Abstract
Objective
To explore associations between early life physical and sexual abuse and subsequent risk of premature mortality (death before age 70 years).
Design
Prospective cohort study.
Setting
The Nurses’ Health Study II (2001-19).
Participants
67 726 female nurses aged 37-54 years when completing a violence victimization questionnaire in 2001.
Main outcome measures
Hazard ratios and 95% confidence intervals for total and cause specific premature mortality by childhood or adolescent physical and sexual abuse, estimated by multivariable Cox proportional hazard models.
Results
2410 premature deaths were identified over 18 years of follow-up. Nurses who experienced severe physical abuse or forced sexual activity in childhood and adolescence had a higher crude premature mortality rate than nurses without such abuse in childhood or adolescence (3.15 v 1.83 and 4.00 v 1.90 per 1000 person years, respectively). The corresponding age adjusted hazard ratios for premature deaths were 1.65 (95% confidence interval 1.45 to 1.87) and 2.04 (1.71 to 2.44), respectively, which were materially unchanged after further adjusting for personal characteristics and early life socioeconomic status (1.53, 1.35 to 1.74, and 1.80, 1.50 to 2.15, respectively). Cause specific analyses indicated that severe physical abuse was associated with a greater risk of mortality due to external causes of injury and poisoning (multivariable adjusted hazard ratio 2.81, 95% confidence interval 1.62 to 4.89), suicide (3.05, 1.41 to 6.60), and diseases of the digestive system (2.40, 1.01 to 5.68). Forced sexual activity as a child and adolescent was associated with greater risk of mortality due to cardiovascular disease (2.48, 1.37 to 4.46), external injury or poisoning (3.25, 1.53 to 6.91), suicide (4.30, 1.74 to 10.61), respiratory disease (3.74, 1.40 to 9.99), and diseases of the digestive system (4.83, 1.77 to 13.21). The association of sexual abuse with premature mortality was stronger among women who smoked or had higher levels of anxiety during adulthood. Smoking, low physical activity, anxiety, and depression each explained 3.9-22.4% of the association between early life abuse and premature mortality.
Conclusion
Early life physical and sexual abuse could be associated with a greater risk of adult premature mortality.
Introduction
Reduction of premature mortality (death before age 70 years), one of the United Nations Sustainable Development Goals, is a major public health focus.1 While the dramatic development of public health and medical treatment over the past several decades has led to a worldwide reduction in age adjusted mortality, premature mortality from non-communicable diseases, injuries, and suicide remains a leading public health challenge.2 In the United States between 1999 and 2013, a surprising increase in premature mortality of 0.5% per year occurred among middle aged white women.1 3 To design effective preventive strategies and prioritize interventions, identifying the risk factors of premature mortality from non-communicable diseases, injuries, and suicides is urgently needed. Besides the well identified risk factors such as unhealthy lifestyles and mental disorders, growing evidence shows that adverse childhood experiences might increase the risk of premature mortality.
Early life abuse, including physical and sexual abuse, is a global public health issue because it is common and substantially contributes to child mortality, and has a range of long term health consequences in adulthood.4 5 In the US in 2020, the estimated total number of children who suffered from physical and sexual abuse was 101 961 and 57 963, respectively.6 Evidence has linked childhood or adolescent physical and sexual abuse to adult hypertension,7 suicide attempts,8 violence or criminal behaviors,9 type 2 diabetes,10 and cardiovascular diseases11 12 as a result of changes in health behaviors, psychiatric function, and biological systems.13 14 15 Meanwhile, several population studies have shown that experiencing individual or combined types of abuse in early life is associated with a greater risk of total and injury related mortality in adulthood.16 17 18 19 20 21 22 However, the association of childhood or adolescent abuse with total and cause specific premature mortality remains unclear. More importantly, while numerous studies have associated early life abuse with adult risk of mental health conditions (eg, depression and anxiety)15 23 and adverse lifestyle patterns (eg, poorer diet, obesity, and smoking),24 25 no study has examined potential interactive and mediating roles of these factors. Therefore, we examined the associations of childhood and adolescent sexual and physical abuse with subsequent risk of total and cause specific premature mortality. We also explored possible interactions between early life abuse and adult anxiety, depression, and unhealthy lifestyle patterns and the potential mediating role of these factors among middle aged female nurses in the Nurses’ Health Study II (NHSII).
Methods
Study population
The NHSII is an ongoing prospective cohort established in 1989 by recruiting 116 429 US female nurses aged 25-42 years without compensation. Nurses have been followed biennially thereafter regardless of their job types and employment status using mailed or electronic questionnaires that collect data on demographic and lifestyle characteristics and health outcomes. The response rate of each follow-up cycle exceeds 90%. We restricted the present study to female nurses who received the Lifetime Exposure to Violence Victimization Questionnaire (n=91 248) and provided data on early life exposure to violence victimization in 2001 (n=68 374; fig S1). We further excluded participants who had missing data on abuse exposure (n=648), leaving 67 726 participants for the current analysis (fig S1). Most age standardized characteristics were similar among included female nurses (n=67 726) and those excluded because of a lack of data on early life abuse (n=47 868; table S1). Study procedures were approved by the institutional review boards of Mass General Brigham and Harvard TH Chan School of Public Health (protocol No 2009-P-002375), and those of participating registries as required. The return of completed questionnaires indicates participants’ consent.
Determination of early life abuse
In 2001, experiences of physical and sexual abuse were separately assessed for childhood (occurring before age 12 years) and adolescence (occurring between ages 12 and 17 years). Physical abuse by parents, step parents, or adult guardians was assessed by the Revised Conflict Tactics Scale,26 which queried five specific acts of physical abuse (kick, bite or punch you; push, grab or shove you; choke or burn you; hit you with something that hurt your body; physically attack you in some other way). Response options for each item included never, once, a few times, and more than a few times. Sexual abuse was measured using modified questions from the Sexual Experiences Survey: “Were you ever touched in a sexual way” or “forced into any sexual activity” by an adult or an older child?27 Response options included never, once, or more than once. To create a summary measure of physical abuse, we used the principal components factor analysis with oblique (Promax) rotation and identified four physical abuse groups in NHSII: none, mild, moderate, and severe (table S2).28 Likewise, NHSII participants were classified into four sexual abuse groups: none; sexual touching as a child or adolescent; forced sexual activity as a child or adolescent; and forced sexual activity as a child and adolescent (table S2).28 Because both abuse experiences were highly correlated, we also used a summary measure of physical and sexual abuse based on a principal components factor analysis, which has been described in detail in our previous study.28 In brief, NHSII participants were categorized into five groups by combining subtypes of physical and sexual abuse occurring in childhood and adolescence: no abuse, mild or moderate single type, mild multiple types or severe single type, moderate chronic or multiple types, and severe chronic or multiple types (table S2).28
The summary measure of childhood physical and emotional abuse before age 12 years was also assessed using the Childhood Trauma Questionnaire short form,29 which asked five questions: “People in my family hit me so hard that it left me with bruises and marks”; “The punishments seemed cruel”; “I was punished with a hard object (eg, belt, board, cord)”; “Someone in my family yelled and screamed at me”; and “People in my family said hurtful or insulting things to me”. Response options for each item included never (0), rarely (1), sometimes (2), often (3), and very often (4). We calculated the total Childhood Trauma Questionnaire score across items and divided it into five categories.30 The Childhood Trauma Questionnaire also assessed social support up to age 11 by a single question: “There was someone in my family who helped me feel that I was important or special”.29
Determination of premature mortality
Deaths were identified by searching state vital statistics records and the National Death Index, supplemented by reports from next of kin or the postal authorities, which were able to correctly identify >98% of the deaths by comparing them against medical records.31 Cause of death was determined by reviewing medical records, autopsy reports, or death certificates based on the International Classification of Diseases, eighth and ninth revisions. We classified participants into 19 major categories according to the Statistics Netherlands’ Database, the US Public Health Service, and the National Center for Health Statistics (table S3).32 33 According to the definition from the World Health Organization, premature mortality was defined as death before age 70 years.34 35 The 107 nurses who died at or after 70 years of age were treated as censored observations for which the event of interest was not observed during follow-up.
Assessment of covariates
Factors in early childhood, including race and ethnicity, age at menarche, parental highest education, home ownership, and profession at the time of the participant’s birth, and the location of birth, were self-reported at baseline or during follow-up. Participants’ body shape at age 5 years was assessed using somatograms (a set of nine drawings ranging from very lean to obese) and was shown to be reliable for assessing childhood body mass index in a previous study.36 Parity (number of pregnancies lasting at least six months) was collected at baseline and updated every two years until 2009 when most nurses had passed their reproductive lifespan. Adulthood cardiovascular disease risk factors (eg, physical activity, diet, smoking status, and alcohol intake), prescription drug use, and depression that might modify the influence of early life abuse were also collected biennially. Body mass index was calculated using baseline height and updated weight for each follow-up cycle. In 1991, 1995, 1999, 2003, 2007, 2011, and 2015, nurses reported how often, on average, they consumed a specified amount of nearly 130 food items over the previous year using a validated semiquantitative food frequency questionnaire. We calculated adherence to the Alternative Health Eating Index 2010 to reflect each nurse’s overall diet quality.37 In 1989, 1991, 1997, 2001, 2005, 2009, and 2013, a validated questionnaire was used to collect nurses’ weekly hours of moderate to vigorous activities requiring at least three metabolic equivalent units per hour (eg, jogging, hiking, running, swimming, bicycling, playing racquetball or tennis, and performing calisthenics).38 Clinician diagnosed depression was self-reported biennially since 2003.39 Phobic anxiety, characterized by an excessive and irrational fear of an object, activity, or specific situations (eg, enclosed spaces, heights, or crowds), was assessed by the Crown-Crisp index that was calculated from eight questions administered in 1993 and 2005.40 Most of these self-reported covariates have been validated in NHSII or NHS.41 42 43 44 45
Statistical analysis
All analyses were conducted using the Statistical Analysis System, version 9.4 (SAS Institute). We used Cox proportional hazards models with time varying age and calendar time (in two year intervals) as the underlying timescale to estimate hazard ratios and 95% confidence intervals for total and cause specific premature mortality according to early life physical and sexual abuse. Person years of follow-up started from the return date of the 2001 questionnaire until the date of death or end of follow-up (30 June 2019), whichever occurred first.
