Key Words: diabetes mellitus; dipeptidyl peptidase 4 inhibitor; exendin-4; glucagon-like peptide-1 receptor agonist; 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; linagliptin; microglia; neuroinflammation; NLRP3 inflammasome; Parkinson’s disease; pyroptosis
Abstract
Use of glucagon-like peptide-1 receptor agonist or dipeptidyl peptidase 4 inhibitor has been shown to lower the incidence of Parkinson’s disease in patients with diabetes mellitus. Therefore, using these two treatments may help treat Parkinson’s disease. To further investigate the mechanisms of action of these two compounds, we established a model of Parkinson’s disease by treating mice with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and then subcutaneously injected them with the glucagon-like peptide-1 receptor agonist exendin-4 or the dipeptidyl peptidase 4 inhibitor linagliptin. We found that both exendin-4 and linagliptin reversed motor dysfunction, glial activation, and dopaminergic neuronal death in this model. In addition, both exendin-4 and linagliptin induced microglial polarization to the anti-inflammatory M2 phenotype and reduced pro-inflammatory cytokine secretion. Moreover, in vitro experiments showed that treatment with exendin-4 and linagliptin inhibited activation of the nucleotide-binding oligomerization domain- and leucine-rich-repeat- and pyrin-domain-containing 3/caspase-1/interleukin-1β pathway and subsequent pyroptosis by decreasing the production of reactive oxygen species. These findings suggest that exendin-4 and linagliptin exert neuroprotective effects by attenuating neuroinflammation through regulation of microglial polarization and the nucleotide-binding oligomerization domain- and leucine-rich-repeat- and pyrin-domain-containing 3/caspase-1/interleukin-1β pathway in a mouse model of Parkinson’s disease induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Therefore, these two drugs may serve as novel anti-inflammatory treatments for Parkinson’s disease.
Introduction
Parkinson’s disease (PD) is the second most prevalent neurodegenerative disease worldwide after Alzheimer’s disease (Parkinson, 2002; Ascherio and Schwarzschild, 2016). The pathological hallmarks of PD are progressive death of dopaminergic neurons and aggregation of misfolded α-synuclein (α-syn) to form eosinophilic inclusions called Lewy bodies or Lewy neurites in resident neurons (Halliday et al., 2011). PD mainly causes symptomatic motor dysfunction, including bradykinesia, muscle rigidity, resting tremor, and even postural instability. Nonmotor symptoms including constipation, depression, anxiety, and disordered rapid eye movement sleep can also occur with disease progression (Schapira et al., 2017; Armstrong and Okun, 2020). Currently, PD treatment is mainly symptomatic, and there is no cure.
Neuroinflammation plays a key role in PD (Calabrese et al., 2018; Li et al., 2022). Microglia are the prominent immune cells in the central nervous system and have been shown to be largely activated in the postmortem brain tissue of PD patients (Imamura et al., 2003; Ransohoff and Cardona, 2010). Under different stimuli, resting microglia polarize into one of two phenotypes, namely the classic pro-inflammatory M1 phenotype, or the alternative anti-inflammatory M2 phenotype (Tang and Le, 2016; Kwon and Koh, 2020). In cellular and animal models of PD, microglia are prone to polarization into the M1 phenotype (Joers et al., 2017). Moreover, excessive activation of the nucleotide-binding oligomerization domain- and leucine-rich-repeat- and pyrin-domain-containing 3 (NLRP3) inflammasome has been observed in the brains of PD animal models and patients with PD (Sarkar et al., 2017; Gordon et al., 2018). NLRP3 belongs to NOD-like receptor (NLR) family. Upon sensing danger-associated molecular patterns or pathogen-associated molecular patterns, NLRP3 recruits pro-caspase-1 and apoptosis-associated speck-like protein containing a caspase recruitment domain to assemble the NLRP3 complex. Inactive pro-caspase-1 is then cleaved into mature, active caspase-1, which further cleaves pro-interleukin-18 and pro-interleukin-1β (pro-IL-1β) to mature interleukin-18 (IL-18) and interleukin-1β (IL-1β), respectively (Guo et al., 2015). However, inhibition of NLRP3 activation can prevent dopaminergic neuron degeneration and ameliorate α-syn pathology (Gordon et al., 2018; Ou et al., 2021). Therefore, targeting microglial polarization and the NLRP3 inflammasome could help treat PD (Haque et al., 2020).
The incretins include glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 (GLP-1). After meals, GLP-1 promotes insulin secretion by pancreatic β cells and inhibits glucagon secretion by pancreatic α cells in a glucose-dependent manner. It also delays gastric emptying through stimulation of vagus nerve and increases the sensation of satiety at the hypothalamus level, thus reducing food intake (Nauck and Meier, 2016). GLP-1 and glucose-dependent insulinotropic polypeptide have a short half-life of only 2–3 minutes in vivo and are quickly degraded by dipeptidyl peptidase 4 (DPP4) into inactive metabolites. Competitive inhibition of DPP4 can prolong the half-life of incretins and enhance their bioavailability and biological function (Drucker, 2007; Jose and Inzucchi, 2012). In addition to its normal physiological function in regulating glucose homeostasis in diabetes treatment, the role of GLP-1 in the central nervous system has been widely studied. GLP-1 receptor agonists (GLP-1Ra) have been revealed to be neuroprotective in some animal models of Alzheimer’s disease, PD, stroke, and multiple sclerosis (Hölscher, 2018; Lee et al., 2018; Shan et al., 2019; Hölscher, 2020). Although activation of the GLP-1 receptor has been demonstrated to reverse motor deficits in PD animal models, the underlying mechanisms remain unknown (Harkavyi et al., 2008).
Our study aimed to explore the effects of the GLP-1Ra exendin-4 and the DPP4 inhibitor (DPP4i) linagliptin in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced mouse model of subacute PD to assess whether they can attenuate neuroinflammation and investigate the potential mechanisms underlying this effect.
Methods
Animals and design
Wild-type 8-week-old C57BL/6J male mice, weighing 22–26 g, were purchased from the Beijing Huafukang Company (license No. SCXK2019-0008). All mice were housed under a 12/12-hour light/dark cycle with standard humidity (55 ± 3%) and temperature (23 ± 3°C) conditions, with five mice to a cage. The animal experiments were approved by the Animal Medical Ethics Committee of Shengjing Hospital of China Medical University on February 21, 2019 (approval No. 2019PS025K). All experiments were designed and reported according to Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines (Percie du Sert et al., 2020).
The MPTP-induced subacute PD mouse model established in male mice, as described previously, because male mice are more sensitive to MPTP than are female mice (Zhang et al., 2017; Mustapha and Mat Taib, 2021). Sixty C57BL6/J mice were randomly assigned to one of four groups (n = 15 per group): normal control group (CON), MPTP model group (MPTP), exendin-4 treatment group (MPTP+EX-4), and linagliptin treatment group (MPTP+LINA). Exendin-4 (MCE, Shanghai, China) and linagliptin (MCE) were dissolved in dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO, USA) to make the stock solutions (100 μg/mL exendin-4 and 10 mg/mL linagliptin). MPTP (Sigma-Aldrich) was injected intraperitoneally at a dose of 30 mg/kg per day, exendin-4 was injected subcutaneously at the back of the neck at a dose of 50 μg/kg per day, linagliptin was injected subcutaneously at the back of the neck at 5 mg/kg per day, and the mice in the normal control group received the same volume of solvent (5 μL/g) subcutaneously at the back of the neck (10% dimethyl sulfoxide, 40% PEG300 (MCE), 5% Tween-80 (MCE), and 45% normal saline (Dazhong, Tianjin, China)) (Kabel et al., 2018; Zhang et al., 2021). The mice were acclimated to the animal housing facility for 7 days before the experiment began. The two drugs were administered for 14 days, and behavioral training was conducted for the first 3 days. MPTP was administered on days 4–8, and behavioral tests were performed on days 9–14; the drugs were administered continuously throughout this period. On the 15th day, mice were sacrificed for follow-up experiments (Zhang et al., 2017; Wang et al., 2019). The experimental design is illustrated in Figure 1A.
Figure 1.
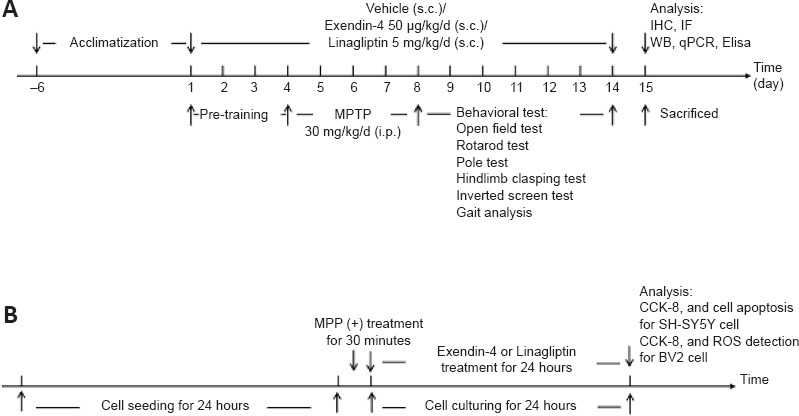
Experimental design.
(A) The timeline for the in vivo experiment. (B) The timeline for the in vitro experiment. CCK-8: Cell counting kit-8; Elisa: enzyme-linked immunosorbent assay; IF: immunofluorescence; IHC: immunohistochemistry; MPP (+): 1-methyl-4-phenylpyridium; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; qPCR: quantitative polymerase chain reaction; ROS: reactive oxygen species; WB: western blot.
SH-SY5Y cell and BV2 cell culture
SH-SY5Y cell was usually used to simulate dopaminergic neurons, and BV2 cell was usually used to simulate microglia. A human neuroblastoma cell line (SH-SY5Y) (Cat# CL-0208, RRID: CVCL-0019) and a mouse microglial cell line (BV2) (Cat# CL-0493, RRID: CVCL-0182) were kindly provided by Procell Life Science&Technology Co., Ltd. (Wuhan, Hubei, China). The identities of the SH-SY5Y and BV2 cell lines were verified by short tandem repeat analysis. The cells were cultured in Dulbecco’s modified Eagle medium/F12 and Dulbecco’s modified Eagle medium (high glucose) medium, respectively, supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin (Gibco, Waltham, MA, USA), at 37°C with 5% CO2 in a humidified incubator (Thermo Fisher Scientific, Waltham, MA, USA). The timeline for the in vitro experiment is shown in Figure 1B.
