Abstract
An imbalance of bacteria in oral environment can lead to a variety of oral diseases, such as periodontal disease, dental caries, and peri-implant inflammation. In the long term, in view of the increasing bacterial resistance, finding suitable alternatives to traditional antibacterial methods is an important research today. With the development of nanotechnology, antibacterial agents based on nanomaterials have attracted much attention in dental field due to their low cost, stable structures, excellent antibacterial properties and broad antibacterial spectrum. Multifunctional nanomaterials can break through the limitations of single therapy and have the functions of remineralization and osteogenesis on the basis of antibacterial, which has made significant progress in the long-term prevention and treatment of oral diseases. In this review, we have summarized the applications of metal and their oxides, organic and composite nanomaterials in oral field in recent five years. These nanomaterials can not only inactivate oral bacteria, but also achieve more efficient treatment and prevention of oral diseases by improving the properties of the materials themselves, enhancing the precision of targeted delivery of drugs and imparting richer functions. Finally, future challenges and untapped potential are elaborated to demonstrate the future prospects of antibacterial nanomaterials in oral field.
Keywords: Dental, Nanomaterials, Antibacterial, Oral, Remineralization
Graphical abstract

1. Introduction
The human oral cavity is a microbial swamp with about more than 700 species of microorganisms [[1], [2], [3]]. Most of the bacteria in the oral cavity are mainly attached to the teeth and mucous membranes in the form of biofilms. The strong survival ability of biofilms in hostile environment makes the treatment much more complicated than free stains, which is related to their different physiology and behavior from free strains [[4], [5], [6]]. When dental materials such as restorations, implants or orthodontic brackets enter the oral cavity, a new balance between microbiota needs to be established to maintain the health of cavity. However, the addition of dental materials tends to accumulate biofilms and results in the imbalance of bacteria and the occurrence of various oral diseases (such as periodontal disease, caries, or peri-implantitis) [[7], [8], [9]]. Therefore, the applications of antibacterial materials are highly required. Traditional antibacterial agents or fillers have many disadvantages such as low drug delivery efficiency, insufficient mechanical property, and unsatisfactory antibacterial effect, which cannot meet the therapeutic needs of patients with complex oral environment [[10], [11], [12]]. In addition, it cannot achieve long-term effective antibacterial due to their easy and rapid degradation or release, which may lead to the occurrence and recurrence of dental diseases, such as peri-implantitis, secondary caries, and pulpitis [13,14]. Therefore, new approaches are highly essential for infection prevention and treatment to achieve enhanced multifunctional performance.
Recently, antibacterial nanomaterials have been applied to dental materials as promising alternatives for traditional antibacterial drugs, bringing countless breakthroughs in treatment modalities. Advances in nanomaterial-based systems can provide novel strategies in prevention and treatment of dental diseases, especially in the control of oral pathogenic bacteria [[15], [16], [17], [18]]. Nano-antibacterial agents have stable antibacterial performance and broad antibacterial spectrum, due to their high specific surface area and high charge density. Nanomaterials with catalytic activities can generate reactive oxygen species (ROS), resulting in lethal oxidative stress [[19], [20], [21]]. It can also release ions, penetrate eukaryotic cells, as well as having high drug loading capacity [11,22,23]. Multiple bactericidal pathways feature the capacities to combat antibacterial more effectively and evade antibiotic resistance mechanisms, offering an opportunity to solve the public safety and health challenge of resistance to antibiotics. Furthermore, the special sizes and physical properties of nanomaterials allow them to target biofilms [24]. Some drug carriers even can achieve therapeutic selectivity and enhancement of delivery efficiency by releasing drugs in a controlled manner [25,26]. Besides effective antibacterial capacity, nanomaterials cater to the special needs of the oral environment [[27], [28], [29]]. Recently, various nanoantibacterial agents have been applied to modify the resin matrix or fillers, bringing further improvements in mechanical and physical performances. For example, nanomaterials containing Ca and P can promote the remineralization as well, due to the process of demineralization always started at the nano-level [22,30,31].
Overall, nanomaterials hold great promise for the treatment of oral bacteria-induced diseases, which have the potential in the long-term stable improvement of oral microenvironment. However, even though great progress has been made in nanotechnology, several problems still remain in the clinical applications [32,33]. Based on the characteristics of the oral mucosal environment and the therapy needs of different oral diseases, corresponding standards should also be followed in the design of antibacterial materials, such as: i) almost non-toxic to the human body; ii) meet the special needs of the oral cavity in different situations, including remineralization, anti-inflammatory; iii) can target specific lesions and penetrate areas that are difficult to reach by ordinary treatment; iv) low-cost and easy to produce [[34], [35], [36], [37]].
In this review, we have summarized the relevant researches on the applications of nano-antibacterial materials in dentistry in the past five years, and introduced them in detail from perspectives of single nano-antibacterial materials and composite nano-antibacterial materials (Fig. 1). We focused on the antibacterial effects and advantages of nano-antibacterial materials, and summarized and prospected the development of oral field.
Fig. 1.
Different types of nanomaterials used for antibacterial therapy in the oral field.
2. Nano-antibacterial materials
2.1. Inorganic nanoparticles
Metals and their oxides play an important role in the field of nano-antibacterial materials. They achieve effective antibacterial effect through multiple bactericidal mechanisms, including direct membrane damage, generation of ROS and binding to intracellular components. Furthermore, they have many special features, including chemical stability, catalytic activity and therapeutic selectivity (Table 1).
Table 1.
Summary of nano inorganic antibacterial agents in the dental field.
| Category | Morphology | Synthesis | Antibacterial | Application | Ref |
|---|---|---|---|---|---|
| nano-Ag | 50 nm | – | S. mutans: inhibition zone: 30 d: 7.88 mm | orthodontic adhesive | [38] |
| nano-Ag | – | from silver nitrate |
S. mutans biofilm: 99.76%; S. sanguis biofilm: 99.93%; L. acidophilus biofilm: 99.61% |
orthodontics fixed retainer | [39] |
| nano–Bi2O3, nano-HA, nano–ZnO, nano–Ag∗ | – | n-HA: a wet chemical method | S. mutans, S. aureus: HA: stable at ∼6.5%; Zn > Ag | PU-based system | [40] |
| DT-Ag-CS + nano-sonosensitizer | – | a photocatalytic method | in vivo: the lowest relative bacterial survival rate of only 19.3% | a multifunctional nanoplatform for treating periodontitis | [41] |
| nano–Ag | silver quartz fiber post | – | S. mutans, S. salivarius, S. sanguis: a fair antibacterial effect | endodontic sealers | [43] |
| Ag/ZnO | rod-like morphology length: 300–500 nm width: 10–20 nm |
ZnO: solvothermal method; Ag/ZnO: deposition-precipitation method |
S. mutans: inhibited 91% of bacteria growth | potentially effective dental antibacterial agents | [45] |
| Ag/ZnO-SPEEK | Ag: about 50–70 nm; ZnO: 120–150 nm | layer-by-layer self-assembly strategy | S. aureus: around 99.3%; E. coli: more than a 99.2% decrease | implant | [46] |
| nano-AgF@Silk fibroin nanofiber | - | Ag: reduction reaction; silk fibroin: electrospinning process | P. gingivalis: 3.1 folds | guided tissue regeneration | [48] |
| nano-silver fluoride | spherical and monodisperse particles | – | S. mutans: MIC: 30 ppm adherence | a new nano-Ag fluoride-containing dentifrice | [81] |
| nano-Sized Silver Ion Particles | disc shapes | – | S. mutans: effective | silver ions in porcelain | [82] |
| Dox NPs nano-Zn | no agglomeration | a polymerization precipitation procedure | multispecies biofilm: Dox-NPs: 72 h: 80%, 7 d: reduced about five times; Zn-NPs: 7 d, 87% | doped PolymP-n active nanoparticles | [50] |
| ZnO NRS | nanorods, nanospheres | a hydrothermal method | S. aureus, E. coli: effective antibacterial effect, in vivo: the least number of bacteria | implant | [51] |
| nano-ZnO | sizes: 20, 40, 140 nm | – | S. mutans, L. fermentum, E. faecalis, C. albicans: 20 nm greatest | – | [52] |
| nano-ZnO | ZnO-A: nanorod; ZnO–B: nanoplate | – | S. mutans, S. sobrinus: A > B | – | [53] |
| nano-ZnO | oval-shaped | chemical precipitation method |
S. mutans: inhibited; P. gingivalis: lower than 20% |
poly-carboxylate cement | [54] |
| nano-ZnO | spherical, hexagonal, long shape | a commercially available nanopowder of ZnO | saliva-derived multi species biofilm: 7.5 wt%: higher; total streptococci: no difference; S. mutans biofilm: 7.5 wt% best |
adhesive resin | [55] |
| PMMA with TiO2 | homogeneous spherical particles | TiO2: a modified sol-gel procedure | C. scotti: completely blocked | PMMA | [57] |
| nano_TiO2 | approximate spherical shape | solvothermalmethod | S. mutans biofilms: affect the viability | secondary caries in adhesive dentistry | [58] |
| Ti–6Al–4V alloys | lots of spherical grains and cavities |
the fiber engraving laser technique | E. coli: viable bacteria: lower | implant | [59] |
| Ti nanotubes | inductively coupled plasma | – | P. gingivalis: remarkably hamper the adhesion | implant | [60] |
| ZIF-8: Ce NPs | – | the obtained geometrical shape | F. nucleatum, P. gingivalis: about 2 log | anti-inflammatory and antibacterial platforms for treating periodontitis | [64] |
| CeO2–Ti | rod-CeO2 cube-CeO2 octa-CeO2 |
hydrothermal methods | S. sanguinis, P. gingivalis, F. nucleatum: decreased up to 2 orders of magnitude; octa > cube > rod; a strong limitation for gram-negativebacteria adhesion | implant | [65] |
| PAI-Cu | 124 nm | photoinitiated inverse-phase microemulsion polymerization |
S. mutans: 7.8 log-reduction, E. faecalis: 3.8 log-reduction, L. acidophilus: 3.5 log-reduction, A. viscosus: 7.6 log-reduction, P. gingivalis: 8.0 log-reduction, F. nucleatum: 7.0 log-reduction, A. actinomycetemcomitans: 7.8 log-reduction, P. intermedia: 7.4 log-reduction |
nanohydrogel | [68] |
| Cu-doped mesoporous bioactive glass nanospheres | rough surfaces | – | S. mutans, A. naeslundii: stronger than that of BG and Si composites | Cu-doped mesoporous bioactive glass nanospheres | [69] |
| Cu-BGn | – | – | E. faecalis: relatively lower | incorporate to the zinc phosphate cement | [71] |
| Cu-CDs | particle size with an average of 4.50 nm | a hydrothermal method | E. coli and S. mutans: kill almost 99%; S. aureus: no bacterial colonies: Cu-CDs: inhibit the biofilm rate: up to 97%; Cu-CDs + H2O2: antibiofilm activity: more than 90% | more convenient and economical daily strategy for oral healthcare | [70] |
| N–CaSiO3 | spherical mesoporous silica particles | hydrolysis of TEOS |
S. pyrogenes: MIC: much smaller; S. aureus: significant bacteriostatic properties, E. coli: very negligible; S. pyrogenes biofilm: much better than S. aureus and E. coli strains |
endodontic and orthopaedic applications | [72] |
| SBMP adhesive + 40% NACP and 5% DMAHDM | – | NACP: a spray-drying technique | multi-species biofilm (S. sanguinis + S. gordonii + S. mutans): much thinner biofilm | adhesive | [73] |
| Fe3O4 magnetic nanoparticles | inverse cubic spinel | – | S. aureus: enhance the destruction | treat peri-implant osteomyelitis | [74] |
| Dex-NZM | range from 30 to 60 nm | CAT-NPs: a solvothermal system | fold intensity of dead bacteria: 5 | a potent and biocompatible antibiofilm agent | [75] |
| bismuth subsalicylate | polygonal shape particle morphology | laser ablation of solids | inhibition growth: A. actinomycetemcomitans: 21.7 μg/mL: 90%; C. gingivalis: 21.7 μg/mL 90.1%; P. gingivalis: 21.7 μg/mL 91%; |
a potential clinical use; antiseptic solutions or mouthwash | [79] |
| GNCs-based mixed-MM-MON | around 1.8 nm | – | reduced the methicillin-resistant S. aureus implant colonization by about 2 logs in vivo | combating implant-associated infections | [80] |
2.1.1. Nano-Ag
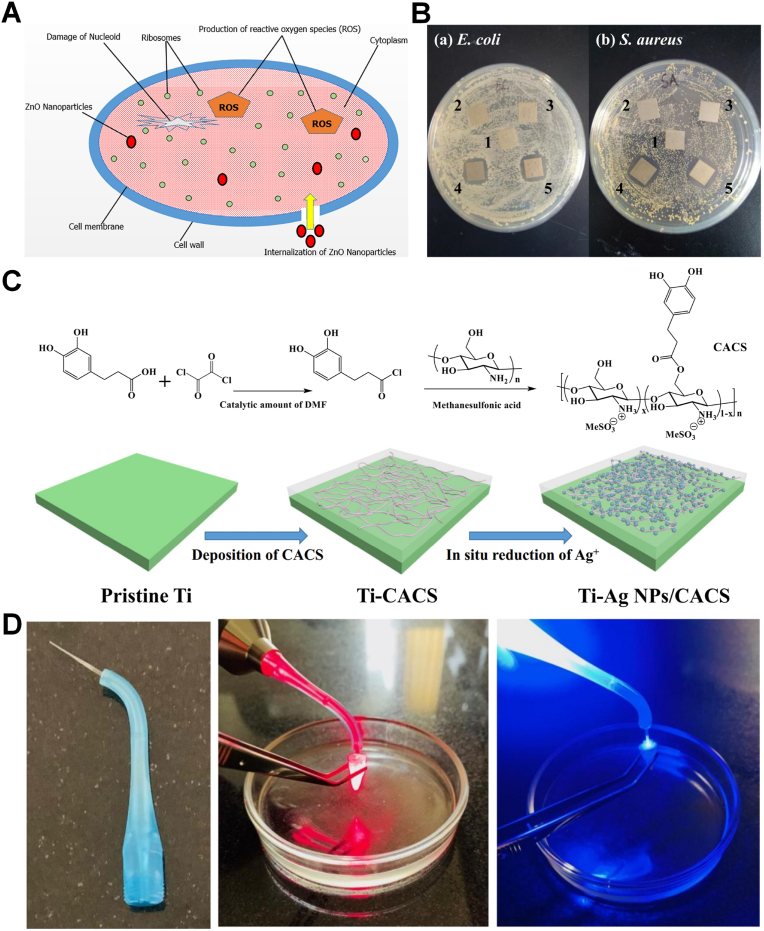
The antibacterial properties of silver were discovered in ancient Greece and recent researches have showed the antibacterial effects of nano-Ag are superior to that of other nano-agents [[38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48]]. Ag has broad spectrum antibacterial activity, which is effective against Gram-positive bacteria, Gram-negative bacteria, fungi and viruses. Recently, nano-Ag have been applied to dental materials (such as adhesives, composite resins, cements, etc.) in order to enhance the antibacterial performance. Mirhashemi et al. showed that the nanocomposite resin sample with 5% concentrations of nano-Ag can achieve reductions of more than 99% in Streptococcus mutans (S. mutans), Streptococcus sanguis (S. sanguis), Lactobacillus acidophilus (L. acidophilus) [39]. Some mechanisms of this antibacterial action have been revealed, but the exact mode remains unclear. Since nano-Ag have more contact surfaces, it is considered that nano-Ag can directly interact with bacteria, affect the mitochondrial functions and cause functional damage to the membrane. Nano-Ag can also combine with DNA and destroy its structure, inhibiting DNA replication. At the same time, it has the capability to release high levels of Ag ions (Ag+). Ag+ interacts with thiol groups (-SH) of antioxidants to generate a large amount of ROS, resulting in the bacteria death. The experiments of Lei et al. also suggested that nano-Ag have the ability to mediate mitochondrial apoptosis by regulating the expression of the B-cell lymphoma 2 (BCL-2) family (Fig. 2A) [40]. Multiple bactericidal pathways of Ag make it nearly impossible for bacteria to develop resistance to it. Xin et al. designed a novel nano-sonosensitizer was prepared for generating robust ROS through growing TiO2 on large pore mesoporous silica nanoparticles (DLMSNs) followed by deposition of Ag (DT-Ag) and modifying with the quaternary ammonium chitosan (DT-Ag-CS+) [41]. The results of showed that DT-Ag-CS + almost completely killed Porphyromonas gingivalis (P. gingivalis) in vitro, and in vivo experiments also showed the low bacterial survival rate of only 19.3%, demonstrating its potential in the treatment of periodontitis.