In the primary analysis, we included the following covariates as potential confounders, including white race or ethnicity, body shape at age 5, age at menarche, parental highest education at participant’s birth, parents owned home at participant’s birth, parents worked as a professional, manager, or executive at participant’s birth, and the tier of birth. In a secondary analysis, we further adjusted for time varying adult characteristics, including body mass index, parity, smoking status, Alternative Healthy Eating Index 2010 dietary score, alcohol intake, menopausal status, oral contraceptive use, and postmenopausal hormone use. For the covariates with missing values, data from the most recent previous survey cycle were carried forward; otherwise, we created missing indicators (<1% for diet), which have been validated to induce minimal or no bias.46
We further estimated the risk of premature mortality according to the joint categories of early life physical abuse, sexual abuse, and social support. We also conducted stratified analysis according to adult body mass index (<25 v ≥25), smoking (never v ever), diet quality (the top 40% v the bottom 60%), physical activity (≥30 v <30 min/day), phobic anxiety symptom scores (<3 v ≥3), and depression (no v yes). Multiplicative interaction between these psychological and lifestyle factors and abuse experiences was evaluated by conducting the Wald test after adding interaction terms into multivariable models.47 The additive interaction was estimated by calculating the relative excess risk due to interaction (RERI).47 48 We also explored to what extent these factors mediated the association between early life abuse and premature mortality using a mediation analysis developed by Lin and colleagues.49 50 Finally, we conducted a series of sensitivity analyses to assess the influence of other potential confounding factors (pack years of smoking and the presence of other early life abuse), selection bias (the inclusion of nurses reporting cardiovascular diseases or cancer in 2001), and alternative definitions of mortality (deaths at any ages or before age 65 years).
Patient and public involvement
Patients and the public are not invited to comment on the study design, develop patient relevant outcomes, interpret the results, or write or edit the manuscript. However, participants are invited to provide constructive feedback on biennial questionnaires across follow-up, which has been incorporated when appropriate. The study has also incorporated suggestions from an internal review panel.
Results
Among 67 726 female nurses included in the current analysis, during childhood, 46.5% (31 460 of 67 726) never experienced physical abuse and 66.5% (45 008 of 67 726) never experienced sexual abuse (table 1). Compared with nurses reporting physical or sexual abuse during childhood or adolescence, those without early life abuse were more likely to have parents who received higher education, owned a home, and worked as professionals, managers, or executives during nurses’ infancy (table 1). Additionally, depression, anxiety, smoking, obesity, and parental history of cardiovascular disease before age 60 years were less common among nurses who never experienced early life physical or sexual abuse (table 1).
Table 1.
Age standardized characteristics in 2001 according to severity of early life physical and sexual abuse among 67 726 female nurses (Nurses’ Health Study II, 2001-19)
| Characteristics* | Severity of physical abuse | Severity of sexual abuse | |||||||
|---|---|---|---|---|---|---|---|---|---|
| None | Mild physical abuse | Moderate physical abuse | Severe physical abuse | None | Sexual touching as child or adolescent | Forced sexual activity as child or adolescent | Forced sexual activity as child and adolescent | ||
| Number | 31 460 | 12 607 | 17 785 | 5874 | 45 008 | 15 045 | 5778 | 1895 | |
| Age (years), mean (SD) | 46.8 (4.7) | 46.9 (4.7) | 46.9 (4.6) | 47 (4.6) | 46.8 (4.7) | 46.9 (4.7) | 46.9 (4.6) | 47.1 (4.6) | |
| White | 29 932 (95.1) | 11 941 (94.7) | 16 380 (92.1) | 5381 (91.6) | 42 652 (94.8) | 13 943 (92.7) | 5339 (92.4) | 1700 (89.7) | |
| Parental highest education above high school | 14 254 (45.3) | 5571 (44.1) | 7025 (39.5) | 2152 (36.8) | 20 005 (44.4) | 6103 (40.7) | 2233 (38.6) | 661 (35.0) | |
| Parents owned home at participant’s birth | 18 436 (58.6) | 6988 (55.3) | 9528 (53.6) | 2827 (48.3) | 25 582 (56.8) | 8245 (54.9) | 3031 (52.4) | 921 (48.9) | |
| Parents worked as a professional, manager, or executive at the participant’s birth | 10 673 (33.9) | 4036 (31.9) | 4953 (27.9) | 1484 (25.4) | 14 771 (32.8) | 4362 (29.1) | 1556 (26.9) | 457 (24.3) | |
| Age at menarche (years), mean (SD) | 12.4 (1.4) | 12.4 (1.4) | 12.4 (1.4) | 12.4 (1.5) | 12.4 (1.4) | 12.3 (1.4) | 12.3 (1.5) | 12.2 (1.6) | |
| Body mass index | |||||||||
| <18.5 | 409 (1.3) | 160 (1.3) | 210 (1.2) | 61 (1.1) | 623 (1.4) | 147 (1.0) | 42 (0.7) | 28 (1.5) | |
| 18.5-24.9 | 15 633 (49.7) | 6204 (49.1) | 8115 (45.7) | 2448 (42.0) | 22 454 (49.8) | 6778 (45.3) | 2504 (43.3) | 664 (35.3) | |
| 25-29.9 | 8448 (26.9) | 3416 (27.1) | 4985 (28.0) | 1572 (26.7) | 12 076 (26.9) | 4174 (27.7) | 1621 (28.1) | 550 (29.0) | |
| ≥30 | 6970 (22.2) | 2827 (22.5) | 4475 (25.1) | 1793 (30.3) | 9855 (21.9) | 3946 (26.1) | 1611 (27.8) | 653 (34.2) | |
| Parental history of CVD before age 60 years | 8524 (27.1) | 3446 (27.4) | 5105 (28.7) | 1911 (32.3) | 12 143 (27.1) | 4384 (29.0) | 1789 (30.9) | 670 (35.0) | |
| Total physical activity (hours/week), mean (SD) | 2.4 (3.1) | 2.5 (3.1) | 2.4 (3.3) | 2.5 (3.6) | 2.5 (3.2) | 2.3 (3.2) | 2.4 (3.3) | 2.4 (3.6) | |
| AHEI-2010 dietary score,† mean (SD) | 55.6 (12.8) | 56.1 (12.7) | 56 (12.9) | 56.8 (13.1) | 55.8 (12.8) | 56.3 (12.9) | 56.2 (13) | 55.8 (12.7) | |
| Alcohol intake (g/day), mean (SD) | 3.8 (6.9) | 4.5 (7.8) | 4.0 (7.5) | 4.2 (8.0) | 4.0 (7.2) | 4.2 (7.6) | 4.1 (7.7) | 3.6 (7.5) | |
| Parity,‡ mean (SD) | 1.9 (1.2) | 1.9 (1.2) | 1.9 (1.2) | 1.8 (1.2) | 1.9 (1.2) | 1.9 (1.2) | 1.9 (1.2) | 1.8 (1.3) | |
| Depression | 7735 (24.6) | 3730 (29.6) | 5892 (33.1) | 2738 (46.5) | 11 656 (25.9) | 5140 (34.1) | 2350 (40.7) | 949 (50.1) | |
| Phobic anxiety symptom scores ≥3 | 7163 (22.8) | 3327 (26.4) | 4886 (27.5) | 1982 (33.7) | 10 934 (24.3) | 3902 (25.9) | 1821 (31.5) | 701 (36.9) | |
| Smoking status | |||||||||
| Never | 22 043 (70.0) | 7929 (62.8) | 11 163 (62.8) | 3184 (54.5) | 30 600 (67.9) | 9465 (63.1) | 3184 (55.1) | 1070 (56.6) | |
| Past | 7219 (23.0) | 3512 (28.0) | 5005 (28.1) | 1936 (32.7) | 10 957 (24.4) | 4271 (28.2) | 1880 (32.5) | 564 (29.6) | |
| Current 1-34 cigarettes/day | 2069 (6.6) | 1097 (8.7) | 1498 (8.4) | 708 (12.1) | 3233 (7.2) | 1221 (8.1) | 679 (11.8) | 239 (12.6) | |
| Current ≥35 cigarettes/day | 100 (0.3) | 59 (0.5) | 88 (0.5) | 43 (0.7) | 163 (0.4) | 75 (0.5) | 31 (0.5) | 21 (1.1) | |
| Oral contraceptive use | |||||||||
| Never | 2805 (8.9) | 1124 (8.8) | 1447 (8.2) | 420 (7.4) | 4048 (8.9) | 1184 (8.2) | 446 (7.7) | 118 (6.3) | |
| Past | 24 024 (76.4) | 9812 (78.0) | 14 109 (79.3) | 4731 (80.3) | 34 396 (76.5) | 11 967 (79.3) | 4728 (81.8) | 1585 (83.6) | |
| Current | 4478 (14.3) | 1618 (12.8) | 2145 (12.0) | 677 (11.5) | 6341 (14.1) | 1827 (12.1) | 574 (9.9) | 176 (9.3) | |
| Tier of residence at birth,§ | |||||||||
| North | 6528 (20.7) | 2383 (18.9) | 3759 (21.1) | 1138 (19.4) | 9116 (20.2) | 3141 (20.9) | 1151 (19.9) | 400 (21.1) | |
| Middle | 7821 (24.9) | 3024 (24.0) | 4536 (25.5) | 1393 (23.7) | 10 716 (23.8) | 4035 (26.8) | 1502 (26.0) | 521 (27.4) | |
| South | 13 922 (44.3) | 5867 (46.6) | 7555 (42.5) | 2632 (44.7) | 20 611 (45.8) | 6162 (40.8) | 2443 (42.3) | 760 (40.3) | |
| Outside of US or uncertain | 3189 (10.1) | 1333 (10.6) | 1935 (10.9) | 711 (12.2) | 4565 (10.1) | 1707 (11.4) | 682 (11.8) | 214 (11.3) | |
AHEI-10=Alternative Health Eating Index 2010; CVD=cardiovascular disease; SD=standard deviation.