Behavioral assessment
Open field test
The open field test can detect the spontaneous motor activity and exploratory behaviors of mice. Mice were acclimated to the environment before testing. During the formal experiment on day 10, the mice were placed in a white box (50 cm × 50 cm × 40 cm) and allowed to run freely for 10 minutes. Computer software (SMART V3.0 Software, Harvard Apparatus, Holliston, MA, USA) was used to record the total distance moved by the mice, the total time spent in the middle of the box, and number of times each mouse reared up on its hind legs (Zhang et al., 2018).
Rotarod test
The rotarod test was used to evaluate the motor coordination of the mice on day 12. The black sensor plate was placed horizontally at the bottom of the rotating rod device (IITC Life Science, Woodland Hills, CA, USA), and the mice were placed in individual channels on the rotating rod. On clicking START, the rotation bar began to rotate, and the speed gradually increased from 0 to 40.18 r/min. The time at which each mouse fell from the rotating rod was recorded. The rod automatically stopped rotating at 300 seconds. For mice that did not fall, the time recorded was 300 seconds (Deacon, 2013).
Pole test
The pole test was used to evaluate the motor coordination of the mice on day 14. A metal rod (50 cm long and 8 mm in diameter) was fixed vertically to a metal base and wrapped with gauze, and a small ball with a diameter of 1 cm was installed at the top of the metal rod. Each mouse was lifted by its tail and placed head up on the small ball at the top of the pole. The mouse was released, and the time spent to climb down from top to bottom of the pole was recorded (Deacon, 2013).
Inverted screen test
The inverted screen test was used to estimate the muscle strength of the mice on day 9. A 43 cm × 43 cm screen was constructed from 12 mm × 12 mm wire mesh. Each mouse was placed in the middle of the mesh, the mesh was turned upside down so that the mouse was on the hanging downward, and the timer was started. The screen was held 40–50 cm above a protective surface, and the time it took each mouse to fall was recorded. Each mouse then received a score based on the time it took to fall: 1–10 seconds = 1 point, 11–25 seconds = 2 points, 26–60 seconds = 3 points, 61–90 seconds = 4 points, and greater than 90 seconds = 5 points (Deacon, 2013).
Hindlimb clasping test
The hindlimb clasping test reflects the progression of neurodegenerative diseases. To perform this test on day 8, each mouse was grasped by its tail and lifted away from all surrounding objects. The position of the hindlimbs was observed for 10 seconds. If both hindlimbs were always spread outwards from the abdomen, the score was 0. If one hindlimb moved toward the abdomen for more than half of the suspension time, the score was 1; if both hindlimbs moved towards the abdomen for more than half of the suspension time, the score was 2; if both hindlimbs were fully retracted and touched the abdomen, the score was 3 (Guyenet et al., 2010).
Gait analysis
To observe differences in gait on day 11, the mice were placed at the beginning of a black track under quiet and non-stimulating conditions and allowed to walk freely through the track under weak red light. High-frequency cameras were used to record the gait of the mice, and computer software was used to calculate maximum amplitude of change in step length, the time taken for passing through the track, average speed, and step sequence (Catwalk System Software XT8.0, Noldus, Wageningen, the Netherlands) (Geldenhuys et al., 2015).
Tissue preparation
After the behavioral tests were performed, all mice were anesthetized with 1% sodium pentobarbital (Shangyao, Shanghai, China) by intraperitoneal injection at a dose of 50 mg/kg. Then, the mice were perfused with normal saline. Half of the mice were then perfused and fixed with 4% paraformaldehyde for 24 hours at 4°C. Next, the brains were dehydrated with 30% sucrose. Sequential coronal sections (30 µm thick) were cut into continuous slices using a sliding microtome (SM2010R, Leica, Deer Park, IL, USA) for histological analysis. The other half of the samples were quick-frozen and stored at −80°C for molecular biological experiments.
Immunohistochemistry
To observe the morphology and number of dopaminergic neurons, microglia, and astrocytes, sections of the striatum and substantia nigra (SN) were heated in a 60°C water bath in citrate buffer (pH 6.0) for antigen retrieval for 30 minutes (Baquet et al., 2009). They were then incubated with 3% H2O2 for 10 minutes to inactivate endogenous peroxidase, followed by incubation with 5% goat serum (Solarbio, Beijing, China) for 1 hour to block non-specific binding sites. Next, the sections were placed on a horizontal shaker and incubated with rabbit anti-TH (1:500, Proteintech, Chicago, IL, USA, Cat# 25859-1-AP, RRID: AB_2716568), rabbit anti-ionized calcium binding adapter molecule 1 (Iba1; 1:500, Wako, Osaka, Japan, Cat# 019-19741, RRID: AB_839504), and rabbit anti-glial fibrillary acidic protein (GFAP; 1:500, Abcam, Cambridge, UK, Cat# ab7260, RRID: AB_305808) primary antibodies at 4°C overnight, followed by biotin-conjugated goat anti-rabbit secondary antibody (1:500, Vector Laboratories, Burlingame, CA, USA, Cat# BA-1000, RRID: AB_2313606) at 25°C for 90 minutes. Finally, the sections were stained with diaminobenzidine and imaged using a light microscope (Nikon, Tokyo, Japan). For cell counting, ImageJ software (V1.8.0; National Institutes of Health, Bethesda, MD, USA) (Schneider et al., 2012) was used to calculate all TH-positive neurons at 100× magnification, as well as Iba1-positive and GFAP-positive cells at 200× magnification, per section in the same region of the SN (Zhang et al., 2017).
Immunofluorescence
Briefly, brain sections were incubated in citrate buffer at 60°C for 30 minutes and in 5% goat serum at room temperature for 1 hour. The sections were then incubated with rabbit anti-CD206 primary antibody (a marker for M2 microglia; 1:500, Abcam, Cat# ab64693, RRID: AB_1523910) at 4°C overnight and Cy3 goat anti-rabbit secondary antibody (1:500, Abcam, Cat# ab6939, RRID: AB_955021) at room temperature for 1 hour. Finally, anti-fluorescence quenching sealing tablets containing 4,6-diamidino-2-phenylindole were used to stain the nuclei, and fluorescent images were taken using a fluorescence microscope (Nikon, Tokyo, Japan).
BV2 cells were treated with the drugs for 24 hours, and then were fixed with 4% paraformaldehyde for 20 minutes, followed by permeabilization and blocking with 0.1% Triton X-100 and 5% donkey serum (Solarbio) at room temperature for 1 hour. The cells were then incubated with rabbit anti-NLRP3 antibody (1:500, Abcam, Cat# ab214185, RRID: AB_2819003) at 4°C overnight followed by incubation with Alexa Fluor 488-conjugated donkey anti-rabbit secondary antibody (1:500, Abcam, Cat# ab150073, RRID: AB_2636877) at room temperature for 2 hours. Finally, 4,6-diamidino-2-phenylindole was used to stain the nuclei, and fluorescent images were taken. The level of NLRP3 was observed using a fluorescence microscope.
Western blot assay
To determine the expression levels of proteins in dopaminergic neurons and the NLRP3 inflammasome pathway, mouse SN tissue was homogenized via sonication in radioimmunoprecipitation assay buffer containing 1% protease and 1% phosphatase inhibitors. Next, the homogenate was centrifuged at 16,000 × g at 4°C for 30 minutes, and the protein concentration of the supernatant was quantified using a bicinchoninic acid method (Takara, Shiga, Japan) (Cortés-Ríos et al., 2020). Then, the supernatant was mixed with a 5× loading buffer and denatured in a water bath at 95°C for 10 minutes. Next, samples with the same amount of protein (20 µg) were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Solarbio), and the proteins were transferred to polyvinylidene fluoride membranes (Millipore, Burlington, MA, USA). After blocking with 5% skim milk in Tris-buffered saline with 0.1% Tween-20 at room temperature for 1 hour, the membranes were incubated with a mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primary antibody (1:20,000, Proteintech, Cat# 60004-1-Ig, RRID: AB_2107436) as an internal control, and rabbit anti-tyrosine hydroxylase (TH; 1:1000, Proteintech, Cat# 25859-1-AP, RRID: AB_2716568), rabbit anti-dopamine transporter (DAT; 1:1000, Proteintech, Cat# 22524-1-AP, RRID: AB_2879116), rabbit anti-NLRP3 (1:1000, Abcam, Cat# ab214185, RRID: AB_2819003), rabbit anti-caspase-1 (1:1000, Proteintech, Cat# 22915-1-AP, RRID: AB_2876874), rabbit anti-IL-1β (1:1000, Abcam, Cambridge, UK, Cat# ab9722, RRID: AB_308765), and rabbit anti-gasdermin D (GSDMD; 1:1000, Cell Signaling Technology, Danvers, MA, USA, Cat#96458, RRID: AB_2894914) antibodies at 4°C overnight. Then, the membranes were washed with Tris-buffered saline containing 0.1% Tween-20 three times and incubated with horseradish peroxidase-conjugated goat anti-mouse (1:7500, Proteintech, Cat# SA00001-1, RRID: AB_2722565) and goat anti-rabbit (1:7500, Proteintech, Cat# SA00001-2, RRID: AB_2722564) secondary antibodies at room temperature for 2 hours. Finally, the protein bands were detected by enhanced chemiluminescence. The band intensities were standardized to that of the GAPDH signal using ImageJ software.
Quantitative polymerase chain reaction
To detect the level of pro-inflammatory and anti-inflammatory cytokines, total RNA from SN tissue was isolated using TRIzol (Invitrogen, Waltham, MA, USA). Reverse transcription of total RNA to complementary DNA and quantitative polymerase chain reaction (qPCR) analyses were performed using TaKaRa kits (Shiga, Japan). The standard procedure for two-step qPCR was as follows: 95°C for 30 seconds, followed by 95°C for 5 seconds and 60°C for 30 seconds for 40 cycles. Relative mRNA expression levels were quantified using the 2–ΔΔCt method and normalized to a reference gene (GAPDH) (Rao et al., 2013). Primers were synthesized by Shenggong (Shanghai, China). The primer sequences for the inflammatory cytokines used in this study are shown in Table 1.
Table 1.
