Key Words: Alzheimer’s disease, blood-brain barrier, cerebrospinal fluid, clinical characteristics, cognitive function, matrix metalloproteinases, matrix metalloproteinase-3, neuropsychiatric symptoms, orexin signaling, sleep insufficiency
Abstract
Previous studies have shown that reduced sleep duration, sleep fragmentation, and decreased sleep quality in patients with Alzheimer’s disease are related to dysfunction in orexin signaling. At the same time, blood-brain barrier disruption is considered an early biomarker of Alzheimer’s disease. However, currently no report has examined how changes in orexin signaling relate to changes in the blood-brain barrier of patients who have Alzheimer’s disease with sleep insufficiency. This cross-sectional study included 50 patients with Alzheimer’s disease who received treatment in 2019 at Beijing Tiantan Hospital. Patients were divided into two groups: those with insufficient sleep (sleep duration ≤ 6 hours, n = 19, age 61.58 ± 8.54 years, 10 men) and those with normal sleep durations (sleep duration > 6 hours, n = 31, age 63.19 ± 10.09 years, 18 men). Demographic variables were collected to evaluate cognitive function, neuropsychiatric symptoms, and activities of daily living. The levels of orexin, its receptor proteins, and several blood-brain barrier factors were measured in cerebrospinal fluid. Sleep insufficiency was associated with impaired overall cognitive function that spanned multiple cognitive domains. Furthermore, levels of orexin and its receptors were upregulated in the cerebrospinal fluid, and the blood–brain barrier was destroyed. Both these events precipitated each other and accelerated the progression of Alzheimer’s disease. These findings describe the clinical characteristics and potential mechanism underlying Alzheimer’s disease accompanied by sleep deprivation. Inhibiting the upregulation of elements within the orexin system or preventing the breakdown of the blood-brain barrier could thus be targets for treating Alzheimer’s disease.
Introduction
Alzheimer’s disease (AD) is the most common cognitive disorder in the older adult population, with pathological features including extracellular neuritic plaques and intracellular neurofibrillary tangles, with β amyloid (Aβ) and hyperphosphorylated tau as the major respective components (Jia et al., 2020). Episodic memory decline is usually the first symptom of AD, which is gradually followed by impairment of overall cognitive function, neuropsychiatric symptoms, and eventually severely compromised activities of daily living (ADL) (Atri, 2019).
Sleep disorders are common in patients with AD, having a global incidence of 25–66% (Guarnieri et al., 2012). Several types of sleep disorders manifest in AD, including sleep insufficiency (SI; total sleep duration ≤ 6 hours), sleep fragmentation, reduced slow wave sleep, reduced rapid eye movement (REM) sleep, sleep-disordered breathing, and excessive daytime sleepiness (Kent et al., 2021). Sleep disorders might begin when patients are at the mild cognitive impairment (MCI) stage of AD and continue throughout disease progression. Pathologically, there is a bidirectional relationship between sleep disorders and AD (Havekes et al., 2019). Continuously deposited Aβ in the brain decreases sleep duration and compromises sleep quality (Spira et al., 2013), while at the same time, wakefulness promotes more Aβ deposition (Holth et al., 2019). However, few studies have investigated the frequency of AD with SI (AD-SI) or the relationship between SI and clinical manifestations of AD, including cognitive function, neuropsychiatric symptoms, or ADL.
The orexin system comprises orexin and its receptors. Orexin is a neuropeptide produced by neurons in the lateral hypothalamus (Soya and Sakurai, 2020; Wang et al., 2021). Both types of orexin—orexin A and orexin B—regulate multiple bodily functions, including the sleep-wake cycle, eating, mood changes, and response to rewards. Regulation occurs through activation of G-protein-coupled receptors orexin receptor 1 (OR1) and orexin receptor 2 (OR2) on cell surfaces (Sakurai, 2014). In patients with AD, orexin signaling has been shown to be impaired, leading to increased wakefulness and decreased REM sleep (Havekes et al., 2019), and consequently increased Aβ deposition and neurodegeneration (Liguori et al., 2017). However, alterations in orexin or its receptors, and the relationship between these alterations and sleep variables in patients with AD-SI has not been explored.
The blood-brain barrier (BBB) is primarily composed of matrix metalloproteinases (MMPs), vascular endothelial cells, astrocytes, neurons, and pericytes (Gong et al., 2022). MMPs regulate the activation of growth factors, zymogen cleavage, and remodeling of extracellular matrix in the brain, which are essential for the integrity of the BBB, neuronal network repair, and tissue formation. In AD, Aβ was reported to destroy the integrity of the BBB by activating MMPs, including MMP-2, MMP-3, and MMP-9 (Liebner et al., 2018). Receptor of advanced glycation endproducts (RAGE) is widely expressed on the surface of vascular endothelial cells, neurons, microglia, and astrocytes of the BBB. In AD, RAGE expression was reported to be markedly high, coexisting with Aβ in neurons and microglia (Deane et al., 2009). Furthermore, another study showed that Aβ inhibited the activity of low density lipoprotein receptor related protein 1 (LRP1), phosphoglycoprotein, and the expression of glucose transporter 1 by inducing RAGE expression. These actions together acted to destroy the ability of the BBB to function (Montagne et al., 2017). Additionally, In AD, Aβ leads to the production of a large amount specific glial fibrillary acidic protein (GFAP) by excessively activating astrocytes. This further harms the integrity of the BBB. Hence, structural and functional disruptions of the BBB appear closely related to AD (Yamazaki and Kanekiyo, 2017; Zenaro et al., 2017; Dias et al., 2022; Zhang et al., 2022). However, no study has yet focused on the relationship between AD-SI and the BBB.
SI might be related to alterations in orexin signaling, which can increase wakefulness (Oeckl et al., 2019). Studies show that SI is associated with compromised BBB integrity and further accelerates progression of AD pathology by significantly intensifying oxidative stress and neuroinflammation, which leads to production of free radicals and neuroinflammatory factors (Irwin and Vitiello, 2019; Vaccaro et al., 2020). However, no study has examined the relationship between orexin signaling and the BBB in patients with AD-SI.
In this study, patients with AD were recruited and divided into AD-SI and AD without SI (AD-nSI) groups based on sleep durations recorded by actigraphy (AD-SI: sleep ≤ 6 hours; AD-nSI: sleep > 6 hours). Demographic variables were also collected. Cognitive function, neuropsychiatric symptoms, and ADL were evaluated using a series of rating scales. Actigraphy was adopted to monitor sleep variables, including sleep duration, sleep latency, wakefulness duration after sleep onset, number of wakefulness attacks during sleep, sleep efficiency, and parameters of sleep-disordered breathing. Levels of orexin, its receptors (orexin A, orexin B, OR1, and OR2) and BBB factors (MMP-2, MMP-3, MMP-9, RAGE, LRP1, and GFAP) were measured in the cerebrospinal fluid (CSF) obtained from participants in the AD-SI and AD-nSI groups. The measures were compared between the two groups, and the correlations among the variables were analyzed in the AD-SI group.
Methods
Ethics statement
All participants or their family members completed the written informed consent (Additional file 1 (162.2KB, pdf) ) to participate in the cross-sectional study before data collection. The study was approved by the Review Board of Beijing Tiantan Hospital, Capital Medical University (approval No. Z2019SY015, approval date April 23, 2019; Additional file 2 (262.7KB, pdf) ). The manuscript was prepared in accordance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement (von Elm et al., 2007; Additional file 3).