Values are numbers (percentages) unless stated otherwise; means (standard deviations) and percentages of all variables except for age are age standardized.
551 (0.8%) nurses had missing data on baseline diet scores (including alcohol intake).
Pregnancies lasting at least six months.
The north tier of states, lying above 41-42° N latitude, includes states in the east column, the central column, the mountain column, and the Pacific column; the middle tier of states has east, central, mountain, and Pacific columns; the south tier, lying south of 37° N latitude comprises states in the east, central, mountain, and Pacific columns.
Over 18 years of follow-up, 2410 deaths before the age of 70 occurred, including 874 from cancer, 154 from cardiovascular disease, 94 from external causes of injury and poisoning, 49 from suicide, and 49 from respiratory disease (table S3). Nurses who as a child and also an adolescent experienced severe physical abuse or forced sexual activity had a higher crude total premature mortality rate during follow-up than nurses without such abuse in childhood or adolescence (3.15 v 1.83 and 4.00 v 1.90 per 1000 person years, respectively; fig 1). The corresponding age adjusted hazard ratios for total premature mortality were 1.65 (95% confidence interval 1.45 to 1.87) and 2.04 (1.71 to 2.44), respectively, which were materially unchanged after further adjusting for childhood covariates (1.53, 1.35 to 1.74, and 1.80, 1.50 to 2.15, respectively; fig 1). The increased total premature mortality risk persisted among nurses simultaneously reporting physical and sexual abuse in both periods (before age 12 years and at age 12-17 years; table S4). A similar pattern of association was observed across the summary categories of increasing severity and chronicity of physical and sexual abuse (fig 1). In the adjusted models, there was a 4% (95% confidence interval of hazard ratios 0.94 to 1.15) greater risk of total premature mortality for nurses reporting one episode of mild or moderate abuse, 9% (0.96 to 1.23) for mild multiple or severe single type of abuse, 24% (1.06 to 1.44) for moderate chronic or multiple types of abuse, and 76% (1.51 to 2.05) for severe chronic or multiple types of abuse compared with those without physical or sexual abuse. Further adjustment for adult covariates, many of which might be considered intermediates on the pathway between child abuse and adult mortality, only slightly weakened these associations (fig 1 and table S4). We also observed an increased risk of total premature mortality among nurses who had higher physical and emotional abuse and those who received rare or no social support during childhood (table S4).
Fig 1.
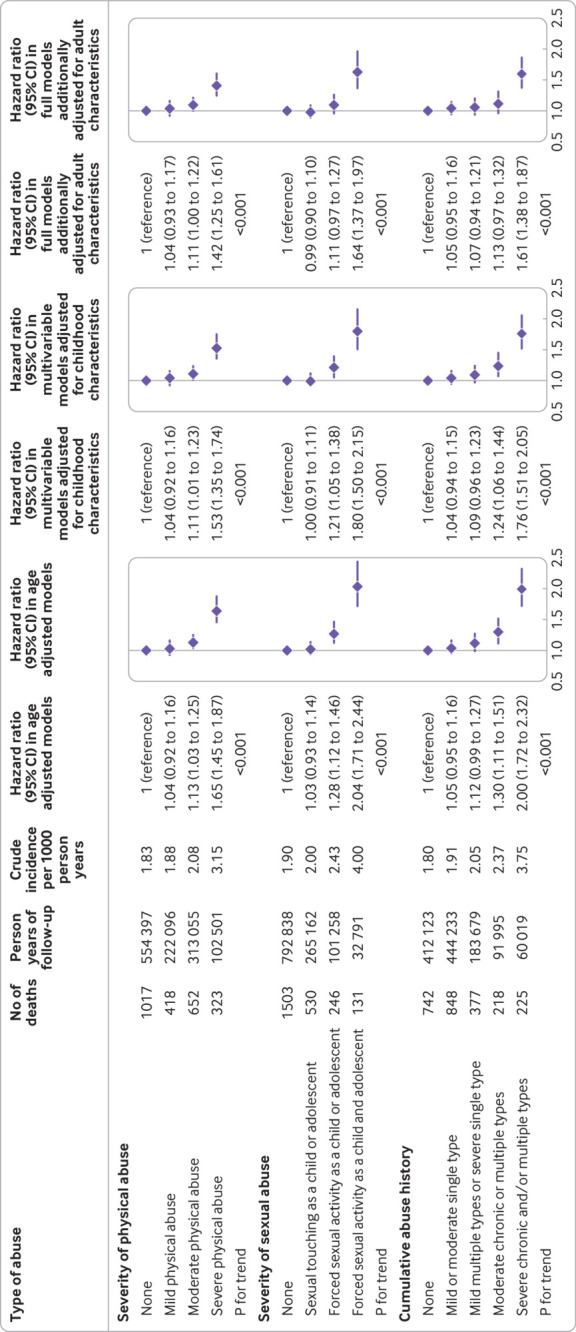
Risk of all cause premature mortality (before age 70 years) according to early life physical and sexual abuse among 67 726 female nurses (Nurses’ Health Study II, 2001-19). P for trend was conducted by modeling categories of increasing severity of physical and sexual abuse as an ordinal level variable using integer values. Cumulative abuse history is summary of childhood and adolescent physical abuse measured by the Revised Conflict Tactics Scale and sexual abuse measured by the Sexual Experiences Survey. In the age adjusted model, age in months (continuous) at the start of follow-up and the calendar year of the current questionnaire cycle were included as stratified variables to control for age, calendar time, and any possible interactions between these two timescales. Based on age adjusted models, multivariable models were further adjusted for white race or ethnicity (no or yes), body shape at age 5 (somatogram figure 1, 2, 3, 4, or ≥5), age at menarche (continuous), parental highest education (less than high school, completed high school, or more than high school), parents owned a home during infancy (no or yes), parents worked as a professional, manager, or executive during infancy (no or yes), and the tier of birth (north, middle, south, or outside of United States or uncertain). Based on multivariable models, full models were further adjusted for time varying body mass index (<24.9, 25-29.9, 30-34.9, or ≥35), smoking status (never, former, current 1-34 cigarettes/day, or current ≥35 cigarettes/day), parity (≤1, 2, or ≥3), Alternative Healthy Eating Index 2010 dietary score (five categories), alcohol intake (0, 1-14, or ≥15 g/day), oral contraceptive use (never, past, or current), menopausal status (premenopausal, postmenopausal, or uncertain), and postmenopausal hormone use (never, past, or current)
When we examined specific premature mortality causes with at least 40 deaths (table 2 and table 3), severe physical abuse was associated with a greater risk of mortality due to external causes of injury and poisoning (hazard ratio 2.81, 95% confidence interval 1.62 to 4.89), suicide (3.05, 1.41 to 6.60), and diseases of the digestive system (2.40, 1.01 to 5.68) with adjustment for childhood covariates; forced sexual activity as a child and an adolescent was associated with a greater risk of mortality due to cardiovascular disease (2.48, 1.37 to 4.46), external causes (3.25, 1.53 to 6.91), suicide (4.30, 1.74 to 10.61), respiratory disease (3.74, 1.40 to 9.99), and diseases of the gastrointestinal system (4.83, 1.77 to 13.21). Severe chronic or multiple types of abuse were associated with a greater risk of mortality due to cardiovascular diseases (1.98, 1.14 to 3.43), external causes of injury and poisoning (3.39, 1.74 to 6.60), suicide (4.32, 1.85 to 10.08), respiratory disease (3.72, 1.54 to 9.00), and diseases of the digestive system (3.30, 1.08 to 10.09; table S5). As with total mortality, most associations were substantially unchanged when we additionally adjusted for adult covariates (table 2, table 3, and table S5). We did not find any evidence of associations for cancer mortality.
Table 2.