Primer sequences of genes used in quantitative polymerase chain reaction
| Gene | Species | Sequence (5’–3’) |
|---|---|---|
| GAPDH | Mouse | Forward: TGT GTC CGT CGT GGA TCT GA |
| Reverse: CCT GCT TCA CCA CCT TCT TGA | ||
| IL-1β | Mouse | Forward: TGT CCT GAT GAG AGC ATC C |
| Reverse: AAG GTC CAC GGG AAA GAC | ||
| TNF-α | Mouse | Forward: CGC TGA GGT CAA TCT GC |
| Reverse: GGC TGG GTA GAG AAT GGA | ||
| iNOS | Mouse | Forward: TCT TTG ACG CTC GGA ACT |
| Reverse: ATG GCC GAC CTG ATG TT | ||
| IL-10 | Mouse | Forward: GCC CTT TGC TAT GGT GTC |
| Reverse: TCT CCC TGG TTT CTC TTC C | ||
| TGF-β | Mouse | Forward: GCA ACA ATT CCT GGC GTT A |
| Reverse: TTC CGT CTC CTT GGT TCA G | ||
| Arg1 | Mouse | Forward: GGC AAG GTG ATG GAA GAG |
| Reverse: AAA GCT CAG GTG AAT CGG |
Arg1: Arginase 1; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; IL-10: interleukin-10; IL-1β : interleukin-1β; iNOS: inducible nitric oxide synthase; TGF-β : transforming growth factor-β; TNF-α: tumor necrosis factor-α.
Enzyme-linked immunosorbent assay
Lactate dehydrogenase (LDH) levels were measured to assess the cell death. Immediately after the mice were anesthetized with 1% sodium pentobarbital on day 15 of the experiment, peripheral blood was collected from the eyeballs and centrifuged at 14,000 × g at 4°C for 10 minutes to isolate the serum. LDH levels were tested using a commercial LDH enzyme-linked immunosorbent assay kit (Meimian, Yancheng, China) in accordance with the manufacturer’s instructions. The LDH concentration was calculated using a multi-functional microplate reader (BioTeke, Beijing, China) at 450 nm with reference to the standard curve.
Cell viability analysis
Cell viability was assessed by cell counting kit-8 (CCK-8; Shangbao, Shanghai, China). SH-SY5Y and BV2 cells were seeded into a 96-well plate and cultured for 24 hours. Then, the cells were treated with 1, 10, 100 nM, and 1 µM exendin-4 and linagliptin for 30 minutes, followed by treatment with 3 mM 1-methyl-4-phenylpyridium (MPP (+); (Sigma-Aldrich). After culturing for 24 hours, 10 µL enhanced CCK-8 reagent diluted in 100 µL serum-free medium was added to each well. Then, the 96-well plate was incubated at 37°C for 2 hours. The optical density (OD) value was measured at 490 nm using a microplate reader. Cell viability (%) = (OD of treated cells – blank OD)/(OD of control cells – blank OD) × 100.
Cell apoptosis assay
Cell apoptosis was detected using an annexin V-fluorescein isothiocyanate/propidium iodide (PI) kit (BD Bioscience, Franklin Lakes, NJ, USA). SH-SY5Y cells were cultured in 6-cm dishes and treated with 100 nM exendin-4 or 100 nM linagliptin and 3 mM MPP (+) for 24 hours. Then, the cells were collected and stained with annexin V-fluorescein isothiocyanate and PI. Finally, cell apoptosis was measured by flow cytometry (BD Bioscience). Annexin V (+) and PI (–) cells (Q4) were defined as early apoptotic cells, while annexin V (+) and PI (+) cells (Q2) were defined as late apoptotic cells.
Reactive oxygen species measurement
Intracellular reactive oxygen species (ROS) production was detected using a ROS Detection Kit (KeyGEN BioTECH, Nanjing, China). After the BV2 cells were treated with the drugs, a 2,7-dichlorodihydrofluorescin diacetate (20 μM) fluorescent probe was added to the cells, which were then incubated at 37°C for 20 minutes. The 2,7-dichlorodihydrofluorescin diacetate was oxidized by ROS into 2,7-dichlorofluorescein. The cells were washed with warm phosphate-buffered saline three times and then collected. The average 2,7-dichlorofluorescein fluorescence intensity was quantified by flow cytometry.
Statistical analysis
No statistical methods were used to predetermine sample sizes for the in vivo study; however, our sample sizes were similar to those reported in a previous publication (Zhang et al., 2017). GraphPad Prism 8.0.2 (GraphPad Software, San Diego, CA, USA, www.graphpad.com) was used for statistical analysis and graphical analysis. The raters were blinded to the group assignments. Data are presented as mean ± standard deviation (SD). Multiple groups were compared by one-way analysis of variance followed by Tukey’s post hoc test. P < 0.05 was considered statistically significant.
Results
Exendin-4 and linagliptin reverse motor dysfunction in an MPTP-induced mouse model of PD
The open field test detects the spontaneous motor activity and exploratory behaviors of mice (Zhang et al., 2018), and the results of the open field test showed that exendin-4 and linagliptin treatment increased the total distance moved and the number of times each mouse reared up on its hind legs, but had no effect on the time in the central area, compared with the MPTP group (Figure 2A–D). These results indicate that the two drugs increased autonomous movement and exploration behavior and improved anxiety and depression in mice. Next, the rotarod and pole tests were used to evaluate motor coordination (Deacon, 2013). The results showed that the MPTP group spent less time on the rotating rod, whereas the exendin-4 and linagliptin treatment groups spent more time on the rotating rod, compared with the MPTP group, suggesting that the two drugs ameliorated the decrease in motor coordination caused by MPTP. The pole test showed that the MPTP group took a longer time to climb down from top to bottom, and neither of the drugs reduced the descent time (Figure 2E and F). The inverted screen test was used to estimate the muscle strength of the mice (Deacon, 2013). The results demonstrated that the mice in the MPTP group fell earlier, and neither exendin-4 nor linagliptin increased the grip time (Figure 2G). The hindlimb clasping test reflects the progression of neurodegenerative diseases (Guyenet et al., 2010). Mice in the normal control group kept their hindlimbs fully extended throughout the test, whereas mice in the MPTP group had their hindlimbs almost completely retracted and touching their abdomen. The retraction of one or both hindlimbs observed in the exendin-4 and linagliptin treatment groups suggests that the two drugs slowed the progression of PD in this model (Figure 2H and Additional Figure 1A (1.3MB, tif) ). We then analyzed the gait of the mice (Geldenhuys et al., 2015). Gait analysis showed that exendin-4 and linagliptin decreased the MPTP-induced increase in maximum running variation (Figure 2I and Additional Figure 1B (1.3MB, tif) ).
Figure 2.
Effects of exendin-4 and linagliptin on mouse behavior.
(A) Representative locomotor activity tracking map in the OFT. Blue boxes indicate the total area, while green boxes indicate the central area. (B–D) Total movement distance, time spent in the central area, and number of times that mice reared up on their hind legs in the OFT (n = 10). (E) Latency in the rotarod test (n = 15). (F) Latency in the pole test (n = 15). (G) Muscle strength score for the inverted screen test (n = 15). (H) Hindlimb clasping test score (n = 15). (I) Maximum running variation from the gait analysis (n = 10). Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, vs. CON; #P < 0.05, ####P < 0.0001, vs. MPTP (one-way analysis of variance followed by Tukey’s post hoc test). CON: Control; EX-4: exendin-4; LINA: linagliptin; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; OFT: open field test.
Exendin-4 and linagliptin reduce dopaminergic neuron death induced by MPTP
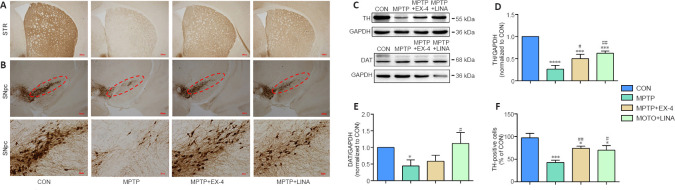
One of the hallmark pathological changes in patients with PD is the progressive death of dopaminergic neurons. TH is the key enzyme involved in dopamine synthesis and can be used as a biomarker for dopaminergic neurons . Immunohistochemical staining for TH-positive cell bodies in the SN pars compacta and cell terminals in the striatum showed a severe loss of dopaminergic neurons caused by MPTP. However, treatment with exendin-4 or linagliptin protected against dopaminergic neuron loss (Figure 3A and B). Furthermore, the western blot results demonstrated that TH and DAT expression levels were decreased in the SN tissue of mice in the MPTP group, whereas a significantly restoration of TH and DAT expression was observed in the exendin-4 and linagliptin treatment groups (Figure 3C–E). Finally, immunohistochemistry-based quantification of TH-positive cells in the SN pars compacta demonstrated that MPTP-induced dopaminergic neuron death in the SN pars compacta was attenuated in the exendin-4 and linagliptin treatment groups (Figure 3F).
Figure 3.
Effects of exendin-4 and linagliptin on dopaminergic neurons in the SN and cell terminals in the STR.
(A, B) Immunohistochemical staining for TH in the SNpc and STR. The MPTP group showed weaker staining for TH than the CON group, while the MPTP + EX-4 and MPTP + LINA groups showed stronger staining for TH than the MPTP group. Scale bar: 200 µm, 40 µm for low and high magnification, respectively. (C–E) Western blot assay for TH and DAT expression in SN tissue. (F) Quantitation of TH-positive cells (/100-fold magnification) in the SNpc as detected by immunohistochemistry. Data are presented as mean ± SD (n = 3). *P < 0.05, ***P < 0.001, ****P < 0.0001, vs. CON; #P < 0.05, ##P < 0.01, vs. MPTP (one-way analysis of variance followed by Tukey’s post hoc test). CON: Control; DAT: dopamine transporter; EX-4: exendin-4; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; LINA: linagliptin; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; SNpc: substantia nigra pars compacta; STR: striatum; TH: tyrosine hydroxylase.
Exendin-4 and linagliptin inhibit MPTP-induced activation of microglia and astrocytes
Chronic neuroinflammation plays a key role in dopaminergic neuron death (Calabrese et al., 2018). To study the effects of exendin-4 and linagliptin on neuroinflammation, we conducted immunohistochemical staining for Iba1 and GFAP, which label microglia and astrocytes, respectively. The results showed that in the SN, especially in the SN reticular region, the number of microglia and astrocytes in the MPTP group was significantly increased, and the cell morphology was consistent with activation (Krashia et al., 2019). Activated microglia appear amoeba-like, with enlarged cell bodies and unclear branches. Activated microglia can further activate astrocytes, resulting in neurotoxicity (Liddelow et al., 2017; Figure 4A and B). However, the number of microglia and astrocytes was markedly decreased in both the exendin-4 and the linagliptin treatment groups (Figure 4C and D).
Figure 4.

Effects of exendin-4 and linagliptin on activation of microglia and astrocytes in the SN.
