Abstract
Group B Streptococcus (GBS) causes substantial morbidity but most individuals exposed to the organism remain healthy. These experiments tested the hypothesis that engagement of the complement receptor 3 (CR3) lectin site would effectively trigger neutrophil-mediated phagocytosis of complement-opsonized type III GBS by nonimmune human sera. Using an opsonophagocytosis assay, saccharides identified as interacting with the CR3 lectin site effectively inhibited neutrophil-mediated killing of type III, strain COH1. Fructose, which does not interact with the lectin site, promoted significantly less inhibition of opsonophagocytosis. Saccharide-mediated inhibition was reversed in a dose-related fashion by addition of type III, GBS capsular polysaccharide-specific immunoglobulin G. When capsule-deficient or asialo mutant type III strains were employed, the lectin site was not required. Structurally defined GBS serotypes with a side chain at least two sugars in length engaged the lectin site, and N-acetyl d-glucosamine was not a required component monosaccharide. Intact type III capsular polysaccharide interacted significantly more efficiently with the lectin site than did oligosaccharides representing approximately 5 or 20 repeating units, respectively. Taken together, these experiments indicate that interaction of type III GBS capsular polysaccharide with the lectin site of CR3 effects phagocytosis of these organisms by nonimmune serum. Use of this mechanism of innate immunity provides a potential explanation for the infrequency with which susceptible individuals exposed to type III GBS develop invasive infection.
Group B Streptococcus (GBS) has been a leading cause of neonatal sepsis and meningitis since the 1970s. In the 1990s the incidence ranged from 0.5 to 3 cases per 1,000 live births (31). Despite advances in neonatal intensive care and antimicrobial therapy, case fatality ratios remain 4 to 6% for cases identified by population-based surveillance (30). A substantial burden of GBS-related illness occurs among nonpregnant adults, and the case fatality rate is 32% in this population (30). Those with immunocompromising conditions such as diabetes mellitus, renal failure, cirrhosis, and cancer are at particular risk for developing invasive infection. This ongoing disease burden highlights the importance of defining further the pathogenesis and immune response to GBS with the goal of formulating effective intervention strategies.
The innate immune response is critical to host defense. This is particularly evident early in the host-pathogen encounter when specific immunity has not yet developed (10). Several pathogens express surface domains that are recognized by and activate host phagocytic cells in the absence of specific antibody (25). These interactions have been shown to have a role in the phagocytosis of Pseudomonas aeruginosa, Escherichia coli, Neisseria meningitidis, mycobacteria, and yeasts, among others (6, 9, 11, 17, 22). Complement receptor 3 (CR3, also known as CD11b/CD18, or Mac-1) is a β2 integrin expressed on the plasma membrane of mammalian polymorphonuclear neutrophils (PMN), most mononuclear phagocytes, and NK cells, and it has the ability to recognize multiple ligands. CR3 possesses an I-domain that binds inactivated C3b (iC3b), intracellular adhesion molecule 1 (ICAM-1), and numerous extracellular matrix proteins (5). It also possesses a lectin site with the capacity to bind certain saccharides, such as β-glucans and those containing N-acetyl d-glucosamine (NADG) (27, 32, 35, 44). Engagement of the CR3 lectin site results in priming of CR3-expressing PMN for cytotoxicity of iC3b-opsonized targets in the absence of specific antibody (36). Thus, the engagement of this site is required for the full activation of CR3. This occurs by a two-step process whereby iC3b-opsonized particles bind to CR3 via the I-domain and a polysaccharide, either exogenous or intrinsic to the opsonized particle, engages the lectin site.
The capsular polysaccharide (CPS) of type III GBS consists of repeating units of glucose, galactose, NADG, and N-acetyl neuraminic acid. The molar ratios and tertiary configuration vary, but the CPSs of GBS serotypes Ia, Ib, II, III, and V all are comprised of a backbone and one or more side chains containing these four sugars (13, 15, 39, 40, 42). In the presence of complement, GBS III CPS-specific antibody optimizes opsonophagocytosis of type III GBS in vitro and protects against invasive infection in young infants (3, 7). Participation by PMN CR1 and CR3 as well as Fcγ receptor II (FcγRII) and FcγRIII mediates these phagocytic interactions. However, opsonophagocytosis of type III GBS can occur in the setting of a deficiency of CPS-specific immunoglobulin G (IgG) (7), and GBS can activate C3 and deposit iC3b in the absence of antibody (2). Furthermore, a majority of newborn infants exposed to a GBS-colonized mother at birth do not develop invasive disease in the face of low concentrations of CPS-specific antibody in serum.
Based on the known saccharide-binding properties of the CR3 lectin site and the defined structure of the CPS of type III GBS, we hypothesized that in nonimmune sera engagement of the CR3 lectin site by GBS CPS would be an effective trigger to phagocytosis and killing of these organisms. We found that type III GBS triggers phagocytosis and killing by binding to the lectin site of CR3 and that type III GBS-specific CPS is a ligand for this interaction. In the presence of a sufficient concentration of type III GBS CPS-specific IgG, use of the lectin site is not required. There were differences among GBS serotypes in use of the lectin site that related to differences in the structures of these polysaccharides. Taken together, interactions of GBS CPS with the CR3 lectin site serves as an important arm of the innate immune response in the susceptible host.
(This work was presented in abstract format at the 36th Annual Meeting of the Infectious Diseases Society of America, Denver, Colo., in November 1998 [abstr. #121].)
MATERIALS AND METHODS
Isolation of neutrophils.
PMN were isolated from fresh whole blood obtained from healthy adult volunteers, anticoagulated with citrate phosphate dextrose (Abbott Laboratories, North Chicago, Ill.), dextran sedimented, and purified over a Ficoll (Sigma Chemical Co., St. Louis, Mo.)-Hypaque (Nycomed Inc., Princeton, N.J.) gradient (33). Cell viability, determined by trypan blue dye exclusion, was consistently more than 92%.
Collection and preparation of sera.
