Abstract
The circumsporozoite protein (CSP) from the surface of sporozoite stage Plasmodium sp. malaria parasites is among the most important of the malaria vaccine candidates. Gene gun injection of genetic vaccines encoding Plasmodium berghei CSP induces a significant protective effect against sporozoite challenge; however, intramuscular injection does not. In the present study we compared the immune responses and protective effects induced by P. berghei CSP genetic vaccines delivered intradermally with a needle or epidermally with a gene gun. Mice were immunized three times at 4-week intervals and challenged by a single infectious mosquito bite. Although 50 times more DNA was administered by needle than by gene gun, the latter method induced significantly greater protection against infection. Intradermal injection of the CSP genetic vaccine induced a strong Th1-type immune response characterized by a dominant CSP-specific immunoglobulin G2a (IgG2a) humoral response and high levels of gamma interferon produced by splenic T cells. Gene gun injection induced a predominantly Th2-type immune response characterized by a high IgG1/IgG2a ratio and significant IgE production. Neither method generated measurable cytotoxic T lymphocyte activity. The results indicate that a gene gun-mediated CS-specific Th2-type response may be best for protecting against malarial sporozoite infection when the route of parasite entry is via mosquito bite.
Vaccination by using “naked” plasmid DNA is revolutionizing vaccine development. Genetic vaccines have been employed in animal studies to induce protective immune responses against a variety of viruses, bacteria, and parasites (5), and preliminary Phase I testing has been conducted in humans (30, 34).
Because genetic vaccination induces a response in both the humoral and cellular arms of the immune system, this approach offers new opportunities in malaria vaccine development. Genetic vaccines encoding the circumsporozoite protein (CSP) gene from Plasmodium yoelii (28) and Plasmodium berghei (17) protected against malaria infection in BALB/c mice. Intramuscular (i.m.) needle injection of the P. yoelii CSP vaccine induced a significant protective effect against an intravenous sporozoite challenge (28). Epidermal (e.d.) injection of the P. berghei CSP vaccine conferred a significant protective effect against a challenge by infectious mosquito, but intramuscular injection did not (17). i.m. immunization with the P. yoelii CSP vaccine initially induced an interleukin 4 (IL-4)-dependent Th2-type response (19) that quickly switched to a Th1-type response characterized by upregulation of gamma interferon (IFN-γ), induction of high titers of immunoglobulin G2a (IgG2a), and cytotoxic leukocyte (CTL) activity after boosting. Epidermal immunization with the P. berghei CSP vaccine resulted in a much slower progression from a Th2-type response toward a Th1-type response, which was measured by IgG1-to-IgG2a isotype switching (17). Although CTLs specific to the known H-2Kd class I epitopes of P. berghei were not detected, the shift from IgG1 to IgG2a indicated that a Th1-type immune response might be important for protection.
In other CSP genetic vaccine studies, protection against sporozoite infection was observed for animals vaccinated with mixtures of plasmids expressing the CSP gene and the granulocyte-macrophage colony-stimulating factor gene injected i.m. (35), mixtures of plasmids expressing different malaria antigens injected i.m. (7), and boosting with recombinant CSP vaccinia virus after priming with a minigene construction injected e.d. by using a gene gun (26) or a CSP genetic vaccine injected i.m. (29).
Intradermal (i.d.) needle injection (25), like i.m. injection, generally induces a strong Th1-type response (8, 13, 22). The induction of Th1-type responses by i.d. and i.m. injection and of Th2-type responses by gene gun injection (10, 11, 21, 32) can be explained by the amount of DNA injected (1).
The objective of this work was to define the type of immune response induced by i.d. injection of a P. berghei CS genetic vaccine and to determine if the response could protect against challenge with malaria by infectious mosquito bite.
MATERIALS AND METHODS
Construction of vectors.
Construction of the expression vector WRG-6518 was reported previously (17). In this vector, the natural CSP signal sequence for P. berghei CSP was replaced with the human tissue plasminogen activator (hTPA) signal sequence. The plasmid pCMV-hTPA/CSP was prepared by using PCR to amplify the hTPA/CSP insert in WRG-6518 (sense primer, 5′-GGGCTCGAGATGGATGCAATGAAG-3′; antisense primer, 5′-CCCGCGGCCGCTTAATTAAAGAATACTAATACTAAT-3′), and the product was cloned into the eukaryotic expression vector pCI (Promega, Madison, Wis.) via the XhoI and NotI restriction sites within the vector's multiple cloning site. The insert's sequence was verified by using an ABI Prism genetic analyzer (Perkin-Elmer, Norwalk, Conn.) and was identical to the CSP gene sequence within WRG-6518. The plasmid was propagated in Escherichia coli Xl1-blue (Stratagene, La Jolla, Calif.). Large-scale purification of the expression vector was conducted with Endo Free Plasmid Giga kits (Qiagen, Hilden, Germany) according to the manufacturer's protocol. The plasmid DNA was stored in endotoxin-free H2O at −20°C.
