Abstract
Myasthenia gravis is an acquired, humoral immunity-mediated autoimmune disease characterized by the production of autoantibodies that impair synaptic transmission at the neuromuscular junction. The intervention-mediated clearance of immunoglobulin G (IgG) was shown to be effective in controlling the progression of the disease. The neonatal Fc receptor (FcRn) plays a key role in prolonging the serum half-life of IgG. Antagonizing FcRn to prevent its binding to IgG can accelerate the catabolism of the latter, resulting in decreased levels of IgG, including pathogenic autoantibodies, thereby achieving a therapeutic effect. In this review, we detail the substantial research progress, both basic and clinical, relating to the use of FcRn inhibitors in the treatment of myasthenia gravis.
Key Words: batoclimab, clinical trial, efgartigimod, FcRn, FcRn inhibitors, immunoglobulin G (IgG), myasthenia gravis, nipocalimab, rozanolixizumab
Introduction
Myasthenia gravis (MG) is an autoimmune disease caused by the binding of autoantibodies to important functional molecules on the postsynaptic membrane at the neuromuscular junction. The typical clinical manifestation of MG is generalized or localized skeletal muscle weakness that affects eye movements, swallowing, speech, mobility, and respiratory functions. Features associated with MG include muscle fatigability and fluctuating muscle weakness (mild weakness in the morning and severe weakness in the evening; worsened by activity and relieved by rest (Barrantes, 2021). Most patients with MG have autoantibodies against the acetylcholine receptor (AChR), while a few have antibodies against muscle-specific tyrosine kinase or lipoprotein receptor-associated protein 4 (Hehir and Silvestri, 2018). These pathogenic antibodies act by functionally blocking or accelerating receptor degradation and activating complement, leading to reduced receptor density on the postsynaptic membrane, impaired neuromuscular transmission, and muscle weakness (Hehir and Silvestri, 2018). Current treatment strategies include reducing immunoglobulin G (IgG) production through the use of immunosuppressive and anti-B-cell drugs and increasing IgG clearance via intravenous immunoglobulin (IVIG) infusion, plasma exchange (PE), and immunoadsorption (IA). The former, although effective, has extensive immunosuppressive effects, and its use is often limited by numerous adverse reactions (Narayanaswami et al., 2021); the latter, meanwhile, typically reduces serum IgG levels by approximately 50% and clinical effects can be seen within days to weeks; however, the associated therapies are difficult to maintain over the long term (Karelis et al., 2019; Liu et al., 2021).
Given that the damage to target organs caused by pathogenic IgG is strongly related to its long half-life, interfering with its half-life/reducing its retention time in the body may represent a better therapeutic option for MG than the above-mentioned methods. The neonatal Fc receptor (FcRn) plays an important role in the distribution, transport, and persistence of IgG. Strategies aimed at preventing the binding of FcRn to IgG can shorten the half-life of the latter and specifically reduce its retention time in the body, thus producing effects similar to those of PE or IA on pathogenic IgG clearance, but with greater potential for long-term maintenance therapy. Several FcRn inhibitors have shown excellent efficacy and broad application prospects in MG-related studies. Indeed, one of these, efgartigimod, was approved by the United States Food and Drug Administration (FDA) for the treatment of generalized myasthenia gravis (gMG) in December 2021 (Heo, 2022). Nevertheless, FcRn inhibitors have yet to be incorporated into conventional treatment regimens. Here, we review the research progress relating to the use of FcRn inhibitors for the treatment of MG.
Retrieval Strategy
The studies cited in this review were retrieved through an electronic search of the Web of Science, Google Scholar, and PubMed databases. The following keyword combinations were used to preliminarily select the articles to be evaluated: myasthenia gravis; IgG; FcRn; FcRn inhibitors; clinical trial; efgartigimod; rozanolixizumab; batoclimab; nipocalimab. Most of the elected studies (80% of all the references) were published between 2012 and 2022. One publication from 1958 was included because of its relevance to the FcRn field.
FcRn Structure
FcRn, also known as the Brambell receptor, is the major histocompatibility complex (MHC) I-related receptor encoded by the FCGRT gene. In the 1960s, Brambell was the first to propose that there might be a receptor capable of mediating the transport of IgG from mother to infant (Brambell, 1958). Subsequently, (Rodewald and Kraehenbuhl, 1984) demonstrated that this IgG transport was pH-dependent. FcRn was first isolated from the intestine of neonatal rats and cloned in 1989 (Simister and Mostov, 1989). This receptor exists as a non-covalently bound heterodimer consisting of an alpha (α) heavy chain of approximately 50 kDa and a β2-microglobulin (β2m) light chain of 12 kDa. The structure of the heavy chain is similar to that of MHC-I class molecules and comprises three extracellular structural domains (α1, α2, and α3), a transmembrane structural domain, and a cytoplasmic tail of 44 amino acids (Simister and Mostov, 1989). Although FcRn is highly similar to MHC-I, the two sites where peptides anchor in the MHC-I molecule are blocked in FcRn by an arginine side chain and a proline residue in the α2 helical region, respectively (Simister and Mostov, 1989). FcRn binds IgG in a 1:1 or a 2:1 ratio and albumin in a 1:1 ratio, and this binding serves to regulate the metabolism of both proteins (Chaudhury et al., 2006; Abdiche et al., 2015). The IgG and albumin binding sites on FcRn are different such that FcRn/IgG interaction is largely unaffected by albumin (Ward et al., 2022). Although known as a neonatal receptor, FcRn is expressed in the liver, kidney, muscle, and vascular endothelial tissues throughout life.
Biological Functions of FcRn
The binding of FcRn and IgG
The molecular basis of FcRn-IgG binding
FcRn interacts with IgG at the CH2 and CH3 structural domain interface of IgG and involves the residues Ile253, His310, and His435 of the IgG molecule (Kim et al., 1999). The binding stoichiometry of FcRn with IgG is 2:1 under conditions of equilibrium and 1:1 under nonequilibrium conditions (Abdiche et al., 2015). The two histidine residues (His310 and His435) become protonated in an acidic environment (pH 6.0 to 6.5), which allows their interaction with FcRn residues and the high-affinity binding of IgG to FcRn; in contrast, in a neutral or alkaline environment (pH 7.0 to 7.5), histidine protonation is gradually lost and the affinity of IgG for FcRn decreases (Kim et al., 1999; Booth et al., 2018; Lim et al., 2020). In summary, the difference in the degree of histidine protonation under different pH environments influences the affinity of FcRn for IgG and, consequently, FcRn-mediated IgG recirculation. Studies using mutated FcRn molecules revealed that two histidines (residues 250 and 251) on the FcRn heavy chain also promote the pH dependence of the FcRn-IgG interaction (Pyzik et al., 2019). Monoclonal antibodies with the same Fc but different Fab regions show different affinities for FcRn, suggesting that the Fab region may also be involved in the interaction between FcRn and IgG; however, the details of the involvement are unclear (Nath et al., 2021). Roopenian and Akilesh (2007) found that IgG catabolism was accelerated, the IgG serum half-life was shortened, and the IgG concentration was reduced in Fcgrt–/– mice. Familial hypercatabolic hypoproteinemia in humans is caused by loss-of-function mutations in the β2m light chain of FcRn, resulting in extremely low serum IgG and albumin levels (Wani et al., 2006). The molecular basis of the interaction between FcRn and IgG provides the basis and entry point for drug development using FcRn as a target.
The mode and pathway of FcRn-mediated IgG transport
In rodents, FcRn is responsible for the transport of maternal IgG into the bloodstream of newborn animals via the intestinal epithelium, thereby providing the neonates with passively acquired immunity (Simister and Mostov, 1989). Similarly, in humans, FcRn mediates the transport of maternal IgG to the fetus via the placenta, thereby enabling acquired immunity in the fetus (Borghi et al., 2020). The expression of FcRn in polarized epithelial cells endows the epithelial barrier with the capacity to transport IgG in both apical-to-basal and basal-to-apical directions, allowing IgG to be transported from the tissue space to the lumen and back to the lamina propria in different tissues (Rath et al., 2012). The ability of FcRn to transport IgG in both directions has been demonstrated both in in vitro models of polarized epithelia, such as those of the intestine, lung, placenta (syncytial trophectoderm), genitourinary system, and kidney, as well as in vivo experiments in mice, non-human primates, and adult humans (Rath et al., 2012; Pyzik et al., 2019). Mucosal surfaces that express FcRn can absorb and transport IgG, which, on the one hand, shows the vital role of FcRn in immune monitoring (Aaen et al., 2021), and, on the other hand, identifies a potentially novel method of drug delivery. Several research teams have achieved the delivery of macromolecular proteins, such as monomeric Epo-Fc and Gag-Fc, using lung mucosal epithelium (Fieux et al., 2021), which may represent a revolution in therapeutic macromolecular protein delivery.
