Abstract
Background and Hypothesis
Risk-taking in specific contexts can be beneficial, leading to rewarding outcomes. Schizophrenia is associated with disadvantageous decision-making, as subjects pursue uncertain risky rewards less than controls. However, it is unclear whether this behavior is associated with more risk sensitivity or less reward incentivization. Matching on demographics and intelligence quotient (IQ), we determined whether risk-taking was more associated with brain activation in regions affiliated with risk evaluation or reward processing.
Study Design
Subjects (30 schizophrenia/schizoaffective disorder, 30 controls) completed a modified, fMRI Balloon Analogue Risk Task. Brain activation was modeled during decisions to pursue risky rewards and parametrically modeled according to risk level.
Study Results
The schizophrenia group exhibited less risky-reward pursuit despite previous adverse outcomes (Average Explosions; F(1,59) = 4.06, P = .048) but the comparable point at which risk-taking was volitionally discontinued (Adjusted Pumps; F(1,59) = 2.65, P = .11). Less activation was found in schizophrenia via whole brain and region of interest (ROI) analyses in the right (F(1,59) = 14.91, P < 0.001) and left (F(1,59) = 16.34, P < 0.001) nucleus accumbens (NAcc) during decisions to pursue rewards relative to riskiness. Risk-taking correlated with IQ in schizophrenia, but not controls. Path analyses of average ROI activation revealed less statistically determined influence of anterior insula upon dorsal anterior cingulate bilaterally (left: χ2 = 12.73, P < .001; right: χ2 = 9.54, P = .002) during risky reward pursuit in schizophrenia.
Conclusions
NAcc activation in schizophrenia varied less according to the relative riskiness of uncertain rewards compared to controls, suggesting aberrations in reward processing. The lack of activation differences in other regions suggests similar risk evaluation. Less insular influence on the anterior cingulate may relate to attenuated salience attribution or inability for risk-related brain region collaboration to sufficiently perceive situational risk.
Keywords: Psychosis, decision-making, cingulate, risk-taking, fMRI, schizoaffective disorder, nucleus accumbens
Introduction
Uncertain risky decision-making in schizophrenia has been interpreted as more “risk averse” than that of nonpsychiatric controls.1–3 However, the construct of risk aversion is difficult to isolate given potentially confounding factors inherent to tasks measuring uncertain risk-taking, including reinforcement learning, reward sensitivity, effort allocation (eg, repeated button presses), or risk imperception.4,5 Schizophrenia is associated with impaired reinforcement learning, low motivation, and defeatist performance beliefs associated with atypical decision-making.6–9 Thus, while behavioral results on uncertain risk-taking tasks consistently indicate less risky reward pursuit in schizophrenia,5 it is unclear which specific psychological processes and neural substrates underlie this decision-making often attributed to risk-averse attitude.
The Balloon Analogue Risk Task (BART10) measures uncertain risk-taking behavior by presenting opportunities for larger monetary rewards accompanied by the higher risk of a null outcome. It is a measure of uncertain, sequential risk-taking as the probability of outcome likelihoods are not explicitly communicated and decisions to pursue risky rewards beget further opportunities for risky rewards.11,12 Despite idiosyncratic features, behavioral and fMRI BART versions are reliable tasks requiring learning to optimize risk-taking for a reward via trial and error.4,13,14
Behavioral BART investigations have consistently found disadvantageous choice behavior in people with schizophrenia compared to controls, with all finding fewer explosions, most finding less adjusted pumps (ie, the average number of inflations on unexploded balloons), and roughly half finding less money earned.1–3,5,15–18 A modified fMRI version of the BART found fewer explosions relative to controls despite no differences in money earned (adjusted pumps were not reported) in male participants with schizophrenia.19 Interestingly, less risk-taking has been observed in bipolar disorder with a history of psychosis but not bipolar disorder generally, potentially related to differing profiles in risk and reward processing.3,20 However, across the literature psychiatric groups have consistently exhibited lower cognitive functioning than controls by at least 10 points (t-scores),5 so is it unclear if behavioral differences are better attributed to general cognitive deficits common to schizophrenia, rather than genuine differences in risk attitudes, risk perception, or reward processing. Despite this, findings suggest that when choosing between lesser guaranteed rewards, or greater uncertain risky rewards, those with schizophrenia generally prefer the former.
Several brain regions have been implicated during risky reward pursuit on the BART, including the anterior insula (AI), dorsal anterior cingulate cortex (dACC), and striatum (differing studies implicate nucleus accumbens (NAcc), caudate, and/or putamen13). Activation in these regions has good test-retest reliability on the BART, as have the dorsolateral prefrontal cortex, thalamus, and occipital lobe.14
The AI is implicated in risk and uncertainty evaluation,21 with activation scaling according to decision uncertainty, associated with real-time updating of risk, and predicting choice behavior.22–25 The AI is also associated with the formulation of prediction errors, with an emphasis on potential losses.26–28 Parametrically modulated AI activation has consistently been found to scale to risk on the BART.14,29,30 Surprisingly, no AI differences have been found in schizophrenia during ambiguous or uncertain risk-taking19,31; however, region of interest (ROI) analyses have found less activation during reward anticipation,32,33 suggesting potential deficits in attentional and salience processing inherent to the uncertainty of monetary gains/losses on cognitive and reward-processing tasks.
The NAcc has a prominent role in risky and uncertain decision-making,34 with greater activation associated with reward expectation and pursuit.25,35,36 During risky choice, NAcc activation has scaled to potential gains/losses,37 and is greater when riskier rewards are pursued.38–40 In chronic schizophrenia and first-episode psychosis, less NAcc activation has been found using reward anticipation,41–43 reversal learning,44 probabilistic decision-making,45 reinforcement, and associative learning tasks,46,47 suggesting a heavy role in affective valuation and prediction error signaling.48,49 However, not all studies of reward anticipation in schizophrenia have found between-group differences in NAcc activation.50–52
It is currently unclear whether those with schizophrenia demonstrate differences in brain activation during risky reward pursuit subserving reward incentivization or risky/uncertain decision-making. Investigating this via the BART, Tikàsz and colleagues19 did not find any activation differences between a psychosis group and controls, however, subjects were entirely male and controls had higher intelligence quotient (IQ). Additionally, the clinical group (n = 47) was double the control group (n = 23). Considering these caveats, it is difficult to interpret or contextualize these findings.
The current investigation is the first to study uncertain risky reward pursuit via the BART in male, female, and non-binary subjects with schizophrenia/schizoaffective disorder matched to controls on IQ, to determine if schizophrenia is associated with differential brain activation in regions associated with reward processing or risk evaluation inherent to uncertain decision-making. These findings will inform theories of whether uncertain risky reward pursuit in schizophrenia is better attributed to risk aversion, blunted reward anticipation, or may highlight a biologically informed common process underlying both.
Methods and Materials
Participants
The final sample included 30 participants with schizophrenia/schizoaffective disorder and 30 controls with no psychiatric history. Subjects were recruited from local mental health centers, paper flyers, and online ads (eg, Facebook, Craigslist, and Reddit) in Bloomington and Indianapolis, IN. Participant transportation and/or travel reimbursement was provided. Controls were matched controls based on age, sex, race, and IQ. Details regarding clinical and neurocognitive assessments are in table 1. For consent and excluded subject information see supplementary materials.
Table 1.
