Abstract
Health-related conditions often differ qualitatively or quantitatively between individuals of different birth-assigned sexes and gender identities, and/or with different gendered experiences, requiring tailored care. Studying the moderating and mediating effects of sex-related and gender-related factors on impairment, disability, wellbeing and health is of paramount importance especially for neurodivergent individuals, who are diagnosed with neurodevelopmental conditions with uneven sex/gender distributions. Researchers have become aware of the myriad influences that sex-related and gender-related variables have on the manifestations of neurodevelopmental conditions, and contemporary work has begun to investigate the mechanisms through which these effects are mediated. Here we describe topical concepts of sex and gender science, summarize current knowledge, and discuss research and clinical challenges related to autism, attention-deficit/hyperactivity disorder and other neurodevelopmental conditions. We consider sex and gender in the context of epidemiology, behavioural phenotypes, neurobiology, genetics, endocrinology and neighbouring disciplines. The available evidence supports the view that sex and gender are important contributors to the biological and behavioural variability in neurodevelopmental conditions. Methodological caveats such as frequent conflation of sex and gender constructs, inappropriate measurement of these constructs and under-representation of specific demographic groups (for example, female and gender minority individuals and people with intellectual disabilities) limit the translational potential of research so far. Future research and clinical implementation should integrate sex and gender into next-generation diagnostics, mechanistic investigations and support practices.
Introduction
Sex, gender identity and gendered experiences are known to result in differing qualities and quantities of psychological and biological characteristics. Hypotheses about moderation of individual differences by sex/gender have existed for roughly 100 years1, and include those regarding the nervous system2–5. Furthermore, these sex/gender differences can be more or less pronounced among individuals with neurological, psychiatric and neurodevelopmental conditions than among the general population6. Understanding the mechanisms whereby sex/gender influence clinical presentation, biology, developmental trajectory and treatment response is of importance for diagnostic assessment, intervention planning, and societal sex and gender equity7. Many authorities and organizations now require that research properly accounts for sex and gender8.
Considering the effects of sex/gender (Box 1) is particularly relevant for conditions with uneven sex/gender distributions (for example, neurodevelopmental conditions9) (Boxes 2,3; Fig. 1). Neurodevelopmental conditions defined by the Diagnostic and Statistical Manual of Mental Disorders, 5th edition, Text Revision (DSM-5-TR)10 and the International Classification of Diseases, 11th edition (ICD-11)11 differ in sex/gender prevalence across diagnoses, with male children being up to four times more likely to be diagnosed than female children, a difference that, interestingly, seems to attenuate in adulthood12–15. Gender diversity has also been associated with some neurodevelopmental conditions16,17 (Box 4). Moreover, behavioural expressions of neurodevelopmental conditions are modulated by sex and gender in nuanced but clinically important ways. Research has generated mixed findings on the moderating effects of sex/gender on genetic processes, and endocrine, neurological and other body systems, in neurodivergent individuals. Many hypotheses have been applied and debated to account for the observed sex/gender effects to achieve better scientific clarity and derive clinically meaningful actions.
Box 1. Sex and gender concepts.
Sex
The umbrella term ‘sex’ includes related but separable biological constructs7,54,324. Genetic sex components exist at conception, with XY and XX sex chromosomes being the most prevalent forms and, much less frequently, various sex chromosome aneuploidies. The SRY gene on the Y chromosome determines the development of the testes (versus the default development of the ovaries) and the subsequent prenatal testosterone surge, which influences (primarily via the regulation of gene transcription) the development of the genitals and reproductive organs, brain organization and systemic physiology. These influences are further modulated during puberty by sex hormone surges, resulting in physiological effects that last for the rest of life. Other differences in chromosome complement (for example, in genes other than SRY), genomic imprinting, X chromosome inactivation and the escape of such in individuals with XX chromosomes, contribute to additional downstream effects across cell types independent of sex hormone effects. Additionally, mosaicism exists across levels. Therefore, a binary sex assigned at birth label is at best a proxy for the joint or averaged effects of multiple sex-related biological variables. Mechanism-relevant multi-categorical or continuous measures of specific sex-related attributes and mosaicism at each level provide a more accurate approach for studying sex-related mechanisms.
Gender
The umbrella term ‘gender’ includes social–relational constructs that range from individual to societal attributes, and include norms and positional power7,55,56,324; these constructs change dynamically over the lifespan. Individuals hold a personal sense of their own gender (that is, gender identity) and present themselves in the same or different gender in daily life (that is, lived or expressed gender); both of these gender-related attributes are multi-categorical and can change over time in an individual. An individual’s behaviours are categorized by historically defined, stereotyped characteristics, which can be continuous dimensions that coexist in one person (masculinity and femininity) or qualitatively different social role categories (gender roles). Implicit and explicit stereotypes about gender (gender stereotypes) are prevalent, can be experienced and held by an individual of any gender or sex assigned at birth (enacted gender stigma or discrimination), and internalized as an individual’s belief (internalized gender stigma). Gendered structures and norms (gender ideology) dictate relational patterns such as social support (gender relations) and power structures such as wage gaps, gendered policies and laws (institutionalized gender). These constructs are important moderators or mediators of health-related outcomes56. They also afford analytical and interpretational angles for research in which sex-related and gender-related attributes have not been sufficiently differentiated in the first place324.
Inter-relatedness
Sex-related and gender-related attributes interact to shape human biology, behaviours and health outcomes. It is also important to consider other demographic correlates and social determinants of health — such as race, ethnicity, socioeconomic status, living conditions, food security, age, sexual orientation and language — that interact with sex and gender to influence outcomes (that is, intersectionality325) and the co-aggregation of clinical conditions (that is, syndemics326), especially in individuals from multiply-marginalized populations (see Fig. 1).
Box 2. Sex, gender and nervous system development.
Sex and brain development
Sex differences in the genetic architecture of human complex traits and mechanisms contribute to sex-differential traits327. Sex differences in brain development depend on gonadal hormonal exposure during fetal and postnatal life, and confer different susceptibilities to genetic and environmental exposures247,268. The sex chromosomes interact synergistically and possibly antagonistically with sex hormones328. SRY promotes fetal testosterone production in the second trimester, which causes masculinized brain organization in animal models247. Sex-differential gene expression is ubiquitous across human tissues but is largely tissue-specific, reflecting the effects of escape from X chromosome inactivation for X-linked genes, as well as hormone-related transcription factor regulation and sex-differentiated distribution of epigenetic markers for autosomal genes329. The organization of the human brain does not show dimorphism, but instead a mosaicism of female-typical and male-typical features on multiple continua2, which are associated with mental health vulnerability330,331. Prenatal sex steroid hormonal exposure is longitudinally associated with sex-typical play behaviours, gender identity, sexual orientation and, to a lesser extent, specific cognitive–psychological traits including empathy, aggression, mental rotation and social dominance4; nevertheless, very few of these sex-differential traits have been robustly linked to measurable sex-differential brain features4. The literature on brain sex/gender differences is inconsistent, although some findings are more established, including on average larger whole-brain, limbic and temporal regional volumes in males, and larger cingulate and prefrontal regional volumes in females5,332. Some differences have been associated with males performing better on average on perceptual and motor tasks and females on analytical intuitive processing and communication abilities, a difference that has been hypothesized to be mediated by prenatal differences in sex hormones332. Sex-differential patterns across brain anatomy, function and gene expression are interrelated, and these patterns imply sex-influenced brain development across species333, which highlights the importance of measuring brain development against the norm of the same sex334.
Nature–nurture interplay
Neurodevelopment is not sex-determined but developmentally shaped by the environment (many environmental contexts are gendered) in sex-dependent ways3. Sex-related or gender-related health vulnerability is underpinned by the nature–nurture interplay and the entwined effects of sex-related and gender-related attributes335. Examples include varied trajectories and increased prevalence of anxiety, mood, stress-related and trauma-related disorders in women and individuals assigned female at birth336, and differences in the onset and trajectory characteristics of cardiovascular disease attributable to sex-based biology and gender-related factors337.
Susceptibility to neurodevelopmental conditions
Evidence indicates that sex hormones affect neurotransmitter systems, neural differentiation and synapse formation338, with critical developmental windows during mid-gestation, shortly after birth and around puberty339. They also modulate neurotransmitter concentrations and regional glucose metabolism later in life338. Alterations in intracellular signalling that influence the neurobiology of attention-deficit/hyperactivity disorder are modulated by sex hormones and the menstrual cycle338. Sex hormones also modulate hypothalamic structure and function, which differ between males and females340. The hypothalamus might be a pivotal structure in Tourette syndrome and other neurodevelopmental conditions340.
Sex/gender differences in dopamine receptor distribution are present in cortical and subcortical regions across humans and animals341. Females exhibit higher regional glutamate levels than males in the striatum, anterior cingulate, sensorimotor cortex and cerebellum342. Alterations in dopaminergic and glutamatergic neurotransmission have been implicated in neurodevelopmental conditions341,342. Studies in rodents have shown alterations in dopamine receptor functioning as a consequence of exposure to stressors and addictive substances, and hyperactivity in association with developmental fluctuation of striatal dopamine receptor densities only in males341. Therefore, certain neurotransmitter systems, such as the dopaminergic system, might be more susceptible to interference in males than in females.
Box 3. A note on anti-ableist and neurodiversity-affirmative language.
In recent years, the language used to describe neurodevelopmental conditions and the individuals diagnosed with these conditions has evolved dramatically, specifically with regard to the shift away from overly medicalized and deficit-based language47,343–345 towards non-pathologizing alternatives, which are rooted in the neurodiversity approach(es) to these conditions39,46,346–348 and informed by the preferences of neurodivergent community members343,344,349–351. For instance, although diagnostic manuals such as DSM-5-TR and ICD-11 use the term ‘neurodevelopmental disorders’ to describe this group of conditions, the more value-neutral term ‘neurodevelopmental conditions’ is used in this Review, as it does not imply that neurodivergent individuals are inherently ‘disordered’ or pathological. Although the term ‘neurodevelopmental disabilities’ is a widely accepted alternative that reflects the often-disabling nature of these conditions, it is notable that Tourette syndrome and tic disorders can be diagnosed even when tics do not produce substantial disability or functional impairment10.
Individuals diagnosed with neurodevelopmental conditions generally are referred to as ‘neurodivergent’ and individuals without these conditions (that is, typically developing individuals) are frequently referred to as ‘neurotypical’. Additional anti-ableist language choices adopted in this Review include referring to core and associated features of neurodevelopmental conditions as traits, characteristics or differences rather than ‘symptoms’ when appropriate, discussing factors that increase the ‘likelihood’ or ‘probability’ of neurodevelopmental conditions rather than ‘risk’, and the use of identity-first language when possible (for example, ‘autistic person’ rather than ‘person with autism’) to describe autism and other conditions where identity-first language is often preferred by stakeholders47,343,352,353 (although we recognize this preference might differ by language and culture354). Although a full discussion of language preferences in neurodivergent communities is beyond the scope of this Review, interested readers are directed to several foundational articles in this area for a deeper understanding of these topics39,343–345,352,355.
Fig. 1 |. Why are sex and gender important for neurodevelopmental conditions?
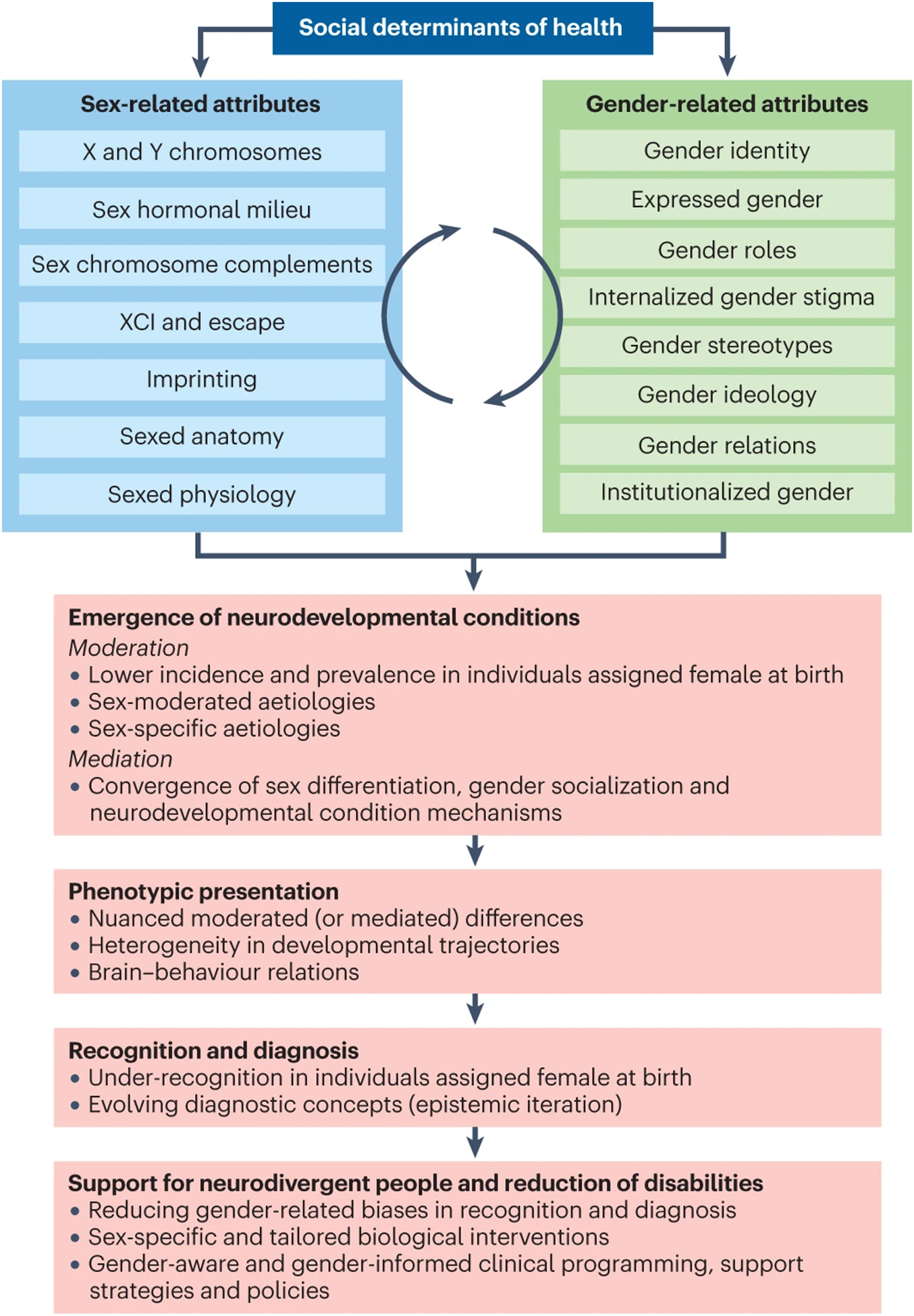
Sex-related biological attributes (blue) and, to a lesser extent, gender-related sociocultural factors (green) serve specific roles in moderating and mediating the sex-differential likelihood of the emergence of neurodevelopmental conditions228,229,236,321, presumably explaining the male preponderance in prevalence123, sex-moderated and sex-specific aetiological factors, and converging mechanisms of neurodivergent development and sex differentiation (such as overlapping downstream molecular pathways277 and neuroendocrine–immune mechanisms268). Sex-related biological and gender socialization mechanisms also converge with mechanisms shaping the lifespan development of neurodivergent individuals322,323. Sex-related and gender-related attributes also influence how neurodevelopmental conditions are diagnosed and how multilevel phenotypic presentations vary among individuals106,133. Specifically, these attributes moderate or mediate the behavioural and cognitive manifestations of neurodevelopmental and co-occurring health conditions, account for developmental heterogeneity, shape (and are shaped by) brain development and neurodivergent biology, and impact how and when neurodevelopmental conditions are recognized and diagnosed (especially owing to implicit and explicit gender biases, gender role expectations and gendered stereotypes of neurodivergent presentations)323. Findings and reflections then drive the nosological evolution and the operationalization of the diagnostic criteria for neurodevelopmental conditions iteratively123. Ultimately, new knowledge generated by sex-informed and gender-informed research will have a clinical impact8 in reducing recognition and diagnostic biases, and in enabling the development and evaluation of sex-specific and tailored biological interventions based on sex-informed neurodivergent biology, and the design and implementation of gender-aware and gender-informed care and support for neurodivergent individuals of all sexes and genders.
Box 4. Gender diversity and neurodevelopmental conditions.
Gender diversity refers to the variations of gender identity and expression that may or may not align with those stereotypically linked to an individual’s sex assigned at birth. When an individual suffers from substantial distress owing to the incongruence between their experienced or expressed gender and their assigned gender (that links to their sex assigned at birth), the diagnostic label of ‘gender dysphoria’ (DSM-5-TR)10 or ‘gender incongruence’ (ICD-11)11 is used to highlight the clinical support needs. In the context of increasing awareness, recognition and referral for clinical care for gender dysphoria (especially in those assigned female at birth) in the general population356,357, rates of gender dysphoria and minority gender identities are reported to be particularly heightened in the neurodivergent population358,359. Studies that used a single item of the Child Behavior Checklist to approximate gender dysphoria or incongruence have shown that 2.5–5.4 of children and adolescents diagnosed with autism, attention-deficit/hyperactivity disorder or intellectual disabilities might have gender-minority identities17,359,360. Studies using dimensional measures have also found that greater self-reported gender diversity and gender incongruence are more common in autistic than in non-autistic children and adolescents, especially in those assigned female at birth361,362. In autistic adults, these rates are even higher363. The presence of gender dysphoria is associated with bullying victimization, poorer mental health and increased suicidal ideation in autistic young people364. Equally, neurodevelopmental conditions are enriched in gender-minority populations across ages365–367. In particular, the pooled estimate of autism diagnosis prevalence in gender-minority individuals is 11%16, much higher than in the current general population prevalence. This co-occurrence of gender diversity and neurodevelopmental conditions has been observed across several sociodemographic groups in the past decade; however, hypotheses regarding the developmental mechanisms underlying such co-occurrence are speculative, ranging from shared prenatal sex steroid organizational effects of the brain368 to an impact of neurodivergent information processing on gender cognition and development16. Regardless, community input to research has resulted in preliminary clinical guidance and service design tailored to the unique needs of neurodivergent gender-minority individuals369,370, aiming to improve care and support for these historically under-recognized and multiply-marginalized subpopulations.
Neurodevelopmental conditions
Neurodevelopmental conditions arise from divergent architecture, maturation and function of the developing nervous system. They are heterogeneous in presentation, neurobiology and aetiology9,18, and research has generated various findings indicative of alterations in brain structure and function in the most common neurodevelopmental conditions19–22. In a US population-based survey performed in 2015–2017, the overall prevalence of neurodevelopmental conditions was estimated at 17.812. According to the ICD-11 (ref.11) and DSM-5-TR10, neurodevelopmental conditions include autism spectrum disorder (hereafter termed autism; see Box 3), attention-deficit/hyperactivity disorder (ADHD), communication (speech and language) disorders, learning disorders (for example, dyslexia), motor disorders (for example, Tourette syndrome) and intellectual disabilities. Although we adopt this definition, broader definitions of neurodevelopmental conditions might also include genetic syndromes (for example, Rett syndrome), congenital brain injury (for example, cerebral palsy), fetal alcohol spectrum disorders, schizophrenia and obsessive–compulsive disorder23,24.
Neurodevelopmental conditions are usually inborn or develop early in life; some present with unspecific ‘prodromal’ features and are thus detected in later childhood, and more recently the possible existence of later-onset variants, such as in ADHD, has been discussed25–27. Neurodevelopmental conditions are defined by characteristic differences in social communication, impulsivity, flexibility, attention, speech and language, sensory processing, learning and motor control that diverge substantially from typical development and tend to co-occur9–11,18,28,29. When these traits interfere with subjective wellbeing and social, educational or vocational functioning, a clinical disorder diagnosis is given. Alterations in cognition, such as executive function, social cognition, bottom-up versus top-down processing, phonological and sensorimotor awareness, numerical cognition or general cognitive abilities, are presumed to underlie the defining clinical features30. Neurodevelopmental conditions are highly heritable31,32 but have multifactorial origins alongside biology–environment interplay33–35. Psychosocial environments, and society’s awareness, attitudes and support have important effects on neurodivergent people’s health, social participation, experiences of acceptance, wellbeing, quality of life and functional outcomes36–39.