Animals and immunization protocol.
Mice used for immunizations were 6- to 8-week-old BALB/c females from the Jackson Laboratory (Bar Harbor, Maine) and Himberg (Himberg, Austria). Sera were collected before the first immunization and at weekly intervals thereafter. Sera were preserved by adding sodium azide (final concentration, 0.2%) and stored at 4°C. Mice were vaccinated three or four times at 4-week intervals. For i.d. needle injection, the backs of anesthetized mice were shaved and injected with 100 μg of plasmid DNA in 100 μl of sterile phosphate-buffered saline (PBS) divided equally between two sites. For gene gun vaccination, plasmid DNA was precipitated onto gold beads (diameter, 1.6 μm) with CaCl2 in the presence of spermidine at a loading rate of 2 μg of DNA per milligram of gold. Mice received a total of 2 μg of DNA, divided between two nonoverlapping areas, on the shaved abdomen, at a helium pressure of 400 lb/in2 as described previously (17).
Analysis of antigen-specific antibody production by ELISA.
Black 96-well high-bind immunoplates (Greiner, Kremsmuenster, Austria) were coated by overnight incubation at room temperature with a synthetic peptide containing three copies of the CSP repeat epitope (DPPPPNPN) at a concentration of 1 μg/ml in PBS. Plates were washed with water using an AW1 automatic enzyme-linked immunosorbent assay (ELISA) plate washer (Anthos Labtec, Salzburg, Austria) and blocked with blocking buffer (0.015 M Na2B4O7 · 10H2O, 0.12 M NaCl, 0.05% Tween 20, 1 mM EDTA, 0.25% bovine serum albumin, 0.05% NaN3 [pH 8.5]) for 2 h at room temperature. Sera were serially diluted in blocking buffer, added to the plates, and incubated for 2 h at room temperature to capture immunoglobulin. Horseradish peroxidase-conjugated detection antibodies prepared with goat anti-mouse IgG (Bio-Rad, Hercules, Calif.), rat anti-mouse IgG2a (PharMingen, San Diego, Calif.), or rat anti-mouse IgG1 (Biosource, Camarillo, Calif.) were diluted 1:1,000 in blocking buffer and reacted for 2 h at room temperature. The assay was developed with Luminol (BM chemiluminescence substrate; Boehringer Mannheim, Mannheim, Germany) diluted 1:2 in H2O. Chemiluminescence (photon counts/second) was quantified by using a Lucy I ELISA-plate Luminometer (Anthos Labtec).
In order to control for plate-to-plate variation, the endpoint titer of CSP-specific IgG in test sera was determined by interpolation from standard curves prepared with high-titered reference antiserum run on each ELISA plate. Wells with a luminescence greater than 3 standard deviations (SD) above the background level were scored as positive. Background levels were calculated by using the average luminescence value from at least 20 wells lacking primary antibodies.
For the detection of serum IgM and IgE, diluted sera were incubated for 3 or 14 h, respectively. Anti-IgE secondary antibody was incubated for 4 h (rat anti-mouse IgE; Biotrade, Vienna, Austria), and sheep anti-mouse IgM (Sigma-Aldrich, Deisenhofen, Germany) was incubated for 2 h.
IFA.
Two weeks after the fourth immunization, antibody titers were determined by indirect immunofluorescence assay (IFA) and P. berghei ANKA strain sporozoites were placed on multispot glass slides, air dried, and stored at −20°C until used. Sporozoites were fixed with ice-cold methanol and incubated with blocking buffer for 1 h at 37°C. Sera were diluted 10-fold in blocking buffer and incubated for 90 min. Slides were washed three times with H2O and incubated for 90 min with fluorescein isothiocyanate-labeled goat anti-mouse IgG (H+L) (Zymed, San Francisco, Calif.). After repeated washings, slides were mounted in PBS containing 50% (vol/vol) glycerol and 50 mM dithioerythritol to reduce bleaching. Digital images of the sporozoites were obtained in a UV microscope and analyzed for integrated fluorescence density as a relative measurement of antibody titer. For each serum sample, the average integrated fluorescence density of more than 20 randomly chosen sporozoites was determined.
Proliferative responses.
Splenocytes were prepared 2 weeks after the final immunization, were resuspended in Dulbecco's modified Eagle's medium supplemented with 100 U of penicillin and streptomycin/ml, 5% heat-inactivated fetal calf serum (FCS), 2 × 10−6 M 2-Me, 1 mM sodium pyruvate, and 2 mM l-glutamine, and were distributed into 96-well, flat-bottom tissue culture plates (Becton Dickinson, Franklin Lakes, N.J.) at a density of 106 cells/well. Wells were treated with 20 μg of peptide/ml containing a class II epitope. Peptides used included CS 57-70, CS 260-279, or CS 242-279 (18). Additionally, CS 242-279 contains an H-2Kd class I epitope and CS 57-70 contains a motif consistent with binding to H-2Kd class I (16). Five replicate wells were stimulated with each peptide for 52 h under conditions of 37°C, 95% relative humidity, and 7.5% CO2. Wells were pulsed with 25 μCi of [3H]thymidine (Amersham, Buckinghamshire, United Kingdom)/ml for an additional 20 h and then harvested with a cell harvester (Skatron, Lier, Norway). [3H]Thymidine incorporation was measured in a liquid scintillation counter (Beckman Coulter, Fullerton, Calif.).