Fig. 2.
(A) Main mechanism responsible for the antibacterial activity and mitochondria-mediated apoptotic pathway [40], Copyright 2021 Royal Society of Chemistry. (B) SEM and TEM image of Ag/ZnO nanocomposite [45]. Copyright 2017 Elsevier. (C) Schematic drawing of the preparation of Ag/ZnO dual-decorated micro-/nanoporous SPEEK, and its bactericidal effect and osteogenic activity. (D) SEM observation of the amount and morphology of E. coli and S. aureus after culturing on the bare SEEK and modified SPEEK surfaces [46]. Copyright 2018 John Wiley and Sons. (E) Schematic of the surface modification process by fiber engraving laser [59]. Copyright 2021 Elsevier. (F) Fabrication of microgrooves and nanotubes on Ti surfaces [60]. Copyright 2020 Elsevier.
Several synthetic routes have been developed to produce nano-Ag. Amid them, hydrothermal method and reduction process are widely used. Nano-Ag may be agglomerated in the presence of proteins due to the large amounts of active sites, thus weakening the antibacterial effect. To avoid agglomeration, the stability can be improved via modifying their specific surface with various polymers such as poly (vinyl alcohol) (PVA) [42]. The shapes and sizes of Ag are main factors related to the antibacterial effect, due to the differences in the number of active sites and surface charge. Nano-Ag usually have different shapes, such as fiber posts, spheres, and rods (Fig. 2B) [[42], [43], [44], [45]]. The antibacterial effect decreases with the increasing size of nano-Ag. However, widespread use of nano-Ag is still limited due to the safety issues. Nano-Ag may lead to the accumulation of silver in internal organs, such as kidney and liver, resulting in injuries. Therefore, it is of great significance to avoid the toxicity problems of nano-Ag. It is well known that the toxicity of nano-Ag is dose-related. In order to safely utilize silver, the concentration of nano-Ag can be effectively reduced by supporting them on low-toxicity or non-toxic antibacterial materials without compromising the antibacterial effect. Recently, the combinations of nano-Ag and nano-ZnO have attracted enormous interest due to their antibacterial properties and biosafety. Deng et al. designed dual Ag/ZnO-decorated micro-/nanoporous sulfonated PEEK can inhibit the growth of Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) with a bactericide rate nearly 99% and the spreading MG-63 on the Ag/Zn-sulfonated PEEK surface were improved compared with single ion immobilized sulfonated PEEK, overcoming the problem of the toxicity of Ag-sulfonated PEEK (Fig. 2C and D) [46]. In addition, many properties affect the biocompatibility of nano-Ag, such as sizes, shapes and methods for preparing nano-Ag. The method of green synthesis of nano-Ag has been reported. The advantages of this route are clean, non-toxic and environmentally acceptable, and it has a variety of sources including plants. Logaranjan et al. successfully synthesized octahedral nanosilver with enhanced antibacterial effects using aloe vera plant extract as a shape-directing agent under microwave irradiation [47].
Furthermore, many dental materials (such as orthodontic brackets, restorations, orthodontic adhesive) have unique requirements for mechanical properties. And the influence on mechanical properties of materials of incorporating Ag should take into consideration. The study suggested shear bond strength (SBS) decreased after incorporation of nano-Ag into the orthodontic adhesive [38]. However, nano silver fluoride (NSF) coated on a novel electrospun silk fibroin (SF) nonwoven mat as a guided tissue regeneration (GTR) membrane significantly enhanced the tensile strength and elasticity and improved the ability to combat Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans) and P. gingivalis [48].
Nano-Ag feature many special physico-chemical properties, which are usually considered as the most promising materials. However, it is of great importance to focus more on the remaining problems, such as toxicity and stability. Furthermore, due to the aesthetic needs of the oral cavity, problems of changes of the color of materials should be avoided, such as the greening effect caused by the incorporation of Ag+ into the porcelain [49]. To overcome these challenges, optimal structures of silver and appropriate concentrations are still worth further studying.
2.1.2. Nano Zn and nano ZnO
Due to their superior biocompatibility and bleaching properties, nano-Zn and nano-ZnO are employed in many fields including dental materials and cosmetics [[50], [51], [52], [53], [54], [55]]. However, the mechanisms of antibacterial action of nano-Zn and nano-ZnO are still unclear. It is generally believed that nano-Zn directly interact with functional groups such as –SH and amine (-NH2) on bacterial proteins and ZnO can generate ROS through oxygen defect sites and release Zn2+, hence interfering with the DNA replication and metabolic paths. In addition, ZnO can contact with the bacterial membrane and affect the permeability, leading to bacteria death [56]. At the same time, as a photoactive material, zinc oxide will generate a large number of free hydroxyl radicals (·OH), hydrogen peroxide (H2O2), superoxide radical (·O2−) when exposed to light with a certain energy, stimulating a series of redox reactions and playing a bactericidal role. Various antibacterial pathways make Zn and ZnO effective against Gram-positive bacteria and Gram-negative bacteria, which have broad antibacterial spectrum and stable performance, which have antibacterial effects on various bacteria including S. mutans, Enterococcus faecalis (E. faecalis), Lactobacillus fermentum (L. fermentum). By applying nano-Zn to adhesion resin, and the measured bacterial death rates were 87% after 7 days, which was much higher than applying doxycycline (DOX) [50]. Wang et al. designed ZnO nanorods-nanospheres hierarchical structure (NRS), which showed excellent antibacterial activities against both E. coli and S. aureus and low cytotoxicity [51]. The Ti–Zr–ZnO NRS sample was implanted into the rats and they injected the bacterial suspension of S. aureus into implant position every day. The samples with ZnO NRS modification had the least number of bacteria, indicating the superior antibacterial effect. However, there is also confirmed that the antibacterial effect on Candida albicans (C. albicans) is not outstanding, which may be related to the participation of Zn2+ as a coenzyme in regulating the metabolic functions and the stability of the structure of the fungal enzymes [52]. The antibacterial properties of ZnO are mainly determined according to the size, shape, and morphology. By comparing 20 nm, 40 nm, and 140 nm nano-ZnO, it was also found that the smaller the size, the stronger antibacterial effect. A custom-made zinc boiling furnace pilot plan was implemented to produce two forms of zinc oxide (rod-shaped nano-ZnO and disc-shaped nano-ZnO) by using different crucible temperatures during the melting process. It showed that the ability of rod-shaped ZnO to combat S. mutans and Streptococcus sobrinus (S. sobrinus) was significantly higher than that of disc-shaped, which was closely related to its ability to release more Zn2+ and generate more ROS [53]. In addition to broad spectrum antibacterial activity, Zn, as one of the trace elements in the human body, plays an indispensable role in the human body and participates in the formation of bones. Therefore, Zn also has the potential to promote angiogenesis and osteogenesis. In addition, nano-ZnO powders have long been used to formulate restorative materials to improve mechanical strength. Incorporating spherical nano-ZnO into cement increased the compressive strength and made it have ability to fight against S. mutans and P. gingivalis [54]. Zn and ZnO can be synthesized in many ways, and many biosynthetic pathways have also been developed to improve their biocompatibility.
2.1.3. Nano TiO2
Titanium dioxide has been frequently used in dentistry, and its mechanical properties and antibacterial properties have been improved at the nanometer scale [[57], [58], [59], [60]]. The applications of titanium are more focused on planting. Nano-modification and nano-coating are popular ways to modify, so seeking better coating solutions and modification solutions is the main research direction in the field of titanium.
Titanium-based materials are a kind of excellent implant and orthopaedic materials. Its superior mechanical properties and low cost make it ideal material for implant and antibacterial fillers. Titanium dioxide has a wide antibacterial spectrum and has antibacterial effect on S. mutans, S. aureus, Fusobacterium nucleatum (F. nucleatum), P. gingivalis, etc [61]. Totu et al. added spherical TiO2 to PMMA denture base to produce antibacterial effect on Candida species [57]. As a photoactive material, a series of redox reactions will occur under the irradiation of ultraviolet rays, attacking polyunsaturated phospholipids of the microorganisms' membranes [62]. However, the damage of ultraviolet light to the oral cavity may limit its use as a photosensitizer. To overcome this disadvantage, Florez et al. doped TiO2 nanoparticles with N, which displayed higher light absorption levels and realized antibacterial efficacy under dark conditions [58]. Nanoscale implant modification is a popular method to reduce the adhesion of bacteria, but it also inhibits the adhesion of osteoblasts. To seek the optimal surface wetting angle, an experimental team used modern fiber engraving laser method to modify the surface of Ti–6Al–4V dental implants at the micro-nano level as shown in Fig. 2E, which reduced the adhesion of E. coli without affecting osteogenesis [59].
TiO2 have a variety of shapes, among which TiO2 nanotubes have many unique properties that can play a role in the modification of implant surfaces [63]. Zhou et al. designed a best implant surface by changing the depth into 3.6 μm and further decorating with 55 nm nanotubes, which had unique structural advantages, enabling them to carry drugs, such as gentamicin, naproxen sodium and providing a new idea for nanocarriers (Fig. 2F) [60].
2.1.4. Nano CeO2
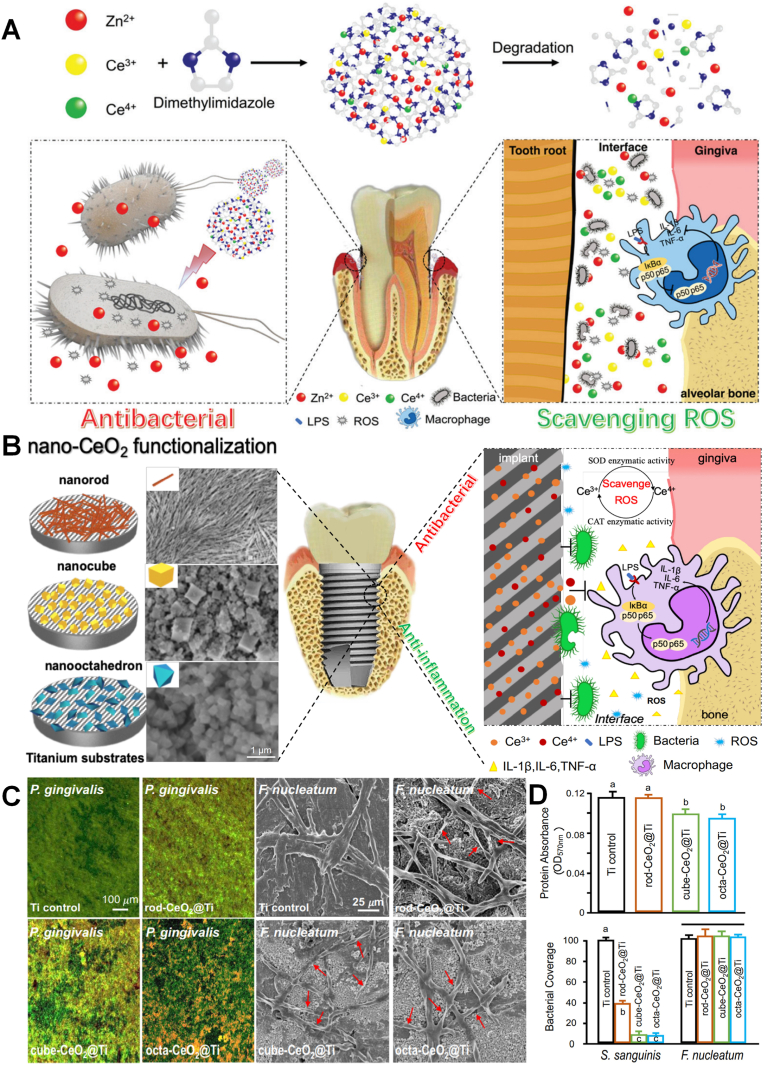
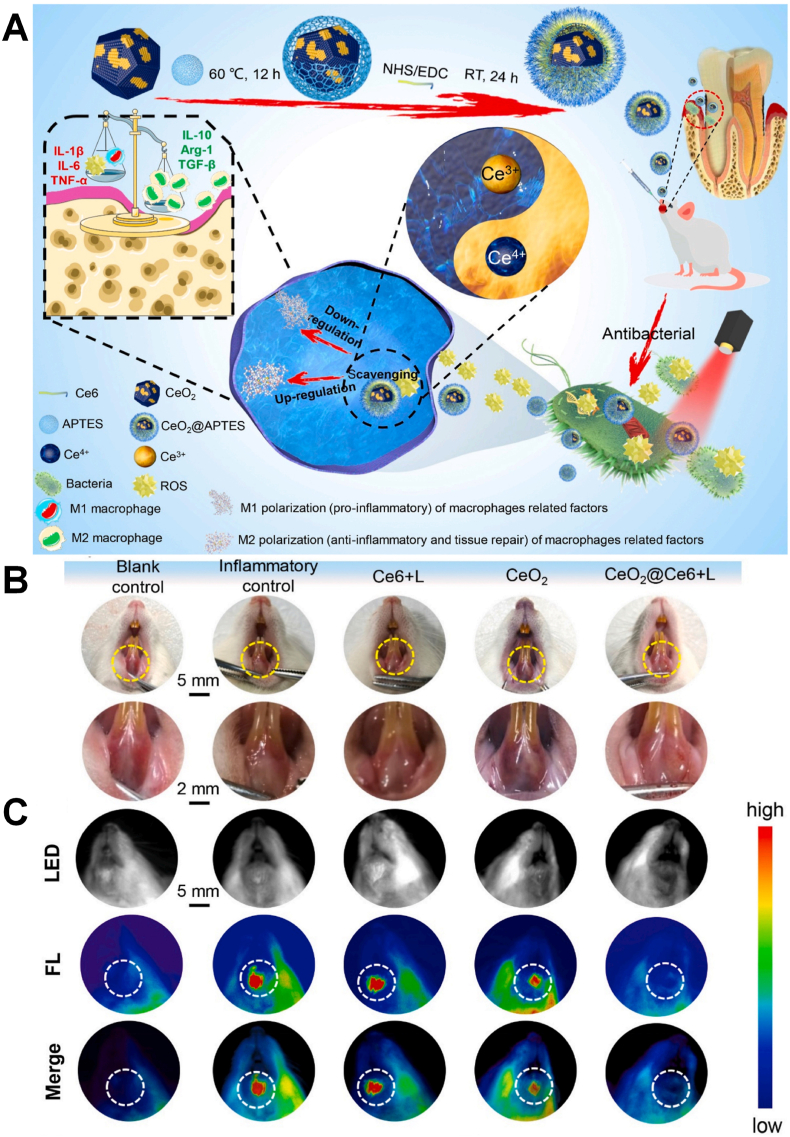
The dual mechanism of antibacterial and anti-inflammatory makes CeO2 promising in the field of bacterial-induced inflammation such as periodontitis and peri-implantitis [[64], [65], [66]]. The interaction between CeO2 and the bacterial surface may lead to the inactivation of bacterial surface proteins through the interaction of –SH, resulting in a decrease in the permeability. Aside from their broad spectrums of antibacterial activity, CeO2 can reduce the expression of TNF-a, IL-6 and IL-1β in macrophages by restraining the NF-kB/p65 subunit translocation, achieving the anti-inflammatory effect. However, the mechanism of Ce is different from other inorganic materials including Ag and Zn to promote the generation of ROS, CeO2 is through the regulation of ROS cycle to scavenge ROS. CeO2 has the ability to simulate the generation of ROS by superoxide dismutase (SOD) and the elimination of ROS by catalase (CAT) through the redox cycling reaction between Ce3+/Ce4+, the catalytic reaction of SOD and H2O2, and the transformation of oxygen vacancies. Li et al. doped nano-Ce into zeolitic imidazolate framework-8 (ZIF-8) [64] (Fig. 3A). Ce could achieve superior antibacterial effect and its ability to scavenge ROS produced from free Zn2+, enabling them to protect cells from oxidative stress. And the results also confirmed that the ZIF-8 could decrease the CFU count of F. nucleatum and P. gingivalis by about 2 log and achieve lower toxicity, indicating it could be a promising application for treating periodontitis. The antibacterial and anti-inflammatory mechanism also proved that the catalytic activity of ceria could be regulated by changing the morphological structure and size of CeO2, due to the ratio of Ce3+ to Ce4+ and the number and mobility of its surface oxygen vacancies [64,67]. Li et al. simultaneously synthesized rod-shaped, cube-shaped and octahedral CeO2 by hydrothermal method [65]. As shown in Fig. 3B–D, the experiment found that the octahedral CeO2 with the highest Ce3+ ratio showed the best antibacterial properties against P. gingivalis, F. nucleatum, and S. sanguis, which was related to the similar function of Ce3+ with SOD. The unique antioxidant function, the reduction of oxidative stress and the expression of pro-inflammatory iNOS protein provide a safe living environment for human cells, demonstrating CeO2 has excellent biocompatibility. A higher proportion of Ce4+ is beneficial to promote the osteogenesis of stem cells and the polarization of macrophages, and osteogenic function makes it promising for modified implants. Based these unique properties, Sun et al. used CeO2@Ce6 nanocomposites to achieve simultaneous sterilization and anti-inflammatory effect via a dual directional regulation effect (Fig. 4A) [66]. The animal models with periodontal diseases in vivo was established and the CeO2@Ce6 nanocomposite was administrated into the infectious site and irradiated upon 630 nm light for 3 min per day to evaluate the effects of CeO2@Ce6 nanocomposite on aPDT-aggravated periodontal inflammation. As shown in Fig. 4B and C, almost all the local inflammation together with redness and swollen disappeared and the fluorescence signals of the fluorescent probe for ROS (DCFH-DA) injected in situ was as weak as the blank control, determining aPDT/antioxidant synergistic therapy can be valuable in stable disease treatment of periodontitis.