Demographics, Clinical Characteristics, and Uncertain Risk-Taking Behavior
| Schizophrenia (n = 30) | Control (n = 30) | Statistic | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 33.13 (8.65) | 32.63 (8.52) | F = 0.05 |
| Assigned female at birth⊕ (%) | 15 (50%) | 15 (50%) | χ 2 < 0.001 |
| Race/Ethnicity A/AA/HLNA/MTO/W | 1/4/0/6/19 | 1/4/3/3/19 | χ 2 = 4.00 |
| MTO: | AA/HLNA (2); AA/W (2); HLNA/W (2) | AA/W (1); HLNA/W (2) | |
| Neurocognitive (WASI) IQ measures | |||
| Full-scale intelligence quotient-2 | 108.67 (10.64) | 109.50 (8.93) | F = 0.11 |
| Vocabulary | 53.50 (9.34) | 55.73 (6.07) | F = 1.21 |
| Matrix reasoning | 56.33 (4.79) | 55.43 (6.84) | F = 0.35 |
| Neurocognitive (WAIS) working memory measures | |||
| Working memory mean (DS+LNS) | 9.75 (2.18) | 11.43 (2.88) | F = 6.49* |
| Digit Symbol (DS) | 8.67 (2.35) | 10.93 (3.00) | F = 10.58** |
| Letter-number sequencing (LNS) | 10.83 (2.98) | 11.93 (3.55) | F = 1.69 |
| Balloon analog risk task (BART) Risk-taking behavior | |||
| Adjusted Pumps | 4.79 (0.96) | 5.18 (0.88) | F = 2.65 |
| Average # Explosions Per Block | 5.05 (2.01) | 6.10 (2.03) | F = 4.06* |
| Psychosis symptom assessments | |||
| Psychosis SZ/SZA_BD/SZA_MDD | 14/7/9 | — | |
| CAPE total count (min = 0, max = 42) | 26. 54 (9.42) | 10.53 (6.63) | F = 57.81*** |
| CAPE total frequency | 2.04 (0.58) | 1.27 (0.17) | U = 829.5*** |
| CAPE total distress† | 2.11 (0.59) | 1.16 (0.25) | U = 835.5*** |
| PANSS positive (min = 7, max = 49) | 16.10 (5.25) | — | |
| PANSS negative (min = 7, max = 49) | 15.00 (4.96) | — | |
| PANSS general (min = 16, max=112) | 29.45 (7.51) | — | |
| YMRS⊗ (n = 5) | 2.00 (1.58) | — | |
A, Asian; AA, African American; W,White; HLNA, Hispanic/Latino/Native American; MTO, more than one; CAPE, Community Assessment of Psychic Experiences (CAPE-42); PANSS, Positive and Negative Syndrome Scale; SZ, schizophrenia; SZA_BD, bipolar schizoaffective disorder; SZA_MDD, unipolar depressive schizoaffective disorder; YMRS, Young Mania Rating Scale.
⊗YMRS was added later in the study protocol, precluding 2 subjects.
⊕Two transgender subjects were included within the SZ group, 1 assigned male at birth identified as non-binary and 1 assigned female at birth identified as male.
†See “Cape Scoring Note” and subscale scores in Supplementary Materials.
*<.05; **<.01; ***<.001.
Modified fMRI Balloon Analog Risk Task
BART trials consisted of a balloon, response cue, wager amount (monetary value of current balloon), and total money earned across previous trials (supplementary figure S1). Subjects decided between either inflating the balloon, causing it to expand, or choosing to “win” by discontinuing inflations and adding the wager amount to the total money. The response cue indicated when participants could respond by turning from red to green. After each decision and an imposed delay of 0, 2, 4, or 6 s, the balloon would inflate, explode, or be cashed out. Following successful inflations, the balloon would increase in size and monetary value, and the response cue would turn red for 1.5, 2, or 2.5 s, forcing subjects to wait before responding. If the inflation was unsuccessful, an exploded balloon was presented for 0.5 s followed by “You Lose!” for 1 s. Following cash-outs “You Win!” was visible for 1 s. After each trial, the screen was blank for 2–4 s, after which a new balloon trial was initiated. Delays were imposed for separate estimations of BOLD response during choice (inflate/win) and outcome (explosion, successful inflation, or “You Win”).
The fMRI BART19,30,53 is modified from the behavioral version in 2 ways: (1) Each balloon could potentially be inflated a maximum of 12 times instead of 128, and (2) each inflation increased the explosion probability and potential monetary value (Pearson Correlation, r = 0.995) exponentially rather than linearly. This means that within each trial later inflations were worth more than early inflations (eg, first inflation = +$0.05; last possible inflation = +$0.90; supplementary table S1). Subjects completed three (one subject only completed 2 task blocks due to time restrictions) 8-min task runs, which began with a 30-s fixation cross.
There are 2 behavioral indices of risk-taking from the fMRI BART. Average Number of Explosions indicates risk-taking as a measure of continued, risky reward pursuit despite previous adverse outcomes.4,54 Whereas Adjusted Pumps (ie, average number of inflations on unexploded balloons), indicates the average point that risk-taking for reward is intentionally discontinued.10 See Supplementary Materials for additional, descriptive BART measures.
Procedures
Participation occurred over 2 laboratory visits consisting of clinical, neurocognitive, and demographic assessments, task practice, MRI scanning, and self-report measures. Urine drug screening ruled out non-prescription use of methamphetamine, cocaine, amphetamine, opioids, or benzodiazepines. Five participants with schizophrenia screened positive for cannabis without abuse/dependence. During the task, practice subjects read instructions on a desktop computer (Supplementary Materials). For practice, subjects inflated several balloons (schizophrenia M = 4.58, SD = 0.89; control M = 4.48, SD = 1.05; p = .79). Task practice was briefer than prior studies,19,53 to familiarize subjects with the task mechanics without allowing uncertainty-related reward learning before experimentation. Practice continued until subjects demonstrated familiarity with delaying button presses, post-button-pressing delays, and gained awareness that a balloon could be inflated at least 4 times (if at least 1 practice balloon was not inflated at least 4 times subjects were given the vague, nonchalant instruction “Go ahead and give this last balloon a few more pumps,” to ensure they were aware the balloon could be inflated at least 4 times, so that if they chose to take risks during the task, brain activation could be adequately modeled).
MRI Data Collection
Images were acquired using a 3T Siemens Prisma MRI scanner with a 64-channel head coil. High-resolution T1-weighted anatomical images were acquired sagittally using an MP-RAGE sequence (TR = 1.8 s; TE = 2.7 ms; inversion time = 0.9 s; flip angle 9°; imaging matrix = 256 × 256; 160 slices; sequential multislice acquisition; voxel size = 1 × 1 × 1 mm3, 1-mm slice thickness). Functional BOLD data were collected using a gradient echo T2-weighted echo planar imaging sequence (TR = 2.0 s; TE = 25 ms; flip angle 70°; imaging matrix = 64 × 64; 35 slices; voxel size = 3.4 × 3.4 × 3.8 mm3; 0-mm gap; 240 volumes).
Image Processing and Event-Related Modeling
T1-weighted anatomical scans were corrected for intensity non-homogeneity, reoriented for AC-PC alignment, and skull-stripped (FreeSurfer v6.0.0). Functional (EPI) scans of each subject were preprocessed using AFNI (v18.3.03) as follows: Despiking, slice-timing correction, motion correction, deobliquing (for better alignment during co-registration), linear co-registration (9 translation, rotation, and scaling parameters; 1st-EPI volume used in alignment; cost function = normalized mutual information), spatial normalization to MNI152 template (3dQwarp), and smoothing (FWHM = 6 mm). Within-subject (ie, first level) analyses were performed in SPM12 (Matlab v2019a) according to a general linear model (GLM) with 9 event-related regressors: 2 choice, 3 outcome, and 4 parametrically modulated regressors. A constant regressor was modeled for each run. If there was a paucity of events (eg, no explosions), runs were collapsed and modeled as a single run. Motion regressors (6 motion, 6 squared motion, 6 differential motion, and 6 squared differential motion regressors) were included for all subjects.