(A) Immunohistochemical staining for the microglia marker Iba1 in the SN. Activated microglia appear amoeba-like, with enlarged cell bodies, in the MPTP group, and the number of this type of cell is decreased in the MPTP + EX-4 and MPTP + LINA groups. (B) Immunohistochemical staining for the astrocyte marker GFAP in the SN. Activated astrocytes have unclear branches in the MPTP group, and the number of this type of cell is decreased in the MPTP + EX-4 and MPTP + LINA groups. Scale bar: 80 µm, 20 µm for low and high magnification, respectively. (C) Quantitation of Iba1-positive cells in the SN. (D) Quantitation of GFAP-positive cells in the SN. Data are shown as mean ± SD (n = 3). **P < 0.01, ***P < 0.001, ****P < 0.0001, vs. CON; #P < 0.05, ##P < 0.01, vs. MPTP (one-way analysis of variance followed by Tukey’s post hoc test). CON: Control; EX-4: exendin-4; GFAP: glial fibrillary acidic protein; Iba-1: ionized calcium binding adapter molecule 1; LINA: linagliptin; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; SN: substantia nigra.
Exendin-4 and linagliptin induce polarization of microglia to the M2 phenotype in an MPTP-induced mouse model of PD
Under normal conditions, microglia remain in a resting state. When exposed to different external stimuli, microglia can polarize into the M1 or M2 phenotype (Pisanu et al., 2014; Joers et al., 2017). Immunofluorescence staining was performed for CD206, a surface biomarker of M2 microglia. The results showed that the number of anti-inflammatory M2 microglia was clearly reduced in the SN of mice in the MPTP group, whereas the number of anti-inflammatory M2 microglia was increased in the exendin-4 and linagliptin treatment groups (Figure 5A and B).
Figure 5.
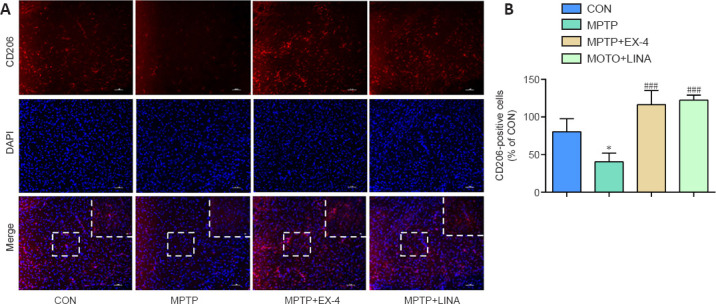
Effects of exendin-4 and linagliptin on microglial polarization in the SN.
(A) Immunofluorescence staining for the M2 phenotype microglia marker CD206 (red, Cy3) in the SN. The intensity of the CD206 signal is weaker in the MPTP group and stronger in the MPTP + EX-4 and MPTP + LINA groups. Scale bar: 50 µm, 20 µm for low and high magnification, respectively. (B) Quantitation of CD206-positive microglia in the SN. Data are shown as mean ± SD (n = 3). *P < 0.05, vs. CON; ###P < 0.001, vs. MPTP (one-way analysis of variance followed by Tukey’s post hoc test). CON: Control; DAPI: 4,6-diamidino-2-phenylindole; EX-4: exendin-4; LINA: linagliptin; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; SN: substantia nigra.
Exendin-4 and linagliptin reduce proinflammatory cytokine secretion and increase anti-inflammatory cytokine secretion in an MPTP-induced mouse model of PD
We performed qPCR analysis of RNA extracted from the SN and found that exendin-4 and linagliptin reduced the mRNA expression levels of pro-inflammatory cytokines, such as IL-1β and tumor necrosis factor-α (TNF-α) (Figure 6A and B), and increased the mRNA expression levels of secreted anti-inflammatory cytokines, including interleukin-10 (IL-10) and transforming growth factor-β (TGF-β) (Figure 6C and D; Pisanu et al., 2014). In addition, the expression levels of iNOS, an M1 microglial marker, were significantly decreased by the two drugs, whereas the expression levels of Arg1, an M2 microglial marker, were increased by linagliptin (Figure 6E and F), which further indicated that exendin-4 and linagliptin can induce the transformation of resting microglia from the pro-inflammatory M1 phenotype to the anti-inflammatory M2 phenotype, thus inhibiting the neuroinflammation induced by MPTP. Notably, linagliptin appeared to have a stronger anti-inflammatory effect than exendin-4 based on the qPCR results.
Figure 6.
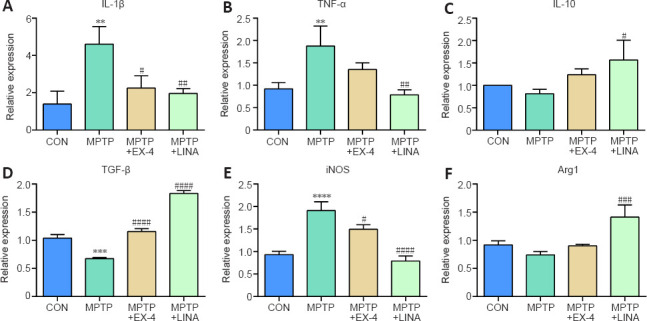
Effects of exendin-4 and linagliptin on the expression of inflammatory cytokines in the SN.
(A, B) mRNA levels of the pro-inflammatory cytokines IL-1β and TNF-α in the SN. (C, D) mRNA levels of the anti-inflammatory cytokines IL-10 and TGF-β in the SN. (E, F) mRNA levels of iNOS, a marker of M1 microglia, and Arg1, a marker of M2 microglia, in the SN. Relative expression was normalized to the CON group. Data are shown as mean ± SD (n = 3). **P < 0.01, ***P < 0.001, ****P < 0.0001, vs. CON; #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001, vs. MPTP (one-way analysis of variance followed by Tukey’s post hoc test). Arg1: Arginase 1; CON: control; EX-4: exendin-4; IL-10: interleukin-10; IL-1β: interleukin-1β; iNOS: inducible nitric oxide synthase; LINA: linagliptin; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; SN: substantia nigra; TGF-β: transforming growth factor-β; TNF-α: tumor necrosis factor-α; SN: substantia nigra.
Exendin-4 and linagliptin inhibit the NLRP3/caspase-1/IL-1β pathway in the SN of an MPTP-induced mouse model of PD
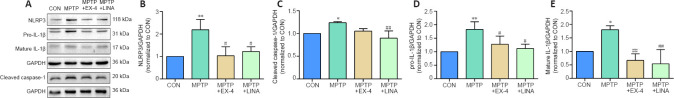
To further explore the mechanism by which exendin-4 and linagliptin inhibited neuroinflammation, we explored the effects of both treatments on the NLRP3 inflammasome signaling pathway by western blot. The expression levels of NLRP3, active cleaved caspase-1, pro-IL-1β, and mature IL-1β were found to have increased in the SN of mice in the MPTP group. However, after exendin-4 and linagliptin treatment, NLRP3, cleaved caspase-1, pro-IL-1β, and IL-1β protein expression levels decreased (Figure 7A–E). These results indicate that exendin-4 and linagliptin suppress MPTP-induced NLRP3/caspase-1/IL-1β pathway activation.
Figure 7.
Effects of exendin-4 and linagliptin on the NLRP3/caspase-1/IL-1β pathway in the SN of Parkinson’s disease mice.
(A) Representative western blot image of NLRP3, pro-IL-1β, mature IL-1β, and cleaved caspase-1 expression in the SN. (B–E) Quantification of NLRP3, cleaved caspase-1, pro-IL-1β, and mature IL-1β protein expression levels. Data are shown as mean ± SD (n = 3). *P < 0.05, **P < 0.01, vs. CON; #P < 0.05, ##P < 0.01, vs. MPTP (one-way analysis of variance followed by Tukey’s post hoc test). CON: Control; EX-4: exendin-4; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; IL-1β: interleukin-1β; LINA: linagliptin; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; NLRP3: nucleotide-binding oligomerization domain- and leucine-rich-repeat- and pyrin-domain-containing 3; SN: substantia nigra.
Exendin-4 and linagliptin attenuate pyroptosis in the SN of an MPTP-induced mouse model of PD
When the NLRP3 inflammasome is activated, mature IL-1β and IL-18 lead to the formation of holes in the cell membrane, causing the cells to swell, lyse, and die, a process known as pyroptosis, which is a form of inflammatory cell death (Lünemann et al., 2021). Pyroptosis is primarily mediated by gasdermins family proteins. Gasdermin D (GSDMD) is cleaved into C-terminal and N-terminal domains after NLRP3 activation. The C-terminal domain is the regulatory domain, and the N-terminal domain is responsible for the formation of holes in the cell membrane (Shi et al., 2017). We found that GSDMD N-terminus expression increased in the SN of mice in the MPTP group but decreased in the exendin-4 and linagliptin treatment groups, as determined by western blot (Figure 8A and B). LDH is released after cell death. We measured the LDH levels in the serum of mice by enzyme-linked immunosorbent assay and found that exendin-4 and linagliptin reduced MPTP-induced LDH production (Figure 8C). These results suggest that exendin-4 and linagliptin inhibit pyroptosis.
Figure 8.
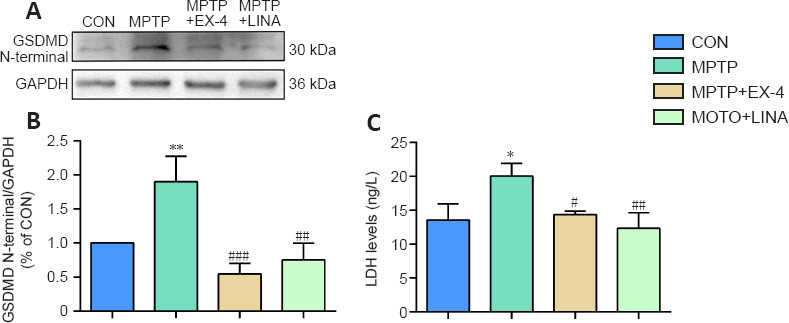
Effects of exendin-4 and linagliptin on pyroptosis in the SN of PD mice.
(A) Representative western blot image of GSDMD N-terminus expression in the SN. (B) Quantification of GSDMD N-terminus protein expression level. (C) LDH content in the serum of mice. Data are shown as mean ± SD (n = 3). *P < 0.05, **P < 0.01, vs. CON; #P < 0.05, ##P < 0.01, ###P < 0.001, vs. MPTP (one-way analysis of variance followed by Tukey’s post hoc test). CON: Control; EX-4: exendin-4; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; GSDMD: gasdermin D; LDH: lactate dehydrogenase; LINA: linagliptin; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; PD: Parkinson’s disease; SN: substantia nigra.
