notochord expression (Schifferl et al., 2021). A small element termed TNE in the 37 kb interval was sufficient to drive specific notochord expression in mouse reporter assays, yet its deletion showed mild to no phenotypic consequences (Schifferl et al., 2021). These pioneering data show that additional regulatory element(s) in addition to TNE contribute to Brachyury/Tbxtb expression specifically in the notochord. Uncovering the cis-regulatory logic of vertebrate Brachyury notochord enhancer(s) will expand our understanding of the evolutionary history of this key developmental regulator and of the mechanisms leading to notochord formation. In particular, comparison to the Ciona Brachyury locus containing two upstream shadow enhancers with well-defined regulatory grammar (Farley et al., 2016; Song et al., 2023) may inform cis-regulatory adaptations at the onset of vertebrate emergence.
Uncovering the regulatory elements responsible for its notochord expression also promises to shed light onto the role of Brachyury in adult human spine health and in chordoma tumors, a rare sarcoma of the spine that is hypothesized to arise from notochord remnants (Nibu et al., 2013; Vujovic et al., 2006; Yakkioui et al., 2014). Native Brachyury-expressing cells in the nucleus pulposus decrease in number with age along with a concomitant increase in cartilage-like cells (Nakamichi and Asahara, 2020; Richardson et al., 2017; Risbud et al., 2010; Tang et al., 2012). What role these long-lasting Brachyury-positive cells play in the adult spine, if they progressively differentiate into cartilage, and how Brachyury gene activity is sustained, remain unknown. Detection of Brachyury protein is a diagnostic marker for chordoma (Vujovic et al., 2006), yet the functional contribution of its re-activated or persistent expression in the tumor remains uncertain (D’Agati et al., 2019; Hu et al., 2014; Presneau et al., 2011; Zhu et al., 2016). Several familial chordomas harbor duplications or further complex amplifications of the Brachyury/T/TBXTB locus that possibly convey chordoma susceptibility to carriers (Bhadra and Casey, 2006; Hsu et al., 2011; Yang et al., 2009). These findings suggest that chordoma-associated Brachyury/T/TBXTB locus amplifications contain, or hijack the action of, cis-regulatory elements possibly driving Brachyury/T/TBXTB expression in chordoma, potentially with Brachyury controlling its own expression as suggested by ChIP-seq findings (Nelson et al., 2012).
Here, we identify the complement of three auto-regulated shadow enhancers T3, C, and I in the Brachyury/T/Tbxtb locus that convey notochord activity. We combined i) genomic data from human chordoma tumor cell lines, human embryonic stem cells, and mouse embryonic stem cells; ii) non-coding element conservation across mammals (human, mouse, Monodelphis) and all vertebrates; iii) transgenic reporter assays in zebrafish, mouse, Axolotl, and Ciona; iv) and enhancer knockouts in mice. In triple-enhancer-knockout mice, we document the selective absence of Brachyury protein in the notochord and subsequent neural tube and trunk defects as linked to notochord perturbations. Using comparative genomics, we uncover the conservation of the enhancers T3, C, and I in the brachyury/tbxtb loci across jawed vertebrates. Our data uncover a deep conservation of shadow enhancers regulating Brachyury expression in the notochord, one of the most prominent developmental structures of the vertebrate body and involved in spine and neural tube defects.
RESULTS
Defining a minimal regulatory region for human Brachyury notochord expression
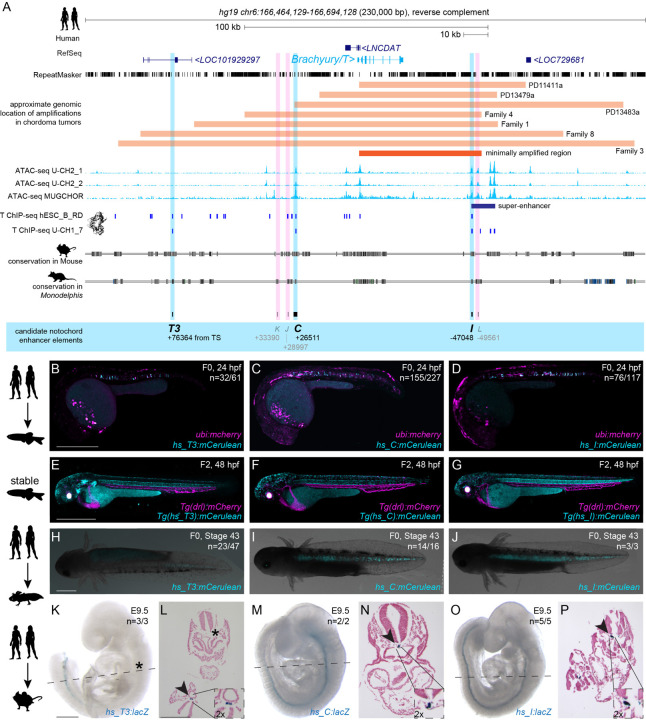
To identify enhancer elements with notochord activity in the human Brachyury/T/TBXTB locus, we analyzed the Brachyury/T/TBXTB locus to narrow down a minimally required genomic region around the Brachyury gene body. Familial and sporadic chordoma feature duplications and/or complex amplifications of Brachyury (Bhadra and Casey, 2006; Hsu et al., 2011; Tarpey et al., 2017; Yang et al., 2009), suggesting that essential cis-regulatory elements for notochord expression lie within the commonly amplified region. Available genomic patient data outlined a minimally amplified region of approximately 50 kb surrounding the human Brachyury gene body, with individual tumors extending their amplifications proximal or distal of this minimal region (Tarpey et al., 2017; Yang et al., 2009) (Fig. 1A). Within this minimal interval and its vicinity, we uncovered several regions that have been charted as open chromatin in the chordoma cell lines U-CH2 and MUGCHOR using ATAC-seq (Nelson et al., 2012; Sharifnia et al., 2019), indicating potential regulatory elements in accessible chromatin (Fig. 1A). These regions include a super-enhancer region previously proposed to be active in chordoma (Sharifnia et al., 2019) (Fig. 1A). Further, mammalian Brachyury has been postulated to control its own notochord expression, as underlined by the TNE enhancer that features putative T-box motifs (Beisaw et al., 2018; Schifferl et al., 2021). Using Brachyury/T ChIP-seq data from the human chordoma tumor cell line U-CH1 and human ES-derived mesoderm progenitor cells (Faial et al., 2015; Nelson et al., 2012), we found discrete Brachyury binding events within the minimal amplification interval and its vicinity (Fig. 1A). Genome alignment of human versus other mammalian species indicated candidate enhancer regions (conserved non-coding elements; CNEs) through non-coding sequence conservation in mouse and the more distant marsupial Monodelphis domestica (Mikkelsen et al., 2007) (Fig. 1A).
Figure 1: Human Brachyury enhancer elements T3, C and I are active in different species.
(A) Human Brachyury/T/TBXTB locus with surrounding gene loci adapted from UCSC genome browser. Repeats marked in black using the RepeatMasker track. Further annotated are approximate amplifications (light orange) and the minimally amplified region (dark orange) in chordoma tumors. ATAC-sequencing (light blue peaks) and T ChIP-sequencing (dark blue lines) suggest enhancer elements (light pink highlight, not active; light blue highlight, active) that are conserved in mouse and the marsupial Monodelphis domestica.
(B,C,D) Representative F0 zebrafish embryos injected with the human enhancer elements hs_T3 (B), hs_C (C), and hs_I (D) showing mosaic mCerulean reporter expression in the notochord at 24 hpf and expression of ubi:mCherry as injection control. N represents the number of animals expressing mCerulean in the notochord relative to the total number of animals expressing mCherry. Scale bar in B: 0.5 mm, applies to B-C.
(E,F,G) Representative images of stable transgenic F2 embryos at 48 hpf for each of the human enhancer elements hs_T3, hs_C, and hs_I crossed to Tg(drl:mCherry) that labels lateral plate mesoderm and later cardiovascular lineages. Transgenic F2 embryos recapitulate the F0 expression pattern in the notochord, with hs_T3 (E) additionally expressing cerulean in the pharyngeal arches and fin, and hs_I (G) in the proximal kidney close to the anal pore. Enhancer element hs_C (F) stable transgenic lines have lower relative notochord reporter activity than hs_T3 and hs_I. Scale bar in E: 0.5 mm, applies to E-G.
(H,I,J) Representative F0 axolotl embryos at peri-hatching stages expressing mCerulean from the human enhancers hs_T3 (G), hs_C (H), hs_I (I). N represent the number of animals expressing mCerulean in the notochord relative to the total number of animals showing any mCerulean expression. Scale bar in H: 1 mm, applies to H-J.
(K,M,O) Representative images of transgenic E9.5 mouse embryos expressing lacZ (encoding beta-galactosidase) under the human enhancers hs_T3 (K), hs_C (M), and hs_I (O) visualized with X-gal whole-mount staining. While hs_C and hs_I express beta-galactosidase in the entire notochord, beta-galactosidase expression from hs_T3 is restricted to the posterior notochord. Black asterisk marks absence of beta-galactosidase in the anterior notochord. N represent the number of animals expressing beta-galactosidase in the notochord relative to the total number of animals with tandem integrations at H11. Dotted lines represent the sectioning plane. Scale bar in K: 0.5 mm, applies to K,M,O.
(L,N,P) Representative images of Fast Red-stained cross sections from embryos shown on the left, hs_T3 (L), hs_C (N), and hs_I (P). Black arrowheads point at notochord, and inserts show notochords at 2x higher magnification. Scale bar in L: 0.25 mm, applies to L,N,P.
From our combined locus analysis, we identified the six initial candidates T3 (the human ortholog of mouse TNE) (Schifferl et al., 2021), K, J, C, I, and L as putative notochord enhancer elements in the vicinity of the human Brachyury gene (Fig. 1A, Supplemental Table 1; all Supplemental Data is included in the Supplemental Data Files archive). While K and J represent conserved sequence to other mammalian genomes, candidates I and L notably lie in the annotated chordoma super-enhancer region (Sharifnia et al., 2019). From this combined analysis, we hypothesized that individual or combined elements among the six enhancer candidates could convey notochord activity to the human Brachyury gene.
Distal enhancers in the human Brachyury locus have autonomous notochord activity
Given the evolutionarily conserved notochord expression of vertebrate Brachyury genes, we hypothesized that the human enhancers may be correctly interpreted in a model vertebrate. We initially tested all six enhancer candidates in zebrafish that allows for highly efficient reporter gene activity screening in developing embryos. To test their activity within a broad evolutionary framework, we cloned the human enhancer element candidates T3, K, J, C, I, and L into reporter vectors coupled with the mouse betaE-globin minimal promoter to express the blue fluorophore mCerulean for enhancer testing in zebrafish embryos (Kemmler et al., 2023). Upon co-injection into one cell-stage zebrafish embryos together with ubi:mCherry as injection control, the human hs_T3, hs_C, and hs_I elements resulted in mCerulean expression in the developing zebrafish notochord during early somitogenesis, followed by strong, selective notochord activity in injected embryos at 24 hours post-fertilization (hpf) (n=32/61, n=155/227, n=76/117; mCerulean-positive notochord/total mCherry-positive embryos) (Fig. 1B-D, Supplemental Table 2). Zebrafish embryos injected with hs_T3, hs_C, and hs_I reporters maintained notochord-specific mCerulean expression throughout our observations until 5 days post-fertilization (dpf). In contrast, we did not observe any specific mCerulean reporter expression at any timepoint with elements hs_K, hs_J, and hs_L (n= 0/68, n=0/63, n=0/254) (Supplemental Table 2). Notably, hs_C was still active when further trimming the sequence 5’ and 3’ (hs_Cshort, n=55/103) (Supplemental Fig. 1A-C, Supplemental Table 2). Germline-transmitted, stable transgenic integrations for mCerulean reporters based on hs_T3, hs_C, and hs_I recapitulated the transient reporter results and consistently showed selective notochord expression, with minimal variability across independent transgenic insertions for each enhancer reporter (followed to at least F3 generation) (Fig. 1E-G). Together, these data indicate that the three enhancer elements hs_T3, hs_C, and hs_I within the human Brachyury/T/TBXTB locus convey notochord activity when tested in zebrafish.
Next, we tested the activity of hs_T3, hs_C, and hs_I in axolotl (Ambystoma mexicanum) as a representative amphibian species (Nowoshilow et al., 2018; Prummel et al., 2019). Upon microinjection, reporters based on hs_T3, hs_C, and hs_I enhancer elements showed consistent reporter expression in the notochord of axolotl embryos (n=23/47, n=14/16, n=3/3) throughout tailbud stages (st. 30–41) and beyond (Fig. 1H-J, Supplemental Fig. 1D-M, Supplemental Table 2). Notably, 50% of hs_T3-positive F0 animals had additional expression in other mesodermal tissues such as trunk muscles. In contrast, 80% and 100% of positive hs_C and hs_I F0 animals, respectively, showed expression exclusively in the notochord. In addition, hs_C and hs_I reporter expression was distributed along the entire rostral-caudal axis in all observed embryos, while hs_T3 reporter expression was frequently restricted to more caudal portions of the notochord. Combined, these results indicate that the human enhancers hs_T3, hs_C, and hs_I also integrate regulatory input for driving notochord activity in amphibians.
