Abstract
Scaffold macroporosity has been shown to be critical for promoting bone regeneration. Although injectable materials are preferred for minimally invasive delivery, conventional macroporous scaffolds were not injectable and do not support homogeneous cell encapsulation. We recently reported a gelatin-based microribbon (μRB) scaffold that offers macroporosity while also supporting homogeneous cell encapsulation. Compared to conventional gelatin hydrogels, macroporous gelatin μRB scaffolds demonstrated great advantage in enhancing mesenchymal stem cell (MSC)-based cartilage formation. However, whether gelatin-based μRBs support MSC osteogenesis and bone formation remains unknown. The goal of this study is to assess the potential of gelatin-based μRBs for supporting MSC-based osteogenesis and bone formation in vitro. Given recent evidence from the literature that osteogenesis is sensitive to substrate stiffness, we further investigate how varying μRB stiffness modulates MSC osteogenesis. We first determine the maximal stiffness range of gelatin μRBs that can be fabricated (13–57 kPa), which supports both retention of μRB shape and macroporosity within scaffolds after inter-cross-linking. Interestingly, varying μRB stiffness across a broad range of stiffness did not significantly impact osteogenesis, with all groups supporting upregulation of bone markers and extensive collagen deposition. All gelatin μRBs also supported a comparable level of cell spreading and upregulation of mechanosensing markers. However, soft μRB (13 kPa) scaffolds did not maintain structural integrity and condensed into a pellet over time. Both intermediate and stiff gelatin μRB-based scaffolds maintained their integrity and supported robust bone formation, leading to a more than 10-fold increase in the compressive moduli of engineered bone after 5 weeks of culture in osteogenic media. Incorporating hydroxyapatite (HA) nanoparticle coating onto the gelatin μRB surface further accelerated the maturation of MSCs into osteoblasts and mineralization. Together, these results validate that gelatin μRBs can support MSC osteogenesis across a broad range of stiffness and offers an injectable macroporous scaffold for enhancing stem-cell-based bone regeneration.
Keywords: macroporous, gelatin, scaffolds, stiffness, bone, mesenchymal stem cells
Graphical Abstract
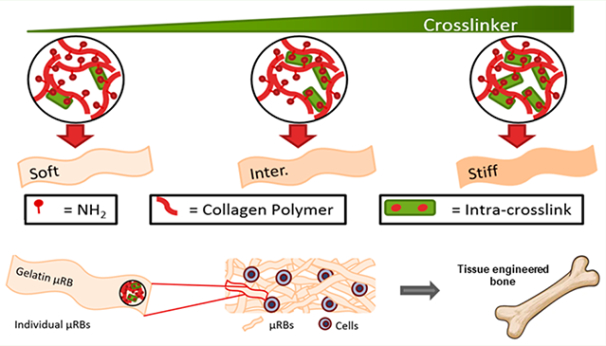
1. INTRODUCTION
More than half a million bone graft procedures are performed in the United States annually, resulting in significant combined costs of $2.5 billion for patient visits and loss of productivity.1 These numbers are forecast to double by 2020 in the United States and globally because of the increased life expectancy and growing needs of Baby Boomers.1 Bone tissue engineering has the potential to reduce our current dependence on bone grafting tissue by offering a promising alternative to conventional treatments to repair bone loss.2 In addition to cells alone, biomaterials can be used as injectable or implantable matrices to provide initial structural support, enhance cell delivery, and promote desirable cell fates and tissue formation. Given the weight-bearing function of bone, it is important that the choice of scaffold can maintain structural integrity in a load-bearing environment.3,4 In addition, macroporosity within scaffolds has been shown to be critical for promoting bone regeneration by facilitating cell proliferation, blood vessel ingrowth, and accelerating new matrix deposition.5–7 Scaffolds lacking macroporosity not only restrict matrix deposition but also limit the restoration of mechanical strength, highlighting the need for interconnected macroporosity throughout the scaffold.8–10
To provide macroporous scaffolds with desirable load-bearing properties, researchers have developed various biomaterials for use as scaffolds for bone tissue engineering including collagen, ceramics, and biodegradable polyester scaffolds. Given that collagen I is the major organic component of bone tissue, collagen sponges are highly biomimetic to native bone extracellular matrix (ECM) in biochemical composition but only have limited tunability in niche cues.11,12 Ceramic-based scaffolds, on the other hand, mimic the mineral composition of bone ECM.13,14 Synthetic biodegradable polyester polymers such as poly(lactic-co-glycolic acid) offer a broader range of tunability and have been shown to support bone regeneration in vitro and in vivo.15–17 Although these previous macroporous scaffolds have shown great promise for bone tissue engineering, they require prefabrication before cell seeding. As a result, it is challenging to achieve uniform cell distribution in scaffolds with clinically relevant dimensions, and the scaffolds can only be implanted, rendering them unsuitable for minimally invasive delivery.18,19
To address the limitations associated with conventional macroporous scaffolds, we recently reported a gelatin-based macroporous scaffold that combines macroporosity with the ability to support direct cell encapsulation. Specifically, methacrylated gelatin precursor solution is wet-spun into microribbon (μRB)-like hydrogel building blocks with tunable width ~40–100 μm, which can be intra-cross-linked first using glutaraldehyde to lock the μRB shape. The μRBs can then be mixed homogeneously with cells, and then inter-cross-linked into a 3D cell-laden scaffold upon exposure to light.9,20 Compared to conventional gelatin hydrogels, macroporous gelatin-based μRB scaffolds substantially enhanced the speed and amount of new cartilage matrix deposition by mesenchymal stem cells (MSCs) in 3D, improved interconnectivity of newly deposited ECM, and enabled a more than 20-fold increase in the compressive moduli of engineered cartilage in just 3 weeks.8
Although our previous study demonstrated the advantage of macroporous gelatin μRB-based scaffolds for accelerating MSC-based cartilage regeneration in vitro, it only used gelatin μRBs with a single stiffness; how varying stiffness of gelatin μRBs would modulate MSC osteogenesis in vitro remains unknown. Unlike electrospun nanofibers, which have subcellular-scale fiber diameters, the width of individual μRBs is larger than individual cells. As such, encapsulated cells attach to the surface of individual μRBs and sense stiffness of μRB like a substrate. Recent reports have shown that MSC differentiation can be directly modulated by substrate stiffness, with increasing substrate stiffness leading to enhanced osteogenesis.21–24 As such, the goal of this study is to synthesize gelatin μRBs with tunable stiffness and evaluate the effects of varying gelatin μRB stiffness in modulating MSC-based osteogenesis in vitro. To fabricate gelatin μRBs with tunable stiffness, we first vary the concentration of glutaraldehyde, the cross-linker used for fixing gelatin into the unique μRB shape after spinning. To assess the effects of varying μRB stiffness on osteogenesis, human MSCs are encapsulated in 3D macroporous scaffolds formed using gelatin μRBs with tunable stiffness (13–57 kPa) and cultured in standard osteogenic media for up to 5 weeks. We further investigate the effect of hydroxyapatite (HA), a major mineral component of native bone, on accelerating maturation of MSC-based osteogenesis in μRB scaffolds. We hypothesize that increasing μRB stiffness enhances osteogenesis, and the incorporation of HA further accelerates the MSC maturation toward bone lineage. Outcomes were analyzed using quantitative gene expression analyses, quantitative biochemical assays for cell proliferation and bone matrix deposition, mechanical testing, and histology.
2. MATERIALS AND METHODS
2.1. Materials.
Porcine-skin-derived gelatin (type-A), dimethyl sulfoxide (DMSO), methacrylic anhydride, glutaraldehyde, l-lysine hydrochloride, and HA nanoparticles were purchased from Sigma-Aldrich (St. Louis, MO). All materials were used as received.
2.2. Fabricating and Characterizing μRBs with Varying Stiffness.
Gelatin μRBs were fabricated using wet-spinning as previously reported.8 To fabricate μRBs with different stiffness, we transferred MA-NHS-functionalized gelatin μRBs to fresh methanol (200 mL) containing varying concentrations of glutaraldehyde (1.25–125% (w/v)) and stirred vigorously for 24 h at room temperature. Unreacted glutaraldehyde was quenched by adding l-lysine hydrochloride (1% in 200 mL phosphate-buffered saline (PBS)) and stirred for 2 h. The product was washed eight times with PBS and three times with deionized water to remove any unreacted reagents before being lyophilized and stored at −20 °C. For cell studies, three formulations of μRBs (fixed with 12.5, 25, and 62.5% glutaraldehyde) were chosen to vary the substrate stiffness. The widest possible range was chosen that could support intra-cross-linking to maintain the μRB shape while also allowing for efficient inter-cross-linking to form a 3D macroporous scaffold.