Many neurodevelopmental conditions are better understood as extreme expressions of traits that are continuously distributed in the population, as opposed to qualitatively distinct entities40–42, and traditional biomedical and nosological conceptions that exclusively aspire to normalize or cure neurodevelopmental conditions have been increasingly criticized43–45. The neurodiversity paradigm acknowledges that neurodevelopmental conditions can both result in disability and confer vulnerability to co-occurring health conditions, but this approach conceives neurodevelopmental conditions as parts of variability of the human CNS, not pathology per se39,43,46,47. Transdiagnostic methods have the potential to promote neurodiversity-affirmative research and practice48, owing to their tendency to incorporate and harmonize both medical and social models of disability39. Viable intervention targets within this approach include promotion of optimum developmental and adaptive skills, prevention and intervention for adverse health and social outcomes, quality of life, increasing opportunities for learning and growing to compensate for difficulties, and altering the environment37,44,49,50. Herein, we favour a strengths-based, non-pathologizing approach to disability to capitalize on an individual’s resources and opportunities, rather than solely focusing on limitations or impairments51.
Sex and gender constructs
Sex and gender are both multi-component umbrella constructs (Box 1); they are distinct but inter-correlated because of interplaying biological and sociological mechanisms52. Sex-related constructs are biological attributes that were initially conceptualized on the basis of the reproduction of organisms and include chromosomal, genetic, gonadal, hormonal, genital and other anatomical and physiological components53. Owing to this conceptual origin, many sex-related attributes are treated as dichotomous (that is, female versus male), but the actual distributions of specific sex-related variables can in some cases be multi-categorical (for example, sex chromosome configurations) or continuous (for example, serum testosterone levels)54. Gender refers to constructs of social relations in human societies; these constructs originated in the reproductive arena and extend to psychological, sociocultural and contextual attributes, and power structures55,56. Ideologies that maintain dominating power structures often closely associate gender with sex and impose a binary view of gender, although empirical data show most gender-related variables to be either multi-categorical (for example, gender identities) or continuous (for example, masculinity and femininity)54–56. Notably, gender-related attributes can influence biological outcomes that are thought to be primarily driven by sex-related attributes (for example, brain biology, hormonal status and related behaviours)57,58. Sex-related and gender-related attributes separately and jointly act as moderators of many human health outcomes7,52.
In this Review, when an empirical finding cannot be clearly delineated as reflecting sex or gender effects specifically, owing to research limitations, we use the term ‘sex/gender’ to highlight this ambiguity in interpretation. Although suboptimal, when studies only report findings by binarized sex/gender, we use the generic terms ‘female(s)’ and ‘male(s)’ to reflect the joint effects of sex and gender attributes, rather than using gender identity-based terms (for example, ‘girls/women’, ‘boys/men’) or claiming that these terms unambiguously represent sex assigned at birth.
Observed differences among individuals of different sexes or gender identities suggest moderating (and in specific cases, mediating) effects of sex and/or gender attributes on typical and atypical development, including neurodevelopmental conditions (Box 2; Fig. 1). Depending on the outcome of interest and the specific sex-related or gender-related variable, such differences vary from dimorphic (that is, existing in two forms) to small differences on a continuum (that is, distributions substantially overlap despite mean differences)4. The specific ways in which sex and gender influence the outcome of interest vary according to the persistence of the influence (across development, experience and environment), context-dependence52 and the interplay of sex components and gender components across individual development and over generations59.
Epidemiology
The estimated prevalence rates and sex/gender ratios of neurodevelopmental conditions vary depending on study design, cohort, age group, informant and country. Meta-analytic or large-scale studies published in the last decade have estimated childhood prevalence as: 5.9% for ADHD60, 1.0% for autism61, 7.7% for speech–language disorders62, 9.7% for learning disorders63, 2.8% for tic disorders64 and 1% for intellectual disabilities65. Far fewer studies have been performed in adult populations. The rates of some neurodevelopmental conditions (for example, ADHD and tic disorders66,67) are commonly lower in adulthood than in childhood, although associated functional impairment might persist. However, for other neurodevelopmental conditions, such as autism, the number of individuals receiving first-time diagnoses in adulthood has increased substantially in recent years61,68.
A sex/gender bias towards higher prevalence in males than in females is observed across all neurodevelopmental conditions and ranges from 1.2:1 to 4:1 (Table 1). In a large US parent report survey in 88,530 children aged 3–17 years, the rate of any neurodevelopmental condition, including ADHD, autism, speech fluency disorder (stuttering), learning disorders, intellectual disabilities or any other developmental delay was 12.6% in females and 22.7% in males12. This sex/gender bias has been found to be more pronounced in childhood than in adulthood in autism, tic disorders and ADHD14,19,69–71, and varies for different subtypes of these conditions14,72. Level of intellectual functioning modulates the sex/gender ratio in the prevalence of some neurodevelopmental conditions; for example, in autism, more balanced ratios are usually found in individuals with so-called profound autism73 and in autistic individuals with co-occurring intellectual disabilities more generally14,61.
Table 1 |.
Neurodevelopmental conditions: summary of prevalence, sex/gender ratio, and average differences in behavioural presentation and co-occurring conditions
| Neurodevelopmental conditiona | Prevalence (%) | Female to male ratio | Moderation by sex/gender | Refs. | |
|---|---|---|---|---|---|
| Behavioural presentation | Co-occurring conditions | ||||
| Allb | Children: 17.8 F:12.6 M: 22.7 |
Children: 1:1.7 | ‐ | F: possibly internalizing problems and later detection of some conditions more likely M: possibly co-occurring externalizing behaviours more likely |
12,132,133,320 |
| Autism | 1.0 | 1:3 | F: less RRB; better social communication, prelinguistic and linguistic functioning, cognitive flexibility and autobiographical memory M: fewer compulsions and less self-injurious behaviour; better impulse inhibition and visuospatial functioning |
F: higher rates of epilepsy, endocrine problems, depression, eating problems and internalizing behaviours M: hyperactivity in childhood and externalizing behaviours more likely |
15,61,78–80,94,104–106,110–119,122,123,125,126,309 |
| ADHD | Children: 5.9c
Adults: 2.8 |
Children: 1:2.3 Adults: 1:1.6 |
F: inattentive presentation more likely (childhood); fewer inhibition and cognitive flexibility problems (childhood); fewer complex attention problems (adulthood) M: hyperactive presentation more likely (childhood) |
F: possibly higher rates of anxiety, depression and other internalizing behaviours; higher rates of cigarette smoking M: possibly higher rates of delinquent behaviours, ODD, conduct disorder and ASPD (adulthood) |
60,72,106,129–133,320 |
| Non-syndromic ID | All: 1.0 Children: 1.8 Adults: 0.5 |
Mild: 1:1.6 Severe: 1:1.2 Children: 1:1.0–2.2 Adults: 1:1.1–1.4 |
‐ | F: depression (all ages) and dementia (later adulthood) more likely M: personality disorder and psychosis (adulthood) more likely |
65,158,160,162 |
| Fragile X syndrome | F: 0.009 M: 0.014 |
1:1.6 | F: milder cognitive difficulties, less evident dysmorphism, and higher adaptive functioning and verbal strengths | F: typically with mild ID or borderline-to-average IQ, co-occurring learning disorder, socioemotional difficulties and psychiatric problems, and 20% have an autism diagnosis M: typically with severe ID and co-occurring ADHD, anxiety and features of autism, and 50–60% have an autism diagnosis |
162–164 |
| Communication disorders | Children: 7.7 | 1:1.7 | F: modest advantage in language acquisition in early childhood; grammar problems in childhood and later referral to services more likely | M: higher association with medical conditions in childhood | 62,141–143 |
| Learning disorders | Lifetime: 9.7 F: 7.1 M: 12.2 |
1:1.7 | F: literacy advantage, better verbal processing speed, working memory, conceptualization, and orthographic and visuospatial coding M: tendency for mathematical skills to be less challenging, better verbal reasoning |
F: higher rates of inattentive and internalizing symptoms M: higher rates of hyperactive and other externalizing behaviours |
63,144–148 |
| Motor disorders | All: 5.0–6.0 DCD, severe: 1.5 DCD, probable: 3.0 SMD: 3.0–4.0 TS: 0.77 (adults, 0.05; M, 1.1; F, 0.3) Tic disorders: 3.0 (M, 4.1; F, 3.1) |
DCD, severe: 1:2 TS and other tic disorders: 1:1.5 |
F: onset of tics with OCD; increase of tics during menstrual cycle; higher social impairment; tics more complex and persistent, with a later onset and a later peak of severity; in DCD, gross motor problems and higher social impact more likely M: onset of tics with rage and ADHD; in DCD, fine motor problems more likely |
F: possibly higher rates of anxiety and mood disorders with tics M: possibly higher rates of ADHD and disruptive behavioural disorders with tics |
64,149,150,154,157 |
ADHD, attention-deficit/hyperactivity disorder; ASPD, antisocial personality disorder; DCD, developmental coordination disorder; F, females; ID, intellectual disabilities; M, males; OCD, obsessive-compulsive disorder; ODD, oppositional defiant disorder; RRB, restricted and repetitive behaviour; SMD, stereotypic movement disorder; TS, Tourette syndrome.
DSM-5-TR or ICD-11 diagnoses.
ADHD, autism, speech fluency disorder (stuttering), learning disorders, intellectual disabilities or any other developmental delay.
Best estimate for children and adolescents.
Co-occurrence of neurodevelopmental conditions with psychiatric and medical conditions is high74–79. Despite the widespread opinion amongst clinicians that the co-occurrence profiles differ between sexes/genders80,81 — with males more likely to show externalizing problems such as oppositional defiant disorder and conduct disorder, and females more likely to show internalizing problems such as anxiety and mood disorders — the evidence is inconsistent. Population-based research, as opposed to clinical studies, has often failed to identify these sex/gender differences in psychiatric illnesses82–84. Alternatively, population-based studies have shown higher rates of suicide in autistic females than in autistic males85,86, and this finding is particularly pronounced among individuals who are in the average IQ range and have co-occurring ADHD87. Sex/gender differences in the causes of premature mortality (which occurs more frequently in autistic and ADHD individuals than in the general population) have also been observed in autism and other developmental disabilities88,89. For example, autistic males have a higher mortality risk from diseases of the circulatory and nervous systems and cancer, and from external causes, and autistic females have a higher risk from similar causes but also from endocrine diseases and congenital malformations compared with sex-matched non-autistic counterparts85,87–89. In ADHD, a trend for greater mortality — owing to both natural causes (for example, cardiovascular disease) and unnatural causes (for example, suicide or accidents) — was observed in females compared with males88,90.
Behavioural phenotypes
Autism
Historically, autistic females have been reported to be more likely to show intellectual disabilities, neurological problems and emotional–behavioural challenges than autistic males91–96. The consequent presumption that autistic females are more likely to have cognitive and neurological impairments might have contributed to the under-recognition15 and delayed diagnosis of autism in some females68,97–102, especially when their autistic features were less obvious103. Large-scale studies published in the last 10 years indicate that cognitive and language abilities vary substantially across all sexes and genders in the autistic population, with the most consistent difference being slightly lower restricted and repetitive behaviour scores in females than in males94,104,105. However, sex-related and gender-related factors are also likely to influence the profiles of some autistic features in subtle ways106. For instance, autistic females tend to show better social communication skills than autistic males in nuanced ways, which means that autistic features in females might not be sufficiently captured by commonly used instruments107,108. These social communication skills involve: social attention and motivation; gender stereotypical play; expression of non-verbal communication; impression management skills rehearsed over time106,109 (Box 5); and (pre)linguistic functioning from communicative gestures, babbling and semantics, to narrative and pragmatic competency110–114. On average, autistic males and females have similar levels of insistence on sameness; autistic males are more likely to show repetitive motor behaviours and speech, and circumscribed interests, whereas autistic females are more likely to experience compulsions and/or self-injurious behaviours115–119.
Box 5. Gender, socialization and social coping.
Like neurotypical individuals, neurodivergent people live in societies with varied sociocultural norms, and they grow up navigating and negotiating with the social world with these norms. This socialization experience throughout the course of development shapes individuals’ social coping, including the process of managing their public self-image(s) in social settings (that is, impression management). Although this kind of experience is ubiquitous across people with and without a neurodevelopmental diagnosis, the mismatch between social expectations and neurodivergent behaviours in the neurotypical majority, alongside the unique information processing styles (and constraints) of neurodivergent individuals, render impression management an extremely challenging endeavour for many neurodivergent individuals, that requires intensive efforts and could serve as a major stressor for some109. Neurodivergent and neurotypical individuals who self-report higher rates of these experiences also typically report worse mental health, although studies have yet to determine the causal direction312. The impression management experiences of neurodivergent individuals who strive to cope socially in the world of the neurotypical majority range from the suppression of tics371, strategies to compensate and mask attention-deficit/hyperactivity disorder presentations132, and the ‘camouflaging’ or ‘passing’ experiences described by some autistic people109,312,313,372 and observed in some children with developmental language disorder373. As gender-related influences such as gender role expectations and gender stereotypes are pervasive and have a profound impact on socialization, female neurodivergent individuals tend to be influenced differently from their male counterparts when it comes to ways and efforts to ‘fit in’ to what the particular society expects of them by suppressing neurodivergent behaviours and forcing the presentation of neurotypical behaviours — all potentially contributing to mental health strain323,374.
Developmentally, a declining outward autistic presentation is more likely to be observed in autistic females than in autistic males. This sex/gender difference exists even among individuals diagnosed in early childhood120,121, suggesting the contribution of both sex-related biological and gender socialization influences. Moderating effects of sex/gender on cognitive features associated with autism are mostly observed in executive function and visuospatial domains122,123: evidence suggests that autistic males are more likely to have challenges with cognitive flexibility and autobiographical memory, whereas autistic females are more likely to have challenges with response inhibition and visuospatial processing124. In terms of health conditions, autistic females are more likely to have epilepsy and endocrine–reproductive problems79,125, depressive disorders78, and disordered eating and sleep126–128, whereas autistic males are more likely to exhibit hyperactivity in childhood124.
ADHD
In children diagnosed with ADHD, males are more likely to express hyperactivity than females, and females are more likely to show inattention as the predominant presentation129,130; similar patterns have also been observed in adults131. Evidence also indicates that females are less likely than males to show ‘classic’ ADHD (for example, displaying disruptive and impulsive behaviours)132,133. This observation should however be considered alongside recognition biases106; for example, ADHD symptom ratings by teachers and parents tend to be higher for male than for female children, despite no differences in directly observed ADHD behaviours134. According to a meta-analysis published in 2021, among children diagnosed with ADHD, males experience more inhibition and cognitive flexibility difficulties than females129; less is known about the differences between adult males and adult females, although the results of another meta-analysis suggest that males are more likely to experience impaired complex attention than females135. Some studies indicate that males diagnosed with ADHD are more likely to show antisocial behaviours and to receive oppositional defiant disorder or conduct disorder diagnoses, whereas females are more likely to experience internalizing and emotion regulation problems106,130. ADHD has been causally associated with a greater risk of cigarette smoking in female than male adolescents136. In a Danish population-based registry study, compared with male individuals diagnosed with ADHD, female individuals diagnosed with ADHD had greater rates of co-occurring autism, intellectual disabilities, oppositional defiant disorder and conduct disorder, personality disorders, schizophrenia, substance use disorders and suicidal behaviour82. In adults specifically, the results of registry-based studies indicate that ADHD is more strongly associated with anxiety, depression, bipolar disorder and personality disorders in females than in males, and more strongly associated with schizophrenia, substance use disorders and hypertension in males than in females137,138.
Communication disorders
Limited research exists on sex/gender influences on language disorders and hardly any is available on stuttering, speech sound disorder or social (pragmatic) communication disorder139. In the general population, recent research unexpectedly revealed that male children produce more ‘protophones’ (that is, speech-like vocalizations) than female children in the first year of life140. On the other hand, there is a modest female advantage in communicative gestures and receptive and expressive vocabulary, especially in the early stages of language acquisition141. However, the existence of sex/gender differences in language is controversial, with evidence published over the last 10 years indicating that these effects are smaller than previously assumed142. In a longitudinal population study of primary school children aged 4–6 years, the association between speech–language dysfunctions and medical diagnoses was higher in males than females, and females diagnosed with language disorders were more likely to have morphosyntactic problems than males143. Importantly, in this study, screening for language disorders identified more males than females; indeed, females are often older than males when they are referred to clinical services143. Overall, there seem to be sex/gender differences in language disorders, which currently appear subtle, but influence the timing and rates of detection and service access.
Learning disorders
The literature regarding clinically relevant effects of sex and gender on learning disorders is scarce. In a population-based study, female children showed a literacy advantage over males, with better spelling and reading fluency144. Regarding mathematical skills, findings are mixed, with a tendency for more female than male children to have challenges in this area144–146. In a clinically enriched community study of children, males showed lower reading performance than females, but this pattern was mostly accounted for by an over-representation of males in the low end of test score distributions; further, this sex/gender difference in reading is fully mediated by differences in processing speed, inhibition and verbal reasoning146. The findings of other cognitive studies in dyslexia also suggest that females have better verbal working memory and conceptualization, and orthographic and visuospatial coding than males147. The co-occurrence of behavioural challenges with dyslexia might differ among sexes/genders. For example, in a study published in 2000, dyslexia was associated with inattentive patterns in both female and male children but was associated with hyperactive and impulsive symptoms only in males; the latter symptoms might be more likely to trigger externalizing behaviours and subsequent clinical referrals in males148.
Motor disorders
The nature of motor, vocal and combined tics seems to be similar across sexes/genders; however, evidence suggests that compared with males, females are less likely to experience simple tics and more often have complex tics149. Tics in females also seem to start later than in males, with a later peak of symptom severity, and are more likely to be persistent and/or worsen with age than in males149. Evidence suggests that complex tics in females can also surge during the oestrogenic phase of the menstrual cycle150,151. Furthermore, some findings suggest that on the simple-to-complex sensory phenomena and motor phenomena continua — from premonitory urges to obsessive urges or thoughts, and from simple tics to compulsive tics — males tend to more commonly manifest simple urge and tic behaviours152, whereas females more commonly manifest obsessive–compulsive-like tic behaviours153 and show an increase in complexity of tics with age154. However, these findings might have been confounded by different age distributions among male and female samples, with females being older153.
The onset of tics in concert with co-occurring conditions might also differ according to sex/gender. Evidence suggests that females more commonly have an onset combined with compulsion, whereas males frequently have tics that co-occur with rage and ADHD150,153,154. Among individuals with Tourette syndrome, females are less likely than males to have co-occurring ADHD but are more likely to have anxiety and mood problems150,154–156. Overall, females seem to experience greater tic-related impairment in social participation and quality of life than males150,156.
In a survey-based study in adults with a high likelihood of developmental coordination disorder, females were more likely to report gross motor and non-motor difficulties and a negative impact on activities and participation, whereas males were more likely to report fine motor difficulties157.
Intellectual disabilities
Little research exists on sex/gender differences in non-syndromic intellectual disabilities. In adolescents and adults with intellectual disabilities, depression seems to be more common in females, and psychotic and personality disorders more common in males158,159. Evidence from a registry study suggests that, in later adulthood, females with intellectual disabilities are more likely to have dementia than males with intellectual disabilities160. The literature on behavioural phenotypes in syndromic intellectual disabilities is comprehensive161 but few studies exist on sex/gender differences. This scarcity is probably because some of these conditions are rare, which results in small sample sizes, or sex-specific (for example, Rett syndrome, which almost exclusively affects females).