Serum, processed to preserve endogenous complement activity, was separated from whole blood from healthy adult volunteers (23). The concentration of CPS-specific IgG for GBS serotypes Ia, II, III, and V was determined by enzyme-linked immunosorbent assay (12). The lower limit of sensitivity of these assays ranged from 0.12 to 0.05 μg/ml. Sera containing a low concentration of CPS-specific IgG (<1 μg/ml) were used as antibody-deficient sources of complement. The IgG-rich fraction of serum was purified from serum containing a high concentration of antibody to GBS III CPS-specific IgG by passage through a QAE-Sephadex A-50 column (Sigma Chemical Co.) (16). The specific antibody content of the IgG-rich fraction purified was 217 μg/ml.
Bacteria.
Encapsulated type III GBS strain COH1, isolated originally from the blood of an infant with sepsis, was studied together with an unencapsulated mutant of this parent (COH1-13) and a mutant strain (COH1-11) that lacks the terminal sialic acid residue of the CPS. These strains were derived by transposon insertion mutagenesis (29) and were provided by Craig E. Rubens, University of Washington, Seattle. Serotype Ia GBS strain 515, type II GBS strain 612, and type V GBS strain G106 were isolated from the blood or cerebrospinal fluid of newborn infants with invasive GBS infection and were provided by Carol J. Baker, Baylor College of Medicine, Houston, Tex. Serotype VI GBS, strain SMU053, was a vaginal isolate from a healthy pregnant woman in Kawasaki, Japan, and was a gift from Catherine Lachenauer, Channing Laboratory, Boston, Mass. Serotype VIII GBS, strain JM9-130013, isolated from an infant in Japan with invasive disease, was provided by Dennis L. Kasper, Channing Laboratory. Aliquots of the stock solution of these GBS isolates were stored at −70°C. Bacteria were grown to log phase in Todd-Hewitt broth (Difco, Detroit, Mich.), washed, and resuspended in phosphate-buffered saline (PBS).
Saccharides and monoclonal antibody.
The saccharides NADG, methyl β-d-mannopyranoside (β-methylmannoside), methyl β-d-glucopyranoside (β-methylglucoside), and fructose were obtained from Sigma Chemical Co. The monoclonal antibody (MAb) OKM1 (anti-CD11b lectin site, IgG2b) was provided by Michelle Mariscalco (Baylor College of Medicine).
Type III GBS capsular poly- and oligosaccharides.
CPS from type III GBS was isolated and purified from broth culture (14). All experiments were performed using lot 10C (16-25) which had a peak Mr of 237,000. This size is within the usual range for intact type III GBS CPS (38). Oligosaccharides with an average Mr of 5,900, representing approximately five repeating units, and an Mr of 22,500, representing approximately 20 repeating units, of the CPS were prepared by ozone depolymerization of type III GBS CPS (lot 8S) in PBS plus bicarbonate by the method of Wang et al. (38). The CPS and oligosaccharides were provided by Dennis L. Kasper and Lawrence C. Paoletti, Channing Laboratory.
Opsonophagocytosis assay.
The opsonophagocytosis assay was modified as follows from one previously described (7). The reaction mixture contained 50 μl of PMN (1.5 × 106), 30 μl of serum containing endogenous complement, 50 μl of GBS suspension (∼5 × 106 CFU), and either 100 μl of PBS or the reagent of the experiment, added at the concentration indicated. The reaction mixtures were incubated at 37°C with end-over-end rotation for 60 min. Pre-and postincubation aliquots were removed, diluted serially, and plated onto blood agar for overnight culture. The results were expressed as the bactericidal index, calculated as the percent reduction in initial inoculum. Each experiment included as a positive control serum from an adult known to have a high concentration of CPS-specific IgG for each GBS serotype as well as a reaction mixture lacking PMN.
Statistical analysis.
Unless otherwise stated, the results represent the mean ± standard error of the mean (SEM) of three to five experiments. Levels of significance for comparisons between samples were determined using Student's unpaired t test (two tailed). P values of <0.05 were considered significant.
RESULTS
Type III GBS utilizes the CR3 lectin site for opsonophagocytosis by nonimmune sera.
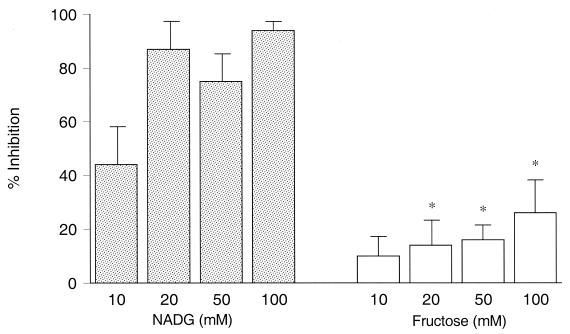
NADG is a sugar known to inhibit the lectin site while fructose does not (32, 35, 44). The potential inhibitory effect of these saccharides upon the phagocytosis and killing of type III GBS was studied. Before addition of a saccharide, the bactericidal activity (mean ± SEM) after 60 min incubation was 61% ± 5% (seven experiments) when type III GBS was opsonized by serum containing a low concentration of III CPS-specific IgG (<0.07 μg/ml). Incubation of type III GBS with increasing concentrations of NADG effected a dose-associated inhibition of opsonophagocytosis. At a 10 mM concentration of NADG, opsonophagocytosis was inhibited by 44% ± 14%, and this reached 94% ± 3% at 100 mM NADG (Fig. 1). Addition of equimolar concentrations of fructose had significantly less inhibitory effect, reaching a maximum of 26% ± 12% at 100 mM (P < 0.003 for NADG versus fructose at 20, 50, or 100 mM concentration).
FIG. 1.
Inhibition of the lectin site of CR3 by NADG or fructose. The extent to which PMN-mediated bactericidal activity was inhibited by NADG (stippled bars) or fructose (open bars) is shown (mean ± SEM [error bars] of seven experiments). At equimolar concentrations, fructose effected significantly less inhibition of opsonophagocytosis, indicated by the asterisks, than did NADG (P < 0.003).
