Abstract
Introduction:
This study compared the cleaning effectiveness of NeoNiTi, 2Shape and Revo_S rotary instruments.
Materials and Methods:
Fifty mandibular molar mesial roots were selected with an angle of curvature less than 20 degrees divided into three groups (n=15). Five samples were selected as negative control group. In all three systems, the final file was 25, 6%. The score of debris and smear layer in three thirds (coronal, middle and apical) of the root canal walls were evaluated using scanning electron microscopic (SEM) magnification. The data were analyzed using the Kruskal Wallis and Mann Whitney U tests for intergroup comparison (P≤0.05) and Freidman and Wilcoxon signed-rank test was employed for intragroup comparison (P≤0.05).
Results:
Residual debris of the 2Shape system in the apical region was significantly higher than the other two systems (P=0.039). Revo_S and 2Shape groups had significantly higher quantities of debris in the apical than the coronal region (P=0.029 and P=0.02, respectively). In the 2Shape group, the amount of mid-region debris was significantly higher (P=0.005) than the coronal. In inter-group comparison there was no significant difference in residual smear layer between the systems. In intra-group comparison in all three systems, the amount of smear layer in the coronal third was significantly higher than in the other two areas. (P=0.017, P<0.001 and P=0.032, respectively).
Conclusion:
2Shape left the highest amount of debris in the apical region. The amount of debris in Revo_S and 2Shape groups in the apical region was significantly higher than in the coronal. The amount of smear layer in all three groups in the coronal area was higher than the middle and apical areas.
Key Words: Root Canal Preparation, Scanning Electron Microscopy, Smear Layer
Introduction
Debris is defined as dentin chips, pulp remnants, and particles loosely attached to the root canal wall [1]. During cleaning and shaping, organic components of pulp tissue and inorganic dentinal debris accumulate on the radicular canal wall, producing an amorphous, irregular smear layer. Apical extrusion of debris is associated with symptomatic apical periodontitis [2]. Accumulation of debris may prevent adequate sealing during root canal filling, and in cases with apical periodontitis reduces the chance of healing [3]. The residual smear layer on the root canal walls may become disintegrated or removed by bacterial byproducts allowing leakage [4]. However according to another concept, the smear layer may prevent bacteria from penetrating the dentin tubules and its contamination [5]. It prevents detergents and sealers from penetrating tubules [6]. Based on a review by Torabinejad et al. [7] the smear layer dramatically affects the micro leakage of apical and coronal areas and consequently the long-term success of the treatment. Therefore, this layer of organic and inorganic materials must be removed before filling the root canal.
For successful root canal treatment, the root canal system needs to be properly cleaned, shaped and filled, while maintaining the original shape with no displacement in the canal path [8, 9]. Rotary nickel-titanium (NiTi) instruments are more flexible than stainless steel files and reduce procedural errors. But there is little information about their cleaning efficacy [10]. There is still no instrumentation technique that can completely clean the root canal system [11]. With further investigation, we can find a system with better cleaning efficiency.
Alobaidy et al. [12] compared the cleaning efficiency of 2Shape, HyFlex EDM, and Protaper Gold rotary systems in removing debris and found that 2Shape system was significantly less effective than HyFlex EDM and Protaper Gold systems in eliminating debris at all root canal levels. Lubna Afreen et al. [13] compared the smear removal efficacy of different rotary files and found that Revo_S gave the best and EDM the poorest results. Feghali et al. [14] found that the mean amount of smear layer was not significantly different with WaveOne Gold and Reciproc Blue in three_thirds of the root canal. Both systems showed more debris and smear layer in the apical third; nevertheless, WaveOne Gold showed better results. Chatterjee et al. [15] evaluated the debris and smear layer formation using three NiTi rotary instruments and found that the highest amount of debris and smear layer was formed by ProTaper Universal rotary files at all root canal levels (cervical, middle and apical thirds) and the least amount by XP Endo file system.
NeoNiTi, 2Shape, and Revo_S rotary instruments have been introduced for root canal preparation. According to the manufacturer, 2Shape (Micro-Mega, Besancon, France) file has an asymmetrical cross-section that offers superior cleaning of the root canal walls with two main and one secondary cutting edge. These edges increase the cutting efficiency of the file and improve debris removal [16]. Three asymmetrical blades of Revo_S (Micro Mega, Besancon, France) cross-section reduce the contact length of the blades on the dentinal walls thus reducing the production of debris and smear layer and increasing the available volume for irrigating solutions and upward debris elimination [17]. Moreover, fewer instruments may negatively affect the production of the smear layer [18]. NeoNiTi (Neolix, Chatres-la-Foret, France) is manufactured with a wire-cut electrical discharge machining (WEDM) process and is used to prepare the root canal system [19]. Furthermore, it produces a rough surface, resulting in abrasive properties that enhance the speed of root canal preparation [19, 20]. The advantages of this (EDM) technique of the NeoNiTi file system are minimal residual stresses, a high level of accuracy, and more advanced surface finishing [21, 22].
