Abstract
Context
Hypothalamic obesity is a rare, treatment-resistant form of obesity. In preliminary studies, the hypothalamic hormone oxytocin (OXT) has shown promise as a potential weight loss therapy.
Objective
To determine whether 8 weeks of intranasal OXT (vs 8 weeks of placebo) promotes weight loss in children, adolescents, and young adults with hypothalamic obesity.
Methods
This randomized, double-blind, placebo-controlled, crossover pilot trial (NCT02849743), conducted at an outpatient academic medical center, included patients aged 10 to 35 years with hypothalamic obesity from hypothalamic/pituitary tumors. Participants received intranasal OXT (Syntocinon, 40 USP units/mL, 4 IU/spray) vs excipient-matched placebo, 16 to 24 IU 3 times daily at mealtimes. Weight loss attributable to OXT vs placebo and safety (adverse events) were assessed.
Results
Of 13 individuals randomized (54% female, 31% pre-pubertal, median age 15.3 years, IQR 13.3-20.6), 10 completed the entire study. We observed a nonsignificant within-subject weight change of −0.6 kg (95% CI: −2.7, 1.5) attributable to OXT vs placebo. A subset (2/18 screened, 5/13 randomized) had prolonged QTc interval on electrocardiography prior to screening and/or in both treatment conditions. Overall, OXT was well-tolerated, and adverse events (epistaxis and nasal irritation, headache, nausea/vomiting, and changes in heart rate, blood pressure, and QTc interval) were similar between OXT and placebo. In exploratory analyses, benefits of OXT for anxiety and impulsivity were observed.
Conclusion
In this pilot study in hypothalamic obesity, we did not detect a significant impact of intranasal OXT on body weight. OXT was well-tolerated, so future larger studies could examine different dosing, combination therapies, and potential psychosocial benefits.
Keywords: oxytocin, neuroendocrinology, hypopituitarism, pediatric endocrinology, hypothalamic obesity, craniopharyngioma
Hypothalamic obesity is a rare form of treatment-resistant obesity that frequently develops in individuals with brain tumors affecting the hypothalamus and pituitary, such as craniopharyngioma [1, 2]. Craniopharyngioma is a rare, grade I tumor, often treated surgically. These tumors themselves and their extensive surgical resection can each damage the hypothalamic regions that govern metabolism, leading to hypothalamic obesity [3]. Affected individuals have low resting energy expenditure and often have uncontrolled appetite, which are important pathogenic factors [4-7]. Excess lifetime mortality occurs in individuals with craniopharyngioma who develop hypothalamic obesity, a relationship that is likely mediated by the excess burden of obesity-related comorbidities, including fatty liver disease, type 2 diabetes mellitus, and cardiovascular disease [8-11]. Unfortunately, there are no therapies approved specifically for treatment of hypothalamic obesity. Lifestyle and pharmacologic therapies for “common” obesity are often less effective in individuals with hypothalamic obesity, and more focused research is needed to understand this heterogeneity in treatment response [12, 13].
The hypothalamic neuropeptide oxytocin (OXT) participates in the regulation of appetite and energy balance. OXT is produced in the paraventricular nucleus (PVN) and supra-optic nucleus (SON) of the hypothalamus; thus, OXT deficiency is plausible in individuals with hypothalamic and pituitary tumors. In preclinical and preliminary clinical studies, OXT has been posited to produce weight loss by multiple mechanisms, including decreased food intake (via modulation of homeostatic, reward-related, and impulse control neural circuitry) and increased energy expenditure [14]. One report found that individuals with hypothalamic/pituitary tumors and obesity had a decreased rise in salivary OXT following meals as compared to individuals with hypothalamic/pituitary tumors without obesity [15], suggesting that mealtime OXT administration might be a viable therapeutic intervention. In a small study of otherwise healthy adults with obesity, 8 weeks of intranasal OXT (24 IU, 4 times daily) led to mean body weight loss of 9% [16]. In a case report, 10 weeks of 6 IU compounded intranasal OXT with carbohydrate restriction, followed by 38 weeks of OXT with naltrexone, led to sustained reductions in body mass index (BMI) Z-score and excess appetite in a 13-year-old boy with craniopharyngioma, hypothalamic obesity, and hyperphagia [17]. Taken together, these findings prompted us to undertake a pilot trial of intranasal OXT in individuals with hypothalamic obesity, with a view to examining both safety and effects on body weight and gaining valuable experience on the potential therapeutic utility of OXT in survivors of hypothalamic/pituitary brain tumors.
Methods
Study Design
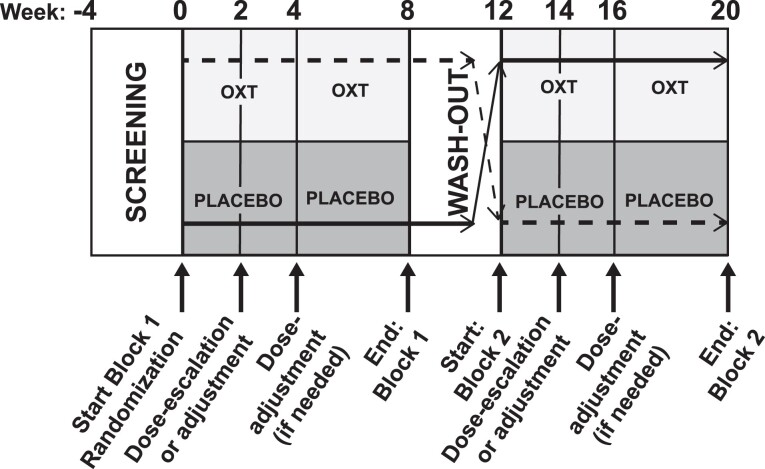
We performed a randomized, double-blind, placebo-controlled, crossover pilot trial (study schema, Fig. 1). Prospective participants were screened by telephone, then underwent in-person screening procedures to confirm eligibility. Eligible participants were randomized to either intranasal OXT (Syntocinon, 40 USP units/mL, 4 IU/spray; Novartis/Mylan, Victoria Pharmacy, Zurich, Switzerland) or excipient-matched intranasal placebo, and then entered the first 8-week treatment block. At the end of block 1 (week 8), participants entered the 4-week “washout” period. At the end of the washout (week 12), subjects crossed over and entered the second 8-week treatment block. At the end of block 2 (week 20), subjects completed participation. Subjects completed in-person evaluations at the start of each treatment block (visits 1 and 5), dose escalation (visits 2 and 6), interim safety (visits 3 and 7), and the end of each treatment block (visits 4 and 8). In addition to the in-person visits, participants completed remote safety assessments (telephone call, local laboratory draw for basic metabolic profile) 24 to 48 hours after initiation and/or increase of OXT/placebo (after visits 1, 2, 3 and 5, 6, and 7). In total, across both treatment blocks, participants completed 9 in-person visits and 6 remote safety assessments.
Figure 1.
Study schema. The study schema for the protocol is shown for an individual randomized to either treatment arm (OXT-placebo or placebo-OXT).
Human Subject Considerations
This trial was performed under a protocol approved by the Institutional Review Board of the Children's Hospital of Philadelphia (CHOP) with reciprocal agreement from the University of Pennsylvania under an Investigational New Drug application (IND129379) and was conducted according to the Declaration of Helsinki. The trial was preregistered at clinicaltrials.gov, NCT02849743. Written informed consent and assent, for those under 18 years, were obtained from all participants prior to participation.
Participant Eligibility
Children, adolescents, and adults (10-35 years of age, inclusive) with obesity after treatment for a hypothalamic/pituitary brain tumor were recruited from the clinical practices at CHOP and University of Pennsylvania, referred from outside physicians, and/or were self-referred. This age range was selected to permit recruitment of sufficient numbers of participants in this rare condition and avoided older adults in whom the burden of comorbidities would be expected to be higher. Participants were required to have a minimum weight of at least 51 kg (to be comparable to previous studies using similar OXT dosing), currently be overweight (BMI ≥ 85th percentile and <95 percentile for age/sex for age 10 to <18 years, BMI ≥ 25 kg/m2 and <30 kg/m2 for age 18-35 years) or have obesity (BMI ≥ 95th percentile for age/sex for age 10 to <18 years, BMI ≥ 30 kg/m2 for age 18-35 years), at least one hypothalamic/pituitary hormone disorder (as evidence of hypothalamic damage) [18], and excess weight gain in association with tumor diagnosis and/or treatment (as per the referring clinician and medical records). We included individuals with BMI in the overweight range for at least 2 reasons. First, anti-obesity interventions may be more effective in individuals who have not yet developed persistent obesity, as is expected given the natural history of this condition. Second, increased cardiometabolic risk is present even at BMI ≥ 85% percentile (children) or BMI ≥ 25 kg/m2 (adults), especially since BMI may not fully capture the extent of excess adiposity. We conferred with referring clinicians to ensure participation was appropriate. Additional inclusion criteria included at least 6 months after completion of brain tumor therapy with stable disease and no evidence of recurrence. Participants were also required to be stable for at least 2 months on pituitary replacement therapy (except for clinically indicated adjustments of < 20%) or appetite modulating medications. Individuals with diabetes insipidus (DI) were required to have intact thirst and their DI was required to be adequately controlled on their current regimen. Individuals with diabetes mellitus (DM) requiring insulin or insulin secretagogue; concurrent use of medications known to prolong the QTc interval; a history of clinically significant cardiovascular (including QTc interval >460 msec), liver, or kidney problems; anemia; history of gastric bypass surgery; supra-physiologic steroid use; current substance use, psychosis, or suicidality; pregnancy; or any seizure history in the previous 12 months were excluded. Female participants were required to have a negative urine/serum pregnancy test for study entry. Postmenarchal female participants were asked to use an acceptable method of contraception, including abstinence if this was their usual practice, a barrier method, or pharmacologic contraceptive for the duration of the study.
Randomization
The study statistician (R.X.) generated a randomization sequence by computer that was implemented via REDCap. Randomization was stratified by sex in consideration of potential sex-specific differences in OXT impact on food intake [19] to ensure balance by sex across treatment arms. To ensure allocation concealment, permuted-block randomization with varying sizes of 2 and 4 was used.
Dose Selection and Washout
We selected a dose of 24 IU at mealtimes, to promote decreased food intake at meals [16, 20]. Participants were counseled that waiting times as short as 15 minutes prior to meals had been shown to impact food intake, as reviewed in [14], and that if possible, they should administer 15 minutes prior to mealtime, recognizing that there may be practical limitations to adhering to this schedule, and that they should prioritize overall adherence and premeal doses. To permit each individual to achieve a maximally tolerated dose, throughout the protocol we had the option to decrease the dose by 4 IU (1 spray) 3 times daily for any Grade 2 adverse event and/or any degree of hyponatremia despite desmopressin adjustment. The potential impact of dose adjustments on treatment outcomes were assessed as covariates in statistical models. For individuals 70 kg and over, we began with 20 IU 3 times daily for 2 weeks, then increased to 24 IU 3 times daily, and for individuals weighing 50 to <70 kg, we started at 16 IU 3 times daily for 2 weeks, then increased to 20 IU 3 times daily. We used an interblock washout interval of 4 weeks based on previous studies [21-24].