The stiffness of individual μRBs was measured using atomic force microscopy. We used a silicon nitride cantilever fitted with a 5.4 μm colloidal probe (AppNano, Mountain View, CA), 0.03–0.08 N/m spring constant, and 4–6 kHz fundamental resonance frequency. All measurements were performed in PBS at 37 °C using an MFP-3D system (Asylum Research, Goleta, CA). A 10 × 10 grid map was applied across the width of the ribbon and measured in force–volume mode on three samples; each grid map yielded 100 force–distance curves recorded at a probing rate of 0.5 Hz. The stiffness value was calculated by fitting the loading part of each force–distance curve to the Hertz model, assuming a Poisson’s ratio of 0.4 for the samples. To characterize the morphology of hydrated μRB scaffolds, we performed environmental scanning electron microscopy using a Hitachi S-3400N (Hitachi, Tokyo, Japan) variable pressure SEM as previously described.8
2.3. Cell Encapsulation and Differentiation.
Passage 2 bone-marrow-derived human MSCs were purchased from Lonza (Basel, Switzerland), and expanded in MSC growth media. Passage 6 MSCs were encapsulated in μRB scaffolds (7.5% w/v) with three different stiffnesses (13, 28, and 57 kPa). Cell viability was assessed 24 h after encapsulation using a LIVE/DEAD Viability/Cytotoxicity Kit (Invitrogen, Carlsbad, CA) for 30 min (37 °C, 5% CO2). Representative 3D images were acquired using a z-stack and merged with maximum projection on a Zeiss fluorescence microscope (Axio Observer 3.1, Zeiss, Oberkochen, Germany) and the ZEN 2 (blue edition) software (Zeiss). Cell morphology was visualized using fluorescently labeled phalloidin (P1951, 1:100, Sigma-Aldrich), which selectively labels F-actin. To characterize the baseline of cell-laden μRB scaffolds at the beginning of the culture, we harvested some samples at day 1 from each group for histology (n = 2), mechanical testing (n = 5), gene expression analysis (n = 3), and biochemical assays (n = 5). The remaining samples were then cultured for up to 5 weeks in osteogenic media consisting of DMEM, 100 nM dexamethasone, 50 μg/mL ascorbic acid-2-phosphate, and 10 mM β-glycerol phosphate disodium salt. Samples were harvested earlier at a final time point of day 31 because of rapid degradation/shrinkage of the soft μRB group to enable comparison across all formulations.
2.4. Immunostaining and Histology.
Samples were harvested for histology and immunostaining on day 7 (n = 2/group) and day 31 (n = 5/group). To assess osteogenesis, we performed staining for alkaline phosphatase (ALP), Masson trichrome, and Alizarin Red S (ARS). ALP staining was performed using Fast Blue RR Salt and Naphthol AS-MX phosphate (FBS25, 855, Sigma-Aldrich). Masson’s Trichrome staining was performed using a staining kit (87019, Thermo Scientific). Mineralization was visualized using ARS staining (A5533 Sigma-Aldrich). To check if MSCs also undergo chondrogenesis, we performed staining for cartilage matrix sulfated glycosaminoglycans (sGAG) using Safranin-O staining (Sigma-Aldrich). For immunostaining, sections were first treated with blocking buffer (2% goat serum and 3% bovine serum albumin in 1X PBS) for 1 h, then incubated with primary antibodies for 18 h at 4 °C. The following primary antibodies were used: rabbit polyclonal antibodies (against type I collagen (ab34710, 1:100), type II (ab34712, 1:80), type X collagen (ab58632, 1:100), and osteopontin (ab8448, 1:100)) and mouse polyclonal antibody against osteocalcin (ab13418, 1:80). All primary antibodies were purchased from Abcam (Cambridge, MA). The sections were then incubated for 1 h at room temperature using species-specific secondary antibodies including Alexa Fluor 488 goat antirabbit (1:100, Invitrogen) and Alexa Fluor 488 goat antimouse (1:100, Invitrogen). For all immunostainings, nuclei were counterstained using Hoechst 33342 stain (Thermo Scientific). All images were taken using the Zeiss fluorescence microscope as mentioned above (Axio Observer 3.1).
2.5. Gene Expression Analyses.
RNA was extracted from 3D cell-laden μRB samples (n = 5) at day 1 and day 14 using previously described methods.8 Quantitative real-time polymerase chain reaction (qRT-PCR) was performed on an Applied Biosystems 7900 Real-Time PCR system using SYBR Green master mix (Applied Biosystems, Carlsbad, CA). To characterize mechanosensing, we assayed the following genes: Ras homologue gene family, member A (RhoA), and rho-associated, coiled-coil-containing protein kinase 1 (ROCK1). To assess osteogenesis, bone markers representing early to late stage differentiation were examined, including runt-related transcription factor 2 (RUNX2), ALP, type I collagen (Col I), and osteonectin (ON). Normalized gene expression was quantified and reported using the ΔΔCt method.25 Specifically, the gene expression of each gene was first normalized to the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH), then further normalized to the day one control to show the fold change of gene expressions as of day 14.
2.6. Characterizing Compressive Moduli of 3D μRB Scaffolds.
The stiffness of 3D μRB scaffolds and resulting tissues were characterized using unconfined compression tests using an Instron 5944 materials testing system (Instron Corporation, Norwood, MA) fitted with a 10 N load cell (Interface Inc., Scottsdale, AZ). Sample constructs were tested on day 1 and 31 of culture (n = 5 per group). The test setup consisted of custom-made aluminum compression platens lined with polytetrafluoroethylene to minimize friction. All tests were conducted in PBS at room temperature. Specimen diameter (~4.5 mm) and thickness (~2 mm) were measured using digital calipers and the material testing system’s position read-out, respectively. Before each test, a preload of approximately 10 mN was applied. The upper plate was then lowered at a rate of 1% strain/s to a maximum strain of 30%. Load and displacement data were recorded at 100 Hz. The compressive modulus was determined for a strain range of 20–30% from a linear curve fit of the stress versus strain curve.
2.7. Coating Gelatin μRBs with HA Nanoparticles.
To assess whether incorporating a mineral component would further enhance the osteoinductivity of gelatin μRBs, we compared osteogenesis in gelatin μRBs with and without HA nanoparticles using gelatin μRBs with intermediate stiffness (28 kPa). For the HA nanoparticle containing group, 1.75% HA nanoparticle was added to PBS containing 0.05% LAP photoinitiator, which was then added to the lyophilized μRBs to form a scaffold. Special attention was given to ensure uniform distribution of HA nanoparticles when hydrating the μRBs.
2.8. Biochemical Assays.
Biochemical assays were conducted to quantify cell proliferation and amount of sGAG deposition (n = 5 per group). The wet weight and dry weight of all samples were measured, and then samples were digested in papainase solution (Worthington Biochemical, Lakewood, NJ) at 60 °C for 16 h. DNA content was quantified using the PicoGreen assay (Molecular Probes, Eugene, OR) with lambda phage DNA as a standard. sGAG content was measured using the 1,9-dimethylmethylene blue dye-binding assay and shark chondroitin sulfate (Sigma-Aldrich) as a standard.
2.9. Statistical Analysis.
All data were expressed as mean ± standard error. Statistical significance was determined via analysis of variance using Student’s t-test with equal variance. P-values (two-tailed) less than 0.05 were considered statistically significant; p-values less than 0.005 were considered highly significant.
3. RESULTS
3.1. Gelatin μRBs with a Broad Range of Stiffness Form Macroporous Scaffolds.
μRB scaffolds are formed through a two-step cross-linking process. First, individual gelatin μRBs were intra-cross-linked via reaction between amine groups on the collagen polymer and glutaraldehyde, which fixed the morphology of the μRBs (Figure 1A). In the second step, scaffolds are formed by mixing multiple μRBs with or without cells, and inter-cross-linking them to form a macroporous 3D scaffold (Figure 1A). We first determined the range of glutaraldehyde that is sufficient to fix μRB morphology, but not too high (too stiff) to prevent successful inter-cross-linking to form a scaffold. Varying glutaraldehyde between 1.25 and 7.5% showed insufficient intra-cross-linking, and the resulting scaffolds failed to maintain individual μRB morphology (Figure S1). On the other extreme, increasing glutaraldehyde concentration to 87.5% prevented successful scaffold formation because of insufficient inter-cross-linking (Figure S1). As such, we determined 12.5– 62.5% to be the range of glutaraldehyde concentration that supports μRB formation. Within this chosen range, increasing glutaraldehyde concentration increased the stiffness of individual μRB, as shown by AFM (Figure 1A). We chose three formulations that represent the broadest range of stiffness of μRBs that can be formed and support scaffold formation (13–57 kPa) and added an intermediate stiffness (28 kPa) in between (Figure 1B).
Figure 1.