Exendin-4 and linagliptin do not directly inhibit MPP (+)-induced apoptosis of SH-SY5Y cells
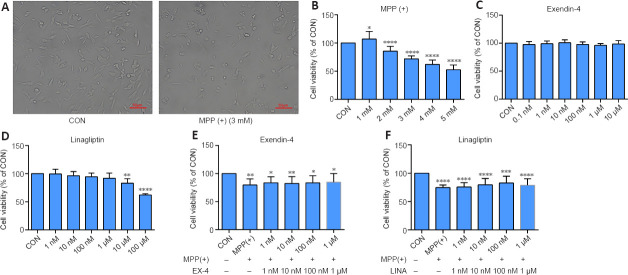
We used SH-SY5Y cells to simulate dopaminergic cells to establish PD models in vitro. The CCK-8 assay results showed that SH-SY5Y cell viability decreased to 70% when treated with 3 mM MPP (+) (Figure 9A and B), and thus this concentration was used in all subsequent experiments. Then, we determined the safe concentration range of exendin-4 and linagliptin for the rescue experiments (Figure 9C and D). Half an hour before adding 3 mM MPP (+) to the cells for the CCK-8 experiment, the drugs were added at different concentrations, and the cells were incubated for 24 hours. The results showed that neither of the two drugs directly rescued the decline in SH-SY5Y cell viability caused by MPP (+) (Figure 9E and F). To further explore the effects of exendin-4 and linagliptin on SH-SY5Y cells, we treated cells with the optimized concentrations of exendin-4 and linagliptin and assessed cell apoptosis by flow cytometry. The results showed that the two drugs had no effect on MPP (+)-induced late apoptosis, which was consistent with the results from the CCK-8 experiment. However, there was a significant increase in the number of early apoptotic cells in the MPP (+) group, and only 100 nM linagliptin slightly reduced the number of early apoptotic cells induced by MPP (+) (Figure 10).
Figure 9.
Effects of exendin-4 and linagliptin on the viability of SH-SY5Y cells treated with MPP (+).
(A) Representative bright field images of the effect of MPP (+) on SH-SY5Y cell morphology. SH-SY5Y cells shrink and died after treatment with 3 mM MPP (+). Scale bars: 50 µm. (B–D) The effect of MPP (+), exendin-4, and linagliptin on SH-SY5Y cell viability, as assessed by CCK-8. (E, F) The effect of exendin-4 and linagliptin on the MPP (+)-induced decrease in SH-SY5Y cell viability, as assessed by CCK-8. Data are expressed as mean ± SD. The experiment was repeated three times. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, vs. CON (one-way analysis of variance followed by Tukey’s post hoc test). CCK-8: Cell counting kit-8; CON: control; EX-4: exendin-4; LINA: linagliptin; MPP (+): 1-methyl-4-phenylpyridium.
Figure 10.
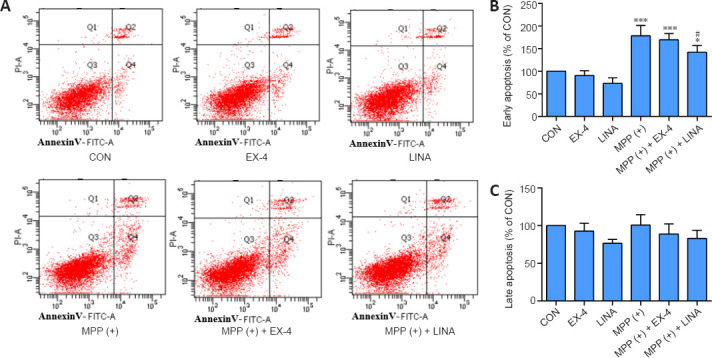
Effects of exendin-4 and linagliptin on MPP (+)-induced SH-SY5Y cell apoptosis.
(A) Apoptosis of SH-SY5Y cells was detected by flow cytometry. There was an increase in early apoptosis in the MPP (+) group, and a decrease in the MPP (+) + EX-4 and MPP (+) + LINA groups. (B) Quantitative analysis of early apoptosis (Q4) of SH-SY5Y cells. (C) Quantitative analysis of late apoptosis (Q2) of SH-SY5Y cells. Data are shown as mean ± SD. The experiment was repeated three times. *P < 0.05, ***P < 0.001, vs. CON; #P < 0.05, vs. MPP (+) (one-way analysis of variance followed by Tukey’s post hoc test). CON: control; EX-4: exendin-4; FITC: fluorescein isothiocyanate; LINA: linagliptin; PI: propidium iodide; MPP (+): 1-methyl-4-phenylpyridium.
Exendin-4 and linagliptin inhibit NLRP3 inflammasome expression by reducing MPP (+)-induced ROS production by BV2 cells
To further investigate the anti-inflammatory properties of exendin-4 and linagliptin in vitro, we detected NLRP3 expression in BV2 cells. MPP (+) treatment significantly increased NLRP3 expression in BV2 cells, while exendin-4 and linagliptin inhibited NLRP3 expression (Figure 11). Then, we used flow cytometry to analyze intracellular ROS levels in BV2 cells. MPP (+) is the toxic metabolite of MPTP, and MPP (+) treatment significantly increases ROS accumulation in BV2 cells (Schildknecht et al., 2017). However, co-treatment with exendin-4 or linagliptin significantly reduced the ROS levels (Figure 12 and Additional Figure 2 (1.3MB, tif) ). Furthermore, intracellular ROS levels were lower in the linagliptin treatment group than in the exendin-4 treatment group.
Figure 11.
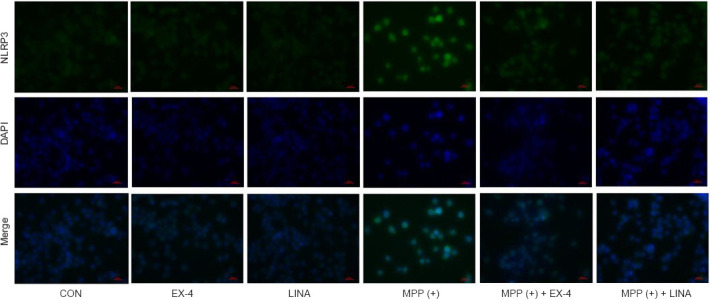
Effects of exendin-4 and linagliptin on MPP (+)-induced NLRP3 expression in BV2 cells.
Expression of NLRP3 (green, Alexa Fluor 488) in BV2 cells, as detected by immunofluorescence. The intensity of the NLRP3 signal was increased in the MPP (+) group and decreased in the MPP (+) + EX-4 and MPP (+) + LINA groups. Scale bar: 50 µm. CON: Control; DAPI: 4,6-diamidino-2-phenylindole; EX-4: exendin-4; LINA: linagliptin; MPP (+): 1-methyl-4-phenylpyridium; NLRP3: nucleotide-binding oligomerization domain- and leucine-rich-repeat- and pyrin-domain-containing 3.
Figure 12.
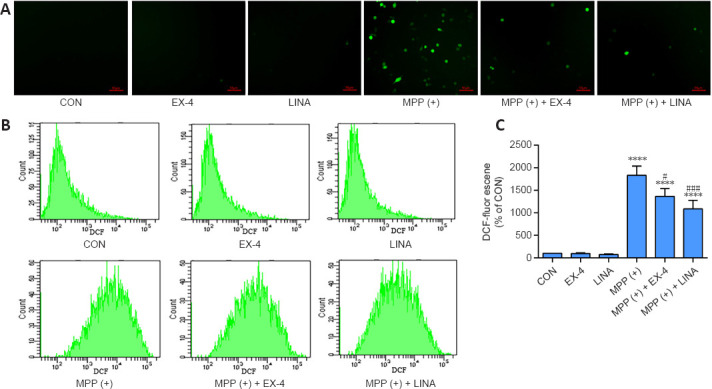
Effects of exendin-4 and linagliptin on MPP (+)-induced ROS production in BV2 cells.
(A) Fluorescence microscopy image of ROS (green) production in BV2 cells. The intensity of the ROS signal was increased in the MPP (+) group and decreased in the MPP (+) + EX-4 and MPP (+) + LINA groups. Scale bar: 50 µm. (B) ROS production in BV2 cells was measured using flow cytometry. (C) Quantitative analysis of ROS level (fluorescence intensity of DCF) in BV2 cells. Data are shown as mean ± SD. The experiment was repeated three times. ****P < 0.0001, vs. CON; #P < 0.05, ###P < 0.001, vs. MPP (+) (one-way analysis of variance followed by Tukey’s post hoc test). CON: Control; DCF: 2,7-dichlorofluorescein; EX-4: exendin-4; LINA: linagliptin; MPP (+): 1-methyl-4-phenylpyridium; ROS: reactive oxygen species.
Discussion
PD is one of the most prevalent neurodegenerative disorders, affecting 1% of people over 60 years of age worldwide (Tysnes and Storstein, 2017). PD is considered incurable, and no disease-modifying drugs are available (Alegre-Cortes et al., 2021). Epidemiological studies have recently proven that there is an association between PD and diabetes mellitus (DM). A meta-analysis by Chohan et al. (2021) showed that any type of DM can slightly increase the risk of PD, whereas type 2 DM significantly increases the morbidity of PD. Moreover, type 2 DM is associated with faster progression of motor dysfunction and cognitive impairment (Chohan et al., 2021). In addition, some common pathological changes are in both DM and PD, including misfolded protein aggregation, oxidative stress, mitochondrial dysfunction, and chronic inflammation (Camargo Maluf et al., 2019). Therefore, antidiabetic drugs can be used to treat PD (Qin et al., 2021).
GLP-1 plays a vital role in glucose and insulin homeostasis. Beyond their normal physiological function, GLP-1Ras have been demonstrated to be neuroprotective in the context of many neurological diseases (Li et al., 2009). The natural GLP-1 analogue exendin-4 and the long-acting GLP-1Ras lixisenatide and liraglutide have exhibited neuroprotective roles in several animal models of PD (Liu et al., 2015; Zhang et al., 2021); therefore, they may reverse motor dysfunction in PD models. GLP-1Ras are only partially neuroprotective, in that they protect dopaminergic neurons, increase neurotrophic factors, and preserve mitochondrial function (Erbil et al., 2019). However, in our study we found that exendin-4 cannot directly inhibit MPP (+)-induced SH-SY5Y cell apoptosis in vitro, in contrast to the findings from a previous study (Zhang et al., 2021). Therefore, we speculate that the neuroprotective role of exendin-4 is due to its attenuation of neuroinflammation. However, the underlying mechanisms by which exendin-4 regulates neuroinflammation remain unclear. So we used BV2 cells for further investigation.