Adherence
Adherence to OXT/placebo was assessed using dosing diaries and confirmed by comparing weights of used and unused vials of OXT/placebo returned to the CHOP Investigational Drug service to weights noted prior to dispensation. If dosing diaries were incomplete or not returned, bottle weights were used to estimate adherence. Adherence was calculated as the percentage of doses administered (vs prescribed) during each treatment block.
Clinical Characteristics
Clinical characteristics were abstracted from the medical record. Hypothalamic injury score, an index of damage to the hypothalamus assessed on most recent surveillance pituitary magnetic resonance imaging (MRI), was also calculated by a single neuro-radiologist (K.S.) as per previous publications (range, 0-7, where a higher score reflects greater injury) [25, 26].
Vital Signs and Electrocardiography
Heart rate and blood pressure were recorded after a 5-minute rest in a quiet area with the participant in a seated position. Electrocardiograms (ECG; 15-lead) were performed at screening, at each study visit, and following monitored doses of OXT/placebo during the first 2 visits of each treatment block. All ECG measurements were reviewed by the study cardiologist (V.L.V.), and QTc intervals were hand-measured using online calipers. QTc interval was calculated using the Bazett correction method.
Anthropometrics
The primary outcome, body weight, was measured by digital electronic scale (Scaletronix, White Plains, NY). Subjects were weighed after an overnight fast (minimum 10 hours), in the morning, wearing hospital scrubs at the beginning and end of each treatment block; at other visits, they were measured in hospital scrubs but we did not require that weight be assessed at a specific time of day and/or while fasting. Stature was measured on a stadiometer (Holtain, Crymych, UK). Age- and sex-specific Z-scores for BMI and height were generated based on United States CDC 2000 growth charts [27]. Waist circumference was measured with a nonstretchable fiberglass tape (0.1 cm; McCoy, Maryland Heights, MO) at the umbilicus by trained personnel in the CHOP Growth & Nutrition Laboratory. All anthropometric measurements were taken and recorded in triplicate and the mean was used in analyses. All measurements follow the methods described in The Anthropometric Standardization Manual of Lohman et al [28].
Test Meal
In men across the weight spectrum, 24 IU of intranasal OXT has been shown to decrease caloric intake at a test meal [20]. Sixty minutes after each administration of each monitored dose of OXT/placebo (first dose, then dose 2 weeks later) we provided participants with a test meal containing two-thirds of estimated recommended daily allowance of calories (based on ideal body weight), with macronutrient content as follows: 55% calories from carbohydrate, 30% calories from fat, and 15% of calories from protein. Meals were prepared according to prespecified dietary preferences for breakfast by registered dieticians in the CHOP Center for Human Phenomic Sciences Bionutrition core. Participants had up to 2 hours to consume as much of the meal as they wished; food was weighed before and after to document the amount of energy consumed, and its macronutrient content (ie, fat, carbohydrate, protein).
Cognitive Restraint
A Stop-Signal Task (programmed with Presentation software [Neurobehavioral Systems, Inc., Berkeley, CA]) was administered to assess the ability to suppress unwanted behavioral impulses 30 minutes after the administration of a monitored dose of OXT/placebo [29, 30]. Participants were instructed to categorize nouns as “animal” or “non-animal” by pressing a left or right response key. In a random 25% of trials, an auditory signal indicated to withhold the response to the word presented. A staircase-tracking procedure was employed to adaptively set the time delay between onset of word and stop signal, depending on the subject's performance. The duration of the stop process (stop-signal reaction time) was derived as a measure of response inhibition [31, 32].
Questionnaires
Several validated questionnaires were used to assess eating behaviors, physical activity, quality of life and family function. A 13-item hyperphagia questionnaire developed for caregivers of individuals with Prader-Willi syndrome (PWS) based on a cohort ages 4 to 51 years [33] was completed by parents/legal guardians to assesses Hyperphagic Drive (range, 4-20), Hyperphagic Behavior (range, 5-25), and Hyperphagic Severity (range, 2-10), for a total score ranging from 11 to 55. A 51-item Eating Inventory questionnaire [34] assessed cognitive restraint with respect to eating (range, 0-20), disinhibition (range, 0-16), and hunger (range, 0-15). The Bone Mineral Density Cohort Study Physical Activity questionnaire (BMDCS-PA) was used to estimate self-reported usual amounts and intensities of physical activity [35]. Four National Institute of Neurological Disorders and Stroke (NINDS) quality of life in neurological disorders (Neuro-QOL) measures [36] were used to track changes in mental and emotional health that could be related to OXT administration. Specifically, these instruments included short forms (8 items each) to assess each of the following domains over the preceding 7 days: i) anxiety; ii) cognitive function; iii) depression; and iv) social interactions. For each of these instruments, there is a pediatric version (ages 8-17) and an adult version (18 years and older). The associated, freely available computerized Assessment Center Scoring Service calculated outcomes for each participant, most notably a standardized T-score relative to reference populations [37]. These questionnaires were administered by the study team. The 12-item Family Assessment Device General Function scale (FAD-GFS) was completed by parents/legal guardians for children and adult participants not living independently, and participants’ scaled scores are from 1 to 4, where a score of 2 or above is suggestive of dysfunction [38].
Adverse Events
Clinical adverse events (AEs) were monitored throughout the study and classified according to Common Terminology Criteria for Adverse Events (CTCAE), version 4.0 [39]. A Safety Monitoring Uniform Report Form (SMURF) based on [40] was designed to systematically assess OXT-specific adverse events and was administered at each in-person visit and telephone check-in. The full SMURF contained a general inquiry, several questions about daily activities (eg, sleep, appetite, energy level, bowel and bladder function), and modified queries specific to OXT. The general inquiry included an open-ended question about any problems or complaints, as well as questions regarding the need for other medications and doctor or health care encounters since the last study visit. The next section included 25 system-specific queries to ensure completeness. For the telephone check-in, a focused subset of the SMURF items was asked—only those related to specific risks of intranasal OXT (eg, nasal irritation)—as well as a general inquiry about any new or concerning symptoms. Serum sodium concentrations (via basic metabolic profile) were checked at each in-person visit and 48 hours after any dose adjustments, as well as with any potential change in symptoms given the structural similarity of OXT to vasopressin and theoretical risk of hyponatremia [41].
Statistical Analysis
The primary outcome was the difference of the posttreatment weight between the 2 periods (treatment vs placebo). Key secondary outcomes included: change in other anthropometric measurements (BMI, waist circumference), and safety/tolerability of OXT based on AE reporting over the same intervals. Additional exploratory outcomes included differences in energy intake at a test meal, changes in hyperphagia (Hyperphagia Questionnaire), hunger, disinhibition of eating, cognitive restraint (Eating Inventory, Stop-Signal Task), physical activity (BMDCS physical activity questionnaire), mental/emotional health and quality of life (Neuro-QOL), and family function (FAD-GFS).
Baseline and demographic characteristics were summarized with standard descriptive statistics, ie, mean and SD or median and interquartile range (IQR) for continuous variables such as age, count and percentage for categorical variables such as gender and Tanner stage. For the main outcome, we performed a linear mixed-effects regression analysis to test the impact of OXT (vs placebo) on weight change over the treatment block, accounting for baseline weight at the start of each block and treatment order (OXT-placebo vs placebo-OXT) as covariates. To test for possible carry-over effect, an interaction term between treatment (OXT vs placebo) and block (1 vs 2) was also included in the main model. For outcomes measured once per block, linear mixed-effects models were constructed with period and treatment included. The linear mixed-effects model accounts for within-subject correlation due to repeated measures and is robust to values missing at random. In sensitivity analyses, we tested the effect of age, sex, and treatment adherence by adding each of them to the main model iteratively to understand the extent to which these may influence the main result. The statistical analysis plan was finalized prior to unblinding (June 30, 2021). Analyses were performed in R Studio (v.1.3.1103). Statistical significance was taken to be a two-sided P value of <.05.
Sample Size Considerations
We initially proposed a sample size of 20 (10 per arm) participants with complete data. For sample size calculations, we used the within-subject difference of the posttreatment weight between the 2 periods (OXT vs placebo) as the primary outcome. A sample size of 20 would have 84% power to detect a mean of paired differences of 0.7×SD (corresponding to a change of ∼3.8 kg over 8 weeks in adults using the observed SD of 5.5 kg) at a significance level of .05 using a two-sided paired t test. This posited effect size was plausible given a published effect size attributable to OXT of ∼1.6×SDs (corresponding to a change of ∼8.9 kg over 8 weeks in adults with the same SD) reported in a previous study [16]; furthermore, our crossover design is expected to yield additional statistical power.
Results
Enrollment and Disposition
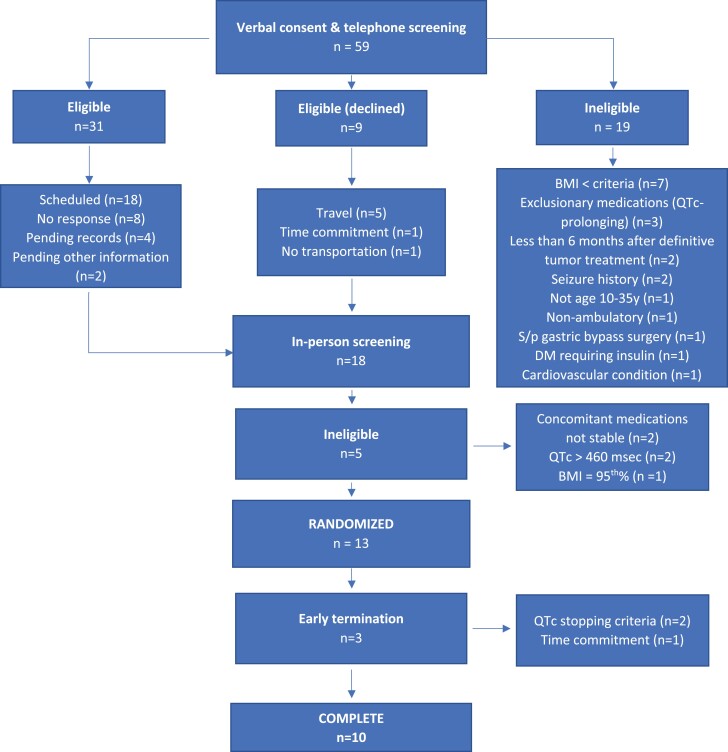
Informed consent for in-person screening was obtained from a total of 18 individuals (consort diagram, Fig. 2). After in-person screening, 5 of 18 were excluded for the following reasons: concomitant medications not stable for 2 months prior to screening (n=2); BMI not in range (n=1); screening QTc interval > 460 msec (n=2). Thirteen of 18 participants were confirmed eligible and were randomized to one of the 2 treatment arms. Ten of the 13 randomized participants completed the entire study while 3 terminated participation early. One participant withdrew related due to time commitment concerns within Block 2 and 2 met stopping criteria (related to prolonged QTc interval) within Block 1. The first participant was enrolled in December 2016, and the last study procedure was completed in April 2021.