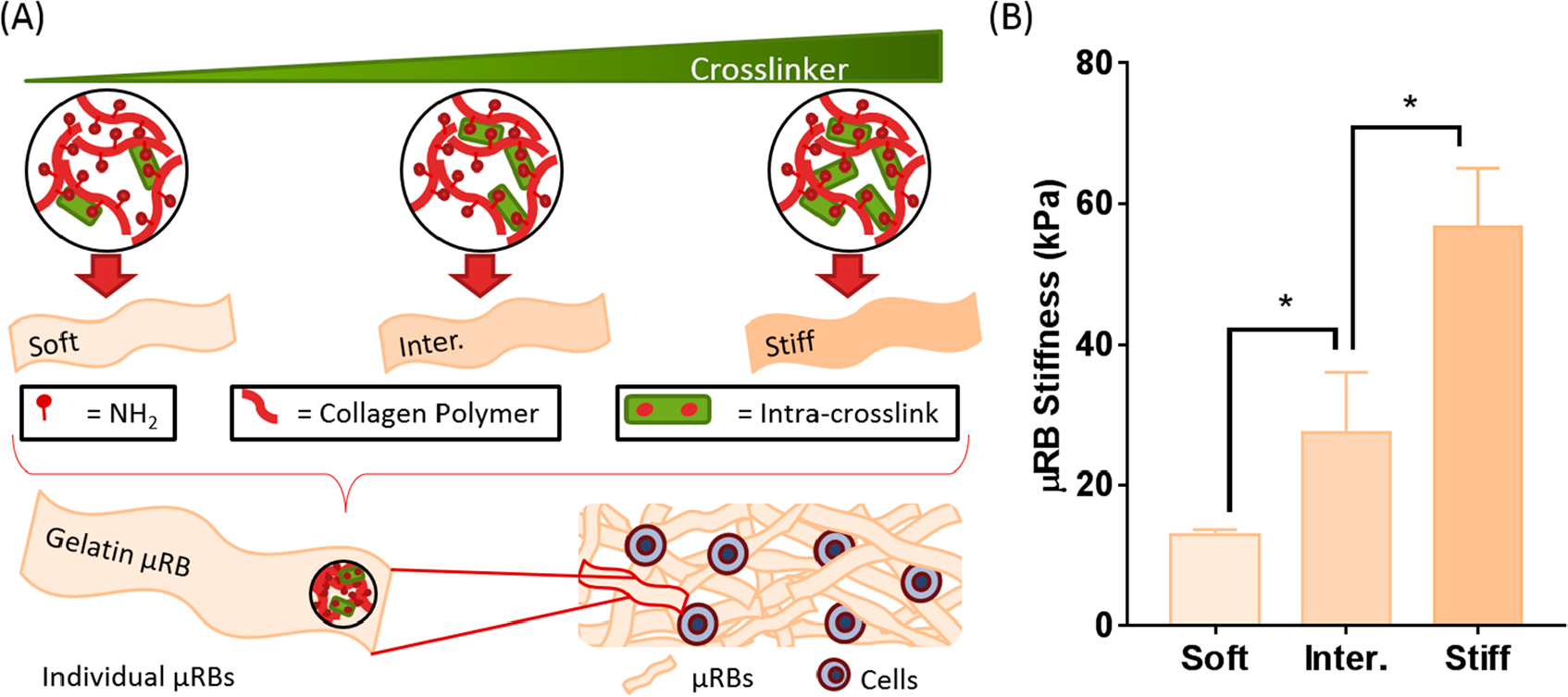
Two-step cross-linking mechanism forms gelatin μRB scaffolds with tunable substrate stiffness, independent from the stiffness of the scaffold, that encapsulated cells can sense. (A) In the first step, individual μRB stiffness is modulated by increasing the concentration of cross-linker, subsequently increasing the degree of intra-cross-linking within collagen polymer. In the second step, individual μRBs of desired stiffness can be mixed with cells and inter-cross-linked to form a bulk 3D scaffold. (B) Individual gelatin μRBs achieved a compressive modulus range from 13 kPa (soft) to 28 kPa (intermediate) to 57 kPa (stiff) as measured by AFM. * = p < 0.05.
3.2. All μRB Stiffnesses Support High Cell Viability and Spreading.
The structure and morphology of μRB scaffolds were characterized using SEM. For all three stiffnesses, the cross-linked μRB scaffolds exhibited highly interconnected macroporosity (Figure 2A). LIVE/DEAD staining at 24 h after cell encapsulation shows comparable levels of high cell viability and homogeneous cell distribution (Figure 2B). Although all three stiffnesses supported rapid cell attachment, μRBs with stiffnesses of 28 and 57 kPa showed more cell spreading compared to the soft μRBs (13 kPa) 24 h after encapsulation (Figure 2B). By day 7, F-actin staining showed all μRB formulations support extensive cell spreading (Figure 2C). Staining of ALP, an early osteogenesis marker, showed positive signals in all groups, with slightly enhanced signals observed in the intermediate and stiff μRB groups (Figure 2D).
Figure 2.
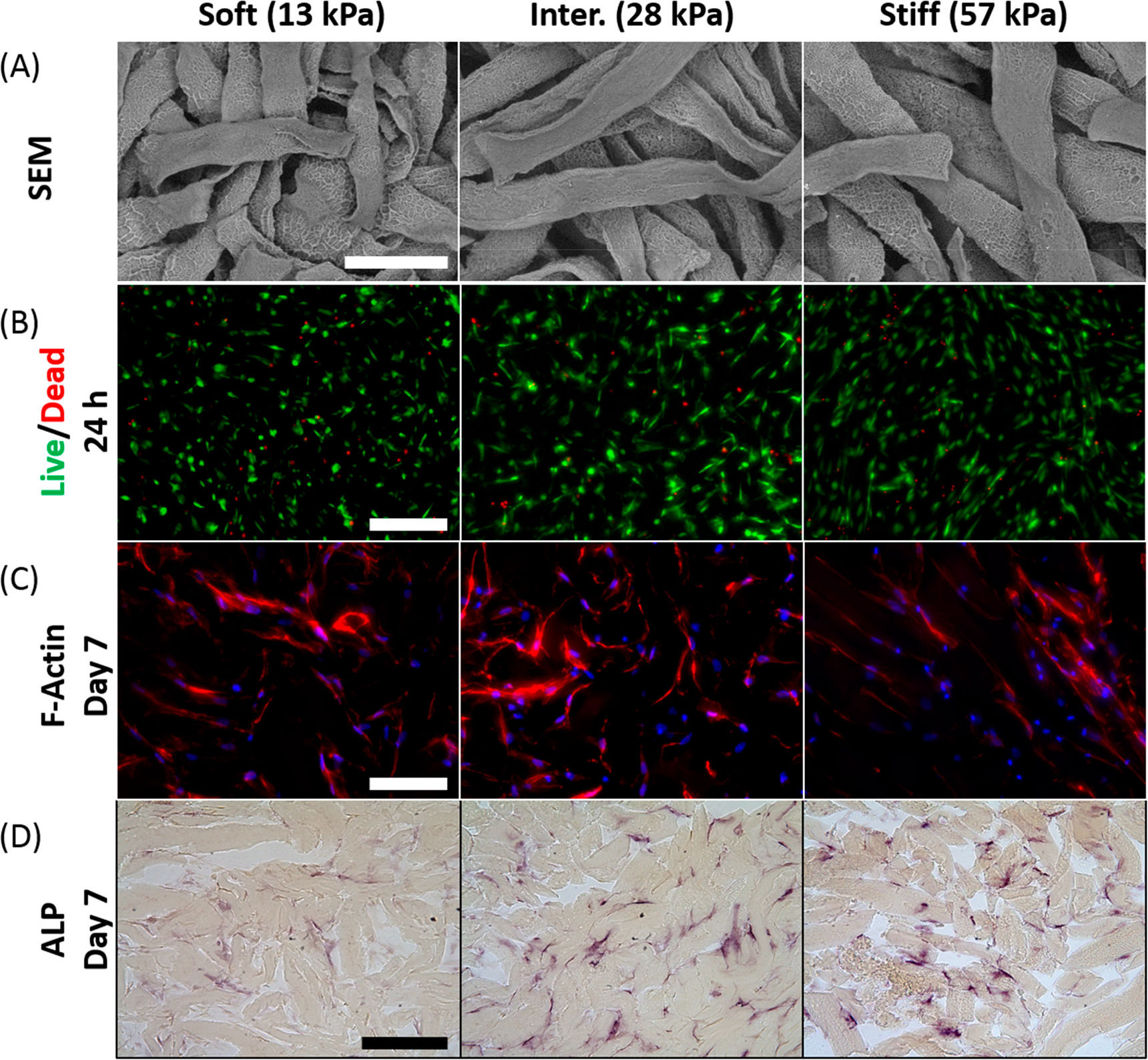
Regardless of stiffness, gelatin μRB scaffolds promote high cell viability and robust cell spreading. (A) SEM micrographs indicated comparable morphology and interconnected macroporosity within the stiffness range tested. Scale bar: 200 μm. (B) Cell viability and morphology as shown by LIVE/DEAD staining 24 h after encapsulation. Green, live cells; red, dead cells; scale bar, 200 μm. (C) Staining for actin cytoskeleton revealed similar cell morphology and cell spreading in all three stiffness groups after 7 days. Red, actin; blue, nuclei; scale bar, 100 μm. (D) ALP staining on day 7. Scale bar, 200 μm.