STROBE Statement—Checklist of items that should be included in reports of cross-sectional studies
| Item No | Recommendation | Page No | |
|---|---|---|---|
| Title and abstract | 1 | (a) Indicate the study’s design with a commonly used term in the title or the abstract | 1 |
|
| |||
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found | 4 | ||
|
| |||
| Introduction | |||
|
| |||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported | 4 |
|
| |||
| Objectives | 3 | State specific objectives, including any prespecified hypotheses | 7 |
|
| |||
| Methods | |||
|
| |||
| Study design | 4 | Present key elements of study design early in the paper | 7 |
|
| |||
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection | 7 |
|
| |||
| Participants | 6 | (a) Give the eligibility criteria, and the sources and methods of selection of participants | 7 |
|
| |||
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable | 7 |
|
| |||
| Data sources/ | 8* | For each variable of interest, give sources of data and details of methods of measurement assessment (measurement). Describe comparability of assessment methods if there is more than one group | 7-11 |
|
| |||
| Bias | 9 | Describe any efforts to address potential sources of bias | / |
|
| |||
| Study size | 10 | Explain how the study size was arrived at | / |
|
| |||
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why | 7 |
|
| |||
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding | 11 |
|
| |||
| (b) Describe any methods used to examine subgroups and interactions | / | ||
|
| |||
| (c) Explain how missing data were addressed | / | ||
|
| |||
| (d) If applicable, describe analytical methods taking account of sampling strategy | / | ||
|
| |||
| (e) Describe any sensitivity analyses | / | ||
|
| |||
| Results | |||
|
| |||
| Participants | 13* | (a) Report numbers of individuals at each stage of study—eg numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analysed | 7 |
|
| |||
| (b) Give reasons for non-participation at each stage | / | ||
|
| |||
| (c) Consider use of a flow diagram | / | ||
|
| |||
| Descriptive data | 14* | (a) Give characteristics of study participants (eg demographic, clinical, social) and information on exposures and potential confounders | 22-25 |
|
| |||
| (b) Indicate number of participants with missing data for each variable of interest | / | ||
|
| |||
| Outcome data | 15* | Report numbers of outcome events or summary measures | 7/22-28 |
|
| |||
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (eg, 95% confidence interval). Make clear which confounders were adjusted for and why they were included | / |
|
| |||
| (b) Report category boundaries when continuous variables were categorized | / | ||
|
| |||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | / | ||
|
| |||
| Other analyses | 17 | Report other analyses done—eg analyses of subgroups and interactions, and sensitivity analyses | / |
|
| |||
| Discussion | |||
|
| |||
| Key results | 18 | Summarise key results with reference to study objectives | 19 |
|
| |||
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias | 18 |
|
| |||
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence | 14-18 |
|
| |||
| Generalisability | 21 | Discuss the generalisability (external validity) of the study results | 18 |
|
| |||
| Other information | |||
|
| |||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based | 3 |
*Give information separately for exposed and unexposed groups. Note: An Explanation and Elaboration article discusses each checklist item and gives methodological background and published examples of transparent reporting. The STROBE checklist is best used in conjunction with this article (freely available on the Web sites of PLoS Medicine at http://www.plosmedicine.org/, Annals of Internal Medicine at http://www.annals.org/, and Epidemiology at http://www.epidem.com/). Information on the STROBE Initiative is available at www.strobe-statement.org.
Participants
In this cross-sectional study, patients were sequentially recruited from 2019 to 2021 at Beijing Tiantan Hospital, Capital Medical University. Fifty patients with AD participated in the study. Inclusion criteria: (1) Diagnosed with mild cognitive impairment (MCI) due to AD (Albert et al., 2011) and AD dementia (McKhann et al., 2011) according to the 2011 National Institute of Aging and Alzheimer’s Association criteria. (2) Consistent sleep duration assessed by actigraphy and evaluated using the Pittsburgh Sleep Quality Index (PSQI) scale, which estimates the sleep duration for a 1-month period (Buysse et al., 1989). Exclusion criteria: (1) the presence of neurological diseases other than AD that might explain cognitive decline, neuropsychiatric symptoms, and poor ADL, including Lewy body disease, Parkinson’s disease, frontotemporal dementia, vitamin B12 deficiency, and brain tumor. (2) Severe systematic diseases, such as heart failure, pulmonary diseases, or other conditions that interfere with sleep quality. (3) Diagnosis of primary sleep disorders or a history of night shift work. (4) The presence of disorders that might affect the BBB, such as stroke, traumatic brain injury, or infectious and inflammatory disorders of the peripheral and central nervous systems. (5) History of drug and alcohol abuse.
According to the monitored sleep duration (see Monitoring sleep variables by actigraphy), the 50 patients who met these criteria were divided into AD-SI (sleep ≤ 6 hours) and AD-nSI (sleep > 6 hours) groups.
Monitoring sleep variables by actigraphy
Actigraphy, a cost-efficient method for collecting sleep-wake states based on movement data, is often used in clinical sleep research because of its ability to monitor sleep in natural settings (Fekedulegn et al., 2020). In this study, sleep variables were monitored by a small wristwatch actigraphy device (DHR998, Dehaier Medical Syst, Beijing, China).
Participants were requested to abstain from sleep medications for at least 12-14 hours if their condition allowed and sleep for at least 7 hours overnight in a quiet environment with monitoring time from 8:00 p.m. to 8:00 a.m. (Camargos et al., 2014). Actigraphy was adopted to monitor the following sleep variables: (1) sleep duration: sleep duration ≤ 6 hours was defined as SI; (2) sleep latency: the time between going to bed to actually falling asleep; (3) wakefulness: amount and number of times being awake after having fallen sleep (maintained for > 1 minute) during the night; (4) sleep efficiency: sleep duration divided by time in bed, expressed as a percentage (Fekedulegn et al., 2020); (5) parameters of sleep-disordered breathing: (a) an apnea hypopnea index evaluating the number of apnea and hypopnea episodes per hour of sleep time, and (b) average oxyhemoglobin saturation and lowest oxyhemoglobin saturation, which measure the oxygen-carrying capacity of blood. The above sleep variables were analyzed by Morpheus Ox software (WideMed Ltd, Herziliya, Israel).
Collection of demographic variables
Demographic variables, including age, age of disease onset, gender, disease duration, and education level of the 50 patients with AD were collected.
Assessments of clinical manifestations
Cognitive function
We assessed overall cognitive function, memory, executive function, attention, language ability, and visuospatial ability. Overall cognitive function was evaluated using the Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MOCA) scales. Both MMSE and MOCA scores range from 0–30, with lower scores indicating poorer cognitive function (Cockrell and Folstein, 1988; Carson et al., 2018). Immediate and delayed auditory memory were evaluated using the Rey Auditory Verbal Learning Test: immediate and delayed recall (Guo et al., 2009). Visual memory was rated with the Complex Figure Test: delayed memory (Guo et al., 2009). Lower scores on these two memory scales indicate poorer auditory verbal and visual memories, respectively. Executive function was indexed by reaction time on the Stroop Color-Word Test-Color A/B/C (word-reading/color-naming /color-word) (Periáñez et al., 2021). Lower scores reflect more severe impairment of executive function. Attention was assessed by the Symbol Digit Modality Test (Ryan et al., 2020). Longer time needed to complete this test indicates worse attention. Language function was evaluated using the 30-item Boston Naming Test (Lin et al., 2014), with lower scores indicating more severe language dysfunction. Visuospatial ability was assessed using the Complex Figure Test-imitation test (Beretta et al., 2022), with lower scores indicating poorer visuospatial ability.