This study has focused on electron microscopic evaluation of cleaning efficiency or comparative evaluation of residual debris and smear layer on the mesiobuccal canal walls of extracted mandibular molars after preparation with NeoNiTi (Neolix, Chatres-la-Foret, France), 2shape (MicroMega, Besancon, France) or Revo_S (Micro Mega, Besancon, France) in coronal, middle and apical levels.
The null hypothesis was that there would be no difference among the three instruments in terms of residual smear layer and debris on the root canal walls.
Materials and Methods
Sample size calculation and tooth preparation
This ex-vivo study was approved in the Research Ethics Committee of Shahid Beheshti University of medical sciences, Tehran, Iran (IR.SBMU.DRC.REC.1399.029).
Based on a previous research (Hulsaman et al. [1]), the study power of 0.9 and an alpha-type error of 0.05 were considered for the sample size calculation. The estimated sample size was 15 teeth in each group.
A total of fifty freshly extracted human mandibular molars were selected and the degree of mesial root curvature was determined by the Schneider technique using a Planmeca X-Ray viewer (Planmeca, Helsinki, Finland) software [23, 24]. The mesio-buccal, mature roots with an angle of curvature of fewer than 20 degrees were included. Teeth were evaluated with magnification and radiographed from both the mesial and buccal sides to assess canal morphology. Teeth with caries, fracture, restorations, resorption, calcification or an immature apex were not included.
Teeth were disinfected with 5.25% NaOCl and kept in 0.5% chlorhexidine solution. Before the preparation, samples were decoronated using a diamond-coated bur supplemented with water cooling to standardize the working length to 15 mm. A standard manual K-File (Mani, Utsunomiya, Japan) size 10 was passed through the canal to investigate the canal path, calcification or pulp stone. Teeth with sclerotic canals, aberrations, apical diameter greater than size 15, or with an altered apex were not included.
A plastic tube was fixed on the coronal part of all roots to create a constant reservoir for irrigation. Finally, a small amount of Carbowax (DOW Chemical Co., Midland, MI, USA) was placed on each root tip to create a closed irrigation system.
Root canal instrumentation
Samples were randomly assigned into three groups (NeoNiTi, 2Shape, Revo_S; n=15 each), and five teeth were considered negative control group with no preparation.
Group 1 : NeoNiTi (Neolix, France) full sequence rotary instrumentation: Rotary instrumentation in the continuous rotation was accomplished in a crown down approach using C1 (25/.12( at the coronal third of the canal, GPS )15/.03( to measure the glide path and check the canal patency, and A1 )25/.06( for shaping canal at WL in a torque-controlled system (Endo-mate DT engine; NSK MIO, Tokyo, Japan) set at 400 rpm and 1.5 N/cm torque.
Group2: 2shape (Micro Mega, France) files were used in a rotational motion. The root canal preparation was performed in a crown-down technique using TS1 and TS2 in a continuous rotary clockwise motion. The progressive movement in three waves (3 up and down movements) with upward circumferential movement was performed. The speed of rotation was set at 300 rpm and torque at 2.5 N/cm as recommended by MICRO-MEGA.
Group 3 : Revo _S (Micro Mega, France) files were used in a rotational motion and crown down technique. SC1 was employed at 2/3rd of WL, SC2 and SCU at WL. The speed of rotation was set at 300 rpm and torque at 2.5 N/cm as recommended by MICRO-MEGA.
Canal preparation was carried out by a single operator. Each root canal was initially negotiated with #10 stainless steel K-file (Mani K-files, Utsunomiya, Japan) until the file was barely visible through the apex, then one mm was subtracted to establish the working length.
The canals were instrumented with NeoNiTi (Neolix, France), 2Shape (Micro-Mega, France) and Revo_S (Micro-Mega, France) according to the manufacturer’s instructions. The files were inserted into the canal and activated using the NSK Endo-Mate DT motor (NSK MIO, Tokyo, Japan) at 250-400 rpm, applying quick pecking motions with slight apical pressure for five seconds. This was repeated until the instrument reached the working length, and the instrument was removed from the canal while it was rotating. Each rotary file was used for a single canal and then discarded. Root canals were irrigated with 5 mL of 5.25% NaOCl (Chloraxid, Cerkamed, Poland) using a single side vented beveled needle of 30 gauge (Tribest, Jiangsu, China) placed 3 mm short of the working length after each instrument. After the final file, the canal was rinsed with 5 mL of normal saline (Samen Industries, Mashad, Iran), then with 5 mL of 17% EDTA solution (Meta Biomed, Korea) and 5 mL of normal saline again.