Choice regressors included choosing inflations (ChooseInflate) or stopping inflations/cashing-out winnings (ChooseWin). Outcome regressors included inflations (SuccessfulInflate), explosions (ExplodeOutcome) followed by loss feedback, and win feedback (WinOutcome). The explosion probabilities of each balloon (supplementary table S1) were included as parametrically modulated (pmod) regressors to determine whether regressor height corresponded with situational uncertainty risk. ChooseInflate_pmod and ChooseWin_pmod were included for choice regressors, while SuccessfulInflate_pmod and ExplodeOutcome_pmod were included for outcome regressors. Pmod analyses model brain activation according to each sequentially risky inflation per balloon. Thus, activation differences according to relative riskiness within trial are modeled regardless of whether groups differ in behavior averaged across trials.
Hypotheses and Analysis Plan
Given behavior-only BART findings,5 schizophrenia subjects were hypothesized to exhibit less risk-taking via Adjusted Pumps and Average Explosions. Only less Average Explosions were reported in a previous BART fMRI investigation.19 As the first investigation to match groups on IQ, Pearson correlations between IQ and risk-taking measures were conducted. Positive correlations were expected only in schizophrenia, as cognitive deficits in schizophrenia have been related to less risky decision-making.2,3,5 As our sample included a broad range of IQs (schizophrenia: 82–125; control: 90–130), we expected stronger correlations in schizophrenia than in previous investigations. For between-group differences in correlation coefficient, within-group correlations are reported; otherwise, correlations across the entire sample are reported. However, all within-group correlations are reported descriptively for comparison to previous findings (supplementary table S9).2,3 Despite individual findings associating BART risk-taking with negative,3 positive,2 and disorganized symptoms,1 none have been replicated.5 Given this, and our modest sample size, symptom correlations were not pursued. Behavioral analyses were performed in SPSS (v28) and R (v4.2.2) and correlations were Bonferroni corrected for multiple comparisons.
Within-group whole-brain activation for each condition was reported descriptively (supplementary tables S5–S8) to contextualize between-group differences or lack thereof. Consistent with previous fMRI BART investigations,53,55 all contrasts during decision and outcome periods are reported. Between-group whole-brain analyses thresholded at a cluster-defining threshold of P = .001 and cluster significance threshold of P < .05, were undertaken for general and parametrically modulated choice and outcome regressors to determine differences and provide full dataset result transparency. Given our interest in risky decision-making, we focus particularly on whole-brain results for ChooseInflate–ChooseWin and ChooseInflate_pmod, which respectively model decisions to pursue risky rewards across trials and risky reward pursuit relative to within-trial riskiness.
As we were interested in decision-making relative to risky rewards,53a priori NAcc, AI, and dACC ROI analyses during ChooseInflate_pmod were undertaken. This approach is consistent with prior ROI-specific findings in the literature, despite the lack of whole-brain activation differences in schizophrenia.32,33,56 Five ROIs were identified as left/right NAcc, left/right AI, and bilateral dACC, based upon anatomically informed regions implicated in risk aversion, reward anticipation, and cognitive control identified in the BART and schizophrenia13,33,55 (supplementary figure S3; Supplementary Materials). Average activation beta weights were extracted (SPM12) and between-group ANOVAs were Bonferroni corrected for 5 comparisons. Given the bilateral NAcc’s specific association with reward prediction signaling,42 decision-making in schizophrenia,57 and fluid intelligence,58 Bonferroni corrected correlations were conducted between NAcc activation, IQ, and risk-taking measures according to the aforementioned convention (entire-sample correlation unless groups differed in correlation coefficient).
Path analysis during general risk-taking (ChooseInflate–ChooseWin) was undertaken to statistically discern whether average activation within a predetermined ROI was influenced by average activation in another through directional model pathways. This is investigated via regression modeling; comparing the relationship between regions in the observed data to those predicted by the model.59 This method does not measure temporally determined brain connectivity. Path analysis directionality was chosen based on well-established, anatomically supported theories that AI and dACC projections to NAcc may temper motivated behavior, uncertainty processing, and valuation-based decision-making.60,61 This model tests the hypothesis that reward-anticipation NAcc activation during risky decision-making in schizophrenia is more influenced by risk-aversion signaling (AI) and if NAcc signals are mediated by cognitive valuation and integration with prior outcomes (dACC62). Path analyses conducted using AMOS (SPSS v28) yielded between-group differences operationalized by path coefficients between ROIs in within-group models.
Results
Behavioral Analyses and Descriptive Correlations
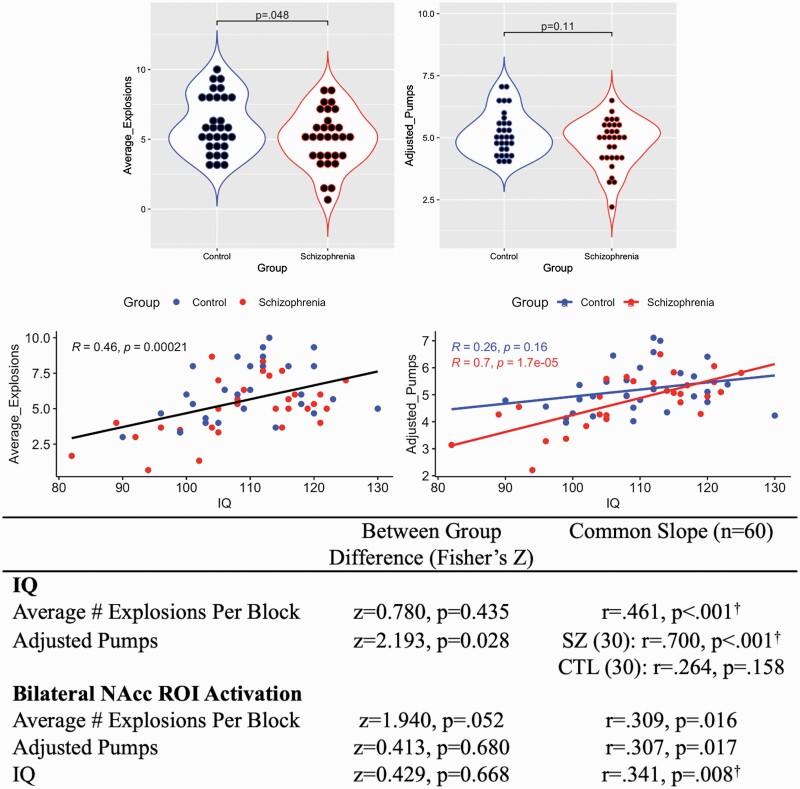
Consistent with previous fMRI BART findings in schizophrenia,19 Average Explosions was greater in controls (F(1,59) = 4.06, P = .048, η2 = 0.065), indicating more risk-taking despite previous adverse outcomes (table 1). There were no between-group differences in Adjusted Pumps (P = .11). Between groups, only the correlation between IQ and Adjusted Pumps was significantly stronger in schizophrenia relative to controls (z = 2.193, P = .028; figure 1). Across the entire sample, IQ positively correlated with Average Explosions.
Fig. 1.