Fig. 3.
(A) Schematic illustration of ZIF-8: Ce NPs and their antibacterial and anti-inflammatory properties [64]. Copyright 2019 Royal Society of Chemistry. (B) Schematic illustration of implant surface modified by nano-CeO2 (rod-CeO2, cube-CeO2, octa-CeO2) for antibacterial and anti-inflammatory properties. (C) Representative live/dead images of 4-day biofilms of P. gingivalis on Ti with different surfaces and SEM images show the obvious F. nucleatum bactericidal effect of CeO2-functionalized Ti disks. (D) Protein adsorption onto different surfaces (n = 6; mean ± sd) and CLSM images and area cover fractions of S. sanguinis and F. nucleatum early colonization onto Ti at 4 h (n = 6; mean ± sd) [65]. Copyright 2018 Elsevier.
Fig. 4.
(A) Schematic illustration of CeO2@Ce6 nanocomposite in synthesis, the antibacterial mechanism and modulating the polarization of macrophages for the treatment of periodontal diseases. (B, C) The different response of local periodontal inflammation to the different treatments in animal models [66]. Copyright 2018 Elsevier.
Ce has excellent antibacterial and anti-inflammatory properties, which can solve many problems that cannot be overcome by classical antibacterial materials, including excessive ROS generation that damages the body's own cells. Cerium with different structures has different ratios of Ce3+/Ce4+, due to Ce3+ and Ce4+ undertake different functions, which can also be used flexibly for different environments and needs. Although the problem of toxicity still remains to be solved, the future application prospects of cerium should not be underestimated.
2.1.5. Nano Cu
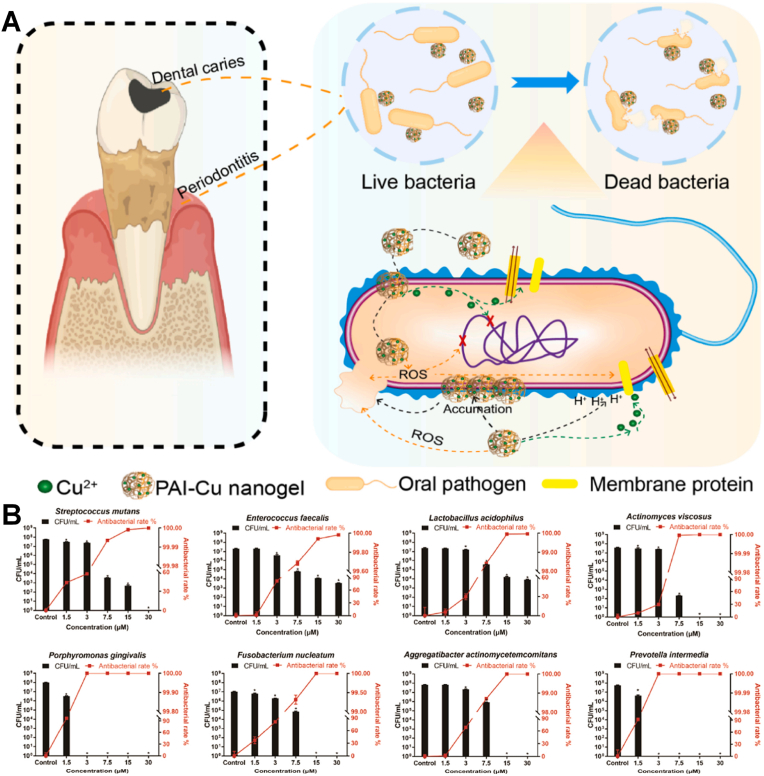
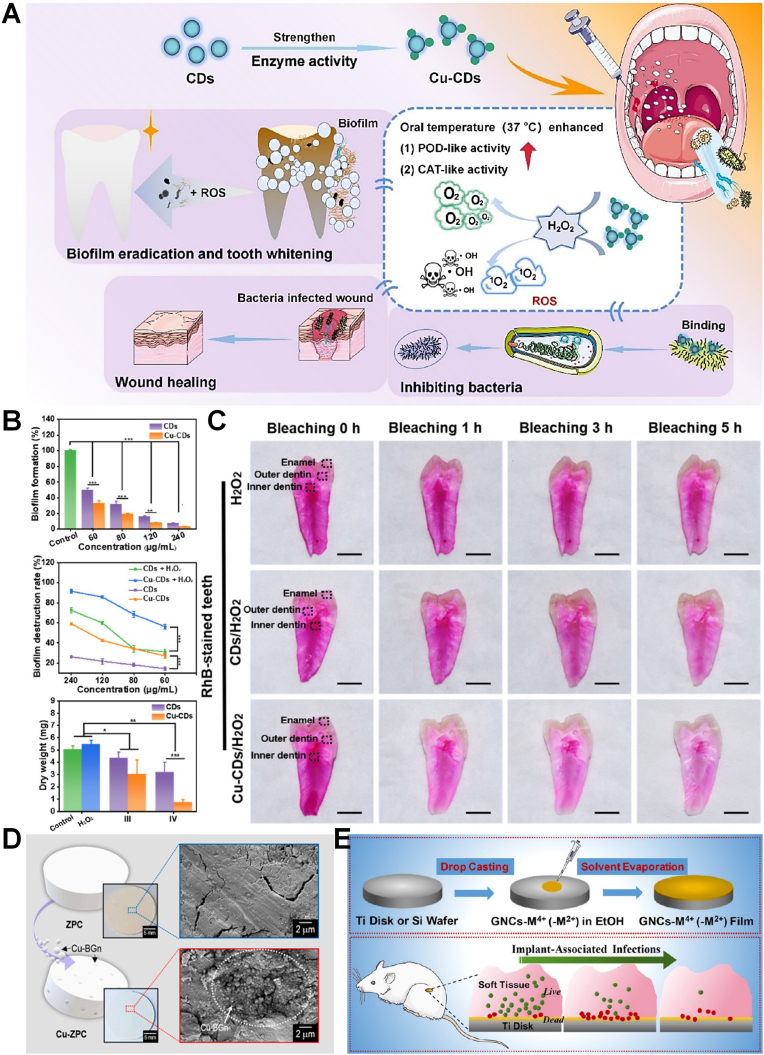
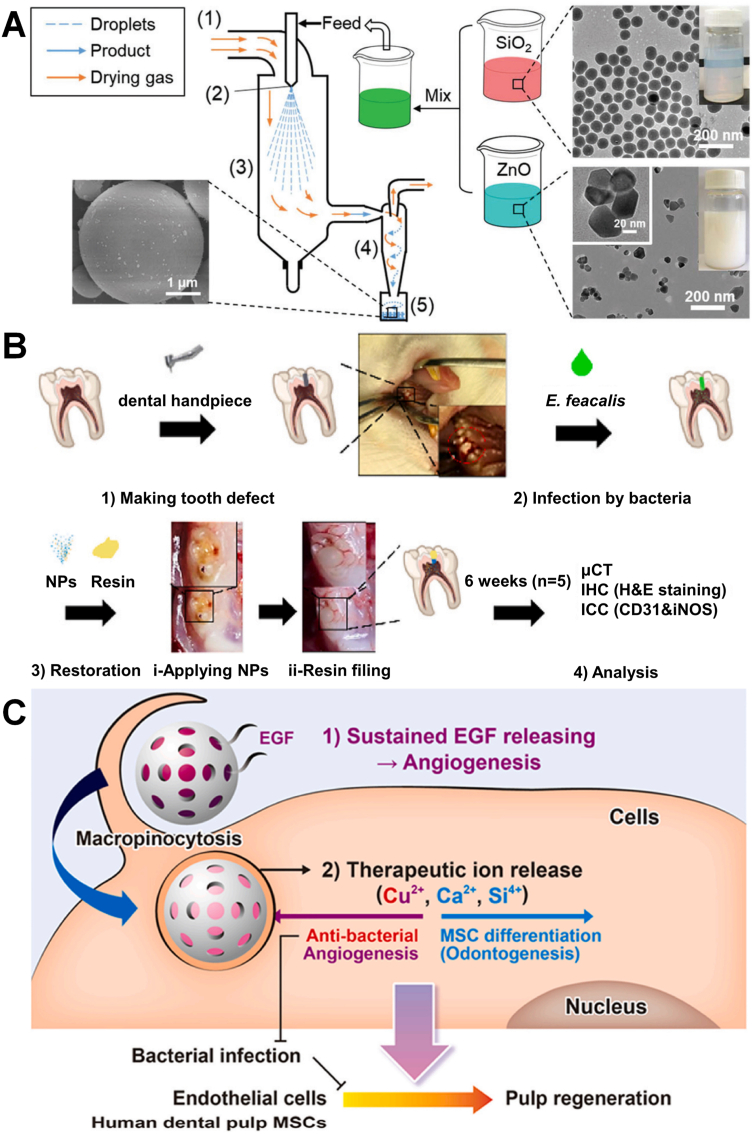
Cu is a kind of essential trace element that involved in multiple enzymatic and cellular processes, which also can prevent bacterial infection of teeth [[68], [69], [70], [71]]. And nano-Cu may exert bactericidal, angiogenic, or pro-osteogenic activities in different doses, due to its multifunctional biological activities. Copper ions (Cu2+) can produce oxidative stress to cause the damage of a bacterial cell membrane or inhibit bacterial metabolism to exhibit excellent anti-bacterial effect. However, nano-Cu can become severely toxic at relatively high concentrations. To overcome its toxicity and optimize it, Zhan et al. composed a trace amount of Cu2+ and nanoscale biocompatible polymer poly (acrylic acid-co-itaconic acid) (PAI-Cu) nanohydrogel, which achieved high antibacterial effectivity and low toxicity [68]. The bactericidal mechanism of nanohydrogel can be divided into damaging the cell wall and generating ⋅OH. Cu2+ accumulated on the surface can affect the membrane permeability of bacteria and break down the microbial electron transport system and material transport system (Fig. 5A and B). Munir et al. designed a copper-doped mesoporous bioactive glass nanospheres (Cu-MBGN) which achieved the release of calcium and Cu2+ for 28 days [69]. And the results showed Cu-MBGN had anti-bacterial effect against free-floating S. mutans and significantly reduced the formation of S. mutans biofilm. Due to the complex environment of dentistry, Liu et al. synthesized biomaterial-based enzyme-powered copper-doped carbon dots (Cu-CDs) as shown in Fig. 6A, which exhibited enhanced catalytic (CAT-like, POD-like) activity in the oral environment for inhibiting initial bacteria (S. mutans) adhesion and for abundant biofilm dissociation without impacting the surrounding oral tissues through O2 and ROS generation [70]. Cu-CDs can bind lipopolysaccharides (or peptidoglycans) derived from bacterial cell walls effectively, and achieve antibacterial effect and eradicate most biofilm through catalytic activation. Using a representative oral biofilm model, the results showed that the Cu-CDs group can inhibit up to 97% biofilm and Cu-CDs + H2O2 group can exhibit over 90% of the antibiofilm activity (Fig. 6B). The enzymatic EPS degradation turned out different degrees of surface destruction of biofilms and dissociation of the skeleton structure. Cu-CDs also have the capacity of promoting wound healing and tooth whitening (Fig. 6C). Furthermore, zinc phosphate cement (ZPC) with Cu doped bioglass nanoparticles (Cu-BGn) exhibited sufficient mechanophysical properties and enhanced antibacterial effect (Fig. 6D) [71]. In general, nano-Cu are widely used in the field of oral antibacterial and have broad prospects.
Fig. 5.
(A) Schematic illustration of the preparation of PAI-Cu nanohydrogels and antibacterial mechanisms of PAI-Cu nanogels on oral pathogens. (B) Colony forming unit counting was conducted to quantitatively evaluate the antibacterial effect [69]. Copyright 2022 Elsevier.
Fig. 6.