Neuropsychiatric symptoms
We evaluated overall neuropsychiatric status, and individually evaluated depression, anxiety, apathy, and agitation. The Neuropsychiatric Inventory Scale (NPS) was used to evaluate overall neuropsychiatric status. Higher NPS scores indicate more severe overall neuropsychiatric symptoms. The 24-item Hamilton Depression Scale (HAMD; Hamilton, 1960) was used to assess depressive symptoms, with scores > 8 reflecting depression. The 14-item Hamilton Anxiety Scale (HAMA; Hamilton, 1959) was used to assess symptoms of anxiety, with scores > 8 reflecting anxiety. The Modified Apathy Estimate Scale (MAES; Starkstein et al., 1992) was used to assess apathy. Higher MAES scores indicate more serious apathy. The Cohen-Mansfield Agitation Inventory (CMAI; Koss et al., 1997) was used to assess agitation. Higher CMAI scores reflect more serious agitation.
ADL
ADL was rated by the 20-item ADL scale, including basic ADL and instrumental ADL. Elevated scores indicate compromised ADL (Katz et al., 1963).
Collection and analysis of CSF samples
Participants were requested to abstain from drugs that block cognitive impairment for at least 12–14 hours if their condition allowed. CSF samples were obtained by lumbar puncture using standard sterile techniques between 7 a.m. and 10 a.m. under fasting condition to minimize the effects of diurnal variations. Five milliliters of CSF were collected in a polypropylene tube (Beijing JingkeHongda Biotechnology Co., Ltd., Beijing, China). CSF samples were immediately centrifuged at 3000 r/min for 10 minutes after collection at 4°C. Each CSF sample was then aliquoted into separate Nunc cryotubes (Beijing JingkeHongda Biotechnology Co., Ltd.) and 0.5 mL was frozen per tube at –80°C until the assay.
Orexinergic proteins
Levels of orexin A and orexin B in the CSF were measured by radioimmunoassay according to the published standard procedures (Bridoux et al., 2013). RK-003-30 and RK-003-31 kits (Phoenix Pharmaceuticals, Burlingame, CA, USA) were used for orexin A and orexin B, respectively. The levels of OR1 and OR2 in the CSF was measured using enzyme-linked immunosorbent assays. EK2497 and EK3718 kits (Signalway Antibody, College Park, MD, USA) were used for OR1 and OR2, respectively.
BBB factors
Levels of BBB factors (MMP-2, MMP-3, MMP-9, RAGE, LRP1, and GFAP) in the CSF were determined using enzyme-linked immunosorbent assays. MMP200, DMP300, DMP900, and DRG00 kits (R&D Systems Company, Minneapolis, MN, USA) were used to evaluate the levels of MMP-2, MMP-3, MMP-9 and RAGE in the CSF, respectively. An MBS772326 kit (MyBioSource Company, San Diego, CA, USA) was used for LRP1 and a NS830 kit (Merck Millipore Company, Darmstadt, Germany) was used for GFAP.
Data analyses
We set the sample size referring to Liguori et al. (2014, 2016). All analyses were performed using SPSS Statistics 24.0 (IBM Corp., Armonk, NY, USA). Differences between the AD-SI and AD-nSI groups were considered statistically significant at P < 0.05.
Demographics variables, sleep variables (via actigraphy), cognitive symptom scores, neuropsychiatric symptom scores, and ADL scores (via rating scales), and levels of orexin, its receptors, and BBB factors in the CSF were compared between AD-SI and AD-nSI groups. Normally distributed measurement data are expressed as mean ± standard deviation (SD) and were compared using the independent-sample t-test. Non-normally distributed data are expressed as median (first quartile, third quartile) and were compared using the Mann-Whitney U test. A Chi-squared test was used to compare categorical variables. A bivariate correlation analysis (Spearman correlation analysis) was performed to investigate the relationship among the variables measured.
Results
AD-SI frequency
In the 50 patients recruited in this study, 19 had SI and the frequency of AD-SI was thus 38%.
Demographic variables and clinical information in the AD-nSI and AD-SI groups
Demographic variables and clinical information were compared between AD-nSI and AD-SI groups (Table 1). There were no significant differences in gender, age, age of disease onset, disease duration, or educational level between the two groups (all P > 0.05).
Table 1.
Demographic variables and clinical information for the AD-nSI and AD-SI groups
| AD-nSI group (n = 31) | AD-SI group (n = 19) | P-value | |
|---|---|---|---|
| Male | 18(58) | 10(53) | 0.707 |
| Age (yr) | 63.19±10.09 | 61.58±8.54 | 0.564 |
| Age of onset (yr) | 51.96±23.55 | 59.65±9.18 | 0.131 |
| Disease duration (yr) | 3.00 (1.00, 5.00) | 2.00 (1.08, 3.75) | 0.76 |
| Educational level | 0.695 | ||
| Primary school and below | 6(19) | 4(21) | |
| Middle and high school | 17(55) | 12(63) | |
| Bachelor’s degree and above | 8(26) | 3(16) |
Sex is expressed as number (percentage), and was analyzed by independent-sample t-test. Age and age of disease onset are expressed as mean ± SD, and were analyzed by independent-sample t-test. Disease durations are expressed as median (first quartile, third quartile), and were analyzed by Mann-Whitney U test. Educational levels are expressed as number (percentage), and were analyzed by Chi-squared test. AD-nSI: Alzheimer’s disease with no sleep insufficiency; AD-SI: Alzheimer’s disease with sleep insufficiency.
Sleep variables in the AD-nSI and AD-SI groups
Sleep duration, sleep latency, awake duration after sleep onset, number of awakenings during sleep, sleep efficiency, apnea hypopnea index, average oxyhemoglobin saturation, and lowest oxyhemoglobin saturation were monitored by actigraphy, and the data were compared between AD-nSI and AD-SI groups (Table 2). The results showed that compared with the AD-nSI group, the AD-SI group had significantly shorter sleep duration (P = 0.000), longer sleep latency (P = 0.012), higher awake duration after sleep onset (P = 0.029), and lower sleep efficiency (P = 0.000). No significant differences were observed between groups in the numbers of awakenings during sleep, apnea hypopnea index, average oxyhemoglobin saturation, or lowest oxyhemoglobin saturation (all P > 0.05).
Table 2.
Sleep variables recorded by actigraphy in the AD-nSI and AD-SI groups
| AD-nSI group (n = 31) | AD-SI group (n = 19) | P-value | |
|---|---|---|---|
| Sleep duration (min) | 381.24±31.42 | 157.03±58.58 | 0 |
| Sleep latency (min) | 10.00 (10.00, 13.00) | 16.00 (10.00, 28.00) | 0.012 |
| Wakefulness duration after sleep onset (min) | 158.00 (119.50, 234.00) | 195.00 (153.00, 282.50) | 0.029 |
| Number of wakefulness | 13.82±1.16 | 15.71±0.70 | 0.189 |
| Sleep efficiency (%) | 65.13±10.06 | 46.04±18.36 | 0 |
| Apnea hypopnea index | 11.20 (5.30, 22.30) | 13.40 (5.90,19.35) | 0.736 |
| Average oxyhemoglobin saturation (%) | 94.05±1.90 | 93.79±2.05 | 0.668 |
| Lowest oxyhemoglobin saturation (%) | 85.00 (81.00, 88.50) | 85.00 (81.00, 88.00) | 0.625 |
Sleep duration, number of times waking up, sleep efficiency, and average oxyhemoglobin saturation are expressed as mean ± SD, and were analyzed by independent-sample t-test. Other data are expressed as median (first quartile, third quartile), and were analyzed by Mann-Whitney U test. AD-nSI: Alzheimer’s disease with no sleep insufficiency; AD-SI: Alzheimer’s disease with sleep insufficiency.