We next tested if human enhancers hs_T3, hs_C and hs_I also drive notochord-specific reporter activity in mouse embryos. For increased specificity and reproducibility, we used a site-directed transgenic integration strategy at the H11 locus (enSERT) (Kvon et al., 2020) to generate mouse embryos harboring enhancer-LacZ reporter transgenes. As observed in zebrafish and axolotl, hs_T3, hs_C and hs_I elements exhibited specific and selective notochord expression in mouse embryos at E9.5 (n=3/3, n=2/2 and n=5/5) (Fig. 1 K,M,O, Supplemental Table 2). Of note, hs_T3 reporter activity appeared predominantly confined to the posterior notochord compared to hs_C or hs_I, which showed reporter activity in the entire mouse notochord. Histological analysis of Nuclear Fast Red-stained transversal sections from transgenic mouse embryos further confirmed reproducible, notochord-specific activity for human notochord enhancer elements hs_T3, hs_C, and hs_I (Fig. 1 L, N, P).
Taken together, we identified three enhancer candidates in the human Brachyury/T/TBXTB locus, including hs_T3 as ortholog of the mouse TNE element (Schifferl et al., 2021), that all display notochord enhancer activity as transgenic reporters when tested in teleost fish, amphibian, and rodent embryos, suggesting pan-bony vertebrate activity and function.
Dependence of human Brachyury enhancers on T-box motifs
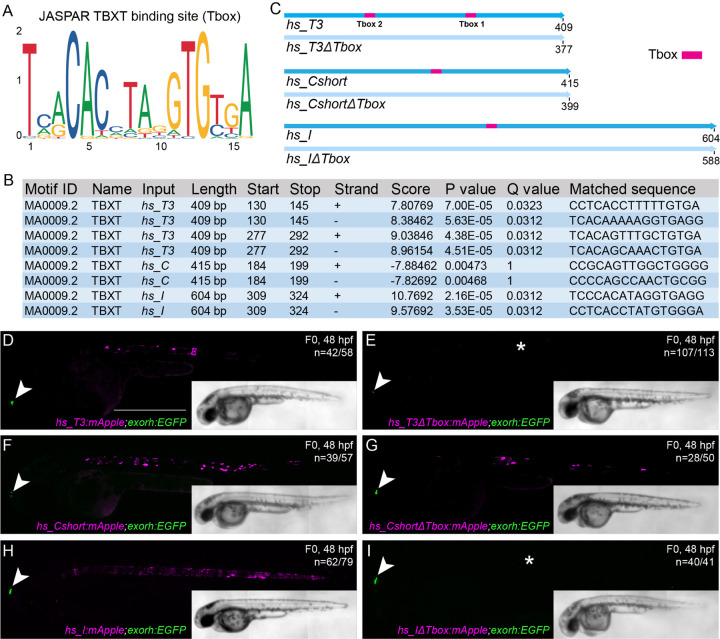
Published ChIP-seq data indicated Brachyury binding at hs_T3, hs_C, and hs_I (Fig 1A), suggesting that notochord expression of the Brachyury/T/Tbxtb gene might be auto-regulated by Brachyury itself (Beisaw et al., 2018; Schifferl et al., 2021). We investigated if the three human notochord enhancer elements contained a TBXT binding motif (short T-box, Fig. 2A) using FIMO (Grant et al., 2011): we found that enhancer element hs_T3 contained two low p-value T-box motifs, hs_I contained one low p-value T-box motif, while enhancer element hs_C contained a possibly degenerate T-box that we only identified when increasing the p-value (Fig. 2B). We then generated the reporter constructs hs_T3ΔTbox:mApple, hs_CshortΔTbox:mApple, and hs_IΔTbox:mApple, in which we deleted the respective T-boxes in the enhancer elements, as well as constructs containing the wildtype enhancer elements in an identical backbone (Fig. 2C). The reporter constructs further harbor the transgenesis marker exorh:EGFP (expression in the pineal gland, Fig. 2D-I) for precise quantification of reporter activity (Kemmler et al., 2023). After injection into zebrafish embryos and in line with the enhancer element activity at 24 hpf (Fig. 1B-D), we observed continued and reproducible notochord expression at 48 hpf with all three wildtype enhancer element reporters T3, C, and I (n=42/58, n=39/57 and n=62/79 (mCerulean-positive notochord/total EGFP pineal gland-positive embryos)) (Fig. 2D,F,H, Supplemental Table 2). However, we observed a complete loss of specific notochord reporter activity in zebrafish embryos injected with the deletion constructs hs_T3ΔTbox:mApple (n=6/113) and hs_IΔTbox:mApple (n=1/41), with positive embryos containing few labelled notochord cells (Fig. 2E,I). In contrast, hs_Cshort ΔTbox retained reporter activity in the notochord in injected zebrafish embryos with mosaic mApple expression throughout the notochord despite deletion of the T-box motif (n=28/50) (Fig. 2G, Supplemental Table 2).
Figure 2: Identified TBXT binding sites in the enhancer elements are essential for reporter activity.
(A) Sequence of the human TBXT binding site (T-box) using JASPAR.
(B) FIMO output with location of the T-box, statistical significance, and matched sequence within the enhancer elements.
(C) Schematic depiction of the wildtype human enhancer elements with the TBXT binding site/T-box (pink box) and the enhancer elements without the respective T-box sites (ΔTbox). All human enhancer elements are depicted in the reverse complement direction.
(D-I) Injection of the wildtype enhancer elements hs_T3 (D), hs_C (F), and hs_I (H) as reporter constructs results in mApple fluorophore expression in the notochord at 48 hpf, whereas injection of hs_T3ΔTbox (E) and hs_IΔTbox (I) show complete loss of notochord expression (asterisks in E,I) in comparison to hs_CshortΔTbox (G), which has residual reporter activity even without the T-box. Arrowheads (D-I) mark EGFP expression in the pineal gland from the transgenesis marker exorh:EGFP. Scale bar in D: 0.5 mm, applies to D-I.
Together, we conclude that the T-box elements in the notochord enhancers hs_T3 and hs_I are critical to the activity of these regulatory elements in reporter assays. In contrast, our mutagenesis of the most-obvious T-box motif in enhancer hs_C did not interfere with its notochord activity, suggesting either additional T-box motifs to be important for its reporter activity (Supplemental Fig. 2 new; need to edit following Supplemental Figs.) or that hs_C responds to other upstream input than Brachyury/T/TBXTB (Song et al., 2023). Nevertheless, these data support the model in which Brachyury/T/TBXTB auto-regulates its own expression in the notochord through select regulatory elements (Beisaw et al., 2018; Schifferl et al., 2021).
The human Brachyury enhancers are conserved across mammals
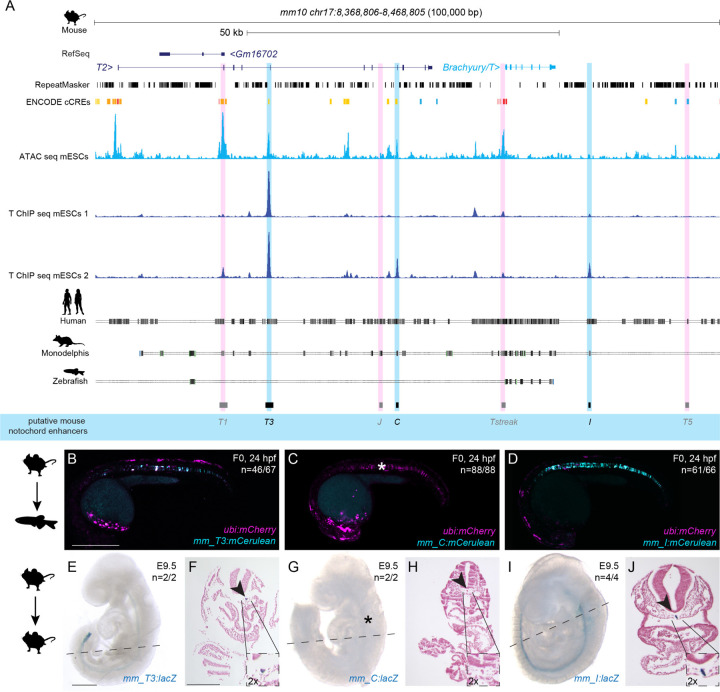
We next sought to determine if other mammalian genomes harbor orthologous T3, C, and I enhancer regions in their Brachyury/T/Tbxtb loci. Here, we focused on the orthologous T3, C, and I enhancer candidate regions from mouse (Fig. 3A). As in the human Brachyury/T/TBXTB locus, we found open chromatin and Brachyury protein binding events at the mouse orthologs of the putative enhancer elements T3, C, and I, as well as the well-characterized murine Brachyury/T/Tbxtb promoter Tstreak (Fig. 3A).
Figure 3: Mouse Brachyury enhancer elements are active in different species.
(A) Mouse Brachyury/T/TBXTB locus adapted from the UCSC genome browser. Repeats marked in black using the RepeatMasker track. Further annotated is the track with ENCODE conserved cis-regulatory elements (cCREs). ATAC-sequencing (light blue peaks) and T ChIP-sequencing (dark blue lines) indicate enhancer elements (light pink highlight, not active; light blue highlight, active) that are conserved in human and Monodelphis.
(B,C,D) Representative F0 zebrafish embryos injected with the mouse enhancer elements mm_T3 (B), mm_C (C), and mm_I (D). mm_T3 and mm_I show mosaic mCerulean reporter expression in the notochord at 24 hpf and mosaic ubi:mCherry expression as injection control. Mouse enhancer element mm_C is not active in the zebrafish notochord (asterisk in C). N represent the number of animals expressing mCerulean in the notochord relative to the total number of animals expressing mCherry. Scale bar in B: 0.5 mm, applies to B-D.
(E,G,I) Representative images of transgenic E9.5 mouse embryos expressing lacZ (encoding beta-galactosidase) under the mouse enhancer elements mm_T3 (E), mm_C (G) and mm_I (I) visualized with X-gal whole mount staining. While mm_T3 and mm_I express beta-galactosidase in the entire notochord, beta-galactosidase expression from mouse mm_C is absent (asterisk in G). N represent the number of animals expressing beta-galactosidase in the notochord relative to the total number of animals with tandem integrations at H11. Dotted lines represent the sectioning plane. Scale bar in E: 0.5 mm, applies to E,G,I.
(F,H,J) Representative images of Fast Red-stained cross sections from embryos shown on the left, mm_T3 (F), mm_C (H), and mm_I (J). Black arrowheads point at notochord, and inserts show notochords at 2x higher magnification. Scale bar in F: 0.25 mm, applies to F,H,J.
Mouse TNE has been established to act as autonomous notochord enhancer when tested in mouse embryos and gastruloid cultures (Schifferl et al., 2021). When tested in zebrafish, both mouse enhancer mm_T3/TNE and mm_I showed reporter activity emerging at the forming shield (Supplemental Fig. 3A-D) before expression in the developing notochord (n=46/67, n=61/66) at 24 hpf (Fig. 3B,D, Supplemental Table 2). In contrast, mouse enhancer mm_C failed to drive any reporter expression in the zebrafish notochord (n=0/88) (Fig. 3C, Supplemental Table 2). Imaging of transgenic zebrafish carrying mouse mm_I as stable reporter documented robust notochord expression, again with little variability across independent transgenic insertions (Supplemental Fig. 3E). The murine Brachyury/T/Tbxtb promoter region Tstreak (Arnold et al., 2000; Clements et al., 1996; Latinkić et al., 1997) showed transient, variable reporter expression in the zebrafish shield (around 6 hpf), with no reporter activity upon somitogenesis (n=79/102) (Supplemental Table 2). We further tested the mouse ortholog of enhancer candidate mm_J, as well as the two lesser conserved elements mm_T1 and mm_T5, none of which showed reporter activity in zebrafish embryos up to 5 dpf (n=0/98, n=0/98, n=0/79) (Supplemental Table 2).
Tested with site-directed reporter transgenesis at H11, mm_T3/TNE and mm_I conveyed specific notochord activity in mouse embryos at E9.5 (n=2/2, n=4/4) (Fig. 3E,G, Supplemental Table 2). In contrast, and consistent with our observations in zebrafish reporter assays, mm_C did not show any detectable reporter activity in the notochord in mouse embryos at E9.5 (n=0/2) (Fig. 3F, Supplemental Table 2).