In fragile X syndrome (FXS), a relatively common syndromic form of intellectual disability caused by a mutation (CGG trinucleotide repeat expansion) in the fragile X messenger ribonucleoprotein 1 (FMR1) gene, the female-to-male ratio is about 1:1.6 (ref.162). Males with FXS often have severe intellectual disability and co-occurring ADHD, anxiety and features of autism. By contrast, females with FXS typically have mild intellectual disability or borderline-to-average IQ, with co-occurring learning disorders, socioemotional difficulties and psychiatric problems163. These differences are likely to be the result of males being hemizygous for the mutation whereas females carry heterozygous mutations and have one functioning copy of the FMR1 gene164. Despite females with FXS having milder cognitive difficulties and dysmorphism, higher adaptive functioning and stronger verbal communication skills, they do not necessarily have better outcomes than males with FXS163.
The effects of sex/gender on behavioural phenotypes of the main neurodevelopmental conditions are summarized in Table 1.
Access and response to intervention
Neurodivergent females and their parents have clearly voiced the need for sex and gender to be considered in relation to therapeutic intervention and support165,166; however, few studies have examined whether access and response to intervention differs between neurodivergent females and males. In ADHD, evidence suggests that female children (but not adults) are less likely than male children to receive medication if no co-occurring externalizing challenges are present167,168. Regarding pharmacodynamic profiles for extended-release formulations of methylphenidate for ADHD, compared with males, females show a better response at 1.5 h after administration and an inferior response at 12 h169. In Tourette syndrome, a study published in 1989 found that haloperidol yields a better treatment response in females than in males170. In autism, trials of social skills group interventions showed better effects on social responsiveness in females than in males171,172, despite many female individuals struggling to fit in in these predominantly male groups. In short, sex-related and gender-related attributes can be important moderators of access and response to therapies and support across biological and social levels, but their exact roles (both statistically and mechanistically) remain to be clarified in well-powered trials.
Brain biology
Autism
Structure.
With regard to autism-associated changes in brain morphology, more evidence supports an influence of sex/gender on the spatial pattern of these changes than simply on their effect size173,174. According to a systematic review, sex/gender-by-diagnosis interaction effects are predominantly observed in brain regions that also tend to vary by sex/gender in typical development; for example, visual, limbic, cerebellar, sensorimotor, ventral attention and default mode network regions175. In these regions, autistic males usually exhibit higher and autistic females lower values in cortical thickness, volume and surface area than their neurotypical counterparts175. According to a large-scale analysis published in 2020, both autistic males and females have greater cortical thickness than neurotypical individuals, although these differences are concentrated in different brain regions in the two sexes176. Another large-scale study published in 2022 used a deep-learning framework that had been trained on structural images of neurotypical brains to predict biological sex. By applying this prediction model to two large samples of structural brain images from autistic people, the researchers identified a replicable shift towards more male-typical brain anatomy in both autistic males and females177. In females this shift was driven mainly by features in regions associated with visual, face and speech processing, whereas in males it was driven mainly by regions associated with reward and motor processing177.
Studies have found that, compared with sex-matched neurotypical children, autistic females but not males have a smaller head circumference in the first year of life178. Brain overgrowth between 1 and 5 years of age has been observed in both autistic males and autistic females, and notably involves partly different brain regions or shows different developmental trajectories in different sexes/genders179–182. In a longitudinal study that spanned early to middle childhood, brain overgrowth of autistic males was predominantly driven by increased grey matter growth, whereas autistic females showed slower grey and white matter growth trajectories compared with neurotypical peers182. In the same cohort, developing brain regions that have monosynaptic connections with the amygdala showed qualitatively different volumetric alterations in autistic females and autistic males183, and amygdala volume correlated with internalizing behaviours only in females184. Studies in adolescents and adults have identified region-specific volumetric sex/gender differences, especially in limbic regions; in addition, greater white matter reductions were observed in autistic females in youth and in autistic males in adulthood175. Alterations in grey matter density covariance (a measure of structural connectivity) in salience, executive control and default mode networks have been found to differ between preschool-aged autistic females and males185. In another study, both autistic males and autistic females showed alterations compared with neurotypical peers in grey-to-white matter boundary sharpness; however, these alterations occurred in different brain regions in females and males and with larger effects in females186.
Replicable sex/gender-by-diagnosis interactions have been identified in fibre tracts (primarily in white matter microstructural properties, such as fractional anisotropy) that commonly show sex/gender differences in neurotypical individuals (for example, the corpus callosum, corona radiata, cingulum and inferior fronto-occipital fasciculus)175,187. White matter tract development deviations from sex/gender-specific age norms have been reported in individuals diagnosed with autism, ADHD and early-stage schizophrenia188. Interestingly, such deviations, especially in tracts connecting prefrontal cortex regions, are shared between autistic individuals and their undiagnosed female siblings189. In early childhood, there is evidence for sex/gender-by-diagnosis interaction effects on callosal structural connectivity but not tract development trajectories190,191. Overall, the evidence indicates that structural brain organization differs between autistic females and males when compared with neurotypical counterparts, especially with regard to the spatial pattern of alterations and their developmental trajectories. Many of these sex/gender differential alterations associated with autism overlap with brain structures that also show sex/gender differences in neurotypical populations.
Functional organization.
Functionally, regions-of-interest studies have found sex/gender-by-diagnosis interaction effects on resting-state functional connectivity of the default mode, salience and ventral attention networks, as well as limbic regions and the cerebellum, although the direction of the effects varies between studies and conditions175,187. Sex/gender differences in amygdala resting-state connectivity were attenuated in autistic compared with neurotypical children192. Voxel-mirrored homotopic connectivity, a proxy for cross-hemispheric processing, showed replicable occipital sex/gender-by-diagnosis interaction effects193. Task-based functional MRI (fMRI) studies have used predominantly social processing tasks and have identified sex/gender-by-diagnosis interaction effects predominantly in limbic and default mode network regions. Where sex/gender-by-diagnosis interactions were found, the most common findings were hypoactivation in autistic males compared with autistic females175,187.
An imbalance between excitatory and inhibitory neural activity has been proposed as a core mechanism of autism194,195. Interestingly, one study that estimated excitation–inhibition balance using resting-state fMRI data found that autistic male adults, but not autistic female adults, showed an excitation–inhibition imbalance towards higher excitation compared with their neurotypical counterparts in default mode network and other areas196. In autistic female adults, excitation–inhibition balance was associated with greater estimated camouflaging196 (Box 5). The results of studies in animal models and gene expression patterns in humans have also led to the speculation that the emergence of autism is driven by brain plastic reactions following genetic and environmental events; such plastic reactions occur more readily in males than in females and involve perceptual or language-related brain regions, leading to the male-preponderant prevalence of autism and autistic cognitive strengths197. Taken together, functional brain alterations in autism seem to differ between females and males, but owing to the heterogeneity of study designs and findings, drawing firm conclusions about how they differ would be premature.
Neurochemistry.
In the light of the excitation–inhibition imbalance hypothesis of autism194,195, studies have investigated the neurochemical correlates of autism, focusing on glutamate and &-aminobutyric acid (GABA), the predominant excitatory and inhibitory neurotransmitters in the CNS. In a study that used magnetic resonance spectroscopy (MRS), neurotypical sex/gender differences in glutamate–glutamine and N-acetyl compounds in bilateral posterior thalamic radiations and the left centrum semiovale were partly attenuated or reversed in autistic individuals198. N-Acetyl compounds are thought to have an important role in longer-term energy expenditure and myelin synthesis198. Another MRS study found a sex/gender-by-diagnosis interaction effect in GABA concentration in the left dorsolateral prefrontal cortex; thalamic GABA concentration was positively correlated with autistic traits in autistic females and negatively correlated with these traits in autistic males199. A study using PET and simultaneous MRI found that, compared with neurotypical individuals, the association between cortical thickness and GABAA receptor binding potential in the left postcentral gyrus was different in autistic male adults but not in autistic female adults200. In summary, studies on sex/gender-specific alterations in autism-related neurochemistry are scarce to date, with some limited evidence for differences in excitatory and inhibitory neurotransmission in particular regions.
ADHD
Structure.
Both males and females diagnosed with ADHD have reductions in the volume of some brain regions compared with their neurotypical peers. Volume reductions in basal ganglia, medial prefrontal and premotor cortex regions have been found predominantly in males, and reductions in lateral premotor and prefrontal regions seem to be more common in females201. In children, reduced total brain volume has been associated with both ADHD and higher polygenic scores for ADHD, with both these effects stronger in males than in females202. Reduced caudate volume in male adults diagnosed with ADHD compared with neurotypical male adults correlated with hyperactive–impulsive symptoms, whereas no such effect was observed in female adults203. In contrast, volume reductions in the putamen and thalamus were associated with ADHD symptoms in female but not in male children diagnosed with ADHD204.
A study in school-aged children identified sex/gender-by-diagnosis interaction effects on measures of white matter microstructure, which also correlated with inhibitory control performance205. More specifically, compared with neurotypical participants, males diagnosed with ADHD had alterations in fractional anisotropy in primary motor regions, whereas ADHD females had alterations in medial orbitofrontal regions. Another study, which assessed callosal fibre tracts, found a sex/gender-by-diagnosis interaction effect on radial diffusivity in the posterior parietal tracts; diffusivity was altered only in females diagnosed with ADHD and correlated with attention control in both sexes/genders206. Taken together, the brain structural correlates of both ADHD diagnosis and traits differ partly between males and females, potentially underlying sex/gender-related differences in ADHD behavioural characteristics.
Functional organization.
Evidence from one study suggests that, compared with neurotypical peers, school-aged ADHD females display more alterations in resting-state functional connectivity between striatal and prefrontal regions than males207. Functional connectivity between these regions also correlated with increased delay discounting of monetary rewards, a proxy for altered reward functioning and impulsivity, that was elevated in ADHD females only. A study that explored lateralization found, in addition to sex/gender-independent effects of ADHD, more leftward lateralization in male individuals diagnosed with ADHD and more rightward lateralization in female individuals diagnosed with ADHD208. In neurotypical adults, inattention and hyperactivity traits were associated with striatal resting-state connectivity to different regions in males and females209. Emerging evidence also indicates sex/gender-specific alterations in functional connectivity between the limbic striatum and frontal default mode network regions in children diagnosed with ADHD, with increased connectivity in ADHD compared with neurotypical children only in males210. Overall, brain functional connectivity alterations associated with a diagnosis of ADHD, specific ADHD traits and reward functioning seem to differ between female and male individuals, especially in the limbic and prefrontal regions.
Neurochemistry.
One small-sample study in children using single-voxel proton MRS found a sex/gender-by-diagnosis interaction effect for N-acetylaspartate concentration in the right dorsolateral frontal white matter, with especially low concentrations in ADHD females, potentially indicating elevated levels of neuronal death or injury211. Another study found sex/gender differences in cerebellar choline and glutamate–glutamine concentrations with lower levels in females and interaction effects between age and sex/gender for choline, creatine and glutamate–glutamine concentrations in the pregenual anterior cingulate cortex in adults diagnosed with ADHD212. Choline is associated with energy metabolism and creatine with myelination. These findings might be potential evidence for sex/gender differences in brain maturation delays linked to ADHD, although the study assessed adults. In sum, there is preliminary evidence for sex/gender-specific neurochemical mechanisms underlying ADHD, affecting not only neurotransmitters but also other metabolites linked to brain maturation or injury.
Dyslexia
The results of some studies indicate that dyslexic female individuals have more frequent or pronounced changes in brain volume at the gross level than dyslexic male individuals147. These changes include reduced whole-brain volume, reduced right hemispheric grey matter and bilateral white matter volume, and an increased left-hemispheric grey matter to white matter ratio, as compared with neurotypical individuals. A study that assessed whole and lobar brain volumes across participants from three countries did not find sex/gender-by-diagnosis interactions213. The results of some studies indicate that some dyslexia-related alterations are specific to female individuals; for example, reductions in right hemispheric grey matter, especially sensorimotor regions (precuneus, cuneus, precentral and postcentral gyrus) and the left visual word form area147,214. In contrast, altered left–right asymmetry of the planum temporale214, and reductions in left-hemispheric grey matter cortical thickness in the middle and superior temporal gyri, orbitofrontal cortex, inferior frontal gyrus, temporoparietal cortex and fusiform gyrus have been observed in dyslexic male individuals compared with neurotypical male individuals147. Preliminary evidence suggests that structural brain alterations are more anatomically widespread in dyslexic females than in dyslexic males, who have a clearer involvement of the classic reading network regions147. One 1H-MRS study assessed neurotransmitter metabolite concentrations in the anterior cingulate cortex and identified higher levels of choline and myoinositol in association with reduced processing speed across dyslexic and neurotypical female children; this association was not observed in male children215. Taken together, there is preliminary evidence that dyslexic females have more widespread brain alterations than males.
Tourette syndrome
Preliminary findings indicate that, compared with neurotypical children, male children with Tourette syndrome have alterations in cortical and callosal thickness and basal ganglia volume; these alterations were not observed in female children with Tourette syndrome149,216. One study found reduced cortical thickness in prefrontal, orbitofrontal and parietal regions in males with Tourette syndrome compared with females with Tourette syndrome, as well as a negative correlation between tic severity and post-central and pre-central regions that was significantly stronger in males than in females217. Another study in children with Tourette syndrome identified a sex/gender-by-diagnosis interaction effect on grey matter volume of the bilateral hypothalamus218. In a study from 2001 that included participants aged 6–63 years, sex/gender differences in parieto-occipital volume (including both grey and white matter) observed in the neurotypical group were diminished in the Tourette syndrome group, especially in adults219. Overall, current evidence suggests that males with Tourette syndrome might have more widespread brain alterations than their neurotypical counterparts than do females with Tourette syndrome, and that typical brain sex/gender differences are reduced in individuals diagnosed with Tourette syndrome.
Intellectual disabilities
Sex/gender differences in the brain correlates of FXS have been observed. Caudate enlargement, a striking feature in FXS, is more pronounced in males than in females164, whereas a decrease in the size of the cerebellar vermis, which correlates with IQ, seems to be similar in females and in males with FXS220. During eye-gaze processing, females with FXS show reduced insula and fusiform gyrus activation, whereas males with FXS show increased amygdala and insula activation, as compared with neurotypical individuals164.
Sex/gender moderation effects on brain structure and function in non-syndromic intellectual disabilities remain largely unexplored. Furthermore, the effects of IQ are usually insufficiently captured in neuroimaging studies of sex/gender in neurodevelopmental conditions188,221, with the majority of studies controlling for IQ as a nuisance covariate or simply excluding participants with intellectual disabilities174. The results of a meta-analysis indicate that whole-brain volume positively correlates with IQ across sexes/genders in both neurotypical and neurodivergent cohorts222. However, in an fMRI study, the resting-state brain connectivity features that predicted higher IQ differed between neurotypical males and females223.
Table 2 summarizes the average sex/gender differences and sex/gender-moderating effects that have been reported for brain structure, function and neurochemistry in neurodevelopmental conditions.
Table 2 |.
Summary of average sex and gender differences in brain structure, function, and neurochemistry in neurodevelopmental conditions
| Category | Autism | ADHD | ID | LDa | MD |
|---|---|---|---|---|---|
| Brain structure | Sex/gender-specific alterations in GM and WM volume, cortical thickness, and white matter tracts notably in regions and tracts with sex/gender differences in neurotypical individuals173,175,187
Sex/gender-specific alterations in callosal structural connectivity191 ↓ WM integrity in autistic female youth and autistic male adults compared with neurotypical peers175 |
F: ADHD associated with ↓ GM volume in lateral premotor and prefrontal regions201, and altered WM microstructure in medial orbitofrontal regions205 and posterior parietal tracts of corpus callosum206; ADHD symptoms associated with ↓ putamen and thalamus volumes204 M: ADHD associated with ↓ GM volume in basal ganglia, medial prefrontal and premotor cortex regions201; stronger total ↓ brain volume in childhood202; reduced caudate volume that correlated with hyperactive-impulsive symptoms203; and altered WM microstructure in primary motor cortex205 |
In FXS: caudate enlargement more pronounced in males than in females164,220 | F: dyslexia associated with more widespread and gross-level volume reductions, ↓ GM in right hemisphere, and ↓ WM in left hemisphere147,214
M: dyslexia associated with altered left-right asymmetry of planum temporale surface area, and ↓ left hemisphere GM and WM volume in reading network regions147,214 |
Normative sex/gender differences in parietooccipital volume diminished in TS, especially in adults219
↓ cortical thickness in prefrontal, orbitofrontal and parietal regions in males compared with females with TS217 M: TS associated with altered cortical and callosal thickness and basal ganglia volume in childhood149,216 |
| Brain function | Different alterations in functional connectivity among sexes/genders, e.g. in default mode and salience/ventral attention networks, limbic regions and cerebellum175 F: autism associated with ↓ voxel-mirrored homotopic connectivity of dorsolateral occipital cortex193 |
In ADHD: more right-ward lateralization of resting-state signal variability in females and more left-ward lateralization in males208 F: ADHD associated with altered resting-state functional connectivity between striatal and prefrontal regions, related to reward processing207 M: ADHD associated with ↑ functional connectivity between limbic striatum and frontal default mode network regions210 |
Neurotypical males and females differ in which resting-state brain connectivity features predict higher IQ scores223
In FXS: During eye-gaze processing, females show reduced insula and fusiform gyrus activation, and males show increased amygdala and insula activation164 |
Insufficient information | Insufficient information |
| Brain neurochemistry | Compared with neurotypical individuals: reversed or reduced sex/gender differences in the concentrations of glutamate-glutamine and N-acetyl compounds in bilateral posterior thalamic radiations and the left centrum semiovale198; females and males show different alterations in GABA concentration in the left dorsolateral prefrontal cortex and opposite direction of correlation between thalamic GABA and autistic traits199
M: association between cortical thickness and GABAA receptor binding potential in the left postcentral gyrus different in autistic compared with typically developing males200 |
Cerebellum choline and glutamate-glutamine concentrations greater in males than in females diagnosed with ADHD212 F: ADHD associated with reduced N-acetylaspartate concentration in right dorsolateral frontal WM in childhood211 |
Insufficient information | F: ↑ choline and myoinositol in the anterior cingulate associated with reduced processing speed in dyslexic and neurotypical children215 | Insufficient information |
ADHD, attention-deficit/hyperactivity disorder; F, females; FXS, fragile X syndrome; GABA, (γ-aminobutyric acid; GM, grey matter; ID, intellectual disabilities; LD, learning disorders; M, males; MD, motor disorders; TS, Tourette syndrome; WM, white matter.
Here dyslexia.
Genomics
Most heritable traits are subject to different liabilities by sex224. In relation to autism, the so-called female protective effect model proposes that autistic females inherit more genetic variants that increase the likelihood of autism compared with autistic males225,226. Higher autism prevalence (compared with that in the general population) occurs among individuals who have a close autistic female relative and the likelihood of autism increases in later-born offspring siblings of autistic females, compared with autistic males (known as the Carter effect)227–229. Similarly, in the context of ADHD, mothers diagnosed with ADHD transmit a greater likelihood of ADHD to offspring than fathers230, although this has not been found in all studies231. Genetic propensity for various neurodevelopmental conditions can be associated with sex-differentiated phenotypic heterogeneity; for example, male siblings of females with anxiety disorders have elevated relative likelihoods of ADHD or any neurodevelopmental condition, although this effect could also represent a diagnostic sex/gender bias232.
Molecular genetic studies indicate that autistic females carry more common genetic variants associated with autism than autistic males. Polygenic scores for autism, based on the weighted sum of susceptibility alleles present, are increased in autistic females without intellectual disabilities229, in non-autistic mothers and female siblings228, and in mothers with broader subdiagnostic autism phenotypes233. In a separate study, no consistent within-trait sex differences in heritability, referring to the proportion of the phenotypic variance explained by all measured single nucleotide polymorphisms (SNP), were found in an analysis of data from 20 psychiatric and neurodevelopmental conditions, including autism and ADHD234. This is despite the existence of several shared sex-specific SNP statistical associations between autism and ADHD. Autistic females are also more likely than autistic males to carry a higher burden of de novo rare variants235,236, which is inversely related to polygenic score load236. In addition to dominant mutations, biallelic recessive mutations are descriptively more prevalent in autistic females than in autistic males237. In relation to structural variants, some neurodevelopmental condition-related copy number variations display phenotypic sex differences; autism is more common in males with 22q11.2 duplication and 16p11.2 deletion syndromes than in females with these syndromes238. These findings suggest that the sex-differential probability of developing autism is relevant to sex-related factors that modify the effects of common and rare variants associated with autism.