Correlations between intelligence quotient (IQ), risk-taking, and NAcc activation. Top: Adjusted pumps are average inflations on unexploded balloons. Average explosions per task run. Middle: IQ and risk-taking scatter plots. Bottom: IQ, risk-taking, and NAcc activation correlations. †indicates correlations surviving Bonferroni correction for 6 analyses.
fMRI Results
Whole-Brain Between-Group Results.
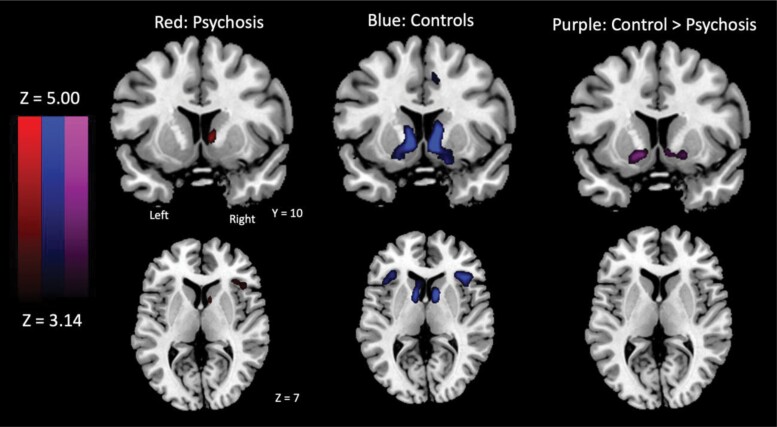
During decision-making, no between-group differences emerged during general risky reward pursuit (ChooseInflate–ChooseWin). However, during decisions to pursue rewards modulated by uncertainty risk (ChooseInflate_pmod), the schizophrenia group exhibited less NAcc activation than controls (figure 2; table 2). Decision-outcomes results are reported in supplementary table S12.
Figure 2.
Whole-brain activation clusters in the striatum and insula during decisions to pursue risky rewards relative to risk (ChooseInflate_pmod).
Table 2.
Whole-Brain Neuroimaging Results for ChooseInflate_pmod
| Region | Laterality | Cluster Size | Peak X | Peak Y | Peak Z | Max stat Z | P Cluster corrected |
|---|---|---|---|---|---|---|---|
| Control group | |||||||
| Caudate/NAcc | R | 177 | 8 | 10 | −4 | 6.21 | <.001 |
| Caudate/NAcc | L | 190 | −8 | 8 | −4 | 5.93 | <.001 |
| Pars orbitalis/anterior insula (45/47) | R | 150 | 40 | 26 | 2 | 5.39 | <.001 |
| Pars orbitalis/anterior insula (45/47) | L | 120 | −38 | 28 | −4 | 5.04 | <.001 |
| White matter | L | 34 | −28 | −50 | 14 | 4.98 | .009 |
| Schizophrenia group | |||||||
| Caudate | R | 24 | 8 | 10 | 2 | 4.43 | .04 |
| Anterior insula (45) | R | 40 | 34 | 28 | 4 | 3.91 | .03 |
| Control group – S chizophrenia group | |||||||
| NAcc | L | 50 | −10 | 2 | −14 | 4.32 | .001 |
| Premotor (6) | L | 29 | −4 | −2 | 80 | 4.05 | .029 |
| NAcc | R | 34 | 8 | 10 | −4 | 3.97 | .013 |
| Occipital (19) | R | 34 | 32 | −88 | −16 | 3.96 | .013 |
Between-Group ROI Analyses and Correlations.
Bonferroni-corrected ROI analyses confirmed less right (F(1,59) = 14.91, P < .001, η2 = 0.205) and left (F(1,59) = 16.34, P< .001, η2 = 0.220) NAcc activation in schizophrenia compared to controls during ChooseInflate_pmod. There were no between-group differences in left/right AI or dACC activation. Groups did not differ in bilateral NAcc activation and risk-taking correlation coefficients, and entire-sample correlations did not survive Bonferroni correction (figure 1). Bilateral NAcc activation across the entire sample positively correlated with IQ.
Path Analysis Results.
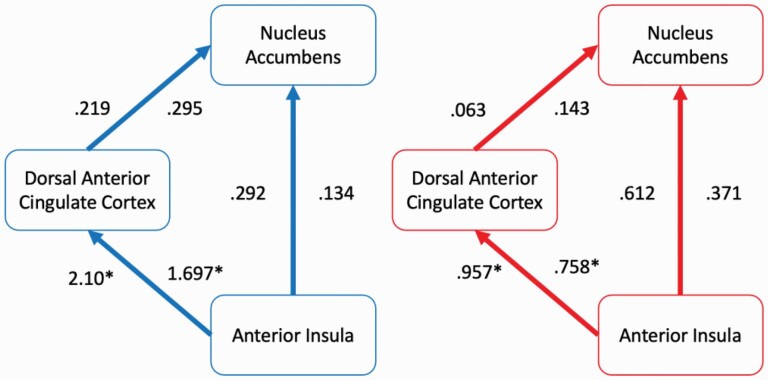
The path fit from AI-to-dACC was lower in schizophrenia in left (χ2 = 12.73, P < .001; figure 3) and right (χ2 = 9.54, P = .002) hemispheres compared to controls. Similarly, indirect NAcc-to-dACC-to-AI path coefficients were lower in left (χ2 = 13.44, P = .001) and right (χ2 = 10.63, P < .001) hemispheres. There were no significant AI-to-NAcc nor dACC-to-NAcc path differences. A model with non-significant AI-to-NAcc and dACC-to-NAcc constrained between groups demonstrated good fit (Left χ2 = 0.78, P = .678, comparative fit index (CFI) = 1.00, incremental fit index (IFI) = 1.013; Right χ2 = 1.17, P = .558, CFI = 1.00, IFI = 1.010). The second model with just the non-significant AI-to-NAcc constrained also demonstrated good fit (Left χ2 = 0.70, P = .405, CFI = 1.000, IFI = 1.003; Right χ2 = 0.90, P = .345, CFI = 1.000, IFI = 1.001).
Fig. 3.
Path analysis results during risk-taking (ChooseInflate–ChooseWin). Anterior insula (AI) and nucleus accumbens regions of interest (ROIs) were unilateral. Anterior cingulate ROI was bilateral. Numbers left of the arrows reflect left hemisphere path coefficients and numbers on the right reflect right hemisphere coefficients. Blue/Left: controls. Red/Right: schizophrenia group.
Discussion
While the behavioral BART investigates uncertain risk-taking, it is unclear whether parallel constructs such as reward anticipation, risk perception, reinforcement learning, motivation, or effort may undergird risky decision-making in schizophrenia.4,5 Using an fMRI-modified BART, we identified NAcc activation differences in schizophrenia associated with risky reward pursuit, modeled parametrically within-trials relative to sequentially increasing balloon explosion probability. The schizophrenia group exhibited less risk-taking despite previous adverse outcomes (Average Explosions), but no difference in average point that risk-taking was volitionally discontinued (Adjusted Pumps).
Behaviorally, results averaged across trials are consistent with previous fMRI BART findings of less risky reward pursuit (fewer Explosions) in schizophrenia despite no differences in other behavioral measures compared to controls.19 Lack of differences in Adjusted Pumps on the fMRI BART is surprising and may relate to alterations from behavioral-only predecessors to accommodate modeling brain responses (eg, restricted balloon inflation range; exponential rather than linear reward/explosion probability). Relatedly, this behavioral result for Adjusted Pumps may be a false-negative due to the modest sample size despite our increased statistical strength by collecting more trials per subject than in previous investigations. While Adjusted Pumps, averaged across trials, were not significantly lower in schizophrenia, our parametric analysis of brain activation well-captured within-trial neural correlates undergirding risky reward pursuit. Between-group differences in NAcc activation without differences in behavior averaged across trials are common across reward processing studies in schizophrenia,42,47,56,63,64 indicating the ability for these tasks to illuminate differences in brain activation without requiring differences in behavior averaged across trials.