(A) Schematic diagram of the procedures of Cu-CDs with oral-adaptive dual-enzyme mimicking catalytic activity for multifunctional prevention and treatment of oral diseases. (B) Antibiofilm properties of Cu-CDs. (C) Tooth internal whitening based on nanozyme activity; photographs of the interior of RhB-stained teeth after bleaching for 0, 1, 3, and 5 h, respectively [70]. Copyright 2022 American Chemical Society. (D) Schematic, optical, and SEM images of the fabricated ZPC and Cu-BGn incorporated ZPC (Cu-ZPC) to observe the Cu-BGn distribution [71]. Copyright 2022 Elsevier. (E) Schematic of gold nanocluster constructed mixed-metal metal-organic network film for combating implant-associated infections [80]. Copyright 2020 American Chemical Society. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2.1.6. Nano Ca
Calcium, as a constituent element of bone, has significant value in osteogenesis and remineralization [40,72,73]. CaSiO3 can cause damage to the proteins of bacterial cell walls and the DNA inside, and it can bind directly to living tissue. Biwas et al. synthesized 100% phase pure calcium silicate nanoparticles (CSNPs) by a cheap and simple method, which have higher hardness, superior Young's moduli values. They can also be antibacterial against Streptococcus pyogenes (S. pyrogenes), S. aureus, and E. coli, making them potentially valuable for orthopaedic and endodontic applications [72]. In addition, CaPO4, CaP, CaF and CaO also have the ability to promote remineralization, and these ionic compounds are often added to resins. Amid them, nano amorphous calcium phosphate (NACP) has attracted much attention. It has been reported that NACP can achieve acid-neutralization function to resist the effect of acid-producing bacteria, and can also release a large amount of Ca and P ions to relieve the demineralization at cariogenic pH [73]. Hydroxyapatite (HA), another compound of Ca, has extremely excellent biocompatibility. It also shows high affinity for histatins, where derivatives of histamines have abilities to improve its antibacterial efficiency against a variety of pathogens. Due to the capability of stimulating the calcium bridge formation at the apex, n-HA was introduced to the sealer, exhibiting stable antibacterial activity [40]. It is showed that, as a slow-release agent of Ca ions (Ca2+), n-HA can induce immature hard tissue formation and have a slow but stable and sustained antibacterial effect on Gram-positive bacteria.
To sum up, Calcium's antibacterial, osteogenetic and remineralizing abilities make it favored by the dental field, and calcium has the potential for widespread use in the implantation and prevention of caries.
2.1.7. Nano iron oxide
Fe is a trace element required by the human body and an essential element for the active sites of many electron transfer and oxygen transport enzymes. In recent years, it has also been introduced into oral materials [74,75]. Iron oxide nanocrystals (IONPs) have attracted widespread attention due to its superparamagnetism and superior biocompatibility, which have been widely used in medical imaging. As a member of IONPs, superparamagnetic iron oxide nanoparticles (SPIONs) have been widely used in magnetic resonance (MRI) contrast agents due to its safe use in vivo. SPIONs can enhance, target and combat pathogens effectively through their unique magnetic properties. Fang et al. have used SPIONs to improve antibiotic efficacy of the treatment of peri-implant osteomyelitis [74]. The results of osteomyelitis rats after 40 days infection have showed that temperature up to 75 °C still do not cause tissue damage and enhance antibiotic penetration by disrupting the biofilm polysaccharide matrix. Furthermore, catalytic iron oxide nanoparticles (CAT-NPs) termed nanozymes have been proved to have the capacity of natural peroxidases, which can activate H2O2 in vitro and achieve the effect of anti-plaque or caries-preventive [76]. To apply them in vivo, Naha et al. designed dextran-coated iron oxide nanoparticles termed nanozymes (Dex-NZM) and the iron cores accounted mostly for the catalytic activity and the catalytic activation of H2O2 degrade exopolysaccharides matrix at acidic pH value [75]. The assessment of the Dex-NZM binding and antibiofilm activity showed that approximately 49% of Dex-NZM were colocalized with bacteria and 51% with the EPS and Dex-NZM penetrated into the biofilm (up to 40 μm penetration vs 56 μm average biofilm height) with a homogeneous distribution, indicating the selectivity and strong antibacterial effect. A well-established rodent model of dental caries showed the excellent in vivo anti-caries treatment efficacy, the superior synergistic antibacterial ability and biocompatibility.
Iron oxide has unique properties, and its targeted capability can improve the efficiency of treatment and reduce the load on the body [77,78]. In addition, as an essential element of the human body, superior biocompatibility can bring opportunities to solve the toxicity problem of composite materials.
2.1.8. Nano bismuth compounds
Bismuth-based compounds have been used in dental materials for more than a decade, and their antibacterial properties have also been widely recognized [79]. The biggest advantage of bismuth is its low toxicity and broad spectrum of antibacterial. A team has used laser ablation of solids to synthesize nano-bismuth subsalicylate (BBS-nano) and confirmed that it had greater sensitivity against Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans), Capnocytophaga gingivalis (C. gingivalis), Prevotella intermedia (P. intermedia). And the toxicity test using gingival fibroblasts also confirmed its good biocompatibility, suggesting that BBS-nano can be used as a dental antibacterial agent [79].
The superior biocompatibility of bismuth is widely recognized, and its broad-spectrum antibacterial properties have been continuously confirmed, suggesting an alternative antibacterial in the oral field.
2.1.9. Nano Au
Gold is an ancient and traditional antibacterial material and introduced as novel applications including nanobiomedicine [80]. Nano-Au have convenient surface bio-conjunction and obvious plasmon resonance optional properties. Gold nanoparticles have inhibitory effect on both Gram-positive and Gram-negative bacteria. The antibacterial mechanism of nano-gold may be adsorbed on the cell membrane through electrostatic interaction, changing its membrane structure, and entering the cell to affect mitochondrial function. Chu et al. prepared para-mercaptobenzoic acid (pMBA)-capped gold nanoclusters (GNCs) and further mixed them with metal−organic network (MM-MON) films on titanium disks, showing the adjustable antibacterial activity [80]. The MM-MON films showed excellent stability due to the robust M4+ (Ti, Zr, Hf; hard Lewis acid) –O bonds and M2+ (Cu, Zn; borderline acid) releasing behavior resulting from the labile M2+−O coordinating. And by integrating the bacteria-responsive character of GNCs, it achieved the ability of killing the adherent bacteria, which was proved by a rat model for subcutaneous implant-associated infection (Fig. 6E). Furthermore, due to its inert properties, gold is generally considered as non-toxic material, which is highly required for dental materials.
2.2. Organic antibacterial material
In the field of organic materials, there are various nano-antibacterial materials with great potential for development. In addition to traditional organic oral antibacterial nanomaterials, multifunctional nano agents including nano quaternary ammonium, nano chitosan and nano-Chinese medicines are gradually emerging, providing more and better choices for oral antibacterial designs (Table 2). The advantages of organic antibacterial materials are numerous, including broad-spectrum antibacterial properties, stable property and safety.
Table 2.
Summary of nano organic antibacterial agents in the dental field.
| Category | Morphology | Synthesis | Antibacterial | Application | Ref |
|---|---|---|---|---|---|
| quaternary ammonium polyethylenimine | – | – | S. mutans and L. casei: >40% reduction;S. mutans monospecies biofilm: >60% reduction | orthodontic brackets | [83] |
| TMC-Lip-DOX NPs | a diameter of around 107.6 nm | – | bacterial biofilm: a small number of free bacteria;in vivo: the bacterial plaque decreased | good potential for use in the treatment of periodontal and other inflammatory diseases | [84] |
| catechol-functionalized chitosan and Ag NPs | homogenous esterification reaction | – | E. coli, S. aureus: adhered bacterial cell; E. coil: dead: 32.2%;S. aureus: dead: 14.3% | coatings for practical biomedical applications | [88] |
| Curcuma xanthorrhiza oil | – | sonication | S. mutans biofilm: 10 min: the lowest value of 5.72 log10 CFU mL−1 compared with distilled water (7.83 log10 CFU mL−1) and Listerine (7.52 log10 CFU mL−1), | nanoemulsion–based mouthwash | [89] |
| N-CUR@ICG- Met | a uniform shape | under ultrasonic conditions | E. faecalis biofilm: aPDT: statistically decrease; aPDT/LED < aPDT/diode | a synergistic advantage with photosensitivity | [90] |
| nCUR- ABBL | length: around 200 nm; width: 50–100 nm | – | S. mutans biofilm: 5%: 60 d: decreased 40% | an excellent ABBL additive in aPDT producer | [91] |
| chlorhexidine | particle diameter: 140 nm; pore: 2.5 nm | CHX swell and assemble to nanoparticles | S. mutans: MIC: 19.5 mg/mL; MBC: 312.5 mg/mL;E. faecalis: MIC: 156 mg/mL; MBC: 1250 mg/mL | a novel and promising anti-biofilm agent | [92] |
| SB and pure chlorhexidine | diameter: 140 nm; pore: 2.5 nm; volume: 1.0 cm3/g | SB was swelled and loaded into mesoporous silica nanoparticle | S. mutans; S. sobrinus; F. nucleatum, A. actinomycetemcomitans: MIC: lower than 50 μg/mL;E. faecalis: MIC: 200 μg/mL; | potential clinical usage | [93] |
| nano cinnamon powder | – | mechanical attrition method | S. mutans: inhibition zone: 3%: 25 mm | orthodontic | [94] |
| Cinnamon modified TiO2 NPs | 10.5 nm | hydrodistillation | P. aeruginosa: large inhibition zone | potential biological applications | [95] |
2.2.1. Nano quaternary ammonium
Quaternary ammonium particles have antibacterial effects on a variety of bacteria, fungi and viruses, which are broad-spectrum antibacterial agents [83,84]. Quaternary ammonium destroys the integrity of the cell membrane by interacting with the cell membrane, causing cytoplasmic leakage and cell death. And when it becomes nanoscale, the antibacterial effect is much better. Quaternary ammonium nanoparticles are easy for polymers to form covalent bonds and stabilize, and can maintain a long time without being released, achieving stable and long-term bacteriostasis [85]. Furthermore, by adding quaternary ammonium nanoparticles into the dental materials, it is able to achieve materials with antibacterial activity without weakening their original morphology and properties. Based on the advantages of quaternary ammonium nanoparticles, a team added nano-quaternary ammonium polyethylenimine (QPEI) into orthodontic brackets to achieve antibacterial effects on S. mutans and Lactobacillus casei (L. casei), which are common bacteria on orthodontic brackets [83]. N,N,N-Trimethyl chitosan (TMC) is the simplest form of quaternary ammonium chitosan derivative and has a permanent positive charge, which has higher antibacterial activity against both gram-positive and gram-negative bacteria than chitosan. Hu et al. synthesized TMC-liposome-doxycycline NPs (TMC-Lip-DOX NPs) [84]. The results of animal experiments proved that the NPs could strongly inhibit biofilms formation and prevent alveolar bone absorption in vivo, indicating the TMC-Lip-DOX NPs have good potential for treating periodontal and other inflammatory diseases.
The advantage of nano quaternary ammonium is that it does not affect other properties of the material, and even optimizes the material. It can play an auxiliary antibacterial role in many fields [86].
2.2.2. Nano chitosan
Natural non-toxic chitosan has biodegradability, biocompatibility properties and can be used as an antibacterial bioactive material in drug or vaccine delivery systems, tumor and other fields. Recent researches have demonstrated the value of chitosan in oral field [87,88]. Chitosan has the unique properties of cationic global charge, which can interact with negatively charged bacterial membranes, resulting in the bacteria death. Due to antibacterial properties, chitosan has been introduced into the field of dental anti-caries. Javed et al. showed chitosan as stabilizing agent impregnated ZnO nanoparticles fabricated from co-precipitation technique has a synergistic antibacterial effect (Fig. 7A) [87]. Furthermore, to prepare a practical application of long-term stability and effectiveness bactericidal coatings, Cheng et al. synthesized the water-soluble catechol-containing chitosan (CACS) and the CACS-coated Ti surfaces were further deposited with Ag NPs via in-situ reduction. The resulting Ag NPs/CACS-coated Ti surfaces exhibited antibacterial properties and can prevent the surface adhesion of E. coli and S. aureus (Fig. 7B and C) [88]. Chitosan has significant effect on antibacterial and anti-adhesion, and can be used more widely in the field of dentistry in the future.
Fig. 7.
(A) Diagrammatic representation of mechanism of bactericidal activity of ZnO nanoparticles [87]. Copyright 2020 Elsevier. (B) Inhibition zone test of the pristine and modified Ti substrates against. (C) Schematic illustration for the preparation of CACS and the fabrication CACS- and Ag NPs/CACS-coated Ti surfaces [88]. Copyright 2019 Elsevier. (D) Three-dimension fiber-optic system; Irradiation of diode laser at wavelength of 810 nm and LED at wavelengths of 450 nm to the root canal with E. faecalis biofilm, respectively [90]. Copyright 2020 Elsevier.
2.2.3. Nano Chinese medicine
In recent years, antibacterial effects of traditional Chinese medicine have been confirmed and nano traditional Chinese medicine have shown to synergize with other ingredients to achieve the purpose of strong antibacterial, making the ancient medicine more widely used in the advanced nanometer way and be accepted internationally. In this section, nano curcumin (CUR), scutellaria baicalensis (SB) and Cinnamon will be introduced respectively [[89], [90], [91], [92], [93], [94], [95]].
CUR can be extracted from the rhizome of turmeric (Curcuma longa L.), which has traditionally been used as a medicinal or food ingredient in China. And nano-CUR have been proven to be antibacterial, anti-inflammatory and non-toxic agents with superior biological activity, pharmacological activity, which can repair and regenerate eukaryotic cells [96]. Nano-CUR can interact with the cell wall of the bacterial cell and break the peptidoglycan layer and penetrated inside the cell, killing the cell through lysis. Cho et al. formulated Curcuma xanthorrhiza oil as an optimal nanoemulsion, exhibiting stable and strong antimicrobial effects against S. mutans [89]. Furthermore, when curcumin is reduced to nanometer size, it overcomes the problems of poor water solubility, poor bioavailability and easy hydrolysis, which greatly improves its application value in the field of aPDT. The team doped nano-curcumin into indocyanine green (ICG) and metformin (Met) for photodynamic therapy and the synergistic effect of the two greatly improved its photosensitivity, confirming that N-CUR@ICG-Met provided a conjugating advantage with photosensitivity in irradiation of diode laser at wavelength of 810 nm and LED at wavelengths of 450 nm, which enhanced the treatment of root canals for E. faecalis biofilm infection (Fig. 7D) [90]. In addition, it has been confirmed that CUR can inhibit the production of acid by S. mutans while inhibiting the adhesion of S. mutans. Pourhajibagher et al. demonstrated that 5% nCUR-Activa BioActive Base/Liner (ABBL) activated by aPDT still had significant anti-biofilm activity against S. mutans within 60 days of aging [91].
SB has been used as a traditional Chinese medicine to treat infectious diseases and inflammation of all the time. SB is anti-inflammatory by decreasing the release ofhistamine activating lymphocytes, the phagocytic function of neutrophils and permeability of capillaries, which enables it to inhibit the development of experimental periodontitis and control periodontal disease. We have further shown that baicalin could modulate P. gingivalis lipopolysaccharide-induced immuno-inflammatory response in oral epithelia On the basis of previous experiments that have proved the antibacterial properties of chlorhexidine [92], a team encapsulated nano-SB and nano-chlorhexidine in a ratio of 9:1 (w/w) with mesoporous silica nanoparticles, and the data showed that a lower MIC significantly affected the composite biofilm (S. mutans, F. nucleatum, A. actinomycetemcomitans and P. gingivalis), enhanced the biocompatibility, and also solved the problems of tooth staining caused by chlorhexidine to a certain extent [93].