Sex chromosome aneuploidies (SCA) are associated with increased prevalence of autism, ADHD, and language and cognitive deficits, particularly in association with male phenotypes239. For example, autism diagnosis prevalence is almost double in individuals with XYY syndrome (on average 30%) than in individuals with XXY syndrome (18%) and XXX syndrome (15%), although it is still higher in individuals with the latter two syndromes than in the general population239. ADHD symptoms and diagnoses are also markedly increased across all these three types of sex chromosome trisomy239. Chromosomal dosage might influence SCA phenotypes owing to excess or reduced sex chromosome number; for example, a supernumerary Y chromosome is particularly associated with reduced social and attentional functioning240.
X chromosome inactivation (XCI) could also account for phenotypic heterogeneity241. X-linked neurodevelopmental conditions, FXS and Rett syndrome, are associated with sex-differentiated behavioural and developmental outcomes in human and animal studies242 although this is not universal for X-linked genes243. Evidence suggests that XCI skewing can cause phenotypic variability among female family members with FXS, but evidence regarding the role of XCI in the Rett syndrome phenotype is inconclusive244. Some genes escape XCI (that is, they continue to be expressed from both the active and the inactive chromosome) and contribute to complex trait variation and SCA phenotypes via dosage effects associated with increased expression of genes that have not been silenced245. Y chromosome genes that could contribute to sex bias in neurodevelopmental conditions include NLGN4Y, an autism candidate gene246, and SRY247, a brain-expressed gene that stimulates male gonadal development and fetal testosterone production. Some autosomal genes show sexually differentiated expression in the context of neurodevelopmental conditions; for example, genes involved in the Wnt signalling pathway in ADHD248 and SHANK1 in autism249. In studies in animal models, the candidate autism genes Chd8 (ref.250) and Cntnap2 (ref.251) show sex-differentiated expression.
In summary, genetic contributions to sex differences in neurodevelopmental conditions are small and polygenic, and large sample sizes are typically required to detect the effects252. Studies investigating the so-called female protective effect in autism have used partly overlapping samples, and independent replication is therefore required225,227–229,236. A publication from 2019 describes a method for the estimation of X-linked gene dosage from the summary statistics of genome-wide association studies, which will aid in the investigation of X-linked variation in complex traits253. Studying XCI and escape genes in humans is methodologically challenging254 and difficult to relate to human brain tissues244, although catalogues of XCI across human tissues have helped identify genes contributing to sex-specific differences255. See Table 3 for a summary of the findings presented in this section.
Table 3 |.
Summary of sex and gender influences in genetic factors and sex hormones associated with neurodevelopmental conditions
| Category | Autism | ADHD | ID | MD |
|---|---|---|---|---|
| Clinical genetics | ↑ Autism prevalence in close relatives of autistic females compared with close relatives of autistic males227–229 | ↑ Likelihood of ADHD in offspring of mothers with ADHD compared with offspring of fathers with ADHD in some studies23
231
↑ ADHD prevalence, ↑ any neurodevelopmental diagnosis in siblings of females with anxiety disorders compared with siblings of males with anxiety disorders232 |
Insufficient information | Insufficient information |
| Common variants | ↑ PGS for autism in autistic females without ID compared with males229, non-autistic mothers compared with fathers, female siblings of autistic individuals compared with male siblings228, and in mothers with broader autism phenotype compared with fathers233 | No specific within-trait sex-specific SNP heritability234
Shared sex-specific SNP associations between ADHD and autism234 |
Insufficient information | Insufficient information |
| Rare variants | ↑ Prevalence of rare de novo variants and biallelic recessive mutations in autistic females compared with autistic males235–237
Autism is a common outcome in males with 22q11.2 duplication and 16p11.2 deletion CNV syndromes238 |
Insufficient information | No associated sex differences in X-linked rare variants243 | Insufficient information |
| Sex chromosomes and autosomes | ↑ Autism prevalence in people with SCA (XXX, XXY, XYY, XXYY)239 Y-linked genes NLGN4Y246 and SRY247 might increase male prevalence Sex differences in SHANK1 gene expression249 Sex differences in Chd8 (ref.250) and Cntnap2 (ref.251) animal models |
↑ ADHD prevalence in people with SCA (XXX, XXY, XYY, XXYY)239. Sex differences in gene expression in Wnt pathway248 |
↑ Language and cognitive deficits in people with SCA (XXX, XXY, XYY, XXYY)239
XCI thought to ↑ phenotype heterogeneity in FXS242 |
Insufficient information |
| Sex hormones | ↓ 2D to 4D digit ratio, ↑ facial landmark masculinity260,261; ↑ androgens and oestradiol in amniotic fluid (prenatally)263,264; low maternal serum oestradiol265, compared with sex-matched neurotypical groups ↑ Autism in offspring of mothers with PCOS, regardless of offspring sex266 |
↓ 2D to 4D digit ratio compared with neurotypical individuals260
↑ ADHD in offspring of mothers with PCOS, regardless of offspring sex266 |
Insufficient information | ↑ Chronic tic disorder in offspring of mothers with PCOS, regardless of offspring sex266 |
| Immune response | Autism or related features ↑ with in utero exposure to sodium valproate in humans and murine models33 and with sex hormone differences in rodents271
↑ Autism in offspring of mothers with obesity272 Weak-to-moderate association between autism and the effect of EDCs on immune cell function275 |
Insufficient information | Insufficient information | Insufficient information |
No sex/gender differences in these factors reported for learning disorders. ADHD, attention-deficit/hyperactivity disorder; CNV, copy number variation; EDCs, endocrine disrupting chemicals; FXS, fragile X syndrome; ID, intellectual disabilities; MD, motor disorders; PCOS, polycystic ovary syndrome; PGS, polygenic score; SCA, sex chromosome aneuploidies; SNP, single nucleotide polymorphisms; XCI, X chromosome inactivation; XXX, triple X syndrome; XXY, Klinefelter syndrome; XYY, XYY syndrome.
Sex hormones
Steroid hormones, particularly androgens, might be biological mediators (and potential moderators for genetic aetiological factors256) of demonstrated sex differences in autism prevalence. The ‘extreme male brain’ and fetal androgen theories257,258 posit that in utero exposure to androgens increases stereotypically male-prevalent cognitive traits (‘systemizing’), which are on-average higher in autistic than in non-autistic individuals, proportionately more than stereotypically female-prevalent cognitive traits (‘empathizing’)259. Supporting these theories, physical markers of in utero androgen exposure such as lower 2D to 4D digit ratio260 or increased facial landmark masculinity261 are associated with increased prevalence of autism and ADHD diagnoses. In utero androgen exposure is associated with neurodevelopmental outcomes in rodent models (via testosterone aromatization to oestradiol)262. However, in primates the mechanism is more complex and yet to be fully clarified242. A small number of studies have found associations between higher concentrations of androgens263 and oestrogens (particularly oestradiol) in amniotic fluid264 and an increased likelihood of an autism diagnosis in male children. In contrast, low maternal serum oestradiol was associated with offspring autism diagnoses265. Maternal polycystic ovary syndrome, a condition associated with elevated testosterone levels, is associated with an increased likelihood of autism, ADHD and chronic tic disorder diagnoses in offspring regardless of sex266. However, there is no consistent association between in utero androgen exposure and autistic traits266,267.
The role of steroid hormones is intricately linked to the placenta, the in utero environment and inflammation268. Indeed, the immune system seems to act bidirectionally with the endocrine system. In animal models, immune-related signalling and cellular actions are implicated in brain masculinization, sex-specific synaptic patterning and social behaviours of the animals269,270. For example, disrupted prostaglandin signalling during a critical period of cerebellar development is associated with deficits in play behaviour in male rodents269. Sodium valproate is an important teratogen associated with cognitive deficits and autism in humans33. Mice exposed to sodium valproate in utero showed sex-specific differences in steroid metabolism in the brain271. Evidence suggests that maternal obesity results in early maturation of the fetal hypothalamic–pituitary–adrenal axis, which might alter fetal neural programming and promote sex-specific neurodevelopmental outcomes272,273. In addition to metabolic and neuroendocrine effects, obesity is associated with a pro-inflammatory state, suggesting a role for maternal immune activation in the development of neurodevelopmental conditions272.
Evidence from animal and human studies also suggests that endocrine-disrupting compounds such as bisphenol A affect neurodevelopmental outcomes via immune cell functioning274, although most human studies are limited by imprecise measures of exposure and more rigorous epidemiological studies are warranted275. Sexually dimorphic outcomes, potentially mediated by disrupted oestrogen receptor-’ signalling, have been associated with altered expression of autism-related genes in the transcriptomes of exposed rat pups276. Converging findings from transcriptomic studies indicate that autistic individuals have increased cerebral cortical expression of genes associated with glial and immune functions, and decreased expression of neuronal and synaptic genes, as compared with neurotypical individuals277. Interestingly, these findings mirror sex-differentiated gene expression in neurotypical individuals: compared with neurotypical females, neurotypical males have increased expression of glial and immune-related genes and decreased expression of neuronal and synaptic genes. This observation provides insight into sex-differentiated neurobiology, but there is a need to overcome limitations such as under-representation of females in these cohorts in order for the findings to be truly informative.
Immunology
Sex/gender differences in immunological responses are present across the lifespan and contribute to differential susceptibility to infections and autoimmune diseases in the general population278. Specific immune alterations such as maternal immune activation and infection, as well as differential cytokine and chemokine profiles, have been linked to neurodevelopmental conditions, particularly autism and ADHD279. Maternal prenatal infections increase the likelihood of autism (though potentially not causally280), intellectual disabilities and ADHD in offspring, and the presence of specific inflammatory disease states during pregnancy (for example, maternal asthma, diabetes or autoimmune diseases) has been associated with autism, ADHD and Tourette syndrome272,281. Evidence of neuroinflammation has been observed in post-mortem studies in individuals who had neurodevelopmental conditions, and gene expression studies indicate microglial over-activation277,282,283. Moreover, parental immunological disorders (for example, ankylosing spondylitis, ulcerative colitis, autoimmune thyroid disease and atopic/allergic diseases) are associated with autism and ADHD in offspring284,285. Shared genetic factors or familial causes of neurodevelopmental conditions and autoimmune disorders have also been identified286.
Nevertheless, research on the intersections of sex/gender and immunology in neurodevelopmental conditions remains scarce279. A Norwegian registry study found higher rates of psoriasis, ulcerative colitis and Crohn’s disease in females than in males diagnosed with ADHD287. Animal studies have generated some evidence that the effects of immune activation are moderated by offspring sex270. For example, male, but not female, offspring of exposed mice exhibited elevated IL-6 cytokine levels and repetitive-restricted behaviours compared with sex-matched offspring of control mice288–290. However, other studies found more pronounced effects of maternal immune activation on female offspring291,292. In short, although solid evidence exists that some immunological mechanisms differ for females and males in the general population, and it is likely that immune processes are altered in neurodevelopmental conditions, the study of sex/gender differences in immune responses and mechanisms in neurodivergent populations is still in its infancy.
Gut–brain axis and microbiome
Evidence suggests that the gut–brain axis and microbiome can modulate brain development and behaviour293, impacting mental health and neurodevelopmental outcomes294 while also interacting with sex-related attributes295. In the general population, some gastrointestinal disorders are more common in males (for example, diarrhoea) and some are more common in females (for example, functional dyspepsia and irritable bowel syndrome); they are also more often associated with anxiety and depression in females296. Associations between neurodevelopmental conditions, particularly autism and ADHD, and gastrointestinal problems are established but specific microbiome signatures have yet to be identified77,297,298. Nevertheless, specific antigenic determinants (epitopes) found on microorganism surfaces have been reported to correlate with sex/gender in autism299. In addition, in animal studies, sex-specific gut microbiota profiles were detected in the BTBR mouse model of autism, and prenatal exposure to elevated levels of microbiome products generated subtle sex differences in social behaviour and activity levels at different points during development291,300. To summarize, although research shows that the gut–brain axis and microbiome influences on mental health issues and neurodevelopmental conditions might affect females and males differently, exact findings on mechanisms are lacking.
Conclusions and future directions
The evidence discussed in this Review demonstrates that multiple sex-related and gender-related factors drive the biological and behavioural variability in neurodevelopmental conditions (see Fig. 2 for summary). The strength of this evidence varies — from established to emerging — and is drawn from epidemiological, behavioural, cognitive, neurobiological, genetic, endocrinological and immunological research. Notably, the mechanisms by which sex-related and gender-related attributes exert their effects on neurodevelopmental outcomes are likely to include a wide range of interacting biological, psychological and sociocultural processes. As neurodevelopmental conditions are operationalized diagnostically via sets of prototypical traits and behaviours, it is important that clinical assessments and subsequent recommendations for services are well informed by the nuanced sex and gender differences in the clinical gestalt of neurodivergent individuals (Table 1). Research has been instrumental in raising clinical awareness of such nuances, especially in autism and ADHD106,133, and improving the timely and effective identification and support of neurodivergent females. Clarifying the biological mechanisms and specific processes that mediate sex/gender influences on neurodevelopmental phenotypes will bring new opportunities for sex-tailored and gender-tailored biological interventions (Fig. 2; Tables 2,3). In autism, there is more support for qualitative sex/gender brain differences, with more widespread and qualitatively different network alterations in females compared with males. Similarly, increasing evidence suggests that the neural correlates of ADHD, dyslexia, Tourette syndrome and FXS might be different in females than in males.
Fig. 2 |. Simplified schema of the influences of sex-related and gender-related attributes on brain development.
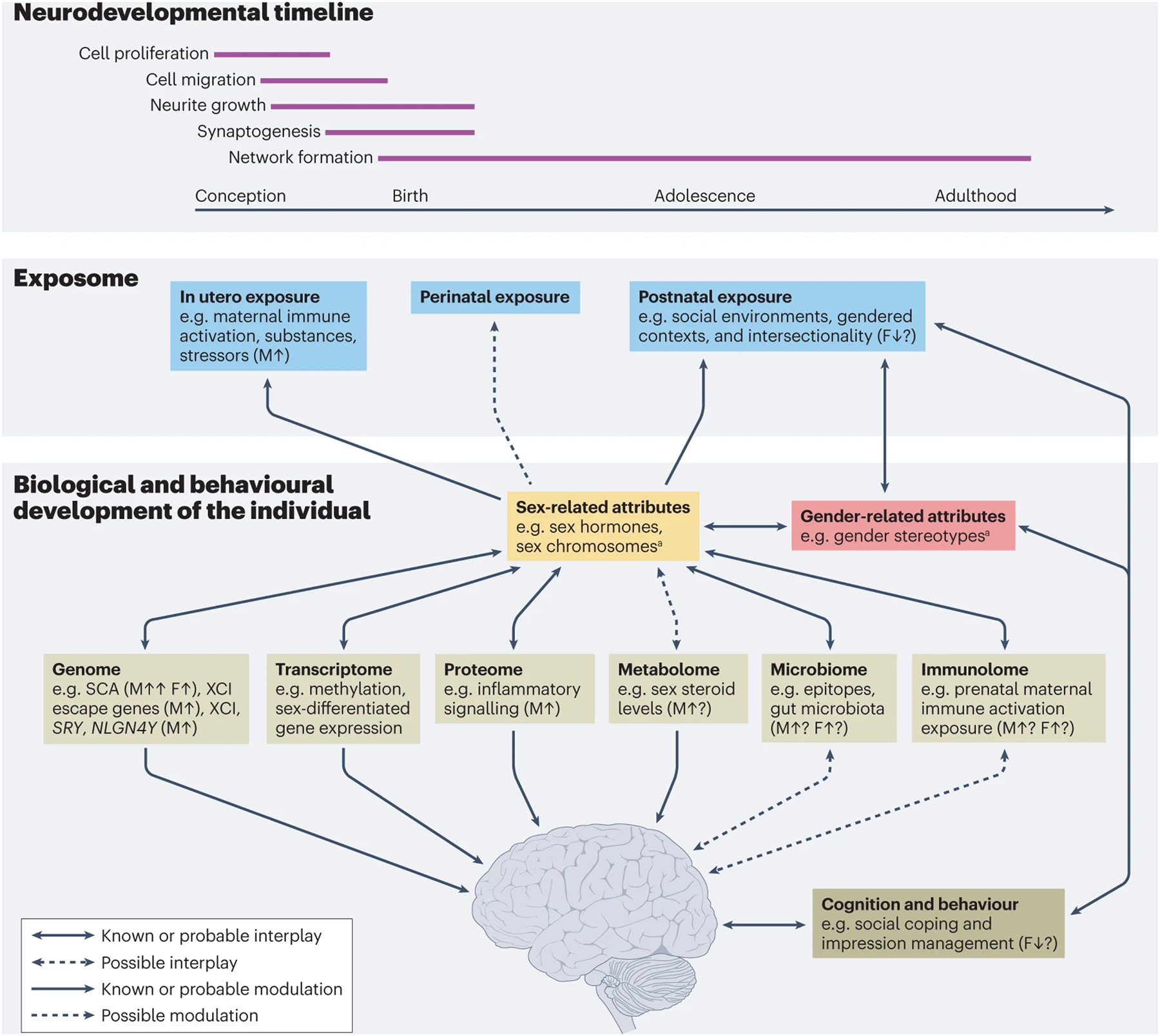
Visualization of different levels at which sex-related and gender-related attributes influence brain development, interacting with environmental factors. Sex-related attributes can influence brain development before or shortly after birth and throughout life, whereas the influence of gender-related attributes occurs after birth and throughout the lifespan. SRY encodes sex-determining region Y protein, which stimulates male gonadal development and fetal testosterone production. NLGN4Y encodes neuroligin-4 and is a candidate autism gene. F, females; M, males; SCA, sex chromosome aneuploidies; XCI, X chromosome inactivation; ↑, factor increases the likelihood of developing and/or presenting neurodevelopmental conditions; ↓, factor decreases the likelihood of developing and/or presenting neurodevelopmental conditions. aSee Fig. 1 for more examples of sex-related and gender-related attributes.
Limitations of existing evidence
Much of the evidence implicating sex hormones in the mechanisms of sex differences in brain development and of neurodevelopmental conditions relies on studies in animals. Research in humans is limited by methodological challenges and an incomplete understanding of the complex interactions between genetic, endocrine, metabolic and immune factors. Mechanistic research is required to characterize the human sex steroid metabolome, the actions of sex steroids across development and their interaction with genes and downstream epigenetic factors, alongside their interplay with gender-related experiential factors in human societies.
There remain major caveats in current research that limit our understanding and the translatability of knowledge. Substantial methodological problems arise from the inappropriate conflation of sex and gender attributes and the systemic under-representation of specific subpopulations. Foremost, although gender terminology is used increasingly in research, most studies have investigated the moderating effect(s) of sex-labels assigned at birth, which only serve as a proxy of the joint effects of sex-related biological factors and gender-related attributes beyond gender identity. It is rather the exception that biomedical research properly assesses gender-related attributes301 (Box 1), and there are very few countries that register sex beyond the male/female binary and gender identity. The convenient but problematic use of one single, binary variable to represent sex or gender (even conflating sex and gender) without delineating specific sex-related and gender-related components has to change302. Further, many studies include small numbers of females and gender-minority individuals and therefore frequently lack the statistical power to conduct sex-focused and gender-focused analyses. Some research has excluded females or treated sex/gender as confounds to adjust for, rather than examining sex and gender attributes as substantive predictors, moderators or mediators in their own right, which is suboptimal187,303.