Taken together, our imaging results do not implicate greater general brain activation, or greater statistically determined influence from brain regions, associated with stronger uncertainty risk processing or aversion in schizophrenia. Rather, they implicate less NAcc activation in schizophrenia relative to risky reward amounts and less average influence (via statistical path analysis) from AI and dACC during general risky reward pursuit. These findings add to the current literature in several ways.
Firstly, while no between-group activation differences were found during general uncertain risky reward pursuit (nonparametric analysis), less NAcc activation in schizophrenia specifically depended upon the amount of risk and reward being pursued (pmod analysis). This distinction is crucial, as it suggests that NAcc responses in schizophrenia did not as starkly differentiate between relative riskiness of potential uncertain rewards. Thus, remediating this difference would not involve upregulating processes underlying activation broadly, but rather improving the ability for NAcc activation to change as a function of situational risky reward. Schizophrenia has been associated with excessive striatal dopamine and less BOLD activation during reward processing, error predictions, and salience attribution, which may contribute to the current NAcc findings.48 Lack of AI and dACC activation differences between groups may suggest similar recruitment of risk-processing regions and preclude the interpretation that schizophrenia is associated with greater uncertainty risk-aversion signaling in the brain.65,66
Secondly, we found significantly stronger correlations between risk-taking (Adjusted Pumps) and IQ in schizophrenia. IQ also correlated with Average Explosions across our entire sample. Matching groups based on cognitive functioning is rare in the risky decision-making literature,5 but may improve understanding of whether behavior or brain differences may be genuinely attributed to risky reward pursuit rather than general cognitive deficits common in schizophrenia literature. Our schizophrenia sample IQ (M = 108) may be disparate to behavioral BART literature, but our findings are consistent with previous fMRI BART findings in schizophrenia subjects with lower IQ (M = 89).19 Our sample may also better relate to people with schizophrenia broadly, rather than a subset of those characterized by impaired cognitive ability, given that poor cognitive ability is not universal in schizophrenia67,68 and brain differences in schizophrenia are related to cognitive ability.69,70
Thirdly, our path analysis results complement previous findings of lower association between the AI and ACC during reward-prediction processing in first-episode psychosis,71 potentially related to attenuated salience attribution during risky reward pursuit or inability for risk-related brain regions to collaborate and perceive uncertain risk. Notably, the direction of this effect is counter to what might be expected if uncertain risk-averse signals from the AI were influencing the dACC and dampening risk-taking for reward in schizophrenia. Thus, despite no between-group activation differences within dACC and AI, nor differences in their statistically determining influence on striatal regions, the influence of average AI activation upon dACC may relate to risk-taking in schizophrenia.
Risky, often suboptimal, decision-making in schizophrenia has been conceptualized according to various frameworks. The predictive coding perspective suggests that new information is integrated with prior beliefs, resulting in prediction errors in the AI, NAcc, and dACC when information violates expectancies.57,72 These signals, used to learn, adapt, and optimize behavior, have been lower in the NAcc in schizophrenia44,73 but not in first-episode psychosis.72 To the best of our knowledge the current work is the first to find differences in NAcc activation according to situational risky reward pursuit in schizophrenia. While our findings may be associated with poor distinction between relevant and irrelevant prediction error formation signal formation in the NAcc, further work is necessary to bolster these theories.74 It may be that disadvantageously cautious risk-taking in behavioral studies,1–3,15–18 and partially observed via fewer Explosions in fMRI BART adaptations and the current dataset,19 in schizophrenia better reflects impairment specific to reward-related information integration nonspecific to uncertain risky decision-making.
Future Directions
Although the human literature on the neural correlates of risky, uncertain decision-making in psychosis is scarce,19,31 rodent studies have found excessive striatal dopamine may result in biased choices inappropriately updated according to uncertain risk.75 Since differing striatal, frontal, and dopaminergic processes relate to risky decision-making in a myriad of ways,76 future work should parse contributions from these neurotransmitter systems on uncertain and risky decision-making in psychosis.
Previous research has found symptom associations between lower effort allocation and impaired reinforcement learning within subgroups of people with schizophrenia high in negative symptomatology.9,51 Considering heterogeneity of schizophrenia symptoms, larger samples may elucidate how brain activation and risk-taking are associated with specific symptom profiles.
Most importantly, there is little research into how people with schizophrenia process everyday risks, or if they differ from those without schizophrenia. Future work must extend beyond contrived laboratory tasks to discern if aberrant uncertain risk-taking infringes upon the quality of life in those with schizophrenia. If so, they may benefit from interventions emphasizing the evaluation of everyday risks (eg, trying something new, expressing opinions to a peer), weighing potential benefits, and integrating information from previous outcomes to improve decision-making.
Limitations
First, despite no correlations between neuroleptic medication and task performance or NAcc activation (supplementary table S13), we are unable to rule out the potential impact of medication on our findings.77–80 Second, the 2 risk-taking measures, Adjusted Pumps and Average Explosions shared considerable variance (Pearson correlation r = 0.461). Third, brain activation and behavior were each averaged within groups, which may undervalue potential within-group heterogeneity. A larger sample would allow for further targeted analyses beyond group averages. Fourth, computational modeling approaches could further parse components contributing to decision-making.81,82 Fifth, path analyses, though sometimes referred to as “effective connectivity,”83 do not provide temporal associations between regions within trials, only elucidating relationships between average ROI activation. Future work should determine this temporal relationship. Finally, while no brain-related differences were found implicating greater risk-aversion signals in schizophrenia, there is no way of discerning whether groups differed in the experiential, psychological process of risk processing from brain data alone.
Supplementary Material
Supplementary material is available at https://academic.oup.com/schizophreniabulletin/.
Acknowledgments
All authors have reviewed and approved the final version of this manuscript. The authors declare no conflicts of interest. For acknowledgements see supplementary materials.
Contributor Information
John R Purcell, Department of Psychological & Brain Sciences, Indiana University, Bloomington, IN, USA; Program in Neuroscience, Indiana University, Bloomington, IN, USA; Department of Psychiatry, Brain Health Institute, Rutgers University, Piscataway, NJ, USA.
Joshua W Brown, Department of Psychological & Brain Sciences, Indiana University, Bloomington, IN, USA; Program in Neuroscience, Indiana University, Bloomington, IN, USA.
Rachel L Tullar, Department of Psychological & Brain Sciences, Indiana University, Bloomington, IN, USA.
Bess F Bloomer, Department of Psychological & Brain Sciences, Indiana University, Bloomington, IN, USA.
Dae-Jin Kim, Department of Psychological & Brain Sciences, Indiana University, Bloomington, IN, USA.
Alexandra B Moussa-Tooks, Department of Psychological & Brain Sciences, Indiana University, Bloomington, IN, USA; Program in Neuroscience, Indiana University, Bloomington, IN, USA; Department of Psychiatry and Behavioral Sciences, Vanderbilt University School of Medicine, Nashville, TN, USA.
Katherine Dolan-Bennett, Department of Psychological & Brain Sciences, Indiana University, Bloomington, IN, USA; Department of Psychological and Brain Science, Washington University, St. Louise, MO, USA.
Brianna M Bangert, Program in Neuroscience, Indiana University, Bloomington, IN, USA; College of Medicine, University of Cincinnati, Cincinnati, OH, USA.