The antibacterial properties of Cinnamon are related to the compounds with antibacterial ability such as cinnamaldehyde, eugenol, benzoic acid, benzaldehyde and cinnamic acid. Cinnamon nanoparticles have the ability to generate ROS, inducing oxidative stress in bacteria. Yaseen et al. incorporated cinnamon nanomaterials into orthodontic composites effectively against S. mutans at concentrations that did not affect SBS [94]. And Maheswari et al. used Cinnamon to modify nano-TiO2 and the results of antibacterial experiments also showed that it could strongly inhibit the growth of Pseudomonas aeruginosa (P. aeruginosa) [95].
The value of nano Chinese medicine is constantly being explored. Their antibacterial ability and superior biocompatibility make them possible to be applied in dental materials. It is expected that more Chinese medicine materials will be added to oral materials in the form of nanometers in the future.
3. Composite antibacterial materials
3.1. Combination of nano-antibacterial agents
In the face of the complex and changeable environment of the oral cavity, it is difficult for a single material to meet the requirements in many aspects including antibacterial effect and safety. While composite materials can combine the advantages of various materials to achieve multi-functional applications or use the synergy between materials to achieve low-concentration antibacterial effects and improve biosafety. At present, nanocomposites are mainly the combinations of nano-antibacterial agents (Table 3). However, the ratio of combining with other materials is still a topic worthy of attention. Under the appropriate ratio, maximizing the advantages of each material is the optimal solution for dental antibacterial materials. Designing suitable combinations, optimal ratios, and superior biocompatibility is the goal of continuous exploration in this field.
Table 3.
Summary of combined application of antibacterial agents in the dental field.
| Category | Morphology | Synthesis | Antibacterial | Application | Ref |
|---|---|---|---|---|---|
| n-ZnO + x·Ag NPs | a spherical shape | microwave solvothermal synthesis | S. salivarius, S. oralis, S. mutans, P. gingivalis and S. mitis: MICs 32–64 ppm | drug delivery systems for dental and craniofacial biomaterials | [42] |
| AgBr/cationic polymer nanocomposites | – | – | S. mutans: much lower | resin | [97] |
| silver-loaded polycation functionalized nanodiamond | less than 100 nm | an in-situ reduction method | S. mutans: 1.0 wt% decreased to 17.9% | resin-based restorative material | [98] |
| SiO2–ZnO | a spherical shape and close packing structure | a spray-drying process |
S. mutans: 4 wt%; ZnO over 99.9% |
dental caries filler for resin | [99] |
| Cu-ZPC | spherical mesoporous nanospheres | alkali-mediated ultrasonic-coupled sol-gel synthesis | E. faecalis: suppressed bacterial growth | nanotherapeutic approach for the regeneration of infected dental pulp tissue defect | [100] |
| bioactive glass nanopowders & ethanol + TEOS + ammonia + Cu-doped calcium silicate | – | the sol-gel method | B. cereus, S. aureus, P. aeruginosa: decrease in growth rate; L. monocytogenes, E. coli, two serotypes of S. enterica: not affected | nanoceramics | [101] |
| bioactive glass containing 50%SiO2–40%CaO–5%P2O5–2%ZnO–2%MgO–1%SrO | granular appearance of highly amorphous microstructure on the surface of glass; NBG: spherical shaped particles | a sol-gel technique | B. cereus, E. coli, C. albicans, S. aureus: a significant zone of inhibition, respectively | bioactive glass | [102] |
| nano-Ag or nano-calcium hydroxide | – | – | total bacterial, total E. faecalis: reduction | intracanal medications | [103] |
The combinations of nano-antibacterial agents are various [[97], [98], [99]], among which nano-Ag are the most popular material selections for the combination of nano-antibacterial agents. Ag plays an important role in the field of antibacterial, but the toxicity is always the limitation. The combined sterilization function of Ag+ and cationic polymer has advantageous in the dimension of nanometers, which can solve the problem of easy aggregation of nanometer silver ions. Photocurable core-shell AgBr/cationic polymer nanocomposites have showed sufficient durable antibacterial properties at low concentrations [97], and the silver-loaded polycation functionalized nanodiamonds (Ag/QNDs) also achieved improvement of mechanics and antibacterial effect, which caused by the combined bactericidal effect of Ag+ and cationic polymers [98]. Furthermore, resin composites containing some inorganic fillers may exhibit enhanced mechanical properties and antibacterial effect. Yang et al. fabricated dental resin composites filled with SiO2–ZnO complex clusters by the spray-drying technology to enhance the antibacterial activity while maintaining the mechanical and aesthetic properties (Fig. 8A) [99].
Fig. 8.
(A) Preparation process of SiO2–ZnO CCs by the spray-drying technology [99]. Copyright 2021 Royal Society of Chemistry. (B) In vivo efficacy of the EGF@Cu-BGn nanotherapeutics for treatment and regeneration of infected/damaged dental pulp. (C) Triple-functional (pro-angiogenic, anti-bacterial and odontogenic) nanotherapeutics of copper doped/EGF loaded mesoporous bioactive glass nanospheres (Cu-BGn) to regenerate bacterial infected dental pulp [100]. Copyright 2021 Elsevier.
What's more, bioactive glass nanoparticles (BGn) are a combination of Na2O, CaO, SiO2, P2O5 with multifunctional bioactive substances (such as antibacterial, remineralization, osteogenesis, etc.), which are mainly synthesized via sol-gel method [[100], [101], [102]]. According to the needs of treatment and prevention, BGn can be loaded with various ions, oxides, making it more suitable for treatment needs. The introduction of copper into bioactive glass is popular [100,101], which is related to the fact that Cu2+ can promote angiogenesis to assist in osteogenesis. A mesoporous bioactive glass nano-delivery system loading –NH2 surface functionalized copper and epidermal growth factor (EGF) achieved antibacterial activity against E. faecalis and the synergistic pro-angiogenic effect (Fig. 8B and C) [100]. It has been reported that the addition of other inorganic components, such as Sr, Mg and Zn, can improve the bioactivity and physicochemical properties of bioactive glass, and can enhance the antibacterial and bone tissue repair capabilities of bioactive glass [102]. The size of nanobioactive glass can be affected by the concentration of TEOS, H2O and ammonia, the quantity and the nature of the alkohol (solvent), and the reaction temperature. may be considered. The higher the ethanol/TEOS ratio and the higher the ammonia content, the stronger the formation of apatite and the overall antibacterial ability, which is related to its smaller size [101].
3.2. Antibacterial drugs loaded on nanocarriers
The oral use of antibiotics is still valuable, but traditional treatments are limited by their inability to achieve a lasting antibacterial effect. Recently, the carrier drug can achieve sustained release and targeted drug delivery, and it also solves the problem of dissolution of some poorly water-soluble substances. This section will introduce the application of nano-drug loading technology in the oral cavity, which is divided into two aspects: organic carriers and inorganic carriers (Table 4).
Table 4.
Summary of nanocarriers loaded antibacterial drugs in the dental field.
| Category | Morphology | Synthesis | Antibacterial | Application | Ref |
|---|---|---|---|---|---|
| Ca–SiO2 (loading ciprofloxacin hydrochlor) | spherical morphology size 230 nm | a sol-gel approach | S. aureus: inhibit greater than 90% | restorative materials for potential dental applications | [104] |
| pH-responsive nano micelle | spherical core-shell morphology | atom transfer radical polymerization | S. mutans: a bactericidal effect at pH about 5 | a good potential for clinical application | [25] |
| vancomycin gelatin nanoparticles & PEEK + polydopamine/P-PEEK | Van-GNPs: obvious granular protrusions | nano-topology PEEK: low-temperature argon plasma modification technology; van-GNPs: two-step desolvation |
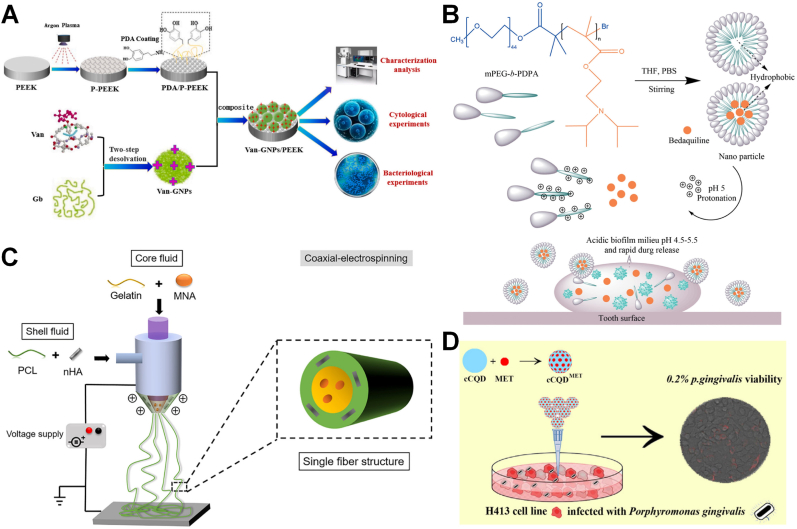
S. aureus and S. mutans: bactericidal ring: significantly greater; the number of bacteria adhering to the surface: significantly lower, respectively | implant | [105] |
| electrospun core-shell nanofibers loaded with metronidazole and nano-HA | nanofibers possess smooth surfaces | electrospinning | E. nucleatum, P. gingivalis: inhibition zones are clearly observed | anti-infective guided bone regeneration membranes | [106] |
| gentamicin loaded fibroin nanoparticles | uniformity of particle size | silane-glutaraldehyde cross-linking method | S. aureus: lowered amount of colonies | implant | [107] |
| poly (lactic-co-glycolic acid) loaded norfloxacin | spherical and a smooth surface | a spray-drying technique | E. coli: a significant decrease | implant | [108] |
| chlorophyll nano metronidazole | cCQD: spherical morphology, 2–4 nm | – | P. gingivalis: decreased approximately 99% | potent nano-antibiotic for intracellular pathogens | [109] |
| poly(ε-caprolactone) nanocapsules | uniform smooth spherical particles with core/shell morphology | – | E. faecalis, S. mutans: in wide inhibition zones | CHX-loaded nanocapsules | [110] |
| polymeric NPs loaded Dox | – | polymerization precipitation | E. faecalis biofilm: dead: 68.68% | endodontic sealers | [111] |
The advantage of inorganic nanomaterial is that most of them have antibacterial properties and excellent mechanical properties, which can protect the drug from physical damage and enhance the stability of the drug and antibacterial effects. The calcium-doped silica nanoparticles loaded ciprofloxacin hydrochloride designed by Zhang et al. were added to the resin to achieve the triple effect of antibacterial, remineralization and improved mechanical properties, and the mesoporous structure of nano-silicon also realized the effect of drug sustained release [104].
Organic carriers are also very popular. The enhanced antibacterial effect of the carriers and the drug is also showed in the organic carriers [25,[105], [106], [107], [108], [109], [110], [111]]. Chen et al. have constructed a tightly binding vancomycin gelatin nanoparticles sustained release system on the PEEK surface, improving the antibacterial performance of PEEK (Fig. 9A) [105]. Another advantage of organic carriers is that they can achieve intelligent release according to environmental changes (such as pH, magnetic field, and pressure, etc.). Zhang et al. synthesized a pH-responsive core-shell nano micelle to release bedaquiline under acidic conditions for intelligent treatment of dental caries (Fig. 9B) [25]. The bedaquiline-loaded nano-micelles inhibited the growth of S. mutans in neutral and acidic environments, and can affect the biofilm. The dual purpose of osteogenic antibiotics can also be achieved in this field. Electrospun core-shell nanofibers loaded with nano-HA and metronidazole as anti-infective GTR membrane (Fig. 9C) [106], and silk protein nanoparticles were used to continuously release gentamicin in vitro as titanium coating material [107] both have been proved to promote both osteogenesis and antibacterial activity. Applying degradable materials to carriers is a promising idea to improve biocompatibility. PLGA is an FDA-approved polymer with biodegradable, controlled-release properties. The use of PLGA to load norfloxacin can improve biosafety while achieving two-stage controlled release, namely, the first 48 h burst release and the subsequent slow release, achieving stable and safe long-term antibacterial effect [108].
Fig. 9.
(A) Preparation and characterisation of PEEK with nano-topology by low-temperature argon plasma modification technology and of Van-GNPs with two-step desolvation method [105]. Copyright 2021 Elsevier. (B) Scheme chemical formulas of diblock copolymer (mPEG-b-PDPA); Schematic representation for the formation and pH-responsive property of micelles; and the proposed mode of pH-responsive of micelles for biofilm penetration property [25]. Copyright 2021 Frontiers. (C) Schematic illustration showing preparation of PH-GM nanofibers with core-shell structure by coaxial electrospinning technique [106]. Copyright 2019 Elsevier. (D) Schematic illustration of the conjugated cCQD-MET in synthesis, the cytotoxicity assay and the antibacterial efficacy [109]. Copyright 2019 Elsevier.
Antibiotic resistance comes from abuse as well as defecting in the antibiotics themselves, such as the inability of many antibiotics to efficiently cross the plasma membrane of bacteria. Therefore, in the face of the major public health safety challenge of bacterial resistance to antibiotics, in addition to finding suitable alternatives, it is also a feasible way to increase the drug delivery rate. Among them, carbon quantum dots (CQDs) with enzyme activity and cell permeability have been confirmed to have the effect of promoting drug delivery. Ardekani et al. significantly enhanced intracellular microbicidal activity by coupling the nanoantibiotic MET to CQDs of chlorophyll nanocarriers, reducing the dose of antibiotics and thus reducing the risk of resistance (Fig. 9D) [109].
Inorganic and organic carriers have their own advantages, and the selection can be flexibly matched according to the needs of patients. In recent years, due to the risk of antibiotic resistance, humans have raised a series of concerns about it. In fact, as long as the new technology of antibiotics is used to improve the way of administration, the advantages of antibiotics such as strong antibacterial effect and fast onset of action can still bring convenience to the lives of patients at this stage.
3.3. Nano antibacterial coating
Coating is a common form of compounding, which distributes antibacterial materials evenly on the surface of the substrate, so that the substrate surface has high nano-irregularity to achieve antibacterial and damage the adhesion of Gram-positive bacteria. Gram-negative bacteria suffer little from this inhibition due to their characteristic extra fluid outer membrane of the peptidoglycan outer layer, however, Gram-positive bacteria tend to be early colonizers, so inhibition of Gram-positive bacteria is more valuable. The principle of nano-coating technology is to make material more effective antibacterial and anti-adhesion, and even anti-inflammatory and osteogenic effects, so as to improve the performance of the product, which enable dental materials to meet various requirements in the field of planting, and are of great value in improving the success rate of planting (Table 5). In this field, better materials, forms and synthesis methods are still being explored, which are worthy further studying [[112], [113], [114], [115], [116]].
Table 5.