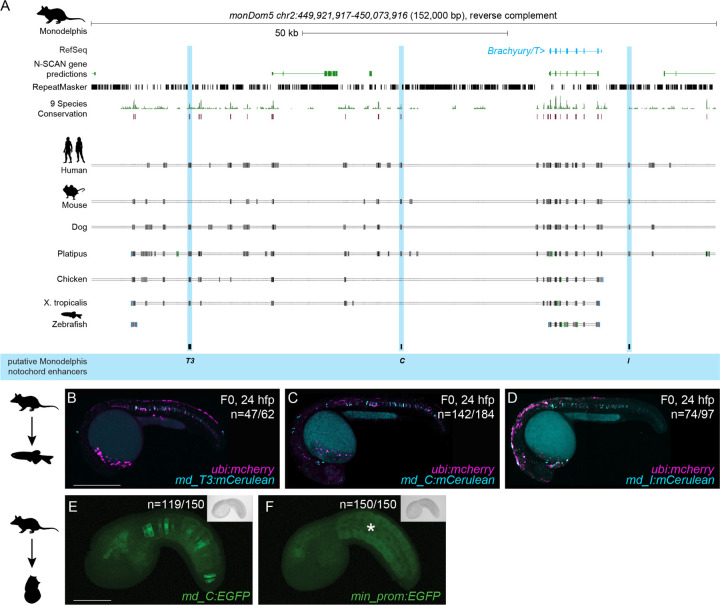
While humans and mice diverged approximately 90 million years ago, marsupials split from eutherians (placental mammals) approximately 160 million years ago (Goodstadt et al., 2007; Kumar et al., 2017; Mikkelsen et al., 2007; Nei et al., 2001; Pippucci et al., 2011). The opossum Monodelphis domestica is a representative marsupial species and provides a more distant comparative species to human and mouse (Supplemental Fig. 4A). Detailed sequence alignments documented dispersed conserved regions along the entire sequences for all three enhancer candidates in Monodelphis (Fig. 4A). When injected into zebrafish embryos as mCerulean reporters, the Monodelphis-derived md_T3, md_C, and md_I enhancer element candidates all conveyed specific notochord activity at 24 hpf (n=47/62, n=142/184, n=74/97) (Fig. 4B-D, Supplemental Table 2), with md_T3 transiently expressing at 80% epiboly (Supplemental Fig. 4B,C). In addition to the notochord activity, md_C reporter-injected zebrafish embryos showed transient reporter expression in the heart whereas md_I reporter-injected embryos showed transient expression in the brain and spinal cord neurons (Fig. 4C,D).
Figure 4: Monodelphis Brachyury enhancer elements are active in different species.
(A) Monodelphis Brachyury/T/TBXTB locus adapted from the UCSC genome browser. Repeats are marked in black using the RepeatMasker track. Further annotated are tracks containing N-SCAN gene predictions and 9 Species Conservation. The light blue highlighted boxes mark the Monodelphis enhancer elements T3, C and I and their conservation in other species.
(B,C,D) Representative F0 zebrafish embryos injected with the Monodelphis enhancer elements md_T3 (B), md_C (C), and md_I (D) showing mosaic mCerulean reporter expression in the zebrafish notochord at 24 hpf. ubi:mCherry was used as injection control. N represent the number of animals expressing mCerulean in the notochord relative to the total number of animals expressing mCherry. Scale bar in B: 0.5 mm, applies to B-C.
(E,F) Representative images of Ciona embryos electroporated with Monodelphis enhancer element md_C (E), and minimal forkhead promoter (fkh) only as control (F). Monodelphis enhancer element md_C expresses EGFP throughout the entire Ciona notochord, compared to minimal fkh promoter only which does not express EGFP at all (asterisk in F). N represent the number of animals expressing EGFP in the notochord relative to the total number of animals. Inserts on the top right represent bright field images of respective embryos. Scale bar in E: 0.05 mm, applies to E,F.
Given the mammalian sequence conservation and differential responses in reporter assays, we next tested the notochord enhancer element candidates in the tunicate Ciona intestinalis as non-vertebrate outgroup. As a chordate, Ciona forms a bona fide notochord (Passamaneck et al., 2009). Testing T3/TNE, C, and I of human, mouse, and Monodelphis by reporter gene assays in Ciona, we found that only Monodelphis-derived md_C showed specific and robust reporter activity in the notochord (n=119/150) compared to all other eight elements (n=0/150) and minimal promoter only control (n=0/150) (Fig. 4E,F, Supplemental Table 2).
Taken together, and extending previous work on the mouse TNE element (Schifferl et al., 2021), our data indicate that three distant elements in the mammalian Brachyury/T/Tbxtb locus with differential activity converge on providing notochord-specific activity in reporter assays across chordates.
Enhancer deletions cause notochord-selective loss of Brachyury/T/Tbxtb expression in mice
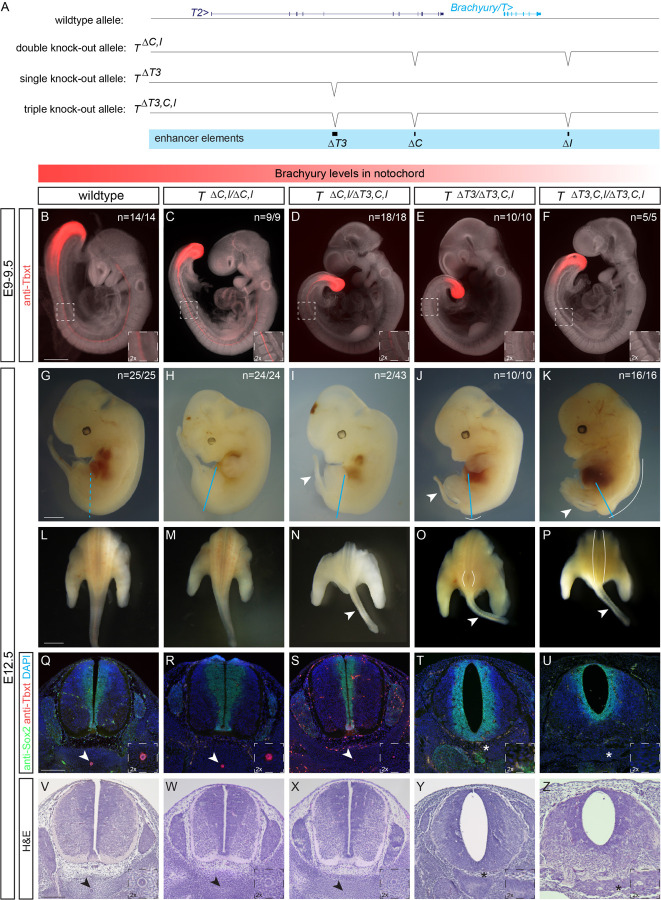
While especially enhancer element C seems to have diverged in activity (or is sensitive to the specific trans environment it was tested in), all three elements T3, C, and I remain conserved and detectable at the sequence level throughout the mammalian clade. In mice, homozygous Brachyury/T/Tbxtb mutations in the gene body cause preimplantation defects leading to embryonic lethality between E9.5 and E10.5 (Gluecksohn-Schoenheimer, 1938; Gluecksohn-Schoenheimer, 1944; Yanagisawa, 1990). Previous work established that deletion of mouse enhancer T3/TNE does not cause a fully penetrant loss of Brachyury/T/Tbxtb expression in the developing notochord, indicating the presence of additional shadow elements interacting with, or compensating for, T3/TNE (Schifferl et al., 2021). To functionally test if the three enhancer elements are involved in Brachyury/T/Tbxtb expression in the mouse notochord, we generated a series of knockout alleles targeting the three mouse enhancer elements T3, C, and I (Fig. 5A).
Figure 5: Deletion of the three enhancer elements T3, C and I results in selective loss of Brachyury protein expression in the notochord at E9.5 and posterior defects at E12.5.
(A) Overview of wildtype mouse Brachyury/T/TBXTB locus adapted from the UCSC genome browser and deletion alleles generated with CRISPR-Cas9 genome editing. Exact coordinates and sequences of target sites, deletions, and genotyping primer sequences can be found in Supplemental Table 5.
(B-F) Brachyury/T antibody staining (red) of E9.5 embryos. White dashed square in panels represents location of right bottom inserts with 2x magnification. Brachyury/T protein expression in the notochord is dose-dependent on the three enhancer elements. Scale bar in B: 1 mm, applies to panels B-F.
(G-K) Overall morphology of E12.5 embryos with different genotypes. Blue lines indicate location of immunofluorescence and H&E sections. Spina bifida and tail defects are dose-dependent. Arrowheads marks small tails. White lines mark spina bifida. Scale bar in G: 1 mm, applies to panels G-K.
(L-P) Dorsal view of embryos (sectioned at blue line in G-K). White lines mark areas of spina bifida. Arrowheads mark small tails compared to tails in wildtype control and double knock-out allele. Scale bar in L: 2.5 mm, applies to panels L-P.
(Q-U) Immunofluorescence of mouse transverse sections. Anti-Sox2 labels the neural plate, anti-Tbxt the notochord, and DAPI marks nuclei. Sox2 expression is comparable amongst all genotypes, even in the genotypes with spina bifida, while there is loss of Brachyury/T staining in the notochord with increased loss of the enhancers. Arrowheads point to notochord. Asterisks mark absent notochord. Scale bar in Q: 0.2 mm, applies to panels Q-U.
(V-Z) H&E staining of transverse sections confirm the dose-dependent loss of the notochord and spina bifida. Arrowheads point to notochord. Asterisks mark absent notochord. Scale bar in V: 0.2 mm, applies to panels V-Z.
We employed CRISPR-Cas9 genome editing using target sites flanking the enhancers and established heterozygous and homozygous mice carrying individual and combined enhancer deletions (Fig. 5A, Supplemental Fig. 5A). Compared to E9.5 wildtype control embryos (Fig. 5B) (n=14/14), neither homozygous deletion of mouse C (TΔC/ΔC) (n=7/7) or I (TΔI/ΔI) (n=7/7) alone, nor heterozygous (T+/ΔC,I) (n=12/12) (Supplemental Fig. 5B-D) or homozygous deletion of both C and I (TΔC,I/ΔC,I) (n=9/9) (Fig. 5C) altered Brachyury/T/Tbxtb expression in the notochord as determined by Brachyury/T antibody staining.
In contrast, we observed reduced Brachyury/T/Tbxtb expression in the notochord of E9.5 embryos in a dose-dependent manner when we combined ΔT3/TNE with ΔC,I deletions. E9.5 embryos heterozygous for the triple knockout chromosome carrying ΔT3,C,I (T+/ΔT3,C,I) in cis appeared normal (n=14/14) (Supplemental Fig. 5E). In contrast, we documented a partial loss of Brachyury/T/Tbxtb protein in trans-heterozygous E9.5 embryos carrying ΔC,I and ΔT3,C,I alleles (TΔC,I/ΔT3,C,I) (n=14/18) (Fig. 5D). Comparably, Brachyury/T /Tbxtb protein levels were even further reduced or lost in the notochord of embryos trans-heterozygous for ΔT3 and ΔT3,C,I alleles (TΔT3/ΔT3,C,I) (n=10/10) (Fig. 5E). These data are consistent with, and expand upon, previous observations that the severity of Brachyury/T/Tbxtb phenotypes correlate with gene dosage (Yanagisawa, 1990). Importantly, the TΔT3/ΔT3,C,I genotype with severely reduced Brachyury/T/Tbxtb protein levels is consistent with the loss of Brachyury/T/Tbxtb protein in the notochord in mice trans-heterozygous for T3/TNE deletion and a large, locus-spanning Brachyury/T/Tbxtb deletion that includes elements C and I (Schifferl et al., 2021), revealing the actual relevant enhancer regions (Fig. 1,3,4) and motifs (Fig. 2). Finally, E9.5 homozygous triple knockout ΔT3,C,I embryos (TΔT3,C,I/ΔT3,C,I) showed a complete absence of Brachyury/T/Tbxtb protein in the prospective notochord region (n=0/5) yet all embryos retained Brachyury/T/Tbxtb protein in the tailbud (n=5/5) (Fig. 5F). Taken together, our data establish the notochord-specific Brachyury/T/Tbxtb loss-of-function mutant in mice by means of deleting three conserved enhancer elements in cis.
Next, we examined phenotypic defects resulting from perturbed Brachyury/T/Tbxtb expression using various allele combinations involving ΔC,I and ΔT3,C,I. Consistent with the phenotypes at E9.5 (Fig. 5B-F), we observed a gradual increase of phenotype severity with deletion of the three different enhancer elements at E12.5 (Fig. 5G-Z). Wildtype control (n=25/25) (Fig. 5G,L), homozygous TΔC,I/ΔC,I embryos (n=24/24) (Fig. 5H,M), heterozygous T+/ΔC,I (n=5/5) and T+/ΔT3,C,I embryos (n=23/23) (Supplemental Fig. 5F,G) appeared grossly normal. In contrast, we observed rudimentary/short/small tails with additional enhancer deletions. Rudimentary/short/small tails appeared in trans-heterozygous TΔC,I/ΔT3,C,I embryos in 4.7 % (n=2/43) (Fig. 5I,N) and was fully penetrant in trans-heterozygous TΔT3/ΔT3,C,I embryos (n=10/10) (Fig. 5J,O), as well as in triple homozygous TΔT3,C,I/ΔT3,C,I embryos (n=16/16) (Fig. 5K,P). In addition, trans-heterozygous TΔT3/ΔT3,C,I embryos displayed caudal spina bifida (n=16/16) (Fig. 5O), as previously reported for homozygous TNE embryos (Schifferl et al., 2021). Finally, triple-homozygous TΔT3,C,I/ΔT3,C,I embryos lacking all three enhancers displayed spina bifida along 3/4 of the spine (n=16/16) (Fig. 5P), reminiscent of previous observations using Brachyury/T/Tbxtb-targeting RNAi in mouse embryos (Pennimpede et al., 2012; Zhu et al., 2016). These results provide compelling phenotypic evidence of the impact of cumulative enhancer deletions on Brachyury/T/Tbxtb expression in the notochord.