The protection of circulating IgG by FcRn
IgG is the most abundant immunoglobulin in the body and has a half-life of approximately 21 days in humans. Its high serum levels and long half-life are largely reliant on FcRn-mediated recapture and recirculation. IgG recovery was reported to be 42% higher than IgG productivity, suggesting that the recovery of IgG, rather than its production, is the primary mechanism underlying the high IgG serum concentrations in humans (Peter et al., 2020). Vascular endothelial cells are the major site where FcRn protects IgG from lysosomal degradation. IgG molecules enter cells via fluid-phase endocytosis and encounter FcRn in endosomes (Junghans and Anderson, 1996; Dalakas and Spaeth, 2021). As endosomes mature, they become more acidic, which increases the affinity of FcRn for IgG (Oganesyan et al., 2014; Dalakas and Spaeth, 2021). After sorting in endosomes, the FcRn-IgG complex is delivered directly to the cell surface, where FcRn separates from IgG under the influence of the neutral extracellular environment, thus allowing IgG to re-enter the circulation. These observations highlight the importance of pH-dependent binding and FcRn-dependent cycling in the maintenance of the long half-life of IgG. The IgG molecules that fail to bind to FcRn, such as when the intracellular IgG level is high enough such that all FcRn molecules are in the bound state, will be transported to the lysosome for degradation (Figure 1; Oganesyan et al., 2014; Dalakas and Spaeth, 2021). This indicates that intracellular FcRn saturation with bound IgG also affects the serum levels of the latter. Other immunoglobulins cannot undergo the above-mentioned processes, which explains why IgG has the longest half-life of the immunoglobulin classes. Blocking the binding of FcRn to IgG was shown to accelerate the clearance of pathogenic IgG in vivo (Blumberg et al., 2019), implying that FcRn may serve as a therapeutic target for the treatment of IgG-mediated autoimmune diseases.
Figure 1.
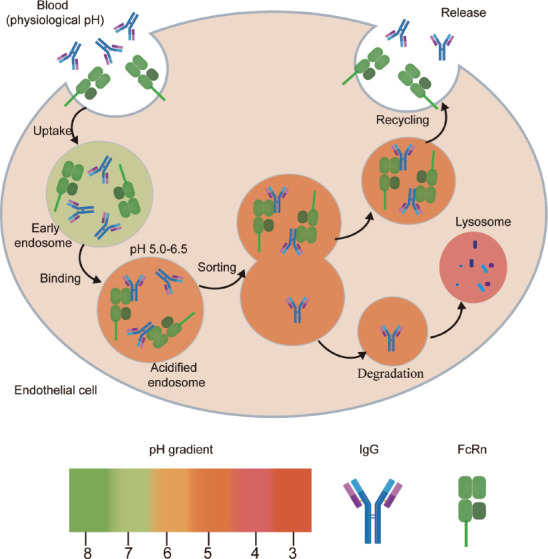
Mechanism of action underlying how FcRn protects IgG from degradation.
FcRn is widely expressed on endothelial cells. IgG enters the cell via fluid-phase endocytosis and, when the endosome is acidified (pH < 6.5), IgG can bind to FcRn, which is followed by endosomal sorting. FcRn-IgG complexes are transported to the cell surface, where the local near-neutral environment promotes the separation of FcRn from IgG and IgG re-enters the circulation. IgG that is not bound to FcRn is transported to the lysosome for degradation. FcRn: Neonatal Fc receptor; IgG: immunoglobulin G.
FcRn binds to albumin
Serum albumin is the most abundant protein in mammalian plasma and has a half-life of approximately 18 days in humans. Albumin consists of three homologous structural domains (DI, DII, and DIII) (Rabbani and Ahn, 2019). The binding interface between albumin and FcRn is mainly located in the DI and DIII domains (Pilati and Howard, 2020). The most striking feature at the DIII binding interface is the insertion of two conserved tryptophan residues (Trp53 and Trp59) in FcRn into the two hydrophobic pockets in the DIII A and B subdomains of albumin (Pilati and Howard, 2020). Meanwhile, the DI domain modulates the optimal binding of albumin to FcRn via two surface-exposed loops that directly contact FcRn (Pilati and Howard, 2020). The albumin binding sites on FcRn differ from those of IgG, which allows albumin and IgG to bind FcRn simultaneously and in a non-competitive manner (Ward et al., 2022). The mechanisms involved in FcRn-mediated recycling and recirculation of albumin, which is essential for the maintenance of albumin homeostasis, are very similar to those for IgG, with both being pH-dependent; the protonation of histidine (His166) on the FcRn heavy chain is the main mechanism underlying the pH-dependent interaction of FcRn with albumin (Andersen et al., 2012). Albumin synthesis mainly occurs in the liver, which is also where the binding of FcRn and albumin occurs (Pyzik et al., 2017). The lack of FcRn in the liver leads to hypoalbuminemia and the loss of albumin into the bile (Pyzik et al., 2017). Albumin is an important transporter of ions, fatty acids, amino acids, heme, bilirubin, and various drugs in the body, all of which compete with FcRn for binding to albumin and affect its metabolism and transport.
FcRn mediates antigen presentation
FcRn is expressed at high levels in antigen-presenting cells such as dendritic cells (DCs), B cells, and macrophages (Challa et al., 2019; Macri et al., 2021), suggesting that FcRn is involved in the antigen presentation process. Antigen presentation comprises two steps, namely, the FcγR-mediated internalization of the antigen-antibody complex (IgG-IC) and intracellular sorting. First, under the neutral pH conditions of the extracellular environment, IgG-IC binds to FcγR on the DC surface, initiating receptor-mediated endocytosis and vesicle formation. Following the recruitment of vesicular ATPase (V-ATPase), the vesicles become more acidic (pH < 6.5) during their maturation, which allows IgG-IC to dissociate from FcγR and bind to FcRn in the vesicles. In the presence of FcRn, IgG-IC can efficiently deliver the antigens to the antigen processing pathway, facilitating their loading onto MHC class I and MHC class II molecules and initiating T-cell-mediated immune responses (Figure 2; Baker et al., 2014). In addition, Baker et al. (2011) found that FcRn was involved in antigen cross-presentation and that inflammatory DCs are highly dependent on FcRn when the antigen is provided as an IgG-IC, with significant cross-presentation occurring at low doses of antigen stimulation. The blocking of FcRn was reported to impair FcRn-mediated antigen presentation and cross-presentation; moreover, blocking FcRn decreased circulating IgG levels and suppressed IgG-IC-mediated immune responses, implying that targeting FcRn may represent a therapeutic strategy for the treatment of autoimmune diseases (Blumberg et al., 2019).
Figure 2.
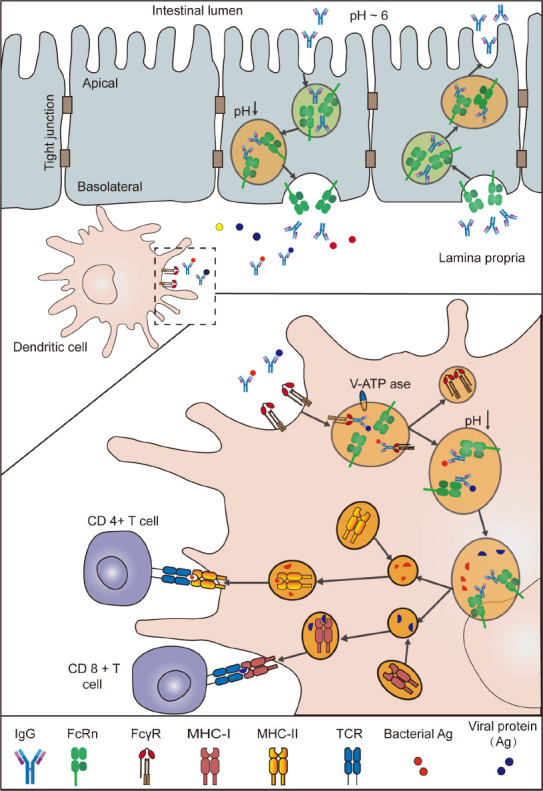
FcRn mediates the transport and immune response of IgG and IgG-IC in the intestine.
In the adult intestine, both intestinal epithelial cells and antigen-presenting cells in the lamina propria express FcRn. Enterocytes translocate intracellular IgG to the intestinal lumen, where it binds to antigen. IgG immune complexes (IgG-IC) are then transported to dendritic cells in the lamina propria. IgG-IC binds to FcγR on the surface of DCs and enters the cells via receptor-mediated endocytosis, forming vesicles. During vesicle maturation, through the recruitment of vesicular ATPase (V-ATPase), the vesicles become more acidic (pH ~6.0), and IgG dissociates from FcγR and binds to FcRn. Sorting of IgG-IC is completed in the presence of FcRn and antigen is loaded onto major histocompatibility complex (MHC)-I and MHC-II, thus activating CD8+ T cells and CD4+ T cells, respectively, and initiating T-cell-mediated adaptive immunity. FcRn: Neonatal Fc receptor; IgG: immunoglobulin G; TCR: T-cell receptor.
FcRn Inhibitors
Overview of FcRn inhibitors
IgG autoantibodies have an important role in the pathogenesis of many autoimmune diseases such as MG, neuromyelitis optica spectrum disorder, and chronic inflammatory demyelinating polyneuropathy (CIDP). The pathogenic effects of IgG autoantibodies may be related to their direct binding to the target antigen or to immune complexes mediated by complement cascade reactions, which leads to target organ damage (Xiao et al., 2021). Treatment modalities currently used to reduce the levels of pathogenic IgG autoantibodies in these diseases include corticosteroids, immunosuppressants, B cell-targeting drugs, IVIG infusions, PE, and IA (Gable and Guptill, 2019; Menon and Bril, 2022). However, these treatment options have different degrees of deficiencies in terms of safety, efficacy, and accessibility. Accordingly, it is imperative to identify safe and effective alternative treatment options to reduce circulating pathogenic IgG content. Targeting FcRn may represent such an option.