Summary of nano-antibacterial coating in the dental field.
| Category | Morphology | Synthesis | Antibacterial | Biocompatibility | Application | Ref |
|---|---|---|---|---|---|---|
| nano-Ag-coated Ti | the small particles coverage on the surface | microwave-assisted synthesis | S. aureus: significant suppressed | human gingival fibroblasts: no significant difference | implant | [112] |
| Polym P-n active nanoparticles & metal ions | spherical and a smooth surface | a polymerization/precipitation process |
S. oralis, A. naeslundii, V. parvula, F.nucleatum, P. gingivalis, A. actinomycetemcomitans biofilms: significantly lower, Ag: antibacterial: highest; Zn: inhibited bacteria coaggregation, markedly |
– | – | [113] |
| nano-Ag coated PEEK | Ag: round and compact | a magnetron sputtering apparatus | S. mutans, S. aureus: greater than 99% | mouse fibroblast cell: good relative growth rate | implant | [114] |
| nano-Ag | uniform-grain-size; distributed on the surface | a hydrothermal method | S. aureus: not statistically reduced | osteogenic precursor cells: strong cytotoxicity | implant | [115] |
| BBF-loaded poly(L-lactic acid) nanospheres | nanospheres | the oil-in-water (O/W) emulsion solvent-evaporation method | P. gingivalis, A. actinomycetemcomitans: antibacterial rate: approximately 92% | – | implant | [116] |
In this field, Ag is a very popular and promising choice, and the coating technology can significantly reduce the concentration of Ag to achieve better and safer biocompatibility. After Odatsu et al. coated the common implant material titanium with microwave-assisted synthesis of silver, the titanium disc significantly inhibited S. aureus [112]. Similarly, the coating modification of the emerging implant PEEK by magnetron sputtering technology of silver [114] and the coating of HA by a polymerization/precipitation process [113] have improved their antibacterial ability. However, some team applied silver to coat titanium by hydrothermal method without improving its antibacterial ability, but reducing the biological toxicity of titanium [115]. This suggests that the ability to produce significant antibacterial ability is related to the synthesis method, so better synthesis methods in this field are expected to be developed. In addition, there are some organic materials that can be used as coating materials. Cheng et al. designed (Z-)-4-bromo-5-(bromomethylene)-2(5H)-furanone (BBF) -loaded poly(L-lactic acid) nanospheres as antibacterial coating [116]. The antibacterial coating could sustain the release of BBF for 60 d, with a slight initial burst release occurring in the first 4 h, which was related to the nanosphere structure. The antibacterial rate of the composite coating for adhering bacteria was over 97% after 1 d and over 90% throughout a 30-day incubation period, and fluorescence staining also showed the vast majority of bacteria was dead.
The application of coating technology is mainly based on implantation. In the face of complex environments such as oral and maxillofacial soft tissues and bone tissues, attention should be paid to the osteogenic ability of the material, the degree of surface wetness, and the ability to cope with a range of problems caused by bacterial infection after implant surgery, such as peri-implantitis-induced bone loss and mucosal inflammation. Many requirements are difficult to meet the implant needs only by simple antibacterial therapy. Therefore, to improve the success rate of implant surgery, breakthroughs must be made in three aspects: promoting osseointegration, anti-inflammatory, antibacterial and anti-adhesion. Based on these requirements, Ce, which has dual functions of antibacterial and anti-inflammatory, was introduced into the implant surface coating. Li et al. used a hydrothermal method to coat titanium with octahedral CeO2, which enhanced the antibacterial and anti-inflammatory properties of traditional Ti implants. The hydrophilic group of Ce and its octahedral multi-angle structure can effectively hinder the adhesion of bacteria without modifying the hydrophilicity [65]. At the same time, there are other reports that Ce can promote new bone formation and osseointegration. Ce is valuable in alleviating and treating peri-implantitis, and its synthesis at this stage is mainly by hydrothermal method. Perhaps, with the development of new synthesis methods and structural forms, Ce will have more advantages in the field of implant.
4. Conclusion and prospect
This article reviews the application in recent 5 years of nano-antibacterial technology in oral materials. The aetiology of pathogenesis in the oral cavity is complex, most oral health problems are caused by the disruption of the balance of oral flora. Acidic bacterial metabolites may cause the demineralization of the tooth structure, including inducing caries. Factors such as lipopolysaccharides (LPS) released by bacterial infection of the biofilm also lead to inflammation. That is why antibacterial is the most basic common requirement in this field. The emergence of nanotechnology has brought oral antibacterial to a new level. The antibacterial advantage of nanoparticles is mainly due to the superior antibacterial activity exhibited by their ultra-high surface area to volume ratio. Many of the nanomaterials presented here have potent antibacterial ability against common oral flora (such as S. mutans, P. gingivalis, and S. aureus), and also show inhibition of initial bacterial colonization and strong confrontation ability of biofilms. However, due to the complex pathogenic factors of oral diseases and the diversity of materials' application environments, it is difficult for a single antibacterial to meet the demands. Therefore, novel composites need to be considered to improve the antibacterial effect. The composite materials have various antibacterial pathways and abundant functions. On the basis of antibacterial, anti-inflammatory, remineralization, and osteogenesis can be realized on demand. The research and development of oral antibacterial materials should adjust the type and concentration of materials according to the differences in the corresponding environment to design the most suitable choice [114,115].
With the continuous maturity of nanotechnology, a growing number of antibacterial materials have been developed according to clinical needs, but it has also caused many concerns based on their safety. As oral antibacterial materials, these agents may enter the human body after prolonged exposure to oral mucosa and teeth. Even more dangerous is the nanoscale structure that makes it possible to evade the body's own defense mechanisms. However, most biocompatibility experiments are still carried out in a petri dish with common cells and nanoparticles in the environment. This is far from the actual situation, and the obtained data is difficult to accurately reflect the actual situation. In order to explore the effect of nanomaterials on the human body, more accurate in vivo experiments are worth designing. Meanwhile, methods to improve biocompatibility (such as biosynthesis, optimization of antibacterial efficiency, etc.) are also worth further researches. There is also a concern that some nanoparticles may induce a hypersensitivity reaction or inflammation in vulnerable patients, because they are immunogenic. This drawback may be found in traditional medicines and medical devices, which is still unknown that whether the risk would be greater in the oral cavity. In addition, as a clinical product, large-scale production is a necessary condition. Nevertheless, most of the nano-products have complex synthetic paths and high costs at the present stage, which hinder their industrialization.
Although there are still many difficulties to be overcome based on the toxicity and production cost of nanoparticles from the laboratory to the clinic, there is still a broad space for further development of oral nanotechnology. In addition, many questions are expected to be further studied, including how orally administered nanoparticles interact with biological fluids, their whole passage from the oral cavity and through the whole GI tract and the phenomena of protein coronas formation in the oral cavity and their influence. So many things unknown that there are still many difficulties in translating nanoproducts into the clinic for the lack of clinical trials. Furthermore, in order to better translate into clinical practice, some improvements should be made in health and safety. Better guidance to practitioners on nano-incorporated products about a patient safety and occupational health should be made, including more rigorous animal experiments. Also, the nano-specific labelling on the many personal dental care products should be improved to clearly identify the nano ingredients, which can made consumer clear to the proposed mode of action and benefits of the new product. Also, new strategies are worth exploring. For example, combining with other emerging fields such as nanobiosensors can be a direction for further exploration. In general, more attention should be paid to the toxicity and other shortcomings of nanotechnology. With the further development of nanotechnology, these problems are expected to be solved, and the applications of nanometer antibacterial materials in the oral cavity will be realized.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grants 82200610), the Basic and Clinical Cooperative Research and Promotion Program of Anhui Medical University (2021xkjT027), and research fund from Anhui Provincial Institute of Translational Medicine (2021zhyx-B15).
Contributor Information
Haisheng Qian, Email: shqian@ahmu.edu.cn.
Yuanyin Wang, Email: wyy1970548@sohu.com.
Wanni Wang, Email: wnwang@ahmu.edu.cn.
Data availability
No data was used for the research described in the article.
References
- 1.Tuominen H., Rautava J. Oral microbiota and cancer development. Pathobiology. 2021;88:116–126. doi: 10.1159/000510979. [DOI] [PubMed] [Google Scholar]
- 2.Pathak J.L., Yan Y., Zhang Q., Wang L., Ge L. The role of oral microbiome in respiratory health and diseases. Respir. Med. 2021;185 doi: 10.1016/j.rmed.2021.106475. [DOI] [PubMed] [Google Scholar]
- 3.Kitamoto S., Nagao-Kitamoto H., Hein R., Schmidt T.M., Kamada N. The bacterial connection between the oral cavity and the gut diseases. J. Dent. Res. 2020;99:1021–1029. doi: 10.1177/0022034520924633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lusiak-Szelachowska M., Weber-Dabrowska B., Gorski A. Bacteriophages and lysins in biofilm control. Virol. Sin. 2020;35:125–133. doi: 10.1007/s12250-019-00192-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Odularu A.T., Afolayan A.J., Sadimenko A.P., Ajibade P.A., Mbese J.Z. Multidrug-Resistant biofilm, quorum sensing, quorum quenching, and antibacterial activities of indole derivatives as potential eradication approaches. BioMed Res. Int. 2022;2022 doi: 10.1155/2022/9048245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo Y., Song Y. Mechanism of antimicrobial peptides: antimicrobial, anti-inflammatory and antibiofilm activities. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms222111401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H., Gu L., Liao B., Zhou X., Cheng L., Ren B. Advances of anti-caries nanomaterials. Molecules. 2020;25 doi: 10.3390/molecules25215047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jakubovics N.S., Goodman S.D., Mashburn-Warren L., Stafford G.P., Cieplik F. The dental plaque biofilm matrix. Periodontol. 2000;86(2021):32–56. doi: 10.1111/prd.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valm A.M. The structure of dental plaque microbial communities in the transition from health to dental caries and periodontal disease. J. Mol. Biol. 2019;431:2957–2969. doi: 10.1016/j.jmb.2019.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrouel F., Viennot S., Ottolenghi L., Gaillard C., Bourgeois D. Nanoparticles as anti-microbial, anti-inflammatory, and remineralizing agents in oral care cosmetics: a review of the current situation. Nanomaterials. 2020;10 doi: 10.3390/nano10010140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makvandi P., Josic U., Delfi M., Pinelli F., Jahed V., Kaya E., Ashrafizadeh M., Zarepour A., Rossi F., Zarrabi A., Agarwal T., Zare E.N., Ghomi M., Kumar Maiti T., Breschi L., Tay F.R. Drug delivery (Nano)Platforms for oral and dental applications: tissue regeneration, infection control, and cancer management. Adv. Sci. 2021;8 doi: 10.1002/advs.202004014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Najeeb S., Khurshid Z., Zafar M.S., Khan A.S., Zohaib S., Marti J.M., Sauro S., Matinlinna J.P., Rehman I.U. Modifications in glass ionomer cements: nano-sized fillers and bioactive nanoceramics. Int. J. Mol. Sci. 2016;17:1134. doi: 10.3390/ijms17071134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo T., Gulati K., Arora H., Han P., Fournier B., Ivanovski S. Orchestrating soft tissue integration at the transmucosal region of titanium implants. Acta Biomater. 2021;124:33–49. doi: 10.1016/j.actbio.2021.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Qi M., Ren X., Li W., Sun Y., Sun X., Li C., Yu S., Xu L., Zhou Y., Song S., Dong B., Wang L. NIR responsive nitric oxide nanogenerator for enhanced biofilm eradication and inflammation immunotherapy against periodontal diseases. Nano Today. 2022;43 [Google Scholar]
- 15.Benoit D.S.W., Sims K.R., Jr., Fraser D. Nanoparticles for oral biofilm treatments. ACS Nano. 2019;13:4869–4875. doi: 10.1021/acsnano.9b02816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Padovani G.C., Feitosa V.P., Sauro S., Tay F.R., Duran G., Paula A.J., Duran N. Advances in dental materials through nanotechnology: facts, perspectives and toxicological aspects. Trends Biotechnol. 2015;33:621–636. doi: 10.1016/j.tibtech.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Xu N., Fu J., Zhao L., Chu P.K., Huo K. Biofunctional elements incorporated nano/microstructured coatings on titanium implants with enhanced osteogenic and antibacterial performance. Adv Healthc Mater. 2020 doi: 10.1002/adhm.202000681. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Q., Wu W., Qian C., Xiao W., Zhu H., Guo J., Meng Z., Zhu J., Ge Z., Cui W. Advanced biomaterials for repairing and reconstruction of mandibular defects. Mater Sci Eng C Mater Biol Appl. 2019;103 doi: 10.1016/j.msec.2019.109858. [DOI] [PubMed] [Google Scholar]
- 19.Makabenta J.M.V., Nabawy A., Li C.H., Schmidt-Malan S., Patel R., Rotello V.M. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 2021;19:23–36. doi: 10.1038/s41579-020-0420-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krol A., Pomastowski P., Rafinska K., Railean-Plugaru V., Buszewski B. Zinc oxide nanoparticles: synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid Interface Sci. 2017;249:37–52. doi: 10.1016/j.cis.2017.07.033. [DOI] [PubMed] [Google Scholar]