Figure 2.
Consort flow diagram. The disposition of all participants completing verbal consent and telephone screening is shown.
Participants
Median age was 15.3 years (IQR, 13.3-20.6), and 9 were under 18 years of age at enrollment. Median age- and sex-specific BMI Z-score for participants under 18 years was 2.21 (Table 1), well above the threshold for pediatric obesity (1.64). For participants aged 18 years and older, 1 had World Health Organization (WHO) Class I obesity, 1 had WHO Class II obesity, and 2 had WHO Class III obesity. The etiology of hypothalamic obesity included craniopharyngioma (n = 9), germinoma (n = 2), optic glioma (n = 1), and pilocytic astrocytoma (n = 1), and the median time since diagnosis was 7.5 years (IQR, 4.3-8.7). Pituitary hormone deficiencies were common, with 9 of 13 having diabetes insipidus, and 12 of 13 having central hypothyroidism. Hypothalamic injury score was a median of 5.5 (IQR, 4-6.5). Median (IQR) FAD score was 1.58 (1.37-2.21); scores above 2.0 (4/11 of those living in family situations in this study) indicate problematic family functioning [42].
Table 1.
Participant characteristics
| Characteristic | Arm 1 (OXT-PBO) n = 6 |
Arm 2 (PBO-OXT) n = 7 |
Overall n = 13 |
|---|---|---|---|
| Demographics | |||
| Age, years | 17.0 [11.9, 21.4] | 15.3 [14.3, 16.0] | 15.3 [13.3, 20.6] |
| Sex, n (%) | |||
| Male | 2 (33.3%) | 4 (57.1%) | 6 (46.2%) |
| Female | 4 (66.7%) | 3 (42.9%) | 7 (53.8%) |
| Race, n (%) | |||
| White | 5 (83%) | 6 (86%) | 11 (85%) |
| >1 race | 1 (17%) | 1 (14%) | 2 (15%) |
| Ethnicity, n (%) | |||
| Hispanic | 0 (0%) | 2 (29%) | 2 (15%) |
| Non-Hispanic | 6 (100%) | 5 (71%) | 11 (85%) |
| Type of tumor, n (%) | |||
| Craniopharyngioma | 3 (50%) | 6 (86%) | 9 (69%) |
| Germinoma | 2 (33%) | 0 (0%) | 2 (15%) |
| Optic glioma | 1 (17%) | 0 (0%) | 1 (8%) |
| Pilocytic astrocytoma | 0 (0%) | 1 (14%) | 1 (8%) |
| Time since diagnosis, years | 7.6 [5.1, 8.5] | 7.1 [4.0, 8.7] | 7.5 [4.3, 8.7] |
| Anthropometrics and vital signs | |||
| Height, cm | 145.9 [144.1, 162.3] | 163.9 [159.9, 169.4] | 161.8 [146.0, 168.5] |
| Height, Z-score | −0.32 (1.66) | 0.16 (1.00) | −0.06 (1.31) |
| Weight, kg | 74.5 [66.4, 100.4] | 95.1 [82.5, 109.2] | 83.6 [72.7, 109.2] |
| BMI, kg/m2 | 32.4 [31.0, 36.7] | 33.5 [30.2, 39.1] | 33.1 [30.8, 37.4] |
| BMI, Z-score (<18y) | 2.18 (0.31) | 2.23 (0.34) | 2.21 (0.32) |
| Heart rate, bpm | 80 [73, 85] | 84 [65, 88] | 84 [65, 85] |
| ECG QTc interval, msec | 437 [414, 463] | 442 [427, 451] | 442 [421, 452] |
| Systolic BP, mm Hg | 107 [105, 108] | 108 [99, 113] | 107 [104, 109] |
| Diastolic BP, mm Hg | 62 [56, 64] | 58 [57, 65] | 60 [55, 64] |
| Tanner stage | |||
| Tanner I, n (%) | 2 (33%) | 2 (29%) | 4 (31%) |
| Tanner II-IV, n (%) | 1 (17%) | 2 (29%) | 3 (23%) |
| Tanner V – n (%) | 3 (50%) | 3 (43%) | 6 (46%) |
| Concomitant medications | |||
| DDAVP – n (%) | 4 (67%) | 5 (71%) | 9 (69%) |
| Growth hormone – n (%) | 2 (33%) | 6 (86%) | 8 (62%) |
| Thyroid hormone, n (%) | 6 (100%) | 6 (86%) | 12 (92%) |
| Hydrocortisone, n (%) | 4 (67%) | 5 (71%) | 9 (69%) |
| Stimulants, n (%) | 2 (33%) | 2 (29%) | 4 (31%) |
| GLP1R agonists, n (%) | 1 (17%) | 0 (0%) | 1 (8%) |
| Hypothalamic injury | |||
| Score (0-7, higher = more injury) | 4.8 [3.3, 5.9] | 5.5 [4.5, 6.5] | 5.5 [4, 6.5] |
Participant characteristics, stratified by treatment arm, are shown.
Data are expressed as mean (SD) for height and BMI Z-scores and median [interquartile range] for continuous variables or count (% of group total) for categorical variables, as appropriate. Abbreviations: BMI, body mass index; BP, blood pressure; bpm, beats per minute; DDAVP, desmopressin; GLP1R, glucagon-like peptide 1 receptor; msec, milliseconds; OXT, oxytocin; PBO, placebo.
Baseline Eating Behavior and Physical Activity
Mean (SD) scores on the Eating Inventory subscales (for which a higher score reflects more of the dimension measured) for dietary restraint (0-20), disinhibition (0-16), and hunger (0-15) were 9.3 (4.2), 8.2 (3.5), and 7.2 (4.6), respectively. Mean scores (SD) for the 3 hyperphagia scales, where a higher number indicates more substantial symptoms [33], were 9.8 (4.0), 9.7 (4.0), and 4.1 (2.2) respectively. Participants reported a median of 1 hour (IQR, 0.4-1.4) of any kind of physical activity weekly.
Adherence to OXT/Placebo
Participants demonstrated adherence ranging from 70% to 100% based on dosing diaries that were consistent with bottle weights; Only 1 participant did not complete the dosing diary for 4 weeks of the study; thus, bottle weights were used to estimate adherence for this portion of the study.
Primary Outcome and Key Secondary Outcomes
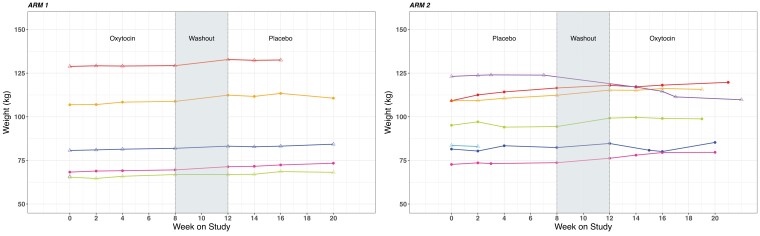
Participant body weights over the course of the study are shown in Fig. 3. Overall, we did not detect an effect of OXT on body weight, nor after adjusting for age, sex, and adherence to study drug on univariate (Table 2) or multivariable (Table 3) analyses. The one participant (in Treatment Arm 2, placebo-OXT, Fig. 3) who demonstrated substantial weight loss in the study began to lose weight at the end of the placebo treatment, through the washout, and while on OXT. We did not find any effect of OXT on BMI, BMI Z-score (children only), or waist circumference (Table 4). We also did not detect an effect of OXT on fasting glucose, though this result is unsurprising since at baseline, fasting glucose was not elevated in this cohort (median 88 mg/dL, IQR 83-92). We also did not detect an effect of OXT on fasting triglycerides, which also were not substantially elevated at baseline (median 134 mg/dL, IQR 88-176).
Figure 3.
Body weights over the course of the study for each participant, by treatment arm. Each participant's values are shown individually (colors), stratified by treatment arm.
Table 2.
Primary and key secondary outcomes, univariate analyses
| Outcome | Overall, median [IQR] n = 11 |
Change with OXT, median [IQR] n = 11 |
Change with PBO, median [IQR] n = 11 |
|---|---|---|---|
| Anthropometrics and vital signs | |||
| Body weight, kg | 97.1 [78.8, 114.4] | 1.2 [0.4, 1.6] | 0.9 [−0.4, 1.4] |
| BMI, kg/m2 | 34.0 [31.7, 40.6] | 0.2 [−0.2, 0.5] | 0.1 [−0.4, 0.7] |
| BMI, Z-score (age <20 years only) | 2.35 [2.12, 2.42] | 0.01 [−0.01, 0.03] | 0 [−0.03, 0.02] |
| Waist circumference, cm | 107.3 [100.3, 119.6] | 1.2 [−0.5, 3.3] | −0.5 [−1.4, 1.9] |
| Heart rate, bpm | 80 [72, 86] | −4 [−6, 2] | 1 [−4, 5] |
| Systolic BP, mm Hg | 110 [105, 115] | 1 [−8, 6] | 2 [−3, 9] |
| Diastolic BP, mm Hg | 62 [56, 65] | 4 [−8, 7] | −1 [−3, 4] |
| ECG QTc interval – msec | 432 [409, 450] | −6 [−15, 5] | −7 [−15, 9] |
| Eating behaviors and physical activity | |||
| Eating Inventory (higher score = more) | |||
| Disinhibition | 6 [4, 11] | 0 [−1, 1.5] | 0 [−1.25, 1] |
| Dietary restraint | 9 [6, 13] | 3 [0.5, 3.5] | 0.5 [−2, 1.25] |
| Hunger | 5 [1, 8] | 0 [−0.5, 3] | 0 [−1, 1.25] |
| Hyperphagia questionnaire (higher score = more) | |||
| Drive | 8 [6, 10] | 0 [−2, 1] | 0 [−1, 1.75] |
| Behavior | 8 [6, 10] | 0 [−8, 0] | 0 [−0.25, 3.25] |
| Severity | 3 [2, 5] | 0 [0, 0.5] | 0.5 [−0.25, 1] |
| Total | 19 [15, 23] | 0 [−2.5, 1.5] | 0 [−1, 1.25] |
| Physical activity, time spent in any activity (hours per day) | 0.9 [0.5, 1.3] | −0.1 [−1.0, 0.1] | −0.2 [−0.6, 0.1] |
| Quality of life and family function | |||
| Quality of life (Neuro-QOL), T-scores (higher score = more) | |||
| Anxiety | 49 [46, 56] | −1 [−5.9, 0]* | 0 [−2.1, 4.2] |
| Depression | 37 [36, 50] | 0 [−0.6, 0] | 0.6 [0, 3.9] |
| Cognition | 51 [43, 57] | 0 [−1.4, 1.9] | 0.6 [−4.1, 2.4] |
| Social function (adults) or peer interaction (children) | 48 [41, 59] | 0 [−3.5, 2.1] | −2.1 [−7.2, 0] |
| Family Assessment Device (N = 10) (higher score = worse function) | 1.4 [1.1, 1.8] | 0 [−0.1, 0.1] | −0.1 [−0.5, 0] |
Median [interquartile range] of the change in each treatment outcome with OXT and PBO are shown. Paired samples Wilcoxon test was used to test for the within-participant difference between the change in each treatment outcome with OXT and PBO for the n = 11 participants who received both OXT and PBO over the course of the crossover trial. Statistical significance is indicated by *P < 0.05 (unadjusted for multiple comparisons).