Risk of cause specific premature mortality (before age 70 years) with at least 40 deaths attributed according to the severity of physical abuse during childhood or adolescence among 67 726 female nurses (Nurses’ Health Study II, 2001-19)
| Cause specific mortality | Severity of physical abuse | P for trend‡ | |||
|---|---|---|---|---|---|
| None | Mild | Moderate | Severe | ||
| Cancer | |||||
| Events/crude incidence per 1000 person years | 393/0.71 | 175/0.79 | 221/0.71 | 85/0.83 | — |
| Multivariable models adjusted for childhood characteristics* | 1.00 (reference) | 1.12 (0.93 to 1.34) | 0.98 (0.83 to 1.15) | 1.04 (0.82 to 1.31) | 0.97 |
| Full models additionally adjusted for adult characteristics† | 1.00 (reference) | 1.14 (0.95 to 1.37) | 1.00 (0.85 to 1.18) | 1.04 (0.82 to 1.31) | 0.86 |
| Cardiovascular disease | |||||
| Events/crude incidence per 1000 person years | 68/0.12 | 19/0.09 | 51/0.16 | 16/0.16 | — |
| Multivariable models adjusted for childhood characteristics* | 1.00 (reference) | 0.70 (0.42 to 1.17) | 1.29 (0.89 to 1.85) | 1.08 (0.62 to 1.87) | 0.30 |
| Full models additionally adjusted for adult characteristics† | 1.00 (reference) | 0.70 (0.42 to 1.17) | 1.29 (0.89 to 1.87) | 0.92 (0.53 to 1.62) | 0.51 |
| External causes of injury and poisoning | |||||
| Events/crude incidence per 1000 person years | 36/0.06 | 14/0.06 | 24/0.08 | 20/0.19 | — |
| Multivariable models adjusted for childhood characteristics* | 1.00 (reference) | 0.95 (0.51 to 1.77) | 1.10 (0.66 to 1.86) | 2.81 (1.62 to 4.89) | 0.007 |
| Full models additionally adjusted for adult characteristics† | 1.00 (reference) | 0.93 (0.50 to 1.73) | 1.06 (0.63 to 1.79) | 2.45 (1.40 to 4.29) | 0.02 |
| Senility and ill-defined diseases | |||||
| Events/crude incidence per 1000 person years | 39/0.07 | 10/0.05 | 28/0.09 | 12/0.12 | — |
| Multivariable models adjusted for childhood characteristics* | 1.00 (reference) | 0.69 (0.34 to 1.39) | 1.31 (0.80 to 2.13) | 1.46 (0.75 to 2.84) | 0.17 |
| Full models additionally adjusted for adult characteristics† | 1.00 (reference) | 0.73 (0.36 to 1.48) | 1.30 (0.79 to 2.14) | 1.27 (0.64 to 2.51) | 0.28 |
| Suicide | |||||
| Events/crude incidence per 1000 person years | 17/0.03 | 7/0.03 | 14/0.04 | 11/0.11 | — |
| Multivariable models adjusted for childhood characteristics* | 1.00 (reference) | 1.00 (0.41 to 2.41) | 1.45 (0.71 to 2.96) | 3.05 (1.41 to 6.60) | 0.01 |
| Full models additionally adjusted for adult characteristics† | 1.00 (reference) | 1.02 (0.42 to 2.52) | 1.52 (0.74 to 3.13) | 2.80 (1.26 to 6.22) | 0.02 |
| Respiratory disease | |||||
| Events/crude incidence per 1000 person years | 18/0.03 | 11/0.05 | 12/0.04 | 8/0.08 | — |
| Multivariable models adjusted for childhood characteristics* | 1.00 (reference) | 1.55 (0.73 to 3.29) | 1.15 (0.55 to 2.39) | 2.08 (0.89 to 4.83) | 0.19 |
| Full models additionally adjusted for adult characteristics† | 1.00 (reference) | 1.41 (0.65 to 3.04) | 1.02 (0.49 to 2.15) | 1.73 (0.73 to 4.08) | 0.40 |
| Diseases of the digestive system | |||||
| Events/crude incidence per 1000 person years | 16/0.03 | 7/0.03 | 11/0.04 | 8/0.08 | — |
| Multivariable models adjusted for childhood characteristics* | 1.00 (reference) | 1.15 (0.47 to 2.80) | 1.16 (0.53 to 2.50) | 2.40 (1.01 to 5.68) | 0.12 |
| Full models additionally adjusted for adult characteristics† | 1.00 (reference) | 0.97 (0.38 to 2.44) | 0.95 (0.43 to 2.10) | 1.83 (0.75 to 4.48) | 0.39 |
Data are hazard ratios (95% confidence intervals).
Model adjusted for age (continuous), white race or ethnicity (no or yes), body shape at age 5 (somatogram figure 1, 2, 3, 4, or ≥5), age at menarche (continuous), parental highest education (less than high school, completed high school, or more than high school), parents owned a home during infancy (no or yes), parents worked as a professional, manager, or executive during infancy (no or yes), the tier of birth (north, middle, south, or outside of United States or uncertain).
Based on multivariable models with further adjustment for time varying body mass index (<24.9, 25-29.9, 30-34.9, or ≥35), smoking status (never, former, current 1-34 cigarettes/day, or current ≥35 cigarettes/day), parity (≤1, 2, or ≥3), Alternative Healthy Eating Index 2010 dietary score (five categories), alcohol intake (0, 1-14, or ≥15 g/day), oral contraceptive use (never, past, or current), menopausal status (premenopausal, postmenopausal, or uncertain), and postmenopausal hormone use (never, past, or current).
P for trend was conducted by modeling the categories of increasing severity of physical abuse as an ordinal level variable using integer values.
Table 3.
Risk of cause specific premature mortality (before age 70 years) with at least 40 deaths attributed according to severity of sexual abuse during childhood or adolescence among 67 726 female nurses (Nurses’ Health Study II, 2001-19)
| Cause specific mortality | Severity of sexual abuse | ||||
|---|---|---|---|---|---|
| None | Sexual touching as a child or adolescent | Forced sexual activity as a child or adolescent | Forced sexual activity as a child and adolescent | P for trend‡ | |
| Cancer | |||||
| Events/crude incidence per 1000 person years | 576/0.73 | 181/0.68 | 91/0.90 | 26/0.79 | — |
| Multivariable models adjusted for childhood characteristics* | 1.00 (reference) | 0.91 (0.77 to 1.07) | 1.17 (0.94 to 1.46) | 0.93 (0.62 to 1.38) | 0.82 |
| Full models additionally adjusted for adult characteristics† | 1.00 (reference) | 0.92 (0.78 to 1.09) | 1.14 (0.91 to 1.43) | 0.92 (0.62 to 1.37) | 0.89 |
| Cardiovascular disease | |||||
| Events/crude incidence per 1000 person years | 95/0.12 | 31/0.12 | 15/0.15 | 13/0.40 | — |
| Multivariable models adjusted for childhood characteristics* | 1.00 (reference) | 0.85 (0.56 to 1.28) | 1.09 (0.63 to 1.89) | 2.48 (1.37 to 4.46) | 0.07 |
| Full models additionally adjusted for adult characteristics† | 1.00 (reference) | 0.81 (0.54 to 1.22) | 0.93 (0.53 to 1.62) | 2.03 (1.11 to 3.72) | 0.30 |
| External causes of injury and poisoning | |||||
| Events/crude incidence per 1000 person years | 53/0.07 | 20/0.08 | 13/0.13 | 8/0.24 | — |
| Multivariable models adjusted for childhood characteristics* | 1.00 (reference) | 1.08 (0.64 to 1.81) | 1.84 (1.00 to 3.40) | 3.25 (1.53 to 6.91) | 0.002 |
| Full models additionally adjusted for adult characteristics† | 1.00 (reference) | 1.04 (0.62 to 1.74) | 1.60 (0.86 to 2.98) | 2.68 (1.24 to 5.79) | 0.02 |
| Senility and ill-defined diseases | |||||
| Events/crude incidence per 1000 person years | 63/0.08 | 16/0.06 | 5/0.05 | 5/0.15 | — |
| Multivariable models adjusted for childhood characteristics* | 1.00 (reference) | 0.73 (0.42 to 1.26) | 0.61 (0.24 to 1.52) | 1.61 (0.64 to 4.03) | 0.68 |
| Full models additionally adjusted for adult characteristics† | 1.00 (reference) | 0.72 (0.41 to 1.25) | 0.46 (0.18 to 1.19) | 1.46 (0.57 to 3.71) | 0.38 |
| Suicide | |||||
| Events/crude incidence per 1000 person years | 29/0.04 | 9/0.03 | 5/0.05 | 6/0.18 | — |
| Multivariable models adjusted for childhood characteristics* | 1.00 (reference) | 0.89 (0.42 to 1.88) | 1.15 (0.44 to 2.99) | 4.30 (1.74 to 10.61) | 0.04 |
| Full models additionally adjusted for adult characteristics† | 1.00 (reference) | 0.93 (0.43 to 2.00) | 1.08 (0.41 to 2.84) | 3.71 (1.45 to 9.48) | 0.07 |
| Respiratory disease | |||||
| Events/crude incidence per 1000 person years | 25/0.03 | 10/0.04 | 9/0.09 | 5/0.15 | — |
| Multivariable models adjusted for childhood characteristics* | 1.00 (reference) | 1.03 (0.49 to 2.15) | 2.42 (1.12 to 5.24) | 3.74 (1.40 to 9.99) | 0.004 |
| Full models additionally adjusted for adult characteristics† | 1.00 (reference) | 0.96 (0.45 to 2.02) | 1.95 (0.88 to 4.30) | 3.11 (1.12 to 8.67) | 0.02 |
| Diseases of the digestive system | |||||
| Events/crude incidence per 1000 person years | 21/0.03 | 10/0.04 | 6/0.06 | 5/0.15 | — |
| Multivariable models adjusted for childhood characteristics* | 1.00 (reference) | 1.38 (0.64 to 2.93) | 2.12 (0.85 to 5.30) | 4.83 (1.77 to 13.21) | 0.003 |
| Full models additionally adjusted for adult characteristics† | 1.00 (reference) | 1.31 (0.60 to 2.85) | 1.73 (0.67 to 4.50) | 4.53 (1.58 to 12.96) | 0.01 |
Data are hazard ratios (95% confidence intervals).
Model adjusted for age (continuous), white race or ethnicity (no or yes), body shape at age 5 (somatogram figure 1, 2, 3, 4, or ≥5), age at menarche (continuous), parental highest education (less than high school, completed high school, more than high school), parents owned a home during infancy (no or yes), parents worked as a professional, manager, or executive during infancy (no or yes), the tier of birth (north, middle, south, or outside of United States or uncertain).
Based on multivariable models with further adjustment for time varying body mass index (<24.9, 25-29.9, 30-34.9, or ≥35), smoking status (never, former, current 1-34 cigarettes/day, or current ≥35 cigarettes/day), parity (≤1, 2, or ≥3), Alternative Healthy Eating Index 2010 dietary score (five categories), alcohol intake (0, 1-14, or ≥15 g/day), oral contraceptive use (never, past, or current), menopausal status (premenopausal, postmenopausal, or uncertain), and postmenopausal hormone use (never, past, or current).
P for trend was conducted by modeling the categories of increasing severity of sexual abuse as an ordinal level variable using integer values.