- 21.Meng Y., Chen L., Chen Y., Shi J., Zhang Z., Wang Y., Wu F., Jiang X., Yang W., Zhang L., Wang C., Meng X., Wu Y., Bu W. Reactive metal boride nanoparticles trap lipopolysaccharide and peptidoglycan for bacteria-infected wound healing. Nat. Commun. 2022;13:7353. doi: 10.1038/s41467-022-35050-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abou Neel E.A., Aljabo A., Strange A., Ibrahim S., Coathup M., Young A.M., Bozec L., Mudera V. Demineralization-remineralization dynamics in teeth and bone. Int. J. Nanomed. 2016;11:4743–4763. doi: 10.2147/IJN.S107624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y., Yang Y., Shi Y., Song H., Yu C. Antibiotic-free antibacterial strategies enabled by nanomaterials: progress and perspectives. Adv. Mater. 2020;32 doi: 10.1002/adma.201904106. [DOI] [PubMed] [Google Scholar]
- 24.Pelgrift R.Y., Friedman A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013;65:1803–1815. doi: 10.1016/j.addr.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Zhang M., Yu Z., Lo E.C.M. A new pH-responsive nano micelle for enhancing the effect of a hydrophobic bactericidal agent on mature Streptococcus mutans biofilm. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.761583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birk S.E., Boisen A., Nielsen L.H. Polymeric nano- and microparticulate drug delivery systems for treatment of biofilms. Adv. Drug Deliv. Rev. 2021;174:30–52. doi: 10.1016/j.addr.2021.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Ferraris S., Vitale A., Bertone E., Guastella S., Cassinelli C., Pan J., Spriano S. Multifunctional commercially pure titanium for the improvement of bone integration: multiscale topography, wettability, corrosion resistance and biological functionalization. Mater Sci Eng C Mater Biol Appl. 2016;60:384–393. doi: 10.1016/j.msec.2015.11.049. [DOI] [PubMed] [Google Scholar]
- 28.Jun S.K., Yang S.A., Kim Y.J., El-Fiqi A., Mandakhbayar N., Kim D.S., Roh J., Sauro S., Kim H.W., Lee J.H., Lee H.H. Multi-functional nano-adhesive releasing therapeutic ions for MMP-deactivation and remineralization. Sci. Rep. 2018;8:5663. doi: 10.1038/s41598-018-23939-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tammaro L., Salle A.D., Calarco A., Luca I., Riccitiello F., Peluso G., Vittoria V., Sorrentino A. Multifunctional bioactive resin for dental restorative materials. Polymers. 2020:12. doi: 10.3390/polym12020332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kyrylenko S., Warchol F., Oleshko O., Husak Y., Kazek-Kesik A., Korniienko V., Deineka V., Sowa M., Maciej A., Michalska J., Jakobik-Kolon A., Matula I., Basiaga M., Hulubnycha V., Stolarczyk A., Pisarek M., Mishchenko O., Pogorielov M., Simka W. Effects of the sources of calcium and phosphorus on the structural and functional properties of ceramic coatings on titanium dental implants produced by plasma electrolytic oxidation. Mater Sci Eng C Mater Biol Appl. 2021;119 doi: 10.1016/j.msec.2020.111607. [DOI] [PubMed] [Google Scholar]
- 31.Bordea I.R., Candrea S., Alexescu G.T., Bran S., Baciut M., Baciut G., Lucaciu O., Dinu C.M., Todea D.A. Nano-hydroxyapatite use in dentistry: a systematic review. Drug Metab. Rev. 2020;52:319–332. doi: 10.1080/03602532.2020.1758713. [DOI] [PubMed] [Google Scholar]
- 32.Natan M., Banin E. From Nano to Micro: using nanotechnology to combat microorganisms and their multidrug resistance. FEMS Microbiol. Rev. 2017;41:302–322. doi: 10.1093/femsre/fux003. [DOI] [PubMed] [Google Scholar]
- 33.Wu Z.L., Zhao J., Xu R. Recent advances in oral nano-antibiotics for bacterial infection therapy. Int. J. Nanomed. 2020;15:9587–9610. doi: 10.2147/IJN.S279652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y., Gulati K., Li Z., Di P., Liu Y. Dental implant nano-engineering: advances, limitations and future directions. Nanomaterials. 2021:11. doi: 10.3390/nano11102489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chopra D., Gulati K., Ivanovski S. Understanding and optimizing the antibacterial functions of anodized nano-engineered titanium implants. Acta Biomater. 2021;127:80–101. doi: 10.1016/j.actbio.2021.03.027. [DOI] [PubMed] [Google Scholar]
- 36.Sun Y., Li R., Yu X., Li X., Han Z., Sun J., Bi W., Liu W., Yu Y., Cui W. Highly active biological dermal acellular tissue scaffold composite with human bone powder for bone regeneration. Mater. Des. 2021;209 [Google Scholar]
- 37.Wan Y., Fang J., Wang Y., Sun J., Sun Y., Sun X., Qi M., Li W., Li C., Zhou Y., Xu L., Dong B., Wang L. Antibacterial zeolite imidazole frameworks with manganese doping for immunomodulation to accelerate infected wound healing. Adv Healthc Mater. 2021;10 doi: 10.1002/adhm.202101515. [DOI] [PubMed] [Google Scholar]
- 38.Eslamian L., Borzabadi-Farahani A., Karimi S., Saadat S., Badiee M.R. Evaluation of the shear bond strength and antibacterial activity of orthodontic adhesive containing silver nanoparticle, an in-vitro study. Nanomaterials. 2020;10:1466. doi: 10.3390/nano10081466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mirhashemi A., Bahador A., Sodagar A., Pourhajibagher M., Amiri A., Gholamrezayi E. Evaluation of antimicrobial properties of nano-silver particles used in orthodontics fixed retainer composites: an experimental in-vitro study. J. Dent. Res. Dent. Clin. Dent. Prospects. 2021;15:87–93. doi: 10.34172/joddd.2021.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lei X., Wang J., Chen J., Gao J., Zhang J., Zhao Q., Tang J., Fang W., Li J., Li Y., Zuo Y. The in vitro evaluation of antibacterial efficacy optimized with cellular apoptosis on multi-functional polyurethane sealers for the root canal treatment. J. Mater. Chem. B. 2021;9:1370–1383. doi: 10.1039/d0tb02504f. [DOI] [PubMed] [Google Scholar]
- 41.Xin Y., Guo Z., Ma A., Shi E., Li Z., Liang Z., Qian Z., Yang L., Wang Y., Cao M., Yang X. A robust ROS generation nanoplatform combating periodontitis via sonodynamic/chemodynamic combination therapy. Chem. Eng. J. 2023;451 [Google Scholar]
- 42.Pokrowiecki R., Wojnarowicz J., Zareba T., Koltsov I., Lojkowski W., Tyski S., Mielczarek A., Zawadzki P. Nanoparticles and human saliva: a step towards drug delivery systems for dental and craniofacial biomaterials. Int. J. Nanomed. 2019;14:9235–9257. doi: 10.2147/IJN.S221608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poggio C., Trovati F., Ceci M., Chiesa M., Colombo M., Pietrocola G. Biological and antibacterial properties of a new silver fiber post: In vitro evaluation. J Clin Exp Dent. 2017;9:e387–e393. doi: 10.4317/jced.53464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paiva L., Fidalgo T.K.S., da Costa L.P., Maia L.C., Balan L., Anselme K., Ploux L., Thire R. Antibacterial properties and compressive strength of new one-step preparation silver nanoparticles in glass ionomer cements (NanoAg-GIC) J. Dent. 2018;69:102–109. doi: 10.1016/j.jdent.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 45.Wang S., Wu J., Yang H., Liu X., Huang Q., Lu Z. Antibacterial activity and mechanism of Ag/ZnO nanocomposite against anaerobic oral pathogen Streptococcus mutans. J. Mater. Sci. Mater. Med. 2017;28:23. doi: 10.1007/s10856-016-5837-8. [DOI] [PubMed] [Google Scholar]
- 46.Deng Y., Yang L., Huang X., Chen J., Shi X., Yang W., Hong M., Wang Y., Dargusch M.S., Chen Z.G. Dual Ag/ZnO-decorated micro-/nanoporous sulfonated polyetheretherketone with superior antibacterial capability and biocompatibility via layer-by-layer self-assembly strategy. Macromol. Biosci. 2018;18 doi: 10.1002/mabi.201800028. [DOI] [PubMed] [Google Scholar]
- 47.Logaranjan K., Raiza A.J., Gopinath S.C., Chen Y., Pandian K. Shape- and size-controlled synthesis of silver nanoparticles using aloe vera plant extract and their antimicrobial activity. Nanoscale Res. Lett. 2016;11:520. doi: 10.1186/s11671-016-1725-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pandey A., Yang T.S., Yang T.I., Belem W.F., Teng N.C., Chen I.W., Huang C.S., Kareiva A., Yang J.C. An insight into nano silver fluoride-coated silk fibroin bioinspired membrane properties for guided tissue regeneration. Polymers. 2021;13 doi: 10.3390/polym13162659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnson C.R., Tran M.N., Michelitsch L.M., Abraham S., Hu J., Gray K.A., Hartmann E.M. Nano-enabled, antimicrobial toothbrushes - how physical and chemical properties relate to antibacterial capabilities. J. Hazard Mater. 2020;396 doi: 10.1016/j.jhazmat.2020.122445. [DOI] [PubMed] [Google Scholar]
- 50.Toledano-Osorio M., Osorio R., Aguilera F.S., Medina-Castillo A.L., Toledano M., Osorio E., Acosta S., Chen R., Aparicio C. Polymeric nanoparticles protect the resin-dentin bonded interface from cariogenic biofilm degradation. Acta Biomater. 2020;111:316–326. doi: 10.1016/j.actbio.2020.05.002. [DOI] [PubMed] [Google Scholar]
- 51.Wang X., Fan H., Zhang F., Zhao S., Liu Y., Xu Y., Wu R., Li D., Yang Y., Liao L., Zhu H., Wang X. Antibacterial properties of bilayer biomimetic nano-ZnO for dental implants. ACS Biomater. Sci. Eng. 2020;6:1880–1886. doi: 10.1021/acsbiomaterials.9b01695. [DOI] [PubMed] [Google Scholar]
- 52.Mirhosseini F., Amiri M., Daneshkazemi A., Zandi H., Javadi Z.S. Antimicrobial effect of different sizes of nano zinc oxide on oral microorganisms. Front Dent. 2019;16:105–112. doi: 10.18502/fid.v16i2.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mohd Bakhori S.K., Mahmud S., Ling C.A., Sirelkhatim A.H., Hasan H., Mohamad D., Masudi S.M., Seeni A., Abd Rahman R. In-vitro efficacy of different morphology zinc oxide nanopowders on Streptococcus sobrinus and Streptococcus mutans. Mater Sci Eng C Mater Biol Appl. 2017;78:868–877. doi: 10.1016/j.msec.2017.04.085. [DOI] [PubMed] [Google Scholar]
- 54.Nguyen T.M.T., Wang P.W., Hsu H.M., Cheng F.Y., Shieh D.B., Wong T.Y., Chang H.J. Dental cement's biological and mechanical properties improved by ZnO nanospheres. Mater Sci Eng C Mater Biol Appl. 2019;97:116–123. doi: 10.1016/j.msec.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 55.Garcia I.M., Balhaddad A.A., Ibrahim M.S., Weir M.D., Xu H.H.K., Collares F.M., Melo M.A.S. Antibacterial response of oral microcosm biofilm to nano-zinc oxide in adhesive resin. Dent. Mater. 2021;37:e182–e193. doi: 10.1016/j.dental.2020.11.022. [DOI] [PubMed] [Google Scholar]
- 56.Fang J., Wan Y., Sun Y., Sun X., Qi M., Cheng S., Li C., Zhou Y., Xu L., Dong B., Wang L. Near-infrared-activated nanohybrid coating with black phosphorus/zinc oxide for efficient biofilm eradication against implant-associated infections. Chem. Eng. J. 2022;435 [Google Scholar]
- 57.Totu E.E., Nechifor A.C., Nechifor G., Aboul-Enein H.Y., Cristache C.M. Poly(methyl methacrylate) with TiO2 nanoparticles inclusion for stereolitographic complete denture manufacturing - the fututre in dental care for elderly edentulous patients? J. Dent. 2017;59:68–77. doi: 10.1016/j.jdent.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 58.Esteban Florez F.L., Hiers R.D., Larson P., Johnson M., O'Rear E., Rondinone A.J., Khajotia S.S. Antibacterial dental adhesive resins containing nitrogen-doped titanium dioxide nanoparticles. Mater Sci Eng C Mater Biol Appl. 2018;93:931–943. doi: 10.1016/j.msec.2018.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eghbali N., Naffakh-Moosavy H., Sadeghi Mohammadi S., Naderi-Manesh H. The influence of laser frequency and groove distance on cell adhesion, cell viability, and antibacterial characteristics of Ti-6Al-4V dental implants treated by modern fiber engraving laser. Dent. Mater. 2021;37:547–558. doi: 10.1016/j.dental.2020.12.007. [DOI] [PubMed] [Google Scholar]
- 60.Zhou P., Long S., Mao F., Huang H., Li H., He F., Zhang R., Ren L., Chen J., Wei S. Controlling cell viability and bacterial attachment through fabricating extracellular matrix-like micro/nanostructured surface on titanium implant. Biomed. Mater. 2020;15 doi: 10.1088/1748-605X/ab70ee. [DOI] [PubMed] [Google Scholar]
- 61.Cheng S., Qi M., Li W., Sun W., Li M., Lin J., Bai X., Sun Y., Dong B., Wang L. Dual-responsive nanocomposites for synergistic antibacterial therapies facilitating bacteria-infected wound healing. Adv Healthc Mater. 2023;12 doi: 10.1002/adhm.202202652. [DOI] [PubMed] [Google Scholar]
- 62.Qi M., Li X., Sun X., Li C., Tay F.R., Weir M.D., Dong B., Zhou Y., Wang L., Xu H.H.K. Novel nanotechnology and near-infrared photodynamic therapy to kill periodontitis-related biofilm pathogens and protect the periodontium. Dent. Mater. 2019;35:1665–1681. doi: 10.1016/j.dental.2019.08.115. [DOI] [PubMed] [Google Scholar]
- 63.Schilling K., Bradford B., Castelli D., Dufour E., Nash J.F., Pape W., Schulte S., Tooley I., van den Bosch J., Schellauf F. Human safety review of "nano" titanium dioxide and zinc oxide. Photochem. Photobiol. Sci. 2010;9:495–509. doi: 10.1039/b9pp00180h. [DOI] [PubMed] [Google Scholar]
- 64.Li X., Qi M., Li C., Dong B., Wang J., Weir M.D., Imazato S., Du L., Lynch C.D., Xu L., Zhou Y., Wang L., Xu H.H.K. Novel nanoparticles of cerium-doped zeolitic imidazolate frameworks with dual benefits of antibacterial and anti-inflammatory functions against periodontitis. J. Mater. Chem. B. 2019;7:6955–6971. doi: 10.1039/c9tb01743g. [DOI] [PubMed] [Google Scholar]
- 65.Li X., Qi M., Sun X., Weir M.D., Tay F.R., Oates T.W., Dong B., Zhou Y., Wang L., Xu H.H.K. Surface treatments on titanium implants via nanostructured ceria for antibacterial and anti-inflammatory capabilities. Acta Biomater. 2019;94:627–643. doi: 10.1016/j.actbio.2019.06.023. [DOI] [PubMed] [Google Scholar]
- 66.Sun Y., Sun X., Li X., Li W., Li C., Zhou Y., Wang L., Dong B. A versatile nanocomposite based on nanoceria for antibacterial enhancement and protection from aPDT-aggravated inflammation via modulation of macrophage polarization. Biomaterials. 2021;268 doi: 10.1016/j.biomaterials.2020.120614. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y., Li C., Wan Y., Qi M., Chen Q., Sun Y., Sun X., Fang J., Fu L., Xu L., Dong B., Wang L. Quercetin-loaded ceria nanocomposite potentiate dual-directional immunoregulation via macrophage polarization against periodontal inflammation. Small. 2021;17 doi: 10.1002/smll.202101505. [DOI] [PubMed] [Google Scholar]
- 68.Zhan L., Qian Q., Zhang Y., Qi Z., Zhang L., Fang H., Tang Z., Zhou Y. Copper functionalized poly (acrylic acid-co-itaconic acid) nanohydrogel: its antibacterial properties on oral pathogens and biocompatibility. Colloids Surf. B Biointerfaces. 2022;218 doi: 10.1016/j.colsurfb.2022.112741. [DOI] [PubMed] [Google Scholar]