Quantification of cytokines in proliferation assay supernatants.
IFN-γ and IL-4 in supernatants from splenocytes stimulated in vitro with CSP peptides were quantified by sandwich ELISA using the OptEIA system (PharMingen). Briefly, cytokines were captured with monoclonal anti-mouse IFN-γ or anti-mouse IL-4 antibodies. Cytokines were identified by adding biotinylated anti-IFN-γ or anti-IL-4 followed by avidin-conjugated horseradish peroxidase. The luminometric assay described above was used to detect reactions, and the cytokines were quantified by extrapolation from a standard curve prepared with recombinant murine IFN-γ or IL-4 (PharMingen, San Diego, Calif.).
Challenge.
Fourteen days after the third immunization, mice were challenged by a single infectious mosquito bite as described previously (17). Infection was determined by the presence of blood stage parasites in Giemsa-stained thin blood smears prepared 7 and 14 days after challenge.
Statistical analysis.
Serology and proliferation assays were performed on groups of four mice each. Data are expressed as the mean ± the standard error of the mean (SEM). Statistical significance was assessed using an unpaired Student's t test. For the challenge experiments, a group size of 10 mice was used. To evaluate the protective effect of the vaccination, Fisher's exact test was used to compare differences between the control group and the immunized groups. This test was also used to compare the different immunized groups.
RESULTS
Immune responses induced by gene gun immunization or needle injection of DNA differ quantitatively.
BALB/c mice were vaccinated four times with pCMV-TPA/CS at 4-week intervals either by gene gun immunization or needle injection. Serum samples were collected weekly and tested for antibodies specific for the CSP repeat epitope (Fig. 1).
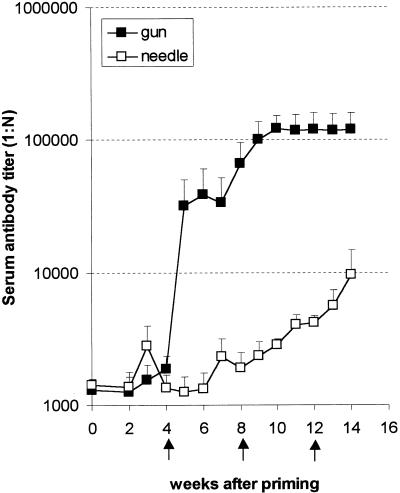
FIG. 1.
Gene gun immunization induces a superior antibody response compared to needle injection into the same tissue. Immunizations were performed four times at 4-week intervals. BALB/c mice (four mice/group) received either 100 μg of pCMV-TPA/CS in saline by needle injection or 2 μg of pCMV-TPA/CS by the gene gun method. Sera were collected from individual mice and analyzed for anti-CS repeat epitope IgG antibody by ELISA. Data are shown as the mean ± the SEM of endpoint titers of each group.
Needle injection induced a twofold increase in titers of antibody by week 3 after priming, and this level returned to baseline by week 4. Boosting at week 4 induced nearly a twofold increase in antibody titer by week 7, but the antibody levels began dropping at week 8. Boosting again at week 8 caused a 50% increase in the levels of antibody by week 11, and boosting at week 12 caused an additional twofold increase in the titer of antibody by week 14. Needle injection induced an overall 10-fold increase over the baseline titer.
Gene gun injection did not significantly increase the titer of antibody until week 4 after priming, and this titer never returned to baseline levels. Boosting at week 4 induced a 20-fold increase in the antibody titer by week 8. Boosting at week 8 caused an additional threefold increase that reached a plateau at week 10 and was not boosted further. Gene gun injection induced an overall 100-fold increase in the antibody titer over the baseline titer.
Determination of IgG by IFA at week 14 revealed that gene gun injection induced approximately 2.5 times more sporozoite-reactive antibody than needle injection. The average integrated density for the gene gun-injected group was 36,770 ± 4243, and that for the needle-injected group was 14,324 ± 3638. The IFA experiment supports the ELISA data and confirms that the antibodies recognize native CSP.
IgG responses induced by gene gun immunization or needle injection of DNA differ qualitatively.
The subclass distribution of serum IgG1 and IgG2a antibodies was examined during the course of immunization and used as an indicator of the type of immune response induced (Th1 versus Th2).