We found multiplicative (P<0.01) and additive (P=0.004) interactions between physical and sexual abuse on the risk of total premature mortality (table S6), in which those with severe abuse of both types were at the highest risk of premature mortality. Meanwhile, although there was no evidence of either multiplicative or additive interaction between physical abuse and social support, we found positive interaction on the additive scale between sexual abuse and social support on the risk of premature mortality (RERI=0.17, 95% confidence interval −0.02 to 0.36; P=0.08). We did not find any evidence of interaction between early life abuse and adult diet quality, body mass index, physical activity, and depression on the risk of total premature mortality (table 4). However, the association of sexual abuse with total premature mortality was stronger among nurses who smoked or had a higher anxiety score during adulthood (RERI=0.63, 0.28 to 0.98; and 0.54, 0.19 to 0.89). Mediation analyses showed that smoking, low levels of physical activity, anxiety, and depression explained 12.9% (95% confidence interval 9.1% to 18.0%), 3.9% (2.7% to 5.7%), 4.7% (2.8% to 7.7%), and 20.7% (14.4% to 28.9%) of the association between physical abuse and premature mortality, respectively (table 4). A similar mediated proportion was observed for the association between sexual abuse and premature mortality (16.2%, 10.8% to 23.5%; 7.3%, 4.9% to 10.9%; 5.9%, 3.5% to 9.9%; and 22.4%, 14.7% to 32.7%, respectively). We found that neither obesity nor diet mediated the associations of early life abuse with premature mortality.
Table 4.
Risk of all cause premature mortality (before age 70 years) according to early life physical and sexual abuse among 67 726 female nurses stratified by psychological and lifestyle factors (Nurses’ Health Study II, 2001-19)*
| Stratified factors | Physical abuse | Forced sexual activity | ||||
|---|---|---|---|---|---|---|
| None or mild | Moderate or severe | Never | Ever | |||
| Diet quality | ||||||
| Top 40% (n=814 deaths) | 1.00 (reference) | 1.58 (1.30 to 1.93) | 1.00 (reference) | 1.42 (1.17 to 1.71) | ||
| Bottom 60% (n=1596 deaths) | 1.00 (reference) | 1.42 (1.22 to 1.64) | 1.00 (reference) | 1.33 (1.16 to 1.52) | ||
| P for multiplicative interaction | 0.21 | — | 0.28 | — | ||
| P for additive interaction | 0.92 | — | 0.94 | — | ||
| RERI (95% CI) | 0.02 (−0.43 to 0.47) | — | 0.02 (−0.37 to 0.40) | — | ||
| Proportion mediated by low diet quality | <0.1% | — | <0.1% | — | ||
| Smoking status | ||||||
| Never (n=1239 deaths) | 1.00 (reference) | 1.36 (1.13 to 1.63) | 1.00 (reference) | 1.08 (0.90 to 1.29) | ||
| Current or past (n=1171 deaths) | 1.00 (reference) | 1.42 (1.22 to 1.66) | 1.00 (reference) | 1.46 (1.26 to 1.68) | ||
| P for multiplicative interaction | 0.62 | — | 0.009 | — | ||
| P for additive interaction | 0.11 | — | <0.001 | — | ||
| RERI (95% CI) | 0.33 (−0.08 to 0.74) | — | 0.63 (0.28 to 0.98) | — | ||
| Proportion mediated by smoking | 12.9% (9.1% to 18.0%) | — | 16.2% (10.8% to 23.5%) | — | ||
| Body mass index | ||||||
| <25 (n=944 deaths) | 1.00 (reference) | 1.68 (1.38 to 2.04) | 1.00 (reference) | 1.39 (1.15 to 1.68) | ||
| ≥25 (n=1466 deaths) | 1.00 (reference) | 1.39 (1.19 to 1.61) | 1.00 (reference) | 1.34 (1.17 to 1.54) | ||
| P for multiplicative interaction | 0.09 | — | 0.70 | — | ||
| P for additive interaction | 0.09 | — | 0.65 | — | ||
| RERI (95% CI) | −0.32 (−0.69 to 0.05) | — | −0.07 (−0.38 to 0.24) | — | ||
| Proportion mediated by overweight or obesity | <0.1% | — | <0.1% | — | ||
| Physical activity | ||||||
| ≥30 min/day (n=750 deaths) | 1.00 (reference) | 1.43 (1.14 to 1.79) | 1.00 (reference) | 1.40 (1.14 to 1.72) | ||
| <30 min/day (n=1660 deaths) | 1.00 (reference) | 1.47 (1.28 to 1.69) | 1.00 (reference) | 1.29 (1.13 to 1.47) | ||
| P for multiplicative interaction | 0.82 | — | 0.40 | — | ||
| P for additive interaction | 0.10 | — | 0.71 | — | ||
| RERI (95% CI) | 0.38 (−0.07 to 0.83) | — | 0.08 (−0.33 to 0.48) | — | ||
| Proportion mediated by physically inactive | 3.9% (2.7% to 5.7%) | — | 7.3% (4.9% to 10.9%) | — | ||
| Phobic anxiety symptom scores | ||||||
| <3 (n=1677 deaths) | 1.00 (reference) | 1.44 (1.24 to 1.68) | 1.00 (reference) | 1.18 (1.02 to 1.37) | ||
| ≥3 (n=733 deaths) | 1.00 (reference) | 1.45 (1.19 to 1.76) | 1.00 (reference) | 1.55 (1.30 to 1.85) | ||
| P for multiplicative interaction | 0.91 | — | 0.008 | — | ||
| P for additive interaction | 0.51 | — | 0.002 | — | ||
| RERI (95% CI) | 0.13 (−0.26 to 0.53) | — | 0.54 (0.19 to 0.89) | — | ||
| Proportion mediated by anxiety | 4.7% (2.8% to 7.7%) | — | 5.9% (3.5% to 9.9%) | — | ||
| Depression | ||||||
| No (n=1557 deaths) | 1.00 (reference) | 1.32 (1.11 to 1.56) | 1.00 (reference) | 1.27 (1.09 to 1.47) | ||
| Yes (n=853 deaths) | 1.00 (reference) | 1.43 (1.20 to 1.69) | 1.00 (reference) | 1.31 (1.11 to 1.54) | ||
| P for multiplicative interaction | 0.74 | — | 0.91 | — | ||
| P for additive interaction | 0.20 | — | 0.35 | — | ||
| RERI (95% CI) | 0.27 (−0.14 to 0.67) | — | 0.18 (−0.19 to 0.54) | — | ||
| Proportion mediated by depression | 20.7% (14.4% to 28.9%) | — | 22.4% (14.7% to 32.7%) | — | ||
Data are adjusted hazard ratios (95% confidence intervals).
RERI=relative excess risk due to interaction.
Model adjusted for age (continuous), white race or ethnicity (no or yes), body shape at age 5 (somatogram figure 1, 2, 3, 4, or ≥5), age at menarche (continuous), parental highest education (less than high school, completed high school, or more than high school), parents owned a home during infancy (no or yes), parents worked as a professional, manager, or executive during infancy (no or yes), and the tier of birth (north, middle, south, or outside of United States or uncertain).
The positive associations between early life physical and sexual abuse and total mortality persisted after excluding nurses reporting a diagnosis of cardiovascular disease or cancer in 2001, redefining premature death as those who died before age 65 years, including deaths at or after 70 years of age, and adjusting for more refined smoking covariates (smoking status, pack years of smoking, and the square of pack years) or the presence of another early life abuse (physical or sexual abuse; table S7).
Discussion
Principal findings
In this large prospective study with 18 years of follow-up, we found an increased risk of premature adult mortality in female nurses reporting moderate to severe physical abuse and forced sexual activity before age 12 and at ages 12-17 years. When the most common causes of premature mortality were considered, severe physical abuse was associated with a greater risk of mortality due to external causes of injury and poisoning, suicide, and diseases of the digestive system, while severe sexual abuse was associated with a greater risk of mortality due to cardiovascular diseases, external causes of injury and poisoning, suicide, respiratory disease, and diseases of the digestive system.
Comparison with previous studies
In support of our results, previous studies reported that adverse childhood experiences, of which physical and sexual abuse constitute only a part of six to eight domain adverse childhood experience measures, were associated with a greater risk of all cause mortality.17 51 52 Early life maltreatment, a combined measure of abuse and neglect, has also been associated with a greater risk of total or injury related mortality during adulthood.16 17 18 19 In a large retrospective cohort study of 331 254 young Australians, Segal and colleagues reported a greater risk of total and cause specific mortality (eg, deaths from suicide, poisonings, or alcohol) between ages 16 and 33 years among those with versus those without child protection service involvement.21 However, few population studies to date have explored the association of individual types of early life abuse with subsequent long term risk of mortality. In a national sample of 3298 US women, Chen and colleagues reported a greater risk of total mortality over two decades of follow-up among women reporting childhood physical abuse (hazard ratio 1.30, 95% confidence interval 1.05 to 1.60 for moderate physical abuse; and 1.58, 1.20 to 2.08 for severe physical abuse) or emotional abuse (1.22, 1.01 to 1.49) compared with those without such an experience; they did not evaluate sexual abuse.22 Similarly, in a British prospective cohort study consisting of 9310 adults, Rogers and colleagues revealed a greater risk of total mortality at ages 44-58 years among adults reporting childhood sexual (hazard ratio 2.64, 95% confidence interval 1.52 to 4.59) or physical abuse (1.73, 1.11 to 2.71) than those without such abuse.20
Our study extends and refines the existing evidence in this area. We included a larger population with detailed information on causes of death and abuse history, which permitted us to separately examine the risk for cause specific mortality in relation to physical and sexual abuse. We found that both early life sexual and physical abuse were associated with a greater risk of mortality. Specifically, severe physical and forced sexual activity in childhood or adolescence were both associated with a greater risk of mortality due to external causes (injury and poisoning), suicide, and diseases of the digestive system. Additionally, we found that forced sexual activity, especially if it occurred in childhood and adolescence, was associated with a greater risk of mortality due to cardiovascular disease and respiratory disease. In support of our findings, early life physical or sexual abuse has been associated with an increased risk of suicide attempts,53 54 cardiovascular diseases,11 14 respiratory diseases,55 and gastrointestinal illnesses in later life,56 which might eventually lead to a greater risk of mortality. Similarly, in a meta-analysis consisting of 37 studies, Hughes and colleagues estimated the risk for 23 outcomes and found that adverse childhood experiences were associated with a greater risk of suicide attempt, illicit or problematic drug use, cardiovascular diseases (eg, heart attack, coronary heart disease, or ischemic heart disease), chronic respiratory diseases (eg, tuberculosis and chronic obstructive pulmonary disease), and digestive diseases (liver disease, jaundice, or hepatitis).57 Several studies showed positive associations between the number of adverse childhood experiences and cancer incidence,58 59 but no study has explored the associations of early life abuse with cancer mortality. In the present study, neither physical nor sexual abuse was associated with cancer mortality. We suspect that many nurses who develop cancer might still have not died due to advances in medical treatment. However, given the limited sample size for some cause specific mortality categories, additional studies are needed to verify our findings.