DPP4is can enhance GLP-1 function and regulate glucose levels. In addition to their effect on the endocrinological system, DPP4is play a protective role in other systems, including the cardiovascular system and central nervous system, owing to their immunosuppressive functions (Mousa and Ayoub, 2019). In a rotenone-induced PD mouse model, vildagliptin, saxagliptin, sitagliptin, and alogliptin reduced dopaminergic neuron degeneration and improved motor performance (Abdelsalam and Safar, 2015; Nassar et al., 2015; Badawi et al., 2019; Safar et al., 2021). In our study, we showed that linagliptin clearly inhibits neuroinflammation, whereas only 100 nM linagliptin slightly reduced MPP (+)-induced early apoptosis of SH-SY5Y cells. These effects may be mediated directly by immunosuppression and indirectly by promoting an increase in GLP-1 secretion.
Age-related chronic inflammation is related to the pathogenesis and progression of neurodegenerative disorders. The NLRP3 inflammasome pathway plays a crucial role in neuroinflammation. In acute and chronic MPTP-induced PD models, the NLRP3 inflammasome pathway is activated (Han et al., 2019). Moreover, Lee et al. (2019) found that activation of the microglial NLRP3 inflammasome is pivotal for dopaminergic neurodegeneration and subsequent motor dysfunction in an MPTP-induced PD model. Therefore, the microglial NLRP3 inflammasome is a potential target for PD treatment (Haque et al., 2020). In our research, we discovered that exendin-4 and linagliptin can inhibit MPTP-induced NLRP3/caspase-1/IL-1β pathway activation. Previous studies found that mitochondrial dysfunction, oxidative stress, and ROS production are upstream priming signals for NLRP3 inflammasome activation (Sarkar et al., 2017; Ahmed et al., 2021; Jayaram and Krishnamurthy, 2021). Some compounds can inhibit NLRP3 inflammasome activation by reducing ROS production (Fan et al., 2017; Chen et al., 2019; Que et al., 2021; Zheng et al., 2021). MPTP can be oxidized into the toxic metabolite MPP (+) under the action of monoamine oxidase B in glia. MPP (+) decreases adenosine triphosphate production and increases ROS production by inhibiting the mitochondrial electron transport chain complex (Schildknecht et al., 2017; Dionísio et al., 2021). We found that NLRP3 expression is reduced in microglia and BV2 cells, which may be due to inhibition of MPTP/MPP (+)-induced ROS production by exendin-4 and linagliptin. When the NLRP3 inflammasome pathway is activated, the proinflammatory cytokines IL-18 and IL-1β are released. Subsequently, resting microglia become activated and polarize into the pro-inflammatory M1 phenotype, which release more proinflammatory cytokines, creating a vicious cycle (Wu et al., 2021). However, we found that exendin-4 and linagliptin induced microglial polarization into the anti-inflammatory M2 phenotype, thus protecting dopaminergic neurons from inflammatory stimuli. This finding may be extended to other neurodegenerative diseases related to neuroinflammation, such as Alzheimer’s disease (Muscogiuri et al., 2017).
Our findings have translational value to clinical practice. In a cohort study with a large population, Brauer et al. (Brauer et al., 2020) found that GLP-1Ra and DPP4i decrease the risk of PD in patients with DM, suggesting that patients with DM can use GLP-1Ra and DPP4i to prevent PD development. Moreover, some clinical trials have been conducted to test the effectiveness and safety of exenatide – a synthetic exendin-4 compound – in PD treatment (Athauda et al., 2017, 2019). However, clinical trials of DDP4i have not yet been conducted. The advantage of DPP4is is that they can be taken orally, while exenatide requires subcutaneous injection, resulting in better compliance with DPP4i-based treatment plans (Ayoub et al., 2018). However, most gliptins are unable to cross the blood-brain barrier, which may limit their application. Therefore, the mechanism of DPP4i needs to be explored before it can be tested in clinical trials in the future, and clinical trials of GLP-1Ras such as exenatide with larger sample sizes and longer treatment durations are needed to determine its efficacy.
Our study had some limitations. Although we proved the neuroprotective effects of exendin-4 and linagliptin, especially regulation of neuroinflammation and the microglial phenotype, through several behavioral tests and histological findings, the specific molecular mechanisms underlying these effects need to be explored further. In particular, the effects of these two drugs should be investigated in microglia-depleted MPTP models to confirm that they act through microglia (Guo et al., 2020). Moreover, the roles that exendin-4 and linagliptin play in misfolded α-syn aggregation, another important pathological feature of PD, remain unclear. A study on multiple system atrophy demonstrated that exendin-4 can inhibit the expression of α-syn (Bassil et al., 2017). Therefore, an animal model of PD overexpressing α-syn is needed to explore the roles of exendin-4 and linagliptin in misfolded α-syn aggregation and degradation. Furthermore, while we found that exendin-4 and linagliptin cannot directly inhibit SH-SY5Y cells apoptosis, BV2 cells and SH-SY5Y cells should be co-cultured to confirm that the neuroprotective effects of these two compounds are due to attenuation of neuroinflammation (Yao et al., 2019).
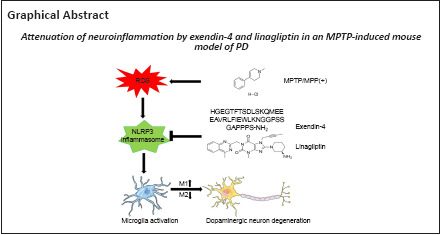
In conclusion, in this study we demonstrated that exendin-4 and linagliptin attenuate neuroinflammation by regulating microglial polarization and the NLRP3 signaling pathway in an MPTP-induced mouse model of PD. These findings provide experimental evidence for the clinical application of exendin-4 and linagliptin for the treatment of PD.
Additional files:
Additional Figure 1 (1.3MB, tif) : Representative figures of the hindlimb clasping test and gait analysis in mice.
Representative figures of the hindlimb clasping test and gait analysis in mice.
(A) Representative performance of the hindlimb clasping test in mice. Hindlimbs of mice in the CON group fully extended, whereas MPTP group almost completely retracted and touching abdomen. (B) Representative image of the gait analysis in mice. Gait of mice in MPTP group was irregular with high maximum running variation, which improved in MPTP + EX-4 and MPTP + LINA groups. CON: Control; EX-4: exendin-4; LINA: linagliptin; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine.
Additional Figure 2 (1.3MB, tif) : Effects of MPP (+), exendin-4, and linagliptin on viability of BV2 cells.
Effects of MPP (+), exendin-4, and linagliptin on viability of BV2 cells.
(A) The effect of MPP (+) on BV2 cell morphology, representative image in bright field. BV-2 cell shrink and died after treatment with 1 mM MPP (+), and cell density slightly decreased after treatment with 500 μM MPP (+). Scale bar: 50 μm. (B-D) The effect of MPP (+), exendin-4, and linagliptin on BV2 cell viability by CCK-8. Data were expressed as mean± SD. The experiment was repeated three times. **P < 0.01, ***P < 0.001, ****P < 0.0001, vs. CON (one-way analysis of variance followed by Tukey's post hoc test). C: Control; CCK-8: cell counting kit-8; CON: control; MPP (+): 1-methyl-4-phenylpyridium.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, Nos. 81771271 (to JF), 31800898 (to WL), 81430025 (to JYL), and U1801681 (to JYL); Key Research and Development Program of Liaoning Province, No. 2020JH2/10300047 (to JF); the Key Field Research Development Program of Guangdong Province, No. 2018B030337001 (to JYL); and the Outstanding Scientific Fund of Shengjing Hospital, No. M0475 (to JF).
Conflicts of interest: The authors declare no conflicts of interest.
Availability of data and materials: All data generated or analyzed during this study are included in this published article and its supplementary information files.