Specimen preparation for scanning electron microscopy
Two longitudinal grooves were prepared on the mesial and distal surfaces of the mesial root with a handpiece (NSK MIO, Tokyo, Japan) and a diamond disk (Sybron Endo, Anaheim, USA). The samples were longitudinally split immediately after instrumentation using vacuum, and examined with scanning electron microscopy (SEM) a chisel and mallet by striking on the longitudinal grooves made initial to the instrumentation.
Specimens were desiccated in ethanol with ascending concentrations, sputter coated with gold platinum under a scanning electron microscope (SEM) (TESCAN VEGA II LMU, Czech Republic). In each specimen, images were taken in three regions: 3 mm (apical third), 7 mm (middle third) and 11 mm (coronal third) from the apical foramen, and the canal cleanliness was blindly evaluated by two calibrated investigators using a 5-point scoring system as described previously [1]. Two endodontists were calibrated before observing the main samples by observing 20 debris samples and 20 smear samples. Calibration was repeated after five days and two observers scored separately. In case of disagreement, the third endodontist was asked for help. Briefly, debris and smear scores were evaluated on 500 and 2000 magnified images, respectively.
The following scale was used:
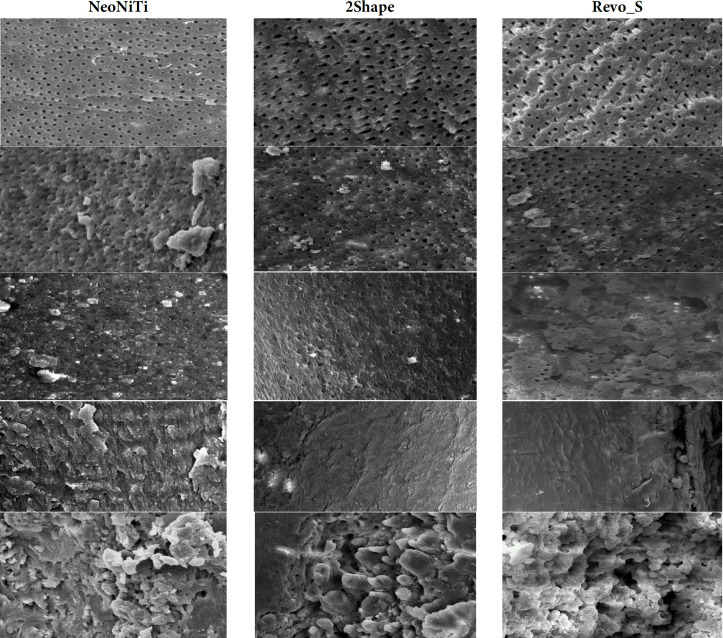
Indices of debris dispersion (Figure 1):
Figure 1.
500× magnification electron microscope images for debris examination (each column represents a file and the rows represent a score based on the Hulsman classification)
Score 1: Root canal walls were clean; only a few debris particles
Score 2: Few conglomerations of debris
Score 3: Many conglomerations of debris covered less than 50% of canal walls
Score 4: Debris covered more than 50% of canal walls
Score 5: Debris covered complete or nearly complete surfaces of canal walls
Indices of smear layer dispersion (Figure 2):
Figure 2.
2000× magnification electron microscope images for smear layer examination (each column represents a file and the rows represent a score based on the Hulsman classification
Score 1: No smear layer, all dentinal tubules open
Score 2: Small amount of smear layer, some dentinal tubules open
Score 3: Homogeneous smear layer coverage, few dentinal tubules open
Score 4: Homogeneous smear layer coverage, no open dentinal tubules
Score 5: Thick and inhomogeneous smear layer cover the entire root canal walls
Statistical analyses
Data were systematically collected and analyzed using SPSS software (SPSS Version 20; SPSS Inc, Chicago, USA). Cohen's kappa coefficient was used to measure inter-rater and also intra-rater reliability. For intergroup comparison, the data were statistically analyzed using the Kruskal-Wallis test and the Mann-Whitney U test, intra-group comparisons were statistically analyzed using Friedman and Wilcoxon signed-rank test, and the significance was set at P=0.05.
Results
Tables 1 and 2 gives a detailed summary of the debris and smear layer scores. The bar-graphs (Figures 3 and 4) show the results for each parameter amongst the three groups and at the three radicular canal areas (coronal, middle, and apical thirds).
Table 1.