FcRn plays a crucial role in the maintenance of IgG levels by salvaging IgG from lysosomal degradation, thereby prolonging its half-life. In Fcgrt–/– mice, the half-life of IgG is reduced from 6 to 8 days to approximately 1 day, and the serum IgG level is decreased to 20–30% of normal (Roopenian and Akilesh, 2007). In rodent models of MG, primary immune thrombocytopenic purpura (ITP), experimental autoimmune encephalomyelitis (EAE), epidermolysis bullosa acquisita, and arthritis, anti-FcRn antibodies can effectively reduce plasma concentrations of pathogenic IgG and exert beneficial effects on disease symptoms (Smith et al., 2019; Kasprick et al., 2020; Remlinger et al., 2022). In non-human primates, anti-FcRn antibodies reduced IgG levels by over 60%, without significant, concomitant changes in the serum content of albumin, IgA, or IgM (Kiessling et al., 2017; Laurence Blumberg et al., 2017; Ulrichts et al., 2018). FcRn inhibitors are mostly humanized anti-FcRn monoclonal antibodies (or Fc fragments) that have a higher affinity for FcRn than wild-type IgG in both acidic and neutral environments. They can efficiently block the binding of pathogenic IgG to FcRn in vivo through competition, thereby achieving the rapid clearance of pathogenic IgG (Figure 3).
Figure 3.
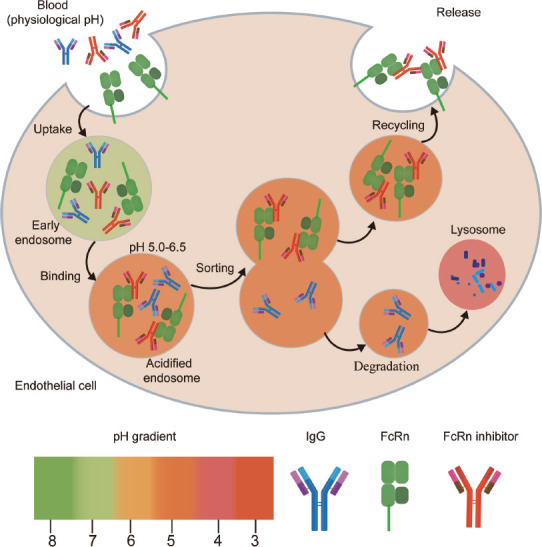
Mechanism of action of FcRn inhibitors.
FcRn inhibitors are anti-FcRn monoclonal antibodies with high affinity for FcRn at both neutral and acidic pH. Inside the cell, FcRn inhibitors compete with IgG for binding to FcRn. Because of their higher affinity, FcRn inhibitors prevent IgG from binding to FcRn, and IgG is transported to the lysosome and degraded, which leads to a decrease in circulating IgG levels. FcRn: Neonatal Fc receptor; IgG: immunoglobulin G.
Basic research on FcRn inhibitors
Abdegs (MST-HN)
Abdegs are engineered variants of human IgG1 that contain amino acid modifications (M252Y/S254T/T256E/H433K/N434F) (Vaccaro et al., 2005). Compared with wild-type IgG, Abdegs display higher affinity for human FcRn (hFcRn) both at pH 6 and pH 7.2. Consequently, they can effectively compete with IgG for FcRn binding, thereby accelerating the degradation of endogenous IgG (Vaccaro et al., 2005). Studies using mouse models of diseases such as arthritis and EAE have suggested that Abdegs can decrease the level of pathogenic IgG antibodies and exert therapeutic effects in vivo without affecting the expression of FcRn (Patel et al., 2011; Remlinger et al., 2022). However, Abdegs cannot target specific tissues and produce predominant local effects; they can only systematically induce the degradation of all IgGs, leading to a reduction in total IgG levels in vivo, thus increasing the risk of infection.
Seldegs
Sun et al. (2021) developed a novel bioagent, Seldegs, a MOG-Fc fusion protein with an N-terminal-targeting antigen. To avoid autoantibody cross-linking, the authors used a monovalent myelin oligodendrocyte glycoprotein (MOG) linked to the N terminus of the Fc of human IgG1. To reduce interaction with FcγRs and enhance binding to FcRn, G236R/L428R (“R”) mutations were inserted in the Fc fragment, while the C-terminus of the Fc fragment was linked to the C2A domain of synaptophysin 1 to allow binding to phosphatidylserine (PS) on the cell surface. Unlike wild-type IgG, Seldegs displayed high affinity for FcRn both at pH 6.0 and 7.4, which allowed them to bind to FcRn, be rapidly internalized, and subsequently enter the lysosomal pathway. Seldegs were shown to selectively clear MOG-specific antibodies with no effect on the levels of other antigen-specific/protective antibodies (Devanaboyina et al., 2017; Sun et al., 2021). In transgenic mice expressing human FcγR, MOG-Seldeg-PS promoted the rapid clearance of a MOG-specific antibody (ch8-18C5) in vivo, with a reduction in the serum levels of ch8-18C5 to 6–7% of the injected dose being recorded within 6 hours of injection; no difference in total serum IgG levels was observed either before or after MOG-Seldeg-PS treatment (Sun et al., 2021). The transfer of MOG-specific antibodies derived from the serum of patients with multiple sclerosis into mice with EAE led to disease exacerbation (Khare et al., 2018), while Seldeg treatment led to a significant reduction in antibody-mediated disease aggravation that lasted until the experimental endpoint. A significant improvement in the disease scores of EAE mice relative to those of control animals was also detected (Sun et al., 2021). In summary, Seldegs selectively degrade antigen-specific autoantibodies by antigen-binding autoantibodies and then by enhancing the binding of Fc to FcRn.
SYN1436 and SYN1327
Mezo et al. (2008a, b) identified a family of five peptides (SYN722–SYN726) that bind hFcRn and block IgG binding. The consensus peptide sequence for binding FcRn was GHFGGXY, where X is preferably a hydrophobic amino acid. SYN1436 is a 26-amino acid peptide dimer (molecular mass: 3.1 kDa) containing the SYN722 core sequence. Given that SYN1436 has a high affinity for FcRn at pH 6.0 and 7.4, it outcompetes IgG for hFcRn binding both intracellularly and on the cell surface, thereby inhibiting the interaction of hFcRn with IgG which, in turn, reduces circulating IgG levels. SYN1436 reduced IgG levels by up to 80% in cynomolgus monkeys without affecting serum albumin levels (Mezo et al., 2008b). Similar effects were observed for SYN1327, a monomeric peptide based on SYN1436 (Mezo et al., 2008a). Further structural studies of peptides could help develop small molecule FcRn inhibitors.
Clinical research on FcRn inhibitors in MG
Several FcRn inhibitors are currently undergoing clinical trials. The existing data indicate that FcRn inhibitors are safe, well tolerated, and can rapidly and persistently clear pathogenic IgG (Peter et al., 2020), resulting in improved survival scores in patients with MG. FcRn inhibitors are expected to be a novel option for the treatment of MG. In the following section, we introduce several FcRn inhibitors that have been clinically evaluated for MG treatment, one of which is an Fc fragment while the other four are monoclonal anti-FcRn antibodies (Table 1).
Table 1.