3.3. All μRB Stiffnesses Showed Comparable Level of Mechanosensing but Differential Response in Osteogenesis.
qRT-PCR of mechanosensing genes showed comparable level of upregulation of RhoA and ROCK1 in all three μRB stiffnesses, suggesting cells sense all three formulations as a stiff substrate in a similar manner (Figure 3). The same trend was observed for RUNX2. The early osteogenic marker ALP showed the greatest relative fold change, with the intermediate stiffness μRB group exhibiting statistically higher upregulation than the other two groups. For more mature bone markers, Col I and ON, increasing μRB stiffness generally led to a stiffness-dependent upregulation (Figure 3).
Figure 3.
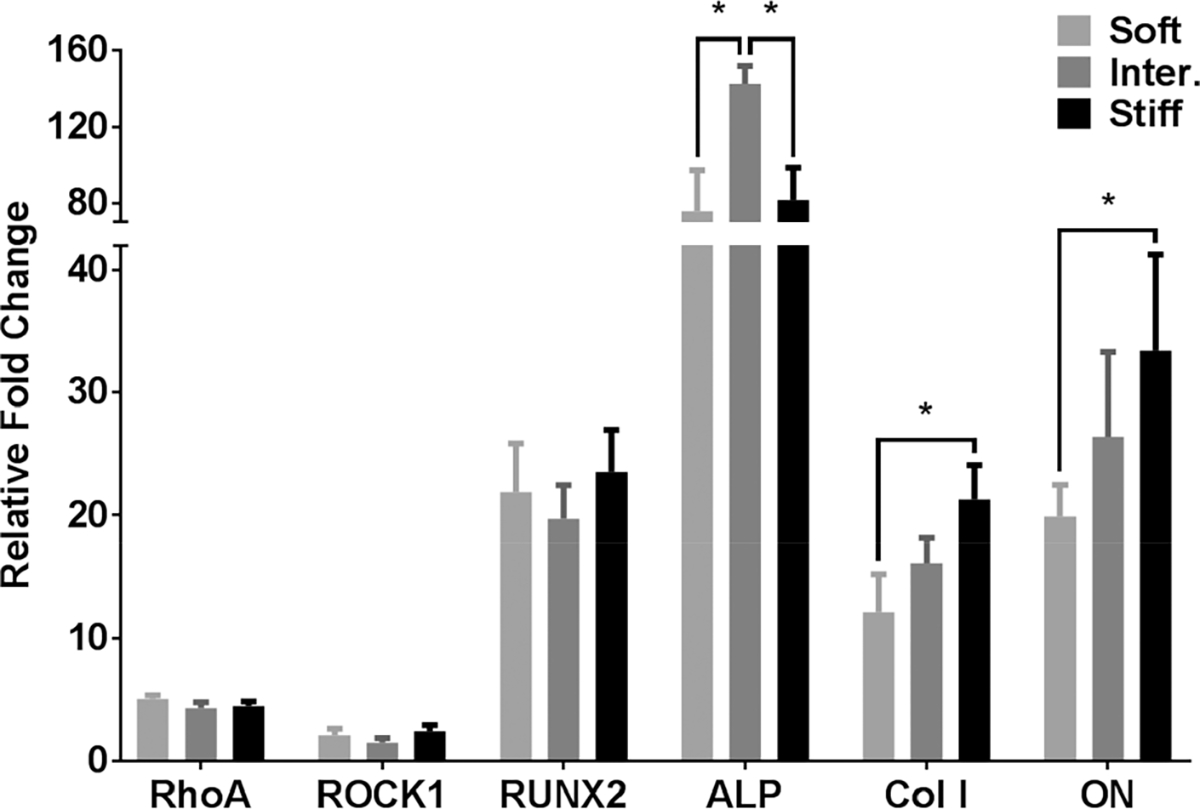
MSCs sensed μRB stiffness similarly within the range tested, but increased stiffness accelerated activation of mature osteogenic genes as measured by qRT-PCR. Gene expression for mechanosensing genes RhoA and ROCK1 and osteogenic markers RUNX2, ALP, Col I, and ON were analyzed on day 14. * = p < 0.05.
3.4. All μRB Stiffnesses Support Collagen Deposition without Mineralization, And soft μRB Scaffolds Fail to Maintain Structural Integrity.
Although all groups started out with comparable size, significant degradation was observed in soft μRB groups and all samples were harvested by day 31 for histology before the acellular soft μRB group completely degraded. The MSC-containing soft μRB group contracted substantially into a small dense cell pellet (Figure 4A), suggesting soft μRBs were not able to resist contractile forces of encapsulated MSCs. Unlike the soft μRB group, the intermediate and stiff cellular μRB groups maintained structural integrity and exhibited increased opacity over time, with the highest opacity observed in the stiff μRB group (Figure 4A). Matrix deposition and morphology was further validated by SEM on day 31 for the intermediate group (Figure S2).
Figure 4.
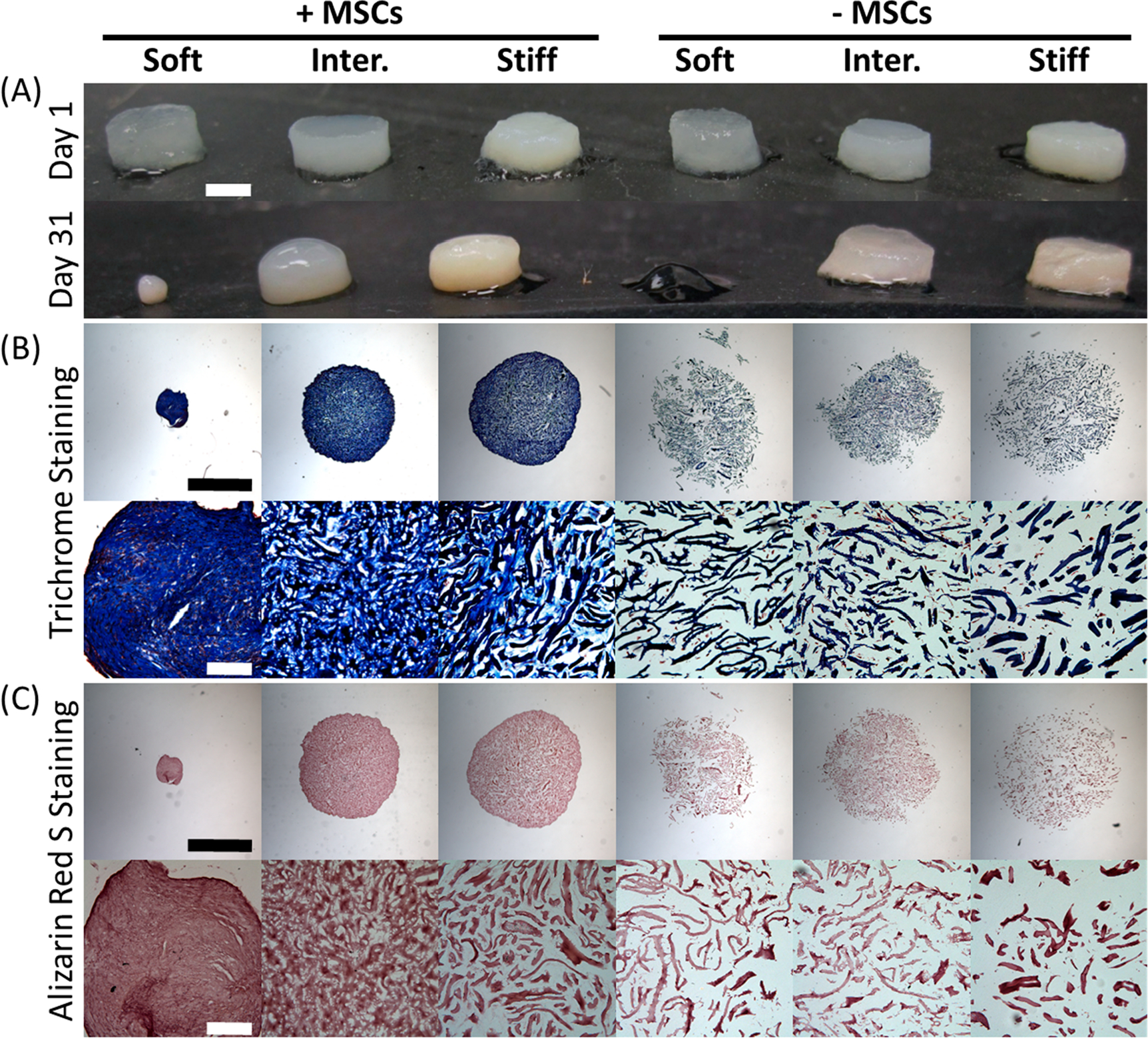
μRB scaffolds promote robust and interconnected collagen regardless of stiffness. (A) Gross morphology of μRB scaffolds with varying stiffness and with or without MSCs on days 1 and 31. Increase in sample opacity suggests matrix deposition. The soft formulation was unable to maintain its physical integrity. Scale bar, 2 mm (B) Masson’s Trichrome staining for total collagen on day 31. (C) ARS staining for bone mineralization. Low-magnification (upper row) and high-magnification (lower row) images are provided for both stains. Scale bars in low-magnification images, 2 mm; scale bars in high-magnification images, 200 μm.