Cognitive function, neuropsychiatric symptoms, and ADL in the AD-nSI and AD-SI groups
Cognitive function, neuropsychiatric symptoms, and ADL were compared between AD-nSI and AD-SI groups (Table 3). The AD-SI group exhibited significantly greater impairments in overall cognitive function (MMSE: 22.42 ± 2.75 vs. 19.79 ± 3.87, P = 0.007, MOCA: 14.55 ± 3.91 vs. 12.11 ± 4.38, P = 0.046), and multiple individual cognitive domains were compromised, including auditory immediate and delayed memory (P = 0.021), delayed visual memory (P = 0.038), language ability (P = 0.032), and visuospatial ability (P = 0.010). No significant differences between groups were observed for executive function or attention, or for neuropsychiatric symptoms, either overall or on the individual scales for depression anxiety, apathy, agitation or ADL (all P > 0.05).
Table 3.
Comparisons of clinical symptoms between AD-nSI and AD-SI groups
| AD-nSI group (n = 31) | AD-SI group (n = 19) | P-value | |
|---|---|---|---|
| Cognitive function | |||
| Overall cognitive function | |||
| MMSE score | 22.42±2.75 | 19.79±3.87 | 0.007 |
| MOCA score | 14.55±3.91 | 12.11±4.38 | 0.046 |
| Cognitive domains | |||
| Memory | |||
| AVLT-immediate recall score | 10.74±4.50 | 7.37±5.41 | 0.021 |
| AVLT-delayed recall score | 1.00 (0.00, 4.00) | 0.00 (0.00, 4.00) | 0.853 |
| Visual memory | |||
| CFT-delayed memory score | 6.00 (0.00, 12.00) | 0.00 (0.00, 4.50) | 0.038 |
| Executive function | |||
| SCWT-A time (s) | 47.16±28.67 | 67.75±76.42 | 0.345 |
| SCWT-B time (s) | 62.10±37.36 | 64.93±35.47 | 0.82 |
| SCWT-C time (s) | 104.68±62.37 | 117.69±66.33 | 0.564 |
| Attention | |||
| SDMT score | 23.84±4.77 | 18.05±8.32 | 0.01 |
| Language | |||
| BNT score | 22.97±3.20 | 20.68±4.04 | 0.032 |
| Visuospatial ability | |||
| CFT-imitation score | 18.00 (1.00, 26.00) | 12.00 (0.00, 19.00) | 0.322 |
| Neuropsychiatric symptoms | |||
| Overall neuropsychiatric status | 0.00 (0.00, 3.00) | 2.00 (0.00, 4.00) | |
| NPI score | 0.102 | ||
| Neuropsychiatric symptoms | |||
| HAMD score | 4.81±2.88 | 5.89±2.10 | 0.16 |
| HAMA score | 5.77±5.29 | 9.38±7.78 | 0.081 |
| MAES score | 17.41±9.50 | 17.96±11.01 | 0.866 |
| CMAI score | 29.00 (29.00, 31.00) | 30.00 (29.00, 33.50) | 0.244 |
| ADL | |||
| ADL score | 25.00 (20.00, 28.00) | 21.00 (20.00, 28.00) | 0.335 |
MMSE, MOCA, AVLT-immediate recall, SCWT-A/B/C time, SDMT, BNT, HAMD, HAMA, and MAES are expressed as mean ± SD, and were analyzed by independent-sample t-test. Other data are expressed as median (first quartile, third quartile), and were analyzed by Mann-Whitney U test. ADL: Activities of daily living; AD-nSI: Alzheimer’s disease with no sleep insufficiency; AD-SI: Alzheimer’s disease with sleep insufficiency; BNT: Boston Naming Test; CFT: Complex Figure Test; SCWT, Stroop Color Word Test; CMAI: Cohen-Mansfield Agitation Inventory; HAMA: Hamilton Anxiety Scale; HAMD: Hamilton Depression Scale; MAES: Modified Apathy Estimate Scale; MMSE: Mini-Mental State Examination; MoCA: Montreal Cognitive Assessment; NPI: Neuropsychiatric Inventory; RAVLT: Rey Auditory Verbal Learning Test; SDMT: Symbol Digit Modality Test.
Levels of orexinergic proteins in the CSF of the AD-nSI and AD-SI groups
CSF samples were collected from all patients with AD and levels of orexin A, orexin B, OR1, and OR2 were measured (Table 4). We found that orexin A and orexin B levels were significantly higher in the AD-SI group than in the AD-nSI group (both P < 0.05). However, levels of OR1 and OR2 did not differ between groups (both P > 0.05).
Table 4.
Levels of orexin and its receptors in the CSF of AD-nSI and AD-SI groups
| AD-nSI group (n = 31) | AD-SI group (n = 19) | P-value | |
|---|---|---|---|
| Orexin A (pg/mL) | 223.71±29.60 | 243.90±29.90 | 0.026 |
| Orexin B (pg/mL) | 317.31±36.06 | 349.53±53.47 | 0.028 |
| OR1 (pg/mL) | 13.85 (3.40, 37.07) | 19.71 (10.90, 27.33) | 0.310 |
| OR2 (ng/mL) | 0.08±0.01 | 0.08±0.02 | 0.779 |
Orexin A, orexin B, and OR2 are expressed as mean ± SD, and were analyzed by independent-sample t-test. OR1 is expressed as median (first quartile, third quartile), and was analyzed by Mann-Whitney U test. AD-nSI: Alzheimer’s disease with no sleep insufficiency; AD-SI: Alzheimer’s disease with sleep insufficiency; OR1: orexin receptor 1; OR2: orexin receptor 2.
Correlation of orexinergic protein levels in the CSF with sleep variables in the AD-SI group
The correlation coefficients were obtained for the correlation between orexinergic protein levels in the CSF and each sleep variable in the AD-SI group (Table 5). The analysis showed that orexin A and orexin B levels were significantly and negatively correlated with total sleep duration in AD-SI group (orexin A: r = −0.523, P = 0.022; orexin B: r = −0.515, P = 0.024), indicating that higher the levels of orexin A and orexin B in the CSF were associated with shorter sleep duration. The correlations with other sleep variables and those between OR1 and OR2 levels in the CSF and all of the sleep variables were not significant (P > 0.05).
Table 5.
Correlations between the levels of orexin and its receptors in the CSF and sleep variables in the AD-SI group
| Sleep duration | Sleep latency | WASO | Sleep efficiency | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| r-value | P-value | r-value | P-value | r-value | P-value | r-value | P-value | |
| Orexin A | –0.523 | 0.022 | 0.081 | 0.749 | 0.162 | 0.503 | –0.307 | 0.201 |
| Orexin B | –0.515 | 0.024 | 0.243 | 0.486 | 0.163 | 0.505 | –0.314 | 0.19 |
| OR1 | –0.117 | 0.69 | 0.252 | 0.188 | –0.028 | 0.925 | 0.078 | 0.791 |
| OR2 | –0.407 | 0.148 | 0.136 | 0.474 | –0.232 | 0.425 | 0.239 | 0.411 |
AD-SI: Alzheimer’s disease with sleep insufficiency; OR1: orexin receptor 1; OR2: orexin receptor 2; WASO: wakefulness duration after sleep onset.
Comparisons of the levels of BBB factors in the CSF between the AD-nSI and AD-SI groups
Analysis showed that among all BBB factors measured in the CSF (MMP-2, MMP-3, MMP-9, RAGE, LRP1, and GFAP), MMP-3 levels were significantly higher in the AD-SI group than in the AD-nSI group (P < 0.01; Table 6). Levels of the other factors did not differ between groups (all P > 0.05).
Table 6.