Abbreviations: BMI, body mass index; BP, blood pressure; bpm, beats per minute; OXT, oxytocin; PBO, placebo.
Table 3.
Within-participant change in body weight, in kg, during each treatment block, accounting for baseline weight at the beginning of each treatment block, testing potential explanatory covariates in each statistical model
| Factor | Model 1 (N = 13) | Model 2 (N = 13) | Model 3 (N = 13) | Model 4 (N = 13) |
|---|---|---|---|---|
| β coefficient (95% CI) |
β coefficient (95% CI) |
β coefficient (95% CI) |
β coefficient (95% CI) |
|
| Treatment (OXT, reference = PBO) | −0.6 (−2.7, 1.5) |
−0.6 (−2.7, 1.5) |
−0.6 (−2.7, 1.5) |
−0.6 (−3.1, 1.8) |
| Block in study (Block 2, reference = Block 1) | −1.4 (−3.5, 0.8) |
−1.4 (−3.5, 0.7) |
−1.4 (−3.4, 0.7) |
−1.3 (−3.7, 1) |
| Weight at beginning of each treatment block, kg | 0 (−0.1, 0) |
0 (−0.1, 0.1) |
0 (−0.1, 0.0) |
0 (−0.1, 0.1) |
| Age, years | - | −0.1 (−0.4, 0.2) |
- | - |
| Sex, (female, reference = male) | - | - | −1.2 (−3.6, 1.2) |
- |
| Adherence to study drug (% of all prescribed doses taken) | - | - | - | 0 (−0.1, 0.1) |
Main model (Model 1) and sensitivity analyses (Models 2-4) of prespecified primary outcome, change in body weight (kg), are shown. To test for possible carry-over effect, an interaction term between treatment (OXT vs PBO) and block (1 vs 2) was included in the main model (Model 1), and the interaction term was not statistically significant (not shown), so models are presented without this interaction term. None of the β coefficients shown above was statistically significant.
Abbreviations: OXT, oxytocin; PBO, placebo.
Table 4.
Within-participant change in other anthropometric measures during each treatment block, accounting for baseline anthropometric measures at the beginning of each treatment block
| Factor | BMI (kg/m2) (N = 13) |
BMI (Z-score) (N = 9, children only) |
Waist circumference (cm) (N = 13) |
|---|---|---|---|
| β coefficient (95% CI) |
β coefficient (95% CI) | β coefficient (95% CI) |
|
| Treatment (OXT, reference = PBO) | −0.2 (−1.1, 0.7) |
0.02 (−0.03, 0.06) |
0.6 (−1.9, 3.1) |
| Block in study (Block 2, reference = Block 1) | −0.4 (−1.3, 0.5) |
−0.02 (−0.07, 0.02) |
−1.5 (−4.0, 1) |
| Outcome measured at the beginning of each treatment block | 0.0 (−0.1, 0.1) |
0.02 (−0.07, 0.10) |
−0.1 (−0.2, 0.1) |
Mixed-effects models of other anthropometric measurements were also constructed. None of the β coefficients shown above was statistically significant.
Abbreviations: BMI, body mass index; OXT, oxytocin; PBO, placebo.
Safety
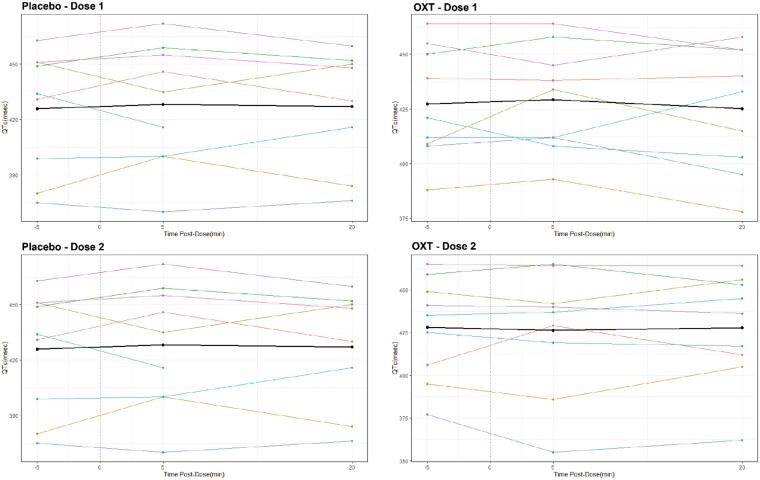
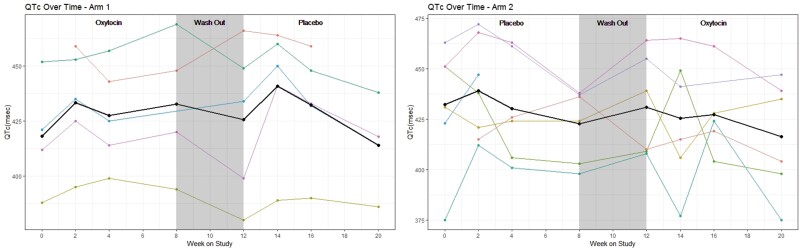
The Data Safety Monitoring Board (DSMB) met throughout and recommended continuing the study. Overall, we found OXT to be safe and well-tolerated, with no AEs higher than Grade 2 in severity. Commonly reported side effects included epistaxis, nasal irritation, headache, and nausea. AE-related dose adjustments occurred equally in OXT and placebo conditions. Of the 13 individuals randomized, 5/13 did not require any dose adjustments, and 2/13 were terminated based on QTc interval prolongation. Dose was decreased for marginal QTc intervals (2 participants during placebo, 1 during OXT), gastrointestinal symptoms (constipation, reflux) (1 in each group), and fatigue (1 during OXT). With respect to QTc intervals, prior to any treatment and in both treatment conditions, a subset of individuals (2/18 screened, 5/13 randomized) had some degree of prolonged QTc interval (>450 msec, CTCAE Grade 1) on ECG (Table 5). No statistically significant effects of either OXT or placebo on QTc were detected around dosing (Fig. 4) or over the course of participation (Fig. 5). Two participants were taken off study (in consultation with Cardiology) for prolonged QTc. One had a QTc interval of 451 msec on screening ECG, 460 msec pre-dose of OXT and then 478 msec at 20 minutes post-dose. The other individual had a QTc interval of 446 msec on screening ECG, and on the second study visit, QTc was 488 msec pre-dose (placebo), thus the dose was not given. The frequency of hyponatremia in the trial was low (15%) and did not differ between OXT and placebo conditions; any hyponatremia was mild (Grade 1), with measured values ≥ 133 mmol/L. Of the 9 individuals with DI, 6 did not have any adjustments to their desmopressin (DDAVP) dose at any point in the study. Of the 3 individuals with DI who did have DDAVP dose adjustments, 2 decreased their DDAVP dose during the placebo block, and 1 decreased their DDAVP dose during the OXT block. Thus, we did not detect an effect of OXT on sodium levels and DDAVP requirements.
Table 5.
Adverse events impacting at least 2 individuals and/or achieving grade 2 severity
| Adverse events | Treatment | ||
|---|---|---|---|
| Oxytocin (n = 12) | Placebo (n = 12) | All (n = 13) | |
| Adverse event of special interest | |||
| Epistaxis | 3 (23%) | 2 (15%) | 3 (23%) |
| Nasal congestion/irritation | 4 (31%) | 0 (0%) | 4 (31%) |
| Nausea/vomiting | 1 (8%) | 3 (23%) | 4 (31%) |
| Headache | 3 (23%) | 4 (31%) | 6 (46%) |
| Rash | 0 (0%) | 1 (8%) | 1 (8%) |
| Hyponatremia | 1 (8%) | 2 (15%) | 2 (15%) |
| Hypertension | 5 (38%) | 5 (38%) | 6 (46%) |
| Hypotension | 1 (8%) | 1 (8%) | 2 (15%) |
| Sinus tachycardia | 4 (31%) | 5 (38%) | 7 (54%) |
| QTc interval prolonged | 2 (15%) | 3 (23%) | 5 (38%) |
| Sinus bradycardia | 2 (15%) | 2 (15%) | 3 (23%) |
| Adverse events leading to treatment discontinuation | |||
| QTc interval prolonged | 1 (8%) | 1 (8%) | 2 (15%) |
| Other adverse events (affecting 2 or more participants and/or CTCAE Grade ≥ 2) | |||
| Fatigue | 2 (15%) | 0 (0%) | 2 (15%) |
| Irritability | 1 (8%) | 1 (8%) | 2 (15%) |
| Constipation | 2 (15%) | 0 (0%) | 2 (15%) |
| Diarrhea | 0 (0%) | 3 (23%) | 3 (23%) |
| Gastrointestinal – other (rectal irritation) | 1 (8%) | 1 (8%) | 1 (8%) |
| Gastrointestinal – other (worsening IBS) | 1 (8%) | 0 (0%) | 1 (8%) |
| Gastrointestinal – other (worsening heartburn) | 0 (0%) | 1 (8%) | 1 (8%) |
| Allergic Rhinitis | 2 (15%) | 0 (0%) | 2 (15%) |
| Sore Throat | 2 (15%) | 2 (15%) | 4 (27%) |
| Insomnia | 1 (8%) | 1 (8%) | 2 (15%) |
| Skin and Subcutaneous Tissue – other (Dermatitis) | 1 (8%) | 0 (0%) | 1 (8%) |
| Skin and Subcutaneous Tissue – other (Dry Lips) | 0 (0%) | 1 (8%) | 1 (8%) |
| Myalgia | 1 (8%) | 1 (8%) | 2 (15%) |
| Infections – other (Pharyngitis, Sinusitis) | 0 (0%) | 1 (8%) | 1 (8%) |
| Investigations – other (Low free T4) | 1 (8%) | 2 (15%) | 3 (23%) |
| Investigations – other (Elevated AST) | 1 (8%) | 0 (0%) | 1 (8%) |
| Investigations – other (Elevated bilirubin) | 0 (0%) | 1 (8%) | 1 (8%) |
Prevalence of adverse events of special interest, leading to treatment discontinuation, and reported by at least 2 individuals and/or reaching CTCAE Grade 2 severity are shown for all individuals who received at least one dose of oxytocin (n = 12), placebo (n = 12), or either (n = 13). All adverse events were Grades 0, 1, or 2 severity. Grade 0 referred to abnormalities outside the reference range, but not sufficiently abnormal to rate.