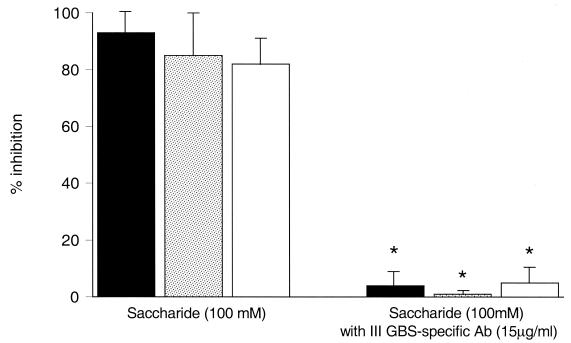
We next evaluated the effect of other sugars shown to function as inhibitors of the lectin site (32, 35). The saccharides β-methylmannoside and β-methylglucoside, used at a 100 mM concentration, inhibited the opsonophagocytosis of III GBS by 82% ± 10% and 85% ± 15%, respectively (Fig. 2).
FIG. 2.
Saccharide-mediated lectin site inhibition and the effect of type III GBS CPS-specific IgG upon inhibition. Shown on the left is a comparison of the mean (± SEM) inhibition of opsonophagocytosis of type III GBS strain COH1 by nonimmune serum mediated by NADG (solid bars), β-methylglucoside (stippled bars), or β-methylmannoside (open bars). The bars on the right show significant reversal of inhibition, indicated by asterisks, when type III GBS CPS-specific IgG (15 μg/ml) was added (P < 0.04 for each sugar).
Effect of III CPS-specific IgG on saccharide-mediated inhibition of the lectin site.
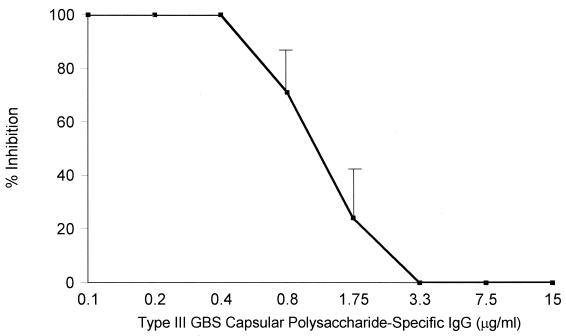
In the presence of sufficient CPS-specific antibody, complement-dependent phagocytosis of type III GBS is achieved using PMN Fc receptors, in particular the transmembrane receptor FcγRII, potentially obviating a requirement for the CR3 lectin site to trigger phagocytosis (23). Thus, it was predicted that III CPS-specific IgG would reverse the inhibitory effect of a lectin site-specific saccharide. As seen in Fig. 2, the addition of III CPS-specific IgG at a concentration of 15 μg/ml negated the inhibitory effect of each of these saccharides (P < 0.04). Figure 3 shows a dose-response curve for this effect. Complete reversal of the inhibitory effect of NADG was observed at a concentration of type III GBS CPS-specific IgG of 3.3 μg/ml or more. Taken together, these data indicate that in the presence of a sufficient concentration of type III GBS CPS-specific IgG, PMN may utilize receptors other than the CR3 lectin site to effect phagocytosis and killing. In contrast, the nonimmune host requires a CR3 lectin site-mediated interaction to trigger phagocytosis of type III GBS.
FIG. 3.
Dose-response effect of type III GBS CPS-specific IgG on mean (± SEM) NADG-mediated lectin site inhibition. Increasing concentrations of type III GBS CPS-specific IgG were added to a reaction mixture in the setting of 100 mM NADG-mediated lectin site inhibition. Inhibition of opsonophagocytosis was complete at type III GBS CPS-specific antibody concentrations of <0.8 μg/ml, was partial at concentrations of 0.8 and 1.5 μg/ml, and was absent at concentrations of ≥3.3 μg/ml.
Role of type III GBS CPS in lectin site interactions.
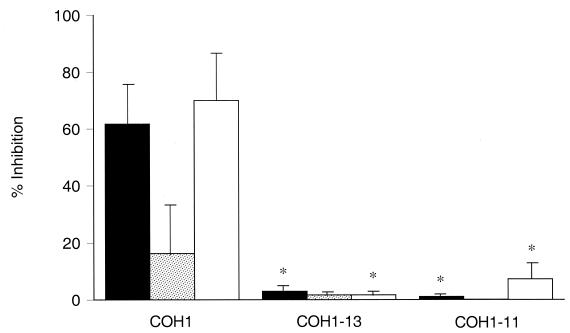
To determine whether the type III GBS CPS is the specific ligand that interacts with the CR3 lectin site, transposon mutant strains of type III GBS with altered capsule were employed. The initial bactericidal index mediated by serum with a low concentration of type III GBS CPS-specific IgG (≤0.07 μg/ml) was 63% ± 7% for the parent strain (COH1) and 97% ± 2% and 98% ± 0.4%, respectively, for the acapsular (COH1-13) and the asialo mutants (COH1-11). As shown in Fig. 4, 50 mM NADG caused 62% ± 11% inhibition, and the MAb OKM1 with specificity for the CD11b lectin site (27), used at a saturating concentration of 10 μg/ml (33), inhibited opsonophagocytosis of COH1 by 70% ± 14%. Fructose inhibited opsonophagocytosis minimally (16% ± 9%). By comparison, neither NADG nor OKM1 had any inhibitory effect upon opsonophagocytosis of the acapsular mutant (COH1-13) or the asialo mutant (COH1-11) in the presence of low III CPS-specific IgG-containing serum. The extent to which inhibition mediated by NADG occurred was significantly less for either mutant compared to the parent strain (P ≤ 0.007). Similarly, the extent to which OKM1 caused inhibition was significantly less for either mutant compared to the parent strain (P ≤ 0.02). The degree of inhibition by fructose with COH1 did not differ significantly from either mutant (P ≥ 0.15). These results indicate that the native type III CPS is required to interact with the CR3 lectin site to trigger phagocytosis and killing of type III GBS.
FIG. 4.