Levels of BBB factors in the CSF of the AD-nSI and AD-SI groups
| AD-nSI group (n = 31) | AD-SI group (n = 19) | P-value | |
|---|---|---|---|
| MMP-2 (ng/mL) | 5.35±3.07 | 10.48±8.50 | 0.138 |
| MMP-3 (ng/mL) | MMP-3 (ng/mL) | 0.59±0.19 | 0.006 |
| MMP-9 (ng/mL) | 6.60±2.09 | 6.49±0.57 | 0.840 |
| RAGE (pg/mL) | 1.91±1.16 | 1.97±0.93 | 0.894 |
| LRP1 (ng/mL) | 33.02 (28.56, 42.20) | 36.20 (25.37, 57.47) | 0.692 |
| GFAP (ng/mL) | 0.08±0.036 | 0.12±0.07 | 0.182 |
MMP-2, MMP-3, MMP-9, RAGE, and GFAP are expressed as mean ± SD, and were analyzed by independent-sample t-test. LRP1 is expressed as median (first quartile, third quartile), and was analyzed by Mann-Whitney U test. AD-SI: Alzheimer‘s disease with sleep insufficiency; BBB: blood-brain barrier; GFAP: glial fibrillary acidic protein; LRP1: lipoprotein receptor related protein 1; MMP-2: matrix metalloproteinase 2; MMP- 3: matrix metalloproteinase 3; MMP-9: matrix metalloproteinase 9; RAGE: receptor of advanced glycation endproducts.
Correlation of orexin A and orexin B levels with MMP-3 levels in the CSF from AD-SI patients
Analysis showed that orexin A and orexin B levels in the CSF of the AD-SSI group were both significantly and positively correlated with MMP-3 levels (orexin A: r = 0.516, P = 0.024; orexin B: r = 0.455, P = 0.049; Figure 1).
Figure 1.
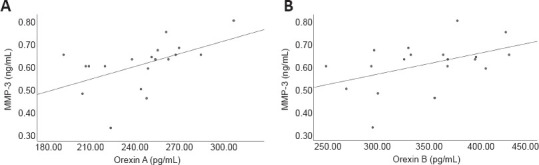
Correlation of orexin and MMP-3 levels in the CSF of the AD-SI group.
(A) Correlation of orexin A and MMP-3 levels (r = 0.516, P = 0.024, Spearman correlation). (B) Correlation of orexin B and MMP-3 levels (r = 0.455, P = 0.049, Spearman correlation). AD-SI: Alzheimer’s disease with sleep insufficiency; CSF: cerebrospinal fluid; MMP-3: matrix metalloproteinase 3.
Discussion
Frequency of AD-SI
SI, which was defined as having a sleep duration ≤ 6 hours (Sabia et al., 2021; Winer et al., 2021), has not attracted great attention from either clinicians or patients with AD. Therefore, the frequency of SI in these patients has been rarely reported. In this study, actigraphy was used to monitor sleep duration. Analysis showed that 19 of 50 patients with AD (38%) had SI, indicating that SI was fairly common among patients with AD.
AD-SI and demographic variables
Sleep duration is affected by multiple factors (Couvineau et al., 2018). A previous study indicated that sleep duration decreases with age (Liguori et al., 2014). In this study, AD-SI and AD-nSI groups did not significantly differ in age or age of disease onset. Other demographic variables, including gender (Liguori et al., 2014), education level (Sabia et al., 2021; Winer et al., 2021) and disease duration did not differ between AD-SI and AD-nSI groups, which was similar to other studies. Taken together, the SI observed in the current patients with AD was not related to the demographic variables. Thus, we can conclude that these variables were not related to any of the observed group differences in clinical characteristics, orexin-related proteins, or BBB factors.
Relationship between SI and sleep variables in patients with AD
We monitored several sleep variables by actigraphy and compared them between AD-nSI and AD-SI groups. We found that sleep duration was significantly longer in the AD-SI group than in the AD-nSI group, and these participants also took significantly longer time to fall sleep, spent dramatically more time being awake after having initially fallen asleep, and displayed remarkably poorer sleep efficiency. Accordingly, the prolonged sleep latency and long duration of wakefulness during sleep highly contributed to SI, and consequently significantly compromised sleep efficiency of the patients with AD.
Effect of SI on clinical symptoms of AD
SI has been reported to cause cognitive decline, produce negative impact on behavior, and eventually lead to dementia in adults (Moraes et al., 2014). The Whitehall II cohort study spanning 30 years showed that people who slept less than 6 hours a night at the age of 50, 60, and 70 years had an increased risk of AD by 22%, 37% and 24%, respectively (Hazard ratios = 1.22, 1.37, and 1.24, respectively) compared with those who slept a normal duration (7 hours). However, the effect of SI on clinical symptoms of AD has been barely reported.
Our results showed that overall cognitive function, as well as the individual cognitive domains of memory, language, and visuospatial ability were poorer in the AD-SI group than in the AD-nSI group. In the brain, neurons and glial cells have a high metabolic rate and produce a large number of toxic substances, including Aβ and tau (Komaroff, 2021). During sleep, a large amount of fluid flows through the lymphoid system, and the brain interstitial fluid increases by 60%. This helps clear Aβ and hyperphosphorylated tau. Thus, in the case of SI, less sleep leads to less clearing of these toxic substances, raising the risk of developing AD and aggravating cognitive impairment (Nedergaard and Goldman, 2020).
In the current study, neuropsychiatric symptoms were compared between AD-SI and AD-nSI groups. A previous study found that SI was related to emotional disorders. In SI, activity of medial prefrontal cortex and its connection with the limbic region were low, and this was accompanied by hyperactivity of the amygdala and prefrontal cortex and higher anxiety (Ben Simon et al., 2020). In the current study, the AD-SI group reported greater anxiety than the AD-nSI group, as reflected by higher scores on the HAMA, but this difference was not statistically significant. This implies that anxiety may not be associated with SI in patients with AD.
Overall neuropsychiatric status and the other neuropsychiatric symptoms did not differ significantly between AD-SI and AD-nSI groups. We speculate that because the participants enrolled in this study had relatively short disease duration, brain regions related to neuropsychiatric symptoms were not significantly involved, or were not correlated with SI-related brain regions. We also suppose that extended SI duration is accompanied by more obvious changes in brain structure, which could lead to neuropsychiatric symptoms in the future. Previous studies showed that ADL in older people was significantly compromised by decreased sleep quality when the people had mild cognitive impairment compared than when they were cognitively unimpaired. However, no reports have examined the effect of SI on ADL in patients with AD. In this study, ADL scores did not significantly differ between groups. We speculate that because the patients with AD recruited in this investigation had relatively short disease duration, the negative impact of SI on ADL had not yet manifested.
Relationship between AD-SI and the orexinergic system
The sleep-wake cycle is regulated by the brainstem, hypothalamus, thalamus, and basal forebrain, which project to the cerebral cortex and release excitatory and inhibitory neurotransmitters that regulate and maintain normal sleep-wake rhythms, and consequently protect cognitive function (Yaffe et al., 2014). In particular, changes in the orexinergic system in the lateral hypothalamus, their impact on sleep and cognition, and potential underlying mechanisms, have drawn increasing attention in the recent years.
After orexinergic neurons are activated, they emit excitatory projections to monoaminergic and cholinergic neurons in the arousal system, and thereby physiologically promote wakefulness (Dauvilliers, 2021). Orexin is released rhythmically, and levels peak in the awaking phase (Kilduff and Peyron, 2000). A previous investigation found that shorter sleep duration, more sleep fragmentation, and compromised sleep quality in patients with AD, especially those in the late stages, were related to alterations of the orexinergic system (Liguori et al., 2014). However, these alterations purported are still controversial. A postmortem study showed that patients with AD had significantly fewer orexinergic neurons in the lateral hypothalamus and decreased orexin in the CSF than did normal controls (Yaffe et al., 2014). However, elevation of orexin in the CSF was found to be significant in moderate to severe AD compared with mild AD and controls (Liguori et al., 2014). The lack of consistency in these studies could be related to the differences in AD stage, the severity of the impairment in the orexinergic system, or sample size. Currently, there is no report on alterations of the orexin signaling in patients with AD-SI.