We further validated these phenotypes with immunohistochemistry and histology. We visualized Brachyury/T/Tbxtb protein in transversal sections of E12.5 embryos together with the neural plate marker Sox2: compared to wildtype (Fig. 5Q), heterozygous T+/ΔC,I, T+/ΔT3,C,I (Supplemental Fig. 5H,I) as well as homozygous TΔC,I/ΔC,I (Fig. 5R) embryos that were all grossly normal, we found decreased Brachyury protein in the notochord of TΔC,I/ΔT3,C,I embryos (Fig. 5S). Strikingly, we observed a complete absence of Brachyury protein in TΔT3/ΔT3,C,I embryos (Fig. 5T) and TΔT3,C,I/ΔT3,C,I embryos (Fig. 5U). In contrast, Sox2 expression was comparable in all embryos (Fig. 5Q-U, Supplemental Fig, 5H,I), even in TΔT3,C,I/ΔT3,C,I embryos that clearly displayed spina bifida along the entire spine (Fig. 5U). Compared to wildtype embryos (Fig. 5V), additional histology assessed by H&E staining confirmed wildtype-looking notochords in T+/ΔC,I, T+/ΔT3,C,I, and homozygous TΔC,I/ΔC,I embryos (Supplemental Fig. 5J,K, Fig. 5W), smaller/rudimentary notochords in TΔC,I/ΔT3,C,I embryos (Fig. 5X), and absent notochords in TΔT3/ΔT3,C,I and TΔT3,C,I/ΔT3,C,I embryos (Fig. 5Y,Z).
We found that the two most severe enhancer mutants are not viable since we did not recover adult triple-homozygous TΔT3,C,I/ΔT3,C,I (n=0/59) or trans-heterozygote TΔT3/ΔT3,C,I (n=0/31) animals at term (Supplemental Fig. 5L), indicating lethality prior to or shortly after birth. In contrast, TΔC,I/ΔT3,C,I (n=46) trans-heterozygotes and homozygous TΔC,I/ΔC,I (n=100) animals survived to adulthood. Notably, a variable percentage of TΔC,I/ΔC,I, TΔC,I/ΔT3,C,I, and T+/ΔT3 animals presented with kinked tails (Supplemental Fig. 5M), with two TΔC,I/ΔT3,C,I animals displaying a short/small tail (Supplemental Fig. 5N), reminiscent of hypomorphic Brachyury/T/Tbxtb mutants and in vivo Brachyury/T/Tbxtb knockdown by siRNA (Dobrovolskaia-Zavadskaia, 1927; Pennimpede et al., 2012; Schifferl et al., 2021; Zhu et al., 2016). Taken together, our data are consistent with the correlation of Brachyury/T/Tbxtb-mutant phenotypes and gene dosage controlled by enhancer activity, as revealed by increasing phenotype severity with an increasing number of combined enhancer deletions in Brachyury/T/Tbxt.
In summary, our data establishes that the combined activity of the enhancers T3/TNE, C, and I in the mouse Brachyury/T/Tbxtb locus are necessary to convey notochord expression of Brachyury/T/Tbxtb. Upon combined loss of these enhancers, the notochord is lost.
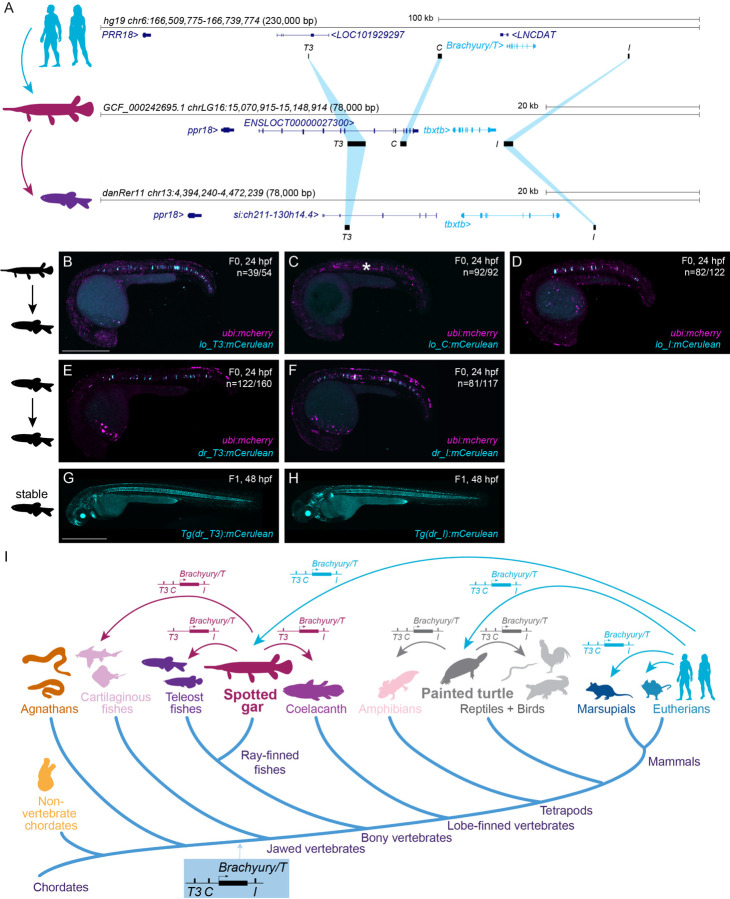
T3, C and I are conserved in sequence and function among jawed vertebrates
The evolutionary trajectory of chordate Brachyury control in the notochord remains unresolved. The notochord-regulatory elements driving Brachyury expression in Ciona are promoter-proximal (Corbo et al., 1997; Nibu et al., 2013; Satoh et al., 2012). Zebrafish tbxta/ntla harbors a −2.1 kb upstream notochord element containing the two smaller elements E1 and E2 (Harvey et al., 2010). In contrast, zebrafish tbxtb descended from the same ancestral Brachyury gene as the single mammalian Tbxtb gene. Further, while zebrafish tbxtb remains expressed in the notochord (Inoue et al., 2017; Martin and Kimelman, 2010), its regulatory elements have not been reported. Using direct sequence comparisons of mammalian T3, C, and I to the zebrafish genome, we did not find any sequences of significant sequence similarity.
Identifying non-coding sequence conservation across vertebrate lineages, whether from human or other tetrapods to the fast-evolving teleost fishes like zebrafish, remains notoriously challenging. Species with slow rates of molecular evolution can help as “genomic bridges” to provide sequence connectivity across distant vertebrate groups (Braasch et al., 2016; Thompson et al., 2021). The spotted gar (Lepisosteus oculatus) is a slowly evolving ray-finned fish that has diverged from zebrafish and other teleosts before a teleost-specific whole-genome duplication, providing a bridge species for genomic comparisons between tetrapods and teleosts (Braasch et al., 2016). Using BLAST searches, we found sequence similarity between human T3/TNE, C, and I and regions of the spotted gar tbxtb locus with equivalent positions relative to the gar tbxtb gene body compared to mammals (Fig. 6A). Next, we used these spotted gar T3/TNE, C, and I regions as BLAST queries to bridge to the genomes of zebrafish and other fish lineages (Supplemental Table 4). This approach uncovered candidate regions for T3/TNE and I, but not C, within the zebrafish tbxtb locus (Fig. 6A).
Figure 6: Bridge species establish the presence of Tbxtb enhancers across jawed vertebrates.
(A) Location of the enhancer elements in the human (top), gar (middle), and zebrafish (bottom) Brachyury/T/Tbxtb loci, adapted from the UCSC browser as established through the “gar bridge”.
(B,C,D) Representative F0 zebrafish embryos injected with the gar enhancer elements Io_T3 (B), Io_C (C), and Io_I (D). T3 and I show mosaic mCerulean reporter expression in the notochord at 24 hpf compared to gar element C with is not active in the zebrafish notochord (asterisk). N represent the number of animals expressing mCerulean in the notochord relative to the total number of animals expressing mosaic ubi:mCherry as injection control. Scale bar in B: 0.5 mm, applies to B-F.
(E,F) Representative F0 zebrafish embryos injected with the conserved zebrafish enhancer elements dr_T3 (E) and dr_I (F). T3 and I show mosaic mCerulean reporter expression in the notochord at 24 hpf. N represent the number of animals expressing mCerulean in the notochord relative to the total number of animals expressing mosaic ubi:mCherry as injection control.
(G,H) Representative images of stable F1 embryos at 2 dpf of zebrafish enhancer elements T3 and I recapitulate the F0 expression pattern in the notochord, with dr_T3 (E) additionally expressing mCerulean in the brain, heart, and fin, and dr_I (G) in the proximal kidney close to the anal pore, pharyngeal arches, heart, fin, and spinal cord neurons. Scale bar in G: 0.5 mm, applies to G,H.
(I) Phylogenetic representation of species investigated using the bridging approach with spotted gar and painted turtle as anchor species within ray-finned fish and tetrapod lineages. Arrows indicate informative phylogenetic comparisons to uncover conservation of enhancer elements T3, I, and C.
Analogous to our tests with mammalian enhancer candidates, we cloned reporter transgenes coupled with the betaE-globin:mCerulean cassette using the T3/TNE, C, and I enhancer elements from the spotted gar tbxtb locus. Upon injection into zebrafish embryos, both spotted gar lo_T3 and lo_I displayed specific and reproducible notochord reporter activity (n=39/54, n=82/122) (Fig. 6B,D, Supplemental Table 2). In contrast, and akin to the mouse mm_C enhancer element, spotted gar element lo_C did not convey any notochord reporter activity in zebrafish embryos (n=0/92) (Fig. 6C, Supplemental Table 2). The zebrafish-derived dr_T3 and dr_I also showed selective notochord activity when tested in zebrafish F) transgenic reporter assays (n=122/160, n=81/117) (Fig. 6E,F, Supplemental Table 2). Further confirming our results, we found robust reporter activity in the notochord of stable transgenic zebrafish lines based on dr_T3 and dr_I (Fig. 6G,H).
Using the three gar elements as queries, in addition to clupeocephalan teleosts (e.g. zebrafish), we found T3 and I also in the other two major teleost lineages elopomorphs (e.g. eel) and osteoglossomorphs (e.g. arowana). However, we did not detect any equivalent sequence for C in any teleosts, indicating that this element has been lost or diverged beyond recognition in the teleost lineage (Fig. 6I). However, we detected orthologs of all three elements, including C, at expected locations around the tbxtb genes in additional non-teleost ray-finned fishes (e.g. bowfin, sturgeon, reedfish) as well as in the more basally diverging cartilaginous fishes (e.g. sharks, skate) (Supplemental Table 4); in contrast, we only detected T3 and I in the lobe-finned coelacanth (Fig. 6I). To explore the presence of the three enhancer elements among tetrapods, we used the painted turtle, characterized by a slow genomic evolutionary rate (Bradley Shaffer et al., 2013; Takezaki, 2018), as an additional bridge species within tetrapods. We found all three elements in the turtle Brachyury/T/Tbxtb locus and through use of the painted turtle as reference also in other reptiles and birds, as well as in amphibians (e.g. axolotl) (Fig. 6I, Supplemental Table 4), but did not detect any of the three elements in the jawless cyclostome (e.g. lamprey, hagfish) genomes. Furthermore, we found that the human T-box motifs, which we identified using FIMO (Fig. 2) in our enhancers, are conserved across tetrapods and fishes as distantly related as ghost shark based on sequence alignments (Supplemental Fig. 6A-C) as well as multi-species FIMO analyses (Supplemental Data File). Cross-species sequence conservation centers at the T-box motifs (Supplemental Fig. 6A-C) which supports both their functional importance as well as their evolutionary ancestry since at least the last common ancestor of jawed vertebrates.
Taken together, our observations provide strong evidence that notochord enhancers T3, I, and C are deeply conserved cis-regulatory elements of the Brachyury/T/Tbxtb gene that were already present in the last common ancestor of jawed vertebrates over 430 million years ago.
DISCUSSION
How the Brachyury/T/Tbxtb gene is controlled during notochord development is fundamental to our understanding of how basic concepts of body plan formation remain conserved or have diverged across species. Shadow enhancers, seemingly redundant transcriptional cis-regulatory elements that regulate the same gene and drive overlapping expression patterns, are a pervasive feature of developmental gene regulation (Kvon et al., 2021). The concept of enhancer redundancy through one or more shadow enhancers acting on the same gene in addition to primary enhancer has been established for numerous loci (Antosova et al., 2016; Cannavò et al., 2015; Hong et al., 2008; Kvon et al., 2021; Letelier et al., 2018; Osterwalder et al., 2018). Shadow enhancers are thought to provide robustness to gene expression and buffer against genetic and environmental variations (Kvon et al., 2021), a hypothesis validated in mammals (Antosova et al., 2016; Osterwalder et al., 2018).