Summary of FcRn inhibitors in clinical studies for MG
| Drug | Mode of administration | Type | Phase | Sample size | Intervention and comparator | Mean maximum reduction in IgG levels | Impact on albumin | TEAEs of interest | Result summary |
|---|---|---|---|---|---|---|---|---|---|
| Efgartigimod (ARGX-113) | IV | IgG1 Fc fragment (M252Y/S254T/T256E/H433K/N434F) | I (NCT03457649) | 62 | SAD • 0.2, 2, 10, 25, 50 mg/kg • Placebo MAD • 10 mg/kg q4d×6 • 10 mg/kg q7d×4 • 25 mg/kg q7d×4 • Placebo |
SAD: 50% MAD: 75% | Not reported | • Headache (18%) • Abnormal differential WBC count (16%) • Increase in CRP levels (16%) |
• Efgartigimod is safe and no serious adverse events related to efgartigimod infusion were observed. |
| II (NCT02965573) | 24 | • 10 mg/kg QW×4 • Placebo |
70.7% week 4 | Not reported | • Headache (33.3%) | • In the efgartigimod group, 75% of patients had a clinically meaningful and statistically significant improvement in the MG-ADL score versus 25% in the placebo group (P = 0.0391). | |||
| III (NCT03669588) | 167 | • 10 mg/kg QW×4 • Placebo |
61.3% week 5 | Not reported | • Headache (29%) • Nasopharyngitis (12%) |
• 68% (44/65) of patients were MG-ADL responders in the efgartigimod group compared with only 30% (19/64) in the placebo group (P < 0.0001). | |||
| Rozanolixizumab (UCB7665) | SC | IgG4 mAb | I (NCT02220153) | 49 | • 1, 4, 7 mg/kg IV • 1, 4, 7 mg/kg SC • Placebo |
50% | Compared with the placebo group, the mean serum albumin levels in the rozanolixizumab group decreased slightly over time; however, the difference was not statistically significant. | • Headache (38.9%) • Vomiting (25%) |
• Rozanolixizumab has good safety and tolerability, and SC administration has better safety and tolerability than IV administration. |
| II (NCT03052751) | 43 | Period 1 (D1–29): • 7 mg/kg QW×3 • Placebo Period 2 (D29–43): • 4 mg/kg QW×3 • 7 mg/kg QW×3 • Placebo |
• Period 1: 61% D22 • Period 2: 68% D50 |
Not reported | • Headache (57%) | • The improvement in QMG scores with rozanolixizumab compared with placebo was not statistically significant (P = 0.221). • MG-ADL showed a statistically significant improvement. |
|||
| III (NCT03971422) | 200 | • 7 mg/kg QW×3 • Placebo |
NA | NA | NA | • The trial met its primary endpoint with a statistically significant and clinically meaningful change from baseline in the MG-ADL total scores at D43. • All secondary endpoints were also met with statistical significance. |
|||
| Batoclimab (RVT-1401) | SC | IgG1 mAb | I (NCT03971916) | 24 | • 340 mg • 510 mg • 680 mg • Placebo |
• 340 mg 21.0% D11 • 510 mg 39.8% D11 • 680 mg 41.2% D11 |
Slight but reversible decrease in the albumin level. | • Influenza-like illness • Rash |
• HBM9161 showed good safety and tolerability. |
| II (NCT04346888) | 30 | Period 1 • 340mg QW×6 • 680mg QW×6 • Placebo Period 2 340mg Q2W×3 |
• 340 mg 57% D43 • 680 mg 74% D43 |
Serum albumin decreased in a dose-dependent manner but recovered 6 weeks after discontinuation. | • Hyper cholesterolemia (6.4%) | • Batoclimab improved the clinical symptoms of patients with MG. | |||
| Nipocalimab (M281) | IV | Aglycosylated IgG1 mAb | I (NCT02828046) | 50 | SAD • 0.3, 3, 10, 30, 60 mg/kg • Placebo MAD • 15, 30 mg/kg QW×4 • Placebo |
SAD: 80% MAD: 85% | Asymptomatic, temporary reductions in total protein and albumin levels. | • Headache (8.3%) • Blood creatine phosphokinase increased (25%) • Viral upper respiratory tract infection (16.7%) |
• M281 exhibited an excellent safety and tolerance profile. |
| II (NCT03772587) | 68 | • 60 mg/kg SAD • 5 mg/kg Q4W×3 • 30 mg/kg Q4W×3 • 60 mg/kg Q2W×5 • Placebo Q2W×5 |
NA | NA | NA | • In the nipocalimab group, 52% of patients had a rapid, evident, and sustained decrease in the MG-ADL score versus 15% in the placebo group (P = 0.017). • Statistically significant relationship between IgG reduction and clinical benefit (P < 0.0001). |
|||
| Orilanolimab (SYNT001) | IV | IgG4 mAb (S241P mutation) | Ia (NCT03643627) | 31 | • 1 mg/kg • 3 mg/kg • 10 mg/kg • 30 mg/kg • Placebo |
30 mg/kg 46.21% D5 | Not reported | • Headache (34.8%) • Chills (4.3%) • Presyncope (4.3%) |
• SYNT001 exhibited an excellent safety and tolerance profile. • SYNT001 decreased the levels of all IgG subtypes and IgG-ICs in the circulation in humans. |
D: Day; FcRn: neonatal Fc receptor; IgG: immunoglobulin G; IgG-IC: IgG immune complex; IV: intravenous; mAb: monoclonal Ab; MAD: multiple ascending doses; MG: myasthenia gravis; MG-ADL: myasthenia gravis activities of daily living; NA: not available; QMG: quantitative myasthenia gravis; QW: quaque week; SAD: single ascending dose; SC: subcutaneous.
Efgartigimod
Efgartigimod is a modified human IgG1-derived Fc fragment harboring amino acid modifications, introduced using Abdeg technology, that enhance its binding affinity for FcRn in both acidic and neutral environments (equilibrium dissociation constant [KD]=14.2 nM at pH 6.0 and 320 nM at pH 7.4) while retaining the pH-dependent interaction with FcRn (Vaccaro et al., 2005; Ulrichts et al., 2018). Efgartigimod can effectively compete with IgG for binding to FcRn, thereby blocking the internal circulation of IgG and reducing the level of pathogenic IgG antibodies. Additionally, efgartigimod can be recycled to some extent by FcRn, which makes maintenance therapy feasible (Howard et al., 2021).
Preclinical studies: Efgartigimod was effective in reducing IgG levels and this effect was dose-dependent. A single infusion of 70 mg/kg efgartigimod decreased the IgG content in cynomolgus monkeys faster and more effectively than 2 g/kg IVIG; single 2, 20, or 200 mg/kg doses of efgartigimod all decreased IgG levels and displayed good safety and tolerability (Ulrichts et al., 2018).
Phase 1 study (NCT03457649): This was a phase 1 clinical trial on 62 healthy volunteers designed to evaluate the safety of efgartigimod through single ascending dose (SAD) and multiple ascending dose (MAD) studies. In the SAD study, the subjects were given 0.2 to 50 mg/kg efgartigimod or placebo, while in the MAD study, the subjects were given 10 or 25 mg/kg efgartigimod or placebo per 4 or 7 days. A single dose reduced IgG levels by 50%, whereas multiple doses reduced IgG levels by 75%. IgG levels returned to baseline levels approximately 8 weeks after the last dose (Ulrichts et al., 2018), indicating that efgartigimod is effective in reducing IgG and has a long-lasting effect. Furthermore, only the levels of IgG were reduced in the serum of the subjects, and no clinically significant reduction in the serum levels of albumin or other immunoglobulins was observed; no serious adverse events (AEs) associated with efgartigimod were reported (Ulrichts et al., 2018).
Phase 2 study (NCT02965573): This was a randomized, double-blind, placebo-controlled, multicenter phase 2 study exploring the safety and efficacy of efgartigimod. A total of 24 patients with anti-AChR antibody positive gMG were randomly assigned to receive four once-weekly intravenous (IV) infusions of 10 mg/kg efgartigimod or matching placebo (Howard et al., 2019). The maximum decrease in total IgG levels in the efgartigimod group was approximately 70.7%; eight weeks after the last infusion, total IgG levels were still 20% lower than baseline. All IgG subclasses showed a rapid, significant, and sustained reduction. Anti-AChR antibody levels were similarly reduced, showing a maximum decrease of approximately 40% to 70%, returning to baseline approximately 8 weeks after the final dose. A significant clinical and statistical improvement in MG activities of daily living (MG-ADL) scores (MG-ADL ≥ 2) was seen in 75% of efgartigimod-treated patients for at least 6 consecutive weeks compared with 25% for placebo-treated patients (P = 0.0391) (Howard et al., 2019). Assessments using four efficacy scales (MG-ADL, quantitative myasthenia gravis [QMG], myasthenia gravis composite [MGC], and revised 15-item Myasthenia Gravis Quality of Life scale [MG-QoL15r] scores) consistently demonstrated rapid and durable disease improvement in 75% of patients, with a temporal evolution that was consistent with the detected total serum IgG and anti-AChR antibody levels. No efgartigimod treatment-associated decreases in IgM, IgA, or albumin levels were observed (Howard et al., 2019).
Phase 3 study (NCT03669588): In a randomized, double-blind, placebo-controlled phase 3 clinical study (ADAPT), 167 patients with gMG were randomly assigned (1:1) to receive four once-weekly infusions (four infusions per cycle) of efgartigimod (10 mg/kg) or matching placebo to demonstrate the efficacy of efgartigimod (Howard et al., 2021). In cycle 1, 68% (44/65) of anti-AChR antibody positive patients in the efgartigimod group were MG-ADL responders, significantly more than the 30% (19/64) in the placebo group (P < 0.0001) (Howard et al., 2021). An improvement in MG-ADL scores was shown to be clinically meaningful during 48.7% of the time between study onset and day 126 in efgartigimod-treated patients compared with 26.6% of the time in the placebo group. Significant reductions in serum levels of different IgG subtypes were observed. One week after the last infusion, the mean maximum decrease in IgG and anti-AChR antibody levels was 61.3% and 57.6%, respectively, in anti-AChR antibody positive patients (Howard et al., 2021). No decrease in serum albumin levels was observed, demonstrating that efgartigimod selectively binds to the IgG binding site of FcRn and does not affect FcRn binding to albumin.
The above study showed that efgartigimod significantly reduced the levels of total IgG and those of IgGs of each subclass without affecting the levels of other immunoglobulins and albumin, and also markedly improved the clinical symptoms and quality of daily life of patients with gMG. The treatment-emergent adverse event (TEAE) common to the phase 2 and 3 studies was headache; however, the symptoms were mild, and no deaths occurred during the trial (Howard et al., 2019, 2021). The findings indicated that efgartigimod has good efficacy and a good safety profile in patients with gMG; accordingly, on December 17, 2021, the drug (trade name: Vyvgart) was the first FcRn inhibitor to be approved by the US FDA for the treatment of anti-AChR antibody positive gMG (Heo, 2022). Although it is primarily used for the treatment of gMG, efgartigimod has also shown some success in phase 2 clinical trials for the treatment of ITP (NCT03102593) (Newland et al., 2020), pemphigus (NCT03334058) CIDP (NCT04280718), and other conditions. China has also completed phase 1 clinical studies on the safety of efgartigimod (CTR20211952 and CTR20211805), thereby initiating the process of targeted interventions for the treatment of pathogenic IgG-mediated severe autoimmune diseases in this country.