In this investigation, compared with AD-nSI group, the CSF of the AD-SI group exhibited significantly higher levels of both orexin A and orexin B, and exhibited a trend towards higher levels of both OR1 and OR2. Furthermore, both orexin A and orexin B levels were significantly and negatively correlated with sleep duration. Accordingly, elevation of orexin A and orexin B in the CSF was correlated with reduced sleep duration in patients with AD. We speculate that upregulation of orexin-related proteins might serve as a potential mechanism underlying AD-SI.
Relationship among AD-SI, the BBB, and the orexinergic system
The BBB is mainly composed of MMPs, LRP1, RAGE and GFAP. Compromise of the BBB might occur in the early stage of AD (Sweeney et al., 2018). A study found that overexpression of MMPs caused excessive opening of the BBB via degraded tight junction proteins, which aggravated neuroinflammation, neuronal demyelination, and neurotoxicity in brain. Thus, BBB disruption is an important event related to the pathogenesis of AD (Van Hove et al., 2012). MMP-3 is a main member of the MMP family. Elevation of MMP-3 in plasma was reported in patients in the early stages of AD, and was associated with a high risk of cognitive decline (Iulita et al., 2019). SI led to aseptic inflammation (Lahtinen et al., 2019), promoted accumulation of metabolites and Aβ in brain, and eventually enhanced the permeability of the BBB (He et al., 2014). In particular, the significant decrease in slow waves (0–0.5 Hz) during deep sleep indicated reduced clearance of harmful substances through the lymphoid system (Lucey et al., 2019), increased BBB leakage, and progression of cognitive impairment in patients with AD (Semyachkina-Glushkovskaya et al., 2020). In this study, MMP-3 levels in the CSF of AD-SI group was significantly higher than those in the AD-nSI group, suggesting that the AD-SI group had more severer BBB impairment, which was related to the lack of sleep (He et al., 2014). In addition, LRP1, RAGE, and GFAP levels in AD-SI group all showed upward trends, indicating that, compared with other BBB factors, MMP-3 plays a key role in the damage to the BBB precipitated by SI in patients with AD.
Orexin plays a pivotal role in the regulation of the sleep-wake cycle. Orexin A has been observed to cross the BBB via free diffusion (Kastin and Akerstrom, 1999). However, there is no report on the relationship between BBB factors and orexin in the CSF of patients with AD-SI. In this study, MMP-3 levels were significantly and positively correlated with levels of both orexin A and orexin B in the CSF of patients with AD-SI. We speculate that SI led to BBB damage, characterized by significantly more MMP-3 in the CSF, which in turn triggered the release of more orexin A and orexin B from the lateral hypothalamus, leading to increased wakefulness and eventually insufficient sleep.
Limitations
This investigation has limitations. First, although sleep was monitored for 24 hours, one night of sleep monitoring is not enough to confirm what kind of sleep impairment a patient with AD has. In the future, we will monitor the sleep of each patient for several consecutive days and obtain the average result of each sleep parameter, which will better reflect the sleep status. Additionally, lumbar puncture was delayed in some patients because of time conflicts with other examinations during hospitalization. In the future, we will strive to improve the procedure to ensure that each patient has the lumbar puncture conducted on the day after sleep monitoring.
Conclusions
The results from this investigation demonstrated that SI is common in patients with AD. SI was associated with greater overall cognitive impairment that was observed in memory, language, and executive functions. Analysis suggests that mechanisms underlying AD-SI include the significant increase of orexin A and orexin B in the CSF, and damage to the BBB reflected by the increase of MMP-3 in the CSF, which precipitate each other and accelerate the progression of AD. Thus, inhibiting upregulation of orexin or its receptors and preventing destruction of the BBB may be the promising therapeutic targets for AD.
Additional files:
Additional file 1 (162.2KB, pdf) : Informed consent form (Chinese).
Additional file 2 (262.7KB, pdf) : Hospital ethics approval (Chinese).
Additional file 3: STROBE checklist.
Additional file 4: Open peer review report 1 (81.6KB, pdf) .
Footnotes
Funding: This work was supported by the National Key Research and Development Program of China, Nos. 2016YFC1306300 (to XMW), 2016YFC1306000; the National Key R&D Program of China-European Commission Horizon 2020, No. 2017YFE0118800-779238 (to YXW); the National Natural Science Foundation of China, Nos. 81970992 (to WZ), 81571229 (to WZ), 81071015 (to WZ), 30770745 (to WZ); Capital’s Funds for Health Improvement and Research (CFH), No. 2022-2-2048 (to WZ); the Key Technology R&D Program of Beijing Municipal Education Commission, No. kz201610025030 (to WZ); the Natural Science Foundation of Beijing, No. 7082032 (to WZ); the Key Project of the Natural Science Foundation of Beijing, No. 4161004 (to WZ); Capital Clinical Characteristic Application Research, No. Z121107001012161 (to WZ); Project of Scientific and Technological Development of Traditional Chinese Medicine in Beijing, No. JJ2018-48 (to WZ); High Level Technical Personnel Training Project of Beijing Health System of China, No. 2009-3-26 (to WZ); Excellent Personnel Training Project of Beijing, No. 20071D0300400076 (to WZ); Important National Science & Technology Specific Project, No. 2011ZX09102-003-01 (to WZ); Beijing Healthcare Research Project, No. JING-15-2 (to WZ); Basic-Clinical Research Cooperation Funding of Capital Medical University of China, Nos. 2015-JL-PT-X04 (to WZ), 10JL49 (to WZ), 14JL15 (to WZ); the Natural Science Foundation of Capital Medical University, Beijing, China, No. PYZ2018077 (to PG); Youth Research Fund of Beijing Tiantan Hospital of Capital Medical University of China, Nos. 2015-YQN-14 (to PG), 2015-YQN-15, 2015-YQN-17.
Conflicts of interest: The authors declare that they have no competing interests.
Availability of data and materials: All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open peer reviewer: Melinda Barkhuizen, Philip Morris International Management SA, Netherlands.