Mean score for debris at the coronal, middle, apical thirds of the canals
| S | C | M | A |
|---|---|---|---|
| NNT | 28.03Aa | 23.63Aa | 19.60Aa |
| R_s | 21.13Aa | 22.37a | 23.80B |
| 2s | 23.20Aa | 27.97Ba | 29.60b |
| C | 37.90Aa | 33.10Aa | 36.00Ab |
| P- | 0.103 | 0.394 | 0.039 |
Values with the same superscript letters were not statistically different at P=0.05. Capital letters are for intragroup comparison and small letters are for intergroup comparison
Table 2.
Mean score for smear layer at the coronal, middle, and apical thirds of the canals
| S | C | M | A |
|---|---|---|---|
| NNT | 28.00B | 22.57A | 18.97Aa |
| R_S | 20.07A | 22.00A | 25.23 |
| 2S | 26.33A | 27.40B | 27.63 |
| C | 31.80A | 39.10A | 39.50Ab |
| P- | 0.213 | 0.090 | 0.033 |
Values with the same superscript letters were not statistically different at P = 0.05. Capital letters are for intragroup comparison and small letters are for intergroup comparison
Figure 3.
Distribution of debris in apical, middle and coronal thirds in NeoNiTi, 2Shape, Revo_S and control groups (colors show scores based on Hulsman criteria and the numbers in each column indicate the percentage prevalence of scores)
Figure 4.
Distribution of smears in 3 apical, middle andoronal thirds in NeoNiTi, 2Shape, Revo_S and control groups (colors show scores based on Hulsman criteria and the numbers in each column indicate the percentage prevalence of score)
Intergroup comparison of debris
Intergroup comparisons using the Kruskal-Wallis test revealed a statistically significant difference in debris score only at apical thirds (P=0.039) of the groups. Mann-Whitney U test showed no significant difference between the debris score of NeoNiTi and Revo_S (P=0.508). However, NeoNiTi left significantly less amount of debris in comparison with 2Shape (P=0.03) and control groups (0.024) in the apical third.
There was no statistically significant difference between the other groups in the apical region (Figure 3).
Intergroup comparison of smear layer
Intergroup comparisons using the Kruskal-Wallis test revealed a statistically significant difference in smear layer score at the apical third (P=0.033) of the groups compared. Despite that, there was no significant difference at the middle (0.09) and coronal (0.213) thirds.
Mann-Whitney U test showed no significant difference between the smear score of NeoNiTi and Revo_S (P=0.289) and also with 2Shape (0.055), but NeoNiTi left significantly less amount of smear layer in comparison with the control group (0.018) in the apical third.
There was no statistically significant difference in the amount of apical smear layer between the control and Revo_S (0.071) and between 2 Shape and control group (0.057) (Figure 4).
Intra group comparison of debris
The Intragroup comparisons were carried out using the Friedman test for the debris revealing a statistically significant difference between three thirds in Revo_S (P=0.022) and in 2Shape (P=0.001). Wilcoxon Rank test showed statistically less debris in the coronal third in comparison with the apical part by Revo S (0.029) and 2Shape (0.02); likewise, there was a significant difference between the coronal and middle third in 2Shape system with less debris in the coronal third (P=0.005). There was no statistically significant difference between the apical, middle and coronal regions by NeoNiTi system (P=0.416).
Intra-group comparison of smear layer
The Intragroup comparisons using the Friedman test for the smear layer showed a statistically significant difference between three thirds in Revo_S (P=0.032), in 2Shape (P=0.017) and in NeoNiTi (P<0.001). Wilcoxon Rank test revealed statistically more smear layer in the coronal third compared with the middle part by 2Shape (0.008). In the NeoNiTi system, there was significantly more smear layer in the coronal in comparison with the middle (P<0.001) and apical thirds (P=0.003).
Discussion
The smear layer is a superficial film of dentin particles and vital or necrotic pulp remnants that are produced when a canal is instrumented [25]. Debris was defined as dentin chips, and vital or necrotic pulp remnants loosely attached to the canal walls [1]. It is considered to be desirable to remove this layer due to its potential deleterious effects [26]. The adverse effect of the smear layer and debris becomes apparent when microorganisms remain inside this layer [27]. In addition, the presence of this layer in dentin tubules may prevent sealants from penetrating the tubules [6, 28].
A way to evaluate the clearance of different systems is to use acrylic blocks that allow standardization of root canal diameter, root canal length, and canal curvature radius. However, the hardness and abrasion of acrylic resin and dentin may not be the same, so we selected human-extracted mandibular first molars for this study. The selected teeth in all three groups were similar in angle, length and dimensions. Only mesio-buccal canal of all the samples was instrumented for standardization. The mesio-buccal canal of the mandibular molar is very narrow apically and usually presents with an apical curvature [29]. However, teeth may show differences in dentin stiffness and root canal morphology, but it seems acceptable to evaluate the cleaning ability of a preparation technique as it has been used in previous studies [14, 30].