Rozanolixizumab (UCB7665)
Rozanolixizumab is a subcutaneously (SC) administered, humanized, anti-FcRn monoclonal antibody (IgG4P) with high affinity for FcRn at both pH 6.0 and pH 7.4 (Smith et al., 2018). Rozanolixizumab selectively inhibits the binding of FcRn and IgG, leading to IgG degradation and clearance via the lysosomal pathway and, consequently, a decrease in serum IgG levels.
Preclinical studies: Rozanolixizumab induced a rapid and sustained reduction in serum IgG levels in transgenic mice expressing hFcRn (Smith et al., 2018). A 13-week toxicology study in cynomolgus monkeys showed that both SC (50 and 150 mg/kg) and IV (150 mg/kg) (every 3 days) rozanolixizumab were well tolerated, inducing a sustained (but reversible) reduction in IgG concentrations of 75–90%, with no AEs being observed (Kiessling et al., 2017).
Phase 1 study (NCT02220153): A phase 1 clinical trial of the safety and tolerability of rozanolixizumab was conducted in 49 healthy volunteers randomized to receive IV or SC rozanolixizumab (n = 36) at the doses of 1, 4, or 7 mg/kg or placebo (n = 13) (Kiessling et al., 2017). In the rozanolixizumab group, serum IgG concentrations were lowered by 50% and serum IgG levels only gradually returned to baseline levels at week 8. A dose-dependent reduction was observed for each of the IgG subclasses, with the most significant reduction being detected in serum IgG3 levels (Kiessling et al., 2017). Compared with the placebo group, mean albumin concentrations decreased slightly with time in the IV and SC administration groups; however, the differences were not significant (Kiessling et al., 2017). Rozanolixizumab had a favorable safety profile at the single doses of 4 mg/kg IV or 7 mg/kg SC. However, at 7 mg/kg IV, three subjects had severe headache and one had back pain (Kiessling et al., 2017). SC administration had better safety and tolerability than IV administration.
Phase 2 study (NCT03052751): In a randomized, double-blind, placebo-controlled phase 2a study, 43 patients with gMG were randomized (1:1) to receive three once-weekly infusions of 7 mg/kg rozanolixizumab or placebo in dosing period 1 (days 1–29); in dosing period 2 (days 29–43), patients were re-randomized to receive three once-weekly infusions of 7 mg/kg or 4 mg/kg rozanolixizumab; days 44–99 comprised an observation period. In period 1, the maximum reductions in serum IgG concentration and anti-AChR antibody titres were 61% and 44%, respectively; in period 2, the maximum reductions in serum IgG reached 68%. Although the primary endpoint (change in the QMG score from baseline to day 29) between the rozanolixizumab and placebo groups did not reach statistical significance, improvements were seen both in MG-ADL scores and the daily functioning of patients (Bril et al., 2021). The most common AE was headache. The incidence of headaches was 57% in the rozanolixizumab group, higher than the 14% seen in the placebo group, and three patients in the treatment group did not complete the study because of headache (Bril et al., 2021).
Phase 3 study (NCT03971422): This was a randomized, double-blind, placebo-controlled phase 3 study evaluating the safety and efficacy of rozanolixizumab in 200 patients with gMG. Results published by UCB showed that the study reached the primary endpoint of a statistically meaningful and clinically significant change from baseline in the MG-ADL score on day 43. In addition, all secondary endpoints also reached statistical significance (UCB, 2021). Rozanolixizumab showed good tolerability (UCB, 2021). Furthermore, rozanolixizumab has demonstrated good therapeutic efficacy in a phase 2 clinical study on ITP (NCT02718716), and an associated phase 3 clinical study (NCT04596995) is ongoing; a phase 2 clinical study on the use of rozanolixizumab for the treatment of CIDP (NCT03861481) is also ongoing.
Batoclimab (HBM9161)
HBM9161 is a fully human, IgG1 monoclonal anti-FcRn antibody, the Fc portion of which was modified to reduce antibody-dependent cell-mediated cytotoxicity (Yap et al., 2021). HBM9161 specifically targets the IgG binding site on FcRn, thereby blocking FcRn from binding to IgG and accelerating IgG clearance. HBM9161 is the first FcRn inhibitor for which evidence has been obtained in clinical studies in China.
Phase 1 study (NCT03971916): A phase 1, randomized, double-blind, dose-escalation study enrolled 24 healthy Chinese volunteers to assess the safety and tolerability of HBM9161 (Yap et al., 2021). This first human study involving HBM9161 among Chinese volunteers demonstrated that HBM9161 was well tolerated and safe in both single (IV or SC) and multiple (SC) doses and showed rapid and consistent reductions in total serum IgG levels and the levels of IgGs in each subclass following multiple SC doses. Subjects receiving HBM9161 had a sustained, dose-dependent reduction in serum IgG concentrations (Yap et al., 2021). The most common AEs were influenza-like symptoms and rash, but none of the AEs were serious (Yap et al., 2021).
Phase 2 study (NCT04346888): A randomized, double-blind, placebo-controlled, parallel phase 2 clinical study enrolled 30 patients with gMG (Yan et al., 2022) randomized to two batoclimab groups (340 mg, n = 10; 680 mg, n = 11; administered once weekly for 6 weeks) or a placebo group (n = 9); during the open-label treatment period, 340 mg batoclimab was administered every 2 weeks for 6 weeks. Batoclimab rapidly and effectively reduced total serum IgG levels on day 7, with 23% and 44% reductions in the 340 mg and 680 mg groups, respectively, compared with 1% in the placebo group; the maximum reduction in serum total IgG levels was reached on day 43, with 57% and 74% in the 340 mg and 680 mg groups, respectively, compared with 2% in the placebo group (Yan et al., 2022). During the double-blind treatment, the primary endpoint (changes in MG-ADL scores from baseline to day 43) was –4.7 ± 0.6, –4.4 ± 1.0, and –2.2 ± 0.9 for 340 mg batoclimab, 680 mg batoclimab, and placebo, respectively; the MGC, QMG, and MG-QoL15r scores showed similar improvements. During this study, no changes in serum IgA, IgM, or IgE levels were observed, and the serum albumin content showed a dose-dependent decrease in the batoclimab group, but returned to normal levels 6 weeks after treatment discontinuation (Yan et al., 2022). Batoclimab exhibited a good safety and tolerability profile, and no associated deaths or serious AEs were observed; the most common TEAE was hypercholesterolemia (Yan et al., 2022). Notably, the incidence of headache in the batoclimab group was lower than that reported for other FcRn inhibitors, with only one case (9%) of headache reported in the 680 mg group throughout the study period (Yan et al., 2022). The results regarding the clinical efficacy and safety of batoclimab in Chinese patients with gMG will facilitate a phase 3 study of this antibody. Batoclimab has also been evaluated for safety and efficacy in the treatment of ITP (NCT04428255), thyroid eye disease (NCT05015127), and neuromyelitis optica spectrum disorder (NCT04227470).
Nipocalimab (M281)
Nipocalimab is an aglycosylated, fully human, IgG1 monoclonal anti-FcRn antibody that shows high affinity for FcRn at both pH 6.0 and pH 7.6 (Ling et al., 2019). M281 specifically binds to the IgG binding site on FcRn and reduces the levels of pathogenic IgG without affecting IgG production.
Preclinical studies: M281 showed specific, dose-dependent, albumin-preserving IgG clearance in Fcgrt null mice expressing human FCGRT and in cynomolgus monkeys. M281 also showed efficacy in mouse models of ITP and collagen antibody-induced arthritic disease (Ling et al., 2015).
Phase 1 study (NCT02828046): A randomized, double-blind, placebo-controlled, phase 1 clinical study enrolled 50 healthy adult volunteers to explore the safety and tolerability of M281 (Ling et al., 2019). In the SAD study, subjects were randomized to receive 0.3, 3, 10, 30, or 60 mg/kg M281 or placebo; in the MAD study, subjects were randomly assigned to receive four weekly doses of 15 or 30 mg/kg M281 or placebo. At doses ≥ 3 mg/kg, receptor occupancy, as measured by flow cytometry, was greater than 90% after 2 hours, indicating that M281 can almost saturate FcRn, leading to a decrease in serum IgG levels (Ling et al., 2019). In the SAD study, dose-dependent reductions in serum IgG levels were seen in all dose groups and were similar in all IgG subclasses, with the highest test dose of 60 mg/kg reducing circulating IgG by approximately 80% from baseline over 2 weeks (Ling et al., 2019). In the MAD study, M281 reduced IgG levels by approximately 85% and maintained a ≥ 75% reduction in IgG levels from baseline for up to 24 days (Ling et al., 2019). No clinically meaningful changes in the levels of serum immunoglobulins, such as IgA, IgM, IgD, and IgE, were observed in the study, and asymptomatic, transient decreases in total protein and albumin content were observed in subjects in the 60 mg/kg group and the 15 and 30 mg/kg multiple-dose groups. M281 was well tolerated and no severe, treatment-associated AEs occurred.