C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Crow E, Yu J, Song LP; T-Editor: Jia Y
References
- 1.Abdelsalam RM, Safar MM. Neuroprotective effects of vildagliptin in rat rotenone Parkinson's disease model:role of RAGE-NFκB and Nrf2-antioxidant signaling pathways. J Neurochem. (2015);133:700–707. doi: 10.1111/jnc.13087. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed S, Kwatra M, Ranjan Panda S, Murty USN, Naidu VGM. Andrographolide suppresses NLRP3 inflammasome activation in microglia through induction of parkin-mediated mitophagy in in-vitro and in-vivo models of Parkinson disease. Brain Behav Immun. (2021);91:142–158. doi: 10.1016/j.bbi.2020.09.017. [DOI] [PubMed] [Google Scholar]
- 3.Alegre-Cortes E, Martínez-Chacón G, Fuentes JM, Yakhine-Diop SMS. The dual role of necrostatin-1 in Parkinson's disease models. Neural Regen Res. (2021);16:2019–2020. doi: 10.4103/1673-5374.308080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong MJ, Okun MS. Diagnosis and treatment of Parkinson disease:a review. JAMA. (2020);323:548–560. doi: 10.1001/jama.2019.22360. [DOI] [PubMed] [Google Scholar]
- 5.Ascherio A, Schwarzschild MA. The epidemiology of Parkinson's disease:risk factors and prevention. The Lancet Neurology. (2016);15:1257–1272. doi: 10.1016/S1474-4422(16)30230-7. [DOI] [PubMed] [Google Scholar]
- 6.Athauda D, Maclagan K, Skene SS, Bajwa-Joseph M, Letchford D, Chowdhury K, Hibbert S, Budnik N, Zampedri L, Dickson J, Li Y, Aviles-Olmos I, Warner TT, Limousin P, Lees AJ, Greig NH, Tebbs S, Foltynie T. Exenatide once weekly versus placebo in Parkinson's disease:a randomised, double-blind, placebo-controlled trial. Lancet. (2017);390:1664–1675. doi: 10.1016/S0140-6736(17)31585-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Athauda D, Gulyani S, Karnati HK, Li Y, Tweedie D, Mustapic M, Chawla S, Chowdhury K, Skene SS, Greig NH, Kapogiannis D, Foltynie T. Utility of neuronal-derived exosomes to examine molecular mechanisms that affect motor function in patients with Parkinson disease:a secondary analysis of the exenatide-PD trial. JAMA Neurol. (2019);76:420–429. doi: 10.1001/jamaneurol.2018.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayoub BM, Mowaka S, Safar MM, Ashoush N, Arafa MG, Michel HE, Tadros MM, Elmazar MM, Mousa SA. Repositioning of omarigliptin as a once-weekly intranasal anti-parkinsonian agent. Sci Rep. (2018);8:8959. doi: 10.1038/s41598-018-27395-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badawi GA, Abd El Fattah MA, Zaki HF, El Sayed MI. Sitagliptin and liraglutide modulate l-dopa effect and attenuate dyskinetic movements in rotenone-lesioned rats. Neurotox Res. (2019);35:635–653. doi: 10.1007/s12640-019-9998-3. [DOI] [PubMed] [Google Scholar]
- 10.Baquet ZC, Williams D, Brody J, Smeyne RJ. A comparison of model-based (2D) and design-based (3D) stereological methods for estimating cell number in the substantia nigra pars compacta (SNpc) of the C57BL/6J mouse. Neuroscience. (2009);161:1082–1090. doi: 10.1016/j.neuroscience.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bassil F, Canron MH, Vital A, Bezard E, Li Y, Greig NH, Gulyani S, Kapogiannis D, Fernagut PO, Meissner WG. Insulin resistance and exendin-4 treatment for multiple system atrophy. Brain. (2017);140:1420–1436. doi: 10.1093/brain/awx044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brauer R, Wei L, Ma T, Athauda D, Girges C, Vijiaratnam N, Auld G, Whittlesea C, Wong I, Foltynie T. Diabetes medications and risk of Parkinson's disease:a cohort study of patients with diabetes. Brain. (2020);143:3067–3076. doi: 10.1093/brain/awaa262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calabrese V, Santoro A, Monti D, Crupi R, Di Paola R, Latteri S, Cuzzocrea S, Zappia M, Giordano J, Calabrese EJ, Franceschi C. Aging and Parkinson's Disease: Inflammaging, neuroinflammation, and biological remodeling as key factors in pathogenesis. Free Radic Biol Med. (2018);115:80–91. doi: 10.1016/j.freeradbiomed.2017.10.379. [DOI] [PubMed] [Google Scholar]
- 14.Camargo Maluf F, Feder D, Alves de Siqueira Carvalho A. Analysis of the relationship between type II diabetes mellitus and Parkinson's disease:a systematic review. Parkinsons Dis. (2019);2019:4951379. doi: 10.1155/2019/4951379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen D, Zhang XY, Sun J, Cong QJ, Chen WX, Ahsan HM, Gao J, Qian JJ. Asiatic acid protects dopaminergic neurons from neuroinflammation by suppressing mitochondrial ros production. Biomol Ther (Seoul) (2019);27:442–449. doi: 10.4062/biomolther.2018.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chohan H, Senkevich K, Patel RK, Bestwick JP, Jacobs BM, Bandres Ciga S, Gan-Or Z, Noyce AJ. Type 2 diabetes as a determinant of Parkinson's disease risk and progression. Mov Disord. (2021);36:1420–1429. doi: 10.1002/mds.28551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cortés-Ríos J, Zárate AM, Figueroa JD, Medina J, Fuentes-Lemus E, Rodríguez-Fernández M, Aliaga M, López-Alarcón C. Protein quantification by bicinchoninic acid (BCA) assay follows complex kinetics and can be performed at short incubation times. Anal Biochem. (2020);608:113904. doi: 10.1016/j.ab.2020.113904. [DOI] [PubMed] [Google Scholar]
- 18.Daubner SC, Le T, Wang S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch Biochem Biophys. (2011);508:1–12. doi: 10.1016/j.abb.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deacon RM. Measuring motor coordination in mice. J Vis Exp. (2013):e2609. doi: 10.3791/2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dionísio PA, Amaral JD, Rodrigues CMP. Oxidative stress and regulated cell death in Parkinson's disease. Ageing Res Rev. (2021);67:101263. doi: 10.1016/j.arr.2021.101263. [DOI] [PubMed] [Google Scholar]
- 21.Drucker DJ. Dipeptidyl peptidase-4 inhibition and the treatment of type 2 diabetes:preclinical biology and mechanisms of action. Diabetes Care. (2007);30:1335–1343. doi: 10.2337/dc07-0228. [DOI] [PubMed] [Google Scholar]
- 22.Erbil D, Eren CY, Demirel C, Küçüker MU, Solaroğlu I, Eser HY. GLP-1's role in neuroprotection:a systematic review. Brain Inj. (2019);33:734–819. doi: 10.1080/02699052.2019.1587000. [DOI] [PubMed] [Google Scholar]
- 23.Fan Z, Liang Z, Yang H, Pan Y, Zheng Y, Wang X. Tenuigenin protects dopaminergic neurons from inflammation via suppressing NLRP3 inflammasome activation in microglia. J Neuroinflammation. (2017);14:256. doi: 10.1186/s12974-017-1036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geldenhuys WJ, Guseman TL, Pienaar IS, Dluzen DE, Young JW. A novel biomechanical analysis of gait changes in the MPTP mouse model of Parkinson's disease. PeerJ. (2015);3:e1175. doi: 10.7717/peerj.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon R, Albornoz EA, Christie DC, Langley MR, Kumar V, Mantovani S, Robertson AAB, Butler MS, Rowe DB, O'Neill LA, Kanthasamy AG, Schroder K, Cooper MA, Woodruff TM. Inflammasome inhibition prevents α-synuclein pathology and dopaminergic neurodegeneration in mice. Sci Transl Med. (2018);10:eaah4066. doi: 10.1126/scitranslmed.aah4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo H, Callaway JB, Ting JP. Inflammasomes:mechanism of action, role in disease, and therapeutics. Nat Med. (2015);21:677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo M, Wang J, Zhao Y, Feng Y, Han S, Dong Q, Cui M, Tieu K. Microglial exosomes facilitate α-synuclein transmission in Parkinson's disease. Brain. (2020);143:1476–1497. doi: 10.1093/brain/awaa090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guyenet SJ, Furrer SA, Damian VM, Baughan TD, La Spada AR, Garden GA. A simple composite phenotype scoring system for evaluating mouse models of cerebellar ataxia. J Vis Exp. (2010):1787. doi: 10.3791/1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halliday G, Lees A, Stern M. Milestones in Parkinson's disease--clinical and pathologic features. Mov Disord. (2011);26:1015–1021. doi: 10.1002/mds.23669. [DOI] [PubMed] [Google Scholar]
- 30.Han X, Sun S, Sun Y, Song Q, Zhu J, Song N, Chen M, Sun T, Xia M, Ding J, Lu M, Yao H, Hu G. Small molecule-driven NLRP3 inflammation inhibition via interplay between ubiquitination and autophagy:implications for Parkinson disease. Autophagy. (2019);15:1860–1881. doi: 10.1080/15548627.2019.1596481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haque ME, Akther M, Jakaria M, Kim IS, Azam S, Choi DK. Targeting the microglial NLRP3 inflammasome and its role in Parkinson's disease. Mov Disord. (2020);35:20–33. doi: 10.1002/mds.27874. [DOI] [PubMed] [Google Scholar]
- 32.Harkavyi A, Abuirmeileh A, Lever R, Kingsbury AE, Biggs CS, Whitton PS. Glucagon-like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson's disease. J Neuroinflammation. (2008);5:19. doi: 10.1186/1742-2094-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hölscher C. Novel dual GLP-1/GIP receptor agonists show neuroprotective effects in Alzheimer's and Parkinson's disease models. Neuropharmacology. (2018);136:251–259. doi: 10.1016/j.neuropharm.2018.01.040. [DOI] [PubMed] [Google Scholar]
- 34.Hölscher C. Brain insulin resistance:role in neurodegenerative disease and potential for targeting. Expert Opin Investig Drugs. (2020);29:333–348. doi: 10.1080/13543784.2020.1738383. [DOI] [PubMed] [Google Scholar]
- 35.Imamura K, Hishikawa N, Sawada M, Nagatsu T, Yoshida M, Hashizume Y. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson's disease brains. Acta Neuropathol. (2003);106:518–526. doi: 10.1007/s00401-003-0766-2. [DOI] [PubMed] [Google Scholar]
- 36.Jayaram S, Krishnamurthy PT. Role of microgliosis, oxidative stress and associated neuroinflammation in the pathogenesis of Parkinson's disease: The therapeutic role of Nrf2 activators. Neurochem Int. (2021);145:105014. doi: 10.1016/j.neuint.2021.105014. [DOI] [PubMed] [Google Scholar]
- 37.Joers V, Tansey MG, Mulas G, Carta AR. Microglial phenotypes in Parkinson's disease and animal models of the disease. Prog Neurobiol. (2017);155:57–75. doi: 10.1016/j.pneurobio.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jose T, Inzucchi SE. Cardiovascular effects of the DPP-4 inhibitors. Diab Vasc Dis Res. (2012);9:109–116. doi: 10.1177/1479164111436236. [DOI] [PubMed] [Google Scholar]