Other caveats and controversies are also prominent. First, most research focuses on autism, and to a lesser extent on ADHD and tic disorders, whereas research for other diagnoses is selective or absent. Beyond specific genetic syndromes (for example, FXS), there is little research on sex/gender effects in intellectual disabilities, which is also reflected in the autism literature insofar as autistic people with intellectual disabilities are disproportionately under-represented304,305. This is especially the case for those with high support needs, whose pronounced cognitive and/or language impairments necessitate round-the-clock care, that is, so-called profound autism73. Second, sex/gender ratios, behavioural and biological phenotypes, and co-occurring conditions change over time, and there are cohort effects306. In ADHD, hyperactivity, impulsivity and inattentive patterns change over the course of development, and research published over the last two decades has proposed the existence of a potential ‘late-onset’ form, although this remains controversial25,27,307,308. The limited number of cross-sectional and especially longitudinal studies on the moderating and mediating effects of specific sex and gender components prevents us from drawing firm conclusions regarding their roles in neurodivergent developmental trajectories175. Third, some widely cited constructs and models require reconsideration and refinement. Discussions about the validity and conceptual clarity of the so-called female (autism) phenotype106,309,310, camouflaging109,311–315 and female protective effect226,316 are ongoing. Fourth, it is important to determine the extent to which the observed sex/gender effects in neurodevelopmental conditions diverge from established sex/gender effects in the general population123,317. Typically, larger sex/gender differences are reported in clinical studies, but infrequently the same patterns emerge in population-based studies78,82–84. Fifth, the intersectionality of ethnocultural and sex/gender influences in neurodevelopmental conditions requires far more attention. For instance, in contrast to popular belief, the largest sex/gender differences in psychological constructs in the general population, and possibly neurodevelopmental conditions, are reported in egalitarian, high-income countries318. Sixth, polarization exists in public and scientific discourses regarding sex/gender issues, including risks of both over-emphasizing and under-emphasizing sex/gender differences and influences for ideological reasons over scientific reasons. Research should seek to achieve a balanced and sober approach, unaffected by populism. Finally, although a pivotal goal of sex/gender-related research is to generate evidence that enables the best possible support for individuals of any sex and gender, studies on sex/gender influences of intervention effects are in their infancy. Inclusive and equitable intervention research to address the question ‘what works for whom’ must generate evidence that is sex/gender-informed, sex/gender-aware and sex/gender-tailored.
Future directions
We should aim to develop a better understanding of sex-informed and gender-informed measurements and outcomes via collaborative and participatory approaches with neurodivergent people and their families. We should recruit large-enough samples with stratification for specific sex-related and gender-related factors to provide the necessary information for sex-tailored and gender-tailored understanding and individualized prediction, and to quantify behaviours and biology in relation to general population sex and gender norms. We should account for specific sex-related and gender-related factors in proposed mechanisms rather than lumping these constructs together. Applying this research strategy is especially important, as hitherto, evidence does not allow robust separation of shared, non-shared and quantitatively different neurodevelopmental features by sex and gender, which muddles mechanistic understanding. To make real progress, future research should avoid using a single, conflated sex/gender label, but rather measure specific sex-related and gender-related attributes more precisely to test theory-driven hypotheses and allow post hoc and data-driven explorations. Study samples need to be diversified to ensure representation not only across sexes and genders, but also across ethnic, socioeconomic and cultural groups to address intersectionality. Inclusion of people with intellectual disabilities across research areas is crucial to ensuring that findings can be generalized to the neurodivergent population at large. Accordingly, new longitudinal studies will be able to capture the heterogeneity of developmental trajectories of the neurodivergent populations and clarify the developmental contributions of sex-related and gender-related factors to different life outcomes. With these improvements, we are optimistic that research will be able to integrate sex and gender into next-generation diagnostic, intervention and support practices favouring personalized profiles29,319.
Key points.
Sex-related and gender-related factors moderate or mediate biological and behavioural variability in neurodevelopmental conditions; they exert influence through diverse mechanisms that act at multiple levels.
Moderating effects of sex and/or gender are supported by epidemiological, behavioural, cognitive, neurobiological, genetic, endocrinological and immunological evidence, especially in autism, but also in attention-deficit/hyperactivity disorder (ADHD), Tourette syndrome, dyslexia and fragile X syndrome.
In autism and ADHD, research has started to raise awareness of clinical nuances to enable better and earlier identification of neurodivergent females.
In neuroimaging studies, the neurobiological correlates of autism and ADHD show some overlap between males and females, but also some qualitative differences; preliminary evidence indicates that there are differences in the presentation of fragile X syndrome, dyslexia and Tourette syndrome in the brain between female and male individuals.
Sex hormones are implicated in the mechanisms underlying sex differences in brain development and in susceptibility to neurodevelopmental conditions, but much of the evidence relies on preclinical studies.
Future research needs to address under-representation of females, gender minorities and specific diagnoses; challenges of interpretation; insufficient understanding of specific sex and gender attributes; developmental variabilities; intersectionality of ethnocultural and sex/gender factors; and sex/gender influences of intervention.
Acknowledgements
The authors thank E.-L. Säätelä and A. Vikingson, Karolinska Institutet Library, for expertise and assistance in the systematic literature search. For this review, J.N. was supported by Riksbankens Jubileumsfonds as a Pro-Futura Scientia fellow; P.B.M. was supported by DFG grant 456967546 and VW IDENTIFIED; Z.J.W. was supported by National Institute on Deafness and Other Communication Disorders grant F30-DC019510 and National Institute of General Medical Sciences grant T32-GM007347; and M.-C.L. was supported by the Academic Scholars Award from the Department of Psychiatry, University of Toronto, and the Canadian Institutes of Health Research Sex and Gender Science Chair (GSB 171373).
Glossary
- Gender diversity
The variations of gender identity and expression in the human populations that may or may not be aligned with those stereotypically linked to a person’s sex assigned at birth.
- Gender-minority individual
A person whose gender identity is different from their gender and sex assigned at birth; for instance, someone who identifies as transgender or non-binary.
- Genomic imprinting
Parent-of-origin-specific allelic expression of genes whereby only one copy of a gene is expressed while the other copy is suppressed; both maternally and paternally inherited genes can be subject to genomic imprinting.
- Impression management
The ubiquitous human tendency to present favourable impressions of oneself in front of others during social interactions, such that one can achieve interpersonal or pragmatic rewards.
- Lateralization
The tendency for selective neural or cognitive processes to be more strongly represented in or specialized to one brain hemisphere.
- Liabilities
The sum of genetic and environmental factors that contribute to the development of a multifactorial phenotype; the genetic liability threshold model proposes that there is a continuous liability distribution within a population for a binary trait outcome and the distribution has a threshold that divides the population into two (that is, those with and those without the trait).
- Morphosyntactic
An alternative term for ‘grammar’; grammar includes morphology and syntax, where morphology is about words and their formation, and syntax is about sentences and their formation.
- Mosaicism
In genetics, mosaicism refers to the situation where an individual or organism has more than one genetic lineage within their cells, which can arise secondary to genetic mutation (including sex chromosomes) in the zygote; regarding neurophenotypes, mosaicism refers to the idea that the degree of typical ‘maleness’/’femaleness’ of specific features in the brain of an individual is not internally consistent.
- Profound autism
A new administrative term proposed by The Lancet Commission on the future of care and clinical research in autism to describe autistic individuals with high support needs (that is, requiring 24-h access to an adult who can care for them if concerns arise, being unable to be left completely alone in a residence, and not being able to take care of basic daily adaptive needs), often owing to substantial intellectual disability, very limited language, or both73.
- Sex assigned at birth
The sex label (also termed ‘sex at birth’ or ‘sex designated at birth’) recorded at a person’s birth (for example, on a person’s birth certificate), typically given based on a child’s reproductive system and related physical characteristics (for example, genital anatomy).
- Sex chromosome aneuploidies
The presence of additional sex chromosome(s) (X or Y) beyond the normal complement, associated with neurogenetic conditions; for example, X0 (Turner syndrome), XXY (Klinefelter syndrome), XXX (trisomy X, triple X syndrome), XYY (XYY syndrome, Jacobs syndrome).
- Transdiagnostic methods
Approaches that reduce adherence to the conventional categorical diagnostic nosology or replace it with new frameworks that characterize medical, psychiatric and neurodevelopmental conditions in terms of relevant trait dimensions instead of discrete categories21.
- X chromosome inactivation
The random silencing of one copy of the X chromosome per cell in individuals with XX chromosomes to compensate for dosage effects.
Footnotes
Competing interests
The authors declare no direct conflict of interest related to this article. S.B. discloses that he has in the last 3 years acted as an author, consultant or lecturer for Medice and Roche. He receives royalties for textbooks and diagnostic tools from Hogrefe, Kohlhammer and UTB, and editorial honorarium from SAGE Publications. S.B. is a shareholder in NeuroSupportSolutions International and SB Education/Psychological Consulting. P.B.M. has received royalties for textbooks from Springer and Urban & Fischer and editorial honorarium from Elsevier. Z.J.W. has received consulting fees from Autism Speaks, the May Institute, and Roche; he also serves on the Autistic Researchers Review Board of the Autism Intervention Research Network for Physical Health (AIR-P). L.G. has acted as a consultant to Kingdom Therapeutics. M.-C.L. has received editorial honorarium from SAGE Publications.
References
- 1.Poeschl G A hundred years of debates on sex differences: developing research for social change. J. Soc. Polit. Psychol 9, 221–235 (2021). [Google Scholar]
- 2.Joel D Beyond the binary: rethinking sex and the brain. Neurosci. Biobehav. Rev 122, 165–175 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Kiesow H et al. 10,000 social brains: sex differentiation in human brain anatomy. Sci. Adv 6, eaaz1170 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hines M Neuroscience and sex/gender: looking back and forward. J. Neurosci 40, 37–43 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeCasien AR, Guma E, Liu S & Raznahan A Sex differences in the human brain: a roadmap for more careful analysis and interpretation of a biological reality. Biol. Sex. Differ 13, 43 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lanzenberger R, Kranz GS & Savic I (eds) Sex Differences in Neurology and Psychiatry (Elsevier, 2020). [Series Eds Aminoff MJ, Boller F & Swaab DF Handbook of Clinical Neurology Vol. 175] [Google Scholar]
- 7.Mauvais-Jarvis F et al. Sex and gender: modifiers of health, disease, and medicine. Lancet 396, 565–582 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White J, Tannenbaum C, Klinge I, Schiebinger L & Clayton J The integration of sex and gender considerations into biomedical research: lessons from international funding agencies. J. Clin. Endocrinol. Metab 106, 3034–3048 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thapar A, Cooper M & Rutter M Neurodevelopmental disorders. Lancet Psychiatry 4, 339–346 (2017). [DOI] [PubMed] [Google Scholar]
- 10.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 5th edn Text Revision (American Psychiatric Association, 2022). [Google Scholar]
- 11.World Health Organization. International Classification of Diseases, eleventh revision (ICD-11). (WHO, 2019). [Google Scholar]
- 12.Zablotsky B et al. Prevalence and trends of developmental disabilities among children in the United States: 2009–2017. Pediatrics 144, e20190811 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merikangas AK & Almasy L Using the tools of genetic epidemiology to understand sex differences in neuropsychiatric disorders. Genes Brain Behav. 19, e12660 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Posserud MB, Skretting Solberg B, Engeland A, Haavik J & Klungsoyr K Male to female ratios in autism spectrum disorders by age, intellectual disability and attention-deficit/hyperactivity disorder. Acta Psychiatr. Scand 144, 635–646 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Loomes R, Hull L & Mandy WPL What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J. Am. Acad. Child Adolesc. Psychiatry 56, 466–474 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Kallitsounaki A & Williams DM Autism spectrum disorder and gender dysphoria/incongruence. A systematic literature review and meta-analysis. J. Autism Dev. Disord 10.1007/s10803-022-05517-y (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McPhate L et al. Gender variance in children and adolescents with neurodevelopmental and psychiatric conditions from Australia. Arch. Sex. Behav 50, 863–871 (2021). [DOI] [PubMed] [Google Scholar]
- 18.Ismail FY & Shapiro BK What are neurodevelopmental disorders? Curr. Opin. Neurol 32, 611–616 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Faraone SV et al. The World Federation of ADHD International Consensus Statement: 208 evidence-based conclusions about the disorder. Neurosci. Biobehav. Rev 128, 789–818 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norton ES, Beach SD & Gabrieli JD Neurobiology of dyslexia. Curr. Opin. Neurobiol 30, 73–78 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Astle DE, Holmes J, Kievit R & Gathercole SE Annual research review: the transdiagnostic revolution in neurodevelopmental disorders. J. Child Psychol. Psychiatry 63, 397–417 (2022). [DOI] [PubMed] [Google Scholar]
- 22.Hoogman M et al. Consortium neuroscience of attention deficit/hyperactivity disorder and autism spectrum disorder: the ENIGMA adventure. Hum. Brain Mapp 43, 37–55 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris-Rosendahl DJ & Crocq MA Neurodevelopmental disorders – the history and future of a diagnostic concept. Dialogues Clin. Neurosci 22, 65–72 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rapoport JL, Giedd JN & Gogtay N Neurodevelopmental model of schizophrenia: update 2012. Mol. Psychiatry 17, 1228–1238 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sibley MH et al. Late-onset ADHD reconsidered with comprehensive repeated assessments between ages 10 and 25. Am. J. Psychiatry 175, 140–149 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agnew-Blais J & Arseneault L Late-onset ADHD: case closed or open question? Am. J. Psychiatry 175, 481–482 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Asherson P & Agnew-Blais J Annual research review: does late-onset attention-deficit/hyperactivity disorder exist? J. Child Psychol. Psychiatry 60, 333–352 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Bölte S et al. Standardised assessment of functioning in ADHD: consensus on the ICF core sets for ADHD. Eur. Child Adolesc. Psychiatry 27, 1261–1281 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Bölte S et al. The gestalt of functioning in autism spectrum disorder: results of the international conference to develop final consensus International Classification of Functioning, Disability and Health core sets. Autism 23, 449–467 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Souza H & Karmiloff-Smith A Neurodevelopmental disorders. Wiley Interdiscip. Rev. Cogn. Sci 8, e1398 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Tick B, Bolton P, Happe F, Rutter M & Rijsdijk F Heritability of autism spectrum disorders: a meta-analysis of twin studies. J. Child Psychol. Psychiatry 57, 585–595 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faraone SV & Larsson H Genetics of attention deficit hyperactivity disorder. Mol. Psychiatry 24, 562–575 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bölte S, Girdler S & Marschik PB The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell Mol. Life Sci 76, 1275–1297 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mandy W & Lai MC Annual research review: the role of the environment in the developmental psychopathology of autism spectrum condition. J. Child Psychol. Psychiatry 57, 271–292 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Kiser DP, Rivero O & Lesch KP Annual research review: the (epi)genetics of neurodevelopmental disorders in the era of whole-genome sequencing – unveiling the dark matter. J. Child Psychol. Psychiatry 56, 278–295 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Jonsson U et al. Annual research review: quality of life and childhood mental and behavioural disorders – a critical review of the research. J. Child Psychol. Psychiatry 58, 439–469 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Lai MC, Anagnostou E, Wiznitzer M, Allison C & Baron-Cohen S Evidence-based support for autistic people across the lifespan: maximising potential, minimising barriers, and optimising the person-environment fit. Lancet Neurol. 19, 434–451 (2020). [DOI] [PubMed] [Google Scholar]
- 38.Kapp SK Social support, well-being, and quality of life among individuals on the autism spectrum. Pediatrics 141, S362–S368 (2018). [DOI] [PubMed] [Google Scholar]
- 39.Dwyer P The neurodiversity approach(es): what are they and what do they mean for researchers? Hum. Dev 66, 73–92 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coghill D & Sonuga-Barke EJ Annual research review: categories versus dimensions in the classification and conceptualisation of child and adolescent mental disorders – implications of recent empirical study. J. Child Psychol. Psychiatry 53, 469–489 (2012). [DOI] [PubMed] [Google Scholar]
- 41.Posner J, Polanczyk GV & Sonuga-Barke E Attention-deficit hyperactivity disorder. Lancet 395, 450–462 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Happé F & Frith U Dimensional or categorical approaches to autism? Both are needed. A reply to Nick Chown and Julia Leatherland. J. Autism Dev. Disord 51, 752–753 (2021). [DOI] [PubMed] [Google Scholar]
- 43.Pellicano E & den Houting J Annual research review: shifting from ‘normal science’ to neurodiversity in autism science. J. Child Psychol. Psychiatry 63, 381–396 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schuck RK et al. Neurodiversity and autism intervention: reconciling perspectives through a naturalistic developmental behavioral intervention framework. J. Autism Dev. Disord 52, 4625–4645 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chapman R Neurodiversity and the social ecology of mental functions. Perspect. Psychol. Sci 16, 1360–1372 (2021). [DOI] [PubMed] [Google Scholar]
- 46.Chapman R in Neurodiversity Studies Ch. 14 (eds Rosqvist H, Chown N & Stenning A) 218–220 (Routledge, 2020). [Google Scholar]
- 47.Dwyer P, Ryan JG, Williams ZJ & Gassner DL First do no harm: suggestions regarding respectful autism language. Pediatrics 149, e2020049437N (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fletcher-Watson S Transdiagnostic research and the neurodiversity paradigm: commentary on the transdiagnostic revolution in neurodevelopmental disorders by Astle et al. J. Child Psychol. Psychiatry 63, 418–420 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leadbitter K, Buckle KL, Ellis C & Dekker M Autistic self-advocacy and the neurodiversity movement: implications for autism early intervention research and practice. Front. Psychol 12, 635690 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown HM et al. The Autism Intervention Research Network on Physical Health Autistic Researcher Review Board. Pediatrics 149, e2020049437F (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wehmeyer ML The future of positive psychology and disability. Front. Psychol 12, 790506(2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joel D & McCarthy MM Incorporating sex as a biological variable in neuropsychiatric research: where are we now and where should we be? Neuropsychopharmacology 42, 379–385 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhargava A et al. Considering sex as a biological variable in basic and clinical studies: an Endocrine Society scientific statement. Endocr. Rev 42, 219–258 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cost KT et al. Checking assumptions: advancing the analysis of sex and gender in human health and psychological sciences. Preprint at OSF Preprints 10.31219/osf.io/c29kg (2022). [DOI] [Google Scholar]