Phase 2 study (NCT03772587): A randomized, double-blind, placebo-controlled, phase 2 clinical study randomized 68 patients with gMG to four nipocalimab treatment groups (5 mg/kg Q4W, 30 mg/kg Q4W, 60 mg/kg Q2W, and 60 mg/kg single dose) and one placebo group (Q2W). Data obtained from the study from Momenta (2020), indicated that across the 4 dosing groups, 52% of patients who received nipocalimab had rapid, significant, and durable improvement in MG-ADL scores (≥ 2 points from baseline for at least 4 consecutive weeks) compared with 15% for placebo-treated patients (P = 0.017). A substantial correlation was observed between IgG reduction and clinical benefit in the nipocalimab group (P < 0.0001) (Momenta, 2020). Nipocalimab showed good safety and efficacy and no serious, nipocalimab-associated AEs were reported (Momenta, 2020). Meanwhile, nipocalimab is also in phase 2 clinical studies relating to warm autoimmune hemolytic anemia (NCT04119050) and early-onset, severe, hemolytic disease of the fetus and newborn (NCT03842189).
SYNT001
SYNT001 is a humanized, de-immunized IgG4 monoclonal antibody harboring a S241P mutation. SYNT001 binds to hFcRn at both neutral and acidic pHs, thereby disrupting IgG homeostasis and achieving a reduction in serum IgG levels (Laurence Blumberg et al., 2017; Blumberg et al., 2019).
Preclinical studies: SYNT001 was well tolerated in non-human primates and induced a dose-dependent decrease in serum IgG (Laurence Blumberg et al., 2017).
Phase 1a study (NCT03643627): This was a SAD, randomized (6:2), double-blind, placebo-controlled phase 1a trial enrolling 31 healthy male volunteers to assess the safety, PK, and PD of SYNT001 (Blumberg et al., 2019). Subjects were randomized to receive 1, 3, 10, or 30 mg/kg SYNT001 or placebo. Total IgG and all IgG subclasses in serum showed a reversible, dose-dependent reduction in healthy volunteers receiving SYNT001, with a minimum reduction of approximately 46.21% in total IgG levels in the 30 mg/kg group at 5 days post-dose and an approximately 27.33% reduction in total IgG levels relative to baseline on day 28 (Blumberg et al., 2019). In addition, SYNT001 also led to a dose-dependent reduction in circulating immune complexes (CICs), with CIC levels declining by 18.98%, 29.41%, and 48.39% in the 3, 10, and 30 mg/kg groups, respectively (Blumberg et al., 2019). This indicated that SYNT001 not only accelerated the degradation of endogenous IgG but also participated in the degradation of CICs. SYNT001 showed good safety and no serious AEs were reported. The most common TEAE was headache.
Phase 2 clinical studies in patients with pemphigus foliaceus and pemphigus vulgaris showed that SYNT001 reduces pathogenic antibody levels and controls disease activity, indicating that it has marked therapeutic potential in the treatment of pemphigus (Werth et al., 2021). However, SYNT001 has not been further evaluated in the treatment of MG.
Conclusions and Outlook
MG is a disabling autoimmune disease that markedly affects the quality of life of patients and imposes a heavy burden on families and society. Existing therapeutic options are restricted by low efficacy and safety; however, research on FcRn inhibitors has begun to address these limitations in the treatment of MG. Humanized FcRn inhibitors have several potential advantages in the treatment of this condition. (1) They can bind FcRn with high affinity on the cell surface; (2) they have a higher affinity for FcRn than wild-type IgG in acidic endosomes; (3) they increase IgG clearance in vivo without affecting IgG production; (4) because IgA, IgD, IgE, and IgM recycling is not dependent on FcRn, targeting FcRn does not affect their serum levels, thus minimizing the risk of infection; (5) they have no effect on albumin levels or the metabolism of circulating albumin (albumin and IgG bind to FcRn at different sites); (6) preliminary studies have not found effects on immune cells (e.g., T cells, NK cells), cytokines, or complement, thus avoiding many of the immunosuppression-related side effects; (7) they do not affect FcRn expression or turnover, and maintenance treatment is achievable; (8) humanized FcRn inhibitors potentially offer a more cost-effective long-term option, particularly if they can be administered subcutaneously, as this can be done in an outpatient setting or at home. These observations highlight the unparalleled advantages of FcRn inhibitors over existing immunotherapeutic drugs.
Despite these advantages, the effects of long-term treatment with FcRn inhibitors remain to be fully elucidated. FcRn inhibitors may lead to as yet unidentified AEs or they may be potentially immunogenic, such as that seen with ABY039, a phase 1 clinical trial that was discontinued in 2020 for this reason. Additionally, during treatment with some FcRn inhibitors (batoclimab and nipocalimab), drug-related reductions in albumin concentrations have been detected, although the decreases were reversible and usually asymptomatic (Ling et al., 2019; Yan et al., 2022). Further studies are needed to clarify the exact mechanisms underlying these AEs and their long-term effects. Although available data suggest that IgA, IgM, complement, T cells, and NK cells may not be affected by FcRn inhibitors, it is unknown whether they can increase the risk of coronavirus infection during the COVID-19 pandemic, and FcRn inhibitors should be used with caution in patients with novel coronavirus pneumonia. Available evidence suggests that most FcRn inhibitor-related AEs are mild and no systemic infectious events have been reported. Nonetheless, FcRn inhibitors remove protective IgG as well as pathogenic IgG, which may increase the risk of infection. Whether FcRn inhibitors can cause infection requires further and extensive assessment, especially in the long term.
In conclusion, FcRn inhibitors have shown great potential and good prospects in the treatment of MG in several studies. However, further research on FcRn inhibitors is needed to clarify their efficacy and determine their potential as therapeutic options for MG.
Footnotes
Funding: This work was supported by the National Natural Science Foundation of China, No. U1604181, the Joint Project of Medical Science and Technology Research Program of Henan Province, No. LHGJ20190078, Henan Medical Education Research Project, No. Wjlx2020531, and Henan Province Key R&D and Promotion Special Project (Science and Technology Tackle), No. 212102310834 (all to JW).
Conflicts of interest: The authors report no competing interests.
C-Editor: Zhao M; S-Editor: Li CH; L-Editors: Li CH, Song LP; T-Editor: Jia Y
References
- 1.Aaen KH, Anthi AK, Sandlie I, Nilsen J, Mester S, Andersen JT. The neonatal Fc receptor in mucosal immune regulation. Scand J Immunol. (2021);93:e13017. doi: 10.1111/sji.13017. [DOI] [PubMed] [Google Scholar]
- 2.Abdiche YN, Yeung YA, Chaparro-Riggers J, Barman I, Strop P, Chin SM, Pham A, Bolton G, McDonough D, Lindquist K, Pons J, Rajpal A. The neonatal Fc receptor (FcRn) binds independently to both sites of the IgG homodimer with identical affinity. mAbs. (2015);7:331–343. doi: 10.1080/19420862.2015.1008353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen JT, Dalhus B, Cameron J, Daba MB, Plumridge A, Evans L, Brennan SO, Gunnarsen KS, Bjørås M, Sleep D, Sandlie I. Structure-based mutagenesis reveals the albumin-binding site of the neonatal Fc receptor. Nat Commun. (2012);3:610. doi: 10.1038/ncomms1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker K, Qiao SW, Kuo TT, Aveson VG, Platzer B, Andersen JT, Sandlie I, Chen Z, de Haar C, Lencer WI, Fiebiger E, Blumberg RS. Neonatal Fc receptor for IgG (FcRn) regulates cross-presentation of IgG immune complexes by CD8-CD11b+dendritic cells. Proc Natl Acad Sci U S A. (2011);108:9927–9932. doi: 10.1073/pnas.1019037108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker K, Rath T, Pyzik M, Blumberg RS. The role of FcRn in antigen presentation. Front Immunol. (2014);5:408. doi: 10.3389/fimmu.2014.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrantes FJ. Possible implications of dysregulated nicotinic acetylcholine receptor diffusion and nanocluster formation in myasthenia gravis. Neural Regen Res. (2021);16:242–246. doi: 10.4103/1673-5374.290880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blumberg LJ, Humphries JE, Jones SD, Pearce LB, Holgate R, Hearn A, Cheung J, Mahmood A, Del Tito B, Graydon JS, Stolz LE, Bitonti A, Purohit S, de Graaf D, Kacena K, Andersen JT, Christianson GJ, Roopenian DC, Hubbard JJ, Gandhi AK, et al. Blocking FcRn in humans reduces circulating IgG levels and inhibits IgG immune complex-mediated immune responses. Sci Adv. (2019);5:eaax9586. doi: 10.1126/sciadv.aax9586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Booth BJ, Ramakrishnan B, Narayan K, Wollacott AM, Babcock GJ, Shriver Z, Viswanathan K. Extending human IgG half-life using structure-guided design. mAbs. (2018);10:1098–1110. doi: 10.1080/19420862.2018.1490119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borghi S, Bournazos S, Thulin NK, Li C, Gajewski A, Sherwood RW, Zhang S, Harris E, Jagannathan P, Wang LX, Ravetch JV, Wang TT. FcRn, but not FcγRs, drives maternal-fetal transplacental transport of human IgG antibodies. Proc Natl Acad Sci U S A. (2020);117:12943–12951. doi: 10.1073/pnas.2004325117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brambell FWR. The passive immunity of the young mammal. Biol Rev. (1958);33:488–531. [Google Scholar]