Here, we discovered a deeply conserved set of three notochord-specific shadow enhancers within the human TBXT locus as ancient cis-regulatory logic. Cross-species enhancer testing reveals that the cis-regulatory grammar of all three human enhancers (T3, I, and C, respectively) is correctly interpreted in other vertebrates including mice, salamanders, and zebrafish, but not in the invertebrate chordate Ciona. The three notochord enhancers described here are not the only conserved non-coding elements across mammalian Brachyury/T/Tbxtb loci (Fig. 1A, 3A, 4A): while our reporter gene assays in zebrafish did not reveal any notochord activity of six tested human enhancer elements besides T3, C and I, we cannot rule out synergistic or interdependent notochord activity conveyed by additional elements. Reporter gene assays indicate that not all three Brachyury/T/Tbxtb notochord enhancers have equal potency. Enhancer element C shows variable activity and remains unrecognized in teleost fishes and Coelacanth. Compared to human C with reproducible notochord activity in all tested models (Fig. 1C,F,I,M) and Monodelphis C that is active in zebrafish and uniquely in Ciona (Fig. 4C,E), mouse C showed no discernible activity in any assay including in mouse embryos (Fig. 3C,G) despite significant sequence conservation. We speculate that while mouse C is not active in isolation, it may contribute together with T3/TNE and I to Brachyury activity in the notochord. This model is consistent with the impact of TNE deletions when combined with larger deletions that include TNE and C in mouse trans-heterozygotes (Schifferl et al., 2021) (Fig. 5). The potential auto-regulation of Brachyury/T/Tbxtb by its protein product via in part conserved T-box motifs in enhancers T3/TNE and I might contribute to the enhancer redundancy and divergent activity of element C when tested in isolation (Fig. 2). Our data propose that enhancer C is an auxiliary element to T3/TNE and might contribute to duration, expression levels, or other features that differ among Brachyury/T/Tbxtb notochord expression across vertebrates. Our combined data proposes a model in which notochord expression of vertebrate Brachyury/T/Tbxtb is cumulatively or cooperatively driven by enhancers T3, C, and I. In this model, sequence variants of T3, C, and I that modulate their individual potency became selected for modulating Brachyury/T levels to species-specific requirements.
The conservation of gene order (micro-synteny) between species can be indicative of the presence of cis-regulatory elements, which are crucial for controlling expression of the physically linked genes (Irimia et al., 2012). The finding of functionally relevant distant enhancers 5’ and 3’ of the Brachyury/T/Tbxtb gene body is further supported by the conserved gene linkage Sftd2-(Prr18)-Brachyury/T/Tbxtb-Pde10a across the entire jawed vertebrate phylogeny. In agreement with a distinct gene linkage surrounding Brachyury/T/Tbxtb in agnathans (Fig. 6I), we were unable to identify any of the three distant enhancers in two species representing this clade. Likewise, a distinct gene linkage associates with Tbxta, the second Tbxtb paralog in fish, which apparently lacks any of the three notochord enhancers described here. tbxta/ntla expression is instead controlled by two mesoderm/notochord enhancers located close to the gene promoter (Harvey et al., 2010), a possible example of evolutionary novelty following ancestral gene duplication. In contrast, the functionally less impactful zebrafish tbxtb/ntlb gene retained the cis-regulatory logic of the Tbxtb gene from the jawed vertebrate ancestor (Fig. 6). We did not find any evidence for sequence conservation of the Tbxtb T3, I, or C regions within vertebrate Tbxta loci or any other genomic regions. Future detailed studies across vertebrate Tbxt paralogs are needed to evaluate whether or not the three Tbxtb regulatory elements identified here were already part of the single Tbxt gene in a vertebrate ancestor. Notably, zebrafish mutants of tbxta/ntla have been widely studied as model for Brachyury function in notochord formation (Amacher et al., 2002; Halpern et al., 1993; Schulte-Merker et al., 1994), while the seemingly less impactful tbxtb has retained ancestral regulation. Why zebrafish, and possibly other fish lineages, use tbxta as their main functional Brachyury paralog, and how the regulatory balance between T3, C, and I plays out across individual vertebrate lineages, warrants future efforts.
We found that Brachyury/T/Tbxtb notochord enhancers T3 and I, and possibly further supported by enhancer C, represent a shadow enhancer combination that contributes to the robust Brachyury/T/Tbxt expression in mammals. In mice, neither deletion of enhancer T3/TNE (Schifferl et al., 2021), nor deletion of enhancer C, I, or C and I, resulted in a discernable notochord phenotype (Fig. 5). Nonetheless, by combining deletions of all three notochord enhancer elements, we showed a dose response for Brachyury/T expression in the notochord. In particular, in embryos where ΔT3 is combined with a chromosome harboring ΔT3,C,I as trans-heterozygotes (TΔT3/ΔT3,C,I) or in triple homozygous knock-out embryos (TΔT3,C,I/ΔT3,C,I), we observed loss of Brachyury/T protein in the notochord as well as notochord-specific phenotypes, such as spina bifida (Fig. 5). The neural tube closure defects are similar to phenotypes observed in Brachyury/T/Tbxtb knockdown embryos (Pennimpede et al., 2012; Zhu et al., 2016) or hypomorphic Brachyury/T/Tbxtb mutants (Dobrovolskaia-Zavadskaia, 1927). These results assign an essential, combinatorial role to the enhancer elements T3/TNE, C and I in regulating Brachyury/T/Tbxtb in the notochord. Notably, previous work (Rennebeck et al., 1995; Rennebeck et al., 1998) has described the T2 mutant caused by a large viral integration 5’ of the mouse Brachyury/Tbxt locus that i) is recessive lethal with phenotypes reminiscent of Brachyury loss, and ii) does complement loss-of-function alleles for Brachyury. T2 has been hypothesized to encode a short protein off a long mRNA (Rennebeck et al., 1995; Rennebeck et al., 1998). The described genomic position of the viral integration in T2 places it in the vicinity and upstream of enhancer element C. We note that various vertebrate Brachyury/tbxtb loci feature annotated long non-coding RNAs upstream of the main gene body that are reminiscent of enhancer RNAs (Fig. 3A, 6A). We therefore hypothesize that the T2 mutation is caused by a disruption of the gene-regulatory landscape of the mouse Brachyury/Tbxt gene by the viral integration, changing the interaction of distant enhancer elements with the promoter. Inspection of the chromatin landscape of the Brachyury/Tbxt locus, also in T2 mutants, could shed light on the architecture of the locus during notochord development.
The significance of Brachyury/T/Tbxtb regulation in the notochord translates to chordoma tumors that feature expression of this T-box transcription factor as key diagnostic readout (Sangoi et al., 2011; Takei and Powell, 2010; Vujovic et al., 2006). Both sporadic and familial chordoma are hypothesized to derive from notochord remnants in the spine that do not convert to nucleus pulposus tissue (Choi et al., 2008; Heaton and Turner, 1985; Vujovic et al., 2006). However, the mechanisms leading to retained or reinitiated Brachyury/T/TBXTB expression in chordoma remain unknown. Our analysis of reported familial and sporadic chordoma amplifications indicate that amplifications invariantly retain the notochord enhancer I together with the gene body including the promoter (Tarpey et al., 2017; Yang et al., 2009). Enhancer I lies within a super-enhancer region identified in chordoma cell lines (Sharifnia et al., 2019), further implicating its transcriptional engagement in chordoma. Amplifications occurring in tandem with the original locus propose a scenario where the retained enhancer I could synergize with C and T3 from the original locus on the newly amplified gene copies, potentially resulting in increased Brachyury/T/TBXTB expression (Fig. 1A). Beyond chordoma, changes in enhancer sequence or relative distance to the Brachyury/T/TBXTB gene body could also impact spine formation and health by altering the robustness of Brachyury expression in the notochord and subsequent nucleus pulposus.
Tremendous progress in in vitro differentiation regimens have resulted in stem cell-derived models for body segmentation and different organ structures (Brink et al., 2020; Lópezlópez-Anguita et al., 2022; Moris et al., 2020; Veenvliet et al., 2020). However, notochord formation has only been reported in more complex systems that recapitulate major hallmarks of embryo patterning (Rito et al., 2023; Xu et al., 2014; Xu et al., 2021). Reporters based on our isolated enhancers potentially provide potent readouts to screen for differentiation regimens that result in notochord fates. Together, our uncovered set of shadow enhancers in Brachyury/T/TBXTB advance our concepts of how this key contributor to notochord formation is regulated and de-regulated in development and disease.
MATERIALS AND METHODS
Brachyury locus annotations
The UCSC genome browser was used to identify and visualize enhancer elements in the human, mouse, and Monodelphis Brachyury locus. *.bed files were generated with the approximate genomic location of human Brachyury amplifications in chordoma tumors from different patients (Tarpey et al., 2017; Yang et al., 2009). Previously published ATAC sequencing data of U-CH2 cells and MUGCHOR cells (Sharifnia et al., 2019), as well as Brachyury/T ChIP sequencing data of human embryonic stem cells (hESCs) (Faial et al., 2015) and U-CH1 cells (Nelson et al., 2012) were added. Further, the repeat masker track and the conservation track for mouse and Monodelphis were added. Ultimately, using this strategy, the human enhancer element candidates T3, K, J, C, I, and L were identified. For detailed information, see Supplemental Table 1 and 3.
The same strategy was applied to find the corresponding mouse enhancer elements. Published ATAC-seq data of mouse ESCs (Tosic et al., 2019) and Brachyury/T-positive fluorescence-activated cell sorted cells from the caudal ends of wild-type mouse embryos (TS12/8 dpc and TS13/8.5 dpc) (Koch et al., 2017), as well as Brachyury/T ChIP sequencing data of mouse ESCs (Beisaw et al., 2018; Koch et al., 2017) were used. Again, the repeat masker track, the ENCODE Candidate Cis-Regulatory Elements (cCREs, combined from all cell types) track, and the Vertebrate Multiz Alignment & Conservation track to check for conservation in human, Monodelphis, and zebrafish, were added. This approach identified the mouse enhancer element candidates T1, T2, T3/TNE, J, C2, C, Tstreak, I, T4, T5, and T6, of which T1, T3/TNE, J, C, Tstreak, I, and T5 were pursued and tested (Supplemental Table 3 and 5).
To find the corresponding Monodelphis elements, the repeat masker and 9-Way Multiz Alignment & Conservation track were included to identify T3, C, and I (Supplemental Table 3 and 5).
Cloning of the enhancer element reporter plasmids
Each Brachyury enhancer element candidate was amplified from either human, mouse, Monodelphis, spotted gar, or zebrafish genomic DNA using the Expand Hi-Fidelity PCR System (11732641001, Roche). Exact coordinates are listed in Supplemental Table 3. Each enhancer candidate was TOPO-cloned into the pENTR5′-TOPO plasmid (K59120, Invitrogen) using halt-volume reactions according to the manufacturer’s instructions (half-volume reactions). Subsequent Multisite Gateway cloning (half-volume reactions) were performed using LR Clonase II Plus (12538120, Invitrogen) according to the manufacturer’s instructions (half-volume reactions) and recommended reaction calculations (Mosimann, 2022). 5’ entry plasmids containing the different enhancer elements were assembled into reporter expression plasmids together with the middle entry plasmid (pME) containing the mouse betaE-globin minimal promoter expressing mCerulean (pSN001) as well as mApple (pCK068), the 3’plasmid #302 (p3E_SV40polyA), and the destination plasmid pDESTTol2A2 containing crybb1:mKate2 (pCB59) and pDESTexorh:EGFP containing EGFP expression in the pineal gland (pCK017) as transgenesis markers (Kemmler et al., 2023). Assembled vectors were verified using restriction digest and Sanger sequencing using standard sequencing primers for Multisite Gateway assemblies (Kemmler et al., 2023; Mosimann, 2022).
Zebrafish husbandry, transgenic reporter assays and stable transgenic lines
Zebrafish animal care and procedures were carried out in accordance with the IACUC of the University of Colorado Anschutz Medical Campus (protocol # 00979), Aurora, Colorado. Zebrafish embryos were raised in E3 embryo medium at 28 °C.
To test the transient activity of our putative enhancer elements, 25 ng/µL Tol2 mRNA, 12.5 ng/µL reporter expression plasmid DNA, and 12.5 ng/µL ubi:mCherry plasmid (Mosimann et al., 2011) as injection control were co-injected into one-cell stage wild type zebrafish embryos (Prummel et al., 2019). At 24 hpf, embryos were anesthetized with 0.016% Tricaine-S (MS-222, Pentair Aquatic Ecosystems Inc.) in E3 embryo medium and embedded in E3 with 1% low-melting-point agarose (A9045, Sigma Aldrich). Embryos were mounted laterally on glass bottom culture dishes (627861, Greiner Bio-One) and confocal imaging was performed with a Zeiss LSM880 using a ×10/0.8 air-objective lens. Fluorescence channels were acquired sequentially with maximum speed in bidirectional mode in 3 µM slices. The range of detection for each channel was adapted to avoid any crosstalk between the channels. Images of acquired Z-stacks were reconstructed with ImageJ/Fiji as a maximum intensity projections.
To generate stable transgenic lines, 25 ng/µL Tol2 mRNA were co-injected with 25 ng/µL reporter expression plasmid DNA (Felker and Mosimann, 2016; Kwan et al., 2007). Multiple F0 founders were screened for specific mCerulean and mKate2 expression, raised to adulthood, and screened for germline transmission. Resulting F1 single-insertion transgenic strains were established and verified through screening for a 50% germline transmission rate outcrosses in the subsequent generations as per our previously outlined procedures (Felker and Mosimann, 2016).
Axolotl husbandry, transgenic reporter assays and immunostaining
Axolotl husbandry and experiments (non-free feeding stages) were performed at the CRTD/Center for Regenerative Therapies Dresden, Dresden, Germany. Transgenic animals were generated using Tol2 transposase following standard protocols (Khattak et al., 2014). For live imaging, the animals were anaesthetized by bathing in 0.01% benzocaine and imaged on an Olympus SZX16 fluorescence stereomicroscope. Embryos were staged as described previously (Armstrong and Malacinski, 1989).