Evaluation of the role of type III GBS CPS in lectin site interactions. The mean (± SEM) inhibitory effect mediated either by NADG (50 mM) (solid bars), fructose (50 mM) (stippled bars), or the MAb OKM1 (10 μg/ml) (open bars) was compared for type III GBS strains. Bactericidal activity for the encapsulated type III GBS parent strain (COH1) was inhibited 62% ± 11% by NADG, 16% ± 9% by fructose, and 70% ± 14% by OKM1. When transposon-derived mutants of COH1 that lack CPS (COH1-13) or terminal sialic acid residues (COH1-11) were used, NADG (P ≤ 0.007 for either mutant versus COH1) or OKM1 effected significantly less inhibition, as indicated by asterisks (P ≤ 0.02 for either mutant versus COH1).
Effect of GBS serotype specificity upon lectin site interactions.
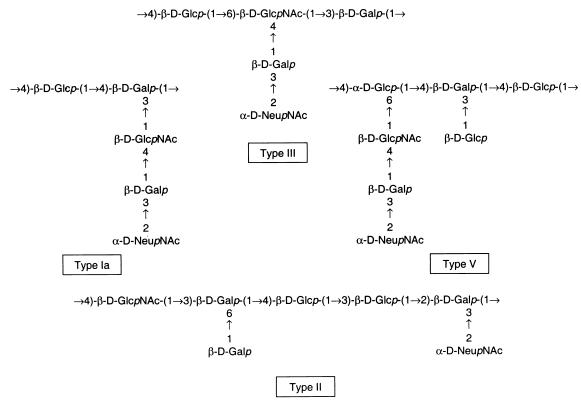
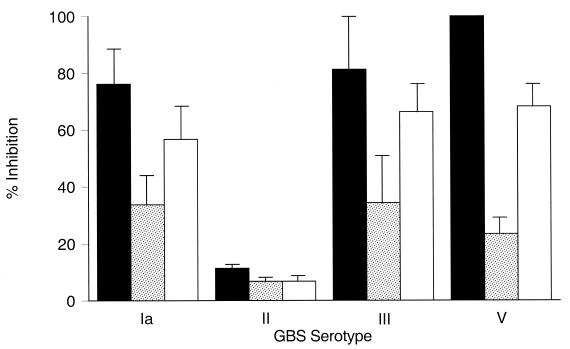
The CPSs of the defined GBS serotypes, while similar in saccharide components, differ in their structural arrangements. The CPSs of GBS serotypes Ia, III, and V each have at least one disaccharide or trisaccharide side chain extending from the backbone. The type II GBS CPS consists of a backbone repeating unit structure with two monosaccharide side chains (Fig. 5). As shown in Fig. 6, the inhibitory effect mediated by NADG was significantly greater for opsonophagocytosis of serotypes Ia, III, and V than for serotype II GBS (P ≤ 0.02). Inhibition mediated by OKM1 was greater for each of these serotypes than for serotype II (P < 0.03). Inhibition mediated by fructose was low for each of the four serotypes. These findings suggested that the length of the CPS side chain or its tertiary structure could be important to lectin site use.
FIG. 5.
Schematics of the repeating unit structures of GBS CPSs Ia (13), III (40, 42), V (39), and II (15).
FIG. 6.
The effect of lectin site inhibition of opsonophagocytosis of GBS serotypes Ia, II, III, and V by NADG (50 mM) (solid bars), fructose (50 mM) (stippled bars), or OKM1 (10 μg/ml) (open bars). The mean (± SEM) inhibition mediated by NADG was significantly greater for serotypes Ia, III, or V than for serotype II (P ≤ 0.02 for each serotype versus type II GBS). Similarly, the mean (± SEM) inhibition mediated by OKM1 was significantly greater for serotypes Ia, III, or V GBS than for type II GBS (P < 0.03).
Lack of a requirement for NADG as a component monosaccharide for lectin site use.
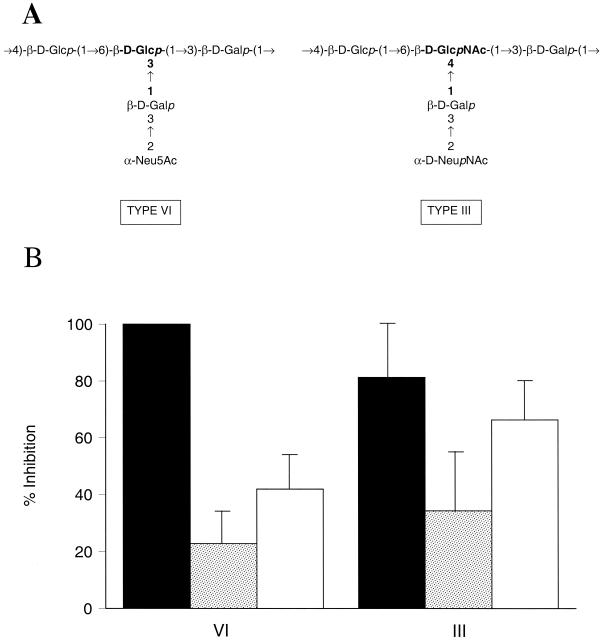
Experiments next questioned whether NADG was required as a component monosaccharide for GBS to engage the CR3 lectin site. The type VI GBS structure has a backbone comprised of repeating units of glucose and galactose, and the disaccharide side chain consists of galactose and sialic acid (Fig. 7A). The only structural difference between the CPSs of types III and VI is that the side chain galactose of the type VI capsule connects to the backbone glucose by a 1→3 linkage, whereas that of type III connects to the backbone NADG by a 1→4 linkage (19). As shown in Fig. 7B, lectin site use was similar for serotypes III and VI GBS. The inhibitory effect mediated by NADG (50 mM) was 100% for type VI and 81% ± 19% for type III (data from Fig. 6). Inhibition mediated by OKM1 was 42% ± 12% (type VI) and 66% ± 14% (type III), respectively. Therefore, interaction of GBS with the CR3 lectin site does not require a NADG-containing structure.
FIG. 7.