- 11.Bril V, Benatar M, Andersen H, Vissing J, Brock M, Greve B, Kiessling P, Woltering F, Griffin L, Van den Bergh P, Investigators MG. Efficacy and safety of rozanolixizumab in moderate to severe generalized myasthenia gravis:a phase 2 randomized control trial. Neurology. (2021);96:e853–865. doi: 10.1212/WNL.0000000000011108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Challa DK, Wang X, Montoyo HP, Velmurugan R, Ober RJ, Ward ES. Neonatal Fc receptor expression in macrophages is indispensable for IgG homeostasis. mAbs. (2019);11:848–860. doi: 10.1080/19420862.2019.1602459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaudhury C, Brooks CL, Carter DC, Robinson JM, Anderson CL. Albumin binding to FcRn:distinct from the FcRn-IgG interaction. Biochemistry. (2006);45:4983–4990. doi: 10.1021/bi052628y. [DOI] [PubMed] [Google Scholar]
- 14.Dalakas MC, Spaeth PJ. The importance of FcRn in neuro-immunotherapies: From IgG catabolism, FCGRT gene polymorphisms, IVIg dosing and efficiency to specific FcRn inhibitors. Ther Adv Neurol Disord. (2021);14:1756286421997381. doi: 10.1177/1756286421997381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devanaboyina SC, Khare P, Challa DK, Ober RJ, Ward ES. Engineered clearing agents for the selective depletion of antigen-specific antibodies. Nat Commun. (2017);8:15314. doi: 10.1038/ncomms15314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fieux M, Le Quellec S, Bartier S, Coste A, Louis B, Giroudon C, Nourredine M, Bequignon E. FcRn as a transporter for nasal delivery of biologics:a systematic review. Int J Mol Sci. (2021);22:6475. doi: 10.3390/ijms22126475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gable KL, Guptill JT. Antagonism of the neonatal Fc receptor as an emerging treatment for myasthenia gravis. Front Immunol. (20191);10:3052. doi: 10.3389/fimmu.2019.03052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hehir MK, Silvestri NJ. Generalized myasthenia gravis:classification, clinical presentation, natural history, and epidemiology. Neurol Clin. (2018);36:253–260. doi: 10.1016/j.ncl.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Heo YA. Efgartigimod:first approval. Drugs. (2022);82:341–348. doi: 10.1007/s40265-022-01678-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howard JF, Jr, Bril V, Burns TM, Mantegazza R, Bilinska M, Szczudlik A, Beydoun S, Garrido FJRR, Piehl F, Rottoli M, Van Damme P, Vu T, Evoli A, Freimer M, Mozaffar T, Ward ES, Dreier T, Ulrichts P, Verschueren K, Guglietta A, et al. Randomized phase 2 study of FcRn antagonist efgartigimod in generalized myasthenia gravis. Neurology. (2019);92:e2661–2673. doi: 10.1212/WNL.0000000000007600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howard JF, Jr, Bril V, Vu T, Karam C, Peric S, Margania T, Murai H, Bilinska M, Shakarishvili R, Smilowski M, Guglietta A, Ulrichts P, Vangeneugden T, Utsugisawa K, Verschuuren J, Mantegazza R ADAPT Investigator Study Group. Safety, efficacy, and tolerability of efgartigimod in patients with generalised myasthenia gravis (ADAPT):a multicentre, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. (2021);20:526–536. doi: 10.1016/S1474-4422(21)00159-9. [DOI] [PubMed] [Google Scholar]
- 22.Junghans RP, Anderson CL. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc Natl Acad Sci U S A. (1996);93:5512–5516. doi: 10.1073/pnas.93.11.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karelis G, Balasa R, De Bleecker JL, Stuchevskaya T, Villa A, Van Damme P, Lagrange E, Heckmann JM, Nicolle M, Vilciu C, Bril V, Mondou E, Griffin R, Chen J, Henriquez W, Garcia B, Camprubi S, Ayguasanosa J. A phase 3 multicenter, prospective, open-label efficacy and safety study of immune globulin (Human) 10% caprylate/chromatography purified in patients with myasthenia gravis exacerbations. Eur Neurol. (2019);81:223–230. doi: 10.1159/000502818. [DOI] [PubMed] [Google Scholar]
- 24.Kasprick A, Hofrichter M, Smith B, Ward P, Bieber K, Shock A, Ludwig RJ, Schmidt E. Treatment with anti-neonatal Fc receptor (FcRn) antibody ameliorates experimental epidermolysis bullosa acquisita in mice. Br J Pharmacol. (2020);177:2381–2392. doi: 10.1111/bph.14986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khare P, Challa DK, Devanaboyina SC, Velmurugan R, Hughes S, Greenberg BM, Ober RJ, Ward ES. Myelin oligodendrocyte glycoprotein-specific antibodies from multiple sclerosis patients exacerbate disease in a humanized mouse model. J Autoimmun. (2018);86:104–115. doi: 10.1016/j.jaut.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Kiessling P, Lledo-Garcia R, Watanabe S, Langdon G, Tran D, Bari M, Christodoulou L, Jones E, Price G, Smith B, Brennan F, White I, Jolles S. The FcRn inhibitor rozanolixizumab reduces human serum IgG concentration: A randomized phase 1 study. Sci Transl Med. (2017);9:eaan1208. doi: 10.1126/scitranslmed.aan1208. [DOI] [PubMed] [Google Scholar]
- 27.Kim JK, Firan M, Radu CG, Kim CH, Ghetie V, Ward ES. Mapping the site on human IgG for binding of the MHC class I-related receptor, FcRn. Eur J Immunol. (1999);29:2819–2825. doi: 10.1002/(SICI)1521-4141(199909)29:09<2819::AID-IMMU2819>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 28.Laurence Blumberg M, John E Humphries M, Kenneth C Lasseter M, Richard S, Blumberg M. SYNT001:a humanized IgG4 monoclonal antibody that disrupts the interaction of FcRn and IgG for the treatment of IgG-mediated autoimmune diseases. Blood. (2017);130((Supplement 1)):3483. [Google Scholar]
- 29.Lim YY, Lim TS, Choong YS. Human IgG1 Fc pH-dependent optimization from a constant pH molecular dynamics simulation analysis. RSC Adv. (2020);10:13066–13075. doi: 10.1039/c9ra10712f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ling LE, Roy S, Daly T, Cochran E, Tyler S, Markowitz L, Bulik D, Choudhury A, Meador J, Parge V, Mekala D, Sipsey S, Gurnani S, Duffner J, Lee S, Washburn N, Meccariello R, Schaeck J, Wang J, Schultes B, et al. M281:a therapeutic anti-FcRn blocking antibody for rapid clearance of IgG and IgG autoantibodies in immune cytopenias and other auto/allo-immune disease. Blood. (2015);126:3472. [Google Scholar]
- 31.Ling LE, Hillson JL, Tiessen RG, Bosje T, van Iersel MP, Nix DJ, Markowitz L, Cilfone NA, Duffner J, Streisand JB, Manning AM, Arroyo S. M281, an anti-FcRn antibody:pharmacodynamics, pharmacokinetics, and safety across the full range of IgG reduction in a first-in-human study. Clin Pharmacol Ther. (2019);105:1031–1039. doi: 10.1002/cpt.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu C, Liu P, Ma M, Yang H, Qi G. Efficacy and safety of double-filtration plasmapheresis treatment of myasthenia gravis: A systematic review and meta-analysis. Medicine. (2021);100:e25622. doi: 10.1097/MD.0000000000025622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Macri C, Morgan H, Villadangos JA, Mintern JD. Regulation of dendritic cell function by Fc-gamma-receptors and the neonatal Fc receptor. Mol Immunol. (2021);139:193–201. doi: 10.1016/j.molimm.2021.07.024. [DOI] [PubMed] [Google Scholar]
- 34.Menon D, Bril V. Pharmacotherapy of generalized myasthenia gravis with special emphasis on newer biologicals. Drugs. (2022);82:865–887. doi: 10.1007/s40265-022-01726-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mezo AR, McDonnell KA, Castro A, Fraley C. Structure-activity relationships of a peptide inhibitor of the human FcRn:human IgG interaction. Bioorg Med Chem. (2008a);16:6394–6405. doi: 10.1016/j.bmc.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Mezo AR, McDonnell KA, Hehir CA, Low SC, Palombella VJ, Stattel JM, Kamphaus GD, Fraley C, Zhang Y, Dumont JA, Bitonti AJ. Reduction of IgG in nonhuman primates by a peptide antagonist of the neonatal Fc receptor FcRn. Proc Natl Acad Sci U S A. (2008b);105:2337–2342. doi: 10.1073/pnas.0708960105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Momenta Pharmaceuticals, Inc. (2020) Momenta Pharmaceuticals Announces Positive Topline Data from Interim Analysis of Phase 2 Vivacity-MG Study of Nipocalimab (M281) in Generalized Myasthenia Gravis (gMG) [Accessed October 2, 2022]. https://www.globenewswire.com/news-release/2020/06/15/2047945/0/en/Momenta-Pharmaceuticals-Announces-Positive-Topline-Data-from-Interim-Analysis-of-Phase-2-Vivacity-MG-Study-of-Nipocalimab-M281-in-Generalized-Myasthenia-Gravis-gMG.html.