Figure 4.
QTc (Bazett) interval (msec) for each participant, before (−5 minutes) and after (5 minutes, 20 minutes) Dose #1 (starting dose) and Dose #2 (dose following size-based escalation, if applicable) of placebo and/or OXT. Each participant's values are shown individually (colors), and the mean is also shown (black).
Figure 5.
QTc (Bazett) interval (msec) for each participant over the course of the study, by treatment arm. Each participant's values are shown individually (colors), and the mean is also shown (black), stratified by treatment arm.
Eating Behaviors, Cognitive Restraint, and Quality of Life
We did not detect any effects of intranasal OXT on eating behaviors, including as reflected by scores on the Eating Inventory (Table 6) and hyperphagia questionnaire (Table 7) subscales as well as the test meal (Table 8). In unadjusted within-participant exploratory analyses (Table 9), the Stop-Signal Task indicated showed shorter stop-signal reaction times (SSRTs) following OXT administration vs placebo (43 msec; 95% CI, 1-85; P < .01), indicating better response inhibition; we also observed that individuals displayed faster SSRTs in the second treatment block regardless of treatment (57 msec; 95% CI, 15-99; P < .01). In additional exploratory analyses (Table 10), we found mild improvements in self-reported anxiety of 3.7 points (95% CI, 0.8-6.6) attributable to OXT, from a median starting value of 49 (range, 0-100), which is nearly aligned with a healthy population reference mean of 50. (The NIH PROMIS instruments were designed to generate scores relative to the healthy population reference.)
Table 6.
Within-participant change in eating inventory subscales during each treatment block, accounting for baseline subscale values at the beginning of each treatment block
| Factor | Model 1 Eating – disinhibition score (N = 13) |
Model 2 Eating – restraint score (N = 13) |
Model 3 Eating – hunger score (N = 13) |
|---|---|---|---|
| β coefficient (95% CI) |
β coefficient (95% CI) |
β coefficient (95% CI) |
|
| Treatment (OXT, reference = PBO) | 0.41 (−1.79, 2.61) |
1.59 (−1.06, 4.23) |
0.32 (−0.87, 1.52) |
| Block in study (Block 2, reference = Block 1) | 0.23 (−2.01, 2.47) |
0.69 (−1.99, 3.37) |
0.72 (−0.63, 2.08) |
| Outcome of interest at the beginning of each treatment block |
0.86*
(0.57, 1.15) |
0.83*
(0.47, 1.18) |
0.52*
(0.27, 0.76) |
| Treatment duration (number of days within the Block when outcome was measured) | 0.01 (−0.07, 0.09) |
−0.02 (−0.14, 0.11) |
−0.04 (−0.13, 0.04) |
Mixed-effects models of other outcomes of interest were also constructed. The “treatment duration” variable reflects the time (in days) in within the treatment Block when the outcome was measured. Statistical significance is indicated by bold text; *P < 0.05.
Abbreviations: OXT, oxytocin; PBO, placebo.
Table 7.
Within-participant change in hyperphagia questionnaire subscales during each treatment block), accounting for baseline subscale values at the beginning of each treatment block
| Factor | Model 1 Hyperphagia – Behavior (N = 13) |
Model 2 Hyperphagia – Severity (N = 13) |
Model 3 Hyperphagia – Drive (N = 13) |
Model 4 Hyperphagia – Total Score (N = 13) |
|---|---|---|---|---|
| β coefficient (95% CI) |
β coefficient (95% CI) |
β coefficient (95% CI) |
β coefficient (95% CI) |
|
| Treatment (OXT, reference = PBO) | −2.39 (−6.19, 1.41) |
0.03 (−0.43, 0.50) |
−0.94 (−2.77, 0.90) |
0.33 (−1.17, 1.82) |
| Block in study (Block 2, reference = Block 1) | 3.43 (−0.39, 7.25) |
−0.02 (−0.49, 0.45) |
0.17 (−1.75, 2.01) |
−1.89 (−3.39, −0.40) |
| Outcome of interest at the beginning of each treatment block | 0.06 (−0.32, 0.45) |
0.03 (−0.23, 0.29) |
0.34*
(0.05, 0.62) |
0.34*
(0.09, 0.69) |
| Treatment duration (number of days within the Block when outcome was measured) | −0.04 (−0.19, 0.11) |
0.02 (−0.03, 0.06) |
−0.01 (−0.12, 0.11) |
0.03 (−0.11, 0.17) |
Mixed-effects models of other outcomes of interest were also constructed. The “treatment duration” variable reflects the time (in days) in within the treatment Block when the outcome was measured. Statistical significance is indicated by bold text; *P < 0.05.
Table 8.
Kcal eaten as a % of total kcal offered during mixed meal tests administered 2 times during each treatment block on 2 different weight-based doses of OXT or placebo
| Factor | Test meal (kcal eaten, as a % of total kcal offered) | |
|---|---|---|
| Visits 1 & 5 (lower dose) |
Visits 2 & 6 (higher dose) | |
| β coefficient (95% CI) |
β coefficient (95% CI) |
|
| Treatment (OXT, reference = PBO) | 1.4 (−7.3, 10.2) |
1.7 (−11.9, 15.2) |
| Block in study (Block 2, reference = Block 1) | 6.0 (−2.8, 14.8) |
−7.0 (−20.6, 6.5) |
None of the β coefficients shown above was statistically significant.
Abbreviations: OXT, oxytocin; PBO, placebo.
Table 9.
Stop-signal task, test of cognitive restraint and capacity for response inhibition
| Factor | Model 1 SSRT (N = 13) |
Model 2 Stop Error (N = 13) |
Model 3 Go RT (N = 13) |
Model 4 Go Error (N = 13) |
|---|---|---|---|---|
| β coefficient (95% CI) |
β coefficient (95% CI) |
β coefficient (95% CI) |
β coefficient (95% CI) |
|
| Treatment (OXT, reference = PBO) |
−43.1**
(−85.3, −1.0) |
1.1 (−3.9, 6.1) |
−62.9 (−268, 142.1) |
0.5 (−0.7, 1.7) |
| Block in study (Block 2, reference = Block 1) |
−57.1**
(−99.4, −14.9) |
−1.5 (−6.5, 3.5) |
−31.2 (−236.9, 174.6) |
−0.2 (−1.4, 1) |
Stop-signal task was performed once during each treatment block. Statistical significance is indicated by bold text; *P < .05; **P < .01. Abbreviations: OXT, oxytocin; PBO, placebo.
Table 10.
Linear mixed-effects model for quality of life scores
| Factor | Model 1 Neuro-QOL – Social Function T-score (N = 13) |
Model 2 Neuro-QOL – Anxiety T-score (N = 13) |
Model 3 Neuro-QOL – Cognition T-score (N = 13) |
Model 4 Neuro-QOL – Depression T-score (N = 13) |
|---|---|---|---|---|
| β coefficient (95% CI) |
β coefficient (95% CI) |
β coefficient (95% CI) |
β coefficient (95% CI) |
|
| Treatment (OXT, reference = PBO) | 1.4 (−5.6, 8.4) |
−3.7*
(−6.6, −0.8) |
−0.1 (−3.6, 3.5) |
−1.3 (−3.2, 0.6) |
| Block in study (Block 2, reference = Block 1) | −1.7 (−8.8, 5.4) |
2.0 (−0.9, 4.9) |
4.5*
(0.9, 8.1) |
−0.5 (−2.4, 1.4) |
| Outcome of interest at the beginning of each treatment block |
0.9*
(0.5, 1.2) |
1.0*
(0.7, 1.2) |
1.4*
(0.9, 1.2) |
1.0*
(0.9, 1.1) |
| Treatment duration (number of days within the Block when outcome was measured) | −0.1 (−0.4, 0.2) |
0.0 (−0.1, 0.1) |
−0.1 (−0.2, 0.1) |
0.1 (0.0, 0.2) |
Change in self-reported quality of life T-scores during each treatment block, accounting for baseline T-scores at the beginning of each treatment block.
Mixed-effects models of other outcomes of interest were also constructed. The “treatment duration” variable reflects the time (in days) in within the treatment Block when the outcome was measured. Statistical significance is indicated by bold text; *P < .05. Abbreviations: OXT, oxytocin; PBO, placebo.
Discussion
In this pilot study, we did not detect an effect of intranasal OXT on body weight in individuals with hypothalamic obesity. Side effects were generally mild and did not differ meaningfully between OXT and placebo. Given the lack of data around potential cardiac effects of OXT, we also assessed ECGs. Strikingly, we found that individuals with hypothalamic obesity can have prolonged QTc intervals even prior to treatment with OXT or placebo. Rates of QTc prolongation in our trial were similar to those from a previously published study in individuals with craniopharyngioma [43], in which half of the cohort (6 of 12) had at least one abnormality of cardiac structure, function, and/or rhythm. The prevalence of prolonged QTc interval in our independent cohort, absent any electrolyte abnormalities, highlights the need to investigate the potentially under-recognized burden of cardiac comorbidities in individuals with hypothalamic obesity, in particular those seeking investigational therapies. Our detailed studies, prompted by the lack of available data in this at-risk population, did not find an effect of OXT on QTc interval.
Although we did not detect an impact of OXT on our primary outcome, body weight, or other related metabolic measures, the 95% CI for our observed effect size (−0.6 kg; 95% CI −2.7 kg, 1.5 kg) includes −2.2 kg, which is the effect size that would have been detectable if we had randomized 20 individuals as originally planned, prior to the pandemic, and observed the same SD of weight change. Thus, we cannot exclude the possibility that a statistically significant effect of OXT on body weight (−2.2 kg) would have been detectable with a larger sample. In exploratory analyses, OXT appeared to decrease individual- and caregiver-reported anxiety and to decrease impulsivity on an assessment of cognitive control. Our experience also demonstrates the value of within-participant crossover randomized controlled trials for rigorous assessment of specific treatment strategies in rare disorders.
A number of factors may have led to lack of weight loss attributable to OXT in this trial, beyond limitations related to sample size. It is possible that individuals seeking clinical trials have exhausted other available treatment options, and so may have particularly recalcitrant forms of hypothalamic obesity. However, our cohort appears representative of individuals with hypothalamic obesity. Specifically, rates of hormone deficiencies were similar to previous epidemiologic studies [44]. Also, MRI-based hypothalamic injury scores were similar to those in a different, larger clinical trial of exenatide in individuals with hypothalamic obesity that found exenatide had benefit relative to placebo for waist circumference and fat mass, although not BMI [26]. Duration of hypothalamic injury in this pilot trial is also similar to previous studies [26]. Interventions implemented within the first 6 months after tumor diagnosis, when obesity often first becomes apparent, may be more efficacious than treatment begun after many years of established obesity, as in this study, where median time since diagnosis was 7.5 years. Another possibility is that individuals with hypothalamic injury lack the ability to respond to intranasal OXT because OXT exerts effects on key hypothalamic nuclei that impact appetite and energy balance. In animal models, however, administration of OXT outside the hypothalamus, in the hindbrain, decreases weight gain via decrease in food intake and also activates catecholamine neurons in nucleus tractus solitarius (NTS) [45]. Thus, based on the shared physiology governing energy balance, exogenous OXT could plausibly exert effects even in individuals with hypothalamic injury. Also, exogenous OXT could act on any residual hypothalamic tissue, and some investigations have posited a “feed-forward” mechanism, where exogenous OXT enhances endogenous secretion [46]. With respect to body weight over this time interval, however, we did not detect an OXT effect.