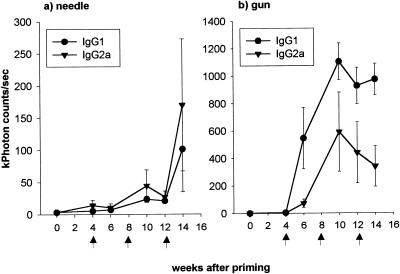
Figure 2 shows that gene gun and i.d. needle administration of pCMV-TPA/CS induced different distributions of IgG1 and IgG2a. Two weeks following the fourth immunization (14 weeks after priming), the IgG1:IgG2a ratio was 0.60:1 for the needle-injected group and 2.86:1 for the gene gun-injected group. A predominant IgG2a response was observed throughout the needle injection regimen. Initially, gene gun injection induced a strongly biased IgG1 response (IgG1:IgG2a ratio ≅ 8:1) that became more balanced with IgG2a (IgG1:IgG2a ratio ≅ 2.86:1) after four immunizations.
FIG. 2.
Effect of the route of immunization on the subclass distribution of antibodies in serum after needle (a) and gene gun (b) immunization. PbCSP-repeat epitope-specific IgG1 and IgG2a levels were measured at weeks 4, 6, 10, 12, and 14. The results are presented as the mean ± the SEM of each group (n = 4 mice), expressed in kilophoton counts/second.
Gene gun immunization induced an earlier CSP-specific IgM response and a stronger CSP-specific IgE antibody response.
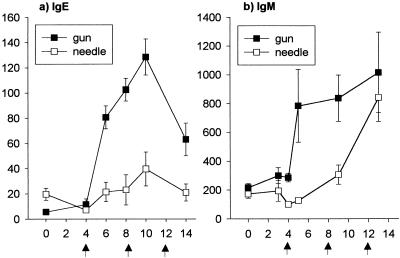
Gene gun vaccination induced a CSP-specific IgM response, but the response did not appear until 1 week after the second immunization, at which time it reached a plateau, where it remained for the duration of the experiment (Fig. 3b). Furthermore, gene gun vaccination induced a CSP-specific IgE response, which appeared after the second immunization, peaked 2 weeks after the third immunization, and began dropping after the last immunization. (Fig. 3a).
FIG. 3.
CS-specific IgE (a) and IgM (b) titers of the respective immunization groups. Sera taken at weeks 0, 4, 6, 8, 10, and 14 for IgE and 0, 3, 4, 5, 9, and 13 for IgM were evaluated by ELISA. The results are presented as the mean ± the SEM of each group (n = 4 mice), expressed in kilophoton counts/second.
Needle vaccination also induced CSP-specific IgM antibody, but the response did not exceed baseline levels until after the fourth immunization (Fig. 3b), at which time it approached the levels induced by gene gun injection. Needle injection may have induced a low level of CSP-specific IgE antibodies, but at their highest levels they were not significantly different from background levels (Fig. 3a).
Neither modality of immunization induced an in vitro proliferative response or a CTL response.
Proliferative responses against all peptides used were weak. Gene gun injection and needle injection of the CSP genetic vaccine yielded stimulation indices ranging from 0.83 to 1.24 and 1.06 to 1.56, respectively, for the T-helper epitopes contained within the peptides CS 57-70, CS 260-279, and CS 242-279; these responses were not significantly different from results for controls (Table 1). No CTL responses to any of the peptides were detected (data not shown).
TABLE 1.
Proliferative response against the Th epitopes CS57-70 and CS 260-279 and peptide CS242-279
| Method of vaccination | Response toa:
|
|||
|---|---|---|---|---|
| CS57-70 | CS260-79 | CS242-79 | Ova | |
| None | 0.76 | 1.26 | 0.95 | 0.95 |
| Needle | 1.13b | 1.06 | 1.56 | 0.84 |
| Gun | 0.83 | 1.24 | 0.85 | 1.00 |
Data shown are average stimulation indices (n = 4 mice). Ovalbumin (Ova) was used as a control protein for stimulation.
Only peptide CS57-70 in the needle group stimulated a significant proliferative response compared to results for the control (P < 0.05).
Only splenocytes from needle-injected mice recall a detectable cytokine response in vitro.
In vitro stimulation with peptides CS 57-70 and CS 242-279, which both contain a class I and a class II epitope, recalled 25 times more IFN-γ from splenic lymphocytes prepared from needle-injected mice than from control mice. However, stimulation of these cells with peptide CS 260-79, which is the C-terminal part of CS 242-279 and contains only a class II epitope, failed to recall this IFN-γ response (Table 2). None of these peptides recalled an IL-4 response from splenocytes obtained from needle-injected mice (Table 2), nor did they recall either an IFN-γ or an IL-4 response from splenocytes obtained from gene gun-vaccinated mice (data not shown).
TABLE 2.
Cytokines in supernatants of proliferation cultures after four immunizations
| Method of vaccination | Cytokine | Response (pg/ml) toa:
|
||
|---|---|---|---|---|
| CS57-70 | CS260-79 | CS242-79 | ||
| Needle | IFN-γ | 3,867 | 151 | 2,328 |
| IL-4 | 4.2 | 0.7 | 5.4 | |
| None | IFN-γ | −89 | 90 | −71 |
| IL-4 | −6.7 | 1.1 | −2.8 | |
Values are the mean increase in medium (n = 4 mice).