- 38.Narayanaswami P, Sanders DB, Wolfe G, Benatar M, Cea G, Evoli A, Gilhus NE, Illa I, Kuntz NL, Massey J, Melms A, Murai H, Nicolle M, Palace J, Richman D, Verschuuren J. International consensus guidance for management of myasthenia gravis:2020 Update. Neurology. (2021);96:114–122. doi: 10.1212/WNL.0000000000011124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nath N, Godat B, Flemming R, Urh M. Deciphering the interaction between neonatal Fc receptor and antibodies using a homogeneous bioluminescent immunoassay. J Immunol. (2021);207:1211–1221. doi: 10.4049/jimmunol.2100181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Newland AC, Sanchez-Gonzalez B, Rejto L, Egyed M, Romanyuk N, Godar M, Verschueren K, Gandini D, Ulrichts P, Beauchamp J, Dreier T, Ward ES, Michel M, Liebman HA, de Haard H, Leupin N, Kuter DJ. Phase 2 study of efgartigimod, a novel FcRn antagonist, in adult patients with primary immune thrombocytopenia. Am J Hematol. (2020);95:178–187. doi: 10.1002/ajh.25680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oganesyan V, Damschroder MM, Cook KE, Li Q, Gao C, Wu H, Dall'Acqua WF. Structural insights into neonatal Fc receptor-based recycling mechanisms. J Biol Chem. (2014);289:7812–7824. doi: 10.1074/jbc.M113.537563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel DA, Puig-Canto A, Challa DK, Perez Montoyo H, Ober RJ, Ward ES. Neonatal Fc receptor blockade by Fc engineering ameliorates arthritis in a murine model. J Immunol. (2011);187:1015–1022. doi: 10.4049/jimmunol.1003780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peter HH, Ochs HD, Cunningham-Rundles C, Vinh DC, Kiessling P, Greve B, Jolles S. Targeting FcRn for immunomodulation: Benefits, risks, and practical considerations. J Allergy Clin Immunol. (2020);146:479–491. doi: 10.1016/j.jaci.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pilati D, Howard KA. Albumin-based drug designs for pharmacokinetic modulation. Expert Opin Drug Metab Toxicol. (2020);16:783–795. doi: 10.1080/17425255.2020.1801633. [DOI] [PubMed] [Google Scholar]
- 45.Pyzik M, Rath T, Kuo TT, Win S, Baker K, Hubbard JJ, Grenha R, Gandhi A, Krämer TD, Mezo AR, Taylor ZS, McDonnell K, Nienaber V, Andersen JT, Mizoguchi A, Blumberg L, Purohit S, Jones SD, Christianson G, Lencer WI, et al. Hepatic FcRn regulates albumin homeostasis and susceptibility to liver injury. Proc Natl Acad Sci U S A. (2017);114:E2862–2871. doi: 10.1073/pnas.1618291114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pyzik M, Sand KMK, Hubbard JJ, Andersen JT, Sandlie I, Blumberg RS. The neonatal Fc receptor (FcRn): A misnomer? Front Immunol. (2019);10:1540. doi: 10.3389/fimmu.2019.01540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rabbani G, Ahn SN. Structure, enzymatic activities, glycation, and therapeutic potential of human serum albumin: A natural cargo. Int J Biol Macromol. (2019);123:979–990. doi: 10.1016/j.ijbiomac.2018.11.053. [DOI] [PubMed] [Google Scholar]
- 48.Rath T, Kuo TT, Baker K, Qiao SW, Kobayashi K, Yoshida M, Roopenian D, Fiebiger E, Lencer WI, Blumberg RS. The immunologic functions of the neonatal Fc receptor for IgG. J Clin Immunol. (2013);33(Suppl 1(Suppl 1)):S9–17. doi: 10.1007/s10875-012-9768-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Remlinger J, Madarasz A, Guse K, Hoepner R, Bagnoud M, Meli I, Feil M, Abegg M, Linington C, Shock A, Boroojerdi B, Kiessling P, Smith B, Enzmann V, Chan A, Salmen A. Antineonatal Fc receptor antibody treatment ameliorates MOG-IgG-associated experimental autoimmune encephalomyelitis. Neurol Neuroimmunol Neuroinflamm. (2022);9:e1134. doi: 10.1212/NXI.0000000000001134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodewald R, Kraehenbuhl JP. Receptor-mediated transport of IgG. J Cell Biol. (1984);99:159s–164s. doi: 10.1083/jcb.99.1.159s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roopenian DC, Akilesh S. FcRn:the neonatal Fc receptor comes of age. Nat Rev Immunol. (2007);7:715–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 52.Simister NE, Mostov KE. An Fc receptor structurally related to MHC class I antigens. Nature. (1989);337:184–187. doi: 10.1038/337184a0. [DOI] [PubMed] [Google Scholar]
- 53.Smith B, Kiessling A, Lledo-Garcia R, Dixon KL, Christodoulou L, Catley MC, Atherfold P, D'Hooghe LE, Finney H, Greenslade K, Hailu H, Kevorkian L, Lightwood D, Meier C, Munro R, Qureshi O, Sarkar K, Shaw SP, Tewari R, Turner A, et al. Generation and characterization of a high affinity anti-human FcRn antibody, rozanolixizumab, and the effects of different molecular formats on the reduction of plasma IgG concentration. MAbs. (2018);10:1111–1130. doi: 10.1080/19420862.2018.1505464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith B, Christodoulou L, Clargo A, Eddleston A, Greenslade K, Lightwood D, Shock A, Tyson K, Brennan FR. Generation of two high affinity anti-mouse FcRn antibodies: Inhibition of IgG recycling in wild type mice and effect in a mouse model of immune thrombocytopenia. Int Immunopharmacol. (2019);66:362–365. doi: 10.1016/j.intimp.2018.11.040. [DOI] [PubMed] [Google Scholar]
- 55.Sun W, Khare P, Wang X, Challa DK, Greenberg BM, Ober RJ, Ward ES. Selective depletion of antigen-specific antibodies for the treatment of demyelinating disease. Mol Ther. (2021);29:1312–1323. doi: 10.1016/j.ymthe.2020.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.UCB (2021) UCB announces positive Phase 3 results for rozanolixizumab in generalized myasthenia gravis. [Accessed October 2, 2022]. https://www.ucb.com/stories-media/Press-Releases/article/UCBannounces-positive-Phase-3-results-for-rozanolixizumab-in-generalized-myastheniagravis.
- 57.Ulrichts P, Guglietta A, Dreier T, Bragt Tv, Hanssens V, Hofman E, Vankerckhoven B, Verheesen P, Ongenae N, Lykhopiy V, Enriquez FJ, Cho J, Ober RJ, Ward ES, Haard Hd, Leupin N. Neonatal Fc receptor antagonist efgartigimod safely and sustainably reduces IgGs in humans. J Clin Invest. (2018);128:4372–4386. doi: 10.1172/JCI97911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vaccaro C, Zhou J, Ober RJ, Ward ES. Engineering the Fc region of immunoglobulin G to modulate in vivo antibody levels. Nat Biotechnol. (2005);23:1283–1288. doi: 10.1038/nbt1143. [DOI] [PubMed] [Google Scholar]
- 59.Wani MA, Haynes LD, Kim J, Bronson CL, Chaudhury C, Mohanty S, Waldmann TA, Robinson JM, Anderson CL. Familial hypercatabolic hypoproteinemia caused by deficiency of the neonatal Fc receptor, FcRn, due to a mutant beta2-microglobulin gene. Proc Natl Acad Sci U S A. (2006);103:5084–5089. doi: 10.1073/pnas.0600548103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ward ES, Gelinas D, Dreesen E, Van Santbergen J, Andersen JT, Silvestri NJ, Kiss JE, Sleep D, Rader DJ, Kastelein JJP, Louagie E, Vidarsson G, Spriet I. Clinical significance of serum albumin and implications of FcRn inhibitor treatment in IgG-mediated autoimmune disorders. Front Immunol. (2022);13:892534. doi: 10.3389/fimmu.2022.892534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Werth VP, Culton DA, Concha JSS, Graydon JS, Blumberg LJ, Okawa J, Pyzik M, Blumberg RS, Hall RP., 3rd Safety, tolerability, and activity of ALXN1830 targeting the neonatal Fc receptor in chronic pemphigus. J Invest Dermatol. (2021);141:2858–2865. doi: 10.1016/j.jid.2021.04.031. [DOI] [PubMed] [Google Scholar]
- 62.Xiao ZX, Miller JS, Zheng SG. An updated advance of autoantibodies in autoimmune diseases. Autoimmun Rev. (2021);20:102743. doi: 10.1016/j.autrev.2020.102743. [DOI] [PubMed] [Google Scholar]
- 63.Yan C, Duan RS, Yang H, Li HF, Zou Z, Zhang H, Zhou H, Li XL, Zhou H, Jiao L, Chen J, Yin J, Du Q, Lee M, Chen Y, Chen X, Zhao C. Therapeutic effects of batoclimab in Chinese patients with generalized myasthenia gravis:a double-blinded, randomized, placebo-controlled phase II study. Neurol Ther. (2022);11:815–834. doi: 10.1007/s40120-022-00345-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yap DYH, Hai J, Lee PCH, Zhou X, Lee M, Zhang Y, Wang M, Chen X. Safety, tolerability, pharmacokinetics, and pharmacodynamics of HBM9161, a novel FcRn inhibitor, in a phase I study for healthy Chinese volunteers. Clin Transl Sci. (2021);14:1769–1779. doi: 10.1111/cts.13019. [DOI] [PMC free article] [PubMed] [Google Scholar]