We also observe evidence of interaction between sexual and physical abuse; the risk of premature mortality was particularly strong among participants who experienced both severe physical abuse and forced sexual activity. Additionally, we found that the association of sexual abuse with premature mortality was stronger among female nurses who smoked and had higher anxiety scores during later adulthood, suggesting that early life sexual abuse could interact synergistically with adult smoking and anxiety disorders to further increase the risk of premature mortality. However, it is somewhat surprising that obesity was not a mediator of the associations of child abuse with adult mortality, given higher rates of obesity among those with a history of child abuse,25 60 the adverse effect of obesity on premature mortality,61 and higher rates of adult obesity in those with severe physical or sexual abuse in the present study. It should be noted that the association of childhood or adolescent physical and sexual abuse with premature mortality observed in the present study was mainly driven by mortality due to external causes and suicide that were less likely to be influenced by obesity. Meanwhile, we found evidence of positive interaction on the additive scale between sexual abuse and social support on the risk of premature mortality, suggesting that the association of sexual abuse with premature mortality is greater among female nurses receiving rare or no social support. Our findings were supported by a recent study of 6078 US adults showing that midlife social support could offset mortality risk associated with early life abuse.62
Possible underlying mechanisms
One explanation underlying the associations of early life abuse with premature mortality due to chronic diseases (cardiovascular disease, respiratory disease, and diseases of the digestive system), injuries, and suicides is that abuse might heighten vulnerability to mental health problems and adverse lifestyle patterns.14 In support of this hypothesis, our study shows that part of the associations between physical and sexual abuse and premature mortality was mediated by smoking, low levels of physical activity, anxiety, and depression during adulthood. Numerous studies have documented associations between early life maltreatment and abuse and subsequent risk of psychological disorders and emotional distress, including depression.15 63 64 Early life abuse has also been associated with an increased risk of unhealthy behaviors, such as smoking and alcohol consumption.24 65 66 These psychological and behavioral problems, in turn, might heighten the risk for later life morbidity or mortality of cardiovascular disease, injury, suicide, respiratory disease, and diseases of the digestive system. Other potential mechanisms are related to biological changes across body systems, including immune and inflammatory function, brain development trajectories, and neuroendocrine stress activities.13 For instance, previous studies from other populations or subsamples of NHSII participants have shown that childhood or adolescent abuse is associated with higher concentrations of inflammatory biomarkers (eg, C reactive protein, interleukin 1β, and interleukin 6),67 68 altered brain structure, function, and network architecture,15 impaired cellular immunity,69 and dysregulated neuroendocrine stress system.13 70 Early life abuse and other stressful life experiences have also been associated with epigenetic modifications,71 which could alter the trajectories of brain development, eventually leading to psychiatric disorders and addictive behaviors in adulthood.72 73 Interestingly, the increased risk of mortality due to cardiovascular disease and respiratory disease was only observed among female nurses reporting forced sexual activity, rather than physical abuse. Given that smoking, physical activity, anxiety, and depression consistently mediated a greater proportion of the associations for forced sexual activity than physical abuse, we suspected that those who experienced sexual violence were more likely to be influenced by subsequent mental disorders and adverse lifestyle patterns, which themselves have been positively associated with cardiovascular diseases and respiratory diseases.74 75
Strengths and limitations
The strengths of this study include its large sample size, prospective follow-up over 18 years with excellent response rates, detailed information on causes of death and abuse history, and the collection of various relevant confounders, psychosocial outcomes (eg, depression), and lifestyle factors (eg, smoking). Our study also has some limitations. Participants recalled their childhood and adolescent abuse in 2001 when they were aged 37-54 years, which might have resulted in recall bias and exposure misclassification. However, we queried specific acts of early life abuse instead of asking participants to characterize their experience as abuse, which should create less bias.11 76 As a result, our cumulative prevalence of moderate to severe physical abuse was within the range of the prevalence of severe parental violence in the UK, the US, New Zealand, Finland, Italy, and Portugal (5-35% v 34.9%, 23 659 of 67 726 in NHSII).4 Similarly, the cumulative prevalence of any (15-30%) and penetrative sexual abuse (5-10%) in developed countries (US, Australia, New Zealand, and Canada) was also comparable to the prevalence in NHSII (33.5%, 22 718 of 67 726; and 11.3%, 7673 of 67 726, respectively).4 The slightly higher overall prevalence of physical and sexual abuse in NHSII is probably reflective of the dramatic decline in childhood abuse rate over the past several decades in the US.77 Meanwhile, several previous studies have shown that adults’ recall of early life abuse is reliable.78 79 However, problems such as forgetting, denial, misunderstanding, and embarrassment are still likely to lead to an underestimated report of early life abuse.4 In this case, since childhood or adolescent abuse was reported before premature mortality, we suspected that the underestimated abuse would be non-differential with respect to adult mortality and that our findings were unlikely to be included by exposure misclassification.
Selection bias cannot be fully excluded given that those who experienced abuse might have refused to return the violence questionnaire. However, we found a similar pattern of age standardized characteristics between included female nurses and those excluded due to missing exposure information in 2001. The NHSII cohort includes primarily non-Hispanic white female nurses. Therefore, our results should be interpreted with caution to the general female population and other more diverse populations who might have different educational attainment, socioeconomic status, social support resources, and risk factors for premature mortality. As with any other observational study, our study cannot show causality. Because randomizing women to experience abuse is impossible, high quality observational studies with extended follow-up are likely to provide the best available evidence to assess the long term effects of early life abuse. It is also likely there are factors (eg, social and structural factors) associated with childhood abuse that could affect early mortality and thus confound the associations we identified.80 81 However, our findings were materially unchanged across the adjustments for socioeconomic status (parental education, home ownership, job types), birthplaces, and time varying adult characteristics, and were robust to a series of sensitivity analyses. We did not collect data on psychological aids or therapies and other adverse childhood experiences (eg, household violence and substance abuse) and adult psychiatric disorders (eg, schizophrenia, substance use disorders, and bipolar disorder) that could also play an interactive or mediating role. Finally, we have limited numbers for some categories of death, such as respiratory diseases and diseases of the digestive track, which limited our ability to generate precise estimates for some causes of death. Additional studies with larger numbers of deaths are needed to verify our findings for cause specific deaths.
Conclusion and policy implications
This large prospective study consisting of 67 726 female nurses showed that moderate to severe physical abuse and forced sexual activity in early life were associated with a greater subsequent risk of total premature mortality. These associations were driven by various reasons for death, including external causes, suicide, cardiovascular disease, respiratory disease, and diseases of the gastrointestinal system, and could be partly mediated by later life smoking, low levels of physical activity, anxiety, and depression. Together, these findings suggest that women who experienced childhood physical and sexual abuse continue to be vulnerable to premature mortality and highlight that efforts to prevent child abuse might have long reaching benefits for population health and longevity by reducing premature mortality. Additionally, we found evidence of an interactive or mediating role of social support, psychological conditions, and lifestyle factors, which emphasizes the importance of social supporting services and psychological or lifestyle interventions in improving health outcomes for those who have experienced child abuse.
What is already known on this topic
Early life abuse is a global public health issue because it substantially contributes to child mortality and a wide range of long term consequences during adulthood
Individual and combined types of early life abuse are associated with a greater risk of total and injury related mortality during adulthood
What this study adds
Childhood and adolescent physical abuse and forced sexual activity were associated with a greater risk of premature mortality due to various causes (eg, external causes, suicide, and cardiovascular diseases)
Women reporting early life physical abuse and forced sexual activity might continue to be vulnerable to premature mortality, highlighting the importance of providing trauma informed care for those who have experienced child abuse
Acknowledgments
The authors would like to acknowledge the contribution to this study from central cancer registries supported through the Centers for Disease Control and Prevention’s National Program of Cancer Registries (NPCR) and/or the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program. Central registries might also be supported by state agencies, universities, and cancer centers. Participating central cancer registries include the following: Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Indiana, Iowa, Kentucky, Louisiana, Massachusetts, Maine, Maryland, Michigan, Mississippi, Montana, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Puerto Rico, Rhode Island, Seattle SEER Registry, South Carolina, Tennessee, Texas, Utah, Virginia, West Virginia, Wyoming.
Web extra.