Gene gun immunization provided significant protection against malaria, but needle injection did not.
BALB/cJ mice were vaccinated three times at 4-week intervals either with WRG-6518 or with pCMV-TPA/CS given by needle injection or gene gun injection. Vaccinated mice were challenged by a single infectious mosquito bite 14 days after the last immunization (Table 3). Infection rates in vaccinated mice were compared with those in cohort-matched naïve control mice. The strong reduction in the infection rate produced by gene gun injection of either WRG-6518 (1 of 10 mice infected) or pCMV-TPA/CS (2 of 9 mice infected) was significant when compared to results for naïve control mice (9 of 10 mice infected; P < 0.05). A weak reduction in the infection rate was produced by needle injection of either plasmid (with WRG-6518, 6 of 9 mice were infected; with pCMV-TPA/CS, 6 of 10 mice were infected), but this effect was not significant when compared to results for naïve control mice (P > 0.05). Overall, the reduction in the infection rate obtained by gene gun injection (3 of 19 mice infected) was significantly greater (P < 0.05) than that obtained by needle injection (12 of 19 mice infected).
TABLE 3.
Challenge results after vaccination with pCMV-TPA/CS and WRG-6518 inoculated i.d. by needle injection or with a gene guna
| Vaccine | Method | I/n | % Efficacy |
|---|---|---|---|
| None | 9/10 | ||
| pCMV-TPA/CS | Gun | 2/9 | 78 (P = 0.005) |
| pCMV-TPA/CS | Needle | 6/10 | 33 (P = 0.152) |
| WRG-6518 | Gun | 1/10 | 89 (P = 0.001) |
| WRG-6518 | Needle | 6/9 | 33 (P = 0.249) |
Mice were immunized on days 0, 28, and 56 and challenged 70 days after priming. I/n is the incidence rate, where I is the number of animals with parasitemia and n is the total number of animals in the group. Efficacy = 1 − lv/lc, where Iv and Ic are the incidence rates in the vaccinated and control groups, respectively. P was calculated via Fisher's exact test (one tail).
DISCUSSION
Multiple factors influence the immune responses induced by genetic vaccination. Early studies identified the immunization site as one of these factors (2–4, 12, 31, 33); however, more recently it has become clear that the amount of plasmid DNA injected, which is related to the amount of CpG motifs administered, also plays an important role (1). Previously, we showed that low doses of DNA targeted to the skin by using gene gun injection gave a stronger protective immunity against sporozoite challenge by infectious mosquito bite than large doses of DNA given in muscle by needle injection (17). In that study, we suggested that the induction of Th1-type immune responses might account for the protective effect of the genetic vaccine, as had been observed by others (19, 28). In the present study, we evaluated the protective efficacy and the nature of the immune responses induced by targeting skin with a malaria genetic vaccine delivered e.d. by gene gun and i.d. by needle. Efficacy was tested by challenging by an infectious mosquito bite. Because e.d. injection of small amounts of DNA induces Th2-type responses (12–15) and i.d. injection of larger amounts of DNA induces Th1-type responses (8, 13, 22), they may differ in their abilities to protect against challenge.
Analysis of the immune responses that were induced showed that the kinetics and levels of immunoglobulin synthesis and types of cellular immune response differed for the two modalities. First, although neither approach induced a significant IgM response after the first immunization, gene gun vaccination induced maximal IgM titers after the second immunization which were maintained throughout the regimen. The IgM response induced by needle injection approached that induced by the gene gun only after the fourth immunization. Second, gene gun vaccination with the regimen used here induced primarily a Th2-type immune response (high IgG1:IgG2a ratio; high IgE), and needle injection induced a Th1-type immune response (low IgG1:IgG2a ratio; no IgE; IFN-γ response in splenocytes stimulated with CSP peptides bearing major histocompatibility complex [MHC] class I epitopes). Third, for a gene gun-injected genetic vaccine, the initial immune response was strongly polarized towards a Th2-type response (IgG1:IgG2a ratio, ∼8:1), which changed slightly upon additional immunization (IgG1:IgG2a ratio, ∼2.8:1) and down regulation of CSP-specific IgE after the third immunization, whereas for needle injection the strong Th1-type immune response was maintained throughout the regimen (constant IgG1:IgG2a ratio, ∼0.6). Finally, the total CSP-specific IgG produced by gene gun vaccination exceeded that induced by needle injection by 2.5-fold in the case of IFA against sporozoites and by 10-fold in the case of ELISA against the peptide corresponding to the repetitive CS epitope. These results are consistent with data obtained by immunofluorescent antibody analysis of the IgG, IgG1, and IgG2a titers in prechallenge sera taken from the challenge cohort (not shown).