(A) The repeating units of the CPS of types VI and III GBS are shown schematically (19, 42). (B) Inhibitory effect of NADG (50 mM) (solid bars), fructose (50 mM) (stippled bars), or OKM1 (10 μg/ml) (open bars) upon opsonophagocytosis. NADG-mediated inhibition was 100% in all experiments for type VI GBS. There were no significant differences in the extent to which NADG, fructose, or OKM1 promoted inhibition of opsonophagocytosis of type VI versus type III GBS (P > 0.1 for each comparison). Error bars, SEMs.
The type VIII CPS recently has been isolated and shown to have a backbone repeating unit consisting of d-glucose, d-galactose, and l-rhamnose with sialic acid as a monosaccharide side chain linked 2→3 to the backbone galactose (18). We predicted that the lack of NADG as a component monosaccharide would not affect lectin site interactions but that the side chain construct would effect poor lectin site use. Inhibition mediated by NADG (50 mM) was 25% ± 7%, by fructose (50 mM) was 11% ± 5%, and by OKM1 was 6% ± 3%. Thus, type VIII resembles type II or the asialo type III mutant in its lectin site use. These data confirmed that side chain length is an important determinant of lectin site use by GBS CPS.
Effect of molecular mass of type III capsule upon lectin site inhibition.
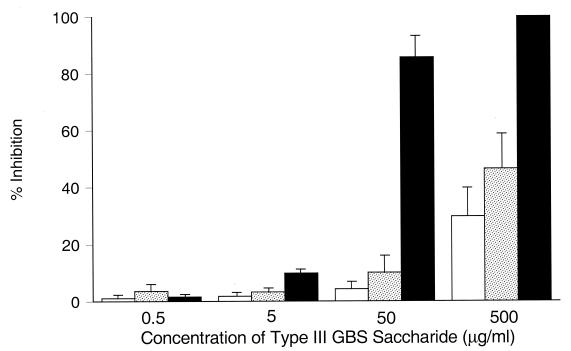
Figure 8 compares the inhibition of opsonophagocytosis by a type III GBS oligosaccharide with an average Mr of 5,900, representing approximately five repeating units; a larger oligosaccharide with an Mr of 22,500, representing approximately 20 units of the CPS; and native type III GBS CPS. At the lower concentrations tested (range, 0.5 to 50 μg/ml), the oligosaccharides mediated minimal inhibition of opsonophagocytosis (≤10% ± 6%). Significantly greater inhibition was mediated by native type III polysaccharide at 50 μg/ml (86% ± 8%) than for either oligosaccharide (P ≤ 0.001). At the highest concentration tested (500 μg/ml), the smaller oligosaccharide mediated 30% ± 10% inhibition and the larger oligosaccharide mediated 46% ± 12% inhibition of opsonophagocytosis. Significantly greater inhibition (100% [P ≤ 0.02 versus either oligosaccharide]) was observed at this concentration by native type III GBS CPS. Thus, increasing numbers of repeating units of type III GBS CPS increase the efficiency of CPS interaction with the CR3 lectin site.
FIG. 8.
Effect of molecular weight of type III capsule upon lectin site inhibition. The extent to which increasing concentrations of type III oligosaccharides with an average Mr of 5,900 (open bars), an Mr of 22,500 (stippled bars), or native (intact) type III GBS CPS (closed bars) inhibited PMN-mediated opsonophagocytosis of type III GBS by nonimmune serum is shown. At 500 μg/ml, native type III GBS CPS effected significantly greater mean (± SEM) inhibition of the lectin site (100%) than did the smaller (30% ± 10% inhibition [P = 0.002]) or larger (46% ± 12% inhibition [P = 0.02]) type III GBS oligosaccharides.
DISCUSSION
The importance of antibody with specificity for the CPS of type III GBS in protective immunity against GBS infections and that of the complement system in mediating optimal opsonophagocytosis of type III organisms are well recognized concepts (7, 8). Some 1 to 2% of newborn infants born to type III GBS CPS IgG-deficient genitally colonized mothers develop invasive infection (30). The immune mechanisms protecting the majority of susceptible infants are poorly elucidated. We have shown that type III GBS triggers PMN-mediated phagocytosis by use of the lectin site of CR3 and that the type III GBS CPS is a ligand for this interaction in sera containing complement and a low concentration of type III CPS-specific IgG.
A number of bacterial species interact with phagocytic cells via surface lectins. One of the best characterized is the mannose–N-acetylglucosamine-specific lectin, or mannose receptor that is expressed on tissue macrophages (25). This lectin receptor is a type I transmembrane glycoprotein consisting of a single subunit with as many as eight adjacent carbohydrate recognition domains, several of which must be engaged to initiate receptor activity (34). The mannose receptor recognizes carbohydrates expressed on the surface of a number of microorganisms, including E. coli, Klebsiella pneumoniae, and P. aeruginosa, and mediates their ingestion. Ofek and Doyle (24) have described such an interaction for GBS with murine peritoneal macrophages that leads to binding of the organisms.
Another prominent phagocytic cell receptor involved in recognizing pathogens belongs to the CD11b/CD18 family of integrins. CR3 is a receptor that mediates both opsonin-mediated and nonopsonic phagocytosis. Ross and associates (27) described the lectin-like properties of CR3, identifying two distinct binding sites in the molecule, one for fixed iC3b that did not trigger functions such as respiratory burst and ingestion and a second function-triggering site that bound zymosan or unopsonized yeast, inducing a superoxide burst. This lectin site of CR3 was inhibited by EDTA or NADG, but not by mannan. Subsequently, CR3 was reported to have a specificity for β-glucan (28). The CR3 lectin site has been mapped to a region of CD11b located COOH terminal to the I domain binding sites for ICAM-1, fibrinogen, and iC3b (35). The sugar specificity of the receptor is broader than originally appreciated, allowing it to interact with a number of monosaccharides, including α- and β-methylmannoside and α- and β-methylglucoside and, with certain polysaccharides containing mannose or NADG, as well as with glucose (35). Other saccharides such as galactose or fructose did not inhibit lectin site-mediated functions (32, 35).