There are different methods for assessing the amount of canal clearance, including histology and Micro-CT which are not able to assess the amount of smear layer. Another method is SEM, which has high sensitivity and specificity for assessing the degree of canal clearance. SEM is the most widely used method to evaluate smear layer removal [31-33].
Efficient cleaning does not necessarily depend on the type of instrument or technique used. To remove debris and smear layer, chemical rinsing solutions with mechanical instrumentation are recommended [34, 35]. The irrigation protocol hereby used was 5.25% NaOCl alternated with EDTA 17% delivered using a single side vented needle of 30 gauge (Tribest, Jiangsu, China). The final rinse was performed with a normal saline solution to neutralize any erosion after using EDTA [36]. The same protocol of irrigation with equal volume and concentration was used while preparing the samples.
Mechanical preparation of the canals was carried out using NeoNiTi (#25-6%), 2Shape (#25-6%) and Revo_S (#25-6%) in clockwise rotational motion. A limited number of studies have evaluated the cleaning efficiency of NeoNiTi, 2Shape and Revo_S, also they are popular and available systems in Iran. The 2Shape and Revo-S systems have an asymmetric design that reduces instrument stress during canal preparation by minimizing interference between files and dentin and increasing the volume available for upward debris removal [37, 38]. NoNiTi was selected because has resulted in good debridement of canals in an earlier study [39].
These systems have different cross-sections and different numbers of preparation devices. However, to eliminate the interfering factor of size and final tapering of clearance, the final file for preparation in all three systems was size 25 with a tapering of 6%.
Advanced instrument designs such as increased taper, altered helical angle and cross-sections, non- cutting tip and specific flute design help to remove vital/necrotic tissue and infected dentin and debris but affect the cleaning effectiveness. There has been a rising trend in the use of NiTi files. NiTi files resulted in fewer procedural errors such as zipping, ledges, or transportation due to their superelasticity, compared with SS files [40, 41]. However, previous studies have shown that NiTi rotary files come into contact with 40% to 45% of the root canal walls during preparation, leaving most of the canal untouched [42, 43].
Instruments with radial land have inferior cleaning efficiency, due to their scraping action on the canal walls rather than a cutting action. Instruments with positive cutting angles may have a superior cleaning efficiency than those with neutral or negative cutting angles [44]. In this study, the amount of smear layer and debris remaining on the canal walls, in three thirds were compared by three different rotary systems Revo-S with three asymmetrical blades, 2Shape with asymmetrical cross-section and NeoNiTi with advanced surface finishing. Because none of the instruments used for canal preparation could produce a completely clean canal, this study was conducted with the aim of comparison between three rotary file systems (NeoNiTi, 2Shape and Revo_S).
In inter-group comparisons, the amount of debris left after using 2Shape file in the apical area was significantly higher than NeoNiTi and Revo_S files. There were similar results in a study by Alobaidy et al. [12] which evaluated the cleanup capabilities of 2Shape, Hyflex EDM, and Pro Taper Gold systems, and concluded that 2Shape's cleaning efficacy was less than that of ProTaper Gold files. The justification for this result could be the presence of a constant cross-section with three cutting blades across the length of the file in the 2Shape creates less room for debris to exit.
The intra-group comparison showed significantly more debris at apical third in 2Shape and Revo_S groups. These results confirm the findings reported by Zouiten et al. [45]. They found that Revo_S and CMA files had significantly more debris in apical third compared with coronal and middle third.
In this study, there was no significant difference in the amount of residual smear between groups in the three apical, middle and coronal thirds after using three rotary files Revo_S, 2Shape and NeoNiTi, which was in line with the results of Zouiten et al. [45]. In their study, they compared the cleaning efficacy of Revo_S and CMA files and found no significant difference between the two systems. Also, in the study by Poggio et al. [46] there was no significant difference in the cleaning efficacy of Mtwo and Revo_S systems.
In the present study, the amount of smear layer in the NeoNiTi group in the coronal area was significantly higher than in the middle and apical areas, and in the 2Shape group, the amount of smear layer in the coronal area was significantly higher than the middle area. This result may be due to the fact that the dentinal tubules become larger and wider from the apical to the coronal area and the amount of smear they contain is higher. This result is consistent with the study of Taha et al. [47]. In contrast to the present study, Zouiten et al. [39] compared the cleaning efficacy of Revo_S and CMA [45] and Dagna compared the cleaning efficacy of Mtwo, Revo-S and HyFlex CM, and found that the amount of smear layer and debris in the apical thirds is higher than the coronal and middle thirds. It was due to the differences in the file type, sample type and its curvature, concentration and the number of irrigants.