The dominance of Th1-type immune responses after i.m. (10) and i.d. (25) genetic immunization and the preferential induction of Th2 responses by gene gun immunization (10) have been reported previously. The induction of the Th1-type responses by genetic vaccination has been attributed in part to the dose of DNA administered (1) and to the presence of immunostimulatory DNA sequences containing CpG motifs (14, 15, 25). Although the CSP gene proper sequence lacks CpG motifs, the pCI plasmid backbone contains 38 motifs (data not shown).
Given that IgM production is usually thought of as being characteristic for a primary immune response, the appearance of CSP-specific IgM only after the second gene gun immunization is intriguing. One possible interpretation of this result is that the threshold level of antigen required to induce an immune response was not achieved until the second gene gun immunization, and once this antigen threshold was reached, further immunizations did not improve the IgM response. It has been observed that a plasmid capable of expressing antigen persists for up to 14 weeks after injection (S. A. Johnston, personal communication). Thus, waiting a sufficient period of time after priming might allow an accumulation of antigen that is sufficient to permit IgM induction without further immunization.
Proliferative cellular responses to three different CSP peptides bearing MHC class II epitopes were not detected after immunization with either method, nor were IL-4 responses recalled. However, peptides CS 57-70 and CS 242-279, both of which contain a class I and a class II T-cell epitope (16, 18), were able to recall CSP-specific IFN-γ responses from splenocytes prepared from needle-vaccinated mice but not from those prepared from gene gun-vaccinated mice. The peptide CS 260-279, which contains a class II epitope but not a class I epitope, did not recall this response from mice vaccinated by either modality. Because both CS 57-70 and CS 242-279 contain a class I epitope, the source of this IFN-γ response may be CSP-specific CD8+ T-cells.
Different approaches to vaccination against sporozoite infection induce different effector responses. Protection induced by immunization with X-irradiated P. berghei sporozoites depends upon the response of antibody, CD8+ T cells, and IFN-γ (27) as well as MHC class I (36) but not CD8+ CTLs (23). Protection induced by immunization with recombinant adenovirus-CSP against P. yoelii sporozoite infection depends upon CD8+ T-cells but not upon IFN-γ (24). Protection induced by i.m. immunization with a P. yoelii CSP genetic vaccine depends upon CD8+ T-cells but not CTL and on non-T-cell-derived cytokines (6). Our data show that i.d. needle injection of a genetic vaccine was the only modality that induced a measurable IFN-γ response, yet the protective effect induced by this approach was not significant compared to results for naïve control animals.
Our present data show that e.d. injection with low doses of plasmid by using a gene gun induced a strong protective effect against sporozoite challenge delivered by a single infectious mosquito bite, whereas i.d. injection with high doses of plasmid by using a needle did not. These results suggest the possibility that the active protective principle may lie within skin-localized Th2-type immunity. There are two options to consider. First, we show for the first time that production of CSP-specific IgE can be induced by genetic vaccination and that the kinetics of this IgE induction correlates with protection against sporozoite infection. Although IgE in association with immune complexes has been reported to contribute to cerebral malaria (20), its effect on the sporozoite stage is not known. Second, the IgG1 titer induced by gene gun vaccination is significantly higher than that induced by i.d. injection. At least part of this increased IgG1 may correspond to that subpopulation whose production is dependent upon IL-4 rather than IL-12. IL-4-dependent IgG1 is able to bind to FcRIII and stimulate mast cell degranulation, but the IL-12-dependent population is not (9).
Although these results and our previous results (17) show that low doses of CS genetic vaccines given by a gene gun induced a predominately Th2-type immune response whereas larger doses of vaccine given i.m. or i.d with a needle induced a predominately Th1-type response, a clear understanding of the protective effector mechanism has not been elucidated. Both studies show that significant protection against challenge by infectious mosquito appeared to be associated with the induction of the Th2-type response; however, it is not known whether this type of response would provide significant protection against the more widely used method of intravenous challenge.
ACKNOWLEDGMENTS
This work was partly supported by the Fonds zur Förderung der wissenschaftlichen Forschung (P13827-Med), the Ludwig-Boltzmann-Institute for Experimental Surgery (O. Boeckl), and the Jubiläumsfondsprojekt 6975/3 of the National Bank of Austria.