For immunostaining, axolotl embryos were fixed in MEMFA at 4 °C overnight, washed in PBS, embedded in 2% low-melting temperature agarose, and sectioned by vibratome into 200 μm-thick sections. Fibronectin was detected using mouse anti-Fibronectin antibody (ab6328, Abcam) at 5 μg/mL. Confocal images were acquired on a Zeiss LSM780-FCS inverted microscope.
Transgenic mouse reporter assays
Research was conducted at the E.O. Lawrence Berkeley National Laboratory (LBNL) and performed under U.S. Department of Energy Contract DE-AC02–05CH11231, University of California (UC). Transgenic mouse assays were performed in Mus musculus FVB strain mice (animal protocol number 290003; reviewed and approved by the Animal Welfare and Research Committee at Lawrence Berkeley National Laboratory). Animals of both sexes were used in these analyses. Sample size selection and randomization strategies were conducted as follows: sample sizes were selected empirically based on previous experience in transgenic mouse assays for >3,000 total putative enhancers (VISTA Enhancer Browser: https://enhancer.lbl.gov/). Mouse embryos were excluded from further analysis if they did not encode the reporter transgene or if the developmental stage was not correct. All transgenic mice were treated with identical experimental conditions. Randomization and experimenter blinding were unnecessary and not performed. For comprehensive analysis of species-specific T3, C and I, enSERT enhancer analysis was used, allowing for site-directed insertion of transgenic constructs at the H11 safe-harbor locus (Hippenmeyer et al., 2010; Tasic et al., 2011). EnSERT is based on co-injection of Cas9 protein and H11-targeted sgRNA in the pronucleus of FVB single cell-stage mouse embryos (E0.5) with the targeting vector encoding a candidate enhancer element upstream of the Shh-promoter-LacZ reporter cassette (Kvon et al., 2020). Enhancer elements were PCR-amplified from human, mouse and Monodelphis genomic DNA and cloned into the respective LacZ expression vector (Osterwalder et al., 2022). Embryos were excluded from further analysis if they did not contain a reporter transgene in tandem. CD-1 females served as pseudo-pregnant recipients for embryo transfer to produce transgenic embryos which were collected at E9.5 and stained with X-gal using standard methods (Osterwalder et al., 2022).
Histological analysis of Nuclear Fast Red-stained sections from transgenic mouse embryos
After LacZ staining, E9.5 transgenic mouse embryos were dehydrated in serial alcohols (1× 70%, 1× 80%, 1× 90%, 2× 96%, 2× 100% ethanol, followed by 1× 100% isopropanol for 20 minutes each) and cleared twice for 30 minutes with Histo-Clear II (HS-202, National Diagnostics) for paraffin wax embedding. 10 µm-thick transverse sections were obtained with a Leica Biosystems RM2245 Semi-Automated Rotary Microtome. Sections were de-waxed, rehydrated, and stained with Nuclear Fast Red (R5463200, Ricca Chemical) for two minutes. After staining, sections were dehydrated and mounted with Omnimount (HS-110, National Diagnostics). Images were obtained using a Leica M205 FA stereo microscope.
Ciona reporter assays
Ciona experiments were performed at UCSD as described previously (Farley et al., 2015; Song et al., 2023). Adult Ciona intestinalis type A aka Ciona robusta (obtained from M-Rep) were maintained under constant illumination in seawater (obtained from Reliant Aquariums) at 18 °C. Briefly, human, mouse and Monodelphis enhancer elements T3, C and I were subcloned into appropriate plasmids suited for expression in Ciona, upstream of a basal Ciona Forkhead promoter driving GFP (Farley et al., 2016; Harafuji et al., 2002). Ciona embryos were electroporated with 70 μg of each plasmid as previously described (Christiaen et al., 2009) and reporter expression was counted blind in 50 embryos per biological repeat, 3 repeats were performed. Images were taken of representative embryos.
Deletion of mouse enhancer elements T3, C and I
All mouse experimental procedures and animal care were approved by the Animal Care Committee of the Institute of Molecular Genetics (IMG), Czech Academy of Sciences, Prague, Czech Republic, and covered under protocol permission number 357/2021. Experiments were performed in compliance with the European Communities Council Directive of November 24, 1986 (86/609/EEC), as well as national and institutional guidelines.
For this study, inbred C57BL/6N mice were used. Mice carrying deletions of enhancer elements T3/TNE, C, and I were generated using CRISPR-Cas9 technology. The cRNAs (purchased from Integrated DNA technologies) were designed to target the 5’ and 3’ ends of the mouse enhancer elements T3/TNE, C and I to delete the genomic regions in between. For genomic location and sequence of the selected target sites, as well as genomic coordinates of the deleted enhancer element sequences, see Supplemental Table 5.
A ribonucleoprotein (RNP) complex of crRNA/TRACR (1072532, Integrated DNA technologies) and SpCas9 protein (1081058, Integrated DNA technologies) was electroporated into fertilized zygotes isolated from C57BL/6N mice. Zygote electroporation and transfer into pseudo-pregnant foster females was performed as previously described (Jenickova et al., 2021). Founder animals from multiple embryo transfers were genotyped from tail biopsies using PCR and Sanger sequencing and the positive animals were backcrossed to C57BL/6N mice.
Independent knockout lines for enhancer element C (ΔC) and I (ΔI) were generated. Heterozygous ΔC and ΔI (T+/ΔC and T+/ΔI) and homozygous ΔC and ΔI (T ΔC/ΔC and T ΔI/ΔI) embryos were investigated for potential overall phenotypes, but appeared phenotypically normal. Pups were born normally and grew up into fertile adults.
To generate a double knockout ΔC,I strain, homozygous T ΔC/ΔC mice were used for electroporation of CRISPR-Cas9 RNP complexes deleting enhancer element I. Pups homozygous for ΔC,I (T ΔC,I/ΔC,I) were born phenotypically normal and developed into fertile adults; however, around 20% of the animals had a kinked tail (Supplemental Fig. 5M,N).
To generate a triple knockout ΔT3,C,I mouse strain, heterozygous ΔC,I (T+/ΔC,I) mice were used for electroporation of CRISPR-Cas9 RNP complexes deleting enhancer element T3 (ΔT3). Heterozygous T+/ΔT3,C,I or trans-heterozygous TΔT3/ΔC,I embryos were phenotypically normal and grew up into fertile adults. To establish a single knockout line for enhancer element T3 (ΔT3), TΔT3/ΔC,I animals were outcrossed to establish T+/ΔT3. TΔC,I/ΔT3,C,I animals were generated by mating ΔC,I (T ΔC,I/ΔC,I) and ΔT3,C,I (T+/ΔT3,C,I) strains and TΔT3/ΔT3,C,I by mating ΔT3 (T +/ΔT3) and ΔT3,C,I (T+/ΔT3,C,I) strains, respectively.
Around 60% of TΔC,I/ΔT3,C,I pups were born with a tail defect and adult animals displayed a kinked tail, with around 2% of the TΔC,I/ΔT3,C,I pups displaying a small tail. In contrast, adult trans-heterozygous TΔT3/ΔT3,C,I and homozygous TΔT3,C,I/ΔT3,C,I animals were never recovered likely due to lethality at around birth or during early postnatal life.
The deletion breakpoints in the individual enhancer alleles were determined by Sanger sequencing. Mice were genotyped using PCR with dedicated primer sets (Supplemental Table 5). Mouse embryos of the given stage were harvested from timed pregnant mice. The day of plug was counted as embryonic day 0.5 (E0.5).
E9.5 whole mount immunostaining and imaging
E9.5 mouse embryos were collected and whole mount immunostaining was done as previously described (Mašek et al., 2016). Brachyury/T/Tbxt expression in E9.5 embryos was visualized using anti-Brachyury (ab209665, Abcam). Images were obtained using a Zeiss AxioZoom V16 macroscope with Apotome with a Zeiss Axiocam 512 mono camera. A qualitative analysis of all investigated embryos can be found in Supplemental Table 6.
E12.5 embryo preparation, immunostaining and imaging
E12.5 mouse embryos were collected and fixed overnight in 4% paraformaldehyde. Whole embryo images were acquired using a Olympus SZX9 stereo microscope with a Olympus DP72 camera. Afterwards, embryos were embedded in paraffin, and 9 µm-thick transverse sections were obtained using a Microtome Leica RM2255. Sections were deparaffinized, rehydrated, and stained with hematoxylin & eosin (H-3502, Vectorlabs) for histology, or anti-Brachyury (ab209665, Abcam), anti-Sox2 Y-17 (sc-17320, Santa Cruz), and DAPI (10236276001, Roche Diagnostics) according to the manufacturer’s instructions. After staining, sections were mounted with Mowiol (81381, Millipore Sigma). Images of sections were obtained using a Leica DM6000 widefield fluorescence microscope with a Leica DFC 9000 camera.
Gar and turtle bridge alignment
To establish genomic connectivity across distant vertebrate lineages, a “bridging approach” that leverages species with slowly evolving genomic sequences, such as spotted gar within ray-finned fishes (Braasch et al., 2016) and painted turtle within tetrapods (Bradley Shaffer et al., 2013), was used. Using human T3, C, and I as queries, BLASTN searches (search sensitivity: distant homologies) at Ensembl.org against the bridge species genomes were performed. Candidate BLAST hit regions were manually inspected for their location in relation to the Tbxtb gene locus for further consideration. Core regions based on the initial BLAST hits in both bridge species were expanded in both directions up to the next annotated repeat element. Once the three elements were established in the bridge species, their sequences were used for as queries for BLASTN searches with genomes representative species across all major vertebrate lineages as targets (see Supplemental Table 4 for species list, genome assemblies, and enhancer element coordinates). Further BLASTN chaining through additional species was performed as needed (e.g., human->gar->goldfish->zebrafish for T3 and I). All BLAST hits were manually inspected for proximity to the Tbxtb gene. Multi-species alignments of the three elements were generated with MAFFT version 1.5.0 (Katoh et al., 2009) and presence of T-box motifs in individual species was established with FIMO version 5.4.1 (Grant et al., 2011) using the human TBXT motif TBXT_MA0009.2.meme obtained from JASPAR as input sequence.
Supplementary Material
Acknowledgements
We thank Christine Archer, Molly Waters, Nikki Tsuji, Ainsley Gilbard, and Olivia Gomez (CU Anschutz), as well as Vesna Barros, Lukas Obernosterer, and Yorgos Panayotu (UZH) for excellent zebrafish husbandry support; Jitka Lachova (IMG) for outstanding expert assistance with mouse embryo preparation and strain maintenance; Beate Gruhl and Anja Wagner (TU Dresden) for excellent axolotl husbandry support; Dr. Alexa Sadier and Dr. Karen Sears (UCLA) for generously providing us with Monodelphis genomic DNA; Dr. Jelena Kresoja and Elena Cabello for bioinformatics input; and all members of our individual laboratories for critical input and discussion on experiments, concepts, and the manuscript. Species silhouettes adapted from PhyloPic.
Financial Support
This work has been supported by an UZH URPP “Translational Cancer Research” seed grant and NIH/NIDDK grant 1R01DK129350-01A1 to A.B.; the Children’s Hospital Colorado Foundation, National Science Foundation Grant 2203311, and Swiss National Science Foundation (SNSF) Sinergia grant CRSII5_180345 to C.M.; a project grant from the Swiss Cancer League, the SwissBridge Award 2016 from the SwissBridge Foundation, Additional Ventures SVRF2021-1048003 grant, and the University of Colorado Anschutz Medical Campus to C.M. and A.B.; NIH/NIGMS 1T32GM141742-01, 3T32GM121742-02S1, and NIH/NHLBI F31HL167580 to H.R.M.; Czech Science Foundation grant 23-07056S to Z.K. and Czech Center for Phenogenomics infrastructure support LM2018126, OP VaVpI CZ.1.05/2.1.00/19.0395, and CZ.1.05/1.1.00 /02.0109 to IMG; NIH grant 5R01DE024745-09 to L.S.; NSF EDGE Award #2029216 to I.B.; SNSF Eccellenza professorship PCEFP3_186993 to M.O.; an Alexander von Humboldt postdoctoral Fellowship to A.C.; Deutsche Forschungsgemeinschaft (DFG) Grants 22137416, 450807335 and 497658823, as well as TUD and CRTD funds to M.H.Y.; NIH grant DP2HG010013 to E.K.F and F.L.; NIH grants R01HG003988, R01DE028599, and R01HL162304 to A.V. and B.J.M.
Footnotes
Competing financial interests
The authors declare no competing financial interests.