P-Reviewer: Barkhuizen M; S-Editors: Yu J, Li CH; L-Editors: Phillips A, Song LP; T-Editor: Jia Y
References
- 1.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer's disease:recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. (2011);7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atri A. The Alzheimer's disease clinical spectrum:diagnosis and management. Med Clin North Am. (2019);103:263–293. doi: 10.1016/j.mcna.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Ben Simon E, Rossi A, Harvey AG, Walker MP. Overanxious and underslept. Nat Hum Behav. (2020);4:100–110. doi: 10.1038/s41562-019-0754-8. [DOI] [PubMed] [Google Scholar]
- 4.Beretta L, Carli G, Caffarra P, Perani D. Distinct brain dysfunctions underlying visuo-constructive deficit in DLB and AD. Brain Imaging Behav. (2022);16:532–537. doi: 10.1007/s11682-021-00515-7. [DOI] [PubMed] [Google Scholar]
- 5.Bridoux A, Moutereau S, Covali-Noroc A, Margarit L, Palfi S, Nguyen JP, Lefaucheur JP, Césaro P, d'Ortho MP, Drouot X. Ventricular orexin-A (hypocretin-1) levels correlate with rapid-eye-movement sleep without atonia in Parkinson's disease. Nat Sci Sleep. (2013);5:87–91. doi: 10.2147/NSS.S41245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index:a new instrument for psychiatric practice and research. Psychiatry Res. (1989);28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 7.Camargos EF, Louzada LL, Quintas JL, Naves JO, Louzada FM, Nóbrega OT. Trazodone improves sleep parameters in Alzheimer disease patients:a randomized, double-blind, and placebo-controlled study. Am J Geriatr Psychiatry. (2014);22:1565–1574. doi: 10.1016/j.jagp.2013.12.174. [DOI] [PubMed] [Google Scholar]
- 8.Carson N, Leach L, Murphy KJ. A re-examination of Montreal Cognitive Assessment (MoCA) cutoff scores. Int J Geriatr Psychiatry. (2018);33:379–388. doi: 10.1002/gps.4756. [DOI] [PubMed] [Google Scholar]
- 9.Cockrell JR, Folstein MF. Mini-Mental State Examination (MMSE) Psychopharmacol Bull. (1988);24:689–692. [PubMed] [Google Scholar]
- 10.Couvineau A, Dayot S, Nicole P, Gratio V, Rebours V, Couvelard A, Voisin T. The anti-tumoral properties of orexin/hypocretin hypothalamic neuropeptides:an unexpected therapeutic role. Front Endocrinol (Lausanne) (2018);9:573. doi: 10.3389/fendo.2018.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dauvilliers Y. Hypocretin/orexin, sleep and Alzheimer's disease. Front Neurol Neurosci. (2021);45:139–149. doi: 10.1159/000514967. [DOI] [PubMed] [Google Scholar]
- 12.Deane R, Bell RD, Sagare A, Zlokovic BV. Clearance of amyloid-beta peptide across the blood-brain barrier:implication for therapies in Alzheimer's disease. CNS Neurol Disord Drug Targets. (2009);8:16–30. doi: 10.2174/187152709787601867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dias IH, Taiwo R, Ma D. The blood-brain barrier models to study apolipoprotein E genotypes in Alzheimer's disease. Neural Regen Res. (2022);17:1973–1974. doi: 10.4103/1673-5374.331538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fekedulegn D, Andrew ME, Shi M, Violanti JM, Knox S, Innes KE. Actigraphy-Based Assessment of Sleep Parameters. Ann Work Expo Health. (2020);64:350–367. doi: 10.1093/annweh/wxaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gong QY, Cai L, Jing Y, Wang W, Yang DX, Chen SW, Tian HL. Urolithin A alleviates blood-brain barrier disruption and attenuates neuronal apoptosis following traumatic brain injury in mice. Neural Regen Res. (2022);17:2007–2013. doi: 10.4103/1673-5374.335163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guarnieri B, Adorni F, Musicco M, Appollonio I, Bonanni E, Caffarra P, Caltagirone C, Cerroni G, Concari L, Cosentino FI, Ferrara S, Fermi S, Ferri R, Gelosa G, Lombardi G, Mazzei D, Mearelli S, Morrone E, Murri L, Nobili FM, et al. Prevalence of sleep disturbances in mild cognitive impairment and dementing disorders:a multicenter Italian clinical cross-sectional study on 431 patients. Dement Geriatr Cogn Disord. (2012);33:50–58. doi: 10.1159/000335363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo Q, Zhao Q, Chen M, Ding D, Hong Z. A comparison study of mild cognitive impairment with 3 memory tests among Chinese individuals. Alzheimer Dis Assoc Disord. (2009);23:253–259. doi: 10.1097/WAD.0b013e3181999e92. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. (1959);32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. (1960);23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Havekes R, Heckman PRA, Wams EJ, Stasiukonyte N, Meerlo P, Eisel ULM. Alzheimer's disease pathogenesis: The role of disturbed sleep in attenuated brain plasticity and neurodegenerative processes. Cell Signal. (2019);64:109420. doi: 10.1016/j.cellsig.2019.109420. [DOI] [PubMed] [Google Scholar]
- 21.He J, Hsuchou H, He Y, Kastin AJ, Wang Y, Pan W. Sleep restriction impairs blood-brain barrier function. J Neurosci. (2014);34:14697–14706. doi: 10.1523/JNEUROSCI.2111-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holth JK, Fritschi SK, Wang C, Pedersen NP, Cirrito JR, Mahan TE, Finn MB, Manis M, Geerling JC, Fuller PM, Lucey BP, Holtzman DM. The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science. (2019);363:880–884. doi: 10.1126/science.aav2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irwin MR, Vitiello MV. Implications of sleep disturbance and inflammation for Alzheimer's disease dementia. Lancet Neurol. (2019);18:296–306. doi: 10.1016/S1474-4422(18)30450-2. [DOI] [PubMed] [Google Scholar]
- 24.Iulita MF, Ganesh A, Pentz R, Flores Aguilar L, Gubert P, Ducatenzeiler A, Christie S, Wilcock GK, Cuello AC. Identification and preliminary validation of a plasma profile associated with cognitive decline in dementia and at-risk individuals:a retrospective cohort analysis. J Alzheimers Dis. (2019);67:327–341. doi: 10.3233/JAD-180970. [DOI] [PubMed] [Google Scholar]
- 25.Jia L, Quan M, Fu Y, Zhao T, Li Y, Wei C, Tang Y, Qin Q, Wang F, Qiao Y, Shi S, Wang YJ, Du Y, Zhang J, Zhang J, Luo B, Qu Q, Zhou C, Gauthier S, Jia J. Dementia in China:epidemiology, clinical management, and research advances. Lancet Neurol. (2020);19:81–92. doi: 10.1016/S1474-4422(19)30290-X. [DOI] [PubMed] [Google Scholar]
- 26.Kastin AJ, Akerstrom V. Orexin A but not orexin B rapidly enters brain from blood by simple diffusion. J Pharmacol Exp Ther. (1999);289:219–223. [PubMed] [Google Scholar]
- 27.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of adl:a standardized measure of biological and psychosocial function. JAMA. (1963);185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 28.Kent BA, Feldman HH, Nygaard HB. Sleep and its regulation: An emerging pathogenic and treatment frontier in Alzheimer's disease. Prog Neurobiol. (2021);197:101902. doi: 10.1016/j.pneurobio.2020.101902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kilduff TS, Peyron C. The hypocretin/orexin ligand-receptor system:implications for sleep and sleep disorders. Trends Neurosci. (2000);23:359–365. doi: 10.1016/s0166-2236(00)01594-0. [DOI] [PubMed] [Google Scholar]
- 30.Komaroff AL. Does Sleep Flush Wastes From the Brain? JAMA. (2021);325:2153–2155. doi: 10.1001/jama.2021.5631. [DOI] [PubMed] [Google Scholar]
- 31.Koss E, Weiner M, Ernesto C, Cohen-Mansfield J, Ferris SH, Grundman M, Schafer K, Sano M, Thal LJ, Thomas R, Whitehouse PJ. Assessing patterns of agitation in Alzheimer's disease patients with the Cohen-Mansfield Agitation Inventory. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord. (1997);11(Suppl 2):S45–50. doi: 10.1097/00002093-199700112-00007. [DOI] [PubMed] [Google Scholar]