With three winning blades across the length of the file, the 2Shape probably leaves less space for debris to exit, while in a file such as Revo_S with different cross-sections, the file core is smaller thus more space remains for the exit of the debris. The authors found that the design of Revo_S files (with modified cross-section along file length, asymmetric-cross section and three cutting edges in different radii) can create, a snake-like movement of the file in the canal and also prepares flutes with more space near the shank than the apical area, which in turn leads to the easier exit of the debris from the coronal part. In the case of the NeoNiTi with the rectangular cross-section, the contact of the file with the canal wall is reduced and there is more space for the exit of the debris.
Regarding the limitations of this study, none of the instrument groups produced a canal surface free from debris and smear layer at all levels of root canals. The null hypothesis was rejected as there was a significant difference observable between the experimental groups in terms of debris and smear layer on canal walls.
Conclusion
In inter-group comparison, 2Shape system had the highest degree of debris in the apical region compared to the other two groups. There was no significant difference in the smear layer between the three systems. In comparison, the amount of debris in both Revo_S and 2 Shape systems in the apical region was significantly higher than in the coronal region.
The amount of smear layer in all three systems was higher in the coronal area than in the middle and apical areas.
Conflict of Interest:
‘None declared’.
References
- 1.Hülsmann M, Rümmelin C, Schäfers F. Root canal cleanliness after preparation with different endodontic handpieces and hand instruments: a comparative SEM investigation. J Endod. 1997;23(5):301–6. doi: 10.1016/S0099-2399(97)80410-4. [DOI] [PubMed] [Google Scholar]
- 2.Nevares G, Romeiro K, Albuquerque D, Xavier F, Fogel H, Freire L, Cunha R. Evaluation of Apically Extruded Debris during Root Canal Retreatment Using ProTaper Next and Reciproc in Severely Curved Canals. Iran Endod J. 2017;12(3):323–8. doi: 10.22037/iej.v12i3.15850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McComb D, Smith DC. A preliminary scanning electron microscopic study of root canals after endodontic procedures. J Endod. 1975;1(7):238–42. doi: 10.1016/S0099-2399(75)80226-3. [DOI] [PubMed] [Google Scholar]
- 4.Sen BH, Wesselink PR, Türkün M. The smear layer: a phenomenon in root canal therapy. Int Endod J. 1995;28(3):141–8. doi: 10.1111/j.1365-2591.1995.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 5.Wang Z, Shen Y, Haapasalo M. Effectiveness of endodontic disinfecting solutions against young and old Enterococcus faecalis biofilms in dentin canals. J Endod. 2012;38(10):1376–9. doi: 10.1016/j.joen.2012.06.035. [DOI] [PubMed] [Google Scholar]
- 6.Kokkas AB, Boutsioukis A, Vassiliadis LP, Stavrianos CK. The influence of the smear layer on dentinal tubule penetration depth by three different root canal sealers: an in vitro study. J Endod. 2004;30(2):100–2. doi: 10.1097/00004770-200402000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Torabinejad M, Handysides R, Khademi AA, Bakland LK. Clinical implications of the smear layer in endodontics: a review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94(6):658–66. doi: 10.1067/moe.2002.128962. [DOI] [PubMed] [Google Scholar]
- 8.Estrela C, Holland R, Estrela CR, Alencar AH, Sousa-Neto MD, Pécora JD. Characterization of successful root canal treatment. Braz Dent J. 2014;25(1):3–11. doi: 10.1590/0103-6440201302356. [DOI] [PubMed] [Google Scholar]
- 9.Silva EJ, Tameirão MD, Belladonna FG, Neves AA, Souza EM, De-Deus G. Quantitative Transportation Assessment in Simulated Curved Canals Prepared with an Adaptive Movement System. J Endod. 2015;41(7):1125–9. doi: 10.1016/j.joen.2015.02.028. [DOI] [PubMed] [Google Scholar]
- 10.Gavini G, Santos MD, Caldeira CL, Machado MEL, Freire LG, Iglecias EF, Peters OA, Candeiro GTM. Nickel-titanium instruments in endodontics: a concise review of the state of the art. Braz Oral Res. 2018;32(suppl 1) doi: 10.1590/1807-3107bor-2018.vol32.0067. [DOI] [PubMed] [Google Scholar]
- 11.Dalton BC, Orstavik D, Phillips C, Pettiette M, Trope M. Bacterial reduction with nickel-titanium rotary instrumentation. J Endod. 1998;24(11):763–7. doi: 10.1016/S0099-2399(98)80170-2. [DOI] [PubMed] [Google Scholar]
- 12.Alobaidy SS, Shukri BMS. Evaluation of the Cleaning Efficiency of 2 Shape, Hyflex EDM and Pro Taper GOLD Systems Using Digital Image Morphometric Analysis (An in Vitro Study) J Forensic Med. 2020:14:2584–91. [Google Scholar]
- 13.Afreen L, Chandra R, Jain J, Mehrotra A. Comparative evaluation of removal of smear layer using newer rotary endodontic files: A scanning electron microscope study. J Conserve Dent. 2021;24(6):616–21. doi: 10.4103/jcd.jcd_92_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feghali M, Jabbour E, Koyess E, Sabbagh J. Scanning electron microscopy evaluation of debris and smear layer generated by two instruments used in reciprocating motion WaveOne Gold® and Reciproc Blue®. Aust Endod J. 2019;45(3):388–93. doi: 10.1111/aej.12338. [DOI] [PubMed] [Google Scholar]
- 15.Chatterjee S, Desai PD, Mukherjee S, Mazumdar P, Sengupta P. Evaluation of debris and smear layer formation using three different NI-TI rotary instrument systems: An in vitro scanning electron microscope study. J Conserv Dent. 2021;24(6):568–75. doi: 10.4103/JCD.JCD_510_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.2Shape. Sequence of 2 shaping instruments in continuous rotation. 2017. Available from: https://micro-mega.com/shaping/2shape/?lang=en.