Krista M Wisner, Department of Psychological & Brain Sciences, Indiana University, Bloomington, IN, USA; Program in Neuroscience, Indiana University, Bloomington, IN, USA.
Nancy B Lundin, Department of Psychological & Brain Sciences, Indiana University, Bloomington, IN, USA; Program in Neuroscience, Indiana University, Bloomington, IN, USA; Department of Psychiatry and Behavioral Health, The Ohio State University, Columbus, OH, USA.
Brian F O’Donnell, Department of Psychological & Brain Sciences, Indiana University, Bloomington, IN, USA; Program in Neuroscience, Indiana University, Bloomington, IN, USA.
William P Hetrick, Department of Psychological & Brain Sciences, Indiana University, Bloomington, IN, USA; Program in Neuroscience, Indiana University, Bloomington, IN, USA.
Funding
This research was supported in part by the National Institute of Mental Health [T32MH103213; F31MH119767; F31MH122122 (NBL; ABM; JRP)], the Indiana Clinical & Translational Science Institute (CTSI) Predoctoral Grant [UL1TR001108; TL1TR001107 (ABM; JRP)], National Science Foundation Graduate Research Fellowship Program [1342962 (NBL)], Indiana CTSI Core Pilot Grant [UL1TR002529 (JRP; WPH)], Hoosier Lottery Problem Gambling Research Fund, a fund of Central Indiana Community Foundation (JRP), and IU Imaging Research Facility Pilot Scan Program (JRP). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH or any other entities.
Authors Contributions
JRP wrote manuscript drafts and produced revisions with substantial contributions and feedback from all authors. Conceptualization: JRP, JWB, and WPH; Data collection: ABM, BFB, JRP, and RFT; Data cleaning/processing: BMB, DJK, JRP, and KDB; Data analyses: ABM, DJK, NBL, JRP, and JWB.
References
- 1. Brown EC, Hack SM, Gold JM, et al. Integrating frequency and magnitude information in decision-making in schizophrenia: an account of patient performance on the Iowa Gambling Task. J Psychiatr Res. 2015;66-67:16–23. doi: 10.1016/j.jpsychires.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Luk M, Chang W, Chong C, et al. Altered risky decision making in patients with early non-affective psychosis. Eur Arch Psychiatry Clin Neurosci. 2021;271(4):723–731. doi: 10.1007/s00406-019-00994-2. [DOI] [PubMed] [Google Scholar]
- 3. Reddy LF, Lee J, Davis MC, et al. Impulsivity and risk taking in bipolar disorder and schizophrenia. Neuropsychopharmacology. 2014;39(2):456–463. doi: 10.1038/npp.2013.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Steiner MD, Frey R.. Representative design in psychological assessment: a case study using the Balloon Analogue Risk Task (BART). J Exp Psychol Gen. 2021;150(10):2117–2136. doi: 10.1037/xge0001036. [DOI] [PubMed] [Google Scholar]
- 5. Purcell JR, Herms EN, Morales J, Hetrick WP, Wisner KM, Brown JW.. A review of risky decision-making in psychosis-spectrum disorders. Clin Psychol Rev. 2022;91:102112. doi: 10.1016/j.cpr.2021.102112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reddy LF, Horan WP, Barch DM, et al. Understanding the association between negative symptoms and performance on effort-based decision-making tasks: the importance of defeatist performance beliefs. Schizophr Bull. 2018;44(6):1217–1226. doi: 10.1093/schbul/sbx156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cicero DC, Martin EA, Becker TM, Kerns JG.. Reinforcement learning deficits in people with schizophrenia persist after extended trials. Psychiatry Res. 2014;220(3):760–764. doi: 10.1016/j.psychres.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gard DE, Sanchez AH, Cooper K, Fisher M, Garrett C, Vinogradov S.. Do people with schizophrenia have difficulty anticipating pleasure, engaging in effortful behavior, or both? J Abnorm Psychol. 2014;123(4):771–782. doi: 10.1037/abn0000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Strauss GP, Whearty KM, Morra LF, Sullivan SK, Ossenfort KL, Frost KH.. Avolition in schizophrenia is associated with reduced willingness to expend effort for reward on a Progressive Ratio task. Schizophr Res. 2016;170(1):198–204. doi: 10.1016/j.schres.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lejuez CW, Read JP, Kahler CW, et al. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART). J Exp Psychol Appl. 2002;8(2):75–84. doi: 10.1037/1076-898X.8.2.75. [DOI] [PubMed] [Google Scholar]
- 11. De Groot K, Thurik R.. Disentangling risk and uncertainty: when risk-taking measures are not about risk. Front Psychol. 2018;9:2194. doi: 10.3389/fpsyg.2018.02194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pleskac TJ, Wallsten TS, Wang P, Lejuez CW.. Development of an automatic response mode to improve the clinical utility of sequential risk-taking tasks. Exp Clin Psychopharmacol. 2008;16(6):555–564. doi: 10.1037/a0014245. [DOI] [PubMed] [Google Scholar]
- 13. Korucuoglu O, Harms MP, Astafiev SV, et al. Test-retest reliability of fMRI-measured brain activity during decision making under risk. NeuroImage. 2020;214:116759. doi: 10.1016/j.neuroimage.2020.116759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li X, Pan Y, Fang Z, et al. Test-retest reliability of brain responses to risk-taking during the balloon analogue risk task. NeuroImage. 2020;209:116495. doi: 10.1016/j.neuroimage.2019.116495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boka E, Pozzo JD, Goetz D, et al. Ineffective risk-reward learning in schizophrenia. Psychiatry Res. 2020;293:113370. doi: 10.1016/j.psychres.2020.113370. [DOI] [PubMed] [Google Scholar]
- 16. Cheng GLF, Tang JCY, Li FWS, Lau EYY, Lee TMC.. Schizophrenia and risk-taking: impaired reward but preserved punishment processing. Schizophr Res. 2012;136(1-3):122–127. doi: 10.1016/j.schres.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 17. Fischer BA, McMahon RP, Kelly DL, et al. Risk-taking in schizophrenia and controls with and without cannabis dependence. Schizophr Res. 2015;161(2-3):471–477. doi: 10.1016/j.schres.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dominguez GB. The relationship between risk-taking, substance abuse and aggression in. Published online 2011;28:1–28. doi: 10.7916/D8GQ74RS. [Google Scholar]
- 19. Tikàsz A, Dumais A, Lipp O, et al. Reward-related decision-making in schizophrenia: a multimodal neuroimaging study. Psychiatry Res Neuroimag. 2019;286:45–52. doi: 10.1016/j.pscychresns.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 20. Wong SCY, Ng MCM, Chan JKN, et al. Altered risk-taking behavior in early-stage bipolar disorder with a history of psychosis. Front Psychiatry. 2021;12:763545. doi: 10.3389/fpsyt.2021.763545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mohr PNC, Biele G, Heekeren HR.. Neural processing of risk. J Neurosci. 2010;30(19):6613–6619. doi: 10.1523/JNEUROSCI.0003-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bossaerts P. Risk and risk prediction error signals in anterior insula. Brain Struct Funct. 2010;214(5-6):645–653. doi: 10.1007/s00429-010-0253-1. [DOI] [PubMed] [Google Scholar]