Three sets of observations led to our hypothesis. First, Antal et al. (2) demonstrated that antibody and complement-independent phagocytosis of GBS by murine peritoneal macrophages was mediated in part by macrophage CR3. Next, an earlier investigation from our laboratory compared inhibitory effects of multiple anti-CD11b antibodies and found that OKM1 significantly inhibited opsonophagocytosis of type Ia or III GBS (33). The inability of OKM1 to inhibit a number of adherence-dependent PMN functions (37), an effect associated with its recognition of an epitope physically removed and distinct from the iC3b binding site (4), suggested that a cooperative interaction of GBS cell wall sugars with fixed iC3b might be taking place. Finally, the panel of saccharides shown to interact with the lectin site included NADG, a component monosaccharide of each GBS serotype that causes most invasive human disease. Vĕtvička et al. (36) had shown that β-glucan induced a primed state of CR3 that could trigger killing of iC3b target cells that were otherwise resistant to cytotoxicity. We reasoned that a similar mechanism could effect phagocytosis for iC3b-primed type III GBS if the CPS was interacting with the lectin site.
The finding that NADG, in concentrations shown by Thornton et al. (35) to block use of the lectin site, inhibited opsonophagocytosis of type III GBS by nonimmune serum affirmed the hypothesis. Significantly less inhibition of opsonophagocytosis was observed using fructose, a saccharide identified as a nonlectin site-binding sugar (32, 44). Use of additional saccharides, β-methylglucoside and β-methylmannoside, shown to inhibit CR3 staining by fluorescein isothiocyanate-labeled zymosan, also inhibited opsonophagocytosis of type III GBS.
We have shown previously that IgG specific for III GBS CPS interacts with PMN FcγRII and FcγRIII (23). By contrast, in the absence of complement or antibody, lectin site interactions are insufficient to trigger uptake or killing of type III GBS by PMN (1). When complement is present, FcγRII is no longer required to mediate phagocytosis. Since CR3 possesses lectin-binding activity and since FcγRIII possesses lectin binding sites, Zhou et al. (44) proposed and proved that the two molecules interact physically at the plasma membrane to mediate biologic functions. This cocapping interaction of the carbohydrate side chains of FcRγIII with the lectin site of CR3 was effectively inhibited by NADG but not by fructose. We reasoned that in the presence of a sufficient concentration of type III GBS CPS-specific IgG, activation of CR3 by lectin site-associated interactions would no longer be required to mediate opsonophagocytosis. CPS-specific IgG could bind to FcγRII, a transmembrane receptor, or to FcγRIII without the requirement of CR3 lectin site cocapping to accomplish phagocytosis. The experiments in Fig. 2 and 3 indicated that type III GBS CPS-specific IgG overrides the requirement for lectin site use.
A length dependency of the repeating unit of the pentasaccharide of type III GBS CPS optimal for antibody binding affinity has been shown (41). A graded increase in affinity of antibody binding was seen as oligosaccharide size increased from 2.6 to 92 repeating units. We questioned whether type III GBS capsule was required for lectin site use to occur and examined the spatial and size relationships optimizing these interactions. Mutant strains derived from type III GBS in which capsule is absent or altered genetically by removal of terminal sialic acid residues failed to interact with the CR3 lectin site. This observation led us to query whether a disaccharide side chain was optimal. It proved that GBS serotypes with at least one disaccharide side chain interact with the lectin site, but those with exclusively monosaccharide side chains do not. Of note, the acapsular and asialo mutants of III GBS have a higher bactericidal index than the encapsulated parent strain. The capsular polysaccharide of type III GBS is a virulence factor, serving as a cloak impairing phagocytosis (21, 26). In addition, it has been shown that acapsular and asialo type III GBS mutants lose their virulence in animal models of sepsis and demonstrate enhanced binding to important immune cells and thus are easier to kill (1, 20, 43). Therefore, we postulate that in order for PMN to phagocytose the more virulent encapsulated strain it utilizes the lectin site, which, while not equivalent to the acquired or antibody-mediated immune response, is a more sophisticated or developmentally advanced arm of the innate immune response than that required for the capsular mutant strains. Type VI GBS, unique in its lack of NADG as a component monosaccharide but possessing a disaccharide side chain, also interacted with the lectin site, dispelling the notion that NADG might be a required structural unit. It recently has been proposed that the conformational epitope of the type III GBS CPS is an extended helical segment of the polysaccharide (45). In addition to structural units, tertiary configuration is an important determinant for CR3 recognition and interactions (5, 35). Thus, the extent to which side chain length affects the final tertiary configurations of GBS CPS could determine their ability to bind to CR3 at the lectin site. As to the effect of relative molecular mass, intact or native type III GBS CPS, with a peak Mr of 237,000, interacted with significantly greater efficiency than oligosaccharides comprised of approximately 5 and 20 repeating units, respectively.
The results of these experiments extend the current understanding of host defense against invasive type III GBS infections in the setting of low concentrations of type III GBS CPS-specific IgG. Our findings define a fundamental role for the lectin site of PMN CR3 in triggering opsonophagocytosis and demonstrate that the type III GBS CPS functions as a ligand to promote PMN CR3 function. When adequate iC3b has been deposited, PMN accomplish phagocytosis by using the type III GBS CPS as the stimulus to initiate CR3-mediated functions. The pathogen itself thus stimulates PMN-mediated phagocytosis, an excellent strategy for the host to avert invasion before the development of specific immunity. This mechanism offers an explanation for the efficiency with which many, but not all, naive hosts encountering type III GBS are able to respond to and ingest this pathogen.
ACKNOWLEDGMENTS
This work was supported by The Streptococcal Initiative, contract N01 AI75326, from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
We thank Lawrence C. Paoletti and Dennis L. Kasper for preparing and providing oligosaccharides from the type III GBS CPS, Claire M. Skeeter and Barbara Reinap for excellent technical assistance, and Carol J. Baker for review of the manuscript.