- 55.Tadiri CP et al. Methods for prospectively incorporating gender into health sciences research. J. Clin. Epidemiol 129, 191–197 (2021). [DOI] [PubMed] [Google Scholar]
- 56.Nielsen MW et al. Gender-related variables for health research. Biol. Sex. Difer 12, 23(2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Anders SM, Steiger J & Goldey KL Effects of gendered behavior on testosterone in women and men. Proc. Natl Acad. Sci. USA 112, 13805–13810 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cortes LR, Cisternas CD & Forger NG Does gender leave an epigenetic imprint on the brain? Front. Neurosci 13, 173 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fine C, Dupre J & Joel D Sex-linked behavior: evolution, stability, and variability. Trends Cogn. Sci 21, 666–673 (2017). [DOI] [PubMed] [Google Scholar]
- 60.Polanczyk GV, Willcutt EG, Salum GA, Kieling C & Rohde LA ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. Int. J. Epidemiol 43, 434–442 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeidan J et al. Global prevalence of autism: a systematic review update. Autism Res. 15, 778–790 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Black LI, Vahratian A & Hoffman HJ Communication disorders and use of intervention services among children aged 3–17 years: United States, 2012. NCHS Data Brief. 205, 1–8 (2015). [PubMed] [Google Scholar]
- 63.Altarac M & Saroha E Lifetime prevalence of learning disability among US children. Pediatrics 119, S77–S83 (2007). [DOI] [PubMed] [Google Scholar]
- 64.Knight T et al. Prevalence of tic disorders: a systematic review and meta-analysis. Pediatr. Neurol 47, 77–90 (2012). [DOI] [PubMed] [Google Scholar]
- 65.Maulik PK, Mascarenhas MN, Mathers CD, Dua T & Saxena S Prevalence of intellectual disability: a meta-analysis of population-based studies. Res. Dev. Disabil 32, 419–436 (2011). [DOI] [PubMed] [Google Scholar]
- 66.Fayyad J et al. The descriptive epidemiology of DSM-IV Adult ADHD in the World Health Organization World Mental Health Surveys. Atten. Defic. Hyperact. Disord 9, 47–65 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Levine JLS, Szejko N & Bloch MH Meta-analysis: adulthood prevalence of Tourette syndrome. Prog. Neuropsychopharmacol. Biol. Psychiatry 95, 109675 (2019). [DOI] [PubMed] [Google Scholar]
- 68.Russell G et al. Time trends in autism diagnosis over 20 years: a UK population-based cohort study. J. Child Psychol. Psychiatry 63, 674–682 (2022). [DOI] [PubMed] [Google Scholar]
- 69.Rutherford M et al. Gender ratio in a clinical population sample, age of diagnosis and duration of assessment in children and adults with autism spectrum disorder. Autism 20, 628–634 (2016). [DOI] [PubMed] [Google Scholar]
- 70.Idring S et al. Changes in prevalence of autism spectrum disorders in 2001–2011: findings from the Stockholm Youth Cohort. J. Autism Dev. Disord 45, 1766–1773 (2015). [DOI] [PubMed] [Google Scholar]
- 71.Yang J et al. The prevalence of diagnosed Tourette syndrome in Canada: a national population-based study. Mov. Disord 31, 1658–1663 (2016). [DOI] [PubMed] [Google Scholar]
- 72.Willcutt EG The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics 9, 490–499 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lord C et al. The Lancet Commission on the future of care and clinical research in autism. Lancet 399, 271–334 (2022). [DOI] [PubMed] [Google Scholar]
- 74.Bölte S, Poustka L & Geurts HM in Oxford Textbook of Attention Defiicit Hyperactivity Disorder Ch. 24 (eds Banaschewski T, Coghill D & Zuddas A) 227–234 (Oxford Univ. Press, 2018). [Google Scholar]
- 75.Brimo K et al. The co-occurrence of neurodevelopmental problems in dyslexia. Dyslexia 27, 277–293 (2021). [DOI] [PubMed] [Google Scholar]
- 76.Pan PY, Bölte S, Kaur P, Jamil S & Jonsson U Neurological disorders in autism: a systematic review and meta-analysis. Autism 25, 812–830 (2021). [DOI] [PubMed] [Google Scholar]
- 77.Pan PY & Bölte S The association between ADHD and physical health: a co-twin control study. Sci. Rep 10, 22388 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lai MC et al. Prevalence of co-occurring mental health diagnoses in the autism population: a systematic review and meta-analysis. Lancet Psychiatry 6, 819–829 (2019). [DOI] [PubMed] [Google Scholar]
- 79.Kassee C et al. Physical health of autistic girls and women: a scoping review. Mol. Autism 11, 84 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lundin K, Mahdi S, Isaksson J & Bölte S Functional gender differences in autism: an international, multidisciplinary expert survey using the International Classification of Functioning, Disability, and Health model. Autism 25, 1020–1035 (2021). [DOI] [PubMed] [Google Scholar]
- 81.de Schipper E et al. Towards an ICF core set for ADHD: a worldwide expert survey on ability and disability. Eur. Child Adolesc. Psychiatry 24, 1509–1521 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ottosen C et al. Sex differences in comorbidity patterns of attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry 58, 412–422 (2019). [DOI] [PubMed] [Google Scholar]
- 83.Simonoff E et al. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J. Am. Acad. Child Adolesc. Psychiatry 47, 921–929 (2008). [DOI] [PubMed] [Google Scholar]
- 84.Martin J et al. Sex differences in anxiety and depression in children with attention deficit hyperactivity disorder: investigating genetic liability and comorbidity. Am. J. Med. Genet. B Neuropsychiatr. Genet 186, 412–422 (2021). [DOI] [PubMed] [Google Scholar]
- 85.Hirvikoski T et al. Premature mortality in autism spectrum disorder. Br. J. Psychiatry 208, 232–238 (2016). [DOI] [PubMed] [Google Scholar]
- 86.Kirby AV et al. A 20-year study of suicide death in a statewide autism population. Autism Res. 12, 658–666 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hirvikoski T et al. Individual risk and familial liability for suicide attempt and suicide in autism: a population-based study. Psychol. Med 50, 1463–1474 (2020). [DOI] [PubMed] [Google Scholar]
- 88.Catala-Lopez F et al. Mortality in persons with autism spectrum disorder or attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. JAMA Pediatr. 176, e216401 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lunsky Y et al. Premature mortality in a population-based cohort of autistic adults in Canada. Autism Res. 15, 1550–1559 (2022). [DOI] [PubMed] [Google Scholar]
- 90.Dalsgaard S, Østergaard SD, Leckman JF, Mortensen PB & Pedersen MG Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet 385, 2190–2196 (2015). [DOI] [PubMed] [Google Scholar]
- 91.Wing L Sex ratios in early childhood autism and related conditions. Psychiatry Res. 5, 129–137 (1981). [DOI] [PubMed] [Google Scholar]
- 92.Tsai LY & Beisler JM The development of sex differences in infantile autism. Br. J. Psychiatry 142, 373–378 (1983). [DOI] [PubMed] [Google Scholar]
- 93.Dworzynski K, Ronald A, Bolton P & Happé F How different are girls and boys above and below the diagnostic threshold for autism spectrum disorders? J. Am. Acad. Child Adolesc. Psychiatry 51, 788–797 (2012). [DOI] [PubMed] [Google Scholar]
- 94.Frazier TW, Georgiades S, Bishop SL & Hardan AY Behavioral and cognitive characteristics of females and males with autism in the Simons Simplex Collection. J. Am. Acad. Child Adolesc. Psychiatry 53, 329–340 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Duvekot J et al. Factors influencing the probability of a diagnosis of autism spectrum disorder in girls versus boys. Autism 21, 646–658 (2017). [DOI] [PubMed] [Google Scholar]
- 96.Rødgaard EM, Jensen K, Miskowiak KW & Mottron L Autism comorbidities show elevated female-to-male odds ratios and are associated with the age of first autism diagnosis. Acta Psychiatr. Scand 144, 475–486 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.May T & Williams K Brief report: gender and age of diagnosis time trends in children with autism using Australian Medicare data. J. Autism Dev. Disord 48, 4056–4062 (2018). [DOI] [PubMed] [Google Scholar]
- 98.McCormick CEB et al. Autism heterogeneity in a densely sampled US population: results from the first 1000 participants in the RI-CART study. Autism Res. 13, 474–488 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang Y et al. Factors associated with age at autism diagnosis in a community sample of Australian adults. Autism Res. 14, 2677–2687 (2021). [DOI] [PubMed] [Google Scholar]
- 100.Kavanaugh BC et al. Moderators of age of diagnosis in >20,000 females with autism in two large US studies. J. Autism Dev. Disord 10.1007/s10803-021-05026-4 (2021). [DOI] [PubMed] [Google Scholar]
- 101.Fusar-Poli L, Brondino N, Politi P & Aguglia E Missed diagnoses and misdiagnoses of adults with autism spectrum disorder. Eur. Arch. Psychiatry Clin. Neurosci 272, 187–198 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bonney E, Abbo C, Ogara C, Villalobos ME & Elison JT Sex differences in age of diagnosis of autism spectrum disorder: preliminary evidence from Uganda. Autism Res. 15, 183–191 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Whitlock A, Fulton K, Lai MC, Pellicano E & Mandy W Recognition of girls on the autism spectrum by primary school educators: an experimental study. Autism Res. 13, 1358–1372 (2020). [DOI] [PubMed] [Google Scholar]
- 104.Tillmann J et al. Evaluating sex and age differences in ADI-R and ADOS scores in a large European multi-site sample of individuals with autism spectrum disorder. J. Autism Dev. Disord 48, 2490–2505 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kaat AJ et al. Sex differences in scores on standardized measures of autism symptoms: a multisite integrative data analysis. J. Child Psychol. Psychiatry 62, 97–106 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lai MC, Lin HY & Ameis SH Towards equitable diagnoses for autism and attention-deficit/hyperactivity disorder across sexes and genders. Curr. Opin. Psychiatry 35, 90–100 (2022). [DOI] [PubMed] [Google Scholar]
- 107.Wood-Downie H, Wong B, Kovshoff H, Cortese S & Hadwin JA Research review: a systematic review and meta-analysis of sex/gender differences in social interaction and communication in autistic and nonautistic children and adolescents. J. Child Psychol. Psychiatry 62, 922–936 (2021). [DOI] [PubMed] [Google Scholar]
- 108.Clarke E et al. Assessing gender differences in autism spectrum disorder using the gendered autism behavioral scale (GABS): an exploratory study. Res. Autism Spectr. Disord 88, 101844 (2021). [Google Scholar]
- 109.Ai W, Cunningham WA & Lai MC Reconsidering autistic ‘camouflaging’ as transactional impression management. Trends Cogn. Sci 26, 631–645 (2022). [DOI] [PubMed] [Google Scholar]
- 110.Yankowitz LD et al. Infants later diagnosed with autism have lower canonical babbling ratios in the first year of life. Mol. Autism 13, 28 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Parish-Morris J et al. Linguistic camouflage in girls with autism spectrum disorder. Mol. Autism 8, 48 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sturrock A, Marsden A, Adams C & Freed J Observational and reported measures of language and pragmatics in young people with autism: a comparison of respondent data and gender profiles. J. Autism Dev. Disord 50, 812–830 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Boorse J et al. Linguistic markers of autism in girls: evidence of a “blended phenotype” during storytelling. Mol. Autism 10, 14 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Conlon O et al. Gender differences in pragmatic communication in school-aged children with autism spectrum disorder (ASD). J. Autism Dev. Disord 49, 1937–1948 (2019). [DOI] [PubMed] [Google Scholar]
- 115.Uljarević M et al. Deconstructing the repetitive behaviour phenotype in autism spectrum disorder through a large population-based analysis. J. Child Psychol. Psychiatry 61, 1030–1042 (2020). [DOI] [PubMed] [Google Scholar]
- 116.Uljarević M et al. Big data approach to characterize restricted and repetitive behaviors in autism. J. Am. Acad. Child Adolesc. Psychiatry 61, 446–457 (2022). [DOI] [PubMed] [Google Scholar]
- 117.Tsirgiotis JM, Young RL & Weber N Sex/gender differences in CARS2 and GARS-3 item scores: evidence of phenotypic differences between males and females with ASD. J. Autism Dev. Disord 52, 3958–3976 (2022). [DOI] [PubMed] [Google Scholar]
- 118.Stephenson KG, Norris M & Butter EM Sex-based differences in autism symptoms in a large, clinically-referred sample of preschool-aged children with ASD. J. Autism Dev. Disord 10.1007/s10803-020-04836-2 (2021). [DOI] [PubMed] [Google Scholar]
- 119.Uljarević M et al. Dimensional assessment of restricted and repetitive behaviors: development and preliminary validation of a new measure. J. Am. Acad. Child Adolesc. Psychiatry 10.1016/j.jaac.2022.07.863 (2022). [DOI] [PubMed] [Google Scholar]
- 120.Szatmari P et al. Developmental trajectories of symptom severity and adaptive functioning in an inception cohort of preschool children with autism spectrum disorder. JAMA Psychiatry 72, 276–283 (2015). [DOI] [PubMed] [Google Scholar]
- 121.Waizbard-Bartov E et al. Trajectories of autism symptom severity change during early childhood. J. Autism Dev. Disord 51, 227–242 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bölte S, Duketis E, Poustka F & Holtmann M Sex differences in cognitive domains and their clinical correlates in higher-functioning autism spectrum disorders. Autism 15, 497–511 (2011). [DOI] [PubMed] [Google Scholar]
- 123.Lai MC, Lombardo MV, Auyeung B, Chakrabarti B & Baron-Cohen S Sex/gender differences and autism: setting the scene for future research. J. Am. Acad. Child Adolesc. Psychiatry 54, 11–24 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hull L, Mandy W & Petrides KV Behavioural and cognitive sex/gender differences in autism spectrum condition and typically developing males and females. Autism 21, 706–727 (2017). [DOI] [PubMed] [Google Scholar]
- 125.Liu X et al. Prevalence of epilepsy in autism spectrum disorders: a systematic review and meta-analysis. Autism 26, 33–50 (2022). [DOI] [PubMed] [Google Scholar]
- 126.Wallace GL, Richard E, Wolff A, Nadeau M & Zucker N Increased emotional eating behaviors in children with autism: sex differences and links with dietary variety. Autism 25, 603–612 (2021). [DOI] [PubMed] [Google Scholar]
- 127.Lundin Remnélius K, Neufeld J, Isaksson J & Bolte S Eating problems in autistic females and males: a co-twin control study. J. Autism Dev. Disord 52, 3153–3168 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Weir E, Allison C, Ong KK & Baron-Cohen S An investigation of the diet, exercise, sleep, BMI, and health outcomes of autistic adults. Mol. Autism 12, 31 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Loyer Carbonneau M, Demers M, Bigras M & Guay MC Meta-analysis of sex differences in ADHD symptoms and associated cognitive deficits. J. Atten. Disord 25, 1640–1656 (2021). [DOI] [PubMed] [Google Scholar]
- 130.Rucklidge JJ Gender differences in attention-deficit/hyperactivity disorder. Psychiatr. Clin. North. Am 33, 357–373 (2010). [DOI] [PubMed] [Google Scholar]
- 131.Cortese S, Faraone SV, Bernardi S, Wang S & Blanco C Gender differences in adult attention-deficit/hyperactivity disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). J. Clin. Psychiatry 77, e421–e428 (2016). [DOI] [PubMed] [Google Scholar]
- 132.Young S et al. Females with ADHD: an expert consensus statement taking a lifespan approach providing guidance for the identification and treatment of attention-deficit/hyperactivity disorder in girls and women. BMC Psychiatry 20, 404 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hinshaw SP, Nguyen PT, O’Grady SM & Rosenthal EA Annual research review: attention-deficit/hyperactivity disorder in girls and women: underrepresentation, longitudinal processes, and key directions. J. Child Psychol. Psychiatry 63, 484–496 (2022). [DOI] [PubMed] [Google Scholar]
- 134.Meyer BJ, Stevenson J & Sonuga-Barke EJS Sex differences in the meaning of parent and teacher ratings of ADHD behaviors: an observational study. J. Atten. Disord 24, 1847–1856 (2020). [DOI] [PubMed] [Google Scholar]
- 135.Balint S et al. Attention deficit hyperactivity disorder (ADHD): gender- and age-related differences in neurocognition. Psychol. Med 39, 1337–1345 (2009). [DOI] [PubMed] [Google Scholar]
- 136.Elkins IJ et al. Increased risk of smoking in female adolescents who had childhood ADHD. Am. J. Psychiatry 175, 63–70 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Solberg BS et al. Gender differences in psychiatric comorbidity: a population-based study of 40 000 adults with attention deficit hyperactivity disorder. Acta Psychiatr. Scand 137, 176–186 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chen Q et al. Common psychiatric and metabolic comorbidity of adult attention-deficit/hyperactivity disorder: a population-based cross-sectional study. PLoS ONE 13, e0204516 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chilosi AM, Brovedani P, Cipriani P & Casalini C Sex differences in early language delay and in developmental language disorder. J. Neurosci. Res 10.1002/jnr.24976 (2021). [DOI] [PubMed] [Google Scholar]
- 140.Oller DK et al. Infant boys are more vocal than infant girls. Curr. Biol 30, R426–R427 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rinaldi P, Pasqualetti P, Volterra V & Caselli MC Gender differences in early stages of language development. Some evidence and possible explanations. J. Neurosci. Res 10.1002/jnr.24914 (2021). [DOI] [PubMed] [Google Scholar]
- 142.Etchell A et al. A systematic literature review of sex differences in childhood language and brain development. Neuropsychologia 114, 19–31 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Norbury CF et al. The impact of nonverbal ability on prevalence and clinical presentation of language disorder: evidence from a population study. J. Child Psychol. Psychiatry 57, 1247–1257 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Landerl K & Moll K Comorbidity of learning disorders: prevalence and familial transmission. J. Child Psychol. Psychiatry 51, 287–294 (2010). [DOI] [PubMed] [Google Scholar]
- 145.Moll K, Kunze S, Neuhoff N, Bruder J & Schulte-Korne G Specific learning disorder: prevalence and gender differences. PLoS ONE 9, e103537 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Arnett AB et al. Explaining the sex difference in dyslexia. J. Child Psychol. Psychiatry 58, 719–727 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Krafnick AJ & Evans TM Neurobiological sex differences in developmental dyslexia. Front. Psychol 9, 2669 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Willcutt EG & Pennington BF Comorbidity of reading disability and attention-deficit/hyperactivity disorder: differences by gender and subtype. J. Learn. Disabil 33, 179–191 (2000). [DOI] [PubMed] [Google Scholar]
- 149.Garris J & Quigg M The female Tourette patient: sex differences in Tourette disorder. Neurosci. Biobehav. Rev 129, 261–268 (2021). [DOI] [PubMed] [Google Scholar]
- 150.Meoni S, Macerollo A & Moro E Sex differences in movement disorders. Nat. Rev. Neurol 16, 84–96 (2020). [DOI] [PubMed] [Google Scholar]
- 151.Schwabe MJ & Konkol RJ Menstrual cycle-related fluctuations of tics in Tourette syndrome. Pediatr. Neurol 8, 43–46 (1992). [DOI] [PubMed] [Google Scholar]
- 152.Leckman JF, Walker DE & Cohen DJ Premonitory urges in Tourette’s syndrome. Am. J. Psychiatry 150, 98–102 (1993). [DOI] [PubMed] [Google Scholar]
- 153.Santangelo SL et al. Tourette’s syndrome: what are the influences of gender and comorbid obsessive-compulsive disorder? J. Am. Acad. Child Adolesc. Psychiatry 33, 795–804 (1994). [DOI] [PubMed] [Google Scholar]
- 154.Baizabal-Carvallo JF & Jankovic J Sex differences in patients with Tourette syndrome. CNS Spectr. 10.1017/S1092852922000074 (2022). [DOI] [PubMed] [Google Scholar]
- 155.Rodgers S et al. Sex-related and non-sex-related comorbidity subtypes of tic disorders: a latent class approach. Eur. J. Neurol 21, 700–707 (2014). [DOI] [PubMed] [Google Scholar]
- 156.Lewin AB et al. A phenomenological investigation of women with Tourette or other chronic tic disorders. Compr. Psychiatry 53, 525–534 (2012). [DOI] [PubMed] [Google Scholar]
- 157.Cleaton MAM, Tal-Saban M, Hill EL & Kirby A Gender and age differences in the presentation of at-risk or probable developmental coordination disorder in adults. Res. Dev. Disabil 115, 104010 (2021). [DOI] [PubMed] [Google Scholar]
- 158.Tsakanikos E, Bouras N, Sturmey P & Holt G Psychiatric co-morbidity and gender differences in intellectual disability. J. Intellect. Disabil. Res 50, 582–587 (2006). [DOI] [PubMed] [Google Scholar]
- 159.Lunsky Y Depressive symptoms in intellectual disability: does gender play a role? J. Intellect. Disabil. Res 47, 417–427 (2003). [DOI] [PubMed] [Google Scholar]