- 23. Huettel SA. Decisions under uncertainty: probabilistic context influences activation of prefrontal and parietal cortices. J Neurosci. 2005;25(13):3304–3311. doi: 10.1523/JNEUROSCI.5070-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Preuschoff K, Quartz SR, Bossaerts P.. Human insula activation reflects risk prediction errors as well as risk. J Neurosci. 2008;28(11):2745–2752. doi: 10.1523/JNEUROSCI.4286-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Preuschoff K, Bossaerts P, Quartz SR.. Neural differentiation of expected reward and risk in human subcortical structures. Neuron. 2006;51(3):381–390. doi: 10.1016/j.neuron.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 26. Levin IP, Xue G, Weller JA, Reimann M, Lauriola M, Bechara A.. A neuropsychological approach to understanding risk-taking for potential gains and losses. Front Neurosci. 2012;6:1–11. doi: 10.3389/fnins.2012.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Palminteri S, Justo D, Jauffret C, et al. Critical roles for anterior insula and dorsal striatum in punishment-based avoidance learning. Neuron. 2012;76(5):998–1009. doi: 10.1016/j.neuron.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 28. Purcell JR, Jahn A, Fine JM, Brown JW.. Neural correlates of visual attention during risky decision evidence integration. NeuroImage. 2021;234:117979. doi: 10.1016/j.neuroimage.2021.117979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pan Y, Lai F, Fang Z, et al. Risk choice and emotional experience: a multi-level comparison between active and passive decision-making. J Risk Res. 2019;22(10):1239–1266. doi: 10.1080/13669877.2018.1459798. [DOI] [Google Scholar]
- 30. Rao H, Korczykowski M, Pluta J, Hoang A, Detre JA.. Neural correlates of voluntary and involuntary risk taking in the human brain: an fMRI study of the Balloon Analog Risk Task (BART). NeuroImage. 2008;42(2):902–910. doi: 10.1016/j.neuroimage.2008.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fujino J, Hirose K, Tei S, et al. Ambiguity aversion in schizophrenia: an fMRI study of decision-making under risk and ambiguity. Schizophr Res. 2016;178(1-3):94–101. doi: 10.1016/j.schres.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 32. Moran EK, Culbreth AJ, Kandala S, Barch DM.. From neuroimaging to daily functioning: a multimethod analysis of reward anticipation in people with schizophrenia. J Abnorm Psychol. 2019;128(7):723–734. doi: 10.1037/abn0000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smucny J, Tully LM, Howell AM, et al. Schizophrenia and bipolar disorder are associated with opposite brain reward anticipation-associated response. Neuropsychopharmacology. 2021;46(6):1152–1160. doi: 10.1038/s41386-020-00940-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu S, Sun S, Camilleri JA, Eickhoff SB, Yu R.. Better the devil you know than the devil you don’t: neural processing of risk and ambiguity. NeuroImage. 2021;236:118109. doi: 10.1016/j.neuroimage.2021.118109. [DOI] [PubMed] [Google Scholar]
- 35. Matthews SC, Simmons AN, Lane SD, Paulus MP.. Selective activation of the nucleus accumbens during risk-taking decision making NeuroReport. 2004;15(13):2123–2127. doi: 10.1097/00001756-200409150-00025. [DOI] [PubMed] [Google Scholar]
- 36. Venkatraman V, Payne JW, Bettman JR, Luce MF, Huettel SA.. Separate neural mechanisms underlie choices and strategic preferences in risky decision making. Neuron. 2009;62(4):593–602. doi: 10.1016/j.neuron.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tom SM, Fox CR, Trepel C, Poldrack RA.. The neural basis of loss aversion in decision-making under risk. Science. 2007;315(5811):515–518. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- 38. Ernst M, Nelson EE, McClure EB, et al. Choice selection and reward anticipation: an fMRI study. Neuropsychologia. 2004;42(12):1585–1597. doi: 10.1016/j.neuropsychologia.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 39. Knutson B, Adams CM, Fong GW, Hommer D.. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21(16):RC159–RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zheng Y, Li Q, Tian M, et al. Deficits in voluntary pursuit and inhibition of risk taking in sensation seeking: risk taking in sensation seeking. Hum Brain Mapp. 2017;38(12):6019–6028. doi: 10.1002/hbm.23807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Esslinger C, Englisch S, Inta D, et al. Ventral striatal activation during attribution of stimulus saliency and reward anticipation is correlated in unmedicated first episode schizophrenia patients. Schizophr Res. 2012;140(1-3):114–121. doi: 10.1016/j.schres.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 42. Juckel G, Schlagenhauf F, Koslowski M, et al. Dysfunction of ventral striatal reward prediction in schizophrenia. NeuroImage. 2006;29(2):409–416. doi: 10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 43. Schwarz K, Moessnang C, Schweiger JI, et al. Transdiagnostic prediction of affective, cognitive, and social function through brain reward anticipation in schizophrenia, bipolar disorder, major depression, and autism spectrum diagnoses. Schizophr Bull. 2020;46(3):592–602. doi: 10.1093/schbul/sbz075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schlagenhauf F, Huys QJM, Deserno L, et al. Striatal dysfunction during reversal learning in unmedicated schizophrenia patients. NeuroImage. 2014;89:171–180. doi: 10.1016/j.neuroimage.2013.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rausch F, Mier D, Eifler S, et al. Reduced activation in ventral striatum and ventral tegmental area during probabilistic decision-making in schizophrenia. Schizophr Res. 2014;156(2–3):143–149. doi: 10.1016/j.schres.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 46. Morris RW, Vercammen A, Lenroot R, et al. Disambiguating ventral striatum fMRI-related bold signal during reward prediction in schizophrenia. Mol Psychiatry. 2012;17(3):280–289. doi: 10.1038/mp.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Murray GK, Corlett PR, Clark L, et al. Substantia nigra/ventral tegmental reward prediction error disruption in psychosis. Mol Psychiatry. 2008;13(3):267–276. doi: 10.1038/sj.mp.4002058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kesby JP, Murray GK, Knolle F.. Neural circuitry of salience and reward processing in psychosis. Biol Psychiatry Glob Open Sci. 3(1):33–46. doi: 10.1016/j.bpsgos.2021.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Radua J, Schmidt A, Borgwardt S, et al. Ventral striatal activation during reward processing in psychosis: a neurofunctional meta-analysis. JAMA Psychiatry. 2015;72(12):12431243. doi: 10.1001/jamapsychiatry.2015.2196. [DOI] [PubMed] [Google Scholar]
- 50. Walter H, Kammerer H, Frasch K, Spitzer M, Abler B.. Altered reward functions in patients on atypical antipsychotic medication in line with the revised dopamine hypothesis of schizophrenia. Psychopharmacology (Berl). 2009;206(1):121–132. doi: 10.1007/s00213-009-1586-4. [DOI] [PubMed] [Google Scholar]
- 51. Waltz JA, Xu Z, Brown EC, Ruiz RR, Frank MJ, Gold JM.. Motivational deficits in schizophrenia are associated with reduced differentiation between gain and loss-avoidance feedback in the striatum. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(3):239–247. doi: 10.1016/j.bpsc.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Reckless GE, Andreassen OA, Server A, Østefjells T, Jensen J.. Negative symptoms in schizophrenia are associated with aberrant striato-cortical connectivity in a rewarded perceptual decision-making task. NeuroImage Clin. 2015;8:290–297. doi: 10.1016/j.nicl.2015.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fukunaga R, Brown JW, Bogg T.. Decision making in the Balloon Analogue Risk Task (BART): anterior cingulate cortex signals loss aversion but not the infrequency of risky choices. Cogn Affect Behav Neurosci. 2012;12(3):479–490. doi: 10.3758/s13415-012-0102-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bornovalova MA, Cashman-Rolls A, O’Donnell JM, et al. Risk taking differences on a behavioral task as a function of potential reward/loss magnitude and individual differences in impulsivity and sensation seeking. Pharmacol Biochem Behav. 2009;93(3):258–262. doi: 10.1016/j.pbb.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 55. Raymond DR, Paneto A, Yoder KK, et al. Does chronic cannabis use impact risky decision-making: an examination of fMRI activation and effective connectivity? Front Psychiatry. 2020;11:599256. doi: 10.3389/fpsyt.2020.599256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. de Leeuw M, Kahn RS, Vink M.. Fronto-striatal dysfunction during reward processing in unaffected siblings of schizophrenia patients. Schizophr Bull. 2015;41(1):94–103. doi: 10.1093/schbul/sbu153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sterzer P, Voss M, Schlagenhauf F, Heinz A.. Decision-making in schizophrenia: a predictive-coding perspective. NeuroImage. 2019;190:133–143. doi: 10.1016/j.neuroimage.2018.05.074. [DOI] [PubMed] [Google Scholar]