To visualize the amount and distribution of collagen deposition by MSCs, we performed Masson’s Trichrome staining. The acellular samples show the background staining from the gelatin μRBs, with interconnected macroporosity among all groups (Figure 4B). Such macroporosity was mostly filled up by cell-produced collagen in MSC-containing μRBs, with the highest intensity of collagen observed in the soft μRB group because of the substantially increased cell density from the sample contraction (Figure 4B). Higher-magnification highlights the robust and interconnected collagen matrix deposition throughout the scaffold. To examine mineralization in the μRB samples, we stained all scaffolds with ARS staining. Regardless of stiffness, all groups were negative for mineralization (Figure 4C). Higher magnification in the acellular samples suggested small amount of automineralization, but MSCs did not produce additional mineralization compared to acellular controls (Figure 4C). To determine whether MSCs also produced any cartilage, we performed safranin-O staining to visualize sulfated glycosaminoglycans. Negative results were observed in all groups, indicating no cartilage formation (Figure S3A). This was further confirmed by minimal staining for cartilage markers, type II and type X collagens (Figures S2B and S2C).
3.5. Intermediate and Stiff μRBs Enabled Rapid Increase in Compressive Moduli of Tissue-Engineered Bone.
We next evaluate how varying μRB stiffness impacts the mechanical properties of engineered bone tissues using unconfined compression testing. At day 1, all groups exhibit comparable levels of compressive moduli regardless of the stiffness of individual μRBs (Figure 5). After 31 days of culture in osteogenic media, the compressive moduli of the acellular scaffolds decreased because of degradation. The biggest drop in compressive modulus was observed in acellular scaffolds made from soft μRBs, which decreased from 11 to 0.1 kPa. Unlike the acellular scaffolds, the compressive moduli of the MSC-seeded scaffolds increased significantly (Figure 5). Although only a mild increase was observed in the soft μRB + MSC group, both intermediate and stiff μRB groups exhibited a more than 10-fold increase in compressive moduli to 261 and 293 kPa, respectively. Given that the soft μRB +MSC group failed to maintain its size and integrity (Figure 4A), this formulation is not suitable for clinical application.
Figure 5.
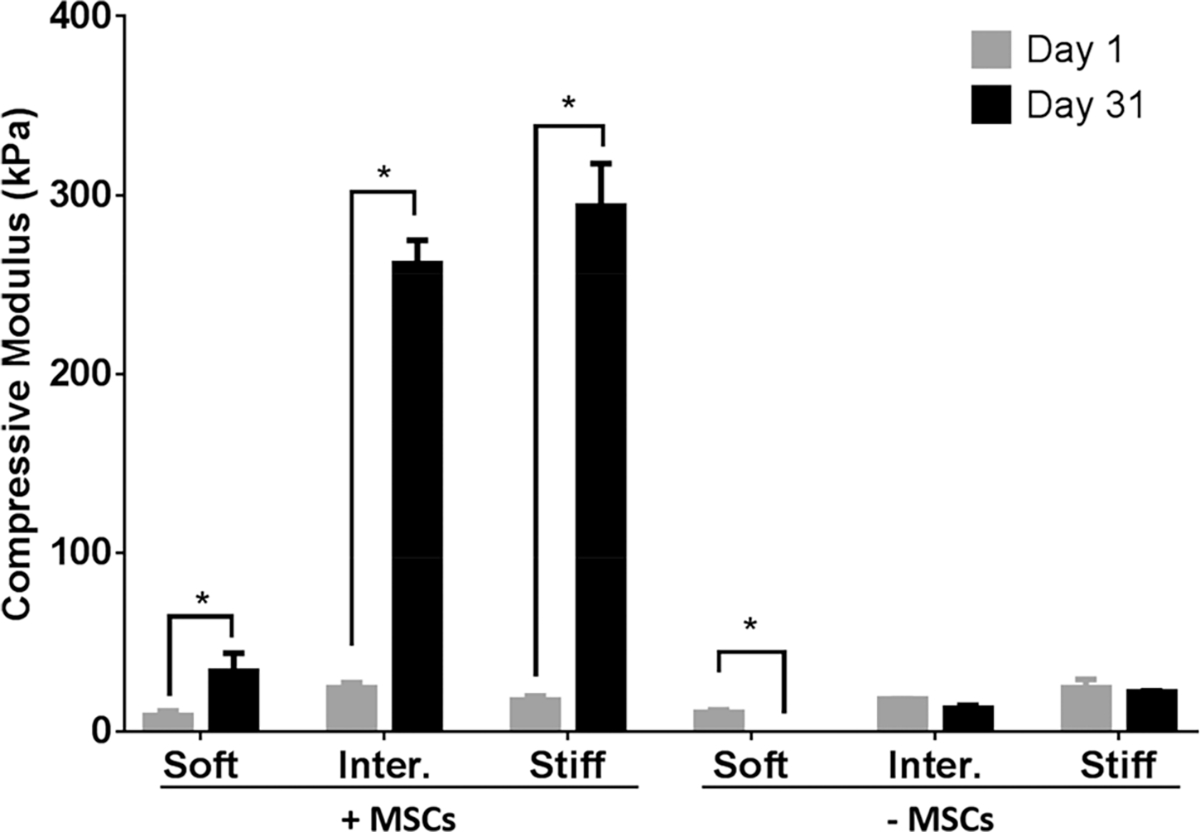
Characterization of compressive moduli of gelatin μRB scaffolds with varying substrate stiffness with or without MSCs. The samples were characterized on days 1 and 31 using unconfined compressive testing. * = p < 0.05.
3.6. Effect of Varying μRB Stiffness on Osteoblast Phenotype.
To further characterize the phenotype of MSC-derived osteoblasts in response to varying μRB stiffness, we performed immunostaining of bone markers including type I collagen, osteopontin, and osteocalcin. All three μRB stiffnesses supported robust type I collagen deposition throughout the scaffold, although the soft μRB sample collapsed into a small dense cell pellet (Figure 6A). However, only low levels of osteopontin, an intermediate osteogenic marker, were detected in the intermediate and stiff μRB groups (Figure 6B). The staining for osteocalcin, a mature bone marker, was negative for all groups, indicating MSCs had not yet fully matured into osteoblasts after 31 days of culture (Figure 6C).
Figure 6.