- 39.Kabel AM, Omar MS, Alhadhrami A, Alharthi SS, Alrobaian MM. Linagliptin potentiates the effect of l-dopa on the behavioural, biochemical, and immunohistochemical changes in experimentally-induced Parkinsonism: Role of toll-like receptor 4, TGF-β1, NF-κB, and glucagon-like peptide 1. Physiol Behav. (2018);188:108–118. doi: 10.1016/j.physbeh.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 40.Krashia P, Cordella A, Nobili A, La Barbera L, Federici M, Leuti A, Campanelli F, Natale G, Marino G, Calabrese V, Vedele F, Ghiglieri V, Picconi B, Di Lazzaro G, Schirinzi T, Sancesario G, Casadei N, Riess O, Bernardini S, Pisani A, et al. Blunting neuroinflammation with resolvin D1 prevents early pathology in a rat model of Parkinson's disease. Nat Commun. (2019);10:3945. doi: 10.1038/s41467-019-11928-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwon HS, Koh SH. Neuroinflammation in neurodegenerative disorders:the roles of microglia and astrocytes. Transl Neurodegener. (2020);9:42. doi: 10.1186/s40035-020-00221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee CH, Jeon SJ, Cho KS, Moon E, Sapkota A, Jun HS, Ryu JH, Choi JW. Activation of glucagon-like peptide-1 receptor promotes neuroprotection in experimental autoimmune encephalomyelitis by reducing neuroinflammatory responses. Mol Neurobiol. (2018);55:3007–3020. doi: 10.1007/s12035-017-0550-2. [DOI] [PubMed] [Google Scholar]
- 43.Lee E, Hwang I, Park S, Hong S, Hwang B, Cho Y, Son J, Yu JW. MPTP-driven NLRP3 inflammasome activation in microglia plays a central role in dopaminergic neurodegeneration. Cell Death Differ. (2019);26:213–228. doi: 10.1038/s41418-018-0124-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li KL, Huang HY, Ren H, Yang XL. Role of exosomes in the pathogenesis of inflammation in Parkinson's disease. Neural Regen Res. (2022);17:1898–1906. doi: 10.4103/1673-5374.335143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, Powers K, Shen H, Egan JM, Sambamurti K, Brossi A, Lahiri DK, Mattson MP, Hoffer BJ, Wang Y, Greig NH. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci U S A. (2009);106:1285–1290. doi: 10.1073/pnas.0806720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. (2017);541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu W, Jalewa J, Sharma M, Li G, Li L, Holscher C. Neuroprotective effects of lixisenatide and liraglutide in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. Neuroscience. (2015);303:42–50. doi: 10.1016/j.neuroscience.2015.06.054. [DOI] [PubMed] [Google Scholar]
- 48.Lünemann JD, Malhotra S, Shinohara ML, Montalban X, Comabella M. Targeting inflammasomes to treat neurological diseases. Ann Neurol. (2021);90:177–188. doi: 10.1002/ana.26158. [DOI] [PubMed] [Google Scholar]
- 49.Mousa SA, Ayoub BM. Repositioning of dipeptidyl peptidase-4 inhibitors and glucagon like peptide-1 agonists as potential neuroprotective agents. Neural Regen Res. (2019);14:745–748. doi: 10.4103/1673-5374.249217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muscogiuri G, DeFronzo RA, Gastaldelli A, Holst JJ. Glucagon-like peptide-1 and the central/peripheral nervous system:crosstalk in diabetes. Trends Endocrinol Metab. (2017);28:88–103. doi: 10.1016/j.tem.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 51.Mustapha M, Mat Taib CN. MPTP-induced mouse model of Parkinson's disease:a promising direction of therapeutic strategies. Bosn J Basic Med Sci. (2021);21:422–433. doi: 10.17305/bjbms.2020.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nassar NN, Al-Shorbagy MY, Arab HH, Abdallah DM. Saxagliptin:a novel antiparkinsonian approach. Neuropharmacology. (2015);89:308–317. doi: 10.1016/j.neuropharm.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 53.Nauck MA, Meier JJ. The incretin effect in healthy individuals and those with type 2 diabetes:physiology, pathophysiology, and response to therapeutic interventions. Lancet Diabetes Endocrinol. (2016);4:525–536. doi: 10.1016/S2213-8587(15)00482-9. [DOI] [PubMed] [Google Scholar]
- 54.Ou Z, Zhou Y, Wang L, Xue L, Zheng J, Chen L, Tong Q. NLRP3 inflammasome inhibition prevents α-synuclein pathology by relieving autophagy dysfunction in chronic MPTP-treated NLRP3 knockout mice. Mol Neurobiol. (2021);58:1303–1311. doi: 10.1007/s12035-020-02198-5. [DOI] [PubMed] [Google Scholar]
- 55.Parkinson J. An essay on the shaking palsy. 1817. J Neuropsychiatry Clin Neurosci. (2002);14:223–236. doi: 10.1176/jnp.14.2.223. discussion 222. [DOI] [PubMed] [Google Scholar]
- 56.Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. (2020);18:e3000410. doi: 10.1371/journal.pbio.3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pisanu A, Lecca D, Mulas G, Wardas J, Simbula G, Spiga S, Carta AR. Dynamic changes in pro- and anti-inflammatory cytokines in microglia after PPAR-γagonist neuroprotective treatment in the MPTPp mouse model of progressive Parkinson's disease. Neurobiol Dis. (2014);71:280–291. doi: 10.1016/j.nbd.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 58.Qin X, Zhang X, Li P, Wang M, Yan L, Bao Z, Liu Q. Association between diabetes medications and the risk of Parkinson's disease:a systematic review and meta-analysis. Front Neurol. (2021);12:678649. doi: 10.3389/fneur.2021.678649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Que R, Zheng J, Chang Z, Zhang W, Li H, Xie Z, Huang Z, Wang HT, Xu J, Jin D, Yang W, Tan EK, Wang Q. Dl-3-n-Butylphthalide rescues dopaminergic neurons in Parkinson's disease models by inhibiting the NLRP3 inflammasome and ameliorating mitochondrial impairment. Front Immunol. (2021);12:794770. doi: 10.3389/fimmu.2021.794770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ransohoff RM, Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature. (2010);468:253–262. doi: 10.1038/nature09615. [DOI] [PubMed] [Google Scholar]
- 61.Rao X, Huang X, Zhou Z, Lin X. An improvement of the 2ˆ(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat Bioinforma Biomath. (2013);3:71–85. [PMC free article] [PubMed] [Google Scholar]
- 62.Safar MM, Abdelkader NF, Ramadan E, Kortam MA, Mohamed AF. Novel mechanistic insights towards the repositioning of alogliptin in Parkinson's disease. Life Sci. (2021);287:120132. doi: 10.1016/j.lfs.2021.120132. [DOI] [PubMed] [Google Scholar]
- 63.Sarkar S, Malovic E, Harishchandra DS, Ghaisas S, Panicker N, Charli A, Palanisamy BN, Rokad D, Jin H, Anantharam V, Kanthasamy A, Kanthasamy AG. Mitochondrial impairment in microglia amplifies NLRP3 inflammasome proinflammatory signaling in cell culture and animal models of Parkinson's disease. NPJ Parkinsons Dis. (2017);3:30. doi: 10.1038/s41531-017-0032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schapira AHV, Chaudhuri KR, Jenner P. Non-motor features of Parkinson disease. Nat Rev Neurosci. (2017);18:435–450. doi: 10.1038/nrn.2017.62. [DOI] [PubMed] [Google Scholar]
- 65.Schildknecht S, Di Monte DA, Pape R, Tieu K, Leist M. Tipping points and endogenous determinants of nigrostriatal degeneration by MPTP. Trends Pharmacol Sci. (2017);38:541–555. doi: 10.1016/j.tips.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 66.Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ:25 years of image analysis. Nat Methods. (2012);9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shan Y, Tan S, Lin Y, Liao S, Zhang B, Chen X, Wang J, Deng Z, Zeng Q, Zhang L, Wang Y, Hu X, Qiu W, Peng L, Lu Z. The glucagon-like peptide-1 receptor agonist reduces inflammation and blood-brain barrier breakdown in an astrocyte-dependent manner in experimental stroke. J Neuroinflammation. (2019);16:242. doi: 10.1186/s12974-019-1638-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shi J, Gao W, Shao F. Pyroptosis:gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. (2017);42:245–254. doi: 10.1016/j.tibs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 69.Tang Y, Le W. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol. (2016);53:1181–1194. doi: 10.1007/s12035-014-9070-5. [DOI] [PubMed] [Google Scholar]
- 70.Tysnes OB, Storstein A. Epidemiology of Parkinson's disease. J Neural Transm (Vienna) (2017);124:901–905. doi: 10.1007/s00702-017-1686-y. [DOI] [PubMed] [Google Scholar]
- 71.Wang LY, Yu X, Li XX, Zhao YN, Wang CY, Wang ZY, He ZY. Catalpol exerts a neuroprotective effect in the MPTP mouse model of Parkinson's disease. Front Aging Neurosci. (2019);11:316. doi: 10.3389/fnagi.2019.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu AG, Zhou XG, Qiao G, Yu L, Tang Y, Yan L, Qiu WQ, Pan R, Yu CL, Law BY, Qin DL, Wu JM. Targeting microglial autophagic degradation in NLRP3 inflammasome-mediated neurodegenerative diseases. Ageing Res Rev. (2021);65:101202. doi: 10.1016/j.arr.2020.101202. [DOI] [PubMed] [Google Scholar]
- 73.Yao S, Li L, Sun X, Hua J, Zhang K, Hao L, Liu L, Shi D, Zhou H. FTY720 Inhibits MPP(+)-induced microglial activation by affecting NLRP3 inflammasome activation. J Neuroimmune Pharmacol. (2019);14:478–492. doi: 10.1007/s11481-019-09843-4. [DOI] [PubMed] [Google Scholar]
- 74.Zhang LY, Jin QQ, Hölscher C, Li L. Glucagon-like peptide-1/glucose-dependent insulinotropic polypeptide dual receptor agonist DA-CH5 is superior to exendin-4 in protecting neurons in the 6-hydroxydopamine rat Parkinson model. Neural Regen Res. (2021);16:1660–1670. doi: 10.4103/1673-5374.303045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang QS, Heng Y, Mou Z, Huang JY, Yuan YH, Chen NH. Reassessment of subacute MPTP-treated mice as animal model of Parkinson's disease. Acta Pharmacol Sin. (2017);38:1317–1328. doi: 10.1038/aps.2017.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang YH, Wang DW, Xu SF, Zhang S, Fan YG, Yang YY, Guo SQ, Wang S, Guo T, Wang ZY, Guo C. α-Lipoic acid improves abnormal behavior by mitigation of oxidative stress, inflammation, ferroptosis, and tauopathy in P301S Tau transgenic mice. Redox Biol. (2018);14:535–548. doi: 10.1016/j.redox.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zheng R, Ruan Y, Yan Y, Lin Z, Xue N, Yan Y, Tian J, Yin X, Pu J, Zhang B. Melatonin attenuates neuroinflammation by down-regulating NLRP3 inflammasome via a SIRT1-dependent pathway in MPTP-induced models of Parkinson's disease. J Inflamm Res. (2021);14:3063–3075. doi: 10.2147/JIR.S317672. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative figures of the hindlimb clasping test and gait analysis in mice.
(A) Representative performance of the hindlimb clasping test in mice. Hindlimbs of mice in the CON group fully extended, whereas MPTP group almost completely retracted and touching abdomen. (B) Representative image of the gait analysis in mice. Gait of mice in MPTP group was irregular with high maximum running variation, which improved in MPTP + EX-4 and MPTP + LINA groups. CON: Control; EX-4: exendin-4; LINA: linagliptin; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine.
Effects of MPP (+), exendin-4, and linagliptin on viability of BV2 cells.
(A) The effect of MPP (+) on BV2 cell morphology, representative image in bright field. BV-2 cell shrink and died after treatment with 1 mM MPP (+), and cell density slightly decreased after treatment with 500 μM MPP (+). Scale bar: 50 μm. (B-D) The effect of MPP (+), exendin-4, and linagliptin on BV2 cell viability by CCK-8. Data were expressed as mean± SD. The experiment was repeated three times. **P < 0.01, ***P < 0.001, ****P < 0.0001, vs. CON (one-way analysis of variance followed by Tukey's post hoc test). C: Control; CCK-8: cell counting kit-8; CON: control; MPP (+): 1-methyl-4-phenylpyridium.