REFERENCES
- 1.Barry M A, Johnston S A. Biological features of genetic immunization. Vaccine. 1997;15:788–791. doi: 10.1016/s0264-410x(96)00265-4. [DOI] [PubMed] [Google Scholar]
- 2.Boyle J S, Silva A, Brady J L, Lew A M. DNA immunization: induction of higher avidity antibody and effect of route on T cell cytotoxicity. Proc Natl Acad Sci USA. 1997;94:14626–14631. doi: 10.1073/pnas.94.26.14626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardoso A I, Sixt N, Vallier A, Fayolle J, Buckland R, Wild T F. Measles virus DNA vaccination: antibody isotype is determined by the method of immunization and by the nature of both the antigen and the coimmunized antigen. J Virol. 1998;72:2516–2518. doi: 10.1128/jvi.72.3.2516-2518.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis H L, Michel M L, Mancini M, Schleef M, Whalen R G. Direct gene transfer in skeletal muscle: plasmid DNA-based immunization against the hepatitis B virus surface antigen. Vaccine. 1994;12:1503–1509. doi: 10.1016/0264-410x(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 5.Donnelly J J, Ulmer J B, Shiver J W, Liu M A. DNA vaccines. Annu Rev Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 6.Doolan D L, Hoffman S L. IL-12 and NK cells are required for antigen-specific adaptive immunity against malaria initiated by CD8+ T cells in the Plasmodium yoelii model. J Immunol. 1999;163:884–892. [PubMed] [Google Scholar]
- 7.Doolan D L, Sedegah M, Hedstrom R C, Hobart P, Charoenvit Y, Hoffman S L. Circumventing genetic restriction of protection against malaria with multigene DNA immunization: CD8+ cell-, interferon gamma-, and nitric oxide-dependent immunity. J Exp Med. 1996;183:1739–1746. doi: 10.1084/jem.183.4.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dupre L, Poulain-Godefroy O, Ban E, Ivanoff N, Mekranfar M, Schacht A M, Capron A, Riveau G. Intradermal immunization of rats with plasmid DNA encoding Schistosoma mansoni 28 kDa glutathione S-transferase. Parasite Immunol. 1997;19:505–513. doi: 10.1046/j.1365-3024.1997.d01-163.x. [DOI] [PubMed] [Google Scholar]
- 9.FaQuim-Mauro E L, Coffman R L, Abrahamsohn I A, Macedo M S. Mouse IgG1 antibodies comprise two functionally distinct types that are differentially regulated by IL-4 and IL-12. J Immunol. 1999;163:3572–3576. [PubMed] [Google Scholar]
- 10.Feltquate D M, Heaney S, Webster R G, Robinson H L. Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization. J Immunol. 1997;158:2278–2284. [PubMed] [Google Scholar]
- 11.Fuller D H, Haynes J R. A qualitative progression in HIV type 1 glycoprotein 120-specific cytotoxic cellular and humoral immune responses in mice receiving a DNA-based glycoprotein 120 vaccine. AIDS Res Hum Retrovir. 1994;10:1433–1441. doi: 10.1089/aid.1994.10.1433. [DOI] [PubMed] [Google Scholar]
- 12.Fynan E F, Webster R G, Fuller D H, Haynes J R, Santoro J C, Robinson H L. DNA vaccines: protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc Natl Acad Sci USA. 1993;90:11478–11482. doi: 10.1073/pnas.90.24.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartl A, Kiesslich J, Weiss R, Bernhaupt A, Mostbock S, Scheiblhofer S, Ebner C, Ferreira F, Thalhamer J. Immune responses after immunization with plasmid DNA encoding Bet v 1, the major allergen of birch pollen. J Allergy Clin Immunol. 1999;103:107–113. doi: 10.1016/s0091-6749(99)70533-6. [DOI] [PubMed] [Google Scholar]
- 14.Klinman D M, Yi A K, Beaucage S L, Conover J, Krieg A M. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci USA. 1996;93:2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krieg A M, Yi A K, Matson S, Waldschmidt T J, Bishop G A, Teasdale R, Koretzky G A, Klinman D M. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 16.Leitner W W, Ying H, Restifo N P. DNA and RNA-based vaccines: principles, progress and prospects. Vaccine. 1999;18:765–777. doi: 10.1016/s0264-410x(99)00271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leitner W W, Seguin M C, Ballou W R, Seitz J P, Schultz A M, Sheehy M J, Lyon J A. Immune responses induced by intramuscular or gene gun injection of protective deoxyribonucleic acid vaccines that express the circumsporozoite protein from Plasmodium berghei malaria parasites. J Immunol. 1997;159:6112–6119. [PubMed] [Google Scholar]
- 18.Migliorini P, Betschart B, Corradin G. Malaria vaccine: immunization of mice with a synthetic T cell helper epitope alone leads to protective immunity. Eur J Immunol. 1993;23:582–585. doi: 10.1002/eji.1830230245. [DOI] [PubMed] [Google Scholar]
- 19.Mor G, Klinman D M, Shapiro S, Hagiwara E, Sedegah M, Norman J A, Hoffman S L, Steinberg A D. Complexity of the cytokine and antibody response elicited by immunizing mice with Plasmodium yoelii circumsporozoite protein plasmid DNA. J Immunol. 1995;155:2039–2046. [PubMed] [Google Scholar]