REFERENCES
- 1.Albanyan E A, Vallejo J G, Smith C W, Edwards M S. Nonopsonic binding of type III group B streptococci to human neutrophils induces interleukin-8 release mediated by the p38 mitogen-activated protein kinase pathway. Infect Immun. 2000;68:2053–2060. doi: 10.1128/iai.68.4.2053-2060.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antal J M, Cunningham J V, Goodrum K J. Opsonin-independent phagocytosis of group B streptococci: role of complement receptor type three. Infect Immun. 1992;60:1114–1121. doi: 10.1128/iai.60.3.1114-1121.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker C J, Kasper D L. Correlation of maternal antibody deficiency with susceptibility to neonatal group B streptococcal infection. N Engl J Med. 1976;294:753–756. doi: 10.1056/NEJM197604012941404. [DOI] [PubMed] [Google Scholar]
- 4.Cain J A, Newman S L, Ross G D. Role of complement receptor type three and serum opsonins in the neutrophil response to yeast. Complement. 1987;4:75–86. doi: 10.1159/000463011. [DOI] [PubMed] [Google Scholar]
- 5.Diamond M S, Garcia-Aguilar J, Bickford J K, Corbi A L, Springer T A. The I domain is a major recognition site on the leukocyte integrin Mac-1 (CD11b/CD18) for four distinct adhesion ligands. J Cell Biol. 1993;120:1031–1043. doi: 10.1083/jcb.120.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong Z M, Murphy J W. Cryptococcal polysaccharides bind to CD18 on human neutrophils. Infect Immun. 1997;65:557–563. doi: 10.1128/iai.65.2.557-563.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards M S, Baker C J, Kasper D L. Opsonic specificity of human antibody to the type III polysaccharide of group B Streptococcus. J Infect Dis. 1979;140:1004–1008. doi: 10.1093/infdis/140.6.1004. [DOI] [PubMed] [Google Scholar]
- 8.Edwards M S, Nicholson-Weller A, Baker C J, Kasper D L. The role of specific antibody in alternative complement pathway-mediated opsonophagocytosis of type III, group B Streptococcus. J Exp Med. 1980;151:1275–1287. doi: 10.1084/jem.151.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Estabrook M M, Zhou D, Apicella M A. Nonopsonic phagocytosis of group C Neisseria meningitidis by human neutrophils. Infect Immun. 1998;66:1028–1036. doi: 10.1128/iai.66.3.1028-1036.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fearon D T, Locksley R M. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50–53. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- 11.Grange P A, Erickson A K, Anderson T J, Francis D H. Characterization of the carbohydrate moiety of intestinal mucin-type sialoglycoprotein receptors for the K88ac fimbrial adhesin of Escherichia coli. Infect Immun. 1998;66:1613–1621. doi: 10.1128/iai.66.4.1613-1621.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guttormsen H-K, Baker C J, Edwards M S, Paoletti L C, Kasper D L. Quantitative determination of antibodies to type III group B streptococcal polysaccharide. J Infect Dis. 1996;173:142–150. doi: 10.1093/infdis/173.1.142. [DOI] [PubMed] [Google Scholar]
- 13.Jennings H J, Katzenellenbogen E, Lugowski C, Kasper D L. Structure of native polysaccharide antigens of type Ia and Ib group B Streptococcus. Biochemistry. 1983;22:1258–1264. doi: 10.1021/bi00274a042. [DOI] [PubMed] [Google Scholar]
- 14.Jennings H J, Rosell K-G, Kasper D L. Structural determination and serology of the native polysaccharide antigen of type-III group B Streptococcus. Can J Biochem. 1980;58:112–120. doi: 10.1139/o80-016. [DOI] [PubMed] [Google Scholar]
- 15.Jennings H J, Rosell K-G, Katzenellenbogen E, Kasper D L. Structural determination of the capsular polysaccharide antigen of type II group B Streptococcus. J Biol Chem. 1983;258:1793–1798. [PubMed] [Google Scholar]
- 16.Joustra M, Lundgren H. Preparation of freeze dried, monomeric and immunochemically pure IgG by a rapid and reproducible chromatographic technique. In: Peeters H, editor. Protides of the biological fluids. 17. Proteins and related subjects. New York, N.Y: Pergamon Press; 1969. pp. 511–515. [Google Scholar]
- 17.Kang B K, Schlesinger L S. Characterization of mannose receptor-dependent phagocytosis mediated by Mycobacterium tuberculosis lipoarabinomannan. Infect Immun. 1998;66:2769–2777. doi: 10.1128/iai.66.6.2769-2777.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kogan G, Uhrín D, Brisson J-R, Paoletti L C, Blodgett A E, Kasper D L, Jennings H J. Structural and immunochemical characterization of the type VIII group B Streptococcus capsular polysaccharide. J Biol Chem. 1996;271:8786–8790. doi: 10.1074/jbc.271.15.8786. [DOI] [PubMed] [Google Scholar]
- 19.Kogan G, Uhrín D, Brisson J-R, Paoletti L C, Kasper D L, von Hunolstein C, Orefici G, Jennings H J. Structure of the type VI group B streptococcus capsular polysaccharide determined by high resolution NMR spectroscopy. J Carbohydr Chem. 1994;13:1071–1078. [Google Scholar]
- 20.Martin T R, Rubens C E, Wilson C B. Lung antibacterial defense mechanisms in infant and adult rats: implications for the pathogenesis of group B streptococcal infections in the neonatal lung. J Infect Dis. 1988;157:91–100. doi: 10.1093/infdis/157.1.91. [DOI] [PubMed] [Google Scholar]
- 21.Martin T R, Ruzinski J T, Rubens C E, Chi E Y, Wilson C B. The effect of type-specific polysaccharide capsule on the clearance of group B streptococci from the lungs of infant and adult rats. J Infect Dis. 1992;165:306–314. doi: 10.1093/infdis/165.2.306. [DOI] [PubMed] [Google Scholar]