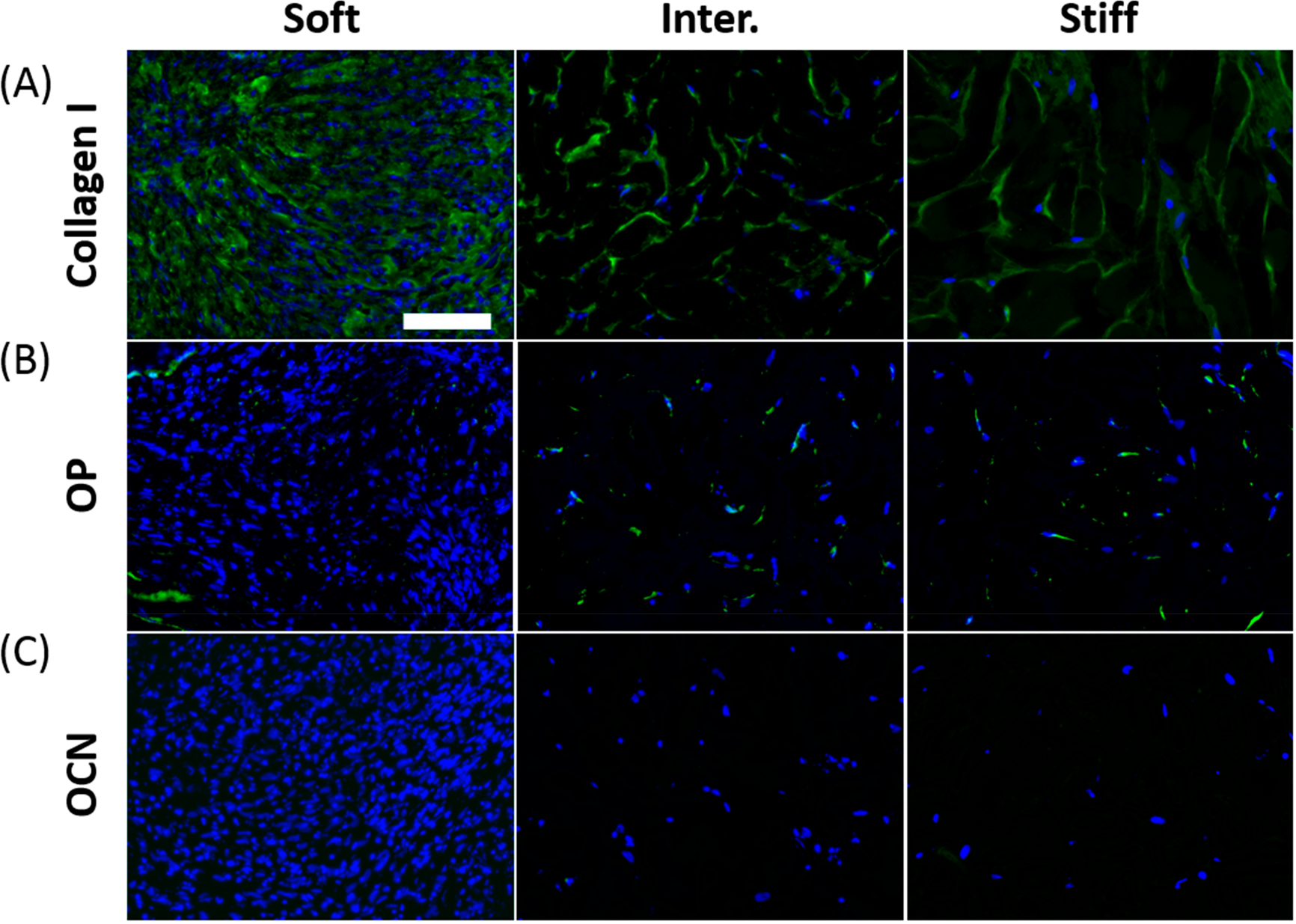
Immunostaining for osteogenic markers on day 31 showed robust collagen type I deposition regardless of stiffness. (A) Type I collagen, (B) intermediate osteogenic marker osteopontin, (C) mature osteogenic maker osteocalcin. Green, marker; blue, nuclei; scale bar, 100 μm.
3.7. Incorporation of HA Nanoparticles into Gelatin μRB Scaffolds Enabled Fast Mineralization by MSCs in 3D.
To accelerate the speed of bone differentiation of MSCs in gelatin μRB scaffolds, we coated μRBs of intermediate stiffness with HA nanoparticles. Both cellular and acellular scaffolds were cultured in osteogenic media for 31 days, and bone formation was assessed by ARS staining, osteopontin, and osteocalcin. Acellular samples with HA showed minimal background of ARS stain from the incorporated HA particles (Figure 7A). Furthermore, staining cellular samples with HA on day 1 for osteopontin and osteocalcin was largely negative, indicating that the HA particles provide no background (Figure S5). In contrast, after 31 days of culture in osteogenic media, HA-coated μRB scaffolds led to robust and extensive mineralization by MSCs throughout the scaffold. Furthermore, MSCs in HA-coated μRB scaffolds also stained positive for mature osteogenic markers including osteopontin and osteocalcin (Figure 7B).
Figure 7.
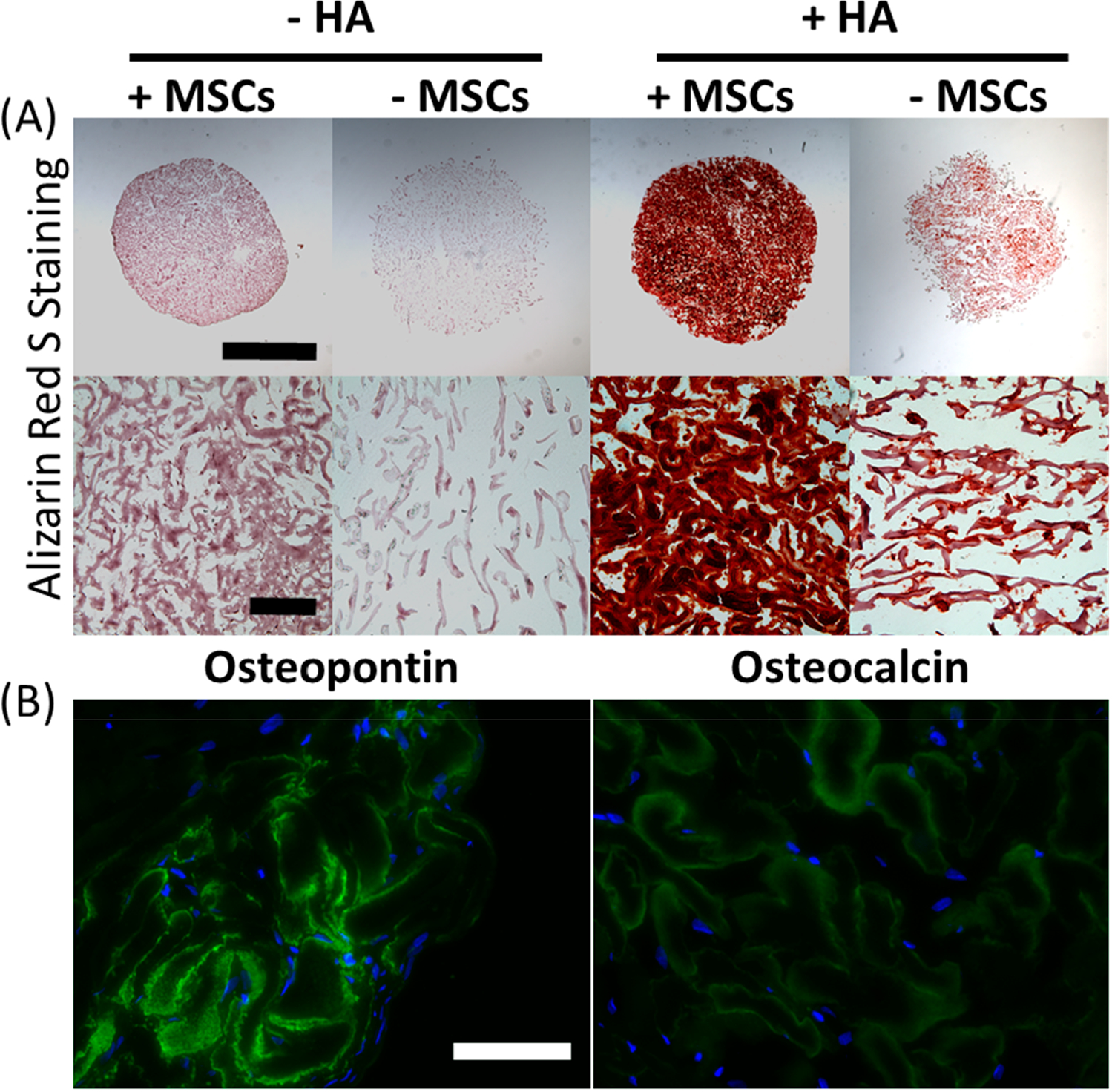
Incorporation of HA into μRB scaffolds promoted robust mineralization and osteogenic maturation after 31 days of culture in osteogenic media. (A) ARS staining for bone mineralization. Scale bars in low-magnification images, 2 mm; scale bars in high-magnification images, 200 μm. (B) Immunostaining for osteopontin and osteocalcin. Green, osteopontin/osteocalcin; blue, nuclei; scale bar, 100 μm.
4. DISCUSSION
In this paper, we first set out to determine the maximal range of stiffness (13–57 kPa) of individual μRBs that can be synthesized (Figure S1). The three stiffnesses we chose in the final study cover the full stiffness range, where “soft” represents the softest μRB that can be synthesized, and “stiff” represents the stiffest μRB that can be synthesized. A further decrease in the intra-cross-linking of gelatin μRBs would lead to too much swelling and result in formation of nanoporous hydrogels rather than macroporous μRB scaffolds. A further increase in the intra-cross-linking of gelatin μRBs would render the product too stiff, not supporting efficient inter-cross-linking to form μRB scaffolds. We then evaluated how varying μRB stiffness modulates MSC-based osteogenesis in vitro (Figure 1B). Contrary to our hypothesis, varying gelatin μRB stiffness did not have a significant impact on modulating MSC osteogenesis. All three μRB stiffnesses supported comparable levels of upregulation of bone markers and extensive collagen deposition throughout the scaffold (Figures 3, 4). Although all three μRB stiffnesses supported MSC osteogenesis, the soft μRB group degraded prematurely, was unable to maintain its size and condensed into a pellet, excluding it from being considered for future in vivo applications. Both intermediate and stiff gelatin μRB scaffolds supported more than 10-fold increases in the compressive moduli of tissue-engineered bone, reaching almost 300 kPa after 31 days of culture in osteogenic media (Figure 5). The increase in compressive moduli was largely contributed by the deposition of collagen deposition, as indicated by the relative low expressions of mature bone markers including osteopontin and osteocalcin (Figure 6). Compared to plain gelatin μRBs, HA nanoparticle-coated μRBs showed significantly accelerated mineralization by MSCs and maturation into osteoblasts (Figure 7). To differentiate the contribution of cell-deposited mineralization from the HA particles from the scaffold itself, we included acellular scaffolds as controls (+ HA, − MSCs). ARS staining on these controls shows uniform HA coating on μRBs, and no mineralization was observed in the porosity in the μRB scaffolds. In contrast, ARS staining from day 31 showed that most of the open pores had been filled with positive staining, which were contributed by cell-deposited mineralization (Figure 7A). Together, these results validate that μRBs support MSC osteogenesis across a broad range of stiffness, and HA nanoparticle incorporation further accelerates the maturation of MSCs and mineralized bone formation.