- 160.Axmon A, Sandberg M & Ahlstrom G Gender differences in psychiatric diagnoses in older people with intellectual disability: a register study. BMC Psychiatry 17, 192 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Society for the Study of Behavioural Phenotypes. Syndrome Sheets: Resources for Clinicians – Syndrome Specific Information and Current Research. SSBP https://ssbp.org.uk/syndrome-sheets/(2022). [Google Scholar]
- 162.Hunter J et al. Epidemiology of fragile X syndrome: a systematic review and meta-analysis. Am. J. Med. Genet. A 164A, 1648–1658 (2014). [DOI] [PubMed] [Google Scholar]
- 163.Bartholomay KL, Lee CH, Bruno JL, Lightbody AA & Reiss AL Closing the gender gap in fragile X syndrome: review on females with FXS and preliminary research findings. Brain Sci. 9, 11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hagerman RJ et al. Fragile X syndrome. Nat. Rev. Dis. Prim 3, 17065 (2017). [DOI] [PubMed] [Google Scholar]
- 165.Tint A & Weiss JA A qualitative study of the service experiences of women with autism spectrum disorder. Autism 22, 928–937 (2018). [DOI] [PubMed] [Google Scholar]
- 166.Mademtzi M, Singh P, Shic F & Koenig K Challenges of females with autism: a parental perspective. J. Autism Dev. Disord 48, 1301–1310 (2018). [DOI] [PubMed] [Google Scholar]
- 167.Mowlem FD et al. Sex differences in predicting ADHD clinical diagnosis and pharmacological treatment. Eur. Child Adolesc. Psychiatry 28, 481–489 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kok FM, Groen Y, Fuermaier ABM & Tucha O The female side of pharmacotherapy for ADHD – a systematic literature review. PLoS ONE 15, e0239257 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sonuga-Barke EJS et al. Sex differences in the response of children with ADHD to once-daily formulations of methylphenidate. J. Am. Acad. Child Adolesc. Psychiatry 46, 701–710 (2007). [DOI] [PubMed] [Google Scholar]
- 170.Schwabe MJ & Konkol RJ Treating Tourette syndrome with haloperidol: predictors of success. Wis. Med. J 88, 23–27 (1989). [PubMed] [Google Scholar]
- 171.Choque Olsson N et al. Social skills training for children and adolescents with autism spectrum disorder: a randomized controlled trial. J. Am. Acad. Child Adolesc. Psychiatry 56, 585–592 (2017). [DOI] [PubMed] [Google Scholar]
- 172.Ko JA, Schuck RK, Jimenez-Munoz M, Penner-Baiden KM & Vernon TW Brief report: sex/gender differences in adolescents with autism: socialization profiles and response to social skills intervention. J. Autism Dev. Disord 52, 2812–2818 (2022). [DOI] [PubMed] [Google Scholar]
- 173.Lai MC et al. Imaging sex/gender and autism in the brain: etiological implications. J. Neurosci. Res 95, 380–397 (2017). [DOI] [PubMed] [Google Scholar]
- 174.Hammill C et al. Quantitative and qualitative sex modulations in the brain anatomy of autism. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 6, 898–909 (2021). [DOI] [PubMed] [Google Scholar]
- 175.Walsh MJM, Wallace GL, Gallegos SM & Braden BB Brain-based sex differences in autism spectrum disorder across the lifespan: a systematic review of structural MRI, fMRI, and DTI findings. Neuroimage Clin. 31, 102719 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bedford SA et al. Large-scale analyses of the relationship between sex, age and intelligence quotient heterogeneity and cortical morphometry in autism spectrum disorder. Mol. Psychiatry 25, 614–628 (2020). [DOI] [PubMed] [Google Scholar]
- 177.Floris DL et al. The link between autism and sex-related neuroanatomy, and associated cognition and gene expression. Am. J. Psychiatry 180, 50–64 (2022). [DOI] [PubMed] [Google Scholar]
- 178.Surén P et al. Early growth patterns in children with autism. Epidemiology 24, 660–670 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sparks BF et al. Brain structural abnormalities in young children with autism spectrum disorder. Neurology 59, 184–192 (2002). [DOI] [PubMed] [Google Scholar]
- 180.Bloss CS & Courchesne E MRI neuroanatomy in young girls with autism: a preliminary study. J. Am. Acad. Child Adolesc. Psychiatry 46, 515–523 (2007). [DOI] [PubMed] [Google Scholar]
- 181.Schumann CM et al. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J. Neurosci 30, 4419–4427 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lee JK et al. Longitudinal evaluation of cerebral growth across childhood in boys and girls with autism spectrum disorder. Biol. Psychiatry 90, 286–294 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lee JK et al. Altered development of amygdala-connected brain regions in males and females with autism. J. Neurosci 42, 6145–6155 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Nordahl CW et al. High psychopathology subgroup in young children with autism: associations with biological sex and amygdala volume. J. Am. Acad. Child Adolesc. Psychiatry 59, 1353–1363.e2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zielinski BA et al. Sex-dependent structure of socioemotional salience, executive control, and default mode networks in preschool-aged children with autism. Neuroimage 257, 119252 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Olafson E et al. Examining the boundary sharpness coefficient as an index of cortical microstructure in autism spectrum disorder. Cereb. Cortex 31, 3338–3352 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mo K et al. Sex/gender differences in the human autistic brains: a systematic review of 20 years of neuroimaging research. Neuroimage Clin. 32, 102811 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chien YL et al. Neurodevelopmental model of schizophrenia revisited: similarity in individual deviation and idiosyncrasy from the normative model of whole-brain white matter tracts and shared brain-cognition covariation with ADHD and ASD. Mol. Psychiatry 27, 3262–3271 (2022). [DOI] [PubMed] [Google Scholar]
- 189.Tung YH et al. Whole brain white matter tract deviation and idiosyncrasy from normative development in autism and ADHD and unaffected siblings link with dimensions of psychopathology and cognition. Am. J. Psychiatry 178, 730–743 (2021). [DOI] [PubMed] [Google Scholar]
- 190.Andrews DS et al. A longitudinal study of white matter development in relation to changes in autism severity across early childhood. Biol. Psychiatry 89, 424–432 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Nordahl CW et al. Sex differences in the corpus callosum in preschool-aged children with autism spectrum disorder. Mol. Autism 6, 26 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lee JK et al. Sex differences in the amygdala resting-state connectome of children with autism spectrum disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 5, 320–329 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Floris DL et al. Towards robust and replicable sex differences in the intrinsic brain function of autism. Mol. Autism 12, 19 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sohal VS & Rubenstein JLR Excitation-inhibition balance as a framework for investigating mechanisms in neuropsychiatric disorders. Mol. Psychiatry 24, 1248–1257 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Dickinson A, Jones M & Milne E Measuring neural excitation and inhibition in autism: different approaches, different findings and different interpretations. Brain Res. 1648, 277–289 (2016). [DOI] [PubMed] [Google Scholar]
- 196.Trakoshis S et al. Intrinsic excitation-inhibition imbalance affects medial prefrontal cortex differently in autistic men versus women. Elife 9, e55684 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mottron L et al. Sex differences in brain plasticity: a new hypothesis for sex ratio bias in autism. Mol. Autism 6, 33 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.O’Neill J et al. Parsing the heterogeneity of brain metabolic disturbances in autism spectrum disorder. Biol. Psychiatry 87, 174–184 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Fung LK et al. Thalamic and prefrontal GABA concentrations but not GABAA receptor densities are altered in high-functioning adults with autism spectrum disorder. Mol. Psychiatry 26, 1634–1646 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.James D, Lam VT, Jo B & Fung LK Region-specific associations between gamma-aminobutyric acid A receptor binding and cortical thickness in high-functioning autistic adults. Autism Res. 15, 1068–1082 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Greven CU, Richards JS & Buitelaar JK in Oxford Textbook of Attention Deficit Hyperactivity Disorder Ch. 16 (eds Banaschewski T, Coghill D & Zuddas A) 154–160 (Oxford Univ. Press, 2018). [Google Scholar]
- 202.Mooney MA et al. Smaller total brain volume but not subcortical structure volume related to common genetic risk for ADHD. Psychol. Med. 51, 1279–1288 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Onnink AM et al. Brain alterations in adult ADHD: effects of gender, treatment and comorbid depression. Eur. Neuropsychopharmacol 24, 397–409 (2014). [DOI] [PubMed] [Google Scholar]
- 204.Rosch KS et al. Reduced subcortical volumes among preschool-age girls and boys with ADHD. Psychiatry Res. Neuroimaging 271, 67–74 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Jacobson LA et al. Sex-based dissociation of white matter microstructure in children with attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry 54, 938–946 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lin Q et al. Sex differences in microstructural alterations in the corpus callosum tracts in drug-naive children with ADHD. Brain Imaging Behav. 16, 1592–1604 (2022). [DOI] [PubMed] [Google Scholar]
- 207.Rosch KS, Mostofsky SH & Nebel MB ADHD-related sex differences in fronto-subcortical intrinsic functional connectivity and associations with delay discounting. J. Neurodev. Disord 10, 34 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zou H & Yang J Exploring the brain lateralization in ADHD based on variability of resting-state fMRI signal. J. Atten. Disord 25, 258–264 (2021). [DOI] [PubMed] [Google Scholar]
- 209.Chen Y, Li G, Ide JS, Luo X & Li CR Sex differences in attention deficit hyperactivity symptom severity and functional connectivity of the dorsal striatum in young adults. Neuroimage Rep. 1, 100025 (2021). [Google Scholar]
- 210.Nikolaidis A, He X, Pekar J, Rosch K & Mostofsky SH Frontal corticostriatal functional connectivity reveals task positive and negative network dysregulation in relation to ADHD, sex, and inhibitory control. Dev. Cogn. Neurosci 54, 101101 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yeo RA et al. Proton magnetic resonance spectroscopy investigation of the right frontal lobe in children with attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry 42, 303–310 (2003). [DOI] [PubMed] [Google Scholar]
- 212.Endres D et al. Neurochemical sex differences in adult ADHD patients: an MRS study. Biol. Sex. Difer 10, 50 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Peyre H et al. Neuroanatomy of dyslexia: an allometric approach. Eur. J. Neurosci 52, 3595–3609 (2020). [DOI] [PubMed] [Google Scholar]
- 214.Ramus F, Altarelli I, Jednoróg K, Zhao J & Scotto di Covella L in Dyslexia and Neuroscience: The Geschwind-Galaburda Hypothesis 30 Years Later Ch. 7 (eds Galaburda AM, Gaab N, Hoeft F & McCardle P) 78–86 (Brookes, 2018). [Google Scholar]
- 215.Horowitz-Kraus T, Brunst KJ & Cecil KM Children with dyslexia and typical readers: sex-based choline differences revealed using proton magnetic resonance spectroscopy acquired within anterior cingulate cortex. Front. Hum. Neurosci 12, 466 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Pinares-Garcia P, Stratikopoulos M, Zagato A, Loke H & Lee J Sex: a significant risk factor for neurodevelopmental and neurodegenerative disorders. Brain Sci. 8, 154 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Fahim C et al. Somatosensory-motor bodily representation cortical thinning in Tourette: effects of tic severity, age and gender. Cortex 46, 750–760 (2010). [DOI] [PubMed] [Google Scholar]
- 218.Greene DJ et al. Brain structure in pediatric Tourette syndrome. Mol. Psychiatry 22, 972–980 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Peterson BS et al. Regional brain and ventricular volumes in Tourette syndrome. Arch. Gen. Psychiatry 58, 427–440 (2001). [DOI] [PubMed] [Google Scholar]
- 220.Lightbody AA & Reiss AL Gene, brain, and behavior relationships in fragile X syndrome: evidence from neuroimaging studies. Dev. Disabil. Res. Rev 15, 343–352 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Gharehgazlou A et al. Cortical gyrification morphology in individuals with ASD and ADHD across the lifespan: a systematic review and meta-analysis. Cereb. Cortex 31, 2653–2669 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Pietschnig J, Penke L, Wicherts JM, Zeiler M & Voracek M Meta-analysis of associations between human brain volume and intelligence differences: how strong are they and what do they mean? Neurosci. Biobehav. Rev 57, 411–432 (2015). [DOI] [PubMed] [Google Scholar]
- 223.Jiang R et al. Gender differences in connectome-based predictions of individualized intelligence quotient and sub-domain scores. Cereb. Cortex 30, 888–900 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Carter CO Genetics of common disorders. Br. Med. Bull 25, 52–57 (1969). [DOI] [PubMed] [Google Scholar]
- 225.Robinson EB, Lichtenstein P, Anckarsater H, Happe F & Ronald A Examining and interpreting the female protective effect against autistic behavior. Proc. Natl Acad. Sci. USA 110, 5258–5262 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Dougherty JD et al. Can the “female protective effect” liability threshold model explain sex differences in autism spectrum disorder? Neuron 110, 3243–3262 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Palmer N et al. Association of sex with recurrence of autism spectrum disorder among siblings. JAMA Pediatr. 171, 1107–1112 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wigdor EM et al. The female protective effect against autism spectrum disorder. Cell Genomics 2, 100134 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Warrier V et al. Genetic correlates of phenotypic heterogeneity in autism. Nat. Genet 54, 1293–1304 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Solberg BS et al. Sex differences in parent-offspring recurrence of attention-deficit/hyperactivity disorder. J. Child Psychol. Psychiatry 62, 1010–1018 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Mouridsen SE, Rich B & Isager T The sex ratio of full and half siblings of people diagnosed with ADHD in childhood and adolescence: a Danish nationwide register-based cohort study. J. Atten. Disord 20, 1017–1022 (2016). [DOI] [PubMed] [Google Scholar]
- 232.Martin J et al. Investigating gender-specific effects of familial risk for attention-deficit hyperactivity disorder and other neurodevelopmental disorders in the Swedish population. BJPsych Open 6, e65 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Nayar K et al. Elevated polygenic burden for autism spectrum disorder is associated with the broad autism phenotype in mothers of individuals with autism spectrum disorder. Biol. Psychiatry 89, 476–485 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Martin J et al. Examining sex differences in neurodevelopmental and psychiatric genetic risk in anxiety and depression. PLoS ONE 16, e0248254 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zhang Y et al. Genetic evidence of gender difference in autism spectrum disorder supports the female-protective effect. Transl. Psychiatry 10, 4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Antaki D et al. A phenotypic spectrum of autism is attributable to the combined effects of rare variants, polygenic risk and sex. Nat. Genet 54, 1284–1292 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Doan RN et al. Recessive gene disruptions in autism spectrum disorder. Nat. Genet 51, 1092–1098 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Polyak A, Rosenfeld JA & Girirajan S An assessment of sex bias in neurodevelopmental disorders. Genome Med. 7, 94 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.van Rijn S A review of neurocognitive functioning and risk for psychopathology in sex chromosome trisomy (47,XXY, 47,XXX, 47,XYY). Curr. Opin. Psychiatry 32, 79–84 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Rau S et al. Patterns of psychopathology and cognition in sex chromosome aneuploidy. J. Neurodev. Disord 13, 61 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lyon MF Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature 190, 372–373 (1961). [DOI] [PubMed] [Google Scholar]
- 242.Worsham W, Dalton S & Bilder DA The prenatal hormone milieu in autism spectrum disorder. Front. Psychiatry 12, 655438 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Martin HC et al. The contribution of X-linked coding variation to severe developmental disorders. Nat. Commun 12, 627 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Brand BA, Blesson AE & Smith-Hicks CL The impact of X-chromosome inactivation on phenotypic expression of X-linked neurodevelopmental disorders. Brain Sci. 11, 904 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Slavney A, Arbiza L, Clark AG & Keinan A Strong constraint on human genes escaping X-inactivation is modulated by their expression level and breadth in both sexes. Mol. Biol. Evol 33, 384–393 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Jamain S et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat. Genet 34, 27–29 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Tahira AC et al. Putative contributions of the sex chromosome proteins SOX3 and SRY to neurodevelopmental disorders. Am. J. Med. Genet. B Neuropsychiatr. Genet 180, 390–414 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Grunblatt E et al. The involvement of the canonical Wnt-signaling receptor LRP5 and LRP6 gene variants with ADHD and sexual dimorphism: association study and meta-analysis. Am. J. Med. Genet. B Neuropsychiatr. Genet 180, 365–376 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Berkel S et al. Sex hormones regulate SHANK expression. Front. Mol. Neurosci 11, 337 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Jung H et al. Sexually dimorphic behavior, neuronal activity, and gene expression in Chd8-mutant mice. Nat. Neurosci 21, 1218–1228 (2018). [DOI] [PubMed] [Google Scholar]
- 251.Panaitof SC, Abrahams BS, Dong H, Geschwind DH & White SA Language-related Cntnap2 gene is differentially expressed in sexually dimorphic song nuclei essential for vocal learning in songbirds. J. Comp. Neurol 518, 1995–2018 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Martin J et al. Examining sex-differentiated genetic effects across neuropsychiatric and behavioral traits. Biol. Psychiatry 89, 1127–1137 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Sidorenko J et al. The effect of X-linked dosage compensation on complex trait variation. Nat. Commun 10, 3009 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Geens M et al. Female human pluripotent stem cells rapidly lose X chromosome inactivation marks and progress to a skewed methylation pattern during culture. Mol. Hum. Reprod 22, 285–298 (2016). [DOI] [PubMed] [Google Scholar]
- 255.Tukiainen T et al. Landscape of X chromosome inactivation across human tissues. Nature 550, 244–248 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Terloyeva D et al. Meconium androgens are correlated with ASD-related phenotypic traits in early childhood in a familial enriched risk cohort. Mol. Autism 11, 93 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Baron-Cohen S The extreme male brain theory of autism. Trends Cogn. Sci 6, 248–254 (2002). [DOI] [PubMed] [Google Scholar]
- 258.Baron-Cohen S et al. Why are autism spectrum conditions more prevalent in males? PLoS Biol. 9, e1001081 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Greenberg DM, Warrier V, Allison C & Baron-Cohen S Testing the empathizing-systemizing theory of sex differences and the extreme male brain theory of autism in half a million people. Proc. Natl Acad. Sci. USA 115, 12152–12157 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Fusar-Poli L et al. Second-to-fourth digit ratio (2D:4D) in psychiatric disorders: a systematic review of case-control studies. Clin. Psychopharmacol. Neurosci 19, 26–45 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.McKenna BG et al. Genetic and morphological estimates of androgen exposure predict social deficits in multiple neurodevelopmental disorder cohorts. Mol. Autism 12, 43 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.McCarthy MM Estradiol and the developing brain. Physiol. Rev 88, 91–124 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Baron-Cohen S et al. Elevated fetal steroidogenic activity in autism. Mol. Psychiatry 20, 369–376 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Baron-Cohen S et al. Foetal oestrogens and autism. Mol. Psychiatry 25, 2970–2978 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Windham GC, Lyall K, Anderson M & Kharrazi M Autism spectrum disorder risk in relation to maternal mid-pregnancy serum hormone and protein markers from prenatal screening in California. J. Autism Dev. Disord 46, 478–488 (2016). [DOI] [PubMed] [Google Scholar]
- 266.Dubey P et al. A systematic review and meta-analysis of the association between maternal polycystic ovary syndrome and neuropsychiatric disorders in children. Transl. Psychiatry 11, 569 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Coscini N et al. Association between early androgens and autistic traits: a systematic review and meta-analysis. Res. Autism Spectr. Disord 85, 101789 (2021). [Google Scholar]