Dose selection is another potential contributor to OXT efficacy or lack thereof. Several considerations add complexity to the selection of dosing of OXT for individuals with hypothalamic obesity. Exogenous OXT may act via either central and/or peripheral mechanisms [14, 47, 48] in a manner that depends both on the route of administration and the dose administered. In preclinical models, labeled OXT administered intranasally to rhesus macaques [49] or rabbits [50] appears to reach the cerebrospinal fluid and brain, respectively. These studies using labeled OXT suggest that exogenous OXT acts directly on the central nervous system, although the possibility has been raised previously that exogenous OXT administration may work by leading to augmented endogenous OXT production [49]. Preclinical and clinical studies have also explored alternate methods of OXT administration, for example, via aerosolization [51], with a view to achieving fewer side effects [52] and/or better ability to reach relevant brain regions [50]. OXT has a short half-life; a longer-acting formulation might be preferable, and/or one more focused on the OXTR (ie, instead of also engaging vasopressin receptors) [52]. When acting centrally, OXT effects are likely related primarily to the magnitude of the dose administered, independent of the size of the individual, because the drug does not require distribution in the peripheral circulation [53]. In contrast, intranasal OXT may also exert peripheral effects that depend on the size-dependent volume of distribution of the drug, although the serum half-life of OXT in the periphery is expected to be relatively short, ∼6 to 17 minutes for the intravenous form [54]. As reviewed in [55], species, sex, energy status, developmental stage, chronicity of administration, and even social context at the time of OXT administration may modulate OXT effects. Also, benefit may only be observable when OXT is administered concurrently with intensive lifestyle modification efforts. Most families reported continuing to engage to the extent possible in the healthful eating and exercise habits that their clinical teams recommended, although we note overall low levels of physical activity in this cohort.
The most appropriate OXT dosing approach in individuals with hypothalamic obesity is also uncertain because there is no consensus regarding a strategy for dose selection. One option, measurement of peripheral OXT concentrations, is technically challenging [56], and the peripheral concentration(s) of OXT needed to be achieved to produce a weight loss effect are unclear. Moreover, peripheral OXT concentrations may not reflect tissue concentrations in the most relevant target tissues, such as the brain [57, 58]. However, recent insights regarding the most appropriate methodological techniques to measure and interpret OXT concentrations in biological samples may make it possible in future to target dosing to tissue-specific OXT levels [59].
Given the current limitations of peripheral OXT measurements for making dosing decisions, the most appropriate dose selection strategy for OXT may instead focus on the individual-specific dose required to achieve the mechanistic and/or clinically relevant outcome that is most sensitive to OXT. While weight loss was the focus of the present investigation, trials of OXT in individuals with other forms of obesity related to hypothalamic impairment, such as PWS, have targeted other related endpoints, including hyperphagia, repetitive behavior, and/or social function [60, 61]. Although hyperphagia can be particularly challenging to assess objectively, multiple studies [7, 62] have concluded that hyperphagia is quite variable in individuals with brain tumor related hypothalamic obesity. Individuals with hypothalamic obesity in our study had similar scores on scales of disinhibited eating and hunger as adolescents with “common” obesity and binge eating disorder (ie, without hypothalamic obesity) [63]. On average, individuals in our study endorsed some hyperphagia, although not to the same extent as a comparator cohort with PWS [33]. Thus, it is possible that a study enrolling exclusively the subset of individuals with hypothalamic obesity and significant hyperphagia might have had different results, particularly in light of potential effects of OXT on impulsivity. Indeed, in one case report of an adolescent boy with hypothalamic obesity and significant hyperphagia, compounded intranasal OXT 6 IU daily along with carbohydrate restriction, and then with the addition of naltrexone, led to amelioration of hyperphagia, and a sustained decrease in BMI Z-score [17]. This individual's course also highlights that combination therapies may be valuable in hypothalamic obesity.
Another set of outcomes that may be sensitive to OXT are related to socioemotional and cognitive function. Individuals and families frequently report concerns related to these domains [64], and in this study, a substantial number of participants and caregivers reported impairment in family function, similar to a previous report in other survivors of childhood brain tumors [42]. Although not directly related to metabolism per se, interventions that mitigate anxiety or increase social engagement could promote the success of weight loss efforts in hypothalamic obesity, for example, by decreasing barriers to participating in group-based physical activity. Several studies have posited an association between endogenous OXT levels and various dimensions of social and cognitive function in individuals with hypopituitarism [65-68]. In a pilot cross-sectional interventional study, a single dose of 24 IU of intranasal OXT was administered to N = 10 individuals with craniopharyngioma, and individuals with lesions limited to anterior hypothalamus (as compared to those with lesions in both anterior and posterior hypothalamus) appeared to improve in their ability to identify emotions, in particular negative emotions [68]. In our exploratory analyses, we found a ∼10% decrease in self-reported anxiety in the OXT-treated group, which merits further focused investigation in light of the posited role for OXT in anxiety behaviors including in preclinical models [69]. We did not observe an increase in behavioral outbursts or irritability attributable to this dose of OXT, unlike a previous study in individuals with Prader-Willi syndrome [23], which may be because our cohort had an overall lower burden of psychiatric comorbidities. Also, less common side effects may only become apparent in larger studies.
Applying the Stop-Signal Task as a well-established objective measure of impulse control (including investigations of disordered eating behaviors) [30], we found that OXT led to a faster suppression of unwanted responses (indicated by a shorter SSRT), highlighting an increased individual capacity to control existing behavioral impulses (reactive control). A previous study in otherwise healthy men with overweight and obesity showed that a single dose of intranasal OXT caused a strategic performance shift that improved control over behavioral impulses (faster go response times together with fewer stop errors) indicative of increased proactive control [29]. While both studies highlight the potential of OXT to improve impulse control, the difference in how OXT affects impulse control might be related to differences in sample characteristics. For example, the strategic proactive control improvement was observed in adults, while the present study showed augmented reactive control in a sample spanning an age range in which impulse control continues to mature. How best to harness this additional capacity for cognitive control to facilitate weight management is another potential future avenue for research. Also, as a recent negative study of OXT in autism illustrates, selection of the most appropriate participants and OXT-sensitive outcomes is essential [70]. Social and/or familial context of OXT administration could also influence outcomes.
Our main limitation is the small sample size. Two key factors limited our enrollment: i) the COVID-19 pandemic and ii) prevalence of prolonged QTc interval on screening (and related to this, the requirement to exclude individuals taking QTc prolonging medications). QTc interval prolongation was also problematic because it impacted participant completion rate. While the COVID-19 pandemic and QTc interval prolongation impacted recruitment and study completion, hypothalamic obesity is itself a rare disorder. As with other rare conditions, sample size is an inherent challenge, and study designs that maximize the insights generated from the available cohort, like the crossover design used here, are essential. With the notable exception of one phase 3 randomized controlled trial that enrolled 42 participants [71], most studies in hypothalamic obesity are small case reports or series with fewer than 10 individuals. A review in 2019 [13] noted only 3 previous randomized controlled trials in hypothalamic obesity, enrolling n = 14, n = 18, and n = 40 respectively, with each cohort divided into 2 treatment groups. Thus, this pilot crossover trial enrolling n = 13 individuals adds to the existing evidence base. The COVID-19 pandemic may have also affected individuals’ weight trajectories by impacting eating behaviors, physical activity, stress, and other factors, although we might expect the impact to be similar in OXT and placebo conditions. Also, the relatively short duration of treatment was deliberate, given the absence of safety data that could justify a longer treatment course, but it is possible that longer treatment might have yielded more discernible benefit in some individuals.
Future work related to the potential role for OXT in hypothalamic obesity should focus, first, on identifying predictors or biomarkers of OXT response so that more refined individualized study eligibility criteria and/or dosing strategies can be used. For example, patient-level factors, such as duration of hypothalamic obesity, may also influence treatment response and could be evaluated in larger, adaptive study designs. Also, as in other, related conditions, such as PWS, addressing excess eating and impulsivity even without impacting body weight would be of value to the subset of individuals with hypothalamic obesity who experience chronic distress related to these behaviors. Some participants in our study had nominal improvements in hyperphagia with OXT which could also be the focus of future studies. In addition, previous studies in preclinical models [72] and humans [73, 16] have suggested that OXT may modulate glucose metabolism, suggesting glycemia as a potential therapeutic target. We did not detect an effect of OXT on fasting glucose or triglycerides, an index of insulin resistance, potentially because these values were not substantively elevated in our cohort. Alternatively, the effect of OXT may depend on the metabolic milieu, and its impact on insulin and glucose could be less in the context of obesity [74].
Targeting the choice of anti-obesity therapy to the predominant obesity phenotype(s), including, for example, hyperphagia or decreased energy expenditure, has shown promise in “common” obesity [75], and may also be useful in hypothalamic obesity, since despite sharing this diagnosis, individuals may differ in its manifestations. In preclinical animal models, OXT has been tested in combination with other treatments to target multiple pathogenic mechanisms (eg, decreased energy intake, increased brown adipose tissue thermogenesis) and avoid compensatory adaptations to weight loss [76]. In humans with “common” obesity, combination therapies may produce these benefits, and may also decrease dose-related adverse events by permitting administration of lower doses, a valuable advantage in individuals with hypothalamic obesity who have multiple comorbidities and are taking many other medications [77, 78]. Thus, testing OXT in combination with other agents may also be a useful approach. Participants with hypothalamic obesity mostly gained weight across treatment arms in our study, highlighting the compelling need to identify effective approaches in this condition.
Acknowledgments
We are grateful to the individuals and their caregivers who chose to participate in this trial, as well as the members of the CHOP Center for Human Phenomic Sciences and Growth & Nutrition Laboratory who performed study procedures. We appreciate Drs. Francisco Perez and Christian Roth instructing us in their techniques for scoring hypothalamic injury via MRI.
Abbreviations
- BMDCS
Bone Mineral Density Cohort Study Physical Activity
- BMI
body mass index
- CHOP
Children’s Hospital of Philadelphia
- CTCAE
Common Terminology Criteria for Adverse Events
- DDAVP
desmopressin
- DI
diabetes insipidus
- ECG
electrocardiography
- FAD-GFS
Family Assessment Device General Function scale
- IQR
interquartile range
- MRI
magnetic resonance imaging
- Neuro-QOL
Quality of Life in Neurological Disorders
- OXT
oxytocin
- PWS
Prader-Willi syndrome
- SMURF
Safety Monitoring Uniform Report Form
- SSRT
stop-signal reaction time
Contributor Information
Shana E McCormack, Email: mccormacks1@chop.edu, Division of Endocrinology & Diabetes, Children's Hospital of Philadelphia, Philadelphia, PA 19104, USA; Department of Pediatrics, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA 19104, USA.