The finding that changing gelatin μRB stiffness across a broad range did not impact MSC osteogenesis was unexpected. Contrary to the result shown here, previous studies have observed that MSC osteogenesis in 3D hydrogels is dependent on hydrogel stiffness, although opposing trends have been observed in physically cross-linked vs covalently cross-linked hydrogels.26,27 Using physically cross-linked alginate hydrogels containing RGD cell adhesive peptide, Huebesch et al. have shown that increasing hydrogel stiffness enhanced MSCs osteogenesis in 3D.27 However, using covalently cross-linked hyaluronic acid hydrogels, Khetan et al. showed that differentiation of MSCs is directed by generation of degradation-mediated cellular traction.26 Regardless of the cross-linking mechanisms, MSCs in these two studies exhibited minimal cell spreading in 3D hydrogels because of physical constraints posed by the nanoporous hydrogel network,26,27 whereas previous literature using 2D substrates has suggested that increased spreading often correlates with enhanced osteogenesis and upregulation of mechanosensing genes such as RhoA and ROCK1.28–30 Unlike conventional nanoporous hydrogels that restrict cell spreading in 3D,8,9 the μRB scaffolds in our work supported robust cell spreading right after encapsulation due to the macroporosity, which encourages MSCs to attach to the flat surface of the μRBs.8 In addition, increasing ligand density alone has been shown to promote cell spreading, and the gelatin material used in the μRBs is a digested form of type I collagen and contains rich cell adhesive ligands.31–33 The unique morphology and biomimetic composition of 3D gelatin μRB scaffolds do not physically restrict cells, unlike conventional 3D hydrogels. In our results, we found that μRBs across all tested stiffnesses support similar levels of upregulation of mechanosensing genes (Figure 3). This corroborated our findings that all stiffness levels also support robust and comparable levels of cell spreading (Figure 2C). Our lab has recently reported that increasing cell adhesive ligand density alone can modulate stem cell mechanosensing.34 Taken together, our results indicate that μRBs across a broad range of stiffnesses can support robust MSC spreading and osteogenesis.28–30
The range of μRB stiffness tested in this study spans from 13 to 57 kPa. We chose the lower limit as it was the softest gelatin μRB that can be fabricated while maintaining μRB morphology (Figure S1). A further decrease in glutaraldehyde, the cross-linker for gelatin, led to insufficient intra-cross-linking, causing gelatin μRBs to lose their shape and form a nanoporous hydrogel network after inter-cross-linking (Figure S1). We have chosen 57 kPa as the upper limit of μRB stiffness, as further increases in μRB stiffness result in poor inter-crosslinking due to reduced surface contact among stiffer μRBs, therefore leading to poor scaffold formation (Figure S1). Our chosen range of μRB stiffness (13 to 57 kPa) overlaps with the middle to high range of substrate stiffness assessed in previous studies which examined the effects of varying substrate stiffness on MSC osteogenesis. These previous studies using conventional hydrogels showed minimal osteogenesis using very soft hydrogels (from 0.1 to 5 kPa),24,35,36 which is below the range of the softest μRB that can be fabricated. This may also explain why even the softest gelatin μRB formulation (13 kPa) is able to support robust osteogenesis.
Although all μRB stiffnesses supported upregulation of bone markers and robust collagen deposition, maturation of MSC into osteoblasts and mineral deposition remained slow (Figures 6 and 7). This limitation was overcome by incorporating HA nanoparticle coatings onto μRBs of intermediate stiffness, which further accelerated the maturation and mineralization by MSCs (Figure 7). The intermediate stiffness was chosen because this formulation was easiest to handle and cross-linked the most robustly. HA has been widely used to coat orthopedic implants to enhance osteointegration, and incorporation of HA into tissue engineering scaffolds has been shown to enhance osteogenic differentiation.37–40 Consistent with previous reports, here we found HA-coating can enhance the osteoinductivity of gelatin-based μRB scaffolds and could further accelerate maturation of MSCs toward bone lineage.
5. CONCLUSION
In summary, here we demonstrate the gelatin-based, macroporous μRB scaffold as a novel 3D stem cell niche for supporting MSC-based bone regeneration in vitro. Importantly, gelatin μRBs across a broad range of stiffness can support robust MSC spreading and comparable levels of bone matrix deposition. Although soft gelatin μRBs (13 kPa) degraded prematurely, both intermediate and stiff μRBs supported MSC-based bone formation with a rapid increase in compressive moduli. Coating gelatin μRBs with HA further accelerated the maturation of MSCs toward bone lineage and mineralization. Future work will evaluate the potential of gelatin μRB-based scaffolds coated with HA in supporting MSC-based bone regeneration in vivo using disease-relevant bone defect models. Although this study focused on MSCs as a model cell type, we envision gelatin μRBs serving as macroporous scaffolds to enhance and accelerate osteogenesis and mineralized bone formation for a broad range of multipotent or pluripotent stem cell types.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank NIH R01DE024772 (F. Y.), NIH 1R01AR074502 (F.Y.), National Science Foundation CAREER award program (CBET-1351289) (F. Y.), California Institute for Regenerative Medicine Tools and Technologies award (Grant TR3-05569) (F.Y.), Stanford Chem-H Institute New Materials for Applications in Biology and Medicine Seed Grant (F.Y.), Stanford Child Health Research Institute (F.Y.), California Institute for Regenerative Medicine predoctoral fellowship (B.C.), and NIH NRSA predoctoral fellowship (5F31DE025788-03) (B.C.) for funding this work. We also thank Dr. Lydia-Marie Joubert from the Cell Sciences Imaging Facility at Stanford for the use of, and assistance with, the Hitachi electron microscope. Finally, we thank Anthony Behn from the Department of Orthopedic Surgery at Stanford for his assistance in mechanical testing.
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsbiomaterials.9b01792.
Figure S1 determines the maximal stiffness range of μRBs that can be formed by varying cross-linker concentration, while still supporting inter-cross-linking to form 3D macroporous scaffolds; Figure S2 shows SEM of cell-laden μRB scaffolds at day 31using μRB with intermediate stiffness; Figure S3 shows negative cartilage marker expression at day 31; Figure S4 shows the initial cell number being encapsulated and proliferation in gelatin μRB scaffolds over 31 days; Figure S5 shows negative osteopontin and osteocalcin staining in samples at day 1 with or without HA incorporation (PDF)
Contributor Information
Bogdan Conrad, Program of Stem Cell Biology and Regenerative Medicine, Stanford University, Stanford, California 94305, United States.
Camila Hayashi, Department of Chemical Engineering, Stanford University, Stanford, California 94305, United States.
Fan Yang, Department of Orthopaedic Surgery Department of Bioengineering, Stanford University, Stanford, California 94305, United States.