REFERENCES
- Amacher S. L., Draper B. W., Summers B. R. and Kimmel C. B. (2002). The zebrafish T-box genes no tail and spadetail are required for development of trunk and tail mesoderm and medial floor plate. Development 3311–3323. [DOI] [PubMed]
- Amemiya C. T., Alfoldi J., Lee A. P., Fan S., Philippe H., MacCallum I., Braasch I., Manousaki T., Schneider I., Rohner N., et al. (2013). The African coelacanth genome provides insights into tetrapod evolution. Nat. 2013 4967445 496, 311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antosova B., Smolikova J., Klimova L., Lachova J., Bendova M., Kozmikova I., Machon O. and Kozmik Z. (2016). The Gene Regulatory Network of Lens Induction Is Wired through Meis-Dependent Shadow Enhancers of Pax6. PLOS Genet. 12, e1006441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. B. and Malacinski G. M. (1989). Developmental biology of the axolotl. 320. [Google Scholar]
- Arnold S. J., Stappert J., Bauer A., Kispert A., Herrmann B. G. and Kemler R. (2000). Brachyury is a target gene of the Wnt/β-catenin signaling pathway. Mech. Dev. 91, 249–258. [DOI] [PubMed] [Google Scholar]
- Bagnat M. and Gray R. S. (2020). Development of a straight vertebrate body axis. Development 147,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisaw A., Tsaytler P., Koch F., Schmitz S. U., Melissari M., Senft A. D., Wittler L., Pennimpede T., Macura K., Herrmann B. G., et al. (2018). BRACHYURY directs histone acetylation to target loci during mesoderm development. EMBO Rep. 19, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhadra A. K. and Casey A. T. (2006). Familial chordoma. A report of two cases. J Bone Jt. Surg Br 88, 634–636. [DOI] [PubMed] [Google Scholar]
- Braasch I., Gehrke A. R., Smith J. J., Kawasaki K., Manousaki T., Pasquier J., Amores A., Desvignes T., Batzel P., Catchen J., et al. (2016). The spotted gar genome illuminates vertebrate evolution and facilitates human-teleost comparisons. Nat. Genet. 48, 427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley Shaffer H., Minx P., Warren D. E., Shedlock A. M., Thomson R. C., Valenzuela N., Abramyan J., Amemiya C. T., Badenhorst D., Biggar K. K., et al. (2013). The western painted turtle genome, a model for the evolution of extreme physiological adaptations in a slowly evolving lineage. Genome Biol. 14,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink S. C. van den, Alemany A., Batenburg V. van, Moris N., Blotenburg M., Vivié J., Baillie-Johnson P., Nichols J., Sonnen K. F., Arias A. M., et al. (2020). Single-cell and spatial transcriptomics reveal somitogenesis in gastruloids. Nat. 2020 1–5. [DOI] [PubMed] [Google Scholar]
- Cannavò E., Khoueiry P., Garfield D. A., Geeleher P., Zichner T., Gustafson E. H., Ciglar L., Korbel J. O. and Furlong E. E. M. (2015). Shadow Enhancers Are Pervasive Features of Developmental Regulatory Networks. Curr. Biol. [DOI] [PMC free article] [PubMed]
- Choi K. S., Cohn M. J. and Harfe B. D. (2008). Identification of nucleus pulposus precursor cells and notochordal remnants in the mouse: implications for disk degeneration and chordoma formation. Dev Dyn 237, 3953–3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiaen L., Wagner E., Shi W. and Levine M. (2009). Electroporation of Transgenic DNAs in the Sea Squirt Ciona. Cold Spring Harb. Protoc. 2009, pdb.prot5345. [DOI] [PubMed] [Google Scholar]
- Clements D., Taylor H. C., Herrmann B. G. and Stott D. (1996). Distinct regulatory control of the Brachyury gene in axial and non-axial mesoderm suggests separation of mesoderm lineages early in mouse gastrulation. Mech. Dev. 56, 139–149. [DOI] [PubMed] [Google Scholar]
- Corallo D., Trapani V. and Bonaldo P. (2015). The notochord: structure and functions. Cell. Mol. Life Sci. 72, 2989–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbo J. C., Levine M. and Zeller R. W. (1997). Characterization of a notochord-specific enhancer from the Brachyury promoter region of the ascidian, Ciona intestinalis. Development 124, 589–602. [DOI] [PubMed] [Google Scholar]
- D’Agati G., Cabello E. M., Frontzek K., Rushing E. J., Klemm R., Robinson M. D., White R. M., Mosimann C. and Burger A. (2019). Active receptor tyrosine kinases, but not Brachyury, are sufficient to trigger chordoma in zebrafish. Dis. Model. Mech. 12,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrovolskaia-Zavadskaia N. (1927). Sur la mortification spontanee de la chez la souris nouveau-nee et sur l’existence d’un caractere (facteur) hereditaire, non-viable. Crit Rev Soc Biol 97, 114–116. [Google Scholar]
- Faial T., Bernardo A. S., Mendjan S., Diamanti E., Ortmann D., Gentsch G. E., Mascetti V. L., Trotter M. W. B., Smith J. C., Pedersen R. A., et al. (2015). Brachyury and SMAD signalling collaboratively orchestrate distinct mesoderm and endoderm gene regulatory networks in differentiating human embryonic stem cells. Development 142, 2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley E. K., Olson K. M., Zhang W., Brandt A. J., Rokhsar D. S. and Levine M. S. (2015). Suboptimization of developmental enhancers. Science 350, 325–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley E. K., Olson K. M., Zhang W., Rokhsar D. S. and Levine M. S. (2016). Syntax compensates for poor binding sites to encode tissue specificity of developmental enhancers. Proc. Natl. Acad. Sci. U. S. A. 113, 6508–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felker A. and Mosimann C. (2016). Contemporary zebrafish transgenesis with Tol2 and application for Cre/lox recombination experiments. Methods Cell Biol. 135, 219–44. [DOI] [PubMed] [Google Scholar]
- Gluecksohn-Schoenheimer S. (1938). THE DEVELOPMENT OF TWO TAILLESS MUTANTS IN THE HOUSE MOUSE. Genetics 23, 573–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluecksohn-Schoenheimer S. (1944). The Development of Normal and Homozygous Brachy (T/T) Mouse Embryos in the Extraembryonic Coelom of the Chick. Proc. Natl. Acad. Sci. 30, 134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodstadt L., Heger A., Webber C. and Ponting C. P. (2007). An analysis of the gene complement of a marsupial, Monodelphis domestica: evolution of lineage-specific genes and giant chromosomes. Genome Res. 17, 969–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant C. E., Bailey T. L. and Noble W. S. (2011). FIMO: scanning for occurrences of a given motif. Bioinformatics 27, 1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern M. E., Ho R. K., Walker C. and Kimmel C. B. (1993). Induction of muscle pioneers and floor plate is distinguished by the zebrafish no tail mutation. Cell 75, 99–111. [PubMed] [Google Scholar]
- Harafuji N., Keys D. N. and Levine M. (2002). Genome-wide identification of tissue-specific enhancers in the Ciona tadpole. Proc. Natl. Acad. Sci. U. S. A. 99, 6802–6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey S. A. A., Tümpel S., Dubrulle J., Schier A. F. F., Smith J. C. C., Tumpel S., Dubrulle J., Schier A. F. F. and Smith J. C. C. (2010). no tail integrates two modes of mesoderm induction. Development 137, 1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton J. M. and Turner D. R. (1985). Reflections on notochordal differentiation arising from a study of chordomas. Histopathology 9, 543–550. [DOI] [PubMed] [Google Scholar]
- Henrique D., Abranches E., Verrier L. and Storey K. G. (2015). Neuromesodermal progenitors and the making of the spinal cord. Development 142, 2864–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann B. G. (1995). The mouse Brachyury (T) gene. Semin. Dev. Biol. 6, 385–394. [Google Scholar]
- Hippenmeyer S., Youn Y. H., Moon H. M., Miyamichi K., Zong H., Wynshaw-Boris A. and Luo L. (2010). Genetic Mosaic Dissection of Lis1 and Ndel1 in Neuronal Migration. Neuron 68, 695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland P. W., Koschorz B., Holland L. Z. and Herrmann B. G. (1995). Conservation of Brachyury (T) genes in amphioxus and vertebrates: developmental and evolutionary implications. Development 121, 4283–4291. [DOI] [PubMed] [Google Scholar]
- Hong J. W., Hendrix D. A. and Levine M. S. (2008). Shadow enhancers as a source of evolutionary novelty. Science (80-. ). 321, 1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu W., Mohyeldin A., Shah S. R., ap Rhys C. M., Johnson L. F., Sedora-Roman N. I., Kosztowski T. A., Awad O. A., McCarthy E. F., Loeb D. M., et al. (2011). Generation of chordoma cell line JHC7 and the identification of Brachyury as a novel molecular target. J Neurosurg 115, 760–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Mintz A., Shah S. R. R., Quinones-Hinojosa A. and Hsu W. (2014). The FGFR/MEK/ERK/brachyury pathway is critical for chordoma cell growth and survival. Carcinogenesis 35, 1491–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue J., Yasuoka Y., Takahashi H. and Satoh N. (2017). The chordate ancestor possessed a single copy of the Brachyury gene for notochord acquisition. Zool. Lett. 3, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irimia M., Tena J. J., Alexis M. S., Fernandez-Miñan A., Maeso I., Bogdanović O., De La Calle-Mustienes E., Roy S. W., Gómez-Skarmeta J. L. and Fraser H. B. (2012). Extensive conservation of ancient microsynteny across metazoans due to cis-regulatory constraints. Genome Res. 22, 2356–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenickova I., Kasparek P., Petrezselyova S., Elias J., Prochazka J., Kopkanova J., Navratil M., Barinka C. and Sedlacek R. (2021). Efficient allele conversion in mouse zygotes and primary cells based on electroporation of Cre protein. Methods 191, 87–94. [DOI] [PubMed] [Google Scholar]
- Katoh K., Asimenos G. and Toh H. (2009). Multiple alignment of DNA sequences with MAFFT. Methods Mol. Biol. 537, 39–64. [DOI] [PubMed] [Google Scholar]
- Kemmler C. L., Moran H. R., Murray B. F., Scoresby A., Klem J. R., Eckert R. L., Lepovsky E., Bertho S., Nieuwenhuize S., Burger S., et al. (2023). Next-generation plasmids for transgenesis in zebrafish and beyond. Development 150,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattak S., Murawala P., Andreas H., Kappert V., Schuez M., Sandoval-Guzmán T., Crawford K. and Tanaka E. M. (2014). Optimized axolotl (Ambystoma mexicanum) husbandry, breeding, metamorphosis, transgenesis and tamoxifen-mediated recombination. Nat. Protoc. 9, 529–540. [DOI] [PubMed] [Google Scholar]
- Koch F., Scholze M., Wittler L., Schifferl D., Sudheer S., Grote P., Timmermann B., Macura K. and Herrmann B. G. (2017). Antagonistic Activities of Sox2 and Brachyury Control the Fate Choice of Neuro-Mesodermal Progenitors. Dev. Cell. [DOI] [PubMed]
- Kumar S., Stecher G., Suleski M. and Blair Hedges S. (2017). TimeTree: A Resource for Timelines, Timetrees, and Divergence Times. Mol. Biol. Evol. 34, 1812–1819. [DOI] [PubMed] [Google Scholar]
- Kvon E. Z., Zhu Y., Kelman G., Novak C. S., Plajzer-Frick I., Kato M., Garvin T. H., Pham Q., Harrington A. N., Hunter R. D., et al. (2020). Comprehensive In Vivo Interrogation Reveals Phenotypic Impact of Human Enhancer Variants. Cell 180, 1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvon E. Z., Waymack R., Elabd M. G., Wunderlich Z., Gad M. and Wunderlich Z. (2021). Enhancer redundancy in development and disease. 22,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan K. M., Fujimoto E., Grabher C., Mangum B. D., Hardy M. E., Campbell D. S., Parant J. M., Yost H. J., Kanki J. P. and Chien C. Bin (2007). The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev Dyn 236, 3088–3099. [DOI] [PubMed] [Google Scholar]
- Latinkić B. V., Umbhauer M., Neal K. A., Lerchner W., Smith J. C. and Cunliffe V. (1997). The Xenopus Brachyury promoter is activated by FGF and low concentrations ofactivinandsuppressed by high concentrationsof activin and by paired-type homeodomain proteins. Genes Dev. 11, 3265–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letelier J., De La Calle-Mustienes E., Pieretti J., Naranjo S., Maeso I., Nakamura T., Pascual-Anaya J., Shubin N. H., Schneider I., Martinez-Morales J. R., et al. (2018). A conserved Shh cis-regulatory module highlights a common developmental origin of unpaired and paired fins. Nat. Genet. 50, 504–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lópezlópez-Anguita N., Gassaloglu S. I., Stö Tzel M., Bolondi A., Conkar D., Typou M., Renébuschow R., Veenvliet J. V and Bulut-Karslioglu A. (2022). Hypoxia induces an early primitive streak signature, enhancing spontaneous elongation and lineage representation in gastruloids. Development 149,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B. L. and Kimelman D. (2008). Regulation of Canonical Wnt Signaling by Brachyury Is Essential for Posterior Mesoderm Formation. Dev. Cell 15, 121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B. L. and Kimelman D. (2010). Brachyury establishes the embryonic mesodermal progenitor niche. Genes Dev. 24, 2778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mašek J., Machoň O., Kořínek V., Taketo M. M. and Kozmik Z. (2016). Tcf7l1 protects the anterior neural fold from adopting the neural crest fate. Development 143, 2206–2216. [DOI] [PubMed] [Google Scholar]
- Mikkelsen T. S., Wakefield M. J., Aken B., Amemiya C. T., Chang J. L., Duke S., Garber M., Gentles A. J., Goodstadt L., Heger A., et al. (2007). Genome of the marsupial Monodelphis domestica reveals innovation in non-coding sequences. Nature 447, 167–77. [DOI] [PubMed] [Google Scholar]
- Moris N., Anlas K., van den Brink S. C., Alemany A., Schröder J., Ghimire S., Balayo T., van Oudenaarden A. and Martinez Arias A. (2020). An in vitro model of early anteroposterior organization during human development. Nature 1–6. [DOI] [PubMed]
- Mosimann C. (2022). Multisite Gateway Calculations: Excel spreadsheet. protocols.io.