- 20.Perlmann P, Perlmann H, El Ghazali G, Blomberg M T. IgE and tumor necrosis factor in malaria infection. Immunol Lett. 1999;65:29–33. doi: 10.1016/s0165-2478(98)00120-5. [DOI] [PubMed] [Google Scholar]
- 21.Pertmer T M, Roberts T R, Haynes J R. Influenza virus nucleoprotein-specific immunoglobulin G subclass and cytokine responses elicited by DNA vaccination are dependent on the route of vector DNA delivery. J Virol. 1996;70:6119–6125. doi: 10.1128/jvi.70.9.6119-6125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raz E, Tighe H, Sato Y, Corr M, Dudler J A, Roman M, Swain S L, Spiegelberg H L, Carson D A. Preferential induction of a Th1 immune response and inhibition of specific IgE antibody formation by plasmid DNA immunization. Proc Natl Acad Sci USA. 1996;93:5141–5145. doi: 10.1073/pnas.93.10.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Renggli J, Hahne M, Matile H, Betschart B, Tschopp J, Corradin G. Elimination of P. berghei liver stages is independent of Fas (CD95/Apo-I) or perforin-mediated cytotoxicity. Parasite Immunol. 1997;19:145–148. doi: 10.1046/j.1365-3024.1997.d01-190.x. [DOI] [PubMed] [Google Scholar]
- 24.Rodrigues E G, Claassen J, Lee S, Wilson J M, Nussenzweig R S, Tsuji M. Interferon-gamma-independent CD8+ T cell-mediated protective anti-malaria immunity elicited by recombinant adenovirus. Parasite Immunol. 2000;22:157–160. doi: 10.1046/j.1365-3024.2000.00289.x. [DOI] [PubMed] [Google Scholar]
- 25.Sato Y, Roman M, Tighe H, Lee D, Corr M, Nguyen M D, Silverman G J, Lotz M, Carson D A, Raz E. Immunostimulatory DNA sequences necessary for effective intradermal gene immunization. Science. 1996;273:352–354. doi: 10.1126/science.273.5273.352. [DOI] [PubMed] [Google Scholar]
- 26.Schneider J, Gilbert S C, Blanchard T J, Hanke T, Robson K J, Hannan C M, Becker M, Sinden R, Smith G L, Hill A V. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat Med. 1998;4:397–402. doi: 10.1038/nm0498-397. [DOI] [PubMed] [Google Scholar]
- 27.Schofield L, Villaquiran J, Ferreira A, Schellekens H, Nussenzweig R, Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987;330:664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- 28.Sedegah M, Hedstrom R, Hobart P, Hoffman S L. Protection against malaria by immunization with plasmid DNA encoding circumsporozoite protein. Proc Natl Acad Sci USA. 1994;91:9866–9870. doi: 10.1073/pnas.91.21.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sedegah M, Jones T R, Kaur M, Hedstrom R, Hobart P, Tine J A, Hoffman S L. Boosting with recombinant vaccinia increases immunogenicity and protective efficacy of malaria DNA vaccine. Proc Natl Acad Sci USA. 1998;95:7648–7653. doi: 10.1073/pnas.95.13.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tacket C O, Roy M J, Widera G, Swain W F, Broome S, Edelman R. Phase 1 safety and immune response studies of a DNA vaccine encoding hepatitis B surface antigen delivered by a gene delivery device. Vaccine. 1999;17:2826–2829. doi: 10.1016/s0264-410x(99)00094-8. [DOI] [PubMed] [Google Scholar]
- 31.Ulmer J B, Donnelly J J, Parker S E, Rhodes G H, Felgner P L, Dwarki V J, Gromkowski S H, Deck R R, DeWitt C M, Friedman A, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 32.Vinner L, Nielsen H V, Bryder K, Corbet S, Nielsen C, Fomsgaard A. Gene gun DNA vaccination with Rev-independent synthetic HIV-1 gp160 envelope gene using mammalian codons. Vaccine. 1999;17:2166–2175. doi: 10.1016/s0264-410x(98)00474-5. [DOI] [PubMed] [Google Scholar]
- 33.Wang B, Ugen K E, Srikantan V, Agadjanyan M G, Dang K, Refaeli Y, Sato A I, Boyer J, Williams W V, Weiner D B. Gene inoculation generates immune responses against human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1993;90:4156–4160. doi: 10.1073/pnas.90.9.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang R, Doolan D L, Le T P, Hedstrom R C, Coonan K M, Charoenvit Y, Jones T R, Hobart P, Margalith M, Ng J, Weiss W R, Sedegah M, de Taisne C, Norman J A, Hoffman S L. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science. 1998;282:476–480. doi: 10.1126/science.282.5388.476. [DOI] [PubMed] [Google Scholar]
- 35.Weiss W R, Ishii K J, Hedstrom R C, Sedegah M, Ichino M, Barnhart K, Klinman D M, Hoffman S L. A plasmid encoding murine granulocyte-macrophage colony-stimulating factor increases protection conferred by a malaria DNA vaccine. J Immunol. 1998;161:2325–2332. [PubMed] [Google Scholar]
- 36.White K L, Snyder H L, Krzych U. MHC class I-dependent presentation of exoerythrocytic antigens to CD8+ T lymphocytes is required for protective immunity against Plasmodium berghei. J Immunol. 1996;156:3374–3381. [PubMed] [Google Scholar]