Zi Wang, Biostatistics & Data Management, Children's Hospital of Philadelphia, Philadelphia, PA 19104, USA.
Kristin L Wade, Division of Endocrinology & Diabetes, Children's Hospital of Philadelphia, Philadelphia, PA 19104, USA.
Anna Dedio, Division of Endocrinology & Diabetes, Children's Hospital of Philadelphia, Philadelphia, PA 19104, USA.
Nicolette Cilenti, Division of Endocrinology & Diabetes, Children's Hospital of Philadelphia, Philadelphia, PA 19104, USA.
Julia Crowley, Division of Endocrinology & Diabetes, Children's Hospital of Philadelphia, Philadelphia, PA 19104, USA.
Franziska Plessow, Neuroendocrine Unit, Department of Medicine, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA.
Vaneeta Bamba, Division of Endocrinology & Diabetes, Children's Hospital of Philadelphia, Philadelphia, PA 19104, USA; Department of Pediatrics, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA 19104, USA.
Jeffrey D Roizen, Division of Endocrinology & Diabetes, Children's Hospital of Philadelphia, Philadelphia, PA 19104, USA; Department of Pediatrics, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA 19104, USA.
Yaoguang Jiang, Department of Psychology, School of Arts and Sciences, University of Pennsylvania, Philadelphia, PA 19104, USA.
Jack Stylli, Georgetown University School of Medicine, Washington, DC 20007, USA.
Arjun Ramakrishnan, Department of Biological Sciences and Bioengineering, Indian Institute of Technology, Kanpur, Uttar Pradesh 208016, India.
Michael L Platt, Department of Psychology, School of Arts and Sciences, University of Pennsylvania, Philadelphia, PA 19104, USA; Department of Neuroscience, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA 19104, USA; Marketing Department, Wharton School of Business, University of Pennsylvania, Philadelphia, PA 19104, USA.
Karuna Shekdar, Division of Neuroradiology, Children's Hospital of Philadelphia, Philadelphia, PA 19104, USA; Department of Radiology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA 19104, USA.
Michael J Fisher, Department of Pediatrics, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA 19104, USA; Center for Childhood Cancer Research and Division of Oncology, Children's Hospital of Philadelphia, Philadelphia, PA 19104, USA.
Victoria L Vetter, Department of Pediatrics, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA 19104, USA; Cardiac Center, Children's Hospital of Philadelphia, Philadelphia, PA 19104, USA.
Matthew Hocking, Center for Childhood Cancer Research and Division of Oncology, Children's Hospital of Philadelphia, Philadelphia, PA 19104, USA; Department of Psychiatry, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA 19104, USA.
Rui Xiao, Center of Clinical Epidemiology & Biostatistics, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA 19104, USA.
Elizabeth A Lawson, Neuroendocrine Unit, Department of Medicine, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA.
Funding
This study was funded by a Doris Duke Clinical Scientist Development Award (DDCF 2016090, SEM). The project described was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences (NIH UL1TR001878). The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
Disclosures
S.E.M. has previously consulted for Rhythm Pharmaceuticals and has served as site PI for trials by Levo Therapeutics (Jan 2019-Sep 2022) and Rhythm Pharmaceuticals (ongoing). E.A.L. was on the scientific advisory board and has/had a financial interest in OXT Therapeutics, a company that developed oxytocin-based therapeutics for obesity and metabolic disease; and received an investigator-initiated grant from Tonix Pharmaceuticals. M.L.P. is a scientific advisory board member, consultant, and/or cofounder of Blue Horizons International, NeuroFlow, Amplio, Cogwear Technologies, Burgeon Labs, and Glassview, and receives research funding from AIIR Consulting, the SEB Group, Mars Inc, Slalom Inc, the Lefkort Family Research Foundation, and Benjamin Franklin Technology Partners. Other authors have nothing to declare.
Data Availability
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
Clinical Trial Information
ClinicalTrials.gov registration no. NCT02849743.
References
- 1. Abuzzahab MJ, Roth CL, Shoemaker AH. Hypothalamic obesity: prologue and promise. Horm Res Paediatr. 2019;912128–-136.. [DOI] [PubMed] [Google Scholar]
- 2. Muller HL. Management of hypothalamic obesity. Endocrinol Metab Clin North Am. 2020;49(3):533‐552. [DOI] [PubMed] [Google Scholar]
- 3. Madsen PJ, Buch VP, Douglas JE, et al. Endoscopic endonasal resection versus open surgery for pediatric craniopharyngioma: comparison of outcomes and complications. J Neurosurg Pediatr. 2019;243236–-245.. [DOI] [PubMed] [Google Scholar]
- 4. Shaikh MG, Grundy RG, Kirk JM. Reductions in basal metabolic rate and physical activity contribute to hypothalamic obesity. J Clin Endocrinol Metab. 2008;93(7):2588‐2593. [DOI] [PubMed] [Google Scholar]
- 5. Cohen M, Syme C, McCrindle BW, Hamilton J. Autonomic nervous system balance in children and adolescents with craniopharyngioma and hypothalamic obesity. Eur J Endocrinol. 2013;168(6):845‐852. [DOI] [PubMed] [Google Scholar]
- 6. Roth C, Wilken B, Hanefeld F, Schroter W, Leonhardt U. Hyperphagia in children with craniopharyngioma is associated with hyperleptinaemia and a failure in the downregulation of appetite. Eur J Endocrinol. 1998;138(1):89‐91. [DOI] [PubMed] [Google Scholar]
- 7. Harz KJ, Muller HL, Waldeck E, Pudel V, Roth C. Obesity in patients with craniopharyngioma: assessment of food intake and movement counts indicating physical activity. J Clin Endocrinol Metab. 2003;88(11):5227‐5231. [DOI] [PubMed] [Google Scholar]
- 8. Olsson DS, Andersson E, Bryngelsson IL, Nilsson AG, Johannsson G. Excess mortality and morbidity in patients with craniopharyngioma, especially in patients with childhood onset: a population-based study in Sweden. J Clin Endocrinol Metab. 2015;100(2):467‐474. [DOI] [PubMed] [Google Scholar]
- 9. Jung SY, Lee YJ, Lee HJ, et al. Nonalcoholic fatty liver disease in long-term survivors of childhood-onset craniopharyngioma. Ann Pediatr Endocrinol Metab. 2017;22(3):189‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yuen KCJ, Mattsson AF, Burman P, et al. Relative risks of contributing factors to morbidity and mortality in adults with craniopharyngioma on growth hormone replacement. J Clin Endocrinol Metab. 2018;103(2):768‐777. [DOI] [PubMed] [Google Scholar]
- 11. Qiao N. Excess mortality after craniopharyngioma treatment: are we making progress? Endocrine. 2019;64(1):31‐37. [DOI] [PubMed] [Google Scholar]
- 12. Rose SR, Horne VE, Bingham N, Jenkins T, Black J, Inge T. Hypothalamic obesity: 4 years of the international registry of hypothalamic obesity disorders. Obesity (Silver Spring). 2018;26(11):1727‐1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Iersel L, Brokke KE, Adan RAH, Bulthuis LCM, van den Akker ELT, van Santen HM. Pathophysiology and individualized treatment of hypothalamic obesity following craniopharyngioma and other suprasellar tumors: a systematic review. Endocr Rev. 2019;40(1):193‐235. [DOI] [PubMed] [Google Scholar]
- 14. McCormack SE, Blevins JE, Lawson EA. Metabolic effects of oxytocin. Endocr Rev. 2020;41(2):121‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Daubenbuchel AM, Hoffmann A, Eveslage M, et al. Oxytocin in survivors of childhood-onset craniopharyngioma. Endocrine. 2016;54(2):524‐531. [DOI] [PubMed] [Google Scholar]
- 16. Zhang H, Wu C, Chen Q, et al. Treatment of obesity and diabetes using oxytocin or analogs in patients and mouse models. PLoS One. 2013;8(5):e61477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hsu EA, Miller JL, Perez FA, Roth CL. Oxytocin and naltrexone successfully treat hypothalamic obesity in a boy post-craniopharyngioma resection. J Clin Endocrinol Metab. 2018;103(2):370‐375. [DOI] [PubMed] [Google Scholar]
- 18. Lustig RH, Hinds PS, Ringwald-Smith K, et al. Octreotide therapy of pediatric hypothalamic obesity: a double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2003;88(6):2586‐2592. [DOI] [PubMed] [Google Scholar]
- 19. Liu CM, Davis EA, Suarez AN, Wood RI, Noble EE, Kanoski SE. Sex differences and estrous influences on oxytocin control of food intake. Neuroscience. 2020;447:63‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lawson EA, Marengi DA, DeSanti RL, Holmes TM, Schoenfeld DA, Tolley CJ. Oxytocin reduces caloric intake in men. Obesity (Silver Spring. 2015;23(5):950‐956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blevins JE, Graham JL, Morton GJ, et al. Chronic oxytocin administration inhibits food intake, increases energy expenditure, and produces weight loss in fructose-fed obese rhesus monkeys. Am J Physiol Regul Integr Comp Physiol. 2015;308(5):R431‐R438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Muin DA, Wolzt M, Marculescu R, et al. Effect of long-term intranasal oxytocin on sexual dysfunction in premenopausal and postmenopausal women: a randomized trial. Fertil Steril. 2015;104(3):715‐723.e4. [DOI] [PubMed] [Google Scholar]
- 23. Einfeld SL, Smith E, McGregor IS, et al. A double-blind randomized controlled trial of oxytocin nasal spray in Prader Willi syndrome. Am J Med Genet A. 2014;164A(9):2232‐2239. [DOI] [PubMed] [Google Scholar]
- 24. Yatawara CJ, Einfeld SL, Hickie IB, Davenport TA, Guastella AJ. The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: a randomized clinical crossover trial. Mol Psychiatry. 2016;21(9):1225‐1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roth CL, Eslamy H, Werny D, et al. Semiquantitative analysis of hypothalamic damage on MRI predicts risk for hypothalamic obesity. Obesity (Silver Spring). 2015;23(6):1226‐1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Perez FA, Elfers C, Yanovski JA, Shoemaker AH, Abuzzahab MJ, Roth CL. MRI Measures of hypothalamic injury are associated with glucagon-like peptide-1 receptor agonist treatment response in people with hypothalamic obesity. Diabetes Obes Metab. 2021;23(7):1532‐1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC Growth charts: United States. Adv Data. 2000;(7):1‐27. [PubMed] [Google Scholar]
- 28. Lohman T, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Human Kinetics Books; 1988. [Google Scholar]
- 29. Plessow F, Marengi DA, Perry SK, Lawson EA. Oxytocin administration increases proactive control in men with overweight or obesity: a randomized, double-blind, placebo-controlled crossover study. Obesity (Silver Spring). 2021;29(1):56‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bartholdy S, Dalton B, O'Daly OG, Campbell IC, Schmidt U. A systematic review of the relationship between eating, weight and inhibitory control using the stop signal task. Neurosci Biobehav Rev. 2016;64:35‐62. [DOI] [PubMed] [Google Scholar]
- 31. Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform. 1984;10(2):276‐291. [DOI] [PubMed] [Google Scholar]
- 32. Logan GD, Van Zandt T, Verbruggen F, Wagenmakers EJ. On the ability to inhibit thought and action: general and special theories of an act of control. Psychol Rev. 2014;121(1):66‐95. [DOI] [PubMed] [Google Scholar]
- 33. Dykens EM, Maxwell MA, Pantino E, Kossler R, Roof E. Assessment of hyperphagia in Prader-Willi syndrome. Obesity (Silver Spring, Md). 2007;15(7):1816‐1826. [DOI] [PubMed] [Google Scholar]
- 34. Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29(1):71‐83. [DOI] [PubMed] [Google Scholar]
- 35. Zemel BS, Leonard MB, Kelly A, et al. Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab. 2010;95(3):1265‐1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Neuro-QoL Technical Report. March 2015.