- Mosimann C., Kaufman C. K., Li P., Pugach E. K., Tamplin O. J. and Zon L. I. (2011). Ubiquitous transgene expression and Cre-based recombination driven by the ubiquitin promoter in zebrafish. Development 138, 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamichi R. and Asahara H. (2020). The transcription factors regulating intervertebral disc development. JOR Spine 3, e1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M., Xu P. and Glazko G. (2001). Estimation of divergence times from multiprotein sequences for a few mammalian species and several distantly related organisms. Proc. Natl. Acad. Sci. 98, 2497–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson A. C., Pillay N., Henderson S., Presneau N. N., Tirabosco R., Halai D., Berisha F., Flicek P., Stemple D. L., Stern C. D., et al. (2012). An integrated functional genomics approach identifies the regulatory network directed by brachyury (T) in chordoma. J. Pathol. 228, 274–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibu Y., Jose-Edwards D. S., Di Gregorio A., Jos??- Edwards D. S. and Di Gregorio A. (2013). From notochord formation to hereditary chordoma: The many roles of brachyury. [DOI] [PMC free article] [PubMed]
- Nowoshilow S., Schloissnig S., Fei J. F., Dahl A., Pang A. W. C., Pippel M., Winkler S., Hastie A. R., Young G., Roscito J. G., et al. (2018). The axolotl genome and the evolution of key tissue formation regulators. Nat. 2018 5547690 554, 50–55. [DOI] [PubMed] [Google Scholar]
- Osterwalder M., Barozzi I., Tissières V., Fukuda-Yuzawa Y., Mannion B. J., Afzal S. Y., Lee E. A., Zhu Y., Plajzer-Frick I., Pickle C. S., et al. (2018). Enhancer redundancy provides phenotypic robustness in mammalian development. Nature 554, 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterwalder M., Tran S., Hunter R. D., Meky E. M., von Maydell K., Harrington A. N., Godoy J., Novak C. S., Plajzer-Frick I., Zhu Y., et al. (2022). Characterization of Mammalian In Vivo Enhancers Using Mouse Transgenesis and CRISPR Genome Editing. Methods Mol. Biol. 2403, 147–186. [DOI] [PubMed] [Google Scholar]
- Passamaneck Y. J., Katikala L., Perrone L., Dunn M. P., Oda-Ishii I. and Di Gregorio A. (2009). Direct activation of a notochord cis-regulatory module by Brachyury and FoxA in the ascidian Ciona intestinalis. Development 136, 3679–3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck S. H., McKee K. K., Tobias J. W., Malhotra N. R., Harfe B. D. and Smith L. J. (2017). Whole Transcriptome Analysis of Notochord-Derived Cells during Embryonic Formation of the Nucleus Pulposus. Sci. Reports 2017 71 7, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennimpede T., Proske J., König A., Vidigal J. A., Morkel M., Bramsen J. B., Herrmann B. G. and Wittler L. (2012). In vivo knockdown of Brachyury results in skeletal defects and urorectal malformations resembling caudal regression syndrome. Dev. Biol. 372, 55–67. [DOI] [PubMed] [Google Scholar]
- Pippucci T., Savoia A., Perrotta S., Pujol-Moix N., Noris P., Castegnaro G., Pecci A., Gnan C., Punzo F., Marconi C., et al. (2011). Mutations in the 5’ UTR of ANKRD26, the ankirin repeat domain 26 gene, cause an autosomal-dominant form of inherited thrombocytopenia, THC2. Am. J. Hum. Genet. 88 1, 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presneau N., Shalaby A., Ye H., Pillay N., Halai D., Idowu B., Tirabosco R., Whitwell D., Jacques T. S., Kindblom L.-G. G., et al. (2011). Role of the transcription factor T (brachyury) in the pathogenesis of sporadic chordoma: a genetic and functional-based study. J Pathol 223, 327–335. [DOI] [PubMed] [Google Scholar]
- Prummel K. D., Hess C., Nieuwenhuize S., Parker H. J., Rogers K. W., Kozmikova I., Racioppi C., Brombacher E. C., Czarkwiani A., Knapp D., et al. (2019). A conserved regulatory program initiates lateral plate mesoderm emergence across chordates. Nat. Commun. 10, 3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennebeck G. M., Lader E., Chen Q., Bohm R. a, Cai Z. S., Faust C., Magnuson T., Pease L. R. and Artzt K. (1995). Is there a Brachyury the Second? Analysis of a transgenic mutation involved in notochord maintenance in mice. Dev. Biol. 172, 206–217. [DOI] [PubMed] [Google Scholar]
- Rennebeck G., Lader E., Fujimoto A., Lei E. P. and Artzt K. (1998). Mouse Brachyury the second (T2) is a gene next to classical T and a candidate gene for tct. Genetics 150, 1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S. M., Ludwinski F. E., Gnanalingham K. K., Atkinson R. A., Freemont A. J. and Hoyland J. A. (2017). Notochordal and nucleus pulposus marker expression is maintained by sub-populations of adult human nucleus pulposus cells through aging and degeneration. Sci. Reports 2017 71 7, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbud M. V, Schaer T. P. and Shapiro I. M. (2010). Toward an understanding of the role of notochordal cells in the adult intervertebral disc: from discord to accord. Dev. Dyn. 239, 2141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rito T., Libby A. R. G., Demuth M. and Briscoe J. (2023). Notochord and axial progenitor generation by timely BMP and NODAL inhibition during vertebrate trunk formation. bioRxiv 2023.02.27.530267.
- Rivera-Pérez J. A. and Magnuson T. (2005). Primitive streak formation in mice is preceded by localized activation of Brachyury and Wnt3. Dev. Biol. 288, 363–371. [DOI] [PubMed] [Google Scholar]
- Sangoi A. R., Karamchandani J., Lane B., Higgins J. P., Rouse R. V, Brooks J. D. and McKenney J. K. (2011). Specificity of brachyury in the distinction of chordoma from clear cell renal cell carcinoma and germ cell tumors: a study of 305 cases. Mod Pathol 24, 425–429. [DOI] [PubMed] [Google Scholar]
- Satoh N., Tagawa K. and Takahashi H. (2012). How was the notochord born? 14, 56–75. [DOI] [PubMed] [Google Scholar]
- Schifferl D., Scholze-Wittler M., Wittler L., Veenvliet J. V., Koch F. and Herrmann B. G. (2021). A 37 kb region upstream of Brachyury comprising a notochord enhancer is essential for notochord and tail development. Development. [DOI] [PMC free article] [PubMed]
- Schulte-Merker S., van Eeden F. J., Halpern M. E., Kimmel C. B. and Nüsslein-Volhard C. (1994). no tail (ntl) is the zebrafish homologue of the mouse T (Brachyury) gene. Development 120, 1009–15. [DOI] [PubMed] [Google Scholar]
- Schwaner M. J., Hsieh S. T., Swalla B. J. and McGowan C. P. (2021). An Introduction to an Evolutionary Tail: EvoDevo, Structure, and Function of Post-Anal Appendages. Integr. Comp. Biol. 61, 352–357. [DOI] [PubMed] [Google Scholar]
- Sebé-Pedrós A., Ariza-Cosano A., Weirauch M. T., Leininger S., Yang A., Torruella G., Adamski M., Adamska M., Hughes T. R., Gómez-Skarmeta J. L., et al. (2013). Early eèolution of the T-box transcription factor family. Proc. Natl. Acad. Sci. U. S. A. 110, 16050–16055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifnia T., Wawer M. J., Chen T., Huang Q.-Y., Weir B. A., Sizemore A., Lawlor M. A., Goodale A., Cowley G. S., Vazquez F., et al. (2019). Small-molecule targeting of brachyury transcription factor addiction in chordoma. Nat. Med. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. C., Price B. M. J. J., Green J. B. A. A., Weigel D. and Herrmann B. G. (1991). Expression of a xenopus homolog of Brachyury (T) is an immediate-early response to mesoderm induction. Cell 67, 79–87. [DOI] [PubMed] [Google Scholar]
- Song B. P., Ragsac M. F., Tellez K., Jindal G. A., Grudzien J. L., Le S. H. and Farley E. K. (2023). Diverse logics and grammar encode notochord enhancers. Cell Rep. 42,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemple D. L. (2005). Structure and function of the notochord: an essential organ for chordate development. Development 132, 2503–12. [DOI] [PubMed] [Google Scholar]
- Stosiek P., Kasper M. and Karsten U. (1988). Expression of cytokeratin and vimentin in nucleus pulposus cells. Differentiation 39, 78–81. [DOI] [PubMed] [Google Scholar]
- Takei H. and Powell S. Z. (2010). Novel immunohistochemical markers in the diagnosis of nonglial tumors of nervous system. Adv Anat Pathol 17, 150–153. [DOI] [PubMed] [Google Scholar]
- Takezaki N. (2018). Global Rate Variation in Bony Vertebrates. Genome Biol. Evol. 10, 1803–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Jing L. and Chen J. (2012). Changes in the Molecular Phenotype of Nucleus Pulposus Cells with Intervertebral Disc Aging. PLoS One 7, 52020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarpey P. S., Behjati S., Young M. D., Martincorena I., Alexandrov L. B., Farndon S. J., Guzzo C., Hardy C., Latimer C., Butler A. P., et al. (2017). The driver landscape of sporadic chordoma. Nat. Commun. 8, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasic B., Hippenmeyer S., Wang C., Gamboa M., Zong H., Chen-Tsai Y. and Luo L. (2011). Site-specific integrase-mediated transgenesis in mice via pronuclear injection. Proc. Natl. Acad. Sci. U. S. A. 108, 7902–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A. W., Hawkins M. B., Parey E., Wcisel D. J., Ota T., Kawasaki K., Funk E., Losilla M., Fitch O. E., Pan Q., et al. (2021). The bowfin genome illuminates the developmental evolution of ray-finned fishes . Nat. Genet. 2021 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosic J., Kim G.-J., Pavlovic M., Schröder C. M., Mersiowsky S.-L., Barg M., Hofherr A., Probst S., Köttgen M., Hein L., et al. (2019). Eomes and Brachyury control pluripotency exit and germ-layer segregation by changing the chromatin state. Nat. Cell Biol. 21, 1518–1531. [DOI] [PubMed] [Google Scholar]
- Veenvliet J. V., Bolondi A., Kretzmer H., Haut L., Scholze-Wittler M., Schifferl D., Koch F., Guignard L., Kumar A. S., Pustet M., et al. (2020). Mouse embryonic stem cells self-organize into trunk-like structures with neural tube and somites. Science (80-. ). 370,. [DOI] [PubMed] [Google Scholar]
- Vujovic S., Henderson S., Presneau N., Odell E., Jacques T. S., Tirabosco R., Boshoff C. and Flanagan A. M. (2006). Brachyury, a crucial regulator of notochordal development, is a novel biomarker for chordomas. J Pathol 209, 157–165. [DOI] [PubMed] [Google Scholar]
- Wang F., Zhang C., Shi R., Xie Z.-Y., Chen L., Wang K., Wang Y.-T., Xie X.-H. and Wu X. T. (2018). The embryonic and evolutionary boundaries between notochord and cartilage: A new look at nucleus pulposus-specific markers. Osteoarthr. Cartil. [DOI] [PubMed]
- Xu P.-F., Houssin N., Ferri-Lagneau K. F., Thisse B. and Thisse C. (2014). Construction of a vertebrate embryo from two opposing morphogen gradients. Science 344, 87–9. [DOI] [PubMed] [Google Scholar]
- Xu P. F., Borges R. M., Fillatre J., de Oliveira-Melo M., Cheng T., Thisse B. and Thisse C. (2021). Construction of a mammalian embryo model from stem cells organized by a morphogen signalling centre. Nat. Commun. 12,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakkioui Y., van Overbeeke J. J., Santegoeds R., van Engeland M. and Temel Y. (2014). The origin of chordoma. Biochim. Biophys. Acta. [DOI] [PubMed]
- Yanagisawa K. O. (1990). Does the T gene determine the anteroposterior axis of a mouse embryo? Japanese J. Genet. 65, 287–297. [DOI] [PubMed] [Google Scholar]
- Yang X. R., Ng D., Alcorta D. A., Liebsch N. J., Sheridan E., Li S., Goldstein A. M., Parry D. M. and Kelley M. J. (2009). T (brachyury) gene duplication confers major susceptibility to familial chordoma. Nat Genet 41, 1176–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Kwan K. M. and Mackem S. (2016). Putative oncogene Brachyury (T) is essential to specify cell fate but dispensable for notochord progenitor proliferation and EMT. Proc. Natl. Acad. Sci. U. S. A. 1601252113-. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.