- 69.Munir A., Marovic D., Nogueira L.P., Simm R., Naemi A.O., Landro S.M., Helgerud M., Zheng K., Par M., Taubock T.T., Attin T., Tarle Z., Boccaccini A.R., Haugen H.J. Using copper-doped mesoporous bioactive glass nanospheres to impart anti-bacterial properties to dental composites. Pharmaceutics. 2022;14:2241. doi: 10.3390/pharmaceutics14102241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu M., Huang L., Xu X., Wei X., Yang X., Li X., Wang B., Xu Y., Li L., Yang Z. Copper doped carbon dots for addressing bacterial biofilm formation, wound infection, and tooth staining. ACS Nano. 2022:9479–9497. doi: 10.1021/acsnano.2c02518. [DOI] [PubMed] [Google Scholar]
- 71.Choe Y.E., Kim Y.J., Jeon S.J., Ahn J.Y., Park J.H., Dashnyam K., Mandakhbayar N., Knowles J.C., Kim H.W., Jun S.K., Lee J.H., Lee H.H. Investigating the mechanophysical and biological characteristics of therapeutic dental cement incorporating copper doped bioglass nanoparticles. Dent. Mater. 2022;38:363–375. doi: 10.1016/j.dental.2021.12.019. [DOI] [PubMed] [Google Scholar]
- 72.Biswas N., Samanta A., Podder S., Ghosh C.K., Ghosh J., Das M., Mallik A.K., Mukhopadhyay A.K. Phase pure, high hardness, biocompatible calcium silicates with excellent anti-bacterial and biofilm inhibition efficacies for endodontic and orthopaedic applications. J. Mech. Behav. Biomed. Mater. 2018;86:264–283. doi: 10.1016/j.jmbbm.2018.06.046. [DOI] [PubMed] [Google Scholar]
- 73.Tao S., Su Z., Xiang Z., Xu H.H.K., Weir M.D., Fan M., Yu Z., Zhou X., Liang K., Li J. Nano-calcium phosphate and dimethylaminohexadecyl methacrylate adhesive for dentin remineralization in a biofilm-challenged environment. Dent. Mater. 2020;36:e316–e328. doi: 10.1016/j.dental.2020.08.001. [DOI] [PubMed] [Google Scholar]
- 74.Fang C.H., Tsai P.I., Huang S.W., Sun J.S., Chang J.Z., Shen H.H., Chen S.Y., Lin F.H., Hsu L.T., Chen Y.C. Magnetic hyperthermia enhance the treatment efficacy of peri-implant osteomyelitis. BMC Infect. Dis. 2017;17:516. doi: 10.1186/s12879-017-2621-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Naha P.C., Liu Y., Hwang G., Huang Y., Gubara S., Jonnakuti V., Simon-Soro A., Kim D., Gao L., Koo H., Cormode D.P. Dextran-coated iron oxide nanoparticles as biomimetic catalysts for localized and pH-activated biofilm disruption. ACS Nano. 2019;13:4960–4971. doi: 10.1021/acsnano.8b08702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gao L., Liu Y., Kim D., Li Y., Hwang G., Naha P.C., Cormode D.P., Koo H. Nanocatalysts promote Streptococcus mutans biofilm matrix degradation and enhance bacterial killing to suppress dental caries in vivo. Biomaterials. 2016;101:272–284. doi: 10.1016/j.biomaterials.2016.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sun X., Wang L., Lynch C.D., Sun X., Li X., Qi M., Ma C., Li C., Dong B., Zhou Y., Xu H.H.K. Nanoparticles having amphiphilic silane containing Chlorin e6 with strong anti-biofilm activity against periodontitis-related pathogens. J. Dent. 2019;81:70–84. doi: 10.1016/j.jdent.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 78.Sun X., Sun J., Sun Y., Li C., Fang J., Zhang T., Wan Y., Xu L., Zhou Y., Wang L., Dong B. Oxygen self-sufficient nanoplatform for enhanced and selective antibacterial photodynamic therapy against anaerobe-induced periodontal disease. Adv. Funct. Mater. 2021;31 [Google Scholar]
- 79.Vega-Jimenez A.L., Almaguer-Flores A., Flores-Castaneda M., Camps E., Uribe-Ramirez M., Aztatzi-Aguilar O.G., De Vizcaya-Ruiz A. Bismuth subsalicylate nanoparticles with anaerobic antibacterial activity for dental applications. Nanotechnology. 2017;28 doi: 10.1088/1361-6528/aa8838. [DOI] [PubMed] [Google Scholar]
- 80.Chu G., Zhang C., Liu Y., Cao Z., Wang L., Chen Y., Zhou W., Gao G., Wang K., Cui D. A gold nanocluster constructed mixed-metal metal-organic network film for combating implant-associated infections. ACS Nano. 2020;14:15633–15645. doi: 10.1021/acsnano.0c06446. [DOI] [PubMed] [Google Scholar]
- 81.Teixeira J.A., Silva A., Dos Santos Junior V.E., de Melo Junior P.C., Arnaud M., Lima M.G., Flores M.A.P., Stamford T.C.M., Dias Pereira J.R., Ribeiro Targino A.G., Galembeck A., Rosenblatt A. Effects of a new nano-silver fluoride-containing dentifrice on demineralization of enamel and Streptococcus mutans adhesion and acidogenicity. Int J Dent. 2018 doi: 10.1155/2018/1351925. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim J.H., Park S.W., Lim H.P., Park C., Yun K.D. Biocompatibility evaluation of feldspathic porcelain with nano-sized silver ion particles. J. Nanosci. Nanotechnol. 2018;18:1237–1240. doi: 10.1166/jnn.2018.14873. [DOI] [PubMed] [Google Scholar]
- 83.Sharon E., Sharabi R., Eden A., Zabrovsky A., Ben-Gal G., Sharon E., Pietrokovski Y., Houri-Haddad Y., Beyth N. Antibacterial activity of orthodontic cement containing quaternary ammonium polyethylenimine nanoparticles adjacent to orthodontic brackets. Int. J. Environ. Res. Publ. Health. 2018;15 doi: 10.3390/ijerph15040606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hu F., Zhou Z., Xu Q., Fan C., Wang L., Ren H., Xu S., Ji Q., Chen X. A novel pH-responsive quaternary ammonium chitosan-liposome nanoparticles for periodontal treatment. Int. J. Biol. Macromol. 2019;129:1113–1119. doi: 10.1016/j.ijbiomac.2018.09.057. [DOI] [PubMed] [Google Scholar]
- 85.Cheng L., Zhang K., Weir M.D., Melo M.A., Zhou X., Xu H.H. Nanotechnology strategies for antibacterial and remineralizing composites and adhesives to tackle dental caries. Nanomedicine. 2015;10:627–641. doi: 10.2217/nnm.14.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cheng L., Zhang K., Zhou C.C., Weir M.D., Zhou X.D., Xu H.H. One-year water-ageing of calcium phosphate composite containing nano-silver and quaternary ammonium to inhibit biofilms. Int. J. Oral Sci. 2016;8:172–181. doi: 10.1038/ijos.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Javed R., Rais F., Fatima H., Haq I.U., Kaleem M., Naz S.S., Ao Q. Chitosan encapsulated ZnO nanocomposites: fabrication, characterization, and functionalization of bio-dental approaches. Mater Sci Eng C Mater Biol Appl. 2020;116 doi: 10.1016/j.msec.2020.111184. [DOI] [PubMed] [Google Scholar]
- 88.Cheng Y.F., Zhang J.Y., Wang Y.B., Li C.M., Lu Z.S., Hu X.F., Xu L.Q. Deposition of catechol-functionalized chitosan and silver nanoparticles on biomedical titanium surfaces for antibacterial application. Mater Sci Eng C Mater Biol Appl. 2019;98:649–656. doi: 10.1016/j.msec.2019.01.019. [DOI] [PubMed] [Google Scholar]
- 89.Cho M.Y., Kang S.M., Lee E.S., Kim B.I. Antimicrobial activity of Curcuma xanthorrhiza nanoemulsions on Streptococcus mutans biofilms. Biofouling. 2020;36:825–833. doi: 10.1080/08927014.2020.1823376. [DOI] [PubMed] [Google Scholar]
- 90.Pourhajibagher M., Plotino G., Chiniforush N., Bahador A. Dual wavelength irradiation antimicrobial photodynamic therapy using indocyanine green and metformin doped with nano-curcumin as an efficient adjunctive endodontic treatment modality. Photodiagnosis Photodyn. Ther. 2020;29 doi: 10.1016/j.pdpdt.2019.101628. [DOI] [PubMed] [Google Scholar]
- 91.Pourhajibagher M., Ranjbar Omrani L., Noroozian M., Ghorbanzadeh Z., Bahador A. In vitro antibacterial activity and durability of a nano-curcumin-containing pulp capping agent combined with antimicrobial photodynamic therapy. Photodiagnosis Photodyn. Ther. 2021;33 doi: 10.1016/j.pdpdt.2020.102150. [DOI] [PubMed] [Google Scholar]
- 92.Seneviratne C.J., Leung K.C., Wong C.H., Lee S.F., Li X., Leung P.C., Lau C.B., Wat E., Jin L. Nanoparticle-encapsulated chlorhexidine against oral bacterial biofilms. PLoS One. 2014;9 doi: 10.1371/journal.pone.0103234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Leung K.C., Seneviratne C.J., Li X., Leung P.C., Lau C.B., Wong C.H., Pang K.Y., Wong C.W., Wat E., Jin L. Synergistic antibacterial effects of nanoparticles encapsulated with scutellaria baicalensis and pure chlorhexidine on oral bacterial biofilms. Nanomaterials. 2016;6:61. doi: 10.3390/nano6040061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yaseen S.N., Taqa A.A., Al-Khatib A.R. The effect of incorporation Nano Cinnamon powder on the shear bond of the orthodontic composite (an in vitro study) J Oral Biol Craniofac Res. 2020;10:128–134. doi: 10.1016/j.jobcr.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maheswari P., Ponnusamy S., Harish S., Muthamizhchelvan C., Hayakawa Y. Syntheses and characterization of Syzygium aromaticum, Elettaria cardamomum and Cinnamomum verum modified TiO2 and their biological applications. Mater. Sci. Semicond. Process. 2020;105 [Google Scholar]
- 96.Wu L., Gao Y., Zhao C., Huang D., Chen W., Lin X., Liu A., Lin L. Synthesis of curcumin-quaternized carbon quantum dots with enhanced broad-spectrum antibacterial activity for promoting infected wound healing. Biomater Adv. 2022;133 doi: 10.1016/j.msec.2021.112608. [DOI] [PubMed] [Google Scholar]
- 97.Cao W., Zhang Y., Wang X., Chen Y., Li Q., Xing X., Xiao Y., Peng X., Ye Z. Development of a novel resin-based dental material with dual biocidal modes and sustained release of Ag(+) ions based on photocurable core-shell AgBr/cationic polymer nanocomposites. J. Mater. Sci. Mater. Med. 2017;28:103. doi: 10.1007/s10856-017-5918-3. [DOI] [PubMed] [Google Scholar]
- 98.Cao W., Wang X., Li Q., Ye Z., Xing X. Mechanical property and antibacterial activity of silver-loaded polycation functionalized nanodiamonds for use in resin-based dental material formulations. Mater. Lett. 2018;220:104–107. [Google Scholar]
- 99.Yang D.L., Cui Y.N., Sun Q., Liu M., Niu H., Wang J.X. Antibacterial activity and reinforcing effect of SiO2-ZnO complex cluster fillers for dental resin composites. Biomater. Sci. 2021;9:1795–1804. doi: 10.1039/d0bm01834a. [DOI] [PubMed] [Google Scholar]
- 100.El-Fiqi A., Mandakhbayar N., Jo S.B., Knowles J.C., Lee J.H., Kim H.W. Nanotherapeutics for regeneration of degenerated tissue infected by bacteria through the multiple delivery of bioactive ions and growth factor with antibacterial/angiogenic and osteogenic/odontogenic capacity. Bioact. Mater. 2021;6:123–136. doi: 10.1016/j.bioactmat.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pouroutzidou G.K., Theodorou G.S., Kontonasaki E., Tsamesidis I., Pantaleo A., Patsiaoura D., Papadopoulou L., Rhoades J., Likotrafiti E., Lioutas C.B., Chrissafis K., Paraskevopoulos K.M. Effect of ethanol/TEOS ratios and amount of ammonia on the properties of copper-doped calcium silicate nanoceramics. J. Mater. Sci. Mater. Med. 2019;30:98. doi: 10.1007/s10856-019-6297-8. [DOI] [PubMed] [Google Scholar]
- 102.Ranga N., Gahlyan S., Duhan S. Antibacterial efficiency of Zn, Mg and Sr doped bioactive glass for bone tissue engineering. J. Nanosci. Nanotechnol. 2020;20:2465–2472. doi: 10.1166/jnn.2020.17336. [DOI] [PubMed] [Google Scholar]
- 103.Fahim M.M., Saber S.E.M., Elkhatib W.F., Nagy M.M., Schafer E. The antibacterial effect and the incidence of post-operative pain after the application of nano-based intracanal medications during endodontic retreatment: a randomized controlled clinical trial. Clin. Oral Invest. 2022;26:2155–2163. doi: 10.1007/s00784-021-04196-w. [DOI] [PubMed] [Google Scholar]
- 104.Zhang Y., Huang C., Chang J. Ca-Doped mesoporous SiO2/dental resin composites with enhanced mechanical properties, bioactivity and antibacterial properties. J. Mater. Chem. B. 2018;6:477–486. doi: 10.1039/c7tb02864d. [DOI] [PubMed] [Google Scholar]
- 105.Chen T., Chen Q., Fu H., Wang D., Gao Y., Zhang M., Liu H. Construction and performance evaluation of a sustained release implant material polyetheretherketone with antibacterial properties. Mater Sci Eng C Mater Biol Appl. 2021;126 doi: 10.1016/j.msec.2021.112109. [DOI] [PubMed] [Google Scholar]
- 106.Wang Y., Jiang Y., Zhang Y., Wen S., Wang Y., Zhang H. Dual functional electrospun core-shell nanofibers for anti-infective guided bone regeneration membranes. Mater Sci Eng C Mater Biol Appl. 2019;98:134–139. doi: 10.1016/j.msec.2018.12.115. [DOI] [PubMed] [Google Scholar]
- 107.Sharma S., Bano S., Ghosh A.S., Mandal M., Kim H.W., Dey T., Kundu S.C. Silk fibroin nanoparticles support in vitro sustained antibiotic release and osteogenesis on titanium surface. Nanomedicine. 2016;12:1193–1204. doi: 10.1016/j.nano.2015.12.385. [DOI] [PubMed] [Google Scholar]
- 108.Baghdan E., Raschpichler M., Lutfi W., Pinnapireddy S.R., Pourasghar M., Schafer J., Schneider M., Bakowsky U. Nano spray dried antibacterial coatings for dental implants. Eur. J. Pharm. Biopharm. 2019;139:59–67. doi: 10.1016/j.ejpb.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 109.Ardekani S.M., Dehghani A., Ye P., Nguyen K.A., Gomes V.G. Conjugated carbon quantum dots: potent nano-antibiotic for intracellular pathogens. J. Colloid Interface Sci. 2019;552:378–387. doi: 10.1016/j.jcis.2019.05.067. [DOI] [PubMed] [Google Scholar]
- 110.Priyadarshini B.M., Selvan S.T., Lu T.B., Xie H., Neo J., Fawzy A.S. Chlorhexidine nanocapsule drug delivery approach to the resin-dentin interface. J. Dent. Res. 2016;95:1065–1072. doi: 10.1177/0022034516656135. [DOI] [PubMed] [Google Scholar]
- 111.Arias-Moliz M.T., Baca P., Solana C., Toledano M., Medina-Castillo A.L., Toledano-Osorio M., Osorio R. Doxycycline-functionalized polymeric nanoparticles inhibit Enterococcus faecalis biofilm formation on dentine. Int. Endod. J. 2021;54:413–426. doi: 10.1111/iej.13436. [DOI] [PubMed] [Google Scholar]
- 112.Odatsu T., Kuroshima S., Sato M., Takase K., Valanezhad A., Naito M., Sawase T. Antibacterial properties of nano-Ag coating on healing abutment: an in vitro and clinical study. Antibiotics. 2020;9:347. doi: 10.3390/antibiotics9060347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sanchez M.C., Toledano-Osorio M., Bueno J., Figuero E., Toledano M., Medina-Castillo A.L., Osorio R., Herrera D., Sanz M. Antibacterial effects of polymeric PolymP-n Active nanoparticles. An in vitro biofilm study. Dent. Mater. 2019;35:156–168. doi: 10.1016/j.dental.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 114.Liu X., Gan K., Liu H., Song X., Chen T., Liu C. Antibacterial properties of nano-silver coated PEEK prepared through magnetron sputtering. Dent. Mater. 2017;33:e348–e360. doi: 10.1016/j.dental.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 115.Shen X.T., Zhang Y.Z., Xiao F., Zhu J., Zheng X.D. Effects on cytotoxicity and antibacterial properties of the incorporations of silver nanoparticles into the surface coating of dental alloys. J. Zhejiang Univ. - Sci. B. 2017;18:615–625. doi: 10.1631/jzus.B1600555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cheng Y., Mei S., Kong X., Liu X., Gao B., Chen B., Wu J. Long-term antibacterial activity of a composite coating on titanium for dental implant application. J. Biomater. Appl. 2021;35:643–654. doi: 10.1177/0885328220963934. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.