- 58. Schlagenhauf F, Rapp MA, Huys QJM, et al. Ventral striatal prediction error signaling is associated with dopamine synthesis capacity and fluid intelligence. Hum Brain Mapp. 2013;34(6):1490–1499. doi: 10.1002/hbm.22000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bullmore E, Horwitz B, Honey G, Brammer M, Williams S, Sharma T.. How good is good enough in path analysis of fMRI data? NeuroImage. 2000;11(4):289–301. doi: 10.1006/nimg.2000.0544. [DOI] [PubMed] [Google Scholar]
- 60. Centanni SW, Janes AC, Haggerty DL, Atwood B, Hopf FW.. Better living through understanding the insula: why subregions can make all the difference. Neuropharmacology. 2021;198:108765. doi: 10.1016/j.neuropharm.2021.108765. [DOI] [PubMed] [Google Scholar]
- 61. Haber SN, Behrens TEJ.. The neural network underlying incentive-based learning: implications for interpreting circuit disruptions in psychiatric disorders. Neuron. 2014;83(5):1019–1039. doi: 10.1016/j.neuron.2014.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cohen MX, Heller AS, Ranganath C.. Functional connectivity with anterior cingulate and orbitofrontal cortices during decision-making. Cogn Brain Res. 2005;23(1):61–70. doi: 10.1016/j.cogbrainres.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 63. Simon JJ, Cordeiro SA, Weber MA, et al. Reward system dysfunction as a neural substrate of symptom expression across the general population and patients with schizophrenia. Schizophr Bull. 2015;41(6):1370–1378. doi: 10.1093/schbul/sbv067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Corlett PR, Murray GK, Honey GD, et al. Disrupted prediction-error signal in psychosis: evidence for an associative account of delusions. Brain. 2007;130(9):2387–2400. doi: 10.1093/brain/awm173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Christopoulos GI, Tobler PN, Bossaerts P, Dolan RJ, Schultz W.. Neural correlates of value, risk, and risk aversion contributing to decision making under risk. J Neurosci. 2009;29(40):12574–12583. doi: 10.1523/JNEUROSCI.2614-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fukunaga R, Purcell JR, Brown JW.. Discriminating formal representations of risk in anterior cingulate cortex and inferior frontal gyrus. Front Neurosci. 2018;12:553. doi: 10.3389/fnins.2018.00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kremen WS, Seidman LJ, Faraone SV, Toomey R, Tsuang MT.. The paradox of normal neuropsychological function in schizophrenia. J Abnorm Psychol. 2000;109(4):743–752 [DOI] [PubMed] [Google Scholar]
- 68. Weickert TW, Goldberg TE, Gold JM, Bigelow LB, Egan MF, Weinberger DR.. Cognitive impairments in patients with schizophrenia displaying preserved and compromised intellect. Arch Gen Psychiatry. 2000;57(9):907. doi: 10.1001/archpsyc.57.9.907. [DOI] [PubMed] [Google Scholar]
- 69. Woodward ND, Heckers S.. Brain structure in neuropsychologically defined subgroups of schizophrenia and psychotic bipolar disorder. Schizophr Bull. 2015;41(6):1349–1359. doi: 10.1093/schbul/sbv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Toulopoulou T, Grech A, Morris RG, et al. The relationship between volumetric brain changes and cognitive function: a family study on schizophrenia. Biol Psychiatry. 2004;56(6):447–453. doi: 10.1016/j.biopsych.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 71. Schmidt A, Palaniyappan L, Smieskova R, et al. Dysfunctional insular connectivity during reward prediction in patients with first-episode psychosis. J Psychiatry Neurosci. 2016;41(6):367–376. doi: 10.1503/jpn.150234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Haarsma J, Fletcher PC, Griffin JD, et al. 2021.. Precision weighting of cortical unsigned prediction error signals benefits learning, is mediated by dopamine, and is impaired in psychosis. Molecular Psychiatry. 2021;26(9):5320-5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yaple ZA, Tolomeo S, Yu R.. Abnormal prediction error processing in schizophrenia and depression. Hum Brain Mapp. 2021;42(11):3547–3560. doi: 10.1002/hbm.25453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Heinz A, Murray GK, Schlagenhauf F, Sterzer P, Grace AA, Waltz JA.. Towards a unifying cognitive, neurophysiological, and computational neuroscience account of schizophrenia. Schizophr Bull. 2019;45(5):1092–1100. doi: 10.1093/schbul/sby154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Stopper CM, Floresco SB.. Dopaminergic circuitry and risk/reward decision making: implications for schizophrenia. Schizophr Bull. 2015;41(1):9–14. doi: 10.1093/schbul/sbu165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bailey MR, Simpson EH, Balsam PD.. Neural substrates underlying effort, time, and risk-based decision making in motivated behavior. Neurobiol Learn Mem. 2016;133:233–256. doi: 10.1016/j.nlm.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kirsch P, Ronshausen S, Mier D, Gallhofer B.. The influence of antipsychotic treatment on brain reward system reactivity in schizophrenia patients. Pharmacopsychiatry. 2007;40(5):196–198. doi: 10.1055/s-2007-984463. [DOI] [PubMed] [Google Scholar]
- 78. Nielsen MO, Rostrup E, Wulff S, et al. Improvement of brain reward abnormalities by antipsychotic monotherapy in schizophrenia. Arch Gen Psychiatry. 2012;69(12):11951195. doi: 10.1001/archgenpsychiatry.2012.847. [DOI] [PubMed] [Google Scholar]
- 79. Diederen KMJ, Ziauddeen H, Vestergaard MD, Spencer T, Schultz W, Fletcher PC.. Dopamine modulates adaptive prediction error coding in the human midbrain and striatum. J Neurosci. 2017;37(7):1708–1720. doi: 10.1523/JNEUROSCI.1979-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD.. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature. 2006;442(7106):1042–1045. doi: 10.1038/nature05051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lasagna CA, Pleskac TJ, Burton CZ, McInnis MG, Taylor SF, Tso IF.. Mathematical modeling of risk-taking in bipolar disorder: evidence of reduced behavioral consistency, with altered loss aversion specific to those with history of substance use disorder. Comput Psychiatry. 2022;6(1):96. doi: 10.5334/cpsy.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Quan P, He L, Mao T, et al. Cerebellum anatomy predicts individual risk-taking behavior and risk tolerance. Neuroimage. 2022;254:119148. doi: 10.1016/j.neuroimage.2022.119148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Moussa-Tooks AB, Kim DJ, Bartolomeo LA, et al. Impaired effective connectivity during a cerebellar-mediated sensorimotor synchronization task in schizophrenia. Schizophr Bull. 2019;45(3):531–541. doi: 10.1093/schbul/sby064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.