Extra material supplied by authors
Web appendix: Supplemental material
Contributors: Y-XW analyzed and drafted the manuscript. YS checked the accuracy of the data analysis. Y-XW, JEC, and JWR-E were involved in the study conception and design. All authors participated in the interpretation of the results and critical revision of the manuscript. JWR-E is the study guarantor and accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This research was funded by the following grants from the National Institutes of Health: U01-HL145386, U01-CA176726, R01-HL034594, R01-HL088521, UM-CA186107, P01-CA87969, R01-CA49449, R01-CA67262, U01-HL145386, U01-CA167552, R01-HL35464, and R24-ES028521-01. The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the National Institutes of Health for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
The lead author (the manuscript’s guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: Study participants are periodically updated on findings from the study through multiple channels including our yearly newsletter, the study website, and our social media feeds.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and the Harvard TH Chan School of Public Health (protocol No: 2009-P-002375). The completion of the self-administered questionnaire was considered to imply informed consent.
Data availability statement
Data described in the manuscript, code book, and analytic code will not be made publicly available. Further information including the procedures for obtaining and accessing data from the Nurses’ Health Studies II is described at https://www.nurseshealthstudy.org/researchers (email: nhsaccess@channing.harvard.edu).
References
- 1. Shiels MS, Chernyavskiy P, Anderson WF, et al. Trends in premature mortality in the USA by sex, race, and ethnicity from 1999 to 2014: an analysis of death certificate data. Lancet 2017;389:1043-54. 10.1016/S0140-6736(17)30187-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Norheim OF, Jha P, Admasu K, et al. Avoiding 40% of the premature deaths in each country, 2010-30: review of national mortality trends to help quantify the UN sustainable development goal for health. Lancet 2015;385:239-52. 10.1016/S0140-6736(14)61591-9. [DOI] [PubMed] [Google Scholar]
- 3. Case A, Deaton A. Rising morbidity and mortality in midlife among white non-Hispanic Americans in the 21st century. Proc Natl Acad Sci U S A 2015;112:15078-83. 10.1073/pnas.1518393112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gilbert R, Widom CS, Browne K, Fergusson D, Webb E, Janson S. Burden and consequences of child maltreatment in high-income countries. Lancet 2009;373:68-81. 10.1016/S0140-6736(08)61706-7. [DOI] [PubMed] [Google Scholar]
- 5. Organization WH. Global Health Observatory data repository—child maltreatment: global estimates. World Health Organization, 2014. [Google Scholar]
- 6.US Department of Health & Human Services Administration for Children and Families Administration on Children YaFCsB. Child maltreatment 2020. https://www.acf.hhs.gov/sites/default/files/documents/cb/cm2020.pdf 2020.
- 7. Obi IE, McPherson KC, Pollock JS. Childhood adversity and mechanistic links to hypertension risk in adulthood. Br J Pharmacol 2019;176:1932-50. 10.1111/bph.14576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dube SR, Anda RF, Felitti VJ, Chapman DP, Williamson DF, Giles WH. Childhood abuse, household dysfunction, and the risk of attempted suicide throughout the life span: findings from the Adverse Childhood Experiences Study. JAMA 2001;286:3089-96. 10.1001/jama.286.24.3089 [DOI] [PubMed] [Google Scholar]
- 9. Cuadra LE, Jaffe AE, Thomas R, DiLillo D. Child maltreatment and adult criminal behavior: does criminal thinking explain the association? Child Abuse Negl 2014;38:1399-408. 10.1016/j.chiabu.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 10. Rich-Edwards JW, Spiegelman D, Lividoti Hibert EN, et al. Abuse in childhood and adolescence as a predictor of type 2 diabetes in adult women. Am J Prev Med 2010;39:529-36. 10.1016/j.amepre.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rich-Edwards JW, Mason S, Rexrode K, et al. Physical and sexual abuse in childhood as predictors of early-onset cardiovascular events in women. Circulation 2012;126:920-7. 10.1161/CIRCULATIONAHA.111.076877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jakubowski KP, Murray V, Stokes N, Thurston RC. Sexual violence and cardiovascular disease risk: A systematic review and meta-analysis. Maturitas 2021;153:48-60. 10.1016/j.maturitas.2021.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Berens AE, Jensen SKG, Nelson CA, 3rd. Biological embedding of childhood adversity: from physiological mechanisms to clinical implications. BMC Med 2017;15:135. 10.1186/s12916-017-0895-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Godoy LC, Frankfurter C, Cooper M, Lay C, Maunder R, Farkouh ME. Association of adverse childhood experiences with cardiovascular disease later in life: a review. JAMA Cardiol 2021;6:228-35. 10.1001/jamacardio.2020.6050. [DOI] [PubMed] [Google Scholar]
- 15. Teicher MH, Samson JA, Anderson CM, Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci 2016;17:652-66. 10.1038/nrn.2016.111. [DOI] [PubMed] [Google Scholar]
- 16. Chandan JS, Okoth K, Gokhale KM, Bandyopadhyay S, Taylor J, Nirantharakumar K. Increased cardiometabolic and mortality risk following childhood maltreatment in the United Kingdom. J Am Heart Assoc 2020;9:e015855. 10.1161/JAHA.119.015855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Johnson J, Chaudieu I, Ritchie K, Scali J, Ancelin ML, Ryan J. The extent to which childhood adversity and recent stress influence all-cause mortality risk in older adults. Psychoneuroendocrinology 2020;111:104492. 10.1016/j.psyneuen.2019.104492. [DOI] [PubMed] [Google Scholar]
- 18. Jonson-Reid M, Drake B, Kohl PL. Childhood maltreatment, public service system contact, and preventable death in young adulthood. Violence Vict 2017;32:93-109. 10.1891/0886-6708.VV-D-14-00133. [DOI] [PubMed] [Google Scholar]
- 19. Lee C, White HR. Effects of childhood maltreatment on violent injuries and premature death during young adulthood among urban high-risk men. Arch Pediatr Adolesc Med 2012;166:814-20. 10.1001/archpediatrics.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rogers NT, Power C, Pinto Pereira SM. Child maltreatment, early life socioeconomic disadvantage and all-cause mortality in mid-adulthood: findings from a prospective British birth cohort. BMJ Open 2021;11:e050914. 10.1136/bmjopen-2021-050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Segal L, Armfield JM, Gnanamanickam ES, et al. Child maltreatment and mortality in young adults. Pediatrics 2021;147:e2020023416. 10.1542/peds.2020-023416. [DOI] [PubMed] [Google Scholar]
- 22. Chen E, Turiano NA, Mroczek DK, Miller GE. Association of reports of childhood abuse and all-cause mortality rates in women. JAMA Psychiatry 2016;73:920-7. 10.1001/jamapsychiatry.2016.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hailes HP, Yu R, Danese A, Fazel S. Long-term outcomes of childhood sexual abuse: an umbrella review. Lancet Psychiatry 2019;6:830-9. 10.1016/S2215-0366(19)30286-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kristman-Valente AN, Brown EC, Herrenkohl TI. Child physical and sexual abuse and cigarette smoking in adolescence and adulthood. J Adolesc Health 2013;53:533-8. 10.1016/j.jadohealth.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boynton-Jarrett R, Rosenberg L, Palmer JR, Boggs DA, Wise LA. Child and adolescent abuse in relation to obesity in adulthood: the Black Women’s Health Study. Pediatrics 2012;130:245-53. 10.1542/peds.2011-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Straus MA, Hamby SL, Boney-McCoy S, et al. The revised conflict tactics scales (CTS2) development and preliminary psychometric data. J Fam Issues 1996;17:283-316 10.1177/019251396017003001. [DOI] [Google Scholar]
- 27. Koss MP, Oros CJ. Sexual Experiences Survey: a research instrument investigating sexual aggression and victimization. J Consult Clin Psychol 1982;50:455-7. 10.1037/0022-006X.50.3.455 [DOI] [PubMed] [Google Scholar]
- 28. Boynton-Jarrett R, Rich-Edwards JW, Jun HJ, Hibert EN, Wright RJ. Abuse in childhood and risk of uterine leiomyoma: the role of emotional support in biologic resilience. Epidemiology 2011;22:6-14. 10.1097/EDE.0b013e3181ffb172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bernstein DP, Fink L, Handelsman L, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry 1994;151:1132-6. 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- 30. Harris HR, Wieser F, Vitonis AF, et al. Early life abuse and risk of endometriosis. Hum Reprod 2018;33:1657-68. 10.1093/humrep/dey248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rich-Edwards JWC, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol 1994;140:1016-9. 10.1093/oxfordjournals.aje.a117191 [DOI] [PubMed] [Google Scholar]
- 32.StatLine. Underlying cause of death (shortlist), sex, age. https://data.overheid.nl/en/dataset/4251-deaths--underlying-cause-of-death--shortlist---sex--age 2021.
- 33. Dean AG, West DJ, Weir WM. Measuring loss of life, health, and income due to disease and injury: a method for combining morbidity, mortality, and direct medical cost into a single measure of disease impact. Public Health Rep 1982;97:38-47. [PMC free article] [PubMed] [Google Scholar]
- 34. Wang YX, Arvizu M, Rich-Edwards JW, et al. Menstrual cycle regularity and length across the reproductive lifespan and risk of premature mortality: prospective cohort study. BMJ 2020;371:m3464. 10.1136/bmj.m3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang YX, Mínguez-Alarcón L, Gaskins AJ, et al. Association of spontaneous abortion with all cause and cause specific premature mortality: prospective cohort study. BMJ 2021;372:n530. 10.1136/bmj.n530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Field AE, Franko DL, Striegel-Moore RH, Schreiber GB, Crawford PB, Daniels SR. Race differences in accuracy of self-reported childhood body size among white and black women. Obes Res 2004;12:1136-44. 10.1038/oby.2004.142. [DOI] [PubMed] [Google Scholar]
- 37. Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142:1009-18. 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang YX, Arvizu M, Rich-Edwards JW, et al. Breastfeeding duration and subsequent risk of mortality among US women: A prospective cohort study. EClinicalMedicine 2022;54:101693. 10.1016/j.eclinm.2022.101693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vetter C, Chang SC, Devore EE, Rohrer F, Okereke OI, Schernhammer ES. Prospective study of chronotype and incident depression among middle- and older-aged women in the Nurses’ Health Study II. J Psychiatr Res 2018;103:156-60. 10.1016/j.jpsychires.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Farvid MS, Qi L, Hu FB, et al. Phobic anxiety symptom scores and incidence of type 2 diabetes in US men and women. Brain Behav Immun 2014;36:176-82. 10.1016/j.bbi.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wolf AMH, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol 1994;23:991-9. 10.1093/ije/23.5.991 [DOI] [PubMed] [Google Scholar]
- 42. Forman JP, Curhan GC, Taylor EN. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension among young women. Hypertension 2008;52:828-32. 10.1161/HYPERTENSIONAHA.108.117630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Colditz GA, Martin P, Stampfer MJ, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol 1986;123:894-900. 10.1093/oxfordjournals.aje.a114319 [DOI] [PubMed] [Google Scholar]
- 44. Troy LM, Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, Willett WC. The validity of recalled weight among younger women. Int J Obes Relat Metab Disord 1995;19:570-2. [PubMed] [Google Scholar]
- 45. Yuan C, Spiegelman D, Rimm EB, et al. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am J Epidemiol 2017;185:570-84. 10.1093/aje/kww104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song M, Zhou X, Pazaris M, et al. The missing covariate indicator method is nearly valid almost always. arXiv preprint arXiv:211100138 2021.