- 17.Xu X, Eng M, Zheng Y, Eng D. Comparative study of torsional and bending properties for six models of nickel-titanium root canal instruments with different cross-sections. J Endod. 2006;32(4):372–5. doi: 10.1016/j.joen.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Poggio C, Dagna A, Chiesa M, Beltrami R, Bianchi S. Cleaning Effectiveness of Three NiTi Rotary Instruments: A Focus on Biomaterial Properties. J Funct Biomater. 2015:2015:66–76. doi: 10.3390/jfb6010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aminsobhani M, Meraji N, Sadri E. Comparison of Cyclic Fatigue Resistance of Five Nickel Titanium Rotary File Systems with Different Manufacturing Techniques. J Dent (Tehran) 2015;12(9):636–46. [PMC free article] [PubMed] [Google Scholar]
- 20.Moazzami F, Khojastepour L, Nabavizadeh M, Seied Habashi M. Cone-Beam Computed Tomography Assessment of Root Canal Transportation by Neoniti and Reciproc Single-File Systems. Iran Endod J. 2016;11(2):96–100. doi: 10.7508/iej.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han F, Jiang J, Yu D. Influence of discharge current on machined surfaces by thermo-analysis in finish cut of WEDM. Int J Mach Tools Manuf. 2007;47(7):1187–96. [Google Scholar]
- 22.Labbaf H, Nazari Moghadam K, Shahab S, Mohammadi Bassir M, Fahimi MA. An In vitro Comparison of Apically Extruded Debris Using Reciproc, ProTaper Universal, Neolix and Hyflex in Curved Canals. Iran Endod J. 2017;12(3):307–11. doi: 10.22037/iej.v12i3.13540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneider SW. A comparison of canal preparations in straight and curved root canals. Oral Surg Oral Med Oral Pathol. 1971;32(2):271–5. doi: 10.1016/0030-4220(71)90230-1. [DOI] [PubMed] [Google Scholar]
- 24.Malur MH, Chandra A. Curvature height and distance of MB canal of mandibular molar with Schneider angle and its comparison with canal access angle. Journal of oral biology and craniofacial research. 2018;8(3):212–6. doi: 10.1016/j.jobcr.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grandini S, Balleri P, Ferrari M. Evaluation of Glyde File Prep in combination with sodium hypochlorite as a root canal irrigant. J Endod. 2002;28(4):300–3. doi: 10.1097/00004770-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Shahravan A, Haghdoost AA, Adl A, Rahimi H, Shadifar F. Effect of smear layer on sealing ability of canal obturation: a systematic review and meta-analysis. J Endod. 2007;33(2):96–105. doi: 10.1016/j.joen.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Bidar M, Moradi S, Forghani M, Bidad S, Azghadi M, Rezvani S, Khoynezhad S. Microscopic evaluation of cleaning efficiency of three different nickel-titanium rotary instruments. Iran Endod J. 2010;5(4):174–8. [PMC free article] [PubMed] [Google Scholar]
- 28.Kuçi A, Alaçam T, Yavaş O, Ergul-Ulger Z, Kayaoglu G. Sealer penetration into dentinal tubules in the presence or absence of smear layer: a confocal laser scanning microscopic study. J Endod. 2014;40(10):1627–31. doi: 10.1016/j.joen.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 29.Vertucci F. Root canal morphology and its relationship to endodontic procedure. Endod Topics. 2005:10:3–29. [Google Scholar]
- 30.Karade P, Chopade R, Patil S, Hoshing U, Rao M, Rane N, chopade A, Kulkarni A. Efficiency of Different Endodontic Irrigation and Activation Systems in Removal of the Smear Layer: A Scanning Electron Microscopy Study. Iran Endod J. 2017;12(4):414–8. doi: 10.22037/iej.v12i4.9571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh N, Chandra A, Tikku AP, Verma P. A comparative evaluation of different irrigation activation systems on smear layer removal from root canal: An in-vitro scanning electron microscope study. J Conserv Dent. 2014;17(2):159–63. doi: 10.4103/0972-0707.128060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caron G, Nham K, Bronnec F, Machtou P. Effectiveness of different final irrigant activation protocols on smear layer removal in curved canals. J Endod. 