- 32.Lahtinen A, Puttonen S, Vanttola P, Viitasalo K, Sulkava S, Pervjakova N, Joensuu A, Salo P, Toivola A, Härmä M, Milani L, Perola M, Paunio T. A distinctive DNA methylation pattern in insufficient sleep. Sci Rep. (2019);9:1193. doi: 10.1038/s41598-018-38009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liebner S, Dijkhuizen RM, Reiss Y, Plate KH, Agalliu D, Constantin G. Functional morphology of the blood-brain barrier in health and disease. Acta Neuropathol. (2018);135:311–336. doi: 10.1007/s00401-018-1815-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liguori C, Chiaravalloti A, Nuccetelli M, Izzi F, Sancesario G, Cimini A, Bernardini S, Schillaci O, Mercuri NB, Fabio P. Hypothalamic dysfunction is related to sleep impairment and CSF biomarkers in Alzheimer's disease. J Neurol. (2017);264:2215–2223. doi: 10.1007/s00415-017-8613-x. [DOI] [PubMed] [Google Scholar]
- 35.Liguori C, Nuccetelli M, Izzi F, Sancesario G, Romigi A, Martorana A, Amoroso C, Bernardini S, Marciani MG, Mercuri NB, Placidi F. Rapid eye movement sleep disruption and sleep fragmentation are associated with increased orexin-A cerebrospinal-fluid levels in mild cognitive impairment due to Alzheimer's disease. Neurobiol Aging. (2016);40:120–126. doi: 10.1016/j.neurobiolaging.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Liguori C, Romigi A, Nuccetelli M, Zannino S, Sancesario G, Martorana A, Albanese M, Mercuri NB, Izzi F, Bernardini S, Nitti A, Sancesario GM, Sica F, Marciani MG, Placidi F. Orexinergic system dysregulation, sleep impairment, and cognitive decline in Alzheimer disease. JAMA Neurol. (2014);71:1498–1505. doi: 10.1001/jamaneurol.2014.2510. [DOI] [PubMed] [Google Scholar]
- 37.Lin CY, Chen TB, Lin KN, Yeh YC, Chen WT, Wang KS, Wang PN. Confrontation naming errors in Alzheimer's disease. Dement Geriatr Cogn Disord. (2014);37:86–94. doi: 10.1159/000354359. [DOI] [PubMed] [Google Scholar]
- 38.Lucey BP, McCullough A, Landsness EC, Toedebusch CD, McLeland JS, Zaza AM, Fagan AM, McCue L, Xiong C, Morris JC, Benzinger TLS, Holtzman DM. Reduced non-rapid eye movement sleep is associated with tau pathology in early Alzheimer's disease. Sci Transl Med. (2019);11:eaau6550. doi: 10.1126/scitranslmed.aau6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer's disease:recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. (2011);7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montagne A, Zhao Z, Zlokovic BV. Alzheimer's disease: A matter of blood-brain barrier dysfunction? J Exp Med. (2017);214:3151–3169. doi: 10.1084/jem.20171406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moraes W, Piovezan R, Poyares D, Bittencourt LR, Santos-Silva R, Tufik S. Effects of aging on sleep structure throughout adulthood:a population-based study. Sleep Med. (2014);15:401–409. doi: 10.1016/j.sleep.2013.11.791. [DOI] [PubMed] [Google Scholar]
- 42.Nedergaard M, Goldman SA. Glymphatic failure as a final common pathway to dementia. Science. (2020);370:50–56. doi: 10.1126/science.abb8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oeckl P, Halbgebauer S, Anderl-Straub S, Steinacker P, Huss AM, Neugebauer H, von Arnim CAF, Diehl-Schmid J, Grimmer T, Kornhuber J, Lewczuk P, Danek A, Ludolph AC, Otto M. Glial fibrillary acidic protein in serum is increased in Alzheimer's disease and correlates with cognitive impairment. J Alzheimers Dis. (2019);67:481–488. doi: 10.3233/JAD-180325. [DOI] [PubMed] [Google Scholar]
- 44.Periáñez JA, Lubrini G, García-Gutiérrez A, Ríos-Lago M. Construct Validity of the stroop Color-Word Test: Influence of Speed of Visual Search, Verbal Fluency, Working Memory, Cognitive Flexibility, and Conflict Monitoring. Arch Clin Neuropsychol. (2021);36:99–111. doi: 10.1093/arclin/acaa034. [DOI] [PubMed] [Google Scholar]
- 45.Ryan J, Storey E, Murray AM, Woods RL, Wolfe R, Reid CM, Nelson MR, Chong TTJ, Williamson JD, Ward SA, Lockery JE, Orchard SG, Trevaks R, Kirpach B, Newman AB, Ernst ME, McNeil JJ, Shah RC. Randomized placebo-controlled trial of the effects of aspirin on dementia and cognitive decline. Neurology. (2020);95:e320–e331. doi: 10.1212/WNL.0000000000009277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sabia S, Fayosse A, Dumurgier J, van Hees VT, Paquet C, Sommerlad A, Kivimäki M, Dugravot A, Singh-Manoux A. Association of sleep duration in middle and old age with incidence of dementia. Nat Commun. (2021);12:2289. doi: 10.1038/s41467-021-22354-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakurai T. The role of orexin in motivated behaviours. Nat Rev Neurosci. (2014);15:719–731. doi: 10.1038/nrn3837. [DOI] [PubMed] [Google Scholar]
- 48.Semyachkina-Glushkovskaya O, Postnov D, Penzel T, Kurths J. Sleep as a novel biomarker and a promising therapeutic target for cerebral small vessel disease:a review focusing on Alzheimer's disease and the blood-brain barrier. Int J Mol Sci. (2020);21:6293. doi: 10.3390/ijms21176293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soya S, Sakurai T. Evolution of orexin neuropeptide system:structure and function. Front Neurosci. (2020);14:691. doi: 10.3389/fnins.2020.00691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, Zhou Y, Wong DF, Ferrucci L, Resnick SM. Self-reported sleep and β-amyloid deposition in community-dwelling older adults. JAMA Neurol. (2013);70:1537–1543. doi: 10.1001/jamaneurol.2013.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Starkstein SE, Mayberg HS, Preziosi TJ, Andrezejewski P, Leiguarda R, Robinson RG. Reliability, validity, and clinical correlates of apathy in Parkinson's disease. J Neuropsychiatry Clin Neurosci. (1992);4:134–139. doi: 10.1176/jnp.4.2.134. [DOI] [PubMed] [Google Scholar]
- 52.Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol. (2018);14:133–150. doi: 10.1038/nrneurol.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vaccaro A, Kaplan Dor Y, Nambara K, Pollina EA, Lin C, Greenberg ME, Rogulja D. Sleep loss can cause death through accumulation of reactive oxygen species in the gut. Cell. (2020);181:1307–1328.e15. doi: 10.1016/j.cell.2020.04.049. [DOI] [PubMed] [Google Scholar]
- 54.Van Hove I, Lemmens K, Van de Velde S, Verslegers M, Moons L. Matrix metalloproteinase-3 in the central nervous system:a look on the bright side. J Neurochem. (2012);123:203–216. doi: 10.1111/j.1471-4159.2012.07900.x. [DOI] [PubMed] [Google Scholar]
- 55.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement:guidelines for reporting observational studies. PLoS Med. (2007);4:e296. doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Q, Cao F, Wu Y. Orexinergic system in neurodegenerative diseases. Front Aging Neurosci. (2021);13:713201. doi: 10.3389/fnagi.2021.713201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winer JR, Deters KD, Kennedy G, Jin M, Goldstein-Piekarski A, Poston KL, Mormino EC. Association of short and long sleep duration with amyloid-βburden and cognition in aging. JAMA Neurol. (2021);78:1187–1196. doi: 10.1001/jamaneurol.2021.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yaffe K, Falvey CM, Hoang T. Connections between sleep and cognition in older adults. Lancet Neurol. (2014);13:1017–1028. doi: 10.1016/S1474-4422(14)70172-3. [DOI] [PubMed] [Google Scholar]
- 59.Yamazaki Y, Kanekiyo T. Blood-brain barrier dysfunction and the pathogenesis of Alzheimer's disease. Int J Mol Sci. (2017);18:1965. doi: 10.3390/ijms18091965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zenaro E, Piacentino G, Constantin G. The blood-brain barrier in Alzheimer's disease. Neurobiol Dis. (2017);107:41–56. doi: 10.1016/j.nbd.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang YL, Wang J, Zhang ZN, Su Q, Guo JH. The relationship between amyloid-beta and brain capillary endothelial cells in Alzheimer's disease. Neural Regen Res. (2022);17:2355–2363. doi: 10.4103/1673-5374.335829. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