- 47. Wang YX, Li Y, Rich-Edwards JW, et al. Associations of birth weight and later life lifestyle factors with risk of cardiovascular disease in the USA: A prospective cohort study. EClinicalMedicine 2022;51:101570. 10.1016/j.eclinm.2022.101570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li R, Chambless L. Test for additive interaction in proportional hazards models. Ann Epidemiol 2007;17:227-36. 10.1016/j.annepidem.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 49. Lin DY, Fleming TR, De Gruttola V. Estimating the proportion of treatment effect explained by a surrogate marker. Stat Med 1997;16:1515-27. . [DOI] [PubMed] [Google Scholar]
- 50. Wang YX, Mínguez-Alarcón L, Gaskins AJ, et al. Pregnancy loss and risk of cardiovascular disease: the Nurses’ Health Study II. Eur Heart J 2022;43:190-9. 10.1093/eurheartj/ehab737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kelly-Irving M, Lepage B, Dedieu D, et al. Adverse childhood experiences and premature all-cause mortality. Eur J Epidemiol 2013;28:721-34. 10.1007/s10654-013-9832-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Brown DW, Anda RF, Tiemeier H, et al. Adverse childhood experiences and the risk of premature mortality. Am J Prev Med 2009;37:389-96. 10.1016/j.amepre.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 53. Ng QX, Yong BZJ, Ho CYX, Lim DY, Yeo WS. Early life sexual abuse is associated with increased suicide attempts: An update meta-analysis. J Psychiatr Res 2018;99:129-41. 10.1016/j.jpsychires.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 54. Wong WH, Kuo WH, Sobolewski C, Bhatia I, Ip P. The association between child abuse and attempted suicide. Crisis 2020;41:196-204. 10.1027/0227-5910/a000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Santaularia J, Johnson M, Hart L, Haskett L, Welsh E, Faseru B. Relationships between sexual violence and chronic disease: a cross-sectional study. BMC Public Health 2014;14:1286. 10.1186/1471-2458-14-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Drossman DA, Talley NJ, Leserman J, Olden KW, Barreiro MA. Sexual and physical abuse and gastrointestinal illness. Review and recommendations. Ann Intern Med 1995;123:782-94. 10.7326/0003-4819-123-10-199511150-00007. [DOI] [PubMed] [Google Scholar]
- 57. Hughes K, Bellis MA, Hardcastle KA, et al. The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. Lancet Public Health 2017;2:e356-66. 10.1016/S2468-2667(17)30118-4. [DOI] [PubMed] [Google Scholar]
- 58. Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med 1998;14:245-58. 10.1016/S0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 59. Bellis MA, Hughes K, Leckenby N, Hardcastle KA, Perkins C, Lowey H. Measuring mortality and the burden of adult disease associated with adverse childhood experiences in England: a national survey. J Public Health (Oxf) 2015;37:445-54. 10.1093/pubmed/fdu065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hemmingsson E, Johansson K, Reynisdottir S. Effects of childhood abuse on adult obesity: a systematic review and meta-analysis. Obes Rev 2014;15:882-93. 10.1111/obr.12216. [DOI] [PubMed] [Google Scholar]
- 61. Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA 2013;309:71-82. 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chiang JJ, Chen E, Miller GE. Midlife self-reported social support as a buffer against premature mortality risks associated with childhood abuse. Nat Hum Behav 2018;2:261-8. 10.1038/s41562-018-0316-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chen LP, Murad MH, Paras ML, et al. Sexual abuse and lifetime diagnosis of psychiatric disorders: systematic review and meta-analysis. Mayo Clin Proc 2010;85:618-29. 10.4065/mcp.2009.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lindert J, von Ehrenstein OS, Grashow R, Gal G, Braehler E, Weisskopf MG. Sexual and physical abuse in childhood is associated with depression and anxiety over the life course: systematic review and meta-analysis. Int J Public Health 2014;59:359-72. 10.1007/s00038-013-0519-5. [DOI] [PubMed] [Google Scholar]
- 65. Kisely S, Abajobir AA, Mills R, et al. Child Maltreatment and Persistent Smoking From Adolescence Into Adulthood: A Birth Cohort Study. Nicotine Tob Res 2020;22:66-73. 10.1093/ntr/ntz039. [DOI] [PubMed] [Google Scholar]
- 66. Jun HJ, Rich-Edwards JW, Boynton-Jarrett R, Austin SB, Frazier AL, Wright RJ. Child abuse and smoking among young women: the importance of severity, accumulation, and timing. J Adolesc Health 2008;43:55-63. 10.1016/j.jadohealth.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kraynak TE, Marsland AL, Hanson JL, Gianaros PJ. Retrospectively reported childhood physical abuse, systemic inflammation, and resting corticolimbic connectivity in midlife adults. Brain Behav Immun 2019;82:203-13. 10.1016/j.bbi.2019.08.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bertone-Johnson ER, Whitcomb BW, Missmer SA, Karlson EW, Rich-Edwards JW. Inflammation and early-life abuse in women. Am J Prev Med 2012;43:611-20. 10.1016/j.amepre.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Slopen N, Koenen KC, Kubzansky LD. Childhood adversity and immune and inflammatory biomarkers associated with cardiovascular risk in youth: a systematic review. Brain Behav Immun 2012;26:239-50. 10.1016/j.bbi.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 70. Orta OR, Huang T, Kubzansky LD, et al. The association between abuse history in childhood and salivary rhythms of cortisol and DHEA in postmenopausal women. Psychoneuroendocrinology 2020;112:104515. 10.1016/j.psyneuen.2019.104515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lang J, McKie J, Smith H, et al. Adverse childhood experiences, epigenetics and telomere length variation in childhood and beyond: a systematic review of the literature. Eur Child Adolesc Psychiatry 2020;29:1329-38. 10.1007/s00787-019-01329-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hyman SE. How adversity gets under the skin. Nat Neurosci 2009;12:241-3. 10.1038/nn0309-241. [DOI] [PubMed] [Google Scholar]
- 73. Perroud N, Zewdie S, Stenz L, et al. Methylation of serotonin receptor 3a in adhd, borderline personality, and bipolar disorders: link with severity of the disorders and childhood maltreatment. Depress Anxiety 2016;33:45-55. 10.1002/da.22406. [DOI] [PubMed] [Google Scholar]
- 74. Williams RB. Psychosocial and biobehavioral factors and their interplay in coronary heart disease. Annu Rev Clin Psychol 2008;4:349-65. 10.1146/annurev.clinpsy.4.022007.141237. [DOI] [PubMed] [Google Scholar]
- 75. Oland AA, Booster GD, Bender BG. Psychological and lifestyle risk factors for asthma exacerbations and morbidity in children. World Allergy Organ J 2017;10:35. 10.1186/s40413-017-0169-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hardt J, Rutter M. Validity of adult retrospective reports of adverse childhood experiences: review of the evidence [published Online First: 2004/02/26]. J Child Psychol Psychiatry 2004;45:260-73. 10.1111/j.1469-7610.2004.00218.x [DOI] [PubMed] [Google Scholar]
- 77. Finkelhor D. Trends in Adverse Childhood Experiences (ACEs) in the United States. Child Abuse Negl 2020;108:104641. 10.1016/j.chiabu.2020.104641. [DOI] [PubMed] [Google Scholar]
- 78. Widom CS, Shepard RL. Accuracy of adult recollections of childhood victimization: Part 1. Childhood physical abuse. Psychol Assess 1996;8:412 10.1037/1040-3590.8.4.412. [DOI] [Google Scholar]
- 79. Widom CS, Morris S. Accuracy of adult recollections of childhood victimization, part 2: childhood sexual abuse. Psychol Assess 1997;9:34 10.1037/1040-3590.9.1.34. [DOI] [Google Scholar]
- 80. Assink M, van der Put CE, Meeuwsen MWCM, et al. Risk factors for child sexual abuse victimization: A meta-analytic review. Psychol Bull 2019;145:459-89. 10.1037/bul0000188. [DOI] [PubMed] [Google Scholar]
- 81. Keenan HT, Cook LJ, Olson LM, Bardsley T, Campbell KA. Social intuition and social information in physical child abuse evaluation and diagnosis. Pediatrics 2017;140:e20171188. 10.1542/peds.2017-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplemental material
Data Availability Statement
Data described in the manuscript, code book, and analytic code will not be made publicly available. Further information including the procedures for obtaining and accessing data from the Nurses’ Health Studies II is described at https://www.nurseshealthstudy.org/researchers (email: nhsaccess@channing.harvard.edu).