- 268.Arambula SE & McCarthy MM Neuroendocrine-immune crosstalk shapes sex-specific brain development. Endocrinology 161, bqaa055 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.McCarthy MM & Wright CL Convergence of sex differences and the neuroimmune system in autism spectrum disorder. Biol. Psychiatry 81, 402–410 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Turano A, McAuley EM, Muench MC & Schwarz JM Examining the impact of neuroimmune dysregulation on social behavior of male and female juvenile rats. Behav. Brain Res 415, 113449 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Cho SH, Chai JH, Chang SY & Kim SA Acute valproate exposure induces sex-specific changes in steroid hormone metabolism in the cerebral cortex of juvenile mice. Neurochem. Res 45, 2044–2051 (2020). [DOI] [PubMed] [Google Scholar]
- 272.Han VX, Patel S, Jones HF & Dale RC Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat. Rev. Neurol 17, 564–579 (2021). [DOI] [PubMed] [Google Scholar]
- 273.Kim DW, Glendining KA, Grattan DR & Jasoni CL Maternal obesity leads to increased proliferation and numbers of astrocytes in the developing fetal and neonatal mouse hypothalamus. Int. J. Dev. Neurosci 53, 18–25 (2016). [DOI] [PubMed] [Google Scholar]
- 274.Lee SC et al. Solving for X: evidence for sex-specific autism biomarkers across multiple transcriptomic studies. Am. J. Med. Genet. B Neuropsychiatr. Genet 180, 377–389 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Marí-Bauset S et al. Systematic review of prenatal exposure to endocrine disrupting chemicals and autism spectrum disorder in offspring. Autism 26, 6–32 (2022). [DOI] [PubMed] [Google Scholar]
- 276.Thongkorn S et al. Sex differences in the effects of prenatal bisphenol A exposure on genes associated with autism spectrum disorder in the hippocampus. Sci. Rep 9, 3038 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Kissel LT & Werling DM Neural transcriptomic analysis of sex differences in autism spectrum disorder: current insights and future directions. Biol. Psychiatry 91, 53–60 (2022). [DOI] [PubMed] [Google Scholar]
- 278.Klein SL & Flanagan KL Sex differences in immune responses. Nat. Rev. Immunol 16, 626–638 (2016). [DOI] [PubMed] [Google Scholar]
- 279.Breach MR & Lenz KM Sex Differences in Neurodevelopmental Disorders: A Key Role for the Immune System (Springer, 2022). [Series Eds Geyer MA et al. Current Topics in Behavioral Neurosciences] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Brynge M et al. Maternal infection during pregnancy and likelihood of autism and intellectual disability in children in Sweden: a negative control and sibling comparison cohort study. Lancet Psychiatry 9, 782–791 (2022). [DOI] [PubMed] [Google Scholar]
- 281.Solek CM, Farooqi N, Verly M, Lim TK & Ruthazer ES Maternal immune activation in neurodevelopmental disorders. Dev. Dyn 247, 588–619 (2018). [DOI] [PubMed] [Google Scholar]
- 282.Lennington JB et al. Transcriptome analysis of the human striatum in Tourette syndrome. Biol. Psychiatry 79, 372–382 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Voineagu I et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 474, 380–384 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Chen SW et al. Maternal autoimmune diseases and the risk of autism spectrum disorders in offspring: a systematic review and meta-analysis. Behav. Brain Res 296, 61–69 (2016). [DOI] [PubMed] [Google Scholar]
- 285.Lee H et al. Risk of attention deficit hyperactivity and autism spectrum disorders among the children of parents with autoimmune diseases: a nationwide birth cohort study. Eur. Child Adolesc. Psychiatry 10.1007/s00787-021-01860-0 (2021). [DOI] [PubMed] [Google Scholar]
- 286.Tylee DS et al. Genetic correlations among psychiatric and immune-related phenotypes based on genome-wide association data. Am. J. Med. Genet. B Neuropsychiatr. Genet 177, 641–657 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Hegvik TA, Instanes JT, Haavik J, Klungsoyr K & Engeland A Associations between attention-deficit/hyperactivity disorder and autoimmune diseases are modified by sex: a population-based cross-sectional study. Eur. Child Adolesc. Psychiatry 27, 663–675 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Xuan IC & Hampson DR Gender-dependent effects of maternal immune activation on the behavior of mouse offspring. PLoS ONE 9, e104433 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Custódio CS et al. Neonatal immune challenge with lipopolysaccharide triggers long-lasting sex- and age-related behavioral and immune/neurotrophic alterations in mice: relevance to autism spectrum disorders. Mol. Neurobiol 55, 3775–3788 (2018). [DOI] [PubMed] [Google Scholar]
- 290.Turano A, Lawrence JH & Schwarz JM Activation of neonatal microglia can be influenced by other neural cells. Neurosci. Lett 657, 32–37 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Coretti L et al. Sex-related alterations of gut microbiota composition in the BTBR mouse model of autism spectrum disorder. Sci. Rep 7, 45356 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Basil P et al. Prenatal maternal immune activation causes epigenetic differences in adolescent mouse brain. Transl. Psychiatry 4, e434 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Diaz Heijtz R et al. Normal gut microbiota modulates brain development and behavior. Proc. Natl Acad. Sci. USA 108, 3047–3052 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Holingue C et al. Sex differences in the gut-brain axis: implications for mental health. Curr. Psychiatry Rep 22, 83 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Jaggar M, Rea K, Spichak S, Dinan TG & Cryan JF You’ve got male: sex and the microbiota-gut-brain axis across the lifespan. Front. Neuroendocrinol 56, 100815 (2020). [DOI] [PubMed] [Google Scholar]
- 296.Narayanan SP, Anderson B & Bharucha AE Sex- and gender-related differences in common functional gastroenterologic disorders. Mayo Clin. Proc 96, 1071–1089 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Isaksson J, Pettersson E, Kostrzewa E, Diaz Heijtz R & Bölte S Brief report: association between autism spectrum disorder, gastrointestinal problems and perinatal risk factors within sibling pairs. J. Autism Dev. Disord 47, 2621–2627 (2017). [DOI] [PubMed] [Google Scholar]
- 298.Andreo-Martínez P, Rubio-Aparicio M, Sánchez-Meca J, Veas A & Martínez-González AE A meta-analysis of gut microbiota in children with autism. J. Autism Dev. Disord 52, 1374–1387 (2022). [DOI] [PubMed] [Google Scholar]
- 299.Wang M et al. Alteration of gut microbiota-associated epitopes in children with autism spectrum disorders. Brain Behav. Immun 75, 192–199 (2019). [DOI] [PubMed] [Google Scholar]
- 300.Foley KA, MacFabe DF, Vaz A, Ossenkopp KP & Kavaliers M Sexually dimorphic effects of prenatal exposure to propionic acid and lipopolysaccharide on social behavior in neonatal, adolescent, and adult rats: implications for autism spectrum disorders. Int. J. Dev. Neurosci 39, 68–78 (2014). [DOI] [PubMed] [Google Scholar]
- 301.Cameron JJ & Stinson DA Gender (mis)measurement: guidelines for respecting gender diversity in psychological research. Soc. Personal. Psychol. Compass 13, e12506 (2019). [Google Scholar]
- 302.Strang JF et al. Both sex- and gender-related factors should be considered in autism research and clinical practice. Autism 24, 539–543 (2020). [DOI] [PubMed] [Google Scholar]
- 303.Rechlin RK, Splinter TFL, Hodges TE, Albert AY & Galea LAM An analysis of neuroscience and psychiatry papers published from 2009 and 2019 outlines opportunities for increasing discovery of sex differences. Nat. Commun 13, 2137 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Russell G et al. Selection bias on intellectual ability in autism research: a cross-sectional review and meta-analysis. Mol. Autism 10, 9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Thurm A et al. Making research possible: barriers and solutions for those with ASD and ID. J. Autism Dev. Disord 52, 4646–4650 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Arvidsson O, Gillberg C, Lichtenstein P & Lundstrom S Secular changes in the symptom level of clinically diagnosed autism. J. Child Psychol. Psychiatry 59, 744–751 (2018). [DOI] [PubMed] [Google Scholar]
- 307.Willcutt EG et al. Validity of DSM-IV attention deficit/hyperactivity disorder symptom dimensions and subtypes. J. Abnorm. Psychol 121, 991–1010 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Taylor LE, Kaplan-Kahn EA, Lighthall RA & Antshel KM Adult-onset ADHD: a critical analysis and alternative explanations. Child Psychiatry Hum. Dev 53, 635–653 (2022). [DOI] [PubMed] [Google Scholar]
- 309.Lai MC & Szatmari P Sex and gender impacts on the behavioural presentation and recognition of autism. Curr. Opin. Psychiatry 33, 117–123 (2020). [DOI] [PubMed] [Google Scholar]
- 310.Hull L, Petrides KV & Mandy W The female autism phenotype and camouflaging: a narrative review. Rev. J. Autism Dev. Disord 7, 306–317 (2020). [Google Scholar]
- 311.Fombonne E Camouflage and autism. J. Child Psychol. Psychiatry 61, 735–738 (2020). [DOI] [PubMed] [Google Scholar]
- 312.Cook J, Hull L, Crane L & Mandy W Camouflaging in autism: a systematic review. Clin. Psychol. Rev 89, 102080 (2021). [DOI] [PubMed] [Google Scholar]
- 313.Libsack EJ et al. A systematic review of passing as non-autistic in autism spectrum disorder. Clin. Child Fam. Psychol. Rev 24, 783–812 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Lai MC et al. Commentary: ‘Camouflaging’ in autistic people – reflection on Fombonne (2020). J. Child Psychol. Psychiatry 62, 1037–1041 (2021). [DOI] [PubMed] [Google Scholar]
- 315.Williams ZJ Commentary: the construct validity of ‘camouflaging’ in autism: psychometric considerations and recommendations for future research – reflection on Lai et al. (2020). J. Child Psychol. Psychiatry 63, 118–121 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Santos S, Ferreira H, Martins J, Goncalves J & Castelo-Branco M Male sex bias in early and late onset neurodevelopmental disorders: shared aspects and differences in autism spectrum disorder, attention deficit/hyperactivity disorder, and schizophrenia. Neurosci. Biobehav. Rev 135, 104577 (2022). [DOI] [PubMed] [Google Scholar]
- 317.Halpern DF Sex Differences in Cognitive Abilities 4th edn (Psychology Press, 2011). [Google Scholar]
- 318.Mac Giolla E & Kajonius PJ Sex differences in personality are larger in gender equal countries: replicating and extending a surprising finding. Int. J. Psychol 54, 705–711 (2019). [DOI] [PubMed] [Google Scholar]
- 319.Buitelaar J et al. Toward precision medicine in ADHD. Front. Behav. Neurosci 16, 900981 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Gershon J A meta-analytic review of gender differences in ADHD. J. Atten. Disord 5, 143–154 (2002). [DOI] [PubMed] [Google Scholar]
- 321.Martin J et al. A genetic investigation of sex bias in the prevalence of attention-deficit/hyperactivity disorder. Biol. Psychiatry 83, 1044–1053 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Cheslack-Postava K & Jordan-Young RM Autism spectrum disorders: toward a gendered embodiment model. Soc. Sci. Med 74, 1667–1674 (2012). [DOI] [PubMed] [Google Scholar]
- 323.Kreiser NL & White SW ASD in females: are we overstating the gender difference in diagnosis? Clin. Child Fam. Psychol. Rev 17, 67–84 (2014). [DOI] [PubMed] [Google Scholar]
- 324.Bauer GR Sex and gender multidimensionality in epidemiological research. Am. J. Epidemiol 10.1093/aje/kwac173 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Bauer GR Incorporating intersectionality theory into population health research methodology: challenges and the potential to advance health equity. Soc. Sci. Med 110, 10–17 (2014). [DOI] [PubMed] [Google Scholar]
- 326.Mendenhall E, Kohrt BA, Logie CH & Tsai AC Syndemics and clinical science. Nat. Med 28, 1359–1362 (2022). [DOI] [PubMed] [Google Scholar]
- 327.Khramtsova EA, Davis LK & Stranger BE The role of sex in the genomics of human complex traits. Nat. Rev. Genet 20, 173–190 (2019). [DOI] [PubMed] [Google Scholar]
- 328.Nguyen TV, Ducharme S & Karama S Effects of sex steroids in the human brain. Mol. Neurobiol 54, 7507–7519 (2017). [DOI] [PubMed] [Google Scholar]
- 329.Oliva M et al. The impact of sex on gene expression across human tissues. Science 369, eaba3066 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Zhang Y et al. The human brain is best described as being on a female/male continuum: evidence from a neuroimaging connectivity study. Cereb. Cortex 31, 3021–3033 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Phillips OR et al. Beyond a binary classification of sex: an examination of brain sex differentiation, psychopathology, and genotype. J. Am. Acad. Child Adolesc. Psychiatry 58, 787–798 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Ostatníková D, Lakatosova S, Babkova J, Hodosy J & Celec P Testosterone and the brain: from cognition to autism. Physiol. Res 69, S403–S419 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Liu S, Seidlitz J, Blumenthal JD, Clasen LS & Raznahan A Integrative structural, functional, and transcriptomic analyses of sex-biased brain organization in humans. Proc. Natl Acad. Sci. USA 117, 18788–18798 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Bethlehem RAI et al. Brain charts for the human lifespan. Nature 604, 525–533 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Juster RP et al. Sex and gender roles in relation to mental health and allostatic load. Psychosom. Med 78, 788–804 (2016). [DOI] [PubMed] [Google Scholar]
- 336.Li SH & Graham BM Why are women so vulnerable to anxiety, trauma-related and stress-related disorders? The potential role of sex hormones. Lancet Psychiatry 4, 73–82 (2017). [DOI] [PubMed] [Google Scholar]
- 337.O’Neil A, Scovelle AJ, Milner AJ & Kavanagh A Gender/sex as a social determinant of cardiovascular risk. Circulation 137, 854–864 (2018). [DOI] [PubMed] [Google Scholar]
- 338.Haimov-Kochman R & Berger I Cognitive functions of regularly cycling women may differ throughout the month, depending on sex hormone status; a possible explanation to conflicting results of studies of ADHD in females. Front. Hum. Neurosci 8, 191 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Manoli DS & Tollkuhn J Gene regulatory mechanisms underlying sex differences in brain development and psychiatric disease. Ann. N. Y. Acad. Sci 1420, 26–45 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Albin RL Tourette syndrome as a disorder of the social decision making network. Front. Psychiatry 10, 742 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Williams OOF, Coppolino M, George SR & Perreault ML Sex differences in dopamine receptors and relevance to neuropsychiatric disorders. Brain Sci. 11, 1199 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Wickens MM, Bangasser DA & Briand LA Sex differences in psychiatric disease: a focus on the glutamate system. Front. Mol. Neurosci 11, 197 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Bottema-Beutel K, Kapp SK, Lester JN, Sasson NJ & Hand BN Avoiding ableist language: suggestions for autism researchers. Autism Adulthood 3, 18–29 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Botha M, Hanlon J & Williams GL Does language matter? Identity-first versus person-first language use in autism research: a response to Vivanti. J. Autism Dev. Disord 10.1007/s10803-020-04858-w (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Gernsbacher MA Editorial perspective: the use of person-first language in scholarly writing may accentuate stigma. J. Child Psychol. Psychiatry 58, 859–861 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Gillespie-Lynch K et al. in Disability Alliances and Allies Vol. 12 (eds Carey AC, Ostrove JM & Fannon T) 189–223 (Emerald, 2020). [Google Scholar]
- 347.Donaldson AL, Krejcha K & McMillin A A strengths-based approach to autism: neurodiversity and partnering with the autism community. Perspect. ASHA Spec. Interest. Groups 2, 56–68 (2017). [Google Scholar]
- 348.Constantino CD What can stutterers learn from the neurodiversity movement? Semin. Speech Lang 39, 382–396 (2018). [DOI] [PubMed] [Google Scholar]
- 349.Kenny L et al. Which terms should be used to describe autism? Perspectives from the UK autism community. Autism 20, 442–462 (2016). [DOI] [PubMed] [Google Scholar]
- 350.Bury SM, Jellett R, Spoor JR & Hedley D “It Defines Who I Am” or “It’s Something I Have”: what language do [Autistic] Australian adults [on the autism spectrum] prefer? J. Autism Dev. Disord 10.1007/s10803-020-04425-3 (2020). [DOI] [PubMed] [Google Scholar]
- 351.Robison JE Talking about autism – thoughts for researchers. Autism Res. 12, 1004–1006 (2019). [DOI] [PubMed] [Google Scholar]
- 352.Best KL, Mortenson WB, Lauziere-Fitzgerald Z & Smith EM Language matters! The long-standing debate between identity-first language and person first language. Assist. Technol 34, 127–128 (2022). [DOI] [PubMed] [Google Scholar]
- 353.Keating CT et al. Autism-related language preferences of English-speaking individuals across the globe: a mixed methods investigation. Autism Res. 10.1002/aur.2864 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Buijsman R, Begeer S & Scheeren AM ‘Autistic person’ or ‘person with autism’? Person-first language preference in Dutch adults with autism and parents. Autism 10.1177/13623613221117914 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Dwyer P Stigma, incommensurability, or both? Pathology-first, person-first, and identity-first language and the challenges of discourse in divided autism communities. J. Dev. Behav. Pediatr 43, 111–113 (2022). [DOI] [PubMed] [Google Scholar]
- 356.Chiniara LN, Bonifacio HJ & Palmert MR Characteristics of adolescents referred to a gender clinic: are youth seen now different from those in initial reports? Horm. Res. Paediatr 89, 434–441 (2018). [DOI] [PubMed] [Google Scholar]
- 357.de Graaf NM, Carmichael P, Steensma TD & Zucker KJ Evidence for a change in the sex ratio of children referred for gender dysphoria: data from the Gender Identity Development Service in London (2000–2017). J. Sex. Med 15, 1381–1383 (2018). [DOI] [PubMed] [Google Scholar]
- 358.Hisle-Gorman E et al. Gender dysphoria in children with autism spectrum disorder. LGBT Health 6, 95–100 (2019). [DOI] [PubMed] [Google Scholar]
- 359.Strang JF et al. Increased gender variance in autism spectrum disorders and attention deficit hyperactivity disorder. Arch. Sex. Behav 43, 1525–1533 (2014). [DOI] [PubMed] [Google Scholar]
- 360.May T, Pang K & Williams KJ Gender variance in children and adolescents with autism spectrum disorder from the National Database for Autism Research. Int. J. Transgend 18, 7–15 (2017). [Google Scholar]
- 361.Brunissen L, Rapoport E, Chawarska K & Adesman A Sex differences in gender-diverse expressions and identities among youth with autism spectrum disorder. Autism Res 14, 143–155 (2021). [DOI] [PubMed] [Google Scholar]
- 362.Corbett BA et al. Greater gender diversity among autistic children by self-report and parent-report. Autism 10.1177/13623613221085337 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Dewinter J, De Graaf H & Begeer S Sexual orientation, gender identity, and romantic relationships in adolescents and adults with autism spectrum disorder. J. Autism Dev. Disord 47, 2927–2934 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Chang JC, Lai MC, Tai YM & Gau SS Mental health correlates and potential childhood predictors for the wish to be of the opposite sex in young autistic adults. Autism 26, 146–159 (2022). [DOI] [PubMed] [Google Scholar]
- 365.Warrier V et al. Elevated rates of autism, other neurodevelopmental and psychiatric diagnoses, and autistic traits in transgender and gender-diverse individuals. Nat. Commun 11, 3959 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Thrower E, Bretherton I, Pang KC, Zajac JD & Cheung AS Prevalence of autism spectrum disorder and attention-deficit hyperactivity disorder amongst individuals with gender dysphoria: a systematic review. J. Autism Dev. Disord 50, 695–706 (2020). [DOI] [PubMed] [Google Scholar]
- 367.Nunes-Moreno M et al. Behavioral health diagnoses in youth with gender dysphoria compared with controls: a PEDSnet study. J. Pediatr 241, 147–153.e1 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Hines M Human gender development. Neurosci. Biobehav. Rev 118, 89–96 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Strang JF et al. Initial clinical guidelines for co-occurring autism spectrum disorder and gender dysphoria or incongruence in adolescents. J. Clin. Child Adolesc. Psychol 47, 105–115 (2018). [DOI] [PubMed] [Google Scholar]
- 370.Strang JF et al. A clinical program for transgender and gender-diverse neurodiverse/autistic adolescents developed through community-based participatory design. J. Clin. Child Adolesc. Psychol 50, 730–745 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Ueda K, Kim S, Greene DJ & Black KJ Correlates and clinical implications of tic suppressibility. Curr. Dev. Disord. Rep 8, 112–120 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Livingston LA, Shah P & Happé F Compensatory strategies below the behavioural surface in autism: a qualitative study. Lancet Psychiatry 6, 766–777 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Hobson HM & Lee A Camouflaging in developmental language disorder: the views of speech and language pathologists and parents. Commun. Disord. Q 10.1177/15257401221120937 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Bernardin CJ, Mason E, Lewis T & Kanne S “You must become a chameleon to survive”: adolescent experiences of camouflaging. J. Autism Dev. Disord 51, 4422–4435 (2021). [DOI] [PubMed] [Google Scholar]