2010;36(8):1361–6. doi: 10.1016/j.joen.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 33.Khademi A, Saatchi M, Shokouhi MM, Baghaei B. Scanning Electron Microscopic Evaluation of Residual Smear Layer Following Preparation of Curved Root Canals Using Hand Instrumentation or Two Engine-Driven Systems. Iran Endod J. 2015;10(4):236–9. doi: 10.7508/iej.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haapasalo M, Shen Y, Wang Z, Gao Y. Irrigation in endodontics. Br Dent J. 2014;216(6):299–303. doi: 10.1038/sj.bdj.2014.204. [DOI] [PubMed] [Google Scholar]
- 35.Metzger Z, Solomonov M, Kfir A. The role of mechanical instrumentation in the cleaning of root canals. Endod Topics. 2013:29. [Google Scholar]
- 36.Wang Z, Maezono H, Shen Y, Haapasalo M. Evaluation of Root Canal Dentin Erosion after Different Irrigation Methods Using Energy-dispersive X-ray Spectroscopy. J Endod. 2016;42(12):1834–9. doi: 10.1016/j.joen.2016.07.024. [DOI] [PubMed] [Google Scholar]
- 37.Ruddle CJ, Machtou P, West JD. The shaping movement: fifth-generation technology. Dent Today. 2013;32(4):94, 6–9. [PubMed] [Google Scholar]
- 38.Faisal I, Saif R, Alsulaiman M, Natto ZS. Shaping ability of 2Shape and NeoNiTi rotary instruments in preparation of curved canals using micro-computed tomography. BMC oral health. 2021;21(1):595. doi: 10.1186/s12903-021-01961-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dagna A, Gastaldo G, Beltrami R, Poggio C. Debris Evaluation after Root Canal Shaping with Rotating and Reciprocating Single-File Systems. J Funct Biomater. 2016;7:4. doi: 10.3390/jfb7040028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walia HM, Brantley WA, Gerstein H. An initial investigation of the bending and torsional properties of Nitinol root canal files. J Endod. 1988;14(7):346–51. doi: 10.1016/s0099-2399(88)80196-1. [DOI] [PubMed] [Google Scholar]
- 41.Liu SB, Fan B, Cheung GS, Peng B, Fan MW, Gutmann JL, Song YL, Fu Q, Bian Z. Cleaning effectiveness and shaping ability of rotary ProTaper compared with rotary GT and manual K-Flexofile. American journal of dentistry. 2006;19(6):353–8. [PubMed] [Google Scholar]
- 42.Zand V, Lotfi M, Rahimi S, Mokhtari H, Kazemi A, Sakhamanesh V. A comparative scanning electron microscopic investigation of the smear layer after the use of sodium hypochlorite gel and solution forms as root canal irrigants. J Endod. 2010;36(7) doi: 10.1016/j.joen.2010.02.033. [DOI] [PubMed] [Google Scholar]
- 43.Eshagh Saberi A, Ebrahimipour S, Saberi M. Apical Debris Extrusion with Conventional Rotary and Reciprocating Instruments. Iran Endod J. 2020;15(1):38–43. doi: 10.22037/iej.v15i1.23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Afreen L, Chandra R, Jain J, Mehrotra A. Comparative evaluation of removal of smear layer using newer rotary endodontic files: A scanning electron microscope study. J Conserv Dent. 2021;24(6):616–21. doi: 10.4103/jcd.jcd_92_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sonia Z, Jemâa M, Dagna A. Scanning Electron Microscopic Evaluation of Debris and Smear Layer after Use of Revo-S and CMA Instruments in Straight Root Canals. J Dent Oral Care. 2015;1 [Google Scholar]
- 46.Poggio C, Dagna A, Chiesa M, Beltrami R, Colombo M. Ultrastructural analysis of the root canal walls after preparation with two rotary nickel-titanium endodontic instruments. Contemporary Clinical Dentistry. 2014;5(3):357–60. doi: 10.4103/0976-237X.137948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taha NA, Ozawa T, Messer HH. Comparison of three techniques for preparing oval-shaped root canals. J Endod. 2010;36(3):532–5. doi: 10.1016/j.joen.2009.11.015. [DOI] [PubMed] [Google Scholar]