REFERENCES
- (1).Amini AR; Laurencin CT; Nukavarapu SP Bone Tissue Engineering: Recent Advances and Challenges. Crit. Rev. Biomed. Eng. 2012, 40 (5), 363–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Henkel J; Woodruff MA; Epari DR; Steck R; Glatt V; Dickinson IC; Choong PFM; Schuetz MA; Hutmacher DW Bone Regeneration Based on Tissue Engineering Conceptions — A 21st Century Perspective. Bone Res. 2013, 1 (3), 216–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Bose S; Roy M; Bandyopadhyay A Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30 (10), 546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Roseti L; Parisi V; Petretta M; Cavallo C; Desando G; Bartolotti I; Grigolo B Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater. Sci. Eng., C 2017, 78, 1246–1262. [DOI] [PubMed] [Google Scholar]
- (5).Teixeira S; Fernandes H; Leusink A; van Blitterswijk C; Ferraz MP; Monteiro FJ; de Boer J In vivo evaluation of highly macroporous ceramic scaffolds for bone tissue engineering. J. Biomed. Mater. Res., Part A 2009, 93A (2), 567–575. [DOI] [PubMed] [Google Scholar]
- (6).Oliveira JM; Silva SS; Malafaya PB; Rodrigues MT; Kotobuki N; Hirose M; Gomes ME; Mano JF; Ohgushi H; Reis RL Macroporous hydroxyapatite scaffolds for bone tissue engineering applications: Physicochemical characterization and assessment of rat bone marrow stromal cell viability. J. Biomed. Mater. Res., Part A 2009, 91A (1), 175–186. [DOI] [PubMed] [Google Scholar]
- (7).Kim HJ; Kim U-J; Vunjak-Novakovic G; Min B-H; Kaplan DL Influence of macroporous protein scaffolds on bone tissue engineering from bone marrow stem cells. Biomaterials 2005, 26 (21), 4442–4452. [DOI] [PubMed] [Google Scholar]
- (8).Conrad B; Han LH; Yang F Gelatin-based microribbon hydrogels accelerate cartilage formation by mesenchymal stem cells in 3D. Tissue Eng., Part A 2018, 24 (21–22), 1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Han LH; Conrad B; Chung MT; Deveza L; Jiang X; Wang A; Butte MJ; Longaker MT; Wan D; Yang F Winner of the Young Investigator Award of the Society for Biomaterials at the 10th World Biomaterials Congress, May 17–22, 2016, Montreal QC, Canada: Microribbon-based hydrogels accelerate stem cell-based bone regeneration in a mouse critical-size cranial defect model. J. Biomed. Mater. Res., Part A 2016, 104 (6), 1321–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Conrad B; Hayashi C; Yang F, Gelatin-Based Microribbon Hydrogels Guided Mesenchymal Stem Cells to Undergo Endochondral Ossification In Vivo with Bone-Mimicking Mechanical Strength. Regenerative Eng. Transl. Med. 2019, DOI: 10.1007/s40883-019-00138-x. [DOI] [Google Scholar]
- (11).Zhang D; Wu X; Chen J; Lin K The development of collagen based composite scaffolds for bone regeneration. Bioactive Materials 2018, 3 (1), 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Toosi S; Naderi-Meshkin H; Kalalinia F; Peivandi MT; HosseinKhani H; Bahrami AR; Heirani-Tabasi A; Mirahmadi M; Behravan J PGA-incorporated collagen: Toward a biodegradable composite scaffold for bone-tissue engineering. J. Biomed. Mater. Res., Part A 2016, 104 (8), 2020–2028. [DOI] [PubMed] [Google Scholar]
- (13).Samavedi S; Whittington AR; Goldstein AS Calcium phosphate ceramics in bone tissue engineering: A review of properties and their influence on cell behavior. Acta Biomater. 2013, 9 (9), 8037–8045. [DOI] [PubMed] [Google Scholar]
- (14).Trombetta R; Inzana JA; Schwarz EM; Kates SL; Awad HA 3D Printing of Calcium Phosphate Ceramics for Bone Tissue Engineering and Drug Delivery. Ann. Biomed. Eng. 2017, 45 (1), 23–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Pan Z; Ding J Poly(lactide-co-glycolide) porous scaffolds for tissue engineering and regenerative medicine. Interface Focus 2012, 2 (3), 366–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Lo DD; Hyun JS; Chung MT; Montoro DT; Zimmermann A; Grova MM; Lee M; Wan DC; Longaker MT Repair of a Critical-sized Calvarial Defect Model Using Adipose-derived Stromal Cells Harvested from Lipoaspirate. J. Visualized Exp. 2012, No. 68, No. e4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Levi B; Hyun JS; Montoro DT; Lo DD; Chan CK; Hu S; Sun N; Lee M; Grova M; Connolly AJ; Wu JC; Gurtner GC; Weissman IL; Wan DC; Longaker MT In vivo directed differentiation of pluripotent stem cells for skeletal regeneration. Proc. Natl. Acad. Sci. U. S. A. 2012, 109 (50), 20379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Melchels FP; Tonnarelli B; Olivares AL; Martin I; Lacroix D; Feijen J; Wendt DJ; Grijpma DW The influence of the scaffold design on the distribution of adhering cells after perfusion cell seeding. Biomaterials 2011, 32 (11), 2878–2884. [DOI] [PubMed] [Google Scholar]
- (19).Kretlow JD; Young S; Klouda L; Wong M; Mikos AG Injectable biomaterials for regenerating complex craniofacial tissues. Adv. Mater. 2009, 21 (32–33), 3368–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Han L-H; Yu S; Wang T; Behn AW; Yang F Tissue Engineering: Microribbon-Like Elastomers for Fabricating Macroporous and Highly Flexible Scaffolds that Support Cell Proliferation in 3D (Adv. Funct. Mater. 3/2013). Adv. Funct. Mater. 2013, 23 (3), 266–266. [Google Scholar]
- (21).O’Brien FJ Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14 (3), 88–95. [Google Scholar]
- (22).Pandit V; Zuidema JM; Venuto KN; Macione J; Dai G; Gilbert RJ; Kotha SP Evaluation of multifunctional polysaccharide hydrogels with varying stiffness for bone tissue engineering. Tissue Eng., Part A 2013, 19 (21–22), 2452–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Tan S; Fang JY; Yang Z; Nimni ME; Han B The synergetic effect of hydrogel stiffness and growth factor on osteogenic differentiation. Biomaterials 2014, 35 (20), 5294–5306. [DOI] [PubMed] [Google Scholar]
- (24).Engler AJ; Sen S; Sweeney HL; Discher DE Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126 (4), 677–689. [DOI] [PubMed] [Google Scholar]
- (25).Schmittgen TD; Livak KJ Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101. [DOI] [PubMed] [Google Scholar]
- (26).Khetan S; Guvendiren M; Legant WR; Cohen DM; Chen CS; Burdick JA Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat. Mater. 2013, 12, 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Huebsch N; Arany PR; Mao AS; Shvartsman D; Ali OA; Bencherif SA; Rivera-Feliciano J; Mooney DJ Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater. 2010, 9, 518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).McBeath R; Pirone DM; Nelson CM; Bhadriraju K; Chen CS Cell Shape, Cytoskeletal Tension, and RhoA Regulate Stem Cell Lineage Commitment. Dev. Cell 2004, 6 (4), 483–495. [DOI] [PubMed] [Google Scholar]
- (29).Kilian KA; Bugarija B; Lahn BT; Mrksich M Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl. Acad. Sci. U. S. A. 2010, 107 (11), 4872–4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Oh S; Brammer KS; Li YSJ; Teng D; Engler AJ; Chien S; Jin S Stem cell fate dictated solely by altered nanotube dimension. Proc. Natl. Acad. Sci. U. S. A. 2009, 106 (7), 2130–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Engler A; Bacakova L; Newman C; Hategan A; Griffin M; Discher D Substrate Compliance versus Ligand Density in Cell on Gel Responses. Biophys. J. 2004, 86 (1), 617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Henry SJ; Crocker JC; Hammer DA Ligand density elicits a phenotypic switch in human neutrophils. Integrative biology: quantitative biosciences from nano to macro 2014, 6 (3), 348–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Reinhart-King CA; Dembo M; Hammer DA The Dynamics and Mechanics of Endothelial Cell Spreading. Biophys. J. 2005, 89 (1), 676–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Stanton AE; Tong X; Lee S; Yang F Biochemical Ligand Density Regulates Yes-Associated Protein Translocation in Stem Cells through Cytoskeletal Tension and Integrins. ACS Appl. Mater. Interfaces 2019, 11 (9), 8849–8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Hwang J-H; Byun MR; Kim AR; Kim KM; Cho HJ; Lee YH; Kim J; Jeong MG; Hwang ES; Hong J-H Extracellular Matrix Stiffness Regulates Osteogenic Differentiation through MAPK Activation. PLoS One 2015, 10 (8), No. e0135519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Mao AS; Shin J-W; Mooney DJ Effects of substrate stiffness and cell-cell contact on mesenchymal stem cell differentiation. Biomaterials 2016, 98, 184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Kuroda K; Okido M Hydroxyapatite Coating of Titanium Implants Using Hydroprocessing and Evaluation of Their Osteo-conductivity. Bioinorg. Chem. Appl. 2012, 2012, 730693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Sun X; Su W; Ma X; Zhang H; Sun Z; Li X Comparison of the osteogenic capability of rat bone mesenchymal stem cells on collagen, collagen/hydroxyapatite, hydroxyapatite and biphasic calcium phosphate. Regenerative Biomaterials 2018, 5 (2), 93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Villa MM; Wang L; Huang J; Rowe DW; Wei M Bone tissue engineering with a collagen–hydroxyapatite scaffold and culture expanded bone marrow stromal cells. J. Biomed. Mater. Res., Part B 2015, 103 (2), 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Wang X-F; Lu P-J; Song Y; Sun Y-C; Wang Y-G; Wang Y Nano hydroxyapatite particles promote osteogenesis in a three-dimensional bio-printing construct consisting of alginate/gelatin/hASCs. RSC Adv. 2016, 6 (8), 6832–6842. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