- 37. National Institute of Neurological Disorders and Stroke (NINDS) . User Manual for the Quality of Life in Neurological Disorders (Neuro-QoL) Measures. March 2015.
- 38. Epstein NB, Baldwin LM, Bishop DS. The McMaster family assessment device. J Marital Family Therapy. 1983;9(2):171‐180. [Google Scholar]
- 39. Institute NC . Common Terminology Criteria for Adverse Events v.3.0 and v.4.0 (CTCAE). Accessed January 7, 2016. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm
- 40. Greenhill LL, Vitiello B, Fisher P, et al. Comparison of increasingly detailed elicitation methods for the assessment of adverse events in pediatric psychopharmacology. J Am Acad Child Adolesc Psychiatry. 2004;43(12):1488‐1496. [DOI] [PubMed] [Google Scholar]
- 41. MacDonald E, Dadds MR, Brennan JL, Williams K, Levy F, Cauchi AJ. A review of safety, side-effects and subjective reactions to intranasal oxytocin in human research. Psychoneuroendocrinology. 2011;36(8):1114‐1126. [DOI] [PubMed] [Google Scholar]
- 42. Hocking MC, Hobbie WL, Deatrick JA, Hardie TL, Barakat LP. Family functioning mediates the association between neurocognitive functioning and health-related quality of life in young adult survivors of childhood brain tumors. J Adolesc Young Adult Oncol. 2015;4(1):18‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mong S, Pomeroy SL, Cecchin F, Juraszek A, Alexander ME. Cardiac risk after craniopharyngioma therapy. Pediatric Neurol. 2008;38(4):256‐260. [DOI] [PubMed] [Google Scholar]
- 44. Muller HL, Gebhardt U, Teske C, et al. Post-operative hypothalamic lesions and obesity in childhood craniopharyngioma: results of the multinational prospective trial KRANIOPHARYNGEOM 2000 after 3-year follow-up. Eur J Endocrinol. 2011;165(1):17‐24. [DOI] [PubMed] [Google Scholar]
- 45. Anekonda VT, Thompson BW, Ho JM, et al. Hindbrain administration of oxytocin reduces food intake, weight gain and activates catecholamine neurons in the hindbrain nucleus of the solitary tract in rats. J Clin Med. 2021;10(21):5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Alaerts K, Steyaert J, Vanaudenaerde B, Wenderoth N, Bernaerts S. Changes in endogenous oxytocin levels after intranasal oxytocin treatment in adult men with autism: an exploratory study with long-term follow-up. Eur Neuropsychopharmacol. 2021;43:147‐152. [DOI] [PubMed] [Google Scholar]
- 47. Blevins JE, Baskin DG. Translational and therapeutic potential of oxytocin as an anti-obesity strategy: insights from rodents, nonhuman primates and humans. Physiol Behav. 2015;152(Pt B):438‐449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kou J, Lan C, Zhang Y, et al. In the nose or on the tongue? Contrasting motivational effects of oral and intranasal oxytocin on arousal and reward during social processing. Transl Psychiatry. 2021;11(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lee MR, Scheidweiler KB, Diao XX, et al. Oxytocin by intranasal and intravenous routes reaches the cerebrospinal fluid in rhesus macaques: determination using a novel oxytocin assay. Mol Psychiatry. 2018;23(1):115‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ishii D, Kageyama M, Umeda S. Cerebral and extracerebral distribution of radioactivity associated with oxytocin in rabbits after intranasal administration: comparison of TTA-121, a newly developed oxytocin formulation, with syntocinon. PloS one. 2021;16(12):e0261451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Modi ME, Connor-Stroud F, Landgraf R, Young LJ, Parr LA. Aerosolized oxytocin increases cerebrospinal fluid oxytocin in rhesus macaques. Psychoneuroendocrinology. 2014;45:49‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dykens EM, Miller J, Angulo M, et al. Intranasal carbetocin reduces hyperphagia in individuals with Prader-Willi syndrome. JCI Insight. 2018;3(12):e98333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5(6):514‐516. [DOI] [PubMed] [Google Scholar]
- 54. Vankrieken L, Godart A, Thomas K. Oxytocin determination by radioimmunoassay. Gynecol Obstet Invest. 1983;16(3):180‐185. [DOI] [PubMed] [Google Scholar]
- 55. Geschwind DH. Oxytocin for autism spectrum disorder - down, but not out. N Engl J Med. 2021;385(16):1524‐1525. [DOI] [PubMed] [Google Scholar]
- 56. Leng G, Sabatier N. Measuring oxytocin and vasopressin: bioassays, immunoassays and random numbers. J Neuroendocrinol. 2016;28(10):10.1111/jne.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lefevre A, Mottolese R, Dirheimer M, Mottolese C, Duhamel JR, Sirigu A. A comparison of methods to measure central and peripheral oxytocin concentrations in human and non-human primates. Sci Rep. 2017;7(1):17222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Valstad M, Alvares GA, Egknud M, et al. The correlation between central and peripheral oxytocin concentrations: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2017;78:117‐124. [DOI] [PubMed] [Google Scholar]
- 59. Tabak BA, Leng G, Szeto A, et al. Advances in human oxytocin measurement: challenges and proposed solutions. Mol Psychiatry. 2023;28(1):127‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Damen L, Grootjen LN, Juriaans AF, et al. Oxytocin in young children with Prader-Willi syndrome: results of a randomized, double-blind, placebo-controlled, crossover trial investigating 3 months of oxytocin. Clin Endocrinol (Oxf). 2021;94(5):774‐785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hollander E, Levine KG, Ferretti CJ, et al. Intranasal oxytocin versus placebo for hyperphagia and repetitive behaviors in children with Prader-Willi syndrome: a randomized controlled pilot trial. J Psychiatr Res. 2021;137:643‐651. [DOI] [PubMed] [Google Scholar]
- 62. Hoffmann A, Postma FP, Sterkenburg AS, Gebhardt U, Muller HL. Eating behavior, weight problems and eating disorders in 101 long-term survivors of childhood-onset craniopharyngioma. J Pediatr Endocrinol Metab. 2015;28(1-2):35‐43. [DOI] [PubMed] [Google Scholar]
- 63. Bishop-Gilyard CT, Berkowitz RI, Wadden TA, Gehrman CA, Cronquist JL, Moore RH. Weight reduction in obese adolescents with and without binge eating. Obesity (Silver Spring). 2011;19(5):982‐987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Craven M, Crowley JH, Chiang L, et al. A survey of patient-relevant outcomes in pediatric craniopharyngioma: focus on hypothalamic obesity. Front Endocrinol (Lausanne). 2022;13:876770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Daughters K, Manstead ASR, Rees DA. Hypopituitarism is associated with lower oxytocin concentrations and reduced empathic ability. Endocrine. 2017;57(1):166‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Aulinas A, Plessow F, Asanza E, et al. Low plasma oxytocin levels and increased psychopathology in hypopituitary men with diabetes insipidus. J Clin Endocrinol Metab. 2019;104(8):3181‐3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Eisenberg Y, Murad S, Casagrande A, et al. Oxytocin alterations and neurocognitive domains in patients with hypopituitarism. Pituitary. 2019;22(2):105‐112. [DOI] [PubMed] [Google Scholar]
- 68. Hoffmann A, Ozyurt J, Lohle K, Reichel J, Thiel CM, Muller HL. First experiences with neuropsychological effects of oxytocin administration in childhood-onset craniopharyngioma. Endocrine. 2017;56(1):175‐185. [DOI] [PubMed] [Google Scholar]
- 69. Li K, Nakajima M, Ibanez-Tallon I, Heintz N. A cortical circuit for sexually dimorphic oxytocin-dependent anxiety behaviors. Cell. 2016;167(1):60‐72.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sikich L, Kolevzon A, King BH, et al. Intranasal oxytocin in children and adolescents with autism spectrum disorder. N Engl J Med. 2021;385(16):1462‐1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Roth CL, Perez FA, Whitlock KB, et al. A phase 3 randomized clinical trial using once-weekly GLP-1 receptor agonist in adolescents and young adults with hypothalamic obesity. Diabetes Obes Metab. 2021;23(2):363‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Altszuler N, Hampshire J. Intranasal instillation of oxytocin increases insulin and glucagon secretion. Proc Soc Exp Biol Med. 1981;168(1):123‐124. [DOI] [PubMed] [Google Scholar]
- 73. Chiodera P, Coiro V, Camellini L, et al. Effect of pharmacological doses of oxytocin on insulin response to glucose in normal man. Hormone Res. 1984;20(2):150‐154. [DOI] [PubMed] [Google Scholar]
- 74. Brede S, Fehr S, Dalla-Man C, et al. Intranasal oxytocin fails to acutely improve glucose metabolism in obese men. Diabetes Obes Metab. 2019;21(2):424‐428. [DOI] [PubMed] [Google Scholar]
- 75. Acosta A, Camilleri M, Abu Dayyeh B, et al. Selection of antiobesity medications based on phenotypes enhances weight loss: a pragmatic trial in an obesity clinic. Obesity (Silver Spring). 2021;29(4):662‐671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Edwards MM, Nguyen HK, Dodson AD, et al. Effects of combined oxytocin and beta-3 receptor agonist (CL 316243) treatment on body weight and adiposity in male diet-induced obese rats. Front Physiol. 2021;12:725912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Camilleri M, Acosta A. Combination therapies for obesity. Metab Syndr Relat Disord. 2018;16(8):390‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kakouri A, Kanti G, Kapantais E, et al. New incretin combination treatments under investigation in obesity and metabolism: a systematic review. Pharmaceuticals (Basel). 2021;14(9):869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.