- 22.Mork T, Hancock R E W. Mechanisms of nonopsonic phagocytosis of Pseudomonas aeruginosa. Infect Immun. 1993;61:3287–3293. doi: 10.1128/iai.61.8.3287-3293.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noya F J D, Baker C J, Edwards M S. Neutrophil Fc receptor participation in phagocytosis of type III group B streptococci. Infect Immun. 1993;61:1415–1420. doi: 10.1128/iai.61.4.1415-1420.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ofek I, Doyle R J. Bacterial adhesion to cells and tissues. London, United Kingdom: Chapman & Hall; 1994. pp. 171–194. [Google Scholar]
- 25.Ofek I, Goldhar J, Keisari Y, Sharon N. Nonopsonic phagocytosis of microorganisms. Annu Rev Microbiol. 1995;49:239–276. doi: 10.1146/annurev.mi.49.100195.001323. [DOI] [PubMed] [Google Scholar]
- 26.Philips J B, III, Li J-X, Gray B M, Pritchard D G, Oliver J R. Role of capsule in pulmonary hypertension induced by group B streptococcus. Pediatr Res. 1992;31:386–390. doi: 10.1203/00006450-199204000-00016. [DOI] [PubMed] [Google Scholar]
- 27.Ross G D, Cain J A, Lachmann P J. Membrane complement receptor type three (CR3) has lectin-like properties analogous to bovine conglutinin and functions as a receptor for zymosan and rabbit erythrocytes as well as a receptor for iC3b. J Immunol. 1985;134:3307–3315. [PubMed] [Google Scholar]
- 28.Ross G D, Cain J A, Myones B L, Newman S L, Lachmann P J. Specificity of membrane complement receptor type three (CR3) for β-glucans. Complement. 1987;4:61–74. doi: 10.1159/000463010. [DOI] [PubMed] [Google Scholar]
- 29.Rubens C E, Wessels M R, Heggen L M, Kasper D L. Transposon mutagenesis of type III group B Streptococcus: correlation of capsule expression with virulence. Proc Natl Acad Sci USA. 1987;84:7208–7212. doi: 10.1073/pnas.84.20.7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schuchat A. Epidemiology of group B streptococcal disease in the United States: shifting paradigms. Clin Microbiol Rev. 1998;11:497–513. doi: 10.1128/cmr.11.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuchat A. Group B streptococcus. Lancet. 1999;353:51–56. doi: 10.1016/S0140-6736(98)07128-1. [DOI] [PubMed] [Google Scholar]
- 32.Sehgal G, Zhang K, Todd III R F, Boxer L A, Petty H R. Lectin-like inhibition of immune complex receptor-mediated stimulation of neutrophils. J Immunol. 1993;150:4571–4580. [PubMed] [Google Scholar]
- 33.Smith C L, Baker C J, Anderson D C, Edwards M S. Role of complement receptors in opsonophagocytosis of group B streptococci by adult and neonatal neutrophils. J Infect Dis. 1990;162:489–495. doi: 10.1093/infdis/162.2.489. [DOI] [PubMed] [Google Scholar]
- 34.Taylor M E, Drickamer K. Structural requirements for high affinity binding of complex ligands by the macrophage mannose receptor. J Biol Chem. 1993;268:399–404. [PubMed] [Google Scholar]
- 35.Thornton B P, Vĕtvička V, Pitman M, Goldman R C, Ross G D. Analysis of the sugar specificity and molecular location of the β-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18) J Immunol. 1996;156:1235–1246. [PubMed] [Google Scholar]
- 36.Vĕtvička V, Thornton B P, Ross G D. Soluble β-glucan polysaccharide binding to the lectin site of neutrophil or natural killer cell complement receptor type 3 (CD11b/CD18) generates a primed state of the receptor capable of mediating cytotoxicity of iC3b-opsonized target cells. J Clin Investig. 1996;98:50–61. doi: 10.1172/JCI118777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wallis W J, Hickstein D D, Schwartz B R, June C H, Ochs H D, Beatty P G, Klebanoff S J, Harlan J M. Monoclonal antibody-defined functional epitopes on the adhesion-promoting glycoprotein complex (CDw18) of human neutrophils. Blood. 1986;67:1007–1013. [PubMed] [Google Scholar]
- 38.Wang Y, Hollingsworth R I, Kasper D L. Ozonolysis for selectively depolymerizing polysaccharides containing β-d-aldosidic linkages. Proc Natl Acad Sci USA. 1998;95:6584–6589. doi: 10.1073/pnas.95.12.6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wessels M R, DiFabio J L, Benedi V-J, Kasper D L, Michon F, Brisson J-R, Jelínková J, Jennings H J. Structural determination and immunochemical characterization of the type V group B Streptococcus capsular polysaccharide. J Biol Chem. 1991;266:6714–6719. [PubMed] [Google Scholar]
- 40.Wessels M R, Kasper D L. Correct structure of repeating unit of group B Streptococcus type III capsular polysaccharide. J Infect Dis. 1990;162:1412. doi: 10.1093/infdis/162.6.1412. [DOI] [PubMed] [Google Scholar]
- 41.Wessels M R, Muñoz A, Kasper D L. A model of high-affinity antibody binding to type III group B Streptococcus capsular polysaccharide. Proc Natl Acad Sci USA. 1987;84:9170–9174. doi: 10.1073/pnas.84.24.9170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wessels M R, Pozsgay V, Kasper D L, Jennings H J. Structure and immunochemistry of an oligosaccharide repeating unit of the capsular polysaccharide of type III group B Streptococcus. A revised structure for the type III group B streptococcal polysaccharide antigen. J Biol Chem. 1987;262:8262–8267. [PubMed] [Google Scholar]
- 43.Wessels M R, Rubens C E, Benedi V J, Kasper D L. Definition of a bacterial virulence factor: sialylation of the group B streptococcal capsule. Proc Natl Acad Sci USA. 1989;86:8983–8987. doi: 10.1073/pnas.86.22.8983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou M-J, Todd III R F, van de Winkel J G J, Petty H R. Cocapping of the leukoadhesin molecules complement receptor type 3 and lymphocyte function-associated antigen-1 with Fcγ receptor III on human neutrophils. J Immunol. 1993;150:3030–3041. [PubMed] [Google Scholar]
- 45.Zou W, Mackenzie R, Thérien L, Hirama T, Yang Q, Gidney M A, Jennings H J. Conformational epitope of the type III group B Streptococcus capsular polysaccharide. J Immunol. 1999;163:820–825. [PubMed] [Google Scholar]